article_id
stringlengths 6
7
| url
stringlengths 19
20
| article_data
listlengths 1
7
| questions
listlengths 0
5
|
|---|---|---|---|
863054 | /viewarticle/863054 | [
{
"authors": "Heather Kesler DeVore, MD; Ali Nawaz Khan, MBBS, FRCS, FRCP, FRCR, LRCP; Sirhan Alvi, MBChB, MRCS(Ed), MRCS(Glasg)",
"content": [
"Editor's Note:\n\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.",
"A 42-year-old Nigerian woman, born and raised in West Africa, recently moved to Manchester, United Kingdom, where she is to undergo a routine but extensive evaluation for a renal transplant. The patient has a history of end-stage renal disease secondary to chronic glomerulonephritis. She is currently on hemodialysis but otherwise has no other active medical conditions. At the request of her nephrologist, she has presented to an outpatient breast clinic to receive a baseline examination and screening mammography.",
"On a review of systems focused on the imaging study to be performed, the patient denies having any weight loss, night sweats, or fevers. Additionally, she denies any tenderness in her breasts, nipple discharge, noticeable breast lumps, or other complaints related to her breasts. Her social history is significant for her previous work as a nurse in Nigeria, where she often treated people in small villages and on farms."
],
"date": "May 12, 2016",
"figures": [],
"markdown": "# A 42-Year-Old Woman Undergoing a Renal Transplant Evaluation\n\n **Authors:** Heather Kesler DeVore, MD; Ali Nawaz Khan, MBBS, FRCS, FRCP, FRCR, LRCP; Sirhan Alvi, MBChB, MRCS(Ed), MRCS(Glasg) \n **Date:** May 12, 2016\n\n ## Content\n\n Editor's Note:\n\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.\nA 42-year-old Nigerian woman, born and raised in West Africa, recently moved to Manchester, United Kingdom, where she is to undergo a routine but extensive evaluation for a renal transplant. The patient has a history of end-stage renal disease secondary to chronic glomerulonephritis. She is currently on hemodialysis but otherwise has no other active medical conditions. At the request of her nephrologist, she has presented to an outpatient breast clinic to receive a baseline examination and screening mammography.\nOn a review of systems focused on the imaging study to be performed, the patient denies having any weight loss, night sweats, or fevers. Additionally, she denies any tenderness in her breasts, nipple discharge, noticeable breast lumps, or other complaints related to her breasts. Her social history is significant for her previous work as a nurse in Nigeria, where she often treated people in small villages and on farms.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 42-Year-Old Woman Undergoing a Renal Transplant Evaluation"
},
{
"authors": "Heather Kesler DeVore, MD; Ali Nawaz Khan, MBBS, FRCS, FRCP, FRCR, LRCP; Sirhan Alvi, MBChB, MRCS(Ed), MRCS(Glasg)",
"content": [
"On physical examination, the patient's vital signs are noted to be normal, with a heart rate of 82 beats/min and a blood pressure of 125/67 mm Hg. Her breathing rate is 10 breaths/min, and she has a normal temperature of 98.2°F. She is a well-appearing woman in no apparent distress. Her lungs are clear, and the cardiac examination reveals a regular rate, without murmurs. The patient's breast examination shows symmetric breasts with no palpable lumps, skin changes, or nipple discharge. No palpable lymphadenopathy is observed. The findings on the rest of the physical examination are also unremarkable.",
"Figure.",
"Figure.",
"No laboratory investigations are performed as part of the baseline examination and screening mammography at the clinic; however, prior to her mammography, the patient underwent laboratory testing as part of her workup for the renal transplant, which revealed a blood urea nitrogen level of 56 mg/dL (20 mmol/L) and a baseline creatinine level of 6.2 mg/dL (548.1 µmol/L). She also has microcytic anemia, with a hemoglobin of 8.2 g/dL (82 g/L). The remainder of the metabolic panel and the complete blood count, as well as other laboratory investigations (including a coagulation profile and liver function panel), are normal.",
"The mammogram is obtained (see Figure)."
],
"date": "May 12, 2016",
"figures": [
{
"caption": "Figure.",
"image_url": "https://img.medscapestatic.com/article/863/054/863054-Thumb1.png"
}
],
"markdown": "# A 42-Year-Old Woman Undergoing a Renal Transplant Evaluation\n\n **Authors:** Heather Kesler DeVore, MD; Ali Nawaz Khan, MBBS, FRCS, FRCP, FRCR, LRCP; Sirhan Alvi, MBChB, MRCS(Ed), MRCS(Glasg) \n **Date:** May 12, 2016\n\n ## Content\n\n On physical examination, the patient's vital signs are noted to be normal, with a heart rate of 82 beats/min and a blood pressure of 125/67 mm Hg. Her breathing rate is 10 breaths/min, and she has a normal temperature of 98.2°F. She is a well-appearing woman in no apparent distress. Her lungs are clear, and the cardiac examination reveals a regular rate, without murmurs. The patient's breast examination shows symmetric breasts with no palpable lumps, skin changes, or nipple discharge. No palpable lymphadenopathy is observed. The findings on the rest of the physical examination are also unremarkable.\nFigure.\nFigure.\nNo laboratory investigations are performed as part of the baseline examination and screening mammography at the clinic; however, prior to her mammography, the patient underwent laboratory testing as part of her workup for the renal transplant, which revealed a blood urea nitrogen level of 56 mg/dL (20 mmol/L) and a baseline creatinine level of 6.2 mg/dL (548.1 µmol/L). She also has microcytic anemia, with a hemoglobin of 8.2 g/dL (82 g/L). The remainder of the metabolic panel and the complete blood count, as well as other laboratory investigations (including a coagulation profile and liver function panel), are normal.\nThe mammogram is obtained (see Figure).\n\n ## Figures\n\n **Figure.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 965309,
"choiceText": "Dracunculiasis",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 965311,
"choiceText": "Dirofilariasis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 965313,
"choiceText": "Onchocerciasis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 965315,
"choiceText": "Loiasis\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 305333,
"questionText": "What is the underlying etiology of the abnormalities noted on the mammogram?<br><br>\r\n<i>Hint: A more common location for this entity is in the lower extremity.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 42-Year-Old Woman Undergoing a Renal Transplant Evaluation"
},
{
"authors": "Heather Kesler DeVore, MD; Ali Nawaz Khan, MBBS, FRCS, FRCP, FRCR, LRCP; Sirhan Alvi, MBChB, MRCS(Ed), MRCS(Glasg)",
"content": [
"Dracunculiasis, or Guinea worm disease, results from infection by the nematode Dracunculus medinensis. The mammogram shows a coiled, whorled-type calcification in the subcutaneous tissues; this finding is characteristic of a dead Guinea worm. In 1986, more than 3.5 million cases of dracunculiasis occurred worldwide.[1] Ten years later, the worldwide annual incidence had declined significantly, with only 152,000 new cases annually, mostly occurring in Sudan. As of 2003, the US Centers for Disease Control and Prevention reported the annual incidence at fewer than 33,000 cases, again mostly originating in the Sudan.[2] This decline was a result of the Global Dracunculiasis Eradication Campaign. Dracunculiasis now occurs in only 13 countries in Africa, the Middle East, and in South Asia, including Nigeria, Cameroon, Ghana, Sudan, India, and Pakistan. Infected areas in Africa lie in a band between the Sahara and the equator.[1,2,3]",
"People acquire dracunculiasis by drinking fresh water contaminated with D medinensis larvae. Exposure can also occur from ingestion of fresh fruits or vegetables washed with contaminated water or from bathing or swimming in infected water. Small water fleas present in the water swallow the D medinensis larvae. The worms continue to mature within the flea. Humans contract the infection by ingesting water that is contaminated with these water fleas. Once inside the body, the stomach acid dissolves the water flea but not the Guinea worm. During the next year, the worms mature to adult size; they mate, and the male dies. At the end of that year, the female worms migrate toward the surface of the body, into the subcutaneous tissue. As a worm migrates, a blister develops on the skin above where the worm resides. The female adult worm eventually emerges from the blister, rupturing the skin. When an infected person comes into contact with water, exposed worms release a milky, white liquid containing millions of immature larvae; these larvae contaminate the water supply. Seasonal variation in exposure to the organism correlates with periods of increased exposure to contaminated water.[3]"
],
"date": "May 12, 2016",
"figures": [],
"markdown": "# A 42-Year-Old Woman Undergoing a Renal Transplant Evaluation\n\n **Authors:** Heather Kesler DeVore, MD; Ali Nawaz Khan, MBBS, FRCS, FRCP, FRCR, LRCP; Sirhan Alvi, MBChB, MRCS(Ed), MRCS(Glasg) \n **Date:** May 12, 2016\n\n ## Content\n\n Dracunculiasis, or Guinea worm disease, results from infection by the nematode Dracunculus medinensis. The mammogram shows a coiled, whorled-type calcification in the subcutaneous tissues; this finding is characteristic of a dead Guinea worm. In 1986, more than 3.5 million cases of dracunculiasis occurred worldwide.[1] Ten years later, the worldwide annual incidence had declined significantly, with only 152,000 new cases annually, mostly occurring in Sudan. As of 2003, the US Centers for Disease Control and Prevention reported the annual incidence at fewer than 33,000 cases, again mostly originating in the Sudan.[2] This decline was a result of the Global Dracunculiasis Eradication Campaign. Dracunculiasis now occurs in only 13 countries in Africa, the Middle East, and in South Asia, including Nigeria, Cameroon, Ghana, Sudan, India, and Pakistan. Infected areas in Africa lie in a band between the Sahara and the equator.[1,2,3]\nPeople acquire dracunculiasis by drinking fresh water contaminated with D medinensis larvae. Exposure can also occur from ingestion of fresh fruits or vegetables washed with contaminated water or from bathing or swimming in infected water. Small water fleas present in the water swallow the D medinensis larvae. The worms continue to mature within the flea. Humans contract the infection by ingesting water that is contaminated with these water fleas. Once inside the body, the stomach acid dissolves the water flea but not the Guinea worm. During the next year, the worms mature to adult size; they mate, and the male dies. At the end of that year, the female worms migrate toward the surface of the body, into the subcutaneous tissue. As a worm migrates, a blister develops on the skin above where the worm resides. The female adult worm eventually emerges from the blister, rupturing the skin. When an infected person comes into contact with water, exposed worms release a milky, white liquid containing millions of immature larvae; these larvae contaminate the water supply. Seasonal variation in exposure to the organism correlates with periods of increased exposure to contaminated water.[3]\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 965309,
"choiceText": "Dracunculiasis",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 965311,
"choiceText": "Dirofilariasis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 965313,
"choiceText": "Onchocerciasis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 965315,
"choiceText": "Loiasis\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 305333,
"questionText": "What is the underlying etiology of the abnormalities noted on the mammogram?<br><br>\r\n<i>Hint: A more common location for this entity is in the lower extremity.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 42-Year-Old Woman Undergoing a Renal Transplant Evaluation"
},
{
"authors": "Heather Kesler DeVore, MD; Ali Nawaz Khan, MBBS, FRCS, FRCP, FRCR, LRCP; Sirhan Alvi, MBChB, MRCS(Ed), MRCS(Glasg)",
"content": [
"Most cases involve worms appearing on the legs and feet, but the worms may occur anywhere on the body; cases involving the arms, breasts, head, and back have been well documented. Less commonly, patients with dracunculiasis can present with worms in other locations, such as the lungs, pancreas, testes, spinal cord, or periorbital tissue.[4,5]",
"A blister typically forms in the epidermis at the site chosen by the female worm to emerge, usually in the lower extremity. Just before blister formation, symptoms similar to an allergic reaction, such as mild respiratory distress with wheezing, urticaria, periorbital edema, and pruritus, may be noted. Affected individuals may also be febrile during this period. As the worm's head continues to emerge, the blister grows in size and becomes erythematous at its edges. The formation of edema around the site causes further pruritus and burning pain. The blister erupts (usually after a few days, although the eruption can occur after as long as 2 weeks), and the worm releases a milky fluid that is teeming with larvae. The swelling and pain will often decrease after the blister erupts. An ulcer forms around the blister site as the adult worm continues to emerge; the definitive diagnosis is often made at this stage, when the head of the worm is identifiable. No other commonly noted physical findings typically develop, although varying degrees of lymphadenopathy may be found at any stage of the illness.",
"The live Guinea worm cannot be identified radiologically, except in the rare instances when iodinated contrast medium is injected into the body of the worm to delineate its full extent; however, after it dies, the Guinea worm may become calcified from cell secretion or necrotic cellular debris. The female D medinensis worm appears as a long, stringlike, serpiginous calcification. The calcification is frequently segmented and beaded as muscle movements break up the body of the worm.",
"If the worm is in the breast, the calcifications may be intramammary, in and around the ducts, in the lobules, in the vascular structures, in interlobular connective tissue, or in the fat. They may also be found in the subcutaneous tissue of the skin. They can appear with or without an associated lesion, and their morphologies and distribution can provide clues to their etiology and to their association with benign or malignant processes. The incidence of breast calcification from Guinea worm infection is difficult to assess because dracunculiasis is rare outside of endemic areas. Cases of dracunculiasis are usually rural and not well documented; however, the breast is probably a relatively rare site of presentation."
],
"date": "May 12, 2016",
"figures": [],
"markdown": "# A 42-Year-Old Woman Undergoing a Renal Transplant Evaluation\n\n **Authors:** Heather Kesler DeVore, MD; Ali Nawaz Khan, MBBS, FRCS, FRCP, FRCR, LRCP; Sirhan Alvi, MBChB, MRCS(Ed), MRCS(Glasg) \n **Date:** May 12, 2016\n\n ## Content\n\n Most cases involve worms appearing on the legs and feet, but the worms may occur anywhere on the body; cases involving the arms, breasts, head, and back have been well documented. Less commonly, patients with dracunculiasis can present with worms in other locations, such as the lungs, pancreas, testes, spinal cord, or periorbital tissue.[4,5]\nA blister typically forms in the epidermis at the site chosen by the female worm to emerge, usually in the lower extremity. Just before blister formation, symptoms similar to an allergic reaction, such as mild respiratory distress with wheezing, urticaria, periorbital edema, and pruritus, may be noted. Affected individuals may also be febrile during this period. As the worm's head continues to emerge, the blister grows in size and becomes erythematous at its edges. The formation of edema around the site causes further pruritus and burning pain. The blister erupts (usually after a few days, although the eruption can occur after as long as 2 weeks), and the worm releases a milky fluid that is teeming with larvae. The swelling and pain will often decrease after the blister erupts. An ulcer forms around the blister site as the adult worm continues to emerge; the definitive diagnosis is often made at this stage, when the head of the worm is identifiable. No other commonly noted physical findings typically develop, although varying degrees of lymphadenopathy may be found at any stage of the illness.\nThe live Guinea worm cannot be identified radiologically, except in the rare instances when iodinated contrast medium is injected into the body of the worm to delineate its full extent; however, after it dies, the Guinea worm may become calcified from cell secretion or necrotic cellular debris. The female D medinensis worm appears as a long, stringlike, serpiginous calcification. The calcification is frequently segmented and beaded as muscle movements break up the body of the worm.\nIf the worm is in the breast, the calcifications may be intramammary, in and around the ducts, in the lobules, in the vascular structures, in interlobular connective tissue, or in the fat. They may also be found in the subcutaneous tissue of the skin. They can appear with or without an associated lesion, and their morphologies and distribution can provide clues to their etiology and to their association with benign or malignant processes. The incidence of breast calcification from Guinea worm infection is difficult to assess because dracunculiasis is rare outside of endemic areas. Cases of dracunculiasis are usually rural and not well documented; however, the breast is probably a relatively rare site of presentation.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 42-Year-Old Woman Undergoing a Renal Transplant Evaluation"
},
{
"authors": "Heather Kesler DeVore, MD; Ali Nawaz Khan, MBBS, FRCS, FRCP, FRCR, LRCP; Sirhan Alvi, MBChB, MRCS(Ed), MRCS(Glasg)",
"content": [
"Dracunculiasis typically has a low mortality rate, although significant morbidity may occur. Death occurring in this setting is not caused by the primary worm infestation but rather from a secondary bacterial infection at the worm's exit site that can lead to sepsis. Secondary infection of the lesions can be severe. The appearance of cellulitis or the formation of an abscess at the worm's exit site requires prompt attention. Morbidity also includes pain at the exit sites, which occurs frequently and can incapacitate patients for long periods of time, especially those patients with multiple worms (typically, individuals experience multiple worm extrusions simultaneously), as well as patients who rely on their ability to stand or walk for their livelihood.",
"Significant loss of productivity with a resultant negative socioeconomic burden on individuals and communities has been documented; for example, farmers with untreated dracunculiasis in Nigeria have been found to miss work for up to 3 months at a time. Another debilitating complication of dracunculiasis is the development of chronic pain and intermittent swelling of the extremities secondary to the calcified encapsulation of the adult worm upon its death. In a small percentage of individuals, permanent scarring or deformity of the lower extremity may occur.",
"Of related interest, the universal symbols of medicine (ie, the asklepios, the snake wrapped around a rod that is attributed the Greek god of healing and medicine, as well as the similar caduceus) are likely a representation of dracunculiasis and its treatment. To this day, accepted treatment remains the same. The adult Guinea worm is wrapped around a stick a few centimeters a day to coax it from a person's skin. Removal of the entire worm may take days to weeks. Metronidazole or thiabendazole may be used as an adjunct to stick therapy. These medications, however, have not been proven effective in controlled clinical trials and may be associated with aberrant migration of the worm; consequently, they should be used with caution. The worm may also be removed surgically, if such facilities are available. Diagnosis of dracunculiasis outside of endemic areas requires consultation with an infectious disease specialist and epidemiologic investigation to prevent additional cases.",
"Special thanks are extended to Dr J. Walls, MBChB, FRCS, for his contributions to the publication of this case."
],
"date": "May 12, 2016",
"figures": [],
"markdown": "# A 42-Year-Old Woman Undergoing a Renal Transplant Evaluation\n\n **Authors:** Heather Kesler DeVore, MD; Ali Nawaz Khan, MBBS, FRCS, FRCP, FRCR, LRCP; Sirhan Alvi, MBChB, MRCS(Ed), MRCS(Glasg) \n **Date:** May 12, 2016\n\n ## Content\n\n Dracunculiasis typically has a low mortality rate, although significant morbidity may occur. Death occurring in this setting is not caused by the primary worm infestation but rather from a secondary bacterial infection at the worm's exit site that can lead to sepsis. Secondary infection of the lesions can be severe. The appearance of cellulitis or the formation of an abscess at the worm's exit site requires prompt attention. Morbidity also includes pain at the exit sites, which occurs frequently and can incapacitate patients for long periods of time, especially those patients with multiple worms (typically, individuals experience multiple worm extrusions simultaneously), as well as patients who rely on their ability to stand or walk for their livelihood.\nSignificant loss of productivity with a resultant negative socioeconomic burden on individuals and communities has been documented; for example, farmers with untreated dracunculiasis in Nigeria have been found to miss work for up to 3 months at a time. Another debilitating complication of dracunculiasis is the development of chronic pain and intermittent swelling of the extremities secondary to the calcified encapsulation of the adult worm upon its death. In a small percentage of individuals, permanent scarring or deformity of the lower extremity may occur.\nOf related interest, the universal symbols of medicine (ie, the asklepios, the snake wrapped around a rod that is attributed the Greek god of healing and medicine, as well as the similar caduceus) are likely a representation of dracunculiasis and its treatment. To this day, accepted treatment remains the same. The adult Guinea worm is wrapped around a stick a few centimeters a day to coax it from a person's skin. Removal of the entire worm may take days to weeks. Metronidazole or thiabendazole may be used as an adjunct to stick therapy. These medications, however, have not been proven effective in controlled clinical trials and may be associated with aberrant migration of the worm; consequently, they should be used with caution. The worm may also be removed surgically, if such facilities are available. Diagnosis of dracunculiasis outside of endemic areas requires consultation with an infectious disease specialist and epidemiologic investigation to prevent additional cases.\nSpecial thanks are extended to Dr J. Walls, MBChB, FRCS, for his contributions to the publication of this case.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 965317,
"choiceText": "The disease is usually acquired by drinking contaminated water",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 965319,
"choiceText": "There is a seasonal variation in the rate of infection",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 965321,
"choiceText": "Small water fleas are the etiologic agent of the disease",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 965323,
"choiceText": "Exposure may occur by ingestion of fresh fruit washed in contaminated water",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 965325,
"choiceText": "Stomach acids are not able to digest Guinea worms when ingested\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "People acquire dracunculiasis by drinking fresh water contaminated with <i>D medinensis larvae</i>. Exposure can also occur from ingestion of fresh fruits or vegetables washed with contaminated water or from bathing or swimming in infected water. Small water fleas present in the water swallow the <i>D medinensis larvae</i>. The worms continue to mature within the flea. Humans contract the infection by ingesting water that is contaminated with these water fleas.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 305335,
"questionText": "Which of the following statements about Guinea worm disease is <i>not</i> true?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 965327,
"choiceText": "Secondary bacterial infection of lesions",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 965329,
"choiceText": "Intermittent swelling of the affected extremities",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 965331,
"choiceText": "Chronic pain",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 965333,
"choiceText": "Development of deep venous thrombosis",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 965335,
"choiceText": "Scarring and deformity of the affected extremities\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Another debilitating complication of dracunculiasis is the development of chronic pain and intermittent swelling of the extremities secondary to the calcified encapsulation of the adult worm upon its death. In a small percentage of individuals, permanent scarring or deformity of the lower extremity may occur.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 305337,
"questionText": "Which of the following morbidities is <i>not</i> associated with dracunculiasis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 42-Year-Old Woman Undergoing a Renal Transplant Evaluation"
},
{
"authors": "Heather Kesler DeVore, MD; Ali Nawaz Khan, MBBS, FRCS, FRCP, FRCR, LRCP; Sirhan Alvi, MBChB, MRCS(Ed), MRCS(Glasg)",
"content": [],
"date": "May 12, 2016",
"figures": [],
"markdown": "# A 42-Year-Old Woman Undergoing a Renal Transplant Evaluation\n\n **Authors:** Heather Kesler DeVore, MD; Ali Nawaz Khan, MBBS, FRCS, FRCP, FRCR, LRCP; Sirhan Alvi, MBChB, MRCS(Ed), MRCS(Glasg) \n **Date:** May 12, 2016\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 965317,
"choiceText": "The disease is usually acquired by drinking contaminated water",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 965319,
"choiceText": "There is a seasonal variation in the rate of infection",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 965321,
"choiceText": "Small water fleas are the etiologic agent of the disease",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 965323,
"choiceText": "Exposure may occur by ingestion of fresh fruit washed in contaminated water",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 965325,
"choiceText": "Stomach acids are not able to digest Guinea worms when ingested\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "People acquire dracunculiasis by drinking fresh water contaminated with <i>D medinensis larvae</i>. Exposure can also occur from ingestion of fresh fruits or vegetables washed with contaminated water or from bathing or swimming in infected water. Small water fleas present in the water swallow the <i>D medinensis larvae</i>. The worms continue to mature within the flea. Humans contract the infection by ingesting water that is contaminated with these water fleas.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 305335,
"questionText": "Which of the following statements about Guinea worm disease is <i>not</i> true?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 965327,
"choiceText": "Secondary bacterial infection of lesions",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 965329,
"choiceText": "Intermittent swelling of the affected extremities",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 965331,
"choiceText": "Chronic pain",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 965333,
"choiceText": "Development of deep venous thrombosis",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 965335,
"choiceText": "Scarring and deformity of the affected extremities\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Another debilitating complication of dracunculiasis is the development of chronic pain and intermittent swelling of the extremities secondary to the calcified encapsulation of the adult worm upon its death. In a small percentage of individuals, permanent scarring or deformity of the lower extremity may occur.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 305337,
"questionText": "Which of the following morbidities is <i>not</i> associated with dracunculiasis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 42-Year-Old Woman Undergoing a Renal Transplant Evaluation"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 965309,
"choiceText": "Dracunculiasis",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 965311,
"choiceText": "Dirofilariasis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 965313,
"choiceText": "Onchocerciasis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 965315,
"choiceText": "Loiasis\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 305333,
"questionText": "What is the underlying etiology of the abnormalities noted on the mammogram?<br><br>\r\n<i>Hint: A more common location for this entity is in the lower extremity.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 965317,
"choiceText": "The disease is usually acquired by drinking contaminated water",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 965319,
"choiceText": "There is a seasonal variation in the rate of infection",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 965321,
"choiceText": "Small water fleas are the etiologic agent of the disease",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 965323,
"choiceText": "Exposure may occur by ingestion of fresh fruit washed in contaminated water",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 965325,
"choiceText": "Stomach acids are not able to digest Guinea worms when ingested\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "People acquire dracunculiasis by drinking fresh water contaminated with <i>D medinensis larvae</i>. Exposure can also occur from ingestion of fresh fruits or vegetables washed with contaminated water or from bathing or swimming in infected water. Small water fleas present in the water swallow the <i>D medinensis larvae</i>. The worms continue to mature within the flea. Humans contract the infection by ingesting water that is contaminated with these water fleas.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 305335,
"questionText": "Which of the following statements about Guinea worm disease is <i>not</i> true?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 965327,
"choiceText": "Secondary bacterial infection of lesions",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 965329,
"choiceText": "Intermittent swelling of the affected extremities",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 965331,
"choiceText": "Chronic pain",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 965333,
"choiceText": "Development of deep venous thrombosis",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 965335,
"choiceText": "Scarring and deformity of the affected extremities\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Another debilitating complication of dracunculiasis is the development of chronic pain and intermittent swelling of the extremities secondary to the calcified encapsulation of the adult worm upon its death. In a small percentage of individuals, permanent scarring or deformity of the lower extremity may occur.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 305337,
"questionText": "Which of the following morbidities is <i>not</i> associated with dracunculiasis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
863053 | /viewarticle/863053 | [
{
"authors": "Juan Carlos Munoz, MD; William J. Salyers, Jr, MD, MPH",
"content": [
"Editor's Note:\n\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.",
"A 47-year-old man with a history of alcohol-induced chronic pancreatitis presents to the emergency department (ED) complaining of a constellation of symptoms, including lightheadedness, fatigue, vague abdominal discomfort, and profuse hematochezia. The patient states that he has been intermittently having loose, \"dark-colored\" stools over the past 2 weeks. He was previously discharged from the hospital 4 weeks ago following management of a communicating pancreatic pseudocyst, with pancreatic duct stent placement via endoscopic retrograde cholangiopancreatography (ERCP) and CT-guided percutaneous drainage of the pseudocyst.",
"His medical history is significant only for chronic pancreatitis complicated by pseudocyst formation. He admits to prior heavy alcohol abuse but also reports abstinence for the past 4 years. The patient's medications at the time of admission include acetaminophen/hydrocodone, metoclopramide, pancrelipase, lansoprazole, promethazine, and octreotide 100 mcg subcutaneously three times per day. He denies any recent nonsteroidal anti-inflammatory drug use."
],
"date": "May 11, 2016",
"figures": [],
"markdown": "# A 47-Year-Old Man With a History of Alcohol-Induced Chronic Pancreatitis\n\n **Authors:** Juan Carlos Munoz, MD; William J. Salyers, Jr, MD, MPH \n **Date:** May 11, 2016\n\n ## Content\n\n Editor's Note:\n\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.\nA 47-year-old man with a history of alcohol-induced chronic pancreatitis presents to the emergency department (ED) complaining of a constellation of symptoms, including lightheadedness, fatigue, vague abdominal discomfort, and profuse hematochezia. The patient states that he has been intermittently having loose, \"dark-colored\" stools over the past 2 weeks. He was previously discharged from the hospital 4 weeks ago following management of a communicating pancreatic pseudocyst, with pancreatic duct stent placement via endoscopic retrograde cholangiopancreatography (ERCP) and CT-guided percutaneous drainage of the pseudocyst.\nHis medical history is significant only for chronic pancreatitis complicated by pseudocyst formation. He admits to prior heavy alcohol abuse but also reports abstinence for the past 4 years. The patient's medications at the time of admission include acetaminophen/hydrocodone, metoclopramide, pancrelipase, lansoprazole, promethazine, and octreotide 100 mcg subcutaneously three times per day. He denies any recent nonsteroidal anti-inflammatory drug use.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 47-Year-Old Man With a History of Alcohol-Induced Chronic Pancreatitis"
},
{
"authors": "Juan Carlos Munoz, MD; William J. Salyers, Jr, MD, MPH",
"content": [
"Figure 1.",
"On physical examination, his oral temperature is 98.6°F (37°C), his pulse is regular but tachycardic at a rate of 107 beats/min, and his blood pressure is low, at 69/37 mm Hg. Significant orthostatic changes are noted in the patient's pulse and blood pressure. The patient is tachypneic, with a respiratory rate of 24 breaths/min, but he is not in any acute respiratory distress. At the time of initial evaluation, he appears pale and weak, and dry oral mucosa is noted. The examination of the head and neck is normal except for pale conjunctiva. His lungs are clear to auscultation. The cardiac evaluation reveals tachycardia, with normal S1 and S2 heart sounds.",
"The abdominal examination is significant for mild epigastric and left upper quadrant tenderness, without rebound or guarding. He is noted to have mild abdominal distension as well as hyperactive bowel sounds. The patient's peripheral arterial pulses in the upper and lower extremities are poorly palpable but equal. A rectal examination reveals dark-red stool in the rectum. The rest of the physical examination is unremarkable, except for external hemorrhoids that are not actively bleeding or thrombosed.",
"The initial workup includes normal chest x-ray and electrocardiography findings that only reveal sinus tachycardia. Laboratory testing on admission reveals a hemoglobin level of 3.9 g/dL (39 g/L; the patient's last hemoglobin test, which was performed 4 weeks ago, was 14.9 g/dL [149 g/L]). His platelet count is measured at 389 × 103/μL (389 × 109/L), prothrombin time is 1.3 seconds, and his partial thromboplastin time is 26 seconds. Values obtained on measurement of the patient's liver enzymes are within normal limits.",
"The patient is treated in the ED with intravenous fluids, packed red blood cell transfusion, and intravenous proton pump inhibitors. A gastroenterologist is consulted for endoscopic evaluation. As a result of profuse bleeding, however, no endoscopy is performed. The patient is instead scheduled for a technetium Tc 99m-labeled red blood cell scintigraphy, which reveals bleeding from the second part of the duodenum. The patient is stabilized hemodynamically, and another transfusion is performed. He is subsequently able to undergo the upper endoscopy.",
"The image seen in the Figure is recorded at the level of the ampulla of Vater."
],
"date": "May 11, 2016",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/863/053/863053-Thumb1.png"
}
],
"markdown": "# A 47-Year-Old Man With a History of Alcohol-Induced Chronic Pancreatitis\n\n **Authors:** Juan Carlos Munoz, MD; William J. Salyers, Jr, MD, MPH \n **Date:** May 11, 2016\n\n ## Content\n\n Figure 1.\nOn physical examination, his oral temperature is 98.6°F (37°C), his pulse is regular but tachycardic at a rate of 107 beats/min, and his blood pressure is low, at 69/37 mm Hg. Significant orthostatic changes are noted in the patient's pulse and blood pressure. The patient is tachypneic, with a respiratory rate of 24 breaths/min, but he is not in any acute respiratory distress. At the time of initial evaluation, he appears pale and weak, and dry oral mucosa is noted. The examination of the head and neck is normal except for pale conjunctiva. His lungs are clear to auscultation. The cardiac evaluation reveals tachycardia, with normal S1 and S2 heart sounds.\nThe abdominal examination is significant for mild epigastric and left upper quadrant tenderness, without rebound or guarding. He is noted to have mild abdominal distension as well as hyperactive bowel sounds. The patient's peripheral arterial pulses in the upper and lower extremities are poorly palpable but equal. A rectal examination reveals dark-red stool in the rectum. The rest of the physical examination is unremarkable, except for external hemorrhoids that are not actively bleeding or thrombosed.\nThe initial workup includes normal chest x-ray and electrocardiography findings that only reveal sinus tachycardia. Laboratory testing on admission reveals a hemoglobin level of 3.9 g/dL (39 g/L; the patient's last hemoglobin test, which was performed 4 weeks ago, was 14.9 g/dL [149 g/L]). His platelet count is measured at 389 × 103/μL (389 × 109/L), prothrombin time is 1.3 seconds, and his partial thromboplastin time is 26 seconds. Values obtained on measurement of the patient's liver enzymes are within normal limits.\nThe patient is treated in the ED with intravenous fluids, packed red blood cell transfusion, and intravenous proton pump inhibitors. A gastroenterologist is consulted for endoscopic evaluation. As a result of profuse bleeding, however, no endoscopy is performed. The patient is instead scheduled for a technetium Tc 99m-labeled red blood cell scintigraphy, which reveals bleeding from the second part of the duodenum. The patient is stabilized hemodynamically, and another transfusion is performed. He is subsequently able to undergo the upper endoscopy.\nThe image seen in the Figure is recorded at the level of the ampulla of Vater.\n\n ## Figures\n\n **Figure 1.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 964611,
"choiceText": "Peptic ulcer disease",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 964613,
"choiceText": "Esophageal varices",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 964615,
"choiceText": "Hemosuccus pancreaticus",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 964617,
"choiceText": "Dieulafoy lesion",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 305105,
"questionText": "What is the diagnosis?<br/><br/>\r\n<em>Hint: Note the history of chronic pancreatitis with pancreatic pseudocyst and intermittent melena.</em>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 47-Year-Old Man With a History of Alcohol-Induced Chronic Pancreatitis"
},
{
"authors": "Juan Carlos Munoz, MD; William J. Salyers, Jr, MD, MPH",
"content": [
"Figure 1.",
"The diagnosis of hemosuccus pancreaticus was made at the time the upper endoscopy was performed following hemodynamic stabilization of the patient. Using a side-viewing endoscope, active bleeding from the ampulla of Vater was visualized (see Figure). In the clinical setting of chronic pancreatitis with a large communicating pancreatic pseudocyst following the recent ERCP, this finding established the diagnosis.",
"Hemosuccus pancreaticus, also known as wirsungorrhagia or pseudohemobilia,[1] is a rare syndrome of bleeding into the pancreatic duct manifested by blood loss through the ampulla of Vater. The first case was described in 1931 by Lower and Ferrell[2]; and, in 1969, Vankemmel proposed the term \"wirsungorrhagia\" (currently used in France).[3] In 1970, Sandblom published 3 cases and coined the term \"hemosuccus pancreaticus\" to describe the similarity of the disorder to the clinical syndrome of hemobilia.[4]",
"Overall, hemosuccus pancreaticus is a rare clinical entity with a frequency of only 1 out of 1500 gastrointestinal (GI) bleeding cases, and less than 100 cases have been reported in the medical literature.[3,5] It most commonly occurs in the setting of chronic pancreatitis with and without pancreatic pseudocysts. It is also seen with acute pancreatitis, neuroendocrine tumors, ectopic pancreas, pancreas divisum, and pancreatolithiasis, as well as being reported as a complication of ERCP and following traumatic abdominal pseudoaneurysm formation.[3,6,7] Hemosuccus pancreaticus usually develops following the rupture of an aneurysm or pseudoaneurysm, which develops in the setting of both pressure necrosis and autodigestion from pancreatic enzymes that lead to progressive vessel wall thinning.[8,9] The splenic artery is most commonly affected (60%-65% of cases), followed by the gastroduodenal artery.[9] Pancreaticoduodenal artery involvement occurs in only 10%-15% of cases, with hepatic artery and left gastric artery involvement also having been reported.[9] Mortality rates as high as 57% have been reported with pseudocyst-associated rupture of pseudoaneurysms.[9]"
],
"date": "May 11, 2016",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/863/053/863053-Thumb1.png"
}
],
"markdown": "# A 47-Year-Old Man With a History of Alcohol-Induced Chronic Pancreatitis\n\n **Authors:** Juan Carlos Munoz, MD; William J. Salyers, Jr, MD, MPH \n **Date:** May 11, 2016\n\n ## Content\n\n Figure 1.\nThe diagnosis of hemosuccus pancreaticus was made at the time the upper endoscopy was performed following hemodynamic stabilization of the patient. Using a side-viewing endoscope, active bleeding from the ampulla of Vater was visualized (see Figure). In the clinical setting of chronic pancreatitis with a large communicating pancreatic pseudocyst following the recent ERCP, this finding established the diagnosis.\nHemosuccus pancreaticus, also known as wirsungorrhagia or pseudohemobilia,[1] is a rare syndrome of bleeding into the pancreatic duct manifested by blood loss through the ampulla of Vater. The first case was described in 1931 by Lower and Ferrell[2]; and, in 1969, Vankemmel proposed the term \"wirsungorrhagia\" (currently used in France).[3] In 1970, Sandblom published 3 cases and coined the term \"hemosuccus pancreaticus\" to describe the similarity of the disorder to the clinical syndrome of hemobilia.[4]\nOverall, hemosuccus pancreaticus is a rare clinical entity with a frequency of only 1 out of 1500 gastrointestinal (GI) bleeding cases, and less than 100 cases have been reported in the medical literature.[3,5] It most commonly occurs in the setting of chronic pancreatitis with and without pancreatic pseudocysts. It is also seen with acute pancreatitis, neuroendocrine tumors, ectopic pancreas, pancreas divisum, and pancreatolithiasis, as well as being reported as a complication of ERCP and following traumatic abdominal pseudoaneurysm formation.[3,6,7] Hemosuccus pancreaticus usually develops following the rupture of an aneurysm or pseudoaneurysm, which develops in the setting of both pressure necrosis and autodigestion from pancreatic enzymes that lead to progressive vessel wall thinning.[8,9] The splenic artery is most commonly affected (60%-65% of cases), followed by the gastroduodenal artery.[9] Pancreaticoduodenal artery involvement occurs in only 10%-15% of cases, with hepatic artery and left gastric artery involvement also having been reported.[9] Mortality rates as high as 57% have been reported with pseudocyst-associated rupture of pseudoaneurysms.[9]\n\n ## Figures\n\n **Figure 1.** \n \n\n\n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 964611,
"choiceText": "Peptic ulcer disease",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 964613,
"choiceText": "Esophageal varices",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 964615,
"choiceText": "Hemosuccus pancreaticus",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 964617,
"choiceText": "Dieulafoy lesion",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 305105,
"questionText": "What is the diagnosis?<br/><br/>\r\n<em>Hint: Note the history of chronic pancreatitis with pancreatic pseudocyst and intermittent melena.</em>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 47-Year-Old Man With a History of Alcohol-Induced Chronic Pancreatitis"
},
{
"authors": "Juan Carlos Munoz, MD; William J. Salyers, Jr, MD, MPH",
"content": [
"Establishing the diagnosis requires clinical suspicion in patients with a medical history of chronic pancreatitis who present with GI bleeding and severe anemia. This may be manifested primarily as intermittent melena without associated hematemesis, although frank hematochezia may occur.[6,10] More insidious presentations have been described with anemia and vague abdominal discomfort, which may indicate intraperitoneal bleeding and/or bleeding within the pseudocyst. Other exceptional forms of presentation include jaundice, nausea with and without vomiting, and a palpable pulsating mass.[2,3,4]",
"The differential diagnosis of hemosuccus pancreaticus is broad and includes other causes of acute upper GI bleeding. Depending on the clinical presentation of the individual patient, other considerations include peptic ulcer disease, esophageal varices, arteriovenous malformations, Mallory-Weiss tears, and tumors. Because bleeding may be intermittent with an initial endoscopic evaluation, relatively obscure causes may also be included in the differential, such as Dieulafoy lesion, aortoenteric fistula, and true hemobilia of biliary origin.[8]",
"Following hemodynamic stabilization of the patient, the initial workup should be aimed at identifying the source of bleeding. Esophagogastroduodenoscopy (EGD) can rule out other causes of upper GI bleeding and may identify the presence of blood clots in the duodenum in the setting of pseudohemobilia[8]; however, active bleeding from the ampulla of Vater is rarely seen because of the intermittent nature of the bleeding. Use of a side-viewing endoscope may help with the visualization of active bleeding from the ampulla of Vater. CT scanning, CT angiography, MRI, and magnetic resonance angiography (MRA) may provide information regarding the presence of a fistula between a peripancreatic aneurysm or pseudoaneurysm and the pancreatic duct, as well as identify the presence of a \"sentinel clot\"[11] (focal, high-density clotted blood) in the pancreatic duct during episodes of intermittent bleeding.[12]",
"Doppler studies performed percutaneously or by endoscopic ultrasonography may be useful in identifying the presence of pancreatic pseudocysts as well as any aneurysmal mass. ERCP may demonstrate the presence of clots in the pancreatic duct as well as pancreatic duct dilation and pseudocyst filling, if present. Finally, a pancreatoscopy can be performed using a mother-daughter system endoscope in select centers. Technetium Tc 99m-labeled red blood cell scintigraphy may help identify the location of the bleeding during periods of active bleeding.[12]",
"Angiography is potentially useful as a part of early diagnostic and therapeutic management strategies, especially in the setting of significant GI bleeding of obscure origin (which is typical in the setting of hemosuccus pancreaticus).[12] Selective angiography of the celiac trunk and the superior mesenteric artery allows for characterization of the anatomic origin of a hemorrhage, as well as identification of any aneurysms or pseudoaneurysms that may be present. It also allows for therapeutic intervention with gel foam or coil embolization of the involved arterial segments.[6,10,12] Additionally, interventional radiologic therapy with the use of a bare metal stent across a splenic artery aneurysm has been described.[10]"
],
"date": "May 11, 2016",
"figures": [],
"markdown": "# A 47-Year-Old Man With a History of Alcohol-Induced Chronic Pancreatitis\n\n **Authors:** Juan Carlos Munoz, MD; William J. Salyers, Jr, MD, MPH \n **Date:** May 11, 2016\n\n ## Content\n\n Establishing the diagnosis requires clinical suspicion in patients with a medical history of chronic pancreatitis who present with GI bleeding and severe anemia. This may be manifested primarily as intermittent melena without associated hematemesis, although frank hematochezia may occur.[6,10] More insidious presentations have been described with anemia and vague abdominal discomfort, which may indicate intraperitoneal bleeding and/or bleeding within the pseudocyst. Other exceptional forms of presentation include jaundice, nausea with and without vomiting, and a palpable pulsating mass.[2,3,4]\nThe differential diagnosis of hemosuccus pancreaticus is broad and includes other causes of acute upper GI bleeding. Depending on the clinical presentation of the individual patient, other considerations include peptic ulcer disease, esophageal varices, arteriovenous malformations, Mallory-Weiss tears, and tumors. Because bleeding may be intermittent with an initial endoscopic evaluation, relatively obscure causes may also be included in the differential, such as Dieulafoy lesion, aortoenteric fistula, and true hemobilia of biliary origin.[8]\nFollowing hemodynamic stabilization of the patient, the initial workup should be aimed at identifying the source of bleeding. Esophagogastroduodenoscopy (EGD) can rule out other causes of upper GI bleeding and may identify the presence of blood clots in the duodenum in the setting of pseudohemobilia[8]; however, active bleeding from the ampulla of Vater is rarely seen because of the intermittent nature of the bleeding. Use of a side-viewing endoscope may help with the visualization of active bleeding from the ampulla of Vater. CT scanning, CT angiography, MRI, and magnetic resonance angiography (MRA) may provide information regarding the presence of a fistula between a peripancreatic aneurysm or pseudoaneurysm and the pancreatic duct, as well as identify the presence of a \"sentinel clot\"[11] (focal, high-density clotted blood) in the pancreatic duct during episodes of intermittent bleeding.[12]\nDoppler studies performed percutaneously or by endoscopic ultrasonography may be useful in identifying the presence of pancreatic pseudocysts as well as any aneurysmal mass. ERCP may demonstrate the presence of clots in the pancreatic duct as well as pancreatic duct dilation and pseudocyst filling, if present. Finally, a pancreatoscopy can be performed using a mother-daughter system endoscope in select centers. Technetium Tc 99m-labeled red blood cell scintigraphy may help identify the location of the bleeding during periods of active bleeding.[12]\nAngiography is potentially useful as a part of early diagnostic and therapeutic management strategies, especially in the setting of significant GI bleeding of obscure origin (which is typical in the setting of hemosuccus pancreaticus).[12] Selective angiography of the celiac trunk and the superior mesenteric artery allows for characterization of the anatomic origin of a hemorrhage, as well as identification of any aneurysms or pseudoaneurysms that may be present. It also allows for therapeutic intervention with gel foam or coil embolization of the involved arterial segments.[6,10,12] Additionally, interventional radiologic therapy with the use of a bare metal stent across a splenic artery aneurysm has been described.[10]\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 47-Year-Old Man With a History of Alcohol-Induced Chronic Pancreatitis"
},
{
"authors": "Juan Carlos Munoz, MD; William J. Salyers, Jr, MD, MPH",
"content": [
"Although specific management of bleeding primarily involves interventional radiologic therapies, surgical intervention must be considered if less invasive strategies are unsuccessful at controlling bleeding. Surgical management includes arterial ligation of involved vessels as well as resection of the pancreatic head or tail and pseudocysts. Also, aneurysm resection with possible splenectomy may be indicated in cases of splenic artery aneurysms.[6,10,12] Additionally, both intraoperative ultrasonography and pancreatoscopy have been used in identifying the origin of bleeding during surgery.[6]",
"Selective angiography was performed on the patient in this case. The angiography revealed active arterial extravasation arising from the pancreaticoduodenal arcade. Coil embolization of the gastroduodenal artery was performed successfully. Follow-up images demonstrated excellent results, with significant stagnation of flow in the gastroduodenal artery and segmental branches without any significant arterial blush noted. Repeat angiography performed the following day demonstrated no further bleeding. The bleeding in this case was caused by vessel rupture within the pancreaticoduodenal arcade, most likely associated with the large pancreatic pseudocyst in the setting of chronic pancreatitis.",
"Although recent pseudocyst management may have played some role in the development of pseudohemobilia, the extent that each intervention contributed to the patient's presentation is uncertain. The patient remained clinically stable throughout the remainder of his hospital course and was discharged to home with continued outpatient follow-up of his chronic pancreatitis."
],
"date": "May 11, 2016",
"figures": [],
"markdown": "# A 47-Year-Old Man With a History of Alcohol-Induced Chronic Pancreatitis\n\n **Authors:** Juan Carlos Munoz, MD; William J. Salyers, Jr, MD, MPH \n **Date:** May 11, 2016\n\n ## Content\n\n Although specific management of bleeding primarily involves interventional radiologic therapies, surgical intervention must be considered if less invasive strategies are unsuccessful at controlling bleeding. Surgical management includes arterial ligation of involved vessels as well as resection of the pancreatic head or tail and pseudocysts. Also, aneurysm resection with possible splenectomy may be indicated in cases of splenic artery aneurysms.[6,10,12] Additionally, both intraoperative ultrasonography and pancreatoscopy have been used in identifying the origin of bleeding during surgery.[6]\nSelective angiography was performed on the patient in this case. The angiography revealed active arterial extravasation arising from the pancreaticoduodenal arcade. Coil embolization of the gastroduodenal artery was performed successfully. Follow-up images demonstrated excellent results, with significant stagnation of flow in the gastroduodenal artery and segmental branches without any significant arterial blush noted. Repeat angiography performed the following day demonstrated no further bleeding. The bleeding in this case was caused by vessel rupture within the pancreaticoduodenal arcade, most likely associated with the large pancreatic pseudocyst in the setting of chronic pancreatitis.\nAlthough recent pseudocyst management may have played some role in the development of pseudohemobilia, the extent that each intervention contributed to the patient's presentation is uncertain. The patient remained clinically stable throughout the remainder of his hospital course and was discharged to home with continued outpatient follow-up of his chronic pancreatitis.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 964619,
"choiceText": "Hemosuccus pancreaticus usually occurs in untreated acute pancreatitis and is less commonly seen in patients with chronic pancreatitis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 964621,
"choiceText": "The pancreaticoduodenal artery is the most common site for aneurysms/pseudoaneurysms associated with bleeding in cases of hemosuccus pancreaticus",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 964623,
"choiceText": "In cases of hemosuccus pancreaticus, active bleeding from the ampulla of Vater is rarely seen because of the intermittent nature of the bleeding",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 964625,
"choiceText": "Management of hemosuccus pancreaticus is typically surgical, as conservative measures are rarely successful",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "EGD can rule out other causes of upper GI bleeding and may identify the presence of blood clots in the duodenum in the setting of pseudohemobilia<sup type=\"ref\">[8]</sup>; however, active bleeding from the ampulla of Vater is rarely seen because of the intermittent nature of the bleeding. Use of a side-viewing endoscope may help with the visualization of active bleeding from the ampulla of Vater. CT scanning, CT angiography, MRI, and MRA may provide information regarding the presence of a fistula between a peripancreatic aneurysm or pseudoaneurysm and the pancreatic duct, as well as identify the presence of a \"sentinel clot\"<sup type=\"ref\">[11]</sup> (focal, high-density clotted blood) in the pancreatic duct during episodes of intermittent bleeding.<sup type=\"ref\">[12]</sup>",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 305107,
"questionText": "You are evaluating a patient with hematochezia and are concerned about the possibility of hemosuccus pancreaticus. Which of the following statements regarding hemosuccus pancreaticus is true?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 964637,
"choiceText": "Chronic hepatitis C with hepatic cirrhosis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 964639,
"choiceText": "Chronic nonsteroidal anti-inflammatory drug use",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 964641,
"choiceText": "Chronic pancreatitis",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 964643,
"choiceText": "<em>Helicobacter pylori</em> infection",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Establishing the diagnosis requires clinical suspicion in patients with a medical history of chronic pancreatitis who present with GI bleeding and severe anemia.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 305111,
"questionText": "Your patient is diagnosed with hemosuccus pancreaticus with an upper GI endoscopy followed by angiographic confirmation. Which of the following conditions is most commonly associated with upper GI bleeding resulting from hemosuccus pancreaticus?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 47-Year-Old Man With a History of Alcohol-Induced Chronic Pancreatitis"
},
{
"authors": "Juan Carlos Munoz, MD; William J. Salyers, Jr, MD, MPH",
"content": [],
"date": "May 11, 2016",
"figures": [],
"markdown": "# A 47-Year-Old Man With a History of Alcohol-Induced Chronic Pancreatitis\n\n **Authors:** Juan Carlos Munoz, MD; William J. Salyers, Jr, MD, MPH \n **Date:** May 11, 2016\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 964619,
"choiceText": "Hemosuccus pancreaticus usually occurs in untreated acute pancreatitis and is less commonly seen in patients with chronic pancreatitis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 964621,
"choiceText": "The pancreaticoduodenal artery is the most common site for aneurysms/pseudoaneurysms associated with bleeding in cases of hemosuccus pancreaticus",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 964623,
"choiceText": "In cases of hemosuccus pancreaticus, active bleeding from the ampulla of Vater is rarely seen because of the intermittent nature of the bleeding",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 964625,
"choiceText": "Management of hemosuccus pancreaticus is typically surgical, as conservative measures are rarely successful",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "EGD can rule out other causes of upper GI bleeding and may identify the presence of blood clots in the duodenum in the setting of pseudohemobilia<sup type=\"ref\">[8]</sup>; however, active bleeding from the ampulla of Vater is rarely seen because of the intermittent nature of the bleeding. Use of a side-viewing endoscope may help with the visualization of active bleeding from the ampulla of Vater. CT scanning, CT angiography, MRI, and MRA may provide information regarding the presence of a fistula between a peripancreatic aneurysm or pseudoaneurysm and the pancreatic duct, as well as identify the presence of a \"sentinel clot\"<sup type=\"ref\">[11]</sup> (focal, high-density clotted blood) in the pancreatic duct during episodes of intermittent bleeding.<sup type=\"ref\">[12]</sup>",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 305107,
"questionText": "You are evaluating a patient with hematochezia and are concerned about the possibility of hemosuccus pancreaticus. Which of the following statements regarding hemosuccus pancreaticus is true?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 964637,
"choiceText": "Chronic hepatitis C with hepatic cirrhosis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 964639,
"choiceText": "Chronic nonsteroidal anti-inflammatory drug use",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 964641,
"choiceText": "Chronic pancreatitis",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 964643,
"choiceText": "<em>Helicobacter pylori</em> infection",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Establishing the diagnosis requires clinical suspicion in patients with a medical history of chronic pancreatitis who present with GI bleeding and severe anemia.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 305111,
"questionText": "Your patient is diagnosed with hemosuccus pancreaticus with an upper GI endoscopy followed by angiographic confirmation. Which of the following conditions is most commonly associated with upper GI bleeding resulting from hemosuccus pancreaticus?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 47-Year-Old Man With a History of Alcohol-Induced Chronic Pancreatitis"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 964611,
"choiceText": "Peptic ulcer disease",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 964613,
"choiceText": "Esophageal varices",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 964615,
"choiceText": "Hemosuccus pancreaticus",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 964617,
"choiceText": "Dieulafoy lesion",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 305105,
"questionText": "What is the diagnosis?<br/><br/>\r\n<em>Hint: Note the history of chronic pancreatitis with pancreatic pseudocyst and intermittent melena.</em>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 964619,
"choiceText": "Hemosuccus pancreaticus usually occurs in untreated acute pancreatitis and is less commonly seen in patients with chronic pancreatitis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 964621,
"choiceText": "The pancreaticoduodenal artery is the most common site for aneurysms/pseudoaneurysms associated with bleeding in cases of hemosuccus pancreaticus",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 964623,
"choiceText": "In cases of hemosuccus pancreaticus, active bleeding from the ampulla of Vater is rarely seen because of the intermittent nature of the bleeding",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 964625,
"choiceText": "Management of hemosuccus pancreaticus is typically surgical, as conservative measures are rarely successful",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "EGD can rule out other causes of upper GI bleeding and may identify the presence of blood clots in the duodenum in the setting of pseudohemobilia<sup type=\"ref\">[8]</sup>; however, active bleeding from the ampulla of Vater is rarely seen because of the intermittent nature of the bleeding. Use of a side-viewing endoscope may help with the visualization of active bleeding from the ampulla of Vater. CT scanning, CT angiography, MRI, and MRA may provide information regarding the presence of a fistula between a peripancreatic aneurysm or pseudoaneurysm and the pancreatic duct, as well as identify the presence of a \"sentinel clot\"<sup type=\"ref\">[11]</sup> (focal, high-density clotted blood) in the pancreatic duct during episodes of intermittent bleeding.<sup type=\"ref\">[12]</sup>",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 305107,
"questionText": "You are evaluating a patient with hematochezia and are concerned about the possibility of hemosuccus pancreaticus. Which of the following statements regarding hemosuccus pancreaticus is true?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 964637,
"choiceText": "Chronic hepatitis C with hepatic cirrhosis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 964639,
"choiceText": "Chronic nonsteroidal anti-inflammatory drug use",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 964641,
"choiceText": "Chronic pancreatitis",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 964643,
"choiceText": "<em>Helicobacter pylori</em> infection",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Establishing the diagnosis requires clinical suspicion in patients with a medical history of chronic pancreatitis who present with GI bleeding and severe anemia.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 305111,
"questionText": "Your patient is diagnosed with hemosuccus pancreaticus with an upper GI endoscopy followed by angiographic confirmation. Which of the following conditions is most commonly associated with upper GI bleeding resulting from hemosuccus pancreaticus?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
863119 | /viewarticle/863119 | [
{
"authors": "Cailey Miller, MS3; Pranatharthi Chandrasekar, MD",
"content": [
"Editor's Note:\n\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.",
"A 49-year-old Nigerian man is referred to an infectious diseases clinic for evaluation. The patient states that he has been working as a taxi driver in the United States since he arrived in 1991. He discloses that he was diagnosed with HIV over a decade ago, prior to immigrating to the United States, but has never received treatment.",
"In addition, the patient has a past medical history of hypertension and type 2 diabetes mellitus. He is a nonsmoker and denies illicit drug use. He is separated from his wife and denies having sexual encounters with male partners. His medications include metformin, glipizide, clonidine, amlodipine, lisinopril, and pravastatin. The patient has been depressed since the HIV diagnosis, isolating himself from others."
],
"date": "May 11, 2016",
"figures": [],
"markdown": "# Challenging Laboratory Findings in a Patient With Untreated HIV\n\n **Authors:** Cailey Miller, MS3; Pranatharthi Chandrasekar, MD \n **Date:** May 11, 2016\n\n ## Content\n\n Editor's Note:\n\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.\nA 49-year-old Nigerian man is referred to an infectious diseases clinic for evaluation. The patient states that he has been working as a taxi driver in the United States since he arrived in 1991. He discloses that he was diagnosed with HIV over a decade ago, prior to immigrating to the United States, but has never received treatment.\nIn addition, the patient has a past medical history of hypertension and type 2 diabetes mellitus. He is a nonsmoker and denies illicit drug use. He is separated from his wife and denies having sexual encounters with male partners. His medications include metformin, glipizide, clonidine, amlodipine, lisinopril, and pravastatin. The patient has been depressed since the HIV diagnosis, isolating himself from others.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "Challenging Laboratory Findings in a Patient With Untreated HIV"
},
{
"authors": "Cailey Miller, MS3; Pranatharthi Chandrasekar, MD",
"content": [
"Physical examination revealed an overweight man with a blood pressure of 169/103 mm Hg, heart rate of 87 beats/min, and oral temperature of 97.2° F. No rashes or other skin lesions or peripheral lymphadenopathy are noted. The remainder of his physical examination was noncontributory.",
"To evaluate for his self-disclosed prior HIV infection diagnosis, HIV-1 viral load, HIV-1/2 serology, and CD4 lymphocyte counts were obtained.",
"His laboratory findings on a return appointment show the following:",
"HIV-1/2 antigen/antibody combination immunoassay: Positive",
"CD4 cell count: 752 cells/mm3; 47%",
"HIV-1 RNA quantitative reverse transcriptase-polymerase chain reaction (RT-PCR): Not detected"
],
"date": "May 11, 2016",
"figures": [],
"markdown": "# Challenging Laboratory Findings in a Patient With Untreated HIV\n\n **Authors:** Cailey Miller, MS3; Pranatharthi Chandrasekar, MD \n **Date:** May 11, 2016\n\n ## Content\n\n Physical examination revealed an overweight man with a blood pressure of 169/103 mm Hg, heart rate of 87 beats/min, and oral temperature of 97.2° F. No rashes or other skin lesions or peripheral lymphadenopathy are noted. The remainder of his physical examination was noncontributory.\nTo evaluate for his self-disclosed prior HIV infection diagnosis, HIV-1 viral load, HIV-1/2 serology, and CD4 lymphocyte counts were obtained.\nHis laboratory findings on a return appointment show the following:\nHIV-1/2 antigen/antibody combination immunoassay: Positive\nCD4 cell count: 752 cells/mm3; 47%\nHIV-1 RNA quantitative reverse transcriptase-polymerase chain reaction (RT-PCR): Not detected\n\n ## Figures\n\n \n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 965077,
"choiceText": "The patient has HIV-1 infection",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 965079,
"choiceText": "The patient may have HIV-2 infection and confirmation is required",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 965081,
"choiceText": "The patient does not have active HIV infection",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 965083,
"choiceText": "The patient has both HIV-1 and HIV-2 infection",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 305267,
"questionText": "What is the correct interpretation of his HIV laboratory tests?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Challenging Laboratory Findings in a Patient With Untreated HIV"
},
{
"authors": "Cailey Miller, MS3; Pranatharthi Chandrasekar, MD",
"content": [
"Figure 1.",
"Patients who have HIV-2 infection have a positive rapid screen but an undetectable HIV-1 RNA RT-PCR. This patient had HIV-2 infection that was discovered with an HIV-1/HIV-2 antibody differentiation immunoassay test. This finding suggests that the patient may have HIV-2 infection that was discovered with an HIV-1/HIV-2 antibody differentiation immunoassay test. A confirmatory test is required. Ideally, this would be done by a PCR assay for HIV-2 RNA, but this may be difficult to acquire. A multi-spot assay or other immunological test to differentiate between HIV-1 and HIV-2 antibodies should be obtained.",
"HIV-2 infection is uncommon in the United States; it is mostly found in West Africa. When suspected HIV-2 infection is encountered, a connection to West Africa should be sought[1,2] HIV-2 cases were first identified in the United States in 1987.[3] As with HIV-1, HIV-2 infection is transferred via blood, sexual contact, and perinatally. HIV-2 is less pathogenic than HIV-1. HIV-2 infection results in lower levels of viremia and immune activation, and is characterized by a longer asymptomatic stage and a slower CD4 cell count decline.[3,4] HIV-2, like HIV-1, was first transferred to human beings from sooty mangabey monkeys living in West Africa. The condition is believed to have been circulating since 1966. Recent data in Gambia and the Ivory Coast indicate that HIV-2 may be as much as 621 times less transmissible than HIV-1.[3]",
"Infections with HIV-1 and HIV-2 differ in their laboratory test findings. Patients who are infected with HIV-2 typically present with higher CD4 cell counts than patients with HIV-1. Patients with HIV-2 infection have a lower viral load than patients with HIV-1 infection. These distinctions are important because HIV-1 and HIV-2 infections typically require different treatment approaches for successful management. The two virus subtypes have helped promote the creation of fourth-generation HIV testing."
],
"date": "May 11, 2016",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/863/119/863119-Thumb1.png"
}
],
"markdown": "# Challenging Laboratory Findings in a Patient With Untreated HIV\n\n **Authors:** Cailey Miller, MS3; Pranatharthi Chandrasekar, MD \n **Date:** May 11, 2016\n\n ## Content\n\n Figure 1.\nPatients who have HIV-2 infection have a positive rapid screen but an undetectable HIV-1 RNA RT-PCR. This patient had HIV-2 infection that was discovered with an HIV-1/HIV-2 antibody differentiation immunoassay test. This finding suggests that the patient may have HIV-2 infection that was discovered with an HIV-1/HIV-2 antibody differentiation immunoassay test. A confirmatory test is required. Ideally, this would be done by a PCR assay for HIV-2 RNA, but this may be difficult to acquire. A multi-spot assay or other immunological test to differentiate between HIV-1 and HIV-2 antibodies should be obtained.\nHIV-2 infection is uncommon in the United States; it is mostly found in West Africa. When suspected HIV-2 infection is encountered, a connection to West Africa should be sought[1,2] HIV-2 cases were first identified in the United States in 1987.[3] As with HIV-1, HIV-2 infection is transferred via blood, sexual contact, and perinatally. HIV-2 is less pathogenic than HIV-1. HIV-2 infection results in lower levels of viremia and immune activation, and is characterized by a longer asymptomatic stage and a slower CD4 cell count decline.[3,4] HIV-2, like HIV-1, was first transferred to human beings from sooty mangabey monkeys living in West Africa. The condition is believed to have been circulating since 1966. Recent data in Gambia and the Ivory Coast indicate that HIV-2 may be as much as 621 times less transmissible than HIV-1.[3]\nInfections with HIV-1 and HIV-2 differ in their laboratory test findings. Patients who are infected with HIV-2 typically present with higher CD4 cell counts than patients with HIV-1. Patients with HIV-2 infection have a lower viral load than patients with HIV-1 infection. These distinctions are important because HIV-1 and HIV-2 infections typically require different treatment approaches for successful management. The two virus subtypes have helped promote the creation of fourth-generation HIV testing.\n\n ## Figures\n\n **Figure 1.** \n \n\n\n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 965077,
"choiceText": "The patient has HIV-1 infection",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 965079,
"choiceText": "The patient may have HIV-2 infection and confirmation is required",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 965081,
"choiceText": "The patient does not have active HIV infection",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 965083,
"choiceText": "The patient has both HIV-1 and HIV-2 infection",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 305267,
"questionText": "What is the correct interpretation of his HIV laboratory tests?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Challenging Laboratory Findings in a Patient With Untreated HIV"
},
{
"authors": "Cailey Miller, MS3; Pranatharthi Chandrasekar, MD",
"content": [
"Third-generation testing using enzyme-linked immunosorbent assay (ELISA) followed by western blots misclassified HIV-2 infection. This testing algorithm missed those with early infection if antibodies had not yet developed. Also, patients with dual infection showed cross-reactivity, and the diagnosis of HIV-2 infection was missed. These tests also showed negative HIV-1 western blot findings, requiring HIV-2 western blot testing. Third-generation testing was also indeterminate in acute HIV-1 infection or chronic HIV-2 infection, requiring further testing.[3]",
"Fourth-generation testing was developed to address these problems. Screening for HIV should be done with an HIV-1/2 Ag/Ab combination. If findings are positive, an HIV-1/2 antibody differentiation immunoassay should be performed. If those results reveal positivity for HIV-2 infection, an HIV-2-specific ELISA, RT-PCR, or western blot test should be performed.[3] Two rapid result tests are available in the United States: the ARCHITECT HIV Ag/Ab combination test (an immunoassay) and the Determine HIV-1/2 Ag/Ab combination test (using lateral flow technology).",
"HIV-1 RT-PCR does not detect presence of HIV-2; therefore, patients infected with HIV-2 may have an \"undetectable\" viral load. The New York State Department of Health Wadsworth laboratory and the University of Washington laboratory medicine community services are the only laboratories currently available to assess HIV-2 viral load in the clinical setting.[5]",
"Treatment for HIV-2 infection shows intrinsic resistance to nonnucleoside RT inhibitors (NNRTIs) and fusion inhibitors. A combination of two nucleoside RT inhibitors (NRTIs) and a boosted protease inhibitor is recommended for treatment. Treatment data on susceptibility to integrase inhibitors are limited. HIV-2 may use different co-receptors; therefore, CCR5 antagonists may have limited effectiveness.[6]",
"Recommended NRTIs include tenofovir and abacavir. Zidovudine is not recommended because only two mutations are required for resistance to develop. A high level of intrinsic resistance to lamivudine/emtricitabine has also been documented.",
"Protease inhibitors show variable results. The following may be used:",
"Lopinavir",
"Darunavir",
"Saquinavir",
"The following should be avoided:",
"Atazanavir",
"Amprenavir",
"Indinavir",
"Nelfinavir",
"Tipranavir",
"Another differential diagnosis to consider in the presence of a high CD4 cell count and undetectable HIV-1 viral load is that the patient is an \"elite controller.\""
],
"date": "May 11, 2016",
"figures": [],
"markdown": "# Challenging Laboratory Findings in a Patient With Untreated HIV\n\n **Authors:** Cailey Miller, MS3; Pranatharthi Chandrasekar, MD \n **Date:** May 11, 2016\n\n ## Content\n\n Third-generation testing using enzyme-linked immunosorbent assay (ELISA) followed by western blots misclassified HIV-2 infection. This testing algorithm missed those with early infection if antibodies had not yet developed. Also, patients with dual infection showed cross-reactivity, and the diagnosis of HIV-2 infection was missed. These tests also showed negative HIV-1 western blot findings, requiring HIV-2 western blot testing. Third-generation testing was also indeterminate in acute HIV-1 infection or chronic HIV-2 infection, requiring further testing.[3]\nFourth-generation testing was developed to address these problems. Screening for HIV should be done with an HIV-1/2 Ag/Ab combination. If findings are positive, an HIV-1/2 antibody differentiation immunoassay should be performed. If those results reveal positivity for HIV-2 infection, an HIV-2-specific ELISA, RT-PCR, or western blot test should be performed.[3] Two rapid result tests are available in the United States: the ARCHITECT HIV Ag/Ab combination test (an immunoassay) and the Determine HIV-1/2 Ag/Ab combination test (using lateral flow technology).\nHIV-1 RT-PCR does not detect presence of HIV-2; therefore, patients infected with HIV-2 may have an \"undetectable\" viral load. The New York State Department of Health Wadsworth laboratory and the University of Washington laboratory medicine community services are the only laboratories currently available to assess HIV-2 viral load in the clinical setting.[5]\nTreatment for HIV-2 infection shows intrinsic resistance to nonnucleoside RT inhibitors (NNRTIs) and fusion inhibitors. A combination of two nucleoside RT inhibitors (NRTIs) and a boosted protease inhibitor is recommended for treatment. Treatment data on susceptibility to integrase inhibitors are limited. HIV-2 may use different co-receptors; therefore, CCR5 antagonists may have limited effectiveness.[6]\nRecommended NRTIs include tenofovir and abacavir. Zidovudine is not recommended because only two mutations are required for resistance to develop. A high level of intrinsic resistance to lamivudine/emtricitabine has also been documented.\nProtease inhibitors show variable results. The following may be used:\nLopinavir\nDarunavir\nSaquinavir\nThe following should be avoided:\nAtazanavir\nAmprenavir\nIndinavir\nNelfinavir\nTipranavir\nAnother differential diagnosis to consider in the presence of a high CD4 cell count and undetectable HIV-1 viral load is that the patient is an \"elite controller.\"\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "Challenging Laboratory Findings in a Patient With Untreated HIV"
},
{
"authors": "Cailey Miller, MS3; Pranatharthi Chandrasekar, MD",
"content": [
"Elite controller is a term used to describe patients with HIV infection who have no clinical evidence of viremia. Such patients are uncommon; they usually present with a high CD4 cell count and undetectable viral loads.[3] Elite controllers are theorized to have a lack of susceptibility to HIV infection of CD4 cells, are infected with replication-defective HIV variants, have an efficient immune response to viral replication, or have reduced inflammation.[7] Data support that these individuals mount a strong HIV host response which controls their infection. Most agree that elite controllers downregulate viral replication in lymphoid tissue so that few HIV cells persist, resulting in low HIV RNA and, therefore, fewer infected CD4 cells.[7]",
"In this patient, HIV-2 RNA detection by PCR was performed; RNA was detected but was below the lower limit of quantification. Chronic HIV-2 infection was determined by a positive rapid test for HIV infection and an undetectable HIV-1 RNA viral load but detectable HIV-2 RNA. The patient was started on a boosted protease inhibitor and two NRTIs (tenofovir and abacavir). He has maintained proper control and followed the recommended treatment indications. He remains asymptomatic at last follow-up."
],
"date": "May 11, 2016",
"figures": [],
"markdown": "# Challenging Laboratory Findings in a Patient With Untreated HIV\n\n **Authors:** Cailey Miller, MS3; Pranatharthi Chandrasekar, MD \n **Date:** May 11, 2016\n\n ## Content\n\n Elite controller is a term used to describe patients with HIV infection who have no clinical evidence of viremia. Such patients are uncommon; they usually present with a high CD4 cell count and undetectable viral loads.[3] Elite controllers are theorized to have a lack of susceptibility to HIV infection of CD4 cells, are infected with replication-defective HIV variants, have an efficient immune response to viral replication, or have reduced inflammation.[7] Data support that these individuals mount a strong HIV host response which controls their infection. Most agree that elite controllers downregulate viral replication in lymphoid tissue so that few HIV cells persist, resulting in low HIV RNA and, therefore, fewer infected CD4 cells.[7]\nIn this patient, HIV-2 RNA detection by PCR was performed; RNA was detected but was below the lower limit of quantification. Chronic HIV-2 infection was determined by a positive rapid test for HIV infection and an undetectable HIV-1 RNA viral load but detectable HIV-2 RNA. The patient was started on a boosted protease inhibitor and two NRTIs (tenofovir and abacavir). He has maintained proper control and followed the recommended treatment indications. He remains asymptomatic at last follow-up.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 965105,
"choiceText": "HIV-2 progresses more slowly than with HIV-1",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 965107,
"choiceText": "HIV-2 is acquired mostly in Southeast Asia and Latin America compared with HIV-1",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 965109,
"choiceText": "HIV-2 is associated with more complications compared with HIV-1",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 965111,
"choiceText": "There are no medications available to treat HIV-2 ",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Patients infected with HIV-2 present with higher CD4 cell counts than those with HIV-1 who have been infected for the same amount of time. HIV-2 does not appear to destroy CD4 cells as quickly as HIV-1; thus, patients with HIV-2 often have normal CD4 cell counts.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 305273,
"questionText": "How does HIV-2 infection differ from HIV-1 infection?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 965113,
"choiceText": "HIV-2 has a high level of resistance to lamivudine/emtricitabine",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 965115,
"choiceText": "HIV-2 is susceptible to NNRTIs",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 965117,
"choiceText": "HIV-2 is variably susceptible to protease inhibitors",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 965119,
"choiceText": "HIV-2 recommended treatments include a boosted protease inhibitor with two NRTIs",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "HIV-2 is intrinsically resistant to NNRTIs. These are not effective medications for controlling HIV2 infections and should be avoided when creating a treatment plan. ",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 305275,
"questionText": "Which of the following is <em>not</em> true regarding the susceptibility pattern of HIV-2 to antiretrovirals?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Challenging Laboratory Findings in a Patient With Untreated HIV"
},
{
"authors": "Cailey Miller, MS3; Pranatharthi Chandrasekar, MD",
"content": [],
"date": "May 11, 2016",
"figures": [],
"markdown": "# Challenging Laboratory Findings in a Patient With Untreated HIV\n\n **Authors:** Cailey Miller, MS3; Pranatharthi Chandrasekar, MD \n **Date:** May 11, 2016\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 965105,
"choiceText": "HIV-2 progresses more slowly than with HIV-1",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 965107,
"choiceText": "HIV-2 is acquired mostly in Southeast Asia and Latin America compared with HIV-1",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 965109,
"choiceText": "HIV-2 is associated with more complications compared with HIV-1",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 965111,
"choiceText": "There are no medications available to treat HIV-2 ",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Patients infected with HIV-2 present with higher CD4 cell counts than those with HIV-1 who have been infected for the same amount of time. HIV-2 does not appear to destroy CD4 cells as quickly as HIV-1; thus, patients with HIV-2 often have normal CD4 cell counts.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 305273,
"questionText": "How does HIV-2 infection differ from HIV-1 infection?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 965113,
"choiceText": "HIV-2 has a high level of resistance to lamivudine/emtricitabine",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 965115,
"choiceText": "HIV-2 is susceptible to NNRTIs",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 965117,
"choiceText": "HIV-2 is variably susceptible to protease inhibitors",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 965119,
"choiceText": "HIV-2 recommended treatments include a boosted protease inhibitor with two NRTIs",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "HIV-2 is intrinsically resistant to NNRTIs. These are not effective medications for controlling HIV2 infections and should be avoided when creating a treatment plan. ",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 305275,
"questionText": "Which of the following is <em>not</em> true regarding the susceptibility pattern of HIV-2 to antiretrovirals?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Challenging Laboratory Findings in a Patient With Untreated HIV"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 965077,
"choiceText": "The patient has HIV-1 infection",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 965079,
"choiceText": "The patient may have HIV-2 infection and confirmation is required",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 965081,
"choiceText": "The patient does not have active HIV infection",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 965083,
"choiceText": "The patient has both HIV-1 and HIV-2 infection",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 305267,
"questionText": "What is the correct interpretation of his HIV laboratory tests?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 965105,
"choiceText": "HIV-2 progresses more slowly than with HIV-1",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 965107,
"choiceText": "HIV-2 is acquired mostly in Southeast Asia and Latin America compared with HIV-1",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 965109,
"choiceText": "HIV-2 is associated with more complications compared with HIV-1",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 965111,
"choiceText": "There are no medications available to treat HIV-2 ",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Patients infected with HIV-2 present with higher CD4 cell counts than those with HIV-1 who have been infected for the same amount of time. HIV-2 does not appear to destroy CD4 cells as quickly as HIV-1; thus, patients with HIV-2 often have normal CD4 cell counts.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 305273,
"questionText": "How does HIV-2 infection differ from HIV-1 infection?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 965113,
"choiceText": "HIV-2 has a high level of resistance to lamivudine/emtricitabine",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 965115,
"choiceText": "HIV-2 is susceptible to NNRTIs",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 965117,
"choiceText": "HIV-2 is variably susceptible to protease inhibitors",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 965119,
"choiceText": "HIV-2 recommended treatments include a boosted protease inhibitor with two NRTIs",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "HIV-2 is intrinsically resistant to NNRTIs. These are not effective medications for controlling HIV2 infections and should be avoided when creating a treatment plan. ",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 305275,
"questionText": "Which of the following is <em>not</em> true regarding the susceptibility pattern of HIV-2 to antiretrovirals?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
862410 | /viewarticle/862410 | [
{
"authors": "Liza Cholin, BS; Jordan Burlen, BS; Umar Darr, MD",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.",
"A 69-year-old woman, recently discharged from the hospital for sepsis secondary to urinary tract infection (UTI), was admitted for acute kidney injury found during laboratory studies performed at a nursing facility. Acute chronic kidney injury secondary to dehydration was diagnosed, and the patient was admitted for rehydration. A sample for urinalysis was collected, and ceftriaxone was started for probable recurrent UTI. The patient's long-term suppressive therapy with doxycycline for left-knee osteomyelitis was also continued.",
"On the third day of admission, the patient experienced sudden right-cheek swelling, tenderness, and mild difficulty in swallowing. She denied fever, chills, or odynophagia; recent travel or sick contacts; and mandibular pain, changes in hearing, or loss of sensation of the overlying skin of the face.",
"The patient's medical history includes stage 3 chronic kidney disease, congestive heart failure, coronary artery disease status post-stent, chronic obstructive pulmonary disease, lower-extremity lymphedema, chronic osteomyelitis of the left knee, recurrent UTIs, and a history of extended-spectrum beta-lactamase–positive UTI. Surgical history includes left knee arthroplasty and gastric bypass. The patient has a 60–pack-year smoking history but is currently not smoking. She denied alcohol or illicit drug use."
],
"date": "April 27, 2016",
"figures": [],
"markdown": "# Sudden-Onset Right-Cheek Swelling in a 69-Year-Old Woman\n\n **Authors:** Liza Cholin, BS; Jordan Burlen, BS; Umar Darr, MD \n **Date:** April 27, 2016\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.\nA 69-year-old woman, recently discharged from the hospital for sepsis secondary to urinary tract infection (UTI), was admitted for acute kidney injury found during laboratory studies performed at a nursing facility. Acute chronic kidney injury secondary to dehydration was diagnosed, and the patient was admitted for rehydration. A sample for urinalysis was collected, and ceftriaxone was started for probable recurrent UTI. The patient's long-term suppressive therapy with doxycycline for left-knee osteomyelitis was also continued.\nOn the third day of admission, the patient experienced sudden right-cheek swelling, tenderness, and mild difficulty in swallowing. She denied fever, chills, or odynophagia; recent travel or sick contacts; and mandibular pain, changes in hearing, or loss of sensation of the overlying skin of the face.\nThe patient's medical history includes stage 3 chronic kidney disease, congestive heart failure, coronary artery disease status post-stent, chronic obstructive pulmonary disease, lower-extremity lymphedema, chronic osteomyelitis of the left knee, recurrent UTIs, and a history of extended-spectrum beta-lactamase–positive UTI. Surgical history includes left knee arthroplasty and gastric bypass. The patient has a 60–pack-year smoking history but is currently not smoking. She denied alcohol or illicit drug use.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "Sudden-Onset Right-Cheek Swelling in a 69-Year-Old Woman"
},
{
"authors": "Liza Cholin, BS; Jordan Burlen, BS; Umar Darr, MD",
"content": [
"On physical examination, the patient's temperature was 97.5°F, heart rate was 87 beats/min, respiratory rate was 16 breaths/min, blood pressure was 126/60 mm Hg, and oxygen saturation was 96% on room air.",
"The patient was alert and oriented to person, place, and time, and was in no apparent distress. The patient's right parotid gland was swollen and measured approximately 4 x 3 cm. The gland was tender, mobile, and firm, without overlying erythema. Purulent fluid was elicited by applying pressure over the gland. The skin was intact.",
"The oral cavity had no lesions, swelling, or drainage. The tongue, gingiva, oropharynx, and soft palate appeared normal. Cranial nerves II-XII were intact. The patient's face was asymmetrical. Findings on cardiovascular, respiratory, and abdominal examination were unremarkable. Her lower extremities had pitting edema bilaterally, with evidence of chronic lymphedema."
],
"date": "April 27, 2016",
"figures": [],
"markdown": "# Sudden-Onset Right-Cheek Swelling in a 69-Year-Old Woman\n\n **Authors:** Liza Cholin, BS; Jordan Burlen, BS; Umar Darr, MD \n **Date:** April 27, 2016\n\n ## Content\n\n On physical examination, the patient's temperature was 97.5°F, heart rate was 87 beats/min, respiratory rate was 16 breaths/min, blood pressure was 126/60 mm Hg, and oxygen saturation was 96% on room air.\nThe patient was alert and oriented to person, place, and time, and was in no apparent distress. The patient's right parotid gland was swollen and measured approximately 4 x 3 cm. The gland was tender, mobile, and firm, without overlying erythema. Purulent fluid was elicited by applying pressure over the gland. The skin was intact.\nThe oral cavity had no lesions, swelling, or drainage. The tongue, gingiva, oropharynx, and soft palate appeared normal. Cranial nerves II-XII were intact. The patient's face was asymmetrical. Findings on cardiovascular, respiratory, and abdominal examination were unremarkable. Her lower extremities had pitting edema bilaterally, with evidence of chronic lymphedema.\n\n ## Figures\n\n \n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 961321,
"choiceText": "Salivary gland neoplasm",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961323,
"choiceText": "Viral parotitis (mumps)",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961325,
"choiceText": "Bacterial (suppurative) parotitis",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961327,
"choiceText": "Sialolithiasis ",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961329,
"choiceText": "Swollen lymph nodes",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961331,
"choiceText": "Dental abscess ",
"correct": false,
"displayOrder": 6,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961333,
"choiceText": "External otitis",
"correct": false,
"displayOrder": 7,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961335,
"choiceText": "Autoimmune parotitis\r\n",
"correct": false,
"displayOrder": 8,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 304035,
"questionText": "On the basis of the history, physical examination, and workup, what is the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Sudden-Onset Right-Cheek Swelling in a 69-Year-Old Woman"
},
{
"authors": "Liza Cholin, BS; Jordan Burlen, BS; Umar Darr, MD",
"content": [
"On the basis of the history and physical examination findings, the most probable diagnosis was determined to be acute suppurative parotitis. The patient had a unilateral, swollen, and tender gland that was draining purulent fluid, which makes an acute bacterial etiology most likely. The patient also had several risk factors for developing infectious parotitis; these include multiple comorbidities and a recent nursing home stay.",
"A salivary gland neoplasm has a more indolent course and is generally painless. Viral parotitis is found more commonly in young children and is now rare, owing to the widespread use of the measles, mumps, and rubella vaccine. Viral parotitis is also more likely to produce a clear discharge.",
"Sialoduct stones, or sialolithiasis, is associated with more intermittent pain around the time that the gland is releasing salivary fluids (during eating). A patient with salivary stones generally appears well otherwise and will not have purulent drainage.",
"Swollen lymph nodes surrounding the parotid gland is another possibility. However, drainage from the gland is not expected, and tenderness is mild if present.",
"Referring pain from adjacent structures should also be taken into account. Owing to the patient's denial of ear pain and normal findings in the ear and oral cavity, external otitis and dental abscess are less likely.",
"Finally, some autoimmune cases of parotitis have been noted. Sarcoidosis and Sjögren syndrome are both associated with parotitis. However, they are associated with a more chronic course, and often the swelling is bilateral, with only mild tenderness. Also, other organ systems would be affected with these diseases. The patient in this case report had a fairly unremarkable physical examination and no history of autoimmune disorders, making these unlikely causes.",
"In this case, on the second day of the parotid swelling, the surgery team was consulted. The team was able to express purulent discharge with gentle massage. They sent the fluid for culture and began treating the patient empirically for acute bacterial parotitis. Intravenous ceftriaxone and doxycycline were continued, and metronidazole was added to that regimen.",
"The parotid gland is one of three major salivary glands; it has a Stensen duct, which drains saliva during mastication, above the angle of the mandible and anterior to the auricle. Infection of this gland is rare, but when it occurs, it can pose significant problems.",
"In the past, acute bacterial parotitis was most commonly found in hospitalized patients after a major procedure. With advances in fluid management and prophylactic antibiotics, this clinical picture has dramatically decreased. Presently, it is more common to see acute bacterial parotitis as a nosocomial infection. Risk factors for acute bacterial parotitis include dehydration, poor oral hygiene, medications that reduce the salivary flow, and an obstruction within the parotid gland (ie, calculi, stricture, mass).[1]"
],
"date": "April 27, 2016",
"figures": [],
"markdown": "# Sudden-Onset Right-Cheek Swelling in a 69-Year-Old Woman\n\n **Authors:** Liza Cholin, BS; Jordan Burlen, BS; Umar Darr, MD \n **Date:** April 27, 2016\n\n ## Content\n\n On the basis of the history and physical examination findings, the most probable diagnosis was determined to be acute suppurative parotitis. The patient had a unilateral, swollen, and tender gland that was draining purulent fluid, which makes an acute bacterial etiology most likely. The patient also had several risk factors for developing infectious parotitis; these include multiple comorbidities and a recent nursing home stay.\nA salivary gland neoplasm has a more indolent course and is generally painless. Viral parotitis is found more commonly in young children and is now rare, owing to the widespread use of the measles, mumps, and rubella vaccine. Viral parotitis is also more likely to produce a clear discharge.\nSialoduct stones, or sialolithiasis, is associated with more intermittent pain around the time that the gland is releasing salivary fluids (during eating). A patient with salivary stones generally appears well otherwise and will not have purulent drainage.\nSwollen lymph nodes surrounding the parotid gland is another possibility. However, drainage from the gland is not expected, and tenderness is mild if present.\nReferring pain from adjacent structures should also be taken into account. Owing to the patient's denial of ear pain and normal findings in the ear and oral cavity, external otitis and dental abscess are less likely.\nFinally, some autoimmune cases of parotitis have been noted. Sarcoidosis and Sjögren syndrome are both associated with parotitis. However, they are associated with a more chronic course, and often the swelling is bilateral, with only mild tenderness. Also, other organ systems would be affected with these diseases. The patient in this case report had a fairly unremarkable physical examination and no history of autoimmune disorders, making these unlikely causes.\nIn this case, on the second day of the parotid swelling, the surgery team was consulted. The team was able to express purulent discharge with gentle massage. They sent the fluid for culture and began treating the patient empirically for acute bacterial parotitis. Intravenous ceftriaxone and doxycycline were continued, and metronidazole was added to that regimen.\nThe parotid gland is one of three major salivary glands; it has a Stensen duct, which drains saliva during mastication, above the angle of the mandible and anterior to the auricle. Infection of this gland is rare, but when it occurs, it can pose significant problems.\nIn the past, acute bacterial parotitis was most commonly found in hospitalized patients after a major procedure. With advances in fluid management and prophylactic antibiotics, this clinical picture has dramatically decreased. Presently, it is more common to see acute bacterial parotitis as a nosocomial infection. Risk factors for acute bacterial parotitis include dehydration, poor oral hygiene, medications that reduce the salivary flow, and an obstruction within the parotid gland (ie, calculi, stricture, mass).[1]\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 961321,
"choiceText": "Salivary gland neoplasm",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961323,
"choiceText": "Viral parotitis (mumps)",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961325,
"choiceText": "Bacterial (suppurative) parotitis",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961327,
"choiceText": "Sialolithiasis ",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961329,
"choiceText": "Swollen lymph nodes",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961331,
"choiceText": "Dental abscess ",
"correct": false,
"displayOrder": 6,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961333,
"choiceText": "External otitis",
"correct": false,
"displayOrder": 7,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961335,
"choiceText": "Autoimmune parotitis\r\n",
"correct": false,
"displayOrder": 8,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 304035,
"questionText": "On the basis of the history, physical examination, and workup, what is the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Sudden-Onset Right-Cheek Swelling in a 69-Year-Old Woman"
},
{
"authors": "Liza Cholin, BS; Jordan Burlen, BS; Umar Darr, MD",
"content": [
"The typical presentation of acute bacterial parotitis consists of sudden onset of severe unilateral pain, tenderness, and erythema of the overlying skin. Owing to the density of the fascia overlying the gland, it is usually not fluctuant, but rather firm. The patient may also present with trismus and dysphagia. In general, high fever, chills, and overall unwell appearance accompany the clinical findings. On physical examination, purulent discharge may be expressed with gentle massage of the gland in about one half of all patients.",
"Even though acute bacterial parotitis is mostly a clinical diagnosis, several diagnostic studies can help to confirm the diagnosis and determine the extent of disease. If purulent drainage is present, it should be sent for Gram stain and culture. However, contamination with oral cavity flora is a risk. Therefore, needle aspiration of the parotid gland via an extraoral route can be performed to yield the most accurate results. This may be chosen if the culture result is inconclusive.",
"Imagining studies are also useful in determining whether an abscess has formed and the extent to which the disease has spread to adjacent structures. Ultrasound, CT, and MRI are the most common imaging modalities used.[2] These studies can detect masses, strictures, and calculi as well. CT is the best imaging test for excluding salivary duct stones. On ultrasound, an abscess can be seen as a hypoechogenic lesion. Sialography can be used to visualize the ductal system; however, it is less commonly performed.",
"When a patient initially presents with a swollen parotid gland, several different causes should be considered.",
"Infectious causes of parotid gland swelling vary. With bacterial causes, the presentation typically includes sudden onset of unilateral parotid gland swelling, erythema, and marked tenderness, along with purulent drainage. However, parotitis in tuberculosis is associated with chronic nontender swelling of one gland.",
"Bacterial causes include the following:",
"Staphylococcus aureus",
"Viridans streptococci",
"Streptococcus pyogenes",
"Streptococcus pneumoniae",
"Haemophilus influenzae",
"Moraxella catarrhalis",
"Pseudomonas aeruginosa",
"Gram-negative bacilli",
"Mycobacteria",
"With viral causes, a prodrome of fever and malaise is typically followed by pain and swelling of the parotid glands. Involvement is typically bilateral. However, HIV parotitis is associated with nonpainful swelling of glands but is otherwise asymptomatic.",
"Viral causes include the following:",
"Paramyxovirus",
"Coxsackievirus",
"Influenza A virus",
"Parainfluenza virus",
"Epstein-Barr virus",
"Herpes simplex virus",
"Cytomegalovirus",
"HIV",
"Candida albicans is a recognized fungal cause.",
"In noninfectious parotitis, patients with Sjögren syndrome have chronic, recurrent parotid gland swelling (unilateral or bilateral) and mild pain. Sjögren syndrome occurs most frequently in women aged 40-60 years and is associated with keratoconjunctivitis sicca, xerostomia, and connective tissue disease.[3] Patients with sarcoidosis demonstrate chronic, bilateral, nontender swelling of the parotid gland. Sarcoidosis has a higher incidence in African American persons aged 20-40 years. When it involves the parotid glands, bilateral nontender enlargement is typically seen.[4]A biopsy reveals noncaseating granulomas. Patients with neoplasm of the salivary gland are usually asymptomatic. Imaging helps differentiate etiology, and definitive diagnosis relies on biopsy. In patients with sialolithiasis (salivary calculi), pain occurs during eating (owing to obstructed salivary flow).",
"Depending on the vaccination status of the patient, viral parotitis or mumps may be possible. The most common virus known to cause parotitis is paramyxovirus; however, several other viruses can cause a similar presentation and include coxsackievirus, parainfluenza virus, influenza A virus, Epstein-Barr virus, herpes simplex virus, and cytomegalovirus.[2]",
"Unlike bacterial parotitis, viral parotitis is associated with a clear discharge. Typically, the patient has a prodromal period of fever, chills, and headache before the salivary gland swells and becomes tender. Other, less common infectious causes include mycobacterial tuberculosis and HIV.",
"All autoimmune causes are associated with a more chronic and indolent course, unlike infectious parotitis.",
"Finally, other diagnoses to consider include salivary calculi (sialolithiasis) and salivary gland neoplasms. As mentioned above, imaging is the modality of choice in making such a diagnosis. Salivary calculi may also be associated with severe pain with eating that resolves shortly afterward."
],
"date": "April 27, 2016",
"figures": [],
"markdown": "# Sudden-Onset Right-Cheek Swelling in a 69-Year-Old Woman\n\n **Authors:** Liza Cholin, BS; Jordan Burlen, BS; Umar Darr, MD \n **Date:** April 27, 2016\n\n ## Content\n\n The typical presentation of acute bacterial parotitis consists of sudden onset of severe unilateral pain, tenderness, and erythema of the overlying skin. Owing to the density of the fascia overlying the gland, it is usually not fluctuant, but rather firm. The patient may also present with trismus and dysphagia. In general, high fever, chills, and overall unwell appearance accompany the clinical findings. On physical examination, purulent discharge may be expressed with gentle massage of the gland in about one half of all patients.\nEven though acute bacterial parotitis is mostly a clinical diagnosis, several diagnostic studies can help to confirm the diagnosis and determine the extent of disease. If purulent drainage is present, it should be sent for Gram stain and culture. However, contamination with oral cavity flora is a risk. Therefore, needle aspiration of the parotid gland via an extraoral route can be performed to yield the most accurate results. This may be chosen if the culture result is inconclusive.\nImagining studies are also useful in determining whether an abscess has formed and the extent to which the disease has spread to adjacent structures. Ultrasound, CT, and MRI are the most common imaging modalities used.[2] These studies can detect masses, strictures, and calculi as well. CT is the best imaging test for excluding salivary duct stones. On ultrasound, an abscess can be seen as a hypoechogenic lesion. Sialography can be used to visualize the ductal system; however, it is less commonly performed.\nWhen a patient initially presents with a swollen parotid gland, several different causes should be considered.\nInfectious causes of parotid gland swelling vary. With bacterial causes, the presentation typically includes sudden onset of unilateral parotid gland swelling, erythema, and marked tenderness, along with purulent drainage. However, parotitis in tuberculosis is associated with chronic nontender swelling of one gland.\nBacterial causes include the following:\nStaphylococcus aureus\nViridans streptococci\nStreptococcus pyogenes\nStreptococcus pneumoniae\nHaemophilus influenzae\nMoraxella catarrhalis\nPseudomonas aeruginosa\nGram-negative bacilli\nMycobacteria\nWith viral causes, a prodrome of fever and malaise is typically followed by pain and swelling of the parotid glands. Involvement is typically bilateral. However, HIV parotitis is associated with nonpainful swelling of glands but is otherwise asymptomatic.\nViral causes include the following:\nParamyxovirus\nCoxsackievirus\nInfluenza A virus\nParainfluenza virus\nEpstein-Barr virus\nHerpes simplex virus\nCytomegalovirus\nHIV\nCandida albicans is a recognized fungal cause.\nIn noninfectious parotitis, patients with Sjögren syndrome have chronic, recurrent parotid gland swelling (unilateral or bilateral) and mild pain. Sjögren syndrome occurs most frequently in women aged 40-60 years and is associated with keratoconjunctivitis sicca, xerostomia, and connective tissue disease.[3] Patients with sarcoidosis demonstrate chronic, bilateral, nontender swelling of the parotid gland. Sarcoidosis has a higher incidence in African American persons aged 20-40 years. When it involves the parotid glands, bilateral nontender enlargement is typically seen.[4]A biopsy reveals noncaseating granulomas. Patients with neoplasm of the salivary gland are usually asymptomatic. Imaging helps differentiate etiology, and definitive diagnosis relies on biopsy. In patients with sialolithiasis (salivary calculi), pain occurs during eating (owing to obstructed salivary flow).\nDepending on the vaccination status of the patient, viral parotitis or mumps may be possible. The most common virus known to cause parotitis is paramyxovirus; however, several other viruses can cause a similar presentation and include coxsackievirus, parainfluenza virus, influenza A virus, Epstein-Barr virus, herpes simplex virus, and cytomegalovirus.[2]\nUnlike bacterial parotitis, viral parotitis is associated with a clear discharge. Typically, the patient has a prodromal period of fever, chills, and headache before the salivary gland swells and becomes tender. Other, less common infectious causes include mycobacterial tuberculosis and HIV.\nAll autoimmune causes are associated with a more chronic and indolent course, unlike infectious parotitis.\nFinally, other diagnoses to consider include salivary calculi (sialolithiasis) and salivary gland neoplasms. As mentioned above, imaging is the modality of choice in making such a diagnosis. Salivary calculi may also be associated with severe pain with eating that resolves shortly afterward.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "Sudden-Onset Right-Cheek Swelling in a 69-Year-Old Woman"
},
{
"authors": "Liza Cholin, BS; Jordan Burlen, BS; Umar Darr, MD",
"content": [
"Empirical treatment should be started after a clinical diagnosis of acute bacterial parotitis has been made. By far the most common organism found is S aureus, followed by S viridans and oral anaerobes.[5] Treatment should be aimed to cover methicillin-sensitive (or methicillin-resistant, depending on the hospital region and patient history) S aureus and oral aerobes and anaerobes until culture results are returned. For immunocompromised patients, consider covering for P aeruginosa and gram-negative bacilli in addition to the standard treatment.[5] A penicillin (ie, nafcillin) or first-generation cephalosporin in combination with an aminoglycoside is an appropriate initial treatment choice.[2]",
"Figure 1.",
"Figure 2.",
"Clindamycin or metronidazole can be used in place of an aminoglycoside as well. Vancomycin or linezolid can be considered if methicillin-resistant S aureus infection is a concern. Pseudomonal coverage can be accomplished by using cefepime or a carbapenem.",
"If treatment does not adequately control the infection, it can spread to surrounding tissues and give rise to several complications. Facial nerve palsy, septicemia, and thrombophlebitis of the jugular vein (Lemierre syndrome) are some of the problems that can arise when medical treatment fails.",
"If the patient does not appear to be responding to conservative management, an otolaryngology specialist should be consulted. Although there are no set guidelines for how soon to obtain a consult are noted, if the patient's condition does not improve within 3 days, more aggressive treatment is generally needed to clear the infection. A specialist is better able to determine the extent of the disease, as well as the next step in management. Sometimes, only incision and drainage is needed; however, parotidectomy is sometimes necessary.",
"The patient in this case report was continued on empirical treatment for acute bacterial parotitis and underwent ultrasound of the head and neck to rule out abscess. The ultrasound (Figure 1) revealed a heterogeneous echotexture of the enlarged gland but no definite cystic or solid lesions. The right parotid gland measured 6.5 x 4.3 x 3.3 cm.",
"Two days after the purulent discharge was sent for culture, the results came back positive for moderate growth of extended-spectrum beta-lactamase–producing Escherichia coli, moderate growth of Enterococcus faecalis, and light growth of C albicans. The patient's antibiotics were changed to meropenem and metronidazole.",
"Extended-spectrum beta-lactamases are an increasingly reported threat to the future of antibiotic effectiveness. Although an exact definition does not exist, the general term is used to describe beta-lactamases that have formed resistance to penicillins; first-, second-, and third-generation cephalosporins; and aztreonam. However, they remain susceptible to clavulanic acid.[6]",
"Two general classification schemes are recognized: one that groups them according to the enzyme structure homology, and another that categorizes them according to functional similarities. The diversity of extended-spectrum beta-lactamase types is rapidly expanding. Several main gene groupings exist, from which many derivatives have emerged. These gene groups include SHV, TEM, CTX-M, Toho, OXA, and PER, as well as other, less common types.[7]Most infections in the United States have been with TEM-types and SHV-types.[6]",
"Most of the TEM-types are considered extended-spectrum beta-lactamases; however, not all have extended-spectrum cephalosporin hydrolytic activity. They are still important to note because they have reduced affinity for beta-lactamase inhibitors. Therefore, when these non–extended-spectrum beta-lactamases TEM-types combine with extended-spectrum beta-lactamase TEM-types, they have inhibitor resistance as well as the ability to hydrolyze third-generation cephalosporins. These combined TEM-types are referred to as \"complex mutants of TEM.\"[6]Extended-spectrum beta-lactamases are most commonly produced by Klebsiella pneumoniae and E coli, although other gram-negative bacilli can form these enzymes as well.",
"Studies on risk factors for acquiring an extended-spectrum beta-lactamase–positive infection have had conflicting results thus far. However, there is agreement that patients at higher risk for such infections include seriously ill patients with prolonged hospital stays and those in whom invasive medical devices were used for an extended duration. Heavy antibiotic use is another risk factor. Although extended-spectrum beta-lactamase–positive organisms were initially thought to occur only in hospitals, the literature shows that community-acquired infections with these organisms are becoming more prevalent.[8]",
"A retrospective study from 2009 to 2010 studied extended-spectrum beta-lactamase UTIs in Spain in hopes of discovering characteristics and risk factors for such UTIs. The conclusions yielded that male sex, hospitalization, diabetes, recurrent UTI, and comorbidity (score based on the Charlson index) were risk factors for extended-spectrum beta-lactamase–positive UTI.[8]",
"Given the large variance in extended-spectrum beta-lactamase–forming organisms from region to region, no standardized treatment has been established yet. However, infection control should be initiated promptly, and antibiotic coverage should be assigned according to organism susceptibility. Carbapenems are usually the drugs of choice for these highly resistant infections.",
"The patient in this case report had no further complications after extended-spectrum beta-lactamase–positive bacterial parotitis was diagnosed. Her antibiotic coverage was adjusted to treat resistant E coli. Her clinical symptoms began to improve, and ultrasound of the head and neck was repeated to ensure that no abscess had formed after the initial diagnosis. The second ultrasound (Figure 2) remained unchanged, and the patient was soon discharged from the hospital. She was sent to an extended-care facility, where she finished a course of ertapenem for 1 week. She was also educated on the importance of oral hygiene and given chewing gum to increase salivary flow."
],
"date": "April 27, 2016",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/862/410/862410-Thumb1.png"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/862/410/862410-Thumb2.png"
}
],
"markdown": "# Sudden-Onset Right-Cheek Swelling in a 69-Year-Old Woman\n\n **Authors:** Liza Cholin, BS; Jordan Burlen, BS; Umar Darr, MD \n **Date:** April 27, 2016\n\n ## Content\n\n Empirical treatment should be started after a clinical diagnosis of acute bacterial parotitis has been made. By far the most common organism found is S aureus, followed by S viridans and oral anaerobes.[5] Treatment should be aimed to cover methicillin-sensitive (or methicillin-resistant, depending on the hospital region and patient history) S aureus and oral aerobes and anaerobes until culture results are returned. For immunocompromised patients, consider covering for P aeruginosa and gram-negative bacilli in addition to the standard treatment.[5] A penicillin (ie, nafcillin) or first-generation cephalosporin in combination with an aminoglycoside is an appropriate initial treatment choice.[2]\nFigure 1.\nFigure 2.\nClindamycin or metronidazole can be used in place of an aminoglycoside as well. Vancomycin or linezolid can be considered if methicillin-resistant S aureus infection is a concern. Pseudomonal coverage can be accomplished by using cefepime or a carbapenem.\nIf treatment does not adequately control the infection, it can spread to surrounding tissues and give rise to several complications. Facial nerve palsy, septicemia, and thrombophlebitis of the jugular vein (Lemierre syndrome) are some of the problems that can arise when medical treatment fails.\nIf the patient does not appear to be responding to conservative management, an otolaryngology specialist should be consulted. Although there are no set guidelines for how soon to obtain a consult are noted, if the patient's condition does not improve within 3 days, more aggressive treatment is generally needed to clear the infection. A specialist is better able to determine the extent of the disease, as well as the next step in management. Sometimes, only incision and drainage is needed; however, parotidectomy is sometimes necessary.\nThe patient in this case report was continued on empirical treatment for acute bacterial parotitis and underwent ultrasound of the head and neck to rule out abscess. The ultrasound (Figure 1) revealed a heterogeneous echotexture of the enlarged gland but no definite cystic or solid lesions. The right parotid gland measured 6.5 x 4.3 x 3.3 cm.\nTwo days after the purulent discharge was sent for culture, the results came back positive for moderate growth of extended-spectrum beta-lactamase–producing Escherichia coli, moderate growth of Enterococcus faecalis, and light growth of C albicans. The patient's antibiotics were changed to meropenem and metronidazole.\nExtended-spectrum beta-lactamases are an increasingly reported threat to the future of antibiotic effectiveness. Although an exact definition does not exist, the general term is used to describe beta-lactamases that have formed resistance to penicillins; first-, second-, and third-generation cephalosporins; and aztreonam. However, they remain susceptible to clavulanic acid.[6]\nTwo general classification schemes are recognized: one that groups them according to the enzyme structure homology, and another that categorizes them according to functional similarities. The diversity of extended-spectrum beta-lactamase types is rapidly expanding. Several main gene groupings exist, from which many derivatives have emerged. These gene groups include SHV, TEM, CTX-M, Toho, OXA, and PER, as well as other, less common types.[7]Most infections in the United States have been with TEM-types and SHV-types.[6]\nMost of the TEM-types are considered extended-spectrum beta-lactamases; however, not all have extended-spectrum cephalosporin hydrolytic activity. They are still important to note because they have reduced affinity for beta-lactamase inhibitors. Therefore, when these non–extended-spectrum beta-lactamases TEM-types combine with extended-spectrum beta-lactamase TEM-types, they have inhibitor resistance as well as the ability to hydrolyze third-generation cephalosporins. These combined TEM-types are referred to as \"complex mutants of TEM.\"[6]Extended-spectrum beta-lactamases are most commonly produced by Klebsiella pneumoniae and E coli, although other gram-negative bacilli can form these enzymes as well.\nStudies on risk factors for acquiring an extended-spectrum beta-lactamase–positive infection have had conflicting results thus far. However, there is agreement that patients at higher risk for such infections include seriously ill patients with prolonged hospital stays and those in whom invasive medical devices were used for an extended duration. Heavy antibiotic use is another risk factor. Although extended-spectrum beta-lactamase–positive organisms were initially thought to occur only in hospitals, the literature shows that community-acquired infections with these organisms are becoming more prevalent.[8]\nA retrospective study from 2009 to 2010 studied extended-spectrum beta-lactamase UTIs in Spain in hopes of discovering characteristics and risk factors for such UTIs. The conclusions yielded that male sex, hospitalization, diabetes, recurrent UTI, and comorbidity (score based on the Charlson index) were risk factors for extended-spectrum beta-lactamase–positive UTI.[8]\nGiven the large variance in extended-spectrum beta-lactamase–forming organisms from region to region, no standardized treatment has been established yet. However, infection control should be initiated promptly, and antibiotic coverage should be assigned according to organism susceptibility. Carbapenems are usually the drugs of choice for these highly resistant infections.\nThe patient in this case report had no further complications after extended-spectrum beta-lactamase–positive bacterial parotitis was diagnosed. Her antibiotic coverage was adjusted to treat resistant E coli. Her clinical symptoms began to improve, and ultrasound of the head and neck was repeated to ensure that no abscess had formed after the initial diagnosis. The second ultrasound (Figure 2) remained unchanged, and the patient was soon discharged from the hospital. She was sent to an extended-care facility, where she finished a course of ertapenem for 1 week. She was also educated on the importance of oral hygiene and given chewing gum to increase salivary flow.\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n\n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 961337,
"choiceText": "<i>S pneumoniae</i>",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961339,
"choiceText": "<i>M catarrhalis </i>",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961341,
"choiceText": "<i>S aureus </i>",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961343,
"choiceText": "<i>H influenzae</i>\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<i>S aureus</i> is the most common organism cultured in patients with acute bacterial parotitis. The most accepted theory for this finding is that decreased salivary flow of the parotid gland allows oral cavity organisms to ascend the Stensen duct.\r\n",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 304037,
"questionText": "What is the most common organism associated with acute bacterial parotitis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 961345,
"choiceText": "Penicillins",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961347,
"choiceText": "Carbapenems ",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961349,
"choiceText": "Third-generation cephalosporins",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961351,
"choiceText": "Monobactams\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Extended-spectrum beta-lactamases have genetically altered sequences from older beta-lactamases that allow a broader range of antibiotic resistance. These extended-spectrum beta-lactamases are capable of hydrolyzing penicillins; first-generation, second-generation, and third-generation cephalosporins; and the monobactam aztreonam. Carbapenem and clavulanic acid, in most cases, remain effective treatment options for these more resistant organisms.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 304039,
"questionText": "To which of the following classes of antibiotics are extended-spectrum beta-lactamases susceptible?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Sudden-Onset Right-Cheek Swelling in a 69-Year-Old Woman"
},
{
"authors": "Liza Cholin, BS; Jordan Burlen, BS; Umar Darr, MD",
"content": [],
"date": "April 27, 2016",
"figures": [],
"markdown": "# Sudden-Onset Right-Cheek Swelling in a 69-Year-Old Woman\n\n **Authors:** Liza Cholin, BS; Jordan Burlen, BS; Umar Darr, MD \n **Date:** April 27, 2016\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 961337,
"choiceText": "<i>S pneumoniae</i>",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961339,
"choiceText": "<i>M catarrhalis </i>",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961341,
"choiceText": "<i>S aureus </i>",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961343,
"choiceText": "<i>H influenzae</i>\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<i>S aureus</i> is the most common organism cultured in patients with acute bacterial parotitis. The most accepted theory for this finding is that decreased salivary flow of the parotid gland allows oral cavity organisms to ascend the Stensen duct.\r\n",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 304037,
"questionText": "What is the most common organism associated with acute bacterial parotitis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 961345,
"choiceText": "Penicillins",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961347,
"choiceText": "Carbapenems ",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961349,
"choiceText": "Third-generation cephalosporins",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961351,
"choiceText": "Monobactams\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Extended-spectrum beta-lactamases have genetically altered sequences from older beta-lactamases that allow a broader range of antibiotic resistance. These extended-spectrum beta-lactamases are capable of hydrolyzing penicillins; first-generation, second-generation, and third-generation cephalosporins; and the monobactam aztreonam. Carbapenem and clavulanic acid, in most cases, remain effective treatment options for these more resistant organisms.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 304039,
"questionText": "To which of the following classes of antibiotics are extended-spectrum beta-lactamases susceptible?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Sudden-Onset Right-Cheek Swelling in a 69-Year-Old Woman"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 961321,
"choiceText": "Salivary gland neoplasm",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961323,
"choiceText": "Viral parotitis (mumps)",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961325,
"choiceText": "Bacterial (suppurative) parotitis",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961327,
"choiceText": "Sialolithiasis ",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961329,
"choiceText": "Swollen lymph nodes",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961331,
"choiceText": "Dental abscess ",
"correct": false,
"displayOrder": 6,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961333,
"choiceText": "External otitis",
"correct": false,
"displayOrder": 7,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961335,
"choiceText": "Autoimmune parotitis\r\n",
"correct": false,
"displayOrder": 8,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 304035,
"questionText": "On the basis of the history, physical examination, and workup, what is the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 961337,
"choiceText": "<i>S pneumoniae</i>",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961339,
"choiceText": "<i>M catarrhalis </i>",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961341,
"choiceText": "<i>S aureus </i>",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961343,
"choiceText": "<i>H influenzae</i>\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<i>S aureus</i> is the most common organism cultured in patients with acute bacterial parotitis. The most accepted theory for this finding is that decreased salivary flow of the parotid gland allows oral cavity organisms to ascend the Stensen duct.\r\n",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 304037,
"questionText": "What is the most common organism associated with acute bacterial parotitis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 961345,
"choiceText": "Penicillins",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961347,
"choiceText": "Carbapenems ",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961349,
"choiceText": "Third-generation cephalosporins",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961351,
"choiceText": "Monobactams\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Extended-spectrum beta-lactamases have genetically altered sequences from older beta-lactamases that allow a broader range of antibiotic resistance. These extended-spectrum beta-lactamases are capable of hydrolyzing penicillins; first-generation, second-generation, and third-generation cephalosporins; and the monobactam aztreonam. Carbapenem and clavulanic acid, in most cases, remain effective treatment options for these more resistant organisms.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 304039,
"questionText": "To which of the following classes of antibiotics are extended-spectrum beta-lactamases susceptible?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
862418 | /viewarticle/862418 | [
{
"authors": "Tami O. Tiamfook-Morgan, MD; Daniel M. Lindberg, MD",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.",
"A 39-year-old Guatemalan man presents to the emergency department (ED) with severe and debilitating back pain. The patient had been evaluated by his primary care provider (PCP) for back pain approximately 3 weeks ago; in addition, he was seen in the ED about 1 week ago for his back pain. The patient's pain started immediately after he caught a heavy bag of ice, and it has progressively worsened.",
"At both his visit to his PCP and in the ED, the patient was diagnosed with musculoskeletal back pain, and he was discharged with a therapeutic regimen that included a nonsteroidal anti-inflammatory drug (ibuprofen). He was advised to follow up with his PCP.",
"At today's presentation to the ED, the patient describes the pain as throbbing and diffuse throughout the lower back, with radiation to his left buttock and upper back. The pain is exacerbated by walking and relieved with rest. He denies having any associated lower-extremity numbness or paresthesias. He also denies any fever, chills, or night sweats. Review of systems is negative for loss of bowel or bladder control, difficulty urinating, or constipation.",
"The patient has no known chronic medical conditions. He smokes half a pack of cigarettes daily. He is currently not taking any medications other than the prescribed ibuprofen, and he denies intravenous drug abuse."
],
"date": "April 27, 2016",
"figures": [],
"markdown": "# A 39-Year-Old Man With Debilitating Back Pain\n\n **Authors:** Tami O. Tiamfook-Morgan, MD; Daniel M. Lindberg, MD \n **Date:** April 27, 2016\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.\nA 39-year-old Guatemalan man presents to the emergency department (ED) with severe and debilitating back pain. The patient had been evaluated by his primary care provider (PCP) for back pain approximately 3 weeks ago; in addition, he was seen in the ED about 1 week ago for his back pain. The patient's pain started immediately after he caught a heavy bag of ice, and it has progressively worsened.\nAt both his visit to his PCP and in the ED, the patient was diagnosed with musculoskeletal back pain, and he was discharged with a therapeutic regimen that included a nonsteroidal anti-inflammatory drug (ibuprofen). He was advised to follow up with his PCP.\nAt today's presentation to the ED, the patient describes the pain as throbbing and diffuse throughout the lower back, with radiation to his left buttock and upper back. The pain is exacerbated by walking and relieved with rest. He denies having any associated lower-extremity numbness or paresthesias. He also denies any fever, chills, or night sweats. Review of systems is negative for loss of bowel or bladder control, difficulty urinating, or constipation.\nThe patient has no known chronic medical conditions. He smokes half a pack of cigarettes daily. He is currently not taking any medications other than the prescribed ibuprofen, and he denies intravenous drug abuse.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 39-Year-Old Man With Debilitating Back Pain"
},
{
"authors": "Tami O. Tiamfook-Morgan, MD; Daniel M. Lindberg, MD",
"content": [
"On physical examination, the patient's temperature is 98.7°F, pulse is 75 beats/min, blood pressure is 138/69 mm Hg, and respiratory rate is 16 breaths/min. Head and neck examination findings are normal, and the lungs are clear to auscultation. Cardiac examination reveals normal S1 and S2 heart sounds, without any murmurs, rubs, or gallops. His abdomen is soft and nontender to palpation, and without any pulsatile masses. Rectal examination shows normal tone and brown, guaiac-negative stool.",
"Figure 1.",
"Figure 2.",
"Mild tenderness to palpation is noted in the midline lower back, at the T11-L1 levels. No significant pain is produced by flexion of the leg at the hip. The patient has normal strength and sensation in both lower extremities, and no sensory level is noted. Normal reflexes are noted in his upper and lower extremities. His gait is antalgic but without ataxia.",
"An anteroposterior lumbar radiograph (Figure 1) is obtained and, on the basis of the interpretation of the plain film, MRI of the spine (Figure 2) is obtained."
],
"date": "April 27, 2016",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/862/418/862418-Thumb1.png"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/862/418/862418-Thumb2.png"
}
],
"markdown": "# A 39-Year-Old Man With Debilitating Back Pain\n\n **Authors:** Tami O. Tiamfook-Morgan, MD; Daniel M. Lindberg, MD \n **Date:** April 27, 2016\n\n ## Content\n\n On physical examination, the patient's temperature is 98.7°F, pulse is 75 beats/min, blood pressure is 138/69 mm Hg, and respiratory rate is 16 breaths/min. Head and neck examination findings are normal, and the lungs are clear to auscultation. Cardiac examination reveals normal S1 and S2 heart sounds, without any murmurs, rubs, or gallops. His abdomen is soft and nontender to palpation, and without any pulsatile masses. Rectal examination shows normal tone and brown, guaiac-negative stool.\nFigure 1.\nFigure 2.\nMild tenderness to palpation is noted in the midline lower back, at the T11-L1 levels. No significant pain is produced by flexion of the leg at the hip. The patient has normal strength and sensation in both lower extremities, and no sensory level is noted. Normal reflexes are noted in his upper and lower extremities. His gait is antalgic but without ataxia.\nAn anteroposterior lumbar radiograph (Figure 1) is obtained and, on the basis of the interpretation of the plain film, MRI of the spine (Figure 2) is obtained.\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 961355,
"choiceText": "Spinal cord hemorrhage",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961357,
"choiceText": "Epidural abscess secondary to tuberculous spondylitis",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961359,
"choiceText": "Spinal malignant metastases\r\n",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961361,
"choiceText": "Epidural abscess secondary to pyogenic spondylitis\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 304041,
"questionText": "On the basis of the history, physical examination, and workup, what is the diagnosis?<br><br>\r\n<i>Hint: Look closely at T11</i>.",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 39-Year-Old Man With Debilitating Back Pain"
},
{
"authors": "Tami O. Tiamfook-Morgan, MD; Daniel M. Lindberg, MD",
"content": [
"This patient had a final diagnosis of an epidural abscess secondary to tuberculous spondylitis (also known as \"Pott disease\" or \"spinal tuberculosis\"). The lumbar plain-film radiographs revealed a compression fracture of the T11 vertebra (Figure 1). A subsequent MRI (Figure 2; sagittal T2-weighted image) revealed severe pathologic compression fractures, multilevel osteomyelitis, and an epidural abscess extending from T10 to T12. Although increased disk signal was noted, particularly in the T10-T11 disk space, the signal was also present at uninvolved levels; this finding was believed to be secondary to normal hydration, because enhancement consistent with diskitis was not demonstrated in contrast-enhanced images (not available) of the involved disk spaces. These radiologic findings suggested tuberculous spondylitis, which was definitively diagnosed after cultures revealed pansusceptible Mycobacterium tuberculosis.",
"Figure 1.",
"Figure 2.",
"Tuberculous spondylitis results from hematogenous spread of M tuberculosis. Percival Pott first described spinal tuberculosis in 1779; however, evidence of the disease can be seen in ancient mummies from Egypt and Peru. Although rare in industrialized countries, this disease continues to be seen in developing countries.",
"Tuberculosis is the world's deadliest infection. Because humans are the only carriers of M tuberculosis, eradication is possible, but tuberculosis control programs have had varied success; not surprisingly, some of the more successful countries include the United States and other industrialized nations. It is particularly important to consider the diagnosis when examining patients from Southeast Asia, India, China, and other endemic regions.",
"Tuberculous spondylitis is seen in approximately 8%-9% of cases of extrapulmonary tuberculosis. The vertebral bodies of the spine are susceptible to seeding from tuberculosis because of high blood flow throughout adulthood. The distribution of the vertebral blood supply also allows multiple adjacent vertebrae to be affected.",
"Pott disease is more commonly associated with late reactivation of tuberculosis than with primary infection. In the United States, Pott disease primarily occurs in adults and most commonly affects the lower thoracic and lumbar regions. In approximately 10% of cases, the cervical region is affected. Cervical and upper thoracic involvement is potentially more disabling[1] and can present with dysphagia, stridor, torticollis, hoarseness, and other neurologic deficits.",
"In tuberculous spondylitis, the infection leads to inflammatory bone destruction and caseating necrosis within the vertebral body.[2] It then spreads via the anterior/posterior longitudinal ligaments to adjacent vertebral bodies; typically, two or more contiguous vertebrae are involved (which is unlike the bony lesions seen in most cancers). This destruction can cause collapse of the vertebral bodies, producing spinal instability, spinal cord compression, and herniation of the disk. Involvement of the anterior and lateral portions of the vertebral body typically causes vertebral collapse, with kyphosis and gibbous deformity; cavitation and extradural masses more often result when the posterior vertebral body is affected.",
"A tuberculous abscess in the epidural region can also compress the spinal cord, frequently causing bilateral symptoms. Abscess formation or bony destruction may cause serious morbidity and permanent neurologic deficits. Uncommonly, cervical involvement can result in extension of disease into the neck or retropharynx. Lumbar disease can similarly track along fascial planes and form calcifications within psoas abscesses or extradural abscesses[3]; this finding is nearly pathognomonic for tuberculous infection."
],
"date": "April 27, 2016",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/862/418/862418-Thumb1.png"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/862/418/862418-Thumb2.png"
}
],
"markdown": "# A 39-Year-Old Man With Debilitating Back Pain\n\n **Authors:** Tami O. Tiamfook-Morgan, MD; Daniel M. Lindberg, MD \n **Date:** April 27, 2016\n\n ## Content\n\n This patient had a final diagnosis of an epidural abscess secondary to tuberculous spondylitis (also known as \"Pott disease\" or \"spinal tuberculosis\"). The lumbar plain-film radiographs revealed a compression fracture of the T11 vertebra (Figure 1). A subsequent MRI (Figure 2; sagittal T2-weighted image) revealed severe pathologic compression fractures, multilevel osteomyelitis, and an epidural abscess extending from T10 to T12. Although increased disk signal was noted, particularly in the T10-T11 disk space, the signal was also present at uninvolved levels; this finding was believed to be secondary to normal hydration, because enhancement consistent with diskitis was not demonstrated in contrast-enhanced images (not available) of the involved disk spaces. These radiologic findings suggested tuberculous spondylitis, which was definitively diagnosed after cultures revealed pansusceptible Mycobacterium tuberculosis.\nFigure 1.\nFigure 2.\nTuberculous spondylitis results from hematogenous spread of M tuberculosis. Percival Pott first described spinal tuberculosis in 1779; however, evidence of the disease can be seen in ancient mummies from Egypt and Peru. Although rare in industrialized countries, this disease continues to be seen in developing countries.\nTuberculosis is the world's deadliest infection. Because humans are the only carriers of M tuberculosis, eradication is possible, but tuberculosis control programs have had varied success; not surprisingly, some of the more successful countries include the United States and other industrialized nations. It is particularly important to consider the diagnosis when examining patients from Southeast Asia, India, China, and other endemic regions.\nTuberculous spondylitis is seen in approximately 8%-9% of cases of extrapulmonary tuberculosis. The vertebral bodies of the spine are susceptible to seeding from tuberculosis because of high blood flow throughout adulthood. The distribution of the vertebral blood supply also allows multiple adjacent vertebrae to be affected.\nPott disease is more commonly associated with late reactivation of tuberculosis than with primary infection. In the United States, Pott disease primarily occurs in adults and most commonly affects the lower thoracic and lumbar regions. In approximately 10% of cases, the cervical region is affected. Cervical and upper thoracic involvement is potentially more disabling[1] and can present with dysphagia, stridor, torticollis, hoarseness, and other neurologic deficits.\nIn tuberculous spondylitis, the infection leads to inflammatory bone destruction and caseating necrosis within the vertebral body.[2] It then spreads via the anterior/posterior longitudinal ligaments to adjacent vertebral bodies; typically, two or more contiguous vertebrae are involved (which is unlike the bony lesions seen in most cancers). This destruction can cause collapse of the vertebral bodies, producing spinal instability, spinal cord compression, and herniation of the disk. Involvement of the anterior and lateral portions of the vertebral body typically causes vertebral collapse, with kyphosis and gibbous deformity; cavitation and extradural masses more often result when the posterior vertebral body is affected.\nA tuberculous abscess in the epidural region can also compress the spinal cord, frequently causing bilateral symptoms. Abscess formation or bony destruction may cause serious morbidity and permanent neurologic deficits. Uncommonly, cervical involvement can result in extension of disease into the neck or retropharynx. Lumbar disease can similarly track along fascial planes and form calcifications within psoas abscesses or extradural abscesses[3]; this finding is nearly pathognomonic for tuberculous infection.\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n\n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 961355,
"choiceText": "Spinal cord hemorrhage",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961357,
"choiceText": "Epidural abscess secondary to tuberculous spondylitis",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961359,
"choiceText": "Spinal malignant metastases\r\n",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961361,
"choiceText": "Epidural abscess secondary to pyogenic spondylitis\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 304041,
"questionText": "On the basis of the history, physical examination, and workup, what is the diagnosis?<br><br>\r\n<i>Hint: Look closely at T11</i>.",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 39-Year-Old Man With Debilitating Back Pain"
},
{
"authors": "Tami O. Tiamfook-Morgan, MD; Daniel M. Lindberg, MD",
"content": [
"Tuberculous spondylitis typically presents with a 3- to 4-month history of achy back pain that gradually intensifies and is sometimes associated with muscle spasm or radicular pain. Fever, weight loss, and elevated white blood cell counts are not typical, presenting in less than 40% of cases.[4] Neurologic abnormalities occur in 50% of patients and may include nerve root pain, cauda equina syndrome, sensory loss, or paresis. The most serious complication of tuberculous spondylitis is spinal cord compression that causes paraplegia; this condition is also known as \"Pott paraplegia.\"",
"Diagnosis of Pott disease can be challenging because of the indolent nature of the disease and the extensive differential diagnosis. A comprehensive history that includes questions about the patient's country of origin, history of tuberculosis exposure, and family history of tuberculosis must be performed. A complete physical examination should also be performed, with careful assessment of the spine, skin, abdomen (looking for a flank mass), and lungs. A complete neurologic examination, including assessment of strength, rectal tone, perineal sensation, and lower-extremity reflexes, is vital. A chest radiograph can be obtained to visualize apical scarring, cavitary disease, or infiltrates; however, the diagnosis should still be investigated despite a negative chest radiograph, especially if clinical suspicion is strong.",
"Tuberculin skin testing (purified protein derivative [PPD]) should be performed because it is positive in 90% of immunocompetent patients with skeletal tuberculosis. Nonetheless, a negative tuberculin skin test does not exclude a diagnosis of tuberculosis. In particular, patients who are immunocompromised are more likely to have a false-negative PPD test; such patients are the most susceptible to Pott disease.[5] The erythrocyte sedimentation rate should also be checked, because it is frequently elevated (> 100 mm/h) in patients with Pott disease.",
"A biopsy of the affected area with positive acid-fast bacillus stain or cultures is diagnostic. Unfortunately, the tubercle bacillus is notably difficult to isolate, with only 50% of biopsies yielding positive cultures.",
"MRI and CT are both excellent studies for visualizing the typical findings of Pott disease. Plain radiographs can also show evidence of tuberculous spondylitis, including osteolytic destruction of the vertebral body, collapse of the vertebral body, increased anterior wedging, and reactive sclerosis. CT can reveal the bony detail of irregular osteolytic lesions, disk collapse, sclerosis, or disruption of bone circumference.",
"MRI is effective at demonstrating neural compression and the presence of epidural abscesses, and differentiating tuberculous spondylitis from pyogenic spondylitis; the latter condition is characterized by a thick and irregular enhancement of the abscess wall, whereas enhancement is typically smooth and thin in tuberculous spondylitis.[6] In addition, tuberculous spondylitis differs radiologically from pyogenic spondylitis in that the disk space is usually secondarily involved or not involved at all; vertebral body involvement with disk-space sparing may be seen. As a result of this pattern of involvement, tuberculous spondylitis may exhibit skip lesions from subligamentous extension, whereas pyogenic spondylitis almost always involves a disk and adjacent vertebral bodies."
],
"date": "April 27, 2016",
"figures": [],
"markdown": "# A 39-Year-Old Man With Debilitating Back Pain\n\n **Authors:** Tami O. Tiamfook-Morgan, MD; Daniel M. Lindberg, MD \n **Date:** April 27, 2016\n\n ## Content\n\n Tuberculous spondylitis typically presents with a 3- to 4-month history of achy back pain that gradually intensifies and is sometimes associated with muscle spasm or radicular pain. Fever, weight loss, and elevated white blood cell counts are not typical, presenting in less than 40% of cases.[4] Neurologic abnormalities occur in 50% of patients and may include nerve root pain, cauda equina syndrome, sensory loss, or paresis. The most serious complication of tuberculous spondylitis is spinal cord compression that causes paraplegia; this condition is also known as \"Pott paraplegia.\"\nDiagnosis of Pott disease can be challenging because of the indolent nature of the disease and the extensive differential diagnosis. A comprehensive history that includes questions about the patient's country of origin, history of tuberculosis exposure, and family history of tuberculosis must be performed. A complete physical examination should also be performed, with careful assessment of the spine, skin, abdomen (looking for a flank mass), and lungs. A complete neurologic examination, including assessment of strength, rectal tone, perineal sensation, and lower-extremity reflexes, is vital. A chest radiograph can be obtained to visualize apical scarring, cavitary disease, or infiltrates; however, the diagnosis should still be investigated despite a negative chest radiograph, especially if clinical suspicion is strong.\nTuberculin skin testing (purified protein derivative [PPD]) should be performed because it is positive in 90% of immunocompetent patients with skeletal tuberculosis. Nonetheless, a negative tuberculin skin test does not exclude a diagnosis of tuberculosis. In particular, patients who are immunocompromised are more likely to have a false-negative PPD test; such patients are the most susceptible to Pott disease.[5] The erythrocyte sedimentation rate should also be checked, because it is frequently elevated (> 100 mm/h) in patients with Pott disease.\nA biopsy of the affected area with positive acid-fast bacillus stain or cultures is diagnostic. Unfortunately, the tubercle bacillus is notably difficult to isolate, with only 50% of biopsies yielding positive cultures.\nMRI and CT are both excellent studies for visualizing the typical findings of Pott disease. Plain radiographs can also show evidence of tuberculous spondylitis, including osteolytic destruction of the vertebral body, collapse of the vertebral body, increased anterior wedging, and reactive sclerosis. CT can reveal the bony detail of irregular osteolytic lesions, disk collapse, sclerosis, or disruption of bone circumference.\nMRI is effective at demonstrating neural compression and the presence of epidural abscesses, and differentiating tuberculous spondylitis from pyogenic spondylitis; the latter condition is characterized by a thick and irregular enhancement of the abscess wall, whereas enhancement is typically smooth and thin in tuberculous spondylitis.[6] In addition, tuberculous spondylitis differs radiologically from pyogenic spondylitis in that the disk space is usually secondarily involved or not involved at all; vertebral body involvement with disk-space sparing may be seen. As a result of this pattern of involvement, tuberculous spondylitis may exhibit skip lesions from subligamentous extension, whereas pyogenic spondylitis almost always involves a disk and adjacent vertebral bodies.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 39-Year-Old Man With Debilitating Back Pain"
},
{
"authors": "Tami O. Tiamfook-Morgan, MD; Daniel M. Lindberg, MD",
"content": [
"Treatment of Pott disease consists of at least a three-drug and, frequently, a four-drug regimen of antituberculous medications. A 6-month course of therapy is recommended for tuberculosis involving all sites (except the meninges) by the American Thoracic Society, the Centers for Disease Control and Prevention, and the Infectious Diseases Society of America.[7]",
"In one study, routine surgery was not shown to be beneficial for the treatment of Pott disease;[8] however, if used judiciously, surgery may improve early mobilization and reduce mortality.[9] Surgery is more clearly indicated for decompression of the spinal cord if the patient has advanced neurologic deficits or neurologic deficits that progress or persist despite medical therapy, if the patient's spinal stability is a concern (with kyphosis greater than 40°), or if an epidural abscess needs to be drained.",
"After treatment, patients should be closely followed for their response to therapy and medication compliance, because these issues can significantly affect their individual outcomes.",
"The patient in this case was admitted to the hospital, where he underwent diskectomies of T10 to L1, corpectomy of T11 and T12, drainage of the epidural abscess, and fusion of T11 and T12. He was definitively diagnosed with tuberculous spondylitis (Pott disease) after cultures revealed pansusceptible M tuberculosis. He was discharged to home with a 9-month course of rifampin, isoniazid, pyridoxine, and pyrazinamide. He was able to ambulate at the time of discharge."
],
"date": "April 27, 2016",
"figures": [],
"markdown": "# A 39-Year-Old Man With Debilitating Back Pain\n\n **Authors:** Tami O. Tiamfook-Morgan, MD; Daniel M. Lindberg, MD \n **Date:** April 27, 2016\n\n ## Content\n\n Treatment of Pott disease consists of at least a three-drug and, frequently, a four-drug regimen of antituberculous medications. A 6-month course of therapy is recommended for tuberculosis involving all sites (except the meninges) by the American Thoracic Society, the Centers for Disease Control and Prevention, and the Infectious Diseases Society of America.[7]\nIn one study, routine surgery was not shown to be beneficial for the treatment of Pott disease;[8] however, if used judiciously, surgery may improve early mobilization and reduce mortality.[9] Surgery is more clearly indicated for decompression of the spinal cord if the patient has advanced neurologic deficits or neurologic deficits that progress or persist despite medical therapy, if the patient's spinal stability is a concern (with kyphosis greater than 40°), or if an epidural abscess needs to be drained.\nAfter treatment, patients should be closely followed for their response to therapy and medication compliance, because these issues can significantly affect their individual outcomes.\nThe patient in this case was admitted to the hospital, where he underwent diskectomies of T10 to L1, corpectomy of T11 and T12, drainage of the epidural abscess, and fusion of T11 and T12. He was definitively diagnosed with tuberculous spondylitis (Pott disease) after cultures revealed pansusceptible M tuberculosis. He was discharged to home with a 9-month course of rifampin, isoniazid, pyridoxine, and pyrazinamide. He was able to ambulate at the time of discharge.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 961363,
"choiceText": "Back pain",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961365,
"choiceText": "Fever",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961367,
"choiceText": "Night sweats",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961369,
"choiceText": "Weight loss",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961371,
"choiceText": "Cough\r\n",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Pott disease typically presents with a 3- to 4-month history of achy back pain that gradually intensifies and is sometimes associated with muscle spasm or radicular pain. Fever, weight loss, and night sweats are uncommon, although these symptoms are present in some cases. Cough is not typically associated with Pott disease; however, it can be seen in patients with pulmonary involvement of tuberculosis.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 304043,
"questionText": "Which of the following symptoms is not typically associated with Pott disease?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 961373,
"choiceText": "Surgery is not always recommended for treatment",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961375,
"choiceText": "MRI and CT are both sensitive for and suggestive of the diagnosis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961377,
"choiceText": "It most commonly affects the cervical region",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961379,
"choiceText": "The most serious complication of Pott disease is Pott paraplegia",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961381,
"choiceText": "An elevated white blood cell count may only be present in 40% of cases",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "\r\nPott disease most commonly affects the lower thoracic and lumbar regions in adults; the cervical region is affected in only 10% of cases. Surgery is not always recommended for treatment; however, if used judiciously, it may improve early mobilization and reduce mortality. MRI or CT is sensitive for and suggestive of the diagnosis; MRI is more sensitive than CT. The most serious complication of Pott disease is spinal cord compression causing paraplegia, also known as \"Pott paraplegia.\" An elevated white blood cell count may only be present in less than 40% of cases of Pott disease.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 304045,
"questionText": "Which of the following statements regarding Pott disease is false?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 39-Year-Old Man With Debilitating Back Pain"
},
{
"authors": "Tami O. Tiamfook-Morgan, MD; Daniel M. Lindberg, MD",
"content": [],
"date": "April 27, 2016",
"figures": [],
"markdown": "# A 39-Year-Old Man With Debilitating Back Pain\n\n **Authors:** Tami O. Tiamfook-Morgan, MD; Daniel M. Lindberg, MD \n **Date:** April 27, 2016\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 961363,
"choiceText": "Back pain",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961365,
"choiceText": "Fever",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961367,
"choiceText": "Night sweats",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961369,
"choiceText": "Weight loss",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961371,
"choiceText": "Cough\r\n",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Pott disease typically presents with a 3- to 4-month history of achy back pain that gradually intensifies and is sometimes associated with muscle spasm or radicular pain. Fever, weight loss, and night sweats are uncommon, although these symptoms are present in some cases. Cough is not typically associated with Pott disease; however, it can be seen in patients with pulmonary involvement of tuberculosis.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 304043,
"questionText": "Which of the following symptoms is not typically associated with Pott disease?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 961373,
"choiceText": "Surgery is not always recommended for treatment",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961375,
"choiceText": "MRI and CT are both sensitive for and suggestive of the diagnosis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961377,
"choiceText": "It most commonly affects the cervical region",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961379,
"choiceText": "The most serious complication of Pott disease is Pott paraplegia",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961381,
"choiceText": "An elevated white blood cell count may only be present in 40% of cases",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "\r\nPott disease most commonly affects the lower thoracic and lumbar regions in adults; the cervical region is affected in only 10% of cases. Surgery is not always recommended for treatment; however, if used judiciously, it may improve early mobilization and reduce mortality. MRI or CT is sensitive for and suggestive of the diagnosis; MRI is more sensitive than CT. The most serious complication of Pott disease is spinal cord compression causing paraplegia, also known as \"Pott paraplegia.\" An elevated white blood cell count may only be present in less than 40% of cases of Pott disease.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 304045,
"questionText": "Which of the following statements regarding Pott disease is false?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 39-Year-Old Man With Debilitating Back Pain"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 961355,
"choiceText": "Spinal cord hemorrhage",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961357,
"choiceText": "Epidural abscess secondary to tuberculous spondylitis",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961359,
"choiceText": "Spinal malignant metastases\r\n",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961361,
"choiceText": "Epidural abscess secondary to pyogenic spondylitis\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 304041,
"questionText": "On the basis of the history, physical examination, and workup, what is the diagnosis?<br><br>\r\n<i>Hint: Look closely at T11</i>.",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 961363,
"choiceText": "Back pain",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961365,
"choiceText": "Fever",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961367,
"choiceText": "Night sweats",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961369,
"choiceText": "Weight loss",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961371,
"choiceText": "Cough\r\n",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Pott disease typically presents with a 3- to 4-month history of achy back pain that gradually intensifies and is sometimes associated with muscle spasm or radicular pain. Fever, weight loss, and night sweats are uncommon, although these symptoms are present in some cases. Cough is not typically associated with Pott disease; however, it can be seen in patients with pulmonary involvement of tuberculosis.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 304043,
"questionText": "Which of the following symptoms is not typically associated with Pott disease?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 961373,
"choiceText": "Surgery is not always recommended for treatment",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961375,
"choiceText": "MRI and CT are both sensitive for and suggestive of the diagnosis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961377,
"choiceText": "It most commonly affects the cervical region",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961379,
"choiceText": "The most serious complication of Pott disease is Pott paraplegia",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961381,
"choiceText": "An elevated white blood cell count may only be present in 40% of cases",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "\r\nPott disease most commonly affects the lower thoracic and lumbar regions in adults; the cervical region is affected in only 10% of cases. Surgery is not always recommended for treatment; however, if used judiciously, it may improve early mobilization and reduce mortality. MRI or CT is sensitive for and suggestive of the diagnosis; MRI is more sensitive than CT. The most serious complication of Pott disease is spinal cord compression causing paraplegia, also known as \"Pott paraplegia.\" An elevated white blood cell count may only be present in less than 40% of cases of Pott disease.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 304045,
"questionText": "Which of the following statements regarding Pott disease is false?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
862420 | /viewarticle/862420 | [
{
"authors": "Emmanuel K. Konstantakos, MD; Lynn A. Crosby, MD",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.",
"A 57-year-old man presents to the emergency department with vague left shoulder pain for the past 3 months after an incident in which a garage door fell on him. The pain is slightly worse with movement, but he denies any limitation in the range of motion. The pain is dull and has recently become increasingly severe. He denies experiencing any prior trauma to the area, as well as any history of arthralgia, myalgia, other associated symptoms, or any prior surgeries or medical problems. He denies weight loss, chills, night sweats, or recent illnesses.",
"The patient smoked about one pack of cigarettes per day for about 30 years, but he quit 8 years ago. He drinks one or two beers every night. He does not use any illicit drugs and does not take any medications other than an occasional nonsteroidal anti-inflammatory drug, which seems to relieve the pain. He has no allergies."
],
"date": "April 27, 2016",
"figures": [],
"markdown": "# A 57-Year-Old Man With Vague Left Shoulder Pain\n\n **Authors:** Emmanuel K. Konstantakos, MD; Lynn A. Crosby, MD \n **Date:** April 27, 2016\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.\nA 57-year-old man presents to the emergency department with vague left shoulder pain for the past 3 months after an incident in which a garage door fell on him. The pain is slightly worse with movement, but he denies any limitation in the range of motion. The pain is dull and has recently become increasingly severe. He denies experiencing any prior trauma to the area, as well as any history of arthralgia, myalgia, other associated symptoms, or any prior surgeries or medical problems. He denies weight loss, chills, night sweats, or recent illnesses.\nThe patient smoked about one pack of cigarettes per day for about 30 years, but he quit 8 years ago. He drinks one or two beers every night. He does not use any illicit drugs and does not take any medications other than an occasional nonsteroidal anti-inflammatory drug, which seems to relieve the pain. He has no allergies.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 57-Year-Old Man With Vague Left Shoulder Pain"
},
{
"authors": "Emmanuel K. Konstantakos, MD; Lynn A. Crosby, MD",
"content": [
"On physical examination, the patient's oral temperature is 99.5°F; his pulse has a regular rhythm, measured at 87 beats/min; and his blood pressure is 136/88 mm Hg. He is noted to be in mild distress as a result of his left shoulder pain. The examination of his head, including inspection of the ears, eyes, and throat, is normal. His lungs are clear to auscultation. Heart sounds are normal, and no murmurs are detected. His abdomen is soft and nontender, and no distention is noted.",
"Figure 1.",
"Figure 2.",
"Figure 3.",
"The patient has full range of motion of the left shoulder and neck. Mild tenderness to palpation over the posterolateral aspect of the left scapula is found, but no lumps or nodules are palpated. He does not have any skin lesions.",
"The patient is neurovascularly intact throughout both of his upper extremities, with symmetric reflexes. No pain is elicited with provocative maneuvers of the shoulder, including the Neer, Hawkins-Kennedy, or O'Brien tests.",
"Laboratory studies, including a complete blood cell count (CBC) and a serum electrolyte panel with calcium levels, are obtained in the emergency department; results are normal. Plain radiographic films of the left shoulder are obtained, which reveal multiple well-circumscribed lytic lesions of the neck and glenoid regions of the scapula (Figures 1-3).",
"The patient is discharged from the emergency department with pain medication and a shoulder sling for comfort. He is asked to follow up with the orthopedic clinic within the following week. Serum immunoglobulin levels and serum and urine protein electrophoresis findings are all normal when obtained in the outpatient clinic. A biopsy is performed, which reveals a clear margin and a narrow zone of transition to normal surrounding bone."
],
"date": "April 27, 2016",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/862/420/862420-Thumb1.png"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/862/420/862420-Thumb2.png"
},
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/862/420/862420-Thumb3.png"
}
],
"markdown": "# A 57-Year-Old Man With Vague Left Shoulder Pain\n\n **Authors:** Emmanuel K. Konstantakos, MD; Lynn A. Crosby, MD \n **Date:** April 27, 2016\n\n ## Content\n\n On physical examination, the patient's oral temperature is 99.5°F; his pulse has a regular rhythm, measured at 87 beats/min; and his blood pressure is 136/88 mm Hg. He is noted to be in mild distress as a result of his left shoulder pain. The examination of his head, including inspection of the ears, eyes, and throat, is normal. His lungs are clear to auscultation. Heart sounds are normal, and no murmurs are detected. His abdomen is soft and nontender, and no distention is noted.\nFigure 1.\nFigure 2.\nFigure 3.\nThe patient has full range of motion of the left shoulder and neck. Mild tenderness to palpation over the posterolateral aspect of the left scapula is found, but no lumps or nodules are palpated. He does not have any skin lesions.\nThe patient is neurovascularly intact throughout both of his upper extremities, with symmetric reflexes. No pain is elicited with provocative maneuvers of the shoulder, including the Neer, Hawkins-Kennedy, or O'Brien tests.\nLaboratory studies, including a complete blood cell count (CBC) and a serum electrolyte panel with calcium levels, are obtained in the emergency department; results are normal. Plain radiographic films of the left shoulder are obtained, which reveal multiple well-circumscribed lytic lesions of the neck and glenoid regions of the scapula (Figures 1-3).\nThe patient is discharged from the emergency department with pain medication and a shoulder sling for comfort. He is asked to follow up with the orthopedic clinic within the following week. Serum immunoglobulin levels and serum and urine protein electrophoresis findings are all normal when obtained in the outpatient clinic. A biopsy is performed, which reveals a clear margin and a narrow zone of transition to normal surrounding bone.\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n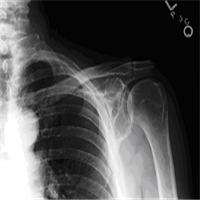 \n\n**Figure 3.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 961383,
"choiceText": "Fibrous dysplasia",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961385,
"choiceText": "Aneurysmal bone cyst",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961387,
"choiceText": "Isolated bone plasmacytoma",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961389,
"choiceText": "Chondrosarcoma\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 304047,
"questionText": "On the basis of the history, physical examination, and workup, what is the diagnosis?<br><br><i>Hint: Note the lytic characteristics of the lesions.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 57-Year-Old Man With Vague Left Shoulder Pain"
},
{
"authors": "Emmanuel K. Konstantakos, MD; Lynn A. Crosby, MD",
"content": [
"Isolated bone plasmacytoma (IBP) refers to a malignant, monoclonal plasma cell tumor growing only in bone. IBP is usually discovered as an incidental finding during routine radiographic studies for a separate, unrelated condition; however, patients may present with a single painful bone lesion.",
"IBP most commonly involves the axial skeleton (primarily, the vertebrae), although any area may be involved. Patients mainly experience painless swelling of the ribs, sternum, or other parts of the axial skeleton. IBP has a male-to-female ratio of 2:1, with a mean age of 55 years. Burt and colleagues[1] showed that the ribs, clavicle, sternum, or scapula were involved in 20% of patients presenting with IBP.",
"Although diagnostic criteria for identifying IBP vary, the presence of at least a single destructive bone lesion on plain films plus a fine-needle aspiration or core biopsy revealing infiltration by plasma cells are generally needed to establish the diagnosis. A skeletal survey with or without a bone scan is also helpful for ruling out other lesions, which could convert the diagnosis to multiple myeloma.",
"Laboratory studies, including a CBC, serum immunoglobulin levels, serum and urine protein electrophoresis, and a serum electrolyte panel with calcium levels, should be performed. A full skeletal survey should also be performed. MRI studies of the thoracic and lumbar spine with bone marrow aspirate should be part of the routine clinical workup in all patients presumed to have IBP. The absence of any further destructive lesions on the skeletal survey (specifically of the axial skeleton) and an absence of clonal plasma cells in a random sample of bone marrow help to differentiate IBP from multiple myeloma.",
"Lack of hypercalcemia, anemia, urinary monoclonal protein, and renal impairment also aids in the diagnosis of IBP. MRI is a noninvasive and useful means for investigating a large volume of bone marrow, and in some cases has revealed multiple myeloma in patients with a presumed diagnosis of IBP. Even as early as 1993, Moulopoulos and colleagues[2] showed that 33% of patients with an apparent isolated lesion were subsequently found to have evidence of multiple myeloma after MRI examination. Therefore, a negative MRI scan of the thoracic and lumbar spine is necessary to rule out multiple myeloma before making a definitive diagnosis of IBP.[3]"
],
"date": "April 27, 2016",
"figures": [],
"markdown": "# A 57-Year-Old Man With Vague Left Shoulder Pain\n\n **Authors:** Emmanuel K. Konstantakos, MD; Lynn A. Crosby, MD \n **Date:** April 27, 2016\n\n ## Content\n\n Isolated bone plasmacytoma (IBP) refers to a malignant, monoclonal plasma cell tumor growing only in bone. IBP is usually discovered as an incidental finding during routine radiographic studies for a separate, unrelated condition; however, patients may present with a single painful bone lesion.\nIBP most commonly involves the axial skeleton (primarily, the vertebrae), although any area may be involved. Patients mainly experience painless swelling of the ribs, sternum, or other parts of the axial skeleton. IBP has a male-to-female ratio of 2:1, with a mean age of 55 years. Burt and colleagues[1] showed that the ribs, clavicle, sternum, or scapula were involved in 20% of patients presenting with IBP.\nAlthough diagnostic criteria for identifying IBP vary, the presence of at least a single destructive bone lesion on plain films plus a fine-needle aspiration or core biopsy revealing infiltration by plasma cells are generally needed to establish the diagnosis. A skeletal survey with or without a bone scan is also helpful for ruling out other lesions, which could convert the diagnosis to multiple myeloma.\nLaboratory studies, including a CBC, serum immunoglobulin levels, serum and urine protein electrophoresis, and a serum electrolyte panel with calcium levels, should be performed. A full skeletal survey should also be performed. MRI studies of the thoracic and lumbar spine with bone marrow aspirate should be part of the routine clinical workup in all patients presumed to have IBP. The absence of any further destructive lesions on the skeletal survey (specifically of the axial skeleton) and an absence of clonal plasma cells in a random sample of bone marrow help to differentiate IBP from multiple myeloma.\nLack of hypercalcemia, anemia, urinary monoclonal protein, and renal impairment also aids in the diagnosis of IBP. MRI is a noninvasive and useful means for investigating a large volume of bone marrow, and in some cases has revealed multiple myeloma in patients with a presumed diagnosis of IBP. Even as early as 1993, Moulopoulos and colleagues[2] showed that 33% of patients with an apparent isolated lesion were subsequently found to have evidence of multiple myeloma after MRI examination. Therefore, a negative MRI scan of the thoracic and lumbar spine is necessary to rule out multiple myeloma before making a definitive diagnosis of IBP.[3]\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 961383,
"choiceText": "Fibrous dysplasia",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961385,
"choiceText": "Aneurysmal bone cyst",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961387,
"choiceText": "Isolated bone plasmacytoma",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961389,
"choiceText": "Chondrosarcoma\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 304047,
"questionText": "On the basis of the history, physical examination, and workup, what is the diagnosis?<br><br><i>Hint: Note the lytic characteristics of the lesions.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 57-Year-Old Man With Vague Left Shoulder Pain"
},
{
"authors": "Emmanuel K. Konstantakos, MD; Lynn A. Crosby, MD",
"content": [
"Radiography will typically show a lytic appearance. Patients with vertebral lesions may present with neurologic compromise as well. Schindler and colleagues[4] reported that IBP may present with signs and symptoms of demyelinating polyneuropathy. Imaging of the spine by MRI is required in the setting of vertebral involvement with neurologic deficit. Bone scanning is usually unreliable. MRI characteristically shows a focal area of bone marrow replacement on both T1- and T2-weighted images. Biopsy reveals a clear margin and a narrow zone of transition to normal surrounding bone.",
"Serum electrophoresis reveals monoclonal protein in the most patients with IBP. This correlates with disease progression, although less so than in multiple myeloma. Monoclonal protein in the serum or urine has been noted in 24%-72% of patients with IBP before treatment is initiated.[5,6]",
"IBP is highly radiosensitive; local radiation to the area of focal bone destruction is the primary treatment modality for IBP. All areas should include at least a 2-cm margin of normal tissue as shown on CT or MRI. A standard dose of 4500 cGy or greater, in 20-25 fractions, is preferred to reduce local recurrence rates. Using this regimen of radiation therapy, the risk for local recurrence is less than 5%.",
"Monoclonal protein levels are considerably reduced after local radiation. Monitoring myeloma protein levels for at least 6 months is required after radiation therapy. In 25%-50% of patients with IBP, the monoclonal protein disappears with radiation therapy.[7]",
"Persistence of monoclonal protein after radiation therapy suggests either the presence of other sites of tumor that were not included in the radiation field or progression to multiple myeloma, which occurs in some patients despite radiation therapy. IBP of the spine may present with rapid development of neurologic sequelae that may require decompressive procedures, such as laminectomy, before radiation.[8] Operative intervention is reserved for tumors that cause structural or neurovascular compromise.[9]",
"Previous reports have suggested decompressive laminectomy, spine fusion, or intramedullary rod fixation in patients who have signs of IBP with evidence of neurologic involvement or an impending fracture in the posterior elements of the vertebrae. Because most lesions appear in the vertebral body, however, reconstructing the anterior column and middle columns is recommended over laminectomy for spine tumors in general. In addition, cement augmentation (via kyphoplasty or vertebroplasty) may be used in cases of excessive collapse of the vertebral bodies.[8,10,11]",
"Recurrent IBP without evidence of multiple myeloma may develop after appropriate radiation therapy. In these cases, careful evaluation of the bone marrow, a bone survey, and MRI of the axial skeleton must again be performed to definitively rule out multiple myeloma.",
"After completion of radiation therapy, patients should be monitored for local recurrence and/or new bone lesions, as well as for any progression to multiple myeloma. Serial serum and urine electrophoresis, CBC, and renal functioning should be evaluated every 4-6 months. A skeletal survey should be performed every 6-12 months to monitor any new bone lesions."
],
"date": "April 27, 2016",
"figures": [],
"markdown": "# A 57-Year-Old Man With Vague Left Shoulder Pain\n\n **Authors:** Emmanuel K. Konstantakos, MD; Lynn A. Crosby, MD \n **Date:** April 27, 2016\n\n ## Content\n\n Radiography will typically show a lytic appearance. Patients with vertebral lesions may present with neurologic compromise as well. Schindler and colleagues[4] reported that IBP may present with signs and symptoms of demyelinating polyneuropathy. Imaging of the spine by MRI is required in the setting of vertebral involvement with neurologic deficit. Bone scanning is usually unreliable. MRI characteristically shows a focal area of bone marrow replacement on both T1- and T2-weighted images. Biopsy reveals a clear margin and a narrow zone of transition to normal surrounding bone.\nSerum electrophoresis reveals monoclonal protein in the most patients with IBP. This correlates with disease progression, although less so than in multiple myeloma. Monoclonal protein in the serum or urine has been noted in 24%-72% of patients with IBP before treatment is initiated.[5,6]\nIBP is highly radiosensitive; local radiation to the area of focal bone destruction is the primary treatment modality for IBP. All areas should include at least a 2-cm margin of normal tissue as shown on CT or MRI. A standard dose of 4500 cGy or greater, in 20-25 fractions, is preferred to reduce local recurrence rates. Using this regimen of radiation therapy, the risk for local recurrence is less than 5%.\nMonoclonal protein levels are considerably reduced after local radiation. Monitoring myeloma protein levels for at least 6 months is required after radiation therapy. In 25%-50% of patients with IBP, the monoclonal protein disappears with radiation therapy.[7]\nPersistence of monoclonal protein after radiation therapy suggests either the presence of other sites of tumor that were not included in the radiation field or progression to multiple myeloma, which occurs in some patients despite radiation therapy. IBP of the spine may present with rapid development of neurologic sequelae that may require decompressive procedures, such as laminectomy, before radiation.[8] Operative intervention is reserved for tumors that cause structural or neurovascular compromise.[9]\nPrevious reports have suggested decompressive laminectomy, spine fusion, or intramedullary rod fixation in patients who have signs of IBP with evidence of neurologic involvement or an impending fracture in the posterior elements of the vertebrae. Because most lesions appear in the vertebral body, however, reconstructing the anterior column and middle columns is recommended over laminectomy for spine tumors in general. In addition, cement augmentation (via kyphoplasty or vertebroplasty) may be used in cases of excessive collapse of the vertebral bodies.[8,10,11]\nRecurrent IBP without evidence of multiple myeloma may develop after appropriate radiation therapy. In these cases, careful evaluation of the bone marrow, a bone survey, and MRI of the axial skeleton must again be performed to definitively rule out multiple myeloma.\nAfter completion of radiation therapy, patients should be monitored for local recurrence and/or new bone lesions, as well as for any progression to multiple myeloma. Serial serum and urine electrophoresis, CBC, and renal functioning should be evaluated every 4-6 months. A skeletal survey should be performed every 6-12 months to monitor any new bone lesions.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 57-Year-Old Man With Vague Left Shoulder Pain"
},
{
"authors": "Emmanuel K. Konstantakos, MD; Lynn A. Crosby, MD",
"content": [
"Most patients with IBP develop multiple myeloma within 2-3 years, and some authors believe IBP to be an early manifestation of multiple myeloma.[12,13] Patients with the best prognosis are those in whom the monoclonal protein is undetectable 1 year after radiation therapy. Elderly patients with IBP or patients who have immunologic compromise have an associated risk for progression to multiple myeloma.[14] New bone lesions, development of marrow plasmacytosis, and an increasing myeloma protein level indicate progression to multiple myeloma.",
"No direct correlation between the dose of local radiation therapy and the possibility of disease progression has been established. Holland and colleagues[15] were able to correlate the size of the lesion (≥ 5 cm) with increased conversion rates to multiple myeloma. Mayr and colleagues[16] showed that adjuvant chemotherapy may prevent IBP from progressing to multiple myeloma. Others have found that adjuvant chemotherapy delays progression to multiple myeloma by 30 months[15]; however, current trends in the literature do not encourage the use of adjuvant chemotherapy for patients with IBP.",
"In addition, early exposure to chemotherapy may also increase resistance to other future chemotherapeutic options if multiple myeloma arises. Wilder and colleagues[17] have shown that the persistence of myeloma protein for more than 1 year after radiation therapy was the only independent prognostic factor indicating that patients with IBP will eventually develop multiple myeloma. With more radiologic and technologic advancements, the diagnosis of IBP should become less common, and earlier detection methods with more sensitive staging procedures may decrease the overall incidence of IBP in the future.",
"In the patient in this case, treatment with 4500 cGy in 25 fractions was performed after core biopsy revealed infiltration by plasma cells. The patient had an initial local MRI scan of the lesion, and extensive imaging ruled out remote disease. The biopsy findings were consistent with IBP. He did not have any detectable monoclonal protein levels at his most recent follow-up visit with the oncology service at 6 months after radiation therapy, and he continues to be in good condition, without any symptoms of shoulder pain. A skeletal survey is planned for his next follow-up appointment."
],
"date": "April 27, 2016",
"figures": [],
"markdown": "# A 57-Year-Old Man With Vague Left Shoulder Pain\n\n **Authors:** Emmanuel K. Konstantakos, MD; Lynn A. Crosby, MD \n **Date:** April 27, 2016\n\n ## Content\n\n Most patients with IBP develop multiple myeloma within 2-3 years, and some authors believe IBP to be an early manifestation of multiple myeloma.[12,13] Patients with the best prognosis are those in whom the monoclonal protein is undetectable 1 year after radiation therapy. Elderly patients with IBP or patients who have immunologic compromise have an associated risk for progression to multiple myeloma.[14] New bone lesions, development of marrow plasmacytosis, and an increasing myeloma protein level indicate progression to multiple myeloma.\nNo direct correlation between the dose of local radiation therapy and the possibility of disease progression has been established. Holland and colleagues[15] were able to correlate the size of the lesion (≥ 5 cm) with increased conversion rates to multiple myeloma. Mayr and colleagues[16] showed that adjuvant chemotherapy may prevent IBP from progressing to multiple myeloma. Others have found that adjuvant chemotherapy delays progression to multiple myeloma by 30 months[15]; however, current trends in the literature do not encourage the use of adjuvant chemotherapy for patients with IBP.\nIn addition, early exposure to chemotherapy may also increase resistance to other future chemotherapeutic options if multiple myeloma arises. Wilder and colleagues[17] have shown that the persistence of myeloma protein for more than 1 year after radiation therapy was the only independent prognostic factor indicating that patients with IBP will eventually develop multiple myeloma. With more radiologic and technologic advancements, the diagnosis of IBP should become less common, and earlier detection methods with more sensitive staging procedures may decrease the overall incidence of IBP in the future.\nIn the patient in this case, treatment with 4500 cGy in 25 fractions was performed after core biopsy revealed infiltration by plasma cells. The patient had an initial local MRI scan of the lesion, and extensive imaging ruled out remote disease. The biopsy findings were consistent with IBP. He did not have any detectable monoclonal protein levels at his most recent follow-up visit with the oncology service at 6 months after radiation therapy, and he continues to be in good condition, without any symptoms of shoulder pain. A skeletal survey is planned for his next follow-up appointment.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 961391,
"choiceText": "MRI of the thoracic and lumbar spine with bone marrow aspirate",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961393,
"choiceText": "Plain radiography showing a single destructive bone lesion, in addition to a fine-needle aspiration or core biopsy revealing infiltration by plasma cells",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961395,
"choiceText": "Serial serum and urine electrophoresis alone revealing elevated immunoglobulin G levels and Bence Jones proteins",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961397,
"choiceText": "Laboratory studies, including a CBC, serum immunoglobulin levels, serum and urine protein electrophoresis, and a serum electrolyte panel with calcium levels",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961399,
"choiceText": "A full skeletal survey, including standard x-rays of the skeleton with anteroposterior and lateral views of the cervical, thoracic and lumbar spine, chest, pelvis, humeri, and femora\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "IBP is usually discovered as an incidental finding during routine radiographic studies for a separate, unrelated condition; however, patients may present with a single painful bone lesion.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 304049,
"questionText": "You suspect that the patient you are examining has IBP, and schedule this patient for various tests. Which of the following diagnostic modalities would be sufficient to diagnose IBP in this patient?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 961409,
"choiceText": "Monoclonal protein levels detectable up to 1 year after radiation therapy",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961411,
"choiceText": "Immunologic compromise",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961413,
"choiceText": "High doses (> 4500 cGy) of local radiation therapy",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961415,
"choiceText": "A lesion of ≥ 5 cm",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "IBP is highly radiosensitive; local radiation to the area of focal bone destruction is the primary treatment modality for IBP. All areas should include at least a 2-cm margin of normal tissue as shown on CT or MRI. A standard dose of 4500 cGy or greater, in 20-25 fractions, is preferred in order to reduce local recurrence rates. Using this regimen of radiation therapy, the risk for local recurrence is less than 5%. Monoclonal protein levels are considerably reduced after local radiation.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 304053,
"questionText": "\r\nA major concern in any patient who is being treated for IBP is eventual progression of the disease to multiple myeloma. Which of the following procedures/patient findings does not indicate an increased risk for conversion to multiple myeloma?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 57-Year-Old Man With Vague Left Shoulder Pain"
},
{
"authors": "Emmanuel K. Konstantakos, MD; Lynn A. Crosby, MD",
"content": [],
"date": "April 27, 2016",
"figures": [],
"markdown": "# A 57-Year-Old Man With Vague Left Shoulder Pain\n\n **Authors:** Emmanuel K. Konstantakos, MD; Lynn A. Crosby, MD \n **Date:** April 27, 2016\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 961391,
"choiceText": "MRI of the thoracic and lumbar spine with bone marrow aspirate",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961393,
"choiceText": "Plain radiography showing a single destructive bone lesion, in addition to a fine-needle aspiration or core biopsy revealing infiltration by plasma cells",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961395,
"choiceText": "Serial serum and urine electrophoresis alone revealing elevated immunoglobulin G levels and Bence Jones proteins",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961397,
"choiceText": "Laboratory studies, including a CBC, serum immunoglobulin levels, serum and urine protein electrophoresis, and a serum electrolyte panel with calcium levels",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961399,
"choiceText": "A full skeletal survey, including standard x-rays of the skeleton with anteroposterior and lateral views of the cervical, thoracic and lumbar spine, chest, pelvis, humeri, and femora\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "IBP is usually discovered as an incidental finding during routine radiographic studies for a separate, unrelated condition; however, patients may present with a single painful bone lesion.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 304049,
"questionText": "You suspect that the patient you are examining has IBP, and schedule this patient for various tests. Which of the following diagnostic modalities would be sufficient to diagnose IBP in this patient?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 961409,
"choiceText": "Monoclonal protein levels detectable up to 1 year after radiation therapy",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961411,
"choiceText": "Immunologic compromise",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961413,
"choiceText": "High doses (> 4500 cGy) of local radiation therapy",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961415,
"choiceText": "A lesion of ≥ 5 cm",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "IBP is highly radiosensitive; local radiation to the area of focal bone destruction is the primary treatment modality for IBP. All areas should include at least a 2-cm margin of normal tissue as shown on CT or MRI. A standard dose of 4500 cGy or greater, in 20-25 fractions, is preferred in order to reduce local recurrence rates. Using this regimen of radiation therapy, the risk for local recurrence is less than 5%. Monoclonal protein levels are considerably reduced after local radiation.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 304053,
"questionText": "\r\nA major concern in any patient who is being treated for IBP is eventual progression of the disease to multiple myeloma. Which of the following procedures/patient findings does not indicate an increased risk for conversion to multiple myeloma?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 57-Year-Old Man With Vague Left Shoulder Pain"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 961383,
"choiceText": "Fibrous dysplasia",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961385,
"choiceText": "Aneurysmal bone cyst",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961387,
"choiceText": "Isolated bone plasmacytoma",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961389,
"choiceText": "Chondrosarcoma\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 304047,
"questionText": "On the basis of the history, physical examination, and workup, what is the diagnosis?<br><br><i>Hint: Note the lytic characteristics of the lesions.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 961391,
"choiceText": "MRI of the thoracic and lumbar spine with bone marrow aspirate",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961393,
"choiceText": "Plain radiography showing a single destructive bone lesion, in addition to a fine-needle aspiration or core biopsy revealing infiltration by plasma cells",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961395,
"choiceText": "Serial serum and urine electrophoresis alone revealing elevated immunoglobulin G levels and Bence Jones proteins",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961397,
"choiceText": "Laboratory studies, including a CBC, serum immunoglobulin levels, serum and urine protein electrophoresis, and a serum electrolyte panel with calcium levels",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961399,
"choiceText": "A full skeletal survey, including standard x-rays of the skeleton with anteroposterior and lateral views of the cervical, thoracic and lumbar spine, chest, pelvis, humeri, and femora\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "IBP is usually discovered as an incidental finding during routine radiographic studies for a separate, unrelated condition; however, patients may present with a single painful bone lesion.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 304049,
"questionText": "You suspect that the patient you are examining has IBP, and schedule this patient for various tests. Which of the following diagnostic modalities would be sufficient to diagnose IBP in this patient?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 961409,
"choiceText": "Monoclonal protein levels detectable up to 1 year after radiation therapy",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961411,
"choiceText": "Immunologic compromise",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961413,
"choiceText": "High doses (> 4500 cGy) of local radiation therapy",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 961415,
"choiceText": "A lesion of ≥ 5 cm",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "IBP is highly radiosensitive; local radiation to the area of focal bone destruction is the primary treatment modality for IBP. All areas should include at least a 2-cm margin of normal tissue as shown on CT or MRI. A standard dose of 4500 cGy or greater, in 20-25 fractions, is preferred in order to reduce local recurrence rates. Using this regimen of radiation therapy, the risk for local recurrence is less than 5%. Monoclonal protein levels are considerably reduced after local radiation.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 304053,
"questionText": "\r\nA major concern in any patient who is being treated for IBP is eventual progression of the disease to multiple myeloma. Which of the following procedures/patient findings does not indicate an increased risk for conversion to multiple myeloma?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
861790 | /viewarticle/861790 | [
{
"authors": "Jamie Shalkow, MD",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.",
"A 3-day-old East African baby boy is brought to the emergency department (ED) by his parents with a 2-day history of feeding intolerance and persistent vomiting of green fluid. Both parents are farmers, and the patient has five older siblings, all without any known medical conditions. He was born full-term at 39 weeks' gestation; however, he was small for his gestational age, measuring 18.1 in (46 cm) and weighing 4.6 lb (2.1 kg).",
"The patient's prenatal history is noncontributory, but it is notable that no antenatal ultrasonography to check for physical, physiologic, or amniotic fluid abnormalities was performed. The patient was delivered vaginally at home without any complications. He initially tolerated breastfeeding well and passed meconium during the first day of life.",
"Since the second day of life, he has not tolerated breastfeeding and has been vomiting recurrently. Initially, the emesis consisted of ingested milk and would occur approximately 30 minutes after having a meal. Subsequently, the vomiting became green, more profuse, and would start only 10 minutes after being fed. On the day of presentation, the vomiting is more frequent and occurs even without oral intake. In addition, he has not passed any stool for the past 24 hours."
],
"date": "April 14, 2016",
"figures": [],
"markdown": "# A 3-Day-Old Boy With Bilious Emesis\n\n **Authors:** Jamie Shalkow, MD \n **Date:** April 14, 2016\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.\nA 3-day-old East African baby boy is brought to the emergency department (ED) by his parents with a 2-day history of feeding intolerance and persistent vomiting of green fluid. Both parents are farmers, and the patient has five older siblings, all without any known medical conditions. He was born full-term at 39 weeks' gestation; however, he was small for his gestational age, measuring 18.1 in (46 cm) and weighing 4.6 lb (2.1 kg).\nThe patient's prenatal history is noncontributory, but it is notable that no antenatal ultrasonography to check for physical, physiologic, or amniotic fluid abnormalities was performed. The patient was delivered vaginally at home without any complications. He initially tolerated breastfeeding well and passed meconium during the first day of life.\nSince the second day of life, he has not tolerated breastfeeding and has been vomiting recurrently. Initially, the emesis consisted of ingested milk and would occur approximately 30 minutes after having a meal. Subsequently, the vomiting became green, more profuse, and would start only 10 minutes after being fed. On the day of presentation, the vomiting is more frequent and occurs even without oral intake. In addition, he has not passed any stool for the past 24 hours.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 3-Day-Old Boy With Bilious Emesis"
},
{
"authors": "Jamie Shalkow, MD",
"content": [
"The physical examination reveals a full-term baby boy who is small for his gestational age and in poor general condition. He is awake but hypotonic and hyporeactive. His temperature is 96.6° F (35.9°C), heart rate is 175 beats/min, respiratory rate is 48 breaths/min, and blood pressure is 75/40 mm Hg. He has a generalized grayish coloration, with acrocyanosis, and appears to be dehydrated. The head is normocephalic, with a depressed anterior fontanel, and the mucous membranes are dry.",
"No congenital anomalies are noted. The trachea is in a central position and there is no jugular venous distension. On chest examination, the respiratory movements are fast and shallow. Both lungs are clear to auscultation. Although tachycardic, the heart rate is regular and no murmurs are heard. The upper abdomen is grossly distended, whereas the lower abdomen appears scaphoid. There is a mild bluish discoloration of the abdominal skin, which also appears shiny and thin. Subcutaneous veins are easily seen. The baby retracts his legs upwards and cries while the abdomen is being palpated. No hepatosplenomegaly or masses are palpated, and no bowel sounds are noted. No rebound tenderness is observed. On rectal examination with a thermometer, bloody mucus is seen. The external genitalia are normal for the patient's age and gender. The extremities are thin and there is skin tenting. The capillary refill time is documented at 4 seconds.",
"Initially, oxygen is administered by nasal cannula at 1 L/min, and a 20 cc/kg bolus of Ringer lactate solution is given. An orogastric tube is passed, and 35 cc of bilious material is evacuated. A Foley catheter is inserted but no urine is initially obtained. After resuscitative measures are started, the patient is taken to the radiology department for a plain abdominal radiograph followed by an upper gastrointestinal contrast study."
],
"date": "April 14, 2016",
"figures": [],
"markdown": "# A 3-Day-Old Boy With Bilious Emesis\n\n **Authors:** Jamie Shalkow, MD \n **Date:** April 14, 2016\n\n ## Content\n\n The physical examination reveals a full-term baby boy who is small for his gestational age and in poor general condition. He is awake but hypotonic and hyporeactive. His temperature is 96.6° F (35.9°C), heart rate is 175 beats/min, respiratory rate is 48 breaths/min, and blood pressure is 75/40 mm Hg. He has a generalized grayish coloration, with acrocyanosis, and appears to be dehydrated. The head is normocephalic, with a depressed anterior fontanel, and the mucous membranes are dry.\nNo congenital anomalies are noted. The trachea is in a central position and there is no jugular venous distension. On chest examination, the respiratory movements are fast and shallow. Both lungs are clear to auscultation. Although tachycardic, the heart rate is regular and no murmurs are heard. The upper abdomen is grossly distended, whereas the lower abdomen appears scaphoid. There is a mild bluish discoloration of the abdominal skin, which also appears shiny and thin. Subcutaneous veins are easily seen. The baby retracts his legs upwards and cries while the abdomen is being palpated. No hepatosplenomegaly or masses are palpated, and no bowel sounds are noted. No rebound tenderness is observed. On rectal examination with a thermometer, bloody mucus is seen. The external genitalia are normal for the patient's age and gender. The extremities are thin and there is skin tenting. The capillary refill time is documented at 4 seconds.\nInitially, oxygen is administered by nasal cannula at 1 L/min, and a 20 cc/kg bolus of Ringer lactate solution is given. An orogastric tube is passed, and 35 cc of bilious material is evacuated. A Foley catheter is inserted but no urine is initially obtained. After resuscitative measures are started, the patient is taken to the radiology department for a plain abdominal radiograph followed by an upper gastrointestinal contrast study.\n\n ## Figures\n\n \n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 957997,
"choiceText": "Malrotation with midgut volvulus",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 957999,
"choiceText": "Intussusception",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 958001,
"choiceText": "Necrotizing enterocolitis\r\n",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 958003,
"choiceText": "Hirschsprung disease",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 302967,
"questionText": "On the basis of the history, physical examination, and workup, what is the diagnosis?<br><br>\r\n<i>\r\nHint: Consider the age of the patient and the color of the vomitus.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 3-Day-Old Boy With Bilious Emesis"
},
{
"authors": "Jamie Shalkow, MD",
"content": [
"Bilious emesis in a newborn should be considered a surgical emergency until proven otherwise. In a previously healthy neonate, this finding should raise suspicion for midgut volvulus, which was the ultimate diagnosis in this infant.",
"During the fourth week of embryonic life, the developing small intestine moves outside the abdominal cavity and into the umbilical cord. After the intestine enlarges and matures, it returns to the abdominal cavity during the 10th week of gestation. Rotation and final placement of the intestinal loops is completed by the eleventh week of pregnancy. This movement comprises a 270° counterclockwise turn that leaves the duodenojejunal junction at the ligament of Treitz fixed to the left of midline and the cecum fixed in the right lower quadrant.[1]",
"Figure 1.",
"Under normal circumstances, the base of the mesentery is both wide and immobile from the left upper to the right lower quadrant, creating a broad attachment that is unlikely to twist. When this movement is not completed in the usual fashion or does not happen at all, the small bowel is fixed and supported only by a narrow base of the mesentery. It can twist in a clockwise direction, causing a bowel obstruction and simultaneously compromising perfusion to the entire midgut, giving it a dark, dusky appearance when viewed surgically (Figure 1).[2,3] This anatomical condition, known as malrotation, is found in 0.5%-2% of asymptomatic patients at autopsy or during an upper gastrointestinal evaluation done for another reason. Malrotation is twice as common in boys as it is in girls.[4]",
"Midgut volvulus is the most common and catastrophic complication of a preexisting malrotation. Thirty percent of cases occur during the first week of life, and more than 50% of cases occur before 1 month of age.[2] Bilious emesis is the hallmark feature of the diagnosis, and more than 95% of patients with volvulus present with this symptom.[5] Initially, the infant may have a flat abdomen because the obstruction occurs fairly proximal at the duodenum; however, if the bowel becomes ischemic (and eventually necrotic), severe distension may result, and the infant may become tachycardic, tachypneic, pale, and diaphoretic. Fluid third-spacing and sequestration into the ischemic bowel produce dehydration and acidosis resulting from generalized hypoperfusion. Intestinal mucosal sloughing may eventually cause abdominal wall discoloration and hematochezia."
],
"date": "April 14, 2016",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/861/790/861790-Thumb1.png"
}
],
"markdown": "# A 3-Day-Old Boy With Bilious Emesis\n\n **Authors:** Jamie Shalkow, MD \n **Date:** April 14, 2016\n\n ## Content\n\n Bilious emesis in a newborn should be considered a surgical emergency until proven otherwise. In a previously healthy neonate, this finding should raise suspicion for midgut volvulus, which was the ultimate diagnosis in this infant.\nDuring the fourth week of embryonic life, the developing small intestine moves outside the abdominal cavity and into the umbilical cord. After the intestine enlarges and matures, it returns to the abdominal cavity during the 10th week of gestation. Rotation and final placement of the intestinal loops is completed by the eleventh week of pregnancy. This movement comprises a 270° counterclockwise turn that leaves the duodenojejunal junction at the ligament of Treitz fixed to the left of midline and the cecum fixed in the right lower quadrant.[1]\nFigure 1.\nUnder normal circumstances, the base of the mesentery is both wide and immobile from the left upper to the right lower quadrant, creating a broad attachment that is unlikely to twist. When this movement is not completed in the usual fashion or does not happen at all, the small bowel is fixed and supported only by a narrow base of the mesentery. It can twist in a clockwise direction, causing a bowel obstruction and simultaneously compromising perfusion to the entire midgut, giving it a dark, dusky appearance when viewed surgically (Figure 1).[2,3] This anatomical condition, known as malrotation, is found in 0.5%-2% of asymptomatic patients at autopsy or during an upper gastrointestinal evaluation done for another reason. Malrotation is twice as common in boys as it is in girls.[4]\nMidgut volvulus is the most common and catastrophic complication of a preexisting malrotation. Thirty percent of cases occur during the first week of life, and more than 50% of cases occur before 1 month of age.[2] Bilious emesis is the hallmark feature of the diagnosis, and more than 95% of patients with volvulus present with this symptom.[5] Initially, the infant may have a flat abdomen because the obstruction occurs fairly proximal at the duodenum; however, if the bowel becomes ischemic (and eventually necrotic), severe distension may result, and the infant may become tachycardic, tachypneic, pale, and diaphoretic. Fluid third-spacing and sequestration into the ischemic bowel produce dehydration and acidosis resulting from generalized hypoperfusion. Intestinal mucosal sloughing may eventually cause abdominal wall discoloration and hematochezia.\n\n ## Figures\n\n **Figure 1.** \n \n\n\n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 957997,
"choiceText": "Malrotation with midgut volvulus",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 957999,
"choiceText": "Intussusception",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 958001,
"choiceText": "Necrotizing enterocolitis\r\n",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 958003,
"choiceText": "Hirschsprung disease",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 302967,
"questionText": "On the basis of the history, physical examination, and workup, what is the diagnosis?<br><br>\r\n<i>\r\nHint: Consider the age of the patient and the color of the vomitus.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 3-Day-Old Boy With Bilious Emesis"
},
{
"authors": "Jamie Shalkow, MD",
"content": [
"The initial management of suspected midgut volvulus should include fluid resuscitation, nasogastric suctioning, and imaging with plain radiography.[2] Radiographic findings may include the \"double bubble\" sign, which is evidence of a proximal small bowel obstruction or a gasless abdomen (Figure 2; image shown is an example of malrotation without volvulus, and not from the actual patient). Blood should be sent to the laboratory for a complete blood cell count (CBC) and metabolic panel. A finding of acidosis should raise suspicion.[6]",
"Figure 2.",
"Figure 3.",
"Figure 4.",
"The confirmatory diagnostic studies of choice for midgut volvulus are either an upper gastrointestinal contrast study or a barium enema. As soon as the diagnosis of volvulus is seriously considered, a general surgeon should be contacted to discuss management and to expedite both confirmatory studies and definitive care. This condition is a true surgical emergency, with a mortality of approximately 15% and, when surgery is delayed, significant morbidity associated with necessary resection of ischemic bowel.",
"Most pediatric surgeons will prefer the upper gastrointestinal study because it confirms the position of the ligament of Treitz and demonstrates small bowel loops hanging completely into the right side of the abdomen (Figure 3).[2] The duodenal loop will be dilated and obstructed (corkscrew appearance), it will lack the classic \"C\" shape, and it won't cross the spine back into its normal left-sided location. A barium enema may demonstrate a cecum that is not in its normal right lower quadrant position (Figure 4). An upper gastrointestinal study is preferable because malrotation includes a spectrum of conditions which may prevent the intestine from being completely nonrotated. In some cases, the bowel may be only partially rotated, which can be missed on a barium enema.[1] Doppler ultrasonography may also be useful for assessing the vascular flow in the superior mesenteric artery and confirming the abnormal position of the superior mesenteric vein (which is normally to the right of the superior mesenteric artery).[5] Ultrasonography can also identify a fluid-filled, distended duodenum and small bowel loops exclusively to the right of the midline."
],
"date": "April 14, 2016",
"figures": [
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/861/790/861790-Thumb2.png"
},
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/861/790/861790-Thumb3.png"
},
{
"caption": "Figure 4.",
"image_url": "https://img.medscapestatic.com/article/861/790/861790-Thumb4.png"
}
],
"markdown": "# A 3-Day-Old Boy With Bilious Emesis\n\n **Authors:** Jamie Shalkow, MD \n **Date:** April 14, 2016\n\n ## Content\n\n The initial management of suspected midgut volvulus should include fluid resuscitation, nasogastric suctioning, and imaging with plain radiography.[2] Radiographic findings may include the \"double bubble\" sign, which is evidence of a proximal small bowel obstruction or a gasless abdomen (Figure 2; image shown is an example of malrotation without volvulus, and not from the actual patient). Blood should be sent to the laboratory for a complete blood cell count (CBC) and metabolic panel. A finding of acidosis should raise suspicion.[6]\nFigure 2.\nFigure 3.\nFigure 4.\nThe confirmatory diagnostic studies of choice for midgut volvulus are either an upper gastrointestinal contrast study or a barium enema. As soon as the diagnosis of volvulus is seriously considered, a general surgeon should be contacted to discuss management and to expedite both confirmatory studies and definitive care. This condition is a true surgical emergency, with a mortality of approximately 15% and, when surgery is delayed, significant morbidity associated with necessary resection of ischemic bowel.\nMost pediatric surgeons will prefer the upper gastrointestinal study because it confirms the position of the ligament of Treitz and demonstrates small bowel loops hanging completely into the right side of the abdomen (Figure 3).[2] The duodenal loop will be dilated and obstructed (corkscrew appearance), it will lack the classic \"C\" shape, and it won't cross the spine back into its normal left-sided location. A barium enema may demonstrate a cecum that is not in its normal right lower quadrant position (Figure 4). An upper gastrointestinal study is preferable because malrotation includes a spectrum of conditions which may prevent the intestine from being completely nonrotated. In some cases, the bowel may be only partially rotated, which can be missed on a barium enema.[1] Doppler ultrasonography may also be useful for assessing the vascular flow in the superior mesenteric artery and confirming the abnormal position of the superior mesenteric vein (which is normally to the right of the superior mesenteric artery).[5] Ultrasonography can also identify a fluid-filled, distended duodenum and small bowel loops exclusively to the right of the midline.\n\n ## Figures\n\n **Figure 2.** \n \n\n**Figure 3.** \n \n\n**Figure 4.** \n \n\n\n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 3-Day-Old Boy With Bilious Emesis"
},
{
"authors": "Jamie Shalkow, MD",
"content": [
"Once the diagnosis is made, surgery must occur as soon as possible. A delay in treatment may result in bowel necrosis of a large section of small intestine (which must then be resected) or even death. A patient should be rushed into the operating room while resuscitation continues. The procedure of choice, the Ladd procedure, was first described by Dr William Ladd in 1932.[1] William Ladd is considered the \"father of pediatric surgery.\" He was the first surgeon to devote his entire practice to the surgical care of children.",
"In the Ladd procedure, the abdomen is approached through a transverse supraumbilical incision. The first step is to untwist or \"devolvulate\" the bowel. On first inspection, it is sometimes difficult to realize in which direction the bowel has twisted. Derotation is always done in a counterclockwise fashion because the bowel always twists in a clockwise direction. The abnormal peritoneal attachments of the duodenum, right colon and cecum (Ladd bands) that create the narrow mesenteric base between the duodenum and cecum are then divided. This step widens the base of the small bowel mesentery by allowing maximal separation between the duodenum and the cecum, thereby preventing further twisting. The bowel is then returned to the abdominal cavity in a nonrotated position (leaving the small bowel in the right abdomen, and the colon on the left).",
"Fixing the bowel loops in this position with sutures has not proven to avoid further incidences of volvulus, and it has increased the incidence of intestinal postoperative complications, so it is not recommended.[1] An incidental appendectomy is also usually performed to avoid diagnostic dilemmas in the future. This can be done in a regular fashion. Finally, an orogastric tube is passed into the duodenum to rule out duodenal atresias or webs, which may occur concomitantly with malrotation. Postoperatively, the patient is kept NPO, with gastric decompression, administration of intravenous fluids, and antibiotic coverage. Depending on the intestinal damage encountered and the expected time to recovery, total parenteral nutrition may be required.",
"As much bowel should be preserved as possible during the procedure in order to avoid short bowel syndrome. No anastomoses should be performed in a bowel with questionable viability so as to avoid the risk of anastomotic leak.[5] Full intestinal resection entails several ethical considerations; these are beyond the scope of this presentation. Postoperative complications may include recurrent volvulus (2%-6%), short bowel syndrome, adhesions causing bowel obstruction, postoperative intussusceptions, and the need for total parenteral nutrition. Mortality rates have been reported to be between 2% and 24%, depending on the extent of bowel necrosis, associated anomalies, and the age of the patient.[3] Less than 10% necrosis at the time of surgery carries nearly a 100% survival rate; however, 75% necrosis at the time of surgery has only a 35% survival rate.[2]",
"In this case, the clinical picture and the initial plain abdominal film were considered enough to support the diagnosis of malrotation with midgut volvulus. The patient was brought to the operating room as resuscitation was continued. The abdomen was entered through a transverse supraumbilical wide incision, and the entire midgut (from the ligament of Treitz to the mid-transverse colon) was completely necrotic. The chances for survival were dismal. The case was discussed with the family. The child's surgical incision was sewn up and he was allowed to expire in a peaceful way under palliative care. This case underscores the catastrophic outcomes that these patients may have if not diagnosed and treated in a timely fashion."
],
"date": "April 14, 2016",
"figures": [],
"markdown": "# A 3-Day-Old Boy With Bilious Emesis\n\n **Authors:** Jamie Shalkow, MD \n **Date:** April 14, 2016\n\n ## Content\n\n Once the diagnosis is made, surgery must occur as soon as possible. A delay in treatment may result in bowel necrosis of a large section of small intestine (which must then be resected) or even death. A patient should be rushed into the operating room while resuscitation continues. The procedure of choice, the Ladd procedure, was first described by Dr William Ladd in 1932.[1] William Ladd is considered the \"father of pediatric surgery.\" He was the first surgeon to devote his entire practice to the surgical care of children.\nIn the Ladd procedure, the abdomen is approached through a transverse supraumbilical incision. The first step is to untwist or \"devolvulate\" the bowel. On first inspection, it is sometimes difficult to realize in which direction the bowel has twisted. Derotation is always done in a counterclockwise fashion because the bowel always twists in a clockwise direction. The abnormal peritoneal attachments of the duodenum, right colon and cecum (Ladd bands) that create the narrow mesenteric base between the duodenum and cecum are then divided. This step widens the base of the small bowel mesentery by allowing maximal separation between the duodenum and the cecum, thereby preventing further twisting. The bowel is then returned to the abdominal cavity in a nonrotated position (leaving the small bowel in the right abdomen, and the colon on the left).\nFixing the bowel loops in this position with sutures has not proven to avoid further incidences of volvulus, and it has increased the incidence of intestinal postoperative complications, so it is not recommended.[1] An incidental appendectomy is also usually performed to avoid diagnostic dilemmas in the future. This can be done in a regular fashion. Finally, an orogastric tube is passed into the duodenum to rule out duodenal atresias or webs, which may occur concomitantly with malrotation. Postoperatively, the patient is kept NPO, with gastric decompression, administration of intravenous fluids, and antibiotic coverage. Depending on the intestinal damage encountered and the expected time to recovery, total parenteral nutrition may be required.\nAs much bowel should be preserved as possible during the procedure in order to avoid short bowel syndrome. No anastomoses should be performed in a bowel with questionable viability so as to avoid the risk of anastomotic leak.[5] Full intestinal resection entails several ethical considerations; these are beyond the scope of this presentation. Postoperative complications may include recurrent volvulus (2%-6%), short bowel syndrome, adhesions causing bowel obstruction, postoperative intussusceptions, and the need for total parenteral nutrition. Mortality rates have been reported to be between 2% and 24%, depending on the extent of bowel necrosis, associated anomalies, and the age of the patient.[3] Less than 10% necrosis at the time of surgery carries nearly a 100% survival rate; however, 75% necrosis at the time of surgery has only a 35% survival rate.[2]\nIn this case, the clinical picture and the initial plain abdominal film were considered enough to support the diagnosis of malrotation with midgut volvulus. The patient was brought to the operating room as resuscitation was continued. The abdomen was entered through a transverse supraumbilical wide incision, and the entire midgut (from the ligament of Treitz to the mid-transverse colon) was completely necrotic. The chances for survival were dismal. The case was discussed with the family. The child's surgical incision was sewn up and he was allowed to expire in a peaceful way under palliative care. This case underscores the catastrophic outcomes that these patients may have if not diagnosed and treated in a timely fashion.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 958005,
"choiceText": "Clinical finding of a dehydrated baby with distended abdomen",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 958007,
"choiceText": "Plain abdominal radiography",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 958009,
"choiceText": "Upper gastrointestinal contrast study",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 958011,
"choiceText": "Ultrasonography",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 958013,
"choiceText": "Barium enema\r\n\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "An upper gastrointestinal contrast study is considered the criterion standard for diagnosis.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 302969,
"questionText": "You are evaluating a child who has been brought to the emergency department by his parents for persistent bilious vomiting, which raises the concern for midgut volvulus. Which of the following studies/procedures is the standard criterion for the diagnosis of malrotation and midgut volvulus?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 958015,
"choiceText": "Proper resuscitation to keep the patient hydrated and well oxygenated to preserve the bowel",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 958017,
"choiceText": "Upper gastrointestinal endoscopy to untwist the bowel\t",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 958019,
"choiceText": "Aggressive intestinal resection",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 958021,
"choiceText": "Ladd procedure\t",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 958023,
"choiceText": "Kimura procedure\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Adequate resuscitation of the patient is an essential part of the treatment; however, the bowel must be untwisted in order to preserve its blood supply. As far as we know, this has never been reported via an upper gastrointestinal endoscopy. Aggressive intestinal resections are best avoided unless absolutely necessary in order to decrease the risk for short gut syndrome. The Kimura procedure is an inverted diamond-shaped duodenoduodenostomy (i-DSD), which is a commonly used surgical technique for duodenal atresia. The procedure of choice for malrotation with midgut volvulus is the Ladd procedure, which includes derotation of the twisted bowel in a counterclockwise fashion, division of the Ladd bands, widening of the mesentery, placing the small bowel into the right abdomen and the colon into the left, ruling out associated duodenal atresia, and prophylactic appendectomy.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 302971,
"questionText": "Based on the results of the diagnostic study/procedure mentioned above, the diagnosis of midgut volvulus is made in the presenting patient. Which of the following would be the treatment of choice for this patient?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 3-Day-Old Boy With Bilious Emesis"
},
{
"authors": "Jamie Shalkow, MD",
"content": [],
"date": "April 14, 2016",
"figures": [],
"markdown": "# A 3-Day-Old Boy With Bilious Emesis\n\n **Authors:** Jamie Shalkow, MD \n **Date:** April 14, 2016\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 958005,
"choiceText": "Clinical finding of a dehydrated baby with distended abdomen",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 958007,
"choiceText": "Plain abdominal radiography",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 958009,
"choiceText": "Upper gastrointestinal contrast study",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 958011,
"choiceText": "Ultrasonography",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 958013,
"choiceText": "Barium enema\r\n\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "An upper gastrointestinal contrast study is considered the criterion standard for diagnosis.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 302969,
"questionText": "You are evaluating a child who has been brought to the emergency department by his parents for persistent bilious vomiting, which raises the concern for midgut volvulus. Which of the following studies/procedures is the standard criterion for the diagnosis of malrotation and midgut volvulus?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 958015,
"choiceText": "Proper resuscitation to keep the patient hydrated and well oxygenated to preserve the bowel",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 958017,
"choiceText": "Upper gastrointestinal endoscopy to untwist the bowel\t",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 958019,
"choiceText": "Aggressive intestinal resection",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 958021,
"choiceText": "Ladd procedure\t",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 958023,
"choiceText": "Kimura procedure\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Adequate resuscitation of the patient is an essential part of the treatment; however, the bowel must be untwisted in order to preserve its blood supply. As far as we know, this has never been reported via an upper gastrointestinal endoscopy. Aggressive intestinal resections are best avoided unless absolutely necessary in order to decrease the risk for short gut syndrome. The Kimura procedure is an inverted diamond-shaped duodenoduodenostomy (i-DSD), which is a commonly used surgical technique for duodenal atresia. The procedure of choice for malrotation with midgut volvulus is the Ladd procedure, which includes derotation of the twisted bowel in a counterclockwise fashion, division of the Ladd bands, widening of the mesentery, placing the small bowel into the right abdomen and the colon into the left, ruling out associated duodenal atresia, and prophylactic appendectomy.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 302971,
"questionText": "Based on the results of the diagnostic study/procedure mentioned above, the diagnosis of midgut volvulus is made in the presenting patient. Which of the following would be the treatment of choice for this patient?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 3-Day-Old Boy With Bilious Emesis"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 957997,
"choiceText": "Malrotation with midgut volvulus",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 957999,
"choiceText": "Intussusception",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 958001,
"choiceText": "Necrotizing enterocolitis\r\n",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 958003,
"choiceText": "Hirschsprung disease",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 302967,
"questionText": "On the basis of the history, physical examination, and workup, what is the diagnosis?<br><br>\r\n<i>\r\nHint: Consider the age of the patient and the color of the vomitus.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 958005,
"choiceText": "Clinical finding of a dehydrated baby with distended abdomen",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 958007,
"choiceText": "Plain abdominal radiography",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 958009,
"choiceText": "Upper gastrointestinal contrast study",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 958011,
"choiceText": "Ultrasonography",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 958013,
"choiceText": "Barium enema\r\n\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "An upper gastrointestinal contrast study is considered the criterion standard for diagnosis.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 302969,
"questionText": "You are evaluating a child who has been brought to the emergency department by his parents for persistent bilious vomiting, which raises the concern for midgut volvulus. Which of the following studies/procedures is the standard criterion for the diagnosis of malrotation and midgut volvulus?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 958015,
"choiceText": "Proper resuscitation to keep the patient hydrated and well oxygenated to preserve the bowel",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 958017,
"choiceText": "Upper gastrointestinal endoscopy to untwist the bowel\t",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 958019,
"choiceText": "Aggressive intestinal resection",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 958021,
"choiceText": "Ladd procedure\t",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 958023,
"choiceText": "Kimura procedure\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Adequate resuscitation of the patient is an essential part of the treatment; however, the bowel must be untwisted in order to preserve its blood supply. As far as we know, this has never been reported via an upper gastrointestinal endoscopy. Aggressive intestinal resections are best avoided unless absolutely necessary in order to decrease the risk for short gut syndrome. The Kimura procedure is an inverted diamond-shaped duodenoduodenostomy (i-DSD), which is a commonly used surgical technique for duodenal atresia. The procedure of choice for malrotation with midgut volvulus is the Ladd procedure, which includes derotation of the twisted bowel in a counterclockwise fashion, division of the Ladd bands, widening of the mesentery, placing the small bowel into the right abdomen and the colon into the left, ruling out associated duodenal atresia, and prophylactic appendectomy.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 302971,
"questionText": "Based on the results of the diagnostic study/procedure mentioned above, the diagnosis of midgut volvulus is made in the presenting patient. Which of the following would be the treatment of choice for this patient?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
861697 | /viewarticle/861697 | [
{
"authors": "Sumaira Nabi, MBBS; Shahzad Ahmed, MBBS; Mazhar Badshah, MBBS; Ali Nomani, MBBS",
"content": [
"Editor's Note:\n\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.",
"A 62-year-old Pakistani man with a medical history of hypertension for 10 years and noncompliance with antihypertensive medication presents with a 1-year history of involuntary movements of the left half of the face. These movements are exaggerated by talking, laughing, and anxiety. These were initially brief and episodic and only involved the ocular muscles.",
"Three months before presentation, the movements intensified to more sustained tonic contractions involving the entire left half of the face. This is associated with pulsatile tinnitus in the right ear and recurrent episodic vertigo. No history of facial pain, hearing loss, visual impairment, or falls is reported. Past history does not include stroke or transient ischemic attacks. He had been taking enteral baclofen, amlodipine, and betahistine dihydrochloride (an antivertigo drug) for 2 months. He is a nonsmoker and denies substance abuse. He has a strong family history of essential hypertension."
],
"date": "April 12, 2016",
"figures": [],
"markdown": "# A 62-Year-Old Man With Involuntary Facial Movements\n\n **Authors:** Sumaira Nabi, MBBS; Shahzad Ahmed, MBBS; Mazhar Badshah, MBBS; Ali Nomani, MBBS \n **Date:** April 12, 2016\n\n ## Content\n\n Editor's Note:\n\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.\nA 62-year-old Pakistani man with a medical history of hypertension for 10 years and noncompliance with antihypertensive medication presents with a 1-year history of involuntary movements of the left half of the face. These movements are exaggerated by talking, laughing, and anxiety. These were initially brief and episodic and only involved the ocular muscles.\nThree months before presentation, the movements intensified to more sustained tonic contractions involving the entire left half of the face. This is associated with pulsatile tinnitus in the right ear and recurrent episodic vertigo. No history of facial pain, hearing loss, visual impairment, or falls is reported. Past history does not include stroke or transient ischemic attacks. He had been taking enteral baclofen, amlodipine, and betahistine dihydrochloride (an antivertigo drug) for 2 months. He is a nonsmoker and denies substance abuse. He has a strong family history of essential hypertension.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 62-Year-Old Man With Involuntary Facial Movements"
},
{
"authors": "Sumaira Nabi, MBBS; Shahzad Ahmed, MBBS; Mazhar Badshah, MBBS; Ali Nomani, MBBS",
"content": [
"On physical examination, the patient is a middle-aged man, conscious, and oriented to time, place, and person. He has a regular pulse of 86 beats/min. His blood pressure is 140/90 mm Hg and respiratory rate is 16/min. His oral temperature is 98.6° F. His score on the Glasgow Coma Scale is 15/15.",
"He has periodic involuntary movements of the left half of the face, with eyelid twitching and deviation of mouth. The cranial nerves are intact and symmetric. Funduscopic examination reveals grade II hypertensive retinopathy. No signs of pyramidal weakness or incoordination are present. Signs of meningeal irritation are absent. His abdomen is soft and nontender. No clinical evidence suggests organomegaly or ascites. His bowel sounds are audible. The patient's precordial examination reveals normal heart sounds. Auscultation of the lung fields reveals normal vesicular breath sounds.",
"Figure 1.",
"Figure 1.",
"Figure 2.",
"Figure 2.",
"Figure 3.",
"Figure 3.",
"The laboratory analysis demonstrates a normal complete blood cell count and erythrocyte sedimentation rate. His liver function test results, renal function test results, blood sugar levels, and electrolyte levels are normal. His urinalysis reveals trace proteinuria. His lipid profile is abnormal, with total cholesterol of 247 mg/dL, low-density lipoprotein (LDL)-cholesterol level of 143 mg/dL, triglyceride level of 190 mg/dL, and high-density lipoprotein (HDL)-cholesterol level of 38 mg/dL.",
"Ultrasonography findings in the abdomen are normal. His chest radiography findings are unremarkable. ECG and echocardiogram are unrevealing. An MRI of the brain with and without contrast demonstrates a severely tortuous, dilated, and ectatic vertebrobasilar system, particularly the left vertebral artery (Figures 1 and 2). The internal carotid and middle cerebral vessels are also dilated (Figure 3). No arteriovenous malformation is seen. An altered magnetic resonance (MR) signal is noted in the periventricular and subcortical white matter, which is suggestive of chronic microvascular angiopathy. The ventricles are normal in size.",
"CT angiography (CTA) of the head reveals elongated, dilated, tortuous vertebral and basilar arteries and branches, whereas the internal carotids and middle cerebral are dilated on both sides but not tortuous. The left posterior circulation is more severely affected."
],
"date": "April 12, 2016",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/861/697/861697-Thumb1.png"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/861/697/861697-Thumb2.png"
},
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/861/697/861697-Thumb3.png"
}
],
"markdown": "# A 62-Year-Old Man With Involuntary Facial Movements\n\n **Authors:** Sumaira Nabi, MBBS; Shahzad Ahmed, MBBS; Mazhar Badshah, MBBS; Ali Nomani, MBBS \n **Date:** April 12, 2016\n\n ## Content\n\n On physical examination, the patient is a middle-aged man, conscious, and oriented to time, place, and person. He has a regular pulse of 86 beats/min. His blood pressure is 140/90 mm Hg and respiratory rate is 16/min. His oral temperature is 98.6° F. His score on the Glasgow Coma Scale is 15/15.\nHe has periodic involuntary movements of the left half of the face, with eyelid twitching and deviation of mouth. The cranial nerves are intact and symmetric. Funduscopic examination reveals grade II hypertensive retinopathy. No signs of pyramidal weakness or incoordination are present. Signs of meningeal irritation are absent. His abdomen is soft and nontender. No clinical evidence suggests organomegaly or ascites. His bowel sounds are audible. The patient's precordial examination reveals normal heart sounds. Auscultation of the lung fields reveals normal vesicular breath sounds.\nFigure 1.\nFigure 1.\nFigure 2.\nFigure 2.\nFigure 3.\nFigure 3.\nThe laboratory analysis demonstrates a normal complete blood cell count and erythrocyte sedimentation rate. His liver function test results, renal function test results, blood sugar levels, and electrolyte levels are normal. His urinalysis reveals trace proteinuria. His lipid profile is abnormal, with total cholesterol of 247 mg/dL, low-density lipoprotein (LDL)-cholesterol level of 143 mg/dL, triglyceride level of 190 mg/dL, and high-density lipoprotein (HDL)-cholesterol level of 38 mg/dL.\nUltrasonography findings in the abdomen are normal. His chest radiography findings are unremarkable. ECG and echocardiogram are unrevealing. An MRI of the brain with and without contrast demonstrates a severely tortuous, dilated, and ectatic vertebrobasilar system, particularly the left vertebral artery (Figures 1 and 2). The internal carotid and middle cerebral vessels are also dilated (Figure 3). No arteriovenous malformation is seen. An altered magnetic resonance (MR) signal is noted in the periventricular and subcortical white matter, which is suggestive of chronic microvascular angiopathy. The ventricles are normal in size.\nCT angiography (CTA) of the head reveals elongated, dilated, tortuous vertebral and basilar arteries and branches, whereas the internal carotids and middle cerebral are dilated on both sides but not tortuous. The left posterior circulation is more severely affected.\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n**Figure 3.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 957151,
"choiceText": "Aneurysm",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 957153,
"choiceText": "Multiple sclerosis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 957155,
"choiceText": "Tuberculoma",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 957157,
"choiceText": "Cranial arterial dolichoectasia",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 302713,
"questionText": "On the basis of the history, physical examination, and workup, what is the diagnosis?\r\n<br><br><i>\r\nHint: Note the history of hemifacial spasm and the typical neuroradiologic findings.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 62-Year-Old Man With Involuntary Facial Movements"
},
{
"authors": "Sumaira Nabi, MBBS; Shahzad Ahmed, MBBS; Mazhar Badshah, MBBS; Ali Nomani, MBBS",
"content": [
"This patient's presentation leads to a wide range of differential considerations, including vascular compression within the cerebellopontine angle, stroke, a demyelinating disease (eg, multiple sclerosis), or a mass lesion-like tumor compressing the seventh nerve. MRI of the brain in this case effectively ruled out stroke, multiple sclerosis, and a mass lesion. However, it revealed dilated tortuous intracranial vasculature, which most likely was the cause of these symptoms.",
"CTA confirmed the diagnosis of cranial arterial dolichoectasia (CADE). The patient was started on antihypertensive and lipid-lowering medications after a thorough literature review. He was also administered botulinum neurotoxin in the left orbicularis oculi and orbicularis oris muscles. The patient was then discharged on close follow-up on outpatient basis.",
"The authors sought a neurosurgical opinion in this case. However, because the benefit of neurovascular intervention is controversial, the patient declined microvascular decompression surgery. At 3-month follow-up, the patient had reduced severity and frequency of facial twitching. After 6 months, he presented to the emergency department with sudden loss of consciousness and experienced fatal intracerebral hemorrhage.",
"Hemifacial spasm represents abnormal twitching movements of the musculature innervated by the facial nerve.[1] It is almost always unilateral and commonly originates in the orbicularis oculi muscle, spreading to involve other muscles of facial expression. The pathophysiology involves irritation of the seventh nerve nucleus or its proximal segment. The most common etiology is compression of the proximal nerve segment by aberrant vasculature.[2] CADE can present with hemifacial spasm in older hypertensive patients due to local compression.",
"CADE, also known as intracranial dilatative arteriopathy, is an uncommon clinical entity, with an estimated annual prevalence of 0.6% to 5.8%; the prevalence increases with age.[3] CADE is more common in males.[4] It is an arteriopathy characterized by abnormal dilatation, elongation, and tortuosity of the intracranial vasculature and preferentially involves the vertebrobasilar circulation, followed by arteries of the anterior circulation (ie, internal carotid, middle cerebral arteries).[5] Involvement of the carotid circulation, however, is rare."
],
"date": "April 12, 2016",
"figures": [],
"markdown": "# A 62-Year-Old Man With Involuntary Facial Movements\n\n **Authors:** Sumaira Nabi, MBBS; Shahzad Ahmed, MBBS; Mazhar Badshah, MBBS; Ali Nomani, MBBS \n **Date:** April 12, 2016\n\n ## Content\n\n This patient's presentation leads to a wide range of differential considerations, including vascular compression within the cerebellopontine angle, stroke, a demyelinating disease (eg, multiple sclerosis), or a mass lesion-like tumor compressing the seventh nerve. MRI of the brain in this case effectively ruled out stroke, multiple sclerosis, and a mass lesion. However, it revealed dilated tortuous intracranial vasculature, which most likely was the cause of these symptoms.\nCTA confirmed the diagnosis of cranial arterial dolichoectasia (CADE). The patient was started on antihypertensive and lipid-lowering medications after a thorough literature review. He was also administered botulinum neurotoxin in the left orbicularis oculi and orbicularis oris muscles. The patient was then discharged on close follow-up on outpatient basis.\nThe authors sought a neurosurgical opinion in this case. However, because the benefit of neurovascular intervention is controversial, the patient declined microvascular decompression surgery. At 3-month follow-up, the patient had reduced severity and frequency of facial twitching. After 6 months, he presented to the emergency department with sudden loss of consciousness and experienced fatal intracerebral hemorrhage.\nHemifacial spasm represents abnormal twitching movements of the musculature innervated by the facial nerve.[1] It is almost always unilateral and commonly originates in the orbicularis oculi muscle, spreading to involve other muscles of facial expression. The pathophysiology involves irritation of the seventh nerve nucleus or its proximal segment. The most common etiology is compression of the proximal nerve segment by aberrant vasculature.[2] CADE can present with hemifacial spasm in older hypertensive patients due to local compression.\nCADE, also known as intracranial dilatative arteriopathy, is an uncommon clinical entity, with an estimated annual prevalence of 0.6% to 5.8%; the prevalence increases with age.[3] CADE is more common in males.[4] It is an arteriopathy characterized by abnormal dilatation, elongation, and tortuosity of the intracranial vasculature and preferentially involves the vertebrobasilar circulation, followed by arteries of the anterior circulation (ie, internal carotid, middle cerebral arteries).[5] Involvement of the carotid circulation, however, is rare.\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 957151,
"choiceText": "Aneurysm",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 957153,
"choiceText": "Multiple sclerosis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 957155,
"choiceText": "Tuberculoma",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 957157,
"choiceText": "Cranial arterial dolichoectasia",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 302713,
"questionText": "On the basis of the history, physical examination, and workup, what is the diagnosis?\r\n<br><br><i>\r\nHint: Note the history of hemifacial spasm and the typical neuroradiologic findings.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 62-Year-Old Man With Involuntary Facial Movements"
},
{
"authors": "Sumaira Nabi, MBBS; Shahzad Ahmed, MBBS; Mazhar Badshah, MBBS; Ali Nomani, MBBS",
"content": [
"The exact pathophysiology of CADE is not known; it is thought to result from irreversible disruption and damage of the connective tissue and muscle cells of the vessel wall due to turbulent blood flow and activation of certain enzymes like metalloproteinases. It has been associated with cardiovascular risk factors like hypertension, dyslipidemia, diabetes, and smoking.[3,4,5,6] Hypertension-induced atherosclerosis is the most commonly implicated etiology. Numerous congenital conditions have been associated with CADE, including tuberous sclerosis,[7] Ehlers-Danlos syndrome,[8] Marfan syndrome,[9] and adult polycystic kidney disease.[10] The process advances through various stages and the severity of the disease depends on the stage at which the patient presents. Initially, the artery dilates with an increase in diameter. This then advances to formation of fusiform and serpentine aneurysms.[3]",
"A wide range of clinical manifestations is reported. Patients can present with symptoms and signs of hemodynamic compromise or thromboembolic phenomena, including ischemic stroke, transient ischemic attacks, repeated falls, or, rarely, intracerebral hemorrhage. Ischemic stroke is the most common clinical symptom, especially involving the brainstem. Intracranial hemorrhage results from rupture of aneurysms or dissection of the dilated, ectatic vessels.",
"Cranial neuropathies can result from direct vascular compression. The nerves most commonly affected are the seventh, fifth, third, eighth, and sixth, in descending order of frequency, respectively. Some patients may have simultaneous involvement of multiple cranial nerves. Patients may sometimes develop brainstem compression or hydrocephalus. Therefore, patients can present with headache, vertigo, tinnitus, visual impairment, and signs of cranial nerve dysfunction like hemifacial spasm or trigeminal neuralgia.[3,4,11,12] Some cases may remain asymptomatic.",
"Modern neuroimaging and vascular imaging aid in confirming the diagnosis; CTA is the imaging modality of choice.[13] It helps with accurate measurement of vascular diameters. Brain MRI is the superior imaging technique in patients with stroke or features of compressive cranial neuropathies. However, MR angiography is less sensitive than CTA. Patients require serial imaging on follow-ups to monitor progression of disease as well as to identify complicating lesions of ischemic or hemorrhagic stroke. Progression of vascular dilatation and elongation is a poor prognostic feature. Smoker and colleagues' criteria can be applied for the diagnosis of posterior circulation ectasia. These criteria incorporate basilar artery diameter, lateral deviation from midline, and height of bifurcation into the scoring. No set of diagnostic criteria has been established for diagnosis of anterior circulation ectasia.[14]"
],
"date": "April 12, 2016",
"figures": [],
"markdown": "# A 62-Year-Old Man With Involuntary Facial Movements\n\n **Authors:** Sumaira Nabi, MBBS; Shahzad Ahmed, MBBS; Mazhar Badshah, MBBS; Ali Nomani, MBBS \n **Date:** April 12, 2016\n\n ## Content\n\n The exact pathophysiology of CADE is not known; it is thought to result from irreversible disruption and damage of the connective tissue and muscle cells of the vessel wall due to turbulent blood flow and activation of certain enzymes like metalloproteinases. It has been associated with cardiovascular risk factors like hypertension, dyslipidemia, diabetes, and smoking.[3,4,5,6] Hypertension-induced atherosclerosis is the most commonly implicated etiology. Numerous congenital conditions have been associated with CADE, including tuberous sclerosis,[7] Ehlers-Danlos syndrome,[8] Marfan syndrome,[9] and adult polycystic kidney disease.[10] The process advances through various stages and the severity of the disease depends on the stage at which the patient presents. Initially, the artery dilates with an increase in diameter. This then advances to formation of fusiform and serpentine aneurysms.[3]\nA wide range of clinical manifestations is reported. Patients can present with symptoms and signs of hemodynamic compromise or thromboembolic phenomena, including ischemic stroke, transient ischemic attacks, repeated falls, or, rarely, intracerebral hemorrhage. Ischemic stroke is the most common clinical symptom, especially involving the brainstem. Intracranial hemorrhage results from rupture of aneurysms or dissection of the dilated, ectatic vessels.\nCranial neuropathies can result from direct vascular compression. The nerves most commonly affected are the seventh, fifth, third, eighth, and sixth, in descending order of frequency, respectively. Some patients may have simultaneous involvement of multiple cranial nerves. Patients may sometimes develop brainstem compression or hydrocephalus. Therefore, patients can present with headache, vertigo, tinnitus, visual impairment, and signs of cranial nerve dysfunction like hemifacial spasm or trigeminal neuralgia.[3,4,11,12] Some cases may remain asymptomatic.\nModern neuroimaging and vascular imaging aid in confirming the diagnosis; CTA is the imaging modality of choice.[13] It helps with accurate measurement of vascular diameters. Brain MRI is the superior imaging technique in patients with stroke or features of compressive cranial neuropathies. However, MR angiography is less sensitive than CTA. Patients require serial imaging on follow-ups to monitor progression of disease as well as to identify complicating lesions of ischemic or hemorrhagic stroke. Progression of vascular dilatation and elongation is a poor prognostic feature. Smoker and colleagues' criteria can be applied for the diagnosis of posterior circulation ectasia. These criteria incorporate basilar artery diameter, lateral deviation from midline, and height of bifurcation into the scoring. No set of diagnostic criteria has been established for diagnosis of anterior circulation ectasia.[14]\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 62-Year-Old Man With Involuntary Facial Movements"
},
{
"authors": "Sumaira Nabi, MBBS; Shahzad Ahmed, MBBS; Mazhar Badshah, MBBS; Ali Nomani, MBBS",
"content": [
"CADE is treated conservatively in most cases, especially in older patients because of widespread involvement of vessels.[14] No set guidelines for management are available. Treatment options primarily depend on the patient's presenting features and severity of involvement of the intracranial vasculature.",
"Medical management includes management of arterial hypertension, hyperlipidemia, and diabetes. Tight blood pressure control may reduce the risk for cerebrovascular accidents. Although thromboembolic events are a risk, the role of antiplatelet therapy and anticoagulation is controversial and limited by the risk for hemorrhagic stroke.[15] Patients with trigeminal neuralgia or hemifacial spasm can be treated with appropriate medication. Hemifacial spasm is best treated medically and symptomatically with botulinum neurotoxin.[2]",
"The role of neurosurgery in CADE is also controversial, with no studies indicating a clear benefit. Surgical procedures mainly manage CADE symptoms (eg, hemifacial spasm) or may be beneficial in treating complications. Procedures include microsurgical decompression and endovascular interventions.[15] Microsurgical procedures involve either alteration of hemodynamics using anastomosis[16] or direct surgical procedures like thrombotic resection and aneurysm suture.[17] Endovascular procedures involve flow-diversion technology: Stents are placed in the arteries harboring aneurysms in order to reduce the blood flow into the aneurysm with subsequent venous stasis, thrombosis, and neointimal coverage.[18] The surrounding arteries have normal flow dynamics. Another endovascular technique is coil-assisted stent reconstruction. In this technique, placement of coils leads to stasis of blood flow and gradual thrombosis.[19]",
"CADE is an uncommon arteriopathy. Few cases have been reported in the literature. This case demonstrates the importance of considering this entity in older hypertensive patients who present with cranial nerve dysfunction. It also highlights the role of neuroradiology in the diagnosis. This is a rare case of dolichoectasia involving both anterior and posterior circulation."
],
"date": "April 12, 2016",
"figures": [],
"markdown": "# A 62-Year-Old Man With Involuntary Facial Movements\n\n **Authors:** Sumaira Nabi, MBBS; Shahzad Ahmed, MBBS; Mazhar Badshah, MBBS; Ali Nomani, MBBS \n **Date:** April 12, 2016\n\n ## Content\n\n CADE is treated conservatively in most cases, especially in older patients because of widespread involvement of vessels.[14] No set guidelines for management are available. Treatment options primarily depend on the patient's presenting features and severity of involvement of the intracranial vasculature.\nMedical management includes management of arterial hypertension, hyperlipidemia, and diabetes. Tight blood pressure control may reduce the risk for cerebrovascular accidents. Although thromboembolic events are a risk, the role of antiplatelet therapy and anticoagulation is controversial and limited by the risk for hemorrhagic stroke.[15] Patients with trigeminal neuralgia or hemifacial spasm can be treated with appropriate medication. Hemifacial spasm is best treated medically and symptomatically with botulinum neurotoxin.[2]\nThe role of neurosurgery in CADE is also controversial, with no studies indicating a clear benefit. Surgical procedures mainly manage CADE symptoms (eg, hemifacial spasm) or may be beneficial in treating complications. Procedures include microsurgical decompression and endovascular interventions.[15] Microsurgical procedures involve either alteration of hemodynamics using anastomosis[16] or direct surgical procedures like thrombotic resection and aneurysm suture.[17] Endovascular procedures involve flow-diversion technology: Stents are placed in the arteries harboring aneurysms in order to reduce the blood flow into the aneurysm with subsequent venous stasis, thrombosis, and neointimal coverage.[18] The surrounding arteries have normal flow dynamics. Another endovascular technique is coil-assisted stent reconstruction. In this technique, placement of coils leads to stasis of blood flow and gradual thrombosis.[19]\nCADE is an uncommon arteriopathy. Few cases have been reported in the literature. This case demonstrates the importance of considering this entity in older hypertensive patients who present with cranial nerve dysfunction. It also highlights the role of neuroradiology in the diagnosis. This is a rare case of dolichoectasia involving both anterior and posterior circulation.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 957159,
"choiceText": "Second",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 957161,
"choiceText": "Third",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 957163,
"choiceText": "Fifth",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 957165,
"choiceText": "Seventh",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 957167,
"choiceText": "Eighth\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Cranial neuropathies can result from direct vascular compression. The nerves most commonly affected are the seventh, fifth, third, eighth, and sixth, in descending order of frequency, respectively.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 302715,
"questionText": "Which of the following cranial nerves is most frequently affected by CADE?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 957169,
"choiceText": "Ischemic stroke",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 957171,
"choiceText": "Cranial neuropathies",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 957173,
"choiceText": "Intracerebral hemorrhage",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 957175,
"choiceText": "Meningitis",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 957177,
"choiceText": "Hydrocephalus",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Patients can present with symptoms and signs of hemodynamic compromise or thromboembolic phenomena, including ischemic stroke, transient ischemic attacks, repeated falls, or, rarely, intracerebral hemorrhage. Ischemic stroke is the most common clinical symptom, especially involving the brainstem. Intracranial hemorrhage results from rupture of aneurysms or dissection of the dilated, ectatic vessels. Some patients may have simultaneous involvement of multiple cranial nerves. Patients may sometimes develop brainstem compression or hydrocephalus. Therefore, patients can present with headache, vertigo, tinnitus, visual impairment and signs of cranial nerve dysfunction like hemifacial spasm or trigeminal neuralgia. Some cases may remain asymptomatic. Signs of meningeal irritation are absent.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 302717,
"questionText": "Which of the following does <i>not</i> commonly present with CADE?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 62-Year-Old Man With Involuntary Facial Movements"
},
{
"authors": "Sumaira Nabi, MBBS; Shahzad Ahmed, MBBS; Mazhar Badshah, MBBS; Ali Nomani, MBBS",
"content": [],
"date": "April 12, 2016",
"figures": [],
"markdown": "# A 62-Year-Old Man With Involuntary Facial Movements\n\n **Authors:** Sumaira Nabi, MBBS; Shahzad Ahmed, MBBS; Mazhar Badshah, MBBS; Ali Nomani, MBBS \n **Date:** April 12, 2016\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 957159,
"choiceText": "Second",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 957161,
"choiceText": "Third",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 957163,
"choiceText": "Fifth",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 957165,
"choiceText": "Seventh",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 957167,
"choiceText": "Eighth\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Cranial neuropathies can result from direct vascular compression. The nerves most commonly affected are the seventh, fifth, third, eighth, and sixth, in descending order of frequency, respectively.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 302715,
"questionText": "Which of the following cranial nerves is most frequently affected by CADE?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 957169,
"choiceText": "Ischemic stroke",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 957171,
"choiceText": "Cranial neuropathies",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 957173,
"choiceText": "Intracerebral hemorrhage",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 957175,
"choiceText": "Meningitis",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 957177,
"choiceText": "Hydrocephalus",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Patients can present with symptoms and signs of hemodynamic compromise or thromboembolic phenomena, including ischemic stroke, transient ischemic attacks, repeated falls, or, rarely, intracerebral hemorrhage. Ischemic stroke is the most common clinical symptom, especially involving the brainstem. Intracranial hemorrhage results from rupture of aneurysms or dissection of the dilated, ectatic vessels. Some patients may have simultaneous involvement of multiple cranial nerves. Patients may sometimes develop brainstem compression or hydrocephalus. Therefore, patients can present with headache, vertigo, tinnitus, visual impairment and signs of cranial nerve dysfunction like hemifacial spasm or trigeminal neuralgia. Some cases may remain asymptomatic. Signs of meningeal irritation are absent.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 302717,
"questionText": "Which of the following does <i>not</i> commonly present with CADE?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 62-Year-Old Man With Involuntary Facial Movements"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 957151,
"choiceText": "Aneurysm",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 957153,
"choiceText": "Multiple sclerosis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 957155,
"choiceText": "Tuberculoma",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 957157,
"choiceText": "Cranial arterial dolichoectasia",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 302713,
"questionText": "On the basis of the history, physical examination, and workup, what is the diagnosis?\r\n<br><br><i>\r\nHint: Note the history of hemifacial spasm and the typical neuroradiologic findings.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 957159,
"choiceText": "Second",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 957161,
"choiceText": "Third",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 957163,
"choiceText": "Fifth",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 957165,
"choiceText": "Seventh",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 957167,
"choiceText": "Eighth\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Cranial neuropathies can result from direct vascular compression. The nerves most commonly affected are the seventh, fifth, third, eighth, and sixth, in descending order of frequency, respectively.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 302715,
"questionText": "Which of the following cranial nerves is most frequently affected by CADE?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 957169,
"choiceText": "Ischemic stroke",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 957171,
"choiceText": "Cranial neuropathies",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 957173,
"choiceText": "Intracerebral hemorrhage",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 957175,
"choiceText": "Meningitis",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 957177,
"choiceText": "Hydrocephalus",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Patients can present with symptoms and signs of hemodynamic compromise or thromboembolic phenomena, including ischemic stroke, transient ischemic attacks, repeated falls, or, rarely, intracerebral hemorrhage. Ischemic stroke is the most common clinical symptom, especially involving the brainstem. Intracranial hemorrhage results from rupture of aneurysms or dissection of the dilated, ectatic vessels. Some patients may have simultaneous involvement of multiple cranial nerves. Patients may sometimes develop brainstem compression or hydrocephalus. Therefore, patients can present with headache, vertigo, tinnitus, visual impairment and signs of cranial nerve dysfunction like hemifacial spasm or trigeminal neuralgia. Some cases may remain asymptomatic. Signs of meningeal irritation are absent.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 302717,
"questionText": "Which of the following does <i>not</i> commonly present with CADE?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
861018 | /viewarticle/861018 | [
{
"authors": "Juan Carlos Munoz, MD; Matthew S. Cole, MD, MPH",
"content": [
"Editor's Note:\n\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us\n.",
"A 64-year-old black woman is directly transferred from her primary care provider's office to the emergency department because she presented with symptomatic anemia and several episodes of painless rectal bleeding. During an initial assessment, the patient states that she \"can hardly walk\" and that she has dyspnea on exertion, chest pain, and \"ringing in the ears.\"",
"She denies any history of gastrointestinal ulcers, liver disease, or bleeding disorder. Although no medication list is available on admission, she denies anticoagulant, aspirin, and nonsteroidal anti-inflammatory drug (NSAID) intake. The patient also denies smoking and alcohol and recreational drug use.",
"The patient's medical history is remarkable for type 2 diabetes mellitus, colonic diverticulosis, chronic anemia, recurrent rectal bleeding, colonic polyps, coronary artery disease, hypertension, and end-stage kidney disease requiring hemodialysis.",
"When further queried about her medical history, the patient mentions two previous hospital admissions for similar problems. She has received multiple blood transfusions, two colonoscopies, two upper gastrointestinal endoscopies, and a negative gastrointestinal bleeding scan."
],
"date": "March 31, 2016",
"figures": [],
"markdown": "# A 64-Year-Old Woman With Recurrent Gastrointestinal Bleeding\n\n **Authors:** Juan Carlos Munoz, MD; Matthew S. Cole, MD, MPH \n **Date:** March 31, 2016\n\n ## Content\n\n Editor's Note:\n\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us\n.\nA 64-year-old black woman is directly transferred from her primary care provider's office to the emergency department because she presented with symptomatic anemia and several episodes of painless rectal bleeding. During an initial assessment, the patient states that she \"can hardly walk\" and that she has dyspnea on exertion, chest pain, and \"ringing in the ears.\"\nShe denies any history of gastrointestinal ulcers, liver disease, or bleeding disorder. Although no medication list is available on admission, she denies anticoagulant, aspirin, and nonsteroidal anti-inflammatory drug (NSAID) intake. The patient also denies smoking and alcohol and recreational drug use.\nThe patient's medical history is remarkable for type 2 diabetes mellitus, colonic diverticulosis, chronic anemia, recurrent rectal bleeding, colonic polyps, coronary artery disease, hypertension, and end-stage kidney disease requiring hemodialysis.\nWhen further queried about her medical history, the patient mentions two previous hospital admissions for similar problems. She has received multiple blood transfusions, two colonoscopies, two upper gastrointestinal endoscopies, and a negative gastrointestinal bleeding scan.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 64-Year-Old Woman With Recurrent Gastrointestinal Bleeding"
},
{
"authors": "Juan Carlos Munoz, MD; Matthew S. Cole, MD, MPH",
"content": [
"Figure 1.",
"Figure 2.",
"On physical examination, the patient's oral temperature is 98.6°F. Her pulse is regular, with a rate of 107 beats/min. Her blood pressure is 144/70 mm Hg. Significant orthostatic changes are noted in the patient's pulse and blood pressure. Her respiratory rate is 24 breaths/min, with mildly increased work of breathing. She appears pale and cachectic, and her oral mucosa is observed to be dry.",
"The examination of the head and neck is normal, with no palpable masses or cervical lymphadenopathy. Her lungs are clear to auscultation, but she is tachypneic. Cardiac evaluation demonstrates normal S1 and S2 heart sounds and a mild systolic murmur. The patient's abdomen is protuberant, obese, and soft; no tenderness to deep palpation, rebound, or guarding is noted. Normal bowel sounds are auscultated. The peripheral arterial pulses in the upper and lower extremities are faintly palpable. A venous shunt is palpated in the right upper extremity, with a normal thrill. Rectal examination reveals dark red blood in the rectum. The rest of the examination is unremarkable, except for external hemorrhoids without visible active bleeding.",
"The initial work-up includes a normal chest radiograph and an ECG showing sinus tachycardia. The remaining findings are nonspecific.",
"The patient's cardiac enzyme examination is within normal limits. Other laboratory examinations, however, reveal a hemoglobin level of 3.6 g/dL, a hematocrit of 12.2%, a platelet count of 295 × 103 cells/µL, a prothrombin time of 12.3 sec, and a partial thromboplastin time of 22.6 sec. Liver enzyme values are within normal limits. The patient is treated in the emergency department with intravenous fluids, packed red blood cells, and intravenous proton pump inhibitors.",
"A gastroenterologist is urgently consulted. Emergent upper endoscopy is performed, which does not reveal the source of bleeding. The patient is admitted to the hospital and subsequently undergoes an autologous red blood cell scan, which is also unremarkable.",
"Anterograde single-balloon enteroscopy with fluoroscopy is performed. This shows an area of active bleeding approximately 160 cm from the incisors. After the area is flushed with tap water, a visible vessel is seen, with normal surrounding mucosa and no evidence of ulceration (Figures 1 and 2)."
],
"date": "March 31, 2016",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/861/018/861018-Thumb1.png"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/861/018/861018-Thumb2.png"
}
],
"markdown": "# A 64-Year-Old Woman With Recurrent Gastrointestinal Bleeding\n\n **Authors:** Juan Carlos Munoz, MD; Matthew S. Cole, MD, MPH \n **Date:** March 31, 2016\n\n ## Content\n\n Figure 1.\nFigure 2.\nOn physical examination, the patient's oral temperature is 98.6°F. Her pulse is regular, with a rate of 107 beats/min. Her blood pressure is 144/70 mm Hg. Significant orthostatic changes are noted in the patient's pulse and blood pressure. Her respiratory rate is 24 breaths/min, with mildly increased work of breathing. She appears pale and cachectic, and her oral mucosa is observed to be dry.\nThe examination of the head and neck is normal, with no palpable masses or cervical lymphadenopathy. Her lungs are clear to auscultation, but she is tachypneic. Cardiac evaluation demonstrates normal S1 and S2 heart sounds and a mild systolic murmur. The patient's abdomen is protuberant, obese, and soft; no tenderness to deep palpation, rebound, or guarding is noted. Normal bowel sounds are auscultated. The peripheral arterial pulses in the upper and lower extremities are faintly palpable. A venous shunt is palpated in the right upper extremity, with a normal thrill. Rectal examination reveals dark red blood in the rectum. The rest of the examination is unremarkable, except for external hemorrhoids without visible active bleeding.\nThe initial work-up includes a normal chest radiograph and an ECG showing sinus tachycardia. The remaining findings are nonspecific.\nThe patient's cardiac enzyme examination is within normal limits. Other laboratory examinations, however, reveal a hemoglobin level of 3.6 g/dL, a hematocrit of 12.2%, a platelet count of 295 × 103 cells/µL, a prothrombin time of 12.3 sec, and a partial thromboplastin time of 22.6 sec. Liver enzyme values are within normal limits. The patient is treated in the emergency department with intravenous fluids, packed red blood cells, and intravenous proton pump inhibitors.\nA gastroenterologist is urgently consulted. Emergent upper endoscopy is performed, which does not reveal the source of bleeding. The patient is admitted to the hospital and subsequently undergoes an autologous red blood cell scan, which is also unremarkable.\nAnterograde single-balloon enteroscopy with fluoroscopy is performed. This shows an area of active bleeding approximately 160 cm from the incisors. After the area is flushed with tap water, a visible vessel is seen, with normal surrounding mucosa and no evidence of ulceration (Figures 1 and 2).\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 954327,
"choiceText": "Esophageal varices",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 954329,
"choiceText": "Gastritis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 954331,
"choiceText": "Dieulafoy lesion",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 954333,
"choiceText": "Diverticular bleeding",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 301855,
"questionText": "On the basis of the history, physical examination, and workup, what is the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 64-Year-Old Woman With Recurrent Gastrointestinal Bleeding"
},
{
"authors": "Juan Carlos Munoz, MD; Matthew S. Cole, MD, MPH",
"content": [
"Figure 1.",
"Figure 2.",
"The findings in this case are consistent with a Dieulafoy lesion of the small bowel, probably in the midjejunum (Figures 1 and 2). Dieulafoy lesion is also known as exulceratio simplex, caliber-persistent artery, gastric arteriosclerosis, submucosal arterial malformation, and cirsoid aneurysm of the gastric vessels. Unlike most other aneurysms, Dieulafoy lesions are thought to be developmental mal uncommon condition, accounting for 1%-5% of all cases of acute gastrointestinal bleeding in adults (depending on the series). In approximately 4%-9% of cases of massive upper gastrointestinal hemorrhage, no demonstrable cause can be found; Dieulafoy lesion is thought to be the cause of acute and chronic upper gastrointestinal bleeding in approximately 1%-2% of these cases. It is a well-recognized cause of recurrent, intermittent, and life-threatening bleeding from the gastrointestinal tract, and it results from the rupture of an unexposed submucosal artery.[3,5,6,7,8,9]",
"In a Dieulafoy lesion, the submucosal artery does not undergo normal ramification into mucosal capillary microvessels. Instead, a caliber-persistent artery at the muscularis mucosae is seen. It is characterized by subintimal fibrosis of the artery and an absence of inflammation at the edge of the mucosal defect. As a result, the caliber of the artery is in the range of 1-5 mm. This is approximately 10 times the normal caliber of mucosal capillaries.",
"Previous descriptions of Dieulafoy lesions emphasized a predilection for the proximal stomach at 6 cm from the gastroesophageal junction, most often located along the lesser curvature (80%-85%); however, it has been found in all areas of the gastrointestinal tract, including the colon and rectum (10%), esophagus (2%), and small intestine (2%).[6]"
],
"date": "March 31, 2016",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/861/018/861018-Thumb1.png"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/861/018/861018-Thumb2.png"
}
],
"markdown": "# A 64-Year-Old Woman With Recurrent Gastrointestinal Bleeding\n\n **Authors:** Juan Carlos Munoz, MD; Matthew S. Cole, MD, MPH \n **Date:** March 31, 2016\n\n ## Content\n\n Figure 1.\nFigure 2.\nThe findings in this case are consistent with a Dieulafoy lesion of the small bowel, probably in the midjejunum (Figures 1 and 2). Dieulafoy lesion is also known as exulceratio simplex, caliber-persistent artery, gastric arteriosclerosis, submucosal arterial malformation, and cirsoid aneurysm of the gastric vessels. Unlike most other aneurysms, Dieulafoy lesions are thought to be developmental mal uncommon condition, accounting for 1%-5% of all cases of acute gastrointestinal bleeding in adults (depending on the series). In approximately 4%-9% of cases of massive upper gastrointestinal hemorrhage, no demonstrable cause can be found; Dieulafoy lesion is thought to be the cause of acute and chronic upper gastrointestinal bleeding in approximately 1%-2% of these cases. It is a well-recognized cause of recurrent, intermittent, and life-threatening bleeding from the gastrointestinal tract, and it results from the rupture of an unexposed submucosal artery.[3,5,6,7,8,9]\nIn a Dieulafoy lesion, the submucosal artery does not undergo normal ramification into mucosal capillary microvessels. Instead, a caliber-persistent artery at the muscularis mucosae is seen. It is characterized by subintimal fibrosis of the artery and an absence of inflammation at the edge of the mucosal defect. As a result, the caliber of the artery is in the range of 1-5 mm. This is approximately 10 times the normal caliber of mucosal capillaries.\nPrevious descriptions of Dieulafoy lesions emphasized a predilection for the proximal stomach at 6 cm from the gastroesophageal junction, most often located along the lesser curvature (80%-85%); however, it has been found in all areas of the gastrointestinal tract, including the colon and rectum (10%), esophagus (2%), and small intestine (2%).[6]\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n\n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 954327,
"choiceText": "Esophageal varices",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 954329,
"choiceText": "Gastritis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 954331,
"choiceText": "Dieulafoy lesion",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 954333,
"choiceText": "Diverticular bleeding",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 301855,
"questionText": "On the basis of the history, physical examination, and workup, what is the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 64-Year-Old Woman With Recurrent Gastrointestinal Bleeding"
},
{
"authors": "Juan Carlos Munoz, MD; Matthew S. Cole, MD, MPH",
"content": [
"The etiology of Dieulafoy lesions is unknown. It was originally thought that Dieulafoy lesions were caused by an aneurysm in one of the vessels within the gastric wall, perhaps in combination with atherosclerosis. It has also been suggested that a congenital or acquired vascular malformation might be the underlying cause. The consensus, however, seems to be that it is caused by an abnormally large-caliber persistent tortuous submucosal artery.",
"Events that can trigger bleeding are also not well understood. Patients who bleed from Dieulafoy lesions are typically men (the male-to-female ratio is 2:1) who have multiple comorbidities, including cardiovascular disease, hypertension, chronic kidney disease, and diabetes. In the setting of gastrointestinal bleeding caused by Dieulafoy lesions, a history of alcohol abuse or NSAID use is generally absent. The most common presenting symptom is recurrent hematemesis with melena, which is present in 51% of cases; hematemesis without melena is present in 28% of cases, and melena alone is seen in 18%.",
"Patients with lesions in the middle or distal jejunum, right colon, left colon, and rectum may present with only hematochezia in 3% of cases. Bleeding is often self-limited, although it is usually recurrent and can be profuse. Because of the small size of the lesion and the normal surrounding mucosa, the diagnosis of a Dieulafoy lesion can be made with confidence just after or during active bleeding in an area without an associated ulcer or mass lesion. An initial evaluation may reveal hemodynamic instability, postural hypotension, and profound anemia.",
"Various radiologic (eg, small-bowel enteroclysis, tagged red blood cell scan, mesenteric angiography) and endoscopic modalities (eg, upper endoscopy; colonoscopy; push enteroscopy; single-balloon enteroscopy; double-balloon enteroscopy; and, most recently, capsule endoscopy) have been used to localize the lesion in the gastrointestinal tract. Video capsule endoscopy (VCE, also known as \"wireless capsule endoscopy\") has a diagnostic yield of 60%-80% in patients with obscure gastrointestinal bleeding. In head-to-head comparisons, the yield of VCE is superior to that of push enteroscopy, small-bowel enteroclysis, and mesenteric angiography. No reliable data currently exist comparing VCE with double-balloon enteroscopy devices.[7,8,9,10]",
"At present, the diagnosis is usually made by endoscopy. Repeat endoscopies are sometimes necessary. Approximately 49% of lesions are identified during the initial endoscopic examination, whereas 33% require more than one endoscopic evaluation (including push, single-balloon, or double-balloon enteroscopy or VCE) for confident identification.",
"The remaining patients require angiographic identification of the Dieulafoy lesions. In some series, however, identification of the lesions was more accurate at the initial endoscopic examination, because Dieulafoy lesions were identified in as many as 95% of cases. This was attributed to the fact that endoscopy was generally performed within the first 2 hours after admission of the patient, allowing recognition of actively bleeding lesions. This might also, however, reflect the increased awareness of the existence of a Dieulafoy lesion and the experience of the endoscopist.",
"The endoscopic appearance of Dieulafoy lesions varies, and it may consist of active arterial spurting, a protruding vessel without active bleeding, or fresh adherent clots. The risk for rebleeding after endoscopic therapy ranges from 9% to 40% in various reports. Endoscopic tattooing with India ink injections has been very helpful for locating the lesion for endoscopic retreatment or intraoperative surgical intervention.[4,6,8,10,11]"
],
"date": "March 31, 2016",
"figures": [],
"markdown": "# A 64-Year-Old Woman With Recurrent Gastrointestinal Bleeding\n\n **Authors:** Juan Carlos Munoz, MD; Matthew S. Cole, MD, MPH \n **Date:** March 31, 2016\n\n ## Content\n\n The etiology of Dieulafoy lesions is unknown. It was originally thought that Dieulafoy lesions were caused by an aneurysm in one of the vessels within the gastric wall, perhaps in combination with atherosclerosis. It has also been suggested that a congenital or acquired vascular malformation might be the underlying cause. The consensus, however, seems to be that it is caused by an abnormally large-caliber persistent tortuous submucosal artery.\nEvents that can trigger bleeding are also not well understood. Patients who bleed from Dieulafoy lesions are typically men (the male-to-female ratio is 2:1) who have multiple comorbidities, including cardiovascular disease, hypertension, chronic kidney disease, and diabetes. In the setting of gastrointestinal bleeding caused by Dieulafoy lesions, a history of alcohol abuse or NSAID use is generally absent. The most common presenting symptom is recurrent hematemesis with melena, which is present in 51% of cases; hematemesis without melena is present in 28% of cases, and melena alone is seen in 18%.\nPatients with lesions in the middle or distal jejunum, right colon, left colon, and rectum may present with only hematochezia in 3% of cases. Bleeding is often self-limited, although it is usually recurrent and can be profuse. Because of the small size of the lesion and the normal surrounding mucosa, the diagnosis of a Dieulafoy lesion can be made with confidence just after or during active bleeding in an area without an associated ulcer or mass lesion. An initial evaluation may reveal hemodynamic instability, postural hypotension, and profound anemia.\nVarious radiologic (eg, small-bowel enteroclysis, tagged red blood cell scan, mesenteric angiography) and endoscopic modalities (eg, upper endoscopy; colonoscopy; push enteroscopy; single-balloon enteroscopy; double-balloon enteroscopy; and, most recently, capsule endoscopy) have been used to localize the lesion in the gastrointestinal tract. Video capsule endoscopy (VCE, also known as \"wireless capsule endoscopy\") has a diagnostic yield of 60%-80% in patients with obscure gastrointestinal bleeding. In head-to-head comparisons, the yield of VCE is superior to that of push enteroscopy, small-bowel enteroclysis, and mesenteric angiography. No reliable data currently exist comparing VCE with double-balloon enteroscopy devices.[7,8,9,10]\nAt present, the diagnosis is usually made by endoscopy. Repeat endoscopies are sometimes necessary. Approximately 49% of lesions are identified during the initial endoscopic examination, whereas 33% require more than one endoscopic evaluation (including push, single-balloon, or double-balloon enteroscopy or VCE) for confident identification.\nThe remaining patients require angiographic identification of the Dieulafoy lesions. In some series, however, identification of the lesions was more accurate at the initial endoscopic examination, because Dieulafoy lesions were identified in as many as 95% of cases. This was attributed to the fact that endoscopy was generally performed within the first 2 hours after admission of the patient, allowing recognition of actively bleeding lesions. This might also, however, reflect the increased awareness of the existence of a Dieulafoy lesion and the experience of the endoscopist.\nThe endoscopic appearance of Dieulafoy lesions varies, and it may consist of active arterial spurting, a protruding vessel without active bleeding, or fresh adherent clots. The risk for rebleeding after endoscopic therapy ranges from 9% to 40% in various reports. Endoscopic tattooing with India ink injections has been very helpful for locating the lesion for endoscopic retreatment or intraoperative surgical intervention.[4,6,8,10,11]\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 64-Year-Old Woman With Recurrent Gastrointestinal Bleeding"
},
{
"authors": "Juan Carlos Munoz, MD; Matthew S. Cole, MD, MPH",
"content": [
"Figure 3.",
"Endoscopic management has become the standard approach for the treatment of Dieulafoy lesions. Several modalities, alone or in combination, are used to control the bleeding. These include injection of epinephrine or a sclerosing agent; hemoclip placement; monopolar, bipolar, heater probe, and argon electrocoagulation; and laser photocoagulation. Endoscopic success rates are reported to be as high as 90%-95%. Endoscopic Doppler ultrasonography has been used to confirm ablation of a Dieulafoy lesion by documenting the absence of blood flow after injection therapy.[4,11]",
"Surgical intervention is reserved for cases in which endoscopic treatment is unsuccessful or the patient has rebleeding despite endoscopic treatment. Wedge resection is preferred to simple oversewing of the lesion by the surgeon because of high rebleeding rates. A combined endoscopic and laparoscopic approach has been described; this approach allows precise location of the aberrant vessel with intraoperative endoscopy, followed by limited laparoscopic surgical resection.",
"Angiography and embolization is another modality that has been reported in patients with active bleeding who are not amenable to endoscopic therapy.",
"The possibility of identifying patients at risk for Dieulafoy lesions is still uncertain. In at least a subset of patients, mucosal injury may unmask caliber-persistent arteries. In other groups of patients, ischemia resulting from decreased perfusion or oxygenation may play a role.",
"In this case, the patient was treated with hemoclips because of the large caliber of the vessel; three hemoclips (Figure 3) obliterated the lesion, and no active bleeding was seen after flushing.",
"The patient was discharged on the fourth day after treatment, without any complications. The patient remained well at 2 months after treatment; her hemoglobin was stable at approximately 10 g/dL, without signs or symptoms of hemorrhagic diathesis."
],
"date": "March 31, 2016",
"figures": [
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/861/018/861018-Thumb3.png"
}
],
"markdown": "# A 64-Year-Old Woman With Recurrent Gastrointestinal Bleeding\n\n **Authors:** Juan Carlos Munoz, MD; Matthew S. Cole, MD, MPH \n **Date:** March 31, 2016\n\n ## Content\n\n Figure 3.\nEndoscopic management has become the standard approach for the treatment of Dieulafoy lesions. Several modalities, alone or in combination, are used to control the bleeding. These include injection of epinephrine or a sclerosing agent; hemoclip placement; monopolar, bipolar, heater probe, and argon electrocoagulation; and laser photocoagulation. Endoscopic success rates are reported to be as high as 90%-95%. Endoscopic Doppler ultrasonography has been used to confirm ablation of a Dieulafoy lesion by documenting the absence of blood flow after injection therapy.[4,11]\nSurgical intervention is reserved for cases in which endoscopic treatment is unsuccessful or the patient has rebleeding despite endoscopic treatment. Wedge resection is preferred to simple oversewing of the lesion by the surgeon because of high rebleeding rates. A combined endoscopic and laparoscopic approach has been described; this approach allows precise location of the aberrant vessel with intraoperative endoscopy, followed by limited laparoscopic surgical resection.\nAngiography and embolization is another modality that has been reported in patients with active bleeding who are not amenable to endoscopic therapy.\nThe possibility of identifying patients at risk for Dieulafoy lesions is still uncertain. In at least a subset of patients, mucosal injury may unmask caliber-persistent arteries. In other groups of patients, ischemia resulting from decreased perfusion or oxygenation may play a role.\nIn this case, the patient was treated with hemoclips because of the large caliber of the vessel; three hemoclips (Figure 3) obliterated the lesion, and no active bleeding was seen after flushing.\nThe patient was discharged on the fourth day after treatment, without any complications. The patient remained well at 2 months after treatment; her hemoglobin was stable at approximately 10 g/dL, without signs or symptoms of hemorrhagic diathesis.\n\n ## Figures\n\n **Figure 3.** \n \n\n\n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 954335,
"choiceText": "< 1%",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 954337,
"choiceText": "1%-5%",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 954339,
"choiceText": "10%",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 954341,
"choiceText": "15%",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Dieulafoy lesion is an uncommon condition, accounting for 1%-5% of all cases of acute gastrointestinal bleeding in adults (depending on the series). In approximately 4%-9% of cases of massive upper gastrointestinal hemorrhage, no demonstrable cause can be found; Dieulafoy lesion is thought to be the cause of acute and chronic upper gastrointestinal bleeding in approximately 1%-2% of these cases.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 301857,
"questionText": "What is the percentage of acute gastrointestinal bleeding secondary to a Dieulafoy lesion?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 954343,
"choiceText": "Bleeding is often self-limited",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 954345,
"choiceText": "A history of recent alcohol or NSAID use is uncommon",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 954347,
"choiceText": "The most common presenting symptom is melena alone",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 954349,
"choiceText": "Thirty-three percent of patients require more than one endoscopic evaluation",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The most common presenting symptom is recurrent hematemesis with melena, which is present in 51% of cases; hematemesis without melena is present in 28% of cases, and melena alone is seen in 18%. ",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 301859,
"questionText": "Which the following statements is <i>not</i> associated with Dieulafoy lesions?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 64-Year-Old Woman With Recurrent Gastrointestinal Bleeding"
},
{
"authors": "Juan Carlos Munoz, MD; Matthew S. Cole, MD, MPH",
"content": [],
"date": "March 31, 2016",
"figures": [],
"markdown": "# A 64-Year-Old Woman With Recurrent Gastrointestinal Bleeding\n\n **Authors:** Juan Carlos Munoz, MD; Matthew S. Cole, MD, MPH \n **Date:** March 31, 2016\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 954335,
"choiceText": "< 1%",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 954337,
"choiceText": "1%-5%",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 954339,
"choiceText": "10%",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 954341,
"choiceText": "15%",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Dieulafoy lesion is an uncommon condition, accounting for 1%-5% of all cases of acute gastrointestinal bleeding in adults (depending on the series). In approximately 4%-9% of cases of massive upper gastrointestinal hemorrhage, no demonstrable cause can be found; Dieulafoy lesion is thought to be the cause of acute and chronic upper gastrointestinal bleeding in approximately 1%-2% of these cases.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 301857,
"questionText": "What is the percentage of acute gastrointestinal bleeding secondary to a Dieulafoy lesion?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 954343,
"choiceText": "Bleeding is often self-limited",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 954345,
"choiceText": "A history of recent alcohol or NSAID use is uncommon",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 954347,
"choiceText": "The most common presenting symptom is melena alone",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 954349,
"choiceText": "Thirty-three percent of patients require more than one endoscopic evaluation",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The most common presenting symptom is recurrent hematemesis with melena, which is present in 51% of cases; hematemesis without melena is present in 28% of cases, and melena alone is seen in 18%. ",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 301859,
"questionText": "Which the following statements is <i>not</i> associated with Dieulafoy lesions?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 64-Year-Old Woman With Recurrent Gastrointestinal Bleeding"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 954327,
"choiceText": "Esophageal varices",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 954329,
"choiceText": "Gastritis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 954331,
"choiceText": "Dieulafoy lesion",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 954333,
"choiceText": "Diverticular bleeding",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 301855,
"questionText": "On the basis of the history, physical examination, and workup, what is the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 954335,
"choiceText": "< 1%",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 954337,
"choiceText": "1%-5%",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 954339,
"choiceText": "10%",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 954341,
"choiceText": "15%",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Dieulafoy lesion is an uncommon condition, accounting for 1%-5% of all cases of acute gastrointestinal bleeding in adults (depending on the series). In approximately 4%-9% of cases of massive upper gastrointestinal hemorrhage, no demonstrable cause can be found; Dieulafoy lesion is thought to be the cause of acute and chronic upper gastrointestinal bleeding in approximately 1%-2% of these cases.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 301857,
"questionText": "What is the percentage of acute gastrointestinal bleeding secondary to a Dieulafoy lesion?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 954343,
"choiceText": "Bleeding is often self-limited",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 954345,
"choiceText": "A history of recent alcohol or NSAID use is uncommon",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 954347,
"choiceText": "The most common presenting symptom is melena alone",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 954349,
"choiceText": "Thirty-three percent of patients require more than one endoscopic evaluation",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The most common presenting symptom is recurrent hematemesis with melena, which is present in 51% of cases; hematemesis without melena is present in 28% of cases, and melena alone is seen in 18%. ",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 301859,
"questionText": "Which the following statements is <i>not</i> associated with Dieulafoy lesions?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
861017 | /viewarticle/861017 | [
{
"authors": "Joe Nemeth, MD, CCFP(EM); Catherine Patocka, MDCM; David Barbic, MD, MSc",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.",
"A 35-year-old man is brought to the emergency department (ED) by the local emergency medical services after a fall from his motorcycle. The patient was on his way to work when, while traveling at 30 mph, his motorcycle caught the edge of the sidewalk, which led to the fall.",
"The patient denies having lost consciousness. He is an otherwise healthy man who is not on any medications and has no history of allergies. He denies having ingested any recreational drugs."
],
"date": "March 30, 2016",
"figures": [],
"markdown": "# A 35-Year-Old Man Who Fell From a Motorcycle\n\n **Authors:** Joe Nemeth, MD, CCFP(EM); Catherine Patocka, MDCM; David Barbic, MD, MSc \n **Date:** March 30, 2016\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.\nA 35-year-old man is brought to the emergency department (ED) by the local emergency medical services after a fall from his motorcycle. The patient was on his way to work when, while traveling at 30 mph, his motorcycle caught the edge of the sidewalk, which led to the fall.\nThe patient denies having lost consciousness. He is an otherwise healthy man who is not on any medications and has no history of allergies. He denies having ingested any recreational drugs.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 35-Year-Old Man Who Fell From a Motorcycle"
},
{
"authors": "Joe Nemeth, MD, CCFP(EM); Catherine Patocka, MDCM; David Barbic, MD, MSc",
"content": [
"Figure 1.",
"Figure 2.",
"Figure 3.",
"On presentation to the ED, the patient is noted to be in moderate discomfort secondary to multiple abrasions and contusions. Upon initial examination, findings are largely unremarkable. He has a regular pulse of 95 beats/min, a respiratory rate of 18 breaths/min, a blood pressure of 115/80 mm Hg, an oxygen saturation of 99% while breathing room air, and an oral temperature of 97°F. He is maintaining his airway, has good breath sounds bilaterally, and has a Glasgow Coma Score of 15.",
"Upon further examination, however, tenderness is noted in his right-shoulder region, with mild swelling of the general shoulder region. Although the shoulder joint is observed to move normally, crepitus is observed upon palpitation.",
"No open wounds and no deformities are noted over the affected area. The peripheral arterial pulses in the right extremity are normal. No neurologic deficits are noted in the extremity, and the axillary nerve is normal. Examinations of the chest wall, scapula, sternum, and the remaining distal regions of the extremity are also unremarkable.",
"All laboratory investigations, including a complete blood cell count and a basic metabolic panel, are within normal limits. Of the imaging studies that the patient undergoes, radiographs of the right shoulder and chest (Figures 1, 2, and 3) are interpreted as normal.",
"The patient is subsequently admitted for observation and pain control; he is then discharged a few days later from the trauma service. At the time of discharge, he is still experiencing right-shoulder pain. A discharge diagnosis of shoulder contusion is assigned, and no further imaging or other investigations are performed on his shoulder."
],
"date": "March 30, 2016",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/861/017/861017-Thumb1.png"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/861/017/861017-Thumb2.png"
},
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/861/017/861017-Thumb3.png"
}
],
"markdown": "# A 35-Year-Old Man Who Fell From a Motorcycle\n\n **Authors:** Joe Nemeth, MD, CCFP(EM); Catherine Patocka, MDCM; David Barbic, MD, MSc \n **Date:** March 30, 2016\n\n ## Content\n\n Figure 1.\nFigure 2.\nFigure 3.\nOn presentation to the ED, the patient is noted to be in moderate discomfort secondary to multiple abrasions and contusions. Upon initial examination, findings are largely unremarkable. He has a regular pulse of 95 beats/min, a respiratory rate of 18 breaths/min, a blood pressure of 115/80 mm Hg, an oxygen saturation of 99% while breathing room air, and an oral temperature of 97°F. He is maintaining his airway, has good breath sounds bilaterally, and has a Glasgow Coma Score of 15.\nUpon further examination, however, tenderness is noted in his right-shoulder region, with mild swelling of the general shoulder region. Although the shoulder joint is observed to move normally, crepitus is observed upon palpitation.\nNo open wounds and no deformities are noted over the affected area. The peripheral arterial pulses in the right extremity are normal. No neurologic deficits are noted in the extremity, and the axillary nerve is normal. Examinations of the chest wall, scapula, sternum, and the remaining distal regions of the extremity are also unremarkable.\nAll laboratory investigations, including a complete blood cell count and a basic metabolic panel, are within normal limits. Of the imaging studies that the patient undergoes, radiographs of the right shoulder and chest (Figures 1, 2, and 3) are interpreted as normal.\nThe patient is subsequently admitted for observation and pain control; he is then discharged a few days later from the trauma service. At the time of discharge, he is still experiencing right-shoulder pain. A discharge diagnosis of shoulder contusion is assigned, and no further imaging or other investigations are performed on his shoulder.\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n**Figure 3.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 954249,
"choiceText": "Acromioclavicular dislocation",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 954251,
"choiceText": "Humeral fracture",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 954253,
"choiceText": "Clavicle fracture",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 954255,
"choiceText": "Avulsion fracture",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 301829,
"questionText": "On the basis of the history, physical examination, and workup, what is the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 35-Year-Old Man Who Fell From a Motorcycle"
},
{
"authors": "Joe Nemeth, MD, CCFP(EM); Catherine Patocka, MDCM; David Barbic, MD, MSc",
"content": [
"Figure 4.",
"The patient returned to the ED the following day and, on further review of the patient's initial radiographs (keeping in mind the context of the physical examination), a repeat shoulder radiograph was obtained. This radiograph clearly showed a clavicle fracture (Figure 4).",
"This case reinforces the following points:",
"When evaluating radiographs, careful attention must be paid to the clinical examination findings.",
"Before any images are obtained, all hospital and nonhospital attachments must be removed from the body part that is being examined.",
"The reported incidence of errors in the interpretation of radiologic studies in the ED widely varies. One study examining the competency of five senior surgical residents compared with radiologists in a Level II trauma center showed a 99.2% rate of agreement; the residents identified 127 injuries, whereas 128 were identified by the radiologists (the radiologists missed one finding and the residents missed two).[1]",
"Other studies have shown error rates ranging from 2% to 16% for plain radiographs interpreted by nonradiologists.[2,3] A study by Russell and colleagues[4] showed error rates as high as 29%.[4] In that study, two of the errors led to serious management issues. Consequently, much reflection is required to determine where errors in radiologic interpretation stem from.",
"Errors in radiology may be broadly classified into the two following categories: perceptual/cognitive errors (false-positive, false-negative, and misinterpretation) and other types of error (eg, complications from procedures and communication lapses).[5] Errors in the ED environment are primarily of the perceptual-cognitive type.",
"A false-positive error is an error of overreading, in which a finding is appreciated by the clinician but is in fact not present. However, false-negative results are more common, in which findings that are true are missed. False-negatives may result from a lack of knowledge or a failure to isolate important material, or they may occur because of limitations in the examination. Misclassification, the final type of perceptual/cognitive error, occurs when an abnormality is recognized but is interpreted incorrectly.",
"In this case, the error would be classified as a false-negative interpretation of the film. The proximal cause of the error, however, was not misinterpretation. Rather, it was the failure to ensure an adequate examination by not removing the extraneous radio-opaque material (the monitoring electrodes) and repeating radiography.",
"Other common examples of this kind of error in the trauma setting include failure to obtain proper views when visualizing a scaphoid fracture on plain radiography, failure to insist on complete inspiration during chest radiography, and failure to obtain adequate views to visualize the C7/T1 interface on lateral cervical-spine films. The American College of Radiology and the Physician Insurers Association of America report that faulty technique is responsible for 10%-30% of radiologic medical-malpractice claims.[6]",
"Such mistakes as these are fairly simple to avoid by implementing a systems-thinking approach, which in this particular case would have been implementation of (and adherence to) a simple rule. In a systems approach, errors are seen as being shaped and provoked by upstream systemic factors, such as organizational culture, risk management, or lack of resources.[7] A longitudinal study of reducing errors in radiographic interpretation by emergency physicians demonstrated that implementing a systemic approach reduced errors from 3% to 0.3% over a 3-year period.[8] In that particular study, a policy was implemented wherein radiographs were brought directly to emergency physicians for interpretation and subsequently reexamined by radiologists within 12 hours. In the event of a clinically significant misinterpretation, the staff contacted the patients and asked them to return.",
"Although the error in this case was missed by both the emergency physician and the radiologist, a systems-based approach to radiograph interpretation, with strict insistence on proper visualization and systematic identification of landmarks during radiographic interpretation, might well have prevented the error. Other errors that fall under the perceptual/cognitive category are multifactorial and, therefore, are more difficult to address."
],
"date": "March 30, 2016",
"figures": [
{
"caption": "Figure 4.",
"image_url": "https://img.medscapestatic.com/article/861/017/861017-Thumb4.png"
}
],
"markdown": "# A 35-Year-Old Man Who Fell From a Motorcycle\n\n **Authors:** Joe Nemeth, MD, CCFP(EM); Catherine Patocka, MDCM; David Barbic, MD, MSc \n **Date:** March 30, 2016\n\n ## Content\n\n Figure 4.\nThe patient returned to the ED the following day and, on further review of the patient's initial radiographs (keeping in mind the context of the physical examination), a repeat shoulder radiograph was obtained. This radiograph clearly showed a clavicle fracture (Figure 4).\nThis case reinforces the following points:\nWhen evaluating radiographs, careful attention must be paid to the clinical examination findings.\nBefore any images are obtained, all hospital and nonhospital attachments must be removed from the body part that is being examined.\nThe reported incidence of errors in the interpretation of radiologic studies in the ED widely varies. One study examining the competency of five senior surgical residents compared with radiologists in a Level II trauma center showed a 99.2% rate of agreement; the residents identified 127 injuries, whereas 128 were identified by the radiologists (the radiologists missed one finding and the residents missed two).[1]\nOther studies have shown error rates ranging from 2% to 16% for plain radiographs interpreted by nonradiologists.[2,3] A study by Russell and colleagues[4] showed error rates as high as 29%.[4] In that study, two of the errors led to serious management issues. Consequently, much reflection is required to determine where errors in radiologic interpretation stem from.\nErrors in radiology may be broadly classified into the two following categories: perceptual/cognitive errors (false-positive, false-negative, and misinterpretation) and other types of error (eg, complications from procedures and communication lapses).[5] Errors in the ED environment are primarily of the perceptual-cognitive type.\nA false-positive error is an error of overreading, in which a finding is appreciated by the clinician but is in fact not present. However, false-negative results are more common, in which findings that are true are missed. False-negatives may result from a lack of knowledge or a failure to isolate important material, or they may occur because of limitations in the examination. Misclassification, the final type of perceptual/cognitive error, occurs when an abnormality is recognized but is interpreted incorrectly.\nIn this case, the error would be classified as a false-negative interpretation of the film. The proximal cause of the error, however, was not misinterpretation. Rather, it was the failure to ensure an adequate examination by not removing the extraneous radio-opaque material (the monitoring electrodes) and repeating radiography.\nOther common examples of this kind of error in the trauma setting include failure to obtain proper views when visualizing a scaphoid fracture on plain radiography, failure to insist on complete inspiration during chest radiography, and failure to obtain adequate views to visualize the C7/T1 interface on lateral cervical-spine films. The American College of Radiology and the Physician Insurers Association of America report that faulty technique is responsible for 10%-30% of radiologic medical-malpractice claims.[6]\nSuch mistakes as these are fairly simple to avoid by implementing a systems-thinking approach, which in this particular case would have been implementation of (and adherence to) a simple rule. In a systems approach, errors are seen as being shaped and provoked by upstream systemic factors, such as organizational culture, risk management, or lack of resources.[7] A longitudinal study of reducing errors in radiographic interpretation by emergency physicians demonstrated that implementing a systemic approach reduced errors from 3% to 0.3% over a 3-year period.[8] In that particular study, a policy was implemented wherein radiographs were brought directly to emergency physicians for interpretation and subsequently reexamined by radiologists within 12 hours. In the event of a clinically significant misinterpretation, the staff contacted the patients and asked them to return.\nAlthough the error in this case was missed by both the emergency physician and the radiologist, a systems-based approach to radiograph interpretation, with strict insistence on proper visualization and systematic identification of landmarks during radiographic interpretation, might well have prevented the error. Other errors that fall under the perceptual/cognitive category are multifactorial and, therefore, are more difficult to address.\n\n ## Figures\n\n **Figure 4.** \n \n\n\n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 954249,
"choiceText": "Acromioclavicular dislocation",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 954251,
"choiceText": "Humeral fracture",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 954253,
"choiceText": "Clavicle fracture",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 954255,
"choiceText": "Avulsion fracture",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 301829,
"questionText": "On the basis of the history, physical examination, and workup, what is the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 35-Year-Old Man Who Fell From a Motorcycle"
},
{
"authors": "Joe Nemeth, MD, CCFP(EM); Catherine Patocka, MDCM; David Barbic, MD, MSc",
"content": [
"Studies by Croskerry and Norman[9] on critical thinking and decision-making in EDs in the United States, Canada, and Australia have identified 25 processes that contribute to medical errors. Apart from the system errors mentioned previously, almost one half of these resulted from individual error (ie, faulty decision-making, mistriage, and cognitive and emotional biases). Croskerry and Norman identified that the manner in which clinicians working the ED environment inherently think, although a necessary approach to care in the ED, may also be a key source of error.",
"Emergency providers combine two thought processes. The first can be simplified as \"pattern recognition,\" and it is characterized by mental shortcuts and reflexive reactions to patients' clinical presentations. This is, in fact, what many would call gestalt: An experienced clinician will recognize certain aspects of a disease presentation, rapidly come to diagnostic closure, and then proceed with his or her plan of action. The second thought process is deductive, rational, rule-based, and low in emotional investment.",
"Both of these processes are essential to the practice of emergency healthcare, and studies have shown that pure reliance on either of the two processes results in more errors.[10,11] Clinicians in the ED must strive to balance both thought processes and use each in the appropriate context in order to deal with critically ill patients. In this case, radiography of the shoulder was ordered, which suggests that injury was suspected; however, there was a failure to follow the rule that extraneous material not be present.",
"To prevent this type of error from recurring, a systems approach would entail an inquiry into why this failure occurred. Was it because the clinicians were unaware of or disagreed with the rule? This would indicate a training issue. Were they too busy? This would point to a workforce scheduling issue. Perhaps the radiology team had become lax about removing hardware because they had not done so in many cases, without harm. That would be an example of \"normalized deviance,\" wherein an unsafe practice becomes accepted as part of everyday routine by the whole team; this is most likely to occur in situations where the consequences of deviation from an accepted practice are ambiguous or seem to be of very low frequency."
],
"date": "March 30, 2016",
"figures": [],
"markdown": "# A 35-Year-Old Man Who Fell From a Motorcycle\n\n **Authors:** Joe Nemeth, MD, CCFP(EM); Catherine Patocka, MDCM; David Barbic, MD, MSc \n **Date:** March 30, 2016\n\n ## Content\n\n Studies by Croskerry and Norman[9] on critical thinking and decision-making in EDs in the United States, Canada, and Australia have identified 25 processes that contribute to medical errors. Apart from the system errors mentioned previously, almost one half of these resulted from individual error (ie, faulty decision-making, mistriage, and cognitive and emotional biases). Croskerry and Norman identified that the manner in which clinicians working the ED environment inherently think, although a necessary approach to care in the ED, may also be a key source of error.\nEmergency providers combine two thought processes. The first can be simplified as \"pattern recognition,\" and it is characterized by mental shortcuts and reflexive reactions to patients' clinical presentations. This is, in fact, what many would call gestalt: An experienced clinician will recognize certain aspects of a disease presentation, rapidly come to diagnostic closure, and then proceed with his or her plan of action. The second thought process is deductive, rational, rule-based, and low in emotional investment.\nBoth of these processes are essential to the practice of emergency healthcare, and studies have shown that pure reliance on either of the two processes results in more errors.[10,11] Clinicians in the ED must strive to balance both thought processes and use each in the appropriate context in order to deal with critically ill patients. In this case, radiography of the shoulder was ordered, which suggests that injury was suspected; however, there was a failure to follow the rule that extraneous material not be present.\nTo prevent this type of error from recurring, a systems approach would entail an inquiry into why this failure occurred. Was it because the clinicians were unaware of or disagreed with the rule? This would indicate a training issue. Were they too busy? This would point to a workforce scheduling issue. Perhaps the radiology team had become lax about removing hardware because they had not done so in many cases, without harm. That would be an example of \"normalized deviance,\" wherein an unsafe practice becomes accepted as part of everyday routine by the whole team; this is most likely to occur in situations where the consequences of deviation from an accepted practice are ambiguous or seem to be of very low frequency.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 35-Year-Old Man Who Fell From a Motorcycle"
},
{
"authors": "Joe Nemeth, MD, CCFP(EM); Catherine Patocka, MDCM; David Barbic, MD, MSc",
"content": [
"A final source of error described by both Croskerry and Norman[9] and Berner and Graber[2] is overconfidence. These researchers advance the notion that clinicians in general underappreciate the likelihood that their diagnoses may be wrong. They cite a lack of clinician-demonstrated knowledge-seeking behavior and failure to adhere to guideline-recommended best practices as indirect indicators of overconfidence.",
"Concrete and definite evidence of overconfidence has been demonstrated in several studies.[12,13] Podbregar and colleagues[14] asked intensive care unit (ICU) clinicians to provide a clinical diagnosis and their level of uncertainty for 126 patients who had died in the ICU.[14] The uncertainty was measured by three levels: Level 1 represented complete certainty, level 2 represented minor uncertainty, and level 3 was major uncertainty. The discrepancy between the clinical and postmortem diagnoses was identical in all the groups. Similar results were found by Landefeld and colleagues.[15] Clearly, overconfidence plays a role in medical errors; the difficulty lies in developing reasonable strategies to reduce these errors.",
"Practical solutions to ensure quality patient care are dependent on adapting a systems approach to error reduction, as well as individual clinicians becoming cognizant of their own clinical thought processes. Hospitals and health systems need to adapt approaches that promote individual and departmental excellence. This process is enhanced by using some of the following steps in daily practice:",
"The clinician should regularly step back from a problem and assess how the current thought process has led to the conclusion.",
"The clinician should engage in regular review and feedback activities in order to identify possible sources of error (ie, morbidity and mortality rounds).",
"The clinician should engage in periodic evaluation of his or her practice habits and trends in order to look for possible sources of error in their own practice methods."
],
"date": "March 30, 2016",
"figures": [],
"markdown": "# A 35-Year-Old Man Who Fell From a Motorcycle\n\n **Authors:** Joe Nemeth, MD, CCFP(EM); Catherine Patocka, MDCM; David Barbic, MD, MSc \n **Date:** March 30, 2016\n\n ## Content\n\n A final source of error described by both Croskerry and Norman[9] and Berner and Graber[2] is overconfidence. These researchers advance the notion that clinicians in general underappreciate the likelihood that their diagnoses may be wrong. They cite a lack of clinician-demonstrated knowledge-seeking behavior and failure to adhere to guideline-recommended best practices as indirect indicators of overconfidence.\nConcrete and definite evidence of overconfidence has been demonstrated in several studies.[12,13] Podbregar and colleagues[14] asked intensive care unit (ICU) clinicians to provide a clinical diagnosis and their level of uncertainty for 126 patients who had died in the ICU.[14] The uncertainty was measured by three levels: Level 1 represented complete certainty, level 2 represented minor uncertainty, and level 3 was major uncertainty. The discrepancy between the clinical and postmortem diagnoses was identical in all the groups. Similar results were found by Landefeld and colleagues.[15] Clearly, overconfidence plays a role in medical errors; the difficulty lies in developing reasonable strategies to reduce these errors.\nPractical solutions to ensure quality patient care are dependent on adapting a systems approach to error reduction, as well as individual clinicians becoming cognizant of their own clinical thought processes. Hospitals and health systems need to adapt approaches that promote individual and departmental excellence. This process is enhanced by using some of the following steps in daily practice:\nThe clinician should regularly step back from a problem and assess how the current thought process has led to the conclusion.\nThe clinician should engage in regular review and feedback activities in order to identify possible sources of error (ie, morbidity and mortality rounds).\nThe clinician should engage in periodic evaluation of his or her practice habits and trends in order to look for possible sources of error in their own practice methods.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 954257,
"choiceText": "Overconfidence",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 954259,
"choiceText": "False-positive error",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 954261,
"choiceText": "False-negative error",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 954263,
"choiceText": "Misinterpretation error",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "This is a case of overreading; a finding (ie, <a href=\"http://emedicine.medscape.com/article/773895-overview\">appendicitis</a>) was initially identified, but was clearly shown to not exist. This is an example of a false-positive error. If the study had been interpreted as normal and the patient was subsequently found to have appendicitis, it would be a false-negative error. Finally, if the study was interpreted as appendicitis and instead a tumor was found during surgery, this would be an example of misinterpretation.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 301831,
"questionText": "An appendix 8 mm in diameter was interpreted on ultrasonography as demonstrating appendicitis, but a normal appendix was noted at surgery and on subsequent pathology reports. What type of error is this an example of?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 954265,
"choiceText": "Stepping back from the problem and assessing how the current thought process has led to the conclusion",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 954267,
"choiceText": "Engaging in regular review and feedback activities to identify sources of error",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 954269,
"choiceText": "Engaging in periodic evaluation of practice habits and trends to look for sources of error",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 954271,
"choiceText": "All of the above",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The above are all practical solutions to promoting individual and departmental excellence. Other important aspects of error reduction include adapting a systems approach to error reduction and becoming cognizant of one's clinical thought processes.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 301833,
"questionText": "Which of the following choices represents a method or set of methods for avoiding medical errors?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 35-Year-Old Man Who Fell From a Motorcycle"
},
{
"authors": "Joe Nemeth, MD, CCFP(EM); Catherine Patocka, MDCM; David Barbic, MD, MSc",
"content": [],
"date": "March 30, 2016",
"figures": [],
"markdown": "# A 35-Year-Old Man Who Fell From a Motorcycle\n\n **Authors:** Joe Nemeth, MD, CCFP(EM); Catherine Patocka, MDCM; David Barbic, MD, MSc \n **Date:** March 30, 2016\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 954257,
"choiceText": "Overconfidence",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 954259,
"choiceText": "False-positive error",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 954261,
"choiceText": "False-negative error",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 954263,
"choiceText": "Misinterpretation error",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "This is a case of overreading; a finding (ie, <a href=\"http://emedicine.medscape.com/article/773895-overview\">appendicitis</a>) was initially identified, but was clearly shown to not exist. This is an example of a false-positive error. If the study had been interpreted as normal and the patient was subsequently found to have appendicitis, it would be a false-negative error. Finally, if the study was interpreted as appendicitis and instead a tumor was found during surgery, this would be an example of misinterpretation.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 301831,
"questionText": "An appendix 8 mm in diameter was interpreted on ultrasonography as demonstrating appendicitis, but a normal appendix was noted at surgery and on subsequent pathology reports. What type of error is this an example of?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 954265,
"choiceText": "Stepping back from the problem and assessing how the current thought process has led to the conclusion",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 954267,
"choiceText": "Engaging in regular review and feedback activities to identify sources of error",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 954269,
"choiceText": "Engaging in periodic evaluation of practice habits and trends to look for sources of error",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 954271,
"choiceText": "All of the above",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The above are all practical solutions to promoting individual and departmental excellence. Other important aspects of error reduction include adapting a systems approach to error reduction and becoming cognizant of one's clinical thought processes.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 301833,
"questionText": "Which of the following choices represents a method or set of methods for avoiding medical errors?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 35-Year-Old Man Who Fell From a Motorcycle"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 954249,
"choiceText": "Acromioclavicular dislocation",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 954251,
"choiceText": "Humeral fracture",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 954253,
"choiceText": "Clavicle fracture",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 954255,
"choiceText": "Avulsion fracture",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 301829,
"questionText": "On the basis of the history, physical examination, and workup, what is the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 954257,
"choiceText": "Overconfidence",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 954259,
"choiceText": "False-positive error",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 954261,
"choiceText": "False-negative error",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 954263,
"choiceText": "Misinterpretation error",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "This is a case of overreading; a finding (ie, <a href=\"http://emedicine.medscape.com/article/773895-overview\">appendicitis</a>) was initially identified, but was clearly shown to not exist. This is an example of a false-positive error. If the study had been interpreted as normal and the patient was subsequently found to have appendicitis, it would be a false-negative error. Finally, if the study was interpreted as appendicitis and instead a tumor was found during surgery, this would be an example of misinterpretation.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 301831,
"questionText": "An appendix 8 mm in diameter was interpreted on ultrasonography as demonstrating appendicitis, but a normal appendix was noted at surgery and on subsequent pathology reports. What type of error is this an example of?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 954265,
"choiceText": "Stepping back from the problem and assessing how the current thought process has led to the conclusion",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 954267,
"choiceText": "Engaging in regular review and feedback activities to identify sources of error",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 954269,
"choiceText": "Engaging in periodic evaluation of practice habits and trends to look for sources of error",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 954271,
"choiceText": "All of the above",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The above are all practical solutions to promoting individual and departmental excellence. Other important aspects of error reduction include adapting a systems approach to error reduction and becoming cognizant of one's clinical thought processes.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 301833,
"questionText": "Which of the following choices represents a method or set of methods for avoiding medical errors?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
860319 | /viewarticle/860319 | [
{
"authors": "Lars Grimm, MD, MHS; Malkeet Gupta, MS, MD; Joshua M. Kosowsky, MD",
"content": [
"Editor's Note:\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.",
"A 38-year-old man with altered mental status is brought to the emergency department (ED) by ambulance. The patient had been found sprawled on the stoop of an apartment building when residents called the police. He was unresponsive when the paramedics arrived, and they were unable to obtain any additional history from the patient or from the onlookers at the scene.",
"The patient was wrapped in blankets and an intravenous line was inserted, which provided 0.5 L of normal saline during transit to the ED. When the patient was found, the temperature outside was about 35°F, with winds reaching speeds of 10-15 mi/hr."
],
"date": "March 15, 2016",
"figures": [],
"markdown": "# An Unresponsive 38-Year-Old Man\n\n **Authors:** Lars Grimm, MD, MHS; Malkeet Gupta, MS, MD; Joshua M. Kosowsky, MD \n **Date:** March 15, 2016\n\n ## Content\n\n Editor's Note:\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.\nA 38-year-old man with altered mental status is brought to the emergency department (ED) by ambulance. The patient had been found sprawled on the stoop of an apartment building when residents called the police. He was unresponsive when the paramedics arrived, and they were unable to obtain any additional history from the patient or from the onlookers at the scene.\nThe patient was wrapped in blankets and an intravenous line was inserted, which provided 0.5 L of normal saline during transit to the ED. When the patient was found, the temperature outside was about 35°F, with winds reaching speeds of 10-15 mi/hr.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "An Unresponsive 38-Year-Old Man"
},
{
"authors": "Lars Grimm, MD, MHS; Malkeet Gupta, MS, MD; Joshua M. Kosowsky, MD",
"content": [
"On arrival at the ED, the patient has a blood pressure of 88/40 mm Hg, heart rate of 38 beats/min, and respiratory rate of 24 breaths/min. An oral thermometer reading is unsuccessful, but a rectal probe records a temperature of 85.4°F (29.7°C). Repeated attempts to obtain an oxygen saturation measurement via a finger pulse oximeter are not successful.",
"Figure 1.",
"Figure 1.",
"Upon initial examination, the patient appears to be a homeless, disheveled man with a faint smell of alcohol on his breath. His clothing is stained and extensively worn. He looks older than the stated age on his identification card.",
"In the ED, the patient is mildly arousable and has trouble following simple commands. He has intact gag and corneal reflexes and does not fight the examiner during the tests. He responds appropriately to painful stimuli. His pupils are equal, round, and reactive to light. No obvious signs of head trauma are noted, and his oropharynx, nares, and ears are unremarkable.",
"Cardiac examination is notable for marked bradycardia of 45 beats/min, but no murmurs, rubs, gallops, or extra heart sounds are noted. A lung examination reveals rhonchi and dullness to percussion in the right lower lung field, but otherwise normal lung sounds. The patient's abdomen is soft, nontender, and nondistended, and normal bowel sounds are heard. His skin is cold, and his fingers and toes have a bluish tinge, with a delayed capillary refill time. A brief skin survey reveals some minor abrasions, but no major wounds or erosions.",
"The patient cannot provide any additional information about his medical, family, medication, social, or allergy history. His pockets contain an identification card, but no other useful information.",
"Routine blood work is ordered. The results of the patient's metabolic panel are all within normal limits. His blood glucose level is 104 mg/dL (5.77 mmol/L). The complete blood cell count reveals a small elevation in the white blood cell count, but is otherwise unremarkable. A portable chest radiograph shows a small consolidation of the right lower lobe that is consistent with pneumonia.",
"An ECG is performed (Figure 1)."
],
"date": "March 15, 2016",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/860/319/860319-Thumb1a.jpg"
}
],
"markdown": "# An Unresponsive 38-Year-Old Man\n\n **Authors:** Lars Grimm, MD, MHS; Malkeet Gupta, MS, MD; Joshua M. Kosowsky, MD \n **Date:** March 15, 2016\n\n ## Content\n\n On arrival at the ED, the patient has a blood pressure of 88/40 mm Hg, heart rate of 38 beats/min, and respiratory rate of 24 breaths/min. An oral thermometer reading is unsuccessful, but a rectal probe records a temperature of 85.4°F (29.7°C). Repeated attempts to obtain an oxygen saturation measurement via a finger pulse oximeter are not successful.\nFigure 1.\nFigure 1.\nUpon initial examination, the patient appears to be a homeless, disheveled man with a faint smell of alcohol on his breath. His clothing is stained and extensively worn. He looks older than the stated age on his identification card.\nIn the ED, the patient is mildly arousable and has trouble following simple commands. He has intact gag and corneal reflexes and does not fight the examiner during the tests. He responds appropriately to painful stimuli. His pupils are equal, round, and reactive to light. No obvious signs of head trauma are noted, and his oropharynx, nares, and ears are unremarkable.\nCardiac examination is notable for marked bradycardia of 45 beats/min, but no murmurs, rubs, gallops, or extra heart sounds are noted. A lung examination reveals rhonchi and dullness to percussion in the right lower lung field, but otherwise normal lung sounds. The patient's abdomen is soft, nontender, and nondistended, and normal bowel sounds are heard. His skin is cold, and his fingers and toes have a bluish tinge, with a delayed capillary refill time. A brief skin survey reveals some minor abrasions, but no major wounds or erosions.\nThe patient cannot provide any additional information about his medical, family, medication, social, or allergy history. His pockets contain an identification card, but no other useful information.\nRoutine blood work is ordered. The results of the patient's metabolic panel are all within normal limits. His blood glucose level is 104 mg/dL (5.77 mmol/L). The complete blood cell count reveals a small elevation in the white blood cell count, but is otherwise unremarkable. A portable chest radiograph shows a small consolidation of the right lower lobe that is consistent with pneumonia.\nAn ECG is performed (Figure 1).\n\n ## Figures\n\n **Figure 1.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 950755,
"choiceText": "Hyperkalemia",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 950757,
"choiceText": "Hypothyroidism",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 950759,
"choiceText": "Hypothermia",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 950761,
"choiceText": "Acute myocardial infarction\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 300795,
"questionText": "On the basis of the history, physical examination, and workup, what is the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "An Unresponsive 38-Year-Old Man"
},
{
"authors": "Lars Grimm, MD, MHS; Malkeet Gupta, MS, MD; Joshua M. Kosowsky, MD",
"content": [
"The patient in this case had severe hypothermia, which was diagnosed on the basis of the core body temperature reading obtained using the rectal temperature probe.",
"Figure 2.",
"Figure 2.",
"The patient's ECG demonstrated classic abnormalities associated with hypothermia; the most notable was profound sinus bradycardia. In addition, all leads showed the classic Osborne waves (also known as \"J waves\"), which were seen at the junction of the QRS complex and the ST segment (Figure 2) and are thought to represent alterations in the initial phase of ventricular repolarization.",
"Classically, Osborne waves are not thought to affect the ST segment; however, an ECG must be interpreted within the appropriate clinical context. In this case, the patient was found intoxicated, outdoors in near-freezing temperatures, and with a low core body temperature; as such, the apparent elevations of the ST segments should not be misinterpreted as evidence of myocardial injury. Other possible ECG findings of hypothermia that were not seen on this tracing include atrial and ventricular dysrhythmia, as well as prolongation of the PR, QRS, and QT intervals.[1]",
"The incidence of hypothermia is difficult to assess because many cases are not treated in medical centers. Between 1979 and 1998, hypothermia was listed as the cause of death for 13,970 people in the United States, an average of roughly 700 people per year.[2] More cases occur in urban settings as a result of the higher incidence of alcoholism, illicit drug use, mental illness, and homelessness. One half of all deaths involve patients older than 65 years of age, with an overall male-to-female ratio of 2.5:1.",
"Regions of the United States with colder temperatures or with greater day-to-night swings in temperature, as is found at high elevations or in deserts, report a higher incidence of hypothermia. The greatest number of cases are reported in Alaska, New Mexico, North Dakota, and Montana.",
"Patients at the highest risk have impaired cognition, inadequate shelter or clothing, or immobility. A smaller, secondary group of individuals at risk are those who venture into the wilderness for work or pleasure and are unprepared or experience an accident. The rapid increase in the popularity of outdoor sporting is causing the size of this secondary group to grow steadily.[3]",
"Body heat is lost via four different mechanisms. Radiation is heat loss from infrared emissions, usually in noninsulated areas of the body. Conduction is heat loss from direct contact, which is the primary means of heat loss in water immersion. Convection removes warmed air from around the body, resulting in increased heat loss during windy conditions. Evaporation cools the body via the removal of warmed water and is prevalent in dry, cold, windy environments.",
"Radiation accounts for 55%-65% of heat loss, conduction and convection each account for an additional 15% loss, and evaporation accounts for the remainder; however, these percentages are highly dependent on the local conditions. In this patient, convection could be expected to play a larger share because of the windy conditions.[3]"
],
"date": "March 15, 2016",
"figures": [
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/860/319/860319-Thumb2a.jpg"
}
],
"markdown": "# An Unresponsive 38-Year-Old Man\n\n **Authors:** Lars Grimm, MD, MHS; Malkeet Gupta, MS, MD; Joshua M. Kosowsky, MD \n **Date:** March 15, 2016\n\n ## Content\n\n The patient in this case had severe hypothermia, which was diagnosed on the basis of the core body temperature reading obtained using the rectal temperature probe.\nFigure 2.\nFigure 2.\nThe patient's ECG demonstrated classic abnormalities associated with hypothermia; the most notable was profound sinus bradycardia. In addition, all leads showed the classic Osborne waves (also known as \"J waves\"), which were seen at the junction of the QRS complex and the ST segment (Figure 2) and are thought to represent alterations in the initial phase of ventricular repolarization.\nClassically, Osborne waves are not thought to affect the ST segment; however, an ECG must be interpreted within the appropriate clinical context. In this case, the patient was found intoxicated, outdoors in near-freezing temperatures, and with a low core body temperature; as such, the apparent elevations of the ST segments should not be misinterpreted as evidence of myocardial injury. Other possible ECG findings of hypothermia that were not seen on this tracing include atrial and ventricular dysrhythmia, as well as prolongation of the PR, QRS, and QT intervals.[1]\nThe incidence of hypothermia is difficult to assess because many cases are not treated in medical centers. Between 1979 and 1998, hypothermia was listed as the cause of death for 13,970 people in the United States, an average of roughly 700 people per year.[2] More cases occur in urban settings as a result of the higher incidence of alcoholism, illicit drug use, mental illness, and homelessness. One half of all deaths involve patients older than 65 years of age, with an overall male-to-female ratio of 2.5:1.\nRegions of the United States with colder temperatures or with greater day-to-night swings in temperature, as is found at high elevations or in deserts, report a higher incidence of hypothermia. The greatest number of cases are reported in Alaska, New Mexico, North Dakota, and Montana.\nPatients at the highest risk have impaired cognition, inadequate shelter or clothing, or immobility. A smaller, secondary group of individuals at risk are those who venture into the wilderness for work or pleasure and are unprepared or experience an accident. The rapid increase in the popularity of outdoor sporting is causing the size of this secondary group to grow steadily.[3]\nBody heat is lost via four different mechanisms. Radiation is heat loss from infrared emissions, usually in noninsulated areas of the body. Conduction is heat loss from direct contact, which is the primary means of heat loss in water immersion. Convection removes warmed air from around the body, resulting in increased heat loss during windy conditions. Evaporation cools the body via the removal of warmed water and is prevalent in dry, cold, windy environments.\nRadiation accounts for 55%-65% of heat loss, conduction and convection each account for an additional 15% loss, and evaporation accounts for the remainder; however, these percentages are highly dependent on the local conditions. In this patient, convection could be expected to play a larger share because of the windy conditions.[3]\n\n ## Figures\n\n **Figure 2.** \n \n\n\n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 950755,
"choiceText": "Hyperkalemia",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 950757,
"choiceText": "Hypothyroidism",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 950759,
"choiceText": "Hypothermia",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 950761,
"choiceText": "Acute myocardial infarction\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 300795,
"questionText": "On the basis of the history, physical examination, and workup, what is the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "An Unresponsive 38-Year-Old Man"
},
{
"authors": "Lars Grimm, MD, MHS; Malkeet Gupta, MS, MD; Joshua M. Kosowsky, MD",
"content": [
"This case is an example of primary hypothermia, or accidental hypothermia from environmental exposure. This generally occurs when an unprepared individual is put in a situation of unanticipated exposure. In primary hypothermia, the body loses heat despite normal thermoregulatory mechanisms. To prevent excessive heat loss, the body's thermoregulatory center in the hypothalamus conserves and produces heat as needed. Shivering and the release of hypermetabolic hormones will increase the basal metabolic rate and produce heat; however, these productive capacities can be overrun if the rate of heat loss is too great or the energy stores are depleted over time.",
"Primary hypothermia must be distinguished from intentional hypothermia, in which an individual is placed in an induced state of hypothermia for neuroprotection.[4] It must also be distinguished from secondary hypothermia, in which the low body temperature is the result of a medical illness that causes the body's temperature set-point to be reduced. Other conditions, including infection, metabolic abnormalities (eg, hypoglycemia), drug or alcohol overdose, and endocrine problems (eg, hypothyroidism), often coexist, although these conditions may be the cause of secondary hypothermia.",
"The clinical presentation is dependent on the degree and duration of hypothermia.[3] Mild hypothermia is defined as a core body temperature of 90-95°F, and patients may initially show signs of shivering, tachycardia, vasoconstriction, and tachypnea. Later signs include apathy, ataxia, impaired judgment, and diuresis. Moderate hypothermia is a core body temperature of 82.4-89.9°F, and patients may stop shivering and shows signs of dysrhythmia, bradycardia, hypotension, J waves on ECG, diminished reflexes, dilated pupils, and a decreased level of consciousness.",
"Severe hypothermia is a core body temperature less than 82.4°F, at which point patients may begin to experience apnea, decreased activity on electroencephalography (EEG), nonreactive pupils, oliguria, pulmonary edema, coagulopathy, hemoconcentration, and more severe arrhythmia. In general, the life-threatening cardiovascular complications of hypothermia are cardiogenic shock and malignant dysrhythmias.[3]",
"Initial stabilization begins with establishing an accurate, continuous means of measuring the core body temperature in order to detect the degree of hypothermia and the response to therapy. Electronic oral thermometers may report an error at low temperatures; therefore, patients may need to have a rectal, esophageal, or bladder thermometer inserted for continuous measurements. Any wet clothing should be removed immediately.",
"Patients in respiratory failure should be intubated; mechanically ventilated; and given warmed, humidified, supplemental oxygen. Oral intubation is preferred because of the risk for epistaxis in these coagulopathic patients. Volume resuscitation should be initiated with warmed (104-113°F) normal saline—not lactated Ringer solution, because the shocked liver cannot metabolize the lactate.[3]"
],
"date": "March 15, 2016",
"figures": [],
"markdown": "# An Unresponsive 38-Year-Old Man\n\n **Authors:** Lars Grimm, MD, MHS; Malkeet Gupta, MS, MD; Joshua M. Kosowsky, MD \n **Date:** March 15, 2016\n\n ## Content\n\n This case is an example of primary hypothermia, or accidental hypothermia from environmental exposure. This generally occurs when an unprepared individual is put in a situation of unanticipated exposure. In primary hypothermia, the body loses heat despite normal thermoregulatory mechanisms. To prevent excessive heat loss, the body's thermoregulatory center in the hypothalamus conserves and produces heat as needed. Shivering and the release of hypermetabolic hormones will increase the basal metabolic rate and produce heat; however, these productive capacities can be overrun if the rate of heat loss is too great or the energy stores are depleted over time.\nPrimary hypothermia must be distinguished from intentional hypothermia, in which an individual is placed in an induced state of hypothermia for neuroprotection.[4] It must also be distinguished from secondary hypothermia, in which the low body temperature is the result of a medical illness that causes the body's temperature set-point to be reduced. Other conditions, including infection, metabolic abnormalities (eg, hypoglycemia), drug or alcohol overdose, and endocrine problems (eg, hypothyroidism), often coexist, although these conditions may be the cause of secondary hypothermia.\nThe clinical presentation is dependent on the degree and duration of hypothermia.[3] Mild hypothermia is defined as a core body temperature of 90-95°F, and patients may initially show signs of shivering, tachycardia, vasoconstriction, and tachypnea. Later signs include apathy, ataxia, impaired judgment, and diuresis. Moderate hypothermia is a core body temperature of 82.4-89.9°F, and patients may stop shivering and shows signs of dysrhythmia, bradycardia, hypotension, J waves on ECG, diminished reflexes, dilated pupils, and a decreased level of consciousness.\nSevere hypothermia is a core body temperature less than 82.4°F, at which point patients may begin to experience apnea, decreased activity on electroencephalography (EEG), nonreactive pupils, oliguria, pulmonary edema, coagulopathy, hemoconcentration, and more severe arrhythmia. In general, the life-threatening cardiovascular complications of hypothermia are cardiogenic shock and malignant dysrhythmias.[3]\nInitial stabilization begins with establishing an accurate, continuous means of measuring the core body temperature in order to detect the degree of hypothermia and the response to therapy. Electronic oral thermometers may report an error at low temperatures; therefore, patients may need to have a rectal, esophageal, or bladder thermometer inserted for continuous measurements. Any wet clothing should be removed immediately.\nPatients in respiratory failure should be intubated; mechanically ventilated; and given warmed, humidified, supplemental oxygen. Oral intubation is preferred because of the risk for epistaxis in these coagulopathic patients. Volume resuscitation should be initiated with warmed (104-113°F) normal saline—not lactated Ringer solution, because the shocked liver cannot metabolize the lactate.[3]\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "An Unresponsive 38-Year-Old Man"
},
{
"authors": "Lars Grimm, MD, MHS; Malkeet Gupta, MS, MD; Joshua M. Kosowsky, MD",
"content": [
"Cardiac reserve is decreased in hypothermic patients and, therefore, judicious volume resuscitation is advised. Cardiac arrhythmia can occur as a consequence of hypothermia, and treatment of cardiac arrhythmia may differ in hypothermic patients. Rewarming the patient is typically sufficient for him or her to regain normal myocardial contractility and rhythm. For patients in shock who do not respond to rewarming and resuscitation with intravenous fluid, low-dose dopamine is the recommended agent because of its inotropic and peripheral vasoconstrictive effects.",
"Atrial dysrhythmia is generally associated with a slow ventricular response; therefore, treatment with digoxin or calcium-channel blockers is not warranted. Bretylium has long been recommended for the treatment and prevention of ventricular dysrhythmias, although little evidence supports this practice and this agent is no longer available for clinical use. In the case of ventricular arrhythmias, amiodarone is a reasonable choice.",
"For refractory bradydysrhythmia, external noninvasive pacing is recommended as a first-line treatment. Transvenous pacing may provoke ventricular dysrhythmias with the insertion of pacing wires into a hypothermic ventricle; however, this concern should not limit its use if transcutaneous and pharmacologic therapies are ineffective.[3]",
"There are three general types of rewarming techniques: passive external, active external, and active internal. Passive external rewarming is reserved for mildly hypothermic patients and involves insulating the patient to allow the intact thermoregulatory mechanisms to reheat the body. Covering the patient in blankets and having him or her inspire humidified air allows slow, steady increases in core body temperature.",
"Active external rewarming techniques include warm-water immersion, the use of forced-air warming systems, or the placement of a patient in a heat cradle. A major complication of active external warming to be aware of is core temperature afterdrop, in which cold peripheral blood rapidly returns to the heart, leading to inaccurate temperature readings and subsequent inappropriate management decisions.",
"Active internal rewarming is reserved for severe hypothermia or for patients who do not respond to less aggressive measures.",
"The rewarming techniques can be classified as either minimally invasive (eg, heated, humidified air and warm intravenous fluids) or more invasive (eg, body cavity lavage, hemodialysis, cardiopulmonary bypass, and extracorporeal blood warming). The choice of the most appropriate rewarming technique is based on the equipment available and the experience of the operator; however, the least invasive means of adequate rewarming is generally best. No concrete evidence suggests that faster rates of rewarming lead to improved outcomes, because comorbidities are the best predictors of mortality. In general, mild or moderate hypothermic patients have an excellent chance of recovery, but patients with severe hypothermia have a mortality rate of roughly 50%.[1,3]",
"The patient in this case was determined to have moderate hypothermia and was immediately stripped of his clothes. He was given a mask with warm, humidified air to breathe, and warm intravenous fluids were administered. A forced-air blanket was used to cover his torso, and warmed blankets were wrapped around his extremities. Peritoneal lavage with warm saline was started, and the patient's core body temperature began to steadily rise. The ECG changes resolved, the patient's volume status returned to normal, and the body temperature returned to a normal range.",
"The patient was admitted for monitoring and initiation of treatment of his right lower lobe pneumonia. Eventually, he was discharged without any major disabilities."
],
"date": "March 15, 2016",
"figures": [],
"markdown": "# An Unresponsive 38-Year-Old Man\n\n **Authors:** Lars Grimm, MD, MHS; Malkeet Gupta, MS, MD; Joshua M. Kosowsky, MD \n **Date:** March 15, 2016\n\n ## Content\n\n Cardiac reserve is decreased in hypothermic patients and, therefore, judicious volume resuscitation is advised. Cardiac arrhythmia can occur as a consequence of hypothermia, and treatment of cardiac arrhythmia may differ in hypothermic patients. Rewarming the patient is typically sufficient for him or her to regain normal myocardial contractility and rhythm. For patients in shock who do not respond to rewarming and resuscitation with intravenous fluid, low-dose dopamine is the recommended agent because of its inotropic and peripheral vasoconstrictive effects.\nAtrial dysrhythmia is generally associated with a slow ventricular response; therefore, treatment with digoxin or calcium-channel blockers is not warranted. Bretylium has long been recommended for the treatment and prevention of ventricular dysrhythmias, although little evidence supports this practice and this agent is no longer available for clinical use. In the case of ventricular arrhythmias, amiodarone is a reasonable choice.\nFor refractory bradydysrhythmia, external noninvasive pacing is recommended as a first-line treatment. Transvenous pacing may provoke ventricular dysrhythmias with the insertion of pacing wires into a hypothermic ventricle; however, this concern should not limit its use if transcutaneous and pharmacologic therapies are ineffective.[3]\nThere are three general types of rewarming techniques: passive external, active external, and active internal. Passive external rewarming is reserved for mildly hypothermic patients and involves insulating the patient to allow the intact thermoregulatory mechanisms to reheat the body. Covering the patient in blankets and having him or her inspire humidified air allows slow, steady increases in core body temperature.\nActive external rewarming techniques include warm-water immersion, the use of forced-air warming systems, or the placement of a patient in a heat cradle. A major complication of active external warming to be aware of is core temperature afterdrop, in which cold peripheral blood rapidly returns to the heart, leading to inaccurate temperature readings and subsequent inappropriate management decisions.\nActive internal rewarming is reserved for severe hypothermia or for patients who do not respond to less aggressive measures.\nThe rewarming techniques can be classified as either minimally invasive (eg, heated, humidified air and warm intravenous fluids) or more invasive (eg, body cavity lavage, hemodialysis, cardiopulmonary bypass, and extracorporeal blood warming). The choice of the most appropriate rewarming technique is based on the equipment available and the experience of the operator; however, the least invasive means of adequate rewarming is generally best. No concrete evidence suggests that faster rates of rewarming lead to improved outcomes, because comorbidities are the best predictors of mortality. In general, mild or moderate hypothermic patients have an excellent chance of recovery, but patients with severe hypothermia have a mortality rate of roughly 50%.[1,3]\nThe patient in this case was determined to have moderate hypothermia and was immediately stripped of his clothes. He was given a mask with warm, humidified air to breathe, and warm intravenous fluids were administered. A forced-air blanket was used to cover his torso, and warmed blankets were wrapped around his extremities. Peritoneal lavage with warm saline was started, and the patient's core body temperature began to steadily rise. The ECG changes resolved, the patient's volume status returned to normal, and the body temperature returned to a normal range.\nThe patient was admitted for monitoring and initiation of treatment of his right lower lobe pneumonia. Eventually, he was discharged without any major disabilities.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 950763,
"choiceText": "Radiation",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 950765,
"choiceText": "Convection",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 950767,
"choiceText": "Conduction",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 950769,
"choiceText": "Evaporation\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Whereas radiation is the mechanism that generally accounts for the greatest percentage of heat loss, conduction is the primary means of heat loss in water immersion. Radiation is generally responsible for approximately 55%-65% of heat loss. Convection and conduction each account for roughly 15%, with evaporation covering the remainder. Radiation occurs in noninsulated, usually exposed, areas of the body, such as the hands and head in unprepared individuals. The mechanism of heat loss is highly dependent on the local conditions.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 300797,
"questionText": "You treat a patient for a case of near-drowning. In addition, the patient has hypothermia. Which of the following mechanisms of heat loss is responsible for the greatest percentage of heat loss in this patient?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 950771,
"choiceText": "Coagulopathy",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 950773,
"choiceText": "Apnea",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 950775,
"choiceText": "Pulmonary edema",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 950777,
"choiceText": "Nonreactive pupils",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 950779,
"choiceText": "All of the above\r\n",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "All of the clinical signs listed may be present in cases of severe hypothermia. In addition, oliguria, lack of activity on EEG, and severe dysrhythmias may also be present. Severe hypothermia is an extremely dangerous state, and even with appropriate treatment, only 50% of patients survive, many with crippling disabilities.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 300799,
"questionText": "You suspect that a patient you are treating has severe hypothermia. Which of the following clinical signs may be present in this patient?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "An Unresponsive 38-Year-Old Man"
},
{
"authors": "Lars Grimm, MD, MHS; Malkeet Gupta, MS, MD; Joshua M. Kosowsky, MD",
"content": [],
"date": "March 15, 2016",
"figures": [],
"markdown": "# An Unresponsive 38-Year-Old Man\n\n **Authors:** Lars Grimm, MD, MHS; Malkeet Gupta, MS, MD; Joshua M. Kosowsky, MD \n **Date:** March 15, 2016\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 950763,
"choiceText": "Radiation",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 950765,
"choiceText": "Convection",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 950767,
"choiceText": "Conduction",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 950769,
"choiceText": "Evaporation\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Whereas radiation is the mechanism that generally accounts for the greatest percentage of heat loss, conduction is the primary means of heat loss in water immersion. Radiation is generally responsible for approximately 55%-65% of heat loss. Convection and conduction each account for roughly 15%, with evaporation covering the remainder. Radiation occurs in noninsulated, usually exposed, areas of the body, such as the hands and head in unprepared individuals. The mechanism of heat loss is highly dependent on the local conditions.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 300797,
"questionText": "You treat a patient for a case of near-drowning. In addition, the patient has hypothermia. Which of the following mechanisms of heat loss is responsible for the greatest percentage of heat loss in this patient?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 950771,
"choiceText": "Coagulopathy",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 950773,
"choiceText": "Apnea",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 950775,
"choiceText": "Pulmonary edema",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 950777,
"choiceText": "Nonreactive pupils",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 950779,
"choiceText": "All of the above\r\n",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "All of the clinical signs listed may be present in cases of severe hypothermia. In addition, oliguria, lack of activity on EEG, and severe dysrhythmias may also be present. Severe hypothermia is an extremely dangerous state, and even with appropriate treatment, only 50% of patients survive, many with crippling disabilities.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 300799,
"questionText": "You suspect that a patient you are treating has severe hypothermia. Which of the following clinical signs may be present in this patient?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "An Unresponsive 38-Year-Old Man"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 950755,
"choiceText": "Hyperkalemia",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 950757,
"choiceText": "Hypothyroidism",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 950759,
"choiceText": "Hypothermia",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 950761,
"choiceText": "Acute myocardial infarction\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 300795,
"questionText": "On the basis of the history, physical examination, and workup, what is the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 950763,
"choiceText": "Radiation",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 950765,
"choiceText": "Convection",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 950767,
"choiceText": "Conduction",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 950769,
"choiceText": "Evaporation\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Whereas radiation is the mechanism that generally accounts for the greatest percentage of heat loss, conduction is the primary means of heat loss in water immersion. Radiation is generally responsible for approximately 55%-65% of heat loss. Convection and conduction each account for roughly 15%, with evaporation covering the remainder. Radiation occurs in noninsulated, usually exposed, areas of the body, such as the hands and head in unprepared individuals. The mechanism of heat loss is highly dependent on the local conditions.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 300797,
"questionText": "You treat a patient for a case of near-drowning. In addition, the patient has hypothermia. Which of the following mechanisms of heat loss is responsible for the greatest percentage of heat loss in this patient?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 950771,
"choiceText": "Coagulopathy",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 950773,
"choiceText": "Apnea",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 950775,
"choiceText": "Pulmonary edema",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 950777,
"choiceText": "Nonreactive pupils",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 950779,
"choiceText": "All of the above\r\n",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "All of the clinical signs listed may be present in cases of severe hypothermia. In addition, oliguria, lack of activity on EEG, and severe dysrhythmias may also be present. Severe hypothermia is an extremely dangerous state, and even with appropriate treatment, only 50% of patients survive, many with crippling disabilities.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 300799,
"questionText": "You suspect that a patient you are treating has severe hypothermia. Which of the following clinical signs may be present in this patient?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
859634 | /viewarticle/859634 | [
{
"authors": "Jordan Burlen, BS; Umar Darr, MD; Zeinab Moussa, MD; Syed Hasan, DO",
"content": [
"Editor's Note:\n\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.",
"A 51-year-old black man with history of HIV infection resistant to tenofovir disoproxil/emtricitabine, renal stones, and recurrent urinary tract infection (UTI) presents to the emergency department with right suprapubic pain radiating to the testicles. The pain woke him about 1 hour before presentation. He describes the pain as sudden in onset, continuous and progressive, severe in intensity, and sharp and aching in character. The pain does not radiate anywhere else. He also complains of nausea, vomiting without blood, neck pain, dizziness, swollen lymph nodes, bone pain, fatigue, ischuria, and dysuria without blood.",
"The patient had been hospitalized 15 days ago for sepsis secondary to enterococcal infection, methicillin-resistant Staphylococcus aureus (MRSA) pyelonephritis, and urolithiasis. His medical history is also significant for benign prostatic hypertrophy, herpes zoster, and constipation. The patient was a former smoker, drinks alcohol occasionally, and lives alone. He also states that he has not been compliant with his medications, including those for his HIV infection."
],
"date": "March 02, 2016",
"figures": [],
"markdown": "# A 51-Year-Old Man With HIV and Suprapubic Pain\n\n **Authors:** Jordan Burlen, BS; Umar Darr, MD; Zeinab Moussa, MD; Syed Hasan, DO \n **Date:** March 02, 2016\n\n ## Content\n\n Editor's Note:\n\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.\nA 51-year-old black man with history of HIV infection resistant to tenofovir disoproxil/emtricitabine, renal stones, and recurrent urinary tract infection (UTI) presents to the emergency department with right suprapubic pain radiating to the testicles. The pain woke him about 1 hour before presentation. He describes the pain as sudden in onset, continuous and progressive, severe in intensity, and sharp and aching in character. The pain does not radiate anywhere else. He also complains of nausea, vomiting without blood, neck pain, dizziness, swollen lymph nodes, bone pain, fatigue, ischuria, and dysuria without blood.\nThe patient had been hospitalized 15 days ago for sepsis secondary to enterococcal infection, methicillin-resistant Staphylococcus aureus (MRSA) pyelonephritis, and urolithiasis. His medical history is also significant for benign prostatic hypertrophy, herpes zoster, and constipation. The patient was a former smoker, drinks alcohol occasionally, and lives alone. He also states that he has not been compliant with his medications, including those for his HIV infection.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 51-Year-Old Man With HIV and Suprapubic Pain"
},
{
"authors": "Jordan Burlen, BS; Umar Darr, MD; Zeinab Moussa, MD; Syed Hasan, DO",
"content": [
"Vital signs on admission include an oral temperature of 98.6°F, blood pressure of 105/65 mm Hg, pulse of 108 beats/min, respiratory rate of 14 breaths/min, and oxygen saturation of 98% on room air. The patient is alert; aware; and oriented to person, place, and time. He appears to be in acute distress.",
"Figure 1.",
"Figure 1.",
"Figure 2.",
"Figure 2.",
"Figure 3.",
"Figure 3.",
"Physical examination reveals a chronically ill, malnourished man. The cardiopulmonary examination is significant only for tachycardia. The abdomen is soft and flat, with no masses or pulsations. Hepatomegaly is noted. The patient has tenderness upon light and deep palpitation in the left lower quadrant and suprapubic area. The Rovsing sign is not observed. No rebound or rigidity is noted. Multiple tender lymph nodes are palpated in the neck, axilla, and groin.",
"A CT renal survey reveals a bilateral, nonobstructive, 4-mm calculus at the left ureterovesical junction, which was unchanged from prior studies. A complete blood cell count revealed incidental normocytic anemia (7.6 g/dL; normal range, 13.9-16.3 g/dL) and thrombocytopenia (77 × 103 cells/µL; normal range, 100-400 × 103 cells/µL).",
"Further investigation reveals the following:",
"Rouleaux formation and plasmacytoid lymphocytes",
"Serum monoclonal M gammopathy",
"Elevated total protein and paraprotein levels",
"Elevated kappa and lambda free light chains",
"Monoclonal bands in the urine",
"Elevated serum viscosity",
"At discharge from his last admission, the patient was found to have an elevated viral load and had a CD4 count of 210 cells/µL. Bone marrow biopsy reveals a solid nodule of plasmacytoid cells that stained positive for CD138 (Figures 1-3). Flow cytometry found a monoclonal population of B cells that was positive for CD19 and kappa, heterogeneous for CD20, and negative for CD5 and CD10."
],
"date": "March 02, 2016",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/859/634/859634-Thumb1.png"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/859/634/859634-Thumb2.png"
},
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/859/634/859634-Thumb3.png"
}
],
"markdown": "# A 51-Year-Old Man With HIV and Suprapubic Pain\n\n **Authors:** Jordan Burlen, BS; Umar Darr, MD; Zeinab Moussa, MD; Syed Hasan, DO \n **Date:** March 02, 2016\n\n ## Content\n\n Vital signs on admission include an oral temperature of 98.6°F, blood pressure of 105/65 mm Hg, pulse of 108 beats/min, respiratory rate of 14 breaths/min, and oxygen saturation of 98% on room air. The patient is alert; aware; and oriented to person, place, and time. He appears to be in acute distress.\nFigure 1.\nFigure 1.\nFigure 2.\nFigure 2.\nFigure 3.\nFigure 3.\nPhysical examination reveals a chronically ill, malnourished man. The cardiopulmonary examination is significant only for tachycardia. The abdomen is soft and flat, with no masses or pulsations. Hepatomegaly is noted. The patient has tenderness upon light and deep palpitation in the left lower quadrant and suprapubic area. The Rovsing sign is not observed. No rebound or rigidity is noted. Multiple tender lymph nodes are palpated in the neck, axilla, and groin.\nA CT renal survey reveals a bilateral, nonobstructive, 4-mm calculus at the left ureterovesical junction, which was unchanged from prior studies. A complete blood cell count revealed incidental normocytic anemia (7.6 g/dL; normal range, 13.9-16.3 g/dL) and thrombocytopenia (77 × 103 cells/µL; normal range, 100-400 × 103 cells/µL).\nFurther investigation reveals the following:\nRouleaux formation and plasmacytoid lymphocytes\nSerum monoclonal M gammopathy\nElevated total protein and paraprotein levels\nElevated kappa and lambda free light chains\nMonoclonal bands in the urine\nElevated serum viscosity\nAt discharge from his last admission, the patient was found to have an elevated viral load and had a CD4 count of 210 cells/µL. Bone marrow biopsy reveals a solid nodule of plasmacytoid cells that stained positive for CD138 (Figures 1-3). Flow cytometry found a monoclonal population of B cells that was positive for CD19 and kappa, heterogeneous for CD20, and negative for CD5 and CD10.\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n**Figure 3.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 947387,
"choiceText": "Sepsis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 947389,
"choiceText": "Waldenström macroglobulinemia",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 947391,
"choiceText": "Multiple myeloma\r\n\r\n",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 947393,
"choiceText": "Sarcoidosis",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 299713,
"questionText": "On the basis of the history, physical examination, and workup, what is the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 51-Year-Old Man With HIV and Suprapubic Pain"
},
{
"authors": "Jordan Burlen, BS; Umar Darr, MD; Zeinab Moussa, MD; Syed Hasan, DO",
"content": [
"This case demonstrates the unique and unexpected sequelae that result from HIV infection and the difficulties in confirming a diagnosis in a patient with several major concurrent diseases. Waldenström macroglobulinemia is seldom seen in patients with HIV, and many of the examination and laboratory findings can be incorrectly attributed to the HIV process. Making the proper diagnosis is critical to initiating plasmapheresis and the appropriate chemotherapy to prevent the complications of Waldenström macroglobulinemia.",
"Figure 1.",
"Figure 1.",
"Figure 2.",
"Figure 2.",
"Figure 3.",
"Figure 3.",
"Waldenström macroglobulinemia is a rarely encountered lymphoplasmacytic lymphoma (LPL), accounting for less than 2% of all hematologic cancers.[1] The incidence is estimated to be 3-4 cases per million people per year.[2] Diagnosis of Waldenström macroglobulinemia requires a combination of clinical judgment, as well as bone marrow involvement of LPL and immunoglobulin (Ig) M paraproteinemia.[3] The presence of IgM and LPL always causes Waldenström macroglobulinemia. Even though LPL is not always associated with Waldenström macroglobulinemia, and a serum IgM spike is not pathognomonic for Waldenström macroglobulinemia, Waldenström macroglobulinemia is closely tied to an M spike of the kappa light chain.[3]",
"Differentiating Waldenström macroglobulinemia from other LPLs requires bone marrow biopsy, immunophenotyping by flow cytometry, and cytogenetic analysis. Waldenström macroglobulinemia cells have been characterized as expressing pan B-cell markers, such as CD19, CD20, CD22, and surface IgM. At the same time, Waldenström macroglobulinemia cells have been shown to not express CD10, CD23, CD38, and cytoplasmic Ig.[4] Expression of CD5 occurs in 5%-20% of cases.[3]",
"Diagnosing Waldenström macroglobulinemia clinically is especially difficult because it is almost indistinguishable from other IgM monoclonal gammopathies. Many patients have no symptoms and are incidentally diagnosed by routine blood work.[5]",
"Waldenström macroglobulinemia symptoms are a result of the presence of the IgM paraprotein and infiltration of the bone marrow and other tissues by the lymphoplasmacytic cells.[4] The most common symptoms are weakness (66%), anorexia (25%), peripheral neuropathy (24%), and weight loss (17%).[5]",
"Waldenström macroglobulinemia has many known complications, including hyperviscosity syndrome, visual disturbances, diarrhea and malabsorption, renal disease, amyloidosis, bleeding irregularities, Raynaud phenomenon, and increased risk for infection from B-cell dysfunction.[5,6] Common physical exam findings include hepatomegaly, splenomegaly, lymphadenopathy, purpura, bleeding manifestations, papilledema, and neuropathy.[5]",
"The pathophysiology of these findings are explained by the monoclonal cells invading tissue; the hyperviscosity state is produced by the paraprotein inducing antigen/antibody reactions; and malfunctioning of the hemostatic system is caused by the paraprotein.[1] The presentation of Waldenström macroglobulinemia can be easily mistaken for multiple myeloma. The clinical difference is that Waldenström macroglobulinemia is commonly associated with organomegaly and usually does not present with lytic bony disease or renal disease.[7] In this case, CD56 testing was negative, which helped to rule out myeloma (Figures 1-3).",
"The median age at diagnosis of Waldenström macroglobulinemia is > 65 years. Men and white person have a higher incidence than women and African American persons.[2]",
"Common findings in Waldenström macroglobulinemia include normochromic, normocytic anemia and thrombocytopenia. Blood smear may show marked rouleaux formation. Elevations in total protein are also common.[4] Monoclonal gammopathy is defined by the monoclonal spike that is seen on serum protein electrophoresis. Serum immunofixation is then used to determine the IgM heavy chain and the type of associated light chain.[5]"
],
"date": "March 02, 2016",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/859/634/859634-Thumb1.png"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/859/634/859634-Thumb2.png"
},
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/859/634/859634-Thumb3.png"
}
],
"markdown": "# A 51-Year-Old Man With HIV and Suprapubic Pain\n\n **Authors:** Jordan Burlen, BS; Umar Darr, MD; Zeinab Moussa, MD; Syed Hasan, DO \n **Date:** March 02, 2016\n\n ## Content\n\n This case demonstrates the unique and unexpected sequelae that result from HIV infection and the difficulties in confirming a diagnosis in a patient with several major concurrent diseases. Waldenström macroglobulinemia is seldom seen in patients with HIV, and many of the examination and laboratory findings can be incorrectly attributed to the HIV process. Making the proper diagnosis is critical to initiating plasmapheresis and the appropriate chemotherapy to prevent the complications of Waldenström macroglobulinemia.\nFigure 1.\nFigure 1.\nFigure 2.\nFigure 2.\nFigure 3.\nFigure 3.\nWaldenström macroglobulinemia is a rarely encountered lymphoplasmacytic lymphoma (LPL), accounting for less than 2% of all hematologic cancers.[1] The incidence is estimated to be 3-4 cases per million people per year.[2] Diagnosis of Waldenström macroglobulinemia requires a combination of clinical judgment, as well as bone marrow involvement of LPL and immunoglobulin (Ig) M paraproteinemia.[3] The presence of IgM and LPL always causes Waldenström macroglobulinemia. Even though LPL is not always associated with Waldenström macroglobulinemia, and a serum IgM spike is not pathognomonic for Waldenström macroglobulinemia, Waldenström macroglobulinemia is closely tied to an M spike of the kappa light chain.[3]\nDifferentiating Waldenström macroglobulinemia from other LPLs requires bone marrow biopsy, immunophenotyping by flow cytometry, and cytogenetic analysis. Waldenström macroglobulinemia cells have been characterized as expressing pan B-cell markers, such as CD19, CD20, CD22, and surface IgM. At the same time, Waldenström macroglobulinemia cells have been shown to not express CD10, CD23, CD38, and cytoplasmic Ig.[4] Expression of CD5 occurs in 5%-20% of cases.[3]\nDiagnosing Waldenström macroglobulinemia clinically is especially difficult because it is almost indistinguishable from other IgM monoclonal gammopathies. Many patients have no symptoms and are incidentally diagnosed by routine blood work.[5]\nWaldenström macroglobulinemia symptoms are a result of the presence of the IgM paraprotein and infiltration of the bone marrow and other tissues by the lymphoplasmacytic cells.[4] The most common symptoms are weakness (66%), anorexia (25%), peripheral neuropathy (24%), and weight loss (17%).[5]\nWaldenström macroglobulinemia has many known complications, including hyperviscosity syndrome, visual disturbances, diarrhea and malabsorption, renal disease, amyloidosis, bleeding irregularities, Raynaud phenomenon, and increased risk for infection from B-cell dysfunction.[5,6] Common physical exam findings include hepatomegaly, splenomegaly, lymphadenopathy, purpura, bleeding manifestations, papilledema, and neuropathy.[5]\nThe pathophysiology of these findings are explained by the monoclonal cells invading tissue; the hyperviscosity state is produced by the paraprotein inducing antigen/antibody reactions; and malfunctioning of the hemostatic system is caused by the paraprotein.[1] The presentation of Waldenström macroglobulinemia can be easily mistaken for multiple myeloma. The clinical difference is that Waldenström macroglobulinemia is commonly associated with organomegaly and usually does not present with lytic bony disease or renal disease.[7] In this case, CD56 testing was negative, which helped to rule out myeloma (Figures 1-3).\nThe median age at diagnosis of Waldenström macroglobulinemia is > 65 years. Men and white person have a higher incidence than women and African American persons.[2]\nCommon findings in Waldenström macroglobulinemia include normochromic, normocytic anemia and thrombocytopenia. Blood smear may show marked rouleaux formation. Elevations in total protein are also common.[4] Monoclonal gammopathy is defined by the monoclonal spike that is seen on serum protein electrophoresis. Serum immunofixation is then used to determine the IgM heavy chain and the type of associated light chain.[5]\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n**Figure 3.** \n \n\n\n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 947387,
"choiceText": "Sepsis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 947389,
"choiceText": "Waldenström macroglobulinemia",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 947391,
"choiceText": "Multiple myeloma\r\n\r\n",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 947393,
"choiceText": "Sarcoidosis",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 299713,
"questionText": "On the basis of the history, physical examination, and workup, what is the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 51-Year-Old Man With HIV and Suprapubic Pain"
},
{
"authors": "Jordan Burlen, BS; Umar Darr, MD; Zeinab Moussa, MD; Syed Hasan, DO",
"content": [
"HIV disease is caused by infection with HIV-1 or HIV-2. The virus is blood-borne and is most often transmitted by sexual intercourse, sharing of intravenous drug paraphernalia, and transmission from mother to child during and after pregnancy.[8] AIDS is defined as a CD4 count below 200 cells/μL in the United States.[9] The incidence of HIV infection in the United States is estimated to be 17.4 per 100,000 population.[10] The incidence in developed countries is higher among men and African American persons.",
"HIV infection is characterized by a depletion of CD4+ helper cells.[8] The loss of this component of the immune system leaves the individual susceptible to opportunistic infections and neoplastic processes.[11] Signs and symptoms of HIV are usually nonspecific and include flu-like illness, fatigue, diarrhea, weight loss, generalized lymphadenopathy, and recurrent infections.[11] HIV infection is diagnosed on the basis of screening with high-sensitivity enzyme-linked immunoabsorbent assay, followed by Western blot assaying for confirmation.[9]",
"The list of opportunistic infections associated with HIV infection is extensive and includes candidiasis, coccidioidomycosis, cytomegalovirus infection, \nMycobacterium avium complex infection, Mycobacterium tuberculosis infection, toxoplasmosis, and recurrent pneumonia. HIV infection also leaves the patient vulnerable to the development of neoplasms, such as Kaposi sarcoma, lymphoma, and cervical and anal cancers.[8,11]",
"The definitive causes of Waldenström macroglobulinemia are not clearly understood. New studies have begun to link genetic and immune-related factors in the development of Waldenström macroglobulinemia.[12] Patients with specific infectious diseases appear to be at increased risk for Waldenström macroglobulinemia. This information suggests that chronic immune stimulation may have an important part in the etiology and pathogenesis.[13]",
"A few studies have provided evidence of somatic immunoglobulin gene mutations.[12] This may suggest that Waldenström macroglobulinemia cells originate from B cells that have repeatedly undergone antigenic stimulation and late-stage selection in the germinal centers of lymphoid follicles.[12]",
"Although neoplastic processes are not uncommon in patients with HIV, Waldenström macroglobulinemia is rare. Including the patient in this case, only three cases have been reported in the medical literature.[14,15] A retrospective study looked at the relationship of chronic immune stimulation on the development of Waldenström macroglobulinemia. The authors observed that three persons out of 14,736 HIV-exposed individuals developed Waldenström macroglobulinemia (relative risk, 12.05; 95% confidence interval, 2.83-51.46).[13]"
],
"date": "March 02, 2016",
"figures": [],
"markdown": "# A 51-Year-Old Man With HIV and Suprapubic Pain\n\n **Authors:** Jordan Burlen, BS; Umar Darr, MD; Zeinab Moussa, MD; Syed Hasan, DO \n **Date:** March 02, 2016\n\n ## Content\n\n HIV disease is caused by infection with HIV-1 or HIV-2. The virus is blood-borne and is most often transmitted by sexual intercourse, sharing of intravenous drug paraphernalia, and transmission from mother to child during and after pregnancy.[8] AIDS is defined as a CD4 count below 200 cells/μL in the United States.[9] The incidence of HIV infection in the United States is estimated to be 17.4 per 100,000 population.[10] The incidence in developed countries is higher among men and African American persons.\nHIV infection is characterized by a depletion of CD4+ helper cells.[8] The loss of this component of the immune system leaves the individual susceptible to opportunistic infections and neoplastic processes.[11] Signs and symptoms of HIV are usually nonspecific and include flu-like illness, fatigue, diarrhea, weight loss, generalized lymphadenopathy, and recurrent infections.[11] HIV infection is diagnosed on the basis of screening with high-sensitivity enzyme-linked immunoabsorbent assay, followed by Western blot assaying for confirmation.[9]\nThe list of opportunistic infections associated with HIV infection is extensive and includes candidiasis, coccidioidomycosis, cytomegalovirus infection, \nMycobacterium avium complex infection, Mycobacterium tuberculosis infection, toxoplasmosis, and recurrent pneumonia. HIV infection also leaves the patient vulnerable to the development of neoplasms, such as Kaposi sarcoma, lymphoma, and cervical and anal cancers.[8,11]\nThe definitive causes of Waldenström macroglobulinemia are not clearly understood. New studies have begun to link genetic and immune-related factors in the development of Waldenström macroglobulinemia.[12] Patients with specific infectious diseases appear to be at increased risk for Waldenström macroglobulinemia. This information suggests that chronic immune stimulation may have an important part in the etiology and pathogenesis.[13]\nA few studies have provided evidence of somatic immunoglobulin gene mutations.[12] This may suggest that Waldenström macroglobulinemia cells originate from B cells that have repeatedly undergone antigenic stimulation and late-stage selection in the germinal centers of lymphoid follicles.[12]\nAlthough neoplastic processes are not uncommon in patients with HIV, Waldenström macroglobulinemia is rare. Including the patient in this case, only three cases have been reported in the medical literature.[14,15] A retrospective study looked at the relationship of chronic immune stimulation on the development of Waldenström macroglobulinemia. The authors observed that three persons out of 14,736 HIV-exposed individuals developed Waldenström macroglobulinemia (relative risk, 12.05; 95% confidence interval, 2.83-51.46).[13]\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 51-Year-Old Man With HIV and Suprapubic Pain"
},
{
"authors": "Jordan Burlen, BS; Umar Darr, MD; Zeinab Moussa, MD; Syed Hasan, DO",
"content": [
"The median survival for Waldenström macroglobulinemia ranges from 5 to 10 years.[3] In certain patient populations, the disease can be more aggressive.[3] The five adverse covariates that have been identified by the International Prognostic Scoring System for determining prognosis in Waldenström macroglobulinemia are as follows:",
"Age > 65 years",
"Hemoglobin level ≤ 11.5 g/dL",
"Platelet count ≤ 100,000 cells/µL",
"Beta-2 microglobulin level > 3 mg/L",
"Serum monoclonal protein concentration > 7 g/dL",
"Each of these variables confers 1 point.[1] Risk groups are stratified as low (1 point), intermediate (2 points), and high (> 2 points); the 5-year survival rates for these groups are 87%, 68%, and 36%, respectively.[3]",
"The current indications for immediate therapy for Waldenström macroglobulinemia are disease-related cytopenia, palpable adenopathy or organomegaly, hyperviscosity causing symptoms, severe neuropathy, amyloidosis, cryoglobulinemia, cold agglutinin disease, or evidence of disease transformation.[16]",
"No consensus treatment regimen for symptomatic Waldenström macroglobulinemia has been identified. Current recommendations include a combination of rituximab with nucleoside analogues and/or alkylating agents or with cyclophosphamide-based therapies; a combination of rituximab and thalidomide has also been recommended.[16]",
"According to the scoring system, the patient in this case was in the high-risk group, with a hemoglobin level of 7.6 g/dL, platelet count of 77,000 cells/µL, and serum monoclonal protein concentration of 10 g/dL.",
"Plasmapheresis was followed by treatment with rituximab, rasburicase, and dexamethasone. The patient's clinical picture and symptoms improved dramatically, and he was discharged with follow-up in the outpatient setting with oncology and infectious disease specialists.",
"This is an exceedingly rare case of Waldenström macroglobulinemia in a patient with HIV disease and presents an opportunity for discussion of the pathogenesis of Waldenström macroglobulinemia, as well as the unique diseases that can arise in noncompliant HIV patients. This case is also an example of the difficulty with further testing and pursuit of diagnosis in noncompliant patients while they are in hospital vs following up in clinic. The pathway determining clonal proliferation and survival must be investigated in order to enhance our understanding of Waldenström macroglobulinemia pathophysiology, elicit knowledge on etiology, and result in more specific and effective treatments for patients."
],
"date": "March 02, 2016",
"figures": [],
"markdown": "# A 51-Year-Old Man With HIV and Suprapubic Pain\n\n **Authors:** Jordan Burlen, BS; Umar Darr, MD; Zeinab Moussa, MD; Syed Hasan, DO \n **Date:** March 02, 2016\n\n ## Content\n\n The median survival for Waldenström macroglobulinemia ranges from 5 to 10 years.[3] In certain patient populations, the disease can be more aggressive.[3] The five adverse covariates that have been identified by the International Prognostic Scoring System for determining prognosis in Waldenström macroglobulinemia are as follows:\nAge > 65 years\nHemoglobin level ≤ 11.5 g/dL\nPlatelet count ≤ 100,000 cells/µL\nBeta-2 microglobulin level > 3 mg/L\nSerum monoclonal protein concentration > 7 g/dL\nEach of these variables confers 1 point.[1] Risk groups are stratified as low (1 point), intermediate (2 points), and high (> 2 points); the 5-year survival rates for these groups are 87%, 68%, and 36%, respectively.[3]\nThe current indications for immediate therapy for Waldenström macroglobulinemia are disease-related cytopenia, palpable adenopathy or organomegaly, hyperviscosity causing symptoms, severe neuropathy, amyloidosis, cryoglobulinemia, cold agglutinin disease, or evidence of disease transformation.[16]\nNo consensus treatment regimen for symptomatic Waldenström macroglobulinemia has been identified. Current recommendations include a combination of rituximab with nucleoside analogues and/or alkylating agents or with cyclophosphamide-based therapies; a combination of rituximab and thalidomide has also been recommended.[16]\nAccording to the scoring system, the patient in this case was in the high-risk group, with a hemoglobin level of 7.6 g/dL, platelet count of 77,000 cells/µL, and serum monoclonal protein concentration of 10 g/dL.\nPlasmapheresis was followed by treatment with rituximab, rasburicase, and dexamethasone. The patient's clinical picture and symptoms improved dramatically, and he was discharged with follow-up in the outpatient setting with oncology and infectious disease specialists.\nThis is an exceedingly rare case of Waldenström macroglobulinemia in a patient with HIV disease and presents an opportunity for discussion of the pathogenesis of Waldenström macroglobulinemia, as well as the unique diseases that can arise in noncompliant HIV patients. This case is also an example of the difficulty with further testing and pursuit of diagnosis in noncompliant patients while they are in hospital vs following up in clinic. The pathway determining clonal proliferation and survival must be investigated in order to enhance our understanding of Waldenström macroglobulinemia pathophysiology, elicit knowledge on etiology, and result in more specific and effective treatments for patients.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 947395,
"choiceText": "Kaposi sarcoma",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 947397,
"choiceText": "Tuberculosis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 947399,
"choiceText": "Non-Hodgkin lymphoma",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 947401,
"choiceText": "Multiple myeloma",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Non-Hodgkin lymphoma is one of the most commonly seen cancers in patients with HIV infection. Risk factors include male sex and noncompliance with medications. To make a definitive diagnosis, blood tests, lymph node and bone marrow biopsy, and chest imaging are required.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 299715,
"questionText": "A man with known HIV infection presents to his primary care physician with fatigue and swelling in the neck and armpits. He has also been waking up with cold sweats in the night and had weight loss of 15 lb in the past month. He has not been compliant with medications for his HIV infection. All other review of systems is negative. Which of the following is the most likely diagnosis? ",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 947403,
"choiceText": "Lytic bone disease",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 947405,
"choiceText": "Anemia",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 947407,
"choiceText": "Renal disease\r\n",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 947409,
"choiceText": "Organomegaly",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The pathophysiology of these findings is explained by the monoclonal cells invading tissue, the hyperviscosity state produced by the paraprotein inducing antigen/antibody reactions, and malfunctioning of the hemostatic system caused by the paraprotein. Waldenström macroglobulinemia is most associated with organomegaly, increased bleeding, cardiac failure, diarrhea, and hyperviscosity syndrome. Renal disease and lytic bone lesions are more common in multiple myeloma. \r\n",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 299717,
"questionText": "A patient returns to the outpatient clinic for follow-up of pancytopenia noted on a recent complete blood count. He had a protein electrophoresis that revealed a monoclonal paraprotein band and Bence Jones proteins on urinalysis. Which of the following clinical findings is associated more with Waldenström macroglobulinemia than multiple myeloma?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 51-Year-Old Man With HIV and Suprapubic Pain"
},
{
"authors": "Jordan Burlen, BS; Umar Darr, MD; Zeinab Moussa, MD; Syed Hasan, DO",
"content": [],
"date": "March 02, 2016",
"figures": [],
"markdown": "# A 51-Year-Old Man With HIV and Suprapubic Pain\n\n **Authors:** Jordan Burlen, BS; Umar Darr, MD; Zeinab Moussa, MD; Syed Hasan, DO \n **Date:** March 02, 2016\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 947395,
"choiceText": "Kaposi sarcoma",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 947397,
"choiceText": "Tuberculosis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 947399,
"choiceText": "Non-Hodgkin lymphoma",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 947401,
"choiceText": "Multiple myeloma",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Non-Hodgkin lymphoma is one of the most commonly seen cancers in patients with HIV infection. Risk factors include male sex and noncompliance with medications. To make a definitive diagnosis, blood tests, lymph node and bone marrow biopsy, and chest imaging are required.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 299715,
"questionText": "A man with known HIV infection presents to his primary care physician with fatigue and swelling in the neck and armpits. He has also been waking up with cold sweats in the night and had weight loss of 15 lb in the past month. He has not been compliant with medications for his HIV infection. All other review of systems is negative. Which of the following is the most likely diagnosis? ",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 947403,
"choiceText": "Lytic bone disease",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 947405,
"choiceText": "Anemia",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 947407,
"choiceText": "Renal disease\r\n",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 947409,
"choiceText": "Organomegaly",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The pathophysiology of these findings is explained by the monoclonal cells invading tissue, the hyperviscosity state produced by the paraprotein inducing antigen/antibody reactions, and malfunctioning of the hemostatic system caused by the paraprotein. Waldenström macroglobulinemia is most associated with organomegaly, increased bleeding, cardiac failure, diarrhea, and hyperviscosity syndrome. Renal disease and lytic bone lesions are more common in multiple myeloma. \r\n",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 299717,
"questionText": "A patient returns to the outpatient clinic for follow-up of pancytopenia noted on a recent complete blood count. He had a protein electrophoresis that revealed a monoclonal paraprotein band and Bence Jones proteins on urinalysis. Which of the following clinical findings is associated more with Waldenström macroglobulinemia than multiple myeloma?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 51-Year-Old Man With HIV and Suprapubic Pain"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 947387,
"choiceText": "Sepsis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 947389,
"choiceText": "Waldenström macroglobulinemia",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 947391,
"choiceText": "Multiple myeloma\r\n\r\n",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 947393,
"choiceText": "Sarcoidosis",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 299713,
"questionText": "On the basis of the history, physical examination, and workup, what is the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 947395,
"choiceText": "Kaposi sarcoma",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 947397,
"choiceText": "Tuberculosis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 947399,
"choiceText": "Non-Hodgkin lymphoma",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 947401,
"choiceText": "Multiple myeloma",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Non-Hodgkin lymphoma is one of the most commonly seen cancers in patients with HIV infection. Risk factors include male sex and noncompliance with medications. To make a definitive diagnosis, blood tests, lymph node and bone marrow biopsy, and chest imaging are required.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 299715,
"questionText": "A man with known HIV infection presents to his primary care physician with fatigue and swelling in the neck and armpits. He has also been waking up with cold sweats in the night and had weight loss of 15 lb in the past month. He has not been compliant with medications for his HIV infection. All other review of systems is negative. Which of the following is the most likely diagnosis? ",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 947403,
"choiceText": "Lytic bone disease",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 947405,
"choiceText": "Anemia",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 947407,
"choiceText": "Renal disease\r\n",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 947409,
"choiceText": "Organomegaly",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The pathophysiology of these findings is explained by the monoclonal cells invading tissue, the hyperviscosity state produced by the paraprotein inducing antigen/antibody reactions, and malfunctioning of the hemostatic system caused by the paraprotein. Waldenström macroglobulinemia is most associated with organomegaly, increased bleeding, cardiac failure, diarrhea, and hyperviscosity syndrome. Renal disease and lytic bone lesions are more common in multiple myeloma. \r\n",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 299717,
"questionText": "A patient returns to the outpatient clinic for follow-up of pancytopenia noted on a recent complete blood count. He had a protein electrophoresis that revealed a monoclonal paraprotein band and Bence Jones proteins on urinalysis. Which of the following clinical findings is associated more with Waldenström macroglobulinemia than multiple myeloma?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
857710 | /viewarticle/857710 | [
{
"authors": "Caroline Tschibelu, MD",
"content": [
"Editor's Note:\n\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.",
"A 65-year-old woman presents to the emergency department (ED) with several days of increasing confusion and decreased coordination. The symptoms started insidiously over the past 2 months, as the patient experienced some confusion and became increasingly forgetful. Over the past week, she has become more off-balance and has had difficulty with purposeful movements.",
"The patient's husband pressed her to come to the ED because she walked into a wall in her house that afternoon and again in the evening. She is a homemaker and has lived in that house for 15 years with her husband. She usually walks without assistance and does not report additional neurologic symptoms. She reports recent weight loss.",
"The patient reports having had a chronic dry cough for months, for which she recently started taking an over-the-counter cough syrup. She denies hemoptysis, night sweats, or fever. Otherwise, she has no known medical history, because she has not seen a doctor regularly in 23 years. She smoked one pack of cigarettes per day for 30 years but stopped when she started coughing. She does not use drugs or drink alcohol and has no allergies or family history of significant illness."
],
"date": "January 27, 2016",
"figures": [],
"markdown": "# A 65-Year-Old Woman With Decreased Coordination\n\n **Authors:** Caroline Tschibelu, MD \n **Date:** January 27, 2016\n\n ## Content\n\n Editor's Note:\n\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.\nA 65-year-old woman presents to the emergency department (ED) with several days of increasing confusion and decreased coordination. The symptoms started insidiously over the past 2 months, as the patient experienced some confusion and became increasingly forgetful. Over the past week, she has become more off-balance and has had difficulty with purposeful movements.\nThe patient's husband pressed her to come to the ED because she walked into a wall in her house that afternoon and again in the evening. She is a homemaker and has lived in that house for 15 years with her husband. She usually walks without assistance and does not report additional neurologic symptoms. She reports recent weight loss.\nThe patient reports having had a chronic dry cough for months, for which she recently started taking an over-the-counter cough syrup. She denies hemoptysis, night sweats, or fever. Otherwise, she has no known medical history, because she has not seen a doctor regularly in 23 years. She smoked one pack of cigarettes per day for 30 years but stopped when she started coughing. She does not use drugs or drink alcohol and has no allergies or family history of significant illness.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 65-Year-Old Woman With Decreased Coordination"
},
{
"authors": "Caroline Tschibelu, MD",
"content": [
"Figure 1",
"Figure 2",
"On physical examination, the patient has a blood pressure of 150/85 mm Hg, pulse of 126 beats/min, respiratory rate of 18 breaths/min, pulse oximetry of 94% on room air, and a temperature of 99.2°F.",
"The patient is a thin woman in no acute distress. The lung examination reveals decreased breath sounds in the right lower lobe. Her heart has a regular rhythm, and a systolic ejection murmur is appreciated.",
"Upon neurologic evaluation, the patient is alert and oriented. She exhibits visual agnosia and is unable to identify a pen or a clock. However, she is unaware of her deficits. She initially denies any problems with her vision or balance when her husband gives details of her recent history. As deficits are found during the examination, she starts wondering what is wrong with her.",
"The patient cannot count fingers and has a right temporal visual-field cut. Extraocular muscles are intact. Her pupils are 2 mm and reactive to light. Fundi are poorly visualized on undilated examination.",
"Sensory examination of the face is unremarkable. Left facial droop is noted, and her tongue and uvula are midline with a positive gag reflex. Hearing is symmetric. Shoulder shrug lags on the left side. Strength is 5/5 in the lower extremities and 4/5 in the upper extremities. Sensory examination is symmetric to light touch, pinprick, temperature, vibration, and proprioception. Reflexes are 2+, except in the left upper extremity, which is 1+. The patient is unable to perform rapid alternating movements. The remainder of her physical examination is unremarkable.",
"Plain-film radiography of the chest reveals a 5.5-cm mass in the right lower lobe associated with a small pleural effusion (Figure 1), findings later confirmed by chest CT. ECG shows normal sinus at a rate of 120 beats/min, without ST-T wave changes. Head CT reveals a left occipital lobe infarct and a smaller right occipital lobe infarct; the left parietal and left cerebellum infarcts appear to be subacute.",
"Laboratory analyses performed in the ED include a complete blood cell count, metabolic panel, hepatic panel with lipase, and troponin. Laboratory test findings are remarkable for a sodium level of 133 mEq/L (normal range, 135-147 mEq/L), white blood cell count of 19.2 × 103 cells/µL (normal range, 4.2-11.0 × 103 cells/µL) with 89% segmented neutrophils (54-62%) and bandemia of 2% (3-5%), and a hemoglobin level of 8.6 g/dL (normal range, 12-15 g/dL for women). Prothrombin time is 14.1 sec (normal range, 11-14 sec), and partial thromboplastin time is 41.5 sec (normal range, 20-40 sec). Cardiac enzymes are significant for a troponin level of 3.94 ng/mL (normal range, 0-0.4 ng/mL). The remainder of laboratory test findings, including a lipid panel, were within normal limits.",
"The patient was diagnosed with a non–ST-segment elevation myocardial infarction that was treated with aspirin, beta-blocker therapy, and anticoagulation with heparin before admission to the medical service. Given her constellation of findings, transthoracic echocardiography was performed (Figure 2). Additional laboratory studies ordered by the admitting team are notable for a lactate dehydrogenase (LDH) level of 777 U/L (normal range, 50-150 U/L). Blood cultures were negative."
],
"date": "January 27, 2016",
"figures": [
{
"caption": "Figure 1",
"image_url": "https://img.medscapestatic.com/article/857/710/857710-Thumb1.png"
},
{
"caption": "Figure 2",
"image_url": "https://img.medscapestatic.com/article/857/710/857710-Thumb2.png"
}
],
"markdown": "# A 65-Year-Old Woman With Decreased Coordination\n\n **Authors:** Caroline Tschibelu, MD \n **Date:** January 27, 2016\n\n ## Content\n\n Figure 1\nFigure 2\nOn physical examination, the patient has a blood pressure of 150/85 mm Hg, pulse of 126 beats/min, respiratory rate of 18 breaths/min, pulse oximetry of 94% on room air, and a temperature of 99.2°F.\nThe patient is a thin woman in no acute distress. The lung examination reveals decreased breath sounds in the right lower lobe. Her heart has a regular rhythm, and a systolic ejection murmur is appreciated.\nUpon neurologic evaluation, the patient is alert and oriented. She exhibits visual agnosia and is unable to identify a pen or a clock. However, she is unaware of her deficits. She initially denies any problems with her vision or balance when her husband gives details of her recent history. As deficits are found during the examination, she starts wondering what is wrong with her.\nThe patient cannot count fingers and has a right temporal visual-field cut. Extraocular muscles are intact. Her pupils are 2 mm and reactive to light. Fundi are poorly visualized on undilated examination.\nSensory examination of the face is unremarkable. Left facial droop is noted, and her tongue and uvula are midline with a positive gag reflex. Hearing is symmetric. Shoulder shrug lags on the left side. Strength is 5/5 in the lower extremities and 4/5 in the upper extremities. Sensory examination is symmetric to light touch, pinprick, temperature, vibration, and proprioception. Reflexes are 2+, except in the left upper extremity, which is 1+. The patient is unable to perform rapid alternating movements. The remainder of her physical examination is unremarkable.\nPlain-film radiography of the chest reveals a 5.5-cm mass in the right lower lobe associated with a small pleural effusion (Figure 1), findings later confirmed by chest CT. ECG shows normal sinus at a rate of 120 beats/min, without ST-T wave changes. Head CT reveals a left occipital lobe infarct and a smaller right occipital lobe infarct; the left parietal and left cerebellum infarcts appear to be subacute.\nLaboratory analyses performed in the ED include a complete blood cell count, metabolic panel, hepatic panel with lipase, and troponin. Laboratory test findings are remarkable for a sodium level of 133 mEq/L (normal range, 135-147 mEq/L), white blood cell count of 19.2 × 103 cells/µL (normal range, 4.2-11.0 × 103 cells/µL) with 89% segmented neutrophils (54-62%) and bandemia of 2% (3-5%), and a hemoglobin level of 8.6 g/dL (normal range, 12-15 g/dL for women). Prothrombin time is 14.1 sec (normal range, 11-14 sec), and partial thromboplastin time is 41.5 sec (normal range, 20-40 sec). Cardiac enzymes are significant for a troponin level of 3.94 ng/mL (normal range, 0-0.4 ng/mL). The remainder of laboratory test findings, including a lipid panel, were within normal limits.\nThe patient was diagnosed with a non–ST-segment elevation myocardial infarction that was treated with aspirin, beta-blocker therapy, and anticoagulation with heparin before admission to the medical service. Given her constellation of findings, transthoracic echocardiography was performed (Figure 2). Additional laboratory studies ordered by the admitting team are notable for a lactate dehydrogenase (LDH) level of 777 U/L (normal range, 50-150 U/L). Blood cultures were negative.\n\n ## Figures\n\n **Figure 1** \n \n\n**Figure 2** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 938383,
"choiceText": "Multi-infarct dementia",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 938385,
"choiceText": "Nonbacterial thrombotic endocarditis",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 938387,
"choiceText": "Tuberculosis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 938389,
"choiceText": "Infective endocarditis",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 938391,
"choiceText": "Atrial myxoma",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 296891,
"questionText": "On the basis of the history, physical examination, and workup, what is the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 65-Year-Old Woman With Decreased Coordination"
},
{
"authors": "Caroline Tschibelu, MD",
"content": [
"Transthoracic echocardiography reveals valvular masses on the mitral valve characteristic of nonbacterial thrombotic endocarditis, especially in the setting of negative blood cultures and without signs of systemic infection.",
"Nonbacterial thrombotic endocarditis (NBTE), also known as marantic, verrucous, or Libman-Sacks endocarditis, is a rare condition characterized by the formation of friable and noninfectious vegetations on the heart valves of affected patients. These vegetations most often affect the mitral and aortic valves, more specifically along the line of closure of the leaflets or cusps,[1] and have a high tendency to embolize, causing extensive systemic infarctions more often than infective endocarditis (IE).[2] The vegetations in NBTE are more easily dislodged than those in IE because of the minimal inflammation involved in their pathophysiology. NBTE is commonly associated with advanced cancer (80% of cases have an underlying cancer), prothrombic states, sepsis, burns, and systemic lupus erythematosus (SLE).",
"The exact pathogenesis of NBTE is unknown, but initial endothelial injury combined with impaired antithrombotic mechanisms, such as those found in cancer, antiphospholipid syndromes, and hypercoagulable states, are thought to be critical to the disease process.[3] The current hypothesis states that interaction among malignant cells, monocytes, and macrophages causes endothelial damage in the absence of inflammation or infection. The deposition of fibrin and circulating platelets onto normal or superficially damaged heart valves leads to the formation of vegetations. NBTE occurs in the absence of bacterial infection and multiple blood cultures are commonly negative, distinguishing it from IE.",
"In this patient's case, NBTE was secondary to underlying cancer, because she presented with metastatic non-small cell lung cancer (NSCLC). She had not received medical care in over 20 years. A peripheral lung mass on chest radiography in the setting of chronic cough, weight loss, and increased LDH level should raise suspicion for lung cancer. NSCLC is often insidious, and patients can become symptomatic when the disease is very advanced.[4]",
"The hypercoagulable state of cancer is secondary to the ability of the tumor itself to activate the coagulation cascade as well the interaction between cancer cells and host cells described earlier.[5] Cases have been reported in which NBTE was the presenting manifestation of stage IV gastric cancer,[6]\novarian cancer, lung adenocarcinoma, and pancreatic cancer.",
"In addition, the patient had experienced a non-ST segment elevation myocardial infarction—another possible manifestation of NBTE.",
"Besides IE, the following are differential diagnoses that were considered but excluded in this case. Tuberculosis has a presentation similar to that of lung cancer; however, upper-lobe involvement is most common, LDH is typically not elevated, and risk factors are usually present (eg, history of travel, immigrant status, crowded living conditions).",
"Atrial myxomas are the most common primary cardiac tumors, accounting for 40%-50%. Myxomas often affect the left atrium and are more likely to become symptomatic if they are left-sided and >5 cm in diameter. These factors, combined with the friable and intravascular nature of myxomas, lead to mechanical interference and potential arterial embolization.[7]",
"Multiple-infarct dementia is a syndrome of vascular dementia, the second most common form of dementia after Alzheimer disease. It has a similar presentation, with cognitive or memory impairment, but is secondary to vascular disease, leading to cortical or subcortical ischemic changes; 50% of patients have a long-standing history of hypertension.[8]"
],
"date": "January 27, 2016",
"figures": [
{
"caption": "",
"image_url": "https://img.medscapestatic.com/article/857/710/857710-Thumb3.jpg"
}
],
"markdown": "# A 65-Year-Old Woman With Decreased Coordination\n\n **Authors:** Caroline Tschibelu, MD \n **Date:** January 27, 2016\n\n ## Content\n\n Transthoracic echocardiography reveals valvular masses on the mitral valve characteristic of nonbacterial thrombotic endocarditis, especially in the setting of negative blood cultures and without signs of systemic infection.\nNonbacterial thrombotic endocarditis (NBTE), also known as marantic, verrucous, or Libman-Sacks endocarditis, is a rare condition characterized by the formation of friable and noninfectious vegetations on the heart valves of affected patients. These vegetations most often affect the mitral and aortic valves, more specifically along the line of closure of the leaflets or cusps,[1] and have a high tendency to embolize, causing extensive systemic infarctions more often than infective endocarditis (IE).[2] The vegetations in NBTE are more easily dislodged than those in IE because of the minimal inflammation involved in their pathophysiology. NBTE is commonly associated with advanced cancer (80% of cases have an underlying cancer), prothrombic states, sepsis, burns, and systemic lupus erythematosus (SLE).\nThe exact pathogenesis of NBTE is unknown, but initial endothelial injury combined with impaired antithrombotic mechanisms, such as those found in cancer, antiphospholipid syndromes, and hypercoagulable states, are thought to be critical to the disease process.[3] The current hypothesis states that interaction among malignant cells, monocytes, and macrophages causes endothelial damage in the absence of inflammation or infection. The deposition of fibrin and circulating platelets onto normal or superficially damaged heart valves leads to the formation of vegetations. NBTE occurs in the absence of bacterial infection and multiple blood cultures are commonly negative, distinguishing it from IE.\nIn this patient's case, NBTE was secondary to underlying cancer, because she presented with metastatic non-small cell lung cancer (NSCLC). She had not received medical care in over 20 years. A peripheral lung mass on chest radiography in the setting of chronic cough, weight loss, and increased LDH level should raise suspicion for lung cancer. NSCLC is often insidious, and patients can become symptomatic when the disease is very advanced.[4]\nThe hypercoagulable state of cancer is secondary to the ability of the tumor itself to activate the coagulation cascade as well the interaction between cancer cells and host cells described earlier.[5] Cases have been reported in which NBTE was the presenting manifestation of stage IV gastric cancer,[6]\novarian cancer, lung adenocarcinoma, and pancreatic cancer.\nIn addition, the patient had experienced a non-ST segment elevation myocardial infarction—another possible manifestation of NBTE.\nBesides IE, the following are differential diagnoses that were considered but excluded in this case. Tuberculosis has a presentation similar to that of lung cancer; however, upper-lobe involvement is most common, LDH is typically not elevated, and risk factors are usually present (eg, history of travel, immigrant status, crowded living conditions).\nAtrial myxomas are the most common primary cardiac tumors, accounting for 40%-50%. Myxomas often affect the left atrium and are more likely to become symptomatic if they are left-sided and >5 cm in diameter. These factors, combined with the friable and intravascular nature of myxomas, lead to mechanical interference and potential arterial embolization.[7]\nMultiple-infarct dementia is a syndrome of vascular dementia, the second most common form of dementia after Alzheimer disease. It has a similar presentation, with cognitive or memory impairment, but is secondary to vascular disease, leading to cortical or subcortical ischemic changes; 50% of patients have a long-standing history of hypertension.[8]\n\n ## Figures\n\n **** \n \n\n\n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 938383,
"choiceText": "Multi-infarct dementia",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 938385,
"choiceText": "Nonbacterial thrombotic endocarditis",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 938387,
"choiceText": "Tuberculosis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 938389,
"choiceText": "Infective endocarditis",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 938391,
"choiceText": "Atrial myxoma",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 296891,
"questionText": "On the basis of the history, physical examination, and workup, what is the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 65-Year-Old Woman With Decreased Coordination"
},
{
"authors": "Caroline Tschibelu, MD",
"content": [
"The exact incidence of NBTE is unknown; it is approximately 1.6%, on the basis of the largest autopsy series available. No pathognomonic signs or symptoms are associated with NBTE; patients are usually asymptomatic until embolization. Therefore, a high degree of suspicion is required, especially in patients who present with embolic strokes and known cancer. The most common sites of embolization include the kidneys, spleen, skin, and extremities. Those patients may have signs of hematuria, rash, and digital ischemia; however, embolic events that affect the coronary arteries or lead to cerebrovascular accidents most often lead the clinician to the diagnosis.",
"Patients with NBTE initially present with multiple systemic or venous embolic events in 30%–50% of cases[9]; stroke is the most common manifestation, occurring in 33% of cases, and myocardial infarction occurs in 7.5% of cases.[10]",
"Physical examination findings can be normal, but symptomatic cases can present with chest pain or the following cardiac murmurs: ejection systolic murmur (crescendo decrescendo), holosystolic murmur due to mitral or tricuspid regurgitation, early diastolic murmur of aortic regurgitation, and mid-diastolic murmur of mitral stenosis.",
"The workup should first aim at distinguishing NBTE from IE, with blood cultures performed before starting antibiotic treatment. A thorough workup should be performed to establish a hypercoagulable state, disseminated intravascular coagulation, SLE, or cancer.",
"Patients with advanced cancer and presenting with a stroke should be evaluated with brain MRI or brain CT. MRI is more sensitive and specific for embolic strokes; therefore, it should be performed first if the suspicion is high. NBTE often presents with small and large ischemic strokes in a wide distribution on brain imaging. However, hemorrhagic transformation can occur de novo or after anticoagulation therapy.",
"Patients with new murmurs or signs of coronary embolism (cardiac enzymes are elevated in 81% of cases), should undergo a TEE. After embolic events, vegetations may be too small to be detected by transthoracic echocardiography; therefore, TEE is the best diagnostic modality despite being an invasive study."
],
"date": "January 27, 2016",
"figures": [],
"markdown": "# A 65-Year-Old Woman With Decreased Coordination\n\n **Authors:** Caroline Tschibelu, MD \n **Date:** January 27, 2016\n\n ## Content\n\n The exact incidence of NBTE is unknown; it is approximately 1.6%, on the basis of the largest autopsy series available. No pathognomonic signs or symptoms are associated with NBTE; patients are usually asymptomatic until embolization. Therefore, a high degree of suspicion is required, especially in patients who present with embolic strokes and known cancer. The most common sites of embolization include the kidneys, spleen, skin, and extremities. Those patients may have signs of hematuria, rash, and digital ischemia; however, embolic events that affect the coronary arteries or lead to cerebrovascular accidents most often lead the clinician to the diagnosis.\nPatients with NBTE initially present with multiple systemic or venous embolic events in 30%–50% of cases[9]; stroke is the most common manifestation, occurring in 33% of cases, and myocardial infarction occurs in 7.5% of cases.[10]\nPhysical examination findings can be normal, but symptomatic cases can present with chest pain or the following cardiac murmurs: ejection systolic murmur (crescendo decrescendo), holosystolic murmur due to mitral or tricuspid regurgitation, early diastolic murmur of aortic regurgitation, and mid-diastolic murmur of mitral stenosis.\nThe workup should first aim at distinguishing NBTE from IE, with blood cultures performed before starting antibiotic treatment. A thorough workup should be performed to establish a hypercoagulable state, disseminated intravascular coagulation, SLE, or cancer.\nPatients with advanced cancer and presenting with a stroke should be evaluated with brain MRI or brain CT. MRI is more sensitive and specific for embolic strokes; therefore, it should be performed first if the suspicion is high. NBTE often presents with small and large ischemic strokes in a wide distribution on brain imaging. However, hemorrhagic transformation can occur de novo or after anticoagulation therapy.\nPatients with new murmurs or signs of coronary embolism (cardiac enzymes are elevated in 81% of cases), should undergo a TEE. After embolic events, vegetations may be too small to be detected by transthoracic echocardiography; therefore, TEE is the best diagnostic modality despite being an invasive study.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 65-Year-Old Woman With Decreased Coordination"
},
{
"authors": "Caroline Tschibelu, MD",
"content": [
"The management of NBTE should be directed toward the underlying cancer or associated condition, and systemic anticoagulation should be given to prevent recurrent embolization. Unfractionated heparin is more effective than other agents at decreasing the incidence of recurrent thromboembolic events. Low-molecular-weight heparin may be useful, but experience is more limited. Warfarin should be avoided because it is associated with a high rate of recurrent embolic events.",
"The prognosis for patients with NBTE associated with cancer is poor, because the disease is often advanced and incurable at the time of diagnosis. Even for these patients, antitumor and anticoagulation therapies have palliative benefits.",
"Surgery for vegetation excision or valve replacement should be considered if severe valvular dysfunction is present, contraindications to anticoagulation are noted, or thromboembolic events recur despite anticoagulation.[10] If surgical excision of the vegetation is warranted, the valve should be preserved if possible, unlike in IE, where complete removal of the infected tissue is important.",
"Diagnosis of NBTE requires a high degree of clinical suspicion; it should be considered in cases of IE that are unresponsive to antibiotics and new or recurrent thromboembolic events in patients with known cancer. Similarly, NBTE without a specific etiology should prompt an evaluation to exclude underlying cancer.",
"In this case, the diagnosis was made on the basis of the clinical presentation and findings on the head CT, chest radiography, and TEE. This patient presented an interesting challenge because of the constellation of symptoms. Additional blood cultures were negative, so she was not started on antibiotics. She was maintained on unfractionated heparin and had no additional thromboembolic events.",
"On further evaluation, the patient was found to have multiple renal and hepatic emboli as well as metastatic disease to her spine and adrenal glands. She was diagnosed with stage IV adenocarcinoma, and palliative care was initiated."
],
"date": "January 27, 2016",
"figures": [],
"markdown": "# A 65-Year-Old Woman With Decreased Coordination\n\n **Authors:** Caroline Tschibelu, MD \n **Date:** January 27, 2016\n\n ## Content\n\n The management of NBTE should be directed toward the underlying cancer or associated condition, and systemic anticoagulation should be given to prevent recurrent embolization. Unfractionated heparin is more effective than other agents at decreasing the incidence of recurrent thromboembolic events. Low-molecular-weight heparin may be useful, but experience is more limited. Warfarin should be avoided because it is associated with a high rate of recurrent embolic events.\nThe prognosis for patients with NBTE associated with cancer is poor, because the disease is often advanced and incurable at the time of diagnosis. Even for these patients, antitumor and anticoagulation therapies have palliative benefits.\nSurgery for vegetation excision or valve replacement should be considered if severe valvular dysfunction is present, contraindications to anticoagulation are noted, or thromboembolic events recur despite anticoagulation.[10] If surgical excision of the vegetation is warranted, the valve should be preserved if possible, unlike in IE, where complete removal of the infected tissue is important.\nDiagnosis of NBTE requires a high degree of clinical suspicion; it should be considered in cases of IE that are unresponsive to antibiotics and new or recurrent thromboembolic events in patients with known cancer. Similarly, NBTE without a specific etiology should prompt an evaluation to exclude underlying cancer.\nIn this case, the diagnosis was made on the basis of the clinical presentation and findings on the head CT, chest radiography, and TEE. This patient presented an interesting challenge because of the constellation of symptoms. Additional blood cultures were negative, so she was not started on antibiotics. She was maintained on unfractionated heparin and had no additional thromboembolic events.\nOn further evaluation, the patient was found to have multiple renal and hepatic emboli as well as metastatic disease to her spine and adrenal glands. She was diagnosed with stage IV adenocarcinoma, and palliative care was initiated.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 938393,
"choiceText": "Warfarin",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 938395,
"choiceText": "Surgical excision of the vegetation",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 938397,
"choiceText": "Valve replacement",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 938399,
"choiceText": "Unfractionated heparin",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Unfractionated heparin is the initial treatment of choice for patients with NBTE. It is more effective than warfarin at decreasing the incidence of recurrent thromboembolic events, and experience with low-molecular-weight heparin is limited.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 296893,
"questionText": "A 30-year-old woman with a history of SLE presents with mental status changes. She is found to have multiple ischemic strokes on MRI and is diagnosed with NBTE by TEE. Which of the following is the most appropriate treatment?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 938401,
"choiceText": "Maintain the patient on unfractionated heparin",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 938403,
"choiceText": "Start the patient on warfarin",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 938405,
"choiceText": "Surgical excision of the vegetation",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 938407,
"choiceText": "Valve replacement",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "This patient just had a recurrent thromboembolic event in addition to a subdural hematoma, which is probably a complication of long-term anticoagulation. These are contraindications to anticoagulation therapy. Surgical excision of the vegetation is the best option for this patient because she does not have any valvular dysfunction.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 296895,
"questionText": "Three months later, the same patient in the previous question presents with slurred speech. Head CT reveals new acute emboli as well as a subdural hematoma. Which of the following is the most appropriate next step?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 65-Year-Old Woman With Decreased Coordination"
},
{
"authors": "Caroline Tschibelu, MD",
"content": [],
"date": "January 27, 2016",
"figures": [],
"markdown": "# A 65-Year-Old Woman With Decreased Coordination\n\n **Authors:** Caroline Tschibelu, MD \n **Date:** January 27, 2016\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 938393,
"choiceText": "Warfarin",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 938395,
"choiceText": "Surgical excision of the vegetation",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 938397,
"choiceText": "Valve replacement",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 938399,
"choiceText": "Unfractionated heparin",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Unfractionated heparin is the initial treatment of choice for patients with NBTE. It is more effective than warfarin at decreasing the incidence of recurrent thromboembolic events, and experience with low-molecular-weight heparin is limited.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 296893,
"questionText": "A 30-year-old woman with a history of SLE presents with mental status changes. She is found to have multiple ischemic strokes on MRI and is diagnosed with NBTE by TEE. Which of the following is the most appropriate treatment?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 938401,
"choiceText": "Maintain the patient on unfractionated heparin",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 938403,
"choiceText": "Start the patient on warfarin",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 938405,
"choiceText": "Surgical excision of the vegetation",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 938407,
"choiceText": "Valve replacement",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "This patient just had a recurrent thromboembolic event in addition to a subdural hematoma, which is probably a complication of long-term anticoagulation. These are contraindications to anticoagulation therapy. Surgical excision of the vegetation is the best option for this patient because she does not have any valvular dysfunction.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 296895,
"questionText": "Three months later, the same patient in the previous question presents with slurred speech. Head CT reveals new acute emboli as well as a subdural hematoma. Which of the following is the most appropriate next step?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 65-Year-Old Woman With Decreased Coordination"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 938383,
"choiceText": "Multi-infarct dementia",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 938385,
"choiceText": "Nonbacterial thrombotic endocarditis",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 938387,
"choiceText": "Tuberculosis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 938389,
"choiceText": "Infective endocarditis",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 938391,
"choiceText": "Atrial myxoma",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 296891,
"questionText": "On the basis of the history, physical examination, and workup, what is the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 938393,
"choiceText": "Warfarin",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 938395,
"choiceText": "Surgical excision of the vegetation",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 938397,
"choiceText": "Valve replacement",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 938399,
"choiceText": "Unfractionated heparin",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Unfractionated heparin is the initial treatment of choice for patients with NBTE. It is more effective than warfarin at decreasing the incidence of recurrent thromboembolic events, and experience with low-molecular-weight heparin is limited.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 296893,
"questionText": "A 30-year-old woman with a history of SLE presents with mental status changes. She is found to have multiple ischemic strokes on MRI and is diagnosed with NBTE by TEE. Which of the following is the most appropriate treatment?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 938401,
"choiceText": "Maintain the patient on unfractionated heparin",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 938403,
"choiceText": "Start the patient on warfarin",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 938405,
"choiceText": "Surgical excision of the vegetation",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 938407,
"choiceText": "Valve replacement",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "This patient just had a recurrent thromboembolic event in addition to a subdural hematoma, which is probably a complication of long-term anticoagulation. These are contraindications to anticoagulation therapy. Surgical excision of the vegetation is the best option for this patient because she does not have any valvular dysfunction.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 296895,
"questionText": "Three months later, the same patient in the previous question presents with slurred speech. Head CT reveals new acute emboli as well as a subdural hematoma. Which of the following is the most appropriate next step?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
857712 | /viewarticle/857712 | [
{
"authors": "Michel E. Rivlin, MD",
"content": [
"Editor's Note:\n\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"An 18-year-old white woman presents to the clinic with a 2-month history of generalized abdominal pain, nausea, decreased appetite, and increased abdominal girth. She is not exhibiting any abnormal urinary or bowel symptoms, but she does complain of an intermittent, low-grade fever that is sometimes accompanied by chills. A review of her past medical, surgical, and family histories is insignificant.",
"Her menstrual history reveals regular cycles. She is nulligravid but is sexually active and is not currently using any contraception. She has no known allergies and does not regularly take any medications."
],
"date": "January 26, 2016",
"figures": [],
"markdown": "# An 18-Year-Old With Nausea and Increased Abdominal Girth\n\n **Authors:** Michel E. Rivlin, MD \n **Date:** January 26, 2016\n\n ## Content\n\n Editor's Note:\n\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nAn 18-year-old white woman presents to the clinic with a 2-month history of generalized abdominal pain, nausea, decreased appetite, and increased abdominal girth. She is not exhibiting any abnormal urinary or bowel symptoms, but she does complain of an intermittent, low-grade fever that is sometimes accompanied by chills. A review of her past medical, surgical, and family histories is insignificant.\nHer menstrual history reveals regular cycles. She is nulligravid but is sexually active and is not currently using any contraception. She has no known allergies and does not regularly take any medications.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "An 18-Year-Old With Nausea and Increased Abdominal Girth"
},
{
"authors": "Michel E. Rivlin, MD",
"content": [
"On physical examination, she is 58 inches (147.32 cm) in height and weighs 115 lb (52.2 kg). Her vital signs include an oral temperature of 99.5° F (37.5° C), a pulse rate of 106 beats/min, and a blood pressure of 98/61 mm Hg. The patient appears to be in no acute distress. The examination of her chest, including auscultation of her heart and lungs, reveals no abnormalities. The peripheral pulses are palpable.",
"The examination of her head and neck, as well as the neurologic examination, are normal. Her abdomen appears visibly distended and there is some degree of lower abdominal tenderness, but no guarding or rigidity is noted. No hepatomegaly or splenomegaly is found. The abdomen is dull to percussion and marked ascites is noted, with shifting dullness. A large, doughy mass is found in the lower abdomen, which is tender on deep palpation. The mass is immobile, firm, does not move with breathing movements, and is nonpulsatile. The overlying skin is normal, with no erythema, pallor, or venous distention. The mass extends from the pelvis in the midline towards the umbilicus. On rectovaginal examination, the mass is found to be filling the pelvis to a size similar to that of a 20-week pregnancy. No evidence of cervical discharge is present, and the vulva, vagina, and cervix appear normal. The rest of the physical examination is normal.",
"Significant laboratory analyses include a hematocrit of 26.7% (0.267), a platelet count of 51 × 103/μL (51 × 109/L), and a white blood cell (WBC) count of 9 × 103/μL (9 × 109/L). Her carcinoembryonic antigen (CEA) level is less than 1 ng/mL (1 μg/L; normal range, 0-10 ng/mL), her human chorionic gonadotropin (hCG) value is < 5 mIU/mL (5 IU/L; normal range for a nonpregnant woman, < 5 mIU/mL), her alpha-fetoprotein (AFP) level is 0.8 ng/mL (0.8 μg/L; normal range, 0-10 ng/mL), her lactate dehydrogenase (LDH) measurement is 534 U/L (normal range, 259-613 U/L), and her cancer antigen (CA) 125 level is 509 U/mL (509 kU/L; normal range, 0-35 U/mL). Her hepatic function tests are normal.",
"Figure 1.",
"Figure 1.",
"Figure 2.",
"Figure 2.",
"Radiographs of the chest and an ECG are ordered and are found to be normal. Pelvic ultrasonography indicates a normal-sized uterus and endometrial stripe; it also shows a complex, midline pelvic mass of 11 × 9 cm in size, with both solid and cystic components (Figure 1). CT scanning confirms the presence of a complex abdominopelvic mass with ascites. The patient is scheduled for an exploratory laparotomy with ovarian cystectomy, but she is also counseled for a hysterectomy and staging procedure, to which she consents. The gynecologic oncologist is aware of the surgery and is available if required.",
"At laparotomy, a large pelvic abscess is encountered and, subsequently, 1700 mL of turbid fluid is drained from the abscess cavity. The abscess extends from the pubic symphysis in the midline to the umbilicus. The small intestine appears to be seeded with small implants, all < 5 mm in diameter. Both fallopian tubes are noted to be grossly dilated, rigid, and have a rougher appearance externally, with the right tube appearing more grossly abnormal. Multiple constrictions are seen along the course of the right tube, with obstruction at the transition area between the isthmus and the ampulla. The uterus, ovaries, and appendix appear to be grossly normal. No abnormalities of the liver, kidneys, or stomach are found. Multiple biopsies and cultures are taken and sent for analysis. Frozen-section analysis demonstrates \"granulomatous reaction compatible with tuberculosis.”",
"Figure 3.",
"Figure 3.",
"Figure 4.",
"Figure 4.",
"Surgery is terminated at this point, with the midline incision being closed without drains following extensive irrigation. All cultures come back negative for bacteria and fungi, with the exception of the abscess, which grows acid-fast bacilli (AFB) on Lowenstein-Jensen medium in about 6 weeks. Testing for drug sensitivities shows no resistance to first-line tuberculosis agents. The biopsies reveal multiple caseating and noncaseating granulomas (Figures 2 and 3), with organisms compatible with Mycobacterium tuberculosis on AFB staining (Figure 4). A Gomori methenamine silver (GMS) stain examination is negative for fungal organisms. Postsurgery HIV tests are also negative; however, a PPD intradermal skin test (Mantoux test) is positive. The health department is notified."
],
"date": "January 26, 2016",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/857/712/857712-Thumb1.png"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/857/712/857712-Thumb2.png"
},
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/857/712/857712-Thumb3.png"
},
{
"caption": "Figure 4.",
"image_url": "https://img.medscapestatic.com/article/857/712/857712-Thumb4.png"
}
],
"markdown": "# An 18-Year-Old With Nausea and Increased Abdominal Girth\n\n **Authors:** Michel E. Rivlin, MD \n **Date:** January 26, 2016\n\n ## Content\n\n On physical examination, she is 58 inches (147.32 cm) in height and weighs 115 lb (52.2 kg). Her vital signs include an oral temperature of 99.5° F (37.5° C), a pulse rate of 106 beats/min, and a blood pressure of 98/61 mm Hg. The patient appears to be in no acute distress. The examination of her chest, including auscultation of her heart and lungs, reveals no abnormalities. The peripheral pulses are palpable.\nThe examination of her head and neck, as well as the neurologic examination, are normal. Her abdomen appears visibly distended and there is some degree of lower abdominal tenderness, but no guarding or rigidity is noted. No hepatomegaly or splenomegaly is found. The abdomen is dull to percussion and marked ascites is noted, with shifting dullness. A large, doughy mass is found in the lower abdomen, which is tender on deep palpation. The mass is immobile, firm, does not move with breathing movements, and is nonpulsatile. The overlying skin is normal, with no erythema, pallor, or venous distention. The mass extends from the pelvis in the midline towards the umbilicus. On rectovaginal examination, the mass is found to be filling the pelvis to a size similar to that of a 20-week pregnancy. No evidence of cervical discharge is present, and the vulva, vagina, and cervix appear normal. The rest of the physical examination is normal.\nSignificant laboratory analyses include a hematocrit of 26.7% (0.267), a platelet count of 51 × 103/μL (51 × 109/L), and a white blood cell (WBC) count of 9 × 103/μL (9 × 109/L). Her carcinoembryonic antigen (CEA) level is less than 1 ng/mL (1 μg/L; normal range, 0-10 ng/mL), her human chorionic gonadotropin (hCG) value is < 5 mIU/mL (5 IU/L; normal range for a nonpregnant woman, < 5 mIU/mL), her alpha-fetoprotein (AFP) level is 0.8 ng/mL (0.8 μg/L; normal range, 0-10 ng/mL), her lactate dehydrogenase (LDH) measurement is 534 U/L (normal range, 259-613 U/L), and her cancer antigen (CA) 125 level is 509 U/mL (509 kU/L; normal range, 0-35 U/mL). Her hepatic function tests are normal.\nFigure 1.\nFigure 1.\nFigure 2.\nFigure 2.\nRadiographs of the chest and an ECG are ordered and are found to be normal. Pelvic ultrasonography indicates a normal-sized uterus and endometrial stripe; it also shows a complex, midline pelvic mass of 11 × 9 cm in size, with both solid and cystic components (Figure 1). CT scanning confirms the presence of a complex abdominopelvic mass with ascites. The patient is scheduled for an exploratory laparotomy with ovarian cystectomy, but she is also counseled for a hysterectomy and staging procedure, to which she consents. The gynecologic oncologist is aware of the surgery and is available if required.\nAt laparotomy, a large pelvic abscess is encountered and, subsequently, 1700 mL of turbid fluid is drained from the abscess cavity. The abscess extends from the pubic symphysis in the midline to the umbilicus. The small intestine appears to be seeded with small implants, all < 5 mm in diameter. Both fallopian tubes are noted to be grossly dilated, rigid, and have a rougher appearance externally, with the right tube appearing more grossly abnormal. Multiple constrictions are seen along the course of the right tube, with obstruction at the transition area between the isthmus and the ampulla. The uterus, ovaries, and appendix appear to be grossly normal. No abnormalities of the liver, kidneys, or stomach are found. Multiple biopsies and cultures are taken and sent for analysis. Frozen-section analysis demonstrates \"granulomatous reaction compatible with tuberculosis.”\nFigure 3.\nFigure 3.\nFigure 4.\nFigure 4.\nSurgery is terminated at this point, with the midline incision being closed without drains following extensive irrigation. All cultures come back negative for bacteria and fungi, with the exception of the abscess, which grows acid-fast bacilli (AFB) on Lowenstein-Jensen medium in about 6 weeks. Testing for drug sensitivities shows no resistance to first-line tuberculosis agents. The biopsies reveal multiple caseating and noncaseating granulomas (Figures 2 and 3), with organisms compatible with Mycobacterium tuberculosis on AFB staining (Figure 4). A Gomori methenamine silver (GMS) stain examination is negative for fungal organisms. Postsurgery HIV tests are also negative; however, a PPD intradermal skin test (Mantoux test) is positive. The health department is notified.\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n**Figure 3.** \n \n\n**Figure 4.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 938347,
"choiceText": "Chronic pelvic inflammation",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 938349,
"choiceText": "Genital tuberculosis with peritonitis",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 938351,
"choiceText": "Mycotic infection",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 938353,
"choiceText": "Ovarian carcinoma\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 296879,
"questionText": "What is the most likely diagnosis in this young patient who presents with a 2-month history of low-grade fever, abdominal distention, and an abdominal mass?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "An 18-Year-Old With Nausea and Increased Abdominal Girth"
},
{
"authors": "Michel E. Rivlin, MD",
"content": [
"Tuberculosis is a major world health problem, with a global prevalence estimated at 32%. In the United States, the percentage of cases occurring among foreign-born persons was 53% in 2003. Female genital tuberculosis is not uncommon in parts of the world where pulmonary tuberculosis is widespread. Tuberculosis is also associated with the HIV epidemic; in particular, extrapulmonary tuberculosis can be found in more than 50% of patients with concurrent AIDS.[1,2]",
"Female genital tract infection may be contracted by hematogenous spread from a pulmonary nidus (the fallopian tube is the predominant site of infection) or the spread may be from gastrointestinal infection, characteristically from the ileocecal region by lymphatic spread to the right tube. Characteristically, the involvement of the fallopian tubes is bilateral (although asymmetric), with the tubes becoming thickened, swollen, and often with a roughened surface and adhesions. The tubes may also become obstructed, most often at the junction of the ampullary region with the isthmus and with multiple constrictions throughout the tubal length.",
"The appearance of the tubes varies; in severe cases, they may be distended with caseous material. In milder cases, they may have only tubercles on the serosa. The fimbrial end of the tube is usually spared and remains patent with the fimbria everted, which produces the \"tobacco pouch\" appearance.[3] Distal tubal disease usually appears secondary to peritubal adhesions. These adhesions disrupt the delicate anatomical relationship between the tube and the ovary and interfere with normal ovulation. Spread from the tubes to the endometrium is common, but the ovaries do not usually show signs of involvement. In addition, involvement of the cervix, vagina, and vulva is uncommon. Tuberculous peritonitis is a variant of genital tuberculosis that results from initial miliary dissemination during primary bacteremia or secondarily during reactivation of pulmonary or extrapulmonary disease. Genital and peritoneal diseases are coexistent in up to 50% of cases.",
"The characteristic tuberculous granuloma consists of a central area of caseous necrosis surrounded by concentric layers of modified epithelial cells and with multiple Langerhans giant cells, all of which is surrounded by a peripheral zone of lymphocytes, monocytes, and fibroblasts. Calcified lymph nodes or irregular calcifications of the adnexa may be noted.",
"The clinical manifestations of genital/peritoneal tuberculosis include abdominal pain, abdominal swelling, persistent low-grade fever, weight loss, malaise, and fatigue. Menstrual disturbances initially include increased and irregular bleeding. Amenorrhea is usually evidence of advanced endometritis, which is secondary to spread from a primary focus in the tubes. Infertility is the most common complaint, and up to 85% of women with tuberculous salpingitis or genital tuberculosis never get pregnant."
],
"date": "January 26, 2016",
"figures": [],
"markdown": "# An 18-Year-Old With Nausea and Increased Abdominal Girth\n\n **Authors:** Michel E. Rivlin, MD \n **Date:** January 26, 2016\n\n ## Content\n\n Tuberculosis is a major world health problem, with a global prevalence estimated at 32%. In the United States, the percentage of cases occurring among foreign-born persons was 53% in 2003. Female genital tuberculosis is not uncommon in parts of the world where pulmonary tuberculosis is widespread. Tuberculosis is also associated with the HIV epidemic; in particular, extrapulmonary tuberculosis can be found in more than 50% of patients with concurrent AIDS.[1,2]\nFemale genital tract infection may be contracted by hematogenous spread from a pulmonary nidus (the fallopian tube is the predominant site of infection) or the spread may be from gastrointestinal infection, characteristically from the ileocecal region by lymphatic spread to the right tube. Characteristically, the involvement of the fallopian tubes is bilateral (although asymmetric), with the tubes becoming thickened, swollen, and often with a roughened surface and adhesions. The tubes may also become obstructed, most often at the junction of the ampullary region with the isthmus and with multiple constrictions throughout the tubal length.\nThe appearance of the tubes varies; in severe cases, they may be distended with caseous material. In milder cases, they may have only tubercles on the serosa. The fimbrial end of the tube is usually spared and remains patent with the fimbria everted, which produces the \"tobacco pouch\" appearance.[3] Distal tubal disease usually appears secondary to peritubal adhesions. These adhesions disrupt the delicate anatomical relationship between the tube and the ovary and interfere with normal ovulation. Spread from the tubes to the endometrium is common, but the ovaries do not usually show signs of involvement. In addition, involvement of the cervix, vagina, and vulva is uncommon. Tuberculous peritonitis is a variant of genital tuberculosis that results from initial miliary dissemination during primary bacteremia or secondarily during reactivation of pulmonary or extrapulmonary disease. Genital and peritoneal diseases are coexistent in up to 50% of cases.\nThe characteristic tuberculous granuloma consists of a central area of caseous necrosis surrounded by concentric layers of modified epithelial cells and with multiple Langerhans giant cells, all of which is surrounded by a peripheral zone of lymphocytes, monocytes, and fibroblasts. Calcified lymph nodes or irregular calcifications of the adnexa may be noted.\nThe clinical manifestations of genital/peritoneal tuberculosis include abdominal pain, abdominal swelling, persistent low-grade fever, weight loss, malaise, and fatigue. Menstrual disturbances initially include increased and irregular bleeding. Amenorrhea is usually evidence of advanced endometritis, which is secondary to spread from a primary focus in the tubes. Infertility is the most common complaint, and up to 85% of women with tuberculous salpingitis or genital tuberculosis never get pregnant.\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 938347,
"choiceText": "Chronic pelvic inflammation",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 938349,
"choiceText": "Genital tuberculosis with peritonitis",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 938351,
"choiceText": "Mycotic infection",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 938353,
"choiceText": "Ovarian carcinoma\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 296879,
"questionText": "What is the most likely diagnosis in this young patient who presents with a 2-month history of low-grade fever, abdominal distention, and an abdominal mass?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "An 18-Year-Old With Nausea and Increased Abdominal Girth"
},
{
"authors": "Michel E. Rivlin, MD",
"content": [
"Symptoms are usually present for weeks or months, and a history of close contact with an infected person, such as other family members, may be reported. Patients may have a personal history of pulmonary or extrapulmonary disorders, such as pleurisy, erythema nodosum, renal disease, and/or bone disease. A physical examination may be unremarkable except for mild weight loss. Ascites is present in as many as 97% of cases of tuberculous peritonitis. The abdomen can feel \"doughy\" and with irregular masses, which may be calcified and visible on abdominopelvic radiographs. A pelvic examination often shows findings similar to nontuberculous pelvic inflammatory disease; however, the bilateral masses are usually less tender and less uniform in consistency. The finding of bilateral inflammatory masses in a virginal female or of ascites in an adolescent, respectively, should raise suspicion for genital or peritoneal tuberculosis.[3,4,5]",
"In this patient, the presence of a pelvic mass with ascites and an elevated CA 125 led to the erroneous presumptive diagnosis of an ovarian malignancy; however, pelvic/peritoneal tuberculosis may be associated with elevated serum and peritoneal fluid CA 125 levels, and should always be kept in mind with such presentations. These levels can return to normal after successful drug therapy. Other conditions may cause granulomas with giant cells, including sarcoidosis, actinomycosis, and foreign-body reactions. Actinomyces, an anaerobic gram-positive bacterium, is only occasionally a cause of pelvic organ infection, usually in the presence of a long-standing intrauterine device. Sarcoidosis rarely involves pelvic organs, and this patient's ethnicity makes sarcoidosis a highly unlikely diagnosis (it is more common in black persons).[5]",
"The diagnosis is best established by successful culture of the organism and demonstrating the AFB with the Ziehl-Neelsen staining technique. Samples tested may be derived from peritoneal fluid, biopsies, pus from the abscess, sputum, urine, and/or menstrual fluid. Histopathologic diagnosis is usually based on premenstrual endometrial biopsy samples or biopsies obtained at laparoscopy or laparotomy (as in this case). Imaging studies are nonspecific and the findings include high-density ascites (which appear more radiopaque rather than radiolucent because the ascitic fluid is thick, usually as a result of blood or a proteinaceous exudate), adenopathy, adnexal masses, and omental and mesenteric thickening.",
"Hysterosalpingography may show characteristic tubal changes, including \"pipe-stem\" appearance and multiple fistulae; however, unlike in chronic pelvic inflammatory disease, the fimbriae are uninvolved. Further evaluation should include HIV status, chest radiography, and renal tract assessment (because 10% of patients with genital lesions have renal tuberculosis and vice versa). Positive cultures should be tested for drug resistance against all first-line agents.[3] Nucleic acid amplification tests (eg, polymerase chain reaction) have been approved in the United States for the diagnosis of tuberculosis in patients with positive sputum smears with high positive and negative predictive value, but in sputum-negative patients, the positive predictive value is only about 50%. Ultimately the diagnosis of tuberculosis involves a synthesis of clinical and laboratory findings.[6]"
],
"date": "January 26, 2016",
"figures": [],
"markdown": "# An 18-Year-Old With Nausea and Increased Abdominal Girth\n\n **Authors:** Michel E. Rivlin, MD \n **Date:** January 26, 2016\n\n ## Content\n\n Symptoms are usually present for weeks or months, and a history of close contact with an infected person, such as other family members, may be reported. Patients may have a personal history of pulmonary or extrapulmonary disorders, such as pleurisy, erythema nodosum, renal disease, and/or bone disease. A physical examination may be unremarkable except for mild weight loss. Ascites is present in as many as 97% of cases of tuberculous peritonitis. The abdomen can feel \"doughy\" and with irregular masses, which may be calcified and visible on abdominopelvic radiographs. A pelvic examination often shows findings similar to nontuberculous pelvic inflammatory disease; however, the bilateral masses are usually less tender and less uniform in consistency. The finding of bilateral inflammatory masses in a virginal female or of ascites in an adolescent, respectively, should raise suspicion for genital or peritoneal tuberculosis.[3,4,5]\nIn this patient, the presence of a pelvic mass with ascites and an elevated CA 125 led to the erroneous presumptive diagnosis of an ovarian malignancy; however, pelvic/peritoneal tuberculosis may be associated with elevated serum and peritoneal fluid CA 125 levels, and should always be kept in mind with such presentations. These levels can return to normal after successful drug therapy. Other conditions may cause granulomas with giant cells, including sarcoidosis, actinomycosis, and foreign-body reactions. Actinomyces, an anaerobic gram-positive bacterium, is only occasionally a cause of pelvic organ infection, usually in the presence of a long-standing intrauterine device. Sarcoidosis rarely involves pelvic organs, and this patient's ethnicity makes sarcoidosis a highly unlikely diagnosis (it is more common in black persons).[5]\nThe diagnosis is best established by successful culture of the organism and demonstrating the AFB with the Ziehl-Neelsen staining technique. Samples tested may be derived from peritoneal fluid, biopsies, pus from the abscess, sputum, urine, and/or menstrual fluid. Histopathologic diagnosis is usually based on premenstrual endometrial biopsy samples or biopsies obtained at laparoscopy or laparotomy (as in this case). Imaging studies are nonspecific and the findings include high-density ascites (which appear more radiopaque rather than radiolucent because the ascitic fluid is thick, usually as a result of blood or a proteinaceous exudate), adenopathy, adnexal masses, and omental and mesenteric thickening.\nHysterosalpingography may show characteristic tubal changes, including \"pipe-stem\" appearance and multiple fistulae; however, unlike in chronic pelvic inflammatory disease, the fimbriae are uninvolved. Further evaluation should include HIV status, chest radiography, and renal tract assessment (because 10% of patients with genital lesions have renal tuberculosis and vice versa). Positive cultures should be tested for drug resistance against all first-line agents.[3] Nucleic acid amplification tests (eg, polymerase chain reaction) have been approved in the United States for the diagnosis of tuberculosis in patients with positive sputum smears with high positive and negative predictive value, but in sputum-negative patients, the positive predictive value is only about 50%. Ultimately the diagnosis of tuberculosis involves a synthesis of clinical and laboratory findings.[6]\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "An 18-Year-Old With Nausea and Increased Abdominal Girth"
},
{
"authors": "Michel E. Rivlin, MD",
"content": [
"Response to drug therapy is excellent for all forms of genital tuberculosis. Surgery is reserved for large tubo-ovarian abscesses, abscesses refractory to antituberculosis treatment, persistent adnexal masses, persistent pain, and drug resistance. Multidrug-resistant strains of tuberculosis (MDR-TB) are defined as those resistant to at least isoniazid and rifampin. The leading risk factors for these strains are coexistent HIV, improper drug selection, inappropriate or incomplete treatment, patient noncompliance with the treatment, or infection spread by an individual who carries a resistant strain.",
"Because sensitivity results may take 6-8 weeks, treatment is started with multidrug regimens that are modified when results become available. If genital tuberculosis is encountered unexpectedly at surgery (as in this case), only biopsy should be performed, as procedures after 3-4 months of drug therapy are technically easier and less prone to complications (especially fistulae). For the same reason, surgery should be delayed until an adequate course of medical therapy has been delivered, whenever possible. Drug therapy should be prolonged for 18-24 months as required.[3] In adults, however, most forms of extrapulmonary tuberculosis can be cured with 6 months of chemotherapy, although the clinical response should guide decisions on treatment duration.[7]",
"In this patient, treatment with rifampin, ethambutol, isoniazid, and pyrazinamide was promptly initiated. Characteristically after laparotomy, the ascites did not recur and her clinical progress was satisfactory. Two months after surgery, she had made remarkable progress. Treatment was continued with the same drugs, as the organism was sensitive to all four agents. Her prognosis was quite good, but unfortunately her prospects for spontaneous pregnancy were poor, as evidenced by the massive scarring in and around her fallopian tubes. The rarity of a successful uterine pregnancy with genital tuberculosis is borne out by a report from India in which nine of 56 treated patients conceived; of these, eight suffered spontaneous abortions and only one had a successful pregnancy.[8] In these cases, however, successful pregnancies have been reported with in vitro fertilization.[5]"
],
"date": "January 26, 2016",
"figures": [],
"markdown": "# An 18-Year-Old With Nausea and Increased Abdominal Girth\n\n **Authors:** Michel E. Rivlin, MD \n **Date:** January 26, 2016\n\n ## Content\n\n Response to drug therapy is excellent for all forms of genital tuberculosis. Surgery is reserved for large tubo-ovarian abscesses, abscesses refractory to antituberculosis treatment, persistent adnexal masses, persistent pain, and drug resistance. Multidrug-resistant strains of tuberculosis (MDR-TB) are defined as those resistant to at least isoniazid and rifampin. The leading risk factors for these strains are coexistent HIV, improper drug selection, inappropriate or incomplete treatment, patient noncompliance with the treatment, or infection spread by an individual who carries a resistant strain.\nBecause sensitivity results may take 6-8 weeks, treatment is started with multidrug regimens that are modified when results become available. If genital tuberculosis is encountered unexpectedly at surgery (as in this case), only biopsy should be performed, as procedures after 3-4 months of drug therapy are technically easier and less prone to complications (especially fistulae). For the same reason, surgery should be delayed until an adequate course of medical therapy has been delivered, whenever possible. Drug therapy should be prolonged for 18-24 months as required.[3] In adults, however, most forms of extrapulmonary tuberculosis can be cured with 6 months of chemotherapy, although the clinical response should guide decisions on treatment duration.[7]\nIn this patient, treatment with rifampin, ethambutol, isoniazid, and pyrazinamide was promptly initiated. Characteristically after laparotomy, the ascites did not recur and her clinical progress was satisfactory. Two months after surgery, she had made remarkable progress. Treatment was continued with the same drugs, as the organism was sensitive to all four agents. Her prognosis was quite good, but unfortunately her prospects for spontaneous pregnancy were poor, as evidenced by the massive scarring in and around her fallopian tubes. The rarity of a successful uterine pregnancy with genital tuberculosis is borne out by a report from India in which nine of 56 treated patients conceived; of these, eight suffered spontaneous abortions and only one had a successful pregnancy.[8] In these cases, however, successful pregnancies have been reported with in vitro fertilization.[5]\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 938355,
"choiceText": "Exploratory laparotomy and surgical drainage",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 938357,
"choiceText": "Biopsy of mass",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 938359,
"choiceText": "Empiric treatment with antituberculosis agents for 3-4 months",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 938361,
"choiceText": "Obtain two sets of sputum cultures",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 938363,
"choiceText": "Obtain blood cultures\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "\r\nHistopathologic diagnosis is usually based on premenstrual endometrial biopsy samples or biopsies obtained at laparoscopy or laparotomy (as in this case).\r\n",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 296881,
"questionText": "A patient with known tuberculosis exposure complains of a several-month history of fevers, progressive abdominal pain, and increasing girth. The patient is found to have a heterogeneous pelvic mass by ultrasonography. What is the recommended first step for diagnosis and/or treatment?\r\n",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 938365,
"choiceText": "Physical examination",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 938367,
"choiceText": "Abdominopelvic radiography",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 938369,
"choiceText": "Culture with examination on Ziehl-Neelsen staining",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 938371,
"choiceText": "Hysterosalpingography\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The diagnosis is best established by successful culture of the organism and demonstrating the AFB with the Ziehl-Neelsen staining technique. Samples tested may be derived from peritoneal fluid, biopsies, pus from the abscess, sputum, urine, and/or menstrual fluid.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 296883,
"questionText": "When examining a patient with genital tuberculosis, which of the following choices would be the best for confirming a diagnosis of tuberculosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "An 18-Year-Old With Nausea and Increased Abdominal Girth"
},
{
"authors": "Michel E. Rivlin, MD",
"content": [],
"date": "January 26, 2016",
"figures": [],
"markdown": "# An 18-Year-Old With Nausea and Increased Abdominal Girth\n\n **Authors:** Michel E. Rivlin, MD \n **Date:** January 26, 2016\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 938355,
"choiceText": "Exploratory laparotomy and surgical drainage",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 938357,
"choiceText": "Biopsy of mass",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 938359,
"choiceText": "Empiric treatment with antituberculosis agents for 3-4 months",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 938361,
"choiceText": "Obtain two sets of sputum cultures",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 938363,
"choiceText": "Obtain blood cultures\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "\r\nHistopathologic diagnosis is usually based on premenstrual endometrial biopsy samples or biopsies obtained at laparoscopy or laparotomy (as in this case).\r\n",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 296881,
"questionText": "A patient with known tuberculosis exposure complains of a several-month history of fevers, progressive abdominal pain, and increasing girth. The patient is found to have a heterogeneous pelvic mass by ultrasonography. What is the recommended first step for diagnosis and/or treatment?\r\n",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 938365,
"choiceText": "Physical examination",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 938367,
"choiceText": "Abdominopelvic radiography",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 938369,
"choiceText": "Culture with examination on Ziehl-Neelsen staining",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 938371,
"choiceText": "Hysterosalpingography\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The diagnosis is best established by successful culture of the organism and demonstrating the AFB with the Ziehl-Neelsen staining technique. Samples tested may be derived from peritoneal fluid, biopsies, pus from the abscess, sputum, urine, and/or menstrual fluid.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 296883,
"questionText": "When examining a patient with genital tuberculosis, which of the following choices would be the best for confirming a diagnosis of tuberculosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "An 18-Year-Old With Nausea and Increased Abdominal Girth"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 938347,
"choiceText": "Chronic pelvic inflammation",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 938349,
"choiceText": "Genital tuberculosis with peritonitis",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 938351,
"choiceText": "Mycotic infection",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 938353,
"choiceText": "Ovarian carcinoma\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 296879,
"questionText": "What is the most likely diagnosis in this young patient who presents with a 2-month history of low-grade fever, abdominal distention, and an abdominal mass?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 938355,
"choiceText": "Exploratory laparotomy and surgical drainage",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 938357,
"choiceText": "Biopsy of mass",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 938359,
"choiceText": "Empiric treatment with antituberculosis agents for 3-4 months",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 938361,
"choiceText": "Obtain two sets of sputum cultures",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 938363,
"choiceText": "Obtain blood cultures\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "\r\nHistopathologic diagnosis is usually based on premenstrual endometrial biopsy samples or biopsies obtained at laparoscopy or laparotomy (as in this case).\r\n",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 296881,
"questionText": "A patient with known tuberculosis exposure complains of a several-month history of fevers, progressive abdominal pain, and increasing girth. The patient is found to have a heterogeneous pelvic mass by ultrasonography. What is the recommended first step for diagnosis and/or treatment?\r\n",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 938365,
"choiceText": "Physical examination",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 938367,
"choiceText": "Abdominopelvic radiography",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 938369,
"choiceText": "Culture with examination on Ziehl-Neelsen staining",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 938371,
"choiceText": "Hysterosalpingography\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The diagnosis is best established by successful culture of the organism and demonstrating the AFB with the Ziehl-Neelsen staining technique. Samples tested may be derived from peritoneal fluid, biopsies, pus from the abscess, sputum, urine, and/or menstrual fluid.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 296883,
"questionText": "When examining a patient with genital tuberculosis, which of the following choices would be the best for confirming a diagnosis of tuberculosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
857143 | /viewarticle/857143 | [
{
"authors": "Maria Romanova, MD",
"content": [
"Editor's Note:\n\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.",
"A 49-year-old man with a medical history of cervical spine fracture, diabetes mellitus, and hearing loss presents to the emergency department with increasing pain at the site of a previous complicated right femoral fracture after a minor fall onto the affected area earlier in the day. No other trauma is noted, and the fall itself is thought to have been mechanical, with no antecedent symptoms (such as lightheadedness).",
"The patient has no significant history of alcohol use or illicit substance use. He had sustained a displaced transverse fracture of the right proximal femur 6 months ago as a result of a car accident. The fracture had been treated with operative reduction and internal fixation; the hardware was removed prematurely 2 months after the accident at an outside facility for unknown reasons (although it was thought to be because of increased pain). Despite an aggressive physical therapy regimen, the patient remained bedridden, with continued pain in the injured extremity."
],
"date": "January 14, 2016",
"figures": [],
"markdown": "# A 49-Year-Old Man With Pain at the Site of a Previous Fracture\n\n **Authors:** Maria Romanova, MD \n **Date:** January 14, 2016\n\n ## Content\n\n Editor's Note:\n\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.\nA 49-year-old man with a medical history of cervical spine fracture, diabetes mellitus, and hearing loss presents to the emergency department with increasing pain at the site of a previous complicated right femoral fracture after a minor fall onto the affected area earlier in the day. No other trauma is noted, and the fall itself is thought to have been mechanical, with no antecedent symptoms (such as lightheadedness).\nThe patient has no significant history of alcohol use or illicit substance use. He had sustained a displaced transverse fracture of the right proximal femur 6 months ago as a result of a car accident. The fracture had been treated with operative reduction and internal fixation; the hardware was removed prematurely 2 months after the accident at an outside facility for unknown reasons (although it was thought to be because of increased pain). Despite an aggressive physical therapy regimen, the patient remained bedridden, with continued pain in the injured extremity.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 49-Year-Old Man With Pain at the Site of a Previous Fracture"
},
{
"authors": "Maria Romanova, MD",
"content": [
"Upon physical examination, the patient has an overall ill appearance, with a heart rate of 110 beats/min, blood pressure of 120/65 mm Hg, respiratory rate of 12 breaths/min, and temperature of 98.6°F. He has a normal oxygen saturation of 100% while breathing room air. The cardiac and pulmonary examinations are within normal limits.",
"Figure 1.",
"Figure 1.",
"Figure 2.",
"Figure 2.",
"Examination of the right leg reveals a healed scar over the lateral thigh, with diffuse tenderness to palpation of the proximal femur. Mild limitation in hip flexion to 100° and severe limitation in knee flexion to 30° are noted, as well as muscle stiffness and atrophy. The lower leg and foot of the affected extremity are well-perfused, with bounding distal pulses. No overlying, chronic skin changes are noted except for a well-healed scar over the site of the previous operative repair. The neurologic examination findings, including motor strength and deep tendon reflexes, are normal in the affected leg.",
"A complete blood cell count and other trauma-related laboratory investigations (including a coagulation profile) are within normal limits. The alkaline phosphatase level is slightly elevated at 131 mg/dL, with an erythrocyte sedimentation rate of 16 mm/h. Plain radiographs of the femur and pelvis are obtained (Figures 1 and 2)."
],
"date": "January 14, 2016",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/857/143/857143-Thumb1.jpg"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/857/143/857143-Thumb2.jpg"
}
],
"markdown": "# A 49-Year-Old Man With Pain at the Site of a Previous Fracture\n\n **Authors:** Maria Romanova, MD \n **Date:** January 14, 2016\n\n ## Content\n\n Upon physical examination, the patient has an overall ill appearance, with a heart rate of 110 beats/min, blood pressure of 120/65 mm Hg, respiratory rate of 12 breaths/min, and temperature of 98.6°F. He has a normal oxygen saturation of 100% while breathing room air. The cardiac and pulmonary examinations are within normal limits.\nFigure 1.\nFigure 1.\nFigure 2.\nFigure 2.\nExamination of the right leg reveals a healed scar over the lateral thigh, with diffuse tenderness to palpation of the proximal femur. Mild limitation in hip flexion to 100° and severe limitation in knee flexion to 30° are noted, as well as muscle stiffness and atrophy. The lower leg and foot of the affected extremity are well-perfused, with bounding distal pulses. No overlying, chronic skin changes are noted except for a well-healed scar over the site of the previous operative repair. The neurologic examination findings, including motor strength and deep tendon reflexes, are normal in the affected leg.\nA complete blood cell count and other trauma-related laboratory investigations (including a coagulation profile) are within normal limits. The alkaline phosphatase level is slightly elevated at 131 mg/dL, with an erythrocyte sedimentation rate of 16 mm/h. Plain radiographs of the femur and pelvis are obtained (Figures 1 and 2).\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 934821,
"choiceText": "Primary hypoparathyroidism",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 934823,
"choiceText": "Paget disease ",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 934825,
"choiceText": "Benign osteopetrosis",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 934827,
"choiceText": "Lead toxicity",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 295739,
"questionText": "What is the likely underlying diagnosis for the patient's fractures? ",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 49-Year-Old Man With Pain at the Site of a Previous Fracture"
},
{
"authors": "Maria Romanova, MD",
"content": [
"Osteopetrosis (marble bone disease) is a rare hereditary disorder of diminished osteoclast function characterized by deficient bone resorption.[1] In this patient, the plain radiograph of the right femur shows a marked, diffuse increase in the density of the osseous structures, and evidence of refracture is noted through the proximal mid-diaphyseal/subtrochanteric region (Figure 1). A similar pattern, with increased bone density (which gives the bones a very bright appearance), is noted on the radiograph of the pelvis (Figure 2).",
"Figure 1.",
"Figure 1.",
"Figure 2.",
"Figure 2.",
"Figure 3.",
"Figure 3.",
"A review of past radiographs of the cervical spine (Figure 3) reveals diffusely increased density of all osseous structures, with a \"sandwich\" appearance (presence of an extreme and uniform increase in radiographic density at the superior and inferior margins) of the upper vertebrae (ie, C2-C4), and a healed posterior spinous process fracture of the sixth and seventh cervical vertebra.",
"As a consequence of deficient bone resorption resulting from diminished osteoclast function, bone modeling and remodeling are impaired in osteopetrosis. Defective bone turnover results in skeletal fragility, despite the increase in bone mass. The condition is a heterogeneous disorder encompassing different molecular lesions (more than 14, some with human homologues, have been identified in the murine model) and a wide range of clinical features in its phenotypic manifestation in affected individuals.[1]",
"In humans, three distinct forms of the disease account for most cases of osteopetrosis; these forms, based on age and clinical features, are adult-onset, infantile, and intermediate. The adult-onset form (benign osteopetrosis, also called \"Albers-Schönberg disease\") is inherited in an autosomal dominant pattern and is often recognized only incidentally. In most cases, it is due to an autosomal dominant single gene mutation of the chloride channel 7 (CLC7) gene, which is involved in a regulatory pathway for bone remodeling. Homozygous mutations of this gene are one cause of the severe infantile form of the disease. Typically, no evidence of bone marrow failure is found.",
"Approximately 50% of patients with infantile osteopetrosis are asymptomatic. The diagnosis is often made incidentally (usually in late adolescence) because radiologic abnormalities start appearing in childhood in the setting of fractures sustained by the individual, or it is made as a result of the development of osteomyelitis, especially of the mandible. In other patients, the diagnosis may be made earlier in life, on the basis of the family history.",
"The term \"benign\" is a misnomer. Bony defects are common and include neuropathies caused by cranial nerve entrapment, with specific problems, including deafness and facial palsy. Carpal tunnel syndrome and osteoarthritis also occur. The bones are fragile and may fracture easily;[2] approximately 40% of patients have recurrent fractures. Bone marrow function is not compromised.[1]",
"Other manifestations include vision impairment caused by retinal degeneration and psychomotor retardation. In benign osteopetrosis, laboratory data may reveal elevated alkaline phosphatase levels, as well as elevated levels of acid phosphatase and creatinine kinase caused by increased release from defective osteoclasts."
],
"date": "January 14, 2016",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/857/143/857143-Thumb1.jpg"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/857/143/857143-Thumb2.jpg"
},
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/857/143/857143-Thumb3.jpg"
}
],
"markdown": "# A 49-Year-Old Man With Pain at the Site of a Previous Fracture\n\n **Authors:** Maria Romanova, MD \n **Date:** January 14, 2016\n\n ## Content\n\n Osteopetrosis (marble bone disease) is a rare hereditary disorder of diminished osteoclast function characterized by deficient bone resorption.[1] In this patient, the plain radiograph of the right femur shows a marked, diffuse increase in the density of the osseous structures, and evidence of refracture is noted through the proximal mid-diaphyseal/subtrochanteric region (Figure 1). A similar pattern, with increased bone density (which gives the bones a very bright appearance), is noted on the radiograph of the pelvis (Figure 2).\nFigure 1.\nFigure 1.\nFigure 2.\nFigure 2.\nFigure 3.\nFigure 3.\nA review of past radiographs of the cervical spine (Figure 3) reveals diffusely increased density of all osseous structures, with a \"sandwich\" appearance (presence of an extreme and uniform increase in radiographic density at the superior and inferior margins) of the upper vertebrae (ie, C2-C4), and a healed posterior spinous process fracture of the sixth and seventh cervical vertebra.\nAs a consequence of deficient bone resorption resulting from diminished osteoclast function, bone modeling and remodeling are impaired in osteopetrosis. Defective bone turnover results in skeletal fragility, despite the increase in bone mass. The condition is a heterogeneous disorder encompassing different molecular lesions (more than 14, some with human homologues, have been identified in the murine model) and a wide range of clinical features in its phenotypic manifestation in affected individuals.[1]\nIn humans, three distinct forms of the disease account for most cases of osteopetrosis; these forms, based on age and clinical features, are adult-onset, infantile, and intermediate. The adult-onset form (benign osteopetrosis, also called \"Albers-Schönberg disease\") is inherited in an autosomal dominant pattern and is often recognized only incidentally. In most cases, it is due to an autosomal dominant single gene mutation of the chloride channel 7 (CLC7) gene, which is involved in a regulatory pathway for bone remodeling. Homozygous mutations of this gene are one cause of the severe infantile form of the disease. Typically, no evidence of bone marrow failure is found.\nApproximately 50% of patients with infantile osteopetrosis are asymptomatic. The diagnosis is often made incidentally (usually in late adolescence) because radiologic abnormalities start appearing in childhood in the setting of fractures sustained by the individual, or it is made as a result of the development of osteomyelitis, especially of the mandible. In other patients, the diagnosis may be made earlier in life, on the basis of the family history.\nThe term \"benign\" is a misnomer. Bony defects are common and include neuropathies caused by cranial nerve entrapment, with specific problems, including deafness and facial palsy. Carpal tunnel syndrome and osteoarthritis also occur. The bones are fragile and may fracture easily;[2] approximately 40% of patients have recurrent fractures. Bone marrow function is not compromised.[1]\nOther manifestations include vision impairment caused by retinal degeneration and psychomotor retardation. In benign osteopetrosis, laboratory data may reveal elevated alkaline phosphatase levels, as well as elevated levels of acid phosphatase and creatinine kinase caused by increased release from defective osteoclasts.\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n**Figure 3.** \n \n\n\n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 934821,
"choiceText": "Primary hypoparathyroidism",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 934823,
"choiceText": "Paget disease ",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 934825,
"choiceText": "Benign osteopetrosis",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 934827,
"choiceText": "Lead toxicity",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 295739,
"questionText": "What is the likely underlying diagnosis for the patient's fractures? ",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 49-Year-Old Man With Pain at the Site of a Previous Fracture"
},
{
"authors": "Maria Romanova, MD",
"content": [
"Infantile and intermediate osteopetrosis are inherited in an autosomal recessive fashion; they carry a poor prognosis and high mortality. These forms of the disease are diagnosed early in life. Bone marrow failure often results, with associated bony abnormalities, hydrocephalus, proptosis, delayed dentition, blindness due to retinal detachment, and extreme bone fragility. Failure to thrive and growth retardation are also symptoms.",
"Nasal stuffiness caused by mastoid and paranasal sinus malformation is often the presenting feature. Neuropathies related to cranial nerve entrapment occur because the foramina of the skull fail to widen completely, with manifestations that include deafness, proptosis, and hydrocephalus. Normal dentition may be delayed, and osteomyelitis of the mandible is commonly seen because of an abnormal blood supply. The bones are fragile and can fracture easily.[1]",
"Bone marrow tends to be replaced by defective osseous tissue, which leads to bone marrow failure with resultant pancytopenia. Patients may have anemia, a tendency to bruise easily, and bleeding resulting from thrombocytopenia; recurrent infections may occur because of inherent defects in the immune system. Extramedullary hematopoiesis may also be seen, with resultant hepatosplenomegaly, hypersplenism, and hemolysis. Other physical findings include short stature, frontal bossing, a large head, nystagmus, and genu valgum. Laboratory findings in infantile osteopetrosis include hypocalcemia, elevated parathyroid hormone levels (secondary hyperparathyroidism), and high acid phosphatase and creatinine kinase levels.[1]",
"Plain radiographs are usually diagnostic and reveal generalized osteosclerosis, although findings may vary by subtype. The cranium is usually thickened and dense, especially at the base, and the paranasal and mastoid sinuses are underpneumatized. On lateral views, vertebral radiographs may show a \"bone-in-bone\" (endobone) configuration or end-plate sclerosis causing a \"rugger-jersey\" appearance. Similarly, other bones may also be uniformly sclerotic, but alternating sclerotic and lucent bands may be noted in the iliac wings and near the ends of the long bones. The radiographs may also show evidence of frequent fractures or osteomyelitis.",
"MRI can be used to assess bones over time, especially after bone marrow transplantation. On histologic examination, failure of osteoclasts to resorb skeletal tissue is the pathognomonic feature of osteopetrosis. Remnants of mineralized primary spongiosa can be seen as islands of calcified cartilage within mature bone. Woven bone is commonly seen. The number of osteoclasts can be increased, normal, or even decreased."
],
"date": "January 14, 2016",
"figures": [],
"markdown": "# A 49-Year-Old Man With Pain at the Site of a Previous Fracture\n\n **Authors:** Maria Romanova, MD \n **Date:** January 14, 2016\n\n ## Content\n\n Infantile and intermediate osteopetrosis are inherited in an autosomal recessive fashion; they carry a poor prognosis and high mortality. These forms of the disease are diagnosed early in life. Bone marrow failure often results, with associated bony abnormalities, hydrocephalus, proptosis, delayed dentition, blindness due to retinal detachment, and extreme bone fragility. Failure to thrive and growth retardation are also symptoms.\nNasal stuffiness caused by mastoid and paranasal sinus malformation is often the presenting feature. Neuropathies related to cranial nerve entrapment occur because the foramina of the skull fail to widen completely, with manifestations that include deafness, proptosis, and hydrocephalus. Normal dentition may be delayed, and osteomyelitis of the mandible is commonly seen because of an abnormal blood supply. The bones are fragile and can fracture easily.[1]\nBone marrow tends to be replaced by defective osseous tissue, which leads to bone marrow failure with resultant pancytopenia. Patients may have anemia, a tendency to bruise easily, and bleeding resulting from thrombocytopenia; recurrent infections may occur because of inherent defects in the immune system. Extramedullary hematopoiesis may also be seen, with resultant hepatosplenomegaly, hypersplenism, and hemolysis. Other physical findings include short stature, frontal bossing, a large head, nystagmus, and genu valgum. Laboratory findings in infantile osteopetrosis include hypocalcemia, elevated parathyroid hormone levels (secondary hyperparathyroidism), and high acid phosphatase and creatinine kinase levels.[1]\nPlain radiographs are usually diagnostic and reveal generalized osteosclerosis, although findings may vary by subtype. The cranium is usually thickened and dense, especially at the base, and the paranasal and mastoid sinuses are underpneumatized. On lateral views, vertebral radiographs may show a \"bone-in-bone\" (endobone) configuration or end-plate sclerosis causing a \"rugger-jersey\" appearance. Similarly, other bones may also be uniformly sclerotic, but alternating sclerotic and lucent bands may be noted in the iliac wings and near the ends of the long bones. The radiographs may also show evidence of frequent fractures or osteomyelitis.\nMRI can be used to assess bones over time, especially after bone marrow transplantation. On histologic examination, failure of osteoclasts to resorb skeletal tissue is the pathognomonic feature of osteopetrosis. Remnants of mineralized primary spongiosa can be seen as islands of calcified cartilage within mature bone. Woven bone is commonly seen. The number of osteoclasts can be increased, normal, or even decreased.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 49-Year-Old Man With Pain at the Site of a Previous Fracture"
},
{
"authors": "Maria Romanova, MD",
"content": [
"On initial presentation, exclusion of other diseases that result in a similar \"bright bone\" appearance on radiography is important. The causes of diffuse osteosclerosis include hypervitaminosis D, hypoparathyroidism, myelofibrosis, Paget disease, lead toxicity, diffuse skeletal metastasis of breast or prostate cancer, pseudohypoparathyroidism, fluoride toxicity, beryllium toxicity, sickle cell disease, and leukemia.",
"A case report has also described abnormal bone modeling and increased bone density, with histologic features of drug-induced osteopetrosis, in a 12-year-old boy treated with bisphosphonates.[3] When given for an extended period, agents that inhibit the recruitment and function of osteoclasts may cause a clinical picture similar to that of heritable osteopetrosis.[4]",
"Once other causes of increased bone density are excluded, patients with osteopetrosis do not require additional testing. Benign osteopetrosis requires no treatment except in patients who present with complications.",
"Infantile osteopetrosis warrants treatment because of the adverse outcomes associated with the disease. Vitamin D (calcitriol) appears to help by stimulating dormant osteoclasts, thereby stimulating bone resorption. Treatment with gamma-interferon has produced long-term benefits by improving leukocyte function and decreasing the incidence of new infections. Combination therapy with calcitriol often results in a decrease in trabecular bone volume and an increase in bone marrow volume. This effect causes increases in hemoglobin, platelet counts, and survival rates. Erythropoietin can be used to correct anemia.",
"Corticosteroids have been used to stimulate bone resorption and treat anemia because of their effect in reducing the destruction of erythrocytes in the reticuloendothelial system. Internal and external fixations have been used for the treatment of fractures, with excellent results.[2,5] Despite increased susceptibility to fractures, bone healing appears to proceed normally, although impaired osteoclast functioning eventually leads to osteopetrotic bone. Healthcare providers caring for adult patients with marble bone disease (osteoporosis) should also be aware of potential bone fragility and an increased propensity to develop osteomyelitis.",
"The patient in this case underwent an extensive evaluation for the noted changes, including several laboratory investigations that were within normal limits, to rule out other etiologies. Tests in the evaluation included parathyroid hormone, calcium, 25-hydroxyvitamin D, 1,25-dihydroxyvitamin D, serum fluoride, total protein, and prostate-specific antigen levels. A bone scan was performed and was negative for skeletal metastases. At this presentation, the patient unfortunately refused a repeat surgical intervention, and he remained wheelchair-bound 4 months later. Follow-up radiographs (not pictured) showed interval healing of the fracture, without complications."
],
"date": "January 14, 2016",
"figures": [],
"markdown": "# A 49-Year-Old Man With Pain at the Site of a Previous Fracture\n\n **Authors:** Maria Romanova, MD \n **Date:** January 14, 2016\n\n ## Content\n\n On initial presentation, exclusion of other diseases that result in a similar \"bright bone\" appearance on radiography is important. The causes of diffuse osteosclerosis include hypervitaminosis D, hypoparathyroidism, myelofibrosis, Paget disease, lead toxicity, diffuse skeletal metastasis of breast or prostate cancer, pseudohypoparathyroidism, fluoride toxicity, beryllium toxicity, sickle cell disease, and leukemia.\nA case report has also described abnormal bone modeling and increased bone density, with histologic features of drug-induced osteopetrosis, in a 12-year-old boy treated with bisphosphonates.[3] When given for an extended period, agents that inhibit the recruitment and function of osteoclasts may cause a clinical picture similar to that of heritable osteopetrosis.[4]\nOnce other causes of increased bone density are excluded, patients with osteopetrosis do not require additional testing. Benign osteopetrosis requires no treatment except in patients who present with complications.\nInfantile osteopetrosis warrants treatment because of the adverse outcomes associated with the disease. Vitamin D (calcitriol) appears to help by stimulating dormant osteoclasts, thereby stimulating bone resorption. Treatment with gamma-interferon has produced long-term benefits by improving leukocyte function and decreasing the incidence of new infections. Combination therapy with calcitriol often results in a decrease in trabecular bone volume and an increase in bone marrow volume. This effect causes increases in hemoglobin, platelet counts, and survival rates. Erythropoietin can be used to correct anemia.\nCorticosteroids have been used to stimulate bone resorption and treat anemia because of their effect in reducing the destruction of erythrocytes in the reticuloendothelial system. Internal and external fixations have been used for the treatment of fractures, with excellent results.[2,5] Despite increased susceptibility to fractures, bone healing appears to proceed normally, although impaired osteoclast functioning eventually leads to osteopetrotic bone. Healthcare providers caring for adult patients with marble bone disease (osteoporosis) should also be aware of potential bone fragility and an increased propensity to develop osteomyelitis.\nThe patient in this case underwent an extensive evaluation for the noted changes, including several laboratory investigations that were within normal limits, to rule out other etiologies. Tests in the evaluation included parathyroid hormone, calcium, 25-hydroxyvitamin D, 1,25-dihydroxyvitamin D, serum fluoride, total protein, and prostate-specific antigen levels. A bone scan was performed and was negative for skeletal metastases. At this presentation, the patient unfortunately refused a repeat surgical intervention, and he remained wheelchair-bound 4 months later. Follow-up radiographs (not pictured) showed interval healing of the fracture, without complications.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 934829,
"choiceText": "Infection",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 934831,
"choiceText": "Nonaccidental trauma\r\n",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 934833,
"choiceText": "Bone marrow failure",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 934835,
"choiceText": "Leukemia",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "In patients with osteopetrosis, bone marrow tends to be replaced by defective osseous tissue, which leads to bone marrow failure with resultant pancytopenia. Patients may have anemia, a tendency to bruise easily, and bleeding resulting from thrombocytopenia. Recurrent infections may occur because of immunocompromise, but the patient's symptoms do not suggest acute infection.<br><br>\r\n\r\nNonaccidental trauma should always be considered when evaluating children for poorly explained injuries; however, gum bleeding, fatigue, and the patient's history of osteopetrosis point to pancytopenia secondary to bone marrow failure as a more likely etiology. Likewise, leukemia is a consideration in this child, but his existing disease is the more likely explanation.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 295741,
"questionText": "A 12-year-old boy with intermediate osteopetrosis presents for a routine visit to his primary care provider. His mother notes that he has been acting increasingly fatigued for the past few months. His examination is significant for bruises in varying stages of healing. His mother had seen the bruises, but assumed they were from playing at school. The patient does not know how he got them. Upon further questioning, he has noted bleeding while brushing his teeth for the past couple of weeks. Which of the following is the most likely explanation for the boy's symptoms?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 934837,
"choiceText": "Bone marrow aspiration",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 934839,
"choiceText": "Whole-body CT",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 934841,
"choiceText": "Plain radiography",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 934843,
"choiceText": "MRI",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The adult-onset form (benign osteopetrosis, also called \"Albers-Schönberg disease\") is inherited in an autosomal dominant pattern and is often only recognized incidentally. Plain radiographs are usually diagnostic and reveal generalized osteosclerosis, although findings may vary by subtype. Typically, there is no evidence of bone marrow failure. MRI can also be used to assess the bones over time but is not indicated as an initial testing modality.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 295743,
"questionText": "A 15-year-old high school cross-country runner presents to the emergency department via ambulance after a fall. He clinically has a femur fracture, but he reports that the fall resulted from tripping over loose rocks and landing on grass, and he described it as \"no big deal.\" While obtaining a family history, he reports that his father has some type of \"bone problem.\" Which of the following tests is most appropriate to initially diagnose both his femur fracture and suspected underlying osteopetrosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 49-Year-Old Man With Pain at the Site of a Previous Fracture"
},
{
"authors": "Maria Romanova, MD",
"content": [],
"date": "January 14, 2016",
"figures": [],
"markdown": "# A 49-Year-Old Man With Pain at the Site of a Previous Fracture\n\n **Authors:** Maria Romanova, MD \n **Date:** January 14, 2016\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 934829,
"choiceText": "Infection",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 934831,
"choiceText": "Nonaccidental trauma\r\n",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 934833,
"choiceText": "Bone marrow failure",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 934835,
"choiceText": "Leukemia",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "In patients with osteopetrosis, bone marrow tends to be replaced by defective osseous tissue, which leads to bone marrow failure with resultant pancytopenia. Patients may have anemia, a tendency to bruise easily, and bleeding resulting from thrombocytopenia. Recurrent infections may occur because of immunocompromise, but the patient's symptoms do not suggest acute infection.<br><br>\r\n\r\nNonaccidental trauma should always be considered when evaluating children for poorly explained injuries; however, gum bleeding, fatigue, and the patient's history of osteopetrosis point to pancytopenia secondary to bone marrow failure as a more likely etiology. Likewise, leukemia is a consideration in this child, but his existing disease is the more likely explanation.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 295741,
"questionText": "A 12-year-old boy with intermediate osteopetrosis presents for a routine visit to his primary care provider. His mother notes that he has been acting increasingly fatigued for the past few months. His examination is significant for bruises in varying stages of healing. His mother had seen the bruises, but assumed they were from playing at school. The patient does not know how he got them. Upon further questioning, he has noted bleeding while brushing his teeth for the past couple of weeks. Which of the following is the most likely explanation for the boy's symptoms?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 934837,
"choiceText": "Bone marrow aspiration",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 934839,
"choiceText": "Whole-body CT",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 934841,
"choiceText": "Plain radiography",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 934843,
"choiceText": "MRI",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The adult-onset form (benign osteopetrosis, also called \"Albers-Schönberg disease\") is inherited in an autosomal dominant pattern and is often only recognized incidentally. Plain radiographs are usually diagnostic and reveal generalized osteosclerosis, although findings may vary by subtype. Typically, there is no evidence of bone marrow failure. MRI can also be used to assess the bones over time but is not indicated as an initial testing modality.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 295743,
"questionText": "A 15-year-old high school cross-country runner presents to the emergency department via ambulance after a fall. He clinically has a femur fracture, but he reports that the fall resulted from tripping over loose rocks and landing on grass, and he described it as \"no big deal.\" While obtaining a family history, he reports that his father has some type of \"bone problem.\" Which of the following tests is most appropriate to initially diagnose both his femur fracture and suspected underlying osteopetrosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 49-Year-Old Man With Pain at the Site of a Previous Fracture"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 934821,
"choiceText": "Primary hypoparathyroidism",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 934823,
"choiceText": "Paget disease ",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 934825,
"choiceText": "Benign osteopetrosis",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 934827,
"choiceText": "Lead toxicity",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 295739,
"questionText": "What is the likely underlying diagnosis for the patient's fractures? ",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 934829,
"choiceText": "Infection",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 934831,
"choiceText": "Nonaccidental trauma\r\n",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 934833,
"choiceText": "Bone marrow failure",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 934835,
"choiceText": "Leukemia",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "In patients with osteopetrosis, bone marrow tends to be replaced by defective osseous tissue, which leads to bone marrow failure with resultant pancytopenia. Patients may have anemia, a tendency to bruise easily, and bleeding resulting from thrombocytopenia. Recurrent infections may occur because of immunocompromise, but the patient's symptoms do not suggest acute infection.<br><br>\r\n\r\nNonaccidental trauma should always be considered when evaluating children for poorly explained injuries; however, gum bleeding, fatigue, and the patient's history of osteopetrosis point to pancytopenia secondary to bone marrow failure as a more likely etiology. Likewise, leukemia is a consideration in this child, but his existing disease is the more likely explanation.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 295741,
"questionText": "A 12-year-old boy with intermediate osteopetrosis presents for a routine visit to his primary care provider. His mother notes that he has been acting increasingly fatigued for the past few months. His examination is significant for bruises in varying stages of healing. His mother had seen the bruises, but assumed they were from playing at school. The patient does not know how he got them. Upon further questioning, he has noted bleeding while brushing his teeth for the past couple of weeks. Which of the following is the most likely explanation for the boy's symptoms?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 934837,
"choiceText": "Bone marrow aspiration",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 934839,
"choiceText": "Whole-body CT",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 934841,
"choiceText": "Plain radiography",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 934843,
"choiceText": "MRI",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The adult-onset form (benign osteopetrosis, also called \"Albers-Schönberg disease\") is inherited in an autosomal dominant pattern and is often only recognized incidentally. Plain radiographs are usually diagnostic and reveal generalized osteosclerosis, although findings may vary by subtype. Typically, there is no evidence of bone marrow failure. MRI can also be used to assess the bones over time but is not indicated as an initial testing modality.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 295743,
"questionText": "A 15-year-old high school cross-country runner presents to the emergency department via ambulance after a fall. He clinically has a femur fracture, but he reports that the fall resulted from tripping over loose rocks and landing on grass, and he described it as \"no big deal.\" While obtaining a family history, he reports that his father has some type of \"bone problem.\" Which of the following tests is most appropriate to initially diagnose both his femur fracture and suspected underlying osteopetrosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
856954 | /viewarticle/856954 | [
{
"authors": "Timothy C. Petersen, MD; Romesh K. Khardori, MD, PhD",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to recognize accurately. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"A 44-year-old white man with a medical history remarkable for hypertension and dyslipidemia presents to a local emergency department with a chief symptom of weakness. He states that he recently recovered from a sore throat that started 1 week ago, but he has since developed fatigue and global weakness.",
"The patient fell 1 day ago and struck his head; he attributes the fall to his weakness. He denies other neurologic changes, including headache, vision changes, sensory changes, focal motor weakness, and dizziness. Fluid and nutrient intake are noted to be normal. He states that he is adherent with his usual home regimen of lisinopril and simvastatin. He smokes 10 cigarettes per day but denies illicit drug use and ethanol intake.",
"A review of systems is negative for fever, appetite changes, chest pain, palpitations, diarrhea, and nausea."
],
"date": "February 14, 2019",
"figures": [],
"markdown": "# A 44-Year-Old Man Who Had a Mysterious Accident\n\n **Authors:** Timothy C. Petersen, MD; Romesh K. Khardori, MD, PhD \n **Date:** February 14, 2019\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to recognize accurately. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nA 44-year-old white man with a medical history remarkable for hypertension and dyslipidemia presents to a local emergency department with a chief symptom of weakness. He states that he recently recovered from a sore throat that started 1 week ago, but he has since developed fatigue and global weakness.\nThe patient fell 1 day ago and struck his head; he attributes the fall to his weakness. He denies other neurologic changes, including headache, vision changes, sensory changes, focal motor weakness, and dizziness. Fluid and nutrient intake are noted to be normal. He states that he is adherent with his usual home regimen of lisinopril and simvastatin. He smokes 10 cigarettes per day but denies illicit drug use and ethanol intake.\nA review of systems is negative for fever, appetite changes, chest pain, palpitations, diarrhea, and nausea.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 44-Year-Old Man Who Had a Mysterious Accident"
},
{
"authors": "Timothy C. Petersen, MD; Romesh K. Khardori, MD, PhD",
"content": [
"Vital signs are remarkable for a blood pressure of 134/77 mm Hg, a heart rate of 67 beats/min, and a temperature of 97.6°F (36.4°C). The physical examination reveals an alert, well-nourished, comfortable-appearing male of lean habitus. He has a minor abrasion on his right forehead with no other traumatic changes, and his mucous membranes are moist. His speech is somewhat slow in cadence and slurred.",
"The patient's visual fields and extraocular movements are grossly intact. The remainder of his neurologic examination is nonfocal, including normal reflexes and gait. No extra heart sounds, jugular venous distention, or peripheral edema is observed.",
"Initial laboratory evaluation demonstrates the following:",
"Sodium level: 104 mmol/L (normal range, 133-145 mmol/L)",
"Chloride level: 72 mmol/L (normal range, 98-110 mmol/L)",
"Potassium level: 4.9 mmol/L (normal range, 3.5-5.5 mmol/L)",
"Bicarbonate level: 21 mmol/L (normal range, 20-32 mmol/L)",
"Creatinine level: 0.5 mg/dL (normal range, 0.5-1.2 mg/dL)",
"Blood glucose level: 37 mg/dL (normal range, 65-99 mg/dL)",
"Blood urea nitrogen level: 9 mg/dL (normal range, 6-22 mg/dL)",
"The laboratory findings are verified on repeat measurement, except for a higher glucose level (106 mg/dL) after treatment.",
"CT of the head was also performed, due to the patient's history of falls and the presence of head trauma. This reveals a 3-cm pituitary adenoma (Figure 1).",
"Figure 1.",
"The patient is given enteral glucose, parenteral dextrose, and intravenous sodium chloride infusion and is admitted to the intensive care unit. The endocrinology service is consulted for further evaluation and management of his pituitary adenoma and hyponatremia.",
"Subsequent laboratory evaluation demonstrated the following:",
"Serum cortisol level: 8.27 µg/dL (12:30 PM)",
"Adrenocorticotropic hormone (ACTH) level: 20.7 pg/mL (normal range, 7.2-63.3 pg/mL)",
"Thyroid-stimulating hormone (TSH) level: 1.29 µU/ mL (normal range, 0.27-4.20 µU/mL)",
"Free thyroxine level: 1 ng/dL (normal range, 0.9-1.8 ng/dL)",
"Insulin-like growth factor 1 level: 72 ng/mL (normal range, 75-216 ng/mL)",
"Prolactin level: 32.8 ng/mL (normal range, 4-15.2 ng/mL)",
"Total testosterone level: 31 ng/dL (normal range, 398-1197 ng/dL)",
"Follicle-stimulating hormone level: 48.4 mIU/mL (normal range, 1.5-12.4 mIU/mL)",
"Serum osmolality level: 242 mOs/kg (normal range, 280-300 mOs/kg)",
"Random urine osmolality level: 431 mOs/kg (normal range, 200-1200 mOs/kg)",
"Random urine sodium level: < 20 mmol/L",
"Serum uric acid level: 4.1 mg/dL (normal range, 3.9-9 mg/dL)",
"MRI of the brain is performed, which corroborates the CT findings (Figure 2). Optic chiasm impingement was noted, but not gross cavernous sinus invasion.",
"Figure 2."
],
"date": "February 14, 2019",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/856/954/856954-Thumb1.png"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/856/954/856954-Thumb2.png"
}
],
"markdown": "# A 44-Year-Old Man Who Had a Mysterious Accident\n\n **Authors:** Timothy C. Petersen, MD; Romesh K. Khardori, MD, PhD \n **Date:** February 14, 2019\n\n ## Content\n\n Vital signs are remarkable for a blood pressure of 134/77 mm Hg, a heart rate of 67 beats/min, and a temperature of 97.6°F (36.4°C). The physical examination reveals an alert, well-nourished, comfortable-appearing male of lean habitus. He has a minor abrasion on his right forehead with no other traumatic changes, and his mucous membranes are moist. His speech is somewhat slow in cadence and slurred.\nThe patient's visual fields and extraocular movements are grossly intact. The remainder of his neurologic examination is nonfocal, including normal reflexes and gait. No extra heart sounds, jugular venous distention, or peripheral edema is observed.\nInitial laboratory evaluation demonstrates the following:\nSodium level: 104 mmol/L (normal range, 133-145 mmol/L)\nChloride level: 72 mmol/L (normal range, 98-110 mmol/L)\nPotassium level: 4.9 mmol/L (normal range, 3.5-5.5 mmol/L)\nBicarbonate level: 21 mmol/L (normal range, 20-32 mmol/L)\nCreatinine level: 0.5 mg/dL (normal range, 0.5-1.2 mg/dL)\nBlood glucose level: 37 mg/dL (normal range, 65-99 mg/dL)\nBlood urea nitrogen level: 9 mg/dL (normal range, 6-22 mg/dL)\nThe laboratory findings are verified on repeat measurement, except for a higher glucose level (106 mg/dL) after treatment.\nCT of the head was also performed, due to the patient's history of falls and the presence of head trauma. This reveals a 3-cm pituitary adenoma (Figure 1).\nFigure 1.\nThe patient is given enteral glucose, parenteral dextrose, and intravenous sodium chloride infusion and is admitted to the intensive care unit. The endocrinology service is consulted for further evaluation and management of his pituitary adenoma and hyponatremia.\nSubsequent laboratory evaluation demonstrated the following:\nSerum cortisol level: 8.27 µg/dL (12:30 PM)\nAdrenocorticotropic hormone (ACTH) level: 20.7 pg/mL (normal range, 7.2-63.3 pg/mL)\nThyroid-stimulating hormone (TSH) level: 1.29 µU/ mL (normal range, 0.27-4.20 µU/mL)\nFree thyroxine level: 1 ng/dL (normal range, 0.9-1.8 ng/dL)\nInsulin-like growth factor 1 level: 72 ng/mL (normal range, 75-216 ng/mL)\nProlactin level: 32.8 ng/mL (normal range, 4-15.2 ng/mL)\nTotal testosterone level: 31 ng/dL (normal range, 398-1197 ng/dL)\nFollicle-stimulating hormone level: 48.4 mIU/mL (normal range, 1.5-12.4 mIU/mL)\nSerum osmolality level: 242 mOs/kg (normal range, 280-300 mOs/kg)\nRandom urine osmolality level: 431 mOs/kg (normal range, 200-1200 mOs/kg)\nRandom urine sodium level: < 20 mmol/L\nSerum uric acid level: 4.1 mg/dL (normal range, 3.9-9 mg/dL)\nMRI of the brain is performed, which corroborates the CT findings (Figure 2). Optic chiasm impingement was noted, but not gross cavernous sinus invasion.\nFigure 2.\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 934263,
"choiceText": "Primary polydipsia",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 934265,
"choiceText": "Adrenal insufficiency",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 934267,
"choiceText": "Syndrome of inappropriate antidiuretic hormone secretion (SIADH)",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 934269,
"choiceText": "\"Tea and toast\" diet\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 295581,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 44-Year-Old Man Who Had a Mysterious Accident"
},
{
"authors": "Timothy C. Petersen, MD; Romesh K. Khardori, MD, PhD",
"content": [
"Hyponatremia is a common clinical entity that is present in as many as 30% of hospitalized patients and as many as 8% of ambulatory patients.[1] Discovering the underlying etiology may be difficult, which makes appropriate management challenging, and the presence of hyponatremia is associated with higher morbidity and mortality compared with eunatremic individuals.[2]",
"Hyponatremia is diagnosed when a patient's serum sodium value is less than the lower limit of normal for the laboratory used, typically less than 136 mmol/L.[3] Causative factors include, but are not limited to, decreased solute intake (such as in malnourished individuals—eg, beer potomania or a \"tea and toast\" diet), increased water intake (eg, primary polydipsia), impaired free water excretion (eg, SIADH), laboratory assay abnormalities (eg, severe hypertriglyceridemia or paraproteinemia), and translocation of solute (eg, hyperglycemia).[3,4]",
"Initial history and physical examination classifies patients into hypovolemic, euvolemic, or hypervolemic status. Workup should involve appropriate laboratory studies, including those listed in the case presentation above, as well as ancillary tests as the clinical picture dictates (eg, echocardiography in a patient with suspected acutely decompensated congestive heart failure). Comparing serum and urine osmolality in association with other laboratory and clinical findings allows for further division of hyponatremia according to overall plasma tonicity (hypotonic, isotonic, or hypertonic).",
"Hypovolemic hyponatremia generally results from low solute intake or increased urinary solute loss; hypervolemic hyponatremia generally results from end-organ dysfunction, such as cardiac, hepatic, or renal failure, or from decreased plasma oncotic pressure (eg, hypoalbuminemia). Pregnancy is also a hypervolemic state.[3]",
"Euvolemic hyponatremia is the most problematic category, given its still-broad differential diagnosis and often overlapping etiologies. A particular challenge is determining the presence or absence of SIADH, which is best considered a diagnosis of exclusion. As a protective measure, release of ADH—which is more appropriately referred to as \"arginine vasopressin\" (AVP)—is often physiologic (ie, appropriate) in certain circumstances, such as pain, nausea, stress, and hypovolemia, because AVP release promotes water and volume retention.[5] In addition, its release may result from conditions that result in relative hypovolemia, such as end-organ damage (as outlined above).[6] Adrenal insufficiency (particularly primary adrenal insufficiency) and hypothyroidism also frequently result in increased AVP secretion."
],
"date": "February 14, 2019",
"figures": [],
"markdown": "# A 44-Year-Old Man Who Had a Mysterious Accident\n\n **Authors:** Timothy C. Petersen, MD; Romesh K. Khardori, MD, PhD \n **Date:** February 14, 2019\n\n ## Content\n\n Hyponatremia is a common clinical entity that is present in as many as 30% of hospitalized patients and as many as 8% of ambulatory patients.[1] Discovering the underlying etiology may be difficult, which makes appropriate management challenging, and the presence of hyponatremia is associated with higher morbidity and mortality compared with eunatremic individuals.[2]\nHyponatremia is diagnosed when a patient's serum sodium value is less than the lower limit of normal for the laboratory used, typically less than 136 mmol/L.[3] Causative factors include, but are not limited to, decreased solute intake (such as in malnourished individuals—eg, beer potomania or a \"tea and toast\" diet), increased water intake (eg, primary polydipsia), impaired free water excretion (eg, SIADH), laboratory assay abnormalities (eg, severe hypertriglyceridemia or paraproteinemia), and translocation of solute (eg, hyperglycemia).[3,4]\nInitial history and physical examination classifies patients into hypovolemic, euvolemic, or hypervolemic status. Workup should involve appropriate laboratory studies, including those listed in the case presentation above, as well as ancillary tests as the clinical picture dictates (eg, echocardiography in a patient with suspected acutely decompensated congestive heart failure). Comparing serum and urine osmolality in association with other laboratory and clinical findings allows for further division of hyponatremia according to overall plasma tonicity (hypotonic, isotonic, or hypertonic).\nHypovolemic hyponatremia generally results from low solute intake or increased urinary solute loss; hypervolemic hyponatremia generally results from end-organ dysfunction, such as cardiac, hepatic, or renal failure, or from decreased plasma oncotic pressure (eg, hypoalbuminemia). Pregnancy is also a hypervolemic state.[3]\nEuvolemic hyponatremia is the most problematic category, given its still-broad differential diagnosis and often overlapping etiologies. A particular challenge is determining the presence or absence of SIADH, which is best considered a diagnosis of exclusion. As a protective measure, release of ADH—which is more appropriately referred to as \"arginine vasopressin\" (AVP)—is often physiologic (ie, appropriate) in certain circumstances, such as pain, nausea, stress, and hypovolemia, because AVP release promotes water and volume retention.[5] In addition, its release may result from conditions that result in relative hypovolemia, such as end-organ damage (as outlined above).[6] Adrenal insufficiency (particularly primary adrenal insufficiency) and hypothyroidism also frequently result in increased AVP secretion.\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 934263,
"choiceText": "Primary polydipsia",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 934265,
"choiceText": "Adrenal insufficiency",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 934267,
"choiceText": "Syndrome of inappropriate antidiuretic hormone secretion (SIADH)",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 934269,
"choiceText": "\"Tea and toast\" diet\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 295581,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 44-Year-Old Man Who Had a Mysterious Accident"
},
{
"authors": "Timothy C. Petersen, MD; Romesh K. Khardori, MD, PhD",
"content": [
"After excluding these and other possible causes of euvolemic hyponatremia with inappropriately concentrated urine (urine sodium > 40 mmol/L, urine osmolality > 100 mOsm/kg), the diagnosis of SIADH may be considered.[7] Appropriate evaluation for the cause of SIADH must then ensue, including careful review of implicated medications and consideration of an AVP-secreting tumor or intracranial pathology.[7]",
"The treatment for hyponatremia is case-specific, and targeted therapy for the underlying etiology accompanies sodium- and water-directed management. The degree of neurologic dysfunction caused by the resultant cerebral edema is another important consideration because it further guides management. Hyponatremic individuals experiencing seizures, coma, or other severe neurologic sequelae generally warrant more aggressive management (eg, with hypertonic saline); those with minor or no neurologic symptoms or signs generally benefit from more conservative management (eg, fluid restriction, controlled crystalloid infusion).[3]",
"Patients with chronic hyponatremia, generally defined as hyponatremia persisting for greater than 48 hours, typically have a more indolent course. Time allows the central nervous system to accommodate the low sodium levels by accumulation of other intracellular organic osmolytes,[8,9] which helps explain the relative clinical stability despite progressively worsening serum sodium status. However, because of this adaptation, rapid correction of serum sodium may result in central pontine myelinolysis, a potentially devastating complication that manifests classically as seizures or encephalopathy followed by pontine-related deficits (eg, dysarthria, dysphagia, oculomotor deficits, focal motor weakness, locked-in syndrome). In addition, characteristic findings are noted on MRI.[10]",
"To avoid central pontine myelinolysis, current guidelines recommend a rate of serum sodium correction to not exceed 0.5 mmol/L per hour, less than 10 mmol/L over 24 hours, and less than 18 mmol/L over 48 hours.[11] Of note, case reports describe central pontine myelinolysis despite sodium correction rates of 9-10 mmol/L per day, leading some to advocate for a slower rate of correction (< 8 mmol/L per day).[3]",
"Acute hyponatremia, in contrast, does not afford the same opportunity for accommodation, which results in relatively greater symptoms despite smaller changes in sodium levels.[3] Rapid correction of acute hyponatremia is usually well-tolerated; however, the absence of firm documentation of recently preceding eunatremia is often absent, and data supporting this approach are limited.[3] Exercising caution, with slow and deliberate correction of sodium, is still prudent—again, so long as severe neurologic compromise is not present."
],
"date": "February 14, 2019",
"figures": [],
"markdown": "# A 44-Year-Old Man Who Had a Mysterious Accident\n\n **Authors:** Timothy C. Petersen, MD; Romesh K. Khardori, MD, PhD \n **Date:** February 14, 2019\n\n ## Content\n\n After excluding these and other possible causes of euvolemic hyponatremia with inappropriately concentrated urine (urine sodium > 40 mmol/L, urine osmolality > 100 mOsm/kg), the diagnosis of SIADH may be considered.[7] Appropriate evaluation for the cause of SIADH must then ensue, including careful review of implicated medications and consideration of an AVP-secreting tumor or intracranial pathology.[7]\nThe treatment for hyponatremia is case-specific, and targeted therapy for the underlying etiology accompanies sodium- and water-directed management. The degree of neurologic dysfunction caused by the resultant cerebral edema is another important consideration because it further guides management. Hyponatremic individuals experiencing seizures, coma, or other severe neurologic sequelae generally warrant more aggressive management (eg, with hypertonic saline); those with minor or no neurologic symptoms or signs generally benefit from more conservative management (eg, fluid restriction, controlled crystalloid infusion).[3]\nPatients with chronic hyponatremia, generally defined as hyponatremia persisting for greater than 48 hours, typically have a more indolent course. Time allows the central nervous system to accommodate the low sodium levels by accumulation of other intracellular organic osmolytes,[8,9] which helps explain the relative clinical stability despite progressively worsening serum sodium status. However, because of this adaptation, rapid correction of serum sodium may result in central pontine myelinolysis, a potentially devastating complication that manifests classically as seizures or encephalopathy followed by pontine-related deficits (eg, dysarthria, dysphagia, oculomotor deficits, focal motor weakness, locked-in syndrome). In addition, characteristic findings are noted on MRI.[10]\nTo avoid central pontine myelinolysis, current guidelines recommend a rate of serum sodium correction to not exceed 0.5 mmol/L per hour, less than 10 mmol/L over 24 hours, and less than 18 mmol/L over 48 hours.[11] Of note, case reports describe central pontine myelinolysis despite sodium correction rates of 9-10 mmol/L per day, leading some to advocate for a slower rate of correction (< 8 mmol/L per day).[3]\nAcute hyponatremia, in contrast, does not afford the same opportunity for accommodation, which results in relatively greater symptoms despite smaller changes in sodium levels.[3] Rapid correction of acute hyponatremia is usually well-tolerated; however, the absence of firm documentation of recently preceding eunatremia is often absent, and data supporting this approach are limited.[3] Exercising caution, with slow and deliberate correction of sodium, is still prudent—again, so long as severe neurologic compromise is not present.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 44-Year-Old Man Who Had a Mysterious Accident"
},
{
"authors": "Timothy C. Petersen, MD; Romesh K. Khardori, MD, PhD",
"content": [
"In the setting of this patient's minimally symptomatic euvolemic hyponatremia, the presence of a pituitary macroadenoma with attendant hormonal deficits (growth hormone deficiency, hypogonadism) warranted concern that an endocrinopathy could be the etiology. Ensuring that thyroid and adrenal function are adequate is paramount in this circumstance.",
"The patient's TSH and free thyroxine values were within normal limits, supporting an intact hypothalamic-pituitary-thyroid axis. Although his serum cortisol and ACTH levels seemed reasonable at first glance, the presence of hypoglycemia (37 mg/dL on a venous blood sample) should have prompted a dramatic increase in ACTH and cortisol.[5] The presence of hypoglycemia and hyponatremia in the setting of a pituitary macroadenoma with relatively low serum cortisol make the diagnosis of adrenal insufficiency likely. A study from an endocrine group in Germany demonstrated that 28 of 139 patients (20.1%) with severe hyponatremia had central adrenal insufficiency as the etiology.[12]",
"Normal potassium level and blood pressure, which may be seen with primary adrenal insufficiency, do not rule out central adrenal insufficiency. This is due to preserved aldosterone secretion from the adrenal cortex, which responds to an intact renin/angiotensin system in addition to ACTH.[13] Hyponatremia in the setting of adrenal insufficiency results from interactions between cortisol and aquaporin channels, which yield decreased free water clearance and thus a dilutional hyponatremia, and a physiologic (not \"inappropriate\") elevation of ADH owing to central mechanisms.[14]",
"As such, a diagnosis of SIADH can only be made in a euvolemic patient once hormone deficiencies are sufficiently ruled out.[15] In addition, this patient's normal (not low) uric acid and blood urea nitrogen levels make this an unlikely diagnosis, as does the low random urine sodium level.",
"A \"tea and toast\" diet diagnosis typically accompanies a clearly malnourished or cachectic individual. Adequate dietary history should provide clues to this pathology, if available.",
"Primary polydipsia is a relatively common phenomenon that must be considered for causes of hyponatremia, particularly in patients with a known history of psychiatric disorders. It typically yields a euvolemic hyponatremia with dilute urine (low urine osmolality and urine sodium levels). The elevated urine osmolality in this case rules this out."
],
"date": "February 14, 2019",
"figures": [],
"markdown": "# A 44-Year-Old Man Who Had a Mysterious Accident\n\n **Authors:** Timothy C. Petersen, MD; Romesh K. Khardori, MD, PhD \n **Date:** February 14, 2019\n\n ## Content\n\n In the setting of this patient's minimally symptomatic euvolemic hyponatremia, the presence of a pituitary macroadenoma with attendant hormonal deficits (growth hormone deficiency, hypogonadism) warranted concern that an endocrinopathy could be the etiology. Ensuring that thyroid and adrenal function are adequate is paramount in this circumstance.\nThe patient's TSH and free thyroxine values were within normal limits, supporting an intact hypothalamic-pituitary-thyroid axis. Although his serum cortisol and ACTH levels seemed reasonable at first glance, the presence of hypoglycemia (37 mg/dL on a venous blood sample) should have prompted a dramatic increase in ACTH and cortisol.[5] The presence of hypoglycemia and hyponatremia in the setting of a pituitary macroadenoma with relatively low serum cortisol make the diagnosis of adrenal insufficiency likely. A study from an endocrine group in Germany demonstrated that 28 of 139 patients (20.1%) with severe hyponatremia had central adrenal insufficiency as the etiology.[12]\nNormal potassium level and blood pressure, which may be seen with primary adrenal insufficiency, do not rule out central adrenal insufficiency. This is due to preserved aldosterone secretion from the adrenal cortex, which responds to an intact renin/angiotensin system in addition to ACTH.[13] Hyponatremia in the setting of adrenal insufficiency results from interactions between cortisol and aquaporin channels, which yield decreased free water clearance and thus a dilutional hyponatremia, and a physiologic (not \"inappropriate\") elevation of ADH owing to central mechanisms.[14]\nAs such, a diagnosis of SIADH can only be made in a euvolemic patient once hormone deficiencies are sufficiently ruled out.[15] In addition, this patient's normal (not low) uric acid and blood urea nitrogen levels make this an unlikely diagnosis, as does the low random urine sodium level.\nA \"tea and toast\" diet diagnosis typically accompanies a clearly malnourished or cachectic individual. Adequate dietary history should provide clues to this pathology, if available.\nPrimary polydipsia is a relatively common phenomenon that must be considered for causes of hyponatremia, particularly in patients with a known history of psychiatric disorders. It typically yields a euvolemic hyponatremia with dilute urine (low urine osmolality and urine sodium levels). The elevated urine osmolality in this case rules this out.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 934287,
"choiceText": "Hypertonic (3%) saline infusion",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 934289,
"choiceText": "Urgent resection of pituitary adenoma",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 934291,
"choiceText": "Normal (0.9%) saline infusion",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 934293,
"choiceText": "Tolvaptan \r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "His remarkable adaptation suggests a chronic, progressive pathology. Hence, therapy for severe hyponatremia complicated by seizures or coma with hypertonic (3%) saline was not warranted. His sodium-avid state (as evidenced by his urine sodium level < 20 mmol/L) and need for gentle correction warranted normal (0.9%) saline infusion. <br><br>\r\nUrgent resection of pituitary adenoma was not advised. Given this patient's relative clinical stability, the risk for postoperative central diabetes insipidus with an attendant rapid rise in sodium indicated that surgery should be withheld until normalization of sodium occurs and persists. <br><br>\r\nTolvaptan is a vasopressin receptor antagonist and was not indicated in this patient with adrenal insufficiency as the likely etiology of his chronic hyponatremia.\r\n",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 295587,
"questionText": "In addition to corticosteroid therapy, which of the following is the most appropriate next step in management for this patient's hyponatremia?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 934295,
"choiceText": "Subclinical seizures",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 934297,
"choiceText": "Medication/iatrogenic",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 934299,
"choiceText": "Prolactinoma ",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 934301,
"choiceText": "Stalk effect\t\r\n",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "\"Stalk effect\" refers to the relatively mild elevation of prolactin that occurs owing to compression of the dopaminergic afferents to the adenohypophysis in the setting of mass effect.<sup>[16]</sup> This patient's pituitary adenoma was of sufficient size and location to cause reduced dopamine inhibition of prolactin secretion, thus resulting in hyperprolactinemia. <br><br>\r\nProlactinomas typically have serum prolactin elevation concordant with the size of the lesion. One would expect a serum prolactin value in the range of 200 ng/mL or greater from a prolactinoma of the size presented here.<sup>[5]</sup> In addition, the absence of clinical signs and symptoms of hyperprolactinemia renders this unlikely. Of note, laboratories using monoclonal immunoradiometric or chemiluminometric assays for serum prolactin measurements are subject to a phenomenon known as the \"hook effect,\" which results in an erroneously low lab value.<sup>[17]</sup><br><br>\r\nMedications may have an impact on serum prolactin values. The greatest offenders are atypical antipsychotics, such as risperidone; agents used for gastroparesis, such as metoclopramide; and verapamil.<sup>[5,18]</sup> This patient is not taking any implicated medications. <br><br>\r\n\r\nSubclinical seizures are generally not a cause of mildly elevated serum prolactin levels, although generalized tonic/clonic or partial complex seizures are.<sup>[19]</sup> The clinical history and presence of a macroadenoma suggest that the stalk effect is a more likely cause of the mild hyperprolactinemia. <br><br>\r\n\r\nThe patient received appropriate corticosteroid therapy and gentle normal saline infusions, along with frequent laboratory monitoring in the critical care setting. He experienced slow normalization of his serum sodium without any untoward sequelae. Surgery for his pituitary adenoma was delayed until at least 5 days of persistent near-normal serum sodium values were documented.<br><br>\r\n\r\nThe patient underwent a successful and uncomplicated transsphenoidal adenoma resection and was discharged home on corticosteroids with a normal serum sodium level. Pathologic examination revealed a pituitary adenoma that stained positive for follicle-stimulating hormone and luteinizing hormone and negative for prolactin.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 295589,
"questionText": "Which of the following is the most likely explanation for the elevated serum prolactin level?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 44-Year-Old Man Who Had a Mysterious Accident"
},
{
"authors": "Timothy C. Petersen, MD; Romesh K. Khardori, MD, PhD",
"content": [],
"date": "February 14, 2019",
"figures": [],
"markdown": "# A 44-Year-Old Man Who Had a Mysterious Accident\n\n **Authors:** Timothy C. Petersen, MD; Romesh K. Khardori, MD, PhD \n **Date:** February 14, 2019\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 934287,
"choiceText": "Hypertonic (3%) saline infusion",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 934289,
"choiceText": "Urgent resection of pituitary adenoma",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 934291,
"choiceText": "Normal (0.9%) saline infusion",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 934293,
"choiceText": "Tolvaptan \r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "His remarkable adaptation suggests a chronic, progressive pathology. Hence, therapy for severe hyponatremia complicated by seizures or coma with hypertonic (3%) saline was not warranted. His sodium-avid state (as evidenced by his urine sodium level < 20 mmol/L) and need for gentle correction warranted normal (0.9%) saline infusion. <br><br>\r\nUrgent resection of pituitary adenoma was not advised. Given this patient's relative clinical stability, the risk for postoperative central diabetes insipidus with an attendant rapid rise in sodium indicated that surgery should be withheld until normalization of sodium occurs and persists. <br><br>\r\nTolvaptan is a vasopressin receptor antagonist and was not indicated in this patient with adrenal insufficiency as the likely etiology of his chronic hyponatremia.\r\n",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 295587,
"questionText": "In addition to corticosteroid therapy, which of the following is the most appropriate next step in management for this patient's hyponatremia?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 934295,
"choiceText": "Subclinical seizures",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 934297,
"choiceText": "Medication/iatrogenic",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 934299,
"choiceText": "Prolactinoma ",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 934301,
"choiceText": "Stalk effect\t\r\n",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "\"Stalk effect\" refers to the relatively mild elevation of prolactin that occurs owing to compression of the dopaminergic afferents to the adenohypophysis in the setting of mass effect.<sup>[16]</sup> This patient's pituitary adenoma was of sufficient size and location to cause reduced dopamine inhibition of prolactin secretion, thus resulting in hyperprolactinemia. <br><br>\r\nProlactinomas typically have serum prolactin elevation concordant with the size of the lesion. One would expect a serum prolactin value in the range of 200 ng/mL or greater from a prolactinoma of the size presented here.<sup>[5]</sup> In addition, the absence of clinical signs and symptoms of hyperprolactinemia renders this unlikely. Of note, laboratories using monoclonal immunoradiometric or chemiluminometric assays for serum prolactin measurements are subject to a phenomenon known as the \"hook effect,\" which results in an erroneously low lab value.<sup>[17]</sup><br><br>\r\nMedications may have an impact on serum prolactin values. The greatest offenders are atypical antipsychotics, such as risperidone; agents used for gastroparesis, such as metoclopramide; and verapamil.<sup>[5,18]</sup> This patient is not taking any implicated medications. <br><br>\r\n\r\nSubclinical seizures are generally not a cause of mildly elevated serum prolactin levels, although generalized tonic/clonic or partial complex seizures are.<sup>[19]</sup> The clinical history and presence of a macroadenoma suggest that the stalk effect is a more likely cause of the mild hyperprolactinemia. <br><br>\r\n\r\nThe patient received appropriate corticosteroid therapy and gentle normal saline infusions, along with frequent laboratory monitoring in the critical care setting. He experienced slow normalization of his serum sodium without any untoward sequelae. Surgery for his pituitary adenoma was delayed until at least 5 days of persistent near-normal serum sodium values were documented.<br><br>\r\n\r\nThe patient underwent a successful and uncomplicated transsphenoidal adenoma resection and was discharged home on corticosteroids with a normal serum sodium level. Pathologic examination revealed a pituitary adenoma that stained positive for follicle-stimulating hormone and luteinizing hormone and negative for prolactin.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 295589,
"questionText": "Which of the following is the most likely explanation for the elevated serum prolactin level?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 44-Year-Old Man Who Had a Mysterious Accident"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 934263,
"choiceText": "Primary polydipsia",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 934265,
"choiceText": "Adrenal insufficiency",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 934267,
"choiceText": "Syndrome of inappropriate antidiuretic hormone secretion (SIADH)",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 934269,
"choiceText": "\"Tea and toast\" diet\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 295581,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 934287,
"choiceText": "Hypertonic (3%) saline infusion",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 934289,
"choiceText": "Urgent resection of pituitary adenoma",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 934291,
"choiceText": "Normal (0.9%) saline infusion",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 934293,
"choiceText": "Tolvaptan \r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "His remarkable adaptation suggests a chronic, progressive pathology. Hence, therapy for severe hyponatremia complicated by seizures or coma with hypertonic (3%) saline was not warranted. His sodium-avid state (as evidenced by his urine sodium level < 20 mmol/L) and need for gentle correction warranted normal (0.9%) saline infusion. <br><br>\r\nUrgent resection of pituitary adenoma was not advised. Given this patient's relative clinical stability, the risk for postoperative central diabetes insipidus with an attendant rapid rise in sodium indicated that surgery should be withheld until normalization of sodium occurs and persists. <br><br>\r\nTolvaptan is a vasopressin receptor antagonist and was not indicated in this patient with adrenal insufficiency as the likely etiology of his chronic hyponatremia.\r\n",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 295587,
"questionText": "In addition to corticosteroid therapy, which of the following is the most appropriate next step in management for this patient's hyponatremia?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 934295,
"choiceText": "Subclinical seizures",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 934297,
"choiceText": "Medication/iatrogenic",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 934299,
"choiceText": "Prolactinoma ",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 934301,
"choiceText": "Stalk effect\t\r\n",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "\"Stalk effect\" refers to the relatively mild elevation of prolactin that occurs owing to compression of the dopaminergic afferents to the adenohypophysis in the setting of mass effect.<sup>[16]</sup> This patient's pituitary adenoma was of sufficient size and location to cause reduced dopamine inhibition of prolactin secretion, thus resulting in hyperprolactinemia. <br><br>\r\nProlactinomas typically have serum prolactin elevation concordant with the size of the lesion. One would expect a serum prolactin value in the range of 200 ng/mL or greater from a prolactinoma of the size presented here.<sup>[5]</sup> In addition, the absence of clinical signs and symptoms of hyperprolactinemia renders this unlikely. Of note, laboratories using monoclonal immunoradiometric or chemiluminometric assays for serum prolactin measurements are subject to a phenomenon known as the \"hook effect,\" which results in an erroneously low lab value.<sup>[17]</sup><br><br>\r\nMedications may have an impact on serum prolactin values. The greatest offenders are atypical antipsychotics, such as risperidone; agents used for gastroparesis, such as metoclopramide; and verapamil.<sup>[5,18]</sup> This patient is not taking any implicated medications. <br><br>\r\n\r\nSubclinical seizures are generally not a cause of mildly elevated serum prolactin levels, although generalized tonic/clonic or partial complex seizures are.<sup>[19]</sup> The clinical history and presence of a macroadenoma suggest that the stalk effect is a more likely cause of the mild hyperprolactinemia. <br><br>\r\n\r\nThe patient received appropriate corticosteroid therapy and gentle normal saline infusions, along with frequent laboratory monitoring in the critical care setting. He experienced slow normalization of his serum sodium without any untoward sequelae. Surgery for his pituitary adenoma was delayed until at least 5 days of persistent near-normal serum sodium values were documented.<br><br>\r\n\r\nThe patient underwent a successful and uncomplicated transsphenoidal adenoma resection and was discharged home on corticosteroids with a normal serum sodium level. Pathologic examination revealed a pituitary adenoma that stained positive for follicle-stimulating hormone and luteinizing hormone and negative for prolactin.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 295589,
"questionText": "Which of the following is the most likely explanation for the elevated serum prolactin level?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
856057 | /viewarticle/856057 | [
{
"authors": "Jaime Shalkow, MD",
"content": [
"Editor's Note:\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.",
"The parents of a 7-month-old boy bring him to the emergency department (ED) owing to recent lethargy and an 18-hour history of pain that his parents believe stems from his abdomen. He is a previously healthy and well-nourished boy without any significant medical history. He was born at term via vaginal delivery, and his neuromotor development is adequate for his age. His vaccinations are up to date.",
"Four weeks ago, he developed a common cold, with runny nose, low-grade fever, and sneezing, which subsided in 3 days with symptomatic treatment (acetaminophen, hydration, and physical measures). The afternoon before arrival to the ED, he appeared to be in significant pain, accompanied by inconsolable crying and drawing up his legs toward the abdomen. Between painful episodes, the child behaved relatively normally and free of pain; he was even able to fall asleep before waking again with pain. Since then, he has vomited gastric contents four times. Two hours before admission, he became lethargic, just waking up to cry during the colic episodes."
],
"date": "December 17, 2015",
"figures": [],
"markdown": "# 7-Month-Old Boy With Abdominal Pain and Rectal Bleeding\n\n **Authors:** Jaime Shalkow, MD \n **Date:** December 17, 2015\n\n ## Content\n\n Editor's Note:\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.\nThe parents of a 7-month-old boy bring him to the emergency department (ED) owing to recent lethargy and an 18-hour history of pain that his parents believe stems from his abdomen. He is a previously healthy and well-nourished boy without any significant medical history. He was born at term via vaginal delivery, and his neuromotor development is adequate for his age. His vaccinations are up to date.\nFour weeks ago, he developed a common cold, with runny nose, low-grade fever, and sneezing, which subsided in 3 days with symptomatic treatment (acetaminophen, hydration, and physical measures). The afternoon before arrival to the ED, he appeared to be in significant pain, accompanied by inconsolable crying and drawing up his legs toward the abdomen. Between painful episodes, the child behaved relatively normally and free of pain; he was even able to fall asleep before waking again with pain. Since then, he has vomited gastric contents four times. Two hours before admission, he became lethargic, just waking up to cry during the colic episodes.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "7-Month-Old Boy With Abdominal Pain and Rectal Bleeding"
},
{
"authors": "Jaime Shalkow, MD",
"content": [
"Upon physical examination, the boy is a well-nourished and well-hydrated infant. His vital signs show a heart rate of 180 beats/min, a respiratory rate of 32 breaths/min, systolic blood pressure of 110 mm Hg, and temperature of 98.6°F .",
"Figure 1.",
"Figure 1.",
"Figure 2.",
"Figure 2.",
"Figure 3.",
"Figure 3.",
"Between episodes of cramps, he sleeps tiredly and his abdomen is soft; during the acute pain phases, he cries inconsolably and draws his legs toward the abdomen, with rigid abdominal muscles. Peristalsis is augmented and high-pitched. No masses are felt, and no rebound tenderness is observed. He passes a small amount of mucous bloody stool (Figure 1). A complete blood cell count demonstrates mild leukocytosis, with a left shift. Electrolytes and the rest of the chemical panel are normal. Plain abdominal radiography and ultrasound images are shown (Figures 2 and 3)."
],
"date": "December 17, 2015",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/856/057/856057-Thumb1.png"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/856/057/856057-Thumb2.png"
},
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/856/057/856057-Thumb3.png"
}
],
"markdown": "# 7-Month-Old Boy With Abdominal Pain and Rectal Bleeding\n\n **Authors:** Jaime Shalkow, MD \n **Date:** December 17, 2015\n\n ## Content\n\n Upon physical examination, the boy is a well-nourished and well-hydrated infant. His vital signs show a heart rate of 180 beats/min, a respiratory rate of 32 breaths/min, systolic blood pressure of 110 mm Hg, and temperature of 98.6°F .\nFigure 1.\nFigure 1.\nFigure 2.\nFigure 2.\nFigure 3.\nFigure 3.\nBetween episodes of cramps, he sleeps tiredly and his abdomen is soft; during the acute pain phases, he cries inconsolably and draws his legs toward the abdomen, with rigid abdominal muscles. Peristalsis is augmented and high-pitched. No masses are felt, and no rebound tenderness is observed. He passes a small amount of mucous bloody stool (Figure 1). A complete blood cell count demonstrates mild leukocytosis, with a left shift. Electrolytes and the rest of the chemical panel are normal. Plain abdominal radiography and ultrasound images are shown (Figures 2 and 3).\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n**Figure 3.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 927005,
"choiceText": "Acute gastroenteritis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 927007,
"choiceText": "Meckel diverticulum",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 927009,
"choiceText": "Intestinal polyps",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 927011,
"choiceText": "Intussusception\r\n",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 927013,
"choiceText": "Intestinal volvulus",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 293035,
"questionText": "On the basis of the history, physical examination, and workup, what is the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "7-Month-Old Boy With Abdominal Pain and Rectal Bleeding"
},
{
"authors": "Jaime Shalkow, MD",
"content": [
"Intussusception is the most common abdominal emergency in children younger than 2 years, and is the most common cause of intestinal obstruction in those aged 6-36 months. The term refers to a segment of intestine (the intussusceptum) that telescopes or invaginates into the lumen of another, immediately adjacent distal segment called the \"intussuscipiens\" (Figure 4).[1]Early diagnosis with fluid resuscitation and treatment results in a mortality rate of less than 1% in children. However, if left untreated, intussusception is uniformly fatal in 2-5 days.",
"Figure 4.",
"Figure 4.",
"Acute gastroenteritis in children is most often viral, thus showing lymphocytosis rather than an elevated neutrophil count. It is also commonly preceded by an upper respiratory tract infection. Low-grade fever may be present, but the pain is persistent. Episodes of colicky pain with the patient crying inconsolably and drawing the legs upward toward the abdomen, intermingled with periods of rest and calm, are characteristic of intussusception.",
"Meckel diverticulum is usually found in older children and is mostly asymptomatic, except in cases complicated with diverticulitis. Meckel diverticulum is often confused with acute appendicitis, and the rectal bleeding is fresh and profuse. Currant-jelly stools are also characteristic of intussusception.",
"Intestinal polyps are often asymptomatic, except in complicated cases that present with rectal bleeding, and are mostly painless. A family history may be recorded.",
"Intestinal volvulus is an acute surgical emergency in babies with malrotation. These individuals present extremely sick, with hemodynamic instability and severe electrolyte imbalance, a scaphoid and sometimes discolored abdomen, and vomit that is characteristically bilious (green)."
],
"date": "December 17, 2015",
"figures": [
{
"caption": "Figure 4.",
"image_url": "https://img.medscapestatic.com/article/856/057/856057-Thumb4.png"
}
],
"markdown": "# 7-Month-Old Boy With Abdominal Pain and Rectal Bleeding\n\n **Authors:** Jaime Shalkow, MD \n **Date:** December 17, 2015\n\n ## Content\n\n Intussusception is the most common abdominal emergency in children younger than 2 years, and is the most common cause of intestinal obstruction in those aged 6-36 months. The term refers to a segment of intestine (the intussusceptum) that telescopes or invaginates into the lumen of another, immediately adjacent distal segment called the \"intussuscipiens\" (Figure 4).[1]Early diagnosis with fluid resuscitation and treatment results in a mortality rate of less than 1% in children. However, if left untreated, intussusception is uniformly fatal in 2-5 days.\nFigure 4.\nFigure 4.\nAcute gastroenteritis in children is most often viral, thus showing lymphocytosis rather than an elevated neutrophil count. It is also commonly preceded by an upper respiratory tract infection. Low-grade fever may be present, but the pain is persistent. Episodes of colicky pain with the patient crying inconsolably and drawing the legs upward toward the abdomen, intermingled with periods of rest and calm, are characteristic of intussusception.\nMeckel diverticulum is usually found in older children and is mostly asymptomatic, except in cases complicated with diverticulitis. Meckel diverticulum is often confused with acute appendicitis, and the rectal bleeding is fresh and profuse. Currant-jelly stools are also characteristic of intussusception.\nIntestinal polyps are often asymptomatic, except in complicated cases that present with rectal bleeding, and are mostly painless. A family history may be recorded.\nIntestinal volvulus is an acute surgical emergency in babies with malrotation. These individuals present extremely sick, with hemodynamic instability and severe electrolyte imbalance, a scaphoid and sometimes discolored abdomen, and vomit that is characteristically bilious (green).\n\n ## Figures\n\n **Figure 4.** \n \n\n\n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 927005,
"choiceText": "Acute gastroenteritis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 927007,
"choiceText": "Meckel diverticulum",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 927009,
"choiceText": "Intestinal polyps",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 927011,
"choiceText": "Intussusception\r\n",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 927013,
"choiceText": "Intestinal volvulus",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 293035,
"questionText": "On the basis of the history, physical examination, and workup, what is the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "7-Month-Old Boy With Abdominal Pain and Rectal Bleeding"
},
{
"authors": "Jaime Shalkow, MD",
"content": [
"Most intussusceptions are idiopathic; only 2%-8% of cases are caused by an underlying disease or condition that creates a pathologic lead point for the intussusception.[2] Although the cause of intussusception is unknown in 90%-95% of children, a viral etiology is suspected because of the seasonal predisposition for intussusception to occur in spring and autumn, as well as a higher incidence of adenoid hypertrophy in children with intussusceptions. Although diarrhea is a common symptom preceding intussusception, recent studies have failed to prove statistical significance of a specific viral infection as a cause for intussusception.[3]",
"Older children with intussusception may have lead points as the cause of the condition. These include Meckel diverticulum, cocaine abuse, laxatives, and antibiotic use. It has also been described in patients with parasites, particularly Ascaris lumbricoides, and Henoch-Schönlein purpura, where mucosal hematomas are thought to act as the lead point.[4,5,6]Acute appendicitis in patients with Burkitt lymphoma has also been reported as an etiology.[2]",
"Figure 5.",
"Figure 5.",
"Figure 1.",
"Figure 1.",
"Peutz-Jeghers syndrome\n\n \n, familial polyposis coli, and juvenile polyposis can also cause intussusceptions.[7]A vermiform appendix may occasionally cause the disease (Figure 5). Familial cases of intussusception have been described. Intestinal lymphomas should be suspected in all children older than 6 years with intussusception.",
"Compression of the mesentery at the point of invagination leads to venous stasis and edema. Goblet cells pour copious amounts of mucus into the intestinal lumen. The engorged hyperemic intestinal mucosa seeps blood, which mixes with the mucus to form the currant-jelly stool that occurs in 60% of patients (Figure 1). Tissue pressure eventually exceeds arterial pressure, and necrosis ensues within 24 hours.",
"The peak age at presentation is between 5 and 10 months; it is more common in males. Less than 1% of intussusceptions are found in neonates. Early diagnosis and prompt treatment prevent catastrophic complications. In 95% of cases, the intussusception is in the ileocecal area. Ileoileal and colocolic intussusceptions are rare.",
"After nonoperative reduction, the recurrence rate of intussusception is usually less than 10% but has been reported to be as high as 15%. Most intussusceptions recur within 72 hours; however, some recurrences have been noted as long as 36 months later. If there is more than one recurrence, a lead point is suggested. The onset of the same symptoms typically signals recurrence. Treatment for a recurrence is similar, unless a lead point is strongly suggested, which would indicate that surgical exploration is needed."
],
"date": "December 17, 2015",
"figures": [
{
"caption": "Figure 5.",
"image_url": "https://img.medscapestatic.com/article/856/057/856057-Thumb5.png"
},
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/856/057/856057-Thumb1.png"
}
],
"markdown": "# 7-Month-Old Boy With Abdominal Pain and Rectal Bleeding\n\n **Authors:** Jaime Shalkow, MD \n **Date:** December 17, 2015\n\n ## Content\n\n Most intussusceptions are idiopathic; only 2%-8% of cases are caused by an underlying disease or condition that creates a pathologic lead point for the intussusception.[2] Although the cause of intussusception is unknown in 90%-95% of children, a viral etiology is suspected because of the seasonal predisposition for intussusception to occur in spring and autumn, as well as a higher incidence of adenoid hypertrophy in children with intussusceptions. Although diarrhea is a common symptom preceding intussusception, recent studies have failed to prove statistical significance of a specific viral infection as a cause for intussusception.[3]\nOlder children with intussusception may have lead points as the cause of the condition. These include Meckel diverticulum, cocaine abuse, laxatives, and antibiotic use. It has also been described in patients with parasites, particularly Ascaris lumbricoides, and Henoch-Schönlein purpura, where mucosal hematomas are thought to act as the lead point.[4,5,6]Acute appendicitis in patients with Burkitt lymphoma has also been reported as an etiology.[2]\nFigure 5.\nFigure 5.\nFigure 1.\nFigure 1.\nPeutz-Jeghers syndrome\n\n \n, familial polyposis coli, and juvenile polyposis can also cause intussusceptions.[7]A vermiform appendix may occasionally cause the disease (Figure 5). Familial cases of intussusception have been described. Intestinal lymphomas should be suspected in all children older than 6 years with intussusception.\nCompression of the mesentery at the point of invagination leads to venous stasis and edema. Goblet cells pour copious amounts of mucus into the intestinal lumen. The engorged hyperemic intestinal mucosa seeps blood, which mixes with the mucus to form the currant-jelly stool that occurs in 60% of patients (Figure 1). Tissue pressure eventually exceeds arterial pressure, and necrosis ensues within 24 hours.\nThe peak age at presentation is between 5 and 10 months; it is more common in males. Less than 1% of intussusceptions are found in neonates. Early diagnosis and prompt treatment prevent catastrophic complications. In 95% of cases, the intussusception is in the ileocecal area. Ileoileal and colocolic intussusceptions are rare.\nAfter nonoperative reduction, the recurrence rate of intussusception is usually less than 10% but has been reported to be as high as 15%. Most intussusceptions recur within 72 hours; however, some recurrences have been noted as long as 36 months later. If there is more than one recurrence, a lead point is suggested. The onset of the same symptoms typically signals recurrence. Treatment for a recurrence is similar, unless a lead point is strongly suggested, which would indicate that surgical exploration is needed.\n\n ## Figures\n\n **Figure 5.** \n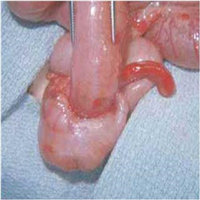 \n\n**Figure 1.** \n \n\n\n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "7-Month-Old Boy With Abdominal Pain and Rectal Bleeding"
},
{
"authors": "Jaime Shalkow, MD",
"content": [
"Intussusception causes a sudden onset of severe colicky abdominal pain. Children appear healthy between paroxysms of pain. As the condition progresses, the child becomes progressively more irritable and lethargic until shock develops. Vomiting occurs in the early phase of the illness and is bilious in 30% of cases. Early in the course of the disease, stools are normal, but they rapidly become bloody and mucoid within the first 12 hours.",
"The classic triad described for intussusception, which consists of colicky abdominal pain, a sausage-shaped palpable abdominal mass, and currant-jelly stools, is actually found in only 20% of cases. Patients may present with the Dance sign (empty right lower quadrant).",
"Figure 6.",
"Figure 6.",
"Figure 7.",
"Figure 7.",
"Figure 8.",
"Figure 8.",
"Abdominal plain films may reveal the head of the intussusceptum projecting into the air-filled colon and scattered air-fluid levels that suggest an ileus or partial obstruction (Figure 6).",
"Contrast fluoroscopy can be diagnostic as well as therapeutic. The classic sign is a coiled-spring appearance caused by the tracking of contrast around the lumen of the edematous intestine (Figure 7). However, air and water enemas are safer than and just as effective contrast fluoroscopy.[8]",
"Ultrasonography is the method of choice to detect intussusception in most institutions. A \"target,\" \"bull's-eye,\" or \"coiled spring\" lesion is seen, representing layers of the intestine within the intestine (Figure 8)."
],
"date": "December 17, 2015",
"figures": [
{
"caption": "Figure 6.",
"image_url": "https://img.medscapestatic.com/article/856/057/856057-Thumb6.png"
},
{
"caption": "Figure 7.",
"image_url": "https://img.medscapestatic.com/article/856/057/856057-Thumb7.png"
},
{
"caption": "Figure 8.",
"image_url": "https://img.medscapestatic.com/article/856/057/856057-Thumb8.png"
}
],
"markdown": "# 7-Month-Old Boy With Abdominal Pain and Rectal Bleeding\n\n **Authors:** Jaime Shalkow, MD \n **Date:** December 17, 2015\n\n ## Content\n\n Intussusception causes a sudden onset of severe colicky abdominal pain. Children appear healthy between paroxysms of pain. As the condition progresses, the child becomes progressively more irritable and lethargic until shock develops. Vomiting occurs in the early phase of the illness and is bilious in 30% of cases. Early in the course of the disease, stools are normal, but they rapidly become bloody and mucoid within the first 12 hours.\nThe classic triad described for intussusception, which consists of colicky abdominal pain, a sausage-shaped palpable abdominal mass, and currant-jelly stools, is actually found in only 20% of cases. Patients may present with the Dance sign (empty right lower quadrant).\nFigure 6.\nFigure 6.\nFigure 7.\nFigure 7.\nFigure 8.\nFigure 8.\nAbdominal plain films may reveal the head of the intussusceptum projecting into the air-filled colon and scattered air-fluid levels that suggest an ileus or partial obstruction (Figure 6).\nContrast fluoroscopy can be diagnostic as well as therapeutic. The classic sign is a coiled-spring appearance caused by the tracking of contrast around the lumen of the edematous intestine (Figure 7). However, air and water enemas are safer than and just as effective contrast fluoroscopy.[8]\nUltrasonography is the method of choice to detect intussusception in most institutions. A \"target,\" \"bull's-eye,\" or \"coiled spring\" lesion is seen, representing layers of the intestine within the intestine (Figure 8).\n\n ## Figures\n\n **Figure 6.** \n \n\n**Figure 7.** \n \n\n**Figure 8.** \n \n\n\n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 927015,
"choiceText": "Intravenous hydration, antibiotics, and observation",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 927017,
"choiceText": "Surgical exploration",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 927019,
"choiceText": "Air enema",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 927021,
"choiceText": "Upper gastrointestinal barium study",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 927023,
"choiceText": "Colonoscopy\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "As was undertaken with this patient, the proper treatment for intussusception is to stabilize the patient's airway, breathing, and circulation (ABCs) and replace large fluid losses. Administer nothing by mouth and place a nasogastric tube to decompress the obstruction from above. The use of antibiotics is appropriate for management of bacterial translocation.\r\n<br><br>\r\nFor stable patients with radiographic evidence of intussusception and no evidence of bowel perforation, nonoperative reduction of the intussusception is recommended rather than surgery. The reduction can be guided by fluoroscopy or ultrasound, and either hydrostatic or pneumatic enemas may be used. <br><br>\r\nThe success rates and risks of these techniques are similar. Ultrasound-guided approaches have the benefit of better identification of pathologic lead points and lower exposure to radiation. Pneumatic reduction is currently considered an optimal first-line treatment.<sup>[9]</sup> Most recurrences occur during the first 72 hours.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 293037,
"questionText": "What is the best next step in the management of this patient?\r\n\r\n",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 927025,
"choiceText": "Admission to the intensive care unit for monitoring and aggressive medical treatment",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 927027,
"choiceText": "Repeat the enema with barium",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 927029,
"choiceText": "Therapeutic endoscopy\r\n",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 927031,
"choiceText": "Surgical exploration",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Barium, water, or air enema reductions are appropriate after surgical consultation if the symptom duration is less than 24 hours and the patient has no signs of peritonitis. If symptoms persist longer than 24 hours, signs of peritonitis appear, or the intussusception cannot be reduced after two enema attempts, or if it recurs, stabilize the child and transport him or her immediately to the operating room, because untreated intussusception is almost always fatal. The recurrence rate is higher after radiographic than after surgical reduction. <br><br>\r\nSurgical treatment is indicated as a primary intervention for patients with suspected intussusception who are acutely ill or have evidence of perforation. Surgery may also be appropriate when the patient is treated in a location where the radiographic facilities and expertise to perform nonoperative reduction are not readily available. It is also necessary for patients in whom nonoperative reduction is unsuccessful or for evaluation or resection of a pathologic lead point. <br><br>\r\nLaparoscopy is currently considered a reasonable approach to pediatric intussusception, even when bowel resection is necessary.<sup>[10]</sup>\r\n<br><br>\r\nAfter two unsuccessful attempts to reduce this patient's intussusception, a transverse laparotomy was performed, and the bowel was manually reduced by squeezing the intussusceptum with warm moist towels. No evidence of bowel necrosis or perforation was found. A mild amount of clear peritoneal fluid was washed out. Blood loss was minimal, and the patient recovered uneventfully, resuming diet the next morning; he was discharged home within 24 hours.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 293039,
"questionText": "If the episode cannot be corrected with an enema, and the patient develops increased abdominal pain, tachycardia, and lethargy, which of the following is the next step?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "7-Month-Old Boy With Abdominal Pain and Rectal Bleeding"
},
{
"authors": "Jaime Shalkow, MD",
"content": [],
"date": "December 17, 2015",
"figures": [],
"markdown": "# 7-Month-Old Boy With Abdominal Pain and Rectal Bleeding\n\n **Authors:** Jaime Shalkow, MD \n **Date:** December 17, 2015\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 927015,
"choiceText": "Intravenous hydration, antibiotics, and observation",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 927017,
"choiceText": "Surgical exploration",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 927019,
"choiceText": "Air enema",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 927021,
"choiceText": "Upper gastrointestinal barium study",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 927023,
"choiceText": "Colonoscopy\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "As was undertaken with this patient, the proper treatment for intussusception is to stabilize the patient's airway, breathing, and circulation (ABCs) and replace large fluid losses. Administer nothing by mouth and place a nasogastric tube to decompress the obstruction from above. The use of antibiotics is appropriate for management of bacterial translocation.\r\n<br><br>\r\nFor stable patients with radiographic evidence of intussusception and no evidence of bowel perforation, nonoperative reduction of the intussusception is recommended rather than surgery. The reduction can be guided by fluoroscopy or ultrasound, and either hydrostatic or pneumatic enemas may be used. <br><br>\r\nThe success rates and risks of these techniques are similar. Ultrasound-guided approaches have the benefit of better identification of pathologic lead points and lower exposure to radiation. Pneumatic reduction is currently considered an optimal first-line treatment.<sup>[9]</sup> Most recurrences occur during the first 72 hours.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 293037,
"questionText": "What is the best next step in the management of this patient?\r\n\r\n",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 927025,
"choiceText": "Admission to the intensive care unit for monitoring and aggressive medical treatment",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 927027,
"choiceText": "Repeat the enema with barium",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 927029,
"choiceText": "Therapeutic endoscopy\r\n",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 927031,
"choiceText": "Surgical exploration",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Barium, water, or air enema reductions are appropriate after surgical consultation if the symptom duration is less than 24 hours and the patient has no signs of peritonitis. If symptoms persist longer than 24 hours, signs of peritonitis appear, or the intussusception cannot be reduced after two enema attempts, or if it recurs, stabilize the child and transport him or her immediately to the operating room, because untreated intussusception is almost always fatal. The recurrence rate is higher after radiographic than after surgical reduction. <br><br>\r\nSurgical treatment is indicated as a primary intervention for patients with suspected intussusception who are acutely ill or have evidence of perforation. Surgery may also be appropriate when the patient is treated in a location where the radiographic facilities and expertise to perform nonoperative reduction are not readily available. It is also necessary for patients in whom nonoperative reduction is unsuccessful or for evaluation or resection of a pathologic lead point. <br><br>\r\nLaparoscopy is currently considered a reasonable approach to pediatric intussusception, even when bowel resection is necessary.<sup>[10]</sup>\r\n<br><br>\r\nAfter two unsuccessful attempts to reduce this patient's intussusception, a transverse laparotomy was performed, and the bowel was manually reduced by squeezing the intussusceptum with warm moist towels. No evidence of bowel necrosis or perforation was found. A mild amount of clear peritoneal fluid was washed out. Blood loss was minimal, and the patient recovered uneventfully, resuming diet the next morning; he was discharged home within 24 hours.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 293039,
"questionText": "If the episode cannot be corrected with an enema, and the patient develops increased abdominal pain, tachycardia, and lethargy, which of the following is the next step?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "7-Month-Old Boy With Abdominal Pain and Rectal Bleeding"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 927005,
"choiceText": "Acute gastroenteritis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 927007,
"choiceText": "Meckel diverticulum",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 927009,
"choiceText": "Intestinal polyps",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 927011,
"choiceText": "Intussusception\r\n",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 927013,
"choiceText": "Intestinal volvulus",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 293035,
"questionText": "On the basis of the history, physical examination, and workup, what is the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 927015,
"choiceText": "Intravenous hydration, antibiotics, and observation",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 927017,
"choiceText": "Surgical exploration",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 927019,
"choiceText": "Air enema",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 927021,
"choiceText": "Upper gastrointestinal barium study",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 927023,
"choiceText": "Colonoscopy\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "As was undertaken with this patient, the proper treatment for intussusception is to stabilize the patient's airway, breathing, and circulation (ABCs) and replace large fluid losses. Administer nothing by mouth and place a nasogastric tube to decompress the obstruction from above. The use of antibiotics is appropriate for management of bacterial translocation.\r\n<br><br>\r\nFor stable patients with radiographic evidence of intussusception and no evidence of bowel perforation, nonoperative reduction of the intussusception is recommended rather than surgery. The reduction can be guided by fluoroscopy or ultrasound, and either hydrostatic or pneumatic enemas may be used. <br><br>\r\nThe success rates and risks of these techniques are similar. Ultrasound-guided approaches have the benefit of better identification of pathologic lead points and lower exposure to radiation. Pneumatic reduction is currently considered an optimal first-line treatment.<sup>[9]</sup> Most recurrences occur during the first 72 hours.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 293037,
"questionText": "What is the best next step in the management of this patient?\r\n\r\n",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 927025,
"choiceText": "Admission to the intensive care unit for monitoring and aggressive medical treatment",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 927027,
"choiceText": "Repeat the enema with barium",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 927029,
"choiceText": "Therapeutic endoscopy\r\n",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 927031,
"choiceText": "Surgical exploration",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Barium, water, or air enema reductions are appropriate after surgical consultation if the symptom duration is less than 24 hours and the patient has no signs of peritonitis. If symptoms persist longer than 24 hours, signs of peritonitis appear, or the intussusception cannot be reduced after two enema attempts, or if it recurs, stabilize the child and transport him or her immediately to the operating room, because untreated intussusception is almost always fatal. The recurrence rate is higher after radiographic than after surgical reduction. <br><br>\r\nSurgical treatment is indicated as a primary intervention for patients with suspected intussusception who are acutely ill or have evidence of perforation. Surgery may also be appropriate when the patient is treated in a location where the radiographic facilities and expertise to perform nonoperative reduction are not readily available. It is also necessary for patients in whom nonoperative reduction is unsuccessful or for evaluation or resection of a pathologic lead point. <br><br>\r\nLaparoscopy is currently considered a reasonable approach to pediatric intussusception, even when bowel resection is necessary.<sup>[10]</sup>\r\n<br><br>\r\nAfter two unsuccessful attempts to reduce this patient's intussusception, a transverse laparotomy was performed, and the bowel was manually reduced by squeezing the intussusceptum with warm moist towels. No evidence of bowel necrosis or perforation was found. A mild amount of clear peritoneal fluid was washed out. Blood loss was minimal, and the patient recovered uneventfully, resuming diet the next morning; he was discharged home within 24 hours.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 293039,
"questionText": "If the episode cannot be corrected with an enema, and the patient develops increased abdominal pain, tachycardia, and lethargy, which of the following is the next step?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
855906 | /viewarticle/855906 | [
{
"authors": "Arun Phophalia, MBBS, MS; Madhu Phophalia, MD, MBBS, MS",
"content": [
"Editor's Note:\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.",
"A 9-year-old boy is brought to the outpatient clinic by his parents with a 2- to 3-year history of intermittent fever, abdominal pain, failure to thrive, tiredness, and weakness. In between times of active illness, the patient is usually asymptomatic, although he gets tired very easily during his sporting activities. When present, the fever and pain usually subside on their own, but, occasionally, an antipyretic or a course of antibiotics is needed.",
"His weight has been constant with no significant height gain over the last year. He does not have any nausea, vomiting, diarrhea, constipation, bloating, or distention. No history of hematuria or stone disease is reported. He does not have any history of surgery, prolonged fever, hospitalization, or any illnesses (except for the present one). The family history is not significant. He has no known allergies, and his vaccinations are up to date. The patient comes from a poor socioeconomic background."
],
"date": "December 16, 2015",
"figures": [],
"markdown": "# Fever, Pain, and Failure to Thrive in a 9-Year-Old Boy\n\n **Authors:** Arun Phophalia, MBBS, MS; Madhu Phophalia, MD, MBBS, MS \n **Date:** December 16, 2015\n\n ## Content\n\n Editor's Note:\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.\nA 9-year-old boy is brought to the outpatient clinic by his parents with a 2- to 3-year history of intermittent fever, abdominal pain, failure to thrive, tiredness, and weakness. In between times of active illness, the patient is usually asymptomatic, although he gets tired very easily during his sporting activities. When present, the fever and pain usually subside on their own, but, occasionally, an antipyretic or a course of antibiotics is needed.\nHis weight has been constant with no significant height gain over the last year. He does not have any nausea, vomiting, diarrhea, constipation, bloating, or distention. No history of hematuria or stone disease is reported. He does not have any history of surgery, prolonged fever, hospitalization, or any illnesses (except for the present one). The family history is not significant. He has no known allergies, and his vaccinations are up to date. The patient comes from a poor socioeconomic background.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "Fever, Pain, and Failure to Thrive in a 9-Year-Old Boy"
},
{
"authors": "Arun Phophalia, MBBS, MS; Madhu Phophalia, MD, MBBS, MS",
"content": [
"The child is well-nourished but appears small for his age. He is friendly and cooperative. His blood pressure is 94/68 mm Hg, pulse is 80 beats/min with a regular rhythm, oral temperature is 98.8°F (37.1°C), and respiration rate is 16 breaths/min. The patient's conjunctiva is pale, the sclera is muddy, the neck veins are not distended, and no pedal edema is noted.",
"Although the abdomen is slightly distended, no organs or lumps are palpable, and the bowel sounds are normal. Slight tenderness is noted in the left lumbar region on deep palpation, and the rest of the abdomen is nontender. The review of his other systems is normal. No free fluid is detected in the abdomen. The general examination does not show any abnormalities, except for slight pallor and grade 1 digital clubbing. No lymphadenopathy is detected, and the hernial sites are normal.",
"Figure 1.",
"Figure 1.",
"A complete blood cell (CBC) count reveals a hemoglobin of 10.7 g/dL (107 g/L), a hematocrit of 32% (0.32), and a red blood cell (RBC) count of 3.5 × 106/µL (3.5 × 1012/L). The liver function tests, kidney function tests, electrolyte panel, and coagulation profile, as well as the remainder of the CBC, are all normal. A routine urine examination shows 3-4 white blood cells (WBCs) per high power field, with no RBCs. A urine culture is performed; within 48 hours, over 100,000 colony forming units/mL of Escherichia coli sensitive to ciprofloxacin, gatifloxacin, gentamicin, and amikacin are found.",
"An intravenous urogram is ordered (Figure 1)."
],
"date": "December 16, 2015",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/855/906/855906-Thumb1.JPG"
}
],
"markdown": "# Fever, Pain, and Failure to Thrive in a 9-Year-Old Boy\n\n **Authors:** Arun Phophalia, MBBS, MS; Madhu Phophalia, MD, MBBS, MS \n **Date:** December 16, 2015\n\n ## Content\n\n The child is well-nourished but appears small for his age. He is friendly and cooperative. His blood pressure is 94/68 mm Hg, pulse is 80 beats/min with a regular rhythm, oral temperature is 98.8°F (37.1°C), and respiration rate is 16 breaths/min. The patient's conjunctiva is pale, the sclera is muddy, the neck veins are not distended, and no pedal edema is noted.\nAlthough the abdomen is slightly distended, no organs or lumps are palpable, and the bowel sounds are normal. Slight tenderness is noted in the left lumbar region on deep palpation, and the rest of the abdomen is nontender. The review of his other systems is normal. No free fluid is detected in the abdomen. The general examination does not show any abnormalities, except for slight pallor and grade 1 digital clubbing. No lymphadenopathy is detected, and the hernial sites are normal.\nFigure 1.\nFigure 1.\nA complete blood cell (CBC) count reveals a hemoglobin of 10.7 g/dL (107 g/L), a hematocrit of 32% (0.32), and a red blood cell (RBC) count of 3.5 × 106/µL (3.5 × 1012/L). The liver function tests, kidney function tests, electrolyte panel, and coagulation profile, as well as the remainder of the CBC, are all normal. A routine urine examination shows 3-4 white blood cells (WBCs) per high power field, with no RBCs. A urine culture is performed; within 48 hours, over 100,000 colony forming units/mL of Escherichia coli sensitive to ciprofloxacin, gatifloxacin, gentamicin, and amikacin are found.\nAn intravenous urogram is ordered (Figure 1).\n\n ## Figures\n\n **Figure 1.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 925039,
"choiceText": "Ureteropelvic junction obstruction",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 925041,
"choiceText": "Neurogenic bladder",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 925043,
"choiceText": "Vesicoureteral reflux",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 925045,
"choiceText": "Ureterovesical junction obstruction\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 292293,
"questionText": "On the basis of the history, physical examination, and workup, what is the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Fever, Pain, and Failure to Thrive in a 9-Year-Old Boy"
},
{
"authors": "Arun Phophalia, MBBS, MS; Madhu Phophalia, MD, MBBS, MS",
"content": [
"On the initial nephrogram images, a large amount of uptake was noted in the right kidney (this is known as a \"prompt nephrogram\"); however, on the left side, a delay of uptake into the kidney parenchyma was observed (known as a \"delayed nephrogram\"). (Please note that the initial film obtained in the first minute of the nephrogram is not available.) A delayed nephrogram is pathognomonic for an obstruction. It does not, however, show exactly where the obstruction is located.",
"Figure 1.",
"Figure 1.",
"Figure 2.",
"Figure 2.",
"Figure 3.",
"Figure 3.",
"Figure 1, a 5-minute delayed film, demonstrated a persistent nephrogram in an enlarged left kidney and delayed excretion of contrast into a dilated collecting system; the right kidney appeared normal. In addition, on other delayed films (Figures 2 and 3), a massively dilated pelvicalyceal system with blunting of the calyces was noted on the left. A cut-off point at the ureteropelvic junction (UPJ) was also noted on that side, without visualization of the left ureter. All of these changes were consistent with localization of the obstruction to the UPJ on the affected side. The left ureter was not seen to opacify on the delayed films. The right pelvicalyceal system, however, was well-defined, and the calyces showed normal sharp cupping (Figure 2). The right ureter appeared thin or delicate, and a column of contrast was noted to reach down to the bladder; this ruled out any right-sided obstruction (Figure 2). The postvoiding film showed almost complete emptying of the bladder. By that point, the contrast had relatively cleared (with some residual contrast still in the collecting system) from the right kidney collecting system; the left side, however, was still full of contrast (Figure 3). These findings were consistent with a radiographically established UPJ obstruction.",
"The ultrasonography (images not available) in this patient showed dilatation of the left pelvicalyceal system, with hydronephrosis of the left kidney and thinning of the cortex. The right kidney and bladder were normal. No abnormalities were seen in the liver, gall bladder, pancreas, or spleen. A renal scan was also performed (images not available), which showed a nonobstructed right kidney with normal function. The left kidney showed hydronephrosis and impaired function suggestive of UPJ obstruction. The total glomerular filtration rate (GFR) was measured at 123.35 mL/min, with a right-kidney GFR of 69.33 mL/min (within normal limits) and a left-kidney GFR of 54.02 mL/min (below normal). The split functions of the kidneys were noted to be 56% for the right kidney and 44% for the left kidney."
],
"date": "December 16, 2015",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/855/906/855906-Thumb1.JPG"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/855/906/855906-Thumb2.JPG"
},
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/855/906/855906-Thumb3.JPG"
}
],
"markdown": "# Fever, Pain, and Failure to Thrive in a 9-Year-Old Boy\n\n **Authors:** Arun Phophalia, MBBS, MS; Madhu Phophalia, MD, MBBS, MS \n **Date:** December 16, 2015\n\n ## Content\n\n On the initial nephrogram images, a large amount of uptake was noted in the right kidney (this is known as a \"prompt nephrogram\"); however, on the left side, a delay of uptake into the kidney parenchyma was observed (known as a \"delayed nephrogram\"). (Please note that the initial film obtained in the first minute of the nephrogram is not available.) A delayed nephrogram is pathognomonic for an obstruction. It does not, however, show exactly where the obstruction is located.\nFigure 1.\nFigure 1.\nFigure 2.\nFigure 2.\nFigure 3.\nFigure 3.\nFigure 1, a 5-minute delayed film, demonstrated a persistent nephrogram in an enlarged left kidney and delayed excretion of contrast into a dilated collecting system; the right kidney appeared normal. In addition, on other delayed films (Figures 2 and 3), a massively dilated pelvicalyceal system with blunting of the calyces was noted on the left. A cut-off point at the ureteropelvic junction (UPJ) was also noted on that side, without visualization of the left ureter. All of these changes were consistent with localization of the obstruction to the UPJ on the affected side. The left ureter was not seen to opacify on the delayed films. The right pelvicalyceal system, however, was well-defined, and the calyces showed normal sharp cupping (Figure 2). The right ureter appeared thin or delicate, and a column of contrast was noted to reach down to the bladder; this ruled out any right-sided obstruction (Figure 2). The postvoiding film showed almost complete emptying of the bladder. By that point, the contrast had relatively cleared (with some residual contrast still in the collecting system) from the right kidney collecting system; the left side, however, was still full of contrast (Figure 3). These findings were consistent with a radiographically established UPJ obstruction.\nThe ultrasonography (images not available) in this patient showed dilatation of the left pelvicalyceal system, with hydronephrosis of the left kidney and thinning of the cortex. The right kidney and bladder were normal. No abnormalities were seen in the liver, gall bladder, pancreas, or spleen. A renal scan was also performed (images not available), which showed a nonobstructed right kidney with normal function. The left kidney showed hydronephrosis and impaired function suggestive of UPJ obstruction. The total glomerular filtration rate (GFR) was measured at 123.35 mL/min, with a right-kidney GFR of 69.33 mL/min (within normal limits) and a left-kidney GFR of 54.02 mL/min (below normal). The split functions of the kidneys were noted to be 56% for the right kidney and 44% for the left kidney.\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n**Figure 3.** \n \n\n\n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 925039,
"choiceText": "Ureteropelvic junction obstruction",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 925041,
"choiceText": "Neurogenic bladder",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 925043,
"choiceText": "Vesicoureteral reflux",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 925045,
"choiceText": "Ureterovesical junction obstruction\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 292293,
"questionText": "On the basis of the history, physical examination, and workup, what is the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Fever, Pain, and Failure to Thrive in a 9-Year-Old Boy"
},
{
"authors": "Arun Phophalia, MBBS, MS; Madhu Phophalia, MD, MBBS, MS",
"content": [
"UPJ obstruction is the most common obstructive lesion in childhood. Prior to the use of prenatal ultrasound, most patients with UPJ obstruction presented with pain, hematuria, urosepsis, failure to thrive, or a palpable mass. Approximately 60% of cases occur on the left side, and the male-to-female ratio is 2:1. In 10% of cases, UPJ obstruction is bilateral. In kidneys with UPJ obstruction, renal function may be significantly impaired as a result of pressure atrophy.[1,2,3]",
"Figure 1.",
"Figure 1.",
"Congenital UPJ obstruction most often results from intrinsic disease. A common defect in congenital UPJ obstruction is an aperistaltic segment of the ureter. In these cases, histopathologic studies have revealed that the spiral musculature normally present has been replaced by abnormal longitudinal muscle bundles or fibrous tissue. This results in failure to develop a normal peristaltic wave for the propagation of urine from the renal pelvis to the ureter. Further investigations into the etiology of UPJ obstruction have shown decreased interstitial cells of Cajal at the UPJ in children.",
"In addition, the cytokine produced in the urothelium is also theorized to exacerbate UPJ obstruction. A less common intrinsic cause of congenital UPJ obstruction is true ureteral stricture. True congenital ureteral strictures are most often found at the UPJ, although they may be situated at sites anywhere along the ureter. Abnormalities of the ureteral musculature have been implicated because of the deposition of too much collagen at the site of the stricture. Intrinsic obstruction at the UPJ may also result from kinks or valves produced by infoldings of the ureteral mucosa and musculature. In these cases, the obstruction may be, essentially, at the level of the proximal ureter. Grossly, this can manifest as external bands or adhesions that appear to cause the obstruction. In most cases, however, these bands or adhesions are likely to be a secondary phenomenon associated with intrinsic obstruction; therefore, operative pyeloplasty would generally be the most effective procedure.[1,2,3,4]",
"The presence of these kinks, valves, bands, or adhesions may also produce angulation of the ureter at the lower margin of the renal pelvis in such a manner that, as the pelvis dilates anteriorly and inferiorly, the ureteral insertion is carried further proximally. In these cases, the most dependent portion of the pelvis is inadequately drained, and the apparent \"high insertion\" of the ureteral ostium is actually a secondary phenomenon. In at least some cases, however, the high insertion itself is likely the primary obstructing lesion because this phenomenon is frequently found in cases of renal ectopia or fusion anomalies. As such, a high insertion can have implications in the ensuing surgical management.",
"An accessory artery and/or vein to the lower pole of the kidney also may cause extrinsic obstruction. Although these lower-pole arteries have often been referred to as aberrant, this variant is found in 20% of people. In some patients, these lower-pole vessels cross the ureter posteriorly and truly have an aberrant course. Although some have speculated that the crossing vessel plays a role in the pathogenesis of UPJ obstruction, the associated vessel is unlikely to cause the primary obstruction on its own. In fact, the true etiology is an intrinsic lesion at the UPJ or proximal ureter that causes dilatation and ballooning of the renal pelvis over the polar or aberrant vessel.[3,4,5]",
"UPJ obstruction may also result from acquired lesions. In children, vesicoureteral reflux can lead to upper tract dilatation, with subsequent elongation, tortuosity, and kinking of the ureter. Other acquired causes of obstruction at the UPJ include benign tumors (such as fibroepithelial polyps), urothelial malignancy, stone disease, and postinflammatory or postoperative scarring or ischemia.[4,5]"
],
"date": "December 16, 2015",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/855/906/855906-Thumb1.JPG"
}
],
"markdown": "# Fever, Pain, and Failure to Thrive in a 9-Year-Old Boy\n\n **Authors:** Arun Phophalia, MBBS, MS; Madhu Phophalia, MD, MBBS, MS \n **Date:** December 16, 2015\n\n ## Content\n\n UPJ obstruction is the most common obstructive lesion in childhood. Prior to the use of prenatal ultrasound, most patients with UPJ obstruction presented with pain, hematuria, urosepsis, failure to thrive, or a palpable mass. Approximately 60% of cases occur on the left side, and the male-to-female ratio is 2:1. In 10% of cases, UPJ obstruction is bilateral. In kidneys with UPJ obstruction, renal function may be significantly impaired as a result of pressure atrophy.[1,2,3]\nFigure 1.\nFigure 1.\nCongenital UPJ obstruction most often results from intrinsic disease. A common defect in congenital UPJ obstruction is an aperistaltic segment of the ureter. In these cases, histopathologic studies have revealed that the spiral musculature normally present has been replaced by abnormal longitudinal muscle bundles or fibrous tissue. This results in failure to develop a normal peristaltic wave for the propagation of urine from the renal pelvis to the ureter. Further investigations into the etiology of UPJ obstruction have shown decreased interstitial cells of Cajal at the UPJ in children.\nIn addition, the cytokine produced in the urothelium is also theorized to exacerbate UPJ obstruction. A less common intrinsic cause of congenital UPJ obstruction is true ureteral stricture. True congenital ureteral strictures are most often found at the UPJ, although they may be situated at sites anywhere along the ureter. Abnormalities of the ureteral musculature have been implicated because of the deposition of too much collagen at the site of the stricture. Intrinsic obstruction at the UPJ may also result from kinks or valves produced by infoldings of the ureteral mucosa and musculature. In these cases, the obstruction may be, essentially, at the level of the proximal ureter. Grossly, this can manifest as external bands or adhesions that appear to cause the obstruction. In most cases, however, these bands or adhesions are likely to be a secondary phenomenon associated with intrinsic obstruction; therefore, operative pyeloplasty would generally be the most effective procedure.[1,2,3,4]\nThe presence of these kinks, valves, bands, or adhesions may also produce angulation of the ureter at the lower margin of the renal pelvis in such a manner that, as the pelvis dilates anteriorly and inferiorly, the ureteral insertion is carried further proximally. In these cases, the most dependent portion of the pelvis is inadequately drained, and the apparent \"high insertion\" of the ureteral ostium is actually a secondary phenomenon. In at least some cases, however, the high insertion itself is likely the primary obstructing lesion because this phenomenon is frequently found in cases of renal ectopia or fusion anomalies. As such, a high insertion can have implications in the ensuing surgical management.\nAn accessory artery and/or vein to the lower pole of the kidney also may cause extrinsic obstruction. Although these lower-pole arteries have often been referred to as aberrant, this variant is found in 20% of people. In some patients, these lower-pole vessels cross the ureter posteriorly and truly have an aberrant course. Although some have speculated that the crossing vessel plays a role in the pathogenesis of UPJ obstruction, the associated vessel is unlikely to cause the primary obstruction on its own. In fact, the true etiology is an intrinsic lesion at the UPJ or proximal ureter that causes dilatation and ballooning of the renal pelvis over the polar or aberrant vessel.[3,4,5]\nUPJ obstruction may also result from acquired lesions. In children, vesicoureteral reflux can lead to upper tract dilatation, with subsequent elongation, tortuosity, and kinking of the ureter. Other acquired causes of obstruction at the UPJ include benign tumors (such as fibroepithelial polyps), urothelial malignancy, stone disease, and postinflammatory or postoperative scarring or ischemia.[4,5]\n\n ## Figures\n\n **Figure 1.** \n \n\n\n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "Fever, Pain, and Failure to Thrive in a 9-Year-Old Boy"
},
{
"authors": "Arun Phophalia, MBBS, MS; Madhu Phophalia, MD, MBBS, MS",
"content": [
"UPJ obstruction, although most often a congenital problem, can present clinically at any time of life. In older children or adults, intermittent abdominal or flank pain is a frequent presenting symptom. This is occasionally associated with nausea or vomiting as well. Hematuria, either spontaneous or associated with otherwise relatively minor trauma, may also be an initial symptom. Laboratory findings of microhematuria, pyuria, or frank urinary tract infection might also bring an otherwise asymptomatic patient to the healthcare system. Rarely, hypertension may be a presenting finding.[2,6]",
"Figure 1.",
"Figure 1.",
"Figure 2.",
"Figure 2.",
"Radiographic studies should be performed with the goal of determining both the anatomic site and the functional significance of an apparent obstruction. Excretory urography remains a reasonable first-line option for radiographic diagnosis. Classically, findings on the affected side include delay in function associated with a dilated pelvicalyceal symptom. Ultrasonography also has an important role in diagnosing UPJ obstruction. It can be used in patients whose poor renal function precludes the use of intravenous contrast studies. Because of its current widespread use, CT scanning more frequently raises the suspicion of UPJ obstruction. The CT scan should first be performed without contrast in order to rule out obstructing stones, followed by a scan with contrast to assess for lack of drainage from the pelvis as well as detect any potential tumors in the collecting system (ureter or pelvis).",
"Nuclear renography can also be performed for all patients with suspected UPJ obstruction. Specifically, a technetium 99m-labeled mercaptoacetyltriglycine (MAG3) scan can differentiate the split functions of both kidneys and can quantify the drainage from each kidney. Specifically, it measures the t, which is the time it takes for half of the radiolabeled MAG3 to wash out of the renal pelvis. In cases of obstruction, the t is typically greater than 15 minutes.[2,3,4]",
"The following entities should be considered in the differential diagnosis: (1) megacalycosis, a congenital nonobstructive dilatation of the calyces without pelvic or ureteric dilatation; (2) vesicoureteral reflux, with marked dilatation and kinking of the ureter; and (3) midureteral or distal ureteral obstruction (when the ureter is not well visualized on the urogram).[2]",
"The patient was admitted to the hospital, and an open dismembered pyeloplasty was planned. The short obstructing segment of the ureter was resected, and the free end of the ureter was sutured to the open renal pelvis. This anastomosis was performed using 4-0 vicryl. A stent bridging the anastomosis was placed from the kidney to the bladder, and a drain in the perinephric space was inserted. The drain was removed on the sixth postoperative day, and the sutures were removed on the tenth day. The internal stent is typically removed in postoperative week 4-6 with an office-based cystoscopy.",
"The postoperative course was uneventful. While retrograde endoscopic approaches have been described, laparoscopic pyeloplasty is rapidly becoming the standard of care. This minimally invasive approach offers the advantages of less pain, a reduced need for pain medications, a shorter hospital stay, quicker convalescence, and better cosmesis. Most importantly, the results of establishing good drainage from the pelvis with laparoscopic pyeloplasty have been shown to be equivalent to those of open pyeloplasty. The patient was doing well after discharge, and he had gained height and weight at both the 3-month and 6-month follow-up.[2]"
],
"date": "December 16, 2015",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/855/906/855906-Thumb1.JPG"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/855/906/855906-Thumb2.JPG"
}
],
"markdown": "# Fever, Pain, and Failure to Thrive in a 9-Year-Old Boy\n\n **Authors:** Arun Phophalia, MBBS, MS; Madhu Phophalia, MD, MBBS, MS \n **Date:** December 16, 2015\n\n ## Content\n\n UPJ obstruction, although most often a congenital problem, can present clinically at any time of life. In older children or adults, intermittent abdominal or flank pain is a frequent presenting symptom. This is occasionally associated with nausea or vomiting as well. Hematuria, either spontaneous or associated with otherwise relatively minor trauma, may also be an initial symptom. Laboratory findings of microhematuria, pyuria, or frank urinary tract infection might also bring an otherwise asymptomatic patient to the healthcare system. Rarely, hypertension may be a presenting finding.[2,6]\nFigure 1.\nFigure 1.\nFigure 2.\nFigure 2.\nRadiographic studies should be performed with the goal of determining both the anatomic site and the functional significance of an apparent obstruction. Excretory urography remains a reasonable first-line option for radiographic diagnosis. Classically, findings on the affected side include delay in function associated with a dilated pelvicalyceal symptom. Ultrasonography also has an important role in diagnosing UPJ obstruction. It can be used in patients whose poor renal function precludes the use of intravenous contrast studies. Because of its current widespread use, CT scanning more frequently raises the suspicion of UPJ obstruction. The CT scan should first be performed without contrast in order to rule out obstructing stones, followed by a scan with contrast to assess for lack of drainage from the pelvis as well as detect any potential tumors in the collecting system (ureter or pelvis).\nNuclear renography can also be performed for all patients with suspected UPJ obstruction. Specifically, a technetium 99m-labeled mercaptoacetyltriglycine (MAG3) scan can differentiate the split functions of both kidneys and can quantify the drainage from each kidney. Specifically, it measures the t, which is the time it takes for half of the radiolabeled MAG3 to wash out of the renal pelvis. In cases of obstruction, the t is typically greater than 15 minutes.[2,3,4]\nThe following entities should be considered in the differential diagnosis: (1) megacalycosis, a congenital nonobstructive dilatation of the calyces without pelvic or ureteric dilatation; (2) vesicoureteral reflux, with marked dilatation and kinking of the ureter; and (3) midureteral or distal ureteral obstruction (when the ureter is not well visualized on the urogram).[2]\nThe patient was admitted to the hospital, and an open dismembered pyeloplasty was planned. The short obstructing segment of the ureter was resected, and the free end of the ureter was sutured to the open renal pelvis. This anastomosis was performed using 4-0 vicryl. A stent bridging the anastomosis was placed from the kidney to the bladder, and a drain in the perinephric space was inserted. The drain was removed on the sixth postoperative day, and the sutures were removed on the tenth day. The internal stent is typically removed in postoperative week 4-6 with an office-based cystoscopy.\nThe postoperative course was uneventful. While retrograde endoscopic approaches have been described, laparoscopic pyeloplasty is rapidly becoming the standard of care. This minimally invasive approach offers the advantages of less pain, a reduced need for pain medications, a shorter hospital stay, quicker convalescence, and better cosmesis. Most importantly, the results of establishing good drainage from the pelvis with laparoscopic pyeloplasty have been shown to be equivalent to those of open pyeloplasty. The patient was doing well after discharge, and he had gained height and weight at both the 3-month and 6-month follow-up.[2]\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n\n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 925047,
"choiceText": "Prenatal",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 925049,
"choiceText": "Infants",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 925051,
"choiceText": "Adults",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 925053,
"choiceText": "Old age",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 925055,
"choiceText": "Any age group\r\n",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "\r\nUPJ obstruction, although most often a congenital problem, can present clinically at any time of life. In older children or adults, intermittent abdominal or flank pain is a frequent presenting symptom.\r\n",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 292295,
"questionText": "In which of the following age groups does UPJ abnormality present?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 925057,
"choiceText": "Delayed nephrogram (or uptake of contrast by the kidney)",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 925059,
"choiceText": "Hydronephrosis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 925061,
"choiceText": "Lack of peristalsis",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 925063,
"choiceText": "Lack of contrast in the ureter on drainage films",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 925065,
"choiceText": ">15 minutes t on a MAG3 study\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "\r\nA delayed nephrogram is pathognomonic for an obstruction. It does not, however, show exactly where the obstruction is located. Hydronephrosis and impaired kidney function are suggestive of UPJ obstruction, as is lack of contrast in the ureter. In cases of UPJ obstruction, the t is typically greater than 15 minutes.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 292297,
"questionText": "Which of following findings on radiographic studies does <i>not</i> help establish the diagnosis of UPJ obstruction?\r\n",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Fever, Pain, and Failure to Thrive in a 9-Year-Old Boy"
},
{
"authors": "Arun Phophalia, MBBS, MS; Madhu Phophalia, MD, MBBS, MS",
"content": [],
"date": "December 16, 2015",
"figures": [],
"markdown": "# Fever, Pain, and Failure to Thrive in a 9-Year-Old Boy\n\n **Authors:** Arun Phophalia, MBBS, MS; Madhu Phophalia, MD, MBBS, MS \n **Date:** December 16, 2015\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 925047,
"choiceText": "Prenatal",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 925049,
"choiceText": "Infants",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 925051,
"choiceText": "Adults",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 925053,
"choiceText": "Old age",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 925055,
"choiceText": "Any age group\r\n",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "\r\nUPJ obstruction, although most often a congenital problem, can present clinically at any time of life. In older children or adults, intermittent abdominal or flank pain is a frequent presenting symptom.\r\n",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 292295,
"questionText": "In which of the following age groups does UPJ abnormality present?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 925057,
"choiceText": "Delayed nephrogram (or uptake of contrast by the kidney)",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 925059,
"choiceText": "Hydronephrosis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 925061,
"choiceText": "Lack of peristalsis",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 925063,
"choiceText": "Lack of contrast in the ureter on drainage films",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 925065,
"choiceText": ">15 minutes t on a MAG3 study\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "\r\nA delayed nephrogram is pathognomonic for an obstruction. It does not, however, show exactly where the obstruction is located. Hydronephrosis and impaired kidney function are suggestive of UPJ obstruction, as is lack of contrast in the ureter. In cases of UPJ obstruction, the t is typically greater than 15 minutes.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 292297,
"questionText": "Which of following findings on radiographic studies does <i>not</i> help establish the diagnosis of UPJ obstruction?\r\n",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Fever, Pain, and Failure to Thrive in a 9-Year-Old Boy"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 925039,
"choiceText": "Ureteropelvic junction obstruction",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 925041,
"choiceText": "Neurogenic bladder",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 925043,
"choiceText": "Vesicoureteral reflux",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 925045,
"choiceText": "Ureterovesical junction obstruction\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 292293,
"questionText": "On the basis of the history, physical examination, and workup, what is the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 925047,
"choiceText": "Prenatal",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 925049,
"choiceText": "Infants",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 925051,
"choiceText": "Adults",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 925053,
"choiceText": "Old age",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 925055,
"choiceText": "Any age group\r\n",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "\r\nUPJ obstruction, although most often a congenital problem, can present clinically at any time of life. In older children or adults, intermittent abdominal or flank pain is a frequent presenting symptom.\r\n",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 292295,
"questionText": "In which of the following age groups does UPJ abnormality present?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 925057,
"choiceText": "Delayed nephrogram (or uptake of contrast by the kidney)",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 925059,
"choiceText": "Hydronephrosis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 925061,
"choiceText": "Lack of peristalsis",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 925063,
"choiceText": "Lack of contrast in the ureter on drainage films",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 925065,
"choiceText": ">15 minutes t on a MAG3 study\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "\r\nA delayed nephrogram is pathognomonic for an obstruction. It does not, however, show exactly where the obstruction is located. Hydronephrosis and impaired kidney function are suggestive of UPJ obstruction, as is lack of contrast in the ureter. In cases of UPJ obstruction, the t is typically greater than 15 minutes.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 292297,
"questionText": "Which of following findings on radiographic studies does <i>not</i> help establish the diagnosis of UPJ obstruction?\r\n",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
855834 | /viewarticle/855834 | [
{
"authors": "Stella Izuchukwu, MD, MPH",
"content": [
"Editor's Note:\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.",
"A 55-year-old woman with a medical history of congestive heart failure, hypertension, hyperlipidemia, asthma, gastroesophageal reflux disease, and lupus anticoagulant syndrome presents to the emergency department with severe, progressive shortness of breath that has lasted for the past 12 hours, with associated chest pressure and wheezing. She denies having any leg swelling, chills, sore throat, coughing, or heartburn.",
"Before reaching the emergency department, she used her albuterol inhaler without relief of symptoms and contacted emergency medical services. She has a 40–pack-year history of tobacco use, as well as a history of alcoholism (with her last consumption being 4 years before presentation) and remote marijuana use. Her medication regimen includes furosemide, fosinopril, isosorbide nitrate, pantoprazole, spironolactone, and enteric-coated aspirin, but she has a well-documented history of nonadherence."
],
"date": "December 14, 2015",
"figures": [],
"markdown": "# A 55-Year-Old Woman With Worsening Shortness of Breath\n\n **Authors:** Stella Izuchukwu, MD, MPH \n **Date:** December 14, 2015\n\n ## Content\n\n Editor's Note:\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.\nA 55-year-old woman with a medical history of congestive heart failure, hypertension, hyperlipidemia, asthma, gastroesophageal reflux disease, and lupus anticoagulant syndrome presents to the emergency department with severe, progressive shortness of breath that has lasted for the past 12 hours, with associated chest pressure and wheezing. She denies having any leg swelling, chills, sore throat, coughing, or heartburn.\nBefore reaching the emergency department, she used her albuterol inhaler without relief of symptoms and contacted emergency medical services. She has a 40–pack-year history of tobacco use, as well as a history of alcoholism (with her last consumption being 4 years before presentation) and remote marijuana use. Her medication regimen includes furosemide, fosinopril, isosorbide nitrate, pantoprazole, spironolactone, and enteric-coated aspirin, but she has a well-documented history of nonadherence.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 55-Year-Old Woman With Worsening Shortness of Breath"
},
{
"authors": "Stella Izuchukwu, MD, MPH",
"content": [
"On physical examination, the patient is afebrile, with a heart rate of 150 beats/min, a blood pressure of 202/126 mm Hg, a respiratory rate of 26 breaths/min, and an oxygen saturation of 81% while breathing room air (which improved to 92% when she was given a non-rebreather face mask). In general, she has labored respirations and is unable to speak in full sentences. She has no jugular venous distention of the neck; however, her lung fields are remarkable for bilateral crackles.",
"Her heart sounds include S1 and S2, but no murmurs, rubs, or gallops are noted. The patient has 1+ pitting edema of the lower extremities bilaterally. Incidentally, an atopic dyshidrotic eczematous rash of the skin is noted on the palmar and plantar surfaces of the patient's hands and feet.",
"Figure 1.",
"Figure 1.",
"An arterial blood gas sample obtained while the patient is breathing room air reveals a pH of 7.27, with a partial oxygen pressure of 54 mm Hg and a partial carbon dioxide pressure of 63 mm Hg. The complete blood cell count, coagulation profile, serum electrolyte panel, and renal function test are unremarkable.",
"ECG is performed (Figure 1), and it shows a tachycardic rhythm of 152 beats/min, with a left bundle branch block (which is noted to be preexisting when compared with a previous ECG). Chest radiography is performed, which reveals evidence of pulmonary edema."
],
"date": "December 14, 2015",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/855/834/855834-Thumb1.png"
}
],
"markdown": "# A 55-Year-Old Woman With Worsening Shortness of Breath\n\n **Authors:** Stella Izuchukwu, MD, MPH \n **Date:** December 14, 2015\n\n ## Content\n\n On physical examination, the patient is afebrile, with a heart rate of 150 beats/min, a blood pressure of 202/126 mm Hg, a respiratory rate of 26 breaths/min, and an oxygen saturation of 81% while breathing room air (which improved to 92% when she was given a non-rebreather face mask). In general, she has labored respirations and is unable to speak in full sentences. She has no jugular venous distention of the neck; however, her lung fields are remarkable for bilateral crackles.\nHer heart sounds include S1 and S2, but no murmurs, rubs, or gallops are noted. The patient has 1+ pitting edema of the lower extremities bilaterally. Incidentally, an atopic dyshidrotic eczematous rash of the skin is noted on the palmar and plantar surfaces of the patient's hands and feet.\nFigure 1.\nFigure 1.\nAn arterial blood gas sample obtained while the patient is breathing room air reveals a pH of 7.27, with a partial oxygen pressure of 54 mm Hg and a partial carbon dioxide pressure of 63 mm Hg. The complete blood cell count, coagulation profile, serum electrolyte panel, and renal function test are unremarkable.\nECG is performed (Figure 1), and it shows a tachycardic rhythm of 152 beats/min, with a left bundle branch block (which is noted to be preexisting when compared with a previous ECG). Chest radiography is performed, which reveals evidence of pulmonary edema.\n\n ## Figures\n\n **Figure 1.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 923079,
"choiceText": "Atrial fibrillation",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 923081,
"choiceText": "Ventricular tachycardia",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 923083,
"choiceText": "Atrial flutter",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 923085,
"choiceText": "Torsades de pointes\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 291551,
"questionText": "On the basis of the history, physical examination, and workup, what is the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 55-Year-Old Woman With Worsening Shortness of Breath"
},
{
"authors": "Stella Izuchukwu, MD, MPH",
"content": [
"The initial ECG (Figure 1) reveals a tachycardic rhythm that is regular and consistent with clockwise typical atrial flutter with a 2:1 atrioventricular conduction block;[1] this is consistent with congestive heart failure. Atrial flutter is a relatively common atrial tachyarrhythmia and is the most significant of the atrial tachyarrhythmias, after sinus tachycardia and atrial fibrillation. Atrial flutter is defined by the presence of stable, uniform atrial activation, which is seen on ECG as flutter waves (most evident in lead II; see Figure 1), and has traditionally been characterized as a macroreentrant arrhythmia, with atrial rates ranging from 240 to 400 beats/min (though the rate is most commonly around 300 beats/min).[1]",
"Figure 1.",
"Figure 1.",
"Depending on the ventricular rate, the rhythm can interfere with cardiac output, leading to heart failure or atrial thrombus formation. Thrombus places the patient at risk for possible systemic embolization and stroke. Atrial flutter usually includes an atrioventricular (AV) block, with the rhythm commonly conducted at a 2:1 or 4:1 ratio; less commonly, a ratio of 3:1 or 5:1 is seen.[1]",
"The pathophysiology of atrial flutter includes multiple reentrant or ectopic atrial waveforms reaching the AV node. Two forms of atrial flutter are recognized: type I and type II. Type I, also called \"typical,\" \"common,\" or \"isthmus-dependent\" atrial flutter, involves a reentrant circuit that encircles the tricuspid annulus of the right atrium, with rates that average 240-340 beats/min. The depolarizing impulse can travel in a \"counterclockwise\" fashion around the tricuspid annulus, resulting in negative flutter waves in the inferior leads, or it can travel in a \"clockwise\" fashion, resulting in positive flutter waves in the inferior leads.",
"Type II atrial flutter, also known as \"atypical\" flutter, is less common and remains uncharacterized except for unusually high heart rates of 340-440 beats/min.[1,2]",
"The exact etiology of atrial flutter is unknown, but it has been found to occur in patients with various conditions, such as heart failure, pericarditis, valvular disease, pulmonary embolism, chronic obstructive pulmonary disease, and hyperthyroidism, as well as after most types of cardiac surgery. An estimated 200,000 new cases of atrial flutter are diagnosed each year in the United States. The condition tends to be more common in elderly men and in patients with the above-mentioned comorbidities; however, it can also occur in patients without identifiable risk factors.[1]"
],
"date": "December 14, 2015",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/855/834/855834-Thumb1.png"
}
],
"markdown": "# A 55-Year-Old Woman With Worsening Shortness of Breath\n\n **Authors:** Stella Izuchukwu, MD, MPH \n **Date:** December 14, 2015\n\n ## Content\n\n The initial ECG (Figure 1) reveals a tachycardic rhythm that is regular and consistent with clockwise typical atrial flutter with a 2:1 atrioventricular conduction block;[1] this is consistent with congestive heart failure. Atrial flutter is a relatively common atrial tachyarrhythmia and is the most significant of the atrial tachyarrhythmias, after sinus tachycardia and atrial fibrillation. Atrial flutter is defined by the presence of stable, uniform atrial activation, which is seen on ECG as flutter waves (most evident in lead II; see Figure 1), and has traditionally been characterized as a macroreentrant arrhythmia, with atrial rates ranging from 240 to 400 beats/min (though the rate is most commonly around 300 beats/min).[1]\nFigure 1.\nFigure 1.\nDepending on the ventricular rate, the rhythm can interfere with cardiac output, leading to heart failure or atrial thrombus formation. Thrombus places the patient at risk for possible systemic embolization and stroke. Atrial flutter usually includes an atrioventricular (AV) block, with the rhythm commonly conducted at a 2:1 or 4:1 ratio; less commonly, a ratio of 3:1 or 5:1 is seen.[1]\nThe pathophysiology of atrial flutter includes multiple reentrant or ectopic atrial waveforms reaching the AV node. Two forms of atrial flutter are recognized: type I and type II. Type I, also called \"typical,\" \"common,\" or \"isthmus-dependent\" atrial flutter, involves a reentrant circuit that encircles the tricuspid annulus of the right atrium, with rates that average 240-340 beats/min. The depolarizing impulse can travel in a \"counterclockwise\" fashion around the tricuspid annulus, resulting in negative flutter waves in the inferior leads, or it can travel in a \"clockwise\" fashion, resulting in positive flutter waves in the inferior leads.\nType II atrial flutter, also known as \"atypical\" flutter, is less common and remains uncharacterized except for unusually high heart rates of 340-440 beats/min.[1,2]\nThe exact etiology of atrial flutter is unknown, but it has been found to occur in patients with various conditions, such as heart failure, pericarditis, valvular disease, pulmonary embolism, chronic obstructive pulmonary disease, and hyperthyroidism, as well as after most types of cardiac surgery. An estimated 200,000 new cases of atrial flutter are diagnosed each year in the United States. The condition tends to be more common in elderly men and in patients with the above-mentioned comorbidities; however, it can also occur in patients without identifiable risk factors.[1]\n\n ## Figures\n\n **Figure 1.** \n \n\n\n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 923079,
"choiceText": "Atrial fibrillation",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 923081,
"choiceText": "Ventricular tachycardia",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 923083,
"choiceText": "Atrial flutter",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 923085,
"choiceText": "Torsades de pointes\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 291551,
"questionText": "On the basis of the history, physical examination, and workup, what is the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 55-Year-Old Woman With Worsening Shortness of Breath"
},
{
"authors": "Stella Izuchukwu, MD, MPH",
"content": [
"After addressing the need for immediate cardioversion in patients with atrial flutter who may be hemodynamically unstable, the general goals for pharmacotherapy include ventricular rate control, conversion to a normal sinus rhythm (NSR), prevention of recurrent episodes, minimization of the risk for thromboembolic complications, and minimization of any adverse effects from pharmacologic therapy.[1,2]",
"Figure 1.",
"Figure 1.",
"Ventricular rate control can alleviate the symptoms associated with a rapid ventricular response. Two classes of medications are routinely used: calcium-channel blockers (eg, diltiazem) and beta-blockers (eg, metoprolol). Both classes of medications work by blocking conduction of the atrial tachydysrhythmia through the AV node. The potential for hypotension associated with the negative inotropic effects of these drugs should be considered when they are used to treat atrial flutter, especially in patients with severe left ventricular dysfunction.",
"After controlling the ventricular rate, the safety of attempting restoration of NSR must be established; addressing the need for anticoagulation therapy for the prevention of thromboembolic phenomenon should be the first consideration. The success rate of direct-current (DC) synchronized cardioversion is > 95% for returning the heart to NSR; however, as with atrial fibrillation, the success rate of sinus rhythm maintenance after 1 year of DC cardioversion without the aid of antiarrhythmic pharmacotherapy varies from 20% to 50%.",
"Atrial flutter generally requires less energy for conversion than atrial fibrillation; in many cases, conversion is achieved with as little as 50 joules. Pharmacologic cardioversion is an alternative to electrical cardioversion, and it offers several choices with regard to specific medications.",
"Procainamide is effective in 0%-13% of patients; flecainide in approximately 10% of patients; and dofetilide in approximately 70%-80% of patients. Ibutilide can convert recent-onset atrial flutter to NSR in 63% of patients with a single infusion. Large, single oral doses of class Ic antiarrhythmic agents, such as propafenone (450-600 mg) or flecainide (200-300 mg), have been shown to be effective in converting recent-onset atrial fibrillation to NSR as well.[2]",
"Oral amiodarone during the loading period (>1 mo) has been shown to convert 18% of cases of atrial fibrillation or flutter to NSR. Intravenous amiodarone is also effective in converting an atrial flutter to NSR, and it slows the ventricular rate in patients with a rapid ventricular response.",
"A final option is atrial overdrive pacing, which can be performed invasively or through the use of a transesophageal electrode to pace the left atrium; this therapy has a success rate of approximately 50%.[2] A combination of DC cardioversion and antiarrhythmic therapy is most commonly used to effectively restore NSR and maintain sinus rhythm.[1,2]"
],
"date": "December 14, 2015",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/855/834/855834-Thumb1.png"
}
],
"markdown": "# A 55-Year-Old Woman With Worsening Shortness of Breath\n\n **Authors:** Stella Izuchukwu, MD, MPH \n **Date:** December 14, 2015\n\n ## Content\n\n After addressing the need for immediate cardioversion in patients with atrial flutter who may be hemodynamically unstable, the general goals for pharmacotherapy include ventricular rate control, conversion to a normal sinus rhythm (NSR), prevention of recurrent episodes, minimization of the risk for thromboembolic complications, and minimization of any adverse effects from pharmacologic therapy.[1,2]\nFigure 1.\nFigure 1.\nVentricular rate control can alleviate the symptoms associated with a rapid ventricular response. Two classes of medications are routinely used: calcium-channel blockers (eg, diltiazem) and beta-blockers (eg, metoprolol). Both classes of medications work by blocking conduction of the atrial tachydysrhythmia through the AV node. The potential for hypotension associated with the negative inotropic effects of these drugs should be considered when they are used to treat atrial flutter, especially in patients with severe left ventricular dysfunction.\nAfter controlling the ventricular rate, the safety of attempting restoration of NSR must be established; addressing the need for anticoagulation therapy for the prevention of thromboembolic phenomenon should be the first consideration. The success rate of direct-current (DC) synchronized cardioversion is > 95% for returning the heart to NSR; however, as with atrial fibrillation, the success rate of sinus rhythm maintenance after 1 year of DC cardioversion without the aid of antiarrhythmic pharmacotherapy varies from 20% to 50%.\nAtrial flutter generally requires less energy for conversion than atrial fibrillation; in many cases, conversion is achieved with as little as 50 joules. Pharmacologic cardioversion is an alternative to electrical cardioversion, and it offers several choices with regard to specific medications.\nProcainamide is effective in 0%-13% of patients; flecainide in approximately 10% of patients; and dofetilide in approximately 70%-80% of patients. Ibutilide can convert recent-onset atrial flutter to NSR in 63% of patients with a single infusion. Large, single oral doses of class Ic antiarrhythmic agents, such as propafenone (450-600 mg) or flecainide (200-300 mg), have been shown to be effective in converting recent-onset atrial fibrillation to NSR as well.[2]\nOral amiodarone during the loading period (>1 mo) has been shown to convert 18% of cases of atrial fibrillation or flutter to NSR. Intravenous amiodarone is also effective in converting an atrial flutter to NSR, and it slows the ventricular rate in patients with a rapid ventricular response.\nA final option is atrial overdrive pacing, which can be performed invasively or through the use of a transesophageal electrode to pace the left atrium; this therapy has a success rate of approximately 50%.[2] A combination of DC cardioversion and antiarrhythmic therapy is most commonly used to effectively restore NSR and maintain sinus rhythm.[1,2]\n\n ## Figures\n\n **Figure 1.** \n \n\n\n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 55-Year-Old Woman With Worsening Shortness of Breath"
},
{
"authors": "Stella Izuchukwu, MD, MPH",
"content": [
"After the initial episode of atrial fibrillation has terminated and any underlying precipitating factors have been treated, some patients may not need any further intervention, except for avoidance of the precipitating factor (eg, alcohol or caffeine); however, as mentioned above, approximately 30% of patients remain in sinus rhythm at 1 year without antiarrhythmic therapy, requiring some sort of maintenance therapy. The guidelines regarding the use of antiarrhythmic agents in atrial flutter are similar to those for using antiarrhythmic agents in atrial fibrillation.",
"In addition, radiofrequency ablation has a high success rate and low complication rate for treating atrial flutter, and in many cases, it is a more favorable option compared with lifelong antiarrhythmic drug therapy because of adverse reactions that can include fatal proarrhythmic events and organ toxicity. Antiarrhythmics used to treat atrial fibrillation have been shown to be effective in treating fibrillation or flutter during 6 to 12 months of follow-up; the specific characteristics and the adverse effects of each antiarrhythmic agent should be considered when selecting which pharmacologic agent to use.",
"Generally speaking, class Ic agents may be used in patients without structural heart disease[3]; however, for patients with left ventricular hypertrophy with or without ischemia or conduction delay, class III agents (specifically, sotalol or amiodarone) are the drugs of choice. For patients with significant systolic dysfunction, dofetilide can be an effective option provided that there is no evidence of renal dysfunction.[1,2]",
"Risk for thromboembolic complications is increased in patients who have been in atrial flutter for more than 48 hours, which may result from episodic atrial fibrillation leading to impaired left atrial appendage function and subsequent thrombus formation. The same anticoagulation strategy used in patients with atrial fibrillation may be applied to patients with atrial flutter.[2]",
"Some reports have demonstrated thrombus in the left atrium appendage in as many as 43% of patients with atrial flutter, with postcardioversion thromboembolic events occurring in up to 7% of patients who were not anticoagulated. These events, if not occurring immediately after cardioversion, typically occur about 3 days after cardioversion, with almost all cases occurring within 10 days after restoration of NSR. Stunning of the left atrial appendage is thought to contribute to thrombogenicity and may play a role for as long as 4 weeks after cardioversion. This is believed to be the source of emboli in patients who have had a thromboembolic event after cardioversion despite no evidence of thrombus on transesophageal electrocardiography (TEE).",
"Anticoagulation is also recommended for at least 1 week after any ablation of an atrial flutter that persists for more than 48 hours. Adequate anticoagulation, as recommended by the American College of Chest Physicians, has been shown to decrease thromboembolic complications in patients with chronic atrial flutter and in patients undergoing cardioversion.[2]",
"Figure 1.",
"Figure 1.",
"Figure 2.",
"Figure 2.",
"The patient in this case was placed on noninvasive positive-pressure ventilation for her pulmonary edema and treated with intravenous diltiazem for the rapid ventricular response to the underlying 2:1 atrial flutter rhythm. Administration of diltiazem resulted in conversion to a variable block that was mainly 4:1, resulting in a heart rate of around 80 beats/min. Additional intravenous pharmacologic therapy with furosemide, nitroglycerin, and morphine sulfate was administered for her congestive heart failure. Heparin was administered as an anticoagulant for the atrial flutter.",
"After several hours, the patient improved notably and was in no evident respiratory distress. The initial examination of cardiac enzymes was unremarkable, and she was admitted to a cardiac unit on a monitored floor for further management of her congestive heart failure, evaluation for possible myocardial ischemia, and workup for her atrial flutter. She continued to improve with intravenous diuretic therapy; on hospital day 2, she underwent TEE that did not reveal structural heart disease or thrombus in the left atrium. She underwent successful DC cardioversion (see the ECG in Figure 2) and was discharged on warfarin therapy with follow-up."
],
"date": "December 14, 2015",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/855/834/855834-Thumb1.png"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/855/834/855834-Thumb2.png"
}
],
"markdown": "# A 55-Year-Old Woman With Worsening Shortness of Breath\n\n **Authors:** Stella Izuchukwu, MD, MPH \n **Date:** December 14, 2015\n\n ## Content\n\n After the initial episode of atrial fibrillation has terminated and any underlying precipitating factors have been treated, some patients may not need any further intervention, except for avoidance of the precipitating factor (eg, alcohol or caffeine); however, as mentioned above, approximately 30% of patients remain in sinus rhythm at 1 year without antiarrhythmic therapy, requiring some sort of maintenance therapy. The guidelines regarding the use of antiarrhythmic agents in atrial flutter are similar to those for using antiarrhythmic agents in atrial fibrillation.\nIn addition, radiofrequency ablation has a high success rate and low complication rate for treating atrial flutter, and in many cases, it is a more favorable option compared with lifelong antiarrhythmic drug therapy because of adverse reactions that can include fatal proarrhythmic events and organ toxicity. Antiarrhythmics used to treat atrial fibrillation have been shown to be effective in treating fibrillation or flutter during 6 to 12 months of follow-up; the specific characteristics and the adverse effects of each antiarrhythmic agent should be considered when selecting which pharmacologic agent to use.\nGenerally speaking, class Ic agents may be used in patients without structural heart disease[3]; however, for patients with left ventricular hypertrophy with or without ischemia or conduction delay, class III agents (specifically, sotalol or amiodarone) are the drugs of choice. For patients with significant systolic dysfunction, dofetilide can be an effective option provided that there is no evidence of renal dysfunction.[1,2]\nRisk for thromboembolic complications is increased in patients who have been in atrial flutter for more than 48 hours, which may result from episodic atrial fibrillation leading to impaired left atrial appendage function and subsequent thrombus formation. The same anticoagulation strategy used in patients with atrial fibrillation may be applied to patients with atrial flutter.[2]\nSome reports have demonstrated thrombus in the left atrium appendage in as many as 43% of patients with atrial flutter, with postcardioversion thromboembolic events occurring in up to 7% of patients who were not anticoagulated. These events, if not occurring immediately after cardioversion, typically occur about 3 days after cardioversion, with almost all cases occurring within 10 days after restoration of NSR. Stunning of the left atrial appendage is thought to contribute to thrombogenicity and may play a role for as long as 4 weeks after cardioversion. This is believed to be the source of emboli in patients who have had a thromboembolic event after cardioversion despite no evidence of thrombus on transesophageal electrocardiography (TEE).\nAnticoagulation is also recommended for at least 1 week after any ablation of an atrial flutter that persists for more than 48 hours. Adequate anticoagulation, as recommended by the American College of Chest Physicians, has been shown to decrease thromboembolic complications in patients with chronic atrial flutter and in patients undergoing cardioversion.[2]\nFigure 1.\nFigure 1.\nFigure 2.\nFigure 2.\nThe patient in this case was placed on noninvasive positive-pressure ventilation for her pulmonary edema and treated with intravenous diltiazem for the rapid ventricular response to the underlying 2:1 atrial flutter rhythm. Administration of diltiazem resulted in conversion to a variable block that was mainly 4:1, resulting in a heart rate of around 80 beats/min. Additional intravenous pharmacologic therapy with furosemide, nitroglycerin, and morphine sulfate was administered for her congestive heart failure. Heparin was administered as an anticoagulant for the atrial flutter.\nAfter several hours, the patient improved notably and was in no evident respiratory distress. The initial examination of cardiac enzymes was unremarkable, and she was admitted to a cardiac unit on a monitored floor for further management of her congestive heart failure, evaluation for possible myocardial ischemia, and workup for her atrial flutter. She continued to improve with intravenous diuretic therapy; on hospital day 2, she underwent TEE that did not reveal structural heart disease or thrombus in the left atrium. She underwent successful DC cardioversion (see the ECG in Figure 2) and was discharged on warfarin therapy with follow-up.\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n\n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 923095,
"choiceText": "Control of the ventricular response",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 923097,
"choiceText": "Conversion to a normal sinus rhythm",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 923099,
"choiceText": "Coronary artery catheterization ",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 923101,
"choiceText": "Prevention of recurrent episodes",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 923103,
"choiceText": "Prevention of thromboembolic complications",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "After addressing the need for immediate cardioversion for the minority of patients with atrial flutter who are hemodynamically unstable, the general goals of pharmacologic treatment of the condition include not only control of the ventricular rate and termination of the rhythm to NSR, but also prevention of recurrent episodes, prevention of thromboembolic complications, and minimization of any adverse effects from the pharmacologic therapy. Typically, coronary artery catheterization is not necessary in the management of a patient with atrial flutter, unless there is a concomitant acute coronary syndrome.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 291555,
"questionText": "Which of the following is <i>not</i> a therapeutic consideration in the management of patients with atrial flutter?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 923113,
"choiceText": "Procainamide ",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 923115,
"choiceText": "Furosemide",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 923117,
"choiceText": "Dofetilide",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 923119,
"choiceText": "Ibutilide ",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 923121,
"choiceText": "Amiodarone ",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Several medications are available for the pharmacologic cardioversion of atrial flutter. Procainamide is effective in 0%-13% of patients, and flecainide is effective in approximately 10% of patients. Dofetilide is effective in approximately 70%-80% of patients, and ibutilide can convert recent-onset atrial flutter to a sinus rhythm in 63% of patients with a single infusion. A large, single oral dose of a class Ic antiarrhythmic agent, such as propafenone (450-600 mg) or flecainide (200-300 mg), has also been shown to be effective in converting recent-onset atrial flutter to NSR.<br><br>\r\n\r\nOral amiodarone during the loading period (>1 mo) has been shown to convert 18% of cases of atrial flutter to NSR. Intravenous amiodarone is also effective in converting atrial flutter to NSR, and it can slow the ventricular rate in patients with a rapid ventricular response. Furosemide is a loop diuretic that is not effective in converting atrial flutter to NSR.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 291559,
"questionText": "Which of the following medications is <i>not</i> typically used for pharmacologic cardioversion?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 55-Year-Old Woman With Worsening Shortness of Breath"
},
{
"authors": "Stella Izuchukwu, MD, MPH",
"content": [],
"date": "December 14, 2015",
"figures": [],
"markdown": "# A 55-Year-Old Woman With Worsening Shortness of Breath\n\n **Authors:** Stella Izuchukwu, MD, MPH \n **Date:** December 14, 2015\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 923095,
"choiceText": "Control of the ventricular response",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 923097,
"choiceText": "Conversion to a normal sinus rhythm",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 923099,
"choiceText": "Coronary artery catheterization ",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 923101,
"choiceText": "Prevention of recurrent episodes",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 923103,
"choiceText": "Prevention of thromboembolic complications",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "After addressing the need for immediate cardioversion for the minority of patients with atrial flutter who are hemodynamically unstable, the general goals of pharmacologic treatment of the condition include not only control of the ventricular rate and termination of the rhythm to NSR, but also prevention of recurrent episodes, prevention of thromboembolic complications, and minimization of any adverse effects from the pharmacologic therapy. Typically, coronary artery catheterization is not necessary in the management of a patient with atrial flutter, unless there is a concomitant acute coronary syndrome.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 291555,
"questionText": "Which of the following is <i>not</i> a therapeutic consideration in the management of patients with atrial flutter?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 923113,
"choiceText": "Procainamide ",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 923115,
"choiceText": "Furosemide",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 923117,
"choiceText": "Dofetilide",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 923119,
"choiceText": "Ibutilide ",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 923121,
"choiceText": "Amiodarone ",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Several medications are available for the pharmacologic cardioversion of atrial flutter. Procainamide is effective in 0%-13% of patients, and flecainide is effective in approximately 10% of patients. Dofetilide is effective in approximately 70%-80% of patients, and ibutilide can convert recent-onset atrial flutter to a sinus rhythm in 63% of patients with a single infusion. A large, single oral dose of a class Ic antiarrhythmic agent, such as propafenone (450-600 mg) or flecainide (200-300 mg), has also been shown to be effective in converting recent-onset atrial flutter to NSR.<br><br>\r\n\r\nOral amiodarone during the loading period (>1 mo) has been shown to convert 18% of cases of atrial flutter to NSR. Intravenous amiodarone is also effective in converting atrial flutter to NSR, and it can slow the ventricular rate in patients with a rapid ventricular response. Furosemide is a loop diuretic that is not effective in converting atrial flutter to NSR.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 291559,
"questionText": "Which of the following medications is <i>not</i> typically used for pharmacologic cardioversion?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 55-Year-Old Woman With Worsening Shortness of Breath"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 923079,
"choiceText": "Atrial fibrillation",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 923081,
"choiceText": "Ventricular tachycardia",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 923083,
"choiceText": "Atrial flutter",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 923085,
"choiceText": "Torsades de pointes\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 291551,
"questionText": "On the basis of the history, physical examination, and workup, what is the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 923095,
"choiceText": "Control of the ventricular response",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 923097,
"choiceText": "Conversion to a normal sinus rhythm",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 923099,
"choiceText": "Coronary artery catheterization ",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 923101,
"choiceText": "Prevention of recurrent episodes",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 923103,
"choiceText": "Prevention of thromboembolic complications",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "After addressing the need for immediate cardioversion for the minority of patients with atrial flutter who are hemodynamically unstable, the general goals of pharmacologic treatment of the condition include not only control of the ventricular rate and termination of the rhythm to NSR, but also prevention of recurrent episodes, prevention of thromboembolic complications, and minimization of any adverse effects from the pharmacologic therapy. Typically, coronary artery catheterization is not necessary in the management of a patient with atrial flutter, unless there is a concomitant acute coronary syndrome.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 291555,
"questionText": "Which of the following is <i>not</i> a therapeutic consideration in the management of patients with atrial flutter?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 923113,
"choiceText": "Procainamide ",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 923115,
"choiceText": "Furosemide",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 923117,
"choiceText": "Dofetilide",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 923119,
"choiceText": "Ibutilide ",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 923121,
"choiceText": "Amiodarone ",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Several medications are available for the pharmacologic cardioversion of atrial flutter. Procainamide is effective in 0%-13% of patients, and flecainide is effective in approximately 10% of patients. Dofetilide is effective in approximately 70%-80% of patients, and ibutilide can convert recent-onset atrial flutter to a sinus rhythm in 63% of patients with a single infusion. A large, single oral dose of a class Ic antiarrhythmic agent, such as propafenone (450-600 mg) or flecainide (200-300 mg), has also been shown to be effective in converting recent-onset atrial flutter to NSR.<br><br>\r\n\r\nOral amiodarone during the loading period (>1 mo) has been shown to convert 18% of cases of atrial flutter to NSR. Intravenous amiodarone is also effective in converting atrial flutter to NSR, and it can slow the ventricular rate in patients with a rapid ventricular response. Furosemide is a loop diuretic that is not effective in converting atrial flutter to NSR.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 291559,
"questionText": "Which of the following medications is <i>not</i> typically used for pharmacologic cardioversion?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
855458 | /viewarticle/855458 | [
{
"authors": "Shradha B. Ahuja, MBBS; Rajiv S. Hira, MBBS; Vijay Panikar, MD",
"content": [
"Editor's Note:\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.",
"A 60-year-old, right-handed woman is brought to the emergency department (ED) for left-sided weakness after an unwitnessed fall. She fell to the floor on her left side while attempting to get out of bed. She realized that her entire left side was weak, and she did not have the strength to stand up on her own.",
"Her family states that she has been sleeping much more than normal and had had a severe occipital headache for the past 3 days, accompanied by 6-7 episodes of vomiting per day. The vomiting is not relieved by antacids and proton pump inhibitors, and it is not associated with nausea. She also complained to her family of feeling feverish for the past few days and had appeared intermittently agitated. She denies any history of head trauma, photophobia, diplopia, convulsions, dizziness, difficulty speaking, or difficulty swallowing.",
"No prior history of stroke, transient ischemic attacks, hypertension, coronary artery disease, rheumatic heart disease, or atrial fibrillation is noted. The patient reports no allergies. On review of her family history, she reports that her husband was diagnosed with pulmonary tuberculosis (TB) 10 months ago. She states that he was treated with antituberculosis medications for 6 months but admits that he had not been compliant with the entire course of treatment. She did not have a tuberculin skin test (TST), interferon gamma-release assay, or chest radiograph when he was diagnosed.",
"The patient does not smoke tobacco, drink alcohol, or use illicit drugs. She has never used oral contraceptive agents. She lives in Mumbai, India, and has never traveled abroad."
],
"date": "December 10, 2015",
"figures": [],
"markdown": "# Left-Sided Weakness in a 60-Year-Old Woman\n\n **Authors:** Shradha B. Ahuja, MBBS; Rajiv S. Hira, MBBS; Vijay Panikar, MD \n **Date:** December 10, 2015\n\n ## Content\n\n Editor's Note:\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.\nA 60-year-old, right-handed woman is brought to the emergency department (ED) for left-sided weakness after an unwitnessed fall. She fell to the floor on her left side while attempting to get out of bed. She realized that her entire left side was weak, and she did not have the strength to stand up on her own.\nHer family states that she has been sleeping much more than normal and had had a severe occipital headache for the past 3 days, accompanied by 6-7 episodes of vomiting per day. The vomiting is not relieved by antacids and proton pump inhibitors, and it is not associated with nausea. She also complained to her family of feeling feverish for the past few days and had appeared intermittently agitated. She denies any history of head trauma, photophobia, diplopia, convulsions, dizziness, difficulty speaking, or difficulty swallowing.\nNo prior history of stroke, transient ischemic attacks, hypertension, coronary artery disease, rheumatic heart disease, or atrial fibrillation is noted. The patient reports no allergies. On review of her family history, she reports that her husband was diagnosed with pulmonary tuberculosis (TB) 10 months ago. She states that he was treated with antituberculosis medications for 6 months but admits that he had not been compliant with the entire course of treatment. She did not have a tuberculin skin test (TST), interferon gamma-release assay, or chest radiograph when he was diagnosed.\nThe patient does not smoke tobacco, drink alcohol, or use illicit drugs. She has never used oral contraceptive agents. She lives in Mumbai, India, and has never traveled abroad.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "Left-Sided Weakness in a 60-Year-Old Woman"
},
{
"authors": "Shradha B. Ahuja, MBBS; Rajiv S. Hira, MBBS; Vijay Panikar, MD",
"content": [
"Upon physical examination, the patient's oral temperature is 99.6°F. Her pulse is regular, with a rate of 60 beats/min. Her blood pressure is 130/80 mm Hg, and her respiratory rate is 10 breaths/min.",
"Auscultation of the heart reveals a normal S1 and S2, with no murmurs or rub. Palpation of the abdomen reveals no tenderness, masses, or enlargement of the liver or spleen. The lungs are clear in all fields except for slight coarse sounds heard over the right lung field.",
"The patient appears to be drowsy and disoriented. She follows commands poorly and withdraws from painful stimulus, and her total Glasgow Coma Scale score is 10. She is spontaneously breathing and appears to be controlling her secretions. No evidence of trauma on head-to-toe examination is noted. Cervical lymph nodes are palpable and are measured at 2-3 cm in diameter. Both pupils are 3 mm and are reactive to light.",
"She is unable to flex her neck (meningismus) and, on forceful flexion, partial flexion of both right and left thighs (positive Brudzinski sign) is observed. Muscle strength in the left upper and lower limbs is decreased at grade 2/5, or full range of motion without gravity, whereas reflexes in the same extremities are brisk and excessive. Both strength and deep tendon reflexes on the right side are normal. The plantar reflex is positive for the Babinski sign bilaterally. Left hemianesthesia and left homonymous hemianopsia are observed. The rest of the cranial nerve examination findings are normal.",
"The laboratory analysis includes a complete blood count that reveals an elevated total leukocyte count of 13.5 × 103 cells/μL (normal range, 4.0-10.0 × 103 cells/μL) with 56% lymphocytes (normal range, 20%-45%) and an elevated erythrocyte sedimentation rate of 80 mm/hr (normal range in women, 0-20 mm/hr). Other biochemical investigations reveal hyponatremia, with sodium levels at 120 mEq/L (normal range, 135-145 mEq/L), and normal potassium and chloride levels. Serum glucose level is 78 mg/dL.",
"Figure 1.",
"Figure 1.",
"Tests for HIV-1 and HIV-2 are nonreactive, and liver function tests are normal. A lumbar puncture is performed that reveals hazy cerebrospinal fluid (CSF), with an elevated protein count at 156 mg/dL (normal range, 10-60 mg/dL) and a decreased glucose level of 35 mg/dL (normal range, 40-80 mg/dL). On microscopic examination, the CSF has an elevated total cell count of 40 cells/µL (normal range, 0-5 cells/µL), with 90% of cells being lymphocytes.",
"CT shows a hypodense area in the distribution of the right middle cerebral artery that is consistent with a cerebral infarction (Figure 1)."
],
"date": "December 10, 2015",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/855/458/855458-Thumb1.JPG"
}
],
"markdown": "# Left-Sided Weakness in a 60-Year-Old Woman\n\n **Authors:** Shradha B. Ahuja, MBBS; Rajiv S. Hira, MBBS; Vijay Panikar, MD \n **Date:** December 10, 2015\n\n ## Content\n\n Upon physical examination, the patient's oral temperature is 99.6°F. Her pulse is regular, with a rate of 60 beats/min. Her blood pressure is 130/80 mm Hg, and her respiratory rate is 10 breaths/min.\nAuscultation of the heart reveals a normal S1 and S2, with no murmurs or rub. Palpation of the abdomen reveals no tenderness, masses, or enlargement of the liver or spleen. The lungs are clear in all fields except for slight coarse sounds heard over the right lung field.\nThe patient appears to be drowsy and disoriented. She follows commands poorly and withdraws from painful stimulus, and her total Glasgow Coma Scale score is 10. She is spontaneously breathing and appears to be controlling her secretions. No evidence of trauma on head-to-toe examination is noted. Cervical lymph nodes are palpable and are measured at 2-3 cm in diameter. Both pupils are 3 mm and are reactive to light.\nShe is unable to flex her neck (meningismus) and, on forceful flexion, partial flexion of both right and left thighs (positive Brudzinski sign) is observed. Muscle strength in the left upper and lower limbs is decreased at grade 2/5, or full range of motion without gravity, whereas reflexes in the same extremities are brisk and excessive. Both strength and deep tendon reflexes on the right side are normal. The plantar reflex is positive for the Babinski sign bilaterally. Left hemianesthesia and left homonymous hemianopsia are observed. The rest of the cranial nerve examination findings are normal.\nThe laboratory analysis includes a complete blood count that reveals an elevated total leukocyte count of 13.5 × 103 cells/μL (normal range, 4.0-10.0 × 103 cells/μL) with 56% lymphocytes (normal range, 20%-45%) and an elevated erythrocyte sedimentation rate of 80 mm/hr (normal range in women, 0-20 mm/hr). Other biochemical investigations reveal hyponatremia, with sodium levels at 120 mEq/L (normal range, 135-145 mEq/L), and normal potassium and chloride levels. Serum glucose level is 78 mg/dL.\nFigure 1.\nFigure 1.\nTests for HIV-1 and HIV-2 are nonreactive, and liver function tests are normal. A lumbar puncture is performed that reveals hazy cerebrospinal fluid (CSF), with an elevated protein count at 156 mg/dL (normal range, 10-60 mg/dL) and a decreased glucose level of 35 mg/dL (normal range, 40-80 mg/dL). On microscopic examination, the CSF has an elevated total cell count of 40 cells/µL (normal range, 0-5 cells/µL), with 90% of cells being lymphocytes.\nCT shows a hypodense area in the distribution of the right middle cerebral artery that is consistent with a cerebral infarction (Figure 1).\n\n ## Figures\n\n **Figure 1.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 920525,
"choiceText": "Acute bacterial meningitis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 920527,
"choiceText": "Tuberculous meningitis with vasculitis",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 920529,
"choiceText": "Meningioma\r\n",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 920531,
"choiceText": "Lymphoma",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 290663,
"questionText": "What is the most likely diagnosis in this patient?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Left-Sided Weakness in a 60-Year-Old Woman"
},
{
"authors": "Shradha B. Ahuja, MBBS; Rajiv S. Hira, MBBS; Vijay Panikar, MD",
"content": [
"Tuberculous meningitis (TBM) was included in the differential diagnosis on the basis of the patient's history and physical examination. The initial prodrome of fever, fatigue, and irritability followed by signs of meningeal irritation, including headache, neck rigidity, vomiting, drowsiness, or altered mental status, suggests the possibility of meningitis. The CSF analysis showed elevated protein, decreased glucose, and lymphocytic pleocytosis, supporting the diagnosis of TBM. TBM may present with hemiparesis or other focal neurologic abnormalities due to a vasculitis-induced arterial infarction.",
"In this case, the constellation of left hemiparesis, left hemianesthesia, and left homonymous hemianopsia suggested involvement of the middle cerebral artery. Serum electrolytes revealed hyponatremia, which is thought to be related either to the syndrome of inappropriate antidiuretic hormone secretion (SIADH) or to secretion of plasma atrial natriuretic factor and may have contributed to the patient's lethargy.",
"Fifteen percent of TB cases have extrapulmonary manifestations. Meningitis is the second most common and by far the most devastating form. Although more common among children between 6 months and 5 years of age, the incidence of TB in the central nervous system (CNS) among adults has increased in the past two decades owing to the spread of HIV/AIDS.[1]",
"Mycobacterium tuberculosis bacilli enter the host by inhalation of 0.5- to 5-µm droplets. As few as 10 TB bacteria can cause a localized infection within the pulmonary alveoli, replicating within the alveolar macrophages and forming the primary site of infection (Ghon complex).[2,3,4] This TB bacteremia then causes seeding to other parts of the body.",
"Dissemination to the brain is more likely if miliary TB develops.[1] If TB reaches the brain, it forms metastatic caseous lesions called \"Rich foci.\" Symptoms of CNS TB depend on the size, location, and whether or not the tubercle ruptures. Should the tubercle rupture into the subarachnoid space, then meningitis results; however, if it enlarges, a tuberculoma or abscess can form, which can then burst into the ventricle.[1,7] The exudates within cerebral arteries cause a vasculitic picture of inflammation, infarction, or obstruction.[1]"
],
"date": "December 10, 2015",
"figures": [],
"markdown": "# Left-Sided Weakness in a 60-Year-Old Woman\n\n **Authors:** Shradha B. Ahuja, MBBS; Rajiv S. Hira, MBBS; Vijay Panikar, MD \n **Date:** December 10, 2015\n\n ## Content\n\n Tuberculous meningitis (TBM) was included in the differential diagnosis on the basis of the patient's history and physical examination. The initial prodrome of fever, fatigue, and irritability followed by signs of meningeal irritation, including headache, neck rigidity, vomiting, drowsiness, or altered mental status, suggests the possibility of meningitis. The CSF analysis showed elevated protein, decreased glucose, and lymphocytic pleocytosis, supporting the diagnosis of TBM. TBM may present with hemiparesis or other focal neurologic abnormalities due to a vasculitis-induced arterial infarction.\nIn this case, the constellation of left hemiparesis, left hemianesthesia, and left homonymous hemianopsia suggested involvement of the middle cerebral artery. Serum electrolytes revealed hyponatremia, which is thought to be related either to the syndrome of inappropriate antidiuretic hormone secretion (SIADH) or to secretion of plasma atrial natriuretic factor and may have contributed to the patient's lethargy.\nFifteen percent of TB cases have extrapulmonary manifestations. Meningitis is the second most common and by far the most devastating form. Although more common among children between 6 months and 5 years of age, the incidence of TB in the central nervous system (CNS) among adults has increased in the past two decades owing to the spread of HIV/AIDS.[1]\nMycobacterium tuberculosis bacilli enter the host by inhalation of 0.5- to 5-µm droplets. As few as 10 TB bacteria can cause a localized infection within the pulmonary alveoli, replicating within the alveolar macrophages and forming the primary site of infection (Ghon complex).[2,3,4] This TB bacteremia then causes seeding to other parts of the body.\nDissemination to the brain is more likely if miliary TB develops.[1] If TB reaches the brain, it forms metastatic caseous lesions called \"Rich foci.\" Symptoms of CNS TB depend on the size, location, and whether or not the tubercle ruptures. Should the tubercle rupture into the subarachnoid space, then meningitis results; however, if it enlarges, a tuberculoma or abscess can form, which can then burst into the ventricle.[1,7] The exudates within cerebral arteries cause a vasculitic picture of inflammation, infarction, or obstruction.[1]\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 920525,
"choiceText": "Acute bacterial meningitis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 920527,
"choiceText": "Tuberculous meningitis with vasculitis",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 920529,
"choiceText": "Meningioma\r\n",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 920531,
"choiceText": "Lymphoma",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 290663,
"questionText": "What is the most likely diagnosis in this patient?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Left-Sided Weakness in a 60-Year-Old Woman"
},
{
"authors": "Shradha B. Ahuja, MBBS; Rajiv S. Hira, MBBS; Vijay Panikar, MD",
"content": [
"The symptoms of TBM may be due to meningeal irritation, cerebral vasculitis, increased intracranial pressure (ICP), obstructive hydrocephalus caused by compression, or basilar cistern obstruction due to an intracranial space-occupying lesion. Meningeal irritation and increased ICP cause headache, neck stiffness, blurred vision, lethargy, confusion, altered mental status, nausea, vomiting, and seizures.[6,7]",
"When vasculitis occurs, it most commonly involves the middle cerebral artery and results in contralateral hemiplegia, hemianesthesia, homonymous hemianopsia, aphasia (dominant hemisphere), anosognosia, or constructional apraxia and hemineglect (nondominant hemisphere). Compression effects can cause cranial nerve palsies, especially of cranial nerve VI, followed by cranial nerves III, IV, and VII.[1] Arachnoiditis of the optic chiasm can cause visual disturbance and papilledema. Spread of the inflammatory process may include the spinal cord-associated vasculature, resulting in spinal cord-associated neurologic deficits.",
"The differential diagnosis of TBM includes other infectious processes (acute/subacute bacterial meningitis, viral infections, cerebral abscesses, tuberculomas, encephalitis, neurocysticercosis), vascular pathologies (vasculitis, vascular infarcts), and other space-occupying lesions (infectious lesions, primary tumors, metastasis). Depending on the region of the brain and spinal cord involved, TB of the CNS can have a varied presentation, which thereby broadens the differential diagnosis.",
"In suspected cases of TBM, the most important diagnostic tests are a full CSF analysis, including CSF adenosine deaminase (ADA) measurement, and radiographic evaluation, including chest radiography, CT, and MRI of the brain. Spinal fluid leukocytosis and lymphocytosis are key diagnostic features of TBM,[1] usually observed in combination with decreased CSF glucose levels and elevated CSF protein levels. Ziehl-Neelsen staining of the CSF may demonstrate acid-fast bacilli, and culture of the CSF for M tuberculosis should be done. Polymerase chain reaction (PCR) can also provide a rapid and reliable diagnosis of TBM. CSF ADA levels have high sensitivity and specificity for the diagnosis of TBM.[1,8,9] The significant cut-off value for CSF ADA is a level > 8.2 U/L. In this patient, the CSF ADA level was 15.3 U/L.",
"When TBM is a concern, imaging studies may be obtained. A posteroanterior and lateral chest radiograph may demonstrate signs of TB, such as infiltrates with or without cavitations, pleural effusion, and lymphadenopathy. CT of the brain characteristically will show nodular lesions with central hypodensity indicating caseation. Contrast enhancement is essential and will initially demonstrate nonencapsulated, isodense lesions with edema out of proportion to the mass effect. As the disease progresses, a hyperdense, well-encapsulated lesion develops, indicative of a tuberculoma.[1,6,10] MRI images demonstrate obliteration of the subarachnoid space along with the varying degrees of edema and mass effect.[1,6,10] Both CT and MRI contrast-enhanced images of the brain will also detect TBM sequelae, including cerebral infarcts, hydrocephalus, and basilar meningeal thickening and enhancement."
],
"date": "December 10, 2015",
"figures": [],
"markdown": "# Left-Sided Weakness in a 60-Year-Old Woman\n\n **Authors:** Shradha B. Ahuja, MBBS; Rajiv S. Hira, MBBS; Vijay Panikar, MD \n **Date:** December 10, 2015\n\n ## Content\n\n The symptoms of TBM may be due to meningeal irritation, cerebral vasculitis, increased intracranial pressure (ICP), obstructive hydrocephalus caused by compression, or basilar cistern obstruction due to an intracranial space-occupying lesion. Meningeal irritation and increased ICP cause headache, neck stiffness, blurred vision, lethargy, confusion, altered mental status, nausea, vomiting, and seizures.[6,7]\nWhen vasculitis occurs, it most commonly involves the middle cerebral artery and results in contralateral hemiplegia, hemianesthesia, homonymous hemianopsia, aphasia (dominant hemisphere), anosognosia, or constructional apraxia and hemineglect (nondominant hemisphere). Compression effects can cause cranial nerve palsies, especially of cranial nerve VI, followed by cranial nerves III, IV, and VII.[1] Arachnoiditis of the optic chiasm can cause visual disturbance and papilledema. Spread of the inflammatory process may include the spinal cord-associated vasculature, resulting in spinal cord-associated neurologic deficits.\nThe differential diagnosis of TBM includes other infectious processes (acute/subacute bacterial meningitis, viral infections, cerebral abscesses, tuberculomas, encephalitis, neurocysticercosis), vascular pathologies (vasculitis, vascular infarcts), and other space-occupying lesions (infectious lesions, primary tumors, metastasis). Depending on the region of the brain and spinal cord involved, TB of the CNS can have a varied presentation, which thereby broadens the differential diagnosis.\nIn suspected cases of TBM, the most important diagnostic tests are a full CSF analysis, including CSF adenosine deaminase (ADA) measurement, and radiographic evaluation, including chest radiography, CT, and MRI of the brain. Spinal fluid leukocytosis and lymphocytosis are key diagnostic features of TBM,[1] usually observed in combination with decreased CSF glucose levels and elevated CSF protein levels. Ziehl-Neelsen staining of the CSF may demonstrate acid-fast bacilli, and culture of the CSF for M tuberculosis should be done. Polymerase chain reaction (PCR) can also provide a rapid and reliable diagnosis of TBM. CSF ADA levels have high sensitivity and specificity for the diagnosis of TBM.[1,8,9] The significant cut-off value for CSF ADA is a level > 8.2 U/L. In this patient, the CSF ADA level was 15.3 U/L.\nWhen TBM is a concern, imaging studies may be obtained. A posteroanterior and lateral chest radiograph may demonstrate signs of TB, such as infiltrates with or without cavitations, pleural effusion, and lymphadenopathy. CT of the brain characteristically will show nodular lesions with central hypodensity indicating caseation. Contrast enhancement is essential and will initially demonstrate nonencapsulated, isodense lesions with edema out of proportion to the mass effect. As the disease progresses, a hyperdense, well-encapsulated lesion develops, indicative of a tuberculoma.[1,6,10] MRI images demonstrate obliteration of the subarachnoid space along with the varying degrees of edema and mass effect.[1,6,10] Both CT and MRI contrast-enhanced images of the brain will also detect TBM sequelae, including cerebral infarcts, hydrocephalus, and basilar meningeal thickening and enhancement.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "Left-Sided Weakness in a 60-Year-Old Woman"
},
{
"authors": "Shradha B. Ahuja, MBBS; Rajiv S. Hira, MBBS; Vijay Panikar, MD",
"content": [
"TB has been treated with combination therapy for over 50 years. Regimens that use only single drugs result in the rapid development of resistance and treatment failure.[11,12] Directly observed therapy, in which a trained healthcare worker observes the patient taking each dose of medication, has been a major strategy in the World Health Organization's global TB eradication program. This treatment and health system guideline improves health and decreases TB drug resistance.",
"The standard treatment for TBM is isoniazid, rifampicin, and pyrazinamide, adding streptomycin (or ethambutol) for 2 months for resistant or undetermined strains, followed by 7-10 months of isoniazid and rifampin[15,16] for fully susceptible strains along with steroids for 4-6 weeks.[14] TBM is the one infectious disease in which steroids have been shown to offer significant benefit.",
"The CSF of patients with treated TBM is commonly abnormal even at 12 months, and the resolution of CSF findings has no correlation with clinical progress or outcome. Repeated sampling of CSF by lumbar puncture to monitor treatment progress is therefore not indicated, nor is extending or repeating treatment on the basis of clinical symptoms.[15,16]",
"Early studies suggested that corticosteroids reduce CSF inflammation as well as time to recovery and mortality in patients with TBM.[12] Care should be used in patients with concurrent HIV infection. Although some studies have questioned the value of steroids, most experts still agree that the benefits outweigh the risks.[13] The dose of dexamethasone in TBM is 8-12 mg daily tapered over 6 weeks. In those who have failed to respond to steroid treatment, thalidomide may be of benefit in TBM and has been used in certain cases.[17]",
"In patients with evidence of obstructive hydrocephalus and neurologic deterioration, placement of a ventricular drain or ventriculoperitoneal or ventriculoatrial shunt should be done as soon as possible. Unless mass effect is compromising vital structures, surgical intervention is rarely required in the treatment of tuberculomas.[1]",
"In this case, a neurologist was consulted and 20% mannitol was administered intravenously at a dosage of 0.5 g/kg over 15 minutes. The patient's hyponatremia due to SIADH caused by the meningitis may reverse automatically on treatment of the underlying cause; however, in this case, the serum sodium levels were dangerously low and required correction. Fluids were limited to about 800-1000 mL/day; a low-dose diuretic and hypertonic saline were given intravenously at a dosage of 200 mL over 3 -4 hours. Dexamethasone was given at a dosage of 8 mg/day and tapered gradually over 2 weeks.",
"Chemotherapy consisted of administration of isoniazid in a dosage of 5 mg/kg body weight, rifampin at 10 mg/kg body weight, ethambutol at 25 mg/kg body weight, and pyrazinamide at 15 mg/kg body weight, each given three times a week for 2 months. The patient remained in the intensive care unit for 2 weeks. Supportive management included rehabilitation of the patient with physiotherapy, nutritional supplementation, and symptomatic treatment.",
"The patient's general condition improved significantly, and she was discharged from the hospital. She came for regular follow-up visits in the outpatient department for 6 weeks thereafter and showed a gradual improvement in muscle power on the left side, which became 4/5 by the sixth week. Upon completion of the 2-month course of antituberculous treatment, she was advised to continue isoniazid and rifampin (three times a week at the above doses) for 10 months."
],
"date": "December 10, 2015",
"figures": [],
"markdown": "# Left-Sided Weakness in a 60-Year-Old Woman\n\n **Authors:** Shradha B. Ahuja, MBBS; Rajiv S. Hira, MBBS; Vijay Panikar, MD \n **Date:** December 10, 2015\n\n ## Content\n\n TB has been treated with combination therapy for over 50 years. Regimens that use only single drugs result in the rapid development of resistance and treatment failure.[11,12] Directly observed therapy, in which a trained healthcare worker observes the patient taking each dose of medication, has been a major strategy in the World Health Organization's global TB eradication program. This treatment and health system guideline improves health and decreases TB drug resistance.\nThe standard treatment for TBM is isoniazid, rifampicin, and pyrazinamide, adding streptomycin (or ethambutol) for 2 months for resistant or undetermined strains, followed by 7-10 months of isoniazid and rifampin[15,16] for fully susceptible strains along with steroids for 4-6 weeks.[14] TBM is the one infectious disease in which steroids have been shown to offer significant benefit.\nThe CSF of patients with treated TBM is commonly abnormal even at 12 months, and the resolution of CSF findings has no correlation with clinical progress or outcome. Repeated sampling of CSF by lumbar puncture to monitor treatment progress is therefore not indicated, nor is extending or repeating treatment on the basis of clinical symptoms.[15,16]\nEarly studies suggested that corticosteroids reduce CSF inflammation as well as time to recovery and mortality in patients with TBM.[12] Care should be used in patients with concurrent HIV infection. Although some studies have questioned the value of steroids, most experts still agree that the benefits outweigh the risks.[13] The dose of dexamethasone in TBM is 8-12 mg daily tapered over 6 weeks. In those who have failed to respond to steroid treatment, thalidomide may be of benefit in TBM and has been used in certain cases.[17]\nIn patients with evidence of obstructive hydrocephalus and neurologic deterioration, placement of a ventricular drain or ventriculoperitoneal or ventriculoatrial shunt should be done as soon as possible. Unless mass effect is compromising vital structures, surgical intervention is rarely required in the treatment of tuberculomas.[1]\nIn this case, a neurologist was consulted and 20% mannitol was administered intravenously at a dosage of 0.5 g/kg over 15 minutes. The patient's hyponatremia due to SIADH caused by the meningitis may reverse automatically on treatment of the underlying cause; however, in this case, the serum sodium levels were dangerously low and required correction. Fluids were limited to about 800-1000 mL/day; a low-dose diuretic and hypertonic saline were given intravenously at a dosage of 200 mL over 3 -4 hours. Dexamethasone was given at a dosage of 8 mg/day and tapered gradually over 2 weeks.\nChemotherapy consisted of administration of isoniazid in a dosage of 5 mg/kg body weight, rifampin at 10 mg/kg body weight, ethambutol at 25 mg/kg body weight, and pyrazinamide at 15 mg/kg body weight, each given three times a week for 2 months. The patient remained in the intensive care unit for 2 weeks. Supportive management included rehabilitation of the patient with physiotherapy, nutritional supplementation, and symptomatic treatment.\nThe patient's general condition improved significantly, and she was discharged from the hospital. She came for regular follow-up visits in the outpatient department for 6 weeks thereafter and showed a gradual improvement in muscle power on the left side, which became 4/5 by the sixth week. Upon completion of the 2-month course of antituberculous treatment, she was advised to continue isoniazid and rifampin (three times a week at the above doses) for 10 months.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 920533,
"choiceText": "Elevated CSF glucose and elevated CSF protein",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 920535,
"choiceText": "Elevated CSF cell count with neutrophilia",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 920537,
"choiceText": "Hypodense lesion with edema out of proportion to the mass effect on contrast-enhanced CT of the brain",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 920539,
"choiceText": "A hyperdense, well-encapsulated lesion on CT of the brain",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 920541,
"choiceText": "A low CSF ADA level\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "CSF findings consistent with TBM are low glucose, elevated protein, elevated cell count with lymphocytosis, and an elevated ADA level. In the early stages of TBM, contrast-enhanced CT will show a hypodense lesion with edema out of proportion to the mass effect. Once a tuberculoma starts to develop later in the course of the disease, a hyperdense, well-encapsulated lesion will be seen on non–contrast-enhanced CT of the brain.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 290665,
"questionText": "You are treating a patient who has a stiff neck, low-grade fever, confusion, hemiplegia, and vision changes. You are concerned about meningitis, elevated ICP, and vasculitis. Which of the following findings are consistent with the early presentation of TBM?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 920543,
"choiceText": "Ziehl-Neelsen staining for acid-fast bacilli",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 920545,
"choiceText": "Tuberculin sensitivity test",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 920547,
"choiceText": "PCR analysis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 920549,
"choiceText": "Serum ADA levels",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 920551,
"choiceText": "CSF ADA levels\r\n",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Although PCR is the most rapid investigation for diagnosis of TB, research has shown that it can produce false-negative results in certain cases. Serum ADA levels, Ziehl-Neelsen staining, and tuberculin testing have been found to have low sensitivity; however, in pleural TB causing effusion and TBM, ADA testing had a higher sensitivity than any other tests.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 290667,
"questionText": "In a patient with findings that are consistent with meningitis, which test would be the most sensitive in ruling out TBM?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Left-Sided Weakness in a 60-Year-Old Woman"
},
{
"authors": "Shradha B. Ahuja, MBBS; Rajiv S. Hira, MBBS; Vijay Panikar, MD",
"content": [],
"date": "December 10, 2015",
"figures": [],
"markdown": "# Left-Sided Weakness in a 60-Year-Old Woman\n\n **Authors:** Shradha B. Ahuja, MBBS; Rajiv S. Hira, MBBS; Vijay Panikar, MD \n **Date:** December 10, 2015\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 920533,
"choiceText": "Elevated CSF glucose and elevated CSF protein",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 920535,
"choiceText": "Elevated CSF cell count with neutrophilia",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 920537,
"choiceText": "Hypodense lesion with edema out of proportion to the mass effect on contrast-enhanced CT of the brain",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 920539,
"choiceText": "A hyperdense, well-encapsulated lesion on CT of the brain",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 920541,
"choiceText": "A low CSF ADA level\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "CSF findings consistent with TBM are low glucose, elevated protein, elevated cell count with lymphocytosis, and an elevated ADA level. In the early stages of TBM, contrast-enhanced CT will show a hypodense lesion with edema out of proportion to the mass effect. Once a tuberculoma starts to develop later in the course of the disease, a hyperdense, well-encapsulated lesion will be seen on non–contrast-enhanced CT of the brain.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 290665,
"questionText": "You are treating a patient who has a stiff neck, low-grade fever, confusion, hemiplegia, and vision changes. You are concerned about meningitis, elevated ICP, and vasculitis. Which of the following findings are consistent with the early presentation of TBM?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 920543,
"choiceText": "Ziehl-Neelsen staining for acid-fast bacilli",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 920545,
"choiceText": "Tuberculin sensitivity test",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 920547,
"choiceText": "PCR analysis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 920549,
"choiceText": "Serum ADA levels",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 920551,
"choiceText": "CSF ADA levels\r\n",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Although PCR is the most rapid investigation for diagnosis of TB, research has shown that it can produce false-negative results in certain cases. Serum ADA levels, Ziehl-Neelsen staining, and tuberculin testing have been found to have low sensitivity; however, in pleural TB causing effusion and TBM, ADA testing had a higher sensitivity than any other tests.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 290667,
"questionText": "In a patient with findings that are consistent with meningitis, which test would be the most sensitive in ruling out TBM?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Left-Sided Weakness in a 60-Year-Old Woman"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 920525,
"choiceText": "Acute bacterial meningitis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 920527,
"choiceText": "Tuberculous meningitis with vasculitis",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 920529,
"choiceText": "Meningioma\r\n",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 920531,
"choiceText": "Lymphoma",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 290663,
"questionText": "What is the most likely diagnosis in this patient?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 920533,
"choiceText": "Elevated CSF glucose and elevated CSF protein",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 920535,
"choiceText": "Elevated CSF cell count with neutrophilia",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 920537,
"choiceText": "Hypodense lesion with edema out of proportion to the mass effect on contrast-enhanced CT of the brain",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 920539,
"choiceText": "A hyperdense, well-encapsulated lesion on CT of the brain",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 920541,
"choiceText": "A low CSF ADA level\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "CSF findings consistent with TBM are low glucose, elevated protein, elevated cell count with lymphocytosis, and an elevated ADA level. In the early stages of TBM, contrast-enhanced CT will show a hypodense lesion with edema out of proportion to the mass effect. Once a tuberculoma starts to develop later in the course of the disease, a hyperdense, well-encapsulated lesion will be seen on non–contrast-enhanced CT of the brain.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 290665,
"questionText": "You are treating a patient who has a stiff neck, low-grade fever, confusion, hemiplegia, and vision changes. You are concerned about meningitis, elevated ICP, and vasculitis. Which of the following findings are consistent with the early presentation of TBM?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 920543,
"choiceText": "Ziehl-Neelsen staining for acid-fast bacilli",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 920545,
"choiceText": "Tuberculin sensitivity test",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 920547,
"choiceText": "PCR analysis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 920549,
"choiceText": "Serum ADA levels",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 920551,
"choiceText": "CSF ADA levels\r\n",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Although PCR is the most rapid investigation for diagnosis of TB, research has shown that it can produce false-negative results in certain cases. Serum ADA levels, Ziehl-Neelsen staining, and tuberculin testing have been found to have low sensitivity; however, in pleural TB causing effusion and TBM, ADA testing had a higher sensitivity than any other tests.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 290667,
"questionText": "In a patient with findings that are consistent with meningitis, which test would be the most sensitive in ruling out TBM?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
855472 | /viewarticle/855472 | [
{
"authors": "Olugbemiga Jegede, MD; Walid Abuhammour, MD",
"content": [
"Editor's Note:\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.",
"A 14-year-old, previously healthy, fully immunized girl is presented to the emergency department with a 2-week history of left ear pain and discharge. She has just completed a 10-day course of antibiotic therapy, prescribed by her primary care provider, for marked left ear pain and swelling with purulent bloody discharge, headache, and left temporal and facial pain. She denies any history of foreign body in the ears, hearing loss, or fever. The review of systems is not contributory. Her medical history is unremarkable, and the patient has no known history of allergy."
],
"date": "December 09, 2015",
"figures": [],
"markdown": "# Double Vision and Ear Discharge in a 14-Year-Old Girl\n\n **Authors:** Olugbemiga Jegede, MD; Walid Abuhammour, MD \n **Date:** December 09, 2015\n\n ## Content\n\n Editor's Note:\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.\nA 14-year-old, previously healthy, fully immunized girl is presented to the emergency department with a 2-week history of left ear pain and discharge. She has just completed a 10-day course of antibiotic therapy, prescribed by her primary care provider, for marked left ear pain and swelling with purulent bloody discharge, headache, and left temporal and facial pain. She denies any history of foreign body in the ears, hearing loss, or fever. The review of systems is not contributory. Her medical history is unremarkable, and the patient has no known history of allergy.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "Double Vision and Ear Discharge in a 14-Year-Old Girl"
},
{
"authors": "Olugbemiga Jegede, MD; Walid Abuhammour, MD",
"content": [
"Upon examination, the patient is a young white girl who is ill-appearing but in no obvious distress. Her vital signs include a temperature of 97.9°F (36.6°C), a regular heart rate of 86 beats/min, a respiratory rate of 16 breaths/min, and a blood pressure of 110/80 mm Hg. On examination of the head, swelling and tenderness are noted behind the left ear lobe, with purulent bloody discharge from the left ear.",
"The central nervous system (CNS) examination reveals an alert and well-oriented young girl with a Glasgow Coma Score of 15/15. She is unable to wrinkle the left side of her forehead, she cannot close her left eyelid, and the left nasolabial fold is flat and with deviation of the mouth to the right. The cardiovascular, respiratory, and abdominal examinations are all normal.",
"The initial laboratory analysis, which includes a complete blood cell count and basic metabolic panel, are normal. CT scanning of the brain confirms a left-sided mastoiditis, but no findings to suggest increased intracranial pressure are noted. An analysis of the cerebrospinal fluid (CSF) shows no significant white or red blood cells or abnormalities in the glucose and protein concentrations. A swab of the purulent discharge from the left ear, blood, and CSF samples are sent for culture.",
"Figure 1.",
"Figure 1.",
"Figure 2.",
"Figure 2.",
"She is admitted for suppurative otitis media, mastoiditis and seventh nerve palsy. Infectious disease, head and neck surgery, and neurology specialists are consulted. Her initial management includes intravenous fluid, pain medications, tympanostomy for drainage and culture, and intravenous meropenem pending the culture and sensitivity results.",
"On admission day 2, however, the patient develops severe left orbital pain, double vision, and the inability to abduct the left eye. The neurologist clinically confirms a sixth nerve palsy, and a request is made for MRI of the temporal bones as well as magnetic resonance angiography (MRA) and magnetic resonance venography (MRV) to rule out venous sinus thrombosis.",
"The MRI report is obtained (see Figures 1 and 2)."
],
"date": "December 09, 2015",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/855/472/855472-Thumb1.JPG"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/855/472/855472-Thumb2.JPG"
}
],
"markdown": "# Double Vision and Ear Discharge in a 14-Year-Old Girl\n\n **Authors:** Olugbemiga Jegede, MD; Walid Abuhammour, MD \n **Date:** December 09, 2015\n\n ## Content\n\n Upon examination, the patient is a young white girl who is ill-appearing but in no obvious distress. Her vital signs include a temperature of 97.9°F (36.6°C), a regular heart rate of 86 beats/min, a respiratory rate of 16 breaths/min, and a blood pressure of 110/80 mm Hg. On examination of the head, swelling and tenderness are noted behind the left ear lobe, with purulent bloody discharge from the left ear.\nThe central nervous system (CNS) examination reveals an alert and well-oriented young girl with a Glasgow Coma Score of 15/15. She is unable to wrinkle the left side of her forehead, she cannot close her left eyelid, and the left nasolabial fold is flat and with deviation of the mouth to the right. The cardiovascular, respiratory, and abdominal examinations are all normal.\nThe initial laboratory analysis, which includes a complete blood cell count and basic metabolic panel, are normal. CT scanning of the brain confirms a left-sided mastoiditis, but no findings to suggest increased intracranial pressure are noted. An analysis of the cerebrospinal fluid (CSF) shows no significant white or red blood cells or abnormalities in the glucose and protein concentrations. A swab of the purulent discharge from the left ear, blood, and CSF samples are sent for culture.\nFigure 1.\nFigure 1.\nFigure 2.\nFigure 2.\nShe is admitted for suppurative otitis media, mastoiditis and seventh nerve palsy. Infectious disease, head and neck surgery, and neurology specialists are consulted. Her initial management includes intravenous fluid, pain medications, tympanostomy for drainage and culture, and intravenous meropenem pending the culture and sensitivity results.\nOn admission day 2, however, the patient develops severe left orbital pain, double vision, and the inability to abduct the left eye. The neurologist clinically confirms a sixth nerve palsy, and a request is made for MRI of the temporal bones as well as magnetic resonance angiography (MRA) and magnetic resonance venography (MRV) to rule out venous sinus thrombosis.\nThe MRI report is obtained (see Figures 1 and 2).\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 920681,
"choiceText": "Foix-Jefferson syndrome",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 920683,
"choiceText": "Vernet syndrome",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 920685,
"choiceText": "Gradenigo syndrome",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 920687,
"choiceText": "Villaret syndrome",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 290717,
"questionText": "On the basis of the history, physical examination, and workup, what is the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Double Vision and Ear Discharge in a 14-Year-Old Girl"
},
{
"authors": "Olugbemiga Jegede, MD; Walid Abuhammour, MD",
"content": [
"Acute suppurative otitis media is defined as a suppurative infection involving the mucosa of the middle ear cleft. By convention, it is termed acute if the infection is less than 3 weeks in duration. Obstruction of the eustachian tube seems to be the most important antecedent event in the pathophysiology of acute suppurative otitis media.[1,2] Most cases of acute suppurative otitis media are triggered by upper respiratory infections, which seed the middle ear cavity through the eustachian tube orifice. Infections involving the nasopharynx can infect the middle ear through the pharyngeal end of the eustachian tube. Most commonly, the infection is viral in origin; however, allergic symptoms may also play an important role in the pathogenesis.",
"Pathogenic bacteria can secondarily infect the middle ear mucosa. The bacteria that commonly cause this disorder are Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis.[3] To become pathogenic, the bacteria must become adherent to the mucosal lining of the middle ear cavity. This is possible with prior viral infection of the middle ear mucosa. If the infection persists beyond a period of 2 weeks, an associated thickening of the mucoperiosteum is noted, especially in the air cells around the periantral area that lead to a blockage of the drainage from the antral cells.",
"The trapped secretions in the mastoid air cell system cause intense pressure, venous stasis, and local acidosis. This acidosis causes dissolution of calcium from the bone, causing decalcification and coalescence of the mastoid air cell system. This condition is known as coalescent mastoiditis.[1] This stage is characterized by the emergence of otalgia and low-grade fever. Erosion of the outer mastoid cortex can lead to the formation of an abscess under the periosteum of the mastoid cortex.[1,2,3] Known complications include the following:",
"Mastoiditis",
"Petrositis or petrous apicitis (Gradenigo syndrome)",
"Facial nerve palsy",
"Meningitis",
"Venous sinus thrombosis"
],
"date": "December 09, 2015",
"figures": [],
"markdown": "# Double Vision and Ear Discharge in a 14-Year-Old Girl\n\n **Authors:** Olugbemiga Jegede, MD; Walid Abuhammour, MD \n **Date:** December 09, 2015\n\n ## Content\n\n Acute suppurative otitis media is defined as a suppurative infection involving the mucosa of the middle ear cleft. By convention, it is termed acute if the infection is less than 3 weeks in duration. Obstruction of the eustachian tube seems to be the most important antecedent event in the pathophysiology of acute suppurative otitis media.[1,2] Most cases of acute suppurative otitis media are triggered by upper respiratory infections, which seed the middle ear cavity through the eustachian tube orifice. Infections involving the nasopharynx can infect the middle ear through the pharyngeal end of the eustachian tube. Most commonly, the infection is viral in origin; however, allergic symptoms may also play an important role in the pathogenesis.\nPathogenic bacteria can secondarily infect the middle ear mucosa. The bacteria that commonly cause this disorder are Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis.[3] To become pathogenic, the bacteria must become adherent to the mucosal lining of the middle ear cavity. This is possible with prior viral infection of the middle ear mucosa. If the infection persists beyond a period of 2 weeks, an associated thickening of the mucoperiosteum is noted, especially in the air cells around the periantral area that lead to a blockage of the drainage from the antral cells.\nThe trapped secretions in the mastoid air cell system cause intense pressure, venous stasis, and local acidosis. This acidosis causes dissolution of calcium from the bone, causing decalcification and coalescence of the mastoid air cell system. This condition is known as coalescent mastoiditis.[1] This stage is characterized by the emergence of otalgia and low-grade fever. Erosion of the outer mastoid cortex can lead to the formation of an abscess under the periosteum of the mastoid cortex.[1,2,3] Known complications include the following:\nMastoiditis\nPetrositis or petrous apicitis (Gradenigo syndrome)\nFacial nerve palsy\nMeningitis\nVenous sinus thrombosis\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 920681,
"choiceText": "Foix-Jefferson syndrome",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 920683,
"choiceText": "Vernet syndrome",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 920685,
"choiceText": "Gradenigo syndrome",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 920687,
"choiceText": "Villaret syndrome",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 290717,
"questionText": "On the basis of the history, physical examination, and workup, what is the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Double Vision and Ear Discharge in a 14-Year-Old Girl"
},
{
"authors": "Olugbemiga Jegede, MD; Walid Abuhammour, MD",
"content": [
"Gradenigo syndrome, also known as petrous apicitis or apical petrositis, is a rare complication of suppurative otitis media, with only a few cases reported (mostly in the otolaryngology literature). The syndrome is a triad of sixth nerve palsy, pain in the distribution of the trigeminal nerve, and otitis media.[2] It was first described by Giuseppe Gradenigo in 1904; the incidence has since diminished with the advent of antibiotic use.",
"In his original description of the disease, only 42% of the cases he described exhibited the classical triad. The neurologic manifestations of Gradenigo syndrome are attributed to the involvement of the fifth and sixth nerves, which are only separated from the inflamed petrous bone apex by the dura matter.[4] The temporal bone not only contains the organs for hearing, balance, and sound conduction, but it also contributes to the cranial vault and zygoma.[1] The temporal bones are situated at the sides and base of the skull and consist of five parts: the squama, mastoid, petrous, tympanic, and styloid process. The petrous portion, called the petrous pyramid, contains the otic and labyrinth. Superiorly, it forms the inferior surface of the middle cranial fossa. Posteriorly, it is bounded by the attachment of the tentorium cerebelli, and together with the mastoid portion it helps to form the anterior face of the posterior cranial fossa. At the petrous apex, there is a hiatus between the tentorium and the petrous that forms a canal for the fifth cranial nerve (Meckel cave). The sixth cranial nerve runs through a notch just below the posterior clinoid process (the medial attachment of the tentorium) and above the articulation of the petrous and sphenoid (Dorello canal).[1]",
"The inflammatory process spreads from the base (mastoid and middle ear) of the pyramid-shaped os petrosum to the top (petrous apex). This explains why the time interval between the onset of otitis media and the manifestation of cranial nerve dysfunction varies between 1 week and 2-3 months.[4]",
"Complications of Gradenigo syndrome include the following:",
"Meningitis",
"Intracranial abscess",
"Spread to the skull base and involvement of the ninth, tenth, and eleventh cranial nerves; known as Vernet syndrome",
"Prevertebral/paravertebral/retropharyngeal abscess",
"Spread to the sympathetic plexus around the carotid sheath",
"Labyrinthitis",
"Venous sinus thrombosis",
"Death",
"The differential diagnosis of Gradenigo syndrome includes tumors of the petrous apex, such as meningioma, sarcoma, trigeminal neuralgia, or metastatic disease. Gradenigo syndrome in children usually results from an infectious etiology. The organisms causing Gradenigo syndrome are often difficult to recover, and cultures from the middle ear and mastoid or petrous tissue may be negative; however, common organisms recovered include group A Streptococcus, pneumococcus, Staphylococcus, Pseudomonas aeruginosa, and Mycobacterium tuberculosis.[3]",
"The evaluation of a patient with suspected Gradenigo syndrome should include neuroimaging. A reasonable initial imaging modality is a CT scan of the head with high resolution to provide detail of the petrous apex, as the test is widely available and can identify abnormalities in bony anatomy. MRI is superior, however, for demonstrating the CNS anatomy and meningeal enhancement, which makes it the ideal test to diagnose Gradenigo syndrome. MRA and/or MRV may be useful in evaluating sigmoid sinus pathology."
],
"date": "December 09, 2015",
"figures": [],
"markdown": "# Double Vision and Ear Discharge in a 14-Year-Old Girl\n\n **Authors:** Olugbemiga Jegede, MD; Walid Abuhammour, MD \n **Date:** December 09, 2015\n\n ## Content\n\n Gradenigo syndrome, also known as petrous apicitis or apical petrositis, is a rare complication of suppurative otitis media, with only a few cases reported (mostly in the otolaryngology literature). The syndrome is a triad of sixth nerve palsy, pain in the distribution of the trigeminal nerve, and otitis media.[2] It was first described by Giuseppe Gradenigo in 1904; the incidence has since diminished with the advent of antibiotic use.\nIn his original description of the disease, only 42% of the cases he described exhibited the classical triad. The neurologic manifestations of Gradenigo syndrome are attributed to the involvement of the fifth and sixth nerves, which are only separated from the inflamed petrous bone apex by the dura matter.[4] The temporal bone not only contains the organs for hearing, balance, and sound conduction, but it also contributes to the cranial vault and zygoma.[1] The temporal bones are situated at the sides and base of the skull and consist of five parts: the squama, mastoid, petrous, tympanic, and styloid process. The petrous portion, called the petrous pyramid, contains the otic and labyrinth. Superiorly, it forms the inferior surface of the middle cranial fossa. Posteriorly, it is bounded by the attachment of the tentorium cerebelli, and together with the mastoid portion it helps to form the anterior face of the posterior cranial fossa. At the petrous apex, there is a hiatus between the tentorium and the petrous that forms a canal for the fifth cranial nerve (Meckel cave). The sixth cranial nerve runs through a notch just below the posterior clinoid process (the medial attachment of the tentorium) and above the articulation of the petrous and sphenoid (Dorello canal).[1]\nThe inflammatory process spreads from the base (mastoid and middle ear) of the pyramid-shaped os petrosum to the top (petrous apex). This explains why the time interval between the onset of otitis media and the manifestation of cranial nerve dysfunction varies between 1 week and 2-3 months.[4]\nComplications of Gradenigo syndrome include the following:\nMeningitis\nIntracranial abscess\nSpread to the skull base and involvement of the ninth, tenth, and eleventh cranial nerves; known as Vernet syndrome\nPrevertebral/paravertebral/retropharyngeal abscess\nSpread to the sympathetic plexus around the carotid sheath\nLabyrinthitis\nVenous sinus thrombosis\nDeath\nThe differential diagnosis of Gradenigo syndrome includes tumors of the petrous apex, such as meningioma, sarcoma, trigeminal neuralgia, or metastatic disease. Gradenigo syndrome in children usually results from an infectious etiology. The organisms causing Gradenigo syndrome are often difficult to recover, and cultures from the middle ear and mastoid or petrous tissue may be negative; however, common organisms recovered include group A Streptococcus, pneumococcus, Staphylococcus, Pseudomonas aeruginosa, and Mycobacterium tuberculosis.[3]\nThe evaluation of a patient with suspected Gradenigo syndrome should include neuroimaging. A reasonable initial imaging modality is a CT scan of the head with high resolution to provide detail of the petrous apex, as the test is widely available and can identify abnormalities in bony anatomy. MRI is superior, however, for demonstrating the CNS anatomy and meningeal enhancement, which makes it the ideal test to diagnose Gradenigo syndrome. MRA and/or MRV may be useful in evaluating sigmoid sinus pathology.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "Double Vision and Ear Discharge in a 14-Year-Old Girl"
},
{
"authors": "Olugbemiga Jegede, MD; Walid Abuhammour, MD",
"content": [
"Treatment of Gradenigo syndrome must be initiated immediately. The underlying infection must be addressed in order to prevent further spread, complications, or permanent abducens nerve injury. The advent of the antibiotic era has facilitated the conservative management of a select cohort of patients with apical petrositis. It is now generally advocated that patients be treated with high-dose, broad-spectrum antibiotics and less-aggressive surgical procedures.[2]",
"Surgical treatment involves a minimum of infectious decompression with myringotomy and ventilation tube placement. Tympanocentesis should be performed to allow for culture-directed intravenous antibiotic therapy. In patients with cranial nerve palsies, steroids have been used to speed recovery by reducing inflammation, edema, and nerve compression.[4]",
"The MRI of the patient in this case showed fluid collection in the left middle ear, mastoid air cells, and the petrous apex, which is consistent with the diagnosis of apical petrositis. This finding, in conjunction with retro-orbital pain, otorrhea, and ipsilateral sixth nerve palsies, led to the final diagnosis of Gradenigo syndrome. Her blood and CSF cultures were negative; however, the middle ear fluid culture showed Stenotrophomonas maltophilia sensitive to trimethoprim-sulfamethoxazole. Her antibiotic prescription was changed from meropenem to intravenous trimethoprim-sulfamethoxazole, and 6 days of intravenous dexamethasone was added to her regimen.",
"The patient started to show improvement on the third day of trimethoprim-sulfamethoxazole and steroid therapy. The sixth and seventh nerve palsies improved slowly, and a decrease in the ear discharge, headache, and left orbital pain were noted. She received both intravenous and oral trimethoprim-sulfamethoxazole for a total of 6 weeks, and she continued physiotherapy for the facial nerve palsy. A follow-up examination showed full recovery, and a repeat MRI a few months later showed full resolution.",
"Gradenigo syndrome should be considered in patients with suppurative otitis media who present with orbital/retro-orbital pain and/or cranial nerve palsies, especially palsy of the sixth nerve. Prompt otolaryngology consultation and initiation of antibiotic therapy are important in curtailing the serious morbidity resulting from this condition."
],
"date": "December 09, 2015",
"figures": [],
"markdown": "# Double Vision and Ear Discharge in a 14-Year-Old Girl\n\n **Authors:** Olugbemiga Jegede, MD; Walid Abuhammour, MD \n **Date:** December 09, 2015\n\n ## Content\n\n Treatment of Gradenigo syndrome must be initiated immediately. The underlying infection must be addressed in order to prevent further spread, complications, or permanent abducens nerve injury. The advent of the antibiotic era has facilitated the conservative management of a select cohort of patients with apical petrositis. It is now generally advocated that patients be treated with high-dose, broad-spectrum antibiotics and less-aggressive surgical procedures.[2]\nSurgical treatment involves a minimum of infectious decompression with myringotomy and ventilation tube placement. Tympanocentesis should be performed to allow for culture-directed intravenous antibiotic therapy. In patients with cranial nerve palsies, steroids have been used to speed recovery by reducing inflammation, edema, and nerve compression.[4]\nThe MRI of the patient in this case showed fluid collection in the left middle ear, mastoid air cells, and the petrous apex, which is consistent with the diagnosis of apical petrositis. This finding, in conjunction with retro-orbital pain, otorrhea, and ipsilateral sixth nerve palsies, led to the final diagnosis of Gradenigo syndrome. Her blood and CSF cultures were negative; however, the middle ear fluid culture showed Stenotrophomonas maltophilia sensitive to trimethoprim-sulfamethoxazole. Her antibiotic prescription was changed from meropenem to intravenous trimethoprim-sulfamethoxazole, and 6 days of intravenous dexamethasone was added to her regimen.\nThe patient started to show improvement on the third day of trimethoprim-sulfamethoxazole and steroid therapy. The sixth and seventh nerve palsies improved slowly, and a decrease in the ear discharge, headache, and left orbital pain were noted. She received both intravenous and oral trimethoprim-sulfamethoxazole for a total of 6 weeks, and she continued physiotherapy for the facial nerve palsy. A follow-up examination showed full recovery, and a repeat MRI a few months later showed full resolution.\nGradenigo syndrome should be considered in patients with suppurative otitis media who present with orbital/retro-orbital pain and/or cranial nerve palsies, especially palsy of the sixth nerve. Prompt otolaryngology consultation and initiation of antibiotic therapy are important in curtailing the serious morbidity resulting from this condition.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 920689,
"choiceText": "Pain in the distribution of trigeminal nerve",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 920691,
"choiceText": "Acute suppurative otitis media",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 920693,
"choiceText": "Facial nerve palsy\r\n",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 920695,
"choiceText": "Abducens nerve palsy",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The classic triad that indicates a diagnosis of Gradenigo syndrome is pain in the distribution of trigeminal nerve, acute suppurative otitis media, and abducens nerve palsy. Facial nerve palsy is a complication of acute suppurative otitis media.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 290719,
"questionText": "You examine a patient who exhibits the classic triad seen in cases of Gradenigo syndrome. Which of the following findings would <i>not</i> be seen in this patient?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 920697,
"choiceText": "MRA",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 920699,
"choiceText": "MRI\r\n",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 920701,
"choiceText": "High-resolution CT scanning",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 920703,
"choiceText": "Skull radiography\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Although CT scanning is better at demonstrating bony anatomy, an MRI scan is superior for demonstrating the CNS anatomy and meningeal enhancement, and it is the most useful imaging study for the evaluation of Gradenigo syndrome. MRA may be useful in evaluating the sigmoid sinus pathology. Radiography of the skull has not been shown to be of benefit.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 290721,
"questionText": "Which of the following diagnostic methods is the best for diagnosing apical petrositis (Gradenigo syndrome) in the above described patient?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Double Vision and Ear Discharge in a 14-Year-Old Girl"
},
{
"authors": "Olugbemiga Jegede, MD; Walid Abuhammour, MD",
"content": [],
"date": "December 09, 2015",
"figures": [],
"markdown": "# Double Vision and Ear Discharge in a 14-Year-Old Girl\n\n **Authors:** Olugbemiga Jegede, MD; Walid Abuhammour, MD \n **Date:** December 09, 2015\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 920689,
"choiceText": "Pain in the distribution of trigeminal nerve",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 920691,
"choiceText": "Acute suppurative otitis media",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 920693,
"choiceText": "Facial nerve palsy\r\n",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 920695,
"choiceText": "Abducens nerve palsy",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The classic triad that indicates a diagnosis of Gradenigo syndrome is pain in the distribution of trigeminal nerve, acute suppurative otitis media, and abducens nerve palsy. Facial nerve palsy is a complication of acute suppurative otitis media.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 290719,
"questionText": "You examine a patient who exhibits the classic triad seen in cases of Gradenigo syndrome. Which of the following findings would <i>not</i> be seen in this patient?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 920697,
"choiceText": "MRA",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 920699,
"choiceText": "MRI\r\n",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 920701,
"choiceText": "High-resolution CT scanning",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 920703,
"choiceText": "Skull radiography\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Although CT scanning is better at demonstrating bony anatomy, an MRI scan is superior for demonstrating the CNS anatomy and meningeal enhancement, and it is the most useful imaging study for the evaluation of Gradenigo syndrome. MRA may be useful in evaluating the sigmoid sinus pathology. Radiography of the skull has not been shown to be of benefit.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 290721,
"questionText": "Which of the following diagnostic methods is the best for diagnosing apical petrositis (Gradenigo syndrome) in the above described patient?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Double Vision and Ear Discharge in a 14-Year-Old Girl"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 920681,
"choiceText": "Foix-Jefferson syndrome",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 920683,
"choiceText": "Vernet syndrome",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 920685,
"choiceText": "Gradenigo syndrome",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 920687,
"choiceText": "Villaret syndrome",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 290717,
"questionText": "On the basis of the history, physical examination, and workup, what is the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 920689,
"choiceText": "Pain in the distribution of trigeminal nerve",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 920691,
"choiceText": "Acute suppurative otitis media",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 920693,
"choiceText": "Facial nerve palsy\r\n",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 920695,
"choiceText": "Abducens nerve palsy",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The classic triad that indicates a diagnosis of Gradenigo syndrome is pain in the distribution of trigeminal nerve, acute suppurative otitis media, and abducens nerve palsy. Facial nerve palsy is a complication of acute suppurative otitis media.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 290719,
"questionText": "You examine a patient who exhibits the classic triad seen in cases of Gradenigo syndrome. Which of the following findings would <i>not</i> be seen in this patient?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 920697,
"choiceText": "MRA",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 920699,
"choiceText": "MRI\r\n",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 920701,
"choiceText": "High-resolution CT scanning",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 920703,
"choiceText": "Skull radiography\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Although CT scanning is better at demonstrating bony anatomy, an MRI scan is superior for demonstrating the CNS anatomy and meningeal enhancement, and it is the most useful imaging study for the evaluation of Gradenigo syndrome. MRA may be useful in evaluating the sigmoid sinus pathology. Radiography of the skull has not been shown to be of benefit.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 290721,
"questionText": "Which of the following diagnostic methods is the best for diagnosing apical petrositis (Gradenigo syndrome) in the above described patient?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
855222 | /viewarticle/855222 | [
{
"authors": "Marc Hare, MD",
"content": [
"Editor's Note:\n\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.",
"A 61-year-old woman presents to the wound center for continued treatment of venous leg ulcers complicated by diabetes mellitus. Her medical history is remarkable for essential hypertension and obesity.",
"The patient also has a history of angioimmunoblastic lymphadenopathy, which occurred over 15 years ago. The patient cannot remember any details of this diagnosis; consultation with her primary care clinician reveals that this was a benign/peripheral presentation. No records are available for review. According to the primary care clinician, the condition went into complete remission after a course of prednisone.",
"The patient notes that she has developed new wounds on both of her thighs. The lesions are black, with surrounding redness and tenderness. She reports no itching. She states that the lesions have been enlarging slowly over the past several days, and many are now several centimeters in diameter.",
"The patient denies having any fever or chills. Her fasting serum glucose readings have been stable in the range of 100-200 mg/dL. She denies experiencing any easy bruising or bleeding. She has not had any recent trauma or procedures in the area of the lesions, and she has not had any recent changes in her medications. She has no history of smoking or alcohol or illicit drug use."
],
"date": "December 02, 2015",
"figures": [],
"markdown": "# A 61-Year-Old Woman With Leg Ulcers\n\n **Authors:** Marc Hare, MD \n **Date:** December 02, 2015\n\n ## Content\n\n Editor's Note:\n\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.\nA 61-year-old woman presents to the wound center for continued treatment of venous leg ulcers complicated by diabetes mellitus. Her medical history is remarkable for essential hypertension and obesity.\nThe patient also has a history of angioimmunoblastic lymphadenopathy, which occurred over 15 years ago. The patient cannot remember any details of this diagnosis; consultation with her primary care clinician reveals that this was a benign/peripheral presentation. No records are available for review. According to the primary care clinician, the condition went into complete remission after a course of prednisone.\nThe patient notes that she has developed new wounds on both of her thighs. The lesions are black, with surrounding redness and tenderness. She reports no itching. She states that the lesions have been enlarging slowly over the past several days, and many are now several centimeters in diameter.\nThe patient denies having any fever or chills. Her fasting serum glucose readings have been stable in the range of 100-200 mg/dL. She denies experiencing any easy bruising or bleeding. She has not had any recent trauma or procedures in the area of the lesions, and she has not had any recent changes in her medications. She has no history of smoking or alcohol or illicit drug use.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 61-Year-Old Woman With Leg Ulcers"
},
{
"authors": "Marc Hare, MD",
"content": [
"Upon physical examination, the patient's oral temperature is 97.8°F. Her pulse is 86 beats/min and regular, and her blood pressure is 128/81 mm Hg. Her respiratory rate is 14 breaths/min. She is in no acute distress, but she notes that the wounds are causing her discomfort. Findings on examination of the head, neck, lungs, heart, and abdomen are unremarkable. The skin of the upper extremities and torso is also unremarkable.",
"The patient has a nonhealing venous ulcer on each of her medial malleolar areas, which are unchanged from previous examination. She has multiple black eschars on her thighs, each surrounded by a centimeter of erythema with a slightly reticular pattern, as well as induration. She has trace edema of the ankles, but no edema in her legs or thighs.",
"Figure 1.",
"Figure 1.",
"The lesions are tender. There are no vesicles or pustules. No confluent or ascending erythema is noted. The largest lesion measures 4.4 × 2.6 cm (Figure 1).",
"Laboratory testing shows a white blood cell (WBC) count of 10.1 × 103 cells/µL (normal range, 4.1-10.9 × 103 cells/µL), a hemoglobin level of 10 g/dL (normal range, 12.0-15.2 g/dL), platelet count of 492 × 103 cells/µL (normal range, 140-450 × 103 cells/µL), and a normal WBC differential. The basic metabolic panel is normal. The hemoglobin A1c value is 6.6% (normal range, 3.8%-6.4%). The albumin level is normal, at 3.5 g/dL, and the erythrocyte sedimentation rate (ESR) is elevated at 80 mm/hr (normal range, 1-25 mm/hr).",
"Samples for biopsy are obtained from the edges of representative lesions. Biopsy demonstrates acute and chronic inflammation in the dermis and subcutaneous fat. No viral cytopathic changes are seen. Small-vessel microthrombi are limited to the base of the ulcer and are not identified in vessels away from the ulcer, which suggests a secondary thrombotic reaction. No specific changes of vasculitis or malignancy are identified."
],
"date": "December 02, 2015",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/855/222/855222-Thumb1.png"
}
],
"markdown": "# A 61-Year-Old Woman With Leg Ulcers\n\n **Authors:** Marc Hare, MD \n **Date:** December 02, 2015\n\n ## Content\n\n Upon physical examination, the patient's oral temperature is 97.8°F. Her pulse is 86 beats/min and regular, and her blood pressure is 128/81 mm Hg. Her respiratory rate is 14 breaths/min. She is in no acute distress, but she notes that the wounds are causing her discomfort. Findings on examination of the head, neck, lungs, heart, and abdomen are unremarkable. The skin of the upper extremities and torso is also unremarkable.\nThe patient has a nonhealing venous ulcer on each of her medial malleolar areas, which are unchanged from previous examination. She has multiple black eschars on her thighs, each surrounded by a centimeter of erythema with a slightly reticular pattern, as well as induration. She has trace edema of the ankles, but no edema in her legs or thighs.\nFigure 1.\nFigure 1.\nThe lesions are tender. There are no vesicles or pustules. No confluent or ascending erythema is noted. The largest lesion measures 4.4 × 2.6 cm (Figure 1).\nLaboratory testing shows a white blood cell (WBC) count of 10.1 × 103 cells/µL (normal range, 4.1-10.9 × 103 cells/µL), a hemoglobin level of 10 g/dL (normal range, 12.0-15.2 g/dL), platelet count of 492 × 103 cells/µL (normal range, 140-450 × 103 cells/µL), and a normal WBC differential. The basic metabolic panel is normal. The hemoglobin A1c value is 6.6% (normal range, 3.8%-6.4%). The albumin level is normal, at 3.5 g/dL, and the erythrocyte sedimentation rate (ESR) is elevated at 80 mm/hr (normal range, 1-25 mm/hr).\nSamples for biopsy are obtained from the edges of representative lesions. Biopsy demonstrates acute and chronic inflammation in the dermis and subcutaneous fat. No viral cytopathic changes are seen. Small-vessel microthrombi are limited to the base of the ulcer and are not identified in vessels away from the ulcer, which suggests a secondary thrombotic reaction. No specific changes of vasculitis or malignancy are identified.\n\n ## Figures\n\n **Figure 1.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 917183,
"choiceText": "Multiple myeloma",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 917185,
"choiceText": "Cryoglobulinemia",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 917187,
"choiceText": "Non-Hodgkin lymphoma",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 917189,
"choiceText": "Giant cell arteritis\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 289497,
"questionText": "On the basis of the history, physical examination, and workup, what is the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 61-Year-Old Woman With Leg Ulcers"
},
{
"authors": "Marc Hare, MD",
"content": [
"Given the patient's history of an immunoproliferative disorder and the atypical presentation of her lesions, consideration was given to a vasculitic or vasculopathic etiology. A panel of additional laboratory studies was ordered. Lupus anticoagulant was not detected, and anticardiolipin was also negative. Antinuclear antibodies and antibodies to neutrophil cytoplasmic antigens were not detected. All hepatitis markers were negative, including for hepatitis C. The test for rheumatoid factor was negative. The cryoglobulin test was positive and quantified as primarily immunoglobulin G. This was subsequently confirmed on a separate test several months later.",
"Cryoglobulins are single or mixed immunoglobulins that reversibly precipitate at low temperatures. Cryoglobulinemia is defined as the presence of cryoglobulins in the serum. This can lead to a syndrome of systemic inflammation caused by immune complexes associated with the cryoglobulins.",
"The mechanism of precipitation is poorly understood. The solubility of cryoglobulins is partially related to the structure of the immunoglobulin heavy and light chains. Alteration in protein conformation resulting from temperature changes may cause decreased solubility and subsequent vasculitic damage. The ratio of antibody to antigen in circulating cryoglobulin aggregates affects the rate of clearance from the circulation and the resultant location of tissue deposition.[1,2]",
"Cryoglobulins are reported in otherwise healthy individuals, so their true prevalence is unknown. Although cryoglobulinemia is thought to be rare, it may be underdiagnosed because of the diversity of clinical presentations. The prevalence of mixed cryoglobulinemia is approximately 1:100,000. The female-to-male ratio is 3:1. The mean age reported is 42-52 years.",
"Cryoglobulinemia is classified on the basis of cryoglobulin type using the Brouet classification. Type I cryoglobulinemia is monoclonal, usually immunoglobulin M (IgM). Types II and III are immunocomplexes formed by monoclonal or polyclonal IgM, respectively. Types II and III have rheumatoid factor activity and bind to polyclonal immunoglobulins. These two types are referred to as \"mixed cryoglobulinemia.\"",
"Type I accounts for 10%-15% of cases, type II is seen in 50%-60%, and type 3 is found in 25%-30%. Atypical cryoglobulins with a microheterogeneous composition that does not fit into any of the classifications have prompted suggestion of a classification called \"type II-III variant.\"[1,2,3]",
"Type I cryoglobulinemia is usually related to an underlying lymphoproliferative disease and may be difficult to distinguish from Waldenström macroglobulinemia, multiple myeloma, or chronic lymphocytic leukemia. Type I cryoglobulinemia may result in hyperviscosity as a result of high levels of circulating monoclonal cryoglobulins, leading to physical obstruction of vessels. In addition, immune complex deposition may cause an inflammatory vasculitis. Specific clinical manifestations include acrocyanosis, retinal hemorrhage, Raynaud phenomenon with digital ulceration, livedo reticularis, purpura, and arterial thrombosis.[1,2,3,4]",
"Types II and III cryoglobulinemia are associated with chronic inflammatory states, such as systemic lupus erythematosus, Sjögren syndrome, and viral infections (particularly hepatitis C). B-cell clonal expansion, particularly rheumatoid factor-secreting cells, is a distinctive feature of many of these disease states. Tissue damage results from immune complex deposition and complement activation. Specific clinical manifestations associated with types II and III cryoglobulinemia include arthralgia (usually of the leg, ankle, and foot), fatigue, myalgia, renal immune complex disease, cutaneous vasculitis, and peripheral neuropathy. The Meltzer triad of purpura, arthralgia, and weakness is seen in 25%-30% of patients.[1,3,4,5]"
],
"date": "December 02, 2015",
"figures": [],
"markdown": "# A 61-Year-Old Woman With Leg Ulcers\n\n **Authors:** Marc Hare, MD \n **Date:** December 02, 2015\n\n ## Content\n\n Given the patient's history of an immunoproliferative disorder and the atypical presentation of her lesions, consideration was given to a vasculitic or vasculopathic etiology. A panel of additional laboratory studies was ordered. Lupus anticoagulant was not detected, and anticardiolipin was also negative. Antinuclear antibodies and antibodies to neutrophil cytoplasmic antigens were not detected. All hepatitis markers were negative, including for hepatitis C. The test for rheumatoid factor was negative. The cryoglobulin test was positive and quantified as primarily immunoglobulin G. This was subsequently confirmed on a separate test several months later.\nCryoglobulins are single or mixed immunoglobulins that reversibly precipitate at low temperatures. Cryoglobulinemia is defined as the presence of cryoglobulins in the serum. This can lead to a syndrome of systemic inflammation caused by immune complexes associated with the cryoglobulins.\nThe mechanism of precipitation is poorly understood. The solubility of cryoglobulins is partially related to the structure of the immunoglobulin heavy and light chains. Alteration in protein conformation resulting from temperature changes may cause decreased solubility and subsequent vasculitic damage. The ratio of antibody to antigen in circulating cryoglobulin aggregates affects the rate of clearance from the circulation and the resultant location of tissue deposition.[1,2]\nCryoglobulins are reported in otherwise healthy individuals, so their true prevalence is unknown. Although cryoglobulinemia is thought to be rare, it may be underdiagnosed because of the diversity of clinical presentations. The prevalence of mixed cryoglobulinemia is approximately 1:100,000. The female-to-male ratio is 3:1. The mean age reported is 42-52 years.\nCryoglobulinemia is classified on the basis of cryoglobulin type using the Brouet classification. Type I cryoglobulinemia is monoclonal, usually immunoglobulin M (IgM). Types II and III are immunocomplexes formed by monoclonal or polyclonal IgM, respectively. Types II and III have rheumatoid factor activity and bind to polyclonal immunoglobulins. These two types are referred to as \"mixed cryoglobulinemia.\"\nType I accounts for 10%-15% of cases, type II is seen in 50%-60%, and type 3 is found in 25%-30%. Atypical cryoglobulins with a microheterogeneous composition that does not fit into any of the classifications have prompted suggestion of a classification called \"type II-III variant.\"[1,2,3]\nType I cryoglobulinemia is usually related to an underlying lymphoproliferative disease and may be difficult to distinguish from Waldenström macroglobulinemia, multiple myeloma, or chronic lymphocytic leukemia. Type I cryoglobulinemia may result in hyperviscosity as a result of high levels of circulating monoclonal cryoglobulins, leading to physical obstruction of vessels. In addition, immune complex deposition may cause an inflammatory vasculitis. Specific clinical manifestations include acrocyanosis, retinal hemorrhage, Raynaud phenomenon with digital ulceration, livedo reticularis, purpura, and arterial thrombosis.[1,2,3,4]\nTypes II and III cryoglobulinemia are associated with chronic inflammatory states, such as systemic lupus erythematosus, Sjögren syndrome, and viral infections (particularly hepatitis C). B-cell clonal expansion, particularly rheumatoid factor-secreting cells, is a distinctive feature of many of these disease states. Tissue damage results from immune complex deposition and complement activation. Specific clinical manifestations associated with types II and III cryoglobulinemia include arthralgia (usually of the leg, ankle, and foot), fatigue, myalgia, renal immune complex disease, cutaneous vasculitis, and peripheral neuropathy. The Meltzer triad of purpura, arthralgia, and weakness is seen in 25%-30% of patients.[1,3,4,5]\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 917183,
"choiceText": "Multiple myeloma",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 917185,
"choiceText": "Cryoglobulinemia",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 917187,
"choiceText": "Non-Hodgkin lymphoma",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 917189,
"choiceText": "Giant cell arteritis\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 289497,
"questionText": "On the basis of the history, physical examination, and workup, what is the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 61-Year-Old Woman With Leg Ulcers"
},
{
"authors": "Marc Hare, MD",
"content": [
"Mortality and morbidity in individuals with cryoglobulinemia often depends on the underlying associated disease, if present. The overall prognosis is worse in patients with associated disease. The mean survival is approximately 50% at 10 years after diagnosis. The risk for renal failure appears to be greater in patients with hepatitis C virus-associated disease.[1,2]",
"Lymphoproliferative disease is more common in individuals with cryoglobulinemia. Patients with mixed cryoglobulinemia may develop benign lymphoid infiltrates in the spleen and bone marrow. Less frequently, patients may develop B-cell non-Hodgkin lymphoma. The incidence of malignant lymphoma in mixed cryoglobulinemia varies from 10% to 40%, with onset 5-10 years after disease diagnosis.[1,4]",
"Cutaneous manifestations are almost always present in cryoglobulinemia. Lesions are most often seen in dependent areas (especially the lower extremities), and they include erythematous macules and purpuric papules (90%-95%) as well as ulcers (10%-25%). Lesions in nondependent areas (head and mucosa) are more common in type I cryoglobulinemia, as are livedo reticularis, Raynaud phenomenon, and ulcers. Nailfold capillary abnormalities are common and include dilatation, altered orientation, capillary shortening, and neoangiogenesis.[3,4]",
"Renal disease may occur secondary to thrombosis (type I cryoglobulinemia) or immune complex deposition (types II and III). The incidence of renal disease varies from 5% to 60%. Histologically, membranoproliferative glomerulonephritis is almost always seen in mixed cryoglobulinemia. Renal involvement is one of the most serious complications of cryoglobulinemia, and it typically manifests early in the course of the disease (within 3-5 years of diagnosis). This may progress to complete renal failure. Hypertension and nephritic-range proteinuria with resultant edema are characteristically seen in cryoglobulinemia.[1,2]",
"A reduction in forced expiratory flow rates and the presence of interstitial infiltrates are common in mixed cryoglobulinemia. Approximately 40%-50% of patients are symptomatic, with dyspnea, cough, or pleuritic pain. Severe pulmonary disease is rare. Neuropathy is common in types II and III disease, affecting 70%-80% of patients. Sensory neuropathy is more common than motor neuropathy (5% of patients). Abdominal pain has been reported in 2%-22% of patients. Vasculitis of the small mesenteric vessels that leads to acute abdomen has been reported. Splenomegaly may be seen in these patients.",
"The differential diagnosis of cryoglobulinemia includes antiphospholipid syndrome, chronic lymphocytic leukemia, Churg-Strauss syndrome, cirrhosis, giant cell arteritis, glomerulonephritis, Goodpasture syndrome, hemolytic-uremic syndrome, hepatitides, non-Hodgkin lymphoma, microscopic polyangiitis, multiple myeloma, polyarteritis nodosa, sarcoidosis, serum sickness, systemic lupus erythematosus, and Waldenström macroglobulinemia.[1,4]",
"Key laboratory studies include evaluation for serum cryoglobulins. The specimen must be obtained in warm tubes (98.6°F) without anticoagulants and then allowed to clot before centrifugation. The serum is then incubated at 39.2°F. Type I tends to precipitate within 24 hours. Type III may require 7 days to precipitate. Specific immunologic assays may be used to identify cryoglobulin components (immunoglobulins, light chains, clonality).",
"Urinalysis, serum creatinine, and electrolytes are important to evaluate for evidence of renal disease. A complete blood cell count should be done to screen for infection, anemia, or leukemia. Abnormal liver function studies and aminotransferase levels may suggest underlying hepatitis. If so, hepatitis serologic studies are indicated. If hepatitis C virus test results are negative and clinical suspicion remains high, serologic testing may be performed on the cryoprecipitate.",
"Rheumatoid factor is positive in types II and III disease. Antinuclear antibody testing is indicated upon clinical suspicion of an underlying connective tissue disease. ESR elevation is nonspecific, and it may occur in several associated inflammatory disorders.",
"Patients may display hypocomplementemia (especially low C4 levels). The clinician should consider serum protein electrophoresis, urine protein electrophoresis, and quantitative immunoglobulin if underlying gammopathy is suspected.[4]",
"Clinical imaging should be ordered as warranted on the basis of suspected underlying disease. Tissue biopsy may be required for diagnosis in patients with vasculitis or renal disease. Purpura is characterized by dermal vasculitis that extends variably to the subcutaneous tissue. Hepatitis C virus-associated proteins have been found in vasculitic skin biopsy specimens. Although samples generally exhibit inflammatory vascular changes, intraluminal cryoglobulin deposits may be observed, especially in renal glomeruli."
],
"date": "December 02, 2015",
"figures": [],
"markdown": "# A 61-Year-Old Woman With Leg Ulcers\n\n **Authors:** Marc Hare, MD \n **Date:** December 02, 2015\n\n ## Content\n\n Mortality and morbidity in individuals with cryoglobulinemia often depends on the underlying associated disease, if present. The overall prognosis is worse in patients with associated disease. The mean survival is approximately 50% at 10 years after diagnosis. The risk for renal failure appears to be greater in patients with hepatitis C virus-associated disease.[1,2]\nLymphoproliferative disease is more common in individuals with cryoglobulinemia. Patients with mixed cryoglobulinemia may develop benign lymphoid infiltrates in the spleen and bone marrow. Less frequently, patients may develop B-cell non-Hodgkin lymphoma. The incidence of malignant lymphoma in mixed cryoglobulinemia varies from 10% to 40%, with onset 5-10 years after disease diagnosis.[1,4]\nCutaneous manifestations are almost always present in cryoglobulinemia. Lesions are most often seen in dependent areas (especially the lower extremities), and they include erythematous macules and purpuric papules (90%-95%) as well as ulcers (10%-25%). Lesions in nondependent areas (head and mucosa) are more common in type I cryoglobulinemia, as are livedo reticularis, Raynaud phenomenon, and ulcers. Nailfold capillary abnormalities are common and include dilatation, altered orientation, capillary shortening, and neoangiogenesis.[3,4]\nRenal disease may occur secondary to thrombosis (type I cryoglobulinemia) or immune complex deposition (types II and III). The incidence of renal disease varies from 5% to 60%. Histologically, membranoproliferative glomerulonephritis is almost always seen in mixed cryoglobulinemia. Renal involvement is one of the most serious complications of cryoglobulinemia, and it typically manifests early in the course of the disease (within 3-5 years of diagnosis). This may progress to complete renal failure. Hypertension and nephritic-range proteinuria with resultant edema are characteristically seen in cryoglobulinemia.[1,2]\nA reduction in forced expiratory flow rates and the presence of interstitial infiltrates are common in mixed cryoglobulinemia. Approximately 40%-50% of patients are symptomatic, with dyspnea, cough, or pleuritic pain. Severe pulmonary disease is rare. Neuropathy is common in types II and III disease, affecting 70%-80% of patients. Sensory neuropathy is more common than motor neuropathy (5% of patients). Abdominal pain has been reported in 2%-22% of patients. Vasculitis of the small mesenteric vessels that leads to acute abdomen has been reported. Splenomegaly may be seen in these patients.\nThe differential diagnosis of cryoglobulinemia includes antiphospholipid syndrome, chronic lymphocytic leukemia, Churg-Strauss syndrome, cirrhosis, giant cell arteritis, glomerulonephritis, Goodpasture syndrome, hemolytic-uremic syndrome, hepatitides, non-Hodgkin lymphoma, microscopic polyangiitis, multiple myeloma, polyarteritis nodosa, sarcoidosis, serum sickness, systemic lupus erythematosus, and Waldenström macroglobulinemia.[1,4]\nKey laboratory studies include evaluation for serum cryoglobulins. The specimen must be obtained in warm tubes (98.6°F) without anticoagulants and then allowed to clot before centrifugation. The serum is then incubated at 39.2°F. Type I tends to precipitate within 24 hours. Type III may require 7 days to precipitate. Specific immunologic assays may be used to identify cryoglobulin components (immunoglobulins, light chains, clonality).\nUrinalysis, serum creatinine, and electrolytes are important to evaluate for evidence of renal disease. A complete blood cell count should be done to screen for infection, anemia, or leukemia. Abnormal liver function studies and aminotransferase levels may suggest underlying hepatitis. If so, hepatitis serologic studies are indicated. If hepatitis C virus test results are negative and clinical suspicion remains high, serologic testing may be performed on the cryoprecipitate.\nRheumatoid factor is positive in types II and III disease. Antinuclear antibody testing is indicated upon clinical suspicion of an underlying connective tissue disease. ESR elevation is nonspecific, and it may occur in several associated inflammatory disorders.\nPatients may display hypocomplementemia (especially low C4 levels). The clinician should consider serum protein electrophoresis, urine protein electrophoresis, and quantitative immunoglobulin if underlying gammopathy is suspected.[4]\nClinical imaging should be ordered as warranted on the basis of suspected underlying disease. Tissue biopsy may be required for diagnosis in patients with vasculitis or renal disease. Purpura is characterized by dermal vasculitis that extends variably to the subcutaneous tissue. Hepatitis C virus-associated proteins have been found in vasculitic skin biopsy specimens. Although samples generally exhibit inflammatory vascular changes, intraluminal cryoglobulin deposits may be observed, especially in renal glomeruli.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 61-Year-Old Woman With Leg Ulcers"
},
{
"authors": "Marc Hare, MD",
"content": [
"The management of cryoglobulinemia should be focused on treating underlying conditions, as well as limiting the precipitation of cryoglobulins and the resultant inflammatory effects. Asymptomatic cryoglobulinemia does not require treatment. When treatment is required, it is based on suppression of the immune response.[1]",
"Figure 2.",
"Figure 2.",
"Figure 3.",
"Figure 3.",
"Nonsteroidal anti-inflammatory drugs are used in patients with arthralgia and fatigue, but may be contraindicated in patients with renal disease. Immunosuppressive medications (eg, corticosteroids, cyclophosphamide, azathioprine) are indicated in cases with evidence of organ involvement, such as vasculitis, renal disease, progressive neurologic findings, or severe skin manifestations.[1]",
"Plasmapheresis is indicated for severe or life-threatening complications related to in vivo cryoprecipitation or serum hyperviscosity. Concomitant use of high-dose corticosteroids and cytotoxic agents is recommended for the reduction of immunoglobulin production.[1]",
"Pegylated interferon alfa combined with ribavirin has been demonstrated to be effective in patients with cryoglobulinemia associated with hepatitis C, and efficacy in patients with chronic myelogenous leukemias and low-grade lymphomas has been reported. Several oral regimens using direct-acting antiviral regimens have achieved cure rates exceeding 90% for hepatitis C. Their high cost has limited their use. In patients with some forms of hepatitis C, use of the oral regimen with ledipasvir/sofosbuvir is recommended for 8-12 weeks, depending on subtype and patient status.",
"Rituximab (anti-CD20 chimeric monoclonal antibody) has shown promise in controlling vasculitis, peripheral neuropathy, arthralgias, low-grade B cell lymphomas, renal disease, and fever, specifically in patients with hepatitis C virus/related mixed cryoglobulinemia that is refractory to or unsuitable for corticosteroids and antiviral therapy. A randomized controlled trial of rituximab for severe cryoglobulinemic vasculitis showed substantial efficacy.[6]",
"Consultation with a rheumatologist or clinical immunologist is appropriate in all cases. The clinician should consider consultation with a nephrologist, hematologist, or a gastroenterologist, depending on any other clinical manifestations.",
"The patient in this case was referred to rheumatology and prescribed a course of oral corticosteroids. She was also referred to dermatology and had azathioprine added to her treatment.",
"The lesions began to slowly shrink and lift at the edges (Figure 2). Serial debridements were performed. Appropriate wound care was provided by her caregiver under instruction and supervision of the wound center. Following this care plan, the patient has had nearly complete resolution of most of her lesions (Figure 3).",
"She developed persistent pancytopenia despite discontinuation of azathioprine. She was referred to a hematologist and now has evidence of multiple myeloma on the basis of bone marrow biopsy, with continued positive cryoglobulins."
],
"date": "December 02, 2015",
"figures": [
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/855/222/855222-Thumb2.png"
},
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/855/222/855222-Thumb3.png"
}
],
"markdown": "# A 61-Year-Old Woman With Leg Ulcers\n\n **Authors:** Marc Hare, MD \n **Date:** December 02, 2015\n\n ## Content\n\n The management of cryoglobulinemia should be focused on treating underlying conditions, as well as limiting the precipitation of cryoglobulins and the resultant inflammatory effects. Asymptomatic cryoglobulinemia does not require treatment. When treatment is required, it is based on suppression of the immune response.[1]\nFigure 2.\nFigure 2.\nFigure 3.\nFigure 3.\nNonsteroidal anti-inflammatory drugs are used in patients with arthralgia and fatigue, but may be contraindicated in patients with renal disease. Immunosuppressive medications (eg, corticosteroids, cyclophosphamide, azathioprine) are indicated in cases with evidence of organ involvement, such as vasculitis, renal disease, progressive neurologic findings, or severe skin manifestations.[1]\nPlasmapheresis is indicated for severe or life-threatening complications related to in vivo cryoprecipitation or serum hyperviscosity. Concomitant use of high-dose corticosteroids and cytotoxic agents is recommended for the reduction of immunoglobulin production.[1]\nPegylated interferon alfa combined with ribavirin has been demonstrated to be effective in patients with cryoglobulinemia associated with hepatitis C, and efficacy in patients with chronic myelogenous leukemias and low-grade lymphomas has been reported. Several oral regimens using direct-acting antiviral regimens have achieved cure rates exceeding 90% for hepatitis C. Their high cost has limited their use. In patients with some forms of hepatitis C, use of the oral regimen with ledipasvir/sofosbuvir is recommended for 8-12 weeks, depending on subtype and patient status.\nRituximab (anti-CD20 chimeric monoclonal antibody) has shown promise in controlling vasculitis, peripheral neuropathy, arthralgias, low-grade B cell lymphomas, renal disease, and fever, specifically in patients with hepatitis C virus/related mixed cryoglobulinemia that is refractory to or unsuitable for corticosteroids and antiviral therapy. A randomized controlled trial of rituximab for severe cryoglobulinemic vasculitis showed substantial efficacy.[6]\nConsultation with a rheumatologist or clinical immunologist is appropriate in all cases. The clinician should consider consultation with a nephrologist, hematologist, or a gastroenterologist, depending on any other clinical manifestations.\nThe patient in this case was referred to rheumatology and prescribed a course of oral corticosteroids. She was also referred to dermatology and had azathioprine added to her treatment.\nThe lesions began to slowly shrink and lift at the edges (Figure 2). Serial debridements were performed. Appropriate wound care was provided by her caregiver under instruction and supervision of the wound center. Following this care plan, the patient has had nearly complete resolution of most of her lesions (Figure 3).\nShe developed persistent pancytopenia despite discontinuation of azathioprine. She was referred to a hematologist and now has evidence of multiple myeloma on the basis of bone marrow biopsy, with continued positive cryoglobulins.\n\n ## Figures\n\n **Figure 2.** \n \n\n**Figure 3.** \n \n\n\n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 917191,
"choiceText": "Underlying lymphoproliferative disorder",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 917193,
"choiceText": "Systemic lupus erythematosus",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 917195,
"choiceText": "B-cell clonal expansion",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 917197,
"choiceText": "Immune complex deposition and complement activation",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 917199,
"choiceText": "Meltzer triad\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Type I cryoglobulinemia is usually related to an underlying lymphoproliferative disease and may be difficult to distinguish from Waldenström macroglobulinemia, multiple myeloma, or chronic lymphocytic leukemia.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 289499,
"questionText": "Type I cryoglobulinemia is most associated with which of the following conditions?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 917201,
"choiceText": "Lesions in dependent areas",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 917203,
"choiceText": "Erythematous macules and purpuric papules",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 917205,
"choiceText": "Ulcers",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 917207,
"choiceText": "Eczematous patches",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 917209,
"choiceText": "Nailfold capillary abnormalities\r\n\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "\r\n\r\nCutaneous manifestations are almost always present in cryoglobulinemia. Lesions are most often seen in dependent areas (especially the lower extremities), and they include erythematous macules and purpuric papules (90%-95%), as well as ulcers (10%-25%). Lesions in nondependent areas are more common in type I cryoglobulinemia. Nailfold capillary abnormalities are common and include dilatation, altered orientation, capillary shortening, and neoangiogenesis.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 289501,
"questionText": "Which of the following choices is <i>not</i> typical of the cutaneous manifestation of cryoglobulinemia?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 61-Year-Old Woman With Leg Ulcers"
},
{
"authors": "Marc Hare, MD",
"content": [],
"date": "December 02, 2015",
"figures": [],
"markdown": "# A 61-Year-Old Woman With Leg Ulcers\n\n **Authors:** Marc Hare, MD \n **Date:** December 02, 2015\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 917191,
"choiceText": "Underlying lymphoproliferative disorder",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 917193,
"choiceText": "Systemic lupus erythematosus",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 917195,
"choiceText": "B-cell clonal expansion",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 917197,
"choiceText": "Immune complex deposition and complement activation",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 917199,
"choiceText": "Meltzer triad\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Type I cryoglobulinemia is usually related to an underlying lymphoproliferative disease and may be difficult to distinguish from Waldenström macroglobulinemia, multiple myeloma, or chronic lymphocytic leukemia.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 289499,
"questionText": "Type I cryoglobulinemia is most associated with which of the following conditions?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 917201,
"choiceText": "Lesions in dependent areas",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 917203,
"choiceText": "Erythematous macules and purpuric papules",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 917205,
"choiceText": "Ulcers",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 917207,
"choiceText": "Eczematous patches",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 917209,
"choiceText": "Nailfold capillary abnormalities\r\n\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "\r\n\r\nCutaneous manifestations are almost always present in cryoglobulinemia. Lesions are most often seen in dependent areas (especially the lower extremities), and they include erythematous macules and purpuric papules (90%-95%), as well as ulcers (10%-25%). Lesions in nondependent areas are more common in type I cryoglobulinemia. Nailfold capillary abnormalities are common and include dilatation, altered orientation, capillary shortening, and neoangiogenesis.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 289501,
"questionText": "Which of the following choices is <i>not</i> typical of the cutaneous manifestation of cryoglobulinemia?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 61-Year-Old Woman With Leg Ulcers"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 917183,
"choiceText": "Multiple myeloma",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 917185,
"choiceText": "Cryoglobulinemia",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 917187,
"choiceText": "Non-Hodgkin lymphoma",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 917189,
"choiceText": "Giant cell arteritis\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 289497,
"questionText": "On the basis of the history, physical examination, and workup, what is the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 917191,
"choiceText": "Underlying lymphoproliferative disorder",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 917193,
"choiceText": "Systemic lupus erythematosus",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 917195,
"choiceText": "B-cell clonal expansion",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 917197,
"choiceText": "Immune complex deposition and complement activation",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 917199,
"choiceText": "Meltzer triad\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Type I cryoglobulinemia is usually related to an underlying lymphoproliferative disease and may be difficult to distinguish from Waldenström macroglobulinemia, multiple myeloma, or chronic lymphocytic leukemia.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 289499,
"questionText": "Type I cryoglobulinemia is most associated with which of the following conditions?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 917201,
"choiceText": "Lesions in dependent areas",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 917203,
"choiceText": "Erythematous macules and purpuric papules",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 917205,
"choiceText": "Ulcers",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 917207,
"choiceText": "Eczematous patches",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 917209,
"choiceText": "Nailfold capillary abnormalities\r\n\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "\r\n\r\nCutaneous manifestations are almost always present in cryoglobulinemia. Lesions are most often seen in dependent areas (especially the lower extremities), and they include erythematous macules and purpuric papules (90%-95%), as well as ulcers (10%-25%). Lesions in nondependent areas are more common in type I cryoglobulinemia. Nailfold capillary abnormalities are common and include dilatation, altered orientation, capillary shortening, and neoangiogenesis.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 289501,
"questionText": "Which of the following choices is <i>not</i> typical of the cutaneous manifestation of cryoglobulinemia?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
854167 | /viewarticle/854167 | [
{
"authors": "Mithula Rao Gopalam, MD, MBBS; Moiz Kasubhai, MD, MRCP",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.",
"A 52-year-old woman with a history of right-sided inflammatory breast cancer presents to the oncology clinic complaining of a 5-day history of headaches and lethargy. She is accompanied by a family member. The patient appears sleepy and is a poor historian, but the family member states that the patient developed a throbbing headache 5 days before presentation; and since the development of her headache, the patient has become increasingly sleepy. Today, she had difficulty awakening.",
"Initially, the intensity of the headache was high in the mornings and lessened when the patient sat upright; however, the headache has become constant over the past day. The patient also complains of nausea, but she has not vomited and has not had any abdominal pain. The family member thinks the patient is having difficulty walking and notes that the patient has stumbled several times. The patient also complained of blurry vision earlier today. The family member notes that the patient appears confused. The patient states that her head hurts, but she cannot provide further information.",
"The patient's medical history is significant for right-sided inflammatory breast cancer stage 3 (based on the TNM staging criteria, which in this case was T4d, N2a, M0). She underwent a right modified radical mastectomy 1 year ago, and she completed six cycles of chemotherapy with doxorubicin and cyclophosphamide at that time. Tamoxifen is the only medication she is currently taking, and she has no known allergies."
],
"date": "January 12, 2018",
"figures": [],
"markdown": "# Depressed Mentation in a 52-Year-Old Woman\n\n **Authors:** Mithula Rao Gopalam, MD, MBBS; Moiz Kasubhai, MD, MRCP \n **Date:** January 12, 2018\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.\nA 52-year-old woman with a history of right-sided inflammatory breast cancer presents to the oncology clinic complaining of a 5-day history of headaches and lethargy. She is accompanied by a family member. The patient appears sleepy and is a poor historian, but the family member states that the patient developed a throbbing headache 5 days before presentation; and since the development of her headache, the patient has become increasingly sleepy. Today, she had difficulty awakening.\nInitially, the intensity of the headache was high in the mornings and lessened when the patient sat upright; however, the headache has become constant over the past day. The patient also complains of nausea, but she has not vomited and has not had any abdominal pain. The family member thinks the patient is having difficulty walking and notes that the patient has stumbled several times. The patient also complained of blurry vision earlier today. The family member notes that the patient appears confused. The patient states that her head hurts, but she cannot provide further information.\nThe patient's medical history is significant for right-sided inflammatory breast cancer stage 3 (based on the TNM staging criteria, which in this case was T4d, N2a, M0). She underwent a right modified radical mastectomy 1 year ago, and she completed six cycles of chemotherapy with doxorubicin and cyclophosphamide at that time. Tamoxifen is the only medication she is currently taking, and she has no known allergies.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "Depressed Mentation in a 52-Year-Old Woman"
},
{
"authors": "Mithula Rao Gopalam, MD, MBBS; Moiz Kasubhai, MD, MRCP",
"content": [
"On physical examination, the patient's blood pressure is 114/80 mm Hg, her pulse is 80 beats/min, her respiratory rate is 14 breaths/min, and her oral temperature is 99°F (37.2°C). The patient appears drowsy, but she is initially able to follow simple commands. Over the course of the office visit, the patient's neurologic function deteriorates. She only opens her eyes to painful stimuli, she makes incomprehensible verbal noises, and she moves all four extremities to painful stimuli; these findings give her a Glasgow coma scale score of 8 (out of 15).",
"Examination of the eyes reveals right and left pupils of 4 mm and 3 mm in size, respectively. The pupils are briskly reactive to light. The funduscopic examination is significant for blurred optic disks bilaterally. No facial asymmetry is noted, and the patient has normal deep tendon reflexes. Examination of the chest is significant for a right mastectomy scar; no palpable lymphadenopathy or masses are present. The heart is regular, without murmurs, and auscultation of the lungs is unremarkable. The abdomen is soft and without masses.",
"While an emergent CT scan of the head is being set up, blood and urine are obtained and sent for testing. Analysis of the blood is unremarkable, revealing a normal complete blood count, basic metabolic panel, and coagulation profile. The urinalysis is positive for leukocytes, nitrates, and bacteria.",
"The emergent CT scan of the head is obtained (see Figure 1).",
"Figure 1."
],
"date": "January 12, 2018",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/854/167/854167-Thumb1.png"
}
],
"markdown": "# Depressed Mentation in a 52-Year-Old Woman\n\n **Authors:** Mithula Rao Gopalam, MD, MBBS; Moiz Kasubhai, MD, MRCP \n **Date:** January 12, 2018\n\n ## Content\n\n On physical examination, the patient's blood pressure is 114/80 mm Hg, her pulse is 80 beats/min, her respiratory rate is 14 breaths/min, and her oral temperature is 99°F (37.2°C). The patient appears drowsy, but she is initially able to follow simple commands. Over the course of the office visit, the patient's neurologic function deteriorates. She only opens her eyes to painful stimuli, she makes incomprehensible verbal noises, and she moves all four extremities to painful stimuli; these findings give her a Glasgow coma scale score of 8 (out of 15).\nExamination of the eyes reveals right and left pupils of 4 mm and 3 mm in size, respectively. The pupils are briskly reactive to light. The funduscopic examination is significant for blurred optic disks bilaterally. No facial asymmetry is noted, and the patient has normal deep tendon reflexes. Examination of the chest is significant for a right mastectomy scar; no palpable lymphadenopathy or masses are present. The heart is regular, without murmurs, and auscultation of the lungs is unremarkable. The abdomen is soft and without masses.\nWhile an emergent CT scan of the head is being set up, blood and urine are obtained and sent for testing. Analysis of the blood is unremarkable, revealing a normal complete blood count, basic metabolic panel, and coagulation profile. The urinalysis is positive for leukocytes, nitrates, and bacteria.\nThe emergent CT scan of the head is obtained (see Figure 1).\nFigure 1.\n\n ## Figures\n\n **Figure 1.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 909721,
"choiceText": "Glioblastoma multiforme",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 909723,
"choiceText": "Subarachnoid hemorrhage",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 909725,
"choiceText": "Epidural hemorrhage",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 909727,
"choiceText": "Obstructive hydrocephalus\r\n",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 286857,
"questionText": "On the basis of the history, physical examination, and workup, what is the diagnosis?<br><br><i>\r\nHint: This is a condition that is associated with breast cancer metastasis.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Depressed Mentation in a 52-Year-Old Woman"
},
{
"authors": "Mithula Rao Gopalam, MD, MBBS; Moiz Kasubhai, MD, MRCP",
"content": [
"Based on the CT scan, the patient was diagnosed with obstructive hydrocephalus. The CT and MRI scans (see Figures 1-8) show enlargement of the third and lateral ventricles secondary to multiple posterior fossa metastases, which are narrowing the cerebral aqueduct, the fourth ventricle, and the fourth ventricular outflow tract. Supratentorial metastasis is also noted. The arrow on Figure 5 indicates the dilated ventricles, while Figure 6 shows a right frontal ventriculostomy. An MRI of the brain also shows multiple metastases, including a posterior fossa lesion causing obstructive hydrocephalus (see Figures 5-8). The arrows in Figure 7 indicate the metastases, and in Figure 8, they point towards the narrowing of the foramen to the third ventricle.",
"Figure 1.",
"Figure 2.",
"Figure 3.",
"Figure 4.",
"The term \"hydrocephalus\" is derived from Greek, wherein \"hydro\" means water and \"cephalus\" means head. Hydrocephalus can be defined broadly as a disturbance of the formation, flow, or absorption of cerebrospinal fluid (CSF) that leads to an increase in the volume occupied by this fluid in the central nervous system (CNS). A hydrodynamic disorder, hydrocephalus does not pertain to the passive accumulation of CSF in vacant space resulting from cerebral atrophy or focal lytic lesions. Normally, CSF is produced in the choroid plexus, and it flows through the CNS by the following route: from the choroid plexus, the CSF flows (1) to the lateral ventricle, (2) through the interventricular foramen of Monro, (3) into the third ventricle, (4) through the cerebral aqueduct of Sylvius, (5) into the fourth ventricle, (6) through the two lateral foramina of Luschka and the single medial foramen of Magendie, and, finally, (7) into the subarachnoid space. In the subarachnoid space, the CSF is absorbed by the arachnoid granulations into the dural sinus and drains into the venous system.",
"Figure 5.",
"Figure 6.",
"Figure 7.",
"Figure 8.",
"Hydrocephalus can be classified as acute or chronic. Acute hydrocephalus occurs over days, subacute hydrocephalus over weeks, and chronic hydrocephalus over months to years.[1] Hydrocephalus can also be categorized as obstructive or nonobstructive (communicating) hydrocephalus.",
"Obstructive hydrocephalus occurs when there is ventricular enlargement under tension as a result of an obstruction of the flow of CSF anywhere in the above-described pathway. Congenital malformations (eg, myelomeningocele) are the most common cause of obstructive hydrocephalus in children; however, children may also acquire hydrocephalus from obstructive tumors, intraventricular hemorrhage, infections, and increased venous sinus pressure.[2] The causes of obstructive hydrocephalus in adults include prior posterior fossa surgery, congenital aqueduct stenosis that has been asymptomatic until adulthood, bacterial meningitis, and benign and malignant tumors of the brain. Although the primary site for breast metastasis is bone, the second most common site for metastasis is the leptomeninges and the brain parenchyma."
],
"date": "January 12, 2018",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/854/167/854167-Thumb1.png"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/854/167/854167-Thumb2.png"
},
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/854/167/854167-Thumb3.png"
},
{
"caption": "Figure 4.",
"image_url": "https://img.medscapestatic.com/article/854/167/854167-Thumb4.png"
},
{
"caption": "Figure 5.",
"image_url": "https://img.medscapestatic.com/article/854/167/854167-Thumb5.png"
},
{
"caption": "Figure 6.",
"image_url": "https://img.medscapestatic.com/article/854/167/854167-Thumb6.png"
},
{
"caption": "Figure 7.",
"image_url": "https://img.medscapestatic.com/article/854/167/854167-Thumb7.png"
},
{
"caption": "Figure 8.",
"image_url": "https://img.medscapestatic.com/article/854/167/854167-Thumb8.png"
}
],
"markdown": "# Depressed Mentation in a 52-Year-Old Woman\n\n **Authors:** Mithula Rao Gopalam, MD, MBBS; Moiz Kasubhai, MD, MRCP \n **Date:** January 12, 2018\n\n ## Content\n\n Based on the CT scan, the patient was diagnosed with obstructive hydrocephalus. The CT and MRI scans (see Figures 1-8) show enlargement of the third and lateral ventricles secondary to multiple posterior fossa metastases, which are narrowing the cerebral aqueduct, the fourth ventricle, and the fourth ventricular outflow tract. Supratentorial metastasis is also noted. The arrow on Figure 5 indicates the dilated ventricles, while Figure 6 shows a right frontal ventriculostomy. An MRI of the brain also shows multiple metastases, including a posterior fossa lesion causing obstructive hydrocephalus (see Figures 5-8). The arrows in Figure 7 indicate the metastases, and in Figure 8, they point towards the narrowing of the foramen to the third ventricle.\nFigure 1.\nFigure 2.\nFigure 3.\nFigure 4.\nThe term \"hydrocephalus\" is derived from Greek, wherein \"hydro\" means water and \"cephalus\" means head. Hydrocephalus can be defined broadly as a disturbance of the formation, flow, or absorption of cerebrospinal fluid (CSF) that leads to an increase in the volume occupied by this fluid in the central nervous system (CNS). A hydrodynamic disorder, hydrocephalus does not pertain to the passive accumulation of CSF in vacant space resulting from cerebral atrophy or focal lytic lesions. Normally, CSF is produced in the choroid plexus, and it flows through the CNS by the following route: from the choroid plexus, the CSF flows (1) to the lateral ventricle, (2) through the interventricular foramen of Monro, (3) into the third ventricle, (4) through the cerebral aqueduct of Sylvius, (5) into the fourth ventricle, (6) through the two lateral foramina of Luschka and the single medial foramen of Magendie, and, finally, (7) into the subarachnoid space. In the subarachnoid space, the CSF is absorbed by the arachnoid granulations into the dural sinus and drains into the venous system.\nFigure 5.\nFigure 6.\nFigure 7.\nFigure 8.\nHydrocephalus can be classified as acute or chronic. Acute hydrocephalus occurs over days, subacute hydrocephalus over weeks, and chronic hydrocephalus over months to years.[1] Hydrocephalus can also be categorized as obstructive or nonobstructive (communicating) hydrocephalus.\nObstructive hydrocephalus occurs when there is ventricular enlargement under tension as a result of an obstruction of the flow of CSF anywhere in the above-described pathway. Congenital malformations (eg, myelomeningocele) are the most common cause of obstructive hydrocephalus in children; however, children may also acquire hydrocephalus from obstructive tumors, intraventricular hemorrhage, infections, and increased venous sinus pressure.[2] The causes of obstructive hydrocephalus in adults include prior posterior fossa surgery, congenital aqueduct stenosis that has been asymptomatic until adulthood, bacterial meningitis, and benign and malignant tumors of the brain. Although the primary site for breast metastasis is bone, the second most common site for metastasis is the leptomeninges and the brain parenchyma.\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n**Figure 3.** \n \n\n**Figure 4.** \n \n\n**Figure 5.** \n \n\n**Figure 6.** \n \n\n**Figure 7.** \n \n\n**Figure 8.** \n \n\n\n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 909721,
"choiceText": "Glioblastoma multiforme",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 909723,
"choiceText": "Subarachnoid hemorrhage",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 909725,
"choiceText": "Epidural hemorrhage",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 909727,
"choiceText": "Obstructive hydrocephalus\r\n",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 286857,
"questionText": "On the basis of the history, physical examination, and workup, what is the diagnosis?<br><br><i>\r\nHint: This is a condition that is associated with breast cancer metastasis.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Depressed Mentation in a 52-Year-Old Woman"
},
{
"authors": "Mithula Rao Gopalam, MD, MBBS; Moiz Kasubhai, MD, MRCP",
"content": [
"In humans, the epidemiology of hydrocephalus is shown to exhibit a bimodal age curve. The first peak in incidence occurs in infancy and is commonly associated with congenital malformations. A second peak is seen during later adulthood, secondary to normal-pressure hydrocephalus, and is diagnosed when enlarged ventricles are seen in the context of a clinical triad of gait disturbance, dementia, and urinary incontinence.[3] In the United States, the incidence of congenital hydrocephalus is 3 cases per 1000 live births; the incidence of acquired hydrocephalus is not known. Males and females appear to be equally affected by the disease, although normal-pressure hydrocephalus has a slight predominance in males.",
"The presentation of obstructive hydrocephalus varies according to the age of the patient and the etiology of the obstruction. Adults frequently complain of headaches that are worse in the morning and that are relieved by sitting up; however, the headaches become continuous as the condition progresses. Nausea and vomiting in the morning are also common symptoms. Increasing confusion, blurred vision, double vision from a sixth nerve palsy, neck pain, difficulty walking, and incontinence may also be present. On physical examination, adult patients may have papilledema, truncal and limb ataxia, failure of upward gaze (Parinaud sign), and a unilateral or bilateral sixth nerve palsy. If hydrocephalus is left untreated, death may occur. Accumulation of the CSF causes elevated intracranial tension, resulting in tonsillar herniation, compression of the brain stem, and subsequent respiratory arrest.",
"In adults, the diagnosis of hydrocephalus is made by either CT scanning or MRI of the brain. The criteria for diagnosing acute hydrocephalus by diagnostic imaging are as follows: The ratio between the largest width of the frontal horns and the internal diameter, from inner-table to inner-table at this level, should be greater than 0.5; the ratio of the largest width of the frontal horns to the maximum biparietal diameter should be greater than 0.3; the temporal horns are enlarged (this is helpful in distinguishing hydrocephalus from atrophy, wherein the temporal horns are typically normal in size); the sulci and interhemispheric and sylvian fissures are narrowed (in atrophy, these are widened); transependymal absorption of CSF is seen as periventricular low density; and, on sagittal images, upward bowing of the corpus callosum and decreased distance between the pons and the mammillary bodies are seen.",
"Common imaging findings for chronic hydrocephalus include less prominent temporal horn dilation, third ventricle herniation, erosion of the sella turcica, macrocrania, and an atrophied corpus callosum. In infants, ultrasonography can be used via the anterior fontanelle to assess for intraventricular hemorrhage and progressive hydrocephalus. Although a lumbar puncture is useful in diagnosing and treating nonobstructive hydrocephalus, the procedure is contraindicated in obstructive hydrocephalus, as the sudden loss of CSF in the spinal column could potentially exacerbate tonsillar herniation."
],
"date": "January 12, 2018",
"figures": [],
"markdown": "# Depressed Mentation in a 52-Year-Old Woman\n\n **Authors:** Mithula Rao Gopalam, MD, MBBS; Moiz Kasubhai, MD, MRCP \n **Date:** January 12, 2018\n\n ## Content\n\n In humans, the epidemiology of hydrocephalus is shown to exhibit a bimodal age curve. The first peak in incidence occurs in infancy and is commonly associated with congenital malformations. A second peak is seen during later adulthood, secondary to normal-pressure hydrocephalus, and is diagnosed when enlarged ventricles are seen in the context of a clinical triad of gait disturbance, dementia, and urinary incontinence.[3] In the United States, the incidence of congenital hydrocephalus is 3 cases per 1000 live births; the incidence of acquired hydrocephalus is not known. Males and females appear to be equally affected by the disease, although normal-pressure hydrocephalus has a slight predominance in males.\nThe presentation of obstructive hydrocephalus varies according to the age of the patient and the etiology of the obstruction. Adults frequently complain of headaches that are worse in the morning and that are relieved by sitting up; however, the headaches become continuous as the condition progresses. Nausea and vomiting in the morning are also common symptoms. Increasing confusion, blurred vision, double vision from a sixth nerve palsy, neck pain, difficulty walking, and incontinence may also be present. On physical examination, adult patients may have papilledema, truncal and limb ataxia, failure of upward gaze (Parinaud sign), and a unilateral or bilateral sixth nerve palsy. If hydrocephalus is left untreated, death may occur. Accumulation of the CSF causes elevated intracranial tension, resulting in tonsillar herniation, compression of the brain stem, and subsequent respiratory arrest.\nIn adults, the diagnosis of hydrocephalus is made by either CT scanning or MRI of the brain. The criteria for diagnosing acute hydrocephalus by diagnostic imaging are as follows: The ratio between the largest width of the frontal horns and the internal diameter, from inner-table to inner-table at this level, should be greater than 0.5; the ratio of the largest width of the frontal horns to the maximum biparietal diameter should be greater than 0.3; the temporal horns are enlarged (this is helpful in distinguishing hydrocephalus from atrophy, wherein the temporal horns are typically normal in size); the sulci and interhemispheric and sylvian fissures are narrowed (in atrophy, these are widened); transependymal absorption of CSF is seen as periventricular low density; and, on sagittal images, upward bowing of the corpus callosum and decreased distance between the pons and the mammillary bodies are seen.\nCommon imaging findings for chronic hydrocephalus include less prominent temporal horn dilation, third ventricle herniation, erosion of the sella turcica, macrocrania, and an atrophied corpus callosum. In infants, ultrasonography can be used via the anterior fontanelle to assess for intraventricular hemorrhage and progressive hydrocephalus. Although a lumbar puncture is useful in diagnosing and treating nonobstructive hydrocephalus, the procedure is contraindicated in obstructive hydrocephalus, as the sudden loss of CSF in the spinal column could potentially exacerbate tonsillar herniation.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "Depressed Mentation in a 52-Year-Old Woman"
},
{
"authors": "Mithula Rao Gopalam, MD, MBBS; Moiz Kasubhai, MD, MRCP",
"content": [
"Obstructive hydrocephalus generally requires surgical treatment, although medical therapy may be used as a temporizing measure until surgery is performed, or it can be used in cases of hydrocephalus that are expected to resolve (as is occasionally seen in meningitis). A medical course of therapy aims to either reduce CSF production or increase CSF absorption. CSF production can be reduced through the use of high-dose acetazolamide (25 mg/kg/d divided into three doses, with a maximum daily dose of 2 g) and furosemide (1 mg/kg/d divided into three doses). Historically, isosorbide has been given for osmotic diuresis, although its effectiveness remains questionable.",
"Patients with brain metastases who present with acute worsening of mentation should be given corticosteroids to decrease cerebral edema. Dexamethasone is the preferred medication, as it has limited mineralocorticoid action and thereby reduces the potential for fluid retention. Typically, dexamethasone is given as a loading dose of 10 mg, followed by 4 mg every 6 hours. Patients who present with elevated intracranial pressure can also be treated with mannitol to reduce cerebral edema, with a starting dose of 0.5-1.0 g/kg. This can be repeated every 4-6 hours. Patients with rapidly declining mental status can also be intubated and hyperventilated to elicit a transient drop in intracranial pressure. Patients may also present with seizures; in these cases, anticonvulsant medications should be given, although the role of prophylactic anticonvulsants remains controversial.[4]",
"Surgical treatment is the preferred and definitive mode of treatment. The principle of shunting is used to establish communication between the CSF (ventricular or lumbar) and a drainage cavity (peritoneum, right atrium, pleura). Ventriculoperitoneal shunts that extend from the lateral ventricle to the peritoneum are most commonly used. In those cases in which ventriculoperitoneal shunts are contraindicated, such as peritonitis, morbid obesity, or after exclusive abdominal surgery, ventriculoatrial shunts may be used. Ventriculoatrial shunts, also called vascular shunts, transfer the CSF from the cerebral ventricles to the cardiac atrium by way of the jugular vein and superior vena cava.",
"A common complication associated with shunt placement is infection of the valve and catheter, which can occasionally cause ventriculitis and bacteremia. Postoperative subdural hygroma or hematoma may occur, as reduced ventricular pressure can cause the bridging veins to stretch and rupture. Ventriculoperitoneal shunts can also result in inguinal hernias, perforation of the abdominal organs, intestinal obstruction, volvulus, and CSF ascites. Other problems encountered include occlusion of the tip of the catheter in the ventricle, orthostatic headaches, and misplacement of a catheter. Another surgical option aims to reduce CSF production by choroid plexectomy or choroid plexus thrombosis. Yet another treatment option is third ventriculostomy, in which a hole is made in the floor of the third ventricle, creating a detour around the blockage that allows the CSF to circulate and be absorbed.[5]",
"In this case, neurosurgeons were consulted, and the patient received an emergent ventriculostomy. The patient then underwent whole-brain radiation in an attempt to decrease the size of the metastasis. Upon admission, the patient was also found to have a urinary tract infection, which was treated with ciprofloxacin. Following treatment of the urinary tract infection and radiation therapy, she underwent ventriculoperitoneal shunt placement. The patient improved considerably and was successfully discharged from the hospital. Unfortunately, the patient has been lost to follow-up."
],
"date": "January 12, 2018",
"figures": [],
"markdown": "# Depressed Mentation in a 52-Year-Old Woman\n\n **Authors:** Mithula Rao Gopalam, MD, MBBS; Moiz Kasubhai, MD, MRCP \n **Date:** January 12, 2018\n\n ## Content\n\n Obstructive hydrocephalus generally requires surgical treatment, although medical therapy may be used as a temporizing measure until surgery is performed, or it can be used in cases of hydrocephalus that are expected to resolve (as is occasionally seen in meningitis). A medical course of therapy aims to either reduce CSF production or increase CSF absorption. CSF production can be reduced through the use of high-dose acetazolamide (25 mg/kg/d divided into three doses, with a maximum daily dose of 2 g) and furosemide (1 mg/kg/d divided into three doses). Historically, isosorbide has been given for osmotic diuresis, although its effectiveness remains questionable.\nPatients with brain metastases who present with acute worsening of mentation should be given corticosteroids to decrease cerebral edema. Dexamethasone is the preferred medication, as it has limited mineralocorticoid action and thereby reduces the potential for fluid retention. Typically, dexamethasone is given as a loading dose of 10 mg, followed by 4 mg every 6 hours. Patients who present with elevated intracranial pressure can also be treated with mannitol to reduce cerebral edema, with a starting dose of 0.5-1.0 g/kg. This can be repeated every 4-6 hours. Patients with rapidly declining mental status can also be intubated and hyperventilated to elicit a transient drop in intracranial pressure. Patients may also present with seizures; in these cases, anticonvulsant medications should be given, although the role of prophylactic anticonvulsants remains controversial.[4]\nSurgical treatment is the preferred and definitive mode of treatment. The principle of shunting is used to establish communication between the CSF (ventricular or lumbar) and a drainage cavity (peritoneum, right atrium, pleura). Ventriculoperitoneal shunts that extend from the lateral ventricle to the peritoneum are most commonly used. In those cases in which ventriculoperitoneal shunts are contraindicated, such as peritonitis, morbid obesity, or after exclusive abdominal surgery, ventriculoatrial shunts may be used. Ventriculoatrial shunts, also called vascular shunts, transfer the CSF from the cerebral ventricles to the cardiac atrium by way of the jugular vein and superior vena cava.\nA common complication associated with shunt placement is infection of the valve and catheter, which can occasionally cause ventriculitis and bacteremia. Postoperative subdural hygroma or hematoma may occur, as reduced ventricular pressure can cause the bridging veins to stretch and rupture. Ventriculoperitoneal shunts can also result in inguinal hernias, perforation of the abdominal organs, intestinal obstruction, volvulus, and CSF ascites. Other problems encountered include occlusion of the tip of the catheter in the ventricle, orthostatic headaches, and misplacement of a catheter. Another surgical option aims to reduce CSF production by choroid plexectomy or choroid plexus thrombosis. Yet another treatment option is third ventriculostomy, in which a hole is made in the floor of the third ventricle, creating a detour around the blockage that allows the CSF to circulate and be absorbed.[5]\nIn this case, neurosurgeons were consulted, and the patient received an emergent ventriculostomy. The patient then underwent whole-brain radiation in an attempt to decrease the size of the metastasis. Upon admission, the patient was also found to have a urinary tract infection, which was treated with ciprofloxacin. Following treatment of the urinary tract infection and radiation therapy, she underwent ventriculoperitoneal shunt placement. The patient improved considerably and was successfully discharged from the hospital. Unfortunately, the patient has been lost to follow-up.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 909729,
"choiceText": "The sylvian and interhemispheric fissures are widened.",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 909731,
"choiceText": "Upward bowing of the corpus callosum is seen on sagittal MRI scans.",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 909733,
"choiceText": "The ratio of the largest width of the frontal horns to the maximum biparietal diameter is greater than 0.3",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 909735,
"choiceText": "Ballooning of the frontal horns of the lateral ventricles and the third ventricle indicating aqueductal obstruction\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The criteria for diagnosing acute hydrocephalus by diagnostic imaging are as follows: The ratio between the largest width of the frontal horns and the internal diameter, from inner-table to inner-table at this level, should be greater than 0.5; the ratio of the largest width of the frontal horns to the maximum biparietal diameter should be greater than 0.3; the temporal horns are enlarged (this is helpful in distinguishing hydrocephalus from atrophy, wherein the temporal horns are typically normal in size); the sulci and interhemispheric and sylvian fissures are narrowed (in atrophy, these are widened); transependymal absorption of CSF is seen as periventricular low density; on sagittal images, upward bowing of the corpus callosum and decreased distance between the pons and the mammillary bodies are seen.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 286859,
"questionText": "Which of the following is NOT a criterion for diagnosing acute hydrocephalus by CT or MRI?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 909745,
"choiceText": "Acetazolamide given at 25 mg/kg/d divided into 3 doses",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 909747,
"choiceText": "Furosemide given at 1 mg/kg/d divided into 3 doses",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 909749,
"choiceText": "Dexamethasone given at 10 mg intravenously (IV), followed by 4 mg IV every 6 hours",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 909751,
"choiceText": "Ventriculoperitoneal shunt",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 909753,
"choiceText": "Intubation with subsequent hyperventilation\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "\r\nSurgical treatment is the preferred and definitive mode of treatment. The principle of shunting is used to establish communication between the CSF (ventricular or lumbar) and a drainage cavity (peritoneum, right atrium, pleura). Ventriculoperitoneal shunts that extend from the lateral ventricle to the peritoneum are most commonly used. In those cases in which ventriculoperitoneal shunts are contraindicated, such as peritonitis, morbid obesity, or after exclusive abdominal surgery, ventriculoatrial shunts may be used. Ventriculoatrial shunts, also called vascular shunts, transfer the CSF from the cerebral ventricles to the cardiac atrium by way of the jugular vein and superior vena cava.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 286863,
"questionText": "Which of the following is the definitive treatment for acute obstructive hydrocephalus in a patient with brain metastases?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Depressed Mentation in a 52-Year-Old Woman"
},
{
"authors": "Mithula Rao Gopalam, MD, MBBS; Moiz Kasubhai, MD, MRCP",
"content": [],
"date": "January 12, 2018",
"figures": [],
"markdown": "# Depressed Mentation in a 52-Year-Old Woman\n\n **Authors:** Mithula Rao Gopalam, MD, MBBS; Moiz Kasubhai, MD, MRCP \n **Date:** January 12, 2018\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 909729,
"choiceText": "The sylvian and interhemispheric fissures are widened.",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 909731,
"choiceText": "Upward bowing of the corpus callosum is seen on sagittal MRI scans.",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 909733,
"choiceText": "The ratio of the largest width of the frontal horns to the maximum biparietal diameter is greater than 0.3",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 909735,
"choiceText": "Ballooning of the frontal horns of the lateral ventricles and the third ventricle indicating aqueductal obstruction\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The criteria for diagnosing acute hydrocephalus by diagnostic imaging are as follows: The ratio between the largest width of the frontal horns and the internal diameter, from inner-table to inner-table at this level, should be greater than 0.5; the ratio of the largest width of the frontal horns to the maximum biparietal diameter should be greater than 0.3; the temporal horns are enlarged (this is helpful in distinguishing hydrocephalus from atrophy, wherein the temporal horns are typically normal in size); the sulci and interhemispheric and sylvian fissures are narrowed (in atrophy, these are widened); transependymal absorption of CSF is seen as periventricular low density; on sagittal images, upward bowing of the corpus callosum and decreased distance between the pons and the mammillary bodies are seen.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 286859,
"questionText": "Which of the following is NOT a criterion for diagnosing acute hydrocephalus by CT or MRI?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 909745,
"choiceText": "Acetazolamide given at 25 mg/kg/d divided into 3 doses",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 909747,
"choiceText": "Furosemide given at 1 mg/kg/d divided into 3 doses",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 909749,
"choiceText": "Dexamethasone given at 10 mg intravenously (IV), followed by 4 mg IV every 6 hours",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 909751,
"choiceText": "Ventriculoperitoneal shunt",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 909753,
"choiceText": "Intubation with subsequent hyperventilation\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "\r\nSurgical treatment is the preferred and definitive mode of treatment. The principle of shunting is used to establish communication between the CSF (ventricular or lumbar) and a drainage cavity (peritoneum, right atrium, pleura). Ventriculoperitoneal shunts that extend from the lateral ventricle to the peritoneum are most commonly used. In those cases in which ventriculoperitoneal shunts are contraindicated, such as peritonitis, morbid obesity, or after exclusive abdominal surgery, ventriculoatrial shunts may be used. Ventriculoatrial shunts, also called vascular shunts, transfer the CSF from the cerebral ventricles to the cardiac atrium by way of the jugular vein and superior vena cava.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 286863,
"questionText": "Which of the following is the definitive treatment for acute obstructive hydrocephalus in a patient with brain metastases?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Depressed Mentation in a 52-Year-Old Woman"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 909721,
"choiceText": "Glioblastoma multiforme",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 909723,
"choiceText": "Subarachnoid hemorrhage",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 909725,
"choiceText": "Epidural hemorrhage",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 909727,
"choiceText": "Obstructive hydrocephalus\r\n",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 286857,
"questionText": "On the basis of the history, physical examination, and workup, what is the diagnosis?<br><br><i>\r\nHint: This is a condition that is associated with breast cancer metastasis.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 909729,
"choiceText": "The sylvian and interhemispheric fissures are widened.",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 909731,
"choiceText": "Upward bowing of the corpus callosum is seen on sagittal MRI scans.",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 909733,
"choiceText": "The ratio of the largest width of the frontal horns to the maximum biparietal diameter is greater than 0.3",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 909735,
"choiceText": "Ballooning of the frontal horns of the lateral ventricles and the third ventricle indicating aqueductal obstruction\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The criteria for diagnosing acute hydrocephalus by diagnostic imaging are as follows: The ratio between the largest width of the frontal horns and the internal diameter, from inner-table to inner-table at this level, should be greater than 0.5; the ratio of the largest width of the frontal horns to the maximum biparietal diameter should be greater than 0.3; the temporal horns are enlarged (this is helpful in distinguishing hydrocephalus from atrophy, wherein the temporal horns are typically normal in size); the sulci and interhemispheric and sylvian fissures are narrowed (in atrophy, these are widened); transependymal absorption of CSF is seen as periventricular low density; on sagittal images, upward bowing of the corpus callosum and decreased distance between the pons and the mammillary bodies are seen.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 286859,
"questionText": "Which of the following is NOT a criterion for diagnosing acute hydrocephalus by CT or MRI?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 909745,
"choiceText": "Acetazolamide given at 25 mg/kg/d divided into 3 doses",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 909747,
"choiceText": "Furosemide given at 1 mg/kg/d divided into 3 doses",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 909749,
"choiceText": "Dexamethasone given at 10 mg intravenously (IV), followed by 4 mg IV every 6 hours",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 909751,
"choiceText": "Ventriculoperitoneal shunt",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 909753,
"choiceText": "Intubation with subsequent hyperventilation\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "\r\nSurgical treatment is the preferred and definitive mode of treatment. The principle of shunting is used to establish communication between the CSF (ventricular or lumbar) and a drainage cavity (peritoneum, right atrium, pleura). Ventriculoperitoneal shunts that extend from the lateral ventricle to the peritoneum are most commonly used. In those cases in which ventriculoperitoneal shunts are contraindicated, such as peritonitis, morbid obesity, or after exclusive abdominal surgery, ventriculoatrial shunts may be used. Ventriculoatrial shunts, also called vascular shunts, transfer the CSF from the cerebral ventricles to the cardiac atrium by way of the jugular vein and superior vena cava.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 286863,
"questionText": "Which of the following is the definitive treatment for acute obstructive hydrocephalus in a patient with brain metastases?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
854135 | /viewarticle/854135 | [
{
"authors": "Sumaira Nabi, MBBS; Shahzad Ahmed, MBBS; Muhammad Tariq, MBBS, FRCP",
"content": [
"Editor's Note:\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.",
"A 30-year-old man presents with a 2-month history of acute weakness in all extremities associated with paresthesia and dysesthesia. One week ago, he suffered vision loss in the right eye and mild ocular pain. Three days before presenting to the hospital, he developed bladder dysfunction.",
"No history of fever, headache, altered sensorium, seizures, or difficulty speaking or swallowing is noted. He has no history of limb weakness, ataxia, or sensory symptoms. He also denies any antecedent diarrheal or coryzal illness. He is a driver by occupation, is married with two children, and belongs to a lower socioeconomic group. The patient is a nonsmoker and denies substance abuse and is not taking any medications on a regular basis. The family history is unremarkable as well."
],
"date": "November 10, 2015",
"figures": [],
"markdown": "# Limb Weakness and Vision Loss in a 30-Year-Old Man\n\n **Authors:** Sumaira Nabi, MBBS; Shahzad Ahmed, MBBS; Muhammad Tariq, MBBS, FRCP \n **Date:** November 10, 2015\n\n ## Content\n\n Editor's Note:\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.\nA 30-year-old man presents with a 2-month history of acute weakness in all extremities associated with paresthesia and dysesthesia. One week ago, he suffered vision loss in the right eye and mild ocular pain. Three days before presenting to the hospital, he developed bladder dysfunction.\nNo history of fever, headache, altered sensorium, seizures, or difficulty speaking or swallowing is noted. He has no history of limb weakness, ataxia, or sensory symptoms. He also denies any antecedent diarrheal or coryzal illness. He is a driver by occupation, is married with two children, and belongs to a lower socioeconomic group. The patient is a nonsmoker and denies substance abuse and is not taking any medications on a regular basis. The family history is unremarkable as well.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "Limb Weakness and Vision Loss in a 30-Year-Old Man"
},
{
"authors": "",
"content": [],
"date": "",
"figures": [],
"markdown": "# \n\n **Authors:** \n **Date:** \n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 2 of 1*",
"pagination": {
"current_page": 2,
"total_pages": 1
},
"questionnaire": [],
"title": ""
},
{
"authors": "Sumaira Nabi, MBBS; Shahzad Ahmed, MBBS; Muhammad Tariq, MBBS, FRCP",
"content": [
"The patient was given pulse therapy with methylprednisolone for 5 days; he was later switched to oral corticosteroids with azathioprine. Improvement in his motor power was negligible. His vision loss persisted. He was then referred to the physiotherapy and rehabilitation unit because he was confined to bed due to severe disability. Unfortunately, because this case occurred in Pakistan, neuromyelitis optica (NMO) antibody testing was not possible. Most serologic assays are not available in the resource-limited settings of the third-world countries.",
"NMO, also known as Devic syndrome or disease, is an idiopathic, inflammatory, demyelinating disease specific to the spinal cord and optic nerves resulting in acute attacks of severe myelitis and acute or subacute unilateral or bilateral optic neuritis.[1] The recovery may be complete or partial. These attacks commonly spare the brain in the early stages.[2] NMO was traditionally thought to be a monophasic illness but is now recognized as a discrete multiphasic or relapsing demyelinating disease.[3]",
"The first account of an association between myelitis and an optic nerve disorder was reported by T.C. Allbutt in 1870.[4] Eugene Devic, a French physician, subsequently described a monophasic illness of bilateral optic neuritis and myelitis occurring in quick succession.[5] In 1894, his student Fernand Gault proposed it to be a distinct clinical entity: neuromyélite optique aiguë.[6] Neuromyelitis optica has a very strong female preponderance, with prevalence reportedly nine times higher in women than in men. Although the first episode of NMO can occur at any time from childhood to old age, the reported mean age of onset is almost 35 years.[7] Worldwide, NMO is more common in nonwhite populations, especially East Asians.",
"The etiology of NMO is the subject of much debate. In some cases, a specific cause has been identified[8,9]; in most cases of uncertain cause, whether it is a variant of multiple sclerosis or a distinct clinical syndrome is unclear. However, optic neuritis, myelitis, and inflammatory demyelination are features of both disorders.[3,5]"
],
"date": "November 10, 2015",
"figures": [],
"markdown": "# Limb Weakness and Vision Loss in a 30-Year-Old Man\n\n **Authors:** Sumaira Nabi, MBBS; Shahzad Ahmed, MBBS; Muhammad Tariq, MBBS, FRCP \n **Date:** November 10, 2015\n\n ## Content\n\n The patient was given pulse therapy with methylprednisolone for 5 days; he was later switched to oral corticosteroids with azathioprine. Improvement in his motor power was negligible. His vision loss persisted. He was then referred to the physiotherapy and rehabilitation unit because he was confined to bed due to severe disability. Unfortunately, because this case occurred in Pakistan, neuromyelitis optica (NMO) antibody testing was not possible. Most serologic assays are not available in the resource-limited settings of the third-world countries.\nNMO, also known as Devic syndrome or disease, is an idiopathic, inflammatory, demyelinating disease specific to the spinal cord and optic nerves resulting in acute attacks of severe myelitis and acute or subacute unilateral or bilateral optic neuritis.[1] The recovery may be complete or partial. These attacks commonly spare the brain in the early stages.[2] NMO was traditionally thought to be a monophasic illness but is now recognized as a discrete multiphasic or relapsing demyelinating disease.[3]\nThe first account of an association between myelitis and an optic nerve disorder was reported by T.C. Allbutt in 1870.[4] Eugene Devic, a French physician, subsequently described a monophasic illness of bilateral optic neuritis and myelitis occurring in quick succession.[5] In 1894, his student Fernand Gault proposed it to be a distinct clinical entity: neuromyélite optique aiguë.[6] Neuromyelitis optica has a very strong female preponderance, with prevalence reportedly nine times higher in women than in men. Although the first episode of NMO can occur at any time from childhood to old age, the reported mean age of onset is almost 35 years.[7] Worldwide, NMO is more common in nonwhite populations, especially East Asians.\nThe etiology of NMO is the subject of much debate. In some cases, a specific cause has been identified[8,9]; in most cases of uncertain cause, whether it is a variant of multiple sclerosis or a distinct clinical syndrome is unclear. However, optic neuritis, myelitis, and inflammatory demyelination are features of both disorders.[3,5]\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 909263,
"choiceText": "Multiple sclerosis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 909265,
"choiceText": "Guillain-Barré syndrome",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 909267,
"choiceText": "Acute disseminated encephalomyelitis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 909269,
"choiceText": "Neuromyelitis optica",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 286711,
"questionText": "On the basis of the history, physical examination, and workup, what is the diagnosis?<br><br><i>\r\nHint: Note the history of optic neuritis with quadriparesis and the normal brain MRI.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Limb Weakness and Vision Loss in a 30-Year-Old Man"
},
{
"authors": "Sumaira Nabi, MBBS; Shahzad Ahmed, MBBS; Muhammad Tariq, MBBS, FRCP",
"content": [
"NMO is characterized by attacks of unilateral or (less often) bilateral optic neuritis and myelitis that usually occur sequentially rather than simultaneously.[2] Although, as Devic observed, these two conditions can follow in quick succession, in some cases they may be separated by an interval of years or even decades.[2,7] Typically, the history is significant for a painful visual loss with severe paraplegia, sensory loss, and bladder dysfunction. Cervical myelitis can extend into the brainstem, leading to intractable hiccups, nausea and vomiting, vertigo, various bulbar symptoms, and acute neurogenic respiratory failure.[10,11] Other symptoms of spinal cord demyelination that are seen in multiple sclerosis, such as flexor spasms and Lhermitte phenomenon, may also be seen in NMO.",
"MRI of the spine typically reveals longitudinally extensive transverse myelitis (LETM), with lesions extending over three or more vertebral segments. These necrotizing and cavitating lesions are predominantly located in the cervical and thoracic cord and are hypointense on T1-weighted imaging and hyperintense on T2-weighted imaging and FLAIR sequences. Cord swelling and gadolinium enhancement are seen in acute cord lesions.[12,13] MRI of the brain is typically normal except for optic nerve enhancement by intravenously administered gadolinium during an acute attack of optic neuritis. Nonspecific white matter lesions may be seen in some patients.[14] Brainstem lesions can occur in isolation or as an extension of cervical myelitis. They can be detected in 60% of patients with NMO later in the course of the disease and are usually clinically silent.[15]",
"Cerebrospinal studies may also aid in the diagnostic process. CSF pleocytosis (>50×106 leukocytes/L) with a neutrophilic predominance is a characteristic of NMO.[16] Oligoclonal bands are usually absent but may be found in 15%-30% of patients with NMO.[17] Identification of NMO immunoglobulin G (IgG) as the pathogenic biomarker has contributed to widening the spectrum of NMO. These antibodies are directed against the aquaporin-4 water channels in the astrocyte foot processes at the blood-brain barrier, pia, subpia, and Virchow-Robin spaces. NMO IgG has a sensitivity of 73% and specificity of 91%.[18]",
"NMO is defined using the revised diagnostic criteria devised by Wingerchuk and colleagues in 2006,[19] which require the presence of optic neuritis and myelitis plus any two of the following: (1) brain MRI scan not satisfying the McDonald criteria; (2) T2-weighted MRI scan showing contiguous spinal cord lesions spanning three or more vertebral segments; and/or (3) positive serology for NMO IgG (anti-aquaporin-4 antibody). Our patient fulfilled these criteria.",
"Since the discovery of NMO IgG, various limited forms of NMO have also been recognized based on NMO-IgG seropositivity; these constitute the NMO spectrum disorders.[20] These include the following clinical presentations: idiopathic single or recurrent attacks of LETM, recurrent or simultaneous bilateral optic neuritis, Asian-type (opticospinal) multiple sclerosis, optic neuritis or LETM associated with systemic autoimmune diseases, and optic neuritis or LETM associated with brain lesions typical of NMO.",
"NMO is now recognized as a discrete demyelinating disorder, with clinical, neuroimaging, and laboratory findings that can distinguish it from multiple sclerosis.[2,20] Multiple sclerosis may involve any white matter tract and has milder attacks. NMO involves the optic nerves and spinal cord only, with more severe attacks. Brain MRI scans are usually normal or nonspecific in NMO, whereas those in multiple sclerosis show periventricular and other white matter lesions. In addition, spinal cord MRI scans reveal longitudinally extensive, central necrotic lesions in NMO, whereas multiple small peripheral lesions are seen in multiple sclerosis. CSF oligoclonal bands are typically absent in NMO, unlike in multiple sclerosis, and NMO may be associated with a coexistent autoimmune disease.[20]"
],
"date": "November 10, 2015",
"figures": [],
"markdown": "# Limb Weakness and Vision Loss in a 30-Year-Old Man\n\n **Authors:** Sumaira Nabi, MBBS; Shahzad Ahmed, MBBS; Muhammad Tariq, MBBS, FRCP \n **Date:** November 10, 2015\n\n ## Content\n\n NMO is characterized by attacks of unilateral or (less often) bilateral optic neuritis and myelitis that usually occur sequentially rather than simultaneously.[2] Although, as Devic observed, these two conditions can follow in quick succession, in some cases they may be separated by an interval of years or even decades.[2,7] Typically, the history is significant for a painful visual loss with severe paraplegia, sensory loss, and bladder dysfunction. Cervical myelitis can extend into the brainstem, leading to intractable hiccups, nausea and vomiting, vertigo, various bulbar symptoms, and acute neurogenic respiratory failure.[10,11] Other symptoms of spinal cord demyelination that are seen in multiple sclerosis, such as flexor spasms and Lhermitte phenomenon, may also be seen in NMO.\nMRI of the spine typically reveals longitudinally extensive transverse myelitis (LETM), with lesions extending over three or more vertebral segments. These necrotizing and cavitating lesions are predominantly located in the cervical and thoracic cord and are hypointense on T1-weighted imaging and hyperintense on T2-weighted imaging and FLAIR sequences. Cord swelling and gadolinium enhancement are seen in acute cord lesions.[12,13] MRI of the brain is typically normal except for optic nerve enhancement by intravenously administered gadolinium during an acute attack of optic neuritis. Nonspecific white matter lesions may be seen in some patients.[14] Brainstem lesions can occur in isolation or as an extension of cervical myelitis. They can be detected in 60% of patients with NMO later in the course of the disease and are usually clinically silent.[15]\nCerebrospinal studies may also aid in the diagnostic process. CSF pleocytosis (>50×106 leukocytes/L) with a neutrophilic predominance is a characteristic of NMO.[16] Oligoclonal bands are usually absent but may be found in 15%-30% of patients with NMO.[17] Identification of NMO immunoglobulin G (IgG) as the pathogenic biomarker has contributed to widening the spectrum of NMO. These antibodies are directed against the aquaporin-4 water channels in the astrocyte foot processes at the blood-brain barrier, pia, subpia, and Virchow-Robin spaces. NMO IgG has a sensitivity of 73% and specificity of 91%.[18]\nNMO is defined using the revised diagnostic criteria devised by Wingerchuk and colleagues in 2006,[19] which require the presence of optic neuritis and myelitis plus any two of the following: (1) brain MRI scan not satisfying the McDonald criteria; (2) T2-weighted MRI scan showing contiguous spinal cord lesions spanning three or more vertebral segments; and/or (3) positive serology for NMO IgG (anti-aquaporin-4 antibody). Our patient fulfilled these criteria.\nSince the discovery of NMO IgG, various limited forms of NMO have also been recognized based on NMO-IgG seropositivity; these constitute the NMO spectrum disorders.[20] These include the following clinical presentations: idiopathic single or recurrent attacks of LETM, recurrent or simultaneous bilateral optic neuritis, Asian-type (opticospinal) multiple sclerosis, optic neuritis or LETM associated with systemic autoimmune diseases, and optic neuritis or LETM associated with brain lesions typical of NMO.\nNMO is now recognized as a discrete demyelinating disorder, with clinical, neuroimaging, and laboratory findings that can distinguish it from multiple sclerosis.[2,20] Multiple sclerosis may involve any white matter tract and has milder attacks. NMO involves the optic nerves and spinal cord only, with more severe attacks. Brain MRI scans are usually normal or nonspecific in NMO, whereas those in multiple sclerosis show periventricular and other white matter lesions. In addition, spinal cord MRI scans reveal longitudinally extensive, central necrotic lesions in NMO, whereas multiple small peripheral lesions are seen in multiple sclerosis. CSF oligoclonal bands are typically absent in NMO, unlike in multiple sclerosis, and NMO may be associated with a coexistent autoimmune disease.[20]\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "Limb Weakness and Vision Loss in a 30-Year-Old Man"
},
{
"authors": "Sumaira Nabi, MBBS; Shahzad Ahmed, MBBS; Muhammad Tariq, MBBS, FRCP",
"content": [
"The initial treatment for acute attacks of optic neuritis or myelitis is mainly high-dose intravenous corticosteroid therapy.[20] Plasmapheresis (1-1.5 L plasma volume per exchange) over a 2-week period may be initiated in patients who do not respond to treatment with corticosteroids.[21] Interferon and natalizumab are not effective for prevention of NMO relapses and may even lead to worsening of symptoms.[22,23,24] Azathioprine, with or without prednisolone, and rituximab are considered the first-line therapies.[25] Monthly pulse cyclophosphamide, mitoxantrone, mycophenolate mofetil, and methotrexate have been suggested as second-line therapies.",
"Cyclosporin A has been used in a small case series for patients with NMO.[26] Evidence for the use of intermittent plasma exchange and intravenous Ig for relapse prevention is insufficient.[25] Other, newer agents under study that have shown promise include eculizumab, a humanized monoclonal antibody against the complement protein C5, and tocilizumab, a humanized anti-interleukin-6 antibody.[27,28] The role of anti-aquaporin-4 monoclonal antibody blocker is also being investigated.[29]",
"This case highlights the fact that with lack of serologic assays, such as detection of NMO IgG antibodies in resource-limited settings, clinicians must rely on clinical judgment, neuroimaging, and other supportive lab tests and must promptly initiate treatment so as to delay and decrease the morbidity and mortality associated with NMO."
],
"date": "November 10, 2015",
"figures": [],
"markdown": "# Limb Weakness and Vision Loss in a 30-Year-Old Man\n\n **Authors:** Sumaira Nabi, MBBS; Shahzad Ahmed, MBBS; Muhammad Tariq, MBBS, FRCP \n **Date:** November 10, 2015\n\n ## Content\n\n The initial treatment for acute attacks of optic neuritis or myelitis is mainly high-dose intravenous corticosteroid therapy.[20] Plasmapheresis (1-1.5 L plasma volume per exchange) over a 2-week period may be initiated in patients who do not respond to treatment with corticosteroids.[21] Interferon and natalizumab are not effective for prevention of NMO relapses and may even lead to worsening of symptoms.[22,23,24] Azathioprine, with or without prednisolone, and rituximab are considered the first-line therapies.[25] Monthly pulse cyclophosphamide, mitoxantrone, mycophenolate mofetil, and methotrexate have been suggested as second-line therapies.\nCyclosporin A has been used in a small case series for patients with NMO.[26] Evidence for the use of intermittent plasma exchange and intravenous Ig for relapse prevention is insufficient.[25] Other, newer agents under study that have shown promise include eculizumab, a humanized monoclonal antibody against the complement protein C5, and tocilizumab, a humanized anti-interleukin-6 antibody.[27,28] The role of anti-aquaporin-4 monoclonal antibody blocker is also being investigated.[29]\nThis case highlights the fact that with lack of serologic assays, such as detection of NMO IgG antibodies in resource-limited settings, clinicians must rely on clinical judgment, neuroimaging, and other supportive lab tests and must promptly initiate treatment so as to delay and decrease the morbidity and mortality associated with NMO.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 909271,
"choiceText": "LETM",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 909273,
"choiceText": "Positive NMO IgG",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 909275,
"choiceText": "Periventricular white matter lesions",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 909277,
"choiceText": "Negative oligoclonal bands",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 909279,
"choiceText": "Presence of other coexistent autoimmune diseases\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "\r\nMultiple sclerosis may involve any white matter tract and has milder attacks. NMO involves the optic nerves and spinal cord only, with more severe attacks. Brain MRI scans are usually normal or nonspecific in NMO, whereas those in multiple sclerosis show periventricular and other white matter lesions. In addition, spinal cord MRI scans reveal longitudinally extensive, central necrotic lesions in NMO, whereas multiple small peripheral lesions are seen in multiple sclerosis. CSF oligoclonal bands are typically absent in NMO, unlike in multiple sclerosis, and NMO may be associated with a coexistent autoimmune disease.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 286713,
"questionText": "Which of the following features is suggestive of multiple sclerosis more than NMO?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 909281,
"choiceText": "Corticosteroids",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 909283,
"choiceText": "Plasmapheresis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 909285,
"choiceText": "Azathioprine",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 909287,
"choiceText": "Interferon",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 909289,
"choiceText": "Rituximab\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Interferon and natalizumab are not effective for prevention of NMO relapses and may even lead to worsening of symptoms.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 286715,
"questionText": "Which of the following therapeutic modalities is thought to worsen the course of disease in NMO?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Limb Weakness and Vision Loss in a 30-Year-Old Man"
},
{
"authors": "Sumaira Nabi, MBBS; Shahzad Ahmed, MBBS; Muhammad Tariq, MBBS, FRCP",
"content": [],
"date": "November 10, 2015",
"figures": [],
"markdown": "# Limb Weakness and Vision Loss in a 30-Year-Old Man\n\n **Authors:** Sumaira Nabi, MBBS; Shahzad Ahmed, MBBS; Muhammad Tariq, MBBS, FRCP \n **Date:** November 10, 2015\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 909271,
"choiceText": "LETM",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 909273,
"choiceText": "Positive NMO IgG",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 909275,
"choiceText": "Periventricular white matter lesions",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 909277,
"choiceText": "Negative oligoclonal bands",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 909279,
"choiceText": "Presence of other coexistent autoimmune diseases\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "\r\nMultiple sclerosis may involve any white matter tract and has milder attacks. NMO involves the optic nerves and spinal cord only, with more severe attacks. Brain MRI scans are usually normal or nonspecific in NMO, whereas those in multiple sclerosis show periventricular and other white matter lesions. In addition, spinal cord MRI scans reveal longitudinally extensive, central necrotic lesions in NMO, whereas multiple small peripheral lesions are seen in multiple sclerosis. CSF oligoclonal bands are typically absent in NMO, unlike in multiple sclerosis, and NMO may be associated with a coexistent autoimmune disease.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 286713,
"questionText": "Which of the following features is suggestive of multiple sclerosis more than NMO?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 909281,
"choiceText": "Corticosteroids",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 909283,
"choiceText": "Plasmapheresis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 909285,
"choiceText": "Azathioprine",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 909287,
"choiceText": "Interferon",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 909289,
"choiceText": "Rituximab\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Interferon and natalizumab are not effective for prevention of NMO relapses and may even lead to worsening of symptoms.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 286715,
"questionText": "Which of the following therapeutic modalities is thought to worsen the course of disease in NMO?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Limb Weakness and Vision Loss in a 30-Year-Old Man"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 909263,
"choiceText": "Multiple sclerosis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 909265,
"choiceText": "Guillain-Barré syndrome",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 909267,
"choiceText": "Acute disseminated encephalomyelitis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 909269,
"choiceText": "Neuromyelitis optica",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 286711,
"questionText": "On the basis of the history, physical examination, and workup, what is the diagnosis?<br><br><i>\r\nHint: Note the history of optic neuritis with quadriparesis and the normal brain MRI.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 909271,
"choiceText": "LETM",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 909273,
"choiceText": "Positive NMO IgG",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 909275,
"choiceText": "Periventricular white matter lesions",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 909277,
"choiceText": "Negative oligoclonal bands",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 909279,
"choiceText": "Presence of other coexistent autoimmune diseases\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "\r\nMultiple sclerosis may involve any white matter tract and has milder attacks. NMO involves the optic nerves and spinal cord only, with more severe attacks. Brain MRI scans are usually normal or nonspecific in NMO, whereas those in multiple sclerosis show periventricular and other white matter lesions. In addition, spinal cord MRI scans reveal longitudinally extensive, central necrotic lesions in NMO, whereas multiple small peripheral lesions are seen in multiple sclerosis. CSF oligoclonal bands are typically absent in NMO, unlike in multiple sclerosis, and NMO may be associated with a coexistent autoimmune disease.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 286713,
"questionText": "Which of the following features is suggestive of multiple sclerosis more than NMO?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 909281,
"choiceText": "Corticosteroids",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 909283,
"choiceText": "Plasmapheresis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 909285,
"choiceText": "Azathioprine",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 909287,
"choiceText": "Interferon",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 909289,
"choiceText": "Rituximab\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Interferon and natalizumab are not effective for prevention of NMO relapses and may even lead to worsening of symptoms.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 286715,
"questionText": "Which of the following therapeutic modalities is thought to worsen the course of disease in NMO?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
853228 | /viewarticle/853228 | [
{
"authors": "Setshedi Makwinja, MD; Ben Numpang, MD; Benjamin R. Aubey, MD, MPH",
"content": [
"Editor's Note:\n\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.",
"A 3-year-old girl is brought to the emergency department by her parents with a fever and refusal to walk secondary to pain in her right leg. The pain is associated with thigh and back pain on the same side as the limp. The day before presentation, the child's mother picked her up from her babysitter, where she noticed that the child was irritable and crying. At that time, the child was pointing at and trying to touch her back, and she was walking with a noticeable limp. The parents called the patient's pediatrician, who recommended an antipyretic with follow-up the next day.",
"The review of symptoms is only remarkable for a 1-week history of a \"cold\" with a runny nose and a cough. The mother denies any history of trauma to the affected extremity, and there is no history of similar problems. The patient's medical history is unremarkable. The child is well-appearing and has been regularly observed by the outpatient pediatric department. The patient's immunization schedule is up to date. The child's developmental milestones are appropriate for her age. The family history is noncontributory."
],
"date": "October 28, 2015",
"figures": [],
"markdown": "# Fever and Limp in a 3-Year-Old Girl\n\n **Authors:** Setshedi Makwinja, MD; Ben Numpang, MD; Benjamin R. Aubey, MD, MPH \n **Date:** October 28, 2015\n\n ## Content\n\n Editor's Note:\n\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.\nA 3-year-old girl is brought to the emergency department by her parents with a fever and refusal to walk secondary to pain in her right leg. The pain is associated with thigh and back pain on the same side as the limp. The day before presentation, the child's mother picked her up from her babysitter, where she noticed that the child was irritable and crying. At that time, the child was pointing at and trying to touch her back, and she was walking with a noticeable limp. The parents called the patient's pediatrician, who recommended an antipyretic with follow-up the next day.\nThe review of symptoms is only remarkable for a 1-week history of a \"cold\" with a runny nose and a cough. The mother denies any history of trauma to the affected extremity, and there is no history of similar problems. The patient's medical history is unremarkable. The child is well-appearing and has been regularly observed by the outpatient pediatric department. The patient's immunization schedule is up to date. The child's developmental milestones are appropriate for her age. The family history is noncontributory.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "Fever and Limp in a 3-Year-Old Girl"
},
{
"authors": "Setshedi Makwinja, MD; Ben Numpang, MD; Benjamin R. Aubey, MD, MPH",
"content": [
"Upon physical examination, the patient's vital signs are stable, with a blood pressure of 103/66 mm Hg, pulse rate of 115 beats/min, respiratory rate of 24 breaths/min, and an oxygen saturation of 100% while breathing room air. Her temperature is 97.5°F (36.4°C). The patient initially refuses to bear weight on the right leg and is unwilling to walk. She is tearful and uncooperative as a result of the pain.",
"Figure 1.",
"Figure 1.",
"Figure 2.",
"Figure 2.",
"The respiratory and cardiac portions of the physical examination are normal. The abdominal examination is also normal, with no palpable masses or tenderness to deep palpation. There is localized exquisite tenderness over the L1-2 region of the back, with slight induration noted in the overlying tissue. The lower extremities are well-perfused, with intact peripheral pulses; no external evidence of trauma is found. There is no limitation in the range of motion at the hip and knee joints bilaterally, with unremarkable obturator and psoas signs. The neurologic examination findings are normal. No lymphadenopathy is detected.",
"Figure 3.",
"Figure 3.",
"Figure 4.",
"Figure 4.",
"The initial laboratory investigations reveal an elevated white blood cell count of 20.5 × 103/μL (20.5 × 109/L; neutrophils, 66.5% [0.66]; lymphocytes, 21% [0.21]; monocytes, 11.3% [0.11]), an elevated erythrocyte sedimentation rate of 87 mm/h, a C-reactive protein (CRP) level of 49.5 mg/L, a creatine kinase of 69 U/L, and a lactate dehydrogenase of 247 U/L. The urinalysis is normal, with no evidence of infection or hematuria. Plain x-rays of the lumbar spine are obtained (see Figures 1 and 2) and are essentially unremarkable. Due to a high suspicion for serious pathology based on the clinical presentation and the results of the laboratory investigations, urgent MRI scans of the spine are also obtained (see Figures 3 and 4)."
],
"date": "October 28, 2015",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/853/228/853228-Thumb1.png"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/853/228/853228-Thumb2.png"
},
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/853/228/853228-Thumb3.png"
},
{
"caption": "Figure 4.",
"image_url": "https://img.medscapestatic.com/article/853/228/853228-Thumb4.png"
}
],
"markdown": "# Fever and Limp in a 3-Year-Old Girl\n\n **Authors:** Setshedi Makwinja, MD; Ben Numpang, MD; Benjamin R. Aubey, MD, MPH \n **Date:** October 28, 2015\n\n ## Content\n\n Upon physical examination, the patient's vital signs are stable, with a blood pressure of 103/66 mm Hg, pulse rate of 115 beats/min, respiratory rate of 24 breaths/min, and an oxygen saturation of 100% while breathing room air. Her temperature is 97.5°F (36.4°C). The patient initially refuses to bear weight on the right leg and is unwilling to walk. She is tearful and uncooperative as a result of the pain.\nFigure 1.\nFigure 1.\nFigure 2.\nFigure 2.\nThe respiratory and cardiac portions of the physical examination are normal. The abdominal examination is also normal, with no palpable masses or tenderness to deep palpation. There is localized exquisite tenderness over the L1-2 region of the back, with slight induration noted in the overlying tissue. The lower extremities are well-perfused, with intact peripheral pulses; no external evidence of trauma is found. There is no limitation in the range of motion at the hip and knee joints bilaterally, with unremarkable obturator and psoas signs. The neurologic examination findings are normal. No lymphadenopathy is detected.\nFigure 3.\nFigure 3.\nFigure 4.\nFigure 4.\nThe initial laboratory investigations reveal an elevated white blood cell count of 20.5 × 103/μL (20.5 × 109/L; neutrophils, 66.5% [0.66]; lymphocytes, 21% [0.21]; monocytes, 11.3% [0.11]), an elevated erythrocyte sedimentation rate of 87 mm/h, a C-reactive protein (CRP) level of 49.5 mg/L, a creatine kinase of 69 U/L, and a lactate dehydrogenase of 247 U/L. The urinalysis is normal, with no evidence of infection or hematuria. Plain x-rays of the lumbar spine are obtained (see Figures 1 and 2) and are essentially unremarkable. Due to a high suspicion for serious pathology based on the clinical presentation and the results of the laboratory investigations, urgent MRI scans of the spine are also obtained (see Figures 3 and 4).\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n**Figure 3.** \n \n\n**Figure 4.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 904611,
"choiceText": "Psoas abscess",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 904613,
"choiceText": "Spinal epidural abscess",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 904615,
"choiceText": "Retroperitoneal schwannoma\r\n",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 904617,
"choiceText": "Retroperitoneal hematoma",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 285151,
"questionText": "On the basis of the history, physical examination, and workup, what is the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Fever and Limp in a 3-Year-Old Girl"
},
{
"authors": "Setshedi Makwinja, MD; Ben Numpang, MD; Benjamin R. Aubey, MD, MPH",
"content": [
"As previously mentioned, the lumbosacral plain films were essentially unrevealing (see Figures 1 and 2). The MRI scan of the lumbosacral region, however, revealed a right multiloculated psoas abscess, with paraspinal extension in the region of L3-4 (see Figures 3 and 4) and possible involvement of the ipsilateral pedicle of L3 (not shown in the images provided). Osteomyelitis could not be ruled out based on the images. The abscess measured 1.1×0.6×2.5 cm.",
"Figure 1.",
"Figure 1.",
"Figure 2.",
"Figure 2.",
"A psoas abscess may be classified as primary or secondary, depending on the presence or absence of an underlying cause. In cases of primary psoas abscess, there is no identifiable source of infection. The psoas muscle is a single structure (\"psoas major\") in 70% of people, but the remaining 30% also have a smaller \"psoas minor,\" which lies anterior to the psoas major and along the same course. In the lower half of the psoas muscle's course, it runs alongside the iliacus muscle, with a common tendon insertion into the lesser trochanter. Together, they are referred to as the iliopsoas. It lies in close proximity to many organs, such as the sigmoid colon, jejunum, appendix, ureters, aorta, renal pelvis, pancreas, iliac lymph nodes, and the spine. Infections in these organs can contiguously spread to the psoas muscle. A rich vascular supply is believed to predispose it to hematogenous spread from sites of occult infection.[1,2,3]",
"Figure 3.",
"Figure 3.",
"Figure 4.",
"Figure 4.",
"A psoas abscess in children classically presents as a triad of fever, limp, and hip pain. It is important to differentiate between primary disease of the hip and a psoas abscess, as close proximity of a psoas abscess to the hip capsule can result in a similar presentation. This contributes to its extreme clinical variability. Passive rotation of the hip joint in flexion is possible in cases of psoas abscess, whereas in primary disease of the hip, resistance would be likely. Dysfunction of this joint, however, widely varies, ranging from complete pseudoparalysis to normal range of motion.",
"In our case, the child presented with the additional finding of tenderness localized to the lower back, which led to an initial incorrect working diagnosis of diskitis. The differential diagnoses in this patient included psoas abscess, pyelonephritis (ruled out by the urine analysis), osteomyelitis, and a neoplastic process. Garner has and colleagues[4] suggested that the incidence of psoas abscess is probably underreported. Primary psoas abscess has a better prognosis than secondary psoas abscess, with a mortality rate of 2.4% (18.9% for secondary abscesses). The median time of diagnosis is 3 days, and the median hospital stay is 27 days. The major cause of death is delayed or inadequate therapy.[5,6]",
"The appropriate imaging studies are important to accurately diagnose this uncommon clinical presentation. Ultrasonography has been recognized as the quickest and least expensive diagnostic imaging modality, as well as being a safe one. It can also differentiate between solids and liquids but, unfortunately, has a low sensitivity. Plain films of the abdomen may show enlargement or loss of definition of the psoas muscle or gas shadows in the soft tissue. Even though findings on plain films correlate poorly with a lesion, especially given the frequent presence of overlying bowel and stool, studies have shown that plain films should be performed before other imaging modalities in patients with suspected psoas abscesses (but may not be necessary if CT scan is already planned). CT scans done with intravenous (IV) contrast show rim-enhancing hypodense areas, with secondary findings of inflammation obliterating surrounding tissue, gas bubbles, and bone destruction when present.",
"Marked wall thickening, rim thickening, or multiple cavities may be noted; this suggests tuberculous rather than pyogenic infection. CT scans are also useful in recognizing potential etiologies such as Crohn disease and appendicitis. MRI (which was the diagnostic study in this case) has been reported to be more sensitive than CT scanning in displaying tissue contrast resolution and in screening out bone interference, as well as showing the extent of disease. A study by Yin and colleagues[7] recommended the use of a bone scan, especially in patients presenting with low back pain and an established fever of unknown origin, as it allows detection of unexpected concomitant infections.[1,7,8]"
],
"date": "October 28, 2015",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/853/228/853228-Thumb1.png"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/853/228/853228-Thumb2.png"
},
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/853/228/853228-Thumb3.png"
},
{
"caption": "Figure 4.",
"image_url": "https://img.medscapestatic.com/article/853/228/853228-Thumb4.png"
}
],
"markdown": "# Fever and Limp in a 3-Year-Old Girl\n\n **Authors:** Setshedi Makwinja, MD; Ben Numpang, MD; Benjamin R. Aubey, MD, MPH \n **Date:** October 28, 2015\n\n ## Content\n\n As previously mentioned, the lumbosacral plain films were essentially unrevealing (see Figures 1 and 2). The MRI scan of the lumbosacral region, however, revealed a right multiloculated psoas abscess, with paraspinal extension in the region of L3-4 (see Figures 3 and 4) and possible involvement of the ipsilateral pedicle of L3 (not shown in the images provided). Osteomyelitis could not be ruled out based on the images. The abscess measured 1.1×0.6×2.5 cm.\nFigure 1.\nFigure 1.\nFigure 2.\nFigure 2.\nA psoas abscess may be classified as primary or secondary, depending on the presence or absence of an underlying cause. In cases of primary psoas abscess, there is no identifiable source of infection. The psoas muscle is a single structure (\"psoas major\") in 70% of people, but the remaining 30% also have a smaller \"psoas minor,\" which lies anterior to the psoas major and along the same course. In the lower half of the psoas muscle's course, it runs alongside the iliacus muscle, with a common tendon insertion into the lesser trochanter. Together, they are referred to as the iliopsoas. It lies in close proximity to many organs, such as the sigmoid colon, jejunum, appendix, ureters, aorta, renal pelvis, pancreas, iliac lymph nodes, and the spine. Infections in these organs can contiguously spread to the psoas muscle. A rich vascular supply is believed to predispose it to hematogenous spread from sites of occult infection.[1,2,3]\nFigure 3.\nFigure 3.\nFigure 4.\nFigure 4.\nA psoas abscess in children classically presents as a triad of fever, limp, and hip pain. It is important to differentiate between primary disease of the hip and a psoas abscess, as close proximity of a psoas abscess to the hip capsule can result in a similar presentation. This contributes to its extreme clinical variability. Passive rotation of the hip joint in flexion is possible in cases of psoas abscess, whereas in primary disease of the hip, resistance would be likely. Dysfunction of this joint, however, widely varies, ranging from complete pseudoparalysis to normal range of motion.\nIn our case, the child presented with the additional finding of tenderness localized to the lower back, which led to an initial incorrect working diagnosis of diskitis. The differential diagnoses in this patient included psoas abscess, pyelonephritis (ruled out by the urine analysis), osteomyelitis, and a neoplastic process. Garner has and colleagues[4] suggested that the incidence of psoas abscess is probably underreported. Primary psoas abscess has a better prognosis than secondary psoas abscess, with a mortality rate of 2.4% (18.9% for secondary abscesses). The median time of diagnosis is 3 days, and the median hospital stay is 27 days. The major cause of death is delayed or inadequate therapy.[5,6]\nThe appropriate imaging studies are important to accurately diagnose this uncommon clinical presentation. Ultrasonography has been recognized as the quickest and least expensive diagnostic imaging modality, as well as being a safe one. It can also differentiate between solids and liquids but, unfortunately, has a low sensitivity. Plain films of the abdomen may show enlargement or loss of definition of the psoas muscle or gas shadows in the soft tissue. Even though findings on plain films correlate poorly with a lesion, especially given the frequent presence of overlying bowel and stool, studies have shown that plain films should be performed before other imaging modalities in patients with suspected psoas abscesses (but may not be necessary if CT scan is already planned). CT scans done with intravenous (IV) contrast show rim-enhancing hypodense areas, with secondary findings of inflammation obliterating surrounding tissue, gas bubbles, and bone destruction when present.\nMarked wall thickening, rim thickening, or multiple cavities may be noted; this suggests tuberculous rather than pyogenic infection. CT scans are also useful in recognizing potential etiologies such as Crohn disease and appendicitis. MRI (which was the diagnostic study in this case) has been reported to be more sensitive than CT scanning in displaying tissue contrast resolution and in screening out bone interference, as well as showing the extent of disease. A study by Yin and colleagues[7] recommended the use of a bone scan, especially in patients presenting with low back pain and an established fever of unknown origin, as it allows detection of unexpected concomitant infections.[1,7,8]\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n**Figure 3.** \n \n\n**Figure 4.** \n \n\n\n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 904611,
"choiceText": "Psoas abscess",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 904613,
"choiceText": "Spinal epidural abscess",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 904615,
"choiceText": "Retroperitoneal schwannoma\r\n",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 904617,
"choiceText": "Retroperitoneal hematoma",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 285151,
"questionText": "On the basis of the history, physical examination, and workup, what is the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Fever and Limp in a 3-Year-Old Girl"
},
{
"authors": "Setshedi Makwinja, MD; Ben Numpang, MD; Benjamin R. Aubey, MD, MPH",
"content": [
"A review of the literature published from 1881 through 1990 revealed that the incidence of psoas abscesses approximates four cases per 100,000 population per year, with a more recent Taiwanese study reporting that the rate of occurrence was 2.5 cases annually.[8] These incidences reflect adult patients. In Asia and Africa, 99.5% of all psoas abscesses are primary, compared with 61% in the United States and Canada and 18.7% in Europe. The causes of psoas abscess in the Western world have changed since the beginning of the 20th century. Primary psoas abscess caused by hematogenous spread from an occult source is common, especially in immunocompromised individuals.",
"In the past, psoas abscess was mainly caused by tuberculosis of the spine (Pott disease); but with the decline in the prevalence of infections caused by Mycobacterium tuberculosis, major pathogens associated with psoas abscesses are those related to diseases of the digestive tract. This is reflective of the role of contiguous sites of infection in the development of a psoas abscess.",
"Common secondary causes of psoas abscess include Crohn disease (60%), appendicitis (16%), ulcerative colitis, diverticulitis, colon cancer (11%), and vertebral osteomyelitis (10%). In secondary psoas abscesses, cultures are often mixed with \nEscherichia coli\n and Bacteroides species predominating. \nStaphylococcus aureus\n is the most common isolated organism in primary disease. Leukocytosis is the most common laboratory finding.[1,3,9]",
"Without localizing tenderness, the presentation of fever and irritable hip could be attributed to primary diseases of the hip; therefore, the clinician must know how to distinguish between psoas abscesses and primary hip diseases. In psoas abscess, the posterior part of the hip joint is not tender, and a fully flexed hip can be rotated without pain; this would be difficult in patients with hip problems. Digital rectal examinations produce tenderness in psoas muscle disease.",
"Percutaneous drainage and antibiotics are the first line of treatment, but recurrence is common. In the past, open drainage of the abscess through a McBurney or iliac crest incision was performed. Open surgical drainage allows simultaneous treatment of the underlying pathology in secondary abscess. The duration of antibiotic therapy must be individualized and graded by the clinical signs and any involvement of other sites.[4,5,10]"
],
"date": "October 28, 2015",
"figures": [],
"markdown": "# Fever and Limp in a 3-Year-Old Girl\n\n **Authors:** Setshedi Makwinja, MD; Ben Numpang, MD; Benjamin R. Aubey, MD, MPH \n **Date:** October 28, 2015\n\n ## Content\n\n A review of the literature published from 1881 through 1990 revealed that the incidence of psoas abscesses approximates four cases per 100,000 population per year, with a more recent Taiwanese study reporting that the rate of occurrence was 2.5 cases annually.[8] These incidences reflect adult patients. In Asia and Africa, 99.5% of all psoas abscesses are primary, compared with 61% in the United States and Canada and 18.7% in Europe. The causes of psoas abscess in the Western world have changed since the beginning of the 20th century. Primary psoas abscess caused by hematogenous spread from an occult source is common, especially in immunocompromised individuals.\nIn the past, psoas abscess was mainly caused by tuberculosis of the spine (Pott disease); but with the decline in the prevalence of infections caused by Mycobacterium tuberculosis, major pathogens associated with psoas abscesses are those related to diseases of the digestive tract. This is reflective of the role of contiguous sites of infection in the development of a psoas abscess.\nCommon secondary causes of psoas abscess include Crohn disease (60%), appendicitis (16%), ulcerative colitis, diverticulitis, colon cancer (11%), and vertebral osteomyelitis (10%). In secondary psoas abscesses, cultures are often mixed with \nEscherichia coli\n and Bacteroides species predominating. \nStaphylococcus aureus\n is the most common isolated organism in primary disease. Leukocytosis is the most common laboratory finding.[1,3,9]\nWithout localizing tenderness, the presentation of fever and irritable hip could be attributed to primary diseases of the hip; therefore, the clinician must know how to distinguish between psoas abscesses and primary hip diseases. In psoas abscess, the posterior part of the hip joint is not tender, and a fully flexed hip can be rotated without pain; this would be difficult in patients with hip problems. Digital rectal examinations produce tenderness in psoas muscle disease.\nPercutaneous drainage and antibiotics are the first line of treatment, but recurrence is common. In the past, open drainage of the abscess through a McBurney or iliac crest incision was performed. Open surgical drainage allows simultaneous treatment of the underlying pathology in secondary abscess. The duration of antibiotic therapy must be individualized and graded by the clinical signs and any involvement of other sites.[4,5,10]\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "Fever and Limp in a 3-Year-Old Girl"
},
{
"authors": "Setshedi Makwinja, MD; Ben Numpang, MD; Benjamin R. Aubey, MD, MPH",
"content": [
"The patient in this case was started on empiric intravenous antibiotic therapy, initially on ceftriaxone 75 mg/kg/day. Ultrasonography obtained shortly after admission was read as a \"negative study of the upper abdomen, perirenal and perivertebral lower thoracic lumbar area.\" Following consultation with the infectious disease service, the antibiotic regimen was changed to cefepime to provide better broad-spectrum coverage.",
"A review of the MRI findings with the interventional radiology department led to the decision that an invasive drainage procedure for a relatively small collection would not be immediately warranted, and a \"wait-and-see\" approach to determine the response to the intravenous antibiotics would be the best course of action. After 3 days of intravenous antibiotic therapy, the child's fever subsided, and she was noted to be moving around without limping and with no residual tenderness on examination. In fact, her mother described her as \"back to her old self.\" A consultation with the neurology service found no neurologic deficits, and the initial limp was attributed to pain. A repeat CRP level was obtained, with a result of 34.5 mg/L. The final results of the blood and urine cultures, as well as a purified protein derivative, were all negative.",
"Because the child was doing well clinically (running and jumping up and down) and had a compliant parent, the patient was discharged on hospital Day 5 with a combination antibiotic regimen of rifampin and amoxicillin/clavulanic acid for 4 weeks, along with appropriate outpatient follow-up with her primary pediatrician and an infectious disease specialist.",
"Repeat MRI scans performed at 1 month and again at 3 months after discharge demonstrated complete resolution of the collections, except for a residual \"abnormal signal intensity in the right pedicle of L3,\" which persisted with no identifiable clinical impact; the patient was noted to have complete resolution of her symptoms. The use of IV antibiotics as the sole modality of therapy in this case was unusual for the management of a psoas abscess."
],
"date": "October 28, 2015",
"figures": [],
"markdown": "# Fever and Limp in a 3-Year-Old Girl\n\n **Authors:** Setshedi Makwinja, MD; Ben Numpang, MD; Benjamin R. Aubey, MD, MPH \n **Date:** October 28, 2015\n\n ## Content\n\n The patient in this case was started on empiric intravenous antibiotic therapy, initially on ceftriaxone 75 mg/kg/day. Ultrasonography obtained shortly after admission was read as a \"negative study of the upper abdomen, perirenal and perivertebral lower thoracic lumbar area.\" Following consultation with the infectious disease service, the antibiotic regimen was changed to cefepime to provide better broad-spectrum coverage.\nA review of the MRI findings with the interventional radiology department led to the decision that an invasive drainage procedure for a relatively small collection would not be immediately warranted, and a \"wait-and-see\" approach to determine the response to the intravenous antibiotics would be the best course of action. After 3 days of intravenous antibiotic therapy, the child's fever subsided, and she was noted to be moving around without limping and with no residual tenderness on examination. In fact, her mother described her as \"back to her old self.\" A consultation with the neurology service found no neurologic deficits, and the initial limp was attributed to pain. A repeat CRP level was obtained, with a result of 34.5 mg/L. The final results of the blood and urine cultures, as well as a purified protein derivative, were all negative.\nBecause the child was doing well clinically (running and jumping up and down) and had a compliant parent, the patient was discharged on hospital Day 5 with a combination antibiotic regimen of rifampin and amoxicillin/clavulanic acid for 4 weeks, along with appropriate outpatient follow-up with her primary pediatrician and an infectious disease specialist.\nRepeat MRI scans performed at 1 month and again at 3 months after discharge demonstrated complete resolution of the collections, except for a residual \"abnormal signal intensity in the right pedicle of L3,\" which persisted with no identifiable clinical impact; the patient was noted to have complete resolution of her symptoms. The use of IV antibiotics as the sole modality of therapy in this case was unusual for the management of a psoas abscess.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 904619,
"choiceText": "MRI has been reported to be more sensitive than CT scanning",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 904621,
"choiceText": "Ultrasonography is more sensitive than CT",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 904623,
"choiceText": "A negative plain film of the area can rule out psoas abscess",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 904625,
"choiceText": "CT scanning is the least expensive and quickest imaging modality\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "MRI (which was the diagnostic study in this case) has been reported to be more sensitive than CT scanning in displaying tissue contrast resolution and in screening out bone interference, as well as showing the extent of disease.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 285153,
"questionText": "Which of the following statements about psoas abscess imaging is true?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 904627,
"choiceText": "The psoas muscle together with the iliacus muscle is referred to as the iliopsoas",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 904629,
"choiceText": "Seventy percent of individuals have a smaller secondary psoas muscle",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 904631,
"choiceText": "The psoas muscle is relatively isolated from other organs",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 904633,
"choiceText": "A poor vascular supply can cause hematogenous spread from sites of occult infection\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "\r\nIn the lower half of the psoas muscle's course, it runs alongside the iliacus muscle, with a common tendon insertion into the lesser trochanter. Together, they are referred to as the iliopsoas.\r\n",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 285155,
"questionText": "Which of the following statements about the psoas muscle's anatomy is true?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Fever and Limp in a 3-Year-Old Girl"
},
{
"authors": "Setshedi Makwinja, MD; Ben Numpang, MD; Benjamin R. Aubey, MD, MPH",
"content": [],
"date": "October 28, 2015",
"figures": [],
"markdown": "# Fever and Limp in a 3-Year-Old Girl\n\n **Authors:** Setshedi Makwinja, MD; Ben Numpang, MD; Benjamin R. Aubey, MD, MPH \n **Date:** October 28, 2015\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 904619,
"choiceText": "MRI has been reported to be more sensitive than CT scanning",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 904621,
"choiceText": "Ultrasonography is more sensitive than CT",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 904623,
"choiceText": "A negative plain film of the area can rule out psoas abscess",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 904625,
"choiceText": "CT scanning is the least expensive and quickest imaging modality\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "MRI (which was the diagnostic study in this case) has been reported to be more sensitive than CT scanning in displaying tissue contrast resolution and in screening out bone interference, as well as showing the extent of disease.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 285153,
"questionText": "Which of the following statements about psoas abscess imaging is true?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 904627,
"choiceText": "The psoas muscle together with the iliacus muscle is referred to as the iliopsoas",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 904629,
"choiceText": "Seventy percent of individuals have a smaller secondary psoas muscle",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 904631,
"choiceText": "The psoas muscle is relatively isolated from other organs",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 904633,
"choiceText": "A poor vascular supply can cause hematogenous spread from sites of occult infection\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "\r\nIn the lower half of the psoas muscle's course, it runs alongside the iliacus muscle, with a common tendon insertion into the lesser trochanter. Together, they are referred to as the iliopsoas.\r\n",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 285155,
"questionText": "Which of the following statements about the psoas muscle's anatomy is true?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Fever and Limp in a 3-Year-Old Girl"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 904611,
"choiceText": "Psoas abscess",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 904613,
"choiceText": "Spinal epidural abscess",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 904615,
"choiceText": "Retroperitoneal schwannoma\r\n",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 904617,
"choiceText": "Retroperitoneal hematoma",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 285151,
"questionText": "On the basis of the history, physical examination, and workup, what is the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 904619,
"choiceText": "MRI has been reported to be more sensitive than CT scanning",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 904621,
"choiceText": "Ultrasonography is more sensitive than CT",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 904623,
"choiceText": "A negative plain film of the area can rule out psoas abscess",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 904625,
"choiceText": "CT scanning is the least expensive and quickest imaging modality\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "MRI (which was the diagnostic study in this case) has been reported to be more sensitive than CT scanning in displaying tissue contrast resolution and in screening out bone interference, as well as showing the extent of disease.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 285153,
"questionText": "Which of the following statements about psoas abscess imaging is true?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 904627,
"choiceText": "The psoas muscle together with the iliacus muscle is referred to as the iliopsoas",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 904629,
"choiceText": "Seventy percent of individuals have a smaller secondary psoas muscle",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 904631,
"choiceText": "The psoas muscle is relatively isolated from other organs",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 904633,
"choiceText": "A poor vascular supply can cause hematogenous spread from sites of occult infection\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "\r\nIn the lower half of the psoas muscle's course, it runs alongside the iliacus muscle, with a common tendon insertion into the lesser trochanter. Together, they are referred to as the iliopsoas.\r\n",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 285155,
"questionText": "Which of the following statements about the psoas muscle's anatomy is true?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
853225 | /viewarticle/853225 | [
{
"authors": "Faisal M. Mawri, MD; Staci Batchelder; Carrie Fales; Ranjini Srinivasan; Walid Abuhammour, MD",
"content": [
"Editor's Note:\n\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.",
"A 40-day-old white male infant with a previously uncomplicated neonatal course presents with a 2-day history of expanding swelling and erythema involving the left angle of the mandible. The infant has been irritable and inconsolable for the 12 hours before presentation. His parents are very distraught. They tried feeding, rocking, and burping the infant, but nothing relieved the crying.",
"According to the parents, the patient had a fever on the day before admission. The infant was a 7.5-lb (3.4-kg), term baby delivered via normal, spontaneous vaginal delivery to a 26-year-old mother who was positive for group B Streptococcus (GBS) at 35 weeks gestation, rubella-immune, and negative for hepatitis B surface antigen. Rupture of the membranes occurred 10 hours before delivery, with clear fluid. The intrapartum medications included two doses of clindamycin for the positive GBS test. No perinatal maternal fever was noted. The Apgar scores were 8 and 9 at 1 and 5 minutes, respectively."
],
"date": "October 28, 2015",
"figures": [],
"markdown": "# Facial Swelling in a 40-Day-Old Boy\n\n **Authors:** Faisal M. Mawri, MD; Staci Batchelder; Carrie Fales; Ranjini Srinivasan; Walid Abuhammour, MD \n **Date:** October 28, 2015\n\n ## Content\n\n Editor's Note:\n\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.\nA 40-day-old white male infant with a previously uncomplicated neonatal course presents with a 2-day history of expanding swelling and erythema involving the left angle of the mandible. The infant has been irritable and inconsolable for the 12 hours before presentation. His parents are very distraught. They tried feeding, rocking, and burping the infant, but nothing relieved the crying.\nAccording to the parents, the patient had a fever on the day before admission. The infant was a 7.5-lb (3.4-kg), term baby delivered via normal, spontaneous vaginal delivery to a 26-year-old mother who was positive for group B Streptococcus (GBS) at 35 weeks gestation, rubella-immune, and negative for hepatitis B surface antigen. Rupture of the membranes occurred 10 hours before delivery, with clear fluid. The intrapartum medications included two doses of clindamycin for the positive GBS test. No perinatal maternal fever was noted. The Apgar scores were 8 and 9 at 1 and 5 minutes, respectively.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "Facial Swelling in a 40-Day-Old Boy"
},
{
"authors": "Faisal M. Mawri, MD; Staci Batchelder; Carrie Fales; Ranjini Srinivasan; Walid Abuhammour, MD",
"content": [
"The physical examination shows an irritable infant with a rectal temperature of 101.5°F (38.6°C). In the emergency department, the infant has a heart rate of 120 beats/min, a respiratory rate of 48 breaths/min, and a blood pressure of 100/58 mm Hg. A 4-cm area of indurated, nonfluctuant, tender swelling and erythema involving the left subauricular and submandibular facial areas and extending along the line of the jaw to the chin is noted (Figure 1). The floor of the mouth is soft, and the uvula is midline and not enlarged. No pharyngeal erythema or exudates are noted. No rhinorrhea is evident. The fontanelle is soft and flat. The lungs are clear to auscultation bilaterally, and no retractions or nasal flaring is observed. No heart murmur is detected, and the heart sounds are normal. The rest of the physical examination is unremarkable.",
"Figure 1.",
"Figure 1.",
"The peripheral leukocyte count at admission is 10.2 × 103/mm3 (10.2 × 109/L), with 13% bands, 61% neutrophils, 13% lymphocytes, and 3% monocytes. Sodium, chloride, potassium, and bicarbonate are within normal limits for the patient's age. A lumbar puncture is performed and reveals cerebrospinal fluid (CSF) with protein of 67 mg/dL, glucose of 59 mg/dL, leukocytes of 2/mm3, and no red blood cells. Gram stain and agglutination studies are negative. The urine analysis and urine culture results are unremarkable."
],
"date": "October 28, 2015",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/853/225/853225-Thumb1.png"
}
],
"markdown": "# Facial Swelling in a 40-Day-Old Boy\n\n **Authors:** Faisal M. Mawri, MD; Staci Batchelder; Carrie Fales; Ranjini Srinivasan; Walid Abuhammour, MD \n **Date:** October 28, 2015\n\n ## Content\n\n The physical examination shows an irritable infant with a rectal temperature of 101.5°F (38.6°C). In the emergency department, the infant has a heart rate of 120 beats/min, a respiratory rate of 48 breaths/min, and a blood pressure of 100/58 mm Hg. A 4-cm area of indurated, nonfluctuant, tender swelling and erythema involving the left subauricular and submandibular facial areas and extending along the line of the jaw to the chin is noted (Figure 1). The floor of the mouth is soft, and the uvula is midline and not enlarged. No pharyngeal erythema or exudates are noted. No rhinorrhea is evident. The fontanelle is soft and flat. The lungs are clear to auscultation bilaterally, and no retractions or nasal flaring is observed. No heart murmur is detected, and the heart sounds are normal. The rest of the physical examination is unremarkable.\nFigure 1.\nFigure 1.\nThe peripheral leukocyte count at admission is 10.2 × 103/mm3 (10.2 × 109/L), with 13% bands, 61% neutrophils, 13% lymphocytes, and 3% monocytes. Sodium, chloride, potassium, and bicarbonate are within normal limits for the patient's age. A lumbar puncture is performed and reveals cerebrospinal fluid (CSF) with protein of 67 mg/dL, glucose of 59 mg/dL, leukocytes of 2/mm3, and no red blood cells. Gram stain and agglutination studies are negative. The urine analysis and urine culture results are unremarkable.\n\n ## Figures\n\n **Figure 1.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 904517,
"choiceText": "Acute parotitis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 904519,
"choiceText": "Cervical lymphadenitis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 904521,
"choiceText": "Traumatic hematoma",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 904523,
"choiceText": "Cellulitis-adenitis syndrome\r\n",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 285123,
"questionText": "On the basis of the history, physical examination, and workup, what is the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Facial Swelling in a 40-Day-Old Boy"
},
{
"authors": "Faisal M. Mawri, MD; Staci Batchelder; Carrie Fales; Ranjini Srinivasan; Walid Abuhammour, MD",
"content": [
"Treatment of a culture-positive GBS mother decreases the likelihood of early-onset GBS infection in the infant; however, preventing late-onset GBS infections, such as cellulitis-adenitis, is not as certain. Therefore, the mother's positive finding for GBS does not really help with differential diagnosis. However, some cases of late-onset GBS disease in neonates and infants have been shown to be associated with colonization in the mother. Cellulitis-adenitis syndrome is a disease of great concern because of the potential for upper airway obstruction and septic shock, which may lead to significant morbidity and mortality. This case exemplifies the importance of including GBS infection within the differential diagnosis of newborn infections.",
"GBS, or Streptococcus agalactiae, are gram-positive encapsulated diplococci. GBS organisms are facultative anaerobes that, when cultured, form chains or diplococci in liquid media and small white-gray colonies on solid media. This organism usually produces a narrow zone of B-hemolysis on blood agar, and most strains are resistant to bacitracin. Definitive identification of GBS is through latex agglutination testing for the Lancefield group B carbohydrate antigen. Individual strains of GBS are serologically classified according to their capsular polysaccharides. Nine serotypes of GBS are currently defined and are classified as types Ia, Ib, II, III, IV, V, VI, VII, and VIII. All serotypes have been implicated in causing GBS early-onset disease (occurring at less than 7 days of age); however, serotypes Ia, Ib, II, III, and V are the most common types identified in the United States.[1] Ninety percent of cases of GBS late-onset disease (occurring at 7 days of age or later), including cellulitis-adenitis syndrome, are caused by serotype III. Compared with other serotypes, GBS serotype III is also more readily taken up by brain endothelial cells in vitro, which helps explain the occurrence of GBS serotype III late-onset meningitis.[2]",
"In the late 1960s, GBS was identified as a major neonatal pathogen. The incidence over the following 2 decades ranged from 1.0 to 5.4 per 1000 live births in the United States.[2] In the 1990s, maternal chemoprophylaxis against GBS resulted in a 65% decrease in the presentation of early-onset disease, from 1.7 to 0.6 per 1000 live births; however, the incidence of late-onset disease remained at 0.4 per 1 000 live births.[2] In 2003, the incidence of early-onset disease had decreased to 0.3 cases per 1 000 live births, equaling the incidence of late-onset disease.[3] Cellulitis-adenitis syndrome, a rare late-onset disease presentation of GBS infection, represents less than 1% of late-onset GBS disease.[4] Because cellulitis-adenitis syndrome is so rare, the incidence of central nervous system involvement is unknown.[5]",
"GBS are common organisms in the maternal genitourinary and gastrointestinal tracts, with approximately 30% colonization among pregnant women.[2] Vaginal and rectal colonization is implicated in the vertical transmission of GBS to infants.[2] Without maternal chemoprophylaxis of GBS-colonized women, about 50% of infants will become infected, with 1%-2% of those developing invasive disease. Without serotype-specific immunoglobulin G (IgG), the capsular polysaccharide of the GBS organism acts as a virulence factor, preventing activation of the alternative complement pathway and blocking subsequent opsonization and phagocytosis. IgG serum levels higher than 1-2 µg/mL are effective in killing the GBS organism; however, only 10%-20% of women have these levels, so most newborns lack serotype-specific antibody protection against GBS.[1]",
"Premature infants have a decreased transplacental transfer of maternal antibodies; therefore, they are at an increased risk for GBS colonization and resultant infection. Other risk factors for colonization include prolonged rupture of membranes (greater than 18 hours), intrapartum fever (temperature greater than 100.4°F [38°C]), maternal GBS bacteremia during pregnancy, a previous infant with GBS disease, absent or low serotype-specific serum antibody, or a high GBS maternal disease burden. Other GBS virulence factors include surface proteins that help adhesion to host cells; C5a peptidase, which inhibits neutrophil recruitment to the site of infection; B-hemolysin, which causes cell injury; and hyaluronidase, which acts as a spreading factor in host tissues.[2] Two possibilities for the pathogenesis of GBS cellulitis-adenitis syndrome have been proposed. The first involves mucus membrane colonization with GBS, followed by bacteremia and seeding of the soft tissues. The second is ipsilateral otitis media, with subsequent lymphatic spread and bacteremia.[5]"
],
"date": "October 28, 2015",
"figures": [],
"markdown": "# Facial Swelling in a 40-Day-Old Boy\n\n **Authors:** Faisal M. Mawri, MD; Staci Batchelder; Carrie Fales; Ranjini Srinivasan; Walid Abuhammour, MD \n **Date:** October 28, 2015\n\n ## Content\n\n Treatment of a culture-positive GBS mother decreases the likelihood of early-onset GBS infection in the infant; however, preventing late-onset GBS infections, such as cellulitis-adenitis, is not as certain. Therefore, the mother's positive finding for GBS does not really help with differential diagnosis. However, some cases of late-onset GBS disease in neonates and infants have been shown to be associated with colonization in the mother. Cellulitis-adenitis syndrome is a disease of great concern because of the potential for upper airway obstruction and septic shock, which may lead to significant morbidity and mortality. This case exemplifies the importance of including GBS infection within the differential diagnosis of newborn infections.\nGBS, or Streptococcus agalactiae, are gram-positive encapsulated diplococci. GBS organisms are facultative anaerobes that, when cultured, form chains or diplococci in liquid media and small white-gray colonies on solid media. This organism usually produces a narrow zone of B-hemolysis on blood agar, and most strains are resistant to bacitracin. Definitive identification of GBS is through latex agglutination testing for the Lancefield group B carbohydrate antigen. Individual strains of GBS are serologically classified according to their capsular polysaccharides. Nine serotypes of GBS are currently defined and are classified as types Ia, Ib, II, III, IV, V, VI, VII, and VIII. All serotypes have been implicated in causing GBS early-onset disease (occurring at less than 7 days of age); however, serotypes Ia, Ib, II, III, and V are the most common types identified in the United States.[1] Ninety percent of cases of GBS late-onset disease (occurring at 7 days of age or later), including cellulitis-adenitis syndrome, are caused by serotype III. Compared with other serotypes, GBS serotype III is also more readily taken up by brain endothelial cells in vitro, which helps explain the occurrence of GBS serotype III late-onset meningitis.[2]\nIn the late 1960s, GBS was identified as a major neonatal pathogen. The incidence over the following 2 decades ranged from 1.0 to 5.4 per 1000 live births in the United States.[2] In the 1990s, maternal chemoprophylaxis against GBS resulted in a 65% decrease in the presentation of early-onset disease, from 1.7 to 0.6 per 1000 live births; however, the incidence of late-onset disease remained at 0.4 per 1 000 live births.[2] In 2003, the incidence of early-onset disease had decreased to 0.3 cases per 1 000 live births, equaling the incidence of late-onset disease.[3] Cellulitis-adenitis syndrome, a rare late-onset disease presentation of GBS infection, represents less than 1% of late-onset GBS disease.[4] Because cellulitis-adenitis syndrome is so rare, the incidence of central nervous system involvement is unknown.[5]\nGBS are common organisms in the maternal genitourinary and gastrointestinal tracts, with approximately 30% colonization among pregnant women.[2] Vaginal and rectal colonization is implicated in the vertical transmission of GBS to infants.[2] Without maternal chemoprophylaxis of GBS-colonized women, about 50% of infants will become infected, with 1%-2% of those developing invasive disease. Without serotype-specific immunoglobulin G (IgG), the capsular polysaccharide of the GBS organism acts as a virulence factor, preventing activation of the alternative complement pathway and blocking subsequent opsonization and phagocytosis. IgG serum levels higher than 1-2 µg/mL are effective in killing the GBS organism; however, only 10%-20% of women have these levels, so most newborns lack serotype-specific antibody protection against GBS.[1]\nPremature infants have a decreased transplacental transfer of maternal antibodies; therefore, they are at an increased risk for GBS colonization and resultant infection. Other risk factors for colonization include prolonged rupture of membranes (greater than 18 hours), intrapartum fever (temperature greater than 100.4°F [38°C]), maternal GBS bacteremia during pregnancy, a previous infant with GBS disease, absent or low serotype-specific serum antibody, or a high GBS maternal disease burden. Other GBS virulence factors include surface proteins that help adhesion to host cells; C5a peptidase, which inhibits neutrophil recruitment to the site of infection; B-hemolysin, which causes cell injury; and hyaluronidase, which acts as a spreading factor in host tissues.[2] Two possibilities for the pathogenesis of GBS cellulitis-adenitis syndrome have been proposed. The first involves mucus membrane colonization with GBS, followed by bacteremia and seeding of the soft tissues. The second is ipsilateral otitis media, with subsequent lymphatic spread and bacteremia.[5]\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 904517,
"choiceText": "Acute parotitis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 904519,
"choiceText": "Cervical lymphadenitis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 904521,
"choiceText": "Traumatic hematoma",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 904523,
"choiceText": "Cellulitis-adenitis syndrome\r\n",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 285123,
"questionText": "On the basis of the history, physical examination, and workup, what is the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Facial Swelling in a 40-Day-Old Boy"
},
{
"authors": "Faisal M. Mawri, MD; Staci Batchelder; Carrie Fales; Ranjini Srinivasan; Walid Abuhammour, MD",
"content": [
"Other possibilities that should be considered in the differential diagnosis of GBS sepsis include neonatal respiratory distress syndrome and transient tachypnea of the newborn. In cases with associated meningitis, other common organisms responsible for infant meningitis, such as \nEscherichia coli\n and \nListeria monocytogenes, must be considered and treated empirically.",
"The typical presentation is divided into early-onset and late-onset GBS infection. Early-onset GBS infection is limited to the first 5 days of an infant's life; most often, the infection appears within the first 24 hours. The infants most at risk for early-onset GBS are those with maternal GBS colonization, low birth weight, and delivery complications, including premature labor, rupture of the membranes more than 24 hours before birth, and maternal fever.",
"The most typical symptom is respiratory distress, which may be followed by apnea and cardiovascular collapse. The child may have sepsis in association with pulmonary infiltrates. Thirty percent of infants will also have meningitis. In such cases, GBS infection is often confused with E coli infection. Acute respiratory distress is the most predictable first sign of infection and is present in 84% of cases of GBS. A low Apgar score at 1 minute, a low absolute neutrophil count, a history of prolonged rupture of membranes, and the presence of GBS in the gastric aspirate are some of the criteria used to differentiate GBS from neonatal respiratory distress syndrome.",
"Late-onset presentation occurs later than 7 days after birth, with many cases presenting around 24 days of age. This form of GBS typically manifests as meningitis (seen in up to 80% of late-onset cases). It may also present as cellulitis-adenitis, which is most common in male infants between 3 and 7 weeks of age. The symptoms include acute suppurative cervical lymphadenitis, fever, otitis media, bacteremia, and poor feeding.",
"Many serious complications are associated with late-onset GBS; these include cortical blindness, central diabetes insipidus, deafness, poor thermal regulation, and subdural effusions. Infants presenting with meningitis may develop hypotension, seizures, coma, and leukopenia, which are poor prognostic factors. Some rare presentations of late-onset GBS infections include asymptomatic bacteremia, otitis media, cellulitis, conjunctivitis, abscess, endocarditis, pericarditis, and osteomyelitis. Bacteremia is most common at 2-4 weeks of age, and it can be diagnosed by blood culture. In one study, a series of nine infants with GBS osteomyelitis showed a propensity for localizing infection to one bone (most often, at the proximal humerus, and also at the proximal and distal femur).[6,7,8]",
"In cases of cellulitis-adenitis, ultrasonography may be used to diagnose suppuration of the lymph nodes. In addition, a fine-needle biopsy, blood cultures, or aspiration of bone or joints to check for osteomyelitis may be indicated. Cultures should show beta-hemolytic bacteria that are resistant to bacitracin. Counterimmunoelectrophoresis and latex particle agglutination (LPA) are sensitive and specific tests for GBS infection. A lumbar puncture with signs of bacterial infection consistent with GBS is diagnostic for meningitis. Although LPA is useful for confirming the diagnosis, it is not routinely available."
],
"date": "October 28, 2015",
"figures": [],
"markdown": "# Facial Swelling in a 40-Day-Old Boy\n\n **Authors:** Faisal M. Mawri, MD; Staci Batchelder; Carrie Fales; Ranjini Srinivasan; Walid Abuhammour, MD \n **Date:** October 28, 2015\n\n ## Content\n\n Other possibilities that should be considered in the differential diagnosis of GBS sepsis include neonatal respiratory distress syndrome and transient tachypnea of the newborn. In cases with associated meningitis, other common organisms responsible for infant meningitis, such as \nEscherichia coli\n and \nListeria monocytogenes, must be considered and treated empirically.\nThe typical presentation is divided into early-onset and late-onset GBS infection. Early-onset GBS infection is limited to the first 5 days of an infant's life; most often, the infection appears within the first 24 hours. The infants most at risk for early-onset GBS are those with maternal GBS colonization, low birth weight, and delivery complications, including premature labor, rupture of the membranes more than 24 hours before birth, and maternal fever.\nThe most typical symptom is respiratory distress, which may be followed by apnea and cardiovascular collapse. The child may have sepsis in association with pulmonary infiltrates. Thirty percent of infants will also have meningitis. In such cases, GBS infection is often confused with E coli infection. Acute respiratory distress is the most predictable first sign of infection and is present in 84% of cases of GBS. A low Apgar score at 1 minute, a low absolute neutrophil count, a history of prolonged rupture of membranes, and the presence of GBS in the gastric aspirate are some of the criteria used to differentiate GBS from neonatal respiratory distress syndrome.\nLate-onset presentation occurs later than 7 days after birth, with many cases presenting around 24 days of age. This form of GBS typically manifests as meningitis (seen in up to 80% of late-onset cases). It may also present as cellulitis-adenitis, which is most common in male infants between 3 and 7 weeks of age. The symptoms include acute suppurative cervical lymphadenitis, fever, otitis media, bacteremia, and poor feeding.\nMany serious complications are associated with late-onset GBS; these include cortical blindness, central diabetes insipidus, deafness, poor thermal regulation, and subdural effusions. Infants presenting with meningitis may develop hypotension, seizures, coma, and leukopenia, which are poor prognostic factors. Some rare presentations of late-onset GBS infections include asymptomatic bacteremia, otitis media, cellulitis, conjunctivitis, abscess, endocarditis, pericarditis, and osteomyelitis. Bacteremia is most common at 2-4 weeks of age, and it can be diagnosed by blood culture. In one study, a series of nine infants with GBS osteomyelitis showed a propensity for localizing infection to one bone (most often, at the proximal humerus, and also at the proximal and distal femur).[6,7,8]\nIn cases of cellulitis-adenitis, ultrasonography may be used to diagnose suppuration of the lymph nodes. In addition, a fine-needle biopsy, blood cultures, or aspiration of bone or joints to check for osteomyelitis may be indicated. Cultures should show beta-hemolytic bacteria that are resistant to bacitracin. Counterimmunoelectrophoresis and latex particle agglutination (LPA) are sensitive and specific tests for GBS infection. A lumbar puncture with signs of bacterial infection consistent with GBS is diagnostic for meningitis. Although LPA is useful for confirming the diagnosis, it is not routinely available.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "Facial Swelling in a 40-Day-Old Boy"
},
{
"authors": "Faisal M. Mawri, MD; Staci Batchelder; Carrie Fales; Ranjini Srinivasan; Walid Abuhammour, MD",
"content": [
"Initial empiric treatment of neonatal GBS infection requires antimicrobial coverage consisting of ampicillin and an aminoglycoside or cefotaxime. For confirmed GBS with documented sensitivities, penicillin G is the antibiotic of choice. The dosage depends on the patient's age. Infants aged 7 days and younger require 250,000-450,000 U/kg/day of intravenous (IV) penicillin G divided into three daily doses. Infants older than 7 days require 450,000-500,000 U/kg/day of IV penicillin G divided into four to six doses daily.[3] The location and extent of infection will dictate the duration of treatment. Bacteremia without a focus requires at least 10 days of treatment, meningitis requires 2-3 weeks, while ventriculitis and osteomyelitis require 4 weeks of treatment.[2] The necessity of a second lumbar puncture after 24-48 hours of therapy to document CSF sterility remains controversial. Additionally, nafcillin is active against GBS in vitro, but it is not effective for GBS meningitis because it does not reach bactericidal CSF concentrations.[5,9]",
"GBS infection is a serious condition. The fatality rate for early-onset neonatal GBS disease is 4.7%, and it is 2.8% for late-onset disease. Up to 30% of infants who survive GBS meningitis have serious long-term neurologic sequelae. Long-term complications can include developmental delays, microcephaly, seizure disorders, blindness, deafness, and quadriplegia.[2]",
"Routine screening for GBS colonization is done with vaginal and rectal GBS cultures at 35-37 weeks gestation for all pregnant women. Women with a prior infant with invasive GBS disease or GBS bacteriuria during a current pregnancy are exempt from routine screening because they will be treated regardless of the culture results. For women found to be culture-positive with an unknown GBS status, delivering at less than 37 weeks gestation, with greater than 18 hours of ruptured of membranes before delivery, or an intrapartum temperature greater than 100.4°F (38°C), treatment is administered during labor. Patients undergoing planned cesarean section without labor or membrane rupture do not require treatment, regardless of the results of the culture.[2]",
"Prevention of GBS infection in infants relies on the intrapartum administration of penicillin G to mothers, given at an initial dose of 5,000,000 U, then 2,500,000 U every 4 hours until delivery; alternatively, ampicillin can be administered. No reports of resistance to penicillin G are known, but GBS resistance to erythromycin and clindamycin has been reported at 15%-30% and 10%-20% of cases, respectively.[3] Cefazolin is an alternative for those women who are allergic to penicillin. Vancomycin is reserved for patients with a high anaphylactic risk and those for whom susceptibility to erythromycin and clindamycin has not been performed or has been found resistant. Administration of antibiotics prior to the onset of labor has been shown to be ineffective for preventing neonatal GBS disease. Unfortunately, while the treatment of GBS has shown a decrease in the incidence of early-onset GBS infections, it has not had an effect on late-onset GBS infections (such as cellulitis-adenitis).",
"Clinical trials have studied the possibility of a GBS vaccine, using the capsular polysaccharide to provide transplacental passage of GBS antibodies to the infant. The vaccine would eliminate the need for routine cultures during pregnancy and antibiotic prophylaxis and would likely have an effect on both early-onset and late-onset GBS disease.[2] Currently, no vaccines are routinely used in the United States for the prevention of GBS disease.",
"GBS cellulitis-adenitis was part of the differential diagnosis upon initial outpatient presentation of the patient in this case. He presented with fever, irritability, and swelling under the left ear. A full septic evaluation was initiated, including a complete blood cell count with differential, electrolytes, urine analysis, urine culture, and CSF studies. The patient was also started on empiric antibiotic treatment with ampicillin and cefotaxime at meningitic doses. Despite the mother being treated with 2 doses of clindamycin prior to vaginal delivery, the infant's blood culture was positive for GBS type III infection. The CSF culture was negative. The treatment was then modified to ceftriaxone for a total of 14 days. The patient had a complete recovery."
],
"date": "October 28, 2015",
"figures": [],
"markdown": "# Facial Swelling in a 40-Day-Old Boy\n\n **Authors:** Faisal M. Mawri, MD; Staci Batchelder; Carrie Fales; Ranjini Srinivasan; Walid Abuhammour, MD \n **Date:** October 28, 2015\n\n ## Content\n\n Initial empiric treatment of neonatal GBS infection requires antimicrobial coverage consisting of ampicillin and an aminoglycoside or cefotaxime. For confirmed GBS with documented sensitivities, penicillin G is the antibiotic of choice. The dosage depends on the patient's age. Infants aged 7 days and younger require 250,000-450,000 U/kg/day of intravenous (IV) penicillin G divided into three daily doses. Infants older than 7 days require 450,000-500,000 U/kg/day of IV penicillin G divided into four to six doses daily.[3] The location and extent of infection will dictate the duration of treatment. Bacteremia without a focus requires at least 10 days of treatment, meningitis requires 2-3 weeks, while ventriculitis and osteomyelitis require 4 weeks of treatment.[2] The necessity of a second lumbar puncture after 24-48 hours of therapy to document CSF sterility remains controversial. Additionally, nafcillin is active against GBS in vitro, but it is not effective for GBS meningitis because it does not reach bactericidal CSF concentrations.[5,9]\nGBS infection is a serious condition. The fatality rate for early-onset neonatal GBS disease is 4.7%, and it is 2.8% for late-onset disease. Up to 30% of infants who survive GBS meningitis have serious long-term neurologic sequelae. Long-term complications can include developmental delays, microcephaly, seizure disorders, blindness, deafness, and quadriplegia.[2]\nRoutine screening for GBS colonization is done with vaginal and rectal GBS cultures at 35-37 weeks gestation for all pregnant women. Women with a prior infant with invasive GBS disease or GBS bacteriuria during a current pregnancy are exempt from routine screening because they will be treated regardless of the culture results. For women found to be culture-positive with an unknown GBS status, delivering at less than 37 weeks gestation, with greater than 18 hours of ruptured of membranes before delivery, or an intrapartum temperature greater than 100.4°F (38°C), treatment is administered during labor. Patients undergoing planned cesarean section without labor or membrane rupture do not require treatment, regardless of the results of the culture.[2]\nPrevention of GBS infection in infants relies on the intrapartum administration of penicillin G to mothers, given at an initial dose of 5,000,000 U, then 2,500,000 U every 4 hours until delivery; alternatively, ampicillin can be administered. No reports of resistance to penicillin G are known, but GBS resistance to erythromycin and clindamycin has been reported at 15%-30% and 10%-20% of cases, respectively.[3] Cefazolin is an alternative for those women who are allergic to penicillin. Vancomycin is reserved for patients with a high anaphylactic risk and those for whom susceptibility to erythromycin and clindamycin has not been performed or has been found resistant. Administration of antibiotics prior to the onset of labor has been shown to be ineffective for preventing neonatal GBS disease. Unfortunately, while the treatment of GBS has shown a decrease in the incidence of early-onset GBS infections, it has not had an effect on late-onset GBS infections (such as cellulitis-adenitis).\nClinical trials have studied the possibility of a GBS vaccine, using the capsular polysaccharide to provide transplacental passage of GBS antibodies to the infant. The vaccine would eliminate the need for routine cultures during pregnancy and antibiotic prophylaxis and would likely have an effect on both early-onset and late-onset GBS disease.[2] Currently, no vaccines are routinely used in the United States for the prevention of GBS disease.\nGBS cellulitis-adenitis was part of the differential diagnosis upon initial outpatient presentation of the patient in this case. He presented with fever, irritability, and swelling under the left ear. A full septic evaluation was initiated, including a complete blood cell count with differential, electrolytes, urine analysis, urine culture, and CSF studies. The patient was also started on empiric antibiotic treatment with ampicillin and cefotaxime at meningitic doses. Despite the mother being treated with 2 doses of clindamycin prior to vaginal delivery, the infant's blood culture was positive for GBS type III infection. The CSF culture was negative. The treatment was then modified to ceftriaxone for a total of 14 days. The patient had a complete recovery.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 904525,
"choiceText": "Type Ia",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 904527,
"choiceText": "Type Ib",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 904529,
"choiceText": "Type II",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 904531,
"choiceText": "Type III",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Ninety percent of cases of GBS late-onset disease (occurring at 7 days of age or later), including cellulitis-adenitis syndrome, are caused by serotype III.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 285125,
"questionText": "Which GBS serotype is responsible for the most cases of late-onset GBS disease?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 904563,
"choiceText": "Vision and hearing problems",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 904565,
"choiceText": "Microcephaly",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 904567,
"choiceText": "Seizure disorders",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 904569,
"choiceText": "Learning disabilities",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 904571,
"choiceText": "All of the above",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Up to 30% of infants who survive GBS meningitis have serious long-term neurologic sequelae. Long-term complications can include developmental delays, microcephaly, seizure disorders, blindness, deafness, and quadriplegia.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 285141,
"questionText": "Which of the following conditions can be late sequelae of GBS meningitis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Facial Swelling in a 40-Day-Old Boy"
},
{
"authors": "Faisal M. Mawri, MD; Staci Batchelder; Carrie Fales; Ranjini Srinivasan; Walid Abuhammour, MD",
"content": [],
"date": "October 28, 2015",
"figures": [],
"markdown": "# Facial Swelling in a 40-Day-Old Boy\n\n **Authors:** Faisal M. Mawri, MD; Staci Batchelder; Carrie Fales; Ranjini Srinivasan; Walid Abuhammour, MD \n **Date:** October 28, 2015\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 904525,
"choiceText": "Type Ia",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 904527,
"choiceText": "Type Ib",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 904529,
"choiceText": "Type II",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 904531,
"choiceText": "Type III",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Ninety percent of cases of GBS late-onset disease (occurring at 7 days of age or later), including cellulitis-adenitis syndrome, are caused by serotype III.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 285125,
"questionText": "Which GBS serotype is responsible for the most cases of late-onset GBS disease?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 904563,
"choiceText": "Vision and hearing problems",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 904565,
"choiceText": "Microcephaly",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 904567,
"choiceText": "Seizure disorders",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 904569,
"choiceText": "Learning disabilities",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 904571,
"choiceText": "All of the above",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Up to 30% of infants who survive GBS meningitis have serious long-term neurologic sequelae. Long-term complications can include developmental delays, microcephaly, seizure disorders, blindness, deafness, and quadriplegia.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 285141,
"questionText": "Which of the following conditions can be late sequelae of GBS meningitis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Facial Swelling in a 40-Day-Old Boy"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 904517,
"choiceText": "Acute parotitis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 904519,
"choiceText": "Cervical lymphadenitis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 904521,
"choiceText": "Traumatic hematoma",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 904523,
"choiceText": "Cellulitis-adenitis syndrome\r\n",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 285123,
"questionText": "On the basis of the history, physical examination, and workup, what is the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 904525,
"choiceText": "Type Ia",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 904527,
"choiceText": "Type Ib",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 904529,
"choiceText": "Type II",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 904531,
"choiceText": "Type III",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Ninety percent of cases of GBS late-onset disease (occurring at 7 days of age or later), including cellulitis-adenitis syndrome, are caused by serotype III.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 285125,
"questionText": "Which GBS serotype is responsible for the most cases of late-onset GBS disease?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 904563,
"choiceText": "Vision and hearing problems",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 904565,
"choiceText": "Microcephaly",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 904567,
"choiceText": "Seizure disorders",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 904569,
"choiceText": "Learning disabilities",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 904571,
"choiceText": "All of the above",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Up to 30% of infants who survive GBS meningitis have serious long-term neurologic sequelae. Long-term complications can include developmental delays, microcephaly, seizure disorders, blindness, deafness, and quadriplegia.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 285141,
"questionText": "Which of the following conditions can be late sequelae of GBS meningitis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
852384 | /viewarticle/852384 | [
{
"authors": "Pradeep Arora, MD; Karen Convay, NP",
"content": [
"Editor's Note:\n\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.",
"A 65-year-old black male with a history of hypertension for more than 30 years was referred to the nephrologist for evaluation of proteinuria. Recently, his hypertension became uncontrolled despite the use of four antihypertensive medications. These included lisinopril (60 mg daily), nifedipine (60 mg daily), atenolol (100 mg daily), and hydrochlorothiazide (50 mg once a day).",
"The patient was diagnosed with type 2 diabetes mellitus when admitted in a hyperosmolar state nearly 20 years ago. Control of his diabetes was suboptimal at that time, with an average A1c level of 9%. He was also diagnosed with infective endocarditis, which was treated with ceftriaxone and methicillin. Within the next year, he was diagnosed with diabetic neuropathy, diabetic retinopathy after laser therapy, gastroparesis, and erectile dysfunction.",
"The patient's serum creatinine level before admission for infective endocarditis was 0.9 mg/dL; he subsequently developed acute kidney injury (AKI). Around 6 years ago, his estimated glomerular filtration rate (eGFR) was 50 mL/min/1.73 m2 with 6 g for proteinuria when referred to nephrology. He has been diagnosed with biopsy-proven chronic active hepatitis, which was not considered to be treatable.",
"Figure 1.",
"Figure 1.",
"He was prescribed lisinopril, advised to restrict salt, and switched to furosemide from hydrochlorothiazide. Despite several educational sessions, the patient's hypertension and blood sugar level remained at the upper level of poor control. His eGFR decreased to 20 mL/min 3 years prior to the current presentation. Last year, he presented to the emergency department with shortness of breath; his leg showed livedo reticularis and violaceous, painful, plaquelike subcutaneous nodules, which progressed to ischemic and necrotic ulcers with eschars (Figure 1).",
"The patient denies a history of coronary artery disease or stroke. His appetite has been fair, without dietary restriction. He denies fever, vomiting, decreased urine output, joint pain, or rash. He reports fatigue and increased nocturnal urinary frequency."
],
"date": "October 12, 2015",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/852/384/852384-Thumb1.png"
}
],
"markdown": "# A 65-Year-Old Man With Hypertension and Proteinuria\n\n **Authors:** Pradeep Arora, MD; Karen Convay, NP \n **Date:** October 12, 2015\n\n ## Content\n\n Editor's Note:\n\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.\nA 65-year-old black male with a history of hypertension for more than 30 years was referred to the nephrologist for evaluation of proteinuria. Recently, his hypertension became uncontrolled despite the use of four antihypertensive medications. These included lisinopril (60 mg daily), nifedipine (60 mg daily), atenolol (100 mg daily), and hydrochlorothiazide (50 mg once a day).\nThe patient was diagnosed with type 2 diabetes mellitus when admitted in a hyperosmolar state nearly 20 years ago. Control of his diabetes was suboptimal at that time, with an average A1c level of 9%. He was also diagnosed with infective endocarditis, which was treated with ceftriaxone and methicillin. Within the next year, he was diagnosed with diabetic neuropathy, diabetic retinopathy after laser therapy, gastroparesis, and erectile dysfunction.\nThe patient's serum creatinine level before admission for infective endocarditis was 0.9 mg/dL; he subsequently developed acute kidney injury (AKI). Around 6 years ago, his estimated glomerular filtration rate (eGFR) was 50 mL/min/1.73 m2 with 6 g for proteinuria when referred to nephrology. He has been diagnosed with biopsy-proven chronic active hepatitis, which was not considered to be treatable.\nFigure 1.\nFigure 1.\nHe was prescribed lisinopril, advised to restrict salt, and switched to furosemide from hydrochlorothiazide. Despite several educational sessions, the patient's hypertension and blood sugar level remained at the upper level of poor control. His eGFR decreased to 20 mL/min 3 years prior to the current presentation. Last year, he presented to the emergency department with shortness of breath; his leg showed livedo reticularis and violaceous, painful, plaquelike subcutaneous nodules, which progressed to ischemic and necrotic ulcers with eschars (Figure 1).\nThe patient denies a history of coronary artery disease or stroke. His appetite has been fair, without dietary restriction. He denies fever, vomiting, decreased urine output, joint pain, or rash. He reports fatigue and increased nocturnal urinary frequency.\n\n ## Figures\n\n **Figure 1.** \n \n\n\n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 65-Year-Old Man With Hypertension and Proteinuria"
},
{
"authors": "Pradeep Arora, MD; Karen Convay, NP",
"content": [
"The physical examination reveals an obese male in no acute distress. His blood pressure is 180/98 mm Hg. His jugular vein is not distended.",
"A lung examination reveals normal breath sounds, with inspiratory rales heard at both lung bases. The heart rhythm is regular, with a grade 3 systolic murmur heard at the cardiac apex. The abdomen is soft and nontender, and no hepatosplenomegaly is noted. The kidneys are not palpable. He has 2+ swelling of the feet.",
"Laboratory workup reveals a hemoglobin level of 9.8 g/dL, a hematocrit of 28%, a mean corpuscular volume of 81 fL, a platelet count of 172,000 cells/µL, and a white blood cell count of 6700 cells/µL. A serum chemistry panel reveals a sodium level of 138 mEq/L, a chloride level of 101 mEq/L, a potassium level of 5.4 mEq/L, and a bicarbonate level of 18 mEq/L. His serum calcium level is 7.8 mg/dL, phosphate level is 5.5 mg/dL, blood urea nitrogen level is 53 mg/dL, and creatinine level is 2.4 mg/dL (eGFR, 28 mL/min).",
"Other laboratory findings include an alkaline phosphatase level of 150 U/mL, an albumin level of 3.3 g/dL, and an intact parathyroid hormone level of 240 pg/mL. Urinalysis reveals 2+ proteinuria, no hematuria, and no red blood cell (RBC) or white blood cell casts. His urinary protein/creatinine ratio is 6. Renal ultrasonography reveals that the right kidney measures 9.6 cm, and the left kidney is absent."
],
"date": "October 12, 2015",
"figures": [],
"markdown": "# A 65-Year-Old Man With Hypertension and Proteinuria\n\n **Authors:** Pradeep Arora, MD; Karen Convay, NP \n **Date:** October 12, 2015\n\n ## Content\n\n The physical examination reveals an obese male in no acute distress. His blood pressure is 180/98 mm Hg. His jugular vein is not distended.\nA lung examination reveals normal breath sounds, with inspiratory rales heard at both lung bases. The heart rhythm is regular, with a grade 3 systolic murmur heard at the cardiac apex. The abdomen is soft and nontender, and no hepatosplenomegaly is noted. The kidneys are not palpable. He has 2+ swelling of the feet.\nLaboratory workup reveals a hemoglobin level of 9.8 g/dL, a hematocrit of 28%, a mean corpuscular volume of 81 fL, a platelet count of 172,000 cells/µL, and a white blood cell count of 6700 cells/µL. A serum chemistry panel reveals a sodium level of 138 mEq/L, a chloride level of 101 mEq/L, a potassium level of 5.4 mEq/L, and a bicarbonate level of 18 mEq/L. His serum calcium level is 7.8 mg/dL, phosphate level is 5.5 mg/dL, blood urea nitrogen level is 53 mg/dL, and creatinine level is 2.4 mg/dL (eGFR, 28 mL/min).\nOther laboratory findings include an alkaline phosphatase level of 150 U/mL, an albumin level of 3.3 g/dL, and an intact parathyroid hormone level of 240 pg/mL. Urinalysis reveals 2+ proteinuria, no hematuria, and no red blood cell (RBC) or white blood cell casts. His urinary protein/creatinine ratio is 6. Renal ultrasonography reveals that the right kidney measures 9.6 cm, and the left kidney is absent.\n\n ## Figures\n\n \n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 898215,
"choiceText": "Diabetic kidney disease",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 898217,
"choiceText": "Hypertensive nephrosclerosis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 898219,
"choiceText": "Unrecovered AKI",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 898221,
"choiceText": "Hepatitis C-related membranoproliferative glomerulonephritis",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 283085,
"questionText": "On the basis of the history, physical examination, and workup, what is the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 65-Year-Old Man With Hypertension and Proteinuria"
},
{
"authors": "Pradeep Arora, MD; Karen Convay, NP",
"content": [
"The patient in this case has chronic kidney disease (CKD), as evidenced by a decreased eGFR for more than 3 months. The etiologies of CKD include diabetic kidney disease, hypertensive nephrosclerosis, glomerulonephritis, polycystic kidney disease, unrecovered AKI, chronic interstitial nephritis, and ischemic nephropathy. Unrecovered AKI and ischemic nephropathy are becoming important causes of CKD.",
"In this patient, the differential diagnoses of CKD included diabetic kidney disease; his history of long-standing diabetes mellitus along with nephrotic-range proteinuria, even in the absence of retinopathy, makes diabetic kidney disease an important consideration. Unrecovered AKI after treatment of infective endocarditis and hepatitis C-related glomerulonephritis are also among the differential diagnoses of CKD in this patient. An immunologic workup was performed and was negative.",
"Ideally, a kidney biopsy should be performed to confirm the diagnosis; however, the patient in this case declined. A tentative diagnosis of diabetic kidney disease was made, because the presence of diabetic retinopathy favored this diagnosis.",
"Once CKD is diagnosed and its etiology is determined, investigation for an acute reversible factor is paramount; these factors include volume depletion, accelerated hypertension, rapid lowering of blood pressure, treatment with nephrotoxic medications, and urinary tract obstruction. In this patient, the blood pressure was uncontrolled, but no clinically evident acute factor for deterioration was identified.",
"After diagnosis, identification of the etiology, and exploration for an acute reversible factor, the progression of CKD needs to be assessed. Most patients with CKD show progression of kidney disease. This may be largely due to secondary factors that are unrelated to the initial disease. These include systemic and intraglomerular hypertension, glomerular hypertrophy, intrarenal precipitation of calcium phosphate, hyperlipidemia, and altered prostanoid metabolism. The major histologic manifestation of these secondary causes of renal injury is focal segmental glomerulosclerosis."
],
"date": "October 12, 2015",
"figures": [],
"markdown": "# A 65-Year-Old Man With Hypertension and Proteinuria\n\n **Authors:** Pradeep Arora, MD; Karen Convay, NP \n **Date:** October 12, 2015\n\n ## Content\n\n The patient in this case has chronic kidney disease (CKD), as evidenced by a decreased eGFR for more than 3 months. The etiologies of CKD include diabetic kidney disease, hypertensive nephrosclerosis, glomerulonephritis, polycystic kidney disease, unrecovered AKI, chronic interstitial nephritis, and ischemic nephropathy. Unrecovered AKI and ischemic nephropathy are becoming important causes of CKD.\nIn this patient, the differential diagnoses of CKD included diabetic kidney disease; his history of long-standing diabetes mellitus along with nephrotic-range proteinuria, even in the absence of retinopathy, makes diabetic kidney disease an important consideration. Unrecovered AKI after treatment of infective endocarditis and hepatitis C-related glomerulonephritis are also among the differential diagnoses of CKD in this patient. An immunologic workup was performed and was negative.\nIdeally, a kidney biopsy should be performed to confirm the diagnosis; however, the patient in this case declined. A tentative diagnosis of diabetic kidney disease was made, because the presence of diabetic retinopathy favored this diagnosis.\nOnce CKD is diagnosed and its etiology is determined, investigation for an acute reversible factor is paramount; these factors include volume depletion, accelerated hypertension, rapid lowering of blood pressure, treatment with nephrotoxic medications, and urinary tract obstruction. In this patient, the blood pressure was uncontrolled, but no clinically evident acute factor for deterioration was identified.\nAfter diagnosis, identification of the etiology, and exploration for an acute reversible factor, the progression of CKD needs to be assessed. Most patients with CKD show progression of kidney disease. This may be largely due to secondary factors that are unrelated to the initial disease. These include systemic and intraglomerular hypertension, glomerular hypertrophy, intrarenal precipitation of calcium phosphate, hyperlipidemia, and altered prostanoid metabolism. The major histologic manifestation of these secondary causes of renal injury is focal segmental glomerulosclerosis.\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 898215,
"choiceText": "Diabetic kidney disease",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 898217,
"choiceText": "Hypertensive nephrosclerosis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 898219,
"choiceText": "Unrecovered AKI",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 898221,
"choiceText": "Hepatitis C-related membranoproliferative glomerulonephritis",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 283085,
"questionText": "On the basis of the history, physical examination, and workup, what is the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 65-Year-Old Man With Hypertension and Proteinuria"
},
{
"authors": "Pradeep Arora, MD; Karen Convay, NP",
"content": [
"Clinical characteristics that predict a faster decline in glomerular filtration rate include greater proteinuria, higher blood pressure, black ethnicity, a lower serum high-density lipoprotein cholesterol level, and a lower serum transferrin level. In this patient, the rate of progression was very fast. Factors that led to faster progression of CKD here included uncontrolled blood pressure, diabetes, nephrotic-range proteinuria, and black ethnicity.[1]The ideal blood pressure goal in patients such as this is < 130/80 mm Hg.",
"Renin-angiotensin system (RAS) blockers are the first choice for treatment, along with sodium restriction and diuretics in patients with proteinuria. However, data on use of RAS blockers in elderly patients with CKD who are nondiabetic and nonproteinuric are lacking. Other measures to slow progression of kidney disease include a low-protein diet, better control of blood sugar, control of proteinuria (goal, < 0.5 g/day), smoking cessation, and lipid control.[2,3]",
"Anemia is a common complication in patients with CKD. The most important cause of anemia is lack of production of erythropoietin by interstitial cells in the kidney. Other causes include decreased RBC survival, bleeding diathesis, iron deficiency, chronic inflammation, and folate deficiency. Treatment includes use of recombinant erythropoietin after replenishing iron to a saturation > 20%.",
"Renal bone disease is a common complication of CKD. It is defined as an abnormality of calcium, phosphorus, parathyroid hormone (PTH), or vitamin D metabolism; an abnormality in bone turnover, mineralization, volume linear growth, or strength; or extraskeletal calcification.",
"Different types of bone disease occur in CKD; these include:",
"High-turnover bone disease from high PTH levels",
"Low-turnover bone disease (adynamic bone disease)",
"Defective mineralization (osteomalacia)",
"Mixed disease",
"Beta-2-microglobulin–associated bone disease",
"Factors associated with secondary hyperparathyroidism include hyperphosphatemia; hypocalcemia; decreased renal synthesis of 1,25-dihydroxycholecalciferol (1,25-dihydroxyvitamin D, or calcitriol); an intrinsic alteration of the parathyroid glands, which gives rise to increased PTH secretion; and increased parathyroid growth and skeletal resistance to PTH.",
"Treatment of secondary hyperparathyroidism includes correction of serum phosphorus and calcium levels. Vitamin D and calcimimetics are used to treat secondary hyperparathyroidism."
],
"date": "October 12, 2015",
"figures": [],
"markdown": "# A 65-Year-Old Man With Hypertension and Proteinuria\n\n **Authors:** Pradeep Arora, MD; Karen Convay, NP \n **Date:** October 12, 2015\n\n ## Content\n\n Clinical characteristics that predict a faster decline in glomerular filtration rate include greater proteinuria, higher blood pressure, black ethnicity, a lower serum high-density lipoprotein cholesterol level, and a lower serum transferrin level. In this patient, the rate of progression was very fast. Factors that led to faster progression of CKD here included uncontrolled blood pressure, diabetes, nephrotic-range proteinuria, and black ethnicity.[1]The ideal blood pressure goal in patients such as this is < 130/80 mm Hg.\nRenin-angiotensin system (RAS) blockers are the first choice for treatment, along with sodium restriction and diuretics in patients with proteinuria. However, data on use of RAS blockers in elderly patients with CKD who are nondiabetic and nonproteinuric are lacking. Other measures to slow progression of kidney disease include a low-protein diet, better control of blood sugar, control of proteinuria (goal, < 0.5 g/day), smoking cessation, and lipid control.[2,3]\nAnemia is a common complication in patients with CKD. The most important cause of anemia is lack of production of erythropoietin by interstitial cells in the kidney. Other causes include decreased RBC survival, bleeding diathesis, iron deficiency, chronic inflammation, and folate deficiency. Treatment includes use of recombinant erythropoietin after replenishing iron to a saturation > 20%.\nRenal bone disease is a common complication of CKD. It is defined as an abnormality of calcium, phosphorus, parathyroid hormone (PTH), or vitamin D metabolism; an abnormality in bone turnover, mineralization, volume linear growth, or strength; or extraskeletal calcification.\nDifferent types of bone disease occur in CKD; these include:\nHigh-turnover bone disease from high PTH levels\nLow-turnover bone disease (adynamic bone disease)\nDefective mineralization (osteomalacia)\nMixed disease\nBeta-2-microglobulin–associated bone disease\nFactors associated with secondary hyperparathyroidism include hyperphosphatemia; hypocalcemia; decreased renal synthesis of 1,25-dihydroxycholecalciferol (1,25-dihydroxyvitamin D, or calcitriol); an intrinsic alteration of the parathyroid glands, which gives rise to increased PTH secretion; and increased parathyroid growth and skeletal resistance to PTH.\nTreatment of secondary hyperparathyroidism includes correction of serum phosphorus and calcium levels. Vitamin D and calcimimetics are used to treat secondary hyperparathyroidism.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 65-Year-Old Man With Hypertension and Proteinuria"
},
{
"authors": "Pradeep Arora, MD; Karen Convay, NP",
"content": [
"This patient had a high phosphorus level with an increased PTH and alkaline phosphatase level, suggesting high-turnover disease. He was started on a phosphate binder (calcium acetate), activated vitamin D (doxercalciferol), and calcimimetics.",
"The patient had calciphylaxis (calcific uremic arteriolopathy). This is characterized by systemic medial calcification of the arteries. It occurs more commonly among patients on dialysis but can be present in individuals with CKD who are not on dialysis. Risk factors include hyperphosphatemia; obesity; female sex; hypercoagulable states; hypoalbuminemia; and use of warfarin, calcium-based phosphate binders, and vitamin D analogues. Lesions classically develop on areas with the greatest adiposity, including the abdomen, buttocks, and thighs.",
"No specific diagnostic tests for calciphylaxis are available. Skin biopsy reveals arterial occlusion and calcification in the absence of vasculitic change. Treatment includes aggressive wound care, oxygen therapy, phosphorus control, better clearance, sodium thiosulfate, and calcimimetics (or parathyroidectomy).[4]",
"Dialysis education should be provided if the eGFR is < 20 mL/min. At this time, placement of an arteriovenous fistula in the arm is recommended in case the patient chooses to begin hemodialysis in the future. Usual indications for dialysis in CKD include uremia-related pericarditis, coagulopathy, anorexia, unexplained weight loss, encephalopathy, volume overload or hypertension that is unresponsive to diuretic therapy, and resistant hyperkalemia. However, the aim of management is avoidance of these complications; dialysis should therefore be initiated when patients are relatively asymptomatic, with an eGFR of 5-9 mL/min. Early dialysis does not improve survival.[5]",
"When the patient's eGFR fell to 20 mL/min, he was advised to undergo placement of an arteriovenous fistula in his arm. The fistula developed nicely, and the patient was started on dialysis while relatively asymptomatic when his eGFR reached 6 mL/min."
],
"date": "October 12, 2015",
"figures": [],
"markdown": "# A 65-Year-Old Man With Hypertension and Proteinuria\n\n **Authors:** Pradeep Arora, MD; Karen Convay, NP \n **Date:** October 12, 2015\n\n ## Content\n\n This patient had a high phosphorus level with an increased PTH and alkaline phosphatase level, suggesting high-turnover disease. He was started on a phosphate binder (calcium acetate), activated vitamin D (doxercalciferol), and calcimimetics.\nThe patient had calciphylaxis (calcific uremic arteriolopathy). This is characterized by systemic medial calcification of the arteries. It occurs more commonly among patients on dialysis but can be present in individuals with CKD who are not on dialysis. Risk factors include hyperphosphatemia; obesity; female sex; hypercoagulable states; hypoalbuminemia; and use of warfarin, calcium-based phosphate binders, and vitamin D analogues. Lesions classically develop on areas with the greatest adiposity, including the abdomen, buttocks, and thighs.\nNo specific diagnostic tests for calciphylaxis are available. Skin biopsy reveals arterial occlusion and calcification in the absence of vasculitic change. Treatment includes aggressive wound care, oxygen therapy, phosphorus control, better clearance, sodium thiosulfate, and calcimimetics (or parathyroidectomy).[4]\nDialysis education should be provided if the eGFR is < 20 mL/min. At this time, placement of an arteriovenous fistula in the arm is recommended in case the patient chooses to begin hemodialysis in the future. Usual indications for dialysis in CKD include uremia-related pericarditis, coagulopathy, anorexia, unexplained weight loss, encephalopathy, volume overload or hypertension that is unresponsive to diuretic therapy, and resistant hyperkalemia. However, the aim of management is avoidance of these complications; dialysis should therefore be initiated when patients are relatively asymptomatic, with an eGFR of 5-9 mL/min. Early dialysis does not improve survival.[5]\nWhen the patient's eGFR fell to 20 mL/min, he was advised to undergo placement of an arteriovenous fistula in his arm. The fistula developed nicely, and the patient was started on dialysis while relatively asymptomatic when his eGFR reached 6 mL/min.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 898297,
"choiceText": "Intravenous erythropoietin ",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 898299,
"choiceText": "Oral iron",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 898301,
"choiceText": "Intravenous iron (to achieve saturation > 20%), then epoetin alfa if hemoglobin is < 10 g/dL",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 898303,
"choiceText": "Intravenous iron (to achieve saturation > 20%), then epoetin alfa if hemoglobin is < 12 g/dL\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The National Kidney Foundation's Kidney Disease Outcomes Quality Initiative issued a clinical practice guideline that recommends using erythropoiesis-stimulating agents, such as epoetin alfa or darbepoetin alfa, when hemoglobin levels are < 10 g/dL and iron saturation is > 20%.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 283109,
"questionText": "Which of the following is the best strategy to treat a patient with CKD who has a hemoglobin level of 5.8 g/dL, iron saturation of 12%, and ferritin level of 300 mg/dL? ",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 898305,
"choiceText": "Relative deficiency of erythropoietin",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 898307,
"choiceText": "Decreased survival of RBC",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 898309,
"choiceText": "Chronic inflammation",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 898311,
"choiceText": "Bleeding diathesis",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 898313,
"choiceText": "All of the above\r\n",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "\r\n\r\nAnemia is a complication of CKD and correlates with eGFR. Causes of anemia in CKD include decreased erythropoietin production by the kidney; decreased RBC survival; chronic inflammation leading to an increased hepcidin level; bleeding diathesis; hyperparathyroidism; and deficiencies of iron, vitamin B12, or folate. Hepcidin is produced by the liver and secreted into circulation. Hepcidin binds and induces degradation of the iron exporter, ferroportin, on duodenal enterocytes, reticuloendothelial macrophages, and hepatocytes to inhibit iron entry into the plasma. Inflammatory cytokines directly induce hepcidin transcription.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 283111,
"questionText": "Which of the following is an important cause of anemia among patients with CKD?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 65-Year-Old Man With Hypertension and Proteinuria"
},
{
"authors": "Pradeep Arora, MD; Karen Convay, NP",
"content": [],
"date": "October 12, 2015",
"figures": [],
"markdown": "# A 65-Year-Old Man With Hypertension and Proteinuria\n\n **Authors:** Pradeep Arora, MD; Karen Convay, NP \n **Date:** October 12, 2015\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 898297,
"choiceText": "Intravenous erythropoietin ",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 898299,
"choiceText": "Oral iron",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 898301,
"choiceText": "Intravenous iron (to achieve saturation > 20%), then epoetin alfa if hemoglobin is < 10 g/dL",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 898303,
"choiceText": "Intravenous iron (to achieve saturation > 20%), then epoetin alfa if hemoglobin is < 12 g/dL\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The National Kidney Foundation's Kidney Disease Outcomes Quality Initiative issued a clinical practice guideline that recommends using erythropoiesis-stimulating agents, such as epoetin alfa or darbepoetin alfa, when hemoglobin levels are < 10 g/dL and iron saturation is > 20%.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 283109,
"questionText": "Which of the following is the best strategy to treat a patient with CKD who has a hemoglobin level of 5.8 g/dL, iron saturation of 12%, and ferritin level of 300 mg/dL? ",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 898305,
"choiceText": "Relative deficiency of erythropoietin",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 898307,
"choiceText": "Decreased survival of RBC",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 898309,
"choiceText": "Chronic inflammation",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 898311,
"choiceText": "Bleeding diathesis",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 898313,
"choiceText": "All of the above\r\n",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "\r\n\r\nAnemia is a complication of CKD and correlates with eGFR. Causes of anemia in CKD include decreased erythropoietin production by the kidney; decreased RBC survival; chronic inflammation leading to an increased hepcidin level; bleeding diathesis; hyperparathyroidism; and deficiencies of iron, vitamin B12, or folate. Hepcidin is produced by the liver and secreted into circulation. Hepcidin binds and induces degradation of the iron exporter, ferroportin, on duodenal enterocytes, reticuloendothelial macrophages, and hepatocytes to inhibit iron entry into the plasma. Inflammatory cytokines directly induce hepcidin transcription.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 283111,
"questionText": "Which of the following is an important cause of anemia among patients with CKD?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 65-Year-Old Man With Hypertension and Proteinuria"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 898215,
"choiceText": "Diabetic kidney disease",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 898217,
"choiceText": "Hypertensive nephrosclerosis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 898219,
"choiceText": "Unrecovered AKI",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 898221,
"choiceText": "Hepatitis C-related membranoproliferative glomerulonephritis",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 283085,
"questionText": "On the basis of the history, physical examination, and workup, what is the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 898297,
"choiceText": "Intravenous erythropoietin ",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 898299,
"choiceText": "Oral iron",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 898301,
"choiceText": "Intravenous iron (to achieve saturation > 20%), then epoetin alfa if hemoglobin is < 10 g/dL",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 898303,
"choiceText": "Intravenous iron (to achieve saturation > 20%), then epoetin alfa if hemoglobin is < 12 g/dL\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The National Kidney Foundation's Kidney Disease Outcomes Quality Initiative issued a clinical practice guideline that recommends using erythropoiesis-stimulating agents, such as epoetin alfa or darbepoetin alfa, when hemoglobin levels are < 10 g/dL and iron saturation is > 20%.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 283109,
"questionText": "Which of the following is the best strategy to treat a patient with CKD who has a hemoglobin level of 5.8 g/dL, iron saturation of 12%, and ferritin level of 300 mg/dL? ",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 898305,
"choiceText": "Relative deficiency of erythropoietin",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 898307,
"choiceText": "Decreased survival of RBC",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 898309,
"choiceText": "Chronic inflammation",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 898311,
"choiceText": "Bleeding diathesis",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 898313,
"choiceText": "All of the above\r\n",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "\r\n\r\nAnemia is a complication of CKD and correlates with eGFR. Causes of anemia in CKD include decreased erythropoietin production by the kidney; decreased RBC survival; chronic inflammation leading to an increased hepcidin level; bleeding diathesis; hyperparathyroidism; and deficiencies of iron, vitamin B12, or folate. Hepcidin is produced by the liver and secreted into circulation. Hepcidin binds and induces degradation of the iron exporter, ferroportin, on duodenal enterocytes, reticuloendothelial macrophages, and hepatocytes to inhibit iron entry into the plasma. Inflammatory cytokines directly induce hepcidin transcription.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 283111,
"questionText": "Which of the following is an important cause of anemia among patients with CKD?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
851822 | /viewarticle/851822 | [
{
"authors": "Peggy Nelson, MD; Ved V. Gossain, MD",
"content": [
"Editor's Note:\n\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please \ncontact us.",
"Figure 1",
"A 79-year-old man with a history of atrial fibrillation currently taking warfarin is presented to the emergency department (ED) following two syncopal episodes. Each episode was without any preceding prodromal symptoms. His first syncopal episode occurred while seated in the bathroom shaving in the morning. He awakened on the bathroom floor after an undetermined amount of time and was able to crawl to the kitchen and drink a glass of orange juice, which led to an improvement of his status. He was evaluated by his cardiologist following this first episode; the cardiologist discontinued his antihypertensive medications (amlodipine/benazepril and triamterene/hydrochlorothiazide) because of a concern that orthostasis may have been responsible.",
"The next morning, the patient suffered a second syncopal episode, for which he is now in the ED. This time, he was found unresponsive on his bedroom floor by family. On evaluation by the emergency medical service, the patient was noted to have a blood glucose of 20 mg/dL (1.11 mmol/L), for which he received 1 ampule of 50% dextrose. He became alert and responsive immediately after being treated with dextrose and stated that he was unaware of the events leading up to his loss of consciousness.",
"The patient has no history of diabetes mellitus, hepatic disease, or renal dysfunction. He denies any current tobacco or alcohol use. The patient has been losing weight unintentionally. In fact, he states that he has been eating more than previously but is still unable to maintain his weight. His current medications include ezetimibe, propafenone, lovastatin, atenolol, diazepam, doxazosin, aspirin, amlodipine/benazepril, triamterene/hydrochlorothiazide, and warfarin. He is also taking folic acid, fish oil, zinc, and vitamin E."
],
"date": "September 30, 2015",
"figures": [
{
"caption": "Figure 1",
"image_url": "https://img.medscapestatic.com/article/851/822/851822-Thumb1.png"
}
],
"markdown": "# A 79-Year-Old Man With Syncope\n\n **Authors:** Peggy Nelson, MD; Ved V. Gossain, MD \n **Date:** September 30, 2015\n\n ## Content\n\n Editor's Note:\n\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please \ncontact us.\nFigure 1\nA 79-year-old man with a history of atrial fibrillation currently taking warfarin is presented to the emergency department (ED) following two syncopal episodes. Each episode was without any preceding prodromal symptoms. His first syncopal episode occurred while seated in the bathroom shaving in the morning. He awakened on the bathroom floor after an undetermined amount of time and was able to crawl to the kitchen and drink a glass of orange juice, which led to an improvement of his status. He was evaluated by his cardiologist following this first episode; the cardiologist discontinued his antihypertensive medications (amlodipine/benazepril and triamterene/hydrochlorothiazide) because of a concern that orthostasis may have been responsible.\nThe next morning, the patient suffered a second syncopal episode, for which he is now in the ED. This time, he was found unresponsive on his bedroom floor by family. On evaluation by the emergency medical service, the patient was noted to have a blood glucose of 20 mg/dL (1.11 mmol/L), for which he received 1 ampule of 50% dextrose. He became alert and responsive immediately after being treated with dextrose and stated that he was unaware of the events leading up to his loss of consciousness.\nThe patient has no history of diabetes mellitus, hepatic disease, or renal dysfunction. He denies any current tobacco or alcohol use. The patient has been losing weight unintentionally. In fact, he states that he has been eating more than previously but is still unable to maintain his weight. His current medications include ezetimibe, propafenone, lovastatin, atenolol, diazepam, doxazosin, aspirin, amlodipine/benazepril, triamterene/hydrochlorothiazide, and warfarin. He is also taking folic acid, fish oil, zinc, and vitamin E.\n\n ## Figures\n\n **Figure 1** \n \n\n\n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 79-Year-Old Man With Syncope"
},
{
"authors": "",
"content": [],
"date": "",
"figures": [],
"markdown": "# \n\n **Authors:** \n **Date:** \n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 2 of 1*",
"pagination": {
"current_page": 2,
"total_pages": 1
},
"questionnaire": [],
"title": ""
},
{
"authors": "",
"content": [],
"date": "",
"figures": [],
"markdown": "# \n\n **Authors:** \n **Date:** \n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 3 of 1*",
"pagination": {
"current_page": 3,
"total_pages": 1
},
"questionnaire": [],
"title": ""
},
{
"authors": "",
"content": [],
"date": "",
"figures": [],
"markdown": "# \n\n **Authors:** \n **Date:** \n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 4 of 1*",
"pagination": {
"current_page": 4,
"total_pages": 1
},
"questionnaire": [],
"title": ""
},
{
"authors": "",
"content": [],
"date": "",
"figures": [],
"markdown": "# \n\n **Authors:** \n **Date:** \n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 5 of 1*",
"pagination": {
"current_page": 5,
"total_pages": 1
},
"questionnaire": [],
"title": ""
},
{
"authors": "",
"content": [],
"date": "",
"figures": [],
"markdown": "# \n\n **Authors:** \n **Date:** \n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 1*",
"pagination": {
"current_page": 6,
"total_pages": 1
},
"questionnaire": [],
"title": ""
}
] | [] |
851820 | /viewarticle/851820 | [
{
"authors": "Muralikrishna Gopalakrishnamoorthy, MBBS, PGY-1; Archana Bhaskaran, MBBS, PGY-1; Rajeswari Anaparthy, MBBS, PGY-1; Ted T. Lin, MS3; Syed Hasan, MBBS",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.",
"A 21-year-old woman presents to the emergency department with a 1-month history of fever; general malaise; and mild, diffuse cramping and abdominal pain. The abdominal pain is generalized, with no specific aggravating or relieving factors, and it is not associated with constipation or diarrhea. She also reports a productive cough, with occasional blood-tinged sputum.",
"Figure 1.",
"Upon further questioning, she states that she has been experiencing night sweats, has subjectively lost weight over the past month, and is becoming progressively more short of breath with mild exertion. The patient denies having sore throat, wheezing, pleuritic chest pain, or rash, as well as lower-extremity swelling or paroxysmal nocturnal dyspnea. She also denies any recent travel, has not been incarcerated or stayed in any shelters, and has not had any known tuberculosis exposures. She does not have any pets. She is a nonsmoker and denies using intravenous drugs.",
"The patient has a medical history of asthma and was recently diagnosed with HIV/AIDS, for which she is currently not on antiretroviral medications. Her current medications include albuterol, fluticasone, and occasional ibuprofen. She received her annual influenza vaccine 1 month ago. She denies a history of allergy to medications."
],
"date": "September 30, 2015",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/851/820/851820-Thumb1.png"
}
],
"markdown": "# A 21-Year-Old Woman With Persistent Fever and Malaise\n\n **Authors:** Muralikrishna Gopalakrishnamoorthy, MBBS, PGY-1; Archana Bhaskaran, MBBS, PGY-1; Rajeswari Anaparthy, MBBS, PGY-1; Ted T. Lin, MS3; Syed Hasan, MBBS \n **Date:** September 30, 2015\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.\nA 21-year-old woman presents to the emergency department with a 1-month history of fever; general malaise; and mild, diffuse cramping and abdominal pain. The abdominal pain is generalized, with no specific aggravating or relieving factors, and it is not associated with constipation or diarrhea. She also reports a productive cough, with occasional blood-tinged sputum.\nFigure 1.\nUpon further questioning, she states that she has been experiencing night sweats, has subjectively lost weight over the past month, and is becoming progressively more short of breath with mild exertion. The patient denies having sore throat, wheezing, pleuritic chest pain, or rash, as well as lower-extremity swelling or paroxysmal nocturnal dyspnea. She also denies any recent travel, has not been incarcerated or stayed in any shelters, and has not had any known tuberculosis exposures. She does not have any pets. She is a nonsmoker and denies using intravenous drugs.\nThe patient has a medical history of asthma and was recently diagnosed with HIV/AIDS, for which she is currently not on antiretroviral medications. Her current medications include albuterol, fluticasone, and occasional ibuprofen. She received her annual influenza vaccine 1 month ago. She denies a history of allergy to medications.\n\n ## Figures\n\n **Figure 1.** \n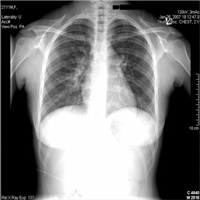 \n\n\n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 21-Year-Old Woman With Persistent Fever and Malaise"
},
{
"authors": "Muralikrishna Gopalakrishnamoorthy, MBBS, PGY-1; Archana Bhaskaran, MBBS, PGY-1; Rajeswari Anaparthy, MBBS, PGY-1; Ted T. Lin, MS3; Syed Hasan, MBBS",
"content": [
"On physical examination, the patient is noted to be tachycardic, with a heart rate of 105 beats/min. She is febrile, with a temperature of 103.3°F; in addition, she has mild tachypnea, with a respiratory rate of 26 breaths/min, and she is noted to have a moderately increased respiratory effort.",
"The patient appears cachectic and fatigued, but she is easily arousable. She is not disoriented and has normal speech and comprehension.",
"Inspiratory and expiratory crackles are heard in the anterior right lung field. No cardiac murmurs or gallops are present. The jugular veins are not elevated. No peripheral edema or lymphadenopathy is appreciated. Her abdomen is soft, nontender, and without palpable masses. No rashes or skin lesions are detected.",
"Figure 1.",
"Figure 2.",
"The remainder of the examination is unremarkable. Diagnostic laboratory studies reveal a white blood cell count of 5 × 103 cells/µL, a hematocrit of 22.5%, a hemoglobin value of 8 g/dL, a mean corpuscular volume of 69.7 fL, and a platelet count of 219 × 103 cells/µL. The CD4 count at presentation was 9 cells/ µL(normal range, 522-1594 cells/ µL), with an HIV branched DNA viral load > 500,000 copies/mL.",
"Electrolyte measurements are normal except for a bicarbonate level of 18 mEq/L. Liver function test results are abnormally elevated, with an alkaline phosphatase value of 301 U/L, an aspartate aminotransferase values of 56 U/L, and an alanine aminotransferase value of 490 U/L. The lactate dehydrogenase (LDH) level is also elevated, at 6450 U/L.",
"Arterial blood gas analysis showed the following values:",
"pH: 7.46",
"pCO2: 29",
"pO2: 154",
"HCO3: 19",
"Chest radiography (Figure 1) and subsequent CT of the chest (Figure 2) are obtained."
],
"date": "September 30, 2015",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/851/820/851820-Thumb1.png"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/851/820/851820-Thumb2.png"
}
],
"markdown": "# A 21-Year-Old Woman With Persistent Fever and Malaise\n\n **Authors:** Muralikrishna Gopalakrishnamoorthy, MBBS, PGY-1; Archana Bhaskaran, MBBS, PGY-1; Rajeswari Anaparthy, MBBS, PGY-1; Ted T. Lin, MS3; Syed Hasan, MBBS \n **Date:** September 30, 2015\n\n ## Content\n\n On physical examination, the patient is noted to be tachycardic, with a heart rate of 105 beats/min. She is febrile, with a temperature of 103.3°F; in addition, she has mild tachypnea, with a respiratory rate of 26 breaths/min, and she is noted to have a moderately increased respiratory effort.\nThe patient appears cachectic and fatigued, but she is easily arousable. She is not disoriented and has normal speech and comprehension.\nInspiratory and expiratory crackles are heard in the anterior right lung field. No cardiac murmurs or gallops are present. The jugular veins are not elevated. No peripheral edema or lymphadenopathy is appreciated. Her abdomen is soft, nontender, and without palpable masses. No rashes or skin lesions are detected.\nFigure 1.\nFigure 2.\nThe remainder of the examination is unremarkable. Diagnostic laboratory studies reveal a white blood cell count of 5 × 103 cells/µL, a hematocrit of 22.5%, a hemoglobin value of 8 g/dL, a mean corpuscular volume of 69.7 fL, and a platelet count of 219 × 103 cells/µL. The CD4 count at presentation was 9 cells/ µL(normal range, 522-1594 cells/ µL), with an HIV branched DNA viral load > 500,000 copies/mL.\nElectrolyte measurements are normal except for a bicarbonate level of 18 mEq/L. Liver function test results are abnormally elevated, with an alkaline phosphatase value of 301 U/L, an aspartate aminotransferase values of 56 U/L, and an alanine aminotransferase value of 490 U/L. The lactate dehydrogenase (LDH) level is also elevated, at 6450 U/L.\nArterial blood gas analysis showed the following values:\npH: 7.46\npCO2: 29\npO2: 154\nHCO3: 19\nChest radiography (Figure 1) and subsequent CT of the chest (Figure 2) are obtained.\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 896539,
"choiceText": "Tuberculosis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 896541,
"choiceText": "<i>Mycobacterium avium-intracellulare</i> (MAI)",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 896543,
"choiceText": "Histoplasmosis\r\n",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 896545,
"choiceText": "Cryptococcosis",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 896547,
"choiceText": "Lymphoma",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 282539,
"questionText": "What is the most likely infectious process this patient is experiencing?<br><br><i>\r\nHint: Note the markedly elevated LDH level.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 21-Year-Old Woman With Persistent Fever and Malaise"
},
{
"authors": "Muralikrishna Gopalakrishnamoorthy, MBBS, PGY-1; Archana Bhaskaran, MBBS, PGY-1; Rajeswari Anaparthy, MBBS, PGY-1; Ted T. Lin, MS3; Syed Hasan, MBBS",
"content": [
"The differential diagnosis in a patient with HIV/AIDS and a 1-month history of nonspecific symptoms of weakness, fatigue, weight loss, and fever is broad. In addition to the usual etiologies in immunocompetent individuals, numerous specific diseases that are more commonly found among immunocompromised patients must be considered, such as disseminated MAI, Pneumocystis jiroveci pneumonia (PJP), tuberculosis, disseminated cryptococcosis, and disseminated histoplasmosis. The presence of markedly elevated LDH levels makes histoplasmosis the most probable diagnosis in this case.[1]",
"An extremely high LDH level, with few or no other conditions, has a relatively high diagnostic accuracy for histoplasmosis (especially in HIV-positive patients). In addition, the pulmonary infiltrates seen on the plain chest radiograph and CT scan in this case are suggestive of histoplasmosis. However, the alternative diagnoses must also be considered.",
"The nonspecific symptoms seen in this patient may suggest disseminated MAI infection; however, such markedly high LDH levels are uncharacteristic of MAI. Moreover, patients with HIV/AIDS who are infected with MAI typically present with extrapulmonary symptoms, rather than with the pulmonary manifestations that predominated in this patient.",
"Although the presence of fever, shortness of breath, cough, and hemoptysis in this patient suggest PJP, the findings of multiorgan involvement make PJP a less likely diagnosis. The duration of symptoms, lack of hypoxia, and characteristic infiltrates on the chest radiograph also make PJP unlikely.",
"The occurrence of chronic pulmonary symptoms in a patient with HIV/AIDS is certainly suggestive of tuberculosis, but the chest radiograph seen here is not classic for tuberculosis, and the diagnosis would require demonstration of a positive acid-fast bacilli smear from respiratory tract specimens or a positive culture (eg, blood, sputum, or bone marrow culture). Another possibility is disseminated cryptococcosis, which can also cause a similar clinical picture; however, significant central nervous system (CNS) involvement and skin lesions (which may be observed in patients with cryptococcal infection) were absent in this patient.[1]",
"Histoplasmosis is a fungal infection caused by Histoplasma capsulatum, a thermally dimorphic fungus found in nature. It grows saprophytically and replicates outside the human host in order to evolve into an infectious form. Its natural habitat is warm, humid soil contaminated with bird droppings or bat excrement (such as that found in old barns, caves, and parks).[2] In the soil, the organism exists in a mycelial form with macroconidia and microconidia. It grows in mold form at 77°F, and as yeast at 98.6°F."
],
"date": "September 30, 2015",
"figures": [],
"markdown": "# A 21-Year-Old Woman With Persistent Fever and Malaise\n\n **Authors:** Muralikrishna Gopalakrishnamoorthy, MBBS, PGY-1; Archana Bhaskaran, MBBS, PGY-1; Rajeswari Anaparthy, MBBS, PGY-1; Ted T. Lin, MS3; Syed Hasan, MBBS \n **Date:** September 30, 2015\n\n ## Content\n\n The differential diagnosis in a patient with HIV/AIDS and a 1-month history of nonspecific symptoms of weakness, fatigue, weight loss, and fever is broad. In addition to the usual etiologies in immunocompetent individuals, numerous specific diseases that are more commonly found among immunocompromised patients must be considered, such as disseminated MAI, Pneumocystis jiroveci pneumonia (PJP), tuberculosis, disseminated cryptococcosis, and disseminated histoplasmosis. The presence of markedly elevated LDH levels makes histoplasmosis the most probable diagnosis in this case.[1]\nAn extremely high LDH level, with few or no other conditions, has a relatively high diagnostic accuracy for histoplasmosis (especially in HIV-positive patients). In addition, the pulmonary infiltrates seen on the plain chest radiograph and CT scan in this case are suggestive of histoplasmosis. However, the alternative diagnoses must also be considered.\nThe nonspecific symptoms seen in this patient may suggest disseminated MAI infection; however, such markedly high LDH levels are uncharacteristic of MAI. Moreover, patients with HIV/AIDS who are infected with MAI typically present with extrapulmonary symptoms, rather than with the pulmonary manifestations that predominated in this patient.\nAlthough the presence of fever, shortness of breath, cough, and hemoptysis in this patient suggest PJP, the findings of multiorgan involvement make PJP a less likely diagnosis. The duration of symptoms, lack of hypoxia, and characteristic infiltrates on the chest radiograph also make PJP unlikely.\nThe occurrence of chronic pulmonary symptoms in a patient with HIV/AIDS is certainly suggestive of tuberculosis, but the chest radiograph seen here is not classic for tuberculosis, and the diagnosis would require demonstration of a positive acid-fast bacilli smear from respiratory tract specimens or a positive culture (eg, blood, sputum, or bone marrow culture). Another possibility is disseminated cryptococcosis, which can also cause a similar clinical picture; however, significant central nervous system (CNS) involvement and skin lesions (which may be observed in patients with cryptococcal infection) were absent in this patient.[1]\nHistoplasmosis is a fungal infection caused by Histoplasma capsulatum, a thermally dimorphic fungus found in nature. It grows saprophytically and replicates outside the human host in order to evolve into an infectious form. Its natural habitat is warm, humid soil contaminated with bird droppings or bat excrement (such as that found in old barns, caves, and parks).[2] In the soil, the organism exists in a mycelial form with macroconidia and microconidia. It grows in mold form at 77°F, and as yeast at 98.6°F.\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 896539,
"choiceText": "Tuberculosis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 896541,
"choiceText": "<i>Mycobacterium avium-intracellulare</i> (MAI)",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 896543,
"choiceText": "Histoplasmosis\r\n",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 896545,
"choiceText": "Cryptococcosis",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 896547,
"choiceText": "Lymphoma",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 282539,
"questionText": "What is the most likely infectious process this patient is experiencing?<br><br><i>\r\nHint: Note the markedly elevated LDH level.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 21-Year-Old Woman With Persistent Fever and Malaise"
},
{
"authors": "Muralikrishna Gopalakrishnamoorthy, MBBS, PGY-1; Archana Bhaskaran, MBBS, PGY-1; Rajeswari Anaparthy, MBBS, PGY-1; Ted T. Lin, MS3; Syed Hasan, MBBS",
"content": [
"Histoplasmosis is most prevalent in the United States, but it is also found in the warm climates of Central and South America, the Caribbean, Africa, and Asia. In the United States, most cases occur within the Ohio and Mississippi River valleys. Approximately 250,000-500,000 cases are reported yearly, although disseminated histoplasmosis occurs in < 0.05% of infections. Farmers, landscapers, construction workers, and people who have contact with birds or bats are especially at risk.",
"The occurrence of histoplasmosis in AIDS patients is 2%-5%. Other risk factors include the extremes of age; chronic obstructive pulmonary disease; primary immunodeficiency or immunosuppressing disorders; and use of immunosuppressive medications, such as corticosteroids, methotrexate, tumor necrosis factor alpha inhibitors, and antirejection therapies in solid-organ transplant recipients.",
"Infection follows inhalation of microconidia (spores), which germinate into yeast in the lungs. An ineffective neutrophil response against the yeast is followed by ingestion of the yeast by macrophages. Macrophages then spread the organism via the lymphatic system and the blood to the adjacent lymph nodes and throughout the reticuloendothelial system. The organisms are confined to the macrophages and are rarely seen within the tissue spaces. In patients with disseminated infection, the organisms can be seen within monocytes in the peripheral blood.",
"In an immunocompetent host, specific immunity develops 10-21 days after infection.[3,4,5]Patients may present shortly after exposure or years later, and they may experience asymptomatic periods interrupted by symptomatic relapses.",
"Progressive disseminated histoplasmosis occurs in two forms: acute and chronic, which are determined in part by the time course of the illness and by the extent of the infection. Acute infection is seen mostly in infants, immunocompromised hosts, and patients treated with tumor necrosis factor alpha inhibitors. The duration of the acute syndrome ranges from a course of 1-5 days to a prolonged course of 10-21 days of severe illness. During the acute syndrome, nonspecific symptoms of fever, myalgia, pleuritic chest pain, and nonproductive cough predominate.",
"Chronic infection is seen more often among older, otherwise immunocompetent adults. Pancytopenia, hepatosplenomegaly, elevated liver enzyme levels, and oropharyngeal or gastrointestinal (GI) lesions are commonly seen in presentations of chronic infection. The symptoms in chronic infection are similar to those of acute infection, but they are often less severe and may be combined with weight loss, fatigue, and night sweats. In severely immunocompromised patients and those receiving immunosuppressive medications, it may present as an overwhelming infection, with shock and multiorgan failure; in these cases, the mortality rate approaches 50%, despite treatment.",
"Manifestations of disseminated disease may occur in several body systems. In immunocompetent hosts, exposure to a large inoculum typically results in a severe acute pulmonary syndrome that is characterized by a prodrome of cough, chest pain, and fever, with subsequent dyspnea, hypoxemia, and coarse breath sounds on examination. In severe cases, the illness may progress to adult respiratory distress syndrome. Pericarditis may develop, either as a result of inflammation in contiguous lymph nodes or, less commonly, true infection of the pericardium. Endocarditis is uncommon in disseminated histoplasmosis, only occurring in approximately 4% of patients.",
"A rheumatologic syndrome of arthritis, arthralgia, and erythema nodosum is seen in approximately 10% of cases. GI symptoms may include dysphagia, abdominal pain, and GI bleeding. Adrenal involvement is very common (80%-90%), although only 10% of patients develop adrenal insufficiency (often as a manifestation of relapsing histoplasmosis years after the initial episode).",
"CNS histoplasmosis manifests most commonly as meningitis in 10% of patients with disseminated disease, but it rarely occurs in immunocompetent patients. The symptoms include fever, headache, and changes in mental status (if accompanied by encephalitis). Seizures and focal neurologic findings may suggest meningitis or focal brain lesions. Localized spinal cord lesions may also occur.",
"Local manifestations of disseminated disease may include chorioretinitis, peritonitis, pancreatitis or cholecystitis, prostatitis, panniculitis, mastitis, osteomyelitis, septic arthritis, epididymitis, or penile/vaginal involvement.[3,4]",
"Histoplasmosis is diagnosed using antigen testing; the Histoplasma antigen is present in the urine in 90% of cases and in the serum in 70% of cases. It is less frequently detected in other sterile body fluids (eg, cerebrospinal fluid [CSF], bronchoalveolar lavage). Histopathology may reveal organisms on hematoxylin/eosin staining of a biopsy sample from infected tissue, although silver and periodic acid/Schiff staining are superior examinations. Serologic tests are of limited value, because they often yield false-negative results in immunocompromised patients. Blood cultures are positive in 50%-70% of cases, but they yield false-negative results in 20% of disseminated cases. Bone marrow cultures are positive in up to 75% of cases, and when combined with blood cultures, markedly increase the likelihood of positive cultures.",
"Biopsy may be performed at any suspected site of involvement, such as the skin or oral mucosa, GI lesions, lymph nodes, adrenal glands, and liver. Chest radiographs are abnormal in 70% of cases, usually showing diffuse interstitial or reticulonodular infiltrates. CSF lymphocytic pleocytosis, protein elevation, and low glucose are suggestive of CNS involvement. Single or multiple enhancing lesions may also be seen on CT.[1,6,7]"
],
"date": "September 30, 2015",
"figures": [],
"markdown": "# A 21-Year-Old Woman With Persistent Fever and Malaise\n\n **Authors:** Muralikrishna Gopalakrishnamoorthy, MBBS, PGY-1; Archana Bhaskaran, MBBS, PGY-1; Rajeswari Anaparthy, MBBS, PGY-1; Ted T. Lin, MS3; Syed Hasan, MBBS \n **Date:** September 30, 2015\n\n ## Content\n\n Histoplasmosis is most prevalent in the United States, but it is also found in the warm climates of Central and South America, the Caribbean, Africa, and Asia. In the United States, most cases occur within the Ohio and Mississippi River valleys. Approximately 250,000-500,000 cases are reported yearly, although disseminated histoplasmosis occurs in < 0.05% of infections. Farmers, landscapers, construction workers, and people who have contact with birds or bats are especially at risk.\nThe occurrence of histoplasmosis in AIDS patients is 2%-5%. Other risk factors include the extremes of age; chronic obstructive pulmonary disease; primary immunodeficiency or immunosuppressing disorders; and use of immunosuppressive medications, such as corticosteroids, methotrexate, tumor necrosis factor alpha inhibitors, and antirejection therapies in solid-organ transplant recipients.\nInfection follows inhalation of microconidia (spores), which germinate into yeast in the lungs. An ineffective neutrophil response against the yeast is followed by ingestion of the yeast by macrophages. Macrophages then spread the organism via the lymphatic system and the blood to the adjacent lymph nodes and throughout the reticuloendothelial system. The organisms are confined to the macrophages and are rarely seen within the tissue spaces. In patients with disseminated infection, the organisms can be seen within monocytes in the peripheral blood.\nIn an immunocompetent host, specific immunity develops 10-21 days after infection.[3,4,5]Patients may present shortly after exposure or years later, and they may experience asymptomatic periods interrupted by symptomatic relapses.\nProgressive disseminated histoplasmosis occurs in two forms: acute and chronic, which are determined in part by the time course of the illness and by the extent of the infection. Acute infection is seen mostly in infants, immunocompromised hosts, and patients treated with tumor necrosis factor alpha inhibitors. The duration of the acute syndrome ranges from a course of 1-5 days to a prolonged course of 10-21 days of severe illness. During the acute syndrome, nonspecific symptoms of fever, myalgia, pleuritic chest pain, and nonproductive cough predominate.\nChronic infection is seen more often among older, otherwise immunocompetent adults. Pancytopenia, hepatosplenomegaly, elevated liver enzyme levels, and oropharyngeal or gastrointestinal (GI) lesions are commonly seen in presentations of chronic infection. The symptoms in chronic infection are similar to those of acute infection, but they are often less severe and may be combined with weight loss, fatigue, and night sweats. In severely immunocompromised patients and those receiving immunosuppressive medications, it may present as an overwhelming infection, with shock and multiorgan failure; in these cases, the mortality rate approaches 50%, despite treatment.\nManifestations of disseminated disease may occur in several body systems. In immunocompetent hosts, exposure to a large inoculum typically results in a severe acute pulmonary syndrome that is characterized by a prodrome of cough, chest pain, and fever, with subsequent dyspnea, hypoxemia, and coarse breath sounds on examination. In severe cases, the illness may progress to adult respiratory distress syndrome. Pericarditis may develop, either as a result of inflammation in contiguous lymph nodes or, less commonly, true infection of the pericardium. Endocarditis is uncommon in disseminated histoplasmosis, only occurring in approximately 4% of patients.\nA rheumatologic syndrome of arthritis, arthralgia, and erythema nodosum is seen in approximately 10% of cases. GI symptoms may include dysphagia, abdominal pain, and GI bleeding. Adrenal involvement is very common (80%-90%), although only 10% of patients develop adrenal insufficiency (often as a manifestation of relapsing histoplasmosis years after the initial episode).\nCNS histoplasmosis manifests most commonly as meningitis in 10% of patients with disseminated disease, but it rarely occurs in immunocompetent patients. The symptoms include fever, headache, and changes in mental status (if accompanied by encephalitis). Seizures and focal neurologic findings may suggest meningitis or focal brain lesions. Localized spinal cord lesions may also occur.\nLocal manifestations of disseminated disease may include chorioretinitis, peritonitis, pancreatitis or cholecystitis, prostatitis, panniculitis, mastitis, osteomyelitis, septic arthritis, epididymitis, or penile/vaginal involvement.[3,4]\nHistoplasmosis is diagnosed using antigen testing; the Histoplasma antigen is present in the urine in 90% of cases and in the serum in 70% of cases. It is less frequently detected in other sterile body fluids (eg, cerebrospinal fluid [CSF], bronchoalveolar lavage). Histopathology may reveal organisms on hematoxylin/eosin staining of a biopsy sample from infected tissue, although silver and periodic acid/Schiff staining are superior examinations. Serologic tests are of limited value, because they often yield false-negative results in immunocompromised patients. Blood cultures are positive in 50%-70% of cases, but they yield false-negative results in 20% of disseminated cases. Bone marrow cultures are positive in up to 75% of cases, and when combined with blood cultures, markedly increase the likelihood of positive cultures.\nBiopsy may be performed at any suspected site of involvement, such as the skin or oral mucosa, GI lesions, lymph nodes, adrenal glands, and liver. Chest radiographs are abnormal in 70% of cases, usually showing diffuse interstitial or reticulonodular infiltrates. CSF lymphocytic pleocytosis, protein elevation, and low glucose are suggestive of CNS involvement. Single or multiple enhancing lesions may also be seen on CT.[1,6,7]\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 21-Year-Old Woman With Persistent Fever and Malaise"
},
{
"authors": "Muralikrishna Gopalakrishnamoorthy, MBBS, PGY-1; Archana Bhaskaran, MBBS, PGY-1; Rajeswari Anaparthy, MBBS, PGY-1; Ted T. Lin, MS3; Syed Hasan, MBBS",
"content": [
"Intravenous amphotericin B is the drug of choice for the initial treatment of patients with disseminated histoplasmosis who are severely ill or immunosuppressed or whose infection involves the CNS. The conventional or the lipid formulation can be used, but the lipid formulation is preferred because of a lower incidence of adverse effects. This regimen can be changed to itraconazole (200 mg twice daily) once clinical improvement is evident, and the latter regimen can be used as the initial therapy in less severely ill patients.",
"Patients with AIDS whose disseminated histoplasmosis has responded to 10 weeks of therapy should receive itraconazole (200 mg/d) for life to prevent relapse. Lifelong maintenance therapy may not be necessary for HIV-infected patients who have received prolonged itraconazole treatment, who have had a sustained response to highly active antiretroviral therapy (HAART), and who no longer have detectable Histoplasma antigen in the serum.",
"If left untreated, disseminated histoplasmosis is progressive and can lead to death within a few weeks.[1,3,8,9]",
"After a sputum culture and an acid-fast bacilli smear were noted to be negative, the diagnosis in this patient was subsequently confirmed by an elevated urine Histoplasma antigen value of 42.5 U (upper limit of normal, 2 U), as well as blood cultures that were positive for H capsulatum. She was treated with a 10-day course of intravenous liposomal amphotericin at a dose of 4 mg/kg body weight (usual dose, 3-5 mg/kg body weight); after she showed signs of clinical improvement, therapy was switched to oral itraconazole at a dose of 200 mg twice a day for 1 year.",
"The patient was not started on antiretroviral therapy at presentation because of concern about immune reconstitution syndrome; however, HAART was initiated at the time of discharge. At the time of the writing of this case report, the patient is doing well on HAART therapy, with recovery of her CD4 count and adequate viral suppression."
],
"date": "September 30, 2015",
"figures": [],
"markdown": "# A 21-Year-Old Woman With Persistent Fever and Malaise\n\n **Authors:** Muralikrishna Gopalakrishnamoorthy, MBBS, PGY-1; Archana Bhaskaran, MBBS, PGY-1; Rajeswari Anaparthy, MBBS, PGY-1; Ted T. Lin, MS3; Syed Hasan, MBBS \n **Date:** September 30, 2015\n\n ## Content\n\n Intravenous amphotericin B is the drug of choice for the initial treatment of patients with disseminated histoplasmosis who are severely ill or immunosuppressed or whose infection involves the CNS. The conventional or the lipid formulation can be used, but the lipid formulation is preferred because of a lower incidence of adverse effects. This regimen can be changed to itraconazole (200 mg twice daily) once clinical improvement is evident, and the latter regimen can be used as the initial therapy in less severely ill patients.\nPatients with AIDS whose disseminated histoplasmosis has responded to 10 weeks of therapy should receive itraconazole (200 mg/d) for life to prevent relapse. Lifelong maintenance therapy may not be necessary for HIV-infected patients who have received prolonged itraconazole treatment, who have had a sustained response to highly active antiretroviral therapy (HAART), and who no longer have detectable Histoplasma antigen in the serum.\nIf left untreated, disseminated histoplasmosis is progressive and can lead to death within a few weeks.[1,3,8,9]\nAfter a sputum culture and an acid-fast bacilli smear were noted to be negative, the diagnosis in this patient was subsequently confirmed by an elevated urine Histoplasma antigen value of 42.5 U (upper limit of normal, 2 U), as well as blood cultures that were positive for H capsulatum. She was treated with a 10-day course of intravenous liposomal amphotericin at a dose of 4 mg/kg body weight (usual dose, 3-5 mg/kg body weight); after she showed signs of clinical improvement, therapy was switched to oral itraconazole at a dose of 200 mg twice a day for 1 year.\nThe patient was not started on antiretroviral therapy at presentation because of concern about immune reconstitution syndrome; however, HAART was initiated at the time of discharge. At the time of the writing of this case report, the patient is doing well on HAART therapy, with recovery of her CD4 count and adequate viral suppression.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 896549,
"choiceText": "Liposomal amphotericin B",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 896551,
"choiceText": "Fluconazole",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 896553,
"choiceText": "Itraconazole",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 896555,
"choiceText": "Griseofulvin",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 896557,
"choiceText": "Caspofungin",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Patients with AIDS whose disseminated histoplasmosis has responded to 10 weeks of therapy should receive itraconazole (200 mg/d) for life to prevent relapse.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 282541,
"questionText": "Which drug is used in maintenance therapy for histoplasmosis in patients with HIV/AIDS?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 896559,
"choiceText": "Serum <i>Histoplasma</i> antigen",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 896561,
"choiceText": "Bone marrow culture",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 896563,
"choiceText": "Blood culture",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 896565,
"choiceText": "CSF <i>Histoplasma</i> antigen",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 896567,
"choiceText": "Urine <i>Histoplasma</i> antigen",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The diagnosis of histoplasmosis is accomplished via antigen testing, because the <i>Histoplasma</i> antigen is present in the urine in 90% of cases and in the serum in 70% of cases.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 282543,
"questionText": "Which of these tests has the highest sensitivity for the diagnosis of histoplasmosis in patients with HIV/AIDS?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 21-Year-Old Woman With Persistent Fever and Malaise"
},
{
"authors": "Muralikrishna Gopalakrishnamoorthy, MBBS, PGY-1; Archana Bhaskaran, MBBS, PGY-1; Rajeswari Anaparthy, MBBS, PGY-1; Ted T. Lin, MS3; Syed Hasan, MBBS",
"content": [],
"date": "September 30, 2015",
"figures": [],
"markdown": "# A 21-Year-Old Woman With Persistent Fever and Malaise\n\n **Authors:** Muralikrishna Gopalakrishnamoorthy, MBBS, PGY-1; Archana Bhaskaran, MBBS, PGY-1; Rajeswari Anaparthy, MBBS, PGY-1; Ted T. Lin, MS3; Syed Hasan, MBBS \n **Date:** September 30, 2015\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 896549,
"choiceText": "Liposomal amphotericin B",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 896551,
"choiceText": "Fluconazole",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 896553,
"choiceText": "Itraconazole",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 896555,
"choiceText": "Griseofulvin",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 896557,
"choiceText": "Caspofungin",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Patients with AIDS whose disseminated histoplasmosis has responded to 10 weeks of therapy should receive itraconazole (200 mg/d) for life to prevent relapse.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 282541,
"questionText": "Which drug is used in maintenance therapy for histoplasmosis in patients with HIV/AIDS?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 896559,
"choiceText": "Serum <i>Histoplasma</i> antigen",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 896561,
"choiceText": "Bone marrow culture",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 896563,
"choiceText": "Blood culture",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 896565,
"choiceText": "CSF <i>Histoplasma</i> antigen",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 896567,
"choiceText": "Urine <i>Histoplasma</i> antigen",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The diagnosis of histoplasmosis is accomplished via antigen testing, because the <i>Histoplasma</i> antigen is present in the urine in 90% of cases and in the serum in 70% of cases.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 282543,
"questionText": "Which of these tests has the highest sensitivity for the diagnosis of histoplasmosis in patients with HIV/AIDS?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 21-Year-Old Woman With Persistent Fever and Malaise"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 896539,
"choiceText": "Tuberculosis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 896541,
"choiceText": "<i>Mycobacterium avium-intracellulare</i> (MAI)",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 896543,
"choiceText": "Histoplasmosis\r\n",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 896545,
"choiceText": "Cryptococcosis",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 896547,
"choiceText": "Lymphoma",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 282539,
"questionText": "What is the most likely infectious process this patient is experiencing?<br><br><i>\r\nHint: Note the markedly elevated LDH level.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 896549,
"choiceText": "Liposomal amphotericin B",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 896551,
"choiceText": "Fluconazole",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 896553,
"choiceText": "Itraconazole",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 896555,
"choiceText": "Griseofulvin",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 896557,
"choiceText": "Caspofungin",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Patients with AIDS whose disseminated histoplasmosis has responded to 10 weeks of therapy should receive itraconazole (200 mg/d) for life to prevent relapse.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 282541,
"questionText": "Which drug is used in maintenance therapy for histoplasmosis in patients with HIV/AIDS?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 896559,
"choiceText": "Serum <i>Histoplasma</i> antigen",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 896561,
"choiceText": "Bone marrow culture",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 896563,
"choiceText": "Blood culture",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 896565,
"choiceText": "CSF <i>Histoplasma</i> antigen",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 896567,
"choiceText": "Urine <i>Histoplasma</i> antigen",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The diagnosis of histoplasmosis is accomplished via antigen testing, because the <i>Histoplasma</i> antigen is present in the urine in 90% of cases and in the serum in 70% of cases.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 282543,
"questionText": "Which of these tests has the highest sensitivity for the diagnosis of histoplasmosis in patients with HIV/AIDS?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
850976 | /viewarticle/850976 | [
{
"authors": "Stephen Johnson, MD, MS; Michael Furman, MD, MS",
"content": [
"Editor's Note:\n\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.",
"A 57-year-old man presents to his primary care provider with gradually worsening lower-extremity pain and fatigue with ambulation. He is able to walk one block and then must sit down and rest owing to \"tiredness\" in his legs. He has intermittent low back pain, which rarely radiates into his left anterior thigh.",
"His medical history includes type 1 diabetes mellitus, hypertension, and chronic obstructive pulmonary disease. Current medications include insulin glargine (Lantus®), lisinopril (Zestril®), aspirin (Bayer®), and a fluticasone propionate inhaler (Flovent®). He smokes one pack of cigarettes per day, with a 20–pack-year history. He does not drink alcohol or use illicit drugs."
],
"date": "September 16, 2015",
"figures": [],
"markdown": "# A 57-Year-Old Man With Difficulty Walking\n\n **Authors:** Stephen Johnson, MD, MS; Michael Furman, MD, MS \n **Date:** September 16, 2015\n\n ## Content\n\n Editor's Note:\n\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.\nA 57-year-old man presents to his primary care provider with gradually worsening lower-extremity pain and fatigue with ambulation. He is able to walk one block and then must sit down and rest owing to \"tiredness\" in his legs. He has intermittent low back pain, which rarely radiates into his left anterior thigh.\nHis medical history includes type 1 diabetes mellitus, hypertension, and chronic obstructive pulmonary disease. Current medications include insulin glargine (Lantus®), lisinopril (Zestril®), aspirin (Bayer®), and a fluticasone propionate inhaler (Flovent®). He smokes one pack of cigarettes per day, with a 20–pack-year history. He does not drink alcohol or use illicit drugs.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 57-Year-Old Man With Difficulty Walking"
},
{
"authors": "Stephen Johnson, MD, MS; Michael Furman, MD, MS",
"content": [
"On physical examination, the patient appears well, with a blood pressure of 170/98 mm Hg, a pulse of 80 beats/min with regular rhythm, a respiration rate of 14 breaths/min, and a temperature of 97.8°F. The cardiovascular examination reveals diminished but palpable posterior tibial and dorsalis pedis pulses. The respiratory examination reveals decreased breath sounds but is otherwise normal.",
"The neurologic examination reveals 5 out of 5 strength throughout the bilateral lower extremities. Reflexes are 2+ at the bilateral patella and 1+ at the bilateral Achilles tendon. Sensation is diminished to light touch in a stocking distribution to the mid-calf bilaterally. The straight leg raise and seated slump test results are negative. The femoral nerve stretch test causes increased low back pain, with radiation to the left anterior thigh.",
"Figure 1.",
"Figure 1.",
"Figure 2.",
"Figure 2.",
"During the musculoskeletal examination, the patient stands with loss of lumbar lordosis and forward trunk lean. Tenderness is noted along the bilateral lumbar paraspinals and posterior superior iliac spines. Forward flexion at the lumbar spine is limited but does not increase pain. Lumbar extension is also limited and causes increased axial low back pain.",
"Gait evaluation reveals a wide based gait. He experiences a vague, \"tired\" feeling in his legs with short-distance ambulation in the exam room. This sensation resolves shortly after sitting down.",
"Figure 3.",
"Figure 3.",
"Figure 4.",
"Figure 4.",
"The ankle/brachial index (ABI) is 1.2 on the right and 1 on the left. Anteroposterior and lateral radiographs of the lumbar spine reveal mild to moderate degenerative scoliosis, loss of lumbar lordosis, disc height loss at all levels, and a grade 1 anterolisthesis of L2-L3. MRI of the lumbar spine reveals rotatory levoconvex scoliosis, advanced degenerative disc disease L2-3 through L5-S1, multilevel facet arthropathy, and severe central canal stenosis at L2-3 and L3-4 (Figures 1-4)."
],
"date": "September 16, 2015",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/850/976/850976-Thumb1.png"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/850/976/850976-Thumb2.png"
},
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/850/976/850976-Thumb3.png"
},
{
"caption": "Figure 4.",
"image_url": "https://img.medscapestatic.com/article/850/976/850976-Thumb4.png"
}
],
"markdown": "# A 57-Year-Old Man With Difficulty Walking\n\n **Authors:** Stephen Johnson, MD, MS; Michael Furman, MD, MS \n **Date:** September 16, 2015\n\n ## Content\n\n On physical examination, the patient appears well, with a blood pressure of 170/98 mm Hg, a pulse of 80 beats/min with regular rhythm, a respiration rate of 14 breaths/min, and a temperature of 97.8°F. The cardiovascular examination reveals diminished but palpable posterior tibial and dorsalis pedis pulses. The respiratory examination reveals decreased breath sounds but is otherwise normal.\nThe neurologic examination reveals 5 out of 5 strength throughout the bilateral lower extremities. Reflexes are 2+ at the bilateral patella and 1+ at the bilateral Achilles tendon. Sensation is diminished to light touch in a stocking distribution to the mid-calf bilaterally. The straight leg raise and seated slump test results are negative. The femoral nerve stretch test causes increased low back pain, with radiation to the left anterior thigh.\nFigure 1.\nFigure 1.\nFigure 2.\nFigure 2.\nDuring the musculoskeletal examination, the patient stands with loss of lumbar lordosis and forward trunk lean. Tenderness is noted along the bilateral lumbar paraspinals and posterior superior iliac spines. Forward flexion at the lumbar spine is limited but does not increase pain. Lumbar extension is also limited and causes increased axial low back pain.\nGait evaluation reveals a wide based gait. He experiences a vague, \"tired\" feeling in his legs with short-distance ambulation in the exam room. This sensation resolves shortly after sitting down.\nFigure 3.\nFigure 3.\nFigure 4.\nFigure 4.\nThe ankle/brachial index (ABI) is 1.2 on the right and 1 on the left. Anteroposterior and lateral radiographs of the lumbar spine reveal mild to moderate degenerative scoliosis, loss of lumbar lordosis, disc height loss at all levels, and a grade 1 anterolisthesis of L2-L3. MRI of the lumbar spine reveals rotatory levoconvex scoliosis, advanced degenerative disc disease L2-3 through L5-S1, multilevel facet arthropathy, and severe central canal stenosis at L2-3 and L3-4 (Figures 1-4).\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n**Figure 3.** \n \n\n**Figure 4.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 888701,
"choiceText": "Vascular claudication (VC)",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 888703,
"choiceText": "Lumbar compression fracture",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 888705,
"choiceText": "Deconditioning syndrome",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 888707,
"choiceText": "Neurogenic intermittent claudication (NIC)\r\n",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 279937,
"questionText": "\r\nOn the basis of the history, physical examination, and workup, what is the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 57-Year-Old Man With Difficulty Walking"
},
{
"authors": "Stephen Johnson, MD, MS; Michael Furman, MD, MS",
"content": [
"NIC was diagnosed on the basis of the clinical symptoms of low back pain and claudication (leg pain upon standing/walking), with relief of symptoms with recumbency. Central canal stenosis and subarticular and foraminal compromise were then confirmed with imaging.",
"The first step is distinguishing between the anatomical observation of lumbar spinal stenosis (LSS) and the clinical syndromes it may incite, including NIC or radiculopathy in the lumbar spine. LSS, particularly central canal stenosis, is a frequent finding in asymptomatic individuals and increases with age. Narrowing of the central canal, often combined with subarticular recess (lateral recess) and foraminal compromise, provide the anatomical potential for the development of the clinical syndrome called NIC.[1]",
"LSS can be congenital or acquired; often, the etiology is a combination of the two. Age-related changes contribute to canal narrowing, including facet hypertrophy, ligamentum flavum hypertrophy, vertebral body osteophytes, and intervertebral disk degeneration. Other, rare causes include epidural lipomatosis and ossification of the posterior longitudinal ligament. Congenital stenosis predisposes patients to NIC because mild degenerative changes take place as a normal part of aging, worsening LSS.",
"LSS and NIC is defined by the 2011 evidence-based practice guideline from the North American Spine Society as a condition in which the space for neural and vascular elements in the lumbar spine is diminished.[2] This is secondary to degenerative changes found in the spinal canal. Gluteal and lower-extremity pain or fatigue may occur, with or without back pain.",
"The most common symptoms reported by patients with LSS include (in order of decreasing prevalence) back pain, claudication, leg pain, weakness, and voiding dysfunction.[3] Physical examination findings are not specific, and a normal neurologic examination is most common."
],
"date": "September 16, 2015",
"figures": [],
"markdown": "# A 57-Year-Old Man With Difficulty Walking\n\n **Authors:** Stephen Johnson, MD, MS; Michael Furman, MD, MS \n **Date:** September 16, 2015\n\n ## Content\n\n NIC was diagnosed on the basis of the clinical symptoms of low back pain and claudication (leg pain upon standing/walking), with relief of symptoms with recumbency. Central canal stenosis and subarticular and foraminal compromise were then confirmed with imaging.\nThe first step is distinguishing between the anatomical observation of lumbar spinal stenosis (LSS) and the clinical syndromes it may incite, including NIC or radiculopathy in the lumbar spine. LSS, particularly central canal stenosis, is a frequent finding in asymptomatic individuals and increases with age. Narrowing of the central canal, often combined with subarticular recess (lateral recess) and foraminal compromise, provide the anatomical potential for the development of the clinical syndrome called NIC.[1]\nLSS can be congenital or acquired; often, the etiology is a combination of the two. Age-related changes contribute to canal narrowing, including facet hypertrophy, ligamentum flavum hypertrophy, vertebral body osteophytes, and intervertebral disk degeneration. Other, rare causes include epidural lipomatosis and ossification of the posterior longitudinal ligament. Congenital stenosis predisposes patients to NIC because mild degenerative changes take place as a normal part of aging, worsening LSS.\nLSS and NIC is defined by the 2011 evidence-based practice guideline from the North American Spine Society as a condition in which the space for neural and vascular elements in the lumbar spine is diminished.[2] This is secondary to degenerative changes found in the spinal canal. Gluteal and lower-extremity pain or fatigue may occur, with or without back pain.\nThe most common symptoms reported by patients with LSS include (in order of decreasing prevalence) back pain, claudication, leg pain, weakness, and voiding dysfunction.[3] Physical examination findings are not specific, and a normal neurologic examination is most common.\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 888701,
"choiceText": "Vascular claudication (VC)",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 888703,
"choiceText": "Lumbar compression fracture",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 888705,
"choiceText": "Deconditioning syndrome",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 888707,
"choiceText": "Neurogenic intermittent claudication (NIC)\r\n",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 279937,
"questionText": "\r\nOn the basis of the history, physical examination, and workup, what is the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 57-Year-Old Man With Difficulty Walking"
},
{
"authors": "Stephen Johnson, MD, MS; Michael Furman, MD, MS",
"content": [
"Despite the significant impact of NIC on the health of older adults, little epidemiologic literature is available. Kalichman and colleagues[4] assessed data from the Framingham study to determine the prevalence of congenital and acquired lumbar central canal stenosis in a community population. Using CT, they found congenital narrowing that resulted in relative stenosis (defined as central canal anterior/posterior dimension of 12 mm) in 4.7% of the population and absolute stenosis (defined as central canal anterior/posterior dimension of 10 mm) in 2.6% of the population. Acquired central canal stenosis that resulted in relative stenosis was seen in 22.5% of the population, and absolute stenosis was seen in 7.3% of the population. Acquired stenosis increased with age.",
"Approximately 5 persons per 1000 population older than 50 years have anatomical spinal stenosis. Extrapolating this information, approximately 250,000-500,000 Americans have symptoms related to spinal stenosis.",
"The pathophysiology of NIC remains controversial. Evidence suggests that venous congestion develops from multiple sites of neural compression. Venous congestion causes a breakdown of the blood/nerve barrier, probably resulting in an inflammatory reaction that causes neural dysfunction and irritability.[5]",
"NIC is a clinical diagnosis that can be supported by spinal imaging. The goal of spinal imaging is to localize the site of pathology, rule out competing diagnoses, and guide conservative or surgical treatments.",
"In 2011, the American College of Physicians released guidelines for the diagnostic imaging of low back pain. The guidelines recommend against routine diagnostic imaging for patients with low back pain. Routine imaging has not been shown to improve patient outcomes and may actually cause harm. Early imaging is only recommended for patients with \"red flag\" symptoms suggestive of cancer, spinal infection, cauda equina syndrome, or worsening neurologic weakness.[6]",
"When symptoms persist, the initial imaging modality is often plain radiography, with MRI considered the criterion standard for evaluation of LSS. When reading MRI findings, most radiologists perform a qualitative assessment of \"crowding\" of the neural elements within the central canal. Mild central canal stenosis is present when one third or less of the expected canal area is compromised; moderate central canal stenosis is present when one third to two thirds of the central canal is compromised; and severe central stenosis is present when more than two thirds of the expected canal area is compromised.[7]",
"Several imaging parameters have been proposed to evaluate central canal, subarticular, and foraminal compromise. No single parameter has proven satisfactory.",
"NIC must be differentiated from VC because the treatments are vastly different. VC is a result of peripheral vascular disease and arteriosclerosis. The symptoms of NIC are often worsened by lumbar extension, such as with standing and walking. Thus, NIC symptoms are rapidly improved with lumbar flexion, such as with sitting or walking uphill.",
"The prominent symptom of VC is leg pain brought on by physical activity. Symptoms often predictably start after a finite walking distance and dissipate more gradually with cessation of walking. In contrast to NIC, the leg pain associated with VC does not improve with uphill walking (lumbar flexion).",
"The diagnosis of VC can be confirmed with arteriography. Noninvasive diagnostic tests include the ABI, which compares the systolic blood pressure at the ankle with that of the arm.",
"ABI is classified as follows:",
">1.4: Arterial calcification",
"1-1.4: Normal",
"0.9-1: Acceptable",
"0.8-0.9: Mild arterial disease",
"0.5-0.8: Moderate arterial disease; and",
"<0.5: Severe arterial disease.",
"Patients with confirmed peripheral arterial disease should be referred to a vascular specialist for risk factor assessment and for potential invasive treatments.[8]"
],
"date": "September 16, 2015",
"figures": [],
"markdown": "# A 57-Year-Old Man With Difficulty Walking\n\n **Authors:** Stephen Johnson, MD, MS; Michael Furman, MD, MS \n **Date:** September 16, 2015\n\n ## Content\n\n Despite the significant impact of NIC on the health of older adults, little epidemiologic literature is available. Kalichman and colleagues[4] assessed data from the Framingham study to determine the prevalence of congenital and acquired lumbar central canal stenosis in a community population. Using CT, they found congenital narrowing that resulted in relative stenosis (defined as central canal anterior/posterior dimension of 12 mm) in 4.7% of the population and absolute stenosis (defined as central canal anterior/posterior dimension of 10 mm) in 2.6% of the population. Acquired central canal stenosis that resulted in relative stenosis was seen in 22.5% of the population, and absolute stenosis was seen in 7.3% of the population. Acquired stenosis increased with age.\nApproximately 5 persons per 1000 population older than 50 years have anatomical spinal stenosis. Extrapolating this information, approximately 250,000-500,000 Americans have symptoms related to spinal stenosis.\nThe pathophysiology of NIC remains controversial. Evidence suggests that venous congestion develops from multiple sites of neural compression. Venous congestion causes a breakdown of the blood/nerve barrier, probably resulting in an inflammatory reaction that causes neural dysfunction and irritability.[5]\nNIC is a clinical diagnosis that can be supported by spinal imaging. The goal of spinal imaging is to localize the site of pathology, rule out competing diagnoses, and guide conservative or surgical treatments.\nIn 2011, the American College of Physicians released guidelines for the diagnostic imaging of low back pain. The guidelines recommend against routine diagnostic imaging for patients with low back pain. Routine imaging has not been shown to improve patient outcomes and may actually cause harm. Early imaging is only recommended for patients with \"red flag\" symptoms suggestive of cancer, spinal infection, cauda equina syndrome, or worsening neurologic weakness.[6]\nWhen symptoms persist, the initial imaging modality is often plain radiography, with MRI considered the criterion standard for evaluation of LSS. When reading MRI findings, most radiologists perform a qualitative assessment of \"crowding\" of the neural elements within the central canal. Mild central canal stenosis is present when one third or less of the expected canal area is compromised; moderate central canal stenosis is present when one third to two thirds of the central canal is compromised; and severe central stenosis is present when more than two thirds of the expected canal area is compromised.[7]\nSeveral imaging parameters have been proposed to evaluate central canal, subarticular, and foraminal compromise. No single parameter has proven satisfactory.\nNIC must be differentiated from VC because the treatments are vastly different. VC is a result of peripheral vascular disease and arteriosclerosis. The symptoms of NIC are often worsened by lumbar extension, such as with standing and walking. Thus, NIC symptoms are rapidly improved with lumbar flexion, such as with sitting or walking uphill.\nThe prominent symptom of VC is leg pain brought on by physical activity. Symptoms often predictably start after a finite walking distance and dissipate more gradually with cessation of walking. In contrast to NIC, the leg pain associated with VC does not improve with uphill walking (lumbar flexion).\nThe diagnosis of VC can be confirmed with arteriography. Noninvasive diagnostic tests include the ABI, which compares the systolic blood pressure at the ankle with that of the arm.\nABI is classified as follows:\n>1.4: Arterial calcification\n1-1.4: Normal\n0.9-1: Acceptable\n0.8-0.9: Mild arterial disease\n0.5-0.8: Moderate arterial disease; and\n<0.5: Severe arterial disease.\nPatients with confirmed peripheral arterial disease should be referred to a vascular specialist for risk factor assessment and for potential invasive treatments.[8]\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 57-Year-Old Man With Difficulty Walking"
},
{
"authors": "Stephen Johnson, MD, MS; Michael Furman, MD, MS",
"content": [
"Deconditioning is also a consideration in patients with NIC. Exercise limitations due to dyspnea are common in patients with chronic obstructive pulmonary disease. The Moser classification is often used to describe functional pulmonary disability.[9] The classification is as follows:",
"Class 1: Normal at rest, dyspnea with strenuous exercise;",
"Class 2: Normal activities of daily life (ADL), dyspnea with stairs and inclines;",
"Class 3: Dyspnea with ADL, able to walk one block at a slow pace;",
"Class 4: Dependent with ADL, dyspnea with minimal exertion; and",
"Class 5: Housebound, dyspnea at rest.",
"Patients in classes 1-4 have no dyspnea at rest.",
"In the patient in this case, the combination of lower-extremity pain and fatigue and low back pain that worsened with walking and was relieved by sitting was most consistent with a diagnosis of NIC. A normal ABI and severe LSS on MRI confirmed the diagnosis.",
"The natural history of LSS is not well understood. Most cases show slow progression, but improvement of symptoms is expected in a significant percentage of patients without intervention.",
"The most concerning complication of LSS is cauda equina syndrome. This is rare even in persons with severe stenosis. Affected individuals often have an acute change in canal diameter secondary to a herniated nucleus pulposus. Gradual development of severe LSS often does not lead to cauda equina syndrome. Symptoms suggestive of cauda equina syndrome include flaccid lower-extremity paralysis, saddle anesthesia, urine retention, and urinary and fecal incontinence.",
"Treatment options for NIC due to LSS are conservative or surgical. Conservative care includes activity modification, physical therapy, nonsteroidal anti-inflammatory drugs, analgesics, antispasmodics, and epidural steroid injections (ESIs). However, a randomized controlled trial of ESIs and LSS has called into question the use of ESIs for the treatment of NIC.[10]",
"Surgical decompression is indicated in the setting of debilitating pain that is unresponsive to conservative care, worsening neurologic deficits, and cauda equina syndrome. Concomitant stabilization is necessary for patients with segmental instability (eg, spondylolisthesis).",
"The patient in this case initially responded well to conservative measures, including physical therapy, oral anti-inflammatory medications, and ESIs. He had complete resolution of his left anterior thigh pain and decreased lower-extremity fatigue for several months after a left sided L3 and L4 transforaminal ESI.",
"Unfortunately, the patient's symptoms recurred and worsened to the point of being unable to stand for longer than a few minutes, with walking tolerance of less than half a block. He underwent successful lumbar decompression at L2-3 and L3-4 with posterior spinal instrumentation and fusion. His walking tolerance improved, and his left lower-extremity radicular pain has not recurred."
],
"date": "September 16, 2015",
"figures": [],
"markdown": "# A 57-Year-Old Man With Difficulty Walking\n\n **Authors:** Stephen Johnson, MD, MS; Michael Furman, MD, MS \n **Date:** September 16, 2015\n\n ## Content\n\n Deconditioning is also a consideration in patients with NIC. Exercise limitations due to dyspnea are common in patients with chronic obstructive pulmonary disease. The Moser classification is often used to describe functional pulmonary disability.[9] The classification is as follows:\nClass 1: Normal at rest, dyspnea with strenuous exercise;\nClass 2: Normal activities of daily life (ADL), dyspnea with stairs and inclines;\nClass 3: Dyspnea with ADL, able to walk one block at a slow pace;\nClass 4: Dependent with ADL, dyspnea with minimal exertion; and\nClass 5: Housebound, dyspnea at rest.\nPatients in classes 1-4 have no dyspnea at rest.\nIn the patient in this case, the combination of lower-extremity pain and fatigue and low back pain that worsened with walking and was relieved by sitting was most consistent with a diagnosis of NIC. A normal ABI and severe LSS on MRI confirmed the diagnosis.\nThe natural history of LSS is not well understood. Most cases show slow progression, but improvement of symptoms is expected in a significant percentage of patients without intervention.\nThe most concerning complication of LSS is cauda equina syndrome. This is rare even in persons with severe stenosis. Affected individuals often have an acute change in canal diameter secondary to a herniated nucleus pulposus. Gradual development of severe LSS often does not lead to cauda equina syndrome. Symptoms suggestive of cauda equina syndrome include flaccid lower-extremity paralysis, saddle anesthesia, urine retention, and urinary and fecal incontinence.\nTreatment options for NIC due to LSS are conservative or surgical. Conservative care includes activity modification, physical therapy, nonsteroidal anti-inflammatory drugs, analgesics, antispasmodics, and epidural steroid injections (ESIs). However, a randomized controlled trial of ESIs and LSS has called into question the use of ESIs for the treatment of NIC.[10]\nSurgical decompression is indicated in the setting of debilitating pain that is unresponsive to conservative care, worsening neurologic deficits, and cauda equina syndrome. Concomitant stabilization is necessary for patients with segmental instability (eg, spondylolisthesis).\nThe patient in this case initially responded well to conservative measures, including physical therapy, oral anti-inflammatory medications, and ESIs. He had complete resolution of his left anterior thigh pain and decreased lower-extremity fatigue for several months after a left sided L3 and L4 transforaminal ESI.\nUnfortunately, the patient's symptoms recurred and worsened to the point of being unable to stand for longer than a few minutes, with walking tolerance of less than half a block. He underwent successful lumbar decompression at L2-3 and L3-4 with posterior spinal instrumentation and fusion. His walking tolerance improved, and his left lower-extremity radicular pain has not recurred.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 888709,
"choiceText": "Central canal anterior/posterior dimension of 12 mm",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 888711,
"choiceText": "Lower-extremity pain and/or fatigue that is worsened by walking and improved by sitting",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 888713,
"choiceText": "Central canal anterior/posterior dimension of 10 mm",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 888715,
"choiceText": "Central canal compromise in more than two thirds of the expected canal area\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "LSS is an anatomical observation. Central canal measurements are used to define the degree of anatomical stenosis. In the lumbar spine, LSS may result in clinical syndromes, including NIC and radiculopathy. NIC is a clinical syndrome in which lower-extremity pain and/or fatigue is worsened by walking and improved by sitting.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 279939,
"questionText": "Which of the following is most suggestive of a diagnosis of NIC?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 888717,
"choiceText": "No improvement in symptoms",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 888719,
"choiceText": "Complete resolution of low back pain ",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 888721,
"choiceText": "Short-term improvement in walking tolerance as a result of decreased radicular pain from inflamed lumbosacral nerve roots",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 888723,
"choiceText": "Complete resolution of lower-extremity pain and/or fatigue\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "\r\nESIs for the treatment of NIC are controversial. Studies have shown conflicting results. ESIs are effective in the short term at reducing lower-extremity radicular pain in patients with NIC. This may allow full participation in a physical therapy program that may otherwise not be possible owing to pain that limits patient involvement. Anecdotally, in the authors' practice, ESIs used for the treatment of NIC often result in improved function, decreased radicular pain, and improved quality of life for months at a time. ",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 279941,
"questionText": "In the treatment of NIC as a result of central canal stenosis, what outcome is most likely after an ESI?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 57-Year-Old Man With Difficulty Walking"
},
{
"authors": "Stephen Johnson, MD, MS; Michael Furman, MD, MS",
"content": [],
"date": "September 16, 2015",
"figures": [],
"markdown": "# A 57-Year-Old Man With Difficulty Walking\n\n **Authors:** Stephen Johnson, MD, MS; Michael Furman, MD, MS \n **Date:** September 16, 2015\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 888709,
"choiceText": "Central canal anterior/posterior dimension of 12 mm",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 888711,
"choiceText": "Lower-extremity pain and/or fatigue that is worsened by walking and improved by sitting",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 888713,
"choiceText": "Central canal anterior/posterior dimension of 10 mm",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 888715,
"choiceText": "Central canal compromise in more than two thirds of the expected canal area\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "LSS is an anatomical observation. Central canal measurements are used to define the degree of anatomical stenosis. In the lumbar spine, LSS may result in clinical syndromes, including NIC and radiculopathy. NIC is a clinical syndrome in which lower-extremity pain and/or fatigue is worsened by walking and improved by sitting.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 279939,
"questionText": "Which of the following is most suggestive of a diagnosis of NIC?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 888717,
"choiceText": "No improvement in symptoms",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 888719,
"choiceText": "Complete resolution of low back pain ",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 888721,
"choiceText": "Short-term improvement in walking tolerance as a result of decreased radicular pain from inflamed lumbosacral nerve roots",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 888723,
"choiceText": "Complete resolution of lower-extremity pain and/or fatigue\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "\r\nESIs for the treatment of NIC are controversial. Studies have shown conflicting results. ESIs are effective in the short term at reducing lower-extremity radicular pain in patients with NIC. This may allow full participation in a physical therapy program that may otherwise not be possible owing to pain that limits patient involvement. Anecdotally, in the authors' practice, ESIs used for the treatment of NIC often result in improved function, decreased radicular pain, and improved quality of life for months at a time. ",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 279941,
"questionText": "In the treatment of NIC as a result of central canal stenosis, what outcome is most likely after an ESI?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 57-Year-Old Man With Difficulty Walking"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 888701,
"choiceText": "Vascular claudication (VC)",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 888703,
"choiceText": "Lumbar compression fracture",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 888705,
"choiceText": "Deconditioning syndrome",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 888707,
"choiceText": "Neurogenic intermittent claudication (NIC)\r\n",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 279937,
"questionText": "\r\nOn the basis of the history, physical examination, and workup, what is the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 888709,
"choiceText": "Central canal anterior/posterior dimension of 12 mm",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 888711,
"choiceText": "Lower-extremity pain and/or fatigue that is worsened by walking and improved by sitting",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 888713,
"choiceText": "Central canal anterior/posterior dimension of 10 mm",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 888715,
"choiceText": "Central canal compromise in more than two thirds of the expected canal area\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "LSS is an anatomical observation. Central canal measurements are used to define the degree of anatomical stenosis. In the lumbar spine, LSS may result in clinical syndromes, including NIC and radiculopathy. NIC is a clinical syndrome in which lower-extremity pain and/or fatigue is worsened by walking and improved by sitting.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 279939,
"questionText": "Which of the following is most suggestive of a diagnosis of NIC?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 888717,
"choiceText": "No improvement in symptoms",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 888719,
"choiceText": "Complete resolution of low back pain ",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 888721,
"choiceText": "Short-term improvement in walking tolerance as a result of decreased radicular pain from inflamed lumbosacral nerve roots",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 888723,
"choiceText": "Complete resolution of lower-extremity pain and/or fatigue\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "\r\nESIs for the treatment of NIC are controversial. Studies have shown conflicting results. ESIs are effective in the short term at reducing lower-extremity radicular pain in patients with NIC. This may allow full participation in a physical therapy program that may otherwise not be possible owing to pain that limits patient involvement. Anecdotally, in the authors' practice, ESIs used for the treatment of NIC often result in improved function, decreased radicular pain, and improved quality of life for months at a time. ",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 279941,
"questionText": "In the treatment of NIC as a result of central canal stenosis, what outcome is most likely after an ESI?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
850959 | /viewarticle/850959 | [
{
"authors": "Fahad M. Iqbal, MD; Preeti Singh, MD; Jayanth Koneru, MD; Vivekananda Pattabiraman, MD",
"content": [
"Editor's Note:\n\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.",
"A 72-year-old man with a medical history significant for chronic obstructive pulmonary disease (COPD), type 2 diabetes mellitus, hypertension, atrial fibrillation, and prior ischemic stroke is admitted for a 1-day history of worsening abdominal pain. The pain started suddenly, is constant and diffuse, and is described by the patient as 7 out of 10 in intensity. It is not associated with oral intake. The pain is not relieved by over-the-counter pain relievers and is not exacerbated with movement.",
"The patient felt some nausea and has had multiple episodes of bilious emesis, but he has not had any hematemesis, hematochezia, diarrhea, or melena. He denies having fever, chills, or night sweats, as well as any history of recent abdominal trauma, weight changes, or surgery. He is currently taking metformin (Glumetza®), amiodarone (Cordarone®), baby aspirin (Bayer®), extended-release metoprolol (Toprol XL®), and amlodipine (Norvasc®).",
"The patient has no allergies or family history of coronary artery disease or cancer. He drinks socially and denies any illicit drug use. He has an approximately 80 pack-year smoking history."
],
"date": "September 15, 2015",
"figures": [],
"markdown": "# A 72-Year-Old Man With Abdominal Pain\n\n **Authors:** Fahad M. Iqbal, MD; Preeti Singh, MD; Jayanth Koneru, MD; Vivekananda Pattabiraman, MD \n **Date:** September 15, 2015\n\n ## Content\n\n Editor's Note:\n\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.\nA 72-year-old man with a medical history significant for chronic obstructive pulmonary disease (COPD), type 2 diabetes mellitus, hypertension, atrial fibrillation, and prior ischemic stroke is admitted for a 1-day history of worsening abdominal pain. The pain started suddenly, is constant and diffuse, and is described by the patient as 7 out of 10 in intensity. It is not associated with oral intake. The pain is not relieved by over-the-counter pain relievers and is not exacerbated with movement.\nThe patient felt some nausea and has had multiple episodes of bilious emesis, but he has not had any hematemesis, hematochezia, diarrhea, or melena. He denies having fever, chills, or night sweats, as well as any history of recent abdominal trauma, weight changes, or surgery. He is currently taking metformin (Glumetza®), amiodarone (Cordarone®), baby aspirin (Bayer®), extended-release metoprolol (Toprol XL®), and amlodipine (Norvasc®).\nThe patient has no allergies or family history of coronary artery disease or cancer. He drinks socially and denies any illicit drug use. He has an approximately 80 pack-year smoking history.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 72-Year-Old Man With Abdominal Pain"
},
{
"authors": "Fahad M. Iqbal, MD; Preeti Singh, MD; Jayanth Koneru, MD; Vivekananda Pattabiraman, MD",
"content": [
"On physical examination, the patient's oral temperature is 99.8°F. His pulse is irregular, with a rate of 95 beats/min and no audible murmurs. His blood pressure is 168/95 mm Hg. He is a moderately obese, barrel-chested man in moderate distress secondary to his generalized abdominal discomfort.",
"The head and neck examination is normal. No scleral or sublingual icterus is observed. The patient's lungs are clear to auscultation, and normal respiratory effort is noted. His abdomen is soft and exhibits only diffuse mild tenderness on deep palpation. No organomegaly, rebound, guarding, or rigidity is noted. The rectal examination reveals normal tone and brown, guaiac-positive stool. The remainder of the physical examination is otherwise unremarkable.",
"Figure 1.",
"Figure 1.",
"Figure 2.",
"Figure 2.",
"The white blood cell (WBC) count is elevated, at 16.8 × 103 cells/µL (normal range, 4.2-11.0 × 103 cells/µL), with bandemia of 15%. The remainder of the laboratory analysis—including the basic metabolic panel, amylase, lipase, hemoglobin, hematocrit, lactic acid, coagulation profile, thyroid-stimulating hormone, urinalysis, and chest radiography—is normal. Initial blood and urine cultures are negative.",
"CT of the abdomen and pelvis using oral and intravenous contrast is performed (Figures 1 and 2)."
],
"date": "September 15, 2015",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/850/959/850959-Thumb1.jpg"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/850/959/850959_thumb2.jpg"
}
],
"markdown": "# A 72-Year-Old Man With Abdominal Pain\n\n **Authors:** Fahad M. Iqbal, MD; Preeti Singh, MD; Jayanth Koneru, MD; Vivekananda Pattabiraman, MD \n **Date:** September 15, 2015\n\n ## Content\n\n On physical examination, the patient's oral temperature is 99.8°F. His pulse is irregular, with a rate of 95 beats/min and no audible murmurs. His blood pressure is 168/95 mm Hg. He is a moderately obese, barrel-chested man in moderate distress secondary to his generalized abdominal discomfort.\nThe head and neck examination is normal. No scleral or sublingual icterus is observed. The patient's lungs are clear to auscultation, and normal respiratory effort is noted. His abdomen is soft and exhibits only diffuse mild tenderness on deep palpation. No organomegaly, rebound, guarding, or rigidity is noted. The rectal examination reveals normal tone and brown, guaiac-positive stool. The remainder of the physical examination is otherwise unremarkable.\nFigure 1.\nFigure 1.\nFigure 2.\nFigure 2.\nThe white blood cell (WBC) count is elevated, at 16.8 × 103 cells/µL (normal range, 4.2-11.0 × 103 cells/µL), with bandemia of 15%. The remainder of the laboratory analysis—including the basic metabolic panel, amylase, lipase, hemoglobin, hematocrit, lactic acid, coagulation profile, thyroid-stimulating hormone, urinalysis, and chest radiography—is normal. Initial blood and urine cultures are negative.\nCT of the abdomen and pelvis using oral and intravenous contrast is performed (Figures 1 and 2).\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 888543,
"choiceText": "Ruptured abdominal aortic aneurysm",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 888545,
"choiceText": "Bowel obstruction",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 888547,
"choiceText": "Pneumatosis intestinalis ",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 888549,
"choiceText": "Pancreatitis\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 279879,
"questionText": "On the basis of the history, physical examination, and work-up, what is the diagnosis?\r\n<br><br><i>\r\nHint: Recognition of this often benign condition can help avoid an unnecessary exploratory laparotomy.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 72-Year-Old Man With Abdominal Pain"
},
{
"authors": "Fahad M. Iqbal, MD; Preeti Singh, MD; Jayanth Koneru, MD; Vivekananda Pattabiraman, MD",
"content": [
"Multiple submucosal, air-filled cysts were visible on the CT scan of the abdomen and pelvis, as well as areas with extraluminal air both in the bowel and the liver that were consistent with a diagnosis of pneumatosis intestinalis (PI) with hepatic portal venous gas (HPVG). PI is the presence of gas-filled cysts localized in the submucosa and/or subserosa of the alimentary tract.[1] The cysts may rupture and cause pneumoperitoneum. Pneumoperitoneum requires emergency laparotomy in 90% of cases, although many causes of free intraperitoneal air (including PI) can be managed conservatively.[2] The extent of the PI-involved bowel does not necessarily correlate with its cause.",
"PI presentations can be grouped into two broad but overlapping categories: an incidental finding on imaging, or in association with an abdominal emergency. Modern imaging techniques have increased the frequency of the diagnosis of PI, which was previously believed to be quite rare. PI occurs most frequently in the small intestine. In 42% of cases, PI affects only the small intestine, whereas the colon is affected in 36% of cases and both the small and large intestine are affected in 22% of cases.[1,2,3,4,5,6]",
"PI may be a sequela of numerous clinical conditions and does not represent an independent disease process. It can be associated with free intraperitoneal, retroperitoneal, or portal venous air, even in the absence of bowel perforation.[7] PI requires close monitoring and frequent assessment of the patient because surgery is not always appropriate.",
"Until recently, PI was a rare diagnosis, with an incidence of approximately 0.03% (based on an autopsy series).[8,9,10] However, it is now believed to be much more common, with studies showing as many as 1000 cases at a single institution.[3] This increased incidence is probably a consequence of the higher sensitivity of modern imaging modalities and their availability and use.",
"The preferred imaging technique for diagnosing PI is CT of the abdomen and pelvis. Oral contrast may be beneficial but is not essential. Lung windows are helpful in visualizing PI, especially in the colon.[10,11] CT may also be useful in detecting other associated findings, such as bowel-wall thickening, mesenteric edema, stratification, mural thrombi, hemorrhage, and bowel dilatation, which will dictate the appropriateness and urgency of surgery.[6]",
"Although PI has characteristic features on both plain radiography (air bubbles or curvilinear/circumferential areas of lucency tracking along the bowel wall and portal venous air) and ultrasonography (intramural echogenic foci with ring-down or dirty shadowing, and portal venous air seen as mobile echogenic foci causing a classic spike or ping on Doppler evaluation), neither of these is an optimal diagnostic modality.",
"The exact prevalence of PI is unknown. PI typically presents in the fifth to eighth decade of life and is three to four times more common in men. It is divided into primary (idiopathic) and secondary types. Primary PI (15% of cases) is a generally benign idiopathic condition that has a higher rate of recurrence than secondary PI. The secondary form (85% of cases) is associated with numerous pathologic processes and can be either benign or life-threatening.[4,12] Mortality rates as high as 75% have been reported when PI is associated with portal gas or diseases that lead to bowel necrosis or perforation.[13,14,15]"
],
"date": "September 15, 2015",
"figures": [],
"markdown": "# A 72-Year-Old Man With Abdominal Pain\n\n **Authors:** Fahad M. Iqbal, MD; Preeti Singh, MD; Jayanth Koneru, MD; Vivekananda Pattabiraman, MD \n **Date:** September 15, 2015\n\n ## Content\n\n Multiple submucosal, air-filled cysts were visible on the CT scan of the abdomen and pelvis, as well as areas with extraluminal air both in the bowel and the liver that were consistent with a diagnosis of pneumatosis intestinalis (PI) with hepatic portal venous gas (HPVG). PI is the presence of gas-filled cysts localized in the submucosa and/or subserosa of the alimentary tract.[1] The cysts may rupture and cause pneumoperitoneum. Pneumoperitoneum requires emergency laparotomy in 90% of cases, although many causes of free intraperitoneal air (including PI) can be managed conservatively.[2] The extent of the PI-involved bowel does not necessarily correlate with its cause.\nPI presentations can be grouped into two broad but overlapping categories: an incidental finding on imaging, or in association with an abdominal emergency. Modern imaging techniques have increased the frequency of the diagnosis of PI, which was previously believed to be quite rare. PI occurs most frequently in the small intestine. In 42% of cases, PI affects only the small intestine, whereas the colon is affected in 36% of cases and both the small and large intestine are affected in 22% of cases.[1,2,3,4,5,6]\nPI may be a sequela of numerous clinical conditions and does not represent an independent disease process. It can be associated with free intraperitoneal, retroperitoneal, or portal venous air, even in the absence of bowel perforation.[7] PI requires close monitoring and frequent assessment of the patient because surgery is not always appropriate.\nUntil recently, PI was a rare diagnosis, with an incidence of approximately 0.03% (based on an autopsy series).[8,9,10] However, it is now believed to be much more common, with studies showing as many as 1000 cases at a single institution.[3] This increased incidence is probably a consequence of the higher sensitivity of modern imaging modalities and their availability and use.\nThe preferred imaging technique for diagnosing PI is CT of the abdomen and pelvis. Oral contrast may be beneficial but is not essential. Lung windows are helpful in visualizing PI, especially in the colon.[10,11] CT may also be useful in detecting other associated findings, such as bowel-wall thickening, mesenteric edema, stratification, mural thrombi, hemorrhage, and bowel dilatation, which will dictate the appropriateness and urgency of surgery.[6]\nAlthough PI has characteristic features on both plain radiography (air bubbles or curvilinear/circumferential areas of lucency tracking along the bowel wall and portal venous air) and ultrasonography (intramural echogenic foci with ring-down or dirty shadowing, and portal venous air seen as mobile echogenic foci causing a classic spike or ping on Doppler evaluation), neither of these is an optimal diagnostic modality.\nThe exact prevalence of PI is unknown. PI typically presents in the fifth to eighth decade of life and is three to four times more common in men. It is divided into primary (idiopathic) and secondary types. Primary PI (15% of cases) is a generally benign idiopathic condition that has a higher rate of recurrence than secondary PI. The secondary form (85% of cases) is associated with numerous pathologic processes and can be either benign or life-threatening.[4,12] Mortality rates as high as 75% have been reported when PI is associated with portal gas or diseases that lead to bowel necrosis or perforation.[13,14,15]\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 888543,
"choiceText": "Ruptured abdominal aortic aneurysm",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 888545,
"choiceText": "Bowel obstruction",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 888547,
"choiceText": "Pneumatosis intestinalis ",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 888549,
"choiceText": "Pancreatitis\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 279879,
"questionText": "On the basis of the history, physical examination, and work-up, what is the diagnosis?\r\n<br><br><i>\r\nHint: Recognition of this often benign condition can help avoid an unnecessary exploratory laparotomy.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 72-Year-Old Man With Abdominal Pain"
},
{
"authors": "Fahad M. Iqbal, MD; Preeti Singh, MD; Jayanth Koneru, MD; Vivekananda Pattabiraman, MD",
"content": [
"PI is most often mistaken for bowel perforation, appendicitis, diverticulitis, or pancreatitis, depending on the severity of the pain and the location of the involved bowel wall. Subserosal cysts further complicate the diagnosis because they are particularly liable to rupture, making it more difficult to distinguish PI from bowel perforation.[16,17] As such, clinical signs and symptoms, in addition to laboratory data and imaging (ultrasonography, radiography, and CT) must be used to assist in diagnostic and treatment decision-making.",
"Multiple theories have been advanced to explain the pathogenesis of PI. These can be categorized as either mechanical or bacterial theories. The mechanical theory proposes that the dissection of gas through the bowel wall occurs via luminal mucosal breaks, via serosal surfaces by tracking along mesenteric blood vessels, or via increased intraluminal pressure.[6,18,19]",
"The mechanical theory has become popular for several reasons. First, PI has been reproduced experimentally by insufflating air into an excised colonic segment in which mucosal incisions have been made. Second, it accounts for the association of PI with conditions that disrupt mucosal integrity (ie, necrotizing enterocolitis, intestinal ischemia, caustic ingestions, inflammatory bowel disease, intestinal infections, intestinal edema, acute and chronic constipation, and numerous other conditions).[20]",
"Third, the mechanical theory offers an explanation for the association of PI with obstructive pulmonary disease. In these patients, coughing may cause alveolar rupture and air may subsequently track along blood vessels into the mediastinum, through the diaphragm, and ultimately to the mesenteric root. Once air has gained access to the mesenteric root, it may course along small mesenteric blood vessels that ultimately penetrate through the bowel wall.",
"Multiple arguments have been made against the mechanical theory. The argument against tracking of air from the lungs into the bowel wall is the absence of a \"gas trail\" from the mediastinum to the intestine in vivo. Examination of multiple patients has found that the cysts contain a mixture of hydrogen, nitrogen, carbon monoxide, and carbon dioxide, a combination of gases that is more consistent with bacterial metabolism than with alveolar air.[4,21,22,23] The bacterial theory proposes that gas-forming bacteria cause PI by either breaching the colonic mucosa directly or by producing excessive gas through fermentation of intraluminal substrates, leading to an increase in colonic pressure and forcing gas through the intestinal mucosa.",
"The bacterial theory has adherents for several reasons. First, PI has been reproduced experimentally by injecting gas-producing bacteria into the bowel wall of mice. Second, PI has been associated with the use of alpha-glucosidase inhibitors, which increase intestinal gas. Third, PI improves rapidly when patients are on elemental diets. Finally, PI treated with antibiotics tends to resolve more quickly.[6,19,21]",
"The argument against the bacterial theory is that the cysts are often sterile and if they rupture, the resulting pneumoperitoneum often follows a benign course without development of peritonitis, unless associated with necrosis or infarction. Furthermore, the hydrogen content of PI cysts has been reported to be as high as 50%, much higher than the 14% found in bowel gas.[8,21,24,25]",
"Despite the debate regarding the etiologies of PI, intraluminal gas alone is clearly insufficient to explain PI.[4] Both theories require the presence of some amount of mucosal damage, whether iatrogenic, from increased intraluminal pressure, or directly from bacterial cytotoxicity. The pathophysiology of PI probably involves some element of both theories and some preexisting mucosal damage. Therefore, one patient could have PI secondary to mesenteric ischemia, whereas another could have colonic flora changes and increased intraluminal pressure from chronic constipation.",
"PI is challenging because it is often an inconsequential finding and is life-threatening in only a minority of patients, although it may be associated with life-threatening diagnoses. Because PI is found primarily on imaging, a correlation must be made with a patient's clinical history, physical examination, and laboratory tests, because this more comprehensive analysis is the best indicator of clinical course.",
"A review of multiple studies and case series indicate that patients with a history and physical findings suggestive of peritonitis, pH < 7.3, WBC count > 12.0 × 103 cells/µL, age > 60 years, lactic acidosis (lactate level > 18.02 mg/dL), metabolic acidosis (serum bicarbonate level < 20 mEq/L), an elevated serum amylase level, frank rectal bleeding, or the presence of HPVG were most likely to require surgical intervention.[1,6,8,10,13,18,26,27,28] Furthermore, patients with PI who also had HPVG, sepsis, or lactic acidosis had the highest risk for death, approaching 80%.[13]"
],
"date": "September 15, 2015",
"figures": [],
"markdown": "# A 72-Year-Old Man With Abdominal Pain\n\n **Authors:** Fahad M. Iqbal, MD; Preeti Singh, MD; Jayanth Koneru, MD; Vivekananda Pattabiraman, MD \n **Date:** September 15, 2015\n\n ## Content\n\n PI is most often mistaken for bowel perforation, appendicitis, diverticulitis, or pancreatitis, depending on the severity of the pain and the location of the involved bowel wall. Subserosal cysts further complicate the diagnosis because they are particularly liable to rupture, making it more difficult to distinguish PI from bowel perforation.[16,17] As such, clinical signs and symptoms, in addition to laboratory data and imaging (ultrasonography, radiography, and CT) must be used to assist in diagnostic and treatment decision-making.\nMultiple theories have been advanced to explain the pathogenesis of PI. These can be categorized as either mechanical or bacterial theories. The mechanical theory proposes that the dissection of gas through the bowel wall occurs via luminal mucosal breaks, via serosal surfaces by tracking along mesenteric blood vessels, or via increased intraluminal pressure.[6,18,19]\nThe mechanical theory has become popular for several reasons. First, PI has been reproduced experimentally by insufflating air into an excised colonic segment in which mucosal incisions have been made. Second, it accounts for the association of PI with conditions that disrupt mucosal integrity (ie, necrotizing enterocolitis, intestinal ischemia, caustic ingestions, inflammatory bowel disease, intestinal infections, intestinal edema, acute and chronic constipation, and numerous other conditions).[20]\nThird, the mechanical theory offers an explanation for the association of PI with obstructive pulmonary disease. In these patients, coughing may cause alveolar rupture and air may subsequently track along blood vessels into the mediastinum, through the diaphragm, and ultimately to the mesenteric root. Once air has gained access to the mesenteric root, it may course along small mesenteric blood vessels that ultimately penetrate through the bowel wall.\nMultiple arguments have been made against the mechanical theory. The argument against tracking of air from the lungs into the bowel wall is the absence of a \"gas trail\" from the mediastinum to the intestine in vivo. Examination of multiple patients has found that the cysts contain a mixture of hydrogen, nitrogen, carbon monoxide, and carbon dioxide, a combination of gases that is more consistent with bacterial metabolism than with alveolar air.[4,21,22,23] The bacterial theory proposes that gas-forming bacteria cause PI by either breaching the colonic mucosa directly or by producing excessive gas through fermentation of intraluminal substrates, leading to an increase in colonic pressure and forcing gas through the intestinal mucosa.\nThe bacterial theory has adherents for several reasons. First, PI has been reproduced experimentally by injecting gas-producing bacteria into the bowel wall of mice. Second, PI has been associated with the use of alpha-glucosidase inhibitors, which increase intestinal gas. Third, PI improves rapidly when patients are on elemental diets. Finally, PI treated with antibiotics tends to resolve more quickly.[6,19,21]\nThe argument against the bacterial theory is that the cysts are often sterile and if they rupture, the resulting pneumoperitoneum often follows a benign course without development of peritonitis, unless associated with necrosis or infarction. Furthermore, the hydrogen content of PI cysts has been reported to be as high as 50%, much higher than the 14% found in bowel gas.[8,21,24,25]\nDespite the debate regarding the etiologies of PI, intraluminal gas alone is clearly insufficient to explain PI.[4] Both theories require the presence of some amount of mucosal damage, whether iatrogenic, from increased intraluminal pressure, or directly from bacterial cytotoxicity. The pathophysiology of PI probably involves some element of both theories and some preexisting mucosal damage. Therefore, one patient could have PI secondary to mesenteric ischemia, whereas another could have colonic flora changes and increased intraluminal pressure from chronic constipation.\nPI is challenging because it is often an inconsequential finding and is life-threatening in only a minority of patients, although it may be associated with life-threatening diagnoses. Because PI is found primarily on imaging, a correlation must be made with a patient's clinical history, physical examination, and laboratory tests, because this more comprehensive analysis is the best indicator of clinical course.\nA review of multiple studies and case series indicate that patients with a history and physical findings suggestive of peritonitis, pH < 7.3, WBC count > 12.0 × 103 cells/µL, age > 60 years, lactic acidosis (lactate level > 18.02 mg/dL), metabolic acidosis (serum bicarbonate level < 20 mEq/L), an elevated serum amylase level, frank rectal bleeding, or the presence of HPVG were most likely to require surgical intervention.[1,6,8,10,13,18,26,27,28] Furthermore, patients with PI who also had HPVG, sepsis, or lactic acidosis had the highest risk for death, approaching 80%.[13]\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 72-Year-Old Man With Abdominal Pain"
},
{
"authors": "Fahad M. Iqbal, MD; Preeti Singh, MD; Jayanth Koneru, MD; Vivekananda Pattabiraman, MD",
"content": [
"When no surgical emergency is identified, conservative, symptomatic therapy is the preferred option in PI. Treatment recommendations, from various reports, consist of bowel rest and decompression, normobaric or hyperbaric oxygen therapy, adequate analgesia, and antibiotic treatment (most commonly metronidazole [Flagyl®]).",
"Prior reports of PI demonstrate that maintaining a prolonged arterial oxygen tension of 200-350 mm Hg accelerated the resolution of PI, but also increased the risk for oxygen toxicity.[6,12,14,16,17,22,23,24] Hyperbaric oxygen may increase the cyst-to-blood diffusion gradient of other gases (hydrogen and nitrogen) and lead to enhanced reabsorption of gas cysts, and has the added benefit of reducing the exposure time to high arterial oxygen tension.[4,21,23,29] Finally, hyperbaric oxygen therapy may have another advantage because of its possible ability to kill gas-forming microorganisms possessing little or no activity of superoxide dismutase.[21,30]",
"One case series[18] speculated that insufflating oxygen into the lumen of the gastrointestinal tract via a nasointestinal tube might accelerate resolution. Antibiotics, given in combination with oxygen, are thought to work by decreasing the amount of gas-producing bacteria in the gut.[6] Finally, exploratory laparotomy should be avoided unless signs of progression or complication are present or the patient has any of the risk factors previously mentioned.",
"An understanding of PI with early recognition and appropriate management is of tantamount importance. Considering the wide range of causes and the outcomes of this condition, physicians are frequently faced with a challenge in identifying patients who actually require surgery.",
"This patient was diagnosed with mesenteric ischemia and possible bowel perforation. Urgent surgical consultation was obtained, and he was started on broad-spectrum antibiotic therapy. His normal vital signs and benign laboratory findings suggested an alternative diagnosis, mesenteric ischemia with secondary PI with HPVG (although he also had COPD). Despite the patient's WBC count and CT-identified HPVG, the surgical team recommended supportive care with a plan for laparotomy if his condition worsened.",
"The patient responded rapidly to conservative therapy, with correction of his elevated WBC count. He was discharged to home with routine follow-up. Repeat CT imaging of the abdomen and pelvis demonstrated resolution of the PI."
],
"date": "September 15, 2015",
"figures": [],
"markdown": "# A 72-Year-Old Man With Abdominal Pain\n\n **Authors:** Fahad M. Iqbal, MD; Preeti Singh, MD; Jayanth Koneru, MD; Vivekananda Pattabiraman, MD \n **Date:** September 15, 2015\n\n ## Content\n\n When no surgical emergency is identified, conservative, symptomatic therapy is the preferred option in PI. Treatment recommendations, from various reports, consist of bowel rest and decompression, normobaric or hyperbaric oxygen therapy, adequate analgesia, and antibiotic treatment (most commonly metronidazole [Flagyl®]).\nPrior reports of PI demonstrate that maintaining a prolonged arterial oxygen tension of 200-350 mm Hg accelerated the resolution of PI, but also increased the risk for oxygen toxicity.[6,12,14,16,17,22,23,24] Hyperbaric oxygen may increase the cyst-to-blood diffusion gradient of other gases (hydrogen and nitrogen) and lead to enhanced reabsorption of gas cysts, and has the added benefit of reducing the exposure time to high arterial oxygen tension.[4,21,23,29] Finally, hyperbaric oxygen therapy may have another advantage because of its possible ability to kill gas-forming microorganisms possessing little or no activity of superoxide dismutase.[21,30]\nOne case series[18] speculated that insufflating oxygen into the lumen of the gastrointestinal tract via a nasointestinal tube might accelerate resolution. Antibiotics, given in combination with oxygen, are thought to work by decreasing the amount of gas-producing bacteria in the gut.[6] Finally, exploratory laparotomy should be avoided unless signs of progression or complication are present or the patient has any of the risk factors previously mentioned.\nAn understanding of PI with early recognition and appropriate management is of tantamount importance. Considering the wide range of causes and the outcomes of this condition, physicians are frequently faced with a challenge in identifying patients who actually require surgery.\nThis patient was diagnosed with mesenteric ischemia and possible bowel perforation. Urgent surgical consultation was obtained, and he was started on broad-spectrum antibiotic therapy. His normal vital signs and benign laboratory findings suggested an alternative diagnosis, mesenteric ischemia with secondary PI with HPVG (although he also had COPD). Despite the patient's WBC count and CT-identified HPVG, the surgical team recommended supportive care with a plan for laparotomy if his condition worsened.\nThe patient responded rapidly to conservative therapy, with correction of his elevated WBC count. He was discharged to home with routine follow-up. Repeat CT imaging of the abdomen and pelvis demonstrated resolution of the PI.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 888551,
"choiceText": "Elevated lipase",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 888553,
"choiceText": "Fever\r\n",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 888555,
"choiceText": "Abdominal pain ",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 888557,
"choiceText": "Hematochezia",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The symptom most consistently associated with PI is diffuse, mild to moderate, nonmigratory abdominal pain. Hematochezia may occasionally be associated with PI, but it is usually a late finding of bowel-wall ischemia and friability (if not infarction). However, fever is usually absent. Amylase may be somewhat elevated depending on the underlying cause of PI, but significant lipase elevation is not expected.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 279881,
"questionText": "Which of the following is most consistently associated with PI?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 888559,
"choiceText": "CT ",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 888561,
"choiceText": "Ultrasonography",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 888563,
"choiceText": "MRI",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 888565,
"choiceText": "PET\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Ultrasonography provides characteristic findings in patients presenting with PI, but it is not as rapidly available in most emergency department settings as CT or MRI. MRI and PET have not been studied in patients presenting with PI, and no PI-specific signs have been reported for either modality. CT is widely available, can be performed rapidly, and can rule out potentially life-threatening alternative diagnoses. This makes CT the optimal imaging modality for PI.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 279883,
"questionText": "Which of the following imaging modalities is best for establishing the diagnosis of PI?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 72-Year-Old Man With Abdominal Pain"
},
{
"authors": "Fahad M. Iqbal, MD; Preeti Singh, MD; Jayanth Koneru, MD; Vivekananda Pattabiraman, MD",
"content": [],
"date": "September 15, 2015",
"figures": [],
"markdown": "# A 72-Year-Old Man With Abdominal Pain\n\n **Authors:** Fahad M. Iqbal, MD; Preeti Singh, MD; Jayanth Koneru, MD; Vivekananda Pattabiraman, MD \n **Date:** September 15, 2015\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 888551,
"choiceText": "Elevated lipase",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 888553,
"choiceText": "Fever\r\n",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 888555,
"choiceText": "Abdominal pain ",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 888557,
"choiceText": "Hematochezia",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The symptom most consistently associated with PI is diffuse, mild to moderate, nonmigratory abdominal pain. Hematochezia may occasionally be associated with PI, but it is usually a late finding of bowel-wall ischemia and friability (if not infarction). However, fever is usually absent. Amylase may be somewhat elevated depending on the underlying cause of PI, but significant lipase elevation is not expected.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 279881,
"questionText": "Which of the following is most consistently associated with PI?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 888559,
"choiceText": "CT ",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 888561,
"choiceText": "Ultrasonography",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 888563,
"choiceText": "MRI",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 888565,
"choiceText": "PET\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Ultrasonography provides characteristic findings in patients presenting with PI, but it is not as rapidly available in most emergency department settings as CT or MRI. MRI and PET have not been studied in patients presenting with PI, and no PI-specific signs have been reported for either modality. CT is widely available, can be performed rapidly, and can rule out potentially life-threatening alternative diagnoses. This makes CT the optimal imaging modality for PI.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 279883,
"questionText": "Which of the following imaging modalities is best for establishing the diagnosis of PI?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 72-Year-Old Man With Abdominal Pain"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 888543,
"choiceText": "Ruptured abdominal aortic aneurysm",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 888545,
"choiceText": "Bowel obstruction",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 888547,
"choiceText": "Pneumatosis intestinalis ",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 888549,
"choiceText": "Pancreatitis\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 279879,
"questionText": "On the basis of the history, physical examination, and work-up, what is the diagnosis?\r\n<br><br><i>\r\nHint: Recognition of this often benign condition can help avoid an unnecessary exploratory laparotomy.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 888551,
"choiceText": "Elevated lipase",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 888553,
"choiceText": "Fever\r\n",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 888555,
"choiceText": "Abdominal pain ",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 888557,
"choiceText": "Hematochezia",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The symptom most consistently associated with PI is diffuse, mild to moderate, nonmigratory abdominal pain. Hematochezia may occasionally be associated with PI, but it is usually a late finding of bowel-wall ischemia and friability (if not infarction). However, fever is usually absent. Amylase may be somewhat elevated depending on the underlying cause of PI, but significant lipase elevation is not expected.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 279881,
"questionText": "Which of the following is most consistently associated with PI?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 888559,
"choiceText": "CT ",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 888561,
"choiceText": "Ultrasonography",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 888563,
"choiceText": "MRI",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 888565,
"choiceText": "PET\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Ultrasonography provides characteristic findings in patients presenting with PI, but it is not as rapidly available in most emergency department settings as CT or MRI. MRI and PET have not been studied in patients presenting with PI, and no PI-specific signs have been reported for either modality. CT is widely available, can be performed rapidly, and can rule out potentially life-threatening alternative diagnoses. This makes CT the optimal imaging modality for PI.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 279883,
"questionText": "Which of the following imaging modalities is best for establishing the diagnosis of PI?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
849321 | /viewarticle/849321 | [
{
"authors": "Sridharan Ramaratnam, MD",
"content": [
"Editor's Note:\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.",
"A 60-year-old woman from northeastern India presents with abnormal behavior, irritability, disorientation, forgetfulness, and unsteadiness while walking, leading to repeated falls. Her symptoms have progressed gradually over the past 3 months; she is now unable to recognize her immediate family or feed herself orally and has become incontinent. She has also developed intermittent, jerking limb movements but has had no tonic-clonic seizures or headaches.",
"She has no history of hypertension or diabetes. She has not undergone any recent surgeries or received any transfusions of blood products in the past. She is the mother of two children and underwent menopause 10 years ago. Her husband has worked in the United Kingdom for the past 20 years while she has lived in India, looking after her family; occasionally she spends a few months at a time visiting with her husband in the UK. She is a vegetarian. She has a family history of heart disease, with both of her parents dying of cardiovascular disease, but no other family history of medical illness."
],
"date": "August 12, 2015",
"figures": [],
"markdown": "# A 60-Year-Old Woman With Worsening Neuropsychiatric Symptoms\n\n **Authors:** Sridharan Ramaratnam, MD \n **Date:** August 12, 2015\n\n ## Content\n\n Editor's Note:\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.\nA 60-year-old woman from northeastern India presents with abnormal behavior, irritability, disorientation, forgetfulness, and unsteadiness while walking, leading to repeated falls. Her symptoms have progressed gradually over the past 3 months; she is now unable to recognize her immediate family or feed herself orally and has become incontinent. She has also developed intermittent, jerking limb movements but has had no tonic-clonic seizures or headaches.\nShe has no history of hypertension or diabetes. She has not undergone any recent surgeries or received any transfusions of blood products in the past. She is the mother of two children and underwent menopause 10 years ago. Her husband has worked in the United Kingdom for the past 20 years while she has lived in India, looking after her family; occasionally she spends a few months at a time visiting with her husband in the UK. She is a vegetarian. She has a family history of heart disease, with both of her parents dying of cardiovascular disease, but no other family history of medical illness.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 60-Year-Old Woman With Worsening Neuropsychiatric Symptoms"
},
{
"authors": "Sridharan Ramaratnam, MD",
"content": [
"The physical examination reveals a well-nourished woman of average build, with a pulse rate of 76 beats/min, blood pressure of 130/78 mm Hg, respiratory rate of 14 breaths/min, and temperature of 98.4° F (36.9° C). She has no thyromegaly, lymphadenopathy, pallor, or jaundice. Cardiac, respiratory, and abdominal examinations are normal. She is incontinent of urine and stool. No signs of meningeal irritation are present.",
"The patient responds to calls but her attention cannot be sustained. She is disoriented to time and place and is unable to identify her husband or her children. Her memory for recent and past events is impaired. She comprehends simple commands. Her speech is slurred. The cranial nerve examination findings are normal. She has intermittent myoclonic jerks involving all of her limbs. She moves all limbs briskly but is unable to sit or stand without assistance and cannot walk, even with assistance. The patient's reflexes are brisk but symmetric. She responds to pain, but the remainder of her sensory examination cannot be reliably assessed.",
"The complete blood cell count, fasting and postprandial blood sugars, urea, creatinine, electrolytes, and liver function examinations are all normal, as are serum ammonia, ceruloplasmin, copper, thyroid, and parathyroid hormone levels. Serum levels of antithyroperoxidase antibodies are normal. The enzyme-linked immunosorbent assay (ELISA) for HIV and the Venereal Disease Research Laboratory (VDRL) test are negative. Serum B12, folate levels, and arterial lactate levels are in the normal range. Testing for antinuclear antibodies (ANA), antineutrophil cytoplasmic autoantibodies (ANCA), and antibodies to double-stranded DNA, as well as for antibodies to thyroid peroxidase and thyroglobulin, are all negative. The toxicology screens for lead, mercury, and arsenic are negative. The patient's ECG, echocardiogram, radiographs of the chest, and ultrasonogram of the abdomen are normal. CT scans of the chest and abdomen do not reveal neoplasms or evidence of other illnesses.",
"Figure.",
"Figure.",
"MRI of the brain with gadolinium enhancement is normal. Cerebrospinal fluid (CSF) analysis reveals acellular fluid, with normal glucose and protein levels. The CSF bacterial cultures, smears for acid-fast bacilli and Cryptococcus, and cytology are negative. Cryptococcal antigen and measles antibodies are not detected in the CSF. The polymerase chain reaction examinations for mycobacterium tuberculosis and herpes simplex virus are negative.",
"An electroencephalogram (EEG) is obtained (see Figure)."
],
"date": "August 12, 2015",
"figures": [
{
"caption": "Figure.",
"image_url": "https://img.medscapestatic.com/article/849/321/849321-Thumb1.png"
}
],
"markdown": "# A 60-Year-Old Woman With Worsening Neuropsychiatric Symptoms\n\n **Authors:** Sridharan Ramaratnam, MD \n **Date:** August 12, 2015\n\n ## Content\n\n The physical examination reveals a well-nourished woman of average build, with a pulse rate of 76 beats/min, blood pressure of 130/78 mm Hg, respiratory rate of 14 breaths/min, and temperature of 98.4° F (36.9° C). She has no thyromegaly, lymphadenopathy, pallor, or jaundice. Cardiac, respiratory, and abdominal examinations are normal. She is incontinent of urine and stool. No signs of meningeal irritation are present.\nThe patient responds to calls but her attention cannot be sustained. She is disoriented to time and place and is unable to identify her husband or her children. Her memory for recent and past events is impaired. She comprehends simple commands. Her speech is slurred. The cranial nerve examination findings are normal. She has intermittent myoclonic jerks involving all of her limbs. She moves all limbs briskly but is unable to sit or stand without assistance and cannot walk, even with assistance. The patient's reflexes are brisk but symmetric. She responds to pain, but the remainder of her sensory examination cannot be reliably assessed.\nThe complete blood cell count, fasting and postprandial blood sugars, urea, creatinine, electrolytes, and liver function examinations are all normal, as are serum ammonia, ceruloplasmin, copper, thyroid, and parathyroid hormone levels. Serum levels of antithyroperoxidase antibodies are normal. The enzyme-linked immunosorbent assay (ELISA) for HIV and the Venereal Disease Research Laboratory (VDRL) test are negative. Serum B12, folate levels, and arterial lactate levels are in the normal range. Testing for antinuclear antibodies (ANA), antineutrophil cytoplasmic autoantibodies (ANCA), and antibodies to double-stranded DNA, as well as for antibodies to thyroid peroxidase and thyroglobulin, are all negative. The toxicology screens for lead, mercury, and arsenic are negative. The patient's ECG, echocardiogram, radiographs of the chest, and ultrasonogram of the abdomen are normal. CT scans of the chest and abdomen do not reveal neoplasms or evidence of other illnesses.\nFigure.\nFigure.\nMRI of the brain with gadolinium enhancement is normal. Cerebrospinal fluid (CSF) analysis reveals acellular fluid, with normal glucose and protein levels. The CSF bacterial cultures, smears for acid-fast bacilli and Cryptococcus, and cytology are negative. Cryptococcal antigen and measles antibodies are not detected in the CSF. The polymerase chain reaction examinations for mycobacterium tuberculosis and herpes simplex virus are negative.\nAn electroencephalogram (EEG) is obtained (see Figure).\n\n ## Figures\n\n **Figure.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 876779,
"choiceText": "Hepatic encephalopathy",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 876781,
"choiceText": "West Nile Virus meningoencephalitis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 876783,
"choiceText": "Absence seizure",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 876785,
"choiceText": "Creutzfeldt-Jakob disease\r\n\r\n",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 275999,
"questionText": "On the basis of the history, physical examination, and workup, what is the diagnosis?<br><br><i>\r\nHint: Look at the periodic discharges.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 60-Year-Old Woman With Worsening Neuropsychiatric Symptoms"
},
{
"authors": "Sridharan Ramaratnam, MD",
"content": [
"The clinical picture was characterized by progressive cognitive dysfunction, behavioral abnormalities, ataxia, and myoclonic jerks. These findings, combined with the EEG demonstrating 1-per-second periodic sharp wave complexes superimposed on a slow background, were highly suggestive of Creutzfeldt-Jakob disease.",
"Periodic sharp wave complexes (PSWCs) are considered characteristic of sporadic Creutzfeldt-Jakob disease and have a sensitivity and specificity of 67% and 87%, respectively, on a single EEG; however, if repeated recordings are obtained, more than 90% of patients with sporadic Creutzfeldt-Jakob disease show PSWCs.[1] PSWCs on the EEG usually indicate a progressive stage of Creutzfeldt-Jakob disease. They typically become obvious around 8-12 weeks after the onset of clinical symptoms; later onset has been reported in a few cases.[2] PSWCs also occasionally occur in cases of familial Creutzfeldt-Jakob disease and variant Creutzfeldt-Jakob disease, but are absent in cases of iatrogenic human growth factor hormone–related Creutzfeldt-Jakob disease, fatal familial insomnia, and Gerstmann-Sträussler-Scheinker syndrome, which are other types of human-transmissible spongiform encephalopathies. The CSF of this patient also showed elevated levels of neuron-specific enolase, which has been recognized as a neurochemical marker for Creutzfeldt-Jakob disease in the appropriate clinical setting.",
"Creutzfeldt-Jakob disease is a subacute progressive neurologic disorder caused by the deposition of prion proteins in the brain. Most cases are sporadic; however, some cases are familial and are caused by mutations in the prion protein gene. Prion protein (PrP) is necessary for the normal transmission of electrical impulses between nerves (synaptic function). Prion disease may result when there is generation of PrPSc, an abnormal prion, which leads to progressive loss of functional PrP (PrPc). The normal prion protein undergoes a conformational change into the abnormal prion protein. These abnormal proteins clump together into protein aggregates which lead to neuronal loss."
],
"date": "August 12, 2015",
"figures": [],
"markdown": "# A 60-Year-Old Woman With Worsening Neuropsychiatric Symptoms\n\n **Authors:** Sridharan Ramaratnam, MD \n **Date:** August 12, 2015\n\n ## Content\n\n The clinical picture was characterized by progressive cognitive dysfunction, behavioral abnormalities, ataxia, and myoclonic jerks. These findings, combined with the EEG demonstrating 1-per-second periodic sharp wave complexes superimposed on a slow background, were highly suggestive of Creutzfeldt-Jakob disease.\nPeriodic sharp wave complexes (PSWCs) are considered characteristic of sporadic Creutzfeldt-Jakob disease and have a sensitivity and specificity of 67% and 87%, respectively, on a single EEG; however, if repeated recordings are obtained, more than 90% of patients with sporadic Creutzfeldt-Jakob disease show PSWCs.[1] PSWCs on the EEG usually indicate a progressive stage of Creutzfeldt-Jakob disease. They typically become obvious around 8-12 weeks after the onset of clinical symptoms; later onset has been reported in a few cases.[2] PSWCs also occasionally occur in cases of familial Creutzfeldt-Jakob disease and variant Creutzfeldt-Jakob disease, but are absent in cases of iatrogenic human growth factor hormone–related Creutzfeldt-Jakob disease, fatal familial insomnia, and Gerstmann-Sträussler-Scheinker syndrome, which are other types of human-transmissible spongiform encephalopathies. The CSF of this patient also showed elevated levels of neuron-specific enolase, which has been recognized as a neurochemical marker for Creutzfeldt-Jakob disease in the appropriate clinical setting.\nCreutzfeldt-Jakob disease is a subacute progressive neurologic disorder caused by the deposition of prion proteins in the brain. Most cases are sporadic; however, some cases are familial and are caused by mutations in the prion protein gene. Prion protein (PrP) is necessary for the normal transmission of electrical impulses between nerves (synaptic function). Prion disease may result when there is generation of PrPSc, an abnormal prion, which leads to progressive loss of functional PrP (PrPc). The normal prion protein undergoes a conformational change into the abnormal prion protein. These abnormal proteins clump together into protein aggregates which lead to neuronal loss.\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 876779,
"choiceText": "Hepatic encephalopathy",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 876781,
"choiceText": "West Nile Virus meningoencephalitis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 876783,
"choiceText": "Absence seizure",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 876785,
"choiceText": "Creutzfeldt-Jakob disease\r\n\r\n",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 275999,
"questionText": "On the basis of the history, physical examination, and workup, what is the diagnosis?<br><br><i>\r\nHint: Look at the periodic discharges.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 60-Year-Old Woman With Worsening Neuropsychiatric Symptoms"
},
{
"authors": "Sridharan Ramaratnam, MD",
"content": [
"Creutzfeldt-Jakob disease affects approximately 1 person per 1 million per year (approximately 250-300 new cases per year in the United States); this estimate may be low due to difficulty in diagnosing the disease. Approximately 85% of cases are sporadic and 15% are familial. A third type of Creutzfeldt-Jakob disease is acquired Creutzfeldt-Jakob disease, which includes iatrogenic and variant forms. Several cases have occurred from contamination via medical procedures, known as iatrogenic Creutzfeldt-Jakob disease. Variant Creutzfeldt-Jakob disease (vCJD) occurs in young people and has been linked to the ingestion of beef tainted with prions from bovine nervous system tissue. Also called bovine spongiform encephalopathy (BSE), most cases of vCJD have occurred in the United Kingdom.[3,4]",
"Diagnosing Creutzfeldt-Jakob disease may be difficult because of the nonspecific nature of the disease and the wide range of clinical symptoms. Typical features of Creutzfeldt-Jakob disease include rapidly progressive dementia, generalized myoclonus, and ataxia. The diagnostic criteria used to define patients with sporadic Creutzfeldt-Jakob disease are as follows:",
"Definite Creutzfeldt-Jakob disease:",
"Characteristic neuropathology",
"Protease-resistant PrP detected by Western blot",
"Probable Creutzfeldt-Jakob disease:",
"Progressive dementia",
"Typical findings on EEG",
"At least two of the following: myoclonus, visual impairment, cerebellar signs, pyramidal or extrapyramidal signs, or akinetic mutism",
"Possible Creutzfeldt-Jakob disease:",
"Progressive dementia",
"Atypical findings on EEG or EEG not available",
"At least two of the following: myoclonus, visual impairment, cerebellar signs, pyramidal or extrapyramidal signs, or akinetic mutism",
"Duration of less than 2 years",
"CT scans of the brain are usually normal or may show atrophic changes that progress rapidly over time. Recent advances in MRI and neurochemical analysis of the CSF have improved the diagnostic accuracy of these examinations.[3,4] MRI may show increased T2 signal intensity in the basal ganglia, thalamus, occipital cortex, or white matter. The sensitivity of increased T2 signal intensity in the basal ganglia has been reported to be only 79%. Diffusion-weighted MRI abnormalities have been shown to be more sensitive than T2-weighted or fluid-attenuated inversion recovery (FLAIR) sequences in detecting lesions in patients who have clinically definite or probable Creutzfeldt-Jakob disease, with a 92.3% specificity and a 93.8% sensitivity.",
"Diffusion weighting is an MRI technique that provides image contrast dependent on the molecular motion of water. Although most commonly used for the detection of early brain infarction, diffusion-weighted MRI can also detect changes in fluid viscosity caused by other disease processes. Diffusion-weighted images in Creutzfeldt-Jakob disease can demonstrate abnormally high signal intensity in the caudate nucleus, putamen, thalamus, and cortex. The cortex is often most involved in early-stage disease. Basal ganglia involvement is typically most prominent in the caudate. Two characteristic radiologic signs have been described: the \"hockey stick\" sign, which refers to increased signal in the putamen and head of the caudate nucleus resembling a hockey stick, and the \"pulvinar\" sign, which corresponds to increased signal in the pulvinar thalamic nuclei, usually bilaterally. The latter sign is more commonly found in patients with vCJD.",
"Diffusion-weighted imaging is very useful in distinguishing Creutzfeldt-Jakob disease from other neurologic causes of dementia, including Alzheimer disease, vascular dementia, and dementia with Lewy bodies. Other causes of similar diffusion-weighted imaging abnormalities include infectious meningoencephalitis, mitochondrial encephalopathy, lactic acidosis, Wilson disease, venous hypertensive encephalopathy, herpes simplex encephalopathy, and Wernicke encephalopathy. These can be distinguished from Creutzfeldt-Jakob disease based on the clinical and CSF findings. The underlying pathology of these diffusion-weighted imaging lesions in Creutzfeldt-Jakob disease is unknown but may represent spongiform changes or prion protein deposits. These lesions often become less apparent with disease progression.[3,4,5]"
],
"date": "August 12, 2015",
"figures": [],
"markdown": "# A 60-Year-Old Woman With Worsening Neuropsychiatric Symptoms\n\n **Authors:** Sridharan Ramaratnam, MD \n **Date:** August 12, 2015\n\n ## Content\n\n Creutzfeldt-Jakob disease affects approximately 1 person per 1 million per year (approximately 250-300 new cases per year in the United States); this estimate may be low due to difficulty in diagnosing the disease. Approximately 85% of cases are sporadic and 15% are familial. A third type of Creutzfeldt-Jakob disease is acquired Creutzfeldt-Jakob disease, which includes iatrogenic and variant forms. Several cases have occurred from contamination via medical procedures, known as iatrogenic Creutzfeldt-Jakob disease. Variant Creutzfeldt-Jakob disease (vCJD) occurs in young people and has been linked to the ingestion of beef tainted with prions from bovine nervous system tissue. Also called bovine spongiform encephalopathy (BSE), most cases of vCJD have occurred in the United Kingdom.[3,4]\nDiagnosing Creutzfeldt-Jakob disease may be difficult because of the nonspecific nature of the disease and the wide range of clinical symptoms. Typical features of Creutzfeldt-Jakob disease include rapidly progressive dementia, generalized myoclonus, and ataxia. The diagnostic criteria used to define patients with sporadic Creutzfeldt-Jakob disease are as follows:\nDefinite Creutzfeldt-Jakob disease:\nCharacteristic neuropathology\nProtease-resistant PrP detected by Western blot\nProbable Creutzfeldt-Jakob disease:\nProgressive dementia\nTypical findings on EEG\nAt least two of the following: myoclonus, visual impairment, cerebellar signs, pyramidal or extrapyramidal signs, or akinetic mutism\nPossible Creutzfeldt-Jakob disease:\nProgressive dementia\nAtypical findings on EEG or EEG not available\nAt least two of the following: myoclonus, visual impairment, cerebellar signs, pyramidal or extrapyramidal signs, or akinetic mutism\nDuration of less than 2 years\nCT scans of the brain are usually normal or may show atrophic changes that progress rapidly over time. Recent advances in MRI and neurochemical analysis of the CSF have improved the diagnostic accuracy of these examinations.[3,4] MRI may show increased T2 signal intensity in the basal ganglia, thalamus, occipital cortex, or white matter. The sensitivity of increased T2 signal intensity in the basal ganglia has been reported to be only 79%. Diffusion-weighted MRI abnormalities have been shown to be more sensitive than T2-weighted or fluid-attenuated inversion recovery (FLAIR) sequences in detecting lesions in patients who have clinically definite or probable Creutzfeldt-Jakob disease, with a 92.3% specificity and a 93.8% sensitivity.\nDiffusion weighting is an MRI technique that provides image contrast dependent on the molecular motion of water. Although most commonly used for the detection of early brain infarction, diffusion-weighted MRI can also detect changes in fluid viscosity caused by other disease processes. Diffusion-weighted images in Creutzfeldt-Jakob disease can demonstrate abnormally high signal intensity in the caudate nucleus, putamen, thalamus, and cortex. The cortex is often most involved in early-stage disease. Basal ganglia involvement is typically most prominent in the caudate. Two characteristic radiologic signs have been described: the \"hockey stick\" sign, which refers to increased signal in the putamen and head of the caudate nucleus resembling a hockey stick, and the \"pulvinar\" sign, which corresponds to increased signal in the pulvinar thalamic nuclei, usually bilaterally. The latter sign is more commonly found in patients with vCJD.\nDiffusion-weighted imaging is very useful in distinguishing Creutzfeldt-Jakob disease from other neurologic causes of dementia, including Alzheimer disease, vascular dementia, and dementia with Lewy bodies. Other causes of similar diffusion-weighted imaging abnormalities include infectious meningoencephalitis, mitochondrial encephalopathy, lactic acidosis, Wilson disease, venous hypertensive encephalopathy, herpes simplex encephalopathy, and Wernicke encephalopathy. These can be distinguished from Creutzfeldt-Jakob disease based on the clinical and CSF findings. The underlying pathology of these diffusion-weighted imaging lesions in Creutzfeldt-Jakob disease is unknown but may represent spongiform changes or prion protein deposits. These lesions often become less apparent with disease progression.[3,4,5]\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 60-Year-Old Woman With Worsening Neuropsychiatric Symptoms"
},
{
"authors": "Sridharan Ramaratnam, MD",
"content": [
"Measurement of brain-specific proteins in the CSF, such as protein 14-3-3, neuron-specific enolase (NSE), S-100b, and tau protein, is supportive. Detection of protein 14-3-3 is a reliable and sensitive marker for sporadic Creutzfeldt-Jakob disease, with a sensitivity and specificity approaching 96%. In vCJD, on the other hand, sensitivity of 14-3-3 protein is 50% and specificity is 91%, while sensitivity of CSF tau protein is 80% and specificity is 94%. A combined determination of 14-3-3 and tau, S100b, or NSE increases the sensitivity to over 93%. Unfortunately, these tests usually take weeks to return, which limits their immediate clinical utility. A multivariate analysis revealed that the sensitivity of all tests was greatest in patients with homozygosity at codon 129 of the prion protein gene, a short disease duration, and age at onset older than 40 years. A repeat lumbar puncture increased the diagnostic sensitivity.[3,4,6]",
"Prions are highly resistant to inactivation. Dry heat (320° F [160° C] for 24 hours), formaldehyde sterilization, and standard steam sterilization do not sterilize prion-contaminated items. CNS tissue contains the highest titer of PrP and should be handled with the greatest care. Other body tissues/fluids are much less likely to pose a risk for infection with Creutzfeldt-Jakob disease. Because prions are not inactivated by formalin, brain tissue samples that have been formalin-fixed should be immersed in 100% formic acid for 1 hour for disinfection. Disposable instruments should be used whenever possible.",
"The guidelines for reprocessing instruments vary in different countries as a result of scientific uncertainties. Surfaces that have been in contact with prion material should be decontaminated with > 5.25% solution of sodium hypochlorite or 1 N sodium hydroxide for 1 hour. Potentially contaminated waste should be autoclaved at 273-279° F (134-137° C) for 20 minutes and then incinerated. vCJD is highly lymphotropic, has been reported to spread by nonleukodepleted blood transfusion, and is present in high titers in lymphatic tissue, the tonsils, and the spleen. The diagnosis of vCJD is routinely made from histopathologic analysis of brain biopsy tissue; however; tonsillar biopsy can potentially establish the diagnosis of vCJD.",
"Although several drugs have been used against Creutzfeldt-Jakob disease (such as quinacrine, chlorpromazine, and D-penicillamine), no effective treatment has been discovered. Usually, a trial of high-dose intravenous steroids is initially administered, to treat potential steroid-responsive encephalopathy, which mimics Creutzfeldt-Jakob disease.[7]",
"The patient in this case was treated with supportive care, and sodium valproate was used for her myoclonic jerks. At the request of the family, a trial of chlorpromazine—and, later, quinacrine—was given. She developed jaundice and abnormal liver function tests and so the quinacrine was discontinued. She has been cared for at home for the past year, with family members taking all precautions while handling her. On a recent follow-up visit, the patient was bedridden, in a vegetative state, and required enteral feeding and constant nursing care."
],
"date": "August 12, 2015",
"figures": [],
"markdown": "# A 60-Year-Old Woman With Worsening Neuropsychiatric Symptoms\n\n **Authors:** Sridharan Ramaratnam, MD \n **Date:** August 12, 2015\n\n ## Content\n\n Measurement of brain-specific proteins in the CSF, such as protein 14-3-3, neuron-specific enolase (NSE), S-100b, and tau protein, is supportive. Detection of protein 14-3-3 is a reliable and sensitive marker for sporadic Creutzfeldt-Jakob disease, with a sensitivity and specificity approaching 96%. In vCJD, on the other hand, sensitivity of 14-3-3 protein is 50% and specificity is 91%, while sensitivity of CSF tau protein is 80% and specificity is 94%. A combined determination of 14-3-3 and tau, S100b, or NSE increases the sensitivity to over 93%. Unfortunately, these tests usually take weeks to return, which limits their immediate clinical utility. A multivariate analysis revealed that the sensitivity of all tests was greatest in patients with homozygosity at codon 129 of the prion protein gene, a short disease duration, and age at onset older than 40 years. A repeat lumbar puncture increased the diagnostic sensitivity.[3,4,6]\nPrions are highly resistant to inactivation. Dry heat (320° F [160° C] for 24 hours), formaldehyde sterilization, and standard steam sterilization do not sterilize prion-contaminated items. CNS tissue contains the highest titer of PrP and should be handled with the greatest care. Other body tissues/fluids are much less likely to pose a risk for infection with Creutzfeldt-Jakob disease. Because prions are not inactivated by formalin, brain tissue samples that have been formalin-fixed should be immersed in 100% formic acid for 1 hour for disinfection. Disposable instruments should be used whenever possible.\nThe guidelines for reprocessing instruments vary in different countries as a result of scientific uncertainties. Surfaces that have been in contact with prion material should be decontaminated with > 5.25% solution of sodium hypochlorite or 1 N sodium hydroxide for 1 hour. Potentially contaminated waste should be autoclaved at 273-279° F (134-137° C) for 20 minutes and then incinerated. vCJD is highly lymphotropic, has been reported to spread by nonleukodepleted blood transfusion, and is present in high titers in lymphatic tissue, the tonsils, and the spleen. The diagnosis of vCJD is routinely made from histopathologic analysis of brain biopsy tissue; however; tonsillar biopsy can potentially establish the diagnosis of vCJD.\nAlthough several drugs have been used against Creutzfeldt-Jakob disease (such as quinacrine, chlorpromazine, and D-penicillamine), no effective treatment has been discovered. Usually, a trial of high-dose intravenous steroids is initially administered, to treat potential steroid-responsive encephalopathy, which mimics Creutzfeldt-Jakob disease.[7]\nThe patient in this case was treated with supportive care, and sodium valproate was used for her myoclonic jerks. At the request of the family, a trial of chlorpromazine—and, later, quinacrine—was given. She developed jaundice and abnormal liver function tests and so the quinacrine was discontinued. She has been cared for at home for the past year, with family members taking all precautions while handling her. On a recent follow-up visit, the patient was bedridden, in a vegetative state, and required enteral feeding and constant nursing care.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 876787,
"choiceText": "Iatrogenic Creutzfeldt-Jakob disease related to human growth hormone",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 876789,
"choiceText": "Gerstmann-Sträussler-Scheinker syndrome\r\n",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 876791,
"choiceText": "Sporadic Creutzfeldt-Jakob disease",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 876793,
"choiceText": "Hashimoto encephalopathy",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 876795,
"choiceText": "Fatal familial insomnia",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "PSWCs superimposed on a slow background are considered characteristic of sporadic Creutzfeldt-Jakob disease and have a sensitivity and specificity of 67% and 87%, respectively, on a single EEG. If repeated recordings are obtained, however, more than 90% of patients with sporadic Creutzfeldt-Jakob disease show PSWCs. PSWCs may appear as early as 3 weeks after the onset of the disease. PSWCs also occasionally occur in cases of familial Creutzfeldt-Jakob disease and variant Creutzfeldt-Jakob disease, but are absent in iatrogenic human growth factor hormone–related Creutzfeldt-Jakob disease, fatal familial insomnia, and Gerstmann-Sträussler-Scheinker syndrome.\r\n",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 276001,
"questionText": "In which of the following conditions is the typical EEG pattern of periodic sharp wave complexes superimposed on a slow background most often seen?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 876797,
"choiceText": "It is increased in patients with a long duration of illness",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 876799,
"choiceText": "It is increased in patients with onset at age < 30 years",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 876801,
"choiceText": "It is increased in patients with heterozygosity at codon 129 of the prion protein gene",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 876803,
"choiceText": "It is increased by repeated studies",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 876805,
"choiceText": "It is less than 40%\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "CSF estimation of brain-specific proteins, such as protein 14-3-3, NSE, S-100b, and tau protein, may be helpful in the appropriate clinical setting. The detection of protein 14-3-3 is a reliable and sensitive marker for sporadic Creutzfeldt-Jakob disease, having a sensitivity and specificity approaching 96%. In vCJD, on the other hand, the sensitivity of 14-3-3 protein is 50%, while for CSF tau protein the sensitivity is 80%. A multivariate analysis showed that the sensitivity of all tests was highest in patients with the shortest disease duration, age at onset > 40 years, and homozygosity at codon 129 of the prion protein gene. A repeat lumbar puncture increases the diagnostic sensitivity. Because these tests may take many weeks to return, they are not immediately helpful in narrowing the differential diagnosis.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 276003,
"questionText": "Which of the following statements about the sensitivity of CSF brain-specific proteins in the diagnosis of Creutzfeldt-Jakob disease is true?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 60-Year-Old Woman With Worsening Neuropsychiatric Symptoms"
},
{
"authors": "Sridharan Ramaratnam, MD",
"content": [],
"date": "August 12, 2015",
"figures": [],
"markdown": "# A 60-Year-Old Woman With Worsening Neuropsychiatric Symptoms\n\n **Authors:** Sridharan Ramaratnam, MD \n **Date:** August 12, 2015\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 876787,
"choiceText": "Iatrogenic Creutzfeldt-Jakob disease related to human growth hormone",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 876789,
"choiceText": "Gerstmann-Sträussler-Scheinker syndrome\r\n",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 876791,
"choiceText": "Sporadic Creutzfeldt-Jakob disease",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 876793,
"choiceText": "Hashimoto encephalopathy",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 876795,
"choiceText": "Fatal familial insomnia",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "PSWCs superimposed on a slow background are considered characteristic of sporadic Creutzfeldt-Jakob disease and have a sensitivity and specificity of 67% and 87%, respectively, on a single EEG. If repeated recordings are obtained, however, more than 90% of patients with sporadic Creutzfeldt-Jakob disease show PSWCs. PSWCs may appear as early as 3 weeks after the onset of the disease. PSWCs also occasionally occur in cases of familial Creutzfeldt-Jakob disease and variant Creutzfeldt-Jakob disease, but are absent in iatrogenic human growth factor hormone–related Creutzfeldt-Jakob disease, fatal familial insomnia, and Gerstmann-Sträussler-Scheinker syndrome.\r\n",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 276001,
"questionText": "In which of the following conditions is the typical EEG pattern of periodic sharp wave complexes superimposed on a slow background most often seen?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 876797,
"choiceText": "It is increased in patients with a long duration of illness",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 876799,
"choiceText": "It is increased in patients with onset at age < 30 years",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 876801,
"choiceText": "It is increased in patients with heterozygosity at codon 129 of the prion protein gene",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 876803,
"choiceText": "It is increased by repeated studies",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 876805,
"choiceText": "It is less than 40%\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "CSF estimation of brain-specific proteins, such as protein 14-3-3, NSE, S-100b, and tau protein, may be helpful in the appropriate clinical setting. The detection of protein 14-3-3 is a reliable and sensitive marker for sporadic Creutzfeldt-Jakob disease, having a sensitivity and specificity approaching 96%. In vCJD, on the other hand, the sensitivity of 14-3-3 protein is 50%, while for CSF tau protein the sensitivity is 80%. A multivariate analysis showed that the sensitivity of all tests was highest in patients with the shortest disease duration, age at onset > 40 years, and homozygosity at codon 129 of the prion protein gene. A repeat lumbar puncture increases the diagnostic sensitivity. Because these tests may take many weeks to return, they are not immediately helpful in narrowing the differential diagnosis.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 276003,
"questionText": "Which of the following statements about the sensitivity of CSF brain-specific proteins in the diagnosis of Creutzfeldt-Jakob disease is true?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 60-Year-Old Woman With Worsening Neuropsychiatric Symptoms"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 876779,
"choiceText": "Hepatic encephalopathy",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 876781,
"choiceText": "West Nile Virus meningoencephalitis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 876783,
"choiceText": "Absence seizure",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 876785,
"choiceText": "Creutzfeldt-Jakob disease\r\n\r\n",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 275999,
"questionText": "On the basis of the history, physical examination, and workup, what is the diagnosis?<br><br><i>\r\nHint: Look at the periodic discharges.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 876787,
"choiceText": "Iatrogenic Creutzfeldt-Jakob disease related to human growth hormone",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 876789,
"choiceText": "Gerstmann-Sträussler-Scheinker syndrome\r\n",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 876791,
"choiceText": "Sporadic Creutzfeldt-Jakob disease",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 876793,
"choiceText": "Hashimoto encephalopathy",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 876795,
"choiceText": "Fatal familial insomnia",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "PSWCs superimposed on a slow background are considered characteristic of sporadic Creutzfeldt-Jakob disease and have a sensitivity and specificity of 67% and 87%, respectively, on a single EEG. If repeated recordings are obtained, however, more than 90% of patients with sporadic Creutzfeldt-Jakob disease show PSWCs. PSWCs may appear as early as 3 weeks after the onset of the disease. PSWCs also occasionally occur in cases of familial Creutzfeldt-Jakob disease and variant Creutzfeldt-Jakob disease, but are absent in iatrogenic human growth factor hormone–related Creutzfeldt-Jakob disease, fatal familial insomnia, and Gerstmann-Sträussler-Scheinker syndrome.\r\n",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 276001,
"questionText": "In which of the following conditions is the typical EEG pattern of periodic sharp wave complexes superimposed on a slow background most often seen?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 876797,
"choiceText": "It is increased in patients with a long duration of illness",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 876799,
"choiceText": "It is increased in patients with onset at age < 30 years",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 876801,
"choiceText": "It is increased in patients with heterozygosity at codon 129 of the prion protein gene",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 876803,
"choiceText": "It is increased by repeated studies",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 876805,
"choiceText": "It is less than 40%\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "CSF estimation of brain-specific proteins, such as protein 14-3-3, NSE, S-100b, and tau protein, may be helpful in the appropriate clinical setting. The detection of protein 14-3-3 is a reliable and sensitive marker for sporadic Creutzfeldt-Jakob disease, having a sensitivity and specificity approaching 96%. In vCJD, on the other hand, the sensitivity of 14-3-3 protein is 50%, while for CSF tau protein the sensitivity is 80%. A multivariate analysis showed that the sensitivity of all tests was highest in patients with the shortest disease duration, age at onset > 40 years, and homozygosity at codon 129 of the prion protein gene. A repeat lumbar puncture increases the diagnostic sensitivity. Because these tests may take many weeks to return, they are not immediately helpful in narrowing the differential diagnosis.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 276003,
"questionText": "Which of the following statements about the sensitivity of CSF brain-specific proteins in the diagnosis of Creutzfeldt-Jakob disease is true?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
848758 | /viewarticle/848758 | [
{
"authors": "Kathy D. Miller, MD; Jill Kremer, MD",
"content": [
"Editor's Note:\n\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.",
"An 80-year-old woman presents for a routine follow-up with her primary care physician. She is found to have new anemia; her hemoglobin level is 10 g/dL, when 1 year ago it was 14.4 g/dL. She notes increased fatigue, and her family agrees that she has been sleeping more throughout the day. She has no abdominal pain and notices no change in bowel habits. The patient also describes chronic waxing and waning bone pain that she attributes to her long-standing arthritis. She thinks her lower back and left hip discomfort may have become marginally worse over the past year but denies any focal areas of severe pain or sudden change in symptoms.",
"The patient's past medical history is significant for coronary artery disease requiring cardiac stents on two occasions (the last was 4 years prior); diet-controlled diabetes; osteoarthritis treated with regular water aerobics and nonsteroidal anti-inflammatory agents; and stage III invasive lobular carcinoma of the breast diagnosed in 2001. Her breast cancer was treated with a right modified radical mastectomy, prophylactic left mastectomy, chemotherapy with anthracycline and taxane regimen, and chest wall radiation. She continues to take the antiestrogen aromatase inhibitor exemestane."
],
"date": "July 30, 2015",
"figures": [],
"markdown": "# Anemia of Unknown Origin in an 80-Year-Old Woman\n\n **Authors:** Kathy D. Miller, MD; Jill Kremer, MD \n **Date:** July 30, 2015\n\n ## Content\n\n Editor's Note:\n\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.\nAn 80-year-old woman presents for a routine follow-up with her primary care physician. She is found to have new anemia; her hemoglobin level is 10 g/dL, when 1 year ago it was 14.4 g/dL. She notes increased fatigue, and her family agrees that she has been sleeping more throughout the day. She has no abdominal pain and notices no change in bowel habits. The patient also describes chronic waxing and waning bone pain that she attributes to her long-standing arthritis. She thinks her lower back and left hip discomfort may have become marginally worse over the past year but denies any focal areas of severe pain or sudden change in symptoms.\nThe patient's past medical history is significant for coronary artery disease requiring cardiac stents on two occasions (the last was 4 years prior); diet-controlled diabetes; osteoarthritis treated with regular water aerobics and nonsteroidal anti-inflammatory agents; and stage III invasive lobular carcinoma of the breast diagnosed in 2001. Her breast cancer was treated with a right modified radical mastectomy, prophylactic left mastectomy, chemotherapy with anthracycline and taxane regimen, and chest wall radiation. She continues to take the antiestrogen aromatase inhibitor exemestane.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "Anemia of Unknown Origin in an 80-Year-Old Woman"
},
{
"authors": "Kathy D. Miller, MD; Jill Kremer, MD",
"content": [
"The physical examination is notable for an older but vibrant woman. No signs of distress are noted. Her vital signs include a temperature of 97.7° F (36.5° C), heart rate of 93 beats/min, blood pressure of 132/69 mm Hg, respirations of 14 breaths/min, and a weight of 139.5 lb (139 lb 1 year prior).",
"Figure 1.",
"Figure 1.",
"Figure 2.",
"Figure 2.",
"The patient has an unremarkable physical examination except for minimal pallor and postsurgical and radiation changes, including significant telangiectasia over the right chest wall and capsular contracture around her implant reconstruction on the left. She has normal bowel sounds with no abdominal tenderness. The liver and spleen are not palpable. A musculoskeletal examination does not reveal any areas of localized tenderness.",
"Further laboratory studies are significant for a sedimentation rate of 88 mm/hr. Bone marrow biopsy and nuclear bone scan are obtained for further workup. Peripheral smears are shown (Figures 1 and 2)."
],
"date": "July 30, 2015",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/848/758/848758-Thumb1.png"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/848/758/848758-Thumb2.png"
}
],
"markdown": "# Anemia of Unknown Origin in an 80-Year-Old Woman\n\n **Authors:** Kathy D. Miller, MD; Jill Kremer, MD \n **Date:** July 30, 2015\n\n ## Content\n\n The physical examination is notable for an older but vibrant woman. No signs of distress are noted. Her vital signs include a temperature of 97.7° F (36.5° C), heart rate of 93 beats/min, blood pressure of 132/69 mm Hg, respirations of 14 breaths/min, and a weight of 139.5 lb (139 lb 1 year prior).\nFigure 1.\nFigure 1.\nFigure 2.\nFigure 2.\nThe patient has an unremarkable physical examination except for minimal pallor and postsurgical and radiation changes, including significant telangiectasia over the right chest wall and capsular contracture around her implant reconstruction on the left. She has normal bowel sounds with no abdominal tenderness. The liver and spleen are not palpable. A musculoskeletal examination does not reveal any areas of localized tenderness.\nFurther laboratory studies are significant for a sedimentation rate of 88 mm/hr. Bone marrow biopsy and nuclear bone scan are obtained for further workup. Peripheral smears are shown (Figures 1 and 2).\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 872453,
"choiceText": "Anemia of chronic disease",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 872455,
"choiceText": "Myelodysplastic syndrome",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 872457,
"choiceText": "Acute myelogenous leukemia ",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 872459,
"choiceText": "Myelophthisic anemia",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 872461,
"choiceText": "Osteoporosis\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 274573,
"questionText": "On the basis of the history, physical examination, and workup, what is the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Anemia of Unknown Origin in an 80-Year-Old Woman"
},
{
"authors": "Kathy D. Miller, MD; Jill Kremer, MD",
"content": [
"The differential diagnosis is broad in an older patient with anemia. Important considerations can be grouped into several broad categories, including the following:",
"Blood loss (acute or chronic)",
"Increased red cell destruction (hemolysis)",
"Decreased or defective red cell production (chronic disease, renal dysfunction, myelodysplastic syndrome, malignancy)",
"Figure 1.",
"Figure 1.",
"Figure 2.",
"Figure 2.",
"Figure 3.",
"Figure 3.",
"In this patient with a prior malignancy and chemotherapy exposure, disease recurrence or treatment-related myelodysplastic syndrome or frank leukemia is a concern. The diagnosis of myelophthisic anemia was initially suspected on the basis of the peripheral smear (Figures 1-3) with tear-drop cells, nucleated red blood cells, and occasional immature myeloid cells. A bone marrow examination was performed which uncovered the underlying etiology of the myelophthisic anemia: a hypocellular specimen with marked decrease in trilineage hematopoiesis and fibrosis, as well as areas of metastatic cancer. Additional testing confirmed that the cancer cells were strongly estrogen– and progesterone receptor–positive, similar to her initial breast cancer. A bone scan found asymmetric distribution of bony lesions, further supporting metastatic disease.",
"Myelophthisic anemia is a common manifestation of the myeloproliferative disorders, especially myelofibrosis.[1] Although myelophthisic anemia can be a feature of hematologic malignancies, it is more common in patients with metastatic involvement from a solid tumor, particularly gastric and prostate cancer.[2] Further evaluation and treatment are based on the underlying disease rather than the myelophthisic process itself.",
"In this case, further evaluation was aimed at determining the extent of her metastatic disease, with CT scans of the chest and abdomen. Significant findings included verification of the bone lesions, thyroid heterogeneity, and multiple indeterminate liver lesions (all less than 2 cm). Her total bilirubin, aspartate, and alanine transaminases levels were normal as was the alkaline phosphatase. Her serum albumin was 4.1 g/dL."
],
"date": "July 30, 2015",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/848/758/848758-Thumb1.png"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/848/758/848758-Thumb2.png"
},
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/848/758/848758-Thumb3.png"
}
],
"markdown": "# Anemia of Unknown Origin in an 80-Year-Old Woman\n\n **Authors:** Kathy D. Miller, MD; Jill Kremer, MD \n **Date:** July 30, 2015\n\n ## Content\n\n The differential diagnosis is broad in an older patient with anemia. Important considerations can be grouped into several broad categories, including the following:\nBlood loss (acute or chronic)\nIncreased red cell destruction (hemolysis)\nDecreased or defective red cell production (chronic disease, renal dysfunction, myelodysplastic syndrome, malignancy)\nFigure 1.\nFigure 1.\nFigure 2.\nFigure 2.\nFigure 3.\nFigure 3.\nIn this patient with a prior malignancy and chemotherapy exposure, disease recurrence or treatment-related myelodysplastic syndrome or frank leukemia is a concern. The diagnosis of myelophthisic anemia was initially suspected on the basis of the peripheral smear (Figures 1-3) with tear-drop cells, nucleated red blood cells, and occasional immature myeloid cells. A bone marrow examination was performed which uncovered the underlying etiology of the myelophthisic anemia: a hypocellular specimen with marked decrease in trilineage hematopoiesis and fibrosis, as well as areas of metastatic cancer. Additional testing confirmed that the cancer cells were strongly estrogen– and progesterone receptor–positive, similar to her initial breast cancer. A bone scan found asymmetric distribution of bony lesions, further supporting metastatic disease.\nMyelophthisic anemia is a common manifestation of the myeloproliferative disorders, especially myelofibrosis.[1] Although myelophthisic anemia can be a feature of hematologic malignancies, it is more common in patients with metastatic involvement from a solid tumor, particularly gastric and prostate cancer.[2] Further evaluation and treatment are based on the underlying disease rather than the myelophthisic process itself.\nIn this case, further evaluation was aimed at determining the extent of her metastatic disease, with CT scans of the chest and abdomen. Significant findings included verification of the bone lesions, thyroid heterogeneity, and multiple indeterminate liver lesions (all less than 2 cm). Her total bilirubin, aspartate, and alanine transaminases levels were normal as was the alkaline phosphatase. Her serum albumin was 4.1 g/dL.\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n**Figure 3.** \n \n\n\n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 872453,
"choiceText": "Anemia of chronic disease",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 872455,
"choiceText": "Myelodysplastic syndrome",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 872457,
"choiceText": "Acute myelogenous leukemia ",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 872459,
"choiceText": "Myelophthisic anemia",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 872461,
"choiceText": "Osteoporosis\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 274573,
"questionText": "On the basis of the history, physical examination, and workup, what is the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Anemia of Unknown Origin in an 80-Year-Old Woman"
},
{
"authors": "Kathy D. Miller, MD; Jill Kremer, MD",
"content": [
"Late recurrence (> 5 years after initial diagnosis) is common in hormone-sensitive breast cancer, with more than half of all recurrences detected after year 5. Metastatic breast cancer is not curable with any standard therapy or known investigational approach. In this situation, the most important goal is to help patients live as well as possible for as long as possible. That always requires negotiating a sometimes difficult balance between maximizing control of the disease while minimizing toxicity of therapy at the same time.",
"A meta-analysis of eight small randomized trials comparing the response rates for chemotherapy with the response rate for endocrine therapy found an advantage for chemotherapy (relative risk, 1.25; 95% confidence interval, 1.01-1.54).[3] However, these trials were all published prior to 1995 and many of the trials enrolled patients with unknown estrogen receptor status. Because hormone therapy would not be expected to have any benefit in patients with estrogen receptor-negative tumors, these trials would be predicted to underestimate the benefit of hormone therapy.",
"Initial chemotherapy is a reasonable option in patients with estrogen receptor‒positive disease who have visceral crisis (defined as end-organ dysfunction, not the mere presence of visceral disease) or rapidly developing symptoms. This patient's initial breast cancer had lobular histology and was strongly estrogen receptor‒positive; continuous expression of the estrogen receptor was confirmed in the metastatic disease in her bone marrow. She had a long disease-free interval and had few symptoms of her disease. All of these features point to a recommendation for additional hormone therapy rather than an immediate move to cytotoxic chemotherapy."
],
"date": "July 30, 2015",
"figures": [],
"markdown": "# Anemia of Unknown Origin in an 80-Year-Old Woman\n\n **Authors:** Kathy D. Miller, MD; Jill Kremer, MD \n **Date:** July 30, 2015\n\n ## Content\n\n Late recurrence (> 5 years after initial diagnosis) is common in hormone-sensitive breast cancer, with more than half of all recurrences detected after year 5. Metastatic breast cancer is not curable with any standard therapy or known investigational approach. In this situation, the most important goal is to help patients live as well as possible for as long as possible. That always requires negotiating a sometimes difficult balance between maximizing control of the disease while minimizing toxicity of therapy at the same time.\nA meta-analysis of eight small randomized trials comparing the response rates for chemotherapy with the response rate for endocrine therapy found an advantage for chemotherapy (relative risk, 1.25; 95% confidence interval, 1.01-1.54).[3] However, these trials were all published prior to 1995 and many of the trials enrolled patients with unknown estrogen receptor status. Because hormone therapy would not be expected to have any benefit in patients with estrogen receptor-negative tumors, these trials would be predicted to underestimate the benefit of hormone therapy.\nInitial chemotherapy is a reasonable option in patients with estrogen receptor‒positive disease who have visceral crisis (defined as end-organ dysfunction, not the mere presence of visceral disease) or rapidly developing symptoms. This patient's initial breast cancer had lobular histology and was strongly estrogen receptor‒positive; continuous expression of the estrogen receptor was confirmed in the metastatic disease in her bone marrow. She had a long disease-free interval and had few symptoms of her disease. All of these features point to a recommendation for additional hormone therapy rather than an immediate move to cytotoxic chemotherapy.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "Anemia of Unknown Origin in an 80-Year-Old Woman"
},
{
"authors": "Kathy D. Miller, MD; Jill Kremer, MD",
"content": [
"Multiple options for additional hormone therapy are available, all with documented activity after progression on an aromatase inhibitor. The choice of hormone therapy can be individualized on the basis of patient-specific concerns for toxicity, mode of administration, and cost. The addition of everolimus to tamoxifen is supported by data from the TAMRAD study.[4] In this randomized, phase 2 trial, the combination increased clinical benefit rate from 42% to 61% and increased time to progression from 4.5 months to 8.6 months compared with tamoxifen alone. The addition of everolimus also increased toxicity, particularly fatigue (72% vs 53% with tamoxifen alone), stomatitis (56% vs 7%), rash (44% vs 7%), anorexia (43% vs 18%), and diarrhea (39% vs 11%).",
"More recently, the PALOMA3 trial evaluated the compared the efficacy of fulvestrant alone or with the cyclin-dependent kinase 4/6 inhibitor palbociclib n patients who had progressed on prior endocrine therapy.[5] PALOMA3 enrolled 521 patients; 60% had visceral disease at study entry. Clinical benefit increased from 19% to 34%, and progression-free survival improved from 3.8 to 9.3 months. Palbociclib does induce myelosuppression with grade > 3 neutropenia in 62% of patients, although infections were uncommon (< 2%). Less than 3% of patients discontinued palbociclib because of toxicity."
],
"date": "July 30, 2015",
"figures": [],
"markdown": "# Anemia of Unknown Origin in an 80-Year-Old Woman\n\n **Authors:** Kathy D. Miller, MD; Jill Kremer, MD \n **Date:** July 30, 2015\n\n ## Content\n\n Multiple options for additional hormone therapy are available, all with documented activity after progression on an aromatase inhibitor. The choice of hormone therapy can be individualized on the basis of patient-specific concerns for toxicity, mode of administration, and cost. The addition of everolimus to tamoxifen is supported by data from the TAMRAD study.[4] In this randomized, phase 2 trial, the combination increased clinical benefit rate from 42% to 61% and increased time to progression from 4.5 months to 8.6 months compared with tamoxifen alone. The addition of everolimus also increased toxicity, particularly fatigue (72% vs 53% with tamoxifen alone), stomatitis (56% vs 7%), rash (44% vs 7%), anorexia (43% vs 18%), and diarrhea (39% vs 11%).\nMore recently, the PALOMA3 trial evaluated the compared the efficacy of fulvestrant alone or with the cyclin-dependent kinase 4/6 inhibitor palbociclib n patients who had progressed on prior endocrine therapy.[5] PALOMA3 enrolled 521 patients; 60% had visceral disease at study entry. Clinical benefit increased from 19% to 34%, and progression-free survival improved from 3.8 to 9.3 months. Palbociclib does induce myelosuppression with grade > 3 neutropenia in 62% of patients, although infections were uncommon (< 2%). Less than 3% of patients discontinued palbociclib because of toxicity.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 872463,
"choiceText": "Fulvestrant",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 872465,
"choiceText": "Fulvestrant and palbociclib",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 872467,
"choiceText": "Paclitaxel",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 872469,
"choiceText": "Tamoxifen",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 872471,
"choiceText": "Tamoxifen and everolimus\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Some may have suggested chemotherapy on the basis of the potential liver involvement on her CT scan. However, exemestane was discontinued, and the patient began treatment with fulvestrant and palbociclib, as supported by the PALOMA3 trial.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 274575,
"questionText": "Which of the following is <i>not</i> a reasonable option for initial therapy for the patient in this case?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 872473,
"choiceText": "No additional therapy",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 872475,
"choiceText": "Radiation to the lumbosacral spine and left hip",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 872477,
"choiceText": "Supplemental calcium and vitamin D",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 872479,
"choiceText": "Denosumab every 4 weeks\r\n",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>Bone is the most common site of breast cancer metastasis. Complications of disease involving bone include pain, pathologic fracture, hypercalcemia, and spinal cord compression. Prevention of skeletal compromise is critical to maintaining quality of life for patients with metastatic bone involvement. The potent osteoclast inhibitors (bisphosphonates and denosumab) decrease the risk for skeletal-related events and are important adjuncts to ongoing antineoplastic therapy. <br><br>\r\n\r\nFor most patients, denosumab is usually the drug of choice because it results in a greater reduction in the risk for skeletal-related events compared with zoledronic acid. In addition, the subcutaneous mode of administration is convenient for patients and is more efficient for clinic staff. However, the difference in efficacy and toxicity is small, suggesting that the bisphosphonates still have a role in patients unable to tolerate or afford denosumab. <br><br>\r\n\r\nRecent data suggest that zoledronic acid can be given every 12 weeks with no loss of efficacy compared with every-4-week administration.<sup type=\"ref\">[6]</sup> Although generally well tolerated, hypocalcemia is a risk, especially in patients with vitamin D deficiency. To minimize the risk for osteonecrosis of the jaw, patients should be up-to-date on their dental hygiene and should continue regular dental evaluations during treatment. The patient in this case was started on denosumab and has maintained ongoing treatment with the hormone therapy as well. </p>",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 274577,
"questionText": "In addition to discontinuing exemestane and receiving fulvestrant and palbociclib, which of the following is also reasonable?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Anemia of Unknown Origin in an 80-Year-Old Woman"
},
{
"authors": "Kathy D. Miller, MD; Jill Kremer, MD",
"content": [],
"date": "July 30, 2015",
"figures": [],
"markdown": "# Anemia of Unknown Origin in an 80-Year-Old Woman\n\n **Authors:** Kathy D. Miller, MD; Jill Kremer, MD \n **Date:** July 30, 2015\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 872463,
"choiceText": "Fulvestrant",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 872465,
"choiceText": "Fulvestrant and palbociclib",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 872467,
"choiceText": "Paclitaxel",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 872469,
"choiceText": "Tamoxifen",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 872471,
"choiceText": "Tamoxifen and everolimus\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Some may have suggested chemotherapy on the basis of the potential liver involvement on her CT scan. However, exemestane was discontinued, and the patient began treatment with fulvestrant and palbociclib, as supported by the PALOMA3 trial.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 274575,
"questionText": "Which of the following is <i>not</i> a reasonable option for initial therapy for the patient in this case?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 872473,
"choiceText": "No additional therapy",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 872475,
"choiceText": "Radiation to the lumbosacral spine and left hip",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 872477,
"choiceText": "Supplemental calcium and vitamin D",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 872479,
"choiceText": "Denosumab every 4 weeks\r\n",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>Bone is the most common site of breast cancer metastasis. Complications of disease involving bone include pain, pathologic fracture, hypercalcemia, and spinal cord compression. Prevention of skeletal compromise is critical to maintaining quality of life for patients with metastatic bone involvement. The potent osteoclast inhibitors (bisphosphonates and denosumab) decrease the risk for skeletal-related events and are important adjuncts to ongoing antineoplastic therapy. <br><br>\r\n\r\nFor most patients, denosumab is usually the drug of choice because it results in a greater reduction in the risk for skeletal-related events compared with zoledronic acid. In addition, the subcutaneous mode of administration is convenient for patients and is more efficient for clinic staff. However, the difference in efficacy and toxicity is small, suggesting that the bisphosphonates still have a role in patients unable to tolerate or afford denosumab. <br><br>\r\n\r\nRecent data suggest that zoledronic acid can be given every 12 weeks with no loss of efficacy compared with every-4-week administration.<sup type=\"ref\">[6]</sup> Although generally well tolerated, hypocalcemia is a risk, especially in patients with vitamin D deficiency. To minimize the risk for osteonecrosis of the jaw, patients should be up-to-date on their dental hygiene and should continue regular dental evaluations during treatment. The patient in this case was started on denosumab and has maintained ongoing treatment with the hormone therapy as well. </p>",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 274577,
"questionText": "In addition to discontinuing exemestane and receiving fulvestrant and palbociclib, which of the following is also reasonable?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Anemia of Unknown Origin in an 80-Year-Old Woman"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 872453,
"choiceText": "Anemia of chronic disease",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 872455,
"choiceText": "Myelodysplastic syndrome",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 872457,
"choiceText": "Acute myelogenous leukemia ",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 872459,
"choiceText": "Myelophthisic anemia",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 872461,
"choiceText": "Osteoporosis\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 274573,
"questionText": "On the basis of the history, physical examination, and workup, what is the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 872463,
"choiceText": "Fulvestrant",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 872465,
"choiceText": "Fulvestrant and palbociclib",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 872467,
"choiceText": "Paclitaxel",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 872469,
"choiceText": "Tamoxifen",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 872471,
"choiceText": "Tamoxifen and everolimus\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Some may have suggested chemotherapy on the basis of the potential liver involvement on her CT scan. However, exemestane was discontinued, and the patient began treatment with fulvestrant and palbociclib, as supported by the PALOMA3 trial.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 274575,
"questionText": "Which of the following is <i>not</i> a reasonable option for initial therapy for the patient in this case?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 872473,
"choiceText": "No additional therapy",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 872475,
"choiceText": "Radiation to the lumbosacral spine and left hip",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 872477,
"choiceText": "Supplemental calcium and vitamin D",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 872479,
"choiceText": "Denosumab every 4 weeks\r\n",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>Bone is the most common site of breast cancer metastasis. Complications of disease involving bone include pain, pathologic fracture, hypercalcemia, and spinal cord compression. Prevention of skeletal compromise is critical to maintaining quality of life for patients with metastatic bone involvement. The potent osteoclast inhibitors (bisphosphonates and denosumab) decrease the risk for skeletal-related events and are important adjuncts to ongoing antineoplastic therapy. <br><br>\r\n\r\nFor most patients, denosumab is usually the drug of choice because it results in a greater reduction in the risk for skeletal-related events compared with zoledronic acid. In addition, the subcutaneous mode of administration is convenient for patients and is more efficient for clinic staff. However, the difference in efficacy and toxicity is small, suggesting that the bisphosphonates still have a role in patients unable to tolerate or afford denosumab. <br><br>\r\n\r\nRecent data suggest that zoledronic acid can be given every 12 weeks with no loss of efficacy compared with every-4-week administration.<sup type=\"ref\">[6]</sup> Although generally well tolerated, hypocalcemia is a risk, especially in patients with vitamin D deficiency. To minimize the risk for osteonecrosis of the jaw, patients should be up-to-date on their dental hygiene and should continue regular dental evaluations during treatment. The patient in this case was started on denosumab and has maintained ongoing treatment with the hormone therapy as well. </p>",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 274577,
"questionText": "In addition to discontinuing exemestane and receiving fulvestrant and palbociclib, which of the following is also reasonable?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
848759 | /viewarticle/848759 | [
{
"authors": "Kristin K. Egan, MD; Douglas K. Hanks, MD; David W. Kim, MD; Andrew H. Murr, MD",
"content": [
"Editor's Note:\n\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.",
"A 42-year-old man presents to the otolaryngology clinic with a right ear mass situated in the conchal bowl. The mass has been present for a few months, without any notable changes in size. The patient's primary concern is his cosmetic appearance. He denies experiencing any pain, warmth, or tenderness associated with the mass.",
"The patient's medical history is significant for a 4-month history of a left-sided cervical neck mass and ipsilateral Horner syndrome, for which he received treatment about 2 years ago. Fine-needle aspiration at that time revealed numerous sinus histiocytes, but further tissue was needed for a definitive diagnosis. MRI done at that time demonstrated that the mass was encasing the carotid artery and showed prominence of nasopharyngeal tissue, which was also seen on clinical examination. The patient was taken to the operating room and underwent biopsies of the nasopharynx and tonsils, which were negative for malignancy.",
"The patient was then treated by the oncology service with dexamethasone 4 mg twice daily for 14 days. He had noted resolution of his ptosis and a decrease in the size of his neck mass. The patient returned 2 months later with improved, but still notable, miosis, as well as a neck mass. He was once again given dexamethasone 4 mg twice daily for 1 month, but he was subsequently lost to follow-up until his current presentation to the otolaryngology clinic. The patient states that he experienced complete resolution of his Horner syndrome from the previous presentation 2 years ago. No other positive pertinent symptoms are noted.",
"The patient is not currently on any medications and has no active medical conditions. He does not chew tobacco, smoke, or use any illicit substances. The family history is unremarkable."
],
"date": "July 30, 2015",
"figures": [],
"markdown": "# A 42-Year-Old Man With a Right Ear Mass\n\n **Authors:** Kristin K. Egan, MD; Douglas K. Hanks, MD; David W. Kim, MD; Andrew H. Murr, MD \n **Date:** July 30, 2015\n\n ## Content\n\n Editor's Note:\n\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.\nA 42-year-old man presents to the otolaryngology clinic with a right ear mass situated in the conchal bowl. The mass has been present for a few months, without any notable changes in size. The patient's primary concern is his cosmetic appearance. He denies experiencing any pain, warmth, or tenderness associated with the mass.\nThe patient's medical history is significant for a 4-month history of a left-sided cervical neck mass and ipsilateral Horner syndrome, for which he received treatment about 2 years ago. Fine-needle aspiration at that time revealed numerous sinus histiocytes, but further tissue was needed for a definitive diagnosis. MRI done at that time demonstrated that the mass was encasing the carotid artery and showed prominence of nasopharyngeal tissue, which was also seen on clinical examination. The patient was taken to the operating room and underwent biopsies of the nasopharynx and tonsils, which were negative for malignancy.\nThe patient was then treated by the oncology service with dexamethasone 4 mg twice daily for 14 days. He had noted resolution of his ptosis and a decrease in the size of his neck mass. The patient returned 2 months later with improved, but still notable, miosis, as well as a neck mass. He was once again given dexamethasone 4 mg twice daily for 1 month, but he was subsequently lost to follow-up until his current presentation to the otolaryngology clinic. The patient states that he experienced complete resolution of his Horner syndrome from the previous presentation 2 years ago. No other positive pertinent symptoms are noted.\nThe patient is not currently on any medications and has no active medical conditions. He does not chew tobacco, smoke, or use any illicit substances. The family history is unremarkable.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 42-Year-Old Man With a Right Ear Mass"
},
{
"authors": "Kristin K. Egan, MD; Douglas K. Hanks, MD; David W. Kim, MD; Andrew H. Murr, MD",
"content": [
"Figure 1",
"Figure 2",
"Figure 3",
"Figure 4",
"Figure 5",
"The patient appears comfortable and is in no obvious distress. His clinic vital signs include a temperature of 98.2°F, a blood pressure of 134/88 mm Hg, a pulse of 89 beats/min, a respiratory rate of 12 breaths/min, and an oxygen saturation of 100% while breathing room air. A focused HEENT (head, eyes, ears, nose, and throat) examination reveals a mass in the right conchal bowl measuring approximately 4-5 cm, with no overlying edema or erythema (Figure 1). The mass is firm to palpation and nontender. The remainder of the physical examination is unremarkable, despite a detailed search for occult adenopathy.",
"Fiberoptic endoscopy reveals no other abnormalities. Surgical excision for pathologic analysis is proposed to the patient, and he agrees to surgical excision of the right auricular mass (Figure 2). Histologic examination shows macrophages with engulfed lymphocytes (Figure 3). The pathologic analysis reveals positive staining for S100 (Figure 4) and CD68 (Figure 5), as well as many large histiocytic cells with abundant amphophilic cytoplasm and vesicular nuclei."
],
"date": "July 30, 2015",
"figures": [
{
"caption": "Figure 1",
"image_url": "https://img.medscapestatic.com/article/848/759/848759-Thumb-1.png"
},
{
"caption": "Figure 2",
"image_url": "https://img.medscapestatic.com/article/848/759/848759-Thumb-2.png"
},
{
"caption": "Figure 3",
"image_url": "https://img.medscapestatic.com/article/848/759/848759-Thumb-3.png"
},
{
"caption": "Figure 4",
"image_url": "https://img.medscapestatic.com/article/848/759/848759-Thumb-4.png"
},
{
"caption": "Figure 5",
"image_url": "https://img.medscapestatic.com/article/848/759/848759-Thumb-5.png"
}
],
"markdown": "# A 42-Year-Old Man With a Right Ear Mass\n\n **Authors:** Kristin K. Egan, MD; Douglas K. Hanks, MD; David W. Kim, MD; Andrew H. Murr, MD \n **Date:** July 30, 2015\n\n ## Content\n\n Figure 1\nFigure 2\nFigure 3\nFigure 4\nFigure 5\nThe patient appears comfortable and is in no obvious distress. His clinic vital signs include a temperature of 98.2°F, a blood pressure of 134/88 mm Hg, a pulse of 89 beats/min, a respiratory rate of 12 breaths/min, and an oxygen saturation of 100% while breathing room air. A focused HEENT (head, eyes, ears, nose, and throat) examination reveals a mass in the right conchal bowl measuring approximately 4-5 cm, with no overlying edema or erythema (Figure 1). The mass is firm to palpation and nontender. The remainder of the physical examination is unremarkable, despite a detailed search for occult adenopathy.\nFiberoptic endoscopy reveals no other abnormalities. Surgical excision for pathologic analysis is proposed to the patient, and he agrees to surgical excision of the right auricular mass (Figure 2). Histologic examination shows macrophages with engulfed lymphocytes (Figure 3). The pathologic analysis reveals positive staining for S100 (Figure 4) and CD68 (Figure 5), as well as many large histiocytic cells with abundant amphophilic cytoplasm and vesicular nuclei.\n\n ## Figures\n\n **Figure 1** \n \n\n**Figure 2** \n \n\n**Figure 3** \n \n\n**Figure 4** \n \n\n**Figure 5** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 872555,
"choiceText": "Non-Hodgkin lymphoma",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 872557,
"choiceText": "Multiple myeloma",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 872559,
"choiceText": "Neuroblastoma",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 872561,
"choiceText": "Rosai-Dorfman disease",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 274597,
"questionText": "What is the most likely diagnosis?<br/><br/>\r\n<em>Hint: Systemic diseases can present in the head and neck examination; also, note the emperipolesis seen on histology.</em>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 42-Year-Old Man With a Right Ear Mass"
},
{
"authors": "Kristin K. Egan, MD; Douglas K. Hanks, MD; David W. Kim, MD; Andrew H. Murr, MD",
"content": [
"Pathologic analysis from the surgical excision enabled a conclusive diagnosis of an auricular presentation of recurrent Rosai-Dorfman disease. The history of a response to steroid treatment also suggested a systemic process, which correlated with the history of cervical lymphadenopathy and an auricular mass. The pathologic findings for Rosai-Dorfman disease most commonly show positive staining for S100 (Figure 4) and CD68 (Figure 5), as well as many large histiocytic cells with abundant, amphophilic cytoplasm and vesicular nuclei.",
"Named after the physicians who discovered it, Rosai-Dorfman disease (sinus histiocytosis with massive lymphadenopathy) is a systemic disease that is a rare, self-limiting benign process. First described in 1969,[1] the incidence is very rare, with less than 1000 cases reported in the literature.",
"The etiology of Rosai-Dorfman disease is unknown, but it has been proposed to be linked to viral infection via human herpesvirus 6 and an undefined immunologic defect.[2] It most commonly involves the cervical lymph nodes; however, approximately 30% of patients have extranodal involvement.[3]",
"Rosai-Dorfman disease typically presents as a painless, massive, bilateral cervical lymphadenopathy associated with fever, leukocytosis with neutrophilia, an elevated erythrocyte sedimentation rate, and polyclonal hypergammaglobulinemia (specifically, of immunoglobulin G). Patients are commonly in their first and second decade of life and have no other underlying medical conditions. It is equally prevalent among the sexes but shows a predominance among black patients."
],
"date": "July 30, 2015",
"figures": [],
"markdown": "# A 42-Year-Old Man With a Right Ear Mass\n\n **Authors:** Kristin K. Egan, MD; Douglas K. Hanks, MD; David W. Kim, MD; Andrew H. Murr, MD \n **Date:** July 30, 2015\n\n ## Content\n\n Pathologic analysis from the surgical excision enabled a conclusive diagnosis of an auricular presentation of recurrent Rosai-Dorfman disease. The history of a response to steroid treatment also suggested a systemic process, which correlated with the history of cervical lymphadenopathy and an auricular mass. The pathologic findings for Rosai-Dorfman disease most commonly show positive staining for S100 (Figure 4) and CD68 (Figure 5), as well as many large histiocytic cells with abundant, amphophilic cytoplasm and vesicular nuclei.\nNamed after the physicians who discovered it, Rosai-Dorfman disease (sinus histiocytosis with massive lymphadenopathy) is a systemic disease that is a rare, self-limiting benign process. First described in 1969,[1] the incidence is very rare, with less than 1000 cases reported in the literature.\nThe etiology of Rosai-Dorfman disease is unknown, but it has been proposed to be linked to viral infection via human herpesvirus 6 and an undefined immunologic defect.[2] It most commonly involves the cervical lymph nodes; however, approximately 30% of patients have extranodal involvement.[3]\nRosai-Dorfman disease typically presents as a painless, massive, bilateral cervical lymphadenopathy associated with fever, leukocytosis with neutrophilia, an elevated erythrocyte sedimentation rate, and polyclonal hypergammaglobulinemia (specifically, of immunoglobulin G). Patients are commonly in their first and second decade of life and have no other underlying medical conditions. It is equally prevalent among the sexes but shows a predominance among black patients.\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 872555,
"choiceText": "Non-Hodgkin lymphoma",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 872557,
"choiceText": "Multiple myeloma",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 872559,
"choiceText": "Neuroblastoma",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 872561,
"choiceText": "Rosai-Dorfman disease",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 274597,
"questionText": "What is the most likely diagnosis?<br/><br/>\r\n<em>Hint: Systemic diseases can present in the head and neck examination; also, note the emperipolesis seen on histology.</em>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 42-Year-Old Man With a Right Ear Mass"
},
{
"authors": "Kristin K. Egan, MD; Douglas K. Hanks, MD; David W. Kim, MD; Andrew H. Murr, MD",
"content": [
"The appearance of extranodal manifestations of Rosai-Dorfman disease has been limited to case reports, and there have been only three other cases involving the ear. These involved bilateral cauliflower ears,[4] middle ear or external auditory canal lesions,[5] and a preauricular mass.[6] In 1990, Foucar, Rosai, and Dorfman[3] published an update on the disease, with 423 cases; this registry showed a 43% rate of extranodal lesions.[7] These lesions were most frequently found in the nasal cavity and paranasal sinuses.",
"Other sites of extranodal involvement include the soft tissues, central nervous system, orbits, skin, oral cavity, and bones. Presentation resulting from bone marrow involvement has not been seen since the disease was first identified. Patients may also present with amyloidosis, anemia, autoimmune lymphoproliferative syndrome, human immunodeficiency virus (HIV) infection, or lymphoma.",
"The disease can initially be identified in many different locations in the body. Central nervous system disease may present as meningitis, seizures, headache, and cranial nerve palsies. Rosai-Dorfman disease of the skin may present as papulonodular or plaque-like lesions. Other common locations and presentations include the kidneys (eg, presence of a renal mass), the oral cavity (eg, hypertrophy of the maxillary alveolus), the breasts (eg, breast mass), the cervix (cervical mass), and the vascular system (ie, development of vasculitis). It can also present in the gastrointestinal system as involvement of the liver, pancreas, or gastrointestinal tract.",
"Presentation in the head and neck region can be quite varied; Rosai-Dorfman disease can manifest in the salivary glands (as a salivary mass in the setting of lupus erythematosus), the larynx (as dysphonia), and the eyes (as uveitis, papilledema, or compressive orbital neuropathy). The most common presentation of this disease, however, is as a painless, massive cervical lymphadenopathy.",
"The main mechanism of lymphadenopathy is secondary to sinus histiocytosis and the stimulation of monocytes and macrophages via macrophage colony-stimulating factor (M-CSF). Monocytes are found in small amounts within the pulp of reactive lymph nodes, which secrete M-CSF. M-CSF acts as a chemotactic factor to recruit more blood monocytes. These monocytes are stimulated to differentiate into mature macrophages by M-CSF, as opposed to differentiating into dendritic cells when exposed to granulocyte/macrophage colony-stimulating factor.[8] Molecular studies have found no evidence of clonal rearrangement, implying that this disease is a reactive condition rather than a neoplastic one.",
"Ultimately, the diagnosis must be confirmed by pathologic analysis. The characteristic histology is that of a greatly enlarged lymph node, with dilatation of the sinuses. The pathologic features of Rosai-Dorfman disease are large histiocytes, with prominent emperipolesis and fine vacuoles in the cytoplasm, scattered in a background of benign lymphocytes, plasma cells, and polymorphonuclear leukocytes.[9]",
"Emperipolesis is a hallmark of Rosai-Dorfman disease and represents engulfment of the lymphocytes by the large histiocytes, as evidenced by a halo around the cell. Immunohistochemical analysis of the large histiocytes often shows strong positive cytoplasmic staining for S-100 protein. These cells do not stain positive for CD1, which is a commonly used marker of Langerhans cells.",
"Crystal deposition was reported as an isolated occurrence in which a skin biopsy showed classic features of cutaneous Rosai-Dorfman disease, along with conspicuous rhomboidal and needle-shaped crystals within the cytoplasm of many lesional plasma cells, histiocytes, and extracellular involvement. The plasma cells were polyclonal, which was determined by light-chain immunostaining. These crystals were probably a result of the reactive plasma cell proliferation. Staining with other markers has also been reported, including CD31, CD11c, CD14, CD33, CD68, and LN5. These cells will be negative on staining for CD1a."
],
"date": "July 30, 2015",
"figures": [],
"markdown": "# A 42-Year-Old Man With a Right Ear Mass\n\n **Authors:** Kristin K. Egan, MD; Douglas K. Hanks, MD; David W. Kim, MD; Andrew H. Murr, MD \n **Date:** July 30, 2015\n\n ## Content\n\n The appearance of extranodal manifestations of Rosai-Dorfman disease has been limited to case reports, and there have been only three other cases involving the ear. These involved bilateral cauliflower ears,[4] middle ear or external auditory canal lesions,[5] and a preauricular mass.[6] In 1990, Foucar, Rosai, and Dorfman[3] published an update on the disease, with 423 cases; this registry showed a 43% rate of extranodal lesions.[7] These lesions were most frequently found in the nasal cavity and paranasal sinuses.\nOther sites of extranodal involvement include the soft tissues, central nervous system, orbits, skin, oral cavity, and bones. Presentation resulting from bone marrow involvement has not been seen since the disease was first identified. Patients may also present with amyloidosis, anemia, autoimmune lymphoproliferative syndrome, human immunodeficiency virus (HIV) infection, or lymphoma.\nThe disease can initially be identified in many different locations in the body. Central nervous system disease may present as meningitis, seizures, headache, and cranial nerve palsies. Rosai-Dorfman disease of the skin may present as papulonodular or plaque-like lesions. Other common locations and presentations include the kidneys (eg, presence of a renal mass), the oral cavity (eg, hypertrophy of the maxillary alveolus), the breasts (eg, breast mass), the cervix (cervical mass), and the vascular system (ie, development of vasculitis). It can also present in the gastrointestinal system as involvement of the liver, pancreas, or gastrointestinal tract.\nPresentation in the head and neck region can be quite varied; Rosai-Dorfman disease can manifest in the salivary glands (as a salivary mass in the setting of lupus erythematosus), the larynx (as dysphonia), and the eyes (as uveitis, papilledema, or compressive orbital neuropathy). The most common presentation of this disease, however, is as a painless, massive cervical lymphadenopathy.\nThe main mechanism of lymphadenopathy is secondary to sinus histiocytosis and the stimulation of monocytes and macrophages via macrophage colony-stimulating factor (M-CSF). Monocytes are found in small amounts within the pulp of reactive lymph nodes, which secrete M-CSF. M-CSF acts as a chemotactic factor to recruit more blood monocytes. These monocytes are stimulated to differentiate into mature macrophages by M-CSF, as opposed to differentiating into dendritic cells when exposed to granulocyte/macrophage colony-stimulating factor.[8] Molecular studies have found no evidence of clonal rearrangement, implying that this disease is a reactive condition rather than a neoplastic one.\nUltimately, the diagnosis must be confirmed by pathologic analysis. The characteristic histology is that of a greatly enlarged lymph node, with dilatation of the sinuses. The pathologic features of Rosai-Dorfman disease are large histiocytes, with prominent emperipolesis and fine vacuoles in the cytoplasm, scattered in a background of benign lymphocytes, plasma cells, and polymorphonuclear leukocytes.[9]\nEmperipolesis is a hallmark of Rosai-Dorfman disease and represents engulfment of the lymphocytes by the large histiocytes, as evidenced by a halo around the cell. Immunohistochemical analysis of the large histiocytes often shows strong positive cytoplasmic staining for S-100 protein. These cells do not stain positive for CD1, which is a commonly used marker of Langerhans cells.\nCrystal deposition was reported as an isolated occurrence in which a skin biopsy showed classic features of cutaneous Rosai-Dorfman disease, along with conspicuous rhomboidal and needle-shaped crystals within the cytoplasm of many lesional plasma cells, histiocytes, and extracellular involvement. The plasma cells were polyclonal, which was determined by light-chain immunostaining. These crystals were probably a result of the reactive plasma cell proliferation. Staining with other markers has also been reported, including CD31, CD11c, CD14, CD33, CD68, and LN5. These cells will be negative on staining for CD1a.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 42-Year-Old Man With a Right Ear Mass"
},
{
"authors": "Kristin K. Egan, MD; Douglas K. Hanks, MD; David W. Kim, MD; Andrew H. Murr, MD",
"content": [
"The differential diagnosis of Rosai-Dorfman disease can be complex. The symptoms and physical findings associated with Rosai-Dorfman disease vary depending on the specific areas of the body that are affected. The differential diagnosis of a chronic inflammatory infiltrate containing many large histiocytes includes granulomatous diseases, such as Wegener granulomatosis, and sarcoid, Hodgkin disease, and Langerhans histiocytosis. Other diseases that should be considered when confronted with a patient whose symptoms may correlate with Rosai-Dorfman disease include lymphoma, rhinoscleroma, meningioma, and other types of cancers.",
"Treatment of Rosai-Dorfman disease most commonly involves radiation therapy, corticosteroids, or acyclovir (Zovirax®); chemotherapy is generally ineffective in treating the disease.[10] Observation of the lesions can be offered if the patient is not experiencing any adverse consequences; however, treatment for Rosai-Dorfman disease should be based on its clinical manifestations. Many lesions are asymptomatic, heal spontaneously, and do not require treatment. When destructive or disseminated disease is present, radiation therapy, surgical excision, systemic glucocorticoids, and alkylating agents may be used.",
"Rosai-Dorfman disease has a generally favorable prognosis, but involvement of a greater number of nodal groups and associated extranodal systems worsens the prognosis. Patients with associated immunologic abnormalities also usually have a less favorable prognosis than do patients without such abnormalities.",
"The patient in our case underwent a course of intravenous steroids in addition to the aforementioned surgical excision. On follow-up, his auricle was noted have healed well, with no local recurrence of the disease. On long-term follow-up several months later, he was noted to be systemically disease-free. In addition to follow-up with otolaryngology, the patient was followed by the oncology service, although no radiation therapy was administered."
],
"date": "July 30, 2015",
"figures": [],
"markdown": "# A 42-Year-Old Man With a Right Ear Mass\n\n **Authors:** Kristin K. Egan, MD; Douglas K. Hanks, MD; David W. Kim, MD; Andrew H. Murr, MD \n **Date:** July 30, 2015\n\n ## Content\n\n The differential diagnosis of Rosai-Dorfman disease can be complex. The symptoms and physical findings associated with Rosai-Dorfman disease vary depending on the specific areas of the body that are affected. The differential diagnosis of a chronic inflammatory infiltrate containing many large histiocytes includes granulomatous diseases, such as Wegener granulomatosis, and sarcoid, Hodgkin disease, and Langerhans histiocytosis. Other diseases that should be considered when confronted with a patient whose symptoms may correlate with Rosai-Dorfman disease include lymphoma, rhinoscleroma, meningioma, and other types of cancers.\nTreatment of Rosai-Dorfman disease most commonly involves radiation therapy, corticosteroids, or acyclovir (Zovirax®); chemotherapy is generally ineffective in treating the disease.[10] Observation of the lesions can be offered if the patient is not experiencing any adverse consequences; however, treatment for Rosai-Dorfman disease should be based on its clinical manifestations. Many lesions are asymptomatic, heal spontaneously, and do not require treatment. When destructive or disseminated disease is present, radiation therapy, surgical excision, systemic glucocorticoids, and alkylating agents may be used.\nRosai-Dorfman disease has a generally favorable prognosis, but involvement of a greater number of nodal groups and associated extranodal systems worsens the prognosis. Patients with associated immunologic abnormalities also usually have a less favorable prognosis than do patients without such abnormalities.\nThe patient in our case underwent a course of intravenous steroids in addition to the aforementioned surgical excision. On follow-up, his auricle was noted have healed well, with no local recurrence of the disease. On long-term follow-up several months later, he was noted to be systemically disease-free. In addition to follow-up with otolaryngology, the patient was followed by the oncology service, although no radiation therapy was administered.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 872563,
"choiceText": "Lymphocytic predominance",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 872565,
"choiceText": "Eosinophil predominance",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 872567,
"choiceText": "Histiocytes with engulfed lymphocytes",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 872569,
"choiceText": "Neutrophil predominance",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 872571,
"choiceText": "Macrophages engulfing eosinophils",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The hallmark of Rosai-Dorfman disease on histologic analysis is histiocytes with engulfed lymphocytes, also known as \"emperipolesis.\"",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 274599,
"questionText": "Rosai-Dorfman disease can be diagnosed histologically by which of the following findings?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 872573,
"choiceText": "Granulocyte/macrophage colony-stimulating factor",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 872575,
"choiceText": "Macrophage colony-stimulating factor",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 872577,
"choiceText": "Erythroid colony-stimulating factor",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 872579,
"choiceText": "Neutrophil colony-stimulating factor",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 872581,
"choiceText": "Interleukin-1",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Monocytes are found in small amounts within the pulp of reactive lymph nodes, which secrete M-CSF. M-CSF acts as chemotactic factor to recruit more blood monocytes. These monocytes are stimulated to differentiate into mature macrophages by M-CSF in Rosai-Dorfman disease.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 274601,
"questionText": "Which of the following factors contributes to lymphadenopathy associated with Rosai-Dorfman disease?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 42-Year-Old Man With a Right Ear Mass"
},
{
"authors": "Kristin K. Egan, MD; Douglas K. Hanks, MD; David W. Kim, MD; Andrew H. Murr, MD",
"content": [],
"date": "July 30, 2015",
"figures": [],
"markdown": "# A 42-Year-Old Man With a Right Ear Mass\n\n **Authors:** Kristin K. Egan, MD; Douglas K. Hanks, MD; David W. Kim, MD; Andrew H. Murr, MD \n **Date:** July 30, 2015\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 872563,
"choiceText": "Lymphocytic predominance",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 872565,
"choiceText": "Eosinophil predominance",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 872567,
"choiceText": "Histiocytes with engulfed lymphocytes",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 872569,
"choiceText": "Neutrophil predominance",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 872571,
"choiceText": "Macrophages engulfing eosinophils",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The hallmark of Rosai-Dorfman disease on histologic analysis is histiocytes with engulfed lymphocytes, also known as \"emperipolesis.\"",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 274599,
"questionText": "Rosai-Dorfman disease can be diagnosed histologically by which of the following findings?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 872573,
"choiceText": "Granulocyte/macrophage colony-stimulating factor",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 872575,
"choiceText": "Macrophage colony-stimulating factor",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 872577,
"choiceText": "Erythroid colony-stimulating factor",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 872579,
"choiceText": "Neutrophil colony-stimulating factor",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 872581,
"choiceText": "Interleukin-1",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Monocytes are found in small amounts within the pulp of reactive lymph nodes, which secrete M-CSF. M-CSF acts as chemotactic factor to recruit more blood monocytes. These monocytes are stimulated to differentiate into mature macrophages by M-CSF in Rosai-Dorfman disease.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 274601,
"questionText": "Which of the following factors contributes to lymphadenopathy associated with Rosai-Dorfman disease?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 42-Year-Old Man With a Right Ear Mass"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 872555,
"choiceText": "Non-Hodgkin lymphoma",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 872557,
"choiceText": "Multiple myeloma",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 872559,
"choiceText": "Neuroblastoma",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 872561,
"choiceText": "Rosai-Dorfman disease",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 274597,
"questionText": "What is the most likely diagnosis?<br/><br/>\r\n<em>Hint: Systemic diseases can present in the head and neck examination; also, note the emperipolesis seen on histology.</em>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 872563,
"choiceText": "Lymphocytic predominance",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 872565,
"choiceText": "Eosinophil predominance",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 872567,
"choiceText": "Histiocytes with engulfed lymphocytes",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 872569,
"choiceText": "Neutrophil predominance",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 872571,
"choiceText": "Macrophages engulfing eosinophils",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The hallmark of Rosai-Dorfman disease on histologic analysis is histiocytes with engulfed lymphocytes, also known as \"emperipolesis.\"",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 274599,
"questionText": "Rosai-Dorfman disease can be diagnosed histologically by which of the following findings?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 872573,
"choiceText": "Granulocyte/macrophage colony-stimulating factor",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 872575,
"choiceText": "Macrophage colony-stimulating factor",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 872577,
"choiceText": "Erythroid colony-stimulating factor",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 872579,
"choiceText": "Neutrophil colony-stimulating factor",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 872581,
"choiceText": "Interleukin-1",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Monocytes are found in small amounts within the pulp of reactive lymph nodes, which secrete M-CSF. M-CSF acts as chemotactic factor to recruit more blood monocytes. These monocytes are stimulated to differentiate into mature macrophages by M-CSF in Rosai-Dorfman disease.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 274601,
"questionText": "Which of the following factors contributes to lymphadenopathy associated with Rosai-Dorfman disease?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
847992 | /viewarticle/847992 | [
{
"authors": "Lars Grimm, MD",
"content": [
"Editor's Note:\n\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.",
"A 34-year-old woman presents to the emergency department (ED) with a 2-hour history of severe abdominal pain, two episodes of light-headedness, and loss of consciousness. She states that she is about 6 weeks pregnant and that the pain started suddenly the morning of presentation. She went to the bathroom and sat on the toilet when she felt light-headed. After her second time passing out, she alerted her husband, who called for an ambulance. She denies any vaginal bleeding or discharge. No nausea or vomiting is reported, and the patient has not experienced any fevers.",
"The patient has visited an obstetrician for prenatal care but has not yet had ultrasonography. The patient is currently G3P1011; she had a spontaneous abortion that complicated a prior pregnancy 4 years ago. She has no history of pelvic inflammatory disease or prior ectopic pregnancy. She is currently not undergoing ovulatory induction. She does not have an IUD. The patient has no chronic medical problems and takes no medications regularly. She does not drink alcohol and does not use any illicit substances."
],
"date": "July 15, 2015",
"figures": [],
"markdown": "# A 34-Year-Old Pregnant Woman With Acute, Severe Abdominal Pain\n\n **Authors:** Lars Grimm, MD \n **Date:** July 15, 2015\n\n ## Content\n\n Editor's Note:\n\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.\nA 34-year-old woman presents to the emergency department (ED) with a 2-hour history of severe abdominal pain, two episodes of light-headedness, and loss of consciousness. She states that she is about 6 weeks pregnant and that the pain started suddenly the morning of presentation. She went to the bathroom and sat on the toilet when she felt light-headed. After her second time passing out, she alerted her husband, who called for an ambulance. She denies any vaginal bleeding or discharge. No nausea or vomiting is reported, and the patient has not experienced any fevers.\nThe patient has visited an obstetrician for prenatal care but has not yet had ultrasonography. The patient is currently G3P1011; she had a spontaneous abortion that complicated a prior pregnancy 4 years ago. She has no history of pelvic inflammatory disease or prior ectopic pregnancy. She is currently not undergoing ovulatory induction. She does not have an IUD. The patient has no chronic medical problems and takes no medications regularly. She does not drink alcohol and does not use any illicit substances.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 34-Year-Old Pregnant Woman With Acute, Severe Abdominal Pain"
},
{
"authors": "Lars Grimm, MD",
"content": [
"On physical examination, her blood pressure is 86/55 mm Hg, and she is noted to be tachycardic, with a regular rhythm at approximately 130 beats/min. She has a normal oral temperature of 98.9° F (37.2° C) and a respiratory rate of 12 breaths/min. She appears pale and visibly frightened. The pulmonary and cardiac examinations are otherwise unremarkable. The patient's abdomen is slightly distended, with diffuse tenderness to palpation that is most severe in the left lower quadrant. An external examination of the perineum is unremarkable for fluid or blood. On pelvic examination, a moderate amount of blood is noted in the vaginal vault, with tenderness to palpation in the left adnexa. The uterus is normal sized. No adnexal masses are palpable. No lower limb edema is noted, and the patient has normal peripheral pulses.",
"Figure 1.",
"Figure 1.",
"The patient is hooked up to central monitoring, and two large-bore, peripheral intravenous lines are placed. A complete set of laboratory investigations is sent, and 2 L of normal saline are rapidly infused. The patient's heart rate responds by decreasing to approximately 99 beats/min, and her blood pressure rebounds to 104/55 mm Hg. A bedside ultrasonographic examination is performed, and it demonstrates a hypoechoic stripe indicative of free fluid in the right upper quadrant between the liver and the kidney (Figure 1). As a result, stat formal ultrasonography is performed to confirm the finding of the bedside one and to identify a specific etiology for it; in the meantime, a stat obstetric consult is called to prepare the patient for the operating room.",
"Figure 2.",
"Figure 2.",
"Figure 3.",
"Figure 3.",
"Her laboratory tests include a basic metabolic panel; the results are normal. Her complete blood cell (CBC) count shows a low hemoglobin of 7.3 g/dL (73 g/L); a slightly elevated white blood cell (WBC) count of 13.8 × 103/µL (13.8 ×109/L), with a normal differential; and a normal platelet count. The basic metabolic panel is unremarkable. A urine pregnancy test is positive. A coagulation profile and a quantitative beta-human chorionic gonadotropin (beta-hCG) are drawn.",
"Images of formal transvaginal ultrasonography are also presented (Figures 2 and 3)."
],
"date": "July 15, 2015",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/847/992/847992-Thumb1.png"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/847/992/847992-Thumb2.png"
},
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/847/992/847992-Thumb3.png"
}
],
"markdown": "# A 34-Year-Old Pregnant Woman With Acute, Severe Abdominal Pain\n\n **Authors:** Lars Grimm, MD \n **Date:** July 15, 2015\n\n ## Content\n\n On physical examination, her blood pressure is 86/55 mm Hg, and she is noted to be tachycardic, with a regular rhythm at approximately 130 beats/min. She has a normal oral temperature of 98.9° F (37.2° C) and a respiratory rate of 12 breaths/min. She appears pale and visibly frightened. The pulmonary and cardiac examinations are otherwise unremarkable. The patient's abdomen is slightly distended, with diffuse tenderness to palpation that is most severe in the left lower quadrant. An external examination of the perineum is unremarkable for fluid or blood. On pelvic examination, a moderate amount of blood is noted in the vaginal vault, with tenderness to palpation in the left adnexa. The uterus is normal sized. No adnexal masses are palpable. No lower limb edema is noted, and the patient has normal peripheral pulses.\nFigure 1.\nFigure 1.\nThe patient is hooked up to central monitoring, and two large-bore, peripheral intravenous lines are placed. A complete set of laboratory investigations is sent, and 2 L of normal saline are rapidly infused. The patient's heart rate responds by decreasing to approximately 99 beats/min, and her blood pressure rebounds to 104/55 mm Hg. A bedside ultrasonographic examination is performed, and it demonstrates a hypoechoic stripe indicative of free fluid in the right upper quadrant between the liver and the kidney (Figure 1). As a result, stat formal ultrasonography is performed to confirm the finding of the bedside one and to identify a specific etiology for it; in the meantime, a stat obstetric consult is called to prepare the patient for the operating room.\nFigure 2.\nFigure 2.\nFigure 3.\nFigure 3.\nHer laboratory tests include a basic metabolic panel; the results are normal. Her complete blood cell (CBC) count shows a low hemoglobin of 7.3 g/dL (73 g/L); a slightly elevated white blood cell (WBC) count of 13.8 × 103/µL (13.8 ×109/L), with a normal differential; and a normal platelet count. The basic metabolic panel is unremarkable. A urine pregnancy test is positive. A coagulation profile and a quantitative beta-human chorionic gonadotropin (beta-hCG) are drawn.\nImages of formal transvaginal ultrasonography are also presented (Figures 2 and 3).\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n**Figure 3.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 869619,
"choiceText": "Ruptured corpus luteum cyst",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 869621,
"choiceText": "Placenta previa",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 869623,
"choiceText": "Threatened spontaneous abortion",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 869625,
"choiceText": "Ruptured ectopic pregnancy \r\n",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 273625,
"questionText": "On the basis of the history, physical examination, and workup, what is the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 34-Year-Old Pregnant Woman With Acute, Severe Abdominal Pain"
},
{
"authors": "Lars Grimm, MD",
"content": [
"The presentation of severe abdominal pain, hemodynamic instability, and transient loss of consciousness in the setting of a nonconfirmed intrauterine pregnancy raised the suspicion for a ruptured ectopic pregnancy. The demonstration of free fluid in the Morrison pouch on bedside ultrasonography confirmed the presence of intra-abdominal hemorrhage, and confirmatory transvaginal ultrasonography demonstrated no viable intrauterine pregnancy. The beta-hCG was confirmed at 12,200 mIU/mL. At surgery, an ectopic pregnancy was located within the left fallopian tube.",
"The incidence of ectopic pregnancy is 19.7 per 1000 pregnancies in the United States; however, the prevalence is between 6% and 16% in patients who present with first-trimester bleeding and/or pain.[1] This rate has increased over the past several decades as a result of better surveillance and the increased use of in vitro fertilization techniques to induce conception. The incidence of ectopic pregnancy increases with age, with the highest rates occurring in women aged 35-44 years. Ectopic pregnancies are responsible for approximately 10% of pregnancy-related deaths, because if they are untreated, ectopic pregnancies may lead to eventual rupture and hemodynamic collapse.[2]",
"The prime window for developing an ectopic pregnancy is approximately 6-8 weeks after the last menstrual period. The classic triad of findings is abdominal pain, vaginal bleeding, and amenorrhea; however, in only 45% of patients are all of these findings present.[3] Hypotension, light-headedness, or other signs or symptoms of hemodynamic instability raise the concern of a rupture.",
"Ectopic pregnancy occurs as a result of extrauterine implantation of the developing blastocyst. Although the vast majority of ectopic pregnancies occur within the fallopian tubes (95%-98%), they may rarely be found in the cervix (1%), abdominal cavity (< 1%), or cesarean scar sites (< 1%). Risk factors for the development of ectopic pregnancy include current intrauterine device usage, tubal surgery, pelvic inflammatory disease, use of assisted reproductive technology, in utero diethylstilbestrol exposure, infertility, cervicitis, and a history of ectopic pregnancy; however, the majority of patients have no known risk factors."
],
"date": "July 15, 2015",
"figures": [],
"markdown": "# A 34-Year-Old Pregnant Woman With Acute, Severe Abdominal Pain\n\n **Authors:** Lars Grimm, MD \n **Date:** July 15, 2015\n\n ## Content\n\n The presentation of severe abdominal pain, hemodynamic instability, and transient loss of consciousness in the setting of a nonconfirmed intrauterine pregnancy raised the suspicion for a ruptured ectopic pregnancy. The demonstration of free fluid in the Morrison pouch on bedside ultrasonography confirmed the presence of intra-abdominal hemorrhage, and confirmatory transvaginal ultrasonography demonstrated no viable intrauterine pregnancy. The beta-hCG was confirmed at 12,200 mIU/mL. At surgery, an ectopic pregnancy was located within the left fallopian tube.\nThe incidence of ectopic pregnancy is 19.7 per 1000 pregnancies in the United States; however, the prevalence is between 6% and 16% in patients who present with first-trimester bleeding and/or pain.[1] This rate has increased over the past several decades as a result of better surveillance and the increased use of in vitro fertilization techniques to induce conception. The incidence of ectopic pregnancy increases with age, with the highest rates occurring in women aged 35-44 years. Ectopic pregnancies are responsible for approximately 10% of pregnancy-related deaths, because if they are untreated, ectopic pregnancies may lead to eventual rupture and hemodynamic collapse.[2]\nThe prime window for developing an ectopic pregnancy is approximately 6-8 weeks after the last menstrual period. The classic triad of findings is abdominal pain, vaginal bleeding, and amenorrhea; however, in only 45% of patients are all of these findings present.[3] Hypotension, light-headedness, or other signs or symptoms of hemodynamic instability raise the concern of a rupture.\nEctopic pregnancy occurs as a result of extrauterine implantation of the developing blastocyst. Although the vast majority of ectopic pregnancies occur within the fallopian tubes (95%-98%), they may rarely be found in the cervix (1%), abdominal cavity (< 1%), or cesarean scar sites (< 1%). Risk factors for the development of ectopic pregnancy include current intrauterine device usage, tubal surgery, pelvic inflammatory disease, use of assisted reproductive technology, in utero diethylstilbestrol exposure, infertility, cervicitis, and a history of ectopic pregnancy; however, the majority of patients have no known risk factors.\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 869619,
"choiceText": "Ruptured corpus luteum cyst",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 869621,
"choiceText": "Placenta previa",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 869623,
"choiceText": "Threatened spontaneous abortion",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 869625,
"choiceText": "Ruptured ectopic pregnancy \r\n",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 273625,
"questionText": "On the basis of the history, physical examination, and workup, what is the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 34-Year-Old Pregnant Woman With Acute, Severe Abdominal Pain"
},
{
"authors": "Lars Grimm, MD",
"content": [
"The differential diagnosis for a pregnant patient with abdominal pain must include obstetric, gynecologic, gastrointestinal, and renal etiologies. The obstetric and gynecologic causes include normal early pregnancy, spontaneous abortion, molar pregnancy, degenerating uterine leiomyoma, pelvic inflammatory disease, ovarian torsion, dysfunctional uterine bleeding, dysmenorrhea, tubo-ovarian abscess, and hemorrhagic corpus luteum cyst. The major gastrointestinal and renal causes include appendicitis, diverticulitis, nephrolithiasis, and urinary tract infections.",
"The major goals of early detection of ectopic pregnancy are to prevent the major morbidities and mortality associated with an eventual rupture and to allow time for effective medical management of the condition. The diagnostic workup begins with a thorough history, with particular attention focused on the above-described risk factors. Although confirmation or exclusion of an ectopic pregnancy is not possible on physical examination alone, valuable findings, such as adnexal fullness and tenderness, may be appreciated. The vital signs must be closely monitored, because a change in hemodynamic status may be the only overt sign of a rupture.",
"Laboratory and imaging studies are necessary for definitive evaluation. A positive urine hCG will confirm pregnancy status, while a low serum hCG value (for the gestational age) advances the case for an ectopic pregnancy.[4] Low serum progesterone levels indicate a nonviable pregnancy, but they are not helpful in determining the pregnancy location. No serum marker exists to accurately distinguish an ectopic from an intrauterine pregnancy.[5] Transabdominal and/or transvaginal ultrasonographic imaging are the most useful diagnostic tests for evaluating pregnancy. These examinations allow for confirmation of an intrauterine pregnancy and may demonstrate direct signs (eg, a developing blastocyst within a fallopian tube, which is seen in only a minority of cases) or indirect signs (eg, free fluid, lack of intrauterine pregnancy) of an ectopic pregnancy; however, because of the large number of potential alternative foci, it may not be possible to locate an ectopic pregnancy.",
"The concept of a discriminatory zone of the beta-hCG level (ie, the level at which an intrauterine pregnancy should be visible; this is 6000-6500 mIU/mL on transabdominal ultrasonography and 1500-1800 mIU/mL for high-resolution, transvaginal ultrasonography) can validate the findings of the ultrasound.[6] If ultrasonographic views at these levels do not demonstrate an intrauterine pregnancy, the pregnancy is most likely abnormal (and can either be an abnormal intrauterine pregnancy or an extrauterine pregnancy).",
"A visualized intrauterine pregnancy does not absolutely exclude an additional ectopic pregnancy. Heterotopic pregnancy is rare, with a rate of 1 in 30,000-80,000 cases for spontaneous pregnancy, but the incidence is greater in patients utilizing assisted reproduction strategies. If the clinical suspicion is very high and an ectopic pregnancy cannot be confirmed or excluded by other means, direct, intraoperative laparoscopic visualization may then be necessary. The combination of serum hCG and transvaginal ultrasonographic findings, however, is enough to confirm the presence of an ectopic pregnancy. Early consultation with colleagues in obstetrics and gynecology is necessary for cases of suspected ectopic pregnancy, with or without rupture."
],
"date": "July 15, 2015",
"figures": [],
"markdown": "# A 34-Year-Old Pregnant Woman With Acute, Severe Abdominal Pain\n\n **Authors:** Lars Grimm, MD \n **Date:** July 15, 2015\n\n ## Content\n\n The differential diagnosis for a pregnant patient with abdominal pain must include obstetric, gynecologic, gastrointestinal, and renal etiologies. The obstetric and gynecologic causes include normal early pregnancy, spontaneous abortion, molar pregnancy, degenerating uterine leiomyoma, pelvic inflammatory disease, ovarian torsion, dysfunctional uterine bleeding, dysmenorrhea, tubo-ovarian abscess, and hemorrhagic corpus luteum cyst. The major gastrointestinal and renal causes include appendicitis, diverticulitis, nephrolithiasis, and urinary tract infections.\nThe major goals of early detection of ectopic pregnancy are to prevent the major morbidities and mortality associated with an eventual rupture and to allow time for effective medical management of the condition. The diagnostic workup begins with a thorough history, with particular attention focused on the above-described risk factors. Although confirmation or exclusion of an ectopic pregnancy is not possible on physical examination alone, valuable findings, such as adnexal fullness and tenderness, may be appreciated. The vital signs must be closely monitored, because a change in hemodynamic status may be the only overt sign of a rupture.\nLaboratory and imaging studies are necessary for definitive evaluation. A positive urine hCG will confirm pregnancy status, while a low serum hCG value (for the gestational age) advances the case for an ectopic pregnancy.[4] Low serum progesterone levels indicate a nonviable pregnancy, but they are not helpful in determining the pregnancy location. No serum marker exists to accurately distinguish an ectopic from an intrauterine pregnancy.[5] Transabdominal and/or transvaginal ultrasonographic imaging are the most useful diagnostic tests for evaluating pregnancy. These examinations allow for confirmation of an intrauterine pregnancy and may demonstrate direct signs (eg, a developing blastocyst within a fallopian tube, which is seen in only a minority of cases) or indirect signs (eg, free fluid, lack of intrauterine pregnancy) of an ectopic pregnancy; however, because of the large number of potential alternative foci, it may not be possible to locate an ectopic pregnancy.\nThe concept of a discriminatory zone of the beta-hCG level (ie, the level at which an intrauterine pregnancy should be visible; this is 6000-6500 mIU/mL on transabdominal ultrasonography and 1500-1800 mIU/mL for high-resolution, transvaginal ultrasonography) can validate the findings of the ultrasound.[6] If ultrasonographic views at these levels do not demonstrate an intrauterine pregnancy, the pregnancy is most likely abnormal (and can either be an abnormal intrauterine pregnancy or an extrauterine pregnancy).\nA visualized intrauterine pregnancy does not absolutely exclude an additional ectopic pregnancy. Heterotopic pregnancy is rare, with a rate of 1 in 30,000-80,000 cases for spontaneous pregnancy, but the incidence is greater in patients utilizing assisted reproduction strategies. If the clinical suspicion is very high and an ectopic pregnancy cannot be confirmed or excluded by other means, direct, intraoperative laparoscopic visualization may then be necessary. The combination of serum hCG and transvaginal ultrasonographic findings, however, is enough to confirm the presence of an ectopic pregnancy. Early consultation with colleagues in obstetrics and gynecology is necessary for cases of suspected ectopic pregnancy, with or without rupture.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 34-Year-Old Pregnant Woman With Acute, Severe Abdominal Pain"
},
{
"authors": "Lars Grimm, MD",
"content": [
"The natural history of ectopic pregnancy is balanced between medical interventions and surgical management to reduce the risk for rupture and subsequent morbidity or mortality.[1] If there is no rupture and the patient is stable, then medical and surgical treatments are available. Medical management of ectopic pregnancy involves either single- or multiple-dose regimens of methotrexate, and it is the preferred alternative whenever possible.",
"Medically managed patients have a slightly increased rate of long-term complications when compared with surgical patients. Surgical intervention usually takes the form of a laparoscopic salpingostomy or salpingectomy, and it has comparable short-term outcomes to medical management; in cases of a ruptured ectopic pregnancy, however, surgical intervention is the most appropriate course of action. It is possible for an ectopic pregnancy to spontaneously regress; in a small subset of patients with very low serum hCG without an identifiable implantation site on ultrasonographic examination (ectopic pregnancy or incomplete spontaneous abortion may be possible in this scenario), expectant management may be considered.",
"This aforementioned patient in this particular case was immediately prepped and taken to the operating room. A laparoscope was placed, and a large amount of clot was identified in the pelvis and bilateral pericolic gutters. The left fallopian tube was visualized and was found to contain a 2-cm ruptured ectopic pregnancy that was actively bleeding. The right adnexal region was found to be grossly normal. Under laparoscopic guidance, a partial salpingectomy of the middle portion of the left fallopian tube was performed to remove the ectopic pregnancy and control the bleeding. Histologic examination of the removed tissue confirmed the ectopic pregnancy. The patient was taken to the recovery room in stable condition and was later transferred to the obstetrics and gynecology floor, where her recovery was unremarkable. At 2 days post-op, the patient was noted to be recovering well. She was sent home with instructions for outpatient follow-up."
],
"date": "July 15, 2015",
"figures": [],
"markdown": "# A 34-Year-Old Pregnant Woman With Acute, Severe Abdominal Pain\n\n **Authors:** Lars Grimm, MD \n **Date:** July 15, 2015\n\n ## Content\n\n The natural history of ectopic pregnancy is balanced between medical interventions and surgical management to reduce the risk for rupture and subsequent morbidity or mortality.[1] If there is no rupture and the patient is stable, then medical and surgical treatments are available. Medical management of ectopic pregnancy involves either single- or multiple-dose regimens of methotrexate, and it is the preferred alternative whenever possible.\nMedically managed patients have a slightly increased rate of long-term complications when compared with surgical patients. Surgical intervention usually takes the form of a laparoscopic salpingostomy or salpingectomy, and it has comparable short-term outcomes to medical management; in cases of a ruptured ectopic pregnancy, however, surgical intervention is the most appropriate course of action. It is possible for an ectopic pregnancy to spontaneously regress; in a small subset of patients with very low serum hCG without an identifiable implantation site on ultrasonographic examination (ectopic pregnancy or incomplete spontaneous abortion may be possible in this scenario), expectant management may be considered.\nThis aforementioned patient in this particular case was immediately prepped and taken to the operating room. A laparoscope was placed, and a large amount of clot was identified in the pelvis and bilateral pericolic gutters. The left fallopian tube was visualized and was found to contain a 2-cm ruptured ectopic pregnancy that was actively bleeding. The right adnexal region was found to be grossly normal. Under laparoscopic guidance, a partial salpingectomy of the middle portion of the left fallopian tube was performed to remove the ectopic pregnancy and control the bleeding. Histologic examination of the removed tissue confirmed the ectopic pregnancy. The patient was taken to the recovery room in stable condition and was later transferred to the obstetrics and gynecology floor, where her recovery was unremarkable. At 2 days post-op, the patient was noted to be recovering well. She was sent home with instructions for outpatient follow-up.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 869627,
"choiceText": "Cervix",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 869629,
"choiceText": "Abdominal cavity",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 869631,
"choiceText": "Fallopian tube",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 869633,
"choiceText": "Old scar site",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 869635,
"choiceText": "Vagina\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The fallopian tube is the site of implantation in approximately 95% of ectopic pregnancies.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 273627,
"questionText": "What is the most common site of implantation for an ectopic pregnancy?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 869637,
"choiceText": "Elevated serum hCG",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 869639,
"choiceText": "Intrauterine pregnancy on ultrasonographic examination",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 869641,
"choiceText": "Normal physical examination",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 869643,
"choiceText": "Low serum progesterone",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 869645,
"choiceText": "None of the above ",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "None of the above answers can definitively exclude an ectopic pregnancy. A low serum hCG may indicate an ectopic pregnancy. Heterotopic pregnancies have implantation sites within the uterus, as well as ectopically. The physical examination can provide only nonspecific information about an ectopic pregnancy. A low serum progesterone only indicates that the pregnancy is not viable. Diagnosis of an ectopic pregnancy requires either direct visualization or a constellation of compatible findings on imaging studies, laboratory studies, patient history, and physical examination.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 273629,
"questionText": "Which of the following conditions excludes the diagnosis of ectopic pregnancy?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 34-Year-Old Pregnant Woman With Acute, Severe Abdominal Pain"
},
{
"authors": "Lars Grimm, MD",
"content": [],
"date": "July 15, 2015",
"figures": [],
"markdown": "# A 34-Year-Old Pregnant Woman With Acute, Severe Abdominal Pain\n\n **Authors:** Lars Grimm, MD \n **Date:** July 15, 2015\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 869627,
"choiceText": "Cervix",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 869629,
"choiceText": "Abdominal cavity",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 869631,
"choiceText": "Fallopian tube",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 869633,
"choiceText": "Old scar site",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 869635,
"choiceText": "Vagina\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The fallopian tube is the site of implantation in approximately 95% of ectopic pregnancies.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 273627,
"questionText": "What is the most common site of implantation for an ectopic pregnancy?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 869637,
"choiceText": "Elevated serum hCG",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 869639,
"choiceText": "Intrauterine pregnancy on ultrasonographic examination",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 869641,
"choiceText": "Normal physical examination",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 869643,
"choiceText": "Low serum progesterone",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 869645,
"choiceText": "None of the above ",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "None of the above answers can definitively exclude an ectopic pregnancy. A low serum hCG may indicate an ectopic pregnancy. Heterotopic pregnancies have implantation sites within the uterus, as well as ectopically. The physical examination can provide only nonspecific information about an ectopic pregnancy. A low serum progesterone only indicates that the pregnancy is not viable. Diagnosis of an ectopic pregnancy requires either direct visualization or a constellation of compatible findings on imaging studies, laboratory studies, patient history, and physical examination.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 273629,
"questionText": "Which of the following conditions excludes the diagnosis of ectopic pregnancy?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 34-Year-Old Pregnant Woman With Acute, Severe Abdominal Pain"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 869619,
"choiceText": "Ruptured corpus luteum cyst",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 869621,
"choiceText": "Placenta previa",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 869623,
"choiceText": "Threatened spontaneous abortion",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 869625,
"choiceText": "Ruptured ectopic pregnancy \r\n",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 273625,
"questionText": "On the basis of the history, physical examination, and workup, what is the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 869627,
"choiceText": "Cervix",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 869629,
"choiceText": "Abdominal cavity",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 869631,
"choiceText": "Fallopian tube",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 869633,
"choiceText": "Old scar site",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 869635,
"choiceText": "Vagina\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The fallopian tube is the site of implantation in approximately 95% of ectopic pregnancies.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 273627,
"questionText": "What is the most common site of implantation for an ectopic pregnancy?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 869637,
"choiceText": "Elevated serum hCG",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 869639,
"choiceText": "Intrauterine pregnancy on ultrasonographic examination",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 869641,
"choiceText": "Normal physical examination",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 869643,
"choiceText": "Low serum progesterone",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 869645,
"choiceText": "None of the above ",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "None of the above answers can definitively exclude an ectopic pregnancy. A low serum hCG may indicate an ectopic pregnancy. Heterotopic pregnancies have implantation sites within the uterus, as well as ectopically. The physical examination can provide only nonspecific information about an ectopic pregnancy. A low serum progesterone only indicates that the pregnancy is not viable. Diagnosis of an ectopic pregnancy requires either direct visualization or a constellation of compatible findings on imaging studies, laboratory studies, patient history, and physical examination.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 273629,
"questionText": "Which of the following conditions excludes the diagnosis of ectopic pregnancy?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
846811 | /viewarticle/846811 | [
{
"authors": "Sidra Aurangzeb, MBBS; Muhammad Tariq, MRCP, FRCP, FRCPE",
"content": [
"Editor's Note:\n\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.",
"A 17-year-old girl with multiple symptoms is brought to an outpatient clinic by her parents. Her parents have noticed that she has developed slowly progressive clumsiness over the past 6 months. She now displays dystonic movement of all of her limbs. In addition, her speech and ability to walk have been deteriorating over several months. She was also recently noted to have excessive salivation.",
"There is no history of fever, headache, focal weakness, visual changes, bladder dysfunction, convulsions, or trauma. The patient has had no recent travel and no history of animal bites or known toxic exposures.",
"One year ago, the patient had jaundice that persisted for 4 months and then spontaneously resolved. A medical workup at that time did not reveal a clear etiology for the jaundice, nor evidence of liver failure, such as coagulopathy, ascites, or altered mental status. She is otherwise healthy, with no chronic medical conditions, and the family history is unremarkable.",
"The patient is the third child of a nonconsanguineous marriage. Her birth history is unremarkable, and she has met the normal developmental milestones. She denies the use of any medications, alcohol, or recreational drugs."
],
"date": "June 24, 2015",
"figures": [],
"markdown": "# Young Girl With Clumsiness, Dystonia, and Speech Difficulty\n\n **Authors:** Sidra Aurangzeb, MBBS; Muhammad Tariq, MRCP, FRCP, FRCPE \n **Date:** June 24, 2015\n\n ## Content\n\n Editor's Note:\n\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.\nA 17-year-old girl with multiple symptoms is brought to an outpatient clinic by her parents. Her parents have noticed that she has developed slowly progressive clumsiness over the past 6 months. She now displays dystonic movement of all of her limbs. In addition, her speech and ability to walk have been deteriorating over several months. She was also recently noted to have excessive salivation.\nThere is no history of fever, headache, focal weakness, visual changes, bladder dysfunction, convulsions, or trauma. The patient has had no recent travel and no history of animal bites or known toxic exposures.\nOne year ago, the patient had jaundice that persisted for 4 months and then spontaneously resolved. A medical workup at that time did not reveal a clear etiology for the jaundice, nor evidence of liver failure, such as coagulopathy, ascites, or altered mental status. She is otherwise healthy, with no chronic medical conditions, and the family history is unremarkable.\nThe patient is the third child of a nonconsanguineous marriage. Her birth history is unremarkable, and she has met the normal developmental milestones. She denies the use of any medications, alcohol, or recreational drugs.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "Young Girl With Clumsiness, Dystonia, and Speech Difficulty"
},
{
"authors": "Sidra Aurangzeb, MBBS; Muhammad Tariq, MRCP, FRCP, FRCPE",
"content": [
"Upon physical examination, the patient appears mildly jaundiced but is otherwise well-appearing and in no distress. Her mucous membranes are moist.",
"The ophthalmic examination shows icteric sclera, normal pupillary reactions to light, normal visual acuity and fields, and normal optic fundi. Dark, brown-colored rings are noted around the periphery of the iris and are visible on naked-eye examination; this is confirmed with a slit-lamp examination.",
"Neurologic examination reveals slow mentation, slurred speech, ataxic gait, diffuse muscle rigidity, and a fine resting tremor. The findings are symmetric, and the patient's reflexes are brisk bilaterally, with flexor plantar responses. The liver, palpated at three finger-breadths below the costal margin, is enlarged, firm, and nontender. The spleen is not palpable, and shifting dullness is not present. No parotid enlargement, palmar erythema, gynecomastia, or spider nevi are observed. The chest and cardiovascular examinations are unremarkable.",
"A complete blood cell count and erythrocyte sedimentation rate are both within normal limits. A hepatic panel reveals a total bilirubin level of 3.6 mg/dL, an alanine aminotransferase of 99 U/L level, an alkaline phosphatase level of 284 U/L, an albumin of 4.4 g/dL, and a prothrombin time of 18 seconds. Markers for hepatitis B and C are negative.",
"Figure 1.",
"MRI of the patient's brain. Image from Kim TJ, Kim IO, Kim WS, et al. MR imaging of the brain in Wilson disease of childhood: findings before and after treatment with clinical correlation. Am J Neuroradiol. 2008;27:1373-1378. ©American Society of Neuroradiology.",
"MRI of the patient's brain. Image from Kim TJ, Kim IO, Kim WS, et al. MR imaging of the brain in Wilson disease of childhood: findings before and after treatment with clinical correlation. Am J Neuroradiol. 2008;27:1373-1378. ©American Society of Neuroradiology.",
"MRI of the brain is performed (Figure 1)."
],
"date": "June 24, 2015",
"figures": [
{
"caption": "Figure 1.MRI of the patient's brain. Image from Kim TJ, Kim IO, Kim WS, et al. MR imaging of the brain in Wilson disease of childhood: findings before and after treatment with clinical correlation. Am J Neuroradiol. 2008;27:1373-1378. ©American Society of Neuroradiology.",
"image_url": "https://img.medscapestatic.com/article/846/811/846811-Thumb1.jpg"
}
],
"markdown": "# Young Girl With Clumsiness, Dystonia, and Speech Difficulty\n\n **Authors:** Sidra Aurangzeb, MBBS; Muhammad Tariq, MRCP, FRCP, FRCPE \n **Date:** June 24, 2015\n\n ## Content\n\n Upon physical examination, the patient appears mildly jaundiced but is otherwise well-appearing and in no distress. Her mucous membranes are moist.\nThe ophthalmic examination shows icteric sclera, normal pupillary reactions to light, normal visual acuity and fields, and normal optic fundi. Dark, brown-colored rings are noted around the periphery of the iris and are visible on naked-eye examination; this is confirmed with a slit-lamp examination.\nNeurologic examination reveals slow mentation, slurred speech, ataxic gait, diffuse muscle rigidity, and a fine resting tremor. The findings are symmetric, and the patient's reflexes are brisk bilaterally, with flexor plantar responses. The liver, palpated at three finger-breadths below the costal margin, is enlarged, firm, and nontender. The spleen is not palpable, and shifting dullness is not present. No parotid enlargement, palmar erythema, gynecomastia, or spider nevi are observed. The chest and cardiovascular examinations are unremarkable.\nA complete blood cell count and erythrocyte sedimentation rate are both within normal limits. A hepatic panel reveals a total bilirubin level of 3.6 mg/dL, an alanine aminotransferase of 99 U/L level, an alkaline phosphatase level of 284 U/L, an albumin of 4.4 g/dL, and a prothrombin time of 18 seconds. Markers for hepatitis B and C are negative.\nFigure 1.\nMRI of the patient's brain. Image from Kim TJ, Kim IO, Kim WS, et al. MR imaging of the brain in Wilson disease of childhood: findings before and after treatment with clinical correlation. Am J Neuroradiol. 2008;27:1373-1378. ©American Society of Neuroradiology.\nMRI of the patient's brain. Image from Kim TJ, Kim IO, Kim WS, et al. MR imaging of the brain in Wilson disease of childhood: findings before and after treatment with clinical correlation. Am J Neuroradiol. 2008;27:1373-1378. ©American Society of Neuroradiology.\nMRI of the brain is performed (Figure 1).\n\n ## Figures\n\n **Figure 1.MRI of the patient's brain. Image from Kim TJ, Kim IO, Kim WS, et al. MR imaging of the brain in Wilson disease of childhood: findings before and after treatment with clinical correlation. Am J Neuroradiol. 2008;27:1373-1378. ©American Society of Neuroradiology.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 861683,
"choiceText": "Wilson disease ",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 861685,
"choiceText": "Multiple sclerosis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 861687,
"choiceText": "Cortical basal ganglionic degeneration",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 861689,
"choiceText": "Progressive supranuclear palsy\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 270907,
"questionText": "What is the diagnosis?<br><br>\r\n<i>\r\nHint: The unusual findings on eye examination are suggestive of this metabolic abnormality.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Young Girl With Clumsiness, Dystonia, and Speech Difficulty"
},
{
"authors": "Sidra Aurangzeb, MBBS; Muhammad Tariq, MRCP, FRCP, FRCPE",
"content": [
"The unusual dark, brownish rings around the periphery of the irises of this patient are known as Kayser-Fleischer (KF) rings. This finding, in conjunction with the noted hepatic dysfunction and the hypodense regions in the basal ganglia (ie, caudate nucleus, putamen, and globus pallidus) on MRI of the brain raised suspicion of a diagnosis of Wilson disease.",
"Figure 1.",
"MRI of the patient's brain. Image from Kim TJ, Kim IO, Kim WS, et al. MR imaging of the brain in Wilson disease of childhood: findings before and after treatment with clinical correlation. Am J Neuroradiol. 2008;27:1373-1378. ©American Society of Neuroradiology.",
"MRI of the patient's brain. Image from Kim TJ, Kim IO, Kim WS, et al. MR imaging of the brain in Wilson disease of childhood: findings before and after treatment with clinical correlation. Am J Neuroradiol. 2008;27:1373-1378. ©American Society of Neuroradiology.",
"The serum copper concentration was elevated, at 187 μg/dL (normal range, 70-150 µg/dL), and the patient was noted to have a low ceruloplasmin level of 12 mg/dL (normal range, 15-60 mg/dL). A 24-hour urine copper value without penicillamine challenge was also markedly elevated, at 1708 µg/24 hours (normal range, 3-35 µg/24 hours). The diagnosis of Wilson disease was established in the patient.",
"Wilson disease (also known as \"hepatolenticular degeneration\") is a rare autosomal recessive disorder of copper metabolism, with a prevalence of about 1 in 30,000 people. The normal estimated total body copper content is 50-100 mg, with an average daily intake of 2-5 mg. It is absorbed primarily by enterocytes of the duodenum and proximal small bowel and transported into hepatocytes, where it is used for local metabolic needs and incorporated into ceruloplasmin; excess copper is sequestered and rendered nontoxic through complexes with metallothioneins. Most ingested copper is excreted in bile, and a small fraction is excreted in urine.",
"Wilson disease is characterized by decreased biliary copper excretion and a defective incorporation of copper into ceruloplasmin. In 1993, the Wilson disease gene ATP7B was identified. This gene, localized to chromosome 13q14, codes for a membrane-bound, P-type copper-transporting ATPase expressed primarily in the liver. ATP7B protein has both a perinuclear location, where it is involved in delivering copper to apoceruloplasmin, and a plasma membrane location, where it is responsible for the efflux of copper from the hepatocyte. As a result of mutations in its function, progressive copper accumulation occurs.",
"The excess copper is initially bound to metallothionein; however, the binding capacity of metallothionein is eventually exceeded. The excess copper acts as a promoter of free radical formation and causes oxidation of lipids and proteins and hepatocyte dysfunction. Eventually, as liver copper levels increase, copper is released into the circulation and deposited in other organs, such as the brain, kidneys, and cornea.[1,2,3,4]",
"Wilson disease may present with various clinical conditions. The most common are liver disease and neuropsychiatric disturbances. None of the clinical signs is individually typical or diagnostic of the condition.",
"One of the most characteristic features of Wilson disease is that no two patients, even within a family, are ever quite alike. Most patients with Wilson disease, however, have some degree of liver disease, regardless of the clinical presentation or presymptomatic status.",
"The most common age of hepatic manifestation is between 8 and 18 years. Cirrhosis may be present in children younger than 5 years, however, and it may not be detected until advanced chronic liver disease is revealed when patients are in their 50s or 60s, without neurologic symptoms and without KF rings.",
"Associated liver disease may mimic all forms of common liver conditions, including asymptomatic elevation of aminotransferase levels, acute or chronic hepatitis, fulminant hepatic failure, and cirrhosis. Acute Wilsonian hepatitis is indistinguishable from other forms of acute (viral or toxic) liver diseases. The disease may rapidly deteriorate and resemble fulminant hepatic failure.",
"Rapid diagnosis may be very difficult. One puzzling feature of fulminant Wilson disease is the greater incidence seen in females (female-to-male ratio, 3-4:1).[1,2,3]"
],
"date": "June 24, 2015",
"figures": [
{
"caption": "Figure 1.MRI of the patient's brain. Image from Kim TJ, Kim IO, Kim WS, et al. MR imaging of the brain in Wilson disease of childhood: findings before and after treatment with clinical correlation. Am J Neuroradiol. 2008;27:1373-1378. ©American Society of Neuroradiology.",
"image_url": "https://img.medscapestatic.com/article/846/811/846811-Thumb1.jpg"
}
],
"markdown": "# Young Girl With Clumsiness, Dystonia, and Speech Difficulty\n\n **Authors:** Sidra Aurangzeb, MBBS; Muhammad Tariq, MRCP, FRCP, FRCPE \n **Date:** June 24, 2015\n\n ## Content\n\n The unusual dark, brownish rings around the periphery of the irises of this patient are known as Kayser-Fleischer (KF) rings. This finding, in conjunction with the noted hepatic dysfunction and the hypodense regions in the basal ganglia (ie, caudate nucleus, putamen, and globus pallidus) on MRI of the brain raised suspicion of a diagnosis of Wilson disease.\nFigure 1.\nMRI of the patient's brain. Image from Kim TJ, Kim IO, Kim WS, et al. MR imaging of the brain in Wilson disease of childhood: findings before and after treatment with clinical correlation. Am J Neuroradiol. 2008;27:1373-1378. ©American Society of Neuroradiology.\nMRI of the patient's brain. Image from Kim TJ, Kim IO, Kim WS, et al. MR imaging of the brain in Wilson disease of childhood: findings before and after treatment with clinical correlation. Am J Neuroradiol. 2008;27:1373-1378. ©American Society of Neuroradiology.\nThe serum copper concentration was elevated, at 187 μg/dL (normal range, 70-150 µg/dL), and the patient was noted to have a low ceruloplasmin level of 12 mg/dL (normal range, 15-60 mg/dL). A 24-hour urine copper value without penicillamine challenge was also markedly elevated, at 1708 µg/24 hours (normal range, 3-35 µg/24 hours). The diagnosis of Wilson disease was established in the patient.\nWilson disease (also known as \"hepatolenticular degeneration\") is a rare autosomal recessive disorder of copper metabolism, with a prevalence of about 1 in 30,000 people. The normal estimated total body copper content is 50-100 mg, with an average daily intake of 2-5 mg. It is absorbed primarily by enterocytes of the duodenum and proximal small bowel and transported into hepatocytes, where it is used for local metabolic needs and incorporated into ceruloplasmin; excess copper is sequestered and rendered nontoxic through complexes with metallothioneins. Most ingested copper is excreted in bile, and a small fraction is excreted in urine.\nWilson disease is characterized by decreased biliary copper excretion and a defective incorporation of copper into ceruloplasmin. In 1993, the Wilson disease gene ATP7B was identified. This gene, localized to chromosome 13q14, codes for a membrane-bound, P-type copper-transporting ATPase expressed primarily in the liver. ATP7B protein has both a perinuclear location, where it is involved in delivering copper to apoceruloplasmin, and a plasma membrane location, where it is responsible for the efflux of copper from the hepatocyte. As a result of mutations in its function, progressive copper accumulation occurs.\nThe excess copper is initially bound to metallothionein; however, the binding capacity of metallothionein is eventually exceeded. The excess copper acts as a promoter of free radical formation and causes oxidation of lipids and proteins and hepatocyte dysfunction. Eventually, as liver copper levels increase, copper is released into the circulation and deposited in other organs, such as the brain, kidneys, and cornea.[1,2,3,4]\nWilson disease may present with various clinical conditions. The most common are liver disease and neuropsychiatric disturbances. None of the clinical signs is individually typical or diagnostic of the condition.\nOne of the most characteristic features of Wilson disease is that no two patients, even within a family, are ever quite alike. Most patients with Wilson disease, however, have some degree of liver disease, regardless of the clinical presentation or presymptomatic status.\nThe most common age of hepatic manifestation is between 8 and 18 years. Cirrhosis may be present in children younger than 5 years, however, and it may not be detected until advanced chronic liver disease is revealed when patients are in their 50s or 60s, without neurologic symptoms and without KF rings.\nAssociated liver disease may mimic all forms of common liver conditions, including asymptomatic elevation of aminotransferase levels, acute or chronic hepatitis, fulminant hepatic failure, and cirrhosis. Acute Wilsonian hepatitis is indistinguishable from other forms of acute (viral or toxic) liver diseases. The disease may rapidly deteriorate and resemble fulminant hepatic failure.\nRapid diagnosis may be very difficult. One puzzling feature of fulminant Wilson disease is the greater incidence seen in females (female-to-male ratio, 3-4:1).[1,2,3]\n\n ## Figures\n\n **Figure 1.MRI of the patient's brain. Image from Kim TJ, Kim IO, Kim WS, et al. MR imaging of the brain in Wilson disease of childhood: findings before and after treatment with clinical correlation. Am J Neuroradiol. 2008;27:1373-1378. ©American Society of Neuroradiology.** \n \n\n\n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 861683,
"choiceText": "Wilson disease ",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 861685,
"choiceText": "Multiple sclerosis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 861687,
"choiceText": "Cortical basal ganglionic degeneration",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 861689,
"choiceText": "Progressive supranuclear palsy\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 270907,
"questionText": "What is the diagnosis?<br><br>\r\n<i>\r\nHint: The unusual findings on eye examination are suggestive of this metabolic abnormality.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Young Girl With Clumsiness, Dystonia, and Speech Difficulty"
},
{
"authors": "Sidra Aurangzeb, MBBS; Muhammad Tariq, MRCP, FRCP, FRCPE",
"content": [
"KF rings are formed by the deposition of copper in the Descemet membrane in the limbus of the cornea. The color may range from greenish-gold to brown, and the rings form bilaterally, initially appearing at the superior pole of the cornea, then the inferior pole and, ultimately, circumferentially. Rings may be readily visible to the naked eye or with an ophthalmoscope set at +40. If the ring is not detected by clinical inspection, the cornea should be examined under a slit lamp by an experienced ophthalmologist.",
"KF rings are observed in up to 90% of individuals with symptomatic Wilson disease, 95% of patients with neurologic symptoms of Wilson disease, 50%-60% of patients without neurologic symptoms, and only 10% of asymptomatic siblings.",
"Although KF rings are a useful diagnostic sign, they are no longer considered pathognomonic for Wilson disease. They may also be observed in patients with carotenemia, arcus senilis, chronic active hepatitis, chronic cholestasis, chronic jaundice, cryptogenic cirrhosis, intraocular foreign body of < 85% copper, multiple myeloma, primary biliary cirrhosis, and trypanosomiasis.[2,3,4]",
"Neurologic symptoms usually develop in patients who are in their mid-teenage years or 20s. The hallmark of neurologic Wilson disease is a progressive movement disorder characterized by dysarthria, dysphagia, apraxia, drooling, and a tremor/rigidity syndrome (juvenile parkinsonism). The initial symptoms may be very subtle, such as a mild asymmetric tremor, which occurs in approximately one half of individuals with Wilson disease. Late manifestations include dystonia, spasticity, grand mal seizures, rigidity, and flexion contractures.",
"About one third of patients present with psychiatric abnormalities, such as reduced performance in school or at work, depression, labile mood, impulsiveness, disinhibition, sexual exhibitionism, self-injurious behavior, and frank psychosis. The reported percentage of patients with psychiatric symptoms as the initial clinical feature is 10%-20%.[2,3]",
"Skeletal involvement, such as osteoporosis, osteomalacia, chondrocalcinosis, osteoarthritis, and joint hypermobility, is commonly seen in cases of Wilson disease, with more than one half of patients exhibiting osteopenia on conventional radiographs. Coombs-negative hemolytic anemia is a recognized complication of the disease, but it is rare (10%-15% of cases). Patients may present like those with Fanconi syndrome or urolithiasis.",
"Skin pigmentation and a bluish discoloration at the base of the fingernails (azure lunulae) are recognized in patients with Wilson disease. Cardiac manifestations, such as rhythm abnormalities and increased autonomic tone, have also been described. Some female patients have repeated spontaneous abortions, and most become amenorrheic before diagnosis.[2,3]",
"Neurologic Wilson disease is usually diagnosed on the basis of clinical findings and laboratory abnormalities. No additional tests are required if KF rings are present and serum ceruloplasmin levels are low. There are a few well-documented cases, however, of neurologic Wilson disease without KF rings. Clinical neurologic examination is more sensitive than any other method for the detection of neurologic abnormalities.[1,5]",
"The diagnosis is more complex in patients presenting with liver diseases. The serum ceruloplasmin level may be in the low to normal range in as many as 45% of patients with hepatic Wilson disease; however, falsely low levels may also be found in a patient with autoimmune hepatitis or other protein deficiency states, including nephrotic syndrome, malabsorption, protein-losing enteropathy, and malnutrition. Ceruloplasmin is an acute-phase reactant and may be increased in response to inflammation, pregnancy, estrogen use, or infection; therefore, in patients with liver disease, a normal ceruloplasmin level cannot exclude Wilson disease, nor is a low level sufficient to diagnose Wilson disease.",
"Urinary copper excretion is increased in patients with Wilson disease; however, its usefulness in clinical practice is limited. The estimation of urinary copper excretion may be misleading as a result of incorrect collection of the 24-hour urinary volume or possible copper contamination. In presymptomatic patients, urinary copper excretion may be normal, but increases after a D-penicillamine challenge.[1,4]",
"Histologic abnormalities on liver biopsy are generally nonspecific and not helpful for the diagnosis of Wilson disease; however, the exclusion of other etiologies may be necessary and require a liver biopsy, which may show classic features of autoimmune hepatitis. The detection of focal copper stores by rhodanine stain is a pathognomonic sign of Wilson disease but is present in only about 10% of patients. The hepatic copper content is increased in 82% of patients with Wilson disease and when exceeding 250 µg/g dry weight provides the strongest evidence for the disease.",
"CT of the brain may show well-defined, slit-like, low-attenuation foci involving the basal ganglia (particularly, the putamen) as well as regions of low attenuation in the basal ganglia, thalamus, or dentate nucleus. MRI of the brain seems to be more sensitive than CT for detecting early lesions of Wilson disease. Appearance on MRI include atrophy and signal change in the gray matter (typically symmetric) and white matter (often asymmetric).",
"The most common area in which abnormal signal on MRI is seen is the putamen, followed by the caudate, thalamus, midbrain, cerebral white matter, pons, and cerebellum. Typically, the abnormal signal is hyperintense on T2-weighted images. Occasionally, abnormal hypointense signal on T2-weighted images is seen. These MRI abnormalities sometimes lead to the \"face of the giant panda\" sign, reflecting hyperintensity in the midbrain tegmentum with relative sparing of the red nuclei (\"eyes\"), part of the pars reticulata of the substantia nigra (\"ears\"), and the hypointensity of the superior colliculus (\"mouth\"). Positron PET reveals a significantly reduced regional cerebral metabolic rate of glucose consumption.[3,4,6]"
],
"date": "June 24, 2015",
"figures": [],
"markdown": "# Young Girl With Clumsiness, Dystonia, and Speech Difficulty\n\n **Authors:** Sidra Aurangzeb, MBBS; Muhammad Tariq, MRCP, FRCP, FRCPE \n **Date:** June 24, 2015\n\n ## Content\n\n KF rings are formed by the deposition of copper in the Descemet membrane in the limbus of the cornea. The color may range from greenish-gold to brown, and the rings form bilaterally, initially appearing at the superior pole of the cornea, then the inferior pole and, ultimately, circumferentially. Rings may be readily visible to the naked eye or with an ophthalmoscope set at +40. If the ring is not detected by clinical inspection, the cornea should be examined under a slit lamp by an experienced ophthalmologist.\nKF rings are observed in up to 90% of individuals with symptomatic Wilson disease, 95% of patients with neurologic symptoms of Wilson disease, 50%-60% of patients without neurologic symptoms, and only 10% of asymptomatic siblings.\nAlthough KF rings are a useful diagnostic sign, they are no longer considered pathognomonic for Wilson disease. They may also be observed in patients with carotenemia, arcus senilis, chronic active hepatitis, chronic cholestasis, chronic jaundice, cryptogenic cirrhosis, intraocular foreign body of < 85% copper, multiple myeloma, primary biliary cirrhosis, and trypanosomiasis.[2,3,4]\nNeurologic symptoms usually develop in patients who are in their mid-teenage years or 20s. The hallmark of neurologic Wilson disease is a progressive movement disorder characterized by dysarthria, dysphagia, apraxia, drooling, and a tremor/rigidity syndrome (juvenile parkinsonism). The initial symptoms may be very subtle, such as a mild asymmetric tremor, which occurs in approximately one half of individuals with Wilson disease. Late manifestations include dystonia, spasticity, grand mal seizures, rigidity, and flexion contractures.\nAbout one third of patients present with psychiatric abnormalities, such as reduced performance in school or at work, depression, labile mood, impulsiveness, disinhibition, sexual exhibitionism, self-injurious behavior, and frank psychosis. The reported percentage of patients with psychiatric symptoms as the initial clinical feature is 10%-20%.[2,3]\nSkeletal involvement, such as osteoporosis, osteomalacia, chondrocalcinosis, osteoarthritis, and joint hypermobility, is commonly seen in cases of Wilson disease, with more than one half of patients exhibiting osteopenia on conventional radiographs. Coombs-negative hemolytic anemia is a recognized complication of the disease, but it is rare (10%-15% of cases). Patients may present like those with Fanconi syndrome or urolithiasis.\nSkin pigmentation and a bluish discoloration at the base of the fingernails (azure lunulae) are recognized in patients with Wilson disease. Cardiac manifestations, such as rhythm abnormalities and increased autonomic tone, have also been described. Some female patients have repeated spontaneous abortions, and most become amenorrheic before diagnosis.[2,3]\nNeurologic Wilson disease is usually diagnosed on the basis of clinical findings and laboratory abnormalities. No additional tests are required if KF rings are present and serum ceruloplasmin levels are low. There are a few well-documented cases, however, of neurologic Wilson disease without KF rings. Clinical neurologic examination is more sensitive than any other method for the detection of neurologic abnormalities.[1,5]\nThe diagnosis is more complex in patients presenting with liver diseases. The serum ceruloplasmin level may be in the low to normal range in as many as 45% of patients with hepatic Wilson disease; however, falsely low levels may also be found in a patient with autoimmune hepatitis or other protein deficiency states, including nephrotic syndrome, malabsorption, protein-losing enteropathy, and malnutrition. Ceruloplasmin is an acute-phase reactant and may be increased in response to inflammation, pregnancy, estrogen use, or infection; therefore, in patients with liver disease, a normal ceruloplasmin level cannot exclude Wilson disease, nor is a low level sufficient to diagnose Wilson disease.\nUrinary copper excretion is increased in patients with Wilson disease; however, its usefulness in clinical practice is limited. The estimation of urinary copper excretion may be misleading as a result of incorrect collection of the 24-hour urinary volume or possible copper contamination. In presymptomatic patients, urinary copper excretion may be normal, but increases after a D-penicillamine challenge.[1,4]\nHistologic abnormalities on liver biopsy are generally nonspecific and not helpful for the diagnosis of Wilson disease; however, the exclusion of other etiologies may be necessary and require a liver biopsy, which may show classic features of autoimmune hepatitis. The detection of focal copper stores by rhodanine stain is a pathognomonic sign of Wilson disease but is present in only about 10% of patients. The hepatic copper content is increased in 82% of patients with Wilson disease and when exceeding 250 µg/g dry weight provides the strongest evidence for the disease.\nCT of the brain may show well-defined, slit-like, low-attenuation foci involving the basal ganglia (particularly, the putamen) as well as regions of low attenuation in the basal ganglia, thalamus, or dentate nucleus. MRI of the brain seems to be more sensitive than CT for detecting early lesions of Wilson disease. Appearance on MRI include atrophy and signal change in the gray matter (typically symmetric) and white matter (often asymmetric).\nThe most common area in which abnormal signal on MRI is seen is the putamen, followed by the caudate, thalamus, midbrain, cerebral white matter, pons, and cerebellum. Typically, the abnormal signal is hyperintense on T2-weighted images. Occasionally, abnormal hypointense signal on T2-weighted images is seen. These MRI abnormalities sometimes lead to the \"face of the giant panda\" sign, reflecting hyperintensity in the midbrain tegmentum with relative sparing of the red nuclei (\"eyes\"), part of the pars reticulata of the substantia nigra (\"ears\"), and the hypointensity of the superior colliculus (\"mouth\"). Positron PET reveals a significantly reduced regional cerebral metabolic rate of glucose consumption.[3,4,6]\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "Young Girl With Clumsiness, Dystonia, and Speech Difficulty"
},
{
"authors": "Sidra Aurangzeb, MBBS; Muhammad Tariq, MRCP, FRCP, FRCPE",
"content": [
"Once Wilson disease is diagnosed in an index patient, an evaluation of his or her family is mandatory. The likelihood of finding a homozygote among the patient's siblings is 25%, and it is 0.5% among the patient's children. Testing of second-degree relatives is useful only if the gene is found in one of the immediate members of the relative's family.",
"No single biochemical test is able to identify affected siblings or heterozygote carriers of the Wilson disease gene with sufficient certainty. Today, testing for ATP7B mutations or haplotype analysis can be used as primary screening of first-degree relatives. In haplotype analysis, numerous highly polymorphic microsatellite markers of dinucleotides or trinucleotides that closely flank the gene allow Wilson disease to be traced in a family. For such an analysis, at least one first-degree relative and the index patient are required.[4]",
"According to the recently updated American Association for the Study of Liver Diseases practice guidelines on Wilson disease, initial treatment for symptomatic patients should include a chelating agent (penicillamine or trientine). Treatment of presymptomatic patients and maintenance therapy in successfully treated symptomatic patients can also be accomplished with these chelating agents or, alternatively, with zinc. Liver transplantation, which corrects the underlying hepatic defect in Wilson disease, is reserved for patients with acute liver failure and those with decompensated liver disease that is resistant to medical therapy.",
"Treatment with penicillamine is still the criterion standard; the major effect of this compound is chelation and subsequent urinary excretion. Most symptomatic patients, whether hepatic, neurologic, or psychiatric, respond within months of starting treatment. Among patients with neurologic Wilson disease, a significant proportion (10%-50%) may experience an initial worsening of symptoms before they improve.",
"Trientine is also a copper chelator that acts primarily by enhancing urinary copper excretion. It is approved for the treatment of Wilson disease and is as effective as penicillamine, with far fewer side effects.",
"Zinc interferes with intestinal absorption of copper and increases fecal excretion of copper. The experience with other drugs is very limited; ammonium tetrathiomolybdate is currently experimental in the United States, but it appears to be useful for the initial treatment of patients with neurologic symptoms. Antioxidants, mainly vitamin E, may also have a role as adjunctive treatment, but no rigorous studies have been conducted.[1,2,3,4]",
"Patients should avoid foods with a high copper content, such as liver, broccoli, legumes, chocolate, nuts, mushrooms, and shellfish (particularly lobster). Drinking water from atypical sources (eg, well water) should be tested for copper concentration and replaced with purified water if greater than 0.2 parts per million of copper are found. Also, patients should avoid alcohol consumption and potentially hepatotoxic drug therapy.[2,3,4]",
"The patient in this case was started on penicillamine 500 mg three times daily, along with dietary restriction of copper-containing foods. She was followed in the neurology outpatient clinic and showed considerable improvement 6 months after therapy."
],
"date": "June 24, 2015",
"figures": [],
"markdown": "# Young Girl With Clumsiness, Dystonia, and Speech Difficulty\n\n **Authors:** Sidra Aurangzeb, MBBS; Muhammad Tariq, MRCP, FRCP, FRCPE \n **Date:** June 24, 2015\n\n ## Content\n\n Once Wilson disease is diagnosed in an index patient, an evaluation of his or her family is mandatory. The likelihood of finding a homozygote among the patient's siblings is 25%, and it is 0.5% among the patient's children. Testing of second-degree relatives is useful only if the gene is found in one of the immediate members of the relative's family.\nNo single biochemical test is able to identify affected siblings or heterozygote carriers of the Wilson disease gene with sufficient certainty. Today, testing for ATP7B mutations or haplotype analysis can be used as primary screening of first-degree relatives. In haplotype analysis, numerous highly polymorphic microsatellite markers of dinucleotides or trinucleotides that closely flank the gene allow Wilson disease to be traced in a family. For such an analysis, at least one first-degree relative and the index patient are required.[4]\nAccording to the recently updated American Association for the Study of Liver Diseases practice guidelines on Wilson disease, initial treatment for symptomatic patients should include a chelating agent (penicillamine or trientine). Treatment of presymptomatic patients and maintenance therapy in successfully treated symptomatic patients can also be accomplished with these chelating agents or, alternatively, with zinc. Liver transplantation, which corrects the underlying hepatic defect in Wilson disease, is reserved for patients with acute liver failure and those with decompensated liver disease that is resistant to medical therapy.\nTreatment with penicillamine is still the criterion standard; the major effect of this compound is chelation and subsequent urinary excretion. Most symptomatic patients, whether hepatic, neurologic, or psychiatric, respond within months of starting treatment. Among patients with neurologic Wilson disease, a significant proportion (10%-50%) may experience an initial worsening of symptoms before they improve.\nTrientine is also a copper chelator that acts primarily by enhancing urinary copper excretion. It is approved for the treatment of Wilson disease and is as effective as penicillamine, with far fewer side effects.\nZinc interferes with intestinal absorption of copper and increases fecal excretion of copper. The experience with other drugs is very limited; ammonium tetrathiomolybdate is currently experimental in the United States, but it appears to be useful for the initial treatment of patients with neurologic symptoms. Antioxidants, mainly vitamin E, may also have a role as adjunctive treatment, but no rigorous studies have been conducted.[1,2,3,4]\nPatients should avoid foods with a high copper content, such as liver, broccoli, legumes, chocolate, nuts, mushrooms, and shellfish (particularly lobster). Drinking water from atypical sources (eg, well water) should be tested for copper concentration and replaced with purified water if greater than 0.2 parts per million of copper are found. Also, patients should avoid alcohol consumption and potentially hepatotoxic drug therapy.[2,3,4]\nThe patient in this case was started on penicillamine 500 mg three times daily, along with dietary restriction of copper-containing foods. She was followed in the neurology outpatient clinic and showed considerable improvement 6 months after therapy.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 861691,
"choiceText": "Although KF rings are diagnostically useful, they are no longer considered pathognomonic for Wilson disease",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 861693,
"choiceText": "KF rings form bilaterally, initially appearing at the lateral margins of the cornea",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 861695,
"choiceText": "KF rings are pathognomic for Wilson disease, and treatment for the condition should be initiated immediately",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 861697,
"choiceText": "KF rings are uncommon in patients with Wilson disease\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Although KF rings are a useful diagnostic sign, they are no longer considered pathognomonic for Wilson disease. They may also be observed in patients with carotenemia, arcus senilis, chronic active hepatitis, chronic cholestasis, chronic jaundice, cryptogenic cirrhosis, intraocular foreign body of < 85% copper, multiple myeloma, primary biliary cirrhosis, and trypanosomiasis.<sup>[2-4]</sup>",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 270909,
"questionText": "While examining a patient, you notice a greenish ring around the patient's cornea. You suspect that this finding is a KF ring. Which of the following statements regarding KF rings is true?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 861699,
"choiceText": "Chelation with penicillamine ",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 861701,
"choiceText": "Gastric lavage",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 861703,
"choiceText": "Liver transplantation",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 861705,
"choiceText": "Dialysis \r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "\r\n\r\nTreatment with penicillamine is still the criterion standard; the major effect of this compound is chelation and subsequent urinary excretion. Most symptomatic patients, whether hepatic, neurologic, or psychiatric, respond within months of starting treatment.\r\n",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 270911,
"questionText": "You conclude that the diagnosis in the patient you are examining is Wilson disease. Which of the following would be the best initial treatment for this patient?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Young Girl With Clumsiness, Dystonia, and Speech Difficulty"
},
{
"authors": "Sidra Aurangzeb, MBBS; Muhammad Tariq, MRCP, FRCP, FRCPE",
"content": [],
"date": "June 24, 2015",
"figures": [],
"markdown": "# Young Girl With Clumsiness, Dystonia, and Speech Difficulty\n\n **Authors:** Sidra Aurangzeb, MBBS; Muhammad Tariq, MRCP, FRCP, FRCPE \n **Date:** June 24, 2015\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 861691,
"choiceText": "Although KF rings are diagnostically useful, they are no longer considered pathognomonic for Wilson disease",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 861693,
"choiceText": "KF rings form bilaterally, initially appearing at the lateral margins of the cornea",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 861695,
"choiceText": "KF rings are pathognomic for Wilson disease, and treatment for the condition should be initiated immediately",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 861697,
"choiceText": "KF rings are uncommon in patients with Wilson disease\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Although KF rings are a useful diagnostic sign, they are no longer considered pathognomonic for Wilson disease. They may also be observed in patients with carotenemia, arcus senilis, chronic active hepatitis, chronic cholestasis, chronic jaundice, cryptogenic cirrhosis, intraocular foreign body of < 85% copper, multiple myeloma, primary biliary cirrhosis, and trypanosomiasis.<sup>[2-4]</sup>",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 270909,
"questionText": "While examining a patient, you notice a greenish ring around the patient's cornea. You suspect that this finding is a KF ring. Which of the following statements regarding KF rings is true?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 861699,
"choiceText": "Chelation with penicillamine ",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 861701,
"choiceText": "Gastric lavage",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 861703,
"choiceText": "Liver transplantation",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 861705,
"choiceText": "Dialysis \r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "\r\n\r\nTreatment with penicillamine is still the criterion standard; the major effect of this compound is chelation and subsequent urinary excretion. Most symptomatic patients, whether hepatic, neurologic, or psychiatric, respond within months of starting treatment.\r\n",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 270911,
"questionText": "You conclude that the diagnosis in the patient you are examining is Wilson disease. Which of the following would be the best initial treatment for this patient?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Young Girl With Clumsiness, Dystonia, and Speech Difficulty"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 861683,
"choiceText": "Wilson disease ",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 861685,
"choiceText": "Multiple sclerosis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 861687,
"choiceText": "Cortical basal ganglionic degeneration",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 861689,
"choiceText": "Progressive supranuclear palsy\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 270907,
"questionText": "What is the diagnosis?<br><br>\r\n<i>\r\nHint: The unusual findings on eye examination are suggestive of this metabolic abnormality.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 861691,
"choiceText": "Although KF rings are diagnostically useful, they are no longer considered pathognomonic for Wilson disease",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 861693,
"choiceText": "KF rings form bilaterally, initially appearing at the lateral margins of the cornea",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 861695,
"choiceText": "KF rings are pathognomic for Wilson disease, and treatment for the condition should be initiated immediately",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 861697,
"choiceText": "KF rings are uncommon in patients with Wilson disease\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Although KF rings are a useful diagnostic sign, they are no longer considered pathognomonic for Wilson disease. They may also be observed in patients with carotenemia, arcus senilis, chronic active hepatitis, chronic cholestasis, chronic jaundice, cryptogenic cirrhosis, intraocular foreign body of < 85% copper, multiple myeloma, primary biliary cirrhosis, and trypanosomiasis.<sup>[2-4]</sup>",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 270909,
"questionText": "While examining a patient, you notice a greenish ring around the patient's cornea. You suspect that this finding is a KF ring. Which of the following statements regarding KF rings is true?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 861699,
"choiceText": "Chelation with penicillamine ",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 861701,
"choiceText": "Gastric lavage",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 861703,
"choiceText": "Liver transplantation",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 861705,
"choiceText": "Dialysis \r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "\r\n\r\nTreatment with penicillamine is still the criterion standard; the major effect of this compound is chelation and subsequent urinary excretion. Most symptomatic patients, whether hepatic, neurologic, or psychiatric, respond within months of starting treatment.\r\n",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 270911,
"questionText": "You conclude that the diagnosis in the patient you are examining is Wilson disease. Which of the following would be the best initial treatment for this patient?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
846862 | /viewarticle/846862 | [
{
"authors": "Jeffrey Siegelman, MD; Daniel M. Lindberg, MD",
"content": [
"Editor's Note:\n\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.",
"A 61-year-old woman presents to the emergency department after being referred from her primary care provider's office for evaluation of tachycardia. She had been seen by her primary care provider for a routine purified protein derivative tuberculin skin test and was incidentally noted to have a pulse of 160 beats/min.",
"The patient denies any specific symptoms other than occasional palpitations. Upon review of her systems, however, she notes having night sweats; a 110-lb weight loss over the preceding 12 months; and 2-3 months of anxiety, diarrhea, and occasional diplopia. She denies having any fever, chills, chest pain, dyspnea, or swelling in her extremities.",
"The patient has a medical history of an unspecified thyroid problem. She does not take any daily medications and has no medication allergies. She has a 50–pack-year smoking history, with occasional alcohol consumption. She had been homeless for a time, but is currently living in an apartment."
],
"date": "June 24, 2015",
"figures": [],
"markdown": "# Tachycardia in a 61-Year-Old Woman\n\n **Authors:** Jeffrey Siegelman, MD; Daniel M. Lindberg, MD \n **Date:** June 24, 2015\n\n ## Content\n\n Editor's Note:\n\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.\nA 61-year-old woman presents to the emergency department after being referred from her primary care provider's office for evaluation of tachycardia. She had been seen by her primary care provider for a routine purified protein derivative tuberculin skin test and was incidentally noted to have a pulse of 160 beats/min.\nThe patient denies any specific symptoms other than occasional palpitations. Upon review of her systems, however, she notes having night sweats; a 110-lb weight loss over the preceding 12 months; and 2-3 months of anxiety, diarrhea, and occasional diplopia. She denies having any fever, chills, chest pain, dyspnea, or swelling in her extremities.\nThe patient has a medical history of an unspecified thyroid problem. She does not take any daily medications and has no medication allergies. She has a 50–pack-year smoking history, with occasional alcohol consumption. She had been homeless for a time, but is currently living in an apartment.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "Tachycardia in a 61-Year-Old Woman"
},
{
"authors": "Jeffrey Siegelman, MD; Daniel M. Lindberg, MD",
"content": [
"Upon examination, the patient is awake and fully oriented. She is diaphoretic but in no apparent distress. Her temperature is 97°F; her pulse is 160 beats/min; her respiratory rate is 24 breaths/min, with an oxygen saturation of 98%; and her blood pressure is 190/117 mm Hg.",
"Figure.",
"Figure.",
"The patient has bilateral exophthalmos with exotropia of the right eye. Her visual acuity is normal, and her extraocular movements are intact.",
"The neck examination reveals a diffuse, nontender goiter, without nodules or thyroid bruits. The heart is tachycardic, intermittently irregular, and without murmurs. The lungs are clear to auscultation bilaterally. The abdomen is nondistended, soft, and nontender, with no palpable masses. No edema is observed in the extremities.",
"The neurologic examination reveals normal mentation, intact cranial nerves, intact motor strength and sensation, and normal reflexes. No tremor is noted.",
"The initial laboratory studies reveal that the patient's complete blood cell count, electrolytes, renal function, and cardiac marker findings are all within normal limits. Plain chest radiography findings are normal. An ECG is obtained (Figure)."
],
"date": "June 24, 2015",
"figures": [
{
"caption": "Figure.",
"image_url": "https://img.medscapestatic.com/article/846/862/846862-Thumb1.jpg"
}
],
"markdown": "# Tachycardia in a 61-Year-Old Woman\n\n **Authors:** Jeffrey Siegelman, MD; Daniel M. Lindberg, MD \n **Date:** June 24, 2015\n\n ## Content\n\n Upon examination, the patient is awake and fully oriented. She is diaphoretic but in no apparent distress. Her temperature is 97°F; her pulse is 160 beats/min; her respiratory rate is 24 breaths/min, with an oxygen saturation of 98%; and her blood pressure is 190/117 mm Hg.\nFigure.\nFigure.\nThe patient has bilateral exophthalmos with exotropia of the right eye. Her visual acuity is normal, and her extraocular movements are intact.\nThe neck examination reveals a diffuse, nontender goiter, without nodules or thyroid bruits. The heart is tachycardic, intermittently irregular, and without murmurs. The lungs are clear to auscultation bilaterally. The abdomen is nondistended, soft, and nontender, with no palpable masses. No edema is observed in the extremities.\nThe neurologic examination reveals normal mentation, intact cranial nerves, intact motor strength and sensation, and normal reflexes. No tremor is noted.\nThe initial laboratory studies reveal that the patient's complete blood cell count, electrolytes, renal function, and cardiac marker findings are all within normal limits. Plain chest radiography findings are normal. An ECG is obtained (Figure).\n\n ## Figures\n\n **Figure.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 863511,
"choiceText": " Viral cardiomyopathy",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 863513,
"choiceText": "Thyrotoxicosis ",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 863515,
"choiceText": "Tuberculosis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 863517,
"choiceText": "Pulmonary embolism\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 271491,
"questionText": "What is the most likely etiology of the patient's tachycardia? <br><br><i>\r\nHint: Note the presence of weight loss, bilateral exophthalmos, and goiter.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Tachycardia in a 61-Year-Old Woman"
},
{
"authors": "Jeffrey Siegelman, MD; Daniel M. Lindberg, MD",
"content": [
"This patient's ECG showed atrial fibrillation with a rapid ventricular response, which is a possible cardiac manifestation of thyrotoxicosis (Figure). No ischemic changes were noted despite her rapid heart rate.",
"Figure.",
"Figure.",
"The patient's laboratory studies confirmed the suspected diagnosis; she had a markedly depressed thyroid-stimulating hormone (TSH) level of 0.006 mIU/L (normal range, 0.5-5.0 mIU/L) and elevated triiodothyronine (T3) and thyroxine (T4) concentrations of 632 ng/dL (normal range, 70-170 ng/dL) and 23.7 µg/dL (normal range, 5-11 µg/dL), respectively.",
"\"Thyrotoxicosis\" refers to an elevated concentration of thyroid hormone as well as its related clinical manifestations. This is differentiated from thyroid storm, a life-threatening manifestation of thyrotoxicosis in which a markedly hypermetabolic state is present.",
"Hyperthyroidism most commonly results from uncontrolled Graves disease, in which autoantibodies to the TSH receptor are produced. This leads to excessive thyroid hormone production from the thyroid gland and a reflexive inhibition of TSH release from the pituitary gland. Other etiologies can include a solitary thyroid adenoma, toxic multinodular goiter, hypersecretory thyroid carcinoma, thyrotropin-secreting pituitary adenoma, struma ovarii, and iodine or amiodarone administration.",
"A precipitating event, such as surgery, trauma, myocardial infarction, pulmonary embolism, diabetic ketoacidosis, childbirth, severe infection, discontinuation of antithyroid medication, or thyroid surgery in a patient with uncontrolled hyperthyroidism, is often needed to push a patient with hyperthyroidism into thyroid storm.[1,2]",
"The incidence of hyperthyroidism in the United States is 0.05%-1.3%, most of which remains undiagnosed. Approximately 1%-2% of these patients progress to thyroid storm at some point. The prevalence is slightly higher in women than men, and in white and Hispanic populations than in black populations. Thyroid storm is most common in the third to sixth decades of life, although it can occur at any age.[1]",
"Thyroid storm is a clinical diagnosis and, considering the acuity of this life-threatening condition, patients with thyrotoxicosis should be treated empirically when the diagnosis is suspected. Symptoms of thyrotoxicosis include weight loss, palpitations, hair loss, diplopia, chest pain, oligomenorrhea, or confusion. The physical examination reveals a hypermetabolic state, with abnormalities involving multiple organ systems. These findings commonly include hyperpyrexia, tachycardia, tachypnea, and hypertension. Other findings may include fine tremor, exophthalmos, ophthalmoplegia, pretibial edema, congestive heart failure, thyromegaly, thyroid bruit, and hyperreflexia.[3]",
"Laboratory studies show a low TSH level and elevated T3 and T4 concentrations. TSH is the most precise indicator of thyroid function because of the very high sensitivity of the thyroid/pituitary feedback loop, and current assays are able to detect levels ≤ 0.02 mIU/L. As such, a normal TSH level largely excludes significant thyroid disease. Other laboratory findings seen in thyrotoxicosis may include hyperglycemia, hypercalcemia, leukocytosis, and elevated liver enzymes.[2]"
],
"date": "June 24, 2015",
"figures": [
{
"caption": "Figure.",
"image_url": "https://img.medscapestatic.com/article/846/862/846862-Thumb1.jpg"
}
],
"markdown": "# Tachycardia in a 61-Year-Old Woman\n\n **Authors:** Jeffrey Siegelman, MD; Daniel M. Lindberg, MD \n **Date:** June 24, 2015\n\n ## Content\n\n This patient's ECG showed atrial fibrillation with a rapid ventricular response, which is a possible cardiac manifestation of thyrotoxicosis (Figure). No ischemic changes were noted despite her rapid heart rate.\nFigure.\nFigure.\nThe patient's laboratory studies confirmed the suspected diagnosis; she had a markedly depressed thyroid-stimulating hormone (TSH) level of 0.006 mIU/L (normal range, 0.5-5.0 mIU/L) and elevated triiodothyronine (T3) and thyroxine (T4) concentrations of 632 ng/dL (normal range, 70-170 ng/dL) and 23.7 µg/dL (normal range, 5-11 µg/dL), respectively.\n\"Thyrotoxicosis\" refers to an elevated concentration of thyroid hormone as well as its related clinical manifestations. This is differentiated from thyroid storm, a life-threatening manifestation of thyrotoxicosis in which a markedly hypermetabolic state is present.\nHyperthyroidism most commonly results from uncontrolled Graves disease, in which autoantibodies to the TSH receptor are produced. This leads to excessive thyroid hormone production from the thyroid gland and a reflexive inhibition of TSH release from the pituitary gland. Other etiologies can include a solitary thyroid adenoma, toxic multinodular goiter, hypersecretory thyroid carcinoma, thyrotropin-secreting pituitary adenoma, struma ovarii, and iodine or amiodarone administration.\nA precipitating event, such as surgery, trauma, myocardial infarction, pulmonary embolism, diabetic ketoacidosis, childbirth, severe infection, discontinuation of antithyroid medication, or thyroid surgery in a patient with uncontrolled hyperthyroidism, is often needed to push a patient with hyperthyroidism into thyroid storm.[1,2]\nThe incidence of hyperthyroidism in the United States is 0.05%-1.3%, most of which remains undiagnosed. Approximately 1%-2% of these patients progress to thyroid storm at some point. The prevalence is slightly higher in women than men, and in white and Hispanic populations than in black populations. Thyroid storm is most common in the third to sixth decades of life, although it can occur at any age.[1]\nThyroid storm is a clinical diagnosis and, considering the acuity of this life-threatening condition, patients with thyrotoxicosis should be treated empirically when the diagnosis is suspected. Symptoms of thyrotoxicosis include weight loss, palpitations, hair loss, diplopia, chest pain, oligomenorrhea, or confusion. The physical examination reveals a hypermetabolic state, with abnormalities involving multiple organ systems. These findings commonly include hyperpyrexia, tachycardia, tachypnea, and hypertension. Other findings may include fine tremor, exophthalmos, ophthalmoplegia, pretibial edema, congestive heart failure, thyromegaly, thyroid bruit, and hyperreflexia.[3]\nLaboratory studies show a low TSH level and elevated T3 and T4 concentrations. TSH is the most precise indicator of thyroid function because of the very high sensitivity of the thyroid/pituitary feedback loop, and current assays are able to detect levels ≤ 0.02 mIU/L. As such, a normal TSH level largely excludes significant thyroid disease. Other laboratory findings seen in thyrotoxicosis may include hyperglycemia, hypercalcemia, leukocytosis, and elevated liver enzymes.[2]\n\n ## Figures\n\n **Figure.** \n \n\n\n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 863511,
"choiceText": " Viral cardiomyopathy",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 863513,
"choiceText": "Thyrotoxicosis ",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 863515,
"choiceText": "Tuberculosis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 863517,
"choiceText": "Pulmonary embolism\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 271491,
"questionText": "What is the most likely etiology of the patient's tachycardia? <br><br><i>\r\nHint: Note the presence of weight loss, bilateral exophthalmos, and goiter.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Tachycardia in a 61-Year-Old Woman"
},
{
"authors": "Jeffrey Siegelman, MD; Daniel M. Lindberg, MD",
"content": [
"Further testing may be indicated as part of a search for the precipitating cause of clinical decompensation, such as infection, myocardial infarction, or diabetic ketoacidosis. Electrocardiography most often reveals sinus tachycardia. Atrial fibrillation\n\n\nis also not uncommon, particularly in elderly patients and in the setting of underlying heart disease.",
"Although thyroid storm requires more rapid and aggressive therapy than thyrotoxicosis, differentiating between the two conditions can sometimes be difficult, as it was in this patient. Burch and Wartofsky[4] developed a scoring system to assist in making this distinction that takes into account thermoregulatory dysfunction, central nervous system effects, gastrointestinal dysfunction, the degree of tachycardia, the extent of congestive heart failure, the presence of atrial fibrillation, and the presence or absence of a precipitating event.",
"Cardiac complications from thyrotoxicosis include arrhythmias, congestive heart failure, and pulmonary hypertension. The most common arrhythmia in thyrotoxicosis is sinus tachycardia; however, atrial fibrillation occurs in 10%-20% of patients with thyrotoxicosis, most often those who are older than 60 years. Risk factors for atrial fibrillation in these patients include male sex, increasing age, coronary heart disease, heart failure, and structural heart or valvular disease.",
"Congestive heart failure in thyrotoxicosis is predominantly caused by either persistent tachyarrhythmias (tachycardia-induced cardiomyopathy) or uncontrolled hypertension as a consequence of thyrotoxicosis. Systolic dysfunction can occur as a consequence of the persistent cardiac arrhythmias, but it usually resolves once the hyperthyroid state is treated. Pulmonary hypertension can also occur in thyrotoxicosis, either as a result of a primary effect of thyroid hormone on pulmonary arteriolar resistance vessels, decompensated left heart failure, or increased pulmonary arterial blood flow (high-output).[1]",
"The differential diagnosis for thyrotoxicosis and thyroid storm may include anxiety, congestive heart failure, heat exhaustion or heatstroke, factitious disorder, neuroleptic malignant syndrome, panic disorder, septic shock, serotonin syndrome, anticholinergic or sympathomimetic toxicity, and alcohol or benzodiazepine withdrawal syndromes.[5] Because infection is a common trigger for thyroid storm, an initial misdiagnosis of sepsis is not uncommon because of similar characteristics, such as tachycardia, fever, and altered mental status."
],
"date": "June 24, 2015",
"figures": [],
"markdown": "# Tachycardia in a 61-Year-Old Woman\n\n **Authors:** Jeffrey Siegelman, MD; Daniel M. Lindberg, MD \n **Date:** June 24, 2015\n\n ## Content\n\n Further testing may be indicated as part of a search for the precipitating cause of clinical decompensation, such as infection, myocardial infarction, or diabetic ketoacidosis. Electrocardiography most often reveals sinus tachycardia. Atrial fibrillation\n\n\nis also not uncommon, particularly in elderly patients and in the setting of underlying heart disease.\nAlthough thyroid storm requires more rapid and aggressive therapy than thyrotoxicosis, differentiating between the two conditions can sometimes be difficult, as it was in this patient. Burch and Wartofsky[4] developed a scoring system to assist in making this distinction that takes into account thermoregulatory dysfunction, central nervous system effects, gastrointestinal dysfunction, the degree of tachycardia, the extent of congestive heart failure, the presence of atrial fibrillation, and the presence or absence of a precipitating event.\nCardiac complications from thyrotoxicosis include arrhythmias, congestive heart failure, and pulmonary hypertension. The most common arrhythmia in thyrotoxicosis is sinus tachycardia; however, atrial fibrillation occurs in 10%-20% of patients with thyrotoxicosis, most often those who are older than 60 years. Risk factors for atrial fibrillation in these patients include male sex, increasing age, coronary heart disease, heart failure, and structural heart or valvular disease.\nCongestive heart failure in thyrotoxicosis is predominantly caused by either persistent tachyarrhythmias (tachycardia-induced cardiomyopathy) or uncontrolled hypertension as a consequence of thyrotoxicosis. Systolic dysfunction can occur as a consequence of the persistent cardiac arrhythmias, but it usually resolves once the hyperthyroid state is treated. Pulmonary hypertension can also occur in thyrotoxicosis, either as a result of a primary effect of thyroid hormone on pulmonary arteriolar resistance vessels, decompensated left heart failure, or increased pulmonary arterial blood flow (high-output).[1]\nThe differential diagnosis for thyrotoxicosis and thyroid storm may include anxiety, congestive heart failure, heat exhaustion or heatstroke, factitious disorder, neuroleptic malignant syndrome, panic disorder, septic shock, serotonin syndrome, anticholinergic or sympathomimetic toxicity, and alcohol or benzodiazepine withdrawal syndromes.[5] Because infection is a common trigger for thyroid storm, an initial misdiagnosis of sepsis is not uncommon because of similar characteristics, such as tachycardia, fever, and altered mental status.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "Tachycardia in a 61-Year-Old Woman"
},
{
"authors": "Jeffrey Siegelman, MD; Daniel M. Lindberg, MD",
"content": [
"Management of thyrotoxicosis consists of a five-pronged, ordered approach, targeting each step in the biosynthetic pathway of thyroid hormone and its activity on target tissues.",
"Treatment begins with administration of propylthiouracil (PTU) or methimazole, both of which act by inhibiting new hormone synthesis. PTU has the added effect of decreasing peripheral T4 to T3 conversion. Beta-blockers are then used to inhibit target activity of thyroid hormone. Propranolol is the preferred agent because it also blocks peripheral conversion of T4. When cardioselective agents are preferred, atenolol or metoprolol may be used. At least 1 hour after administration of PTU or methimazole, the patient may be given iodide to inhibit further thyroid hormone release.",
"Iodine must be given only after synthesis of new hormone is blocked because iodide administration can have the undesired effect of increasing new hormone synthesis. Potassium iodide or Lugol solution of iodine is recommended. Peripheral conversion of T4 to T3 is blocked, as noted above, and dexamethasone may be used as well. Further treatment is supportive and may include acetaminophen for fever and hydrocortisone if the patient is hypotensive as a result of adrenal insufficiency. Salicylates are contraindicated because they displace bound thyroid hormone in the blood.[2,3]",
"Of note, PTU, methimazole, and iodide solutions are all classified as pregnancy class D and, as such, should not be used in pregnancy.",
"With regard to the management of cardiac symptoms related to thyrotoxicosis, treatment is focused on reducing adrenergic drive to the heart and restoring normal cardiac rhythm. As mentioned above, beta-blockers are very effective for rapid hemodynamic improvement. Either propranolol or metoprolol given intravenously can be used to improve heart rate control in either sinus tachycardia or atrial fibrillation. In severe cases, a continuous infusion of esmolol may be required for rate control. Amiodarone should be avoided when treating atrial fibrillation from thyrotoxicosis because of its high iodine content, which may induce or exacerbate thyroid storm.",
"If a patient is hemodynamically unstable from atrial fibrillation, direct current cardioversion should be used. If symptoms of pulmonary congestion appear, diuretics may be used. Other drugs for heart failure (angiotensin-converting inhibitors, angiotensin receptor blockers, or aldosterone receptor antagonists) are reasonable agents in patients who have depressed left ventricular systolic function.",
"Anticoagulation is recommended for patients in atrial fibrillation secondary to thyrotoxicosis. The American College of Cardiology/American Heart Association/European Society of Cardiology guidelines recommend anticoagulation with warfarin to an international normalized ratio of 2-3 until the patient is euthyroid, after which recommendations and risk stratification are the same as those for atrial fibrillation without thyrotoxicosis.[3]",
"This patient's clinical presentation bordered between thyrotoxicosis and thyroid storm, and she was hemodynamically stable. She was treated immediately with propranolol and PTU and was admitted to a monitored bed in the medical service. Because her clinical condition improved and she remained stable, the admitting team chose to forgo further therapy with iodine and steroids. Her atrial fibrillation resolved within 12 hours, but an echocardiogram revealed cardiomyopathy with a left ventricular ejection fraction of 45% that was attributed to her long-standing hyperthyroidism.",
"A diagnosis of Graves disease was made. Treatment with methimazole and metoprolol was continued, and anticoagulation was initiated. Five months later, the patient was still taking methimazole. Her thyroid function had normalized, her cardiomyopathy had reversed, and her anticoagulation was discontinued, because atrial fibrillation did not recur. Incidentally, tuberculosis was ruled during her admission with three induced sputum samples."
],
"date": "June 24, 2015",
"figures": [],
"markdown": "# Tachycardia in a 61-Year-Old Woman\n\n **Authors:** Jeffrey Siegelman, MD; Daniel M. Lindberg, MD \n **Date:** June 24, 2015\n\n ## Content\n\n Management of thyrotoxicosis consists of a five-pronged, ordered approach, targeting each step in the biosynthetic pathway of thyroid hormone and its activity on target tissues.\nTreatment begins with administration of propylthiouracil (PTU) or methimazole, both of which act by inhibiting new hormone synthesis. PTU has the added effect of decreasing peripheral T4 to T3 conversion. Beta-blockers are then used to inhibit target activity of thyroid hormone. Propranolol is the preferred agent because it also blocks peripheral conversion of T4. When cardioselective agents are preferred, atenolol or metoprolol may be used. At least 1 hour after administration of PTU or methimazole, the patient may be given iodide to inhibit further thyroid hormone release.\nIodine must be given only after synthesis of new hormone is blocked because iodide administration can have the undesired effect of increasing new hormone synthesis. Potassium iodide or Lugol solution of iodine is recommended. Peripheral conversion of T4 to T3 is blocked, as noted above, and dexamethasone may be used as well. Further treatment is supportive and may include acetaminophen for fever and hydrocortisone if the patient is hypotensive as a result of adrenal insufficiency. Salicylates are contraindicated because they displace bound thyroid hormone in the blood.[2,3]\nOf note, PTU, methimazole, and iodide solutions are all classified as pregnancy class D and, as such, should not be used in pregnancy.\nWith regard to the management of cardiac symptoms related to thyrotoxicosis, treatment is focused on reducing adrenergic drive to the heart and restoring normal cardiac rhythm. As mentioned above, beta-blockers are very effective for rapid hemodynamic improvement. Either propranolol or metoprolol given intravenously can be used to improve heart rate control in either sinus tachycardia or atrial fibrillation. In severe cases, a continuous infusion of esmolol may be required for rate control. Amiodarone should be avoided when treating atrial fibrillation from thyrotoxicosis because of its high iodine content, which may induce or exacerbate thyroid storm.\nIf a patient is hemodynamically unstable from atrial fibrillation, direct current cardioversion should be used. If symptoms of pulmonary congestion appear, diuretics may be used. Other drugs for heart failure (angiotensin-converting inhibitors, angiotensin receptor blockers, or aldosterone receptor antagonists) are reasonable agents in patients who have depressed left ventricular systolic function.\nAnticoagulation is recommended for patients in atrial fibrillation secondary to thyrotoxicosis. The American College of Cardiology/American Heart Association/European Society of Cardiology guidelines recommend anticoagulation with warfarin to an international normalized ratio of 2-3 until the patient is euthyroid, after which recommendations and risk stratification are the same as those for atrial fibrillation without thyrotoxicosis.[3]\nThis patient's clinical presentation bordered between thyrotoxicosis and thyroid storm, and she was hemodynamically stable. She was treated immediately with propranolol and PTU and was admitted to a monitored bed in the medical service. Because her clinical condition improved and she remained stable, the admitting team chose to forgo further therapy with iodine and steroids. Her atrial fibrillation resolved within 12 hours, but an echocardiogram revealed cardiomyopathy with a left ventricular ejection fraction of 45% that was attributed to her long-standing hyperthyroidism.\nA diagnosis of Graves disease was made. Treatment with methimazole and metoprolol was continued, and anticoagulation was initiated. Five months later, the patient was still taking methimazole. Her thyroid function had normalized, her cardiomyopathy had reversed, and her anticoagulation was discontinued, because atrial fibrillation did not recur. Incidentally, tuberculosis was ruled during her admission with three induced sputum samples.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 863579,
"choiceText": "Thyroid radionucleotide uptake scan",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 863581,
"choiceText": "TSH",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 863583,
"choiceText": "T<sub>3</sub>",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 863585,
"choiceText": "T<sub>4</sub>",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 863587,
"choiceText": "Free T<sub>4</sub>\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Release of TSH from the anterior pituitary gland is controlled by a very sensitive feedback loop. Even undetectable changes in thyroid hormone levels can have very noticeable effects on TSH levels. In addition, the half-life of TSH is 1 hour, whereas that of T<sub>4</sub> is closer to 1 week. Therefore, TSH is a more precise measure of thyroid disease at the moment when the patient is being tested.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 271519,
"questionText": "You are examining a patient with heart palpitations, diplopia, chest pain, and recent weight loss. You suspect that the thyroid may be involved. Which of the following choices is the most sensitive test for thyroid disease?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 863621,
"choiceText": "Lugol solution of iodine, PTU, propranolol, dexamethasone, and acetaminophen",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 863623,
"choiceText": "Methimazole, propranolol, potassium iodide, aspirin, and dexamethasone",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 863625,
"choiceText": "PTU, propranolol, potassium iodide, dexamethasone, and acetaminophen",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 863627,
"choiceText": "Potassium iodide, propranolol, PTU, dexamethasone, and aspirin",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 863629,
"choiceText": "Dexamethasone, potassium iodide, methimazole, propranolol, and acetaminophen",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Two important points are elucidated here. Iodide must be administered at least 30-60 minutes after blockade of new hormone synthesis is achieved with either PTU or methimazole. This is because iodide has the undesired effect of increased hormone production. Also, salicylates should be avoided in thyrotoxicosis because they displace bound thyroid hormone in the blood.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 271529,
"questionText": "You conclude that the above-described patient has thyroid storm, on the basis of the symptoms and laboratory examinations. Which of the following choices is a proper treatment sequence if thyroid storm is suspected?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Tachycardia in a 61-Year-Old Woman"
},
{
"authors": "Jeffrey Siegelman, MD; Daniel M. Lindberg, MD",
"content": [],
"date": "June 24, 2015",
"figures": [],
"markdown": "# Tachycardia in a 61-Year-Old Woman\n\n **Authors:** Jeffrey Siegelman, MD; Daniel M. Lindberg, MD \n **Date:** June 24, 2015\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 863579,
"choiceText": "Thyroid radionucleotide uptake scan",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 863581,
"choiceText": "TSH",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 863583,
"choiceText": "T<sub>3</sub>",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 863585,
"choiceText": "T<sub>4</sub>",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 863587,
"choiceText": "Free T<sub>4</sub>\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Release of TSH from the anterior pituitary gland is controlled by a very sensitive feedback loop. Even undetectable changes in thyroid hormone levels can have very noticeable effects on TSH levels. In addition, the half-life of TSH is 1 hour, whereas that of T<sub>4</sub> is closer to 1 week. Therefore, TSH is a more precise measure of thyroid disease at the moment when the patient is being tested.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 271519,
"questionText": "You are examining a patient with heart palpitations, diplopia, chest pain, and recent weight loss. You suspect that the thyroid may be involved. Which of the following choices is the most sensitive test for thyroid disease?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 863621,
"choiceText": "Lugol solution of iodine, PTU, propranolol, dexamethasone, and acetaminophen",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 863623,
"choiceText": "Methimazole, propranolol, potassium iodide, aspirin, and dexamethasone",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 863625,
"choiceText": "PTU, propranolol, potassium iodide, dexamethasone, and acetaminophen",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 863627,
"choiceText": "Potassium iodide, propranolol, PTU, dexamethasone, and aspirin",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 863629,
"choiceText": "Dexamethasone, potassium iodide, methimazole, propranolol, and acetaminophen",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Two important points are elucidated here. Iodide must be administered at least 30-60 minutes after blockade of new hormone synthesis is achieved with either PTU or methimazole. This is because iodide has the undesired effect of increased hormone production. Also, salicylates should be avoided in thyrotoxicosis because they displace bound thyroid hormone in the blood.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 271529,
"questionText": "You conclude that the above-described patient has thyroid storm, on the basis of the symptoms and laboratory examinations. Which of the following choices is a proper treatment sequence if thyroid storm is suspected?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Tachycardia in a 61-Year-Old Woman"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 863511,
"choiceText": " Viral cardiomyopathy",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 863513,
"choiceText": "Thyrotoxicosis ",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 863515,
"choiceText": "Tuberculosis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 863517,
"choiceText": "Pulmonary embolism\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 271491,
"questionText": "What is the most likely etiology of the patient's tachycardia? <br><br><i>\r\nHint: Note the presence of weight loss, bilateral exophthalmos, and goiter.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 863579,
"choiceText": "Thyroid radionucleotide uptake scan",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 863581,
"choiceText": "TSH",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 863583,
"choiceText": "T<sub>3</sub>",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 863585,
"choiceText": "T<sub>4</sub>",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 863587,
"choiceText": "Free T<sub>4</sub>\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Release of TSH from the anterior pituitary gland is controlled by a very sensitive feedback loop. Even undetectable changes in thyroid hormone levels can have very noticeable effects on TSH levels. In addition, the half-life of TSH is 1 hour, whereas that of T<sub>4</sub> is closer to 1 week. Therefore, TSH is a more precise measure of thyroid disease at the moment when the patient is being tested.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 271519,
"questionText": "You are examining a patient with heart palpitations, diplopia, chest pain, and recent weight loss. You suspect that the thyroid may be involved. Which of the following choices is the most sensitive test for thyroid disease?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 863621,
"choiceText": "Lugol solution of iodine, PTU, propranolol, dexamethasone, and acetaminophen",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 863623,
"choiceText": "Methimazole, propranolol, potassium iodide, aspirin, and dexamethasone",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 863625,
"choiceText": "PTU, propranolol, potassium iodide, dexamethasone, and acetaminophen",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 863627,
"choiceText": "Potassium iodide, propranolol, PTU, dexamethasone, and aspirin",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 863629,
"choiceText": "Dexamethasone, potassium iodide, methimazole, propranolol, and acetaminophen",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Two important points are elucidated here. Iodide must be administered at least 30-60 minutes after blockade of new hormone synthesis is achieved with either PTU or methimazole. This is because iodide has the undesired effect of increased hormone production. Also, salicylates should be avoided in thyrotoxicosis because they displace bound thyroid hormone in the blood.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 271529,
"questionText": "You conclude that the above-described patient has thyroid storm, on the basis of the symptoms and laboratory examinations. Which of the following choices is a proper treatment sequence if thyroid storm is suspected?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
845960 | /viewarticle/845960 | [
{
"authors": "Nicolas Grundmann, MD, MBA",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.",
"A 28-year-old man presents to the emergency department with 8 hours of nausea, vomiting, and diarrhea. He states that the emesis is not bloody or dark green. The stool consists mainly of watery fluids and mucus without blood (Figure 1). He has had more than six bowel movements in the past 8 hours and is unsure how many times he has vomited, simply stating \"a lot.\" He reports that he cannot eat any food or drink liquids without vomiting.",
"Figure 1.",
"On questioning, the patient has no significant medical history and takes no medication. He denies recent travel, camping, or antibiotic use. He claims that this has never happened to him before. He has taken no medications to help his symptoms and reports no allergies."
],
"date": "June 10, 2015",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/845/960/845960-Thumb1.png"
}
],
"markdown": "# 28-Year-Old Man With Nausea, Vomiting, and Diarrhea\n\n **Authors:** Nicolas Grundmann, MD, MBA \n **Date:** June 10, 2015\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.\nA 28-year-old man presents to the emergency department with 8 hours of nausea, vomiting, and diarrhea. He states that the emesis is not bloody or dark green. The stool consists mainly of watery fluids and mucus without blood (Figure 1). He has had more than six bowel movements in the past 8 hours and is unsure how many times he has vomited, simply stating \"a lot.\" He reports that he cannot eat any food or drink liquids without vomiting.\nFigure 1.\nOn questioning, the patient has no significant medical history and takes no medication. He denies recent travel, camping, or antibiotic use. He claims that this has never happened to him before. He has taken no medications to help his symptoms and reports no allergies.\n\n ## Figures\n\n **Figure 1.** \n \n\n\n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "28-Year-Old Man With Nausea, Vomiting, and Diarrhea"
},
{
"authors": "Nicolas Grundmann, MD, MBA",
"content": [
"Upon examination, the patient appears uncomfortable. He states that he feels like he is going to vomit again. Relevant findings are as follows:",
"Blood pressure: 130/85 mm Hg",
"Heart rate: 95 beats/min",
"Respiratory rate: 18 breaths/min",
"Oral temperature: 98.3°F",
"Oxygen saturation: 94% on room air",
"Point-of-care fingerstick: Normal",
"An evaluation of the patient's head, ears, eyes, nose, and throat reveals only dry mucus membranes. A cardiovascular examination reveals low-grade tachycardia with a regular rhythm and normal S1 and S2 sounds. No murmurs, rubs, or gallops are noted. Upon pulmonary evaluation, clear breath sounds are heard bilaterally, with no wheezes or crackles.",
"Evaluation of the abdomen reveals hyperactive bowel sounds, very mild diffuse tenderness that does not localize to any one area, and a negative Murphy sign, with no guarding or rebound. Pressing deep in the epigastric area makes the patient nauseous. No skin rashes are found.",
"A basic chemistry panel is ordered, and an intravenous line is started for fluid administration."
],
"date": "June 10, 2015",
"figures": [],
"markdown": "# 28-Year-Old Man With Nausea, Vomiting, and Diarrhea\n\n **Authors:** Nicolas Grundmann, MD, MBA \n **Date:** June 10, 2015\n\n ## Content\n\n Upon examination, the patient appears uncomfortable. He states that he feels like he is going to vomit again. Relevant findings are as follows:\nBlood pressure: 130/85 mm Hg\nHeart rate: 95 beats/min\nRespiratory rate: 18 breaths/min\nOral temperature: 98.3°F\nOxygen saturation: 94% on room air\nPoint-of-care fingerstick: Normal\nAn evaluation of the patient's head, ears, eyes, nose, and throat reveals only dry mucus membranes. A cardiovascular examination reveals low-grade tachycardia with a regular rhythm and normal S1 and S2 sounds. No murmurs, rubs, or gallops are noted. Upon pulmonary evaluation, clear breath sounds are heard bilaterally, with no wheezes or crackles.\nEvaluation of the abdomen reveals hyperactive bowel sounds, very mild diffuse tenderness that does not localize to any one area, and a negative Murphy sign, with no guarding or rebound. Pressing deep in the epigastric area makes the patient nauseous. No skin rashes are found.\nA basic chemistry panel is ordered, and an intravenous line is started for fluid administration.\n\n ## Figures\n\n \n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 852543,
"choiceText": "Organophosphate poisoning",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 852545,
"choiceText": "Gastroenteritis",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 852547,
"choiceText": "Appendicitis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 852549,
"choiceText": "Bowel obstruction",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 267911,
"questionText": "On the basis of the history, physical examination, and workup, what is the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "28-Year-Old Man With Nausea, Vomiting, and Diarrhea"
},
{
"authors": "Nicolas Grundmann, MD, MBA",
"content": [
"Foodborne disease typically manifests as a mixture of nausea, vomiting, fever, abdominal pain, and diarrhea along with inflammation in the stomach (Figures 2 and 3). Acute gastroenteritis is a clinical diagnosis; laboratory tests and stool samples are not routinely required. Most pathogens are viral, and routine bacterial stool cultures reveal a pathogen in only 1%-10% of cases.[1] The vast majority of cases spontaneously resolve. The patient must stay hydrated. Treatment is symptomatic, except in cases of specifically identifiable pathogens. Stool samples should be considered in immunocompromised or pregnant patients and in those who have bloody diarrhea or chronic (> 14 days) diarrhea.",
"Figure 2.",
"Figure 3.",
"In developed countries, strict food-handling regulations and water sanitation systems dictate the etiology of disease. The exact incidence of this disease burden widely varies, depending on population, and is often underreported; patients infrequently seek medical care for mild cases.",
"The Centers for Disease Control and Prevention estimates that foodborne diseases account for approximately 8 million cases, 128,000 hospitalizations, and 3000 deaths each year in the United States.[2]",
"Although obtaining a food history is an important step in the diagnosis of gastroenteritis and foodborne pathogens, an exact source of contamination is often not discovered, even with careful history-taking. Depending on the type of pathogen causing the symptoms, the incubation period can range from 1 hour since ingestion (preformed toxins) to days or weeks (invasive pathogens)."
],
"date": "June 10, 2015",
"figures": [
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/845/960/845960-Thumb2.png"
},
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/845/960/845960-Thumb3.png"
}
],
"markdown": "# 28-Year-Old Man With Nausea, Vomiting, and Diarrhea\n\n **Authors:** Nicolas Grundmann, MD, MBA \n **Date:** June 10, 2015\n\n ## Content\n\n Foodborne disease typically manifests as a mixture of nausea, vomiting, fever, abdominal pain, and diarrhea along with inflammation in the stomach (Figures 2 and 3). Acute gastroenteritis is a clinical diagnosis; laboratory tests and stool samples are not routinely required. Most pathogens are viral, and routine bacterial stool cultures reveal a pathogen in only 1%-10% of cases.[1] The vast majority of cases spontaneously resolve. The patient must stay hydrated. Treatment is symptomatic, except in cases of specifically identifiable pathogens. Stool samples should be considered in immunocompromised or pregnant patients and in those who have bloody diarrhea or chronic (> 14 days) diarrhea.\nFigure 2.\nFigure 3.\nIn developed countries, strict food-handling regulations and water sanitation systems dictate the etiology of disease. The exact incidence of this disease burden widely varies, depending on population, and is often underreported; patients infrequently seek medical care for mild cases.\nThe Centers for Disease Control and Prevention estimates that foodborne diseases account for approximately 8 million cases, 128,000 hospitalizations, and 3000 deaths each year in the United States.[2]\nAlthough obtaining a food history is an important step in the diagnosis of gastroenteritis and foodborne pathogens, an exact source of contamination is often not discovered, even with careful history-taking. Depending on the type of pathogen causing the symptoms, the incubation period can range from 1 hour since ingestion (preformed toxins) to days or weeks (invasive pathogens).\n\n ## Figures\n\n **Figure 2.** \n \n\n**Figure 3.** \n \n\n\n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 852543,
"choiceText": "Organophosphate poisoning",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 852545,
"choiceText": "Gastroenteritis",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 852547,
"choiceText": "Appendicitis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 852549,
"choiceText": "Bowel obstruction",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 267911,
"questionText": "On the basis of the history, physical examination, and workup, what is the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "28-Year-Old Man With Nausea, Vomiting, and Diarrhea"
},
{
"authors": "Nicolas Grundmann, MD, MBA",
"content": [
"The following are three important elements of the history clinicians should consider while trying to determine the differential diagnosis of foodborne diseases:",
"Presenting symptoms;",
"Exposure to a particular type of food associated with foodborne disease—determine what the person ate in the week before they became sick; and",
"The interval between exposure to the suspect food and the onset of symptoms.",
"The most frequent presentation of a foodborne disease is a combination of nausea, vomiting, fever, abdominal pain, and diarrhea. Nongastrointestinal foodborne illnesses (eg, botulism, ciguatera/scombroid fish) present with a wide variety of clinical pictures. Symptoms concerning for more serious infection include signs of dehydration, bloody stools, fever, and severe abdominal pain.",
"Diarrhea is an important distinguishing factor for causes of foodborne illness. A lack of diarrhea may suggest that the patient presented early in the course of the disease, the pathogen they ingested was a preformed toxin, or another disease process is occurring (eg, chemical poisoning, bowel obstruction, gastroparesis, gastritis). Bloody diarrhea is a sign of an invasive pathogen and should increase the suspicion for hemorrhagic \nEscherichia coli, \nShigella, \nCampylobacter, and \nSalmonella infection with treatment modalities adjusted accordingly.",
"If a specific food exposure is identified, then the interval between exposure and symptom onset can help determine the etiology of the organism. Broadly, the natural disease process of foodborne pathogens can be divided into three subcategories: preformed toxins, pathogens that produce toxins once ingested, and invasive pathogens.",
"Preformed toxins are organisms that generate a toxin while growing in the food before its consumption. These are associated with a rapid onset of symptoms (6-12 hours) and are predominantly associated with frequent emesis and only sometimes involve diarrhea. Pathogens that make toxins once they have been ingested usually cause symptoms around 24 hours after exposure and are associated with watery or bloody diarrhea.",
"Invasive pathogens are microbes that damage the epithelial cell surface directly or invade across the intestinal epithelial cell barrier. These pathogens produce a wide spectrum of clinical presentations, from watery diarrhea to inflammatory diarrhea or systemic disease."
],
"date": "June 10, 2015",
"figures": [],
"markdown": "# 28-Year-Old Man With Nausea, Vomiting, and Diarrhea\n\n **Authors:** Nicolas Grundmann, MD, MBA \n **Date:** June 10, 2015\n\n ## Content\n\n The following are three important elements of the history clinicians should consider while trying to determine the differential diagnosis of foodborne diseases:\nPresenting symptoms;\nExposure to a particular type of food associated with foodborne disease—determine what the person ate in the week before they became sick; and\nThe interval between exposure to the suspect food and the onset of symptoms.\nThe most frequent presentation of a foodborne disease is a combination of nausea, vomiting, fever, abdominal pain, and diarrhea. Nongastrointestinal foodborne illnesses (eg, botulism, ciguatera/scombroid fish) present with a wide variety of clinical pictures. Symptoms concerning for more serious infection include signs of dehydration, bloody stools, fever, and severe abdominal pain.\nDiarrhea is an important distinguishing factor for causes of foodborne illness. A lack of diarrhea may suggest that the patient presented early in the course of the disease, the pathogen they ingested was a preformed toxin, or another disease process is occurring (eg, chemical poisoning, bowel obstruction, gastroparesis, gastritis). Bloody diarrhea is a sign of an invasive pathogen and should increase the suspicion for hemorrhagic \nEscherichia coli, \nShigella, \nCampylobacter, and \nSalmonella infection with treatment modalities adjusted accordingly.\nIf a specific food exposure is identified, then the interval between exposure and symptom onset can help determine the etiology of the organism. Broadly, the natural disease process of foodborne pathogens can be divided into three subcategories: preformed toxins, pathogens that produce toxins once ingested, and invasive pathogens.\nPreformed toxins are organisms that generate a toxin while growing in the food before its consumption. These are associated with a rapid onset of symptoms (6-12 hours) and are predominantly associated with frequent emesis and only sometimes involve diarrhea. Pathogens that make toxins once they have been ingested usually cause symptoms around 24 hours after exposure and are associated with watery or bloody diarrhea.\nInvasive pathogens are microbes that damage the epithelial cell surface directly or invade across the intestinal epithelial cell barrier. These pathogens produce a wide spectrum of clinical presentations, from watery diarrhea to inflammatory diarrhea or systemic disease.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "28-Year-Old Man With Nausea, Vomiting, and Diarrhea"
},
{
"authors": "Nicolas Grundmann, MD, MBA",
"content": [
"Additional history in patients with foodborne diseases should focus on elements that help localize the epidemiology of possible of food exposure, as well as the potential sequelae of infection. This includes recent travel, camping, and antibiotic use or hospitalization, as well as an immunocompromised or pregnancy state.",
"Recent travel, particularly to resource-limited countries, significantly increases the likelihood of enterotoxigenic \nE coli exposure, often referred to as \"traveler's diarrhea.\" These cases may warrant treatment with antibiotics, such as ciprofloxacin or azithromycin, depending on the native resistance patterns of bacteria in the countries visited.",
"Recent antibiotic use or hospitalization increases the likelihood of gastrointestinal microbe virulence or the incidence of resistant bacterial diseases. \nClostridium difficile infections are of great concern in elderly or recently hospitalized patients who have diarrhea without emesis. These cases require a significantly different treatment course and are not considered foodborne diseases.",
"Medical comorbidities greatly affect the consequences of foodborne illness. Patients with diabetes should be screened to ensure that the illness has not triggered a hyperglycemic state. Elderly persons and infants and children should have special attention focused on their hydration status because their underlying nutritional status can be less robust than that of otherwise healthy patients. Immunocompromised patients should be assumed to have infectious or invasive gastroenteritis; the pathogens in these patients vary significantly from routine foodborne illnesses with an increased incidence of salmonella, cytomegalovirus, herpes simplex virus colitis, and parasitic infections. Pregnant women should be screened for listeriosis and treated as necessary.",
"General treatment should focus on symptom improvement, with hydration and consideration of antiemetic agents. Care should be taken to ensure that patients are able to tolerate food and fluids by mouth before being discharged home. The patient in this case was released after sufficient improvement of symptoms and a demonstration of food and fluid tolerance."
],
"date": "June 10, 2015",
"figures": [],
"markdown": "# 28-Year-Old Man With Nausea, Vomiting, and Diarrhea\n\n **Authors:** Nicolas Grundmann, MD, MBA \n **Date:** June 10, 2015\n\n ## Content\n\n Additional history in patients with foodborne diseases should focus on elements that help localize the epidemiology of possible of food exposure, as well as the potential sequelae of infection. This includes recent travel, camping, and antibiotic use or hospitalization, as well as an immunocompromised or pregnancy state.\nRecent travel, particularly to resource-limited countries, significantly increases the likelihood of enterotoxigenic \nE coli exposure, often referred to as \"traveler's diarrhea.\" These cases may warrant treatment with antibiotics, such as ciprofloxacin or azithromycin, depending on the native resistance patterns of bacteria in the countries visited.\nRecent antibiotic use or hospitalization increases the likelihood of gastrointestinal microbe virulence or the incidence of resistant bacterial diseases. \nClostridium difficile infections are of great concern in elderly or recently hospitalized patients who have diarrhea without emesis. These cases require a significantly different treatment course and are not considered foodborne diseases.\nMedical comorbidities greatly affect the consequences of foodborne illness. Patients with diabetes should be screened to ensure that the illness has not triggered a hyperglycemic state. Elderly persons and infants and children should have special attention focused on their hydration status because their underlying nutritional status can be less robust than that of otherwise healthy patients. Immunocompromised patients should be assumed to have infectious or invasive gastroenteritis; the pathogens in these patients vary significantly from routine foodborne illnesses with an increased incidence of salmonella, cytomegalovirus, herpes simplex virus colitis, and parasitic infections. Pregnant women should be screened for listeriosis and treated as necessary.\nGeneral treatment should focus on symptom improvement, with hydration and consideration of antiemetic agents. Care should be taken to ensure that patients are able to tolerate food and fluids by mouth before being discharged home. The patient in this case was released after sufficient improvement of symptoms and a demonstration of food and fluid tolerance.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 852551,
"choiceText": "<i>Bacillus cereus</i>",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 852553,
"choiceText": "<i>Salmonella</i>",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 852555,
"choiceText": "Norovirus ",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 852557,
"choiceText": "<i>Giardia</i>",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>Viral gastroenteritis (such as the family of noroviruses) is the most common type of gastroenteritis in developed countries.<sup type=\"ref\">[3]</sup> The disease course is usually mild, with nonbloody emesis and diarrhea, and self-resolves in 24-72 hours.</p>\r\n\r\n<p><em>Bacillus cereus</em> usually causes rapid onset (1-6 hours) of nausea and emesis through preformed toxin, is not associated with diarrhea, and is self-limiting; it is most commonly associated with the consumption of old rice. <em>Salmonella</em> most frequently causes bloody inflammatory diarrhea in developed countries, with fever and severe abdominal pain. The incubation period is 1-3 days.</p>\r\n\r\n<p><em>Giardia</em> is infrequently associated with emesis. This organism is uncommon in the United States owing to water sanitation methods. A history of the patient drinking water while hiking or camping could prompt consideration, especially if frequent green, smelly stools with a great deal of flatulence are reported.</p>",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 267913,
"questionText": "Which of the following organisms is the most likely pathogen in the patient described in this case?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 855213,
"choiceText": "Hydration",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 855215,
"choiceText": "Ciprofloxacin",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 855217,
"choiceText": "CT of the abdomen ",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 855219,
"choiceText": "Discharge home",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>Dehydration secondary to frequent emesis, diarrhea, and an inability to tolerate liquids or solids by mouth are common. If dry mucus membranes and low-grade tachycardia is observed, as in this case, the patient should be hydrated before discharge home. Oral and intravenous hydration are functionally equivalent for acute gastroenteritis, as long as the patient is able to tolerate oral hydration. Antiemetics, such as ondansetron (Zofran®), can be used to control symptoms. Metoclopramide (Reglan®) should be avoided because it increases gastrointestinal motility. </p>\r\n\r\n<p>Antibiotics are not required for single, uncomplicated cases of gastroenteritis. Advanced imaging studies are not necessary unless the clinician has a strong suspicion for another disease process. This patient should be hydrated and should be able to demonstrate the ability to tolerate foods and liquids by mouth before being sent home.</p>",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 268747,
"questionText": "Which of the following is the next best step for medical management of a patient with suspected gastroenteritis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "28-Year-Old Man With Nausea, Vomiting, and Diarrhea"
},
{
"authors": "Nicolas Grundmann, MD, MBA",
"content": [],
"date": "June 10, 2015",
"figures": [],
"markdown": "# 28-Year-Old Man With Nausea, Vomiting, and Diarrhea\n\n **Authors:** Nicolas Grundmann, MD, MBA \n **Date:** June 10, 2015\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 852551,
"choiceText": "<i>Bacillus cereus</i>",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 852553,
"choiceText": "<i>Salmonella</i>",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 852555,
"choiceText": "Norovirus ",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 852557,
"choiceText": "<i>Giardia</i>",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>Viral gastroenteritis (such as the family of noroviruses) is the most common type of gastroenteritis in developed countries.<sup type=\"ref\">[3]</sup> The disease course is usually mild, with nonbloody emesis and diarrhea, and self-resolves in 24-72 hours.</p>\r\n\r\n<p><em>Bacillus cereus</em> usually causes rapid onset (1-6 hours) of nausea and emesis through preformed toxin, is not associated with diarrhea, and is self-limiting; it is most commonly associated with the consumption of old rice. <em>Salmonella</em> most frequently causes bloody inflammatory diarrhea in developed countries, with fever and severe abdominal pain. The incubation period is 1-3 days.</p>\r\n\r\n<p><em>Giardia</em> is infrequently associated with emesis. This organism is uncommon in the United States owing to water sanitation methods. A history of the patient drinking water while hiking or camping could prompt consideration, especially if frequent green, smelly stools with a great deal of flatulence are reported.</p>",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 267913,
"questionText": "Which of the following organisms is the most likely pathogen in the patient described in this case?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 855213,
"choiceText": "Hydration",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 855215,
"choiceText": "Ciprofloxacin",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 855217,
"choiceText": "CT of the abdomen ",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 855219,
"choiceText": "Discharge home",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>Dehydration secondary to frequent emesis, diarrhea, and an inability to tolerate liquids or solids by mouth are common. If dry mucus membranes and low-grade tachycardia is observed, as in this case, the patient should be hydrated before discharge home. Oral and intravenous hydration are functionally equivalent for acute gastroenteritis, as long as the patient is able to tolerate oral hydration. Antiemetics, such as ondansetron (Zofran®), can be used to control symptoms. Metoclopramide (Reglan®) should be avoided because it increases gastrointestinal motility. </p>\r\n\r\n<p>Antibiotics are not required for single, uncomplicated cases of gastroenteritis. Advanced imaging studies are not necessary unless the clinician has a strong suspicion for another disease process. This patient should be hydrated and should be able to demonstrate the ability to tolerate foods and liquids by mouth before being sent home.</p>",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 268747,
"questionText": "Which of the following is the next best step for medical management of a patient with suspected gastroenteritis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "28-Year-Old Man With Nausea, Vomiting, and Diarrhea"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 852543,
"choiceText": "Organophosphate poisoning",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 852545,
"choiceText": "Gastroenteritis",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 852547,
"choiceText": "Appendicitis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 852549,
"choiceText": "Bowel obstruction",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 267911,
"questionText": "On the basis of the history, physical examination, and workup, what is the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 852551,
"choiceText": "<i>Bacillus cereus</i>",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 852553,
"choiceText": "<i>Salmonella</i>",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 852555,
"choiceText": "Norovirus ",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 852557,
"choiceText": "<i>Giardia</i>",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>Viral gastroenteritis (such as the family of noroviruses) is the most common type of gastroenteritis in developed countries.<sup type=\"ref\">[3]</sup> The disease course is usually mild, with nonbloody emesis and diarrhea, and self-resolves in 24-72 hours.</p>\r\n\r\n<p><em>Bacillus cereus</em> usually causes rapid onset (1-6 hours) of nausea and emesis through preformed toxin, is not associated with diarrhea, and is self-limiting; it is most commonly associated with the consumption of old rice. <em>Salmonella</em> most frequently causes bloody inflammatory diarrhea in developed countries, with fever and severe abdominal pain. The incubation period is 1-3 days.</p>\r\n\r\n<p><em>Giardia</em> is infrequently associated with emesis. This organism is uncommon in the United States owing to water sanitation methods. A history of the patient drinking water while hiking or camping could prompt consideration, especially if frequent green, smelly stools with a great deal of flatulence are reported.</p>",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 267913,
"questionText": "Which of the following organisms is the most likely pathogen in the patient described in this case?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 855213,
"choiceText": "Hydration",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 855215,
"choiceText": "Ciprofloxacin",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 855217,
"choiceText": "CT of the abdomen ",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 855219,
"choiceText": "Discharge home",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>Dehydration secondary to frequent emesis, diarrhea, and an inability to tolerate liquids or solids by mouth are common. If dry mucus membranes and low-grade tachycardia is observed, as in this case, the patient should be hydrated before discharge home. Oral and intravenous hydration are functionally equivalent for acute gastroenteritis, as long as the patient is able to tolerate oral hydration. Antiemetics, such as ondansetron (Zofran®), can be used to control symptoms. Metoclopramide (Reglan®) should be avoided because it increases gastrointestinal motility. </p>\r\n\r\n<p>Antibiotics are not required for single, uncomplicated cases of gastroenteritis. Advanced imaging studies are not necessary unless the clinician has a strong suspicion for another disease process. This patient should be hydrated and should be able to demonstrate the ability to tolerate foods and liquids by mouth before being sent home.</p>",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 268747,
"questionText": "Which of the following is the next best step for medical management of a patient with suspected gastroenteritis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
845434 | /viewarticle/845434 | [
{
"authors": "Sumaira Nabi, MBBS; Zakir Jan, MBBS, FCPS Neurology; Muhammad Irshad, MBBS, FCPS Neurology",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"A 34-year-old Pakistani woman presents with a 4-year history of psychotic behavior and neurocognitive decline. She has been under the care of different psychiatrists and has been treated for schizophrenia for 3 years. For the last 6 months, she has had progressive motor regression, loss of speech, and bowel and bladder incontinence. The patient has been bed-bound during this time as well.",
"The patient has no history of fever, headache, altered sensorium, seizures, or swallowing difficulty. She also has no history of visual impediment, limb weakness, ataxia, or sensory symptoms. She has 2 daughters, both of whom were born through normal vaginal delivery; the last birth was 5 years ago. She is a nonsmoker and is not taking any medications on a regular basis. Her family history is unremarkable."
],
"date": "April 23, 2018",
"figures": [],
"markdown": "# Neurocognitive Decline and Motor Regression in a 34-Year-Old Woman\n\n **Authors:** Sumaira Nabi, MBBS; Zakir Jan, MBBS, FCPS Neurology; Muhammad Irshad, MBBS, FCPS Neurology \n **Date:** April 23, 2018\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nA 34-year-old Pakistani woman presents with a 4-year history of psychotic behavior and neurocognitive decline. She has been under the care of different psychiatrists and has been treated for schizophrenia for 3 years. For the last 6 months, she has had progressive motor regression, loss of speech, and bowel and bladder incontinence. The patient has been bed-bound during this time as well.\nThe patient has no history of fever, headache, altered sensorium, seizures, or swallowing difficulty. She also has no history of visual impediment, limb weakness, ataxia, or sensory symptoms. She has 2 daughters, both of whom were born through normal vaginal delivery; the last birth was 5 years ago. She is a nonsmoker and is not taking any medications on a regular basis. Her family history is unremarkable.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "Neurocognitive Decline and Motor Regression in a 34-Year-Old Woman"
},
{
"authors": "Sumaira Nabi, MBBS; Zakir Jan, MBBS, FCPS Neurology; Muhammad Irshad, MBBS, FCPS Neurology",
"content": [
"Upon physical examination, the patient is overweight, agitated, and uncooperative. Her oral temperature is 98.6°F. She has a regular pulse of 78 beats/min. Her blood pressure is 110/80 mm Hg, and her respiratory rate is 14 breaths/min. Her Glasgow Coma Scale (GCS) is 12/15, with speech limited to incomprehensible sounds only. Her cranial nerves are grossly intact.",
"Funduscopic examination is consistent with mild, bilateral optic disc pallor. She has a spastic quadriparesis with hyperreflexia and bilateral extensor plantar response. Cerebellar signs and gait cannot be assessed. Signs of meningeal irritation are absent. Her abdomen is soft and nontender. No clinical evidence suggests organomegaly or ascites. Her bowel sounds are audible. The patient's precordial examination reveals normal heart sounds. The lung fields are clear to auscultation.",
"The laboratory analysis reveals a normal complete blood count, with an erythrocyte sedimentation rate of 10 mm/hour. Her liver function test results, renal function test results, muscle enzyme findings, electrocardiography images, chest x-ray, and abdominal ultrasonography are unremarkable. Her nerve conduction studies are inconclusive. Cerebrospinal fluid (CSF) analysis is normal; CSF oligoclonal band findings are also negative. Her thyroid function test results are normal as well. Antinuclear antibody and rheumatoid factor test results are negative.",
"MRI of the brain with contrast reveals areas of abnormal signal intensity diffusely in the white matter of both cerebral hemispheres, appearing hypointense on T1-weighted images and hyperintense on T2-weighted images and fluid-attenuated inversion recovery (FLAIR) sequences (Figures 1-6).",
"Figure 1.",
"Figure 2.",
"Figure 3.",
"Figure 4.",
"Figure 5.",
"Figure 6.",
"These reveal involvement of the centrum semiovale and periventricular regions (predominantly in the posterior parietal lobe and the entire occipital lobe). Subcortical white matter is relatively spared. Mild, generalized cerebral atrophy is also observed. MRI of the cervicodorsal spine with contrast is unremarkable."
],
"date": "April 23, 2018",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/845/434/845434-Thumb1.png"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/845/434/845434-Thumb2.png"
},
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/845/434/845434-Thumb3.png"
},
{
"caption": "Figure 4.",
"image_url": "https://img.medscapestatic.com/article/845/434/845434-Thumb4.png"
},
{
"caption": "Figure 5.",
"image_url": "https://img.medscapestatic.com/article/845/434/845434-Thumb5.png"
},
{
"caption": "Figure 6.",
"image_url": "https://img.medscapestatic.com/article/845/434/845434-Thumb6.png"
}
],
"markdown": "# Neurocognitive Decline and Motor Regression in a 34-Year-Old Woman\n\n **Authors:** Sumaira Nabi, MBBS; Zakir Jan, MBBS, FCPS Neurology; Muhammad Irshad, MBBS, FCPS Neurology \n **Date:** April 23, 2018\n\n ## Content\n\n Upon physical examination, the patient is overweight, agitated, and uncooperative. Her oral temperature is 98.6°F. She has a regular pulse of 78 beats/min. Her blood pressure is 110/80 mm Hg, and her respiratory rate is 14 breaths/min. Her Glasgow Coma Scale (GCS) is 12/15, with speech limited to incomprehensible sounds only. Her cranial nerves are grossly intact.\nFunduscopic examination is consistent with mild, bilateral optic disc pallor. She has a spastic quadriparesis with hyperreflexia and bilateral extensor plantar response. Cerebellar signs and gait cannot be assessed. Signs of meningeal irritation are absent. Her abdomen is soft and nontender. No clinical evidence suggests organomegaly or ascites. Her bowel sounds are audible. The patient's precordial examination reveals normal heart sounds. The lung fields are clear to auscultation.\nThe laboratory analysis reveals a normal complete blood count, with an erythrocyte sedimentation rate of 10 mm/hour. Her liver function test results, renal function test results, muscle enzyme findings, electrocardiography images, chest x-ray, and abdominal ultrasonography are unremarkable. Her nerve conduction studies are inconclusive. Cerebrospinal fluid (CSF) analysis is normal; CSF oligoclonal band findings are also negative. Her thyroid function test results are normal as well. Antinuclear antibody and rheumatoid factor test results are negative.\nMRI of the brain with contrast reveals areas of abnormal signal intensity diffusely in the white matter of both cerebral hemispheres, appearing hypointense on T1-weighted images and hyperintense on T2-weighted images and fluid-attenuated inversion recovery (FLAIR) sequences (Figures 1-6).\nFigure 1.\nFigure 2.\nFigure 3.\nFigure 4.\nFigure 5.\nFigure 6.\nThese reveal involvement of the centrum semiovale and periventricular regions (predominantly in the posterior parietal lobe and the entire occipital lobe). Subcortical white matter is relatively spared. Mild, generalized cerebral atrophy is also observed. MRI of the cervicodorsal spine with contrast is unremarkable.\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n**Figure 3.** \n \n\n**Figure 4.** \n \n\n**Figure 5.** \n \n\n**Figure 6.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 849917,
"choiceText": "Neuromyelitis optica",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 849919,
"choiceText": "Multiple sclerosis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 849921,
"choiceText": "Acute disseminated encephalomyelitis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 849923,
"choiceText": "Leukodystrophy",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 267059,
"questionText": "On the basis of the history, physical examination, and workup, what is the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Neurocognitive Decline and Motor Regression in a 34-Year-Old Woman"
},
{
"authors": "Sumaira Nabi, MBBS; Zakir Jan, MBBS, FCPS Neurology; Muhammad Irshad, MBBS, FCPS Neurology",
"content": [
"This patient's radiologic data and clinical picture were consistent with metachromatic leukodystrophy (MLD). Because the patient had already progressed to a bedridden, quadriparetic state, she was referred for rehabilitation, and the family was counseled about the guarded prognosis and probable course of the disease. They were also counseled for genetic testing. No curative treatment is available for MLD; therefore, only supportive treatment could be offered. She was given atypical antipsychotics for her agitated and restless behavior.",
"The leukodystrophies are a set of rare, progressive diseases that affect the growth or maintenance of white matter (ie, myelin).[1] Most leukodystrophies are genetic disorders and are inherited in a recessive, dominant, or X-linked manner, depending on the type of disease. However, some leukodystrophies appear to be sporadic rather than inherited.",
"Many leukodystrophies have adult-onset variants.[2] These include MLD, adrenoleukodystrophy, and Krabbe disease, in addition to many other rare varieties. MLD is one of the most serious and rare inherited demyelination disorders and eventually progresses to a bed-bound, quadriplegic state, with loss of speech and comprehension.[3] A lysosomal storage disease, it is transmitted as an autosomal recessive trait and is characterized by demyelination of the white matter in the central nervous system and peripheral nerves.[1,4]",
"MLD results from a mutation in the ARSA gene, which codes for a deficiency of the enzyme arylsulfatase A. More rarely, MLD results from a mutation in the PSAP gene, which codes for the activator protein saposin B. Arylsulfatase A converts sulfatide to cerebroside (a major component of myelin).[5] Thus, the deficiency of arylsulfatase A results in sulfatide accumulation in the lysosomes of the brain and peripheral nerves. Accumulation of sulfatide affects the oligodendrocytes and Schwann cells, causing progressive demyelination. The incidence of MLD is estimated to be 1 case per 40,000 births.[1]",
"MLD is clinically heterogeneous with respect to the age of onset, effects on the peripheral and central nervous systems, and disease progression. It can manifest in the late infantile period, early juvenile period, late juvenile period, or adulthood.[1,6,7] It usually has its onset between ages 1 and 4 years. It is characterized in this age group by progressive motor regression with gait difficulties, in association with neurocognitive decline and reduced output of speech. However, seizures are rare. A variable degree of neuropathy may be present. One third of the affected children have optic atrophy. The course of the disease is rapid and the outcome fatal, with a mean survival of 3-4 years."
],
"date": "April 23, 2018",
"figures": [],
"markdown": "# Neurocognitive Decline and Motor Regression in a 34-Year-Old Woman\n\n **Authors:** Sumaira Nabi, MBBS; Zakir Jan, MBBS, FCPS Neurology; Muhammad Irshad, MBBS, FCPS Neurology \n **Date:** April 23, 2018\n\n ## Content\n\n This patient's radiologic data and clinical picture were consistent with metachromatic leukodystrophy (MLD). Because the patient had already progressed to a bedridden, quadriparetic state, she was referred for rehabilitation, and the family was counseled about the guarded prognosis and probable course of the disease. They were also counseled for genetic testing. No curative treatment is available for MLD; therefore, only supportive treatment could be offered. She was given atypical antipsychotics for her agitated and restless behavior.\nThe leukodystrophies are a set of rare, progressive diseases that affect the growth or maintenance of white matter (ie, myelin).[1] Most leukodystrophies are genetic disorders and are inherited in a recessive, dominant, or X-linked manner, depending on the type of disease. However, some leukodystrophies appear to be sporadic rather than inherited.\nMany leukodystrophies have adult-onset variants.[2] These include MLD, adrenoleukodystrophy, and Krabbe disease, in addition to many other rare varieties. MLD is one of the most serious and rare inherited demyelination disorders and eventually progresses to a bed-bound, quadriplegic state, with loss of speech and comprehension.[3] A lysosomal storage disease, it is transmitted as an autosomal recessive trait and is characterized by demyelination of the white matter in the central nervous system and peripheral nerves.[1,4]\nMLD results from a mutation in the ARSA gene, which codes for a deficiency of the enzyme arylsulfatase A. More rarely, MLD results from a mutation in the PSAP gene, which codes for the activator protein saposin B. Arylsulfatase A converts sulfatide to cerebroside (a major component of myelin).[5] Thus, the deficiency of arylsulfatase A results in sulfatide accumulation in the lysosomes of the brain and peripheral nerves. Accumulation of sulfatide affects the oligodendrocytes and Schwann cells, causing progressive demyelination. The incidence of MLD is estimated to be 1 case per 40,000 births.[1]\nMLD is clinically heterogeneous with respect to the age of onset, effects on the peripheral and central nervous systems, and disease progression. It can manifest in the late infantile period, early juvenile period, late juvenile period, or adulthood.[1,6,7] It usually has its onset between ages 1 and 4 years. It is characterized in this age group by progressive motor regression with gait difficulties, in association with neurocognitive decline and reduced output of speech. However, seizures are rare. A variable degree of neuropathy may be present. One third of the affected children have optic atrophy. The course of the disease is rapid and the outcome fatal, with a mean survival of 3-4 years.\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 849917,
"choiceText": "Neuromyelitis optica",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 849919,
"choiceText": "Multiple sclerosis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 849921,
"choiceText": "Acute disseminated encephalomyelitis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 849923,
"choiceText": "Leukodystrophy",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 267059,
"questionText": "On the basis of the history, physical examination, and workup, what is the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Neurocognitive Decline and Motor Regression in a 34-Year-Old Woman"
},
{
"authors": "Sumaira Nabi, MBBS; Zakir Jan, MBBS, FCPS Neurology; Muhammad Irshad, MBBS, FCPS Neurology",
"content": [
"The juvenile form of MLD has its onset between ages 4 and 12 years. In these cases, the clinical course is a protracted one, and manifestations vary from peripheral nerve involvement to learning disability and behavioral dysfunction. The mean survival period is 7-9 years.[7]",
"The adult variant of MLD is quite rare and has a slowly progressive course, with a mean survival of at least 12 years.[4] The most common presenting symptoms of the adult form, which begins in the late teens, are mental deterioration and behavioral abnormalities. The other symptoms of adult MLD include emotional lability; psychiatric symptoms, such as delusions or hallucinations; ataxia; extrapyramidal signs; progressive dementia; epileptic seizures; and signs of peripheral neuropathy.[7] The patient in this case initially had psychotic features and remained under the care of psychiatrists, having been diagnosed with schizophrenia.",
"Hyde and colleagues,[8] in a review of 129 published case reports on MLD, found psychosis to exist in 53% of patients with juvenile and adult-onset MLD, often as the initial manifestation of the disease. The investigators concluded that psychiatric symptoms, such as complex auditory hallucinations and bizarre delusions, are significant characteristics of MLD, presenting in patients aged 12-30 years. The report indicated that disruption of corticocortical and corticosubcortical connections, particularly in the frontal lobes, may give rise to psychosis. Age was also proposed to play an important role in the development of psychosis. In another case series, three cases of late-onset MLD within a family were reported. The patients presented with psychiatric disturbances diagnosed as schizophrenia and dementia.[9]",
"Schizophrenia-like symptoms were the initial manifestation of MLD in this patient, and they persisted for 3.5 years. Later, progressive neurocognitive decline and motor regression set in. Her first symptoms were inappropriate behavior, self-neglect, lack of insight, disorganized thoughts, inappropriate affect with outbursts of laughter, social withdrawal, and auditory hallucinations. The exact onset of dementia could not be identified because it was probably masked by the schizophrenia-like symptoms; however, dementia was not the initial manifestation of MLD in this patient.",
"Cases presenting with cognitive impairment have been reported. Marcão and colleagues[10] described a 37-year-old woman with severely impaired cognitive and mental functions. This patient also displayed frontal signs, including the grasp reflex and perseveration. In some cases, patients instead present with early motor impairment, with psychiatric and cognitive impairment setting in later."
],
"date": "April 23, 2018",
"figures": [],
"markdown": "# Neurocognitive Decline and Motor Regression in a 34-Year-Old Woman\n\n **Authors:** Sumaira Nabi, MBBS; Zakir Jan, MBBS, FCPS Neurology; Muhammad Irshad, MBBS, FCPS Neurology \n **Date:** April 23, 2018\n\n ## Content\n\n The juvenile form of MLD has its onset between ages 4 and 12 years. In these cases, the clinical course is a protracted one, and manifestations vary from peripheral nerve involvement to learning disability and behavioral dysfunction. The mean survival period is 7-9 years.[7]\nThe adult variant of MLD is quite rare and has a slowly progressive course, with a mean survival of at least 12 years.[4] The most common presenting symptoms of the adult form, which begins in the late teens, are mental deterioration and behavioral abnormalities. The other symptoms of adult MLD include emotional lability; psychiatric symptoms, such as delusions or hallucinations; ataxia; extrapyramidal signs; progressive dementia; epileptic seizures; and signs of peripheral neuropathy.[7] The patient in this case initially had psychotic features and remained under the care of psychiatrists, having been diagnosed with schizophrenia.\nHyde and colleagues,[8] in a review of 129 published case reports on MLD, found psychosis to exist in 53% of patients with juvenile and adult-onset MLD, often as the initial manifestation of the disease. The investigators concluded that psychiatric symptoms, such as complex auditory hallucinations and bizarre delusions, are significant characteristics of MLD, presenting in patients aged 12-30 years. The report indicated that disruption of corticocortical and corticosubcortical connections, particularly in the frontal lobes, may give rise to psychosis. Age was also proposed to play an important role in the development of psychosis. In another case series, three cases of late-onset MLD within a family were reported. The patients presented with psychiatric disturbances diagnosed as schizophrenia and dementia.[9]\nSchizophrenia-like symptoms were the initial manifestation of MLD in this patient, and they persisted for 3.5 years. Later, progressive neurocognitive decline and motor regression set in. Her first symptoms were inappropriate behavior, self-neglect, lack of insight, disorganized thoughts, inappropriate affect with outbursts of laughter, social withdrawal, and auditory hallucinations. The exact onset of dementia could not be identified because it was probably masked by the schizophrenia-like symptoms; however, dementia was not the initial manifestation of MLD in this patient.\nCases presenting with cognitive impairment have been reported. Marcão and colleagues[10] described a 37-year-old woman with severely impaired cognitive and mental functions. This patient also displayed frontal signs, including the grasp reflex and perseveration. In some cases, patients instead present with early motor impairment, with psychiatric and cognitive impairment setting in later.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "Neurocognitive Decline and Motor Regression in a 34-Year-Old Woman"
},
{
"authors": "Sumaira Nabi, MBBS; Zakir Jan, MBBS, FCPS Neurology; Muhammad Irshad, MBBS, FCPS Neurology",
"content": [
"Due to its high sensitivity, MRI of the brain is the primary neuroimaging strategy used for the diagnosis of MLD, detection of the extent and etiology of white-matter involvement, and monitoring of the disease's progression.[11] Some leukodystrophies like MLD have typical MRI patterns. Brain MRI reveals demyelination, predominantly within the centrum semiovale.[12,13] The demyelination is most severe in the periventricular white matter, diminishing in regions closer to the cortex. The subcortical U fibers tend to be spared early in the course of the disease. Variable degrees of cerebral atrophy may be present. The prevalent region of demyelination in the late-onset group is a posterior one involving the occipital lobes more than the frontal lobes, as seen in this patient.[14] MR spectroscopy reveals reduced N-acetylaspartate, increased myoinositol, and increased lactate.[11]",
"In addition to the MRI changes, typical histopathologic findings can be demonstrated on biopsy of a peripheral nerve. Metachromatic granules are found within glial cells and macrophages.[4] In addition, sulfatides are also periodic acid-Schiff (PAS) positive in frozen section. The diagnostic laboratory findings include elevated CSF protein, marked increase in sulfatide in urine levels,[15] and an absence of arylsulfatase A in white blood cells, serum, and cultured fibroblasts.[1,5] In this patient, due to unavailability, tests to assess her enzyme and urine sulfatide levels were not performed.",
"MLD is currently considered to be incurable. Hematopoietic stem cell transplantation, gene therapy and enzyme replacement therapy have shown positive results in animal models and have been investigated in clinical trials. The benefit of hematopoietic stem cell/bone marrow transplantation has not been consistently demonstrated or shown to be sustained. This is likely related to the rapid progression of the disease. A presymptomatic or early symptomatic individual is the most appropriate candidate.[16,17,18,19,20,21,22,23] Restoration of arylsulfatase A activity via retroviral vector-mediated gene transfer is also being investigated. Little is known about the symptomatic treatment of psychotic symptoms and dementia in MLD, although atypical antipsychotics have been tried for the psychotic features.[4]",
"This case highlights an organic disease as a cause of what initially appeared to be a fairly straightforward case of schizophrenia. It demonstrates that, in cases of psychosis with associated cognitive decline and motor deterioration, an organic cause must be ruled out."
],
"date": "April 23, 2018",
"figures": [],
"markdown": "# Neurocognitive Decline and Motor Regression in a 34-Year-Old Woman\n\n **Authors:** Sumaira Nabi, MBBS; Zakir Jan, MBBS, FCPS Neurology; Muhammad Irshad, MBBS, FCPS Neurology \n **Date:** April 23, 2018\n\n ## Content\n\n Due to its high sensitivity, MRI of the brain is the primary neuroimaging strategy used for the diagnosis of MLD, detection of the extent and etiology of white-matter involvement, and monitoring of the disease's progression.[11] Some leukodystrophies like MLD have typical MRI patterns. Brain MRI reveals demyelination, predominantly within the centrum semiovale.[12,13] The demyelination is most severe in the periventricular white matter, diminishing in regions closer to the cortex. The subcortical U fibers tend to be spared early in the course of the disease. Variable degrees of cerebral atrophy may be present. The prevalent region of demyelination in the late-onset group is a posterior one involving the occipital lobes more than the frontal lobes, as seen in this patient.[14] MR spectroscopy reveals reduced N-acetylaspartate, increased myoinositol, and increased lactate.[11]\nIn addition to the MRI changes, typical histopathologic findings can be demonstrated on biopsy of a peripheral nerve. Metachromatic granules are found within glial cells and macrophages.[4] In addition, sulfatides are also periodic acid-Schiff (PAS) positive in frozen section. The diagnostic laboratory findings include elevated CSF protein, marked increase in sulfatide in urine levels,[15] and an absence of arylsulfatase A in white blood cells, serum, and cultured fibroblasts.[1,5] In this patient, due to unavailability, tests to assess her enzyme and urine sulfatide levels were not performed.\nMLD is currently considered to be incurable. Hematopoietic stem cell transplantation, gene therapy and enzyme replacement therapy have shown positive results in animal models and have been investigated in clinical trials. The benefit of hematopoietic stem cell/bone marrow transplantation has not been consistently demonstrated or shown to be sustained. This is likely related to the rapid progression of the disease. A presymptomatic or early symptomatic individual is the most appropriate candidate.[16,17,18,19,20,21,22,23] Restoration of arylsulfatase A activity via retroviral vector-mediated gene transfer is also being investigated. Little is known about the symptomatic treatment of psychotic symptoms and dementia in MLD, although atypical antipsychotics have been tried for the psychotic features.[4]\nThis case highlights an organic disease as a cause of what initially appeared to be a fairly straightforward case of schizophrenia. It demonstrates that, in cases of psychosis with associated cognitive decline and motor deterioration, an organic cause must be ruled out.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 849925,
"choiceText": "X-linked recessive",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 849927,
"choiceText": "Autosomal dominant",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 849929,
"choiceText": "Autosomal recessive",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 849931,
"choiceText": "X-linked dominant",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 849933,
"choiceText": "Sporadic",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "MLD is a lysosomal storage disease that is transmitted as an autosomal recessive trait and is characterized by demyelination of the white matter in the central nervous system and peripheral nerves.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 267061,
"questionText": "Which of the following is the mode of transmission in MLD?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 849935,
"choiceText": "Brainstem",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 849937,
"choiceText": "Frontal lobe",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 849939,
"choiceText": "Thalamus ",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 849941,
"choiceText": "Occipital lobe",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 849943,
"choiceText": "Cerebellum",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The prevalent region of demyelination in late-onset MLD is a posterior one involving the occipital lobes more than the frontal lobes.\r\n",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 267063,
"questionText": "Which of the following parts of the brain is predominantly affected on MRI in patients with adult-onset MLD?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Neurocognitive Decline and Motor Regression in a 34-Year-Old Woman"
},
{
"authors": "Sumaira Nabi, MBBS; Zakir Jan, MBBS, FCPS Neurology; Muhammad Irshad, MBBS, FCPS Neurology",
"content": [],
"date": "April 23, 2018",
"figures": [],
"markdown": "# Neurocognitive Decline and Motor Regression in a 34-Year-Old Woman\n\n **Authors:** Sumaira Nabi, MBBS; Zakir Jan, MBBS, FCPS Neurology; Muhammad Irshad, MBBS, FCPS Neurology \n **Date:** April 23, 2018\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 849925,
"choiceText": "X-linked recessive",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 849927,
"choiceText": "Autosomal dominant",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 849929,
"choiceText": "Autosomal recessive",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 849931,
"choiceText": "X-linked dominant",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 849933,
"choiceText": "Sporadic",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "MLD is a lysosomal storage disease that is transmitted as an autosomal recessive trait and is characterized by demyelination of the white matter in the central nervous system and peripheral nerves.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 267061,
"questionText": "Which of the following is the mode of transmission in MLD?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 849935,
"choiceText": "Brainstem",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 849937,
"choiceText": "Frontal lobe",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 849939,
"choiceText": "Thalamus ",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 849941,
"choiceText": "Occipital lobe",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 849943,
"choiceText": "Cerebellum",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The prevalent region of demyelination in late-onset MLD is a posterior one involving the occipital lobes more than the frontal lobes.\r\n",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 267063,
"questionText": "Which of the following parts of the brain is predominantly affected on MRI in patients with adult-onset MLD?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Neurocognitive Decline and Motor Regression in a 34-Year-Old Woman"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 849917,
"choiceText": "Neuromyelitis optica",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 849919,
"choiceText": "Multiple sclerosis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 849921,
"choiceText": "Acute disseminated encephalomyelitis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 849923,
"choiceText": "Leukodystrophy",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 267059,
"questionText": "On the basis of the history, physical examination, and workup, what is the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 849925,
"choiceText": "X-linked recessive",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 849927,
"choiceText": "Autosomal dominant",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 849929,
"choiceText": "Autosomal recessive",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 849931,
"choiceText": "X-linked dominant",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 849933,
"choiceText": "Sporadic",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "MLD is a lysosomal storage disease that is transmitted as an autosomal recessive trait and is characterized by demyelination of the white matter in the central nervous system and peripheral nerves.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 267061,
"questionText": "Which of the following is the mode of transmission in MLD?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 849935,
"choiceText": "Brainstem",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 849937,
"choiceText": "Frontal lobe",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 849939,
"choiceText": "Thalamus ",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 849941,
"choiceText": "Occipital lobe",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 849943,
"choiceText": "Cerebellum",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The prevalent region of demyelination in late-onset MLD is a posterior one involving the occipital lobes more than the frontal lobes.\r\n",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 267063,
"questionText": "Which of the following parts of the brain is predominantly affected on MRI in patients with adult-onset MLD?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
844654 | /viewarticle/844654 | [
{
"authors": "Jason S. Chang, MD; Carin M. Van Gelder, MD",
"content": [
"Editor's Note: \nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.",
"Background",
"A 19-year-old white male presents to the emergency department (ED) in Connecticut after an episode of shortness of breath and syncope while at home. He reports having experienced recurrent episodes of palpitations and fatigue in the week before presentation.",
"Yesterday, the patient sought medical attention for these symptoms at his pediatrician's office. An ECG was performed, but the findings were normal; the patient was sent home wearing a Holter monitor (also known as an \"ambulatory ECG device\").",
"Figure 1.",
"Today, while mowing the lawn, the patient again felt a sudden onset of palpitations, accompanied by shortness of breath and light-headedness. He went inside the house, where he suddenly \"passed out,\" according to the patient's girlfriend. The girlfriend also states that he was unresponsive for a couple of minutes and that the patient exhibited no seizure-like activity or incontinence. She noted that he was \"pretty much himself\" once he regained consciousness. He was then brought by ambulance to the ED; a rhythm strip was acquired en route (Figure 1). Prophylactic transcutaneous pacer pads were placed by the emergency medical services team.",
"In the ED, the patient describes his palpitations as irregular, forceful beats, with a sensation of a racing heart. They occur spontaneously, without any clear inciting factors. He denies having any chest pain or shortness of breath at the time of questioning in the ED. He has not experienced any similar events before these, and he is usually active and athletic.",
"The patient denies having any recent fever, upper respiratory infections, cough, or sore throat. He denies having any recent headaches, neck stiffness, tinnitus, vertigo, or focal neurologic deficits. He also denies experiencing any bleeding (eg, hematochezia or melena).",
"The patient has been eating a regular diet and has not had any recent weight loss. His medical history is significant only for a cervical spine fracture secondary to a diving accident that occurred 3 years ago. He has no residual deficits or physical limitations. The patient is otherwise healthy, with no known cardiac, neurologic, or pulmonary disease. He has no known family history of sudden death or premature cardiac disease. He does not take any regular medications, and he denies any drug abuse, tobacco use, or alcohol consumption.",
"The Holter monitor had been removed before the patient began to mow the lawn; therefore, no results were available to ED staff."
],
"date": "May 14, 2015",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/844/654/844654-thumb-1.jpg"
}
],
"markdown": "# Palpitations in a 19-Year-Old With a Rash\n\n **Authors:** Jason S. Chang, MD; Carin M. Van Gelder, MD \n **Date:** May 14, 2015\n\n ## Content\n\n Editor's Note: \nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.\nBackground\nA 19-year-old white male presents to the emergency department (ED) in Connecticut after an episode of shortness of breath and syncope while at home. He reports having experienced recurrent episodes of palpitations and fatigue in the week before presentation.\nYesterday, the patient sought medical attention for these symptoms at his pediatrician's office. An ECG was performed, but the findings were normal; the patient was sent home wearing a Holter monitor (also known as an \"ambulatory ECG device\").\nFigure 1.\nToday, while mowing the lawn, the patient again felt a sudden onset of palpitations, accompanied by shortness of breath and light-headedness. He went inside the house, where he suddenly \"passed out,\" according to the patient's girlfriend. The girlfriend also states that he was unresponsive for a couple of minutes and that the patient exhibited no seizure-like activity or incontinence. She noted that he was \"pretty much himself\" once he regained consciousness. He was then brought by ambulance to the ED; a rhythm strip was acquired en route (Figure 1). Prophylactic transcutaneous pacer pads were placed by the emergency medical services team.\nIn the ED, the patient describes his palpitations as irregular, forceful beats, with a sensation of a racing heart. They occur spontaneously, without any clear inciting factors. He denies having any chest pain or shortness of breath at the time of questioning in the ED. He has not experienced any similar events before these, and he is usually active and athletic.\nThe patient denies having any recent fever, upper respiratory infections, cough, or sore throat. He denies having any recent headaches, neck stiffness, tinnitus, vertigo, or focal neurologic deficits. He also denies experiencing any bleeding (eg, hematochezia or melena).\nThe patient has been eating a regular diet and has not had any recent weight loss. His medical history is significant only for a cervical spine fracture secondary to a diving accident that occurred 3 years ago. He has no residual deficits or physical limitations. The patient is otherwise healthy, with no known cardiac, neurologic, or pulmonary disease. He has no known family history of sudden death or premature cardiac disease. He does not take any regular medications, and he denies any drug abuse, tobacco use, or alcohol consumption.\nThe Holter monitor had been removed before the patient began to mow the lawn; therefore, no results were available to ED staff.\n\n ## Figures\n\n **Figure 1.** \n \n\n\n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "Palpitations in a 19-Year-Old With a Rash"
},
{
"authors": "Jason S. Chang, MD; Carin M. Van Gelder, MD",
"content": [
"Physical Examination and Workup",
"On physical examination, the patient appears to be in no acute distress, but he is noted to have moderate anxiety. He does not appear ill and is alert.",
"Figure 2.",
"The patient's vital signs include a temperature of 96.7°F (35.9°C), a pulse rate of 40-120 beats/min, a respiratory rate of 16 breaths/min, and a blood pressure of 102/46 mm Hg. His oxygen saturation is 96% while breathing room air. His head and neck examination is unremarkable. No jugular venous distention or carotid bruits are noted. His lungs are clear to auscultation bilaterally.",
"The patient's cardiac examination reveals an irregular, tachycardic rhythm. No discernible murmur is observed with or without Valsalva maneuvers or hand clenching. His abdomen is soft, nontender, and nondistended. No clubbing, cyanosis, or edema is noted in his extremities. His neurologic examination findings are normal. On skin examination, multiple, bilateral macular erythematous lesions with large central pallor are noted on his thighs.",
"An ECG is obtained (Figure 2)."
],
"date": "May 14, 2015",
"figures": [
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/844/654/844654-thumb-2.jpg"
}
],
"markdown": "# Palpitations in a 19-Year-Old With a Rash\n\n **Authors:** Jason S. Chang, MD; Carin M. Van Gelder, MD \n **Date:** May 14, 2015\n\n ## Content\n\n Physical Examination and Workup\nOn physical examination, the patient appears to be in no acute distress, but he is noted to have moderate anxiety. He does not appear ill and is alert.\nFigure 2.\nThe patient's vital signs include a temperature of 96.7°F (35.9°C), a pulse rate of 40-120 beats/min, a respiratory rate of 16 breaths/min, and a blood pressure of 102/46 mm Hg. His oxygen saturation is 96% while breathing room air. His head and neck examination is unremarkable. No jugular venous distention or carotid bruits are noted. His lungs are clear to auscultation bilaterally.\nThe patient's cardiac examination reveals an irregular, tachycardic rhythm. No discernible murmur is observed with or without Valsalva maneuvers or hand clenching. His abdomen is soft, nontender, and nondistended. No clubbing, cyanosis, or edema is noted in his extremities. His neurologic examination findings are normal. On skin examination, multiple, bilateral macular erythematous lesions with large central pallor are noted on his thighs.\nAn ECG is obtained (Figure 2).\n\n ## Figures\n\n **Figure 2.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 844339,
"choiceText": "Acute myocardial infarction",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 844341,
"choiceText": "Disseminated Lyme disease",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 844343,
"choiceText": "Tertiary syphilis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 844345,
"choiceText": "Viral myocarditis",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 265473,
"questionText": "On the basis of the history, physical examination, and workup, what is the diagnosis?<br><br><i>Hint: The patient's rash and geographic location are of particular importance.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Palpitations in a 19-Year-Old With a Rash"
},
{
"authors": "Jason S. Chang, MD; Carin M. Van Gelder, MD",
"content": [
"The ECG (Figure 2) demonstrates atrioventricular dissociation. Close examination of the ECG reveals two independent rhythms: a junctional tachycardia at 130 beats/min and a sinus tachycardia at 100 beats/min. The rhythm strip (Figure 1) demonstrates an interval of nonconducted P waves indicative of heart block. Taken together, the data suggest extensive conduction system disease.",
"In light of the findings on this ECG and of the high incidence of Lyme disease in Connecticut, further history was solicited from the patient. When asked specifically about outdoor activities, he reported that he had been on a hiking trip 1 month before this visit to the ED, and he remembered being bitten by a tick. He also stated that a rash appeared on his legs several days later, but that he thought little of it and applied some over-the-counter cream in an effort to make it go away.",
"Figure 1.",
"Figure 2.",
"Lyme disease is an infectious, tick-borne process endemic to Wisconsin, Minnesota, and the northeastern United States. It was first recognized in 1975 after chronic infections by the spirochete Borrelia burgdorferi were discovered in three Connecticut communities, where an epidemic of oligoarticular arthritis was noted. The organism is transferred by the Ixodes (dammini) scapularis deer tick. The incidence is approximately 6-8 cases per 100,000 people in the United States, according to estimates by the Centers for Disease Control and Prevention; 93% of cases occur in the above-mentioned endemic areas.[1] Approximately 20,000 new cases are reported every year.",
"Patients presenting with symptoms of Lyme disease may or may not remember removing a tick from their body; the ticks often go unnoticed because they are about the size of a pencil tip. With increased awareness and treatment in the first stage of Lyme disease, extracutaneous manifestations have decreased.",
"Although Lyme disease presents with erythema chronicum migrans (ECM) in 70% of cases, extracutaneous disease is well known (eg, heart block by itself occurs in < 1% of cases). The disease may involve the skin, central nervous system (CNS), heart, joints, and eyes. Lyme disease results in few deaths; they are typically limited to patients who are also infected with babesiosis and ehrlichiosis (also known as human granulocytic anaplasmosis).[2]"
],
"date": "May 14, 2015",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/844/654/844654-thumb-1.jpg"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/844/654/844654-thumb-2.jpg"
}
],
"markdown": "# Palpitations in a 19-Year-Old With a Rash\n\n **Authors:** Jason S. Chang, MD; Carin M. Van Gelder, MD \n **Date:** May 14, 2015\n\n ## Content\n\n The ECG (Figure 2) demonstrates atrioventricular dissociation. Close examination of the ECG reveals two independent rhythms: a junctional tachycardia at 130 beats/min and a sinus tachycardia at 100 beats/min. The rhythm strip (Figure 1) demonstrates an interval of nonconducted P waves indicative of heart block. Taken together, the data suggest extensive conduction system disease.\nIn light of the findings on this ECG and of the high incidence of Lyme disease in Connecticut, further history was solicited from the patient. When asked specifically about outdoor activities, he reported that he had been on a hiking trip 1 month before this visit to the ED, and he remembered being bitten by a tick. He also stated that a rash appeared on his legs several days later, but that he thought little of it and applied some over-the-counter cream in an effort to make it go away.\nFigure 1.\nFigure 2.\nLyme disease is an infectious, tick-borne process endemic to Wisconsin, Minnesota, and the northeastern United States. It was first recognized in 1975 after chronic infections by the spirochete Borrelia burgdorferi were discovered in three Connecticut communities, where an epidemic of oligoarticular arthritis was noted. The organism is transferred by the Ixodes (dammini) scapularis deer tick. The incidence is approximately 6-8 cases per 100,000 people in the United States, according to estimates by the Centers for Disease Control and Prevention; 93% of cases occur in the above-mentioned endemic areas.[1] Approximately 20,000 new cases are reported every year.\nPatients presenting with symptoms of Lyme disease may or may not remember removing a tick from their body; the ticks often go unnoticed because they are about the size of a pencil tip. With increased awareness and treatment in the first stage of Lyme disease, extracutaneous manifestations have decreased.\nAlthough Lyme disease presents with erythema chronicum migrans (ECM) in 70% of cases, extracutaneous disease is well known (eg, heart block by itself occurs in < 1% of cases). The disease may involve the skin, central nervous system (CNS), heart, joints, and eyes. Lyme disease results in few deaths; they are typically limited to patients who are also infected with babesiosis and ehrlichiosis (also known as human granulocytic anaplasmosis).[2]\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n\n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 844339,
"choiceText": "Acute myocardial infarction",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 844341,
"choiceText": "Disseminated Lyme disease",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 844343,
"choiceText": "Tertiary syphilis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 844345,
"choiceText": "Viral myocarditis",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 265473,
"questionText": "On the basis of the history, physical examination, and workup, what is the diagnosis?<br><br><i>Hint: The patient's rash and geographic location are of particular importance.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Palpitations in a 19-Year-Old With a Rash"
},
{
"authors": "Jason S. Chang, MD; Carin M. Van Gelder, MD",
"content": [
"Lyme disease has three stages: early localized, early disseminated, and late. The early localized stage of the infection manifests with a nontoxic, nonspecific febrile illness (low-grade fever, myalgias, or fatigue) and the classic ECM lesion, described as a red patch with central pallor (also called a target lesion). A single ECM lesion or multiple lesions may be present at the site of inoculation. This may be the only symptom a patient experiences, and it is diagnostic (necessitating treatment even without serologic proof of the disease).",
"The joints may be involved in early localized disease with arthralgia, often described as a migratory joint pain caused by bursitis, tendonitis, synovitis, or myositis. The spirochete is able to invade human fibroblasts and remain dormant for months to years. In a small percentage of infected patients, untreated Lyme disease may lead to the development of further organ-system involvement.",
"Early disseminated disease typically occurs within weeks to months after the initial infection. It manifests as multiple ECM lesions beyond the site of inoculation, with accompanying extracutaneous manifestations. The neurologic system may be affected, with patients describing headaches heralding CNS penetration and possible meningitis. Bell palsy may occur, with facial nerve involvement leading to unilateral facial droop affecting the lower and upper portions of the face.",
"Cardiac manifestations typically occur within the early disseminated stage of the infection. Approximately 8%-10% of patients with Lyme disease have cardiac involvement.[3] The presenting symptoms may include light-headedness, palpitations, or syncope, and they are typically caused by a conduction defect ranging from first-degree block to complete atrioventricular dissociation.",
"With treatment of the underlying infection, most conduction defects are reversible processes. In addition to conduction defects, other cardiac manifestations include myopericarditis, ventricular dysfunction, cardiomegaly, and pericardial effusion with possible tamponade.[3]",
"Finally, ocular manifestation in early disseminated disease is typically limited to conjunctivitis, described by patients as red, itchy eyes. Rarely, ocular involvement may present as ophthalmitis with unilateral blindness in later stages.",
"Latent disease typically presents months to years later, with arthritis (frank arthritis with painful swelling and redness, typically involving the large joints) or CNS complications. In this stage of the disease, any portion of the CNS and peripheral nervous system may be affected; the manifestations range from chronic encephalopathy to peripheral neuropathies or radiculopathy.",
"The disease is most often diagnosed by history, physical examination, and clinical suspicion. Serologic studies by enzyme-linked immunosorbent assay or Western blot are typically performed, but negative results do not rule out infection. The timing of the sample is crucial to obtaining a reliable result. Early in the infection, serologic studies are often negative. Seropositivity may not develop for several weeks.[4] In addition, patients inoculated in the past may have persistent seropositivity for years after inoculation; therefore, neither a positive result nor a negative result can definitively establish or rule out the presence or absence of an active infection.",
"Other useful laboratory studies may include a complete blood cell count, serum chemistries, erythrocyte sedimentation rate, blood cultures, and specific testing for such organisms as babesiosis and ehrlichiosis. Further studies, as indicated on presentation, may include cerebrospinal studies or joint fluid aspiration.",
"Although there are no definitive radiologic studies, some studies may be warranted to rule out other causes of pain or neurologic symptoms. An ECG should be obtained as part of the basic workup for any patient in whom the diagnosis of Lyme disease is being entertained. Irrespective of whether the patient's history or physical examination findings support cardiac involvement, any evidence of conduction system disease must be ruled out."
],
"date": "May 14, 2015",
"figures": [],
"markdown": "# Palpitations in a 19-Year-Old With a Rash\n\n **Authors:** Jason S. Chang, MD; Carin M. Van Gelder, MD \n **Date:** May 14, 2015\n\n ## Content\n\n Lyme disease has three stages: early localized, early disseminated, and late. The early localized stage of the infection manifests with a nontoxic, nonspecific febrile illness (low-grade fever, myalgias, or fatigue) and the classic ECM lesion, described as a red patch with central pallor (also called a target lesion). A single ECM lesion or multiple lesions may be present at the site of inoculation. This may be the only symptom a patient experiences, and it is diagnostic (necessitating treatment even without serologic proof of the disease).\nThe joints may be involved in early localized disease with arthralgia, often described as a migratory joint pain caused by bursitis, tendonitis, synovitis, or myositis. The spirochete is able to invade human fibroblasts and remain dormant for months to years. In a small percentage of infected patients, untreated Lyme disease may lead to the development of further organ-system involvement.\nEarly disseminated disease typically occurs within weeks to months after the initial infection. It manifests as multiple ECM lesions beyond the site of inoculation, with accompanying extracutaneous manifestations. The neurologic system may be affected, with patients describing headaches heralding CNS penetration and possible meningitis. Bell palsy may occur, with facial nerve involvement leading to unilateral facial droop affecting the lower and upper portions of the face.\nCardiac manifestations typically occur within the early disseminated stage of the infection. Approximately 8%-10% of patients with Lyme disease have cardiac involvement.[3] The presenting symptoms may include light-headedness, palpitations, or syncope, and they are typically caused by a conduction defect ranging from first-degree block to complete atrioventricular dissociation.\nWith treatment of the underlying infection, most conduction defects are reversible processes. In addition to conduction defects, other cardiac manifestations include myopericarditis, ventricular dysfunction, cardiomegaly, and pericardial effusion with possible tamponade.[3]\nFinally, ocular manifestation in early disseminated disease is typically limited to conjunctivitis, described by patients as red, itchy eyes. Rarely, ocular involvement may present as ophthalmitis with unilateral blindness in later stages.\nLatent disease typically presents months to years later, with arthritis (frank arthritis with painful swelling and redness, typically involving the large joints) or CNS complications. In this stage of the disease, any portion of the CNS and peripheral nervous system may be affected; the manifestations range from chronic encephalopathy to peripheral neuropathies or radiculopathy.\nThe disease is most often diagnosed by history, physical examination, and clinical suspicion. Serologic studies by enzyme-linked immunosorbent assay or Western blot are typically performed, but negative results do not rule out infection. The timing of the sample is crucial to obtaining a reliable result. Early in the infection, serologic studies are often negative. Seropositivity may not develop for several weeks.[4] In addition, patients inoculated in the past may have persistent seropositivity for years after inoculation; therefore, neither a positive result nor a negative result can definitively establish or rule out the presence or absence of an active infection.\nOther useful laboratory studies may include a complete blood cell count, serum chemistries, erythrocyte sedimentation rate, blood cultures, and specific testing for such organisms as babesiosis and ehrlichiosis. Further studies, as indicated on presentation, may include cerebrospinal studies or joint fluid aspiration.\nAlthough there are no definitive radiologic studies, some studies may be warranted to rule out other causes of pain or neurologic symptoms. An ECG should be obtained as part of the basic workup for any patient in whom the diagnosis of Lyme disease is being entertained. Irrespective of whether the patient's history or physical examination findings support cardiac involvement, any evidence of conduction system disease must be ruled out.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "Palpitations in a 19-Year-Old With a Rash"
},
{
"authors": "Jason S. Chang, MD; Carin M. Van Gelder, MD",
"content": [
"The treatment of Lyme disease is managed according to the patient history or evidence of systemic involvement. First-line antibiotics used to treat Lyme disease include amoxicillin, doxycycline, and ceftriaxone. Second-line agents include cefuroxime, erythromycin, tetracycline, and azithromycin. The treatment algorithm varies according to the extent of infection. With an early localized infection (ie, a single ECM lesion), a course of a single-agent antibiotic is recommended for 10-21 days.",
"First-choice treatment for early localized infection should be amoxicillin (Moxatag®) or doxycycline (Oracea®).[2,5] For early disseminated infection without CNS infection or third-degree heart block, single-agent oral antibiotics are also appropriate (eg, 21-30 days of oral doxycycline or amoxicillin). A simple seventh cranial nerve palsy associated with B burgdorferi infection but without evidence of meningitis may be treated with a 14-day course of oral antibiotics[2]; however, if CNS involvement or a high-grade conduction defect is noted, parenteral therapy is indicated. The recommended first-line treatment is intravenous ceftriaxone (Rocephin®) for 14 days. Patients with high-grade heart block and hemodynamic instability should undergo transcutaneous pacing and should be monitored in the intensive care unit until they are not dependent on a pacemaker.",
"Latent neurologic Lyme disease requires 2-4 weeks of ceftriaxone, whereas Lyme disease arthritis can be treated with an oral antibiotic for 4 weeks.[2] Persistent arthritic symptoms may necessitate parenteral therapy and should be treated in consultation with the rheumatology department.",
"General precautions for avoiding tick bites in endemic areas include keeping skin covered when outdoors, using insect repellent, and examining oneself for attached ticks after outdoor activities. A tick that has been attached for less than 24 hours has a low likelihood of transmitting infection. Prophylactic treatment for a tick bite is not routinely recommended[2]; however, for patients in hyperendemic areas who present with an engorged tick that has been attached to their skin for longer than 36 hours, treatment with a single dose of doxycycline (200 mg), given within 72 hours of the tick bite, is highly effective in preventing the development of Lyme disease.[2,6]",
"The prognosis for treated Lyme disease is excellent. The patient in our case remained hemodynamically stable, with a normal blood pressure in the ED, and he did not require transcutaneous pacing. He received intravenous ceftriaxone and was admitted to the cardiac care unit for Lyme disease-associated third-degree heart block. The block resolved over the treatment course, and the patient was discharged to home in good condition."
],
"date": "May 14, 2015",
"figures": [],
"markdown": "# Palpitations in a 19-Year-Old With a Rash\n\n **Authors:** Jason S. Chang, MD; Carin M. Van Gelder, MD \n **Date:** May 14, 2015\n\n ## Content\n\n The treatment of Lyme disease is managed according to the patient history or evidence of systemic involvement. First-line antibiotics used to treat Lyme disease include amoxicillin, doxycycline, and ceftriaxone. Second-line agents include cefuroxime, erythromycin, tetracycline, and azithromycin. The treatment algorithm varies according to the extent of infection. With an early localized infection (ie, a single ECM lesion), a course of a single-agent antibiotic is recommended for 10-21 days.\nFirst-choice treatment for early localized infection should be amoxicillin (Moxatag®) or doxycycline (Oracea®).[2,5] For early disseminated infection without CNS infection or third-degree heart block, single-agent oral antibiotics are also appropriate (eg, 21-30 days of oral doxycycline or amoxicillin). A simple seventh cranial nerve palsy associated with B burgdorferi infection but without evidence of meningitis may be treated with a 14-day course of oral antibiotics[2]; however, if CNS involvement or a high-grade conduction defect is noted, parenteral therapy is indicated. The recommended first-line treatment is intravenous ceftriaxone (Rocephin®) for 14 days. Patients with high-grade heart block and hemodynamic instability should undergo transcutaneous pacing and should be monitored in the intensive care unit until they are not dependent on a pacemaker.\nLatent neurologic Lyme disease requires 2-4 weeks of ceftriaxone, whereas Lyme disease arthritis can be treated with an oral antibiotic for 4 weeks.[2] Persistent arthritic symptoms may necessitate parenteral therapy and should be treated in consultation with the rheumatology department.\nGeneral precautions for avoiding tick bites in endemic areas include keeping skin covered when outdoors, using insect repellent, and examining oneself for attached ticks after outdoor activities. A tick that has been attached for less than 24 hours has a low likelihood of transmitting infection. Prophylactic treatment for a tick bite is not routinely recommended[2]; however, for patients in hyperendemic areas who present with an engorged tick that has been attached to their skin for longer than 36 hours, treatment with a single dose of doxycycline (200 mg), given within 72 hours of the tick bite, is highly effective in preventing the development of Lyme disease.[2,6]\nThe prognosis for treated Lyme disease is excellent. The patient in our case remained hemodynamically stable, with a normal blood pressure in the ED, and he did not require transcutaneous pacing. He received intravenous ceftriaxone and was admitted to the cardiac care unit for Lyme disease-associated third-degree heart block. The block resolved over the treatment course, and the patient was discharged to home in good condition.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 844347,
"choiceText": "Peripheral neuropathies",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 844349,
"choiceText": "Myopericarditis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 844351,
"choiceText": "Cardiac conduction defects",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 844353,
"choiceText": "Multiple ECM lesions beyond the site of inoculation",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "In latent Lyme disease, any portion of the CNS and peripheral nervous system can be affected; this includes manifestations that can range from peripheral neuropathies or radiculopathy. Single or multiple ECM lesions may be present during the early localized phase. Cardiac manifestations in the early disseminated phase can include myopericarditis, ventricular dysfunction, cardiomegaly, and pericardial effusion with possible tamponade but not conduction defects.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 265475,
"questionText": "Which of the following manifestations or complications is not typically seen in early disseminated Lyme disease?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 844355,
"choiceText": "Ceftriaxone",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 844357,
"choiceText": "Amoxicillin",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 844359,
"choiceText": "Vancomycin",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 844361,
"choiceText": "Doxycycline",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The first-line antibiotics used to treat Lyme disease include amoxicillin, doxycycline, and ceftriaxone. Second-line agents include cefuroxime, erythromycin, tetracycline, and azithromycin.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 265477,
"questionText": "Which of the following antibiotics is not typically used in the treatment of Lyme disease?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Palpitations in a 19-Year-Old With a Rash"
},
{
"authors": "Jason S. Chang, MD; Carin M. Van Gelder, MD",
"content": [],
"date": "May 14, 2015",
"figures": [],
"markdown": "# Palpitations in a 19-Year-Old With a Rash\n\n **Authors:** Jason S. Chang, MD; Carin M. Van Gelder, MD \n **Date:** May 14, 2015\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 844347,
"choiceText": "Peripheral neuropathies",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 844349,
"choiceText": "Myopericarditis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 844351,
"choiceText": "Cardiac conduction defects",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 844353,
"choiceText": "Multiple ECM lesions beyond the site of inoculation",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "In latent Lyme disease, any portion of the CNS and peripheral nervous system can be affected; this includes manifestations that can range from peripheral neuropathies or radiculopathy. Single or multiple ECM lesions may be present during the early localized phase. Cardiac manifestations in the early disseminated phase can include myopericarditis, ventricular dysfunction, cardiomegaly, and pericardial effusion with possible tamponade but not conduction defects.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 265475,
"questionText": "Which of the following manifestations or complications is not typically seen in early disseminated Lyme disease?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 844355,
"choiceText": "Ceftriaxone",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 844357,
"choiceText": "Amoxicillin",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 844359,
"choiceText": "Vancomycin",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 844361,
"choiceText": "Doxycycline",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The first-line antibiotics used to treat Lyme disease include amoxicillin, doxycycline, and ceftriaxone. Second-line agents include cefuroxime, erythromycin, tetracycline, and azithromycin.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 265477,
"questionText": "Which of the following antibiotics is not typically used in the treatment of Lyme disease?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Palpitations in a 19-Year-Old With a Rash"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 844339,
"choiceText": "Acute myocardial infarction",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 844341,
"choiceText": "Disseminated Lyme disease",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 844343,
"choiceText": "Tertiary syphilis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 844345,
"choiceText": "Viral myocarditis",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 265473,
"questionText": "On the basis of the history, physical examination, and workup, what is the diagnosis?<br><br><i>Hint: The patient's rash and geographic location are of particular importance.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 844347,
"choiceText": "Peripheral neuropathies",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 844349,
"choiceText": "Myopericarditis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 844351,
"choiceText": "Cardiac conduction defects",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 844353,
"choiceText": "Multiple ECM lesions beyond the site of inoculation",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "In latent Lyme disease, any portion of the CNS and peripheral nervous system can be affected; this includes manifestations that can range from peripheral neuropathies or radiculopathy. Single or multiple ECM lesions may be present during the early localized phase. Cardiac manifestations in the early disseminated phase can include myopericarditis, ventricular dysfunction, cardiomegaly, and pericardial effusion with possible tamponade but not conduction defects.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 265475,
"questionText": "Which of the following manifestations or complications is not typically seen in early disseminated Lyme disease?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 844355,
"choiceText": "Ceftriaxone",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 844357,
"choiceText": "Amoxicillin",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 844359,
"choiceText": "Vancomycin",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 844361,
"choiceText": "Doxycycline",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The first-line antibiotics used to treat Lyme disease include amoxicillin, doxycycline, and ceftriaxone. Second-line agents include cefuroxime, erythromycin, tetracycline, and azithromycin.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 265477,
"questionText": "Which of the following antibiotics is not typically used in the treatment of Lyme disease?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
844658 | /viewarticle/844658 | [
{
"authors": "Faisal M. Mawri, MD; Peter Stoyanoff, MD; Meaghan Misiasz, BA; Jillian Ewing, BS; David Ishiyama",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.",
"Background",
"A 9-year-old girl with no significant medical history presents to the pediatric emergency department with a chief symptom of several episodes of vomiting. The vomiting started early in the morning on the day of presentation. The emesis has been nonbloody, nonbilious, and nonprojectile. A total of five episodes have occurred in the 6 hours before presentation.",
"Upon further questioning, the patient states that she has been frequently experiencing bilateral throbbing headaches over the past 2 weeks, which she describes as being worse in the morning and associated with nausea. The duration of the headaches ranges from 2-4 hours, with a recent increase in duration and severity. Coughing, sneezing, or changing the position of the head has had no effect on the headache intensity.",
"The patient denies having any recent trauma, fever, neck pain, cough, nasal discharge, chest pain, shortness of breath, or abdominal pain. She has also been noted to have a change in her gait, with unsteadiness and occasional falls (particularly at night). The remainder of her review of systems is negative.",
"The patient's school performance has reportedly deteriorated over the past year, with no clear explanation. She is not currently taking any medications and has no history of allergies. The patient's family history is significant only for diabetes mellitus and hypertension in her maternal grandfather."
],
"date": "May 14, 2015",
"figures": [],
"markdown": "# A 9-Year-Old Girl With Declining School Performance\n\n **Authors:** Faisal M. Mawri, MD; Peter Stoyanoff, MD; Meaghan Misiasz, BA; Jillian Ewing, BS; David Ishiyama \n **Date:** May 14, 2015\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.\nBackground\nA 9-year-old girl with no significant medical history presents to the pediatric emergency department with a chief symptom of several episodes of vomiting. The vomiting started early in the morning on the day of presentation. The emesis has been nonbloody, nonbilious, and nonprojectile. A total of five episodes have occurred in the 6 hours before presentation.\nUpon further questioning, the patient states that she has been frequently experiencing bilateral throbbing headaches over the past 2 weeks, which she describes as being worse in the morning and associated with nausea. The duration of the headaches ranges from 2-4 hours, with a recent increase in duration and severity. Coughing, sneezing, or changing the position of the head has had no effect on the headache intensity.\nThe patient denies having any recent trauma, fever, neck pain, cough, nasal discharge, chest pain, shortness of breath, or abdominal pain. She has also been noted to have a change in her gait, with unsteadiness and occasional falls (particularly at night). The remainder of her review of systems is negative.\nThe patient's school performance has reportedly deteriorated over the past year, with no clear explanation. She is not currently taking any medications and has no history of allergies. The patient's family history is significant only for diabetes mellitus and hypertension in her maternal grandfather.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 9-Year-Old Girl With Declining School Performance"
},
{
"authors": "Faisal M. Mawri, MD; Peter Stoyanoff, MD; Meaghan Misiasz, BA; Jillian Ewing, BS; David Ishiyama",
"content": [
"Upon physical examination, the patient is afebrile and has a pulse of 78 beats/min, a blood pressure of 105/85 mm Hg, and a respiratory rate of 16 breaths/min. She appears her stated age and is in no acute distress.",
"Examination of the head, ears, and nose is unremarkable. The patient's pupils are equal, round, and reactive to light. No maxillary or frontal sinus tenderness is noted. Her neck is supple, with full range of motion and no meningismus.",
"No audible murmurs, rubs, or gallops are observed during heart examination. The lungs are clear to auscultation bilaterally, and the abdomen is soft and nontender to deep palpation.",
"Figure 1.",
"Neurologic examination shows that the patient has trouble balancing during attempts to stand upright with her eyes closed and reveals an unsteady, wide-based gait; abnormal finger-to-nose coordination; and horizontal diplopia. Her strength and sensory examinations are normal. She has normal speech and recall.",
"Laboratory testing is performed, including a complete blood cell count and comprehensive metabolic panel, but the results are unremarkable.",
"MRI of the brain is performed (Figure 1)."
],
"date": "May 14, 2015",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/844/658/844658-Thumb1s.png"
}
],
"markdown": "# A 9-Year-Old Girl With Declining School Performance\n\n **Authors:** Faisal M. Mawri, MD; Peter Stoyanoff, MD; Meaghan Misiasz, BA; Jillian Ewing, BS; David Ishiyama \n **Date:** May 14, 2015\n\n ## Content\n\n Upon physical examination, the patient is afebrile and has a pulse of 78 beats/min, a blood pressure of 105/85 mm Hg, and a respiratory rate of 16 breaths/min. She appears her stated age and is in no acute distress.\nExamination of the head, ears, and nose is unremarkable. The patient's pupils are equal, round, and reactive to light. No maxillary or frontal sinus tenderness is noted. Her neck is supple, with full range of motion and no meningismus.\nNo audible murmurs, rubs, or gallops are observed during heart examination. The lungs are clear to auscultation bilaterally, and the abdomen is soft and nontender to deep palpation.\nFigure 1.\nNeurologic examination shows that the patient has trouble balancing during attempts to stand upright with her eyes closed and reveals an unsteady, wide-based gait; abnormal finger-to-nose coordination; and horizontal diplopia. Her strength and sensory examinations are normal. She has normal speech and recall.\nLaboratory testing is performed, including a complete blood cell count and comprehensive metabolic panel, but the results are unremarkable.\nMRI of the brain is performed (Figure 1).\n\n ## Figures\n\n **Figure 1.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 844387,
"choiceText": "Herpes encephalitis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 844389,
"choiceText": "Pontine glioma\r\n",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 844391,
"choiceText": "Friedreich ataxia",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 844393,
"choiceText": "Toxic ingestion",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 844395,
"choiceText": "Intramedullary mass lesion of the spinal cord",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 265485,
"questionText": "On the basis of the history, physical examination, and workup, what is the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 9-Year-Old Girl With Declining School Performance"
},
{
"authors": "Faisal M. Mawri, MD; Peter Stoyanoff, MD; Meaghan Misiasz, BA; Jillian Ewing, BS; David Ishiyama",
"content": [
"Brain tumors are the second most common childhood cancer (after leukemia) and are the leading cause of childhood morbidity and mortality from cancer, affecting 2.5-4 per 100,000 children each year. The incidence of childhood brain tumors has increased by 35% in the past 20 years. The reasons for this increase are the subject of much debate; it may reflect improvements in imaging, specifically with newer MRI technologies.[1] The presenting symptoms of specific tumors depend on the nature and location of the lesion.",
"The patient in this case presented with vague symptoms of increased intracranial pressure (vomiting and headaches). Signs of increased intracranial pressure are not uncommon in the presentation of brainstem gliomas, resulting from obstruction of cerebrospinal fluid outflow. Cerebellar signs, such as those present in this patient (a positive Romberg sign, unsteady wide-based gait, abnormal finger-to-nose coordination), are also typical at presentation, particularly for children with diffuse brainstem gliomas, because these tumors are located in the posterior fossa.",
"Notably, this patient was also reported to have a 1-year history of declining school performance, with no attributable cause. In rare cases, declining school performance, especially related to visual disturbances or symptoms of hydrocephalus, may be the only presenting sign of a brainstem glioma.[2]",
"Figure 1.",
"In this case, the diagnosis was ultimately made with MRI; the axial T2-weighted imaging showed ill-defined, bilateral pontine signal hyperintensity in a heterogeneous pattern, with asymmetric involvement of the right half of the pons (Figure 1). These findings are consistent with diffuse intrinsic pontine glioma (DIPG).",
"Unlike adult brain tumors, most childhood tumors are primary central nervous system (CNS) tumors. They are also infratentorial. The most common childhood brain tumors are the primitive neuroectodermal tumors, such as medulloblastoma, which most commonly arise in the posterior fossa and the pineal region.",
"Glial tumors account for 50%-60% of all childhood brain tumors.[3] Glial tumors can arise from any part of the CNS, but they are most commonly found in the supratentorial region and the brainstem. Brainstem gliomas account for 10%-20% of all childhood brain tumors. Approximately 80% of all brainstem gliomas occur in patients younger than 21 years, with the incidence peaking at age 7 years. Both sexes are affected equally, although some studies have suggested a slight male predominance.[4] Some predilection for tumors to arise on the left side has been noted.",
"The term \"brainstem glioma\" describes an intrinsic brainstem tumor which may be a ganglioglioma, pilocytic astrocytoma, fibrillary astrocytoma, anaplastic astrocytoma, or glioblastoma multiforme, depending on the pathology. The simplest classification of brainstem gliomas divides these tumors into two categories: diffusely infiltrative brainstem gliomas and focal brainstem gliomas.[5]",
"The World Health Organization (WHO) further classifies brain tumors on the basis of their pathologic characteristics, including the level of differentiation and number of mitoses. The grades range from I to IV, with grade I being the least aggressive and grade IV being the most aggressive.",
"Diffusely infiltrative brainstem gliomas are high-grade tumors that most commonly originate in the pons and often diffusely expand to other brainstem sites, including the cerebellum and thalamus. These tumors are most often fibrillary astrocytoma (WHO grade II), anaplastic astrocytoma (WHO grade III), or glioblastoma multiforme (WHO grade IV). Focal brainstem gliomas, however, are more likely to be well circumscribed and focal and may be exophytic (growing outside of the brainstem). They more frequently originate in the midbrain or medulla and are typically classified as pilocytic astrocytoma (WHO grade I); ganglioglioma (WHO grade I); or, rarely, fibrillary astrocytoma (WHO grade II).[4,5,6]"
],
"date": "May 14, 2015",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/844/658/844658-Thumb1s.png"
}
],
"markdown": "# A 9-Year-Old Girl With Declining School Performance\n\n **Authors:** Faisal M. Mawri, MD; Peter Stoyanoff, MD; Meaghan Misiasz, BA; Jillian Ewing, BS; David Ishiyama \n **Date:** May 14, 2015\n\n ## Content\n\n Brain tumors are the second most common childhood cancer (after leukemia) and are the leading cause of childhood morbidity and mortality from cancer, affecting 2.5-4 per 100,000 children each year. The incidence of childhood brain tumors has increased by 35% in the past 20 years. The reasons for this increase are the subject of much debate; it may reflect improvements in imaging, specifically with newer MRI technologies.[1] The presenting symptoms of specific tumors depend on the nature and location of the lesion.\nThe patient in this case presented with vague symptoms of increased intracranial pressure (vomiting and headaches). Signs of increased intracranial pressure are not uncommon in the presentation of brainstem gliomas, resulting from obstruction of cerebrospinal fluid outflow. Cerebellar signs, such as those present in this patient (a positive Romberg sign, unsteady wide-based gait, abnormal finger-to-nose coordination), are also typical at presentation, particularly for children with diffuse brainstem gliomas, because these tumors are located in the posterior fossa.\nNotably, this patient was also reported to have a 1-year history of declining school performance, with no attributable cause. In rare cases, declining school performance, especially related to visual disturbances or symptoms of hydrocephalus, may be the only presenting sign of a brainstem glioma.[2]\nFigure 1.\nIn this case, the diagnosis was ultimately made with MRI; the axial T2-weighted imaging showed ill-defined, bilateral pontine signal hyperintensity in a heterogeneous pattern, with asymmetric involvement of the right half of the pons (Figure 1). These findings are consistent with diffuse intrinsic pontine glioma (DIPG).\nUnlike adult brain tumors, most childhood tumors are primary central nervous system (CNS) tumors. They are also infratentorial. The most common childhood brain tumors are the primitive neuroectodermal tumors, such as medulloblastoma, which most commonly arise in the posterior fossa and the pineal region.\nGlial tumors account for 50%-60% of all childhood brain tumors.[3] Glial tumors can arise from any part of the CNS, but they are most commonly found in the supratentorial region and the brainstem. Brainstem gliomas account for 10%-20% of all childhood brain tumors. Approximately 80% of all brainstem gliomas occur in patients younger than 21 years, with the incidence peaking at age 7 years. Both sexes are affected equally, although some studies have suggested a slight male predominance.[4] Some predilection for tumors to arise on the left side has been noted.\nThe term \"brainstem glioma\" describes an intrinsic brainstem tumor which may be a ganglioglioma, pilocytic astrocytoma, fibrillary astrocytoma, anaplastic astrocytoma, or glioblastoma multiforme, depending on the pathology. The simplest classification of brainstem gliomas divides these tumors into two categories: diffusely infiltrative brainstem gliomas and focal brainstem gliomas.[5]\nThe World Health Organization (WHO) further classifies brain tumors on the basis of their pathologic characteristics, including the level of differentiation and number of mitoses. The grades range from I to IV, with grade I being the least aggressive and grade IV being the most aggressive.\nDiffusely infiltrative brainstem gliomas are high-grade tumors that most commonly originate in the pons and often diffusely expand to other brainstem sites, including the cerebellum and thalamus. These tumors are most often fibrillary astrocytoma (WHO grade II), anaplastic astrocytoma (WHO grade III), or glioblastoma multiforme (WHO grade IV). Focal brainstem gliomas, however, are more likely to be well circumscribed and focal and may be exophytic (growing outside of the brainstem). They more frequently originate in the midbrain or medulla and are typically classified as pilocytic astrocytoma (WHO grade I); ganglioglioma (WHO grade I); or, rarely, fibrillary astrocytoma (WHO grade II).[4,5,6]\n\n ## Figures\n\n **Figure 1.** \n \n\n\n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 844387,
"choiceText": "Herpes encephalitis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 844389,
"choiceText": "Pontine glioma\r\n",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 844391,
"choiceText": "Friedreich ataxia",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 844393,
"choiceText": "Toxic ingestion",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 844395,
"choiceText": "Intramedullary mass lesion of the spinal cord",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 265485,
"questionText": "On the basis of the history, physical examination, and workup, what is the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 9-Year-Old Girl With Declining School Performance"
},
{
"authors": "Faisal M. Mawri, MD; Peter Stoyanoff, MD; Meaghan Misiasz, BA; Jillian Ewing, BS; David Ishiyama",
"content": [
"The diagnosis of brainstem gliomas is based on the presenting clinical symptoms as well as the findings on imaging studies. A careful and detailed history should be obtained from both the parent and the child.",
"Children with diffuse brainstem gliomas often present with a subacute course involving a typical symptom duration of less than 6 months.[6] Multiple cranial nerve signs; pyramidal deficits; cerebellar signs; or signs of increased intracranial pressure, such as headache (often worse in the morning), vomiting, gait disturbance, papilledema, blurred vision, and diplopia, are common. A careful family history for neurofibromatosis should also be obtained because neurofibromatosis type 1 is associated with low-grade brainstem gliomas, which typically have an indolent course and a more favorable prognosis.[2]",
"Focal gliomas are generally low-grade and, accordingly, their onset is more prolonged (months to years). The specific location of the tumor often determines the symptomatic presentation of the patient. Upper brainstem tumors are most likely to present with hydrocephalus, oculomotor dysfunction, or cerebellar findings, whereas lower brainstem tumors present with lower cranial nerve deficits.",
"Focal tumors of the tectum (tectal gliomas) typically exhibit low-grade behavior, but they can cause significant neurologic symptoms when they enlarge and compress the cerebral aqueduct, consequently producing obstructive hydrocephalus. Tegmental tumors commonly present with hydrocephalus and oculomotor paresis.",
"Pontine lesions can cause facial paresis, hearing loss, or long tract findings (such as hyperreflexia/clonus and spasticity). Tumors of the medulla present with lower cranial nerve deficits, which may manifest as changes in the voice, swallowing difficulty, or frequent pneumonias resulting from microaspiration. Focal pontine gliomas generally have a poorer prognosis, whereas focal tumors of the medulla have a more favorable prognosis.[2]",
"With the above neurologic symptoms, other pediatric tumors, such as ependymoma, glioblastoma multiforme, low-grade astrocytoma, and medulloblastoma, should be considered. Although unlikely, the possibility of metastatic disease should also be explored.",
"In combination with physical examination, CT and MRI are used to assess the site of origin, extent, and mass effect of these lesions. On CT, gliomas appear as hypoattenuating masses with variable enhancement depending on the grade of the glioma.",
"Relative to CT, however, the higher multiplanar resolution of MRI is necessary for the precise localization, differentiation, and management of brainstem gliomas. Focal brainstem gliomas appear as well-demarcated lesions that occupy less than 50% of the axial diameter of the segment of brainstem in which it resides. In contrast, diffuse brainstem gliomas are poorly marginated and typically occupy more than 50% of the axial diameter of the pons.",
"Furthermore, diffuse brainstem gliomas often impinge upon the basilar artery. Both focal and diffuse brainstem gliomas appear as hypointense masses on T1-weighted MRI and hyperintense masses on T2-weighted MRI. The diagnosis of diffuse brainstem gliomas is based on specific MRI findings, and presently, histologic findings do not augment the current standards of diagnosis. In some cases, however, biopsy and pathologic evaluation may be necessary. Genetic and molecular markers for brainstem gliomas are being investigated.[2,5]",
"Figure 2.",
"Figure 3.",
"The near-total encasement of the basilar artery, a common finding of DIPG, is important to note, as well as the encroachment on the fourth ventricle without invasion seen on the imaging scans of this patient. In addition, DIPG invasion of the midbrain is further evident on the axial fluid attenuation inversion recovery sequence, which demonstrates diffuse, ill-defined, bilateral pontine signal hyperintensity, with asymmetric involvement of the right half of the pons and involvement of the cerebellar peduncles (Figure 2).",
"Finally, a sagittal view on the axial T1-weighted MRI demonstrates the diffuse, ill-defined, heterogeneously low-signal midbrain expansion typical of DIPG (Figure 3). Note the effacement of the pontomedullary notch."
],
"date": "May 14, 2015",
"figures": [
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/844/658/844658-Thumb2s.png"
},
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/844/658/844658-Thumb3s.png"
}
],
"markdown": "# A 9-Year-Old Girl With Declining School Performance\n\n **Authors:** Faisal M. Mawri, MD; Peter Stoyanoff, MD; Meaghan Misiasz, BA; Jillian Ewing, BS; David Ishiyama \n **Date:** May 14, 2015\n\n ## Content\n\n The diagnosis of brainstem gliomas is based on the presenting clinical symptoms as well as the findings on imaging studies. A careful and detailed history should be obtained from both the parent and the child.\nChildren with diffuse brainstem gliomas often present with a subacute course involving a typical symptom duration of less than 6 months.[6] Multiple cranial nerve signs; pyramidal deficits; cerebellar signs; or signs of increased intracranial pressure, such as headache (often worse in the morning), vomiting, gait disturbance, papilledema, blurred vision, and diplopia, are common. A careful family history for neurofibromatosis should also be obtained because neurofibromatosis type 1 is associated with low-grade brainstem gliomas, which typically have an indolent course and a more favorable prognosis.[2]\nFocal gliomas are generally low-grade and, accordingly, their onset is more prolonged (months to years). The specific location of the tumor often determines the symptomatic presentation of the patient. Upper brainstem tumors are most likely to present with hydrocephalus, oculomotor dysfunction, or cerebellar findings, whereas lower brainstem tumors present with lower cranial nerve deficits.\nFocal tumors of the tectum (tectal gliomas) typically exhibit low-grade behavior, but they can cause significant neurologic symptoms when they enlarge and compress the cerebral aqueduct, consequently producing obstructive hydrocephalus. Tegmental tumors commonly present with hydrocephalus and oculomotor paresis.\nPontine lesions can cause facial paresis, hearing loss, or long tract findings (such as hyperreflexia/clonus and spasticity). Tumors of the medulla present with lower cranial nerve deficits, which may manifest as changes in the voice, swallowing difficulty, or frequent pneumonias resulting from microaspiration. Focal pontine gliomas generally have a poorer prognosis, whereas focal tumors of the medulla have a more favorable prognosis.[2]\nWith the above neurologic symptoms, other pediatric tumors, such as ependymoma, glioblastoma multiforme, low-grade astrocytoma, and medulloblastoma, should be considered. Although unlikely, the possibility of metastatic disease should also be explored.\nIn combination with physical examination, CT and MRI are used to assess the site of origin, extent, and mass effect of these lesions. On CT, gliomas appear as hypoattenuating masses with variable enhancement depending on the grade of the glioma.\nRelative to CT, however, the higher multiplanar resolution of MRI is necessary for the precise localization, differentiation, and management of brainstem gliomas. Focal brainstem gliomas appear as well-demarcated lesions that occupy less than 50% of the axial diameter of the segment of brainstem in which it resides. In contrast, diffuse brainstem gliomas are poorly marginated and typically occupy more than 50% of the axial diameter of the pons.\nFurthermore, diffuse brainstem gliomas often impinge upon the basilar artery. Both focal and diffuse brainstem gliomas appear as hypointense masses on T1-weighted MRI and hyperintense masses on T2-weighted MRI. The diagnosis of diffuse brainstem gliomas is based on specific MRI findings, and presently, histologic findings do not augment the current standards of diagnosis. In some cases, however, biopsy and pathologic evaluation may be necessary. Genetic and molecular markers for brainstem gliomas are being investigated.[2,5]\nFigure 2.\nFigure 3.\nThe near-total encasement of the basilar artery, a common finding of DIPG, is important to note, as well as the encroachment on the fourth ventricle without invasion seen on the imaging scans of this patient. In addition, DIPG invasion of the midbrain is further evident on the axial fluid attenuation inversion recovery sequence, which demonstrates diffuse, ill-defined, bilateral pontine signal hyperintensity, with asymmetric involvement of the right half of the pons and involvement of the cerebellar peduncles (Figure 2).\nFinally, a sagittal view on the axial T1-weighted MRI demonstrates the diffuse, ill-defined, heterogeneously low-signal midbrain expansion typical of DIPG (Figure 3). Note the effacement of the pontomedullary notch.\n\n ## Figures\n\n **Figure 2.** \n \n\n**Figure 3.** \n \n\n\n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 9-Year-Old Girl With Declining School Performance"
},
{
"authors": "Faisal M. Mawri, MD; Peter Stoyanoff, MD; Meaghan Misiasz, BA; Jillian Ewing, BS; David Ishiyama",
"content": [
"The prognosis of patients with diffuse intrinsic brainstem gliomas is poor, with a typical survival of less than 12 months after diagnosis. Treatment modalities are limited and have thus far failed to yield significant increases in survival. Given the location and degree of infiltration, most diffuse brainstem gliomas cannot be surgically treated.",
"The standard treatment currently involves radiation therapy, which may produce temporary neurologic improvement but does not improve overall survival. Although several agents are currently under study, no effective chemotherapeutic agent for the treatment of brainstem glioma has yet been identified.[5,6]",
"Once the diagnosis was made, the patient in this case was admitted to the pediatric intensive care unit (ICU) for further evaluation and monitoring. No biopsy was performed, because the MRI findings clearly established the diagnosis and biopsy would not have yielded any additional information applicable to her care but would have considerably increased morbidity.",
"Oral dexamethasone (Ozurdex®) was given to reduce brain edema around the tumor. While in the pediatric ICU, the patient's ataxia, vomiting, and headaches improved, and she was subsequently transferred to the hematology/oncology ward. On the basis of the location of the pontine glioma, the mass was thought to be inoperable; therefore, radiation therapy was initiated on an inpatient basis. Nausea, headache, and abdominal pain were reported after radiation therapy and were treated with acetaminophen, ondansetron, and metoclopramide.",
"The child's prognosis was thought to be poor, with an estimated life expectancy of 12-15 months. Radiation therapy was continued on an outpatient basis.",
"Palliative care and social support resources, which are of key importance to patients and families affected by the diagnosis of brainstem glioma, were offered to the child and her parents. The patient lost her hair and had significant weight loss, and she became depressed. She died 6 months after her diagnosis.",
"The differential diagnosis for a child with poor school performance is extensive; it encompasses psychosocial, neuropsychiatric, and organic disease causes. In children who present with unexplained poor performance in school or changes in personality, a thorough workup that includes a detailed neurologic examination is vastly important. For over a year, the only symptom of the growing intracranial neoplasm in this child was her deteriorating school performance. She went from being an exemplary student to failing classes, even with help from a tutor."
],
"date": "May 14, 2015",
"figures": [],
"markdown": "# A 9-Year-Old Girl With Declining School Performance\n\n **Authors:** Faisal M. Mawri, MD; Peter Stoyanoff, MD; Meaghan Misiasz, BA; Jillian Ewing, BS; David Ishiyama \n **Date:** May 14, 2015\n\n ## Content\n\n The prognosis of patients with diffuse intrinsic brainstem gliomas is poor, with a typical survival of less than 12 months after diagnosis. Treatment modalities are limited and have thus far failed to yield significant increases in survival. Given the location and degree of infiltration, most diffuse brainstem gliomas cannot be surgically treated.\nThe standard treatment currently involves radiation therapy, which may produce temporary neurologic improvement but does not improve overall survival. Although several agents are currently under study, no effective chemotherapeutic agent for the treatment of brainstem glioma has yet been identified.[5,6]\nOnce the diagnosis was made, the patient in this case was admitted to the pediatric intensive care unit (ICU) for further evaluation and monitoring. No biopsy was performed, because the MRI findings clearly established the diagnosis and biopsy would not have yielded any additional information applicable to her care but would have considerably increased morbidity.\nOral dexamethasone (Ozurdex®) was given to reduce brain edema around the tumor. While in the pediatric ICU, the patient's ataxia, vomiting, and headaches improved, and she was subsequently transferred to the hematology/oncology ward. On the basis of the location of the pontine glioma, the mass was thought to be inoperable; therefore, radiation therapy was initiated on an inpatient basis. Nausea, headache, and abdominal pain were reported after radiation therapy and were treated with acetaminophen, ondansetron, and metoclopramide.\nThe child's prognosis was thought to be poor, with an estimated life expectancy of 12-15 months. Radiation therapy was continued on an outpatient basis.\nPalliative care and social support resources, which are of key importance to patients and families affected by the diagnosis of brainstem glioma, were offered to the child and her parents. The patient lost her hair and had significant weight loss, and she became depressed. She died 6 months after her diagnosis.\nThe differential diagnosis for a child with poor school performance is extensive; it encompasses psychosocial, neuropsychiatric, and organic disease causes. In children who present with unexplained poor performance in school or changes in personality, a thorough workup that includes a detailed neurologic examination is vastly important. For over a year, the only symptom of the growing intracranial neoplasm in this child was her deteriorating school performance. She went from being an exemplary student to failing classes, even with help from a tutor.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 844397,
"choiceText": "Supratentorial",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 844399,
"choiceText": "Intraventricular",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 844401,
"choiceText": "Peripheral nervous system",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 844403,
"choiceText": "Infratentorial",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 844405,
"choiceText": "Along the optic nerve\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Unlike adult brain tumors, most childhood tumors are primary CNS tumors. They are also infratentorial. ",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 265487,
"questionText": "A 6-year-old child presents with an unsteady gait. He also complains of headaches that are more frequent in the morning and are associated with nausea and vomiting. Imaging is most likely to reveal a lesion in which of the following regions?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 844407,
"choiceText": "The tumor is poorly marginated on imaging",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 844409,
"choiceText": "The tumor occupies more that 50% of the axial diameter of the pons",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 844411,
"choiceText": "The tumor is impinging on the ophthalmic artery",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 844413,
"choiceText": "On T1-weighted MRI, the tumor appears as a hypointense mass",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Diffuse brainstem gliomas are poorly marginated and typically occupy more than 50% of the axial diameter of the pons. Both focal and diffuse brainstem gliomas appear as hypointense masses on T1-weighted MRI and hyperintense masses on T2-weighted MRI.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 265489,
"questionText": "In a child with diffuse brainstem glioma, which of the following findings would least likely be present?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 9-Year-Old Girl With Declining School Performance"
},
{
"authors": "Faisal M. Mawri, MD; Peter Stoyanoff, MD; Meaghan Misiasz, BA; Jillian Ewing, BS; David Ishiyama",
"content": [],
"date": "May 14, 2015",
"figures": [],
"markdown": "# A 9-Year-Old Girl With Declining School Performance\n\n **Authors:** Faisal M. Mawri, MD; Peter Stoyanoff, MD; Meaghan Misiasz, BA; Jillian Ewing, BS; David Ishiyama \n **Date:** May 14, 2015\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 844397,
"choiceText": "Supratentorial",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 844399,
"choiceText": "Intraventricular",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 844401,
"choiceText": "Peripheral nervous system",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 844403,
"choiceText": "Infratentorial",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 844405,
"choiceText": "Along the optic nerve\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Unlike adult brain tumors, most childhood tumors are primary CNS tumors. They are also infratentorial. ",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 265487,
"questionText": "A 6-year-old child presents with an unsteady gait. He also complains of headaches that are more frequent in the morning and are associated with nausea and vomiting. Imaging is most likely to reveal a lesion in which of the following regions?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 844407,
"choiceText": "The tumor is poorly marginated on imaging",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 844409,
"choiceText": "The tumor occupies more that 50% of the axial diameter of the pons",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 844411,
"choiceText": "The tumor is impinging on the ophthalmic artery",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 844413,
"choiceText": "On T1-weighted MRI, the tumor appears as a hypointense mass",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Diffuse brainstem gliomas are poorly marginated and typically occupy more than 50% of the axial diameter of the pons. Both focal and diffuse brainstem gliomas appear as hypointense masses on T1-weighted MRI and hyperintense masses on T2-weighted MRI.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 265489,
"questionText": "In a child with diffuse brainstem glioma, which of the following findings would least likely be present?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 9-Year-Old Girl With Declining School Performance"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 844387,
"choiceText": "Herpes encephalitis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 844389,
"choiceText": "Pontine glioma\r\n",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 844391,
"choiceText": "Friedreich ataxia",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 844393,
"choiceText": "Toxic ingestion",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 844395,
"choiceText": "Intramedullary mass lesion of the spinal cord",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 265485,
"questionText": "On the basis of the history, physical examination, and workup, what is the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 844397,
"choiceText": "Supratentorial",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 844399,
"choiceText": "Intraventricular",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 844401,
"choiceText": "Peripheral nervous system",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 844403,
"choiceText": "Infratentorial",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 844405,
"choiceText": "Along the optic nerve\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Unlike adult brain tumors, most childhood tumors are primary CNS tumors. They are also infratentorial. ",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 265487,
"questionText": "A 6-year-old child presents with an unsteady gait. He also complains of headaches that are more frequent in the morning and are associated with nausea and vomiting. Imaging is most likely to reveal a lesion in which of the following regions?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 844407,
"choiceText": "The tumor is poorly marginated on imaging",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 844409,
"choiceText": "The tumor occupies more that 50% of the axial diameter of the pons",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 844411,
"choiceText": "The tumor is impinging on the ophthalmic artery",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 844413,
"choiceText": "On T1-weighted MRI, the tumor appears as a hypointense mass",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Diffuse brainstem gliomas are poorly marginated and typically occupy more than 50% of the axial diameter of the pons. Both focal and diffuse brainstem gliomas appear as hypointense masses on T1-weighted MRI and hyperintense masses on T2-weighted MRI.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 265489,
"questionText": "In a child with diffuse brainstem glioma, which of the following findings would least likely be present?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
843819 | /viewarticle/843819 | [
{
"authors": "Helen Kenealy, BHB, MBchB",
"content": [
"Editor's Note: \nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.",
"Background",
"A 79-year-old woman presents to the emergency department with left hip pain. She mildly twisted her left leg 3 days ago and has since had increasing pain and difficulty walking. The pain is sharp and radiates down to her left knee. It is mild when she is at rest, but it becomes severe when she attempts to walk. She has not had any weakness or numbness, and denies having had any fever. She has not had any direct trauma to the hip or falls, and there has not been any notable swelling of the leg, skin changes, or rash.",
"The patient has unintentionally lost 8.8 lb over the past 6 months. She denies having any nausea, vomiting, night sweats, cough, or shortness of breath. She has a history of multiple rib fractures that resulted from vigorous coughing 2 years ago; at that time, she was diagnosed with osteoporosis as the cause of these fractures. She denies having had any other previous fractures.",
"The patient's medical history also includes chronic obstructive pulmonary disease, although she has never been a smoker. She also has known osteoarthritis of the hips, scoliosis of the thoracic spine, and a presumptive diagnosis of Paget disease on the basis of a single elevated serum alkaline phosphatase level.",
"The patient's medications include budesonide/formoterol (Symbicort®) and terbutaline inhalers, oral calcium and vitamin D supplements, and weekly alendronate (Fosamax®). A bisphosphonate was started after dual-energy x-ray absorptiometry (DXA) showed a T score of -2, which is consistent with osteopenia.",
"Both her mother and sister broke their hips later in their lives.",
"The patient lives independently and is still driving. She denies experiencing physical abuse, and the family members who have accompanied her in the emergency department show concern for her."
],
"date": "April 30, 2015",
"figures": [],
"markdown": "# Femoral Neck Fracture in a 79-Year-Old Woman\n\n **Authors:** Helen Kenealy, BHB, MBchB \n **Date:** April 30, 2015\n\n ## Content\n\n Editor's Note: \nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.\nBackground\nA 79-year-old woman presents to the emergency department with left hip pain. She mildly twisted her left leg 3 days ago and has since had increasing pain and difficulty walking. The pain is sharp and radiates down to her left knee. It is mild when she is at rest, but it becomes severe when she attempts to walk. She has not had any weakness or numbness, and denies having had any fever. She has not had any direct trauma to the hip or falls, and there has not been any notable swelling of the leg, skin changes, or rash.\nThe patient has unintentionally lost 8.8 lb over the past 6 months. She denies having any nausea, vomiting, night sweats, cough, or shortness of breath. She has a history of multiple rib fractures that resulted from vigorous coughing 2 years ago; at that time, she was diagnosed with osteoporosis as the cause of these fractures. She denies having had any other previous fractures.\nThe patient's medical history also includes chronic obstructive pulmonary disease, although she has never been a smoker. She also has known osteoarthritis of the hips, scoliosis of the thoracic spine, and a presumptive diagnosis of Paget disease on the basis of a single elevated serum alkaline phosphatase level.\nThe patient's medications include budesonide/formoterol (Symbicort®) and terbutaline inhalers, oral calcium and vitamin D supplements, and weekly alendronate (Fosamax®). A bisphosphonate was started after dual-energy x-ray absorptiometry (DXA) showed a T score of -2, which is consistent with osteopenia.\nBoth her mother and sister broke their hips later in their lives.\nThe patient lives independently and is still driving. She denies experiencing physical abuse, and the family members who have accompanied her in the emergency department show concern for her.\n\n ## Figures\n\n \n*Page 1 of 5*",
"pagination": {
"current_page": 1,
"total_pages": 5
},
"questionnaire": [],
"title": "Femoral Neck Fracture in a 79-Year-Old Woman"
},
{
"authors": "Helen Kenealy, BHB, MBchB",
"content": [
"Physical Examination and Work-up",
"Upon physical examination, the patient appears well and is lying comfortably in bed. Her heart rate is 84 beats/min and regular; her blood pressure is 140/80 mm Hg. Her temperature is normal, at 98.9°F, and her respiratory rate is 14 breaths/min. Her cardiovascular, respiratory, and abdominal examination findings are all normal.",
"Figure.",
"The patient has moderate pain with any movement of the left leg and has groin tenderness on palpation. The left leg is shortened and externally rotated. Her peripheral pulses are palpable, and she has normal distal strength and sensation in the left lower extremity.",
"Blood tests reveal a normal complete blood cell count; however, evaluation of electrolytes shows a significantly depleted phosphate concentration of 0.9 mg/dL (normal range, 3-4.5 mg/dL).",
"Additional laboratory results include the following:",
"Creatinine level: 0.68 mg/dL (normal range, <1.5 mg/dL)",
"Adjusted calcium level: 8.88 mg/dL (normal range, 9.0-10.5 mg/dL)",
"Albumin level: 3.6 g/dL (normal range, 3.5-5.5 g/dL)",
"Alkaline phosphatase level: 350 U/L (normal range, 30-120 U/L)",
"Vitamin D (25-hydroxy) level: 22.04 ng/mL (normal range, 10-68 ng/mL)",
"Parathyroid hormone level: 107.3 pg/mL (normal range, 10-60 pg/mL)",
"A chest radiograph reveals multiple healed rib fractures. Radiographs of the pelvis and left hip are obtained, which reveal a left femoral neck fracture (Figure 1)."
],
"date": "April 30, 2015",
"figures": [
{
"caption": "Figure.",
"image_url": "https://img.medscapestatic.com/article/843/819/843819-thumb.jpg"
}
],
"markdown": "# Femoral Neck Fracture in a 79-Year-Old Woman\n\n **Authors:** Helen Kenealy, BHB, MBchB \n **Date:** April 30, 2015\n\n ## Content\n\n Physical Examination and Work-up\nUpon physical examination, the patient appears well and is lying comfortably in bed. Her heart rate is 84 beats/min and regular; her blood pressure is 140/80 mm Hg. Her temperature is normal, at 98.9°F, and her respiratory rate is 14 breaths/min. Her cardiovascular, respiratory, and abdominal examination findings are all normal.\nFigure.\nThe patient has moderate pain with any movement of the left leg and has groin tenderness on palpation. The left leg is shortened and externally rotated. Her peripheral pulses are palpable, and she has normal distal strength and sensation in the left lower extremity.\nBlood tests reveal a normal complete blood cell count; however, evaluation of electrolytes shows a significantly depleted phosphate concentration of 0.9 mg/dL (normal range, 3-4.5 mg/dL).\nAdditional laboratory results include the following:\nCreatinine level: 0.68 mg/dL (normal range, <1.5 mg/dL)\nAdjusted calcium level: 8.88 mg/dL (normal range, 9.0-10.5 mg/dL)\nAlbumin level: 3.6 g/dL (normal range, 3.5-5.5 g/dL)\nAlkaline phosphatase level: 350 U/L (normal range, 30-120 U/L)\nVitamin D (25-hydroxy) level: 22.04 ng/mL (normal range, 10-68 ng/mL)\nParathyroid hormone level: 107.3 pg/mL (normal range, 10-60 pg/mL)\nA chest radiograph reveals multiple healed rib fractures. Radiographs of the pelvis and left hip are obtained, which reveal a left femoral neck fracture (Figure 1).\n\n ## Figures\n\n **Figure.** \n \n\n\n*Page 2 of 5*",
"pagination": {
"current_page": 2,
"total_pages": 5
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 838593,
"choiceText": "Vitamin D deficiency",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 838595,
"choiceText": "Oncogenic osteomalacia",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 838597,
"choiceText": "Fanconi syndrome",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 838599,
"choiceText": "Secondary hyperparathyroidism",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 263695,
"questionText": "On the basis of the history, physical examination, and work-up, what is the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Femoral Neck Fracture in a 79-Year-Old Woman"
},
{
"authors": "Helen Kenealy, BHB, MBchB",
"content": [
"Discussion",
"The pelvic radiograph clearly demonstrated osteomalacia. The patient was admitted to the hospital for open reduction and internal fixation of her fractured left femoral neck. Evaluation of her left femoral neck revealed suspicion of a pseudofracture, which was also fixed. Of note were multiple pseudofractures in the pubic rami and pelvic ring.",
"The low serum phosphate concentration was the primary clue to the underlying cause. No previous serum phosphate measurements were available for comparison. To determine the cause of hypophosphatemia, a urinary phosphate measurement must be obtained. This is best done with the patient fasting, and it may be done with a single specimen.",
"In this patient, the urinary calcium/creatinine ratio was normal at 0.4, and the urinary phosphate/creatinine ratio was elevated at 5.4. A calculation of tubular reabsorption of phosphate showed a low value of 0.325 (normal, 0.82-0.95), confirming renal phosphate wasting.",
"Numerous causes for increased urinary phosphate excretion had to be considered. Primary and secondary hyperparathyroidism were essentially excluded by the normal serum calcium and creatinine levels. Vitamin D deficiency or resistance was excluded by an adequate serum vitamin D level. Fanconi syndrome was excluded by the negative myeloma screen, normal uric acid level, and absence of glucosuria. Hereditary hypophosphatemic rickets (X-linked, autosomal dominant, or recessive) was highly unlikely because of the patient's advanced age.",
"The serum and urine studies were consistent with a diagnosis of oncogenic (or tumor-induced) osteomalacia. This acquired renal phosphate wasting syndrome is caused by increased serum levels of fibroblast growth factor (FGF)-23. The pathognomonic signs of this condition include adult-onset osteomalacia with a low serum phosphate concentration, a normal serum calcium level, and renal phosphate wasting. The calcitriol level is low as a result of the weak inhibitory effect of FGF-23 on the renal 1-alpha-hydroxylase enzyme; however, calcitriol need not be measured to establish the diagnosis.",
"The serum parathyroid hormone was mildly elevated as a result of the mild decrease in active vitamin D. The mechanism of bone loss is incompletely understood; it is caused in part by disturbed phosphate metabolism and, to a lesser degree, abnormal vitamin D metabolism.",
"The exact epidemiology of this condition is unknown. It is certainly rare; by 1999, only approximately 100 cases had been reported in the literature.[1] On the basis of the description of these cases, the clinical presentation appears to be wide and ranges from musculoskeletal pain, proximal muscle weakness, and bony tenderness to frank osteomalacia and pathologic fractures. It is vital to check the serum phosphate level in any patient with unexplained musculoskeletal symptoms or pathologic fractures. The diagnosis can be confirmed with serum FGF-23 measurement.",
"FGF-23 is a phosphatonin secreted autonomously from tumor cells in oncogenic osteomalacia. FGF-23 inhibits the sodium-phosphate cotransporter in the renal tubules, such that less phosphate is reabsorbed, resulting in urinary phosphate wasting. Of the phosphate that the kidneys filter each day, 85%-90% is reabsorbed by the sodium-phosphate cotransporters in the renal tubules. Autosomal dominant hypophosphatemic rickets is caused by mutations in the FGF-23 gene, located on chromosome 12p13. Part of the metabolism of FGF-23 is degradation by PHEX, a membrane-bound endopeptidase; however, uncontrolled secretion of FGF-23 by certain tumor cells saturates the PHEX degradation capability, which allows FGF-23 action to be unlimited.",
"Serum FGF-23 levels are almost universally elevated in oncogenic osteomalacia, and these can be monitored after treatment to identify disease recurrence.[2] Other putative phosphatonins are the topic of active research."
],
"date": "April 30, 2015",
"figures": [],
"markdown": "# Femoral Neck Fracture in a 79-Year-Old Woman\n\n **Authors:** Helen Kenealy, BHB, MBchB \n **Date:** April 30, 2015\n\n ## Content\n\n Discussion\nThe pelvic radiograph clearly demonstrated osteomalacia. The patient was admitted to the hospital for open reduction and internal fixation of her fractured left femoral neck. Evaluation of her left femoral neck revealed suspicion of a pseudofracture, which was also fixed. Of note were multiple pseudofractures in the pubic rami and pelvic ring.\nThe low serum phosphate concentration was the primary clue to the underlying cause. No previous serum phosphate measurements were available for comparison. To determine the cause of hypophosphatemia, a urinary phosphate measurement must be obtained. This is best done with the patient fasting, and it may be done with a single specimen.\nIn this patient, the urinary calcium/creatinine ratio was normal at 0.4, and the urinary phosphate/creatinine ratio was elevated at 5.4. A calculation of tubular reabsorption of phosphate showed a low value of 0.325 (normal, 0.82-0.95), confirming renal phosphate wasting.\nNumerous causes for increased urinary phosphate excretion had to be considered. Primary and secondary hyperparathyroidism were essentially excluded by the normal serum calcium and creatinine levels. Vitamin D deficiency or resistance was excluded by an adequate serum vitamin D level. Fanconi syndrome was excluded by the negative myeloma screen, normal uric acid level, and absence of glucosuria. Hereditary hypophosphatemic rickets (X-linked, autosomal dominant, or recessive) was highly unlikely because of the patient's advanced age.\nThe serum and urine studies were consistent with a diagnosis of oncogenic (or tumor-induced) osteomalacia. This acquired renal phosphate wasting syndrome is caused by increased serum levels of fibroblast growth factor (FGF)-23. The pathognomonic signs of this condition include adult-onset osteomalacia with a low serum phosphate concentration, a normal serum calcium level, and renal phosphate wasting. The calcitriol level is low as a result of the weak inhibitory effect of FGF-23 on the renal 1-alpha-hydroxylase enzyme; however, calcitriol need not be measured to establish the diagnosis.\nThe serum parathyroid hormone was mildly elevated as a result of the mild decrease in active vitamin D. The mechanism of bone loss is incompletely understood; it is caused in part by disturbed phosphate metabolism and, to a lesser degree, abnormal vitamin D metabolism.\nThe exact epidemiology of this condition is unknown. It is certainly rare; by 1999, only approximately 100 cases had been reported in the literature.[1] On the basis of the description of these cases, the clinical presentation appears to be wide and ranges from musculoskeletal pain, proximal muscle weakness, and bony tenderness to frank osteomalacia and pathologic fractures. It is vital to check the serum phosphate level in any patient with unexplained musculoskeletal symptoms or pathologic fractures. The diagnosis can be confirmed with serum FGF-23 measurement.\nFGF-23 is a phosphatonin secreted autonomously from tumor cells in oncogenic osteomalacia. FGF-23 inhibits the sodium-phosphate cotransporter in the renal tubules, such that less phosphate is reabsorbed, resulting in urinary phosphate wasting. Of the phosphate that the kidneys filter each day, 85%-90% is reabsorbed by the sodium-phosphate cotransporters in the renal tubules. Autosomal dominant hypophosphatemic rickets is caused by mutations in the FGF-23 gene, located on chromosome 12p13. Part of the metabolism of FGF-23 is degradation by PHEX, a membrane-bound endopeptidase; however, uncontrolled secretion of FGF-23 by certain tumor cells saturates the PHEX degradation capability, which allows FGF-23 action to be unlimited.\nSerum FGF-23 levels are almost universally elevated in oncogenic osteomalacia, and these can be monitored after treatment to identify disease recurrence.[2] Other putative phosphatonins are the topic of active research.\n\n ## Figures\n\n \n*Page 3 of 5*",
"pagination": {
"current_page": 3,
"total_pages": 5
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 838593,
"choiceText": "Vitamin D deficiency",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 838595,
"choiceText": "Oncogenic osteomalacia",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 838597,
"choiceText": "Fanconi syndrome",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 838599,
"choiceText": "Secondary hyperparathyroidism",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 263695,
"questionText": "On the basis of the history, physical examination, and work-up, what is the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Femoral Neck Fracture in a 79-Year-Old Woman"
},
{
"authors": "Helen Kenealy, BHB, MBchB",
"content": [
"The vast majority of the causative tumors are small, benign, and themselves asymptomatic,[3] which can make them difficult to locate. T2-weighted short-tau inversion-recovery (STIR) MRI is recommended as the first step in locating the tissues. Any abnormal areas should be scanned in more detail to find the causative tumor. Once identified, the tumor must be removed. The serum FGF-23 level and serum phosphate concentration should normalize postoperatively; if they do not normalize, further exploration for the causative tumor is indicated.",
"Histologic examination usually reveals a phosphaturic mesenchymal tumor (mixed connective tissue variant) or a hemangiopericytoma. In most cases, the nuclear grade is low. Mitotic activity is usually low or absent, and the cellularity is also low. Although most cases appear relatively well-circumscribed on low-power magnification, even benign tumors may infiltrate the surrounding connective tissues. The finding of osteoid-type tissue within the tumor is common in both soft-tissue and bone samples. The matrix may be highly calcified, which correlates with the finding of numerous osteoclast-like giant cells. More than 50% of the soft-tissue samples show a partial shell of woven bone.",
"In one series, only three samples (< 10%) fit the criteria for being labeled \"malignant,\" indicating that this is a relatively rare event. As such, this condition is rarely fatal, and morbidity can be avoided by timely diagnosis and tumor removal.[3] If STIR MRI is unhelpful, an octreotide scan can be performed for localization of the tumor.",
"This patient was treated with an oral high-dose phosphate supplement to normalize her serum phosphate concentration before surgery. She then had an open reduction and internal fixation, with an uncomplicated postoperative course. She successfully completed a course of inpatient rehabilitation and was discharged to home without incident.",
"The patient's preoperative serum FGF-23 was measured and found to be elevated, at 355 U/mL (normal range, 3-45 U/mL). A STIR MRI image indicated an area of abnormality in her left ethmoid sinus. CT of her sinuses revealed a mass lesion in the left posterior ethmoid sinus obstructing the left sphenoidal sinus, with no sign of bony destruction.",
"Review of the literature revealed that sinus tumors cause 5%-10% of cases of oncogenic osteomalacia. [3] This patient underwent an uneventful resection of this tumor, which was a mesenchymal hemangiopericytoma of the phosphaturic type. She made a full clinical recovery, with normalization of the serum phosphate level postoperatively. Serum FGF-23 does not need to be measured, provided that the phosphate level remains normal. If the serum phosphate level remains low, then FGF-23 should be rechecked, and if the level is persistently elevated, a further search for the causative tumor should be made. DXA was not repeated in this patient because the diagnosis of osteomalacia made it redundant."
],
"date": "April 30, 2015",
"figures": [],
"markdown": "# Femoral Neck Fracture in a 79-Year-Old Woman\n\n **Authors:** Helen Kenealy, BHB, MBchB \n **Date:** April 30, 2015\n\n ## Content\n\n The vast majority of the causative tumors are small, benign, and themselves asymptomatic,[3] which can make them difficult to locate. T2-weighted short-tau inversion-recovery (STIR) MRI is recommended as the first step in locating the tissues. Any abnormal areas should be scanned in more detail to find the causative tumor. Once identified, the tumor must be removed. The serum FGF-23 level and serum phosphate concentration should normalize postoperatively; if they do not normalize, further exploration for the causative tumor is indicated.\nHistologic examination usually reveals a phosphaturic mesenchymal tumor (mixed connective tissue variant) or a hemangiopericytoma. In most cases, the nuclear grade is low. Mitotic activity is usually low or absent, and the cellularity is also low. Although most cases appear relatively well-circumscribed on low-power magnification, even benign tumors may infiltrate the surrounding connective tissues. The finding of osteoid-type tissue within the tumor is common in both soft-tissue and bone samples. The matrix may be highly calcified, which correlates with the finding of numerous osteoclast-like giant cells. More than 50% of the soft-tissue samples show a partial shell of woven bone.\nIn one series, only three samples (< 10%) fit the criteria for being labeled \"malignant,\" indicating that this is a relatively rare event. As such, this condition is rarely fatal, and morbidity can be avoided by timely diagnosis and tumor removal.[3] If STIR MRI is unhelpful, an octreotide scan can be performed for localization of the tumor.\nThis patient was treated with an oral high-dose phosphate supplement to normalize her serum phosphate concentration before surgery. She then had an open reduction and internal fixation, with an uncomplicated postoperative course. She successfully completed a course of inpatient rehabilitation and was discharged to home without incident.\nThe patient's preoperative serum FGF-23 was measured and found to be elevated, at 355 U/mL (normal range, 3-45 U/mL). A STIR MRI image indicated an area of abnormality in her left ethmoid sinus. CT of her sinuses revealed a mass lesion in the left posterior ethmoid sinus obstructing the left sphenoidal sinus, with no sign of bony destruction.\nReview of the literature revealed that sinus tumors cause 5%-10% of cases of oncogenic osteomalacia. [3] This patient underwent an uneventful resection of this tumor, which was a mesenchymal hemangiopericytoma of the phosphaturic type. She made a full clinical recovery, with normalization of the serum phosphate level postoperatively. Serum FGF-23 does not need to be measured, provided that the phosphate level remains normal. If the serum phosphate level remains low, then FGF-23 should be rechecked, and if the level is persistently elevated, a further search for the causative tumor should be made. DXA was not repeated in this patient because the diagnosis of osteomalacia made it redundant.\n\n ## Figures\n\n \n*Page 4 of 5*",
"pagination": {
"current_page": 4,
"total_pages": 5
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 838601,
"choiceText": "Raised serum phosphate, lowered urinary phosphate/creatinine ratio, normal serum calcium",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 838603,
"choiceText": "Low serum phosphate, low serum calcium, raised urinary phosphate/creatinine ratio",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 838605,
"choiceText": "Low serum phosphate, raised serum alkaline phosphatase, low serum calcium",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 838607,
"choiceText": "Low serum phosphate, raised urinary phosphate/creatinine ratio, normal serum calcium",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 838609,
"choiceText": "None of the above",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Oncogenic or tumor-induced osteomalacia, an acquired renal phosphate wasting syndrome, is caused by an increased serum FGF-23 level. The pathognomonic signs of this condition include adult-onset osteomalacia with a low serum phosphate concentration, a normal serum calcium level, and renal phosphate wasting. The calcitriol level are low as a result of the weak inhibitory effect of FGF-23 on the renal 1-alpha-hydroxylase enzyme; however, calcitriol need not be measured to establish the diagnosis. The serum parathyroid hormone is mildly elevated as a result of a mild decrease in active vitamin D.<br>\r\n<br>\r\nThe mechanism of bone loss is incompletely understood; it is caused in part by disturbed phosphate metabolism and, to a lesser degree, abnormal vitamin D metabolism.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 263697,
"questionText": "If a patient presented with one of the following biochemical abnormalities, which set of findings would be most likely to lead to a suspicion of oncogenic osteomalacia?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 838611,
"choiceText": "Full skeletal survey by radiography",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 838613,
"choiceText": "CT of the fractured bone",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 838615,
"choiceText": "Octreotide scan",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 838617,
"choiceText": "T2-weighted STIR MRI",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 838619,
"choiceText": "T1-weighted STIR MRI",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "T2-weighted STIR MRI is recommended as the first step in locating the tissues. Any abnormal areas should be scanned in more detail to find the causative tumor. Once identified, the tumor must be removed. The serum FGF-23 level and serum phosphate concentration should normalize postoperatively; if they do not normalize, further exploration for the causative tumor is indicated. ",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 263699,
"questionText": "You diagnose the patient you are examining with osteomalacia and suspect that the disease may be oncogenic in nature. Which of the following methods is considered the best for finding the tumor responsible for oncogenic osteomalacia?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Femoral Neck Fracture in a 79-Year-Old Woman"
},
{
"authors": "Helen Kenealy, BHB, MBchB",
"content": [],
"date": "April 30, 2015",
"figures": [],
"markdown": "# Femoral Neck Fracture in a 79-Year-Old Woman\n\n **Authors:** Helen Kenealy, BHB, MBchB \n **Date:** April 30, 2015\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 5 of 5*",
"pagination": {
"current_page": 5,
"total_pages": 5
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 838601,
"choiceText": "Raised serum phosphate, lowered urinary phosphate/creatinine ratio, normal serum calcium",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 838603,
"choiceText": "Low serum phosphate, low serum calcium, raised urinary phosphate/creatinine ratio",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 838605,
"choiceText": "Low serum phosphate, raised serum alkaline phosphatase, low serum calcium",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 838607,
"choiceText": "Low serum phosphate, raised urinary phosphate/creatinine ratio, normal serum calcium",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 838609,
"choiceText": "None of the above",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Oncogenic or tumor-induced osteomalacia, an acquired renal phosphate wasting syndrome, is caused by an increased serum FGF-23 level. The pathognomonic signs of this condition include adult-onset osteomalacia with a low serum phosphate concentration, a normal serum calcium level, and renal phosphate wasting. The calcitriol level are low as a result of the weak inhibitory effect of FGF-23 on the renal 1-alpha-hydroxylase enzyme; however, calcitriol need not be measured to establish the diagnosis. The serum parathyroid hormone is mildly elevated as a result of a mild decrease in active vitamin D.<br>\r\n<br>\r\nThe mechanism of bone loss is incompletely understood; it is caused in part by disturbed phosphate metabolism and, to a lesser degree, abnormal vitamin D metabolism.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 263697,
"questionText": "If a patient presented with one of the following biochemical abnormalities, which set of findings would be most likely to lead to a suspicion of oncogenic osteomalacia?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 838611,
"choiceText": "Full skeletal survey by radiography",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 838613,
"choiceText": "CT of the fractured bone",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 838615,
"choiceText": "Octreotide scan",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 838617,
"choiceText": "T2-weighted STIR MRI",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 838619,
"choiceText": "T1-weighted STIR MRI",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "T2-weighted STIR MRI is recommended as the first step in locating the tissues. Any abnormal areas should be scanned in more detail to find the causative tumor. Once identified, the tumor must be removed. The serum FGF-23 level and serum phosphate concentration should normalize postoperatively; if they do not normalize, further exploration for the causative tumor is indicated. ",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 263699,
"questionText": "You diagnose the patient you are examining with osteomalacia and suspect that the disease may be oncogenic in nature. Which of the following methods is considered the best for finding the tumor responsible for oncogenic osteomalacia?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Femoral Neck Fracture in a 79-Year-Old Woman"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 838593,
"choiceText": "Vitamin D deficiency",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 838595,
"choiceText": "Oncogenic osteomalacia",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 838597,
"choiceText": "Fanconi syndrome",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 838599,
"choiceText": "Secondary hyperparathyroidism",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 263695,
"questionText": "On the basis of the history, physical examination, and work-up, what is the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 838601,
"choiceText": "Raised serum phosphate, lowered urinary phosphate/creatinine ratio, normal serum calcium",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 838603,
"choiceText": "Low serum phosphate, low serum calcium, raised urinary phosphate/creatinine ratio",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 838605,
"choiceText": "Low serum phosphate, raised serum alkaline phosphatase, low serum calcium",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 838607,
"choiceText": "Low serum phosphate, raised urinary phosphate/creatinine ratio, normal serum calcium",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 838609,
"choiceText": "None of the above",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Oncogenic or tumor-induced osteomalacia, an acquired renal phosphate wasting syndrome, is caused by an increased serum FGF-23 level. The pathognomonic signs of this condition include adult-onset osteomalacia with a low serum phosphate concentration, a normal serum calcium level, and renal phosphate wasting. The calcitriol level are low as a result of the weak inhibitory effect of FGF-23 on the renal 1-alpha-hydroxylase enzyme; however, calcitriol need not be measured to establish the diagnosis. The serum parathyroid hormone is mildly elevated as a result of a mild decrease in active vitamin D.<br>\r\n<br>\r\nThe mechanism of bone loss is incompletely understood; it is caused in part by disturbed phosphate metabolism and, to a lesser degree, abnormal vitamin D metabolism.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 263697,
"questionText": "If a patient presented with one of the following biochemical abnormalities, which set of findings would be most likely to lead to a suspicion of oncogenic osteomalacia?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 838611,
"choiceText": "Full skeletal survey by radiography",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 838613,
"choiceText": "CT of the fractured bone",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 838615,
"choiceText": "Octreotide scan",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 838617,
"choiceText": "T2-weighted STIR MRI",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 838619,
"choiceText": "T1-weighted STIR MRI",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "T2-weighted STIR MRI is recommended as the first step in locating the tissues. Any abnormal areas should be scanned in more detail to find the causative tumor. Once identified, the tumor must be removed. The serum FGF-23 level and serum phosphate concentration should normalize postoperatively; if they do not normalize, further exploration for the causative tumor is indicated. ",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 263699,
"questionText": "You diagnose the patient you are examining with osteomalacia and suspect that the disease may be oncogenic in nature. Which of the following methods is considered the best for finding the tumor responsible for oncogenic osteomalacia?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
842828 | /viewarticle/842828 | [
{
"authors": "Zain Ul Abideen Asad, MD, MBBS",
"content": [
"Editor's Note: \nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"In response to user comments, this case was updated with additional information to clarify the diagnostic approach.",
"Background",
"A 35-year-old-woman presents with a 10-month history of fatigue that has progressively worsened to the point that it is limiting her ability to work as a waitress. It is accompanied by muscle stiffness, joint pain, recurrent headaches, and an inability to concentrate. Adequate rest and over-the-counter analgesics have failed to relieve her symptoms, and she feels tired all the time. She snores at night and has difficulty staying asleep.",
"She has no history of tick bite or skin rash, and the region has low incidence of tick-borne illnesses. She reports no history of alcohol or drug abuse but did have multiple unprotected sexual encounters in the past 5 years. She denies any suicidal or homicidal ideation. She has visited multiple physicians in the last few months with the same symptoms and is not satisfied with the work-up done so far. She is stressed by her symptoms and concerned that she might lose her job. Her medical history is significant for hyperlipidemia, and she has taken atorvastatin (40 mg daily) for the past year."
],
"date": "May 09, 2017",
"figures": [],
"markdown": "# A 35-Year-Old Woman With Fatigue and Joint Pain\n\n **Authors:** Zain Ul Abideen Asad, MD, MBBS \n **Date:** May 09, 2017\n\n ## Content\n\n Editor's Note: \nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nIn response to user comments, this case was updated with additional information to clarify the diagnostic approach.\nBackground\nA 35-year-old-woman presents with a 10-month history of fatigue that has progressively worsened to the point that it is limiting her ability to work as a waitress. It is accompanied by muscle stiffness, joint pain, recurrent headaches, and an inability to concentrate. Adequate rest and over-the-counter analgesics have failed to relieve her symptoms, and she feels tired all the time. She snores at night and has difficulty staying asleep.\nShe has no history of tick bite or skin rash, and the region has low incidence of tick-borne illnesses. She reports no history of alcohol or drug abuse but did have multiple unprotected sexual encounters in the past 5 years. She denies any suicidal or homicidal ideation. She has visited multiple physicians in the last few months with the same symptoms and is not satisfied with the work-up done so far. She is stressed by her symptoms and concerned that she might lose her job. Her medical history is significant for hyperlipidemia, and she has taken atorvastatin (40 mg daily) for the past year.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 35-Year-Old Woman With Fatigue and Joint Pain"
},
{
"authors": "Zain Ul Abideen Asad, MD, MBBS",
"content": [
"Physical Examination and Work-up",
"The patient has a body mass index of 33 kg/m2 (Figure 1). Her vital signs are within normal limits. No edema, hair loss, or skin changes are noted. She has pain on passive movements of joints but no deformities, swelling, or erythema. Her neck circumference is 16 inches, without evidence of thyroid enlargement or jugular venous distention. No vision changes are reported. All cranial nerves are grossly intact, and reflexes are 2+ throughout. Muscle strength is 4/5 in the upper and lower extremities. Her gait and range of motion for all extremities is normal.",
"Figure 1.",
"The patient's complete blood count (CBC), basic metabolic panel, and urinalysis are unremarkable. Other findings are as follows:",
"Erythrocyte sedimentation rate (ESR): 15 mm/h (normal range, 0-20 mm/h);",
"C-reactive protein (CRP) - 0.6 mg/dL (normal range, 0.1-1 mg/dL);",
"Thyroid-stimulating hormone level: 4 µU/mL (normal range, 0.5-5.0 µU/mL);",
"Free T4: Within the normal range;",
"Creatine kinase level: within the normal range; and",
"Antinuclear antibody (ANA) titer: 1:160 (low-positive range, 1:40-1:60).",
"Although statin myopathy was considered, it typically presents as proximal muscle weakness. The patient here described muscle stiffness, and the creatine kinase level was normal. The patient is up to date on immunizations and Pap smear. Enzyme-linked immunoassays for HIV and Lyme disease and tests for viral hepatitis are negative. Sleep disorders are ruled out because they do not typically cause joint pain."
],
"date": "May 09, 2017",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/842/828/842828-thumb-1.jpg"
}
],
"markdown": "# A 35-Year-Old Woman With Fatigue and Joint Pain\n\n **Authors:** Zain Ul Abideen Asad, MD, MBBS \n **Date:** May 09, 2017\n\n ## Content\n\n Physical Examination and Work-up\nThe patient has a body mass index of 33 kg/m2 (Figure 1). Her vital signs are within normal limits. No edema, hair loss, or skin changes are noted. She has pain on passive movements of joints but no deformities, swelling, or erythema. Her neck circumference is 16 inches, without evidence of thyroid enlargement or jugular venous distention. No vision changes are reported. All cranial nerves are grossly intact, and reflexes are 2+ throughout. Muscle strength is 4/5 in the upper and lower extremities. Her gait and range of motion for all extremities is normal.\nFigure 1.\nThe patient's complete blood count (CBC), basic metabolic panel, and urinalysis are unremarkable. Other findings are as follows:\nErythrocyte sedimentation rate (ESR): 15 mm/h (normal range, 0-20 mm/h);\nC-reactive protein (CRP) - 0.6 mg/dL (normal range, 0.1-1 mg/dL);\nThyroid-stimulating hormone level: 4 µU/mL (normal range, 0.5-5.0 µU/mL);\nFree T4: Within the normal range;\nCreatine kinase level: within the normal range; and\nAntinuclear antibody (ANA) titer: 1:160 (low-positive range, 1:40-1:60).\nAlthough statin myopathy was considered, it typically presents as proximal muscle weakness. The patient here described muscle stiffness, and the creatine kinase level was normal. The patient is up to date on immunizations and Pap smear. Enzyme-linked immunoassays for HIV and Lyme disease and tests for viral hepatitis are negative. Sleep disorders are ruled out because they do not typically cause joint pain.\n\n ## Figures\n\n **Figure 1.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 833097,
"choiceText": "Depression",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 833099,
"choiceText": "Somatoform disorder",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 833101,
"choiceText": "Hypothyroidism",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 833103,
"choiceText": "Obstructive sleep apnea",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 833105,
"choiceText": "Chronic fatigue syndrome",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 833107,
"choiceText": "Fibromyalgia",
"correct": false,
"displayOrder": 6,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 262025,
"questionText": "On the basis of the history, physical examination, and work-up, what is the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 35-Year-Old Woman With Fatigue and Joint Pain"
},
{
"authors": "Zain Ul Abideen Asad, MD, MBBS",
"content": [
"Discussion",
"This patient's predominant symptom is fatigue that is limiting her social and occupational life. It is accompanied by a number of other symptoms, including recurrent headaches, inability to concentrate, muscle stiffness, and joint pain. This combination is hard to explain with a single diagnosis.",
"Many physicians have investigated her condition but have failed to make a definitive diagnosis. Chronic medical conditions, including hypothyroidism, HIV, and viral hepatitis were ruled out by diagnostic tests.",
"Figure 2.",
"The difference between chronic fatigue syndrome and fibromyalgia is the intensity of fatigue and pain. Chronic fatigue syndrome involves severe fatigue and mild pain; fibromyalgia involves severe pain and mild fatigue. Also, fibromyalgia is accompanied by tenderness in multiple areas of the body (Figure 2).",
"Chronic fatigue syndrome is a diagnosis of exclusion. Fatigue is one of the most common presenting symptoms among patients and is often due to an existing organic illness; however, if results of the physical examination and diagnostic tests are normal, chronic fatigue syndrome should be considered.",
"According to the Centers for Disease Control and Prevention's 1994 case definition, chronic fatigue syndrome is defined as fatigue that lasts for long than 6 months that is not explained by an existing medical condition. This fatigue must affect activities of living or work and be accompanied by at least four out of the following eight symptoms:",
"Muscle pain;",
"Joint pain without swelling or erythema;",
"Headache of new type or severity;",
"Frequent or recurring sore throat;",
"Painful lymphadenopathy of cervical or axillary region;",
"Postexertional malaise lasting more than 1 day;",
"Impairment of concentration or short-term memory; and",
"Unrefreshing sleep.",
"In 2015, the Institute of Medicine renamed this condition \"systemic exertion intolerance disease\" (SEID).[1] Diagnosis requires the presence of three major symptoms, as follows:",
"A substantial reduction or impairment in the ability to engage in preillness levels of occupational, educational, social, or personal activities, that persists for more than 6 months and is accompanied by fatigue, which is often profound, is of new or definite onset (not lifelong), is not the result of ongoing excessive exertion, and is not substantially alleviated by rest;",
"Postexertion malaise; and",
"Unrefreshing sleep.",
"Diagnosis also requires at least one of the two following manifestations:",
"Cognitive impairment or",
"Orthostatic intolerance",
"Prevalence estimates for chronic fatigue syndrome vary widely, from 0.23% to 2.6% in some studies.[2,3] It is more than four times more common in women (373 per 100,000) than men (83 per 100,000).[2] The discrepancy between different prevalence estimates is due to lack of a consensus on the definition, pathogenesis, and symptoms; the mainly subjective nature of the diagnostic criteria; and variation in the types of questionnaires used."
],
"date": "May 09, 2017",
"figures": [
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/842/828/842828-thumb-2.jpg"
}
],
"markdown": "# A 35-Year-Old Woman With Fatigue and Joint Pain\n\n **Authors:** Zain Ul Abideen Asad, MD, MBBS \n **Date:** May 09, 2017\n\n ## Content\n\n Discussion\nThis patient's predominant symptom is fatigue that is limiting her social and occupational life. It is accompanied by a number of other symptoms, including recurrent headaches, inability to concentrate, muscle stiffness, and joint pain. This combination is hard to explain with a single diagnosis.\nMany physicians have investigated her condition but have failed to make a definitive diagnosis. Chronic medical conditions, including hypothyroidism, HIV, and viral hepatitis were ruled out by diagnostic tests.\nFigure 2.\nThe difference between chronic fatigue syndrome and fibromyalgia is the intensity of fatigue and pain. Chronic fatigue syndrome involves severe fatigue and mild pain; fibromyalgia involves severe pain and mild fatigue. Also, fibromyalgia is accompanied by tenderness in multiple areas of the body (Figure 2).\nChronic fatigue syndrome is a diagnosis of exclusion. Fatigue is one of the most common presenting symptoms among patients and is often due to an existing organic illness; however, if results of the physical examination and diagnostic tests are normal, chronic fatigue syndrome should be considered.\nAccording to the Centers for Disease Control and Prevention's 1994 case definition, chronic fatigue syndrome is defined as fatigue that lasts for long than 6 months that is not explained by an existing medical condition. This fatigue must affect activities of living or work and be accompanied by at least four out of the following eight symptoms:\nMuscle pain;\nJoint pain without swelling or erythema;\nHeadache of new type or severity;\nFrequent or recurring sore throat;\nPainful lymphadenopathy of cervical or axillary region;\nPostexertional malaise lasting more than 1 day;\nImpairment of concentration or short-term memory; and\nUnrefreshing sleep.\nIn 2015, the Institute of Medicine renamed this condition \"systemic exertion intolerance disease\" (SEID).[1] Diagnosis requires the presence of three major symptoms, as follows:\nA substantial reduction or impairment in the ability to engage in preillness levels of occupational, educational, social, or personal activities, that persists for more than 6 months and is accompanied by fatigue, which is often profound, is of new or definite onset (not lifelong), is not the result of ongoing excessive exertion, and is not substantially alleviated by rest;\nPostexertion malaise; and\nUnrefreshing sleep.\nDiagnosis also requires at least one of the two following manifestations:\nCognitive impairment or\nOrthostatic intolerance\nPrevalence estimates for chronic fatigue syndrome vary widely, from 0.23% to 2.6% in some studies.[2,3] It is more than four times more common in women (373 per 100,000) than men (83 per 100,000).[2] The discrepancy between different prevalence estimates is due to lack of a consensus on the definition, pathogenesis, and symptoms; the mainly subjective nature of the diagnostic criteria; and variation in the types of questionnaires used.\n\n ## Figures\n\n **Figure 2.** \n \n\n\n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 833097,
"choiceText": "Depression",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 833099,
"choiceText": "Somatoform disorder",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 833101,
"choiceText": "Hypothyroidism",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 833103,
"choiceText": "Obstructive sleep apnea",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 833105,
"choiceText": "Chronic fatigue syndrome",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 833107,
"choiceText": "Fibromyalgia",
"correct": false,
"displayOrder": 6,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 262025,
"questionText": "On the basis of the history, physical examination, and work-up, what is the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 35-Year-Old Woman With Fatigue and Joint Pain"
},
{
"authors": "Zain Ul Abideen Asad, MD, MBBS",
"content": [
"Typical age of onset of chronic fatigue syndrome is 29-35 years, and the usual trigger is a stressor; these can include infection, such as flulike illness or infectious mononucleosis; surgery; pregnancy; or psychological stress. However, the condition is largely multifactorial in origin.[4] Other uncommon symptoms include nausea, jaw pain, abdominal pain, alcohol intolerance, chest pain, shortness of breath, morning stiffness, irritability, anxiety, and weight loss.",
"The etiology of chronic fatigue syndrome is unknown, but many explanations have been speculated, including infectious, autonomic, immunologic, allergic, and psychiatric origins. Epstein-Barr virus was thought to be causal because symptoms overlap with those of infectious mononucleosis; however, further studies have proved otherwise.[5] Other viruses that have been implicated include cytomegalovirus, measles, human herpesvirus 6, human T-cell lymphotropic virus, and coxsackievirus. No conclusive data supports a causal relationship.",
"Inconsistent studies show increased lymphocyte markers, decreased natural killer cell activity, immune deregulation, and increased interferon activity, and a viral infection has been hypothesized as a trigger to a cascade of immune abnormalities that ultimately cause chronic fatigue. Because the syndrome is characterized by cognitive defects, some studies indicate that it might be mediated by the central nervous system. Cerebral white-matter lesions in frontal lobes have been identified using MRI and regional cerebral blood flow abnormalities as compared with controls.[6]",
"Because of a lack of objectivity in the diagnosis, many clinicians disregard chronic fatigue symptoms as a manifestation of clinical depression. Although depression does overlap, some symptoms not typical of depression are also present: for example, lymphadenopathy, sore throat, and postexertional malaise. In addition, anhedonia and guilt are not seen in patients with chronic fatigue syndrome and are commonly seen in depression.",
"Patients with chronic fatigue syndrome have a higher incidence of atopic disease, elevated levels of eosinophilic cationic proteins, and positive skin tests to allergens, which suggests a possible role of allergens in the pathogenesis.",
"Diagnostic testing includes a CBC; complete metabolic profile; blood glucose, ESR, and CRP measurement; and ANA titer to rule out common conditions, such as anemia, diabetes, kidney disease, and autoimmune disease. Because the main purpose of diagnostic testing is to rule out other causes of chronic fatigue syndrome, the work-up includes tests for endocrine disorders (hypothyroidism, adrenal insufficiency), rheumatologic disorders (systemic lupus erythematosus, rheumatoid arthritis, fibromyalgia), infectious causes (hepatitis, AIDS, tuberculosis) and cancer (lymphoma, leukemia).",
"Polysomnography for sleep disorders and tests for assessment of cognition should be considered. Decreased cortisol levels after exercise, erratic breathing pattern, measurement of maximal oxygen consumption in cardiopulmonary exercise testing, low natural killer cell counts, immunoglobulin deficiency, and elevated interferon alpha levels have been implicated in patients with chronic fatigue syndrome.",
"Possible complications of chronic fatigue syndrome include lifestyle restriction, limitation of physical activity, social isolation, poor job performance, anxiety, and depression."
],
"date": "May 09, 2017",
"figures": [],
"markdown": "# A 35-Year-Old Woman With Fatigue and Joint Pain\n\n **Authors:** Zain Ul Abideen Asad, MD, MBBS \n **Date:** May 09, 2017\n\n ## Content\n\n Typical age of onset of chronic fatigue syndrome is 29-35 years, and the usual trigger is a stressor; these can include infection, such as flulike illness or infectious mononucleosis; surgery; pregnancy; or psychological stress. However, the condition is largely multifactorial in origin.[4] Other uncommon symptoms include nausea, jaw pain, abdominal pain, alcohol intolerance, chest pain, shortness of breath, morning stiffness, irritability, anxiety, and weight loss.\nThe etiology of chronic fatigue syndrome is unknown, but many explanations have been speculated, including infectious, autonomic, immunologic, allergic, and psychiatric origins. Epstein-Barr virus was thought to be causal because symptoms overlap with those of infectious mononucleosis; however, further studies have proved otherwise.[5] Other viruses that have been implicated include cytomegalovirus, measles, human herpesvirus 6, human T-cell lymphotropic virus, and coxsackievirus. No conclusive data supports a causal relationship.\nInconsistent studies show increased lymphocyte markers, decreased natural killer cell activity, immune deregulation, and increased interferon activity, and a viral infection has been hypothesized as a trigger to a cascade of immune abnormalities that ultimately cause chronic fatigue. Because the syndrome is characterized by cognitive defects, some studies indicate that it might be mediated by the central nervous system. Cerebral white-matter lesions in frontal lobes have been identified using MRI and regional cerebral blood flow abnormalities as compared with controls.[6]\nBecause of a lack of objectivity in the diagnosis, many clinicians disregard chronic fatigue symptoms as a manifestation of clinical depression. Although depression does overlap, some symptoms not typical of depression are also present: for example, lymphadenopathy, sore throat, and postexertional malaise. In addition, anhedonia and guilt are not seen in patients with chronic fatigue syndrome and are commonly seen in depression.\nPatients with chronic fatigue syndrome have a higher incidence of atopic disease, elevated levels of eosinophilic cationic proteins, and positive skin tests to allergens, which suggests a possible role of allergens in the pathogenesis.\nDiagnostic testing includes a CBC; complete metabolic profile; blood glucose, ESR, and CRP measurement; and ANA titer to rule out common conditions, such as anemia, diabetes, kidney disease, and autoimmune disease. Because the main purpose of diagnostic testing is to rule out other causes of chronic fatigue syndrome, the work-up includes tests for endocrine disorders (hypothyroidism, adrenal insufficiency), rheumatologic disorders (systemic lupus erythematosus, rheumatoid arthritis, fibromyalgia), infectious causes (hepatitis, AIDS, tuberculosis) and cancer (lymphoma, leukemia).\nPolysomnography for sleep disorders and tests for assessment of cognition should be considered. Decreased cortisol levels after exercise, erratic breathing pattern, measurement of maximal oxygen consumption in cardiopulmonary exercise testing, low natural killer cell counts, immunoglobulin deficiency, and elevated interferon alpha levels have been implicated in patients with chronic fatigue syndrome.\nPossible complications of chronic fatigue syndrome include lifestyle restriction, limitation of physical activity, social isolation, poor job performance, anxiety, and depression.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 35-Year-Old Woman With Fatigue and Joint Pain"
},
{
"authors": "Zain Ul Abideen Asad, MD, MBBS",
"content": [
"Treatment options include cognitive-behavioral therapy, graded exercise therapy, corticosteroids, antidepressants, dietary supplements, oral nicotinamide adenine dinucleotide (NADH), and immunotherapy. All of these focus on symptom control because no cure has been identified. Usually, multiple modalities are tried, with limited success.",
"Cognitive-behavioral therapy and graded exercise therapy are the only interventions found to be beneficial.[7,8] Cognitive-behavioral therapy involves the patient developing a greater understanding of the necessary lifestyle changes, motivation, identification of and change in fatigue-related cognitive problems, and careful planning of daily activities, in an attempt to enable the patient to acquire gradual control over symptoms.",
"Similarly, graded exercise therapy involves a balance between activity and rest. It aims at starting physical activity slowly and gradually increasing it while being careful to avoid extremes. The duration is usually a trial-and-error approach; the goal is to stop before extreme fatigue. Although both cognitive-behavioral therapy and graded exercise therapy have been studied in randomized controlled trials and systematic reviews reinforce their benefit, many doubts surround these interventions. Prolonged rest is not indicated; it has shown no benefits and may worsen fatigue and other symptoms.",
"Drug therapy is usually targeted to address pain and sleep disturbances. Three randomized controlled trials found that the benefits of corticosteroids were short-lived.[9,10,11] Randomized controlled trials failed to show evidence for benefit of antidepressants in chronic fatigue syndrome. Fluoxetine, 20 mg daily, did not improve depression or insomnia in chronic fatigue syndrome.[12] Limited benefit and significant side effects were observed in major randomized control trials that explored the use of immunotherapy (immunoglobulin G).[13,14,15]",
"Of note, interferon-2a was found to improve quality of life for a subgroup of patients with chronic fatigue syndrome who had baseline decreased natural killer cell function.[16] Oral NADH showed limited benefit in a single randomized controlled trial with a small sample; the rationale was that it facilitates generation of adenosine triphosphate, which might be depleted in patients with chronic fatigue syndrome.[17] Narcotics are not indicated for chronic fatigue syndrome associated pain; other choices should be limited to acetaminophen, aspirin, or nonsteroidal anti-inflammatory drugs.",
"This patient was followed up at 3-month intervals for 1 year. Her symptoms were under good control with cognitive-behavioral therapy, and she managed to adapt to a new lifestyle and recognize her activity limitations."
],
"date": "May 09, 2017",
"figures": [],
"markdown": "# A 35-Year-Old Woman With Fatigue and Joint Pain\n\n **Authors:** Zain Ul Abideen Asad, MD, MBBS \n **Date:** May 09, 2017\n\n ## Content\n\n Treatment options include cognitive-behavioral therapy, graded exercise therapy, corticosteroids, antidepressants, dietary supplements, oral nicotinamide adenine dinucleotide (NADH), and immunotherapy. All of these focus on symptom control because no cure has been identified. Usually, multiple modalities are tried, with limited success.\nCognitive-behavioral therapy and graded exercise therapy are the only interventions found to be beneficial.[7,8] Cognitive-behavioral therapy involves the patient developing a greater understanding of the necessary lifestyle changes, motivation, identification of and change in fatigue-related cognitive problems, and careful planning of daily activities, in an attempt to enable the patient to acquire gradual control over symptoms.\nSimilarly, graded exercise therapy involves a balance between activity and rest. It aims at starting physical activity slowly and gradually increasing it while being careful to avoid extremes. The duration is usually a trial-and-error approach; the goal is to stop before extreme fatigue. Although both cognitive-behavioral therapy and graded exercise therapy have been studied in randomized controlled trials and systematic reviews reinforce their benefit, many doubts surround these interventions. Prolonged rest is not indicated; it has shown no benefits and may worsen fatigue and other symptoms.\nDrug therapy is usually targeted to address pain and sleep disturbances. Three randomized controlled trials found that the benefits of corticosteroids were short-lived.[9,10,11] Randomized controlled trials failed to show evidence for benefit of antidepressants in chronic fatigue syndrome. Fluoxetine, 20 mg daily, did not improve depression or insomnia in chronic fatigue syndrome.[12] Limited benefit and significant side effects were observed in major randomized control trials that explored the use of immunotherapy (immunoglobulin G).[13,14,15]\nOf note, interferon-2a was found to improve quality of life for a subgroup of patients with chronic fatigue syndrome who had baseline decreased natural killer cell function.[16] Oral NADH showed limited benefit in a single randomized controlled trial with a small sample; the rationale was that it facilitates generation of adenosine triphosphate, which might be depleted in patients with chronic fatigue syndrome.[17] Narcotics are not indicated for chronic fatigue syndrome associated pain; other choices should be limited to acetaminophen, aspirin, or nonsteroidal anti-inflammatory drugs.\nThis patient was followed up at 3-month intervals for 1 year. Her symptoms were under good control with cognitive-behavioral therapy, and she managed to adapt to a new lifestyle and recognize her activity limitations.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 833109,
"choiceText": "Corticosteroids",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 833111,
"choiceText": "Interferon 2a in patients with normal natural killer cell activity",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 833113,
"choiceText": "Cognitive-behavioral therapy in already depressed patients",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 833115,
"choiceText": "Antihistamines",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 833117,
"choiceText": "Prolonged rest",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Prolonged rest surprisingly showed no benefit, and indirect evidence suggested that it worsens fatigue and other symptoms.<sup>18</sup> This was demonstrated for people who developed chronic fatigue syndrome after infectious mononucleosis. Physical inactivity leads to deconditioning, which aggravates symptoms and is accompanied by low energy and mood.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 262027,
"questionText": "Which of the following treatment modalities for chronic fatigue syndrome showed no benefit and indirect evidence of harm?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 833119,
"choiceText": "Polymyalgia rheumatica",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 833121,
"choiceText": "Ulcerative colitis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 833123,
"choiceText": "Irritable bowel syndrome",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 833125,
"choiceText": "Major depression",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Studies have shown that two thirds of patients with chronic fatigue syndrome have signs of major depressive illness. One half of all patients with chronic fatigue syndrome have experienced at least one episode of major depression.<sup>19,20</sup>",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 262029,
"questionText": "The incidence of which of the following conditions is considerably higher in patients with chronic fatigue syndrome compared with the general population?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 35-Year-Old Woman With Fatigue and Joint Pain"
},
{
"authors": "Zain Ul Abideen Asad, MD, MBBS",
"content": [],
"date": "May 09, 2017",
"figures": [],
"markdown": "# A 35-Year-Old Woman With Fatigue and Joint Pain\n\n **Authors:** Zain Ul Abideen Asad, MD, MBBS \n **Date:** May 09, 2017\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 833109,
"choiceText": "Corticosteroids",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 833111,
"choiceText": "Interferon 2a in patients with normal natural killer cell activity",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 833113,
"choiceText": "Cognitive-behavioral therapy in already depressed patients",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 833115,
"choiceText": "Antihistamines",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 833117,
"choiceText": "Prolonged rest",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Prolonged rest surprisingly showed no benefit, and indirect evidence suggested that it worsens fatigue and other symptoms.<sup>18</sup> This was demonstrated for people who developed chronic fatigue syndrome after infectious mononucleosis. Physical inactivity leads to deconditioning, which aggravates symptoms and is accompanied by low energy and mood.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 262027,
"questionText": "Which of the following treatment modalities for chronic fatigue syndrome showed no benefit and indirect evidence of harm?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 833119,
"choiceText": "Polymyalgia rheumatica",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 833121,
"choiceText": "Ulcerative colitis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 833123,
"choiceText": "Irritable bowel syndrome",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 833125,
"choiceText": "Major depression",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Studies have shown that two thirds of patients with chronic fatigue syndrome have signs of major depressive illness. One half of all patients with chronic fatigue syndrome have experienced at least one episode of major depression.<sup>19,20</sup>",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 262029,
"questionText": "The incidence of which of the following conditions is considerably higher in patients with chronic fatigue syndrome compared with the general population?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 35-Year-Old Woman With Fatigue and Joint Pain"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 833097,
"choiceText": "Depression",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 833099,
"choiceText": "Somatoform disorder",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 833101,
"choiceText": "Hypothyroidism",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 833103,
"choiceText": "Obstructive sleep apnea",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 833105,
"choiceText": "Chronic fatigue syndrome",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 833107,
"choiceText": "Fibromyalgia",
"correct": false,
"displayOrder": 6,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 262025,
"questionText": "On the basis of the history, physical examination, and work-up, what is the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 833109,
"choiceText": "Corticosteroids",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 833111,
"choiceText": "Interferon 2a in patients with normal natural killer cell activity",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 833113,
"choiceText": "Cognitive-behavioral therapy in already depressed patients",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 833115,
"choiceText": "Antihistamines",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 833117,
"choiceText": "Prolonged rest",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Prolonged rest surprisingly showed no benefit, and indirect evidence suggested that it worsens fatigue and other symptoms.<sup>18</sup> This was demonstrated for people who developed chronic fatigue syndrome after infectious mononucleosis. Physical inactivity leads to deconditioning, which aggravates symptoms and is accompanied by low energy and mood.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 262027,
"questionText": "Which of the following treatment modalities for chronic fatigue syndrome showed no benefit and indirect evidence of harm?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 833119,
"choiceText": "Polymyalgia rheumatica",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 833121,
"choiceText": "Ulcerative colitis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 833123,
"choiceText": "Irritable bowel syndrome",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 833125,
"choiceText": "Major depression",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Studies have shown that two thirds of patients with chronic fatigue syndrome have signs of major depressive illness. One half of all patients with chronic fatigue syndrome have experienced at least one episode of major depression.<sup>19,20</sup>",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 262029,
"questionText": "The incidence of which of the following conditions is considerably higher in patients with chronic fatigue syndrome compared with the general population?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
842600 | /viewarticle/842600 | [
{
"authors": "Gideon Lorber, MD; Ofer Nathan Gofrit, MD, PhD",
"content": [
"Editor's Note: \nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.",
"Background",
"A 60-year-old woman is referred to the emergency department because of a recent event of painless macroscopic hematuria. She reports having experienced several similar episodes during the past year, all of which spontaneously resolved. She regards these episodes as being of gynecologic origin because she is 5 years postmenopausal.",
"She describes a general feeling of malaise in the days preceding the current episode but denies having any fever, dysuria, or increased frequency or urgency of urination. The patient also describes an unintentional weight loss of 11 lb (5 kg) during the past 2 years.",
"The patient's medical history includes hypothyroidism that was treated medically with thyroxine. Her surgical history includes two treatments of dilatation and curettage and a tonsillectomy.",
"She has no known drug or food allergies, and she denies smoking, drug use, or alcohol consumption. She has no history of kidney stones or recurrent urinary tract infections."
],
"date": "August 18, 2016",
"figures": [],
"markdown": "# Painless Bloody Urination in a 60-Year-Old Woman\n\n **Authors:** Gideon Lorber, MD; Ofer Nathan Gofrit, MD, PhD \n **Date:** August 18, 2016\n\n ## Content\n\n Editor's Note: \nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.\nBackground\nA 60-year-old woman is referred to the emergency department because of a recent event of painless macroscopic hematuria. She reports having experienced several similar episodes during the past year, all of which spontaneously resolved. She regards these episodes as being of gynecologic origin because she is 5 years postmenopausal.\nShe describes a general feeling of malaise in the days preceding the current episode but denies having any fever, dysuria, or increased frequency or urgency of urination. The patient also describes an unintentional weight loss of 11 lb (5 kg) during the past 2 years.\nThe patient's medical history includes hypothyroidism that was treated medically with thyroxine. Her surgical history includes two treatments of dilatation and curettage and a tonsillectomy.\nShe has no known drug or food allergies, and she denies smoking, drug use, or alcohol consumption. She has no history of kidney stones or recurrent urinary tract infections.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "Painless Bloody Urination in a 60-Year-Old Woman"
},
{
"authors": "Gideon Lorber, MD; Ofer Nathan Gofrit, MD, PhD",
"content": [
"Physical Examination and Work-up",
"On physical examination, the patient appears well. She has a temperature of 98.8°F, a pulse rate of 71 beats/min, and a blood pressure of 150/86 mm Hg.",
"Head and neck examination findings are normal. Lung auscultation reveals normal breath sounds bilaterally, without wheezing or crackles. The patient's heart sounds are regular, with a 2/6 systolic murmur maximally auscultated over the right second intercostal space. The abdomen is nondistended and nontender, no masses are palpated, and no signs of peritoneal irritation are present. No peripheral edema is noticed, peripheral pulses are palpated, and the neurologic examination is normal. A gynecologic evaluation that includes a speculum examination and transvaginal ultrasonography is performed, which reveals no pathologic findings.",
"Figure 1.",
"A laboratory analysis, including a complete blood cell count, coagulation studies, and a basic metabolic panel, shows a normal hemoglobin level, normal platelet count, and no coagulopathy. No electrolyte abnormalities are present. A urine dipstick test shows no signs of hematuria, and a urine culture is negative.",
"Urine cytology is positive for malignant cells. Cystoscopy is performed, which demonstrates a normal urethra, leading to a urinary bladder covered by normal mucosa, with no exophytic lesions and no active bleeding. CT of the abdomen and pelvis with intravenous contrast is obtained (Figure 1)."
],
"date": "August 18, 2016",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/842/600/842600-thumb-1.jpg"
}
],
"markdown": "# Painless Bloody Urination in a 60-Year-Old Woman\n\n **Authors:** Gideon Lorber, MD; Ofer Nathan Gofrit, MD, PhD \n **Date:** August 18, 2016\n\n ## Content\n\n Physical Examination and Work-up\nOn physical examination, the patient appears well. She has a temperature of 98.8°F, a pulse rate of 71 beats/min, and a blood pressure of 150/86 mm Hg.\nHead and neck examination findings are normal. Lung auscultation reveals normal breath sounds bilaterally, without wheezing or crackles. The patient's heart sounds are regular, with a 2/6 systolic murmur maximally auscultated over the right second intercostal space. The abdomen is nondistended and nontender, no masses are palpated, and no signs of peritoneal irritation are present. No peripheral edema is noticed, peripheral pulses are palpated, and the neurologic examination is normal. A gynecologic evaluation that includes a speculum examination and transvaginal ultrasonography is performed, which reveals no pathologic findings.\nFigure 1.\nA laboratory analysis, including a complete blood cell count, coagulation studies, and a basic metabolic panel, shows a normal hemoglobin level, normal platelet count, and no coagulopathy. No electrolyte abnormalities are present. A urine dipstick test shows no signs of hematuria, and a urine culture is negative.\nUrine cytology is positive for malignant cells. Cystoscopy is performed, which demonstrates a normal urethra, leading to a urinary bladder covered by normal mucosa, with no exophytic lesions and no active bleeding. CT of the abdomen and pelvis with intravenous contrast is obtained (Figure 1).\n\n ## Figures\n\n **Figure 1.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 833021,
"choiceText": "Renal stone",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 833023,
"choiceText": "Urothelial carcinoma",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 833025,
"choiceText": "External renal compression",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 833027,
"choiceText": "Complicated renal cyst",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 261991,
"questionText": "On the basis of the history, physical examination, and work-up, what is the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Painless Bloody Urination in a 60-Year-Old Woman"
},
{
"authors": "Gideon Lorber, MD; Ofer Nathan Gofrit, MD, PhD",
"content": [
"Discussion",
"The axial images of the CT urograph, as well as the associated sagittal reformation, demonstrated a large filling defect in the right renal pelvis. The filling defect extended into and replaced most of the right renal pelvis, resulting in a mass effect and a relative delay in the kidney's enhancement during the nephrographic phase (Figures 2 and 3). The combination of this radiographic appearance with a history of painless hematuria and positive urine cytology was indicative of upper-tract urothelial carcinoma.",
"Figure 2.",
"Figure 3.",
"\"Upper-tract urothelial carcinoma\" refers to malignant changes of the urothelial cells lining the urinary tract from the renal calyces to the ureteral orifice. These carcinomas are relatively uncommon, accounting for only about 5%-7% of all renal tumors and about 5% of all urothelial tumors.",
"The true incidence of upper-tract tumors is increasing as the population ages. Upper-tract cancer is typically seen at an older age than bladder cancer; it rarely presents before the age of 40 years, and it has an average age of presentation of 65 years. The incidence of upper-tract tumors is 10 cases per 100,000 population per year. The highest incidence, however, appears to occur in Balkan countries, where urothelial cancers represent 40% of all renal cancers.",
"Figure 4.",
"Men are twice as likely as women to develop upper-tract tumors. White persons have a 2:1 risk of developing upper-tract tumors compared with black persons; however, data indicate that disease-specific annual mortality is greater in black men than in white men (7.4% vs 4.9%) and greater in women than in men (6.1% vs 4.4%).",
"Proposed causes of upper-tract urothelial carcinoma are similar to those for bladder cancer and include environmental factors (cigarette smoking), occupational exposures (aniline dyes), and treatment with anti-inflammatory (phenacetin) or chemotherapy (cyclophosphamide, ifosfamide) agents. A familial association has been identified in patients with Balkan nephropathy, although an unidentified environmental trigger may be the underlying etiology in these cases.[1,2,3]",
"The most common presenting symptom or finding that leads to the diagnosis of upper-tract urothelial tumors is hematuria, which occurs in 56%-98% of patients. The second most common symptom is flank pain, which occurs in 30% of cases. The pain may range from a dull sensation resulting from gradual obstruction of the collecting system to an acute pain mimicking renal colic, believed to result from an acute obstruction by a thrombus. About 15% of patients are asymptomatic at presentation, and disease is diagnosed incidentally by an imaging study performed for a different indication."
],
"date": "August 18, 2016",
"figures": [
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/842/600/842600-thumb-2.jpg"
},
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/842/600/842600-thumb-3.jpg"
},
{
"caption": "Figure 4.",
"image_url": "https://img.medscapestatic.com/article/842/600/842600-thumb-4.jpg"
}
],
"markdown": "# Painless Bloody Urination in a 60-Year-Old Woman\n\n **Authors:** Gideon Lorber, MD; Ofer Nathan Gofrit, MD, PhD \n **Date:** August 18, 2016\n\n ## Content\n\n Discussion\nThe axial images of the CT urograph, as well as the associated sagittal reformation, demonstrated a large filling defect in the right renal pelvis. The filling defect extended into and replaced most of the right renal pelvis, resulting in a mass effect and a relative delay in the kidney's enhancement during the nephrographic phase (Figures 2 and 3). The combination of this radiographic appearance with a history of painless hematuria and positive urine cytology was indicative of upper-tract urothelial carcinoma.\nFigure 2.\nFigure 3.\n\"Upper-tract urothelial carcinoma\" refers to malignant changes of the urothelial cells lining the urinary tract from the renal calyces to the ureteral orifice. These carcinomas are relatively uncommon, accounting for only about 5%-7% of all renal tumors and about 5% of all urothelial tumors.\nThe true incidence of upper-tract tumors is increasing as the population ages. Upper-tract cancer is typically seen at an older age than bladder cancer; it rarely presents before the age of 40 years, and it has an average age of presentation of 65 years. The incidence of upper-tract tumors is 10 cases per 100,000 population per year. The highest incidence, however, appears to occur in Balkan countries, where urothelial cancers represent 40% of all renal cancers.\nFigure 4.\nMen are twice as likely as women to develop upper-tract tumors. White persons have a 2:1 risk of developing upper-tract tumors compared with black persons; however, data indicate that disease-specific annual mortality is greater in black men than in white men (7.4% vs 4.9%) and greater in women than in men (6.1% vs 4.4%).\nProposed causes of upper-tract urothelial carcinoma are similar to those for bladder cancer and include environmental factors (cigarette smoking), occupational exposures (aniline dyes), and treatment with anti-inflammatory (phenacetin) or chemotherapy (cyclophosphamide, ifosfamide) agents. A familial association has been identified in patients with Balkan nephropathy, although an unidentified environmental trigger may be the underlying etiology in these cases.[1,2,3]\nThe most common presenting symptom or finding that leads to the diagnosis of upper-tract urothelial tumors is hematuria, which occurs in 56%-98% of patients. The second most common symptom is flank pain, which occurs in 30% of cases. The pain may range from a dull sensation resulting from gradual obstruction of the collecting system to an acute pain mimicking renal colic, believed to result from an acute obstruction by a thrombus. About 15% of patients are asymptomatic at presentation, and disease is diagnosed incidentally by an imaging study performed for a different indication.\n\n ## Figures\n\n **Figure 2.** \n \n\n**Figure 3.** \n \n\n**Figure 4.** \n \n\n\n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 833021,
"choiceText": "Renal stone",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 833023,
"choiceText": "Urothelial carcinoma",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 833025,
"choiceText": "External renal compression",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 833027,
"choiceText": "Complicated renal cyst",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 261991,
"questionText": "On the basis of the history, physical examination, and work-up, what is the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Painless Bloody Urination in a 60-Year-Old Woman"
},
{
"authors": "Gideon Lorber, MD; Ofer Nathan Gofrit, MD, PhD",
"content": [
"Patients presenting with advanced urothelial carcinoma may have loss of appetite, weight loss, a flank or abdominal mass, and bone pain. Traditionally, intravenous pyelography was used to diagnose upper-tract tumors; however, CT urography (CT with a delayed urographic phase) is now the primary diagnostic modality. With CT urography, the sensitivity for detecting upper-tract malignant disease has been reported to approach 100%, with a specificity of 60% and a negative predictive value of 100%. CT urography does, however, expose the patient to higher doses of radiation and requires intravenous administration of contrast material.",
"Radiolucent filling defects, obstruction or incomplete filling of a part of the upper tract, and nonvisualization of the collecting system are the typical findings suggestive of an upper-tract tumor. Filling defects may represent stones, blood clots, external compression, or a fungus ball. Stones can be ruled out by detecting calcifications on renal ultrasonography or noncontrast CT. The clinician should always assess the contralateral kidney for possible bilaterality and to determine its functioning.[1,2]",
"If the diagnosis remains in question or the treatment plan may be adjusted on the basis of ureteroscopic evaluation, endoscopy with or without biopsy should be performed. Ureteroscopy is a valuable tool in the evaluation of upper-tract urothelial carcinoma. As a result of advancements in optics, flexible ureteroscopes, and endoscopic equipment, visualization and sampling of the tumor have improved.",
"The greatest prognostic factors in the management of upper-tract urothelial carcinoma are pathologic grade and stage. Histologic correlations of up to 90% have been established between the initial ureteroscopic biopsy and the final pathologic specimen; however, because of the small size of the biopsy specimen and the depth of tissue sampling, outcomes with tumor stage have not demonstrated such a strong correlation. In 40 urothelial tumors staged in one series, 45% of tumors thought to be pathologic Ta were upstaged to T1-T3 at the time of complete resection. Ureteroscopic biopsy cannot reliably predict tumor stage; therefore, a combination of tumor grade, endoscopic visual appearance of the tumor, and radiologic appearance are required for the best prediction of tumor stage.[1,2,3]",
"Because voided urine cytology is nonspecific, its usefulness is limited. Even when selectively obtained ureteral cytology specimens are used, the diagnostic yield is still only 60% accurate. Improved diagnostic yield has been demonstrated with the use of saline washing or brush biopsy, with an approximate sensitivity of 90% and a specificity near 90%.[2]"
],
"date": "August 18, 2016",
"figures": [],
"markdown": "# Painless Bloody Urination in a 60-Year-Old Woman\n\n **Authors:** Gideon Lorber, MD; Ofer Nathan Gofrit, MD, PhD \n **Date:** August 18, 2016\n\n ## Content\n\n Patients presenting with advanced urothelial carcinoma may have loss of appetite, weight loss, a flank or abdominal mass, and bone pain. Traditionally, intravenous pyelography was used to diagnose upper-tract tumors; however, CT urography (CT with a delayed urographic phase) is now the primary diagnostic modality. With CT urography, the sensitivity for detecting upper-tract malignant disease has been reported to approach 100%, with a specificity of 60% and a negative predictive value of 100%. CT urography does, however, expose the patient to higher doses of radiation and requires intravenous administration of contrast material.\nRadiolucent filling defects, obstruction or incomplete filling of a part of the upper tract, and nonvisualization of the collecting system are the typical findings suggestive of an upper-tract tumor. Filling defects may represent stones, blood clots, external compression, or a fungus ball. Stones can be ruled out by detecting calcifications on renal ultrasonography or noncontrast CT. The clinician should always assess the contralateral kidney for possible bilaterality and to determine its functioning.[1,2]\nIf the diagnosis remains in question or the treatment plan may be adjusted on the basis of ureteroscopic evaluation, endoscopy with or without biopsy should be performed. Ureteroscopy is a valuable tool in the evaluation of upper-tract urothelial carcinoma. As a result of advancements in optics, flexible ureteroscopes, and endoscopic equipment, visualization and sampling of the tumor have improved.\nThe greatest prognostic factors in the management of upper-tract urothelial carcinoma are pathologic grade and stage. Histologic correlations of up to 90% have been established between the initial ureteroscopic biopsy and the final pathologic specimen; however, because of the small size of the biopsy specimen and the depth of tissue sampling, outcomes with tumor stage have not demonstrated such a strong correlation. In 40 urothelial tumors staged in one series, 45% of tumors thought to be pathologic Ta were upstaged to T1-T3 at the time of complete resection. Ureteroscopic biopsy cannot reliably predict tumor stage; therefore, a combination of tumor grade, endoscopic visual appearance of the tumor, and radiologic appearance are required for the best prediction of tumor stage.[1,2,3]\nBecause voided urine cytology is nonspecific, its usefulness is limited. Even when selectively obtained ureteral cytology specimens are used, the diagnostic yield is still only 60% accurate. Improved diagnostic yield has been demonstrated with the use of saline washing or brush biopsy, with an approximate sensitivity of 90% and a specificity near 90%.[2]\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "Painless Bloody Urination in a 60-Year-Old Woman"
},
{
"authors": "Gideon Lorber, MD; Ofer Nathan Gofrit, MD, PhD",
"content": [
"The standard treatment for upper-tract urothelial carcinoma has been nephroureterectomy with excision of the bladder cuff. Advancements in percutaneous and endoscopic techniques, however, have allowed more conservative nephron-sparing procedures for patients with solitary kidneys or those who are otherwise not ideal candidates for extirpative surgery. Considered the criterion standard, radical nephroureterectomy with resection of the bladder cuff is the treatment of choice for large, high-grade, single or multifocal, noninvasive or invasive renal pelvis or proximal ureter tumors. The risk for multifocality and a high incidence of ipsilateral recurrence after partial resection are the reasons that this radical approach is preferable.",
"Several approaches may be used, including totally open, totally laparoscopic, or a combination of both (eg, laparoscopic nephroureterectomy combined with an open distal intramural ureteral resection).[1,2,3,4] Robot-assisted nephroureterectomy has also been found safe and feasible as a minimally invasive procedure.",
"Management of the distal ureter through endoscopic means has been implicated in higher local recurrence rates than taking the distal ureter open. Patients with a solitary kidney, bilateral disease, renal failure, or significant comorbidities preventing a large abdominal surgery are candidates for endoscopic resection. An additional group of patients who may benefit from endoscopic treatment are those with small, low-grade lesions in the presence of a normal contralateral kidney. Tumors located at the distal ureter may be treated with a rigid ureteroscope, whereas those situated in the upper urinary tract may be reached by a flexible ureteroscope or by an antegrade percutaneous approach.",
"Initially, biopsies are done, and then ablation is achieved using electrocautery or laser energy. Historically, open nephron-sparing surgery for upper0tract urothelial carcinoma was used in patients with a large renal pelvis tumor in a solitary kidney or in cases of synchronous bilateral tumors. Advancements in endourologic techniques, particularly percutaneous antegrade renal surgery, have largely replaced this approach for the conservative management of renal pelvis tumors. Segmental (partial) ureteral resection with end-to-end anastomosis or bladder reimplantation is performed in cases with low-grade, noninvasive tumors of the proximal or mid-ureter that cannot be managed endoscopically when nephron-sparing is crucial for the preservation of renal function.[1,2,3]",
"The recurrence pattern after treatment of upper-tract urothelial carcinoma may be divided into vesical and extravesical recurrence. The higher the grade and stage of the upper-tract urothelial carcinoma, the higher the risk for extravesical recurrence. The recommended follow-up for patients treated for upper-tract urothelial carcinoma should consist of periodic history and physical examination, urine cytology, and surveillance cystoscopy, occurring every 3 months for the first 2 years after treatment, every 6 months for the next 2 years, and yearly thereafter if the patient is free of disease recurrence.",
"In cases of high-grade urothelial carcinoma, radiographic studies, including chest radiography and abdominopelvic CT, should be performed every 6 months for the first 2 years and then yearly thereafter. Ipsilateral endoscopy for patients who undergo organ-sparing treatment should occur every 6 months for the first 2-3 years and then yearly thereafter, provided that the patient is free of disease. Bone scans should be performed only if the patient has bone pain or an elevated alkaline phosphatase level.[1,3,5]",
"In this patient, cystoscopy was performed upon admission, which showed a normal bladder without any exophytic tumor. The combination of a large filling defect in the right renal pelvis seen on CT urography and positive urine cytology made the diagnosis of upper-tract urothelial carcinoma.",
"The patient received a comprehensive explanation of the available treatment modalities and elected to undergo nephroureterectomy. The surgery involved a combined laparoscopic and open approach. The right kidney and ureter were removed entirely, including a bladder cuff around the right distal ureter. The postoperative phase was uneventful, and the patient was discharged on the seventh postoperative day. She has shown no evidence of recurrence."
],
"date": "August 18, 2016",
"figures": [],
"markdown": "# Painless Bloody Urination in a 60-Year-Old Woman\n\n **Authors:** Gideon Lorber, MD; Ofer Nathan Gofrit, MD, PhD \n **Date:** August 18, 2016\n\n ## Content\n\n The standard treatment for upper-tract urothelial carcinoma has been nephroureterectomy with excision of the bladder cuff. Advancements in percutaneous and endoscopic techniques, however, have allowed more conservative nephron-sparing procedures for patients with solitary kidneys or those who are otherwise not ideal candidates for extirpative surgery. Considered the criterion standard, radical nephroureterectomy with resection of the bladder cuff is the treatment of choice for large, high-grade, single or multifocal, noninvasive or invasive renal pelvis or proximal ureter tumors. The risk for multifocality and a high incidence of ipsilateral recurrence after partial resection are the reasons that this radical approach is preferable.\nSeveral approaches may be used, including totally open, totally laparoscopic, or a combination of both (eg, laparoscopic nephroureterectomy combined with an open distal intramural ureteral resection).[1,2,3,4] Robot-assisted nephroureterectomy has also been found safe and feasible as a minimally invasive procedure.\nManagement of the distal ureter through endoscopic means has been implicated in higher local recurrence rates than taking the distal ureter open. Patients with a solitary kidney, bilateral disease, renal failure, or significant comorbidities preventing a large abdominal surgery are candidates for endoscopic resection. An additional group of patients who may benefit from endoscopic treatment are those with small, low-grade lesions in the presence of a normal contralateral kidney. Tumors located at the distal ureter may be treated with a rigid ureteroscope, whereas those situated in the upper urinary tract may be reached by a flexible ureteroscope or by an antegrade percutaneous approach.\nInitially, biopsies are done, and then ablation is achieved using electrocautery or laser energy. Historically, open nephron-sparing surgery for upper0tract urothelial carcinoma was used in patients with a large renal pelvis tumor in a solitary kidney or in cases of synchronous bilateral tumors. Advancements in endourologic techniques, particularly percutaneous antegrade renal surgery, have largely replaced this approach for the conservative management of renal pelvis tumors. Segmental (partial) ureteral resection with end-to-end anastomosis or bladder reimplantation is performed in cases with low-grade, noninvasive tumors of the proximal or mid-ureter that cannot be managed endoscopically when nephron-sparing is crucial for the preservation of renal function.[1,2,3]\nThe recurrence pattern after treatment of upper-tract urothelial carcinoma may be divided into vesical and extravesical recurrence. The higher the grade and stage of the upper-tract urothelial carcinoma, the higher the risk for extravesical recurrence. The recommended follow-up for patients treated for upper-tract urothelial carcinoma should consist of periodic history and physical examination, urine cytology, and surveillance cystoscopy, occurring every 3 months for the first 2 years after treatment, every 6 months for the next 2 years, and yearly thereafter if the patient is free of disease recurrence.\nIn cases of high-grade urothelial carcinoma, radiographic studies, including chest radiography and abdominopelvic CT, should be performed every 6 months for the first 2 years and then yearly thereafter. Ipsilateral endoscopy for patients who undergo organ-sparing treatment should occur every 6 months for the first 2-3 years and then yearly thereafter, provided that the patient is free of disease. Bone scans should be performed only if the patient has bone pain or an elevated alkaline phosphatase level.[1,3,5]\nIn this patient, cystoscopy was performed upon admission, which showed a normal bladder without any exophytic tumor. The combination of a large filling defect in the right renal pelvis seen on CT urography and positive urine cytology made the diagnosis of upper-tract urothelial carcinoma.\nThe patient received a comprehensive explanation of the available treatment modalities and elected to undergo nephroureterectomy. The surgery involved a combined laparoscopic and open approach. The right kidney and ureter were removed entirely, including a bladder cuff around the right distal ureter. The postoperative phase was uneventful, and the patient was discharged on the seventh postoperative day. She has shown no evidence of recurrence.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 833037,
"choiceText": "Men are twice as likely as women to develop an upper-tract tumor.",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 833039,
"choiceText": "Upper-tract tumors rarely present before the age of 40 years.",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 833041,
"choiceText": "Disease-specific annual mortality is greater in men than in women.",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 833043,
"choiceText": "Upper-tract urothelial carcinoma accounts for 5%-7% of all renal tumors.",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Upper-tract urothelial carcinoma accounts for 5%-7% of all renal tumors. Upper-tract cancer rarely presents before age of 40 years, and it has an average age of presentation of 65 years. Although men are twice as likely as women to develop upper-tract tumors, data suggest that the disease-specific annual mortality is greater in woman than in men (6.1% vs 4.4%). Women who develop upper-tract cancer are 25% more likely than men to die of the cancer.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 261995,
"questionText": "Which of the following statements are not true?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 833045,
"choiceText": "Intravenous pyelography",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 833047,
"choiceText": "CT urography",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 833049,
"choiceText": "Abdominal ultrasonography",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 833051,
"choiceText": "Abdominal MRI",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Traditionally, intravenous pyelography was used to diagnose upper-tract tumors; however, CT urography (CT with a delayed urographic phase) is now the primary diagnostic modality. With CT urography, the sensitivity for detecting upper-tract malignant disease has been reported to approach 100%, with a specificity of 60% and a negative predictive value of 100%.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 261997,
"questionText": "Which of the following examinations is today regarded as being the imaging modality of choice for the diagnosis of upper-tract lesions?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Painless Bloody Urination in a 60-Year-Old Woman"
},
{
"authors": "Gideon Lorber, MD; Ofer Nathan Gofrit, MD, PhD",
"content": [],
"date": "August 18, 2016",
"figures": [],
"markdown": "# Painless Bloody Urination in a 60-Year-Old Woman\n\n **Authors:** Gideon Lorber, MD; Ofer Nathan Gofrit, MD, PhD \n **Date:** August 18, 2016\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 833037,
"choiceText": "Men are twice as likely as women to develop an upper-tract tumor.",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 833039,
"choiceText": "Upper-tract tumors rarely present before the age of 40 years.",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 833041,
"choiceText": "Disease-specific annual mortality is greater in men than in women.",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 833043,
"choiceText": "Upper-tract urothelial carcinoma accounts for 5%-7% of all renal tumors.",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Upper-tract urothelial carcinoma accounts for 5%-7% of all renal tumors. Upper-tract cancer rarely presents before age of 40 years, and it has an average age of presentation of 65 years. Although men are twice as likely as women to develop upper-tract tumors, data suggest that the disease-specific annual mortality is greater in woman than in men (6.1% vs 4.4%). Women who develop upper-tract cancer are 25% more likely than men to die of the cancer.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 261995,
"questionText": "Which of the following statements are not true?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 833045,
"choiceText": "Intravenous pyelography",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 833047,
"choiceText": "CT urography",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 833049,
"choiceText": "Abdominal ultrasonography",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 833051,
"choiceText": "Abdominal MRI",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Traditionally, intravenous pyelography was used to diagnose upper-tract tumors; however, CT urography (CT with a delayed urographic phase) is now the primary diagnostic modality. With CT urography, the sensitivity for detecting upper-tract malignant disease has been reported to approach 100%, with a specificity of 60% and a negative predictive value of 100%.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 261997,
"questionText": "Which of the following examinations is today regarded as being the imaging modality of choice for the diagnosis of upper-tract lesions?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Painless Bloody Urination in a 60-Year-Old Woman"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 833021,
"choiceText": "Renal stone",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 833023,
"choiceText": "Urothelial carcinoma",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 833025,
"choiceText": "External renal compression",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 833027,
"choiceText": "Complicated renal cyst",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 261991,
"questionText": "On the basis of the history, physical examination, and work-up, what is the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 833037,
"choiceText": "Men are twice as likely as women to develop an upper-tract tumor.",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 833039,
"choiceText": "Upper-tract tumors rarely present before the age of 40 years.",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 833041,
"choiceText": "Disease-specific annual mortality is greater in men than in women.",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 833043,
"choiceText": "Upper-tract urothelial carcinoma accounts for 5%-7% of all renal tumors.",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Upper-tract urothelial carcinoma accounts for 5%-7% of all renal tumors. Upper-tract cancer rarely presents before age of 40 years, and it has an average age of presentation of 65 years. Although men are twice as likely as women to develop upper-tract tumors, data suggest that the disease-specific annual mortality is greater in woman than in men (6.1% vs 4.4%). Women who develop upper-tract cancer are 25% more likely than men to die of the cancer.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 261995,
"questionText": "Which of the following statements are not true?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 833045,
"choiceText": "Intravenous pyelography",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 833047,
"choiceText": "CT urography",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 833049,
"choiceText": "Abdominal ultrasonography",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 833051,
"choiceText": "Abdominal MRI",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Traditionally, intravenous pyelography was used to diagnose upper-tract tumors; however, CT urography (CT with a delayed urographic phase) is now the primary diagnostic modality. With CT urography, the sensitivity for detecting upper-tract malignant disease has been reported to approach 100%, with a specificity of 60% and a negative predictive value of 100%.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 261997,
"questionText": "Which of the following examinations is today regarded as being the imaging modality of choice for the diagnosis of upper-tract lesions?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
840962 | /viewarticle/840962 | [
{
"authors": "Suzan Sanavi, MD; Reza Afshar, MD",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"A 32-year-old man presents to the emergency department with sudden onset of hematemesis. He reports having vomited bright-red blood three times during the past 4 hours; the last incident occurred about 1 hour ago. He estimates that he has vomited about a half-cup (approximately 118 mL) of blood each time. He has been nauseous for several days.",
"He denies having any abdominal pain or any previous episodes of hematemesis; however, he states that he has had black, tarry stools and decreasing urine output for the past several days. He last urinated about 14 hours ago and he currently has no urge to urinate.",
"He denies any significant past medical history. He denies smoking, drinking alcohol, or using illicit drugs. He does not take any medications, including over-the-counter nonsteroidal anti-inflammatory drugs, and is not taking herbal supplements. He denies any prior surgeries. During the past 2 weeks, the patient noticed some swelling in the glands along his neck, but he has no other complaints."
],
"date": "March 12, 2019",
"figures": [],
"markdown": "# A 32-Year-Old Man Vomiting Blood\n\n **Authors:** Suzan Sanavi, MD; Reza Afshar, MD \n **Date:** March 12, 2019\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nA 32-year-old man presents to the emergency department with sudden onset of hematemesis. He reports having vomited bright-red blood three times during the past 4 hours; the last incident occurred about 1 hour ago. He estimates that he has vomited about a half-cup (approximately 118 mL) of blood each time. He has been nauseous for several days.\nHe denies having any abdominal pain or any previous episodes of hematemesis; however, he states that he has had black, tarry stools and decreasing urine output for the past several days. He last urinated about 14 hours ago and he currently has no urge to urinate.\nHe denies any significant past medical history. He denies smoking, drinking alcohol, or using illicit drugs. He does not take any medications, including over-the-counter nonsteroidal anti-inflammatory drugs, and is not taking herbal supplements. He denies any prior surgeries. During the past 2 weeks, the patient noticed some swelling in the glands along his neck, but he has no other complaints.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 32-Year-Old Man Vomiting Blood"
},
{
"authors": "Suzan Sanavi, MD; Reza Afshar, MD",
"content": [
"Upon physical examination, the patient is ill-appearing. His temperature is 98.6° F (37°C). He has a heart rate of 120 beats/min, with a regular rhythm, a blood pressure of 100/70 mm Hg, and a respiratory rate of 16 breaths/min. The examination of his head and neck reveals conjunctival pallor. He has enlarged lymph nodes along his bilateral anterior cervical chains. His lungs are clear to auscultation. Auscultation of his heart demonstrates normal sounds. He has a soft, nontender abdomen, without appreciable masses or organomegaly. His rectal examination shows normal tone and black, guaiac-positive stool. The axillary and inguinal lymph nodes are enlarged bilaterally, and he has normal peripheral pulses.",
"The patient's laboratory studies reveal a hemoglobin level of 9 g/dL (90 g/L), markedly elevated BUN level of 180 mg/dL (64.26 mmol/L), serum creatinine level of 11.2 mg/dL (990 µmol/L), lactate dehydrogenase (LDH) level of 2550 Units/L, uric acid level of 41 mg/dL (2438.7 µmol/L), phosphorus level of 19.6 mg/dL (6.3 mmol/L), and an erythrocyte sedimentation rate (ESR) of 30 mm/h. Urine sediment reveals hematuria, leukocyte casts, and many uric acid crystals. The liver function test results and serologic and complement assay findings are within normal limits.",
"On the basis of his clinical appearance and the laboratory analysis, the patient is diagnosed with acute upper gastrointestinal (GI) bleeding and acute renal failure. He undergoes prompt treatment with vigorous intravenous (IV) rehydration, blood transfusion for worsening anemia, and IV ranitidine therapy. The patient is then admitted to the hospital's intensive care unit for further management.",
"After stabilization, abdominal ultrasonography is performed, which demonstrates enlarged kidneys (right kidney, 12.5 cm, and left kidney, 12.2 cm) without urinary obstruction. His renal parenchyma is hypoechogenic. He then undergoes endoscopy, which demonstrates erosive gastritis. His laboratory values improve with therapy. Multiple biopsies are then taken of his axillary and inguinal lymph nodes, bone marrow, and kidneys. The axillary and inguinal biopsies are reported as histologically reactive. His bone marrow biopsy demonstrates erythroid hyperplasia. A slide from his renal biopsy is shown below.",
"Figure."
],
"date": "March 12, 2019",
"figures": [
{
"caption": "Figure.",
"image_url": "https://img.medscapestatic.com/article/840/962/840962-thumb.jpg"
}
],
"markdown": "# A 32-Year-Old Man Vomiting Blood\n\n **Authors:** Suzan Sanavi, MD; Reza Afshar, MD \n **Date:** March 12, 2019\n\n ## Content\n\n Upon physical examination, the patient is ill-appearing. His temperature is 98.6° F (37°C). He has a heart rate of 120 beats/min, with a regular rhythm, a blood pressure of 100/70 mm Hg, and a respiratory rate of 16 breaths/min. The examination of his head and neck reveals conjunctival pallor. He has enlarged lymph nodes along his bilateral anterior cervical chains. His lungs are clear to auscultation. Auscultation of his heart demonstrates normal sounds. He has a soft, nontender abdomen, without appreciable masses or organomegaly. His rectal examination shows normal tone and black, guaiac-positive stool. The axillary and inguinal lymph nodes are enlarged bilaterally, and he has normal peripheral pulses.\nThe patient's laboratory studies reveal a hemoglobin level of 9 g/dL (90 g/L), markedly elevated BUN level of 180 mg/dL (64.26 mmol/L), serum creatinine level of 11.2 mg/dL (990 µmol/L), lactate dehydrogenase (LDH) level of 2550 Units/L, uric acid level of 41 mg/dL (2438.7 µmol/L), phosphorus level of 19.6 mg/dL (6.3 mmol/L), and an erythrocyte sedimentation rate (ESR) of 30 mm/h. Urine sediment reveals hematuria, leukocyte casts, and many uric acid crystals. The liver function test results and serologic and complement assay findings are within normal limits.\nOn the basis of his clinical appearance and the laboratory analysis, the patient is diagnosed with acute upper gastrointestinal (GI) bleeding and acute renal failure. He undergoes prompt treatment with vigorous intravenous (IV) rehydration, blood transfusion for worsening anemia, and IV ranitidine therapy. The patient is then admitted to the hospital's intensive care unit for further management.\nAfter stabilization, abdominal ultrasonography is performed, which demonstrates enlarged kidneys (right kidney, 12.5 cm, and left kidney, 12.2 cm) without urinary obstruction. His renal parenchyma is hypoechogenic. He then undergoes endoscopy, which demonstrates erosive gastritis. His laboratory values improve with therapy. Multiple biopsies are then taken of his axillary and inguinal lymph nodes, bone marrow, and kidneys. The axillary and inguinal biopsies are reported as histologically reactive. His bone marrow biopsy demonstrates erythroid hyperplasia. A slide from his renal biopsy is shown below.\nFigure.\n\n ## Figures\n\n **Figure.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 819521,
"choiceText": "Idiopathic GI bleeding",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 819523,
"choiceText": "Wilms tumor",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 819525,
"choiceText": "Non-Hodgkin lymphoma",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 819527,
"choiceText": "Acute myelocytic leukemia",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 257381,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 32-Year-Old Man Vomiting Blood"
},
{
"authors": "Suzan Sanavi, MD; Reza Afshar, MD",
"content": [
"This patient's clinical picture coupled with the biopsy results pointed to a diagnosis of non-Hodgkin lymphoma. Although the patient's acute renal failure could potentially be explained by hypotension stemming from a GI bleed, he had many other clinical and laboratory abnormalities that pointed to an additional diagnosis. He had lymphadenopathy on examination, elevated serum uric acid, phosphorus, and LDH levels without evidence of hemolysis or liver dysfunction. These pointed to a possible tumor lysis syndrome from a malignancy. Unfortunately, his lymph node biopsies did not yield any diagnostic clues, but that is not uncommon in the early stages of some lymphomas. The renal biopsy, however, eventually led to the underlying cause of renal failure, which was determined to be non-Hodgkin lymphoma (specifically, diffuse large B-cell lymphoma).",
"Lymphomas are a heterogeneous group of malignancies that are classified as either non-Hodgkin lymphoma or Hodgkin disease. Most malignant lymphomas (approximately 85%) are non-Hodgkin lymphomas. Non-Hodgkin lymphomas include a diverse group of lymphoproliferative malignancies that originate outside the bone marrow. These malignancies differ in many basic characteristics. Some types, such as follicular small cleaved cell lymphomas, are almost always disseminated with bone marrow involvement upon presentation, whereas diffuse large noncleaved cell lymphomas may present with disease limited to one to two lymph node areas in as many as 30% of cases. Approximately 25% of all cases of non-Hodgkin lymphoma present at an extranodal site without systemic involvement. The malignancies also differ in terms of aggressiveness, with some having indolent courses and others rapidly progressing. They also differ in the type of treatment options and success rates.[1]",
"In this context of clinical diversity, accurate classification of non-Hodgkin lymphoma is essential. Ideally, a classification system divides non-Hodgkin lymphoma into entities that are relatively homogeneous from a morphologic, immunologic, and clinical point of view. Several classification systems are available, such as Rappaport, New Working Formulation, Revised European American (REAL) classification, and the most popular and practical, the World Health Organization (WHO) classification system of lymphoid malignancies. The classification of the lymphoma is clinically significant in assessing the prognosis and guiding treatment.[1,2]"
],
"date": "March 12, 2019",
"figures": [],
"markdown": "# A 32-Year-Old Man Vomiting Blood\n\n **Authors:** Suzan Sanavi, MD; Reza Afshar, MD \n **Date:** March 12, 2019\n\n ## Content\n\n This patient's clinical picture coupled with the biopsy results pointed to a diagnosis of non-Hodgkin lymphoma. Although the patient's acute renal failure could potentially be explained by hypotension stemming from a GI bleed, he had many other clinical and laboratory abnormalities that pointed to an additional diagnosis. He had lymphadenopathy on examination, elevated serum uric acid, phosphorus, and LDH levels without evidence of hemolysis or liver dysfunction. These pointed to a possible tumor lysis syndrome from a malignancy. Unfortunately, his lymph node biopsies did not yield any diagnostic clues, but that is not uncommon in the early stages of some lymphomas. The renal biopsy, however, eventually led to the underlying cause of renal failure, which was determined to be non-Hodgkin lymphoma (specifically, diffuse large B-cell lymphoma).\nLymphomas are a heterogeneous group of malignancies that are classified as either non-Hodgkin lymphoma or Hodgkin disease. Most malignant lymphomas (approximately 85%) are non-Hodgkin lymphomas. Non-Hodgkin lymphomas include a diverse group of lymphoproliferative malignancies that originate outside the bone marrow. These malignancies differ in many basic characteristics. Some types, such as follicular small cleaved cell lymphomas, are almost always disseminated with bone marrow involvement upon presentation, whereas diffuse large noncleaved cell lymphomas may present with disease limited to one to two lymph node areas in as many as 30% of cases. Approximately 25% of all cases of non-Hodgkin lymphoma present at an extranodal site without systemic involvement. The malignancies also differ in terms of aggressiveness, with some having indolent courses and others rapidly progressing. They also differ in the type of treatment options and success rates.[1]\nIn this context of clinical diversity, accurate classification of non-Hodgkin lymphoma is essential. Ideally, a classification system divides non-Hodgkin lymphoma into entities that are relatively homogeneous from a morphologic, immunologic, and clinical point of view. Several classification systems are available, such as Rappaport, New Working Formulation, Revised European American (REAL) classification, and the most popular and practical, the World Health Organization (WHO) classification system of lymphoid malignancies. The classification of the lymphoma is clinically significant in assessing the prognosis and guiding treatment.[1,2]\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 819521,
"choiceText": "Idiopathic GI bleeding",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 819523,
"choiceText": "Wilms tumor",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 819525,
"choiceText": "Non-Hodgkin lymphoma",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 819527,
"choiceText": "Acute myelocytic leukemia",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 257381,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 32-Year-Old Man Vomiting Blood"
},
{
"authors": "Suzan Sanavi, MD; Reza Afshar, MD",
"content": [
"Non-Hodgkin lymphomas are tumors of lymphoid tissues that have progressive clonal expansion of B cells, T cells, and/or natural killer (NK) cells. Most are of B-cell origin; those that are not are derived from T cells, NK cells, and, rarely, from macrophages. The tumors are classified pathologically by their level of differentiation, the size of the cell of origin, the rate of proliferation, and the histologic growth pattern. Among B-cell subtypes, the size of the cell and the growth pattern suggest the degree of tumor aggressiveness. Tumors that grow in a nodular pattern are typically less aggressive than those that proliferate in a diffuse pattern. Additionally, lymphomas derived from small lymphocytes often have a more indolent course than those from large lymphocytes.[1,2]",
"Primary renal lymphoma (PRL) as a clinical entity is controversial because the kidneys do not contain lymphatic tissue and the mechanism of development of PRLs is unclear. Most reported cases showed rapid systemic progression and a poor prognosis. Although no clearly defined diagnostic criteria are recognized for renal lymphomas, abdominal and thoracic CT scanning as well as renal and bone marrow biopsies are potentially beneficial diagnostic tools.[2,3]",
"Non-Hodgkin lymphoma is the most prevalent hematopoietic neoplasm and accounts for approximately 4% of cancers worldwide. The 5-year survival rate has steadily improved over the past 20 years and is now estimated to be 63%. The development of novel therapeutic strategies, such as monoclonal antibodies, validation of response biomarkers, and introduction of patient-specific treatment, are largely responsible for this significant improvement in mortality.[1,2]",
"Evaluating patients with non-Hodgkin lymphoma starts with a careful history-taking and physical examination. Of particular importance is the presence or absence of B-symptoms. These symptoms are uncommon at the initial presentation of low-grade lymphomas, but they are present in approximately 30%-40% of those with intermediate- and high-grade disease. B-symptoms include night sweats, unintentional weight loss, unexplained fevers, and sometimes pruritus. Fatigue and generalized weakness are also more common in those with advanced disease.",
"Physical signs may widely vary and depend on the severity of illness at presentation. Findings include peripheral adenopathy, splenomegaly, and hepatomegaly. A large abdominal mass may be palpated in Burkitt lymphoma. Skin lesions are seen in cutaneous T-cell lymphoma (mycosis fungoides), anaplastic large-cell lymphoma, and angioimmunoblastic lymphoma. Signs such as pallor, ecchymoses, or petechiae may be suggestive of a hematologic etiology for the patient's illness. Additionally, palpating for masses (enlarged lymph nodes, abdominal masses, thyroid masses) may be useful. Regression of enlarged lymph nodes may occur in low-grade lymphoma, which may misguide the diagnosis to an infectious cause. Over one third of patients present with extranodal disease, including involvement of the GI tract, skin, bone marrow, sinuses, genitourinary tract, thyroid, and central nervous system.[1,4]"
],
"date": "March 12, 2019",
"figures": [],
"markdown": "# A 32-Year-Old Man Vomiting Blood\n\n **Authors:** Suzan Sanavi, MD; Reza Afshar, MD \n **Date:** March 12, 2019\n\n ## Content\n\n Non-Hodgkin lymphomas are tumors of lymphoid tissues that have progressive clonal expansion of B cells, T cells, and/or natural killer (NK) cells. Most are of B-cell origin; those that are not are derived from T cells, NK cells, and, rarely, from macrophages. The tumors are classified pathologically by their level of differentiation, the size of the cell of origin, the rate of proliferation, and the histologic growth pattern. Among B-cell subtypes, the size of the cell and the growth pattern suggest the degree of tumor aggressiveness. Tumors that grow in a nodular pattern are typically less aggressive than those that proliferate in a diffuse pattern. Additionally, lymphomas derived from small lymphocytes often have a more indolent course than those from large lymphocytes.[1,2]\nPrimary renal lymphoma (PRL) as a clinical entity is controversial because the kidneys do not contain lymphatic tissue and the mechanism of development of PRLs is unclear. Most reported cases showed rapid systemic progression and a poor prognosis. Although no clearly defined diagnostic criteria are recognized for renal lymphomas, abdominal and thoracic CT scanning as well as renal and bone marrow biopsies are potentially beneficial diagnostic tools.[2,3]\nNon-Hodgkin lymphoma is the most prevalent hematopoietic neoplasm and accounts for approximately 4% of cancers worldwide. The 5-year survival rate has steadily improved over the past 20 years and is now estimated to be 63%. The development of novel therapeutic strategies, such as monoclonal antibodies, validation of response biomarkers, and introduction of patient-specific treatment, are largely responsible for this significant improvement in mortality.[1,2]\nEvaluating patients with non-Hodgkin lymphoma starts with a careful history-taking and physical examination. Of particular importance is the presence or absence of B-symptoms. These symptoms are uncommon at the initial presentation of low-grade lymphomas, but they are present in approximately 30%-40% of those with intermediate- and high-grade disease. B-symptoms include night sweats, unintentional weight loss, unexplained fevers, and sometimes pruritus. Fatigue and generalized weakness are also more common in those with advanced disease.\nPhysical signs may widely vary and depend on the severity of illness at presentation. Findings include peripheral adenopathy, splenomegaly, and hepatomegaly. A large abdominal mass may be palpated in Burkitt lymphoma. Skin lesions are seen in cutaneous T-cell lymphoma (mycosis fungoides), anaplastic large-cell lymphoma, and angioimmunoblastic lymphoma. Signs such as pallor, ecchymoses, or petechiae may be suggestive of a hematologic etiology for the patient's illness. Additionally, palpating for masses (enlarged lymph nodes, abdominal masses, thyroid masses) may be useful. Regression of enlarged lymph nodes may occur in low-grade lymphoma, which may misguide the diagnosis to an infectious cause. Over one third of patients present with extranodal disease, including involvement of the GI tract, skin, bone marrow, sinuses, genitourinary tract, thyroid, and central nervous system.[1,4]\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 32-Year-Old Man Vomiting Blood"
},
{
"authors": "Suzan Sanavi, MD; Reza Afshar, MD",
"content": [
"Initial laboratory studies in non-Hodgkin lymphomas may include a CBC count and peripheral smear, ESR, chemistry studies reflecting major organ function, and a serum LDH. HIV serology should also be considered. Additional studies that may be used include serum beta2-microglobulin (B2M); serum protein electrophoresis; CT scans of the chest, abdomen, and pelvis; gallium scan; and a bone marrow biopsy.[2]",
"Imaging studies can provide anatomic information that will help determine a patient's International Prognostic Index (IPI). This is a powerful predictor of outcome in all patients with non-Hodgkin lymphoma. The factors used to determine the IPI include age, serum LDH, performance status, Ann Arbor staging, and extranodal involvement. The IPI, combined with other factors, helps determine the patient's treatment protocols. Treatment options are complex and may vary substantially given the patient's functional status, age, and type of malignancy.[1,2]",
"A rare but interesting initial manifestation of non-Hodgkin lymphoma is acute renal failure. While lymphoma involves the kidney in 30%-40% of cases if untreated, the incidence of renal involvement at initial presentation is 2.7%-6%. Mostly, it is in the form of insidious renal failure. Primary renal lymphoma is rare. Although initial renal involvement and primary renal lymphoma are rare, many causes of acute renal failure are related to non-Hodgkin lymphoma, including hypoperfusion caused by renal vein thrombosis, urinary tract obstruction caused by lymph nodes and tumor growth, and, more commonly, tumor lysis syndrome. Renal failure as a result of tumor lysis syndrome is often the presenting condition that leads to the diagnosis of non-Hodgkin lymphoma.[3,4,5,6,7]",
"In the patient in this case, the imaging study and biopsies eventually demonstrated renal infiltration of his lymphoma. The fractional uric acid excretion of < 1 meant that decreased renal elimination, rather than increased production of uric acid from a tumor lysis syndrome, likely caused the elevated uric acid levels. Due to the patient's impaired renal function, allopurinol, which undergoes renal elimination, was not initially administered. Instead, the patient underwent hemodialysis.",
"After hemodialysis was discontinued, allopurinol was dosed on the basis of the patient's glomerular filtration rate. A chemotherapy protocol of cyclophosphamide, doxorubicin, vincristine, and prednisone was started with renal dosing. The patient was observed for worsening tumor lysis syndrome following chemotherapy. Hemodialysis was used regularly. Eventually, the dose-adjusted chemotherapy led to renal function improvement, with a serum creatinine level of 1.7 mg/dL (150.28 µmol/L) and diminished renal size (right kidney, 12 cm, and left kidney, 11.5 cm).",
"The patient was discharged in good condition but, unfortunately, he died 2 months later as a result of dissemination of the large B-cell lymphoma, including extensive bone marrow involvement."
],
"date": "March 12, 2019",
"figures": [],
"markdown": "# A 32-Year-Old Man Vomiting Blood\n\n **Authors:** Suzan Sanavi, MD; Reza Afshar, MD \n **Date:** March 12, 2019\n\n ## Content\n\n Initial laboratory studies in non-Hodgkin lymphomas may include a CBC count and peripheral smear, ESR, chemistry studies reflecting major organ function, and a serum LDH. HIV serology should also be considered. Additional studies that may be used include serum beta2-microglobulin (B2M); serum protein electrophoresis; CT scans of the chest, abdomen, and pelvis; gallium scan; and a bone marrow biopsy.[2]\nImaging studies can provide anatomic information that will help determine a patient's International Prognostic Index (IPI). This is a powerful predictor of outcome in all patients with non-Hodgkin lymphoma. The factors used to determine the IPI include age, serum LDH, performance status, Ann Arbor staging, and extranodal involvement. The IPI, combined with other factors, helps determine the patient's treatment protocols. Treatment options are complex and may vary substantially given the patient's functional status, age, and type of malignancy.[1,2]\nA rare but interesting initial manifestation of non-Hodgkin lymphoma is acute renal failure. While lymphoma involves the kidney in 30%-40% of cases if untreated, the incidence of renal involvement at initial presentation is 2.7%-6%. Mostly, it is in the form of insidious renal failure. Primary renal lymphoma is rare. Although initial renal involvement and primary renal lymphoma are rare, many causes of acute renal failure are related to non-Hodgkin lymphoma, including hypoperfusion caused by renal vein thrombosis, urinary tract obstruction caused by lymph nodes and tumor growth, and, more commonly, tumor lysis syndrome. Renal failure as a result of tumor lysis syndrome is often the presenting condition that leads to the diagnosis of non-Hodgkin lymphoma.[3,4,5,6,7]\nIn the patient in this case, the imaging study and biopsies eventually demonstrated renal infiltration of his lymphoma. The fractional uric acid excretion of < 1 meant that decreased renal elimination, rather than increased production of uric acid from a tumor lysis syndrome, likely caused the elevated uric acid levels. Due to the patient's impaired renal function, allopurinol, which undergoes renal elimination, was not initially administered. Instead, the patient underwent hemodialysis.\nAfter hemodialysis was discontinued, allopurinol was dosed on the basis of the patient's glomerular filtration rate. A chemotherapy protocol of cyclophosphamide, doxorubicin, vincristine, and prednisone was started with renal dosing. The patient was observed for worsening tumor lysis syndrome following chemotherapy. Hemodialysis was used regularly. Eventually, the dose-adjusted chemotherapy led to renal function improvement, with a serum creatinine level of 1.7 mg/dL (150.28 µmol/L) and diminished renal size (right kidney, 12 cm, and left kidney, 11.5 cm).\nThe patient was discharged in good condition but, unfortunately, he died 2 months later as a result of dissemination of the large B-cell lymphoma, including extensive bone marrow involvement.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 819529,
"choiceText": "Hyperperfusion resulting from renal vein thrombosis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 819531,
"choiceText": "Urinary tract obstruction from tumor mass",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 819533,
"choiceText": "Hypertensive emergency",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 819535,
"choiceText": "Tumor lysis syndrome",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Many causes of acute renal failure are related to non-Hodgkin lymphoma, including hypoperfusion caused by renal vein thrombosis, urinary tract obstruction caused by lymph nodes and tumor growth, and, most commonly, tumor lysis syndrome. Renal failure as a result of tumor lysis syndrome is often the presenting condition that leads to the diagnosis of non-Hodgkin lymphoma.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 257383,
"questionText": "Which of the following conditions is most likely the cause of renal dysfunction in a patient with non-Hodgkin lymphoma?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 819537,
"choiceText": "Age, serum LDH, performance status, Ann Arbor staging, and extranodal involvement",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 819539,
"choiceText": "Weight change, BUN, performance status, Ann Arbor staging, and bone scintigraphy findings",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 819541,
"choiceText": "Urinary output, serum LDH, blood pressure, WHO classification, and extranodal involvement",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 819543,
"choiceText": "Guaiac test for fecal occult blood, serum potassium, WHO classification, and CT scan findings",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The IPI score is a powerful predictor of outcome in all patients with non-Hodgkin lymphoma. The factors used to determine the IPI include age, serum LDH, performance status, Ann Arbor staging, and extranodal involvement. The IPI, combined with other factors, helps determine the patient's treatment protocols. Treatment options are complex and may vary substantially given the patient's functional status, age, and type of malignancy.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 257385,
"questionText": "What factors should be evaluated to determine a patient's IPI score? ",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 32-Year-Old Man Vomiting Blood"
},
{
"authors": "Suzan Sanavi, MD; Reza Afshar, MD",
"content": [],
"date": "March 12, 2019",
"figures": [],
"markdown": "# A 32-Year-Old Man Vomiting Blood\n\n **Authors:** Suzan Sanavi, MD; Reza Afshar, MD \n **Date:** March 12, 2019\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 819529,
"choiceText": "Hyperperfusion resulting from renal vein thrombosis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 819531,
"choiceText": "Urinary tract obstruction from tumor mass",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 819533,
"choiceText": "Hypertensive emergency",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 819535,
"choiceText": "Tumor lysis syndrome",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Many causes of acute renal failure are related to non-Hodgkin lymphoma, including hypoperfusion caused by renal vein thrombosis, urinary tract obstruction caused by lymph nodes and tumor growth, and, most commonly, tumor lysis syndrome. Renal failure as a result of tumor lysis syndrome is often the presenting condition that leads to the diagnosis of non-Hodgkin lymphoma.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 257383,
"questionText": "Which of the following conditions is most likely the cause of renal dysfunction in a patient with non-Hodgkin lymphoma?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 819537,
"choiceText": "Age, serum LDH, performance status, Ann Arbor staging, and extranodal involvement",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 819539,
"choiceText": "Weight change, BUN, performance status, Ann Arbor staging, and bone scintigraphy findings",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 819541,
"choiceText": "Urinary output, serum LDH, blood pressure, WHO classification, and extranodal involvement",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 819543,
"choiceText": "Guaiac test for fecal occult blood, serum potassium, WHO classification, and CT scan findings",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The IPI score is a powerful predictor of outcome in all patients with non-Hodgkin lymphoma. The factors used to determine the IPI include age, serum LDH, performance status, Ann Arbor staging, and extranodal involvement. The IPI, combined with other factors, helps determine the patient's treatment protocols. Treatment options are complex and may vary substantially given the patient's functional status, age, and type of malignancy.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 257385,
"questionText": "What factors should be evaluated to determine a patient's IPI score? ",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 32-Year-Old Man Vomiting Blood"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 819521,
"choiceText": "Idiopathic GI bleeding",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 819523,
"choiceText": "Wilms tumor",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 819525,
"choiceText": "Non-Hodgkin lymphoma",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 819527,
"choiceText": "Acute myelocytic leukemia",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 257381,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 819529,
"choiceText": "Hyperperfusion resulting from renal vein thrombosis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 819531,
"choiceText": "Urinary tract obstruction from tumor mass",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 819533,
"choiceText": "Hypertensive emergency",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 819535,
"choiceText": "Tumor lysis syndrome",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Many causes of acute renal failure are related to non-Hodgkin lymphoma, including hypoperfusion caused by renal vein thrombosis, urinary tract obstruction caused by lymph nodes and tumor growth, and, most commonly, tumor lysis syndrome. Renal failure as a result of tumor lysis syndrome is often the presenting condition that leads to the diagnosis of non-Hodgkin lymphoma.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 257383,
"questionText": "Which of the following conditions is most likely the cause of renal dysfunction in a patient with non-Hodgkin lymphoma?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 819537,
"choiceText": "Age, serum LDH, performance status, Ann Arbor staging, and extranodal involvement",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 819539,
"choiceText": "Weight change, BUN, performance status, Ann Arbor staging, and bone scintigraphy findings",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 819541,
"choiceText": "Urinary output, serum LDH, blood pressure, WHO classification, and extranodal involvement",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 819543,
"choiceText": "Guaiac test for fecal occult blood, serum potassium, WHO classification, and CT scan findings",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The IPI score is a powerful predictor of outcome in all patients with non-Hodgkin lymphoma. The factors used to determine the IPI include age, serum LDH, performance status, Ann Arbor staging, and extranodal involvement. The IPI, combined with other factors, helps determine the patient's treatment protocols. Treatment options are complex and may vary substantially given the patient's functional status, age, and type of malignancy.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 257385,
"questionText": "What factors should be evaluated to determine a patient's IPI score? ",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
840976 | /viewarticle/840976 | [
{
"authors": "Federico J. Teran, MD; Eric E. Simon, MD; Vecihi Batuman, MD",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"Background",
"A 46-year-old man presents for a follow-up visit after being discharged from hospital. The patient was initially admitted to neurosurgery 35 days ago after falling from a ladder approximately 20 feet high and landing on a concrete apron. The patient had a brief period of confusion that was attributed to a concussion, which resolved during the first day of his admission.",
"Radiologic studies during that admission included CT scanning of the head and spine; the scans revealed two simple fractures in the lower cervical vertebrae and no intracranial bleeding or any other abnormality. He did not have any neurologic deficits. The patient was immobilized at the time, and his pain was controlled by opiate-containing analgesics. He did not require surgery and was discharged with a cervical collar brace and prescribed acetaminophen and oxycodone on an as-needed basis. He is on no other medications.",
"The patient's medical history is significant for a kidney stone 10 years prior but no other health issues. He has smoked about a pack of cigarettes daily for the past 20 years and consumed alcohol in social settings. Before discharge, the patient's intern noted that he had a serum sodium concentration of 132-134 mEq/L. No further investigations were performed, and the patient was advised not to drink too much water."
],
"date": "July 19, 2017",
"figures": [],
"markdown": "# Hyponatremia in a 46-Year-Old Man After Head Trauma\n\n **Authors:** Federico J. Teran, MD; Eric E. Simon, MD; Vecihi Batuman, MD \n **Date:** July 19, 2017\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nBackground\nA 46-year-old man presents for a follow-up visit after being discharged from hospital. The patient was initially admitted to neurosurgery 35 days ago after falling from a ladder approximately 20 feet high and landing on a concrete apron. The patient had a brief period of confusion that was attributed to a concussion, which resolved during the first day of his admission.\nRadiologic studies during that admission included CT scanning of the head and spine; the scans revealed two simple fractures in the lower cervical vertebrae and no intracranial bleeding or any other abnormality. He did not have any neurologic deficits. The patient was immobilized at the time, and his pain was controlled by opiate-containing analgesics. He did not require surgery and was discharged with a cervical collar brace and prescribed acetaminophen and oxycodone on an as-needed basis. He is on no other medications.\nThe patient's medical history is significant for a kidney stone 10 years prior but no other health issues. He has smoked about a pack of cigarettes daily for the past 20 years and consumed alcohol in social settings. Before discharge, the patient's intern noted that he had a serum sodium concentration of 132-134 mEq/L. No further investigations were performed, and the patient was advised not to drink too much water.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "Hyponatremia in a 46-Year-Old Man After Head Trauma"
},
{
"authors": "Federico J. Teran, MD; Eric E. Simon, MD; Vecihi Batuman, MD",
"content": [
"Physical Examination",
"During the follow-up visit, the patient was noted to have a serum sodium concentration of 128 mEq/L and was referred to nephrology for evaluation for hyponatremia. During his visit to the nephrology clinic, the patient complained of occasional dizziness and continued pain in the neck area but had no other complaints. He was alert, oriented, and well developed and appeared in mild distress with pain, wearing a neck collar brace. His weight was 87 kg, height was 174 cm, body mass index was 28.7 kg/m2, blood pressure was 128/76 mm Hg, pulse was 87 beats/min, and heart and lung examination findings were normal. No edema was noted, and the rest of his physical examination was unremarkable.",
"Repeat laboratory studies showed the following:",
"Sodium level—126 mEq/L",
"Potassium level—3.9 mEq/L",
"Chloride level—93 mEq/L",
"Total CO2—23",
"Blood urea nitrogen (BUN) level—10 mg/dL",
"Creatinine level—0.6 mg/dL",
"Glucose level—99 mg/dL",
"Uric acid level—1.6 mg/dL",
"Plasma osmolality—260 mOsm/kg",
"Urine studies revealed the following:",
"Sodium level—173 mEq/L",
"Potassium level—45 mEq/L",
"Creatinine level—168 mg/dL",
"Uric acid level—52 mg/dL",
"Urine osmolality—565 mOsm/kg",
"Complete blood count findings were normal, and nonfasting lipid profile found the following:",
"Total cholesterol—223 mg/dL",
"Triglyceride level—188 mg/dL",
"Low-density lipoprotein level—170 mg/dL",
"High-density lipoprotein level—46 mg/dL",
"Thyroid study findings were normal.",
"The patient declined hospital admission for investigation and treatment of his hyponatremia because he had urgent personal matters to attend to. However, 2 days later, the patient is brought to the emergency department because of increased confusion and seizure. Repeat chemistries showed the following:",
"Serum sodium level—113 mEq/L",
"Plasma osmolality—235 mOsm/kg",
"Urine osmolality—1057 mOsm/kg",
"Urine sodium level—196 mEq/L",
"A CT scan of the head revealed brain edema (Figure 1). The patient is admitted to the intensive care unit and urgent treatment is initiated.",
"Figure 1."
],
"date": "July 19, 2017",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/840/976/840976-thumb-1.jpg"
}
],
"markdown": "# Hyponatremia in a 46-Year-Old Man After Head Trauma\n\n **Authors:** Federico J. Teran, MD; Eric E. Simon, MD; Vecihi Batuman, MD \n **Date:** July 19, 2017\n\n ## Content\n\n Physical Examination\nDuring the follow-up visit, the patient was noted to have a serum sodium concentration of 128 mEq/L and was referred to nephrology for evaluation for hyponatremia. During his visit to the nephrology clinic, the patient complained of occasional dizziness and continued pain in the neck area but had no other complaints. He was alert, oriented, and well developed and appeared in mild distress with pain, wearing a neck collar brace. His weight was 87 kg, height was 174 cm, body mass index was 28.7 kg/m2, blood pressure was 128/76 mm Hg, pulse was 87 beats/min, and heart and lung examination findings were normal. No edema was noted, and the rest of his physical examination was unremarkable.\nRepeat laboratory studies showed the following:\nSodium level—126 mEq/L\nPotassium level—3.9 mEq/L\nChloride level—93 mEq/L\nTotal CO2—23\nBlood urea nitrogen (BUN) level—10 mg/dL\nCreatinine level—0.6 mg/dL\nGlucose level—99 mg/dL\nUric acid level—1.6 mg/dL\nPlasma osmolality—260 mOsm/kg\nUrine studies revealed the following:\nSodium level—173 mEq/L\nPotassium level—45 mEq/L\nCreatinine level—168 mg/dL\nUric acid level—52 mg/dL\nUrine osmolality—565 mOsm/kg\nComplete blood count findings were normal, and nonfasting lipid profile found the following:\nTotal cholesterol—223 mg/dL\nTriglyceride level—188 mg/dL\nLow-density lipoprotein level—170 mg/dL\nHigh-density lipoprotein level—46 mg/dL\nThyroid study findings were normal.\nThe patient declined hospital admission for investigation and treatment of his hyponatremia because he had urgent personal matters to attend to. However, 2 days later, the patient is brought to the emergency department because of increased confusion and seizure. Repeat chemistries showed the following:\nSerum sodium level—113 mEq/L\nPlasma osmolality—235 mOsm/kg\nUrine osmolality—1057 mOsm/kg\nUrine sodium level—196 mEq/L\nA CT scan of the head revealed brain edema (Figure 1). The patient is admitted to the intensive care unit and urgent treatment is initiated.\nFigure 1.\n\n ## Figures\n\n **Figure 1.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 819569,
"choiceText": "Cerebral salt wasting with appropriate antidiuretic syndrome secretion due to hypovolemia",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 819571,
"choiceText": "Fanconi syndrome with uric acid wasting",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 819573,
"choiceText": "Renal salt wasting",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 819575,
"choiceText": "Syndrome of inappropriate antidiuretic hormone secretion",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 819577,
"choiceText": "Pseudohyponatremia",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 257393,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Hyponatremia in a 46-Year-Old Man After Head Trauma"
},
{
"authors": "Federico J. Teran, MD; Eric E. Simon, MD; Vecihi Batuman, MD",
"content": [
"Discussion",
"The patient's hyponatremia with low plasma osmolality and high urine osmolality suggests syndrome of inappropriate antidiuretic hormone (SIADH) secretion.[1] When the plasma osmolality is low, the appropriate kidney response is to dilute the urine below the osmolality of the plasma, usually to maximally dilute levels of 50-60 mOsm/kg. This patient's measured plasma osmolality is low and close to the calculated osmolality (2 x [sodium] + [glucose]/18 + [BUN/2.8]), which rules out pseudohyponatremia. Pseudohyponatremia may be seen in patients with high levels of lipids or proteins, which displace serum water, resulting in an erroneous measurement of serum sodium.",
"Cerebral salt-wasting syndrome is more difficult to distinguish from SIADH. Some investigators consider it a variant of SIADH. The high urine sodium level and marked hypouricemia with renal urate wasting are consistent with cerebral salt wasting. Although cerebral disease is not a prerequisite of this syndrome, the patient does have a history of head trauma. Maesaka and colleagues[2,3,4] have argued that the term cerebral salt wasting should be replaced by renal salt wasting, as indeed the kidney is the organ responsible for the salt wasting. The proposed pathophysiology is relentless salt wasting in the kidney, possibly due to a salt-wasting factor or hormone, resulting in hypovolemia and triggering release of antidiuretic hormone. This syndrome, similar to SIADH, is also associated with hypouricemia and hyperuricosuria; however, unlike SIADH, both the hypouricemia and hyperuricosuria remain irreversible (fixed), which could be the distinguishing feature.[4] In this patient, repeat urine uric acid measurements were not performed; therefore, a salt-wasting syndrome cannot be definitively ruled out. However, such patients are often clinically hypovolemic and occasionally have orthostatic hypotension, which were absent in this patient, again favoring the diagnosis of SIADH.",
"SIADH is a disorder of water balance characterized by hypotonic hyponatremia and impaired water excretion in euvolemic individuals.[5,6] It is a common cause of hyponatremia and can be seen in diverse settings, including lung cancer (small cell), drug use (carbamazepine, certain selective serotonin reuptake inhibitors [SSRIs]), neurologic diseases (strokes, encephalitis, trauma), pulmonary diseases (pneumonia, tuberculosis), endocrine disorders (eg, hypothyroidism, pituitary disorders), and pain.[6]",
"Diagnosis is based on the exclusion of other hyponatremic conditions, the presence of hypo-osmolality with inappropriately high urine osmolality (less than maximally dilute) that is often hyperosmolar to serum (typically ≥400-500 mOsm/kg), and a urine sodium concentration usually above 30 mEq/L. Low serum urea levels and low serum uric acid levels with increased fractional excretion of uric acid (>9%-10%) are commonly associated with SIADH and may be helpful in confirming the diagnosis.[5,6]"
],
"date": "July 19, 2017",
"figures": [],
"markdown": "# Hyponatremia in a 46-Year-Old Man After Head Trauma\n\n **Authors:** Federico J. Teran, MD; Eric E. Simon, MD; Vecihi Batuman, MD \n **Date:** July 19, 2017\n\n ## Content\n\n Discussion\nThe patient's hyponatremia with low plasma osmolality and high urine osmolality suggests syndrome of inappropriate antidiuretic hormone (SIADH) secretion.[1] When the plasma osmolality is low, the appropriate kidney response is to dilute the urine below the osmolality of the plasma, usually to maximally dilute levels of 50-60 mOsm/kg. This patient's measured plasma osmolality is low and close to the calculated osmolality (2 x [sodium] + [glucose]/18 + [BUN/2.8]), which rules out pseudohyponatremia. Pseudohyponatremia may be seen in patients with high levels of lipids or proteins, which displace serum water, resulting in an erroneous measurement of serum sodium.\nCerebral salt-wasting syndrome is more difficult to distinguish from SIADH. Some investigators consider it a variant of SIADH. The high urine sodium level and marked hypouricemia with renal urate wasting are consistent with cerebral salt wasting. Although cerebral disease is not a prerequisite of this syndrome, the patient does have a history of head trauma. Maesaka and colleagues[2,3,4] have argued that the term cerebral salt wasting should be replaced by renal salt wasting, as indeed the kidney is the organ responsible for the salt wasting. The proposed pathophysiology is relentless salt wasting in the kidney, possibly due to a salt-wasting factor or hormone, resulting in hypovolemia and triggering release of antidiuretic hormone. This syndrome, similar to SIADH, is also associated with hypouricemia and hyperuricosuria; however, unlike SIADH, both the hypouricemia and hyperuricosuria remain irreversible (fixed), which could be the distinguishing feature.[4] In this patient, repeat urine uric acid measurements were not performed; therefore, a salt-wasting syndrome cannot be definitively ruled out. However, such patients are often clinically hypovolemic and occasionally have orthostatic hypotension, which were absent in this patient, again favoring the diagnosis of SIADH.\nSIADH is a disorder of water balance characterized by hypotonic hyponatremia and impaired water excretion in euvolemic individuals.[5,6] It is a common cause of hyponatremia and can be seen in diverse settings, including lung cancer (small cell), drug use (carbamazepine, certain selective serotonin reuptake inhibitors [SSRIs]), neurologic diseases (strokes, encephalitis, trauma), pulmonary diseases (pneumonia, tuberculosis), endocrine disorders (eg, hypothyroidism, pituitary disorders), and pain.[6]\nDiagnosis is based on the exclusion of other hyponatremic conditions, the presence of hypo-osmolality with inappropriately high urine osmolality (less than maximally dilute) that is often hyperosmolar to serum (typically ≥400-500 mOsm/kg), and a urine sodium concentration usually above 30 mEq/L. Low serum urea levels and low serum uric acid levels with increased fractional excretion of uric acid (>9%-10%) are commonly associated with SIADH and may be helpful in confirming the diagnosis.[5,6]\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 819569,
"choiceText": "Cerebral salt wasting with appropriate antidiuretic syndrome secretion due to hypovolemia",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 819571,
"choiceText": "Fanconi syndrome with uric acid wasting",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 819573,
"choiceText": "Renal salt wasting",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 819575,
"choiceText": "Syndrome of inappropriate antidiuretic hormone secretion",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 819577,
"choiceText": "Pseudohyponatremia",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 257393,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Hyponatremia in a 46-Year-Old Man After Head Trauma"
},
{
"authors": "Federico J. Teran, MD; Eric E. Simon, MD; Vecihi Batuman, MD",
"content": [
"The patient described had hyponatremia for longer than a month. This puts him in the category of chronic hyponatremia, which may influence management. Nevertheless, on his second presentation, the patient had profound hyponatremia, with symptoms due to brain edema (Figure 1), which constitutes a medical emergency and an indication for emergent treatment.",
"Figure 1.",
"A potentially serious pitfall during treatment of symptomatic hyponatremia is correction of hyponatremia that is too rapid; this can result in osmotic demyelination syndrome, which can be fatal (Figure 2).",
"Figure 2.",
"Hyponatremia must be corrected by a combination of water intake restriction to levels usually less than 500 mL/d. In an individual with a massive excess of water that is causing brain edema, restriction of water intake to zero may be a reasonable intervention.",
"Administration of hypertonic salt solutions is also indicated. The rate of correction can be calculated and should be carefully gauged. Extensive data suggest that the serum sodium should be raised by no more than 10 mEq/L over 24 hours.[1,7] Correction by 6 mEq/L in 24 hours has been dubbed the \"rule of sixes.\"[8,9] The rule of sixes is as follows: \"Six-a-day makes sense for safety. Six in 6 hours for severe symptoms and stop.\"",
"Correction that is too rapid and consequent osmotic demyelination can occur in instances of hyponatremia caused by cerebral salt wasting or with beer potomania, although the pathophysiology of hyponatremia in these disorders is different from that of SIADH.[10] Individuals with hypovolemia who replace their volume losses mostly by hypotonic fluids or electrolyte-free water and become hyponatremic are especially at risk for overcorrection. When such individuals are given crystalloid solutions to correct their hyponatremia and their volume losses are restored, the high antidiuretic hormone levels triggered by hypovolemia return to baseline, and the patients may start excreting copious amounts of maximally dilute urine, resulting in correction that is too rapid. In such situations, the overcorrection should be reversed to avoid osmotic demyelination and potential death. This can be accomplished by administration of electrolyte-free water to partially lower the serum sodium concentration and by simultaneously administering desmopressin to avoid too rapid dilution of urine.[11]"
],
"date": "July 19, 2017",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/840/976/840976-thumb-1.jpg"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/840/976/840976-thumb-2.jpg"
}
],
"markdown": "# Hyponatremia in a 46-Year-Old Man After Head Trauma\n\n **Authors:** Federico J. Teran, MD; Eric E. Simon, MD; Vecihi Batuman, MD \n **Date:** July 19, 2017\n\n ## Content\n\n The patient described had hyponatremia for longer than a month. This puts him in the category of chronic hyponatremia, which may influence management. Nevertheless, on his second presentation, the patient had profound hyponatremia, with symptoms due to brain edema (Figure 1), which constitutes a medical emergency and an indication for emergent treatment.\nFigure 1.\nA potentially serious pitfall during treatment of symptomatic hyponatremia is correction of hyponatremia that is too rapid; this can result in osmotic demyelination syndrome, which can be fatal (Figure 2).\nFigure 2.\nHyponatremia must be corrected by a combination of water intake restriction to levels usually less than 500 mL/d. In an individual with a massive excess of water that is causing brain edema, restriction of water intake to zero may be a reasonable intervention.\nAdministration of hypertonic salt solutions is also indicated. The rate of correction can be calculated and should be carefully gauged. Extensive data suggest that the serum sodium should be raised by no more than 10 mEq/L over 24 hours.[1,7] Correction by 6 mEq/L in 24 hours has been dubbed the \"rule of sixes.\"[8,9] The rule of sixes is as follows: \"Six-a-day makes sense for safety. Six in 6 hours for severe symptoms and stop.\"\nCorrection that is too rapid and consequent osmotic demyelination can occur in instances of hyponatremia caused by cerebral salt wasting or with beer potomania, although the pathophysiology of hyponatremia in these disorders is different from that of SIADH.[10] Individuals with hypovolemia who replace their volume losses mostly by hypotonic fluids or electrolyte-free water and become hyponatremic are especially at risk for overcorrection. When such individuals are given crystalloid solutions to correct their hyponatremia and their volume losses are restored, the high antidiuretic hormone levels triggered by hypovolemia return to baseline, and the patients may start excreting copious amounts of maximally dilute urine, resulting in correction that is too rapid. In such situations, the overcorrection should be reversed to avoid osmotic demyelination and potential death. This can be accomplished by administration of electrolyte-free water to partially lower the serum sodium concentration and by simultaneously administering desmopressin to avoid too rapid dilution of urine.[11]\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n\n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "Hyponatremia in a 46-Year-Old Man After Head Trauma"
},
{
"authors": "Federico J. Teran, MD; Eric E. Simon, MD; Vecihi Batuman, MD",
"content": [
"For patients with relatively asymptomatic chronic hyponatremia, medical management becomes more challenging because these individuals may be seen in the ambulatory setting and not in an inpatient facility. Restricting fluid intake to low levels is difficult due to poor patient compliance and inadequate understanding of which fluids contain water. Therefore, turning to medications to force water loss is a viable option. Urea causes an osmotic diuresis, and urea tablets (not available in the United States) have been used in Belgium to treat SIADH; however, their bitter taste and pill burden result in poor patient acceptance.[12,13] Similarly, sodium chloride tablets and furosemide have been used in the ambulatory setting, causing a natriuresis/diuresis; again, their large pill burden and sour taste contribute to poor patient compliance. Demeclocycline causes reversible diabetes insipidus and has been used for years to treat SIADH, although side effects such as photosensitivity, allergic reactions, or liver or kidney toxicity can limit its use.[14,15]",
"The \"vaptans,\" such as tolvaptan, have emerged as a well-tolerated option to treat SIADH by blocking the effects of antidiuretic hormone.[16,17] Tolvaptan has had much clinical use since it was approved by the US Food and Drug Administration (FDA) in 2009 for the treatment of hyponatremia caused by SIADH or by heart failure, and this drug is available for the treatment of SIADH in selected cases.[1] The orally available form of tolvaptan has been used successfully for the treatment of hyponatremia caused by SIADH in a series of 10 patients with small cell lung cancer.[18] This is a viable option, but it is expensive and may cause liver injury in those on the medication for more than a month. Tolvaptan is not recommended for more than 30-day use because of potential liver toxicity; however, one report described once-weekly safe use at a low dose for a prolonged period in the outpatient setting in an elderly patient with symptomatic chronic hyponatremia due to SIADH.[19] More likely, a combination of oral agents is needed to manage chronic SIADH, with water restriction and patient education."
],
"date": "July 19, 2017",
"figures": [],
"markdown": "# Hyponatremia in a 46-Year-Old Man After Head Trauma\n\n **Authors:** Federico J. Teran, MD; Eric E. Simon, MD; Vecihi Batuman, MD \n **Date:** July 19, 2017\n\n ## Content\n\n For patients with relatively asymptomatic chronic hyponatremia, medical management becomes more challenging because these individuals may be seen in the ambulatory setting and not in an inpatient facility. Restricting fluid intake to low levels is difficult due to poor patient compliance and inadequate understanding of which fluids contain water. Therefore, turning to medications to force water loss is a viable option. Urea causes an osmotic diuresis, and urea tablets (not available in the United States) have been used in Belgium to treat SIADH; however, their bitter taste and pill burden result in poor patient acceptance.[12,13] Similarly, sodium chloride tablets and furosemide have been used in the ambulatory setting, causing a natriuresis/diuresis; again, their large pill burden and sour taste contribute to poor patient compliance. Demeclocycline causes reversible diabetes insipidus and has been used for years to treat SIADH, although side effects such as photosensitivity, allergic reactions, or liver or kidney toxicity can limit its use.[14,15]\nThe \"vaptans,\" such as tolvaptan, have emerged as a well-tolerated option to treat SIADH by blocking the effects of antidiuretic hormone.[16,17] Tolvaptan has had much clinical use since it was approved by the US Food and Drug Administration (FDA) in 2009 for the treatment of hyponatremia caused by SIADH or by heart failure, and this drug is available for the treatment of SIADH in selected cases.[1] The orally available form of tolvaptan has been used successfully for the treatment of hyponatremia caused by SIADH in a series of 10 patients with small cell lung cancer.[18] This is a viable option, but it is expensive and may cause liver injury in those on the medication for more than a month. Tolvaptan is not recommended for more than 30-day use because of potential liver toxicity; however, one report described once-weekly safe use at a low dose for a prolonged period in the outpatient setting in an elderly patient with symptomatic chronic hyponatremia due to SIADH.[19] More likely, a combination of oral agents is needed to manage chronic SIADH, with water restriction and patient education.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 819579,
"choiceText": "Immediately administer tolvaptan (30 mg)",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 819581,
"choiceText": "Restrict water intake to zero, monitor urine volume and sodium levels, and replace urine sodium",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 819583,
"choiceText": "Administer 100-mL bolus of 3% saline intravenously and repeat twice as needed to raise serum sodium by 6 mEq/L during the first 6 hours",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 819585,
"choiceText": "Administer 1 L of 3% saline to rapidly raise serum sodium levels to the normal range",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 819587,
"choiceText": "Administer furosemide 20 mg intravenously every 4 hours, monitor urine sodium levels, and replace urine sodium losses",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "In the setting of profound symptomatic hyponatremia, 3% saline is the only treatment option. Raising the serum sodium levels by 6 mEq/L is usually adequate to sufficiently decrease brain swelling to stop the central nervous system effects (ie, seizures).\r\n<br><br>\r\nAdministering 1 L of 3% saline to raise the serum sodium to normal levels predisposes the patient to neurologic complications of rapid correction, such as osmotic demyelination syndrome. \r\n<br><br>\r\nAlthough furosemide does diminish the concentration gradient in the renal medulla and increase free water losses, it is not recommended in the acute setting because it could lead to excess volume depletion and compromise an already impaired central nervous system circulation.<br><br>Water restriction alone would not be sufficient to treat an acute episode, such as in this patient. Vaptans are contraindicated in acute symptomatic hyponatremia.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 257395,
"questionText": "How would you design an appropriate treatment for this patient in the intensive care unit?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 819589,
"choiceText": "Serum uric acid level of 2.2 mg/dL",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 819591,
"choiceText": "Urine sodium concentration of 13 mEq/L",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 819593,
"choiceText": "Urine osmolality of 200 mOsm/kg",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 819595,
"choiceText": "Urine uric acid level of 48 mg/dL",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 819597,
"choiceText": "Blood pressure of 115/68 mm Hg",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "A urine sodium level of 13 mEq/L suggests volume depletion or ineffective blood volume state, which may trigger antidiuretic hormone release through volume stimulus and is inconsistent with the diagnosis of SIADH. Euvolemia is among the criteria for diagnosis of SIADH. Therefore, this is the best answer to this question, although rare instances do occur in which urine sodium concentrations may be low in a euvolemic person who is on a low-sodium intake. \r\n<br><br>\r\nThe uric acid level of 2.2 mg/dL, although not markedly decreased, may still be associated with SIADH and cannot be interpreted as evidence against that diagnosis.\r\n<br><br>\r\nAlthough in SIADH the urine osmolality is typically much higher than plasma, urine with an osmolality of 200 mOsm/kg is inappropriately less than maximally dilute and represents a defect in free-water clearance by the kidney; thus it is consistent with SIADH.\r\n<br><br>\r\nThe urine uric acid appears on the high side and thus favors the diagnosis of SIADH. \r\n<br><br>\r\nThe blood pressure is normal and is consistent with euvolemia, which is one of the criteria for the diagnosis of SIADH.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 257397,
"questionText": "A 38-year-old woman with a history of psychiatric disorder and chronic liver disease presents with serum sodium of 128 mEq/L. Which of the following findings would rule out SIADH? ",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Hyponatremia in a 46-Year-Old Man After Head Trauma"
},
{
"authors": "Federico J. Teran, MD; Eric E. Simon, MD; Vecihi Batuman, MD",
"content": [],
"date": "July 19, 2017",
"figures": [],
"markdown": "# Hyponatremia in a 46-Year-Old Man After Head Trauma\n\n **Authors:** Federico J. Teran, MD; Eric E. Simon, MD; Vecihi Batuman, MD \n **Date:** July 19, 2017\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 819579,
"choiceText": "Immediately administer tolvaptan (30 mg)",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 819581,
"choiceText": "Restrict water intake to zero, monitor urine volume and sodium levels, and replace urine sodium",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 819583,
"choiceText": "Administer 100-mL bolus of 3% saline intravenously and repeat twice as needed to raise serum sodium by 6 mEq/L during the first 6 hours",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 819585,
"choiceText": "Administer 1 L of 3% saline to rapidly raise serum sodium levels to the normal range",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 819587,
"choiceText": "Administer furosemide 20 mg intravenously every 4 hours, monitor urine sodium levels, and replace urine sodium losses",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "In the setting of profound symptomatic hyponatremia, 3% saline is the only treatment option. Raising the serum sodium levels by 6 mEq/L is usually adequate to sufficiently decrease brain swelling to stop the central nervous system effects (ie, seizures).\r\n<br><br>\r\nAdministering 1 L of 3% saline to raise the serum sodium to normal levels predisposes the patient to neurologic complications of rapid correction, such as osmotic demyelination syndrome. \r\n<br><br>\r\nAlthough furosemide does diminish the concentration gradient in the renal medulla and increase free water losses, it is not recommended in the acute setting because it could lead to excess volume depletion and compromise an already impaired central nervous system circulation.<br><br>Water restriction alone would not be sufficient to treat an acute episode, such as in this patient. Vaptans are contraindicated in acute symptomatic hyponatremia.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 257395,
"questionText": "How would you design an appropriate treatment for this patient in the intensive care unit?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 819589,
"choiceText": "Serum uric acid level of 2.2 mg/dL",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 819591,
"choiceText": "Urine sodium concentration of 13 mEq/L",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 819593,
"choiceText": "Urine osmolality of 200 mOsm/kg",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 819595,
"choiceText": "Urine uric acid level of 48 mg/dL",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 819597,
"choiceText": "Blood pressure of 115/68 mm Hg",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "A urine sodium level of 13 mEq/L suggests volume depletion or ineffective blood volume state, which may trigger antidiuretic hormone release through volume stimulus and is inconsistent with the diagnosis of SIADH. Euvolemia is among the criteria for diagnosis of SIADH. Therefore, this is the best answer to this question, although rare instances do occur in which urine sodium concentrations may be low in a euvolemic person who is on a low-sodium intake. \r\n<br><br>\r\nThe uric acid level of 2.2 mg/dL, although not markedly decreased, may still be associated with SIADH and cannot be interpreted as evidence against that diagnosis.\r\n<br><br>\r\nAlthough in SIADH the urine osmolality is typically much higher than plasma, urine with an osmolality of 200 mOsm/kg is inappropriately less than maximally dilute and represents a defect in free-water clearance by the kidney; thus it is consistent with SIADH.\r\n<br><br>\r\nThe urine uric acid appears on the high side and thus favors the diagnosis of SIADH. \r\n<br><br>\r\nThe blood pressure is normal and is consistent with euvolemia, which is one of the criteria for the diagnosis of SIADH.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 257397,
"questionText": "A 38-year-old woman with a history of psychiatric disorder and chronic liver disease presents with serum sodium of 128 mEq/L. Which of the following findings would rule out SIADH? ",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Hyponatremia in a 46-Year-Old Man After Head Trauma"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 819569,
"choiceText": "Cerebral salt wasting with appropriate antidiuretic syndrome secretion due to hypovolemia",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 819571,
"choiceText": "Fanconi syndrome with uric acid wasting",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 819573,
"choiceText": "Renal salt wasting",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 819575,
"choiceText": "Syndrome of inappropriate antidiuretic hormone secretion",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 819577,
"choiceText": "Pseudohyponatremia",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 257393,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 819579,
"choiceText": "Immediately administer tolvaptan (30 mg)",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 819581,
"choiceText": "Restrict water intake to zero, monitor urine volume and sodium levels, and replace urine sodium",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 819583,
"choiceText": "Administer 100-mL bolus of 3% saline intravenously and repeat twice as needed to raise serum sodium by 6 mEq/L during the first 6 hours",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 819585,
"choiceText": "Administer 1 L of 3% saline to rapidly raise serum sodium levels to the normal range",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 819587,
"choiceText": "Administer furosemide 20 mg intravenously every 4 hours, monitor urine sodium levels, and replace urine sodium losses",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "In the setting of profound symptomatic hyponatremia, 3% saline is the only treatment option. Raising the serum sodium levels by 6 mEq/L is usually adequate to sufficiently decrease brain swelling to stop the central nervous system effects (ie, seizures).\r\n<br><br>\r\nAdministering 1 L of 3% saline to raise the serum sodium to normal levels predisposes the patient to neurologic complications of rapid correction, such as osmotic demyelination syndrome. \r\n<br><br>\r\nAlthough furosemide does diminish the concentration gradient in the renal medulla and increase free water losses, it is not recommended in the acute setting because it could lead to excess volume depletion and compromise an already impaired central nervous system circulation.<br><br>Water restriction alone would not be sufficient to treat an acute episode, such as in this patient. Vaptans are contraindicated in acute symptomatic hyponatremia.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 257395,
"questionText": "How would you design an appropriate treatment for this patient in the intensive care unit?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 819589,
"choiceText": "Serum uric acid level of 2.2 mg/dL",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 819591,
"choiceText": "Urine sodium concentration of 13 mEq/L",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 819593,
"choiceText": "Urine osmolality of 200 mOsm/kg",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 819595,
"choiceText": "Urine uric acid level of 48 mg/dL",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 819597,
"choiceText": "Blood pressure of 115/68 mm Hg",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "A urine sodium level of 13 mEq/L suggests volume depletion or ineffective blood volume state, which may trigger antidiuretic hormone release through volume stimulus and is inconsistent with the diagnosis of SIADH. Euvolemia is among the criteria for diagnosis of SIADH. Therefore, this is the best answer to this question, although rare instances do occur in which urine sodium concentrations may be low in a euvolemic person who is on a low-sodium intake. \r\n<br><br>\r\nThe uric acid level of 2.2 mg/dL, although not markedly decreased, may still be associated with SIADH and cannot be interpreted as evidence against that diagnosis.\r\n<br><br>\r\nAlthough in SIADH the urine osmolality is typically much higher than plasma, urine with an osmolality of 200 mOsm/kg is inappropriately less than maximally dilute and represents a defect in free-water clearance by the kidney; thus it is consistent with SIADH.\r\n<br><br>\r\nThe urine uric acid appears on the high side and thus favors the diagnosis of SIADH. \r\n<br><br>\r\nThe blood pressure is normal and is consistent with euvolemia, which is one of the criteria for the diagnosis of SIADH.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 257397,
"questionText": "A 38-year-old woman with a history of psychiatric disorder and chronic liver disease presents with serum sodium of 128 mEq/L. Which of the following findings would rule out SIADH? ",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
840157 | /viewarticle/840157 | [
{
"authors": "Maria Silvana Horenstein, MD; Eduardo Franklin Horenstein, MD",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"A 2-year-old girl presents to her pediatrician with a 1-week history of a worsening papular rash associated with intense pruritus on the trunk, arms, and hands (see Figure 1 ).",
"Figure.",
"The itching is worse at night. She had not used any oral or topical medications before the onset of pruritus. Her parents deny any history of allergies, concurrent fever, or other illness. Her vaccination schedule is up-to-date. The patient's recent social history includes a summer vacation to the mountains 3 weeks before her parents noticed the skin rash."
],
"date": "July 17, 2017",
"figures": [
{
"caption": "Figure.",
"image_url": "https://img.medscapestatic.com/article/840/157/840157-thumb.jpg"
}
],
"markdown": "# A 2-Year-Old Girl With Intense Itching\n\n **Authors:** Maria Silvana Horenstein, MD; Eduardo Franklin Horenstein, MD \n **Date:** July 17, 2017\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nA 2-year-old girl presents to her pediatrician with a 1-week history of a worsening papular rash associated with intense pruritus on the trunk, arms, and hands (see Figure 1 ).\nFigure.\nThe itching is worse at night. She had not used any oral or topical medications before the onset of pruritus. Her parents deny any history of allergies, concurrent fever, or other illness. Her vaccination schedule is up-to-date. The patient's recent social history includes a summer vacation to the mountains 3 weeks before her parents noticed the skin rash.\n\n ## Figures\n\n **Figure.** \n \n\n\n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 2-Year-Old Girl With Intense Itching"
},
{
"authors": "Maria Silvana Horenstein, MD; Eduardo Franklin Horenstein, MD",
"content": [
"Physical Examination",
"Upon physical examination, the patient is a healthy-looking girl in no distress, in the 50th percentile for weight and 75th percentile for height. Physical examination findings are normal except for the skin. Numerous erythematous papules are evident on the trunk, face, and arms, with heavy involvement of the palms and soles, including the webbing of the fingers, but none on the back. Faint excoriations are also noted in the same anatomic locations. The child withdrew her hand when the lesions were palpated. The remainder of the skin examination was unremarkable.",
"The patient is prescribed a topical antibiotic ointment to prevent infection and an oral antihistamine to decrease the pruritus; however, 1 week later, the patient returns to the clinic with an increased number of lesions and persistent symptoms. Upon further questioning during this repeat visit, the patient's parents note that they had stayed in multiple hotels during their summer vacation. The mother did not have any skin lesions, but the father, not present during the child's visit, was a mechanic who had some papular lesions on his hands that he felt were work related."
],
"date": "July 17, 2017",
"figures": [],
"markdown": "# A 2-Year-Old Girl With Intense Itching\n\n **Authors:** Maria Silvana Horenstein, MD; Eduardo Franklin Horenstein, MD \n **Date:** July 17, 2017\n\n ## Content\n\n Physical Examination\nUpon physical examination, the patient is a healthy-looking girl in no distress, in the 50th percentile for weight and 75th percentile for height. Physical examination findings are normal except for the skin. Numerous erythematous papules are evident on the trunk, face, and arms, with heavy involvement of the palms and soles, including the webbing of the fingers, but none on the back. Faint excoriations are also noted in the same anatomic locations. The child withdrew her hand when the lesions were palpated. The remainder of the skin examination was unremarkable.\nThe patient is prescribed a topical antibiotic ointment to prevent infection and an oral antihistamine to decrease the pruritus; however, 1 week later, the patient returns to the clinic with an increased number of lesions and persistent symptoms. Upon further questioning during this repeat visit, the patient's parents note that they had stayed in multiple hotels during their summer vacation. The mother did not have any skin lesions, but the father, not present during the child's visit, was a mechanic who had some papular lesions on his hands that he felt were work related.\n\n ## Figures\n\n \n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 817235,
"choiceText": "Impetigo",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 817237,
"choiceText": "Scabies",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 817239,
"choiceText": "Psoriasis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 817241,
"choiceText": "Contact dermatitis",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 256565,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 2-Year-Old Girl With Intense Itching"
},
{
"authors": "Maria Silvana Horenstein, MD; Eduardo Franklin Horenstein, MD",
"content": [
"Discussion",
"A skin scraping was performed and examined under microscopy. The scraping demonstrated mites, ova, and scybala (fecal debris) consistent with a diagnosis of scabies. Scabies is a parasitic infestation of the skin caused by the arthropod Sarcoptes scabiei var. hominis, a very small parasite measuring 0.3 mm to 0.9 mm in size. Variants of the scabies mite that affect other animals (eg, dogs, cats, pigs) are unable to reproduce in humans, but they can cause a transient dermatitis. Scabies was well known to the Greeks and Romans. The word scabies is derived from the Latin word scabere, which means to scratch. The Italian biologist Giovanni Cosimo Bonomo described the mite and linked it to the disease in the 17th century; however, it wasn't until the 19th century that the skeptical scientific community finally accepted that scabies was caused by the mite Sarcoptes scabiei.[1,2]",
"In the 21st century, scabies continues to be a worldwide health problem. Its prevalence is higher in developing countries, where overcrowding and poverty lead to an increase in transmission. It is estimated that 300 million people are affected worldwide.",
"Scabies is transmitted by prolonged skin-to-skin contact with an infected person. Children (especially those of poor or underserved communities), sexually active individuals, and institutionalized patients are all at increased risk of acquiring the disease because these groups have an increased chance of human contact. Scabies is also considered a sexually transmitted disease. It is infrequently acquired through the use of infested clothes, bed linen, and towels. A scabies infestation occurs when an impregnated female parasite enters the skin, tunnels into the stratum corneum, and excavates burrows to lay and deposit its eggs. The larvae hatch and begin to move 3-10 days later. The female mite can sometimes be observed at the end of the burrow. Male mites usually roam on the surface of the skin, but female mites may also surface occasionally (especially at night), at which time they can be washed or scratched off.[1,2,3]",
"Intense pruritus is a classic symptom of scabies, and it is usually more severe at night. Symptoms are caused by a delayed type IV hypersensitivity reaction to the mites, ova, and scybala (feces) under the skin. The symptoms occur 4-6 weeks after the initial infection. Previously sensitized patients may have symptoms within hours of reinfection.[1,2]"
],
"date": "July 17, 2017",
"figures": [],
"markdown": "# A 2-Year-Old Girl With Intense Itching\n\n **Authors:** Maria Silvana Horenstein, MD; Eduardo Franklin Horenstein, MD \n **Date:** July 17, 2017\n\n ## Content\n\n Discussion\nA skin scraping was performed and examined under microscopy. The scraping demonstrated mites, ova, and scybala (fecal debris) consistent with a diagnosis of scabies. Scabies is a parasitic infestation of the skin caused by the arthropod Sarcoptes scabiei var. hominis, a very small parasite measuring 0.3 mm to 0.9 mm in size. Variants of the scabies mite that affect other animals (eg, dogs, cats, pigs) are unable to reproduce in humans, but they can cause a transient dermatitis. Scabies was well known to the Greeks and Romans. The word scabies is derived from the Latin word scabere, which means to scratch. The Italian biologist Giovanni Cosimo Bonomo described the mite and linked it to the disease in the 17th century; however, it wasn't until the 19th century that the skeptical scientific community finally accepted that scabies was caused by the mite Sarcoptes scabiei.[1,2]\nIn the 21st century, scabies continues to be a worldwide health problem. Its prevalence is higher in developing countries, where overcrowding and poverty lead to an increase in transmission. It is estimated that 300 million people are affected worldwide.\nScabies is transmitted by prolonged skin-to-skin contact with an infected person. Children (especially those of poor or underserved communities), sexually active individuals, and institutionalized patients are all at increased risk of acquiring the disease because these groups have an increased chance of human contact. Scabies is also considered a sexually transmitted disease. It is infrequently acquired through the use of infested clothes, bed linen, and towels. A scabies infestation occurs when an impregnated female parasite enters the skin, tunnels into the stratum corneum, and excavates burrows to lay and deposit its eggs. The larvae hatch and begin to move 3-10 days later. The female mite can sometimes be observed at the end of the burrow. Male mites usually roam on the surface of the skin, but female mites may also surface occasionally (especially at night), at which time they can be washed or scratched off.[1,2,3]\nIntense pruritus is a classic symptom of scabies, and it is usually more severe at night. Symptoms are caused by a delayed type IV hypersensitivity reaction to the mites, ova, and scybala (feces) under the skin. The symptoms occur 4-6 weeks after the initial infection. Previously sensitized patients may have symptoms within hours of reinfection.[1,2]\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 817235,
"choiceText": "Impetigo",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 817237,
"choiceText": "Scabies",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 817239,
"choiceText": "Psoriasis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 817241,
"choiceText": "Contact dermatitis",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 256565,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 2-Year-Old Girl With Intense Itching"
},
{
"authors": "Maria Silvana Horenstein, MD; Eduardo Franklin Horenstein, MD",
"content": [
"Scabies can mimic numerous skin conditions, such as insect bites, impetigo, atopic dermatitis, contact dermatitis, and herpetic dermatitis, among others. Not uncommonly, scabies is misdiagnosed as one of these other skin conditions, allowing transmission to continue to occur until the correct diagnosis is established. Scabies can be diagnosed by visualizing the burrows or \"tracks\" left by the female mite as she moves through the stratus corneum of the skin. Initially, these burrows may appear as short, zigzagging, and hardly noticeable scratchlike marks with a slightly shiny hue, and they may be connected to an erythematous papule. This papule harbors the female mite and feces. As a result of the intense pruritus associated with scabies, the burrows may be difficult to differentiate from the scratch marks produced by the patient. Adult patients may show lesions between their fingers or on their wrists, axillae, areolae, elbows, knees, genitals, and buttocks. Alternately, children may have any part of their skin affected.",
"The diagnosis can be confirmed by finding the mite, ova, or fecal debris of a lesion scraping under microscopy. The skin scraping is obtained by placing a drop of mineral oil on a vesicle or burrow lesion, scraping with a number-15 blade, and placing the scraping on a glass slide; multiple scrapings may be necessary to positively make the diagnosis. Another method is pinching a lesion between the thumb and index finger and superficially shaving the dermis. Also popular is the \"adhesive tape test,\" using transparent tape applied to a skin lesion and rapidly pulling the tape off. The tape is then placed on a glass slide for microscopic examination. In rare cases, skin biopsies may be obtained to both positively identify the mite or characteristic histopathology and to rule out other dermatoses.[1]",
"Related to scratching, patients with scabies may also become superinfected with Streptococcus pyogenes and Staphylococcus aureus . The complication of superinfection can lead to specific infections, such as impetigo, cellulitis, acute poststreptococcal glomerulonephritis, rheumatic fever, and even sepsis.",
"Immunosuppressed patients, particularly those with AIDS, may present with a severe form of scabies known as crusted scabies or Norwegian scabies. This is a widespread hyperkeratotic rash, with thick scaling and crusting and, at times, minimal to no pruritus. During this type of infection, up to millions of mites may survive for up to 1 week. In nonimmunocompromised hosts, an average of 5-15 mites cause infestation and survive for only 2-3 days. Crusted scabies appears to be the result of a high number of infiltrating CD8+ T lymphocytes in the dermis, with minimal helper T lymphocytes (CD4+), which is the opposite of that of a normal immune reaction to scabies. This detail may account for the body's inability to mount an appropriate immune response against the scabies mites.[1,2]"
],
"date": "July 17, 2017",
"figures": [],
"markdown": "# A 2-Year-Old Girl With Intense Itching\n\n **Authors:** Maria Silvana Horenstein, MD; Eduardo Franklin Horenstein, MD \n **Date:** July 17, 2017\n\n ## Content\n\n Scabies can mimic numerous skin conditions, such as insect bites, impetigo, atopic dermatitis, contact dermatitis, and herpetic dermatitis, among others. Not uncommonly, scabies is misdiagnosed as one of these other skin conditions, allowing transmission to continue to occur until the correct diagnosis is established. Scabies can be diagnosed by visualizing the burrows or \"tracks\" left by the female mite as she moves through the stratus corneum of the skin. Initially, these burrows may appear as short, zigzagging, and hardly noticeable scratchlike marks with a slightly shiny hue, and they may be connected to an erythematous papule. This papule harbors the female mite and feces. As a result of the intense pruritus associated with scabies, the burrows may be difficult to differentiate from the scratch marks produced by the patient. Adult patients may show lesions between their fingers or on their wrists, axillae, areolae, elbows, knees, genitals, and buttocks. Alternately, children may have any part of their skin affected.\nThe diagnosis can be confirmed by finding the mite, ova, or fecal debris of a lesion scraping under microscopy. The skin scraping is obtained by placing a drop of mineral oil on a vesicle or burrow lesion, scraping with a number-15 blade, and placing the scraping on a glass slide; multiple scrapings may be necessary to positively make the diagnosis. Another method is pinching a lesion between the thumb and index finger and superficially shaving the dermis. Also popular is the \"adhesive tape test,\" using transparent tape applied to a skin lesion and rapidly pulling the tape off. The tape is then placed on a glass slide for microscopic examination. In rare cases, skin biopsies may be obtained to both positively identify the mite or characteristic histopathology and to rule out other dermatoses.[1]\nRelated to scratching, patients with scabies may also become superinfected with Streptococcus pyogenes and Staphylococcus aureus . The complication of superinfection can lead to specific infections, such as impetigo, cellulitis, acute poststreptococcal glomerulonephritis, rheumatic fever, and even sepsis.\nImmunosuppressed patients, particularly those with AIDS, may present with a severe form of scabies known as crusted scabies or Norwegian scabies. This is a widespread hyperkeratotic rash, with thick scaling and crusting and, at times, minimal to no pruritus. During this type of infection, up to millions of mites may survive for up to 1 week. In nonimmunocompromised hosts, an average of 5-15 mites cause infestation and survive for only 2-3 days. Crusted scabies appears to be the result of a high number of infiltrating CD8+ T lymphocytes in the dermis, with minimal helper T lymphocytes (CD4+), which is the opposite of that of a normal immune reaction to scabies. This detail may account for the body's inability to mount an appropriate immune response against the scabies mites.[1,2]\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 2-Year-Old Girl With Intense Itching"
},
{
"authors": "Maria Silvana Horenstein, MD; Eduardo Franklin Horenstein, MD",
"content": [
"The cornerstone of medical treatment for scabies consists of administering a scabicidal agent in combination with an antipruritic agent. An antimicrobial agent may be added if the lesions have become infected with bacteria. The most effective topical scabicidal agent is permethrin 5% cream, recommended as a first-line therapy by the Centers for Disease Control and Prevention. Other agents, such as lindane, malathion, sulfur in petrolatum (usually 6%), crotamiton, and benzyl benzoate, are less effective. Permethrin is applied from chin to toes and for a period of 10-12 hours, after which it should be washed off. The treatment is repeated in 1 week. Hypersensitivity reactions to permethrin have been documented; minimal systemic toxicity is caused by minimal skin absorption. Permethrin is the recommended drug of choice for infants (>2 months), children, pregnant women (Class B), and nursing mothers. Antihistamines and topical antibiotics may be used as well to decrease pruritus and treat any skin infection.",
"Ivermectin, an oral scabicide, is used in the United States for refractory patients or patients who cannot tolerate topical treatments. The dose is 200 µg/kg given on days 1, 2, 8, 9, and 15.",
"Preventing scabies re-infestation in family members and close contacts to the affected person is paramount; therefore, all household members should be treated regardless of their symptoms (or lack thereof). In addition, all clothing and linen that may have been in contact with the affected person should be washed in hot water and machine-dried. This process should be repeated 1 week later. Carpets and upholstered furniture should be vacuumed; vacuum bags and canister contents should be disposed of immediately. Patients should be re-examined 2 weeks after treatment to ascertain the treatment effectiveness.[1]",
"In this patient, treatment with topical permethrin 5% cured the infestation; however, she developed an allergic reaction to the permethrin cream that manifested as widespread pruritic, papular, 1-mm lesions on her knees and elbows. Her condition responded to a 1-week course of topical steroids. Although they were asymptomatic, all household contacts were treated, along with the family members who had been on vacation with the patient."
],
"date": "July 17, 2017",
"figures": [],
"markdown": "# A 2-Year-Old Girl With Intense Itching\n\n **Authors:** Maria Silvana Horenstein, MD; Eduardo Franklin Horenstein, MD \n **Date:** July 17, 2017\n\n ## Content\n\n The cornerstone of medical treatment for scabies consists of administering a scabicidal agent in combination with an antipruritic agent. An antimicrobial agent may be added if the lesions have become infected with bacteria. The most effective topical scabicidal agent is permethrin 5% cream, recommended as a first-line therapy by the Centers for Disease Control and Prevention. Other agents, such as lindane, malathion, sulfur in petrolatum (usually 6%), crotamiton, and benzyl benzoate, are less effective. Permethrin is applied from chin to toes and for a period of 10-12 hours, after which it should be washed off. The treatment is repeated in 1 week. Hypersensitivity reactions to permethrin have been documented; minimal systemic toxicity is caused by minimal skin absorption. Permethrin is the recommended drug of choice for infants (>2 months), children, pregnant women (Class B), and nursing mothers. Antihistamines and topical antibiotics may be used as well to decrease pruritus and treat any skin infection.\nIvermectin, an oral scabicide, is used in the United States for refractory patients or patients who cannot tolerate topical treatments. The dose is 200 µg/kg given on days 1, 2, 8, 9, and 15.\nPreventing scabies re-infestation in family members and close contacts to the affected person is paramount; therefore, all household members should be treated regardless of their symptoms (or lack thereof). In addition, all clothing and linen that may have been in contact with the affected person should be washed in hot water and machine-dried. This process should be repeated 1 week later. Carpets and upholstered furniture should be vacuumed; vacuum bags and canister contents should be disposed of immediately. Patients should be re-examined 2 weeks after treatment to ascertain the treatment effectiveness.[1]\nIn this patient, treatment with topical permethrin 5% cured the infestation; however, she developed an allergic reaction to the permethrin cream that manifested as widespread pruritic, papular, 1-mm lesions on her knees and elbows. Her condition responded to a 1-week course of topical steroids. Although they were asymptomatic, all household contacts were treated, along with the family members who had been on vacation with the patient.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 817243,
"choiceText": "Scattered pruriginous, erythematous papular lesions on the wrists unilaterally, with an amber crust",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 817245,
"choiceText": "Pruriginous papules and vesicles with an erythematous base on an infant's cheeks",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 817247,
"choiceText": "A grouping of fluid-filled vesicles on erythematous, dry skin, with intense pruritus surrounding the wrist unilaterally, where jewelry has been removed for better examination",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 817249,
"choiceText": "Erythematous papules associated with burrows located on the child's cheeks",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 817251,
"choiceText": "A cluster of vesicles on an erythematous base on the forehead of an adolescent male with an antecedent history of pruritus and pain",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Scabies can be diagnosed by visualizing the burrows or \"tracks\" left by the female mite as she moves through the stratus corneum of the skin. Initially, these burrows may appear as short, zigzagging, and hardly noticeable scratchlike marks with a slightly shiny hue, and they may be connected to an erythematous papule. This papule harbors the female mite and feces.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 256567,
"questionText": "Which of the following findings is typical in a child who is diagnosed with scabies from <i>Sarcoptes scabiei</i> var. <i>hominis</i>?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": false,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 817253,
"choiceText": "Higher prevalence in children",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 817255,
"choiceText": "Infestation through close contact with infected animals",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 817257,
"choiceText": "Secondary bacterial infection of lesions caused by scratching",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 817259,
"choiceText": "Infestation of household members",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 817261,
"choiceText": "Same prevalence in both sexes",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Variants of the scabies mite that affect other animals (eg, dogs, cats, pigs) are unable to reproduce in humans, but they can cause a transient dermatitis.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 256569,
"questionText": "Which of the following is <i>not </i>characteristic of human scabies?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 2-Year-Old Girl With Intense Itching"
},
{
"authors": "Maria Silvana Horenstein, MD; Eduardo Franklin Horenstein, MD",
"content": [],
"date": "July 17, 2017",
"figures": [],
"markdown": "# A 2-Year-Old Girl With Intense Itching\n\n **Authors:** Maria Silvana Horenstein, MD; Eduardo Franklin Horenstein, MD \n **Date:** July 17, 2017\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 817243,
"choiceText": "Scattered pruriginous, erythematous papular lesions on the wrists unilaterally, with an amber crust",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 817245,
"choiceText": "Pruriginous papules and vesicles with an erythematous base on an infant's cheeks",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 817247,
"choiceText": "A grouping of fluid-filled vesicles on erythematous, dry skin, with intense pruritus surrounding the wrist unilaterally, where jewelry has been removed for better examination",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 817249,
"choiceText": "Erythematous papules associated with burrows located on the child's cheeks",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 817251,
"choiceText": "A cluster of vesicles on an erythematous base on the forehead of an adolescent male with an antecedent history of pruritus and pain",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Scabies can be diagnosed by visualizing the burrows or \"tracks\" left by the female mite as she moves through the stratus corneum of the skin. Initially, these burrows may appear as short, zigzagging, and hardly noticeable scratchlike marks with a slightly shiny hue, and they may be connected to an erythematous papule. This papule harbors the female mite and feces.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 256567,
"questionText": "Which of the following findings is typical in a child who is diagnosed with scabies from <i>Sarcoptes scabiei</i> var. <i>hominis</i>?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": false,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 817253,
"choiceText": "Higher prevalence in children",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 817255,
"choiceText": "Infestation through close contact with infected animals",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 817257,
"choiceText": "Secondary bacterial infection of lesions caused by scratching",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 817259,
"choiceText": "Infestation of household members",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 817261,
"choiceText": "Same prevalence in both sexes",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Variants of the scabies mite that affect other animals (eg, dogs, cats, pigs) are unable to reproduce in humans, but they can cause a transient dermatitis.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 256569,
"questionText": "Which of the following is <i>not </i>characteristic of human scabies?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 2-Year-Old Girl With Intense Itching"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 817235,
"choiceText": "Impetigo",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 817237,
"choiceText": "Scabies",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 817239,
"choiceText": "Psoriasis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 817241,
"choiceText": "Contact dermatitis",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 256565,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 817243,
"choiceText": "Scattered pruriginous, erythematous papular lesions on the wrists unilaterally, with an amber crust",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 817245,
"choiceText": "Pruriginous papules and vesicles with an erythematous base on an infant's cheeks",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 817247,
"choiceText": "A grouping of fluid-filled vesicles on erythematous, dry skin, with intense pruritus surrounding the wrist unilaterally, where jewelry has been removed for better examination",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 817249,
"choiceText": "Erythematous papules associated with burrows located on the child's cheeks",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 817251,
"choiceText": "A cluster of vesicles on an erythematous base on the forehead of an adolescent male with an antecedent history of pruritus and pain",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Scabies can be diagnosed by visualizing the burrows or \"tracks\" left by the female mite as she moves through the stratus corneum of the skin. Initially, these burrows may appear as short, zigzagging, and hardly noticeable scratchlike marks with a slightly shiny hue, and they may be connected to an erythematous papule. This papule harbors the female mite and feces.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 256567,
"questionText": "Which of the following findings is typical in a child who is diagnosed with scabies from <i>Sarcoptes scabiei</i> var. <i>hominis</i>?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": false,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 817253,
"choiceText": "Higher prevalence in children",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 817255,
"choiceText": "Infestation through close contact with infected animals",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 817257,
"choiceText": "Secondary bacterial infection of lesions caused by scratching",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 817259,
"choiceText": "Infestation of household members",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 817261,
"choiceText": "Same prevalence in both sexes",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Variants of the scabies mite that affect other animals (eg, dogs, cats, pigs) are unable to reproduce in humans, but they can cause a transient dermatitis.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 256569,
"questionText": "Which of the following is <i>not </i>characteristic of human scabies?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
839441 | /viewarticle/839441 | [
{
"authors": "Andrew E. Green, MD",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.",
"Background",
"A 52-year-old obese white woman (gravida 2, para 2) presents to the emergency department with shortness of breath and crampy abdominal pain. She was seen 3 weeks ago by her family practitioner for similar pain. Her examination findings were normal at that time, per her report. She was referred to a gastroenterologist. From that appointment, she reports normal examination findings. She had a screening colonoscopy 1 week ago and reports no issues; she has not had her follow-up appointment.",
"Upon further questioning, she reports that her appetite is decreased and that she has been experiencing early satiety for the past 1-2 weeks. She reports having normal bowel movements and urinary habits. She has a medical history of hypercholesterolemia and mild hypertension. She had a screen for diabetes last year that was negative. She has a surgical history of a laparoscopic cholecystectomy and a hysterectomy. She takes metoprolol and atorvastatin. She has no known allergies. She reports no smoking, drinking, or illicit drug use. She has a family history of hypertension and breast cancer."
],
"date": "January 16, 2018",
"figures": [],
"markdown": "# Shortness of Breath and Abdominal Pain in an Obese Woman\n\n **Authors:** Andrew E. Green, MD \n **Date:** January 16, 2018\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.\nBackground\nA 52-year-old obese white woman (gravida 2, para 2) presents to the emergency department with shortness of breath and crampy abdominal pain. She was seen 3 weeks ago by her family practitioner for similar pain. Her examination findings were normal at that time, per her report. She was referred to a gastroenterologist. From that appointment, she reports normal examination findings. She had a screening colonoscopy 1 week ago and reports no issues; she has not had her follow-up appointment.\nUpon further questioning, she reports that her appetite is decreased and that she has been experiencing early satiety for the past 1-2 weeks. She reports having normal bowel movements and urinary habits. She has a medical history of hypercholesterolemia and mild hypertension. She had a screen for diabetes last year that was negative. She has a surgical history of a laparoscopic cholecystectomy and a hysterectomy. She takes metoprolol and atorvastatin. She has no known allergies. She reports no smoking, drinking, or illicit drug use. She has a family history of hypertension and breast cancer.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "Shortness of Breath and Abdominal Pain in an Obese Woman"
},
{
"authors": "Andrew E. Green, MD",
"content": [
"Physical Examination and Workup",
"Upon physical examination, she is in no acute distress but appears to have mild dyspnea after prolonged discussion. She is obese. Her vitals are as follows:",
"• Blood pressure: 140/85 mm Hg",
"• Pulse: 92 beats/min",
"• Respiratory rate: 22 breaths/min",
"• Temperature: 98.7°F",
"Pertinent physical examination findings include regular heart rhythm. She has crackles at the right lung base. Her abdominal examination is benign. Pelvic examination findings are normal, but the examination is limited by her body habitus. Chest radiography was performed and shows a right pleural effusion (Figure 1).",
"Figure 1.",
"An emergency department laboratory panel was performed. Pertinent findings were:",
"• Hemoglobin level: 9.1 g/dL",
"• Albumin level: 2.3 g/dL",
"• Sodium level: 131 mEq/L",
"• Potassium level: 3.3 mEq/L",
"This led to a CT scan of the chest, which confirmed the effusion and showed no pericardial effusion or masses in the chest. It did show ascites in the abdomen. A CT scan of the abdomen and pelvis showed ascites (Figure 2), omental caking, and a 7-cm right pelvic mass.",
"Figure 2."
],
"date": "January 16, 2018",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/839/441/839441-thumb-1.jpg"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/839/441/839441-thumb-2.jpg"
}
],
"markdown": "# Shortness of Breath and Abdominal Pain in an Obese Woman\n\n **Authors:** Andrew E. Green, MD \n **Date:** January 16, 2018\n\n ## Content\n\n Physical Examination and Workup\nUpon physical examination, she is in no acute distress but appears to have mild dyspnea after prolonged discussion. She is obese. Her vitals are as follows:\n• Blood pressure: 140/85 mm Hg\n• Pulse: 92 beats/min\n• Respiratory rate: 22 breaths/min\n• Temperature: 98.7°F\nPertinent physical examination findings include regular heart rhythm. She has crackles at the right lung base. Her abdominal examination is benign. Pelvic examination findings are normal, but the examination is limited by her body habitus. Chest radiography was performed and shows a right pleural effusion (Figure 1).\nFigure 1.\nAn emergency department laboratory panel was performed. Pertinent findings were:\n• Hemoglobin level: 9.1 g/dL\n• Albumin level: 2.3 g/dL\n• Sodium level: 131 mEq/L\n• Potassium level: 3.3 mEq/L\nThis led to a CT scan of the chest, which confirmed the effusion and showed no pericardial effusion or masses in the chest. It did show ascites in the abdomen. A CT scan of the abdomen and pelvis showed ascites (Figure 2), omental caking, and a 7-cm right pelvic mass.\nFigure 2.\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 812173,
"choiceText": "Tubal cancer",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 812175,
"choiceText": "Abdominal peritoneal tuberculosis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 812177,
"choiceText": "Metastatic gastrointestinal carcinoma",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 812179,
"choiceText": "Ovarian cancer",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 254919,
"questionText": "On the basis of the history, physical examination, and workup, what is the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Shortness of Breath and Abdominal Pain in an Obese Woman"
},
{
"authors": "Andrew E. Green, MD",
"content": [
"Discussion",
"A gynecologic oncologist was consulted who recommended paracentesis. This revealed a serous adenocarcinoma, consistent with ovarian cancer.",
"Ovarian cancer is a difficult cancer to treat because it is most often diagnosed at stage IIIC or higher. This patient has a typical presentation. Many women often see two to three other doctors before they are diagnosed and referred to a gynecologic oncologist. As in this case, they often undergo numerous tests prior to diagnosis as well. A high degree of suspicion is required to diagnose this disease, especially in the absence of more severe symptoms.",
"Ovarian, fallopian tube, and primary peritoneal cancers are all malignancies that are treated in a similar fashion. Recent data suggest that much of what has been considered ovarian cancer in the past was actually fallopian tube cancer. Although mostly a pathologic nomenclature issue, this does have significant implications for prevention."
],
"date": "January 16, 2018",
"figures": [],
"markdown": "# Shortness of Breath and Abdominal Pain in an Obese Woman\n\n **Authors:** Andrew E. Green, MD \n **Date:** January 16, 2018\n\n ## Content\n\n Discussion\nA gynecologic oncologist was consulted who recommended paracentesis. This revealed a serous adenocarcinoma, consistent with ovarian cancer.\nOvarian cancer is a difficult cancer to treat because it is most often diagnosed at stage IIIC or higher. This patient has a typical presentation. Many women often see two to three other doctors before they are diagnosed and referred to a gynecologic oncologist. As in this case, they often undergo numerous tests prior to diagnosis as well. A high degree of suspicion is required to diagnose this disease, especially in the absence of more severe symptoms.\nOvarian, fallopian tube, and primary peritoneal cancers are all malignancies that are treated in a similar fashion. Recent data suggest that much of what has been considered ovarian cancer in the past was actually fallopian tube cancer. Although mostly a pathologic nomenclature issue, this does have significant implications for prevention.\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 812173,
"choiceText": "Tubal cancer",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 812175,
"choiceText": "Abdominal peritoneal tuberculosis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 812177,
"choiceText": "Metastatic gastrointestinal carcinoma",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 812179,
"choiceText": "Ovarian cancer",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 254919,
"questionText": "On the basis of the history, physical examination, and workup, what is the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Shortness of Breath and Abdominal Pain in an Obese Woman"
},
{
"authors": "Andrew E. Green, MD",
"content": [
"Nearly 20,000 cases of ovarian cancer occur annually, and a woman has a 1:90 risk of developing one of these tumors in her lifetime.[1] Risk factors include conditions that promote ovulation, including age, early menarche, late menopause, and nulliparity. Family history also plays an important role in individuals with BRCA mutation.",
"For patients diagnosed with ovarian cancer, several important considerations must be made in the initial phases of treatment. The physicians most qualified to do this are gynecologic oncologists. Data are clear that if a patient's care is directed by a gynecologic oncologist, survival rates are better.[2] An early decision is whether to (1) include the use of surgery upfront followed by adjuvant intraperitoneal (IP) and/or intravenous (IV) chemotherapy or (2) start with neoadjuvant intravenous chemotherapy and then perform interval debulking followed by IP/IV or IV chemotherapy.",
"The decision processes are complex, but the major consideration is the ability of the surgeon to optimally perform debulking. The current definition of this is to remove all visible tumor. Leaving no nodules larger than 2 cm behind is the older definition. This has evolved over time, and no visible disease remaining is now the current standard. This is the only modifiable factor in the disease process. Patients whose disease is resected to no visible residual disease have an increased survival rate over those who have disease left behind.",
"Frontline chemotherapy currently consists of a combination of IP and IV chemotherapy or IV therapy alone. Studies have shown significantly increased survival with IP (> 1 year) over IV; this route of chemotherapy should be strongly considered upfront in all patients.[3,4] As long as a patient remains sensitive to it, platinum-based drugs used in doublets with taxanes, gemcitabine, pegylated liposomal doxorubicin, and others should be considered the standard of care. When a patient becomes platinum-resistant, single agents are used and life expectancy is shortened."
],
"date": "January 16, 2018",
"figures": [],
"markdown": "# Shortness of Breath and Abdominal Pain in an Obese Woman\n\n **Authors:** Andrew E. Green, MD \n **Date:** January 16, 2018\n\n ## Content\n\n Nearly 20,000 cases of ovarian cancer occur annually, and a woman has a 1:90 risk of developing one of these tumors in her lifetime.[1] Risk factors include conditions that promote ovulation, including age, early menarche, late menopause, and nulliparity. Family history also plays an important role in individuals with BRCA mutation.\nFor patients diagnosed with ovarian cancer, several important considerations must be made in the initial phases of treatment. The physicians most qualified to do this are gynecologic oncologists. Data are clear that if a patient's care is directed by a gynecologic oncologist, survival rates are better.[2] An early decision is whether to (1) include the use of surgery upfront followed by adjuvant intraperitoneal (IP) and/or intravenous (IV) chemotherapy or (2) start with neoadjuvant intravenous chemotherapy and then perform interval debulking followed by IP/IV or IV chemotherapy.\nThe decision processes are complex, but the major consideration is the ability of the surgeon to optimally perform debulking. The current definition of this is to remove all visible tumor. Leaving no nodules larger than 2 cm behind is the older definition. This has evolved over time, and no visible disease remaining is now the current standard. This is the only modifiable factor in the disease process. Patients whose disease is resected to no visible residual disease have an increased survival rate over those who have disease left behind.\nFrontline chemotherapy currently consists of a combination of IP and IV chemotherapy or IV therapy alone. Studies have shown significantly increased survival with IP (> 1 year) over IV; this route of chemotherapy should be strongly considered upfront in all patients.[3,4] As long as a patient remains sensitive to it, platinum-based drugs used in doublets with taxanes, gemcitabine, pegylated liposomal doxorubicin, and others should be considered the standard of care. When a patient becomes platinum-resistant, single agents are used and life expectancy is shortened.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "Shortness of Breath and Abdominal Pain in an Obese Woman"
},
{
"authors": "Andrew E. Green, MD",
"content": [
"At this time, ovarian cancers cannot be detected at an early stage on a consistent basis; thus, focus must be on efforts at prevention. Oral contraceptives are a mainstay in this effort. If a woman takes oral contraceptives for 10 years, her risk for ovarian cancer is cut approximately in half.[5] Women undergoing surgical sterilization can also significantly reduce their risk by choosing bilateral salpingectomy (removal of the tubes), rather than just undergoing tubal ligation.[6]",
"Routine removal of the ovaries is not recommended, especially in light of recent data indicating an increased risk for heart disease, even among postmenopausal women undergoing bilateral salpingo-oophorectomy.[7] The BRCA mutation–positive population is an exception to this; such women should undergo bilateral salpingectomy as soon as childbearing is complete.[8] Removal of the ovaries has been recommended at this time as well. However, evolving data suggest that this may be able to be delayed to protect the heart."
],
"date": "January 16, 2018",
"figures": [],
"markdown": "# Shortness of Breath and Abdominal Pain in an Obese Woman\n\n **Authors:** Andrew E. Green, MD \n **Date:** January 16, 2018\n\n ## Content\n\n At this time, ovarian cancers cannot be detected at an early stage on a consistent basis; thus, focus must be on efforts at prevention. Oral contraceptives are a mainstay in this effort. If a woman takes oral contraceptives for 10 years, her risk for ovarian cancer is cut approximately in half.[5] Women undergoing surgical sterilization can also significantly reduce their risk by choosing bilateral salpingectomy (removal of the tubes), rather than just undergoing tubal ligation.[6]\nRoutine removal of the ovaries is not recommended, especially in light of recent data indicating an increased risk for heart disease, even among postmenopausal women undergoing bilateral salpingo-oophorectomy.[7] The BRCA mutation–positive population is an exception to this; such women should undergo bilateral salpingectomy as soon as childbearing is complete.[8] Removal of the ovaries has been recommended at this time as well. However, evolving data suggest that this may be able to be delayed to protect the heart.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 812181,
"choiceText": "Follow-up ultrasonography in 6 weeks to re-evaluate the mass",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 812183,
"choiceText": "Admission for urgent surgery",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 812185,
"choiceText": "Referral to a gynecologic oncologist for further evaluation",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 812187,
"choiceText": "Follow-up appointment with her family practitioner",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The initial phase of treatment for ovarian cancer is best handled by a gynecologic oncologist. Improved survival rates among women receiving treatment directed by these physicians has been documented.<sup>2</sup>",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 254921,
"questionText": "Consider a separate case from the one above in which a woman presents with abdominal pain. A CT scan shows a 9-cm cystic and solid mass, with internal blood flow. She has a strong family history of early-onset breast cancer. Upon discharge, which of the following is indicated?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 812189,
"choiceText": "Carboplatin IV and paclitaxel IV",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 812191,
"choiceText": "Cisplatin IV and topotecan IV",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 812193,
"choiceText": "Cisplatin IP and paclitaxel IV/IP",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 812195,
"choiceText": "Carboplatin and gemcitabine",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Frontline chemotherapy is a combination of IP and IV treatment. IP treatment has shown increased survival rates over IV and should be strongly considered in all patients upfront.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 254923,
"questionText": "Consider another case in which a woman has undergone surgical removal of a malignant ovarian tumor and has had all visible disease removed. She is now discussing chemotherapy with her gynecologic oncologist. Which of the following regimens gives her the best chance at long-term survival, if she can tolerate the treatment?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Shortness of Breath and Abdominal Pain in an Obese Woman"
},
{
"authors": "Andrew E. Green, MD",
"content": [],
"date": "January 16, 2018",
"figures": [],
"markdown": "# Shortness of Breath and Abdominal Pain in an Obese Woman\n\n **Authors:** Andrew E. Green, MD \n **Date:** January 16, 2018\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 812181,
"choiceText": "Follow-up ultrasonography in 6 weeks to re-evaluate the mass",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 812183,
"choiceText": "Admission for urgent surgery",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 812185,
"choiceText": "Referral to a gynecologic oncologist for further evaluation",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 812187,
"choiceText": "Follow-up appointment with her family practitioner",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The initial phase of treatment for ovarian cancer is best handled by a gynecologic oncologist. Improved survival rates among women receiving treatment directed by these physicians has been documented.<sup>2</sup>",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 254921,
"questionText": "Consider a separate case from the one above in which a woman presents with abdominal pain. A CT scan shows a 9-cm cystic and solid mass, with internal blood flow. She has a strong family history of early-onset breast cancer. Upon discharge, which of the following is indicated?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 812189,
"choiceText": "Carboplatin IV and paclitaxel IV",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 812191,
"choiceText": "Cisplatin IV and topotecan IV",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 812193,
"choiceText": "Cisplatin IP and paclitaxel IV/IP",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 812195,
"choiceText": "Carboplatin and gemcitabine",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Frontline chemotherapy is a combination of IP and IV treatment. IP treatment has shown increased survival rates over IV and should be strongly considered in all patients upfront.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 254923,
"questionText": "Consider another case in which a woman has undergone surgical removal of a malignant ovarian tumor and has had all visible disease removed. She is now discussing chemotherapy with her gynecologic oncologist. Which of the following regimens gives her the best chance at long-term survival, if she can tolerate the treatment?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Shortness of Breath and Abdominal Pain in an Obese Woman"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 812173,
"choiceText": "Tubal cancer",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 812175,
"choiceText": "Abdominal peritoneal tuberculosis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 812177,
"choiceText": "Metastatic gastrointestinal carcinoma",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 812179,
"choiceText": "Ovarian cancer",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 254919,
"questionText": "On the basis of the history, physical examination, and workup, what is the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 812181,
"choiceText": "Follow-up ultrasonography in 6 weeks to re-evaluate the mass",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 812183,
"choiceText": "Admission for urgent surgery",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 812185,
"choiceText": "Referral to a gynecologic oncologist for further evaluation",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 812187,
"choiceText": "Follow-up appointment with her family practitioner",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The initial phase of treatment for ovarian cancer is best handled by a gynecologic oncologist. Improved survival rates among women receiving treatment directed by these physicians has been documented.<sup>2</sup>",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 254921,
"questionText": "Consider a separate case from the one above in which a woman presents with abdominal pain. A CT scan shows a 9-cm cystic and solid mass, with internal blood flow. She has a strong family history of early-onset breast cancer. Upon discharge, which of the following is indicated?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 812189,
"choiceText": "Carboplatin IV and paclitaxel IV",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 812191,
"choiceText": "Cisplatin IV and topotecan IV",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 812193,
"choiceText": "Cisplatin IP and paclitaxel IV/IP",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 812195,
"choiceText": "Carboplatin and gemcitabine",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Frontline chemotherapy is a combination of IP and IV treatment. IP treatment has shown increased survival rates over IV and should be strongly considered in all patients upfront.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 254923,
"questionText": "Consider another case in which a woman has undergone surgical removal of a malignant ovarian tumor and has had all visible disease removed. She is now discussing chemotherapy with her gynecologic oncologist. Which of the following regimens gives her the best chance at long-term survival, if she can tolerate the treatment?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
838735 | /viewarticle/838735 | [
{
"authors": "Sadaf Khattak, MBBS; Sumaira Nabi, MBBS; Irfan Khattak, MBBS; Mazhar Badshah, MD, MBBS",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"Background",
"A 30-year-old Pakistani man presents to the emergency department with a 6-month history of intermittent headache (more on neck flexion) and difficulty in walking, with a tendency to fall on the left side. He also complains of double vision for the last 2 months without associated ocular pain, swelling, or decreased vision. No history of vomiting, fever, photophobia, phonophobia, dysarthria, dysphagia, seizures, cognitive decline, hearing impairment, joint pains, weight loss, or weakness of any limb is reported.",
"His history is significant for a motor vehicle accident approximately 3 months prior and also for repeated fractures (total, four) following trivial trauma. He is not using any medications and has no known allergies. His family history is unremarkable. He is a nonsmoker and denies use of illicit drugs. He is unmarried, a teacher by profession, and lives in a joint family system with a poor socioeconomic background."
],
"date": "February 09, 2017",
"figures": [],
"markdown": "# A Young Man With Difficulty Walking and Double Vision\n\n **Authors:** Sadaf Khattak, MBBS; Sumaira Nabi, MBBS; Irfan Khattak, MBBS; Mazhar Badshah, MD, MBBS \n **Date:** February 09, 2017\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nBackground\nA 30-year-old Pakistani man presents to the emergency department with a 6-month history of intermittent headache (more on neck flexion) and difficulty in walking, with a tendency to fall on the left side. He also complains of double vision for the last 2 months without associated ocular pain, swelling, or decreased vision. No history of vomiting, fever, photophobia, phonophobia, dysarthria, dysphagia, seizures, cognitive decline, hearing impairment, joint pains, weight loss, or weakness of any limb is reported.\nHis history is significant for a motor vehicle accident approximately 3 months prior and also for repeated fractures (total, four) following trivial trauma. He is not using any medications and has no known allergies. His family history is unremarkable. He is a nonsmoker and denies use of illicit drugs. He is unmarried, a teacher by profession, and lives in a joint family system with a poor socioeconomic background.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A Young Man With Difficulty Walking and Double Vision"
},
{
"authors": "Sadaf Khattak, MBBS; Sumaira Nabi, MBBS; Irfan Khattak, MBBS; Mazhar Badshah, MD, MBBS",
"content": [
"Upon physical examination he is conscious and alert, with a regular pulse of 80 beats/minute, blood pressure of 110/70 mm Hg, and a respiratory rate of 12 breaths/minute. His oral temperature is 98.6°F. He is noted to have a short neck, blue sclera, and increased anteroposterior diameter of his skull. Neurologic examination reveals an oriented male with a Glasgow Coma Score of 15/15, intact higher cognitive functions, and scanning speech. His pupils are round, regular, and reactive to light with no relative afferent pupillary defect (RAPD). Using the measurement of meters (6/6 is the equivalent of 20/20 using US customary units of feet), visual acuity is 6/6, with normal fundi and no field defects.",
"Figure 1.",
"Figure 2.",
"His neck is supple. Increased tone is noted in his lower limbs, with grade 3 reflexes and flexor plantar response. Bulk, power, and sensory examination findings are normal. Upper limb examination findings are normal. Coarse horizontal nystagmus is noted, and heel-knee-shin and finger-to-nose coordination is impaired on left side. Cranial nerve examination reveals bilateral lateral rectus, and a lower motor neuron type left facial palsy (Figures 1 and 2). The rest of the cranial nerves, including CN VIII, IX, and X, are intact and symmetric.",
"Auscultation of the chest reveals normal vesicular breathing, and heart sounds are normal on precordial examination with no murmurs. He has a soft, nontender abdomen, with audible bowel sounds and no evidence of free fluid or organomegaly.",
"The laboratory analysis reveals a normal complete blood cell count, sedimentation rate, and negative antinuclear antibody and rheumatoid factor findings. His liver function test findings show a raised alkaline phosphatase with normal bilirubin and amino transferase levels. His renal function test results, serum electrolyte levels, and serum glucose and serum lactate levels are within normal limits. His serum calcium and phosphorus levels are normal, as are urinary calcium and parathyroid hormone levels. Vitamin D deficiency is noted, with levels of less than 3 ng/mL. Chest radiography and ECG findings are unremarkable.",
"Figure 3.",
"Figure 4.",
"Figure 5.",
"Bone density scanning reveals osteoporosis, with a T-score of -4.9 SD in the lumbar spine. Bone scan findings are suggestive of osteoporotic fractures, with low likelihood of metastasis (Figure 3). MRI of the brain with contrast reveals superiorly displaced basiocciput, with dens of C2 projecting 21 mm above the Chamberlain line and upward migration of the cervical cord and foramen magnum into the cranial cavity (Figures 4 and 5)."
],
"date": "February 09, 2017",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/838/735/838735-thumb-1.jpg"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/838/735/838735-thumb-2.jpg"
},
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/838/735/838735-thumb-3.jpg"
},
{
"caption": "Figure 4.",
"image_url": "https://img.medscapestatic.com/article/838/735/838735-thumb-4.jpg"
},
{
"caption": "Figure 5.",
"image_url": "https://img.medscapestatic.com/article/838/735/838735-thumb-5.jpg"
}
],
"markdown": "# A Young Man With Difficulty Walking and Double Vision\n\n **Authors:** Sadaf Khattak, MBBS; Sumaira Nabi, MBBS; Irfan Khattak, MBBS; Mazhar Badshah, MD, MBBS \n **Date:** February 09, 2017\n\n ## Content\n\n Upon physical examination he is conscious and alert, with a regular pulse of 80 beats/minute, blood pressure of 110/70 mm Hg, and a respiratory rate of 12 breaths/minute. His oral temperature is 98.6°F. He is noted to have a short neck, blue sclera, and increased anteroposterior diameter of his skull. Neurologic examination reveals an oriented male with a Glasgow Coma Score of 15/15, intact higher cognitive functions, and scanning speech. His pupils are round, regular, and reactive to light with no relative afferent pupillary defect (RAPD). Using the measurement of meters (6/6 is the equivalent of 20/20 using US customary units of feet), visual acuity is 6/6, with normal fundi and no field defects.\nFigure 1.\nFigure 2.\nHis neck is supple. Increased tone is noted in his lower limbs, with grade 3 reflexes and flexor plantar response. Bulk, power, and sensory examination findings are normal. Upper limb examination findings are normal. Coarse horizontal nystagmus is noted, and heel-knee-shin and finger-to-nose coordination is impaired on left side. Cranial nerve examination reveals bilateral lateral rectus, and a lower motor neuron type left facial palsy (Figures 1 and 2). The rest of the cranial nerves, including CN VIII, IX, and X, are intact and symmetric.\nAuscultation of the chest reveals normal vesicular breathing, and heart sounds are normal on precordial examination with no murmurs. He has a soft, nontender abdomen, with audible bowel sounds and no evidence of free fluid or organomegaly.\nThe laboratory analysis reveals a normal complete blood cell count, sedimentation rate, and negative antinuclear antibody and rheumatoid factor findings. His liver function test findings show a raised alkaline phosphatase with normal bilirubin and amino transferase levels. His renal function test results, serum electrolyte levels, and serum glucose and serum lactate levels are within normal limits. His serum calcium and phosphorus levels are normal, as are urinary calcium and parathyroid hormone levels. Vitamin D deficiency is noted, with levels of less than 3 ng/mL. Chest radiography and ECG findings are unremarkable.\nFigure 3.\nFigure 4.\nFigure 5.\nBone density scanning reveals osteoporosis, with a T-score of -4.9 SD in the lumbar spine. Bone scan findings are suggestive of osteoporotic fractures, with low likelihood of metastasis (Figure 3). MRI of the brain with contrast reveals superiorly displaced basiocciput, with dens of C2 projecting 21 mm above the Chamberlain line and upward migration of the cervical cord and foramen magnum into the cranial cavity (Figures 4 and 5).\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n**Figure 3.** \n \n\n**Figure 4.** \n \n\n**Figure 5.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 809969,
"choiceText": "Arnold Chiari malformation",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 809971,
"choiceText": "Basilar impression",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 809973,
"choiceText": "Brainstem stroke",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 809975,
"choiceText": "Brain tumor",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 254219,
"questionText": "On the basis of the history, physical examination, and workup, what is the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A Young Man With Difficulty Walking and Double Vision"
},
{
"authors": "Sadaf Khattak, MBBS; Sumaira Nabi, MBBS; Irfan Khattak, MBBS; Mazhar Badshah, MD, MBBS",
"content": [
"Basilar impression, often grouped in with a similar condition (basilar invagination), is a very common craniocervical junction anomaly, in which the top of the C2 vertebra is displaced upward and posteriorly, prolapsing into the foramen magnum and causing its narrowing. The two terms, basilar impression and basilar invagination, are used interchangeably because both are caused by upward displacement of the cervical spine into foramen magnum. The only difference between the two is that upward migration of cervical vertebrae in basilar impression is the result of bony softening at base of the skull, whereas in basilar invagination, it occurs in the presence of normal bone.[1,2,3,4,5,6] The term basilar impression was first described by anatomists as a postmortem entity in the 18th century, but it was not until the early 20thcentury that the first antemortem cases in patients were noted and described in Europe. In American literature, the term emerged in 1939, with the establishment of diagnostic criteria by Chamberlain.[6]",
"Basilar impression is frequently associated with other craniocervical junction malformations, such as atlanto-occipital assimilation, incomplete ring of C1, and hypoplasia of the basiocciput, occipital condyles, and atlas. Chiari malformation, syringomyelia, syringobulbia, and hydrocephalus are among the common neural axis associates of basilar impression.[1,2,3,4,5] It is seen in around 40% of all adults treated for symptomatic Arnold-Chiari malformation,[7,8] and herniation of cerebellar tonsil may occur in both primary and secondary basilar impression.[9]",
"Primary basilar impression is a congenital anomaly inherited as an autosomal dominant trait with incomplete penetrance and is a diagnosis of exclusion.[6] Metabolic or developmental skeletal abnormalities causing generalized bone disease, such as Paget disease, osteogenesis imperfecta, osteomalacia, rickets, osteoporosis, rheumatoid arthritis, and hyperparathyroidism result in secondary basilar impression.[2,10,11] Trauma secondary to falls or vehicular accidents can also result in basilar impression.",
"The onset of symptoms of primary basilar impression is usually in the third to fifth decade.[10,12] Basilar impression causes a wide variety of clinical manifestations, depending on the structures involved. The structures that may be affected include the upper spinal cord, mid brain, pons, medulla, medial cerebellum, and the vertebrobasilar system. Patients become symptomatic when the displaced cervical segment causes sufficient pressure on the upper cervical cord, brainstem, nerves, or their blood supply. These symptoms may be progressive, transient, or even fatal.[12]"
],
"date": "February 09, 2017",
"figures": [],
"markdown": "# A Young Man With Difficulty Walking and Double Vision\n\n **Authors:** Sadaf Khattak, MBBS; Sumaira Nabi, MBBS; Irfan Khattak, MBBS; Mazhar Badshah, MD, MBBS \n **Date:** February 09, 2017\n\n ## Content\n\n Basilar impression, often grouped in with a similar condition (basilar invagination), is a very common craniocervical junction anomaly, in which the top of the C2 vertebra is displaced upward and posteriorly, prolapsing into the foramen magnum and causing its narrowing. The two terms, basilar impression and basilar invagination, are used interchangeably because both are caused by upward displacement of the cervical spine into foramen magnum. The only difference between the two is that upward migration of cervical vertebrae in basilar impression is the result of bony softening at base of the skull, whereas in basilar invagination, it occurs in the presence of normal bone.[1,2,3,4,5,6] The term basilar impression was first described by anatomists as a postmortem entity in the 18th century, but it was not until the early 20thcentury that the first antemortem cases in patients were noted and described in Europe. In American literature, the term emerged in 1939, with the establishment of diagnostic criteria by Chamberlain.[6]\nBasilar impression is frequently associated with other craniocervical junction malformations, such as atlanto-occipital assimilation, incomplete ring of C1, and hypoplasia of the basiocciput, occipital condyles, and atlas. Chiari malformation, syringomyelia, syringobulbia, and hydrocephalus are among the common neural axis associates of basilar impression.[1,2,3,4,5] It is seen in around 40% of all adults treated for symptomatic Arnold-Chiari malformation,[7,8] and herniation of cerebellar tonsil may occur in both primary and secondary basilar impression.[9]\nPrimary basilar impression is a congenital anomaly inherited as an autosomal dominant trait with incomplete penetrance and is a diagnosis of exclusion.[6] Metabolic or developmental skeletal abnormalities causing generalized bone disease, such as Paget disease, osteogenesis imperfecta, osteomalacia, rickets, osteoporosis, rheumatoid arthritis, and hyperparathyroidism result in secondary basilar impression.[2,10,11] Trauma secondary to falls or vehicular accidents can also result in basilar impression.\nThe onset of symptoms of primary basilar impression is usually in the third to fifth decade.[10,12] Basilar impression causes a wide variety of clinical manifestations, depending on the structures involved. The structures that may be affected include the upper spinal cord, mid brain, pons, medulla, medial cerebellum, and the vertebrobasilar system. Patients become symptomatic when the displaced cervical segment causes sufficient pressure on the upper cervical cord, brainstem, nerves, or their blood supply. These symptoms may be progressive, transient, or even fatal.[12]\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 809969,
"choiceText": "Arnold Chiari malformation",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 809971,
"choiceText": "Basilar impression",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 809973,
"choiceText": "Brainstem stroke",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 809975,
"choiceText": "Brain tumor",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 254219,
"questionText": "On the basis of the history, physical examination, and workup, what is the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A Young Man With Difficulty Walking and Double Vision"
},
{
"authors": "Sadaf Khattak, MBBS; Sumaira Nabi, MBBS; Irfan Khattak, MBBS; Mazhar Badshah, MD, MBBS",
"content": [
"The most common documented symptom of basilar impression is nuchal pain and headache, mainly in the occipital region, usually occurring in the morning.[9,13,14] The pain may be triggered by movement and exercise and is sometimes so severe that the patient has to hold the head rigid in order to minimize the pain. Headache-precipitating actions such as coughing, sneezing, and straining can also result in vertigo or imbalance. The exact mechanism of cough headache remains elusive, and some argue against raised intracranial pressure as a cause.[15,16]",
"The most common neurologic sign in symptomatic basilar impression is horizontal nystagmus, with the fast component in the direction of gaze. A marked bi-directionality to the nystagmus almost always denotes a central cause; however, it has poor localizing value because it can occur in both brainstem and cerebellar lesions.[17] The presence of downbeat nystagmus, on the other hand, is indicative of an abnormality at the craniovertebral junction but is more common in Arnold-Chiari malformations than basilar impression alone.",
"Involvement of the lower cranial nerves has also been described in basilar impression but is reported to be relatively uncommon.[14,18 ]The most common nerve involved is the fifth nerve, probably because of its large size and extension of the nerve and its root in the pontomedullary region. Sensory symptoms in the distribution of nerve have been reported to be as high as 30%-50% in some case series, but motor involvement is uncommon.[8] Facial nerve palsy is rare, but facial nerve spasm is more common, presumably due to posterior circulation abnormalities induced by the basilar impression.[19,20]",
"Trigeminal neuralgia has been reported in several cases and has a vascular etiology similar to facial spasm.[19] Bladder disorders are rarely reported in the literature. Raised intracranial pressure has been documented in few case reports. Other symptoms include dysarthria, dysphagia, and periods of confusion, loss of balance, loss of coordination, dizziness, orthostatic hypotension, numbness/tingling in the extremities, paralysis, and even death from brainstem compression. Symptoms may become worse with flexion of the head because it further drapes the spinal cord over the upper portion of C2.",
"In order to elucidate the probable cause and origin of basilar invagination, researchers have put forward various theories, including genetic abnormalities, mechanical or embryologic dysgenesis, and viral infections.[21,22,23,24] For many years, anatomists were of the opinion that the deformation had a mechanical cause and therefore gave it the name impressio baseos cranii or basilar impression.[25] The pathogenesis of neurologic symptoms and signs can be attributed to a number of factors. First, the direct pressure effect of the clivus, the anterior lip of the foramen magnum, the body of C1, and dens on the anterior brainstem results in pontomedullary region angulation and early involvement of the corticospinal tracts. Second, the interaction of minor trauma from head movements with the abnormal anatomy causes chronic low-grade inflammation, leading to arachnoid adhesions and increased vascularization in the region of the foramen magnum.",
"Trigeminal neuralgia has reportedly been relieved by removing these adhesions from the trigeminal roots.[26] Finally, mechanical stresses and inflammation also result in vascular abnormalities. The transient nature of some of the symptoms also points toward a vascular etiology. Early angiographic studies of the vertebral system in basilar impression suggested an abnormal dorsal displacement of the terminal segment of the vertebral and initial parts of the basilar artery.[20] This is because of the \"kinking\" of normal vessels in order to adapt to a posterior fossa shortened by the process of basilar impression. In addition, an abnormal clivus is likely to accentuate this arching of the vessel.[12]"
],
"date": "February 09, 2017",
"figures": [],
"markdown": "# A Young Man With Difficulty Walking and Double Vision\n\n **Authors:** Sadaf Khattak, MBBS; Sumaira Nabi, MBBS; Irfan Khattak, MBBS; Mazhar Badshah, MD, MBBS \n **Date:** February 09, 2017\n\n ## Content\n\n The most common documented symptom of basilar impression is nuchal pain and headache, mainly in the occipital region, usually occurring in the morning.[9,13,14] The pain may be triggered by movement and exercise and is sometimes so severe that the patient has to hold the head rigid in order to minimize the pain. Headache-precipitating actions such as coughing, sneezing, and straining can also result in vertigo or imbalance. The exact mechanism of cough headache remains elusive, and some argue against raised intracranial pressure as a cause.[15,16]\nThe most common neurologic sign in symptomatic basilar impression is horizontal nystagmus, with the fast component in the direction of gaze. A marked bi-directionality to the nystagmus almost always denotes a central cause; however, it has poor localizing value because it can occur in both brainstem and cerebellar lesions.[17] The presence of downbeat nystagmus, on the other hand, is indicative of an abnormality at the craniovertebral junction but is more common in Arnold-Chiari malformations than basilar impression alone.\nInvolvement of the lower cranial nerves has also been described in basilar impression but is reported to be relatively uncommon.[14,18 ]The most common nerve involved is the fifth nerve, probably because of its large size and extension of the nerve and its root in the pontomedullary region. Sensory symptoms in the distribution of nerve have been reported to be as high as 30%-50% in some case series, but motor involvement is uncommon.[8] Facial nerve palsy is rare, but facial nerve spasm is more common, presumably due to posterior circulation abnormalities induced by the basilar impression.[19,20]\nTrigeminal neuralgia has been reported in several cases and has a vascular etiology similar to facial spasm.[19] Bladder disorders are rarely reported in the literature. Raised intracranial pressure has been documented in few case reports. Other symptoms include dysarthria, dysphagia, and periods of confusion, loss of balance, loss of coordination, dizziness, orthostatic hypotension, numbness/tingling in the extremities, paralysis, and even death from brainstem compression. Symptoms may become worse with flexion of the head because it further drapes the spinal cord over the upper portion of C2.\nIn order to elucidate the probable cause and origin of basilar invagination, researchers have put forward various theories, including genetic abnormalities, mechanical or embryologic dysgenesis, and viral infections.[21,22,23,24] For many years, anatomists were of the opinion that the deformation had a mechanical cause and therefore gave it the name impressio baseos cranii or basilar impression.[25] The pathogenesis of neurologic symptoms and signs can be attributed to a number of factors. First, the direct pressure effect of the clivus, the anterior lip of the foramen magnum, the body of C1, and dens on the anterior brainstem results in pontomedullary region angulation and early involvement of the corticospinal tracts. Second, the interaction of minor trauma from head movements with the abnormal anatomy causes chronic low-grade inflammation, leading to arachnoid adhesions and increased vascularization in the region of the foramen magnum.\nTrigeminal neuralgia has reportedly been relieved by removing these adhesions from the trigeminal roots.[26] Finally, mechanical stresses and inflammation also result in vascular abnormalities. The transient nature of some of the symptoms also points toward a vascular etiology. Early angiographic studies of the vertebral system in basilar impression suggested an abnormal dorsal displacement of the terminal segment of the vertebral and initial parts of the basilar artery.[20] This is because of the \"kinking\" of normal vessels in order to adapt to a posterior fossa shortened by the process of basilar impression. In addition, an abnormal clivus is likely to accentuate this arching of the vessel.[12]\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A Young Man With Difficulty Walking and Double Vision"
},
{
"authors": "Sadaf Khattak, MBBS; Sumaira Nabi, MBBS; Irfan Khattak, MBBS; Mazhar Badshah, MD, MBBS",
"content": [
"Diagnosis of basilar impression prior to operation depends primarily on radiography.[13] The abnormality may be missed clinically because of presence of deceptive symptoms and signs or absence of symptoms. MRI is the criterion standard, and flexion extension MRIs have a higher yield. Frontal and lateral skull plain radiography, as well as coronal and mid-sagittal reconstructed CT scanning, can also document this, but MRI provides better information. Somatosensory evoked potentials (SSEPs) may have false-positive results and are not routinely recommended. Radiologically, basilar impression is defined by reference to numerous parameters. These include :",
"The Chamberlain line. This is defined as the line joining the posterior edge of hard palate to the posterior lip of the foramen, caudal to which all parts of the atlas and axis should lie. If the dens is more than 3 mm above this line, the patient has basilar invagination. The dorsal lip, however, can be difficult to define radiologically and can itself become invaginated.",
"The McGregor line. This is a modification of the Chamberlain line proposed by McGregor in 1948. It is drawn from the back of the hard palate to the lowest point of the occipital squama and normally passes just above the tip of the dens peg. In diagnosing basilar invagination, the dens should be more than 4.5 mm above this line.",
"The McCrae line. This is drawn from the middle of the anterior lip of the foramen magnum to the posterior lip of the foramen magnum. This landmark was proposed by McRae in 1953. The dens tip should lie below this point.",
"The Bull angle. This is the angle between the plane of the hard palate and the line formed by joining the midpoints of the anterior and posterior arches of first cervical vertebrae in a lateral radiograph of the skull and cervical spine. In 1955, Bull and colleagues performed a comparative study of 120 patients, measuring the distance of the dens tip above the Chamberlain line and McGregor line, and postulated that exceeding three standard deviations in any one of these criteria is sufficient to diagnose basilar impression.[1,3,4,6,7,8]",
"Treatment of basilar impression depends on the symptomatology of the patient. In the absence of symptoms, a conservative approach may be undertaken, consisting of a collar, nonsteroidal anti-inflammatory drugs (NSAIDs), and simple neck traction.In the presence of neurologic symptoms and signs and confirmation of cord compression on neuroimaging, surgery is recommended.",
"Neurosurgical treatment is difficult and involves anterior decompression, followed by posterior stabilization for irreducible invagination. For reducible invagination, neurosurgical treatment involves posterior decompression and stabilization. In persons with Chiari malformation, occipital decompression surgery may also be necessary.[1] In patients who are considered poor surgical risks, neurologic progression is likely and the 1-year prognosis is poor.",
"The patient in this case was diagnosed with basilar impression. He had a history and examination findings suspicious for osteogenesis imperfecta, including blue sclera and a history of repeated fractures. Skin biopsy for biochemical testing or DNA sequencing for associated gene mutations could have been pursued for diagnostic confirmation and counseling. He was subsequently referred to the neurosurgery department for surgical decompression."
],
"date": "February 09, 2017",
"figures": [],
"markdown": "# A Young Man With Difficulty Walking and Double Vision\n\n **Authors:** Sadaf Khattak, MBBS; Sumaira Nabi, MBBS; Irfan Khattak, MBBS; Mazhar Badshah, MD, MBBS \n **Date:** February 09, 2017\n\n ## Content\n\n Diagnosis of basilar impression prior to operation depends primarily on radiography.[13] The abnormality may be missed clinically because of presence of deceptive symptoms and signs or absence of symptoms. MRI is the criterion standard, and flexion extension MRIs have a higher yield. Frontal and lateral skull plain radiography, as well as coronal and mid-sagittal reconstructed CT scanning, can also document this, but MRI provides better information. Somatosensory evoked potentials (SSEPs) may have false-positive results and are not routinely recommended. Radiologically, basilar impression is defined by reference to numerous parameters. These include :\nThe Chamberlain line. This is defined as the line joining the posterior edge of hard palate to the posterior lip of the foramen, caudal to which all parts of the atlas and axis should lie. If the dens is more than 3 mm above this line, the patient has basilar invagination. The dorsal lip, however, can be difficult to define radiologically and can itself become invaginated.\nThe McGregor line. This is a modification of the Chamberlain line proposed by McGregor in 1948. It is drawn from the back of the hard palate to the lowest point of the occipital squama and normally passes just above the tip of the dens peg. In diagnosing basilar invagination, the dens should be more than 4.5 mm above this line.\nThe McCrae line. This is drawn from the middle of the anterior lip of the foramen magnum to the posterior lip of the foramen magnum. This landmark was proposed by McRae in 1953. The dens tip should lie below this point.\nThe Bull angle. This is the angle between the plane of the hard palate and the line formed by joining the midpoints of the anterior and posterior arches of first cervical vertebrae in a lateral radiograph of the skull and cervical spine. In 1955, Bull and colleagues performed a comparative study of 120 patients, measuring the distance of the dens tip above the Chamberlain line and McGregor line, and postulated that exceeding three standard deviations in any one of these criteria is sufficient to diagnose basilar impression.[1,3,4,6,7,8]\nTreatment of basilar impression depends on the symptomatology of the patient. In the absence of symptoms, a conservative approach may be undertaken, consisting of a collar, nonsteroidal anti-inflammatory drugs (NSAIDs), and simple neck traction.In the presence of neurologic symptoms and signs and confirmation of cord compression on neuroimaging, surgery is recommended.\nNeurosurgical treatment is difficult and involves anterior decompression, followed by posterior stabilization for irreducible invagination. For reducible invagination, neurosurgical treatment involves posterior decompression and stabilization. In persons with Chiari malformation, occipital decompression surgery may also be necessary.[1] In patients who are considered poor surgical risks, neurologic progression is likely and the 1-year prognosis is poor.\nThe patient in this case was diagnosed with basilar impression. He had a history and examination findings suspicious for osteogenesis imperfecta, including blue sclera and a history of repeated fractures. Skin biopsy for biochemical testing or DNA sequencing for associated gene mutations could have been pursued for diagnostic confirmation and counseling. He was subsequently referred to the neurosurgery department for surgical decompression.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 809977,
"choiceText": "Lying down",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 809979,
"choiceText": "Extension of head",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 809981,
"choiceText": "Keeping the head in rigid position",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 809983,
"choiceText": "Neck flexion",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 809985,
"choiceText": "Taking a hot bath",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Because neck flexion further drapes the spinal cord over the upper portion of C2, symptoms of basilar impression may worsen.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 254221,
"questionText": "Symptoms of basilar impression worsen upon which of the following?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 809987,
"choiceText": "NSAIDs",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 809989,
"choiceText": "Cervical collar",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 809991,
"choiceText": "Surgery",
"correct": true,
"displayOrder": 3,
"explanation": "For patients who demonstrate neurologic signs and symptoms due to basilar impression, neuroimaging is indicated to confirm cord compression. Once confirmed, the recommended treatment is anterior decompression followed by posterior stabilization for irreducible invagination, and posterior decompression and stabilization for reducible invagination.",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 809993,
"choiceText": "Corticosteroids",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 809995,
"choiceText": "Radiotherapy",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "For patients who demonstrate neurologic signs and symptoms due to basilar impression, neuroimaging is indicated to confirm cord compression. Once confirmed, the recommended treatment is anterior decompression followed by posterior stabilization for irreducible invagination and posterior decompression and stabilization for reducible invagination.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 254223,
"questionText": "The treatment for symptomatic basilar impression is which of the following?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A Young Man With Difficulty Walking and Double Vision"
},
{
"authors": "Sadaf Khattak, MBBS; Sumaira Nabi, MBBS; Irfan Khattak, MBBS; Mazhar Badshah, MD, MBBS",
"content": [],
"date": "February 09, 2017",
"figures": [],
"markdown": "# A Young Man With Difficulty Walking and Double Vision\n\n **Authors:** Sadaf Khattak, MBBS; Sumaira Nabi, MBBS; Irfan Khattak, MBBS; Mazhar Badshah, MD, MBBS \n **Date:** February 09, 2017\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 809977,
"choiceText": "Lying down",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 809979,
"choiceText": "Extension of head",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 809981,
"choiceText": "Keeping the head in rigid position",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 809983,
"choiceText": "Neck flexion",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 809985,
"choiceText": "Taking a hot bath",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Because neck flexion further drapes the spinal cord over the upper portion of C2, symptoms of basilar impression may worsen.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 254221,
"questionText": "Symptoms of basilar impression worsen upon which of the following?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 809987,
"choiceText": "NSAIDs",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 809989,
"choiceText": "Cervical collar",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 809991,
"choiceText": "Surgery",
"correct": true,
"displayOrder": 3,
"explanation": "For patients who demonstrate neurologic signs and symptoms due to basilar impression, neuroimaging is indicated to confirm cord compression. Once confirmed, the recommended treatment is anterior decompression followed by posterior stabilization for irreducible invagination, and posterior decompression and stabilization for reducible invagination.",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 809993,
"choiceText": "Corticosteroids",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 809995,
"choiceText": "Radiotherapy",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "For patients who demonstrate neurologic signs and symptoms due to basilar impression, neuroimaging is indicated to confirm cord compression. Once confirmed, the recommended treatment is anterior decompression followed by posterior stabilization for irreducible invagination and posterior decompression and stabilization for reducible invagination.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 254223,
"questionText": "The treatment for symptomatic basilar impression is which of the following?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A Young Man With Difficulty Walking and Double Vision"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 809969,
"choiceText": "Arnold Chiari malformation",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 809971,
"choiceText": "Basilar impression",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 809973,
"choiceText": "Brainstem stroke",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 809975,
"choiceText": "Brain tumor",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 254219,
"questionText": "On the basis of the history, physical examination, and workup, what is the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 809977,
"choiceText": "Lying down",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 809979,
"choiceText": "Extension of head",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 809981,
"choiceText": "Keeping the head in rigid position",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 809983,
"choiceText": "Neck flexion",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 809985,
"choiceText": "Taking a hot bath",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Because neck flexion further drapes the spinal cord over the upper portion of C2, symptoms of basilar impression may worsen.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 254221,
"questionText": "Symptoms of basilar impression worsen upon which of the following?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 809987,
"choiceText": "NSAIDs",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 809989,
"choiceText": "Cervical collar",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 809991,
"choiceText": "Surgery",
"correct": true,
"displayOrder": 3,
"explanation": "For patients who demonstrate neurologic signs and symptoms due to basilar impression, neuroimaging is indicated to confirm cord compression. Once confirmed, the recommended treatment is anterior decompression followed by posterior stabilization for irreducible invagination, and posterior decompression and stabilization for reducible invagination.",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 809993,
"choiceText": "Corticosteroids",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 809995,
"choiceText": "Radiotherapy",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "For patients who demonstrate neurologic signs and symptoms due to basilar impression, neuroimaging is indicated to confirm cord compression. Once confirmed, the recommended treatment is anterior decompression followed by posterior stabilization for irreducible invagination and posterior decompression and stabilization for reducible invagination.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 254223,
"questionText": "The treatment for symptomatic basilar impression is which of the following?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
837726 | /viewarticle/837726 | [
{
"authors": "John L. Brusch, MD",
"content": [
"Editor's Note: \nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions.",
"Background",
"A 40-year-old male intravenous drug user is admitted to the hospital with a body temperature of 103.2°F. He strongly denies recent use of illicit drugs. He began using cocaine at 18 years of age. Within 1 year, he was no longer able to afford his habit. At that point, he switched to heroin.",
"Three years later, the patient was admitted to the acute care hospital for treatment of a deep-seated soft-tissue infection of his left arm that complicated a frequently used injection site. Treatment consisted of surgical debridement, as well as antibiotic coverage of mouth flora (anaerobic staphylococci and streptococci and Moraxella). His caregivers were surprised by this set of organisms. Upon questioning, he admitted that, as many addicts do, he would lick his injection needle for good luck.",
"Upon discharge, the patient resumed injecting heroin but did not continue to contaminate his needles. Ten years ago, he was treated for hepatitis C virus infection but did not respond. At that point, he stopped using heroin. He abstained from the drug for many years, until he lost his job, at which point he reverted to using heroin.",
"The patient smokes two packs of cigarettes daily. He abstains from alcohol. His medical history is otherwise unremarkable."
],
"date": "January 12, 2015",
"figures": [],
"markdown": "# Fever in a 40-Year-Old Intravenous Drug User\n\n **Authors:** John L. Brusch, MD \n **Date:** January 12, 2015\n\n ## Content\n\n Editor's Note: \nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions.\nBackground\nA 40-year-old male intravenous drug user is admitted to the hospital with a body temperature of 103.2°F. He strongly denies recent use of illicit drugs. He began using cocaine at 18 years of age. Within 1 year, he was no longer able to afford his habit. At that point, he switched to heroin.\nThree years later, the patient was admitted to the acute care hospital for treatment of a deep-seated soft-tissue infection of his left arm that complicated a frequently used injection site. Treatment consisted of surgical debridement, as well as antibiotic coverage of mouth flora (anaerobic staphylococci and streptococci and Moraxella). His caregivers were surprised by this set of organisms. Upon questioning, he admitted that, as many addicts do, he would lick his injection needle for good luck.\nUpon discharge, the patient resumed injecting heroin but did not continue to contaminate his needles. Ten years ago, he was treated for hepatitis C virus infection but did not respond. At that point, he stopped using heroin. He abstained from the drug for many years, until he lost his job, at which point he reverted to using heroin.\nThe patient smokes two packs of cigarettes daily. He abstains from alcohol. His medical history is otherwise unremarkable.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "Fever in a 40-Year-Old Intravenous Drug User"
},
{
"authors": "John L. Brusch, MD",
"content": [
"Physical Examination and Workup",
"The patient's white blood cell count was 22,000 cells/μL, with 85% neutrophils. His liver function test results were mildly abnormal; the alkaline phosphatase level was 150 IU/L, the alanine aminotransferase level was 72 U/L, the aspartate aminotransferase level was 85 U/L, and bilirubin was 1.4 mg/dL. Blood urea nitrogen and creatinine levels were within the reference range.",
"Chest radiography revealed findings consistent with pulmonary emboli. Transesophageal echocardiography revealed vegetation 2.1 cm in diameter on the anterior cusp of the tricuspid valve. Empirical therapy was begun with vancomycin 15 mg/kg every 12 hours. Within 18 hours, both bottles of all three sets of blood cultures grew methicillin-resistant Staphylococcus aureus (MRSA).",
"The patient steadily improved, with normalization of his fever and decrease in his white blood cell count. The vancomycin trough level 5 days into treatment was 18 μg/mL.",
"Figure 1.",
"Figure 2.",
"Figure 3.",
"Because of the nature of his infectious organism, as well as the presence of septic emboli associated with large vegetation, intravenous treatment was indicated (Figures 1-3). Blood cultures were repeated 3 days into treatment; all findings were negative.",
"During the patient's hospitalization, he had a constant stream of visitors. In the early morning of his seventh hospital day, he developed shaking chills and fever, with a temperature of 104°F. A 3/6 diastolic murmur was observed in the aortic area. Blood cultures were obtained.",
"Transesophageal echocardiography revealed massive vegetation on the posterior leaflet of the aortic valve. The patient's creatinine level had increased to 1.6 mg/dL. His white blood cell count was 15,000 cells/μL, with 90% polymorphonuclear leukocytes.",
"In the early afternoon, the patient began to have severe left leg pain. Upon examination, his leg was pale and cool to the touch, with no pulses detectable below the iliacs. An emergency vascular surgery consultation recommended selective arteriography, which revealed a large clot in the left femoral artery. A thrombectomy was performed.",
"The next day, the patient experienced a massive cerebral embolus. He developed Cheyne-Stokes respiration and was placed on a ventilator. The following day, he was declared brain dead, and life support was discontinued in accordance with his stated wishes expressed and advance directives.",
"While the patient's belongings were being cleared out, drug injection paraphernalia was discovered, along with a supply of brown heroin. At this point, the pathology laboratory called with the results of the examination of the retrieved thrombus."
],
"date": "January 12, 2015",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/837/726/837726-thumb-1.jpg"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/837/726/837726-thumb-2.jpg"
},
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/837/726/837726-thumb-3.jpg"
}
],
"markdown": "# Fever in a 40-Year-Old Intravenous Drug User\n\n **Authors:** John L. Brusch, MD \n **Date:** January 12, 2015\n\n ## Content\n\n Physical Examination and Workup\nThe patient's white blood cell count was 22,000 cells/μL, with 85% neutrophils. His liver function test results were mildly abnormal; the alkaline phosphatase level was 150 IU/L, the alanine aminotransferase level was 72 U/L, the aspartate aminotransferase level was 85 U/L, and bilirubin was 1.4 mg/dL. Blood urea nitrogen and creatinine levels were within the reference range.\nChest radiography revealed findings consistent with pulmonary emboli. Transesophageal echocardiography revealed vegetation 2.1 cm in diameter on the anterior cusp of the tricuspid valve. Empirical therapy was begun with vancomycin 15 mg/kg every 12 hours. Within 18 hours, both bottles of all three sets of blood cultures grew methicillin-resistant Staphylococcus aureus (MRSA).\nThe patient steadily improved, with normalization of his fever and decrease in his white blood cell count. The vancomycin trough level 5 days into treatment was 18 μg/mL.\nFigure 1.\nFigure 2.\nFigure 3.\nBecause of the nature of his infectious organism, as well as the presence of septic emboli associated with large vegetation, intravenous treatment was indicated (Figures 1-3). Blood cultures were repeated 3 days into treatment; all findings were negative.\nDuring the patient's hospitalization, he had a constant stream of visitors. In the early morning of his seventh hospital day, he developed shaking chills and fever, with a temperature of 104°F. A 3/6 diastolic murmur was observed in the aortic area. Blood cultures were obtained.\nTransesophageal echocardiography revealed massive vegetation on the posterior leaflet of the aortic valve. The patient's creatinine level had increased to 1.6 mg/dL. His white blood cell count was 15,000 cells/μL, with 90% polymorphonuclear leukocytes.\nIn the early afternoon, the patient began to have severe left leg pain. Upon examination, his leg was pale and cool to the touch, with no pulses detectable below the iliacs. An emergency vascular surgery consultation recommended selective arteriography, which revealed a large clot in the left femoral artery. A thrombectomy was performed.\nThe next day, the patient experienced a massive cerebral embolus. He developed Cheyne-Stokes respiration and was placed on a ventilator. The following day, he was declared brain dead, and life support was discontinued in accordance with his stated wishes expressed and advance directives.\nWhile the patient's belongings were being cleared out, drug injection paraphernalia was discovered, along with a supply of brown heroin. At this point, the pathology laboratory called with the results of the examination of the retrieved thrombus.\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n**Figure 3.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 806445,
"choiceText": "Relapsing MRSA valvular infection",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 806447,
"choiceText": "Liver failure due to hepatitis C infection",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 806449,
"choiceText": "Polyarteritis secondary to hepatitis C infection",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 806451,
"choiceText": "Fungal endocarditis",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 253049,
"questionText": "On the basis of the history, physical examination, and workup, what is the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Fever in a 40-Year-Old Intravenous Drug User"
},
{
"authors": "John L. Brusch, MD",
"content": [
"Discussion",
"These events probably did not represent a relapse of MRSA endocarditis. The organism was treated with an appropriate antibiotic, and the bacteremia had cleared. In such a situation, breakthrough bacteremia is quite unusual. Macroembolization is not part of polyarteritis or liver failure.",
"Overall, fungal endocarditis is uncommon. Less than 1% of organisms retrieved from cases of infective endocarditis (IE) are Candida species. The rise in cases of Candida IE parallels that of healthcare infections in general; 25%-50% of cases are acquired in healthcare institutions, and Candida species are the most common fungi isolated. Risk factors for the development of fungal IE include the presence of prosthetic valves (45% of cases), central venous catheters (34% of cases), and intravenous drug use (4.1% to ≥ 12% of cases).",
"Often overlooked is the fact that in 20% of patients, the major risk factor is the prolonged use of broad-spectrum antibiotics, which promotes an overgrowth of candidal species in the gut lumen. Parenteral nutrition is involved in less than 1% of cases. The entire spectrum of immunosuppressive states is associated with only 7% of fungal IE cases. Many affected individuals have more than one risk factor. The average number of risk factors is 2.5.",
"Candida species account for 46% of all fungal IE cases. About 50% of candidal isolates are Candida albicans. The specific Candida species is closely connected with the medical profile of the patient. C albicans is unusually isolated from cases of IE in intravenous drug users. In these individuals, the major isolates are Candida parapsilosis (> 50% of cases) and Candida tropicalis. The major exception is, as demonstrated in this patient, users of brown heroin, in whom C albicans is the most common type.[1]",
"Other Candida species less frequently implicated in valvular infection include Candida krusei, Candida guilliermondii, Candida stellatoidea, and Candida lusitaniae.",
"Candidal IE results from a bloodstream infection that deposits the pathogen on a native or prosthetic valve. The most frequent source of candidemia is from proliferation of candidal species within the lumen of the gastrointestinal tract. When the growth of yeasts reaches a certain concentration, the yeasts convert into hyphal forms that are able to penetrate the gut wall and enter into the bloodstream.",
"Infected intravascular lines are overtaking the gastrointestinal tract as the primary source of candidemia. A high rate of prosthetic valve endocarditis due to nosocomial candidemia has been reported (approximately 25%).",
"Virulence factors of Candida isolates include their ability to attach themselves to the epithelial cells of the gastrointestinal tract and dermis. This, combined with their production of lipases and proteinases, promotes clear invasion of the bloodstream. C albicans in particular produces biofilm on various types of prosthetic material, such as vascular lines, prosthetic valves, and pacemakers. Within these biofilms, C albicans appears to be resistant to fluconazole and amphotericin B. This may be due to either poor penetration by antifungals into the biofilm or acquisition of resistance of the fungus within this plankton-type structure. Non-C\nalbicans isolates frequently have intrinsic resistance to several antifungal compounds."
],
"date": "January 12, 2015",
"figures": [],
"markdown": "# Fever in a 40-Year-Old Intravenous Drug User\n\n **Authors:** John L. Brusch, MD \n **Date:** January 12, 2015\n\n ## Content\n\n Discussion\nThese events probably did not represent a relapse of MRSA endocarditis. The organism was treated with an appropriate antibiotic, and the bacteremia had cleared. In such a situation, breakthrough bacteremia is quite unusual. Macroembolization is not part of polyarteritis or liver failure.\nOverall, fungal endocarditis is uncommon. Less than 1% of organisms retrieved from cases of infective endocarditis (IE) are Candida species. The rise in cases of Candida IE parallels that of healthcare infections in general; 25%-50% of cases are acquired in healthcare institutions, and Candida species are the most common fungi isolated. Risk factors for the development of fungal IE include the presence of prosthetic valves (45% of cases), central venous catheters (34% of cases), and intravenous drug use (4.1% to ≥ 12% of cases).\nOften overlooked is the fact that in 20% of patients, the major risk factor is the prolonged use of broad-spectrum antibiotics, which promotes an overgrowth of candidal species in the gut lumen. Parenteral nutrition is involved in less than 1% of cases. The entire spectrum of immunosuppressive states is associated with only 7% of fungal IE cases. Many affected individuals have more than one risk factor. The average number of risk factors is 2.5.\nCandida species account for 46% of all fungal IE cases. About 50% of candidal isolates are Candida albicans. The specific Candida species is closely connected with the medical profile of the patient. C albicans is unusually isolated from cases of IE in intravenous drug users. In these individuals, the major isolates are Candida parapsilosis (> 50% of cases) and Candida tropicalis. The major exception is, as demonstrated in this patient, users of brown heroin, in whom C albicans is the most common type.[1]\nOther Candida species less frequently implicated in valvular infection include Candida krusei, Candida guilliermondii, Candida stellatoidea, and Candida lusitaniae.\nCandidal IE results from a bloodstream infection that deposits the pathogen on a native or prosthetic valve. The most frequent source of candidemia is from proliferation of candidal species within the lumen of the gastrointestinal tract. When the growth of yeasts reaches a certain concentration, the yeasts convert into hyphal forms that are able to penetrate the gut wall and enter into the bloodstream.\nInfected intravascular lines are overtaking the gastrointestinal tract as the primary source of candidemia. A high rate of prosthetic valve endocarditis due to nosocomial candidemia has been reported (approximately 25%).\nVirulence factors of Candida isolates include their ability to attach themselves to the epithelial cells of the gastrointestinal tract and dermis. This, combined with their production of lipases and proteinases, promotes clear invasion of the bloodstream. C albicans in particular produces biofilm on various types of prosthetic material, such as vascular lines, prosthetic valves, and pacemakers. Within these biofilms, C albicans appears to be resistant to fluconazole and amphotericin B. This may be due to either poor penetration by antifungals into the biofilm or acquisition of resistance of the fungus within this plankton-type structure. Non-C\nalbicans isolates frequently have intrinsic resistance to several antifungal compounds.\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 806445,
"choiceText": "Relapsing MRSA valvular infection",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 806447,
"choiceText": "Liver failure due to hepatitis C infection",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 806449,
"choiceText": "Polyarteritis secondary to hepatitis C infection",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 806451,
"choiceText": "Fungal endocarditis",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 253049,
"questionText": "On the basis of the history, physical examination, and workup, what is the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Fever in a 40-Year-Old Intravenous Drug User"
},
{
"authors": "John L. Brusch, MD",
"content": [
"The clinical course of fungal endocarditis is generally similar to that of bacterial endocarditis. Overall, 75% of patients are febrile; 51% develop a new or a change in a preexistent heart murmur. Focal and generalized neurologic abnormalities are found in 31% of cases. Heart failure develops in 24% of patients. Often, the infection may be subacute, with a delay in diagnosis of as long as 10 weeks. However, macroembolization (36%) is likely; 50% of these affect the intracranial blood vessels, with less frequent involvement of the legs and gastrointestinal tract. As many as 25% of patients develop endophthalmitis.",
"In puzzling cases, ophthalmologic examination can detect the pathognomonic appearance of Candida emboli. Similarly, examination of a retrievable peripheral macroembolus can provide both histopathologic and culture evidence of Candida. The pathognomonic signs of subacute bacterial endocarditis, such as Janeway lesions and Osler nodes, and the suggestive findings of Roth spots are not present in candidal endocarditis. These entities result from the immunologic aspects of subacute bacterial endocarditis. Such immune complexes do not occur in fungal valvular infections.[2,3]",
"Across the board, 75% of cases of IE in intravenous drug users involve previously normal hearts. The rate of underlying valvular pathology and the distribution between right-sided and left-sided candidal IE is not established. However, particulate material contained in the injected drug may cause progressive damage to the tricuspid valve. This material is small enough to migrate across the pulmonary capillary bed to the arterial circulation and ultimately scar the mitral or aortic valves. The end result of this process is formation on the valve of a sterile fibrin thrombus (nonbacterial thrombotic endocarditis) to which circulating Candida could attach. Alternatively, increased stimulation of the immune system by repeated exposure to these injected materials could also result in production of nonbacterial thrombotic endocarditis.",
"The most challenging type of candidal IE appears to be candidal prosthetic valve endocarditis, because the infected prosthetic material must be removed and the replacement must be inserted. Fungal prosthetic valve endocarditis that occurs within 60 days of implantation is classified as early. The infection becomes established during the surgery or in the perioperative period. Late prosthetic valve endocarditis occurs 12 months or more after surgery. Intermediate cases are diagnosed between 60 days and 12 months after implantation. High rates (11%-25%) of prosthetic valve endocarditis cases arise from nosocomial fungemia, in which the endocarditis eventually becomes clinically apparent after 26-690 days.",
"The cornerstone of diagnosis of any type of valvular infection is documenting the sustained presence in the bloodstream of the suspected pathogen (bacterial or fungal). The diagnosis of candidal IE is based primarily on blood culture evidence of a continuous fungemia. A continuous bloodstream infection is defined as at least two positive blood culture findings for the same pathogen obtained at least 12 hours apart or three positive blood culture findings positive for the same pathogen, with a first and last finding obtained at least 1 hour apart. The continuous fungemia separates valvular infection from candidemia, which represents intermittent entry of the fungus into the bloodstream from nonvalvular sources, such as lung or kidney.",
"Even if vegetations are detected by transthoracic echocardiography (TTE), transesophageal echocardiography (TEE) should always be performed to detect complications of Candida infections, such as paravalvular abscess or large vegetation. The diagnostic usefulness of pathologic study of retrievable peripheral emboli and the presence of endophthalmitis have already been discussed. Serologic testing has not proven useful as a diagnostic approach.",
"A mean delay in diagnosis of Candida IE of approximately 32 days is noted. The inability to mount an inflammatory response, such as fever, because of immunosuppression in at-risk patients can significantly hinder its expeditious diagnosis."
],
"date": "January 12, 2015",
"figures": [],
"markdown": "# Fever in a 40-Year-Old Intravenous Drug User\n\n **Authors:** John L. Brusch, MD \n **Date:** January 12, 2015\n\n ## Content\n\n The clinical course of fungal endocarditis is generally similar to that of bacterial endocarditis. Overall, 75% of patients are febrile; 51% develop a new or a change in a preexistent heart murmur. Focal and generalized neurologic abnormalities are found in 31% of cases. Heart failure develops in 24% of patients. Often, the infection may be subacute, with a delay in diagnosis of as long as 10 weeks. However, macroembolization (36%) is likely; 50% of these affect the intracranial blood vessels, with less frequent involvement of the legs and gastrointestinal tract. As many as 25% of patients develop endophthalmitis.\nIn puzzling cases, ophthalmologic examination can detect the pathognomonic appearance of Candida emboli. Similarly, examination of a retrievable peripheral macroembolus can provide both histopathologic and culture evidence of Candida. The pathognomonic signs of subacute bacterial endocarditis, such as Janeway lesions and Osler nodes, and the suggestive findings of Roth spots are not present in candidal endocarditis. These entities result from the immunologic aspects of subacute bacterial endocarditis. Such immune complexes do not occur in fungal valvular infections.[2,3]\nAcross the board, 75% of cases of IE in intravenous drug users involve previously normal hearts. The rate of underlying valvular pathology and the distribution between right-sided and left-sided candidal IE is not established. However, particulate material contained in the injected drug may cause progressive damage to the tricuspid valve. This material is small enough to migrate across the pulmonary capillary bed to the arterial circulation and ultimately scar the mitral or aortic valves. The end result of this process is formation on the valve of a sterile fibrin thrombus (nonbacterial thrombotic endocarditis) to which circulating Candida could attach. Alternatively, increased stimulation of the immune system by repeated exposure to these injected materials could also result in production of nonbacterial thrombotic endocarditis.\nThe most challenging type of candidal IE appears to be candidal prosthetic valve endocarditis, because the infected prosthetic material must be removed and the replacement must be inserted. Fungal prosthetic valve endocarditis that occurs within 60 days of implantation is classified as early. The infection becomes established during the surgery or in the perioperative period. Late prosthetic valve endocarditis occurs 12 months or more after surgery. Intermediate cases are diagnosed between 60 days and 12 months after implantation. High rates (11%-25%) of prosthetic valve endocarditis cases arise from nosocomial fungemia, in which the endocarditis eventually becomes clinically apparent after 26-690 days.\nThe cornerstone of diagnosis of any type of valvular infection is documenting the sustained presence in the bloodstream of the suspected pathogen (bacterial or fungal). The diagnosis of candidal IE is based primarily on blood culture evidence of a continuous fungemia. A continuous bloodstream infection is defined as at least two positive blood culture findings for the same pathogen obtained at least 12 hours apart or three positive blood culture findings positive for the same pathogen, with a first and last finding obtained at least 1 hour apart. The continuous fungemia separates valvular infection from candidemia, which represents intermittent entry of the fungus into the bloodstream from nonvalvular sources, such as lung or kidney.\nEven if vegetations are detected by transthoracic echocardiography (TTE), transesophageal echocardiography (TEE) should always be performed to detect complications of Candida infections, such as paravalvular abscess or large vegetation. The diagnostic usefulness of pathologic study of retrievable peripheral emboli and the presence of endophthalmitis have already been discussed. Serologic testing has not proven useful as a diagnostic approach.\nA mean delay in diagnosis of Candida IE of approximately 32 days is noted. The inability to mount an inflammatory response, such as fever, because of immunosuppression in at-risk patients can significantly hinder its expeditious diagnosis.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "Fever in a 40-Year-Old Intravenous Drug User"
},
{
"authors": "John L. Brusch, MD",
"content": [
"The accepted therapy for candidal IE is a lipid formulation of amphotericin B (3-5 mg/kg daily intravenously). Flucytosine (100 mg/kg/day in 4 divided doses) is synergistic with amphotericin. Serum flucytosine levels should be followed because of potential bone marrow toxicity associated with high levels of the drug. Frequent monitoring of renal function tests to detect amphotericin-associated renal failure that could lead to toxic levels of flucytosine is recommended.",
"The use of amphotericin alone should be discouraged because the fibrin meshwork of the vegetation protects Candida species from the fungicidal action of this drug. These recommendations pertain specifically to IE caused by C albicans, C tropicalis, or C parapsilosis. C krusei and Candida glabrata are uncommon causes of candidal valvular infection but may be resistant to amphotericin. Some may be resistant to fluconazole.",
"A major therapeutic challenge is the unreliability or unavailability of sensitivity testing for antifungal therapy. Some evidence suggests that the use of caspofungin, an echinocandin that is fungicidal, can be curative. The difficulty is the shortcomings antifungal sensitivity testing.",
"Currently, for most circumstances, removal of the infected native valve or prosthetic valve as well as drainage of any associated abscesses is preferred. No rationale supports extensive antifungal pretreatment before surgery. Postoperatively, the antifungal should be continued for at least 6 weeks, especially if intracardiac complications, such as paravalvular abscess, are noted. If the patient's clinical response is good and the bloodstream is cleared of organisms, then changing the regimen to fluconazole alone can be considered.",
"In addition, all intravascular lines should be removed at the beginning of therapy as potential sources of the underlying candidemia. A search for metastatic candidal infection, such as brain abscess or vertebral osteomyelitis, should be performed. The mortality rate of candidal prosthetic valve endocarditis and native valve IE has currently decreased to approximately 30%.",
"Likewise, in patients who cannot undergo valvular resection, lifetime suppression with fluconazole should follow the initial treatment with amphotericin. Flucytosine could be used in this role if the isolate to be suppressed is resistant to fluconazole. Such long-term suppression is also used when intracardiac spread of infection is such that risk of relapse is high despite valve resection.",
"A major unanswered question is how to deal with transient candidemia in the setting of a prosthetic valve, which has a 25% chance of being infected. Parenteral antifungal agents may be considered until the bloodstream is cleared.[4]"
],
"date": "January 12, 2015",
"figures": [],
"markdown": "# Fever in a 40-Year-Old Intravenous Drug User\n\n **Authors:** John L. Brusch, MD \n **Date:** January 12, 2015\n\n ## Content\n\n The accepted therapy for candidal IE is a lipid formulation of amphotericin B (3-5 mg/kg daily intravenously). Flucytosine (100 mg/kg/day in 4 divided doses) is synergistic with amphotericin. Serum flucytosine levels should be followed because of potential bone marrow toxicity associated with high levels of the drug. Frequent monitoring of renal function tests to detect amphotericin-associated renal failure that could lead to toxic levels of flucytosine is recommended.\nThe use of amphotericin alone should be discouraged because the fibrin meshwork of the vegetation protects Candida species from the fungicidal action of this drug. These recommendations pertain specifically to IE caused by C albicans, C tropicalis, or C parapsilosis. C krusei and Candida glabrata are uncommon causes of candidal valvular infection but may be resistant to amphotericin. Some may be resistant to fluconazole.\nA major therapeutic challenge is the unreliability or unavailability of sensitivity testing for antifungal therapy. Some evidence suggests that the use of caspofungin, an echinocandin that is fungicidal, can be curative. The difficulty is the shortcomings antifungal sensitivity testing.\nCurrently, for most circumstances, removal of the infected native valve or prosthetic valve as well as drainage of any associated abscesses is preferred. No rationale supports extensive antifungal pretreatment before surgery. Postoperatively, the antifungal should be continued for at least 6 weeks, especially if intracardiac complications, such as paravalvular abscess, are noted. If the patient's clinical response is good and the bloodstream is cleared of organisms, then changing the regimen to fluconazole alone can be considered.\nIn addition, all intravascular lines should be removed at the beginning of therapy as potential sources of the underlying candidemia. A search for metastatic candidal infection, such as brain abscess or vertebral osteomyelitis, should be performed. The mortality rate of candidal prosthetic valve endocarditis and native valve IE has currently decreased to approximately 30%.\nLikewise, in patients who cannot undergo valvular resection, lifetime suppression with fluconazole should follow the initial treatment with amphotericin. Flucytosine could be used in this role if the isolate to be suppressed is resistant to fluconazole. Such long-term suppression is also used when intracardiac spread of infection is such that risk of relapse is high despite valve resection.\nA major unanswered question is how to deal with transient candidemia in the setting of a prosthetic valve, which has a 25% chance of being infected. Parenteral antifungal agents may be considered until the bloodstream is cleared.[4]\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 806453,
"choiceText": "Echocardiography",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 806455,
"choiceText": "Eye examination",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 806457,
"choiceText": "Beta-D-glucan assay",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 806459,
"choiceText": "Examination of a peripheral macroembolus",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Although helpful in diagnosing candidemia (especially invasive candidemia), beta-D-glucan assay has not been demonstrated to increase the sensitivity or specificity of blood cultures. This is because of the very high rate of positive blood cultures in candidal valvular infections.<br>\r\n<br>\r\nEchocardiography is seldom required for diagnosis but is very important to detect complications of candidal IE. A thorough eye examination focused on the periphery of the retina can detect pathognomonic <i>Candida</i> emboli. Occasionally, pathologic examination and culture of peripheral <i>Candida</i> emboli is the first indicator of candidal IE.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 253051,
"questionText": "Which of the following is the least appropriate diagnostic test for candidal IE?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 806461,
"choiceText": "Add gentamicin for synergism against MRSA",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 806463,
"choiceText": "Place the patient on heparin",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 806465,
"choiceText": "Perform emergency valve replacement",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 806467,
"choiceText": "Empirically add antifungal therapy",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "In this patient, before embolization to the leg occurred, massive vegetation was documented of the posterior leaflet of the aortic valve. This embolism was unlikely to have arisen from the right side of the heart because it was systemic. The only viable approach to prevent further embolization is removal of the valve as soon as possible.<br>\r\n<br>\r\nAdding gentamicin for synergy against MRSA offers no therapeutic advantage, although it was previously the standard of care. Anticoagulating a patient with this type of vegetation only promotes embolization. Although it was candidal vegetation in this case, adding antifungal therapy at this point does nothing to minimize the immediate risk for further embolization.<br>\r\n<br>\r\nCharacteristics associated with embolization of vegetations during therapy for IE include the following:<sup>5-8</sup>\r\n\r\n<ul><li>Vegetations > 10 mm in diameter;</li>\r\n<li>Vegetations > 8 mm in diameter and growing;</li>\r\n<li>Multiple vegetations;</li>\r\n<li>Mobile pedunculated vegetations;</li>\r\n<li>Vegetations of the mitral valve;</li>\r\n<li>Vegetations due to <i>S aureus</i>, <i>Abiotrophia</i> species, or <i>Haemophilus parainfluenzae</i>;</li>\r\n<li>Vegetations due to fungi;</li>\r\n<li>Noncalcified vegetations; and</li>\r\n<li>Prolapsing vegetations.</li></ul>",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 253053,
"questionText": "At the point of macroembolization to the leg, which of the following is the most appropriate next therapeutic step?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Fever in a 40-Year-Old Intravenous Drug User"
},
{
"authors": "John L. Brusch, MD",
"content": [],
"date": "January 12, 2015",
"figures": [],
"markdown": "# Fever in a 40-Year-Old Intravenous Drug User\n\n **Authors:** John L. Brusch, MD \n **Date:** January 12, 2015\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 806453,
"choiceText": "Echocardiography",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 806455,
"choiceText": "Eye examination",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 806457,
"choiceText": "Beta-D-glucan assay",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 806459,
"choiceText": "Examination of a peripheral macroembolus",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Although helpful in diagnosing candidemia (especially invasive candidemia), beta-D-glucan assay has not been demonstrated to increase the sensitivity or specificity of blood cultures. This is because of the very high rate of positive blood cultures in candidal valvular infections.<br>\r\n<br>\r\nEchocardiography is seldom required for diagnosis but is very important to detect complications of candidal IE. A thorough eye examination focused on the periphery of the retina can detect pathognomonic <i>Candida</i> emboli. Occasionally, pathologic examination and culture of peripheral <i>Candida</i> emboli is the first indicator of candidal IE.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 253051,
"questionText": "Which of the following is the least appropriate diagnostic test for candidal IE?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 806461,
"choiceText": "Add gentamicin for synergism against MRSA",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 806463,
"choiceText": "Place the patient on heparin",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 806465,
"choiceText": "Perform emergency valve replacement",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 806467,
"choiceText": "Empirically add antifungal therapy",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "In this patient, before embolization to the leg occurred, massive vegetation was documented of the posterior leaflet of the aortic valve. This embolism was unlikely to have arisen from the right side of the heart because it was systemic. The only viable approach to prevent further embolization is removal of the valve as soon as possible.<br>\r\n<br>\r\nAdding gentamicin for synergy against MRSA offers no therapeutic advantage, although it was previously the standard of care. Anticoagulating a patient with this type of vegetation only promotes embolization. Although it was candidal vegetation in this case, adding antifungal therapy at this point does nothing to minimize the immediate risk for further embolization.<br>\r\n<br>\r\nCharacteristics associated with embolization of vegetations during therapy for IE include the following:<sup>5-8</sup>\r\n\r\n<ul><li>Vegetations > 10 mm in diameter;</li>\r\n<li>Vegetations > 8 mm in diameter and growing;</li>\r\n<li>Multiple vegetations;</li>\r\n<li>Mobile pedunculated vegetations;</li>\r\n<li>Vegetations of the mitral valve;</li>\r\n<li>Vegetations due to <i>S aureus</i>, <i>Abiotrophia</i> species, or <i>Haemophilus parainfluenzae</i>;</li>\r\n<li>Vegetations due to fungi;</li>\r\n<li>Noncalcified vegetations; and</li>\r\n<li>Prolapsing vegetations.</li></ul>",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 253053,
"questionText": "At the point of macroembolization to the leg, which of the following is the most appropriate next therapeutic step?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Fever in a 40-Year-Old Intravenous Drug User"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 806445,
"choiceText": "Relapsing MRSA valvular infection",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 806447,
"choiceText": "Liver failure due to hepatitis C infection",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 806449,
"choiceText": "Polyarteritis secondary to hepatitis C infection",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 806451,
"choiceText": "Fungal endocarditis",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 253049,
"questionText": "On the basis of the history, physical examination, and workup, what is the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 806453,
"choiceText": "Echocardiography",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 806455,
"choiceText": "Eye examination",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 806457,
"choiceText": "Beta-D-glucan assay",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 806459,
"choiceText": "Examination of a peripheral macroembolus",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Although helpful in diagnosing candidemia (especially invasive candidemia), beta-D-glucan assay has not been demonstrated to increase the sensitivity or specificity of blood cultures. This is because of the very high rate of positive blood cultures in candidal valvular infections.<br>\r\n<br>\r\nEchocardiography is seldom required for diagnosis but is very important to detect complications of candidal IE. A thorough eye examination focused on the periphery of the retina can detect pathognomonic <i>Candida</i> emboli. Occasionally, pathologic examination and culture of peripheral <i>Candida</i> emboli is the first indicator of candidal IE.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 253051,
"questionText": "Which of the following is the least appropriate diagnostic test for candidal IE?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 806461,
"choiceText": "Add gentamicin for synergism against MRSA",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 806463,
"choiceText": "Place the patient on heparin",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 806465,
"choiceText": "Perform emergency valve replacement",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 806467,
"choiceText": "Empirically add antifungal therapy",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "In this patient, before embolization to the leg occurred, massive vegetation was documented of the posterior leaflet of the aortic valve. This embolism was unlikely to have arisen from the right side of the heart because it was systemic. The only viable approach to prevent further embolization is removal of the valve as soon as possible.<br>\r\n<br>\r\nAdding gentamicin for synergy against MRSA offers no therapeutic advantage, although it was previously the standard of care. Anticoagulating a patient with this type of vegetation only promotes embolization. Although it was candidal vegetation in this case, adding antifungal therapy at this point does nothing to minimize the immediate risk for further embolization.<br>\r\n<br>\r\nCharacteristics associated with embolization of vegetations during therapy for IE include the following:<sup>5-8</sup>\r\n\r\n<ul><li>Vegetations > 10 mm in diameter;</li>\r\n<li>Vegetations > 8 mm in diameter and growing;</li>\r\n<li>Multiple vegetations;</li>\r\n<li>Mobile pedunculated vegetations;</li>\r\n<li>Vegetations of the mitral valve;</li>\r\n<li>Vegetations due to <i>S aureus</i>, <i>Abiotrophia</i> species, or <i>Haemophilus parainfluenzae</i>;</li>\r\n<li>Vegetations due to fungi;</li>\r\n<li>Noncalcified vegetations; and</li>\r\n<li>Prolapsing vegetations.</li></ul>",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 253053,
"questionText": "At the point of macroembolization to the leg, which of the following is the most appropriate next therapeutic step?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
836276 | /viewarticle/836276 | [
{
"authors": "Tomislav Dragovich, MD, PhD; Shailja B. Amin, PA-C",
"content": [
"Editor's Note: \nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions.",
"Background",
"A 70-year-old man presents to a clinic with unintentional weight loss, worsening fatigue, and abdominal cramping. He has a history of breast cancer (estrogen and progesterone receptor-positive and HER2-negative) for which he underwent a right mastectomy 9 years ago. This was followed by adjuvant chemotherapy and a 5-year course of tamoxifen. He also has a distant history of superficial melanoma, which was treated with wide excision 15 years ago. In addition, his medical history is relevant for superficial bladder cancer and irritable bowel syndrome.",
"The patient's family history is notable for lung cancer in his mother in her mid-70s. His current medications include aspirin, omeprazole, and fenofibrate. He has a 20–pack-year history of smoking but quit at age 51 years."
],
"date": "December 19, 2014",
"figures": [],
"markdown": "# Fatigue, Weight Loss, and Abdominal Cramping in a 70-Year-Old Man\n\n **Authors:** Tomislav Dragovich, MD, PhD; Shailja B. Amin, PA-C \n **Date:** December 19, 2014\n\n ## Content\n\n Editor's Note: \nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions.\nBackground\nA 70-year-old man presents to a clinic with unintentional weight loss, worsening fatigue, and abdominal cramping. He has a history of breast cancer (estrogen and progesterone receptor-positive and HER2-negative) for which he underwent a right mastectomy 9 years ago. This was followed by adjuvant chemotherapy and a 5-year course of tamoxifen. He also has a distant history of superficial melanoma, which was treated with wide excision 15 years ago. In addition, his medical history is relevant for superficial bladder cancer and irritable bowel syndrome.\nThe patient's family history is notable for lung cancer in his mother in her mid-70s. His current medications include aspirin, omeprazole, and fenofibrate. He has a 20–pack-year history of smoking but quit at age 51 years.\n\n ## Figures\n\n \n*Page 1 of 5*",
"pagination": {
"current_page": 1,
"total_pages": 5
},
"questionnaire": [],
"title": "Fatigue, Weight Loss, and Abdominal Cramping in a 70-Year-Old Man"
},
{
"authors": "Tomislav Dragovich, MD, PhD; Shailja B. Amin, PA-C",
"content": [
"Physical Examination and Work-up",
"At presentation, the patient appears to be in no acute distress. His vital signs include blood pressure of 142/80 mm Hg, heart rate of 72 beats/min, temperature of 98.2°F, respiration rate of 18 breaths/min, height of 179 cm, and weight of 84 kg. Examination of the head, ears, nose, and throat is unremarkable. The neck is supple, with no evidence of an enlarged thyroid gland. Chest expansions are symmetrical, with clear pulmonary auscultations. The cardiac rhythm is regular, with no murmurs or gallops identified.",
"The abdomen is soft and nontender. Generalized distention is noted upon palpation of the abdomen. The liver edge is palpable 2 cm below the right costal margin. The extremities show no evidence of cyanosis or edema.",
"Laboratory analysis shows a white blood cell count of 6100 cells/mL (normal range, 4000-11,000 cells/mL), a hemoglobin level of 11.9 g/dL (normal range, 13-17.5 g/dL), and a platelet count of 176,000 cells/mL (normal range, 130,000-450,000 cells/mL). The complete metabolic panel is within normal limits.",
"Further work-up included CT of the abdomen and pelvis with contrast, which revealed a 3.7-cm mass within the body of the pancreas. A moderate amount of ascites was present. The patient was referred for an upper endoscopic ultrasonography (EUS)-guided biopsy of the pancreatic mass."
],
"date": "December 19, 2014",
"figures": [],
"markdown": "# Fatigue, Weight Loss, and Abdominal Cramping in a 70-Year-Old Man\n\n **Authors:** Tomislav Dragovich, MD, PhD; Shailja B. Amin, PA-C \n **Date:** December 19, 2014\n\n ## Content\n\n Physical Examination and Work-up\nAt presentation, the patient appears to be in no acute distress. His vital signs include blood pressure of 142/80 mm Hg, heart rate of 72 beats/min, temperature of 98.2°F, respiration rate of 18 breaths/min, height of 179 cm, and weight of 84 kg. Examination of the head, ears, nose, and throat is unremarkable. The neck is supple, with no evidence of an enlarged thyroid gland. Chest expansions are symmetrical, with clear pulmonary auscultations. The cardiac rhythm is regular, with no murmurs or gallops identified.\nThe abdomen is soft and nontender. Generalized distention is noted upon palpation of the abdomen. The liver edge is palpable 2 cm below the right costal margin. The extremities show no evidence of cyanosis or edema.\nLaboratory analysis shows a white blood cell count of 6100 cells/mL (normal range, 4000-11,000 cells/mL), a hemoglobin level of 11.9 g/dL (normal range, 13-17.5 g/dL), and a platelet count of 176,000 cells/mL (normal range, 130,000-450,000 cells/mL). The complete metabolic panel is within normal limits.\nFurther work-up included CT of the abdomen and pelvis with contrast, which revealed a 3.7-cm mass within the body of the pancreas. A moderate amount of ascites was present. The patient was referred for an upper endoscopic ultrasonography (EUS)-guided biopsy of the pancreatic mass.\n\n ## Figures\n\n \n*Page 2 of 5*",
"pagination": {
"current_page": 2,
"total_pages": 5
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 802433,
"choiceText": "Pancreatic lymphoma",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 802435,
"choiceText": "Breast cancer metastasis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 802437,
"choiceText": "Primary pancreatic cancer",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 802439,
"choiceText": "Focal pancreatitis",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 802441,
"choiceText": "Melanoma metastasis",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 802443,
"choiceText": "Pancreatic neuroendocrine tumor",
"correct": false,
"displayOrder": 6,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 251675,
"questionText": "On the basis of the history, physical examination, and work-up, what is the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Fatigue, Weight Loss, and Abdominal Cramping in a 70-Year-Old Man"
},
{
"authors": "Tomislav Dragovich, MD, PhD; Shailja B. Amin, PA-C",
"content": [
"Discussion",
"The patient presented with unintentional weight loss and abdominal cramping. The physical examination was remarkable for hepatomegaly and abdominal ascites. CT of the abdomen and pelvis revealed a 3.7-cm mass within the body of the pancreas, which was further evaluated by esophagogastroduodenoscopy with EUS.",
"The EUS study confirmed the presence of a solid lesion measuring 4 cm x 3 cm (Figures 1-3). The pathologic interpretation of the EUS-guided fine-needle aspiration biopsy was consistent with atypical glandular cells suspicious for malignancy.",
"Figure 1.",
"Figure 2.",
"Figure 3.",
"Because the findings were only suspicious for and not diagnostic of malignancy, the patient underwent staging laparoscopy with biopsies. Upon exploration, diffuse peritoneal and omental carcinomatosis were found. The mass from the body of the pancreas was abutting the common hepatic artery. Pathology of the peritoneum implants and omentum was consistent with metastatic adenocarcinoma. The intraperitoneal fluid that was collected for cytology was positive for malignancy. The serum level of the tumor maker CA 19-9 was elevated, at 3650 IU/mL (normal range, < 35.9 IU/mL).",
"Pancreatic cancer is the fourth leading cause of cancer related death in the United States and the second leading cause of digestive cancer-related death.[1] The incidence of pancreatic cancer increases at age 50 years and peaks in the seventh decade. Male sex and African American race confer higher risk and a higher mortality rate.[2] The 5-year survival rate is about 25%-30% for node-negative disease and 10% for node-positive disease.[2]",
"The pancreas has both endocrine and exocrine functions. Exocrine pancreatic neoplasms include pancreatic ductal, acinar cell, and stem cell neoplasms. About 85% of malignant pancreatic cancer arises as ductal adenocarcinomas. Several subtypes of ductal adenocarcinomas share a poor prognosis; the exception is colloid carcinomas, which have a better prognosis. Endocrine neoplasms include pancreatic neuroendocrine islet cell tumors, which consist of about 5% of pancreatic neoplasms.[3]",
"Diabetes mellitus can be both a symptom of and a risk factor for pancreatic cancer. Studies show that 1% of patients who are diagnosed with diabetes after 50 years of age develop pancreatic cancer within 3 years of being diagnosed with diabetes.[4]Chronic pancreatitis or long-term inflammation of the pancreas can be caused by greater alcohol consumption, leading to a risk for pancreatic cancer that is increased up to 16-fold.[5] Patients with chronic pancreatitis caused by hereditary pancreatitis have up to 50-fold increased risk for developing pancreatic cancer.[6]",
"Hereditary pancreatic cancer accounts for 2% of total pancreatic cancers. Some of these genetic conditions include familial atypical mole and melanoma syndrome (FAMMM), hereditary nonpolyposis colorectal cancer (HNPCC), Peutz-Jeghers syndrome, familial adenomatous polyposis, BRCA2 mutation, and hereditary pancreatitis.[7] Other nonhereditary factors shown to increase risk for pancreatic cancer include peptic ulcer surgery or partial gastrectomy, smoking, obesity, and physical inactivity. Occupational chemicals, such as petrochemicals, have also been linked to pancreatic cancer.[8]",
"The initial presentation of pancreatic cancer varies and depends on tumor location. As many as 60%-70% of tumors arise in the head of the pancreas, which causes symptoms of duct obstruction, jaundice, and pain. Jaundice is most often due to obstruction of the common bile duct by a mass in the head of the pancreas. Only 20%-25% arise in the body or tail; these are less likely to cause symptoms until metastasis has occurred.[7]",
"Other clinical presentations include recent onset of atypical diabetes mellitus or unexplained thrombophlebitis. Signs of metastatic disease include ascites and lymphadenopathy. Common sites of metastasis are the liver; peritoneum; lungs; and, less commonly, the bones.",
"Initial laboratory tests include measurement of serum aminotransferases, alkaline phosphatase, and bilirubin. Imaging should include endoscopic retrograde cholangiopancreatography and EUS to identify the extent of disease for tumor and nodal staging.[2] These techniques are also useful to detect tumor invasion of the portal vein, identify hepatic lesions, and obtain tissue samples. A fine-needle aspiration sample for pathologic diagnosis can be obtained.",
"Dual-phase, contrast, helical CT has a sensitivity of 67% for lesions smaller than 1.5 cm and 100% for tumors larger than 1.5 cm.[2] This scan is also used to investigate major vessel involvement, which includes the superior mesenteric artery, inferior vena cava, aorta, celiac axis, or hepatic artery. A serum CA 19-9 level is not diagnostic but can be useful in patients who are potentially cured after surgery or those who are receiving chemotherapy.[9]"
],
"date": "December 19, 2014",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/836/276/836276-thumb-1.jpg"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/836/276/836276-thumb-2.jpg"
},
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/836/276/836276-thumb-3.jpg"
}
],
"markdown": "# Fatigue, Weight Loss, and Abdominal Cramping in a 70-Year-Old Man\n\n **Authors:** Tomislav Dragovich, MD, PhD; Shailja B. Amin, PA-C \n **Date:** December 19, 2014\n\n ## Content\n\n Discussion\nThe patient presented with unintentional weight loss and abdominal cramping. The physical examination was remarkable for hepatomegaly and abdominal ascites. CT of the abdomen and pelvis revealed a 3.7-cm mass within the body of the pancreas, which was further evaluated by esophagogastroduodenoscopy with EUS.\nThe EUS study confirmed the presence of a solid lesion measuring 4 cm x 3 cm (Figures 1-3). The pathologic interpretation of the EUS-guided fine-needle aspiration biopsy was consistent with atypical glandular cells suspicious for malignancy.\nFigure 1.\nFigure 2.\nFigure 3.\nBecause the findings were only suspicious for and not diagnostic of malignancy, the patient underwent staging laparoscopy with biopsies. Upon exploration, diffuse peritoneal and omental carcinomatosis were found. The mass from the body of the pancreas was abutting the common hepatic artery. Pathology of the peritoneum implants and omentum was consistent with metastatic adenocarcinoma. The intraperitoneal fluid that was collected for cytology was positive for malignancy. The serum level of the tumor maker CA 19-9 was elevated, at 3650 IU/mL (normal range, < 35.9 IU/mL).\nPancreatic cancer is the fourth leading cause of cancer related death in the United States and the second leading cause of digestive cancer-related death.[1] The incidence of pancreatic cancer increases at age 50 years and peaks in the seventh decade. Male sex and African American race confer higher risk and a higher mortality rate.[2] The 5-year survival rate is about 25%-30% for node-negative disease and 10% for node-positive disease.[2]\nThe pancreas has both endocrine and exocrine functions. Exocrine pancreatic neoplasms include pancreatic ductal, acinar cell, and stem cell neoplasms. About 85% of malignant pancreatic cancer arises as ductal adenocarcinomas. Several subtypes of ductal adenocarcinomas share a poor prognosis; the exception is colloid carcinomas, which have a better prognosis. Endocrine neoplasms include pancreatic neuroendocrine islet cell tumors, which consist of about 5% of pancreatic neoplasms.[3]\nDiabetes mellitus can be both a symptom of and a risk factor for pancreatic cancer. Studies show that 1% of patients who are diagnosed with diabetes after 50 years of age develop pancreatic cancer within 3 years of being diagnosed with diabetes.[4]Chronic pancreatitis or long-term inflammation of the pancreas can be caused by greater alcohol consumption, leading to a risk for pancreatic cancer that is increased up to 16-fold.[5] Patients with chronic pancreatitis caused by hereditary pancreatitis have up to 50-fold increased risk for developing pancreatic cancer.[6]\nHereditary pancreatic cancer accounts for 2% of total pancreatic cancers. Some of these genetic conditions include familial atypical mole and melanoma syndrome (FAMMM), hereditary nonpolyposis colorectal cancer (HNPCC), Peutz-Jeghers syndrome, familial adenomatous polyposis, BRCA2 mutation, and hereditary pancreatitis.[7] Other nonhereditary factors shown to increase risk for pancreatic cancer include peptic ulcer surgery or partial gastrectomy, smoking, obesity, and physical inactivity. Occupational chemicals, such as petrochemicals, have also been linked to pancreatic cancer.[8]\nThe initial presentation of pancreatic cancer varies and depends on tumor location. As many as 60%-70% of tumors arise in the head of the pancreas, which causes symptoms of duct obstruction, jaundice, and pain. Jaundice is most often due to obstruction of the common bile duct by a mass in the head of the pancreas. Only 20%-25% arise in the body or tail; these are less likely to cause symptoms until metastasis has occurred.[7]\nOther clinical presentations include recent onset of atypical diabetes mellitus or unexplained thrombophlebitis. Signs of metastatic disease include ascites and lymphadenopathy. Common sites of metastasis are the liver; peritoneum; lungs; and, less commonly, the bones.\nInitial laboratory tests include measurement of serum aminotransferases, alkaline phosphatase, and bilirubin. Imaging should include endoscopic retrograde cholangiopancreatography and EUS to identify the extent of disease for tumor and nodal staging.[2] These techniques are also useful to detect tumor invasion of the portal vein, identify hepatic lesions, and obtain tissue samples. A fine-needle aspiration sample for pathologic diagnosis can be obtained.\nDual-phase, contrast, helical CT has a sensitivity of 67% for lesions smaller than 1.5 cm and 100% for tumors larger than 1.5 cm.[2] This scan is also used to investigate major vessel involvement, which includes the superior mesenteric artery, inferior vena cava, aorta, celiac axis, or hepatic artery. A serum CA 19-9 level is not diagnostic but can be useful in patients who are potentially cured after surgery or those who are receiving chemotherapy.[9]\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n**Figure 3.** \n \n\n\n*Page 3 of 5*",
"pagination": {
"current_page": 3,
"total_pages": 5
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 802433,
"choiceText": "Pancreatic lymphoma",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 802435,
"choiceText": "Breast cancer metastasis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 802437,
"choiceText": "Primary pancreatic cancer",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 802439,
"choiceText": "Focal pancreatitis",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 802441,
"choiceText": "Melanoma metastasis",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 802443,
"choiceText": "Pancreatic neuroendocrine tumor",
"correct": false,
"displayOrder": 6,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 251675,
"questionText": "On the basis of the history, physical examination, and work-up, what is the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Fatigue, Weight Loss, and Abdominal Cramping in a 70-Year-Old Man"
},
{
"authors": "Tomislav Dragovich, MD, PhD; Shailja B. Amin, PA-C",
"content": [
"Resectable disease accounts for less than 10% of pancreatic cancer cases (stage I-IIB). The tumor must be confined to the pancreas and peripancreatic nodes and not encase the celiac axis or superior mesenteric artery.[10] A Whipple procedure (pancreaticoduodenectomy) entails resection of the distal third of the stomach, gallbladder, cystic and common bile ducts, duodenum, and proximal jejunum. A modified Whipple procedure is a pylorus-preserving pancreaticoduodenectomy.[10]",
"Even after resection, the risk for local recurrence is more than 70%, which suggests a strong role for adjuvant therapy.[2] Resection is usually followed by adjuvant chemoradiation (with continuous 5-fluorouracil or capecitabine as a radiation sensitizer) and/or adjuvant gemcitabine-based chemotherapy for 4-6 months.[11]",
"Some cases are classified as borderline resectable, with focal tumor abutment of the visceral arteries or veins.[11] Although this is not a contraindication to resection, many pancreatic cancer centers advocate the use of neoadjuvant chemotherapy or chemoradiation therapy followed by resection.[12]",
"For true locally advanced unresectable disease, induction with infusional fluorouracil plus leucovorin, irinotecan, and oxaliplatin (FOLFIRINOX) or gemcitabine combination therapy is often followed by chemoradiation therapy.[11] Unfortunately, only a small fraction of patients (<10%) are downstaged and are able to undergo R0 or R1 resection.[13]",
"Around 50% of patients with newly diagnosed pancreatic cancer present with metastatic disease. In this setting, palliative chemotherapy is recommended.[2] First-line treatment options are influenced by the patient's performance status and age. Options include FOLFIRINOX, gemcitabine plus nab-paclitaxel, or gemcitabine monotherapy for elderly and frail patients.[13]",
"This case is of interest because of the patient's personal history of breast cancer, melanoma, and pancreatic cancer. Although the proportion of pancreatic cancers linked to these mutations is small, numerous studies have been aimed at finding optimized screening and treatments for this population.[14]",
"BRCA2 is a tumor suppressor gene involved in DNA repair. BRCA2 mutation predisposes individuals to breast cancer; the risk for male breast cancer is 5%-10%. A 3.5-fold, or 7%, risk of developing pancreatic cancer is also noted. BRCA2 is the most common known cause of familial pancreatic cancer, accounting for 17% of cases. A BRCA tumor suppressor gene is involved in DNA cross-linking damage; pancreatic cancer cell lines with BRCA mutations are sensitive to cross-linking agents, such as mitomycin C and cisplatin chemotherapy.[15]",
"Preclinical data also shows that targeting poly(ADP-ribose) polymerase may be effective against BRCA-mutated cells. In tumor cells, inhibition of PARP results in the development of DNA lesions that require intact BRCA for repair; thus, tumor cells that have defective BRCA lack the ability to repair DNA, rendering them more susceptible to cell death. Ongoing studies are combining PARP inhibitors with DNA-damaging chemotherapy for targeting tumors with BRCA mutations.[16]",
"PALB2 is a gene that encodes BRCA2-binding proteins and, therefore, has a molecular relationship with BRCA2. PALB2 mutations have been shown to be associated with an increased risk for familial pancreatic cancer and have a prevalence in 1%-2% of patients with familial breast cancer.[16]",
"A study of 94 patients with a personal history of BRCA1/2-negative breast cancer or a family history of pancreatic cancer found a prevalence of 2.1% for PALB2 mutation. Although further studies are needed to determine the clinical use of PALB2 mutations in patients diagnosed with pancreatic cancer, it may be useful for early screening in high-risk individuals.[16]",
"Another consideration is FAMMM, which is caused by a p16 tumor suppressor gene mutation. It is characterized by familial occurrence of malignant melanoma of the skin and pancreatic cancer. A retrospective study conducted from the Dutch FAMMM registry concluded that the estimated risk for pancreatic cancer in p16 gene mutations by age 75 years was 17%.[17] Although this syndrome is a consideration, a stronger family history of cancer is more common than is found in this case.",
"Currently, no screening tests are routinely recommended for average-risk individuals. Certain groups can benefit from screening to detect early pancreatic neoplasms, which include hereditary pancreatitis and inherited cancer susceptibility syndromes. Two precursor lesions to pancreatic cancer have been identified: intraductal papillary mucinous neoplasm and high-grade pancreatic intraepithelial neoplasia.",
"A prospective study from the American Cancer of the Pancreas Screening Consortium used CT, MRI, and EUS to screen 225 high-risk asymptomatic individuals; 92 (43%) were found to have a pancreatic mass or dilated pancreatic duct.[18] Of those, 82 were intraductal papillary mucinous neoplasms and three were pancreatic neuroendocrine tumors. Potential candidates for screening include patients with Peutz-Jeghers syndrome; those with hereditary pancreatitis; those who have three or more first-, second-, or third-degree relatives with pancreatic cancer; or known carriers of mutations associated with high risk for pancreatic cancer.[14]",
"In this case, although no strong family history is noted, the coincidence of breast and pancreatic cancer diagnosis warrants additional genetic risk evaluation and possibly further testing for BRCA2 and PALB2 germline mutations."
],
"date": "December 19, 2014",
"figures": [],
"markdown": "# Fatigue, Weight Loss, and Abdominal Cramping in a 70-Year-Old Man\n\n **Authors:** Tomislav Dragovich, MD, PhD; Shailja B. Amin, PA-C \n **Date:** December 19, 2014\n\n ## Content\n\n Resectable disease accounts for less than 10% of pancreatic cancer cases (stage I-IIB). The tumor must be confined to the pancreas and peripancreatic nodes and not encase the celiac axis or superior mesenteric artery.[10] A Whipple procedure (pancreaticoduodenectomy) entails resection of the distal third of the stomach, gallbladder, cystic and common bile ducts, duodenum, and proximal jejunum. A modified Whipple procedure is a pylorus-preserving pancreaticoduodenectomy.[10]\nEven after resection, the risk for local recurrence is more than 70%, which suggests a strong role for adjuvant therapy.[2] Resection is usually followed by adjuvant chemoradiation (with continuous 5-fluorouracil or capecitabine as a radiation sensitizer) and/or adjuvant gemcitabine-based chemotherapy for 4-6 months.[11]\nSome cases are classified as borderline resectable, with focal tumor abutment of the visceral arteries or veins.[11] Although this is not a contraindication to resection, many pancreatic cancer centers advocate the use of neoadjuvant chemotherapy or chemoradiation therapy followed by resection.[12]\nFor true locally advanced unresectable disease, induction with infusional fluorouracil plus leucovorin, irinotecan, and oxaliplatin (FOLFIRINOX) or gemcitabine combination therapy is often followed by chemoradiation therapy.[11] Unfortunately, only a small fraction of patients (<10%) are downstaged and are able to undergo R0 or R1 resection.[13]\nAround 50% of patients with newly diagnosed pancreatic cancer present with metastatic disease. In this setting, palliative chemotherapy is recommended.[2] First-line treatment options are influenced by the patient's performance status and age. Options include FOLFIRINOX, gemcitabine plus nab-paclitaxel, or gemcitabine monotherapy for elderly and frail patients.[13]\nThis case is of interest because of the patient's personal history of breast cancer, melanoma, and pancreatic cancer. Although the proportion of pancreatic cancers linked to these mutations is small, numerous studies have been aimed at finding optimized screening and treatments for this population.[14]\nBRCA2 is a tumor suppressor gene involved in DNA repair. BRCA2 mutation predisposes individuals to breast cancer; the risk for male breast cancer is 5%-10%. A 3.5-fold, or 7%, risk of developing pancreatic cancer is also noted. BRCA2 is the most common known cause of familial pancreatic cancer, accounting for 17% of cases. A BRCA tumor suppressor gene is involved in DNA cross-linking damage; pancreatic cancer cell lines with BRCA mutations are sensitive to cross-linking agents, such as mitomycin C and cisplatin chemotherapy.[15]\nPreclinical data also shows that targeting poly(ADP-ribose) polymerase may be effective against BRCA-mutated cells. In tumor cells, inhibition of PARP results in the development of DNA lesions that require intact BRCA for repair; thus, tumor cells that have defective BRCA lack the ability to repair DNA, rendering them more susceptible to cell death. Ongoing studies are combining PARP inhibitors with DNA-damaging chemotherapy for targeting tumors with BRCA mutations.[16]\nPALB2 is a gene that encodes BRCA2-binding proteins and, therefore, has a molecular relationship with BRCA2. PALB2 mutations have been shown to be associated with an increased risk for familial pancreatic cancer and have a prevalence in 1%-2% of patients with familial breast cancer.[16]\nA study of 94 patients with a personal history of BRCA1/2-negative breast cancer or a family history of pancreatic cancer found a prevalence of 2.1% for PALB2 mutation. Although further studies are needed to determine the clinical use of PALB2 mutations in patients diagnosed with pancreatic cancer, it may be useful for early screening in high-risk individuals.[16]\nAnother consideration is FAMMM, which is caused by a p16 tumor suppressor gene mutation. It is characterized by familial occurrence of malignant melanoma of the skin and pancreatic cancer. A retrospective study conducted from the Dutch FAMMM registry concluded that the estimated risk for pancreatic cancer in p16 gene mutations by age 75 years was 17%.[17] Although this syndrome is a consideration, a stronger family history of cancer is more common than is found in this case.\nCurrently, no screening tests are routinely recommended for average-risk individuals. Certain groups can benefit from screening to detect early pancreatic neoplasms, which include hereditary pancreatitis and inherited cancer susceptibility syndromes. Two precursor lesions to pancreatic cancer have been identified: intraductal papillary mucinous neoplasm and high-grade pancreatic intraepithelial neoplasia.\nA prospective study from the American Cancer of the Pancreas Screening Consortium used CT, MRI, and EUS to screen 225 high-risk asymptomatic individuals; 92 (43%) were found to have a pancreatic mass or dilated pancreatic duct.[18] Of those, 82 were intraductal papillary mucinous neoplasms and three were pancreatic neuroendocrine tumors. Potential candidates for screening include patients with Peutz-Jeghers syndrome; those with hereditary pancreatitis; those who have three or more first-, second-, or third-degree relatives with pancreatic cancer; or known carriers of mutations associated with high risk for pancreatic cancer.[14]\nIn this case, although no strong family history is noted, the coincidence of breast and pancreatic cancer diagnosis warrants additional genetic risk evaluation and possibly further testing for BRCA2 and PALB2 germline mutations.\n\n ## Figures\n\n \n*Page 4 of 5*",
"pagination": {
"current_page": 4,
"total_pages": 5
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 802461,
"choiceText": "FAMMM",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 802463,
"choiceText": "Hereditary pancreatitis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 802465,
"choiceText": "<ii>PALB</i> mutation",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 802467,
"choiceText": "<i>BRCA2</i> mutation",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 802469,
"choiceText": "Familial adenomatous polyposis",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "A mutation in <i>BRCA2</i> is the most common known cause of familial pancreatic cancer; it accounts for 17% of cases.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 251681,
"questionText": "Which of the following is the most common cause of familial pancreatic cancer?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 802479,
"choiceText": "MRI",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 802481,
"choiceText": "CA19-9 blood test",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 802483,
"choiceText": "EUS",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 802485,
"choiceText": "PET",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "EUS is a highly sensitive method to detect vessel or nodal involvement when staging pancreatic cancers. Pancreatic-protocol CT is also useful.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 251685,
"questionText": "Which imaging modality is best for local staging in pancreatic cancers?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Fatigue, Weight Loss, and Abdominal Cramping in a 70-Year-Old Man"
},
{
"authors": "Tomislav Dragovich, MD, PhD; Shailja B. Amin, PA-C",
"content": [],
"date": "December 19, 2014",
"figures": [],
"markdown": "# Fatigue, Weight Loss, and Abdominal Cramping in a 70-Year-Old Man\n\n **Authors:** Tomislav Dragovich, MD, PhD; Shailja B. Amin, PA-C \n **Date:** December 19, 2014\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 5 of 5*",
"pagination": {
"current_page": 5,
"total_pages": 5
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 802461,
"choiceText": "FAMMM",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 802463,
"choiceText": "Hereditary pancreatitis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 802465,
"choiceText": "<ii>PALB</i> mutation",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 802467,
"choiceText": "<i>BRCA2</i> mutation",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 802469,
"choiceText": "Familial adenomatous polyposis",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "A mutation in <i>BRCA2</i> is the most common known cause of familial pancreatic cancer; it accounts for 17% of cases.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 251681,
"questionText": "Which of the following is the most common cause of familial pancreatic cancer?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 802479,
"choiceText": "MRI",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 802481,
"choiceText": "CA19-9 blood test",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 802483,
"choiceText": "EUS",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 802485,
"choiceText": "PET",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "EUS is a highly sensitive method to detect vessel or nodal involvement when staging pancreatic cancers. Pancreatic-protocol CT is also useful.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 251685,
"questionText": "Which imaging modality is best for local staging in pancreatic cancers?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Fatigue, Weight Loss, and Abdominal Cramping in a 70-Year-Old Man"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 802433,
"choiceText": "Pancreatic lymphoma",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 802435,
"choiceText": "Breast cancer metastasis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 802437,
"choiceText": "Primary pancreatic cancer",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 802439,
"choiceText": "Focal pancreatitis",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 802441,
"choiceText": "Melanoma metastasis",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 802443,
"choiceText": "Pancreatic neuroendocrine tumor",
"correct": false,
"displayOrder": 6,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 251675,
"questionText": "On the basis of the history, physical examination, and work-up, what is the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 802461,
"choiceText": "FAMMM",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 802463,
"choiceText": "Hereditary pancreatitis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 802465,
"choiceText": "<ii>PALB</i> mutation",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 802467,
"choiceText": "<i>BRCA2</i> mutation",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 802469,
"choiceText": "Familial adenomatous polyposis",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "A mutation in <i>BRCA2</i> is the most common known cause of familial pancreatic cancer; it accounts for 17% of cases.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 251681,
"questionText": "Which of the following is the most common cause of familial pancreatic cancer?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 802479,
"choiceText": "MRI",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 802481,
"choiceText": "CA19-9 blood test",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 802483,
"choiceText": "EUS",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 802485,
"choiceText": "PET",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "EUS is a highly sensitive method to detect vessel or nodal involvement when staging pancreatic cancers. Pancreatic-protocol CT is also useful.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 251685,
"questionText": "Which imaging modality is best for local staging in pancreatic cancers?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
835752 | /viewarticle/835752 | [
{
"authors": "Roberta Capp, MD; Natan Noviski, MD",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"Background",
"A 17-year-old male presents to the emergency department with a headache that has lasted for the past 2 days. The patient states that he returned to the United States 2 days ago after spending 3 weeks in Nigeria. According to the patient, he felt well when he initially arrived in the United States, but developed a severe headache soon after. The headache is constant and throbbing, lasts throughout the day, and is relieved with ibuprofen. He is experiencing subjective fevers and intermittent sweats, especially at night, but he has not taken his temperature.",
"Today, while preparing to board a plane, the patient developed a worsening headache, bilious emesis, palpitations, and sweats. He decided to delay his trip and has now presented to the local emergency department for further evaluation. The patient denies having any trauma, seizures, abdominal pain, stiff neck, or photophobia. He has no significant medical history, and his only medication use is the ibuprofen that he has taken over the past 2 days."
],
"date": "December 11, 2017",
"figures": [],
"markdown": "# A Traveler's Fever\n\n **Authors:** Roberta Capp, MD; Natan Noviski, MD \n **Date:** December 11, 2017\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nBackground\nA 17-year-old male presents to the emergency department with a headache that has lasted for the past 2 days. The patient states that he returned to the United States 2 days ago after spending 3 weeks in Nigeria. According to the patient, he felt well when he initially arrived in the United States, but developed a severe headache soon after. The headache is constant and throbbing, lasts throughout the day, and is relieved with ibuprofen. He is experiencing subjective fevers and intermittent sweats, especially at night, but he has not taken his temperature.\nToday, while preparing to board a plane, the patient developed a worsening headache, bilious emesis, palpitations, and sweats. He decided to delay his trip and has now presented to the local emergency department for further evaluation. The patient denies having any trauma, seizures, abdominal pain, stiff neck, or photophobia. He has no significant medical history, and his only medication use is the ibuprofen that he has taken over the past 2 days.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A Traveler's Fever"
},
{
"authors": "Roberta Capp, MD; Natan Noviski, MD",
"content": [
"Physical Examination and Work-up",
"Upon physical examination, the patient's temperature is 103.0°F, pulse is 83 beats/min, blood pressure is 120/70 mm Hg, respiratory rate is 16 breaths/min, and oxygen saturation is 96% while breathing room air. The patient is generally well-appearing, alert, and oriented. Examination of the head, eyes, and pupils is unremarkable. The neck is supple, without any lymphadenopathy.",
"During auscultation, the lungs are clear; the patient's heart has a regular rate and rhythm, without any murmurs. Examination of the abdomen reveals normal bowel sounds, with mild tenderness to palpation in the right and left upper quadrants. The spleen is noted to be 3 cm below the costal margin. Neurologic examination is unremarkable.",
"The patient is treated with intravenous fluids. ECG shows a normal sinus rhythm, with a corrected QT interval (QTc) of 390 msec. Serum laboratory tests are done and are significant for the following values:",
"Creatinine: 1.2 mg/dL;",
"Magnesium: 1.2 mg/dL;",
"Alanine aminotransferase (ALT): 110 U/L (normal range, 0-35 U/L);",
"Aspartate aminotransferase (AST): 159 U/L (normal range, 0-35 U/L);",
"Total bilirubin: 2.7 mg/dL (normal range, <1.20 mg/dL);",
"Direct bilirubin: 0.9 mg/dL (15.39 μmol/L) (normal range, <0.20 mg/dL); and",
"Alkaline phosphatase, 152 U/L (normal range, 30-130 U/L).",
"A complete blood count reveals a white blood cell count of 5.7 × 103 cells/µL, a hematocrit of 44% (0.44), and platelet count of 34 × 103 cells/µL. A blood smear is obtained (Figure 1).",
"Figure 1."
],
"date": "December 11, 2017",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/835/752/835752-thumb-1.jpg"
}
],
"markdown": "# A Traveler's Fever\n\n **Authors:** Roberta Capp, MD; Natan Noviski, MD \n **Date:** December 11, 2017\n\n ## Content\n\n Physical Examination and Work-up\nUpon physical examination, the patient's temperature is 103.0°F, pulse is 83 beats/min, blood pressure is 120/70 mm Hg, respiratory rate is 16 breaths/min, and oxygen saturation is 96% while breathing room air. The patient is generally well-appearing, alert, and oriented. Examination of the head, eyes, and pupils is unremarkable. The neck is supple, without any lymphadenopathy.\nDuring auscultation, the lungs are clear; the patient's heart has a regular rate and rhythm, without any murmurs. Examination of the abdomen reveals normal bowel sounds, with mild tenderness to palpation in the right and left upper quadrants. The spleen is noted to be 3 cm below the costal margin. Neurologic examination is unremarkable.\nThe patient is treated with intravenous fluids. ECG shows a normal sinus rhythm, with a corrected QT interval (QTc) of 390 msec. Serum laboratory tests are done and are significant for the following values:\nCreatinine: 1.2 mg/dL;\nMagnesium: 1.2 mg/dL;\nAlanine aminotransferase (ALT): 110 U/L (normal range, 0-35 U/L);\nAspartate aminotransferase (AST): 159 U/L (normal range, 0-35 U/L);\nTotal bilirubin: 2.7 mg/dL (normal range, <1.20 mg/dL);\nDirect bilirubin: 0.9 mg/dL (15.39 μmol/L) (normal range, <0.20 mg/dL); and\nAlkaline phosphatase, 152 U/L (normal range, 30-130 U/L).\nA complete blood count reveals a white blood cell count of 5.7 × 103 cells/µL, a hematocrit of 44% (0.44), and platelet count of 34 × 103 cells/µL. A blood smear is obtained (Figure 1).\nFigure 1.\n\n ## Figures\n\n **Figure 1.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 795875,
"choiceText": "Malaria",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 795877,
"choiceText": "Babesiosis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 795879,
"choiceText": "Lyme disease",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 795881,
"choiceText": "Ehrlichiosis",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 249485,
"questionText": "What is the most likely diagnosis?<br><br><i>Hint: Pay close attention to the finding on the blood smear.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A Traveler's Fever"
},
{
"authors": "Roberta Capp, MD; Natan Noviski, MD",
"content": [
"Discussion",
"This case is an example of malaria caused by Plasmodium falciparum. In the context of the patient's recent travel to Nigeria and presentation with fever, malaria was strongly suspected. The patient's blood smear was notable for 15% parasitemia, most likely P falciparum (as evidenced by the circles within some of the red blood cells in the smear in Figure 2).",
"Figure 2.",
"The other laboratory findings, such as the thrombocytopenia and elevated bilirubin level, correlated with the disease burden. The elevated creatinine value was also noted, because this finding is sometimes seen in more aggressive cases of the disease; however, this usually resolves with time.",
"Worldwide, approximately 300-500 million new cases of malaria are recorded annually.[1] Malaria is the most deadly vector-borne illness in the world, causing 3.5-5 million deaths annually. On average, 40% of the 50 million people who travel from industrialized to developing countries each year report some illness associated with their travel. Approximately 1200 cases of malaria are reported each year in the United States among travelers; therefore, malaria should be considered as a possible cause of fever in the returning traveler.",
"Malaria results from an infection caused by any of the following 5 protozoa of the genus Plasmodium: P falciparum, P vivax, P ovale, P knowlesi, and P malariae.[2] Transmission of the parasite occurs via the bite of the Anopheles mosquito. Once the protozoa are injected into the bloodstream, they enter the hepatic cells and reproduce; eventually, the hepatic cells erupt and release more protozoa into the host's circulation. These parasites then remain in the bloodstream, periodically invading erythrocytes, causing hemolysis, and infecting new red blood cells.",
"The incubation period tends to be 9-18 days for P falciparum, P vivax, P ovale, and P knowlesi, but P malariae has an incubation period of 18-40 days. The most common parasites seen in the United States are P falciparum, which are often found in travelers returning from sub-Saharan Africa, and P vivax, which are found in those returning from Asia, Eastern Europe, and Latin America.",
"The clinical presentation varies between these two parasites. P falciparum often causes symptoms within the first month after the travel period and can be fatal; of patients infected with P vivax, 50% have symptoms within 1 month after travel, and approximately 2% have symptoms 1 year after exposure. Most patients infected with either parasite are usually symptomatic within the first 3 months after they return to the United States."
],
"date": "December 11, 2017",
"figures": [
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/835/752/835752-thumb-2.jpg"
}
],
"markdown": "# A Traveler's Fever\n\n **Authors:** Roberta Capp, MD; Natan Noviski, MD \n **Date:** December 11, 2017\n\n ## Content\n\n Discussion\nThis case is an example of malaria caused by Plasmodium falciparum. In the context of the patient's recent travel to Nigeria and presentation with fever, malaria was strongly suspected. The patient's blood smear was notable for 15% parasitemia, most likely P falciparum (as evidenced by the circles within some of the red blood cells in the smear in Figure 2).\nFigure 2.\nThe other laboratory findings, such as the thrombocytopenia and elevated bilirubin level, correlated with the disease burden. The elevated creatinine value was also noted, because this finding is sometimes seen in more aggressive cases of the disease; however, this usually resolves with time.\nWorldwide, approximately 300-500 million new cases of malaria are recorded annually.[1] Malaria is the most deadly vector-borne illness in the world, causing 3.5-5 million deaths annually. On average, 40% of the 50 million people who travel from industrialized to developing countries each year report some illness associated with their travel. Approximately 1200 cases of malaria are reported each year in the United States among travelers; therefore, malaria should be considered as a possible cause of fever in the returning traveler.\nMalaria results from an infection caused by any of the following 5 protozoa of the genus Plasmodium: P falciparum, P vivax, P ovale, P knowlesi, and P malariae.[2] Transmission of the parasite occurs via the bite of the Anopheles mosquito. Once the protozoa are injected into the bloodstream, they enter the hepatic cells and reproduce; eventually, the hepatic cells erupt and release more protozoa into the host's circulation. These parasites then remain in the bloodstream, periodically invading erythrocytes, causing hemolysis, and infecting new red blood cells.\nThe incubation period tends to be 9-18 days for P falciparum, P vivax, P ovale, and P knowlesi, but P malariae has an incubation period of 18-40 days. The most common parasites seen in the United States are P falciparum, which are often found in travelers returning from sub-Saharan Africa, and P vivax, which are found in those returning from Asia, Eastern Europe, and Latin America.\nThe clinical presentation varies between these two parasites. P falciparum often causes symptoms within the first month after the travel period and can be fatal; of patients infected with P vivax, 50% have symptoms within 1 month after travel, and approximately 2% have symptoms 1 year after exposure. Most patients infected with either parasite are usually symptomatic within the first 3 months after they return to the United States.\n\n ## Figures\n\n **Figure 2.** \n \n\n\n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 795875,
"choiceText": "Malaria",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 795877,
"choiceText": "Babesiosis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 795879,
"choiceText": "Lyme disease",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 795881,
"choiceText": "Ehrlichiosis",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 249485,
"questionText": "What is the most likely diagnosis?<br><br><i>Hint: Pay close attention to the finding on the blood smear.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A Traveler's Fever"
},
{
"authors": "Roberta Capp, MD; Natan Noviski, MD",
"content": [
"The clinical presentation of malaria can vary widely and depends on the species of Plasmodium involved. Common symptoms include fever, malaise, myalgia, and headache, which may be accompanied by cough, abdominal pain, or diarrhea. Because these symptoms are nonspecific, malaria should be considered in all febrile travelers, regardless of their clinical presentation. In fact, approximately 78%-100% of patients presenting with malaria are febrile when they are first examined.",
"The classically described fever patterns are rarely observed; however, when these fevers do occur at 48-hour to 72-hour intervals, this finding is virtually pathognomonic for P vivax, P ovale, and P malariae infections. This cyclical pattern of symptoms coincides with the regular interval of erythrocyte hemolysis.",
"Upon examination, splenomegaly is found in 24%-48% of patients, and patients may experience abdominal pain. Severe malaria, usually caused by P falciparum, causes several manifestations, including prostration, impaired consciousness or coma, respiratory distress caused by pulmonary edema and the acute respiratory distress syndrome, seizures, circulatory collapse, abnormal bleeding (including disseminated intravascular coagulopathy), splenic rupture, jaundice, severe anemia, acute renal failure, and acidosis. The level of parasitemia often exceeds 5%.",
"P knowlesi has been reported to cause sporadic cases of traveler's malaria, mostly in fringe areas in Southeast Asia. Symptoms may be atypical. Organ failure may occur, and deaths have been reported. P knowlesi is not associated with relapse.",
"The diagnosis of malaria is made by examination of both thin and thick blood smears. These smears are used to quantify the level of parasitemia, which is used to guide treatment. If the first smear is negative, it should be repeated at 12- to 24-hour intervals for 48-72 hours. If the diagnosis is clinically suspected and a sufficient laboratory diagnosis is not possible, empirical treatment for P falciparum should be started because the disease can be fatal if left untreated. Rapid detection assays and molecular tools, such as polymerase chain reaction, may also be used to confirm the diagnosis.",
"Other laboratory findings, such as normocytic anemia, thrombocytopenia, low white blood cell count, elevated lactate dehydrogenase level, and elevated bilirubin level, are nonspecific but may provide clues to the diagnosis."
],
"date": "December 11, 2017",
"figures": [],
"markdown": "# A Traveler's Fever\n\n **Authors:** Roberta Capp, MD; Natan Noviski, MD \n **Date:** December 11, 2017\n\n ## Content\n\n The clinical presentation of malaria can vary widely and depends on the species of Plasmodium involved. Common symptoms include fever, malaise, myalgia, and headache, which may be accompanied by cough, abdominal pain, or diarrhea. Because these symptoms are nonspecific, malaria should be considered in all febrile travelers, regardless of their clinical presentation. In fact, approximately 78%-100% of patients presenting with malaria are febrile when they are first examined.\nThe classically described fever patterns are rarely observed; however, when these fevers do occur at 48-hour to 72-hour intervals, this finding is virtually pathognomonic for P vivax, P ovale, and P malariae infections. This cyclical pattern of symptoms coincides with the regular interval of erythrocyte hemolysis.\nUpon examination, splenomegaly is found in 24%-48% of patients, and patients may experience abdominal pain. Severe malaria, usually caused by P falciparum, causes several manifestations, including prostration, impaired consciousness or coma, respiratory distress caused by pulmonary edema and the acute respiratory distress syndrome, seizures, circulatory collapse, abnormal bleeding (including disseminated intravascular coagulopathy), splenic rupture, jaundice, severe anemia, acute renal failure, and acidosis. The level of parasitemia often exceeds 5%.\nP knowlesi has been reported to cause sporadic cases of traveler's malaria, mostly in fringe areas in Southeast Asia. Symptoms may be atypical. Organ failure may occur, and deaths have been reported. P knowlesi is not associated with relapse.\nThe diagnosis of malaria is made by examination of both thin and thick blood smears. These smears are used to quantify the level of parasitemia, which is used to guide treatment. If the first smear is negative, it should be repeated at 12- to 24-hour intervals for 48-72 hours. If the diagnosis is clinically suspected and a sufficient laboratory diagnosis is not possible, empirical treatment for P falciparum should be started because the disease can be fatal if left untreated. Rapid detection assays and molecular tools, such as polymerase chain reaction, may also be used to confirm the diagnosis.\nOther laboratory findings, such as normocytic anemia, thrombocytopenia, low white blood cell count, elevated lactate dehydrogenase level, and elevated bilirubin level, are nonspecific but may provide clues to the diagnosis.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A Traveler's Fever"
},
{
"authors": "Roberta Capp, MD; Natan Noviski, MD",
"content": [
"The treatment options for malaria vary according to the region where the traveler was likely to have acquired the infection.[3] This is based on the local prevalence and antimalarial drug susceptibility of particular Plasmodium species. Chloroquine is the treatment of choice for patients infected with P vivax, P ovale, and P malariae.",
"Patients infected with either P vivax or P ovale must also take primaquine, because these species may lie dormant within the liver and are not eradicated by chloroquine alone. Failure to treat P vivax and P ovale with both agents frequently leads to recrudescence of the disease.",
"If P falciparum has been eliminated as the causative agent, patients may be treated in the ambulatory setting. When P falciparum infection is suspected, treatment should be initiated as soon as possible, because severe illness or death can occur in as little as 1-2 days after presentation. The Centers for Disease Control and Prevention recommends hospitalization in order to ensure that patients are able to tolerate oral therapy and have an appropriate response to treatment.[4]",
"For patients returning from areas where Plasmodium species have known chloroquine resistance, such as southern Asia, sub-Saharan Africa, and Oceania, four treatment options are available: (1) oral quinine along with tetracycline, doxycycline, or clindamycin; (2) atovaquone/proguanil; (3) mefloquine along with doxycycline; or (4) artemether-lumefantrine. Due to toxicity issues and difficulty in obtaining quinine, atovaquone/proguanil or artemether-lumefantrine may be preferred. Depending on the severity of the disease and the region where the parasite was acquired, different lengths of treatment are recommended; these vary from 3 to 7 days of quinine therapy and 7 days of antibiotics.",
"For patients with severe malaria, an initial course of doxycycline with quinidine, an intravenous isomer of quinine, is recommended until the parasite burden is less than 1%, at which point oral therapy should be initiated. Unfortunately, quinine and quinidine have several side effects, and patients must be closely monitored for cardiac dysrhythmias caused by prolongation of the QTc, profound asymptomatic hypoglycemia, and respiratory distress.",
"In this case, malaria resulting from P falciparum infection was promptly diagnosed on the basis of the patient's travel history and the severity of his symptoms. He was admitted to the pediatric intensive care unit for close observation because of the potentially dangerous side effects of treatment. The patient received a bolus of intravenous quinidine and clindamycin. His parasitemia level was less than 0.5% on the second day of treatment, and the patient was switched to oral quinine therapy and clindamycin. His platelet and creatinine levels improved on hospital day 5, and he was discharged to home on oral therapy for a total of 7 days."
],
"date": "December 11, 2017",
"figures": [],
"markdown": "# A Traveler's Fever\n\n **Authors:** Roberta Capp, MD; Natan Noviski, MD \n **Date:** December 11, 2017\n\n ## Content\n\n The treatment options for malaria vary according to the region where the traveler was likely to have acquired the infection.[3] This is based on the local prevalence and antimalarial drug susceptibility of particular Plasmodium species. Chloroquine is the treatment of choice for patients infected with P vivax, P ovale, and P malariae.\nPatients infected with either P vivax or P ovale must also take primaquine, because these species may lie dormant within the liver and are not eradicated by chloroquine alone. Failure to treat P vivax and P ovale with both agents frequently leads to recrudescence of the disease.\nIf P falciparum has been eliminated as the causative agent, patients may be treated in the ambulatory setting. When P falciparum infection is suspected, treatment should be initiated as soon as possible, because severe illness or death can occur in as little as 1-2 days after presentation. The Centers for Disease Control and Prevention recommends hospitalization in order to ensure that patients are able to tolerate oral therapy and have an appropriate response to treatment.[4]\nFor patients returning from areas where Plasmodium species have known chloroquine resistance, such as southern Asia, sub-Saharan Africa, and Oceania, four treatment options are available: (1) oral quinine along with tetracycline, doxycycline, or clindamycin; (2) atovaquone/proguanil; (3) mefloquine along with doxycycline; or (4) artemether-lumefantrine. Due to toxicity issues and difficulty in obtaining quinine, atovaquone/proguanil or artemether-lumefantrine may be preferred. Depending on the severity of the disease and the region where the parasite was acquired, different lengths of treatment are recommended; these vary from 3 to 7 days of quinine therapy and 7 days of antibiotics.\nFor patients with severe malaria, an initial course of doxycycline with quinidine, an intravenous isomer of quinine, is recommended until the parasite burden is less than 1%, at which point oral therapy should be initiated. Unfortunately, quinine and quinidine have several side effects, and patients must be closely monitored for cardiac dysrhythmias caused by prolongation of the QTc, profound asymptomatic hypoglycemia, and respiratory distress.\nIn this case, malaria resulting from P falciparum infection was promptly diagnosed on the basis of the patient's travel history and the severity of his symptoms. He was admitted to the pediatric intensive care unit for close observation because of the potentially dangerous side effects of treatment. The patient received a bolus of intravenous quinidine and clindamycin. His parasitemia level was less than 0.5% on the second day of treatment, and the patient was switched to oral quinine therapy and clindamycin. His platelet and creatinine levels improved on hospital day 5, and he was discharged to home on oral therapy for a total of 7 days.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 795893,
"choiceText": "<i>P vivax\t</i>",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 795895,
"choiceText": "<i>P falciparum\t</i>",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 795897,
"choiceText": "<i>P ovale</i>",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 795899,
"choiceText": "<i>P malariae</i>",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 795901,
"choiceText": "<i>P knowlesi</i>",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "If the diagnosis of malaria is clinically suspected and a sufficient laboratory diagnosis is not possible, empirical treatment for <i>P falciparum</i> should be started because the disease can be fatal if left untreated.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 249499,
"questionText": "Which of the parasites that cause malaria is most likely to be fatal if not treated early?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 795903,
"choiceText": "To ensure that the patient does not have an anaphylactic reaction to the medication",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 795905,
"choiceText": "To ensure that the patient does not spread the disease to others in the community",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 795907,
"choiceText": "To ensure that the parasite is sensitive to the medication",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 795909,
"choiceText": "To monitor the patient for QTc prolongation",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 795911,
"choiceText": "To ensure that the patient is compliant with taking the medication",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "For patients with severe malaria, an initial course of doxycycline with quinidine, an intravenous isomer of quinine, is recommended until the parasite burden is less than 1%, at which point oral therapy should be initiated. Unfortunately, quinine and quinidine have several side effects, and patients must be closely monitored for cardiac dysrhythmias caused by prolongation of the QTc, profound asymptomatic hypoglycemia, and respiratory distress.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 249503,
"questionText": "Patients receiving quinidine therapy should be admitted to the hospital for which of the following reasons?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A Traveler's Fever"
},
{
"authors": "Roberta Capp, MD; Natan Noviski, MD",
"content": [],
"date": "December 11, 2017",
"figures": [],
"markdown": "# A Traveler's Fever\n\n **Authors:** Roberta Capp, MD; Natan Noviski, MD \n **Date:** December 11, 2017\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 795893,
"choiceText": "<i>P vivax\t</i>",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 795895,
"choiceText": "<i>P falciparum\t</i>",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 795897,
"choiceText": "<i>P ovale</i>",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 795899,
"choiceText": "<i>P malariae</i>",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 795901,
"choiceText": "<i>P knowlesi</i>",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "If the diagnosis of malaria is clinically suspected and a sufficient laboratory diagnosis is not possible, empirical treatment for <i>P falciparum</i> should be started because the disease can be fatal if left untreated.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 249499,
"questionText": "Which of the parasites that cause malaria is most likely to be fatal if not treated early?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 795903,
"choiceText": "To ensure that the patient does not have an anaphylactic reaction to the medication",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 795905,
"choiceText": "To ensure that the patient does not spread the disease to others in the community",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 795907,
"choiceText": "To ensure that the parasite is sensitive to the medication",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 795909,
"choiceText": "To monitor the patient for QTc prolongation",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 795911,
"choiceText": "To ensure that the patient is compliant with taking the medication",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "For patients with severe malaria, an initial course of doxycycline with quinidine, an intravenous isomer of quinine, is recommended until the parasite burden is less than 1%, at which point oral therapy should be initiated. Unfortunately, quinine and quinidine have several side effects, and patients must be closely monitored for cardiac dysrhythmias caused by prolongation of the QTc, profound asymptomatic hypoglycemia, and respiratory distress.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 249503,
"questionText": "Patients receiving quinidine therapy should be admitted to the hospital for which of the following reasons?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A Traveler's Fever"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 795875,
"choiceText": "Malaria",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 795877,
"choiceText": "Babesiosis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 795879,
"choiceText": "Lyme disease",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 795881,
"choiceText": "Ehrlichiosis",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 249485,
"questionText": "What is the most likely diagnosis?<br><br><i>Hint: Pay close attention to the finding on the blood smear.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 795893,
"choiceText": "<i>P vivax\t</i>",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 795895,
"choiceText": "<i>P falciparum\t</i>",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 795897,
"choiceText": "<i>P ovale</i>",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 795899,
"choiceText": "<i>P malariae</i>",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 795901,
"choiceText": "<i>P knowlesi</i>",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "If the diagnosis of malaria is clinically suspected and a sufficient laboratory diagnosis is not possible, empirical treatment for <i>P falciparum</i> should be started because the disease can be fatal if left untreated.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 249499,
"questionText": "Which of the parasites that cause malaria is most likely to be fatal if not treated early?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 795903,
"choiceText": "To ensure that the patient does not have an anaphylactic reaction to the medication",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 795905,
"choiceText": "To ensure that the patient does not spread the disease to others in the community",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 795907,
"choiceText": "To ensure that the parasite is sensitive to the medication",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 795909,
"choiceText": "To monitor the patient for QTc prolongation",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 795911,
"choiceText": "To ensure that the patient is compliant with taking the medication",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "For patients with severe malaria, an initial course of doxycycline with quinidine, an intravenous isomer of quinine, is recommended until the parasite burden is less than 1%, at which point oral therapy should be initiated. Unfortunately, quinine and quinidine have several side effects, and patients must be closely monitored for cardiac dysrhythmias caused by prolongation of the QTc, profound asymptomatic hypoglycemia, and respiratory distress.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 249503,
"questionText": "Patients receiving quinidine therapy should be admitted to the hospital for which of the following reasons?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
835183 | /viewarticle/835183 | [
{
"authors": "Gerald W. Chodak, MD",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"A 71-year-old man in generally good health begins to notice urinary problems. He gets up twice each night to urinate (nocturia), has a slow urinary stream, and has a sense of incomplete emptying. He denies urinary burning (dysuria) or blood in the urine (hematuria). Otherwise, he has no known medical conditions and takes no medication. Two years prior, he had an insurance examination, at which time his prostate-specific antigen (PSA) test result was 1.5 ng/mL."
],
"date": "March 14, 2017",
"figures": [],
"markdown": "# A 71-Year-Old Man With Urinary Problems\n\n **Authors:** Gerald W. Chodak, MD \n **Date:** March 14, 2017\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nA 71-year-old man in generally good health begins to notice urinary problems. He gets up twice each night to urinate (nocturia), has a slow urinary stream, and has a sense of incomplete emptying. He denies urinary burning (dysuria) or blood in the urine (hematuria). Otherwise, he has no known medical conditions and takes no medication. Two years prior, he had an insurance examination, at which time his prostate-specific antigen (PSA) test result was 1.5 ng/mL.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 71-Year-Old Man With Urinary Problems"
},
{
"authors": "Gerald W. Chodak, MD",
"content": [
"His family doctor orders a PSA test and performs a digital rectal examination (DRE), and feels that the gland is asymmetric. The PSA test result was 2.4 ng/mL.",
"Figure 1."
],
"date": "March 14, 2017",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/835/183/835183-thumb-1.jpg"
}
],
"markdown": "# A 71-Year-Old Man With Urinary Problems\n\n **Authors:** Gerald W. Chodak, MD \n **Date:** March 14, 2017\n\n ## Content\n\n His family doctor orders a PSA test and performs a digital rectal examination (DRE), and feels that the gland is asymmetric. The PSA test result was 2.4 ng/mL.\nFigure 1.\n\n ## Figures\n\n **Figure 1.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 791145,
"choiceText": "Prostate cancer",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 791147,
"choiceText": "Prostatitis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 791149,
"choiceText": "Benign prostatic hypertrophy",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 791151,
"choiceText": "Overactive bladder",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 247935,
"questionText": "On the basis of the history, physical examination, and work-up, what is the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 71-Year-Old Man With Urinary Problems"
},
{
"authors": "Gerald W. Chodak, MD",
"content": [
"Because the patient was bothered by his loss of sleep, the urologist prescribed an alpha-blocker and a 5-alpha reductase inhibitor (finasteride) and scheduled a follow-up examination in 1 month. The alpha-blocker is used to decrease the outlet resistance of the bladder and relax the pelvic musculature. The 5-alpha reductase inhibitor shrinks the prostate but may take 3-5 months to start working. These two drugs are often prescribed together to maximize the effects of both. The patient was instructed that taking finasteride will decrease the PSA by 50%; this needs to be taken into consideration at the next blood test.",
"At follow-up, the patient's urinary symptoms had improved. He continued on the medications and was asked to return in 6 months. At that visit, his symptoms remained stable. He was asked to return again 6 months later.",
"During the next visit, the urologist performed another PSA test; the result was 3.8 ng/mL. The patient stated that he had sexual intercourse the night before the examination. He returned in 1 week after refraining from sex for 1 day, and the PSA test was repeated; the result was 3.6 ng/mL.[1]",
"A prostate biopsy was recommended because the patient's PSA level did not actually decline. Normally, finasteride should drop the PSA by about 50%, thus yielding a \"real\" PSA of 7.2 ng/mL; this value is a dramatic increase from 2.4 ng/mL.",
"A 12-core biopsy was performed after suitable anesthesia and a prebiopsy antibiotic. The results showed a Gleason score of 3+3 cancer in two cores, each occupying less than 50% of the core (Figure 2). The PSA density was calculated at 0.14. No staging tests were performed.[2]",
"Figure 2.",
"The reason for performing staging tests is to determine whether a patient is suitable for local therapy. Whereas routine testing for metastases was formerly performed on every new case of prostate cancer, doctors have started to question whether such tests are necessary. Now, obtaining these tests makes little sense, given that the odds of finding metastases are lower than the false-negative rates of the tests. On the basis of PSA measurement, DRE, and biopsy results, the odds of cancer that spreads to the bones is less than 1%."
],
"date": "March 14, 2017",
"figures": [
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/835/183/835183-thumb-2.jpg"
}
],
"markdown": "# A 71-Year-Old Man With Urinary Problems\n\n **Authors:** Gerald W. Chodak, MD \n **Date:** March 14, 2017\n\n ## Content\n\n Because the patient was bothered by his loss of sleep, the urologist prescribed an alpha-blocker and a 5-alpha reductase inhibitor (finasteride) and scheduled a follow-up examination in 1 month. The alpha-blocker is used to decrease the outlet resistance of the bladder and relax the pelvic musculature. The 5-alpha reductase inhibitor shrinks the prostate but may take 3-5 months to start working. These two drugs are often prescribed together to maximize the effects of both. The patient was instructed that taking finasteride will decrease the PSA by 50%; this needs to be taken into consideration at the next blood test.\nAt follow-up, the patient's urinary symptoms had improved. He continued on the medications and was asked to return in 6 months. At that visit, his symptoms remained stable. He was asked to return again 6 months later.\nDuring the next visit, the urologist performed another PSA test; the result was 3.8 ng/mL. The patient stated that he had sexual intercourse the night before the examination. He returned in 1 week after refraining from sex for 1 day, and the PSA test was repeated; the result was 3.6 ng/mL.[1]\nA prostate biopsy was recommended because the patient's PSA level did not actually decline. Normally, finasteride should drop the PSA by about 50%, thus yielding a \"real\" PSA of 7.2 ng/mL; this value is a dramatic increase from 2.4 ng/mL.\nA 12-core biopsy was performed after suitable anesthesia and a prebiopsy antibiotic. The results showed a Gleason score of 3+3 cancer in two cores, each occupying less than 50% of the core (Figure 2). The PSA density was calculated at 0.14. No staging tests were performed.[2]\nFigure 2.\nThe reason for performing staging tests is to determine whether a patient is suitable for local therapy. Whereas routine testing for metastases was formerly performed on every new case of prostate cancer, doctors have started to question whether such tests are necessary. Now, obtaining these tests makes little sense, given that the odds of finding metastases are lower than the false-negative rates of the tests. On the basis of PSA measurement, DRE, and biopsy results, the odds of cancer that spreads to the bones is less than 1%.\n\n ## Figures\n\n **Figure 2.** \n \n\n\n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 791145,
"choiceText": "Prostate cancer",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 791147,
"choiceText": "Prostatitis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 791149,
"choiceText": "Benign prostatic hypertrophy",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 791151,
"choiceText": "Overactive bladder",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 247935,
"questionText": "On the basis of the history, physical examination, and work-up, what is the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 71-Year-Old Man With Urinary Problems"
},
{
"authors": "Gerald W. Chodak, MD",
"content": [
"Many online prostate risk calculators can be used to determine risk for prostate cancer on the basis of patient race, age, family history, PSA level, DRE, and previous biopsy status. These are convenient when speaking with and counseling patients.",
"The management of localized prostate cancer has undergone considerable modification over the past 10 years. Before then, few doctors ever discussed conservative therapy with their patients. Generally, surgeons would recommend surgery, and radiation therapists would advise brachytherapy or external radiation. In recent years, that attitude has changed. Now, many patients with low-risk cancer (PSA < 10 ng/mL, Gleason score 3+3, stage T1C or T2A) do not need immediate treatment.[3] The reason is because the vast majority of those tumors will never cause harm.",
"Treating these men exposes them to the risk for various side effects, with little or no chance of extending their life. Key questions patients should ask to help them decide on a therapy include the following:",
"What are the odds I will live longer with this treatment?",
"What are the odds that cancer will recur?",
"What are the odds of developing each of the major side effects?",
"The problem with active surveillance for many of these men is the psychological burden of knowing they have cancer. Also, too often they are not being provided the information in a way in which they can process it. Hence, too many men with low-risk cancer are getting immediate local therapy. Genetic testing may make this decision easier in the future.",
"For now, all of the treatment options can be considered, with several important caveats. First, neither cryosurgery nor high-intensity focused ultrasonography has long-term survival data. For that reason, no one can determine the relative results compared with surgery or radiation; patients should be made aware of these shortcomings."
],
"date": "March 14, 2017",
"figures": [],
"markdown": "# A 71-Year-Old Man With Urinary Problems\n\n **Authors:** Gerald W. Chodak, MD \n **Date:** March 14, 2017\n\n ## Content\n\n Many online prostate risk calculators can be used to determine risk for prostate cancer on the basis of patient race, age, family history, PSA level, DRE, and previous biopsy status. These are convenient when speaking with and counseling patients.\nThe management of localized prostate cancer has undergone considerable modification over the past 10 years. Before then, few doctors ever discussed conservative therapy with their patients. Generally, surgeons would recommend surgery, and radiation therapists would advise brachytherapy or external radiation. In recent years, that attitude has changed. Now, many patients with low-risk cancer (PSA < 10 ng/mL, Gleason score 3+3, stage T1C or T2A) do not need immediate treatment.[3] The reason is because the vast majority of those tumors will never cause harm.\nTreating these men exposes them to the risk for various side effects, with little or no chance of extending their life. Key questions patients should ask to help them decide on a therapy include the following:\nWhat are the odds I will live longer with this treatment?\nWhat are the odds that cancer will recur?\nWhat are the odds of developing each of the major side effects?\nThe problem with active surveillance for many of these men is the psychological burden of knowing they have cancer. Also, too often they are not being provided the information in a way in which they can process it. Hence, too many men with low-risk cancer are getting immediate local therapy. Genetic testing may make this decision easier in the future.\nFor now, all of the treatment options can be considered, with several important caveats. First, neither cryosurgery nor high-intensity focused ultrasonography has long-term survival data. For that reason, no one can determine the relative results compared with surgery or radiation; patients should be made aware of these shortcomings.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 71-Year-Old Man With Urinary Problems"
},
{
"authors": "Gerald W. Chodak, MD",
"content": [
"Proton-beam therapy is being advocated by physicians at proton centers; however, despite the claims of fewer side effects, no valid evidence can validate these claims, and no evidence has suggested that it provides better outcomes than other forms of radiation.[4] For now, it simply is not worth the higher cost. That leaves conservative therapy, radical prostatectomy, brachytherapy, and external radiation.",
"To date, no study has compared radiation with conservative therapy, although the ProtecT study in Great Britain may be able to provide some insight.[5] To date, only two well-designed randomized, controlled trials have been reported. A Scandinavian trial of watchful waiting compared with radical prostatectomy found a 5.4% higher survival in the men undergoing surgery at 12 years.[6] However, the difference in survival only applied to men younger than 65 years. This study contained few men with T1C disease, making it hard to broadly apply the results to men diagnosed in the United States.",
"The PIVOT trial primarily enrolled men with T1C disease and found a 2.9% higher survival in the surgery group, although it was not statistically significant.[7] In subset analyses, a slightly higher overall survival occurred in the group receiving conservative therapy, but this too was not significant. Men with low-risk disease did not have any better survival with surgery.",
"As for surgery vs either method of radiation, no randomized study has compared these options. Therefore, they are reasonable to consider; however, it cannot be argued that one of these therapies is more effective in treating localized disease.[8]",
"Clearly, more randomized studies are needed to further identify which patients benefit from definitive therapy and which men do not. Until then, doctors should provide a careful explanation of the pros and cons of the various options, so that they can help each patient make an appropriate choice that is right for them.",
"A trial looking at the use of 5-alpha reductase inhibitors yielded interesting results. Although the risk for prostate cancer decreased in men taking the drug, those who were found to have prostate cancer had a higher grade of cancer. Men who take these drugs should be counseled appropriately on these risks."
],
"date": "March 14, 2017",
"figures": [],
"markdown": "# A 71-Year-Old Man With Urinary Problems\n\n **Authors:** Gerald W. Chodak, MD \n **Date:** March 14, 2017\n\n ## Content\n\n Proton-beam therapy is being advocated by physicians at proton centers; however, despite the claims of fewer side effects, no valid evidence can validate these claims, and no evidence has suggested that it provides better outcomes than other forms of radiation.[4] For now, it simply is not worth the higher cost. That leaves conservative therapy, radical prostatectomy, brachytherapy, and external radiation.\nTo date, no study has compared radiation with conservative therapy, although the ProtecT study in Great Britain may be able to provide some insight.[5] To date, only two well-designed randomized, controlled trials have been reported. A Scandinavian trial of watchful waiting compared with radical prostatectomy found a 5.4% higher survival in the men undergoing surgery at 12 years.[6] However, the difference in survival only applied to men younger than 65 years. This study contained few men with T1C disease, making it hard to broadly apply the results to men diagnosed in the United States.\nThe PIVOT trial primarily enrolled men with T1C disease and found a 2.9% higher survival in the surgery group, although it was not statistically significant.[7] In subset analyses, a slightly higher overall survival occurred in the group receiving conservative therapy, but this too was not significant. Men with low-risk disease did not have any better survival with surgery.\nAs for surgery vs either method of radiation, no randomized study has compared these options. Therefore, they are reasonable to consider; however, it cannot be argued that one of these therapies is more effective in treating localized disease.[8]\nClearly, more randomized studies are needed to further identify which patients benefit from definitive therapy and which men do not. Until then, doctors should provide a careful explanation of the pros and cons of the various options, so that they can help each patient make an appropriate choice that is right for them.\nA trial looking at the use of 5-alpha reductase inhibitors yielded interesting results. Although the risk for prostate cancer decreased in men taking the drug, those who were found to have prostate cancer had a higher grade of cancer. Men who take these drugs should be counseled appropriately on these risks.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 791161,
"choiceText": "Active surveillance with periodic monitoring",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 791163,
"choiceText": "Radical prostatectomy",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 791165,
"choiceText": "Radiation therapy with external beam or seed implantation",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 791167,
"choiceText": "Any of the above",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The odds this patient will die of <a href=\"http://emedicine.medscape.com/article/1967731-overview\">prostate cancer</a> in the next 10 years without therapy is only 6%. Ultimately, the patient must decide by weighing the risks and benefits of each option and factoring in their personal goals and fears. A thorough discussion of the risks of any treatment should be had with these men. Risks for <a href=\"http://emedicine.medscape.com/article/444220-overview\">erectile dysfunction</a>, urinary control, and need for subsequent procedures should be discussed with all men confronted with a diagnosis of prostate cancer.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 247939,
"questionText": "Given the findings above, which of the following is the best treatment for this patient?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 791153,
"choiceText": "Repeat PSA measurement in 3-6 months",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 791155,
"choiceText": "Recommend surgery or radiation",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 791157,
"choiceText": "Perform a repeat prostate biopsy",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 791159,
"choiceText": "Initiate luteinizing hormone-releasing hormone (LHRH) agonist therapy",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The management of men on active surveillance is still evolving. Some doctors recommend a repeat biopsy in 1 year to determine whether the cancer has increased in size or has become more aggressive. Others advise continued monitoring unless the PSA is doubling in less than 3-4 years. Still others will recommend treatment for any rise in PSA. Current guidelines recommend LHRH agonists for initial hormonal management of androgen-sensitive, metastatic, recurrent, or progressive prostate cancer.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 247937,
"questionText": "One year later, the patient's PSA level has increased to 4.5 ng/mL. Which of the following is <i>not</i> a potential next step?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 71-Year-Old Man With Urinary Problems"
},
{
"authors": "Gerald W. Chodak, MD",
"content": [],
"date": "March 14, 2017",
"figures": [],
"markdown": "# A 71-Year-Old Man With Urinary Problems\n\n **Authors:** Gerald W. Chodak, MD \n **Date:** March 14, 2017\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 791161,
"choiceText": "Active surveillance with periodic monitoring",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 791163,
"choiceText": "Radical prostatectomy",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 791165,
"choiceText": "Radiation therapy with external beam or seed implantation",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 791167,
"choiceText": "Any of the above",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The odds this patient will die of <a href=\"http://emedicine.medscape.com/article/1967731-overview\">prostate cancer</a> in the next 10 years without therapy is only 6%. Ultimately, the patient must decide by weighing the risks and benefits of each option and factoring in their personal goals and fears. A thorough discussion of the risks of any treatment should be had with these men. Risks for <a href=\"http://emedicine.medscape.com/article/444220-overview\">erectile dysfunction</a>, urinary control, and need for subsequent procedures should be discussed with all men confronted with a diagnosis of prostate cancer.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 247939,
"questionText": "Given the findings above, which of the following is the best treatment for this patient?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 791153,
"choiceText": "Repeat PSA measurement in 3-6 months",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 791155,
"choiceText": "Recommend surgery or radiation",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 791157,
"choiceText": "Perform a repeat prostate biopsy",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 791159,
"choiceText": "Initiate luteinizing hormone-releasing hormone (LHRH) agonist therapy",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The management of men on active surveillance is still evolving. Some doctors recommend a repeat biopsy in 1 year to determine whether the cancer has increased in size or has become more aggressive. Others advise continued monitoring unless the PSA is doubling in less than 3-4 years. Still others will recommend treatment for any rise in PSA. Current guidelines recommend LHRH agonists for initial hormonal management of androgen-sensitive, metastatic, recurrent, or progressive prostate cancer.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 247937,
"questionText": "One year later, the patient's PSA level has increased to 4.5 ng/mL. Which of the following is <i>not</i> a potential next step?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 71-Year-Old Man With Urinary Problems"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 791145,
"choiceText": "Prostate cancer",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 791147,
"choiceText": "Prostatitis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 791149,
"choiceText": "Benign prostatic hypertrophy",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 791151,
"choiceText": "Overactive bladder",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 247935,
"questionText": "On the basis of the history, physical examination, and work-up, what is the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 791161,
"choiceText": "Active surveillance with periodic monitoring",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 791163,
"choiceText": "Radical prostatectomy",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 791165,
"choiceText": "Radiation therapy with external beam or seed implantation",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 791167,
"choiceText": "Any of the above",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The odds this patient will die of <a href=\"http://emedicine.medscape.com/article/1967731-overview\">prostate cancer</a> in the next 10 years without therapy is only 6%. Ultimately, the patient must decide by weighing the risks and benefits of each option and factoring in their personal goals and fears. A thorough discussion of the risks of any treatment should be had with these men. Risks for <a href=\"http://emedicine.medscape.com/article/444220-overview\">erectile dysfunction</a>, urinary control, and need for subsequent procedures should be discussed with all men confronted with a diagnosis of prostate cancer.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 247939,
"questionText": "Given the findings above, which of the following is the best treatment for this patient?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 791153,
"choiceText": "Repeat PSA measurement in 3-6 months",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 791155,
"choiceText": "Recommend surgery or radiation",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 791157,
"choiceText": "Perform a repeat prostate biopsy",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 791159,
"choiceText": "Initiate luteinizing hormone-releasing hormone (LHRH) agonist therapy",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The management of men on active surveillance is still evolving. Some doctors recommend a repeat biopsy in 1 year to determine whether the cancer has increased in size or has become more aggressive. Others advise continued monitoring unless the PSA is doubling in less than 3-4 years. Still others will recommend treatment for any rise in PSA. Current guidelines recommend LHRH agonists for initial hormonal management of androgen-sensitive, metastatic, recurrent, or progressive prostate cancer.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 247937,
"questionText": "One year later, the patient's PSA level has increased to 4.5 ng/mL. Which of the following is <i>not</i> a potential next step?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
835251 | /viewarticle/835251 | [
{
"authors": "Salwa Ibrahim, MD",
"content": [
"Editor's Note: \nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions.",
"Background",
"A 45-year-old man presents to the outpatient clinic with a 2-month history of painless bilateral ankle swelling. He also has a long-standing history of recurrent fevers that have reached 101.3° F (38.5° C) and has been suffering from a productive cough with blood-tinged sputum for more than 1 year. He has previously sought medical advice for his symptoms; at the time of his previous medical evaluation, chest radiographs and sputum analysis and culture were performed. He received empirical antituberculosis treatment for 6 months because cavitary lung lesions were seen on the chest radiographs. His sputum cultures, however, were negative, and no acid-fast bacilli were detected.",
"Despite treatment, his cough and fevers have persisted. The patient also complains of anorexia, an unintentional weight loss of 22 lb (10 kg) over the past year, and recurrent epistaxis. He has visited several otolaryngology clinics and was given some \"nasal drops,\" without any improvement. No cauterization or nasal packing was ever needed. The patient had no history of easy bruising or bleeding before the episodes of epistaxis began. He recently noticed a change in the appearance of his nose and has developed a loss of smell and diminished taste. He does not smoke or drink alcohol, and he denies any illicit drug use. He denies any known exposure to tuberculosis or to experiencing any risk factors for tuberculosis exposure, such as incarceration, travel to high-risk areas, or employment in a medical facility."
],
"date": "November 24, 2014",
"figures": [],
"markdown": "# Recurrent Hemoptysis and Renal Impairment in a 45-Year-Old Man\n\n **Authors:** Salwa Ibrahim, MD \n **Date:** November 24, 2014\n\n ## Content\n\n Editor's Note: \nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions.\nBackground\nA 45-year-old man presents to the outpatient clinic with a 2-month history of painless bilateral ankle swelling. He also has a long-standing history of recurrent fevers that have reached 101.3° F (38.5° C) and has been suffering from a productive cough with blood-tinged sputum for more than 1 year. He has previously sought medical advice for his symptoms; at the time of his previous medical evaluation, chest radiographs and sputum analysis and culture were performed. He received empirical antituberculosis treatment for 6 months because cavitary lung lesions were seen on the chest radiographs. His sputum cultures, however, were negative, and no acid-fast bacilli were detected.\nDespite treatment, his cough and fevers have persisted. The patient also complains of anorexia, an unintentional weight loss of 22 lb (10 kg) over the past year, and recurrent epistaxis. He has visited several otolaryngology clinics and was given some \"nasal drops,\" without any improvement. No cauterization or nasal packing was ever needed. The patient had no history of easy bruising or bleeding before the episodes of epistaxis began. He recently noticed a change in the appearance of his nose and has developed a loss of smell and diminished taste. He does not smoke or drink alcohol, and he denies any illicit drug use. He denies any known exposure to tuberculosis or to experiencing any risk factors for tuberculosis exposure, such as incarceration, travel to high-risk areas, or employment in a medical facility.\n\n ## Figures\n\n \n*Page 1 of 5*",
"pagination": {
"current_page": 1,
"total_pages": 5
},
"questionnaire": [],
"title": "Recurrent Hemoptysis and Renal Impairment in a 45-Year-Old Man"
},
{
"authors": "Salwa Ibrahim, MD",
"content": [
"Physical Examination and Workup",
"On physical examination, he appears cachectic and pale but in no acute distress. His body mass index is low, at 17 kg/m2. His vital signs include a blood pressure of 150/98 mm Hg, a pulse rate of 102 beats/min, a respiratory rate of 18 breaths/min, and an oral temperature of 99.7° F (37.6° C). He has a saddle nose deformity, with loss of dorsal height. Mild ulceration of the nasal septum is seen from both nares, with no active epistaxis, perforation, or hematoma. The oropharynx and tympanic membranes appear normal. No cervical lymphadenopathy or jugular venous distention is noted during the neck examination. Scattered, coarse breath sounds are auscultated over the mid and lower lung fields but no wheezes are heard. The cardiac examination reveals an apical impulse of normal size and location, with no murmur or gallops on auscultation. A slightly firm and enlarged liver is palpated. No ascites is present. Bilateral ankle swelling is present without any tenderness to palpation, joint effusion, or increased warmth. His neurologic examination is normal, except for the loss of smell and moderate bilateral conductive hearing loss.",
"Figure 1.",
"Figure 2.",
"An initial laboratory workup reveals moderate, normochromic, normocytic anemia, an elevated erythrocyte sedimentation rate of 120 mm/hr, an elevated serum creatinine of 2.4 mg/dL (212.2 µmol/L), and a blood urea nitrogen of 50 mg/dL (17.85 mmol/L). A hepatic panel shows a low serum albumin concentration of 2.2 g/dL (22 g/L), but it is otherwise normal. The urine analysis is remarkable for 30-40 red blood cells per high-power field (HPF), 10-25 white blood cells per HPF, a large albumin value, and no casts. Urine culture and sensitivity testing reveals no growth. The 24-hour urine protein excretion is 1900 mg/24 hr (1.9 g/24 hr). On sputum examination, \nPseudomonas aeruginosa\n is detected. Chest radiographs and CT examinations are obtained, which reveal multiple cavitary lesions (Figures 1 and 2). Three consecutive sputum analyses for acid-fast bacilli are taken and are all negative. An abdominal ultrasound shows normal-sized kidneys with moderate hydronephrosis. Renal and lung biopsy are scheduled soon after admission."
],
"date": "November 24, 2014",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/835/251/835251-thumb-1.jpg"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/835/251/835251-thumb-2.jpg"
}
],
"markdown": "# Recurrent Hemoptysis and Renal Impairment in a 45-Year-Old Man\n\n **Authors:** Salwa Ibrahim, MD \n **Date:** November 24, 2014\n\n ## Content\n\n Physical Examination and Workup\nOn physical examination, he appears cachectic and pale but in no acute distress. His body mass index is low, at 17 kg/m2. His vital signs include a blood pressure of 150/98 mm Hg, a pulse rate of 102 beats/min, a respiratory rate of 18 breaths/min, and an oral temperature of 99.7° F (37.6° C). He has a saddle nose deformity, with loss of dorsal height. Mild ulceration of the nasal septum is seen from both nares, with no active epistaxis, perforation, or hematoma. The oropharynx and tympanic membranes appear normal. No cervical lymphadenopathy or jugular venous distention is noted during the neck examination. Scattered, coarse breath sounds are auscultated over the mid and lower lung fields but no wheezes are heard. The cardiac examination reveals an apical impulse of normal size and location, with no murmur or gallops on auscultation. A slightly firm and enlarged liver is palpated. No ascites is present. Bilateral ankle swelling is present without any tenderness to palpation, joint effusion, or increased warmth. His neurologic examination is normal, except for the loss of smell and moderate bilateral conductive hearing loss.\nFigure 1.\nFigure 2.\nAn initial laboratory workup reveals moderate, normochromic, normocytic anemia, an elevated erythrocyte sedimentation rate of 120 mm/hr, an elevated serum creatinine of 2.4 mg/dL (212.2 µmol/L), and a blood urea nitrogen of 50 mg/dL (17.85 mmol/L). A hepatic panel shows a low serum albumin concentration of 2.2 g/dL (22 g/L), but it is otherwise normal. The urine analysis is remarkable for 30-40 red blood cells per high-power field (HPF), 10-25 white blood cells per HPF, a large albumin value, and no casts. Urine culture and sensitivity testing reveals no growth. The 24-hour urine protein excretion is 1900 mg/24 hr (1.9 g/24 hr). On sputum examination, \nPseudomonas aeruginosa\n is detected. Chest radiographs and CT examinations are obtained, which reveal multiple cavitary lesions (Figures 1 and 2). Three consecutive sputum analyses for acid-fast bacilli are taken and are all negative. An abdominal ultrasound shows normal-sized kidneys with moderate hydronephrosis. Renal and lung biopsy are scheduled soon after admission.\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n\n*Page 2 of 5*",
"pagination": {
"current_page": 2,
"total_pages": 5
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 791775,
"choiceText": "Tuberculosis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 791777,
"choiceText": "Goodpasture syndrome",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 791779,
"choiceText": "Systemic lupus erythematosus",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 791781,
"choiceText": "Granulomatosis with polyangiitis (Wegener granulomatosis)",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 248119,
"questionText": "On the basis of the history, physical examination, and workup, what is the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Recurrent Hemoptysis and Renal Impairment in a 45-Year-Old Man"
},
{
"authors": "Salwa Ibrahim, MD",
"content": [
"Discussion",
"Granulomatosis with polyangiitis is a systemic inflammatory disease characterized by the presence of granulomas, necrosis, and/or vasculitis. Although this granulomatosis typically affects the upper and lower airways and the kidneys, it may involve any organ system. The histologic hallmark of granulomatous inflammation is tissue infiltration with activated macrophages. Granulomas are small collections of modified macrophages usually surrounded by a rim of lymphocytes. Typical histologic findings from open lung biopsy are found in more than 80% of specimens. A focal, segmental, necrotizing, crescentic glomerulonephritis is the classic renal lesion seen in granulomatosis with polyangiitis. It is typically associated with scant or no immunocomplex deposits (pauci-immune).[1,2,3]",
"The most common initial symptoms typically involve structures of the ear, nose, and throat (ENT). These may be associated with systemic symptoms, such as fevers, weight loss, polyarthralgia, and myalgia. Vasculitic abnormalities such as digital infarcts, rash, and gangrene may be found in the extremities and skin. Nasal mucosal inflammation leads to obstruction, crusting, pain, epistaxis, and serosanguineous or purulent discharge. Nasal chondritis often results in collapse of the nasal bridge, with a saddle nose deformity. Chronic sinusitis causes facial pain and swelling. Ear involvement may lead to chondritis, otitis media, and sensorineural deafness. Upper airway symptoms include cough, stridor, and hoarseness of the voice. Lung involvement may include focal or diffuse infiltrates, nodules (which are often cavitary), endobronchial stenosis, and pleurisy. Asymptomatic pulmonary findings are likely to influence the assessment of disease severity and treatment. The most severe pulmonary features are a result of diffuse capillaritis, usually associated with dyspnea, hemoptysis, and anemia. Skin manifestations include palpable purpura caused by leukocytoclastic vasculitis, tender subcutaneous nodules, vesicles, ulcers, and digital infarcts.[2,3,4]",
"The diagnosis of granulomatosis with polyangiitis is based on a combination of clinical, laboratory, and pathologic features. If a typical clinical picture is associated with a positive antineutrophil cytoplasmic antibody (ANCA) test with specificity for proteinase 3 (PR3), the diagnosis of granulomatosis with polyangiitis can be presumed. The American College of Rheumatology proposed specific criteria for the classification of granulomatosis with polyangiitis.[4] The presence of two or more of the outlined criteria was associated with a sensitivity of 88.2% and a specificity of 92% in distinguishing granulomatosis with polyangiitis from other forms of vasculitis. The four criteria are as follows[5]:",
"Abnormal urinary sediment (red cell casts, more than five red blood cells per HPF);",
"Abnormal findings on chest radiography (nodules, cavities, or infiltrates);",
"Oral ulcers or nasal discharge; and",
"Granulomatous inflammation on biopsy.",
"ANCA examinations have two district patterns: C-ANCA indicates cytoplasmic staining and refers to a coarse, granular, centrally accentuated, and cytoplasmic fluorescence pattern, whereas P-ANCA refers to a perinuclear fluorescence pattern. The C-ANCA pattern is usually caused by antibodies against PR3. About 80%-90% of all ANCAs found in granulomatosis with polyangiitis is C-ANCA-specific and PR3-specific. The sensitivity of PR3-ANCA in granulomatosis with polyangiitis depends, in part, on the disease activity and severity. About 80%-95% of patients with severe active disease and 55%-75% of patients with milder, limited active disease are ANCA positive. The presence of red blood cell casts in the urine sediment can be used as a reliable surrogate marker for the diagnosis of glomerulonephritis, and it may eliminate the need for renal biopsy in some patients.[4]",
"The differential diagnosis of granulomatosis with polyangiitis depends on the clinical presentation. When the presenting features are characterized by a pulmonary renal syndrome, granulomatosis with polyangiitis must be distinguished from Goodpasture syndrome, systemic lupus erythematosus, microscopic polyangiitis, and Churg-Strauss syndrome. These features, in addition to a prior or persistent history of upper airway disease and PR3-ANCA positivity, make the diagnosis of granulomatosis with polyangiitis very likely. The presence of granulomatous inflammation within a biopsy specimen, although not an absolute requirement for confirming granulomatosis with polyangiitis, represents an important distinction between granulomatosis with polyangiitis and other vasculitis conditions. Other causes of lung granulomas include sarcoidosis, tuberculosis, and lymphoma.[1,2]"
],
"date": "November 24, 2014",
"figures": [],
"markdown": "# Recurrent Hemoptysis and Renal Impairment in a 45-Year-Old Man\n\n **Authors:** Salwa Ibrahim, MD \n **Date:** November 24, 2014\n\n ## Content\n\n Discussion\nGranulomatosis with polyangiitis is a systemic inflammatory disease characterized by the presence of granulomas, necrosis, and/or vasculitis. Although this granulomatosis typically affects the upper and lower airways and the kidneys, it may involve any organ system. The histologic hallmark of granulomatous inflammation is tissue infiltration with activated macrophages. Granulomas are small collections of modified macrophages usually surrounded by a rim of lymphocytes. Typical histologic findings from open lung biopsy are found in more than 80% of specimens. A focal, segmental, necrotizing, crescentic glomerulonephritis is the classic renal lesion seen in granulomatosis with polyangiitis. It is typically associated with scant or no immunocomplex deposits (pauci-immune).[1,2,3]\nThe most common initial symptoms typically involve structures of the ear, nose, and throat (ENT). These may be associated with systemic symptoms, such as fevers, weight loss, polyarthralgia, and myalgia. Vasculitic abnormalities such as digital infarcts, rash, and gangrene may be found in the extremities and skin. Nasal mucosal inflammation leads to obstruction, crusting, pain, epistaxis, and serosanguineous or purulent discharge. Nasal chondritis often results in collapse of the nasal bridge, with a saddle nose deformity. Chronic sinusitis causes facial pain and swelling. Ear involvement may lead to chondritis, otitis media, and sensorineural deafness. Upper airway symptoms include cough, stridor, and hoarseness of the voice. Lung involvement may include focal or diffuse infiltrates, nodules (which are often cavitary), endobronchial stenosis, and pleurisy. Asymptomatic pulmonary findings are likely to influence the assessment of disease severity and treatment. The most severe pulmonary features are a result of diffuse capillaritis, usually associated with dyspnea, hemoptysis, and anemia. Skin manifestations include palpable purpura caused by leukocytoclastic vasculitis, tender subcutaneous nodules, vesicles, ulcers, and digital infarcts.[2,3,4]\nThe diagnosis of granulomatosis with polyangiitis is based on a combination of clinical, laboratory, and pathologic features. If a typical clinical picture is associated with a positive antineutrophil cytoplasmic antibody (ANCA) test with specificity for proteinase 3 (PR3), the diagnosis of granulomatosis with polyangiitis can be presumed. The American College of Rheumatology proposed specific criteria for the classification of granulomatosis with polyangiitis.[4] The presence of two or more of the outlined criteria was associated with a sensitivity of 88.2% and a specificity of 92% in distinguishing granulomatosis with polyangiitis from other forms of vasculitis. The four criteria are as follows[5]:\nAbnormal urinary sediment (red cell casts, more than five red blood cells per HPF);\nAbnormal findings on chest radiography (nodules, cavities, or infiltrates);\nOral ulcers or nasal discharge; and\nGranulomatous inflammation on biopsy.\nANCA examinations have two district patterns: C-ANCA indicates cytoplasmic staining and refers to a coarse, granular, centrally accentuated, and cytoplasmic fluorescence pattern, whereas P-ANCA refers to a perinuclear fluorescence pattern. The C-ANCA pattern is usually caused by antibodies against PR3. About 80%-90% of all ANCAs found in granulomatosis with polyangiitis is C-ANCA-specific and PR3-specific. The sensitivity of PR3-ANCA in granulomatosis with polyangiitis depends, in part, on the disease activity and severity. About 80%-95% of patients with severe active disease and 55%-75% of patients with milder, limited active disease are ANCA positive. The presence of red blood cell casts in the urine sediment can be used as a reliable surrogate marker for the diagnosis of glomerulonephritis, and it may eliminate the need for renal biopsy in some patients.[4]\nThe differential diagnosis of granulomatosis with polyangiitis depends on the clinical presentation. When the presenting features are characterized by a pulmonary renal syndrome, granulomatosis with polyangiitis must be distinguished from Goodpasture syndrome, systemic lupus erythematosus, microscopic polyangiitis, and Churg-Strauss syndrome. These features, in addition to a prior or persistent history of upper airway disease and PR3-ANCA positivity, make the diagnosis of granulomatosis with polyangiitis very likely. The presence of granulomatous inflammation within a biopsy specimen, although not an absolute requirement for confirming granulomatosis with polyangiitis, represents an important distinction between granulomatosis with polyangiitis and other vasculitis conditions. Other causes of lung granulomas include sarcoidosis, tuberculosis, and lymphoma.[1,2]\n\n ## Figures\n\n \n*Page 3 of 5*",
"pagination": {
"current_page": 3,
"total_pages": 5
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 791775,
"choiceText": "Tuberculosis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 791777,
"choiceText": "Goodpasture syndrome",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 791779,
"choiceText": "Systemic lupus erythematosus",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 791781,
"choiceText": "Granulomatosis with polyangiitis (Wegener granulomatosis)",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 248119,
"questionText": "On the basis of the history, physical examination, and workup, what is the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Recurrent Hemoptysis and Renal Impairment in a 45-Year-Old Man"
},
{
"authors": "Salwa Ibrahim, MD",
"content": [
"Therapy for granulomatosis with polyangiitis includes glucocorticoids and cyclophosphamide. The initial therapy should consist of cyclophosphamide 2 mg/kg/day together with prednisolone 1 mg/kg/day.",
"If improvement is evident after 4 weeks of therapy, prednisolone can be slowly tapered over 6-9 months and then discontinued. Cyclophosphamide is maintained for 6 months and is usually replaced by azathioprine or methotrexate after remission is achieved. The side effects of cyclophosphamide include hemorrhagic cystitis, severe infections, infertility, and bladder cancer. Trimethoprim/sulfamethoxazole has been use to decrease the rate of ENT infections such as sinusitis.[4]",
"In this patient, granulomatosis with polyangiitis was the presumptive diagnosis, which was made based on the history of recurrent ENT conditions, hemoptysis, cavitary lung lesions, and renal insufficiency. His antinuclear antibody test result was positive, with a speckled pattern. The ANCA test was also positive and had a cytoplasmic florescence pattern. A nasal mucosal biopsy revealed crust infiltrated with excess neutrophil, dense submucosal fibrosis with chronic nonspecific cellular infiltrates, and thickened blood vessel walls. Lung biopsies showed extensive parenchymal necrosis, with cavitations admixed with fibrinoid material, excess neutrophils, and few eosinophils involving both lung parenchyma and bronchi (Figure 3). The renal biopsy examination revealed focal segmental sclerosis (Figures 4, 5), with periglomerular fibrosis, hyaline casts within swollen renal tubules, interstitial fibrosis, and lymphocytic infiltration with focal perivascular infiltrate. No active vasculitis was seen. The renal biopsy also lacked the presence of the necrotizing lesions commonly reported in active granulomatosis with polyangiitis.",
"Figure 3.",
"Figure 4.",
"Figure 5.",
"Crescentic glomerulonephritis has been reported in patients with granulomatosis with polyangiitis. Segmental and global glomerulosclerosis were the only findings in some patients with granulomatosis with polyangiitis in a clinicopathological analysis conducted by Hauer and colleagues.[6] The healing process of active glomerular lesions may result in glomerular scarring and interstitial fibrosis.",
"The patient in the present case was prescribed prednisolone (50 mg/day) and cyclophosphamide (100 mg/day) for 1 month, as well as antibiotics, according to the results of the sputum culture and sensitivity test. After 3 weeks the patient had no hemoptysis or arthralgias. He progressively felt better and gained weight as his appetite improved. His hemoglobin level increased to 10.2 g/dL (102 g/L) and his serum creatinine decreased from 2.4 mg/dL (212.2 µmol/L) to 1.6 mg/dL (141.4 µmol/L). The urine analysis showed only trace albumin with 3-5 red blood cells per HPF. Additionally, some resolution of the cavitary lesions in the lungs was seen on subsequent chest radiographs. He was discharged in good condition and was given an outpatient appointment in 1 month for follow-up."
],
"date": "November 24, 2014",
"figures": [
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/835/251/835251-thumb-3.jpg"
},
{
"caption": "Figure 4.",
"image_url": "https://img.medscapestatic.com/article/835/251/835251-thumb-4.jpg"
},
{
"caption": "Figure 5.",
"image_url": "https://img.medscapestatic.com/article/835/251/835251-thumb-5.jpg"
}
],
"markdown": "# Recurrent Hemoptysis and Renal Impairment in a 45-Year-Old Man\n\n **Authors:** Salwa Ibrahim, MD \n **Date:** November 24, 2014\n\n ## Content\n\n Therapy for granulomatosis with polyangiitis includes glucocorticoids and cyclophosphamide. The initial therapy should consist of cyclophosphamide 2 mg/kg/day together with prednisolone 1 mg/kg/day.\nIf improvement is evident after 4 weeks of therapy, prednisolone can be slowly tapered over 6-9 months and then discontinued. Cyclophosphamide is maintained for 6 months and is usually replaced by azathioprine or methotrexate after remission is achieved. The side effects of cyclophosphamide include hemorrhagic cystitis, severe infections, infertility, and bladder cancer. Trimethoprim/sulfamethoxazole has been use to decrease the rate of ENT infections such as sinusitis.[4]\nIn this patient, granulomatosis with polyangiitis was the presumptive diagnosis, which was made based on the history of recurrent ENT conditions, hemoptysis, cavitary lung lesions, and renal insufficiency. His antinuclear antibody test result was positive, with a speckled pattern. The ANCA test was also positive and had a cytoplasmic florescence pattern. A nasal mucosal biopsy revealed crust infiltrated with excess neutrophil, dense submucosal fibrosis with chronic nonspecific cellular infiltrates, and thickened blood vessel walls. Lung biopsies showed extensive parenchymal necrosis, with cavitations admixed with fibrinoid material, excess neutrophils, and few eosinophils involving both lung parenchyma and bronchi (Figure 3). The renal biopsy examination revealed focal segmental sclerosis (Figures 4, 5), with periglomerular fibrosis, hyaline casts within swollen renal tubules, interstitial fibrosis, and lymphocytic infiltration with focal perivascular infiltrate. No active vasculitis was seen. The renal biopsy also lacked the presence of the necrotizing lesions commonly reported in active granulomatosis with polyangiitis.\nFigure 3.\nFigure 4.\nFigure 5.\nCrescentic glomerulonephritis has been reported in patients with granulomatosis with polyangiitis. Segmental and global glomerulosclerosis were the only findings in some patients with granulomatosis with polyangiitis in a clinicopathological analysis conducted by Hauer and colleagues.[6] The healing process of active glomerular lesions may result in glomerular scarring and interstitial fibrosis.\nThe patient in the present case was prescribed prednisolone (50 mg/day) and cyclophosphamide (100 mg/day) for 1 month, as well as antibiotics, according to the results of the sputum culture and sensitivity test. After 3 weeks the patient had no hemoptysis or arthralgias. He progressively felt better and gained weight as his appetite improved. His hemoglobin level increased to 10.2 g/dL (102 g/L) and his serum creatinine decreased from 2.4 mg/dL (212.2 µmol/L) to 1.6 mg/dL (141.4 µmol/L). The urine analysis showed only trace albumin with 3-5 red blood cells per HPF. Additionally, some resolution of the cavitary lesions in the lungs was seen on subsequent chest radiographs. He was discharged in good condition and was given an outpatient appointment in 1 month for follow-up.\n\n ## Figures\n\n **Figure 3.** \n \n\n**Figure 4.** \n \n\n**Figure 5.** \n \n\n\n*Page 4 of 5*",
"pagination": {
"current_page": 4,
"total_pages": 5
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 791783,
"choiceText": "Trimethoprim/sulfamethoxazole",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 791785,
"choiceText": "Cyclophosphamide/prednisolone",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 791787,
"choiceText": "Azathioprine",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 791789,
"choiceText": "Methotrexate",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 248121,
"questionText": "Which of the following treatments is currently considered the best initial drug therapy for patients with granulomatosis with polyangiitis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 791791,
"choiceText": "Low complement component 3 level",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 791793,
"choiceText": "Positive C-ANCA level",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 791795,
"choiceText": "High erythrocyte sedimentation rate",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 791797,
"choiceText": "High serum creatinine level",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 791799,
"choiceText": "Anemia",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 248123,
"questionText": "Which of the following laboratory parameters is not associated with granulomatosis with polyangiitis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Recurrent Hemoptysis and Renal Impairment in a 45-Year-Old Man"
},
{
"authors": "Salwa Ibrahim, MD",
"content": [],
"date": "November 24, 2014",
"figures": [],
"markdown": "# Recurrent Hemoptysis and Renal Impairment in a 45-Year-Old Man\n\n **Authors:** Salwa Ibrahim, MD \n **Date:** November 24, 2014\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 5 of 5*",
"pagination": {
"current_page": 5,
"total_pages": 5
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 791783,
"choiceText": "Trimethoprim/sulfamethoxazole",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 791785,
"choiceText": "Cyclophosphamide/prednisolone",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 791787,
"choiceText": "Azathioprine",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 791789,
"choiceText": "Methotrexate",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 248121,
"questionText": "Which of the following treatments is currently considered the best initial drug therapy for patients with granulomatosis with polyangiitis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 791791,
"choiceText": "Low complement component 3 level",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 791793,
"choiceText": "Positive C-ANCA level",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 791795,
"choiceText": "High erythrocyte sedimentation rate",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 791797,
"choiceText": "High serum creatinine level",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 791799,
"choiceText": "Anemia",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 248123,
"questionText": "Which of the following laboratory parameters is not associated with granulomatosis with polyangiitis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Recurrent Hemoptysis and Renal Impairment in a 45-Year-Old Man"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 791775,
"choiceText": "Tuberculosis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 791777,
"choiceText": "Goodpasture syndrome",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 791779,
"choiceText": "Systemic lupus erythematosus",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 791781,
"choiceText": "Granulomatosis with polyangiitis (Wegener granulomatosis)",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 248119,
"questionText": "On the basis of the history, physical examination, and workup, what is the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 791783,
"choiceText": "Trimethoprim/sulfamethoxazole",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 791785,
"choiceText": "Cyclophosphamide/prednisolone",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 791787,
"choiceText": "Azathioprine",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 791789,
"choiceText": "Methotrexate",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 248121,
"questionText": "Which of the following treatments is currently considered the best initial drug therapy for patients with granulomatosis with polyangiitis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 791791,
"choiceText": "Low complement component 3 level",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 791793,
"choiceText": "Positive C-ANCA level",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 791795,
"choiceText": "High erythrocyte sedimentation rate",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 791797,
"choiceText": "High serum creatinine level",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 791799,
"choiceText": "Anemia",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 248123,
"questionText": "Which of the following laboratory parameters is not associated with granulomatosis with polyangiitis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
834368 | /viewarticle/834368 | [
{
"authors": "Alyssa Abbey, MCMS, PA-C; Martin I. Newman, MD",
"content": [
"Editor's Note: \nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions.",
"Background",
"A 32-year-old woman presents to the emergency department with several flesh-colored papules on her face, trunk, and upper extremities (Figure).",
"Figure 1.",
"She first noticed the lesions at approximately 10 years of age; however, over the past 5 years, the lesions have increased in number and have become uncomfortable. She primarily complains of irritation from the lesions along her bra line. She underwent excision of similar skin lesions 5 years ago, but they have since recurred. She denies having any discharge, pain, trauma, contact with individuals with atypical skin lesions or rashes, travel out of the country, unusual exposure to animals, or a history of sexually transmitted diseases.",
"The patient's medical and surgical history includes environmental allergies, frequent episodes of bronchitis, and the aforementioned excisions. She has no known drug allergies, and she takes cetirizine hydrochloride and fluticasone propionate for seasonal allergies. Her family history is significant for coronary artery disease, hypertension, diabetes mellitus, and glaucoma, but no family history of similar lesions is reported. She does not smoke and drinks alcohol only occasionally. The review of her systems is otherwise unremarkable."
],
"date": "November 11, 2014",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/834/368/834368-thumb.jpg"
}
],
"markdown": "# Fleshy Lesions on a 32-Year-Old Woman\n\n **Authors:** Alyssa Abbey, MCMS, PA-C; Martin I. Newman, MD \n **Date:** November 11, 2014\n\n ## Content\n\n Editor's Note: \nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions.\nBackground\nA 32-year-old woman presents to the emergency department with several flesh-colored papules on her face, trunk, and upper extremities (Figure).\nFigure 1.\nShe first noticed the lesions at approximately 10 years of age; however, over the past 5 years, the lesions have increased in number and have become uncomfortable. She primarily complains of irritation from the lesions along her bra line. She underwent excision of similar skin lesions 5 years ago, but they have since recurred. She denies having any discharge, pain, trauma, contact with individuals with atypical skin lesions or rashes, travel out of the country, unusual exposure to animals, or a history of sexually transmitted diseases.\nThe patient's medical and surgical history includes environmental allergies, frequent episodes of bronchitis, and the aforementioned excisions. She has no known drug allergies, and she takes cetirizine hydrochloride and fluticasone propionate for seasonal allergies. Her family history is significant for coronary artery disease, hypertension, diabetes mellitus, and glaucoma, but no family history of similar lesions is reported. She does not smoke and drinks alcohol only occasionally. The review of her systems is otherwise unremarkable.\n\n ## Figures\n\n **Figure 1.** \n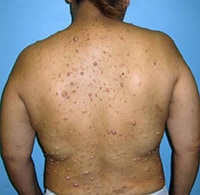 \n\n\n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "Fleshy Lesions on a 32-Year-Old Woman"
},
{
"authors": "Alyssa Abbey, MCMS, PA-C; Martin I. Newman, MD",
"content": [
"Physical Examination and Work-up",
"The physical examination reveals dozens of 0.5-2.0 cm fleshy nodules spread over the patient's trunk, face, and upper extremities. The nodules are nontender to palpation and nonerythematous, and they produce no discharge, crusting, or scaling. Several tan oval macules measuring 1.5-3 cm and patches with well-defined borders are located on her trunk and upper extremities (Figure).",
"Figure 1."
],
"date": "November 11, 2014",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/834/368/834368-thumb.jpg"
}
],
"markdown": "# Fleshy Lesions on a 32-Year-Old Woman\n\n **Authors:** Alyssa Abbey, MCMS, PA-C; Martin I. Newman, MD \n **Date:** November 11, 2014\n\n ## Content\n\n Physical Examination and Work-up\nThe physical examination reveals dozens of 0.5-2.0 cm fleshy nodules spread over the patient's trunk, face, and upper extremities. The nodules are nontender to palpation and nonerythematous, and they produce no discharge, crusting, or scaling. Several tan oval macules measuring 1.5-3 cm and patches with well-defined borders are located on her trunk and upper extremities (Figure).\nFigure 1.\n\n ## Figures\n\n **Figure 1.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 786043,
"choiceText": "Neurofibromatosis",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 786045,
"choiceText": "McCune-Albright syndrome",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 786047,
"choiceText": "Vitiligo",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 786049,
"choiceText": "Hansen disease",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 246289,
"questionText": "On the basis of the history, physical examination, and work-up, what is the diagnosis?<br>\r\n<i>Hint: Tan macules or patches, known as \"café au lait spots,\" are characteristic of this genetic disorder.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Fleshy Lesions on a 32-Year-Old Woman"
},
{
"authors": "Alyssa Abbey, MCMS, PA-C; Martin I. Newman, MD",
"content": [
"Discussion",
"Neurofibromatosis (NF) is an autosomal dominant disorder with numerous presentations that can affect nearly every organ system. The two major subtypes of NF are type 1 (NF1), also historically referred to as peripheral NF, and type 2 (NF2), which is also known as central NF. These terms are not completely accurate, however, because NF1 also can include central nervous system (CNS) abnormalities.",
"About 50% of cases of NF are familial; the other 50% are caused by spontaneous gene mutation. NF1, also known as von Recklinghausen disease, is a common genetic disorder involving a mutation in the gene that produces neurofibromin on chromosome 17; the condition affects one in every 3000-4000 births. All races are affected, and the two sexes are affected equally.",
"Two or more of the following seven criteria must be present in order to make the diagnosis of NF1[1]:",
"Six or more café au lait spots (irregularly shaped, evenly pigmented, brown macules) that are greater than 5 mm in diameter in prepubertal patients or greater than 15 mm in diameter in postpubertal patients;",
"Two or more neurofibromas;",
"Axillary or inguinal freckling;",
"Lisch nodules (hamartomas of the iris);",
"Optic nerve gliomas;",
"Various types of osseous lesions; and",
"A first-degree relative with the condition.",
"Symptomatic NF1 typically initially manifests as café au lait spots that may be present at birth or that appear over time during childhood. Axillary or inguinal freckles are not usually present at birth, but rather appear throughout childhood and adolescence. Neurofibromas are rarely seen in young children but usually appear over time in older children, adolescents, and adults.",
"The patient may have as few as three or as many as thousands of these benign lesions, which consist of Schwann cells, neural fibroblasts, mast cells, and vascular elements. The neurofibromas may occur anywhere in the body and can potentially lead to marked disfigurement. If the lesions are deep, they may be detected only through palpation; cutaneous lesions may initially appear as small papules on the trunk, the extremities, the scalp, or the face.",
"A specific type of these lesions is a plexiform neurofibroma, which is a more diffuse type of growth that can be locally invasive and quite deep. These lesions may be associated with bony erosion and pain, and they may also be accompanied by overlying hyperpigmentation or hypertrichosis.",
"The onset of puberty or pregnancy may be associated with an increased number of neurofibromas, as well as an increase in the speed of preexisting lesion growth. Lesions along visual, auditory, or CNS nerve pathways may result in blindness, deafness, or neurologic deficits.",
"On histology, the neurofibromas are generally well-differentiated tumors that contain elongated spindle-shaped cells and pleomorphic fibroblast-like cells. Some lesions may contain inflammatory cells.",
"Occasionally, a large neurofibroma, a deep plexiform neurofibroma, or a peripheral nerve sheath tumor residing within the brachial or pelvic plexus may undergo malignant transformation into a neurofibrosarcoma. Unlike benign neurofibromas, neurofibrosarcomas are characteristically hypercellular, with giant cells, increased numbers of mitoses, and vascular proliferation. In addition, small masses of malignant cells may be present within larger masses of benign cells (eg, as in a plexiform neurofibroma)."
],
"date": "November 11, 2014",
"figures": [],
"markdown": "# Fleshy Lesions on a 32-Year-Old Woman\n\n **Authors:** Alyssa Abbey, MCMS, PA-C; Martin I. Newman, MD \n **Date:** November 11, 2014\n\n ## Content\n\n Discussion\nNeurofibromatosis (NF) is an autosomal dominant disorder with numerous presentations that can affect nearly every organ system. The two major subtypes of NF are type 1 (NF1), also historically referred to as peripheral NF, and type 2 (NF2), which is also known as central NF. These terms are not completely accurate, however, because NF1 also can include central nervous system (CNS) abnormalities.\nAbout 50% of cases of NF are familial; the other 50% are caused by spontaneous gene mutation. NF1, also known as von Recklinghausen disease, is a common genetic disorder involving a mutation in the gene that produces neurofibromin on chromosome 17; the condition affects one in every 3000-4000 births. All races are affected, and the two sexes are affected equally.\nTwo or more of the following seven criteria must be present in order to make the diagnosis of NF1[1]:\nSix or more café au lait spots (irregularly shaped, evenly pigmented, brown macules) that are greater than 5 mm in diameter in prepubertal patients or greater than 15 mm in diameter in postpubertal patients;\nTwo or more neurofibromas;\nAxillary or inguinal freckling;\nLisch nodules (hamartomas of the iris);\nOptic nerve gliomas;\nVarious types of osseous lesions; and\nA first-degree relative with the condition.\nSymptomatic NF1 typically initially manifests as café au lait spots that may be present at birth or that appear over time during childhood. Axillary or inguinal freckles are not usually present at birth, but rather appear throughout childhood and adolescence. Neurofibromas are rarely seen in young children but usually appear over time in older children, adolescents, and adults.\nThe patient may have as few as three or as many as thousands of these benign lesions, which consist of Schwann cells, neural fibroblasts, mast cells, and vascular elements. The neurofibromas may occur anywhere in the body and can potentially lead to marked disfigurement. If the lesions are deep, they may be detected only through palpation; cutaneous lesions may initially appear as small papules on the trunk, the extremities, the scalp, or the face.\nA specific type of these lesions is a plexiform neurofibroma, which is a more diffuse type of growth that can be locally invasive and quite deep. These lesions may be associated with bony erosion and pain, and they may also be accompanied by overlying hyperpigmentation or hypertrichosis.\nThe onset of puberty or pregnancy may be associated with an increased number of neurofibromas, as well as an increase in the speed of preexisting lesion growth. Lesions along visual, auditory, or CNS nerve pathways may result in blindness, deafness, or neurologic deficits.\nOn histology, the neurofibromas are generally well-differentiated tumors that contain elongated spindle-shaped cells and pleomorphic fibroblast-like cells. Some lesions may contain inflammatory cells.\nOccasionally, a large neurofibroma, a deep plexiform neurofibroma, or a peripheral nerve sheath tumor residing within the brachial or pelvic plexus may undergo malignant transformation into a neurofibrosarcoma. Unlike benign neurofibromas, neurofibrosarcomas are characteristically hypercellular, with giant cells, increased numbers of mitoses, and vascular proliferation. In addition, small masses of malignant cells may be present within larger masses of benign cells (eg, as in a plexiform neurofibroma).\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 786043,
"choiceText": "Neurofibromatosis",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 786045,
"choiceText": "McCune-Albright syndrome",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 786047,
"choiceText": "Vitiligo",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 786049,
"choiceText": "Hansen disease",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 246289,
"questionText": "On the basis of the history, physical examination, and work-up, what is the diagnosis?<br>\r\n<i>Hint: Tan macules or patches, known as \"café au lait spots,\" are characteristic of this genetic disorder.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Fleshy Lesions on a 32-Year-Old Woman"
},
{
"authors": "Alyssa Abbey, MCMS, PA-C; Martin I. Newman, MD",
"content": [
"Optic nerve tumors occur primarily in children younger than 5 years. The most common presenting symptom is asymmetric, noncorrectable visual loss, but subtle peripheral field defects, optic nerve pallor, color-discrimination difficulties, or proptosis may be present without visual acuity being affected. A slow-growing optic nerve glioma (ONG) may lead to vision problems in some older children and adolescents; these patients should be monitored for vision difficulties throughout childhood and adulthood.",
"In adults, a visually insignificant ONG may be detected incidentally on head imaging studies. Although Lisch nodules are usually not visible without use of a slit lamp, they may occasionally be seen with a direct or indirect ophthalmoscope, especially in individuals with light-colored irises. Choroidal abnormalities with a patchy appearance or retinal corkscrew vascular changes may also be noted on funduscopic examination.",
"Orthopedic manifestations are also commonly encountered. Congenital pseudarthrosis may be evident at birth; bowing of the tibia is the most typical presentation. Thinning and angulation of the long bones can occur throughout early childhood and adolescence, with prominence of the anterior tibia and progressive deformity; less commonly, bowing of the forearm can occur.",
"Scoliosis with or without kyphosis may become evident in childhood or adolescence; when this finding is present in children younger than 10 years, scoliosis is associated with a poor prognosis and is likely to progress rapidly. Scoliosis detected during adolescence should still be monitored clinically but is much less likely to require orthopedic intervention.",
"Sphenoid bone dysplasia is usually asymptomatic, but it can occasionally be associated with herniation through the bony defect. Other skeletal anomalies, such as fibrous dysplasia, subperiosteal bone cysts, or vertebral scalloping, can also be found.[2]",
"Rare complications of NF1 include renal artery stenosis and pheochromocytoma, either of which can present with hypertension.[3]",
"NF2 is a progressive genetic disorder that is present in one in every 33,000-40,000 births. Patients with NF2 (which results from an abnormality on chromosome 22) typically present with acoustic neuromas or vestibular schwannomas. In contrast to NF1, patients with NF2 do not typically have cognitive issues nor significant numbers of café au lait spots.",
"The clinical manifestations of NF2 include tinnitus, balance disorders, and progressive hearing loss. Affected patients may also have meningiomas, juvenile cataracts, or schwannomas of the dorsal roots of the spinal cord. The diagnosis of NF2 is typically based on the presence of bilateral acoustic neuromas or a unilateral acoustic neuroma in a first-degree relative with NF2.",
"Criteria have been modified to increase sensitivity and may also be fulfilled by having a first-degree relative with NF2 and any two of the following:",
"Meningioma",
"Schwannoma",
"Glioma",
"Neurofibroma",
"Posterior subcapsular lenticular opacities",
"The criteria may also be fulfilled by unilateral acoustic neuroma and any two of the following:",
"Meningioma",
"Schwannoma",
"Glioma",
"Neurofibroma",
"Posterior subcapsular lenticular opacities",
"Finally, the criteria may also be fulfilled by multiple meningiomas and unilateral acoustic neuroma or any two of schwannoma, glioma, or neurofibroma/cataract.[3]"
],
"date": "November 11, 2014",
"figures": [],
"markdown": "# Fleshy Lesions on a 32-Year-Old Woman\n\n **Authors:** Alyssa Abbey, MCMS, PA-C; Martin I. Newman, MD \n **Date:** November 11, 2014\n\n ## Content\n\n Optic nerve tumors occur primarily in children younger than 5 years. The most common presenting symptom is asymmetric, noncorrectable visual loss, but subtle peripheral field defects, optic nerve pallor, color-discrimination difficulties, or proptosis may be present without visual acuity being affected. A slow-growing optic nerve glioma (ONG) may lead to vision problems in some older children and adolescents; these patients should be monitored for vision difficulties throughout childhood and adulthood.\nIn adults, a visually insignificant ONG may be detected incidentally on head imaging studies. Although Lisch nodules are usually not visible without use of a slit lamp, they may occasionally be seen with a direct or indirect ophthalmoscope, especially in individuals with light-colored irises. Choroidal abnormalities with a patchy appearance or retinal corkscrew vascular changes may also be noted on funduscopic examination.\nOrthopedic manifestations are also commonly encountered. Congenital pseudarthrosis may be evident at birth; bowing of the tibia is the most typical presentation. Thinning and angulation of the long bones can occur throughout early childhood and adolescence, with prominence of the anterior tibia and progressive deformity; less commonly, bowing of the forearm can occur.\nScoliosis with or without kyphosis may become evident in childhood or adolescence; when this finding is present in children younger than 10 years, scoliosis is associated with a poor prognosis and is likely to progress rapidly. Scoliosis detected during adolescence should still be monitored clinically but is much less likely to require orthopedic intervention.\nSphenoid bone dysplasia is usually asymptomatic, but it can occasionally be associated with herniation through the bony defect. Other skeletal anomalies, such as fibrous dysplasia, subperiosteal bone cysts, or vertebral scalloping, can also be found.[2]\nRare complications of NF1 include renal artery stenosis and pheochromocytoma, either of which can present with hypertension.[3]\nNF2 is a progressive genetic disorder that is present in one in every 33,000-40,000 births. Patients with NF2 (which results from an abnormality on chromosome 22) typically present with acoustic neuromas or vestibular schwannomas. In contrast to NF1, patients with NF2 do not typically have cognitive issues nor significant numbers of café au lait spots.\nThe clinical manifestations of NF2 include tinnitus, balance disorders, and progressive hearing loss. Affected patients may also have meningiomas, juvenile cataracts, or schwannomas of the dorsal roots of the spinal cord. The diagnosis of NF2 is typically based on the presence of bilateral acoustic neuromas or a unilateral acoustic neuroma in a first-degree relative with NF2.\nCriteria have been modified to increase sensitivity and may also be fulfilled by having a first-degree relative with NF2 and any two of the following:\nMeningioma\nSchwannoma\nGlioma\nNeurofibroma\nPosterior subcapsular lenticular opacities\nThe criteria may also be fulfilled by unilateral acoustic neuroma and any two of the following:\nMeningioma\nSchwannoma\nGlioma\nNeurofibroma\nPosterior subcapsular lenticular opacities\nFinally, the criteria may also be fulfilled by multiple meningiomas and unilateral acoustic neuroma or any two of schwannoma, glioma, or neurofibroma/cataract.[3]\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "Fleshy Lesions on a 32-Year-Old Woman"
},
{
"authors": "Alyssa Abbey, MCMS, PA-C; Martin I. Newman, MD",
"content": [
"For both NF1 and NF2, the diagnosis is primarily based on the physical findings and a positive family history. Plain radiologic studies, which may detect many bony abnormalities (such as modeling defects of the long bones or ribs, bony erosion secondary to an adjacent plexiform neurofibroma, or scoliosis), may be useful diagnostic tests. Gadolinium-enhanced MRI of the brain is the preferred diagnostic imaging study of the head; however, neuroimaging in NF1 is typically only performed if symptoms or signs of abnormality occur, rather than as standard screening.",
"MRI scanning of the brain assists in evaluating the optic nerves or optic chiasm; however, ophthalmologic examinations are used to first screen for these abnormalities. Eye examinations are recommended annually in early childhood and less often in older children or adults with NF1.",
"MRI studies have been shown to frequently detect unidentified bright objects in the brain parenchyma of patients with NF1. These bright spots do not have clinical significance and may lead to unnecessary repeated testing; in general, they do not enhance, cause no mass effect, and often resolve as the patient gets older. It has been theorized that these masses may represent benign hamartomas.",
"MRI is also useful for diagnosing and evaluating other internal lesions, such as mediastinal masses, spinal cord tumors, deep plexiform neurofibromas, neurofibromas of the brachial or sacral plexus, and abdominopelvic lesions. Genetic analyses and psychological or developmental assessments should also be part of the evaluation of patients with neurofibromas.[4]",
"Genetic testing of at-risk family members of patients with NF2 is recommended; screening for acoustic neuromas and the associated progressive hearing loss that can take place is indicated. Brain MRI is recommended on an annual basis from age 10-12 years through the fourth decade of life, as is hearing evaluation with brainstem auditory evoked response testing, which may demonstrate changes in auditory nerve function before changes on MRI may be seen.[3]",
"No cure exists for NF1 or NF2, although various medical treatments are being evaluated in trials. The recommendations for follow-up include referral to support groups, psychological counseling, and evaluation for learning disorders; potential surgical excision of the lesions; and regular monitoring by a primary care provider for any lesion changes (patients with NF1 are at a somewhat increased risk for malignancy).",
"Annual ocular examinations are recommended. Genetic testing is also available for patients with NF who wish to have children; it is transmitted in an autosomal dominant fashion, although with variability in the severity of features even among family members.",
"Historically, surgery has been a successful treatment for the lesions themselves; however, there is often recurrence, and nerve damage is a risk in cases in which the lesions are located along neural pathways.[5]"
],
"date": "November 11, 2014",
"figures": [],
"markdown": "# Fleshy Lesions on a 32-Year-Old Woman\n\n **Authors:** Alyssa Abbey, MCMS, PA-C; Martin I. Newman, MD \n **Date:** November 11, 2014\n\n ## Content\n\n For both NF1 and NF2, the diagnosis is primarily based on the physical findings and a positive family history. Plain radiologic studies, which may detect many bony abnormalities (such as modeling defects of the long bones or ribs, bony erosion secondary to an adjacent plexiform neurofibroma, or scoliosis), may be useful diagnostic tests. Gadolinium-enhanced MRI of the brain is the preferred diagnostic imaging study of the head; however, neuroimaging in NF1 is typically only performed if symptoms or signs of abnormality occur, rather than as standard screening.\nMRI scanning of the brain assists in evaluating the optic nerves or optic chiasm; however, ophthalmologic examinations are used to first screen for these abnormalities. Eye examinations are recommended annually in early childhood and less often in older children or adults with NF1.\nMRI studies have been shown to frequently detect unidentified bright objects in the brain parenchyma of patients with NF1. These bright spots do not have clinical significance and may lead to unnecessary repeated testing; in general, they do not enhance, cause no mass effect, and often resolve as the patient gets older. It has been theorized that these masses may represent benign hamartomas.\nMRI is also useful for diagnosing and evaluating other internal lesions, such as mediastinal masses, spinal cord tumors, deep plexiform neurofibromas, neurofibromas of the brachial or sacral plexus, and abdominopelvic lesions. Genetic analyses and psychological or developmental assessments should also be part of the evaluation of patients with neurofibromas.[4]\nGenetic testing of at-risk family members of patients with NF2 is recommended; screening for acoustic neuromas and the associated progressive hearing loss that can take place is indicated. Brain MRI is recommended on an annual basis from age 10-12 years through the fourth decade of life, as is hearing evaluation with brainstem auditory evoked response testing, which may demonstrate changes in auditory nerve function before changes on MRI may be seen.[3]\nNo cure exists for NF1 or NF2, although various medical treatments are being evaluated in trials. The recommendations for follow-up include referral to support groups, psychological counseling, and evaluation for learning disorders; potential surgical excision of the lesions; and regular monitoring by a primary care provider for any lesion changes (patients with NF1 are at a somewhat increased risk for malignancy).\nAnnual ocular examinations are recommended. Genetic testing is also available for patients with NF who wish to have children; it is transmitted in an autosomal dominant fashion, although with variability in the severity of features even among family members.\nHistorically, surgery has been a successful treatment for the lesions themselves; however, there is often recurrence, and nerve damage is a risk in cases in which the lesions are located along neural pathways.[5]\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 786051,
"choiceText": "Café au lait spots",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 786053,
"choiceText": "Lisch nodule on the iris",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 786055,
"choiceText": "First-degree relative with the condition",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 786057,
"choiceText": "Acute renal failure",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 786059,
"choiceText": "Axillary or inguinal freckling",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Two or more of the following seven criteria must be present in order to make the diagnosis of NF1: six or more café au lait spots (irregularly shaped, evenly pigmented, brown macules), two or more neurofibromas, axillary or inguinal freckling, Lisch nodules on the iris, ONG, various types of osseous lesions, or a first-degree relative with the condition.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 246291,
"questionText": "Which of the following conditions is <i>not</i> one of the seven diagnostic clinical criteria required for the diagnosis of NF1?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 786061,
"choiceText": "Optic nerve tumors primarily occur in adolescents",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 786063,
"choiceText": "An ONG may lead to worsening vision",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 786065,
"choiceText": "Lisch nodules are located on the iris",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 786067,
"choiceText": "Choroidal abnormalities typically have a patchy appearance",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 786069,
"choiceText": "Corkscrew-shaped vascular changes may be found on the retina",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Optic nerve tumors occur primarily in children younger than 5 years. Asymmetric, noncorrectable visual loss is the most common presenting symptom, but subtle peripheral field defects, color-discrimination difficulties, optic nerve pallor, or proptosis may also occur, without visual acuity problems.<br>\r\n<br>\r\nSome older children and adolescents may present with worsening vision secondary to a slow-growing ONG; therefore, monitoring these patients for vision difficulties should continue throughout childhood and adulthood. Adults may have a visually insignificant ONG detected incidentally on a head imaging study.<br>\r\n<br>\r\nAlthough Lisch nodules occasionally can be seen with a direct or indirect ophthalmoscope, especially in individuals with a light-colored iris, they are usually not visible without using a slit lamp. Choroidal abnormalities with a patchy appearance or retinal corkscrew vascular changes may also be noted on funduscopic examination.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 246293,
"questionText": "Involvement of the structures of the eye and associated impairment of normal vision are prominent features of NF. Which of the following statements is incorrect?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Fleshy Lesions on a 32-Year-Old Woman"
},
{
"authors": "Alyssa Abbey, MCMS, PA-C; Martin I. Newman, MD",
"content": [
"For other resources and access to support networks, contact the webpages of the Children's Tumor Foundation and the Neurofibromatosis Network."
],
"date": "November 11, 2014",
"figures": [],
"markdown": "# Fleshy Lesions on a 32-Year-Old Woman\n\n **Authors:** Alyssa Abbey, MCMS, PA-C; Martin I. Newman, MD \n **Date:** November 11, 2014\n\n ## Content\n\n For other resources and access to support networks, contact the webpages of the Children's Tumor Foundation and the Neurofibromatosis Network.\n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 786051,
"choiceText": "Café au lait spots",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 786053,
"choiceText": "Lisch nodule on the iris",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 786055,
"choiceText": "First-degree relative with the condition",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 786057,
"choiceText": "Acute renal failure",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 786059,
"choiceText": "Axillary or inguinal freckling",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Two or more of the following seven criteria must be present in order to make the diagnosis of NF1: six or more café au lait spots (irregularly shaped, evenly pigmented, brown macules), two or more neurofibromas, axillary or inguinal freckling, Lisch nodules on the iris, ONG, various types of osseous lesions, or a first-degree relative with the condition.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 246291,
"questionText": "Which of the following conditions is <i>not</i> one of the seven diagnostic clinical criteria required for the diagnosis of NF1?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 786061,
"choiceText": "Optic nerve tumors primarily occur in adolescents",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 786063,
"choiceText": "An ONG may lead to worsening vision",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 786065,
"choiceText": "Lisch nodules are located on the iris",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 786067,
"choiceText": "Choroidal abnormalities typically have a patchy appearance",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 786069,
"choiceText": "Corkscrew-shaped vascular changes may be found on the retina",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Optic nerve tumors occur primarily in children younger than 5 years. Asymmetric, noncorrectable visual loss is the most common presenting symptom, but subtle peripheral field defects, color-discrimination difficulties, optic nerve pallor, or proptosis may also occur, without visual acuity problems.<br>\r\n<br>\r\nSome older children and adolescents may present with worsening vision secondary to a slow-growing ONG; therefore, monitoring these patients for vision difficulties should continue throughout childhood and adulthood. Adults may have a visually insignificant ONG detected incidentally on a head imaging study.<br>\r\n<br>\r\nAlthough Lisch nodules occasionally can be seen with a direct or indirect ophthalmoscope, especially in individuals with a light-colored iris, they are usually not visible without using a slit lamp. Choroidal abnormalities with a patchy appearance or retinal corkscrew vascular changes may also be noted on funduscopic examination.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 246293,
"questionText": "Involvement of the structures of the eye and associated impairment of normal vision are prominent features of NF. Which of the following statements is incorrect?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Fleshy Lesions on a 32-Year-Old Woman"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 786043,
"choiceText": "Neurofibromatosis",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 786045,
"choiceText": "McCune-Albright syndrome",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 786047,
"choiceText": "Vitiligo",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 786049,
"choiceText": "Hansen disease",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 246289,
"questionText": "On the basis of the history, physical examination, and work-up, what is the diagnosis?<br>\r\n<i>Hint: Tan macules or patches, known as \"café au lait spots,\" are characteristic of this genetic disorder.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 786051,
"choiceText": "Café au lait spots",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 786053,
"choiceText": "Lisch nodule on the iris",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 786055,
"choiceText": "First-degree relative with the condition",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 786057,
"choiceText": "Acute renal failure",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 786059,
"choiceText": "Axillary or inguinal freckling",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Two or more of the following seven criteria must be present in order to make the diagnosis of NF1: six or more café au lait spots (irregularly shaped, evenly pigmented, brown macules), two or more neurofibromas, axillary or inguinal freckling, Lisch nodules on the iris, ONG, various types of osseous lesions, or a first-degree relative with the condition.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 246291,
"questionText": "Which of the following conditions is <i>not</i> one of the seven diagnostic clinical criteria required for the diagnosis of NF1?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 786061,
"choiceText": "Optic nerve tumors primarily occur in adolescents",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 786063,
"choiceText": "An ONG may lead to worsening vision",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 786065,
"choiceText": "Lisch nodules are located on the iris",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 786067,
"choiceText": "Choroidal abnormalities typically have a patchy appearance",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 786069,
"choiceText": "Corkscrew-shaped vascular changes may be found on the retina",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Optic nerve tumors occur primarily in children younger than 5 years. Asymmetric, noncorrectable visual loss is the most common presenting symptom, but subtle peripheral field defects, color-discrimination difficulties, optic nerve pallor, or proptosis may also occur, without visual acuity problems.<br>\r\n<br>\r\nSome older children and adolescents may present with worsening vision secondary to a slow-growing ONG; therefore, monitoring these patients for vision difficulties should continue throughout childhood and adulthood. Adults may have a visually insignificant ONG detected incidentally on a head imaging study.<br>\r\n<br>\r\nAlthough Lisch nodules occasionally can be seen with a direct or indirect ophthalmoscope, especially in individuals with a light-colored iris, they are usually not visible without using a slit lamp. Choroidal abnormalities with a patchy appearance or retinal corkscrew vascular changes may also be noted on funduscopic examination.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 246293,
"questionText": "Involvement of the structures of the eye and associated impairment of normal vision are prominent features of NF. Which of the following statements is incorrect?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
833929 | /viewarticle/833929 | [
{
"authors": "Abdul Saied Calvino, MD; N. Joseph Espat, MD, MS",
"content": [
"Editor's Note: \nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"Background",
"A 73-year-old woman presents with dysphagia and unintentional weight loss totaling approximately 10-15 lb over 3 months. The dysphagia is mild to moderate, with the transient feeling that food gets \"stuck\" in her chest, which only happens with solids. She denies fever, chills, nausea, emesis, abdominal pain, diarrhea, hematochezia, or melena but reports increasing fatigue and weakness.",
"The patient's medical history is significant for hypertension controlled with beta-blockers, as well as gastroesophageal reflux disease (GERD), which is well controlled with proton pump inhibitors. Previous surgical history is relevant only for a hysterectomy performed in response to benign tumors. The family history is not significant. The patient reports a smoking history of one pack a day for 25 years; she quit 10 years ago. Alcohol intake is occasional, approximately two to three glasses of wine with dinner three times a week."
],
"date": "January 09, 2017",
"figures": [],
"markdown": "# Dysphagia and Weight Loss in a 73-Year-Old Woman\n\n **Authors:** Abdul Saied Calvino, MD; N. Joseph Espat, MD, MS \n **Date:** January 09, 2017\n\n ## Content\n\n Editor's Note: \nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nBackground\nA 73-year-old woman presents with dysphagia and unintentional weight loss totaling approximately 10-15 lb over 3 months. The dysphagia is mild to moderate, with the transient feeling that food gets \"stuck\" in her chest, which only happens with solids. She denies fever, chills, nausea, emesis, abdominal pain, diarrhea, hematochezia, or melena but reports increasing fatigue and weakness.\nThe patient's medical history is significant for hypertension controlled with beta-blockers, as well as gastroesophageal reflux disease (GERD), which is well controlled with proton pump inhibitors. Previous surgical history is relevant only for a hysterectomy performed in response to benign tumors. The family history is not significant. The patient reports a smoking history of one pack a day for 25 years; she quit 10 years ago. Alcohol intake is occasional, approximately two to three glasses of wine with dinner three times a week.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "Dysphagia and Weight Loss in a 73-Year-Old Woman"
},
{
"authors": "Abdul Saied Calvino, MD; N. Joseph Espat, MD, MS",
"content": [
"Physical Examination and Work-up",
"The patient is a thin, pale woman with good functional status. She is not in acute distress and is afebrile. The rest of her vital signs are within normal limits. Mental status and general examination are normal. No lymph nodes are palpable in the neck, axilla, or inguinal regions. Neck and thyroid examination findings are normal. The abdominal examination does not reveal tenderness to palpation or evidence of palpable masses, organomegaly, or ascites. Her extremities have no edema or skin changes.",
"Basic metabolic panel results are within normal limits. A complete white blood cell count reveals normocytic anemia, with a hematocrit of 26% and hemoglobin of 9.5 g/dL. Chest radiograph findings are normal. A barium swallow study was performed and revealed an irregular area with narrowing of the distal esophagus."
],
"date": "January 09, 2017",
"figures": [],
"markdown": "# Dysphagia and Weight Loss in a 73-Year-Old Woman\n\n **Authors:** Abdul Saied Calvino, MD; N. Joseph Espat, MD, MS \n **Date:** January 09, 2017\n\n ## Content\n\n Physical Examination and Work-up\nThe patient is a thin, pale woman with good functional status. She is not in acute distress and is afebrile. The rest of her vital signs are within normal limits. Mental status and general examination are normal. No lymph nodes are palpable in the neck, axilla, or inguinal regions. Neck and thyroid examination findings are normal. The abdominal examination does not reveal tenderness to palpation or evidence of palpable masses, organomegaly, or ascites. Her extremities have no edema or skin changes.\nBasic metabolic panel results are within normal limits. A complete white blood cell count reveals normocytic anemia, with a hematocrit of 26% and hemoglobin of 9.5 g/dL. Chest radiograph findings are normal. A barium swallow study was performed and revealed an irregular area with narrowing of the distal esophagus.\n\n ## Figures\n\n \n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 782485,
"choiceText": "Benign esophageal stricture secondary to GERD",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 782487,
"choiceText": "Achalasia",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 782489,
"choiceText": "Benign esophageal tumor leiomyoma",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 782491,
"choiceText": "Esophageal cancer",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 782493,
"choiceText": "Peptic ulcer disease",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 245183,
"questionText": "Which of the following is the likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Dysphagia and Weight Loss in a 73-Year-Old Woman"
},
{
"authors": "Abdul Saied Calvino, MD; N. Joseph Espat, MD, MS",
"content": [
"Discussion",
"This scenario is a common clinical presentation for patients with esophageal obstruction secondary to esophageal cancer. Patients who present with dysphagia should always arouse suspicion for esophageal cancer. Other benign conditions could also present with similar symptoms; however, the scenario gives a presentation of dysphagia with weight loss in a patient with risk factors for esophageal cancer.",
"The physical examination findings are often negative, with no evidence of gross abnormality. If the disease is found in an advanced stage, palpable lymph nodes may be present. The most common palpable lymph nodes are found in the cervical and supraclavicular areas.[1] Often, the nodes are not palpable but can be visualized on CT scan or upon endoscopic evaluation. The initial laboratory work-up may be completely normal, or, as seen in this case, normocytic anemia can be found secondary to inadvertent bleeding or chronic disease–associated anemia.",
"The two most common malignant esophageal tumors are squamous cell carcinoma (SCC) and adenocarcinoma. In Western countries, the incidence of adenocarcinoma has increased significantly in the past 20 years, representing more than 60% of the esophageal cancers in the United States.[1] In Eastern countries, SCC is still most prevalent. In the United States, the incidence has increased in parallel with an increase in such risk factors as obesity and GERD.[1,2]",
"SCC originates in the epithelium that covers the esophagus. It is more common in males, with a 3:1 male-to-female ratio. The most common site is the mid-esophagus. The major risk factors are smoking and alcohol consumption.[3] The adenocarcinoma type originates from the glandular cells at the gastroesophageal junction; therefore, adenocarcinomas are more commonly located in the distal esophagus. Adenocarcinomas are more common in males than females (7:1). The main risk factors are Barrett esophagus (intestinal metaplasia), acid reflux, obesity, and smoking.[3]"
],
"date": "January 09, 2017",
"figures": [],
"markdown": "# Dysphagia and Weight Loss in a 73-Year-Old Woman\n\n **Authors:** Abdul Saied Calvino, MD; N. Joseph Espat, MD, MS \n **Date:** January 09, 2017\n\n ## Content\n\n Discussion\nThis scenario is a common clinical presentation for patients with esophageal obstruction secondary to esophageal cancer. Patients who present with dysphagia should always arouse suspicion for esophageal cancer. Other benign conditions could also present with similar symptoms; however, the scenario gives a presentation of dysphagia with weight loss in a patient with risk factors for esophageal cancer.\nThe physical examination findings are often negative, with no evidence of gross abnormality. If the disease is found in an advanced stage, palpable lymph nodes may be present. The most common palpable lymph nodes are found in the cervical and supraclavicular areas.[1] Often, the nodes are not palpable but can be visualized on CT scan or upon endoscopic evaluation. The initial laboratory work-up may be completely normal, or, as seen in this case, normocytic anemia can be found secondary to inadvertent bleeding or chronic disease–associated anemia.\nThe two most common malignant esophageal tumors are squamous cell carcinoma (SCC) and adenocarcinoma. In Western countries, the incidence of adenocarcinoma has increased significantly in the past 20 years, representing more than 60% of the esophageal cancers in the United States.[1] In Eastern countries, SCC is still most prevalent. In the United States, the incidence has increased in parallel with an increase in such risk factors as obesity and GERD.[1,2]\nSCC originates in the epithelium that covers the esophagus. It is more common in males, with a 3:1 male-to-female ratio. The most common site is the mid-esophagus. The major risk factors are smoking and alcohol consumption.[3] The adenocarcinoma type originates from the glandular cells at the gastroesophageal junction; therefore, adenocarcinomas are more commonly located in the distal esophagus. Adenocarcinomas are more common in males than females (7:1). The main risk factors are Barrett esophagus (intestinal metaplasia), acid reflux, obesity, and smoking.[3]\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 782485,
"choiceText": "Benign esophageal stricture secondary to GERD",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 782487,
"choiceText": "Achalasia",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 782489,
"choiceText": "Benign esophageal tumor leiomyoma",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 782491,
"choiceText": "Esophageal cancer",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 782493,
"choiceText": "Peptic ulcer disease",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 245183,
"questionText": "Which of the following is the likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Dysphagia and Weight Loss in a 73-Year-Old Woman"
},
{
"authors": "Abdul Saied Calvino, MD; N. Joseph Espat, MD, MS",
"content": [
"Imaging work-up for dysphagia is usually initiated with a noninvasive swallow study, as in this case. The imaging may reveal a stricture, esophageal lumen abnormalities, or both. Any abnormality found is usually followed by an esophagogastroduodenoscopy (EGD). EGD provides clear visualization of the esophageal mucosa and can also be used to obtain tissue sampling (biopsy) if a suspicious lesion is found (Figure 1).",
"Figure 1.",
"Figure 2.",
"Figure 3.",
"Endoscopic ultrasonography (EUS) is also helpful in delineating the tumor invasion and presence of enlarged mediastinal lymph nodes (Figure 2).",
"In addition, fine-needle aspiration of the enlarged lymph node can be performed under EUS guidance. This is important, if cancer is present, for staging purposes and future treatment options. CT scanning can be used initially if esophageal tumor perforation is suspected; however, it is not commonly used as the first diagnostic tool. Once the diagnosis has been established, a CT scan of the chest and upper abdomen is obtained to evaluate local invasion and distant metastatic disease.",
"After the diagnosis is established, the next step is to appropriately stage the disease. The most commonly used staging system is the TNM system of the American Joint Committee on Cancer In 2010, two separate staging system groups were created on the basis of the different histologic types. Locoregional staging is usually performed with EUS. It has been shown to be the most accurate technique, with an overall accuracy for tumor (T) and node staging (N) ranging from 80% to 90%,depending on the operator.[4] The most common areas of distant metastases are the lungs, liver, bone, and adrenal glands.[5] The distant metastases staging (M) is usually completed using a CT or PET scan. In clinical trials the PET scan has shown to be superior to the CT scan in detecting distant metastases (Figure 3).[6]"
],
"date": "January 09, 2017",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/833/929/833929-thumb-1.jpg"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/833/929/833929-thumb-2.jpg"
},
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/833/929/833929-thumb-3.jpg"
}
],
"markdown": "# Dysphagia and Weight Loss in a 73-Year-Old Woman\n\n **Authors:** Abdul Saied Calvino, MD; N. Joseph Espat, MD, MS \n **Date:** January 09, 2017\n\n ## Content\n\n Imaging work-up for dysphagia is usually initiated with a noninvasive swallow study, as in this case. The imaging may reveal a stricture, esophageal lumen abnormalities, or both. Any abnormality found is usually followed by an esophagogastroduodenoscopy (EGD). EGD provides clear visualization of the esophageal mucosa and can also be used to obtain tissue sampling (biopsy) if a suspicious lesion is found (Figure 1).\nFigure 1.\nFigure 2.\nFigure 3.\nEndoscopic ultrasonography (EUS) is also helpful in delineating the tumor invasion and presence of enlarged mediastinal lymph nodes (Figure 2).\nIn addition, fine-needle aspiration of the enlarged lymph node can be performed under EUS guidance. This is important, if cancer is present, for staging purposes and future treatment options. CT scanning can be used initially if esophageal tumor perforation is suspected; however, it is not commonly used as the first diagnostic tool. Once the diagnosis has been established, a CT scan of the chest and upper abdomen is obtained to evaluate local invasion and distant metastatic disease.\nAfter the diagnosis is established, the next step is to appropriately stage the disease. The most commonly used staging system is the TNM system of the American Joint Committee on Cancer In 2010, two separate staging system groups were created on the basis of the different histologic types. Locoregional staging is usually performed with EUS. It has been shown to be the most accurate technique, with an overall accuracy for tumor (T) and node staging (N) ranging from 80% to 90%,depending on the operator.[4] The most common areas of distant metastases are the lungs, liver, bone, and adrenal glands.[5] The distant metastases staging (M) is usually completed using a CT or PET scan. In clinical trials the PET scan has shown to be superior to the CT scan in detecting distant metastases (Figure 3).[6]\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n**Figure 3.** \n \n\n\n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "Dysphagia and Weight Loss in a 73-Year-Old Woman"
},
{
"authors": "Abdul Saied Calvino, MD; N. Joseph Espat, MD, MS",
"content": [
"The treatment of esophageal cancers is stage-dependent. In the case of early-stage tumors (Tis, T1aN0M0), endoscopic mucosal resection or endoscopic submucosal dissection can be performed at centers with experience in these techniques. For the group of patients with T1bN0M0, formal resection could be offered as an initial modality. However, patients with T2 stage or beyond benefit from a multimodality treatment approach. A multidisciplinary evaluation of patients with esophageal cancer is important to delineate the best possible treatment plan for each patient. The use of chemoradiation therapy before surgery (neoadjuvant) resulted in pathologic complete response (pCR)—meaning that there was no evidence of viable tumor in the specimen after surgery—in 15%-30% of patients.[7] The 3-year survival rate for patients with pCR is 50%, compared with 27% for patients without pCR.[7] Most patients with SCC or adenocarcinoma of the esophagus undergo a combination of chemotherapy, radiation, and surgery.",
"The 73-year-old woman in this case scenario went on to receive multimodality treatment, including neoadjuvant chemoradiation therapy with subsequent Ivor-Lewis esophageal resection."
],
"date": "January 09, 2017",
"figures": [],
"markdown": "# Dysphagia and Weight Loss in a 73-Year-Old Woman\n\n **Authors:** Abdul Saied Calvino, MD; N. Joseph Espat, MD, MS \n **Date:** January 09, 2017\n\n ## Content\n\n The treatment of esophageal cancers is stage-dependent. In the case of early-stage tumors (Tis, T1aN0M0), endoscopic mucosal resection or endoscopic submucosal dissection can be performed at centers with experience in these techniques. For the group of patients with T1bN0M0, formal resection could be offered as an initial modality. However, patients with T2 stage or beyond benefit from a multimodality treatment approach. A multidisciplinary evaluation of patients with esophageal cancer is important to delineate the best possible treatment plan for each patient. The use of chemoradiation therapy before surgery (neoadjuvant) resulted in pathologic complete response (pCR)—meaning that there was no evidence of viable tumor in the specimen after surgery—in 15%-30% of patients.[7] The 3-year survival rate for patients with pCR is 50%, compared with 27% for patients without pCR.[7] Most patients with SCC or adenocarcinoma of the esophagus undergo a combination of chemotherapy, radiation, and surgery.\nThe 73-year-old woman in this case scenario went on to receive multimodality treatment, including neoadjuvant chemoradiation therapy with subsequent Ivor-Lewis esophageal resection.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 782511,
"choiceText": "CT scan of the chest and abdomen",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 782513,
"choiceText": "PET scan",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 782515,
"choiceText": "EGD with tissue samples of the lesion",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 782517,
"choiceText": "Evaluation for chemoradiation therapy",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 782519,
"choiceText": "Esophageal resection",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "EGD with biopsies is the next step in the management of this patient. Once the diagnosis has been established, then EUS, CT scanning, or PET scanning are appropriate for staging.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 245189,
"questionText": "Which of the following is the next step in the management of this patient after the barium swallow?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 782521,
"choiceText": "Esophageal resection",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 782523,
"choiceText": "Radiation therapy",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 782525,
"choiceText": "Endoscopic mucosal resection",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 782527,
"choiceText": "Neoadjuvant chemoradiation therapy with esophageal resection",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 782529,
"choiceText": "Systemic chemotherapy",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Multidimensional approaches may be required. Neoadjuvant chemoradiation therapy with esophageal resection is the most appropriate treatment modality for a T2N1M0 esophageal adenocarcinoma.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 245191,
"questionText": "After the diagnosis of esophageal adenocarcinoma is made, which of the following is the most appropriate treatment approach for a T2N1M0 cancer?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Dysphagia and Weight Loss in a 73-Year-Old Woman"
},
{
"authors": "Abdul Saied Calvino, MD; N. Joseph Espat, MD, MS",
"content": [],
"date": "January 09, 2017",
"figures": [],
"markdown": "# Dysphagia and Weight Loss in a 73-Year-Old Woman\n\n **Authors:** Abdul Saied Calvino, MD; N. Joseph Espat, MD, MS \n **Date:** January 09, 2017\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 782511,
"choiceText": "CT scan of the chest and abdomen",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 782513,
"choiceText": "PET scan",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 782515,
"choiceText": "EGD with tissue samples of the lesion",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 782517,
"choiceText": "Evaluation for chemoradiation therapy",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 782519,
"choiceText": "Esophageal resection",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "EGD with biopsies is the next step in the management of this patient. Once the diagnosis has been established, then EUS, CT scanning, or PET scanning are appropriate for staging.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 245189,
"questionText": "Which of the following is the next step in the management of this patient after the barium swallow?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 782521,
"choiceText": "Esophageal resection",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 782523,
"choiceText": "Radiation therapy",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 782525,
"choiceText": "Endoscopic mucosal resection",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 782527,
"choiceText": "Neoadjuvant chemoradiation therapy with esophageal resection",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 782529,
"choiceText": "Systemic chemotherapy",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Multidimensional approaches may be required. Neoadjuvant chemoradiation therapy with esophageal resection is the most appropriate treatment modality for a T2N1M0 esophageal adenocarcinoma.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 245191,
"questionText": "After the diagnosis of esophageal adenocarcinoma is made, which of the following is the most appropriate treatment approach for a T2N1M0 cancer?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Dysphagia and Weight Loss in a 73-Year-Old Woman"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 782485,
"choiceText": "Benign esophageal stricture secondary to GERD",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 782487,
"choiceText": "Achalasia",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 782489,
"choiceText": "Benign esophageal tumor leiomyoma",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 782491,
"choiceText": "Esophageal cancer",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 782493,
"choiceText": "Peptic ulcer disease",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 245183,
"questionText": "Which of the following is the likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 782511,
"choiceText": "CT scan of the chest and abdomen",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 782513,
"choiceText": "PET scan",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 782515,
"choiceText": "EGD with tissue samples of the lesion",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 782517,
"choiceText": "Evaluation for chemoradiation therapy",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 782519,
"choiceText": "Esophageal resection",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "EGD with biopsies is the next step in the management of this patient. Once the diagnosis has been established, then EUS, CT scanning, or PET scanning are appropriate for staging.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 245189,
"questionText": "Which of the following is the next step in the management of this patient after the barium swallow?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 782521,
"choiceText": "Esophageal resection",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 782523,
"choiceText": "Radiation therapy",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 782525,
"choiceText": "Endoscopic mucosal resection",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 782527,
"choiceText": "Neoadjuvant chemoradiation therapy with esophageal resection",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 782529,
"choiceText": "Systemic chemotherapy",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Multidimensional approaches may be required. Neoadjuvant chemoradiation therapy with esophageal resection is the most appropriate treatment modality for a T2N1M0 esophageal adenocarcinoma.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 245191,
"questionText": "After the diagnosis of esophageal adenocarcinoma is made, which of the following is the most appropriate treatment approach for a T2N1M0 cancer?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
833918 | /viewarticle/833918 | [
{
"authors": "Joshua M. Kosowsky, MD",
"content": [
"Editor's Note:\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"A 65-year-old man presents to the emergency department with difficulty breathing. He describes worsening dyspnea on exertion that is associated with chest tightness, wheezing, and coughing. The patient's dyspnea has worsened to the point that he can hardly walk from his couch to the bathroom without becoming extremely short of breath. He recently recovered from a cold, with several days of nasal congestion, clear rhinorrhea, and a nonproductive cough.",
"The patient reports having been healthy his whole life and has not been to see a physician in at least two decades; however, he does admit that he has gradually curtailed his physical activities, such as gardening, shoveling snow, and walking in the mall, because he has been increasingly \"getting winded.\" He smokes two packs of cigarettes daily, a habit he has been trying to break for at least 30 years.",
"Upon physical examination, the patient is alert but appears to be in mild respiratory distress, with moderate retractions and pursed-lipped breathing. He is afebrile. His blood pressure is 140/85 mm Hg; his pulse is 103 beats/min and mostly regular. His respiratory rate is 28 breaths/min, and a pulse oximetry reading shows 85% while the patient is breathing room air. His breath sounds are diminished throughout, with a markedly prolonged expiratory phase and faint expiratory wheezes in the upper lung fields.",
"The cardiac examination reveals distant heart sounds, with a somewhat prominent P2. He has no murmur, gallop, or pericardial rub. His skin is cool and dry. He has trace edema at his ankles, but no cyanosis or clubbing. An ECG is performed (Figures 1 and 2).",
"Figure 1.",
"Figure 2."
],
"date": "April 09, 2020",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/833/918/833918-thumb-1.jpg"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/833/918/833918-thumb-2.jpg"
}
],
"markdown": "# A 65-Year-Old Man With a Cough and Worsening Dyspnea\n\n **Authors:** Joshua M. Kosowsky, MD \n **Date:** April 09, 2020\n\n ## Content\n\n Editor's Note:\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nA 65-year-old man presents to the emergency department with difficulty breathing. He describes worsening dyspnea on exertion that is associated with chest tightness, wheezing, and coughing. The patient's dyspnea has worsened to the point that he can hardly walk from his couch to the bathroom without becoming extremely short of breath. He recently recovered from a cold, with several days of nasal congestion, clear rhinorrhea, and a nonproductive cough.\nThe patient reports having been healthy his whole life and has not been to see a physician in at least two decades; however, he does admit that he has gradually curtailed his physical activities, such as gardening, shoveling snow, and walking in the mall, because he has been increasingly \"getting winded.\" He smokes two packs of cigarettes daily, a habit he has been trying to break for at least 30 years.\nUpon physical examination, the patient is alert but appears to be in mild respiratory distress, with moderate retractions and pursed-lipped breathing. He is afebrile. His blood pressure is 140/85 mm Hg; his pulse is 103 beats/min and mostly regular. His respiratory rate is 28 breaths/min, and a pulse oximetry reading shows 85% while the patient is breathing room air. His breath sounds are diminished throughout, with a markedly prolonged expiratory phase and faint expiratory wheezes in the upper lung fields.\nThe cardiac examination reveals distant heart sounds, with a somewhat prominent P2. He has no murmur, gallop, or pericardial rub. His skin is cool and dry. He has trace edema at his ankles, but no cyanosis or clubbing. An ECG is performed (Figures 1 and 2).\nFigure 1.\nFigure 2.\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n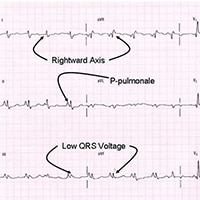 \n\n\n*Page 1 of 5*",
"pagination": {
"current_page": 1,
"total_pages": 5
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 782023,
"choiceText": "Pulmonary embolus\t",
"correct": false,
"displayOrder": 1,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 782025,
"choiceText": "Chronic obstructive pulmonary disease (COPD)",
"correct": true,
"displayOrder": 2,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 782027,
"choiceText": "Acute bacterial pneumonia",
"correct": false,
"displayOrder": 3,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 782029,
"choiceText": "Stable angina",
"correct": false,
"displayOrder": 4,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "",
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": "",
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 245051,
"questionText": "On the basis of the history, physical examination, and work-up, which of the following is the most likely diagnosis? <br /><br /><em>Hint: The ECG results suggest a chronic condition</em>.",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 65-Year-Old Man With a Cough and Worsening Dyspnea"
},
{
"authors": "Joshua M. Kosowsky, MD",
"content": [
"The ECG demonstrates a constellation of findings that suggest chronic obstructive pulmonary disease (COPD), including sinus tachycardia, a rightward axis, P pulmonale (a P-wave amplitude > 2.5 mV in the inferior leads), a low QRS voltage, and a right bundle branch block (RBBB).",
"In addition, the slight ST-segment depressions in the inferior leads are suggestive of prominent atrial repolarization abnormalities and are seen in COPD.",
"COPD is among the leading causes of death in the United States. It is defined as a disease state characterized by airflow obstruction caused by chronic bronchitis or emphysema. The airflow obstruction generally is progressive, and it may be accompanied by partially reversible airway hyperreactivity.",
"The condition was first described in western Europe in the early 19th century by Badham (1808) and Laennec (1827), who made the classic description of chronic bronchitis and emphysema. A British medical textbook of the 1860s described the familiar clinical picture of chronic bronchitis as an advanced disease, with repeated bronchial infections, that ended in right-heart failure. The modern definition of chronic bronchitis and emphysema that incorporated the concept of airflow obstruction was proposed by participants of the Ciba symposium of 1958.",
"Chronic bronchitis is defined, in clinical terms, as the presence of a chronic productive cough for 2 consecutive years, lasting for at least 3 months during each year, at the exclusion of other etiologies. Emphysema is defined, in terms of anatomical pathology, as an abnormal, permanent enlargement of the air spaces distal to the terminal bronchioles, accompanied by destruction of their walls and without obvious fibrosis.",
"The characteristic histopathology of chronic bronchitis is mucous gland hyperplasia with bronchial wall thickening, focal squamous metaplasia, ciliary abnormalities, and variable amounts of airway smooth-muscle hyperplasia and inflammation. These changes lead to luminal occlusion, causing airflow limitation by allowing airway walls to deform and narrow the airway lumen.",
"Emphysema has three morphologic patterns:",
"Centriacinar emphysema, in which focal destruction is limited to the respiratory bronchioles and the central portions of acinus. It is associated with cigarette smoking.",
"Panacinar emphysema involves the entire alveolus distal to the terminal bronchiole. It generally develops in patients with homozygous alpha-1–antitrypsin deficiency.",
"Distal acinar emphysema or paraseptal emphysema is the least common form, involving distal airway structures, alveolar ducts, and sacs. It is localized to fibrous septa or to the pleura and leads to formation of bullae, leading to pneumothorax.",
"Patients with COPD are susceptible to many conditions that can rapidly lead to acute deterioration superimposed on chronic disease, including COVID-19. Quick and accurate recognition of these patients, along with aggressive and prompt intervention, may be the only action that prevents frank respiratory failure."
],
"date": "April 09, 2020",
"figures": [],
"markdown": "# A 65-Year-Old Man With a Cough and Worsening Dyspnea\n\n **Authors:** Joshua M. Kosowsky, MD \n **Date:** April 09, 2020\n\n ## Content\n\n The ECG demonstrates a constellation of findings that suggest chronic obstructive pulmonary disease (COPD), including sinus tachycardia, a rightward axis, P pulmonale (a P-wave amplitude > 2.5 mV in the inferior leads), a low QRS voltage, and a right bundle branch block (RBBB).\nIn addition, the slight ST-segment depressions in the inferior leads are suggestive of prominent atrial repolarization abnormalities and are seen in COPD.\nCOPD is among the leading causes of death in the United States. It is defined as a disease state characterized by airflow obstruction caused by chronic bronchitis or emphysema. The airflow obstruction generally is progressive, and it may be accompanied by partially reversible airway hyperreactivity.\nThe condition was first described in western Europe in the early 19th century by Badham (1808) and Laennec (1827), who made the classic description of chronic bronchitis and emphysema. A British medical textbook of the 1860s described the familiar clinical picture of chronic bronchitis as an advanced disease, with repeated bronchial infections, that ended in right-heart failure. The modern definition of chronic bronchitis and emphysema that incorporated the concept of airflow obstruction was proposed by participants of the Ciba symposium of 1958.\nChronic bronchitis is defined, in clinical terms, as the presence of a chronic productive cough for 2 consecutive years, lasting for at least 3 months during each year, at the exclusion of other etiologies. Emphysema is defined, in terms of anatomical pathology, as an abnormal, permanent enlargement of the air spaces distal to the terminal bronchioles, accompanied by destruction of their walls and without obvious fibrosis.\nThe characteristic histopathology of chronic bronchitis is mucous gland hyperplasia with bronchial wall thickening, focal squamous metaplasia, ciliary abnormalities, and variable amounts of airway smooth-muscle hyperplasia and inflammation. These changes lead to luminal occlusion, causing airflow limitation by allowing airway walls to deform and narrow the airway lumen.\nEmphysema has three morphologic patterns:\nCentriacinar emphysema, in which focal destruction is limited to the respiratory bronchioles and the central portions of acinus. It is associated with cigarette smoking.\nPanacinar emphysema involves the entire alveolus distal to the terminal bronchiole. It generally develops in patients with homozygous alpha-1–antitrypsin deficiency.\nDistal acinar emphysema or paraseptal emphysema is the least common form, involving distal airway structures, alveolar ducts, and sacs. It is localized to fibrous septa or to the pleura and leads to formation of bullae, leading to pneumothorax.\nPatients with COPD are susceptible to many conditions that can rapidly lead to acute deterioration superimposed on chronic disease, including COVID-19. Quick and accurate recognition of these patients, along with aggressive and prompt intervention, may be the only action that prevents frank respiratory failure.\n\n ## Figures\n\n \n*Page 2 of 5*",
"pagination": {
"current_page": 2,
"total_pages": 5
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 782023,
"choiceText": "Pulmonary embolus\t",
"correct": false,
"displayOrder": 1,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 782025,
"choiceText": "Chronic obstructive pulmonary disease (COPD)",
"correct": true,
"displayOrder": 2,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 782027,
"choiceText": "Acute bacterial pneumonia",
"correct": false,
"displayOrder": 3,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 782029,
"choiceText": "Stable angina",
"correct": false,
"displayOrder": 4,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "",
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": "",
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 245051,
"questionText": "On the basis of the history, physical examination, and work-up, which of the following is the most likely diagnosis? <br /><br /><em>Hint: The ECG results suggest a chronic condition</em>.",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 65-Year-Old Man With a Cough and Worsening Dyspnea"
},
{
"authors": "Joshua M. Kosowsky, MD",
"content": [
"The disease is not generally diagnosed on the basis of ECG findings; however, the signs and symptoms of cardiac and pulmonary disease may overlap substantially, and ancillary testing can be useful in establishing the diagnosis.[1] In fact, it may not be unusual for ECG to be the first diagnostic test performed in patients with long-standing COPD, if patients present with shortness of breath as part of a general work-up for a possible cardiac etiology for the symptoms. Knowledge of the usual ECG manifestations of COPD enables the clinician to recognize uncharacteristic abnormalities, which often represent the effects of superimposed illnesses or drug toxicity.[2]",
"A tachycardic rhythm is common in individuals who are experiencing exacerbations of their COPD as a compensatory mechanism for hypoxia or poor right ventricular function (in the setting of cor pulmonale). Sinus tachycardia is the most common form reported in the literature, but other supraventricular arrhythmias, such as atrial tachycardia (unifocal or multifocal), atrial fibrillation, and atrial flutter, can also be present.[3,4]",
"P pulmonale (ie, a P-wave amplitude > 2.5 mm) is frequently reported but is a relatively insensitive predictor of right atrial enlargement.[5] In patients with COPD, the amplitude of the P wave is in fact dynamic, and it tends to be more prominent during acute exacerbation than at other times.[6]",
"A vertical or rightward axis is another manifestation of pulmonary hypertension.[7] Similarly, complete or incomplete RBBB, right ventricular hypertrophy, or both commonly occur in patients with cor pulmonale.",
"Low voltage, particularly in the limb leads, is another ECG characteristic of patients with COPD. This finding is classically attributed to increased impedance through a hyperinflated chest; however, low voltage is not directly correlated with hyperinflation, and it is neither sensitive nor specific for COPD.[8]"
],
"date": "April 09, 2020",
"figures": [],
"markdown": "# A 65-Year-Old Man With a Cough and Worsening Dyspnea\n\n **Authors:** Joshua M. Kosowsky, MD \n **Date:** April 09, 2020\n\n ## Content\n\n The disease is not generally diagnosed on the basis of ECG findings; however, the signs and symptoms of cardiac and pulmonary disease may overlap substantially, and ancillary testing can be useful in establishing the diagnosis.[1] In fact, it may not be unusual for ECG to be the first diagnostic test performed in patients with long-standing COPD, if patients present with shortness of breath as part of a general work-up for a possible cardiac etiology for the symptoms. Knowledge of the usual ECG manifestations of COPD enables the clinician to recognize uncharacteristic abnormalities, which often represent the effects of superimposed illnesses or drug toxicity.[2]\nA tachycardic rhythm is common in individuals who are experiencing exacerbations of their COPD as a compensatory mechanism for hypoxia or poor right ventricular function (in the setting of cor pulmonale). Sinus tachycardia is the most common form reported in the literature, but other supraventricular arrhythmias, such as atrial tachycardia (unifocal or multifocal), atrial fibrillation, and atrial flutter, can also be present.[3,4]\nP pulmonale (ie, a P-wave amplitude > 2.5 mm) is frequently reported but is a relatively insensitive predictor of right atrial enlargement.[5] In patients with COPD, the amplitude of the P wave is in fact dynamic, and it tends to be more prominent during acute exacerbation than at other times.[6]\nA vertical or rightward axis is another manifestation of pulmonary hypertension.[7] Similarly, complete or incomplete RBBB, right ventricular hypertrophy, or both commonly occur in patients with cor pulmonale.\nLow voltage, particularly in the limb leads, is another ECG characteristic of patients with COPD. This finding is classically attributed to increased impedance through a hyperinflated chest; however, low voltage is not directly correlated with hyperinflation, and it is neither sensitive nor specific for COPD.[8]\n\n ## Figures\n\n \n*Page 3 of 5*",
"pagination": {
"current_page": 3,
"total_pages": 5
},
"questionnaire": [],
"title": "A 65-Year-Old Man With a Cough and Worsening Dyspnea"
},
{
"authors": "Joshua M. Kosowsky, MD",
"content": [
"The mainstays of therapy for acute exacerbations of COPD, as in this case, are oxygen, bronchodilators, and steroids. Adequate oxygen should be given to relieve the hypoxia. Supplemental oxygen should maintain an oxygen saturation to above 90%. Bronchodilator therapy with both beta-agonist and anticholinergic nebulizer therapy should be administered promptly. The need for intubation should be established and performed if indicated (eg, hypoxia not relieved with supplemental oxygen, severe respiratory distress, CO2 retention with obtundation). If necessary and available, noninvasive continuous positive airway pressure may be used. Other therapies that are indicated in specific situations may include antibiotics, magnesium, and Heliox (a mixture of helium and oxygen that leads to increased oxygen delivery as a result of improved laminar flow).",
"The patient in this case was given a combination beta-agonist and anticholinergic nebulizer therapy for his respiratory distress and hypoxia. A chest radiograph was performed, which revealed a moderately sized basilar infiltrate. Antibiotics appropriate for community-acquired pneumonia coverage and intravenous steroids were administered, and the patient was admitted to the hospital for continued inpatient therapy and monitoring.",
"On the morning of hospital day 3, the patient was noted to be breathing easier and was discharged to home with prescriptions for maintenance inhaled steroid therapy, and for beta-agonist and anticholinergic metered-dose inhalers. Appropriate follow-up was arranged for continued outpatient management."
],
"date": "April 09, 2020",
"figures": [],
"markdown": "# A 65-Year-Old Man With a Cough and Worsening Dyspnea\n\n **Authors:** Joshua M. Kosowsky, MD \n **Date:** April 09, 2020\n\n ## Content\n\n The mainstays of therapy for acute exacerbations of COPD, as in this case, are oxygen, bronchodilators, and steroids. Adequate oxygen should be given to relieve the hypoxia. Supplemental oxygen should maintain an oxygen saturation to above 90%. Bronchodilator therapy with both beta-agonist and anticholinergic nebulizer therapy should be administered promptly. The need for intubation should be established and performed if indicated (eg, hypoxia not relieved with supplemental oxygen, severe respiratory distress, CO2 retention with obtundation). If necessary and available, noninvasive continuous positive airway pressure may be used. Other therapies that are indicated in specific situations may include antibiotics, magnesium, and Heliox (a mixture of helium and oxygen that leads to increased oxygen delivery as a result of improved laminar flow).\nThe patient in this case was given a combination beta-agonist and anticholinergic nebulizer therapy for his respiratory distress and hypoxia. A chest radiograph was performed, which revealed a moderately sized basilar infiltrate. Antibiotics appropriate for community-acquired pneumonia coverage and intravenous steroids were administered, and the patient was admitted to the hospital for continued inpatient therapy and monitoring.\nOn the morning of hospital day 3, the patient was noted to be breathing easier and was discharged to home with prescriptions for maintenance inhaled steroid therapy, and for beta-agonist and anticholinergic metered-dose inhalers. Appropriate follow-up was arranged for continued outpatient management.\n\n ## Figures\n\n \n*Page 4 of 5*",
"pagination": {
"current_page": 4,
"total_pages": 5
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 782175,
"choiceText": "Atrial fibrillation",
"correct": false,
"displayOrder": 1,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 782177,
"choiceText": "Multifocal atrial tachycardia",
"correct": false,
"displayOrder": 2,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 782179,
"choiceText": "Sinus tachycardia",
"correct": false,
"displayOrder": 3,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 782181,
"choiceText": "Ventricular tachycardia",
"correct": true,
"displayOrder": 4,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 782183,
"choiceText": "Atrial flutter",
"correct": false,
"displayOrder": 5,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "A tachycardic rhythm is common in patients who are experiencing exacerbations of their COPD as a compensatory mechanism for hypoxia or poor right ventricular function. Sinus tachycardia is the most common tachycardia noted, but other supraventricular arrhythmias, such as atrial tachycardia (unifocal or multifocal), atrial fibrillation, and atrial flutter, can also be seen. Ventricular tachyarrhythmias are not typically seen as a result of COPD.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": "",
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 245101,
"questionText": "Which of the following tachydysrhythmias is <em>not</em> typically associated with COPD?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 782185,
"choiceText": "Rightward axis",
"correct": false,
"displayOrder": 1,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 782187,
"choiceText": "P pulmonale",
"correct": false,
"displayOrder": 2,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 782189,
"choiceText": "Low QRS voltage",
"correct": false,
"displayOrder": 3,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 782191,
"choiceText": "Left ventricular hypertrophy",
"correct": true,
"displayOrder": 4,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 782193,
"choiceText": "Right bundle branch block",
"correct": false,
"displayOrder": 5,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The ECG in a patient with COPD often demonstrates a constellation of findings that suggest the diagnosis, including sinus tachycardia, a vertical or rightward axis, P pulmonale, a low QRS voltage (particularly in the limb leads), and a right bundle branch blck (RBBB). The development of left ventricular hypertrophy is typically not a result of COPD.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": "",
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 245103,
"questionText": "Which of the following tachydysrhythmias is <em>not</em> typically associated with COPD?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 65-Year-Old Man With a Cough and Worsening Dyspnea"
},
{
"authors": "Joshua M. Kosowsky, MD",
"content": [
"For more information on COPD, see the Medscape Drugs & Diseases articles Chronic Obstructive Pulmonary Disease and Chronic Obstructive Pulmonary Disease and Emphysema.",
"Acknowledgment: Special thanks are extended to John Vozenilek, MD, for his contributions to the publication of this case."
],
"date": "April 09, 2020",
"figures": [],
"markdown": "# A 65-Year-Old Man With a Cough and Worsening Dyspnea\n\n **Authors:** Joshua M. Kosowsky, MD \n **Date:** April 09, 2020\n\n ## Content\n\n For more information on COPD, see the Medscape Drugs & Diseases articles Chronic Obstructive Pulmonary Disease and Chronic Obstructive Pulmonary Disease and Emphysema.\nAcknowledgment: Special thanks are extended to John Vozenilek, MD, for his contributions to the publication of this case.\n\n ## Figures\n\n \n*Page 5 of 5*",
"pagination": {
"current_page": 5,
"total_pages": 5
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 782175,
"choiceText": "Atrial fibrillation",
"correct": false,
"displayOrder": 1,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 782177,
"choiceText": "Multifocal atrial tachycardia",
"correct": false,
"displayOrder": 2,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 782179,
"choiceText": "Sinus tachycardia",
"correct": false,
"displayOrder": 3,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 782181,
"choiceText": "Ventricular tachycardia",
"correct": true,
"displayOrder": 4,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 782183,
"choiceText": "Atrial flutter",
"correct": false,
"displayOrder": 5,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "A tachycardic rhythm is common in patients who are experiencing exacerbations of their COPD as a compensatory mechanism for hypoxia or poor right ventricular function. Sinus tachycardia is the most common tachycardia noted, but other supraventricular arrhythmias, such as atrial tachycardia (unifocal or multifocal), atrial fibrillation, and atrial flutter, can also be seen. Ventricular tachyarrhythmias are not typically seen as a result of COPD.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": "",
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 245101,
"questionText": "Which of the following tachydysrhythmias is <em>not</em> typically associated with COPD?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 782185,
"choiceText": "Rightward axis",
"correct": false,
"displayOrder": 1,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 782187,
"choiceText": "P pulmonale",
"correct": false,
"displayOrder": 2,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 782189,
"choiceText": "Low QRS voltage",
"correct": false,
"displayOrder": 3,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 782191,
"choiceText": "Left ventricular hypertrophy",
"correct": true,
"displayOrder": 4,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 782193,
"choiceText": "Right bundle branch block",
"correct": false,
"displayOrder": 5,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The ECG in a patient with COPD often demonstrates a constellation of findings that suggest the diagnosis, including sinus tachycardia, a vertical or rightward axis, P pulmonale, a low QRS voltage (particularly in the limb leads), and a right bundle branch blck (RBBB). The development of left ventricular hypertrophy is typically not a result of COPD.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": "",
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 245103,
"questionText": "Which of the following tachydysrhythmias is <em>not</em> typically associated with COPD?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 65-Year-Old Man With a Cough and Worsening Dyspnea"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 782023,
"choiceText": "Pulmonary embolus\t",
"correct": false,
"displayOrder": 1,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 782025,
"choiceText": "Chronic obstructive pulmonary disease (COPD)",
"correct": true,
"displayOrder": 2,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 782027,
"choiceText": "Acute bacterial pneumonia",
"correct": false,
"displayOrder": 3,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 782029,
"choiceText": "Stable angina",
"correct": false,
"displayOrder": 4,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "",
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": "",
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 245051,
"questionText": "On the basis of the history, physical examination, and work-up, which of the following is the most likely diagnosis? <br /><br /><em>Hint: The ECG results suggest a chronic condition</em>.",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 782175,
"choiceText": "Atrial fibrillation",
"correct": false,
"displayOrder": 1,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 782177,
"choiceText": "Multifocal atrial tachycardia",
"correct": false,
"displayOrder": 2,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 782179,
"choiceText": "Sinus tachycardia",
"correct": false,
"displayOrder": 3,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 782181,
"choiceText": "Ventricular tachycardia",
"correct": true,
"displayOrder": 4,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 782183,
"choiceText": "Atrial flutter",
"correct": false,
"displayOrder": 5,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "A tachycardic rhythm is common in patients who are experiencing exacerbations of their COPD as a compensatory mechanism for hypoxia or poor right ventricular function. Sinus tachycardia is the most common tachycardia noted, but other supraventricular arrhythmias, such as atrial tachycardia (unifocal or multifocal), atrial fibrillation, and atrial flutter, can also be seen. Ventricular tachyarrhythmias are not typically seen as a result of COPD.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": "",
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 245101,
"questionText": "Which of the following tachydysrhythmias is <em>not</em> typically associated with COPD?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 782185,
"choiceText": "Rightward axis",
"correct": false,
"displayOrder": 1,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 782187,
"choiceText": "P pulmonale",
"correct": false,
"displayOrder": 2,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 782189,
"choiceText": "Low QRS voltage",
"correct": false,
"displayOrder": 3,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 782191,
"choiceText": "Left ventricular hypertrophy",
"correct": true,
"displayOrder": 4,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 782193,
"choiceText": "Right bundle branch block",
"correct": false,
"displayOrder": 5,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The ECG in a patient with COPD often demonstrates a constellation of findings that suggest the diagnosis, including sinus tachycardia, a vertical or rightward axis, P pulmonale, a low QRS voltage (particularly in the limb leads), and a right bundle branch blck (RBBB). The development of left ventricular hypertrophy is typically not a result of COPD.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": "",
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 245103,
"questionText": "Which of the following tachydysrhythmias is <em>not</em> typically associated with COPD?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
832280 | /viewarticle/832280 | [
{
"authors": "Dieu-Thu Nguyen-Khoa, MD; Mona Sabeti, MD; Anh H. Au, MD",
"content": [
"Editor's Note: \nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions.",
"Background",
"A 24-year-old man with Down syndrome presents to the emergency department with a four-day history of bilateral flank pain, hematuria, and shortness of breath. The patient is accompanied by his mother in the examination room; she gives most of the patient's history. He denies radiation of the flank pain to any other part of his body. He also denies any urinary symptoms, such as dysuria or increased frequency, and he has not experienced any nausea or vomiting.",
"The patient has no history of cough or hemoptysis; fevers, chills, or night sweats; or recent trauma. His bowel and bladder function have been normal. He has a history of bilateral cryptorchidism in childhood, for which he underwent orchiopexy of both undescended testicles at the age of three years. There is no history of recent travel, and the mother reports no recent weight loss. He has no other significant surgical or medical history.",
"The patient does not smoke, drink alcohol, or use illicit drugs intravenously. He is currently not on any medications and does not have any allergies to medications."
],
"date": "September 30, 2014",
"figures": [],
"markdown": "# A 24-Year-Old Man With Down Syndrome and Shortness of Breath\n\n **Authors:** Dieu-Thu Nguyen-Khoa, MD; Mona Sabeti, MD; Anh H. Au, MD \n **Date:** September 30, 2014\n\n ## Content\n\n Editor's Note: \nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions.\nBackground\nA 24-year-old man with Down syndrome presents to the emergency department with a four-day history of bilateral flank pain, hematuria, and shortness of breath. The patient is accompanied by his mother in the examination room; she gives most of the patient's history. He denies radiation of the flank pain to any other part of his body. He also denies any urinary symptoms, such as dysuria or increased frequency, and he has not experienced any nausea or vomiting.\nThe patient has no history of cough or hemoptysis; fevers, chills, or night sweats; or recent trauma. His bowel and bladder function have been normal. He has a history of bilateral cryptorchidism in childhood, for which he underwent orchiopexy of both undescended testicles at the age of three years. There is no history of recent travel, and the mother reports no recent weight loss. He has no other significant surgical or medical history.\nThe patient does not smoke, drink alcohol, or use illicit drugs intravenously. He is currently not on any medications and does not have any allergies to medications.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 24-Year-Old Man With Down Syndrome and Shortness of Breath"
},
{
"authors": "Dieu-Thu Nguyen-Khoa, MD; Mona Sabeti, MD; Anh H. Au, MD",
"content": [
"Physical Examination and Work-up",
"On physical examination, the patient is noted to be a well-nourished man, with the classic dysmorphic facial features associated with Down syndrome. His oral temperature is 96.9°F, blood pressure is 116/66 mm Hg, and heart rate is 102 beats/min. His respiratory rate is 16 breaths/min, and his oxygen saturation is 92% while breathing room air. He has a normal respiratory effort, and his lung sounds are clear to auscultation bilaterally with deep inspiration.",
"The heart examination is mildly tachycardic, with a regular rhythm; normal S1 and S2 heart sounds; and no murmurs, rubs, or gallops. The patient's abdomen is soft, nondistended, and nontender; no masses or organomegaly is noted. There is no costovertebral angle tenderness on palpation and no evidence of lymphadenopathy. A genital examination and digital rectal examination are deferred. His extremities do not exhibit any clubbing, cyanosis, or edema.",
"Figure 1.",
"The initial laboratory tests show a normal urinalysis, with no evidence of blood or infection. The basic metabolic panel and complete blood count are also unremarkable. A liver panel shows a normal aspartate aminotransferase value of 36 U/L (normal range, 15-41 U/L) and an alanine aminotransferase value of 27 U/L (normal range, 14-54 U/L). There is, however, a mild elevation of alkaline phosphatase, at 257 U/L (normal range, 38-126 U/L) and a low albumin level of 2.9 g/dL (29 g/L; normal range, 3.5-4.8 g/dL). The lactate dehydrogenase (LDH) level is elevated at 1229 U/L (normal range, 98-192 U/L).",
"On coagulation studies, the prothrombin time value is 16.1 sec (normal range, 11.4-14.1 sec) and the international normalized ratio is slightly elevated, at 1.33.",
"A chest radiograph is obtained to further evaluate the patient's dyspnea (Figure 1)."
],
"date": "September 30, 2014",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/832/280/832280-thumb-1.jpg"
}
],
"markdown": "# A 24-Year-Old Man With Down Syndrome and Shortness of Breath\n\n **Authors:** Dieu-Thu Nguyen-Khoa, MD; Mona Sabeti, MD; Anh H. Au, MD \n **Date:** September 30, 2014\n\n ## Content\n\n Physical Examination and Work-up\nOn physical examination, the patient is noted to be a well-nourished man, with the classic dysmorphic facial features associated with Down syndrome. His oral temperature is 96.9°F, blood pressure is 116/66 mm Hg, and heart rate is 102 beats/min. His respiratory rate is 16 breaths/min, and his oxygen saturation is 92% while breathing room air. He has a normal respiratory effort, and his lung sounds are clear to auscultation bilaterally with deep inspiration.\nThe heart examination is mildly tachycardic, with a regular rhythm; normal S1 and S2 heart sounds; and no murmurs, rubs, or gallops. The patient's abdomen is soft, nondistended, and nontender; no masses or organomegaly is noted. There is no costovertebral angle tenderness on palpation and no evidence of lymphadenopathy. A genital examination and digital rectal examination are deferred. His extremities do not exhibit any clubbing, cyanosis, or edema.\nFigure 1.\nThe initial laboratory tests show a normal urinalysis, with no evidence of blood or infection. The basic metabolic panel and complete blood count are also unremarkable. A liver panel shows a normal aspartate aminotransferase value of 36 U/L (normal range, 15-41 U/L) and an alanine aminotransferase value of 27 U/L (normal range, 14-54 U/L). There is, however, a mild elevation of alkaline phosphatase, at 257 U/L (normal range, 38-126 U/L) and a low albumin level of 2.9 g/dL (29 g/L; normal range, 3.5-4.8 g/dL). The lactate dehydrogenase (LDH) level is elevated at 1229 U/L (normal range, 98-192 U/L).\nOn coagulation studies, the prothrombin time value is 16.1 sec (normal range, 11.4-14.1 sec) and the international normalized ratio is slightly elevated, at 1.33.\nA chest radiograph is obtained to further evaluate the patient's dyspnea (Figure 1).\n\n ## Figures\n\n **Figure 1.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 775003,
"choiceText": "Pulmonary sarcoidosis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 775005,
"choiceText": "Bilateral round pneumonias",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 775007,
"choiceText": "Metastatic testicular cancer",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 775009,
"choiceText": "Wegener granulomatosis",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 242755,
"questionText": "What is the diagnosis?<br>\r\n<i>Hint: The past medical history increases the risk of developing the underlying condition.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 24-Year-Old Man With Down Syndrome and Shortness of Breath"
},
{
"authors": "Dieu-Thu Nguyen-Khoa, MD; Mona Sabeti, MD; Anh H. Au, MD",
"content": [
"Discussion",
"The patient's chest radiograph showed bilateral lung masses of varying sizes, including multiple \"cannonball\" lesions (Figure 1).",
"The left lung had a laterally located 9 x 6 cm mass, in addition to a 7-cm mass in the left lung base; a 7.5-cm mass was seen in the right lung. After the patient's chest radiograph was reviewed, metastatic cancer was considered because of the nature of the lesions.",
"Figure 1.",
"Figure 2.",
"Figure 3.",
"A more thorough physical examination was done, and the patient was found to have a firm, nontender mass in the left testicle. Ultrasonography of the testicles was performed and demonstrated a 4.4 x 4.8 x 2.6 cm heterogeneous lobulated mass in the left testicle, with areas of flow to some portions and lack of flow to other portions (Figures 2 and 3).",
"This mass was highly suggestive of testicular cancer. There was also extensive associated microlithiasis and a small hydrocele in the affected testicle. Further laboratory testing revealed elevated levels of serum tumor markers; the beta-human chorionic gonadotropin (hCG) level was 6992 mIU/mL (normal range, < 5 mIU/mL), and the alpha-fetoprotein (AFP) level was 1789 ng/mL (normal range, 0-20 ng/mL). A diagnosis of testicular cancer with lung metastases was established.",
"Testicular cancer is a rapidly progressive cancer that, despite being rare and accounting for only 1% of all types of cancer in men, is still the most common solid tumor in men aged 15-35 years. Testicular cancer must always be considered in any adolescent, young adult, or middle-aged man who presents with a testicular mass. Other risk factors for testicular cancer are family history, gonadal dysgenesis, Klinefelter syndrome, Down syndrome, or a history of cryptorchidism.",
"Orchiopexy does not prevent the occurrence of cancer, but it facilitates diagnosis by allowing the surgically descended testes to be palpated and assessed for masses. Patients who receive orchiopexy after 12 years of age have a higher relative risk for testicular cancer than those who undergo corrective surgery at an earlier age.[1] Men with a history of testicular cancer have an increased risk for cancer in the other testicle up to 25 years after diagnosis. Infertility may be linked with testicular cancer; most men have an abnormal semen analysis at the time of diagnosis.[2,3]",
"The most common presentation in patients with testicular cancer is a painless lump felt in the testicle by the man or his partner. Unilateral testicular enlargement can also be a sign of testicular cancer. The patient may have nonspecific back pain at initial presentation caused by retroperitoneal lymphadenopathy. If lung metastases have developed, the patient may present with dyspnea or hemoptysis. Other signs and symptoms, such as testicular redness, swelling, discomfort, and pain, can occur as well. Other testicular conditions, such as epididymitis, hematocele, and varicocele, have been noted to have a similar presentation as testicular cancer.[3]"
],
"date": "September 30, 2014",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/832/280/832280-thumb-1.jpg"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/832/280/832280-thumb-2.jpg"
},
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/832/280/832280-thumb-3.jpg"
}
],
"markdown": "# A 24-Year-Old Man With Down Syndrome and Shortness of Breath\n\n **Authors:** Dieu-Thu Nguyen-Khoa, MD; Mona Sabeti, MD; Anh H. Au, MD \n **Date:** September 30, 2014\n\n ## Content\n\n Discussion\nThe patient's chest radiograph showed bilateral lung masses of varying sizes, including multiple \"cannonball\" lesions (Figure 1).\nThe left lung had a laterally located 9 x 6 cm mass, in addition to a 7-cm mass in the left lung base; a 7.5-cm mass was seen in the right lung. After the patient's chest radiograph was reviewed, metastatic cancer was considered because of the nature of the lesions.\nFigure 1.\nFigure 2.\nFigure 3.\nA more thorough physical examination was done, and the patient was found to have a firm, nontender mass in the left testicle. Ultrasonography of the testicles was performed and demonstrated a 4.4 x 4.8 x 2.6 cm heterogeneous lobulated mass in the left testicle, with areas of flow to some portions and lack of flow to other portions (Figures 2 and 3).\nThis mass was highly suggestive of testicular cancer. There was also extensive associated microlithiasis and a small hydrocele in the affected testicle. Further laboratory testing revealed elevated levels of serum tumor markers; the beta-human chorionic gonadotropin (hCG) level was 6992 mIU/mL (normal range, < 5 mIU/mL), and the alpha-fetoprotein (AFP) level was 1789 ng/mL (normal range, 0-20 ng/mL). A diagnosis of testicular cancer with lung metastases was established.\nTesticular cancer is a rapidly progressive cancer that, despite being rare and accounting for only 1% of all types of cancer in men, is still the most common solid tumor in men aged 15-35 years. Testicular cancer must always be considered in any adolescent, young adult, or middle-aged man who presents with a testicular mass. Other risk factors for testicular cancer are family history, gonadal dysgenesis, Klinefelter syndrome, Down syndrome, or a history of cryptorchidism.\nOrchiopexy does not prevent the occurrence of cancer, but it facilitates diagnosis by allowing the surgically descended testes to be palpated and assessed for masses. Patients who receive orchiopexy after 12 years of age have a higher relative risk for testicular cancer than those who undergo corrective surgery at an earlier age.[1] Men with a history of testicular cancer have an increased risk for cancer in the other testicle up to 25 years after diagnosis. Infertility may be linked with testicular cancer; most men have an abnormal semen analysis at the time of diagnosis.[2,3]\nThe most common presentation in patients with testicular cancer is a painless lump felt in the testicle by the man or his partner. Unilateral testicular enlargement can also be a sign of testicular cancer. The patient may have nonspecific back pain at initial presentation caused by retroperitoneal lymphadenopathy. If lung metastases have developed, the patient may present with dyspnea or hemoptysis. Other signs and symptoms, such as testicular redness, swelling, discomfort, and pain, can occur as well. Other testicular conditions, such as epididymitis, hematocele, and varicocele, have been noted to have a similar presentation as testicular cancer.[3]\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n**Figure 3.** \n \n\n\n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 775003,
"choiceText": "Pulmonary sarcoidosis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 775005,
"choiceText": "Bilateral round pneumonias",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 775007,
"choiceText": "Metastatic testicular cancer",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 775009,
"choiceText": "Wegener granulomatosis",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 242755,
"questionText": "What is the diagnosis?<br>\r\n<i>Hint: The past medical history increases the risk of developing the underlying condition.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 24-Year-Old Man With Down Syndrome and Shortness of Breath"
},
{
"authors": "Dieu-Thu Nguyen-Khoa, MD; Mona Sabeti, MD; Anh H. Au, MD",
"content": [
"Ultrasonography of the testicles is the diagnostic modality of choice and can characterize the lesion and specifically identify the presence of a mass. If the clinical course, history, and symptoms (eg, acute onset, sexual history, fever, chills, nausea) are suggestive of orchitis, a trial of antibiotics (such as ceftriaxone and azithromycin, which are focused on sexually transmitted diseases, for patients aged < 35 years, or fluoroquinolone, which is focused on enteric organisms, for patients aged ≥ 35 years) can be attempted, with a plan for close follow-up to assess for resolution of symptoms or the need for additional work-up. Testicular masses that are suspicious for cancer need to be further evaluated with testicular ultrasonography and measurement of serum tumor markers (beta-hCG, LDH, AFP), which will be elevated in the setting of testicular cancer.",
"If ultrasonography and tumor markers suggest cancer, then orchiectomy is indicated, with subsequent histopathologic evaluation. Cases that are ambiguous or cannot otherwise be resolved by ultrasonography may require MRI for further evaluation. CT of the chest, abdomen, and pelvis is also recommended (especially if dyspnea or back pain is present) to investigate for metastases and for staging of the disease.",
"Serum tumor markers are helpful because they can also disclose cancers that are too small to detect on the physical examination. Serum tumor markers are essential not only for diagnosis but also for prognosis and assessment of disease response to chemotherapy. High levels of serum tumor markers indicate an increased likelihood for metastatic disease and a poor prognosis. These tumor marker levels should decrease with therapy; failure to do so usually indicates a resistance to therapy. Similarly, a resurgence of these markers can signify relapse of cancer after cessation of treatment.[3,4]",
"Testicular cancers are almost always germ cell tumors. Other types of cancers, such as lymphoma, Leydig cell carcinoma, and Sertoli cell carcinoma, are very rare.",
"Germ cell tumors are further categorized into seminomas and nonseminomas. Germ cell tumors that consist of only seminomas are called \"pure seminomas\"; nonseminomas consist of embryonal carcinomas, yolk sac tumors (also called \"endodermal sinus tumors\"), choriocarcinomas, and teratomas. Mixed germ cell tumors can contain any combination of nonseminomas and seminomas; however, if a tumor has a portion of any of the nonseminomatous cell lines in it, it is considered a nonseminoma because the nonseminomatous component most accurately reflects the response to treatment and overall prognosis.",
"Different tumor markers are associated with different types of testicular cancers. High serum AFP levels are not usually seen in pure seminomas. Serum beta-hCG can be elevated in both seminomas and nonseminomas. Tumors with seminoma in the histopathology and an elevated AFP should be treated as nonseminomas.[3]",
"The prognosis of most testicular cancers is excellent, even when metastatic disease is present. The para-aortic lymph nodes are the initial site of metastasis. Patients with CT scans that are negative for lymphadenopathy have a 25%-30% chance of having microscopic involvement of the lymph nodes. Nonseminomatous germ cell tumors are more likely than seminomas to spread hematogenously; the most common sites of hematogenous spread are the lungs, liver, and brain, as well as the para-aortic lymph nodes. Teratomas are the most benign form of nonseminoma, and they are the least likely to metastasize. Choriocarcinomas, which are the least differentiated, are most likely to metastasize. All other nonseminomas have an intermediate likelihood for metastasis.[2,3,5]"
],
"date": "September 30, 2014",
"figures": [],
"markdown": "# A 24-Year-Old Man With Down Syndrome and Shortness of Breath\n\n **Authors:** Dieu-Thu Nguyen-Khoa, MD; Mona Sabeti, MD; Anh H. Au, MD \n **Date:** September 30, 2014\n\n ## Content\n\n Ultrasonography of the testicles is the diagnostic modality of choice and can characterize the lesion and specifically identify the presence of a mass. If the clinical course, history, and symptoms (eg, acute onset, sexual history, fever, chills, nausea) are suggestive of orchitis, a trial of antibiotics (such as ceftriaxone and azithromycin, which are focused on sexually transmitted diseases, for patients aged < 35 years, or fluoroquinolone, which is focused on enteric organisms, for patients aged ≥ 35 years) can be attempted, with a plan for close follow-up to assess for resolution of symptoms or the need for additional work-up. Testicular masses that are suspicious for cancer need to be further evaluated with testicular ultrasonography and measurement of serum tumor markers (beta-hCG, LDH, AFP), which will be elevated in the setting of testicular cancer.\nIf ultrasonography and tumor markers suggest cancer, then orchiectomy is indicated, with subsequent histopathologic evaluation. Cases that are ambiguous or cannot otherwise be resolved by ultrasonography may require MRI for further evaluation. CT of the chest, abdomen, and pelvis is also recommended (especially if dyspnea or back pain is present) to investigate for metastases and for staging of the disease.\nSerum tumor markers are helpful because they can also disclose cancers that are too small to detect on the physical examination. Serum tumor markers are essential not only for diagnosis but also for prognosis and assessment of disease response to chemotherapy. High levels of serum tumor markers indicate an increased likelihood for metastatic disease and a poor prognosis. These tumor marker levels should decrease with therapy; failure to do so usually indicates a resistance to therapy. Similarly, a resurgence of these markers can signify relapse of cancer after cessation of treatment.[3,4]\nTesticular cancers are almost always germ cell tumors. Other types of cancers, such as lymphoma, Leydig cell carcinoma, and Sertoli cell carcinoma, are very rare.\nGerm cell tumors are further categorized into seminomas and nonseminomas. Germ cell tumors that consist of only seminomas are called \"pure seminomas\"; nonseminomas consist of embryonal carcinomas, yolk sac tumors (also called \"endodermal sinus tumors\"), choriocarcinomas, and teratomas. Mixed germ cell tumors can contain any combination of nonseminomas and seminomas; however, if a tumor has a portion of any of the nonseminomatous cell lines in it, it is considered a nonseminoma because the nonseminomatous component most accurately reflects the response to treatment and overall prognosis.\nDifferent tumor markers are associated with different types of testicular cancers. High serum AFP levels are not usually seen in pure seminomas. Serum beta-hCG can be elevated in both seminomas and nonseminomas. Tumors with seminoma in the histopathology and an elevated AFP should be treated as nonseminomas.[3]\nThe prognosis of most testicular cancers is excellent, even when metastatic disease is present. The para-aortic lymph nodes are the initial site of metastasis. Patients with CT scans that are negative for lymphadenopathy have a 25%-30% chance of having microscopic involvement of the lymph nodes. Nonseminomatous germ cell tumors are more likely than seminomas to spread hematogenously; the most common sites of hematogenous spread are the lungs, liver, and brain, as well as the para-aortic lymph nodes. Teratomas are the most benign form of nonseminoma, and they are the least likely to metastasize. Choriocarcinomas, which are the least differentiated, are most likely to metastasize. All other nonseminomas have an intermediate likelihood for metastasis.[2,3,5]\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 24-Year-Old Man With Down Syndrome and Shortness of Breath"
},
{
"authors": "Dieu-Thu Nguyen-Khoa, MD; Mona Sabeti, MD; Anh H. Au, MD",
"content": [
"Treatment for both seminomas and nonseminomas is stage-dependent. Standard treatment for most nonseminomas is orchiectomy, potentially followed by chemotherapy with bleomycin, etoposide, and cisplatin. Retroperitoneal lymph node dissection is also a consideration in selected patients. Nonseminomas vary in prognosis, depending on the stage and the tumor marker levels at diagnosis; 56%-61% of nonseminomas have a good prognosis, with a five-year survival rate of 92%-94%. Even nonseminomas considered to have a poor prognosis, which account for 16%-26% of tumors, have a five-year survival rate of 71%.",
"Seminomas are initially treated with orchiectomy. Additional treatment with chemotherapy, radiation, or active surveillance is guided by stage and prognostic features at the time of diagnosis. Seminomas have an overall better prognosis when stage-matched with nonseminomas. Patients with seminomas are never categorized as having poor prognoses. Seminomas that are diagnosed in the early stages have an almost 100% cure rate, and seminomas of any stage have a cure rate of more than 90%.[2,3,4]",
"Most tumors recur within the first two years after treatment. Close interval follow-up with serum markers and imaging studies is recommended. The follow-up interval varies, depending on whether the tumor was seminoma or nonseminoma.[6] Some cases of later relapse are reported; lifelong periodic physical, radiologic, and laboratory examinations for these patients is reasonable.[3]",
"Figure 4.",
"After this patient was admitted to the hospital for further evaluation, he underwent orchiectomy of his left testicle. Histopathology demonstrated a stage III nonseminomatous testicular cancer, specifically a mixed germ cell tumor consisting of embryonal carcinoma and yolk sac tumor. There was extensive necrosis and lymphovascular invasion of cancer within the left testicle. CT of the abdomen showed significant retroperitoneal lymphadenopathy that was probably causing his back pain.",
"The patient was treated with multiple cycles of bleomycin, etoposide, and cisplatin chemotherapy. After treatment, a repeat chest radiograph (Figure 4) and CT (not available) demonstrated near-complete resolution of his lung metastases and no residual retroperitoneal lymphadenopathy.",
"At the follow-up examination, the patient's serum tumor markers were noted to have improved, with an AFP of 6 ng/mL (normal range, 0-20 ng/mL), a beta-hCG < 2 mIU/mL (normal range, < 5 mIU/mL), and an LDH of 151 U/L (normal range, 98-192 U/L)."
],
"date": "September 30, 2014",
"figures": [
{
"caption": "Figure 4.",
"image_url": "https://img.medscapestatic.com/article/832/280/832280-thumb-4.jpg"
}
],
"markdown": "# A 24-Year-Old Man With Down Syndrome and Shortness of Breath\n\n **Authors:** Dieu-Thu Nguyen-Khoa, MD; Mona Sabeti, MD; Anh H. Au, MD \n **Date:** September 30, 2014\n\n ## Content\n\n Treatment for both seminomas and nonseminomas is stage-dependent. Standard treatment for most nonseminomas is orchiectomy, potentially followed by chemotherapy with bleomycin, etoposide, and cisplatin. Retroperitoneal lymph node dissection is also a consideration in selected patients. Nonseminomas vary in prognosis, depending on the stage and the tumor marker levels at diagnosis; 56%-61% of nonseminomas have a good prognosis, with a five-year survival rate of 92%-94%. Even nonseminomas considered to have a poor prognosis, which account for 16%-26% of tumors, have a five-year survival rate of 71%.\nSeminomas are initially treated with orchiectomy. Additional treatment with chemotherapy, radiation, or active surveillance is guided by stage and prognostic features at the time of diagnosis. Seminomas have an overall better prognosis when stage-matched with nonseminomas. Patients with seminomas are never categorized as having poor prognoses. Seminomas that are diagnosed in the early stages have an almost 100% cure rate, and seminomas of any stage have a cure rate of more than 90%.[2,3,4]\nMost tumors recur within the first two years after treatment. Close interval follow-up with serum markers and imaging studies is recommended. The follow-up interval varies, depending on whether the tumor was seminoma or nonseminoma.[6] Some cases of later relapse are reported; lifelong periodic physical, radiologic, and laboratory examinations for these patients is reasonable.[3]\nFigure 4.\nAfter this patient was admitted to the hospital for further evaluation, he underwent orchiectomy of his left testicle. Histopathology demonstrated a stage III nonseminomatous testicular cancer, specifically a mixed germ cell tumor consisting of embryonal carcinoma and yolk sac tumor. There was extensive necrosis and lymphovascular invasion of cancer within the left testicle. CT of the abdomen showed significant retroperitoneal lymphadenopathy that was probably causing his back pain.\nThe patient was treated with multiple cycles of bleomycin, etoposide, and cisplatin chemotherapy. After treatment, a repeat chest radiograph (Figure 4) and CT (not available) demonstrated near-complete resolution of his lung metastases and no residual retroperitoneal lymphadenopathy.\nAt the follow-up examination, the patient's serum tumor markers were noted to have improved, with an AFP of 6 ng/mL (normal range, 0-20 ng/mL), a beta-hCG < 2 mIU/mL (normal range, < 5 mIU/mL), and an LDH of 151 U/L (normal range, 98-192 U/L).\n\n ## Figures\n\n **Figure 4.** \n \n\n\n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 775011,
"choiceText": "Urinalysis and complete blood count",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 775013,
"choiceText": "Ultrasonography and serum testing for tumor markers",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 775015,
"choiceText": "Plain radiography",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 775017,
"choiceText": "PET",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 242757,
"questionText": "You are examining a 24-year-old man who presents with unilateral scrotal swelling. Palpation of the testicles reveals a small, painless mass. Which of the following examinations should you use to definitively establish a diagnosis of testicular cancer in this patient?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 775019,
"choiceText": "Orchiectomy and chemotherapy",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 775021,
"choiceText": "Chemotherapy alone",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 775023,
"choiceText": "Orchiectomy and radiation therapy",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 775025,
"choiceText": "Orchiectomy, chemotherapy, and radiation therapy",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 775027,
"choiceText": "None of the above",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 242759,
"questionText": "Further examination of the above-described patient reveals a pure seminoma. On the basis of this finding, what treatment course would you pursue for this patient?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 24-Year-Old Man With Down Syndrome and Shortness of Breath"
},
{
"authors": "Dieu-Thu Nguyen-Khoa, MD; Mona Sabeti, MD; Anh H. Au, MD",
"content": [
"For more information on testicular cancer, see the Medscape Drugs & Diseases articles Testicular Cancer, Nonseminomatous Testicular Tumors, and Testicular Seminoma."
],
"date": "September 30, 2014",
"figures": [],
"markdown": "# A 24-Year-Old Man With Down Syndrome and Shortness of Breath\n\n **Authors:** Dieu-Thu Nguyen-Khoa, MD; Mona Sabeti, MD; Anh H. Au, MD \n **Date:** September 30, 2014\n\n ## Content\n\n For more information on testicular cancer, see the Medscape Drugs & Diseases articles Testicular Cancer, Nonseminomatous Testicular Tumors, and Testicular Seminoma.\n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 775011,
"choiceText": "Urinalysis and complete blood count",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 775013,
"choiceText": "Ultrasonography and serum testing for tumor markers",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 775015,
"choiceText": "Plain radiography",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 775017,
"choiceText": "PET",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 242757,
"questionText": "You are examining a 24-year-old man who presents with unilateral scrotal swelling. Palpation of the testicles reveals a small, painless mass. Which of the following examinations should you use to definitively establish a diagnosis of testicular cancer in this patient?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 775019,
"choiceText": "Orchiectomy and chemotherapy",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 775021,
"choiceText": "Chemotherapy alone",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 775023,
"choiceText": "Orchiectomy and radiation therapy",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 775025,
"choiceText": "Orchiectomy, chemotherapy, and radiation therapy",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 775027,
"choiceText": "None of the above",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 242759,
"questionText": "Further examination of the above-described patient reveals a pure seminoma. On the basis of this finding, what treatment course would you pursue for this patient?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 24-Year-Old Man With Down Syndrome and Shortness of Breath"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 775003,
"choiceText": "Pulmonary sarcoidosis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 775005,
"choiceText": "Bilateral round pneumonias",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 775007,
"choiceText": "Metastatic testicular cancer",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 775009,
"choiceText": "Wegener granulomatosis",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 242755,
"questionText": "What is the diagnosis?<br>\r\n<i>Hint: The past medical history increases the risk of developing the underlying condition.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 775011,
"choiceText": "Urinalysis and complete blood count",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 775013,
"choiceText": "Ultrasonography and serum testing for tumor markers",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 775015,
"choiceText": "Plain radiography",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 775017,
"choiceText": "PET",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 242757,
"questionText": "You are examining a 24-year-old man who presents with unilateral scrotal swelling. Palpation of the testicles reveals a small, painless mass. Which of the following examinations should you use to definitively establish a diagnosis of testicular cancer in this patient?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 775019,
"choiceText": "Orchiectomy and chemotherapy",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 775021,
"choiceText": "Chemotherapy alone",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 775023,
"choiceText": "Orchiectomy and radiation therapy",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 775025,
"choiceText": "Orchiectomy, chemotherapy, and radiation therapy",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 775027,
"choiceText": "None of the above",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 242759,
"questionText": "Further examination of the above-described patient reveals a pure seminoma. On the basis of this finding, what treatment course would you pursue for this patient?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
831294 | /viewarticle/831294 | [
{
"authors": "Koyamangalath Krishnan, MD",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"Background",
"A 52-year-old man presents to the emergency department with generalized fatigue and weakness over the past couple of months. He explains that the symptoms are becoming progressively worse, with decreased exercise tolerance. He also reports night sweats and a 20-lb weight loss over the past 6 months. He describes a dull heaviness in abdomen on the left side and early satiety. He denies having cough, chest pain, nausea, vomiting, headaches, dysphagia, rashes, lumps, hemoptysis, hematuria, or blood in stools.",
"The patient's bowel and bladder habits are normal. His medical history is unremarkable other than hypertension. He has had no surgeries in the past. He has a 15-pack-year smoking history but quit 10 years ago. He drinks occasional wine and denies illicit drug use. His home medications include lisinopril and acetaminophen as needed."
],
"date": "December 11, 2017",
"figures": [],
"markdown": "# A 52-Year-Old Man With Fatigue, Leukocytosis, and Moderate Splenomegaly\n\n **Authors:** Koyamangalath Krishnan, MD \n **Date:** December 11, 2017\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nBackground\nA 52-year-old man presents to the emergency department with generalized fatigue and weakness over the past couple of months. He explains that the symptoms are becoming progressively worse, with decreased exercise tolerance. He also reports night sweats and a 20-lb weight loss over the past 6 months. He describes a dull heaviness in abdomen on the left side and early satiety. He denies having cough, chest pain, nausea, vomiting, headaches, dysphagia, rashes, lumps, hemoptysis, hematuria, or blood in stools.\nThe patient's bowel and bladder habits are normal. His medical history is unremarkable other than hypertension. He has had no surgeries in the past. He has a 15-pack-year smoking history but quit 10 years ago. He drinks occasional wine and denies illicit drug use. His home medications include lisinopril and acetaminophen as needed.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 52-Year-Old Man With Fatigue, Leukocytosis, and Moderate Splenomegaly"
},
{
"authors": "Koyamangalath Krishnan, MD",
"content": [
"Physical Examination and Work-up",
"The physical examination reveals that his oral temperature is 98.8°F, pulse is 80 beats/min, blood pressure is 144/80 mm Hg, respiratory rate is 16 breaths/min, and oxygen saturation is 97% on room air. He is alert and oriented to person, place, and time and not in distress. Head and neck examination findings are normal. Examination reveals a palpable spleen about 7 cm below the left costal margin. The remainder of the systemic examination findings are normal.",
"Laboratory tests reveal a complete blood count (CBC) with a white blood cell (WBC) count of 42,000 cells/µL, hemoglobin level of 10.8 g/dL, hematocrit of 32.6%, and a platelet count of 140,000 cells/µL. The differential count reveals neutrophils at 43%, lymphocytes at 13%, monocytes at 6%, eosinophils at 6%, basophils at 12%, and bands at 20%. Chemistry panel findings are within normal limits. The lactate dehydrogenase level is 690 IU/L, the phosphorus level is 4.8 mg/dL, and the uric acid level is 8.7 mg/dL. Coagulation panel findings are within normal limits. Urine analysis findings are negative.",
"Peripheral smear and bone marrow findings are shown in figures 1 and 2.",
"Figure 1.",
"Figure 2."
],
"date": "December 11, 2017",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/831/294/831294-thumb-1.jpg"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/831/294/831294-thumb-2.jpg"
}
],
"markdown": "# A 52-Year-Old Man With Fatigue, Leukocytosis, and Moderate Splenomegaly\n\n **Authors:** Koyamangalath Krishnan, MD \n **Date:** December 11, 2017\n\n ## Content\n\n Physical Examination and Work-up\nThe physical examination reveals that his oral temperature is 98.8°F, pulse is 80 beats/min, blood pressure is 144/80 mm Hg, respiratory rate is 16 breaths/min, and oxygen saturation is 97% on room air. He is alert and oriented to person, place, and time and not in distress. Head and neck examination findings are normal. Examination reveals a palpable spleen about 7 cm below the left costal margin. The remainder of the systemic examination findings are normal.\nLaboratory tests reveal a complete blood count (CBC) with a white blood cell (WBC) count of 42,000 cells/µL, hemoglobin level of 10.8 g/dL, hematocrit of 32.6%, and a platelet count of 140,000 cells/µL. The differential count reveals neutrophils at 43%, lymphocytes at 13%, monocytes at 6%, eosinophils at 6%, basophils at 12%, and bands at 20%. Chemistry panel findings are within normal limits. The lactate dehydrogenase level is 690 IU/L, the phosphorus level is 4.8 mg/dL, and the uric acid level is 8.7 mg/dL. Coagulation panel findings are within normal limits. Urine analysis findings are negative.\nPeripheral smear and bone marrow findings are shown in figures 1 and 2.\nFigure 1.\nFigure 2.\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 767341,
"choiceText": "Essential thrombocythemia",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 767343,
"choiceText": "Splenic marginal zone lymphoma",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 767345,
"choiceText": "Chronic myeloid leukemia",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 767347,
"choiceText": "Myelofibrosis",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 767349,
"choiceText": "Acute leukemia",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 240309,
"questionText": "On the basis of history, physical examination, and work-up, what is the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 52-Year-Old Man With Fatigue, Leukocytosis, and Moderate Splenomegaly"
},
{
"authors": "Koyamangalath Krishnan, MD",
"content": [
"Discussion",
"This patient presents with constitutional symptoms reflective of a hypercatabolic state, with additional symptoms related to splenomegaly (abdominal fullness and early satiety). This is typical of the chronic phase of chronic myelogenous leukemia (CML). Both splenomegaly and painless hepatomegaly can be features at presentation. Less often, patients can present with thrombotic and hemorrhagic complications. Males with leukostasis related to high WBC count can present with priapism.",
"Other disorders that can cause splenomegaly should also be considered in the differential diagnosis and include myeloproliferative disorders, such as polycythemia vera, essential thrombocythemia, and idiopathic myelofibrosis, and overlap syndromes, especially CML. The diagnosis is confirmed by a peripheral smear review in conjunction with a bone marrow examination and cytogenetic and molecular studies.[1,2]",
"Most patients with CML present in the chronic phase. Some patients are asymptomatic and are found to have incidental leukocytosis, which on further molecular testing bears the molecular signature of CML.",
"Occasionally, patients present with the accelerated or blastic phase of CML. The features that suggest accelerated-phase CML include progressive splenomegaly, presence of splenic infarcts, significant weight loss, unexplained fever, or bone pain. The features that indicate blastic-phase CML include bleeding, bruising, purpura, infections, prominent constitutional symptoms, massive splenomegaly, and extramedullary disease.",
"The diagnosis of chronic-phase CML was confirmed in this patient by bone marrow morphology and molecular studies that included bone marrow cytogenetics and fluorescence in situ hybridization (FISH) analysis in addition to routine morphology. Peripheral blood polymerase chain reaction (PCR) for the BCR-ABL fusion transcript was also performed to obtain a baseline for treatment monitoring.",
"The most efficient way to confirm the diagnosis of CML is to assay the peripheral blood for the BCR-ABL fusion gene either by FISH or reverse-transcriptase (RT) PCR. Both techniques can identify cryptic translocations that can be missed by conventional cytogenetic analysis. RT-PCR is the preferred molecular assay for peripheral blood because it can differentiate whether the fusion product is the p210 BCR-ABL or the p190 BCR-ABL transcript. In addition, RT-PCR provides a method to monitor for minimal residual disease in patients who are started on tyrosine kinase inhibitor therapy.[1]",
"In classic chronic-phase CML, the Philadelphia chromosome is detectable using cytogenetics assay, and the 9;22 translocation is detectable using FISH analysis. The BCR-ABL fusion transcript can also be identified in this setting. In a small percentage of patients, the cytogenetics assay may be normal but the fusion transcript is found on RT-PCR; this is so-called Ph-negative BCR-ABL CML. The course of this variant is the same as the typical disease."
],
"date": "December 11, 2017",
"figures": [],
"markdown": "# A 52-Year-Old Man With Fatigue, Leukocytosis, and Moderate Splenomegaly\n\n **Authors:** Koyamangalath Krishnan, MD \n **Date:** December 11, 2017\n\n ## Content\n\n Discussion\nThis patient presents with constitutional symptoms reflective of a hypercatabolic state, with additional symptoms related to splenomegaly (abdominal fullness and early satiety). This is typical of the chronic phase of chronic myelogenous leukemia (CML). Both splenomegaly and painless hepatomegaly can be features at presentation. Less often, patients can present with thrombotic and hemorrhagic complications. Males with leukostasis related to high WBC count can present with priapism.\nOther disorders that can cause splenomegaly should also be considered in the differential diagnosis and include myeloproliferative disorders, such as polycythemia vera, essential thrombocythemia, and idiopathic myelofibrosis, and overlap syndromes, especially CML. The diagnosis is confirmed by a peripheral smear review in conjunction with a bone marrow examination and cytogenetic and molecular studies.[1,2]\nMost patients with CML present in the chronic phase. Some patients are asymptomatic and are found to have incidental leukocytosis, which on further molecular testing bears the molecular signature of CML.\nOccasionally, patients present with the accelerated or blastic phase of CML. The features that suggest accelerated-phase CML include progressive splenomegaly, presence of splenic infarcts, significant weight loss, unexplained fever, or bone pain. The features that indicate blastic-phase CML include bleeding, bruising, purpura, infections, prominent constitutional symptoms, massive splenomegaly, and extramedullary disease.\nThe diagnosis of chronic-phase CML was confirmed in this patient by bone marrow morphology and molecular studies that included bone marrow cytogenetics and fluorescence in situ hybridization (FISH) analysis in addition to routine morphology. Peripheral blood polymerase chain reaction (PCR) for the BCR-ABL fusion transcript was also performed to obtain a baseline for treatment monitoring.\nThe most efficient way to confirm the diagnosis of CML is to assay the peripheral blood for the BCR-ABL fusion gene either by FISH or reverse-transcriptase (RT) PCR. Both techniques can identify cryptic translocations that can be missed by conventional cytogenetic analysis. RT-PCR is the preferred molecular assay for peripheral blood because it can differentiate whether the fusion product is the p210 BCR-ABL or the p190 BCR-ABL transcript. In addition, RT-PCR provides a method to monitor for minimal residual disease in patients who are started on tyrosine kinase inhibitor therapy.[1]\nIn classic chronic-phase CML, the Philadelphia chromosome is detectable using cytogenetics assay, and the 9;22 translocation is detectable using FISH analysis. The BCR-ABL fusion transcript can also be identified in this setting. In a small percentage of patients, the cytogenetics assay may be normal but the fusion transcript is found on RT-PCR; this is so-called Ph-negative BCR-ABL CML. The course of this variant is the same as the typical disease.\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 767341,
"choiceText": "Essential thrombocythemia",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 767343,
"choiceText": "Splenic marginal zone lymphoma",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 767345,
"choiceText": "Chronic myeloid leukemia",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 767347,
"choiceText": "Myelofibrosis",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 767349,
"choiceText": "Acute leukemia",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 240309,
"questionText": "On the basis of history, physical examination, and work-up, what is the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 52-Year-Old Man With Fatigue, Leukocytosis, and Moderate Splenomegaly"
},
{
"authors": "Koyamangalath Krishnan, MD",
"content": [
"Laboratory features also help in the identification of accelerated- and blastic-phase disease. The signature hematopathologic indicator of the blastic phase is a blood or marrow blast percentage greater than 20%.",
"The following are recommended in the work-up for a patient suspected of having CML[2]:",
"History and physical examination, including spleen size",
"CBC with differential and platelet count",
"Chemistry profile",
"Human leukocyte antigen testing (if allogenic hematopoietic stem cell transplantation is being considered)",
"Bone marrow aspiration and biopsy",
"Percentage of blasts",
"Percentage of basophils",
"Bone marrow cytogenetic analysis",
"FISH studies",
"Quantitative RT-PCR using the international scale on peripheral blood or bone marrow (peripheral blood is preferred because the test needs to be repeated during the course of treatment and offers a baseline)",
"Risk score using either the Sokal index or Hasford score",
"Two prognostic scoring systems are used: the Sokal index[3] and the Hasford score.[4] Although these scores were tested in the pre-tyrosine kinase inhibitor era, they are still applicable. Both systems include the percentage of circulating blasts, spleen size, platelet count, and age. In addition, the Sokal index includes cytogenetic clonal evolution, whereas the Hasford score includes percentage of eosinophils and basophils instead. The systems identify low-, intermediate-, and high-risk CML on the basis of the above factors.",
"Some disorders that cause leukocytosis and splenomegaly are Ph-negative and BCR-ABL negative, are not typical CML, and do not respond to tyrosine kinase inhibitors. Atypical CML is such a disorder. A second mimic is chronic neutrophilic leukemia, which is BCR-ABL negative; it needs to be differentiated from neutrophilic CML, which is a rare variant of CML characterized by the presence of a p230 BCR-ABL fusion transcript.",
"The incidence of CML is 1-2 cases per 100,000 persons, with a slight male preponderance. The median age is 50-60 years. CML has been reported in children aged 10-14 years, which accounts for 2% of childhood leukemia. Etiologic studies have excluded an association between CML and exposure to solvents, pesticides, or alkylating agents. Radiation may be causative (eg, Chernobyl nuclear fallout).[1,5]",
"Tyrosine kinase inhibitors have radically transformed the treatment landscape and survival in CML.[1,6] Most patients with CML now remain in clinical or cytogenetic remission for 5-7 years or longer. In select patients, allogeneic stem cell transplantation offers a chance of cure.",
"Available treatment options include the following:",
"Tyrosine kinase inhibitors",
"Allogeneic stem cell transplantation",
"Clinical trial enrollment"
],
"date": "December 11, 2017",
"figures": [],
"markdown": "# A 52-Year-Old Man With Fatigue, Leukocytosis, and Moderate Splenomegaly\n\n **Authors:** Koyamangalath Krishnan, MD \n **Date:** December 11, 2017\n\n ## Content\n\n Laboratory features also help in the identification of accelerated- and blastic-phase disease. The signature hematopathologic indicator of the blastic phase is a blood or marrow blast percentage greater than 20%.\nThe following are recommended in the work-up for a patient suspected of having CML[2]:\nHistory and physical examination, including spleen size\nCBC with differential and platelet count\nChemistry profile\nHuman leukocyte antigen testing (if allogenic hematopoietic stem cell transplantation is being considered)\nBone marrow aspiration and biopsy\nPercentage of blasts\nPercentage of basophils\nBone marrow cytogenetic analysis\nFISH studies\nQuantitative RT-PCR using the international scale on peripheral blood or bone marrow (peripheral blood is preferred because the test needs to be repeated during the course of treatment and offers a baseline)\nRisk score using either the Sokal index or Hasford score\nTwo prognostic scoring systems are used: the Sokal index[3] and the Hasford score.[4] Although these scores were tested in the pre-tyrosine kinase inhibitor era, they are still applicable. Both systems include the percentage of circulating blasts, spleen size, platelet count, and age. In addition, the Sokal index includes cytogenetic clonal evolution, whereas the Hasford score includes percentage of eosinophils and basophils instead. The systems identify low-, intermediate-, and high-risk CML on the basis of the above factors.\nSome disorders that cause leukocytosis and splenomegaly are Ph-negative and BCR-ABL negative, are not typical CML, and do not respond to tyrosine kinase inhibitors. Atypical CML is such a disorder. A second mimic is chronic neutrophilic leukemia, which is BCR-ABL negative; it needs to be differentiated from neutrophilic CML, which is a rare variant of CML characterized by the presence of a p230 BCR-ABL fusion transcript.\nThe incidence of CML is 1-2 cases per 100,000 persons, with a slight male preponderance. The median age is 50-60 years. CML has been reported in children aged 10-14 years, which accounts for 2% of childhood leukemia. Etiologic studies have excluded an association between CML and exposure to solvents, pesticides, or alkylating agents. Radiation may be causative (eg, Chernobyl nuclear fallout).[1,5]\nTyrosine kinase inhibitors have radically transformed the treatment landscape and survival in CML.[1,6] Most patients with CML now remain in clinical or cytogenetic remission for 5-7 years or longer. In select patients, allogeneic stem cell transplantation offers a chance of cure.\nAvailable treatment options include the following:\nTyrosine kinase inhibitors\nAllogeneic stem cell transplantation\nClinical trial enrollment\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 52-Year-Old Man With Fatigue, Leukocytosis, and Moderate Splenomegaly"
},
{
"authors": "Koyamangalath Krishnan, MD",
"content": [
"The treatment of choice in this otherwise reasonably healthy 52-year-old man is tyrosine kinase inhibitors. Options include the first-generation tyrosine kinase inhibitor imatinib mesylate, or a second-generation inhibitor (dasatinib or nilotinib).",
"The National Comprehensive Cancer Network recommends imatinib, dasatinib, and nilotinib as category 1 medications for chronic-phase CML. Imatinib has the longest survival data (>8 years) on the basis of the IRIS trial.[7,8]",
"Second-generation tyrosine kinase inhibitors have only 36-48 months of data, and long-term survival data are lacking. Molecular remissions are achieved earlier with the second-generation inhibitors[9] and can be considered preferentially in patients with intermediate- to high-risk prognostic scores on the Sokal or Hasford system. For patients who cannot tolerate imatinib, dasatinib, or nilotinib, options include bosutinib, interferon, or stem cell transplantation.[10]",
"On the basis of previous studies, milestones have been identified that help in the management of patients receiving tyrosine kinase inhibitors. These milestones help with ascertaining timeliness in responses and in determining when resistance or failure to respond has occurred.",
"The most important determinant of prognosis is response to therapy with tyrosine kinase inhibitors. Patients with CML treated with these inhibitors achieve 3 types of responses: hematologic, cytogenetic, and molecular. The milestones explain when such responses should occur.",
"With imatinib therapy, best outcomes are predicted on achieving a complete hematologic response at 3 months, at least a major cytogenetic response at 12 months, and a complete cytogenetic response at 18 months. \"Major molecular response\" refers to a more than 3-log reduction in the BCR/ABL transcript number on RT-PCR analysis. More than 50% of patients who achieve complete cytogenic response at 18 months have a major molecular response.[11,12,13]",
"In addition, 2 other molecular observations have a role in predicting long-term outcomes: achievement of a less than 10% BCR-ABL transcript level at 3 months, and recognition that earlier and deeper molecular responses are better. A 4.5-log reduction in BCR-ABL transcript number using RT-PCR at 18 months predict better outcomes and longer survival.",
"Patients who do not meet these milestones need to be restaged and evaluated for accelerated or blastic transformation, tested for resistance (BCR-ABL kinase domain mutation testing for the T315I mutation), and presented with a possible change in therapy. For example, in patients who acquire the T315I mutation on tyrosine kinase inhibitor therapy, ponatinib may be indicated.[14]",
"The standard approach is to continue tyrosine kinase inhibitors indefinitely in patients with CML. Emerging data seem to suggest that with low prognostic scores on the Sokal index or Hasford score, patients on such inhibitors as imatinib for longer than 50 months who are in complete molecular response for 2 years and are male may be able to discontinue tyrosine kinase inhibitor therapy, followed by close surveillance. This is not the criterion standard, and further studies are needed to confirm these observations.[1]",
"Patients treated with imatinib may initially have a complete cytogenetic response. Over time, this response may be lost. Acquired T315I mutation is one of the causes of acquired imatinib resistance."
],
"date": "December 11, 2017",
"figures": [],
"markdown": "# A 52-Year-Old Man With Fatigue, Leukocytosis, and Moderate Splenomegaly\n\n **Authors:** Koyamangalath Krishnan, MD \n **Date:** December 11, 2017\n\n ## Content\n\n The treatment of choice in this otherwise reasonably healthy 52-year-old man is tyrosine kinase inhibitors. Options include the first-generation tyrosine kinase inhibitor imatinib mesylate, or a second-generation inhibitor (dasatinib or nilotinib).\nThe National Comprehensive Cancer Network recommends imatinib, dasatinib, and nilotinib as category 1 medications for chronic-phase CML. Imatinib has the longest survival data (>8 years) on the basis of the IRIS trial.[7,8]\nSecond-generation tyrosine kinase inhibitors have only 36-48 months of data, and long-term survival data are lacking. Molecular remissions are achieved earlier with the second-generation inhibitors[9] and can be considered preferentially in patients with intermediate- to high-risk prognostic scores on the Sokal or Hasford system. For patients who cannot tolerate imatinib, dasatinib, or nilotinib, options include bosutinib, interferon, or stem cell transplantation.[10]\nOn the basis of previous studies, milestones have been identified that help in the management of patients receiving tyrosine kinase inhibitors. These milestones help with ascertaining timeliness in responses and in determining when resistance or failure to respond has occurred.\nThe most important determinant of prognosis is response to therapy with tyrosine kinase inhibitors. Patients with CML treated with these inhibitors achieve 3 types of responses: hematologic, cytogenetic, and molecular. The milestones explain when such responses should occur.\nWith imatinib therapy, best outcomes are predicted on achieving a complete hematologic response at 3 months, at least a major cytogenetic response at 12 months, and a complete cytogenetic response at 18 months. \"Major molecular response\" refers to a more than 3-log reduction in the BCR/ABL transcript number on RT-PCR analysis. More than 50% of patients who achieve complete cytogenic response at 18 months have a major molecular response.[11,12,13]\nIn addition, 2 other molecular observations have a role in predicting long-term outcomes: achievement of a less than 10% BCR-ABL transcript level at 3 months, and recognition that earlier and deeper molecular responses are better. A 4.5-log reduction in BCR-ABL transcript number using RT-PCR at 18 months predict better outcomes and longer survival.\nPatients who do not meet these milestones need to be restaged and evaluated for accelerated or blastic transformation, tested for resistance (BCR-ABL kinase domain mutation testing for the T315I mutation), and presented with a possible change in therapy. For example, in patients who acquire the T315I mutation on tyrosine kinase inhibitor therapy, ponatinib may be indicated.[14]\nThe standard approach is to continue tyrosine kinase inhibitors indefinitely in patients with CML. Emerging data seem to suggest that with low prognostic scores on the Sokal index or Hasford score, patients on such inhibitors as imatinib for longer than 50 months who are in complete molecular response for 2 years and are male may be able to discontinue tyrosine kinase inhibitor therapy, followed by close surveillance. This is not the criterion standard, and further studies are needed to confirm these observations.[1]\nPatients treated with imatinib may initially have a complete cytogenetic response. Over time, this response may be lost. Acquired T315I mutation is one of the causes of acquired imatinib resistance.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 767351,
"choiceText": "Bosutinib",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 767353,
"choiceText": "Nilotinib",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 767355,
"choiceText": "Ponatinib",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 767357,
"choiceText": "Dasatinib",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 767359,
"choiceText": "Higher dose of imatinib",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Ponatinib is a third-line tyrosine kinase inhibitor. The specific purpose in developing ponatinib was to address imatinib resistance in light of the <i>ABL1</i> T315I-resistance mutation.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 240311,
"questionText": "The drug of choice for T315I-mutated CML is which of the following?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 767361,
"choiceText": "Imatinib has the longest survival data",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 767363,
"choiceText": "Second-generation tyrosine kinase inhibitors (eg, dasatinib, nilotinib) are preferred for patients with CML who have high Sokal scores",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 767365,
"choiceText": "Deeper molecular responses (>4-log reduction in <i>BCR-ABL</i> fusion transcript) are associated with better outcome",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 767367,
"choiceText": "Bosutinib has been approved by the US Food and Drug Administration (FDA) for first-line use in chronic-phase CML",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 767369,
"choiceText": "In patients with incomplete molecular response to tyrosine kinase inhibitors, the current standard is to continue therapy indefinitely",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "In September 2012, the FDA approved bosutinib for the treatment of chronic-, accelerated-, or blast-phase Philadelphia chromosome-positive CML, specifically in adults who demonstrated resistance or intolerance to previous therapies.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 240313,
"questionText": "Patients with CML should be treated with tyrosine kinase inhibitors. Which of the following is <i>not</i> correct regarding tyrosine kinase inhibitors?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 52-Year-Old Man With Fatigue, Leukocytosis, and Moderate Splenomegaly"
},
{
"authors": "Koyamangalath Krishnan, MD",
"content": [],
"date": "December 11, 2017",
"figures": [],
"markdown": "# A 52-Year-Old Man With Fatigue, Leukocytosis, and Moderate Splenomegaly\n\n **Authors:** Koyamangalath Krishnan, MD \n **Date:** December 11, 2017\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 767351,
"choiceText": "Bosutinib",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 767353,
"choiceText": "Nilotinib",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 767355,
"choiceText": "Ponatinib",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 767357,
"choiceText": "Dasatinib",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 767359,
"choiceText": "Higher dose of imatinib",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Ponatinib is a third-line tyrosine kinase inhibitor. The specific purpose in developing ponatinib was to address imatinib resistance in light of the <i>ABL1</i> T315I-resistance mutation.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 240311,
"questionText": "The drug of choice for T315I-mutated CML is which of the following?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 767361,
"choiceText": "Imatinib has the longest survival data",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 767363,
"choiceText": "Second-generation tyrosine kinase inhibitors (eg, dasatinib, nilotinib) are preferred for patients with CML who have high Sokal scores",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 767365,
"choiceText": "Deeper molecular responses (>4-log reduction in <i>BCR-ABL</i> fusion transcript) are associated with better outcome",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 767367,
"choiceText": "Bosutinib has been approved by the US Food and Drug Administration (FDA) for first-line use in chronic-phase CML",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 767369,
"choiceText": "In patients with incomplete molecular response to tyrosine kinase inhibitors, the current standard is to continue therapy indefinitely",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "In September 2012, the FDA approved bosutinib for the treatment of chronic-, accelerated-, or blast-phase Philadelphia chromosome-positive CML, specifically in adults who demonstrated resistance or intolerance to previous therapies.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 240313,
"questionText": "Patients with CML should be treated with tyrosine kinase inhibitors. Which of the following is <i>not</i> correct regarding tyrosine kinase inhibitors?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 52-Year-Old Man With Fatigue, Leukocytosis, and Moderate Splenomegaly"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 767341,
"choiceText": "Essential thrombocythemia",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 767343,
"choiceText": "Splenic marginal zone lymphoma",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 767345,
"choiceText": "Chronic myeloid leukemia",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 767347,
"choiceText": "Myelofibrosis",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 767349,
"choiceText": "Acute leukemia",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 240309,
"questionText": "On the basis of history, physical examination, and work-up, what is the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 767351,
"choiceText": "Bosutinib",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 767353,
"choiceText": "Nilotinib",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 767355,
"choiceText": "Ponatinib",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 767357,
"choiceText": "Dasatinib",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 767359,
"choiceText": "Higher dose of imatinib",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Ponatinib is a third-line tyrosine kinase inhibitor. The specific purpose in developing ponatinib was to address imatinib resistance in light of the <i>ABL1</i> T315I-resistance mutation.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 240311,
"questionText": "The drug of choice for T315I-mutated CML is which of the following?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 767361,
"choiceText": "Imatinib has the longest survival data",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 767363,
"choiceText": "Second-generation tyrosine kinase inhibitors (eg, dasatinib, nilotinib) are preferred for patients with CML who have high Sokal scores",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 767365,
"choiceText": "Deeper molecular responses (>4-log reduction in <i>BCR-ABL</i> fusion transcript) are associated with better outcome",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 767367,
"choiceText": "Bosutinib has been approved by the US Food and Drug Administration (FDA) for first-line use in chronic-phase CML",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 767369,
"choiceText": "In patients with incomplete molecular response to tyrosine kinase inhibitors, the current standard is to continue therapy indefinitely",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "In September 2012, the FDA approved bosutinib for the treatment of chronic-, accelerated-, or blast-phase Philadelphia chromosome-positive CML, specifically in adults who demonstrated resistance or intolerance to previous therapies.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 240313,
"questionText": "Patients with CML should be treated with tyrosine kinase inhibitors. Which of the following is <i>not</i> correct regarding tyrosine kinase inhibitors?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
831320 | /viewarticle/831320 | [
{
"authors": "Danielle D. DeCourcey, MD; Mark Wainwright, MD, PhD; Jason M. Kane, MD",
"content": [
"Editorial Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"Background",
"A 14-year-old boy presents to the emergency department (ED) with a 10-day history of progressive weakness. The patient reports experiencing rhinorrhea, cough, and malaise approximately 3 weeks before admission. He developed lower-extremity weakness and difficulty walking 8 days after the onset of the upper respiratory tract infection symptoms. He was evaluated at a local hospital, where he was diagnosed with dehydration, treated with intravenous fluids, and discharged to home. Despite these measures, his lower-extremity weakness did not improve.",
"Over the following 7 days, he began experiencing diffuse muscle pain and progressive weakness that extended to his upper extremities. During the 3 days before this presentation, he developed a hoarse voice and shortness of breath. He also notes that he is now having difficulty urinating and has decreased oral intake. He currently denies having any fever, cough, vomiting, or diarrhea.",
"The patient's past medical history is significant only for attention deficit hyperactivity disorder (ADHD), for which he takes methylphenidate. He has had no previous hospitalizations, has no known drug allergies, and has had all recommended childhood immunizations. His family history is noncontributory."
],
"date": "April 02, 2018",
"figures": [],
"markdown": "# Progressive Weakness and Dyspnea in a 14-Year-Old Boy\n\n **Authors:** Danielle D. DeCourcey, MD; Mark Wainwright, MD, PhD; Jason M. Kane, MD \n **Date:** April 02, 2018\n\n ## Content\n\n Editorial Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nBackground\nA 14-year-old boy presents to the emergency department (ED) with a 10-day history of progressive weakness. The patient reports experiencing rhinorrhea, cough, and malaise approximately 3 weeks before admission. He developed lower-extremity weakness and difficulty walking 8 days after the onset of the upper respiratory tract infection symptoms. He was evaluated at a local hospital, where he was diagnosed with dehydration, treated with intravenous fluids, and discharged to home. Despite these measures, his lower-extremity weakness did not improve.\nOver the following 7 days, he began experiencing diffuse muscle pain and progressive weakness that extended to his upper extremities. During the 3 days before this presentation, he developed a hoarse voice and shortness of breath. He also notes that he is now having difficulty urinating and has decreased oral intake. He currently denies having any fever, cough, vomiting, or diarrhea.\nThe patient's past medical history is significant only for attention deficit hyperactivity disorder (ADHD), for which he takes methylphenidate. He has had no previous hospitalizations, has no known drug allergies, and has had all recommended childhood immunizations. His family history is noncontributory.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "Progressive Weakness and Dyspnea in a 14-Year-Old Boy"
},
{
"authors": "Danielle D. DeCourcey, MD; Mark Wainwright, MD, PhD; Jason M. Kane, MD",
"content": [
"Physical Examination and Work-up",
"The physical examination reveals an afebrile, ill-appearing teenager, with a heart rate of 118 beats/min, a respiratory rate of 28 breaths/min, a blood pressure of 168/122 mm Hg, and an oxygen saturation of 93% while breathing room air. Auscultation of the lungs reveals diffuse, poor aeration. His heart sounds are normal, without any appreciable murmur. His strength is symmetric but diminished to 2/5 in his lower extremities and 4/5 in his upper extremities (5/5 being normal strength). The patient's sensation is intact to light touch, but he has a loss of vibratory sense. He has no deep tendon reflexes in his lower extremities, diminished deep tendon reflexes (1+) in his upper extremities, and absent plantar reflexes. Cranial nerves II-XII are intact; however, he has a weak cough and gag reflex, with impaired handling of secretions. The remainder of his examination is unremarkable.",
"The patient is intubated for progressive respiratory distress and loss of airway-protective reflexes. He is fluid-resuscitated with a liter of intravenous normal saline. An electrocardiogram (ECG) is obtained, which demonstrates sinus tachycardia. The initial laboratory analysis, including a complete blood cell (CBC) count and a basic metabolic panel, is within normal limits. A lumbar puncture is performed, with an opening pressure of 15 cm H20. The cell count and Gram stain of the cerebrospinal fluid (CSF) demonstrates 2 white blood cells per high power field, 4 red blood cells per high power field, and no organisms. Additional analysis of the CSF shows a protein concentration of 96 mg/dL (960 mg/L) and glucose concentration of 72 mg/dL (3.99 mmol/L). The patient is sent for MRI of his brain and spine (see Figure 1) and is transported to the pediatric intensive care unit (ICU) for further management.",
"Figure 1."
],
"date": "April 02, 2018",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/831/320/831320-thumb-1.jpg"
}
],
"markdown": "# Progressive Weakness and Dyspnea in a 14-Year-Old Boy\n\n **Authors:** Danielle D. DeCourcey, MD; Mark Wainwright, MD, PhD; Jason M. Kane, MD \n **Date:** April 02, 2018\n\n ## Content\n\n Physical Examination and Work-up\nThe physical examination reveals an afebrile, ill-appearing teenager, with a heart rate of 118 beats/min, a respiratory rate of 28 breaths/min, a blood pressure of 168/122 mm Hg, and an oxygen saturation of 93% while breathing room air. Auscultation of the lungs reveals diffuse, poor aeration. His heart sounds are normal, without any appreciable murmur. His strength is symmetric but diminished to 2/5 in his lower extremities and 4/5 in his upper extremities (5/5 being normal strength). The patient's sensation is intact to light touch, but he has a loss of vibratory sense. He has no deep tendon reflexes in his lower extremities, diminished deep tendon reflexes (1+) in his upper extremities, and absent plantar reflexes. Cranial nerves II-XII are intact; however, he has a weak cough and gag reflex, with impaired handling of secretions. The remainder of his examination is unremarkable.\nThe patient is intubated for progressive respiratory distress and loss of airway-protective reflexes. He is fluid-resuscitated with a liter of intravenous normal saline. An electrocardiogram (ECG) is obtained, which demonstrates sinus tachycardia. The initial laboratory analysis, including a complete blood cell (CBC) count and a basic metabolic panel, is within normal limits. A lumbar puncture is performed, with an opening pressure of 15 cm H20. The cell count and Gram stain of the cerebrospinal fluid (CSF) demonstrates 2 white blood cells per high power field, 4 red blood cells per high power field, and no organisms. Additional analysis of the CSF shows a protein concentration of 96 mg/dL (960 mg/L) and glucose concentration of 72 mg/dL (3.99 mmol/L). The patient is sent for MRI of his brain and spine (see Figure 1) and is transported to the pediatric intensive care unit (ICU) for further management.\nFigure 1.\n\n ## Figures\n\n **Figure 1.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 765409,
"choiceText": "Spinal epidural abscess",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 765411,
"choiceText": "Guillain-Barré syndrome",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 765413,
"choiceText": "Transverse myelitis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 765415,
"choiceText": "Multiple sclerosis",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 239669,
"questionText": "What is the patient's condition as verified by the MRI?<br>\r\n<i>Hint: Look closely at the cauda equina on the MRI images.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Progressive Weakness and Dyspnea in a 14-Year-Old Boy"
},
{
"authors": "Danielle D. DeCourcey, MD; Mark Wainwright, MD, PhD; Jason M. Kane, MD",
"content": [
"Discussion",
"The lumbrosacral MRIs (see Figures 1 and 2) demonstrate nerve root enhancement of the cauda equina on axial post-contrast T1-weighted sequences.",
"Figure 1.",
"Figure 2.",
"The localization of progressive weakness includes spinal cord lesions (such as transverse myelitis or anterior spinal artery syndrome), peripheral neuropathies (such as those caused by heavy metals), neuromuscular junction diseases (such as that caused by organophosphate pesticides, myasthenia gravis, botulism, and myopathies (such as dermatomyositis). The presence of progressive ascending weakness, areflexia, autonomic dysfunction, elevated CSF protein without pleocytosis, and enhancement of the cauda equina nerve roots on lumbrosacral MRIs make the diagnosis of Guillain-Barré syndrome most probable in this patient.",
"Guillain-Barré syndrome is an acute, idiopathic, monophasic, acquired inflammatory demyelinating polyradiculoneuropathy (AIDP) that affects both children and adults. It is a heterogeneous syndrome, with several variant forms. AIDP is the prototype of Guillain-Barré syndrome, and it is the most common form in North America, Europe, and most of the developed world (where it accounts for about 85-90% of cases).",
"Guillain-Barré syndrome can occur at any age, but there appears to be a bimodal distribution, with peaks in young adulthood (15-35 y) and in the elderly (50-75 y). The cause of Guillain-Barré syndrome is unknown, but the disorder is thought to result from a postinfectious immune-mediated process called molecular mimicry that predominantly damages the myelin sheath of peripheral nerves. Approximately two thirds of patients report a history of an antecedent respiratory tract or gastrointestinal infection 2-4 weeks before the onset of neurologic symptoms. Various infectious agents have been associated with Guillain-Barré syndrome, although Campylobacter is the most frequent. Other organisms that commonly precede Guillain-Barré syndrome include cytomegalovirus, Epstein-Barr virus, Haemophilus influenzae, Mycoplasma pneumoniae, the enterovirus family, hepatitis A and B, herpes simplex virus, and Chlamydophila (formerly Chlamydia) pneumoniae. Zika virus has been linked to Guillain-Barré syndrome. Increased risk of Guillain-Barré syndrome after influenza vaccination has not been consistently demonstrated."
],
"date": "April 02, 2018",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/831/320/831320-thumb-1.jpg"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/831/320/831320-thumb-2.jpg"
}
],
"markdown": "# Progressive Weakness and Dyspnea in a 14-Year-Old Boy\n\n **Authors:** Danielle D. DeCourcey, MD; Mark Wainwright, MD, PhD; Jason M. Kane, MD \n **Date:** April 02, 2018\n\n ## Content\n\n Discussion\nThe lumbrosacral MRIs (see Figures 1 and 2) demonstrate nerve root enhancement of the cauda equina on axial post-contrast T1-weighted sequences.\nFigure 1.\nFigure 2.\nThe localization of progressive weakness includes spinal cord lesions (such as transverse myelitis or anterior spinal artery syndrome), peripheral neuropathies (such as those caused by heavy metals), neuromuscular junction diseases (such as that caused by organophosphate pesticides, myasthenia gravis, botulism, and myopathies (such as dermatomyositis). The presence of progressive ascending weakness, areflexia, autonomic dysfunction, elevated CSF protein without pleocytosis, and enhancement of the cauda equina nerve roots on lumbrosacral MRIs make the diagnosis of Guillain-Barré syndrome most probable in this patient.\nGuillain-Barré syndrome is an acute, idiopathic, monophasic, acquired inflammatory demyelinating polyradiculoneuropathy (AIDP) that affects both children and adults. It is a heterogeneous syndrome, with several variant forms. AIDP is the prototype of Guillain-Barré syndrome, and it is the most common form in North America, Europe, and most of the developed world (where it accounts for about 85-90% of cases).\nGuillain-Barré syndrome can occur at any age, but there appears to be a bimodal distribution, with peaks in young adulthood (15-35 y) and in the elderly (50-75 y). The cause of Guillain-Barré syndrome is unknown, but the disorder is thought to result from a postinfectious immune-mediated process called molecular mimicry that predominantly damages the myelin sheath of peripheral nerves. Approximately two thirds of patients report a history of an antecedent respiratory tract or gastrointestinal infection 2-4 weeks before the onset of neurologic symptoms. Various infectious agents have been associated with Guillain-Barré syndrome, although Campylobacter is the most frequent. Other organisms that commonly precede Guillain-Barré syndrome include cytomegalovirus, Epstein-Barr virus, Haemophilus influenzae, Mycoplasma pneumoniae, the enterovirus family, hepatitis A and B, herpes simplex virus, and Chlamydophila (formerly Chlamydia) pneumoniae. Zika virus has been linked to Guillain-Barré syndrome. Increased risk of Guillain-Barré syndrome after influenza vaccination has not been consistently demonstrated.\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n\n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 765409,
"choiceText": "Spinal epidural abscess",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 765411,
"choiceText": "Guillain-Barré syndrome",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 765413,
"choiceText": "Transverse myelitis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 765415,
"choiceText": "Multiple sclerosis",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 239669,
"questionText": "What is the patient's condition as verified by the MRI?<br>\r\n<i>Hint: Look closely at the cauda equina on the MRI images.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Progressive Weakness and Dyspnea in a 14-Year-Old Boy"
},
{
"authors": "Danielle D. DeCourcey, MD; Mark Wainwright, MD, PhD; Jason M. Kane, MD",
"content": [
"The typical presentation of Guillain-Barré syndrome is fine paresthesias in the toes and fingertips, followed by symmetric lower-extremity weakness that may ascend, over hours to days, to involve the arms and the muscles of respiration. Pain, predominately back, lower-limb and abdominal pain, is often a prominent feature of the syndrome.[1] The physical examination reveals symmetric weakness, with diminished or absent reflexes and variable loss of sensation in a stocking-glove distribution. Signs of autonomic dysfunction are present in 50% of patients, and they include cardiac dysrhythmias, orthostatic hypotension, transient or persistent hypertension, ileus, constipation, and bladder dysfunction.[2] Deviation from the classic presentation of ascending progression of weakness is not uncommon. In what is known as the Miller-Fisher variant, cranial nerves are affected in 30-40% of patients at any time in the course of the syndrome. This form of the disease is also characterized by areflexia, ataxia and ophthalmoplegia. The facial nerves are most commonly involved, resulting in bilateral facial weakness.",
"Although the associated autonomic dysfunction may produce life-threatening complications, mortality from Guillain-Barré syndrome is largely secondary to respiratory failure associated with respiratory muscle weakness. Approximately 20% of children with Guillain-Barré syndrome require mechanical ventilation for respiratory failure. The need for intubation should be anticipated early so that it can be done nonemergently in a controlled environment. Progression to respiratory failure has been predicted in patients with rapid disease progression, bulbar dysfunction, bilateral facial weakness, or dysautonomia. Emergent intubation should be performed in any patient with loss of the gag reflex, declining respiratory function, or pharyngeal dysfunction. Care should be taken during intubation, as autonomic dysfunction may complicate the use of vasoactive and sedative drugs.",
"After the first week of symptoms, analysis of the CSF typically reveals normal opening pressures, fewer than 10 white blood cells per high power field (typically mononuclear), and an elevated protein concentration (greater than 45 mg/dL). This finding, also known as albuminocytologic dissociation, may be delayed. As a result, a repeat lumbar puncture may be required as the protein values may not rise for 1-2 weeks, and maximum protein values may not be seen for 4-5 weeks. In addition, gadolinium-enhanced lumbosacral MRI may demonstrate enhancement of the cauda equina nerve roots. This imaging modality has been described to be 83% sensitive for acute Guillain-Barré syndrome, and abnormalities are present in 95% of typical cases.[3]",
"Electrophysiologic studies are the most specific and sensitive tests for confirming the diagnosis. Most patients demonstrate slowing of nerve conduction 2-3 weeks after the onset of symptoms. Various abnormalities seen in Guillain-Barré syndrome indicate evolving multifocal axonal demyelination in peripheral nerves, spinal roots and/or cranial nerves. Abnormalities seen on electromyography include partial motor conduction block, slowed nerve conduction velocities, abnormal temporal dispersion, and prolonged distal latencies.[4] The earliest finding, which may be present within days of symptom onset, is prolongation or absence of the F responses, which indicates demyelination involving the proximal nerve roots.",
"Any patient who presents with a clinical picture consistent with Guillain-Barré syndrome requires immediate hospitalization. The indications for admission to the ICU include, but are not limited to, respiratory insufficiency or failure, loss of airway-protective reflexes, and severe autonomic instability."
],
"date": "April 02, 2018",
"figures": [],
"markdown": "# Progressive Weakness and Dyspnea in a 14-Year-Old Boy\n\n **Authors:** Danielle D. DeCourcey, MD; Mark Wainwright, MD, PhD; Jason M. Kane, MD \n **Date:** April 02, 2018\n\n ## Content\n\n The typical presentation of Guillain-Barré syndrome is fine paresthesias in the toes and fingertips, followed by symmetric lower-extremity weakness that may ascend, over hours to days, to involve the arms and the muscles of respiration. Pain, predominately back, lower-limb and abdominal pain, is often a prominent feature of the syndrome.[1] The physical examination reveals symmetric weakness, with diminished or absent reflexes and variable loss of sensation in a stocking-glove distribution. Signs of autonomic dysfunction are present in 50% of patients, and they include cardiac dysrhythmias, orthostatic hypotension, transient or persistent hypertension, ileus, constipation, and bladder dysfunction.[2] Deviation from the classic presentation of ascending progression of weakness is not uncommon. In what is known as the Miller-Fisher variant, cranial nerves are affected in 30-40% of patients at any time in the course of the syndrome. This form of the disease is also characterized by areflexia, ataxia and ophthalmoplegia. The facial nerves are most commonly involved, resulting in bilateral facial weakness.\nAlthough the associated autonomic dysfunction may produce life-threatening complications, mortality from Guillain-Barré syndrome is largely secondary to respiratory failure associated with respiratory muscle weakness. Approximately 20% of children with Guillain-Barré syndrome require mechanical ventilation for respiratory failure. The need for intubation should be anticipated early so that it can be done nonemergently in a controlled environment. Progression to respiratory failure has been predicted in patients with rapid disease progression, bulbar dysfunction, bilateral facial weakness, or dysautonomia. Emergent intubation should be performed in any patient with loss of the gag reflex, declining respiratory function, or pharyngeal dysfunction. Care should be taken during intubation, as autonomic dysfunction may complicate the use of vasoactive and sedative drugs.\nAfter the first week of symptoms, analysis of the CSF typically reveals normal opening pressures, fewer than 10 white blood cells per high power field (typically mononuclear), and an elevated protein concentration (greater than 45 mg/dL). This finding, also known as albuminocytologic dissociation, may be delayed. As a result, a repeat lumbar puncture may be required as the protein values may not rise for 1-2 weeks, and maximum protein values may not be seen for 4-5 weeks. In addition, gadolinium-enhanced lumbosacral MRI may demonstrate enhancement of the cauda equina nerve roots. This imaging modality has been described to be 83% sensitive for acute Guillain-Barré syndrome, and abnormalities are present in 95% of typical cases.[3]\nElectrophysiologic studies are the most specific and sensitive tests for confirming the diagnosis. Most patients demonstrate slowing of nerve conduction 2-3 weeks after the onset of symptoms. Various abnormalities seen in Guillain-Barré syndrome indicate evolving multifocal axonal demyelination in peripheral nerves, spinal roots and/or cranial nerves. Abnormalities seen on electromyography include partial motor conduction block, slowed nerve conduction velocities, abnormal temporal dispersion, and prolonged distal latencies.[4] The earliest finding, which may be present within days of symptom onset, is prolongation or absence of the F responses, which indicates demyelination involving the proximal nerve roots.\nAny patient who presents with a clinical picture consistent with Guillain-Barré syndrome requires immediate hospitalization. The indications for admission to the ICU include, but are not limited to, respiratory insufficiency or failure, loss of airway-protective reflexes, and severe autonomic instability.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "Progressive Weakness and Dyspnea in a 14-Year-Old Boy"
},
{
"authors": "Danielle D. DeCourcey, MD; Mark Wainwright, MD, PhD; Jason M. Kane, MD",
"content": [
"The main modalities of therapy for Guillain-Barré syndrome include plasma exchange and intravenously administered immunoglobulin (IVIG). Corticosteroids have not been shown to be beneficial.[5] The American Academy of Neurology issued a practice parameter regarding immunotherapy for Guillain-Barré syndrome that concluded IVIG and plasma exchange are options for children with severe disease and should be reserved for those with the following findings:[6]",
"Rapidly progressing weakness",
"Worsening respiratory status or need for mechanical ventilation",
"Significant bulbar weakness",
"Inability to walk unaided",
"Several trials have demonstrated that IVIG is at least as effective as plasma exchange in the treatment of Guillain-Barré syndrome and is associated with a lower rate of complications.[7] IVIG administered at 0.4 g/kg/day for 5 days has been shown to hasten recovery and lower the relapse rate. Doses of 1 g/kg/day over 2 days have also been demonstrated to hasten recovery time, but early relapses are more prevalent.[8] The combination of plasma exchange and IVIG does not improve outcomes or shorten the duration of illness.[6]",
"Complications associated with Guillain-Barré syndrome include arrhythmia, sepsis, pneumonia, ileus, deep venous thrombosis and pulmonary embolism. The risk of sepsis and infection may be decreased by aggressive physiotherapy and mechanical ventilation with positive end expiratory pressure (PEEP). Administration of anticoagulant therapy and intermittent pneumatic compression devices may lower the risk of deep venous thrombosis and pulmonary embolism. Cardiac telemetry is useful to monitor for arrhythmias, which are a common cause of morbidity and mortality in Guillain-Barré syndrome. In addition, physical and occupational therapy should be initiated early and may be beneficial in helping patients to regain their baseline functional status.[9]",
"More than 90% of patients reach the nadir of their function within 4 weeks of the onset of symptoms, with return of normal function occurring slowly over the course of weeks to months. Most patients with Guillain-Barré syndrome achieve a full and functional recovery within 6-12 months. The clinical course of Guillain-Barré syndrome in children is shorter than it is in adults, and recovery is more complete.[10]",
"Upon transferring this patient to the ICU, a dialysis catheter was placed and plasmapheresis was initiated. His hypertension was controlled with a nicardipine drip. Prophylaxis for deep venous thrombosis was started. On hospital day 3, the patient had improved strength in his lower extremities and pressure support trials on the ventilator were initiated. On hospital day 6, he was extubated successfully to room air after receiving a total of 5 sessions of plasmapheresis. He was transferred to a pediatric ward 1 week after admission with intensive physical and occupational therapies. At the time of the transfer, the patient's bulbar symptoms were resolved, and his strength was 3/5 in his lower extremities and 4/5 in his upper extremities."
],
"date": "April 02, 2018",
"figures": [],
"markdown": "# Progressive Weakness and Dyspnea in a 14-Year-Old Boy\n\n **Authors:** Danielle D. DeCourcey, MD; Mark Wainwright, MD, PhD; Jason M. Kane, MD \n **Date:** April 02, 2018\n\n ## Content\n\n The main modalities of therapy for Guillain-Barré syndrome include plasma exchange and intravenously administered immunoglobulin (IVIG). Corticosteroids have not been shown to be beneficial.[5] The American Academy of Neurology issued a practice parameter regarding immunotherapy for Guillain-Barré syndrome that concluded IVIG and plasma exchange are options for children with severe disease and should be reserved for those with the following findings:[6]\nRapidly progressing weakness\nWorsening respiratory status or need for mechanical ventilation\nSignificant bulbar weakness\nInability to walk unaided\nSeveral trials have demonstrated that IVIG is at least as effective as plasma exchange in the treatment of Guillain-Barré syndrome and is associated with a lower rate of complications.[7] IVIG administered at 0.4 g/kg/day for 5 days has been shown to hasten recovery and lower the relapse rate. Doses of 1 g/kg/day over 2 days have also been demonstrated to hasten recovery time, but early relapses are more prevalent.[8] The combination of plasma exchange and IVIG does not improve outcomes or shorten the duration of illness.[6]\nComplications associated with Guillain-Barré syndrome include arrhythmia, sepsis, pneumonia, ileus, deep venous thrombosis and pulmonary embolism. The risk of sepsis and infection may be decreased by aggressive physiotherapy and mechanical ventilation with positive end expiratory pressure (PEEP). Administration of anticoagulant therapy and intermittent pneumatic compression devices may lower the risk of deep venous thrombosis and pulmonary embolism. Cardiac telemetry is useful to monitor for arrhythmias, which are a common cause of morbidity and mortality in Guillain-Barré syndrome. In addition, physical and occupational therapy should be initiated early and may be beneficial in helping patients to regain their baseline functional status.[9]\nMore than 90% of patients reach the nadir of their function within 4 weeks of the onset of symptoms, with return of normal function occurring slowly over the course of weeks to months. Most patients with Guillain-Barré syndrome achieve a full and functional recovery within 6-12 months. The clinical course of Guillain-Barré syndrome in children is shorter than it is in adults, and recovery is more complete.[10]\nUpon transferring this patient to the ICU, a dialysis catheter was placed and plasmapheresis was initiated. His hypertension was controlled with a nicardipine drip. Prophylaxis for deep venous thrombosis was started. On hospital day 3, the patient had improved strength in his lower extremities and pressure support trials on the ventilator were initiated. On hospital day 6, he was extubated successfully to room air after receiving a total of 5 sessions of plasmapheresis. He was transferred to a pediatric ward 1 week after admission with intensive physical and occupational therapies. At the time of the transfer, the patient's bulbar symptoms were resolved, and his strength was 3/5 in his lower extremities and 4/5 in his upper extremities.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 765417,
"choiceText": "Elevated opening pressures",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 765419,
"choiceText": "Fewer than 10 white blood cells per high power field (typically mononuclear)",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 765421,
"choiceText": "Elevated protein concentration (usually greater than 45 mg/dL)",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 765423,
"choiceText": "Normal glucose concentration",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "After the first week of symptoms, analysis of the CSF typically reveals normal opening pressures, fewer than 10 white blood cells per high power field (typically mononuclear), and an elevated protein concentration (greater than 45 mg/dL). This finding, also known as albuminocytologic dissociation, may be delayed. As a result, a repeat lumbar puncture may be required as the protein values may not rise for 1-2 weeks, and maximum protein values may not be seen for 4-5 weeks. ",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 239671,
"questionText": "Which of the following findings is <em>not</em> likely to be seen on analysis of the CSF of a patient with Guillain-Barré syndrome who has had symptoms for 3 weeks?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 765425,
"choiceText": "Rapid disease progression",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 765427,
"choiceText": "Bulbar dysfunction",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 765429,
"choiceText": "Absent deep tendon reflexes in the lower extremities",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 765431,
"choiceText": "Dysautonomia",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 765433,
"choiceText": "Pharyngeal dysfunction with loss of the gag reflex",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Progression to respiratory failure has been predicted in patients with rapid disease progression, bulbar dysfunction, bilateral facial weakness, or dysautonomia. Absent deep tendon reflexes in the lower extremities has not been explicitly associated with a progression to respiratory failure.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 239673,
"questionText": "Which of the following symptoms is <em>not</em> associated with a progression to respiratory failure in children with the above presentation?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Progressive Weakness and Dyspnea in a 14-Year-Old Boy"
},
{
"authors": "Danielle D. DeCourcey, MD; Mark Wainwright, MD, PhD; Jason M. Kane, MD",
"content": [],
"date": "April 02, 2018",
"figures": [],
"markdown": "# Progressive Weakness and Dyspnea in a 14-Year-Old Boy\n\n **Authors:** Danielle D. DeCourcey, MD; Mark Wainwright, MD, PhD; Jason M. Kane, MD \n **Date:** April 02, 2018\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 765417,
"choiceText": "Elevated opening pressures",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 765419,
"choiceText": "Fewer than 10 white blood cells per high power field (typically mononuclear)",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 765421,
"choiceText": "Elevated protein concentration (usually greater than 45 mg/dL)",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 765423,
"choiceText": "Normal glucose concentration",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "After the first week of symptoms, analysis of the CSF typically reveals normal opening pressures, fewer than 10 white blood cells per high power field (typically mononuclear), and an elevated protein concentration (greater than 45 mg/dL). This finding, also known as albuminocytologic dissociation, may be delayed. As a result, a repeat lumbar puncture may be required as the protein values may not rise for 1-2 weeks, and maximum protein values may not be seen for 4-5 weeks. ",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 239671,
"questionText": "Which of the following findings is <em>not</em> likely to be seen on analysis of the CSF of a patient with Guillain-Barré syndrome who has had symptoms for 3 weeks?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 765425,
"choiceText": "Rapid disease progression",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 765427,
"choiceText": "Bulbar dysfunction",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 765429,
"choiceText": "Absent deep tendon reflexes in the lower extremities",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 765431,
"choiceText": "Dysautonomia",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 765433,
"choiceText": "Pharyngeal dysfunction with loss of the gag reflex",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Progression to respiratory failure has been predicted in patients with rapid disease progression, bulbar dysfunction, bilateral facial weakness, or dysautonomia. Absent deep tendon reflexes in the lower extremities has not been explicitly associated with a progression to respiratory failure.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 239673,
"questionText": "Which of the following symptoms is <em>not</em> associated with a progression to respiratory failure in children with the above presentation?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Progressive Weakness and Dyspnea in a 14-Year-Old Boy"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 765409,
"choiceText": "Spinal epidural abscess",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 765411,
"choiceText": "Guillain-Barré syndrome",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 765413,
"choiceText": "Transverse myelitis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 765415,
"choiceText": "Multiple sclerosis",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 239669,
"questionText": "What is the patient's condition as verified by the MRI?<br>\r\n<i>Hint: Look closely at the cauda equina on the MRI images.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 765417,
"choiceText": "Elevated opening pressures",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 765419,
"choiceText": "Fewer than 10 white blood cells per high power field (typically mononuclear)",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 765421,
"choiceText": "Elevated protein concentration (usually greater than 45 mg/dL)",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 765423,
"choiceText": "Normal glucose concentration",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "After the first week of symptoms, analysis of the CSF typically reveals normal opening pressures, fewer than 10 white blood cells per high power field (typically mononuclear), and an elevated protein concentration (greater than 45 mg/dL). This finding, also known as albuminocytologic dissociation, may be delayed. As a result, a repeat lumbar puncture may be required as the protein values may not rise for 1-2 weeks, and maximum protein values may not be seen for 4-5 weeks. ",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 239671,
"questionText": "Which of the following findings is <em>not</em> likely to be seen on analysis of the CSF of a patient with Guillain-Barré syndrome who has had symptoms for 3 weeks?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 765425,
"choiceText": "Rapid disease progression",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 765427,
"choiceText": "Bulbar dysfunction",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 765429,
"choiceText": "Absent deep tendon reflexes in the lower extremities",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 765431,
"choiceText": "Dysautonomia",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 765433,
"choiceText": "Pharyngeal dysfunction with loss of the gag reflex",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Progression to respiratory failure has been predicted in patients with rapid disease progression, bulbar dysfunction, bilateral facial weakness, or dysautonomia. Absent deep tendon reflexes in the lower extremities has not been explicitly associated with a progression to respiratory failure.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 239673,
"questionText": "Which of the following symptoms is <em>not</em> associated with a progression to respiratory failure in children with the above presentation?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
828053 | /viewarticle/828053 | [
{
"authors": "Mary L. Windle, PharmD; Francisco Talavera, PharmD, PhD; Jenna Woods, BS, PharmD Candidate 2015",
"content": [
"Medscape's Pill Identifier helps you to ID generic and brand-name prescription drugs, OTCs, and supplements. Review these patient scenarios and select the relevant medications."
],
"date": "July 11, 2014",
"figures": [],
"markdown": "# Medication Emergencies: What Drug Is This?\n\n **Authors:** Mary L. Windle, PharmD; Francisco Talavera, PharmD, PhD; Jenna Woods, BS, PharmD Candidate 2015 \n **Date:** July 11, 2014\n\n ## Content\n\n Medscape's Pill Identifier helps you to ID generic and brand-name prescription drugs, OTCs, and supplements. Review these patient scenarios and select the relevant medications.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 744447,
"choiceText": "Temazepam and losartan",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 744449,
"choiceText": "Fosinopril and irbesartan/hydrochlorothiazide",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 744451,
"choiceText": "Carisoprodol and tramadol",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 744453,
"choiceText": "Hydrocodone/acetaminophen and lisinopril",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 232867,
"questionText": "Mr. Nelson is at the clinic with complaints of dizziness. You ask him how he has been taking his medications, and he hands you his pill organizer. It contains 2 tablets. One is white and round, with imprints of \"202\" and \"IG\"; the other is oblong and cream-colored, with an imprint of \"L059.\" What are these medications?<br>\r\n<br>Hint: Name the medication with <a href=\"http://reference.medscape.com/pill-identifier\" target=\"_blank\">Medscape’s Pill Identifier</a>.<br>\r\n<br><img width=\"200\" height=\"301\" border=\"1\" src=\"https://img.medscapestatic.com/article/828/053/828053-Figure-1.jpg\" alt=\"\" />",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Medication Emergencies: What Drug Is This?"
},
{
"authors": "Mary L. Windle, PharmD; Francisco Talavera, PharmD, PhD; Jenna Woods, BS, PharmD Candidate 2015",
"content": [
"The physician identifies the white tablets as fosinopril 40 mg and the cream-colored tablets as irbesartan/hydrochlorothiazide 300-12.5mg. He explains to the patient that both of these medications lower blood pressure. The physician had given Mr. Nelson irbesartan/hydrochlorothiazide samples and instructed him to discontinue the fosinopril, but the patient forgot to take the fosinopril out of his pill organizer.",
"Read about fosinopril here.",
"Read about irbesartan/hydrochlorothiazide here."
],
"date": "July 11, 2014",
"figures": [],
"markdown": "# Medication Emergencies: What Drug Is This?\n\n **Authors:** Mary L. Windle, PharmD; Francisco Talavera, PharmD, PhD; Jenna Woods, BS, PharmD Candidate 2015 \n **Date:** July 11, 2014\n\n ## Content\n\n The physician identifies the white tablets as fosinopril 40 mg and the cream-colored tablets as irbesartan/hydrochlorothiazide 300-12.5mg. He explains to the patient that both of these medications lower blood pressure. The physician had given Mr. Nelson irbesartan/hydrochlorothiazide samples and instructed him to discontinue the fosinopril, but the patient forgot to take the fosinopril out of his pill organizer.\nRead about fosinopril here.\nRead about irbesartan/hydrochlorothiazide here.\n\n ## Figures\n\n \n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 744447,
"choiceText": "Temazepam and losartan",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 744449,
"choiceText": "Fosinopril and irbesartan/hydrochlorothiazide",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 744451,
"choiceText": "Carisoprodol and tramadol",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 744453,
"choiceText": "Hydrocodone/acetaminophen and lisinopril",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 232867,
"questionText": "Mr. Nelson is at the clinic with complaints of dizziness. You ask him how he has been taking his medications, and he hands you his pill organizer. It contains 2 tablets. One is white and round, with imprints of \"202\" and \"IG\"; the other is oblong and cream-colored, with an imprint of \"L059.\" What are these medications?<br>\r\n<br>Hint: Name the medication with <a href=\"http://reference.medscape.com/pill-identifier\" target=\"_blank\">Medscape’s Pill Identifier</a>.<br>\r\n<br><img width=\"200\" height=\"301\" border=\"1\" src=\"https://img.medscapestatic.com/article/828/053/828053-Figure-1.jpg\" alt=\"\" />",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Medication Emergencies: What Drug Is This?"
},
{
"authors": "Mary L. Windle, PharmD; Francisco Talavera, PharmD, PhD; Jenna Woods, BS, PharmD Candidate 2015",
"content": [
"The pharmacist was able to identify the peach capsule as a Tikosyn (dofetilide) 250-μg capsule and notified the US Food and Drug Administration.",
"Read about dofetilide here."
],
"date": "July 11, 2014",
"figures": [],
"markdown": "# Medication Emergencies: What Drug Is This?\n\n **Authors:** Mary L. Windle, PharmD; Francisco Talavera, PharmD, PhD; Jenna Woods, BS, PharmD Candidate 2015 \n **Date:** July 11, 2014\n\n ## Content\n\n The pharmacist was able to identify the peach capsule as a Tikosyn (dofetilide) 250-μg capsule and notified the US Food and Drug Administration.\nRead about dofetilide here.\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 744455,
"choiceText": "Ciprofloxacin",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 744457,
"choiceText": "Venlafaxine",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 744459,
"choiceText": "Diltiazem",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 744461,
"choiceText": "Dofetilide",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 232869,
"questionText": "A pharmacist is refilling a patient's monthly prescription for Effexor XR (venlafaxine) 150 mg. She opens a new, sealed bottle and pours the capsules onto a tray to be counted. She notices that one capsule does not look like the others. Twenty-nine of the capsules were Effexor XR (dark orange capsule, with imprints of \"W,\" \"Effexor,\" and \"150 mg\"). One capsule was peach, with imprints of \"Pfizer\" and \"TKN 250.\" What is the peach capsule?<br>\r\n<br>\r\nHint: Name the medication with <a href=\"http://reference.medscape.com/pill-identifier\" target=\"_blank\">Medscape’s Pill Identifier</a>.<br>\r\n<br><img width=\"200\" height=\"302\" border=\"1\" src=\"https://img.medscapestatic.com/article/828/053/828053-Figure-2.jpg\" alt=\"\" />",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Medication Emergencies: What Drug Is This?"
},
{
"authors": "Mary L. Windle, PharmD; Francisco Talavera, PharmD, PhD; Jenna Woods, BS, PharmD Candidate 2015",
"content": [
"The mother calls the poison control center, and they identify the tablets as benazepril/hydrochlorothiazide 10-12.5 mg.",
"Read about benazepril/hydrochlorothiazide here."
],
"date": "July 11, 2014",
"figures": [],
"markdown": "# Medication Emergencies: What Drug Is This?\n\n **Authors:** Mary L. Windle, PharmD; Francisco Talavera, PharmD, PhD; Jenna Woods, BS, PharmD Candidate 2015 \n **Date:** July 11, 2014\n\n ## Content\n\n The mother calls the poison control center, and they identify the tablets as benazepril/hydrochlorothiazide 10-12.5 mg.\nRead about benazepril/hydrochlorothiazide here.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 744463,
"choiceText": "Benazepril/hydrochlorothiazide",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 744465,
"choiceText": "Metoprolol succinate",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 744467,
"choiceText": "Atorvastatin",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 744469,
"choiceText": "Losartan",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 232871,
"questionText": "While sorting the laundry, the mother of a 7-year-old girl finds 3 tablets in the back pocket of her daughter's jeans. They had spent the day at a family member's home, and the mother was concerned that her daughter may have found these in the medicine cabinet there. The tablets were pink, oblong, and scored, and had an imprint of \"E 204\" on one side. What are the tablets?<br>\r\n<br>Hint: Name the medication with <a href=\"http://reference.medscape.com/pill-identifier\" target=\"_blank\">Medscape’s Pill Identifier</a>.<br>\r\n<br><img width=\"250\" height=\"186\" border=\"1\" src=\"https://img.medscapestatic.com/article/828/053/828053-Figure-3.jpg\" alt=\"\" />",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Medication Emergencies: What Drug Is This?"
},
{
"authors": "Mary L. Windle, PharmD; Francisco Talavera, PharmD, PhD; Jenna Woods, BS, PharmD Candidate 2015",
"content": [
"The nurse identifies the white tablet as ciprofloxacin 250 mg and the lavender tablet as warfarin 2 mg. The nurse recognizes that ciprofloxacin could be inhibiting the metabolism of the warfarin and, therefore, increasing the patient's international normalized ratio. The nurse notifies the medical team.",
"Read about ciprofloxacin here.",
"Read about warfarin here."
],
"date": "July 11, 2014",
"figures": [],
"markdown": "# Medication Emergencies: What Drug Is This?\n\n **Authors:** Mary L. Windle, PharmD; Francisco Talavera, PharmD, PhD; Jenna Woods, BS, PharmD Candidate 2015 \n **Date:** July 11, 2014\n\n ## Content\n\n The nurse identifies the white tablet as ciprofloxacin 250 mg and the lavender tablet as warfarin 2 mg. The nurse recognizes that ciprofloxacin could be inhibiting the metabolism of the warfarin and, therefore, increasing the patient's international normalized ratio. The nurse notifies the medical team.\nRead about ciprofloxacin here.\nRead about warfarin here.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 744471,
"choiceText": "Ibuprofen and clopidogrel",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 744473,
"choiceText": "Metoprolol tartrate and anagrelide",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 744475,
"choiceText": "Ciprofloxacin and warfarin",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 744477,
"choiceText": "Clonazepam and naproxen",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 4,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 232873,
"questionText": "While on a week-long vacation, Mrs. Johnson visits the local emergency department because of blood in her stool. She hands the nurse a bottle of pills she has put together for the week. The nurse can identify 2 distinct tablets in the bottle. One tablet is round and white, with imprints of \"C\" and \"95.\" The other tablet is lavender and scored, with imprints of \"AN\" on one side and \"762 2\" on the other. What are these medications?<br>\r\n<br>Hint: Name the medication with <a href=\"http://reference.medscape.com/pill-identifier\" target=\"_blank\">Medscape’s Pill Identifier</a>.<br>\r\n<br><img width=\"200\" height=\"306\" border=\"1\" src=\"https://img.medscapestatic.com/article/828/053/828053-Figure-4.jpg\" alt=\"\" />",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Medication Emergencies: What Drug Is This?"
},
{
"authors": "Mary L. Windle, PharmD; Francisco Talavera, PharmD, PhD; Jenna Woods, BS, PharmD Candidate 2015",
"content": [
"The student health center identifies this tablet as amphetamine/dextroamphetamine 10 mg.",
"Read about amphetamine/dextroamphetamine here."
],
"date": "July 11, 2014",
"figures": [],
"markdown": "# Medication Emergencies: What Drug Is This?\n\n **Authors:** Mary L. Windle, PharmD; Francisco Talavera, PharmD, PhD; Jenna Woods, BS, PharmD Candidate 2015 \n **Date:** July 11, 2014\n\n ## Content\n\n The student health center identifies this tablet as amphetamine/dextroamphetamine 10 mg.\nRead about amphetamine/dextroamphetamine here.\n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 744479,
"choiceText": "Ibuprofen",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 744481,
"choiceText": "Amphetamine/dextroamphetamine",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 744483,
"choiceText": "Carvedilol",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 744485,
"choiceText": "Bupropion ",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 5,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 232875,
"questionText": "A campus custodian is sweeping the floor of an algebra classroom one evening, when he comes across a round, blue tablet. The tablet is blue and scored, and it has an imprint of \"cor\" and \"132\" on one side. What is this tablet?<br>\r\n<br>Hint: Name the medication with <a href=\"http://reference.medscape.com/pill-identifier\" target=\"_blank\">Medscape’s Pill Identifier</a>.<br>\r\n<br><img width=\"250\" height=\"188\" border=\"1\" src=\"https://img.medscapestatic.com/article/828/053/828053-Figure-5.jpg\" alt=\"\" />",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Medication Emergencies: What Drug Is This?"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 744447,
"choiceText": "Temazepam and losartan",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 744449,
"choiceText": "Fosinopril and irbesartan/hydrochlorothiazide",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 744451,
"choiceText": "Carisoprodol and tramadol",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 744453,
"choiceText": "Hydrocodone/acetaminophen and lisinopril",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 232867,
"questionText": "Mr. Nelson is at the clinic with complaints of dizziness. You ask him how he has been taking his medications, and he hands you his pill organizer. It contains 2 tablets. One is white and round, with imprints of \"202\" and \"IG\"; the other is oblong and cream-colored, with an imprint of \"L059.\" What are these medications?<br>\r\n<br>Hint: Name the medication with <a href=\"http://reference.medscape.com/pill-identifier\" target=\"_blank\">Medscape’s Pill Identifier</a>.<br>\r\n<br><img width=\"200\" height=\"301\" border=\"1\" src=\"https://img.medscapestatic.com/article/828/053/828053-Figure-1.jpg\" alt=\"\" />",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 744455,
"choiceText": "Ciprofloxacin",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 744457,
"choiceText": "Venlafaxine",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 744459,
"choiceText": "Diltiazem",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 744461,
"choiceText": "Dofetilide",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 232869,
"questionText": "A pharmacist is refilling a patient's monthly prescription for Effexor XR (venlafaxine) 150 mg. She opens a new, sealed bottle and pours the capsules onto a tray to be counted. She notices that one capsule does not look like the others. Twenty-nine of the capsules were Effexor XR (dark orange capsule, with imprints of \"W,\" \"Effexor,\" and \"150 mg\"). One capsule was peach, with imprints of \"Pfizer\" and \"TKN 250.\" What is the peach capsule?<br>\r\n<br>\r\nHint: Name the medication with <a href=\"http://reference.medscape.com/pill-identifier\" target=\"_blank\">Medscape’s Pill Identifier</a>.<br>\r\n<br><img width=\"200\" height=\"302\" border=\"1\" src=\"https://img.medscapestatic.com/article/828/053/828053-Figure-2.jpg\" alt=\"\" />",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 744463,
"choiceText": "Benazepril/hydrochlorothiazide",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 744465,
"choiceText": "Metoprolol succinate",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 744467,
"choiceText": "Atorvastatin",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 744469,
"choiceText": "Losartan",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 232871,
"questionText": "While sorting the laundry, the mother of a 7-year-old girl finds 3 tablets in the back pocket of her daughter's jeans. They had spent the day at a family member's home, and the mother was concerned that her daughter may have found these in the medicine cabinet there. The tablets were pink, oblong, and scored, and had an imprint of \"E 204\" on one side. What are the tablets?<br>\r\n<br>Hint: Name the medication with <a href=\"http://reference.medscape.com/pill-identifier\" target=\"_blank\">Medscape’s Pill Identifier</a>.<br>\r\n<br><img width=\"250\" height=\"186\" border=\"1\" src=\"https://img.medscapestatic.com/article/828/053/828053-Figure-3.jpg\" alt=\"\" />",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 744471,
"choiceText": "Ibuprofen and clopidogrel",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 744473,
"choiceText": "Metoprolol tartrate and anagrelide",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 744475,
"choiceText": "Ciprofloxacin and warfarin",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 744477,
"choiceText": "Clonazepam and naproxen",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 4,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 232873,
"questionText": "While on a week-long vacation, Mrs. Johnson visits the local emergency department because of blood in her stool. She hands the nurse a bottle of pills she has put together for the week. The nurse can identify 2 distinct tablets in the bottle. One tablet is round and white, with imprints of \"C\" and \"95.\" The other tablet is lavender and scored, with imprints of \"AN\" on one side and \"762 2\" on the other. What are these medications?<br>\r\n<br>Hint: Name the medication with <a href=\"http://reference.medscape.com/pill-identifier\" target=\"_blank\">Medscape’s Pill Identifier</a>.<br>\r\n<br><img width=\"200\" height=\"306\" border=\"1\" src=\"https://img.medscapestatic.com/article/828/053/828053-Figure-4.jpg\" alt=\"\" />",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 744479,
"choiceText": "Ibuprofen",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 744481,
"choiceText": "Amphetamine/dextroamphetamine",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 744483,
"choiceText": "Carvedilol",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 744485,
"choiceText": "Bupropion ",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 5,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 232875,
"questionText": "A campus custodian is sweeping the floor of an algebra classroom one evening, when he comes across a round, blue tablet. The tablet is blue and scored, and it has an imprint of \"cor\" and \"132\" on one side. What is this tablet?<br>\r\n<br>Hint: Name the medication with <a href=\"http://reference.medscape.com/pill-identifier\" target=\"_blank\">Medscape’s Pill Identifier</a>.<br>\r\n<br><img width=\"250\" height=\"188\" border=\"1\" src=\"https://img.medscapestatic.com/article/828/053/828053-Figure-5.jpg\" alt=\"\" />",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
827352 | /viewarticle/827352 | [
{
"authors": "",
"content": [
"Laparoscopic appendectomy for acute appendicitis can reduce pain, allow earlier resumption of diet, and shorten hospital stay. This clinical video highlights key steps in the procedure.",
"For more details on this procedure, see Laparoscopic Appendectomy in Medscape Reference.",
"Single-Port Appendectomy",
"Open Appendectomy",
"Appendicitis",
"Pediatric Appendicitis"
],
"date": "July 01, 2014",
"figures": [],
"markdown": "# Key Steps in Laparoscopic Appendectomy\n\n **Authors:** \n **Date:** July 01, 2014\n\n ## Content\n\n Laparoscopic appendectomy for acute appendicitis can reduce pain, allow earlier resumption of diet, and shorten hospital stay. This clinical video highlights key steps in the procedure.\nFor more details on this procedure, see Laparoscopic Appendectomy in Medscape Reference.\nSingle-Port Appendectomy\nOpen Appendectomy\nAppendicitis\nPediatric Appendicitis\n\n ## Figures\n\n \n*Page 1 of 1*",
"pagination": {
"current_page": 1,
"total_pages": 1
},
"questionnaire": [],
"title": "Key Steps in Laparoscopic Appendectomy"
}
] | [] |
826346 | /viewarticle/826346 | [
{
"authors": "Sumaira Nabi, MBBS; Sadaf Khattak, MBBS; Mansoor Iqbal, MBBS, FCPS",
"content": [
"Editorial Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions.",
"Background",
"Figure 1.",
"A 36-year-old Pakistani female presents to the emergency department with a one-month history of painful, rapidly progressive swelling of her right eye. The patient is unable to open her eyelid. She denies any foreign-body sensation, fever, joint pains, rash, heat intolerance, weight loss, or easy fatigability. There is no diurnal variation in her symptoms. She is a housewife with 3 children and belongs to a lower socioeconomic group. The patient is a nonsmoker and denies substance abuse. She has been taking multiple analgesics prescribed by her local general practitioner, with no improvement in her symptoms. Other than that, her drug history is insignificant. The family history is unremarkable as well."
],
"date": "June 11, 2014",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/826/346/826346-thumb-1.jpg"
}
],
"markdown": "# A 36-Year-Old Woman With Painful Swelling and Ophthalmoplegia of the Right Eye\n\n **Authors:** Sumaira Nabi, MBBS; Sadaf Khattak, MBBS; Mansoor Iqbal, MBBS, FCPS \n **Date:** June 11, 2014\n\n ## Content\n\n Editorial Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions.\nBackground\nFigure 1.\nA 36-year-old Pakistani female presents to the emergency department with a one-month history of painful, rapidly progressive swelling of her right eye. The patient is unable to open her eyelid. She denies any foreign-body sensation, fever, joint pains, rash, heat intolerance, weight loss, or easy fatigability. There is no diurnal variation in her symptoms. She is a housewife with 3 children and belongs to a lower socioeconomic group. The patient is a nonsmoker and denies substance abuse. She has been taking multiple analgesics prescribed by her local general practitioner, with no improvement in her symptoms. Other than that, her drug history is insignificant. The family history is unremarkable as well.\n\n ## Figures\n\n **Figure 1.** \n \n\n\n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 36-Year-Old Woman With Painful Swelling and Ophthalmoplegia of the Right Eye"
},
{
"authors": "Sumaira Nabi, MBBS; Sadaf Khattak, MBBS; Mansoor Iqbal, MBBS, FCPS",
"content": [
"Physical Examination and Workup",
"On physical examination she is an awake, alert young lady who is uncomfortable due to pain. Her vital signs include an oral temperature of 98.6°F, a regular pulse of 80 beats/min, and a blood pressure of 110/70 mm Hg. Her respiratory rate is 14 breaths/min. Her Glasgow Coma Scale score is 15/15. On ocular examination, she has a right complete external ophthalmoplegia (figures 1 and 2) with moderate proptosis (24 mm axial; figure 3). There is no chemosis, conjunctival injection, or crusting on the lashes. The globe is mildly tender to palpation. Her pupils are reactive to light from 4 mm to 2 mm, with no relative afferent pupillary defect (RAPD). Visual acuity is 6/12 (ie, 20/40) on the right side and 6/6 (ie, 20/20) on the left, with no visual-field defects.",
"Figure 1.",
"Figure 2.",
"Figure 3.",
"On funduscopic examination, the disc margins are blurred on the right side. There are no signs of pyramidal weakness or incoordination. The rest of her cranial nerves (CNs) are intact and symmetrical, including CN V, VIII, IX, and X. Her neck is supple, with full range of motion and no adenopathy or masses. Her abdomen is soft and nontender. There is no clinical evidence of organomegaly or ascites. Her bowel sounds are audible. The patient's precordial examination reveals normal heart sounds. Auscultation of the lung fields shows normal vesicular breathing.",
"Figure 4.",
"Figure 5.",
"Figure 6.",
"Laboratory analysis demonstrates a normal complete blood count (CBC) and erythrocyte sedimentation rate. The patient's liver function tests, renal function tests, serum glucose levels, and chest radiograph are unremarkable. Her antinuclear antibody (ANA), rheumatoid factor (RF), anti ̶ double-stranded DNA (anti-dsDNA), HIV serology, hepatitis B surface antigen (HBsAg), anti ̶ hepatitis C virus (anti-HCV) antibody, cytoplasmic antineutrophil cytoplasmic antibody (cANCA), and perinuclear ANCA (pANCA) are negative. Her thyroid function tests are also normal, and her antithyroid antibodies (anti-Tg and anti-TPO) are negative. Routine examination of her cerebrospinal fluid (CSF) is normal, and so are her serum angiotensin-converting enzyme (ACE) levels. Repetitive nerve stimulation is normal, and anti-acetylcholinesterase antibodies are negative.",
"Magnetic resonance imaging (MRI) of the brain and orbit with contrast reveals diffuse thickening and swelling of all of the extraocular muscles of the right orbit, with involvement of the tendinous insertions. (Figures 4 and 5.) No edematous changes are seen in the muscles. However, the right globe is proptotic. (Figure 6.) The right optic nerve is normal. The cavernous sinuses and the brain are also normal."
],
"date": "June 11, 2014",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/826/346/826346-thumb-1.jpg"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/826/346/826346-thumb-2.jpg"
},
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/826/346/826346-thumb-3.jpg"
},
{
"caption": "Figure 4.",
"image_url": "https://img.medscapestatic.com/article/826/346/826346-thumb-4.jpg"
},
{
"caption": "Figure 5.",
"image_url": "https://img.medscapestatic.com/article/826/346/826346-thumb-5.jpg"
},
{
"caption": "Figure 6.",
"image_url": "https://img.medscapestatic.com/article/826/346/826346-thumb-6.jpg"
}
],
"markdown": "# A 36-Year-Old Woman With Painful Swelling and Ophthalmoplegia of the Right Eye\n\n **Authors:** Sumaira Nabi, MBBS; Sadaf Khattak, MBBS; Mansoor Iqbal, MBBS, FCPS \n **Date:** June 11, 2014\n\n ## Content\n\n Physical Examination and Workup\nOn physical examination she is an awake, alert young lady who is uncomfortable due to pain. Her vital signs include an oral temperature of 98.6°F, a regular pulse of 80 beats/min, and a blood pressure of 110/70 mm Hg. Her respiratory rate is 14 breaths/min. Her Glasgow Coma Scale score is 15/15. On ocular examination, she has a right complete external ophthalmoplegia (figures 1 and 2) with moderate proptosis (24 mm axial; figure 3). There is no chemosis, conjunctival injection, or crusting on the lashes. The globe is mildly tender to palpation. Her pupils are reactive to light from 4 mm to 2 mm, with no relative afferent pupillary defect (RAPD). Visual acuity is 6/12 (ie, 20/40) on the right side and 6/6 (ie, 20/20) on the left, with no visual-field defects.\nFigure 1.\nFigure 2.\nFigure 3.\nOn funduscopic examination, the disc margins are blurred on the right side. There are no signs of pyramidal weakness or incoordination. The rest of her cranial nerves (CNs) are intact and symmetrical, including CN V, VIII, IX, and X. Her neck is supple, with full range of motion and no adenopathy or masses. Her abdomen is soft and nontender. There is no clinical evidence of organomegaly or ascites. Her bowel sounds are audible. The patient's precordial examination reveals normal heart sounds. Auscultation of the lung fields shows normal vesicular breathing.\nFigure 4.\nFigure 5.\nFigure 6.\nLaboratory analysis demonstrates a normal complete blood count (CBC) and erythrocyte sedimentation rate. The patient's liver function tests, renal function tests, serum glucose levels, and chest radiograph are unremarkable. Her antinuclear antibody (ANA), rheumatoid factor (RF), anti ̶ double-stranded DNA (anti-dsDNA), HIV serology, hepatitis B surface antigen (HBsAg), anti ̶ hepatitis C virus (anti-HCV) antibody, cytoplasmic antineutrophil cytoplasmic antibody (cANCA), and perinuclear ANCA (pANCA) are negative. Her thyroid function tests are also normal, and her antithyroid antibodies (anti-Tg and anti-TPO) are negative. Routine examination of her cerebrospinal fluid (CSF) is normal, and so are her serum angiotensin-converting enzyme (ACE) levels. Repetitive nerve stimulation is normal, and anti-acetylcholinesterase antibodies are negative.\nMagnetic resonance imaging (MRI) of the brain and orbit with contrast reveals diffuse thickening and swelling of all of the extraocular muscles of the right orbit, with involvement of the tendinous insertions. (Figures 4 and 5.) No edematous changes are seen in the muscles. However, the right globe is proptotic. (Figure 6.) The right optic nerve is normal. The cavernous sinuses and the brain are also normal.\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n**Figure 3.** \n \n\n**Figure 4.** \n \n\n**Figure 5.** \n \n\n**Figure 6.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 735069,
"choiceText": "Cavernous sinus thrombosis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 735071,
"choiceText": "Myasthenia gravis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 735073,
"choiceText": "Graves ophthalmopathy",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 735075,
"choiceText": "Orbital pseudotumor",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 229735,
"questionText": "Based on the history, physical examination, and workup, what is the likely diagnosis?<br><br>\r\n<i>Hint: Note the typical MRI orbital findings.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 36-Year-Old Woman With Painful Swelling and Ophthalmoplegia of the Right Eye"
},
{
"authors": "Sumaira Nabi, MBBS; Sadaf Khattak, MBBS; Mansoor Iqbal, MBBS, FCPS",
"content": [
"Discussion",
"This patient was administered high-dose oral corticosteroid therapy in the form of prednisolone at 2 mg/kg/day. Her symptoms resolved within 48 hours, with significant improvement in proptosis and ptosis. However, her ocular motility showed only mild improvement. She was then referred to an ophthalmology hospital for specialized treatment. The consultant ophthalmologist confirmed the diagnosis of orbital pseudotumor on the basis of clinical and radiologic evidence, as the patient did not give consent for biopsy and histopathology.",
"Figure 1.",
"Figure 2.",
"Figure 3.",
"Orbital pseudotumor, also called “idiopathic orbital inflammatory syndrome” or “idiopathic nonspecific orbital inflammation,” is an inflammatory condition of the orbit characterized by a mixed infiltrate and fibrosis. The lacrimal glands (the most frequently affected structures), globe, extraocular muscles, and optic nerve are among the many structures that can be affected by the disease. The condition may result in diffuse orbital involvement or, alternatively, it can have more focused consequences, affecting specific orbital structures, such as the lacrimal glands or specific muscles.[1,2] Orbital pseudotumor can therefore be subclassified on the basis of the anatomic target areas within the orbit, clinically presenting itself primarily as lacrimal pseudotumor, anterior pseudotumor, posterior pseudotumor, diffuse pseudotumor, and myositic pseudotumor (which mimics thyroid-associated orbitopathy, as seen in our patient).[3,4,5]",
"Figure 4.",
"Figure 5.",
"Figure 6.",
"The disease’s symptoms tend to be nonspecific ones that simulate those of a number of intraorbital tumors, rather than manifesting as the classic triad of pain, ophthalmoparesis, and proptosis.[2] Thus, the clinical features of orbital pseudotumor—which include inflammation, infiltration, and/or signs of mass effect—demonstrate wide variation. Ocular manifestations commonly include pain, proptosis, lid edema, erythema, local swelling, and conjunctival injection; other symptoms can include vision loss, diplopia, ptosis, and extraocular dysmotility.[3,4,5,6,7,8] Symptoms usually develop over a period of hours or days, but development has been known to take place over weeks, or even months. Orbital pseudotumor often occurs in just one eye, but it is not unusual for bilateral symptoms to occur.[8]",
"One of the most common orbital diseases, orbital pseudotumor occurs mainly in adults but has no race or sex predilection.[8,9,10,11] It was first described by Birch-Hirschfeld, in 1905.[12]",
"The disease’s pathogenesis has not been fully elucidated, although immune-mediated processes are thought to be the likely underlying ocular mechanism. The etiology of orbital pseudotumor is also unknown, although infection, autoimmune disease, and aberrant wound healing have been suggested in the literature as possible underlying causes. Infectious diseases (eg, streptococcal pharyngitis, viral upper respiratory infection, and infection with the spirochete \nBorrelia burgdorferi\n) may also be linked to orbital pseudotumor.[13,14,15] Nonspecific findings—benign lymphoid hyperplasia and inflammatory cell infiltration with necrotizing vasculitis—may be the only pathologic results.[15,16]",
"The diagnosis is challenging, since the differential diagnosis is extremely varied, ranging from orbital cellulitis to Graves disease.[8] Therefore, orbital pseudotumor is diagnosed through exclusion, based on the history of the case and on the examination, clinical course, and radiologic, serologic, and histologic findings, as well as on the patient’s response to steroid therapy."
],
"date": "June 11, 2014",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/826/346/826346-thumb-1.jpg"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/826/346/826346-thumb-2.jpg"
},
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/826/346/826346-thumb-3.jpg"
},
{
"caption": "Figure 4.",
"image_url": "https://img.medscapestatic.com/article/826/346/826346-thumb-4.jpg"
},
{
"caption": "Figure 5.",
"image_url": "https://img.medscapestatic.com/article/826/346/826346-thumb-5.jpg"
},
{
"caption": "Figure 6.",
"image_url": "https://img.medscapestatic.com/article/826/346/826346-thumb-6.jpg"
}
],
"markdown": "# A 36-Year-Old Woman With Painful Swelling and Ophthalmoplegia of the Right Eye\n\n **Authors:** Sumaira Nabi, MBBS; Sadaf Khattak, MBBS; Mansoor Iqbal, MBBS, FCPS \n **Date:** June 11, 2014\n\n ## Content\n\n Discussion\nThis patient was administered high-dose oral corticosteroid therapy in the form of prednisolone at 2 mg/kg/day. Her symptoms resolved within 48 hours, with significant improvement in proptosis and ptosis. However, her ocular motility showed only mild improvement. She was then referred to an ophthalmology hospital for specialized treatment. The consultant ophthalmologist confirmed the diagnosis of orbital pseudotumor on the basis of clinical and radiologic evidence, as the patient did not give consent for biopsy and histopathology.\nFigure 1.\nFigure 2.\nFigure 3.\nOrbital pseudotumor, also called “idiopathic orbital inflammatory syndrome” or “idiopathic nonspecific orbital inflammation,” is an inflammatory condition of the orbit characterized by a mixed infiltrate and fibrosis. The lacrimal glands (the most frequently affected structures), globe, extraocular muscles, and optic nerve are among the many structures that can be affected by the disease. The condition may result in diffuse orbital involvement or, alternatively, it can have more focused consequences, affecting specific orbital structures, such as the lacrimal glands or specific muscles.[1,2] Orbital pseudotumor can therefore be subclassified on the basis of the anatomic target areas within the orbit, clinically presenting itself primarily as lacrimal pseudotumor, anterior pseudotumor, posterior pseudotumor, diffuse pseudotumor, and myositic pseudotumor (which mimics thyroid-associated orbitopathy, as seen in our patient).[3,4,5]\nFigure 4.\nFigure 5.\nFigure 6.\nThe disease’s symptoms tend to be nonspecific ones that simulate those of a number of intraorbital tumors, rather than manifesting as the classic triad of pain, ophthalmoparesis, and proptosis.[2] Thus, the clinical features of orbital pseudotumor—which include inflammation, infiltration, and/or signs of mass effect—demonstrate wide variation. Ocular manifestations commonly include pain, proptosis, lid edema, erythema, local swelling, and conjunctival injection; other symptoms can include vision loss, diplopia, ptosis, and extraocular dysmotility.[3,4,5,6,7,8] Symptoms usually develop over a period of hours or days, but development has been known to take place over weeks, or even months. Orbital pseudotumor often occurs in just one eye, but it is not unusual for bilateral symptoms to occur.[8]\nOne of the most common orbital diseases, orbital pseudotumor occurs mainly in adults but has no race or sex predilection.[8,9,10,11] It was first described by Birch-Hirschfeld, in 1905.[12]\nThe disease’s pathogenesis has not been fully elucidated, although immune-mediated processes are thought to be the likely underlying ocular mechanism. The etiology of orbital pseudotumor is also unknown, although infection, autoimmune disease, and aberrant wound healing have been suggested in the literature as possible underlying causes. Infectious diseases (eg, streptococcal pharyngitis, viral upper respiratory infection, and infection with the spirochete \nBorrelia burgdorferi\n) may also be linked to orbital pseudotumor.[13,14,15] Nonspecific findings—benign lymphoid hyperplasia and inflammatory cell infiltration with necrotizing vasculitis—may be the only pathologic results.[15,16]\nThe diagnosis is challenging, since the differential diagnosis is extremely varied, ranging from orbital cellulitis to Graves disease.[8] Therefore, orbital pseudotumor is diagnosed through exclusion, based on the history of the case and on the examination, clinical course, and radiologic, serologic, and histologic findings, as well as on the patient’s response to steroid therapy.\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n**Figure 3.** \n \n\n**Figure 4.** \n \n\n**Figure 5.** \n \n\n**Figure 6.** \n \n\n\n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 735069,
"choiceText": "Cavernous sinus thrombosis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 735071,
"choiceText": "Myasthenia gravis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 735073,
"choiceText": "Graves ophthalmopathy",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 735075,
"choiceText": "Orbital pseudotumor",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 229735,
"questionText": "Based on the history, physical examination, and workup, what is the likely diagnosis?<br><br>\r\n<i>Hint: Note the typical MRI orbital findings.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 36-Year-Old Woman With Painful Swelling and Ophthalmoplegia of the Right Eye"
},
{
"authors": "Sumaira Nabi, MBBS; Sadaf Khattak, MBBS; Mansoor Iqbal, MBBS, FCPS",
"content": [
"Orbital MRI is the most important diagnostic test, with the findings varying according to the affected orbital structures. For example, extraocular muscle involvement causes enlargement of muscles and tendons, as in this case, producing an abnormal tubular configuration (a characteristic not found in thyroid ophthalmopathy, in which the extraocular tendons are normal).[8,17,18] Imaging studies may also reveal infiltration occurring in the orbital fat or along the optic nerve, the presence of a diffuse orbital mass, enhancement of the sclera, and an increase in the size of the Tenon space.[8]",
"Figure 1.",
"Figure 2.",
"Figure 3.",
"While not typically necessary in orbital pseudotumor, biopsy can be used in patients if there is a great deal of doubt regarding the diagnosis or if the condition is recurrent or unresponsive to steroids. Biopsy specimens reveal nonspecific, polymorphic, lymphocytic infiltrates with macrophages, polymorphonuclear leukocytes, and eosinophils. In sclerosing orbital pseudotumor, the biopsy reveals extensive fibrosis formation.[1,2,15,16]",
"A rare condition, calcifying orbital pseudotumor, may develop when the orbit is affected by a chronic, idiopathic inflammatory process. Chronic granulomatous types of orbital pseudotumor can give rise to the sclerosing form of the disease.[1,2,15,16] Serologic studies are required to rule out a systemic cause of orbital pseudotumor.[8]",
"Figure 4.",
"Figure 5.",
"Figure 6.",
"Corticosteroid therapy typically causes symptoms to quickly regress (more than two thirds of patients demonstrate great improvement within 24-48 hours of treatment) and reduces the proportion of patients who suffer permanent disability from sclerosis.[3,4,7,8] Starting dosages of prednisone of 1-2 mg/kg/day are usually sufficient.[15] Once improvement is noted, dosages should be slowly tapered according to the clinical response. Alternatively, patients can initially be treated with IV pulse steroid therapy, followed by gradually tapered dosages of oral steroids.",
"Inflammation may improve in orbital pseudotumor even in the absence of treatment, but patients commonly suffer recurrent inflammatory episodes and residual fibrosis.[2,8] Recurrence is not unusual even in treated cases, occurring in 23-56% of patients who have undergone therapy.[2,8] Recurrence is especially common with bilateral disease.",
"Other treatment options include cytotoxic agents (cyclophosphamide, chlorambucil), immunosuppressants (methotrexate, cyclosporine, azathioprine), IV immunoglobulins, tumor necrosis factor alpha (TNF-α) inhibitor, monoclonal antibody (infliximab, adalimumab), and mycophenolate mofetil.[2,15] Surgical debulking is a possibility for large, sclerotic lesions.[8,19] Low-dose radiation is an option for patients who are elderly or do not respond to systemic corticosteroids, or when steroids are contraindicated. Radiation doses of 20 Gy in 10 fractions seem to reduce the symptoms of orbital myositis but do not offer effective long-term symptom control.[15,20]"
],
"date": "June 11, 2014",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/826/346/826346-thumb-1.jpg"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/826/346/826346-thumb-2.jpg"
},
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/826/346/826346-thumb-3.jpg"
},
{
"caption": "Figure 4.",
"image_url": "https://img.medscapestatic.com/article/826/346/826346-thumb-4.jpg"
},
{
"caption": "Figure 5.",
"image_url": "https://img.medscapestatic.com/article/826/346/826346-thumb-5.jpg"
},
{
"caption": "Figure 6.",
"image_url": "https://img.medscapestatic.com/article/826/346/826346-thumb-6.jpg"
}
],
"markdown": "# A 36-Year-Old Woman With Painful Swelling and Ophthalmoplegia of the Right Eye\n\n **Authors:** Sumaira Nabi, MBBS; Sadaf Khattak, MBBS; Mansoor Iqbal, MBBS, FCPS \n **Date:** June 11, 2014\n\n ## Content\n\n Orbital MRI is the most important diagnostic test, with the findings varying according to the affected orbital structures. For example, extraocular muscle involvement causes enlargement of muscles and tendons, as in this case, producing an abnormal tubular configuration (a characteristic not found in thyroid ophthalmopathy, in which the extraocular tendons are normal).[8,17,18] Imaging studies may also reveal infiltration occurring in the orbital fat or along the optic nerve, the presence of a diffuse orbital mass, enhancement of the sclera, and an increase in the size of the Tenon space.[8]\nFigure 1.\nFigure 2.\nFigure 3.\nWhile not typically necessary in orbital pseudotumor, biopsy can be used in patients if there is a great deal of doubt regarding the diagnosis or if the condition is recurrent or unresponsive to steroids. Biopsy specimens reveal nonspecific, polymorphic, lymphocytic infiltrates with macrophages, polymorphonuclear leukocytes, and eosinophils. In sclerosing orbital pseudotumor, the biopsy reveals extensive fibrosis formation.[1,2,15,16]\nA rare condition, calcifying orbital pseudotumor, may develop when the orbit is affected by a chronic, idiopathic inflammatory process. Chronic granulomatous types of orbital pseudotumor can give rise to the sclerosing form of the disease.[1,2,15,16] Serologic studies are required to rule out a systemic cause of orbital pseudotumor.[8]\nFigure 4.\nFigure 5.\nFigure 6.\nCorticosteroid therapy typically causes symptoms to quickly regress (more than two thirds of patients demonstrate great improvement within 24-48 hours of treatment) and reduces the proportion of patients who suffer permanent disability from sclerosis.[3,4,7,8] Starting dosages of prednisone of 1-2 mg/kg/day are usually sufficient.[15] Once improvement is noted, dosages should be slowly tapered according to the clinical response. Alternatively, patients can initially be treated with IV pulse steroid therapy, followed by gradually tapered dosages of oral steroids.\nInflammation may improve in orbital pseudotumor even in the absence of treatment, but patients commonly suffer recurrent inflammatory episodes and residual fibrosis.[2,8] Recurrence is not unusual even in treated cases, occurring in 23-56% of patients who have undergone therapy.[2,8] Recurrence is especially common with bilateral disease.\nOther treatment options include cytotoxic agents (cyclophosphamide, chlorambucil), immunosuppressants (methotrexate, cyclosporine, azathioprine), IV immunoglobulins, tumor necrosis factor alpha (TNF-α) inhibitor, monoclonal antibody (infliximab, adalimumab), and mycophenolate mofetil.[2,15] Surgical debulking is a possibility for large, sclerotic lesions.[8,19] Low-dose radiation is an option for patients who are elderly or do not respond to systemic corticosteroids, or when steroids are contraindicated. Radiation doses of 20 Gy in 10 fractions seem to reduce the symptoms of orbital myositis but do not offer effective long-term symptom control.[15,20]\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n**Figure 3.** \n \n\n**Figure 4.** \n \n\n**Figure 5.** \n \n\n**Figure 6.** \n \n\n\n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 36-Year-Old Woman With Painful Swelling and Ophthalmoplegia of the Right Eye"
},
{
"authors": "Sumaira Nabi, MBBS; Sadaf Khattak, MBBS; Mansoor Iqbal, MBBS, FCPS",
"content": [
"The literature indicates that patient outcomes are good in close to 80% of cases,[21] with symptomatic resolution, improved eyelid closure, and ocular motility, and with radiographic regression of the disease process in the follow-up period. The patient in this case regained full range of eye motion."
],
"date": "June 11, 2014",
"figures": [],
"markdown": "# A 36-Year-Old Woman With Painful Swelling and Ophthalmoplegia of the Right Eye\n\n **Authors:** Sumaira Nabi, MBBS; Sadaf Khattak, MBBS; Mansoor Iqbal, MBBS, FCPS \n **Date:** June 11, 2014\n\n ## Content\n\n The literature indicates that patient outcomes are good in close to 80% of cases,[21] with symptomatic resolution, improved eyelid closure, and ocular motility, and with radiographic regression of the disease process in the follow-up period. The patient in this case regained full range of eye motion.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 735077,
"choiceText": "Proptosis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 735079,
"choiceText": "Extraocular muscle belly involvement",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 735081,
"choiceText": "Extraocular muscle tendon involvement",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 735083,
"choiceText": "Optic nerve involvement",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 735085,
"choiceText": "Orbital soft tissue involvement",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 229737,
"questionText": "Which of the following is a characteristic radiologic feature of orbital pseudotumor but not of thyroid-associated orbitopathy?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 735087,
"choiceText": "IV immunoglobulin",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 735089,
"choiceText": "Cyclophosphamide",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 735091,
"choiceText": "Radiotherapy",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 735093,
"choiceText": "Corticosteroids",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 735095,
"choiceText": "Surgery",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 229739,
"questionText": "What is the treatment of choice for orbital pseudotumor?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 36-Year-Old Woman With Painful Swelling and Ophthalmoplegia of the Right Eye"
},
{
"authors": "Sumaira Nabi, MBBS; Sadaf Khattak, MBBS; Mansoor Iqbal, MBBS, FCPS",
"content": [],
"date": "June 11, 2014",
"figures": [],
"markdown": "# A 36-Year-Old Woman With Painful Swelling and Ophthalmoplegia of the Right Eye\n\n **Authors:** Sumaira Nabi, MBBS; Sadaf Khattak, MBBS; Mansoor Iqbal, MBBS, FCPS \n **Date:** June 11, 2014\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 735077,
"choiceText": "Proptosis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 735079,
"choiceText": "Extraocular muscle belly involvement",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 735081,
"choiceText": "Extraocular muscle tendon involvement",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 735083,
"choiceText": "Optic nerve involvement",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 735085,
"choiceText": "Orbital soft tissue involvement",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 229737,
"questionText": "Which of the following is a characteristic radiologic feature of orbital pseudotumor but not of thyroid-associated orbitopathy?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 735087,
"choiceText": "IV immunoglobulin",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 735089,
"choiceText": "Cyclophosphamide",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 735091,
"choiceText": "Radiotherapy",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 735093,
"choiceText": "Corticosteroids",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 735095,
"choiceText": "Surgery",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 229739,
"questionText": "What is the treatment of choice for orbital pseudotumor?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 36-Year-Old Woman With Painful Swelling and Ophthalmoplegia of the Right Eye"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 735069,
"choiceText": "Cavernous sinus thrombosis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 735071,
"choiceText": "Myasthenia gravis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 735073,
"choiceText": "Graves ophthalmopathy",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 735075,
"choiceText": "Orbital pseudotumor",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 229735,
"questionText": "Based on the history, physical examination, and workup, what is the likely diagnosis?<br><br>\r\n<i>Hint: Note the typical MRI orbital findings.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 735077,
"choiceText": "Proptosis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 735079,
"choiceText": "Extraocular muscle belly involvement",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 735081,
"choiceText": "Extraocular muscle tendon involvement",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 735083,
"choiceText": "Optic nerve involvement",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 735085,
"choiceText": "Orbital soft tissue involvement",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 229737,
"questionText": "Which of the following is a characteristic radiologic feature of orbital pseudotumor but not of thyroid-associated orbitopathy?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 735087,
"choiceText": "IV immunoglobulin",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 735089,
"choiceText": "Cyclophosphamide",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 735091,
"choiceText": "Radiotherapy",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 735093,
"choiceText": "Corticosteroids",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 735095,
"choiceText": "Surgery",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 229739,
"questionText": "What is the treatment of choice for orbital pseudotumor?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
824942 | /viewarticle/824942 | [
{
"authors": "Sumaira Nabi, MBBS; Rao Suhail, MBBS, FCPS Neurology; Shahzad Ahmed, MBBS",
"content": [
"Background",
"A 55-year-old Pakistani male presents to the emergency department with a 10-month history of numbness, tingling, and pain in both lower extremities and a 5-month history of similar symptoms in both arms. He also complains of having progressive, asymmetrical weakness in both legs, without sphincteric dysfunction, for two months.",
"There is no history of fever, cough, diarrhea, or constipation or of joint pains, weight loss, photosensitivity, polyuria, or polydipsia. The patient is an ex-driver by occupation who has eight children and belongs to a lower socioeconomic group. He is a nonsmoker and denies alcoholism.",
"He has had essential hypertension for five years and takes tablet bisoprolol 5 mg daily on a regular basis. Other than that, his medical history is insignificant. Family history is unremarkable."
],
"date": "May 19, 2014",
"figures": [],
"markdown": "# Pain, Tingling, and Numbness in the Extremities With Foot Drop and Progressive Weakening of the Legs\n\n **Authors:** Sumaira Nabi, MBBS; Rao Suhail, MBBS, FCPS Neurology; Shahzad Ahmed, MBBS \n **Date:** May 19, 2014\n\n ## Content\n\n Background\nA 55-year-old Pakistani male presents to the emergency department with a 10-month history of numbness, tingling, and pain in both lower extremities and a 5-month history of similar symptoms in both arms. He also complains of having progressive, asymmetrical weakness in both legs, without sphincteric dysfunction, for two months.\nThere is no history of fever, cough, diarrhea, or constipation or of joint pains, weight loss, photosensitivity, polyuria, or polydipsia. The patient is an ex-driver by occupation who has eight children and belongs to a lower socioeconomic group. He is a nonsmoker and denies alcoholism.\nHe has had essential hypertension for five years and takes tablet bisoprolol 5 mg daily on a regular basis. Other than that, his medical history is insignificant. Family history is unremarkable.\n\n ## Figures\n\n \n*Page 1 of 5*",
"pagination": {
"current_page": 1,
"total_pages": 5
},
"questionnaire": [],
"title": "Pain, Tingling, and Numbness in the Extremities With Foot Drop and Progressive Weakening of the Legs"
},
{
"authors": "Sumaira Nabi, MBBS; Rao Suhail, MBBS, FCPS Neurology; Shahzad Ahmed, MBBS",
"content": [
"Physical Examination and Workup",
"On physical examination, the patient is found to be alert and oriented to time, place, and person. The patient's oral temperature is 98.6°F. He has a regular pulse of 68 beats/minute. His blood pressure is 130/80 mm Hg, with no postural drop, and his respiratory rate is 14 breaths/minute. His Glasgow Coma Scale score is 15. His cranial nerves are intact and symmetrical. He has a left foot drop and paraparesis with areflexia and bilateral mute plantar response. (Figure 1)",
"Figure 1.",
"Figure 2.",
"Figure 3.",
"The patient also has a bilateral glove-and-stocking loss of sensations in the upper and lower extremities, with normal perianal sensation, and a high-stepping gait. Signs of meningeal irritation are absent. His abdomen is soft and nontender. There is no clinical evidence of palpable masses, organomegaly, or ascites. His bowel sounds are audible. The patient's precordial examination reveals normal heart sounds. Auscultation of the lung fields reveals normal breath sounds bilaterally.",
"Laboratory analysis demonstrates a normal complete blood count (CBC) and erythrocyte sedimentation rate. Liver function tests, renal function tests, serum glucose level, muscle enzymes, electrocardiogram, and chest radiograph are unremarkable. His nerve conduction studies and electromyography are consistent with a chronic demyelinating polyneuropathy. (Figure 2)",
"Lumbar puncture for cerebrospinal fluid (CSF) analysis is performed, revealing a mildly elevated protein level of 0.52 g/L. All other CSF studies are normal. His hemoglobin A1C, thyroid function tests, and serum B-12 levels are normal. Results for antinuclear antibodies, rheumatoid factor, HIV serology, hepatitis B surface antigens, and hepatitis C antibodies are negative. Computed tomography (CT) scan with contrast of the chest, abdomen, and pelvis is unremarkable. His bone marrow biopsy, bone scan, skeletal survey, and urine test for Bence-Jones protein are negative. His serum protein electrophoresis reveals a discrete, narrow spike in the gamma-globulin region of 1.96 g/L, consistent with monoclonal protein (M-protein; also called paraprotein), and serum immunoglobulin-M (IgM) levels are markedly raised at 13.6 g/L (normal range 0.4-2.3 g/L). (Figure 3)"
],
"date": "May 19, 2014",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/824/942/824942-thumb-1.jpg"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/824/942/824942-thumb-2.jpg"
},
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/824/942/824942-thumb-3.jpg"
}
],
"markdown": "# Pain, Tingling, and Numbness in the Extremities With Foot Drop and Progressive Weakening of the Legs\n\n **Authors:** Sumaira Nabi, MBBS; Rao Suhail, MBBS, FCPS Neurology; Shahzad Ahmed, MBBS \n **Date:** May 19, 2014\n\n ## Content\n\n Physical Examination and Workup\nOn physical examination, the patient is found to be alert and oriented to time, place, and person. The patient's oral temperature is 98.6°F. He has a regular pulse of 68 beats/minute. His blood pressure is 130/80 mm Hg, with no postural drop, and his respiratory rate is 14 breaths/minute. His Glasgow Coma Scale score is 15. His cranial nerves are intact and symmetrical. He has a left foot drop and paraparesis with areflexia and bilateral mute plantar response. (Figure 1)\nFigure 1.\nFigure 2.\nFigure 3.\nThe patient also has a bilateral glove-and-stocking loss of sensations in the upper and lower extremities, with normal perianal sensation, and a high-stepping gait. Signs of meningeal irritation are absent. His abdomen is soft and nontender. There is no clinical evidence of palpable masses, organomegaly, or ascites. His bowel sounds are audible. The patient's precordial examination reveals normal heart sounds. Auscultation of the lung fields reveals normal breath sounds bilaterally.\nLaboratory analysis demonstrates a normal complete blood count (CBC) and erythrocyte sedimentation rate. Liver function tests, renal function tests, serum glucose level, muscle enzymes, electrocardiogram, and chest radiograph are unremarkable. His nerve conduction studies and electromyography are consistent with a chronic demyelinating polyneuropathy. (Figure 2)\nLumbar puncture for cerebrospinal fluid (CSF) analysis is performed, revealing a mildly elevated protein level of 0.52 g/L. All other CSF studies are normal. His hemoglobin A1C, thyroid function tests, and serum B-12 levels are normal. Results for antinuclear antibodies, rheumatoid factor, HIV serology, hepatitis B surface antigens, and hepatitis C antibodies are negative. Computed tomography (CT) scan with contrast of the chest, abdomen, and pelvis is unremarkable. His bone marrow biopsy, bone scan, skeletal survey, and urine test for Bence-Jones protein are negative. His serum protein electrophoresis reveals a discrete, narrow spike in the gamma-globulin region of 1.96 g/L, consistent with monoclonal protein (M-protein; also called paraprotein), and serum immunoglobulin-M (IgM) levels are markedly raised at 13.6 g/L (normal range 0.4-2.3 g/L). (Figure 3)\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n**Figure 3.** \n \n\n\n*Page 2 of 5*",
"pagination": {
"current_page": 2,
"total_pages": 5
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 727683,
"choiceText": "Waldenström macroglobulinemia",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 727685,
"choiceText": "Lymphoma",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 727687,
"choiceText": "Multiple myeloma",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 727689,
"choiceText": "Monoclonal gammopathy of undetermined significance (MGUS)",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 227283,
"questionText": "Based on the history, physical examination, and workup, what is the likely diagnosis?<br>\r\n<br>\r\n<i>Hint: Note the M spike with a normal bone marrow biopsy report and skeletal survey.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Pain, Tingling, and Numbness in the Extremities With Foot Drop and Progressive Weakening of the Legs"
},
{
"authors": "Sumaira Nabi, MBBS; Rao Suhail, MBBS, FCPS Neurology; Shahzad Ahmed, MBBS",
"content": [
"Discussion",
"This patient’s serum showed findings consistent with IgM kappa monoclonal gammopathy. Therefore, the final diagnosis of paraproteinemic neuropathy associated with monoclonal gammopathy of undetermined significance (MGUS) was made.",
"Figure 1.",
"Figure 2.",
"Figure 3.",
"MGUS, an asymptomatic, premalignant clonal plasma cell disorder, is the most common of the plasma cell dyscrasias. MGUS occurs in over 5.3% of persons in the general population over age 70 years, and the incidence increases with age.[1] It is more common in men than women. The disorder is typically detected incidentally when patients undergo protein electrophoresis as part of an evaluation for unrelated indications. There are 3 distinct clinical variants of MGUS: non-IgM MGUS, IgM MGUS, and light-chain MGUS.[2,3] Each of these subtypes has a small risk of progressing to a malignant plasma cell dyscrasia or lymphoproliferative disorder.[4,5]",
"Non-IgM and IgM MGUS are characterized by the presence of a serum M-protein at below 3 g/dL, the existence of less than 10% of monoclonal plasma cells in the bone marrow, no or only small amounts of Bence-Jones protein in the urine, and the absence of lytic bone lesions, as well as the absence of any end organ damage, such as anemia, hypercalcemia, or renal failure, related to the proliferative process.[1]",
"MGUS was once considered benign, but it is now known that approximately 20% of patients will in time acquire a malignant plasma cell disorder, usually myeloma. (Typically, 0.6-3.4% of cases of MGUS progress to multiple myeloma annually.[6,7]) Patients with MGUS also have an increased risk of axial bone fractures and thromboembolism, as well as the development of secondary malignancies after multiple myeloma.[8,9,10] Nonetheless, the median survival of patients with MGUS is only slightly shorter than that of the general population.",
"There is no known role for chemotherapy in the management of MGUS, and in fact, no treatment is recommended or required for individuals with this condition. However, because the disease may progress, patients must be followed over time, using history and clinical assessment to determine whether such progression has occurred. All patients should undergo laboratory evaluation—including assessment of serum and urinary M-protein, creatinine, and serum calcium, as well as a CBC—6 months after diagnosis.",
"Periodic laboratory testing should then be performed in high-risk patients. A risk stratification system can be used to determine which patients fall into this category, since not all persons with MGUS have the same risk of disease progression.[11,12,13] The management of a patient with MGUS requires an understanding of the risk of progression to symptomatic disease requiring therapy.",
"In addition to being incidentally discovered, MGUS is also diagnosed during the course of workup for polyneuropathies. Paraproteinemic neuropathies—which are associated with the presence of M-protein (a so-called M spike) in the serum—are thought to constitute approximately 10% of idiopathic polyneuropathies, with two thirds of all paraproteinemic neuropathy cases believed to be associated with MGUS.[14,15,16] Other conditions associated with paraproteins, including multiple myeloma, Waldenström macroglobulinemia, osteosclerotic myeloma, primary amyloidosis, cryoglobulinemia, non-Hodgkin lymphoma, Castleman disease and related lymphatic diseases, and chronic leukemias, are also typically associated with neuropathies.",
"M-proteins are derived from a single clone of plasma cells. Some M-proteins behave as antibodies for components of myelin and axolemma. Nerve damage from M-proteins may also be secondary to deposition of the amyloid byproduct of the circulating paraprotein. Although IgG is the most common class of paraproteins in patients with MGUS, IgM is more frequent in those with neuropathy (60%).[17,18] Patients with MGUS predominantly have a kappa light-chain component."
],
"date": "May 19, 2014",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/824/942/824942-thumb-1.jpg"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/824/942/824942-thumb-2.jpg"
},
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/824/942/824942-thumb-3.jpg"
}
],
"markdown": "# Pain, Tingling, and Numbness in the Extremities With Foot Drop and Progressive Weakening of the Legs\n\n **Authors:** Sumaira Nabi, MBBS; Rao Suhail, MBBS, FCPS Neurology; Shahzad Ahmed, MBBS \n **Date:** May 19, 2014\n\n ## Content\n\n Discussion\nThis patient’s serum showed findings consistent with IgM kappa monoclonal gammopathy. Therefore, the final diagnosis of paraproteinemic neuropathy associated with monoclonal gammopathy of undetermined significance (MGUS) was made.\nFigure 1.\nFigure 2.\nFigure 3.\nMGUS, an asymptomatic, premalignant clonal plasma cell disorder, is the most common of the plasma cell dyscrasias. MGUS occurs in over 5.3% of persons in the general population over age 70 years, and the incidence increases with age.[1] It is more common in men than women. The disorder is typically detected incidentally when patients undergo protein electrophoresis as part of an evaluation for unrelated indications. There are 3 distinct clinical variants of MGUS: non-IgM MGUS, IgM MGUS, and light-chain MGUS.[2,3] Each of these subtypes has a small risk of progressing to a malignant plasma cell dyscrasia or lymphoproliferative disorder.[4,5]\nNon-IgM and IgM MGUS are characterized by the presence of a serum M-protein at below 3 g/dL, the existence of less than 10% of monoclonal plasma cells in the bone marrow, no or only small amounts of Bence-Jones protein in the urine, and the absence of lytic bone lesions, as well as the absence of any end organ damage, such as anemia, hypercalcemia, or renal failure, related to the proliferative process.[1]\nMGUS was once considered benign, but it is now known that approximately 20% of patients will in time acquire a malignant plasma cell disorder, usually myeloma. (Typically, 0.6-3.4% of cases of MGUS progress to multiple myeloma annually.[6,7]) Patients with MGUS also have an increased risk of axial bone fractures and thromboembolism, as well as the development of secondary malignancies after multiple myeloma.[8,9,10] Nonetheless, the median survival of patients with MGUS is only slightly shorter than that of the general population.\nThere is no known role for chemotherapy in the management of MGUS, and in fact, no treatment is recommended or required for individuals with this condition. However, because the disease may progress, patients must be followed over time, using history and clinical assessment to determine whether such progression has occurred. All patients should undergo laboratory evaluation—including assessment of serum and urinary M-protein, creatinine, and serum calcium, as well as a CBC—6 months after diagnosis.\nPeriodic laboratory testing should then be performed in high-risk patients. A risk stratification system can be used to determine which patients fall into this category, since not all persons with MGUS have the same risk of disease progression.[11,12,13] The management of a patient with MGUS requires an understanding of the risk of progression to symptomatic disease requiring therapy.\nIn addition to being incidentally discovered, MGUS is also diagnosed during the course of workup for polyneuropathies. Paraproteinemic neuropathies—which are associated with the presence of M-protein (a so-called M spike) in the serum—are thought to constitute approximately 10% of idiopathic polyneuropathies, with two thirds of all paraproteinemic neuropathy cases believed to be associated with MGUS.[14,15,16] Other conditions associated with paraproteins, including multiple myeloma, Waldenström macroglobulinemia, osteosclerotic myeloma, primary amyloidosis, cryoglobulinemia, non-Hodgkin lymphoma, Castleman disease and related lymphatic diseases, and chronic leukemias, are also typically associated with neuropathies.\nM-proteins are derived from a single clone of plasma cells. Some M-proteins behave as antibodies for components of myelin and axolemma. Nerve damage from M-proteins may also be secondary to deposition of the amyloid byproduct of the circulating paraprotein. Although IgG is the most common class of paraproteins in patients with MGUS, IgM is more frequent in those with neuropathy (60%).[17,18] Patients with MGUS predominantly have a kappa light-chain component.\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n**Figure 3.** \n \n\n\n*Page 3 of 5*",
"pagination": {
"current_page": 3,
"total_pages": 5
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 727683,
"choiceText": "Waldenström macroglobulinemia",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 727685,
"choiceText": "Lymphoma",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 727687,
"choiceText": "Multiple myeloma",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 727689,
"choiceText": "Monoclonal gammopathy of undetermined significance (MGUS)",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 227283,
"questionText": "Based on the history, physical examination, and workup, what is the likely diagnosis?<br>\r\n<br>\r\n<i>Hint: Note the M spike with a normal bone marrow biopsy report and skeletal survey.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Pain, Tingling, and Numbness in the Extremities With Foot Drop and Progressive Weakening of the Legs"
},
{
"authors": "Sumaira Nabi, MBBS; Rao Suhail, MBBS, FCPS Neurology; Shahzad Ahmed, MBBS",
"content": [
"These neuropathies most often affect men over age 50 years. Patients usually present with sensory symptoms such as numbness, paresthesia, dysesthesia, and aching or lancinating pain, with these predominantly involving the lower extremities. Touch, joint position, and vibratory sensation are mostly affected. Gait difficulties, imbalance, and ataxia progress over a period of months.[19,20] Weakness of the distal leg muscles with variable atrophy occurs as the disease progresses. A pure motor disorder simulating motor neuron disease is seen in a very small number of patients.[21]",
"Figure 1.",
"Figure 2.",
"Figure 3.",
"The literature shows that peripheral neuropathy associated with IgM monoclonal gammopathy presents mainly as a chronic demyelinating sensory polyneuropathy, with predominant tremor, sensory loss, and ataxia. Polyneuropathies reported in association with multiple myeloma or with IgG or IgA monoclonal gammopathy are more heterogeneous (mainly axonal or mixed).[22,23,24]",
"The study of MGUS neuropathy has been confounded by its relation to chronic inflammatory demyelinating polyneuropathy (CIDP), with about one quarter of patients with CIDP also having a paraproteinemia. However, the meaning of this association is unclear. Conduction blocks, which are the cardinal electromyographic feature of CIDP, sometimes occur in MGUS as well,[19,20] and the CSF protein level is usually elevated in both conditions. Moreover, both neuropathies respond to immunomodulating treatments. However, IgM paraproteinemic neuropathies are more refractory to therapy. In some cases, monoclonal gammopathy is a coincidental finding and is unrelated to a patient’s neuropathy.",
"The optimal therapy for MGUS neuropathies has not been established. About one third of patients with IgG or IgA MGUS improve symptomatically within days or weeks of the administration of high-dose intravenous immunoglobulin (IVIG; 0.4 g/kg of body weight daily for 5 days), plasma exchange (a total of 220 mL/kg, given in 4 or 5 treatments), or therapy with corticosteroids, often in combination with immunosuppressants.[25] However, only the benefit of plasma exchange has been confirmed in a controlled clinical trial.[26,27]",
"Although the IgM neuropathies tend to be the most refractory to therapy, some improvement can be expected with the same regimens used for the IgG and IgA neuropathies, particularly if cyclophosphamide or chlorambucil is added in doses sufficient to reduce the amount of M-protein.[28,29]",
"An alternative treatment in paraproteinemic neuropathies is immunoadsorption, but its role has not been fully substantiated through trials.[30] Interferon alfa was beneficial in one trial.[31] However, these treatments generally produce only transient improvement and require repetition every several months, depending on the patient’s response and general condition."
],
"date": "May 19, 2014",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/824/942/824942-thumb-1.jpg"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/824/942/824942-thumb-2.jpg"
},
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/824/942/824942-thumb-3.jpg"
}
],
"markdown": "# Pain, Tingling, and Numbness in the Extremities With Foot Drop and Progressive Weakening of the Legs\n\n **Authors:** Sumaira Nabi, MBBS; Rao Suhail, MBBS, FCPS Neurology; Shahzad Ahmed, MBBS \n **Date:** May 19, 2014\n\n ## Content\n\n These neuropathies most often affect men over age 50 years. Patients usually present with sensory symptoms such as numbness, paresthesia, dysesthesia, and aching or lancinating pain, with these predominantly involving the lower extremities. Touch, joint position, and vibratory sensation are mostly affected. Gait difficulties, imbalance, and ataxia progress over a period of months.[19,20] Weakness of the distal leg muscles with variable atrophy occurs as the disease progresses. A pure motor disorder simulating motor neuron disease is seen in a very small number of patients.[21]\nFigure 1.\nFigure 2.\nFigure 3.\nThe literature shows that peripheral neuropathy associated with IgM monoclonal gammopathy presents mainly as a chronic demyelinating sensory polyneuropathy, with predominant tremor, sensory loss, and ataxia. Polyneuropathies reported in association with multiple myeloma or with IgG or IgA monoclonal gammopathy are more heterogeneous (mainly axonal or mixed).[22,23,24]\nThe study of MGUS neuropathy has been confounded by its relation to chronic inflammatory demyelinating polyneuropathy (CIDP), with about one quarter of patients with CIDP also having a paraproteinemia. However, the meaning of this association is unclear. Conduction blocks, which are the cardinal electromyographic feature of CIDP, sometimes occur in MGUS as well,[19,20] and the CSF protein level is usually elevated in both conditions. Moreover, both neuropathies respond to immunomodulating treatments. However, IgM paraproteinemic neuropathies are more refractory to therapy. In some cases, monoclonal gammopathy is a coincidental finding and is unrelated to a patient’s neuropathy.\nThe optimal therapy for MGUS neuropathies has not been established. About one third of patients with IgG or IgA MGUS improve symptomatically within days or weeks of the administration of high-dose intravenous immunoglobulin (IVIG; 0.4 g/kg of body weight daily for 5 days), plasma exchange (a total of 220 mL/kg, given in 4 or 5 treatments), or therapy with corticosteroids, often in combination with immunosuppressants.[25] However, only the benefit of plasma exchange has been confirmed in a controlled clinical trial.[26,27]\nAlthough the IgM neuropathies tend to be the most refractory to therapy, some improvement can be expected with the same regimens used for the IgG and IgA neuropathies, particularly if cyclophosphamide or chlorambucil is added in doses sufficient to reduce the amount of M-protein.[28,29]\nAn alternative treatment in paraproteinemic neuropathies is immunoadsorption, but its role has not been fully substantiated through trials.[30] Interferon alfa was beneficial in one trial.[31] However, these treatments generally produce only transient improvement and require repetition every several months, depending on the patient’s response and general condition.\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n**Figure 3.** \n \n\n\n*Page 4 of 5*",
"pagination": {
"current_page": 4,
"total_pages": 5
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 727691,
"choiceText": "IgG",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 727693,
"choiceText": "IgA",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 727695,
"choiceText": "IgM",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 727697,
"choiceText": "IgD",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 727699,
"choiceText": "Biclonal",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 227285,
"questionText": "Which is most common type of M-protein associated with MGUS-associated neuropathy?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 727701,
"choiceText": "Serum M-protein value < 3 g/dL",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 727703,
"choiceText": "Normal renal functions",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 727705,
"choiceText": "< 10% plasma cells in the bone marrow",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 727707,
"choiceText": "Lytic bone lesions",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 727709,
"choiceText": "No Bence-Jones proteins in urine",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 227287,
"questionText": "The presence of which of the following will favor the diagnosis of multiple myeloma rather than MGUS?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Pain, Tingling, and Numbness in the Extremities With Foot Drop and Progressive Weakening of the Legs"
},
{
"authors": "Sumaira Nabi, MBBS; Rao Suhail, MBBS, FCPS Neurology; Shahzad Ahmed, MBBS",
"content": [
"The patient in this case was given a trial of 5 sessions of plasmapheresis. Although mild improvement in his sensory symptoms was observed, his left foot drop persisted, so he was given an ankle-foot orthosis (AFO). He is undergoing physiotherapy and has been advised to have regular follow-ups in the neurology and oncology clinics. The plan is to start immunomodulatory therapy.",
"Neuropathies associated with paraproteinemias have heterogeneous clinical, neurophysiologic, neuropathologic, and hematologic features. These necessitate prompt and appropriate workup and management to avoid permanent disability."
],
"date": "May 19, 2014",
"figures": [],
"markdown": "# Pain, Tingling, and Numbness in the Extremities With Foot Drop and Progressive Weakening of the Legs\n\n **Authors:** Sumaira Nabi, MBBS; Rao Suhail, MBBS, FCPS Neurology; Shahzad Ahmed, MBBS \n **Date:** May 19, 2014\n\n ## Content\n\n The patient in this case was given a trial of 5 sessions of plasmapheresis. Although mild improvement in his sensory symptoms was observed, his left foot drop persisted, so he was given an ankle-foot orthosis (AFO). He is undergoing physiotherapy and has been advised to have regular follow-ups in the neurology and oncology clinics. The plan is to start immunomodulatory therapy.\nNeuropathies associated with paraproteinemias have heterogeneous clinical, neurophysiologic, neuropathologic, and hematologic features. These necessitate prompt and appropriate workup and management to avoid permanent disability.\n\n ## Figures\n\n \n*Page 5 of 5*",
"pagination": {
"current_page": 5,
"total_pages": 5
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 727691,
"choiceText": "IgG",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 727693,
"choiceText": "IgA",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 727695,
"choiceText": "IgM",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 727697,
"choiceText": "IgD",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 727699,
"choiceText": "Biclonal",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 227285,
"questionText": "Which is most common type of M-protein associated with MGUS-associated neuropathy?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 727701,
"choiceText": "Serum M-protein value < 3 g/dL",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 727703,
"choiceText": "Normal renal functions",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 727705,
"choiceText": "< 10% plasma cells in the bone marrow",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 727707,
"choiceText": "Lytic bone lesions",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 727709,
"choiceText": "No Bence-Jones proteins in urine",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 227287,
"questionText": "The presence of which of the following will favor the diagnosis of multiple myeloma rather than MGUS?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Pain, Tingling, and Numbness in the Extremities With Foot Drop and Progressive Weakening of the Legs"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 727683,
"choiceText": "Waldenström macroglobulinemia",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 727685,
"choiceText": "Lymphoma",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 727687,
"choiceText": "Multiple myeloma",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 727689,
"choiceText": "Monoclonal gammopathy of undetermined significance (MGUS)",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 227283,
"questionText": "Based on the history, physical examination, and workup, what is the likely diagnosis?<br>\r\n<br>\r\n<i>Hint: Note the M spike with a normal bone marrow biopsy report and skeletal survey.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 727691,
"choiceText": "IgG",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 727693,
"choiceText": "IgA",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 727695,
"choiceText": "IgM",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 727697,
"choiceText": "IgD",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 727699,
"choiceText": "Biclonal",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 227285,
"questionText": "Which is most common type of M-protein associated with MGUS-associated neuropathy?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 727701,
"choiceText": "Serum M-protein value < 3 g/dL",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 727703,
"choiceText": "Normal renal functions",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 727705,
"choiceText": "< 10% plasma cells in the bone marrow",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 727707,
"choiceText": "Lytic bone lesions",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 727709,
"choiceText": "No Bence-Jones proteins in urine",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 227287,
"questionText": "The presence of which of the following will favor the diagnosis of multiple myeloma rather than MGUS?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
824288 | /viewarticle/824288 | [
{
"authors": "Sumaira Nabi, MBBS; Muhammad Irshad, MBBS, FCPS Neurology; Haris Rajput, MBBS, FCPS Neurology",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to recognize accurately. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"A 34-year-old woman presents to the emergency department with a 10-day history of weakness of the bilateral lower extremities, as well as urinary retention and constipation. She also complains of vomiting and dysphagia with liquids, along with drooling and nasal regurgitation for 3 weeks.",
"She has no history of fever, headache, altered sensorium, or seizures. She has no medical history, in particular no history of visual impediment, limb weakness, ataxia, or sensory symptoms.",
"The patient has 3 children, all of whom were born through normal vaginal delivery; the last child was born about 2 months before presentation. The woman is a nonsmoker and is not taking any medications on a regular basis. Her family history is unremarkable as well."
],
"date": "December 11, 2018",
"figures": [],
"markdown": "# A 34-Year-Old Woman With Vomiting and Bulbar Weakness\n\n **Authors:** Sumaira Nabi, MBBS; Muhammad Irshad, MBBS, FCPS Neurology; Haris Rajput, MBBS, FCPS Neurology \n **Date:** December 11, 2018\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to recognize accurately. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nA 34-year-old woman presents to the emergency department with a 10-day history of weakness of the bilateral lower extremities, as well as urinary retention and constipation. She also complains of vomiting and dysphagia with liquids, along with drooling and nasal regurgitation for 3 weeks.\nShe has no history of fever, headache, altered sensorium, or seizures. She has no medical history, in particular no history of visual impediment, limb weakness, ataxia, or sensory symptoms.\nThe patient has 3 children, all of whom were born through normal vaginal delivery; the last child was born about 2 months before presentation. The woman is a nonsmoker and is not taking any medications on a regular basis. Her family history is unremarkable as well.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 34-Year-Old Woman With Vomiting and Bulbar Weakness"
},
{
"authors": "Sumaira Nabi, MBBS; Muhammad Irshad, MBBS, FCPS Neurology; Haris Rajput, MBBS, FCPS Neurology",
"content": [
"During the physical examination, the patient appears ill but is conscious and oriented to time, place, and person. Her oral temperature is 98.6°F (37°C). She has a regular pulse of 86 beats/minute. Her blood pressure is 110/80 mm Hg and her respiratory rate is 20 breaths/minute. Her Glasgow Coma Scale score is 15. She has dysphonia, with a nasal twang to her speech.",
"On examination of the cranial nerves, she has a pendular nystagmus in all directions of gaze and reduced palatal movements on the right side. She has flaccid paraplegia with normal reflexes and bilateral mute plantar response. Her sensations are intact. Signs of meningeal irritation are absent. Her abdomen is soft and nontender. She has no evidence of organomegaly or ascites. Her bowel sounds are audible and normal. The patient’s precordial examination reveals normal heart sounds. Auscultation reveals coarse crackles throughout both lung fields.",
"Laboratory analysis demonstrates a normal complete blood count, with an erythrocyte sedimentation rate of 20 mm/hour. Her liver function test results, renal function test results, creatinine kinase level, electrocardiogram findings, and abdominal ultrasonogram findings are unremarkable. Her chest radiograph findings are consistent with aspiration pneumonia. Nerve conduction studies are inconclusive. Because the patient is critically ill with a provisional diagnosis of Guillain-Barré syndrome, plasmapheresis is initiated. MRI of the brain with contrast reveals a subtle oblong area with high signal intensity in the medulla on T2-weighted fluid-attenuated inversion recovery (FLAIR) sequences (Figures 1-3).",
"Figure 1.",
"Figure 2.",
"Figure 3.",
"Lumbar puncture for cerebrospinal fluid (CSF) analysis demonstrates a mildly elevated CSF protein level of 0.55 g/L, lymphocytic pleocytosis (104 white blood cells/mm3, with 98% lymphocytes), and a normal glucose concentration. CSF Gram staining, culture, and cytology, as well as acid-fast bacillus staining and oligoclonal bands findings, are all negative. MRI of the thoracic spine with contrast reveals abnormal magnetic resonance signals extending from the level of the T3-T4 to the T8-T9 intervertebral disc; intensity is low on T1-weighted imaging and high on T2-weighted imaging and FLAIR, and shows subtle postcontrast enhancement (Figures 4-5).",
"Figure 4.",
"Figure 5.",
"Visual evoked potential is normal. The patient’s extended work-up includes a positive antinuclear antibody result; however, the results for rheumatoid arthritis factor and anti–double-stranded DNA are negative. Her extractable nuclear antigen profile is pending."
],
"date": "December 11, 2018",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/824/288/824288-thumb-1.jpg"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/824/288/824288-thumb-2.jpg"
},
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/824/288/824288-thumb-3.jpg"
},
{
"caption": "Figure 4.",
"image_url": "https://img.medscapestatic.com/article/824/288/824288-thumb-4.jpg"
},
{
"caption": "Figure 5.",
"image_url": "https://img.medscapestatic.com/article/824/288/824288-thumb-5.jpg"
}
],
"markdown": "# A 34-Year-Old Woman With Vomiting and Bulbar Weakness\n\n **Authors:** Sumaira Nabi, MBBS; Muhammad Irshad, MBBS, FCPS Neurology; Haris Rajput, MBBS, FCPS Neurology \n **Date:** December 11, 2018\n\n ## Content\n\n During the physical examination, the patient appears ill but is conscious and oriented to time, place, and person. Her oral temperature is 98.6°F (37°C). She has a regular pulse of 86 beats/minute. Her blood pressure is 110/80 mm Hg and her respiratory rate is 20 breaths/minute. Her Glasgow Coma Scale score is 15. She has dysphonia, with a nasal twang to her speech.\nOn examination of the cranial nerves, she has a pendular nystagmus in all directions of gaze and reduced palatal movements on the right side. She has flaccid paraplegia with normal reflexes and bilateral mute plantar response. Her sensations are intact. Signs of meningeal irritation are absent. Her abdomen is soft and nontender. She has no evidence of organomegaly or ascites. Her bowel sounds are audible and normal. The patient’s precordial examination reveals normal heart sounds. Auscultation reveals coarse crackles throughout both lung fields.\nLaboratory analysis demonstrates a normal complete blood count, with an erythrocyte sedimentation rate of 20 mm/hour. Her liver function test results, renal function test results, creatinine kinase level, electrocardiogram findings, and abdominal ultrasonogram findings are unremarkable. Her chest radiograph findings are consistent with aspiration pneumonia. Nerve conduction studies are inconclusive. Because the patient is critically ill with a provisional diagnosis of Guillain-Barré syndrome, plasmapheresis is initiated. MRI of the brain with contrast reveals a subtle oblong area with high signal intensity in the medulla on T2-weighted fluid-attenuated inversion recovery (FLAIR) sequences (Figures 1-3).\nFigure 1.\nFigure 2.\nFigure 3.\nLumbar puncture for cerebrospinal fluid (CSF) analysis demonstrates a mildly elevated CSF protein level of 0.55 g/L, lymphocytic pleocytosis (104 white blood cells/mm3, with 98% lymphocytes), and a normal glucose concentration. CSF Gram staining, culture, and cytology, as well as acid-fast bacillus staining and oligoclonal bands findings, are all negative. MRI of the thoracic spine with contrast reveals abnormal magnetic resonance signals extending from the level of the T3-T4 to the T8-T9 intervertebral disc; intensity is low on T1-weighted imaging and high on T2-weighted imaging and FLAIR, and shows subtle postcontrast enhancement (Figures 4-5).\nFigure 4.\nFigure 5.\nVisual evoked potential is normal. The patient’s extended work-up includes a positive antinuclear antibody result; however, the results for rheumatoid arthritis factor and anti–double-stranded DNA are negative. Her extractable nuclear antigen profile is pending.\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n**Figure 3.** \n \n\n**Figure 4.** \n \n\n**Figure 5.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 724409,
"choiceText": "Guillain-Barré syndrome",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 724411,
"choiceText": "Multiple sclerosis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 724413,
"choiceText": "Acute disseminated encephalomyelitis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 724415,
"choiceText": "Sjögren syndrome",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 226167,
"questionText": "Based on the history, physical examination, and workup, which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 34-Year-Old Woman With Vomiting and Bulbar Weakness"
},
{
"authors": "Sumaira Nabi, MBBS; Muhammad Irshad, MBBS, FCPS Neurology; Haris Rajput, MBBS, FCPS Neurology",
"content": [
"The extractable nuclear antigen profile of the patient showed positive anti-Ro and anti-La antibodies. She had already undergone 2 sessions of plasmapheresis, with no real improvement in her condition. Pulse steroid therapy was initiated. After receiving 5 injections, the bulbar weakness was remarkably improved, and she started tolerating oral feeding. Her feeding tube was removed, but her limb weakness persisted. A rheumatology consultation was obtained, and monthly cyclophosphamide therapy was suggested. The patient was diagnosed with Sjögren syndrome (SS) with neurologic manifestations. She had no sicca features.",
"SS is a chronic inflammatory disorder characterized mainly by sicca symptoms such as xerophthalmia and xerostomia secondary to decreased lacrimal and salivary gland functions.[1] It is an autoimmune exocrinopathy and is classified as primary or secondary, depending on the severity of the sicca complex and whether an autoimmune connective tissue disorder, such as rheumatoid arthritis is also present. Rheumatoid arthritis is the most common rheumatologic disorder associated with secondary SS.[2,3,4]",
"The clinical manifestations of SS are divided into exocrine glandular and extraglandular features. Primary SS (pSS) includes a broad variety of clinical manifestations, as well as neurologic manifestations (0-70%). The neurologic manifestations of pSS can be divided into central nervous system (CNS) manifestations and neuromuscular (peripheral nervous system) manifestations.[5,6,7,8,9,10] In the CNS, any part of the brain or spinal cord can be affected. The pattern is generally one of recurrent episodes that often are separated by long asymptomatic periods."
],
"date": "December 11, 2018",
"figures": [],
"markdown": "# A 34-Year-Old Woman With Vomiting and Bulbar Weakness\n\n **Authors:** Sumaira Nabi, MBBS; Muhammad Irshad, MBBS, FCPS Neurology; Haris Rajput, MBBS, FCPS Neurology \n **Date:** December 11, 2018\n\n ## Content\n\n The extractable nuclear antigen profile of the patient showed positive anti-Ro and anti-La antibodies. She had already undergone 2 sessions of plasmapheresis, with no real improvement in her condition. Pulse steroid therapy was initiated. After receiving 5 injections, the bulbar weakness was remarkably improved, and she started tolerating oral feeding. Her feeding tube was removed, but her limb weakness persisted. A rheumatology consultation was obtained, and monthly cyclophosphamide therapy was suggested. The patient was diagnosed with Sjögren syndrome (SS) with neurologic manifestations. She had no sicca features.\nSS is a chronic inflammatory disorder characterized mainly by sicca symptoms such as xerophthalmia and xerostomia secondary to decreased lacrimal and salivary gland functions.[1] It is an autoimmune exocrinopathy and is classified as primary or secondary, depending on the severity of the sicca complex and whether an autoimmune connective tissue disorder, such as rheumatoid arthritis is also present. Rheumatoid arthritis is the most common rheumatologic disorder associated with secondary SS.[2,3,4]\nThe clinical manifestations of SS are divided into exocrine glandular and extraglandular features. Primary SS (pSS) includes a broad variety of clinical manifestations, as well as neurologic manifestations (0-70%). The neurologic manifestations of pSS can be divided into central nervous system (CNS) manifestations and neuromuscular (peripheral nervous system) manifestations.[5,6,7,8,9,10] In the CNS, any part of the brain or spinal cord can be affected. The pattern is generally one of recurrent episodes that often are separated by long asymptomatic periods.\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 724409,
"choiceText": "Guillain-Barré syndrome",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 724411,
"choiceText": "Multiple sclerosis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 724413,
"choiceText": "Acute disseminated encephalomyelitis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 724415,
"choiceText": "Sjögren syndrome",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 226167,
"questionText": "Based on the history, physical examination, and workup, which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 34-Year-Old Woman With Vomiting and Bulbar Weakness"
},
{
"authors": "Sumaira Nabi, MBBS; Muhammad Irshad, MBBS, FCPS Neurology; Haris Rajput, MBBS, FCPS Neurology",
"content": [
"The pathogenesis of neurologic involvement in SS has not been fully elucidated. Several hypotheses have been proposed in different studies, including direct infiltration of the CNS by mononuclear cells, vascular involvement, and ischemia secondary to small-vessel vasculitis.[10,11,12] Antineuronal antibodies also have been described in patients with pSS with neurologic involvement; however, these antibodies have an unknown pathogenic role.",
"CNS involvement in series of patients with pSS ranges from 0% to 68% and may be focal or diffuse.[11,13,14,15,16,17,18,19] Focal CNS involvement results in seizures, motor or sensory deficits, and cerebellar syndromes. Motor and sensory deficits depend on the area of the CNS involved. Multifocal lesions result in cognitive impairment, dementia, aseptic meningitis, encephalopathy, psychiatric disorders, and conditions that mimic multiple sclerosis, as was the case with the patient presented here. Spinal cord involvement can result in acute transverse myelitis or a chronic progressive myelopathy. Cases of neuromyelitis optica also have been reported.[20] Movement disorders, such as chorea, have been documented in the literature.[21]",
"Nervous system involvement can manifest as pure sensory neuropathy, pure motor neuropathy, mononeuritis multiplex, trigeminal and other cranial neuropathies, autonomic neuropathy, and radiculoneuropathy.[12,22,23,24,25,26,27,28] Axonal polyneuropathy is the most common neuropathy seen in pSS; it includes distal sensorimotor and sensory polyneuropathies. The disease usually starts with distal and symmetrical involvement. Patients may sometimes present with autonomic dysfunction that manifests as pupillary abnormalities, anhidrosis, tachycardia, and orthostatic hypotension. Autonomic symptoms may be explained by both ganglion neuronopathy and vasculitis.",
"Trigeminal neuropathy occurs most often in patients with compromised cranial nerves, followed by facial and oculomotor nerve palsies. Trigeminal neuropathy usually presents with unilateral sensory involvement, which may subsequently become bilateral. Motor involvement of the fifth nerve is uncommon. Myopathies and myositis also have been reported.[29]",
"This polymorphism accounts for the delay in the diagnosis of pSS with neurologic involvement. Neurologic manifestations may precede sicca symptoms in 40% to 93% of cases, with full-blown disease developing later, as perhaps was the case with the patient in this case. According to one study, 93% of patients were diagnosed with pSS after the appearance of neuropathic symptoms and signs.",
"Many modalities have been used for the diagnosis of neurologic involvement in pSS. Spinal tap and CSF examination may be helpful. Lymphocytes may be found (usually <50 cells/mm3). CSF also is necessary to differentiate pSS from conditions such as CNS infection and multiple sclerosis. Oligoclonal bands are suggestive of multiple sclerosis (reported in about 20-25% of patients with pSS, compared with >90% of patients with multiple sclerosis).[30,31,32]",
"In patients with pSS, MRI of the brain usually demonstrates hyperintense areas in the subcortical and periventricular white matter on T2-weighted and FLAIR sequences.[32] These lesions are usually less pronounced in patients with pSS than they are in those with multiple sclerosis.",
"Nerve conduction studies are helpful in diagnosing and differentiating between the 2 major types of neuropathies (axonal and demyelinating), as well as motor and sensory neuropathies. Other electrophysiologic studies include electroencephalography, evoked response testing, and brainstem visual and somatosensory evoked potential studies, depending on the signs and symptoms of the patient.",
"Sural nerve biopsy may be performed in patients with neuropathy. Histopathology usually reveals vascular or perivascular inflammation of small epineurial vessels. Sometimes a necrotizing vasculitis may be observed. Skin biopsy is useful in diagnosing small-fiber neuropathy. The literature has reported that 40% of patients with pSS experience chronic neuropathic pain with normal electrodiagnostic studies. In these cases, skin biopsy has been validated as a diagnostic tool.[5]",
"Multiple diagnostic tests have been used to establish the diagnosis of SS: tests to confirm keratoconjunctivitis sicca, tests to quantify xerostomia, salivary gland biopsy, serologic tests for antibodies to Ro/SSA and La/SSB, and MRI of the salivary glands.[1,2,3] In patients who present primarily with neurologic manifestations, these tests may all initially be negative, especially if sicca symptoms are absent. Anti-Ro and anti-La antibodies seem to be less frequent in patients with pSS with neurologic involvement (40%) than in patients without neurologic manifestations (60%). Thus, new markers are being investigated in pSS to better diagnose patients with neurologic involvement.",
"SS has been confused with multiple sclerosis, especially given the similar features found in these illnesses.[33,34] As in the patient in this case, however, pSS and peripheral nervous system disease can occur simultaneously, which is quite rare in multiple sclerosis. CSF oligoclonal bands are also a handy tool for differentiating between the 2 conditions."
],
"date": "December 11, 2018",
"figures": [],
"markdown": "# A 34-Year-Old Woman With Vomiting and Bulbar Weakness\n\n **Authors:** Sumaira Nabi, MBBS; Muhammad Irshad, MBBS, FCPS Neurology; Haris Rajput, MBBS, FCPS Neurology \n **Date:** December 11, 2018\n\n ## Content\n\n The pathogenesis of neurologic involvement in SS has not been fully elucidated. Several hypotheses have been proposed in different studies, including direct infiltration of the CNS by mononuclear cells, vascular involvement, and ischemia secondary to small-vessel vasculitis.[10,11,12] Antineuronal antibodies also have been described in patients with pSS with neurologic involvement; however, these antibodies have an unknown pathogenic role.\nCNS involvement in series of patients with pSS ranges from 0% to 68% and may be focal or diffuse.[11,13,14,15,16,17,18,19] Focal CNS involvement results in seizures, motor or sensory deficits, and cerebellar syndromes. Motor and sensory deficits depend on the area of the CNS involved. Multifocal lesions result in cognitive impairment, dementia, aseptic meningitis, encephalopathy, psychiatric disorders, and conditions that mimic multiple sclerosis, as was the case with the patient presented here. Spinal cord involvement can result in acute transverse myelitis or a chronic progressive myelopathy. Cases of neuromyelitis optica also have been reported.[20] Movement disorders, such as chorea, have been documented in the literature.[21]\nNervous system involvement can manifest as pure sensory neuropathy, pure motor neuropathy, mononeuritis multiplex, trigeminal and other cranial neuropathies, autonomic neuropathy, and radiculoneuropathy.[12,22,23,24,25,26,27,28] Axonal polyneuropathy is the most common neuropathy seen in pSS; it includes distal sensorimotor and sensory polyneuropathies. The disease usually starts with distal and symmetrical involvement. Patients may sometimes present with autonomic dysfunction that manifests as pupillary abnormalities, anhidrosis, tachycardia, and orthostatic hypotension. Autonomic symptoms may be explained by both ganglion neuronopathy and vasculitis.\nTrigeminal neuropathy occurs most often in patients with compromised cranial nerves, followed by facial and oculomotor nerve palsies. Trigeminal neuropathy usually presents with unilateral sensory involvement, which may subsequently become bilateral. Motor involvement of the fifth nerve is uncommon. Myopathies and myositis also have been reported.[29]\nThis polymorphism accounts for the delay in the diagnosis of pSS with neurologic involvement. Neurologic manifestations may precede sicca symptoms in 40% to 93% of cases, with full-blown disease developing later, as perhaps was the case with the patient in this case. According to one study, 93% of patients were diagnosed with pSS after the appearance of neuropathic symptoms and signs.\nMany modalities have been used for the diagnosis of neurologic involvement in pSS. Spinal tap and CSF examination may be helpful. Lymphocytes may be found (usually <50 cells/mm3). CSF also is necessary to differentiate pSS from conditions such as CNS infection and multiple sclerosis. Oligoclonal bands are suggestive of multiple sclerosis (reported in about 20-25% of patients with pSS, compared with >90% of patients with multiple sclerosis).[30,31,32]\nIn patients with pSS, MRI of the brain usually demonstrates hyperintense areas in the subcortical and periventricular white matter on T2-weighted and FLAIR sequences.[32] These lesions are usually less pronounced in patients with pSS than they are in those with multiple sclerosis.\nNerve conduction studies are helpful in diagnosing and differentiating between the 2 major types of neuropathies (axonal and demyelinating), as well as motor and sensory neuropathies. Other electrophysiologic studies include electroencephalography, evoked response testing, and brainstem visual and somatosensory evoked potential studies, depending on the signs and symptoms of the patient.\nSural nerve biopsy may be performed in patients with neuropathy. Histopathology usually reveals vascular or perivascular inflammation of small epineurial vessels. Sometimes a necrotizing vasculitis may be observed. Skin biopsy is useful in diagnosing small-fiber neuropathy. The literature has reported that 40% of patients with pSS experience chronic neuropathic pain with normal electrodiagnostic studies. In these cases, skin biopsy has been validated as a diagnostic tool.[5]\nMultiple diagnostic tests have been used to establish the diagnosis of SS: tests to confirm keratoconjunctivitis sicca, tests to quantify xerostomia, salivary gland biopsy, serologic tests for antibodies to Ro/SSA and La/SSB, and MRI of the salivary glands.[1,2,3] In patients who present primarily with neurologic manifestations, these tests may all initially be negative, especially if sicca symptoms are absent. Anti-Ro and anti-La antibodies seem to be less frequent in patients with pSS with neurologic involvement (40%) than in patients without neurologic manifestations (60%). Thus, new markers are being investigated in pSS to better diagnose patients with neurologic involvement.\nSS has been confused with multiple sclerosis, especially given the similar features found in these illnesses.[33,34] As in the patient in this case, however, pSS and peripheral nervous system disease can occur simultaneously, which is quite rare in multiple sclerosis. CSF oligoclonal bands are also a handy tool for differentiating between the 2 conditions.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 34-Year-Old Woman With Vomiting and Bulbar Weakness"
},
{
"authors": "Sumaira Nabi, MBBS; Muhammad Irshad, MBBS, FCPS Neurology; Haris Rajput, MBBS, FCPS Neurology",
"content": [
"No validated guidelines regarding the specific treatment of neurologic involvement in pSS are available. However, therapy is directed toward the vasculitis that leads to the neurologic manifestations.",
"In general, corticosteroid therapy is initiated in patients with either CNS or peripheral nervous system involvement. CNS involvement usually responds well to high-dose corticosteroid therapy.[35] For the treatment of acute and chronic myelopathies, a combination of steroids and monthly cyclophosphamide has been used, with a fairly good rate of remission.[36] However, some cases may progress. For the most part, peripheral neuropathy in pSS responds poorly to treatment.[28]"
],
"date": "December 11, 2018",
"figures": [],
"markdown": "# A 34-Year-Old Woman With Vomiting and Bulbar Weakness\n\n **Authors:** Sumaira Nabi, MBBS; Muhammad Irshad, MBBS, FCPS Neurology; Haris Rajput, MBBS, FCPS Neurology \n **Date:** December 11, 2018\n\n ## Content\n\n No validated guidelines regarding the specific treatment of neurologic involvement in pSS are available. However, therapy is directed toward the vasculitis that leads to the neurologic manifestations.\nIn general, corticosteroid therapy is initiated in patients with either CNS or peripheral nervous system involvement. CNS involvement usually responds well to high-dose corticosteroid therapy.[35] For the treatment of acute and chronic myelopathies, a combination of steroids and monthly cyclophosphamide has been used, with a fairly good rate of remission.[36] However, some cases may progress. For the most part, peripheral neuropathy in pSS responds poorly to treatment.[28]\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 724417,
"choiceText": "Vagus nerve",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 724419,
"choiceText": "Facial nerve",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 724421,
"choiceText": "Trigeminal nerve",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 724423,
"choiceText": "Accessory nerve",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 724425,
"choiceText": "Oculomotor nerve",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Trigeminal neuropathy usually presents with unilateral sensory involvement, which may subsequently become bilateral. Motor involvement of the fifth nerve is uncommon. Myopathies and myositis also have been reported in pSS.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 226169,
"questionText": "Which cranial nerve is most commonly affected in primary Sjögren syndrome?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 724767,
"choiceText": "Systemic lupus erythematosus",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 724769,
"choiceText": "Scleroderma",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 724771,
"choiceText": "Polymyositis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 724773,
"choiceText": "Rheumatoid arthritis",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 724795,
"choiceText": "Dermatomyositis",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "CNS complications are potentially serious, but some are treatable with immunosuppressive medications. Awareness of the neurologic manifestations of SS is important to avoid long-term complications associated with delayed or denied treatment. Neurologic manifestations may precede sicca features.",
"displayOrder": 4,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 226265,
"questionText": "Which is the most common rheumatologic disorder associated with secondary Sjögren syndrome?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 34-Year-Old Woman With Vomiting and Bulbar Weakness"
},
{
"authors": "Sumaira Nabi, MBBS; Muhammad Irshad, MBBS, FCPS Neurology; Haris Rajput, MBBS, FCPS Neurology",
"content": [],
"date": "December 11, 2018",
"figures": [],
"markdown": "# A 34-Year-Old Woman With Vomiting and Bulbar Weakness\n\n **Authors:** Sumaira Nabi, MBBS; Muhammad Irshad, MBBS, FCPS Neurology; Haris Rajput, MBBS, FCPS Neurology \n **Date:** December 11, 2018\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 724417,
"choiceText": "Vagus nerve",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 724419,
"choiceText": "Facial nerve",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 724421,
"choiceText": "Trigeminal nerve",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 724423,
"choiceText": "Accessory nerve",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 724425,
"choiceText": "Oculomotor nerve",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Trigeminal neuropathy usually presents with unilateral sensory involvement, which may subsequently become bilateral. Motor involvement of the fifth nerve is uncommon. Myopathies and myositis also have been reported in pSS.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 226169,
"questionText": "Which cranial nerve is most commonly affected in primary Sjögren syndrome?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 724767,
"choiceText": "Systemic lupus erythematosus",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 724769,
"choiceText": "Scleroderma",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 724771,
"choiceText": "Polymyositis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 724773,
"choiceText": "Rheumatoid arthritis",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 724795,
"choiceText": "Dermatomyositis",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "CNS complications are potentially serious, but some are treatable with immunosuppressive medications. Awareness of the neurologic manifestations of SS is important to avoid long-term complications associated with delayed or denied treatment. Neurologic manifestations may precede sicca features.",
"displayOrder": 4,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 226265,
"questionText": "Which is the most common rheumatologic disorder associated with secondary Sjögren syndrome?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 34-Year-Old Woman With Vomiting and Bulbar Weakness"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 724409,
"choiceText": "Guillain-Barré syndrome",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 724411,
"choiceText": "Multiple sclerosis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 724413,
"choiceText": "Acute disseminated encephalomyelitis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 724415,
"choiceText": "Sjögren syndrome",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 226167,
"questionText": "Based on the history, physical examination, and workup, which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 724417,
"choiceText": "Vagus nerve",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 724419,
"choiceText": "Facial nerve",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 724421,
"choiceText": "Trigeminal nerve",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 724423,
"choiceText": "Accessory nerve",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 724425,
"choiceText": "Oculomotor nerve",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Trigeminal neuropathy usually presents with unilateral sensory involvement, which may subsequently become bilateral. Motor involvement of the fifth nerve is uncommon. Myopathies and myositis also have been reported in pSS.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 226169,
"questionText": "Which cranial nerve is most commonly affected in primary Sjögren syndrome?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 724767,
"choiceText": "Systemic lupus erythematosus",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 724769,
"choiceText": "Scleroderma",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 724771,
"choiceText": "Polymyositis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 724773,
"choiceText": "Rheumatoid arthritis",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 724795,
"choiceText": "Dermatomyositis",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "CNS complications are potentially serious, but some are treatable with immunosuppressive medications. Awareness of the neurologic manifestations of SS is important to avoid long-term complications associated with delayed or denied treatment. Neurologic manifestations may precede sicca features.",
"displayOrder": 4,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 226265,
"questionText": "Which is the most common rheumatologic disorder associated with secondary Sjögren syndrome?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
822266 | /viewarticle/822266 | [
{
"authors": "Caroline Tschibelu, MD",
"content": [
"Editorial Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"A 48-year-old man presents to the emergency department with coughing, nonradiating chest pressure, and increasing difficulty breathing over the past month. He had dyspnea upon exertion at first, but now he feels out of breath with minimal exertion. The patient states that over the past month he has developed a cough that was intermittent at first, but is now \"productive of white sputum\" and almost constant. Simultaneously, he has noticed progressive facial and bilateral arm swelling.",
"In addition, over the past 2 weeks he has had frequent headaches, dizziness, and a few days of blurred vision especially in his right eye. He denies chest pain, palpitations, and dysphagia.",
"The patient just returned from Puerto Rico, where he had spent 2 months on vacation with his family; he had shortened his trip because his symptoms had worsened.",
"His past medical history is notable for a recent diagnosis of hypertension, for which he was started on lisinopril 2 months ago, and for hyperlipidemia, treated with atorvastatin. He has smoked 1 pack of cigarettes daily for 25 years, and he drinks alcohol socially."
],
"date": "June 01, 2018",
"figures": [],
"markdown": "# A 48-Year-Old Man With Dyspnea and Cyanotic Skin\n\n **Authors:** Caroline Tschibelu, MD \n **Date:** June 01, 2018\n\n ## Content\n\n Editorial Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nA 48-year-old man presents to the emergency department with coughing, nonradiating chest pressure, and increasing difficulty breathing over the past month. He had dyspnea upon exertion at first, but now he feels out of breath with minimal exertion. The patient states that over the past month he has developed a cough that was intermittent at first, but is now \"productive of white sputum\" and almost constant. Simultaneously, he has noticed progressive facial and bilateral arm swelling.\nIn addition, over the past 2 weeks he has had frequent headaches, dizziness, and a few days of blurred vision especially in his right eye. He denies chest pain, palpitations, and dysphagia.\nThe patient just returned from Puerto Rico, where he had spent 2 months on vacation with his family; he had shortened his trip because his symptoms had worsened.\nHis past medical history is notable for a recent diagnosis of hypertension, for which he was started on lisinopril 2 months ago, and for hyperlipidemia, treated with atorvastatin. He has smoked 1 pack of cigarettes daily for 25 years, and he drinks alcohol socially.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 48-Year-Old Man With Dyspnea and Cyanotic Skin"
},
{
"authors": "Caroline Tschibelu, MD",
"content": [
"The patient is an overweight male, who is in mild respiratory distress but able to speak in full sentences. He needs to sit up straight to breathe adequately. Vitals are notable for an oxygen saturation of 90% on room air, for which he is immediately placed on a nonrebreather. His other vital signs include a regular pulse of 92 beats/min, respiratory rate of 24 breaths/min, and blood pressure of 110/80 mm Hg. The patient is afebrile.",
"The patient’s face is plethoric and diffusely cyanotic. His voice is hoarse. The patient’s right pupil is 1 mm in diameter and not reactive to light; his left pupil is 3 mm in diameter and reactive to light. Right upper eyelid ptosis is noted (Figure 1).",
"Figure 1.",
"No substantial oropharyngeal edema is noted, and jugular venous distention is difficult to assess due to swelling. Pitting edema of the trunk and bilateral upper extremities is noticeable, with the right side more affected than the left. The patient’s skin is cyanotic, and superficial veins are visible (Figure 2).",
"Figure 2.",
"Heart sounds are regular, with no murmurs, rubs, or gallop appreciated. Lung examination reveals reduced levels of breath sounds in the right upper lung, lower lung, and bibasilar rhonchi. Lower extremities are not edematous. The patient has no focal neurologic deficits otherwise.",
"In addition to the administration of supplemental oxygen, urgent management included cardiac monitoring and ultrasound-guided intravenous (IV) access. Laboratory tests included complete blood count and a chemistry and coagulation profile, which are all within normal limits. An electrocardiogram (ECG) was obtained and compared to a prior tracing. The ECG does not show any acute ST-T wave changes.",
"The patient is now comfortable, protecting his airway. His oxygen saturation is 97%. A chest radiograph and chest CT scan are obtained (Figure 3).",
"Figure 3."
],
"date": "June 01, 2018",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/822/266/822266-thumb-1.jpg"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/822/266/822266-thumb-2.jpg"
},
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/822/266/822266-thumb-3.jpg"
}
],
"markdown": "# A 48-Year-Old Man With Dyspnea and Cyanotic Skin\n\n **Authors:** Caroline Tschibelu, MD \n **Date:** June 01, 2018\n\n ## Content\n\n The patient is an overweight male, who is in mild respiratory distress but able to speak in full sentences. He needs to sit up straight to breathe adequately. Vitals are notable for an oxygen saturation of 90% on room air, for which he is immediately placed on a nonrebreather. His other vital signs include a regular pulse of 92 beats/min, respiratory rate of 24 breaths/min, and blood pressure of 110/80 mm Hg. The patient is afebrile.\nThe patient’s face is plethoric and diffusely cyanotic. His voice is hoarse. The patient’s right pupil is 1 mm in diameter and not reactive to light; his left pupil is 3 mm in diameter and reactive to light. Right upper eyelid ptosis is noted (Figure 1).\nFigure 1.\nNo substantial oropharyngeal edema is noted, and jugular venous distention is difficult to assess due to swelling. Pitting edema of the trunk and bilateral upper extremities is noticeable, with the right side more affected than the left. The patient’s skin is cyanotic, and superficial veins are visible (Figure 2).\nFigure 2.\nHeart sounds are regular, with no murmurs, rubs, or gallop appreciated. Lung examination reveals reduced levels of breath sounds in the right upper lung, lower lung, and bibasilar rhonchi. Lower extremities are not edematous. The patient has no focal neurologic deficits otherwise.\nIn addition to the administration of supplemental oxygen, urgent management included cardiac monitoring and ultrasound-guided intravenous (IV) access. Laboratory tests included complete blood count and a chemistry and coagulation profile, which are all within normal limits. An electrocardiogram (ECG) was obtained and compared to a prior tracing. The ECG does not show any acute ST-T wave changes.\nThe patient is now comfortable, protecting his airway. His oxygen saturation is 97%. A chest radiograph and chest CT scan are obtained (Figure 3).\nFigure 3.\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n**Figure 3.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 715613,
"choiceText": "Aortic artery dissection",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 715615,
"choiceText": "Tuberculosis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 715617,
"choiceText": "Lymphedema secondary to filariasis infection",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 715619,
"choiceText": "Superior vena cava syndrome",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 715621,
"choiceText": "Hodgkin lymphoma",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 715623,
"choiceText": "Angioedema",
"correct": false,
"displayOrder": 6,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 223301,
"questionText": "Based on the history, physical examination, and radiograph, what is the likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 48-Year-Old Man With Dyspnea and Cyanotic Skin"
},
{
"authors": "Caroline Tschibelu, MD",
"content": [
"Superior vena cava (SVC) syndrome is a clinical diagnosis, in this case made based on the characteristic physical findings and the patient's medical history. The chest radiograph revealed a large right-sided hilar consolidation of the right lung with lung collapse and bilateral pleural effusions.",
"Presenting symptoms result from SVC obstruction and usually consist of dyspnea, facial and neck swelling, arm edema, distended neck and chest veins,[1] cough, visual symptoms, hoarseness, stridor, headaches, and cyanosis. The patient in this case presented with most of these symptoms. More concerning signs and symptoms are dizziness, confusion, and obtundation, as they are signs of cerebral edema, which, while rare, is usually fatal. Approximately 15,000 new cases of SVC syndrome occur annually in the United States.[2]",
"The differential diagnosis of SVC syndrome includes cardiac tamponade; tuberculosis (especially reactivated tuberculosis); syphilis; Bancroftian filariasis; bacterial, fungal, or viral pneumonia; non-Hodgkin lymphoma; angioedema; mediastinitis; and vasculitic disorders. In addition, aortic dissection can present as SVC syndrome.[3] Hodgkin lymphoma rarely causes SVC syndrome.",
"SVC syndrome used to have predominantly infectious etiologies. The first case, documented by William Hunter in 1757, was of a young man who died of a syphilitic aortic aneurysm compressing the superior vena cava.[4] Other causes included granulomatous mediastinal diseases, notably mediastinitis from tuberculosis.[5] The prevalence of infectious cases of SVC syndrome has significantly decreased with the advent of antibiotics. Malignancies are now the leading causes of SVC syndrome, accounting for 90% of all SVC syndrome cases. The remainder of the cases are due to nonmalignant etiologies such as aortic aneurysm, thrombosis secondary to increasing use of indwelling vascular devices,[6] fibrosing mediastinitis, and vasculitic disorders."
],
"date": "June 01, 2018",
"figures": [],
"markdown": "# A 48-Year-Old Man With Dyspnea and Cyanotic Skin\n\n **Authors:** Caroline Tschibelu, MD \n **Date:** June 01, 2018\n\n ## Content\n\n Superior vena cava (SVC) syndrome is a clinical diagnosis, in this case made based on the characteristic physical findings and the patient's medical history. The chest radiograph revealed a large right-sided hilar consolidation of the right lung with lung collapse and bilateral pleural effusions.\nPresenting symptoms result from SVC obstruction and usually consist of dyspnea, facial and neck swelling, arm edema, distended neck and chest veins,[1] cough, visual symptoms, hoarseness, stridor, headaches, and cyanosis. The patient in this case presented with most of these symptoms. More concerning signs and symptoms are dizziness, confusion, and obtundation, as they are signs of cerebral edema, which, while rare, is usually fatal. Approximately 15,000 new cases of SVC syndrome occur annually in the United States.[2]\nThe differential diagnosis of SVC syndrome includes cardiac tamponade; tuberculosis (especially reactivated tuberculosis); syphilis; Bancroftian filariasis; bacterial, fungal, or viral pneumonia; non-Hodgkin lymphoma; angioedema; mediastinitis; and vasculitic disorders. In addition, aortic dissection can present as SVC syndrome.[3] Hodgkin lymphoma rarely causes SVC syndrome.\nSVC syndrome used to have predominantly infectious etiologies. The first case, documented by William Hunter in 1757, was of a young man who died of a syphilitic aortic aneurysm compressing the superior vena cava.[4] Other causes included granulomatous mediastinal diseases, notably mediastinitis from tuberculosis.[5] The prevalence of infectious cases of SVC syndrome has significantly decreased with the advent of antibiotics. Malignancies are now the leading causes of SVC syndrome, accounting for 90% of all SVC syndrome cases. The remainder of the cases are due to nonmalignant etiologies such as aortic aneurysm, thrombosis secondary to increasing use of indwelling vascular devices,[6] fibrosing mediastinitis, and vasculitic disorders.\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 715613,
"choiceText": "Aortic artery dissection",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 715615,
"choiceText": "Tuberculosis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 715617,
"choiceText": "Lymphedema secondary to filariasis infection",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 715619,
"choiceText": "Superior vena cava syndrome",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 715621,
"choiceText": "Hodgkin lymphoma",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 715623,
"choiceText": "Angioedema",
"correct": false,
"displayOrder": 6,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 223301,
"questionText": "Based on the history, physical examination, and radiograph, what is the likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 48-Year-Old Man With Dyspnea and Cyanotic Skin"
},
{
"authors": "Caroline Tschibelu, MD",
"content": [
"Intrathoracic malignancies cause 60%-85% of SVC syndrome cases, with non-small cell lung cancer (NSCLC) being the most common (50% of cases); small cell lung cancer is found in 10% of cases.[7]",
"Nearly 10% of SCLC cases cause SVC syndrome. However, only 4% of other cancers cause SVC syndrome. This is likely due to SCLC’s aggressive nature, its frequent central location, and its tendency to directly invade the SVC more often than other forms of lung cancer do.[5]",
"Males are more likely to be affected by SVC syndrome than females, due to the higher incidence of lung cancer in the male population. Racial disparities in SVC syndrome diagnosis are dependent on the frequency of lung cancer and lymphoma in the studied populations. SVC syndrome of malignant etiologies mainly affects patients who are aged 40-60 years, whereas SVC syndrome of benign etiologies mainly affects patients who are aged 30-40 years.",
"Pediatric cases of SVC syndrome are rare. The most common etiology of SVC syndrome in the pediatric population is non-Hodgkin lymphoma, followed by T-cell acute lymphoblastic leukemia, Hodgkin lymphoma, and tubercular mediastinitis. A rare case was reported of an adolescent who presented with SVC syndrome due to a mediastinal mixed germ cell tumor with predominant yolk cell component.[8]",
"Four percent of patients with non-Hodgkin lymphoma will develop SVC syndrome. Diffuse large-cell and lymphoblastic lymphoma are the most common causes, but 60% of those are due to mediastinal large B-cell lymphoma with sclerosis, an unusually aggressive type of lymphoma. Other malignancies that are less likely to cause SVC syndrome include teratoma and mediastinal germ cell tumors, mesothelioma, and thymoma.",
"The diagnosis of SVC syndrome is primarily clinical. When SVC syndrome is suspected, imaging is indicated. Most patients with SVC syndrome have abnormal findings on chest radiography. A mediastinal mass or widening is seen in 64% of patients, and pleural effusion in 26% of SVC syndrome patients.[2] However, the most useful imaging modality is a chest CT scanning with IV contrast. CT scanning allows the cause and extent of obstruction and the collateral vessels to be visualized. This is necessary to plan for endovascular or radiation treatment.[3] Additionally, it provides information necessary for diagnosing the underlying tumor, its size, and its location. MRI is an alternative imaging method for patients who cannot tolerate IV dye. Contrast-enhanced head CT or MRI can also help exclude brain metastases and stage the tumor. Positron emission tomography can be used to determine nodal and mediastinal involvement. Venography should be considered only when surgical intervention, such as stent placement, is planned. CT venography may be superior to digital subtraction venography.[9]"
],
"date": "June 01, 2018",
"figures": [],
"markdown": "# A 48-Year-Old Man With Dyspnea and Cyanotic Skin\n\n **Authors:** Caroline Tschibelu, MD \n **Date:** June 01, 2018\n\n ## Content\n\n Intrathoracic malignancies cause 60%-85% of SVC syndrome cases, with non-small cell lung cancer (NSCLC) being the most common (50% of cases); small cell lung cancer is found in 10% of cases.[7]\nNearly 10% of SCLC cases cause SVC syndrome. However, only 4% of other cancers cause SVC syndrome. This is likely due to SCLC’s aggressive nature, its frequent central location, and its tendency to directly invade the SVC more often than other forms of lung cancer do.[5]\nMales are more likely to be affected by SVC syndrome than females, due to the higher incidence of lung cancer in the male population. Racial disparities in SVC syndrome diagnosis are dependent on the frequency of lung cancer and lymphoma in the studied populations. SVC syndrome of malignant etiologies mainly affects patients who are aged 40-60 years, whereas SVC syndrome of benign etiologies mainly affects patients who are aged 30-40 years.\nPediatric cases of SVC syndrome are rare. The most common etiology of SVC syndrome in the pediatric population is non-Hodgkin lymphoma, followed by T-cell acute lymphoblastic leukemia, Hodgkin lymphoma, and tubercular mediastinitis. A rare case was reported of an adolescent who presented with SVC syndrome due to a mediastinal mixed germ cell tumor with predominant yolk cell component.[8]\nFour percent of patients with non-Hodgkin lymphoma will develop SVC syndrome. Diffuse large-cell and lymphoblastic lymphoma are the most common causes, but 60% of those are due to mediastinal large B-cell lymphoma with sclerosis, an unusually aggressive type of lymphoma. Other malignancies that are less likely to cause SVC syndrome include teratoma and mediastinal germ cell tumors, mesothelioma, and thymoma.\nThe diagnosis of SVC syndrome is primarily clinical. When SVC syndrome is suspected, imaging is indicated. Most patients with SVC syndrome have abnormal findings on chest radiography. A mediastinal mass or widening is seen in 64% of patients, and pleural effusion in 26% of SVC syndrome patients.[2] However, the most useful imaging modality is a chest CT scanning with IV contrast. CT scanning allows the cause and extent of obstruction and the collateral vessels to be visualized. This is necessary to plan for endovascular or radiation treatment.[3] Additionally, it provides information necessary for diagnosing the underlying tumor, its size, and its location. MRI is an alternative imaging method for patients who cannot tolerate IV dye. Contrast-enhanced head CT or MRI can also help exclude brain metastases and stage the tumor. Positron emission tomography can be used to determine nodal and mediastinal involvement. Venography should be considered only when surgical intervention, such as stent placement, is planned. CT venography may be superior to digital subtraction venography.[9]\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 48-Year-Old Man With Dyspnea and Cyanotic Skin"
},
{
"authors": "Caroline Tschibelu, MD",
"content": [
"Specific recommendations for SVC syndrome management are based on case series, due to the lack of well-designed randomized trials. The primary goal in management is to alleviate the symptoms of SVC syndrome and then treat the underlying disease. Critical airway and circulatory management should be prioritized, followed by diagnostics. Venogram and urgent stenting should be arranged immediately for critical cases. Supportive care should be adopted, including elevating the patient's head to decrease hydrostatic pressure and thus the edema.",
"Glucocorticoid therapy is generally prescribed as dexamethasone (4 mg every 6 hours). Glucocorticoids are more effective at reducing obstruction and symptoms in patients with lymphoma and thymoma, which are steroid-sensitive.[3] Steroids are also beneficial in patients with laryngeal edema and those undergoing radiotherapy to prevent postradiation swelling.",
"Loop diuretics are recommended, although whether small changes in right atrial pressure also affect venous pressure distal to the obstruction is still debated. An observational study evaluated the rate of clinical improvement in 107 patients treated with steroids, diuretics, or neither therapy. Patients had SVC syndrome of various etiologies, and 84% of the patients showed clinical improvement.[10]",
"Definitive therapy for SVC syndrome is treatment of the underlying disease. Biopsies for histological examination should be obtained when the patient is clinically stable to establish the diagnosis, determine the extent and stage of the tumor, and finally guide treatment for the specific underlying malignancy (ie, radiotherapy and/or chemotherapy).[11] The possibility of obtaining a peripheral biopsy should be assessed first, such as in the case of a patient with palpable supraclavicular lymph nodes, before proceeding with invasive procedures. Thoracentesis and cytologic analysis have a diagnostic yield of only 50%, although they are both simple and fast procedures. Diagnostic bronchoscopy can detect endoluminal tumor growth and peripheral/central airway infiltration. Tissue can be obtained via forceps biopsy which has a diagnostic yield of 65.5%, or via cryobiopsy to increase the diagnostic yield to 89.1%.[12] Mediastinoscopy has a yield of greater than 90%, which is advantageous for lymphoma because an adequate tissue sample is needed to determine nodal architecture, cell type, and immunochemistry, as well as to confirm the subtype of lymphoma.",
"Tumor type and stage are then used to guide therapy. Definitive treatment options include chemotherapy, radiotherapy, or both. Surgery alone or in combination with other therapies is also an option. Patients with SCLC, lymphoma, or germ cell tumor show rapid clinical response to aggressive systemic chemotherapy alone. For patients with NSCLC, the clinical response depends upon the method of cancer treatment. Patients with stage III NSCLC respond to a combination of chemotherapy and radiotherapy. Patients with stage IV NSCLC respond to chemotherapy, but the clinical response is less rapid than in patients with the other types of cancer.",
"If SVC syndrome is secondary to central venous obstruction, patients should receive anticoagulation for 3-6 months to prevent pulmonary embolism.",
"In this case, the patient’s chest CT scan (Figure 3) showed significant narrowing of the right main pulmonary artery and the distal left brachiocephalic vein, as well as the complete obstruction of the distal right brachiocephalic vein.",
"Figure 3.",
"Extensive vascular collateralization of the right hemithorax was noted. Finally, the CT scan showed a normal size heart with a moderate pericardial effusion in addition to bilateral pleural effusion. Due to the extent of his pathology, our patient underwent stenting shortly after admission. He showed substantial improvement of symptoms, with near-complete resolution of swelling within 1 week."
],
"date": "June 01, 2018",
"figures": [
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/822/266/822266-thumb-3.jpg"
}
],
"markdown": "# A 48-Year-Old Man With Dyspnea and Cyanotic Skin\n\n **Authors:** Caroline Tschibelu, MD \n **Date:** June 01, 2018\n\n ## Content\n\n Specific recommendations for SVC syndrome management are based on case series, due to the lack of well-designed randomized trials. The primary goal in management is to alleviate the symptoms of SVC syndrome and then treat the underlying disease. Critical airway and circulatory management should be prioritized, followed by diagnostics. Venogram and urgent stenting should be arranged immediately for critical cases. Supportive care should be adopted, including elevating the patient's head to decrease hydrostatic pressure and thus the edema.\nGlucocorticoid therapy is generally prescribed as dexamethasone (4 mg every 6 hours). Glucocorticoids are more effective at reducing obstruction and symptoms in patients with lymphoma and thymoma, which are steroid-sensitive.[3] Steroids are also beneficial in patients with laryngeal edema and those undergoing radiotherapy to prevent postradiation swelling.\nLoop diuretics are recommended, although whether small changes in right atrial pressure also affect venous pressure distal to the obstruction is still debated. An observational study evaluated the rate of clinical improvement in 107 patients treated with steroids, diuretics, or neither therapy. Patients had SVC syndrome of various etiologies, and 84% of the patients showed clinical improvement.[10]\nDefinitive therapy for SVC syndrome is treatment of the underlying disease. Biopsies for histological examination should be obtained when the patient is clinically stable to establish the diagnosis, determine the extent and stage of the tumor, and finally guide treatment for the specific underlying malignancy (ie, radiotherapy and/or chemotherapy).[11] The possibility of obtaining a peripheral biopsy should be assessed first, such as in the case of a patient with palpable supraclavicular lymph nodes, before proceeding with invasive procedures. Thoracentesis and cytologic analysis have a diagnostic yield of only 50%, although they are both simple and fast procedures. Diagnostic bronchoscopy can detect endoluminal tumor growth and peripheral/central airway infiltration. Tissue can be obtained via forceps biopsy which has a diagnostic yield of 65.5%, or via cryobiopsy to increase the diagnostic yield to 89.1%.[12] Mediastinoscopy has a yield of greater than 90%, which is advantageous for lymphoma because an adequate tissue sample is needed to determine nodal architecture, cell type, and immunochemistry, as well as to confirm the subtype of lymphoma.\nTumor type and stage are then used to guide therapy. Definitive treatment options include chemotherapy, radiotherapy, or both. Surgery alone or in combination with other therapies is also an option. Patients with SCLC, lymphoma, or germ cell tumor show rapid clinical response to aggressive systemic chemotherapy alone. For patients with NSCLC, the clinical response depends upon the method of cancer treatment. Patients with stage III NSCLC respond to a combination of chemotherapy and radiotherapy. Patients with stage IV NSCLC respond to chemotherapy, but the clinical response is less rapid than in patients with the other types of cancer.\nIf SVC syndrome is secondary to central venous obstruction, patients should receive anticoagulation for 3-6 months to prevent pulmonary embolism.\nIn this case, the patient’s chest CT scan (Figure 3) showed significant narrowing of the right main pulmonary artery and the distal left brachiocephalic vein, as well as the complete obstruction of the distal right brachiocephalic vein.\nFigure 3.\nExtensive vascular collateralization of the right hemithorax was noted. Finally, the CT scan showed a normal size heart with a moderate pericardial effusion in addition to bilateral pleural effusion. Due to the extent of his pathology, our patient underwent stenting shortly after admission. He showed substantial improvement of symptoms, with near-complete resolution of swelling within 1 week.\n\n ## Figures\n\n **Figure 3.** \n \n\n\n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 715625,
"choiceText": "Large B-cell lymphoma",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 715627,
"choiceText": "Non-small cell carcinoma",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 715629,
"choiceText": "Primary mediastinal germ cell tumors",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 715631,
"choiceText": "Small cell carcinoma",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 715633,
"choiceText": "Mesothelioma",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Intrathoracic malignancies cause 60%-85% of SVC syndrome cases, with NSCLC being the most common (50% of cases); SCLC is found in 10% of cases. Nearly 10% of SCLC cases cause SVC syndrome. However, only 4% of other cancers cause SVC syndrome. This is likely due to SCLC's aggressive nature, its frequent central location, and its tendency to directly invade the SVC more often than other forms of lung cancer do.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 223303,
"questionText": "A 55-year-old male patient, who is also a heavy smoker, presents with a few weeks of characteristic signs and symptoms of SVC syndrome.<br><br>Diagnosis is confirmed with MRI due to the patient's allergic reaction to IV dye. Laboratory investigations reveal the following electrolyte panel:<br> \r\n<ul><li>Sodium level - 115 mEq/L</li>\r\n<li>Potassium level - 4.6 mEq/L</li>\r\n<li>Chloride level - 95 mEq/L</li>\r\n<li>Bicarbonate level - 29.5 mEq/L</li>\r\n<li>Blood urea nitrogen level - 23 mg/dL</li><li>Creatinine level - 0.8 mg/dL</li>\r\n<li>Glucose level - 102 mg/dL</li>\r\n</ul>\r\nWhich carcinoma would a peripheral biopsy likely show?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 715635,
"choiceText": "Endovascular stenting",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 715637,
"choiceText": "Chemotherapy alone",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 715639,
"choiceText": "Chemotherapy combined with radiotherapy",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 715641,
"choiceText": "Palliative care",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 715643,
"choiceText": "Anticoagulation for 3-6 months",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "SCLC is very responsive to aggressive chemotherapy alone. Complete relief of symptoms is seen in 80% of patients with SCLC, compared with 40% of patients with NSCLC. NSCLC associated with SVC syndrome has a poorer prognosis, and definitive treatment is dictated by staging of the malignancy. Therefore, treatment could consist of chemotherapy alone or chemotherapy combined with radiotherapy and surgery. \r\n<br><br>\r\nEndovascular stenting is useful for immediate relief of severe symptoms, because it does not require prior biopsy. However, the rapid response to radiotherapy or chemotherapy seen in tumors sensitive to those therapies does not usually warrant stent placement. Palliative care and anticoagulation would not be appropriate therapies for this patient at this stage in his disease.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 223305,
"questionText": "Based on the above patient's diagnosis, what is the most appropriate definitive therapy?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 48-Year-Old Man With Dyspnea and Cyanotic Skin"
},
{
"authors": "Caroline Tschibelu, MD",
"content": [],
"date": "June 01, 2018",
"figures": [],
"markdown": "# A 48-Year-Old Man With Dyspnea and Cyanotic Skin\n\n **Authors:** Caroline Tschibelu, MD \n **Date:** June 01, 2018\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 715625,
"choiceText": "Large B-cell lymphoma",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 715627,
"choiceText": "Non-small cell carcinoma",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 715629,
"choiceText": "Primary mediastinal germ cell tumors",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 715631,
"choiceText": "Small cell carcinoma",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 715633,
"choiceText": "Mesothelioma",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Intrathoracic malignancies cause 60%-85% of SVC syndrome cases, with NSCLC being the most common (50% of cases); SCLC is found in 10% of cases. Nearly 10% of SCLC cases cause SVC syndrome. However, only 4% of other cancers cause SVC syndrome. This is likely due to SCLC's aggressive nature, its frequent central location, and its tendency to directly invade the SVC more often than other forms of lung cancer do.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 223303,
"questionText": "A 55-year-old male patient, who is also a heavy smoker, presents with a few weeks of characteristic signs and symptoms of SVC syndrome.<br><br>Diagnosis is confirmed with MRI due to the patient's allergic reaction to IV dye. Laboratory investigations reveal the following electrolyte panel:<br> \r\n<ul><li>Sodium level - 115 mEq/L</li>\r\n<li>Potassium level - 4.6 mEq/L</li>\r\n<li>Chloride level - 95 mEq/L</li>\r\n<li>Bicarbonate level - 29.5 mEq/L</li>\r\n<li>Blood urea nitrogen level - 23 mg/dL</li><li>Creatinine level - 0.8 mg/dL</li>\r\n<li>Glucose level - 102 mg/dL</li>\r\n</ul>\r\nWhich carcinoma would a peripheral biopsy likely show?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 715635,
"choiceText": "Endovascular stenting",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 715637,
"choiceText": "Chemotherapy alone",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 715639,
"choiceText": "Chemotherapy combined with radiotherapy",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 715641,
"choiceText": "Palliative care",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 715643,
"choiceText": "Anticoagulation for 3-6 months",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "SCLC is very responsive to aggressive chemotherapy alone. Complete relief of symptoms is seen in 80% of patients with SCLC, compared with 40% of patients with NSCLC. NSCLC associated with SVC syndrome has a poorer prognosis, and definitive treatment is dictated by staging of the malignancy. Therefore, treatment could consist of chemotherapy alone or chemotherapy combined with radiotherapy and surgery. \r\n<br><br>\r\nEndovascular stenting is useful for immediate relief of severe symptoms, because it does not require prior biopsy. However, the rapid response to radiotherapy or chemotherapy seen in tumors sensitive to those therapies does not usually warrant stent placement. Palliative care and anticoagulation would not be appropriate therapies for this patient at this stage in his disease.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 223305,
"questionText": "Based on the above patient's diagnosis, what is the most appropriate definitive therapy?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 48-Year-Old Man With Dyspnea and Cyanotic Skin"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 715613,
"choiceText": "Aortic artery dissection",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 715615,
"choiceText": "Tuberculosis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 715617,
"choiceText": "Lymphedema secondary to filariasis infection",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 715619,
"choiceText": "Superior vena cava syndrome",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 715621,
"choiceText": "Hodgkin lymphoma",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 715623,
"choiceText": "Angioedema",
"correct": false,
"displayOrder": 6,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 223301,
"questionText": "Based on the history, physical examination, and radiograph, what is the likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 715625,
"choiceText": "Large B-cell lymphoma",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 715627,
"choiceText": "Non-small cell carcinoma",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 715629,
"choiceText": "Primary mediastinal germ cell tumors",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 715631,
"choiceText": "Small cell carcinoma",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 715633,
"choiceText": "Mesothelioma",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Intrathoracic malignancies cause 60%-85% of SVC syndrome cases, with NSCLC being the most common (50% of cases); SCLC is found in 10% of cases. Nearly 10% of SCLC cases cause SVC syndrome. However, only 4% of other cancers cause SVC syndrome. This is likely due to SCLC's aggressive nature, its frequent central location, and its tendency to directly invade the SVC more often than other forms of lung cancer do.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 223303,
"questionText": "A 55-year-old male patient, who is also a heavy smoker, presents with a few weeks of characteristic signs and symptoms of SVC syndrome.<br><br>Diagnosis is confirmed with MRI due to the patient's allergic reaction to IV dye. Laboratory investigations reveal the following electrolyte panel:<br> \r\n<ul><li>Sodium level - 115 mEq/L</li>\r\n<li>Potassium level - 4.6 mEq/L</li>\r\n<li>Chloride level - 95 mEq/L</li>\r\n<li>Bicarbonate level - 29.5 mEq/L</li>\r\n<li>Blood urea nitrogen level - 23 mg/dL</li><li>Creatinine level - 0.8 mg/dL</li>\r\n<li>Glucose level - 102 mg/dL</li>\r\n</ul>\r\nWhich carcinoma would a peripheral biopsy likely show?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 715635,
"choiceText": "Endovascular stenting",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 715637,
"choiceText": "Chemotherapy alone",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 715639,
"choiceText": "Chemotherapy combined with radiotherapy",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 715641,
"choiceText": "Palliative care",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 715643,
"choiceText": "Anticoagulation for 3-6 months",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "SCLC is very responsive to aggressive chemotherapy alone. Complete relief of symptoms is seen in 80% of patients with SCLC, compared with 40% of patients with NSCLC. NSCLC associated with SVC syndrome has a poorer prognosis, and definitive treatment is dictated by staging of the malignancy. Therefore, treatment could consist of chemotherapy alone or chemotherapy combined with radiotherapy and surgery. \r\n<br><br>\r\nEndovascular stenting is useful for immediate relief of severe symptoms, because it does not require prior biopsy. However, the rapid response to radiotherapy or chemotherapy seen in tumors sensitive to those therapies does not usually warrant stent placement. Palliative care and anticoagulation would not be appropriate therapies for this patient at this stage in his disease.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 223305,
"questionText": "Based on the above patient's diagnosis, what is the most appropriate definitive therapy?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
757389 | /viewarticle/757389 | [
{
"authors": "James J. McCombie, MB ChB",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to recognize accurately. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"An 85-year-old man is presented to the emergency department (ED) by ambulance with severe abdominal pain that began suddenly 2 hours before presentation. The pain is mainly in his back and radiates toward his left inguinal region. The patient felt light-headed at the onset of the pain to the extent that he had to grip the sink to steady himself. He also reports significant nausea, although he has not vomited.",
"He has no urinary symptoms. His medical history includes type 2 diabetes, hypertension, hyperlipidemia, ischemic heart disease, and intermittent claudication of his lower extremities. His medications include metformin, ramipril, simvastatin, aspirin, and a glyceryl trinitrate pump spray. He smoked 10-20 cigarettes a day for over 60 years but stopped 5 years ago."
],
"date": "October 11, 2018",
"figures": [],
"markdown": "# An 85-Year-Old With Sudden-Onset Severe Abdominal Pain\n\n **Authors:** James J. McCombie, MB ChB \n **Date:** October 11, 2018\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to recognize accurately. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nAn 85-year-old man is presented to the emergency department (ED) by ambulance with severe abdominal pain that began suddenly 2 hours before presentation. The pain is mainly in his back and radiates toward his left inguinal region. The patient felt light-headed at the onset of the pain to the extent that he had to grip the sink to steady himself. He also reports significant nausea, although he has not vomited.\nHe has no urinary symptoms. His medical history includes type 2 diabetes, hypertension, hyperlipidemia, ischemic heart disease, and intermittent claudication of his lower extremities. His medications include metformin, ramipril, simvastatin, aspirin, and a glyceryl trinitrate pump spray. He smoked 10-20 cigarettes a day for over 60 years but stopped 5 years ago.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "An 85-Year-Old With Sudden-Onset Severe Abdominal Pain"
},
{
"authors": "James J. McCombie, MB ChB",
"content": [
"The physical examination reveals tenderness in the left iliac fossa and left costovertebral angle. His vital signs are remarkable for a heart rate of 105 beats/min and a blood pressure of 110/90 mm Hg. The patient is prescribed further analgesia and 1 L of crystalloid is administered. He is then referred to the surgical assessment unit (SAU) as a case of possible ureteric colic, with an abdominal radiograph taken during transit (Figure 1).",
"Figure 1.",
"On arrival to the SAU, the patient and his abdominal film are reviewed by the on-call surgical doctor. After assessment of his airway, breathing, circulation, level of consciousness, and exposure for examination of his abdomen, oxygen is given via a face mask, intravenous access is obtained at 2 sites, and a urinary catheter is inserted. Blood is drawn for laboratory analysis, including blood urea nitrogen, electrolytes, a complete blood cell count, coagulation panel, and cross matching. The patient's electrolytes, complete blood cell count, and coagulation panel results are unremarkable. Arterial blood gas analysis is obtained and reveals a metabolic acidosis.",
"Intravenous fluids are continued and the patient is sent for an urgent CT scan of the abdomen. Once completed, he is transferred by ambulance to a regional center for immediate intervention."
],
"date": "October 11, 2018",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/754/949/754949-thumb1.png"
}
],
"markdown": "# An 85-Year-Old With Sudden-Onset Severe Abdominal Pain\n\n **Authors:** James J. McCombie, MB ChB \n **Date:** October 11, 2018\n\n ## Content\n\n The physical examination reveals tenderness in the left iliac fossa and left costovertebral angle. His vital signs are remarkable for a heart rate of 105 beats/min and a blood pressure of 110/90 mm Hg. The patient is prescribed further analgesia and 1 L of crystalloid is administered. He is then referred to the surgical assessment unit (SAU) as a case of possible ureteric colic, with an abdominal radiograph taken during transit (Figure 1).\nFigure 1.\nOn arrival to the SAU, the patient and his abdominal film are reviewed by the on-call surgical doctor. After assessment of his airway, breathing, circulation, level of consciousness, and exposure for examination of his abdomen, oxygen is given via a face mask, intravenous access is obtained at 2 sites, and a urinary catheter is inserted. Blood is drawn for laboratory analysis, including blood urea nitrogen, electrolytes, a complete blood cell count, coagulation panel, and cross matching. The patient's electrolytes, complete blood cell count, and coagulation panel results are unremarkable. Arterial blood gas analysis is obtained and reveals a metabolic acidosis.\nIntravenous fluids are continued and the patient is sent for an urgent CT scan of the abdomen. Once completed, he is transferred by ambulance to a regional center for immediate intervention.\n\n ## Figures\n\n **Figure 1.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 476051,
"choiceText": "Ureteric colic",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 476053,
"choiceText": "Acute mesenteric ischemia",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 476055,
"choiceText": "Ruptured abdominal aortic aneurysm",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 476057,
"choiceText": "Perforated diverticulum",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 143217,
"questionText": "What is the diagnosis?<br><br><i>Hint: Pay attention to the distribution of the pain and the history of presyncope at its onset, together with a careful review of the abdominal radiograph.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "An 85-Year-Old With Sudden-Onset Severe Abdominal Pain"
},
{
"authors": "James J. McCombie, MB ChB",
"content": [
"The CT scan revealed the presence of an abdominal aortic aneurysm (AAA) with a calcified wall and mural thrombus, with extraluminal blood indicating a rupture of the aneurysm (Figure 2).",
"Figure 2.",
"An aneurysm is defined as a focal increase in the diameter of a vessel to greater than 50% of normal; anything less is considered arteriomegaly. The abdominal aorta is approximately 2 cm in diameter, and an AAA is usually said to be present when a segment of aorta has a diameter of greater than 3 cm.",
"AAA has a prevalence of 1.3%-8.9% in men and 1%-2.2% in women older than 55 years. It is responsible for 1%-3% of all deaths among men aged 65-85 years in developed countries.[1] The incidence is higher in select groups; 5% of patients with coronary artery disease and as many as 50% of those with popliteal or femoral artery aneurysms have concomitant AAA.[2] Data from a 7-year prospective study of a cohort of 4345 patients, which identified 119 cases of AAA, identified male gender and increasing age as strong risk factors for the development of AAA.[3] Another study demonstrated an association between short leukocyte telomere length and AAA, further supporting the notion that vascular biological aging is indeed an etiology of AAA.[4] Smoking, hypertension, and hypercholesterolemia are also associated with a significantly increased risk for AAA.[3] Interestingly, diabetes is usually protective against aneurysm formation.",
"AAA may cause various symptoms, including abdominal, back, and/or groin pain; however, the vast majority of AAAs are asymptomatic. Symptoms are usually a result of rapid aneurysm sac expansion or rupture. Pain may be mild to severe. A patient may present reporting a mass or swelling in the abdomen, or an awareness of the pulsation of the AAA. Complications of AAA include internal thrombosis, distal embolization, and aortoenteric fistula. AAA rupture is the most significant, immediate cause of mortality. Complaints of feeling light-headed or syncope, in association with back or abdominal pain, should alert the clinician to the possibility of AAA rupture. Approximately 50% of patients die soon after rupture, and of those surviving to surgery, the mortality is approximately 54%.[5] Most early deaths result from free intraperitoneal rupture and exsanguination. Extraperitoneal rupture is associated with a more favorable course, as the bleeding may be temporarily tamponaded by peri-aortic tissue.",
"Given the asymptomatic nature of most AAAs, as well as the dismal outcome after rupture, significant attention has been paid to screening for AAA. The first study to examine AAA screening across a large population was the MASS study. This study involved a population sample of 67,800 men aged 65-74 years. Patients were randomly assigned to receive either screening ultrasonography or no intervention. Over 10 years, 155 deaths related to AAA occurred in the screening group vs 296 in the group that did not receive screening. This equates to a relative risk reduction of 48% (95% confidence interval, 37%-57%).[6] This study also reported that the cost-effectiveness of such a screening program was favorable. A Cochrane review found evidence of a significant reduction in mortality from AAA rupture in men aged 65-79 years who undergo screening.[7]",
"These findings have prompted the initiation of screening programs in several nations. For example, in the United Kingdom, the National Health Service (NHS) AAA screening program invites all men for a screening ultrasound at 65 years. Men older than 65 years who have not been screened or treated for AAA can personally make a request for screening. In the United States, the SAAAVE Act allows for AAA screening ultrasound in patients with a family history of AAA or a history of smoking more than 100 cigarettes, as long as they participate in their \"Welcome to Medicare\" physical examination within 6 months of turning 65."
],
"date": "October 11, 2018",
"figures": [
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/754/949/754949-thumb2.png"
}
],
"markdown": "# An 85-Year-Old With Sudden-Onset Severe Abdominal Pain\n\n **Authors:** James J. McCombie, MB ChB \n **Date:** October 11, 2018\n\n ## Content\n\n The CT scan revealed the presence of an abdominal aortic aneurysm (AAA) with a calcified wall and mural thrombus, with extraluminal blood indicating a rupture of the aneurysm (Figure 2).\nFigure 2.\nAn aneurysm is defined as a focal increase in the diameter of a vessel to greater than 50% of normal; anything less is considered arteriomegaly. The abdominal aorta is approximately 2 cm in diameter, and an AAA is usually said to be present when a segment of aorta has a diameter of greater than 3 cm.\nAAA has a prevalence of 1.3%-8.9% in men and 1%-2.2% in women older than 55 years. It is responsible for 1%-3% of all deaths among men aged 65-85 years in developed countries.[1] The incidence is higher in select groups; 5% of patients with coronary artery disease and as many as 50% of those with popliteal or femoral artery aneurysms have concomitant AAA.[2] Data from a 7-year prospective study of a cohort of 4345 patients, which identified 119 cases of AAA, identified male gender and increasing age as strong risk factors for the development of AAA.[3] Another study demonstrated an association between short leukocyte telomere length and AAA, further supporting the notion that vascular biological aging is indeed an etiology of AAA.[4] Smoking, hypertension, and hypercholesterolemia are also associated with a significantly increased risk for AAA.[3] Interestingly, diabetes is usually protective against aneurysm formation.\nAAA may cause various symptoms, including abdominal, back, and/or groin pain; however, the vast majority of AAAs are asymptomatic. Symptoms are usually a result of rapid aneurysm sac expansion or rupture. Pain may be mild to severe. A patient may present reporting a mass or swelling in the abdomen, or an awareness of the pulsation of the AAA. Complications of AAA include internal thrombosis, distal embolization, and aortoenteric fistula. AAA rupture is the most significant, immediate cause of mortality. Complaints of feeling light-headed or syncope, in association with back or abdominal pain, should alert the clinician to the possibility of AAA rupture. Approximately 50% of patients die soon after rupture, and of those surviving to surgery, the mortality is approximately 54%.[5] Most early deaths result from free intraperitoneal rupture and exsanguination. Extraperitoneal rupture is associated with a more favorable course, as the bleeding may be temporarily tamponaded by peri-aortic tissue.\nGiven the asymptomatic nature of most AAAs, as well as the dismal outcome after rupture, significant attention has been paid to screening for AAA. The first study to examine AAA screening across a large population was the MASS study. This study involved a population sample of 67,800 men aged 65-74 years. Patients were randomly assigned to receive either screening ultrasonography or no intervention. Over 10 years, 155 deaths related to AAA occurred in the screening group vs 296 in the group that did not receive screening. This equates to a relative risk reduction of 48% (95% confidence interval, 37%-57%).[6] This study also reported that the cost-effectiveness of such a screening program was favorable. A Cochrane review found evidence of a significant reduction in mortality from AAA rupture in men aged 65-79 years who undergo screening.[7]\nThese findings have prompted the initiation of screening programs in several nations. For example, in the United Kingdom, the National Health Service (NHS) AAA screening program invites all men for a screening ultrasound at 65 years. Men older than 65 years who have not been screened or treated for AAA can personally make a request for screening. In the United States, the SAAAVE Act allows for AAA screening ultrasound in patients with a family history of AAA or a history of smoking more than 100 cigarettes, as long as they participate in their \"Welcome to Medicare\" physical examination within 6 months of turning 65.\n\n ## Figures\n\n **Figure 2.** \n \n\n\n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 476051,
"choiceText": "Ureteric colic",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 476053,
"choiceText": "Acute mesenteric ischemia",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 476055,
"choiceText": "Ruptured abdominal aortic aneurysm",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 476057,
"choiceText": "Perforated diverticulum",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 143217,
"questionText": "What is the diagnosis?<br><br><i>Hint: Pay attention to the distribution of the pain and the history of presyncope at its onset, together with a careful review of the abdominal radiograph.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "An 85-Year-Old With Sudden-Onset Severe Abdominal Pain"
},
{
"authors": "James J. McCombie, MB ChB",
"content": [
"Multiple imaging modalities, including CT and ultrasonography, have been used to both screen and detail AAA. Three dimensional (3D) reconstruction CT (orthogonal CT) provides a truly accurate model of aneurysm architecture. Angulation of the aneurysm can make traditional axial CT images misleading. When ultrasound and CT are compared with orthogonal CT, ultrasound produces the most accurate estimation of size overall.[8] Traditional CT can be used if the aorta cannot be visualized with ultrasound after repeated efforts.",
"A potential AAA screening test is measurement of baseline insulin-like growth factor I (IGF-I). In a prospective, long-term study, Lindholt et al demonstrated that IGF-I is a novel biomarker for AAA.[9] Baseline levels of serum IGF-I not only had a positive correlation with the size and growth rate of the aneurysms, but they also predicted the need for future surgery.[9] Decision-making regarding operative management vs observation for AAA is informed mainly by the results of the UKSAT trial[10] and the ADAM trial.[2] The UKSAT randomized 1090 small aneurysms (4-5.5 cm) to operation or surveillance. No significant difference in overall mortality was demonstrated for those offered early surgery. Similar findings were found in the ADAM trial, and these 2 studies led to the recommendation that surgical repair should be considered in all aneurysms over 5.5 cm; those patients with AAAs less than 5.5 cm should be enrolled on an ultrasonographic surveillance program. Surgical AAA repair should also be considered when the aneurysm expands by more than 0.6-0.8 cm in a single 12-month period.[11] The threshold for surgical repair in women is typically 5 cm, given a relatively smaller normal aortic size compared with men.",
"These recommendations are rooted in the physics of transmural pressure and the higher rupture rates associated with larger aneurysms. The law of LaPlace states that the tension in the wall of a sphere is proportional to the product of the transmural pressure across the wall and the radius of the sphere, and inversely proportional to the thickness of its wall; it can also be interpreted as stating that in a long pliable tube the site of largest diameter requires the least pressure to distend.[12] This explains why an aneurysm tends to enlarge and why larger aneurysms rupture more frequently (it is because of the higher wall tension). As a result of the tendency of larger aneurysms to expand at a faster rate, AAAs of 4.5-5.4 cm in diameter will need 6-month ultrasound follow-up, and AAAs of 3-4.5 cm in diameter need yearly ultrasound follow-up; these intervals are practiced in the NHS (United Kingdom).[1,2,3]",
"Around 90% of detected aneurysms are below the threshold of 5.5 cm required for intervention and require surveillance to monitor expansion into the interventional range. Numerous approaches designed to limit expansion have been suggested. Large studies have indicated that propranolol does not inhibit aneurysm expansion.[14] Numerous studies agree that tobacco use is associated with an increased rate of aneurysm expansion.[14] Level B and C evidence is available to suggest that 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors (statins) may inhibit aneurysm expansion.[14,15] Some animal data, but no human data, suggest that angiotensin-converting enzyme inhibitors or losartan, an angiotensin receptor blocker, decrease the rate of AAA expansion, when used to treat concomitant hypertension.[14,16] Optimizing glycemic control is also associated with a reduction in the rate of expansion of AAA in patients with diabetes.[16] Weight loss, regular exercise if possible, and a healthy diet should also be encouraged.",
"Open surgical repair or endovascular aneurysm repair (EVAR) are the current operative options available. EVAR is a less-invasive approach to addressing AAA. EVAR involves transluminal placement of a modular stent-graft system into the AAA via remote arterial access (usually, the femoral artery) under local, regional, or, most commonly, general anesthetic. Concerns about the proximity of the renal arteries and other vessels to the aneurysm have been overcome by the use of fenestrated grafts. However, the proximal neck of the aneurysm is considered unfavorable anatomically for EVAR if 1 or more of the following situations exist: angulation >60°, diameter >30 mm, short length <15 mm, conical shape, and extensive thrombus or calcification.[17] In addition, surgical access through the femoral vessels in the groin can be difficult in the setting of extensive iliac artery occlusive disease or vessel tortuosity, thereby limiting the applicability of this procedure in some patients or requiring the use of adjunctive, open surgical, or endovascular techniques to gain access to the aorta.",
"The EVAR 1 trial randomly assigned 1082 elective patients (patients from multiple centers that were also fit for open repair) to either open repair or EVAR. The 30-day operative mortality was 1.6% in the EVAR group and 4.6% in the open-repair group.[18] However, those in the EVAR group were at a significantly higher risk for graft complication (hazard ratio [HR], 4.39) and graft-related intervention (HR, 2.86) during follow-up; no difference in all-cause or aneurysm-related mortality was noted after 8 years of follow-up between the groups, with the initial benefit from EVAR erased in long-term follow-up.[19] Since the study, a steady increase has been observed in the proportion of EVAR compared with open repair performed in the United Kingdom[20] and elsewhere.",
"The EVAR 2 trial randomly assigned 338 patients (from 1999 to 2003) older than 60 years with at least 5.5-cm aneurysms who were unfit for open repair to no intervention or EVAR. This study demonstrated a considerable 30-day mortality (9%) in those treated with EVAR compared with no intervention, and showed no significant difference between the EVAR group and the no intervention group for all-cause mortality (HR, 1.21).[21] These findings are in contrast with other studies that claim appreciable long-term benefits are associated with EVAR in patients unfit for surgery, at a cost of a small 30-day mortality rate of between 1.6% and 2.9%.[22,23] Some of the longer benefit may be due to a statistically higher rate of antiplatelet and statin prescription in the newer studies, as well as the increased expertise available in higher-volume centers, and newer-generation grafts.[22,23] The Cronenwett model[24] suggests that patients with AAAs >5.5 cm with low operative risk and poor anatomic suitability for EVAR who are younger than 70 years should have open repair, and those at high risk operatively with excellent anatomy for EVAR and who are older than 70 years should have EVAR. These two extreme examples seem obvious, and many patients fall between the two; informed patient preference and local expertise influence the final treatment in such cases."
],
"date": "October 11, 2018",
"figures": [],
"markdown": "# An 85-Year-Old With Sudden-Onset Severe Abdominal Pain\n\n **Authors:** James J. McCombie, MB ChB \n **Date:** October 11, 2018\n\n ## Content\n\n Multiple imaging modalities, including CT and ultrasonography, have been used to both screen and detail AAA. Three dimensional (3D) reconstruction CT (orthogonal CT) provides a truly accurate model of aneurysm architecture. Angulation of the aneurysm can make traditional axial CT images misleading. When ultrasound and CT are compared with orthogonal CT, ultrasound produces the most accurate estimation of size overall.[8] Traditional CT can be used if the aorta cannot be visualized with ultrasound after repeated efforts.\nA potential AAA screening test is measurement of baseline insulin-like growth factor I (IGF-I). In a prospective, long-term study, Lindholt et al demonstrated that IGF-I is a novel biomarker for AAA.[9] Baseline levels of serum IGF-I not only had a positive correlation with the size and growth rate of the aneurysms, but they also predicted the need for future surgery.[9] Decision-making regarding operative management vs observation for AAA is informed mainly by the results of the UKSAT trial[10] and the ADAM trial.[2] The UKSAT randomized 1090 small aneurysms (4-5.5 cm) to operation or surveillance. No significant difference in overall mortality was demonstrated for those offered early surgery. Similar findings were found in the ADAM trial, and these 2 studies led to the recommendation that surgical repair should be considered in all aneurysms over 5.5 cm; those patients with AAAs less than 5.5 cm should be enrolled on an ultrasonographic surveillance program. Surgical AAA repair should also be considered when the aneurysm expands by more than 0.6-0.8 cm in a single 12-month period.[11] The threshold for surgical repair in women is typically 5 cm, given a relatively smaller normal aortic size compared with men.\nThese recommendations are rooted in the physics of transmural pressure and the higher rupture rates associated with larger aneurysms. The law of LaPlace states that the tension in the wall of a sphere is proportional to the product of the transmural pressure across the wall and the radius of the sphere, and inversely proportional to the thickness of its wall; it can also be interpreted as stating that in a long pliable tube the site of largest diameter requires the least pressure to distend.[12] This explains why an aneurysm tends to enlarge and why larger aneurysms rupture more frequently (it is because of the higher wall tension). As a result of the tendency of larger aneurysms to expand at a faster rate, AAAs of 4.5-5.4 cm in diameter will need 6-month ultrasound follow-up, and AAAs of 3-4.5 cm in diameter need yearly ultrasound follow-up; these intervals are practiced in the NHS (United Kingdom).[1,2,3]\nAround 90% of detected aneurysms are below the threshold of 5.5 cm required for intervention and require surveillance to monitor expansion into the interventional range. Numerous approaches designed to limit expansion have been suggested. Large studies have indicated that propranolol does not inhibit aneurysm expansion.[14] Numerous studies agree that tobacco use is associated with an increased rate of aneurysm expansion.[14] Level B and C evidence is available to suggest that 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors (statins) may inhibit aneurysm expansion.[14,15] Some animal data, but no human data, suggest that angiotensin-converting enzyme inhibitors or losartan, an angiotensin receptor blocker, decrease the rate of AAA expansion, when used to treat concomitant hypertension.[14,16] Optimizing glycemic control is also associated with a reduction in the rate of expansion of AAA in patients with diabetes.[16] Weight loss, regular exercise if possible, and a healthy diet should also be encouraged.\nOpen surgical repair or endovascular aneurysm repair (EVAR) are the current operative options available. EVAR is a less-invasive approach to addressing AAA. EVAR involves transluminal placement of a modular stent-graft system into the AAA via remote arterial access (usually, the femoral artery) under local, regional, or, most commonly, general anesthetic. Concerns about the proximity of the renal arteries and other vessels to the aneurysm have been overcome by the use of fenestrated grafts. However, the proximal neck of the aneurysm is considered unfavorable anatomically for EVAR if 1 or more of the following situations exist: angulation >60°, diameter >30 mm, short length <15 mm, conical shape, and extensive thrombus or calcification.[17] In addition, surgical access through the femoral vessels in the groin can be difficult in the setting of extensive iliac artery occlusive disease or vessel tortuosity, thereby limiting the applicability of this procedure in some patients or requiring the use of adjunctive, open surgical, or endovascular techniques to gain access to the aorta.\nThe EVAR 1 trial randomly assigned 1082 elective patients (patients from multiple centers that were also fit for open repair) to either open repair or EVAR. The 30-day operative mortality was 1.6% in the EVAR group and 4.6% in the open-repair group.[18] However, those in the EVAR group were at a significantly higher risk for graft complication (hazard ratio [HR], 4.39) and graft-related intervention (HR, 2.86) during follow-up; no difference in all-cause or aneurysm-related mortality was noted after 8 years of follow-up between the groups, with the initial benefit from EVAR erased in long-term follow-up.[19] Since the study, a steady increase has been observed in the proportion of EVAR compared with open repair performed in the United Kingdom[20] and elsewhere.\nThe EVAR 2 trial randomly assigned 338 patients (from 1999 to 2003) older than 60 years with at least 5.5-cm aneurysms who were unfit for open repair to no intervention or EVAR. This study demonstrated a considerable 30-day mortality (9%) in those treated with EVAR compared with no intervention, and showed no significant difference between the EVAR group and the no intervention group for all-cause mortality (HR, 1.21).[21] These findings are in contrast with other studies that claim appreciable long-term benefits are associated with EVAR in patients unfit for surgery, at a cost of a small 30-day mortality rate of between 1.6% and 2.9%.[22,23] Some of the longer benefit may be due to a statistically higher rate of antiplatelet and statin prescription in the newer studies, as well as the increased expertise available in higher-volume centers, and newer-generation grafts.[22,23] The Cronenwett model[24] suggests that patients with AAAs >5.5 cm with low operative risk and poor anatomic suitability for EVAR who are younger than 70 years should have open repair, and those at high risk operatively with excellent anatomy for EVAR and who are older than 70 years should have EVAR. These two extreme examples seem obvious, and many patients fall between the two; informed patient preference and local expertise influence the final treatment in such cases.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "An 85-Year-Old With Sudden-Onset Severe Abdominal Pain"
},
{
"authors": "James J. McCombie, MB ChB",
"content": [
"The Society for Vascular Surgery practice guidelines for the Care of Patients with AAA include specific recommendations for surveillance following repair, as follows[25]:",
"Surveillance during the first year post surgery should include CT angiography at 1 month and 12 months.",
"If no endoleak or AAA enlargement is documented after the first year, color duplex ultrasound is suggested as an alternative to CT imaging for annual postoperative surveillance of EVAR.",
"Color duplex ultrasonography and a noncontrast CT scan are recommended as a substitute for CT angiography for post-EVAR surveillance of patients with renal insufficiency.",
"Noncontrast CT scanning of the entire aorta is recommended at 5-year intervals after open surgical repair or EVAR.",
"Open repair of AAA is of proven durability with a 36-year population-based study confirming that the vast majority of patients (91.6%) remain free of any significant graft-related complication during their remaining lifetime.[26] The noted complications included anastomotic pseudoaneurysm, graft thrombosis, graft enteric erosion/fistula, graft infection, anastomotic hemorrhage, colonic ischemia, and atheroembolism; 2.6% of patient complications were recognized within 30 days of the procedure, and the later complications were noted at a median of 6.1 years.",
"Clinical and radiologic follow-up should be geared to recognize these complications and should follow the recommendations detailed by such bodies as the Society for Vascular Surgery. As noted above, persons with AAA are at risk for synchronous and metachronous aneurysm elsewhere, and follow-up should take this into account. EVAR does not have the risks of anastomotic pseudoaneurysm, anastomotic hemorrhage, but it does have those of graft migration and endoleak in addition to the other risks. Endoleak describes the situation of incomplete isolation of the aneurysm sack by the graft, leading to continued flow and pressurization of the sac. Again, follow-up should be planned according to the recommendations for detecting these complications.",
"Emergency repair of ruptured or leaking aneurysms can be performed with EVAR if anatomic conditions are optimal and the patients hemodynamic status allows; if not, open repair should be used as proximal aortic control can be obtained swiftly. If these conditions are met, data suggest benefits for such patients undergoing EVAR, such as a reduction in the mortality, prolonged intensive care requirement and total hospital stay compared with open repair.[2,3,4,5,6,7] Highly symptomatic but unruptured aneurysms can be managed with EVAR and open repair in the same manner as asymptomatic aneurysms (but on a more urgent basis), the threshold is often less than 5.5 cm for repair in these cases.[28]"
],
"date": "October 11, 2018",
"figures": [],
"markdown": "# An 85-Year-Old With Sudden-Onset Severe Abdominal Pain\n\n **Authors:** James J. McCombie, MB ChB \n **Date:** October 11, 2018\n\n ## Content\n\n The Society for Vascular Surgery practice guidelines for the Care of Patients with AAA include specific recommendations for surveillance following repair, as follows[25]:\nSurveillance during the first year post surgery should include CT angiography at 1 month and 12 months.\nIf no endoleak or AAA enlargement is documented after the first year, color duplex ultrasound is suggested as an alternative to CT imaging for annual postoperative surveillance of EVAR.\nColor duplex ultrasonography and a noncontrast CT scan are recommended as a substitute for CT angiography for post-EVAR surveillance of patients with renal insufficiency.\nNoncontrast CT scanning of the entire aorta is recommended at 5-year intervals after open surgical repair or EVAR.\nOpen repair of AAA is of proven durability with a 36-year population-based study confirming that the vast majority of patients (91.6%) remain free of any significant graft-related complication during their remaining lifetime.[26] The noted complications included anastomotic pseudoaneurysm, graft thrombosis, graft enteric erosion/fistula, graft infection, anastomotic hemorrhage, colonic ischemia, and atheroembolism; 2.6% of patient complications were recognized within 30 days of the procedure, and the later complications were noted at a median of 6.1 years.\nClinical and radiologic follow-up should be geared to recognize these complications and should follow the recommendations detailed by such bodies as the Society for Vascular Surgery. As noted above, persons with AAA are at risk for synchronous and metachronous aneurysm elsewhere, and follow-up should take this into account. EVAR does not have the risks of anastomotic pseudoaneurysm, anastomotic hemorrhage, but it does have those of graft migration and endoleak in addition to the other risks. Endoleak describes the situation of incomplete isolation of the aneurysm sack by the graft, leading to continued flow and pressurization of the sac. Again, follow-up should be planned according to the recommendations for detecting these complications.\nEmergency repair of ruptured or leaking aneurysms can be performed with EVAR if anatomic conditions are optimal and the patients hemodynamic status allows; if not, open repair should be used as proximal aortic control can be obtained swiftly. If these conditions are met, data suggest benefits for such patients undergoing EVAR, such as a reduction in the mortality, prolonged intensive care requirement and total hospital stay compared with open repair.[2,3,4,5,6,7] Highly symptomatic but unruptured aneurysms can be managed with EVAR and open repair in the same manner as asymptomatic aneurysms (but on a more urgent basis), the threshold is often less than 5.5 cm for repair in these cases.[28]\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 476059,
"choiceText": ">5.5 cm",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 476061,
"choiceText": ">2 cm",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 476063,
"choiceText": ">3 cm",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 476065,
"choiceText": ">4 cm",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The UKSAT and the ADAM trials led to the practice that aneurysms less than 5.5 cm are surveyed with ultrasound and those larger than 5.5 cm are repaired. The NHS screening program surveys AAAs of 3-4.4 cm yearly and those between 4.5 cm and 5.5 cm biannually. The normal aorta is around 2 cm in diameter, and it is considered aneurysmal when more than 3 cm.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 143219,
"questionText": "You are examining a patient with a known AAA. At the most recent visit, the AAA seems to have expanded. At which of the following sizes should an asymptomatic AAA cease being under surveillance and be considered for repair?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 476067,
"choiceText": "Graft thrombosis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 476069,
"choiceText": "Graft infection",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 476071,
"choiceText": "Colonic ischemia",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 476073,
"choiceText": "Endoleak",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The term endoleak describes when a graft fails to isolate the aneurysm sac, leading to continued pressurization and aneurysm sac expansion. Type I endoleak occurs at the proximal or distal edge of the endograft, if the proximal or distal seal zones between the stent-graft and blood vessel wall are not adequate. Type II endoleak results from a blood vessel that is filling the original aneurysm sac in a retrograde fashion. Type III endoleak results from a leak between overlapping, modular components of a stent-graft system. Type IV endoleak is very similar to type III endoleak, in that it results from blood flowing from within the endograft through the stent, but this time it results from a flaw in the graft material itself. Type V endoleaks have been described as being due to \"endotension\" with an enlarging aneurysm sac without a visible endoleak, which can be dealt with by reinforcing the indwelling stent graft or re-lining the graft.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 143221,
"questionText": "You are considering management options for a patient with an AAA requiring surgical intervention. Which of the following complications of EVAR is <em>not</em> commonly seen in open repair of AAA?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "An 85-Year-Old With Sudden-Onset Severe Abdominal Pain"
},
{
"authors": "James J. McCombie, MB ChB",
"content": [],
"date": "October 11, 2018",
"figures": [],
"markdown": "# An 85-Year-Old With Sudden-Onset Severe Abdominal Pain\n\n **Authors:** James J. McCombie, MB ChB \n **Date:** October 11, 2018\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 476059,
"choiceText": ">5.5 cm",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 476061,
"choiceText": ">2 cm",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 476063,
"choiceText": ">3 cm",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 476065,
"choiceText": ">4 cm",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The UKSAT and the ADAM trials led to the practice that aneurysms less than 5.5 cm are surveyed with ultrasound and those larger than 5.5 cm are repaired. The NHS screening program surveys AAAs of 3-4.4 cm yearly and those between 4.5 cm and 5.5 cm biannually. The normal aorta is around 2 cm in diameter, and it is considered aneurysmal when more than 3 cm.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 143219,
"questionText": "You are examining a patient with a known AAA. At the most recent visit, the AAA seems to have expanded. At which of the following sizes should an asymptomatic AAA cease being under surveillance and be considered for repair?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 476067,
"choiceText": "Graft thrombosis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 476069,
"choiceText": "Graft infection",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 476071,
"choiceText": "Colonic ischemia",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 476073,
"choiceText": "Endoleak",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The term endoleak describes when a graft fails to isolate the aneurysm sac, leading to continued pressurization and aneurysm sac expansion. Type I endoleak occurs at the proximal or distal edge of the endograft, if the proximal or distal seal zones between the stent-graft and blood vessel wall are not adequate. Type II endoleak results from a blood vessel that is filling the original aneurysm sac in a retrograde fashion. Type III endoleak results from a leak between overlapping, modular components of a stent-graft system. Type IV endoleak is very similar to type III endoleak, in that it results from blood flowing from within the endograft through the stent, but this time it results from a flaw in the graft material itself. Type V endoleaks have been described as being due to \"endotension\" with an enlarging aneurysm sac without a visible endoleak, which can be dealt with by reinforcing the indwelling stent graft or re-lining the graft.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 143221,
"questionText": "You are considering management options for a patient with an AAA requiring surgical intervention. Which of the following complications of EVAR is <em>not</em> commonly seen in open repair of AAA?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "An 85-Year-Old With Sudden-Onset Severe Abdominal Pain"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 476051,
"choiceText": "Ureteric colic",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 476053,
"choiceText": "Acute mesenteric ischemia",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 476055,
"choiceText": "Ruptured abdominal aortic aneurysm",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 476057,
"choiceText": "Perforated diverticulum",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 143217,
"questionText": "What is the diagnosis?<br><br><i>Hint: Pay attention to the distribution of the pain and the history of presyncope at its onset, together with a careful review of the abdominal radiograph.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 476059,
"choiceText": ">5.5 cm",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 476061,
"choiceText": ">2 cm",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 476063,
"choiceText": ">3 cm",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 476065,
"choiceText": ">4 cm",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The UKSAT and the ADAM trials led to the practice that aneurysms less than 5.5 cm are surveyed with ultrasound and those larger than 5.5 cm are repaired. The NHS screening program surveys AAAs of 3-4.4 cm yearly and those between 4.5 cm and 5.5 cm biannually. The normal aorta is around 2 cm in diameter, and it is considered aneurysmal when more than 3 cm.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 143219,
"questionText": "You are examining a patient with a known AAA. At the most recent visit, the AAA seems to have expanded. At which of the following sizes should an asymptomatic AAA cease being under surveillance and be considered for repair?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 476067,
"choiceText": "Graft thrombosis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 476069,
"choiceText": "Graft infection",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 476071,
"choiceText": "Colonic ischemia",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 476073,
"choiceText": "Endoleak",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The term endoleak describes when a graft fails to isolate the aneurysm sac, leading to continued pressurization and aneurysm sac expansion. Type I endoleak occurs at the proximal or distal edge of the endograft, if the proximal or distal seal zones between the stent-graft and blood vessel wall are not adequate. Type II endoleak results from a blood vessel that is filling the original aneurysm sac in a retrograde fashion. Type III endoleak results from a leak between overlapping, modular components of a stent-graft system. Type IV endoleak is very similar to type III endoleak, in that it results from blood flowing from within the endograft through the stent, but this time it results from a flaw in the graft material itself. Type V endoleaks have been described as being due to \"endotension\" with an enlarging aneurysm sac without a visible endoleak, which can be dealt with by reinforcing the indwelling stent graft or re-lining the graft.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 143221,
"questionText": "You are considering management options for a patient with an AAA requiring surgical intervention. Which of the following complications of EVAR is <em>not</em> commonly seen in open repair of AAA?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
756811 | /viewarticle/756811 | [
{
"authors": "Benjamin O. Cornwell, DO; Jignesh Modi, MD",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to recognize accurately. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"A 44-year-old man is referred for neurosurgical evaluation secondary to a 6-month history of progressive bilateral lower extremity numbness and weakness. Additionally, he reports a history of back pain over the past 4 years that he describes as an occasional sharp shooting pain down his right thigh.",
"The only medication that he has taken is ibuprofen for pain relief. His surgical history only includes an appendectomy at age 14 years. He denies tobacco, alcohol, or illicit drug use."
],
"date": "November 26, 2018",
"figures": [],
"markdown": "# A 44-Year-Old Man With Progressive Weakness and Back Pain\n\n **Authors:** Benjamin O. Cornwell, DO; Jignesh Modi, MD \n **Date:** November 26, 2018\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to recognize accurately. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nA 44-year-old man is referred for neurosurgical evaluation secondary to a 6-month history of progressive bilateral lower extremity numbness and weakness. Additionally, he reports a history of back pain over the past 4 years that he describes as an occasional sharp shooting pain down his right thigh.\nThe only medication that he has taken is ibuprofen for pain relief. His surgical history only includes an appendectomy at age 14 years. He denies tobacco, alcohol, or illicit drug use.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 44-Year-Old Man With Progressive Weakness and Back Pain"
},
{
"authors": "Benjamin O. Cornwell, DO; Jignesh Modi, MD",
"content": [
"Upon physical examination, his oral temperature is 98.6°F (37°C). His pulse has a regular rhythm, with a rate of 68 beats/min. His blood pressure is 123/89 mm Hg. He is awake, alert, and oriented to time, person, place, and situation.",
"The cranial nerves II-XII are grossly intact, and the patient's pupils are 3 mm and reactive to 2 mm. His extraocular movements are intact. Face, tongue, uvula, and shoulder shrug are symmetric. The motor examination of the lower extremities reveals 5/5 strength in his bilateral hip flexors and knee extensors. He has 4/5 strength with right dorsal and plantar flexion and 5/5 strength with left dorsal and plantar flexion.",
"Sensory examination reveals intact sensation to vibration, but he has reduced pinprick sensation from the midcalf and below bilaterally. Deep tendon reflexes are 1-2+, with absent bilateral ankle clonus. He has an equivocal left Babinski response with downgoing toes on the right. The patient's gait is normal.",
"MRI with and without contrast of the thoracic and lumbar spine is performed and reveals a posterior epidural mass involving the T12-L1 level (Figures 1-3).",
"Figure 1.",
"Figure 2.",
"Figure 3.",
"Signal characteristics of the mass include homogeneous isointense signal intensity on T1, homogeneous high signal on T2, and uniform homogeneous postcontrast enhancement. Associated mass effect is noted on the dorsal aspect of the spinal cord without signal changes within the cord to suggest edema or myelomalacia. The mass does not extend into the adjacent neuroforamina nor does it involve the osseous structures and the adjacent intervertebral disks, which have normal signal and appearance. The paraspinal musculature and soft tissues have a normal appearance. The visualized portions of the thorax and abdomen are unremarkable. Laboratory analysis findings, including a complete blood count and a basic metabolic panel, are normal."
],
"date": "November 26, 2018",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/754/294/754294-thumb1.jpg"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/754/294/754294-thumb2.jpg"
},
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/754/294/754294-thumb3.jpg"
}
],
"markdown": "# A 44-Year-Old Man With Progressive Weakness and Back Pain\n\n **Authors:** Benjamin O. Cornwell, DO; Jignesh Modi, MD \n **Date:** November 26, 2018\n\n ## Content\n\n Upon physical examination, his oral temperature is 98.6°F (37°C). His pulse has a regular rhythm, with a rate of 68 beats/min. His blood pressure is 123/89 mm Hg. He is awake, alert, and oriented to time, person, place, and situation.\nThe cranial nerves II-XII are grossly intact, and the patient's pupils are 3 mm and reactive to 2 mm. His extraocular movements are intact. Face, tongue, uvula, and shoulder shrug are symmetric. The motor examination of the lower extremities reveals 5/5 strength in his bilateral hip flexors and knee extensors. He has 4/5 strength with right dorsal and plantar flexion and 5/5 strength with left dorsal and plantar flexion.\nSensory examination reveals intact sensation to vibration, but he has reduced pinprick sensation from the midcalf and below bilaterally. Deep tendon reflexes are 1-2+, with absent bilateral ankle clonus. He has an equivocal left Babinski response with downgoing toes on the right. The patient's gait is normal.\nMRI with and without contrast of the thoracic and lumbar spine is performed and reveals a posterior epidural mass involving the T12-L1 level (Figures 1-3).\nFigure 1.\nFigure 2.\nFigure 3.\nSignal characteristics of the mass include homogeneous isointense signal intensity on T1, homogeneous high signal on T2, and uniform homogeneous postcontrast enhancement. Associated mass effect is noted on the dorsal aspect of the spinal cord without signal changes within the cord to suggest edema or myelomalacia. The mass does not extend into the adjacent neuroforamina nor does it involve the osseous structures and the adjacent intervertebral disks, which have normal signal and appearance. The paraspinal musculature and soft tissues have a normal appearance. The visualized portions of the thorax and abdomen are unremarkable. Laboratory analysis findings, including a complete blood count and a basic metabolic panel, are normal.\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n**Figure 3.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 472183,
"choiceText": "Epidural cavernous hemangioma",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 472185,
"choiceText": "Epidural abscess",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 472187,
"choiceText": "Ependymoma",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 472189,
"choiceText": "Epidural hematoma",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 141783,
"questionText": "Which of the following is the most likely diagnosis?<br />\r\n<br />\r\n<i>Hint: Note the location and the postcontrast characteristics of the mass.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 44-Year-Old Man With Progressive Weakness and Back Pain"
},
{
"authors": "Benjamin O. Cornwell, DO; Jignesh Modi, MD",
"content": [
"The diagnosis of epidural cavernous hemangioma was made by pathology following surgical removal of the mass. Cavernous hemangiomas within the epidural space of the spine are exceedingly rare and can only be confidently diagnosed in this region of the spine by histology. The patient's symptoms, backache, and progressive weakness and numbness in the lower extremities are a common presentation.[1] Additionally, the findings on MRI, including the homogeneous isointense signal intensity on T1, homogeneous high signal on T2, and uniform homogeneous postcontrast enhancement are typical for an epidural cavernous hemangioma.[1,2]",
"Pure epidural cavernous hemangiomas are rare and are believed to only comprise 4% of all epidural tumors and 12% of all intraspinal hemangiomas.[2] The cavernous hemangioma is a conglomeration of closely packed, dilated, thin-walled vessels that lack a true elastic lamina. The tumor has no malignant potential, but it causes problems secondary to mass effect on the spinal cord or nerve roots. Symptoms of spinal epidural cavernous hemangiomas include spinal cord syndromes and radiculopathies.[3] The affected part of the neuroaxis depends on where the tumor is located within the spine.",
"These tumors have been documented to originate in the cervical, thoracic, and lumbar spine.[3] They also may grow within the ventral, dorsal, or lateral epidural space and involve the adjacent neuroforamina. Cavernous hemangiomas also have a propensity to bleed, and acute hemorrhage can cause acute compression of the spinal cord leading to acute onset of symptoms. As in this case, patients can present with more chronic symptoms if the cavernous hemangioma grows slowly and is not accompanied by hemorrhage. Patients may also suffer from bladder dysfunction; however, this is not as common as pain syndromes or sensorimotor paresis.[3]",
"The differential diagnosis of an epidural cavernous hemangioma includes meningioma, lymphoma, schwannoma, angiolipoma, disk herniation, synovial cysts, granulomatous infection, pure epidural hematoma, and extramedullary hematopoiesis.[2] The combination of history, physical exam findings, laboratory data, and MRI can be used to narrow the differential. History can help distinguish epidural cavernous hemangioma from certain other lesions. For instance, a history of trauma, coagulopathy, or intervention would favor a pure epidural hematoma. If a patient reports pain with lifting or exertion, disk herniation would be more likely.",
"The physical examination may be helpful because patients with epidural infection may present with fever and tenderness to palpation. Patients who have conditions such as sickle cell disease may be more likely to develop extramedullary hematopoiesis, and there should be associated signal abnormalities in the adjacent bone marrow on MRI. Although pure epidural hemangiomas are rare, findings that may help to distinguish these lesions conditions, such as disk prolapse, more common meningiomas, and nerve sheath tumors include \"its ovoid shape, uniform T2 hyperintense signal, and lack of anatomic connection with the neighboring intervertebral disk or the exiting nerve root.\"[4,5]"
],
"date": "November 26, 2018",
"figures": [],
"markdown": "# A 44-Year-Old Man With Progressive Weakness and Back Pain\n\n **Authors:** Benjamin O. Cornwell, DO; Jignesh Modi, MD \n **Date:** November 26, 2018\n\n ## Content\n\n The diagnosis of epidural cavernous hemangioma was made by pathology following surgical removal of the mass. Cavernous hemangiomas within the epidural space of the spine are exceedingly rare and can only be confidently diagnosed in this region of the spine by histology. The patient's symptoms, backache, and progressive weakness and numbness in the lower extremities are a common presentation.[1] Additionally, the findings on MRI, including the homogeneous isointense signal intensity on T1, homogeneous high signal on T2, and uniform homogeneous postcontrast enhancement are typical for an epidural cavernous hemangioma.[1,2]\nPure epidural cavernous hemangiomas are rare and are believed to only comprise 4% of all epidural tumors and 12% of all intraspinal hemangiomas.[2] The cavernous hemangioma is a conglomeration of closely packed, dilated, thin-walled vessels that lack a true elastic lamina. The tumor has no malignant potential, but it causes problems secondary to mass effect on the spinal cord or nerve roots. Symptoms of spinal epidural cavernous hemangiomas include spinal cord syndromes and radiculopathies.[3] The affected part of the neuroaxis depends on where the tumor is located within the spine.\nThese tumors have been documented to originate in the cervical, thoracic, and lumbar spine.[3] They also may grow within the ventral, dorsal, or lateral epidural space and involve the adjacent neuroforamina. Cavernous hemangiomas also have a propensity to bleed, and acute hemorrhage can cause acute compression of the spinal cord leading to acute onset of symptoms. As in this case, patients can present with more chronic symptoms if the cavernous hemangioma grows slowly and is not accompanied by hemorrhage. Patients may also suffer from bladder dysfunction; however, this is not as common as pain syndromes or sensorimotor paresis.[3]\nThe differential diagnosis of an epidural cavernous hemangioma includes meningioma, lymphoma, schwannoma, angiolipoma, disk herniation, synovial cysts, granulomatous infection, pure epidural hematoma, and extramedullary hematopoiesis.[2] The combination of history, physical exam findings, laboratory data, and MRI can be used to narrow the differential. History can help distinguish epidural cavernous hemangioma from certain other lesions. For instance, a history of trauma, coagulopathy, or intervention would favor a pure epidural hematoma. If a patient reports pain with lifting or exertion, disk herniation would be more likely.\nThe physical examination may be helpful because patients with epidural infection may present with fever and tenderness to palpation. Patients who have conditions such as sickle cell disease may be more likely to develop extramedullary hematopoiesis, and there should be associated signal abnormalities in the adjacent bone marrow on MRI. Although pure epidural hemangiomas are rare, findings that may help to distinguish these lesions conditions, such as disk prolapse, more common meningiomas, and nerve sheath tumors include \"its ovoid shape, uniform T2 hyperintense signal, and lack of anatomic connection with the neighboring intervertebral disk or the exiting nerve root.\"[4,5]\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 472183,
"choiceText": "Epidural cavernous hemangioma",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 472185,
"choiceText": "Epidural abscess",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 472187,
"choiceText": "Ependymoma",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 472189,
"choiceText": "Epidural hematoma",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 141783,
"questionText": "Which of the following is the most likely diagnosis?<br />\r\n<br />\r\n<i>Hint: Note the location and the postcontrast characteristics of the mass.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 44-Year-Old Man With Progressive Weakness and Back Pain"
},
{
"authors": "Benjamin O. Cornwell, DO; Jignesh Modi, MD",
"content": [
"MRI is the best diagnostic examination in the workup of an epidural cavernous hemangioma. Classic MRI findings include a mass within the epidural space that is usually lobulated in contour and may extend into the adjacent neuroforamina. T1-weighted images demonstrate the mass as generally isointense in signal to the adjacent intervertebral disk or isointense with foci of hyperintense signal. T2-weighted images most commonly demonstrate homogeneously increased signal or heterogeneously increased signal. Postcontrast images generally produce avid homogeneous or heterogeneous enhancement.",
"Some cavernous hemangiomas also have been reported to have a low signal rim on T1-weighted images, T2-weighted images, and postcontrast images[2]; however, a low-signal rim on T2-weighted images is the most common pattern.[2] Some cavernous hemangiomas also have been shown to have a dural tail sign. A dural tail sign is considered to be present if a thin dural enhancement is noted near the mass with a broad angle.[2] If the cavernous hemangioma is located laterally within the epidural space, it may result in widening of the neural foramina.[6]",
"Intramedullary cavernous hemangiomas occur more commonly than epidural (extramedullary) hemangiomas. Surgical removal of the lesions is currently recommended secondary to the risk for hemorrhage, which has been estimated at approximately 1.4%-4.5% per year. However, if patients have a history of hemorrhage, the risk for rebleeding is estimated at 66% per year.[3] Preoperative hemorrhage of the cavernous hemangioma has been associated with a worse postoperative prognosis secondary to damage to the cord from mass effect from the hemorrhage.",
"Even in the absence of hemorrhage, intramedullary and extramedullary cavernous hemangiomas have been associated with progressive clinical deterioration suggesting that observation is less favorable in most cases. Postoperatively, extramedullary cavernous hemangiomas have been associated with a better clinical outcome than intramedullary cavernous hemangiomas. This is assumed to be secondary to the fact that intramedullary cavernous hemangiomas grow within the spinal cord and, therefore, require a myelotomy for removal, whereas an extramedullary cavernous hemangioma is located outside the spinal cord and can be removed while leaving the cord intact.",
"In one study, of 290 reported cases of patients who underwent surgery to remove an intramedullary cavernous hemangioma, 62% improved, 29% stayed the same, and 9% reported worsening symptoms following surgery.[3] Of the 60 reported cases of patients who underwent surgery to remove an extramedullary cavernous hemangioma, 90% improved, 8% stayed the same, and 2% reported worsening symptoms following surgery. In a Japanese case report, microsurgical resection of a thoracic epidural cavernous hemangioma that extended into the neuroforamen was successful in an elderly male who had presented with neuropathic pain and bilateral lower limb muscle weakness.[7] Following a partial laminectomy and limited medial foraminotomy, surgeons resected the tumor within the neuroforamen without the necessity of a spinal stabilization procedure."
],
"date": "November 26, 2018",
"figures": [],
"markdown": "# A 44-Year-Old Man With Progressive Weakness and Back Pain\n\n **Authors:** Benjamin O. Cornwell, DO; Jignesh Modi, MD \n **Date:** November 26, 2018\n\n ## Content\n\n MRI is the best diagnostic examination in the workup of an epidural cavernous hemangioma. Classic MRI findings include a mass within the epidural space that is usually lobulated in contour and may extend into the adjacent neuroforamina. T1-weighted images demonstrate the mass as generally isointense in signal to the adjacent intervertebral disk or isointense with foci of hyperintense signal. T2-weighted images most commonly demonstrate homogeneously increased signal or heterogeneously increased signal. Postcontrast images generally produce avid homogeneous or heterogeneous enhancement.\nSome cavernous hemangiomas also have been reported to have a low signal rim on T1-weighted images, T2-weighted images, and postcontrast images[2]; however, a low-signal rim on T2-weighted images is the most common pattern.[2] Some cavernous hemangiomas also have been shown to have a dural tail sign. A dural tail sign is considered to be present if a thin dural enhancement is noted near the mass with a broad angle.[2] If the cavernous hemangioma is located laterally within the epidural space, it may result in widening of the neural foramina.[6]\nIntramedullary cavernous hemangiomas occur more commonly than epidural (extramedullary) hemangiomas. Surgical removal of the lesions is currently recommended secondary to the risk for hemorrhage, which has been estimated at approximately 1.4%-4.5% per year. However, if patients have a history of hemorrhage, the risk for rebleeding is estimated at 66% per year.[3] Preoperative hemorrhage of the cavernous hemangioma has been associated with a worse postoperative prognosis secondary to damage to the cord from mass effect from the hemorrhage.\nEven in the absence of hemorrhage, intramedullary and extramedullary cavernous hemangiomas have been associated with progressive clinical deterioration suggesting that observation is less favorable in most cases. Postoperatively, extramedullary cavernous hemangiomas have been associated with a better clinical outcome than intramedullary cavernous hemangiomas. This is assumed to be secondary to the fact that intramedullary cavernous hemangiomas grow within the spinal cord and, therefore, require a myelotomy for removal, whereas an extramedullary cavernous hemangioma is located outside the spinal cord and can be removed while leaving the cord intact.\nIn one study, of 290 reported cases of patients who underwent surgery to remove an intramedullary cavernous hemangioma, 62% improved, 29% stayed the same, and 9% reported worsening symptoms following surgery.[3] Of the 60 reported cases of patients who underwent surgery to remove an extramedullary cavernous hemangioma, 90% improved, 8% stayed the same, and 2% reported worsening symptoms following surgery. In a Japanese case report, microsurgical resection of a thoracic epidural cavernous hemangioma that extended into the neuroforamen was successful in an elderly male who had presented with neuropathic pain and bilateral lower limb muscle weakness.[7] Following a partial laminectomy and limited medial foraminotomy, surgeons resected the tumor within the neuroforamen without the necessity of a spinal stabilization procedure.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 44-Year-Old Man With Progressive Weakness and Back Pain"
},
{
"authors": "Benjamin O. Cornwell, DO; Jignesh Modi, MD",
"content": [
"Several types of hemangiomas can occur within the epidural space of the spine. They are classified by the predominant type of vascular channel. The different types include cavernous, capillary, arteriovenous, or venous. The most common type is cavernous, which was the type of hemangioma seen in this patient's case. Little information is available in the literature about capillary, arteriovenous, and venous hemangiomas. In a Canadian case report, a 57-year-old male with a spinal epidural capillary hemangioma initially presented with low thoracic spine pain; extension of the strongly enhancing mass was seen on MRI into the adjacent foramina at 2 levels on 1 side.[8] Although no significant changes were noted radiologically, progressive myelopathy occurred over a 2.5-year period, which was treated surgically, with good recovery.[8] Arteriovenous and venous hemangiomas are frequently detected as small cystic masses, whereas the cavernous and capillary types have been reported to usually appear as solid hypervascular masses.[2]",
"The patient in this case reported progressive bilateral lower extremity weakness and numbness for 6 months prior to presentation. He also had been experiencing low back pain that would radiate down his right leg for approximately 4 years. He had several associated abnormal findings on his neurologic examination. Following surgery, the patient remained in the hospital for 2 days and did not experience any postoperative complications. At his first outpatient follow-up examination 9 days after surgery, he reported mild residual decreased sensation in his feet and mild weakness in his right great toe. When he returned 27 days after surgery, he had completely recovered sensation and strength in his lower extremities."
],
"date": "November 26, 2018",
"figures": [],
"markdown": "# A 44-Year-Old Man With Progressive Weakness and Back Pain\n\n **Authors:** Benjamin O. Cornwell, DO; Jignesh Modi, MD \n **Date:** November 26, 2018\n\n ## Content\n\n Several types of hemangiomas can occur within the epidural space of the spine. They are classified by the predominant type of vascular channel. The different types include cavernous, capillary, arteriovenous, or venous. The most common type is cavernous, which was the type of hemangioma seen in this patient's case. Little information is available in the literature about capillary, arteriovenous, and venous hemangiomas. In a Canadian case report, a 57-year-old male with a spinal epidural capillary hemangioma initially presented with low thoracic spine pain; extension of the strongly enhancing mass was seen on MRI into the adjacent foramina at 2 levels on 1 side.[8] Although no significant changes were noted radiologically, progressive myelopathy occurred over a 2.5-year period, which was treated surgically, with good recovery.[8] Arteriovenous and venous hemangiomas are frequently detected as small cystic masses, whereas the cavernous and capillary types have been reported to usually appear as solid hypervascular masses.[2]\nThe patient in this case reported progressive bilateral lower extremity weakness and numbness for 6 months prior to presentation. He also had been experiencing low back pain that would radiate down his right leg for approximately 4 years. He had several associated abnormal findings on his neurologic examination. Following surgery, the patient remained in the hospital for 2 days and did not experience any postoperative complications. At his first outpatient follow-up examination 9 days after surgery, he reported mild residual decreased sensation in his feet and mild weakness in his right great toe. When he returned 27 days after surgery, he had completely recovered sensation and strength in his lower extremities.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 472191,
"choiceText": "Intramedullary, increased signal on T2-weighted images, and postcontrast enhancement",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 472193,
"choiceText": "Intramedullary, decreased signal on T2-weighted images, and postcontrast enhancement",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 472195,
"choiceText": "Extramedullary, increased signal on T2-weighted images, and postcontrast enhancement",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 472197,
"choiceText": "Extramedullary, increased signal on T2-weighted images, and no postcontrast enhancement",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 472199,
"choiceText": "Extramedullary, decreased signal on T2-weighted images, and no postcontrast enhancement",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Epidural cavernous hemangiomas most commonly have the following characteristics on MRI: extramedullary, increased signal on T2-weighted images, isointense to adjacent intervertebral disks on T1-weighted images, and avid postcontrast enhancement.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 141785,
"questionText": "You discover that your patient with neurologic deficits of the lower extremities has an intraspinal tumor. Which of the following common characteristics is most likely on MRI if this tumor is an <a href=\"http://emedicine.medscape.com/article/779872-overview\">epidural cavernous hemangioma</a>?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 472201,
"choiceText": "Epidural cavernous hemangioma",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 472203,
"choiceText": "Epidural abscess",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 472205,
"choiceText": "Meningioma",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 472207,
"choiceText": "Epidural hematoma",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 472209,
"choiceText": "Lymphoma",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The presence of fever and back pain should raise the suspicion of epidural abscess. This can be diagnosed by MRI. The remaining possibilities can also result in pain; however, they should not be associated with fever. Additionally, the history does not include trauma or a bleeding diathesis that is suggestive of epidural hematoma.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 141787,
"questionText": "A patient presents to your clinic with fever and a 1-week history of back pain. The patient does not report experiencing similar symptoms in the past, but does report a history of recent intravenous drug abuse. Which of the following choices is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 44-Year-Old Man With Progressive Weakness and Back Pain"
},
{
"authors": "Benjamin O. Cornwell, DO; Jignesh Modi, MD",
"content": [],
"date": "November 26, 2018",
"figures": [],
"markdown": "# A 44-Year-Old Man With Progressive Weakness and Back Pain\n\n **Authors:** Benjamin O. Cornwell, DO; Jignesh Modi, MD \n **Date:** November 26, 2018\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 472191,
"choiceText": "Intramedullary, increased signal on T2-weighted images, and postcontrast enhancement",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 472193,
"choiceText": "Intramedullary, decreased signal on T2-weighted images, and postcontrast enhancement",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 472195,
"choiceText": "Extramedullary, increased signal on T2-weighted images, and postcontrast enhancement",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 472197,
"choiceText": "Extramedullary, increased signal on T2-weighted images, and no postcontrast enhancement",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 472199,
"choiceText": "Extramedullary, decreased signal on T2-weighted images, and no postcontrast enhancement",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Epidural cavernous hemangiomas most commonly have the following characteristics on MRI: extramedullary, increased signal on T2-weighted images, isointense to adjacent intervertebral disks on T1-weighted images, and avid postcontrast enhancement.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 141785,
"questionText": "You discover that your patient with neurologic deficits of the lower extremities has an intraspinal tumor. Which of the following common characteristics is most likely on MRI if this tumor is an <a href=\"http://emedicine.medscape.com/article/779872-overview\">epidural cavernous hemangioma</a>?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 472201,
"choiceText": "Epidural cavernous hemangioma",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 472203,
"choiceText": "Epidural abscess",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 472205,
"choiceText": "Meningioma",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 472207,
"choiceText": "Epidural hematoma",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 472209,
"choiceText": "Lymphoma",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The presence of fever and back pain should raise the suspicion of epidural abscess. This can be diagnosed by MRI. The remaining possibilities can also result in pain; however, they should not be associated with fever. Additionally, the history does not include trauma or a bleeding diathesis that is suggestive of epidural hematoma.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 141787,
"questionText": "A patient presents to your clinic with fever and a 1-week history of back pain. The patient does not report experiencing similar symptoms in the past, but does report a history of recent intravenous drug abuse. Which of the following choices is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 44-Year-Old Man With Progressive Weakness and Back Pain"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 472183,
"choiceText": "Epidural cavernous hemangioma",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 472185,
"choiceText": "Epidural abscess",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 472187,
"choiceText": "Ependymoma",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 472189,
"choiceText": "Epidural hematoma",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 141783,
"questionText": "Which of the following is the most likely diagnosis?<br />\r\n<br />\r\n<i>Hint: Note the location and the postcontrast characteristics of the mass.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 472191,
"choiceText": "Intramedullary, increased signal on T2-weighted images, and postcontrast enhancement",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 472193,
"choiceText": "Intramedullary, decreased signal on T2-weighted images, and postcontrast enhancement",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 472195,
"choiceText": "Extramedullary, increased signal on T2-weighted images, and postcontrast enhancement",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 472197,
"choiceText": "Extramedullary, increased signal on T2-weighted images, and no postcontrast enhancement",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 472199,
"choiceText": "Extramedullary, decreased signal on T2-weighted images, and no postcontrast enhancement",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Epidural cavernous hemangiomas most commonly have the following characteristics on MRI: extramedullary, increased signal on T2-weighted images, isointense to adjacent intervertebral disks on T1-weighted images, and avid postcontrast enhancement.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 141785,
"questionText": "You discover that your patient with neurologic deficits of the lower extremities has an intraspinal tumor. Which of the following common characteristics is most likely on MRI if this tumor is an <a href=\"http://emedicine.medscape.com/article/779872-overview\">epidural cavernous hemangioma</a>?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 472201,
"choiceText": "Epidural cavernous hemangioma",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 472203,
"choiceText": "Epidural abscess",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 472205,
"choiceText": "Meningioma",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 472207,
"choiceText": "Epidural hematoma",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 472209,
"choiceText": "Lymphoma",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The presence of fever and back pain should raise the suspicion of epidural abscess. This can be diagnosed by MRI. The remaining possibilities can also result in pain; however, they should not be associated with fever. Additionally, the history does not include trauma or a bleeding diathesis that is suggestive of epidural hematoma.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 141787,
"questionText": "A patient presents to your clinic with fever and a 1-week history of back pain. The patient does not report experiencing similar symptoms in the past, but does report a history of recent intravenous drug abuse. Which of the following choices is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
754845 | /viewarticle/754845 | [
{
"authors": "Szekeres Csilla-Cecília, MD; Pelok Benedek, MD; Pap Csilla, MD",
"content": [
"Editor's Note:\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions.",
"A 67-year-old woman is brought to the emergency department (ED) for severe right-sided weakness and difficulty speaking that was observed by her husband upon awakening 6 hours before arrival in the ED. Her daughter discloses that the patient is diabetic, hypertensive, and suffers from ischemic cardiomyopathy. She is missing the distal phalanx of her right hallux because it was amputated secondary to a remote history of diabetic ulcer.",
"Figure 1.",
"She is on chronic insulin and angiotensin-converting enzyme inhibitor treatment. Her general practitioner also prescribed antiplatelet therapy (acetylsalicylic acid at 100 mg/day), but she discontinued this therapy months ago. The patient's daughter denies any head trauma, fever, speech difficulties, or motor weakness in the past weeks. She is retired. Her family denies any illicit drug use, tobacco use, or excessive alcohol consumption. Her family history is not remarkable."
],
"date": "May 28, 2015",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/753/622/753622-thumb1.png"
}
],
"markdown": "# Severe Weakness and Speech Disturbance\n\n **Authors:** Szekeres Csilla-Cecília, MD; Pelok Benedek, MD; Pap Csilla, MD \n **Date:** May 28, 2015\n\n ## Content\n\n Editor's Note:\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions.\nA 67-year-old woman is brought to the emergency department (ED) for severe right-sided weakness and difficulty speaking that was observed by her husband upon awakening 6 hours before arrival in the ED. Her daughter discloses that the patient is diabetic, hypertensive, and suffers from ischemic cardiomyopathy. She is missing the distal phalanx of her right hallux because it was amputated secondary to a remote history of diabetic ulcer.\nFigure 1.\nShe is on chronic insulin and angiotensin-converting enzyme inhibitor treatment. Her general practitioner also prescribed antiplatelet therapy (acetylsalicylic acid at 100 mg/day), but she discontinued this therapy months ago. The patient's daughter denies any head trauma, fever, speech difficulties, or motor weakness in the past weeks. She is retired. Her family denies any illicit drug use, tobacco use, or excessive alcohol consumption. Her family history is not remarkable.\n\n ## Figures\n\n **Figure 1.** \n \n\n\n*Page 1 of 5*",
"pagination": {
"current_page": 1,
"total_pages": 5
},
"questionnaire": [],
"title": "Severe Weakness and Speech Disturbance"
},
{
"authors": "Szekeres Csilla-Cecília, MD; Pelok Benedek, MD; Pap Csilla, MD",
"content": [
"Physical Examination and Work-up",
"On physical examination, her temperature is 97.7°F (36.5°C) and her pulse is regular with a rate of 72 beats/min. Her blood pressure is 140/90 mm Hg. She is an obese white female. She appears to be in moderate distress. The examination of her head and neck is normal. Her lungs are clear to auscultation and normal respiratory effort is noted. Her abdomen is tender and there is a mass located along the midline between the pubis and the umbilicus. Ureterovesical catheterization is performed and 1000 mL of yellowish, clear urine is obtained. She has been vomiting.",
"Figure 1.",
"Despite her agitation, she has a decreased level of consciousness and, although she is responsive to verbal and painful stimuli, she is intermittently somnolent and unresponsive. There are no meningeal signs. She is globally aphasic. She has conjugate forced eye deviation to the left. She has a right-sided facial droop, with flattening of the nasolabial fold. She has decreased muscle tone on the right side (as compared with the left side) involving both her upper and lower extremities and is barely able to resist gravity. Deep tendon reflexes are decreased on the right, but she has a positive Babinski sign on the right side. The sensory and vestibulocerebellar systems cannot be examined secondary to depressed mental status.",
"Her glucose level at admission is 80 mg/dL, which is within the normal range. Laboratory analyses, including a complete blood cell count, metabolic panel, and coagulation panel, are normal. A noncontrast cerebral CT scan is performed (Figure 1)."
],
"date": "May 28, 2015",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/753/622/753622-thumb1.png"
}
],
"markdown": "# Severe Weakness and Speech Disturbance\n\n **Authors:** Szekeres Csilla-Cecília, MD; Pelok Benedek, MD; Pap Csilla, MD \n **Date:** May 28, 2015\n\n ## Content\n\n Physical Examination and Work-up\nOn physical examination, her temperature is 97.7°F (36.5°C) and her pulse is regular with a rate of 72 beats/min. Her blood pressure is 140/90 mm Hg. She is an obese white female. She appears to be in moderate distress. The examination of her head and neck is normal. Her lungs are clear to auscultation and normal respiratory effort is noted. Her abdomen is tender and there is a mass located along the midline between the pubis and the umbilicus. Ureterovesical catheterization is performed and 1000 mL of yellowish, clear urine is obtained. She has been vomiting.\nFigure 1.\nDespite her agitation, she has a decreased level of consciousness and, although she is responsive to verbal and painful stimuli, she is intermittently somnolent and unresponsive. There are no meningeal signs. She is globally aphasic. She has conjugate forced eye deviation to the left. She has a right-sided facial droop, with flattening of the nasolabial fold. She has decreased muscle tone on the right side (as compared with the left side) involving both her upper and lower extremities and is barely able to resist gravity. Deep tendon reflexes are decreased on the right, but she has a positive Babinski sign on the right side. The sensory and vestibulocerebellar systems cannot be examined secondary to depressed mental status.\nHer glucose level at admission is 80 mg/dL, which is within the normal range. Laboratory analyses, including a complete blood cell count, metabolic panel, and coagulation panel, are normal. A noncontrast cerebral CT scan is performed (Figure 1).\n\n ## Figures\n\n **Figure 1.** \n \n\n\n*Page 2 of 5*",
"pagination": {
"current_page": 2,
"total_pages": 5
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 455959,
"choiceText": "Hypoglycemia with focal neurologic signs",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 455961,
"choiceText": "Left hemispheric ischemic stroke",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 455963,
"choiceText": "Meningoencephalitis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 455965,
"choiceText": "Brainstem ischemic stroke",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 455967,
"choiceText": "Right hemispheric intracerebral hemorrhage",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 136309,
"questionText": "<p>What is the diagnosis?<br />\r\n<br />\r\n<i>Hint: Note that the CT scan was performed 6 hours after the observation of symptoms.</i></p>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Severe Weakness and Speech Disturbance"
},
{
"authors": "Szekeres Csilla-Cecília, MD; Pelok Benedek, MD; Pap Csilla, MD",
"content": [
"The diagnosis of left hemispheric ischemic stroke was made based on the clinical appearance of the patient, who experienced a sudden onset of severe right-sided hemiparesis and global aphasia corroborated with the apparently negative CT scan. Her National Institutes of Health (NIH) stroke scale was 22.",
"Urgent noncontrast CT (or MRI in centers that can obtain one on an urgent basis) of the brain is performed in all patients presenting with symptoms that could represent ischemic stroke. Patients presenting within 24 hours of ischemic stroke may demonstrate minimal or nonexistent signs of stroke on CT; however, CT is useful in diagnosing other mimics of ischemic stroke, such as intracerebral hemorrhage or tumor.",
"Figure 1.",
"Figure 2.",
"Hypoglycemia can mimic ischemic stroke and can cause focal neurologic signs and impairment of consciousness. It occurs with more frequency in patients with a history of diabetes that are on insulin or antihyperglycemic therapy. In this case, hypoglyemia was ruled out. Meningoencephalitis was considered as well and was thought to be an unlikely cause of the patient's symptoms because there was no fever and no meningismus. This patient's presentation, which involved hemiparesis and aphasia, as well as facial palsy, led to a strong suspicion for a cortical stroke along the distribution of the left middle cerebral artery (MCA).",
"The frequency of MCA stroke in the United States is more than 80 cases per 100,000 people. Most strokes occur in the MCA territory of the cerebral circulation. A systematic review of stroke incidence worldwide showed that between 1970 and 2008, stroke incidence decreased in high-income countries and increased in low- to middle-income nations. The risk for stroke increases with age. The highest incidence of stroke is in the seventh and eighth decades of life. Men are affected more often than women, with a male-to-female ratio of 3:1. However, because women live longer than men, more women have strokes than men. Ethnic minorities, specifically blacks and Mexican Americans, are at a significantly higher risk for ischemic stroke.[1]",
"The clinical presentation of main trunk MCA occlusion of either side includes contralateral hemiplegia, eye deviation toward the side of the infarct, contralateral hemianopsia, and contralateral hemianesthesia. In a study that evaluated the clinical features and prognostic significance of wrong-way deviation (contralateral conjugate eye deviation) in acute supratentorial stroke, investigators noted wrong-way deviation can occur in ischemic stroke if the stroke is extensive and that immediate surgical decompression may be needed to improve the prognosis in affected patients.[2]",
"MCA occlusion involving the dominant hemisphere (left in about 85% of the population) typically causes aphasia. Involvement of the nondominant hemisphere causes impaired perception of deficits (anosognosia) and neglect of the opposite side. MCA division infarcts lead to contralateral deficits with significant involvement of the upper extremity and face and partial sparing of the contralateral leg and foot. Such infarcts on either side also cause a homonymous hemianopia. Right-sided infarcts may also lead to left visual neglect. Finally, resultant temporal lobe damage can lead to an agitated and confused state.[1]",
"Stroke mimics commonly confound the clinical diagnosis of stroke. One study reported that 19% of patients diagnosed with acute ischemic stroke by neurologists before cranial CT actually had noncerebrovascular causes for their symptoms. The most frequent stroke mimics include seizures, systemic infection, brain tumor, hyponatremia, and vertigo. Hypoglycemia should also be considered. Less frequent stroke mimics to be excluded are Bell's palsy, benign positional vertigo, brain abscess, epidural hematoma, and syncope.[3]",
"Aside from standard vital signs and blood glucose testing, several diagnostic studies should be immediately ordered in patients with potential stroke, such as ECG to evaluate for arrhythmias that would predispose a patient to embolic events (eg, atrial fibrillation), complete blood cell count, metabolic studies (including electrolytes and creatinine/blood urea nitrogen levels), and a coagulation profile. Cardiac biomarker testing may also be useful because primary or secondary cardiac events can occur in association with acute ischemic stroke. Additional tests may also be indicated to assess for associated conditions, such as deep venous thrombosis, pulmonary embolism, dysphagia, and urinary dysfunction. CT of the head is the preferred initial imaging study because it is accessible, fast, and highly sensitive for excluding or confirming hemorrhage. However, it is less sensitive than MRI for the diagnosis of stroke within the first few hours of symptoms.[4]"
],
"date": "May 28, 2015",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/753/622/753622-thumb1.png"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/753/622/753622-thumb2.png"
}
],
"markdown": "# Severe Weakness and Speech Disturbance\n\n **Authors:** Szekeres Csilla-Cecília, MD; Pelok Benedek, MD; Pap Csilla, MD \n **Date:** May 28, 2015\n\n ## Content\n\n The diagnosis of left hemispheric ischemic stroke was made based on the clinical appearance of the patient, who experienced a sudden onset of severe right-sided hemiparesis and global aphasia corroborated with the apparently negative CT scan. Her National Institutes of Health (NIH) stroke scale was 22.\nUrgent noncontrast CT (or MRI in centers that can obtain one on an urgent basis) of the brain is performed in all patients presenting with symptoms that could represent ischemic stroke. Patients presenting within 24 hours of ischemic stroke may demonstrate minimal or nonexistent signs of stroke on CT; however, CT is useful in diagnosing other mimics of ischemic stroke, such as intracerebral hemorrhage or tumor.\nFigure 1.\nFigure 2.\nHypoglycemia can mimic ischemic stroke and can cause focal neurologic signs and impairment of consciousness. It occurs with more frequency in patients with a history of diabetes that are on insulin or antihyperglycemic therapy. In this case, hypoglyemia was ruled out. Meningoencephalitis was considered as well and was thought to be an unlikely cause of the patient's symptoms because there was no fever and no meningismus. This patient's presentation, which involved hemiparesis and aphasia, as well as facial palsy, led to a strong suspicion for a cortical stroke along the distribution of the left middle cerebral artery (MCA).\nThe frequency of MCA stroke in the United States is more than 80 cases per 100,000 people. Most strokes occur in the MCA territory of the cerebral circulation. A systematic review of stroke incidence worldwide showed that between 1970 and 2008, stroke incidence decreased in high-income countries and increased in low- to middle-income nations. The risk for stroke increases with age. The highest incidence of stroke is in the seventh and eighth decades of life. Men are affected more often than women, with a male-to-female ratio of 3:1. However, because women live longer than men, more women have strokes than men. Ethnic minorities, specifically blacks and Mexican Americans, are at a significantly higher risk for ischemic stroke.[1]\nThe clinical presentation of main trunk MCA occlusion of either side includes contralateral hemiplegia, eye deviation toward the side of the infarct, contralateral hemianopsia, and contralateral hemianesthesia. In a study that evaluated the clinical features and prognostic significance of wrong-way deviation (contralateral conjugate eye deviation) in acute supratentorial stroke, investigators noted wrong-way deviation can occur in ischemic stroke if the stroke is extensive and that immediate surgical decompression may be needed to improve the prognosis in affected patients.[2]\nMCA occlusion involving the dominant hemisphere (left in about 85% of the population) typically causes aphasia. Involvement of the nondominant hemisphere causes impaired perception of deficits (anosognosia) and neglect of the opposite side. MCA division infarcts lead to contralateral deficits with significant involvement of the upper extremity and face and partial sparing of the contralateral leg and foot. Such infarcts on either side also cause a homonymous hemianopia. Right-sided infarcts may also lead to left visual neglect. Finally, resultant temporal lobe damage can lead to an agitated and confused state.[1]\nStroke mimics commonly confound the clinical diagnosis of stroke. One study reported that 19% of patients diagnosed with acute ischemic stroke by neurologists before cranial CT actually had noncerebrovascular causes for their symptoms. The most frequent stroke mimics include seizures, systemic infection, brain tumor, hyponatremia, and vertigo. Hypoglycemia should also be considered. Less frequent stroke mimics to be excluded are Bell's palsy, benign positional vertigo, brain abscess, epidural hematoma, and syncope.[3]\nAside from standard vital signs and blood glucose testing, several diagnostic studies should be immediately ordered in patients with potential stroke, such as ECG to evaluate for arrhythmias that would predispose a patient to embolic events (eg, atrial fibrillation), complete blood cell count, metabolic studies (including electrolytes and creatinine/blood urea nitrogen levels), and a coagulation profile. Cardiac biomarker testing may also be useful because primary or secondary cardiac events can occur in association with acute ischemic stroke. Additional tests may also be indicated to assess for associated conditions, such as deep venous thrombosis, pulmonary embolism, dysphagia, and urinary dysfunction. CT of the head is the preferred initial imaging study because it is accessible, fast, and highly sensitive for excluding or confirming hemorrhage. However, it is less sensitive than MRI for the diagnosis of stroke within the first few hours of symptoms.[4]\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n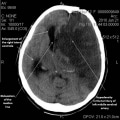 \n\n\n*Page 3 of 5*",
"pagination": {
"current_page": 3,
"total_pages": 5
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 455959,
"choiceText": "Hypoglycemia with focal neurologic signs",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 455961,
"choiceText": "Left hemispheric ischemic stroke",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 455963,
"choiceText": "Meningoencephalitis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 455965,
"choiceText": "Brainstem ischemic stroke",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 455967,
"choiceText": "Right hemispheric intracerebral hemorrhage",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 136309,
"questionText": "<p>What is the diagnosis?<br />\r\n<br />\r\n<i>Hint: Note that the CT scan was performed 6 hours after the observation of symptoms.</i></p>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Severe Weakness and Speech Disturbance"
},
{
"authors": "Szekeres Csilla-Cecília, MD; Pelok Benedek, MD; Pap Csilla, MD",
"content": [
"The NIH Stroke Scale is an extremely valuable tool in assessing patients in the acute phase of [5] Points are assigned based on the level of disability for each of several axes of function, as follows:",
"Assess level of consciousness (Alert = 0, coma = 3)",
"Assess orientation to time and place: Month, Age (1 point per bad answer)",
"Follow commands (1 point per command NOT obeyed)\n \nOpen and close eyes\nMake fist and release",
"Open and close eyes",
"Make fist and release",
"Follow my finger (Normal = 0, Forced deviation = 2)",
"Visual field (0 = Normal, 2 = hemianopia, 3 = bilateral loss)",
"Facial palsy (0 = Normal, 3 = Complete)",
"Motor Strength for each of 4 limbs\n \nNo drift: 0\nUnable to resist gravity: 2\nNo movement: 4\nAmputation or fused joint: 9",
"No drift: 0",
"Unable to resist gravity: 2",
"No movement: 4",
"Amputation or fused joint: 9",
"Coordination or limb ataxia (Absent = 0, both limbs = 2)",
"Sensory (Normal = 0, Severe loss = 2)",
"Language (No aphasia = 0, mute = 3)",
"Speech clarity while reading word list: Grading\n \nNormal articulation: 0\nNearly unintelligible: 2\nIntubated or other physical barrier: 9",
"Normal articulation: 0",
"Nearly unintelligible: 2",
"Intubated or other physical barrier: 9",
"Extinction and Inattention (None = 0, Complete = 2)",
"The modified Rankin scale is a tool for the evaluation of the outcome and long-term prognosis of patients in the chronic phase of stroke.[5] The score is assigned as follows:",
"No symptoms at all",
"1: No significant disability despite symptoms; able to carry out all usual duties and activities",
"2: Slight disability; unable to carry out all previous activities, but able to look after own affairs without assistance",
"3: Moderate disability; requiring some help, but able to walk without assistance",
"4: Moderately severe disability; unable to walk without assistance and unable to attend to own bodily needs without assistance",
"5: Severe disability; bedridden, incontinent, and requiring constant nursing care and attention",
"6: Dead",
"Please refer to this chart for assessment and treatment steps (Figure 3).",
"Figure 3.",
"Treatment for acute ischemic stroke is limited. Aside from supportive care, the only intervention shown to have provided any outcome benefit is the timely administration of thrombolytic therapy. The current window for administration of thrombolysis is 4.5 hours. Candidates for thrombolysis should be selected carefully based on criteria that accounts for the severity of neurologic symptoms as well as exclusion criteria. The main risk of thrombolysis is hemorrhage at both intracerebral and at extracerebral sites. Hemorrhage into the bed of the neurologic tissue involved in the ischemic stroke may occur as frequently as 6% of the time, although many of these cases do not result in clinically important changes in neurologic function.[4]",
"The patient in this case was admitted to the neurology department. She was not eligible for thrombolytic therapy because her symptoms started more than 4.5 hours before her arrival to the ED. She received antiplatelet therapy in the form of aspirin (325 mg orally), intravenous fluids, and also management of her cholesterol; her previous insulin treatment was continued and the patient was placed on an insulin sliding scale with special attention paid to tight glucose control in the post-stroke time period. Special care was taken to avoid excessively aggressive control of blood pressure in the post-stroke period. She exhibited signs of increased intracranial pressure (persistent nausea and vomiting), for which hyperosmolar treatment (20% mannitol solution IV at 6 hours for 5 days) was administered.",
"Figure 2.",
"In the first 3 days of hospitalization, her level of consciousness decreased progressively, but she was thought not to be a candidate for decompressive craniectomy due to her age and overall medical condition. Because of her persistent urinary retention, a permanent bladder catheter was needed. A nasogastric tube was inserted for her severe dysphagia. Passive mobilization of the limbs was effectuated for prevention of decubitus ulcers and deep vein thrombosis. The brain CT scan was repeated after 7 days of hospitalization (Figure 2), which revealed a large hypodense lesion in the left MCA territory with pronounced cerebral edema and mass effect, shift of the median line to the right, compression of the third ventricle, and secondary enlargement of the frontal and temporal horns of the right lateral ventricle. The consciousness of the patient improved from day 10, but she was bedridden because of the severe right hemiparesis and completely aphasic. She was discharged from the hospital after 24 days with a Rankin scale score of 5."
],
"date": "May 28, 2015",
"figures": [
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/754/845/754845-thumb-3.jpg"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/753/622/753622-thumb2.png"
}
],
"markdown": "# Severe Weakness and Speech Disturbance\n\n **Authors:** Szekeres Csilla-Cecília, MD; Pelok Benedek, MD; Pap Csilla, MD \n **Date:** May 28, 2015\n\n ## Content\n\n The NIH Stroke Scale is an extremely valuable tool in assessing patients in the acute phase of [5] Points are assigned based on the level of disability for each of several axes of function, as follows:\nAssess level of consciousness (Alert = 0, coma = 3)\nAssess orientation to time and place: Month, Age (1 point per bad answer)\nFollow commands (1 point per command NOT obeyed)\n \nOpen and close eyes\nMake fist and release\nOpen and close eyes\nMake fist and release\nFollow my finger (Normal = 0, Forced deviation = 2)\nVisual field (0 = Normal, 2 = hemianopia, 3 = bilateral loss)\nFacial palsy (0 = Normal, 3 = Complete)\nMotor Strength for each of 4 limbs\n \nNo drift: 0\nUnable to resist gravity: 2\nNo movement: 4\nAmputation or fused joint: 9\nNo drift: 0\nUnable to resist gravity: 2\nNo movement: 4\nAmputation or fused joint: 9\nCoordination or limb ataxia (Absent = 0, both limbs = 2)\nSensory (Normal = 0, Severe loss = 2)\nLanguage (No aphasia = 0, mute = 3)\nSpeech clarity while reading word list: Grading\n \nNormal articulation: 0\nNearly unintelligible: 2\nIntubated or other physical barrier: 9\nNormal articulation: 0\nNearly unintelligible: 2\nIntubated or other physical barrier: 9\nExtinction and Inattention (None = 0, Complete = 2)\nThe modified Rankin scale is a tool for the evaluation of the outcome and long-term prognosis of patients in the chronic phase of stroke.[5] The score is assigned as follows:\nNo symptoms at all\n1: No significant disability despite symptoms; able to carry out all usual duties and activities\n2: Slight disability; unable to carry out all previous activities, but able to look after own affairs without assistance\n3: Moderate disability; requiring some help, but able to walk without assistance\n4: Moderately severe disability; unable to walk without assistance and unable to attend to own bodily needs without assistance\n5: Severe disability; bedridden, incontinent, and requiring constant nursing care and attention\n6: Dead\nPlease refer to this chart for assessment and treatment steps (Figure 3).\nFigure 3.\nTreatment for acute ischemic stroke is limited. Aside from supportive care, the only intervention shown to have provided any outcome benefit is the timely administration of thrombolytic therapy. The current window for administration of thrombolysis is 4.5 hours. Candidates for thrombolysis should be selected carefully based on criteria that accounts for the severity of neurologic symptoms as well as exclusion criteria. The main risk of thrombolysis is hemorrhage at both intracerebral and at extracerebral sites. Hemorrhage into the bed of the neurologic tissue involved in the ischemic stroke may occur as frequently as 6% of the time, although many of these cases do not result in clinically important changes in neurologic function.[4]\nThe patient in this case was admitted to the neurology department. She was not eligible for thrombolytic therapy because her symptoms started more than 4.5 hours before her arrival to the ED. She received antiplatelet therapy in the form of aspirin (325 mg orally), intravenous fluids, and also management of her cholesterol; her previous insulin treatment was continued and the patient was placed on an insulin sliding scale with special attention paid to tight glucose control in the post-stroke time period. Special care was taken to avoid excessively aggressive control of blood pressure in the post-stroke period. She exhibited signs of increased intracranial pressure (persistent nausea and vomiting), for which hyperosmolar treatment (20% mannitol solution IV at 6 hours for 5 days) was administered.\nFigure 2.\nIn the first 3 days of hospitalization, her level of consciousness decreased progressively, but she was thought not to be a candidate for decompressive craniectomy due to her age and overall medical condition. Because of her persistent urinary retention, a permanent bladder catheter was needed. A nasogastric tube was inserted for her severe dysphagia. Passive mobilization of the limbs was effectuated for prevention of decubitus ulcers and deep vein thrombosis. The brain CT scan was repeated after 7 days of hospitalization (Figure 2), which revealed a large hypodense lesion in the left MCA territory with pronounced cerebral edema and mass effect, shift of the median line to the right, compression of the third ventricle, and secondary enlargement of the frontal and temporal horns of the right lateral ventricle. The consciousness of the patient improved from day 10, but she was bedridden because of the severe right hemiparesis and completely aphasic. She was discharged from the hospital after 24 days with a Rankin scale score of 5.\n\n ## Figures\n\n **Figure 3.** \n \n\n**Figure 2.** \n \n\n\n*Page 4 of 5*",
"pagination": {
"current_page": 4,
"total_pages": 5
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 455979,
"choiceText": "Neglect",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 455981,
"choiceText": "Apraxia",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 455983,
"choiceText": "Aphasia",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 455985,
"choiceText": "Fever",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 455987,
"choiceText": "Neck stiffness",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The clinical presentation of main trunk MCA occlusion of either side causes contralateral hemiplegia, eye deviation toward the side of the infarct, contralateral hemianopsia, and contralateral hemianesthesia. Trunk occlusion involving the dominant hemisphere causes global aphasia. Involvement of the nondominant hemisphere causes impaired perception of deficits (anosognosia) and neglect.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 136313,
"questionText": "You are examining a patient who you suspect may be suffering from a blockage in the MCA. Which of the following symptoms is typically seen in patients with a dominant hemisphere MCA occlusion?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 455989,
"choiceText": "Hypoglycemia",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 455991,
"choiceText": "Subdural hematoma",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 455993,
"choiceText": "Pneumonia",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 455995,
"choiceText": "Myocardial infarction",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 455997,
"choiceText": "Brain abscess",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The most frequent stroke mimics include seizures, systemic infection, brain tumor, hyponatremia, and positional vertigo. Hypoglycemia should also be considered. Less frequent stroke mimics to be excluded are Bell's palsy, benign positional vertigo, brain abscess, epidural hematoma, and syncope.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 136315,
"questionText": "The previously described patient has a history of diabetes. Which of the following is the most frequent stroke-mimic in diabetic patients treated with insulin therapy?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Severe Weakness and Speech Disturbance"
},
{
"authors": "Szekeres Csilla-Cecília, MD; Pelok Benedek, MD; Pap Csilla, MD",
"content": [],
"date": "May 28, 2015",
"figures": [],
"markdown": "# Severe Weakness and Speech Disturbance\n\n **Authors:** Szekeres Csilla-Cecília, MD; Pelok Benedek, MD; Pap Csilla, MD \n **Date:** May 28, 2015\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 5 of 5*",
"pagination": {
"current_page": 5,
"total_pages": 5
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 455979,
"choiceText": "Neglect",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 455981,
"choiceText": "Apraxia",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 455983,
"choiceText": "Aphasia",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 455985,
"choiceText": "Fever",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 455987,
"choiceText": "Neck stiffness",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The clinical presentation of main trunk MCA occlusion of either side causes contralateral hemiplegia, eye deviation toward the side of the infarct, contralateral hemianopsia, and contralateral hemianesthesia. Trunk occlusion involving the dominant hemisphere causes global aphasia. Involvement of the nondominant hemisphere causes impaired perception of deficits (anosognosia) and neglect.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 136313,
"questionText": "You are examining a patient who you suspect may be suffering from a blockage in the MCA. Which of the following symptoms is typically seen in patients with a dominant hemisphere MCA occlusion?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 455989,
"choiceText": "Hypoglycemia",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 455991,
"choiceText": "Subdural hematoma",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 455993,
"choiceText": "Pneumonia",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 455995,
"choiceText": "Myocardial infarction",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 455997,
"choiceText": "Brain abscess",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The most frequent stroke mimics include seizures, systemic infection, brain tumor, hyponatremia, and positional vertigo. Hypoglycemia should also be considered. Less frequent stroke mimics to be excluded are Bell's palsy, benign positional vertigo, brain abscess, epidural hematoma, and syncope.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 136315,
"questionText": "The previously described patient has a history of diabetes. Which of the following is the most frequent stroke-mimic in diabetic patients treated with insulin therapy?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Severe Weakness and Speech Disturbance"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 455959,
"choiceText": "Hypoglycemia with focal neurologic signs",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 455961,
"choiceText": "Left hemispheric ischemic stroke",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 455963,
"choiceText": "Meningoencephalitis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 455965,
"choiceText": "Brainstem ischemic stroke",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 455967,
"choiceText": "Right hemispheric intracerebral hemorrhage",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 136309,
"questionText": "<p>What is the diagnosis?<br />\r\n<br />\r\n<i>Hint: Note that the CT scan was performed 6 hours after the observation of symptoms.</i></p>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 455979,
"choiceText": "Neglect",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 455981,
"choiceText": "Apraxia",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 455983,
"choiceText": "Aphasia",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 455985,
"choiceText": "Fever",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 455987,
"choiceText": "Neck stiffness",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The clinical presentation of main trunk MCA occlusion of either side causes contralateral hemiplegia, eye deviation toward the side of the infarct, contralateral hemianopsia, and contralateral hemianesthesia. Trunk occlusion involving the dominant hemisphere causes global aphasia. Involvement of the nondominant hemisphere causes impaired perception of deficits (anosognosia) and neglect.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 136313,
"questionText": "You are examining a patient who you suspect may be suffering from a blockage in the MCA. Which of the following symptoms is typically seen in patients with a dominant hemisphere MCA occlusion?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 455989,
"choiceText": "Hypoglycemia",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 455991,
"choiceText": "Subdural hematoma",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 455993,
"choiceText": "Pneumonia",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 455995,
"choiceText": "Myocardial infarction",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 455997,
"choiceText": "Brain abscess",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The most frequent stroke mimics include seizures, systemic infection, brain tumor, hyponatremia, and positional vertigo. Hypoglycemia should also be considered. Less frequent stroke mimics to be excluded are Bell's palsy, benign positional vertigo, brain abscess, epidural hematoma, and syncope.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 136315,
"questionText": "The previously described patient has a history of diabetes. Which of the following is the most frequent stroke-mimic in diabetic patients treated with insulin therapy?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
753619 | /viewarticle/753619 | [
{
"authors": "Rearabiloe Sebata, MB ChB; Naveed Afzal, MB BS; Ananda Chakrabarti, MB BS",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"A 35-year-old man presents to the emergency department (ED) with complaints of acute-onset penile pain and swelling that started while in the act of sexual intercourse with his female partner 36 hours before presentation. During intercourse, he missed the vaginal introitus and hit the perineum with some force on attempting to re-enter the vagina with the penis while performing thrusting movements. His partner recalls hearing a cracking sound. He experienced instant penile pain, immediate loss of erection, and almost instantaneous swelling of the penis.",
"About half an hour later he noticed blood at the urethral meatus. He presented to the ED 36 hours after the injury, by which time his entire penis was bruised, with progressive swelling. He has been unable to urinate since the injury; every time he has tried, blood oozes from his urethral meatus. As a result, he is also experiencing great discomfort from urinary retention.",
"In the period before presentation, he applied ice packs and lay in a tub with warm water in an attempt to relieve the symptoms. The patient was reluctant to present for medical care and waited before seeking medical attention in the hope that his symptoms would improve."
],
"date": "May 15, 2018",
"figures": [],
"markdown": "# A 35-Year-Old Man Unable to Urinate and in Severe Pain\n\n **Authors:** Rearabiloe Sebata, MB ChB; Naveed Afzal, MB BS; Ananda Chakrabarti, MB BS \n **Date:** May 15, 2018\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nA 35-year-old man presents to the emergency department (ED) with complaints of acute-onset penile pain and swelling that started while in the act of sexual intercourse with his female partner 36 hours before presentation. During intercourse, he missed the vaginal introitus and hit the perineum with some force on attempting to re-enter the vagina with the penis while performing thrusting movements. His partner recalls hearing a cracking sound. He experienced instant penile pain, immediate loss of erection, and almost instantaneous swelling of the penis.\nAbout half an hour later he noticed blood at the urethral meatus. He presented to the ED 36 hours after the injury, by which time his entire penis was bruised, with progressive swelling. He has been unable to urinate since the injury; every time he has tried, blood oozes from his urethral meatus. As a result, he is also experiencing great discomfort from urinary retention.\nIn the period before presentation, he applied ice packs and lay in a tub with warm water in an attempt to relieve the symptoms. The patient was reluctant to present for medical care and waited before seeking medical attention in the hope that his symptoms would improve.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 35-Year-Old Man Unable to Urinate and in Severe Pain"
},
{
"authors": "Rearabiloe Sebata, MB ChB; Naveed Afzal, MB BS; Ananda Chakrabarti, MB BS",
"content": [
"Upon physical examination, he is noted to be in significant discomfort. His vital signs are all within normal range. His systemic examination is essentially unremarkable.",
"On abdominal examination, he has a palpably full urinary bladder that is midway between his symphysis pubis and the umbilicus. On urologic genital examination, his entire uncircumcised penis is swollen with an ecchymosed appearance, particularly on the ventral surface. Some dry blood is observed at the urethral meatus. His entire penis is extremely tender to touch. No scrotal involvement is noted. His testicles are of normal size and nontender.",
"The initial management includes a 10-mg dose of morphine intravenously. A suprapubic catheter is then inserted with 2% lidocaine local anesthesia, from which 1500 cc of bloody urine are drained. Blood samples are drawn for routine testing, including a complete blood cell count, chemistry, and coagulation profile; all findings are within normal limits.",
"MRI of the penis is requested, without delaying surgical exploration (Figures 1-2). The history and physical examination findings are very concerning. Immediate surgical exploration is necessary.",
"Figure 1.",
"Figure 2."
],
"date": "May 15, 2018",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/752/697/752697-thumb1.png"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/752/697/752697-thumb2.png"
}
],
"markdown": "# A 35-Year-Old Man Unable to Urinate and in Severe Pain\n\n **Authors:** Rearabiloe Sebata, MB ChB; Naveed Afzal, MB BS; Ananda Chakrabarti, MB BS \n **Date:** May 15, 2018\n\n ## Content\n\n Upon physical examination, he is noted to be in significant discomfort. His vital signs are all within normal range. His systemic examination is essentially unremarkable.\nOn abdominal examination, he has a palpably full urinary bladder that is midway between his symphysis pubis and the umbilicus. On urologic genital examination, his entire uncircumcised penis is swollen with an ecchymosed appearance, particularly on the ventral surface. Some dry blood is observed at the urethral meatus. His entire penis is extremely tender to touch. No scrotal involvement is noted. His testicles are of normal size and nontender.\nThe initial management includes a 10-mg dose of morphine intravenously. A suprapubic catheter is then inserted with 2% lidocaine local anesthesia, from which 1500 cc of bloody urine are drained. Blood samples are drawn for routine testing, including a complete blood cell count, chemistry, and coagulation profile; all findings are within normal limits.\nMRI of the penis is requested, without delaying surgical exploration (Figures 1-2). The history and physical examination findings are very concerning. Immediate surgical exploration is necessary.\nFigure 1.\nFigure 2.\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 446029,
"choiceText": "Severe gonococcal urethritis and epididymitis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 446031,
"choiceText": "Penile fracture",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 446033,
"choiceText": "Prostatic hypertrophy",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 446035,
"choiceText": "Testicular torsion",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 132757,
"questionText": "What is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 35-Year-Old Man Unable to Urinate and in Severe Pain"
},
{
"authors": "Rearabiloe Sebata, MB ChB; Naveed Afzal, MB BS; Ananda Chakrabarti, MB BS",
"content": [
"Penile fracture is a true urologic emergency that generally requires urgent surgical repair; therefore, prompt diagnosis is required and the patient should be referred to a urologic surgeon with experience in the repair of this type of injury. This patient's presentation and physical findings immediately led to suspicion for penile fracture. The cracking sound reported by the patient's partner, with instantaneous pain, loss of erection, and immediate swelling, all pointed to a diagnosis of penile fracture.",
"This center has seen 3 cases of penile fracture in recent years, with differing management and outcomes. The first patient was rushed to the operating room without any imaging. Although the final outcome was excellent, undertaking a penile exploration procedure blindly without any idea of the exact site and extent of the injuries, including urethral involvement, made for a challenging procedure. The penis is a very vascular structure that bleeds heavily when injured, which makes blind exploration technically difficult and more traumatic.",
"In the second case, preoperative MRI was performed. The site and extent of injury were localized prior to surgery, which made for a more straightforward surgical exploration. In this patient, MRI was initially obtained. The findings of meatal blood and urinary retention suggested urethral involvement; a suprapubic catheter was placed in the emergency room prior to performing MRI. The MRI confirmed a penile fracture and also demonstrated a complete transaction of the midurethra (Figure 1). The findings were confirmed on surgical exploration (Figures 3-6).",
"Figure 1.",
"Figure 3.",
"Figure 4.",
"Figure 5.",
"Figure 6.",
"Surgical exploration involved first performing a circumcision followed by degloving of the entire penile shaft. A rugged, 1-cm midshaft defect of the tunica albuginea was discovered on the ventral surface. A urethral catheter was then placed retrograde and appeared midshaft through the disruption. The area was irrigated with normal saline and the proximal stump of the urethra was identified; the catheter was then cannulated through to the proximal urethral end and into the urinary bladder. Further exploration revealed a complete disruption of the corpus spongiosum and partial disruption of both corpora cavernosa. The urethra was then repaired with 3-0 VICRYL™ over a silastic catheter. The rest of the penile injuries were repaired in layers with 2-0 VICRYL, including the tunica albuginea defect. The urethral catheter was left in situ and the suprapubic catheter removed in the operating room."
],
"date": "May 15, 2018",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/752/697/752697-thumb1.png"
},
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/752/697/752697-thumb3.png"
},
{
"caption": "Figure 4.",
"image_url": "https://img.medscapestatic.com/article/752/697/752697-thumb4.png"
},
{
"caption": "Figure 5.",
"image_url": "https://img.medscapestatic.com/article/752/697/752697-thumb5.png"
},
{
"caption": "Figure 6.",
"image_url": "https://img.medscapestatic.com/article/752/697/752697-thumb6.png"
}
],
"markdown": "# A 35-Year-Old Man Unable to Urinate and in Severe Pain\n\n **Authors:** Rearabiloe Sebata, MB ChB; Naveed Afzal, MB BS; Ananda Chakrabarti, MB BS \n **Date:** May 15, 2018\n\n ## Content\n\n Penile fracture is a true urologic emergency that generally requires urgent surgical repair; therefore, prompt diagnosis is required and the patient should be referred to a urologic surgeon with experience in the repair of this type of injury. This patient's presentation and physical findings immediately led to suspicion for penile fracture. The cracking sound reported by the patient's partner, with instantaneous pain, loss of erection, and immediate swelling, all pointed to a diagnosis of penile fracture.\nThis center has seen 3 cases of penile fracture in recent years, with differing management and outcomes. The first patient was rushed to the operating room without any imaging. Although the final outcome was excellent, undertaking a penile exploration procedure blindly without any idea of the exact site and extent of the injuries, including urethral involvement, made for a challenging procedure. The penis is a very vascular structure that bleeds heavily when injured, which makes blind exploration technically difficult and more traumatic.\nIn the second case, preoperative MRI was performed. The site and extent of injury were localized prior to surgery, which made for a more straightforward surgical exploration. In this patient, MRI was initially obtained. The findings of meatal blood and urinary retention suggested urethral involvement; a suprapubic catheter was placed in the emergency room prior to performing MRI. The MRI confirmed a penile fracture and also demonstrated a complete transaction of the midurethra (Figure 1). The findings were confirmed on surgical exploration (Figures 3-6).\nFigure 1.\nFigure 3.\nFigure 4.\nFigure 5.\nFigure 6.\nSurgical exploration involved first performing a circumcision followed by degloving of the entire penile shaft. A rugged, 1-cm midshaft defect of the tunica albuginea was discovered on the ventral surface. A urethral catheter was then placed retrograde and appeared midshaft through the disruption. The area was irrigated with normal saline and the proximal stump of the urethra was identified; the catheter was then cannulated through to the proximal urethral end and into the urinary bladder. Further exploration revealed a complete disruption of the corpus spongiosum and partial disruption of both corpora cavernosa. The urethra was then repaired with 3-0 VICRYL™ over a silastic catheter. The rest of the penile injuries were repaired in layers with 2-0 VICRYL, including the tunica albuginea defect. The urethral catheter was left in situ and the suprapubic catheter removed in the operating room.\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 3.** \n \n\n**Figure 4.** \n \n\n**Figure 5.** \n \n\n**Figure 6.** \n \n\n\n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 446029,
"choiceText": "Severe gonococcal urethritis and epididymitis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 446031,
"choiceText": "Penile fracture",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 446033,
"choiceText": "Prostatic hypertrophy",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 446035,
"choiceText": "Testicular torsion",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 132757,
"questionText": "What is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 35-Year-Old Man Unable to Urinate and in Severe Pain"
},
{
"authors": "Rearabiloe Sebata, MB ChB; Naveed Afzal, MB BS; Ananda Chakrabarti, MB BS",
"content": [
"Penile fracture occurs as a result of forceful bending of an erect penis. This happens most commonly during sexual intercourse, particularly when it is intense or energetic. In a small retrospective study that evaluated the specific social situations that caused penile fractures in patients who required surgical intervention, Kramer determined that stressful situations such as extramarital affairs and engaging in sexual intercourse in unusual locations increased the likelihood of these injuries.[1] Other reported cases have occurred during masturbation, falling on an erect penis, and rolling onto a nocturnally erect penis.[2] Penile fracture is often accompanied by an audible snap and followed immediately by severe pain, detumescence, swelling, and ecchymosis. In the erect penis, the tunica albuginea thins down from 2 mm to 0.5 mm and easily snaps when a violent bending of the penis suddenly increases the intracorporeal pressure. A flaccid penis generally will not fracture.[3]",
"Penile fracture ranges from a simple unilateral corpus cavernosa tear with minimal disruption to severe injury with transection of the urethra. Except for blood at the urethral meatus, discerning the extent of injury via physical examination alone is not possible. Significant penile fractures generally involve the corpora cavernosa and their covering sheath, the tunica albuginea. Lacerations rarely extend to involve the urethra (only around 10%), and complete transection of the urethra and corpus spongiosum is extremely rare. If the Buck's fascia remains intact, the swelling and ecchymosis are confined to the penile shaft; if the fascia dos not remain intact, blood and urine may dissect into the scrotum, perineum, and suprapubic spaces.[4]",
"The major complications of penile fracture are loss of penile length, deformity, impotence, and urethral structuring.[5] Most patients who experience long-term complications are those who are managed nonsurgically. In a case series of 25 patients, all cases in which conservative (nonsurgical) management was the first treatment option suffered late complications (penile aneurysm, induration, penile curvature, erectile dysfunction) and the final results were often poor.[5]",
"In a study that evaluated perioperative risk factors of erectile dysfunction and penile vascular changes in 180 patients following surgical repair of penile fracture, El-Assmy et al reported that long-term postsurgical sequela of psychologic or vascular erectile dysfunction may occur—with aging, age older than 50 years, and bilateral corporal involvement at presentation as the main risk factors.[6] Of the patient population, only 2 patients had risk factors for systemic disease (1 had diabetes mellitus, 1 had hypertension) but no patients had a previous history of erectile dysfunction.[6]",
"A few reports have described how best to image penile injuries prior to surgery. Some state that in the presence of obvious clinical signs consistent with a penile fracture, penile exploration can be undertaken without any imaging. The literature emphasizes the need for urgent operative management[7] and suggests that the earlier the operation is done (preferably within 12 h), the lower the risk for complications. Various imaging techniques have been used to determine the location and extent of the injury. Cavernosography has been reported to be good at identifying a corpus cavernosal tear, but it is an invasive procedure[8]; it has also been reported to cause postprocedural priapism.[5] Ultrasonography can locate the exact site of a tear in the tunica albuginea, is easily available, and is noninvasive, but its use can be limited by gross penile swelling and often severe pain. Intraoperative urethrography has also been reported in cases without preoperative imaging. Urethrography will only provide evidence of urethral involvement.",
"MRI is an excellent tool in assessing the exact site and extent of injury in penile fractures and should be taken as the imaging modality of choice when available.[9] MRI is noninvasive, nonradiating, and provides excellent images allowing for determination of the extent of corporal tears and assessment of urethral involvement.[10] Some surgeons prefer to have imaging performed prior to surgery to assess the location of the injury. MRI can accurately depict the presence, location, and extent of tunical tear, which manifests as discontinuity of the tunica albuginea.[10] Moreover, because the tunica albuginea is well demonstrated as a low-signal-intensity structure on both T1- and T2-weighted images, MRI is optimal for the evaluation of the integrity of this anatomic structure. Not all cases of blunt penile trauma with bruising and swelling are associated with significant corpora injury requiring surgery, and the degree of swelling or bruising is in no way suggestive of the severity of the injury. MRI can demonstrate an intact tunica albuginea and the presence of intracavernosal or extratunical hematoma,[10] which does not require surgical management.",
"The time delay for obtaining MRI is not likely significant enough to affect the outcome of surgery. This patient made a full recovery with no complications."
],
"date": "May 15, 2018",
"figures": [],
"markdown": "# A 35-Year-Old Man Unable to Urinate and in Severe Pain\n\n **Authors:** Rearabiloe Sebata, MB ChB; Naveed Afzal, MB BS; Ananda Chakrabarti, MB BS \n **Date:** May 15, 2018\n\n ## Content\n\n Penile fracture occurs as a result of forceful bending of an erect penis. This happens most commonly during sexual intercourse, particularly when it is intense or energetic. In a small retrospective study that evaluated the specific social situations that caused penile fractures in patients who required surgical intervention, Kramer determined that stressful situations such as extramarital affairs and engaging in sexual intercourse in unusual locations increased the likelihood of these injuries.[1] Other reported cases have occurred during masturbation, falling on an erect penis, and rolling onto a nocturnally erect penis.[2] Penile fracture is often accompanied by an audible snap and followed immediately by severe pain, detumescence, swelling, and ecchymosis. In the erect penis, the tunica albuginea thins down from 2 mm to 0.5 mm and easily snaps when a violent bending of the penis suddenly increases the intracorporeal pressure. A flaccid penis generally will not fracture.[3]\nPenile fracture ranges from a simple unilateral corpus cavernosa tear with minimal disruption to severe injury with transection of the urethra. Except for blood at the urethral meatus, discerning the extent of injury via physical examination alone is not possible. Significant penile fractures generally involve the corpora cavernosa and their covering sheath, the tunica albuginea. Lacerations rarely extend to involve the urethra (only around 10%), and complete transection of the urethra and corpus spongiosum is extremely rare. If the Buck's fascia remains intact, the swelling and ecchymosis are confined to the penile shaft; if the fascia dos not remain intact, blood and urine may dissect into the scrotum, perineum, and suprapubic spaces.[4]\nThe major complications of penile fracture are loss of penile length, deformity, impotence, and urethral structuring.[5] Most patients who experience long-term complications are those who are managed nonsurgically. In a case series of 25 patients, all cases in which conservative (nonsurgical) management was the first treatment option suffered late complications (penile aneurysm, induration, penile curvature, erectile dysfunction) and the final results were often poor.[5]\nIn a study that evaluated perioperative risk factors of erectile dysfunction and penile vascular changes in 180 patients following surgical repair of penile fracture, El-Assmy et al reported that long-term postsurgical sequela of psychologic or vascular erectile dysfunction may occur—with aging, age older than 50 years, and bilateral corporal involvement at presentation as the main risk factors.[6] Of the patient population, only 2 patients had risk factors for systemic disease (1 had diabetes mellitus, 1 had hypertension) but no patients had a previous history of erectile dysfunction.[6]\nA few reports have described how best to image penile injuries prior to surgery. Some state that in the presence of obvious clinical signs consistent with a penile fracture, penile exploration can be undertaken without any imaging. The literature emphasizes the need for urgent operative management[7] and suggests that the earlier the operation is done (preferably within 12 h), the lower the risk for complications. Various imaging techniques have been used to determine the location and extent of the injury. Cavernosography has been reported to be good at identifying a corpus cavernosal tear, but it is an invasive procedure[8]; it has also been reported to cause postprocedural priapism.[5] Ultrasonography can locate the exact site of a tear in the tunica albuginea, is easily available, and is noninvasive, but its use can be limited by gross penile swelling and often severe pain. Intraoperative urethrography has also been reported in cases without preoperative imaging. Urethrography will only provide evidence of urethral involvement.\nMRI is an excellent tool in assessing the exact site and extent of injury in penile fractures and should be taken as the imaging modality of choice when available.[9] MRI is noninvasive, nonradiating, and provides excellent images allowing for determination of the extent of corporal tears and assessment of urethral involvement.[10] Some surgeons prefer to have imaging performed prior to surgery to assess the location of the injury. MRI can accurately depict the presence, location, and extent of tunical tear, which manifests as discontinuity of the tunica albuginea.[10] Moreover, because the tunica albuginea is well demonstrated as a low-signal-intensity structure on both T1- and T2-weighted images, MRI is optimal for the evaluation of the integrity of this anatomic structure. Not all cases of blunt penile trauma with bruising and swelling are associated with significant corpora injury requiring surgery, and the degree of swelling or bruising is in no way suggestive of the severity of the injury. MRI can demonstrate an intact tunica albuginea and the presence of intracavernosal or extratunical hematoma,[10] which does not require surgical management.\nThe time delay for obtaining MRI is not likely significant enough to affect the outcome of surgery. This patient made a full recovery with no complications.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 35-Year-Old Man Unable to Urinate and in Severe Pain"
},
{
"authors": "Rearabiloe Sebata, MB ChB; Naveed Afzal, MB BS; Ananda Chakrabarti, MB BS",
"content": [
"All 3 patients treated at this facility underwent emergency circumcision, penile exploration, and repair. All 3 operations were supervised by the same consultant urologic surgeon. In the case presented today, the patient had a preoperative suprapubic urinary catheter placed. This was immediately removed at the end of surgery. He left the operating theatre with a Foley urethral catheter. The catheter was only removed when he returned for follow-up 2 weeks later.",
"The other 2 patients had Foley urethral catheters that were removed after 48 hours and prior to discharge from hospital. They all reported painless nocturnal erections with no deformity at between 2 and 3 weeks postsurgery. They all resumed sexual activity at 6 weeks postsurgery. None of these patients experienced urine voiding difficulties. Follow-up urethrograms at 6 months revealed no evidence of urethral stricturing."
],
"date": "May 15, 2018",
"figures": [],
"markdown": "# A 35-Year-Old Man Unable to Urinate and in Severe Pain\n\n **Authors:** Rearabiloe Sebata, MB ChB; Naveed Afzal, MB BS; Ananda Chakrabarti, MB BS \n **Date:** May 15, 2018\n\n ## Content\n\n All 3 patients treated at this facility underwent emergency circumcision, penile exploration, and repair. All 3 operations were supervised by the same consultant urologic surgeon. In the case presented today, the patient had a preoperative suprapubic urinary catheter placed. This was immediately removed at the end of surgery. He left the operating theatre with a Foley urethral catheter. The catheter was only removed when he returned for follow-up 2 weeks later.\nThe other 2 patients had Foley urethral catheters that were removed after 48 hours and prior to discharge from hospital. They all reported painless nocturnal erections with no deformity at between 2 and 3 weeks postsurgery. They all resumed sexual activity at 6 weeks postsurgery. None of these patients experienced urine voiding difficulties. Follow-up urethrograms at 6 months revealed no evidence of urethral stricturing.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 446065,
"choiceText": "MRI",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 446067,
"choiceText": "CT scanning",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 446069,
"choiceText": "Plain radiographs",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 446071,
"choiceText": "Ultrasonography",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "MRI is an excellent tool in assessing the exact site and extent of injury in penile fractures and should be taken as the imaging modality of choice when available. MRI is noninvasive, nonradiating, and provides excellent images allowing for determination of the extent of corporal tears and assessment of urethral involvement. Some surgeons prefer to have imaging performed prior to surgery to assess the location of the injury. The limitations of the other imaging modalities make them less than ideal.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 132773,
"questionText": "You are examining a teenage patient who describes experiencing severe pain and swelling in his penis following a fall onto his erect penis from his bed in the middle of the night. Which of the following imaging modalities would best illustrate the location and type of injury experienced by this patient?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 446081,
"choiceText": "Conservative management",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 446085,
"choiceText": "Conservative management until swelling subsides followed by interval surgery",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 446087,
"choiceText": "Urgent surgical exploration",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 446089,
"choiceText": "Letting the injury heal without intervention",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Nonoperative therapy consists of cold compresses, penile splinting, and anti-inflammatory medications. The use of pressure dressings is limited by pain. In cases of suspected urethral injury, urinary diversion with a suprapubic catheter was once used with delayed repair of urethral injuries. This results in incredibly high rates of urethral stricturing. In cases of injuries in which MRI shows an intact anatomy with only intracorporeal hematoma, conservative management may still have a place; however, surgical exploration and repair is the treatment of choice. It results in better penile shape and function and a much speedier recovery.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 132777,
"questionText": "If the above-described patient were diagnosed with a penile fracture, what would be the recommended treatment of choice?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 35-Year-Old Man Unable to Urinate and in Severe Pain"
},
{
"authors": "Rearabiloe Sebata, MB ChB; Naveed Afzal, MB BS; Ananda Chakrabarti, MB BS",
"content": [],
"date": "May 15, 2018",
"figures": [],
"markdown": "# A 35-Year-Old Man Unable to Urinate and in Severe Pain\n\n **Authors:** Rearabiloe Sebata, MB ChB; Naveed Afzal, MB BS; Ananda Chakrabarti, MB BS \n **Date:** May 15, 2018\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 446065,
"choiceText": "MRI",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 446067,
"choiceText": "CT scanning",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 446069,
"choiceText": "Plain radiographs",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 446071,
"choiceText": "Ultrasonography",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "MRI is an excellent tool in assessing the exact site and extent of injury in penile fractures and should be taken as the imaging modality of choice when available. MRI is noninvasive, nonradiating, and provides excellent images allowing for determination of the extent of corporal tears and assessment of urethral involvement. Some surgeons prefer to have imaging performed prior to surgery to assess the location of the injury. The limitations of the other imaging modalities make them less than ideal.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 132773,
"questionText": "You are examining a teenage patient who describes experiencing severe pain and swelling in his penis following a fall onto his erect penis from his bed in the middle of the night. Which of the following imaging modalities would best illustrate the location and type of injury experienced by this patient?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 446081,
"choiceText": "Conservative management",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 446085,
"choiceText": "Conservative management until swelling subsides followed by interval surgery",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 446087,
"choiceText": "Urgent surgical exploration",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 446089,
"choiceText": "Letting the injury heal without intervention",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Nonoperative therapy consists of cold compresses, penile splinting, and anti-inflammatory medications. The use of pressure dressings is limited by pain. In cases of suspected urethral injury, urinary diversion with a suprapubic catheter was once used with delayed repair of urethral injuries. This results in incredibly high rates of urethral stricturing. In cases of injuries in which MRI shows an intact anatomy with only intracorporeal hematoma, conservative management may still have a place; however, surgical exploration and repair is the treatment of choice. It results in better penile shape and function and a much speedier recovery.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 132777,
"questionText": "If the above-described patient were diagnosed with a penile fracture, what would be the recommended treatment of choice?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 35-Year-Old Man Unable to Urinate and in Severe Pain"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 446029,
"choiceText": "Severe gonococcal urethritis and epididymitis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 446031,
"choiceText": "Penile fracture",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 446033,
"choiceText": "Prostatic hypertrophy",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 446035,
"choiceText": "Testicular torsion",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 132757,
"questionText": "What is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 446065,
"choiceText": "MRI",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 446067,
"choiceText": "CT scanning",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 446069,
"choiceText": "Plain radiographs",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 446071,
"choiceText": "Ultrasonography",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "MRI is an excellent tool in assessing the exact site and extent of injury in penile fractures and should be taken as the imaging modality of choice when available. MRI is noninvasive, nonradiating, and provides excellent images allowing for determination of the extent of corporal tears and assessment of urethral involvement. Some surgeons prefer to have imaging performed prior to surgery to assess the location of the injury. The limitations of the other imaging modalities make them less than ideal.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 132773,
"questionText": "You are examining a teenage patient who describes experiencing severe pain and swelling in his penis following a fall onto his erect penis from his bed in the middle of the night. Which of the following imaging modalities would best illustrate the location and type of injury experienced by this patient?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 446081,
"choiceText": "Conservative management",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 446085,
"choiceText": "Conservative management until swelling subsides followed by interval surgery",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 446087,
"choiceText": "Urgent surgical exploration",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 446089,
"choiceText": "Letting the injury heal without intervention",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Nonoperative therapy consists of cold compresses, penile splinting, and anti-inflammatory medications. The use of pressure dressings is limited by pain. In cases of suspected urethral injury, urinary diversion with a suprapubic catheter was once used with delayed repair of urethral injuries. This results in incredibly high rates of urethral stricturing. In cases of injuries in which MRI shows an intact anatomy with only intracorporeal hematoma, conservative management may still have a place; however, surgical exploration and repair is the treatment of choice. It results in better penile shape and function and a much speedier recovery.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 132777,
"questionText": "If the above-described patient were diagnosed with a penile fracture, what would be the recommended treatment of choice?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
752607 | /viewarticle/752607 | [
{
"authors": "Kaleem Ullah Toori, MBBS; Sumaira Nabi, MBBS",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"An 80-year-old Pakistani man presents to the local emergency department with a 5-day history of mild-to-moderate, nonradiating epigastric pain associated with nonprojectile, nonbilious vomiting. For the past day he has been drowsy and confused.",
"No history of hematemesis, constipation, fever, headache, seizure, or weakness of the limbs is noted. He has been taking nonsteroidal anti-inflammatory drugs (NSAIDS) for osteoarthritis of his knees for the last year. He is a nonsmoker with no history of illicit drug use."
],
"date": "April 12, 2017",
"figures": [],
"markdown": "# Slurred Speech and Dysphagia Leading to Spastic Quadriplegia Following Treatment\n\n **Authors:** Kaleem Ullah Toori, MBBS; Sumaira Nabi, MBBS \n **Date:** April 12, 2017\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nAn 80-year-old Pakistani man presents to the local emergency department with a 5-day history of mild-to-moderate, nonradiating epigastric pain associated with nonprojectile, nonbilious vomiting. For the past day he has been drowsy and confused.\nNo history of hematemesis, constipation, fever, headache, seizure, or weakness of the limbs is noted. He has been taking nonsteroidal anti-inflammatory drugs (NSAIDS) for osteoarthritis of his knees for the last year. He is a nonsmoker with no history of illicit drug use.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "Slurred Speech and Dysphagia Leading to Spastic Quadriplegia Following Treatment"
},
{
"authors": "Kaleem Ullah Toori, MBBS; Sumaira Nabi, MBBS",
"content": [
"Upon physical examination, he is a frail, elderly man. He is drowsy but arouses to verbal command. He is oriented to person but not to time or place. His pupils are regular and normally reactive to light. The patient's oral temperature is 98.6°F. He has a regular pulse of 88 beats/minute. His blood pressure is 100/70 mm Hg and he appears dehydrated, with dry mucus membranes and an absence of underarm sweat.",
"He is noted to have pallor but no clubbing, jaundice, or cyanosis. His abdomen is soft, with mild epigastric tenderness to deep palpation. No clinical evidence suggests organomegaly or ascites. His bowel sounds are audible. Digital rectal examination is unremarkable. The patient's precordial examination reveals normal heart sounds. His lungs are clear to auscultation, with a normal respiratory rate and effort. He is moving all extremities with bilateral flexor planter response. Signs of meningeal irritation are absent.",
"The laboratory analysis demonstrates a normal complete blood cell count, normal erythrocyte sedimentation rate, and normal serum amylase and lipase levels. His liver function tests, ECG, chest radiograph, and abdominal ultrasound are also unremarkable. His blood urea nitrogen level is normal, but his sodium level is 99 mEq/L (reference range, 135-150 mEq/L), his potassium level is 3 mEq/L (reference range, 3.5-5 mEq/L) and his chloride level is 62 mEq/L (reference range, 96-106 mEq/L).",
"He is diagnosed as having NSAID-induced acute gastritis and delirium caused by severe hyponatremia, which is attributed to persistent vomiting. He is treated with intravenous proton pump inhibitors, antiemetics, and intravenous fluids with potassium supplementation. The hyponatremia is corrected with 3% hypertonic saline. After 24 hours, his pain and vomiting improve and he shows remarkable general improvement. Repeated testing of serum electrolyte level reveals a sodium level of 129 mEq/L and a potassium level of 3.5 mEq/L.",
"Figure 1.",
"After 2 days, difficulty swallowing and slurred speech develop, followed by the development of spastic quadriplegia. An MRI of the brain is obtained (see Figure 1), which reveals several small, high signal-intensity areas in the central pons on T2/fluid attenuated inversion recovery (FLAIR) sequences."
],
"date": "April 12, 2017",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/752/607/752607-Thumb1a.jpg"
}
],
"markdown": "# Slurred Speech and Dysphagia Leading to Spastic Quadriplegia Following Treatment\n\n **Authors:** Kaleem Ullah Toori, MBBS; Sumaira Nabi, MBBS \n **Date:** April 12, 2017\n\n ## Content\n\n Upon physical examination, he is a frail, elderly man. He is drowsy but arouses to verbal command. He is oriented to person but not to time or place. His pupils are regular and normally reactive to light. The patient's oral temperature is 98.6°F. He has a regular pulse of 88 beats/minute. His blood pressure is 100/70 mm Hg and he appears dehydrated, with dry mucus membranes and an absence of underarm sweat.\nHe is noted to have pallor but no clubbing, jaundice, or cyanosis. His abdomen is soft, with mild epigastric tenderness to deep palpation. No clinical evidence suggests organomegaly or ascites. His bowel sounds are audible. Digital rectal examination is unremarkable. The patient's precordial examination reveals normal heart sounds. His lungs are clear to auscultation, with a normal respiratory rate and effort. He is moving all extremities with bilateral flexor planter response. Signs of meningeal irritation are absent.\nThe laboratory analysis demonstrates a normal complete blood cell count, normal erythrocyte sedimentation rate, and normal serum amylase and lipase levels. His liver function tests, ECG, chest radiograph, and abdominal ultrasound are also unremarkable. His blood urea nitrogen level is normal, but his sodium level is 99 mEq/L (reference range, 135-150 mEq/L), his potassium level is 3 mEq/L (reference range, 3.5-5 mEq/L) and his chloride level is 62 mEq/L (reference range, 96-106 mEq/L).\nHe is diagnosed as having NSAID-induced acute gastritis and delirium caused by severe hyponatremia, which is attributed to persistent vomiting. He is treated with intravenous proton pump inhibitors, antiemetics, and intravenous fluids with potassium supplementation. The hyponatremia is corrected with 3% hypertonic saline. After 24 hours, his pain and vomiting improve and he shows remarkable general improvement. Repeated testing of serum electrolyte level reveals a sodium level of 129 mEq/L and a potassium level of 3.5 mEq/L.\nFigure 1.\nAfter 2 days, difficulty swallowing and slurred speech develop, followed by the development of spastic quadriplegia. An MRI of the brain is obtained (see Figure 1), which reveals several small, high signal-intensity areas in the central pons on T2/fluid attenuated inversion recovery (FLAIR) sequences.\n\n ## Figures\n\n **Figure 1.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 440404,
"choiceText": "Pseudobulbar palsy",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 440405,
"choiceText": "Multiple sclerosis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 440406,
"choiceText": "Central pontine myelinolysis",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 440407,
"choiceText": "Space-occupying lesion of the brain",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 130711,
"questionText": "What is the diagnosis?<br />\r\n<br />\r\n<i>Hint: Note the history of hyponatremia.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Slurred Speech and Dysphagia Leading to Spastic Quadriplegia Following Treatment"
},
{
"authors": "Kaleem Ullah Toori, MBBS; Sumaira Nabi, MBBS",
"content": [
"This patient had rapid correction of his hyponatremia followed by the development of neurologic symptoms, including dysphagia and dysarthria. This was followed by spastic quadriplegia, which evolved over 2 days. The initial impression was that of a probable brainstem stroke; central pontine myelinolysis (CPM) was also considered in the differential diagnosis. An MRI of the brain with contrast revealed findings consistent with CPM.",
"CPM is a neurologic disorder caused by sudden, severe, noninflammatory demyelination of the brainstem, more precisely in the area of the pons. It is now typically recognized as a complication of rapid correction of hyponatremia.[1] It was first described by Adams, Victor, and Mancall[2] in 1959 when they observed a fulminant central nervous system disorder in alcoholic and malnourished patients in the form of spastic quadriplegia, pseudobulbar palsy, and varying grades of altered mentation. The association with extrapontine myelinolysis was made in 1962, and the link with rapid correction of hyponatremia was made in 1982.[3]",
"The exact mechanism of CPM remains elusive, but it is thought that the rapid correction of hyponatremia causes an osmotic shift of water out of the neurons, leading to shrinkage of their myelin sheaths. This, in turn, leads to compression of the tract fibers and demyelination.[4,5,6,7] The area of involvement is commonly the basis pontis, but extrapontine sites, including the midbrain, basal nuclei, thalamus, and cerebellum, may also be affected.[2,3,8] These areas are thought to be susceptible because of the close proximity of neurons, glial cells, and myelin sheaths.[9] The condition is thought to be more frequently seen in females than in males, but there is no known racial or genetic susceptibility.",
"Other risk factors for CPM include alcoholism, hypokalemia, malnutrition, Wilson disease, and liver transplant surgery or hematopoietic stem cell transplantation.[10,11,12] CPM may also be seen in severely burned patients.[13]",
"The risk for CPM by rapid correction of hyponatremia increases if the patient has a serum sodium level < 120 mEq/L for more than 48 hours, which is then briskly corrected by more than 12 mEq/L in the next 24 hours. The absolute magnitude of the change in serum sodium concentration is more important than the rate of correction. CPM is not seen in every patient who undergoes rapid sodium correction[14]; other subtle risk factors may contribute, including other electrolyte abnormalities such as hypokalemia (as was noted in this patient)."
],
"date": "April 12, 2017",
"figures": [],
"markdown": "# Slurred Speech and Dysphagia Leading to Spastic Quadriplegia Following Treatment\n\n **Authors:** Kaleem Ullah Toori, MBBS; Sumaira Nabi, MBBS \n **Date:** April 12, 2017\n\n ## Content\n\n This patient had rapid correction of his hyponatremia followed by the development of neurologic symptoms, including dysphagia and dysarthria. This was followed by spastic quadriplegia, which evolved over 2 days. The initial impression was that of a probable brainstem stroke; central pontine myelinolysis (CPM) was also considered in the differential diagnosis. An MRI of the brain with contrast revealed findings consistent with CPM.\nCPM is a neurologic disorder caused by sudden, severe, noninflammatory demyelination of the brainstem, more precisely in the area of the pons. It is now typically recognized as a complication of rapid correction of hyponatremia.[1] It was first described by Adams, Victor, and Mancall[2] in 1959 when they observed a fulminant central nervous system disorder in alcoholic and malnourished patients in the form of spastic quadriplegia, pseudobulbar palsy, and varying grades of altered mentation. The association with extrapontine myelinolysis was made in 1962, and the link with rapid correction of hyponatremia was made in 1982.[3]\nThe exact mechanism of CPM remains elusive, but it is thought that the rapid correction of hyponatremia causes an osmotic shift of water out of the neurons, leading to shrinkage of their myelin sheaths. This, in turn, leads to compression of the tract fibers and demyelination.[4,5,6,7] The area of involvement is commonly the basis pontis, but extrapontine sites, including the midbrain, basal nuclei, thalamus, and cerebellum, may also be affected.[2,3,8] These areas are thought to be susceptible because of the close proximity of neurons, glial cells, and myelin sheaths.[9] The condition is thought to be more frequently seen in females than in males, but there is no known racial or genetic susceptibility.\nOther risk factors for CPM include alcoholism, hypokalemia, malnutrition, Wilson disease, and liver transplant surgery or hematopoietic stem cell transplantation.[10,11,12] CPM may also be seen in severely burned patients.[13]\nThe risk for CPM by rapid correction of hyponatremia increases if the patient has a serum sodium level < 120 mEq/L for more than 48 hours, which is then briskly corrected by more than 12 mEq/L in the next 24 hours. The absolute magnitude of the change in serum sodium concentration is more important than the rate of correction. CPM is not seen in every patient who undergoes rapid sodium correction[14]; other subtle risk factors may contribute, including other electrolyte abnormalities such as hypokalemia (as was noted in this patient).\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 440404,
"choiceText": "Pseudobulbar palsy",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 440405,
"choiceText": "Multiple sclerosis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 440406,
"choiceText": "Central pontine myelinolysis",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 440407,
"choiceText": "Space-occupying lesion of the brain",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 130711,
"questionText": "What is the diagnosis?<br />\r\n<br />\r\n<i>Hint: Note the history of hyponatremia.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Slurred Speech and Dysphagia Leading to Spastic Quadriplegia Following Treatment"
},
{
"authors": "Kaleem Ullah Toori, MBBS; Sumaira Nabi, MBBS",
"content": [
"After correction of hyponatremia, a brief period of improvement in the associated initial confusion and obtundation occurs, followed by a gradual deterioration in the patient's general condition. A wide spectrum of symptoms and signs, ranging from simple confusion to quadriplegia, pseudobulbar palsy, gaze palsies, and coma, may be noted.[1,2,3] Upper motor neuron signs consistent with the involvement of corticospinal fibers may be seen in the form of limb weakness, spasticity, or focal neurologic signs. Patients may also have a weak or absent gag reflex, dysarthria, and horizontal and vertical gaze palsy depending on the involvement of the pons or midbrain. Sensory involvement is rare.",
"The patient in this case had quadriplegia, dysphagia, and dysarthria, which were suggestive of involvement of both corticospinal and corticobulbar fibers. Some patients may experience a \"locked-in-syndrome,\" in which quadriplegia occurs with loss of the lower cranial nerves. The condition leads to a wide spectrum of long-term disability and eventual death in many patients.",
"The diagnosis of CPM typically requires MRI because other diagnostic modalities are not as sensitive. Cerebrospinal fluid analysis may be normal or may demonstrate a high opening pressure and high protein content. CT scanning of the brain may also be normal. Electroencephalogram and evoked potentials may be abnormal as well but are insensitive when compared with MRI. An MRI of the brain with contrast is the imaging modality of choice. A normal MRI, however, should not preclude the diagnosis of CPM. Typically, T1-weighted images show hypointense areas, which then appear hyperintense on T2-weighted and FLAIR images, typically in the basis pontis, as a result of increased water content secondary to osmotic shift.[15,16,17] The lesions do not enhance with contrast. Demyelination is symmetric. MRI changes may be delayed, and repeat imaging may be indicated. Histopathology of autopsy specimens has shown myelin degeneration in the area of the base of pons.",
"Treatment for CPM is supportive and involves prolonged rehabilitation, including physiotherapy, speech and swallow therapy, and prevention of complications.[1,2,3] In a case report of 2 patients, Ludwig et al suggested that early recognition of CPM following liver transplantation in conjunction with administration of plasmapheresis and intravenous immune globulin (IVIG) may help to control progression of this condition and improve long-term neurologic outcome.[18] The underlying cause of the electrolyte disturbance should be identified and addressed accordingly.[19] The nutritional status of alcoholic patients should be assessed and vitamin supplementation should be considered.",
"Complete recovery is seldom achieved. Many patients, depending on the extent of neurologic injury, succumb to complications associated with chronic neurologic disability, including aspiration pneumonia and urosepsis. Other complications include stress ulcers, pressure sores, deep venous thrombosis, and pulmonary embolism. In this case, the patient had significant disability and was completely dependent on his attendants for nursing care.",
"Kallakatta et al described possible predictive prognostic factors for patients with osmotic demyelination syndrome (5 patients with CPM, 7 patients with extrapontine myelinolysis, and 13 with both).[20] The investigators determined that poor outcome could be predicted by hyponatremia of 115 mEq or less, the presence of superadded hypokalemia, and low Glasgow Coma Scale score at presentation, as well as poorer inpatient scores on the Functional Independent Measure (FIM) and Disability Rating Scale (DRS).[20]"
],
"date": "April 12, 2017",
"figures": [],
"markdown": "# Slurred Speech and Dysphagia Leading to Spastic Quadriplegia Following Treatment\n\n **Authors:** Kaleem Ullah Toori, MBBS; Sumaira Nabi, MBBS \n **Date:** April 12, 2017\n\n ## Content\n\n After correction of hyponatremia, a brief period of improvement in the associated initial confusion and obtundation occurs, followed by a gradual deterioration in the patient's general condition. A wide spectrum of symptoms and signs, ranging from simple confusion to quadriplegia, pseudobulbar palsy, gaze palsies, and coma, may be noted.[1,2,3] Upper motor neuron signs consistent with the involvement of corticospinal fibers may be seen in the form of limb weakness, spasticity, or focal neurologic signs. Patients may also have a weak or absent gag reflex, dysarthria, and horizontal and vertical gaze palsy depending on the involvement of the pons or midbrain. Sensory involvement is rare.\nThe patient in this case had quadriplegia, dysphagia, and dysarthria, which were suggestive of involvement of both corticospinal and corticobulbar fibers. Some patients may experience a \"locked-in-syndrome,\" in which quadriplegia occurs with loss of the lower cranial nerves. The condition leads to a wide spectrum of long-term disability and eventual death in many patients.\nThe diagnosis of CPM typically requires MRI because other diagnostic modalities are not as sensitive. Cerebrospinal fluid analysis may be normal or may demonstrate a high opening pressure and high protein content. CT scanning of the brain may also be normal. Electroencephalogram and evoked potentials may be abnormal as well but are insensitive when compared with MRI. An MRI of the brain with contrast is the imaging modality of choice. A normal MRI, however, should not preclude the diagnosis of CPM. Typically, T1-weighted images show hypointense areas, which then appear hyperintense on T2-weighted and FLAIR images, typically in the basis pontis, as a result of increased water content secondary to osmotic shift.[15,16,17] The lesions do not enhance with contrast. Demyelination is symmetric. MRI changes may be delayed, and repeat imaging may be indicated. Histopathology of autopsy specimens has shown myelin degeneration in the area of the base of pons.\nTreatment for CPM is supportive and involves prolonged rehabilitation, including physiotherapy, speech and swallow therapy, and prevention of complications.[1,2,3] In a case report of 2 patients, Ludwig et al suggested that early recognition of CPM following liver transplantation in conjunction with administration of plasmapheresis and intravenous immune globulin (IVIG) may help to control progression of this condition and improve long-term neurologic outcome.[18] The underlying cause of the electrolyte disturbance should be identified and addressed accordingly.[19] The nutritional status of alcoholic patients should be assessed and vitamin supplementation should be considered.\nComplete recovery is seldom achieved. Many patients, depending on the extent of neurologic injury, succumb to complications associated with chronic neurologic disability, including aspiration pneumonia and urosepsis. Other complications include stress ulcers, pressure sores, deep venous thrombosis, and pulmonary embolism. In this case, the patient had significant disability and was completely dependent on his attendants for nursing care.\nKallakatta et al described possible predictive prognostic factors for patients with osmotic demyelination syndrome (5 patients with CPM, 7 patients with extrapontine myelinolysis, and 13 with both).[20] The investigators determined that poor outcome could be predicted by hyponatremia of 115 mEq or less, the presence of superadded hypokalemia, and low Glasgow Coma Scale score at presentation, as well as poorer inpatient scores on the Functional Independent Measure (FIM) and Disability Rating Scale (DRS).[20]\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "Slurred Speech and Dysphagia Leading to Spastic Quadriplegia Following Treatment"
},
{
"authors": "Kaleem Ullah Toori, MBBS; Sumaira Nabi, MBBS",
"content": [
"Prevention of CPM is by judicious correction of hyponatremia. It is recommended that before administering any fluid, an assessment of the amplitude and rate of correction should be performed.[19,21,22] A useful formula in this regard, which roughly guides sodium replacement, is the Adrogue-Madias formula: Change in Na (mEq/L ) = (Na in infusate - serum Na)/(total body water + 1).",
"Total body water is estimated from body weight and is expressed in liters; it is roughly 0.5 × female weight in kilograms and 0.6 × male weight in kilograms. The change in serum sodium level should not exceed 10 mEq/L in the first 24 hours, and for 48 hours replacement the change should not exceed 18 mEq/L. In cases of suspected chronic hyponatremia, the rate of correction should not exceed 0.5-1 mEq/L/hour. The initial goal of care in patients with severe hyponatremia should be an increase to no greater than 120 mEq/L rather than immediately attempting to correct to normal. An increase of this magnitude should be adequate to alleviate any severe symptoms while avoiding the development of CPM.",
"The above formula provides a rough replacement plan; however, it does not take into account the ongoing loss of fluid and electrolytes, and some variability in the rate of correction should be expected. Some investigators state that aggressive correction should be avoided in many patients and that water restriction and cessation of diuretics is enough to correct symptomatic hyponatremia and avoid CPM.[23,24]",
"In this case, the patient had rapid and aggressive correction with hypertonic saline. Although this may have been justified, a controlled and limited increase in serum sodium with frequent monitoring of serum sodium level (every 2 h) would have likely prevented the development of CPM.",
"In conclusion, CPM (or osmotic demyelination syndrome) is a preventable complication resulting from rapid correction of profound hyponatremia and occurs as a consequence of a rapid change in serum osmolality."
],
"date": "April 12, 2017",
"figures": [],
"markdown": "# Slurred Speech and Dysphagia Leading to Spastic Quadriplegia Following Treatment\n\n **Authors:** Kaleem Ullah Toori, MBBS; Sumaira Nabi, MBBS \n **Date:** April 12, 2017\n\n ## Content\n\n Prevention of CPM is by judicious correction of hyponatremia. It is recommended that before administering any fluid, an assessment of the amplitude and rate of correction should be performed.[19,21,22] A useful formula in this regard, which roughly guides sodium replacement, is the Adrogue-Madias formula: Change in Na (mEq/L ) = (Na in infusate - serum Na)/(total body water + 1).\nTotal body water is estimated from body weight and is expressed in liters; it is roughly 0.5 × female weight in kilograms and 0.6 × male weight in kilograms. The change in serum sodium level should not exceed 10 mEq/L in the first 24 hours, and for 48 hours replacement the change should not exceed 18 mEq/L. In cases of suspected chronic hyponatremia, the rate of correction should not exceed 0.5-1 mEq/L/hour. The initial goal of care in patients with severe hyponatremia should be an increase to no greater than 120 mEq/L rather than immediately attempting to correct to normal. An increase of this magnitude should be adequate to alleviate any severe symptoms while avoiding the development of CPM.\nThe above formula provides a rough replacement plan; however, it does not take into account the ongoing loss of fluid and electrolytes, and some variability in the rate of correction should be expected. Some investigators state that aggressive correction should be avoided in many patients and that water restriction and cessation of diuretics is enough to correct symptomatic hyponatremia and avoid CPM.[23,24]\nIn this case, the patient had rapid and aggressive correction with hypertonic saline. Although this may have been justified, a controlled and limited increase in serum sodium with frequent monitoring of serum sodium level (every 2 h) would have likely prevented the development of CPM.\nIn conclusion, CPM (or osmotic demyelination syndrome) is a preventable complication resulting from rapid correction of profound hyponatremia and occurs as a consequence of a rapid change in serum osmolality.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 440408,
"choiceText": "Critical illness neuromyopathy",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 440409,
"choiceText": "Liver disease",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 440410,
"choiceText": "Rapid correction of hyponatremia",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 440411,
"choiceText": "Hyperosmolality",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 440412,
"choiceText": "Hypokalemia",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "CPM after rapid correction of hyponatremia is thought to be a consequence of osmotic shift of water out of neurons, leading to them shrinking away from their myelin sheaths. This in turn leads to compression of axon fibers and demyelination. The area of involvement is commonly the basis pontis, but extrapontine sites may also be affected by demyelination. ",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 130712,
"questionText": "A patient is being treated for severe hyponatremia associated with the patient's alcoholism. The patient initially recovers quickly, but soon exhibits problems speaking and general confusion. Which of the following is the likely cause of CPM?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 440413,
"choiceText": "Noncontrast CT scan of the brain",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 440414,
"choiceText": "CT scan of the brain with contrast",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 440415,
"choiceText": "Cerebrospinal fluid studies",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 440416,
"choiceText": "MRI of the brain",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 440417,
"choiceText": "Electroencephalography",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "An MRI of the brain with contrast is the imaging modality of choice. Most cases of CPM demonstrate T1-weighted images with hypointense areas which then appear hyperintense on T2-weighted and FLAIR images, typically in the basis pontis. These findings are thought to be the result of increased water content of these areas secondary to osmotic shift. MRI may be normal in some patients, and findings may be delayed, and repeat testing may be indicated.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 130713,
"questionText": "In the above described case, which investigation modality should be used to definitively establish a diagnosis of CPM?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Slurred Speech and Dysphagia Leading to Spastic Quadriplegia Following Treatment"
},
{
"authors": "Kaleem Ullah Toori, MBBS; Sumaira Nabi, MBBS",
"content": [],
"date": "April 12, 2017",
"figures": [],
"markdown": "# Slurred Speech and Dysphagia Leading to Spastic Quadriplegia Following Treatment\n\n **Authors:** Kaleem Ullah Toori, MBBS; Sumaira Nabi, MBBS \n **Date:** April 12, 2017\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 440408,
"choiceText": "Critical illness neuromyopathy",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 440409,
"choiceText": "Liver disease",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 440410,
"choiceText": "Rapid correction of hyponatremia",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 440411,
"choiceText": "Hyperosmolality",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 440412,
"choiceText": "Hypokalemia",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "CPM after rapid correction of hyponatremia is thought to be a consequence of osmotic shift of water out of neurons, leading to them shrinking away from their myelin sheaths. This in turn leads to compression of axon fibers and demyelination. The area of involvement is commonly the basis pontis, but extrapontine sites may also be affected by demyelination. ",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 130712,
"questionText": "A patient is being treated for severe hyponatremia associated with the patient's alcoholism. The patient initially recovers quickly, but soon exhibits problems speaking and general confusion. Which of the following is the likely cause of CPM?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 440413,
"choiceText": "Noncontrast CT scan of the brain",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 440414,
"choiceText": "CT scan of the brain with contrast",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 440415,
"choiceText": "Cerebrospinal fluid studies",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 440416,
"choiceText": "MRI of the brain",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 440417,
"choiceText": "Electroencephalography",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "An MRI of the brain with contrast is the imaging modality of choice. Most cases of CPM demonstrate T1-weighted images with hypointense areas which then appear hyperintense on T2-weighted and FLAIR images, typically in the basis pontis. These findings are thought to be the result of increased water content of these areas secondary to osmotic shift. MRI may be normal in some patients, and findings may be delayed, and repeat testing may be indicated.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 130713,
"questionText": "In the above described case, which investigation modality should be used to definitively establish a diagnosis of CPM?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Slurred Speech and Dysphagia Leading to Spastic Quadriplegia Following Treatment"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 440404,
"choiceText": "Pseudobulbar palsy",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 440405,
"choiceText": "Multiple sclerosis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 440406,
"choiceText": "Central pontine myelinolysis",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 440407,
"choiceText": "Space-occupying lesion of the brain",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 130711,
"questionText": "What is the diagnosis?<br />\r\n<br />\r\n<i>Hint: Note the history of hyponatremia.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 440408,
"choiceText": "Critical illness neuromyopathy",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 440409,
"choiceText": "Liver disease",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 440410,
"choiceText": "Rapid correction of hyponatremia",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 440411,
"choiceText": "Hyperosmolality",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 440412,
"choiceText": "Hypokalemia",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "CPM after rapid correction of hyponatremia is thought to be a consequence of osmotic shift of water out of neurons, leading to them shrinking away from their myelin sheaths. This in turn leads to compression of axon fibers and demyelination. The area of involvement is commonly the basis pontis, but extrapontine sites may also be affected by demyelination. ",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 130712,
"questionText": "A patient is being treated for severe hyponatremia associated with the patient's alcoholism. The patient initially recovers quickly, but soon exhibits problems speaking and general confusion. Which of the following is the likely cause of CPM?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 440413,
"choiceText": "Noncontrast CT scan of the brain",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 440414,
"choiceText": "CT scan of the brain with contrast",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 440415,
"choiceText": "Cerebrospinal fluid studies",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 440416,
"choiceText": "MRI of the brain",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 440417,
"choiceText": "Electroencephalography",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "An MRI of the brain with contrast is the imaging modality of choice. Most cases of CPM demonstrate T1-weighted images with hypointense areas which then appear hyperintense on T2-weighted and FLAIR images, typically in the basis pontis. These findings are thought to be the result of increased water content of these areas secondary to osmotic shift. MRI may be normal in some patients, and findings may be delayed, and repeat testing may be indicated.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 130713,
"questionText": "In the above described case, which investigation modality should be used to definitively establish a diagnosis of CPM?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
751176 | /viewarticle/751176 | [
{
"authors": "Manish Chadha, MS (Ortho); Vivek Kochar, MBBS",
"content": [
"Editor's Note:\n\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.",
"Figure 1.",
"A 10-year-old boy is presented to the outpatient clinic with a 1-month history of painful and progressively enlarging swelling over the upper back and the left side of the chest associated with restriction of movement of the left shoulder. He has no history of fever or trauma.",
"The patient has a history of similar episodes of painful swellings in the back, neck, and right thigh. These episodes resolved in a few months without any treatment; however, residual painless swellings persisted (Figure 1). He has no history of any spontaneous bleeding from the gums or of any upper or lower gastrointestinal bleeding. He denies experiencing any prolonged cough or expectoration. He does not take regular medications. His appetite and sleeping patterns have remained normal. He is an only child."
],
"date": "September 28, 2016",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/751/176/751176-thumb1.png"
}
],
"markdown": "# Painful, Persistent Swelling Restricting Range of Motion in a Child\n\n **Authors:** Manish Chadha, MS (Ortho); Vivek Kochar, MBBS \n **Date:** September 28, 2016\n\n ## Content\n\n Editor's Note:\n\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.\nFigure 1.\nA 10-year-old boy is presented to the outpatient clinic with a 1-month history of painful and progressively enlarging swelling over the upper back and the left side of the chest associated with restriction of movement of the left shoulder. He has no history of fever or trauma.\nThe patient has a history of similar episodes of painful swellings in the back, neck, and right thigh. These episodes resolved in a few months without any treatment; however, residual painless swellings persisted (Figure 1). He has no history of any spontaneous bleeding from the gums or of any upper or lower gastrointestinal bleeding. He denies experiencing any prolonged cough or expectoration. He does not take regular medications. His appetite and sleeping patterns have remained normal. He is an only child.\n\n ## Figures\n\n **Figure 1.** \n \n\n\n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "Painful, Persistent Swelling Restricting Range of Motion in a Child"
},
{
"authors": "Manish Chadha, MS (Ortho); Vivek Kochar, MBBS",
"content": [
"Figure 2.",
"Figure 3.",
"Figure 4.",
"Figure 5.",
"Figure 6.",
"Upon physical examination, the patient appears to be his stated age and he seems slightly anxious about his condition. His vitals at the time of presentation are an oral temperature of 98.6°F (37.0°C), regular pulse of 78 beats/min, and a blood pressure of 110/72 mm Hg. He is in moderate distress secondary to his painful swelling.",
"On further examination, mild pallor is noted but no jaundice, significant lymphadenopathy, or increased jugular venous pressure is found. His thyroid is not palpable, the neck is supple and soft, and the trachea is midline with no shift.",
"His bilateral great toes and thumbs are shorter than the rest of the digits (Figure 2). The cardiovascular and chest examinations reveal no significant findings. Gastrointestinal examination reveals a scaphoid abdomen that is soft, nontender, and without dullness to percussion. Positive bowel sounds are heard. The external genitalia are normal. Peripheral pulses are palpable and no pedal edema is observed.",
"His central nervous system examination reveals intact mental status with a Glasgow Coma Score of 15/15; additionally, normal motor power, tone, reflexes, and intact sensation are noted. The cranial nerves are intact. Local examination reveals a diffuse swelling in the left scapular region measuring 10 × 12 cm that is firm (hard in some areas) and tender. The local temperature over the swelling is raised but no dilated overlying veins are noted. The overlying skin is not fixed to the swelling but the swelling is immobile over the underlying structures. No discoloration or discharge from the swelling, no pulsations, and no fluctuance is noted. Multiple other nontender, hard bony swellings are present over the neck in the midline and upper back. Movement of the neck and left shoulder are significantly restricted.",
"The routine laboratory analysis is normal, with a total leukocyte count of 8.5 × 103/mm3 and a differential count of polymorphonuclear cells of 56% and lymphocytes of 44%. His erythrocyte sedimentation rate is 30 mm in the first hour. Radiographs of the cervical spine (Figure 3), hand (Figure 4), and pelvis (Figure 5) taken along with a CT scan for the swelling are shown. Radiographs of the thigh and knee are also obtained, where bony hard swelling is palpable (Figure 6)."
],
"date": "September 28, 2016",
"figures": [
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/751/176/751176-thumb2.png"
},
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/751/176/751176-thumb3.png"
},
{
"caption": "Figure 4.",
"image_url": "https://img.medscapestatic.com/article/751/176/751176-thumb4.png"
},
{
"caption": "Figure 5.",
"image_url": "https://img.medscapestatic.com/article/751/176/751176-thumb5.png"
},
{
"caption": "Figure 6.",
"image_url": "https://img.medscapestatic.com/article/751/176/751176-thumb6.png"
}
],
"markdown": "# Painful, Persistent Swelling Restricting Range of Motion in a Child\n\n **Authors:** Manish Chadha, MS (Ortho); Vivek Kochar, MBBS \n **Date:** September 28, 2016\n\n ## Content\n\n Figure 2.\nFigure 3.\nFigure 4.\nFigure 5.\nFigure 6.\nUpon physical examination, the patient appears to be his stated age and he seems slightly anxious about his condition. His vitals at the time of presentation are an oral temperature of 98.6°F (37.0°C), regular pulse of 78 beats/min, and a blood pressure of 110/72 mm Hg. He is in moderate distress secondary to his painful swelling.\nOn further examination, mild pallor is noted but no jaundice, significant lymphadenopathy, or increased jugular venous pressure is found. His thyroid is not palpable, the neck is supple and soft, and the trachea is midline with no shift.\nHis bilateral great toes and thumbs are shorter than the rest of the digits (Figure 2). The cardiovascular and chest examinations reveal no significant findings. Gastrointestinal examination reveals a scaphoid abdomen that is soft, nontender, and without dullness to percussion. Positive bowel sounds are heard. The external genitalia are normal. Peripheral pulses are palpable and no pedal edema is observed.\nHis central nervous system examination reveals intact mental status with a Glasgow Coma Score of 15/15; additionally, normal motor power, tone, reflexes, and intact sensation are noted. The cranial nerves are intact. Local examination reveals a diffuse swelling in the left scapular region measuring 10 × 12 cm that is firm (hard in some areas) and tender. The local temperature over the swelling is raised but no dilated overlying veins are noted. The overlying skin is not fixed to the swelling but the swelling is immobile over the underlying structures. No discoloration or discharge from the swelling, no pulsations, and no fluctuance is noted. Multiple other nontender, hard bony swellings are present over the neck in the midline and upper back. Movement of the neck and left shoulder are significantly restricted.\nThe routine laboratory analysis is normal, with a total leukocyte count of 8.5 × 103/mm3 and a differential count of polymorphonuclear cells of 56% and lymphocytes of 44%. His erythrocyte sedimentation rate is 30 mm in the first hour. Radiographs of the cervical spine (Figure 3), hand (Figure 4), and pelvis (Figure 5) taken along with a CT scan for the swelling are shown. Radiographs of the thigh and knee are also obtained, where bony hard swelling is palpable (Figure 6).\n\n ## Figures\n\n **Figure 2.** \n \n\n**Figure 3.** \n \n\n**Figure 4.** \n \n\n**Figure 5.** \n \n\n**Figure 6.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 435979,
"choiceText": "Chondrosarcoma",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 435980,
"choiceText": "Fibrodysplasia ossificans progressiva",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 435981,
"choiceText": "Leukemia",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 435982,
"choiceText": "Soft tissue sarcoma",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 129160,
"questionText": "What is the likely diagnosis?<br />\r\n<br />\r\n<i>Hint: Note the bilateral short great toes.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Painful, Persistent Swelling Restricting Range of Motion in a Child"
},
{
"authors": "Manish Chadha, MS (Ortho); Vivek Kochar, MBBS",
"content": [
"Figure 3.",
"Figure 5.",
"Fibrodysplasia (myositis) ossificans progressiva (FOP) is a rare disorder characterized by symmetrical congenital malformation of the first digit of the hands and feet and by progressive heterotopic ossification.[1] The radiologic investigations revealed typical findings of heterotopic ossification in the ligamentum nuchae, paraspinal muscles, and right thigh (Figure 3 and Figure 5). The radiographs of the pelvis showed bilateral femurs with short and broad necks (Figure 5), which has been reported to be almost diagnostic of this condition.[1] The child was diagnosed as having FOP based on the clinical and radiologic picture. No invasive investigations were performed to confirm the diagnosis because even an intramuscular injection has been shown to precipitate an acute flare up of the lesions, with resultant morbidity.[2]",
"FOP is a rare autosomal dominant genetic condition with a worldwide prevalence of approximately 1 case per 2 million individuals.[1] No predilection for either sex is known and it occurs with equal prevalence in all races. The average age of onset is 5 years, with reported onset ranging from fetal life to 25 years. Most cases arise as a result of a spontaneous new mutation. The genetic cause of FOP has now been identified and has been mapped to chromosome 2q23-24. It has been identified as a recurrent missense mutation in the GS activation domain of activin receptor Ia/activin-like kinase 2 (ACVR1/ALK2), a bone morphogenetic protein type I receptor.[3,4] Recently, additional mutations have been identified in the GS domain and kinase domain of ACVR1 in individuals with atypical forms of FOP.[4]",
"Immunologic causes have also been hypothesized for FOP. This is suggested by the presence of inflammatory cells in early lesions, macrophage- and lymphocyte-associated death of skeletal muscle, flare-ups following viral infections, the intermittent timing of flare-ups, and the positive response of early flare-ups to corticosteroids.",
"Most individuals with FOP are normal at birth except for characteristic malformations of the great toes, which is present in all classically affected individuals.[5] Episodic, painful soft-tissue swellings or exacerbations usually develop in the preteen years.[6] Flare-ups may develop spontaneously or as a result of minor trauma, such as intramuscular immunizations, mandibular blocks, muscle fatigue, falls, or viral illnesses; these flare-ups lead to progressive heterotopic ossification.[1] Although some exacerbations spontaneously regress, most transform soft connective tissues (including aponeurosis, fascia, ligaments, tendons, and skeletal muscles) into mature bone. Heterotopic ossification is typically seen first in the dorsal, axial, cranial, and proximal regions of the body and later in the ventral, appendicular, caudal, and distal regions. Cardiac muscle, smooth muscle, and skeletal muscle of the diaphragm, tongue, and extraocular muscles are not affected by heterotopic ossification."
],
"date": "September 28, 2016",
"figures": [
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/751/176/751176-thumb3.png"
},
{
"caption": "Figure 5.",
"image_url": "https://img.medscapestatic.com/article/751/176/751176-thumb5.png"
}
],
"markdown": "# Painful, Persistent Swelling Restricting Range of Motion in a Child\n\n **Authors:** Manish Chadha, MS (Ortho); Vivek Kochar, MBBS \n **Date:** September 28, 2016\n\n ## Content\n\n Figure 3.\nFigure 5.\nFibrodysplasia (myositis) ossificans progressiva (FOP) is a rare disorder characterized by symmetrical congenital malformation of the first digit of the hands and feet and by progressive heterotopic ossification.[1] The radiologic investigations revealed typical findings of heterotopic ossification in the ligamentum nuchae, paraspinal muscles, and right thigh (Figure 3 and Figure 5). The radiographs of the pelvis showed bilateral femurs with short and broad necks (Figure 5), which has been reported to be almost diagnostic of this condition.[1] The child was diagnosed as having FOP based on the clinical and radiologic picture. No invasive investigations were performed to confirm the diagnosis because even an intramuscular injection has been shown to precipitate an acute flare up of the lesions, with resultant morbidity.[2]\nFOP is a rare autosomal dominant genetic condition with a worldwide prevalence of approximately 1 case per 2 million individuals.[1] No predilection for either sex is known and it occurs with equal prevalence in all races. The average age of onset is 5 years, with reported onset ranging from fetal life to 25 years. Most cases arise as a result of a spontaneous new mutation. The genetic cause of FOP has now been identified and has been mapped to chromosome 2q23-24. It has been identified as a recurrent missense mutation in the GS activation domain of activin receptor Ia/activin-like kinase 2 (ACVR1/ALK2), a bone morphogenetic protein type I receptor.[3,4] Recently, additional mutations have been identified in the GS domain and kinase domain of ACVR1 in individuals with atypical forms of FOP.[4]\nImmunologic causes have also been hypothesized for FOP. This is suggested by the presence of inflammatory cells in early lesions, macrophage- and lymphocyte-associated death of skeletal muscle, flare-ups following viral infections, the intermittent timing of flare-ups, and the positive response of early flare-ups to corticosteroids.\nMost individuals with FOP are normal at birth except for characteristic malformations of the great toes, which is present in all classically affected individuals.[5] Episodic, painful soft-tissue swellings or exacerbations usually develop in the preteen years.[6] Flare-ups may develop spontaneously or as a result of minor trauma, such as intramuscular immunizations, mandibular blocks, muscle fatigue, falls, or viral illnesses; these flare-ups lead to progressive heterotopic ossification.[1] Although some exacerbations spontaneously regress, most transform soft connective tissues (including aponeurosis, fascia, ligaments, tendons, and skeletal muscles) into mature bone. Heterotopic ossification is typically seen first in the dorsal, axial, cranial, and proximal regions of the body and later in the ventral, appendicular, caudal, and distal regions. Cardiac muscle, smooth muscle, and skeletal muscle of the diaphragm, tongue, and extraocular muscles are not affected by heterotopic ossification.\n\n ## Figures\n\n **Figure 3.** \n \n\n**Figure 5.** \n \n\n\n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 435979,
"choiceText": "Chondrosarcoma",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 435980,
"choiceText": "Fibrodysplasia ossificans progressiva",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 435981,
"choiceText": "Leukemia",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 435982,
"choiceText": "Soft tissue sarcoma",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 129160,
"questionText": "What is the likely diagnosis?<br />\r\n<br />\r\n<i>Hint: Note the bilateral short great toes.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Painful, Persistent Swelling Restricting Range of Motion in a Child"
},
{
"authors": "Manish Chadha, MS (Ortho); Vivek Kochar, MBBS",
"content": [
"An early finding in patients with FOP is stiffness of the neck due to associated anomalies of the cervical spine. This can precede the appearance of heterotopic ossification at that site. Deformity of the great toes, such as shortened toes and hallux valgus, is characteristically present in all patients with FOP.[5] Other associated skeletal anomalies include short malformed thumbs, clinodactyly, short and broad femoral necks, and proximal medial tibial osteochondromas. Ankylosis of the jaw and rigid fixation of the chest wall can lead to serious complications such as severe weight loss and complications of rigid fixation of the chest wall. Most patients are confined to a wheelchair by the third decade of life and require lifelong assistance in performing activities of daily living.[7]",
"FOP must be distinguished from other genetic conditions of heterotopic ossification as well as from nonhereditary heterotopic ossification. Progressive osseous heteroplasia is a rare genetic condition of progressive ectopic ossification.[8] The presence of congenital malformation of the great toes, preosseous tumorlike inflammation or flare-ups, and the lack of cutaneous ossification differentiates FOP from progressive osseous heteroplasia.",
"Nonhereditary heterotopic ossification follows trauma or other injury in most cases. It can occur at any age but is rare in children younger than 10 years. Other differentials to be considered are aggressive juvenile fibromatosis, isolated congenital malformations, juvenile bunions, brachydactyly, lymphoma, desmoid tumor, and soft tissue sarcoma. About 60%-70% of individuals with FOP are misdiagnosed worldwide, leading to unnecessary and harmful diagnostic biopsies that exacerbate the progression of the condition (especially in the neck, back, or jaw, wherein asymmetric heterotopic ossification can lead to rapidly progressive spinal deformity, exacerbation of thoracic insufficiency syndrome, or rapid ankylosis of the temporomandibular joints).[7]",
"Routine biochemical evaluations of bone mineral metabolism are usually normal, although the serum alkaline phosphatase activity and the erythrocyte sedimentation rate may be increased (especially during disease flare-ups). Imaging modalities, such as radiography, bone scanning, CT scanning, and MRI, help in confirming the diagnosis; however, the definitive diagnosis of FOP can be made by simple clinical evaluation that associates rapidly appearing soft tissue lesions with malformations of the great toes. Definitive genetic testing for FOP is now available.[9] Clinical suspicion of FOP early in life on the basis of malformed great toes can lead to an early clinical diagnosis, confirmatory diagnostic genetic testing (if appropriate), and the avoidance of harmful diagnostic and treatment procedures."
],
"date": "September 28, 2016",
"figures": [],
"markdown": "# Painful, Persistent Swelling Restricting Range of Motion in a Child\n\n **Authors:** Manish Chadha, MS (Ortho); Vivek Kochar, MBBS \n **Date:** September 28, 2016\n\n ## Content\n\n An early finding in patients with FOP is stiffness of the neck due to associated anomalies of the cervical spine. This can precede the appearance of heterotopic ossification at that site. Deformity of the great toes, such as shortened toes and hallux valgus, is characteristically present in all patients with FOP.[5] Other associated skeletal anomalies include short malformed thumbs, clinodactyly, short and broad femoral necks, and proximal medial tibial osteochondromas. Ankylosis of the jaw and rigid fixation of the chest wall can lead to serious complications such as severe weight loss and complications of rigid fixation of the chest wall. Most patients are confined to a wheelchair by the third decade of life and require lifelong assistance in performing activities of daily living.[7]\nFOP must be distinguished from other genetic conditions of heterotopic ossification as well as from nonhereditary heterotopic ossification. Progressive osseous heteroplasia is a rare genetic condition of progressive ectopic ossification.[8] The presence of congenital malformation of the great toes, preosseous tumorlike inflammation or flare-ups, and the lack of cutaneous ossification differentiates FOP from progressive osseous heteroplasia.\nNonhereditary heterotopic ossification follows trauma or other injury in most cases. It can occur at any age but is rare in children younger than 10 years. Other differentials to be considered are aggressive juvenile fibromatosis, isolated congenital malformations, juvenile bunions, brachydactyly, lymphoma, desmoid tumor, and soft tissue sarcoma. About 60%-70% of individuals with FOP are misdiagnosed worldwide, leading to unnecessary and harmful diagnostic biopsies that exacerbate the progression of the condition (especially in the neck, back, or jaw, wherein asymmetric heterotopic ossification can lead to rapidly progressive spinal deformity, exacerbation of thoracic insufficiency syndrome, or rapid ankylosis of the temporomandibular joints).[7]\nRoutine biochemical evaluations of bone mineral metabolism are usually normal, although the serum alkaline phosphatase activity and the erythrocyte sedimentation rate may be increased (especially during disease flare-ups). Imaging modalities, such as radiography, bone scanning, CT scanning, and MRI, help in confirming the diagnosis; however, the definitive diagnosis of FOP can be made by simple clinical evaluation that associates rapidly appearing soft tissue lesions with malformations of the great toes. Definitive genetic testing for FOP is now available.[9] Clinical suspicion of FOP early in life on the basis of malformed great toes can lead to an early clinical diagnosis, confirmatory diagnostic genetic testing (if appropriate), and the avoidance of harmful diagnostic and treatment procedures.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "Painful, Persistent Swelling Restricting Range of Motion in a Child"
},
{
"authors": "Manish Chadha, MS (Ortho); Vivek Kochar, MBBS",
"content": [
"No effective treatment is available for FOP. Surgery is not an option for removing the excess bone because surgery often results in more bone formation. Although there is no definitive evidence for the use of radiation therapy in FOP, this treatment may interfere in the ossification process.[10] There is a case report of successful treatment of the condition with several courses of fractionated radiotherapy.[10]",
"Medical management in the form of steroids has a role in the management of flare-ups, but their use should be restricted to flare-ups that affect the major joints of the body or the jaw. With more information regarding the human genome and decoding of the gene responsible for FOP, as well as better understanding of pathophysiology of the disease, newer products are being synthesized with genetic engineering. Trials are underway but, as yet, few positive reports are available.[11]",
"The above patient was diagnosed clinically as having FOP and was admitted in the orthopedic ward. He was given oral prednisone (30 mg/day) for 1 week, which led to the swelling in the left scapular region subsiding and symptomatic relief. Biopsy and other procedures were avoided. The patient was discharged on oral nonsteroidal anti-inflammatory drugs (NSAIDs) and steroids for a period of 2 weeks. At last follow-up (6 months after the acute flare-up), the patient's symptoms had remarkably reduced. The swelling was greatly reduced and was replaced by a hard mass measuring 3 × 3 cm. Although there was still some restriction, his shoulder movements had improved."
],
"date": "September 28, 2016",
"figures": [],
"markdown": "# Painful, Persistent Swelling Restricting Range of Motion in a Child\n\n **Authors:** Manish Chadha, MS (Ortho); Vivek Kochar, MBBS \n **Date:** September 28, 2016\n\n ## Content\n\n No effective treatment is available for FOP. Surgery is not an option for removing the excess bone because surgery often results in more bone formation. Although there is no definitive evidence for the use of radiation therapy in FOP, this treatment may interfere in the ossification process.[10] There is a case report of successful treatment of the condition with several courses of fractionated radiotherapy.[10]\nMedical management in the form of steroids has a role in the management of flare-ups, but their use should be restricted to flare-ups that affect the major joints of the body or the jaw. With more information regarding the human genome and decoding of the gene responsible for FOP, as well as better understanding of pathophysiology of the disease, newer products are being synthesized with genetic engineering. Trials are underway but, as yet, few positive reports are available.[11]\nThe above patient was diagnosed clinically as having FOP and was admitted in the orthopedic ward. He was given oral prednisone (30 mg/day) for 1 week, which led to the swelling in the left scapular region subsiding and symptomatic relief. Biopsy and other procedures were avoided. The patient was discharged on oral nonsteroidal anti-inflammatory drugs (NSAIDs) and steroids for a period of 2 weeks. At last follow-up (6 months after the acute flare-up), the patient's symptoms had remarkably reduced. The swelling was greatly reduced and was replaced by a hard mass measuring 3 × 3 cm. Although there was still some restriction, his shoulder movements had improved.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 435983,
"choiceText": "Fibrodysplasia ossificans progressiva",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 435984,
"choiceText": "Myositis ossificans traumatica",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 435985,
"choiceText": "Chondrosarcoma",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 435986,
"choiceText": "Soft tissue malignant tumor",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The classical history in patients suffering from FOP is the spontaneous episodes of acute flare-ups typically involving the muscles of the back. The acute episodes subside on their own but a local ossified mass remains. Repeated episodes result in progressive restriction of movement. By the time patients are 30 years of age, they may suffer from significant restriction of chest expansion and repeated episodes of pneumonitis. Survival beyond age 30 years is a rarity.<br />\r\n<br />\r\nTraumatic myositis usually occurs in a localized region, and a history of significant trauma with swelling or a history of massage therapy is usually present. Malignant bone or soft tissue tumors are also considered to be a differential diagnosis but they are usually localized and will not show spontaneous regression of local symptoms.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 129161,
"questionText": "A child is presented to you with repeated episodes of painful swellings in the back that subside in a few weeks time on their own and are replaced with bony hard swellings. On general physical examination, he is noted to have bilateral short great toes with restricted cervical spine movements. What is the probable diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 436018,
"choiceText": "Fine needle aspiration cytology",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 436019,
"choiceText": "Open biopsy",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 436020,
"choiceText": "Bone marrow aspiration",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 436021,
"choiceText": "Skeletal survey (including radiographs of cervical spine, pelvis, limbs, and feet)",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Typically one should <i>not</i> order any invasive investigations because even minimal trauma that occurs with a fine needle may precipitate an acute flare-up and may worsen the symptoms. Noninvasive investigations in the form of radiographs and newer modalities like CT scanning and MRI are useful in establishing the diagnosis. Radiographs typically will show ossification of the ligamentum nuchae. The femoral neck is typically short and broad and the first metatarsals are short. Though the axial skeleton is involved early, evidence of myositis may be observed in radiographs of the limbs as well.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 129169,
"questionText": "Based on your clinical suspicion of the case described in the question above, which test or tests would you order?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Painful, Persistent Swelling Restricting Range of Motion in a Child"
},
{
"authors": "Manish Chadha, MS (Ortho); Vivek Kochar, MBBS",
"content": [],
"date": "September 28, 2016",
"figures": [],
"markdown": "# Painful, Persistent Swelling Restricting Range of Motion in a Child\n\n **Authors:** Manish Chadha, MS (Ortho); Vivek Kochar, MBBS \n **Date:** September 28, 2016\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 435983,
"choiceText": "Fibrodysplasia ossificans progressiva",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 435984,
"choiceText": "Myositis ossificans traumatica",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 435985,
"choiceText": "Chondrosarcoma",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 435986,
"choiceText": "Soft tissue malignant tumor",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The classical history in patients suffering from FOP is the spontaneous episodes of acute flare-ups typically involving the muscles of the back. The acute episodes subside on their own but a local ossified mass remains. Repeated episodes result in progressive restriction of movement. By the time patients are 30 years of age, they may suffer from significant restriction of chest expansion and repeated episodes of pneumonitis. Survival beyond age 30 years is a rarity.<br />\r\n<br />\r\nTraumatic myositis usually occurs in a localized region, and a history of significant trauma with swelling or a history of massage therapy is usually present. Malignant bone or soft tissue tumors are also considered to be a differential diagnosis but they are usually localized and will not show spontaneous regression of local symptoms.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 129161,
"questionText": "A child is presented to you with repeated episodes of painful swellings in the back that subside in a few weeks time on their own and are replaced with bony hard swellings. On general physical examination, he is noted to have bilateral short great toes with restricted cervical spine movements. What is the probable diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 436018,
"choiceText": "Fine needle aspiration cytology",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 436019,
"choiceText": "Open biopsy",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 436020,
"choiceText": "Bone marrow aspiration",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 436021,
"choiceText": "Skeletal survey (including radiographs of cervical spine, pelvis, limbs, and feet)",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Typically one should <i>not</i> order any invasive investigations because even minimal trauma that occurs with a fine needle may precipitate an acute flare-up and may worsen the symptoms. Noninvasive investigations in the form of radiographs and newer modalities like CT scanning and MRI are useful in establishing the diagnosis. Radiographs typically will show ossification of the ligamentum nuchae. The femoral neck is typically short and broad and the first metatarsals are short. Though the axial skeleton is involved early, evidence of myositis may be observed in radiographs of the limbs as well.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 129169,
"questionText": "Based on your clinical suspicion of the case described in the question above, which test or tests would you order?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Painful, Persistent Swelling Restricting Range of Motion in a Child"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 435979,
"choiceText": "Chondrosarcoma",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 435980,
"choiceText": "Fibrodysplasia ossificans progressiva",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 435981,
"choiceText": "Leukemia",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 435982,
"choiceText": "Soft tissue sarcoma",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 129160,
"questionText": "What is the likely diagnosis?<br />\r\n<br />\r\n<i>Hint: Note the bilateral short great toes.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 435983,
"choiceText": "Fibrodysplasia ossificans progressiva",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 435984,
"choiceText": "Myositis ossificans traumatica",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 435985,
"choiceText": "Chondrosarcoma",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 435986,
"choiceText": "Soft tissue malignant tumor",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The classical history in patients suffering from FOP is the spontaneous episodes of acute flare-ups typically involving the muscles of the back. The acute episodes subside on their own but a local ossified mass remains. Repeated episodes result in progressive restriction of movement. By the time patients are 30 years of age, they may suffer from significant restriction of chest expansion and repeated episodes of pneumonitis. Survival beyond age 30 years is a rarity.<br />\r\n<br />\r\nTraumatic myositis usually occurs in a localized region, and a history of significant trauma with swelling or a history of massage therapy is usually present. Malignant bone or soft tissue tumors are also considered to be a differential diagnosis but they are usually localized and will not show spontaneous regression of local symptoms.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 129161,
"questionText": "A child is presented to you with repeated episodes of painful swellings in the back that subside in a few weeks time on their own and are replaced with bony hard swellings. On general physical examination, he is noted to have bilateral short great toes with restricted cervical spine movements. What is the probable diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 436018,
"choiceText": "Fine needle aspiration cytology",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 436019,
"choiceText": "Open biopsy",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 436020,
"choiceText": "Bone marrow aspiration",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 436021,
"choiceText": "Skeletal survey (including radiographs of cervical spine, pelvis, limbs, and feet)",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Typically one should <i>not</i> order any invasive investigations because even minimal trauma that occurs with a fine needle may precipitate an acute flare-up and may worsen the symptoms. Noninvasive investigations in the form of radiographs and newer modalities like CT scanning and MRI are useful in establishing the diagnosis. Radiographs typically will show ossification of the ligamentum nuchae. The femoral neck is typically short and broad and the first metatarsals are short. Though the axial skeleton is involved early, evidence of myositis may be observed in radiographs of the limbs as well.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 129169,
"questionText": "Based on your clinical suspicion of the case described in the question above, which test or tests would you order?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
750480 | /viewarticle/750480 | [
{
"authors": "Zoran Rajković, MD; Dino Papeš, MD",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to recognize accurately. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"A 16-year-old boy is examined in the emergency department (ED) for acute-onset left knee pain and swelling. Two months ago, he suffered a patellar dislocation that was treated with rest, cryotherapy (ice), elevation, and immobilization. He recovered completely from this episode.",
"The rest of his medical and surgical history is unremarkable. The family history is negative for knee problems or dislocations. He takes no regular medications and has no allergies. He is a high school student and a semiprofessional soccer player.",
"This episode of knee pain started a week ago following a soccer match. The knee is moderately swollen and painful. At the onset of symptoms, the patient treated himself with cryotherapy, rest, and an elastic bandage, with mild improvement; however, the symptoms reappeared 3 days ago and have progressively worsened, prompting this visit to the ED."
],
"date": "February 05, 2019",
"figures": [],
"markdown": "# A 16-Year-Old Boy With Concerning, Recurrent Knee Problems\n\n **Authors:** Zoran Rajković, MD; Dino Papeš, MD \n **Date:** February 05, 2019\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to recognize accurately. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nA 16-year-old boy is examined in the emergency department (ED) for acute-onset left knee pain and swelling. Two months ago, he suffered a patellar dislocation that was treated with rest, cryotherapy (ice), elevation, and immobilization. He recovered completely from this episode.\nThe rest of his medical and surgical history is unremarkable. The family history is negative for knee problems or dislocations. He takes no regular medications and has no allergies. He is a high school student and a semiprofessional soccer player.\nThis episode of knee pain started a week ago following a soccer match. The knee is moderately swollen and painful. At the onset of symptoms, the patient treated himself with cryotherapy, rest, and an elastic bandage, with mild improvement; however, the symptoms reappeared 3 days ago and have progressively worsened, prompting this visit to the ED.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 16-Year-Old Boy With Concerning, Recurrent Knee Problems"
},
{
"authors": "Zoran Rajković, MD; Dino Papeš, MD",
"content": [
"Upon examination, the patient appears to be in good condition. He is awake, alert, and in no acute distress. His vital signs show him to be afebrile, with a blood pressure of 125/75 mm Hg, a pulse of 75 beats/min, a respiratory rate of 15 breaths/min, and an oxygen saturation of 98% on room air. His skin is slightly pale but with normal skin turgor. Examination of his oral cavity shows dry mucus membranes. He has normal heart sounds. Auscultation reveals clear lungs without wheezes, rhonchi, or rales. The abdomen is soft and without tenderness, distention, masses, or hernias.",
"Examination of the left hip is unremarkable, with normal range of motion noted. Examination of the left knee shows swelling with positive patellar ballottement indicating the presence of an effusion. The patient is holding his knee in mild flexion. On testing the range of motion, both terminal extension and flexion cannot be tested due to pain. Physical examination tests of the meniscus and ligaments are negative.",
"The patient's workup includes a complete blood count (CBC), which is normal. In addition, x-rays of the knee are obtained. An example similar to the findings in this patient are shown in Figure 1. Arthrocentesis is also performed, yielding 30 mL of old blood. This is sent for cell count and culture, but no evidence of infection is discovered.",
"Figure 1."
],
"date": "February 05, 2019",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/750/480/750480-thumb1a.png"
}
],
"markdown": "# A 16-Year-Old Boy With Concerning, Recurrent Knee Problems\n\n **Authors:** Zoran Rajković, MD; Dino Papeš, MD \n **Date:** February 05, 2019\n\n ## Content\n\n Upon examination, the patient appears to be in good condition. He is awake, alert, and in no acute distress. His vital signs show him to be afebrile, with a blood pressure of 125/75 mm Hg, a pulse of 75 beats/min, a respiratory rate of 15 breaths/min, and an oxygen saturation of 98% on room air. His skin is slightly pale but with normal skin turgor. Examination of his oral cavity shows dry mucus membranes. He has normal heart sounds. Auscultation reveals clear lungs without wheezes, rhonchi, or rales. The abdomen is soft and without tenderness, distention, masses, or hernias.\nExamination of the left hip is unremarkable, with normal range of motion noted. Examination of the left knee shows swelling with positive patellar ballottement indicating the presence of an effusion. The patient is holding his knee in mild flexion. On testing the range of motion, both terminal extension and flexion cannot be tested due to pain. Physical examination tests of the meniscus and ligaments are negative.\nThe patient's workup includes a complete blood count (CBC), which is normal. In addition, x-rays of the knee are obtained. An example similar to the findings in this patient are shown in Figure 1. Arthrocentesis is also performed, yielding 30 mL of old blood. This is sent for cell count and culture, but no evidence of infection is discovered.\nFigure 1.\n\n ## Figures\n\n **Figure 1.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 434394,
"choiceText": "Osteochondromatosis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 434395,
"choiceText": "Juvenile rheumatoid arthritis with loose osteophyte",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 434396,
"choiceText": "Osgood-Schlatter disease",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 434397,
"choiceText": "Osteochondral defect",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 128609,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 16-Year-Old Boy With Concerning, Recurrent Knee Problems"
},
{
"authors": "Zoran Rajković, MD; Dino Papeš, MD",
"content": [
"The patient's age and activity in high-performance sports point to the possibility of an osteochondral defect (OCD). The plain x-ray shows a narrow, well-calcified lesion off the femoral condyle, which could support this diagnosis (Figure 1). The intraoperative photo confirms the diagnosis (Figure 2).",
"Figure 1.",
"Figure 2.",
"OCD is characterized by the separation of an area of subchondral bone. This may result in the formation of cracks in the overlying articular cartilage; the damaged cartilage may then separate from the surrounding cartilage, followed by separation of the osteochondral fragment from the epiphysis.[1] Although \"osteochondritis\" implies inflammation, the lack of inflammatory cells in histological examination suggests a noninflammatory cause. The pathophysiology of OCD is not yet clear, as both trauma and ischemia have been thought to be involved. If separation of an osteochondral fragment from the articular surface occurs, this fragment can be present in situ, partially detached, or entirely detached.[1] A completely detached fragment becomes a loose body. Injury to the articular cartilage allows an influx of synovial fluid into the epiphysis, creating a subchondral cyst.",
"In humans, OCD is a rare disease, occurring in 15-30 per 100,000 persons in the general population each year.[2] OCD tends to affect young patients, with an average age at presentation of 10-20 years (but it may occur in persons of any age). It is more common in the male population, with a male-to-female ratio of 2:1.[2,3,4] Other studies quote a male-to-female ratio of 5:3.[5] The number of reported cases has increased along with an increasing level of participation in competitive sports among both boys and girls.[5] Two forms are recognized: a juvenile form, which appears before closure of the physes, and an adult form.[5]",
"High-impact sports, such as gymnastics, soccer, basketball, lacrosse, football, tennis, and baseball, have been associated with a higher risk for OCD in stressed joints.[5,6] Other factors, such as rapid growth during puberty, deficiencies and imbalances in the ratio of calcium to phosphorus, and anomalies of bone formation, have been suggested to have a role in the development of OCD, but clear evidence is lacking.[7,8,9] Some case reports have suggested a genetic predisposition.[8,10,11]"
],
"date": "February 05, 2019",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/750/480/750480-thumb1a.png"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/749/267/749267-thumb2.png"
}
],
"markdown": "# A 16-Year-Old Boy With Concerning, Recurrent Knee Problems\n\n **Authors:** Zoran Rajković, MD; Dino Papeš, MD \n **Date:** February 05, 2019\n\n ## Content\n\n The patient's age and activity in high-performance sports point to the possibility of an osteochondral defect (OCD). The plain x-ray shows a narrow, well-calcified lesion off the femoral condyle, which could support this diagnosis (Figure 1). The intraoperative photo confirms the diagnosis (Figure 2).\nFigure 1.\nFigure 2.\nOCD is characterized by the separation of an area of subchondral bone. This may result in the formation of cracks in the overlying articular cartilage; the damaged cartilage may then separate from the surrounding cartilage, followed by separation of the osteochondral fragment from the epiphysis.[1] Although \"osteochondritis\" implies inflammation, the lack of inflammatory cells in histological examination suggests a noninflammatory cause. The pathophysiology of OCD is not yet clear, as both trauma and ischemia have been thought to be involved. If separation of an osteochondral fragment from the articular surface occurs, this fragment can be present in situ, partially detached, or entirely detached.[1] A completely detached fragment becomes a loose body. Injury to the articular cartilage allows an influx of synovial fluid into the epiphysis, creating a subchondral cyst.\nIn humans, OCD is a rare disease, occurring in 15-30 per 100,000 persons in the general population each year.[2] OCD tends to affect young patients, with an average age at presentation of 10-20 years (but it may occur in persons of any age). It is more common in the male population, with a male-to-female ratio of 2:1.[2,3,4] Other studies quote a male-to-female ratio of 5:3.[5] The number of reported cases has increased along with an increasing level of participation in competitive sports among both boys and girls.[5] Two forms are recognized: a juvenile form, which appears before closure of the physes, and an adult form.[5]\nHigh-impact sports, such as gymnastics, soccer, basketball, lacrosse, football, tennis, and baseball, have been associated with a higher risk for OCD in stressed joints.[5,6] Other factors, such as rapid growth during puberty, deficiencies and imbalances in the ratio of calcium to phosphorus, and anomalies of bone formation, have been suggested to have a role in the development of OCD, but clear evidence is lacking.[7,8,9] Some case reports have suggested a genetic predisposition.[8,10,11]\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n\n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 434394,
"choiceText": "Osteochondromatosis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 434395,
"choiceText": "Juvenile rheumatoid arthritis with loose osteophyte",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 434396,
"choiceText": "Osgood-Schlatter disease",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 434397,
"choiceText": "Osteochondral defect",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 128609,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 16-Year-Old Boy With Concerning, Recurrent Knee Problems"
},
{
"authors": "Zoran Rajković, MD; Dino Papeš, MD",
"content": [
"The knee is the most commonly affected joint; OCD affects the knee in 75% of cases, the elbow in 6% of cases, and the ankle in 4% of cases. In the knee, OCD involves the lateral aspect of the medial femoral condyle in 75% of cases, the weight-bearing surface of the medial condyle in 10% of cases, the weight-bearing surface of the lateral condyle in 10% of cases, and the anterior intercondylar groove or patella in 5% of cases.[5,12,13,14,15,16,17,18,19,20] Some authors underline that OCD of the talus is being diagnosed more frequently, as CT and MRI are becoming widely used and, in future series, may represent the most frequent site of OCD.[20]",
"The symptoms of OCD include joint pain, crepitation, swelling, and stiffness. Symptoms may be aggravated with physical activities, such as playing sports. When a lower extremity is involved, patients often present with a limp. With complete fragment separation, locking symptoms may occur. Symptoms usually improve with protected immobilization of the joint. Clinical signs include effusion, tenderness over the lesion, quadriceps atrophy, and weakness. The patient may lack full knee extension when compared with the contralateral knee.",
"Occasionally, a loose body may be palpable. The Wilson test is sometimes performed as part of the examination of the knee when OCD is suspected. The examiner flexes the knee to 90° while internally rotating the tibia. A positive Wilson sign occurs when pain is elicited at 30° of flexion and is relieved with external rotation.[21] However, one study found it to be of very little clinical value as 24 of 32 study patients with OCD lesions on x-rays had negative Wilson signs.[21]",
"Several diagnostic methods are available for the diagnosis and staging of OCD, including plain films (anteroposterior, lateral, and notch/tunnel views), arthrography, ultrasonography, MRI (with or without gadolinium contrast), CT scanning, technetium bone scanning, and knee arthroscopy. Plain films may be normal early in the course. Notch views are the most effective view for evaluating the lateral aspect of the medial femoral condyle.[4]",
"MRI is the best noninvasive imaging modality and can assess for lesion stability. Intra-articular gadolinium may improve lesion characterization.[16,22,23] CT can be performed if MRI is not available. Knee arthroscopy can be used both diagnostically and therapeutically.[3,4,20] Arthroscopic OCD stages are defined by the International Cartilage Repair Society.[24] MRI has proven useful in evaluating lesion instability in adults but is less accurate in children.[25,26]"
],
"date": "February 05, 2019",
"figures": [],
"markdown": "# A 16-Year-Old Boy With Concerning, Recurrent Knee Problems\n\n **Authors:** Zoran Rajković, MD; Dino Papeš, MD \n **Date:** February 05, 2019\n\n ## Content\n\n The knee is the most commonly affected joint; OCD affects the knee in 75% of cases, the elbow in 6% of cases, and the ankle in 4% of cases. In the knee, OCD involves the lateral aspect of the medial femoral condyle in 75% of cases, the weight-bearing surface of the medial condyle in 10% of cases, the weight-bearing surface of the lateral condyle in 10% of cases, and the anterior intercondylar groove or patella in 5% of cases.[5,12,13,14,15,16,17,18,19,20] Some authors underline that OCD of the talus is being diagnosed more frequently, as CT and MRI are becoming widely used and, in future series, may represent the most frequent site of OCD.[20]\nThe symptoms of OCD include joint pain, crepitation, swelling, and stiffness. Symptoms may be aggravated with physical activities, such as playing sports. When a lower extremity is involved, patients often present with a limp. With complete fragment separation, locking symptoms may occur. Symptoms usually improve with protected immobilization of the joint. Clinical signs include effusion, tenderness over the lesion, quadriceps atrophy, and weakness. The patient may lack full knee extension when compared with the contralateral knee.\nOccasionally, a loose body may be palpable. The Wilson test is sometimes performed as part of the examination of the knee when OCD is suspected. The examiner flexes the knee to 90° while internally rotating the tibia. A positive Wilson sign occurs when pain is elicited at 30° of flexion and is relieved with external rotation.[21] However, one study found it to be of very little clinical value as 24 of 32 study patients with OCD lesions on x-rays had negative Wilson signs.[21]\nSeveral diagnostic methods are available for the diagnosis and staging of OCD, including plain films (anteroposterior, lateral, and notch/tunnel views), arthrography, ultrasonography, MRI (with or without gadolinium contrast), CT scanning, technetium bone scanning, and knee arthroscopy. Plain films may be normal early in the course. Notch views are the most effective view for evaluating the lateral aspect of the medial femoral condyle.[4]\nMRI is the best noninvasive imaging modality and can assess for lesion stability. Intra-articular gadolinium may improve lesion characterization.[16,22,23] CT can be performed if MRI is not available. Knee arthroscopy can be used both diagnostically and therapeutically.[3,4,20] Arthroscopic OCD stages are defined by the International Cartilage Repair Society.[24] MRI has proven useful in evaluating lesion instability in adults but is less accurate in children.[25,26]\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 16-Year-Old Boy With Concerning, Recurrent Knee Problems"
},
{
"authors": "Zoran Rajković, MD; Dino Papeš, MD",
"content": [
"The treatment of OCD of the knee depends on the age of the patient and the grade of the disease. The general rule is that the younger the patient, the better the prognosis; this is especially true in children with open physes, who are generally felt to do better than adolescent or adult patients in whom the physes are already closed. Nonetheless, it is possible for children with open physes to experience long-term problems from their lesions.[5]",
"In skeletally immature children with nondisplaced fragments, initial treatment includes limitation of activity with the use of crutches and restricted range of motion (eg, knee immobilizer, range-of-motion brace), cryotherapy, and oral analgesics.[5] Earlier surgical intervention should be considered for lesions in children who are approaching physeal closure and with a higher grade of OCD. A trial of nonsurgical treatment can be recommended for 3-6 months. If symptoms persist or failure to unite is observed on x-rays, patients should be treated surgically.",
"Adults may also be given a trial of conservative treatment for knee OCD; however, they are less likely to improve without surgical intervention. Therefore, in adults, the threshold for surgery should be lower.[5] Surgery can be arthroscopic or open. Several surgical options are available, including drilling of the fragment to stimulate healing, pin or screw fixation of the fragment, removal of loose bodies, osteochondral autograft or allograft transplantation, and autologous chondrocyte implantation.[5] The success rates of the above-mentioned procedures vary in different studies and depend on the patient's age and OCD grade.",
"A promising alternative to osteochondral cylinder transfer or conventional autologous chondrocyte implantation (ACI) for knee OCD may be concomitant reconstruction of deep osteochondral defects of the knee with monocortical cancellous cylinders and matrix-associated ACI.[27] Ochs et al reported their 2- to 5-year results in 26 patients with International Cartilage Repair Society grade III/IV OCD who underwent the novel, biologic, 1-step procedure. All clinical rating scores had significant improvement in these patients, and there was concomitant and timed tissue remodeling with clinical improvement.[27] In addition, good results were also observed on MRI.",
"In this case, the patient underwent arthroscopy, as MRI was unavailable. On arthroscopy, a large defect of the patella was found (3 × 1 cm), so conversion to mini arthrotomy was performed (Figure 2).",
"Figure 2.",
"The bone fragment was fixed with 2 screws and 2 resorptive pins. Control x-rays during follow-up showed normal healing of the fracture, and after physical therapy, the patient recovered full knee function."
],
"date": "February 05, 2019",
"figures": [
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/749/267/749267-thumb2.png"
}
],
"markdown": "# A 16-Year-Old Boy With Concerning, Recurrent Knee Problems\n\n **Authors:** Zoran Rajković, MD; Dino Papeš, MD \n **Date:** February 05, 2019\n\n ## Content\n\n The treatment of OCD of the knee depends on the age of the patient and the grade of the disease. The general rule is that the younger the patient, the better the prognosis; this is especially true in children with open physes, who are generally felt to do better than adolescent or adult patients in whom the physes are already closed. Nonetheless, it is possible for children with open physes to experience long-term problems from their lesions.[5]\nIn skeletally immature children with nondisplaced fragments, initial treatment includes limitation of activity with the use of crutches and restricted range of motion (eg, knee immobilizer, range-of-motion brace), cryotherapy, and oral analgesics.[5] Earlier surgical intervention should be considered for lesions in children who are approaching physeal closure and with a higher grade of OCD. A trial of nonsurgical treatment can be recommended for 3-6 months. If symptoms persist or failure to unite is observed on x-rays, patients should be treated surgically.\nAdults may also be given a trial of conservative treatment for knee OCD; however, they are less likely to improve without surgical intervention. Therefore, in adults, the threshold for surgery should be lower.[5] Surgery can be arthroscopic or open. Several surgical options are available, including drilling of the fragment to stimulate healing, pin or screw fixation of the fragment, removal of loose bodies, osteochondral autograft or allograft transplantation, and autologous chondrocyte implantation.[5] The success rates of the above-mentioned procedures vary in different studies and depend on the patient's age and OCD grade.\nA promising alternative to osteochondral cylinder transfer or conventional autologous chondrocyte implantation (ACI) for knee OCD may be concomitant reconstruction of deep osteochondral defects of the knee with monocortical cancellous cylinders and matrix-associated ACI.[27] Ochs et al reported their 2- to 5-year results in 26 patients with International Cartilage Repair Society grade III/IV OCD who underwent the novel, biologic, 1-step procedure. All clinical rating scores had significant improvement in these patients, and there was concomitant and timed tissue remodeling with clinical improvement.[27] In addition, good results were also observed on MRI.\nIn this case, the patient underwent arthroscopy, as MRI was unavailable. On arthroscopy, a large defect of the patella was found (3 × 1 cm), so conversion to mini arthrotomy was performed (Figure 2).\nFigure 2.\nThe bone fragment was fixed with 2 screws and 2 resorptive pins. Control x-rays during follow-up showed normal healing of the fracture, and after physical therapy, the patient recovered full knee function.\n\n ## Figures\n\n **Figure 2.** \n \n\n\n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 434398,
"choiceText": "Treatment is more likely to succeed in older children and adults",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 434399,
"choiceText": "The main cause is genetic predisposition combined with trauma",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 434400,
"choiceText": "OCD mainly occurs in the knee",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 434401,
"choiceText": "It is a common disease",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The knee is the most affected joint as OCD occurs in the knee 75% of the time, the elbow 6% of the time, and the ankle 4% of the time. However, as CT and MRI are becoming widely used, some authors consider that OCD of the talus may represent the most frequent site of OCD in the future.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 128610,
"questionText": "You examine a teenage athlete with progressive elbow pain and trouble with elbow movement. Among other conditions, you suspect OCD. If this patient were diagnosed with OCD, which of the following statements about his condition would be correct?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 434402,
"choiceText": "If MRI is unavailable, arthrography is the preferred method",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 434403,
"choiceText": "If MRI is unavailable, CT is the preferred method",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 434404,
"choiceText": "Arthroscopy is always the method of choice",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 434405,
"choiceText": "Technetium scintigraphy",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 434406,
"choiceText": "Ultrasound",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "If MRI is unavailable, CT scanning is the method of choice. Scintigraphy has been used as an indicator of potential healing of the osteochondral fragment. Greater uptake means higher osteoblastic activity and the greater the likelihood for healing with conservative management. Technetium imaging may also reveal occult bilateral involvement. Sonography is an alternative to CT scanning if an expert sonographer is available. The advantages of sonography are decreased cost compared with MRI and CT scanning, and dynamic scanning with motion of the affected joint.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 128611,
"questionText": "You examine a teenage athlete with x-ray findings that are highly suspicious of OCD. Which of the following is the recommended diagnostic method?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 16-Year-Old Boy With Concerning, Recurrent Knee Problems"
},
{
"authors": "Zoran Rajković, MD; Dino Papeš, MD",
"content": [],
"date": "February 05, 2019",
"figures": [],
"markdown": "# A 16-Year-Old Boy With Concerning, Recurrent Knee Problems\n\n **Authors:** Zoran Rajković, MD; Dino Papeš, MD \n **Date:** February 05, 2019\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 434398,
"choiceText": "Treatment is more likely to succeed in older children and adults",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 434399,
"choiceText": "The main cause is genetic predisposition combined with trauma",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 434400,
"choiceText": "OCD mainly occurs in the knee",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 434401,
"choiceText": "It is a common disease",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The knee is the most affected joint as OCD occurs in the knee 75% of the time, the elbow 6% of the time, and the ankle 4% of the time. However, as CT and MRI are becoming widely used, some authors consider that OCD of the talus may represent the most frequent site of OCD in the future.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 128610,
"questionText": "You examine a teenage athlete with progressive elbow pain and trouble with elbow movement. Among other conditions, you suspect OCD. If this patient were diagnosed with OCD, which of the following statements about his condition would be correct?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 434402,
"choiceText": "If MRI is unavailable, arthrography is the preferred method",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 434403,
"choiceText": "If MRI is unavailable, CT is the preferred method",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 434404,
"choiceText": "Arthroscopy is always the method of choice",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 434405,
"choiceText": "Technetium scintigraphy",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 434406,
"choiceText": "Ultrasound",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "If MRI is unavailable, CT scanning is the method of choice. Scintigraphy has been used as an indicator of potential healing of the osteochondral fragment. Greater uptake means higher osteoblastic activity and the greater the likelihood for healing with conservative management. Technetium imaging may also reveal occult bilateral involvement. Sonography is an alternative to CT scanning if an expert sonographer is available. The advantages of sonography are decreased cost compared with MRI and CT scanning, and dynamic scanning with motion of the affected joint.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 128611,
"questionText": "You examine a teenage athlete with x-ray findings that are highly suspicious of OCD. Which of the following is the recommended diagnostic method?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 16-Year-Old Boy With Concerning, Recurrent Knee Problems"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 434394,
"choiceText": "Osteochondromatosis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 434395,
"choiceText": "Juvenile rheumatoid arthritis with loose osteophyte",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 434396,
"choiceText": "Osgood-Schlatter disease",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 434397,
"choiceText": "Osteochondral defect",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 128609,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 434398,
"choiceText": "Treatment is more likely to succeed in older children and adults",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 434399,
"choiceText": "The main cause is genetic predisposition combined with trauma",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 434400,
"choiceText": "OCD mainly occurs in the knee",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 434401,
"choiceText": "It is a common disease",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The knee is the most affected joint as OCD occurs in the knee 75% of the time, the elbow 6% of the time, and the ankle 4% of the time. However, as CT and MRI are becoming widely used, some authors consider that OCD of the talus may represent the most frequent site of OCD in the future.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 128610,
"questionText": "You examine a teenage athlete with progressive elbow pain and trouble with elbow movement. Among other conditions, you suspect OCD. If this patient were diagnosed with OCD, which of the following statements about his condition would be correct?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 434402,
"choiceText": "If MRI is unavailable, arthrography is the preferred method",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 434403,
"choiceText": "If MRI is unavailable, CT is the preferred method",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 434404,
"choiceText": "Arthroscopy is always the method of choice",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 434405,
"choiceText": "Technetium scintigraphy",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 434406,
"choiceText": "Ultrasound",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "If MRI is unavailable, CT scanning is the method of choice. Scintigraphy has been used as an indicator of potential healing of the osteochondral fragment. Greater uptake means higher osteoblastic activity and the greater the likelihood for healing with conservative management. Technetium imaging may also reveal occult bilateral involvement. Sonography is an alternative to CT scanning if an expert sonographer is available. The advantages of sonography are decreased cost compared with MRI and CT scanning, and dynamic scanning with motion of the affected joint.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 128611,
"questionText": "You examine a teenage athlete with x-ray findings that are highly suspicious of OCD. Which of the following is the recommended diagnostic method?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
749567 | /viewarticle/749567 | [
{
"authors": "Eric Winkel, OMS-3; Mark A. Sanders, DO, JD, MPH, LLM; Anada I. Gunn-Sanders, JD, MPH, CFC, CMI-II",
"content": [
"Editor's Note:\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions.",
"Background",
"Figure 1.",
"Figure 2.",
"A 75-year-old man presents to the emergency department (ED) with a 6-week history of worsening lower abdominal pain. He describes the pain as sharp, constant, and associated with a weight loss of approximately 20 lb over the past month. The patient states that for the past few weeks he has also noticed bilateral lower-extremity swelling and abdominal distention. The abdominal distention has been severe enough to prevent him from bending over. He experienced an increased frequency of urination until 3 days ago; he has been anuric since then. For the past week he has not been able to tolerate solid foods, resulting in an entirely liquid diet. As of the day of presentation even liquids have become intolerable. The patient also has a history of numbness and tingling in both legs and an unsteady gait during the last week. He reports a feeling of confusion but is fully oriented to person, place, and time. His medical history is significant for hypertension under treatment and mitral valve insufficiency. He denies any surgical history. His current medications include daily aspirin and nifedipine. The patient has an age-appropriate level of physical activity and lives alone. He does not smoke or drink."
],
"date": "October 10, 2014",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/748/107/748107-thumb1.png"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/748/107/748107-thumb2.png"
}
],
"markdown": "# Insidious Abdominal Symptoms With Urinary Disturbances in an Elderly Man\n\n **Authors:** Eric Winkel, OMS-3; Mark A. Sanders, DO, JD, MPH, LLM; Anada I. Gunn-Sanders, JD, MPH, CFC, CMI-II \n **Date:** October 10, 2014\n\n ## Content\n\n Editor's Note:\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions.\nBackground\nFigure 1.\nFigure 2.\nA 75-year-old man presents to the emergency department (ED) with a 6-week history of worsening lower abdominal pain. He describes the pain as sharp, constant, and associated with a weight loss of approximately 20 lb over the past month. The patient states that for the past few weeks he has also noticed bilateral lower-extremity swelling and abdominal distention. The abdominal distention has been severe enough to prevent him from bending over. He experienced an increased frequency of urination until 3 days ago; he has been anuric since then. For the past week he has not been able to tolerate solid foods, resulting in an entirely liquid diet. As of the day of presentation even liquids have become intolerable. The patient also has a history of numbness and tingling in both legs and an unsteady gait during the last week. He reports a feeling of confusion but is fully oriented to person, place, and time. His medical history is significant for hypertension under treatment and mitral valve insufficiency. He denies any surgical history. His current medications include daily aspirin and nifedipine. The patient has an age-appropriate level of physical activity and lives alone. He does not smoke or drink.\n\n ## Figures\n\n **Figure 1.** \n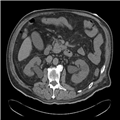 \n\n**Figure 2.** \n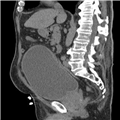 \n\n\n*Page 1 of 5*",
"pagination": {
"current_page": 1,
"total_pages": 5
},
"questionnaire": [],
"title": "Insidious Abdominal Symptoms With Urinary Disturbances in an Elderly Man"
},
{
"authors": "Eric Winkel, OMS-3; Mark A. Sanders, DO, JD, MPH, LLM; Anada I. Gunn-Sanders, JD, MPH, CFC, CMI-II",
"content": [
"On physical examination, the patient is a well-oriented elderly man in no acute distress. His vital signs include an oral temperature of 97.4°F (36.3°C), a blood pressure of 211/92 mm Hg, a pulse of 104 beats/min, an unlabored respiratory rate of 20 breaths/min, and an oxygen saturation of 97% on room air. Head and neck examination reveals dry oral mucous membranes with a supple neck and no jugular venous distention. The chest examination is clear to auscultation bilaterally, with normal breath sounds and normal S1 and S2 heart sounds without murmurs, rubs, or gallops. The abdominal examination is significant for distention, mostly in the hypogastric and umbilical regions, and for the presence of a reducible umbilical hernia. Normal bowel sounds are appreciated in all quadrants. The patient's lower abdomen is diffusely tender, with slight tenderness noted in the upper abdomen as well. There is no rebound tenderness or guarding and Lloyd's sign is negative. Extremity examination is significant for bilateral pitting edema of the lower extremities. The patient has no focal neurologic abnormalities (although gait testing is deferred).",
"Figure 1.",
"Figure 2.",
"Initial laboratory investigations include a complete blood count (CBC) and basic metabolic panel. No significant findings were revealed on the CBC. The metabolic panel reveals a serum sodium level of 130 mEq/L (normal range, 136-145 mEq/L), potassium of 4.2 mEq/L (normal range, 3.5-5.0 mEq/L), chloride of 83 mEq/L (normal range, 95-105 mEq/L), bicarbonate of 16 mEq/L (normal range, 22-28 mEq/L), and glucose of 92 mg/dL (normal range, 70-125 mg/dL). Serum creatinine is 21.2 mg/dL (normal range, 0.6-1.2 mg/dL) and blood urea nitrogen is 120 mg/dL (normal range, 7-18 mg/dL). The calculated glomerular filtration rate (GFR) is 3 mL/min/1.73 m2 (normal, ≥ 90 mL/min/1.73 m2). A CT scan of the abdomen and pelvis is obtained (Figures 1 and 2)."
],
"date": "October 10, 2014",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/748/107/748107-thumb1.png"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/748/107/748107-thumb2.png"
}
],
"markdown": "# Insidious Abdominal Symptoms With Urinary Disturbances in an Elderly Man\n\n **Authors:** Eric Winkel, OMS-3; Mark A. Sanders, DO, JD, MPH, LLM; Anada I. Gunn-Sanders, JD, MPH, CFC, CMI-II \n **Date:** October 10, 2014\n\n ## Content\n\n On physical examination, the patient is a well-oriented elderly man in no acute distress. His vital signs include an oral temperature of 97.4°F (36.3°C), a blood pressure of 211/92 mm Hg, a pulse of 104 beats/min, an unlabored respiratory rate of 20 breaths/min, and an oxygen saturation of 97% on room air. Head and neck examination reveals dry oral mucous membranes with a supple neck and no jugular venous distention. The chest examination is clear to auscultation bilaterally, with normal breath sounds and normal S1 and S2 heart sounds without murmurs, rubs, or gallops. The abdominal examination is significant for distention, mostly in the hypogastric and umbilical regions, and for the presence of a reducible umbilical hernia. Normal bowel sounds are appreciated in all quadrants. The patient's lower abdomen is diffusely tender, with slight tenderness noted in the upper abdomen as well. There is no rebound tenderness or guarding and Lloyd's sign is negative. Extremity examination is significant for bilateral pitting edema of the lower extremities. The patient has no focal neurologic abnormalities (although gait testing is deferred).\nFigure 1.\nFigure 2.\nInitial laboratory investigations include a complete blood count (CBC) and basic metabolic panel. No significant findings were revealed on the CBC. The metabolic panel reveals a serum sodium level of 130 mEq/L (normal range, 136-145 mEq/L), potassium of 4.2 mEq/L (normal range, 3.5-5.0 mEq/L), chloride of 83 mEq/L (normal range, 95-105 mEq/L), bicarbonate of 16 mEq/L (normal range, 22-28 mEq/L), and glucose of 92 mg/dL (normal range, 70-125 mg/dL). Serum creatinine is 21.2 mg/dL (normal range, 0.6-1.2 mg/dL) and blood urea nitrogen is 120 mg/dL (normal range, 7-18 mg/dL). The calculated glomerular filtration rate (GFR) is 3 mL/min/1.73 m2 (normal, ≥ 90 mL/min/1.73 m2). A CT scan of the abdomen and pelvis is obtained (Figures 1 and 2).\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n\n*Page 2 of 5*",
"pagination": {
"current_page": 2,
"total_pages": 5
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 430965,
"choiceText": "Malignant hypertension",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 430966,
"choiceText": "Obstructive uropathy",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 430967,
"choiceText": "Renal artery stenosis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 430968,
"choiceText": "Abdominal aortic aneurysm",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 430969,
"choiceText": "Renal cell carcinoma",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 127467,
"questionText": "What is the most likely diagnosis?<br />\r\n<br />\r\n<i>Hint: Note the decreased GFR in light of the CT findings.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Insidious Abdominal Symptoms With Urinary Disturbances in an Elderly Man"
},
{
"authors": "Eric Winkel, OMS-3; Mark A. Sanders, DO, JD, MPH, LLM; Anada I. Gunn-Sanders, JD, MPH, CFC, CMI-II",
"content": [
"(Enlarge Image)Figure 1.CT scan showing enlarged kidneys, perinephric fat stranding, and distended ureters.\n(Enlarge Image)Figure 2.CT scan showing bladder distention.",
"Figure 1.",
"CT scan showing enlarged kidneys, perinephric fat stranding, and distended ureters.",
"CT scan showing enlarged kidneys, perinephric fat stranding, and distended ureters.",
"Figure 2.",
"CT scan showing bladder distention.",
"CT scan showing bladder distention.",
"The patient's CT scans both with and without contrast revealed moderate bilateral hydroureteronephrosis without evidence of an obstructing stone in the ureters. The kidneys were enlarged and perinephric fat stranding was noted (Figure 1). The prostate was also found to be enlarged and the bladder was noted to be extremely enlarged (Figure 2). A diagnosis of obstructive uropathy resulting from an enlarged prostate was made.",
"Total lower urinary tract obstruction (UTO) should be suspected when a patient presents with acute anuria and other causes, such as a past history of end stage renal disease (ESRD) or shock with prerenal failure, have been excluded. The most common cause of lower UTO in men is benign prostatic hyperplasia (BPH).[1] Other possible causes include phimosis, urethral strictures, and neurogenic bladder. Treatment of prostate cancer by external beam radiation, surgery, and especially brachytherapy can also result in urinary retention, especially in the initial weeks following therapy.[2] Anticholinergic medications are a well-known cause of urinary retention. Diphenhydramine has a similar mechanism and should be administered with caution in the elderly. Causes of unilateral ureteral obstruction include blood clots, sloughed renal papillae (as can occur in diabetic nephropathy, analgesic nephropathy, renal tuberculosis, and sickle cell disease), and renal calculi.[1] Compression of the ureters can be due to neoplasms of adjacent viscera or aneurysms of the aorta or iliac vessels. Accidental ureteral damage can occur with gynecologic surgery. Ureteral stasis and compression are common during pregnancy and usually occur on the right side; however, in rare cases they can present bilaterally. In children, congenital anomalies such as severe vesicoureteral reflux or posterior urethral valves should be suspected.[3]",
"The clinical features of complete bilateral UTO include abdominal pain resulting from distention of the renal (Gerota) fascia or the urinary bladder, difficulty voiding, and hematuria.[4]Hypertension can result from increased renal secretion of renin.[3] Pain is less pronounced and may even go unnoticed in cases in which the obstruction progresses gradually. This can result in patients presenting after irreversible loss of renal function has already occurred. Frequent urinary tract infections or infections in uncommon populations, such as males and children, should raise suspicion for a partial or intermittent obstruction. Urine culture in these patients may reveal atypical organisms (such as Pseudomonas species).",
"Paradoxically, partial or intermittent UTO may result in polyuria. Extracellular volume expansion leads to an increased level of atrial natriuretic peptide (ANP), which inhibits sodium reabsorption at the proximal tubule. Decreased sodium reabsorption diminishes the medullary interstitial gradient, thereby reducing the concentrating power of the nephron. Further reduction in renal concentrating power results from a downregulation of the urea transporters responsible for creating this concentration gradient.[5] A dysfunction of this transporter is also seen in a subset of patients with congenital diabetes insipidus (DI).[6] Finally, increased ureteral pressure damages the distal nephron where vasopressin acts to concentrate the urine, creating an acquired nephrogenic DI.[7]\nHypernatremia resulting from obstructive uropathy can be found in sporadic case reports, but clinicians should be more circumspect of this electrolyte abnormality immediately following relief of the obstruction.[8,9,10] In the above case, the patient was hyponatremic at the time of presentation, likely from expansion of the effective circulating volume.",
"UTO can also lead to hyperkalemic renal tubular acidosis (RTA) or type IV RTA. In fact, the differential diagnosis of hyperkalemic RTA should include an investigation for UTO. This condition is characterized by hyperkalemia, metabolic acidosis, and impairment of ammonia excretion (increased urine anion gap). The mechanism of this sequela of UTO is aldosterone resistance, aldosterone deficiency, or a combination of both. As the distal renal tubule is damaged, it progressively becomes unresponsive to aldosterone stimulation. Aldosterone deficiency occurs as interstitial renal damage impairs the stimulation of renin secretion, thereby suppressing the renin-angiotensin-aldosterone axis.[11]"
],
"date": "October 10, 2014",
"figures": [
{
"caption": "Figure 1.CT scan showing enlarged kidneys, perinephric fat stranding, and distended ureters.",
"image_url": "https://img.medscapestatic.com/article/748/107/748107-thumb1.png"
},
{
"caption": "Figure 2.CT scan showing bladder distention.",
"image_url": "https://img.medscapestatic.com/article/748/107/748107-thumb2.png"
}
],
"markdown": "# Insidious Abdominal Symptoms With Urinary Disturbances in an Elderly Man\n\n **Authors:** Eric Winkel, OMS-3; Mark A. Sanders, DO, JD, MPH, LLM; Anada I. Gunn-Sanders, JD, MPH, CFC, CMI-II \n **Date:** October 10, 2014\n\n ## Content\n\n (Enlarge Image)Figure 1.CT scan showing enlarged kidneys, perinephric fat stranding, and distended ureters.\n(Enlarge Image)Figure 2.CT scan showing bladder distention.\nFigure 1.\nCT scan showing enlarged kidneys, perinephric fat stranding, and distended ureters.\nCT scan showing enlarged kidneys, perinephric fat stranding, and distended ureters.\nFigure 2.\nCT scan showing bladder distention.\nCT scan showing bladder distention.\nThe patient's CT scans both with and without contrast revealed moderate bilateral hydroureteronephrosis without evidence of an obstructing stone in the ureters. The kidneys were enlarged and perinephric fat stranding was noted (Figure 1). The prostate was also found to be enlarged and the bladder was noted to be extremely enlarged (Figure 2). A diagnosis of obstructive uropathy resulting from an enlarged prostate was made.\nTotal lower urinary tract obstruction (UTO) should be suspected when a patient presents with acute anuria and other causes, such as a past history of end stage renal disease (ESRD) or shock with prerenal failure, have been excluded. The most common cause of lower UTO in men is benign prostatic hyperplasia (BPH).[1] Other possible causes include phimosis, urethral strictures, and neurogenic bladder. Treatment of prostate cancer by external beam radiation, surgery, and especially brachytherapy can also result in urinary retention, especially in the initial weeks following therapy.[2] Anticholinergic medications are a well-known cause of urinary retention. Diphenhydramine has a similar mechanism and should be administered with caution in the elderly. Causes of unilateral ureteral obstruction include blood clots, sloughed renal papillae (as can occur in diabetic nephropathy, analgesic nephropathy, renal tuberculosis, and sickle cell disease), and renal calculi.[1] Compression of the ureters can be due to neoplasms of adjacent viscera or aneurysms of the aorta or iliac vessels. Accidental ureteral damage can occur with gynecologic surgery. Ureteral stasis and compression are common during pregnancy and usually occur on the right side; however, in rare cases they can present bilaterally. In children, congenital anomalies such as severe vesicoureteral reflux or posterior urethral valves should be suspected.[3]\nThe clinical features of complete bilateral UTO include abdominal pain resulting from distention of the renal (Gerota) fascia or the urinary bladder, difficulty voiding, and hematuria.[4]Hypertension can result from increased renal secretion of renin.[3] Pain is less pronounced and may even go unnoticed in cases in which the obstruction progresses gradually. This can result in patients presenting after irreversible loss of renal function has already occurred. Frequent urinary tract infections or infections in uncommon populations, such as males and children, should raise suspicion for a partial or intermittent obstruction. Urine culture in these patients may reveal atypical organisms (such as Pseudomonas species).\nParadoxically, partial or intermittent UTO may result in polyuria. Extracellular volume expansion leads to an increased level of atrial natriuretic peptide (ANP), which inhibits sodium reabsorption at the proximal tubule. Decreased sodium reabsorption diminishes the medullary interstitial gradient, thereby reducing the concentrating power of the nephron. Further reduction in renal concentrating power results from a downregulation of the urea transporters responsible for creating this concentration gradient.[5] A dysfunction of this transporter is also seen in a subset of patients with congenital diabetes insipidus (DI).[6] Finally, increased ureteral pressure damages the distal nephron where vasopressin acts to concentrate the urine, creating an acquired nephrogenic DI.[7]\nHypernatremia resulting from obstructive uropathy can be found in sporadic case reports, but clinicians should be more circumspect of this electrolyte abnormality immediately following relief of the obstruction.[8,9,10] In the above case, the patient was hyponatremic at the time of presentation, likely from expansion of the effective circulating volume.\nUTO can also lead to hyperkalemic renal tubular acidosis (RTA) or type IV RTA. In fact, the differential diagnosis of hyperkalemic RTA should include an investigation for UTO. This condition is characterized by hyperkalemia, metabolic acidosis, and impairment of ammonia excretion (increased urine anion gap). The mechanism of this sequela of UTO is aldosterone resistance, aldosterone deficiency, or a combination of both. As the distal renal tubule is damaged, it progressively becomes unresponsive to aldosterone stimulation. Aldosterone deficiency occurs as interstitial renal damage impairs the stimulation of renin secretion, thereby suppressing the renin-angiotensin-aldosterone axis.[11]\n\n ## Figures\n\n **Figure 1.CT scan showing enlarged kidneys, perinephric fat stranding, and distended ureters.** \n \n\n**Figure 2.CT scan showing bladder distention.** \n \n\n\n*Page 3 of 5*",
"pagination": {
"current_page": 3,
"total_pages": 5
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 430965,
"choiceText": "Malignant hypertension",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 430966,
"choiceText": "Obstructive uropathy",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 430967,
"choiceText": "Renal artery stenosis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 430968,
"choiceText": "Abdominal aortic aneurysm",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 430969,
"choiceText": "Renal cell carcinoma",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 127467,
"questionText": "What is the most likely diagnosis?<br />\r\n<br />\r\n<i>Hint: Note the decreased GFR in light of the CT findings.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Insidious Abdominal Symptoms With Urinary Disturbances in an Elderly Man"
},
{
"authors": "Eric Winkel, OMS-3; Mark A. Sanders, DO, JD, MPH, LLM; Anada I. Gunn-Sanders, JD, MPH, CFC, CMI-II",
"content": [
"(Enlarge Image)Figure 1.CT scan showing enlarged kidneys, perinephric fat stranding, and distended ureters.\n(Enlarge Image)Figure 2.CT scan showing bladder distention.",
"Figure 1.",
"CT scan showing enlarged kidneys, perinephric fat stranding, and distended ureters.",
"CT scan showing enlarged kidneys, perinephric fat stranding, and distended ureters.",
"Figure 2.",
"CT scan showing bladder distention.",
"CT scan showing bladder distention.",
"The diagnosis of UTO begins with the insertion of a bladder catheter; resulting diuresis suggests obstruction below the bladder. If catheterizing the bladder does not produce diuresis, a renal ultrasound can be used to confirm hydronephrosis with a sensitivity and specificity of 90%. False negatives on ultrasonography result from concomitant volume depletion, retroperitoneal fibrosis, obstructing malignancy, or if imaging is done too soon after the onset of an acute obstruction.[12] False positives occur in the setting of congenital anomalies and during diuresis.[13] In the setting of flank pain and suspicion for renal calculus without evidence of renal impairment, kidneys-ureters-bladder radiography detects approximately 90% of renal stones.[3] Antegrade and retrograde urography can be used to determine the location of the urinary obstruction.",
"The management of UTO is directed by the underlying cause; however, regardless of the cause, relief of a prolonged period of obstruction with anuria is often accompanied by postobstructive diuresis. In most cases, this polyuria is transient and may be an adaptive response to fluid overload from the anuria, possibly compounded by iatrogenic fluid administration prior to the diagnosis of UTO. Routine monitoring of vital signs and serum electrolytes is important to assess possible volume depletion or the development of acquired DI.[14] The mechanism of this latter phenomenon is the same as that of the polyuria of partial UTO, manifesting as hypernatremia resistant to vasopressin. Alternatively, large sodium losses from tubular dysfunction will result in hyponatremia. If tachycardia and/or orthostatic hypotension develop following relief of the obstruction, pathologic hypovolemia secondary to diuresis and tubular damage should be suspected. In either of these instances, fluid and electrolyte correction are warranted; otherwise, fluid administration may only be serving to prolong the diuretic phase of the postobstructive recovery.[3,14]",
"The prognosis for the recovery of renal function is dependent upon the severity and duration of the obstruction. In addition, patients with obstruction complicated by pyelonephritis generally have a much worse outcome. Longstanding UTO leads to tubulointerstitial damage, which can ultimately result in ESRD. In the pediatric population, congenital urologic anomalies causing obstruction are the major cause of ESRD requiring dialysis.[15] With the exception of these risk factors, there is no way of accurately predicting to what degree renal function will be restored after an obstruction is removed.",
"Following relief of the urinary obstruction, hemodynamic stabilization, and treatment of electrolyte abnormalities, the next step is monitoring. The follow-up labs should include measurement of serum creatinine to assess renal function in an effort to guide treatment following the initial management of BPH.",
"Monitoring of patients for symptoms of worsening BPH, which could result in a repeat UTO, is essential. Clinicians should be vigilant of herbal and over-the-counter medication use in patients with BPH, as some of these can lead to urinary retention with UTO.[16] In a study that assessed the use of alfuzosin, an alpha blocker, in the treatment of men with lower urinary tract symptoms suggestive of BPH for 12 months, investigators reported that all parameters of the International Prostate Symptom Score (IPSS) improved in the patient cohorts (ie, voiding and storage symptoms improved), regardless of their scores on the bladder outlet obstructive index (BOOI) or bladder contractility index (BCI).[17]",
"In the case presented, a Foley catheter was initially inserted, 2200 mL of urine were emptied, and the catheter was clamped. An hour later, 1700 mL were drained and the catheter was again clamped. A third drainage 50 minutes later yielded another 1000 mL. In total, 4900 mL of red-tinged urine was drained. The patient's abdominal distention promptly resolved with evacuation of the bladder, and the lower extremity edema regressed over the course of the patient's 4-day hospital stay. A review of the literature yielded case reports of similarly exaggerated urinary retention, including one case of a patient retaining more than 16 L over a 10-day period of anuria.[18] The patient's creatinine levels dropped dramatically by the time of discharge and were within the normal reference range on follow-up 1 week after discharge."
],
"date": "October 10, 2014",
"figures": [
{
"caption": "Figure 1.CT scan showing enlarged kidneys, perinephric fat stranding, and distended ureters.",
"image_url": "https://img.medscapestatic.com/article/748/107/748107-thumb1.png"
},
{
"caption": "Figure 2.CT scan showing bladder distention.",
"image_url": "https://img.medscapestatic.com/article/748/107/748107-thumb2.png"
}
],
"markdown": "# Insidious Abdominal Symptoms With Urinary Disturbances in an Elderly Man\n\n **Authors:** Eric Winkel, OMS-3; Mark A. Sanders, DO, JD, MPH, LLM; Anada I. Gunn-Sanders, JD, MPH, CFC, CMI-II \n **Date:** October 10, 2014\n\n ## Content\n\n (Enlarge Image)Figure 1.CT scan showing enlarged kidneys, perinephric fat stranding, and distended ureters.\n(Enlarge Image)Figure 2.CT scan showing bladder distention.\nFigure 1.\nCT scan showing enlarged kidneys, perinephric fat stranding, and distended ureters.\nCT scan showing enlarged kidneys, perinephric fat stranding, and distended ureters.\nFigure 2.\nCT scan showing bladder distention.\nCT scan showing bladder distention.\nThe diagnosis of UTO begins with the insertion of a bladder catheter; resulting diuresis suggests obstruction below the bladder. If catheterizing the bladder does not produce diuresis, a renal ultrasound can be used to confirm hydronephrosis with a sensitivity and specificity of 90%. False negatives on ultrasonography result from concomitant volume depletion, retroperitoneal fibrosis, obstructing malignancy, or if imaging is done too soon after the onset of an acute obstruction.[12] False positives occur in the setting of congenital anomalies and during diuresis.[13] In the setting of flank pain and suspicion for renal calculus without evidence of renal impairment, kidneys-ureters-bladder radiography detects approximately 90% of renal stones.[3] Antegrade and retrograde urography can be used to determine the location of the urinary obstruction.\nThe management of UTO is directed by the underlying cause; however, regardless of the cause, relief of a prolonged period of obstruction with anuria is often accompanied by postobstructive diuresis. In most cases, this polyuria is transient and may be an adaptive response to fluid overload from the anuria, possibly compounded by iatrogenic fluid administration prior to the diagnosis of UTO. Routine monitoring of vital signs and serum electrolytes is important to assess possible volume depletion or the development of acquired DI.[14] The mechanism of this latter phenomenon is the same as that of the polyuria of partial UTO, manifesting as hypernatremia resistant to vasopressin. Alternatively, large sodium losses from tubular dysfunction will result in hyponatremia. If tachycardia and/or orthostatic hypotension develop following relief of the obstruction, pathologic hypovolemia secondary to diuresis and tubular damage should be suspected. In either of these instances, fluid and electrolyte correction are warranted; otherwise, fluid administration may only be serving to prolong the diuretic phase of the postobstructive recovery.[3,14]\nThe prognosis for the recovery of renal function is dependent upon the severity and duration of the obstruction. In addition, patients with obstruction complicated by pyelonephritis generally have a much worse outcome. Longstanding UTO leads to tubulointerstitial damage, which can ultimately result in ESRD. In the pediatric population, congenital urologic anomalies causing obstruction are the major cause of ESRD requiring dialysis.[15] With the exception of these risk factors, there is no way of accurately predicting to what degree renal function will be restored after an obstruction is removed.\nFollowing relief of the urinary obstruction, hemodynamic stabilization, and treatment of electrolyte abnormalities, the next step is monitoring. The follow-up labs should include measurement of serum creatinine to assess renal function in an effort to guide treatment following the initial management of BPH.\nMonitoring of patients for symptoms of worsening BPH, which could result in a repeat UTO, is essential. Clinicians should be vigilant of herbal and over-the-counter medication use in patients with BPH, as some of these can lead to urinary retention with UTO.[16] In a study that assessed the use of alfuzosin, an alpha blocker, in the treatment of men with lower urinary tract symptoms suggestive of BPH for 12 months, investigators reported that all parameters of the International Prostate Symptom Score (IPSS) improved in the patient cohorts (ie, voiding and storage symptoms improved), regardless of their scores on the bladder outlet obstructive index (BOOI) or bladder contractility index (BCI).[17]\nIn the case presented, a Foley catheter was initially inserted, 2200 mL of urine were emptied, and the catheter was clamped. An hour later, 1700 mL were drained and the catheter was again clamped. A third drainage 50 minutes later yielded another 1000 mL. In total, 4900 mL of red-tinged urine was drained. The patient's abdominal distention promptly resolved with evacuation of the bladder, and the lower extremity edema regressed over the course of the patient's 4-day hospital stay. A review of the literature yielded case reports of similarly exaggerated urinary retention, including one case of a patient retaining more than 16 L over a 10-day period of anuria.[18] The patient's creatinine levels dropped dramatically by the time of discharge and were within the normal reference range on follow-up 1 week after discharge.\n\n ## Figures\n\n **Figure 1.CT scan showing enlarged kidneys, perinephric fat stranding, and distended ureters.** \n \n\n**Figure 2.CT scan showing bladder distention.** \n \n\n\n*Page 4 of 5*",
"pagination": {
"current_page": 4,
"total_pages": 5
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 430970,
"choiceText": "Right-sided flank pain with 102.1°F fever",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 430971,
"choiceText": "Abdominal distention and anuria",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 430972,
"choiceText": "Hematuria and dysuria",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 430973,
"choiceText": "Polyuria and polydipsia",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 430974,
"choiceText": "Straining during urination with enlarged prostate on digital rectal examination",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "A patient with right-sided flank pain and a high-grade fever probably has pyelonephritis complicating their UTO, which usually implies a worse overall prognosis. Abdominal distention and anuria are likely caused by complete UTO, whereas polyuria and polydipsia are presumably a result of the acquired diabetes insipidus seen in partial UTO.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 127468,
"questionText": "In a patient presenting with a history and physical examination findings suggestive of UTO, which of the following combinations of signs and/or symptoms is most worrisome?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 430975,
"choiceText": "Nicotine gum",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 430976,
"choiceText": "Cetirizine",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 430977,
"choiceText": "Orlistat",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 430978,
"choiceText": "Ibuprofen",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 430979,
"choiceText": "Omeprazole",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Antihistamines have antimuscarinic properties that can cause urinary retention by suppressing postjunctional excitatory muscarinic receptors (M<sub>2</sub>/M<sub>3</sub>) in the detrusor muscle.<sup type=\"ref\">[19]</sup> Herbal medications such as scopolia root should also be avoided for the same reason. The remaining drugs listed do not have antimuscarinic effects.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 127469,
"questionText": "You are examining a patient with diagnosed benign prostatic hyperplasia. Which of the following over-the-counter medications should this patient avoid?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Insidious Abdominal Symptoms With Urinary Disturbances in an Elderly Man"
},
{
"authors": "Eric Winkel, OMS-3; Mark A. Sanders, DO, JD, MPH, LLM; Anada I. Gunn-Sanders, JD, MPH, CFC, CMI-II",
"content": [
"For more information on UTO, see the Medscape Drugs & Diseases article here."
],
"date": "October 10, 2014",
"figures": [],
"markdown": "# Insidious Abdominal Symptoms With Urinary Disturbances in an Elderly Man\n\n **Authors:** Eric Winkel, OMS-3; Mark A. Sanders, DO, JD, MPH, LLM; Anada I. Gunn-Sanders, JD, MPH, CFC, CMI-II \n **Date:** October 10, 2014\n\n ## Content\n\n For more information on UTO, see the Medscape Drugs & Diseases article here.\n\n ## Figures\n\n \n*Page 5 of 5*",
"pagination": {
"current_page": 5,
"total_pages": 5
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 430970,
"choiceText": "Right-sided flank pain with 102.1°F fever",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 430971,
"choiceText": "Abdominal distention and anuria",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 430972,
"choiceText": "Hematuria and dysuria",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 430973,
"choiceText": "Polyuria and polydipsia",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 430974,
"choiceText": "Straining during urination with enlarged prostate on digital rectal examination",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "A patient with right-sided flank pain and a high-grade fever probably has pyelonephritis complicating their UTO, which usually implies a worse overall prognosis. Abdominal distention and anuria are likely caused by complete UTO, whereas polyuria and polydipsia are presumably a result of the acquired diabetes insipidus seen in partial UTO.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 127468,
"questionText": "In a patient presenting with a history and physical examination findings suggestive of UTO, which of the following combinations of signs and/or symptoms is most worrisome?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 430975,
"choiceText": "Nicotine gum",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 430976,
"choiceText": "Cetirizine",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 430977,
"choiceText": "Orlistat",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 430978,
"choiceText": "Ibuprofen",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 430979,
"choiceText": "Omeprazole",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Antihistamines have antimuscarinic properties that can cause urinary retention by suppressing postjunctional excitatory muscarinic receptors (M<sub>2</sub>/M<sub>3</sub>) in the detrusor muscle.<sup type=\"ref\">[19]</sup> Herbal medications such as scopolia root should also be avoided for the same reason. The remaining drugs listed do not have antimuscarinic effects.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 127469,
"questionText": "You are examining a patient with diagnosed benign prostatic hyperplasia. Which of the following over-the-counter medications should this patient avoid?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Insidious Abdominal Symptoms With Urinary Disturbances in an Elderly Man"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 430965,
"choiceText": "Malignant hypertension",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 430966,
"choiceText": "Obstructive uropathy",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 430967,
"choiceText": "Renal artery stenosis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 430968,
"choiceText": "Abdominal aortic aneurysm",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 430969,
"choiceText": "Renal cell carcinoma",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 127467,
"questionText": "What is the most likely diagnosis?<br />\r\n<br />\r\n<i>Hint: Note the decreased GFR in light of the CT findings.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 430970,
"choiceText": "Right-sided flank pain with 102.1°F fever",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 430971,
"choiceText": "Abdominal distention and anuria",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 430972,
"choiceText": "Hematuria and dysuria",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 430973,
"choiceText": "Polyuria and polydipsia",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 430974,
"choiceText": "Straining during urination with enlarged prostate on digital rectal examination",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "A patient with right-sided flank pain and a high-grade fever probably has pyelonephritis complicating their UTO, which usually implies a worse overall prognosis. Abdominal distention and anuria are likely caused by complete UTO, whereas polyuria and polydipsia are presumably a result of the acquired diabetes insipidus seen in partial UTO.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 127468,
"questionText": "In a patient presenting with a history and physical examination findings suggestive of UTO, which of the following combinations of signs and/or symptoms is most worrisome?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 430975,
"choiceText": "Nicotine gum",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 430976,
"choiceText": "Cetirizine",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 430977,
"choiceText": "Orlistat",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 430978,
"choiceText": "Ibuprofen",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 430979,
"choiceText": "Omeprazole",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Antihistamines have antimuscarinic properties that can cause urinary retention by suppressing postjunctional excitatory muscarinic receptors (M<sub>2</sub>/M<sub>3</sub>) in the detrusor muscle.<sup type=\"ref\">[19]</sup> Herbal medications such as scopolia root should also be avoided for the same reason. The remaining drugs listed do not have antimuscarinic effects.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 127469,
"questionText": "You are examining a patient with diagnosed benign prostatic hyperplasia. Which of the following over-the-counter medications should this patient avoid?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
748780 | /viewarticle/748780 | [
{
"authors": "Antonio Mendoza Ladd, MD; Alexander Chun, MD; Nataliya Mar, MD",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"A 73-year-old woman is referred to the hospital for evaluation of iron-deficiency anemia and heme-positive stool. She has a history of hypertension, hypercholesterolemia, and lobular breast carcinoma for which she underwent a modified right mastectomy 3 years ago. The patient complains of generalized weakness and lack of appetite. She denies any history of shortness of breath, chest pain, nausea, vomiting, obstipation, hematemesis, hematochezia, or melena. Her medications at the time of admission include amlodipine, valsartan, and simvastatin. She reports no history of tobacco, alcohol, or illicit drug use. She is not taking any nonsteroidal anti-inflammatory drugs (NSAIDs)."
],
"date": "February 01, 2018",
"figures": [],
"markdown": "# Ominous Symptoms in an Elderly Cancer Survivor\n\n **Authors:** Antonio Mendoza Ladd, MD; Alexander Chun, MD; Nataliya Mar, MD \n **Date:** February 01, 2018\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nA 73-year-old woman is referred to the hospital for evaluation of iron-deficiency anemia and heme-positive stool. She has a history of hypertension, hypercholesterolemia, and lobular breast carcinoma for which she underwent a modified right mastectomy 3 years ago. The patient complains of generalized weakness and lack of appetite. She denies any history of shortness of breath, chest pain, nausea, vomiting, obstipation, hematemesis, hematochezia, or melena. Her medications at the time of admission include amlodipine, valsartan, and simvastatin. She reports no history of tobacco, alcohol, or illicit drug use. She is not taking any nonsteroidal anti-inflammatory drugs (NSAIDs).\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "Ominous Symptoms in an Elderly Cancer Survivor"
},
{
"authors": "Antonio Mendoza Ladd, MD; Alexander Chun, MD; Nataliya Mar, MD",
"content": [
"Upon admission, the patient appears to be a frail, pale, and malnourished elderly woman. Her vital signs are a heart rate of 106 beats/min, temperature of 98.9°F (37.2°C), blood pressure of 156/76 mm Hg, and respiratory rate of 14 breaths/min. Examination of the head and neck is normal. Pulmonary auscultation reveals normal breath sounds bilaterally. The cardiac rhythm is regular and there is a grade II/VI systolic murmur present at the base. Her abdomen is soft and without distention or tenderness. No hepatosplenomegaly is appreciated. The rectal examination is unremarkable except for brown, heme-positive stool. No edema is present in her extremities.",
"Laboratory analysis reveals a white blood cell count of 9400/mm3 (normal range, 4500-11,000/mm3), hemoglobin of 6.8 g/dL (normal range, 12.0-16.0 g/dL), mean corpuscular volume of 63 fL (normal range, 80-100 fL), ferritin of 86 ng/mL (normal range, 12-150 ng/mL), and platelets of 644,000/mm3 (normal range, 150,000-400,000/mm3). The results of the basic metabolic panel are within normal limits.",
"The patient receives 2 units of packed red blood cells and undergoes endoscopic evaluation. Esophagogastroduodenoscopy and colonoscopy fail to expose a bleeding source. Capsule endoscopy is then performed and is reported as normal. Subsequent abdominal CT scanning shows asymmetric nodular wall thickening of a short segment of the mid/distal jejunum. This loop of the jejunum is abnormally dilated, measuring up to 4 cm in diameter (Figure 1). In light of these radiologic findings, the small bowel capsule endoscopy images are reviewed again at a lower speed. Guided by the CT results, the review focuses on the jejunal images. After careful examination, a lesion is indeed seen in a location compatible with the one outlined by the CT scan (Figure 2). The patient is then referred for surgery.",
"Figure 1.",
"Figure 2."
],
"date": "February 01, 2018",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/747/382/747382-thumb1.png"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/747/382/747382-thumb2.png"
}
],
"markdown": "# Ominous Symptoms in an Elderly Cancer Survivor\n\n **Authors:** Antonio Mendoza Ladd, MD; Alexander Chun, MD; Nataliya Mar, MD \n **Date:** February 01, 2018\n\n ## Content\n\n Upon admission, the patient appears to be a frail, pale, and malnourished elderly woman. Her vital signs are a heart rate of 106 beats/min, temperature of 98.9°F (37.2°C), blood pressure of 156/76 mm Hg, and respiratory rate of 14 breaths/min. Examination of the head and neck is normal. Pulmonary auscultation reveals normal breath sounds bilaterally. The cardiac rhythm is regular and there is a grade II/VI systolic murmur present at the base. Her abdomen is soft and without distention or tenderness. No hepatosplenomegaly is appreciated. The rectal examination is unremarkable except for brown, heme-positive stool. No edema is present in her extremities.\nLaboratory analysis reveals a white blood cell count of 9400/mm3 (normal range, 4500-11,000/mm3), hemoglobin of 6.8 g/dL (normal range, 12.0-16.0 g/dL), mean corpuscular volume of 63 fL (normal range, 80-100 fL), ferritin of 86 ng/mL (normal range, 12-150 ng/mL), and platelets of 644,000/mm3 (normal range, 150,000-400,000/mm3). The results of the basic metabolic panel are within normal limits.\nThe patient receives 2 units of packed red blood cells and undergoes endoscopic evaluation. Esophagogastroduodenoscopy and colonoscopy fail to expose a bleeding source. Capsule endoscopy is then performed and is reported as normal. Subsequent abdominal CT scanning shows asymmetric nodular wall thickening of a short segment of the mid/distal jejunum. This loop of the jejunum is abnormally dilated, measuring up to 4 cm in diameter (Figure 1). In light of these radiologic findings, the small bowel capsule endoscopy images are reviewed again at a lower speed. Guided by the CT results, the review focuses on the jejunal images. After careful examination, a lesion is indeed seen in a location compatible with the one outlined by the CT scan (Figure 2). The patient is then referred for surgery.\nFigure 1.\nFigure 2.\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n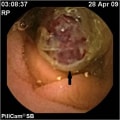 \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 424598,
"choiceText": "Peptic ulcer disease",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 424599,
"choiceText": "Arteriovenous malformation",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 424600,
"choiceText": "Small bowel tumor",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 424601,
"choiceText": "Meckel diverticulum",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 424602,
"choiceText": "Gastritis",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 125316,
"questionText": "What is the most likely etiology of the patient's gastrointestinal bleed?<br />\r\n<br />\r\n<i>Hint:</i> Note the patient's medical history.",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Ominous Symptoms in an Elderly Cancer Survivor"
},
{
"authors": "Antonio Mendoza Ladd, MD; Alexander Chun, MD; Nataliya Mar, MD",
"content": [
"The patient in this case presented with iron-deficiency anemia. This is the most likely explanation for her generalized weakness and malaise. On examination, the patient had heme-positive stool, thereby indicating the gastrointestinal (GI) tract as the likely source of her problem. When the initial colonoscopy and esophagogastroduodenoscopy failed to reveal a source of bleeding, a diagnosis of occult GI bleed was made and attention was directed to the small bowel. Although the small bowel capsule endoscopy initially was reported as normal, cross-sectional imaging identified an asymmetric nodular wall thickening of a short segment of jejunum. It also identified a 0.8-cm, low-attenuation lesion in the middle portion of the right kidney, along with bilateral adrenal nodules.",
"Subsequent review of the prior small bowel endoscopy video confirmed the jejunal lesion. The patient was then referred for surgery. Intraoperatively, a 6-cm exophytic ulcerated mass occupying 90% of the jejunal lumen was identified and resected. Microscopic examination revealed 2 morphologically and immunophenotypically distinct neoplasms. The main tumor was a partially obstructing mass that extended from the mucosa to the subserosa. The other neoplasm consisted of cell cluster nests located within the subserosal soft tissue. The final pathologic diagnosis was high-grade undifferentiated carcinoma of renal origin for the main obstructing mass and breast adenocarcinoma with ductal differentiation for the cell clusters found in the subserosa (Figures 3, 4, and 5).",
"Figure 3.",
"Figure 4.",
"Figure 5.",
"The characteristics of this patient's tumor are compatible with a synchronous tumor in the small bowel. This lesion was composed of both renal cell and ductal breast carcinomas. Of note, the breast component of this patient's small bowel mass was histologically different from her previous lobular carcinoma, leading to the hypothesis that this tumor arose from a silent ductal breast neoplasm. Meanwhile, although the lesion found on CT scan of the right kidney was small, the origin of the main obstructing component of the mass could not be anything other than the kidney; however, the exact mechanisms that led both neoplasms to metastasize simultaneously to the same unusual site remain unclear."
],
"date": "February 01, 2018",
"figures": [
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/747/382/747382-thumb3.png"
},
{
"caption": "Figure 4.",
"image_url": "https://img.medscapestatic.com/article/747/382/747382-thumb4.png"
},
{
"caption": "Figure 5.",
"image_url": "https://img.medscapestatic.com/article/747/382/747382-thumb5.png"
}
],
"markdown": "# Ominous Symptoms in an Elderly Cancer Survivor\n\n **Authors:** Antonio Mendoza Ladd, MD; Alexander Chun, MD; Nataliya Mar, MD \n **Date:** February 01, 2018\n\n ## Content\n\n The patient in this case presented with iron-deficiency anemia. This is the most likely explanation for her generalized weakness and malaise. On examination, the patient had heme-positive stool, thereby indicating the gastrointestinal (GI) tract as the likely source of her problem. When the initial colonoscopy and esophagogastroduodenoscopy failed to reveal a source of bleeding, a diagnosis of occult GI bleed was made and attention was directed to the small bowel. Although the small bowel capsule endoscopy initially was reported as normal, cross-sectional imaging identified an asymmetric nodular wall thickening of a short segment of jejunum. It also identified a 0.8-cm, low-attenuation lesion in the middle portion of the right kidney, along with bilateral adrenal nodules.\nSubsequent review of the prior small bowel endoscopy video confirmed the jejunal lesion. The patient was then referred for surgery. Intraoperatively, a 6-cm exophytic ulcerated mass occupying 90% of the jejunal lumen was identified and resected. Microscopic examination revealed 2 morphologically and immunophenotypically distinct neoplasms. The main tumor was a partially obstructing mass that extended from the mucosa to the subserosa. The other neoplasm consisted of cell cluster nests located within the subserosal soft tissue. The final pathologic diagnosis was high-grade undifferentiated carcinoma of renal origin for the main obstructing mass and breast adenocarcinoma with ductal differentiation for the cell clusters found in the subserosa (Figures 3, 4, and 5).\nFigure 3.\nFigure 4.\nFigure 5.\nThe characteristics of this patient's tumor are compatible with a synchronous tumor in the small bowel. This lesion was composed of both renal cell and ductal breast carcinomas. Of note, the breast component of this patient's small bowel mass was histologically different from her previous lobular carcinoma, leading to the hypothesis that this tumor arose from a silent ductal breast neoplasm. Meanwhile, although the lesion found on CT scan of the right kidney was small, the origin of the main obstructing component of the mass could not be anything other than the kidney; however, the exact mechanisms that led both neoplasms to metastasize simultaneously to the same unusual site remain unclear.\n\n ## Figures\n\n **Figure 3.** \n \n\n**Figure 4.** \n \n\n**Figure 5.** \n \n\n\n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 424598,
"choiceText": "Peptic ulcer disease",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 424599,
"choiceText": "Arteriovenous malformation",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 424600,
"choiceText": "Small bowel tumor",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 424601,
"choiceText": "Meckel diverticulum",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 424602,
"choiceText": "Gastritis",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 125316,
"questionText": "What is the most likely etiology of the patient's gastrointestinal bleed?<br />\r\n<br />\r\n<i>Hint:</i> Note the patient's medical history.",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Ominous Symptoms in an Elderly Cancer Survivor"
},
{
"authors": "Antonio Mendoza Ladd, MD; Alexander Chun, MD; Nataliya Mar, MD",
"content": [
"Both primary and metastatic tumors can affect the small bowel. The incidence of small bowel tumors appears to be increasing, with most recent research estimating it to be 14.8 per 100,000 population.[1] Primary malignant small bowel tumors commonly include, in order of frequency, adenocarcinomas, carcinoids, and gastrointestinal stromal tumors (GISTs).[2] Adenocarcinomas account for up to 40% of diagnosed primary small bowel tumors. These tumors are usually found in the duodenum, but an exception to this rule is seen in patients with Crohn disease.[3] In these patients, small bowel adenocarcinomas arise in the ileum, the primary site of the inflammatory process.[4]",
"Carcinoid tumors account for 29%-40% of primary small bowel tumors, and their incidence appears to be rising within the United States.[5] In contrast to adenocarcinomas, the majority of these tumors occur in the ileum, within 60 cm of the ileocecal valve.[6] Size is a critical prognostic factor in carcinoid tumors. Lesions < 1 cm have an approximate 15% risk for metastatic disease at the time of diagnosis; for tumors > 2 cm, the risk for metastasis reaches 50%.",
"When carcinoid tumors metastasize, the most common targets are the liver, lungs, and bones.[7] GISTs mainly affect the distal jejunum and the ileum, with 25%-30% of all GISTs occurring in these locations.[8] The clinical behavior of these tumors is highly variable, but currently all GISTs are regarded as potentially malignant tumors. The degree of aggressiveness for a GIST is determined by 2 main criteria: size and mitotic count per high-power field (hpf). In general, lesions > 5 cm with a mitotic count > 5 per 50 hpf are considered high-risk lesions.[9]",
"Tumors that commonly metastasize to the small bowel include melanomas and breast, lung, and cervical adenocarcinomas.[10] The small intestine is the most frequent site of metastatic melanoma in the GI tract because of its rich blood supply. Metastatic lesions have been reported up to 21 years after apparently definitive treatment of the primary tumor.[11,12] In contrast, the true incidence of breast and lung cancer metastases to the gastrointestinal tract is unclear. In a large series by McLemore and colleagues,[13] breast cancer was found to metastasize to the small bowel in < 1% of cases. In such cases, the most commonly affected portion was the duodenum.[13,14,15] The type of breast cancer seems to play an important role in this phenomenon. The metastatic patterns of lobular vs ductal carcinoma have been reported to differ considerably, with most series reporting a greater propensity for lobular carcinoma to metastasize to the GI tract and gynecologic organs.[16,17,18] The literature concerning the clinical presentation and outcome of patients with lung carcinoma metastases to the small bowel consists of individual case reports or small series. Very few reports of renal cell cancer metastasizing to the small bowel have been published.[19,20] Metastasis from renal cell carcinoma causing small bowel tumor seems to be relatively rare; in a 2006 case report of a small bowel metastasis from renal cell carcinoma leading to intussusceptions, the authors discovered only 20 other case reports of renal cell carcinoma metastases to the small bowel.[21]",
"Rarely, metastatic lesions are composed of more than 1 kind of malignant cells, giving rise to synchronous tumors. The term \"synchronous tumor\" refers to the simultaneous presence of 2 independent and morphologically distinct neoplasms at the same site. Such tumors may be adjacent to each other, with an area of co-invasion at the site of contact, in which case they are called collision tumors.[22] Cells within such tumors are positioned side by side, but they are not intermixed outside the small area of contact.[23] Another type of synchronous tumor is represented by a phenomenon known as tumor-to-tumor metastasis. In such cases, one neoplasm acts as a cell \"donor\" and the other as a \"recipient.\"[24] Common \"donors\" include carcinomas of the lung followed by breast, GI tract, prostate, and thyroid.[25]",
"Although several recent reports show renal cell carcinomas metastatic to various other tumors, such as thyroid tumors,[26] pleural tumors,[27] and pancreatic endocrine neoplasm,[28] renal cell carcinoma is reported to be the most common recipient, most often from primary lung cancer.[28,29] In such cases, the metastatic neoplasm has to demonstrate real growth within the host tumor and not contiguous invasion, collision, or tumor emboli. In patients with synchronous tumors of multiple organs, the diagnosis of a genetic syndrome, such as multiple endocrine neoplasm, hereditary nonpolyposis colon cancer, or familial adenomatous polyposis, should be considered.",
"Regardless of their origin, small bowel tumors may present as occult GI bleeds. They are frequently difficult to assess with traditional endoscopic procedures and tend to be diagnosed only after extensive investigation. The role of capsule endoscopy in the diagnosis of small bowel tumors causing occult GI bleed has been reported.[30,31,32] The data show that as few as 8%-9% of occult GI bleed cases with small bowel tumors are diagnosed with capsule endoscopy.[33] Capsule endoscopy has inherent clinical and technical limitations, such as incomplete small bowel examination and gaps in the recording of the images.[34,35] Evidence also suggests that capsule endoscopy can miss such tumors as adenocarcinomas and GISTs.[36,37,38] In patients with a nondiagnostic test, a \"second-look\" capsule endoscopy may detect lesions in as many as 48% of cases, which raises concern as to which is the best diagnostic test following a negative capsule endoscopy.[39] This issue is a matter of ongoing debate and has led clinicians to pursue various alternative imaging modalities.",
"Modern technology has improved the accuracy of cross-sectional imaging in the evaluation of the small bowel. Computed tomographic enterography (CTE) is one of the emerging modalities gaining favor in the evaluation of occult GI bleed. This technique uses multidetector CT scanning combined with luminal distention of the small bowel with neutral oral contrast material. The ability of this method to exquisitely depict bowel wall abnormalities, as well as extraintestinal pathology, has made it an important tool in the evaluation of small bowel disease.[40,41,42] Preliminary data show that CTE may identify small bowel tumors missed by capsule endoscopy, but no trials have been conducted to compare the detection rates for both modalities in occult gastrointestinal bleed.",
"The quality of CTE images and their ability to diagnose luminal pathology depend greatly on the CT scanner used, the usage of the appropriate protocol, and the experience of the radiologist. Similarly, a gastroenterologist who takes extra time to read a capsule study is likely to have a better yield. MR enterography may be another option available in some centers. In addition, single- or double-balloon enteroscopy should be arranged in order to obtain a biopsy diagnosis prior to surgery."
],
"date": "February 01, 2018",
"figures": [],
"markdown": "# Ominous Symptoms in an Elderly Cancer Survivor\n\n **Authors:** Antonio Mendoza Ladd, MD; Alexander Chun, MD; Nataliya Mar, MD \n **Date:** February 01, 2018\n\n ## Content\n\n Both primary and metastatic tumors can affect the small bowel. The incidence of small bowel tumors appears to be increasing, with most recent research estimating it to be 14.8 per 100,000 population.[1] Primary malignant small bowel tumors commonly include, in order of frequency, adenocarcinomas, carcinoids, and gastrointestinal stromal tumors (GISTs).[2] Adenocarcinomas account for up to 40% of diagnosed primary small bowel tumors. These tumors are usually found in the duodenum, but an exception to this rule is seen in patients with Crohn disease.[3] In these patients, small bowel adenocarcinomas arise in the ileum, the primary site of the inflammatory process.[4]\nCarcinoid tumors account for 29%-40% of primary small bowel tumors, and their incidence appears to be rising within the United States.[5] In contrast to adenocarcinomas, the majority of these tumors occur in the ileum, within 60 cm of the ileocecal valve.[6] Size is a critical prognostic factor in carcinoid tumors. Lesions < 1 cm have an approximate 15% risk for metastatic disease at the time of diagnosis; for tumors > 2 cm, the risk for metastasis reaches 50%.\nWhen carcinoid tumors metastasize, the most common targets are the liver, lungs, and bones.[7] GISTs mainly affect the distal jejunum and the ileum, with 25%-30% of all GISTs occurring in these locations.[8] The clinical behavior of these tumors is highly variable, but currently all GISTs are regarded as potentially malignant tumors. The degree of aggressiveness for a GIST is determined by 2 main criteria: size and mitotic count per high-power field (hpf). In general, lesions > 5 cm with a mitotic count > 5 per 50 hpf are considered high-risk lesions.[9]\nTumors that commonly metastasize to the small bowel include melanomas and breast, lung, and cervical adenocarcinomas.[10] The small intestine is the most frequent site of metastatic melanoma in the GI tract because of its rich blood supply. Metastatic lesions have been reported up to 21 years after apparently definitive treatment of the primary tumor.[11,12] In contrast, the true incidence of breast and lung cancer metastases to the gastrointestinal tract is unclear. In a large series by McLemore and colleagues,[13] breast cancer was found to metastasize to the small bowel in < 1% of cases. In such cases, the most commonly affected portion was the duodenum.[13,14,15] The type of breast cancer seems to play an important role in this phenomenon. The metastatic patterns of lobular vs ductal carcinoma have been reported to differ considerably, with most series reporting a greater propensity for lobular carcinoma to metastasize to the GI tract and gynecologic organs.[16,17,18] The literature concerning the clinical presentation and outcome of patients with lung carcinoma metastases to the small bowel consists of individual case reports or small series. Very few reports of renal cell cancer metastasizing to the small bowel have been published.[19,20] Metastasis from renal cell carcinoma causing small bowel tumor seems to be relatively rare; in a 2006 case report of a small bowel metastasis from renal cell carcinoma leading to intussusceptions, the authors discovered only 20 other case reports of renal cell carcinoma metastases to the small bowel.[21]\nRarely, metastatic lesions are composed of more than 1 kind of malignant cells, giving rise to synchronous tumors. The term \"synchronous tumor\" refers to the simultaneous presence of 2 independent and morphologically distinct neoplasms at the same site. Such tumors may be adjacent to each other, with an area of co-invasion at the site of contact, in which case they are called collision tumors.[22] Cells within such tumors are positioned side by side, but they are not intermixed outside the small area of contact.[23] Another type of synchronous tumor is represented by a phenomenon known as tumor-to-tumor metastasis. In such cases, one neoplasm acts as a cell \"donor\" and the other as a \"recipient.\"[24] Common \"donors\" include carcinomas of the lung followed by breast, GI tract, prostate, and thyroid.[25]\nAlthough several recent reports show renal cell carcinomas metastatic to various other tumors, such as thyroid tumors,[26] pleural tumors,[27] and pancreatic endocrine neoplasm,[28] renal cell carcinoma is reported to be the most common recipient, most often from primary lung cancer.[28,29] In such cases, the metastatic neoplasm has to demonstrate real growth within the host tumor and not contiguous invasion, collision, or tumor emboli. In patients with synchronous tumors of multiple organs, the diagnosis of a genetic syndrome, such as multiple endocrine neoplasm, hereditary nonpolyposis colon cancer, or familial adenomatous polyposis, should be considered.\nRegardless of their origin, small bowel tumors may present as occult GI bleeds. They are frequently difficult to assess with traditional endoscopic procedures and tend to be diagnosed only after extensive investigation. The role of capsule endoscopy in the diagnosis of small bowel tumors causing occult GI bleed has been reported.[30,31,32] The data show that as few as 8%-9% of occult GI bleed cases with small bowel tumors are diagnosed with capsule endoscopy.[33] Capsule endoscopy has inherent clinical and technical limitations, such as incomplete small bowel examination and gaps in the recording of the images.[34,35] Evidence also suggests that capsule endoscopy can miss such tumors as adenocarcinomas and GISTs.[36,37,38] In patients with a nondiagnostic test, a \"second-look\" capsule endoscopy may detect lesions in as many as 48% of cases, which raises concern as to which is the best diagnostic test following a negative capsule endoscopy.[39] This issue is a matter of ongoing debate and has led clinicians to pursue various alternative imaging modalities.\nModern technology has improved the accuracy of cross-sectional imaging in the evaluation of the small bowel. Computed tomographic enterography (CTE) is one of the emerging modalities gaining favor in the evaluation of occult GI bleed. This technique uses multidetector CT scanning combined with luminal distention of the small bowel with neutral oral contrast material. The ability of this method to exquisitely depict bowel wall abnormalities, as well as extraintestinal pathology, has made it an important tool in the evaluation of small bowel disease.[40,41,42] Preliminary data show that CTE may identify small bowel tumors missed by capsule endoscopy, but no trials have been conducted to compare the detection rates for both modalities in occult gastrointestinal bleed.\nThe quality of CTE images and their ability to diagnose luminal pathology depend greatly on the CT scanner used, the usage of the appropriate protocol, and the experience of the radiologist. Similarly, a gastroenterologist who takes extra time to read a capsule study is likely to have a better yield. MR enterography may be another option available in some centers. In addition, single- or double-balloon enteroscopy should be arranged in order to obtain a biopsy diagnosis prior to surgery.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "Ominous Symptoms in an Elderly Cancer Survivor"
},
{
"authors": "Antonio Mendoza Ladd, MD; Alexander Chun, MD; Nataliya Mar, MD",
"content": [
"Currently, patients with a presentation and history much like those encountered in this case are likely to undergo a fluorodeoxyglucose (FDG) positron emission tomography (PET) scan. “Hot spots” on the image—ie, bright spots created by the absorption of large amounts of FDG by cancer cells—would indicate the presence of one or more small bowel tumors. In many cases of gastrointestinal imaging, PET and CT scanning are combined for greater diagnostic accuracy.",
"In a recent small German study, investigators suggested that in the rare scenario that endoscopy is unable to provide appropriate information, ultrasound-guided percutaneous needle biopsy is a safe and effective diagnostic tool for evaluating suspicious GI tract tumors that have been revealed by abdominal sonograms but for which endoscopy is either negative or incomplete. [43] The sensitivity is reported to be from 92%-96% with 100% specificity with a complication rate <1%; the investigators indicated that percutaneous ultrasound-guided biopsy may be able to help clinicians reach the diagnosis and treatment decisions for these GI tract lesions more quickly, as well as decrease the costs involved.[43]",
"After resection, the patient had an uneventful recovery. She received a total of 4 units of packed red blood cells during her 9-day stay in the hospital and was prescribed oral iron upon discharge. She returned 1 month later for follow-up, at which time her hemoglobin was 10.9 g/dL and no further bleeding episodes were reported. She was then sent to her oncologist for follow-up appointments. To our knowledge, this is the first reported case of a synchronous metastatic tumor of the small bowel composed of both renal cell and ductal breast carcinomas. Although rare, metastatic tumors should be considered in the differential diagnosis of occult gastrointestinal bleed in patients with a history of cancer."
],
"date": "February 01, 2018",
"figures": [],
"markdown": "# Ominous Symptoms in an Elderly Cancer Survivor\n\n **Authors:** Antonio Mendoza Ladd, MD; Alexander Chun, MD; Nataliya Mar, MD \n **Date:** February 01, 2018\n\n ## Content\n\n Currently, patients with a presentation and history much like those encountered in this case are likely to undergo a fluorodeoxyglucose (FDG) positron emission tomography (PET) scan. “Hot spots” on the image—ie, bright spots created by the absorption of large amounts of FDG by cancer cells—would indicate the presence of one or more small bowel tumors. In many cases of gastrointestinal imaging, PET and CT scanning are combined for greater diagnostic accuracy.\nIn a recent small German study, investigators suggested that in the rare scenario that endoscopy is unable to provide appropriate information, ultrasound-guided percutaneous needle biopsy is a safe and effective diagnostic tool for evaluating suspicious GI tract tumors that have been revealed by abdominal sonograms but for which endoscopy is either negative or incomplete. [43] The sensitivity is reported to be from 92%-96% with 100% specificity with a complication rate <1%; the investigators indicated that percutaneous ultrasound-guided biopsy may be able to help clinicians reach the diagnosis and treatment decisions for these GI tract lesions more quickly, as well as decrease the costs involved.[43]\nAfter resection, the patient had an uneventful recovery. She received a total of 4 units of packed red blood cells during her 9-day stay in the hospital and was prescribed oral iron upon discharge. She returned 1 month later for follow-up, at which time her hemoglobin was 10.9 g/dL and no further bleeding episodes were reported. She was then sent to her oncologist for follow-up appointments. To our knowledge, this is the first reported case of a synchronous metastatic tumor of the small bowel composed of both renal cell and ductal breast carcinomas. Although rare, metastatic tumors should be considered in the differential diagnosis of occult gastrointestinal bleed in patients with a history of cancer.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 424615,
"choiceText": "They are easily detected using standard endoscopic techniques",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 424616,
"choiceText": "Capsule endoscopy has a high degree of sensitivity for ruling them out",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 424617,
"choiceText": "Metastases from the brain are a common cause",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 424618,
"choiceText": "Adenocarcinomas are usually found in the ileum",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 424654,
"choiceText": "Lobular breast carcinomas are more likely to cause them than ductal breast carcinomas",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Studies have shown that lobular carcinoma of the breast has a higher likelihood to metastasize to the GI tract than ductal breast carcinoma. It is interesting to note that the patient in the above case was an exception to this finding. Despite having a history of lobular breast carcinoma, a ductal breast carcinoma metastasis was present in the small intestine. Evidence suggests that following a negative capsule endoscopy, a repeat study may detect lesions in as many as 48% of patients, thereby making it a poor technique to rule out small bowel tumors if used in isolation. Adenocarcinomas of the small intestine usually arise in the duodenum, except in cases of Crohn disease, in which the ileum is the most common site.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 125320,
"questionText": "You discover a small bowel tumor in a woman who has a history of breast cancer. Which of the following statements regarding tumors of the small bowel is most accurate?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 424619,
"choiceText": "Repeat esophagogastroduodenoscopy and colonoscopy",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 424620,
"choiceText": "Repeat capsule endoscopy",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 424621,
"choiceText": "MR enterography",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 424622,
"choiceText": "CT enterograph",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 424623,
"choiceText": "Single- or double-balloon enteroscopy",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 424629,
"choiceText": "Intraoperative enteroscopy",
"correct": false,
"displayOrder": 6,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 424630,
"choiceText": "Proceed conservatively with continued observation without any intervention",
"correct": false,
"displayOrder": 7,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 424631,
"choiceText": "Any of the above except proceed conservatively with continued observation without any intervention",
"correct": true,
"displayOrder": 8,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Again, the message here is that a single capsule study of the small intestine has poor sensitivity, and some significant lesions could be missed. Of note, a significant number of gastric or colonic lesions are detected on repeat endoscopy or enteroscopy. The examination of choice may depend on local expertise and accessibility. Such patients should be referred to tertiary care centers.<br />\r\n<br />\r\nCT enterography is a relatively new imaging modality that is becoming an important investigation for cases of occult gastrointestinal bleed. Observation alone is unacceptable because the source of the bleeding, once identified, may likely require further intervention to prevent progression of symptoms.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 125321,
"questionText": "Your patient presents with an occult GI bleed with severe anemia, a heme-positive stool test, and negative esophagogastroduodenoscopy, colonoscopy, and capsule endoscopy studies. What is the next most appropriate diagnostic step?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Ominous Symptoms in an Elderly Cancer Survivor"
},
{
"authors": "Antonio Mendoza Ladd, MD; Alexander Chun, MD; Nataliya Mar, MD",
"content": [],
"date": "February 01, 2018",
"figures": [],
"markdown": "# Ominous Symptoms in an Elderly Cancer Survivor\n\n **Authors:** Antonio Mendoza Ladd, MD; Alexander Chun, MD; Nataliya Mar, MD \n **Date:** February 01, 2018\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 424615,
"choiceText": "They are easily detected using standard endoscopic techniques",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 424616,
"choiceText": "Capsule endoscopy has a high degree of sensitivity for ruling them out",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 424617,
"choiceText": "Metastases from the brain are a common cause",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 424618,
"choiceText": "Adenocarcinomas are usually found in the ileum",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 424654,
"choiceText": "Lobular breast carcinomas are more likely to cause them than ductal breast carcinomas",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Studies have shown that lobular carcinoma of the breast has a higher likelihood to metastasize to the GI tract than ductal breast carcinoma. It is interesting to note that the patient in the above case was an exception to this finding. Despite having a history of lobular breast carcinoma, a ductal breast carcinoma metastasis was present in the small intestine. Evidence suggests that following a negative capsule endoscopy, a repeat study may detect lesions in as many as 48% of patients, thereby making it a poor technique to rule out small bowel tumors if used in isolation. Adenocarcinomas of the small intestine usually arise in the duodenum, except in cases of Crohn disease, in which the ileum is the most common site.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 125320,
"questionText": "You discover a small bowel tumor in a woman who has a history of breast cancer. Which of the following statements regarding tumors of the small bowel is most accurate?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 424619,
"choiceText": "Repeat esophagogastroduodenoscopy and colonoscopy",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 424620,
"choiceText": "Repeat capsule endoscopy",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 424621,
"choiceText": "MR enterography",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 424622,
"choiceText": "CT enterograph",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 424623,
"choiceText": "Single- or double-balloon enteroscopy",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 424629,
"choiceText": "Intraoperative enteroscopy",
"correct": false,
"displayOrder": 6,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 424630,
"choiceText": "Proceed conservatively with continued observation without any intervention",
"correct": false,
"displayOrder": 7,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 424631,
"choiceText": "Any of the above except proceed conservatively with continued observation without any intervention",
"correct": true,
"displayOrder": 8,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Again, the message here is that a single capsule study of the small intestine has poor sensitivity, and some significant lesions could be missed. Of note, a significant number of gastric or colonic lesions are detected on repeat endoscopy or enteroscopy. The examination of choice may depend on local expertise and accessibility. Such patients should be referred to tertiary care centers.<br />\r\n<br />\r\nCT enterography is a relatively new imaging modality that is becoming an important investigation for cases of occult gastrointestinal bleed. Observation alone is unacceptable because the source of the bleeding, once identified, may likely require further intervention to prevent progression of symptoms.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 125321,
"questionText": "Your patient presents with an occult GI bleed with severe anemia, a heme-positive stool test, and negative esophagogastroduodenoscopy, colonoscopy, and capsule endoscopy studies. What is the next most appropriate diagnostic step?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Ominous Symptoms in an Elderly Cancer Survivor"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 424598,
"choiceText": "Peptic ulcer disease",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 424599,
"choiceText": "Arteriovenous malformation",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 424600,
"choiceText": "Small bowel tumor",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 424601,
"choiceText": "Meckel diverticulum",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 424602,
"choiceText": "Gastritis",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 125316,
"questionText": "What is the most likely etiology of the patient's gastrointestinal bleed?<br />\r\n<br />\r\n<i>Hint:</i> Note the patient's medical history.",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 424615,
"choiceText": "They are easily detected using standard endoscopic techniques",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 424616,
"choiceText": "Capsule endoscopy has a high degree of sensitivity for ruling them out",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 424617,
"choiceText": "Metastases from the brain are a common cause",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 424618,
"choiceText": "Adenocarcinomas are usually found in the ileum",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 424654,
"choiceText": "Lobular breast carcinomas are more likely to cause them than ductal breast carcinomas",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Studies have shown that lobular carcinoma of the breast has a higher likelihood to metastasize to the GI tract than ductal breast carcinoma. It is interesting to note that the patient in the above case was an exception to this finding. Despite having a history of lobular breast carcinoma, a ductal breast carcinoma metastasis was present in the small intestine. Evidence suggests that following a negative capsule endoscopy, a repeat study may detect lesions in as many as 48% of patients, thereby making it a poor technique to rule out small bowel tumors if used in isolation. Adenocarcinomas of the small intestine usually arise in the duodenum, except in cases of Crohn disease, in which the ileum is the most common site.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 125320,
"questionText": "You discover a small bowel tumor in a woman who has a history of breast cancer. Which of the following statements regarding tumors of the small bowel is most accurate?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 424619,
"choiceText": "Repeat esophagogastroduodenoscopy and colonoscopy",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 424620,
"choiceText": "Repeat capsule endoscopy",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 424621,
"choiceText": "MR enterography",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 424622,
"choiceText": "CT enterograph",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 424623,
"choiceText": "Single- or double-balloon enteroscopy",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 424629,
"choiceText": "Intraoperative enteroscopy",
"correct": false,
"displayOrder": 6,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 424630,
"choiceText": "Proceed conservatively with continued observation without any intervention",
"correct": false,
"displayOrder": 7,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 424631,
"choiceText": "Any of the above except proceed conservatively with continued observation without any intervention",
"correct": true,
"displayOrder": 8,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Again, the message here is that a single capsule study of the small intestine has poor sensitivity, and some significant lesions could be missed. Of note, a significant number of gastric or colonic lesions are detected on repeat endoscopy or enteroscopy. The examination of choice may depend on local expertise and accessibility. Such patients should be referred to tertiary care centers.<br />\r\n<br />\r\nCT enterography is a relatively new imaging modality that is becoming an important investigation for cases of occult gastrointestinal bleed. Observation alone is unacceptable because the source of the bleeding, once identified, may likely require further intervention to prevent progression of symptoms.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 125321,
"questionText": "Your patient presents with an occult GI bleed with severe anemia, a heme-positive stool test, and negative esophagogastroduodenoscopy, colonoscopy, and capsule endoscopy studies. What is the next most appropriate diagnostic step?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
748055 | /viewarticle/748055 | [
{
"authors": "Matthew T. Houdek; Elizabeth A. Lindgren; Conrad J. Clemens, MD, MPH",
"content": [
"Editor's Note:\n\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.",
"An 8-year-old boy presents to a tertiary care facility with a 1-day history of right knee pain. The patient's mother states that a week ago he experienced symptoms of an upper respiratory tract infection associated with low-grade fever; however, these symptoms have since resolved. According to the mother, the boy's knee pain started the previous night, and because he was playing with his friends outside, she thought that it was \"growing pains.\" When he awoke early this morning, he was unable to bear weight on his right lower extremity. She reports swelling and extreme pain in his knee, along with a fever of 103°F (39.4°C).",
"Due to his symptoms, his mother took him to see his primary care physician where he was noted to have a temperature of 101.4°F (38.6°C). Due to his fever, knee pain, and inability to bear weight on his right lower extremity, he was sent to this facility for further evaluation and management."
],
"date": "March 02, 2016",
"figures": [],
"markdown": "# An Unexpected Cause of Severe Knee Pain in an 8-Year-Old\n\n **Authors:** Matthew T. Houdek; Elizabeth A. Lindgren; Conrad J. Clemens, MD, MPH \n **Date:** March 02, 2016\n\n ## Content\n\n Editor's Note:\n\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.\nAn 8-year-old boy presents to a tertiary care facility with a 1-day history of right knee pain. The patient's mother states that a week ago he experienced symptoms of an upper respiratory tract infection associated with low-grade fever; however, these symptoms have since resolved. According to the mother, the boy's knee pain started the previous night, and because he was playing with his friends outside, she thought that it was \"growing pains.\" When he awoke early this morning, he was unable to bear weight on his right lower extremity. She reports swelling and extreme pain in his knee, along with a fever of 103°F (39.4°C).\nDue to his symptoms, his mother took him to see his primary care physician where he was noted to have a temperature of 101.4°F (38.6°C). Due to his fever, knee pain, and inability to bear weight on his right lower extremity, he was sent to this facility for further evaluation and management.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "An Unexpected Cause of Severe Knee Pain in an 8-Year-Old"
},
{
"authors": "Matthew T. Houdek; Elizabeth A. Lindgren; Conrad J. Clemens, MD, MPH",
"content": [
"On presentation, the patient is anxious and refuses to bear weight on his right leg. He describes a sharp pain, rated as 10 out of 10, distributed over the anterior and posterior aspects of his right knee. On physical examination, his temperature is 101°F (38.3°C), heart rate is 122 beats/min, and blood pressure is 106/58 mm Hg. The right knee is painful to palpation, and it is kept in a flexed position with his right hip externally rotated. There is a mild effusion without overlying erythema; however, the skin over the knee is warm to the touch. There is pain with both flexion and extension of his right knee. The patient is able to achieve full extension; however, flexion is limited to 30° due to pain. Motion of his right hip and ankle does not elicit pain.",
"Figure 1.",
"Figure 2.",
"Laboratory examination reveals a white blood cell count of 13.1 × 103/mm3 (normal range, 4.5-11 × 103/mm3), C-reactive protein of 4.1 mg/L (normal range, 0-4 mg/L), and an erythrocyte sedimentation rate of 56 mm/hr (normal range, 0-15 mm/hr). An ultrasound of his right knee shows a small amount of fluid in the suprapatellar bursa but no fluid in the popliteal fossa. Aspiration of the right knee is unsuccessful and no joint fluid is obtained. At this point, an MR scan of his right knee with gadolinium contrast is ordered (Figures 1 and 2) and he is started empirically on intravenous (IV) clindamycin."
],
"date": "March 02, 2016",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/746/515/746515-thumb1.png"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/746/515/746515-thumb2.png"
}
],
"markdown": "# An Unexpected Cause of Severe Knee Pain in an 8-Year-Old\n\n **Authors:** Matthew T. Houdek; Elizabeth A. Lindgren; Conrad J. Clemens, MD, MPH \n **Date:** March 02, 2016\n\n ## Content\n\n On presentation, the patient is anxious and refuses to bear weight on his right leg. He describes a sharp pain, rated as 10 out of 10, distributed over the anterior and posterior aspects of his right knee. On physical examination, his temperature is 101°F (38.3°C), heart rate is 122 beats/min, and blood pressure is 106/58 mm Hg. The right knee is painful to palpation, and it is kept in a flexed position with his right hip externally rotated. There is a mild effusion without overlying erythema; however, the skin over the knee is warm to the touch. There is pain with both flexion and extension of his right knee. The patient is able to achieve full extension; however, flexion is limited to 30° due to pain. Motion of his right hip and ankle does not elicit pain.\nFigure 1.\nFigure 2.\nLaboratory examination reveals a white blood cell count of 13.1 × 103/mm3 (normal range, 4.5-11 × 103/mm3), C-reactive protein of 4.1 mg/L (normal range, 0-4 mg/L), and an erythrocyte sedimentation rate of 56 mm/hr (normal range, 0-15 mm/hr). An ultrasound of his right knee shows a small amount of fluid in the suprapatellar bursa but no fluid in the popliteal fossa. Aspiration of the right knee is unsuccessful and no joint fluid is obtained. At this point, an MR scan of his right knee with gadolinium contrast is ordered (Figures 1 and 2) and he is started empirically on intravenous (IV) clindamycin.\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 422572,
"choiceText": "Septic arthritis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 422573,
"choiceText": "Pyomyositis",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 422574,
"choiceText": "Osteomyelitis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 422575,
"choiceText": "Myositis",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 124571,
"questionText": "On the basis of the MR images, what is the most likely diagnosis?<br />\r\n<br />\r\nHint: <i>Notice the lack of an effusion within the joint and the increased signal in the head of the gastrocnemius muscle.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "An Unexpected Cause of Severe Knee Pain in an 8-Year-Old"
},
{
"authors": "Matthew T. Houdek; Elizabeth A. Lindgren; Conrad J. Clemens, MD, MPH",
"content": [
"Figure 3.",
"Sagittal MR image of the right knee after the administration of gadolinium contrast. Synovitis along with inflammation (star) of the patient's posterior popliteal soft tissues is seen, with small areas of phlegmon and microabscesses (arrow) mainly at the origin of the lateral head of the gastrocnemius muscle.",
"Sagittal MR image of the right knee after the administration of gadolinium contrast. Synovitis along with inflammation (star) of the patient's posterior popliteal soft tissues is seen, with small areas of phlegmon and microabscesses (arrow) mainly at the origin of the lateral head of the gastrocnemius muscle.",
"Figure 4.",
"Axial MR image of the right knee after the administration of gadolinium contrast. Synovitis along with inflammation (star) of the patient's posterior popliteal soft tissues is seen, with small areas of phlegmon and microabscesses (arrow) mainly at the origin of the lateral head of the gastrocnemius muscle.",
"Axial MR image of the right knee after the administration of gadolinium contrast. Synovitis along with inflammation (star) of the patient's posterior popliteal soft tissues is seen, with small areas of phlegmon and microabscesses (arrow) mainly at the origin of the lateral head of the gastrocnemius muscle.",
"The MR scan revealed synovitis along with inflammation of the patient's posterior popliteal soft tissues, mainly at the origin of the lateral head of the gastrocnemius muscle. There were also small areas of phlegmon and microabscesses located in the lateral head of the gastrocnemius, consistent with a diagnosis of pyomyositis (Figures 3 and 4).",
"Pyomyositis is a subacute bacterial infection affecting the skeletal muscles, typically occurring with the formation of an abscess; however, cases of myonecrosis and frank sepsis have been reported.[1,2,3,4,5,6,7] The diagnosis of pyomyositis is commonly made through the use of MRI, although it can also be made through ultrasound and CT scans.[3,6] In this case, the ultrasound was negative; this examination is rarely diagnostic in the early stages of pyomyositis because, typically, there is no large fluid collection.[3]Pyomyositis presents in 1 of 3 distinct stages, for which a patient's symptoms and MRI findings are used for classification.[4,6] The first stage is known as the invasive stage.[6] This stage is marked by pain and swelling of the muscle, with minimal changes noted on the skin and in the soft tissue.[6] The suppurative stage follows, and it is linked to the formation of a deep collection of pus with marked pain, fever, and soft-tissue edema.[6] The late stage is the final stage, and it is characterized by the formation of a deep abscess along with myonecrosis and possibly septic shock.[6] On the basis of these classifications, this patient presented between the invasive and suppurative stages of infection, with only microabscesses noted but no deep abscesses that required incision and drainage.",
"After being described in Japan in 1885 by Scriba, the condition has commonly been encountered in tropical climates, giving it the name myositis tropicans or tropical pyomyositis.[3,5]Pyomyositis accounts for 4% of all hospital admissions in tropical countries; however, in temperate climates it occurs in 1 in 3000 pediatric admissions.[3,4] Cases of pyomyositis have increased over the past 10 years in part due to the increase in methicillin-resistant \nStaphylococcus aureus (MRSA)\n, with studies showing that 90% of cases are caused by S aureus, although gram-negative organisms have also been encountered.[1,4,6] In a study by Pannaraj and colleagues, S aureus accounted for 56% of pyomyositis cases in children, with group A Streptococcus\n accounting for only 2% of infections.[8] An explanation for the recent increase in pyomyositis may be due to the virulence factors of certain strains of MRSA.[8] The USA300 strain of MRSA, which now represents > 70% of the MRSA isolates in some areas, is associated with the Panton-Valentine leukocidin (PVL) virulence factor. This factor is an exotoxin that induces apoptosis in leukocytes and is associated with skin, bone, joint, and deep soft-tissue infections and abscess formation.[8]",
"In a small report from Kuwait, of 8 children with postvaricella musculoskeletal complications, 3 children had pyomyositis[9]; the investigators also noted cellulitis in 3 children, osteomyelitis in 2 children, and gangrene in 1 child, with an average of 8.8 days between onset of the primary varicella infection and the complication. Although all of the children had negative blood cultures, of 2 children with S aureus-positive pus cultures, 1 was infected with MRSA.[9]"
],
"date": "March 02, 2016",
"figures": [
{
"caption": "Figure 3.Sagittal MR image of the right knee after the administration of gadolinium contrast. Synovitis along with inflammation (star) of the patient's posterior popliteal soft tissues is seen, with small areas of phlegmon and microabscesses (arrow) mainly at the origin of the lateral head of the gastrocnemius muscle.",
"image_url": "https://img.medscapestatic.com/article/746/515/746515-thumb3.png"
},
{
"caption": "Figure 4.Axial MR image of the right knee after the administration of gadolinium contrast. Synovitis along with inflammation (star) of the patient's posterior popliteal soft tissues is seen, with small areas of phlegmon and microabscesses (arrow) mainly at the origin of the lateral head of the gastrocnemius muscle.",
"image_url": "https://img.medscapestatic.com/article/746/515/746515-thumb4.png"
}
],
"markdown": "# An Unexpected Cause of Severe Knee Pain in an 8-Year-Old\n\n **Authors:** Matthew T. Houdek; Elizabeth A. Lindgren; Conrad J. Clemens, MD, MPH \n **Date:** March 02, 2016\n\n ## Content\n\n Figure 3.\nSagittal MR image of the right knee after the administration of gadolinium contrast. Synovitis along with inflammation (star) of the patient's posterior popliteal soft tissues is seen, with small areas of phlegmon and microabscesses (arrow) mainly at the origin of the lateral head of the gastrocnemius muscle.\nSagittal MR image of the right knee after the administration of gadolinium contrast. Synovitis along with inflammation (star) of the patient's posterior popliteal soft tissues is seen, with small areas of phlegmon and microabscesses (arrow) mainly at the origin of the lateral head of the gastrocnemius muscle.\nFigure 4.\nAxial MR image of the right knee after the administration of gadolinium contrast. Synovitis along with inflammation (star) of the patient's posterior popliteal soft tissues is seen, with small areas of phlegmon and microabscesses (arrow) mainly at the origin of the lateral head of the gastrocnemius muscle.\nAxial MR image of the right knee after the administration of gadolinium contrast. Synovitis along with inflammation (star) of the patient's posterior popliteal soft tissues is seen, with small areas of phlegmon and microabscesses (arrow) mainly at the origin of the lateral head of the gastrocnemius muscle.\nThe MR scan revealed synovitis along with inflammation of the patient's posterior popliteal soft tissues, mainly at the origin of the lateral head of the gastrocnemius muscle. There were also small areas of phlegmon and microabscesses located in the lateral head of the gastrocnemius, consistent with a diagnosis of pyomyositis (Figures 3 and 4).\nPyomyositis is a subacute bacterial infection affecting the skeletal muscles, typically occurring with the formation of an abscess; however, cases of myonecrosis and frank sepsis have been reported.[1,2,3,4,5,6,7] The diagnosis of pyomyositis is commonly made through the use of MRI, although it can also be made through ultrasound and CT scans.[3,6] In this case, the ultrasound was negative; this examination is rarely diagnostic in the early stages of pyomyositis because, typically, there is no large fluid collection.[3]Pyomyositis presents in 1 of 3 distinct stages, for which a patient's symptoms and MRI findings are used for classification.[4,6] The first stage is known as the invasive stage.[6] This stage is marked by pain and swelling of the muscle, with minimal changes noted on the skin and in the soft tissue.[6] The suppurative stage follows, and it is linked to the formation of a deep collection of pus with marked pain, fever, and soft-tissue edema.[6] The late stage is the final stage, and it is characterized by the formation of a deep abscess along with myonecrosis and possibly septic shock.[6] On the basis of these classifications, this patient presented between the invasive and suppurative stages of infection, with only microabscesses noted but no deep abscesses that required incision and drainage.\nAfter being described in Japan in 1885 by Scriba, the condition has commonly been encountered in tropical climates, giving it the name myositis tropicans or tropical pyomyositis.[3,5]Pyomyositis accounts for 4% of all hospital admissions in tropical countries; however, in temperate climates it occurs in 1 in 3000 pediatric admissions.[3,4] Cases of pyomyositis have increased over the past 10 years in part due to the increase in methicillin-resistant \nStaphylococcus aureus (MRSA)\n, with studies showing that 90% of cases are caused by S aureus, although gram-negative organisms have also been encountered.[1,4,6] In a study by Pannaraj and colleagues, S aureus accounted for 56% of pyomyositis cases in children, with group A Streptococcus\n accounting for only 2% of infections.[8] An explanation for the recent increase in pyomyositis may be due to the virulence factors of certain strains of MRSA.[8] The USA300 strain of MRSA, which now represents > 70% of the MRSA isolates in some areas, is associated with the Panton-Valentine leukocidin (PVL) virulence factor. This factor is an exotoxin that induces apoptosis in leukocytes and is associated with skin, bone, joint, and deep soft-tissue infections and abscess formation.[8]\nIn a small report from Kuwait, of 8 children with postvaricella musculoskeletal complications, 3 children had pyomyositis[9]; the investigators also noted cellulitis in 3 children, osteomyelitis in 2 children, and gangrene in 1 child, with an average of 8.8 days between onset of the primary varicella infection and the complication. Although all of the children had negative blood cultures, of 2 children with S aureus-positive pus cultures, 1 was infected with MRSA.[9]\n\n ## Figures\n\n **Figure 3.Sagittal MR image of the right knee after the administration of gadolinium contrast. Synovitis along with inflammation (star) of the patient's posterior popliteal soft tissues is seen, with small areas of phlegmon and microabscesses (arrow) mainly at the origin of the lateral head of the gastrocnemius muscle.** \n \n\n**Figure 4.Axial MR image of the right knee after the administration of gadolinium contrast. Synovitis along with inflammation (star) of the patient's posterior popliteal soft tissues is seen, with small areas of phlegmon and microabscesses (arrow) mainly at the origin of the lateral head of the gastrocnemius muscle.** \n \n\n\n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 422572,
"choiceText": "Septic arthritis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 422573,
"choiceText": "Pyomyositis",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 422574,
"choiceText": "Osteomyelitis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 422575,
"choiceText": "Myositis",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 124571,
"questionText": "On the basis of the MR images, what is the most likely diagnosis?<br />\r\n<br />\r\nHint: <i>Notice the lack of an effusion within the joint and the increased signal in the head of the gastrocnemius muscle.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "An Unexpected Cause of Severe Knee Pain in an 8-Year-Old"
},
{
"authors": "Matthew T. Houdek; Elizabeth A. Lindgren; Conrad J. Clemens, MD, MPH",
"content": [
"In muscle tissue, iron is largely sequestered in myoglobin. Iron has been thought to be essential for bacterial reproduction; therefore, muscle tissue has been thought to be rather resistant to infection.[6] As such, clinical cases such as this more commonly raise concern for septic arthritis or osteomyelitis. However, in more tropical climates, pyomyositis may contribute to significant morbidity.[1,2,3,4,5,6,7,8,9,10]",
"Pyomyositis is thought to be caused by the hematogenous spread of bacteria from an occult source,[5] with a majority of patients having a history of a skin barrier breakage or recent trauma.[8] In over 75% of all patients with pyomyositis, a concurrent immunodeficiency (either HIV, cancer, or an autoimmune disease) is also present.[4,5,6] Trauma to the muscle allows the bacteria to access the iron in myoglobin, and the formation of a hematoma following a traumatic event provides an ideal environment for bacterial replication and sequestration.[9] Malnutrition, bacteremia, and chronic diseases such as Crohn disease and diabetes mellitus are also risk factors for pyomyositis.[4,5,6]",
"Figure 3.",
"Figure 4.",
"In tropical countries, the average age of presentation is 2-5 years; however, the disease can affect any age group.[5,9] In the United States, the characteristics of pyomyositis cases are generally similar to those seen in tropical climates, except that it is more commonly seen in immunocompromised patients.[10] Patients typically present with pain; fever; swelling of a muscle; and, if the infection is located in a muscle of the lower extremities (as in this patient), a limp.[8]Pyomyositis is commonly found in the muscles of the pelvic girdle and the large muscles of the lower limbs, with the iliopsoas being the most common site of infection.[4] The infections are usually unifocal, but 10%-20% of patients will present with multifocal disease.[8] Typically, patients will have symptoms for 2-3 days before they present to a physician; however, the diagnosis is frequently not established until the fourth day following presentation.[8] As in this patient, the diagnosis of primary pyomyositis is commonly delayed while diagnoses of septic arthritis, osteomyelitis, and thrombophlebitis are ruled out.[1,2,3,4,5,6,7] Other disease processes that need to be included in the differential diagnosis include myositis, septic bursitis, occult fracture, muscle rupture, muscle spasm, and cellulitis.[1,2,3,4,5,6,7,8]Pyomyositis may also mimic other infective processes depending on the location of the abscess; an infection of the psoas presents with symptoms similar to appendicitis; an infection of the iliacus may mimic septic arthritis of the hip; and an abscess of the paraspinal muscles can present similar to an epidural abscess.[10]"
],
"date": "March 02, 2016",
"figures": [
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/746/515/746515-thumb3.png"
},
{
"caption": "Figure 4.",
"image_url": "https://img.medscapestatic.com/article/746/515/746515-thumb4.png"
}
],
"markdown": "# An Unexpected Cause of Severe Knee Pain in an 8-Year-Old\n\n **Authors:** Matthew T. Houdek; Elizabeth A. Lindgren; Conrad J. Clemens, MD, MPH \n **Date:** March 02, 2016\n\n ## Content\n\n In muscle tissue, iron is largely sequestered in myoglobin. Iron has been thought to be essential for bacterial reproduction; therefore, muscle tissue has been thought to be rather resistant to infection.[6] As such, clinical cases such as this more commonly raise concern for septic arthritis or osteomyelitis. However, in more tropical climates, pyomyositis may contribute to significant morbidity.[1,2,3,4,5,6,7,8,9,10]\nPyomyositis is thought to be caused by the hematogenous spread of bacteria from an occult source,[5] with a majority of patients having a history of a skin barrier breakage or recent trauma.[8] In over 75% of all patients with pyomyositis, a concurrent immunodeficiency (either HIV, cancer, or an autoimmune disease) is also present.[4,5,6] Trauma to the muscle allows the bacteria to access the iron in myoglobin, and the formation of a hematoma following a traumatic event provides an ideal environment for bacterial replication and sequestration.[9] Malnutrition, bacteremia, and chronic diseases such as Crohn disease and diabetes mellitus are also risk factors for pyomyositis.[4,5,6]\nFigure 3.\nFigure 4.\nIn tropical countries, the average age of presentation is 2-5 years; however, the disease can affect any age group.[5,9] In the United States, the characteristics of pyomyositis cases are generally similar to those seen in tropical climates, except that it is more commonly seen in immunocompromised patients.[10] Patients typically present with pain; fever; swelling of a muscle; and, if the infection is located in a muscle of the lower extremities (as in this patient), a limp.[8]Pyomyositis is commonly found in the muscles of the pelvic girdle and the large muscles of the lower limbs, with the iliopsoas being the most common site of infection.[4] The infections are usually unifocal, but 10%-20% of patients will present with multifocal disease.[8] Typically, patients will have symptoms for 2-3 days before they present to a physician; however, the diagnosis is frequently not established until the fourth day following presentation.[8] As in this patient, the diagnosis of primary pyomyositis is commonly delayed while diagnoses of septic arthritis, osteomyelitis, and thrombophlebitis are ruled out.[1,2,3,4,5,6,7] Other disease processes that need to be included in the differential diagnosis include myositis, septic bursitis, occult fracture, muscle rupture, muscle spasm, and cellulitis.[1,2,3,4,5,6,7,8]Pyomyositis may also mimic other infective processes depending on the location of the abscess; an infection of the psoas presents with symptoms similar to appendicitis; an infection of the iliacus may mimic septic arthritis of the hip; and an abscess of the paraspinal muscles can present similar to an epidural abscess.[10]\n\n ## Figures\n\n **Figure 3.** \n \n\n**Figure 4.** \n \n\n\n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "An Unexpected Cause of Severe Knee Pain in an 8-Year-Old"
},
{
"authors": "Matthew T. Houdek; Elizabeth A. Lindgren; Conrad J. Clemens, MD, MPH",
"content": [
"The stages of infection are also used to designate the treatment course.[3] In the first stage of infection, administration of empirical antibiotics typically leads to the resolution of symptoms, as was seen in this case.[3] Patients without bone or joint involvement who respond to antibiotic treatment should remain on antibiotics for 3 weeks.[8] In this patient, he continued to clinically improve on the IV clindamycin. On the second day following the administration of clindamycin, he was ambulating with minimal pain, and the decision was made to place a peripherally inserted central catheter (PICC) line. He was discharged to home on IV clindamycin for a total of 3 weeks of treatment.",
"For the second and third stages of the infection, percutaneous drainage or incision and drainage are necessary to relieve the abscess formation.[3] In over 90% of cases wherein the abscess is drained, the causative bacteria can be isolated and cultured, allowing the physician to obtain antibiotic sensitivities.[8] For empirical microbial coverage, choose an antibiotic with community-acquired MRSA coverage, such as clindamycin or vancomycin.[8] In this case, clindamycin was used for empirical coverage for MRSA due to the low resistance rate found in the institution's community, accounting for only 10%-15% of cases. If there is a high prevalence of clindamycin-resistant MRSA in the community (50% of cases in other areas of the country[11]), then other medications such as trimethoprim-sulfamethoxazole, doxycycline, minocycline, vancomycin (IV) or oral linezolid are good alternatives.[8] Due to the resistance of MRSA to clindamycin, it is important to know the patient dynamics in your community when deciding on treatment options. If methicillin-susceptible S aureus (MSSA) or streptococcus is isolated, switch to a beta-lactam antibiotic such as a penicillin (with beta-lactamase inhibitor for MSSA) or first-generation cephalosporin because these antibiotics are bacteriocidal and well tolerated.",
"In conclusion, pyomyositis is an atypical cause of limb pain in temperate climates. This case presents the need to consider pyomyositis as a diagnosis if there is the clinical suspicion for an infectious process and both septic arthritis and osteomyelitis have been ruled out. It is important to note that if the infection is allowed to progress to the late stage, myonecrosis can occur and potentially lead to septic shock and death."
],
"date": "March 02, 2016",
"figures": [],
"markdown": "# An Unexpected Cause of Severe Knee Pain in an 8-Year-Old\n\n **Authors:** Matthew T. Houdek; Elizabeth A. Lindgren; Conrad J. Clemens, MD, MPH \n **Date:** March 02, 2016\n\n ## Content\n\n The stages of infection are also used to designate the treatment course.[3] In the first stage of infection, administration of empirical antibiotics typically leads to the resolution of symptoms, as was seen in this case.[3] Patients without bone or joint involvement who respond to antibiotic treatment should remain on antibiotics for 3 weeks.[8] In this patient, he continued to clinically improve on the IV clindamycin. On the second day following the administration of clindamycin, he was ambulating with minimal pain, and the decision was made to place a peripherally inserted central catheter (PICC) line. He was discharged to home on IV clindamycin for a total of 3 weeks of treatment.\nFor the second and third stages of the infection, percutaneous drainage or incision and drainage are necessary to relieve the abscess formation.[3] In over 90% of cases wherein the abscess is drained, the causative bacteria can be isolated and cultured, allowing the physician to obtain antibiotic sensitivities.[8] For empirical microbial coverage, choose an antibiotic with community-acquired MRSA coverage, such as clindamycin or vancomycin.[8] In this case, clindamycin was used for empirical coverage for MRSA due to the low resistance rate found in the institution's community, accounting for only 10%-15% of cases. If there is a high prevalence of clindamycin-resistant MRSA in the community (50% of cases in other areas of the country[11]), then other medications such as trimethoprim-sulfamethoxazole, doxycycline, minocycline, vancomycin (IV) or oral linezolid are good alternatives.[8] Due to the resistance of MRSA to clindamycin, it is important to know the patient dynamics in your community when deciding on treatment options. If methicillin-susceptible S aureus (MSSA) or streptococcus is isolated, switch to a beta-lactam antibiotic such as a penicillin (with beta-lactamase inhibitor for MSSA) or first-generation cephalosporin because these antibiotics are bacteriocidal and well tolerated.\nIn conclusion, pyomyositis is an atypical cause of limb pain in temperate climates. This case presents the need to consider pyomyositis as a diagnosis if there is the clinical suspicion for an infectious process and both septic arthritis and osteomyelitis have been ruled out. It is important to note that if the infection is allowed to progress to the late stage, myonecrosis can occur and potentially lead to septic shock and death.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 422576,
"choiceText": "History of diabetes",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 422577,
"choiceText": "History of recent injury to the muscle",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 422578,
"choiceText": "Environmental climate of his hometown",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 422579,
"choiceText": "History of a recent skin infection on his ipsilateral hand",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Pyomyositis is typically a tropical disease; however, there has been an increase in cases in temperate climates. It was once believed that a history of a disease that alters immune function increases the risk for pyomyositis; recent studies have shown that this may not be the case. A history of a recent trauma to the muscle group is seen in up to 50% of patients who are diagnosed with pyomyositis. Direct trauma to the muscle is often seen, although cases of pyomyositis can also be seen following vigorous exercise.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 124572,
"questionText": "An 11-year-old boy with a history of diabetes is diagnosed with pyomyositis of the left psoas after sustaining a \"pulled left leg muscle\" while hiking in his home state of Colorado. What factor is most associated with the development of pyomyositis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 422580,
"choiceText": "Elevated leukocyte count",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 422581,
"choiceText": "Negative blood cultures",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 422582,
"choiceText": "Elevated ESR and CRP",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 422583,
"choiceText": "Lack of an abscess",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Laboratory tests are often normal. The white blood cell count is elevated in over half of these cases, and in many the ESR and CRP are also elevated. These values are not, however, specific. In significant pyomyositis, serum creatinine kinase levels may also be elevated, but a normal level cannot be used to exclude pyomyositis. Approximately two thirds of patients with pyomyositis have positive blood cultures, but the treatment course is based on the stage of infection. If an abscess is present, percutaneous or surgical incision and drainage of the pus should be accomplished along with the initiation of empirical antibiotics. If there is no abscess present, patients can typically be treated with intravenous or oral antibiotics for 3 weeks as long as clinical symptoms improve.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 124573,
"questionText": "The patient is found to have a white blood cell count of 18.0 x 10<sup>3</sup>/mm<sup>3</sup>, an erythrocyte sedimentation rate (ESR) of 50.0 mm/hr, a C-reactive protein (CRP) level of 6 mg/L, and negative blood cultures with an MR image that does not show a definitive abscess in the psoas muscle. What factor has the greatest influence on treatment planning?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "An Unexpected Cause of Severe Knee Pain in an 8-Year-Old"
},
{
"authors": "Matthew T. Houdek; Elizabeth A. Lindgren; Conrad J. Clemens, MD, MPH",
"content": [],
"date": "March 02, 2016",
"figures": [],
"markdown": "# An Unexpected Cause of Severe Knee Pain in an 8-Year-Old\n\n **Authors:** Matthew T. Houdek; Elizabeth A. Lindgren; Conrad J. Clemens, MD, MPH \n **Date:** March 02, 2016\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 422576,
"choiceText": "History of diabetes",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 422577,
"choiceText": "History of recent injury to the muscle",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 422578,
"choiceText": "Environmental climate of his hometown",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 422579,
"choiceText": "History of a recent skin infection on his ipsilateral hand",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Pyomyositis is typically a tropical disease; however, there has been an increase in cases in temperate climates. It was once believed that a history of a disease that alters immune function increases the risk for pyomyositis; recent studies have shown that this may not be the case. A history of a recent trauma to the muscle group is seen in up to 50% of patients who are diagnosed with pyomyositis. Direct trauma to the muscle is often seen, although cases of pyomyositis can also be seen following vigorous exercise.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 124572,
"questionText": "An 11-year-old boy with a history of diabetes is diagnosed with pyomyositis of the left psoas after sustaining a \"pulled left leg muscle\" while hiking in his home state of Colorado. What factor is most associated with the development of pyomyositis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 422580,
"choiceText": "Elevated leukocyte count",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 422581,
"choiceText": "Negative blood cultures",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 422582,
"choiceText": "Elevated ESR and CRP",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 422583,
"choiceText": "Lack of an abscess",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Laboratory tests are often normal. The white blood cell count is elevated in over half of these cases, and in many the ESR and CRP are also elevated. These values are not, however, specific. In significant pyomyositis, serum creatinine kinase levels may also be elevated, but a normal level cannot be used to exclude pyomyositis. Approximately two thirds of patients with pyomyositis have positive blood cultures, but the treatment course is based on the stage of infection. If an abscess is present, percutaneous or surgical incision and drainage of the pus should be accomplished along with the initiation of empirical antibiotics. If there is no abscess present, patients can typically be treated with intravenous or oral antibiotics for 3 weeks as long as clinical symptoms improve.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 124573,
"questionText": "The patient is found to have a white blood cell count of 18.0 x 10<sup>3</sup>/mm<sup>3</sup>, an erythrocyte sedimentation rate (ESR) of 50.0 mm/hr, a C-reactive protein (CRP) level of 6 mg/L, and negative blood cultures with an MR image that does not show a definitive abscess in the psoas muscle. What factor has the greatest influence on treatment planning?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "An Unexpected Cause of Severe Knee Pain in an 8-Year-Old"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 422572,
"choiceText": "Septic arthritis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 422573,
"choiceText": "Pyomyositis",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 422574,
"choiceText": "Osteomyelitis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 422575,
"choiceText": "Myositis",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 124571,
"questionText": "On the basis of the MR images, what is the most likely diagnosis?<br />\r\n<br />\r\nHint: <i>Notice the lack of an effusion within the joint and the increased signal in the head of the gastrocnemius muscle.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 422576,
"choiceText": "History of diabetes",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 422577,
"choiceText": "History of recent injury to the muscle",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 422578,
"choiceText": "Environmental climate of his hometown",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 422579,
"choiceText": "History of a recent skin infection on his ipsilateral hand",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Pyomyositis is typically a tropical disease; however, there has been an increase in cases in temperate climates. It was once believed that a history of a disease that alters immune function increases the risk for pyomyositis; recent studies have shown that this may not be the case. A history of a recent trauma to the muscle group is seen in up to 50% of patients who are diagnosed with pyomyositis. Direct trauma to the muscle is often seen, although cases of pyomyositis can also be seen following vigorous exercise.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 124572,
"questionText": "An 11-year-old boy with a history of diabetes is diagnosed with pyomyositis of the left psoas after sustaining a \"pulled left leg muscle\" while hiking in his home state of Colorado. What factor is most associated with the development of pyomyositis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 422580,
"choiceText": "Elevated leukocyte count",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 422581,
"choiceText": "Negative blood cultures",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 422582,
"choiceText": "Elevated ESR and CRP",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 422583,
"choiceText": "Lack of an abscess",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Laboratory tests are often normal. The white blood cell count is elevated in over half of these cases, and in many the ESR and CRP are also elevated. These values are not, however, specific. In significant pyomyositis, serum creatinine kinase levels may also be elevated, but a normal level cannot be used to exclude pyomyositis. Approximately two thirds of patients with pyomyositis have positive blood cultures, but the treatment course is based on the stage of infection. If an abscess is present, percutaneous or surgical incision and drainage of the pus should be accomplished along with the initiation of empirical antibiotics. If there is no abscess present, patients can typically be treated with intravenous or oral antibiotics for 3 weeks as long as clinical symptoms improve.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 124573,
"questionText": "The patient is found to have a white blood cell count of 18.0 x 10<sup>3</sup>/mm<sup>3</sup>, an erythrocyte sedimentation rate (ESR) of 50.0 mm/hr, a C-reactive protein (CRP) level of 6 mg/L, and negative blood cultures with an MR image that does not show a definitive abscess in the psoas muscle. What factor has the greatest influence on treatment planning?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
744833 | /viewarticle/744833 | [
{
"authors": "Burr J. Loew, MD",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.",
"A 68-year-old woman with a 4-month history of intermittent episodes of epigastric pain with associated nausea and vomiting presents to the emergency department (ED) during an episode. Prior episodes typically lasted for 36-72 hours before resolving on their own. Her medical history is notable only for a splenic marginal zone lymphoma approximately 2 years ago for which she is not currently receiving any specific therapy. She has no other medical problems, and her only prior abdominal surgery was a recent cholecystectomy for presumed symptomatic gallstones that has done nothing to resolve her attacks of abdominal pain. Her only medications are vitamins: calcium, vitamin C, and vitamin D. She does not smoke or drink alcohol, and there is no family history of similar abdominal problems.",
"During one of the prior episodes, she also presented to the ED, and a CT scan taken at that time revealed an edematous and thickened duodenum and jejunum, with a transition point appearing near the midjejunum. A small bowel follow-through performed 2 weeks later was normal. A push enteroscopy to approximately the midjejunum was normal and did not confirm the abnormalities seen on the CT scan. Random biopsies of the jejunum were also normal. A 68-year-old woman with a 4-month history of intermittent episodes of epigastric pain with associated nausea and vomiting presents to the emergency department (ED) during an episode. Prior episodes typically lasted for 36-72 hours before resolving on their own. Her medical history is notable only for a splenic marginal zone lymphoma approximately 2 years ago for which she is not currently receiving any specific therapy. She has no other medical problems, and her only prior abdominal surgery was a recent cholecystectomy for presumed symptomatic gallstones that has done nothing to resolve her attacks of abdominal pain. Her only medications are vitamins: calcium, vitamin C, and vitamin D. She does not smoke or drink alcohol, and there is no family history of similar abdominal problems.",
"During one of the prior episodes, she also presented to the ED, and a CT scan taken at that time revealed an edematous and thickened duodenum and jejunum, with a transition point appearing near the midjejunum. A small bowel follow-through performed 2 weeks later was normal. A push enteroscopy to approximately the midjejunum was normal and did not confirm the abnormalities seen on the CT scan. Random biopsies of the jejunum were also normal."
],
"date": "July 17, 2015",
"figures": [],
"markdown": "# Mysterious Recurrent Abdominal Pain With Nausea and Vomiting\n\n **Authors:** Burr J. Loew, MD \n **Date:** July 17, 2015\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.\nA 68-year-old woman with a 4-month history of intermittent episodes of epigastric pain with associated nausea and vomiting presents to the emergency department (ED) during an episode. Prior episodes typically lasted for 36-72 hours before resolving on their own. Her medical history is notable only for a splenic marginal zone lymphoma approximately 2 years ago for which she is not currently receiving any specific therapy. She has no other medical problems, and her only prior abdominal surgery was a recent cholecystectomy for presumed symptomatic gallstones that has done nothing to resolve her attacks of abdominal pain. Her only medications are vitamins: calcium, vitamin C, and vitamin D. She does not smoke or drink alcohol, and there is no family history of similar abdominal problems.\nDuring one of the prior episodes, she also presented to the ED, and a CT scan taken at that time revealed an edematous and thickened duodenum and jejunum, with a transition point appearing near the midjejunum. A small bowel follow-through performed 2 weeks later was normal. A push enteroscopy to approximately the midjejunum was normal and did not confirm the abnormalities seen on the CT scan. Random biopsies of the jejunum were also normal. A 68-year-old woman with a 4-month history of intermittent episodes of epigastric pain with associated nausea and vomiting presents to the emergency department (ED) during an episode. Prior episodes typically lasted for 36-72 hours before resolving on their own. Her medical history is notable only for a splenic marginal zone lymphoma approximately 2 years ago for which she is not currently receiving any specific therapy. She has no other medical problems, and her only prior abdominal surgery was a recent cholecystectomy for presumed symptomatic gallstones that has done nothing to resolve her attacks of abdominal pain. Her only medications are vitamins: calcium, vitamin C, and vitamin D. She does not smoke or drink alcohol, and there is no family history of similar abdominal problems.\nDuring one of the prior episodes, she also presented to the ED, and a CT scan taken at that time revealed an edematous and thickened duodenum and jejunum, with a transition point appearing near the midjejunum. A small bowel follow-through performed 2 weeks later was normal. A push enteroscopy to approximately the midjejunum was normal and did not confirm the abnormalities seen on the CT scan. Random biopsies of the jejunum were also normal.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "Mysterious Recurrent Abdominal Pain With Nausea and Vomiting"
},
{
"authors": "Burr J. Loew, MD",
"content": [
"On physical examination, her oral temperature is 98.6°F (37°C); her pulse is regular at 70 beats/min; and her blood pressure is 130/74 mm Hg. She is in mild distress secondary to epigastric discomfort. The head and neck examination, including a check for icteric sclerae, is normal. Her lungs are clear to auscultation with a normal respiratory effort. The heart rate is regular, with normal S1 and S2 heart sounds. Her abdomen is soft, moderately distended, and mildly tender to deep palpation in the epigastric region; moderate tympany to percussion is noted. The spleen is palpable in the left midabdomen, and the bowel sounds are decreased though present. The rectal examination reveals normal tone and brown, guaiac-negative stool.",
"Figure 1.",
"Figure 2.",
"The laboratory analysis, including a complete blood cell count and a basic metabolic panel, yields normal results; however, the serum lipase level is elevated at 730 U/L (normal range, 30-110 U/L). A nasogastric tube is placed; intravenous fluids are administered; and the patient is ordered nothing by mouth. A CT scan is performed, and although the pancreas appears normal, an edematous and thickened jejunum and duodenum with an adjacent small amount of free fluid as well as splenomegaly are noted (Figures 1 and 2). This CT scan appears similar to the one that she had on her prior ED visit. She has a surgical consult before being admitted to the medical floor of the hospital; the next day, her symptoms improve significantly."
],
"date": "July 17, 2015",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/743/937/743937-thumb1.png"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/743/937/743937-thumb2.png"
}
],
"markdown": "# Mysterious Recurrent Abdominal Pain With Nausea and Vomiting\n\n **Authors:** Burr J. Loew, MD \n **Date:** July 17, 2015\n\n ## Content\n\n On physical examination, her oral temperature is 98.6°F (37°C); her pulse is regular at 70 beats/min; and her blood pressure is 130/74 mm Hg. She is in mild distress secondary to epigastric discomfort. The head and neck examination, including a check for icteric sclerae, is normal. Her lungs are clear to auscultation with a normal respiratory effort. The heart rate is regular, with normal S1 and S2 heart sounds. Her abdomen is soft, moderately distended, and mildly tender to deep palpation in the epigastric region; moderate tympany to percussion is noted. The spleen is palpable in the left midabdomen, and the bowel sounds are decreased though present. The rectal examination reveals normal tone and brown, guaiac-negative stool.\nFigure 1.\nFigure 2.\nThe laboratory analysis, including a complete blood cell count and a basic metabolic panel, yields normal results; however, the serum lipase level is elevated at 730 U/L (normal range, 30-110 U/L). A nasogastric tube is placed; intravenous fluids are administered; and the patient is ordered nothing by mouth. A CT scan is performed, and although the pancreas appears normal, an edematous and thickened jejunum and duodenum with an adjacent small amount of free fluid as well as splenomegaly are noted (Figures 1 and 2). This CT scan appears similar to the one that she had on her prior ED visit. She has a surgical consult before being admitted to the medical floor of the hospital; the next day, her symptoms improve significantly.\n\n ## Figures\n\n **Figure 1.** \n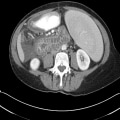 \n\n**Figure 2.** \n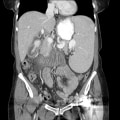 \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 413177,
"choiceText": "Acquired C1-inhibitor (C1-INH) deficiency",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 413178,
"choiceText": "Acute relapsing pancreatitis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 413179,
"choiceText": "Hereditary angioedema (HAE)",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 413180,
"choiceText": "Mesenteric ischemia",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 121299,
"questionText": "On the basis of the patient's clinical history and CT scan findings, what is the diagnosis?<br />\r\n<br />\r\n<i>Hint:</i> The history of a lymphoproliferative disorder is associated with this condition.",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Mysterious Recurrent Abdominal Pain With Nausea and Vomiting"
},
{
"authors": "Burr J. Loew, MD",
"content": [
"The diagnosis of acquired C1-INH deficiency was suspected on the basis of her clinical history; the presence of a concomitant lymphoproliferative disorder; and the classic radiographic findings showing a thickened bowel with low attenuation, intramural edema, and free intraperitoneal fluid. On further questioning, she reported an episode of tongue and lip swelling occurring shortly after one of her abdominal attacks, which further supported the diagnosis. Subsequent testing described below confirmed the diagnosis. The moderately elevated lipase in this case was the result of the acute intestinal obstruction from her edematous bowel; the clinical presentation and imaging did not support the diagnosis of acute pancreatitis.",
"Acquired angioedema (AAE) and HAE present with similar symptoms and manifestations; however, the literature is relatively much more refined for HAE. HAE was initially described in 1888 by William Osler. Deficiency in antigenic and/or C1-INH is present in both disorders. C1-INH is a serine protease inhibitor that regulates several interrelated proinflammatory pathways; it is the primary inhibitor of the classic complement pathway and contact system proteases (ie, kallikrein) and a minor inhibitor of the fibrinolytic (plasmin) and coagulation (factor XIIa) pathways.",
"The pathogenesis of this disorder is suspected to be related to unopposed activation of the contact pathway by the initial generation of kallikrein and/or clotting factor XII by damaged endothelial cells. The end product of this cascade, bradykinin, is produced in large amounts and is believed to be the predominant mediator leading to increased vascular permeability and vasodilation that induces typical angioedema \"attacks\".[1] AAE results from low C1-INH levels as well, but through a different mechanism. In AAE, the malignant tissue appears to consume C1-INH through a paraneoplastic process and/or immune response. Consequently, hyperactivation of the classic pathway of human complement occurs. As a consequence, these patients have almost undetectable serum levels and/or activity of C1-INH; C4; C2; and C1q, r, and s.[2]",
"AAE and HAE are characterized by recurrent episodes of nonpruritic subcutaneous or submucosal edema predominantly of the skin, upper respiratory tract, and gastrointestinal system, although other organs can be involved. Of note, urticaria (the raised erythematous lesions involving the more superficial dermis), which is characteristic of the more common allergic mast cell-mediated conditions, is absent in AAE and HAE. Episodic attacks usually follow a predictable course, with patients often having a prodrome of a tingling sensation, mood changes, or anxiety several hours before the onset of classic edema-related symptoms. Swelling typically worsens slowly over 24 hours and then gradually subsides over the subsequent 48-72 hours. Most attacks involve only one site at a time, although a combination of sites can be seen.",
"Virtually all patients will at some point experience both a cutaneous and a gastrointestinal episode during their lifetime. Although cutaneous manifestations are often physically and functionally disabling, they rarely require hospitalization and can therefore be managed conservatively. Oropharyngeal involvement is more ominous, with concerns for airway obstruction and asphyxiation; therefore, patients should be placed in a setting where rapid intubation and cricothyrotomy is possible. Although most laryngeal episodes resolve before complete airway obstruction, > 30% of HAE mortality in the past was secondary to airway obstruction; even now, patients occasionally die from asphyxiation, especially when they have been misdiagnosed.",
"Abdominal attacks represent about 50% of all episodes. The symptoms are characterized by severe pain, nausea, and vomiting as a result of bowel wall edema, which often mimics a bowel obstruction. Guarding and rebound may be present and can lead to unnecessary surgery. Diarrhea is seen in about 50% of gastrointestinal attacks. Fluid shifts into the interstitium or peritoneal cavity may also occur and occasionally even cause hypotension; circulatory collapse occurs in approximately 4% of abdominal episodes.[1]"
],
"date": "July 17, 2015",
"figures": [],
"markdown": "# Mysterious Recurrent Abdominal Pain With Nausea and Vomiting\n\n **Authors:** Burr J. Loew, MD \n **Date:** July 17, 2015\n\n ## Content\n\n The diagnosis of acquired C1-INH deficiency was suspected on the basis of her clinical history; the presence of a concomitant lymphoproliferative disorder; and the classic radiographic findings showing a thickened bowel with low attenuation, intramural edema, and free intraperitoneal fluid. On further questioning, she reported an episode of tongue and lip swelling occurring shortly after one of her abdominal attacks, which further supported the diagnosis. Subsequent testing described below confirmed the diagnosis. The moderately elevated lipase in this case was the result of the acute intestinal obstruction from her edematous bowel; the clinical presentation and imaging did not support the diagnosis of acute pancreatitis.\nAcquired angioedema (AAE) and HAE present with similar symptoms and manifestations; however, the literature is relatively much more refined for HAE. HAE was initially described in 1888 by William Osler. Deficiency in antigenic and/or C1-INH is present in both disorders. C1-INH is a serine protease inhibitor that regulates several interrelated proinflammatory pathways; it is the primary inhibitor of the classic complement pathway and contact system proteases (ie, kallikrein) and a minor inhibitor of the fibrinolytic (plasmin) and coagulation (factor XIIa) pathways.\nThe pathogenesis of this disorder is suspected to be related to unopposed activation of the contact pathway by the initial generation of kallikrein and/or clotting factor XII by damaged endothelial cells. The end product of this cascade, bradykinin, is produced in large amounts and is believed to be the predominant mediator leading to increased vascular permeability and vasodilation that induces typical angioedema \"attacks\".[1] AAE results from low C1-INH levels as well, but through a different mechanism. In AAE, the malignant tissue appears to consume C1-INH through a paraneoplastic process and/or immune response. Consequently, hyperactivation of the classic pathway of human complement occurs. As a consequence, these patients have almost undetectable serum levels and/or activity of C1-INH; C4; C2; and C1q, r, and s.[2]\nAAE and HAE are characterized by recurrent episodes of nonpruritic subcutaneous or submucosal edema predominantly of the skin, upper respiratory tract, and gastrointestinal system, although other organs can be involved. Of note, urticaria (the raised erythematous lesions involving the more superficial dermis), which is characteristic of the more common allergic mast cell-mediated conditions, is absent in AAE and HAE. Episodic attacks usually follow a predictable course, with patients often having a prodrome of a tingling sensation, mood changes, or anxiety several hours before the onset of classic edema-related symptoms. Swelling typically worsens slowly over 24 hours and then gradually subsides over the subsequent 48-72 hours. Most attacks involve only one site at a time, although a combination of sites can be seen.\nVirtually all patients will at some point experience both a cutaneous and a gastrointestinal episode during their lifetime. Although cutaneous manifestations are often physically and functionally disabling, they rarely require hospitalization and can therefore be managed conservatively. Oropharyngeal involvement is more ominous, with concerns for airway obstruction and asphyxiation; therefore, patients should be placed in a setting where rapid intubation and cricothyrotomy is possible. Although most laryngeal episodes resolve before complete airway obstruction, > 30% of HAE mortality in the past was secondary to airway obstruction; even now, patients occasionally die from asphyxiation, especially when they have been misdiagnosed.\nAbdominal attacks represent about 50% of all episodes. The symptoms are characterized by severe pain, nausea, and vomiting as a result of bowel wall edema, which often mimics a bowel obstruction. Guarding and rebound may be present and can lead to unnecessary surgery. Diarrhea is seen in about 50% of gastrointestinal attacks. Fluid shifts into the interstitium or peritoneal cavity may also occur and occasionally even cause hypotension; circulatory collapse occurs in approximately 4% of abdominal episodes.[1]\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 413177,
"choiceText": "Acquired C1-inhibitor (C1-INH) deficiency",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 413178,
"choiceText": "Acute relapsing pancreatitis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 413179,
"choiceText": "Hereditary angioedema (HAE)",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 413180,
"choiceText": "Mesenteric ischemia",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 121299,
"questionText": "On the basis of the patient's clinical history and CT scan findings, what is the diagnosis?<br />\r\n<br />\r\n<i>Hint:</i> The history of a lymphoproliferative disorder is associated with this condition.",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Mysterious Recurrent Abdominal Pain With Nausea and Vomiting"
},
{
"authors": "Burr J. Loew, MD",
"content": [
"HAE is an autosomal dominant condition, but it is not fully penetrant, with most cases being due to single base-pair mutations; however, partial gene deletions or duplications and new mutations make up a sizable minority of cases. By contrast, AAE is typically acquired in the setting of underlying B-cell lymphoproliferative disorders, including splenic marginal zone lymphoma.",
"In addition, scattered reports describe acquired C1-INH deficiency associated with nonhematologic neoplasm, infections, and certain autoimmune diseases. A minority of cases have no identifiable underlying cause.[3] Patients with HAE typically present during childhood or adolescence (75% by 15 years of age in one report), and the majority have a family history of angioedema.[4] De novo mutations may develop in as many as 25% of cases.[4] The prevalence of this disorder has been estimated to be approximately 1 in 50,000 population. AAE, however, usually presents in middle age or older with known malignancy; its exact prevalence is unknown, but it appears to be much less common than HAE.",
"The triggers for attacks are poorly understood; these may include trauma, insect or bee stings, or certain foods or medications. Oral or dental surgery is a common offender for oropharyngeal episodes. Medications, including exogenous estrogens and angiotensin-converting enzyme inhibitors, can provoke attacks. The eradication of \nHelicobacter pylori\n has been reported to improve the frequency of abdominal attacks.[3,5]",
"Both disorders are underdiagnosed in clinical practice. A 1976 survey found a mean delay in diagnosis of 22 years for HAE. In a more recent survey, the mean delay in diagnosis was still over 9 years.[6]In this case, the clinical diagnosis was not initially suspected even though the first CT scan demonstrated classic findings. The condition is not often suspected until after recurrent episodes of angioedema have occurred. The diagnosis can be confirmed with measurements of C1-INH, C4, and C1q levels. Most authorities recommend C4 levels as an initial screening test because levels are low in AAE and all but the rarest forms of HAE (HAE type III).",
"Two types of HAE are more common: Type I is characterized by low C1-INH and low C4 but normal C1q levels; type II HAE, in comparison, has nonfunctional C1-INH proteins and is characterized by normal C1-INH, low C4, and normal C1q levels. AAE, on the other hand, is characterized by almost undetectable levels of C1-INH, C4, and C1q due to the rapid destruction through hyperactivation of the classic complement pathway.[1,3]AAE is also recognized as having 2 forms, termed type I and type II. AAE type I is most commonly associated with other diseases, such as B-cell lymphoproliferative disorders. AAE type II is an autoimmune process defined by the presence of an autoantibody directed against the C1-INH.[7]"
],
"date": "July 17, 2015",
"figures": [],
"markdown": "# Mysterious Recurrent Abdominal Pain With Nausea and Vomiting\n\n **Authors:** Burr J. Loew, MD \n **Date:** July 17, 2015\n\n ## Content\n\n HAE is an autosomal dominant condition, but it is not fully penetrant, with most cases being due to single base-pair mutations; however, partial gene deletions or duplications and new mutations make up a sizable minority of cases. By contrast, AAE is typically acquired in the setting of underlying B-cell lymphoproliferative disorders, including splenic marginal zone lymphoma.\nIn addition, scattered reports describe acquired C1-INH deficiency associated with nonhematologic neoplasm, infections, and certain autoimmune diseases. A minority of cases have no identifiable underlying cause.[3] Patients with HAE typically present during childhood or adolescence (75% by 15 years of age in one report), and the majority have a family history of angioedema.[4] De novo mutations may develop in as many as 25% of cases.[4] The prevalence of this disorder has been estimated to be approximately 1 in 50,000 population. AAE, however, usually presents in middle age or older with known malignancy; its exact prevalence is unknown, but it appears to be much less common than HAE.\nThe triggers for attacks are poorly understood; these may include trauma, insect or bee stings, or certain foods or medications. Oral or dental surgery is a common offender for oropharyngeal episodes. Medications, including exogenous estrogens and angiotensin-converting enzyme inhibitors, can provoke attacks. The eradication of \nHelicobacter pylori\n has been reported to improve the frequency of abdominal attacks.[3,5]\nBoth disorders are underdiagnosed in clinical practice. A 1976 survey found a mean delay in diagnosis of 22 years for HAE. In a more recent survey, the mean delay in diagnosis was still over 9 years.[6]In this case, the clinical diagnosis was not initially suspected even though the first CT scan demonstrated classic findings. The condition is not often suspected until after recurrent episodes of angioedema have occurred. The diagnosis can be confirmed with measurements of C1-INH, C4, and C1q levels. Most authorities recommend C4 levels as an initial screening test because levels are low in AAE and all but the rarest forms of HAE (HAE type III).\nTwo types of HAE are more common: Type I is characterized by low C1-INH and low C4 but normal C1q levels; type II HAE, in comparison, has nonfunctional C1-INH proteins and is characterized by normal C1-INH, low C4, and normal C1q levels. AAE, on the other hand, is characterized by almost undetectable levels of C1-INH, C4, and C1q due to the rapid destruction through hyperactivation of the classic complement pathway.[1,3]AAE is also recognized as having 2 forms, termed type I and type II. AAE type I is most commonly associated with other diseases, such as B-cell lymphoproliferative disorders. AAE type II is an autoimmune process defined by the presence of an autoantibody directed against the C1-INH.[7]\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "Mysterious Recurrent Abdominal Pain With Nausea and Vomiting"
},
{
"authors": "Burr J. Loew, MD",
"content": [
"Treatment can be divided into prophylaxis and therapy during acute attacks. Prophylaxis includes avoiding known triggers as well as administering prophylactic medications prior to intubation, oral surgery, general surgery, or dental work. Specific prophylactic medications that increase C1-INH through varying and poorly understood mechanisms include attenuated androgens such as danazol, antifibrinolytics such as tranexamic acid and aminocaproic acid, and plasma products containing C1-INH such as fresh frozen plasma.",
"Therapy for acute attacks includes plasma products as well as newer medications such as recombinant C1-INH protein, ecallantide (kallikrein inhibitor), and icatibant (bradykinin receptor antagonist). In a study by Wasserman et al, a weight-based dose (20 U/kg) of esterase inhibitor (C1-INH) concentrate was successful in rapidly and safely treating subsequent acute facial and abdominal HAE episodes.[8] The median time to relief onset for all attacks and for abdominal attacks was 19.8 minutes; for facial attacks, it was 28.2 minutes. The median time to complete resolution for all attacks was 11 hours.[8]",
"Studies on patients with AAE are lacking given the rarity of the condition. The traditional agents that act by increasing C1-INH levels are believed to be less effective for AAE than they are for HAE because any additional C1-INH that is administered will likely be rapidly consumed via the same paraneoplastic process that causes the disease. Ecallantide, an inhibitor of kallikrein, and icatibant, a bradykinin receptor antagonist, are both agents that inhibit the development of angioedema downstream and, therefore, bypass the problem of C1-INH catabolism. These agents theoretically would be useful in the treatment of AAE. C1-INH replacement is still a reasonable therapy for a patient with AAE during an acute episode; higher doses than those for HAE may be required. Although antihistamines, epinephrine, and corticosteroids are given for other causes of angioedema, they are not effective agents in HAE or AAE given that the underlying mechanism is not an allergic mast cell-mediated reaction.[1,3]",
"In this case, further advice was sought from an allergist as well as the patient's hematologist. Given the frequency of symptoms and lack of any potential triggers, she was started on an androgen (danazol) at 200 mg/day. Over the following 3 months, she did not experience any further attacks. The acquired C1-INH deficiency was believed to be secondary to her diagnosed splenic marginal zone lymphoma. The temporal relationship of her acute attack after the push enteroscopy was likely secondary to luminal trauma during the endoscopy. Given the generally indolent course of her lymphoma, no oncologic therapy was planned as long as her angioedema symptoms remained transient and treatable."
],
"date": "July 17, 2015",
"figures": [],
"markdown": "# Mysterious Recurrent Abdominal Pain With Nausea and Vomiting\n\n **Authors:** Burr J. Loew, MD \n **Date:** July 17, 2015\n\n ## Content\n\n Treatment can be divided into prophylaxis and therapy during acute attacks. Prophylaxis includes avoiding known triggers as well as administering prophylactic medications prior to intubation, oral surgery, general surgery, or dental work. Specific prophylactic medications that increase C1-INH through varying and poorly understood mechanisms include attenuated androgens such as danazol, antifibrinolytics such as tranexamic acid and aminocaproic acid, and plasma products containing C1-INH such as fresh frozen plasma.\nTherapy for acute attacks includes plasma products as well as newer medications such as recombinant C1-INH protein, ecallantide (kallikrein inhibitor), and icatibant (bradykinin receptor antagonist). In a study by Wasserman et al, a weight-based dose (20 U/kg) of esterase inhibitor (C1-INH) concentrate was successful in rapidly and safely treating subsequent acute facial and abdominal HAE episodes.[8] The median time to relief onset for all attacks and for abdominal attacks was 19.8 minutes; for facial attacks, it was 28.2 minutes. The median time to complete resolution for all attacks was 11 hours.[8]\nStudies on patients with AAE are lacking given the rarity of the condition. The traditional agents that act by increasing C1-INH levels are believed to be less effective for AAE than they are for HAE because any additional C1-INH that is administered will likely be rapidly consumed via the same paraneoplastic process that causes the disease. Ecallantide, an inhibitor of kallikrein, and icatibant, a bradykinin receptor antagonist, are both agents that inhibit the development of angioedema downstream and, therefore, bypass the problem of C1-INH catabolism. These agents theoretically would be useful in the treatment of AAE. C1-INH replacement is still a reasonable therapy for a patient with AAE during an acute episode; higher doses than those for HAE may be required. Although antihistamines, epinephrine, and corticosteroids are given for other causes of angioedema, they are not effective agents in HAE or AAE given that the underlying mechanism is not an allergic mast cell-mediated reaction.[1,3]\nIn this case, further advice was sought from an allergist as well as the patient's hematologist. Given the frequency of symptoms and lack of any potential triggers, she was started on an androgen (danazol) at 200 mg/day. Over the following 3 months, she did not experience any further attacks. The acquired C1-INH deficiency was believed to be secondary to her diagnosed splenic marginal zone lymphoma. The temporal relationship of her acute attack after the push enteroscopy was likely secondary to luminal trauma during the endoscopy. Given the generally indolent course of her lymphoma, no oncologic therapy was planned as long as her angioedema symptoms remained transient and treatable.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 413181,
"choiceText": "C3 level",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 413182,
"choiceText": "C4 level",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 413183,
"choiceText": "C1-inhibitor (C1-INH) protein level",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 413184,
"choiceText": "C1q level",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Virtually all patients with HAE and AAE will have low levels of C4, which is why this is recommended as an initial screening test. In rare patients with type III HAE, the C4 level is normal (also called HAE with normal C1-INH). However, this represents < 1% of all patients with HAE. If clinically suspected, mutations in other genes may be sought (ie, gain of function of factor XII or decreased function of proteins involved in bradykinin catabolism, such as angiotensin-converting enzyme inhibitors and aminopeptidase P). The use of C1-INH antigenic levels is used to discriminate between the two types of HAE, although this does not have any clinical consequence.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 121300,
"questionText": "A 22-year-old patient reports 10 years of self-limited episodes of nausea, vomiting, and abdominal pain as well as a history of episodic facial swelling. He reports that his brother was recently afflicted with similar symptoms. You suspect hereditary angioedema (HAE).<br />\r\n<br />\r\nWhat would be the appropriate screening test?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 413185,
"choiceText": "Cutaneous",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 413186,
"choiceText": "Oropharynx",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 413187,
"choiceText": "Joints",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 413188,
"choiceText": "Larynx",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "With oropharyngeal involvement, practitioners must be particularly concerned about airway obstruction. Up to 30% of mortality from the disease has been attributed to airway obstruction. This is particularly concerning in a condition that is often under- or misdiagnosed. Close to 100% of all patients with HAE and AAE will experience a cutaneous or gastrointestinal manifestation during their lifetime. Laryngeal involvement occurs in approximately 50% of patients. The remaining sites of involvement include the tongue, brain, bladder, chest, muscles, joints, kidneys, and esophagus. Each of these manifests in < 10% of patients with this condition.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 121301,
"questionText": "The patient has low levels of C4 and you suspect HAE. At which of the following sites are symptoms of an attack most worrisome?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Mysterious Recurrent Abdominal Pain With Nausea and Vomiting"
},
{
"authors": "Burr J. Loew, MD",
"content": [],
"date": "July 17, 2015",
"figures": [],
"markdown": "# Mysterious Recurrent Abdominal Pain With Nausea and Vomiting\n\n **Authors:** Burr J. Loew, MD \n **Date:** July 17, 2015\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 413181,
"choiceText": "C3 level",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 413182,
"choiceText": "C4 level",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 413183,
"choiceText": "C1-inhibitor (C1-INH) protein level",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 413184,
"choiceText": "C1q level",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Virtually all patients with HAE and AAE will have low levels of C4, which is why this is recommended as an initial screening test. In rare patients with type III HAE, the C4 level is normal (also called HAE with normal C1-INH). However, this represents < 1% of all patients with HAE. If clinically suspected, mutations in other genes may be sought (ie, gain of function of factor XII or decreased function of proteins involved in bradykinin catabolism, such as angiotensin-converting enzyme inhibitors and aminopeptidase P). The use of C1-INH antigenic levels is used to discriminate between the two types of HAE, although this does not have any clinical consequence.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 121300,
"questionText": "A 22-year-old patient reports 10 years of self-limited episodes of nausea, vomiting, and abdominal pain as well as a history of episodic facial swelling. He reports that his brother was recently afflicted with similar symptoms. You suspect hereditary angioedema (HAE).<br />\r\n<br />\r\nWhat would be the appropriate screening test?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 413185,
"choiceText": "Cutaneous",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 413186,
"choiceText": "Oropharynx",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 413187,
"choiceText": "Joints",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 413188,
"choiceText": "Larynx",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "With oropharyngeal involvement, practitioners must be particularly concerned about airway obstruction. Up to 30% of mortality from the disease has been attributed to airway obstruction. This is particularly concerning in a condition that is often under- or misdiagnosed. Close to 100% of all patients with HAE and AAE will experience a cutaneous or gastrointestinal manifestation during their lifetime. Laryngeal involvement occurs in approximately 50% of patients. The remaining sites of involvement include the tongue, brain, bladder, chest, muscles, joints, kidneys, and esophagus. Each of these manifests in < 10% of patients with this condition.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 121301,
"questionText": "The patient has low levels of C4 and you suspect HAE. At which of the following sites are symptoms of an attack most worrisome?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Mysterious Recurrent Abdominal Pain With Nausea and Vomiting"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 413177,
"choiceText": "Acquired C1-inhibitor (C1-INH) deficiency",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 413178,
"choiceText": "Acute relapsing pancreatitis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 413179,
"choiceText": "Hereditary angioedema (HAE)",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 413180,
"choiceText": "Mesenteric ischemia",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 121299,
"questionText": "On the basis of the patient's clinical history and CT scan findings, what is the diagnosis?<br />\r\n<br />\r\n<i>Hint:</i> The history of a lymphoproliferative disorder is associated with this condition.",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 413181,
"choiceText": "C3 level",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 413182,
"choiceText": "C4 level",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 413183,
"choiceText": "C1-inhibitor (C1-INH) protein level",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 413184,
"choiceText": "C1q level",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Virtually all patients with HAE and AAE will have low levels of C4, which is why this is recommended as an initial screening test. In rare patients with type III HAE, the C4 level is normal (also called HAE with normal C1-INH). However, this represents < 1% of all patients with HAE. If clinically suspected, mutations in other genes may be sought (ie, gain of function of factor XII or decreased function of proteins involved in bradykinin catabolism, such as angiotensin-converting enzyme inhibitors and aminopeptidase P). The use of C1-INH antigenic levels is used to discriminate between the two types of HAE, although this does not have any clinical consequence.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 121300,
"questionText": "A 22-year-old patient reports 10 years of self-limited episodes of nausea, vomiting, and abdominal pain as well as a history of episodic facial swelling. He reports that his brother was recently afflicted with similar symptoms. You suspect hereditary angioedema (HAE).<br />\r\n<br />\r\nWhat would be the appropriate screening test?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 413185,
"choiceText": "Cutaneous",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 413186,
"choiceText": "Oropharynx",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 413187,
"choiceText": "Joints",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 413188,
"choiceText": "Larynx",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "With oropharyngeal involvement, practitioners must be particularly concerned about airway obstruction. Up to 30% of mortality from the disease has been attributed to airway obstruction. This is particularly concerning in a condition that is often under- or misdiagnosed. Close to 100% of all patients with HAE and AAE will experience a cutaneous or gastrointestinal manifestation during their lifetime. Laryngeal involvement occurs in approximately 50% of patients. The remaining sites of involvement include the tongue, brain, bladder, chest, muscles, joints, kidneys, and esophagus. Each of these manifests in < 10% of patients with this condition.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 121301,
"questionText": "The patient has low levels of C4 and you suspect HAE. At which of the following sites are symptoms of an attack most worrisome?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
743848 | /viewarticle/743848 | [
{
"authors": "Anargyros Skalimis, MD; Ioannis Aidonis, MD",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.",
"A 5-year-old girl presents to the outpatient pediatric clinic with an 8-month history of progressive malodorous nasal discharge. According to her parents, her nasal breathing is slightly affected, with signs of partial obstruction, including sleep disturbances and remarkable snoring.",
"She has had no nasal bleeding, recent illnesses, fevers, history of trauma, history of similar illness, or other complaints. She has not taken any medications recently. Six months before this presentation, her parents brought her to a specialist for similar symptoms, and she was prescribed intranasal antibiotics, after which the symptoms temporarily subsided. However, her symptoms returned and seem to be worsening; this concerns her parents, who are now seeking further medical advice.",
"She has no significant medical or surgical history. She is up-to-date on all her immunizations. She attends school and lives at home with her parents."
],
"date": "June 11, 2018",
"figures": [],
"markdown": "# A 5-Year-Old Girl With Breathing Difficulties\n\n **Authors:** Anargyros Skalimis, MD; Ioannis Aidonis, MD \n **Date:** June 11, 2018\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.\nA 5-year-old girl presents to the outpatient pediatric clinic with an 8-month history of progressive malodorous nasal discharge. According to her parents, her nasal breathing is slightly affected, with signs of partial obstruction, including sleep disturbances and remarkable snoring.\nShe has had no nasal bleeding, recent illnesses, fevers, history of trauma, history of similar illness, or other complaints. She has not taken any medications recently. Six months before this presentation, her parents brought her to a specialist for similar symptoms, and she was prescribed intranasal antibiotics, after which the symptoms temporarily subsided. However, her symptoms returned and seem to be worsening; this concerns her parents, who are now seeking further medical advice.\nShe has no significant medical or surgical history. She is up-to-date on all her immunizations. She attends school and lives at home with her parents.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 5-Year-Old Girl With Breathing Difficulties"
},
{
"authors": "Anargyros Skalimis, MD; Ioannis Aidonis, MD",
"content": [
"Upon physical examination, her vital signs include a temperature of 98.5°F (36.9°C), heart rate of 90 beats/min, respiratory rate of 20 breaths/min, and a blood pressure of 100/60 mm Hg. She appears well developed, well nourished, and in no acute distress. She is appropriately alert and interactive with her parents. Otoscopy shows normal external auditory canals and intact tympanic membranes with sufficient ventilation of the middle ear cavities.",
"Rhinoscopy reveals a bilateral, nonpurulent, malodorous nasal discharge. A further detailed examination with a flexible nasendoscopy is not possible due to poor patient cooperation. Her oropharynx is normal, with no palpable cervical or postauricular lymph nodes. She has a supple neck without other abnormalities. Her lungs are clear to auscultation bilaterally. Her heart rate and rhythm are normal with no murmurs, rubs, or gallops on auscultation. Her abdominal examination is unremarkable. She has no rashes.",
"The patient undergoes tympanograms that both result normal (type A). A culture specimen from the malodorous discharge results positive for Pseudomonas aeruginosa, explaining the presence of the foul smell. Although the infection is identified, no diagnosis explains the obstructive symptoms. A CT scan of the nose and paranasal cavities is obtained (see Figure 1).",
"Figure 1."
],
"date": "June 11, 2018",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/743/408/743408-thumb1.png"
}
],
"markdown": "# A 5-Year-Old Girl With Breathing Difficulties\n\n **Authors:** Anargyros Skalimis, MD; Ioannis Aidonis, MD \n **Date:** June 11, 2018\n\n ## Content\n\n Upon physical examination, her vital signs include a temperature of 98.5°F (36.9°C), heart rate of 90 beats/min, respiratory rate of 20 breaths/min, and a blood pressure of 100/60 mm Hg. She appears well developed, well nourished, and in no acute distress. She is appropriately alert and interactive with her parents. Otoscopy shows normal external auditory canals and intact tympanic membranes with sufficient ventilation of the middle ear cavities.\nRhinoscopy reveals a bilateral, nonpurulent, malodorous nasal discharge. A further detailed examination with a flexible nasendoscopy is not possible due to poor patient cooperation. Her oropharynx is normal, with no palpable cervical or postauricular lymph nodes. She has a supple neck without other abnormalities. Her lungs are clear to auscultation bilaterally. Her heart rate and rhythm are normal with no murmurs, rubs, or gallops on auscultation. Her abdominal examination is unremarkable. She has no rashes.\nThe patient undergoes tympanograms that both result normal (type A). A culture specimen from the malodorous discharge results positive for Pseudomonas aeruginosa, explaining the presence of the foul smell. Although the infection is identified, no diagnosis explains the obstructive symptoms. A CT scan of the nose and paranasal cavities is obtained (see Figure 1).\nFigure 1.\n\n ## Figures\n\n **Figure 1.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 409124,
"choiceText": "Choanal atresia",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 409125,
"choiceText": "Rhinolithiasis",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 409126,
"choiceText": "Mycetoma",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 409127,
"choiceText": "Hamartoma of nasopharynx",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 119913,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 5-Year-Old Girl With Breathing Difficulties"
},
{
"authors": "Anargyros Skalimis, MD; Ioannis Aidonis, MD",
"content": [
"The CT scan seen in Figure 1 revealed a radiopaque mass impacted in the left nasal choana affecting the left antrum. This finding prompted endoscopic examination under general anesthesia. General anesthesia is considered the norm for this kind of operation because the cooperation of the patient is a necessity, and the rigidity of the mass limits the ability to maneuver atraumatically. A large nasal mass was atraumatically removed following careful endoscopic manipulation by pushing it with an old Eustachian tube catheter to the nasopharynx and subsequently to the oropharynx. At the end of these maneuvers, it was collected with forceps from the oral cavity. The catheter used, although outdated, is thought to minimize trauma to the surrounding sensitive tissue. The mass removed confirmed the definitive diagnosis of a rhinolith (Figure 2). No other masses were found in either nostril.",
"Figure 1.",
"Figure 2.",
"The preliminary diagnosis, based on the history, physical examination, and patient age, was that of a foreign body in the nasal cavity with a superimposed infection causing the malodorous discharge. Nasal discharge could be caused by many common ENT problems in the pediatric population, such as allergic rhinitis, sinusitis, the common cold. The persistence of symptoms as well as the malodorous character of the discharge suggested an atypical explanation.",
"The clinical presentation typically consists of unilateral or bilateral rhinorrhea; although bilateral rhinorrhea is a less common finding, it is more specific for rhinolithiasis. The rhinorrhea, which is initially mucoid, progresses to mucopurulent and finally odiferous, mucopurulent discharge and, in rare cases, it may be streaked with blood. Nasal obstruction may mimic sinusitis when inflammation of the ostia causes a subsequent infection of the paranasal sinuses.[6]"
],
"date": "June 11, 2018",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/743/408/743408-thumb1.png"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/743/408/743408-thumb2.png"
}
],
"markdown": "# A 5-Year-Old Girl With Breathing Difficulties\n\n **Authors:** Anargyros Skalimis, MD; Ioannis Aidonis, MD \n **Date:** June 11, 2018\n\n ## Content\n\n The CT scan seen in Figure 1 revealed a radiopaque mass impacted in the left nasal choana affecting the left antrum. This finding prompted endoscopic examination under general anesthesia. General anesthesia is considered the norm for this kind of operation because the cooperation of the patient is a necessity, and the rigidity of the mass limits the ability to maneuver atraumatically. A large nasal mass was atraumatically removed following careful endoscopic manipulation by pushing it with an old Eustachian tube catheter to the nasopharynx and subsequently to the oropharynx. At the end of these maneuvers, it was collected with forceps from the oral cavity. The catheter used, although outdated, is thought to minimize trauma to the surrounding sensitive tissue. The mass removed confirmed the definitive diagnosis of a rhinolith (Figure 2). No other masses were found in either nostril.\nFigure 1.\nFigure 2.\nThe preliminary diagnosis, based on the history, physical examination, and patient age, was that of a foreign body in the nasal cavity with a superimposed infection causing the malodorous discharge. Nasal discharge could be caused by many common ENT problems in the pediatric population, such as allergic rhinitis, sinusitis, the common cold. The persistence of symptoms as well as the malodorous character of the discharge suggested an atypical explanation.\nThe clinical presentation typically consists of unilateral or bilateral rhinorrhea; although bilateral rhinorrhea is a less common finding, it is more specific for rhinolithiasis. The rhinorrhea, which is initially mucoid, progresses to mucopurulent and finally odiferous, mucopurulent discharge and, in rare cases, it may be streaked with blood. Nasal obstruction may mimic sinusitis when inflammation of the ostia causes a subsequent infection of the paranasal sinuses.[6]\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n\n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 409124,
"choiceText": "Choanal atresia",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 409125,
"choiceText": "Rhinolithiasis",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 409126,
"choiceText": "Mycetoma",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 409127,
"choiceText": "Hamartoma of nasopharynx",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 119913,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 5-Year-Old Girl With Breathing Difficulties"
},
{
"authors": "Anargyros Skalimis, MD; Ioannis Aidonis, MD",
"content": [
"Other nonspecific clinical symptoms have been noted, including headache, sleep disorders, snoring, oral breathing, and malaise. Dacryocystitis, or an infection of the nasolacrimal sac usually caused by nasolacrimal duct obstruction, may also occur. An unusual case of naso-oral fistula in a middle-aged male due to rhinolithiasis has also been reported.[7] Other findings that may accompany rhinolithiasis include chronic vestibulitis, septum deviation, and squamous cell carcinoma.[8] Rarely, patients may be asymptomatic and the diagnosis may be discovered either via imaging, as a surgical finding, or incidentally during a routine examination for an unrelated complaint.[4]",
"The differential diagnosis for rhinolithiasis includes choanal atresia, mycetoma, calcified nasal polyps, impacted teeth, and other space-occupying lesions (hamartoma of the nasopharynx and other benign or malignant tumors, as well as organic or inorganic foreign bodies). Choanal atresia is a congenital malformation between the nasal chamber and the posterior oropharynx, which can cause unilateral nasal obstructive symptoms that would share many of the same signs and symptoms of rhinolithiasis.[9] Bilateral choanal atresia presents at birth with severe respiratory distress and cyanosis, but unilateral atresia can present in childhood.",
"Although CT scans can help confirm the diagnosis, only by visualization during surgery may the final diagnosis of atresia be established.[9] Under general anesthesia various maneuvers will differentiate between an immobile or mobile obstruction and determine if the structure is easily excised. Cases of mycetomas may be misdiagnosed as soft-tissue tumors because they can present as solid masses.[10]",
"Causative organisms include Aspergillus species, Fusarium species, Bipolaris species, Curvularia lunata, and Pseudallescheria boydii. These may present as more friable masses and the diagnosis may be suggested by positive fungal culture. Calcified nasal polyps coexist with generalized polyposis, which is usually bilateral and not found in the pediatric population.",
"The diagnosis of an impacted tooth can only be ruled out after the operation and removal has taken place. Tumors that needed to be considered in this particular case were ones that were relevant to the age of our patient: hamartoma of the nasopharynx, hemangioma, teratoma, odontoma, and nasopharyngeal carcinoma (although this is rare).[11,12] CT findings can be helpful in ruling out tumors, as tumors are slightly to very irregularly bordered and are less radiopaque in comparison to rhinoliths, which are well-defined and more radiopaque."
],
"date": "June 11, 2018",
"figures": [],
"markdown": "# A 5-Year-Old Girl With Breathing Difficulties\n\n **Authors:** Anargyros Skalimis, MD; Ioannis Aidonis, MD \n **Date:** June 11, 2018\n\n ## Content\n\n Other nonspecific clinical symptoms have been noted, including headache, sleep disorders, snoring, oral breathing, and malaise. Dacryocystitis, or an infection of the nasolacrimal sac usually caused by nasolacrimal duct obstruction, may also occur. An unusual case of naso-oral fistula in a middle-aged male due to rhinolithiasis has also been reported.[7] Other findings that may accompany rhinolithiasis include chronic vestibulitis, septum deviation, and squamous cell carcinoma.[8] Rarely, patients may be asymptomatic and the diagnosis may be discovered either via imaging, as a surgical finding, or incidentally during a routine examination for an unrelated complaint.[4]\nThe differential diagnosis for rhinolithiasis includes choanal atresia, mycetoma, calcified nasal polyps, impacted teeth, and other space-occupying lesions (hamartoma of the nasopharynx and other benign or malignant tumors, as well as organic or inorganic foreign bodies). Choanal atresia is a congenital malformation between the nasal chamber and the posterior oropharynx, which can cause unilateral nasal obstructive symptoms that would share many of the same signs and symptoms of rhinolithiasis.[9] Bilateral choanal atresia presents at birth with severe respiratory distress and cyanosis, but unilateral atresia can present in childhood.\nAlthough CT scans can help confirm the diagnosis, only by visualization during surgery may the final diagnosis of atresia be established.[9] Under general anesthesia various maneuvers will differentiate between an immobile or mobile obstruction and determine if the structure is easily excised. Cases of mycetomas may be misdiagnosed as soft-tissue tumors because they can present as solid masses.[10]\nCausative organisms include Aspergillus species, Fusarium species, Bipolaris species, Curvularia lunata, and Pseudallescheria boydii. These may present as more friable masses and the diagnosis may be suggested by positive fungal culture. Calcified nasal polyps coexist with generalized polyposis, which is usually bilateral and not found in the pediatric population.\nThe diagnosis of an impacted tooth can only be ruled out after the operation and removal has taken place. Tumors that needed to be considered in this particular case were ones that were relevant to the age of our patient: hamartoma of the nasopharynx, hemangioma, teratoma, odontoma, and nasopharyngeal carcinoma (although this is rare).[11,12] CT findings can be helpful in ruling out tumors, as tumors are slightly to very irregularly bordered and are less radiopaque in comparison to rhinoliths, which are well-defined and more radiopaque.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 5-Year-Old Girl With Breathing Difficulties"
},
{
"authors": "Anargyros Skalimis, MD; Ioannis Aidonis, MD",
"content": [
"Imaging is a key factor in establishing the diagnosis. MacIntyre first described the x-ray appearance of a rhinolith in 1900.[13] A CT scan is very sensitive for recognizing even small amounts of calcification, as well as the effect on nearby anatomical structures.[14] A CT scan helps to distinguish foreign bodies and resulting rhinoliths from other clinical entities by giving us useful information regarding the shape, size, extent, and location of the rhinolith and its relation to the surrounding tissues.",
"Histopathology can further confirm the mass' nature and reveal stratified calcification around a nidus.[15] In this case, the core was an inorganic substance originating from a plastic toy that was heart-shaped. This foreign body was likely introduced into the nasal cavity accidentally several months before the symptom onset and presentation."
],
"date": "June 11, 2018",
"figures": [],
"markdown": "# A 5-Year-Old Girl With Breathing Difficulties\n\n **Authors:** Anargyros Skalimis, MD; Ioannis Aidonis, MD \n **Date:** June 11, 2018\n\n ## Content\n\n Imaging is a key factor in establishing the diagnosis. MacIntyre first described the x-ray appearance of a rhinolith in 1900.[13] A CT scan is very sensitive for recognizing even small amounts of calcification, as well as the effect on nearby anatomical structures.[14] A CT scan helps to distinguish foreign bodies and resulting rhinoliths from other clinical entities by giving us useful information regarding the shape, size, extent, and location of the rhinolith and its relation to the surrounding tissues.\nHistopathology can further confirm the mass' nature and reveal stratified calcification around a nidus.[15] In this case, the core was an inorganic substance originating from a plastic toy that was heart-shaped. This foreign body was likely introduced into the nasal cavity accidentally several months before the symptom onset and presentation.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 409128,
"choiceText": "Endoscopic examination under local anesthesia",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 409129,
"choiceText": "Endoscopic examination with the child conscious to protect the airway reflexes",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 409130,
"choiceText": "Endoscopic examination under general anesthesia",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 409131,
"choiceText": "Anterior rhinoscopy under local anesthesia",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 409804,
"choiceText": "None of the above",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "General anesthesia is the best option as it provides good conditions for mobilization and removal of the foreign body. In pediatric populations, patient compliance can be difficult, and local anesthesia is neither sufficient nor appropriate for either the endoscopic or anterior approach. If local anesthesia affects the reflexes while the airway is not secured, dislodgment of a rigid mass to the respiratory track may cause a serious airway obstruction.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 119914,
"questionText": "An uncooperative child presents with unilateral, odoriferous, mucopurulent drainage and you are concerned about a nasal foreign body, but the child is unable to tolerate an endoscopic examination. Which of the following should be the next diagnostic examination?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 409132,
"choiceText": "Although this appears to be a rhinolith, the CT findings are very general and it really could be anything",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 409133,
"choiceText": "This is formed around a foreign body in the nose, and the findings on CT are very typical for this",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 409134,
"choiceText": "This is could be a rhinolith or cancer",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 409135,
"choiceText": "This is a rhinolith but it might also be choanal atresia",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Choanal atresia, or a congenital malformation of bony plates in the choana, must be included on the list of differential diagnoses for a rhinolith. Rhinoliths are produced from neglected and unidentified inorganic foreign bodies in the nose and they may be in situ for long periods before producing symptoms. Sometimes they may even be discovered accidentally. Rhinolithiasis is relatively uncommon although nasal foreign bodies in children are common. Psychiatric patients or even children with psychological problems are a small fraction of this patient group. For the diagnosis of rhinolithiasis, CT scan shows a characteristic intranasal radiopaque space-occupying mass and is usually very typical for rhinolithiasis, excluding a majority of other possibilities.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 119915,
"questionText": "The parents of your patient are concerned about the cause of a mass identified on CT scan and her future prognosis. Given that the CT findings are consistent with a rhinolith, which of the following is the most appropriate response?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 5-Year-Old Girl With Breathing Difficulties"
},
{
"authors": "Anargyros Skalimis, MD; Ioannis Aidonis, MD",
"content": [],
"date": "June 11, 2018",
"figures": [],
"markdown": "# A 5-Year-Old Girl With Breathing Difficulties\n\n **Authors:** Anargyros Skalimis, MD; Ioannis Aidonis, MD \n **Date:** June 11, 2018\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 409128,
"choiceText": "Endoscopic examination under local anesthesia",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 409129,
"choiceText": "Endoscopic examination with the child conscious to protect the airway reflexes",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 409130,
"choiceText": "Endoscopic examination under general anesthesia",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 409131,
"choiceText": "Anterior rhinoscopy under local anesthesia",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 409804,
"choiceText": "None of the above",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "General anesthesia is the best option as it provides good conditions for mobilization and removal of the foreign body. In pediatric populations, patient compliance can be difficult, and local anesthesia is neither sufficient nor appropriate for either the endoscopic or anterior approach. If local anesthesia affects the reflexes while the airway is not secured, dislodgment of a rigid mass to the respiratory track may cause a serious airway obstruction.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 119914,
"questionText": "An uncooperative child presents with unilateral, odoriferous, mucopurulent drainage and you are concerned about a nasal foreign body, but the child is unable to tolerate an endoscopic examination. Which of the following should be the next diagnostic examination?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 409132,
"choiceText": "Although this appears to be a rhinolith, the CT findings are very general and it really could be anything",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 409133,
"choiceText": "This is formed around a foreign body in the nose, and the findings on CT are very typical for this",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 409134,
"choiceText": "This is could be a rhinolith or cancer",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 409135,
"choiceText": "This is a rhinolith but it might also be choanal atresia",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Choanal atresia, or a congenital malformation of bony plates in the choana, must be included on the list of differential diagnoses for a rhinolith. Rhinoliths are produced from neglected and unidentified inorganic foreign bodies in the nose and they may be in situ for long periods before producing symptoms. Sometimes they may even be discovered accidentally. Rhinolithiasis is relatively uncommon although nasal foreign bodies in children are common. Psychiatric patients or even children with psychological problems are a small fraction of this patient group. For the diagnosis of rhinolithiasis, CT scan shows a characteristic intranasal radiopaque space-occupying mass and is usually very typical for rhinolithiasis, excluding a majority of other possibilities.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 119915,
"questionText": "The parents of your patient are concerned about the cause of a mass identified on CT scan and her future prognosis. Given that the CT findings are consistent with a rhinolith, which of the following is the most appropriate response?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 5-Year-Old Girl With Breathing Difficulties"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 409124,
"choiceText": "Choanal atresia",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 409125,
"choiceText": "Rhinolithiasis",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 409126,
"choiceText": "Mycetoma",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 409127,
"choiceText": "Hamartoma of nasopharynx",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 119913,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 409128,
"choiceText": "Endoscopic examination under local anesthesia",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 409129,
"choiceText": "Endoscopic examination with the child conscious to protect the airway reflexes",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 409130,
"choiceText": "Endoscopic examination under general anesthesia",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 409131,
"choiceText": "Anterior rhinoscopy under local anesthesia",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 409804,
"choiceText": "None of the above",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "General anesthesia is the best option as it provides good conditions for mobilization and removal of the foreign body. In pediatric populations, patient compliance can be difficult, and local anesthesia is neither sufficient nor appropriate for either the endoscopic or anterior approach. If local anesthesia affects the reflexes while the airway is not secured, dislodgment of a rigid mass to the respiratory track may cause a serious airway obstruction.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 119914,
"questionText": "An uncooperative child presents with unilateral, odoriferous, mucopurulent drainage and you are concerned about a nasal foreign body, but the child is unable to tolerate an endoscopic examination. Which of the following should be the next diagnostic examination?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 409132,
"choiceText": "Although this appears to be a rhinolith, the CT findings are very general and it really could be anything",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 409133,
"choiceText": "This is formed around a foreign body in the nose, and the findings on CT are very typical for this",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 409134,
"choiceText": "This is could be a rhinolith or cancer",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 409135,
"choiceText": "This is a rhinolith but it might also be choanal atresia",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Choanal atresia, or a congenital malformation of bony plates in the choana, must be included on the list of differential diagnoses for a rhinolith. Rhinoliths are produced from neglected and unidentified inorganic foreign bodies in the nose and they may be in situ for long periods before producing symptoms. Sometimes they may even be discovered accidentally. Rhinolithiasis is relatively uncommon although nasal foreign bodies in children are common. Psychiatric patients or even children with psychological problems are a small fraction of this patient group. For the diagnosis of rhinolithiasis, CT scan shows a characteristic intranasal radiopaque space-occupying mass and is usually very typical for rhinolithiasis, excluding a majority of other possibilities.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 119915,
"questionText": "The parents of your patient are concerned about the cause of a mass identified on CT scan and her future prognosis. Given that the CT findings are consistent with a rhinolith, which of the following is the most appropriate response?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
742701 | /viewarticle/742701 | [
{
"authors": "Noha M. El Husseiny, MD",
"content": [
"Editor's Note:\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions.",
"A 30-year-old woman presents with a 2-month history of progressive dyspnea on mild exertion. The condition is associated with rapid regular palpitations but no orthopnea, nocturnal dyspnea, chest pain, cough, or hemoptysis. Two weeks earlier, the patient had experienced painless bilateral lower extremity swelling that reached her mid leg, with no erythema or warmth. She has also recently noted diffuse abdominal enlargement and dull, aching, right-sided abdominal pain without jaundice, alteration in her bowel habits, oliguria, polyuria, or hematuria. She received diuretic medications at that time, with resolution of her edema but without improvement in her dyspnea. A year before this presentation, she had recurrent attacks of pain and bluish discoloration of her fingers and toes on exposure to cold. During one episode, her symptoms progressed to the point that fixed color changes developed on the tip of the right big toe, with subsequent gangrene and loss of the digit. In a separate episode, she reported recurrent bleeding following tooth extraction but noted no history of purpura or cutaneous or mucus membrane bleeding. She reports no photosensitivity, hair loss, arthritis, arthralgia, or oral ulcers. She is gravida 6 para 3, with a history of 3 miscarriages."
],
"date": "December 08, 2014",
"figures": [],
"markdown": "# Abdominal Pain, Progressive Dyspnea, and Palpitations in a 30-Year-Old Woman\n\n **Authors:** Noha M. El Husseiny, MD \n **Date:** December 08, 2014\n\n ## Content\n\n Editor's Note:\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions.\nA 30-year-old woman presents with a 2-month history of progressive dyspnea on mild exertion. The condition is associated with rapid regular palpitations but no orthopnea, nocturnal dyspnea, chest pain, cough, or hemoptysis. Two weeks earlier, the patient had experienced painless bilateral lower extremity swelling that reached her mid leg, with no erythema or warmth. She has also recently noted diffuse abdominal enlargement and dull, aching, right-sided abdominal pain without jaundice, alteration in her bowel habits, oliguria, polyuria, or hematuria. She received diuretic medications at that time, with resolution of her edema but without improvement in her dyspnea. A year before this presentation, she had recurrent attacks of pain and bluish discoloration of her fingers and toes on exposure to cold. During one episode, her symptoms progressed to the point that fixed color changes developed on the tip of the right big toe, with subsequent gangrene and loss of the digit. In a separate episode, she reported recurrent bleeding following tooth extraction but noted no history of purpura or cutaneous or mucus membrane bleeding. She reports no photosensitivity, hair loss, arthritis, arthralgia, or oral ulcers. She is gravida 6 para 3, with a history of 3 miscarriages.\n\n ## Figures\n\n \n*Page 1 of 5*",
"pagination": {
"current_page": 1,
"total_pages": 5
},
"questionnaire": [],
"title": "Abdominal Pain, Progressive Dyspnea, and Palpitations in a 30-Year-Old Woman"
},
{
"authors": "Noha M. El Husseiny, MD",
"content": [
"On physical examination, she has a regular pulse of 90 beats/min, a respiratory rate of 14 breaths/min, and a temperature of 98.2°F (36.8°C). Her blood pressure is noted to be 130/80 mm Hg. Her neck veins are not distended. The extremity examination reveals bilateral clubbing of the upper digits. Both feet show sluggish capillary circulation, peripheral cyanosis, loss of nail luster (and the lost tip of the right big toe), and 2 small necrotic ulcers on the shin of the tibia. No lower extremity edema is noted and her calf muscles are nontender. Examination for the Homans' sign is negative. The cardiac examination reveals an accentuated S2 and a grade III/VI pansystolic murmur best heard over the tricuspid area, which increases with inspiration. The chest examination is unremarkable. The abdominal examination reveals hepatosplenomegaly without ascites.",
"The laboratory investigations reveal an erythrocyte sedimentation rate of 140 mm/hr, a complete blood cell count demonstrating a normocytic normochromic anemia (hemoglobin 9.8 g/dL), and thrombocytopenia (30,000/μL). The lactate dehydrogenase level is found to be normal, and a Coombs test is negative. Kidney and liver function testing are normal and the cardiac biomarkers are negative. ECG reveals P-pulmonale, right ventricular enlargement, and extreme right axis deviation (Figure 1).",
"Figure 1.",
"Figure 2.",
"Figure 3.",
"Chest radiography demonstrates right ventricular enlargement. Transthoracic echocardiography reveals a normal-sized left ventricle with good overall contractility and no segmental wall motion abnormalities at rest. Normal septal motion is observed. Normal mitral, aortic, and pulmonary valves are noted, but the tricuspid valve demonstrated severe tricuspid regurgitation, with possible vegetation. The estimated pulmonary artery systolic pressure is 57 mm Hg. Given these findings, a transesophageal echocardiogram is obtained that shows a moderately dilated right atrium and ventricle, with sizable vegetation on the tricuspid valve.",
"CT scanning of the chest with contrast reveals a markedly dilated right pulmonary arterial branch (Figure 2). Venous duplex ultrasonography of the lower extremities shows absent flow with wall thickening consistent with chronic thrombosis of both the right and left deep venous systems (Figure 3).",
"A bone marrow aspirate shows moderately hypercellular bone marrow with erythroid and megakaryocytic hyperplasia. The megakaryocytes demonstrate normal lobulation, granulation, and platelet budding."
],
"date": "December 08, 2014",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/741/964/741964-thumb1.png"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/741/964/741964-thumb2.png"
},
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/741/964/741964-thumb3.png"
}
],
"markdown": "# Abdominal Pain, Progressive Dyspnea, and Palpitations in a 30-Year-Old Woman\n\n **Authors:** Noha M. El Husseiny, MD \n **Date:** December 08, 2014\n\n ## Content\n\n On physical examination, she has a regular pulse of 90 beats/min, a respiratory rate of 14 breaths/min, and a temperature of 98.2°F (36.8°C). Her blood pressure is noted to be 130/80 mm Hg. Her neck veins are not distended. The extremity examination reveals bilateral clubbing of the upper digits. Both feet show sluggish capillary circulation, peripheral cyanosis, loss of nail luster (and the lost tip of the right big toe), and 2 small necrotic ulcers on the shin of the tibia. No lower extremity edema is noted and her calf muscles are nontender. Examination for the Homans' sign is negative. The cardiac examination reveals an accentuated S2 and a grade III/VI pansystolic murmur best heard over the tricuspid area, which increases with inspiration. The chest examination is unremarkable. The abdominal examination reveals hepatosplenomegaly without ascites.\nThe laboratory investigations reveal an erythrocyte sedimentation rate of 140 mm/hr, a complete blood cell count demonstrating a normocytic normochromic anemia (hemoglobin 9.8 g/dL), and thrombocytopenia (30,000/μL). The lactate dehydrogenase level is found to be normal, and a Coombs test is negative. Kidney and liver function testing are normal and the cardiac biomarkers are negative. ECG reveals P-pulmonale, right ventricular enlargement, and extreme right axis deviation (Figure 1).\nFigure 1.\nFigure 2.\nFigure 3.\nChest radiography demonstrates right ventricular enlargement. Transthoracic echocardiography reveals a normal-sized left ventricle with good overall contractility and no segmental wall motion abnormalities at rest. Normal septal motion is observed. Normal mitral, aortic, and pulmonary valves are noted, but the tricuspid valve demonstrated severe tricuspid regurgitation, with possible vegetation. The estimated pulmonary artery systolic pressure is 57 mm Hg. Given these findings, a transesophageal echocardiogram is obtained that shows a moderately dilated right atrium and ventricle, with sizable vegetation on the tricuspid valve.\nCT scanning of the chest with contrast reveals a markedly dilated right pulmonary arterial branch (Figure 2). Venous duplex ultrasonography of the lower extremities shows absent flow with wall thickening consistent with chronic thrombosis of both the right and left deep venous systems (Figure 3).\nA bone marrow aspirate shows moderately hypercellular bone marrow with erythroid and megakaryocytic hyperplasia. The megakaryocytes demonstrate normal lobulation, granulation, and platelet budding.\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n**Figure 3.** \n \n\n\n*Page 2 of 5*",
"pagination": {
"current_page": 2,
"total_pages": 5
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 405421,
"choiceText": "Antiphospholipid syndrome (APS)",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 405422,
"choiceText": "Infective endocarditis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 405423,
"choiceText": "Cardiac myxoma",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 405424,
"choiceText": "Thrombotic thrombocytopenic purpura (TTP)",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 118553,
"questionText": "What is the diagnosis?<br><br><i>Hint: The patient has thrombocytopenia and venous thromboembolism.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Abdominal Pain, Progressive Dyspnea, and Palpitations in a 30-Year-Old Woman"
},
{
"authors": "Noha M. El Husseiny, MD",
"content": [
"APS was the most likely diagnosis in this patient. APS may be associated with extensive arterial and/or venous thromboembolic disease, which did manifest in this case. Recurrent pulmonary thromboembolism can result in pulmonary hypertension, which is the most likely cause of this patient's progressive dyspnea. APS is known to be associated with thrombocytopenia, as was evident in this case. Recurrent miscarriages, which are thought to be secondary to derangements in clotting, can also be a component of this disease. Other diagnoses were considered but ultimately excluded. The patient was not febrile, had multiple negative blood cultures, and had no stigmata of endocarditis.",
"TTP is characterized by microangiopathic hemolytic anemia, thrombocytopenia, fever, neurologic manifestations, and renal dysfunction. It is a fatal, progressive disease without treatment. The course of illness in this case did not fit that of TTP, and her anemia was not microangiopathic in nature. APS was confirmed by an immunologic profile that revealed the anticardiolipin immunoglobulin G (aCL IgG) was 65 GPLU/mL (normal range, up to 23 GPLU/mL) with a negative aCL IgM. Lupus anticoagulant (LA) was 180 seconds (normal range, 33-45 seconds). An antinuclear antibody test was positive, while an anti-double stranded DNA test was negative. The elevation of aCL IgG persisted after 6 weeks.",
"The actual frequency of APS in the general population is unknown. Antiphospholipid antibodies (LA and aCL antibodies) are present in 1%-5% of the general population, and in about 50% of patients with systemic lupus erythematosus (SLE) and other autoimmune diseases.[1] There are 2 forms of APS. Primary APS involves thrombosis and/or obstetric complications in association with antiphospholipid antibodies, but without signs of connective tissue disease; secondary APS refers to those with SLE who also have antiphospholipid antibodies. Antiphospholipid antibodies may develop in patients receiving drugs such as phenytoin, chlorpromazine, dilantin, quinidine, procainamide, and some antibiotics. A miscellaneous group of patients with a variety of diseases, including malignancy, HIV, and other viral infections, may also be positive for antiphospholipid antibodies, although these patients are not at risk for thrombotic complications.",
"A correlation between the strength of the antiphospholipid (aPL) titer and the number of positive aPL serology tests has been noted; these tests include IgG and IgM antiphospholipid antibodies; LA, which manifests as a prolongation of the partial thromboplastin time that can be reversed with phospholipid neutralization; and anti-B2 glycoprotein (GP). A female predominance has been documented, particularly for secondary APS. This parallels the association of APS with SLE and other connective-tissue diseases, which also have a female predominance. APS is more common in young to middle-aged adults; however, it also manifests in children and elderly people. Disease onset has been reported in children as young as 8 months.[2]",
"Recently, the discovery of shared peptide sequences between B2-GPI and some microorganisms, particularly Saccharomyces cerevisiae, has led to the hypothesis that at least some cases of APS may not be of primary autoimmune origin but instead may be molecular mimicry. B2-GPI also has complex interactions with the coagulation system that are poorly understood at present.[3]",
"APS can affect any system of the body, and the clinical picture may demonstrate many different sequelae of thromboembolic disease involving the peripheral venous system (deep venous thrombosis) and central nervous system (cerebrovascular accident, sinus thrombosis). Hematologic (thrombocytopenia, hemolytic anemia), pulmonary (pulmonary embolism, pulmonary hypertension), and dermatologic (digital cyanosis and gangrene, livedo reticularis, discoid rash and chronic ulcers) complications are common. Photosensitivity and cardiac (Libman-Sacks valvulopathy, myocardial ischemia), ocular (amaurosis, retinal thrombosis), adrenal (infarction/hemorrhage), and musculoskeletal (avascular necrosis of bone) manifestations may also occur.[4]",
"APS and LA are associated with pregnancy complications that include fetal loss, fetal growth restriction, preeclampsia, thrombosis, and autoimmune thrombocytopenia, and it is distinct from SLE and other connective tissue disorders."
],
"date": "December 08, 2014",
"figures": [],
"markdown": "# Abdominal Pain, Progressive Dyspnea, and Palpitations in a 30-Year-Old Woman\n\n **Authors:** Noha M. El Husseiny, MD \n **Date:** December 08, 2014\n\n ## Content\n\n APS was the most likely diagnosis in this patient. APS may be associated with extensive arterial and/or venous thromboembolic disease, which did manifest in this case. Recurrent pulmonary thromboembolism can result in pulmonary hypertension, which is the most likely cause of this patient's progressive dyspnea. APS is known to be associated with thrombocytopenia, as was evident in this case. Recurrent miscarriages, which are thought to be secondary to derangements in clotting, can also be a component of this disease. Other diagnoses were considered but ultimately excluded. The patient was not febrile, had multiple negative blood cultures, and had no stigmata of endocarditis.\nTTP is characterized by microangiopathic hemolytic anemia, thrombocytopenia, fever, neurologic manifestations, and renal dysfunction. It is a fatal, progressive disease without treatment. The course of illness in this case did not fit that of TTP, and her anemia was not microangiopathic in nature. APS was confirmed by an immunologic profile that revealed the anticardiolipin immunoglobulin G (aCL IgG) was 65 GPLU/mL (normal range, up to 23 GPLU/mL) with a negative aCL IgM. Lupus anticoagulant (LA) was 180 seconds (normal range, 33-45 seconds). An antinuclear antibody test was positive, while an anti-double stranded DNA test was negative. The elevation of aCL IgG persisted after 6 weeks.\nThe actual frequency of APS in the general population is unknown. Antiphospholipid antibodies (LA and aCL antibodies) are present in 1%-5% of the general population, and in about 50% of patients with systemic lupus erythematosus (SLE) and other autoimmune diseases.[1] There are 2 forms of APS. Primary APS involves thrombosis and/or obstetric complications in association with antiphospholipid antibodies, but without signs of connective tissue disease; secondary APS refers to those with SLE who also have antiphospholipid antibodies. Antiphospholipid antibodies may develop in patients receiving drugs such as phenytoin, chlorpromazine, dilantin, quinidine, procainamide, and some antibiotics. A miscellaneous group of patients with a variety of diseases, including malignancy, HIV, and other viral infections, may also be positive for antiphospholipid antibodies, although these patients are not at risk for thrombotic complications.\nA correlation between the strength of the antiphospholipid (aPL) titer and the number of positive aPL serology tests has been noted; these tests include IgG and IgM antiphospholipid antibodies; LA, which manifests as a prolongation of the partial thromboplastin time that can be reversed with phospholipid neutralization; and anti-B2 glycoprotein (GP). A female predominance has been documented, particularly for secondary APS. This parallels the association of APS with SLE and other connective-tissue diseases, which also have a female predominance. APS is more common in young to middle-aged adults; however, it also manifests in children and elderly people. Disease onset has been reported in children as young as 8 months.[2]\nRecently, the discovery of shared peptide sequences between B2-GPI and some microorganisms, particularly Saccharomyces cerevisiae, has led to the hypothesis that at least some cases of APS may not be of primary autoimmune origin but instead may be molecular mimicry. B2-GPI also has complex interactions with the coagulation system that are poorly understood at present.[3]\nAPS can affect any system of the body, and the clinical picture may demonstrate many different sequelae of thromboembolic disease involving the peripheral venous system (deep venous thrombosis) and central nervous system (cerebrovascular accident, sinus thrombosis). Hematologic (thrombocytopenia, hemolytic anemia), pulmonary (pulmonary embolism, pulmonary hypertension), and dermatologic (digital cyanosis and gangrene, livedo reticularis, discoid rash and chronic ulcers) complications are common. Photosensitivity and cardiac (Libman-Sacks valvulopathy, myocardial ischemia), ocular (amaurosis, retinal thrombosis), adrenal (infarction/hemorrhage), and musculoskeletal (avascular necrosis of bone) manifestations may also occur.[4]\nAPS and LA are associated with pregnancy complications that include fetal loss, fetal growth restriction, preeclampsia, thrombosis, and autoimmune thrombocytopenia, and it is distinct from SLE and other connective tissue disorders.\n\n ## Figures\n\n \n*Page 3 of 5*",
"pagination": {
"current_page": 3,
"total_pages": 5
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 405421,
"choiceText": "Antiphospholipid syndrome (APS)",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 405422,
"choiceText": "Infective endocarditis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 405423,
"choiceText": "Cardiac myxoma",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 405424,
"choiceText": "Thrombotic thrombocytopenic purpura (TTP)",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 118553,
"questionText": "What is the diagnosis?<br><br><i>Hint: The patient has thrombocytopenia and venous thromboembolism.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Abdominal Pain, Progressive Dyspnea, and Palpitations in a 30-Year-Old Woman"
},
{
"authors": "Noha M. El Husseiny, MD",
"content": [
"Th Sapporo APS classification criteria are commonly used for APS diagnosis. Based on these criteria, a diagnosis of APS requires both (1) thrombosis in any organ or tissue or pregnancy event (1 or more miscarriages after the 10 weeks' gestation, 3 or more miscarriages before the 10 weeks' gestation, or 1 or more premature deliveries before the 34 weeks' gestation because of eclampsia) and (2) a persistently positive aPL test (> 12 wk apart in time), positive LA test result, moderate-to-high titer anticardiolipin antibodies, or moderate-to-high titer B2-GPI antibodies.[5] Whether or not testing for aPL can be undertaken in patients receiving anticoagulation, either from heparin or warfarin, is controversial. Although false-positive tests are common in some series (≤10%), titers are usually quite low and do not persist.",
"An abnormal LA is the laboratory test result that confers the strongest risk for thrombosis. The postulated biologic effects mediated by human antiphospholipid antibodies include (1) reactivity with endothelial structures, which disturbs the balance of prostaglandin E2/thromboxane production, (2) interaction with platelet prostaglandins, with consequent upregulation of platelet aggregation, (3) dysregulation of complement activation, (4) interaction of aPL with phosphatidylserine exposed during trophoblast syncytium formation, which raises the possibility of a more direct effect of these autoantibodies on placental structures,[6] and (5) autoantibodies against the fibrinolytic receptor annexin 2.[7]",
"Rarely, patients with APS may present with acute multiorgan involvement, including signs and symptoms of encephalopathy, seizures, livedo reticularis, renal insufficiency, pulmonary failure, and multiple thrombi involving both large and small vessel occlusions. The term \"catastrophic APS\" and \"Asherson syndrome\" has been applied to this constellation, which is associated with high-titered antiphospholipid antibodies and a high mortality rate (approximately 50%).[8]",
"The international consensus statement is commonly used for establishing a diagnosis of catastrophic APS (CAPS). Based on this statement, a definite CAPS diagnosis requires (1) vascular thrombosis in 3 or more organs or tissues, (2) the development of manifestations simultaneously or in less than a week, (3) evidence of small vessel thrombosis in at least 1 organ or tissue, and (4) laboratory confirmation of the presence of aPL.[9]",
"The differential diagnosis of APS includes disseminated intravascular coagulation, infective endocarditis, TTP, heparin-induced thrombocytopenia, and inherited or acquired hypercoagulable states.",
"Appropriate procedures to evaluate specific thrombotic events differ according to the clinical situation and the vascular location involved. These include CT scanning or MRI of the brain (for the identification and characterization of cerebrovascular accident), CT angiography of the chest (pulmonary embolism), and CT scanning of the abdomen with contrast to identify thromboembolic disease in the intestinal or hepatic circulation (Budd-Chiari syndrome). Doppler ultrasound studies are recommended for detecting deep vein thrombosis. Two-dimensional echocardiography findings may demonstrate asymptomatic valve thickening, vegetations, or valvular insufficiency; aortic or mitral insufficiency is the most common valvular defect found in persons with Libman-Sacks endocarditis.",
"Treatment for APS largely depends on the clinical scenario and the severity of disease. Patients with active thrombotic disease should be on appropriate anticoagulation with an international normalized ratio (INR) goal of 2-3 if warfarin anticoagulation is used. Hydroxychloroquine has been shown in several studies to have intrinsic antithrombotic effects and may be considered in patients who have had a history of clot. Aspirin is added to most treatment regimens although evidence supporting its use is meager. Similarly, clopidogrel has been used in place of and in addition to aspirin. In a review that evaluated current knowledge on the pathogenesis of miscarriage in APS and the impact of different antithrombotic therapy strategies, Hoppe et al reported that the combined use of heparin and aspirin rather than either agent alone decreased the miscarriage risk in women with APS and recurrent abortions.[10] To determine the preferred therapeutic strategy and whether detailed characterization of aPL specificities would be beneficial in individualizing treatment, the investigators suggested that well-designed trials and improved profiling of at-risk patients are necessary.[10]",
"The patient in this case started corticosteroid therapy at a dose of 40 mg/day aiming to elevate her platelet count before beginning anticoagulation. At a platelet count of 50,000/μL, anticoagulation therapy was started in the form of low-molecular-weight heparin (LMWH), and warfarin was added 5 days later. LMWH was discontinued when her INR was 3. Low-dose aspirin was added. Nifedipine was added at a dose of 80 mg/day, with gradual improvement in the patient's dyspnea. A low-dose thiazide diuretic was also added, and the patient continues to be followed at monthly follow-up appointments."
],
"date": "December 08, 2014",
"figures": [],
"markdown": "# Abdominal Pain, Progressive Dyspnea, and Palpitations in a 30-Year-Old Woman\n\n **Authors:** Noha M. El Husseiny, MD \n **Date:** December 08, 2014\n\n ## Content\n\n Th Sapporo APS classification criteria are commonly used for APS diagnosis. Based on these criteria, a diagnosis of APS requires both (1) thrombosis in any organ or tissue or pregnancy event (1 or more miscarriages after the 10 weeks' gestation, 3 or more miscarriages before the 10 weeks' gestation, or 1 or more premature deliveries before the 34 weeks' gestation because of eclampsia) and (2) a persistently positive aPL test (> 12 wk apart in time), positive LA test result, moderate-to-high titer anticardiolipin antibodies, or moderate-to-high titer B2-GPI antibodies.[5] Whether or not testing for aPL can be undertaken in patients receiving anticoagulation, either from heparin or warfarin, is controversial. Although false-positive tests are common in some series (≤10%), titers are usually quite low and do not persist.\nAn abnormal LA is the laboratory test result that confers the strongest risk for thrombosis. The postulated biologic effects mediated by human antiphospholipid antibodies include (1) reactivity with endothelial structures, which disturbs the balance of prostaglandin E2/thromboxane production, (2) interaction with platelet prostaglandins, with consequent upregulation of platelet aggregation, (3) dysregulation of complement activation, (4) interaction of aPL with phosphatidylserine exposed during trophoblast syncytium formation, which raises the possibility of a more direct effect of these autoantibodies on placental structures,[6] and (5) autoantibodies against the fibrinolytic receptor annexin 2.[7]\nRarely, patients with APS may present with acute multiorgan involvement, including signs and symptoms of encephalopathy, seizures, livedo reticularis, renal insufficiency, pulmonary failure, and multiple thrombi involving both large and small vessel occlusions. The term \"catastrophic APS\" and \"Asherson syndrome\" has been applied to this constellation, which is associated with high-titered antiphospholipid antibodies and a high mortality rate (approximately 50%).[8]\nThe international consensus statement is commonly used for establishing a diagnosis of catastrophic APS (CAPS). Based on this statement, a definite CAPS diagnosis requires (1) vascular thrombosis in 3 or more organs or tissues, (2) the development of manifestations simultaneously or in less than a week, (3) evidence of small vessel thrombosis in at least 1 organ or tissue, and (4) laboratory confirmation of the presence of aPL.[9]\nThe differential diagnosis of APS includes disseminated intravascular coagulation, infective endocarditis, TTP, heparin-induced thrombocytopenia, and inherited or acquired hypercoagulable states.\nAppropriate procedures to evaluate specific thrombotic events differ according to the clinical situation and the vascular location involved. These include CT scanning or MRI of the brain (for the identification and characterization of cerebrovascular accident), CT angiography of the chest (pulmonary embolism), and CT scanning of the abdomen with contrast to identify thromboembolic disease in the intestinal or hepatic circulation (Budd-Chiari syndrome). Doppler ultrasound studies are recommended for detecting deep vein thrombosis. Two-dimensional echocardiography findings may demonstrate asymptomatic valve thickening, vegetations, or valvular insufficiency; aortic or mitral insufficiency is the most common valvular defect found in persons with Libman-Sacks endocarditis.\nTreatment for APS largely depends on the clinical scenario and the severity of disease. Patients with active thrombotic disease should be on appropriate anticoagulation with an international normalized ratio (INR) goal of 2-3 if warfarin anticoagulation is used. Hydroxychloroquine has been shown in several studies to have intrinsic antithrombotic effects and may be considered in patients who have had a history of clot. Aspirin is added to most treatment regimens although evidence supporting its use is meager. Similarly, clopidogrel has been used in place of and in addition to aspirin. In a review that evaluated current knowledge on the pathogenesis of miscarriage in APS and the impact of different antithrombotic therapy strategies, Hoppe et al reported that the combined use of heparin and aspirin rather than either agent alone decreased the miscarriage risk in women with APS and recurrent abortions.[10] To determine the preferred therapeutic strategy and whether detailed characterization of aPL specificities would be beneficial in individualizing treatment, the investigators suggested that well-designed trials and improved profiling of at-risk patients are necessary.[10]\nThe patient in this case started corticosteroid therapy at a dose of 40 mg/day aiming to elevate her platelet count before beginning anticoagulation. At a platelet count of 50,000/μL, anticoagulation therapy was started in the form of low-molecular-weight heparin (LMWH), and warfarin was added 5 days later. LMWH was discontinued when her INR was 3. Low-dose aspirin was added. Nifedipine was added at a dose of 80 mg/day, with gradual improvement in the patient's dyspnea. A low-dose thiazide diuretic was also added, and the patient continues to be followed at monthly follow-up appointments.\n\n ## Figures\n\n \n*Page 4 of 5*",
"pagination": {
"current_page": 4,
"total_pages": 5
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 405425,
"choiceText": "Livedo reticularis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 405426,
"choiceText": "Leg ulcers",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 405427,
"choiceText": "Discoid rash",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 405428,
"choiceText": "Erythema nodosum",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 405429,
"choiceText": "Digital gangrene",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Dermatologic manifestations of APS include digital cyanosis, livedo reticularis, digital gangrene, and leg ulceration. Photosensitivity and discoid rash are also typical. Older lesions may be atrophic. Erythema nodosum is not a manifestation of APS.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 118554,
"questionText": "Which of the following features is NOT likely to occur in a patient with primary APS?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 405430,
"choiceText": "Plasmapheresis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 405431,
"choiceText": "Aspirin",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 405432,
"choiceText": "Warfarin",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 405433,
"choiceText": "Heparin",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 405434,
"choiceText": "All of the above",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "APS is often treated with aspirin to inhibit platelet activation and/or warfarin as an anticoagulant. The goal of treatment was once thought to require an INR of 3-4, but the consensus is now to maintain the INR at the higher end of 2-3. During pregnancy, LMWH and low-dose aspirin are used instead of warfarin because of warfarin's risk of teratogenicity. Women with recurrent miscarriage are often advised to take aspirin and to start LMWH treatment after missing a menstrual cycle. In refractory cases, plasmapheresis may be employed.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 118555,
"questionText": "Which of the following therapeutic choices may be used for the treatment of APS?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Abdominal Pain, Progressive Dyspnea, and Palpitations in a 30-Year-Old Woman"
},
{
"authors": "Noha M. El Husseiny, MD",
"content": [],
"date": "December 08, 2014",
"figures": [],
"markdown": "# Abdominal Pain, Progressive Dyspnea, and Palpitations in a 30-Year-Old Woman\n\n **Authors:** Noha M. El Husseiny, MD \n **Date:** December 08, 2014\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 5 of 5*",
"pagination": {
"current_page": 5,
"total_pages": 5
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 405425,
"choiceText": "Livedo reticularis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 405426,
"choiceText": "Leg ulcers",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 405427,
"choiceText": "Discoid rash",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 405428,
"choiceText": "Erythema nodosum",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 405429,
"choiceText": "Digital gangrene",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Dermatologic manifestations of APS include digital cyanosis, livedo reticularis, digital gangrene, and leg ulceration. Photosensitivity and discoid rash are also typical. Older lesions may be atrophic. Erythema nodosum is not a manifestation of APS.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 118554,
"questionText": "Which of the following features is NOT likely to occur in a patient with primary APS?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 405430,
"choiceText": "Plasmapheresis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 405431,
"choiceText": "Aspirin",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 405432,
"choiceText": "Warfarin",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 405433,
"choiceText": "Heparin",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 405434,
"choiceText": "All of the above",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "APS is often treated with aspirin to inhibit platelet activation and/or warfarin as an anticoagulant. The goal of treatment was once thought to require an INR of 3-4, but the consensus is now to maintain the INR at the higher end of 2-3. During pregnancy, LMWH and low-dose aspirin are used instead of warfarin because of warfarin's risk of teratogenicity. Women with recurrent miscarriage are often advised to take aspirin and to start LMWH treatment after missing a menstrual cycle. In refractory cases, plasmapheresis may be employed.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 118555,
"questionText": "Which of the following therapeutic choices may be used for the treatment of APS?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Abdominal Pain, Progressive Dyspnea, and Palpitations in a 30-Year-Old Woman"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 405421,
"choiceText": "Antiphospholipid syndrome (APS)",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 405422,
"choiceText": "Infective endocarditis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 405423,
"choiceText": "Cardiac myxoma",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 405424,
"choiceText": "Thrombotic thrombocytopenic purpura (TTP)",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 118553,
"questionText": "What is the diagnosis?<br><br><i>Hint: The patient has thrombocytopenia and venous thromboembolism.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 405425,
"choiceText": "Livedo reticularis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 405426,
"choiceText": "Leg ulcers",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 405427,
"choiceText": "Discoid rash",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 405428,
"choiceText": "Erythema nodosum",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 405429,
"choiceText": "Digital gangrene",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Dermatologic manifestations of APS include digital cyanosis, livedo reticularis, digital gangrene, and leg ulceration. Photosensitivity and discoid rash are also typical. Older lesions may be atrophic. Erythema nodosum is not a manifestation of APS.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 118554,
"questionText": "Which of the following features is NOT likely to occur in a patient with primary APS?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 405430,
"choiceText": "Plasmapheresis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 405431,
"choiceText": "Aspirin",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 405432,
"choiceText": "Warfarin",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 405433,
"choiceText": "Heparin",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 405434,
"choiceText": "All of the above",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "APS is often treated with aspirin to inhibit platelet activation and/or warfarin as an anticoagulant. The goal of treatment was once thought to require an INR of 3-4, but the consensus is now to maintain the INR at the higher end of 2-3. During pregnancy, LMWH and low-dose aspirin are used instead of warfarin because of warfarin's risk of teratogenicity. Women with recurrent miscarriage are often advised to take aspirin and to start LMWH treatment after missing a menstrual cycle. In refractory cases, plasmapheresis may be employed.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 118555,
"questionText": "Which of the following therapeutic choices may be used for the treatment of APS?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
741905 | /viewarticle/741905 | [
{
"authors": "Jose Nicolas Codolosa, MD; Ricardo Balestra, MD; Martine David, MD; Glenn Eiger, MD; Kimberly M. Jegel, MD",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to recognize accurately. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"This case is presented in partnership with the Albert Einstein Medical Center Department of Internal Medicine, a recognized Medscape Academic Partner.",
"A 59-year-old man with a history of hypertension presents for an elective subtotal colectomy for colon cancer. A recent workup identified a near-obstructing mass at the patient's splenic flexure. As an outpatient, he underwent a colonoscopy with a biopsy of the mass which revealed a moderately differentiated adenocarcinoma. The colonoscopy also revealed a second lesion in the cecum.",
"At the time of admission, he denies any symptoms of constipation, diarrhea, abdominal pain, weight loss, or hematochezia. He has no history of heavy alcohol intake, tobacco use, or illicit drug use. He does not have a family history of malignancy. His medications include telmisartan, hydrochlorothiazide, and amlodipine.",
"The patient undergoes a successful subtotal colectomy and ileocolic anastomosis, without any signs of complication. His immediate postoperative state is stable, but on postoperative day 4 he develops sudden-onset shortness of breath. He denies having any chest pain, palpitations, nausea, or diaphoresis."
],
"date": "August 27, 2018",
"figures": [],
"markdown": "# A 59-Year-Old Man With Shortness of Breath After Colectomy\n\n **Authors:** Jose Nicolas Codolosa, MD; Ricardo Balestra, MD; Martine David, MD; Glenn Eiger, MD; Kimberly M. Jegel, MD \n **Date:** August 27, 2018\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to recognize accurately. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nThis case is presented in partnership with the Albert Einstein Medical Center Department of Internal Medicine, a recognized Medscape Academic Partner.\nA 59-year-old man with a history of hypertension presents for an elective subtotal colectomy for colon cancer. A recent workup identified a near-obstructing mass at the patient's splenic flexure. As an outpatient, he underwent a colonoscopy with a biopsy of the mass which revealed a moderately differentiated adenocarcinoma. The colonoscopy also revealed a second lesion in the cecum.\nAt the time of admission, he denies any symptoms of constipation, diarrhea, abdominal pain, weight loss, or hematochezia. He has no history of heavy alcohol intake, tobacco use, or illicit drug use. He does not have a family history of malignancy. His medications include telmisartan, hydrochlorothiazide, and amlodipine.\nThe patient undergoes a successful subtotal colectomy and ileocolic anastomosis, without any signs of complication. His immediate postoperative state is stable, but on postoperative day 4 he develops sudden-onset shortness of breath. He denies having any chest pain, palpitations, nausea, or diaphoresis.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 59-Year-Old Man With Shortness of Breath After Colectomy"
},
{
"authors": "Jose Nicolas Codolosa, MD; Ricardo Balestra, MD; Martine David, MD; Glenn Eiger, MD; Kimberly M. Jegel, MD",
"content": [
"Upon physical examination, his blood pressure is 138/68 mm Hg; his pulse is regular, with a rate of 110 beats/min, and his respiratory rate is 30 breaths/min. His temperature is 97.7°F (36.5°C) and his oxygen saturation is 92% on room air, which improves to 98% on 2 L of oxygen via nasal cannula. He is in mild respiratory distress but is able to speak in full sentences. He is not recruiting the accessory muscles of respiration.",
"The examination of his head and neck is normal. He has mildly decreased breath sounds at his right lung base. His heart examination demonstrates a normal S1 and S2 without murmurs or gallops. His abdomen is soft, nontender, and mildly distended with good bowel sounds; a midline incision scar is clean and nontender. He has palpable peripheral arterial pulses in his upper and lower extremities. The patient did not have edema or tenderness in the lower extremities.",
"Laboratory findings, including a complete blood cell count and basic metabolic panel, are normal. An arterial blood gas on room air demonstrates a pH of 7.45, a pCO2 of 32 mm Hg, and a pO2 of 62 mm Hg, with an oxygen saturation of 93%. A chest x-ray reveals bibasilar subsegmental atelectasis. He is encouraged to perform incentive spirometry; however, his oxygen saturation deteriorates progressively. On postoperative day 5 his hypoxemia is refractory to oxygen via a non-rebreather mask and he is intubated for hypoxemic respiratory distress. He is transferred to the ICU. His ECG is pictured here (Figure 1).",
"Figure 1."
],
"date": "August 27, 2018",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/741/509/741509-thumb1.png"
}
],
"markdown": "# A 59-Year-Old Man With Shortness of Breath After Colectomy\n\n **Authors:** Jose Nicolas Codolosa, MD; Ricardo Balestra, MD; Martine David, MD; Glenn Eiger, MD; Kimberly M. Jegel, MD \n **Date:** August 27, 2018\n\n ## Content\n\n Upon physical examination, his blood pressure is 138/68 mm Hg; his pulse is regular, with a rate of 110 beats/min, and his respiratory rate is 30 breaths/min. His temperature is 97.7°F (36.5°C) and his oxygen saturation is 92% on room air, which improves to 98% on 2 L of oxygen via nasal cannula. He is in mild respiratory distress but is able to speak in full sentences. He is not recruiting the accessory muscles of respiration.\nThe examination of his head and neck is normal. He has mildly decreased breath sounds at his right lung base. His heart examination demonstrates a normal S1 and S2 without murmurs or gallops. His abdomen is soft, nontender, and mildly distended with good bowel sounds; a midline incision scar is clean and nontender. He has palpable peripheral arterial pulses in his upper and lower extremities. The patient did not have edema or tenderness in the lower extremities.\nLaboratory findings, including a complete blood cell count and basic metabolic panel, are normal. An arterial blood gas on room air demonstrates a pH of 7.45, a pCO2 of 32 mm Hg, and a pO2 of 62 mm Hg, with an oxygen saturation of 93%. A chest x-ray reveals bibasilar subsegmental atelectasis. He is encouraged to perform incentive spirometry; however, his oxygen saturation deteriorates progressively. On postoperative day 5 his hypoxemia is refractory to oxygen via a non-rebreather mask and he is intubated for hypoxemic respiratory distress. He is transferred to the ICU. His ECG is pictured here (Figure 1).\nFigure 1.\n\n ## Figures\n\n **Figure 1.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 403448,
"choiceText": "Malignant pericardial effusion with tamponade",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 403449,
"choiceText": "Massive pulmonary embolism",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 403450,
"choiceText": "Atelectasis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 403451,
"choiceText": "Acute coronary syndrome",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 403452,
"choiceText": "Pneumonia",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 117902,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 59-Year-Old Man With Shortness of Breath After Colectomy"
},
{
"authors": "Jose Nicolas Codolosa, MD; Ricardo Balestra, MD; Martine David, MD; Glenn Eiger, MD; Kimberly M. Jegel, MD",
"content": [
"A presumptive diagnosis of massive pulmonary embolism was made on the basis of the patient's medical history, current physical findings, and the ECG findings. The radiographic finding of mild subsegmental atelectasis is not adequate to explain the significant and refractory hypoxemia. A diagnosis of malignant pericardial effusion was entertained, but an echocardiogram showed no effusion, a normal left ventricular ejection fraction, and a normal diastolic function. Furthermore, the right ventricle was dilated and had severe systolic dysfunction. A CT angiogram of the chest showed saddle pulmonary embolism (Figures 2 and 3) and right ventricular strain.",
"Figure 2.",
"Figure 3.",
"Pulmonary embolism is a relatively common condition that can be potentially lethal. It affects all age groups. Prompt diagnosis and treatment can reduce the morbidity and mortality associated with pulmonary embolism; however, the diagnosis is often missed because the symptoms are frequently vague and nonspecific.[1]",
"Thrombi responsible for pulmonary emboli usually arise in the deep venous system of the lower extremities; however, they may rarely originate in veins in the pelvis, kidneys, upper extremities, or the right heart chambers. After traveling to the lung, large thrombi can lodge at the bifurcation of the main pulmonary artery or the lobar branches and cause hemodynamic compromise. Typically, smaller thrombi occlude smaller vessels in the lung periphery. These are more likely to produce pleuritic chest pain by initiating an inflammatory response adjacent to the parietal pleura.",
"Most pulmonary emboli are associated with multiple embolic events, and the lower lobes are more commonly involved.[1]",
"The incidence of pulmonary embolism in the United States is estimated at 1 case per 1000 persons per year, and it appears to be significantly higher in patients of African ancestry than in patients in other racial groups. Mortality rates from pulmonary embolism for blacks have been 50% higher than those for whites, and those for whites have been 50% higher than those for people of other races (eg, Asians, Native Americans).[1] Males and females seem to be equally affected; however, recurrent thromboembolic events are more common in men than in women. Venous thromboembolism and pulmonary embolism are diseases associated with advancing age. This may well be the result of a cumulative effect of risk factors that patients acquire with aging.[1]"
],
"date": "August 27, 2018",
"figures": [
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/741/509/741509-thumb2.png"
},
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/741/509/741509-thumb3.png"
}
],
"markdown": "# A 59-Year-Old Man With Shortness of Breath After Colectomy\n\n **Authors:** Jose Nicolas Codolosa, MD; Ricardo Balestra, MD; Martine David, MD; Glenn Eiger, MD; Kimberly M. Jegel, MD \n **Date:** August 27, 2018\n\n ## Content\n\n A presumptive diagnosis of massive pulmonary embolism was made on the basis of the patient's medical history, current physical findings, and the ECG findings. The radiographic finding of mild subsegmental atelectasis is not adequate to explain the significant and refractory hypoxemia. A diagnosis of malignant pericardial effusion was entertained, but an echocardiogram showed no effusion, a normal left ventricular ejection fraction, and a normal diastolic function. Furthermore, the right ventricle was dilated and had severe systolic dysfunction. A CT angiogram of the chest showed saddle pulmonary embolism (Figures 2 and 3) and right ventricular strain.\nFigure 2.\nFigure 3.\nPulmonary embolism is a relatively common condition that can be potentially lethal. It affects all age groups. Prompt diagnosis and treatment can reduce the morbidity and mortality associated with pulmonary embolism; however, the diagnosis is often missed because the symptoms are frequently vague and nonspecific.[1]\nThrombi responsible for pulmonary emboli usually arise in the deep venous system of the lower extremities; however, they may rarely originate in veins in the pelvis, kidneys, upper extremities, or the right heart chambers. After traveling to the lung, large thrombi can lodge at the bifurcation of the main pulmonary artery or the lobar branches and cause hemodynamic compromise. Typically, smaller thrombi occlude smaller vessels in the lung periphery. These are more likely to produce pleuritic chest pain by initiating an inflammatory response adjacent to the parietal pleura.\nMost pulmonary emboli are associated with multiple embolic events, and the lower lobes are more commonly involved.[1]\nThe incidence of pulmonary embolism in the United States is estimated at 1 case per 1000 persons per year, and it appears to be significantly higher in patients of African ancestry than in patients in other racial groups. Mortality rates from pulmonary embolism for blacks have been 50% higher than those for whites, and those for whites have been 50% higher than those for people of other races (eg, Asians, Native Americans).[1] Males and females seem to be equally affected; however, recurrent thromboembolic events are more common in men than in women. Venous thromboembolism and pulmonary embolism are diseases associated with advancing age. This may well be the result of a cumulative effect of risk factors that patients acquire with aging.[1]\n\n ## Figures\n\n **Figure 2.** \n \n\n**Figure 3.** \n \n\n\n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 403448,
"choiceText": "Malignant pericardial effusion with tamponade",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 403449,
"choiceText": "Massive pulmonary embolism",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 403450,
"choiceText": "Atelectasis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 403451,
"choiceText": "Acute coronary syndrome",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 403452,
"choiceText": "Pneumonia",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 117902,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 59-Year-Old Man With Shortness of Breath After Colectomy"
},
{
"authors": "Jose Nicolas Codolosa, MD; Ricardo Balestra, MD; Martine David, MD; Glenn Eiger, MD; Kimberly M. Jegel, MD",
"content": [
"The presentation of pulmonary embolism may vary from gradually progressive dyspnea to sudden catastrophic hemodynamic collapse with sudden death or syncope. The diagnosis of pulmonary embolism should be considered in patients with respiratory symptoms unexplained by an alternate diagnosis. The symptoms of pulmonary embolism are nonspecific; therefore, a high index of suspicion is required, particularly when a patient has risk factors for the condition. The presentation of patients with pulmonary embolism can be categorized into multiple classes on the basis of the acuity and severity of pulmonary arterial occlusion.[1]",
"Large emboli compromise sufficient pulmonary circulation to produce circulatory collapse and shock. Symptoms of massive pulmonary embolism include hypotension, tachycardia, weakness, pallor, diaphoresis, oliguria, and altered mentation.",
"Approximately 10% of patients with pulmonary emboli have occlusion of a pulmonary artery, causing parenchymal infarction.[1] Symptoms include acute-onset pleuritic chest pain, breathlessness, and hemoptysis. The chest pain may be indistinguishable from acute ischemic coronary syndrome. Patients with acute embolism without infarction may have nonspecific symptoms of unexplained dyspnea and/or substernal discomfort or atypical/pleuritic chest pain.",
"The most common symptoms of pulmonary embolism in the Prospective Investigation of Pulmonary Embolism Diagnosis (PIOPED) study were dyspnea (73%), pleuritic chest pain (66%), cough (37%), and hemoptysis (13%).[1]",
"The ECG in Figure 1 demonstrates sinus tachycardia at a rate of 110 beats/min with a prominent S-wave in lead I, a prominent Q-wave in lead III, and a biphasic T wave in lead III (known as the S1Q3T3 pattern). A right bundle branch lock is also present, which is a new finding for this patient, and a premature ventricular contraction is evident.",
"Figure 1.",
"Manifestations of acute right ventricular overload, such as the S1Q3T3 pattern, right bundle branch block, P-wave pulmonale, or new right-axis deviation, are often transient or new findings on ECG and are considered to be more common with massive embolism than with smaller emboli. Unfortunately, these findings are not sensitive or specific enough to make the diagnosis by ECG alone. However, an observational subgroup of the PIOPED study that included 117 patients with pulmonary embolism concluded that the ECG was abnormal in as much as 70% of patients with pulmonary embolism. Nonspecific ST segment or T-wave abnormalities occurred in 49% of the patients, whereas the aforementioned signs of right ventricular overload were present in only 6% of the patients.[2] Tachyarrhythmia (sinus tachycardia, atrial flutter, atrial fibrillation, or another supraventricular tachycardia) is the rhythm most commonly associated with pulmonary embolism. Unexplained, new-onset tachycardia in any patient with risk factors should prompt suspicion for pulmonary embolus.",
"Both the postoperative state and the presence of malignancy are associated with pulmonary embolus. A study performed by Kakkar and colleagues compared the incidence of autopsy-confirmed fatal pulmonary embolism, death, and bleeding in cancer patients (n = 6124) with that in noncancer patients (n = 16, 954). Fatal pulmonary embolism was significantly more frequent in cancer patients than in noncancer patients (relative risk [RR], 3.7; 95% confidence interval [CI], 1.80-7.77; P = .0001). Perioperative mortality was also significantly higher in cancer patients than in noncancer patients (RR, 4.54; 95% CI, 3.59-5.76; P = .0001).[3]",
"Other classic risk factors for the development of venous thromboembolism include the use of oral contraceptives (especially in young females), immobilization, prolonged travel, and pregnancy. Congenital hypercoagulable states have also been described in this setting, most commonly resulting from a mutation in factor V Leiden, and also acquired deficiencies in protein C, protein S, and antithrombin III.[1]",
"Different diagnostic modalities can be used to diagnose pulmonary embolism. When clinical prediction rule results indicate that the patient has a low pretest probability of pulmonary embolism, D-dimer testing is the usual next step because negative results reliably exclude pulmonary embolism. CT angiography is the initial imaging modality of choice for stable patients with suspected pulmonary embolism. In centers inexperienced in interpreting CT angiography, or in patients who cannot undergo the study, ventilation-perfusion scanning is a reasonable option. Doppler ultrasound of the lower extremities has excellent sensitivity and specificity to diagnose deep venous thrombosis (DVT) but its sensitivity to diagnose pulmonary embolism is much lower, especially in patients without symptoms of DVT.[1]"
],
"date": "August 27, 2018",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/741/509/741509-thumb1.png"
}
],
"markdown": "# A 59-Year-Old Man With Shortness of Breath After Colectomy\n\n **Authors:** Jose Nicolas Codolosa, MD; Ricardo Balestra, MD; Martine David, MD; Glenn Eiger, MD; Kimberly M. Jegel, MD \n **Date:** August 27, 2018\n\n ## Content\n\n The presentation of pulmonary embolism may vary from gradually progressive dyspnea to sudden catastrophic hemodynamic collapse with sudden death or syncope. The diagnosis of pulmonary embolism should be considered in patients with respiratory symptoms unexplained by an alternate diagnosis. The symptoms of pulmonary embolism are nonspecific; therefore, a high index of suspicion is required, particularly when a patient has risk factors for the condition. The presentation of patients with pulmonary embolism can be categorized into multiple classes on the basis of the acuity and severity of pulmonary arterial occlusion.[1]\nLarge emboli compromise sufficient pulmonary circulation to produce circulatory collapse and shock. Symptoms of massive pulmonary embolism include hypotension, tachycardia, weakness, pallor, diaphoresis, oliguria, and altered mentation.\nApproximately 10% of patients with pulmonary emboli have occlusion of a pulmonary artery, causing parenchymal infarction.[1] Symptoms include acute-onset pleuritic chest pain, breathlessness, and hemoptysis. The chest pain may be indistinguishable from acute ischemic coronary syndrome. Patients with acute embolism without infarction may have nonspecific symptoms of unexplained dyspnea and/or substernal discomfort or atypical/pleuritic chest pain.\nThe most common symptoms of pulmonary embolism in the Prospective Investigation of Pulmonary Embolism Diagnosis (PIOPED) study were dyspnea (73%), pleuritic chest pain (66%), cough (37%), and hemoptysis (13%).[1]\nThe ECG in Figure 1 demonstrates sinus tachycardia at a rate of 110 beats/min with a prominent S-wave in lead I, a prominent Q-wave in lead III, and a biphasic T wave in lead III (known as the S1Q3T3 pattern). A right bundle branch lock is also present, which is a new finding for this patient, and a premature ventricular contraction is evident.\nFigure 1.\nManifestations of acute right ventricular overload, such as the S1Q3T3 pattern, right bundle branch block, P-wave pulmonale, or new right-axis deviation, are often transient or new findings on ECG and are considered to be more common with massive embolism than with smaller emboli. Unfortunately, these findings are not sensitive or specific enough to make the diagnosis by ECG alone. However, an observational subgroup of the PIOPED study that included 117 patients with pulmonary embolism concluded that the ECG was abnormal in as much as 70% of patients with pulmonary embolism. Nonspecific ST segment or T-wave abnormalities occurred in 49% of the patients, whereas the aforementioned signs of right ventricular overload were present in only 6% of the patients.[2] Tachyarrhythmia (sinus tachycardia, atrial flutter, atrial fibrillation, or another supraventricular tachycardia) is the rhythm most commonly associated with pulmonary embolism. Unexplained, new-onset tachycardia in any patient with risk factors should prompt suspicion for pulmonary embolus.\nBoth the postoperative state and the presence of malignancy are associated with pulmonary embolus. A study performed by Kakkar and colleagues compared the incidence of autopsy-confirmed fatal pulmonary embolism, death, and bleeding in cancer patients (n = 6124) with that in noncancer patients (n = 16, 954). Fatal pulmonary embolism was significantly more frequent in cancer patients than in noncancer patients (relative risk [RR], 3.7; 95% confidence interval [CI], 1.80-7.77; P = .0001). Perioperative mortality was also significantly higher in cancer patients than in noncancer patients (RR, 4.54; 95% CI, 3.59-5.76; P = .0001).[3]\nOther classic risk factors for the development of venous thromboembolism include the use of oral contraceptives (especially in young females), immobilization, prolonged travel, and pregnancy. Congenital hypercoagulable states have also been described in this setting, most commonly resulting from a mutation in factor V Leiden, and also acquired deficiencies in protein C, protein S, and antithrombin III.[1]\nDifferent diagnostic modalities can be used to diagnose pulmonary embolism. When clinical prediction rule results indicate that the patient has a low pretest probability of pulmonary embolism, D-dimer testing is the usual next step because negative results reliably exclude pulmonary embolism. CT angiography is the initial imaging modality of choice for stable patients with suspected pulmonary embolism. In centers inexperienced in interpreting CT angiography, or in patients who cannot undergo the study, ventilation-perfusion scanning is a reasonable option. Doppler ultrasound of the lower extremities has excellent sensitivity and specificity to diagnose deep venous thrombosis (DVT) but its sensitivity to diagnose pulmonary embolism is much lower, especially in patients without symptoms of DVT.[1]\n\n ## Figures\n\n **Figure 1.** \n \n\n\n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 59-Year-Old Man With Shortness of Breath After Colectomy"
},
{
"authors": "Jose Nicolas Codolosa, MD; Ricardo Balestra, MD; Martine David, MD; Glenn Eiger, MD; Kimberly M. Jegel, MD",
"content": [
"Treatment includes immediate, full anticoagulation for all patients suspected to have DVT or pulmonary embolism. In fact, diagnostic investigations should not delay empirical anticoagulant therapy. Current guidelines recommend starting unfractionated heparin (UFH), low-molecular-weight heparin (LMWH), or fondaparinux (all grade 1A recommendations) in addition to an oral anticoagulant (warfarin) at the time of diagnosis, and to discontinue UFH, LMWH, or fondaparinux only after the international normalized ratio is 2.0 for at least 24 hours, but no sooner than 5 days after warfarin therapy has been started (grade 1C recommendation). The recommended duration of UFH, LMWH, and fondaparinux concomitant to warfarin therapy is based on evidence suggesting that early monotherapy with warfarin may provoke a paradoxical hypercoagulable state. The current grade 1A recommendation is that patients with acute pulmonary embolism should not routinely receive vena cava filters in addition to anticoagulants. Inferior vena cava (IVC) filter (Greenfield filter) is only indicated in patients with acute venous thromboembolism who have an absolute contraindication to anticoagulant therapy (eg, recent surgery, hemorrhagic stroke, significant active or recent bleeding), those with massive pulmonary embolism who survived but in whom recurrent embolism invariably will be fatal, and in those who have objectively documented recurrent venous thromboembolism, adequate anticoagulant therapy notwithstanding.[1]",
"In a retrospective analysis, Stein et al suggested that in hemodynamically stable patients with acute pulmonary embolism, levels of cardiac biomarkers creatine kinase isoenzyme MB (CK-MB) and cardiac troponin I (cTnI) combined with right ventricular size have possible prognostic value.[4] High levels of both cardiac biomarkers and the presence of right ventricular dilatation were each associated with increased mortality; however, the combination of all 3 of these factors had a tendency toward the highest mortality. A trend toward higher mortality was noted in stable patients with acute pulmonary embolism and high CK-MB levels compared with those with high levels of cTnI or right ventricular dilatation; despite this, the value of CK-MB as a single prognostic indicator was limited due to its low prevalence.[4] In addition, CK-MB and cTnI levels only had prognostic value in the presence of right ventricular dilatation. Due to the small sample size, these results require further investigation.",
"A patient with a first thromboembolic event occurring in the setting of reversible risk factors such as immobilization, surgery, or trauma should receive warfarin therapy for at least 3 months. Among patients with an idiopathic (or unprovoked) thromboembolic event, the current recommendation is anticoagulation for at least 3 months, and the need for extending the duration of anticoagulation should be reevaluated at that time.",
"Warfarin treatment for longer than 6 months is indicated in patients with recurrent venous thromboembolism or in those in whom a continuing risk factor for venous thromboembolism exists, including malignancy, immobilization, or morbid obesity.[5]",
"A meta-analysis published in 2008 addressed the question of UFH vs LMWH for thromboprophylaxis. No differences in mortality in patients receiving LMWH compared with UFH (RR, 0.89; 95% CI, 0.61-1.28) or in the incidence of clinically suspected DVT (RR, 0.73; 95% CI, 0.23- 2.28) were noted.[6]",
"The duration of thromboprophylaxis for cancer patients undergoing surgery has also been discussed in literature. Three randomized controlled trials were included in a systematic review published in 2008.[7] No significant difference was observed between extended (4 weeks) and limited duration thromboprophylaxis in terms of death at 3 months (RR, 0.49; 95% CI, 0.12-1.94). However, an extended regimen was associated with a significantly lower risk for asymptomatic DVT (RR, 0.21; 95% CI, 0.05-0.94). On the basis of these data, the American College of Chest Physicians (ACCP) included in its guidelines a recommendation to consider thromboprophylaxis for up to 28 days after hospital discharge.[8] Regarding other aspects of thromboprophylaxis, mechanical methods should be used primarily in patients at high risk for bleeding or possibly as an adjunct to anticoagulant-based thromboprophylaxis. Mechanical thromboprophylaxis can be achieved with properly fitted graduated compression stockings or intermittent pneumatic compression. Aspirin is not an adequate method of thromboprophylaxis.[8]",
"Thrombolysis is indicated for hemodynamically unstable patients with pulmonary embolism. Current recommendations based on the ACCP guidelines limit the use of thrombolysis for patients with hemodynamic compromise and occasionally for high-risk patients with a low risk for bleeding.[5] In regard to the case described above, the patient was not a candidate for intravenous tissue plasminogen activator (tPA) due to recent abdominal surgery. The interventional radiology team attempted mechanical thrombectomy and local tPA infusion and achieved a partial radiologic response. An inferior vena cava filter was inserted. There are no randomized controlled trials to evaluate interventional techniques to treat massive pulmonary embolism. Case series have confirmed the safety and potential benefits of these methods. One such study included 18 patients with massive pulmonary embolism and failed thrombolysis who underwent thrombus fragmentation and aspiration. Significant improvements in the hemodynamic parameters were noted, including systolic systemic blood pressure, mean pulmonary arterial pressure, and oxygen saturation.[9]",
"The patient in this case remained hypotensive despite the treatment described above and was taken emergently to the operating room to undergo pulmonary embolectomy. The surgical technique used for acute pulmonary embolectomy is a variation of the modified Trendelenburg procedure used by many surgeons.[10] Historical literature emphasized a near prohibitive mortality rate (57%-85%) in patients undergoing pulmonary embolectomy during or after cardiopulmonary arrest for salvage. In view of its use as a last resort, a systematic review from 2007 analyzed historic case series and included data from 1300 patients. In patients operated on before 1985, the average mortality was 32%, compared with 20% of patients from 1985 to 2005. In patients who experienced cardiac arrest before pulmonary embolectomy, the operative mortality was 59% compared with 29% in patients who did not have preoperative cardiac arrest.[11] A report from the Mayo Clinic showed 30-day survival rates of 83%.[6] ACCP guidelines suggest that pulmonary embolectomy may be used in highly compromised patients who are unable to receive thrombolytics because of a bleeding risk or whose critical status does not allow time for systemic thrombolytics to be effective.[12]",
"Despite undergoing pulmonary embolectomy, the patient in this case remained hypotensive and was started on extracorporeal membrane oxygenation (ECMO). ECMO is a modality that provides respiratory and cardiac support in patients who are unresponsive to conventional therapeutic interventions. In summary, blood is removed from the venous system via cannulation of a femoral vein or centrally via cannulation of the right atrium, the carbon dioxide is extracted and the blood is oxygenated, and then it is returned to the arterial circulation either via a femoral artery or centrally via the ascending aorta. Alternatively, the oxygenated blood can be returned to the venous circulation in situations in which only respiratory support is required (ie, acute respiratory distress syndrome). The most common indication for ECMO as a cardiac support device is failure to wean from cardiopulmonary bypass. The Extracorporeal Life Support Organization maintains a registry of all known cases in which ECMO was used. Data show that cardiac support cases have accounted for less than 1% of the total cases in which ECMO was used. Survival to discharge was 33%.[13] Its role in massive pulmonary embolism is based on case reports.[14,15]",
"Unfortunately, the patient in this case developed multiorgan failure, including acute kidney injury requiring hemodialysis and an ischemic bowel, and died 24 hours after the institution of ECMO."
],
"date": "August 27, 2018",
"figures": [],
"markdown": "# A 59-Year-Old Man With Shortness of Breath After Colectomy\n\n **Authors:** Jose Nicolas Codolosa, MD; Ricardo Balestra, MD; Martine David, MD; Glenn Eiger, MD; Kimberly M. Jegel, MD \n **Date:** August 27, 2018\n\n ## Content\n\n Treatment includes immediate, full anticoagulation for all patients suspected to have DVT or pulmonary embolism. In fact, diagnostic investigations should not delay empirical anticoagulant therapy. Current guidelines recommend starting unfractionated heparin (UFH), low-molecular-weight heparin (LMWH), or fondaparinux (all grade 1A recommendations) in addition to an oral anticoagulant (warfarin) at the time of diagnosis, and to discontinue UFH, LMWH, or fondaparinux only after the international normalized ratio is 2.0 for at least 24 hours, but no sooner than 5 days after warfarin therapy has been started (grade 1C recommendation). The recommended duration of UFH, LMWH, and fondaparinux concomitant to warfarin therapy is based on evidence suggesting that early monotherapy with warfarin may provoke a paradoxical hypercoagulable state. The current grade 1A recommendation is that patients with acute pulmonary embolism should not routinely receive vena cava filters in addition to anticoagulants. Inferior vena cava (IVC) filter (Greenfield filter) is only indicated in patients with acute venous thromboembolism who have an absolute contraindication to anticoagulant therapy (eg, recent surgery, hemorrhagic stroke, significant active or recent bleeding), those with massive pulmonary embolism who survived but in whom recurrent embolism invariably will be fatal, and in those who have objectively documented recurrent venous thromboembolism, adequate anticoagulant therapy notwithstanding.[1]\nIn a retrospective analysis, Stein et al suggested that in hemodynamically stable patients with acute pulmonary embolism, levels of cardiac biomarkers creatine kinase isoenzyme MB (CK-MB) and cardiac troponin I (cTnI) combined with right ventricular size have possible prognostic value.[4] High levels of both cardiac biomarkers and the presence of right ventricular dilatation were each associated with increased mortality; however, the combination of all 3 of these factors had a tendency toward the highest mortality. A trend toward higher mortality was noted in stable patients with acute pulmonary embolism and high CK-MB levels compared with those with high levels of cTnI or right ventricular dilatation; despite this, the value of CK-MB as a single prognostic indicator was limited due to its low prevalence.[4] In addition, CK-MB and cTnI levels only had prognostic value in the presence of right ventricular dilatation. Due to the small sample size, these results require further investigation.\nA patient with a first thromboembolic event occurring in the setting of reversible risk factors such as immobilization, surgery, or trauma should receive warfarin therapy for at least 3 months. Among patients with an idiopathic (or unprovoked) thromboembolic event, the current recommendation is anticoagulation for at least 3 months, and the need for extending the duration of anticoagulation should be reevaluated at that time.\nWarfarin treatment for longer than 6 months is indicated in patients with recurrent venous thromboembolism or in those in whom a continuing risk factor for venous thromboembolism exists, including malignancy, immobilization, or morbid obesity.[5]\nA meta-analysis published in 2008 addressed the question of UFH vs LMWH for thromboprophylaxis. No differences in mortality in patients receiving LMWH compared with UFH (RR, 0.89; 95% CI, 0.61-1.28) or in the incidence of clinically suspected DVT (RR, 0.73; 95% CI, 0.23- 2.28) were noted.[6]\nThe duration of thromboprophylaxis for cancer patients undergoing surgery has also been discussed in literature. Three randomized controlled trials were included in a systematic review published in 2008.[7] No significant difference was observed between extended (4 weeks) and limited duration thromboprophylaxis in terms of death at 3 months (RR, 0.49; 95% CI, 0.12-1.94). However, an extended regimen was associated with a significantly lower risk for asymptomatic DVT (RR, 0.21; 95% CI, 0.05-0.94). On the basis of these data, the American College of Chest Physicians (ACCP) included in its guidelines a recommendation to consider thromboprophylaxis for up to 28 days after hospital discharge.[8] Regarding other aspects of thromboprophylaxis, mechanical methods should be used primarily in patients at high risk for bleeding or possibly as an adjunct to anticoagulant-based thromboprophylaxis. Mechanical thromboprophylaxis can be achieved with properly fitted graduated compression stockings or intermittent pneumatic compression. Aspirin is not an adequate method of thromboprophylaxis.[8]\nThrombolysis is indicated for hemodynamically unstable patients with pulmonary embolism. Current recommendations based on the ACCP guidelines limit the use of thrombolysis for patients with hemodynamic compromise and occasionally for high-risk patients with a low risk for bleeding.[5] In regard to the case described above, the patient was not a candidate for intravenous tissue plasminogen activator (tPA) due to recent abdominal surgery. The interventional radiology team attempted mechanical thrombectomy and local tPA infusion and achieved a partial radiologic response. An inferior vena cava filter was inserted. There are no randomized controlled trials to evaluate interventional techniques to treat massive pulmonary embolism. Case series have confirmed the safety and potential benefits of these methods. One such study included 18 patients with massive pulmonary embolism and failed thrombolysis who underwent thrombus fragmentation and aspiration. Significant improvements in the hemodynamic parameters were noted, including systolic systemic blood pressure, mean pulmonary arterial pressure, and oxygen saturation.[9]\nThe patient in this case remained hypotensive despite the treatment described above and was taken emergently to the operating room to undergo pulmonary embolectomy. The surgical technique used for acute pulmonary embolectomy is a variation of the modified Trendelenburg procedure used by many surgeons.[10] Historical literature emphasized a near prohibitive mortality rate (57%-85%) in patients undergoing pulmonary embolectomy during or after cardiopulmonary arrest for salvage. In view of its use as a last resort, a systematic review from 2007 analyzed historic case series and included data from 1300 patients. In patients operated on before 1985, the average mortality was 32%, compared with 20% of patients from 1985 to 2005. In patients who experienced cardiac arrest before pulmonary embolectomy, the operative mortality was 59% compared with 29% in patients who did not have preoperative cardiac arrest.[11] A report from the Mayo Clinic showed 30-day survival rates of 83%.[6] ACCP guidelines suggest that pulmonary embolectomy may be used in highly compromised patients who are unable to receive thrombolytics because of a bleeding risk or whose critical status does not allow time for systemic thrombolytics to be effective.[12]\nDespite undergoing pulmonary embolectomy, the patient in this case remained hypotensive and was started on extracorporeal membrane oxygenation (ECMO). ECMO is a modality that provides respiratory and cardiac support in patients who are unresponsive to conventional therapeutic interventions. In summary, blood is removed from the venous system via cannulation of a femoral vein or centrally via cannulation of the right atrium, the carbon dioxide is extracted and the blood is oxygenated, and then it is returned to the arterial circulation either via a femoral artery or centrally via the ascending aorta. Alternatively, the oxygenated blood can be returned to the venous circulation in situations in which only respiratory support is required (ie, acute respiratory distress syndrome). The most common indication for ECMO as a cardiac support device is failure to wean from cardiopulmonary bypass. The Extracorporeal Life Support Organization maintains a registry of all known cases in which ECMO was used. Data show that cardiac support cases have accounted for less than 1% of the total cases in which ECMO was used. Survival to discharge was 33%.[13] Its role in massive pulmonary embolism is based on case reports.[14,15]\nUnfortunately, the patient in this case developed multiorgan failure, including acute kidney injury requiring hemodialysis and an ischemic bowel, and died 24 hours after the institution of ECMO.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 403453,
"choiceText": "tPA",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 403454,
"choiceText": "Heparin",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 403455,
"choiceText": "Compression stocking",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 403456,
"choiceText": "IVC filter insertion",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "IVC filters in pulmonary embolism are indicated for DVT or PE when a contradiction to anticoagulation, recurrent PE in spite of anticoagulation, or the presence of an anticoagulation-related complication is noted.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 117903,
"questionText": "A patient presents with pulmonary embolism. He had a hemorrhagic stroke 2 weeks ago. Which of the following is the best management option?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 403457,
"choiceText": "Compression stockings",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 403458,
"choiceText": "Subcutaneous UFH for 1 week",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 403459,
"choiceText": "Subcutaneous LMWH for 1 month",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 403460,
"choiceText": "Aspirin",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "On the basis of data that found that an extended regimen of thromboprophylaxis was associated with a significantly lower risk for asymptomatic DVT in cancer patients who underwent surgery, the ACCP included a recommendation in its guidelines to consider thromboprophylaxis for up to 28 days after hospital discharge. Another meta-analysis found no differences in mortality in patients receiving LMWH compared with UFH or in the incidence of clinically suspected DVT.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 117904,
"questionText": "A patient with a diagnosis of lung cancer undergoes a pneumonectomy and will be discharged home. Which of the following is the best way to prevent a thromboembolic event?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 59-Year-Old Man With Shortness of Breath After Colectomy"
},
{
"authors": "Jose Nicolas Codolosa, MD; Ricardo Balestra, MD; Martine David, MD; Glenn Eiger, MD; Kimberly M. Jegel, MD",
"content": [],
"date": "August 27, 2018",
"figures": [],
"markdown": "# A 59-Year-Old Man With Shortness of Breath After Colectomy\n\n **Authors:** Jose Nicolas Codolosa, MD; Ricardo Balestra, MD; Martine David, MD; Glenn Eiger, MD; Kimberly M. Jegel, MD \n **Date:** August 27, 2018\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 403453,
"choiceText": "tPA",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 403454,
"choiceText": "Heparin",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 403455,
"choiceText": "Compression stocking",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 403456,
"choiceText": "IVC filter insertion",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "IVC filters in pulmonary embolism are indicated for DVT or PE when a contradiction to anticoagulation, recurrent PE in spite of anticoagulation, or the presence of an anticoagulation-related complication is noted.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 117903,
"questionText": "A patient presents with pulmonary embolism. He had a hemorrhagic stroke 2 weeks ago. Which of the following is the best management option?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 403457,
"choiceText": "Compression stockings",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 403458,
"choiceText": "Subcutaneous UFH for 1 week",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 403459,
"choiceText": "Subcutaneous LMWH for 1 month",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 403460,
"choiceText": "Aspirin",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "On the basis of data that found that an extended regimen of thromboprophylaxis was associated with a significantly lower risk for asymptomatic DVT in cancer patients who underwent surgery, the ACCP included a recommendation in its guidelines to consider thromboprophylaxis for up to 28 days after hospital discharge. Another meta-analysis found no differences in mortality in patients receiving LMWH compared with UFH or in the incidence of clinically suspected DVT.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 117904,
"questionText": "A patient with a diagnosis of lung cancer undergoes a pneumonectomy and will be discharged home. Which of the following is the best way to prevent a thromboembolic event?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 59-Year-Old Man With Shortness of Breath After Colectomy"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 403448,
"choiceText": "Malignant pericardial effusion with tamponade",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 403449,
"choiceText": "Massive pulmonary embolism",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 403450,
"choiceText": "Atelectasis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 403451,
"choiceText": "Acute coronary syndrome",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 403452,
"choiceText": "Pneumonia",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 117902,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 403453,
"choiceText": "tPA",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 403454,
"choiceText": "Heparin",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 403455,
"choiceText": "Compression stocking",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 403456,
"choiceText": "IVC filter insertion",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "IVC filters in pulmonary embolism are indicated for DVT or PE when a contradiction to anticoagulation, recurrent PE in spite of anticoagulation, or the presence of an anticoagulation-related complication is noted.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 117903,
"questionText": "A patient presents with pulmonary embolism. He had a hemorrhagic stroke 2 weeks ago. Which of the following is the best management option?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 403457,
"choiceText": "Compression stockings",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 403458,
"choiceText": "Subcutaneous UFH for 1 week",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 403459,
"choiceText": "Subcutaneous LMWH for 1 month",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 403460,
"choiceText": "Aspirin",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "On the basis of data that found that an extended regimen of thromboprophylaxis was associated with a significantly lower risk for asymptomatic DVT in cancer patients who underwent surgery, the ACCP included a recommendation in its guidelines to consider thromboprophylaxis for up to 28 days after hospital discharge. Another meta-analysis found no differences in mortality in patients receiving LMWH compared with UFH or in the incidence of clinically suspected DVT.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 117904,
"questionText": "A patient with a diagnosis of lung cancer undergoes a pneumonectomy and will be discharged home. Which of the following is the best way to prevent a thromboembolic event?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
741382 | /viewarticle/741382 | [
{
"authors": "Juan Carlos Munoz, MD; Nina Y. Singh, MD; Suraj A. Naik, MD",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to recognize accurately. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"A 43-year-old Hispanic man with no significant medical history is referred from his primary care provider to the emergency department for evaluation of severe intermittent headaches, subjective fever, chills, weakness, abdominal pain, reduced appetite, early satiety, and a 30-lb weight loss over the last few months. He was in normal health until approximately 8 months ago, when he developed nonspecific abdominal pain with partial response to over-the-counter calcium carbonate and ranitidine.",
"He had been living in the United States for several years but returned to his home country in Central America 2 months ago, after which the abdominal pain intensified and localized to the right upper quadrant of his abdomen. The patient decided to return to the United States for a complete medical evaluation.",
"His medical history is significant for migraine headaches, which are well controlled with ibuprofen. He does not take any prescription medications. His current over-the-counter medications include ibuprofen, calcium carbonate, and ranitidine. He has no significant surgical history. He denies smoking, drinking alcohol, and illegal drug use."
],
"date": "October 01, 2018",
"figures": [],
"markdown": "# An 8-Month History of Vague Systemic Symptoms\n\n **Authors:** Juan Carlos Munoz, MD; Nina Y. Singh, MD; Suraj A. Naik, MD \n **Date:** October 01, 2018\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to recognize accurately. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nA 43-year-old Hispanic man with no significant medical history is referred from his primary care provider to the emergency department for evaluation of severe intermittent headaches, subjective fever, chills, weakness, abdominal pain, reduced appetite, early satiety, and a 30-lb weight loss over the last few months. He was in normal health until approximately 8 months ago, when he developed nonspecific abdominal pain with partial response to over-the-counter calcium carbonate and ranitidine.\nHe had been living in the United States for several years but returned to his home country in Central America 2 months ago, after which the abdominal pain intensified and localized to the right upper quadrant of his abdomen. The patient decided to return to the United States for a complete medical evaluation.\nHis medical history is significant for migraine headaches, which are well controlled with ibuprofen. He does not take any prescription medications. His current over-the-counter medications include ibuprofen, calcium carbonate, and ranitidine. He has no significant surgical history. He denies smoking, drinking alcohol, and illegal drug use.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "An 8-Month History of Vague Systemic Symptoms"
},
{
"authors": "Juan Carlos Munoz, MD; Nina Y. Singh, MD; Suraj A. Naik, MD",
"content": [
"Upon examination, the patient appears to be uncomfortable and has a headache and abdominal pain. His oral temperature is 96.6°F (37°C). His pulse is regular at a rate of 126 beats/min. His blood pressure is 120/82 mm Hg. His respiratory rate is 16 breaths/min.",
"During the initial physical examination, no skin rashes, cyanosis, pallor, or jaundice are noted. The examination of the head and neck is unremarkable. His lungs are clear to auscultation. His cardiac evaluation demonstrates rapid S1 and S2 heart sounds without any murmurs.",
"His abdomen is protuberant, with mild-to-moderate tenderness over the epigastrium and right upper quadrant along with mild voluntary guarding (but no rebound guarding). He has palpable hepatomegaly but no splenomegaly. No clinical evidence suggests ascites. No surgical scars are noted. Bowel sounds are present. Examination of his extremities reveals normal findings.",
"The initial laboratory analysis reveals a sodium level of 137 mEq/L, potassium level of 4.6 mEq/L, blood urea nitrogen level of 14 mg/dL, creatinine level of 0.72, white blood cell count of 17.4 × 103/µL, platelet count of 140 × 103/µL, hemoglobin level of 10.2 g/dL, and hematocrit level 31.6%. The liver function tests show an albumin level of 2.6 g/dL, total bilirubin level of 0.5 mg/dL, alkaline phosphatase level of 449 U/L, aspartate aminotransferase level of 49 U/L, and an alanine aminotransferase level of 13 U/L.",
"An initial chest x-ray shows minimal right basilar subsegmental atelectasis; otherwise, no signs of acute cardiopulmonary disease are noted. Initial CT scanning of the head without contrast is normal. MRI of the brain with and without contrast is unremarkable. CT scanning of the abdomen shows many low-attenuating lesions in the liver. These are of varied size with ill-defined borders, some with water density and some with proteinaceous density. Some of these lesions demonstrate mild enhancement. Enlarged lymph nodes are seen in the left gastric region, the peripancreatic region, at the level of the celiac axis, and in the porta hepatis (Figures 1 and 2.) A gastroenterologist is consulted and recommends an EGD (Figure 3) and an MRI of the liver for further characterization of the liver lesions.",
"Figure 1.",
"Figure 2.",
"Figure 3."
],
"date": "October 01, 2018",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/741/382/741382-thumb1.png"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/741/382/741382-thumb2.png"
},
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/741/382/741382-thumb3.png"
}
],
"markdown": "# An 8-Month History of Vague Systemic Symptoms\n\n **Authors:** Juan Carlos Munoz, MD; Nina Y. Singh, MD; Suraj A. Naik, MD \n **Date:** October 01, 2018\n\n ## Content\n\n Upon examination, the patient appears to be uncomfortable and has a headache and abdominal pain. His oral temperature is 96.6°F (37°C). His pulse is regular at a rate of 126 beats/min. His blood pressure is 120/82 mm Hg. His respiratory rate is 16 breaths/min.\nDuring the initial physical examination, no skin rashes, cyanosis, pallor, or jaundice are noted. The examination of the head and neck is unremarkable. His lungs are clear to auscultation. His cardiac evaluation demonstrates rapid S1 and S2 heart sounds without any murmurs.\nHis abdomen is protuberant, with mild-to-moderate tenderness over the epigastrium and right upper quadrant along with mild voluntary guarding (but no rebound guarding). He has palpable hepatomegaly but no splenomegaly. No clinical evidence suggests ascites. No surgical scars are noted. Bowel sounds are present. Examination of his extremities reveals normal findings.\nThe initial laboratory analysis reveals a sodium level of 137 mEq/L, potassium level of 4.6 mEq/L, blood urea nitrogen level of 14 mg/dL, creatinine level of 0.72, white blood cell count of 17.4 × 103/µL, platelet count of 140 × 103/µL, hemoglobin level of 10.2 g/dL, and hematocrit level 31.6%. The liver function tests show an albumin level of 2.6 g/dL, total bilirubin level of 0.5 mg/dL, alkaline phosphatase level of 449 U/L, aspartate aminotransferase level of 49 U/L, and an alanine aminotransferase level of 13 U/L.\nAn initial chest x-ray shows minimal right basilar subsegmental atelectasis; otherwise, no signs of acute cardiopulmonary disease are noted. Initial CT scanning of the head without contrast is normal. MRI of the brain with and without contrast is unremarkable. CT scanning of the abdomen shows many low-attenuating lesions in the liver. These are of varied size with ill-defined borders, some with water density and some with proteinaceous density. Some of these lesions demonstrate mild enhancement. Enlarged lymph nodes are seen in the left gastric region, the peripancreatic region, at the level of the celiac axis, and in the porta hepatis (Figures 1 and 2.) A gastroenterologist is consulted and recommends an EGD (Figure 3) and an MRI of the liver for further characterization of the liver lesions.\nFigure 1.\nFigure 2.\nFigure 3.\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n**Figure 3.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 402744,
"choiceText": "Polycystic liver disease",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 402745,
"choiceText": "Hepatic cystadenocarcinoma",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 402746,
"choiceText": "Metastatic esophageal cancer",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 402747,
"choiceText": "Metastatic gastric cancer",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 402748,
"choiceText": "Hepatic abscess",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 117640,
"questionText": "Which of the following is most likely the correct diagnosis?<br />\r\n<br />\r\n<i>Hint: Note the patient's ethnicity, history of worsening abdominal pain, poor appetite, and progressive weight loss.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "An 8-Month History of Vague Systemic Symptoms"
},
{
"authors": "Juan Carlos Munoz, MD; Nina Y. Singh, MD; Suraj A. Naik, MD",
"content": [
"The patient's history, ethnic background, physical examination, and abdominal CT scan were concerning for metastatic gastric cancer. The liver MRI (not shown) demonstrated hepatic masses with findings compatible with metastatic disease. The EGD with biopsy revealed a large, submucosal, noncircumferential mass without stigmata of recent bleeding in the gastric fundus (Figure 3).",
"Figure 3.",
"Pathologic examination of the biopsy sample revealed a poorly differentiated adenocarcinoma. Colonoscopy was unremarkable. However, the carcinoembryonic antigen level was 36.39 ng/mL (reference range, 0.0-3.8 ng/mL). An ultrasound-guided, core-needle liver biopsy with imprint cytology also revealed a poorly differentiated adenocarcinoma morphologically consistent with gastric adenocarcinoma. The patient was scheduled to receive a combination of capecitabine and oxaliplatin.",
"Gastric cancer (stomach cancer) is the fifth most common malignancy worldwide and is the third leading cause of cancer-related death each year. In the United States, gastric cancer ranks fifteenth in incidence among the major types of cancer. Estimates of new cases and death in the United States in 2018 were 26,240 and 10,800, respectively. Gastric cancer is more common among men than women and among other races and ethnicities than non-Hispanic white individuals. Most patients are over 60 years of age. Cancer of the distal part of the stomach (body and antrum) has been decreasing since the 1930s; however, in the last 2 decades the incidence of more proximal gastric cancer (fundus and cardia) has increased dramatically.[1] The overall 5-year survival rate of people with gastric cancer in the United States is about 31%. One reason for this is that most gastric cancers are found at an advanced stage. The prognosis is better if the cancer is in the distal stomach.[2]",
"The exact etiology of gastric cancer is unknown. The risk for gastric cancer varies among regions of the world. East Asia, Southern and Eastern Europe, South America, and Central America are considered high-risk areas for gastric cancer.[3] The geographic and racial variation in the incidence of gastric cancer is most likely due to differences in diet, a major carcinogenic determinant in gastric carcinoma. A diet rich in animal proteins or high in preserved, smoked, salted, pickled, grilled, barbecued, or poorly refrigerated foods increases the risk of developing gastric cancer. Protective factors described in the literature include adequate consumption of fresh fruit, vegetables (in particular brassica vegetables, such as broccoli, cauliflower, and cabbages), and allium vegetables (such as onions and garlic).[4] Obesity also increases the risk of developing gastric cancer.[5]",
"Studies of Japanese immigrants in the United States have confirmed that early exposure to environmental rather than genetic factors has a great influence on the mortality and incidence rate of gastric cancer. The mortality rate of gastric cancer in the second and third generation of Japanese immigrants tends to decline to that of the native population of the United States.[6] Other risk factors for the development of gastric cancer include persistent inflammation of the gastric lining, such as in Helicobacter pylori infection; pernicious anemia; chronic gastritis, such as in Ménétrier disease; a history of gastric surgery; and a history of smoking.[7,8] Certain genetic disorders, such as hereditary diffuse gastric cancer, hereditary nonpolyposis colorectal cancer (Lynch syndrome), and familial adenomatous polyposis, have been associated with increased risk for stomach cancer.[9,10]",
"In a small study, Kim et al found a significantly positive correlation between cyclin-dependent kinase (CDK) 8 and β-catenin expression and the oncogenesis and progression of gastric adenocarcinoma, particularly lymph node metastasis.[11] The investigators suggested not only that: (1) the detection of CDK8 and delocalization of β-catenin may indicate a poor prognosis, but also (2) because CDK8 suppresses β-catenin activation in gastric adenocarcinoma, this finding may lead to a potential treatment in this disease.[11]"
],
"date": "October 01, 2018",
"figures": [
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/741/382/741382-thumb3.png"
}
],
"markdown": "# An 8-Month History of Vague Systemic Symptoms\n\n **Authors:** Juan Carlos Munoz, MD; Nina Y. Singh, MD; Suraj A. Naik, MD \n **Date:** October 01, 2018\n\n ## Content\n\n The patient's history, ethnic background, physical examination, and abdominal CT scan were concerning for metastatic gastric cancer. The liver MRI (not shown) demonstrated hepatic masses with findings compatible with metastatic disease. The EGD with biopsy revealed a large, submucosal, noncircumferential mass without stigmata of recent bleeding in the gastric fundus (Figure 3).\nFigure 3.\nPathologic examination of the biopsy sample revealed a poorly differentiated adenocarcinoma. Colonoscopy was unremarkable. However, the carcinoembryonic antigen level was 36.39 ng/mL (reference range, 0.0-3.8 ng/mL). An ultrasound-guided, core-needle liver biopsy with imprint cytology also revealed a poorly differentiated adenocarcinoma morphologically consistent with gastric adenocarcinoma. The patient was scheduled to receive a combination of capecitabine and oxaliplatin.\nGastric cancer (stomach cancer) is the fifth most common malignancy worldwide and is the third leading cause of cancer-related death each year. In the United States, gastric cancer ranks fifteenth in incidence among the major types of cancer. Estimates of new cases and death in the United States in 2018 were 26,240 and 10,800, respectively. Gastric cancer is more common among men than women and among other races and ethnicities than non-Hispanic white individuals. Most patients are over 60 years of age. Cancer of the distal part of the stomach (body and antrum) has been decreasing since the 1930s; however, in the last 2 decades the incidence of more proximal gastric cancer (fundus and cardia) has increased dramatically.[1] The overall 5-year survival rate of people with gastric cancer in the United States is about 31%. One reason for this is that most gastric cancers are found at an advanced stage. The prognosis is better if the cancer is in the distal stomach.[2]\nThe exact etiology of gastric cancer is unknown. The risk for gastric cancer varies among regions of the world. East Asia, Southern and Eastern Europe, South America, and Central America are considered high-risk areas for gastric cancer.[3] The geographic and racial variation in the incidence of gastric cancer is most likely due to differences in diet, a major carcinogenic determinant in gastric carcinoma. A diet rich in animal proteins or high in preserved, smoked, salted, pickled, grilled, barbecued, or poorly refrigerated foods increases the risk of developing gastric cancer. Protective factors described in the literature include adequate consumption of fresh fruit, vegetables (in particular brassica vegetables, such as broccoli, cauliflower, and cabbages), and allium vegetables (such as onions and garlic).[4] Obesity also increases the risk of developing gastric cancer.[5]\nStudies of Japanese immigrants in the United States have confirmed that early exposure to environmental rather than genetic factors has a great influence on the mortality and incidence rate of gastric cancer. The mortality rate of gastric cancer in the second and third generation of Japanese immigrants tends to decline to that of the native population of the United States.[6] Other risk factors for the development of gastric cancer include persistent inflammation of the gastric lining, such as in Helicobacter pylori infection; pernicious anemia; chronic gastritis, such as in Ménétrier disease; a history of gastric surgery; and a history of smoking.[7,8] Certain genetic disorders, such as hereditary diffuse gastric cancer, hereditary nonpolyposis colorectal cancer (Lynch syndrome), and familial adenomatous polyposis, have been associated with increased risk for stomach cancer.[9,10]\nIn a small study, Kim et al found a significantly positive correlation between cyclin-dependent kinase (CDK) 8 and β-catenin expression and the oncogenesis and progression of gastric adenocarcinoma, particularly lymph node metastasis.[11] The investigators suggested not only that: (1) the detection of CDK8 and delocalization of β-catenin may indicate a poor prognosis, but also (2) because CDK8 suppresses β-catenin activation in gastric adenocarcinoma, this finding may lead to a potential treatment in this disease.[11]\n\n ## Figures\n\n **Figure 3.** \n \n\n\n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 402744,
"choiceText": "Polycystic liver disease",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 402745,
"choiceText": "Hepatic cystadenocarcinoma",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 402746,
"choiceText": "Metastatic esophageal cancer",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 402747,
"choiceText": "Metastatic gastric cancer",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 402748,
"choiceText": "Hepatic abscess",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 117640,
"questionText": "Which of the following is most likely the correct diagnosis?<br />\r\n<br />\r\n<i>Hint: Note the patient's ethnicity, history of worsening abdominal pain, poor appetite, and progressive weight loss.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "An 8-Month History of Vague Systemic Symptoms"
},
{
"authors": "Juan Carlos Munoz, MD; Nina Y. Singh, MD; Suraj A. Naik, MD",
"content": [
"Gastric cancer tends to develop slowly over many years. During the early stages no specific signs or symptoms may be present. Early symptoms may include abdominal discomfort or pain, indigestion, heartburn, nausea, vomiting, early satiety, bloating, diarrhea or constipation, loss of appetite, and/or a sensation that food gets \"stuck\" at the level of the epigastrium. In advanced stages, patients may complain of weakness, fatigue, dysphagia, hematemesis, melena, more severe and more localized abdominal pain, unexplained weight loss, and increased abdominal girth due to ascites or tumor growth.",
"Before a true cancer develops, precancerous changes often occur in the mucosal lining of the stomach. Approximately 25% of patients have a history of gastric ulcer.[12] Tumor spread typically occurs via direct invasion of adjacent organs, via the lymphatic vessels, and in advanced cases through the bloodstream. Common metastatic distributions of gastric cancer include the left supraclavicular lymph node (Virchow node), the ovaries (Krukenberg tumor), the rectouterine/rectovesical cul-de-sac (Blumer shelf), or the peritoneum (manifesting as an ascitic fluid collection). A palpable umbilical nodule (Sister Mary Joseph nodule) may represent metastasis of gastric carcinoma to the pelvis or abdomen.",
"Early diagnosis improves the prognosis. EGD is the diagnostic method of choice; it is easy to perform, safe, and allows for sampling of tissue. Endoscopic ultrasonography can aid in determining the prognosis by evaluating the depth and extension of the tumor to adjacent structures (lymph nodes) or organs. An upper gastrointestinal series is only used when endoscopic evaluation is not available. CT scan and/or MRI can be used to assess local and potential areas of spread. Positron-emission tomography (PET) scan using 18-fluorodeoxyglucose is probably a more sensitive test than CT scan for the detection of distant metastases.[13,14] Ninety to ninety-five percent of gastric cancers are adenocarcinomas; about 4% are lymphomas, including the slow-growing, mucosal-associated lymphoid tissue (MALT) lymphoma, and about 2% are gastrointestinal stromal tumors (GISTs). Carcinoid tumors, squamous carcinoma, adenoacanthomas, and others account for the remainder of gastric cancers.",
"Gastric carcinomas can be classified in several ways, including by gross appearance and microscopic appearance. Upon gross appearance, gastric cancers can be ulcerative, polypoid, scirrhous (ie, diffuse linitis plastica), superficial spreading, multicentric, or Barrett ectopic adenocarcinoma. Scirrhous carcinoma, a poorly differentiated combination of mucin-producing carcinoma cells, can infiltrate the muscle wall and result in rigid, leathery scar tissue that is difficult to manipulate during endoscopic evaluation.",
"On the microscopic level, undifferentiated lesions or tubular, papillary, mucinous, or signet-ring cells will be seen. The Borrmann classification system categorizes gastric cancer into type I tumors, which are fungating or polypoid; type II tumors, which are ulcerating lesions with elevated borders; type III tumors, which display ulceration and invade the gastric wall; type IV tumors, which are diffusely infiltrating (ie, linitis plastica); and type V lesions, which cannot be classified. The Lauren system sorts gastric cancer histopathology as either epidemic or endemic, and it is useful in that the descriptive histopathologic entities have clinically relevant differences. Type I are expansive, epidemic-type gastric cancers of the intestine (can also be classified as Bormann types I or II) that are associated with a retained glandular structure, chronic atrophic gastritis, a clearly defined margin, and a low propensity for invasiveness. They are associated with most environmental risk factors, have a better prognosis, and are not associated with a familial history. Type II tumors, which are diffuse, poorly differentiated, and affect younger patients, are more invasive and more aggressive in females. This type has been associated with blood type A and can manifest in several members of the same family. They are also associated with mutations in the E-cadherin.[15] There are 2 staging systems: the Japanese and the more commonly used TNM classification system.[16,17]"
],
"date": "October 01, 2018",
"figures": [],
"markdown": "# An 8-Month History of Vague Systemic Symptoms\n\n **Authors:** Juan Carlos Munoz, MD; Nina Y. Singh, MD; Suraj A. Naik, MD \n **Date:** October 01, 2018\n\n ## Content\n\n Gastric cancer tends to develop slowly over many years. During the early stages no specific signs or symptoms may be present. Early symptoms may include abdominal discomfort or pain, indigestion, heartburn, nausea, vomiting, early satiety, bloating, diarrhea or constipation, loss of appetite, and/or a sensation that food gets \"stuck\" at the level of the epigastrium. In advanced stages, patients may complain of weakness, fatigue, dysphagia, hematemesis, melena, more severe and more localized abdominal pain, unexplained weight loss, and increased abdominal girth due to ascites or tumor growth.\nBefore a true cancer develops, precancerous changes often occur in the mucosal lining of the stomach. Approximately 25% of patients have a history of gastric ulcer.[12] Tumor spread typically occurs via direct invasion of adjacent organs, via the lymphatic vessels, and in advanced cases through the bloodstream. Common metastatic distributions of gastric cancer include the left supraclavicular lymph node (Virchow node), the ovaries (Krukenberg tumor), the rectouterine/rectovesical cul-de-sac (Blumer shelf), or the peritoneum (manifesting as an ascitic fluid collection). A palpable umbilical nodule (Sister Mary Joseph nodule) may represent metastasis of gastric carcinoma to the pelvis or abdomen.\nEarly diagnosis improves the prognosis. EGD is the diagnostic method of choice; it is easy to perform, safe, and allows for sampling of tissue. Endoscopic ultrasonography can aid in determining the prognosis by evaluating the depth and extension of the tumor to adjacent structures (lymph nodes) or organs. An upper gastrointestinal series is only used when endoscopic evaluation is not available. CT scan and/or MRI can be used to assess local and potential areas of spread. Positron-emission tomography (PET) scan using 18-fluorodeoxyglucose is probably a more sensitive test than CT scan for the detection of distant metastases.[13,14] Ninety to ninety-five percent of gastric cancers are adenocarcinomas; about 4% are lymphomas, including the slow-growing, mucosal-associated lymphoid tissue (MALT) lymphoma, and about 2% are gastrointestinal stromal tumors (GISTs). Carcinoid tumors, squamous carcinoma, adenoacanthomas, and others account for the remainder of gastric cancers.\nGastric carcinomas can be classified in several ways, including by gross appearance and microscopic appearance. Upon gross appearance, gastric cancers can be ulcerative, polypoid, scirrhous (ie, diffuse linitis plastica), superficial spreading, multicentric, or Barrett ectopic adenocarcinoma. Scirrhous carcinoma, a poorly differentiated combination of mucin-producing carcinoma cells, can infiltrate the muscle wall and result in rigid, leathery scar tissue that is difficult to manipulate during endoscopic evaluation.\nOn the microscopic level, undifferentiated lesions or tubular, papillary, mucinous, or signet-ring cells will be seen. The Borrmann classification system categorizes gastric cancer into type I tumors, which are fungating or polypoid; type II tumors, which are ulcerating lesions with elevated borders; type III tumors, which display ulceration and invade the gastric wall; type IV tumors, which are diffusely infiltrating (ie, linitis plastica); and type V lesions, which cannot be classified. The Lauren system sorts gastric cancer histopathology as either epidemic or endemic, and it is useful in that the descriptive histopathologic entities have clinically relevant differences. Type I are expansive, epidemic-type gastric cancers of the intestine (can also be classified as Bormann types I or II) that are associated with a retained glandular structure, chronic atrophic gastritis, a clearly defined margin, and a low propensity for invasiveness. They are associated with most environmental risk factors, have a better prognosis, and are not associated with a familial history. Type II tumors, which are diffuse, poorly differentiated, and affect younger patients, are more invasive and more aggressive in females. This type has been associated with blood type A and can manifest in several members of the same family. They are also associated with mutations in the E-cadherin.[15] There are 2 staging systems: the Japanese and the more commonly used TNM classification system.[16,17]\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "An 8-Month History of Vague Systemic Symptoms"
},
{
"authors": "Juan Carlos Munoz, MD; Nina Y. Singh, MD; Suraj A. Naik, MD",
"content": [
"Gastric cancer treatment depends on tumor type, stage, and general health of the patient. The overall prognosis of gastric cancer is generally poor due to late presentation and diagnosis. Surgery is the mainstay of treatment for nonmetastatic disease. Early disease is defined as adenocarcinoma limited to the gastric mucosa and submucosa regardless of whether regional lymph nodes are involved (T1NxMx). The prognosis for early disease is excellent, with a 5-year survival rate between 85% and 90% as compared with 28%-40% in advanced gastric cancer.[2,18,19,20] Endoscopic mucosal or submucosal resection may be curative in early gastric cancer without the need for surgery and with a similar long-term prognosis.[19,20]",
"Surgery is successful in 70%-80% of cases of newly diagnosed gastric cancer; however, recurrence occurs in 50%-60% of cases. Patients with recurrence mainly present with distant metastasis, but localized recurrence is seen in 20%-40% of cases and involves the \"gastric bed,\" the lymph nodes and/or the anastomosis, or the duodenal stump. Localized recurrences are probably related to the biological characteristics of the tumor but, in some cases, may result from an incomplete surgical procedure.[21,22] Total gastrectomy, with the exception of distal tumors that can be treated by subtotal gastrectomy, is the procedure of choice. Splenectomy is indicated for proximal advanced tumors. Distal pancreatectomy should be avoided. Controversy exists about the optimal extent of lymphadenectomy in these patients.",
"Unfortunately, most patients in clinical practice present with advanced or metastatic gastric cancer. For metastatic disease, chemotherapy is mainly palliative. There is no consensus on the recommended first-line treatment regimen. Combination treatment with (1) 5-fluorouracil, cisplatin, and epirubicin, (2) docetaxel, cisplatin, and 5-fluorouracil, or (3) capecitabine, oxaliplatin, and epirubicin has been used in clinical practice because of better partial responses. Studies that have evaluated the role of multimodality treatment options for these patients include postoperative chemoradiation and perioperative chemotherapy.[18,23,24,25] The Intergroup 0116 trial demonstrated that patients treated with surgery and postoperative chemoradiation had a significantly higher overall survival rate compared with patients treated with surgery alone.[24] The MAGIC (Medical Research Council Adjuvant Gastric Infusional Chemotherapy) trial showed that patients treated with perioperative epirubicin, cisplatin, and 5-fluorouracil had a significantly higher overall survival rate compared with patients treated with surgery alone.",
"Multidisciplinary evaluation plays a crucial role in the management of these patients.[25,26,27] In addition to the more conventional modalities, several molecular targeting agents are under investigation. HER2 protein is overexpressed in approximately 22% of patients with gastric cancer. Trastuzumab, a recombinant humanized anti-HER2 monoclonal antibody that specially targets the HER2 protein, is the first biologic therapy that has shown a survival improvement in gastric carcinoma (11 months vs 13 months); therefore, trastuzumab in combination with chemotherapy is a treatment option for patients with HER2-positive advanced gastric cancer.[28,29]",
"Tumor size, histology, depth of invasion, and lymph node metastasis are the most significant prognosis factors in gastric cancer.[30,31] Early detection and curative resection with radical lymph node dissection, followed by radiation/chemotherapy, should be the standard treatment.[30,31,32]",
"Returning to this case, the patient was scheduled to begin chemotherapy with a combination of capecitabine and oxaliplatin."
],
"date": "October 01, 2018",
"figures": [],
"markdown": "# An 8-Month History of Vague Systemic Symptoms\n\n **Authors:** Juan Carlos Munoz, MD; Nina Y. Singh, MD; Suraj A. Naik, MD \n **Date:** October 01, 2018\n\n ## Content\n\n Gastric cancer treatment depends on tumor type, stage, and general health of the patient. The overall prognosis of gastric cancer is generally poor due to late presentation and diagnosis. Surgery is the mainstay of treatment for nonmetastatic disease. Early disease is defined as adenocarcinoma limited to the gastric mucosa and submucosa regardless of whether regional lymph nodes are involved (T1NxMx). The prognosis for early disease is excellent, with a 5-year survival rate between 85% and 90% as compared with 28%-40% in advanced gastric cancer.[2,18,19,20] Endoscopic mucosal or submucosal resection may be curative in early gastric cancer without the need for surgery and with a similar long-term prognosis.[19,20]\nSurgery is successful in 70%-80% of cases of newly diagnosed gastric cancer; however, recurrence occurs in 50%-60% of cases. Patients with recurrence mainly present with distant metastasis, but localized recurrence is seen in 20%-40% of cases and involves the \"gastric bed,\" the lymph nodes and/or the anastomosis, or the duodenal stump. Localized recurrences are probably related to the biological characteristics of the tumor but, in some cases, may result from an incomplete surgical procedure.[21,22] Total gastrectomy, with the exception of distal tumors that can be treated by subtotal gastrectomy, is the procedure of choice. Splenectomy is indicated for proximal advanced tumors. Distal pancreatectomy should be avoided. Controversy exists about the optimal extent of lymphadenectomy in these patients.\nUnfortunately, most patients in clinical practice present with advanced or metastatic gastric cancer. For metastatic disease, chemotherapy is mainly palliative. There is no consensus on the recommended first-line treatment regimen. Combination treatment with (1) 5-fluorouracil, cisplatin, and epirubicin, (2) docetaxel, cisplatin, and 5-fluorouracil, or (3) capecitabine, oxaliplatin, and epirubicin has been used in clinical practice because of better partial responses. Studies that have evaluated the role of multimodality treatment options for these patients include postoperative chemoradiation and perioperative chemotherapy.[18,23,24,25] The Intergroup 0116 trial demonstrated that patients treated with surgery and postoperative chemoradiation had a significantly higher overall survival rate compared with patients treated with surgery alone.[24] The MAGIC (Medical Research Council Adjuvant Gastric Infusional Chemotherapy) trial showed that patients treated with perioperative epirubicin, cisplatin, and 5-fluorouracil had a significantly higher overall survival rate compared with patients treated with surgery alone.\nMultidisciplinary evaluation plays a crucial role in the management of these patients.[25,26,27] In addition to the more conventional modalities, several molecular targeting agents are under investigation. HER2 protein is overexpressed in approximately 22% of patients with gastric cancer. Trastuzumab, a recombinant humanized anti-HER2 monoclonal antibody that specially targets the HER2 protein, is the first biologic therapy that has shown a survival improvement in gastric carcinoma (11 months vs 13 months); therefore, trastuzumab in combination with chemotherapy is a treatment option for patients with HER2-positive advanced gastric cancer.[28,29]\nTumor size, histology, depth of invasion, and lymph node metastasis are the most significant prognosis factors in gastric cancer.[30,31] Early detection and curative resection with radical lymph node dissection, followed by radiation/chemotherapy, should be the standard treatment.[30,31,32]\nReturning to this case, the patient was scheduled to begin chemotherapy with a combination of capecitabine and oxaliplatin.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 402749,
"choiceText": "Gastric cancer is the second most common malignancy worldwide.",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 402750,
"choiceText": "The incidence of proximal cancer has decreased since the 1930s.",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 402751,
"choiceText": "The overall 5-year survival for people with gastric cancer in the United States is less than 30%.",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 402753,
"choiceText": "Gastric cancer tends to show nonspecific symptoms early on and it develops slowly over many years.",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>Gastric cancer tends to develop slowly over many years. During the early stages no specific signs or symptoms may be present. If symptoms are present, they may be vague, such as abdominal discomfort or pain, indigestion, heartburn, nausea, vomiting, early satiety, bloating, diarrhea or constipation, and loss of appetite.</p> \r\n<p>In advanced stages, patients may complain of weakness, fatigue, dysphagia, hematemesis, melena, more severe and more localized abdominal pain, unexplained weight loss, and increased abdominal girth due to ascites or tumor growth. These are all reasons why the disease may not be discovered earlier.</p>",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 117641,
"questionText": "A patient recently diagnosed with gastric cancer is concerned about the fact that the cancer wasn’t detected earlier. Which of the following statements best explains why diagnosing this disease at an early and more treatable stage is difficult?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 402754,
"choiceText": "Infection with <i>H pylori</i>",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 402755,
"choiceText": "Pernicious anemia",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 402756,
"choiceText": "Positive history of smoking",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 402757,
"choiceText": "Diet rich in vegetables and fruits",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 402758,
"choiceText": "An excess of body weight",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Protective factors described in the literature include adequate consumption of fresh fruits and vegetables (in particular, brassica vegetables such as broccoli, cauliflower, and cabbages, and allium vegetables such as onions and garlic).",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 117642,
"questionText": "Which of the following is generally <em>not</em> considered a contributing factor in the development of gastric cancer?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "An 8-Month History of Vague Systemic Symptoms"
},
{
"authors": "Juan Carlos Munoz, MD; Nina Y. Singh, MD; Suraj A. Naik, MD",
"content": [],
"date": "October 01, 2018",
"figures": [],
"markdown": "# An 8-Month History of Vague Systemic Symptoms\n\n **Authors:** Juan Carlos Munoz, MD; Nina Y. Singh, MD; Suraj A. Naik, MD \n **Date:** October 01, 2018\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 402749,
"choiceText": "Gastric cancer is the second most common malignancy worldwide.",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 402750,
"choiceText": "The incidence of proximal cancer has decreased since the 1930s.",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 402751,
"choiceText": "The overall 5-year survival for people with gastric cancer in the United States is less than 30%.",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 402753,
"choiceText": "Gastric cancer tends to show nonspecific symptoms early on and it develops slowly over many years.",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>Gastric cancer tends to develop slowly over many years. During the early stages no specific signs or symptoms may be present. If symptoms are present, they may be vague, such as abdominal discomfort or pain, indigestion, heartburn, nausea, vomiting, early satiety, bloating, diarrhea or constipation, and loss of appetite.</p> \r\n<p>In advanced stages, patients may complain of weakness, fatigue, dysphagia, hematemesis, melena, more severe and more localized abdominal pain, unexplained weight loss, and increased abdominal girth due to ascites or tumor growth. These are all reasons why the disease may not be discovered earlier.</p>",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 117641,
"questionText": "A patient recently diagnosed with gastric cancer is concerned about the fact that the cancer wasn’t detected earlier. Which of the following statements best explains why diagnosing this disease at an early and more treatable stage is difficult?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 402754,
"choiceText": "Infection with <i>H pylori</i>",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 402755,
"choiceText": "Pernicious anemia",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 402756,
"choiceText": "Positive history of smoking",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 402757,
"choiceText": "Diet rich in vegetables and fruits",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 402758,
"choiceText": "An excess of body weight",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Protective factors described in the literature include adequate consumption of fresh fruits and vegetables (in particular, brassica vegetables such as broccoli, cauliflower, and cabbages, and allium vegetables such as onions and garlic).",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 117642,
"questionText": "Which of the following is generally <em>not</em> considered a contributing factor in the development of gastric cancer?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "An 8-Month History of Vague Systemic Symptoms"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 402744,
"choiceText": "Polycystic liver disease",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 402745,
"choiceText": "Hepatic cystadenocarcinoma",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 402746,
"choiceText": "Metastatic esophageal cancer",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 402747,
"choiceText": "Metastatic gastric cancer",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 402748,
"choiceText": "Hepatic abscess",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 117640,
"questionText": "Which of the following is most likely the correct diagnosis?<br />\r\n<br />\r\n<i>Hint: Note the patient's ethnicity, history of worsening abdominal pain, poor appetite, and progressive weight loss.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 402749,
"choiceText": "Gastric cancer is the second most common malignancy worldwide.",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 402750,
"choiceText": "The incidence of proximal cancer has decreased since the 1930s.",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 402751,
"choiceText": "The overall 5-year survival for people with gastric cancer in the United States is less than 30%.",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 402753,
"choiceText": "Gastric cancer tends to show nonspecific symptoms early on and it develops slowly over many years.",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>Gastric cancer tends to develop slowly over many years. During the early stages no specific signs or symptoms may be present. If symptoms are present, they may be vague, such as abdominal discomfort or pain, indigestion, heartburn, nausea, vomiting, early satiety, bloating, diarrhea or constipation, and loss of appetite.</p> \r\n<p>In advanced stages, patients may complain of weakness, fatigue, dysphagia, hematemesis, melena, more severe and more localized abdominal pain, unexplained weight loss, and increased abdominal girth due to ascites or tumor growth. These are all reasons why the disease may not be discovered earlier.</p>",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 117641,
"questionText": "A patient recently diagnosed with gastric cancer is concerned about the fact that the cancer wasn’t detected earlier. Which of the following statements best explains why diagnosing this disease at an early and more treatable stage is difficult?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 402754,
"choiceText": "Infection with <i>H pylori</i>",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 402755,
"choiceText": "Pernicious anemia",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 402756,
"choiceText": "Positive history of smoking",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 402757,
"choiceText": "Diet rich in vegetables and fruits",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 402758,
"choiceText": "An excess of body weight",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Protective factors described in the literature include adequate consumption of fresh fruits and vegetables (in particular, brassica vegetables such as broccoli, cauliflower, and cabbages, and allium vegetables such as onions and garlic).",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 117642,
"questionText": "Which of the following is generally <em>not</em> considered a contributing factor in the development of gastric cancer?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
740944 | /viewarticle/740944 | [
{
"authors": "Musa Ibrahim Kurawa, MB BS",
"content": [
"Editor's Note:\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions.",
"A 21-year-old Fulani man presents to a local hospital in Kano, Nigeria with a two-month history of recurrent, mild abdominal discomfort. The patient also complains of weakness, weight loss, occasional diarrhea, and anal itching. He has no history of fever, headache, cough, subcutaneous nodules, or altered skin pigmentation. He has visited various clinics, at which he received multiple unknown medications for his condition. The patient resorted to attempting traditional remedies when he felt that he had not improved with prescription medications. Of note, the patient has observed occasional instances of a \"threadlike worm\" measuring a half inch to a few inches in length in his stool (Figures 1-2).",
"Figure 1.",
"Figure 2.",
"He has no history of hospital admissions, blood transfusion, or surgery. He lives with his extended family in proximity to livestock. The patient is single. He denies drinking alcohol, smoking, and using any prescribed drugs. He has no known drug allergies."
],
"date": "May 29, 2015",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/740/944/740944-thumb1.png"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/740/944/740944-thumb2.png"
}
],
"markdown": "# Recurrent Abdominal Discomfort and Gastric Disturbances in a Young Man\n\n **Authors:** Musa Ibrahim Kurawa, MB BS \n **Date:** May 29, 2015\n\n ## Content\n\n Editor's Note:\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions.\nA 21-year-old Fulani man presents to a local hospital in Kano, Nigeria with a two-month history of recurrent, mild abdominal discomfort. The patient also complains of weakness, weight loss, occasional diarrhea, and anal itching. He has no history of fever, headache, cough, subcutaneous nodules, or altered skin pigmentation. He has visited various clinics, at which he received multiple unknown medications for his condition. The patient resorted to attempting traditional remedies when he felt that he had not improved with prescription medications. Of note, the patient has observed occasional instances of a \"threadlike worm\" measuring a half inch to a few inches in length in his stool (Figures 1-2).\nFigure 1.\nFigure 2.\nHe has no history of hospital admissions, blood transfusion, or surgery. He lives with his extended family in proximity to livestock. The patient is single. He denies drinking alcohol, smoking, and using any prescribed drugs. He has no known drug allergies.\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n\n*Page 1 of 5*",
"pagination": {
"current_page": 1,
"total_pages": 5
},
"questionnaire": [],
"title": "Recurrent Abdominal Discomfort and Gastric Disturbances in a Young Man"
},
{
"authors": "Musa Ibrahim Kurawa, MB BS",
"content": [
"Upon physical examination, the patient is a tall, lean, young man who appears apprehensive and mildly pale. He has a pulse rate of 75 beats/min with a regular rhythm, a blood pressure of 120/80 mm Hg, a respiratory rate of 14 breaths/min without respiratory distress, and a normal temperature. He is 5 ft 7 in (1.7 m) tall and weighs 104 lb (47 kg).",
"The patient has no palpable lymphadenopathy and no scleral icterus or edema is observed. The patient has a normal thyroid examination and no rashes or subcutaneous nodules are noted. Normal breath sounds are found on the pulmonary examination. The cardiac examination demonstrates normal S1 and S2 heart sounds with no murmur, gallops, or rubs. No evidence of jugular venous distension is noted. The abdomen is soft and without organomegaly. Rectal examination reveals no masses, with dark stool noted. He is cooperative and has normal mentation. The patient demonstrates no focal findings on neurologic examination, including no cranial nerve palsies, muscle weakness, or loss of sensation; additionally, normal reflexes are elicited.",
"The laboratory investigations include a complete blood cell count with a hematocrit of 26% and an eosinophil count of 0.5 x 109/L. Other hematologic parameters examined are within the normal range. Abdominal ultrasonography findings are normal. On stool microscopy, small, white, rice-like bits are noted."
],
"date": "May 29, 2015",
"figures": [],
"markdown": "# Recurrent Abdominal Discomfort and Gastric Disturbances in a Young Man\n\n **Authors:** Musa Ibrahim Kurawa, MB BS \n **Date:** May 29, 2015\n\n ## Content\n\n Upon physical examination, the patient is a tall, lean, young man who appears apprehensive and mildly pale. He has a pulse rate of 75 beats/min with a regular rhythm, a blood pressure of 120/80 mm Hg, a respiratory rate of 14 breaths/min without respiratory distress, and a normal temperature. He is 5 ft 7 in (1.7 m) tall and weighs 104 lb (47 kg).\nThe patient has no palpable lymphadenopathy and no scleral icterus or edema is observed. The patient has a normal thyroid examination and no rashes or subcutaneous nodules are noted. Normal breath sounds are found on the pulmonary examination. The cardiac examination demonstrates normal S1 and S2 heart sounds with no murmur, gallops, or rubs. No evidence of jugular venous distension is noted. The abdomen is soft and without organomegaly. Rectal examination reveals no masses, with dark stool noted. He is cooperative and has normal mentation. The patient demonstrates no focal findings on neurologic examination, including no cranial nerve palsies, muscle weakness, or loss of sensation; additionally, normal reflexes are elicited.\nThe laboratory investigations include a complete blood cell count with a hematocrit of 26% and an eosinophil count of 0.5 x 109/L. Other hematologic parameters examined are within the normal range. Abdominal ultrasonography findings are normal. On stool microscopy, small, white, rice-like bits are noted.\n\n ## Figures\n\n \n*Page 2 of 5*",
"pagination": {
"current_page": 2,
"total_pages": 5
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 401209,
"choiceText": "<i>Ascaris lumbricoides</i>",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 401210,
"choiceText": "Pinworm infection",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 401211,
"choiceText": "<i>Taenia saginata</i>",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 401212,
"choiceText": "Appendicitis",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 117136,
"questionText": "What is the diagnosis?<br /><br />\r\n<i>Hint: The diagnosis was based on the patient's history, physical examination, and laboratory investigations.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Recurrent Abdominal Discomfort and Gastric Disturbances in a Young Man"
},
{
"authors": "Musa Ibrahim Kurawa, MB BS",
"content": [
"The white bits in the patient's stool were identified as segments (termed proglottids) of Taenia saginata (a beef tapeworm). A diagnosis of taeniasis with mild anemia was made. The patient presented with a history of recurrent abdominal discomfort and weight loss despite the fact that he was eating normally. He had a history of the passage of threadlike worms in the feces, pruritus ani, and generalized body weakness. The patient had visited other clinics where the treatments provided temporary or no relief. The environment in which the patient lived suggested the diagnosis. His family raised cattle and had a habit of open defecation without proper toilet facilities. Human excrement was sometimes used as fertilizer for the cultivation of crops.[1] Poor personal hygiene and consumption of undercooked beef meat is common where the patient lived.",
"T saginata is the most common large tapeworm that causes disease in humans. Transmission occurs through cysticerci in beef (\"measly beef\").[2] Large worms may grow by 15 to 30 cm a day in the human gut and pass 10 segments daily. The segments may convey up to a million eggs a day into the environment throughout the long lifespan of the worm.[2] Taeniasis occurs in areas where cattle are raised by infected humans who have poor hygiene,[3] human feces is improperly disposed of,[1,4] meat inspection programs are poor, and meat is eaten without proper cooking. All races and both sexes are equally affected.[5] All ages are susceptible to infection, with a predominance in children.",
"Partially cooked, smoked, or pickled beef can transmit the cysticerci, although raw beef (steak tartare) is the most commonly responsible foodstuff. Infection with this tapeworm is most prevalent in subSaharan African and Middle Eastern countries. T saginata asiatica is a variant of T saginata found in Asia in which pigs are the intermediate host.[5] Notably, the frequency of taeniasisis higher in countries such as Ethiopia and Argentina, where raw or undercooked beef is often eaten.[2] In the United States, T saginata infection is primarily an imported disease with a prevalence of less than 1% because most cattle in the United States are free of the parasite.[5] Approximately 50 million people worldwide are infected by T saginata or T solium. The areas with the highest prevalence (>10%) are central Asia, the Near East, and central and eastern Africa.[5]",
"The most prominent symptom of T saginata infection is the passage of proglottids through the anus. Crawling of the segments around the anal region causes the itching (pruritus ani) found in these cases. Patients are usually apprehensive about passing the proglottids.[2] The patient may experience mild-to-moderate abdominal pain or discomfort. The pain is unlikely to be severe except when there are associated complications, such as intestinal perforation or obstruction.[4] Patients may experience nausea, vomiting, diarrhea, change of appetite, or constipation. In comparison, the symptoms of headache, dizziness, hyperexcitability, convulsions, and subcutaneous nodules typically seen in cysticercosis caused by T solium (pork tapeworm) are much less common in cases of T saginata.[5] Except in certain cases of cysticercosis or complications arising from it, no direct mortality has been recorded."
],
"date": "May 29, 2015",
"figures": [],
"markdown": "# Recurrent Abdominal Discomfort and Gastric Disturbances in a Young Man\n\n **Authors:** Musa Ibrahim Kurawa, MB BS \n **Date:** May 29, 2015\n\n ## Content\n\n The white bits in the patient's stool were identified as segments (termed proglottids) of Taenia saginata (a beef tapeworm). A diagnosis of taeniasis with mild anemia was made. The patient presented with a history of recurrent abdominal discomfort and weight loss despite the fact that he was eating normally. He had a history of the passage of threadlike worms in the feces, pruritus ani, and generalized body weakness. The patient had visited other clinics where the treatments provided temporary or no relief. The environment in which the patient lived suggested the diagnosis. His family raised cattle and had a habit of open defecation without proper toilet facilities. Human excrement was sometimes used as fertilizer for the cultivation of crops.[1] Poor personal hygiene and consumption of undercooked beef meat is common where the patient lived.\nT saginata is the most common large tapeworm that causes disease in humans. Transmission occurs through cysticerci in beef (\"measly beef\").[2] Large worms may grow by 15 to 30 cm a day in the human gut and pass 10 segments daily. The segments may convey up to a million eggs a day into the environment throughout the long lifespan of the worm.[2] Taeniasis occurs in areas where cattle are raised by infected humans who have poor hygiene,[3] human feces is improperly disposed of,[1,4] meat inspection programs are poor, and meat is eaten without proper cooking. All races and both sexes are equally affected.[5] All ages are susceptible to infection, with a predominance in children.\nPartially cooked, smoked, or pickled beef can transmit the cysticerci, although raw beef (steak tartare) is the most commonly responsible foodstuff. Infection with this tapeworm is most prevalent in subSaharan African and Middle Eastern countries. T saginata asiatica is a variant of T saginata found in Asia in which pigs are the intermediate host.[5] Notably, the frequency of taeniasisis higher in countries such as Ethiopia and Argentina, where raw or undercooked beef is often eaten.[2] In the United States, T saginata infection is primarily an imported disease with a prevalence of less than 1% because most cattle in the United States are free of the parasite.[5] Approximately 50 million people worldwide are infected by T saginata or T solium. The areas with the highest prevalence (>10%) are central Asia, the Near East, and central and eastern Africa.[5]\nThe most prominent symptom of T saginata infection is the passage of proglottids through the anus. Crawling of the segments around the anal region causes the itching (pruritus ani) found in these cases. Patients are usually apprehensive about passing the proglottids.[2] The patient may experience mild-to-moderate abdominal pain or discomfort. The pain is unlikely to be severe except when there are associated complications, such as intestinal perforation or obstruction.[4] Patients may experience nausea, vomiting, diarrhea, change of appetite, or constipation. In comparison, the symptoms of headache, dizziness, hyperexcitability, convulsions, and subcutaneous nodules typically seen in cysticercosis caused by T solium (pork tapeworm) are much less common in cases of T saginata.[5] Except in certain cases of cysticercosis or complications arising from it, no direct mortality has been recorded.\n\n ## Figures\n\n \n*Page 3 of 5*",
"pagination": {
"current_page": 3,
"total_pages": 5
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 401209,
"choiceText": "<i>Ascaris lumbricoides</i>",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 401210,
"choiceText": "Pinworm infection",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 401211,
"choiceText": "<i>Taenia saginata</i>",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 401212,
"choiceText": "Appendicitis",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 117136,
"questionText": "What is the diagnosis?<br /><br />\r\n<i>Hint: The diagnosis was based on the patient's history, physical examination, and laboratory investigations.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Recurrent Abdominal Discomfort and Gastric Disturbances in a Young Man"
},
{
"authors": "Musa Ibrahim Kurawa, MB BS",
"content": [
"Atypical cases may present with signs and symptoms related to obstruction of bile or pancreatic ducts. The tapeworm can grow in ectopic loci, such as the subretinal space, vitreous space, middle ear, intraventricular spaces, or uterine cavity.[5] The diagnosis is made through careful evaluation of the patient's history, physical examination, and laboratory analysis[6] with a view to ruling out an alternative diagnosis. Investigations that can assist in making the diagnosis are stool microscopy, culture and sensitivity, and complete blood cell count.",
"A potential diagnostic tool for CNS infection by this parasite is the use of immunoblot assay to detect immunoglobulin G (IgG) antibodies to T saginata metacestode antigenic fractions without affinity to concanavalin A (ConA).[9] In one study, immunoblot assay showed 100% sensitivity and specificity for the ConA-unbound fraction relative to the 90% sensitivity and 93.1% sensitivity of enzyme-linked immunosorbent assay (ELISA) for specific antigens in human neurocystercicosis.[9] Other investigations, such as x-ray and CT, are done to exclude complications or an alternative diagnosis.",
"In this case, the patient was worried about the frequent passage of proglottids from his anus. The pruritus ani associated with the condition was so distressing to him that he at times isolated himself from his peers. He was found to be pale based on the examination of the conjunctiva, tongue, and palms. The generalized weakness the patient experienced was presumably due to his chronic anemia. The complete blood cell count confirmed anemia and also identified eosinophilia. Anemia is not a usual finding in cases of T saginata and its detection is noteworthy. A possible explanation for the anemia could be a direct result of infection by the attachment of the tapeworm to the wall of the small intestine via its suckers[4] as well as poor nutrition due to the loss of appetite[2] and diarrhea caused by the parasite.",
"The age of the patient is atypical for cases of T saginata. Although all ages are susceptible, the condition is more common among children.[5,7] The atypical age could be explained by recurrent infection due to the nature of the environment in which the patient lived, or it could be a persistent infection since childhood.[5]",
"Praziquantel (Biltricide®) is the drug of choice for most tapeworm infections. Dosing depends on the species. Dosing for taeniasis and diphyllobothriasis is 5-10 mg/kg orally (single dose), although efficacy for T saginata infections has been well demonstrated at doses as low as 2.5 mg/kg.",
"Following the diagnosis, the patient was given praziquantel 10 mg/kg.[8] He was told to take it with a liquid after a meal and that it should be swallowed without chewing. Subsequently, the patient was able to pass the tapeworm in his stool. Oral hematinics were also prescribed to address the anemia. The patient was advised on proper nutrition and proper personal hygiene to avoid reinfection. Proper cooking of meat was encouraged and the use of human feces as fertilizer was discouraged. The patient's condition did not require hospital admission. During the follow-up visit, he was looking much better. His worries were relieved, he started gaining weight, and the anemia had improved. A long-term follow-up schedule was established to check for possible reinfection.[2]",
"Niclosamide (Niclocide®) is used as an alternative treatment for tapeworms in other countries if praziquantel is not available. However, niclosamide is currently not available in the United States. Dosing consists of four tablets (500 mg) in a single dose (2 g) for adults, two tablets (1 g) for children who weigh 11-34 kg, and three tablets (1.5 g) for children who weigh less than 34 kg. Other treatments to be considered include meglumine/diatrizoate sodium.[9]"
],
"date": "May 29, 2015",
"figures": [],
"markdown": "# Recurrent Abdominal Discomfort and Gastric Disturbances in a Young Man\n\n **Authors:** Musa Ibrahim Kurawa, MB BS \n **Date:** May 29, 2015\n\n ## Content\n\n Atypical cases may present with signs and symptoms related to obstruction of bile or pancreatic ducts. The tapeworm can grow in ectopic loci, such as the subretinal space, vitreous space, middle ear, intraventricular spaces, or uterine cavity.[5] The diagnosis is made through careful evaluation of the patient's history, physical examination, and laboratory analysis[6] with a view to ruling out an alternative diagnosis. Investigations that can assist in making the diagnosis are stool microscopy, culture and sensitivity, and complete blood cell count.\nA potential diagnostic tool for CNS infection by this parasite is the use of immunoblot assay to detect immunoglobulin G (IgG) antibodies to T saginata metacestode antigenic fractions without affinity to concanavalin A (ConA).[9] In one study, immunoblot assay showed 100% sensitivity and specificity for the ConA-unbound fraction relative to the 90% sensitivity and 93.1% sensitivity of enzyme-linked immunosorbent assay (ELISA) for specific antigens in human neurocystercicosis.[9] Other investigations, such as x-ray and CT, are done to exclude complications or an alternative diagnosis.\nIn this case, the patient was worried about the frequent passage of proglottids from his anus. The pruritus ani associated with the condition was so distressing to him that he at times isolated himself from his peers. He was found to be pale based on the examination of the conjunctiva, tongue, and palms. The generalized weakness the patient experienced was presumably due to his chronic anemia. The complete blood cell count confirmed anemia and also identified eosinophilia. Anemia is not a usual finding in cases of T saginata and its detection is noteworthy. A possible explanation for the anemia could be a direct result of infection by the attachment of the tapeworm to the wall of the small intestine via its suckers[4] as well as poor nutrition due to the loss of appetite[2] and diarrhea caused by the parasite.\nThe age of the patient is atypical for cases of T saginata. Although all ages are susceptible, the condition is more common among children.[5,7] The atypical age could be explained by recurrent infection due to the nature of the environment in which the patient lived, or it could be a persistent infection since childhood.[5]\nPraziquantel (Biltricide®) is the drug of choice for most tapeworm infections. Dosing depends on the species. Dosing for taeniasis and diphyllobothriasis is 5-10 mg/kg orally (single dose), although efficacy for T saginata infections has been well demonstrated at doses as low as 2.5 mg/kg.\nFollowing the diagnosis, the patient was given praziquantel 10 mg/kg.[8] He was told to take it with a liquid after a meal and that it should be swallowed without chewing. Subsequently, the patient was able to pass the tapeworm in his stool. Oral hematinics were also prescribed to address the anemia. The patient was advised on proper nutrition and proper personal hygiene to avoid reinfection. Proper cooking of meat was encouraged and the use of human feces as fertilizer was discouraged. The patient's condition did not require hospital admission. During the follow-up visit, he was looking much better. His worries were relieved, he started gaining weight, and the anemia had improved. A long-term follow-up schedule was established to check for possible reinfection.[2]\nNiclosamide (Niclocide®) is used as an alternative treatment for tapeworms in other countries if praziquantel is not available. However, niclosamide is currently not available in the United States. Dosing consists of four tablets (500 mg) in a single dose (2 g) for adults, two tablets (1 g) for children who weigh 11-34 kg, and three tablets (1.5 g) for children who weigh less than 34 kg. Other treatments to be considered include meglumine/diatrizoate sodium.[9]\n\n ## Figures\n\n \n*Page 4 of 5*",
"pagination": {
"current_page": 4,
"total_pages": 5
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 401213,
"choiceText": "Weight loss",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 401214,
"choiceText": "Pallor",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 401215,
"choiceText": "Temperature of 98.6°F (37°C)",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 401216,
"choiceText": "Pulse rate of 75 bpm",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The pallor seen in this patient was due to anemia, which is not a usual finding in cases of <i>T saginata</i> infection. Other factors discussed in this case could explain the anemia seen as well. Weight loss is among the symptoms seen in cases of taeniasis, while a pulse rate of 75 bpm and temperature of 98.6°F (37°C) are normal findings that are expected in this condition.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 117137,
"questionText": "Which of the following clinical findings seen in this patient was considered unusual in light of the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 401217,
"choiceText": "Stool microscopy",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 401218,
"choiceText": "Abdominal ultrasound scan",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 401219,
"choiceText": "Complete blood cell count",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 401220,
"choiceText": "X-ray",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Stool microscopy is the simplest and single most important investigation for establishing a diagnosis of taeniasis. Differentiating between the <i>Taenia</i> species involves fixing the gravid proglottids in 10% formaldehyde solution and injecting the uterine branches with India ink. Abdominal ultrasound may only show certain complications, such as appendicitis. A complete blood cell count can show anemia and eosinophilia, which are not pathognomonic of the disease. X-ray is very useful in cases of cysticercosis.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 117138,
"questionText": "Which of the following laboratory investigations is the single most important investigation for making the diagnosis in patients with <i>T saginata</i>?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Recurrent Abdominal Discomfort and Gastric Disturbances in a Young Man"
},
{
"authors": "Musa Ibrahim Kurawa, MB BS",
"content": [],
"date": "May 29, 2015",
"figures": [],
"markdown": "# Recurrent Abdominal Discomfort and Gastric Disturbances in a Young Man\n\n **Authors:** Musa Ibrahim Kurawa, MB BS \n **Date:** May 29, 2015\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 5 of 5*",
"pagination": {
"current_page": 5,
"total_pages": 5
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 401213,
"choiceText": "Weight loss",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 401214,
"choiceText": "Pallor",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 401215,
"choiceText": "Temperature of 98.6°F (37°C)",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 401216,
"choiceText": "Pulse rate of 75 bpm",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The pallor seen in this patient was due to anemia, which is not a usual finding in cases of <i>T saginata</i> infection. Other factors discussed in this case could explain the anemia seen as well. Weight loss is among the symptoms seen in cases of taeniasis, while a pulse rate of 75 bpm and temperature of 98.6°F (37°C) are normal findings that are expected in this condition.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 117137,
"questionText": "Which of the following clinical findings seen in this patient was considered unusual in light of the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 401217,
"choiceText": "Stool microscopy",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 401218,
"choiceText": "Abdominal ultrasound scan",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 401219,
"choiceText": "Complete blood cell count",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 401220,
"choiceText": "X-ray",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Stool microscopy is the simplest and single most important investigation for establishing a diagnosis of taeniasis. Differentiating between the <i>Taenia</i> species involves fixing the gravid proglottids in 10% formaldehyde solution and injecting the uterine branches with India ink. Abdominal ultrasound may only show certain complications, such as appendicitis. A complete blood cell count can show anemia and eosinophilia, which are not pathognomonic of the disease. X-ray is very useful in cases of cysticercosis.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 117138,
"questionText": "Which of the following laboratory investigations is the single most important investigation for making the diagnosis in patients with <i>T saginata</i>?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Recurrent Abdominal Discomfort and Gastric Disturbances in a Young Man"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 401209,
"choiceText": "<i>Ascaris lumbricoides</i>",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 401210,
"choiceText": "Pinworm infection",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 401211,
"choiceText": "<i>Taenia saginata</i>",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 401212,
"choiceText": "Appendicitis",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 117136,
"questionText": "What is the diagnosis?<br /><br />\r\n<i>Hint: The diagnosis was based on the patient's history, physical examination, and laboratory investigations.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 401213,
"choiceText": "Weight loss",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 401214,
"choiceText": "Pallor",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 401215,
"choiceText": "Temperature of 98.6°F (37°C)",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 401216,
"choiceText": "Pulse rate of 75 bpm",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The pallor seen in this patient was due to anemia, which is not a usual finding in cases of <i>T saginata</i> infection. Other factors discussed in this case could explain the anemia seen as well. Weight loss is among the symptoms seen in cases of taeniasis, while a pulse rate of 75 bpm and temperature of 98.6°F (37°C) are normal findings that are expected in this condition.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 117137,
"questionText": "Which of the following clinical findings seen in this patient was considered unusual in light of the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 401217,
"choiceText": "Stool microscopy",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 401218,
"choiceText": "Abdominal ultrasound scan",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 401219,
"choiceText": "Complete blood cell count",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 401220,
"choiceText": "X-ray",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Stool microscopy is the simplest and single most important investigation for establishing a diagnosis of taeniasis. Differentiating between the <i>Taenia</i> species involves fixing the gravid proglottids in 10% formaldehyde solution and injecting the uterine branches with India ink. Abdominal ultrasound may only show certain complications, such as appendicitis. A complete blood cell count can show anemia and eosinophilia, which are not pathognomonic of the disease. X-ray is very useful in cases of cysticercosis.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 117138,
"questionText": "Which of the following laboratory investigations is the single most important investigation for making the diagnosis in patients with <i>T saginata</i>?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
740003 | /viewarticle/740003 | [
{
"authors": "Antonio Giovanni Richetta, MD, PhD; Simona Giancristoforo, MD; Carlo Mattozzi, MD; Sara D'Epiro, MD; Stefano Calvieri, MD",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"Figure 1.",
"An 84-year-old woman is admitted to the hospital with a 6-month history of asymptomatic, yet rapidly increasing hyperkeratotic patches on her legs. Two months after developing these macules, a red nodule developed on her right shoulder with central ulceration (Figure 1). Her medical history is significant for hypertension, polycythemia vera treated with idrossicarbamide, and gastritis."
],
"date": "December 09, 2016",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/740/003/740003-Thumb-1.png"
}
],
"markdown": "# Asymptomatic Macules and An Ulcerated Shoulder in an Elderly Woman\n\n **Authors:** Antonio Giovanni Richetta, MD, PhD; Simona Giancristoforo, MD; Carlo Mattozzi, MD; Sara D'Epiro, MD; Stefano Calvieri, MD \n **Date:** December 09, 2016\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nFigure 1.\nAn 84-year-old woman is admitted to the hospital with a 6-month history of asymptomatic, yet rapidly increasing hyperkeratotic patches on her legs. Two months after developing these macules, a red nodule developed on her right shoulder with central ulceration (Figure 1). Her medical history is significant for hypertension, polycythemia vera treated with idrossicarbamide, and gastritis.\n\n ## Figures\n\n **Figure 1.** \n \n\n\n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "Asymptomatic Macules and An Ulcerated Shoulder in an Elderly Woman"
},
{
"authors": "Antonio Giovanni Richetta, MD, PhD; Simona Giancristoforo, MD; Carlo Mattozzi, MD; Sara D'Epiro, MD; Stefano Calvieri, MD",
"content": [
"Blood tests performed during her hospitalization show no abnormal values. During her hospital stay, cultures and microscopic evaluation of the wound exudate are found to contain coagulase negative-Staphylococcus and 2 separate Pseudomonas aeruginosa strains. Based on these observations, the patient is treated with intravenous antibiotic therapy (cefepime) for 10 days and advanced topical dressings. A skin biopsy of the ulcer shows an atrophic epidermis and an infiltrate of lymphocytes at the dermal-epidermal junction, with marked epidermotropism. Both immunohistochemical staining and flow cytometric analysis reveal neoplastic cells positive for CD3+, CD8+, CD30+, and negative for CD4, CD56, with several cells CD20+.",
"The patient then undergoes brain, chest, abdomen, and pelvis CT scans, which reveal bilateral subcentimetric lymph nodes in the laterocervical area, a patchy thyroid, small ilo-mediastinal lymph nodes, and a 20-mm lymph node in the right axilla with conserved morphology, as well as some lymph nodes in the left axilla of varying size (maximum 15-mm diameter). The liver does not have vascular abnormalities. The patient undergoes an ultrasound of the abdomen showing a spleen that is increased in volume at the upper reference range limit (4 cm), small exorenal cystic lesions, and small subcentimetric lymph nodes in the retroperitoneal lumboaortic space and bilateral inguinal areas. A bone marrow biopsy does not show any pathologic features. A SPECT scan highlights increased pathologic metabolism at a right parapharyngeal lymph node and superficial soft tissues of the right shoulder, left lower extremity, and back."
],
"date": "December 09, 2016",
"figures": [],
"markdown": "# Asymptomatic Macules and An Ulcerated Shoulder in an Elderly Woman\n\n **Authors:** Antonio Giovanni Richetta, MD, PhD; Simona Giancristoforo, MD; Carlo Mattozzi, MD; Sara D'Epiro, MD; Stefano Calvieri, MD \n **Date:** December 09, 2016\n\n ## Content\n\n Blood tests performed during her hospitalization show no abnormal values. During her hospital stay, cultures and microscopic evaluation of the wound exudate are found to contain coagulase negative-Staphylococcus and 2 separate Pseudomonas aeruginosa strains. Based on these observations, the patient is treated with intravenous antibiotic therapy (cefepime) for 10 days and advanced topical dressings. A skin biopsy of the ulcer shows an atrophic epidermis and an infiltrate of lymphocytes at the dermal-epidermal junction, with marked epidermotropism. Both immunohistochemical staining and flow cytometric analysis reveal neoplastic cells positive for CD3+, CD8+, CD30+, and negative for CD4, CD56, with several cells CD20+.\nThe patient then undergoes brain, chest, abdomen, and pelvis CT scans, which reveal bilateral subcentimetric lymph nodes in the laterocervical area, a patchy thyroid, small ilo-mediastinal lymph nodes, and a 20-mm lymph node in the right axilla with conserved morphology, as well as some lymph nodes in the left axilla of varying size (maximum 15-mm diameter). The liver does not have vascular abnormalities. The patient undergoes an ultrasound of the abdomen showing a spleen that is increased in volume at the upper reference range limit (4 cm), small exorenal cystic lesions, and small subcentimetric lymph nodes in the retroperitoneal lumboaortic space and bilateral inguinal areas. A bone marrow biopsy does not show any pathologic features. A SPECT scan highlights increased pathologic metabolism at a right parapharyngeal lymph node and superficial soft tissues of the right shoulder, left lower extremity, and back.\n\n ## Figures\n\n \n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 399369,
"choiceText": "Parapsoriasis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 399370,
"choiceText": "Sézary syndrome",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 399371,
"choiceText": "Mycosis fungoides",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 399372,
"choiceText": "Primary cutaneous aggressive epidermotropic CD8+ cytotoxic T-cell lymphoma",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 116420,
"questionText": "If you have a patient with rapidly progressive skin lesions and biopsy findings as described above, what is your most likely diagnosis?<br /><br /><i>Hint: Note the history of clinical manifestation and skin biopsy.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Asymptomatic Macules and An Ulcerated Shoulder in an Elderly Woman"
},
{
"authors": "Antonio Giovanni Richetta, MD, PhD; Simona Giancristoforo, MD; Carlo Mattozzi, MD; Sara D'Epiro, MD; Stefano Calvieri, MD",
"content": [
"The diagnosis of primary cutaneous aggressive epidermotropic CD8+ cytotoxic T- cell lymphoma was established according to the World Health Organization (WHO) and European Organization for Research and Treatment of Cancer (EORTC) classification. The WHO-EORTC classification for primary cutaneous lymphoma includes mycosis fungoides, Sézary syndrome, the group of the primary cutaneous CD30 lymphoproliferations, and subcutaneous panniculitis-like T-cell lymphomas.",
"Each diagnosis above is recognized as a distinct, well-defined entity, which together constitute approximately 85% of all primary cutaneous T-cell lymphomas (CTCL). In the WHO-EORTC classification, most cases of primary CTCL that do not belong to one of above entities are included in the group of unspecified primary cutaneous peripheral T-cell lymphomas (PTLs), and rare cases such as extranodal NK/T-cell lymphoma, nasal type.[1] In the unspecified group, primary cutaneous aggressive epidermotropic CD8+ cytotoxic T-cell lymphoma, cutaneous gamma/delta T-cell lymphoma, and primary cutaneous small-medium CD4+ T-cell lymphoma are separate provisional entities. For the remaining diseases that do not fit into either of these provisional entities, the designation unspecified primary cutaneous PTL is used.",
"Primary cutaneous aggressive epidermotropic CD8+ cytotoxic T-cell lymphoma is a rare subtype of CTCL. It is a distinct and clinically aggressive form of CTCL that accounts for less than 1% of all cases of cutaneous lymphoma and carries a very poor prognosis.[2,3,4] The clinical course is extremely rapidly progressive, with an average 5-year survival estimated at 18% compared with the typical 88% in the more indolent mycosis fungoides. The EORTC Cutaneous Lymphoma Task Force's data reported that all 4 patients died from their disease within 3 years of diagnosis, with a median survival of only 22.5 months.[4] Patients affected by primary cutaneous aggressive epidermotropic CD8+ cytotoxic T-cell lymphoma typically have rapid progression with extra-cutaneous dissemination that affects quality-of-life and the overall prognosis. The pathogenesis or genetic characteristics of this disease are not known as this type of lymphoma is extremely rare and only a limited number of cases have been reported.",
"Clinically, the disease usually presents with localized or disseminated eruptive papules, nodules, and tumors showing central ulceration and necrosis, or with superficial, hyperkeratotic patches and plaques.[2,3] Unlike classic mycosis fungoides, patients do not generally follow the typical progression through patch, plaque, and finally tumor-stage disease, but rather present from the onset with widespread plaque- and tumor-stage disease.[4] However, mycosis fungoides, pagetoid reticulosis, and Sézary syndrome are included in the differential diagnosis. The clinical features of this disease are in fact very similar to those observed in patients with a cutaneous gamma/delta T-cell lymphoma and cases described as generalized pagetoid reticulosis (Ketron-Goodman type) in the past.[1]"
],
"date": "December 09, 2016",
"figures": [],
"markdown": "# Asymptomatic Macules and An Ulcerated Shoulder in an Elderly Woman\n\n **Authors:** Antonio Giovanni Richetta, MD, PhD; Simona Giancristoforo, MD; Carlo Mattozzi, MD; Sara D'Epiro, MD; Stefano Calvieri, MD \n **Date:** December 09, 2016\n\n ## Content\n\n The diagnosis of primary cutaneous aggressive epidermotropic CD8+ cytotoxic T- cell lymphoma was established according to the World Health Organization (WHO) and European Organization for Research and Treatment of Cancer (EORTC) classification. The WHO-EORTC classification for primary cutaneous lymphoma includes mycosis fungoides, Sézary syndrome, the group of the primary cutaneous CD30 lymphoproliferations, and subcutaneous panniculitis-like T-cell lymphomas.\nEach diagnosis above is recognized as a distinct, well-defined entity, which together constitute approximately 85% of all primary cutaneous T-cell lymphomas (CTCL). In the WHO-EORTC classification, most cases of primary CTCL that do not belong to one of above entities are included in the group of unspecified primary cutaneous peripheral T-cell lymphomas (PTLs), and rare cases such as extranodal NK/T-cell lymphoma, nasal type.[1] In the unspecified group, primary cutaneous aggressive epidermotropic CD8+ cytotoxic T-cell lymphoma, cutaneous gamma/delta T-cell lymphoma, and primary cutaneous small-medium CD4+ T-cell lymphoma are separate provisional entities. For the remaining diseases that do not fit into either of these provisional entities, the designation unspecified primary cutaneous PTL is used.\nPrimary cutaneous aggressive epidermotropic CD8+ cytotoxic T-cell lymphoma is a rare subtype of CTCL. It is a distinct and clinically aggressive form of CTCL that accounts for less than 1% of all cases of cutaneous lymphoma and carries a very poor prognosis.[2,3,4] The clinical course is extremely rapidly progressive, with an average 5-year survival estimated at 18% compared with the typical 88% in the more indolent mycosis fungoides. The EORTC Cutaneous Lymphoma Task Force's data reported that all 4 patients died from their disease within 3 years of diagnosis, with a median survival of only 22.5 months.[4] Patients affected by primary cutaneous aggressive epidermotropic CD8+ cytotoxic T-cell lymphoma typically have rapid progression with extra-cutaneous dissemination that affects quality-of-life and the overall prognosis. The pathogenesis or genetic characteristics of this disease are not known as this type of lymphoma is extremely rare and only a limited number of cases have been reported.\nClinically, the disease usually presents with localized or disseminated eruptive papules, nodules, and tumors showing central ulceration and necrosis, or with superficial, hyperkeratotic patches and plaques.[2,3] Unlike classic mycosis fungoides, patients do not generally follow the typical progression through patch, plaque, and finally tumor-stage disease, but rather present from the onset with widespread plaque- and tumor-stage disease.[4] However, mycosis fungoides, pagetoid reticulosis, and Sézary syndrome are included in the differential diagnosis. The clinical features of this disease are in fact very similar to those observed in patients with a cutaneous gamma/delta T-cell lymphoma and cases described as generalized pagetoid reticulosis (Ketron-Goodman type) in the past.[1]\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 399369,
"choiceText": "Parapsoriasis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 399370,
"choiceText": "Sézary syndrome",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 399371,
"choiceText": "Mycosis fungoides",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 399372,
"choiceText": "Primary cutaneous aggressive epidermotropic CD8+ cytotoxic T-cell lymphoma",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 116420,
"questionText": "If you have a patient with rapidly progressive skin lesions and biopsy findings as described above, what is your most likely diagnosis?<br /><br /><i>Hint: Note the history of clinical manifestation and skin biopsy.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Asymptomatic Macules and An Ulcerated Shoulder in an Elderly Woman"
},
{
"authors": "Antonio Giovanni Richetta, MD, PhD; Simona Giancristoforo, MD; Carlo Mattozzi, MD; Sara D'Epiro, MD; Stefano Calvieri, MD",
"content": [
"Another condition to consider in the differential diagnosis of primary cutaneous aggressive epidermotropic CD8+ cytotoxic T-cell lymphoma is a cytotoxic variant of lymphomatoid papulosis (a cutaneous CD30+ lymphoproliferative disorder).[5] Investigators proposed the term lymphomatoid papulosis type D for this lymphomatoid papulosis variant that has histopathologic features which may not only be difficult to distinguish from primary cutaneous aggressive epidermotropic CD8+ cytotoxic T-cell lymphoma, but may also impact the clinical management and prognosis.[5]",
"The histologic appearance of primary cutaneous aggressive epidermotropic CD81 T-cell lymphoma lesions varies. Epidermotropism is seen in all stages of disease, with tumor cells predominately confined to the epidermis, most extensively involving the basal layer.[1,6] This pronounced epidermotropism may follow a linear distribution or a pagetoid pattern throughout the epidermis. In all cases of aggressive epidermotropic CD8 T-cell lymphoma, the epidermis itself may be acanthotic or atrophic, with necrotic keratinocytes, ulceration, variable spongiosis, and occasional blister formation.[2,7]",
"Single necrotic keratinocytes may be seen at the dermoepidermal junction or, in some cases, the basal necrotic keratinocytes may be confluent at the basal layer, giving rise to a dermoepidermal cleft. Invasion and destruction of adnexal skin structures, including pilosebaceous units, eccrine ducts, and hair follicles, is frequently seen, as well as perivascular distribution of neoplastic cells with or without signs of angioinvasion.[1,2,7] Nerves are generally spared whereas intercellular edema and necrosis are commonly present in the central part of lesions. The actual tumor cells are medium-to-large pleomorphic T cells or blastic cells, and they are accompanied by a variable number of reactive macrophages and dendritic cells, rare eosinophils, and a few plasma cells.[1,7] The immunophenotype of tumor cells is characterized by βF1+, CD3+, CD81, CD4-, granzyme B+, perforin+, TIA-1+, CD45RA+, and CD45RO-.[1,6,7,10] CD56 expression is variable, as is that of CD2, CD7, and CD5.[2,7,8,9,11]",
"In this clinical case, the patient had pathologic findings of intraepidermal infiltrates of atypical lymphoid cells. Moreover, the immunohistologic phenotype of these cells led to the diagnosis of cutaneous lymphoma. Interestingly, the patient's primary disease manifestation was rapidly spreading maculopapular eruptions on her leg and arms. Extracutaneous dissemination is common, with metastasis to unusual sites (eg, lung, testes, CNS, oral cavity); however, lymph nodes are often spared. Because of the aggressive clinical course of primary cutaneous aggressive epidermotropic CD8+ T-cell lymphoma, patients should be evaluated with a thorough physical examination, peripheral blood evaluation (complete blood cell count, liver function tests, lactate dehydrogenase, flow cytometry), and staging scans.[4]"
],
"date": "December 09, 2016",
"figures": [],
"markdown": "# Asymptomatic Macules and An Ulcerated Shoulder in an Elderly Woman\n\n **Authors:** Antonio Giovanni Richetta, MD, PhD; Simona Giancristoforo, MD; Carlo Mattozzi, MD; Sara D'Epiro, MD; Stefano Calvieri, MD \n **Date:** December 09, 2016\n\n ## Content\n\n Another condition to consider in the differential diagnosis of primary cutaneous aggressive epidermotropic CD8+ cytotoxic T-cell lymphoma is a cytotoxic variant of lymphomatoid papulosis (a cutaneous CD30+ lymphoproliferative disorder).[5] Investigators proposed the term lymphomatoid papulosis type D for this lymphomatoid papulosis variant that has histopathologic features which may not only be difficult to distinguish from primary cutaneous aggressive epidermotropic CD8+ cytotoxic T-cell lymphoma, but may also impact the clinical management and prognosis.[5]\nThe histologic appearance of primary cutaneous aggressive epidermotropic CD81 T-cell lymphoma lesions varies. Epidermotropism is seen in all stages of disease, with tumor cells predominately confined to the epidermis, most extensively involving the basal layer.[1,6] This pronounced epidermotropism may follow a linear distribution or a pagetoid pattern throughout the epidermis. In all cases of aggressive epidermotropic CD8 T-cell lymphoma, the epidermis itself may be acanthotic or atrophic, with necrotic keratinocytes, ulceration, variable spongiosis, and occasional blister formation.[2,7]\nSingle necrotic keratinocytes may be seen at the dermoepidermal junction or, in some cases, the basal necrotic keratinocytes may be confluent at the basal layer, giving rise to a dermoepidermal cleft. Invasion and destruction of adnexal skin structures, including pilosebaceous units, eccrine ducts, and hair follicles, is frequently seen, as well as perivascular distribution of neoplastic cells with or without signs of angioinvasion.[1,2,7] Nerves are generally spared whereas intercellular edema and necrosis are commonly present in the central part of lesions. The actual tumor cells are medium-to-large pleomorphic T cells or blastic cells, and they are accompanied by a variable number of reactive macrophages and dendritic cells, rare eosinophils, and a few plasma cells.[1,7] The immunophenotype of tumor cells is characterized by βF1+, CD3+, CD81, CD4-, granzyme B+, perforin+, TIA-1+, CD45RA+, and CD45RO-.[1,6,7,10] CD56 expression is variable, as is that of CD2, CD7, and CD5.[2,7,8,9,11]\nIn this clinical case, the patient had pathologic findings of intraepidermal infiltrates of atypical lymphoid cells. Moreover, the immunohistologic phenotype of these cells led to the diagnosis of cutaneous lymphoma. Interestingly, the patient's primary disease manifestation was rapidly spreading maculopapular eruptions on her leg and arms. Extracutaneous dissemination is common, with metastasis to unusual sites (eg, lung, testes, CNS, oral cavity); however, lymph nodes are often spared. Because of the aggressive clinical course of primary cutaneous aggressive epidermotropic CD8+ T-cell lymphoma, patients should be evaluated with a thorough physical examination, peripheral blood evaluation (complete blood cell count, liver function tests, lactate dehydrogenase, flow cytometry), and staging scans.[4]\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "Asymptomatic Macules and An Ulcerated Shoulder in an Elderly Woman"
},
{
"authors": "Antonio Giovanni Richetta, MD, PhD; Simona Giancristoforo, MD; Carlo Mattozzi, MD; Sara D'Epiro, MD; Stefano Calvieri, MD",
"content": [
"Figure 2.",
"Figure 3.",
"Figure 4.",
"Treatment of primary cutaneous aggressive epidermotropic CD8+ T-cell lymphoma has proven extremely challenging, with even the most aggressive modalities proving ineffective in establishing a long-term cure. In addition, statistically significant evaluations of the different treatment options are not possible due to the rarity of the diagnosis. In general, initial treatment with skin-directed therapy including topical chemotherapy, psoralen plus UV-A (PUVA), and local radiotherapy is ineffective, and patients often require escalation to systemic, multiagent chemotherapy. Generally, doxorubicin-based regimens are used, including cyclophosphamide, doxorubicin, vincristine, and prednisone, but patients often fail to respond to even the most aggressive polychemotherapy regimens.",
"Histone deacetylase (HDAC) inhibitors have activity in CTCL. Romidepsin has demonstrated overall response rates of 35% in CTCL and is approved by the US Food and Drug Administration for the treatment of CTCL in patients with progressive, persistent, or recurrent disease on or following at least 1 prior systemic therapy.",
"A multi-institutional phase II trial evaluated the use of single-agent intravenous romidepsin in 71 patients with CTCL.The overall and complete response rates were 34% and 6%,[12] respectively. The median duration of response was 11.1 months. Among those with a major response, the median time to progression was 15.1 months. Severely affected patients (grade 3 of 4) saw drug-related toxicities of granulocytopenia and lymphopenia in 10% and 21% of instances, respectively. The nonhematologic side effects were generally mild but included nausea, vomiting, fatigue, electrolyte abnormalities, and electrocardiographic changes.",
"Another study evaluated the use of romidepsin in 96 patients with CTCL who had received a median of 2 prior therapies. Overall and complete response rates were 34% and 6%, respectively. The median duration of response was 14.9 months.[13]",
"Vorinostat is another HDAC inhibitor that has activity in CTCL. In one study it did provide pruritus relief in 32% of patients. Toxicities of vorinostat included diarrhea (46%), fatigue (49%), nausea (43%), anorexia (26%), and dysgeusia (24%).[14] Hematologic abnormalities include anemia, thrombocytopenia (22%), and neutropenia, most of which were grade 1 or 2 in severity. The most common severe adverse event was pulmonary embolus, seen in 4 patients (5%).[14]",
"HDAC inhibitors should be used with caution in patients with cardiac arrhythmias and should not be used in patients with prolonged QT intervals.",
"The patient was scheduled to undergo medical treatment with bexarotene 450 mg a day. During the treatment, the brownish macules healed and disappeared while the nodular lesion on her shoulder remained ulcerated. The skin ulcer was treated with advanced dressing to prevent bacterial superinfection, and a partial clinical improvement was seen. After 7 months of bexarotene therapy, this therapy had to be discontinued due to alterations in her the lipid profile (Figure 2). Subsequently, the patient developed a severe recurrence of the disease, leading to a progressive ulceration of her lesion with exposure of muscles and tendinous structures (as seen in Figure 3). She was then started on gemcitabine 1000 mg a week for 3 weeks and, after the first infusion, notable improvement of the wound with significant reduction in the size of the lesion was observed. Purine analogs, such as gemcitabine, do not have a high immunosuppressive capacity and may be the most appropriate agents for these patients.",
"The protocol was repeated 4 times and, ultimately, 4 months later she had complete resolution of the skin ulcer (Figure 4). Currently, the patient remains without any signs or symptoms of disease recurrence, and all side effects of the treatment protocols have resolved."
],
"date": "December 09, 2016",
"figures": [
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/740/003/740003-Thumb-2.png"
},
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/740/003/740003-Thumb-3.png"
},
{
"caption": "Figure 4.",
"image_url": "https://img.medscapestatic.com/article/740/003/740003-Thumb-4.png"
}
],
"markdown": "# Asymptomatic Macules and An Ulcerated Shoulder in an Elderly Woman\n\n **Authors:** Antonio Giovanni Richetta, MD, PhD; Simona Giancristoforo, MD; Carlo Mattozzi, MD; Sara D'Epiro, MD; Stefano Calvieri, MD \n **Date:** December 09, 2016\n\n ## Content\n\n Figure 2.\nFigure 3.\nFigure 4.\nTreatment of primary cutaneous aggressive epidermotropic CD8+ T-cell lymphoma has proven extremely challenging, with even the most aggressive modalities proving ineffective in establishing a long-term cure. In addition, statistically significant evaluations of the different treatment options are not possible due to the rarity of the diagnosis. In general, initial treatment with skin-directed therapy including topical chemotherapy, psoralen plus UV-A (PUVA), and local radiotherapy is ineffective, and patients often require escalation to systemic, multiagent chemotherapy. Generally, doxorubicin-based regimens are used, including cyclophosphamide, doxorubicin, vincristine, and prednisone, but patients often fail to respond to even the most aggressive polychemotherapy regimens.\nHistone deacetylase (HDAC) inhibitors have activity in CTCL. Romidepsin has demonstrated overall response rates of 35% in CTCL and is approved by the US Food and Drug Administration for the treatment of CTCL in patients with progressive, persistent, or recurrent disease on or following at least 1 prior systemic therapy.\nA multi-institutional phase II trial evaluated the use of single-agent intravenous romidepsin in 71 patients with CTCL.The overall and complete response rates were 34% and 6%,[12] respectively. The median duration of response was 11.1 months. Among those with a major response, the median time to progression was 15.1 months. Severely affected patients (grade 3 of 4) saw drug-related toxicities of granulocytopenia and lymphopenia in 10% and 21% of instances, respectively. The nonhematologic side effects were generally mild but included nausea, vomiting, fatigue, electrolyte abnormalities, and electrocardiographic changes.\nAnother study evaluated the use of romidepsin in 96 patients with CTCL who had received a median of 2 prior therapies. Overall and complete response rates were 34% and 6%, respectively. The median duration of response was 14.9 months.[13]\nVorinostat is another HDAC inhibitor that has activity in CTCL. In one study it did provide pruritus relief in 32% of patients. Toxicities of vorinostat included diarrhea (46%), fatigue (49%), nausea (43%), anorexia (26%), and dysgeusia (24%).[14] Hematologic abnormalities include anemia, thrombocytopenia (22%), and neutropenia, most of which were grade 1 or 2 in severity. The most common severe adverse event was pulmonary embolus, seen in 4 patients (5%).[14]\nHDAC inhibitors should be used with caution in patients with cardiac arrhythmias and should not be used in patients with prolonged QT intervals.\nThe patient was scheduled to undergo medical treatment with bexarotene 450 mg a day. During the treatment, the brownish macules healed and disappeared while the nodular lesion on her shoulder remained ulcerated. The skin ulcer was treated with advanced dressing to prevent bacterial superinfection, and a partial clinical improvement was seen. After 7 months of bexarotene therapy, this therapy had to be discontinued due to alterations in her the lipid profile (Figure 2). Subsequently, the patient developed a severe recurrence of the disease, leading to a progressive ulceration of her lesion with exposure of muscles and tendinous structures (as seen in Figure 3). She was then started on gemcitabine 1000 mg a week for 3 weeks and, after the first infusion, notable improvement of the wound with significant reduction in the size of the lesion was observed. Purine analogs, such as gemcitabine, do not have a high immunosuppressive capacity and may be the most appropriate agents for these patients.\nThe protocol was repeated 4 times and, ultimately, 4 months later she had complete resolution of the skin ulcer (Figure 4). Currently, the patient remains without any signs or symptoms of disease recurrence, and all side effects of the treatment protocols have resolved.\n\n ## Figures\n\n **Figure 2.** \n \n\n**Figure 3.** \n \n\n**Figure 4.** \n \n\n\n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 399373,
"choiceText": "Lung and central nervous system",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 399374,
"choiceText": "Bone",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 399375,
"choiceText": "Liver",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 399376,
"choiceText": "Nerves",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The prognosis in aggressive CD8+ T-cell lymphoma is very poor. Rapid progression with extracutaneous dissemination is typical with metastasis to unusual sites, including the lung, testes, central nervous system, and oral cavity, but generally sparing the lymph nodes.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 116421,
"questionText": "Extracutaneous dissemination is typical in patients affected by T-cell lymphoma, although some localization has been seen (albeit considerably rarely). Where are the most common locations for extracutaneous dissemination of T-cell lymphomas?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 399377,
"choiceText": "Gemcitabine",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 399378,
"choiceText": "Doxorubicin",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 399379,
"choiceText": "Cyclophosphamide",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 399380,
"choiceText": "Docetaxel",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "If the course of disease in primary cutaneous aggressive epidermotropic CD8+ T-cell lymphoma is associated with severe immunodeficiency, purine analogs, such as (eg, gemcitabine) that do not have a high immunosuppressive capacity may be the most appropriate chemotherapeutic agents for these patients.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 116422,
"questionText": "Patients affected by primary cutaneous aggressive epidermotropic CD8+ cytotoxic T-cell lymphoma often require escalation to systemic, multiagent chemotherapy. However, immunosuppression represents an obstacle in the choice of the appropriate agent. Which of the following is the most appropriate?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Asymptomatic Macules and An Ulcerated Shoulder in an Elderly Woman"
},
{
"authors": "Antonio Giovanni Richetta, MD, PhD; Simona Giancristoforo, MD; Carlo Mattozzi, MD; Sara D'Epiro, MD; Stefano Calvieri, MD",
"content": [],
"date": "December 09, 2016",
"figures": [],
"markdown": "# Asymptomatic Macules and An Ulcerated Shoulder in an Elderly Woman\n\n **Authors:** Antonio Giovanni Richetta, MD, PhD; Simona Giancristoforo, MD; Carlo Mattozzi, MD; Sara D'Epiro, MD; Stefano Calvieri, MD \n **Date:** December 09, 2016\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 399373,
"choiceText": "Lung and central nervous system",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 399374,
"choiceText": "Bone",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 399375,
"choiceText": "Liver",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 399376,
"choiceText": "Nerves",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The prognosis in aggressive CD8+ T-cell lymphoma is very poor. Rapid progression with extracutaneous dissemination is typical with metastasis to unusual sites, including the lung, testes, central nervous system, and oral cavity, but generally sparing the lymph nodes.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 116421,
"questionText": "Extracutaneous dissemination is typical in patients affected by T-cell lymphoma, although some localization has been seen (albeit considerably rarely). Where are the most common locations for extracutaneous dissemination of T-cell lymphomas?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 399377,
"choiceText": "Gemcitabine",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 399378,
"choiceText": "Doxorubicin",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 399379,
"choiceText": "Cyclophosphamide",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 399380,
"choiceText": "Docetaxel",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "If the course of disease in primary cutaneous aggressive epidermotropic CD8+ T-cell lymphoma is associated with severe immunodeficiency, purine analogs, such as (eg, gemcitabine) that do not have a high immunosuppressive capacity may be the most appropriate chemotherapeutic agents for these patients.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 116422,
"questionText": "Patients affected by primary cutaneous aggressive epidermotropic CD8+ cytotoxic T-cell lymphoma often require escalation to systemic, multiagent chemotherapy. However, immunosuppression represents an obstacle in the choice of the appropriate agent. Which of the following is the most appropriate?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Asymptomatic Macules and An Ulcerated Shoulder in an Elderly Woman"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 399369,
"choiceText": "Parapsoriasis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 399370,
"choiceText": "Sézary syndrome",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 399371,
"choiceText": "Mycosis fungoides",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 399372,
"choiceText": "Primary cutaneous aggressive epidermotropic CD8+ cytotoxic T-cell lymphoma",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 116420,
"questionText": "If you have a patient with rapidly progressive skin lesions and biopsy findings as described above, what is your most likely diagnosis?<br /><br /><i>Hint: Note the history of clinical manifestation and skin biopsy.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 399373,
"choiceText": "Lung and central nervous system",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 399374,
"choiceText": "Bone",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 399375,
"choiceText": "Liver",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 399376,
"choiceText": "Nerves",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The prognosis in aggressive CD8+ T-cell lymphoma is very poor. Rapid progression with extracutaneous dissemination is typical with metastasis to unusual sites, including the lung, testes, central nervous system, and oral cavity, but generally sparing the lymph nodes.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 116421,
"questionText": "Extracutaneous dissemination is typical in patients affected by T-cell lymphoma, although some localization has been seen (albeit considerably rarely). Where are the most common locations for extracutaneous dissemination of T-cell lymphomas?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 399377,
"choiceText": "Gemcitabine",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 399378,
"choiceText": "Doxorubicin",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 399379,
"choiceText": "Cyclophosphamide",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 399380,
"choiceText": "Docetaxel",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "If the course of disease in primary cutaneous aggressive epidermotropic CD8+ T-cell lymphoma is associated with severe immunodeficiency, purine analogs, such as (eg, gemcitabine) that do not have a high immunosuppressive capacity may be the most appropriate chemotherapeutic agents for these patients.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 116422,
"questionText": "Patients affected by primary cutaneous aggressive epidermotropic CD8+ cytotoxic T-cell lymphoma often require escalation to systemic, multiagent chemotherapy. However, immunosuppression represents an obstacle in the choice of the appropriate agent. Which of the following is the most appropriate?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
739338 | /viewarticle/739338 | [
{
"authors": "Samantha Nicholson-Spence, MB BS; Frederick Williams, MD",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to recognize accurately. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"An 80-year-old man presents to the emergency department (ED) with a 2-day history of right-sided chest pain. The pain was sudden in onset, is constant, and is exacerbated by coughing and deep inspiration. He reports baseline shortness of breath secondary to bronchiectasis, chronic obstructive pulmonary disease (COPD), and congestive heart failure with New York Heart Association (NYHA) class III function. His dyspnea has not worsened since the onset of his pain.",
"He had a worsening cough 1 week ago, with brown sputum, and noted low-grade temperatures of 99°F to 100°F (37.2°-37.7°C). He took ciprofloxacin for these symptoms, which resulted in a return to baseline. He does not use bronchodilators because he reports that they are \"difficult to tolerate.\" He performs postural drainage twice daily and takes guaifenesin 4 times daily. He denies having any fever, chills, or hemoptysis.",
"He has no history of exposure to tuberculosis. He denies experiencing any leg swelling or calf pain, but he does have a past medical history significant for deep vein thrombosis and pulmonary embolism 4 months prior to this admission, for which he takes warfarin. He has never smoked and has no history of occupational exposures. No one in his family is affected by lung disease."
],
"date": "January 08, 2019",
"figures": [],
"markdown": "# Sudden-Onset Chest Pain in an 80-Year-Old Man With COPD\n\n **Authors:** Samantha Nicholson-Spence, MB BS; Frederick Williams, MD \n **Date:** January 08, 2019\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to recognize accurately. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nAn 80-year-old man presents to the emergency department (ED) with a 2-day history of right-sided chest pain. The pain was sudden in onset, is constant, and is exacerbated by coughing and deep inspiration. He reports baseline shortness of breath secondary to bronchiectasis, chronic obstructive pulmonary disease (COPD), and congestive heart failure with New York Heart Association (NYHA) class III function. His dyspnea has not worsened since the onset of his pain.\nHe had a worsening cough 1 week ago, with brown sputum, and noted low-grade temperatures of 99°F to 100°F (37.2°-37.7°C). He took ciprofloxacin for these symptoms, which resulted in a return to baseline. He does not use bronchodilators because he reports that they are \"difficult to tolerate.\" He performs postural drainage twice daily and takes guaifenesin 4 times daily. He denies having any fever, chills, or hemoptysis.\nHe has no history of exposure to tuberculosis. He denies experiencing any leg swelling or calf pain, but he does have a past medical history significant for deep vein thrombosis and pulmonary embolism 4 months prior to this admission, for which he takes warfarin. He has never smoked and has no history of occupational exposures. No one in his family is affected by lung disease.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "Sudden-Onset Chest Pain in an 80-Year-Old Man With COPD"
},
{
"authors": "Samantha Nicholson-Spence, MB BS; Frederick Williams, MD",
"content": [
"His vital signs on admission include a temperature of 98.3°F (36.8°C), heart rate of 93 bpm, blood pressure of 170/90 mm Hg, and a respiratory rate of 26 breaths/min with a pulse oximetry of 93% on room air. He does not appear distressed and, despite being tachypneic, is able to speak in full sentences.",
"Examination of the head, neck, central nervous system, and abdomen are all within normal limits. He has no jugular venous distention or peripheral edema. No breath sounds are noted in the right upper and mid-lung zones and diffuse rhonchi in both lung fields with bibasilar inspiratory crackles are noted. His cardiac examination reveals a grade 3/6 pansystolic murmur heard all over the precordium that is loudest at the apex. His lower extremities are symmetrical and no calf tenderness is detected.",
"His complete blood cell count and basic metabolic panel are within normal limits, except for a white blood cell count of 14.3 × 103/μL (the patient has chronic leukocytosis secondary to his bronchiectasis). His international normalized ratio (INR) is 2.7, and his brain natriuretic peptide (BNP) and cardiac enzyme examinations are unremarkable. The arterial blood gas shows a pO2 of 79 mm Hg on 21% FiO2, a pH of 7.47, pCO2 of 39 mm Hg, and an HCO3 of 28 mEq/L. A chest x-ray shows bilateral airspace disease, a right lower-lobe nodule, and cardiomegaly. A chest CT scan is performed (see Figure 1).",
"Figure 1."
],
"date": "January 08, 2019",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/739/338/739338-thumb1.png"
}
],
"markdown": "# Sudden-Onset Chest Pain in an 80-Year-Old Man With COPD\n\n **Authors:** Samantha Nicholson-Spence, MB BS; Frederick Williams, MD \n **Date:** January 08, 2019\n\n ## Content\n\n His vital signs on admission include a temperature of 98.3°F (36.8°C), heart rate of 93 bpm, blood pressure of 170/90 mm Hg, and a respiratory rate of 26 breaths/min with a pulse oximetry of 93% on room air. He does not appear distressed and, despite being tachypneic, is able to speak in full sentences.\nExamination of the head, neck, central nervous system, and abdomen are all within normal limits. He has no jugular venous distention or peripheral edema. No breath sounds are noted in the right upper and mid-lung zones and diffuse rhonchi in both lung fields with bibasilar inspiratory crackles are noted. His cardiac examination reveals a grade 3/6 pansystolic murmur heard all over the precordium that is loudest at the apex. His lower extremities are symmetrical and no calf tenderness is detected.\nHis complete blood cell count and basic metabolic panel are within normal limits, except for a white blood cell count of 14.3 × 103/μL (the patient has chronic leukocytosis secondary to his bronchiectasis). His international normalized ratio (INR) is 2.7, and his brain natriuretic peptide (BNP) and cardiac enzyme examinations are unremarkable. The arterial blood gas shows a pO2 of 79 mm Hg on 21% FiO2, a pH of 7.47, pCO2 of 39 mm Hg, and an HCO3 of 28 mEq/L. A chest x-ray shows bilateral airspace disease, a right lower-lobe nodule, and cardiomegaly. A chest CT scan is performed (see Figure 1).\nFigure 1.\n\n ## Figures\n\n **Figure 1.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 397688,
"choiceText": "Foreign body",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 397689,
"choiceText": "Pulmonary embolism",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 397690,
"choiceText": "Pneumothorax",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 397691,
"choiceText": "Large mediastinal mass",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 115830,
"questionText": "What diagnosis is best demonstrated on the CT scan?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Sudden-Onset Chest Pain in an 80-Year-Old Man With COPD"
},
{
"authors": "Samantha Nicholson-Spence, MB BS; Frederick Williams, MD",
"content": [
"This patient's chest CT scan demonstrated a right-sided pneumothorax. He also had a left-sided pulmonary artery thrombus and a suspicious left lower-lobe nodule shown on other images from this CT scan (not shown). Bilateral lower-extremity ultrasound studies were negative for deep vein thrombosis.",
"Pneumothorax is a collection of air or gas in the pleural space. It can occur either spontaneously or due to trauma (including iatrogenic injury). The normal pleural space has a negative pressure of -5 cm H2O (relative to atmospheric pressure) due to the recoil force of the lungs, which helps to prevent the lung from total collapse even at the end of expiration.[1] In pneumothorax, the air creates a positive intrapleural pressure, thereby preventing lung expansion and causing compressive atelectasis and even collapse, depending on the severity. A pneumothorax can even progress to the point that it compresses the mediastinum and impairs venous return, and may thereby induce hypotension and shock.",
"Spontaneous pneumothorax is defined by the absence of a preceding intrathoracic trauma or invasive procedure that breaches the thoracic cavity. A spontaneous pneumothorax in a patient with no underlying lung disease is considered a primary spontaneous pneumothorax (PSP). A spontaneous pneumothorax in a person with underlying lung disease (eg, COPD) is considered a secondary spontaneous pneumothorax (SSP). PSP has been attributed to the rupture of a subpleural bleb from congenital anomalies, inflammation of the bronchioles, and disturbances of the collateral ventilation.[2] PSP has a predilection for tall, thin males; the incidence is increased in individuals with Marfan syndrome and homocystinuria.",
"The relative risk for PSP is 7 times higher in light smokers (1-12 cigarettes/day) and up to 102 times higher in heavy smokers (> 22 cigarettes/day), compared with nonsmokers.[3] SSP is attributed to the rupture of alveoli or bullae, most commonly as a result of COPD. SSP is particularly detrimental, as it affects patients with underlying lung disease and pre-existing respiratory compromise."
],
"date": "January 08, 2019",
"figures": [],
"markdown": "# Sudden-Onset Chest Pain in an 80-Year-Old Man With COPD\n\n **Authors:** Samantha Nicholson-Spence, MB BS; Frederick Williams, MD \n **Date:** January 08, 2019\n\n ## Content\n\n This patient's chest CT scan demonstrated a right-sided pneumothorax. He also had a left-sided pulmonary artery thrombus and a suspicious left lower-lobe nodule shown on other images from this CT scan (not shown). Bilateral lower-extremity ultrasound studies were negative for deep vein thrombosis.\nPneumothorax is a collection of air or gas in the pleural space. It can occur either spontaneously or due to trauma (including iatrogenic injury). The normal pleural space has a negative pressure of -5 cm H2O (relative to atmospheric pressure) due to the recoil force of the lungs, which helps to prevent the lung from total collapse even at the end of expiration.[1] In pneumothorax, the air creates a positive intrapleural pressure, thereby preventing lung expansion and causing compressive atelectasis and even collapse, depending on the severity. A pneumothorax can even progress to the point that it compresses the mediastinum and impairs venous return, and may thereby induce hypotension and shock.\nSpontaneous pneumothorax is defined by the absence of a preceding intrathoracic trauma or invasive procedure that breaches the thoracic cavity. A spontaneous pneumothorax in a patient with no underlying lung disease is considered a primary spontaneous pneumothorax (PSP). A spontaneous pneumothorax in a person with underlying lung disease (eg, COPD) is considered a secondary spontaneous pneumothorax (SSP). PSP has been attributed to the rupture of a subpleural bleb from congenital anomalies, inflammation of the bronchioles, and disturbances of the collateral ventilation.[2] PSP has a predilection for tall, thin males; the incidence is increased in individuals with Marfan syndrome and homocystinuria.\nThe relative risk for PSP is 7 times higher in light smokers (1-12 cigarettes/day) and up to 102 times higher in heavy smokers (> 22 cigarettes/day), compared with nonsmokers.[3] SSP is attributed to the rupture of alveoli or bullae, most commonly as a result of COPD. SSP is particularly detrimental, as it affects patients with underlying lung disease and pre-existing respiratory compromise.\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 397688,
"choiceText": "Foreign body",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 397689,
"choiceText": "Pulmonary embolism",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 397690,
"choiceText": "Pneumothorax",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 397691,
"choiceText": "Large mediastinal mass",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 115830,
"questionText": "What diagnosis is best demonstrated on the CT scan?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Sudden-Onset Chest Pain in an 80-Year-Old Man With COPD"
},
{
"authors": "Samantha Nicholson-Spence, MB BS; Frederick Williams, MD",
"content": [
"A 10% pneumothorax in a patient with moderate-to-severe COPD may present very dramatically with respiratory failure and hypotension, whereas a 15%-20% pneumothorax in a patient with normal lung physiology may cause relatively mild respiratory discomfort. Although COPD is the leading cause of SSP, many other lung diseases can predispose a patient to the development of SSP. These include asthma, cystic fibrosis, sarcoidosis, tuberculosis, histiocytosis X, and Pneumocystis jirovecii pneumonia (more commonly seen in patients with HIV/AIDS).[4]",
"Traumatic pneumothorax may result from penetrating, blunt, or blast thoracic injury. Iatrogenic traumatic pneumothorax is most common after invasive procedures such as thoracentesis, transbronchial biopsy, or transthoracic aspiration, but it may also result from barotrauma in the mechanically ventilated patient. Tension pneumothorax, a particularly important type of pneumothorax, commonly occurs in the context of trauma. Tension pneumothorax occurs when air in the pleural space compresses mediastinal structures and limits central venous return. This decrease in central venous return induces hypotension and may lead to cardiac arrest, including pulseless electrical activity. A tension pneumothorax is a medical emergency; the diagnosis is made clinically by the presence of respiratory distress, diminished or absent breath sounds on the affected side, hypotension, and tracheal deviation. Immediate decompression by needle thoracostomy should be followed by insertion of a chest tube, without delaying to obtain chest films.",
"A pneumothorax often presents with acute unilateral pleuritic chest pain and/or dyspnea. In any patient with underlying lung disease, such as COPD or cystic fibrosis, the diagnosis should be considered for any sudden decline in respiratory function, especially when accompanied by chest pain. Hyperresonance, decreased tactile vocal fremitus, and decreased air entry are signs of pneumothorax. Of note, physical signs are not sensitive enough to rule out a pneumothorax, especially in patients with underlying emphysema, who tend to have hyperinflated lungs at baseline.",
"The diagnosis of pneumothorax is most commonly confirmed by x-rays (preferably upright) or CT scans of the chest. Chest x-rays can miss small pneumothoraces in patients with underlying lung disease, especially when multiple thin-walled bullae are present. Supine chest x-rays also frequently miss pneumothoraces. Classic chest x-ray findings include a lack of lung markings, lung asymmetry, pleural line, and, in a tension pneumothorax, mediastinal shift. Most patients with a first episode of spontaneous pneumothorax do not need CT scans; however, they are helpful in evaluating lung parenchyma, in differentiating primary from secondary disease, and in assessing the recurrence risk. Thoracic ultrasonography can identify a pneumothorax with excellent sensitivity.[5]",
"Patients with a pneumothorax should be immediately placed on oxygen therapy. Observation is only appropriate for small primary pneumothoraces.[6] Simple aspiration may also be sufficient in small primary pneumothoraces; however, most patients with SSP should have tube thoracostomy.[7] If the patient develops respiratory failure requiring mechanical ventilation, definitive chest tube placement is preferable to simple aspiration because positive pressure ventilation will enlarge a pre-existing pneumothorax and may lead to rapid decline.[8] In the case of a tension pneumothorax with signs and symptoms of shock, it is crucial to perform needle decompression emergently, followed by placement of a chest tube."
],
"date": "January 08, 2019",
"figures": [],
"markdown": "# Sudden-Onset Chest Pain in an 80-Year-Old Man With COPD\n\n **Authors:** Samantha Nicholson-Spence, MB BS; Frederick Williams, MD \n **Date:** January 08, 2019\n\n ## Content\n\n A 10% pneumothorax in a patient with moderate-to-severe COPD may present very dramatically with respiratory failure and hypotension, whereas a 15%-20% pneumothorax in a patient with normal lung physiology may cause relatively mild respiratory discomfort. Although COPD is the leading cause of SSP, many other lung diseases can predispose a patient to the development of SSP. These include asthma, cystic fibrosis, sarcoidosis, tuberculosis, histiocytosis X, and Pneumocystis jirovecii pneumonia (more commonly seen in patients with HIV/AIDS).[4]\nTraumatic pneumothorax may result from penetrating, blunt, or blast thoracic injury. Iatrogenic traumatic pneumothorax is most common after invasive procedures such as thoracentesis, transbronchial biopsy, or transthoracic aspiration, but it may also result from barotrauma in the mechanically ventilated patient. Tension pneumothorax, a particularly important type of pneumothorax, commonly occurs in the context of trauma. Tension pneumothorax occurs when air in the pleural space compresses mediastinal structures and limits central venous return. This decrease in central venous return induces hypotension and may lead to cardiac arrest, including pulseless electrical activity. A tension pneumothorax is a medical emergency; the diagnosis is made clinically by the presence of respiratory distress, diminished or absent breath sounds on the affected side, hypotension, and tracheal deviation. Immediate decompression by needle thoracostomy should be followed by insertion of a chest tube, without delaying to obtain chest films.\nA pneumothorax often presents with acute unilateral pleuritic chest pain and/or dyspnea. In any patient with underlying lung disease, such as COPD or cystic fibrosis, the diagnosis should be considered for any sudden decline in respiratory function, especially when accompanied by chest pain. Hyperresonance, decreased tactile vocal fremitus, and decreased air entry are signs of pneumothorax. Of note, physical signs are not sensitive enough to rule out a pneumothorax, especially in patients with underlying emphysema, who tend to have hyperinflated lungs at baseline.\nThe diagnosis of pneumothorax is most commonly confirmed by x-rays (preferably upright) or CT scans of the chest. Chest x-rays can miss small pneumothoraces in patients with underlying lung disease, especially when multiple thin-walled bullae are present. Supine chest x-rays also frequently miss pneumothoraces. Classic chest x-ray findings include a lack of lung markings, lung asymmetry, pleural line, and, in a tension pneumothorax, mediastinal shift. Most patients with a first episode of spontaneous pneumothorax do not need CT scans; however, they are helpful in evaluating lung parenchyma, in differentiating primary from secondary disease, and in assessing the recurrence risk. Thoracic ultrasonography can identify a pneumothorax with excellent sensitivity.[5]\nPatients with a pneumothorax should be immediately placed on oxygen therapy. Observation is only appropriate for small primary pneumothoraces.[6] Simple aspiration may also be sufficient in small primary pneumothoraces; however, most patients with SSP should have tube thoracostomy.[7] If the patient develops respiratory failure requiring mechanical ventilation, definitive chest tube placement is preferable to simple aspiration because positive pressure ventilation will enlarge a pre-existing pneumothorax and may lead to rapid decline.[8] In the case of a tension pneumothorax with signs and symptoms of shock, it is crucial to perform needle decompression emergently, followed by placement of a chest tube.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "Sudden-Onset Chest Pain in an 80-Year-Old Man With COPD"
},
{
"authors": "Samantha Nicholson-Spence, MB BS; Frederick Williams, MD",
"content": [
"The recurrence rate for pneumothorax is high; this is especially true in SSP, which has a rate of approximately 45% (compared with 30% in PSP). For this reason, the American College of Chest Physicians (ACCP) consensus statement on pneumothorax recommends chest tubes for all patients and pleurodesis with the first episode of a secondary spontaneous pneumothorax to prevent recurrences. The ACCP recommends medical thoracoscopy or video-assisted thorascopic surgery (VATS) as the primary procedure, and a limited axillary thoracotomy with pleural abrasion as a secondary approach.[4]",
"Chemical pleurodesis with talc or doxycycline may be performed via tube thoracostomy or thoracoscopy. VATS with bullae resection, stapling, and mechanical pleurodesis is a highly effective modality for preventing recurrence, but because the procedure requires induced collapse of 1 lung, patients with very poor lung reserve are not candidates for this procedure. In addition, general anesthetic risks must also be considered when electing for VATS. Success rates with chemical pleurodesis are 78%-91%, but they are 95%-100% with surgical interventions; therefore, the latter technique is preferred in patients with relatively good lung reserve.[6]",
"A retrospective of review of 569 patients treated from 1992 to 2008 with VATS for PSP and SSP showed that freedom from further surgery was seen in 98.1% of PSP patients at 5 years and 97.8% at 10 years after VATS, and in 96.1% of SSP patients at both 5 years and 10 years after VATS.[9] In both groups, no significant differences in results at 10 years were seen based on the pleurodesis technique used (ie, abrasion, chemical, or pleurectomy).",
"The patient in this case did not show any decline in respiratory function (pain rather than dyspnea was his presenting complaint); therefore, a tube thoracostomy was not performed. The procedure was also deferred because the patient was placed on heparin for his new pulmonary thrombus and he was also taking warfarin for his previous pulmonary embolus; both medications increase the risk for excessive bleeding with tube thoracostomy. He was observed in the intensive care unit with serial x-rays and had progressive spontaneous resolution over the next week, without need for an acute intervention. He was treated with unfractionated heparin for his pulmonary thrombus and an inferior vena cava filter was placed. After discharge, he was maintained on low-molecular-weight heparin because he had failed warfarin therapy. He was scheduled to undergo VATS to prevent recurrent pneumothorax."
],
"date": "January 08, 2019",
"figures": [],
"markdown": "# Sudden-Onset Chest Pain in an 80-Year-Old Man With COPD\n\n **Authors:** Samantha Nicholson-Spence, MB BS; Frederick Williams, MD \n **Date:** January 08, 2019\n\n ## Content\n\n The recurrence rate for pneumothorax is high; this is especially true in SSP, which has a rate of approximately 45% (compared with 30% in PSP). For this reason, the American College of Chest Physicians (ACCP) consensus statement on pneumothorax recommends chest tubes for all patients and pleurodesis with the first episode of a secondary spontaneous pneumothorax to prevent recurrences. The ACCP recommends medical thoracoscopy or video-assisted thorascopic surgery (VATS) as the primary procedure, and a limited axillary thoracotomy with pleural abrasion as a secondary approach.[4]\nChemical pleurodesis with talc or doxycycline may be performed via tube thoracostomy or thoracoscopy. VATS with bullae resection, stapling, and mechanical pleurodesis is a highly effective modality for preventing recurrence, but because the procedure requires induced collapse of 1 lung, patients with very poor lung reserve are not candidates for this procedure. In addition, general anesthetic risks must also be considered when electing for VATS. Success rates with chemical pleurodesis are 78%-91%, but they are 95%-100% with surgical interventions; therefore, the latter technique is preferred in patients with relatively good lung reserve.[6]\nA retrospective of review of 569 patients treated from 1992 to 2008 with VATS for PSP and SSP showed that freedom from further surgery was seen in 98.1% of PSP patients at 5 years and 97.8% at 10 years after VATS, and in 96.1% of SSP patients at both 5 years and 10 years after VATS.[9] In both groups, no significant differences in results at 10 years were seen based on the pleurodesis technique used (ie, abrasion, chemical, or pleurectomy).\nThe patient in this case did not show any decline in respiratory function (pain rather than dyspnea was his presenting complaint); therefore, a tube thoracostomy was not performed. The procedure was also deferred because the patient was placed on heparin for his new pulmonary thrombus and he was also taking warfarin for his previous pulmonary embolus; both medications increase the risk for excessive bleeding with tube thoracostomy. He was observed in the intensive care unit with serial x-rays and had progressive spontaneous resolution over the next week, without need for an acute intervention. He was treated with unfractionated heparin for his pulmonary thrombus and an inferior vena cava filter was placed. After discharge, he was maintained on low-molecular-weight heparin because he had failed warfarin therapy. He was scheduled to undergo VATS to prevent recurrent pneumothorax.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 397692,
"choiceText": "Upright chest x-ray",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 397693,
"choiceText": "Supine chest x-ray",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 397694,
"choiceText": "CT scan",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 397695,
"choiceText": "Thoracic ultrasound",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The diagnosis of pneumothorax is most commonly confirmed by x-rays (preferably upright) or CT scans of the chest. Chest x-rays can miss small pneumothoraces in patients with underlying lung disease, especially when multiple thin-walled bullae are present. Supine chest x-rays also frequently miss pneumothoraces. Thoracic ultrasonography can be used to identify a pneumothorax with excellent sensitivity.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 115831,
"questionText": "<p>A 65-year-old man with severe COPD presents to the ED with sudden worsening of his dyspnea since this morning. He has diminished left-sided breath sounds and acute pain. An ECG reveals sinus tachycardia, but it is otherwise unchanged from baseline and does not show acute ischemia. Although multiple possible etiologies for this patient's pain are possible, pneumothorax is strongly suspected.</p><p>Which of the following imaging modalities is <b><i>least preferable</i></b> for confirming the diagnosis of pneumothorax?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 397696,
"choiceText": "No further intervention is necessary",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 397697,
"choiceText": "Chemical pleurodesis via tube thoracostomy",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 397698,
"choiceText": "VATS with bullae stapling",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 397699,
"choiceText": "Thoracotomy with bullae resection",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Per ACCP guidelines, this patient should have an intervention to prevent recurrence because a second episode of pneumothorax could be catastrophic. His severe COPD and limited life expectancy makes him a poor candidate for VATS or thoracotomy. In this patient, chemical pleurodesis would be the least invasive option while still being effective and associated with the least mortality and morbidity. ",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 115832,
"questionText": "The CT scan of the chest in the patient described above demonstrates multiple bullae. In addition to tube thoracostomy, which of the following should be performed in this patient?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Sudden-Onset Chest Pain in an 80-Year-Old Man With COPD"
},
{
"authors": "Samantha Nicholson-Spence, MB BS; Frederick Williams, MD",
"content": [],
"date": "January 08, 2019",
"figures": [],
"markdown": "# Sudden-Onset Chest Pain in an 80-Year-Old Man With COPD\n\n **Authors:** Samantha Nicholson-Spence, MB BS; Frederick Williams, MD \n **Date:** January 08, 2019\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 397692,
"choiceText": "Upright chest x-ray",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 397693,
"choiceText": "Supine chest x-ray",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 397694,
"choiceText": "CT scan",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 397695,
"choiceText": "Thoracic ultrasound",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The diagnosis of pneumothorax is most commonly confirmed by x-rays (preferably upright) or CT scans of the chest. Chest x-rays can miss small pneumothoraces in patients with underlying lung disease, especially when multiple thin-walled bullae are present. Supine chest x-rays also frequently miss pneumothoraces. Thoracic ultrasonography can be used to identify a pneumothorax with excellent sensitivity.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 115831,
"questionText": "<p>A 65-year-old man with severe COPD presents to the ED with sudden worsening of his dyspnea since this morning. He has diminished left-sided breath sounds and acute pain. An ECG reveals sinus tachycardia, but it is otherwise unchanged from baseline and does not show acute ischemia. Although multiple possible etiologies for this patient's pain are possible, pneumothorax is strongly suspected.</p><p>Which of the following imaging modalities is <b><i>least preferable</i></b> for confirming the diagnosis of pneumothorax?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 397696,
"choiceText": "No further intervention is necessary",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 397697,
"choiceText": "Chemical pleurodesis via tube thoracostomy",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 397698,
"choiceText": "VATS with bullae stapling",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 397699,
"choiceText": "Thoracotomy with bullae resection",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Per ACCP guidelines, this patient should have an intervention to prevent recurrence because a second episode of pneumothorax could be catastrophic. His severe COPD and limited life expectancy makes him a poor candidate for VATS or thoracotomy. In this patient, chemical pleurodesis would be the least invasive option while still being effective and associated with the least mortality and morbidity. ",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 115832,
"questionText": "The CT scan of the chest in the patient described above demonstrates multiple bullae. In addition to tube thoracostomy, which of the following should be performed in this patient?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Sudden-Onset Chest Pain in an 80-Year-Old Man With COPD"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 397688,
"choiceText": "Foreign body",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 397689,
"choiceText": "Pulmonary embolism",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 397690,
"choiceText": "Pneumothorax",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 397691,
"choiceText": "Large mediastinal mass",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 115830,
"questionText": "What diagnosis is best demonstrated on the CT scan?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 397692,
"choiceText": "Upright chest x-ray",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 397693,
"choiceText": "Supine chest x-ray",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 397694,
"choiceText": "CT scan",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 397695,
"choiceText": "Thoracic ultrasound",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The diagnosis of pneumothorax is most commonly confirmed by x-rays (preferably upright) or CT scans of the chest. Chest x-rays can miss small pneumothoraces in patients with underlying lung disease, especially when multiple thin-walled bullae are present. Supine chest x-rays also frequently miss pneumothoraces. Thoracic ultrasonography can be used to identify a pneumothorax with excellent sensitivity.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 115831,
"questionText": "<p>A 65-year-old man with severe COPD presents to the ED with sudden worsening of his dyspnea since this morning. He has diminished left-sided breath sounds and acute pain. An ECG reveals sinus tachycardia, but it is otherwise unchanged from baseline and does not show acute ischemia. Although multiple possible etiologies for this patient's pain are possible, pneumothorax is strongly suspected.</p><p>Which of the following imaging modalities is <b><i>least preferable</i></b> for confirming the diagnosis of pneumothorax?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 397696,
"choiceText": "No further intervention is necessary",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 397697,
"choiceText": "Chemical pleurodesis via tube thoracostomy",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 397698,
"choiceText": "VATS with bullae stapling",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 397699,
"choiceText": "Thoracotomy with bullae resection",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Per ACCP guidelines, this patient should have an intervention to prevent recurrence because a second episode of pneumothorax could be catastrophic. His severe COPD and limited life expectancy makes him a poor candidate for VATS or thoracotomy. In this patient, chemical pleurodesis would be the least invasive option while still being effective and associated with the least mortality and morbidity. ",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 115832,
"questionText": "The CT scan of the chest in the patient described above demonstrates multiple bullae. In addition to tube thoracostomy, which of the following should be performed in this patient?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
738832 | /viewarticle/738832 | [
{
"authors": "Miriam Kinai, MB ChB",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to recognize accurately. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"A 27-year-old African-American woman presents with a history of white spots on her face for the past 2 months (see Figure 1).",
"Figure 1.",
"She reports that she has been applying concealer to cover the lesions as they have been progressively increasing in number and size. She states that the patches are neither painful nor itchy, but they have caused her considerable emotional stress as her livelihood depends upon her appearance. She admits dying her hair, which has begun to turn gray. The patient also reports having a flawless complexion prior to the development of these lesions and enjoying good health since childhood. She has not applied any prescription or over-the-counter medications to the lesions. The patient denies using any skin lightening products or undergoing any cosmetic procedures, such as microdermabrasion or laser skin resurfacing, in the past 6 months.",
"She has no family history of any similar skin diseases, hypertension, asthma, or any other significant illnesses. She has no known allergies and is not taking any medications. She is single and has no children. The patient denies drinking alcohol, smoking cigarettes, or using illicit drugs."
],
"date": "September 14, 2018",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/738/832/738832-thumb1.png"
}
],
"markdown": "# A 27-Year-Old Woman With White Spots on Her Face\n\n **Authors:** Miriam Kinai, MB ChB \n **Date:** September 14, 2018\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to recognize accurately. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nA 27-year-old African-American woman presents with a history of white spots on her face for the past 2 months (see Figure 1).\nFigure 1.\nShe reports that she has been applying concealer to cover the lesions as they have been progressively increasing in number and size. She states that the patches are neither painful nor itchy, but they have caused her considerable emotional stress as her livelihood depends upon her appearance. She admits dying her hair, which has begun to turn gray. The patient also reports having a flawless complexion prior to the development of these lesions and enjoying good health since childhood. She has not applied any prescription or over-the-counter medications to the lesions. The patient denies using any skin lightening products or undergoing any cosmetic procedures, such as microdermabrasion or laser skin resurfacing, in the past 6 months.\nShe has no family history of any similar skin diseases, hypertension, asthma, or any other significant illnesses. She has no known allergies and is not taking any medications. She is single and has no children. The patient denies drinking alcohol, smoking cigarettes, or using illicit drugs.\n\n ## Figures\n\n **Figure 1.** \n \n\n\n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 27-Year-Old Woman With White Spots on Her Face"
},
{
"authors": "Miriam Kinai, MB ChB",
"content": [
"Upon physical examination, the patient is a thin, young female. Her vital signs include a blood pressure of 110/70 mm Hg, a respiratory rate of 16 breaths/min, and a temperature of 98.6°F (37°C). Her pulse has a regular rhythm with a rate of 110 beats/min. Several sharply demarcated, depigmented, nonscaly, nonerythematous macules and patches are observed above her left eyebrow in a linear distribution. They appear chalky white when examined using a Wood's lamp.",
"The patient’s eyebrows and eyelashes are intact but grey-white in color. She also has premature graying of her hair, but no alopecia or other skin lesions are detected. Her lungs are clear on auscultation and the S1 and S2 hearts sounds are normal. Her thyroid is not enlarged and no thyroid bruit is noted. Her central nervous system examination findings are normal.",
"Laboratory investigations reveal a normal complete blood cell count as well as a normal basic metabolic panel. Her total thyroxine (total T4) is within normal limits at 10.0 µg/dL (reference range, 4.5-12.3 µg/dL) as is her triiodothyronine (total T3) at 160 ng/dL (reference range, 60-181 ng/dL). An ultrasound of her thyroid reveals an enlarged gland with decreased echogenicity."
],
"date": "September 14, 2018",
"figures": [],
"markdown": "# A 27-Year-Old Woman With White Spots on Her Face\n\n **Authors:** Miriam Kinai, MB ChB \n **Date:** September 14, 2018\n\n ## Content\n\n Upon physical examination, the patient is a thin, young female. Her vital signs include a blood pressure of 110/70 mm Hg, a respiratory rate of 16 breaths/min, and a temperature of 98.6°F (37°C). Her pulse has a regular rhythm with a rate of 110 beats/min. Several sharply demarcated, depigmented, nonscaly, nonerythematous macules and patches are observed above her left eyebrow in a linear distribution. They appear chalky white when examined using a Wood's lamp.\nThe patient’s eyebrows and eyelashes are intact but grey-white in color. She also has premature graying of her hair, but no alopecia or other skin lesions are detected. Her lungs are clear on auscultation and the S1 and S2 hearts sounds are normal. Her thyroid is not enlarged and no thyroid bruit is noted. Her central nervous system examination findings are normal.\nLaboratory investigations reveal a normal complete blood cell count as well as a normal basic metabolic panel. Her total thyroxine (total T4) is within normal limits at 10.0 µg/dL (reference range, 4.5-12.3 µg/dL) as is her triiodothyronine (total T3) at 160 ng/dL (reference range, 60-181 ng/dL). An ultrasound of her thyroid reveals an enlarged gland with decreased echogenicity.\n\n ## Figures\n\n \n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 396501,
"choiceText": "Idiopathic guttate hypomelanosis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 396502,
"choiceText": "Tuberculoid leprosy",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 396503,
"choiceText": "Tinea versicolor",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 396504,
"choiceText": "Vitiligo",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 115418,
"questionText": "<p>Which of the following is the most likely diagnosis?</p>\r\n<p><em>Hint: Note the unilateral, linear distribution of the macules and patches.</em></p>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 27-Year-Old Woman With White Spots on Her Face"
},
{
"authors": "Miriam Kinai, MB ChB",
"content": [
"The diagnosis of vitiligo was made clinically on the basis of the patient’s characteristic history and the physical examination findings. The painless, sharply demarcated, demelanotic macules and patches appeared in a linear distribution above her right eyebrow, in keeping with the diagnosis of segmental vitiligo, which affects one side of the body. These hypopigmented macules and patches were also nonscaly, as is classically described. The patient’s history and physical examination ruled out a diagnosis of idiopathic guttate hypomelanosis, as this condition primarily affects the middle-aged and elderly population.",
"Patients typically present with hypopigmented macules of 2-5 mm on sun-exposed areas of the upper and lower extremities, such as the extensor surfaces of the forearms and legs. These lesions also have a slight xerotic scaling and remain the same size, though their numbers increase. The physical examination also ruled out a diagnosis of tinea versicolor, which presents with small circular macules, usually on the upper back and chest wall, which have a white scale when scratched and golden fluorescence when examined under a Wood's lamp. The normal nervous system examination findings ruled out a diagnosis of tuberculoid leprosy, as these patients have asymmetric macules or patches of hypomelanosis that are insensitive to pain, heat, and touch. Associated anhidrosis and alopecia is also present. The elevated total T3 and T4 findings supported the diagnosis of vitiligo because many patients with vitiligo have an associated autoimmune disease, such as Graves disease or Hashimoto thyroiditis.",
"Vitiligo is a pigmentation disorder of unknown etiology in which melanocytes in the affected skin and mucous membranes are destroyed. This destruction is thought to be caused by an autoimmune process because patients with vitiligo have a high incidence of antibodies to melanocytes.[1] Vitiligo affects approximately 1% of the world’s population.[2] Furthermore, approximately 30% of vitiligo patients have a positive family history of the disorder.",
"In an analysis of genome-wide data that tested 33 candidate genes for generalized vitiligo, investigators found an association with TSLP, XBP1, and FOXP3.[3] When the investigators evaluated CTLA4, the association with generalized vitiligo appeared to have a secondary epidemiologic association with other concomitant autoimmune diseases. Furthermore, linkage disequilibrium with major primary signals in the major histocompatibility complex (MHC) I and II regions at 6p21.33 may be the source of an association between TAP1-PSMB8 and generalized vitiligo.[3]",
"Depigmentation can begin in childhood, and more than 75% of patients present before age 30 years. The disease is characterized by sharply demarcated, depigmented, nonscaly round or oval macules and patches on the skin and mucous membranes that range from a few millimeters to several centimeters in size. The condition is usually nonerythematous. The initial lesions occur most commonly on sun-exposed areas, such as the face, forearms, and dorsal surfaces of the hands and feet. Vitiligo has a periorificial predilection, with areas around the mouth, nose, eyes, nipples, umbilicus and anus commonly being affected. The axillae and genitalia are also commonly involved. Vitiligo may also develop at sites of trauma, such as cuts or burns (Koebner phenomenon). Sweating is increased in the depigmented areas while there may also be leukotrichia or depigmentation of the scalp hair, eyebrows, eyelashes, beard, and pubic hair. Patients with localized segmental vitiligo and poliosis usually do not have associated autoimmune diseases."
],
"date": "September 14, 2018",
"figures": [],
"markdown": "# A 27-Year-Old Woman With White Spots on Her Face\n\n **Authors:** Miriam Kinai, MB ChB \n **Date:** September 14, 2018\n\n ## Content\n\n The diagnosis of vitiligo was made clinically on the basis of the patient’s characteristic history and the physical examination findings. The painless, sharply demarcated, demelanotic macules and patches appeared in a linear distribution above her right eyebrow, in keeping with the diagnosis of segmental vitiligo, which affects one side of the body. These hypopigmented macules and patches were also nonscaly, as is classically described. The patient’s history and physical examination ruled out a diagnosis of idiopathic guttate hypomelanosis, as this condition primarily affects the middle-aged and elderly population.\nPatients typically present with hypopigmented macules of 2-5 mm on sun-exposed areas of the upper and lower extremities, such as the extensor surfaces of the forearms and legs. These lesions also have a slight xerotic scaling and remain the same size, though their numbers increase. The physical examination also ruled out a diagnosis of tinea versicolor, which presents with small circular macules, usually on the upper back and chest wall, which have a white scale when scratched and golden fluorescence when examined under a Wood's lamp. The normal nervous system examination findings ruled out a diagnosis of tuberculoid leprosy, as these patients have asymmetric macules or patches of hypomelanosis that are insensitive to pain, heat, and touch. Associated anhidrosis and alopecia is also present. The elevated total T3 and T4 findings supported the diagnosis of vitiligo because many patients with vitiligo have an associated autoimmune disease, such as Graves disease or Hashimoto thyroiditis.\nVitiligo is a pigmentation disorder of unknown etiology in which melanocytes in the affected skin and mucous membranes are destroyed. This destruction is thought to be caused by an autoimmune process because patients with vitiligo have a high incidence of antibodies to melanocytes.[1] Vitiligo affects approximately 1% of the world’s population.[2] Furthermore, approximately 30% of vitiligo patients have a positive family history of the disorder.\nIn an analysis of genome-wide data that tested 33 candidate genes for generalized vitiligo, investigators found an association with TSLP, XBP1, and FOXP3.[3] When the investigators evaluated CTLA4, the association with generalized vitiligo appeared to have a secondary epidemiologic association with other concomitant autoimmune diseases. Furthermore, linkage disequilibrium with major primary signals in the major histocompatibility complex (MHC) I and II regions at 6p21.33 may be the source of an association between TAP1-PSMB8 and generalized vitiligo.[3]\nDepigmentation can begin in childhood, and more than 75% of patients present before age 30 years. The disease is characterized by sharply demarcated, depigmented, nonscaly round or oval macules and patches on the skin and mucous membranes that range from a few millimeters to several centimeters in size. The condition is usually nonerythematous. The initial lesions occur most commonly on sun-exposed areas, such as the face, forearms, and dorsal surfaces of the hands and feet. Vitiligo has a periorificial predilection, with areas around the mouth, nose, eyes, nipples, umbilicus and anus commonly being affected. The axillae and genitalia are also commonly involved. Vitiligo may also develop at sites of trauma, such as cuts or burns (Koebner phenomenon). Sweating is increased in the depigmented areas while there may also be leukotrichia or depigmentation of the scalp hair, eyebrows, eyelashes, beard, and pubic hair. Patients with localized segmental vitiligo and poliosis usually do not have associated autoimmune diseases.\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 396501,
"choiceText": "Idiopathic guttate hypomelanosis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 396502,
"choiceText": "Tuberculoid leprosy",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 396503,
"choiceText": "Tinea versicolor",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 396504,
"choiceText": "Vitiligo",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 115418,
"questionText": "<p>Which of the following is the most likely diagnosis?</p>\r\n<p><em>Hint: Note the unilateral, linear distribution of the macules and patches.</em></p>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 27-Year-Old Woman With White Spots on Her Face"
},
{
"authors": "Miriam Kinai, MB ChB",
"content": [
"Vitiligo can be classified as localized, generalized, or universal. Patients with localized vitiligo have unilateral segmental areas of depigmentation limited to one part of the body (such as one side of the face or one limb). These lesions may have a dermatome-like distribution and, although vitiligo is usually chronic and progressive, in some patients with segmental vitiligo the depigmented macules and patches remain localized and do not spread. Patients with generalized vitiligo have widely distributed depigmented macules and patches, usually on both sides of the body. This is the most common pattern and the lesions are usually symmetrical but may be asymmetrical. Patients with universal vitiligo have total or near-total depigmentation of their skin.",
"Morphologic variations of vitiligo include the following:",
"Trichrome vitiligo, in which patients have 3 zones of different colors; these range from the central achromic zone, to its surrounding hypochromic zone, to the outermost peripheral normal colored skin zone",
"Quadrichrome vitiligo, in which a fourth dark color is present in areas undergoing perifollicular hyperpigmentation",
"Pentachrome vitiligo, with 5 zones of different colors",
"Inflammatory vitiligo, in which an erythematous border surrounds the vitiliginous area",
"In addition to the dermatologic effects, vitiligo tends to have an emotional and psychological impact. Patients with vitiligo, particularly adolescents who have dark skin types, can experience emotional stress, develop low self-esteem, stigmatization, social anxiety, and even depression. This is especially true if the depigmented patches are on the genitals or on highly visible areas, such as the face and hands.",
"The diagnosis of vitiligo is generally made clinically based on the history and physical examination. A Wood's lamp examination accentuates the ivory or chalk-white colored vitiliginous depigmented macules and patches, thereby helping to differentiate them from hypopigmented lesions. A Wood’s lamp is also useful in revealing the extent of vitiligo in patients with type I and II skin types.[4] A skin biopsy can help differentiate vitiligo from hypopigmented lesions because it demonstrates a complete absence of melanocytes in the affected skin.",
"Patients with vitiligo have an increased incidence of Graves disease, Hashimoto thyroiditis, Addison disease, alopecia areata, diabetes mellitus, pernicious anemia, and other autoimmune diseases. Therefore, laboratory tests in vitiligo should be determined by associated signs or symptoms suggesting other autoimmune disease; these tests may include the following: complete blood count (CBC), vitamin B-12 levels and antiparietal cell antibodies tests for associated pernicious anemia, thyroid function tests, antithyroglobulin tests, fasting blood glucose tests for associated diabetes mellitus in patients with polydipsia or polyuria, and antinuclear antibody tests.",
"The differential diagnosis of vitiligo includes idiopathic guttate hypomelanosis, tinea versicolor, halo nevus, leprosy, postinflammatory hypopigmentation, pityriasis alba, ash leaf macules of tuberous sclerosis, nevus depigmentosus, chemical leukoderma, and piebaldism.",
"Although spontaneous repigmentation does occur in a few cases, the treatment of vitiligo is challenging because no single treatment results in the repigmentation of all patients. This fact should be communicated to patients, especially those with vitiligo in areas known to be resistant to treatment (such as the hands and feet). The patient’s response to treatment should be monitored with serial clinical photography. The choice of therapy depends on the size, number, and location of the hypopigmented patches and can be classified as medical, surgical, or adjunctive."
],
"date": "September 14, 2018",
"figures": [],
"markdown": "# A 27-Year-Old Woman With White Spots on Her Face\n\n **Authors:** Miriam Kinai, MB ChB \n **Date:** September 14, 2018\n\n ## Content\n\n Vitiligo can be classified as localized, generalized, or universal. Patients with localized vitiligo have unilateral segmental areas of depigmentation limited to one part of the body (such as one side of the face or one limb). These lesions may have a dermatome-like distribution and, although vitiligo is usually chronic and progressive, in some patients with segmental vitiligo the depigmented macules and patches remain localized and do not spread. Patients with generalized vitiligo have widely distributed depigmented macules and patches, usually on both sides of the body. This is the most common pattern and the lesions are usually symmetrical but may be asymmetrical. Patients with universal vitiligo have total or near-total depigmentation of their skin.\nMorphologic variations of vitiligo include the following:\nTrichrome vitiligo, in which patients have 3 zones of different colors; these range from the central achromic zone, to its surrounding hypochromic zone, to the outermost peripheral normal colored skin zone\nQuadrichrome vitiligo, in which a fourth dark color is present in areas undergoing perifollicular hyperpigmentation\nPentachrome vitiligo, with 5 zones of different colors\nInflammatory vitiligo, in which an erythematous border surrounds the vitiliginous area\nIn addition to the dermatologic effects, vitiligo tends to have an emotional and psychological impact. Patients with vitiligo, particularly adolescents who have dark skin types, can experience emotional stress, develop low self-esteem, stigmatization, social anxiety, and even depression. This is especially true if the depigmented patches are on the genitals or on highly visible areas, such as the face and hands.\nThe diagnosis of vitiligo is generally made clinically based on the history and physical examination. A Wood's lamp examination accentuates the ivory or chalk-white colored vitiliginous depigmented macules and patches, thereby helping to differentiate them from hypopigmented lesions. A Wood’s lamp is also useful in revealing the extent of vitiligo in patients with type I and II skin types.[4] A skin biopsy can help differentiate vitiligo from hypopigmented lesions because it demonstrates a complete absence of melanocytes in the affected skin.\nPatients with vitiligo have an increased incidence of Graves disease, Hashimoto thyroiditis, Addison disease, alopecia areata, diabetes mellitus, pernicious anemia, and other autoimmune diseases. Therefore, laboratory tests in vitiligo should be determined by associated signs or symptoms suggesting other autoimmune disease; these tests may include the following: complete blood count (CBC), vitamin B-12 levels and antiparietal cell antibodies tests for associated pernicious anemia, thyroid function tests, antithyroglobulin tests, fasting blood glucose tests for associated diabetes mellitus in patients with polydipsia or polyuria, and antinuclear antibody tests.\nThe differential diagnosis of vitiligo includes idiopathic guttate hypomelanosis, tinea versicolor, halo nevus, leprosy, postinflammatory hypopigmentation, pityriasis alba, ash leaf macules of tuberous sclerosis, nevus depigmentosus, chemical leukoderma, and piebaldism.\nAlthough spontaneous repigmentation does occur in a few cases, the treatment of vitiligo is challenging because no single treatment results in the repigmentation of all patients. This fact should be communicated to patients, especially those with vitiligo in areas known to be resistant to treatment (such as the hands and feet). The patient’s response to treatment should be monitored with serial clinical photography. The choice of therapy depends on the size, number, and location of the hypopigmented patches and can be classified as medical, surgical, or adjunctive.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 27-Year-Old Woman With White Spots on Her Face"
},
{
"authors": "Miriam Kinai, MB ChB",
"content": [
"Medical management of vitiligo is the preferred first-line intervention, and the available options include the following:",
"Topical corticosteroids",
"Topical calcineurin inhibitors",
"Vitamin D analogues",
"Narrow-band ultraviolet B (UV-B) light therapy",
"Topical psoralen ointment with ultraviolet A light therapy (PUVA)",
"Oral psoralen with ultraviolet A light therapy (PUVA) or systemic psoralen photochemotherapy",
"Laser therapy with a 308 nm excimer laser",
"In very select cases, depigmentation of normal surrounding skin with monobenzyl ether of hydroquinone (MBEH)",
"Topical PUVA, corticosteroids, and calcineurin inhibitors are used to treat patients with a few localized patches of vitiligo. Oral PUVA is usually reserved for patients with vitiligo affecting more than 20% of their skin surface area, laser therapy is used when less than 30% of the skin is involved, and monobenzyl ether of hydroquinone is used to treat selected patients with extensive vitiligo affecting more than 50% of their skin, as it will depigment normal, unaffected skin. The safest and most effective of the medical therapies for vitiligo are class 3 topical corticosteroids and ultraviolet B (UV-B) therapy.[2] Narrow band ultraviolet B (UV-B) therapy is superior even to oral PUVA in treating nonsegmental vitiligo.[5]",
"Surgical interventions can be applied in patients who fail or have an inadequate response to medical treatment. Furthermore, they should only be considered in patients with localized stable vitiligo that does not Koebnerize and is in a cosmetically sensitive area.[4] The available options include split skin grafts, skin grafts using blisters, minigrafting, cultured or noncultured autologous epidermal suspensions, and micropigmentation or tattooing to repigment skin. The Erbium:YAG laser can be used for disepithelialization of large vitiliginous patches prior to graft application.[7] Split skin grafting is the best surgical option, whereas minigrafting has the most adverse effects (which include a cobblestone appearance in over 25% of patients).[4]",
"Adjunctive therapies are useful in most patients as they address the physiologic as well as the psychologic effect that result from having vitiligo. Several adjunctive treatment options are available. Broad-spectrum sunscreens that offer protection from ultraviolet A (UV-A) and ultraviolet B (UV-B) light and have a sun protection factor (SPF) of 30 or higher should be used because the depigmented lesions are at increased risk for sunburn and subsequent skin cancers. Patients should also be advised to wear protective clothing and minimize sun exposure. Camouflage cosmetics, which include makeup, dyes and self-tanning lotions, are also beneficial. Psychological treatments, including cognitive therapy and counseling, are important because they improve the coping mechanisms of patients with vitiligo. Patient support groups are useful for enhancing emotional well-being. The parents of children with vitiligo should also receive counseling.",
"The patient in the case above was started on topical fluticasone propionate cream. She was also advised to use sunscreen with a sun protection factor (SPF) of at least 30."
],
"date": "September 14, 2018",
"figures": [],
"markdown": "# A 27-Year-Old Woman With White Spots on Her Face\n\n **Authors:** Miriam Kinai, MB ChB \n **Date:** September 14, 2018\n\n ## Content\n\n Medical management of vitiligo is the preferred first-line intervention, and the available options include the following:\nTopical corticosteroids\nTopical calcineurin inhibitors\nVitamin D analogues\nNarrow-band ultraviolet B (UV-B) light therapy\nTopical psoralen ointment with ultraviolet A light therapy (PUVA)\nOral psoralen with ultraviolet A light therapy (PUVA) or systemic psoralen photochemotherapy\nLaser therapy with a 308 nm excimer laser\nIn very select cases, depigmentation of normal surrounding skin with monobenzyl ether of hydroquinone (MBEH)\nTopical PUVA, corticosteroids, and calcineurin inhibitors are used to treat patients with a few localized patches of vitiligo. Oral PUVA is usually reserved for patients with vitiligo affecting more than 20% of their skin surface area, laser therapy is used when less than 30% of the skin is involved, and monobenzyl ether of hydroquinone is used to treat selected patients with extensive vitiligo affecting more than 50% of their skin, as it will depigment normal, unaffected skin. The safest and most effective of the medical therapies for vitiligo are class 3 topical corticosteroids and ultraviolet B (UV-B) therapy.[2] Narrow band ultraviolet B (UV-B) therapy is superior even to oral PUVA in treating nonsegmental vitiligo.[5]\nSurgical interventions can be applied in patients who fail or have an inadequate response to medical treatment. Furthermore, they should only be considered in patients with localized stable vitiligo that does not Koebnerize and is in a cosmetically sensitive area.[4] The available options include split skin grafts, skin grafts using blisters, minigrafting, cultured or noncultured autologous epidermal suspensions, and micropigmentation or tattooing to repigment skin. The Erbium:YAG laser can be used for disepithelialization of large vitiliginous patches prior to graft application.[7] Split skin grafting is the best surgical option, whereas minigrafting has the most adverse effects (which include a cobblestone appearance in over 25% of patients).[4]\nAdjunctive therapies are useful in most patients as they address the physiologic as well as the psychologic effect that result from having vitiligo. Several adjunctive treatment options are available. Broad-spectrum sunscreens that offer protection from ultraviolet A (UV-A) and ultraviolet B (UV-B) light and have a sun protection factor (SPF) of 30 or higher should be used because the depigmented lesions are at increased risk for sunburn and subsequent skin cancers. Patients should also be advised to wear protective clothing and minimize sun exposure. Camouflage cosmetics, which include makeup, dyes and self-tanning lotions, are also beneficial. Psychological treatments, including cognitive therapy and counseling, are important because they improve the coping mechanisms of patients with vitiligo. Patient support groups are useful for enhancing emotional well-being. The parents of children with vitiligo should also receive counseling.\nThe patient in the case above was started on topical fluticasone propionate cream. She was also advised to use sunscreen with a sun protection factor (SPF) of at least 30.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 396505,
"choiceText": "Complete absence of melanocytes",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 396506,
"choiceText": "An abrupt decrease in epidermal melanin content",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 396507,
"choiceText": "Multiple hyphae and round spores",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 396508,
"choiceText": "Granulomas in the dermis",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>Patients with tuberculoid leprosy present with hypopigmented macules or patches with a surrounding elevated and erythematous border. The other differentials, which include vitiligo, idiopathic guttate hypomelanosis, and tinea versicolor, all have normal sensation. Vitiliginous patches are nonscaly whereas those of tinea versicolor have a white scale. Idiopathic guttate hypomelanosis usually affects the legs and arms of the elderly and the lesions have a slight xerotic scaling.</p><p>The above presentation suggests a diagnosis of vitiligo, which on biopsy would reveal a complete absence of melanocytes. An abrupt decrease in epidermal melanin content is seen on biopsying the hypopigmented macules of idiopathic guttate hypomelanosis. Multiple hyphae with round spores are found in the hypopigmented lesions of tinea versicolor, which is caused by the yeast <i>Pityrosporum orbiculare (Malassezia furfur)</i>. Dermal noncaseating granulomas are seen in tuberculoid leprosy biopsies.</p>",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 115419,
"questionText": "A 35-year-old woman presents with a slowly enlarging skin lesion. She reports that 6 weeks prior to presentation she vacationed in a tropical country. Upon examination, you note a single 3-cm, nonscaly, nonerythematous, hypopigmented patch with normal sensation on the dorsal surface of her right hand. Which of the following would you expect to find on biopsying the lesion?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 396509,
"choiceText": "Camouflage cosmetics and sunscreen",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 396510,
"choiceText": "Topical corticosteroids",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 396511,
"choiceText": "P-(benzyloxy) phenol",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 396512,
"choiceText": "Oral psoralen and ultraviolet A light therapy (PUVA)",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Topical corticosteroids are the most appropriate treatment option for this patient because oral PUVA is usually used to treat patients with vitiligo affecting more than 20% of their skin surface area, and p-(benzyloxy) phenol is used for depigmentation in patients with vitiligo affecting more than 50% of their skin. Camouflage cosmetics and sunscreen would not be appropriate as the sole therapy for this patient because her condition is progressive; these should be used as adjuncts to more definitive treatment methods.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 115420,
"questionText": "Upon confirming the diagnosis in the previous patient, which of the following is the most appropriate treatment option for this patient?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 27-Year-Old Woman With White Spots on Her Face"
},
{
"authors": "Miriam Kinai, MB ChB",
"content": [],
"date": "September 14, 2018",
"figures": [],
"markdown": "# A 27-Year-Old Woman With White Spots on Her Face\n\n **Authors:** Miriam Kinai, MB ChB \n **Date:** September 14, 2018\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 396505,
"choiceText": "Complete absence of melanocytes",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 396506,
"choiceText": "An abrupt decrease in epidermal melanin content",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 396507,
"choiceText": "Multiple hyphae and round spores",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 396508,
"choiceText": "Granulomas in the dermis",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>Patients with tuberculoid leprosy present with hypopigmented macules or patches with a surrounding elevated and erythematous border. The other differentials, which include vitiligo, idiopathic guttate hypomelanosis, and tinea versicolor, all have normal sensation. Vitiliginous patches are nonscaly whereas those of tinea versicolor have a white scale. Idiopathic guttate hypomelanosis usually affects the legs and arms of the elderly and the lesions have a slight xerotic scaling.</p><p>The above presentation suggests a diagnosis of vitiligo, which on biopsy would reveal a complete absence of melanocytes. An abrupt decrease in epidermal melanin content is seen on biopsying the hypopigmented macules of idiopathic guttate hypomelanosis. Multiple hyphae with round spores are found in the hypopigmented lesions of tinea versicolor, which is caused by the yeast <i>Pityrosporum orbiculare (Malassezia furfur)</i>. Dermal noncaseating granulomas are seen in tuberculoid leprosy biopsies.</p>",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 115419,
"questionText": "A 35-year-old woman presents with a slowly enlarging skin lesion. She reports that 6 weeks prior to presentation she vacationed in a tropical country. Upon examination, you note a single 3-cm, nonscaly, nonerythematous, hypopigmented patch with normal sensation on the dorsal surface of her right hand. Which of the following would you expect to find on biopsying the lesion?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 396509,
"choiceText": "Camouflage cosmetics and sunscreen",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 396510,
"choiceText": "Topical corticosteroids",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 396511,
"choiceText": "P-(benzyloxy) phenol",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 396512,
"choiceText": "Oral psoralen and ultraviolet A light therapy (PUVA)",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Topical corticosteroids are the most appropriate treatment option for this patient because oral PUVA is usually used to treat patients with vitiligo affecting more than 20% of their skin surface area, and p-(benzyloxy) phenol is used for depigmentation in patients with vitiligo affecting more than 50% of their skin. Camouflage cosmetics and sunscreen would not be appropriate as the sole therapy for this patient because her condition is progressive; these should be used as adjuncts to more definitive treatment methods.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 115420,
"questionText": "Upon confirming the diagnosis in the previous patient, which of the following is the most appropriate treatment option for this patient?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 27-Year-Old Woman With White Spots on Her Face"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 396501,
"choiceText": "Idiopathic guttate hypomelanosis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 396502,
"choiceText": "Tuberculoid leprosy",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 396503,
"choiceText": "Tinea versicolor",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 396504,
"choiceText": "Vitiligo",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 115418,
"questionText": "<p>Which of the following is the most likely diagnosis?</p>\r\n<p><em>Hint: Note the unilateral, linear distribution of the macules and patches.</em></p>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 396505,
"choiceText": "Complete absence of melanocytes",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 396506,
"choiceText": "An abrupt decrease in epidermal melanin content",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 396507,
"choiceText": "Multiple hyphae and round spores",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 396508,
"choiceText": "Granulomas in the dermis",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>Patients with tuberculoid leprosy present with hypopigmented macules or patches with a surrounding elevated and erythematous border. The other differentials, which include vitiligo, idiopathic guttate hypomelanosis, and tinea versicolor, all have normal sensation. Vitiliginous patches are nonscaly whereas those of tinea versicolor have a white scale. Idiopathic guttate hypomelanosis usually affects the legs and arms of the elderly and the lesions have a slight xerotic scaling.</p><p>The above presentation suggests a diagnosis of vitiligo, which on biopsy would reveal a complete absence of melanocytes. An abrupt decrease in epidermal melanin content is seen on biopsying the hypopigmented macules of idiopathic guttate hypomelanosis. Multiple hyphae with round spores are found in the hypopigmented lesions of tinea versicolor, which is caused by the yeast <i>Pityrosporum orbiculare (Malassezia furfur)</i>. Dermal noncaseating granulomas are seen in tuberculoid leprosy biopsies.</p>",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 115419,
"questionText": "A 35-year-old woman presents with a slowly enlarging skin lesion. She reports that 6 weeks prior to presentation she vacationed in a tropical country. Upon examination, you note a single 3-cm, nonscaly, nonerythematous, hypopigmented patch with normal sensation on the dorsal surface of her right hand. Which of the following would you expect to find on biopsying the lesion?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 396509,
"choiceText": "Camouflage cosmetics and sunscreen",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 396510,
"choiceText": "Topical corticosteroids",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 396511,
"choiceText": "P-(benzyloxy) phenol",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 396512,
"choiceText": "Oral psoralen and ultraviolet A light therapy (PUVA)",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Topical corticosteroids are the most appropriate treatment option for this patient because oral PUVA is usually used to treat patients with vitiligo affecting more than 20% of their skin surface area, and p-(benzyloxy) phenol is used for depigmentation in patients with vitiligo affecting more than 50% of their skin. Camouflage cosmetics and sunscreen would not be appropriate as the sole therapy for this patient because her condition is progressive; these should be used as adjuncts to more definitive treatment methods.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 115420,
"questionText": "Upon confirming the diagnosis in the previous patient, which of the following is the most appropriate treatment option for this patient?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
738063 | /viewarticle/738063 | [
{
"authors": "George T. Griffing, MD; Naga Neelima Nallapaneni, MB BS",
"content": [
"Editorial Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"A 49-year-old man with a history of osteopenia and HIV infection presents for follow-up. He had been seen in the emergency department about 8 months ago after several days of dull thigh pain followed by a sudden, intense pain after making a twisting motion when walking. He was diagnosed with a midshaft femoral fracture. He has had no previous bone fractures.",
"He has long-standing HIV infection and is taking antiretroviral medications. He is also on supplemental calcium, vitamin D, and 8 years of alendronate therapy as part of a clinical HIV study. He has no known history of cancer. The patient does not have any known allergies and the family medical history is unremarkable."
],
"date": "April 06, 2018",
"figures": [],
"markdown": "# Mysterious Femur Fracture in a Middle-Aged Man\n\n **Authors:** George T. Griffing, MD; Naga Neelima Nallapaneni, MB BS \n **Date:** April 06, 2018\n\n ## Content\n\n Editorial Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nA 49-year-old man with a history of osteopenia and HIV infection presents for follow-up. He had been seen in the emergency department about 8 months ago after several days of dull thigh pain followed by a sudden, intense pain after making a twisting motion when walking. He was diagnosed with a midshaft femoral fracture. He has had no previous bone fractures.\nHe has long-standing HIV infection and is taking antiretroviral medications. He is also on supplemental calcium, vitamin D, and 8 years of alendronate therapy as part of a clinical HIV study. He has no known history of cancer. The patient does not have any known allergies and the family medical history is unremarkable.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "Mysterious Femur Fracture in a Middle-Aged Man"
},
{
"authors": "George T. Griffing, MD; Naga Neelima Nallapaneni, MB BS",
"content": [
"Upon physical examination, his vital signs are normal. His height is about the same length as his arm span. The patient's physical appearance is remarkable for HIV-treatment–induced lipodystrophy and a Cushingoid habitus (Figure 1).",
"Figure 1.",
"The sclera and tympanic membranes are clear. Gonadal examination demonstrates bilaterally descended and normal-sized testicles. Upon laboratory investigations, the complete blood cell count, urinalysis, complete metabolic panel, vitamin D level, thyroid-stimulating hormone level, and testosterone level are normal. Urinary free cortisol and an overnight dexamethasone suppression test for Cushing syndrome are also normal. He has an undetectable HIV viral load and a normal CD4 count. He has normal plasma and urine calcium, serum phosphorus, alkaline phosphate, and parathyroid hormone levels.",
"The patient had been enrolled in an HIV alendronate study 7 years prior to injury. Bone mineral density (BMD) testing at that time showed osteopenia. The patient was then started on alendronate therapy. Repeat BMD testing 4 years before the injury showed normal density scores at both his hip and spine. Now, 8 months after his injury, his BMD is still normal although it is decreased from his levels 4 years previously (Table 1).",
"Table 1. Bone Mineral Density*",
"*Densities reported in g/cm2"
],
"date": "April 06, 2018",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/738/063/738063-thumb1.png"
}
],
"markdown": "# Mysterious Femur Fracture in a Middle-Aged Man\n\n **Authors:** George T. Griffing, MD; Naga Neelima Nallapaneni, MB BS \n **Date:** April 06, 2018\n\n ## Content\n\n Upon physical examination, his vital signs are normal. His height is about the same length as his arm span. The patient's physical appearance is remarkable for HIV-treatment–induced lipodystrophy and a Cushingoid habitus (Figure 1).\nFigure 1.\nThe sclera and tympanic membranes are clear. Gonadal examination demonstrates bilaterally descended and normal-sized testicles. Upon laboratory investigations, the complete blood cell count, urinalysis, complete metabolic panel, vitamin D level, thyroid-stimulating hormone level, and testosterone level are normal. Urinary free cortisol and an overnight dexamethasone suppression test for Cushing syndrome are also normal. He has an undetectable HIV viral load and a normal CD4 count. He has normal plasma and urine calcium, serum phosphorus, alkaline phosphate, and parathyroid hormone levels.\nThe patient had been enrolled in an HIV alendronate study 7 years prior to injury. Bone mineral density (BMD) testing at that time showed osteopenia. The patient was then started on alendronate therapy. Repeat BMD testing 4 years before the injury showed normal density scores at both his hip and spine. Now, 8 months after his injury, his BMD is still normal although it is decreased from his levels 4 years previously (Table 1).\nTable 1. Bone Mineral Density*\n*Densities reported in g/cm2\n\n ## Figures\n\n **Figure 1.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 394622,
"choiceText": "Bony metastases",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 394623,
"choiceText": "Osteomalacia",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 394624,
"choiceText": "Pycnodysostosis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 394625,
"choiceText": "Primary hyperparathyroidism",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 394626,
"choiceText": "Alendronate therapy",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 114734,
"questionText": "Which of the following most likely contributed to this man's atypical midshaft femoral fracture?<br /><br /><i>Hint: Note the fracture pattern on the femoral x-ray.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Mysterious Femur Fracture in a Middle-Aged Man"
},
{
"authors": "George T. Griffing, MD; Naga Neelima Nallapaneni, MB BS",
"content": [
"In this case, the patient, who was in relatively stable medical health, experienced an atypical midshaft femoral fracture (Figures 1 and 2).",
"Figure 1.",
"Figure 2.",
"This fracture met all major and several of the minor criteria for an atypical femur fracture (Table 2). The specific criteria this fracture met include a midshaft location, lack of trauma, short oblique configuration, noncomminuted, presence of a medial spike (also referred to as unicortical beak), increased diaphyseal cortical thickness, prodromal leg pain near the area of the fracture, and a 7-year history of alendronate use. Given the patient's history and study results, alendronate seemed the most likely cause.",
"Table 2. Atypical Femoral Fracture: Major and Minor Features*[1]",
"Located anywhere along the femur from just distal to the lesser trochanter to just proximal to the supracondylar flare",
"Associated with no trauma or minimal trauma, as in a fall from a standing height or less",
"Transverse or short oblique configuration",
"Noncomminuted",
"Complete fractures extend through both cortices and may be associated with a medial spike; incomplete fractures involve only the lateral cortex",
"Localized periosteal reaction of the lateral cortex‡",
"Generalized increase in cortical thickness of the diaphysis",
"Prodromal symptoms such as dull or aching pain in the groin or thigh",
"Bilateral fractures and symptoms",
"Delayed healing",
"Comorbid conditions (eg, vitamin D deficiency, RA, hypophosphatasia)",
"Use of pharmaceutical agents (eg, BPs, GCs, proton pump inhibitors)",
"All the other features listed can cause atypical fractures but were less likely in this patient. This patient had no evidence of malignancy. He had none of the laboratory data associated with osteomalacia or primary hyperparathyroidism (ie, normal plasma and urine calcium, serum phosphorus, alkaline phosphate, vitamin D, and parathyroid hormone), nor did he have the physical stigmata and repeated bone fracture history of pycnodysostosis or osteogenesis imperfecta.",
"This patient had been taking alendronate as part of an HIV bone-protection study. Alendronate (and possibly other bisphosphonates) have been linked to atypical femoral fractures. In fact, 94% of 310 cases of atypical femur fracture studied by an American Society for Bone and Mineral Research Atypical Femoral Fracture Task Force were associated with bisphosphonate usage (mostly alendronate).[2] The task force stopped short of declaring these medications to be the cause of the fractures because similar fractures have been documented in patients who are not on bisphosphonate therapy. However, this report led to the US Food and Drug Administration (FDA) adding a warning about atypical fractures to bisphosphonate package labeling.[2] Both the task force and the FDA believe that this is a drug class (bisphosphonates) association and that the association with alendronate is the strongest because it has the largest usage and longest approval time."
],
"date": "April 06, 2018",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/738/063/738063-thumb1.png"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/738/063/738063-thumb2.png"
}
],
"markdown": "# Mysterious Femur Fracture in a Middle-Aged Man\n\n **Authors:** George T. Griffing, MD; Naga Neelima Nallapaneni, MB BS \n **Date:** April 06, 2018\n\n ## Content\n\n In this case, the patient, who was in relatively stable medical health, experienced an atypical midshaft femoral fracture (Figures 1 and 2).\nFigure 1.\nFigure 2.\nThis fracture met all major and several of the minor criteria for an atypical femur fracture (Table 2). The specific criteria this fracture met include a midshaft location, lack of trauma, short oblique configuration, noncomminuted, presence of a medial spike (also referred to as unicortical beak), increased diaphyseal cortical thickness, prodromal leg pain near the area of the fracture, and a 7-year history of alendronate use. Given the patient's history and study results, alendronate seemed the most likely cause.\nTable 2. Atypical Femoral Fracture: Major and Minor Features*[1]\nLocated anywhere along the femur from just distal to the lesser trochanter to just proximal to the supracondylar flare\nAssociated with no trauma or minimal trauma, as in a fall from a standing height or less\nTransverse or short oblique configuration\nNoncomminuted\nComplete fractures extend through both cortices and may be associated with a medial spike; incomplete fractures involve only the lateral cortex\nLocalized periosteal reaction of the lateral cortex‡\nGeneralized increase in cortical thickness of the diaphysis\nProdromal symptoms such as dull or aching pain in the groin or thigh\nBilateral fractures and symptoms\nDelayed healing\nComorbid conditions (eg, vitamin D deficiency, RA, hypophosphatasia)\nUse of pharmaceutical agents (eg, BPs, GCs, proton pump inhibitors)\nAll the other features listed can cause atypical fractures but were less likely in this patient. This patient had no evidence of malignancy. He had none of the laboratory data associated with osteomalacia or primary hyperparathyroidism (ie, normal plasma and urine calcium, serum phosphorus, alkaline phosphate, vitamin D, and parathyroid hormone), nor did he have the physical stigmata and repeated bone fracture history of pycnodysostosis or osteogenesis imperfecta.\nThis patient had been taking alendronate as part of an HIV bone-protection study. Alendronate (and possibly other bisphosphonates) have been linked to atypical femoral fractures. In fact, 94% of 310 cases of atypical femur fracture studied by an American Society for Bone and Mineral Research Atypical Femoral Fracture Task Force were associated with bisphosphonate usage (mostly alendronate).[2] The task force stopped short of declaring these medications to be the cause of the fractures because similar fractures have been documented in patients who are not on bisphosphonate therapy. However, this report led to the US Food and Drug Administration (FDA) adding a warning about atypical fractures to bisphosphonate package labeling.[2] Both the task force and the FDA believe that this is a drug class (bisphosphonates) association and that the association with alendronate is the strongest because it has the largest usage and longest approval time.\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n\n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 394622,
"choiceText": "Bony metastases",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 394623,
"choiceText": "Osteomalacia",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 394624,
"choiceText": "Pycnodysostosis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 394625,
"choiceText": "Primary hyperparathyroidism",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 394626,
"choiceText": "Alendronate therapy",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 114734,
"questionText": "Which of the following most likely contributed to this man's atypical midshaft femoral fracture?<br /><br /><i>Hint: Note the fracture pattern on the femoral x-ray.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Mysterious Femur Fracture in a Middle-Aged Man"
},
{
"authors": "George T. Griffing, MD; Naga Neelima Nallapaneni, MB BS",
"content": [
"The cause of this fracture may be related to alendronate's mechanism of action. Alendronate inhibits the osteoclast mevalonate pathway, resulting in decreased bone resorption and increased apoptosis. Reducing bone resorption is the mechanism by which this drug reduces osteoporotic fractures. Because osteoblastic bone formation follows osteoclastic bone resorption, overall bone turnover and bone remodeling decreases. Bone remodeling, however, is the primary repair mechanism for microdamage occurring in the bone. Increased evidence of microdamage has been demonstrated in bone biopsies from both animal and human subjects treated with long-term alendronate (5 years).[3] Accumulation of this microdamage can reduce bone strength, thereby increasing the risk for fracture, especially in areas of high mechanical stresses (eg, femur).",
"Most atypical femoral fractures show a common radiographic pattern. Conventional x-rays in the anteroposterior and lateral projections will show a transverse or oblique fracture. Diffuse cortical thickening can be present, particularly laterally, wherein the fracture often initiates. When cortical thickening is focal and substantial, an appearance of \"beaking\" or \"flaring\" adjacent to the transverse fracture line may be noted. As the fracture evolves and propagates medially, ultimately displacing and becoming a complete fracture, an oblique component may be observed as a prominent medial \"spike.\" Discrete linear lateral cortical translucencies may be observed in the prefracture-displacement phase, often with adjacent focal cortical thickening from periosteal new-bone apposition.",
"Atypical fractures are often preceded by prodromal symptoms of aching, deep thigh or groin pain, and normal x-rays (as they were in this case). Radionuclide bone scintigraphy may be used to document the presence of an evolving stress or atypical fracture. In these cases, the scintigraphic appearance is that of increased uptake in a broad diffuse zone and a centrally located focal region of more intense uptake, usually in the lateral cortex. Like bone scintigraphy, MRI may detect an evolving stress or insufficiency fracture. These MRI findings manifest as diffuse decreased signal on T1-weighted images and diffuse increased signal on T2-weighted images related to the associated inflammation and hyperemia.",
"The evolving fracture line in the lateral cortex may be seen. Spiral CT imaging occasionally detects subtle reactive periosteal new-bone formation and the small discrete radiolucency of an evolving fracture. Although more costly, MRI and CT scanning have superior sensitivity and specificity for detecting the early stages of stress or atypical fractures. Even the lower-resolution images of dual x-ray absorptiometry may occasionally detect the hypertrophic new-bone formation of an evolving proximal subtrochanteric femoral shaft fracture and aid in the differentiation of proximal thigh pain in this condition.",
"The rarity of atypical femoral fractures makes them difficult to study, and there is controversy regarding causality with bisphosphonates.[4] These fractures may share the same epidemiology with osteoporosis and may be a marker for otherwise ill health.[5,6] A large nationwide cohort study by Vestergaard and colleagues[7] also suggested that an increased risk for femoral shaft and subtrochanteric fractures seen with the use of bisphosphonate agents may be a confounding effect of a patient's underlying disease. The investigators noted there was an increased risk for such fractures both before and after the administration of these drugs.[7]",
"This patient, however, was relatively young and in good health compared with other reports of patients with atypical femoral fracture. Despite this, alendronate may not have been his only risk factor. An additional risk may relate to the presence of HIV-associated lipodystrophy syndrome. Evidence supports the possibility that this syndrome is caused by tissue hypercortisolism resulting in a Cushing's appearance, even though blood and urine cortisol levels are normal. The tissue hypercortisolism is the result of increased production of a tissue enzyme (11 beta-hydroxysteroid dehydrogenase) that converts cortisone (inactive) to cortisol (active).[8] Pharmacologic hypercortisolism is known to increase the risk for atypical femoral fracture fivefold, and this patient's lipodystrophy syndrome may confer a risk similar to that of using exogenous glucocorticoids.[9,10] Therefore, the additive risk of alendronate usage and tissue lipodystrophy-associated hypercortisolism may explain the occurrence of this fracture in an otherwise relatively healthy middle-aged man."
],
"date": "April 06, 2018",
"figures": [],
"markdown": "# Mysterious Femur Fracture in a Middle-Aged Man\n\n **Authors:** George T. Griffing, MD; Naga Neelima Nallapaneni, MB BS \n **Date:** April 06, 2018\n\n ## Content\n\n The cause of this fracture may be related to alendronate's mechanism of action. Alendronate inhibits the osteoclast mevalonate pathway, resulting in decreased bone resorption and increased apoptosis. Reducing bone resorption is the mechanism by which this drug reduces osteoporotic fractures. Because osteoblastic bone formation follows osteoclastic bone resorption, overall bone turnover and bone remodeling decreases. Bone remodeling, however, is the primary repair mechanism for microdamage occurring in the bone. Increased evidence of microdamage has been demonstrated in bone biopsies from both animal and human subjects treated with long-term alendronate (5 years).[3] Accumulation of this microdamage can reduce bone strength, thereby increasing the risk for fracture, especially in areas of high mechanical stresses (eg, femur).\nMost atypical femoral fractures show a common radiographic pattern. Conventional x-rays in the anteroposterior and lateral projections will show a transverse or oblique fracture. Diffuse cortical thickening can be present, particularly laterally, wherein the fracture often initiates. When cortical thickening is focal and substantial, an appearance of \"beaking\" or \"flaring\" adjacent to the transverse fracture line may be noted. As the fracture evolves and propagates medially, ultimately displacing and becoming a complete fracture, an oblique component may be observed as a prominent medial \"spike.\" Discrete linear lateral cortical translucencies may be observed in the prefracture-displacement phase, often with adjacent focal cortical thickening from periosteal new-bone apposition.\nAtypical fractures are often preceded by prodromal symptoms of aching, deep thigh or groin pain, and normal x-rays (as they were in this case). Radionuclide bone scintigraphy may be used to document the presence of an evolving stress or atypical fracture. In these cases, the scintigraphic appearance is that of increased uptake in a broad diffuse zone and a centrally located focal region of more intense uptake, usually in the lateral cortex. Like bone scintigraphy, MRI may detect an evolving stress or insufficiency fracture. These MRI findings manifest as diffuse decreased signal on T1-weighted images and diffuse increased signal on T2-weighted images related to the associated inflammation and hyperemia.\nThe evolving fracture line in the lateral cortex may be seen. Spiral CT imaging occasionally detects subtle reactive periosteal new-bone formation and the small discrete radiolucency of an evolving fracture. Although more costly, MRI and CT scanning have superior sensitivity and specificity for detecting the early stages of stress or atypical fractures. Even the lower-resolution images of dual x-ray absorptiometry may occasionally detect the hypertrophic new-bone formation of an evolving proximal subtrochanteric femoral shaft fracture and aid in the differentiation of proximal thigh pain in this condition.\nThe rarity of atypical femoral fractures makes them difficult to study, and there is controversy regarding causality with bisphosphonates.[4] These fractures may share the same epidemiology with osteoporosis and may be a marker for otherwise ill health.[5,6] A large nationwide cohort study by Vestergaard and colleagues[7] also suggested that an increased risk for femoral shaft and subtrochanteric fractures seen with the use of bisphosphonate agents may be a confounding effect of a patient's underlying disease. The investigators noted there was an increased risk for such fractures both before and after the administration of these drugs.[7]\nThis patient, however, was relatively young and in good health compared with other reports of patients with atypical femoral fracture. Despite this, alendronate may not have been his only risk factor. An additional risk may relate to the presence of HIV-associated lipodystrophy syndrome. Evidence supports the possibility that this syndrome is caused by tissue hypercortisolism resulting in a Cushing's appearance, even though blood and urine cortisol levels are normal. The tissue hypercortisolism is the result of increased production of a tissue enzyme (11 beta-hydroxysteroid dehydrogenase) that converts cortisone (inactive) to cortisol (active).[8] Pharmacologic hypercortisolism is known to increase the risk for atypical femoral fracture fivefold, and this patient's lipodystrophy syndrome may confer a risk similar to that of using exogenous glucocorticoids.[9,10] Therefore, the additive risk of alendronate usage and tissue lipodystrophy-associated hypercortisolism may explain the occurrence of this fracture in an otherwise relatively healthy middle-aged man.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "Mysterious Femur Fracture in a Middle-Aged Man"
},
{
"authors": "George T. Griffing, MD; Naga Neelima Nallapaneni, MB BS",
"content": [
"Despite the possible association between bisphosphonates and these atypical fractures, atypical femoral fractures are still relatively rare compared with hip fractures, and the benefits of osteoporotic fracture protection still outweigh this risk.[11] For example, the occurrence of hip fractures in women is approximately 100/10,000 patient-years, but atypical femoral fractures occur in only about 1/10,000 patient-years.[11] If, for instance, alendronate prevents 50% of these hip fractures, the benefit of preventing 50 of these osteoporotic hip fractures offsets the risk of 1 atypical femoral fracture. The short-term benefits of hip-fracture protection begin well before the long-term risk of an atypical femoral fracture. In a study of 102 cases of atypical femoral fractures, 97 patients had taken a bisphosphonate, and the risk for fracture increased with the duration of use (2/100,000 patients/year after 2 years, and 78/100,000 patients/year after 8 years of use).[12] Therefore, shorter durations of alendronate therapy may be useful to maximize the benefits and reduce the risks. Thus, further data are required to determine the optimal duration of alendronate therapy.",
"Given the state of our imperfect knowledge of this problem, following the recommendations of experts is reasonable.[13] In patients without an osteoporotic fracture, the recommendations are as follows:",
"Limit the use of alendronate (and other bisphosphonates) to 5 years or when bone markers show that bone remodeling has stopped (continue calcium and vitamin D).",
"Restart bisphosphonate therapy if bone loss resumes, but do so at the lowest possible dose.",
"Bisphosphonate-treated patients with thigh pain may need to be evaluated for a prodromal stress fracture (eg, x-ray, MRI, and/or bone scanning).",
"When an atypical fracture occurs, stop the alendronate and treat with an anabolic agent (eg, teriparatide) to prevent a contralateral fracture.",
"In patients with an osteoporotic fracture, the benefits of continuing bisphosphonate therapy outweigh the risks of stopping it (in most cases).",
"Ultimately, this patient had an open reduction and internal fixation of the femoral fracture, and he had an uncomplicated postoperative course. Alendronate therapy was stopped, and he was started on teriparatide. A repeat BMD after 15 months showed osteopenia. He has had no further fractures or leg pain."
],
"date": "April 06, 2018",
"figures": [],
"markdown": "# Mysterious Femur Fracture in a Middle-Aged Man\n\n **Authors:** George T. Griffing, MD; Naga Neelima Nallapaneni, MB BS \n **Date:** April 06, 2018\n\n ## Content\n\n Despite the possible association between bisphosphonates and these atypical fractures, atypical femoral fractures are still relatively rare compared with hip fractures, and the benefits of osteoporotic fracture protection still outweigh this risk.[11] For example, the occurrence of hip fractures in women is approximately 100/10,000 patient-years, but atypical femoral fractures occur in only about 1/10,000 patient-years.[11] If, for instance, alendronate prevents 50% of these hip fractures, the benefit of preventing 50 of these osteoporotic hip fractures offsets the risk of 1 atypical femoral fracture. The short-term benefits of hip-fracture protection begin well before the long-term risk of an atypical femoral fracture. In a study of 102 cases of atypical femoral fractures, 97 patients had taken a bisphosphonate, and the risk for fracture increased with the duration of use (2/100,000 patients/year after 2 years, and 78/100,000 patients/year after 8 years of use).[12] Therefore, shorter durations of alendronate therapy may be useful to maximize the benefits and reduce the risks. Thus, further data are required to determine the optimal duration of alendronate therapy.\nGiven the state of our imperfect knowledge of this problem, following the recommendations of experts is reasonable.[13] In patients without an osteoporotic fracture, the recommendations are as follows:\nLimit the use of alendronate (and other bisphosphonates) to 5 years or when bone markers show that bone remodeling has stopped (continue calcium and vitamin D).\nRestart bisphosphonate therapy if bone loss resumes, but do so at the lowest possible dose.\nBisphosphonate-treated patients with thigh pain may need to be evaluated for a prodromal stress fracture (eg, x-ray, MRI, and/or bone scanning).\nWhen an atypical fracture occurs, stop the alendronate and treat with an anabolic agent (eg, teriparatide) to prevent a contralateral fracture.\nIn patients with an osteoporotic fracture, the benefits of continuing bisphosphonate therapy outweigh the risks of stopping it (in most cases).\nUltimately, this patient had an open reduction and internal fixation of the femoral fracture, and he had an uncomplicated postoperative course. Alendronate therapy was stopped, and he was started on teriparatide. A repeat BMD after 15 months showed osteopenia. He has had no further fractures or leg pain.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 394627,
"choiceText": "Long duration of alendronate therapy",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 394628,
"choiceText": "Femoral diaphysis location of the fracture",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 394629,
"choiceText": "Radiographic characteristics of a transverse fracture pattern with beaking of the cortex and cortical hypertrophy",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 394630,
"choiceText": "Absence of other causes of low-energy femoral fractures",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 394631,
"choiceText": "The fracture is comminuted",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "A noncomminuted fracture is one of the major criteria for an atypical femoral fracture. The other factors are all expected with atypical fractures.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 114735,
"questionText": "You suspect alendronate was associated with a femoral fracture in one of your patients. Which of the following factors, if seen in this fracture, would make alendronate use the least likely cause?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 394632,
"choiceText": "Decreased vitamin D levels leading to osteomalacia",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 394633,
"choiceText": "Decreased bone remodeling leading to the accumulation of microfractures",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 394634,
"choiceText": "Increased bone remodeling leading to weakening of cortical bone",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 394635,
"choiceText": "A renal phosphate leak leading to increased calcium losses in the urine",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The prolonged inhibitory action of bisphosphonates on bone remodeling is thought to decrease the repair process, leading to the accumulation and eventual coalescence of microfractures into a complete transverse fracture.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 114736,
"questionText": "Alendronate therapy is strongly suspected as the culprit in an atraumatic femoral fracture in one of your patients. What is the putative mechanism for alendronate-associated atypical femoral fractures?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Mysterious Femur Fracture in a Middle-Aged Man"
},
{
"authors": "George T. Griffing, MD; Naga Neelima Nallapaneni, MB BS",
"content": [],
"date": "April 06, 2018",
"figures": [],
"markdown": "# Mysterious Femur Fracture in a Middle-Aged Man\n\n **Authors:** George T. Griffing, MD; Naga Neelima Nallapaneni, MB BS \n **Date:** April 06, 2018\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 394627,
"choiceText": "Long duration of alendronate therapy",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 394628,
"choiceText": "Femoral diaphysis location of the fracture",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 394629,
"choiceText": "Radiographic characteristics of a transverse fracture pattern with beaking of the cortex and cortical hypertrophy",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 394630,
"choiceText": "Absence of other causes of low-energy femoral fractures",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 394631,
"choiceText": "The fracture is comminuted",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "A noncomminuted fracture is one of the major criteria for an atypical femoral fracture. The other factors are all expected with atypical fractures.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 114735,
"questionText": "You suspect alendronate was associated with a femoral fracture in one of your patients. Which of the following factors, if seen in this fracture, would make alendronate use the least likely cause?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 394632,
"choiceText": "Decreased vitamin D levels leading to osteomalacia",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 394633,
"choiceText": "Decreased bone remodeling leading to the accumulation of microfractures",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 394634,
"choiceText": "Increased bone remodeling leading to weakening of cortical bone",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 394635,
"choiceText": "A renal phosphate leak leading to increased calcium losses in the urine",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The prolonged inhibitory action of bisphosphonates on bone remodeling is thought to decrease the repair process, leading to the accumulation and eventual coalescence of microfractures into a complete transverse fracture.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 114736,
"questionText": "Alendronate therapy is strongly suspected as the culprit in an atraumatic femoral fracture in one of your patients. What is the putative mechanism for alendronate-associated atypical femoral fractures?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Mysterious Femur Fracture in a Middle-Aged Man"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 394622,
"choiceText": "Bony metastases",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 394623,
"choiceText": "Osteomalacia",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 394624,
"choiceText": "Pycnodysostosis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 394625,
"choiceText": "Primary hyperparathyroidism",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 394626,
"choiceText": "Alendronate therapy",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 114734,
"questionText": "Which of the following most likely contributed to this man's atypical midshaft femoral fracture?<br /><br /><i>Hint: Note the fracture pattern on the femoral x-ray.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 394627,
"choiceText": "Long duration of alendronate therapy",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 394628,
"choiceText": "Femoral diaphysis location of the fracture",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 394629,
"choiceText": "Radiographic characteristics of a transverse fracture pattern with beaking of the cortex and cortical hypertrophy",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 394630,
"choiceText": "Absence of other causes of low-energy femoral fractures",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 394631,
"choiceText": "The fracture is comminuted",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "A noncomminuted fracture is one of the major criteria for an atypical femoral fracture. The other factors are all expected with atypical fractures.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 114735,
"questionText": "You suspect alendronate was associated with a femoral fracture in one of your patients. Which of the following factors, if seen in this fracture, would make alendronate use the least likely cause?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 394632,
"choiceText": "Decreased vitamin D levels leading to osteomalacia",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 394633,
"choiceText": "Decreased bone remodeling leading to the accumulation of microfractures",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 394634,
"choiceText": "Increased bone remodeling leading to weakening of cortical bone",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 394635,
"choiceText": "A renal phosphate leak leading to increased calcium losses in the urine",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The prolonged inhibitory action of bisphosphonates on bone remodeling is thought to decrease the repair process, leading to the accumulation and eventual coalescence of microfractures into a complete transverse fracture.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 114736,
"questionText": "Alendronate therapy is strongly suspected as the culprit in an atraumatic femoral fracture in one of your patients. What is the putative mechanism for alendronate-associated atypical femoral fractures?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
736892 | /viewarticle/736892 | [
{
"authors": "Kaleem Ullah Toori, MB BS; Sumaira Nabi, MB BS; Sadaf Khattak, MB BS; Herbert S Diamond, MD",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to recognize accurately. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"A 26-year-old Pakistani woman presents to the emergency department with a 1-week history of nausea, vomiting, diarrhea, lower abdominal pain, and dysuria. She was previously treated for pulmonary tuberculosis about 2 years ago.",
"She has 3 children, all of who were normal vaginal deliveries and were born healthy. She has a 1-year history of menstrual irregularity and dysmenorrhea. She is a nonsmoker and is not taking any medications on a regular basis. Her family history is unremarkable."
],
"date": "August 16, 2018",
"figures": [],
"markdown": "# A 26-Year-Old Woman With Pleural Effusion, Hydronephrosis, and Ascites\n\n **Authors:** Kaleem Ullah Toori, MB BS; Sumaira Nabi, MB BS; Sadaf Khattak, MB BS; Herbert S Diamond, MD \n **Date:** August 16, 2018\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to recognize accurately. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nA 26-year-old Pakistani woman presents to the emergency department with a 1-week history of nausea, vomiting, diarrhea, lower abdominal pain, and dysuria. She was previously treated for pulmonary tuberculosis about 2 years ago.\nShe has 3 children, all of who were normal vaginal deliveries and were born healthy. She has a 1-year history of menstrual irregularity and dysmenorrhea. She is a nonsmoker and is not taking any medications on a regular basis. Her family history is unremarkable.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 26-Year-Old Woman With Pleural Effusion, Hydronephrosis, and Ascites"
},
{
"authors": "Kaleem Ullah Toori, MB BS; Sumaira Nabi, MB BS; Sadaf Khattak, MB BS; Herbert S Diamond, MD",
"content": [
"Upon physical examination, the patient appears toxic. Her oral temperature is 100°F (37.8°C). Her pulse is regular with a rate of 100 beats/min. Her blood pressure is 160/100 mm Hg. She is noted to have generalized pallor, but no clubbing, jaundice, or cyanosis is detected. Her abdominal examination reveals distention with mild generalized tenderness. A mild-to-moderate amount of ascites are noted. The chest examination is suggestive of bilateral basal pleural effusion that is greater on the left. The remainder of the physical examination is unremarkable.",
"The patient’s initial laboratory investigations reveal a normocytic, normochromic anemia with a hemoglobin of 8 mg/dL, an elevated erythrocyte sedimentation rate (ESR) of 65 mm/h, normal liver and renal functioning, and a urinalysis that is positive for red blood cells (RBCs), white blood cells (WBCs), and protein. A chest x-ray confirms the finding of bilateral basilar pleural effusions (that are greater on the left). Mantoux skin testing is negative. Ultrasonography of the abdomen and pelvis demonstrates bilateral hydronephrosis (Figure 1).",
"Figure 1.",
"Abdominal ultrasonogram showing bilateral hydronephrosis.",
"The mesentery and gut wall appears thickened; peritoneal deposits and moderate ascites are found. A sample of the ascitic fluid sent for analysis reveals an exudative ascites, with a predominance of lymphocytes (80%). No organism is found on culture or Gram staining, and no malignant cells are noted on cytology.",
"Carcinoembryonic antigen (CEA) levels are found to be normal, but CA-125 is elevated at 93.5 μg/mL (normal range, 0-35 μg/mL). CT scanning of the abdomen and pelvis is performed, which confirms the presence of moderate ascites and peritoneal nodularity (Figure 2). The bladder and intestinal walls are thickened (Figure 3). Bilateral hydronephrosis and multiple enlarged para-aortic lymph nodes are also seen (Figure 4).",
"Figure 2.",
"A contrast-enhanced CT scan of the pelvis showing pelvic free fluid.",
"Figure 3.",
"A contrast-enhanced CT scan of the abdomen showing thickened bowel loops.",
"Figure 4.",
"A contrast-enhanced CT of the abdomen showing bilateral hydronephrosis.",
"In light of the above findings and past history of tuberculosis, a provisional diagnosis of tuberculous peritonitis is made, and the patient is started on antituberculous therapy (ATT). However, the patient’s symptoms do not abate, and her fevers and abdominal pain continue even after 3 weeks of ATT. Subsequently, the patient develops arthralgia involving both hands and wrists, and her ESR rises to 130 mm/h."
],
"date": "August 16, 2018",
"figures": [
{
"caption": "Figure 1.\n\nAbdominal ultrasonogram showing bilateral hydronephrosis.",
"image_url": "https://img.medscapestatic.com/article/736/892/736892-thumb1.png"
},
{
"caption": "Figure 2.\n\nA contrast-enhanced CT scan of the pelvis showing pelvic free fluid.",
"image_url": "https://img.medscapestatic.com/article/736/892/736892-thumb2.png"
},
{
"caption": "Figure 3.\n\nA contrast-enhanced CT scan of the abdomen showing thickened bowel loops.",
"image_url": "https://img.medscapestatic.com/article/736/892/736892-thumb3.png"
},
{
"caption": "Figure 4.\n\nA contrast-enhanced CT of the abdomen showing bilateral hydronephrosis.",
"image_url": "https://img.medscapestatic.com/article/736/892/736892-thumb4.png"
}
],
"markdown": "# A 26-Year-Old Woman With Pleural Effusion, Hydronephrosis, and Ascites\n\n **Authors:** Kaleem Ullah Toori, MB BS; Sumaira Nabi, MB BS; Sadaf Khattak, MB BS; Herbert S Diamond, MD \n **Date:** August 16, 2018\n\n ## Content\n\n Upon physical examination, the patient appears toxic. Her oral temperature is 100°F (37.8°C). Her pulse is regular with a rate of 100 beats/min. Her blood pressure is 160/100 mm Hg. She is noted to have generalized pallor, but no clubbing, jaundice, or cyanosis is detected. Her abdominal examination reveals distention with mild generalized tenderness. A mild-to-moderate amount of ascites are noted. The chest examination is suggestive of bilateral basal pleural effusion that is greater on the left. The remainder of the physical examination is unremarkable.\nThe patient’s initial laboratory investigations reveal a normocytic, normochromic anemia with a hemoglobin of 8 mg/dL, an elevated erythrocyte sedimentation rate (ESR) of 65 mm/h, normal liver and renal functioning, and a urinalysis that is positive for red blood cells (RBCs), white blood cells (WBCs), and protein. A chest x-ray confirms the finding of bilateral basilar pleural effusions (that are greater on the left). Mantoux skin testing is negative. Ultrasonography of the abdomen and pelvis demonstrates bilateral hydronephrosis (Figure 1).\nFigure 1.\nAbdominal ultrasonogram showing bilateral hydronephrosis.\nThe mesentery and gut wall appears thickened; peritoneal deposits and moderate ascites are found. A sample of the ascitic fluid sent for analysis reveals an exudative ascites, with a predominance of lymphocytes (80%). No organism is found on culture or Gram staining, and no malignant cells are noted on cytology.\nCarcinoembryonic antigen (CEA) levels are found to be normal, but CA-125 is elevated at 93.5 μg/mL (normal range, 0-35 μg/mL). CT scanning of the abdomen and pelvis is performed, which confirms the presence of moderate ascites and peritoneal nodularity (Figure 2). The bladder and intestinal walls are thickened (Figure 3). Bilateral hydronephrosis and multiple enlarged para-aortic lymph nodes are also seen (Figure 4).\nFigure 2.\nA contrast-enhanced CT scan of the pelvis showing pelvic free fluid.\nFigure 3.\nA contrast-enhanced CT scan of the abdomen showing thickened bowel loops.\nFigure 4.\nA contrast-enhanced CT of the abdomen showing bilateral hydronephrosis.\nIn light of the above findings and past history of tuberculosis, a provisional diagnosis of tuberculous peritonitis is made, and the patient is started on antituberculous therapy (ATT). However, the patient’s symptoms do not abate, and her fevers and abdominal pain continue even after 3 weeks of ATT. Subsequently, the patient develops arthralgia involving both hands and wrists, and her ESR rises to 130 mm/h.\n\n ## Figures\n\n **Figure 1.\n\nAbdominal ultrasonogram showing bilateral hydronephrosis.** \n \n\n**Figure 2.\n\nA contrast-enhanced CT scan of the pelvis showing pelvic free fluid.** \n \n\n**Figure 3.\n\nA contrast-enhanced CT scan of the abdomen showing thickened bowel loops.** \n \n\n**Figure 4.\n\nA contrast-enhanced CT of the abdomen showing bilateral hydronephrosis.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 390651,
"choiceText": "Metastatic ovarian carcinoma",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 390652,
"choiceText": "Peritoneal tuberculosis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 390653,
"choiceText": "Systemic lupus erythematosus",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 390654,
"choiceText": "Rheumatoid arthritis",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 113412,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 26-Year-Old Woman With Pleural Effusion, Hydronephrosis, and Ascites"
},
{
"authors": "Kaleem Ullah Toori, MB BS; Sumaira Nabi, MB BS; Sadaf Khattak, MB BS; Herbert S Diamond, MD",
"content": [
"The patient in this case presented with abdominal and urinary complaints and her laboratory and radiologic data revealed bilateral pleural effusions, bilateral hydronephrosis with a thickened bladder wall, and lymphocytic exudative ascites along with elevated inflammatory markers. Tuberculosis was initially considered as the etiology given the previous history of treated pulmonary tuberculosis and the patient’s residence in an endemic country; however, no improvement in the patient’s clinical condition was noted after 3 weeks of appropriate ATT. She also developed arthralgias involving both hands, and her ESR rose to 130 mm/h.",
"The absence of improvement after 3 weeks of appropriate antituberculosis treatment and negative Mantoux test and culture results strongly suggest a diagnosis other than tuberculosis. Although the presentation was atypical, the development of arthralgia raised the possibility of systemic lupus erythematosus (SLE) with manifestations of serositis, arthralgia, anemia, renal disease, and lymphadenopathy. Although ovarian carcinoma can cause serositis, thickening of the peritoneal cavity, lymphadenopathy and hydronephrosis, the absence of an ovarian mass on CT scanning and the presence of fever made this diagnosis unlikely. Rheumatoid arthritis is an unlikely cause of the findings in this case, especially in the absence of clinical arthritis.",
"At this stage, the diagnosis was reviewed and a detailed connective tissue disorder profile was requested. The antinuclear antibody test was found to be strongly positive and with a homogenous pattern. The patient was also found to have an elevated anti-double stranded DNA antibody finding (>50 IU/L). Considering her clinical picture as well as her rheumatologic data, a diagnosis of SLE was made and the patient was started on high-dose corticosteroids along with cytotoxic therapy. Subsequent to the initiation of this therapy, the patient experienced an improvement in her symptoms."
],
"date": "August 16, 2018",
"figures": [],
"markdown": "# A 26-Year-Old Woman With Pleural Effusion, Hydronephrosis, and Ascites\n\n **Authors:** Kaleem Ullah Toori, MB BS; Sumaira Nabi, MB BS; Sadaf Khattak, MB BS; Herbert S Diamond, MD \n **Date:** August 16, 2018\n\n ## Content\n\n The patient in this case presented with abdominal and urinary complaints and her laboratory and radiologic data revealed bilateral pleural effusions, bilateral hydronephrosis with a thickened bladder wall, and lymphocytic exudative ascites along with elevated inflammatory markers. Tuberculosis was initially considered as the etiology given the previous history of treated pulmonary tuberculosis and the patient’s residence in an endemic country; however, no improvement in the patient’s clinical condition was noted after 3 weeks of appropriate ATT. She also developed arthralgias involving both hands, and her ESR rose to 130 mm/h.\nThe absence of improvement after 3 weeks of appropriate antituberculosis treatment and negative Mantoux test and culture results strongly suggest a diagnosis other than tuberculosis. Although the presentation was atypical, the development of arthralgia raised the possibility of systemic lupus erythematosus (SLE) with manifestations of serositis, arthralgia, anemia, renal disease, and lymphadenopathy. Although ovarian carcinoma can cause serositis, thickening of the peritoneal cavity, lymphadenopathy and hydronephrosis, the absence of an ovarian mass on CT scanning and the presence of fever made this diagnosis unlikely. Rheumatoid arthritis is an unlikely cause of the findings in this case, especially in the absence of clinical arthritis.\nAt this stage, the diagnosis was reviewed and a detailed connective tissue disorder profile was requested. The antinuclear antibody test was found to be strongly positive and with a homogenous pattern. The patient was also found to have an elevated anti-double stranded DNA antibody finding (>50 IU/L). Considering her clinical picture as well as her rheumatologic data, a diagnosis of SLE was made and the patient was started on high-dose corticosteroids along with cytotoxic therapy. Subsequent to the initiation of this therapy, the patient experienced an improvement in her symptoms.\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 390651,
"choiceText": "Metastatic ovarian carcinoma",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 390652,
"choiceText": "Peritoneal tuberculosis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 390653,
"choiceText": "Systemic lupus erythematosus",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 390654,
"choiceText": "Rheumatoid arthritis",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 113412,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 26-Year-Old Woman With Pleural Effusion, Hydronephrosis, and Ascites"
},
{
"authors": "Kaleem Ullah Toori, MB BS; Sumaira Nabi, MB BS; Sadaf Khattak, MB BS; Herbert S Diamond, MD",
"content": [
"SLE is an inflammatory disorder that can involve virtually any organ system. The prevalence of SLE varies with ethnicity and location, reflecting the interaction of genetic and environmental influences. The best estimates in white individuals is approximately 30 cases/100,000 population, whereas the rate in black individuals is closer to 100 cases/100,000 population.[1,2,3] The disease targets females 9 times as frequently as males and mainly affects women of reproductive age, although it can occur at any age.[4]",
"SLE is characterized by immune system deregulation, leading to the production of antinuclear and other autoantibodies. These can form circulating immune complexes or bind directly to tissues.[4] Many immune disturbances are observed, including genetic associations with HLA type, immunoregulatory genes, complement genes, T-cell function, and clearance of apoptotic debris. This disease can involve any part of the body, with cutaneous, renal, neurologic, cardiac, pulmonary, and/or hematologic manifestations. Joint inflammation causing arthralgia and arthritis and dermatologic manifestations are the most common presentations.",
"Gastrointestinal (GI) symptoms occur in as many as 50% of cases.[5] In 1895, Sir William Osler was the first researcher to emphasize the myriad abdominal complaints seen in SLE, and the ability of SLE to mimic other diseases.[6] The most common symptoms include anorexia, nausea, vomiting, diarrhea and abdominal pain. Complications such as lupus vasculitis that involves the vasculature of the GI tract, chronic intestinal pseudo-obstruction, and sterile peritonitis due to serositis can manifest as chronic abdominal pain and distension and, rarely, as an acute abdomen.[7]",
"GI vasculitis in SLE can involve any part of the gut from the esophagus to the colon, with a variable prevalence of 0.2%-53%.[8] However, most cases of GI vasculitis involve the superior mesenteric artery, (ie, the small intestine).[9] Symptoms are often nonspecific and may include bloating, anorexia, nausea, vomiting, diarrhea, or rarely as an acute abdomen. It is almost always accompanied by clinical and biochemical evidence of active disease.[7,8,9,10]",
"Some patients present with chronic intestinal pseudo-obstruction due to intestinal hypomotility (as was seen in this patient).[6] It is a rare clinical syndrome of unknown pathophysiology and can be caused by the involvement of the visceral smooth muscle, the enteric nerves, or the visceral autonomic nervous system.[10,11] Similar complications may be seen in systemic sclerosis and other related disorders.[12]",
"Genitourinary manifestations are rare and are often associated with GI disorders. The pathogenesis of lupus cystitis is proposed to be an immune-complex mediated vasculitis. The resulting chronic inflammation and urologic dysmotility leads to bladder wall thickening, obstructive uropathy and hydronephrosis.[13] The patient in this case had bilateral hydronephrosis with a thickened bladder wall on ultrasonography and CT scanning, which was later confirmed on cystoscopy. These are the most common urologic manifestations of SLE and the vasculitic phenomenon.[13,14]"
],
"date": "August 16, 2018",
"figures": [],
"markdown": "# A 26-Year-Old Woman With Pleural Effusion, Hydronephrosis, and Ascites\n\n **Authors:** Kaleem Ullah Toori, MB BS; Sumaira Nabi, MB BS; Sadaf Khattak, MB BS; Herbert S Diamond, MD \n **Date:** August 16, 2018\n\n ## Content\n\n SLE is an inflammatory disorder that can involve virtually any organ system. The prevalence of SLE varies with ethnicity and location, reflecting the interaction of genetic and environmental influences. The best estimates in white individuals is approximately 30 cases/100,000 population, whereas the rate in black individuals is closer to 100 cases/100,000 population.[1,2,3] The disease targets females 9 times as frequently as males and mainly affects women of reproductive age, although it can occur at any age.[4]\nSLE is characterized by immune system deregulation, leading to the production of antinuclear and other autoantibodies. These can form circulating immune complexes or bind directly to tissues.[4] Many immune disturbances are observed, including genetic associations with HLA type, immunoregulatory genes, complement genes, T-cell function, and clearance of apoptotic debris. This disease can involve any part of the body, with cutaneous, renal, neurologic, cardiac, pulmonary, and/or hematologic manifestations. Joint inflammation causing arthralgia and arthritis and dermatologic manifestations are the most common presentations.\nGastrointestinal (GI) symptoms occur in as many as 50% of cases.[5] In 1895, Sir William Osler was the first researcher to emphasize the myriad abdominal complaints seen in SLE, and the ability of SLE to mimic other diseases.[6] The most common symptoms include anorexia, nausea, vomiting, diarrhea and abdominal pain. Complications such as lupus vasculitis that involves the vasculature of the GI tract, chronic intestinal pseudo-obstruction, and sterile peritonitis due to serositis can manifest as chronic abdominal pain and distension and, rarely, as an acute abdomen.[7]\nGI vasculitis in SLE can involve any part of the gut from the esophagus to the colon, with a variable prevalence of 0.2%-53%.[8] However, most cases of GI vasculitis involve the superior mesenteric artery, (ie, the small intestine).[9] Symptoms are often nonspecific and may include bloating, anorexia, nausea, vomiting, diarrhea, or rarely as an acute abdomen. It is almost always accompanied by clinical and biochemical evidence of active disease.[7,8,9,10]\nSome patients present with chronic intestinal pseudo-obstruction due to intestinal hypomotility (as was seen in this patient).[6] It is a rare clinical syndrome of unknown pathophysiology and can be caused by the involvement of the visceral smooth muscle, the enteric nerves, or the visceral autonomic nervous system.[10,11] Similar complications may be seen in systemic sclerosis and other related disorders.[12]\nGenitourinary manifestations are rare and are often associated with GI disorders. The pathogenesis of lupus cystitis is proposed to be an immune-complex mediated vasculitis. The resulting chronic inflammation and urologic dysmotility leads to bladder wall thickening, obstructive uropathy and hydronephrosis.[13] The patient in this case had bilateral hydronephrosis with a thickened bladder wall on ultrasonography and CT scanning, which was later confirmed on cystoscopy. These are the most common urologic manifestations of SLE and the vasculitic phenomenon.[13,14]\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 26-Year-Old Woman With Pleural Effusion, Hydronephrosis, and Ascites"
},
{
"authors": "Kaleem Ullah Toori, MB BS; Sumaira Nabi, MB BS; Sadaf Khattak, MB BS; Herbert S Diamond, MD",
"content": [
"The patient's ascitic fluid examination showed an exudative picture with predominant lymphocytes which is typical for both SLE and tuberculous ascites; however, ascitic fluid analysis in this patient did not reveal any evidence of infectious disease or malignancy. The CA 125 levels were elevated; however, this is not specific for ovarian carcinoma, and this marker may be elevated in immune or rheumatologic disease or in chronic inflammatory processes involving the peritoneum.[15]",
"Pakistan is a TB-endemic country. This past history of tuberculosis as well as her clinical, radiologic, and laboratory data initially pointed towards genitourinary tuberculosis and tuberculous peritonitis, which warranted a trial of antituberculous therapy. However, after 3 weeks the patient had not improved, and she developed a nondeforming arthralgia involving the small joints of the hands. An alternate diagnosis was sought at that point.",
"A persistently high ESR, high titers of ANA with a homogenous pattern, and very high titers of anti-double stranded-DNA antibodies along with low complement levels are highly suggestive of SLE. Although her presentation with polyserositis, symptoms suggesting intestinal obstruction, high fever and hydronephrosis was unusual, she fulfilled the Revised American Rheumatism Association Criteria for SLE on the basis of arthritis, serositis, and renal involvement in the form of persistent proteinuria and high titers of ANA and anti-double stranded-DNA.[16]",
"This patient was initially treated with high-dose corticosteroids and cytotoxic therapy and then maintained on oral steroids. Periodic follow-up on a monthly basis with laboratory testing, including CBC with ESR, renal function and urinalysis were ordered to monitor the response and adverse reactions to therapy and to detect signs and symptoms of new organ-system involvement. The response to treatment was good and no adverse drug reactions were documented.",
"In conclusion, SLE can be challenging to diagnose. Rheumatologic disease should be strongly considered in situations where findings are nonspecific and the work-up has failed to reveal a definite diagnosis."
],
"date": "August 16, 2018",
"figures": [],
"markdown": "# A 26-Year-Old Woman With Pleural Effusion, Hydronephrosis, and Ascites\n\n **Authors:** Kaleem Ullah Toori, MB BS; Sumaira Nabi, MB BS; Sadaf Khattak, MB BS; Herbert S Diamond, MD \n **Date:** August 16, 2018\n\n ## Content\n\n The patient's ascitic fluid examination showed an exudative picture with predominant lymphocytes which is typical for both SLE and tuberculous ascites; however, ascitic fluid analysis in this patient did not reveal any evidence of infectious disease or malignancy. The CA 125 levels were elevated; however, this is not specific for ovarian carcinoma, and this marker may be elevated in immune or rheumatologic disease or in chronic inflammatory processes involving the peritoneum.[15]\nPakistan is a TB-endemic country. This past history of tuberculosis as well as her clinical, radiologic, and laboratory data initially pointed towards genitourinary tuberculosis and tuberculous peritonitis, which warranted a trial of antituberculous therapy. However, after 3 weeks the patient had not improved, and she developed a nondeforming arthralgia involving the small joints of the hands. An alternate diagnosis was sought at that point.\nA persistently high ESR, high titers of ANA with a homogenous pattern, and very high titers of anti-double stranded-DNA antibodies along with low complement levels are highly suggestive of SLE. Although her presentation with polyserositis, symptoms suggesting intestinal obstruction, high fever and hydronephrosis was unusual, she fulfilled the Revised American Rheumatism Association Criteria for SLE on the basis of arthritis, serositis, and renal involvement in the form of persistent proteinuria and high titers of ANA and anti-double stranded-DNA.[16]\nThis patient was initially treated with high-dose corticosteroids and cytotoxic therapy and then maintained on oral steroids. Periodic follow-up on a monthly basis with laboratory testing, including CBC with ESR, renal function and urinalysis were ordered to monitor the response and adverse reactions to therapy and to detect signs and symptoms of new organ-system involvement. The response to treatment was good and no adverse drug reactions were documented.\nIn conclusion, SLE can be challenging to diagnose. Rheumatologic disease should be strongly considered in situations where findings are nonspecific and the work-up has failed to reveal a definite diagnosis.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 390664,
"choiceText": "Volvulus",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 390665,
"choiceText": "Adhesive bands",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 390666,
"choiceText": "Smooth muscle hypomotility",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 390667,
"choiceText": "Internal hernia",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 390668,
"choiceText": "Hypokalemia",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Some patients present with chronic intestinal pseudo-obstruction due to intestinal hypomotility, as did the patient in this case. It is a rare clinical syndrome of unknown pathophysiology and can be caused by the involvement of the visceral smooth muscle, the enteric nerves, or the visceral autonomic nervous system. Similar complications may be seen in systemic sclerosis and other related disorders.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 113415,
"questionText": "The patient described in this case had abdominal symptoms suggestive of intestinal obstruction. Which of the following would be the likely cause of intestinal obstruction in her case?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 390673,
"choiceText": "Congenital ureteropelvic junction obstruction",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 390674,
"choiceText": "Ptosis of the kidney",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 390675,
"choiceText": "Stones",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 390676,
"choiceText": "Vasculitis",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 390677,
"choiceText": "Tumor",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The pathogenesis of lupus cystitis is proposed to be an immune-complex mediated vasculitis. GI vasculitis in SLE can involve any part of the gut from the esophagus to the colon, with a variable prevalence of 0.2%-53%. However, most cases of GI vasculitis involve the superior mesenteric artery, (ie, the small intestine). Symptoms are often nonspecific, including bloating, anorexia, nausea, vomiting, diarrhea, or rarely as an acute abdomen. It is almost always accompanied by clinical and biochemical evidence of active disease.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 113417,
"questionText": "This patient had bilateral hydronephrosis with a thickened bladder wall on ultrasonography and CT scanning, which was later confirmed on cystoscopy. Which of the following would be the most likely cause of hydronephrosis in her case?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 26-Year-Old Woman With Pleural Effusion, Hydronephrosis, and Ascites"
},
{
"authors": "Kaleem Ullah Toori, MB BS; Sumaira Nabi, MB BS; Sadaf Khattak, MB BS; Herbert S Diamond, MD",
"content": [],
"date": "August 16, 2018",
"figures": [],
"markdown": "# A 26-Year-Old Woman With Pleural Effusion, Hydronephrosis, and Ascites\n\n **Authors:** Kaleem Ullah Toori, MB BS; Sumaira Nabi, MB BS; Sadaf Khattak, MB BS; Herbert S Diamond, MD \n **Date:** August 16, 2018\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 390664,
"choiceText": "Volvulus",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 390665,
"choiceText": "Adhesive bands",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 390666,
"choiceText": "Smooth muscle hypomotility",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 390667,
"choiceText": "Internal hernia",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 390668,
"choiceText": "Hypokalemia",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Some patients present with chronic intestinal pseudo-obstruction due to intestinal hypomotility, as did the patient in this case. It is a rare clinical syndrome of unknown pathophysiology and can be caused by the involvement of the visceral smooth muscle, the enteric nerves, or the visceral autonomic nervous system. Similar complications may be seen in systemic sclerosis and other related disorders.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 113415,
"questionText": "The patient described in this case had abdominal symptoms suggestive of intestinal obstruction. Which of the following would be the likely cause of intestinal obstruction in her case?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 390673,
"choiceText": "Congenital ureteropelvic junction obstruction",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 390674,
"choiceText": "Ptosis of the kidney",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 390675,
"choiceText": "Stones",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 390676,
"choiceText": "Vasculitis",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 390677,
"choiceText": "Tumor",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The pathogenesis of lupus cystitis is proposed to be an immune-complex mediated vasculitis. GI vasculitis in SLE can involve any part of the gut from the esophagus to the colon, with a variable prevalence of 0.2%-53%. However, most cases of GI vasculitis involve the superior mesenteric artery, (ie, the small intestine). Symptoms are often nonspecific, including bloating, anorexia, nausea, vomiting, diarrhea, or rarely as an acute abdomen. It is almost always accompanied by clinical and biochemical evidence of active disease.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 113417,
"questionText": "This patient had bilateral hydronephrosis with a thickened bladder wall on ultrasonography and CT scanning, which was later confirmed on cystoscopy. Which of the following would be the most likely cause of hydronephrosis in her case?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 26-Year-Old Woman With Pleural Effusion, Hydronephrosis, and Ascites"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 390651,
"choiceText": "Metastatic ovarian carcinoma",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 390652,
"choiceText": "Peritoneal tuberculosis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 390653,
"choiceText": "Systemic lupus erythematosus",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 390654,
"choiceText": "Rheumatoid arthritis",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 113412,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 390664,
"choiceText": "Volvulus",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 390665,
"choiceText": "Adhesive bands",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 390666,
"choiceText": "Smooth muscle hypomotility",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 390667,
"choiceText": "Internal hernia",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 390668,
"choiceText": "Hypokalemia",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Some patients present with chronic intestinal pseudo-obstruction due to intestinal hypomotility, as did the patient in this case. It is a rare clinical syndrome of unknown pathophysiology and can be caused by the involvement of the visceral smooth muscle, the enteric nerves, or the visceral autonomic nervous system. Similar complications may be seen in systemic sclerosis and other related disorders.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 113415,
"questionText": "The patient described in this case had abdominal symptoms suggestive of intestinal obstruction. Which of the following would be the likely cause of intestinal obstruction in her case?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 390673,
"choiceText": "Congenital ureteropelvic junction obstruction",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 390674,
"choiceText": "Ptosis of the kidney",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 390675,
"choiceText": "Stones",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 390676,
"choiceText": "Vasculitis",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 390677,
"choiceText": "Tumor",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The pathogenesis of lupus cystitis is proposed to be an immune-complex mediated vasculitis. GI vasculitis in SLE can involve any part of the gut from the esophagus to the colon, with a variable prevalence of 0.2%-53%. However, most cases of GI vasculitis involve the superior mesenteric artery, (ie, the small intestine). Symptoms are often nonspecific, including bloating, anorexia, nausea, vomiting, diarrhea, or rarely as an acute abdomen. It is almost always accompanied by clinical and biochemical evidence of active disease.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 113417,
"questionText": "This patient had bilateral hydronephrosis with a thickened bladder wall on ultrasonography and CT scanning, which was later confirmed on cystoscopy. Which of the following would be the most likely cause of hydronephrosis in her case?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
735800 | /viewarticle/735800 | [
{
"authors": "Shradha B. Ahuja, MB BS; Rajiv S. Hira, MB BS; Vijay Panikar, MD, DNB, FCPS",
"content": [
"Editor's Note:\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions.",
"A 60-year-old, right-handed woman is brought to the emergency department (ED) for left-sided weakness following an unwitnessed fall. She fell to the floor on her left side while attempting to get out of bed. She realized that her entire left side was weak, and she did not have the strength to stand up on her own. Her family states that she has been sleeping much more than normal and had complained of a severe occipital headache for the last 3 days, accompanied by 6-7 episodes of vomiting per day. The vomiting is not relieved by antacids and proton-pump inhibitors and is not associated with nausea. She also complained to her family of feeling feverish for the past few days and had appeared intermittently agitated. She denies any history of head trauma, photophobia, diplopia, convulsions, dizziness, difficulty speaking, or difficulty swallowing.",
"Figure 1.",
"Her past medical history is significant for a hysterectomy 15 years ago and an appendectomy at age 13 years. No prior history of stroke, transient ischemic attacks, hypertension, coronary artery disease, rheumatic heart disease, or atrial fibrillation. She reports no allergies. On review of her family history, she reports that her husband was diagnosed with pulmonary tuberculosis 10 months ago. She states that he was treated with antituberculosis medications for a period of 6 months but admits that he had not been compliant with the entire course of treatment.",
"She does not smoke tobacco, drink alcohol, or use illicit drugs. She has never used oral contraceptive agents. She lives in Mumbai, India, and has never travelled abroad."
],
"date": "December 17, 2014",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/735/800/735800-thumb1.png"
}
],
"markdown": "# Sudden Left-Sided Weakness, Somnolence, and Episodic Vomiting\n\n **Authors:** Shradha B. Ahuja, MB BS; Rajiv S. Hira, MB BS; Vijay Panikar, MD, DNB, FCPS \n **Date:** December 17, 2014\n\n ## Content\n\n Editor's Note:\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions.\nA 60-year-old, right-handed woman is brought to the emergency department (ED) for left-sided weakness following an unwitnessed fall. She fell to the floor on her left side while attempting to get out of bed. She realized that her entire left side was weak, and she did not have the strength to stand up on her own. Her family states that she has been sleeping much more than normal and had complained of a severe occipital headache for the last 3 days, accompanied by 6-7 episodes of vomiting per day. The vomiting is not relieved by antacids and proton-pump inhibitors and is not associated with nausea. She also complained to her family of feeling feverish for the past few days and had appeared intermittently agitated. She denies any history of head trauma, photophobia, diplopia, convulsions, dizziness, difficulty speaking, or difficulty swallowing.\nFigure 1.\nHer past medical history is significant for a hysterectomy 15 years ago and an appendectomy at age 13 years. No prior history of stroke, transient ischemic attacks, hypertension, coronary artery disease, rheumatic heart disease, or atrial fibrillation. She reports no allergies. On review of her family history, she reports that her husband was diagnosed with pulmonary tuberculosis 10 months ago. She states that he was treated with antituberculosis medications for a period of 6 months but admits that he had not been compliant with the entire course of treatment.\nShe does not smoke tobacco, drink alcohol, or use illicit drugs. She has never used oral contraceptive agents. She lives in Mumbai, India, and has never travelled abroad.\n\n ## Figures\n\n **Figure 1.** \n \n\n\n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "Sudden Left-Sided Weakness, Somnolence, and Episodic Vomiting"
},
{
"authors": "Shradha B. Ahuja, MB BS; Rajiv S. Hira, MB BS; Vijay Panikar, MD, DNB, FCPS",
"content": [
"On physical examination, her oral temperature is 99.6°F (37.6°C). Her pulse is regular, with a rate of 60 beats/min. Her blood pressure is 130/80 mm Hg, and her respiratory rate is 10 breaths/min. Auscultation of the heart reveals a normal S1 and S2, with no murmurs or rub. Palpation of the abdomen reveals no tenderness, masses, or enlargement of the liver or spleen. The lungs are clear in all fields except for slight coarse sounds heard over the right lung field.",
"The patient appears to be drowsy and disoriented. She follows commands poorly, withdraws from painful stimulus, and her total Glasgow Coma Scale score is 10. She is breathing spontaneously and appears to be controlling her secretions. There is no evidence of trauma on head-to-toe examination. Cervical lymph nodes are palpable and are measured at 2-3 cm in diameter. Both pupils are 3 mm in size and are reactive to light. She is unable to flex her neck (meningismus) and, on forceful flexion, there is partial flexion of both right and left thighs (positive Brudzinski sign). Muscle strength in the left upper and lower limbs is decreased at grade 2/5, with full range of motion along with gravity, while reflexes in the same extremities are brisk and excessive. Both the strength and the deep tendon reflexes on the right side are normal. The plantar reflex is positive for Babinski's sign bilaterally. Left hemianesthesia and left homonymous hemianopsia are also noted. The rest of the cranial nerve examination findings were normal.",
"Figure 1.",
"The laboratory analysis includes a complete blood count that reveals an elevated total leukocyte count of 13.5 x 103/μL (normal range = 4.0-10.0 x 103/μL) with 56% lymphocytes (normal range = 20%-45%) and an elevated erythrocyte sedimentation rate of 80 mm/hour (normal range = 0-20 mm/hour in females). Other biochemical investigations reveal hyponatremia, with sodium levels at 120 mEq/L (normal range, 135-145 mEq/L), while potassium and chloride levels are normal. Serum glucose is 78 mg/dL. Tests for HIV I and II are nonreactive, and liver function tests are normal. A lumbar puncture is performed that reveals hazy cerebrospinal fluid (CSF), with an elevated protein count at 156 mg/dL (normal range, 10-60 mg/dL) and a decreased glucose level of 35 mg/dL (normal range = 40-80 mg/dL). On microscopic examination, the CSF has an elevated total cell count of 40 cells/mm3 (normal range, 0-5 cells/mm3), with 90% of cells being lymphocytes.",
"A CT scan shows a hypodense area in the distribution of the right middle cerebral artery that is consistent with a cerebral infarction (Figure 1)."
],
"date": "December 17, 2014",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/735/800/735800-thumb1.png"
}
],
"markdown": "# Sudden Left-Sided Weakness, Somnolence, and Episodic Vomiting\n\n **Authors:** Shradha B. Ahuja, MB BS; Rajiv S. Hira, MB BS; Vijay Panikar, MD, DNB, FCPS \n **Date:** December 17, 2014\n\n ## Content\n\n On physical examination, her oral temperature is 99.6°F (37.6°C). Her pulse is regular, with a rate of 60 beats/min. Her blood pressure is 130/80 mm Hg, and her respiratory rate is 10 breaths/min. Auscultation of the heart reveals a normal S1 and S2, with no murmurs or rub. Palpation of the abdomen reveals no tenderness, masses, or enlargement of the liver or spleen. The lungs are clear in all fields except for slight coarse sounds heard over the right lung field.\nThe patient appears to be drowsy and disoriented. She follows commands poorly, withdraws from painful stimulus, and her total Glasgow Coma Scale score is 10. She is breathing spontaneously and appears to be controlling her secretions. There is no evidence of trauma on head-to-toe examination. Cervical lymph nodes are palpable and are measured at 2-3 cm in diameter. Both pupils are 3 mm in size and are reactive to light. She is unable to flex her neck (meningismus) and, on forceful flexion, there is partial flexion of both right and left thighs (positive Brudzinski sign). Muscle strength in the left upper and lower limbs is decreased at grade 2/5, with full range of motion along with gravity, while reflexes in the same extremities are brisk and excessive. Both the strength and the deep tendon reflexes on the right side are normal. The plantar reflex is positive for Babinski's sign bilaterally. Left hemianesthesia and left homonymous hemianopsia are also noted. The rest of the cranial nerve examination findings were normal.\nFigure 1.\nThe laboratory analysis includes a complete blood count that reveals an elevated total leukocyte count of 13.5 x 103/μL (normal range = 4.0-10.0 x 103/μL) with 56% lymphocytes (normal range = 20%-45%) and an elevated erythrocyte sedimentation rate of 80 mm/hour (normal range = 0-20 mm/hour in females). Other biochemical investigations reveal hyponatremia, with sodium levels at 120 mEq/L (normal range, 135-145 mEq/L), while potassium and chloride levels are normal. Serum glucose is 78 mg/dL. Tests for HIV I and II are nonreactive, and liver function tests are normal. A lumbar puncture is performed that reveals hazy cerebrospinal fluid (CSF), with an elevated protein count at 156 mg/dL (normal range, 10-60 mg/dL) and a decreased glucose level of 35 mg/dL (normal range = 40-80 mg/dL). On microscopic examination, the CSF has an elevated total cell count of 40 cells/mm3 (normal range, 0-5 cells/mm3), with 90% of cells being lymphocytes.\nA CT scan shows a hypodense area in the distribution of the right middle cerebral artery that is consistent with a cerebral infarction (Figure 1).\n\n ## Figures\n\n **Figure 1.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 387868,
"choiceText": "Acute bacterial meningitis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 387869,
"choiceText": "Tuberculous meningitis with vasculitis",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 387870,
"choiceText": "Meningioma",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 387871,
"choiceText": "Lymphoma",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 112417,
"questionText": "What is the most likely diagnosis for the patient above?<br /><br /><i>Hint: The symptoms, physical findings, and family history are all significant in this case.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Sudden Left-Sided Weakness, Somnolence, and Episodic Vomiting"
},
{
"authors": "Shradha B. Ahuja, MB BS; Rajiv S. Hira, MB BS; Vijay Panikar, MD, DNB, FCPS",
"content": [
"The diagnosis of tuberculous meningitis (TBM) was included in the differential based on the patient's history and physical examination. The initial prodrome of fever, fatigue, and irritability followed by signs of meningeal irritation, including headache, neck rigidity, vomiting, drowsiness, or altered mental status, suggests the possibility of meningitis. The CSF analysis showed elevated protein, decreased glucose, and lymphocytic pleocytosis supporting the diagnosis of TBM. TBM may present with hemiparesis or other focal neurologic abnormalities due to a vasculitis-induced arterial infarction. In this case, the constellation of left hemiparesis, left hemianesthesia, and left homonymous hemianopsia (HHH syndrome) suggested involvement of the middle cerebral artery. Serum electrolytes revealed hyponatremia, which is thought to be related either to syndrome of inappropriate antidiuretic hormone (SIADH) or to the secretion of plasma atrial natriuretic factor and may have contributed to the patient's lethargy.",
"Tuberculosis (TB) is the seventh leading cause of death and disability worldwide.[1] Almost 2 billion people are infected with TB, and 8.9 million new cases of TB were reported in 2004, with 80% of them being in the high-burden regions of South East Asia and Sub-Saharan Africa.[2,3] In 2004, 1.7 million people died of TB, 98% of which occurred in low-income countries.[3] In India alone, 1.8 million new cases and over 370,000 deaths are due to TB.[2]",
"Fifteen percent of TB cases have extrapulmonary manifestations. Meningitis is the second most common and by far the most devastating form. Although more common among children between 6 months and 5 years of age, the incidence of TB in the central nervous system (CNS) among adults has seen a recent increase in the past 2 decades due to the spread of HIV-AIDS.[1]"
],
"date": "December 17, 2014",
"figures": [],
"markdown": "# Sudden Left-Sided Weakness, Somnolence, and Episodic Vomiting\n\n **Authors:** Shradha B. Ahuja, MB BS; Rajiv S. Hira, MB BS; Vijay Panikar, MD, DNB, FCPS \n **Date:** December 17, 2014\n\n ## Content\n\n The diagnosis of tuberculous meningitis (TBM) was included in the differential based on the patient's history and physical examination. The initial prodrome of fever, fatigue, and irritability followed by signs of meningeal irritation, including headache, neck rigidity, vomiting, drowsiness, or altered mental status, suggests the possibility of meningitis. The CSF analysis showed elevated protein, decreased glucose, and lymphocytic pleocytosis supporting the diagnosis of TBM. TBM may present with hemiparesis or other focal neurologic abnormalities due to a vasculitis-induced arterial infarction. In this case, the constellation of left hemiparesis, left hemianesthesia, and left homonymous hemianopsia (HHH syndrome) suggested involvement of the middle cerebral artery. Serum electrolytes revealed hyponatremia, which is thought to be related either to syndrome of inappropriate antidiuretic hormone (SIADH) or to the secretion of plasma atrial natriuretic factor and may have contributed to the patient's lethargy.\nTuberculosis (TB) is the seventh leading cause of death and disability worldwide.[1] Almost 2 billion people are infected with TB, and 8.9 million new cases of TB were reported in 2004, with 80% of them being in the high-burden regions of South East Asia and Sub-Saharan Africa.[2,3] In 2004, 1.7 million people died of TB, 98% of which occurred in low-income countries.[3] In India alone, 1.8 million new cases and over 370,000 deaths are due to TB.[2]\nFifteen percent of TB cases have extrapulmonary manifestations. Meningitis is the second most common and by far the most devastating form. Although more common among children between 6 months and 5 years of age, the incidence of TB in the central nervous system (CNS) among adults has seen a recent increase in the past 2 decades due to the spread of HIV-AIDS.[1]\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 387868,
"choiceText": "Acute bacterial meningitis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 387869,
"choiceText": "Tuberculous meningitis with vasculitis",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 387870,
"choiceText": "Meningioma",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 387871,
"choiceText": "Lymphoma",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 112417,
"questionText": "What is the most likely diagnosis for the patient above?<br /><br /><i>Hint: The symptoms, physical findings, and family history are all significant in this case.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Sudden Left-Sided Weakness, Somnolence, and Episodic Vomiting"
},
{
"authors": "Shradha B. Ahuja, MB BS; Rajiv S. Hira, MB BS; Vijay Panikar, MD, DNB, FCPS",
"content": [
"Mycobacterium tuberculosis bacilli enter the host by inhalation of 0.5-5 µm droplets. As few as 10 TB bacteria can cause a localized infection within pulmonary alveoli, replicating within the alveolar macrophages and forming the primary site of infection (Ghon complex).[4,5,6] This TB bacteremia then causes seeding to other parts of the body. Dissemination to the brain is more likely if miliary TB develops.[1] If TB does reach the brain, it forms metastatic caseous lesions called Rich foci. Symptoms of CNS TB depend on the size, location, and whether or not the tubercle ruptures. Should the tubercle rupture into the subarachnoid space, then meningitis results; however, if it enlarges, a tuberculoma or abscess can form, which can then burst into the ventricle.[1,7] The exudates within cerebral arteries cause a vasculitic picture of inflammation, infarction, or obstruction.[1]",
"The symptoms of TBM may be due to meningeal irritation, cerebral vasculitis, increased intracranial pressure (ICP), obstructive hydrocephalus caused by compression, or basilar cistern obstruction due to an intracranial space-occupying lesion. Meningeal irritation and increased ICP cause headache, neck stiffness, blurred vision, lethargy, confusion, altered mental status, nausea, vomiting, and seizures.[8,9] When vasculitis occurs, it most commonly involves the middle cerebral artery and results in contralateral hemiplegia, hemianesthesia, homonymous hemianopsia, aphasia (dominant hemisphere), anosognosia, and/or constructional apraxia and hemineglect (nondominant hemisphere). Compression effects can cause cranial nerve palsies, especially of cranial nerve VI, followed by cranial nerves III, IV, and VII.[1]\nArachnoiditis of the optic chiasm can cause visual disturbance and papilledema. Spread of the inflammatory process may include the spinal cord-associated vasculature, resulting in spinal cord-associated neurologic deficits.",
"The differential diagnosis of TBM includes other infectious processes (acute/subacute bacterial meningitis, viral infections, cerebral abscesses, tuberculomas, encephalitis, neurocysticercosis), vascular pathologies (vasculitis, vascular infarcts), and other space-occupying lesions (infectious lesions, primary tumors, metastasis). Depending on the region of the brain and spinal cord involved, TB of the CNS can have a varied presentation, which thereby broadens the differential diagnosis.",
"In suspected cases of TBM, the most important diagnostic tests are a full CSF analysis including CSF adenosine deaminase (ADA) levels and radiographic evaluation including chest radiographs, CT scanning, and MRI of the brain. Spinal fluid leukocytosis and lymphocytosis are key diagnostic features of TBM usually observed in combination with decreased CSF glucose levels and elevated CSF protein levels.[1] Ziehl-Neelsen staining of the CSF may demonstrate acid-fast bacilli, and culture of the CSF for M tuberculosis should be done. Polymerase chain reaction (PCR) can also provide a rapid and reliable diagnosis of TBM. CSF ADA levels have high sensitivity and specificity for the diagnosis of TBM.[1,10,11] The significant cut-off for CSF ADA is an ADA finding of more than 8.2 U/L. In this patient, the CSF ADA level was 15.3 U/L.",
"When TBM is a concern, imaging studies may be obtained. A posteroanterior and lateral chest radiograph may demonstrate signs of TB, such as infiltrates with or without cavitations, pleural effusion, and lymphadenopathy. CT scanning of the brain characteristically will show nodular lesions with central hypodensity indicating caseation. Contrast enhancement is essential and will initially demonstrate nonencapsulated, isodense lesions with edema out of proportion to the mass effect.",
"As the disease progresses, a hyperdense, well-encapsulated lesion develops, indicative of a tuberculoma.[1,8,12] MRI images demonstrate obliteration of the subarachnoid space along with the varying degrees of edema and mass effect.[1,8,12] Both CT and MRI contrast-enhanced images of the brain will also detect TBM sequelae, including cerebral infarcts, hydrocephalus, and basilar meningeal thickening and enhancement."
],
"date": "December 17, 2014",
"figures": [],
"markdown": "# Sudden Left-Sided Weakness, Somnolence, and Episodic Vomiting\n\n **Authors:** Shradha B. Ahuja, MB BS; Rajiv S. Hira, MB BS; Vijay Panikar, MD, DNB, FCPS \n **Date:** December 17, 2014\n\n ## Content\n\n Mycobacterium tuberculosis bacilli enter the host by inhalation of 0.5-5 µm droplets. As few as 10 TB bacteria can cause a localized infection within pulmonary alveoli, replicating within the alveolar macrophages and forming the primary site of infection (Ghon complex).[4,5,6] This TB bacteremia then causes seeding to other parts of the body. Dissemination to the brain is more likely if miliary TB develops.[1] If TB does reach the brain, it forms metastatic caseous lesions called Rich foci. Symptoms of CNS TB depend on the size, location, and whether or not the tubercle ruptures. Should the tubercle rupture into the subarachnoid space, then meningitis results; however, if it enlarges, a tuberculoma or abscess can form, which can then burst into the ventricle.[1,7] The exudates within cerebral arteries cause a vasculitic picture of inflammation, infarction, or obstruction.[1]\nThe symptoms of TBM may be due to meningeal irritation, cerebral vasculitis, increased intracranial pressure (ICP), obstructive hydrocephalus caused by compression, or basilar cistern obstruction due to an intracranial space-occupying lesion. Meningeal irritation and increased ICP cause headache, neck stiffness, blurred vision, lethargy, confusion, altered mental status, nausea, vomiting, and seizures.[8,9] When vasculitis occurs, it most commonly involves the middle cerebral artery and results in contralateral hemiplegia, hemianesthesia, homonymous hemianopsia, aphasia (dominant hemisphere), anosognosia, and/or constructional apraxia and hemineglect (nondominant hemisphere). Compression effects can cause cranial nerve palsies, especially of cranial nerve VI, followed by cranial nerves III, IV, and VII.[1]\nArachnoiditis of the optic chiasm can cause visual disturbance and papilledema. Spread of the inflammatory process may include the spinal cord-associated vasculature, resulting in spinal cord-associated neurologic deficits.\nThe differential diagnosis of TBM includes other infectious processes (acute/subacute bacterial meningitis, viral infections, cerebral abscesses, tuberculomas, encephalitis, neurocysticercosis), vascular pathologies (vasculitis, vascular infarcts), and other space-occupying lesions (infectious lesions, primary tumors, metastasis). Depending on the region of the brain and spinal cord involved, TB of the CNS can have a varied presentation, which thereby broadens the differential diagnosis.\nIn suspected cases of TBM, the most important diagnostic tests are a full CSF analysis including CSF adenosine deaminase (ADA) levels and radiographic evaluation including chest radiographs, CT scanning, and MRI of the brain. Spinal fluid leukocytosis and lymphocytosis are key diagnostic features of TBM usually observed in combination with decreased CSF glucose levels and elevated CSF protein levels.[1] Ziehl-Neelsen staining of the CSF may demonstrate acid-fast bacilli, and culture of the CSF for M tuberculosis should be done. Polymerase chain reaction (PCR) can also provide a rapid and reliable diagnosis of TBM. CSF ADA levels have high sensitivity and specificity for the diagnosis of TBM.[1,10,11] The significant cut-off for CSF ADA is an ADA finding of more than 8.2 U/L. In this patient, the CSF ADA level was 15.3 U/L.\nWhen TBM is a concern, imaging studies may be obtained. A posteroanterior and lateral chest radiograph may demonstrate signs of TB, such as infiltrates with or without cavitations, pleural effusion, and lymphadenopathy. CT scanning of the brain characteristically will show nodular lesions with central hypodensity indicating caseation. Contrast enhancement is essential and will initially demonstrate nonencapsulated, isodense lesions with edema out of proportion to the mass effect.\nAs the disease progresses, a hyperdense, well-encapsulated lesion develops, indicative of a tuberculoma.[1,8,12] MRI images demonstrate obliteration of the subarachnoid space along with the varying degrees of edema and mass effect.[1,8,12] Both CT and MRI contrast-enhanced images of the brain will also detect TBM sequelae, including cerebral infarcts, hydrocephalus, and basilar meningeal thickening and enhancement.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "Sudden Left-Sided Weakness, Somnolence, and Episodic Vomiting"
},
{
"authors": "Shradha B. Ahuja, MB BS; Rajiv S. Hira, MB BS; Vijay Panikar, MD, DNB, FCPS",
"content": [
"TB has been treated with combination therapy for over 50 years. Regimens that use only single drugs result in the rapid development of resistance and treatment failure.[13,14] For example, in one study, isoniazid resistance on initial susceptibility testing was associated with subsequent death during the treatment of TBM.[15] Death during treatment was particularly significant in patients with TBM who had positive CSF cultures and initial isoniazid resistance. No such findings were demonstrated with advancing age, race, and HIV status; that is, there was no association between these factors and initial isoniazid resistance and death.[15] The investigators recommended more randomized controlled studies to assess the optimal empirical regimen for treating patients with TBM who are at high risk for initial isoniazid resistance and poor clinical outcomes.[15] Directly observed therapy, in which a trained healthcare worker observes the patient taking each dose of medication, has been a major strategy in the World Health Organization's global TB eradication program. This treatment and health system guideline improves health and decreases TB drug resistance.",
"The standard treatment for TBM is isoniazid, rifampicin, pyrazinamide, and streptomycin (or ethambutol) for 2 months, followed by 7-10 months of isoniazid and rifampin[16,17] for fully susceptible strains along with steroids for 4-6 weeks.[18,19] The CSF of patients with treated TBM is commonly abnormal even at 12 months, and the resolution of CSF findings has no correlation with clinical progress or outcome, so repeated sampling of CSF by lumbar puncture to monitor treatment progress is not indicated, nor is extending or repeating treatment based on clinical symptoms.[20,21]",
"Early studies suggested that corticosteroids reduce cerebrospinal fluid inflammation as well as time to recovery and mortality in patients with TBM.[14] Care should be used in patients with a concurrent HIV infection. Although controversy exists regarding the use of corticosteroids in the treatment of CNS TB, most researchers agree that the benefits outweigh the risks.[16] The dose of dexamethasone in TBM is 8-12 mg daily tapered over 6 weeks. In those who have failed to respond to steroid treatment, thalidomide may be of benefit in TBM and has been used in certain cases.[22]",
"In patients with evidence of obstructive hydrocephalus and neurologic deterioration, placement of a ventricular drain or ventriculoperitoneal or ventriculoatrial shunt should be done as soon as possible. Unless mass effect is compromising vital structures, surgical intervention is rarely required in the treatment of tuberculomas.[1]",
"In this case, a neurologist was consulted and 20% mannitol was administered intravenously in a dose of 0.5g/kg over 15 minutes. The patient's hyponatremia due to SIADH caused by the meningitis may reverse automatically on treatment of the underlying cause; however, in this case, the serum sodium levels were dangerously low and required correction. Fluids were limited to about 800-1000 mL/day; a low-dose diuretic and hypertonic saline were given intravenously in a dose of 200 mL over 3 to 4 hours. Dexamethasone was given in a dose of 8 mg/day and tapered gradually over 2 weeks. Chemotherapy consisted of administration of isoniazid in a dose of 5 mg/kg body weight, rifampin at 10 mg/kg body weight, ethambutol at 25 mg/kg body weight, and pyrazinamide at 15 mg/kg body weight, each given 3 times a week for 2 months. The patient remained in the intensive care unit for a period of 2 weeks.",
"Supportive management included rehabilitation of the patient with physiotherapy, nutritional supplementation, and symptomatic treatment. The patient's general condition improved significantly, and she was discharged from the hospital. She came for regular follow-up visits in the outpatient department for 6 weeks thereafter and showed a gradual improvement in muscle power on the left side, which became 4/5 by the sixth week. Upon completion of the 2-month course of antituberculous treatment, she was advised to continue isoniazid and rifampin (3 times a week in above doses) for a period of 10 months."
],
"date": "December 17, 2014",
"figures": [],
"markdown": "# Sudden Left-Sided Weakness, Somnolence, and Episodic Vomiting\n\n **Authors:** Shradha B. Ahuja, MB BS; Rajiv S. Hira, MB BS; Vijay Panikar, MD, DNB, FCPS \n **Date:** December 17, 2014\n\n ## Content\n\n TB has been treated with combination therapy for over 50 years. Regimens that use only single drugs result in the rapid development of resistance and treatment failure.[13,14] For example, in one study, isoniazid resistance on initial susceptibility testing was associated with subsequent death during the treatment of TBM.[15] Death during treatment was particularly significant in patients with TBM who had positive CSF cultures and initial isoniazid resistance. No such findings were demonstrated with advancing age, race, and HIV status; that is, there was no association between these factors and initial isoniazid resistance and death.[15] The investigators recommended more randomized controlled studies to assess the optimal empirical regimen for treating patients with TBM who are at high risk for initial isoniazid resistance and poor clinical outcomes.[15] Directly observed therapy, in which a trained healthcare worker observes the patient taking each dose of medication, has been a major strategy in the World Health Organization's global TB eradication program. This treatment and health system guideline improves health and decreases TB drug resistance.\nThe standard treatment for TBM is isoniazid, rifampicin, pyrazinamide, and streptomycin (or ethambutol) for 2 months, followed by 7-10 months of isoniazid and rifampin[16,17] for fully susceptible strains along with steroids for 4-6 weeks.[18,19] The CSF of patients with treated TBM is commonly abnormal even at 12 months, and the resolution of CSF findings has no correlation with clinical progress or outcome, so repeated sampling of CSF by lumbar puncture to monitor treatment progress is not indicated, nor is extending or repeating treatment based on clinical symptoms.[20,21]\nEarly studies suggested that corticosteroids reduce cerebrospinal fluid inflammation as well as time to recovery and mortality in patients with TBM.[14] Care should be used in patients with a concurrent HIV infection. Although controversy exists regarding the use of corticosteroids in the treatment of CNS TB, most researchers agree that the benefits outweigh the risks.[16] The dose of dexamethasone in TBM is 8-12 mg daily tapered over 6 weeks. In those who have failed to respond to steroid treatment, thalidomide may be of benefit in TBM and has been used in certain cases.[22]\nIn patients with evidence of obstructive hydrocephalus and neurologic deterioration, placement of a ventricular drain or ventriculoperitoneal or ventriculoatrial shunt should be done as soon as possible. Unless mass effect is compromising vital structures, surgical intervention is rarely required in the treatment of tuberculomas.[1]\nIn this case, a neurologist was consulted and 20% mannitol was administered intravenously in a dose of 0.5g/kg over 15 minutes. The patient's hyponatremia due to SIADH caused by the meningitis may reverse automatically on treatment of the underlying cause; however, in this case, the serum sodium levels were dangerously low and required correction. Fluids were limited to about 800-1000 mL/day; a low-dose diuretic and hypertonic saline were given intravenously in a dose of 200 mL over 3 to 4 hours. Dexamethasone was given in a dose of 8 mg/day and tapered gradually over 2 weeks. Chemotherapy consisted of administration of isoniazid in a dose of 5 mg/kg body weight, rifampin at 10 mg/kg body weight, ethambutol at 25 mg/kg body weight, and pyrazinamide at 15 mg/kg body weight, each given 3 times a week for 2 months. The patient remained in the intensive care unit for a period of 2 weeks.\nSupportive management included rehabilitation of the patient with physiotherapy, nutritional supplementation, and symptomatic treatment. The patient's general condition improved significantly, and she was discharged from the hospital. She came for regular follow-up visits in the outpatient department for 6 weeks thereafter and showed a gradual improvement in muscle power on the left side, which became 4/5 by the sixth week. Upon completion of the 2-month course of antituberculous treatment, she was advised to continue isoniazid and rifampin (3 times a week in above doses) for a period of 10 months.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 387872,
"choiceText": "Elevated CSF glucose and elevated CSF protein",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 387873,
"choiceText": "Elevated CSF cell count with neutrophilia",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 387874,
"choiceText": "Hypodense lesion with edema out of proportion to the mass effect on contrast-enhanced CT of the brain",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 387875,
"choiceText": "A hyper-dense, well-encapsulated lesion seen on CT of the brain",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 387876,
"choiceText": "A low CSF ADA level",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "CSF findings consistent with tuberculous meningitis are low glucose, elevated protein, elevated cell count with lymphocytosis, and an elevated ADA level. In the early stages of TBM, contrast-enhanced CT scanning will show a hypodense lesion with edema out of proportion to the mass effect. Once a tuberculoma starts to develop later in the course of the disease, a hyper-dense, well-encapsulated lesion will be seen on noncontrast enhanced CT scanning of the brain.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 112418,
"questionText": "You are treating a patient who has a stiff neck, a low-grade fever, confusion, hemiplegia, and visual changes. You are concerned about meningitis, elevated ICP, and vasculitis. Which of the following findings are consistent with the early presentation of TBM?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 387877,
"choiceText": "Ziehl-Neelsen staining for acid-fast bacilli",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 387878,
"choiceText": "Tuberculin sensitivity test",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 387879,
"choiceText": "PCR analysis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 387880,
"choiceText": "Serum ADA levels",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 387881,
"choiceText": "CSF ADA levels",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Although PCR analysis is the most rapid investigation for the diagnosis of TB, research has shown that it can produce false-negative results in certain cases. Serum ADA levels, Ziehl-Neelsen staining, and tuberculin testing have been found to have low sensitivity; however, in pleural TB causing effusion and TBM, ADA testing had a higher sensitivity than any other tests.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 112419,
"questionText": " In a patient with findings that are consistent with meningitis, which test would be the most sensitive in ruling out TBM?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Sudden Left-Sided Weakness, Somnolence, and Episodic Vomiting"
},
{
"authors": "Shradha B. Ahuja, MB BS; Rajiv S. Hira, MB BS; Vijay Panikar, MD, DNB, FCPS",
"content": [],
"date": "December 17, 2014",
"figures": [],
"markdown": "# Sudden Left-Sided Weakness, Somnolence, and Episodic Vomiting\n\n **Authors:** Shradha B. Ahuja, MB BS; Rajiv S. Hira, MB BS; Vijay Panikar, MD, DNB, FCPS \n **Date:** December 17, 2014\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 387872,
"choiceText": "Elevated CSF glucose and elevated CSF protein",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 387873,
"choiceText": "Elevated CSF cell count with neutrophilia",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 387874,
"choiceText": "Hypodense lesion with edema out of proportion to the mass effect on contrast-enhanced CT of the brain",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 387875,
"choiceText": "A hyper-dense, well-encapsulated lesion seen on CT of the brain",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 387876,
"choiceText": "A low CSF ADA level",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "CSF findings consistent with tuberculous meningitis are low glucose, elevated protein, elevated cell count with lymphocytosis, and an elevated ADA level. In the early stages of TBM, contrast-enhanced CT scanning will show a hypodense lesion with edema out of proportion to the mass effect. Once a tuberculoma starts to develop later in the course of the disease, a hyper-dense, well-encapsulated lesion will be seen on noncontrast enhanced CT scanning of the brain.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 112418,
"questionText": "You are treating a patient who has a stiff neck, a low-grade fever, confusion, hemiplegia, and visual changes. You are concerned about meningitis, elevated ICP, and vasculitis. Which of the following findings are consistent with the early presentation of TBM?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 387877,
"choiceText": "Ziehl-Neelsen staining for acid-fast bacilli",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 387878,
"choiceText": "Tuberculin sensitivity test",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 387879,
"choiceText": "PCR analysis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 387880,
"choiceText": "Serum ADA levels",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 387881,
"choiceText": "CSF ADA levels",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Although PCR analysis is the most rapid investigation for the diagnosis of TB, research has shown that it can produce false-negative results in certain cases. Serum ADA levels, Ziehl-Neelsen staining, and tuberculin testing have been found to have low sensitivity; however, in pleural TB causing effusion and TBM, ADA testing had a higher sensitivity than any other tests.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 112419,
"questionText": " In a patient with findings that are consistent with meningitis, which test would be the most sensitive in ruling out TBM?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Sudden Left-Sided Weakness, Somnolence, and Episodic Vomiting"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 387868,
"choiceText": "Acute bacterial meningitis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 387869,
"choiceText": "Tuberculous meningitis with vasculitis",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 387870,
"choiceText": "Meningioma",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 387871,
"choiceText": "Lymphoma",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 112417,
"questionText": "What is the most likely diagnosis for the patient above?<br /><br /><i>Hint: The symptoms, physical findings, and family history are all significant in this case.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 387872,
"choiceText": "Elevated CSF glucose and elevated CSF protein",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 387873,
"choiceText": "Elevated CSF cell count with neutrophilia",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 387874,
"choiceText": "Hypodense lesion with edema out of proportion to the mass effect on contrast-enhanced CT of the brain",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 387875,
"choiceText": "A hyper-dense, well-encapsulated lesion seen on CT of the brain",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 387876,
"choiceText": "A low CSF ADA level",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "CSF findings consistent with tuberculous meningitis are low glucose, elevated protein, elevated cell count with lymphocytosis, and an elevated ADA level. In the early stages of TBM, contrast-enhanced CT scanning will show a hypodense lesion with edema out of proportion to the mass effect. Once a tuberculoma starts to develop later in the course of the disease, a hyper-dense, well-encapsulated lesion will be seen on noncontrast enhanced CT scanning of the brain.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 112418,
"questionText": "You are treating a patient who has a stiff neck, a low-grade fever, confusion, hemiplegia, and visual changes. You are concerned about meningitis, elevated ICP, and vasculitis. Which of the following findings are consistent with the early presentation of TBM?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 387877,
"choiceText": "Ziehl-Neelsen staining for acid-fast bacilli",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 387878,
"choiceText": "Tuberculin sensitivity test",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 387879,
"choiceText": "PCR analysis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 387880,
"choiceText": "Serum ADA levels",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 387881,
"choiceText": "CSF ADA levels",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Although PCR analysis is the most rapid investigation for the diagnosis of TB, research has shown that it can produce false-negative results in certain cases. Serum ADA levels, Ziehl-Neelsen staining, and tuberculin testing have been found to have low sensitivity; however, in pleural TB causing effusion and TBM, ADA testing had a higher sensitivity than any other tests.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 112419,
"questionText": " In a patient with findings that are consistent with meningitis, which test would be the most sensitive in ruling out TBM?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
735590 | /viewarticle/735590 | [
{
"authors": "Gamal A. Bebars, MD, FRCS; Evelyn P. Bebars, MD, FPCR",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to recognize accurately. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"A 70-year-old man presents to the emergency department (ED) with generalized abdominal pain and distention. The distention has increased progressively over the last 4 days, with a substantial increase today. His mental status has progressively deteriorated, and he cannot provide additional information regarding the onset, course, and character of the pain. The patient's family states that he has not had a bowel movement in 2 days and has never experienced similar symptoms.",
"The patient has hypertension and has been on oral medication for his blood pressure for the past 18 years. He does not have any history of diabetes, myocardial infarction, or stroke. He has no past history of any abdominal surgery. He also has no history of travel. The patient is a nonsmoker, and he does not consume alcohol."
],
"date": "October 23, 2018",
"figures": [],
"markdown": "# A 70-Year-Old Man With Deteriorating Mental Status and Pain\n\n **Authors:** Gamal A. Bebars, MD, FRCS; Evelyn P. Bebars, MD, FPCR \n **Date:** October 23, 2018\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to recognize accurately. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nA 70-year-old man presents to the emergency department (ED) with generalized abdominal pain and distention. The distention has increased progressively over the last 4 days, with a substantial increase today. His mental status has progressively deteriorated, and he cannot provide additional information regarding the onset, course, and character of the pain. The patient's family states that he has not had a bowel movement in 2 days and has never experienced similar symptoms.\nThe patient has hypertension and has been on oral medication for his blood pressure for the past 18 years. He does not have any history of diabetes, myocardial infarction, or stroke. He has no past history of any abdominal surgery. He also has no history of travel. The patient is a nonsmoker, and he does not consume alcohol.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 70-Year-Old Man With Deteriorating Mental Status and Pain"
},
{
"authors": "Gamal A. Bebars, MD, FRCS; Evelyn P. Bebars, MD, FPCR",
"content": [
"Upon physical examination, the patient is an obese (approximately 242.5 lb [110 kg]), elderly male, who is obtunded. He has a patent airway, bilateral rales, and diminished air entry into both lung bases, normal S1 and S2 heart sounds, pallor, poor capillary refill, and a weak, rapid radial pulse. His heart rate is 124 beats/min. The patient is febrile, with a temperature of 102°F (38.9°C). His blood pressure is 85/65 mm Hg, and his respiratory rate is 26 breaths/min.",
"The patient's abdomen is grossly distended and hyperresonant, particularly above the umbilicus. Bowel sounds are totally absent; digital rectal examination reveals hard stools in the rectum. The patient is resuscitated in the ED using an intravenous infusion of lactated Ringer solution. Orotracheal intubation is performed and mechanical ventilation maintained. Intravenous antibiotics are started and a surgical consultation ordered, and then the patient is transported to the intensive care unit.",
"Laboratory results reveal the following:",
"White blood cell count - 22,500 cells/mm3 (reference range, 4,000-11,000 cells/mm3), with 82% neutrophils (reference range, 40%-75%)",
"Serum hemoglobin level - 9.2 g/dL (reference range, 13.5-18 g/dL)",
"Hematocrit level - 27.5% (reference range, 40%-54%)",
"Serum alanine aminotransferase (ALT) level - 78 U/L (reference range, 5-40 U/L)",
"Serum bilirubin level - 2.3 mg/dL (reference range, 0.2-1.2 mg/dL)",
"Serum C-reactive protein level - 28 mg/dL (reference value, < 1.2 mg/dL)",
"Serum creatinine level - 4.8 mg/dL (reference range, 0.6-1.4 mg/dL)",
"Serum urea level - 79 mg/dL (reference range, 17-50 mg/dL)",
"A plain chest x-ray shows a large air-filled bowel loop in the left hemithorax, with displacement of the mediastinum towards the right side (Figure 1). A supine abdominal x-ray reveals distended small and large bowel loops (Figure 2). A CT scan of the abdomen using both oral and intravenous contrast shows eventration of the left hemidiaphragm and a small right inguinal hernia with a gas shadow amongst its contents (Figure 3).",
"Figure 1.",
"Figure 2.",
"Figure 3.",
"The CT scan also demonstrates thickening of the bowel wall and omentum in addition to multiple abscesses between the bowel loops and within the pelvis. The appendix cannot be visualized in the right iliac fossa, and mesenteric vascular occlusion is not evident."
],
"date": "October 23, 2018",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/735/590/735590-thumb1.png"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/735/590/735590-thumb2.png"
},
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/735/590/735590-thumb3.png"
}
],
"markdown": "# A 70-Year-Old Man With Deteriorating Mental Status and Pain\n\n **Authors:** Gamal A. Bebars, MD, FRCS; Evelyn P. Bebars, MD, FPCR \n **Date:** October 23, 2018\n\n ## Content\n\n Upon physical examination, the patient is an obese (approximately 242.5 lb [110 kg]), elderly male, who is obtunded. He has a patent airway, bilateral rales, and diminished air entry into both lung bases, normal S1 and S2 heart sounds, pallor, poor capillary refill, and a weak, rapid radial pulse. His heart rate is 124 beats/min. The patient is febrile, with a temperature of 102°F (38.9°C). His blood pressure is 85/65 mm Hg, and his respiratory rate is 26 breaths/min.\nThe patient's abdomen is grossly distended and hyperresonant, particularly above the umbilicus. Bowel sounds are totally absent; digital rectal examination reveals hard stools in the rectum. The patient is resuscitated in the ED using an intravenous infusion of lactated Ringer solution. Orotracheal intubation is performed and mechanical ventilation maintained. Intravenous antibiotics are started and a surgical consultation ordered, and then the patient is transported to the intensive care unit.\nLaboratory results reveal the following:\nWhite blood cell count - 22,500 cells/mm3 (reference range, 4,000-11,000 cells/mm3), with 82% neutrophils (reference range, 40%-75%)\nSerum hemoglobin level - 9.2 g/dL (reference range, 13.5-18 g/dL)\nHematocrit level - 27.5% (reference range, 40%-54%)\nSerum alanine aminotransferase (ALT) level - 78 U/L (reference range, 5-40 U/L)\nSerum bilirubin level - 2.3 mg/dL (reference range, 0.2-1.2 mg/dL)\nSerum C-reactive protein level - 28 mg/dL (reference value, < 1.2 mg/dL)\nSerum creatinine level - 4.8 mg/dL (reference range, 0.6-1.4 mg/dL)\nSerum urea level - 79 mg/dL (reference range, 17-50 mg/dL)\nA plain chest x-ray shows a large air-filled bowel loop in the left hemithorax, with displacement of the mediastinum towards the right side (Figure 1). A supine abdominal x-ray reveals distended small and large bowel loops (Figure 2). A CT scan of the abdomen using both oral and intravenous contrast shows eventration of the left hemidiaphragm and a small right inguinal hernia with a gas shadow amongst its contents (Figure 3).\nFigure 1.\nFigure 2.\nFigure 3.\nThe CT scan also demonstrates thickening of the bowel wall and omentum in addition to multiple abscesses between the bowel loops and within the pelvis. The appendix cannot be visualized in the right iliac fossa, and mesenteric vascular occlusion is not evident.\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n**Figure 3.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 387325,
"choiceText": "Small bowel ischemia with peritonitis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 387326,
"choiceText": "Diaphragmatic hernia",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 387327,
"choiceText": "Sigmoid volvulus",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 387328,
"choiceText": "Complicated inguinal hernia with the appendix inside the hernial sac (Amyand hernia)",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 112231,
"questionText": "Which of the following is the most likely diagnosis?<br /><br /><i>Hint: Review the findings of the CT scan.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 70-Year-Old Man With Deteriorating Mental Status and Pain"
},
{
"authors": "Gamal A. Bebars, MD, FRCS; Evelyn P. Bebars, MD, FPCR",
"content": [
"This patient was in septic shock. The patient's supine plain chest x-ray excluded pulmonary septic pathology; however, it raised the suspicion of a diaphragmatic hernia in the left hemithorax. This possibility was excluded by the CT scan, which showed an intact left hemidiaphragm. Abdominal x-ray and CT scanning both excluded the presence of sigmoid volvulus. Contrast-enhanced CT scanning further revealed intact bowel circulation in both the arterial and venous phases, eliminating the possibility of mesenteric occlusion as the cause of the abdominal signs.",
"The presence or absence of free air in the peritoneal cavity could not be ascertained on the plain film because of the gross bowel distention. This was also the reason abdominal ultrasonography was not used, as the excessive distention would have reduced its diagnostic efficacy. Although the CT scan of the abdomen could not visualize the vermiform appendix in the right lower quadrant, it clearly detected a small right inguinal hernia with an air-filled space among the hernia's contents.",
"This hernia could not be identified during the physical examination because of the small size, inability to elicit a cough impulse, the use of mechanical ventilation, and the patient's obesity. Preoperatively, the etiology of the patient's sepsis could not be confirmed, but the working diagnosis was that of a strangulated inguinal hernia resulting in generalized peritonitis and ileus. The diagnosis of a complicated Amyand hernia associated with the perforation of an inflamed appendix was made only following laparotomy, as is the case in almost all the reported case series.[1,2]",
"An Amyand hernia is an inguinal hernia that contains the vermiform appendix within its hernial sac; it is named after English surgeon, Claudius Amyand. Only approximately 0.13% of cases of Amyand hernia cases are associated with appendicitis hernia, whereas a noninflamed appendix is found in about 1% of all hernia repairs. The differential diagnoses for Amyand hernia should include strangulated hernia, strangulated omentocele, Richter hernia, testicular tumor with hemorrhage, acute hydrocele, and inguinal adenitis."
],
"date": "October 23, 2018",
"figures": [],
"markdown": "# A 70-Year-Old Man With Deteriorating Mental Status and Pain\n\n **Authors:** Gamal A. Bebars, MD, FRCS; Evelyn P. Bebars, MD, FPCR \n **Date:** October 23, 2018\n\n ## Content\n\n This patient was in septic shock. The patient's supine plain chest x-ray excluded pulmonary septic pathology; however, it raised the suspicion of a diaphragmatic hernia in the left hemithorax. This possibility was excluded by the CT scan, which showed an intact left hemidiaphragm. Abdominal x-ray and CT scanning both excluded the presence of sigmoid volvulus. Contrast-enhanced CT scanning further revealed intact bowel circulation in both the arterial and venous phases, eliminating the possibility of mesenteric occlusion as the cause of the abdominal signs.\nThe presence or absence of free air in the peritoneal cavity could not be ascertained on the plain film because of the gross bowel distention. This was also the reason abdominal ultrasonography was not used, as the excessive distention would have reduced its diagnostic efficacy. Although the CT scan of the abdomen could not visualize the vermiform appendix in the right lower quadrant, it clearly detected a small right inguinal hernia with an air-filled space among the hernia's contents.\nThis hernia could not be identified during the physical examination because of the small size, inability to elicit a cough impulse, the use of mechanical ventilation, and the patient's obesity. Preoperatively, the etiology of the patient's sepsis could not be confirmed, but the working diagnosis was that of a strangulated inguinal hernia resulting in generalized peritonitis and ileus. The diagnosis of a complicated Amyand hernia associated with the perforation of an inflamed appendix was made only following laparotomy, as is the case in almost all the reported case series.[1,2]\nAn Amyand hernia is an inguinal hernia that contains the vermiform appendix within its hernial sac; it is named after English surgeon, Claudius Amyand. Only approximately 0.13% of cases of Amyand hernia cases are associated with appendicitis hernia, whereas a noninflamed appendix is found in about 1% of all hernia repairs. The differential diagnoses for Amyand hernia should include strangulated hernia, strangulated omentocele, Richter hernia, testicular tumor with hemorrhage, acute hydrocele, and inguinal adenitis.\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 387325,
"choiceText": "Small bowel ischemia with peritonitis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 387326,
"choiceText": "Diaphragmatic hernia",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 387327,
"choiceText": "Sigmoid volvulus",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 387328,
"choiceText": "Complicated inguinal hernia with the appendix inside the hernial sac (Amyand hernia)",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 112231,
"questionText": "Which of the following is the most likely diagnosis?<br /><br /><i>Hint: Review the findings of the CT scan.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 70-Year-Old Man With Deteriorating Mental Status and Pain"
},
{
"authors": "Gamal A. Bebars, MD, FRCS; Evelyn P. Bebars, MD, FPCR",
"content": [
"Amyand hernias can be classified into 4 types: type I, with a normal appendix; type II, with an acute appendicitis localized in the hernial sac; type III, with localized peritonitis; and type IV, with generalized peritonitis. The most common presentation of Amyand hernia with appendicitis is a painful irreducible inguinal or inguinoscrotal swelling. Patients with irreducible or incarcerated Amyand hernias present with clinical manifestations of bowel obstruction or perforation.[3] Published articles regarding preoperative diagnosis of Amyand hernia by ultrasonography or CT scanning are extremely rare, and the condition is almost always discovered intraoperatively.[4,5]",
"Reported conditions in patients presenting with incarcerated Amyand hernias include mucocele of the appendix associated with coexisting colon cancer, fecaliths of the appendix with coexisting colonic diverticulitis, adenocarcinoma of the appendix, and inguinal appendicocele with pseudomyxoma peritonei. A high index of suspicion accompanied by CT scans can avoid delays in management by helping the detection of any coexisting conditions and the planning of the most appropriate procedure, thereby improving patient outcomes.[2,4,5] Although helpful, obtaining CT scans must not delay surgical consultation or intervention in critically ill patients with a surgical abdomen. The most important aspect of surgical intervention in Amyand hernia is to limit any septic spread that can result from perforation of the appendix. Once the septic process involves the peritoneum, it becomes more difficult to manage and is associated with increased mortality.",
"The surgical management of Amyand hernia should be decided on a case-by-case basis according to the type of hernia and the patient's condition. Options include reduction of the appendix and mesh hernioplasty for type I, and appendectomy followed by endogenous repair without mesh for type II. The management of types III and IV Amyand hernia require more complex procedures, such as exploratory laparotomy, orchiectomy, right hemicolectomy, and debridement of any necrotic bowel. Hernioplasty is contraindicated and should be deferred if the patient's condition is poor or life expectancy is limited.[2,3]",
"When considering appendectomy, the surgeon must be mindful of the ease of its reduction and the presence or absence of appendicitis, in addition to the type of Amyand hernia. If significant trauma occurs to the appendix during a difficult reduction, an appendectomy is indicated because traumatic injury to the appendix increases the risk for postoperative appendicitis.[6] If appendicitis or incipient necrosis of the appendix are present, a transherniotomy appendectomy should be performed.[7]",
"Mullinax and colleagues described the management of an Amyand hernia through laparoscopic techniques in an elderly woman. Preoperative CT scanning to rule out possible incarcerated femoral hernia or appendicitis demonstrated distal appendiceal incarceration in a right inguinal hernia. Concomitant laparoscopic inguinal hernia repair and appendectomy were successfully performed, and the authors recommend that this approach be considered when evaluating surgical options for patients suffering from Amyand hernia.[8] In another report, Cankorkmaz L and colleagues described their 8-year institutional experience managing cases of Amyand hernia in the pediatric population, in which they found the status of the inflamed appendix may be used to determine the type of hernial repair and the operative approach in these patients.[9] Although the investigators did not recommend incidental appendectomy in children with an inflamed appendix, appendectomy via the hernia sac and hernia repair were successful."
],
"date": "October 23, 2018",
"figures": [],
"markdown": "# A 70-Year-Old Man With Deteriorating Mental Status and Pain\n\n **Authors:** Gamal A. Bebars, MD, FRCS; Evelyn P. Bebars, MD, FPCR \n **Date:** October 23, 2018\n\n ## Content\n\n Amyand hernias can be classified into 4 types: type I, with a normal appendix; type II, with an acute appendicitis localized in the hernial sac; type III, with localized peritonitis; and type IV, with generalized peritonitis. The most common presentation of Amyand hernia with appendicitis is a painful irreducible inguinal or inguinoscrotal swelling. Patients with irreducible or incarcerated Amyand hernias present with clinical manifestations of bowel obstruction or perforation.[3] Published articles regarding preoperative diagnosis of Amyand hernia by ultrasonography or CT scanning are extremely rare, and the condition is almost always discovered intraoperatively.[4,5]\nReported conditions in patients presenting with incarcerated Amyand hernias include mucocele of the appendix associated with coexisting colon cancer, fecaliths of the appendix with coexisting colonic diverticulitis, adenocarcinoma of the appendix, and inguinal appendicocele with pseudomyxoma peritonei. A high index of suspicion accompanied by CT scans can avoid delays in management by helping the detection of any coexisting conditions and the planning of the most appropriate procedure, thereby improving patient outcomes.[2,4,5] Although helpful, obtaining CT scans must not delay surgical consultation or intervention in critically ill patients with a surgical abdomen. The most important aspect of surgical intervention in Amyand hernia is to limit any septic spread that can result from perforation of the appendix. Once the septic process involves the peritoneum, it becomes more difficult to manage and is associated with increased mortality.\nThe surgical management of Amyand hernia should be decided on a case-by-case basis according to the type of hernia and the patient's condition. Options include reduction of the appendix and mesh hernioplasty for type I, and appendectomy followed by endogenous repair without mesh for type II. The management of types III and IV Amyand hernia require more complex procedures, such as exploratory laparotomy, orchiectomy, right hemicolectomy, and debridement of any necrotic bowel. Hernioplasty is contraindicated and should be deferred if the patient's condition is poor or life expectancy is limited.[2,3]\nWhen considering appendectomy, the surgeon must be mindful of the ease of its reduction and the presence or absence of appendicitis, in addition to the type of Amyand hernia. If significant trauma occurs to the appendix during a difficult reduction, an appendectomy is indicated because traumatic injury to the appendix increases the risk for postoperative appendicitis.[6] If appendicitis or incipient necrosis of the appendix are present, a transherniotomy appendectomy should be performed.[7]\nMullinax and colleagues described the management of an Amyand hernia through laparoscopic techniques in an elderly woman. Preoperative CT scanning to rule out possible incarcerated femoral hernia or appendicitis demonstrated distal appendiceal incarceration in a right inguinal hernia. Concomitant laparoscopic inguinal hernia repair and appendectomy were successfully performed, and the authors recommend that this approach be considered when evaluating surgical options for patients suffering from Amyand hernia.[8] In another report, Cankorkmaz L and colleagues described their 8-year institutional experience managing cases of Amyand hernia in the pediatric population, in which they found the status of the inflamed appendix may be used to determine the type of hernial repair and the operative approach in these patients.[9] Although the investigators did not recommend incidental appendectomy in children with an inflamed appendix, appendectomy via the hernia sac and hernia repair were successful.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 70-Year-Old Man With Deteriorating Mental Status and Pain"
},
{
"authors": "Gamal A. Bebars, MD, FRCS; Evelyn P. Bebars, MD, FPCR",
"content": [
"The patient in this case underwent exploratory laparotomy using a long midline incision rather than a limited inguinal incision. This choice allowed for satisfactory exploration of the entire abdomen, incision and drainage of all the intraperitoneal and pelvic abscesses, and generous peritoneal lavage. Intraoperatively, the cecum was found firmly adherent to the anterior abdominal peritoneum in the right inguinal region. With gentle traction of the cecum, the appendix was extracted from the inguinal hernial sac through the internal ring; approximately 15 mL of greenish, fecal-smelling pus were extracted as well.",
"The appendix was severely inflamed, gangrenous, and perforated in many locations. Appendectomy was performed and the peritoneal cavity was irrigated with copious amounts of warm normal saline solution. Hernioplasty was not performed as the presence of pus or perforation of the appendix is an absolute contraindication to the placement of a mesh for hernia repair because this greatly increases the chances of postoperative surgical site infection.[7] A rectal tube was introduced at the conclusion of surgery to facilitate closure of the abdominal incision and was removed shortly after the patient's recovery from anesthesia.",
"The patient's postoperative course was without incident. Bowel movements were present starting on the second postoperative day and the patient gradually began tolerating oral feeding. Postoperative investigations revealed a consistent improvement of all major systems. The patient was discharged on the eighth postoperative day in good overall condition."
],
"date": "October 23, 2018",
"figures": [],
"markdown": "# A 70-Year-Old Man With Deteriorating Mental Status and Pain\n\n **Authors:** Gamal A. Bebars, MD, FRCS; Evelyn P. Bebars, MD, FPCR \n **Date:** October 23, 2018\n\n ## Content\n\n The patient in this case underwent exploratory laparotomy using a long midline incision rather than a limited inguinal incision. This choice allowed for satisfactory exploration of the entire abdomen, incision and drainage of all the intraperitoneal and pelvic abscesses, and generous peritoneal lavage. Intraoperatively, the cecum was found firmly adherent to the anterior abdominal peritoneum in the right inguinal region. With gentle traction of the cecum, the appendix was extracted from the inguinal hernial sac through the internal ring; approximately 15 mL of greenish, fecal-smelling pus were extracted as well.\nThe appendix was severely inflamed, gangrenous, and perforated in many locations. Appendectomy was performed and the peritoneal cavity was irrigated with copious amounts of warm normal saline solution. Hernioplasty was not performed as the presence of pus or perforation of the appendix is an absolute contraindication to the placement of a mesh for hernia repair because this greatly increases the chances of postoperative surgical site infection.[7] A rectal tube was introduced at the conclusion of surgery to facilitate closure of the abdominal incision and was removed shortly after the patient's recovery from anesthesia.\nThe patient's postoperative course was without incident. Bowel movements were present starting on the second postoperative day and the patient gradually began tolerating oral feeding. Postoperative investigations revealed a consistent improvement of all major systems. The patient was discharged on the eighth postoperative day in good overall condition.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 387329,
"choiceText": "The diagnosis is most often made intraoperatively",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 387330,
"choiceText": "Most cases are associated with appendicitis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 387331,
"choiceText": "CT scans do not aid in either the diagnosis or management",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 387332,
"choiceText": "T1-weighted MRI is the diagnostic investigation of choice",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 387333,
"choiceText": "It often reduces spontaneously and is therefore best managed with watchful waiting",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The diagnosis of Amyand hernia is most often made intraoperatively. Appendicitis is present in only a small fraction of all Amyand hernia cases. CT scans are most useful in identifying the presence of any coexisting conditions and are only rarely capable of making an exact preoperative diagnosis. Amyand hernias are usually incarcerated at presentation and not amenable to watchful waiting.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 112232,
"questionText": "You are treating an elderly man who is critically ill and has peritoneal signs on abdominal examination. A surgeon is called, and intravenous antibiotics and fluids are started. The patient is stabilized and undergoes an abdominal CT scan. The combination of his presentation and the images raises concern for a possible Amyand hernia. Which of the following statements regarding Amyand hernia is most accurate?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 387334,
"choiceText": "Difficulty during reduction of the appendix has no bearing on management",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 387335,
"choiceText": "Necrosis of the appendix is not an indication for appendectomy",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 387336,
"choiceText": "The patient's age or current medical condition does not change surgical management",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 387337,
"choiceText": "Appendectomy is indicated in both type I and type III Amyand hernias",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 387338,
"choiceText": "Hernioplasty is contraindicated if the appendix is perforated",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Hernioplasty is contraindicated if the appendix is perforated. Mesh placement in these patients greatly increases the chances of surgical site infection. These patients are therefore best managed with repair using only endogenous tissue. A difficult reduction of the appendix can cause traumatic injury to the appendix, thereby increasing the chances of postoperative appendicitis. Necrosis of the appendix is an absolute indication for appendectomy. Another consideration when debating appendectomy is the patient's lifelong risk of developing acute appendicitis. Pediatric or adolescent patients are much more likely to suffer from acute appendicitis compared with middle-aged or elderly patients in whom the appendix can more safely be retained.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 112233,
"questionText": "The above patient goes to the operating room and has an incarcerated inguinal hernia with involvement of the appendix (Amyand hernia). Which of the following statements regarding the management of this patient's Amyand hernia is most accurate?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 70-Year-Old Man With Deteriorating Mental Status and Pain"
},
{
"authors": "Gamal A. Bebars, MD, FRCS; Evelyn P. Bebars, MD, FPCR",
"content": [],
"date": "October 23, 2018",
"figures": [],
"markdown": "# A 70-Year-Old Man With Deteriorating Mental Status and Pain\n\n **Authors:** Gamal A. Bebars, MD, FRCS; Evelyn P. Bebars, MD, FPCR \n **Date:** October 23, 2018\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 387329,
"choiceText": "The diagnosis is most often made intraoperatively",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 387330,
"choiceText": "Most cases are associated with appendicitis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 387331,
"choiceText": "CT scans do not aid in either the diagnosis or management",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 387332,
"choiceText": "T1-weighted MRI is the diagnostic investigation of choice",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 387333,
"choiceText": "It often reduces spontaneously and is therefore best managed with watchful waiting",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The diagnosis of Amyand hernia is most often made intraoperatively. Appendicitis is present in only a small fraction of all Amyand hernia cases. CT scans are most useful in identifying the presence of any coexisting conditions and are only rarely capable of making an exact preoperative diagnosis. Amyand hernias are usually incarcerated at presentation and not amenable to watchful waiting.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 112232,
"questionText": "You are treating an elderly man who is critically ill and has peritoneal signs on abdominal examination. A surgeon is called, and intravenous antibiotics and fluids are started. The patient is stabilized and undergoes an abdominal CT scan. The combination of his presentation and the images raises concern for a possible Amyand hernia. Which of the following statements regarding Amyand hernia is most accurate?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 387334,
"choiceText": "Difficulty during reduction of the appendix has no bearing on management",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 387335,
"choiceText": "Necrosis of the appendix is not an indication for appendectomy",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 387336,
"choiceText": "The patient's age or current medical condition does not change surgical management",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 387337,
"choiceText": "Appendectomy is indicated in both type I and type III Amyand hernias",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 387338,
"choiceText": "Hernioplasty is contraindicated if the appendix is perforated",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Hernioplasty is contraindicated if the appendix is perforated. Mesh placement in these patients greatly increases the chances of surgical site infection. These patients are therefore best managed with repair using only endogenous tissue. A difficult reduction of the appendix can cause traumatic injury to the appendix, thereby increasing the chances of postoperative appendicitis. Necrosis of the appendix is an absolute indication for appendectomy. Another consideration when debating appendectomy is the patient's lifelong risk of developing acute appendicitis. Pediatric or adolescent patients are much more likely to suffer from acute appendicitis compared with middle-aged or elderly patients in whom the appendix can more safely be retained.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 112233,
"questionText": "The above patient goes to the operating room and has an incarcerated inguinal hernia with involvement of the appendix (Amyand hernia). Which of the following statements regarding the management of this patient's Amyand hernia is most accurate?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 70-Year-Old Man With Deteriorating Mental Status and Pain"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 387325,
"choiceText": "Small bowel ischemia with peritonitis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 387326,
"choiceText": "Diaphragmatic hernia",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 387327,
"choiceText": "Sigmoid volvulus",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 387328,
"choiceText": "Complicated inguinal hernia with the appendix inside the hernial sac (Amyand hernia)",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 112231,
"questionText": "Which of the following is the most likely diagnosis?<br /><br /><i>Hint: Review the findings of the CT scan.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 387329,
"choiceText": "The diagnosis is most often made intraoperatively",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 387330,
"choiceText": "Most cases are associated with appendicitis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 387331,
"choiceText": "CT scans do not aid in either the diagnosis or management",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 387332,
"choiceText": "T1-weighted MRI is the diagnostic investigation of choice",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 387333,
"choiceText": "It often reduces spontaneously and is therefore best managed with watchful waiting",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The diagnosis of Amyand hernia is most often made intraoperatively. Appendicitis is present in only a small fraction of all Amyand hernia cases. CT scans are most useful in identifying the presence of any coexisting conditions and are only rarely capable of making an exact preoperative diagnosis. Amyand hernias are usually incarcerated at presentation and not amenable to watchful waiting.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 112232,
"questionText": "You are treating an elderly man who is critically ill and has peritoneal signs on abdominal examination. A surgeon is called, and intravenous antibiotics and fluids are started. The patient is stabilized and undergoes an abdominal CT scan. The combination of his presentation and the images raises concern for a possible Amyand hernia. Which of the following statements regarding Amyand hernia is most accurate?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 387334,
"choiceText": "Difficulty during reduction of the appendix has no bearing on management",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 387335,
"choiceText": "Necrosis of the appendix is not an indication for appendectomy",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 387336,
"choiceText": "The patient's age or current medical condition does not change surgical management",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 387337,
"choiceText": "Appendectomy is indicated in both type I and type III Amyand hernias",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 387338,
"choiceText": "Hernioplasty is contraindicated if the appendix is perforated",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Hernioplasty is contraindicated if the appendix is perforated. Mesh placement in these patients greatly increases the chances of surgical site infection. These patients are therefore best managed with repair using only endogenous tissue. A difficult reduction of the appendix can cause traumatic injury to the appendix, thereby increasing the chances of postoperative appendicitis. Necrosis of the appendix is an absolute indication for appendectomy. Another consideration when debating appendectomy is the patient's lifelong risk of developing acute appendicitis. Pediatric or adolescent patients are much more likely to suffer from acute appendicitis compared with middle-aged or elderly patients in whom the appendix can more safely be retained.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 112233,
"questionText": "The above patient goes to the operating room and has an incarcerated inguinal hernia with involvement of the appendix (Amyand hernia). Which of the following statements regarding the management of this patient's Amyand hernia is most accurate?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
733326 | /viewarticle/733326 | [
{
"authors": "Zoe J. Oliver, MD, CCFP",
"content": [
"Editor's Note:\n\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.",
"A 73-year-old man presents to the emergency department with a 3-day history of mild headache and somnolence. His wife notes that he has periods when he seems \"off,\" that he has been napping frequently, and that he has had a poor appetite. They deny any history of trauma, seizures, or focal neurologic deficits.",
"Symptomatic review of the cardiopulmonary, gastrointestinal, and genitourinary systems is unremarkable. The patient drinks 5-6 ounces of hard liquor per day, but he does not smoke or use illicit drugs. He has not travelled recently, and no changes in his daily routine are reported. He takes a baby aspirin daily for unknown reasons but no other medications. He denies allergies or any other medical conditions. He has never had major surgery."
],
"date": "March 15, 2016",
"figures": [],
"markdown": "# Something Is \"Off\" In This Elderly Man\n\n **Authors:** Zoe J. Oliver, MD, CCFP \n **Date:** March 15, 2016\n\n ## Content\n\n Editor's Note:\n\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.\nA 73-year-old man presents to the emergency department with a 3-day history of mild headache and somnolence. His wife notes that he has periods when he seems \"off,\" that he has been napping frequently, and that he has had a poor appetite. They deny any history of trauma, seizures, or focal neurologic deficits.\nSymptomatic review of the cardiopulmonary, gastrointestinal, and genitourinary systems is unremarkable. The patient drinks 5-6 ounces of hard liquor per day, but he does not smoke or use illicit drugs. He has not travelled recently, and no changes in his daily routine are reported. He takes a baby aspirin daily for unknown reasons but no other medications. He denies allergies or any other medical conditions. He has never had major surgery.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "Something Is \"Off\" In This Elderly Man"
},
{
"authors": "Zoe J. Oliver, MD, CCFP",
"content": [
"Figure 1.",
"On physical examination, the patient appears well. Despite the fact that the patient is slightly vague and tangential in answering questions, his Glasgow Coma Scale score is 15. His blood pressure is 180/90 mm Hg, and his heart rate is 80 bpm and regular. The respiratory rate is 16 breaths/min. He is afebrile, with a recorded temperature of 98.1°F (36.7°C). His heart sounds are normal, and he is euvolemic. The lungs are clear on auscultation, and his abdomen is soft, nontender, and without any masses.",
"A detailed cranial nerve examination demonstrates no abnormalities, and funduscopy is normal. The patient's neck is supple, and his cerebellar function tests are normal. He lacks any pronator drift and has a negative Romberg sign. Motor and sensory examination of all 4 limbs is normal; however, he is slightly hyperreflexic at his biceps and patellar tendons.",
"A workup of the patient's altered mental status is initiated. A complete blood cell count, chest radiography, liver enzymes examination, thyroid function test, electrocardiogram, and urinalysis findings are all normal. Electrolytes levels are within the reference range except for a sodium of 129 mEq/L (12 mmol/L).",
"CT scan of the head is performed (see Figure 1). The brain is asymmetric, and a suspected lesion is noted on the right side. Signs of increased intracranial pressure (ICP), including effacement of the right-sided ventricles, obliteration of the right-sided sulci, and midline shift, are apparent. The brain parenchyma on the left is noted to be atrophic, with prominent sulci. The right-sided lesion is hypodense-to-isodense compared with the surrounding parenchyma."
],
"date": "March 15, 2016",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/732/960/732960-thumb1.png"
}
],
"markdown": "# Something Is \"Off\" In This Elderly Man\n\n **Authors:** Zoe J. Oliver, MD, CCFP \n **Date:** March 15, 2016\n\n ## Content\n\n Figure 1.\nOn physical examination, the patient appears well. Despite the fact that the patient is slightly vague and tangential in answering questions, his Glasgow Coma Scale score is 15. His blood pressure is 180/90 mm Hg, and his heart rate is 80 bpm and regular. The respiratory rate is 16 breaths/min. He is afebrile, with a recorded temperature of 98.1°F (36.7°C). His heart sounds are normal, and he is euvolemic. The lungs are clear on auscultation, and his abdomen is soft, nontender, and without any masses.\nA detailed cranial nerve examination demonstrates no abnormalities, and funduscopy is normal. The patient's neck is supple, and his cerebellar function tests are normal. He lacks any pronator drift and has a negative Romberg sign. Motor and sensory examination of all 4 limbs is normal; however, he is slightly hyperreflexic at his biceps and patellar tendons.\nA workup of the patient's altered mental status is initiated. A complete blood cell count, chest radiography, liver enzymes examination, thyroid function test, electrocardiogram, and urinalysis findings are all normal. Electrolytes levels are within the reference range except for a sodium of 129 mEq/L (12 mmol/L).\nCT scan of the head is performed (see Figure 1). The brain is asymmetric, and a suspected lesion is noted on the right side. Signs of increased intracranial pressure (ICP), including effacement of the right-sided ventricles, obliteration of the right-sided sulci, and midline shift, are apparent. The brain parenchyma on the left is noted to be atrophic, with prominent sulci. The right-sided lesion is hypodense-to-isodense compared with the surrounding parenchyma.\n\n ## Figures\n\n **Figure 1.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 378708,
"choiceText": "Epidural hematoma",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 378709,
"choiceText": "Acute subdural hematoma",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 378710,
"choiceText": "Subacute/chronic subdural hematoma",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 378711,
"choiceText": "Intracranial tumor",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 378712,
"choiceText": "Subarachnoid hemorrhage",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 109228,
"questionText": "What is the diagnosis?<br><br><i>Hint: Consider the density of the right-sided lesion seen on the CT scan.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Something Is \"Off\" In This Elderly Man"
},
{
"authors": "Zoe J. Oliver, MD, CCFP",
"content": [
"Figure 1.",
"This case of subacute/chronic subdural hematoma underscores the seriousness of even subtle neurologic changes in the elderly. A CT scan was ordered due to the subjective confusion reported by the patient, in the context of advanced age and significant alcohol consumption. As with this patient, a history of trauma is lacking in 30%-50% of cases of chronic subdural hematoma.[1] In this patient, signs of midline shift were not subtle, but the density of the clot was very similar to the brain parenchyma. Had the lesion been too small to cause the shift, it could have been easily missed on CT scans without contrast.",
"Subdural hematomas are extra-axial blood collections between the dura mater and the brain parenchyma. Epidural hematomas are usually ipsilateral to the side of the trauma, while subdural hematomas are typically on the contralateral side. Subdural hematomas form when bridging vessels are damaged by shear forces during an acceleration-deceleration of the head, causing the initial impact coup and secondary contra-coup injury. Patients with atrophic brains are more prone to subdural hematomas as the bridging vessels are of greater length and more susceptible to shear. As a result of the slow, low-pressure venous oozing typical in these injuries, subdural hematomas can develop slowly, and presentation can be delayed by days to weeks. This is particularly true in patients with increased brain atrophy, whose craniums can more readily accommodate additional intracranial blood volume with minimal neurologic sequelae."
],
"date": "March 15, 2016",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/732/960/732960-thumb1.png"
}
],
"markdown": "# Something Is \"Off\" In This Elderly Man\n\n **Authors:** Zoe J. Oliver, MD, CCFP \n **Date:** March 15, 2016\n\n ## Content\n\n Figure 1.\nThis case of subacute/chronic subdural hematoma underscores the seriousness of even subtle neurologic changes in the elderly. A CT scan was ordered due to the subjective confusion reported by the patient, in the context of advanced age and significant alcohol consumption. As with this patient, a history of trauma is lacking in 30%-50% of cases of chronic subdural hematoma.[1] In this patient, signs of midline shift were not subtle, but the density of the clot was very similar to the brain parenchyma. Had the lesion been too small to cause the shift, it could have been easily missed on CT scans without contrast.\nSubdural hematomas are extra-axial blood collections between the dura mater and the brain parenchyma. Epidural hematomas are usually ipsilateral to the side of the trauma, while subdural hematomas are typically on the contralateral side. Subdural hematomas form when bridging vessels are damaged by shear forces during an acceleration-deceleration of the head, causing the initial impact coup and secondary contra-coup injury. Patients with atrophic brains are more prone to subdural hematomas as the bridging vessels are of greater length and more susceptible to shear. As a result of the slow, low-pressure venous oozing typical in these injuries, subdural hematomas can develop slowly, and presentation can be delayed by days to weeks. This is particularly true in patients with increased brain atrophy, whose craniums can more readily accommodate additional intracranial blood volume with minimal neurologic sequelae.\n\n ## Figures\n\n **Figure 1.** \n \n\n\n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 378708,
"choiceText": "Epidural hematoma",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 378709,
"choiceText": "Acute subdural hematoma",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 378710,
"choiceText": "Subacute/chronic subdural hematoma",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 378711,
"choiceText": "Intracranial tumor",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 378712,
"choiceText": "Subarachnoid hemorrhage",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 109228,
"questionText": "What is the diagnosis?<br><br><i>Hint: Consider the density of the right-sided lesion seen on the CT scan.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Something Is \"Off\" In This Elderly Man"
},
{
"authors": "Zoe J. Oliver, MD, CCFP",
"content": [
"Figure 1.",
"Subdural hematomas may present with many different clinical pictures. Rapid accumulation of extra-axial blood, nonatrophic brain parenchyma, and the presence of other traumatic brain injuries correspond to a worsened neurologic status on presentation.[2] Neurologic deficits can result from increased ICP, sequelae from a herniation syndrome, direct pressure from the clot, or other associated injuries.",
"Chronic subdural hematomas present more often in the elderly, with the incidence in one series estimated as being 7.35 cases out of 100,000 patients in the 70-79 year age group.[3] The most common presentation of chronic subdural hematoma in the elderly is altered mental status, but hemiparesis is also a common finding.[1]\nHeadache and a history of trauma, especially a minor head injury, are other common features on presentation.[4] Another series reported that 24% of elderly patients with chronic subdural hematomas were taking anticoagulants or antiplatelet agents.[5] Other risk factors include male gender and presence of hydrocephalus.",
"Acute subdural hematomas are more common in younger patients, and a history of significant trauma is often noted in those cases.[6] Because a younger brain is rarely atrophic, even small volumes of extra-axial blood can increase the ICP and result in severe neurologic deficits. Patients with an acute subdural hematoma and severe neurologic deficits also have a worse outcome when compared with other types of hematomas. In a study of patients with fixed and dilated pupils, those patients with an acute subdural hematoma suffered a mortality of 64%, whereas those with epidural hematomas only had an 18% mortality rate.[7]",
"In the pediatric population, birth trauma can be a cause of subdural hematoma; however, the presence of acute or chronic subdural hematoma should also raise suspicion for child abuse.[8] Physicians should search for other signs of \"shaken baby syndrome,\" including retinal hemorrhage, rib fracture, and long bone fracture. Infants with increased ICP might present with a bulging fontanel, enlarged head circumference, emesis, failure to thrive, and seizure.",
"Up to 6% of subdural hematomas present with a seizure, usually when the lesion is large. Transient neurologic deficits occur in 1%-12% of patients, and cranial nerve palsies and nystagmus have also been described.[9] Gerstmann syndrome (right-left disorientation, finger agnosia, agraphia, and acalculia) has also been reported in the clinical presentation of subdural hematoma.[10]",
"The clinician must generate a broad differential diagnosis for any elderly patient presenting with weakness or confusion because chronic subdural hematoma can mimic many other disease entities. As such, CT scanning of the brain should be considered in any elderly patient with a change in mentation. Subdural hematoma might initially be easily mistaken for other neurologic injuries, such as ischemic stroke, epidural hematoma, tumor, or subarachnoid hemorrhage.",
"CT scanning is the mainstay of diagnosis, and CT findings vary depending on the age of the lesion. In the acute phase (days 0-7), the lesion appears hyperdense relative to the parenchyma. The clot is fresh and jelly-like.[2] Subacutely, between days 7 and 21, the lesion is isodense or hypodense as a result of fibrinolysis occurring within the hematoma.[11] Intravenous contrast may be helpful in illuminating isodense lesions.[11] Chronic subdural hematomas (> 2 weeks) are usually hypodense and extend past suture lines.[11] Typically, acute subdural hematomas are concave in shape, as opposed to epidural hematomas, which are convex; however, in a small number of patients, particularly those with a past history of brain surgery, subdural hematomas may appear convex due to intracranial adhesions.[12]",
"As a subdural hematoma grows, mass effect becomes evident. CT findings may include midline shift, effacement of the sulci, obliteration of the basal cisterns, and ventricular compression.[2] Bilateral hematomas are fairly common, leading to medial compression of both ventricles and causing them to appear slit-like. This finding is called \"squeezed ventricle\" or the \"rabbit's ears\" sign.[1] Small isodense hematomas at the vertex, the base of the skull, and in the posterior fossa can be particularly difficult to visualize. In these cases, MRI can be of assistance in making the diagnosis.[11]"
],
"date": "March 15, 2016",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/732/960/732960-thumb1.png"
}
],
"markdown": "# Something Is \"Off\" In This Elderly Man\n\n **Authors:** Zoe J. Oliver, MD, CCFP \n **Date:** March 15, 2016\n\n ## Content\n\n Figure 1.\nSubdural hematomas may present with many different clinical pictures. Rapid accumulation of extra-axial blood, nonatrophic brain parenchyma, and the presence of other traumatic brain injuries correspond to a worsened neurologic status on presentation.[2] Neurologic deficits can result from increased ICP, sequelae from a herniation syndrome, direct pressure from the clot, or other associated injuries.\nChronic subdural hematomas present more often in the elderly, with the incidence in one series estimated as being 7.35 cases out of 100,000 patients in the 70-79 year age group.[3] The most common presentation of chronic subdural hematoma in the elderly is altered mental status, but hemiparesis is also a common finding.[1]\nHeadache and a history of trauma, especially a minor head injury, are other common features on presentation.[4] Another series reported that 24% of elderly patients with chronic subdural hematomas were taking anticoagulants or antiplatelet agents.[5] Other risk factors include male gender and presence of hydrocephalus.\nAcute subdural hematomas are more common in younger patients, and a history of significant trauma is often noted in those cases.[6] Because a younger brain is rarely atrophic, even small volumes of extra-axial blood can increase the ICP and result in severe neurologic deficits. Patients with an acute subdural hematoma and severe neurologic deficits also have a worse outcome when compared with other types of hematomas. In a study of patients with fixed and dilated pupils, those patients with an acute subdural hematoma suffered a mortality of 64%, whereas those with epidural hematomas only had an 18% mortality rate.[7]\nIn the pediatric population, birth trauma can be a cause of subdural hematoma; however, the presence of acute or chronic subdural hematoma should also raise suspicion for child abuse.[8] Physicians should search for other signs of \"shaken baby syndrome,\" including retinal hemorrhage, rib fracture, and long bone fracture. Infants with increased ICP might present with a bulging fontanel, enlarged head circumference, emesis, failure to thrive, and seizure.\nUp to 6% of subdural hematomas present with a seizure, usually when the lesion is large. Transient neurologic deficits occur in 1%-12% of patients, and cranial nerve palsies and nystagmus have also been described.[9] Gerstmann syndrome (right-left disorientation, finger agnosia, agraphia, and acalculia) has also been reported in the clinical presentation of subdural hematoma.[10]\nThe clinician must generate a broad differential diagnosis for any elderly patient presenting with weakness or confusion because chronic subdural hematoma can mimic many other disease entities. As such, CT scanning of the brain should be considered in any elderly patient with a change in mentation. Subdural hematoma might initially be easily mistaken for other neurologic injuries, such as ischemic stroke, epidural hematoma, tumor, or subarachnoid hemorrhage.\nCT scanning is the mainstay of diagnosis, and CT findings vary depending on the age of the lesion. In the acute phase (days 0-7), the lesion appears hyperdense relative to the parenchyma. The clot is fresh and jelly-like.[2] Subacutely, between days 7 and 21, the lesion is isodense or hypodense as a result of fibrinolysis occurring within the hematoma.[11] Intravenous contrast may be helpful in illuminating isodense lesions.[11] Chronic subdural hematomas (> 2 weeks) are usually hypodense and extend past suture lines.[11] Typically, acute subdural hematomas are concave in shape, as opposed to epidural hematomas, which are convex; however, in a small number of patients, particularly those with a past history of brain surgery, subdural hematomas may appear convex due to intracranial adhesions.[12]\nAs a subdural hematoma grows, mass effect becomes evident. CT findings may include midline shift, effacement of the sulci, obliteration of the basal cisterns, and ventricular compression.[2] Bilateral hematomas are fairly common, leading to medial compression of both ventricles and causing them to appear slit-like. This finding is called \"squeezed ventricle\" or the \"rabbit's ears\" sign.[1] Small isodense hematomas at the vertex, the base of the skull, and in the posterior fossa can be particularly difficult to visualize. In these cases, MRI can be of assistance in making the diagnosis.[11]\n\n ## Figures\n\n **Figure 1.** \n \n\n\n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "Something Is \"Off\" In This Elderly Man"
},
{
"authors": "Zoe J. Oliver, MD, CCFP",
"content": [
"Clinically significant subdural hematomas are treated surgically, although cases of spontaneous resorption during observation have been reported.[13] The current consensus is that subdural hematomas >1 cm or with a midline shift of >5 mm should be surgically drained, regardless of the Glasgow Coma Scale score.[6] Small hematomas (a few millimeters thick at maximum width) can be closely monitored if asymptomatic, but the CT scan should be repeated as soon as any signs of clinical deterioration appear.[2,14]",
"Surgical therapy involves drainage by twist drill/burr hole craniostomy, craniotomy, and subduroperitoneal shunt placement.[2] Craniotomy is often used for patients with reaccumulated subdural hematomas, hematomas with membranes, or solid hematomas.[15] The most common postoperative complication in chronic subdural hematomas is hematoma reaccumulation.[4] Although some residual fluid is seen in up to 80% of patients, only 8%-37% of patients are symptomatic.[1]\nIntracerebral hemorrhage is an uncommon but deadly complication occurring after evacuation of chronic subdural hematomas. Hyperemia in the rapidly re-expanding brain might be the cause of such postoperative bleeding.[16]Seizures and tension pneumocephalus are also rare complications.[4]",
"Mortality after subdural hematomas is determined by numerous factors, including age, presenting neurologic condition, presence of a lucid interval, midline shift, and promptness of surgical intervention.[1] Although mortality above the age of 80 years has been cited as 100%, a recent case of successful evacuation of acute subdural hematomas in a 102-year-old woman was reported.[17]",
"The patient in this case was successfully treated with burr-hole evacuation of the hematoma, with return to his baseline neurologic status. He suffered no postoperative complications and was discharged to home 4 days later."
],
"date": "March 15, 2016",
"figures": [],
"markdown": "# Something Is \"Off\" In This Elderly Man\n\n **Authors:** Zoe J. Oliver, MD, CCFP \n **Date:** March 15, 2016\n\n ## Content\n\n Clinically significant subdural hematomas are treated surgically, although cases of spontaneous resorption during observation have been reported.[13] The current consensus is that subdural hematomas >1 cm or with a midline shift of >5 mm should be surgically drained, regardless of the Glasgow Coma Scale score.[6] Small hematomas (a few millimeters thick at maximum width) can be closely monitored if asymptomatic, but the CT scan should be repeated as soon as any signs of clinical deterioration appear.[2,14]\nSurgical therapy involves drainage by twist drill/burr hole craniostomy, craniotomy, and subduroperitoneal shunt placement.[2] Craniotomy is often used for patients with reaccumulated subdural hematomas, hematomas with membranes, or solid hematomas.[15] The most common postoperative complication in chronic subdural hematomas is hematoma reaccumulation.[4] Although some residual fluid is seen in up to 80% of patients, only 8%-37% of patients are symptomatic.[1]\nIntracerebral hemorrhage is an uncommon but deadly complication occurring after evacuation of chronic subdural hematomas. Hyperemia in the rapidly re-expanding brain might be the cause of such postoperative bleeding.[16]Seizures and tension pneumocephalus are also rare complications.[4]\nMortality after subdural hematomas is determined by numerous factors, including age, presenting neurologic condition, presence of a lucid interval, midline shift, and promptness of surgical intervention.[1] Although mortality above the age of 80 years has been cited as 100%, a recent case of successful evacuation of acute subdural hematomas in a 102-year-old woman was reported.[17]\nThe patient in this case was successfully treated with burr-hole evacuation of the hematoma, with return to his baseline neurologic status. He suffered no postoperative complications and was discharged to home 4 days later.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 378713,
"choiceText": "Acute subdural hematoma",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 378714,
"choiceText": "Acute epidural hematoma",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 378715,
"choiceText": "Chronic subdural hematoma",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 378716,
"choiceText": "Subarachnoid hemorrhage",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 378717,
"choiceText": "Acute, subacute, and chronic subdural hematoma",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The presence of varying densities within the hematoma indicates that the chronic hematoma has now been complicated by an acute bleed. Hypodense fluid making up the chronic hematoma is seen nearest the brain, while a small amount of hyperdense acute bleeding is noted next to the cranium. An isodense ring on the outside of the right hemisphere represents a subacute hematoma.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 109229,
"questionText": "You are examining an 84-year-old woman in the ED with a dense left-sided hemiparesis. A noncontrast CT scan of the head is obtained, which shows a 1.5-cm right-sided fluid collection. The fluid is primarily hypodense near the brain, hyperdense near the cranium, and an isodense ring is noted outside of the right hemisphere. Given this limited, preliminary description, which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 378722,
"choiceText": "Younger patients with this condition usually do better",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 378723,
"choiceText": "Even with the most severe of neurologic deficits, patients with a subdural hematoma have a better outcome than those with an epidural hematoma",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 378724,
"choiceText": "Prompt surgical drainage improves outcome for hematomas of this size",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 378725,
"choiceText": "Subdural hematomas are always fatal in patients greater than 75 years old.",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Rapid clot expansion next to a nonatrophic parenchyma will cause a significant increase in intracranial pressure, which offsets the other advantages of youth in patients with subdural hematoma. While many elderly patients have a poor prognosis, there are reported cases of very elderly individuals returning to their neurologic baseline after surgical intervention. With the most severe neurologic deficit (fixed and dilated pupils), patients with subdural hematoma have a worse prognosis than those with an epidural hematoma. Prompt surgical drainage improves outcome, particularly when the patient is symptomatic, and in patients wherein the hematoma is > 1 cm or causes a 5-mm midline shift.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 109231,
"questionText": "Your medical student states the patient's family is concerned about her prognosis. Which statement regarding patient outcome in subdural hematoma is true?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Something Is \"Off\" In This Elderly Man"
},
{
"authors": "Zoe J. Oliver, MD, CCFP",
"content": [],
"date": "March 15, 2016",
"figures": [],
"markdown": "# Something Is \"Off\" In This Elderly Man\n\n **Authors:** Zoe J. Oliver, MD, CCFP \n **Date:** March 15, 2016\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 378713,
"choiceText": "Acute subdural hematoma",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 378714,
"choiceText": "Acute epidural hematoma",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 378715,
"choiceText": "Chronic subdural hematoma",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 378716,
"choiceText": "Subarachnoid hemorrhage",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 378717,
"choiceText": "Acute, subacute, and chronic subdural hematoma",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The presence of varying densities within the hematoma indicates that the chronic hematoma has now been complicated by an acute bleed. Hypodense fluid making up the chronic hematoma is seen nearest the brain, while a small amount of hyperdense acute bleeding is noted next to the cranium. An isodense ring on the outside of the right hemisphere represents a subacute hematoma.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 109229,
"questionText": "You are examining an 84-year-old woman in the ED with a dense left-sided hemiparesis. A noncontrast CT scan of the head is obtained, which shows a 1.5-cm right-sided fluid collection. The fluid is primarily hypodense near the brain, hyperdense near the cranium, and an isodense ring is noted outside of the right hemisphere. Given this limited, preliminary description, which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 378722,
"choiceText": "Younger patients with this condition usually do better",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 378723,
"choiceText": "Even with the most severe of neurologic deficits, patients with a subdural hematoma have a better outcome than those with an epidural hematoma",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 378724,
"choiceText": "Prompt surgical drainage improves outcome for hematomas of this size",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 378725,
"choiceText": "Subdural hematomas are always fatal in patients greater than 75 years old.",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Rapid clot expansion next to a nonatrophic parenchyma will cause a significant increase in intracranial pressure, which offsets the other advantages of youth in patients with subdural hematoma. While many elderly patients have a poor prognosis, there are reported cases of very elderly individuals returning to their neurologic baseline after surgical intervention. With the most severe neurologic deficit (fixed and dilated pupils), patients with subdural hematoma have a worse prognosis than those with an epidural hematoma. Prompt surgical drainage improves outcome, particularly when the patient is symptomatic, and in patients wherein the hematoma is > 1 cm or causes a 5-mm midline shift.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 109231,
"questionText": "Your medical student states the patient's family is concerned about her prognosis. Which statement regarding patient outcome in subdural hematoma is true?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Something Is \"Off\" In This Elderly Man"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 378708,
"choiceText": "Epidural hematoma",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 378709,
"choiceText": "Acute subdural hematoma",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 378710,
"choiceText": "Subacute/chronic subdural hematoma",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 378711,
"choiceText": "Intracranial tumor",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 378712,
"choiceText": "Subarachnoid hemorrhage",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 109228,
"questionText": "What is the diagnosis?<br><br><i>Hint: Consider the density of the right-sided lesion seen on the CT scan.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 378713,
"choiceText": "Acute subdural hematoma",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 378714,
"choiceText": "Acute epidural hematoma",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 378715,
"choiceText": "Chronic subdural hematoma",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 378716,
"choiceText": "Subarachnoid hemorrhage",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 378717,
"choiceText": "Acute, subacute, and chronic subdural hematoma",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The presence of varying densities within the hematoma indicates that the chronic hematoma has now been complicated by an acute bleed. Hypodense fluid making up the chronic hematoma is seen nearest the brain, while a small amount of hyperdense acute bleeding is noted next to the cranium. An isodense ring on the outside of the right hemisphere represents a subacute hematoma.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 109229,
"questionText": "You are examining an 84-year-old woman in the ED with a dense left-sided hemiparesis. A noncontrast CT scan of the head is obtained, which shows a 1.5-cm right-sided fluid collection. The fluid is primarily hypodense near the brain, hyperdense near the cranium, and an isodense ring is noted outside of the right hemisphere. Given this limited, preliminary description, which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 378722,
"choiceText": "Younger patients with this condition usually do better",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 378723,
"choiceText": "Even with the most severe of neurologic deficits, patients with a subdural hematoma have a better outcome than those with an epidural hematoma",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 378724,
"choiceText": "Prompt surgical drainage improves outcome for hematomas of this size",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 378725,
"choiceText": "Subdural hematomas are always fatal in patients greater than 75 years old.",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Rapid clot expansion next to a nonatrophic parenchyma will cause a significant increase in intracranial pressure, which offsets the other advantages of youth in patients with subdural hematoma. While many elderly patients have a poor prognosis, there are reported cases of very elderly individuals returning to their neurologic baseline after surgical intervention. With the most severe neurologic deficit (fixed and dilated pupils), patients with subdural hematoma have a worse prognosis than those with an epidural hematoma. Prompt surgical drainage improves outcome, particularly when the patient is symptomatic, and in patients wherein the hematoma is > 1 cm or causes a 5-mm midline shift.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 109231,
"questionText": "Your medical student states the patient's family is concerned about her prognosis. Which statement regarding patient outcome in subdural hematoma is true?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
732114 | /viewarticle/732114 | [
{
"authors": "Craig A Goolsby, MD",
"content": [
"Editor's Note:\n\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.",
"Figure 1.",
"A clinic refers a 36-year-old African man to the emergency department (ED) for vomiting. He has had episodes of vomiting for about a month, and the emesis occurs both when eating food and between meals. He often vomits more than 10 times daily. His vomitus is nonbloody and nonbilious. The patient's vomiting is accompanied by a constant, mild epigastric pain, with some radiation to his right upper quadrant. He characterizes the pain as a mild ache. There has been no associated chest pain or shortness of breath. The pain does not change with exertion. He has not had any diarrhea, fever, or night sweats and, other than during the past month, he has not suffered from similar symptoms. He denies experiencing any trauma. During the past several months, he has noticed an unintentional weight loss of approximately 20 lb. He denies having any prior medical problems, past surgical history, or taking any medications. The patient works as a security guard. He has not recently traveled to Africa or any other foreign destinations, and today is the first time he has sought treatment for his condition."
],
"date": "February 10, 2016",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/711/354/711354-thumb1.png"
}
],
"markdown": "# The Month of Frequent Vomiting\n\n **Authors:** Craig A Goolsby, MD \n **Date:** February 10, 2016\n\n ## Content\n\n Editor's Note:\n\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.\nFigure 1.\nA clinic refers a 36-year-old African man to the emergency department (ED) for vomiting. He has had episodes of vomiting for about a month, and the emesis occurs both when eating food and between meals. He often vomits more than 10 times daily. His vomitus is nonbloody and nonbilious. The patient's vomiting is accompanied by a constant, mild epigastric pain, with some radiation to his right upper quadrant. He characterizes the pain as a mild ache. There has been no associated chest pain or shortness of breath. The pain does not change with exertion. He has not had any diarrhea, fever, or night sweats and, other than during the past month, he has not suffered from similar symptoms. He denies experiencing any trauma. During the past several months, he has noticed an unintentional weight loss of approximately 20 lb. He denies having any prior medical problems, past surgical history, or taking any medications. The patient works as a security guard. He has not recently traveled to Africa or any other foreign destinations, and today is the first time he has sought treatment for his condition.\n\n ## Figures\n\n **Figure 1.** \n \n\n\n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "The Month of Frequent Vomiting"
},
{
"authors": "Craig A Goolsby, MD",
"content": [
"Figure 1.",
"On physical examination, the patient has an oral temperature of 99.4°F (37.4°C). His heart has a regular rhythm with a rate of 110 bpm, his blood pressure is 106/72 mm Hg, and his respiratory rate is 16 breaths/min. He has a pulse oximetry reading of 99% while breathing room air. In general, the patient appears chronically ill; however, he is in no acute distress and is appropriately alert and oriented. He does appear dehydrated. He is noted to have cachexia, with temporal wasting and dry mucous membranes. Clear lung sounds are found bilaterally. His abdomen is mildly tender at the epigastrium, without any peritoneal signs. His extremities are well-perfused, and he has no rash. No focal neurologic deficits are found.",
"An intravenous (IV) line is placed, and fluid resuscitation is started with a liter of normal saline. The patient is given 4 mg of ondansetron for his nausea and vomiting, and he declines pain medications. Laboratory studies, including electrolytes, transaminases, total bilirubin, lipase, and a complete blood cell count (CBC) are ordered and sent. Radiography of the chest is performed and is normal. An abdominal computed tomography (CT) scan shows a large gastric mass causing gastric outlet obstruction (not shown). An electrocardiogram (ECG) is ordered (see Figure 1)."
],
"date": "February 10, 2016",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/711/354/711354-thumb1.png"
}
],
"markdown": "# The Month of Frequent Vomiting\n\n **Authors:** Craig A Goolsby, MD \n **Date:** February 10, 2016\n\n ## Content\n\n Figure 1.\nOn physical examination, the patient has an oral temperature of 99.4°F (37.4°C). His heart has a regular rhythm with a rate of 110 bpm, his blood pressure is 106/72 mm Hg, and his respiratory rate is 16 breaths/min. He has a pulse oximetry reading of 99% while breathing room air. In general, the patient appears chronically ill; however, he is in no acute distress and is appropriately alert and oriented. He does appear dehydrated. He is noted to have cachexia, with temporal wasting and dry mucous membranes. Clear lung sounds are found bilaterally. His abdomen is mildly tender at the epigastrium, without any peritoneal signs. His extremities are well-perfused, and he has no rash. No focal neurologic deficits are found.\nAn intravenous (IV) line is placed, and fluid resuscitation is started with a liter of normal saline. The patient is given 4 mg of ondansetron for his nausea and vomiting, and he declines pain medications. Laboratory studies, including electrolytes, transaminases, total bilirubin, lipase, and a complete blood cell count (CBC) are ordered and sent. Radiography of the chest is performed and is normal. An abdominal computed tomography (CT) scan shows a large gastric mass causing gastric outlet obstruction (not shown). An electrocardiogram (ECG) is ordered (see Figure 1).\n\n ## Figures\n\n **Figure 1.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 375193,
"choiceText": "Begin potassium chloride replacement",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 375194,
"choiceText": "Start the patient on 1 L free water fluid restriction daily",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 375195,
"choiceText": "Give the patient 2 amps of sodium bicarbonate",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 375196,
"choiceText": "Give calcium gluconate immediately",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 108013,
"questionText": "What treatment should be initiated based on this patient's ECG?<br /><br />\r\n<i>Hint: Intractable vomiting can be the cause of this condition.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "The Month of Frequent Vomiting"
},
{
"authors": "Craig A Goolsby, MD",
"content": [
"Figure 1.",
"This patient's ECG demonstrated changes consistent with profound hypokalemia. The patient's history of intractable vomiting occurring for many weeks raised a clinical suspicion for hypokalemia, which was confirmed by the ECG findings. The ECG demonstrated a markedly prolonged QT interval, with the ECG computer interpretation calculating a corrected QT interval (QTc) of 590 msec (which is greatly prolonged). Additionally, the ECG showed slight \"thumbprint-like” ST depressions in many leads, including II, III, aVF, and V4-V6, which represented another sign of hypokalemia. ST elevations were seen in V1 and V2; however, the patient's clinical presentation was not consistent with myocardial ischemia or infarction.",
"Hypokalemia is a common clinical problem. Less than 1% of people on no medications are estimated to have a serum potassium less than 3.5 mEq/L (3.5 mmol/L)[1]; however, up to 21% of hospitalized patients, 50% of patients on non–potassium-sparing diuretics, and 19% of patients with eating disorders may have hypokalemia.[1] Given the prevalence of these conditions and other causes of hypokalemia, many people are affected. In most patients, the degree of hypokalemia is not of clinical significance; however, because symptoms of hypokalemia may be vague or absent, potassium levels should be monitored in patients at risk for developing hypokalemia. The definition of hypokalemia is a serum potassium level of less than 3.5 mEq/L (3.5 mmol/L). Bedside potassium measurements are now available in many facilities. If an immediate test is needed and a delay is anticipated, an ECG can serve as a surrogate marker. If acute hypokalemia is a concern, a normal ECG can be reassuring, whereas an abnormal ECG can suggest hypokalemia, but not a specific level."
],
"date": "February 10, 2016",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/711/354/711354-thumb1.png"
}
],
"markdown": "# The Month of Frequent Vomiting\n\n **Authors:** Craig A Goolsby, MD \n **Date:** February 10, 2016\n\n ## Content\n\n Figure 1.\nThis patient's ECG demonstrated changes consistent with profound hypokalemia. The patient's history of intractable vomiting occurring for many weeks raised a clinical suspicion for hypokalemia, which was confirmed by the ECG findings. The ECG demonstrated a markedly prolonged QT interval, with the ECG computer interpretation calculating a corrected QT interval (QTc) of 590 msec (which is greatly prolonged). Additionally, the ECG showed slight \"thumbprint-like” ST depressions in many leads, including II, III, aVF, and V4-V6, which represented another sign of hypokalemia. ST elevations were seen in V1 and V2; however, the patient's clinical presentation was not consistent with myocardial ischemia or infarction.\nHypokalemia is a common clinical problem. Less than 1% of people on no medications are estimated to have a serum potassium less than 3.5 mEq/L (3.5 mmol/L)[1]; however, up to 21% of hospitalized patients, 50% of patients on non–potassium-sparing diuretics, and 19% of patients with eating disorders may have hypokalemia.[1] Given the prevalence of these conditions and other causes of hypokalemia, many people are affected. In most patients, the degree of hypokalemia is not of clinical significance; however, because symptoms of hypokalemia may be vague or absent, potassium levels should be monitored in patients at risk for developing hypokalemia. The definition of hypokalemia is a serum potassium level of less than 3.5 mEq/L (3.5 mmol/L). Bedside potassium measurements are now available in many facilities. If an immediate test is needed and a delay is anticipated, an ECG can serve as a surrogate marker. If acute hypokalemia is a concern, a normal ECG can be reassuring, whereas an abnormal ECG can suggest hypokalemia, but not a specific level.\n\n ## Figures\n\n **Figure 1.** \n \n\n\n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 375193,
"choiceText": "Begin potassium chloride replacement",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 375194,
"choiceText": "Start the patient on 1 L free water fluid restriction daily",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 375195,
"choiceText": "Give the patient 2 amps of sodium bicarbonate",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 375196,
"choiceText": "Give calcium gluconate immediately",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 108013,
"questionText": "What treatment should be initiated based on this patient's ECG?<br /><br />\r\n<i>Hint: Intractable vomiting can be the cause of this condition.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "The Month of Frequent Vomiting"
},
{
"authors": "Craig A Goolsby, MD",
"content": [
"Figure 1.",
"Hypokalemia has numerous causes, which can be generally divided into poor intake, increased excretion, and shifting potassium from the extracellular to intracellular spaces.[1] Poor intake can be caused by mechanical problems (eg, inability to swallow), eating disorders (eg, bulimia), and hospitalization (eg, inappropriate potassium in parenteral nutrition).[1] Increased excretion occurs in multiple settings. Some examples are an excess of endogenous mineralocorticoids causing increased renal losses, congenital disorders that affect the kidney's distal tubule (such as Bartter and Gitelman syndromes), medications (particularly diuretics), and diarrhea, among other causes.[1] Finally, potassium can shift intracellularly and cause hypokalemia. Potassium shift occurs with alkalosis, albuterol or insulin administration, and during refeeding syndrome.",
"Clinical manifestations of hypokalemia are wide ranging. For mild hypokalemia (potassium levels above 3 mEq/L [3 mmol/L]), patients are typically asymptomatic, unless the level falls rapidly or there are other comorbidities.[4] Once the levels are below 3 mEq/L (3 mmol/L), patients may experience various symptoms that can be difficult to link directly to a particular level and depend more on the individual patient and their medications and comorbidities. Muscle weakness typically manifests below levels of 2.5 mEq/L (2.5 mmol/L), and it tends to begin slowly in the lower extremities and progress to the trunk and upper extremities.[4]",
"Decreased gastrointestinal motility leading to ileus may occur and, if severe, respiratory depression or alkalosis can occur.[4] In addition, rhabdomyolysis can develop as a result of potassium depletion. Potassium release from muscle cells during exertion normally mediates blood flow to muscles during exercise. Decreased potassium release causes less blood flow to the muscles. This decreased blood flow can lead to rhabdomyolysis and the associated cascade of metabolic problems.[4]",
"The most potentially serious complications from hypokalemia involve cardiac dysrhythmia. These may be relatively benign abnormalities, such as premature atrial and ventricular beats, or they can be life-threatening problems, such as atrioventricular block, ventricular tachycardia, or ventricular fibrillation.[4] Because hypokalemia is typically associated with concomitant hypokalemic, torsades des pointes can occur.[4] Also, hypokalemia occurring with digitalis toxicity or underlying illnesses (such as coronary ischemia) can make these illnesses more severe.[4] Potassium levels below 3 mEq/L (3 mmol/L) are associated with a 2-fold increase in ventricular dysrhythmia.[4]",
"Determining the etiology of hypokalemia depends on the clinical context. A history of diuretic use is suggestive of causation. For a patient on no medications, without vomiting or diarrhea and who has normal food intake, establishing the cause may be much more difficult. A diagnostic evaluation may include looking for surreptitious diuretic use in the patient's urine or measuring 24-hour aldosterone and cortisol levels, among many other possibilities.[4] Mild fluctuations in potassium levels are common, and an extensive work-up of the cause in the setting of mild hypokalemia is not typically necessary. Instead, repeat levels can be performed to monitor for correction or progression."
],
"date": "February 10, 2016",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/711/354/711354-thumb1.png"
}
],
"markdown": "# The Month of Frequent Vomiting\n\n **Authors:** Craig A Goolsby, MD \n **Date:** February 10, 2016\n\n ## Content\n\n Figure 1.\nHypokalemia has numerous causes, which can be generally divided into poor intake, increased excretion, and shifting potassium from the extracellular to intracellular spaces.[1] Poor intake can be caused by mechanical problems (eg, inability to swallow), eating disorders (eg, bulimia), and hospitalization (eg, inappropriate potassium in parenteral nutrition).[1] Increased excretion occurs in multiple settings. Some examples are an excess of endogenous mineralocorticoids causing increased renal losses, congenital disorders that affect the kidney's distal tubule (such as Bartter and Gitelman syndromes), medications (particularly diuretics), and diarrhea, among other causes.[1] Finally, potassium can shift intracellularly and cause hypokalemia. Potassium shift occurs with alkalosis, albuterol or insulin administration, and during refeeding syndrome.\nClinical manifestations of hypokalemia are wide ranging. For mild hypokalemia (potassium levels above 3 mEq/L [3 mmol/L]), patients are typically asymptomatic, unless the level falls rapidly or there are other comorbidities.[4] Once the levels are below 3 mEq/L (3 mmol/L), patients may experience various symptoms that can be difficult to link directly to a particular level and depend more on the individual patient and their medications and comorbidities. Muscle weakness typically manifests below levels of 2.5 mEq/L (2.5 mmol/L), and it tends to begin slowly in the lower extremities and progress to the trunk and upper extremities.[4]\nDecreased gastrointestinal motility leading to ileus may occur and, if severe, respiratory depression or alkalosis can occur.[4] In addition, rhabdomyolysis can develop as a result of potassium depletion. Potassium release from muscle cells during exertion normally mediates blood flow to muscles during exercise. Decreased potassium release causes less blood flow to the muscles. This decreased blood flow can lead to rhabdomyolysis and the associated cascade of metabolic problems.[4]\nThe most potentially serious complications from hypokalemia involve cardiac dysrhythmia. These may be relatively benign abnormalities, such as premature atrial and ventricular beats, or they can be life-threatening problems, such as atrioventricular block, ventricular tachycardia, or ventricular fibrillation.[4] Because hypokalemia is typically associated with concomitant hypokalemic, torsades des pointes can occur.[4] Also, hypokalemia occurring with digitalis toxicity or underlying illnesses (such as coronary ischemia) can make these illnesses more severe.[4] Potassium levels below 3 mEq/L (3 mmol/L) are associated with a 2-fold increase in ventricular dysrhythmia.[4]\nDetermining the etiology of hypokalemia depends on the clinical context. A history of diuretic use is suggestive of causation. For a patient on no medications, without vomiting or diarrhea and who has normal food intake, establishing the cause may be much more difficult. A diagnostic evaluation may include looking for surreptitious diuretic use in the patient's urine or measuring 24-hour aldosterone and cortisol levels, among many other possibilities.[4] Mild fluctuations in potassium levels are common, and an extensive work-up of the cause in the setting of mild hypokalemia is not typically necessary. Instead, repeat levels can be performed to monitor for correction or progression.\n\n ## Figures\n\n **Figure 1.** \n \n\n\n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "The Month of Frequent Vomiting"
},
{
"authors": "Craig A Goolsby, MD",
"content": [
"Hypokalemia is treated with potassium repletion and correction of the underlying problem causing the hypokalemia. A large amount of potassium may be required to replace severe hypokalemia, becasue serum potassium reflects total body potassium stores. For example, a drop in serum potassium of just 1 mEq/L (1 mmol/L) in a patient without an acute acid-base disorder may represent a 370 mEq (370 mmol) deficit of total body potassium.[6] The potassium level can increase as much as 1-1.5 mEq/L (1-1.5 mmol/L) transiently after an oral dose of 40-60 mEq (40-60 mmol), so laboratory monitoring and care must be used to avoid hyperkalemia.[4]",
"Potassium replacement can be accomplished via enteral or intravenous replacement. Oral replacement is preferred to intravenous replacement because of the lesser risk of iatrogenic hyperkalemia, the ability to give larger doses of potassium replacement quickly, and burning at the infusion site if potassium is given via peripheral IV.[6] For mild-to-moderate hypokalemia (potassium between 3 and 3.5 mEq/L [3 and 3.5 mmol/L]), potassium can usually be replaced orally. Replacing the lost potassium can be accomplished with 10-20 mEq (10-20 mmol) of potassium chloride dosed 2-4 times daily.[4] For severe hypokalemia (potassium less than 3 mEq/L [3 mmol/L]), oral doses of 40-60 mEq (40-60 mmol) can be given 3-4 times daily.[4]",
"For patients who cannot tolerate oral medications or are acutely ill, intravenous replacement can be considered. Generally, the rate of repletion should be no more than 10-20 mEq/hour (10-20 mmol/hour) to prevent complications related to hyperkalemia or burning at the infusion site.[4,6] Replacement rates exceeding 20 mEq/hour (20 mmol/hour) require cardiac monitoring and central venous access.[6] Patients with hypokalemia almost always have concurrent hypomagnesemia, so empiric magnesium repletion is also recommended. If the magnesium deficit is not corrected, the hypokalemia may persist.[4] It should be noted that serum magnesium levels may not accurately reflect total body stores, so correction should be done empirically even in the setting of a normal laboratory reading.",
"Most hypokalemic patients can be safely discharged to home with primary care follow-up to manage potassium repletion and potassium level monitoring. Severely hypokalemic or symptomatic patients will require admission and, possibly, cardiac monitoring.",
"The laboratory studies for the patient in this case were notable for a potassium level of less than 2 mEq/L (2 mmol/L), as well as a chloride level of 66 mEq/L (66 mmol/L), a creatinine of 1.5 mg/dL (132.6 μmol/L), a total bilirubin of 3.7 mg/dL (63.27 μmol/L), and negative cardiac enzymes. The potassium level was confirmed on a repeat study. Treatment began simultaneously with both oral and intravenous potassium chloride replacement, and oral and intravenous magnesium replacement. The patient was admitted to the intensive care unit for cardiac monitoring, fluid and electrolyte resuscitation, and further evaluation of the etiology of the gastric mass."
],
"date": "February 10, 2016",
"figures": [],
"markdown": "# The Month of Frequent Vomiting\n\n **Authors:** Craig A Goolsby, MD \n **Date:** February 10, 2016\n\n ## Content\n\n Hypokalemia is treated with potassium repletion and correction of the underlying problem causing the hypokalemia. A large amount of potassium may be required to replace severe hypokalemia, becasue serum potassium reflects total body potassium stores. For example, a drop in serum potassium of just 1 mEq/L (1 mmol/L) in a patient without an acute acid-base disorder may represent a 370 mEq (370 mmol) deficit of total body potassium.[6] The potassium level can increase as much as 1-1.5 mEq/L (1-1.5 mmol/L) transiently after an oral dose of 40-60 mEq (40-60 mmol), so laboratory monitoring and care must be used to avoid hyperkalemia.[4]\nPotassium replacement can be accomplished via enteral or intravenous replacement. Oral replacement is preferred to intravenous replacement because of the lesser risk of iatrogenic hyperkalemia, the ability to give larger doses of potassium replacement quickly, and burning at the infusion site if potassium is given via peripheral IV.[6] For mild-to-moderate hypokalemia (potassium between 3 and 3.5 mEq/L [3 and 3.5 mmol/L]), potassium can usually be replaced orally. Replacing the lost potassium can be accomplished with 10-20 mEq (10-20 mmol) of potassium chloride dosed 2-4 times daily.[4] For severe hypokalemia (potassium less than 3 mEq/L [3 mmol/L]), oral doses of 40-60 mEq (40-60 mmol) can be given 3-4 times daily.[4]\nFor patients who cannot tolerate oral medications or are acutely ill, intravenous replacement can be considered. Generally, the rate of repletion should be no more than 10-20 mEq/hour (10-20 mmol/hour) to prevent complications related to hyperkalemia or burning at the infusion site.[4,6] Replacement rates exceeding 20 mEq/hour (20 mmol/hour) require cardiac monitoring and central venous access.[6] Patients with hypokalemia almost always have concurrent hypomagnesemia, so empiric magnesium repletion is also recommended. If the magnesium deficit is not corrected, the hypokalemia may persist.[4] It should be noted that serum magnesium levels may not accurately reflect total body stores, so correction should be done empirically even in the setting of a normal laboratory reading.\nMost hypokalemic patients can be safely discharged to home with primary care follow-up to manage potassium repletion and potassium level monitoring. Severely hypokalemic or symptomatic patients will require admission and, possibly, cardiac monitoring.\nThe laboratory studies for the patient in this case were notable for a potassium level of less than 2 mEq/L (2 mmol/L), as well as a chloride level of 66 mEq/L (66 mmol/L), a creatinine of 1.5 mg/dL (132.6 μmol/L), a total bilirubin of 3.7 mg/dL (63.27 μmol/L), and negative cardiac enzymes. The potassium level was confirmed on a repeat study. Treatment began simultaneously with both oral and intravenous potassium chloride replacement, and oral and intravenous magnesium replacement. The patient was admitted to the intensive care unit for cardiac monitoring, fluid and electrolyte resuscitation, and further evaluation of the etiology of the gastric mass.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 375203,
"choiceText": "Peaked T-waves",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 375204,
"choiceText": "Absent U-waves",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 375205,
"choiceText": "Decreased T-waves",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 375206,
"choiceText": "Osborn waves",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 375207,
"choiceText": "Sine waves",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The hallmark findings of severe hypokalemia on an ECG are diminished T-waves and prominent U-waves. Peaked T-waves are a characteristic finding in hyperkalemia, with sine waves seen in cases of severe hyperkalemia. Osborn waves are seen in severe hypothermia, not hypokalemia.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 108015,
"questionText": "A patient with a history of bulimia and recent recurrent vomiting presents to the ED with generalized weakness and dehydration, raising suspicion of hypokalemia. Which of the following ECG findings would be expected in a patient with severe hypokalemia?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 375208,
"choiceText": "Magnesium",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 375209,
"choiceText": "Sodium",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 375210,
"choiceText": "Calcium",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 375211,
"choiceText": "Phosphorous",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 375212,
"choiceText": "Chloride",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Patients with hypokalemia almost always have coincident hypomagnesemia and should be treated empirically with magnesium repletion. Until the magnesium deficit is corrected, potassium will not return to normal levels despite the administration of appropriate potassium.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 108016,
"questionText": "You are treating a patient with known hypokalemia with potassium repletion. A deficit of which of the following can cause refractory hypokalemia, despite potassium replacement?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "The Month of Frequent Vomiting"
},
{
"authors": "Craig A Goolsby, MD",
"content": [],
"date": "February 10, 2016",
"figures": [],
"markdown": "# The Month of Frequent Vomiting\n\n **Authors:** Craig A Goolsby, MD \n **Date:** February 10, 2016\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 375203,
"choiceText": "Peaked T-waves",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 375204,
"choiceText": "Absent U-waves",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 375205,
"choiceText": "Decreased T-waves",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 375206,
"choiceText": "Osborn waves",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 375207,
"choiceText": "Sine waves",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The hallmark findings of severe hypokalemia on an ECG are diminished T-waves and prominent U-waves. Peaked T-waves are a characteristic finding in hyperkalemia, with sine waves seen in cases of severe hyperkalemia. Osborn waves are seen in severe hypothermia, not hypokalemia.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 108015,
"questionText": "A patient with a history of bulimia and recent recurrent vomiting presents to the ED with generalized weakness and dehydration, raising suspicion of hypokalemia. Which of the following ECG findings would be expected in a patient with severe hypokalemia?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 375208,
"choiceText": "Magnesium",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 375209,
"choiceText": "Sodium",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 375210,
"choiceText": "Calcium",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 375211,
"choiceText": "Phosphorous",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 375212,
"choiceText": "Chloride",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Patients with hypokalemia almost always have coincident hypomagnesemia and should be treated empirically with magnesium repletion. Until the magnesium deficit is corrected, potassium will not return to normal levels despite the administration of appropriate potassium.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 108016,
"questionText": "You are treating a patient with known hypokalemia with potassium repletion. A deficit of which of the following can cause refractory hypokalemia, despite potassium replacement?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "The Month of Frequent Vomiting"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 375193,
"choiceText": "Begin potassium chloride replacement",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 375194,
"choiceText": "Start the patient on 1 L free water fluid restriction daily",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 375195,
"choiceText": "Give the patient 2 amps of sodium bicarbonate",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 375196,
"choiceText": "Give calcium gluconate immediately",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 108013,
"questionText": "What treatment should be initiated based on this patient's ECG?<br /><br />\r\n<i>Hint: Intractable vomiting can be the cause of this condition.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 375203,
"choiceText": "Peaked T-waves",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 375204,
"choiceText": "Absent U-waves",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 375205,
"choiceText": "Decreased T-waves",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 375206,
"choiceText": "Osborn waves",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 375207,
"choiceText": "Sine waves",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The hallmark findings of severe hypokalemia on an ECG are diminished T-waves and prominent U-waves. Peaked T-waves are a characteristic finding in hyperkalemia, with sine waves seen in cases of severe hyperkalemia. Osborn waves are seen in severe hypothermia, not hypokalemia.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 108015,
"questionText": "A patient with a history of bulimia and recent recurrent vomiting presents to the ED with generalized weakness and dehydration, raising suspicion of hypokalemia. Which of the following ECG findings would be expected in a patient with severe hypokalemia?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 375208,
"choiceText": "Magnesium",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 375209,
"choiceText": "Sodium",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 375210,
"choiceText": "Calcium",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 375211,
"choiceText": "Phosphorous",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 375212,
"choiceText": "Chloride",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Patients with hypokalemia almost always have coincident hypomagnesemia and should be treated empirically with magnesium repletion. Until the magnesium deficit is corrected, potassium will not return to normal levels despite the administration of appropriate potassium.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 108016,
"questionText": "You are treating a patient with known hypokalemia with potassium repletion. A deficit of which of the following can cause refractory hypokalemia, despite potassium replacement?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
730514 | /viewarticle/730514 | [
{
"authors": "Chad A. Whyte, MD",
"content": [
"Editorial Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"A 19-year-old woman presents to the clinic after a hospital visit for headaches. She has had headaches for approximately 5 years and they have been progressively increasing in frequency. She experiences a daily headache that she rates as 5 out of 10 in severity, with a more severe one occurring once a week. The severe headaches are accompanied by nausea, photophobia, and an increase in pain with movement. She is worried because the daily headaches are located in the occipital region, whereas the severe headaches typically occur behind her eyes. She was started on divalproex sodium (valproic acid) in the emergency department.",
"Her past medical history is significant for Wyburn-Mason syndrome, a phakomatosis characterized by multiple arteriovenous malformations (AVMs) located above the neck. She has had various laser ablations to attempt to minimize bleeding complications. Besides divalproex sodium (valproic acid), she is taking duloxetine and methylphenidate, as well as over-the-counter nonsteroidal anti-inflammatory drugs (NSAIDs) more than twice a week. Her family history reveals that both parents experience headaches. She does not use alcohol or tobacco, but she does consume approximately 6 cans of caffeinated cola per day."
],
"date": "October 26, 2017",
"figures": [],
"markdown": "# Headache in a 19-Year-Old Woman With a Genetic Disorder\n\n **Authors:** Chad A. Whyte, MD \n **Date:** October 26, 2017\n\n ## Content\n\n Editorial Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nA 19-year-old woman presents to the clinic after a hospital visit for headaches. She has had headaches for approximately 5 years and they have been progressively increasing in frequency. She experiences a daily headache that she rates as 5 out of 10 in severity, with a more severe one occurring once a week. The severe headaches are accompanied by nausea, photophobia, and an increase in pain with movement. She is worried because the daily headaches are located in the occipital region, whereas the severe headaches typically occur behind her eyes. She was started on divalproex sodium (valproic acid) in the emergency department.\nHer past medical history is significant for Wyburn-Mason syndrome, a phakomatosis characterized by multiple arteriovenous malformations (AVMs) located above the neck. She has had various laser ablations to attempt to minimize bleeding complications. Besides divalproex sodium (valproic acid), she is taking duloxetine and methylphenidate, as well as over-the-counter nonsteroidal anti-inflammatory drugs (NSAIDs) more than twice a week. Her family history reveals that both parents experience headaches. She does not use alcohol or tobacco, but she does consume approximately 6 cans of caffeinated cola per day.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "Headache in a 19-Year-Old Woman With a Genetic Disorder"
},
{
"authors": "Chad A. Whyte, MD",
"content": [
"Upon presentation, the patient does not appear to be in acute distress. Her vital signs include a blood pressure of 110/60 mm Hg, a pulse rate of 88 beats/min, and a respiratory rate of 14 breaths/min. She is afebrile. On inspection, a large subcutaneous AVM is apparent on the left side of her face; a right corneal AVM is noted as well. Also visible on her face are tiny ulcers in various stages of healing. Musculoskeletal examination reveals severe tenderness to palpation of the cervical paraspinous muscles, with moderate spasm. The patient has a normal mental status with intact cranial nerves, except for some facial numbness at the area of the left facial AVM. Her strength is 5/5 (5 representing normal strength) bilaterally in both upper and lower extremities, with normal bulk and tone. Deep tendon reflexes are 2+ and symmetric in all 4 extremities. In the rest of the body, sensation is intact to pinprick, temperature, vibration, and proprioception. Her coordination and gait are normal.",
"Laboratory investigations reveal normal complete blood cell count findings, a comprehensive metabolic panel, normal thyroid-stimulating hormone level, and normal coagulation levels. A 3-dimensional reconstructed image from a CT angiogram (CTA) demonstrates a large, complex facial AVM (see Figure), as well as one in the basal ganglia (not shown). No vascular stenosis is noted."
],
"date": "October 26, 2017",
"figures": [
{
"caption": "",
"image_url": "https://img.medscapestatic.com/article/730/007/730007-thumb1.png"
}
],
"markdown": "# Headache in a 19-Year-Old Woman With a Genetic Disorder\n\n **Authors:** Chad A. Whyte, MD \n **Date:** October 26, 2017\n\n ## Content\n\n Upon presentation, the patient does not appear to be in acute distress. Her vital signs include a blood pressure of 110/60 mm Hg, a pulse rate of 88 beats/min, and a respiratory rate of 14 breaths/min. She is afebrile. On inspection, a large subcutaneous AVM is apparent on the left side of her face; a right corneal AVM is noted as well. Also visible on her face are tiny ulcers in various stages of healing. Musculoskeletal examination reveals severe tenderness to palpation of the cervical paraspinous muscles, with moderate spasm. The patient has a normal mental status with intact cranial nerves, except for some facial numbness at the area of the left facial AVM. Her strength is 5/5 (5 representing normal strength) bilaterally in both upper and lower extremities, with normal bulk and tone. Deep tendon reflexes are 2+ and symmetric in all 4 extremities. In the rest of the body, sensation is intact to pinprick, temperature, vibration, and proprioception. Her coordination and gait are normal.\nLaboratory investigations reveal normal complete blood cell count findings, a comprehensive metabolic panel, normal thyroid-stimulating hormone level, and normal coagulation levels. A 3-dimensional reconstructed image from a CT angiogram (CTA) demonstrates a large, complex facial AVM (see Figure), as well as one in the basal ganglia (not shown). No vascular stenosis is noted.\n\n ## Figures\n\n **** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 371623,
"choiceText": "Idiopathic intracranial hypertension",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 371624,
"choiceText": "Headache attributed to cranial or cervical vascular disorder",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 371625,
"choiceText": "Migraine without aura",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 371626,
"choiceText": "Psychogenic headache",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 106680,
"questionText": "Which of the following is the most likely diagnosis?<br /><br /> <i>Hint: Consider the patient's entire history.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Headache in a 19-Year-Old Woman With a Genetic Disorder"
},
{
"authors": "Chad A. Whyte, MD",
"content": [
"The patient in this case had a rare disorder as well as a common presentation of a common disease. The genetic disorder seen in the Figure is likely a “red herring.” Migraine affects approximately 12% of the population and often goes unrecognized and undertreated.[1] Many patients miss more leisure activities than work activities because of the nature of each attack. The criteria for migraine without aura from the International Classification of Headache Disorders are as follows[2]:",
"At least 5 attacks fulfilling the following criteria:",
"Headache attacks lasting 4-72 hours when untreated or unsuccessfully treated",
"The headache is accompanied by at least 2 of the following:",
"Unilateral location",
"Pulsating quality",
"Aggravated by or causing avoidance of routine physical activity",
"Moderate or severe pain intensity",
"During a headache, at least one of the following occurs:",
"Nausea and/or vomiting",
"Photophobia and phonophobia",
"Not attributed to another disorder",
"Many patients have probable migraine, which is diagnosed when the patient has had fewer than 5 attacks."
],
"date": "October 26, 2017",
"figures": [],
"markdown": "# Headache in a 19-Year-Old Woman With a Genetic Disorder\n\n **Authors:** Chad A. Whyte, MD \n **Date:** October 26, 2017\n\n ## Content\n\n The patient in this case had a rare disorder as well as a common presentation of a common disease. The genetic disorder seen in the Figure is likely a “red herring.” Migraine affects approximately 12% of the population and often goes unrecognized and undertreated.[1] Many patients miss more leisure activities than work activities because of the nature of each attack. The criteria for migraine without aura from the International Classification of Headache Disorders are as follows[2]:\nAt least 5 attacks fulfilling the following criteria:\nHeadache attacks lasting 4-72 hours when untreated or unsuccessfully treated\nThe headache is accompanied by at least 2 of the following:\nUnilateral location\nPulsating quality\nAggravated by or causing avoidance of routine physical activity\nModerate or severe pain intensity\nDuring a headache, at least one of the following occurs:\nNausea and/or vomiting\nPhotophobia and phonophobia\nNot attributed to another disorder\nMany patients have probable migraine, which is diagnosed when the patient has had fewer than 5 attacks.\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 371623,
"choiceText": "Idiopathic intracranial hypertension",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 371624,
"choiceText": "Headache attributed to cranial or cervical vascular disorder",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 371625,
"choiceText": "Migraine without aura",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 371626,
"choiceText": "Psychogenic headache",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 106680,
"questionText": "Which of the following is the most likely diagnosis?<br /><br /> <i>Hint: Consider the patient's entire history.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Headache in a 19-Year-Old Woman With a Genetic Disorder"
},
{
"authors": "Chad A. Whyte, MD",
"content": [
"Originally, migraine was theorized to be a vascular disorder because of the results of experiments performed in the 1940s and 1950s on patients suffering from migraine with aura (a reversible neurologic symptom that accompanies migraine in a minority of patients). A phenomenon called cortical spreading depression (CSD), in which a wave of hyperemia precedes oligemia in the cerebrum, is thought to be the cause of aura, although it can occur in migraine without aura. For many years, however, CSD was thought to be the cause of migraine.",
"Multiple studies have shown that migraine may be generated in the brainstem. First, positron emission tomography (PET) scanning during migraine attacks has revealed persistent activation of the brainstem even after abortive treatment.[3] Second, glutamate, an excitatory neurotransmitter, is elevated in the plasma of patients with migraine. This suggests that the brain is hyperexcitable during an attack of migraine. Also, a subtype of migraine with aura -- called familial hemiplegic migraine -- is caused by ion-channel mutation and dysfunction with either increased excitatory substances or reduced inhibitory pathways.[4]",
"Once the migraine is triggered, the sphenopalatine ganglion is activated, which sends signals to the meningeal arteries that are thought to cause inflammation and pain. This is called the trigeminovascular system. Various vasodilating substances, including calcitonin gene-related peptide (CGRP), substance P, and neurokinin A, are released and cause neurogenic inflammation.[5]",
"CGRP is thought to be the most potent vasodilator in the central nervous system and an important substance involved in signaling during an episode of migraine. Activation of CGRP receptors leads to activation of N-methyl-D-aspartate (NMDA) receptors via glutamate, which is thought to indirectly cause CSD. Also, reduction of serum CGRP is associated with relief from migraine.[6] Currently, CGRP inhibitors have been tested and are awaiting FDA approval.",
"Epidemiologic studies have revealed that if migraine is left alone as an episodic disorder, the chance per year that it will evolve into chronic migraine or migraine occurring on more than 15 days per month over at least 3 months is 2.5%.[7] A few events can cause transformation into chronic migraine quicker than expected, including mild head trauma, neurosurgery, or a disruption in hormone balance. Perhaps the most common reason why patients see a headache specialist for chronic daily headache is symptomatic medication overuse, also known as rebound headache or medication overuse headache (MOH). This seems to be more common in those with migraine. Bigal and colleagues[7] described a difference in the odds ratio of developing MOH with different abortive medicines. Patients taking compounds containing butalbital developed MOH when using the medication for 5 days a month, whereas those taking opiates required 8 days. Patients receiving NSAIDs or triptans took about 10 days a month to develop MOH. MOH can lead to reduced response not only to acute medicines but also to preventative (prophylactic) drugs. MOH can cause significant neck pain as well as headaches capable of disrupting sleep, which can be quite distressing. Ingesting large amounts of caffeine daily is known to be associated with chronic headaches, although caffeine itself is an effective migraine pain reliever.[8] Overuse of any pain-relieving substance, including caffeine and over-the-counter medications, can lead to MOH."
],
"date": "October 26, 2017",
"figures": [],
"markdown": "# Headache in a 19-Year-Old Woman With a Genetic Disorder\n\n **Authors:** Chad A. Whyte, MD \n **Date:** October 26, 2017\n\n ## Content\n\n Originally, migraine was theorized to be a vascular disorder because of the results of experiments performed in the 1940s and 1950s on patients suffering from migraine with aura (a reversible neurologic symptom that accompanies migraine in a minority of patients). A phenomenon called cortical spreading depression (CSD), in which a wave of hyperemia precedes oligemia in the cerebrum, is thought to be the cause of aura, although it can occur in migraine without aura. For many years, however, CSD was thought to be the cause of migraine.\nMultiple studies have shown that migraine may be generated in the brainstem. First, positron emission tomography (PET) scanning during migraine attacks has revealed persistent activation of the brainstem even after abortive treatment.[3] Second, glutamate, an excitatory neurotransmitter, is elevated in the plasma of patients with migraine. This suggests that the brain is hyperexcitable during an attack of migraine. Also, a subtype of migraine with aura -- called familial hemiplegic migraine -- is caused by ion-channel mutation and dysfunction with either increased excitatory substances or reduced inhibitory pathways.[4]\nOnce the migraine is triggered, the sphenopalatine ganglion is activated, which sends signals to the meningeal arteries that are thought to cause inflammation and pain. This is called the trigeminovascular system. Various vasodilating substances, including calcitonin gene-related peptide (CGRP), substance P, and neurokinin A, are released and cause neurogenic inflammation.[5]\nCGRP is thought to be the most potent vasodilator in the central nervous system and an important substance involved in signaling during an episode of migraine. Activation of CGRP receptors leads to activation of N-methyl-D-aspartate (NMDA) receptors via glutamate, which is thought to indirectly cause CSD. Also, reduction of serum CGRP is associated with relief from migraine.[6] Currently, CGRP inhibitors have been tested and are awaiting FDA approval.\nEpidemiologic studies have revealed that if migraine is left alone as an episodic disorder, the chance per year that it will evolve into chronic migraine or migraine occurring on more than 15 days per month over at least 3 months is 2.5%.[7] A few events can cause transformation into chronic migraine quicker than expected, including mild head trauma, neurosurgery, or a disruption in hormone balance. Perhaps the most common reason why patients see a headache specialist for chronic daily headache is symptomatic medication overuse, also known as rebound headache or medication overuse headache (MOH). This seems to be more common in those with migraine. Bigal and colleagues[7] described a difference in the odds ratio of developing MOH with different abortive medicines. Patients taking compounds containing butalbital developed MOH when using the medication for 5 days a month, whereas those taking opiates required 8 days. Patients receiving NSAIDs or triptans took about 10 days a month to develop MOH. MOH can lead to reduced response not only to acute medicines but also to preventative (prophylactic) drugs. MOH can cause significant neck pain as well as headaches capable of disrupting sleep, which can be quite distressing. Ingesting large amounts of caffeine daily is known to be associated with chronic headaches, although caffeine itself is an effective migraine pain reliever.[8] Overuse of any pain-relieving substance, including caffeine and over-the-counter medications, can lead to MOH.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "Headache in a 19-Year-Old Woman With a Genetic Disorder"
},
{
"authors": "Chad A. Whyte, MD",
"content": [
"Current treatment of migraine involves not only drugs to abort an active attack but also preventive medicines to be used prophylactically when attacks are frequent. Voxel-based morphometry studies have shown focal reductions in cortical volume in episodic migraine, as well as an increase in focal reductions with increased attack frequency.[9,10] Also, a high frequency of attacks leads to central nervous system hypometabolism and hyperexcitability.[11] The frequency of migraine attacks may be associated with chronic structural changes in the brain.",
"The only FDA-approved medications that have been designed specifically for migraine are the triptan class. They are 5-HT1 agonists that inhibit the release of neuroinflammatory substances and also constrict meningeal vessels. They are available in pill form and oral-dissolving, nasal, and subcutaneous preparations for those who have severe pain or nausea at the very beginning of an attack. Dihydroergotamine (DHE) is another first-line treatment that has existed since 1945 and is very effective intravenously. Contraindications to both triptans and DHE include coronary artery disease, cerebrovascular disease, and uncontrolled hypertension. These medicines were not used in the treatment of the patient in this case because of the vascular anomalies in her brain. Other abortive treatments include NSAIDs, acetaminophen, antiemetics (especially when used in conjunction with NSAIDs), and corticosteroids.",
"Various preventive medicines have been found to be successful in decreasing the frequency of migraine attacks. Although nothing has been designed for migraine, many classes have been used over the last 50 years, including antiseizure, antihypertensive, and antidepressant medications, as well as natural supplements. Currently, the only FDA-approved drugs for migraine prevention are propranolol, timolol, divalproex sodium (valproic acid), and topiramate; however, many other medicines are used and have been found successful vs placebo in adults, including gabapentin, verapamil, angiotensin-converting enzyme inhibitors, angiotensin-receptor blockers, venlafaxine, tricyclic antidepressants, magnesium, riboflavin, and cervicocranial botulinum toxin injections. A randomized, double-blind study in children and adolescents aged 8-17 years did not find a significant difference between placebo and amitriptyline or topiramate; however, the response rate to placebo (50% or more decrease in number of headache days) was 61%.[12]",
"Quite often, patients do not respond to pharmacologic therapy and need nonpharmacologic therapy to help control their pain. It is important to ensure that other comorbidities do not complicate patient improvement. Depression, insomnia, and anxiety are much more common in those who suffer from migraine, and psychiatric therapy can be helpful in managing these issues. Biofeedback is used with success, especially in patients in whom medication therapy is not ideal (eg, pregnant women).",
"The patient in this case had MOH as her secondary headache, probably initially from caffeine overuse and then from NSAIDs. Once she stopped her NSAID and caffeine use and was started on the appropriate regimen, the patient did very well. She at first needed a transitional course of prednisone, which was given on a taper over 12 days to avoid the withdrawal headache while the preventative medication, topiramate, was increased in her system. The abortives used were a combination of tizanidine, acetaminophen, and metoclopramide."
],
"date": "October 26, 2017",
"figures": [],
"markdown": "# Headache in a 19-Year-Old Woman With a Genetic Disorder\n\n **Authors:** Chad A. Whyte, MD \n **Date:** October 26, 2017\n\n ## Content\n\n Current treatment of migraine involves not only drugs to abort an active attack but also preventive medicines to be used prophylactically when attacks are frequent. Voxel-based morphometry studies have shown focal reductions in cortical volume in episodic migraine, as well as an increase in focal reductions with increased attack frequency.[9,10] Also, a high frequency of attacks leads to central nervous system hypometabolism and hyperexcitability.[11] The frequency of migraine attacks may be associated with chronic structural changes in the brain.\nThe only FDA-approved medications that have been designed specifically for migraine are the triptan class. They are 5-HT1 agonists that inhibit the release of neuroinflammatory substances and also constrict meningeal vessels. They are available in pill form and oral-dissolving, nasal, and subcutaneous preparations for those who have severe pain or nausea at the very beginning of an attack. Dihydroergotamine (DHE) is another first-line treatment that has existed since 1945 and is very effective intravenously. Contraindications to both triptans and DHE include coronary artery disease, cerebrovascular disease, and uncontrolled hypertension. These medicines were not used in the treatment of the patient in this case because of the vascular anomalies in her brain. Other abortive treatments include NSAIDs, acetaminophen, antiemetics (especially when used in conjunction with NSAIDs), and corticosteroids.\nVarious preventive medicines have been found to be successful in decreasing the frequency of migraine attacks. Although nothing has been designed for migraine, many classes have been used over the last 50 years, including antiseizure, antihypertensive, and antidepressant medications, as well as natural supplements. Currently, the only FDA-approved drugs for migraine prevention are propranolol, timolol, divalproex sodium (valproic acid), and topiramate; however, many other medicines are used and have been found successful vs placebo in adults, including gabapentin, verapamil, angiotensin-converting enzyme inhibitors, angiotensin-receptor blockers, venlafaxine, tricyclic antidepressants, magnesium, riboflavin, and cervicocranial botulinum toxin injections. A randomized, double-blind study in children and adolescents aged 8-17 years did not find a significant difference between placebo and amitriptyline or topiramate; however, the response rate to placebo (50% or more decrease in number of headache days) was 61%.[12]\nQuite often, patients do not respond to pharmacologic therapy and need nonpharmacologic therapy to help control their pain. It is important to ensure that other comorbidities do not complicate patient improvement. Depression, insomnia, and anxiety are much more common in those who suffer from migraine, and psychiatric therapy can be helpful in managing these issues. Biofeedback is used with success, especially in patients in whom medication therapy is not ideal (eg, pregnant women).\nThe patient in this case had MOH as her secondary headache, probably initially from caffeine overuse and then from NSAIDs. Once she stopped her NSAID and caffeine use and was started on the appropriate regimen, the patient did very well. She at first needed a transitional course of prednisone, which was given on a taper over 12 days to avoid the withdrawal headache while the preventative medication, topiramate, was increased in her system. The abortives used were a combination of tizanidine, acetaminophen, and metoclopramide.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 371627,
"choiceText": "Substance P",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 371628,
"choiceText": "Nitric oxide",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 371629,
"choiceText": "Calcitonin gene-related peptide",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 371630,
"choiceText": "Neurokinin A",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Calcitonin gene-related peptide (CGRP) is located at all sites in the central nervous system involved in migraine. It is perhaps the most potent vasodilator. CGRP inhibitors have shown efficacy in aborting migraine attacks and are currently in the process of being approved for use in migraine. ",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 106681,
"questionText": "A 39-year-old mother of 4 comes to you with a history of intermittent headaches for the past 6 months. She is progressively unable to do even the smallest chores when one of these headaches strikes. It usually lasts for 1 full day and is unilateral. She prefers to lie down in a quiet, dark room for the entire duration of the headache. Which of the following is the most important target of future therapy for this patient's migraines?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 371631,
"choiceText": "Hydrocodone",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 371632,
"choiceText": "Sumatriptan",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 371633,
"choiceText": "Aspirin-acetaminophen-caffeine",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 371634,
"choiceText": "Butalbital-APAP-caffeine",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Butalbital-containing mixtures have been shown to significantly increase the risk for medication overuse headache, thought to be due to the long half-life of butalbital and the short half-lives of APAP and caffeine. No studies to date have confirmed or refuted the efficacy of these drugs in the acute treatment of migraine.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 106682,
"questionText": "A 28-year-old white woman was diagnosed 3 months ago with migraine without aura. She is a pharmacology student and uses her migraine medicines very regularly, even when headache is not present, \"so the headache doesn't happen and I can concentrate on my exam.\" When questioned, she admits to drinking multiple cups of coffee daily. Which of the following pain-relieving substances is most associated with medication overuse headache?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Headache in a 19-Year-Old Woman With a Genetic Disorder"
},
{
"authors": "Chad A. Whyte, MD",
"content": [],
"date": "October 26, 2017",
"figures": [],
"markdown": "# Headache in a 19-Year-Old Woman With a Genetic Disorder\n\n **Authors:** Chad A. Whyte, MD \n **Date:** October 26, 2017\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 371627,
"choiceText": "Substance P",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 371628,
"choiceText": "Nitric oxide",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 371629,
"choiceText": "Calcitonin gene-related peptide",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 371630,
"choiceText": "Neurokinin A",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Calcitonin gene-related peptide (CGRP) is located at all sites in the central nervous system involved in migraine. It is perhaps the most potent vasodilator. CGRP inhibitors have shown efficacy in aborting migraine attacks and are currently in the process of being approved for use in migraine. ",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 106681,
"questionText": "A 39-year-old mother of 4 comes to you with a history of intermittent headaches for the past 6 months. She is progressively unable to do even the smallest chores when one of these headaches strikes. It usually lasts for 1 full day and is unilateral. She prefers to lie down in a quiet, dark room for the entire duration of the headache. Which of the following is the most important target of future therapy for this patient's migraines?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 371631,
"choiceText": "Hydrocodone",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 371632,
"choiceText": "Sumatriptan",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 371633,
"choiceText": "Aspirin-acetaminophen-caffeine",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 371634,
"choiceText": "Butalbital-APAP-caffeine",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Butalbital-containing mixtures have been shown to significantly increase the risk for medication overuse headache, thought to be due to the long half-life of butalbital and the short half-lives of APAP and caffeine. No studies to date have confirmed or refuted the efficacy of these drugs in the acute treatment of migraine.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 106682,
"questionText": "A 28-year-old white woman was diagnosed 3 months ago with migraine without aura. She is a pharmacology student and uses her migraine medicines very regularly, even when headache is not present, \"so the headache doesn't happen and I can concentrate on my exam.\" When questioned, she admits to drinking multiple cups of coffee daily. Which of the following pain-relieving substances is most associated with medication overuse headache?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Headache in a 19-Year-Old Woman With a Genetic Disorder"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 371623,
"choiceText": "Idiopathic intracranial hypertension",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 371624,
"choiceText": "Headache attributed to cranial or cervical vascular disorder",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 371625,
"choiceText": "Migraine without aura",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 371626,
"choiceText": "Psychogenic headache",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 106680,
"questionText": "Which of the following is the most likely diagnosis?<br /><br /> <i>Hint: Consider the patient's entire history.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 371627,
"choiceText": "Substance P",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 371628,
"choiceText": "Nitric oxide",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 371629,
"choiceText": "Calcitonin gene-related peptide",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 371630,
"choiceText": "Neurokinin A",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Calcitonin gene-related peptide (CGRP) is located at all sites in the central nervous system involved in migraine. It is perhaps the most potent vasodilator. CGRP inhibitors have shown efficacy in aborting migraine attacks and are currently in the process of being approved for use in migraine. ",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 106681,
"questionText": "A 39-year-old mother of 4 comes to you with a history of intermittent headaches for the past 6 months. She is progressively unable to do even the smallest chores when one of these headaches strikes. It usually lasts for 1 full day and is unilateral. She prefers to lie down in a quiet, dark room for the entire duration of the headache. Which of the following is the most important target of future therapy for this patient's migraines?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 371631,
"choiceText": "Hydrocodone",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 371632,
"choiceText": "Sumatriptan",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 371633,
"choiceText": "Aspirin-acetaminophen-caffeine",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 371634,
"choiceText": "Butalbital-APAP-caffeine",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Butalbital-containing mixtures have been shown to significantly increase the risk for medication overuse headache, thought to be due to the long half-life of butalbital and the short half-lives of APAP and caffeine. No studies to date have confirmed or refuted the efficacy of these drugs in the acute treatment of migraine.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 106682,
"questionText": "A 28-year-old white woman was diagnosed 3 months ago with migraine without aura. She is a pharmacology student and uses her migraine medicines very regularly, even when headache is not present, \"so the headache doesn't happen and I can concentrate on my exam.\" When questioned, she admits to drinking multiple cups of coffee daily. Which of the following pain-relieving substances is most associated with medication overuse headache?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
729080 | /viewarticle/729080 | [
{
"authors": "Craig A. Goolsby, MD",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"A 76-year-old woman presents to the emergency department (ED) with intense abdominal pain. She describes the pain as a diffuse, severe, constant ache throughout her abdomen. The pain woke her from sleep several hours ago; she felt well when going to bed last night, with no discomfort or other preceding symptoms. Today, she feels nauseous and has had 2 liquid bowel movements that were dark brown in color.",
"She has never had similar pain before. She denies having any fever, trauma, urinary symptoms, vaginal discharge, or bleeding. No chest pain, shortness of breath, or back pain has been noted. She has a medical history of atrial fibrillation, a myocardial infarction 2 years ago with 2 stents placed, hypertension, and chronic obstructive pulmonary disease (COPD).",
"She admits to being a lifelong smoker and has smoked 1-2 packs daily for at least 50 years. She has a glass of sherry nightly but denies ever using drugs. She has no history of abdominal surgery. She is a widowed homemaker who lives alone but has 2 daughters who live nearby. She has been prescribed multiple medications, including warfarin, atenolol, an ipratropium/salbutamol inhaler, lisinopril, a multivitamin, and hydrochlorothiazide. She admits to frequently missing doses of her medications and recently ran out of warfarin. She has no known drug allergies."
],
"date": "October 02, 2017",
"figures": [],
"markdown": "# A 76-Year-Old Woman With Abdominal Pain\n\n **Authors:** Craig A. Goolsby, MD \n **Date:** October 02, 2017\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nA 76-year-old woman presents to the emergency department (ED) with intense abdominal pain. She describes the pain as a diffuse, severe, constant ache throughout her abdomen. The pain woke her from sleep several hours ago; she felt well when going to bed last night, with no discomfort or other preceding symptoms. Today, she feels nauseous and has had 2 liquid bowel movements that were dark brown in color.\nShe has never had similar pain before. She denies having any fever, trauma, urinary symptoms, vaginal discharge, or bleeding. No chest pain, shortness of breath, or back pain has been noted. She has a medical history of atrial fibrillation, a myocardial infarction 2 years ago with 2 stents placed, hypertension, and chronic obstructive pulmonary disease (COPD).\nShe admits to being a lifelong smoker and has smoked 1-2 packs daily for at least 50 years. She has a glass of sherry nightly but denies ever using drugs. She has no history of abdominal surgery. She is a widowed homemaker who lives alone but has 2 daughters who live nearby. She has been prescribed multiple medications, including warfarin, atenolol, an ipratropium/salbutamol inhaler, lisinopril, a multivitamin, and hydrochlorothiazide. She admits to frequently missing doses of her medications and recently ran out of warfarin. She has no known drug allergies.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 76-Year-Old Woman With Abdominal Pain"
},
{
"authors": "Craig A. Goolsby, MD",
"content": [
"Upon physical examination, the patient appears to be in intense pain. She has an oral temperature of 99.4°F (37.4°C). She has an irregular heart rhythm, with a rate of 118 beats/min. Her blood pressure is 101/68 mm Hg, and her respiratory rate is 22 breaths/min. Her pulse oximetry reading is 93% on room air.",
"In general, she appears older than her stated age. She is appropriately alert and oriented. She has slight expiratory wheezing throughout the bilateral lung fields. No murmurs are appreciated on cardiac auscultation, and her abdomen has only mild tenderness to palpation diffusely. No peritoneal signs on examination, palpable masses, or abnormal pulsations are noted. Rectal examination reveals dark-brown stool that is negative for occult blood. She has no costovertebral angle tenderness. Her extremities are warm, with 1+ bilateral dorsalis pedis pulses.",
"The patient is placed on a cardiac monitor, 2 large-bore peripheral intravenous lines are placed, and fluid resuscitation with normal saline is started. The patient is given two 8-mg doses of morphine, with some pain improvement. She is given 4 mg of ondansetron for her nausea. A bedside laboratory test shows a hemoglobin level of 12.1 g/dL (121 g/L). Bedside ultrasonography reveals no free fluid in the abdomen. An electrocardiogram (ECG) demonstrates rapid atrial fibrillation, without an acute injury pattern. A stat upright chest radiograph is obtained that demonstrates hyperinflated lung fields, but no free air under the diaphragm or other acute abnormalities are seen.",
"Laboratory studies, including electrolytes, a hepatic panel, lipase, cardiac enzymes, and a complete blood count, are performed and found to be without significant abnormalities. Her international normalized ratio (INR) is subtherapeutic at 1.4. An abdominal CT angiography scan is obtained; representative images are shown (Figures 1 and 2).",
"Figure 1.",
"Figure 2."
],
"date": "October 02, 2017",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/728/262/728262-thumb2.png"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/728/262/728262-thumb1.png"
}
],
"markdown": "# A 76-Year-Old Woman With Abdominal Pain\n\n **Authors:** Craig A. Goolsby, MD \n **Date:** October 02, 2017\n\n ## Content\n\n Upon physical examination, the patient appears to be in intense pain. She has an oral temperature of 99.4°F (37.4°C). She has an irregular heart rhythm, with a rate of 118 beats/min. Her blood pressure is 101/68 mm Hg, and her respiratory rate is 22 breaths/min. Her pulse oximetry reading is 93% on room air.\nIn general, she appears older than her stated age. She is appropriately alert and oriented. She has slight expiratory wheezing throughout the bilateral lung fields. No murmurs are appreciated on cardiac auscultation, and her abdomen has only mild tenderness to palpation diffusely. No peritoneal signs on examination, palpable masses, or abnormal pulsations are noted. Rectal examination reveals dark-brown stool that is negative for occult blood. She has no costovertebral angle tenderness. Her extremities are warm, with 1+ bilateral dorsalis pedis pulses.\nThe patient is placed on a cardiac monitor, 2 large-bore peripheral intravenous lines are placed, and fluid resuscitation with normal saline is started. The patient is given two 8-mg doses of morphine, with some pain improvement. She is given 4 mg of ondansetron for her nausea. A bedside laboratory test shows a hemoglobin level of 12.1 g/dL (121 g/L). Bedside ultrasonography reveals no free fluid in the abdomen. An electrocardiogram (ECG) demonstrates rapid atrial fibrillation, without an acute injury pattern. A stat upright chest radiograph is obtained that demonstrates hyperinflated lung fields, but no free air under the diaphragm or other acute abnormalities are seen.\nLaboratory studies, including electrolytes, a hepatic panel, lipase, cardiac enzymes, and a complete blood count, are performed and found to be without significant abnormalities. Her international normalized ratio (INR) is subtherapeutic at 1.4. An abdominal CT angiography scan is obtained; representative images are shown (Figures 1 and 2).\nFigure 1.\nFigure 2.\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 366464,
"choiceText": "Emergency vascular surgery consultation",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 366465,
"choiceText": "Systemic thrombolysis with tissue-plasminogen activator",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 366466,
"choiceText": "Start the patient on systemic beta-blockers to control vascular shear stress",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 366467,
"choiceText": "Immediate transfusion of 2 units of packed red blood cells",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 104934,
"questionText": "Based on the findings seen in the CT image, what is the most appropriate immediate action?<br /><br /><i>Hint: This is a high-mortality condition that requires early intervention.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 76-Year-Old Woman With Abdominal Pain"
},
{
"authors": "Craig A. Goolsby, MD",
"content": [
"The patient in this case had many factors in her history and physical examination that raised concern for mesenteric ischemia. She had sudden, poorly localized abdominal pain that seemed subjectively much worse than her abdominal examination suggested. She also had atrial fibrillation and known vascular disease, with admitted poor treatment compliance. CT angiography showed an embolus in the patient’s superior mesenteric artery (Figure 2), with perienteric fat stranding, ascites, and wall thickening (Figure 3), as well as pneumatosis intestinalis and superior mesenteric vein (SMV) air (Figure 1) and portal venous air (Figure 4). While treatment options for mesenteric ischemia vary, an emergency vascular surgery consultation is required to arrange definitive care and early treatment of this potentially catastrophic condition.",
"Figure 1.",
"Figure 2.",
"Figure 3.",
"Figure 4.",
"Acute mesenteric ischemia (AMI) is a relatively uncommon condition that affects an estimated 0.1% of all hospital admissions.[1] AMI comprises 4 clinical entities: mesenteric venous thrombosis (MVT), acute mesenteric arterial thrombosis (AMAT), acute mesenteric arterial embolus (AMAE), and nonocclusive mesenteric ischemia (NOMI). Occlusive mesenteric arterial ischemia (OMAI) is an umbrella term that includes both AMAT and AMAE.[1] The final common pathway of these entities is bowel ischemia, eventually leading to bowel wall gangrene.[1] It can be a difficult disease to diagnose and treat, and it has a poor prognosis, with mortality rates ranging from 60%-100%.[2]"
],
"date": "October 02, 2017",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/728/262/728262-thumb2.png"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/728/262/728262-thumb1.png"
},
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/728/262/728262-thumb3.png"
},
{
"caption": "Figure 4.",
"image_url": "https://img.medscapestatic.com/article/728/262/728262-thumb4.png"
}
],
"markdown": "# A 76-Year-Old Woman With Abdominal Pain\n\n **Authors:** Craig A. Goolsby, MD \n **Date:** October 02, 2017\n\n ## Content\n\n The patient in this case had many factors in her history and physical examination that raised concern for mesenteric ischemia. She had sudden, poorly localized abdominal pain that seemed subjectively much worse than her abdominal examination suggested. She also had atrial fibrillation and known vascular disease, with admitted poor treatment compliance. CT angiography showed an embolus in the patient’s superior mesenteric artery (Figure 2), with perienteric fat stranding, ascites, and wall thickening (Figure 3), as well as pneumatosis intestinalis and superior mesenteric vein (SMV) air (Figure 1) and portal venous air (Figure 4). While treatment options for mesenteric ischemia vary, an emergency vascular surgery consultation is required to arrange definitive care and early treatment of this potentially catastrophic condition.\nFigure 1.\nFigure 2.\nFigure 3.\nFigure 4.\nAcute mesenteric ischemia (AMI) is a relatively uncommon condition that affects an estimated 0.1% of all hospital admissions.[1] AMI comprises 4 clinical entities: mesenteric venous thrombosis (MVT), acute mesenteric arterial thrombosis (AMAT), acute mesenteric arterial embolus (AMAE), and nonocclusive mesenteric ischemia (NOMI). Occlusive mesenteric arterial ischemia (OMAI) is an umbrella term that includes both AMAT and AMAE.[1] The final common pathway of these entities is bowel ischemia, eventually leading to bowel wall gangrene.[1] It can be a difficult disease to diagnose and treat, and it has a poor prognosis, with mortality rates ranging from 60%-100%.[2]\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n**Figure 3.** \n \n\n**Figure 4.** \n \n\n\n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 366464,
"choiceText": "Emergency vascular surgery consultation",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 366465,
"choiceText": "Systemic thrombolysis with tissue-plasminogen activator",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 366466,
"choiceText": "Start the patient on systemic beta-blockers to control vascular shear stress",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 366467,
"choiceText": "Immediate transfusion of 2 units of packed red blood cells",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 104934,
"questionText": "Based on the findings seen in the CT image, what is the most appropriate immediate action?<br /><br /><i>Hint: This is a high-mortality condition that requires early intervention.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 76-Year-Old Woman With Abdominal Pain"
},
{
"authors": "Craig A. Goolsby, MD",
"content": [
"AMI has many causes, depending on the underlying pathology. Emboli can come from cardiac thrombi that develop after myocardial infarction or in association with atrial fibrillation or valvular disease. Thrombi can come from atherosclerotic disease, aortic dissection or aneurysm, and decreased output from heart failure.[1]",
"NOMI occurs in the setting of any low-flow state, such as congestive heart failure, sepsis, or cocaine use (vasoconstriction).[1] Additionally, hypercoagulable states, such as protein-C or -S deficiency, pregnancy, cancer, or intra-abdominal infection, also predispose patients to developing thrombosis.[1]",
"The most common presenting symptom in patients with AMI present is abdominal pain, which is typically severe, constant, and poorly localized.[1,2] Pain onset varies depending on the underlying pathology; for example, embolic disease usually results in abrupt pain onset, whereas NOMI may cause subacute symptom progression over several days.[1]",
"The hallmark finding of AMI is pain out of proportion to physical findings, as patients rarely manifest the peritoneal signs or focal tenderness normally associated with abdominal emergencies.[1] Diarrhea occurs in up to 50% of patients; nausea and vomiting are also frequently present.[2] Similar to patients with cardiac ischemia, 20%-50% of patients may experience abdominal angina prior to developing AMI.[1] Abdominal angina is a clinical syndrome of postprandial abdominal pain lasting up to 3 hours after meals, and is associated with a change in bowel movements, early satiety, and weight loss.[1] However, postprandial pain and weight loss are more often seen in patients with chronic mesenteric ischemia, as opposed to patients with AMI.",
"Patients may also present with concurrent disease causing their AMI. For example, a congestive heart failure exacerbation or dehydration secondary to gastroenteritis may trigger patients to develop NOMI.[1]",
"Diagnosing AMI involves a combination of clinical suspicion and imaging. Laboratory tests are typically nonspecific and not helpful in diagnosing AMI.[1] Leukocytosis often develops as the disease progresses, and more than 75% of patients eventually develop white blood cell counts greater than 15,000 cells/mm3.[2] Lactate levels generally rise late in the clinical course; levels that remain persistently normal suggest another diagnosis is more likely than AMI.[1] However, levels within the reference range are frequently seen in the acute phase of the disease. Lactate levels also have poor specificity, at 42%-77%, even when positive.[3]",
"Elevated serum amylase and potassium levels may be noted because the intestine produces amylase in response to injury and ischemia releases intracellular potassium. Metabolic acidosis and elevated LDH may also be present. Radiography is usually not helpful in diagnosing AMI; however, they may be helpful in ruling out other causes of abdominal catastrophes. In someone with excruciating pain associated with AMI, plain radiography can rule out a perforated viscus may be warranted.[1]",
"The most important test for identifying mesenteric ischemia is angiography. CT angiography has largely supplanted traditional angiography due to its increased ease of use and minimally invasive approach.[1] CT angiography has sensitivities for AMI reported between 96%-100%, and specificities between 89%-94%.[2] Ultrasonography has shown good potential for identifying and evaluating mesenteric ischemia. It has a high specificity of 92%-100%, but suffers poor sensitivities of 70%-89%.[1] Like other abdominal ultrasonography, the results vary depending on operator skill, patient body habitus, and bowel gas patterns.[2] Magnetic resonance (MR) angiography has been shown to have sensitivities and specificities similar to CT angiography[1]; however, CT angiography has higher spatial resolution and faster acquisition times, and better identification of calcified plaques.[4] In addition, MRA is limited by both time and expense constraints, and it cannot be recommended as a first-line diagnostic choice, except in cases where CT contrast cannot be tolerated.[1,2,4] Traditional angiography are seldom used for diagnosing AMI in an ED setting, but when it is used, a lateral aortogram is the best view to image the mesenteric vessels as they arise from the aorta anteriorly."
],
"date": "October 02, 2017",
"figures": [],
"markdown": "# A 76-Year-Old Woman With Abdominal Pain\n\n **Authors:** Craig A. Goolsby, MD \n **Date:** October 02, 2017\n\n ## Content\n\n AMI has many causes, depending on the underlying pathology. Emboli can come from cardiac thrombi that develop after myocardial infarction or in association with atrial fibrillation or valvular disease. Thrombi can come from atherosclerotic disease, aortic dissection or aneurysm, and decreased output from heart failure.[1]\nNOMI occurs in the setting of any low-flow state, such as congestive heart failure, sepsis, or cocaine use (vasoconstriction).[1] Additionally, hypercoagulable states, such as protein-C or -S deficiency, pregnancy, cancer, or intra-abdominal infection, also predispose patients to developing thrombosis.[1]\nThe most common presenting symptom in patients with AMI present is abdominal pain, which is typically severe, constant, and poorly localized.[1,2] Pain onset varies depending on the underlying pathology; for example, embolic disease usually results in abrupt pain onset, whereas NOMI may cause subacute symptom progression over several days.[1]\nThe hallmark finding of AMI is pain out of proportion to physical findings, as patients rarely manifest the peritoneal signs or focal tenderness normally associated with abdominal emergencies.[1] Diarrhea occurs in up to 50% of patients; nausea and vomiting are also frequently present.[2] Similar to patients with cardiac ischemia, 20%-50% of patients may experience abdominal angina prior to developing AMI.[1] Abdominal angina is a clinical syndrome of postprandial abdominal pain lasting up to 3 hours after meals, and is associated with a change in bowel movements, early satiety, and weight loss.[1] However, postprandial pain and weight loss are more often seen in patients with chronic mesenteric ischemia, as opposed to patients with AMI.\nPatients may also present with concurrent disease causing their AMI. For example, a congestive heart failure exacerbation or dehydration secondary to gastroenteritis may trigger patients to develop NOMI.[1]\nDiagnosing AMI involves a combination of clinical suspicion and imaging. Laboratory tests are typically nonspecific and not helpful in diagnosing AMI.[1] Leukocytosis often develops as the disease progresses, and more than 75% of patients eventually develop white blood cell counts greater than 15,000 cells/mm3.[2] Lactate levels generally rise late in the clinical course; levels that remain persistently normal suggest another diagnosis is more likely than AMI.[1] However, levels within the reference range are frequently seen in the acute phase of the disease. Lactate levels also have poor specificity, at 42%-77%, even when positive.[3]\nElevated serum amylase and potassium levels may be noted because the intestine produces amylase in response to injury and ischemia releases intracellular potassium. Metabolic acidosis and elevated LDH may also be present. Radiography is usually not helpful in diagnosing AMI; however, they may be helpful in ruling out other causes of abdominal catastrophes. In someone with excruciating pain associated with AMI, plain radiography can rule out a perforated viscus may be warranted.[1]\nThe most important test for identifying mesenteric ischemia is angiography. CT angiography has largely supplanted traditional angiography due to its increased ease of use and minimally invasive approach.[1] CT angiography has sensitivities for AMI reported between 96%-100%, and specificities between 89%-94%.[2] Ultrasonography has shown good potential for identifying and evaluating mesenteric ischemia. It has a high specificity of 92%-100%, but suffers poor sensitivities of 70%-89%.[1] Like other abdominal ultrasonography, the results vary depending on operator skill, patient body habitus, and bowel gas patterns.[2] Magnetic resonance (MR) angiography has been shown to have sensitivities and specificities similar to CT angiography[1]; however, CT angiography has higher spatial resolution and faster acquisition times, and better identification of calcified plaques.[4] In addition, MRA is limited by both time and expense constraints, and it cannot be recommended as a first-line diagnostic choice, except in cases where CT contrast cannot be tolerated.[1,2,4] Traditional angiography are seldom used for diagnosing AMI in an ED setting, but when it is used, a lateral aortogram is the best view to image the mesenteric vessels as they arise from the aorta anteriorly.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 76-Year-Old Woman With Abdominal Pain"
},
{
"authors": "Craig A. Goolsby, MD",
"content": [
"Multiple treatments should be initiated for patients with either suspected or confirmed AMI. First, supportive care should be initiated. Supplemental oxygen with potential airway intervention should be considered. Due to the underlying ischemia, the patient’s tissues may benefit from improved blood oxygen content. Fluid resuscitation, improving cardiac parameters for patients with decompensated heart failure, broad-spectrum antibiotics, and liberal pain control should all be considered.[2]",
"Due to the high mortality of AMI, consultation with a vascular surgeon should be obtained early, often before obtaining definitive diagnostic testing.[1,2] Definitive treatment options are numerous and depend on the type of AMI present. Options include papaverine infusion in NOMI, surgical embolectomy, intra-arterial thrombolysis, bypass grafting, stenting, anticoagulation, and--in NOMI (ie, no pressors, maximized cardiac function)--medically optimizing the patient’s vascular flow.[1,2] Treatment choices will vary depending on the underlying cause of the AMI and the preferences of the consulting vascular surgeons and interventional radiologists involved in the patient’s care.",
"Patients diagnosed with AMI must be admitted to the hospital, probably to an intensive care unit (ICU) or directly to an operating room or angiography suite (when indicated). Aggressive medical optimization in the ED setting is warranted, pending definitive treatment.",
"In the above case, a vascular surgeon was called, based on the patient’s clinical presentation. The CT angiography was obtained while the vascular surgeon traveled to the hospital. After review of the test results and the patient’s clinical appearance, the patient was admitted to the hospital for anticipated intra-arterial thrombolysis and further supportive critical care."
],
"date": "October 02, 2017",
"figures": [],
"markdown": "# A 76-Year-Old Woman With Abdominal Pain\n\n **Authors:** Craig A. Goolsby, MD \n **Date:** October 02, 2017\n\n ## Content\n\n Multiple treatments should be initiated for patients with either suspected or confirmed AMI. First, supportive care should be initiated. Supplemental oxygen with potential airway intervention should be considered. Due to the underlying ischemia, the patient’s tissues may benefit from improved blood oxygen content. Fluid resuscitation, improving cardiac parameters for patients with decompensated heart failure, broad-spectrum antibiotics, and liberal pain control should all be considered.[2]\nDue to the high mortality of AMI, consultation with a vascular surgeon should be obtained early, often before obtaining definitive diagnostic testing.[1,2] Definitive treatment options are numerous and depend on the type of AMI present. Options include papaverine infusion in NOMI, surgical embolectomy, intra-arterial thrombolysis, bypass grafting, stenting, anticoagulation, and--in NOMI (ie, no pressors, maximized cardiac function)--medically optimizing the patient’s vascular flow.[1,2] Treatment choices will vary depending on the underlying cause of the AMI and the preferences of the consulting vascular surgeons and interventional radiologists involved in the patient’s care.\nPatients diagnosed with AMI must be admitted to the hospital, probably to an intensive care unit (ICU) or directly to an operating room or angiography suite (when indicated). Aggressive medical optimization in the ED setting is warranted, pending definitive treatment.\nIn the above case, a vascular surgeon was called, based on the patient’s clinical presentation. The CT angiography was obtained while the vascular surgeon traveled to the hospital. After review of the test results and the patient’s clinical appearance, the patient was admitted to the hospital for anticipated intra-arterial thrombolysis and further supportive critical care.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 366468,
"choiceText": "Ultrasonography",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 366469,
"choiceText": "Magnetic resonance angiography",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 366470,
"choiceText": "CT angiography",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 366471,
"choiceText": "Traditional angiography",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "While multiple imaging modalities can lead to the diagnosis of AMI, CT angiography has the advantage of speed, minimal invasiveness, and high sensitivity and specificity that make it ideal for the emergency diagnosis of AMI.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 104935,
"questionText": "An elderly patient with a history of peripheral vascular disease presents with diffuse abdominal pain but no significant abdominal tenderness, raising concern for acute mesenteric ischemia (AMI). What is the preferred imaging modality for diagnosing AMI?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 366472,
"choiceText": "Pain out of proportion to physical exam",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 366473,
"choiceText": "Rapid onset of crampy abdominal pain, vomiting, and diarrhea in a patient with poorly controlled atrial fibrillation",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 366474,
"choiceText": "Severe diffuse abdominal pain radiating to the back",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 366475,
"choiceText": "Abdominal pain in conjunction with low-grade fevers and bloody stools",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 366476,
"choiceText": "Nausea and vomiting without passage of stool or flatus",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Patients with AMI can often have a difficult clinical picture. However, the classic hallmark sign is poorly localized, intense abdominal pain that seems to be out of proportion with the physical exam.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 104936,
"questionText": "Abdominal pain is a frequent complaint among patients and has numerous potential causes of wide-ranging severity. Which of the following is a clinical finding most worrisome for AMI?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 76-Year-Old Woman With Abdominal Pain"
},
{
"authors": "Craig A. Goolsby, MD",
"content": [],
"date": "October 02, 2017",
"figures": [],
"markdown": "# A 76-Year-Old Woman With Abdominal Pain\n\n **Authors:** Craig A. Goolsby, MD \n **Date:** October 02, 2017\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 366468,
"choiceText": "Ultrasonography",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 366469,
"choiceText": "Magnetic resonance angiography",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 366470,
"choiceText": "CT angiography",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 366471,
"choiceText": "Traditional angiography",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "While multiple imaging modalities can lead to the diagnosis of AMI, CT angiography has the advantage of speed, minimal invasiveness, and high sensitivity and specificity that make it ideal for the emergency diagnosis of AMI.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 104935,
"questionText": "An elderly patient with a history of peripheral vascular disease presents with diffuse abdominal pain but no significant abdominal tenderness, raising concern for acute mesenteric ischemia (AMI). What is the preferred imaging modality for diagnosing AMI?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 366472,
"choiceText": "Pain out of proportion to physical exam",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 366473,
"choiceText": "Rapid onset of crampy abdominal pain, vomiting, and diarrhea in a patient with poorly controlled atrial fibrillation",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 366474,
"choiceText": "Severe diffuse abdominal pain radiating to the back",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 366475,
"choiceText": "Abdominal pain in conjunction with low-grade fevers and bloody stools",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 366476,
"choiceText": "Nausea and vomiting without passage of stool or flatus",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Patients with AMI can often have a difficult clinical picture. However, the classic hallmark sign is poorly localized, intense abdominal pain that seems to be out of proportion with the physical exam.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 104936,
"questionText": "Abdominal pain is a frequent complaint among patients and has numerous potential causes of wide-ranging severity. Which of the following is a clinical finding most worrisome for AMI?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 76-Year-Old Woman With Abdominal Pain"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 366464,
"choiceText": "Emergency vascular surgery consultation",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 366465,
"choiceText": "Systemic thrombolysis with tissue-plasminogen activator",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 366466,
"choiceText": "Start the patient on systemic beta-blockers to control vascular shear stress",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 366467,
"choiceText": "Immediate transfusion of 2 units of packed red blood cells",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 104934,
"questionText": "Based on the findings seen in the CT image, what is the most appropriate immediate action?<br /><br /><i>Hint: This is a high-mortality condition that requires early intervention.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 366468,
"choiceText": "Ultrasonography",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 366469,
"choiceText": "Magnetic resonance angiography",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 366470,
"choiceText": "CT angiography",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 366471,
"choiceText": "Traditional angiography",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "While multiple imaging modalities can lead to the diagnosis of AMI, CT angiography has the advantage of speed, minimal invasiveness, and high sensitivity and specificity that make it ideal for the emergency diagnosis of AMI.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 104935,
"questionText": "An elderly patient with a history of peripheral vascular disease presents with diffuse abdominal pain but no significant abdominal tenderness, raising concern for acute mesenteric ischemia (AMI). What is the preferred imaging modality for diagnosing AMI?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 366472,
"choiceText": "Pain out of proportion to physical exam",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 366473,
"choiceText": "Rapid onset of crampy abdominal pain, vomiting, and diarrhea in a patient with poorly controlled atrial fibrillation",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 366474,
"choiceText": "Severe diffuse abdominal pain radiating to the back",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 366475,
"choiceText": "Abdominal pain in conjunction with low-grade fevers and bloody stools",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 366476,
"choiceText": "Nausea and vomiting without passage of stool or flatus",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Patients with AMI can often have a difficult clinical picture. However, the classic hallmark sign is poorly localized, intense abdominal pain that seems to be out of proportion with the physical exam.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 104936,
"questionText": "Abdominal pain is a frequent complaint among patients and has numerous potential causes of wide-ranging severity. Which of the following is a clinical finding most worrisome for AMI?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
728248 | /viewarticle/728248 | [
{
"authors": "Zhanna Servetnyk, MD; Constantine Albany, MD; Ira S. Meisels, MD",
"content": [
"Editorial Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions.",
"A 41-year-old black woman presents to the emergency department (ED) with a 5-day history of increasing shortness of breath along with a mild, nonproductive cough. She denies having any fever, chills, chest pain, or palpitations. No sick contacts are identified, and she denies any recent travel. On further questioning, the patient reports that she first experienced shortness of breath 1 year ago, and that it has been gradually progressive since then. Her shortness of breath has particularly worsened over the last 5 days, to the point that it is limiting her daily activities; this has prompted her to come to the ED. She does not attribute the shortness of breath to any precipitating event. Her past medical history is significant for asthma and hypertension, as well as for several episodes of presumed bronchitis/pneumonia last year; she recalls being treated with antibiotics as an outpatient by her primary care provider. She also has a history of heavy menstrual periods, for which she has been on oral contraceptive pills (OCPs). Her family history is positive for diabetes mellitus and ischemic heart disease. Except for the OCPs, she is nonadherent to the rest of her regular medications, which include an angiotensin-converting enzyme (ACE) inhibitor, an inhaled corticosteroid, and a beta-2 agonist. She quit smoking 10 years ago, after a 20-pack-year history. She denies any alcohol or illicit drug use, and she has no known allergies (drug or otherwise).",
"Figure 1.",
"Figure 2.",
"Figure 3."
],
"date": "July 30, 2014",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/727/495/727495-thumb1.png"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/727/495/727495-thumb2.png"
},
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/727/495/727495-thumb3.png"
}
],
"markdown": "# A 41-Year-Old Woman With Shortness of Breath, Hematuria, and Lymphadenopathy\n\n **Authors:** Zhanna Servetnyk, MD; Constantine Albany, MD; Ira S. Meisels, MD \n **Date:** July 30, 2014\n\n ## Content\n\n Editorial Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions.\nA 41-year-old black woman presents to the emergency department (ED) with a 5-day history of increasing shortness of breath along with a mild, nonproductive cough. She denies having any fever, chills, chest pain, or palpitations. No sick contacts are identified, and she denies any recent travel. On further questioning, the patient reports that she first experienced shortness of breath 1 year ago, and that it has been gradually progressive since then. Her shortness of breath has particularly worsened over the last 5 days, to the point that it is limiting her daily activities; this has prompted her to come to the ED. She does not attribute the shortness of breath to any precipitating event. Her past medical history is significant for asthma and hypertension, as well as for several episodes of presumed bronchitis/pneumonia last year; she recalls being treated with antibiotics as an outpatient by her primary care provider. She also has a history of heavy menstrual periods, for which she has been on oral contraceptive pills (OCPs). Her family history is positive for diabetes mellitus and ischemic heart disease. Except for the OCPs, she is nonadherent to the rest of her regular medications, which include an angiotensin-converting enzyme (ACE) inhibitor, an inhaled corticosteroid, and a beta-2 agonist. She quit smoking 10 years ago, after a 20-pack-year history. She denies any alcohol or illicit drug use, and she has no known allergies (drug or otherwise).\nFigure 1.\nFigure 2.\nFigure 3.\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n**Figure 3.** \n \n\n\n*Page 1 of 5*",
"pagination": {
"current_page": 1,
"total_pages": 5
},
"questionnaire": [],
"title": "A 41-Year-Old Woman With Shortness of Breath, Hematuria, and Lymphadenopathy"
},
{
"authors": "Zhanna Servetnyk, MD; Constantine Albany, MD; Ira S. Meisels, MD",
"content": [
"On physical examination, she appears to be in no acute distress. She is morbidly obese, with a body mass index (BMI) of 45. Her blood pressure is 170/95 mm Hg, her heart rate is 106 bpm, her respiratory rate is 18 breaths/min, and her temperature is 98.6°F (37°C). She has an oxygen saturation of 97% while breathing room air. Pertinent findings on chest examination include fine crackles at the lung bases, with decreased vocal fremitus. Auscultation of the rest of the chest reveals no abnormalities. Her cardiovascular examination shows normal first and second heart sounds, with no jugular venous distention, murmurs, rubs, or gallops. There are, however, several enlarged, nontender cervical and axillary lymph nodes bilaterally. She has no rashes. The neurologic examination is nonfocal. Her peripheral pulses are palpable. Examination of her lower extremities elicits mild bilateral pitting pedal edema. The rest of her examination reveals no significant findings.",
"An electrocardiogram is performed that is remarkable for sinus tachycardia. The initial laboratory workup reveals a creatinine of 1.6 mg/dL (141.44 µmol/L), proteinuria (>300 g/L), and hematuria (50-100 red blood cells per high-power field). She is found to have anemia, with a hemoglobin and hematocrit of 8.1 g/dL (81 g/L) and 25.4% (0.254), respectively. Her mean corpuscular volume is 77 µm3 (77 fL), with iron levels of less than 10 g/dL, a total iron binding capacity of 197 μg/dL (35.26 µmol/L), and a ferritin level of 68 ng/mL (152.8 pmol/L). The D-dimer is positive at 4.73 μg/mL (4.73 mg/L), and the erythrocyte sedimentation rate (ESR) is elevated at 50 mm/hr. A chest x-ray (Figure 1) obtained during the ED visit shows a right-sided pleural effusion and a patchy linear opacity at the base of the left lung that is consistent with atelectasis/scar tissue. Computed tomography (CT) scanning of the thorax shows bilateral small pleural effusions that are greater on the right than the left, significantly enlarged axillary and subpectoral lymph nodes bilaterally, and a small pericardial effusion. Ultrasonography of the kidneys, ureters, and bladder reveals no obstruction.",
"Figure 1.",
"Figure 2.",
"Figure 3."
],
"date": "July 30, 2014",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/727/495/727495-thumb1.png"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/727/495/727495-thumb2.png"
},
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/727/495/727495-thumb3.png"
}
],
"markdown": "# A 41-Year-Old Woman With Shortness of Breath, Hematuria, and Lymphadenopathy\n\n **Authors:** Zhanna Servetnyk, MD; Constantine Albany, MD; Ira S. Meisels, MD \n **Date:** July 30, 2014\n\n ## Content\n\n On physical examination, she appears to be in no acute distress. She is morbidly obese, with a body mass index (BMI) of 45. Her blood pressure is 170/95 mm Hg, her heart rate is 106 bpm, her respiratory rate is 18 breaths/min, and her temperature is 98.6°F (37°C). She has an oxygen saturation of 97% while breathing room air. Pertinent findings on chest examination include fine crackles at the lung bases, with decreased vocal fremitus. Auscultation of the rest of the chest reveals no abnormalities. Her cardiovascular examination shows normal first and second heart sounds, with no jugular venous distention, murmurs, rubs, or gallops. There are, however, several enlarged, nontender cervical and axillary lymph nodes bilaterally. She has no rashes. The neurologic examination is nonfocal. Her peripheral pulses are palpable. Examination of her lower extremities elicits mild bilateral pitting pedal edema. The rest of her examination reveals no significant findings.\nAn electrocardiogram is performed that is remarkable for sinus tachycardia. The initial laboratory workup reveals a creatinine of 1.6 mg/dL (141.44 µmol/L), proteinuria (>300 g/L), and hematuria (50-100 red blood cells per high-power field). She is found to have anemia, with a hemoglobin and hematocrit of 8.1 g/dL (81 g/L) and 25.4% (0.254), respectively. Her mean corpuscular volume is 77 µm3 (77 fL), with iron levels of less than 10 g/dL, a total iron binding capacity of 197 μg/dL (35.26 µmol/L), and a ferritin level of 68 ng/mL (152.8 pmol/L). The D-dimer is positive at 4.73 μg/mL (4.73 mg/L), and the erythrocyte sedimentation rate (ESR) is elevated at 50 mm/hr. A chest x-ray (Figure 1) obtained during the ED visit shows a right-sided pleural effusion and a patchy linear opacity at the base of the left lung that is consistent with atelectasis/scar tissue. Computed tomography (CT) scanning of the thorax shows bilateral small pleural effusions that are greater on the right than the left, significantly enlarged axillary and subpectoral lymph nodes bilaterally, and a small pericardial effusion. Ultrasonography of the kidneys, ureters, and bladder reveals no obstruction.\nFigure 1.\nFigure 2.\nFigure 3.\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n**Figure 3.** \n \n\n\n*Page 2 of 5*",
"pagination": {
"current_page": 2,
"total_pages": 5
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 364421,
"choiceText": "COPD exacerbation",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 364422,
"choiceText": "Pulmonary embolism",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 364423,
"choiceText": "Systemic lupus erythematosus",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 364424,
"choiceText": "Sarcoidosis",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 364425,
"choiceText": "Congestive heart failure",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 104235,
"questionText": "Keeping the history in mind, what is the most likely diagnosis?<br /><br /><i>Hint: Asymptomatic azotemia and proteinuria are often a part of the picture in this disease.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 41-Year-Old Woman With Shortness of Breath, Hematuria, and Lymphadenopathy"
},
{
"authors": "Zhanna Servetnyk, MD; Constantine Albany, MD; Ira S. Meisels, MD",
"content": [
"The constellation of serositis, hypertension, diffuse lymphadenopathy, azotemia, and proteinuria in this patient was highly suspicious for a systemic autoimmune disease. Further laboratory workup revealed a positive result for antinuclear antibodies (ANA; titer of 1:640), including those against double-stranded DNA (ds-DNA); an IgG titer of 300 IU/mL; and an anti-Smith finding (156 EU/mL). These results, when correlated with the clinical picture, are consistent with a diagnosis of systemic lupus erythematosus. Other relevant findings included a decreased complement level, with C3 and C4 levels of 46.2 mg/dL (0.462 g/L) and 5.3 mg/dL (0.053 g/L), respectively; a positive Coombs test; hypoalbuminemia (3.1 g/dL [31 g/L]); and a negative lupus anticoagulant antibody finding. Biopsy of the kidney was performed, which showed diffuse proliferative lupus nephritis class IV, with moderate activity and no chronicity; moderate interstitial inflammation was also seen (Figures 2 and 3).",
"Figure 1.",
"Figure 2.",
"Figure 3."
],
"date": "July 30, 2014",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/727/495/727495-thumb1.png"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/727/495/727495-thumb2.png"
},
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/727/495/727495-thumb3.png"
}
],
"markdown": "# A 41-Year-Old Woman With Shortness of Breath, Hematuria, and Lymphadenopathy\n\n **Authors:** Zhanna Servetnyk, MD; Constantine Albany, MD; Ira S. Meisels, MD \n **Date:** July 30, 2014\n\n ## Content\n\n The constellation of serositis, hypertension, diffuse lymphadenopathy, azotemia, and proteinuria in this patient was highly suspicious for a systemic autoimmune disease. Further laboratory workup revealed a positive result for antinuclear antibodies (ANA; titer of 1:640), including those against double-stranded DNA (ds-DNA); an IgG titer of 300 IU/mL; and an anti-Smith finding (156 EU/mL). These results, when correlated with the clinical picture, are consistent with a diagnosis of systemic lupus erythematosus. Other relevant findings included a decreased complement level, with C3 and C4 levels of 46.2 mg/dL (0.462 g/L) and 5.3 mg/dL (0.053 g/L), respectively; a positive Coombs test; hypoalbuminemia (3.1 g/dL [31 g/L]); and a negative lupus anticoagulant antibody finding. Biopsy of the kidney was performed, which showed diffuse proliferative lupus nephritis class IV, with moderate activity and no chronicity; moderate interstitial inflammation was also seen (Figures 2 and 3).\nFigure 1.\nFigure 2.\nFigure 3.\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n**Figure 3.** \n \n\n\n*Page 3 of 5*",
"pagination": {
"current_page": 3,
"total_pages": 5
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 364421,
"choiceText": "COPD exacerbation",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 364422,
"choiceText": "Pulmonary embolism",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 364423,
"choiceText": "Systemic lupus erythematosus",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 364424,
"choiceText": "Sarcoidosis",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 364425,
"choiceText": "Congestive heart failure",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 104235,
"questionText": "Keeping the history in mind, what is the most likely diagnosis?<br /><br /><i>Hint: Asymptomatic azotemia and proteinuria are often a part of the picture in this disease.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 41-Year-Old Woman With Shortness of Breath, Hematuria, and Lymphadenopathy"
},
{
"authors": "Zhanna Servetnyk, MD; Constantine Albany, MD; Ira S. Meisels, MD",
"content": [
"Systemic lupus erythematosus (SLE) is an autoimmune disorder of unknown cause with chronic inflammation. Multiple organ systems, including the skin, joints, kidneys, lungs, nervous system, serous membranes, and/or other organs of the body, may be affected. As is typical of other autoimmune disorders, the immune system attacks the body’s own cells and tissue, which results in a continuous inflammatory response and subsequent tissue damage.",
"Figure 1.",
"Figure 2.",
"Figure 3.",
"SLE is more common, and more severe, in nonwhite patients. The highest prevalence among ethnic groups is in blacks.[1] SLE is up to 10 times more common in women than in men and typically occurs during childbearing age, with a peak age of onset between 15 and 45 years. The disease course is as variable as it is unpredictable, with periods of illness, or \"flares,\" alternating with periods of remission. Estrogen may play a role in the pathophysiology of SLE, but this has not yet been proven conclusively. Women exposed to estrogen-containing OCPs, however, do have an increased risk of developing SLE, especially if they started using them recently.[2]",
"SLE is characterized by a wide spectrum of signs and symptoms due to the ongoing presence of a variety of antigens, auto-antibodies, and immune complexes, which result in accumulated tissue damage to the point of clinical manifestation. According to the American College of Rheumatology's 2012 revision of SLE classification criteria, a diagnosis of SLE requires a patient to have biopsy-proven lupus nephritis with ANA or anti-dsDNA antibodies or to have 4 of the diagnostic criteria for the disease (either serially or simultaneously), including at least 1 clinical and 1 immunologic criterion. These criteria include the following[3]:",
"Acute or subacute cutaneous lupus (eg, lupus malar rash, photosensitive lupus rash)",
"Chronic cutaneous lupus (eg, classic discoid rash, hypertrophic [verrucous] lupus)",
"Oral or nasal ulcers",
"Nonscarring alopecia",
"Synovitis in at least two joints or tenderness in at least two joints with at least half an hour of morning stiffness",
"Serositis",
"Renal manifestations",
"Neurologic manifestations (eg, seizures, psychosis)",
"Hemolytic anemia",
"Leukopenia or lymphopenia",
"Thrombocytopenia",
"The immunologic criteria include the following:",
"ANA level above the laboratory reference range",
"Anti-dsDNA antibody level above the laboratory reference range",
"Anti-Smith antibody–positive",
"Antiphospholipid antibody–positive",
"Low complement level (C3, C4, CH50)",
"Direct Coombs test",
"About 80% of patients have skin involvement manifesting as photosensitivity, malar (butterfly) and discoid rash (thick, red, scaly patches on the skin), ulcers in the oral and nasal cavities, or alopecia, all of which are part of ACR diagnostic criteria. However, an absence of skin manifestation, as described in this patient, should not lower clinical suspicion for lupus because its symptoms vary and come and go unpredictably. Diagnosing SLE can thus be elusive, with some patients suffering from unexplained symptoms of untreated SLE for years, such as the initial (and chronic) complaints of fever, malaise, joint pains, myalgias, and fatigue, as well as temporary loss of cognitive abilities.",
"When SLE is diagnosed, it is important to establish the severity and potential reversibility of the illness in order to institute appropriate therapy. Treatment options usually focus on the suppression of symptoms rather than treating the cause, since there is not an actual \"cure\" for the disease. Renal disease remains the most serious complication of SLE. It affects 30%-60% of patients.[4] Renal involvement is characterized by proteinuria (> 0.5 g/24h) and/or active urinary sediment (> 5 red blood cells per high-power field, pyuria, or cell casts). In patients with inactive sediment and > 500 mg/day of proteinuria, monitoring is recommended with urinalysis every 3-6 months for 3 years; every 3 months is preferred in patients with anti–double-stranded DNA antibodies and/or hypocomplementemia.[5]",
"Prompt renal biopsy is required in all lupus patients with evidence of kidney involvement to determine the histologic subtype of lupus nephritis.[6] The World Health Organization (WHO) classification of glomerulonephritis in SLE includes class I to VI.[7] Pathology reports also reflect the extent of inflammatory (reversible) and chronic (irreversible scarring) changes. In general, treatment for lupus nephritis is not recommended in patients with class I or II disease or in those with extensive irreversible changes. In contrast, aggressive immunosuppression is recommended for patients with disease class III, IV, or V with inflammatory proliferative lesions, because a majority of those individuals, if untreated, develop end-stage renal disease (ESRD).[8] However, ESRD is seen in fewer than 5% of SLE cases, due to earlier detection and subsequent prompt management of the disease. Lupus nephritis tends to be an ongoing disease, with flares that may require repeat biopsy and repeated treatment over time.",
"Pleural inflammation is a common feature of SLE and is the most common pulmonary manifestation of SLE. It causes chest pain, shortness of breath, and cough. Pleural effusions are typical findings and are usually exudates.[9] Less frequent pulmonary complications of SLE include interstitial lung disease and pulmonary hypertension.",
"The most common hematologic manifestation of SLE is anemia, which is often overlooked in young menstruating women. Iron deficiency may also develop. Leukopenia and thrombocytopenia are common as well, which may be due to the primary disease process or may be a side effect of the subsequent pharmacologic treatment for the disease. Leukopenia almost always consists of lymphopenia, not granulocytopenia.[10] Patients with SLE may also have signs and symptoms of a thrombotic disorder known as antiphospholipid syndrome, wherein autoantibodies to phospholipids are present in the serum. Serum levels of anticardiolipin antibodies, anti-β2-glycoprotein I antibodies, and lupus anticoagulant should also be checked in patients suspected of having SLE. The presence of these antibodies may result in a false positive test for syphilis. Lymphadenopathy is not an uncommon presentation in SLE; it occurs in 15%-26% of patients. However, diffuse lymphadenopathy is very rare and is reported in very few cases.[11,12] It is not one of the ACR criteria for the diagnosis of SLE. Lymph node biopsy may be warranted to exclude alternative diagnoses. The patient in this case underwent lymph node biopsy, which showed no evidence of lymphoproliferative disorder.",
"A number of research protocols (Systemic Lupus Activity Measure [SLAM], SLE Disease Activity Index [SLEDAI], British Isles Lupus Assessment Group [BILAG], etc) have been designed in order to accurately monitor disease activity;[13,14,15] all of them use combinations of history, physical examination findings, and laboratory data. Meanwhile, the search for reliable markers of disease activity continues. Employment of complement activation products, soluble T-cell activation markers, antibodies to C1q, and nucleosomes have been reported, but these have not been validated for universal clinical use.[16] Currently, widely used indicators include anti–double-stranded DNA antibodies, complement levels, hemoglobin levels, platelet count, urine analysis, and serum levels of creatinine and albumin. Because there is great variability in SLE's presentation and course, flare prediction in a particular patient should take into account laboratory findings that correlate with past flares.",
"Treatment of SLE is aimed at controlling acute flares and using maintenance strategies that suppress symptoms to an acceptable level, while preventing further organ damage. For milder manifestations of SLE, such as arthritis, dermatitis, and constitutional symptoms, NSAIDs, hydroxychloroquine, and low-dose steroids have been used with success. Severe forms of the disease, including those with more life-threatening courses (including lupus nephritis), require a combination therapy of high-dose systemic glucocorticoids and cyclophosphamide or mycophenolate mofetil to induce remission, followed by longer-term, intense immunosuppressive therapy to maintain response.[17] The immunosuppressive agent azathioprine, which can be used for maintenance in severe SLE, is also often administered to induce remission and as a steroid-sparing drug in patients with mild to moderate forms. Methotrexate and leflunomide can be used to treat mild to moderate arthritis and rash. Mepacrine (quinacrine/atabrine) also can be used to treat rash.[18] In 2011, the monoclonal antibody belimumab received US Food and Drug Administration (FDA) approval for the treatment of ANA- or anti-dsDNA–positive SLE that remains active despite standard treatment.[19]",
"The patient in this case began treatment with high-dose oral corticosteroids and mycophenolate mofetil. After 1 week, her condition improved, with significantly decreased shortness of breath. She was discharged to home with renal, rheumatologic, and primary care outpatient follow-up."
],
"date": "July 30, 2014",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/727/495/727495-thumb1.png"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/727/495/727495-thumb2.png"
},
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/727/495/727495-thumb3.png"
}
],
"markdown": "# A 41-Year-Old Woman With Shortness of Breath, Hematuria, and Lymphadenopathy\n\n **Authors:** Zhanna Servetnyk, MD; Constantine Albany, MD; Ira S. Meisels, MD \n **Date:** July 30, 2014\n\n ## Content\n\n Systemic lupus erythematosus (SLE) is an autoimmune disorder of unknown cause with chronic inflammation. Multiple organ systems, including the skin, joints, kidneys, lungs, nervous system, serous membranes, and/or other organs of the body, may be affected. As is typical of other autoimmune disorders, the immune system attacks the body’s own cells and tissue, which results in a continuous inflammatory response and subsequent tissue damage.\nFigure 1.\nFigure 2.\nFigure 3.\nSLE is more common, and more severe, in nonwhite patients. The highest prevalence among ethnic groups is in blacks.[1] SLE is up to 10 times more common in women than in men and typically occurs during childbearing age, with a peak age of onset between 15 and 45 years. The disease course is as variable as it is unpredictable, with periods of illness, or \"flares,\" alternating with periods of remission. Estrogen may play a role in the pathophysiology of SLE, but this has not yet been proven conclusively. Women exposed to estrogen-containing OCPs, however, do have an increased risk of developing SLE, especially if they started using them recently.[2]\nSLE is characterized by a wide spectrum of signs and symptoms due to the ongoing presence of a variety of antigens, auto-antibodies, and immune complexes, which result in accumulated tissue damage to the point of clinical manifestation. According to the American College of Rheumatology's 2012 revision of SLE classification criteria, a diagnosis of SLE requires a patient to have biopsy-proven lupus nephritis with ANA or anti-dsDNA antibodies or to have 4 of the diagnostic criteria for the disease (either serially or simultaneously), including at least 1 clinical and 1 immunologic criterion. These criteria include the following[3]:\nAcute or subacute cutaneous lupus (eg, lupus malar rash, photosensitive lupus rash)\nChronic cutaneous lupus (eg, classic discoid rash, hypertrophic [verrucous] lupus)\nOral or nasal ulcers\nNonscarring alopecia\nSynovitis in at least two joints or tenderness in at least two joints with at least half an hour of morning stiffness\nSerositis\nRenal manifestations\nNeurologic manifestations (eg, seizures, psychosis)\nHemolytic anemia\nLeukopenia or lymphopenia\nThrombocytopenia\nThe immunologic criteria include the following:\nANA level above the laboratory reference range\nAnti-dsDNA antibody level above the laboratory reference range\nAnti-Smith antibody–positive\nAntiphospholipid antibody–positive\nLow complement level (C3, C4, CH50)\nDirect Coombs test\nAbout 80% of patients have skin involvement manifesting as photosensitivity, malar (butterfly) and discoid rash (thick, red, scaly patches on the skin), ulcers in the oral and nasal cavities, or alopecia, all of which are part of ACR diagnostic criteria. However, an absence of skin manifestation, as described in this patient, should not lower clinical suspicion for lupus because its symptoms vary and come and go unpredictably. Diagnosing SLE can thus be elusive, with some patients suffering from unexplained symptoms of untreated SLE for years, such as the initial (and chronic) complaints of fever, malaise, joint pains, myalgias, and fatigue, as well as temporary loss of cognitive abilities.\nWhen SLE is diagnosed, it is important to establish the severity and potential reversibility of the illness in order to institute appropriate therapy. Treatment options usually focus on the suppression of symptoms rather than treating the cause, since there is not an actual \"cure\" for the disease. Renal disease remains the most serious complication of SLE. It affects 30%-60% of patients.[4] Renal involvement is characterized by proteinuria (> 0.5 g/24h) and/or active urinary sediment (> 5 red blood cells per high-power field, pyuria, or cell casts). In patients with inactive sediment and > 500 mg/day of proteinuria, monitoring is recommended with urinalysis every 3-6 months for 3 years; every 3 months is preferred in patients with anti–double-stranded DNA antibodies and/or hypocomplementemia.[5]\nPrompt renal biopsy is required in all lupus patients with evidence of kidney involvement to determine the histologic subtype of lupus nephritis.[6] The World Health Organization (WHO) classification of glomerulonephritis in SLE includes class I to VI.[7] Pathology reports also reflect the extent of inflammatory (reversible) and chronic (irreversible scarring) changes. In general, treatment for lupus nephritis is not recommended in patients with class I or II disease or in those with extensive irreversible changes. In contrast, aggressive immunosuppression is recommended for patients with disease class III, IV, or V with inflammatory proliferative lesions, because a majority of those individuals, if untreated, develop end-stage renal disease (ESRD).[8] However, ESRD is seen in fewer than 5% of SLE cases, due to earlier detection and subsequent prompt management of the disease. Lupus nephritis tends to be an ongoing disease, with flares that may require repeat biopsy and repeated treatment over time.\nPleural inflammation is a common feature of SLE and is the most common pulmonary manifestation of SLE. It causes chest pain, shortness of breath, and cough. Pleural effusions are typical findings and are usually exudates.[9] Less frequent pulmonary complications of SLE include interstitial lung disease and pulmonary hypertension.\nThe most common hematologic manifestation of SLE is anemia, which is often overlooked in young menstruating women. Iron deficiency may also develop. Leukopenia and thrombocytopenia are common as well, which may be due to the primary disease process or may be a side effect of the subsequent pharmacologic treatment for the disease. Leukopenia almost always consists of lymphopenia, not granulocytopenia.[10] Patients with SLE may also have signs and symptoms of a thrombotic disorder known as antiphospholipid syndrome, wherein autoantibodies to phospholipids are present in the serum. Serum levels of anticardiolipin antibodies, anti-β2-glycoprotein I antibodies, and lupus anticoagulant should also be checked in patients suspected of having SLE. The presence of these antibodies may result in a false positive test for syphilis. Lymphadenopathy is not an uncommon presentation in SLE; it occurs in 15%-26% of patients. However, diffuse lymphadenopathy is very rare and is reported in very few cases.[11,12] It is not one of the ACR criteria for the diagnosis of SLE. Lymph node biopsy may be warranted to exclude alternative diagnoses. The patient in this case underwent lymph node biopsy, which showed no evidence of lymphoproliferative disorder.\nA number of research protocols (Systemic Lupus Activity Measure [SLAM], SLE Disease Activity Index [SLEDAI], British Isles Lupus Assessment Group [BILAG], etc) have been designed in order to accurately monitor disease activity;[13,14,15] all of them use combinations of history, physical examination findings, and laboratory data. Meanwhile, the search for reliable markers of disease activity continues. Employment of complement activation products, soluble T-cell activation markers, antibodies to C1q, and nucleosomes have been reported, but these have not been validated for universal clinical use.[16] Currently, widely used indicators include anti–double-stranded DNA antibodies, complement levels, hemoglobin levels, platelet count, urine analysis, and serum levels of creatinine and albumin. Because there is great variability in SLE's presentation and course, flare prediction in a particular patient should take into account laboratory findings that correlate with past flares.\nTreatment of SLE is aimed at controlling acute flares and using maintenance strategies that suppress symptoms to an acceptable level, while preventing further organ damage. For milder manifestations of SLE, such as arthritis, dermatitis, and constitutional symptoms, NSAIDs, hydroxychloroquine, and low-dose steroids have been used with success. Severe forms of the disease, including those with more life-threatening courses (including lupus nephritis), require a combination therapy of high-dose systemic glucocorticoids and cyclophosphamide or mycophenolate mofetil to induce remission, followed by longer-term, intense immunosuppressive therapy to maintain response.[17] The immunosuppressive agent azathioprine, which can be used for maintenance in severe SLE, is also often administered to induce remission and as a steroid-sparing drug in patients with mild to moderate forms. Methotrexate and leflunomide can be used to treat mild to moderate arthritis and rash. Mepacrine (quinacrine/atabrine) also can be used to treat rash.[18] In 2011, the monoclonal antibody belimumab received US Food and Drug Administration (FDA) approval for the treatment of ANA- or anti-dsDNA–positive SLE that remains active despite standard treatment.[19]\nThe patient in this case began treatment with high-dose oral corticosteroids and mycophenolate mofetil. After 1 week, her condition improved, with significantly decreased shortness of breath. She was discharged to home with renal, rheumatologic, and primary care outpatient follow-up.\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n**Figure 3.** \n \n\n\n*Page 4 of 5*",
"pagination": {
"current_page": 4,
"total_pages": 5
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 364426,
"choiceText": "Photosensitivity",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 364427,
"choiceText": "Arthritis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 364428,
"choiceText": "Proteinuria",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 364429,
"choiceText": "Lymphadenopathy",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 364430,
"choiceText": "Anemia",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The ACR diagnostic criteria includes serositis (pleuritis, pericarditis), oral ulcers, arthritis (nonerosive), photosensitivity (exposure to UV radiation causes skin manifestations), blood dyscrasias (hemolytic anemia, leucopenia, thrombocytopenia), renal manifestations (proteinuria, casts), ANA positive, immunologic disorder (anti-Smith antibodies positive, anti-phospholipid antibody positive, false positive test for syphilis, presence of anti–double-stranded DNA antibodies), neurologic manifestations (seizures, focal signs, psychosis), malar (butterfly) rash, and discoid rash. A patient is considered to have SLE if any 4 of the above manifestations are present simultaneously, or on 2 separate occasions serially.",
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 104236,
"questionText": "You are evaluating a patient who presents with a number of symptoms indicating possible SLE. Which of the following symptoms would NOT cause you to suspect SLE in this patient?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 364431,
"choiceText": "When she develops symptoms",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 364432,
"choiceText": "In 1 month",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 364433,
"choiceText": "In 3 months",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 364434,
"choiceText": "In 12 months",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 364435,
"choiceText": "First perform a biopsy, and then arrange for follow-up every three months",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "In a patient with known SLE who shows no renal findings on urinalysis or a finding of > 500 mg of protein/day in the urine, follow-up is advised every 3-6 months, for at least 3 years.",
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 104237,
"questionText": "You are examining a patient who was diagnosed with SLE last year. Today, at your office, she is asymptomatic and her urine analysis shows no proteinuria and 1-3 RBCs per high-power field, with no casts. What is the appropriate time frame for her kidney function follow-up?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 41-Year-Old Woman With Shortness of Breath, Hematuria, and Lymphadenopathy"
},
{
"authors": "Zhanna Servetnyk, MD; Constantine Albany, MD; Ira S. Meisels, MD",
"content": [],
"date": "July 30, 2014",
"figures": [],
"markdown": "# A 41-Year-Old Woman With Shortness of Breath, Hematuria, and Lymphadenopathy\n\n **Authors:** Zhanna Servetnyk, MD; Constantine Albany, MD; Ira S. Meisels, MD \n **Date:** July 30, 2014\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 5 of 5*",
"pagination": {
"current_page": 5,
"total_pages": 5
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 364426,
"choiceText": "Photosensitivity",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 364427,
"choiceText": "Arthritis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 364428,
"choiceText": "Proteinuria",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 364429,
"choiceText": "Lymphadenopathy",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 364430,
"choiceText": "Anemia",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The ACR diagnostic criteria includes serositis (pleuritis, pericarditis), oral ulcers, arthritis (nonerosive), photosensitivity (exposure to UV radiation causes skin manifestations), blood dyscrasias (hemolytic anemia, leucopenia, thrombocytopenia), renal manifestations (proteinuria, casts), ANA positive, immunologic disorder (anti-Smith antibodies positive, anti-phospholipid antibody positive, false positive test for syphilis, presence of anti–double-stranded DNA antibodies), neurologic manifestations (seizures, focal signs, psychosis), malar (butterfly) rash, and discoid rash. A patient is considered to have SLE if any 4 of the above manifestations are present simultaneously, or on 2 separate occasions serially.",
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 104236,
"questionText": "You are evaluating a patient who presents with a number of symptoms indicating possible SLE. Which of the following symptoms would NOT cause you to suspect SLE in this patient?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 364431,
"choiceText": "When she develops symptoms",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 364432,
"choiceText": "In 1 month",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 364433,
"choiceText": "In 3 months",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 364434,
"choiceText": "In 12 months",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 364435,
"choiceText": "First perform a biopsy, and then arrange for follow-up every three months",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "In a patient with known SLE who shows no renal findings on urinalysis or a finding of > 500 mg of protein/day in the urine, follow-up is advised every 3-6 months, for at least 3 years.",
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 104237,
"questionText": "You are examining a patient who was diagnosed with SLE last year. Today, at your office, she is asymptomatic and her urine analysis shows no proteinuria and 1-3 RBCs per high-power field, with no casts. What is the appropriate time frame for her kidney function follow-up?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 41-Year-Old Woman With Shortness of Breath, Hematuria, and Lymphadenopathy"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 364421,
"choiceText": "COPD exacerbation",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 364422,
"choiceText": "Pulmonary embolism",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 364423,
"choiceText": "Systemic lupus erythematosus",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 364424,
"choiceText": "Sarcoidosis",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 364425,
"choiceText": "Congestive heart failure",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 104235,
"questionText": "Keeping the history in mind, what is the most likely diagnosis?<br /><br /><i>Hint: Asymptomatic azotemia and proteinuria are often a part of the picture in this disease.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 364426,
"choiceText": "Photosensitivity",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 364427,
"choiceText": "Arthritis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 364428,
"choiceText": "Proteinuria",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 364429,
"choiceText": "Lymphadenopathy",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 364430,
"choiceText": "Anemia",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The ACR diagnostic criteria includes serositis (pleuritis, pericarditis), oral ulcers, arthritis (nonerosive), photosensitivity (exposure to UV radiation causes skin manifestations), blood dyscrasias (hemolytic anemia, leucopenia, thrombocytopenia), renal manifestations (proteinuria, casts), ANA positive, immunologic disorder (anti-Smith antibodies positive, anti-phospholipid antibody positive, false positive test for syphilis, presence of anti–double-stranded DNA antibodies), neurologic manifestations (seizures, focal signs, psychosis), malar (butterfly) rash, and discoid rash. A patient is considered to have SLE if any 4 of the above manifestations are present simultaneously, or on 2 separate occasions serially.",
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 104236,
"questionText": "You are evaluating a patient who presents with a number of symptoms indicating possible SLE. Which of the following symptoms would NOT cause you to suspect SLE in this patient?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 364431,
"choiceText": "When she develops symptoms",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 364432,
"choiceText": "In 1 month",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 364433,
"choiceText": "In 3 months",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 364434,
"choiceText": "In 12 months",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 364435,
"choiceText": "First perform a biopsy, and then arrange for follow-up every three months",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "In a patient with known SLE who shows no renal findings on urinalysis or a finding of > 500 mg of protein/day in the urine, follow-up is advised every 3-6 months, for at least 3 years.",
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 104237,
"questionText": "You are examining a patient who was diagnosed with SLE last year. Today, at your office, she is asymptomatic and her urine analysis shows no proteinuria and 1-3 RBCs per high-power field, with no casts. What is the appropriate time frame for her kidney function follow-up?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
727508 | /viewarticle/727508 | [
{
"authors": "Gautam Dehadrai, MD",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"A 76-year-old man presents to the emergency department (ED) due to a sudden onset of abdominal pain. The pain started about 4 hours before presentation to the ED and has been persistent; it is present in the upper abdomen and is centered in the epigastrium. He describes the pain as deep and burning. No associated nausea or vomiting is reported. He does not report any changes in his bowel habits and has not experienced any recent fevers. The review of systems is also negative for any recent unintended weight loss or trauma.",
"The patient also reports having had \"indigestion\" in the past that caused pain similar to what he is currently experiencing, although much less in intensity. His past medical history is significant for coronary artery disease and hypertension. He takes two medications, both for his high blood pressure, but does not drink excessively and does not smoke."
],
"date": "April 12, 2017",
"figures": [],
"markdown": "# Acute Onset of Abdominal Pain in a 76-Year-Old Man\n\n **Authors:** Gautam Dehadrai, MD \n **Date:** April 12, 2017\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nA 76-year-old man presents to the emergency department (ED) due to a sudden onset of abdominal pain. The pain started about 4 hours before presentation to the ED and has been persistent; it is present in the upper abdomen and is centered in the epigastrium. He describes the pain as deep and burning. No associated nausea or vomiting is reported. He does not report any changes in his bowel habits and has not experienced any recent fevers. The review of systems is also negative for any recent unintended weight loss or trauma.\nThe patient also reports having had \"indigestion\" in the past that caused pain similar to what he is currently experiencing, although much less in intensity. His past medical history is significant for coronary artery disease and hypertension. He takes two medications, both for his high blood pressure, but does not drink excessively and does not smoke.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "Acute Onset of Abdominal Pain in a 76-Year-Old Man"
},
{
"authors": "Gautam Dehadrai, MD",
"content": [
"Upon physical examination, the patient is pale and in obvious severe discomfort. His heart rate is 122 beats/min and his blood pressure is 110/65 mm Hg. He is breathing with rapid shallow breaths at a rate greater than 30 breaths/min. His temperature is normal at 99.2ºF (37.3ºC), and his pulse oximetry reading while breathing room air shows a saturation rate of 100%.",
"The cardiovascular and respiratory findings are unremarkable. The patient has significant tenderness in the epigastric region, with a rigid abdomen. Little to no tenderness to palpation is noted in the lower quadrants; a reliable assessment of the upper quadrants is not possible because of the tenderness in the epigastric region. Hyperactive bowel sounds are heard on auscultation. The patient's stool is brown and guaiac positive.",
"Figure 1.",
"Figure 2.",
"An electrocardiogram is performed and is noted to be unremarkable except for sinus tachycardia. A complete blood count (CBC) and a chemistry panel are sent. The CBC reveals mild anemia, with a hemoglobin concentration of 127 g/L (12.7 g/dL). On the chemistry panel, evidence suggests a slight azotemia, with a blood urea nitrogen level of 17.1 mmol/L (48 mg/dL) and a creatinine value of 106 µmol/L (1.2 mg/dL). The remainder of the laboratory investigations is unremarkable. Plain radiographs of the abdomen are performed (see Figures 1 and 2)."
],
"date": "April 12, 2017",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/727/508/727508-Thumb1.png"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/727/508/727508-Thumb2.png"
}
],
"markdown": "# Acute Onset of Abdominal Pain in a 76-Year-Old Man\n\n **Authors:** Gautam Dehadrai, MD \n **Date:** April 12, 2017\n\n ## Content\n\n Upon physical examination, the patient is pale and in obvious severe discomfort. His heart rate is 122 beats/min and his blood pressure is 110/65 mm Hg. He is breathing with rapid shallow breaths at a rate greater than 30 breaths/min. His temperature is normal at 99.2ºF (37.3ºC), and his pulse oximetry reading while breathing room air shows a saturation rate of 100%.\nThe cardiovascular and respiratory findings are unremarkable. The patient has significant tenderness in the epigastric region, with a rigid abdomen. Little to no tenderness to palpation is noted in the lower quadrants; a reliable assessment of the upper quadrants is not possible because of the tenderness in the epigastric region. Hyperactive bowel sounds are heard on auscultation. The patient's stool is brown and guaiac positive.\nFigure 1.\nFigure 2.\nAn electrocardiogram is performed and is noted to be unremarkable except for sinus tachycardia. A complete blood count (CBC) and a chemistry panel are sent. The CBC reveals mild anemia, with a hemoglobin concentration of 127 g/L (12.7 g/dL). On the chemistry panel, evidence suggests a slight azotemia, with a blood urea nitrogen level of 17.1 mmol/L (48 mg/dL) and a creatinine value of 106 µmol/L (1.2 mg/dL). The remainder of the laboratory investigations is unremarkable. Plain radiographs of the abdomen are performed (see Figures 1 and 2).\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 363120,
"choiceText": "Abdominal aortic aneurysm rupture",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 363121,
"choiceText": "Pancreatitis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 363122,
"choiceText": "Pneumoperitoneum from duodenal ulcer perforation",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 363123,
"choiceText": "Acute coronary syndrome",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 103808,
"questionText": "Which of the following is the most likely diagnosis?<br /><br />\r\n<i>Hint: Both the inner and the outer walls of the bowel are visible.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Acute Onset of Abdominal Pain in a 76-Year-Old Man"
},
{
"authors": "Gautam Dehadrai, MD",
"content": [
"Clear definition of both the inner wall and outer wall of the bowel on Figure 3 (the Rigler sign; see below for explanation) and the presence of free air under the right hemidiaphragm on Figure 4 demonstrate pneumoperitoneum. The term \"pneumoperitoneum\" refers to air in the peritoneal cavity. The differential diagnosis of pneumoperitoneum includes iatrogenic causes (eg, peritoneal dialysis; abdominal surgery, including laparoscopy and laparotomy, after which pneumoperitoneum can persist for up to 28 days), blunt or penetrating trauma, perforation of the hollow viscera (eg, gastric ulcer, duodenal ulcer), pneumatosis intestinalis or pneumatosis coli, vaginal insufflation, and gas from the mediastinum (eg, from barotrauma).[1]",
"Figure 3.",
"Figure 4.",
"Although many patients with perforated peptic ulcers initially present with severe abdominal pain as well as epigastric tenderness and classic signs of peritonitis, patients who are elderly, are immunocompromised, or who have altered mental status may have only minimal signs and symptoms. In one study of patients older than 60 years with perforated ulcers, only 70% had abdominal pain. Other patients reported symptoms that included dyspepsia, anorexia, nausea, and vomiting. Severe abdominal pain was present in only 16% of patients.[2]",
"About 6% of patients with perforated ulcers have no abdominal findings. In most cases of perforation from ulcer disease, gastric and duodenal contents (ie, bile, ingested food, swallowed bacteria) leak into the peritoneum, resulting in peritonitis and increased risk of infection and abscess formation. In addition to sepsis, subsequent third-spacing of fluid into the peritoneal cavity caused by the peritonitis can lead to hypotension and shock.[1]"
],
"date": "April 12, 2017",
"figures": [
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/727/508/727508-Thumb3.png"
},
{
"caption": "Figure 4.",
"image_url": "https://img.medscapestatic.com/article/727/508/727508-Thumb4.png"
}
],
"markdown": "# Acute Onset of Abdominal Pain in a 76-Year-Old Man\n\n **Authors:** Gautam Dehadrai, MD \n **Date:** April 12, 2017\n\n ## Content\n\n Clear definition of both the inner wall and outer wall of the bowel on Figure 3 (the Rigler sign; see below for explanation) and the presence of free air under the right hemidiaphragm on Figure 4 demonstrate pneumoperitoneum. The term \"pneumoperitoneum\" refers to air in the peritoneal cavity. The differential diagnosis of pneumoperitoneum includes iatrogenic causes (eg, peritoneal dialysis; abdominal surgery, including laparoscopy and laparotomy, after which pneumoperitoneum can persist for up to 28 days), blunt or penetrating trauma, perforation of the hollow viscera (eg, gastric ulcer, duodenal ulcer), pneumatosis intestinalis or pneumatosis coli, vaginal insufflation, and gas from the mediastinum (eg, from barotrauma).[1]\nFigure 3.\nFigure 4.\nAlthough many patients with perforated peptic ulcers initially present with severe abdominal pain as well as epigastric tenderness and classic signs of peritonitis, patients who are elderly, are immunocompromised, or who have altered mental status may have only minimal signs and symptoms. In one study of patients older than 60 years with perforated ulcers, only 70% had abdominal pain. Other patients reported symptoms that included dyspepsia, anorexia, nausea, and vomiting. Severe abdominal pain was present in only 16% of patients.[2]\nAbout 6% of patients with perforated ulcers have no abdominal findings. In most cases of perforation from ulcer disease, gastric and duodenal contents (ie, bile, ingested food, swallowed bacteria) leak into the peritoneum, resulting in peritonitis and increased risk of infection and abscess formation. In addition to sepsis, subsequent third-spacing of fluid into the peritoneal cavity caused by the peritonitis can lead to hypotension and shock.[1]\n\n ## Figures\n\n **Figure 3.** \n \n\n**Figure 4.** \n \n\n\n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 363120,
"choiceText": "Abdominal aortic aneurysm rupture",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 363121,
"choiceText": "Pancreatitis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 363122,
"choiceText": "Pneumoperitoneum from duodenal ulcer perforation",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 363123,
"choiceText": "Acute coronary syndrome",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 103808,
"questionText": "Which of the following is the most likely diagnosis?<br /><br />\r\n<i>Hint: Both the inner and the outer walls of the bowel are visible.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Acute Onset of Abdominal Pain in a 76-Year-Old Man"
},
{
"authors": "Gautam Dehadrai, MD",
"content": [
"The underlying pathophysiology of a duodenal ulcer is a common condition that is characterized by the presence of a well-demarcated mucosal defect in the duodenum. Approximately 95% of duodenal ulcers are found in the first part of the duodenum; most are smaller than 1 cm in diameter. A prompt and accurate diagnosis combined with treatment can prevent potentially serious complications, such as perforation (which occurred in this case).",
"The duodenal mucosa resists damage from the effects of gastric acid and the proteolytic enzyme pepsin because of the protective qualities of the mucous/gel layer produced by the mucus-secreting epithelial cells, bicarbonate secretions from other gastric and duodenal cellular components, and protective prostaglandins. If gastric acid and pepsin penetrate the mucous layer and reach the epithelial cells, ion pumps in the basolateral cell membrane regulate intracellular pH by removing excess hydrogen ions; healthy epithelial cells migrate to the site of the injury; and mucosal blood flow serves to remove any excess acid diffused through the injured mucosa.",
"Despite these barriers and mechanisms to prevent permanent injury, ulcerations can occur. Any pathophysiologic or iatrogenic process that increases gastric acidity (eg, disease states with increased maximal and basal acid output), decreases prostaglandin production (eg, nonsteroidal anti-inflammatory drug [NSAID] use, which inhibits the cyclooxygenase-1 [COX-1] pathway), or interferes with the mucous layer (eg, Helicobacter pylori infection leading to stimulation of gastric acid production) can result in the formation of peptic ulcer disease.[1,3]",
"In the United States, the prevalence of duodenal ulcer is estimated to be 6%-15% in the general population. Most individuals with duodenal ulcers do not have clinically significant disease. The prevalence is closely linked to the presence of H pylori infection. Among individuals infected with H pylori, the lifetime prevalence is approximately 20%. Internationally, the prevalence of the disease varies among countries and, as in the US, is linked to rates of H pylori infection. [3,4]",
"Duodenal ulcers can result in significant morbidity and mortality. Their main complication is pain; however, serious complications can include ulcer hemorrhage, perforation, penetration, and obstruction. Complications and mortality are generally greater in elderly patients than in other patient populations, possibly because of a higher incidence of comorbid disease and an increased use of NSAIDs in this group.[3,4]",
"When a perforation is suspected, as in this case, an upright chest or left lateral decubitus radiograph can detect as little as 1-2 mL of gas under the diaphragm or lateral margin of the liver, especially if strict positioning techniques are used. Therefore, these studies should be the first diagnostic modalities used. Supine abdominal radiographs are generally of limited value in diagnosing pneumoperitoneum. CT scanning is highly sensitive for depicting pneumoperitoneum, and it has the added benefit of assisting the clinician in identifying the underlying etiology in many patients.[1,5,6]",
"Numerous signs are described for diagnosing pneumoperitoneum on plain radiographs. One of the best known, the Rigler sign (also known as the double-wall or bas-relief sign), is a visualization of the outer surface of a bowel loop wall resulting from free air in the peritoneal cavity. The intraluminal gas provides negative contrast and outlines the internal wall. The cupola sign, typically seen on supine radiographs, is an inverted cup-shaped arcuate lucency overlying the lower thoracic spine and projecting caudally to the heart. This sign is formed as air accumulates anteriorly in the median subphrenic space under the central leaf of the diaphragm. The umbilical ligaments, the urachus, and particularly the falciform ligament are sometimes identified as linear radiopaque structures in the presence of free air.[1,3,5,7]",
"Another common sign is a collection of gas in the right upper quadrant adjacent to the liver, lying mainly in the subhepatic space and in the hepatorenal fossa, which is visible as an oval or triangular gas shadow not in obvious continuity with the rest of the bowel. This collection is usually present in the medial aspect of the right upper quadrant, with a superomedial to inferolateral orientation.[1,3,5,7]",
"The football sign is visualization of the entire peritoneal cavity as an oval gas shadow, with the vertically oriented falciform ligament representing the seam of an American football. This sign is most often seen in the pediatric patient but not in the adult patient, because in the adult patient, there is usually not enough air relative to the size of the peritoneal cavity.[1,7]"
],
"date": "April 12, 2017",
"figures": [],
"markdown": "# Acute Onset of Abdominal Pain in a 76-Year-Old Man\n\n **Authors:** Gautam Dehadrai, MD \n **Date:** April 12, 2017\n\n ## Content\n\n The underlying pathophysiology of a duodenal ulcer is a common condition that is characterized by the presence of a well-demarcated mucosal defect in the duodenum. Approximately 95% of duodenal ulcers are found in the first part of the duodenum; most are smaller than 1 cm in diameter. A prompt and accurate diagnosis combined with treatment can prevent potentially serious complications, such as perforation (which occurred in this case).\nThe duodenal mucosa resists damage from the effects of gastric acid and the proteolytic enzyme pepsin because of the protective qualities of the mucous/gel layer produced by the mucus-secreting epithelial cells, bicarbonate secretions from other gastric and duodenal cellular components, and protective prostaglandins. If gastric acid and pepsin penetrate the mucous layer and reach the epithelial cells, ion pumps in the basolateral cell membrane regulate intracellular pH by removing excess hydrogen ions; healthy epithelial cells migrate to the site of the injury; and mucosal blood flow serves to remove any excess acid diffused through the injured mucosa.\nDespite these barriers and mechanisms to prevent permanent injury, ulcerations can occur. Any pathophysiologic or iatrogenic process that increases gastric acidity (eg, disease states with increased maximal and basal acid output), decreases prostaglandin production (eg, nonsteroidal anti-inflammatory drug [NSAID] use, which inhibits the cyclooxygenase-1 [COX-1] pathway), or interferes with the mucous layer (eg, Helicobacter pylori infection leading to stimulation of gastric acid production) can result in the formation of peptic ulcer disease.[1,3]\nIn the United States, the prevalence of duodenal ulcer is estimated to be 6%-15% in the general population. Most individuals with duodenal ulcers do not have clinically significant disease. The prevalence is closely linked to the presence of H pylori infection. Among individuals infected with H pylori, the lifetime prevalence is approximately 20%. Internationally, the prevalence of the disease varies among countries and, as in the US, is linked to rates of H pylori infection. [3,4]\nDuodenal ulcers can result in significant morbidity and mortality. Their main complication is pain; however, serious complications can include ulcer hemorrhage, perforation, penetration, and obstruction. Complications and mortality are generally greater in elderly patients than in other patient populations, possibly because of a higher incidence of comorbid disease and an increased use of NSAIDs in this group.[3,4]\nWhen a perforation is suspected, as in this case, an upright chest or left lateral decubitus radiograph can detect as little as 1-2 mL of gas under the diaphragm or lateral margin of the liver, especially if strict positioning techniques are used. Therefore, these studies should be the first diagnostic modalities used. Supine abdominal radiographs are generally of limited value in diagnosing pneumoperitoneum. CT scanning is highly sensitive for depicting pneumoperitoneum, and it has the added benefit of assisting the clinician in identifying the underlying etiology in many patients.[1,5,6]\nNumerous signs are described for diagnosing pneumoperitoneum on plain radiographs. One of the best known, the Rigler sign (also known as the double-wall or bas-relief sign), is a visualization of the outer surface of a bowel loop wall resulting from free air in the peritoneal cavity. The intraluminal gas provides negative contrast and outlines the internal wall. The cupola sign, typically seen on supine radiographs, is an inverted cup-shaped arcuate lucency overlying the lower thoracic spine and projecting caudally to the heart. This sign is formed as air accumulates anteriorly in the median subphrenic space under the central leaf of the diaphragm. The umbilical ligaments, the urachus, and particularly the falciform ligament are sometimes identified as linear radiopaque structures in the presence of free air.[1,3,5,7]\nAnother common sign is a collection of gas in the right upper quadrant adjacent to the liver, lying mainly in the subhepatic space and in the hepatorenal fossa, which is visible as an oval or triangular gas shadow not in obvious continuity with the rest of the bowel. This collection is usually present in the medial aspect of the right upper quadrant, with a superomedial to inferolateral orientation.[1,3,5,7]\nThe football sign is visualization of the entire peritoneal cavity as an oval gas shadow, with the vertically oriented falciform ligament representing the seam of an American football. This sign is most often seen in the pediatric patient but not in the adult patient, because in the adult patient, there is usually not enough air relative to the size of the peritoneal cavity.[1,7]\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "Acute Onset of Abdominal Pain in a 76-Year-Old Man"
},
{
"authors": "Gautam Dehadrai, MD",
"content": [
"Nonsurgical management of a perforated ulcer is associated with prohibitive morbidity and mortality rates, especially in high-risk groups, such as immunocompromised patients and the elderly.",
"Surgical management is generally indicated. Initial management includes gastric decompression with a nasogastric tube, pain control, intravenous hydration, and administration of broad-spectrum antibiotics. Closure with a piece of omentum (Graham patch) and truncal vagotomy with pyloroplasty (by incorporating the perforation) are two common approaches to the surgical management of a perforated duodenal ulcer.",
"The patient in this case underwent an omental patch repair for the duodenal perforation."
],
"date": "April 12, 2017",
"figures": [],
"markdown": "# Acute Onset of Abdominal Pain in a 76-Year-Old Man\n\n **Authors:** Gautam Dehadrai, MD \n **Date:** April 12, 2017\n\n ## Content\n\n Nonsurgical management of a perforated ulcer is associated with prohibitive morbidity and mortality rates, especially in high-risk groups, such as immunocompromised patients and the elderly.\nSurgical management is generally indicated. Initial management includes gastric decompression with a nasogastric tube, pain control, intravenous hydration, and administration of broad-spectrum antibiotics. Closure with a piece of omentum (Graham patch) and truncal vagotomy with pyloroplasty (by incorporating the perforation) are two common approaches to the surgical management of a perforated duodenal ulcer.\nThe patient in this case underwent an omental patch repair for the duodenal perforation.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 363124,
"choiceText": "Upright chest radiography",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 363125,
"choiceText": "Supine abdominal ",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 363126,
"choiceText": "MRI",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 363127,
"choiceText": "Abdominal CT scan with contrast",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "When a perforation is suspected, an upright chest or left lateral decubitus radiography can detect as little as 1-2 mL of gas under the diaphragm or lateral margin of the liver, especially if strict positioning techniques are used. Therefore, these studies should be the first diagnostic modalities used. Supine abdominal radiographs are generally of limited value in diagnosing pneumoperitoneum. CT scanning is highly sensitive for depicting pneumoperitoneum, and this modality has the added benefit of assisting the clinician in identifying the underlying etiology in many patients. Contrast is not necessary to identify free air on the CT scan. In addition, because radiography is generally much more readily available than CT scanning, radiography should be obtained initially to avoid a delay in diagnosis.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 103809,
"questionText": "A patient with a history of peptic ulcer disease presents with acute abdominal pain, rigidity, and tenderness. The patient may have pneumoperitoneum resulting from a perforated ulcer. Which of the following choices should be the initial imaging modality when evaluating this patient?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 363128,
"choiceText": "Surgical management, broad-spectrum antibiotics, pain control, IV hydration",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 363129,
"choiceText": "Nonsurgical management, pain control, IV hydration",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 363130,
"choiceText": "Nonsurgical management, broad-spectrum antibiotics",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 363131,
"choiceText": "Surgical management, IV hydration",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Nonsurgical management of a perforated ulcer is associated with prohibitive morbidity and mortality rates, especially in high-risk groups, such as immunocompromised patients and the elderly. Surgical management is generally indicated. Initial management includes gastric decompression with a nasogastric tube, pain control, intravenous hydration, and administration of broad-spectrum antibiotics.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 103810,
"questionText": "Assuming the above patient has a perforated peptic ulcer, which of the following management strategies is most appropriate?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Acute Onset of Abdominal Pain in a 76-Year-Old Man"
},
{
"authors": "Gautam Dehadrai, MD",
"content": [],
"date": "April 12, 2017",
"figures": [],
"markdown": "# Acute Onset of Abdominal Pain in a 76-Year-Old Man\n\n **Authors:** Gautam Dehadrai, MD \n **Date:** April 12, 2017\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 363124,
"choiceText": "Upright chest radiography",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 363125,
"choiceText": "Supine abdominal ",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 363126,
"choiceText": "MRI",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 363127,
"choiceText": "Abdominal CT scan with contrast",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "When a perforation is suspected, an upright chest or left lateral decubitus radiography can detect as little as 1-2 mL of gas under the diaphragm or lateral margin of the liver, especially if strict positioning techniques are used. Therefore, these studies should be the first diagnostic modalities used. Supine abdominal radiographs are generally of limited value in diagnosing pneumoperitoneum. CT scanning is highly sensitive for depicting pneumoperitoneum, and this modality has the added benefit of assisting the clinician in identifying the underlying etiology in many patients. Contrast is not necessary to identify free air on the CT scan. In addition, because radiography is generally much more readily available than CT scanning, radiography should be obtained initially to avoid a delay in diagnosis.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 103809,
"questionText": "A patient with a history of peptic ulcer disease presents with acute abdominal pain, rigidity, and tenderness. The patient may have pneumoperitoneum resulting from a perforated ulcer. Which of the following choices should be the initial imaging modality when evaluating this patient?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 363128,
"choiceText": "Surgical management, broad-spectrum antibiotics, pain control, IV hydration",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 363129,
"choiceText": "Nonsurgical management, pain control, IV hydration",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 363130,
"choiceText": "Nonsurgical management, broad-spectrum antibiotics",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 363131,
"choiceText": "Surgical management, IV hydration",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Nonsurgical management of a perforated ulcer is associated with prohibitive morbidity and mortality rates, especially in high-risk groups, such as immunocompromised patients and the elderly. Surgical management is generally indicated. Initial management includes gastric decompression with a nasogastric tube, pain control, intravenous hydration, and administration of broad-spectrum antibiotics.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 103810,
"questionText": "Assuming the above patient has a perforated peptic ulcer, which of the following management strategies is most appropriate?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Acute Onset of Abdominal Pain in a 76-Year-Old Man"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 363120,
"choiceText": "Abdominal aortic aneurysm rupture",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 363121,
"choiceText": "Pancreatitis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 363122,
"choiceText": "Pneumoperitoneum from duodenal ulcer perforation",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 363123,
"choiceText": "Acute coronary syndrome",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 103808,
"questionText": "Which of the following is the most likely diagnosis?<br /><br />\r\n<i>Hint: Both the inner and the outer walls of the bowel are visible.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 363124,
"choiceText": "Upright chest radiography",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 363125,
"choiceText": "Supine abdominal ",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 363126,
"choiceText": "MRI",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 363127,
"choiceText": "Abdominal CT scan with contrast",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "When a perforation is suspected, an upright chest or left lateral decubitus radiography can detect as little as 1-2 mL of gas under the diaphragm or lateral margin of the liver, especially if strict positioning techniques are used. Therefore, these studies should be the first diagnostic modalities used. Supine abdominal radiographs are generally of limited value in diagnosing pneumoperitoneum. CT scanning is highly sensitive for depicting pneumoperitoneum, and this modality has the added benefit of assisting the clinician in identifying the underlying etiology in many patients. Contrast is not necessary to identify free air on the CT scan. In addition, because radiography is generally much more readily available than CT scanning, radiography should be obtained initially to avoid a delay in diagnosis.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 103809,
"questionText": "A patient with a history of peptic ulcer disease presents with acute abdominal pain, rigidity, and tenderness. The patient may have pneumoperitoneum resulting from a perforated ulcer. Which of the following choices should be the initial imaging modality when evaluating this patient?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 363128,
"choiceText": "Surgical management, broad-spectrum antibiotics, pain control, IV hydration",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 363129,
"choiceText": "Nonsurgical management, pain control, IV hydration",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 363130,
"choiceText": "Nonsurgical management, broad-spectrum antibiotics",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 363131,
"choiceText": "Surgical management, IV hydration",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Nonsurgical management of a perforated ulcer is associated with prohibitive morbidity and mortality rates, especially in high-risk groups, such as immunocompromised patients and the elderly. Surgical management is generally indicated. Initial management includes gastric decompression with a nasogastric tube, pain control, intravenous hydration, and administration of broad-spectrum antibiotics.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 103810,
"questionText": "Assuming the above patient has a perforated peptic ulcer, which of the following management strategies is most appropriate?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
727165 | /viewarticle/727165 | [
{
"authors": "Andréa B. Lese, MD, MA; Rick G. Kulkarni, MD",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to recognize accurately. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"A 33-year-old man is brought by ambulance to the emergency department (ED) with altered mental status and agitation. He had called 911 from a pay phone, stating that people were trying to shoot him. Upon arrival of the police to the scene, the patient was reported to be visibly paranoid, so Emergency Medical Services (EMS) was called.",
"En route to the hospital, the patient became more confused and combative. EMS requested and received authorization to give intramuscular midazolam. A total of 4 mg was given, but 8 people were still needed to control the patient and get him into the ED and onto a gurney.",
"On arrival, the patient is unable to provide much meaningful history of his present illness, but he is coherent enough to deny any previous medical problems, allergies, or prescription medication use (other than methadone). He does admit to using cocaine."
],
"date": "October 05, 2018",
"figures": [],
"markdown": "# Altered Mental Status in a Young Man Picked Up On the Street\n\n **Authors:** Andréa B. Lese, MD, MA; Rick G. Kulkarni, MD \n **Date:** October 05, 2018\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to recognize accurately. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nA 33-year-old man is brought by ambulance to the emergency department (ED) with altered mental status and agitation. He had called 911 from a pay phone, stating that people were trying to shoot him. Upon arrival of the police to the scene, the patient was reported to be visibly paranoid, so Emergency Medical Services (EMS) was called.\nEn route to the hospital, the patient became more confused and combative. EMS requested and received authorization to give intramuscular midazolam. A total of 4 mg was given, but 8 people were still needed to control the patient and get him into the ED and onto a gurney.\nOn arrival, the patient is unable to provide much meaningful history of his present illness, but he is coherent enough to deny any previous medical problems, allergies, or prescription medication use (other than methadone). He does admit to using cocaine.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "Altered Mental Status in a Young Man Picked Up On the Street"
},
{
"authors": "Andréa B. Lese, MD, MA; Rick G. Kulkarni, MD",
"content": [
"Upon physical examination, he is extremely diaphoretic and, soon after arrival, the patient begins convulsing. His pupils are dilated but equal and reactive to light. His vital signs are notable for a blood pressure of 80/40 mm Hg, a heart rate of 180-190 beats/min, a respiratory rate of 32 breaths/min, and an oxygen saturation of 63% while using a nonrebreather mask. The patient's rectal temperature is measured at 108.1°F (42.3°C).",
"While preparations are made to intubate the patient, his blood pressure drops to 60/40 mm Hg. Cardiac monitoring reveals the patient to be in a wide-complex tachycardia indicative of ventricular tachycardia vs supraventricular tachycardia with aberrancy. Because he is unstable, Advanced Cardiac Life Support (ACLS) guidelines are followed, and he is defibrillated with 50 Joules biphasic, without response. The initial defibrillation attempt is followed by a second attempt at 100 Joules, also without resolution of the tachycardia. A 3-lead rhythm strip is then obtained (Figure 1) that demonstrates a narrow-complex tachycardia.",
"Figure 1.",
"Rapid-sequence intubation is initiated with 20 mg intravenous (IV) etomidate and 10 mg IV vecuronium. Once the endotracheal tube is successfully placed and the position confirmed, 4 mg IV lorazepam is administered for ongoing sedation.",
"A presumptive diagnosis of heatstroke caused by cocaine toxicity is made. Ice packs are applied to the patient's groin and axillae, and a cooling blanket is placed on the patient. Cooled IV saline is infused, and a Foley catheter with a temperature probe is placed. The patient's temperature drops to 102°F (38.9°C). After sedation, intubation, aggressive cooling measures, and IV hydration have been initiated, the patient's heart rate is noted to slow to around 130 beats/min, and his blood pressure and oxygen saturation both normalize. The patient is admitted to the medical intensive care unit (ICU)."
],
"date": "October 05, 2018",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/726/722/726722-thumb1.png"
}
],
"markdown": "# Altered Mental Status in a Young Man Picked Up On the Street\n\n **Authors:** Andréa B. Lese, MD, MA; Rick G. Kulkarni, MD \n **Date:** October 05, 2018\n\n ## Content\n\n Upon physical examination, he is extremely diaphoretic and, soon after arrival, the patient begins convulsing. His pupils are dilated but equal and reactive to light. His vital signs are notable for a blood pressure of 80/40 mm Hg, a heart rate of 180-190 beats/min, a respiratory rate of 32 breaths/min, and an oxygen saturation of 63% while using a nonrebreather mask. The patient's rectal temperature is measured at 108.1°F (42.3°C).\nWhile preparations are made to intubate the patient, his blood pressure drops to 60/40 mm Hg. Cardiac monitoring reveals the patient to be in a wide-complex tachycardia indicative of ventricular tachycardia vs supraventricular tachycardia with aberrancy. Because he is unstable, Advanced Cardiac Life Support (ACLS) guidelines are followed, and he is defibrillated with 50 Joules biphasic, without response. The initial defibrillation attempt is followed by a second attempt at 100 Joules, also without resolution of the tachycardia. A 3-lead rhythm strip is then obtained (Figure 1) that demonstrates a narrow-complex tachycardia.\nFigure 1.\nRapid-sequence intubation is initiated with 20 mg intravenous (IV) etomidate and 10 mg IV vecuronium. Once the endotracheal tube is successfully placed and the position confirmed, 4 mg IV lorazepam is administered for ongoing sedation.\nA presumptive diagnosis of heatstroke caused by cocaine toxicity is made. Ice packs are applied to the patient's groin and axillae, and a cooling blanket is placed on the patient. Cooled IV saline is infused, and a Foley catheter with a temperature probe is placed. The patient's temperature drops to 102°F (38.9°C). After sedation, intubation, aggressive cooling measures, and IV hydration have been initiated, the patient's heart rate is noted to slow to around 130 beats/min, and his blood pressure and oxygen saturation both normalize. The patient is admitted to the medical intensive care unit (ICU).\n\n ## Figures\n\n **Figure 1.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 361641,
"choiceText": "Acute myocardial infarction",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 361642,
"choiceText": "Hypomagnesemia",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 361643,
"choiceText": "Cerebral T waves",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 361644,
"choiceText": "Hyperkalemia",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 103299,
"questionText": "What is the potentially life-threatening finding seen on the electrocardiogram (ECG) that is a result of this patient's condition?<br /><br /><em>Hint: Play close attention to the T waves.</em>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Altered Mental Status in a Young Man Picked Up On the Street"
},
{
"authors": "Andréa B. Lese, MD, MA; Rick G. Kulkarni, MD",
"content": [
"The rhythm strip demonstrated sinus tachycardia, with peaked symmetric T waves that are consistent with hyperkalemia. In addition, hyperkalemia can often be associated with small or absent P waves and a widening of the QRS complex. In the setting of tachycardia, this can give the appearance of bundle branch block (BBB) (aberrancy) or ventricular tachycardia (as was the concern here, given the patient's hemodynamic instability). ECG abnormalities can reveal a cardiac effect of hyperkalemia, but they do not always correlate with the severity of the hyperkalemia; therefore, it is important to corroborate the ECG evidence with the laboratory values.",
"The hyperthermia and convulsions in this patient likely led to a severe metabolic acidemia and rhabdomyolysis, with resultant hyperkalemia. The laboratory analysis showed a white blood cell (WBC) count of 19.1 × 103/µL (19.1 × 109/L), with no left shift. The coagulation tests and cardiac troponin results were normal. The urine microscopy and chest x-ray were performed to investigate for a source of infection; findings were normal. A serum toxicology screen, including for aspirin and acetaminophen, was negative; however, a urine toxicology screen was positive for cocaine, methadone, benzodiazepines, and opiates (but it was negative for amphetamines, barbiturates, phencyclidine [PCP], and oxycodone). The chemistry panel showed a potassium level elevated at 6.9 mEq/L (6.9 mmol/L; normal range is <5.0 mEq/L), a carbon dioxide level of <5 mEq/L (<5 mmol/L), and a creatinine level of 1.7 mg/dL (150.28 µmol/L). The patient's serum glucose level was measured at 170 mg/dL (9.44 mmol/L), and his serum lactic acid level was 25.23 mg/dL (2.8 mmol/L). The creatine kinase level was elevated at 710 units/L (710 U/L), as were the aspartate transaminase (AST) level at 86 units/L (86 U/L) and the alanine transaminase (ALT) level at 123 units/L (123 U/L). His urine, while initially negative, turned red on a repeat specimen (while still in the ED). Treatment for hyperkalemia was initiated with calcium gluconate, dextrose plus insulin, and sodium bicarbonate. Copious IV fluids were also given."
],
"date": "October 05, 2018",
"figures": [],
"markdown": "# Altered Mental Status in a Young Man Picked Up On the Street\n\n **Authors:** Andréa B. Lese, MD, MA; Rick G. Kulkarni, MD \n **Date:** October 05, 2018\n\n ## Content\n\n The rhythm strip demonstrated sinus tachycardia, with peaked symmetric T waves that are consistent with hyperkalemia. In addition, hyperkalemia can often be associated with small or absent P waves and a widening of the QRS complex. In the setting of tachycardia, this can give the appearance of bundle branch block (BBB) (aberrancy) or ventricular tachycardia (as was the concern here, given the patient's hemodynamic instability). ECG abnormalities can reveal a cardiac effect of hyperkalemia, but they do not always correlate with the severity of the hyperkalemia; therefore, it is important to corroborate the ECG evidence with the laboratory values.\nThe hyperthermia and convulsions in this patient likely led to a severe metabolic acidemia and rhabdomyolysis, with resultant hyperkalemia. The laboratory analysis showed a white blood cell (WBC) count of 19.1 × 103/µL (19.1 × 109/L), with no left shift. The coagulation tests and cardiac troponin results were normal. The urine microscopy and chest x-ray were performed to investigate for a source of infection; findings were normal. A serum toxicology screen, including for aspirin and acetaminophen, was negative; however, a urine toxicology screen was positive for cocaine, methadone, benzodiazepines, and opiates (but it was negative for amphetamines, barbiturates, phencyclidine [PCP], and oxycodone). The chemistry panel showed a potassium level elevated at 6.9 mEq/L (6.9 mmol/L; normal range is <5.0 mEq/L), a carbon dioxide level of <5 mEq/L (<5 mmol/L), and a creatinine level of 1.7 mg/dL (150.28 µmol/L). The patient's serum glucose level was measured at 170 mg/dL (9.44 mmol/L), and his serum lactic acid level was 25.23 mg/dL (2.8 mmol/L). The creatine kinase level was elevated at 710 units/L (710 U/L), as were the aspartate transaminase (AST) level at 86 units/L (86 U/L) and the alanine transaminase (ALT) level at 123 units/L (123 U/L). His urine, while initially negative, turned red on a repeat specimen (while still in the ED). Treatment for hyperkalemia was initiated with calcium gluconate, dextrose plus insulin, and sodium bicarbonate. Copious IV fluids were also given.\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 361641,
"choiceText": "Acute myocardial infarction",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 361642,
"choiceText": "Hypomagnesemia",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 361643,
"choiceText": "Cerebral T waves",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 361644,
"choiceText": "Hyperkalemia",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 103299,
"questionText": "What is the potentially life-threatening finding seen on the electrocardiogram (ECG) that is a result of this patient's condition?<br /><br /><em>Hint: Play close attention to the T waves.</em>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Altered Mental Status in a Young Man Picked Up On the Street"
},
{
"authors": "Andréa B. Lese, MD, MA; Rick G. Kulkarni, MD",
"content": [
"The underlying etiology of the patient's problems was heatstroke. This condition is defined as the combination of hyperthermia (core temperature >105°F [40.6°C]) and neurologic impairment.[1] Two types of heatstroke are recognized: classic nonexertional heatstroke (NEHS) and exertional heatstroke (EHS).[1] From 1979 to 1997, the National Centers for Health Statistics (NCHS) reported 7,046 deaths secondary to heat, or 371 deaths per year; this is presumed to underestimate the true numbers of deaths. Those older than age 65 years make up almost half of these cases.[1] Heatstroke is seen less often in subtropical areas than in temperate climates. The 2003 heat wave in France is attributed for the deaths of more than 10,000 people.[2]",
"The 80% mortality rate associated with heatstroke can be reduced to as low as 10% if heatstroke is recognized early and cooling treatment is initiated promptly. The damage caused by this condition is directly related to the temperature and the length of time it remains elevated. The classic presentation is that of hyperthermia, anhidrosis, and neurologic deficit. Some patients, however, still sweat, and others begin to cool en route to the hospital; these patients might not meet the classic temperature criteria.[1]",
"Classic NEHS occurs most frequently in very young and very old individuals during heat waves. Individuals with alcoholism are also at risk for NEHS, the chronically or mentally ill, and those who are dehydrated. Patients present with high temperatures, lack of sweating, and altered mental status. Neurologic complaints may include irritability, lethargy, confusion, ataxia, seizures, and coma. Psychiatric-type symptoms, such as delusions or hallucinations, can also occur. The classic triad is not always present. Sweating may occur, and patients may present with temperatures <105.8°F (41°C).[1] Additional symptoms and signs may include vomiting, diarrhea, tachycardia, hypotension, and tachypnea.",
"EHS typically occurs in healthy young patients participating in vigorous physical activity in hot environments,[1] but it can occur at ambient temperatures as low as 70°F (21.1°C) as well.[3] Those affected with EHS present with hyperthermia, altered mental status, and diaphoresis. They can exhibit strange behaviors or syncope during physical activity, as well as experience abdominal cramps, nausea, vomiting, diarrhea, headache, and dizziness. Because these patients retain their ability to sweat, their temperatures may drop after physical activity has stopped but before presentation at the ED. Risk factors for EHS include viral infections, fatigue, dehydration, the use of stimulants, and not being acclimatized to higher temperatures.",
"In addition to elevated temperatures and neurologic signs and symptoms, other organ systems may also be affected in EHS, including the cardiovascular, pulmonary, gastrointestinal, hepatic, renal, and musculoskeletal systems.[1] The stress caused to the cardiovascular system can be particularly worrisome in the elderly. Tachycardia at 130-140 beats/min and greater is common, as is hypotension resulting from dehydration, redistribution of blood to the skin, and collapse of vascular tone. Patients can be tachypneic and hypoxic for various reasons, including atelectasis, noncardiogenic pulmonary edema, and aspiration.[1] Liver and renal failure can pose serious risks to those surviving the neurologic insult.[3] Rhabdomyolysis is common and, along with hypotension, often leads to renal failure. Liver failure also commonly occurs, possibly secondary to direct heat injury and hypoxia.[2,3,4,5] Disseminated intravascular coagulation may also occur.[5]",
"Multisystem damage is a result of the widespread deadly cellular effects of heat and the body's resultant inflammatory response. Multisystem injury leads to increased morbidity and mortality. In a retrospective study on heatstroke victims, Varghese et al found that the overall mortality rate was 70% for all cases, but it was 85% in patients with multiorgan failure. These investigators found that elevation of creatine kinase, elevation of liver transaminases, and metabolic acidosis were each predictors of poor patient prognosis.[2]",
"The differential diagnosis of heatstroke includes entities in many categories, including thyroid storm, pheochromocytoma, sepsis, meningitis/encephalitis, alcohol withdrawal, and abuse of stimulants (among others). Anticholinergic and antipsychotic drugs rarely cause fever, except in rare cases of neuroleptic malignant syndrome, but these agents can place a patient at risk for heatstroke by preventing sweating. Such drugs include antihistamines, antiparkinsonian drugs, neuroleptics, and others.[6] Another life-threatening condition secondary to exposure to certain drugs is malignant hyperthermia (MH). This condition can lead to an uncontrolled rise in body temperature in susceptible individuals from exposure to volatile anesthetics during general anesthesia, nearly all gas anesthetics, and the neuromuscular blocking agent succinylcholine. The clinician must keep a high level of suspicion for heatstroke because, in some cases, it may present insidiously and valuable time may be lost to investigating other possible diagnoses (as shown in a study by Varghese et al, in which patients presented a mean of 4.1 days after the onset of elevated temperature).[2]",
"Given the possibility of multisystem failure, many diagnostic tests are appropriate for suspected heatstroke; these include complete blood cell (CBC) count, chemistries, liver function tests, creatine kinase, troponin, urinalysis and urine culture, blood cultures, chest radiography, and ECG. A CT scan of the brain may identify alternative causes of altered mental status.[1] A toxicology screen may, in some cases, be useful as well.",
"Hypokalemia can occur early on in heatstroke; however, hyperkalemia can possibly occur secondary to muscle damage. Moreover, rhabdomyolysis can lead to creatine kinase levels greater than 100,000 U/L. A urinalysis showing marked hematuria on urine dipstick interpretation but few red blood cells (RBCs) on microscopic analysis is consistent with myoglobin in the urine and rhabdomyolysis. Blood urea nitrogen (BUN) and creatinine measurements may detect renal failure, which may result from dehydration, heat injury to the kidney, and rhabdomyolysis (or even all three). Liver transaminases can be elevated into the thousands or even tens of thousands as a result of liver and/or muscle injury. A rise in troponin level is not uncommon in NEHS, particularly in the elderly; in fact, it was associated, among other parameters, with a higher level of mortality in a study of victims of the 2003 French heat wave.[7] The etiology of elevated troponin in this setting remains unclear.[8]",
"Among illicit drugs, cocaine has been associated with a higher incidence of death secondary to hyperthermia. Marzuk et al concluded that, of patients younger than 55 years dying from hyperthermia in New York City, one-fourth had taken cocaine immediately beforehand. Moreover, these investigators showed that there were one third more deaths secondary to cocaine use on days hotter than 88°F (31.1°C). They concluded that cocaine is singular among drugs in its association with hyperthermia and mortality.[9] A small study of 8 patients presenting with heatstroke during the 1998 New Orleans heat wave found cocaine was associated more often with heat-related illness than were other drugs and medications.[6]"
],
"date": "October 05, 2018",
"figures": [],
"markdown": "# Altered Mental Status in a Young Man Picked Up On the Street\n\n **Authors:** Andréa B. Lese, MD, MA; Rick G. Kulkarni, MD \n **Date:** October 05, 2018\n\n ## Content\n\n The underlying etiology of the patient's problems was heatstroke. This condition is defined as the combination of hyperthermia (core temperature >105°F [40.6°C]) and neurologic impairment.[1] Two types of heatstroke are recognized: classic nonexertional heatstroke (NEHS) and exertional heatstroke (EHS).[1] From 1979 to 1997, the National Centers for Health Statistics (NCHS) reported 7,046 deaths secondary to heat, or 371 deaths per year; this is presumed to underestimate the true numbers of deaths. Those older than age 65 years make up almost half of these cases.[1] Heatstroke is seen less often in subtropical areas than in temperate climates. The 2003 heat wave in France is attributed for the deaths of more than 10,000 people.[2]\nThe 80% mortality rate associated with heatstroke can be reduced to as low as 10% if heatstroke is recognized early and cooling treatment is initiated promptly. The damage caused by this condition is directly related to the temperature and the length of time it remains elevated. The classic presentation is that of hyperthermia, anhidrosis, and neurologic deficit. Some patients, however, still sweat, and others begin to cool en route to the hospital; these patients might not meet the classic temperature criteria.[1]\nClassic NEHS occurs most frequently in very young and very old individuals during heat waves. Individuals with alcoholism are also at risk for NEHS, the chronically or mentally ill, and those who are dehydrated. Patients present with high temperatures, lack of sweating, and altered mental status. Neurologic complaints may include irritability, lethargy, confusion, ataxia, seizures, and coma. Psychiatric-type symptoms, such as delusions or hallucinations, can also occur. The classic triad is not always present. Sweating may occur, and patients may present with temperatures <105.8°F (41°C).[1] Additional symptoms and signs may include vomiting, diarrhea, tachycardia, hypotension, and tachypnea.\nEHS typically occurs in healthy young patients participating in vigorous physical activity in hot environments,[1] but it can occur at ambient temperatures as low as 70°F (21.1°C) as well.[3] Those affected with EHS present with hyperthermia, altered mental status, and diaphoresis. They can exhibit strange behaviors or syncope during physical activity, as well as experience abdominal cramps, nausea, vomiting, diarrhea, headache, and dizziness. Because these patients retain their ability to sweat, their temperatures may drop after physical activity has stopped but before presentation at the ED. Risk factors for EHS include viral infections, fatigue, dehydration, the use of stimulants, and not being acclimatized to higher temperatures.\nIn addition to elevated temperatures and neurologic signs and symptoms, other organ systems may also be affected in EHS, including the cardiovascular, pulmonary, gastrointestinal, hepatic, renal, and musculoskeletal systems.[1] The stress caused to the cardiovascular system can be particularly worrisome in the elderly. Tachycardia at 130-140 beats/min and greater is common, as is hypotension resulting from dehydration, redistribution of blood to the skin, and collapse of vascular tone. Patients can be tachypneic and hypoxic for various reasons, including atelectasis, noncardiogenic pulmonary edema, and aspiration.[1] Liver and renal failure can pose serious risks to those surviving the neurologic insult.[3] Rhabdomyolysis is common and, along with hypotension, often leads to renal failure. Liver failure also commonly occurs, possibly secondary to direct heat injury and hypoxia.[2,3,4,5] Disseminated intravascular coagulation may also occur.[5]\nMultisystem damage is a result of the widespread deadly cellular effects of heat and the body's resultant inflammatory response. Multisystem injury leads to increased morbidity and mortality. In a retrospective study on heatstroke victims, Varghese et al found that the overall mortality rate was 70% for all cases, but it was 85% in patients with multiorgan failure. These investigators found that elevation of creatine kinase, elevation of liver transaminases, and metabolic acidosis were each predictors of poor patient prognosis.[2]\nThe differential diagnosis of heatstroke includes entities in many categories, including thyroid storm, pheochromocytoma, sepsis, meningitis/encephalitis, alcohol withdrawal, and abuse of stimulants (among others). Anticholinergic and antipsychotic drugs rarely cause fever, except in rare cases of neuroleptic malignant syndrome, but these agents can place a patient at risk for heatstroke by preventing sweating. Such drugs include antihistamines, antiparkinsonian drugs, neuroleptics, and others.[6] Another life-threatening condition secondary to exposure to certain drugs is malignant hyperthermia (MH). This condition can lead to an uncontrolled rise in body temperature in susceptible individuals from exposure to volatile anesthetics during general anesthesia, nearly all gas anesthetics, and the neuromuscular blocking agent succinylcholine. The clinician must keep a high level of suspicion for heatstroke because, in some cases, it may present insidiously and valuable time may be lost to investigating other possible diagnoses (as shown in a study by Varghese et al, in which patients presented a mean of 4.1 days after the onset of elevated temperature).[2]\nGiven the possibility of multisystem failure, many diagnostic tests are appropriate for suspected heatstroke; these include complete blood cell (CBC) count, chemistries, liver function tests, creatine kinase, troponin, urinalysis and urine culture, blood cultures, chest radiography, and ECG. A CT scan of the brain may identify alternative causes of altered mental status.[1] A toxicology screen may, in some cases, be useful as well.\nHypokalemia can occur early on in heatstroke; however, hyperkalemia can possibly occur secondary to muscle damage. Moreover, rhabdomyolysis can lead to creatine kinase levels greater than 100,000 U/L. A urinalysis showing marked hematuria on urine dipstick interpretation but few red blood cells (RBCs) on microscopic analysis is consistent with myoglobin in the urine and rhabdomyolysis. Blood urea nitrogen (BUN) and creatinine measurements may detect renal failure, which may result from dehydration, heat injury to the kidney, and rhabdomyolysis (or even all three). Liver transaminases can be elevated into the thousands or even tens of thousands as a result of liver and/or muscle injury. A rise in troponin level is not uncommon in NEHS, particularly in the elderly; in fact, it was associated, among other parameters, with a higher level of mortality in a study of victims of the 2003 French heat wave.[7] The etiology of elevated troponin in this setting remains unclear.[8]\nAmong illicit drugs, cocaine has been associated with a higher incidence of death secondary to hyperthermia. Marzuk et al concluded that, of patients younger than 55 years dying from hyperthermia in New York City, one-fourth had taken cocaine immediately beforehand. Moreover, these investigators showed that there were one third more deaths secondary to cocaine use on days hotter than 88°F (31.1°C). They concluded that cocaine is singular among drugs in its association with hyperthermia and mortality.[9] A small study of 8 patients presenting with heatstroke during the 1998 New Orleans heat wave found cocaine was associated more often with heat-related illness than were other drugs and medications.[6]\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "Altered Mental Status in a Young Man Picked Up On the Street"
},
{
"authors": "Andréa B. Lese, MD, MA; Rick G. Kulkarni, MD",
"content": [
"Heatstroke requires emergent treatment with active cooling measures. Ice packs should be placed on the groin and axillae, and cooled IV saline should be given. The mainstay of treatment in most centers is through the process of evaporation; tepid water is sprayed onto the patient, preferably as a mist, followed by the use of fans to evaporate the water.[10,11] If no fans are available, use of a cooling blanket can be considered, although this technique is less effective than evaporative cooling. Ice-water immersion is effective, but this technique introduces difficulties in monitoring and resuscitating patients; in addition, theoretical concerns surround peripheral vasoconstriction with this treatment; however, these are outweighed by the technique's therapeutic benefit.[11]",
"Other, more aggressive cooling techniques include cold lavage in the peritoneal, thoracic, rectal, and gastric cavities. This treatment has not been well studied, however, and can cause water intoxication.[11] In the most severe cases, cardiopulmonary bypass has been suggested.",
"Antipyretics such as acetaminophen and ibuprofen are not useful in heatstroke; however, until other causes of disease (such as infection) are ruled out, antipyretics may be used. Dantrolene has not proven to be effective in lowering body temperature.[1] Benzodiazepines are helpful in preventing the generation of additional heat by shivering, agitation, or seizures.[1] Cooling measures should be stopped when the patient's temperature reaches 102.2°F (39°C), to prevent overshoot hypothermia.[1]",
"In addition to cooling the patient, supportive care for other manifestations resulting from high temperatures is necessary. Hypovolemia should be treated with fluids. Rhabdomyolysis should be treated primarily with aggressive volume resuscitation. The addition of sodium bicarbonate can be considered, although no conclusive data support its use. Liver failure is usually mild or moderate and amenable to conservative therapy.[3,5] Expectant management of frank liver failure may be the best approach. Three patients with acute liver failure who met the criteria for transplantation underwent transplantation, and all had fatal outcomes. However, case reports have described patients meeting these same criteria who fully recovered after only conservative treatment.[3,5]",
"The patient in this case was intubated and admitted to the medical ICU. After clinicians noted continued normalization of his temperature and an improved mental status, he was successfully extubated the next day. His chest x-rays continued to show clear lungs. Treatment for his rhabdomyolysis was continued with IV fluids containing bicarbonate. His creatine kinase level continued to rise, peaking on day 2 at 130,000 units/L, then began to fall. His urine output remained adequate and, although his creatinine level peaked at 2.5 mg/dL on hospital day 4, he never required dialysis. His troponin level, within normal limits at first, peaked on hospital day 2 at 0.89 ng/mL (0.89 µg/L), then began to fall. His liver enzymes peaked on hospital day 3, with an AST level of 3870 unit/L (3870 U/L) and an ALT level of 2080 units/L (2080 U/L), then began to resolve spontaneously. He eventually stabilized by hospital day 3, and then he began to improve; at discharge, he had no evidence of any permanent neurologic damage."
],
"date": "October 05, 2018",
"figures": [],
"markdown": "# Altered Mental Status in a Young Man Picked Up On the Street\n\n **Authors:** Andréa B. Lese, MD, MA; Rick G. Kulkarni, MD \n **Date:** October 05, 2018\n\n ## Content\n\n Heatstroke requires emergent treatment with active cooling measures. Ice packs should be placed on the groin and axillae, and cooled IV saline should be given. The mainstay of treatment in most centers is through the process of evaporation; tepid water is sprayed onto the patient, preferably as a mist, followed by the use of fans to evaporate the water.[10,11] If no fans are available, use of a cooling blanket can be considered, although this technique is less effective than evaporative cooling. Ice-water immersion is effective, but this technique introduces difficulties in monitoring and resuscitating patients; in addition, theoretical concerns surround peripheral vasoconstriction with this treatment; however, these are outweighed by the technique's therapeutic benefit.[11]\nOther, more aggressive cooling techniques include cold lavage in the peritoneal, thoracic, rectal, and gastric cavities. This treatment has not been well studied, however, and can cause water intoxication.[11] In the most severe cases, cardiopulmonary bypass has been suggested.\nAntipyretics such as acetaminophen and ibuprofen are not useful in heatstroke; however, until other causes of disease (such as infection) are ruled out, antipyretics may be used. Dantrolene has not proven to be effective in lowering body temperature.[1] Benzodiazepines are helpful in preventing the generation of additional heat by shivering, agitation, or seizures.[1] Cooling measures should be stopped when the patient's temperature reaches 102.2°F (39°C), to prevent overshoot hypothermia.[1]\nIn addition to cooling the patient, supportive care for other manifestations resulting from high temperatures is necessary. Hypovolemia should be treated with fluids. Rhabdomyolysis should be treated primarily with aggressive volume resuscitation. The addition of sodium bicarbonate can be considered, although no conclusive data support its use. Liver failure is usually mild or moderate and amenable to conservative therapy.[3,5] Expectant management of frank liver failure may be the best approach. Three patients with acute liver failure who met the criteria for transplantation underwent transplantation, and all had fatal outcomes. However, case reports have described patients meeting these same criteria who fully recovered after only conservative treatment.[3,5]\nThe patient in this case was intubated and admitted to the medical ICU. After clinicians noted continued normalization of his temperature and an improved mental status, he was successfully extubated the next day. His chest x-rays continued to show clear lungs. Treatment for his rhabdomyolysis was continued with IV fluids containing bicarbonate. His creatine kinase level continued to rise, peaking on day 2 at 130,000 units/L, then began to fall. His urine output remained adequate and, although his creatinine level peaked at 2.5 mg/dL on hospital day 4, he never required dialysis. His troponin level, within normal limits at first, peaked on hospital day 2 at 0.89 ng/mL (0.89 µg/L), then began to fall. His liver enzymes peaked on hospital day 3, with an AST level of 3870 unit/L (3870 U/L) and an ALT level of 2080 units/L (2080 U/L), then began to resolve spontaneously. He eventually stabilized by hospital day 3, and then he began to improve; at discharge, he had no evidence of any permanent neurologic damage.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 361645,
"choiceText": "Damage to the tissues is caused by the thermal insult directly and the body's inflammatory response to the thermal insult.",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 361646,
"choiceText": "Peaked T waves are possible on ECG.",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 361647,
"choiceText": "Before treating for heatstroke, the clinician should first rule out all other diagnoses.",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 361648,
"choiceText": "Multiple organ system failure is a poor prognostic indicator.",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 361649,
"choiceText": "Renal failure is a common complication of patients after heatstroke.",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The key to success in treating heatstroke is rapid cooling as early as possible because any tissue damage incurred is directly related to the amount of time that hyperthermia is present. Other diagnoses must be considered, but priority should be given to rapid cooling of the patient. Tissue damage is caused directly by the thermal insult and indirectly by the body's response to the insult. Peaked T waves may be seen on a patient's ECG because the hyperthermia and muscle activity can lead to rhabdomyolysis and renal failure, which can then lead to hyperkalemia. Renal and liver failures are common complications of patients suffering from heatstroke, and multiple organ system failure leads to higher morbidity and mortality.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 103300,
"questionText": "You are examining a patient in the ED with a very high temperature and apparent heatstroke. Which of the following should <em>not</em> be guiding factors in your treatment of this patient?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 361650,
"choiceText": "The patient must have neurologic impairment.",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 361651,
"choiceText": "The patient must have a core temperature >105°F (40.6°C) at presentation.",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 361652,
"choiceText": "The ambient temperature must be >86°F (30°C).",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 361653,
"choiceText": "The patient must have stopped sweating.",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 361654,
"choiceText": "The patient must have normal labs, tox screen, and CT of the brain to rule out all other causes in order to attribute his symptoms to heatstroke.",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The definition of heatstroke includes the manifestation of neurologic impairment, including states ranging from irritability to coma. Altered mental status may also present with psychiatric symptoms, such as hallucinations. Although the definition of heatstroke also includes hyperthermia, with a temperature of >106°F (41.1°C), it is possible that at presentation the patient may already have begun to cool. Heatstroke can occur with vigorous activity at moderate ambient temperatures. Blood tests are useful in indicating multiple organ system failure and very well may be abnormal in the patient with heatstroke. A CT scan can be helpful in evaluating other possible causes of altered mental status. Heatstroke can be associated with some medications and illicit drugs, such as cocaine. These examinations should not delay the timely initiation of cooling.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 103301,
"questionText": "Which of the following characteristics must your patient have in order to be diagnosed with heatstroke?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Altered Mental Status in a Young Man Picked Up On the Street"
},
{
"authors": "Andréa B. Lese, MD, MA; Rick G. Kulkarni, MD",
"content": [],
"date": "October 05, 2018",
"figures": [],
"markdown": "# Altered Mental Status in a Young Man Picked Up On the Street\n\n **Authors:** Andréa B. Lese, MD, MA; Rick G. Kulkarni, MD \n **Date:** October 05, 2018\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 361645,
"choiceText": "Damage to the tissues is caused by the thermal insult directly and the body's inflammatory response to the thermal insult.",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 361646,
"choiceText": "Peaked T waves are possible on ECG.",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 361647,
"choiceText": "Before treating for heatstroke, the clinician should first rule out all other diagnoses.",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 361648,
"choiceText": "Multiple organ system failure is a poor prognostic indicator.",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 361649,
"choiceText": "Renal failure is a common complication of patients after heatstroke.",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The key to success in treating heatstroke is rapid cooling as early as possible because any tissue damage incurred is directly related to the amount of time that hyperthermia is present. Other diagnoses must be considered, but priority should be given to rapid cooling of the patient. Tissue damage is caused directly by the thermal insult and indirectly by the body's response to the insult. Peaked T waves may be seen on a patient's ECG because the hyperthermia and muscle activity can lead to rhabdomyolysis and renal failure, which can then lead to hyperkalemia. Renal and liver failures are common complications of patients suffering from heatstroke, and multiple organ system failure leads to higher morbidity and mortality.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 103300,
"questionText": "You are examining a patient in the ED with a very high temperature and apparent heatstroke. Which of the following should <em>not</em> be guiding factors in your treatment of this patient?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 361650,
"choiceText": "The patient must have neurologic impairment.",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 361651,
"choiceText": "The patient must have a core temperature >105°F (40.6°C) at presentation.",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 361652,
"choiceText": "The ambient temperature must be >86°F (30°C).",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 361653,
"choiceText": "The patient must have stopped sweating.",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 361654,
"choiceText": "The patient must have normal labs, tox screen, and CT of the brain to rule out all other causes in order to attribute his symptoms to heatstroke.",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The definition of heatstroke includes the manifestation of neurologic impairment, including states ranging from irritability to coma. Altered mental status may also present with psychiatric symptoms, such as hallucinations. Although the definition of heatstroke also includes hyperthermia, with a temperature of >106°F (41.1°C), it is possible that at presentation the patient may already have begun to cool. Heatstroke can occur with vigorous activity at moderate ambient temperatures. Blood tests are useful in indicating multiple organ system failure and very well may be abnormal in the patient with heatstroke. A CT scan can be helpful in evaluating other possible causes of altered mental status. Heatstroke can be associated with some medications and illicit drugs, such as cocaine. These examinations should not delay the timely initiation of cooling.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 103301,
"questionText": "Which of the following characteristics must your patient have in order to be diagnosed with heatstroke?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Altered Mental Status in a Young Man Picked Up On the Street"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 361641,
"choiceText": "Acute myocardial infarction",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 361642,
"choiceText": "Hypomagnesemia",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 361643,
"choiceText": "Cerebral T waves",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 361644,
"choiceText": "Hyperkalemia",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 103299,
"questionText": "What is the potentially life-threatening finding seen on the electrocardiogram (ECG) that is a result of this patient's condition?<br /><br /><em>Hint: Play close attention to the T waves.</em>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 361645,
"choiceText": "Damage to the tissues is caused by the thermal insult directly and the body's inflammatory response to the thermal insult.",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 361646,
"choiceText": "Peaked T waves are possible on ECG.",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 361647,
"choiceText": "Before treating for heatstroke, the clinician should first rule out all other diagnoses.",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 361648,
"choiceText": "Multiple organ system failure is a poor prognostic indicator.",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 361649,
"choiceText": "Renal failure is a common complication of patients after heatstroke.",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The key to success in treating heatstroke is rapid cooling as early as possible because any tissue damage incurred is directly related to the amount of time that hyperthermia is present. Other diagnoses must be considered, but priority should be given to rapid cooling of the patient. Tissue damage is caused directly by the thermal insult and indirectly by the body's response to the insult. Peaked T waves may be seen on a patient's ECG because the hyperthermia and muscle activity can lead to rhabdomyolysis and renal failure, which can then lead to hyperkalemia. Renal and liver failures are common complications of patients suffering from heatstroke, and multiple organ system failure leads to higher morbidity and mortality.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 103300,
"questionText": "You are examining a patient in the ED with a very high temperature and apparent heatstroke. Which of the following should <em>not</em> be guiding factors in your treatment of this patient?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 361650,
"choiceText": "The patient must have neurologic impairment.",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 361651,
"choiceText": "The patient must have a core temperature >105°F (40.6°C) at presentation.",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 361652,
"choiceText": "The ambient temperature must be >86°F (30°C).",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 361653,
"choiceText": "The patient must have stopped sweating.",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 361654,
"choiceText": "The patient must have normal labs, tox screen, and CT of the brain to rule out all other causes in order to attribute his symptoms to heatstroke.",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The definition of heatstroke includes the manifestation of neurologic impairment, including states ranging from irritability to coma. Altered mental status may also present with psychiatric symptoms, such as hallucinations. Although the definition of heatstroke also includes hyperthermia, with a temperature of >106°F (41.1°C), it is possible that at presentation the patient may already have begun to cool. Heatstroke can occur with vigorous activity at moderate ambient temperatures. Blood tests are useful in indicating multiple organ system failure and very well may be abnormal in the patient with heatstroke. A CT scan can be helpful in evaluating other possible causes of altered mental status. Heatstroke can be associated with some medications and illicit drugs, such as cocaine. These examinations should not delay the timely initiation of cooling.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 103301,
"questionText": "Which of the following characteristics must your patient have in order to be diagnosed with heatstroke?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
726484 | /viewarticle/726484 | [
{
"authors": "Adrian Ioan Toma, MD, PhD; Crenguta Albu, MD, PhD; Dinu Florin Albu, MD; Adriana Stan; Emilia Severin, PhD",
"content": [
"Editor's Note:\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.",
"A 29-year-old woman has given birth to a boy with a dysmorphic facial appearance. The boy was born at full-term gestation by cesarean delivery. The mother has epilepsy characterized by tonic-clonic seizures, with her first episode occurring in her early teenage years. She pursued a planned pregnancy knowing that a child born to a woman with epilepsy on anticonvulsants is at a higher risk for birth defects.",
"Before conceiving the baby, the mother took 5 mg of folic acid per day. During her pregnancy, she had been taking 750 mg of valproic acid (VPA) twice a day (the upper limit of the safe dose) to control her seizures. She requested genetic counseling to determine whether her unborn child would be affected. At 12 weeks’ gestation she was referred for routine prenatal ultrasonography, and she agreed to undergo a quad screening test (alpha-fetoprotein, human chorionic gonadotropin, and unconjugated estriol markers).",
"Fetal monitoring was performed with ultrasonography to gauge fetal growth, check for possible congenital malformations, and assess the amniotic fluid volume. The ultrasound examination at 17 weeks’ gestation revealed a few abnormal features suggesting a numeric chromosomal disorder, but the fetus was also found to be small for gestational age. As a result, amniotic fluid samples were taken to perform prenatal cytogenetic diagnosis. Pregnancy options, including termination, were discussed with the parents, with the decision by the parents to continue the pregnancy."
],
"date": "November 10, 2015",
"figures": [],
"markdown": "# A Neonate With Dysmorphic Facial Features\n\n **Authors:** Adrian Ioan Toma, MD, PhD; Crenguta Albu, MD, PhD; Dinu Florin Albu, MD; Adriana Stan; Emilia Severin, PhD \n **Date:** November 10, 2015\n\n ## Content\n\n Editor's Note:\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.\nA 29-year-old woman has given birth to a boy with a dysmorphic facial appearance. The boy was born at full-term gestation by cesarean delivery. The mother has epilepsy characterized by tonic-clonic seizures, with her first episode occurring in her early teenage years. She pursued a planned pregnancy knowing that a child born to a woman with epilepsy on anticonvulsants is at a higher risk for birth defects.\nBefore conceiving the baby, the mother took 5 mg of folic acid per day. During her pregnancy, she had been taking 750 mg of valproic acid (VPA) twice a day (the upper limit of the safe dose) to control her seizures. She requested genetic counseling to determine whether her unborn child would be affected. At 12 weeks’ gestation she was referred for routine prenatal ultrasonography, and she agreed to undergo a quad screening test (alpha-fetoprotein, human chorionic gonadotropin, and unconjugated estriol markers).\nFetal monitoring was performed with ultrasonography to gauge fetal growth, check for possible congenital malformations, and assess the amniotic fluid volume. The ultrasound examination at 17 weeks’ gestation revealed a few abnormal features suggesting a numeric chromosomal disorder, but the fetus was also found to be small for gestational age. As a result, amniotic fluid samples were taken to perform prenatal cytogenetic diagnosis. Pregnancy options, including termination, were discussed with the parents, with the decision by the parents to continue the pregnancy.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A Neonate With Dysmorphic Facial Features"
},
{
"authors": "Adrian Ioan Toma, MD, PhD; Crenguta Albu, MD, PhD; Dinu Florin Albu, MD; Adriana Stan; Emilia Severin, PhD",
"content": [
"The result of the quad test was positive. Quantitative fluorescent polymerase chain reaction was used for prenatal detection of chromosomes 13, 18, and 21 and of X and Y aneuploidies. This test was chosen because it provides reliable results that are available in a few hours after sampling; in addition, it requires only small fetal samples (1.5 mL amniotic fluid). The test showed a normal number of chromosomes. The conventional karyotype indicated a normal male: 46, XY. The ultrasound scans excluded malformations of the heart and internal organs.",
"Figure 1.",
"Figure 1.",
"Figure 2.",
"Figure 2.",
"Figure 3.",
"Figure 3.",
"On physical examination following birth, the baby has a distinctive facial appearance that includes a tall and prominent central forehead; a low hairline; median deficiency of eyebrows; ocular hypertelorism; epicanthal folds; a low nasal bridge, with a short upturned nose; midface hypoplasia; low-set ears; and micrognathia. The child is also noted to have a short neck and broad hands and feet (Figures 1, 2, and 3). The neurologic examination reveals the presence of both central and peripheral hypotonia."
],
"date": "November 10, 2015",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/726/242/726242-thumb1.png"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/726/242/726242-thumb2.png"
},
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/726/242/726242-thumb3.png"
}
],
"markdown": "# A Neonate With Dysmorphic Facial Features\n\n **Authors:** Adrian Ioan Toma, MD, PhD; Crenguta Albu, MD, PhD; Dinu Florin Albu, MD; Adriana Stan; Emilia Severin, PhD \n **Date:** November 10, 2015\n\n ## Content\n\n The result of the quad test was positive. Quantitative fluorescent polymerase chain reaction was used for prenatal detection of chromosomes 13, 18, and 21 and of X and Y aneuploidies. This test was chosen because it provides reliable results that are available in a few hours after sampling; in addition, it requires only small fetal samples (1.5 mL amniotic fluid). The test showed a normal number of chromosomes. The conventional karyotype indicated a normal male: 46, XY. The ultrasound scans excluded malformations of the heart and internal organs.\nFigure 1.\nFigure 1.\nFigure 2.\nFigure 2.\nFigure 3.\nFigure 3.\nOn physical examination following birth, the baby has a distinctive facial appearance that includes a tall and prominent central forehead; a low hairline; median deficiency of eyebrows; ocular hypertelorism; epicanthal folds; a low nasal bridge, with a short upturned nose; midface hypoplasia; low-set ears; and micrognathia. The child is also noted to have a short neck and broad hands and feet (Figures 1, 2, and 3). The neurologic examination reveals the presence of both central and peripheral hypotonia.\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n**Figure 3.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 358347,
"choiceText": "Fetal valproate syndrome",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 358348,
"choiceText": "Trisomy 18 (Edwards syndrome)",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 358349,
"choiceText": "Structural chromosome abnormality syndrome",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 358350,
"choiceText": "Fetal alcohol syndrome",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 102224,
"questionText": "What is the likely diagnosis?<br /><br /><em>Hint: Consider the baby’s potential teratogen exposure during pregnancy</em>.",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A Neonate With Dysmorphic Facial Features"
},
{
"authors": "Adrian Ioan Toma, MD, PhD; Crenguta Albu, MD, PhD; Dinu Florin Albu, MD; Adriana Stan; Emilia Severin, PhD",
"content": [
"In this case, fetal valproate syndrome (FVS) was prenatally detected by ultrasonography, without spina bifida or heart anomalies. At birth, the diagnosis was confirmed by the clinical findings. Prenatal exposure to valproate during the first trimester of pregnancy could have resulted in the disorder, even though the neonate did not manifest all of the major diagnostic criteria, such as neural tube defects (eg, spina bifida), congenital cardiovascular abnormalities, genital abnormalities (eg, hypospadias), or finger and toe defects.",
"An increased incidence of major and minor congenital abnormalities is seen in infants born to epileptic mothers (6-7% compared with 2-3% in the general population).[1] Factors which may contribute to this include seizures leading to hypoxia during pregnancy, an inherited predisposition to malformations owing to intrinsic maternal factors, and the teratogenic effects of anticonvulsants.[2] FVS results when the fetus is exposed to either VPA or sodium valproate in utero.[3]"
],
"date": "November 10, 2015",
"figures": [],
"markdown": "# A Neonate With Dysmorphic Facial Features\n\n **Authors:** Adrian Ioan Toma, MD, PhD; Crenguta Albu, MD, PhD; Dinu Florin Albu, MD; Adriana Stan; Emilia Severin, PhD \n **Date:** November 10, 2015\n\n ## Content\n\n In this case, fetal valproate syndrome (FVS) was prenatally detected by ultrasonography, without spina bifida or heart anomalies. At birth, the diagnosis was confirmed by the clinical findings. Prenatal exposure to valproate during the first trimester of pregnancy could have resulted in the disorder, even though the neonate did not manifest all of the major diagnostic criteria, such as neural tube defects (eg, spina bifida), congenital cardiovascular abnormalities, genital abnormalities (eg, hypospadias), or finger and toe defects.\nAn increased incidence of major and minor congenital abnormalities is seen in infants born to epileptic mothers (6-7% compared with 2-3% in the general population).[1] Factors which may contribute to this include seizures leading to hypoxia during pregnancy, an inherited predisposition to malformations owing to intrinsic maternal factors, and the teratogenic effects of anticonvulsants.[2] FVS results when the fetus is exposed to either VPA or sodium valproate in utero.[3]\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 358347,
"choiceText": "Fetal valproate syndrome",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 358348,
"choiceText": "Trisomy 18 (Edwards syndrome)",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 358349,
"choiceText": "Structural chromosome abnormality syndrome",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 358350,
"choiceText": "Fetal alcohol syndrome",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 102224,
"questionText": "What is the likely diagnosis?<br /><br /><em>Hint: Consider the baby’s potential teratogen exposure during pregnancy</em>.",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A Neonate With Dysmorphic Facial Features"
},
{
"authors": "Adrian Ioan Toma, MD, PhD; Crenguta Albu, MD, PhD; Dinu Florin Albu, MD; Adriana Stan; Emilia Severin, PhD",
"content": [
"VPA is a well-documented potential teratogen. Various factors contribute to the teratogenicity of valproic acid. These include the number of drugs that are coadministered, drug dosage, differences in maternal and/or infant metabolism, and the gestational age of the fetus at exposure.[4] In addition, researchers have suggested that epilepsy in the mother could be teratogenic in and of itself. Infants of epileptic mothers are particularly prone to birth defects.",
"Of major concern is the potential for teratogenesis due to exposure to antiseizure medications. As a general rule, certain teratogens often cause specific patterns of birth defects. The pattern of abnormalities encountered with FVS is not yet well-defined, and a broad phenotype of major and occasional malformations has been described over the past 25 years.",
"In humans, VPA use during the first trimester of pregnancy increases risks for spina bifida; heart, limb, and craniofacial abnormalities; and cleft palate.[5,6,7] The dose and timing of VPA exposure can influence the outcome of pregnancy. Exposure during the first trimester is more likely to cause abnormalities because this is the main period of structural development in the fetus.[8,9] VPA crosses the placenta where it then can exert its teratogenic effects. VPA serum concentrations are higher in the fetus than in the mother,[2] and high concentrations increase the embryotoxic potential of VPA.",
"The effects seem to be dose-related. In addition to neural tube defects, dose-related effects associated with VPA include dysmorphic facial features and a reduction in verbal IQ. Generally, dosages of > 1000 mg/d have been implicated in all of these abnormalities.[10] In mice and rabbits, VPA causes neural tube defects and limb anomalies, the appearance of which is affected by genetic background or modifiers that apparently operate both parentally and embryonically.[11] The exact mechanism of teratogenicity is unclear, but VPA has been shown to cause in vitro changes in HOX gene expression (which regulate patterns of development in animals and humans) as well as mutations of these genes, which can produce easily visible phenotypic changes.[11]",
"The estimated frequency of facial and minor malformations after VPA exposure in utero is 50-75%.[12,13] The risk for neural tube defects after in utero VPA exposure has been estimated as 1-5%.[12] Most reports have described spina bifida occurring more commonly than anencephaly,[11] while some published cases of VPA-related spina bifida have also reported family histories of neural tube defects.[8,15] The association of VPA exposure with maternal zinc deficiency has also been suggested as a possible cause of neural tube defects.[5] As administration of folic acid helps protect against human neural tube abnormalities,[17] and VPA interferes with folic acid metabolism, patients taking this medication may benefit from folic acid administration prior to conception.",
"Hypotonia in FVS often occurs between 12 and 48 hours after birth.[14] The association between VPA exposure and developmental delay has been confirmed in several reports[2,6] and also correlates with this case. Postnatal growth often appears normal. In this case, the infant had moderate postnatal growth delay."
],
"date": "November 10, 2015",
"figures": [],
"markdown": "# A Neonate With Dysmorphic Facial Features\n\n **Authors:** Adrian Ioan Toma, MD, PhD; Crenguta Albu, MD, PhD; Dinu Florin Albu, MD; Adriana Stan; Emilia Severin, PhD \n **Date:** November 10, 2015\n\n ## Content\n\n VPA is a well-documented potential teratogen. Various factors contribute to the teratogenicity of valproic acid. These include the number of drugs that are coadministered, drug dosage, differences in maternal and/or infant metabolism, and the gestational age of the fetus at exposure.[4] In addition, researchers have suggested that epilepsy in the mother could be teratogenic in and of itself. Infants of epileptic mothers are particularly prone to birth defects.\nOf major concern is the potential for teratogenesis due to exposure to antiseizure medications. As a general rule, certain teratogens often cause specific patterns of birth defects. The pattern of abnormalities encountered with FVS is not yet well-defined, and a broad phenotype of major and occasional malformations has been described over the past 25 years.\nIn humans, VPA use during the first trimester of pregnancy increases risks for spina bifida; heart, limb, and craniofacial abnormalities; and cleft palate.[5,6,7] The dose and timing of VPA exposure can influence the outcome of pregnancy. Exposure during the first trimester is more likely to cause abnormalities because this is the main period of structural development in the fetus.[8,9] VPA crosses the placenta where it then can exert its teratogenic effects. VPA serum concentrations are higher in the fetus than in the mother,[2] and high concentrations increase the embryotoxic potential of VPA.\nThe effects seem to be dose-related. In addition to neural tube defects, dose-related effects associated with VPA include dysmorphic facial features and a reduction in verbal IQ. Generally, dosages of > 1000 mg/d have been implicated in all of these abnormalities.[10] In mice and rabbits, VPA causes neural tube defects and limb anomalies, the appearance of which is affected by genetic background or modifiers that apparently operate both parentally and embryonically.[11] The exact mechanism of teratogenicity is unclear, but VPA has been shown to cause in vitro changes in HOX gene expression (which regulate patterns of development in animals and humans) as well as mutations of these genes, which can produce easily visible phenotypic changes.[11]\nThe estimated frequency of facial and minor malformations after VPA exposure in utero is 50-75%.[12,13] The risk for neural tube defects after in utero VPA exposure has been estimated as 1-5%.[12] Most reports have described spina bifida occurring more commonly than anencephaly,[11] while some published cases of VPA-related spina bifida have also reported family histories of neural tube defects.[8,15] The association of VPA exposure with maternal zinc deficiency has also been suggested as a possible cause of neural tube defects.[5] As administration of folic acid helps protect against human neural tube abnormalities,[17] and VPA interferes with folic acid metabolism, patients taking this medication may benefit from folic acid administration prior to conception.\nHypotonia in FVS often occurs between 12 and 48 hours after birth.[14] The association between VPA exposure and developmental delay has been confirmed in several reports[2,6] and also correlates with this case. Postnatal growth often appears normal. In this case, the infant had moderate postnatal growth delay.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A Neonate With Dysmorphic Facial Features"
},
{
"authors": "Adrian Ioan Toma, MD, PhD; Crenguta Albu, MD, PhD; Dinu Florin Albu, MD; Adriana Stan; Emilia Severin, PhD",
"content": [
"Approximately 2% of pregnant women who take VPA give birth to children with FVS and the symptoms of the disorder may vary, even between siblings. Folic acid may affect a variety of epigenetic mechanisms and may act as an environmental agent that contributes to phenotypic variability.[10,13] Thus, variability in clinical presentation may be influenced by many factors, such as maternal seizures that lead to periods of hypoxia during pregnancy, folic acid intake, and dose of and timing of exposure to VPA.",
"Genetic differences in susceptibility to malformations (ie, predisposition to malformation owing to maternal genetic metabolic profile, which may interact with the antiseizure medication and cause a birth defect) may also influence clinical presentation variability.[15] The long-term effects of FVS as children develop include autism, speech, language, and learning difficulties. When the diagnosis is made, the child should be referred to early intervention services through the school system. These services include physical therapy, occupational therapy, speech therapy, and special education intervention.",
"In this case, a woman with epilepsy and her healthy partner (both without a family history of epilepsy) were informed that women with epilepsy are at a higher risk for conceiving a child with FVS, a rare congenital disorder. Because she experienced tonic-clonic seizures, the woman was concerned about whether she could conceive a healthy child. Together with her neurologist and obstetrician, the woman carefully planned her pregnancy and sought ways to minimize the risks for birth defects and complications. Before her pregnancy, she took 5 mg of folic acid per day and avoided smoking, caffeine, alcohol, and stress. She consulted with a genetic counselor to determine possible risks. During the pregnancy, she received VPA monotherapy to control her seizures. Although the pregnancy was normal, the results of the prenatal tests indicated the presence of FVS. The couple was informed about the affected fetus and chose to continue the pregnancy. At full-term, the woman gave birth to a baby with dysmorphic features consistent with FVS. The parents accepted the condition of their child and asked for assistance with perinatal care. After birth, the baby presented with central and peripheral hypotonia and growth delay. The long-term prognosis in cases of FVS depends on the presence of associated major defects (spina bifida, oral clefts, and cardiac abnormalities)."
],
"date": "November 10, 2015",
"figures": [],
"markdown": "# A Neonate With Dysmorphic Facial Features\n\n **Authors:** Adrian Ioan Toma, MD, PhD; Crenguta Albu, MD, PhD; Dinu Florin Albu, MD; Adriana Stan; Emilia Severin, PhD \n **Date:** November 10, 2015\n\n ## Content\n\n Approximately 2% of pregnant women who take VPA give birth to children with FVS and the symptoms of the disorder may vary, even between siblings. Folic acid may affect a variety of epigenetic mechanisms and may act as an environmental agent that contributes to phenotypic variability.[10,13] Thus, variability in clinical presentation may be influenced by many factors, such as maternal seizures that lead to periods of hypoxia during pregnancy, folic acid intake, and dose of and timing of exposure to VPA.\nGenetic differences in susceptibility to malformations (ie, predisposition to malformation owing to maternal genetic metabolic profile, which may interact with the antiseizure medication and cause a birth defect) may also influence clinical presentation variability.[15] The long-term effects of FVS as children develop include autism, speech, language, and learning difficulties. When the diagnosis is made, the child should be referred to early intervention services through the school system. These services include physical therapy, occupational therapy, speech therapy, and special education intervention.\nIn this case, a woman with epilepsy and her healthy partner (both without a family history of epilepsy) were informed that women with epilepsy are at a higher risk for conceiving a child with FVS, a rare congenital disorder. Because she experienced tonic-clonic seizures, the woman was concerned about whether she could conceive a healthy child. Together with her neurologist and obstetrician, the woman carefully planned her pregnancy and sought ways to minimize the risks for birth defects and complications. Before her pregnancy, she took 5 mg of folic acid per day and avoided smoking, caffeine, alcohol, and stress. She consulted with a genetic counselor to determine possible risks. During the pregnancy, she received VPA monotherapy to control her seizures. Although the pregnancy was normal, the results of the prenatal tests indicated the presence of FVS. The couple was informed about the affected fetus and chose to continue the pregnancy. At full-term, the woman gave birth to a baby with dysmorphic features consistent with FVS. The parents accepted the condition of their child and asked for assistance with perinatal care. After birth, the baby presented with central and peripheral hypotonia and growth delay. The long-term prognosis in cases of FVS depends on the presence of associated major defects (spina bifida, oral clefts, and cardiac abnormalities).\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 358377,
"choiceText": "Prenatal folic acid exposure",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 358378,
"choiceText": "Maternal diabetes",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 358379,
"choiceText": "Maternal VPA ingestion",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 358380,
"choiceText": "Maternal seizures in pregnancy",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "VPA is a well-known potential teratogen. Prenatal exposure to VPA during the first trimester of pregnancy is the cause of FVS. VPA crosses the human placenta where it may exert a teratogenic effect. These effects appear to be dose related. Maternal seizures have also been associated with birth defects.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 102234,
"questionText": "You are examining a neonatal patient with spina bifida, a cleft palate, and noted heart defects. The child's mother has a history of epileptic seizures. Based on the clinical appearance and findings, you suspect a diagnosis of FVS. Which of the following choices is associated with possible fetal damage and should prompt you to investigate for this condition further?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 358385,
"choiceText": "Amniocentesis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 358386,
"choiceText": "Prenatal ultrasound for major organ malformations",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 358387,
"choiceText": "History of VPA exposure in utero",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 358388,
"choiceText": "VPA serum concentration at birth",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 358389,
"choiceText": "All of the above",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "FVS is a diagnosis of exclusion. While none of the above choices alone can be used to definitively establish a diagnosis of FVS, they can be used together to determine the correct diagnosis. A history of VPA exposure in utero and ultrasonographic findings could suggest the diagnosis of FVS. Specific malformations can be found on ultrasonography in this disorder, but they are also features of other dysmorphic syndromes. Amniocentesis can be used to exclude other dysmorphic syndromes.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 102236,
"questionText": "Which of the following investigations is necessary to definitively establish the diagnosis of FVS in the patient described in the previous question?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A Neonate With Dysmorphic Facial Features"
},
{
"authors": "Adrian Ioan Toma, MD, PhD; Crenguta Albu, MD, PhD; Dinu Florin Albu, MD; Adriana Stan; Emilia Severin, PhD",
"content": [],
"date": "November 10, 2015",
"figures": [],
"markdown": "# A Neonate With Dysmorphic Facial Features\n\n **Authors:** Adrian Ioan Toma, MD, PhD; Crenguta Albu, MD, PhD; Dinu Florin Albu, MD; Adriana Stan; Emilia Severin, PhD \n **Date:** November 10, 2015\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 358377,
"choiceText": "Prenatal folic acid exposure",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 358378,
"choiceText": "Maternal diabetes",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 358379,
"choiceText": "Maternal VPA ingestion",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 358380,
"choiceText": "Maternal seizures in pregnancy",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "VPA is a well-known potential teratogen. Prenatal exposure to VPA during the first trimester of pregnancy is the cause of FVS. VPA crosses the human placenta where it may exert a teratogenic effect. These effects appear to be dose related. Maternal seizures have also been associated with birth defects.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 102234,
"questionText": "You are examining a neonatal patient with spina bifida, a cleft palate, and noted heart defects. The child's mother has a history of epileptic seizures. Based on the clinical appearance and findings, you suspect a diagnosis of FVS. Which of the following choices is associated with possible fetal damage and should prompt you to investigate for this condition further?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 358385,
"choiceText": "Amniocentesis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 358386,
"choiceText": "Prenatal ultrasound for major organ malformations",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 358387,
"choiceText": "History of VPA exposure in utero",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 358388,
"choiceText": "VPA serum concentration at birth",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 358389,
"choiceText": "All of the above",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "FVS is a diagnosis of exclusion. While none of the above choices alone can be used to definitively establish a diagnosis of FVS, they can be used together to determine the correct diagnosis. A history of VPA exposure in utero and ultrasonographic findings could suggest the diagnosis of FVS. Specific malformations can be found on ultrasonography in this disorder, but they are also features of other dysmorphic syndromes. Amniocentesis can be used to exclude other dysmorphic syndromes.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 102236,
"questionText": "Which of the following investigations is necessary to definitively establish the diagnosis of FVS in the patient described in the previous question?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A Neonate With Dysmorphic Facial Features"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 358347,
"choiceText": "Fetal valproate syndrome",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 358348,
"choiceText": "Trisomy 18 (Edwards syndrome)",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 358349,
"choiceText": "Structural chromosome abnormality syndrome",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 358350,
"choiceText": "Fetal alcohol syndrome",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 102224,
"questionText": "What is the likely diagnosis?<br /><br /><em>Hint: Consider the baby’s potential teratogen exposure during pregnancy</em>.",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 358377,
"choiceText": "Prenatal folic acid exposure",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 358378,
"choiceText": "Maternal diabetes",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 358379,
"choiceText": "Maternal VPA ingestion",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 358380,
"choiceText": "Maternal seizures in pregnancy",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "VPA is a well-known potential teratogen. Prenatal exposure to VPA during the first trimester of pregnancy is the cause of FVS. VPA crosses the human placenta where it may exert a teratogenic effect. These effects appear to be dose related. Maternal seizures have also been associated with birth defects.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 102234,
"questionText": "You are examining a neonatal patient with spina bifida, a cleft palate, and noted heart defects. The child's mother has a history of epileptic seizures. Based on the clinical appearance and findings, you suspect a diagnosis of FVS. Which of the following choices is associated with possible fetal damage and should prompt you to investigate for this condition further?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 358385,
"choiceText": "Amniocentesis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 358386,
"choiceText": "Prenatal ultrasound for major organ malformations",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 358387,
"choiceText": "History of VPA exposure in utero",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 358388,
"choiceText": "VPA serum concentration at birth",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 358389,
"choiceText": "All of the above",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "FVS is a diagnosis of exclusion. While none of the above choices alone can be used to definitively establish a diagnosis of FVS, they can be used together to determine the correct diagnosis. A history of VPA exposure in utero and ultrasonographic findings could suggest the diagnosis of FVS. Specific malformations can be found on ultrasonography in this disorder, but they are also features of other dysmorphic syndromes. Amniocentesis can be used to exclude other dysmorphic syndromes.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 102236,
"questionText": "Which of the following investigations is necessary to definitively establish the diagnosis of FVS in the patient described in the previous question?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
725757 | /viewarticle/725757 | [
{
"authors": "Szekeres Csilla-Cecília, MD; Pap Csilla, MD; Pelok Benedek, MD",
"content": [
"Editorial Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"An ambulance brings a 39-year-old woman complaining of a severe occipital headache and vomiting from her workplace to the emergency department (ED). She describes the headache as “the worst ever headache of my life” and states that it started suddenly, after a stressful situation at work. The intensity of the headache did not diminish after taking 200 mg of ibuprofen. She denies any head trauma, intense physical exertion, fever, changes in vision, photophobia, or any other associated symptoms (other than vomiting). She mentions that 2 days before presentation, she had a headache of similar intensity, but it only lasted a few seconds before resolving spontaneously.",
"Her medical history is remarkable for 2 normal pregnancies with uncomplicated deliveries. She denies any prior surgeries, and her only medications are oral contraceptives, which she has taken for about 15 years. She works as a dressmaker and denies illicit drug use, tobacco use, or alcohol consumption. She does not recall any significant medical problems in her family history."
],
"date": "April 02, 2018",
"figures": [],
"markdown": "# A Severe Headache in a Young Woman\n\n **Authors:** Szekeres Csilla-Cecília, MD; Pap Csilla, MD; Pelok Benedek, MD \n **Date:** April 02, 2018\n\n ## Content\n\n Editorial Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nAn ambulance brings a 39-year-old woman complaining of a severe occipital headache and vomiting from her workplace to the emergency department (ED). She describes the headache as “the worst ever headache of my life” and states that it started suddenly, after a stressful situation at work. The intensity of the headache did not diminish after taking 200 mg of ibuprofen. She denies any head trauma, intense physical exertion, fever, changes in vision, photophobia, or any other associated symptoms (other than vomiting). She mentions that 2 days before presentation, she had a headache of similar intensity, but it only lasted a few seconds before resolving spontaneously.\nHer medical history is remarkable for 2 normal pregnancies with uncomplicated deliveries. She denies any prior surgeries, and her only medications are oral contraceptives, which she has taken for about 15 years. She works as a dressmaker and denies illicit drug use, tobacco use, or alcohol consumption. She does not recall any significant medical problems in her family history.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A Severe Headache in a Young Woman"
},
{
"authors": "Szekeres Csilla-Cecília, MD; Pap Csilla, MD; Pelok Benedek, MD",
"content": [
"Upon physical examination, she is obese and appears her stated age. She is in mild distress due to pain. Her axillary temperature is 97.8°F (36.6°C). Her pulse is regular at 78 beats/min, her blood pressure is 160/80 mm Hg (which she states is high for her), and her respiratory rate is 20 breaths/min.",
"Her head is normal on inspection, without any areas of tenderness. The ears, nose, and throat are clear. Her pupils are both small at 2 mm, but they are reactive to light. Otherwise, the eyes appear normal, with normal extraocular movements and no photophobia or nystagmus. The fundi and optic discs appear normal. No masses are detected on examination of her neck, but significant nuchal rigidity is noted. The chest examination is normal, with lungs clear to auscultation bilaterally and normal respiratory effort.",
"The heart sounds are also normal. Her abdomen is soft and nontender. The neurologic examination reveals that she is fully alert, oriented, and mildly anxious. Her cranial nerves are intact. Motor strength is symmetrical, with brisk and symmetric deep tendon reflexes without clonus. Cerebellar function and sensory systems are normal.",
"Laboratory analyses, including a complete blood count, metabolic panel, and urine analysis, are normal. A noncontrast cerebral CT scan is performed (Figure 1).",
"Figure 1."
],
"date": "April 02, 2018",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/725/757/725755-thumb1.png"
}
],
"markdown": "# A Severe Headache in a Young Woman\n\n **Authors:** Szekeres Csilla-Cecília, MD; Pap Csilla, MD; Pelok Benedek, MD \n **Date:** April 02, 2018\n\n ## Content\n\n Upon physical examination, she is obese and appears her stated age. She is in mild distress due to pain. Her axillary temperature is 97.8°F (36.6°C). Her pulse is regular at 78 beats/min, her blood pressure is 160/80 mm Hg (which she states is high for her), and her respiratory rate is 20 breaths/min.\nHer head is normal on inspection, without any areas of tenderness. The ears, nose, and throat are clear. Her pupils are both small at 2 mm, but they are reactive to light. Otherwise, the eyes appear normal, with normal extraocular movements and no photophobia or nystagmus. The fundi and optic discs appear normal. No masses are detected on examination of her neck, but significant nuchal rigidity is noted. The chest examination is normal, with lungs clear to auscultation bilaterally and normal respiratory effort.\nThe heart sounds are also normal. Her abdomen is soft and nontender. The neurologic examination reveals that she is fully alert, oriented, and mildly anxious. Her cranial nerves are intact. Motor strength is symmetrical, with brisk and symmetric deep tendon reflexes without clonus. Cerebellar function and sensory systems are normal.\nLaboratory analyses, including a complete blood count, metabolic panel, and urine analysis, are normal. A noncontrast cerebral CT scan is performed (Figure 1).\nFigure 1.\n\n ## Figures\n\n **Figure 1.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 354836,
"choiceText": "Migraine headache",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 354837,
"choiceText": "Subarachnoid hemorrhage",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 354838,
"choiceText": "Meningitis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 354839,
"choiceText": "Vertebral artery dissection",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 354840,
"choiceText": "Cerebral venous sinus thrombosis",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 101053,
"questionText": "What is the diagnosis?<br><br>\r\n<em>Hint: The patient has no prior history of similar headaches.</em>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A Severe Headache in a Young Woman"
},
{
"authors": "Szekeres Csilla-Cecília, MD; Pap Csilla, MD; Pelok Benedek, MD",
"content": [
"The diagnosis of spontaneous subarachnoid hemorrhage (SAH) was made based on the hyperdensity seen in the subarachnoid space on the noncontrast-enhanced cerebral CT scan (Figure 2).",
"Figure 2.",
"Once the CT diagnosis was made, the other diagnoses in the differential above were effectively ruled out, as the likelihood of 2 simultaneous processes is very low. The history did not reveal significant head trauma, so it was concluded that this was a spontaneous (nontraumatic) SAH. After making the diagnosis and controlling the patient’s pain, urgent neurosurgical consultation was obtained.",
"Spontaneous SAH is a neurosurgical emergency and a life-threatening condition. It can progress to coma, permanent brain damage, and death. It is characterized by extravasation of blood into the subarachnoid space, most commonly from a berry aneurysm or other vascular malformation. The incidence of SAH is 2-49 cases per 100,000 people per year internationally, and 10%-15% of patients die before reaching the hospital. People of African heritage are at higher risk than whites by a ratio of 2.1 to 1. Asians are also at higher risk, and the incidence of SAH is higher in women. Other risk factors include polycystic kidney disease, lupus, Ehlers-Danlos syndrome, and tobacco use. The mean age of onset is 50 years.[1,2]"
],
"date": "April 02, 2018",
"figures": [
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/725/757/725755-thumb2.png"
}
],
"markdown": "# A Severe Headache in a Young Woman\n\n **Authors:** Szekeres Csilla-Cecília, MD; Pap Csilla, MD; Pelok Benedek, MD \n **Date:** April 02, 2018\n\n ## Content\n\n The diagnosis of spontaneous subarachnoid hemorrhage (SAH) was made based on the hyperdensity seen in the subarachnoid space on the noncontrast-enhanced cerebral CT scan (Figure 2).\nFigure 2.\nOnce the CT diagnosis was made, the other diagnoses in the differential above were effectively ruled out, as the likelihood of 2 simultaneous processes is very low. The history did not reveal significant head trauma, so it was concluded that this was a spontaneous (nontraumatic) SAH. After making the diagnosis and controlling the patient’s pain, urgent neurosurgical consultation was obtained.\nSpontaneous SAH is a neurosurgical emergency and a life-threatening condition. It can progress to coma, permanent brain damage, and death. It is characterized by extravasation of blood into the subarachnoid space, most commonly from a berry aneurysm or other vascular malformation. The incidence of SAH is 2-49 cases per 100,000 people per year internationally, and 10%-15% of patients die before reaching the hospital. People of African heritage are at higher risk than whites by a ratio of 2.1 to 1. Asians are also at higher risk, and the incidence of SAH is higher in women. Other risk factors include polycystic kidney disease, lupus, Ehlers-Danlos syndrome, and tobacco use. The mean age of onset is 50 years.[1,2]\n\n ## Figures\n\n **Figure 2.** \n \n\n\n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 354836,
"choiceText": "Migraine headache",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 354837,
"choiceText": "Subarachnoid hemorrhage",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 354838,
"choiceText": "Meningitis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 354839,
"choiceText": "Vertebral artery dissection",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 354840,
"choiceText": "Cerebral venous sinus thrombosis",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 101053,
"questionText": "What is the diagnosis?<br><br>\r\n<em>Hint: The patient has no prior history of similar headaches.</em>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A Severe Headache in a Young Woman"
},
{
"authors": "Szekeres Csilla-Cecília, MD; Pap Csilla, MD; Pelok Benedek, MD",
"content": [
"The typical presentation of a nontraumatic SAH is marked by the sudden onset of a severe headache, which has been dubbed “thunderclap headache.”[2] The rapid onset of the headache is the most important historical feature. Patients usually refer to an SAH as the worst headache of their life; however, patients presenting to an ED with headache of any etiology will often have the same complaint. Headache from SAH tends to be occipital in location. In patients with a prior history of cephalgia, headache from SAH is usually, but not always, described as different from prior headaches. Sentinel headaches are SAHs that do not cause catastrophic damage. If recognized, a sentinel headache provides an opportunity for intervention before a larger, more debilitating SAH occurs. Patients may also experience epileptic seizures, nausea or vomiting, neck stiffness, photophobia, and loss of consciousness.",
"On physical examination, neurologic and vital-sign abnormalities may be present; these include cranial nerve signs, motor deficits, seizures, coma, ophthalmologic signs (papilledema, retinal hemorrhage), and mild-to-moderate blood pressure elevation.[1] A history of headache prior to a fall or syncopal episode or an uncertain history of trauma should increase suspicion for spontaneous SAH.",
"The severity of an SAH is most commonly graded using the Hunt-Hess scale, as follows[2]:",
"0: Unruptured aneurysm",
"1: Asymptomatic or minimal headache without neck rigidity",
"2: Moderate-to-severe headache, neck rigidity, cranial nerve palsy",
"3: Drowsy, confused, mild focal deficit",
"4: Stupor, moderate-to-severe deficit, hemiparesis",
"5: Deep coma, decerebrate rigidity, moribund",
"The differential diagnosis of SAH is very large. It includes encephalitis, meningitis, primary headaches (migraine, cluster headache), temporal arteritis, hypertensive encephalopathy, spontaneous intracerebral hemorrhage, ischemic stroke, transient ischemic attack, panic attack, craniocervical dissections, and cerebral venous sinus thrombosis, as well as other causes of headache.[1]",
"The most useful initial diagnostic tool to confirm the diagnosis of SAH is a noncontrast brain CT scan, which shows the hyperdense collection of blood in the subarachnoid space. The sensitivity of CT scanning of the brain is considered to be 90%-95% within 24 hours of symptom onset, 80% at 3 days, and 50% at 1 week.[1] In one study, high-resolution CT scanning was positive for SAH in all cases examined within 12 hours from the onset and in 93% of patients who presented within 24 hours of onset.[3] Brain CT scan is also useful because it may demonstrate associated complications of SAH, such as hydrocephalus, ischemic strokes due to vasospasm, mass effect, and signs of impending herniation. A falsely negative CT scan may result if there is only a very small sentinel bleed. These patients will usually be Hunt-Hess grade 1, and they are also the patients with the best prognosis if they are diagnosed and treated before a catastrophic SAH. Other possible causes of a false-negative CT scan include a delay of more than 12 hours from symptom onset, anemia (with a hemoglobin < 10 g/dL), and movement artifacts. The clinician should always ask the radiologist if a careful review for blood in the interpeduncular cistern was performed. This area sits posteriorly and includes the circle of Willis; forming a natural “cup,” it may collect just enough blood to be seen on CT scan when an SAH is very small.",
"The criterion standard diagnostic test for SAH is a lumbar puncture (LP) with cerebrospinal fluid (CSF) analysis. An LP must be performed after a negative brain CT scan whenever there is a suspicion of SAH and no contraindications to the procedure. As opposed to CT scanning, the sensitivity of LP for SAH initially improves as the time from onset increases. In addition, an important alternate diagnosis, such as meningitis or encephalitis, may be made. The most important CSF findings in SAH are consistently elevated red blood cell counts in 2 or more tubes, as well as xanthochromia (ie, yellow discoloration due to hemoglobin breakdown and presence of bilirubin), which is seen by 12 hours after the onset of bleeding. The opening pressure should be measured, as it is often elevated in cases of SAH . A false-negative LP may occur if the procedure is done too early, before enough time has elapsed for blood from the brain to circulate down into the lumbar area. Although it is not recommended that LP be delayed because of this reason, it is important to know that sensitivity decreases if the LP is performed before 12 hours from symptom onset. Fortunately, this is exactly the time frame in which the CT scan is most sensitive. In addition, LP loses sensitivity after 2 weeks from symptom onset."
],
"date": "April 02, 2018",
"figures": [],
"markdown": "# A Severe Headache in a Young Woman\n\n **Authors:** Szekeres Csilla-Cecília, MD; Pap Csilla, MD; Pelok Benedek, MD \n **Date:** April 02, 2018\n\n ## Content\n\n The typical presentation of a nontraumatic SAH is marked by the sudden onset of a severe headache, which has been dubbed “thunderclap headache.”[2] The rapid onset of the headache is the most important historical feature. Patients usually refer to an SAH as the worst headache of their life; however, patients presenting to an ED with headache of any etiology will often have the same complaint. Headache from SAH tends to be occipital in location. In patients with a prior history of cephalgia, headache from SAH is usually, but not always, described as different from prior headaches. Sentinel headaches are SAHs that do not cause catastrophic damage. If recognized, a sentinel headache provides an opportunity for intervention before a larger, more debilitating SAH occurs. Patients may also experience epileptic seizures, nausea or vomiting, neck stiffness, photophobia, and loss of consciousness.\nOn physical examination, neurologic and vital-sign abnormalities may be present; these include cranial nerve signs, motor deficits, seizures, coma, ophthalmologic signs (papilledema, retinal hemorrhage), and mild-to-moderate blood pressure elevation.[1] A history of headache prior to a fall or syncopal episode or an uncertain history of trauma should increase suspicion for spontaneous SAH.\nThe severity of an SAH is most commonly graded using the Hunt-Hess scale, as follows[2]:\n0: Unruptured aneurysm\n1: Asymptomatic or minimal headache without neck rigidity\n2: Moderate-to-severe headache, neck rigidity, cranial nerve palsy\n3: Drowsy, confused, mild focal deficit\n4: Stupor, moderate-to-severe deficit, hemiparesis\n5: Deep coma, decerebrate rigidity, moribund\nThe differential diagnosis of SAH is very large. It includes encephalitis, meningitis, primary headaches (migraine, cluster headache), temporal arteritis, hypertensive encephalopathy, spontaneous intracerebral hemorrhage, ischemic stroke, transient ischemic attack, panic attack, craniocervical dissections, and cerebral venous sinus thrombosis, as well as other causes of headache.[1]\nThe most useful initial diagnostic tool to confirm the diagnosis of SAH is a noncontrast brain CT scan, which shows the hyperdense collection of blood in the subarachnoid space. The sensitivity of CT scanning of the brain is considered to be 90%-95% within 24 hours of symptom onset, 80% at 3 days, and 50% at 1 week.[1] In one study, high-resolution CT scanning was positive for SAH in all cases examined within 12 hours from the onset and in 93% of patients who presented within 24 hours of onset.[3] Brain CT scan is also useful because it may demonstrate associated complications of SAH, such as hydrocephalus, ischemic strokes due to vasospasm, mass effect, and signs of impending herniation. A falsely negative CT scan may result if there is only a very small sentinel bleed. These patients will usually be Hunt-Hess grade 1, and they are also the patients with the best prognosis if they are diagnosed and treated before a catastrophic SAH. Other possible causes of a false-negative CT scan include a delay of more than 12 hours from symptom onset, anemia (with a hemoglobin < 10 g/dL), and movement artifacts. The clinician should always ask the radiologist if a careful review for blood in the interpeduncular cistern was performed. This area sits posteriorly and includes the circle of Willis; forming a natural “cup,” it may collect just enough blood to be seen on CT scan when an SAH is very small.\nThe criterion standard diagnostic test for SAH is a lumbar puncture (LP) with cerebrospinal fluid (CSF) analysis. An LP must be performed after a negative brain CT scan whenever there is a suspicion of SAH and no contraindications to the procedure. As opposed to CT scanning, the sensitivity of LP for SAH initially improves as the time from onset increases. In addition, an important alternate diagnosis, such as meningitis or encephalitis, may be made. The most important CSF findings in SAH are consistently elevated red blood cell counts in 2 or more tubes, as well as xanthochromia (ie, yellow discoloration due to hemoglobin breakdown and presence of bilirubin), which is seen by 12 hours after the onset of bleeding. The opening pressure should be measured, as it is often elevated in cases of SAH . A false-negative LP may occur if the procedure is done too early, before enough time has elapsed for blood from the brain to circulate down into the lumbar area. Although it is not recommended that LP be delayed because of this reason, it is important to know that sensitivity decreases if the LP is performed before 12 hours from symptom onset. Fortunately, this is exactly the time frame in which the CT scan is most sensitive. In addition, LP loses sensitivity after 2 weeks from symptom onset.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A Severe Headache in a Young Woman"
},
{
"authors": "Szekeres Csilla-Cecília, MD; Pap Csilla, MD; Pelok Benedek, MD",
"content": [
"Once the diagnosis of SAH has been confirmed either by CT or LP, medical stabilization should be instituted. This is followed by an urgent examination of the intracerebral blood vessel anatomy for early visualization of the bleeding source (if it exists), which most often is a berry aneurysm or arteriovenous malformation (AVM). Medical stabilization is aimed at preventing early complications, including brain edema, hydrocephalus, hyponatremia, and rebleeding, as well as the late complication of vasospasm. Treatment options include bed rest with elevation of the head of the bed to 30 degrees, nimodipine (a calcium channel blocker to prevent vasospasm), seizure prophylaxis, antiemetics, analgesia, and labetalol or other agents as needed for blood pressure control.",
"Vascular malformations leading to SAH can be identified by conventional cerebral angiography, CT angiography, or by magnetic resonance angiography of the cerebral vasculature. Other indications for one of these tests would be if LP is not possible or is refused by the patient, or if the work-up is negative but more than 2 weeks since symptom onset have passed. Some experts also recommend angiography in certain high-risk patients despite a negative CT and LP, such as those with polycystic kidney disease, Marfan syndrome, a family history of SAH, or suspicion for vascular dissection. The clinician should not recommend angiography in lieu of LP, however, because it is neither extremely sensitive nor specific for the diagnosis of SAH. Angiography is only 80%-90% sensitive for SAH.[4] In addition, approximately 1%-2% of the population has an asymptomatic berry aneurysm.[2] Unless they are large or have bled, these aneurysms do not require treatment. Making the diagnosis of berry aneurysm without an LP showing an SAH may lead to confusion about the best course of action or even to unnecessary surgery.",
"Digital-subtraction cerebral angiography is invasive, but it has a great sensitivity for showing vascular anatomy and current bleeding site and can visualize other aneurysms or AVMs. Magnetic resonance angiography is noninvasive, but it has lower sensitivity for visualization of the intracerebral vessels, requires more time to perform the examination, has a greater risk of movement artifacts, and is more examiner-dependent. CT angiography has a similar sensitivity but exposes the patient to a greater dose of radiation and is also examiner-dependent.[1] In 10%-20% of patients with SAH, no source of bleeding is evident after imaging assessment of the cerebral vessels (nonaneurysmal SAH).[4] The outcome of nonaneurysmal SAH is better than that of aneurysm rupture or AVM. Depending on the localization of the subarachnoid blood, 2 nonaneurysmal SAH patterns can be considered to mimic true aneurysmal rupture: the perimesencephalic hemorrhage pattern and the nonperimesencephalic hemorrhage pattern, which includes sylvian, interhemispheric, intraventricular, and convexal bleeding distributions.[5]",
"In patients with a nonperimesencephalic SAH, if the initial angiogram is normal, many practitioners will order a repeat angiogram within two weeks, and sometimes a third one after that, out of concern that an aneurysm or AVM may exist but, for various reasons, was not visible at first. However, protocols for ordering these repeat angiograms have not been clearly defined. When angiography is positive, early neurosurgical intervention with clipping, coiling, or glue injection into the vascular malformation improves the outcome of patients and decreases the risk of rebleeding (a major complication of SAH).",
"The patient in this case was diagnosed with a Hunt-Hess Scale grade 2 SAH based on her severe headache and the nuchal rigidity found on the physical examination. She was referred to the neurosurgical department after the CT scan results were evaluated. Urgent cerebral angiography was performed, which was negative for intracerebral aneurysm or AVM. MRA was performed the next day and was also negative. The patient remained in the neurosurgical department for close observation. She received intravenous fluids, nimodipine, analgesics, and laxatives. She was monitored for cerebral vasospasm by transcranial Doppler sonography and was kept on bed rest for the first 10 days of her admission, with progressive active mobilization thereafter. She developed mild, subclinical vasospasm on day 5 of hospitalization, which resolved completely afterward. She was transferred to the neurologic department for further conservative treatment on day 9 and discharged from the hospital on day 17. Her clinical condition improved continuously during her admission, with resolution of her headache, nuchal rigidity, and miosis and with no development of other neurologic signs during her observation. A CT scan performed 1 month later (Figure 3) showed complete absorption of the blood from the subarachnoid space, with no sign of hydrocephalus or other complications.",
"Figure 3.",
"At discharge, sexual abstinence was recommended for 6 weeks in order to decrease the chance of rebleeding. She was also counseled to avoid strenuous activity for the first 6 weeks after her discharge from the hospital. After 3 months, the patient’s clinical condition remained excellent."
],
"date": "April 02, 2018",
"figures": [
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/725/757/725755-thumb3.png"
}
],
"markdown": "# A Severe Headache in a Young Woman\n\n **Authors:** Szekeres Csilla-Cecília, MD; Pap Csilla, MD; Pelok Benedek, MD \n **Date:** April 02, 2018\n\n ## Content\n\n Once the diagnosis of SAH has been confirmed either by CT or LP, medical stabilization should be instituted. This is followed by an urgent examination of the intracerebral blood vessel anatomy for early visualization of the bleeding source (if it exists), which most often is a berry aneurysm or arteriovenous malformation (AVM). Medical stabilization is aimed at preventing early complications, including brain edema, hydrocephalus, hyponatremia, and rebleeding, as well as the late complication of vasospasm. Treatment options include bed rest with elevation of the head of the bed to 30 degrees, nimodipine (a calcium channel blocker to prevent vasospasm), seizure prophylaxis, antiemetics, analgesia, and labetalol or other agents as needed for blood pressure control.\nVascular malformations leading to SAH can be identified by conventional cerebral angiography, CT angiography, or by magnetic resonance angiography of the cerebral vasculature. Other indications for one of these tests would be if LP is not possible or is refused by the patient, or if the work-up is negative but more than 2 weeks since symptom onset have passed. Some experts also recommend angiography in certain high-risk patients despite a negative CT and LP, such as those with polycystic kidney disease, Marfan syndrome, a family history of SAH, or suspicion for vascular dissection. The clinician should not recommend angiography in lieu of LP, however, because it is neither extremely sensitive nor specific for the diagnosis of SAH. Angiography is only 80%-90% sensitive for SAH.[4] In addition, approximately 1%-2% of the population has an asymptomatic berry aneurysm.[2] Unless they are large or have bled, these aneurysms do not require treatment. Making the diagnosis of berry aneurysm without an LP showing an SAH may lead to confusion about the best course of action or even to unnecessary surgery.\nDigital-subtraction cerebral angiography is invasive, but it has a great sensitivity for showing vascular anatomy and current bleeding site and can visualize other aneurysms or AVMs. Magnetic resonance angiography is noninvasive, but it has lower sensitivity for visualization of the intracerebral vessels, requires more time to perform the examination, has a greater risk of movement artifacts, and is more examiner-dependent. CT angiography has a similar sensitivity but exposes the patient to a greater dose of radiation and is also examiner-dependent.[1] In 10%-20% of patients with SAH, no source of bleeding is evident after imaging assessment of the cerebral vessels (nonaneurysmal SAH).[4] The outcome of nonaneurysmal SAH is better than that of aneurysm rupture or AVM. Depending on the localization of the subarachnoid blood, 2 nonaneurysmal SAH patterns can be considered to mimic true aneurysmal rupture: the perimesencephalic hemorrhage pattern and the nonperimesencephalic hemorrhage pattern, which includes sylvian, interhemispheric, intraventricular, and convexal bleeding distributions.[5]\nIn patients with a nonperimesencephalic SAH, if the initial angiogram is normal, many practitioners will order a repeat angiogram within two weeks, and sometimes a third one after that, out of concern that an aneurysm or AVM may exist but, for various reasons, was not visible at first. However, protocols for ordering these repeat angiograms have not been clearly defined. When angiography is positive, early neurosurgical intervention with clipping, coiling, or glue injection into the vascular malformation improves the outcome of patients and decreases the risk of rebleeding (a major complication of SAH).\nThe patient in this case was diagnosed with a Hunt-Hess Scale grade 2 SAH based on her severe headache and the nuchal rigidity found on the physical examination. She was referred to the neurosurgical department after the CT scan results were evaluated. Urgent cerebral angiography was performed, which was negative for intracerebral aneurysm or AVM. MRA was performed the next day and was also negative. The patient remained in the neurosurgical department for close observation. She received intravenous fluids, nimodipine, analgesics, and laxatives. She was monitored for cerebral vasospasm by transcranial Doppler sonography and was kept on bed rest for the first 10 days of her admission, with progressive active mobilization thereafter. She developed mild, subclinical vasospasm on day 5 of hospitalization, which resolved completely afterward. She was transferred to the neurologic department for further conservative treatment on day 9 and discharged from the hospital on day 17. Her clinical condition improved continuously during her admission, with resolution of her headache, nuchal rigidity, and miosis and with no development of other neurologic signs during her observation. A CT scan performed 1 month later (Figure 3) showed complete absorption of the blood from the subarachnoid space, with no sign of hydrocephalus or other complications.\nFigure 3.\nAt discharge, sexual abstinence was recommended for 6 weeks in order to decrease the chance of rebleeding. She was also counseled to avoid strenuous activity for the first 6 weeks after her discharge from the hospital. After 3 months, the patient’s clinical condition remained excellent.\n\n ## Figures\n\n **Figure 3.** \n \n\n\n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 354841,
"choiceText": "Sudden, rapid onset of headache",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 354842,
"choiceText": "Vertigo",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 354843,
"choiceText": "Associated vomiting",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 354844,
"choiceText": "Fever",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 354845,
"choiceText": "Frequent, similar headaches in the past",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The typical presentation of a nontraumatic subarachnoid hemorrhage is marked by the sudden onset of a severe headache, which has been dubbed \"thunderclap headache.\" The rapid onset of the headache is the most important historical feature. Patients usually refer to a subarachnoid hemorrhage as the worst headache of their life; however, patients presenting to an ED with headache of any etiology will often have the same complaint. Vomiting is associated with many etiologies of headache and is not unique to subarachnoid hemorrhage. Fever and frequent, similar headaches in the past suggest an alternate diagnosis, such as meningitis or migraine, respectively.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 101054,
"questionText": "You are seeing a patient with a severe headache and suspect a subarachnoid bleed. Which of the following historical features is most suggestive of subarachnoid hemorrhage?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 354846,
"choiceText": "Brain CT scan with intravenous contrast",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 354847,
"choiceText": "Noncontrast brain CT scan",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 354848,
"choiceText": "Funduscopic examination of the retina",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 354849,
"choiceText": "MRI of the brain",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The most useful initial diagnostic tool for a suspected subarachnoid hemorrhage is an urgent noncontrast brain CT scan, which would reveal the hyperdense blood in the subarachnoid space. A brain CT scan also may demonstrate associated lesions, such as hydrocephalus, ischemic stroke due to vasospasm, mass effect, and signs of impending herniation. A contrast-enhanced brain CT scan may obscure evidence of subarachnoid hemorrhage. An MRI is an excellent tool to detect intracranial bleeding; however, limited access at many centers and a long evaluation time make it less useful as an initial diagnostic tool. Funduscopic examination is not sensitive or specific for subarachnoid hemorrhage.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 101055,
"questionText": "You are seeing a patient who presents with a thunderclap-type headache. Which of the following is the most useful initial diagnostic tool to evaluate for a subarachnoid hemorrhage?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A Severe Headache in a Young Woman"
},
{
"authors": "Szekeres Csilla-Cecília, MD; Pap Csilla, MD; Pelok Benedek, MD",
"content": [],
"date": "April 02, 2018",
"figures": [],
"markdown": "# A Severe Headache in a Young Woman\n\n **Authors:** Szekeres Csilla-Cecília, MD; Pap Csilla, MD; Pelok Benedek, MD \n **Date:** April 02, 2018\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 354841,
"choiceText": "Sudden, rapid onset of headache",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 354842,
"choiceText": "Vertigo",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 354843,
"choiceText": "Associated vomiting",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 354844,
"choiceText": "Fever",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 354845,
"choiceText": "Frequent, similar headaches in the past",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The typical presentation of a nontraumatic subarachnoid hemorrhage is marked by the sudden onset of a severe headache, which has been dubbed \"thunderclap headache.\" The rapid onset of the headache is the most important historical feature. Patients usually refer to a subarachnoid hemorrhage as the worst headache of their life; however, patients presenting to an ED with headache of any etiology will often have the same complaint. Vomiting is associated with many etiologies of headache and is not unique to subarachnoid hemorrhage. Fever and frequent, similar headaches in the past suggest an alternate diagnosis, such as meningitis or migraine, respectively.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 101054,
"questionText": "You are seeing a patient with a severe headache and suspect a subarachnoid bleed. Which of the following historical features is most suggestive of subarachnoid hemorrhage?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 354846,
"choiceText": "Brain CT scan with intravenous contrast",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 354847,
"choiceText": "Noncontrast brain CT scan",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 354848,
"choiceText": "Funduscopic examination of the retina",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 354849,
"choiceText": "MRI of the brain",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The most useful initial diagnostic tool for a suspected subarachnoid hemorrhage is an urgent noncontrast brain CT scan, which would reveal the hyperdense blood in the subarachnoid space. A brain CT scan also may demonstrate associated lesions, such as hydrocephalus, ischemic stroke due to vasospasm, mass effect, and signs of impending herniation. A contrast-enhanced brain CT scan may obscure evidence of subarachnoid hemorrhage. An MRI is an excellent tool to detect intracranial bleeding; however, limited access at many centers and a long evaluation time make it less useful as an initial diagnostic tool. Funduscopic examination is not sensitive or specific for subarachnoid hemorrhage.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 101055,
"questionText": "You are seeing a patient who presents with a thunderclap-type headache. Which of the following is the most useful initial diagnostic tool to evaluate for a subarachnoid hemorrhage?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A Severe Headache in a Young Woman"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 354836,
"choiceText": "Migraine headache",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 354837,
"choiceText": "Subarachnoid hemorrhage",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 354838,
"choiceText": "Meningitis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 354839,
"choiceText": "Vertebral artery dissection",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 354840,
"choiceText": "Cerebral venous sinus thrombosis",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 101053,
"questionText": "What is the diagnosis?<br><br>\r\n<em>Hint: The patient has no prior history of similar headaches.</em>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 354841,
"choiceText": "Sudden, rapid onset of headache",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 354842,
"choiceText": "Vertigo",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 354843,
"choiceText": "Associated vomiting",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 354844,
"choiceText": "Fever",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 354845,
"choiceText": "Frequent, similar headaches in the past",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The typical presentation of a nontraumatic subarachnoid hemorrhage is marked by the sudden onset of a severe headache, which has been dubbed \"thunderclap headache.\" The rapid onset of the headache is the most important historical feature. Patients usually refer to a subarachnoid hemorrhage as the worst headache of their life; however, patients presenting to an ED with headache of any etiology will often have the same complaint. Vomiting is associated with many etiologies of headache and is not unique to subarachnoid hemorrhage. Fever and frequent, similar headaches in the past suggest an alternate diagnosis, such as meningitis or migraine, respectively.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 101054,
"questionText": "You are seeing a patient with a severe headache and suspect a subarachnoid bleed. Which of the following historical features is most suggestive of subarachnoid hemorrhage?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 354846,
"choiceText": "Brain CT scan with intravenous contrast",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 354847,
"choiceText": "Noncontrast brain CT scan",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 354848,
"choiceText": "Funduscopic examination of the retina",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 354849,
"choiceText": "MRI of the brain",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The most useful initial diagnostic tool for a suspected subarachnoid hemorrhage is an urgent noncontrast brain CT scan, which would reveal the hyperdense blood in the subarachnoid space. A brain CT scan also may demonstrate associated lesions, such as hydrocephalus, ischemic stroke due to vasospasm, mass effect, and signs of impending herniation. A contrast-enhanced brain CT scan may obscure evidence of subarachnoid hemorrhage. An MRI is an excellent tool to detect intracranial bleeding; however, limited access at many centers and a long evaluation time make it less useful as an initial diagnostic tool. Funduscopic examination is not sensitive or specific for subarachnoid hemorrhage.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 101055,
"questionText": "You are seeing a patient who presents with a thunderclap-type headache. Which of the following is the most useful initial diagnostic tool to evaluate for a subarachnoid hemorrhage?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
724695 | /viewarticle/724695 | [
{
"authors": "Brian K. Felice, MD; Daniel S. Richardson, MD",
"content": [
"Editor's Note:\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.",
"A 94-year-old male resident of an extended care facility presents to the emergency department (ED) with altered mental status and confusion. He is unable to provide a medical history, so a collateral history is obtained from EMS, available medical records, and the patient's nephew. The patient has recently been hospitalized for pneumonia, chronic kidney disease, congestive heart failure, hypothyroidism, and a myocardial infarction. The patient’s medications are amiodarone, amlodipine, clopidogrel, levothyroxine, lisinopril, metoprolol, propafenone, and simvastatin. It is initially unclear which of these he is currently taking.",
"A review of systems is unattainable because of the patient’s mental status changes. A chart review reveals an iodine allergy and remote tobacco use. The EMS report details that, according to the patient’s nephew, the patient is generally alert and oriented to person and place; however, over the last 24 hours, he has become less responsive and increasingly confused. EMS personnel confirm decreased responsiveness and document a heart rate of 30s to 40s en route to the ED."
],
"date": "October 12, 2015",
"figures": [],
"markdown": "# Lethargy and Mental Status Changes in a 94-Year-Old Man\n\n **Authors:** Brian K. Felice, MD; Daniel S. Richardson, MD \n **Date:** October 12, 2015\n\n ## Content\n\n Editor's Note:\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.\nA 94-year-old male resident of an extended care facility presents to the emergency department (ED) with altered mental status and confusion. He is unable to provide a medical history, so a collateral history is obtained from EMS, available medical records, and the patient's nephew. The patient has recently been hospitalized for pneumonia, chronic kidney disease, congestive heart failure, hypothyroidism, and a myocardial infarction. The patient’s medications are amiodarone, amlodipine, clopidogrel, levothyroxine, lisinopril, metoprolol, propafenone, and simvastatin. It is initially unclear which of these he is currently taking.\nA review of systems is unattainable because of the patient’s mental status changes. A chart review reveals an iodine allergy and remote tobacco use. The EMS report details that, according to the patient’s nephew, the patient is generally alert and oriented to person and place; however, over the last 24 hours, he has become less responsive and increasingly confused. EMS personnel confirm decreased responsiveness and document a heart rate of 30s to 40s en route to the ED.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "Lethargy and Mental Status Changes in a 94-Year-Old Man"
},
{
"authors": "Brian K. Felice, MD; Daniel S. Richardson, MD",
"content": [
"On presentation, the patient's blood pressure is 109/76 mm Hg, heart rate is 49 beats/min, respiratory rate is 19 breaths/min, rectal temperature is 91.22°F (32.9°C), and oxygen saturation is 98%. The patient is noncommunicative and in mild distress. His pupils are equal, round, and sluggishly reactive to light.",
"Figure 1.",
"The cardiovascular examination is notable for a bradycardic regular rhythm, without murmurs. The distal pulses are faint but appreciable bilaterally. His respiratory rate and effort are normal and the lungs are clear to auscultation bilaterally. The abdomen is soft, nontender, nondistended, and with normal bowel sounds. The rectal examination reveals guaiac-positive brown stool. The patient's skin is cool, dry, and nonedematous. He is nonverbal, but does turn his head in response to his name.",
"The initial work-up includes an electrocardiogram (ECG), chest radiograph, complete blood cell count (CBC), basic metabolic panel (BMP), urinalysis, and cardiac enzymes. The ECG (Figure 1) shows a bradycardic junctional rhythm at a rate of 49 bpm (compared with previous ECGs showing a normal sinus rhythm with a rate in the 80s) and a prolonged QT interval. The CBC shows a normal white blood cell and platelet count, but a hemoglobin of 8.5 g/dL (85 g/L; decreased approximately 2 g/dL from a previous admission). The BMP shows stable stage 4 chronic kidney disease and a blood glucose level of 102 mg/dL (5.7 mmol/L). The cardiac enzyme examination and urinalysis are normal. Chest radiography shows mild vascular congestion and no infiltrates. CT scanning of the head reveals no acute disease.",
"The patient's heart rate throughout the initial ED evaluation ranges from the high 40s to the low 60s. The patient receives a transfusion of packed red blood cells (RBCs). His hypothermia is treated with the application of warming blankets. The patient is re-evaluated and found to have a Glasgow Coma Scale (GCS) rating of 9. His blood glucose is rechecked and is 49 mg/dL (2.72 mmol/L). The low blood glucose is treated but the patient's mental status does not improve. He continues to be hypothermic and bradycardic. He is endotracheally intubated for airway protection and admitted to the medical intensive care unit (MICU)."
],
"date": "October 12, 2015",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/724/695/724695-thumb.png"
}
],
"markdown": "# Lethargy and Mental Status Changes in a 94-Year-Old Man\n\n **Authors:** Brian K. Felice, MD; Daniel S. Richardson, MD \n **Date:** October 12, 2015\n\n ## Content\n\n On presentation, the patient's blood pressure is 109/76 mm Hg, heart rate is 49 beats/min, respiratory rate is 19 breaths/min, rectal temperature is 91.22°F (32.9°C), and oxygen saturation is 98%. The patient is noncommunicative and in mild distress. His pupils are equal, round, and sluggishly reactive to light.\nFigure 1.\nThe cardiovascular examination is notable for a bradycardic regular rhythm, without murmurs. The distal pulses are faint but appreciable bilaterally. His respiratory rate and effort are normal and the lungs are clear to auscultation bilaterally. The abdomen is soft, nontender, nondistended, and with normal bowel sounds. The rectal examination reveals guaiac-positive brown stool. The patient's skin is cool, dry, and nonedematous. He is nonverbal, but does turn his head in response to his name.\nThe initial work-up includes an electrocardiogram (ECG), chest radiograph, complete blood cell count (CBC), basic metabolic panel (BMP), urinalysis, and cardiac enzymes. The ECG (Figure 1) shows a bradycardic junctional rhythm at a rate of 49 bpm (compared with previous ECGs showing a normal sinus rhythm with a rate in the 80s) and a prolonged QT interval. The CBC shows a normal white blood cell and platelet count, but a hemoglobin of 8.5 g/dL (85 g/L; decreased approximately 2 g/dL from a previous admission). The BMP shows stable stage 4 chronic kidney disease and a blood glucose level of 102 mg/dL (5.7 mmol/L). The cardiac enzyme examination and urinalysis are normal. Chest radiography shows mild vascular congestion and no infiltrates. CT scanning of the head reveals no acute disease.\nThe patient's heart rate throughout the initial ED evaluation ranges from the high 40s to the low 60s. The patient receives a transfusion of packed red blood cells (RBCs). His hypothermia is treated with the application of warming blankets. The patient is re-evaluated and found to have a Glasgow Coma Scale (GCS) rating of 9. His blood glucose is rechecked and is 49 mg/dL (2.72 mmol/L). The low blood glucose is treated but the patient's mental status does not improve. He continues to be hypothermic and bradycardic. He is endotracheally intubated for airway protection and admitted to the medical intensive care unit (MICU).\n\n ## Figures\n\n **Figure 1.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 351138,
"choiceText": "Sepsis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 351139,
"choiceText": "Gastrointestinal bleed",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 351140,
"choiceText": "Myxedema coma",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 351141,
"choiceText": "Acute stroke",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 99725,
"questionText": "Based on the constellation of symptoms, what is the most likely primary diagnosis?\r\n<br /><br />\r\n<em>Hint: Pay special attention to presenting complaint, current medications, and vital signs.</em>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Lethargy and Mental Status Changes in a 94-Year-Old Man"
},
{
"authors": "Brian K. Felice, MD; Daniel S. Richardson, MD",
"content": [
"After reviewing the patient's presenting findings, including the altered mental status, hypothermia (rectal temperature, 91.2°F [32.9°C]), bradycardia (junctional rhythm with a rate in the 40s), and hypoglycemia (a blood sugar of 49 mg/dL [2.72 mmol/L]), and correlating them with his past medical history, current medications, and current laboratory studies, a working diagnosis of myxedema coma was made. Thyroid function tests were ordered, and treatment was begun with 4 mg dexamethasone intravenous and 250 mcg levothyroxine intravenous prior to MICU admission. A chart check revealed that the patient had started amiodarone for paroxysmal atrial fibrillation 3 months previously. Also, although the patient was reportedly asymptomatic, on a previous admission, his thyroid stimulating hormone (TSH) was elevated and he'd been started on levothyroxine.",
"Figure 1.",
"The ECG (Figure 1) showed a bradycardic junctional rhythm (with a rate of 49 bpm) and a prolonged QT interval. Although the ECG findings could have been caused by the patient's polypharmacy, they are also consistent with myxedema. The patient returned to a sinus rhythm with a heart rate of 65 beats/min shortly following administration of intravenous levothyroxine.",
"Other information that reduced the likelihood of alternate diagnoses included a normal white blood cell count, no obvious source of infection (pneumonia, urinary tract, skin, or soft tissues) and a CT scan of the brain showing no acute processes. Although the patient's stool was guaiac-positive and he was anemic, he had a normal blood pressure and bradycardia, and no melena was found. As the patient was being transported from the ED to the MICU, the thyroid function tests returned, which provided laboratory evidence supporting our clinical diagnosis of myxedema coma. His TSH level was elevated, at 83.37 µU/mL, and free T3 and free T4 were low, at 1.0 pg/dL (0.0154 pmol/L) and 0.8 ng/dL (10.3 pmol/L), respectively."
],
"date": "October 12, 2015",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/724/695/724695-thumb.png"
}
],
"markdown": "# Lethargy and Mental Status Changes in a 94-Year-Old Man\n\n **Authors:** Brian K. Felice, MD; Daniel S. Richardson, MD \n **Date:** October 12, 2015\n\n ## Content\n\n After reviewing the patient's presenting findings, including the altered mental status, hypothermia (rectal temperature, 91.2°F [32.9°C]), bradycardia (junctional rhythm with a rate in the 40s), and hypoglycemia (a blood sugar of 49 mg/dL [2.72 mmol/L]), and correlating them with his past medical history, current medications, and current laboratory studies, a working diagnosis of myxedema coma was made. Thyroid function tests were ordered, and treatment was begun with 4 mg dexamethasone intravenous and 250 mcg levothyroxine intravenous prior to MICU admission. A chart check revealed that the patient had started amiodarone for paroxysmal atrial fibrillation 3 months previously. Also, although the patient was reportedly asymptomatic, on a previous admission, his thyroid stimulating hormone (TSH) was elevated and he'd been started on levothyroxine.\nFigure 1.\nThe ECG (Figure 1) showed a bradycardic junctional rhythm (with a rate of 49 bpm) and a prolonged QT interval. Although the ECG findings could have been caused by the patient's polypharmacy, they are also consistent with myxedema. The patient returned to a sinus rhythm with a heart rate of 65 beats/min shortly following administration of intravenous levothyroxine.\nOther information that reduced the likelihood of alternate diagnoses included a normal white blood cell count, no obvious source of infection (pneumonia, urinary tract, skin, or soft tissues) and a CT scan of the brain showing no acute processes. Although the patient's stool was guaiac-positive and he was anemic, he had a normal blood pressure and bradycardia, and no melena was found. As the patient was being transported from the ED to the MICU, the thyroid function tests returned, which provided laboratory evidence supporting our clinical diagnosis of myxedema coma. His TSH level was elevated, at 83.37 µU/mL, and free T3 and free T4 were low, at 1.0 pg/dL (0.0154 pmol/L) and 0.8 ng/dL (10.3 pmol/L), respectively.\n\n ## Figures\n\n **Figure 1.** \n \n\n\n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 351138,
"choiceText": "Sepsis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 351139,
"choiceText": "Gastrointestinal bleed",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 351140,
"choiceText": "Myxedema coma",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 351141,
"choiceText": "Acute stroke",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 99725,
"questionText": "Based on the constellation of symptoms, what is the most likely primary diagnosis?\r\n<br /><br />\r\n<em>Hint: Pay special attention to presenting complaint, current medications, and vital signs.</em>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Lethargy and Mental Status Changes in a 94-Year-Old Man"
},
{
"authors": "Brian K. Felice, MD; Daniel S. Richardson, MD",
"content": [
"Myxedema coma is rare and establishing the diagnosis requires a high index of suspicion. Myxedema coma represents the severest form of hypothyroidism and has an associated mortality rate of 30%-40%.[1] It can occur due to long-standing, untreated hypothyroidism, but is often linked to a precipitant, such as acute infection, myocardial infarction, congestive heart failure, cerebral vascular accident, trauma, or drug toxicity.[2,3,4]",
"The signs and symptoms of hypothyroidism are exaggerated as the patient approaches myxedema coma. The manifestations include metabolic derangements and neurologic and cardiovascular disturbances. Decreased metabolic function leads to hypothermia, hypoglycemia, hypoventilation, and hyponatremia. As metabolism decreases, a concomitant decrease in thermogenesis causes hypothermia.[1] While hypoglycemia is thought to occur from multiple contributing factors, including hypothyroidism itself, it can also result from adrenal insufficiency with decreased gluconeogenesis.[1,4] Patients with myxedema coma are often hypercapnic due to decreased muscle strength and diminished ventilatory drive causing hypoventilation.[1,4] Hyponatremia is seen in approximately 50% of patients with myxedema coma.[1] With decreased metabolic function, patients often have decreased free water clearance because of impaired renal function or vasopressin secretion, which causes dilutional hyponatremia.[1,4] Patients may be confused and lethargic or frankly obtunded and comatose.",
"Cardiovascular findings in myxedema coma can be profound, with bradycardia, decreased cardiac output, decreased myocardial contractility, diastolic hypertension, and occasionally hypotension.[1,4] This is partly caused by an overall reduction of beta-adrenergic responsiveness resulting in decreased chronotropic and inotropic activity, and also by a relative increase in alpha-adrenergic receptor responsiveness.[4] All of these abnormalities can be corrected to varying degrees with thyroid hormone replacement therapy.",
"Several medications can cause hypothyroidism, and patients taking them must be carefully monitored. These medications include amiodarone, lithium, and sedatives.[1,3,4] Amiodarone can cause both thyrotoxicosis and hypothyroidism.[5,6,7] Roughly 37% of amiodarone by weight is organic iodine; a maintenance dose of 200 mg/day yields a daily iodine intake of approximately 75 mg.[7] This is greater than 100 times the daily requirement of iodine. One proposed mechanism for the pathogenesis of amiodarone-induced hypothyroidism is the Wolff-Chaikoff effect.[7] Amiodarone metabolism results in a large iodide release, which inhibits TSH production.[7] As iodide exposure continues, TSH production resumes, possibly due to reduced iodide within the thyroid, since iodide is not easily transported across the intrathyroidal membrane. This TSH production rebound is called \"escape from the Wolff-Chaikoff effect,\" and persists because of an underlying defect in thyroid hormonogenesis.[6,7]",
"Due to these interactions and other feedback mechanisms, amiodarone has multiple significant effects on thyroid physiology, and subsequently on other organ systems. When amiodarone therapy is begun, baseline thyroid function values should be established. They should be repeated after 3 months.[5,6,7] If elevated TSH and decreased T4 levels are detected, treat with levothyroxine. The goal of treatment is not to normalize thyroid function studies, but rather to increase thyroxine (T4) levels to a high-normal, or slightly above normal range.[7] This will result in a TSH reduction, although it will likely remain elevated. An alternative approach is to avoid amiodarone in patients with underlying thyroid disease, possibly using antiarrhythmics which do not contain iodine, such as dronedarone."
],
"date": "October 12, 2015",
"figures": [],
"markdown": "# Lethargy and Mental Status Changes in a 94-Year-Old Man\n\n **Authors:** Brian K. Felice, MD; Daniel S. Richardson, MD \n **Date:** October 12, 2015\n\n ## Content\n\n Myxedema coma is rare and establishing the diagnosis requires a high index of suspicion. Myxedema coma represents the severest form of hypothyroidism and has an associated mortality rate of 30%-40%.[1] It can occur due to long-standing, untreated hypothyroidism, but is often linked to a precipitant, such as acute infection, myocardial infarction, congestive heart failure, cerebral vascular accident, trauma, or drug toxicity.[2,3,4]\nThe signs and symptoms of hypothyroidism are exaggerated as the patient approaches myxedema coma. The manifestations include metabolic derangements and neurologic and cardiovascular disturbances. Decreased metabolic function leads to hypothermia, hypoglycemia, hypoventilation, and hyponatremia. As metabolism decreases, a concomitant decrease in thermogenesis causes hypothermia.[1] While hypoglycemia is thought to occur from multiple contributing factors, including hypothyroidism itself, it can also result from adrenal insufficiency with decreased gluconeogenesis.[1,4] Patients with myxedema coma are often hypercapnic due to decreased muscle strength and diminished ventilatory drive causing hypoventilation.[1,4] Hyponatremia is seen in approximately 50% of patients with myxedema coma.[1] With decreased metabolic function, patients often have decreased free water clearance because of impaired renal function or vasopressin secretion, which causes dilutional hyponatremia.[1,4] Patients may be confused and lethargic or frankly obtunded and comatose.\nCardiovascular findings in myxedema coma can be profound, with bradycardia, decreased cardiac output, decreased myocardial contractility, diastolic hypertension, and occasionally hypotension.[1,4] This is partly caused by an overall reduction of beta-adrenergic responsiveness resulting in decreased chronotropic and inotropic activity, and also by a relative increase in alpha-adrenergic receptor responsiveness.[4] All of these abnormalities can be corrected to varying degrees with thyroid hormone replacement therapy.\nSeveral medications can cause hypothyroidism, and patients taking them must be carefully monitored. These medications include amiodarone, lithium, and sedatives.[1,3,4] Amiodarone can cause both thyrotoxicosis and hypothyroidism.[5,6,7] Roughly 37% of amiodarone by weight is organic iodine; a maintenance dose of 200 mg/day yields a daily iodine intake of approximately 75 mg.[7] This is greater than 100 times the daily requirement of iodine. One proposed mechanism for the pathogenesis of amiodarone-induced hypothyroidism is the Wolff-Chaikoff effect.[7] Amiodarone metabolism results in a large iodide release, which inhibits TSH production.[7] As iodide exposure continues, TSH production resumes, possibly due to reduced iodide within the thyroid, since iodide is not easily transported across the intrathyroidal membrane. This TSH production rebound is called \"escape from the Wolff-Chaikoff effect,\" and persists because of an underlying defect in thyroid hormonogenesis.[6,7]\nDue to these interactions and other feedback mechanisms, amiodarone has multiple significant effects on thyroid physiology, and subsequently on other organ systems. When amiodarone therapy is begun, baseline thyroid function values should be established. They should be repeated after 3 months.[5,6,7] If elevated TSH and decreased T4 levels are detected, treat with levothyroxine. The goal of treatment is not to normalize thyroid function studies, but rather to increase thyroxine (T4) levels to a high-normal, or slightly above normal range.[7] This will result in a TSH reduction, although it will likely remain elevated. An alternative approach is to avoid amiodarone in patients with underlying thyroid disease, possibly using antiarrhythmics which do not contain iodine, such as dronedarone.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "Lethargy and Mental Status Changes in a 94-Year-Old Man"
},
{
"authors": "Brian K. Felice, MD; Daniel S. Richardson, MD",
"content": [
"In myxedema coma a precipitating factor often underlies the patient's decline. It is crucial to identify and treat this underlying cause, while simultaneously correcting the effects of profound hypothyroidism. If a myxedematous patient is on amiodarone, it should be stopped and other antiarrhythmics used as needed. Treat sepsis or infection aggressively. Diagnose and manage congestive heart failure or myocardial infarction.",
"No consensus exists on specific thyroid hormone replacement regimens for myxedema coma. Most experts agree that a large intravenous bolus of levothyroxine should be administered (200 - 400 mcg), followed by daily doses of 50 - 100 mcg, based on the patient's weight and comorbidities.[1] Other experts advocate the use of triiodothyronine (T3) or a combination of both T3 and T4.[1,3] In addition to thyroid replacement therapy, it is important to detect coexisting adrenal insufficiency and treat patients with stress-dose steroids to avoid precipitating adrenal crisis.[1,2,4]",
"Myxedema coma patients also require good supportive care in order to ensure a favorable outcome. They often require mechanical ventilation secondary to hypoventilation and hypercapnia, rewarming for hypothermia, and careful fluid and electrolyte management to correct hypoglycemia and hyponatremia.[1,2,3,4] Myxedema coma patients should be admitted to the intensive care unit for close monitoring and aggressive thyroid hormone replacement.",
"In this case, the patient had recently been started on amiodarone for atrial fibrillation rate control. It was immediately discontinued once the diagnosis of myxedema coma was established. Endotracheal intubation and mechanical ventilation were quickly instituted, as was passive rewarming with warming blankets (since active rewarming risks vasodilation and hypotension).[1] Stress-dose steroid therapy and levothyroxine administration continued during the patient's MICU course. He gradually became more alert and responsive. His heart rate increased, his blood sugars stabilized, and his thyroid function studies improved. He was extubated on hospital day 5, with a return to his baseline mental status, and was able to communicate with family and caregivers."
],
"date": "October 12, 2015",
"figures": [],
"markdown": "# Lethargy and Mental Status Changes in a 94-Year-Old Man\n\n **Authors:** Brian K. Felice, MD; Daniel S. Richardson, MD \n **Date:** October 12, 2015\n\n ## Content\n\n In myxedema coma a precipitating factor often underlies the patient's decline. It is crucial to identify and treat this underlying cause, while simultaneously correcting the effects of profound hypothyroidism. If a myxedematous patient is on amiodarone, it should be stopped and other antiarrhythmics used as needed. Treat sepsis or infection aggressively. Diagnose and manage congestive heart failure or myocardial infarction.\nNo consensus exists on specific thyroid hormone replacement regimens for myxedema coma. Most experts agree that a large intravenous bolus of levothyroxine should be administered (200 - 400 mcg), followed by daily doses of 50 - 100 mcg, based on the patient's weight and comorbidities.[1] Other experts advocate the use of triiodothyronine (T3) or a combination of both T3 and T4.[1,3] In addition to thyroid replacement therapy, it is important to detect coexisting adrenal insufficiency and treat patients with stress-dose steroids to avoid precipitating adrenal crisis.[1,2,4]\nMyxedema coma patients also require good supportive care in order to ensure a favorable outcome. They often require mechanical ventilation secondary to hypoventilation and hypercapnia, rewarming for hypothermia, and careful fluid and electrolyte management to correct hypoglycemia and hyponatremia.[1,2,3,4] Myxedema coma patients should be admitted to the intensive care unit for close monitoring and aggressive thyroid hormone replacement.\nIn this case, the patient had recently been started on amiodarone for atrial fibrillation rate control. It was immediately discontinued once the diagnosis of myxedema coma was established. Endotracheal intubation and mechanical ventilation were quickly instituted, as was passive rewarming with warming blankets (since active rewarming risks vasodilation and hypotension).[1] Stress-dose steroid therapy and levothyroxine administration continued during the patient's MICU course. He gradually became more alert and responsive. His heart rate increased, his blood sugars stabilized, and his thyroid function studies improved. He was extubated on hospital day 5, with a return to his baseline mental status, and was able to communicate with family and caregivers.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 351142,
"choiceText": "Active rewarming with warmed IV fluids",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 351143,
"choiceText": "Intravenous levothyroxine bolus",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 351144,
"choiceText": "Institute insulin therapy",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 351145,
"choiceText": "Admission to a regular medical floor",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "In addition to treating the underlying cause of myxedema coma (in this case, urinary tract infection), the most important treatment modality is thyroid hormone replacement therapy. In most cases, hypotension, bradycardia, and other symptoms will resolve as thyroid hormones return to normal levels. Active rewarming is not recommended due to the possibility of peripheral vasodilation and worsening hypotension. Patients in myxedema coma are often hypoglycemic. Hypoglycemia is thought to occur secondarily to multiple contributing factors, including hypothyroidism itself, but it can also result from adrenal insufficiency with decreased gluconeogenesis Her glucose would need to be checked, and possibly supplemented. Myxedema coma patients require management in intensive care units due to their high mortality rate (30-40%) and complex medical needs. Aggressive treatment and monitoring is required to reverse their comatose states and to ensure favorable outcomes.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 99726,
"questionText": "An obtunded 75-year-old woman presents with hypothermia, hypotension, and bradycardia. She is found to have a urinary tract infection (UTI). You make a diagnosis of myxedema coma. In addition to starting intravenous antibiotics for her infection, which of the following treatment options is best?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 351173,
"choiceText": "Diuretics",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 351174,
"choiceText": "Free water",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 351175,
"choiceText": "Amiodarone",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 351176,
"choiceText": "Stress-dose steroids",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "In a patient presenting with myxedema coma, it is important to rule out underlying adrenal insufficiency or hypothyroidism secondary to hypopituitarism. Patients must be empirically started on stress-dose steroids to prevent adrenal crises while these conditions are being evaluated. Most of the patient's symptoms should improve as the patient's thyroid hormone levels begin to normalize. Hyponatremia is seen in approximately 50% of patients with myxedema coma. With decreased metabolic function, patients often have decreased free water clearance from impaired renal function or vasopressin secretion, which causes dilutional hyponatremia. Amiodarone is often implicated as causing myxedema coma; it should not be started in this patient. The patient is hypotensive, so diuretics are not indicated. Treating precipitating ailments, providing supportive care, replacing thyroid hormones and providing stress dose steroids are the essential therapies for myxedema coma.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 99735,
"questionText": "The above patient required mechanical ventilation in the emergency department and admission to the medical intensive care unit for close monitoring and further treatment. Which of the following additional therapies should be initiated?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Lethargy and Mental Status Changes in a 94-Year-Old Man"
},
{
"authors": "Brian K. Felice, MD; Daniel S. Richardson, MD",
"content": [],
"date": "October 12, 2015",
"figures": [],
"markdown": "# Lethargy and Mental Status Changes in a 94-Year-Old Man\n\n **Authors:** Brian K. Felice, MD; Daniel S. Richardson, MD \n **Date:** October 12, 2015\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 351142,
"choiceText": "Active rewarming with warmed IV fluids",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 351143,
"choiceText": "Intravenous levothyroxine bolus",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 351144,
"choiceText": "Institute insulin therapy",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 351145,
"choiceText": "Admission to a regular medical floor",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "In addition to treating the underlying cause of myxedema coma (in this case, urinary tract infection), the most important treatment modality is thyroid hormone replacement therapy. In most cases, hypotension, bradycardia, and other symptoms will resolve as thyroid hormones return to normal levels. Active rewarming is not recommended due to the possibility of peripheral vasodilation and worsening hypotension. Patients in myxedema coma are often hypoglycemic. Hypoglycemia is thought to occur secondarily to multiple contributing factors, including hypothyroidism itself, but it can also result from adrenal insufficiency with decreased gluconeogenesis Her glucose would need to be checked, and possibly supplemented. Myxedema coma patients require management in intensive care units due to their high mortality rate (30-40%) and complex medical needs. Aggressive treatment and monitoring is required to reverse their comatose states and to ensure favorable outcomes.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 99726,
"questionText": "An obtunded 75-year-old woman presents with hypothermia, hypotension, and bradycardia. She is found to have a urinary tract infection (UTI). You make a diagnosis of myxedema coma. In addition to starting intravenous antibiotics for her infection, which of the following treatment options is best?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 351173,
"choiceText": "Diuretics",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 351174,
"choiceText": "Free water",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 351175,
"choiceText": "Amiodarone",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 351176,
"choiceText": "Stress-dose steroids",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "In a patient presenting with myxedema coma, it is important to rule out underlying adrenal insufficiency or hypothyroidism secondary to hypopituitarism. Patients must be empirically started on stress-dose steroids to prevent adrenal crises while these conditions are being evaluated. Most of the patient's symptoms should improve as the patient's thyroid hormone levels begin to normalize. Hyponatremia is seen in approximately 50% of patients with myxedema coma. With decreased metabolic function, patients often have decreased free water clearance from impaired renal function or vasopressin secretion, which causes dilutional hyponatremia. Amiodarone is often implicated as causing myxedema coma; it should not be started in this patient. The patient is hypotensive, so diuretics are not indicated. Treating precipitating ailments, providing supportive care, replacing thyroid hormones and providing stress dose steroids are the essential therapies for myxedema coma.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 99735,
"questionText": "The above patient required mechanical ventilation in the emergency department and admission to the medical intensive care unit for close monitoring and further treatment. Which of the following additional therapies should be initiated?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Lethargy and Mental Status Changes in a 94-Year-Old Man"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 351138,
"choiceText": "Sepsis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 351139,
"choiceText": "Gastrointestinal bleed",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 351140,
"choiceText": "Myxedema coma",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 351141,
"choiceText": "Acute stroke",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 99725,
"questionText": "Based on the constellation of symptoms, what is the most likely primary diagnosis?\r\n<br /><br />\r\n<em>Hint: Pay special attention to presenting complaint, current medications, and vital signs.</em>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 351142,
"choiceText": "Active rewarming with warmed IV fluids",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 351143,
"choiceText": "Intravenous levothyroxine bolus",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 351144,
"choiceText": "Institute insulin therapy",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 351145,
"choiceText": "Admission to a regular medical floor",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "In addition to treating the underlying cause of myxedema coma (in this case, urinary tract infection), the most important treatment modality is thyroid hormone replacement therapy. In most cases, hypotension, bradycardia, and other symptoms will resolve as thyroid hormones return to normal levels. Active rewarming is not recommended due to the possibility of peripheral vasodilation and worsening hypotension. Patients in myxedema coma are often hypoglycemic. Hypoglycemia is thought to occur secondarily to multiple contributing factors, including hypothyroidism itself, but it can also result from adrenal insufficiency with decreased gluconeogenesis Her glucose would need to be checked, and possibly supplemented. Myxedema coma patients require management in intensive care units due to their high mortality rate (30-40%) and complex medical needs. Aggressive treatment and monitoring is required to reverse their comatose states and to ensure favorable outcomes.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 99726,
"questionText": "An obtunded 75-year-old woman presents with hypothermia, hypotension, and bradycardia. She is found to have a urinary tract infection (UTI). You make a diagnosis of myxedema coma. In addition to starting intravenous antibiotics for her infection, which of the following treatment options is best?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 351173,
"choiceText": "Diuretics",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 351174,
"choiceText": "Free water",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 351175,
"choiceText": "Amiodarone",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 351176,
"choiceText": "Stress-dose steroids",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "In a patient presenting with myxedema coma, it is important to rule out underlying adrenal insufficiency or hypothyroidism secondary to hypopituitarism. Patients must be empirically started on stress-dose steroids to prevent adrenal crises while these conditions are being evaluated. Most of the patient's symptoms should improve as the patient's thyroid hormone levels begin to normalize. Hyponatremia is seen in approximately 50% of patients with myxedema coma. With decreased metabolic function, patients often have decreased free water clearance from impaired renal function or vasopressin secretion, which causes dilutional hyponatremia. Amiodarone is often implicated as causing myxedema coma; it should not be started in this patient. The patient is hypotensive, so diuretics are not indicated. Treating precipitating ailments, providing supportive care, replacing thyroid hormones and providing stress dose steroids are the essential therapies for myxedema coma.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 99735,
"questionText": "The above patient required mechanical ventilation in the emergency department and admission to the medical intensive care unit for close monitoring and further treatment. Which of the following additional therapies should be initiated?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
724356 | /viewarticle/724356 | [
{
"authors": "Kristin A. Olson, MD; Sarah M. Dry, MD",
"content": [
"Editor's Note:\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions.",
"A 61-year-old white man presents to the emergency department with a 1-week history of diffuse \"crampy\" abdominal pain. For the past 2 months, he has had diarrhea and has been passing blood with every bowel movement, either on the stool surface or separately from the stool. Recently, he has had 10-15 loose stools per day, with associated urgency and tenesmus. He has lost 4 lb in the past week. He feels lightheaded upon standing and is experiencing fatigue and mild shortness of breath. He takes atorvastatin for hypercholesteremia and acetaminophen for intermittent back pain and knee pain. He is allergic to penicillin. He has no history of gastrointestinal tract disease; screening colonoscopy performed 2 years ago was unremarkable. His father died of myocardial infarction when he was in his 60s, and his mother died of breast cancer when she was in her 40s. He has no siblings. He is retired from his work as an accountant and lives with his wife. He has not travelled recently. He has a 25-pack-year smoking history but has not smoked for approximately 10 years. He drinks alcohol on social occasions and on weekends, and he denies illicit drug abuse.",
"On physical examination, the patient is a pale, diaphoretic man in some distress due to abdominal pain. His oral temperature is 101.5°F (38.6°C), blood pressure is 110/62 mm Hg, pulse is 107 beats/min, and respiratory rate is 24 breaths/min. His body mass index is 29.7 kg/m2. Examination of his head and neck is unremarkable aside from pale mucosal membranes. Lung auscultation reveals normal breath sounds, with no crackles or rhonchi. His heart rate is tachycardic, and his heart rhythm is regular. There are no murmurs or extra heart sounds. His abdomen is distended and firm, with diffuse rebound tenderness that seems worse in the lower left quadrant. Bowel sounds are diminished. No hepatosplenomegaly, ascites, or palpable masses are appreciated. Digital rectal examination demonstrates normal rectal tone, \"velvety\" rectal mucosa, and bright red blood on withdrawal of the examining finger. There is pitting edema of the legs and feet."
],
"date": "November 11, 2014",
"figures": [],
"markdown": "# A 61-Year-Old Man With Crampy Abdominal Pain\n\n **Authors:** Kristin A. Olson, MD; Sarah M. Dry, MD \n **Date:** November 11, 2014\n\n ## Content\n\n Editor's Note:\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions.\nA 61-year-old white man presents to the emergency department with a 1-week history of diffuse \"crampy\" abdominal pain. For the past 2 months, he has had diarrhea and has been passing blood with every bowel movement, either on the stool surface or separately from the stool. Recently, he has had 10-15 loose stools per day, with associated urgency and tenesmus. He has lost 4 lb in the past week. He feels lightheaded upon standing and is experiencing fatigue and mild shortness of breath. He takes atorvastatin for hypercholesteremia and acetaminophen for intermittent back pain and knee pain. He is allergic to penicillin. He has no history of gastrointestinal tract disease; screening colonoscopy performed 2 years ago was unremarkable. His father died of myocardial infarction when he was in his 60s, and his mother died of breast cancer when she was in her 40s. He has no siblings. He is retired from his work as an accountant and lives with his wife. He has not travelled recently. He has a 25-pack-year smoking history but has not smoked for approximately 10 years. He drinks alcohol on social occasions and on weekends, and he denies illicit drug abuse.\nOn physical examination, the patient is a pale, diaphoretic man in some distress due to abdominal pain. His oral temperature is 101.5°F (38.6°C), blood pressure is 110/62 mm Hg, pulse is 107 beats/min, and respiratory rate is 24 breaths/min. His body mass index is 29.7 kg/m2. Examination of his head and neck is unremarkable aside from pale mucosal membranes. Lung auscultation reveals normal breath sounds, with no crackles or rhonchi. His heart rate is tachycardic, and his heart rhythm is regular. There are no murmurs or extra heart sounds. His abdomen is distended and firm, with diffuse rebound tenderness that seems worse in the lower left quadrant. Bowel sounds are diminished. No hepatosplenomegaly, ascites, or palpable masses are appreciated. Digital rectal examination demonstrates normal rectal tone, \"velvety\" rectal mucosa, and bright red blood on withdrawal of the examining finger. There is pitting edema of the legs and feet.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 61-Year-Old Man With Crampy Abdominal Pain"
},
{
"authors": "Kristin A. Olson, MD; Sarah M. Dry, MD",
"content": [
"Laboratory analysis includes a complete blood count (CBC), which demonstrates anemia (hemoglobin concentration of 6.7 g/dL [67 g/L]), leukocytosis (leukocyte count of 14.2 x 103 cells/μL [14.2 x 109 cells/L]), and thrombocytosis (platelet count of 540 x 103 cells/µL [540 x 109 cells/L]). Additional findings include elevations in the erythrocyte sedimentation rate (78 mm/h) and C-reactive protein level (114 mg/L). His albumin level is low, at 2 g/dL (20 g/L). The basic metabolic panel is unremarkable. Repeat stool cultures and samples for ova and parasites are negative. Flexible sigmoidoscopy shows diffusely erythematous and friable colonic mucosa with large ulcerated patches, abundant yellow-white exudate, and oozing blood. These findings extend in a proximal and continuous fashion from the rectum to at least the middle of the sigmoid colon. Tissue biopsy samples sent for histopathologic examination reveal lymphoplasmacytic inflammation along the base of the crypts; neutrophils that infiltrate the crypt epithelium and collect in the depths of the colonic crypts to form \"crypt abscesses\"; and focal chronic reactive changes, including crypt branching, gland atrophy, and goblet-cell mucin depletion. No granulomas are identified.",
"Figure 1.",
"Figure 2.",
"Figure 3."
],
"date": "November 11, 2014",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/724/355/724355-thumb1.png"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/724/355/724355-thumb2.png"
},
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/724/355/724355-thumb3.png"
}
],
"markdown": "# A 61-Year-Old Man With Crampy Abdominal Pain\n\n **Authors:** Kristin A. Olson, MD; Sarah M. Dry, MD \n **Date:** November 11, 2014\n\n ## Content\n\n Laboratory analysis includes a complete blood count (CBC), which demonstrates anemia (hemoglobin concentration of 6.7 g/dL [67 g/L]), leukocytosis (leukocyte count of 14.2 x 103 cells/μL [14.2 x 109 cells/L]), and thrombocytosis (platelet count of 540 x 103 cells/µL [540 x 109 cells/L]). Additional findings include elevations in the erythrocyte sedimentation rate (78 mm/h) and C-reactive protein level (114 mg/L). His albumin level is low, at 2 g/dL (20 g/L). The basic metabolic panel is unremarkable. Repeat stool cultures and samples for ova and parasites are negative. Flexible sigmoidoscopy shows diffusely erythematous and friable colonic mucosa with large ulcerated patches, abundant yellow-white exudate, and oozing blood. These findings extend in a proximal and continuous fashion from the rectum to at least the middle of the sigmoid colon. Tissue biopsy samples sent for histopathologic examination reveal lymphoplasmacytic inflammation along the base of the crypts; neutrophils that infiltrate the crypt epithelium and collect in the depths of the colonic crypts to form \"crypt abscesses\"; and focal chronic reactive changes, including crypt branching, gland atrophy, and goblet-cell mucin depletion. No granulomas are identified.\nFigure 1.\nFigure 2.\nFigure 3.\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n**Figure 3.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 350623,
"choiceText": "Colorectal cancer",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 350624,
"choiceText": "Microscopic colitis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 350625,
"choiceText": "Inflammatory bowel disease",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 350626,
"choiceText": "Hemorrhoids",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 350627,
"choiceText": "<em>Clostridium difficile</em> colitis",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 99538,
"questionText": "What is the most likely diagnosis?\r\n<br><br>\r\n<em>Hint: Note the frequent passage of bloody loose stools for more than 2 months in the setting of fever, tachycardia, and anemia.</em>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 61-Year-Old Man With Crampy Abdominal Pain"
},
{
"authors": "Kristin A. Olson, MD; Sarah M. Dry, MD",
"content": [
"The diagnosis of fulminant ulcerative colitis, an idiopathic inflammatory disease of the bowel, was initially suspected on the basis of the patient's history, physical examination, and laboratory test results; endoscopic and biopsy findings confirmed the diagnosis. The rectal bleeding was bright red, indicating that the source of the bleeding was most likely the lower gastrointestinal tract. The concurrent presence of crampy abdominal pain, diarrhea, tenesmus, and fever suggested that an inflammatory process had damaged the colorectal mucosa. The unremarkable colonoscopy findings 2 years before presentation rendered a neoplasm unlikely. Although not impossible, colon cancer rarely presents with diarrhea. Most compelling was the continuity and character of the mucosal damage identified on endoscopy and the histopathologic findings, which were diagnostic of ulcerative colitis. Symptom duration suggested a chronic inflammatory process and infectious causes of colitis had been ruled out."
],
"date": "November 11, 2014",
"figures": [],
"markdown": "# A 61-Year-Old Man With Crampy Abdominal Pain\n\n **Authors:** Kristin A. Olson, MD; Sarah M. Dry, MD \n **Date:** November 11, 2014\n\n ## Content\n\n The diagnosis of fulminant ulcerative colitis, an idiopathic inflammatory disease of the bowel, was initially suspected on the basis of the patient's history, physical examination, and laboratory test results; endoscopic and biopsy findings confirmed the diagnosis. The rectal bleeding was bright red, indicating that the source of the bleeding was most likely the lower gastrointestinal tract. The concurrent presence of crampy abdominal pain, diarrhea, tenesmus, and fever suggested that an inflammatory process had damaged the colorectal mucosa. The unremarkable colonoscopy findings 2 years before presentation rendered a neoplasm unlikely. Although not impossible, colon cancer rarely presents with diarrhea. Most compelling was the continuity and character of the mucosal damage identified on endoscopy and the histopathologic findings, which were diagnostic of ulcerative colitis. Symptom duration suggested a chronic inflammatory process and infectious causes of colitis had been ruled out.\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 350623,
"choiceText": "Colorectal cancer",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 350624,
"choiceText": "Microscopic colitis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 350625,
"choiceText": "Inflammatory bowel disease",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 350626,
"choiceText": "Hemorrhoids",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 350627,
"choiceText": "<em>Clostridium difficile</em> colitis",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 99538,
"questionText": "What is the most likely diagnosis?\r\n<br><br>\r\n<em>Hint: Note the frequent passage of bloody loose stools for more than 2 months in the setting of fever, tachycardia, and anemia.</em>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 61-Year-Old Man With Crampy Abdominal Pain"
},
{
"authors": "Kristin A. Olson, MD; Sarah M. Dry, MD",
"content": [
"Ulcerative colitis is estimated to respectively have an incidence of 7.3 and prevalence of 116 per 100,000 people in the United States.[1] The peak age at onset is 15-25 years, with a second peak at 40-60 years.[2] Individuals of Ashkenazi Jewish or Scandinavian descent are more often affected, with men and women experiencing a similar disease incidence. Smoking seems to play a protective role against the development of ulcerative colitis, and it has been proposed that the second incidence peak in part represents patients who stopped smoking at a later age.[3] Most research suggests that the etiology and pathogenesis of ulcerative colitis are multifactorial, with a combination of genetic susceptibility, bacterial antigens, and alteration of mucosal immunity responsible for the development of the disease.[4]",
"Figure 1.",
"Figure 2.",
"Figure 3.",
"Histopathologically, ulcerative colitis is characterized by lymphoplasmacytic inflammation of the mucosa and submucosa, with scattered neutrophils in the lamina propria (Figure 1). The neutrophils typically injure the crypt epithelium and may collect in the base of the crypts, forming crypt abscesses (Figure 2). Within weeks of disease onset, features of crypt architectural distortion (such as crypt branching and shortening) develop; these chronic changes are a nonspecific regenerative response to previous injury. Deep granulomas and transmural inflammation, 2 hallmarks of Crohn disease, do not occur.[5]",
"Classically, ulcerative colitis is insidious in onset. Affected patients present with frequent passage of bloody, loose stools and tenesmus. The intensity of the symptoms generally correlates with the extent of anatomic involvement, allowing classification of disease as mild, moderate, or severe/fulminant. The majority of patients have mild, indolent disease limited to the rectum and sigmoid colon that is characterized by diarrhea, intermittent rectal bleeding, and tenesmus. The physical examination is often normal aside from bright red blood within the rectum. Other patients present with systemic symptoms, including more frequent bowel movements, crampy abdominal pain, decreased bowel sounds, high-grade fever, tachycardia, anemia, orthostatic hypotension, and weight loss. Extraintestinal manifestations, such as acute arthropathy, episcleritis, erythema nodosum, and pyoderma gangrenosum may also arise. Fewer than 10% of patients with ulcerative colitis initially present with fulminant disease, with older individuals represented in greater numbers. Fulminant ulcerative colitis is more abrupt in onset and is usually characterized by extensive colonic involvement (\"pancolitis\"), with rectal bleeding that may be extensive enough to necessitate blood transfusion. Abdominal distention and tenderness to palpation with signs of peritoneal inflammation (eg, rebound tenderness) may be observed in these patients. Of greatest concern in patients with fulminant disease is the prospect of massive hemorrhage, toxic megacolon, or bowel perforation. Immediate hospitalization is often necessary in these patients.",
"The differential diagnosis of bright red blood in the rectum associated with diarrhea includes infectious colitis, ischemic colitis, and nonsteroidal anti-inflammatory drug enteropathy. Other causes of colonic bleeding, such as diverticular disease, can present with loose stools, because blood is a cathartic. Stool studies (eg, culture, ova, and parasites) and a \nClostridium difficile\n toxin screen should be considered to eliminate the possibility of infectious colitis. A complete blood cell count should be obtained to evaluate for anemia or leukocytosis. Low albumin levels signify poor nutritional status and protein-losing enteropathy. A basic metabolic panel may demonstrate electrolyte abnormalities in the setting of severe prolonged diarrhea. Inflammatory markers, such as the erythrocyte sedimentation rate and C-reactive protein level, are typically elevated in patients with inflammatory bowel disease. Radiographic studies may be used as an adjunct in diagnosing complications of ulcerative colitis (eg, plain films can establish the presence of toxic megacolon). In the setting of acute flares, other radiologic studies, such as computed tomography (CT), may be done to evaluate for an alternative diagnosis. Common CT findings in ulcerative colitis include thickening of the colonic wall, pericolonic fat stranding, and a \"target\" appearance of the rectum. Most important in confirming the diagnosis of ulcerative colitis is flexible sigmoidoscopy with biopsy; colonoscopy may also be used in some settings, but it is associated with a higher risk for perforation and is contraindicated in cases of suspected toxic megacolon. Typical endoscopic findings in ulcerative colitis patients include diffuse erythema; edema; friability; and granularity of the mucosa, with loss of the normal vascular pattern. Ulceration with exudate and pseudopolyps are frequently identified as well. These findings invariably begin in the rectum and extend proximally in a continuous manner, up to and including the cecum, in cases of pancolitis.[6]"
],
"date": "November 11, 2014",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/724/355/724355-thumb1.png"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/724/355/724355-thumb2.png"
},
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/724/355/724355-thumb3.png"
}
],
"markdown": "# A 61-Year-Old Man With Crampy Abdominal Pain\n\n **Authors:** Kristin A. Olson, MD; Sarah M. Dry, MD \n **Date:** November 11, 2014\n\n ## Content\n\n Ulcerative colitis is estimated to respectively have an incidence of 7.3 and prevalence of 116 per 100,000 people in the United States.[1] The peak age at onset is 15-25 years, with a second peak at 40-60 years.[2] Individuals of Ashkenazi Jewish or Scandinavian descent are more often affected, with men and women experiencing a similar disease incidence. Smoking seems to play a protective role against the development of ulcerative colitis, and it has been proposed that the second incidence peak in part represents patients who stopped smoking at a later age.[3] Most research suggests that the etiology and pathogenesis of ulcerative colitis are multifactorial, with a combination of genetic susceptibility, bacterial antigens, and alteration of mucosal immunity responsible for the development of the disease.[4]\nFigure 1.\nFigure 2.\nFigure 3.\nHistopathologically, ulcerative colitis is characterized by lymphoplasmacytic inflammation of the mucosa and submucosa, with scattered neutrophils in the lamina propria (Figure 1). The neutrophils typically injure the crypt epithelium and may collect in the base of the crypts, forming crypt abscesses (Figure 2). Within weeks of disease onset, features of crypt architectural distortion (such as crypt branching and shortening) develop; these chronic changes are a nonspecific regenerative response to previous injury. Deep granulomas and transmural inflammation, 2 hallmarks of Crohn disease, do not occur.[5]\nClassically, ulcerative colitis is insidious in onset. Affected patients present with frequent passage of bloody, loose stools and tenesmus. The intensity of the symptoms generally correlates with the extent of anatomic involvement, allowing classification of disease as mild, moderate, or severe/fulminant. The majority of patients have mild, indolent disease limited to the rectum and sigmoid colon that is characterized by diarrhea, intermittent rectal bleeding, and tenesmus. The physical examination is often normal aside from bright red blood within the rectum. Other patients present with systemic symptoms, including more frequent bowel movements, crampy abdominal pain, decreased bowel sounds, high-grade fever, tachycardia, anemia, orthostatic hypotension, and weight loss. Extraintestinal manifestations, such as acute arthropathy, episcleritis, erythema nodosum, and pyoderma gangrenosum may also arise. Fewer than 10% of patients with ulcerative colitis initially present with fulminant disease, with older individuals represented in greater numbers. Fulminant ulcerative colitis is more abrupt in onset and is usually characterized by extensive colonic involvement (\"pancolitis\"), with rectal bleeding that may be extensive enough to necessitate blood transfusion. Abdominal distention and tenderness to palpation with signs of peritoneal inflammation (eg, rebound tenderness) may be observed in these patients. Of greatest concern in patients with fulminant disease is the prospect of massive hemorrhage, toxic megacolon, or bowel perforation. Immediate hospitalization is often necessary in these patients.\nThe differential diagnosis of bright red blood in the rectum associated with diarrhea includes infectious colitis, ischemic colitis, and nonsteroidal anti-inflammatory drug enteropathy. Other causes of colonic bleeding, such as diverticular disease, can present with loose stools, because blood is a cathartic. Stool studies (eg, culture, ova, and parasites) and a \nClostridium difficile\n toxin screen should be considered to eliminate the possibility of infectious colitis. A complete blood cell count should be obtained to evaluate for anemia or leukocytosis. Low albumin levels signify poor nutritional status and protein-losing enteropathy. A basic metabolic panel may demonstrate electrolyte abnormalities in the setting of severe prolonged diarrhea. Inflammatory markers, such as the erythrocyte sedimentation rate and C-reactive protein level, are typically elevated in patients with inflammatory bowel disease. Radiographic studies may be used as an adjunct in diagnosing complications of ulcerative colitis (eg, plain films can establish the presence of toxic megacolon). In the setting of acute flares, other radiologic studies, such as computed tomography (CT), may be done to evaluate for an alternative diagnosis. Common CT findings in ulcerative colitis include thickening of the colonic wall, pericolonic fat stranding, and a \"target\" appearance of the rectum. Most important in confirming the diagnosis of ulcerative colitis is flexible sigmoidoscopy with biopsy; colonoscopy may also be used in some settings, but it is associated with a higher risk for perforation and is contraindicated in cases of suspected toxic megacolon. Typical endoscopic findings in ulcerative colitis patients include diffuse erythema; edema; friability; and granularity of the mucosa, with loss of the normal vascular pattern. Ulceration with exudate and pseudopolyps are frequently identified as well. These findings invariably begin in the rectum and extend proximally in a continuous manner, up to and including the cecum, in cases of pancolitis.[6]\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n**Figure 3.** \n \n\n\n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 61-Year-Old Man With Crampy Abdominal Pain"
},
{
"authors": "Kristin A. Olson, MD; Sarah M. Dry, MD",
"content": [
"In most cases, ulcerative colitis can be managed in the outpatient setting. Treatment is designed to achieve and maintain disease remission. Topical therapy and 5-aminosalicylic acid (ASA) agents are the first-line means of treating ulcerative colitis, with steroids for acute flares, biologics (such as infliximab, adalimumab, golimumab, or vedolizumab) for both induction and maintenance of remission and immunomodulators (azathioprine or 6-mercaptopurine) as maintenance therapy. Fulminant ulcerative colitis requires careful attention to the development of toxic megacolon. Parenteral corticosteroids; rehydration; bowel rest; nutritional supplementation; anticoagulation with low-dose heparin to prevent venous thrombosis, which is common in patients with ulcerative colitis; and monitoring of hemoglobin values (with transfusion sometimes required) are recommended. If remission is achieved, a maintenance regimen consisting of immunomodulators and/or 5-ASA should be initiated. In patients with severe/fulminant ulcerative colitis that is refractory to first-line therapy, infusion of a biologic agent or cyclosporine should be considered. However, in some cases, colectomy may be necessary.[7]",
"The patient in this case was immediately admitted to the hospital because of concerns that he was at risk for massive hemorrhage, toxic megacolon, or bowel perforation. Three units of cross-matched blood were transfused, and he was prescribed bowel rest, peripheral hyperalimentation, and prednisolone. Shortly thereafter, 5-ASA was added to his therapeutic regimen. His symptoms resolved gradually, and he was discharged to home. Over the next few years, he experienced several disease flares, and the benefits and risks of immunomodulators and biologic agents versus colectomy were reviewed with him during a particularly severe flare. After considerable reflection, he decided in favor of colectomy; the patient tolerated the operation well. Gross examination of the colectomy specimen demonstrated involvement of the entire colon from cecum to anus. Multiple irregular ulcers with an associated yellow-white exudate were scattered throughout the colon, and diffuse pseudopolyps (Figure 3) were present. Findings consistent with Crohn's disease (eg, fissures, fistulas, perianal involvement, \"creeping fat,\" and segmental disease) were not identified. As expected, the procedure resulted in complete remission of the patient's symptoms. After ileal pouch anal anastomosis surgery, most patients can expect to have 4-6 loose bowel movements per day."
],
"date": "November 11, 2014",
"figures": [],
"markdown": "# A 61-Year-Old Man With Crampy Abdominal Pain\n\n **Authors:** Kristin A. Olson, MD; Sarah M. Dry, MD \n **Date:** November 11, 2014\n\n ## Content\n\n In most cases, ulcerative colitis can be managed in the outpatient setting. Treatment is designed to achieve and maintain disease remission. Topical therapy and 5-aminosalicylic acid (ASA) agents are the first-line means of treating ulcerative colitis, with steroids for acute flares, biologics (such as infliximab, adalimumab, golimumab, or vedolizumab) for both induction and maintenance of remission and immunomodulators (azathioprine or 6-mercaptopurine) as maintenance therapy. Fulminant ulcerative colitis requires careful attention to the development of toxic megacolon. Parenteral corticosteroids; rehydration; bowel rest; nutritional supplementation; anticoagulation with low-dose heparin to prevent venous thrombosis, which is common in patients with ulcerative colitis; and monitoring of hemoglobin values (with transfusion sometimes required) are recommended. If remission is achieved, a maintenance regimen consisting of immunomodulators and/or 5-ASA should be initiated. In patients with severe/fulminant ulcerative colitis that is refractory to first-line therapy, infusion of a biologic agent or cyclosporine should be considered. However, in some cases, colectomy may be necessary.[7]\nThe patient in this case was immediately admitted to the hospital because of concerns that he was at risk for massive hemorrhage, toxic megacolon, or bowel perforation. Three units of cross-matched blood were transfused, and he was prescribed bowel rest, peripheral hyperalimentation, and prednisolone. Shortly thereafter, 5-ASA was added to his therapeutic regimen. His symptoms resolved gradually, and he was discharged to home. Over the next few years, he experienced several disease flares, and the benefits and risks of immunomodulators and biologic agents versus colectomy were reviewed with him during a particularly severe flare. After considerable reflection, he decided in favor of colectomy; the patient tolerated the operation well. Gross examination of the colectomy specimen demonstrated involvement of the entire colon from cecum to anus. Multiple irregular ulcers with an associated yellow-white exudate were scattered throughout the colon, and diffuse pseudopolyps (Figure 3) were present. Findings consistent with Crohn's disease (eg, fissures, fistulas, perianal involvement, \"creeping fat,\" and segmental disease) were not identified. As expected, the procedure resulted in complete remission of the patient's symptoms. After ileal pouch anal anastomosis surgery, most patients can expect to have 4-6 loose bowel movements per day.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 350653,
"choiceText": "Pseudopolyps",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 350654,
"choiceText": "Segmental disease (\"skip lesions\")",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 350655,
"choiceText": "Perianal involvement",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 350656,
"choiceText": "Transmural inflammation",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 350657,
"choiceText": "Deep granulomas",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Pseudopolyps are a common alteration of the colonic mucosa in patients with long-standing active ulcerative colitis; they are raised areas of inflamed, regenerative tissue arising in a background of ulceration. When present in significant numbers, pseudopolyps can cause cobblestoning of the mucosa that does not regress, even with treatment. Segmental disease (\"skip lesions\"), perianal involvement, transmural inflammation, and granulomas are all manifestations of Crohn's disease, another form of inflammatory bowel disease.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 99550,
"questionText": "You are caring for a patient with abdominal pain, fever, and hematochezia and are concerned that the patient has ulcerative colitis. Which finding is most consistent with a diagnosis of ulcerative colitis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 350658,
"choiceText": "Hematochezia",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 350659,
"choiceText": "Diarrhea",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 350660,
"choiceText": "Sacroiliitis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 350661,
"choiceText": "High-grade fever",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 350662,
"choiceText": "Tenesmus",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "High-grade fever is a systemic sign characteristically seen in patients with moderate or severe ulcerative colitis. Other examples of systemic manifestations common in patients with moderate or severe ulcerative colitis include tachycardia, anemia, orthostatic hypotension, and weight loss. A sudden stop in rectal output would be worrisome for development of toxic megacolon. Hematochezia, diarrhea, sacroiliitis, and tenesmus are findings common to almost all patients with ulcerative colitis, including those with mild disease.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 99551,
"questionText": "You are caring for a patient with known ulcerative colitis. Which of the following clinical manifestations is more worrisome for severe or fulminant ulcerative colitis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 61-Year-Old Man With Crampy Abdominal Pain"
},
{
"authors": "Kristin A. Olson, MD; Sarah M. Dry, MD",
"content": [],
"date": "November 11, 2014",
"figures": [],
"markdown": "# A 61-Year-Old Man With Crampy Abdominal Pain\n\n **Authors:** Kristin A. Olson, MD; Sarah M. Dry, MD \n **Date:** November 11, 2014\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 350653,
"choiceText": "Pseudopolyps",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 350654,
"choiceText": "Segmental disease (\"skip lesions\")",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 350655,
"choiceText": "Perianal involvement",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 350656,
"choiceText": "Transmural inflammation",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 350657,
"choiceText": "Deep granulomas",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Pseudopolyps are a common alteration of the colonic mucosa in patients with long-standing active ulcerative colitis; they are raised areas of inflamed, regenerative tissue arising in a background of ulceration. When present in significant numbers, pseudopolyps can cause cobblestoning of the mucosa that does not regress, even with treatment. Segmental disease (\"skip lesions\"), perianal involvement, transmural inflammation, and granulomas are all manifestations of Crohn's disease, another form of inflammatory bowel disease.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 99550,
"questionText": "You are caring for a patient with abdominal pain, fever, and hematochezia and are concerned that the patient has ulcerative colitis. Which finding is most consistent with a diagnosis of ulcerative colitis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 350658,
"choiceText": "Hematochezia",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 350659,
"choiceText": "Diarrhea",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 350660,
"choiceText": "Sacroiliitis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 350661,
"choiceText": "High-grade fever",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 350662,
"choiceText": "Tenesmus",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "High-grade fever is a systemic sign characteristically seen in patients with moderate or severe ulcerative colitis. Other examples of systemic manifestations common in patients with moderate or severe ulcerative colitis include tachycardia, anemia, orthostatic hypotension, and weight loss. A sudden stop in rectal output would be worrisome for development of toxic megacolon. Hematochezia, diarrhea, sacroiliitis, and tenesmus are findings common to almost all patients with ulcerative colitis, including those with mild disease.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 99551,
"questionText": "You are caring for a patient with known ulcerative colitis. Which of the following clinical manifestations is more worrisome for severe or fulminant ulcerative colitis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 61-Year-Old Man With Crampy Abdominal Pain"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 350623,
"choiceText": "Colorectal cancer",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 350624,
"choiceText": "Microscopic colitis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 350625,
"choiceText": "Inflammatory bowel disease",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 350626,
"choiceText": "Hemorrhoids",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 350627,
"choiceText": "<em>Clostridium difficile</em> colitis",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 99538,
"questionText": "What is the most likely diagnosis?\r\n<br><br>\r\n<em>Hint: Note the frequent passage of bloody loose stools for more than 2 months in the setting of fever, tachycardia, and anemia.</em>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 350653,
"choiceText": "Pseudopolyps",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 350654,
"choiceText": "Segmental disease (\"skip lesions\")",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 350655,
"choiceText": "Perianal involvement",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 350656,
"choiceText": "Transmural inflammation",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 350657,
"choiceText": "Deep granulomas",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Pseudopolyps are a common alteration of the colonic mucosa in patients with long-standing active ulcerative colitis; they are raised areas of inflamed, regenerative tissue arising in a background of ulceration. When present in significant numbers, pseudopolyps can cause cobblestoning of the mucosa that does not regress, even with treatment. Segmental disease (\"skip lesions\"), perianal involvement, transmural inflammation, and granulomas are all manifestations of Crohn's disease, another form of inflammatory bowel disease.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 99550,
"questionText": "You are caring for a patient with abdominal pain, fever, and hematochezia and are concerned that the patient has ulcerative colitis. Which finding is most consistent with a diagnosis of ulcerative colitis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 350658,
"choiceText": "Hematochezia",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 350659,
"choiceText": "Diarrhea",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 350660,
"choiceText": "Sacroiliitis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 350661,
"choiceText": "High-grade fever",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 350662,
"choiceText": "Tenesmus",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "High-grade fever is a systemic sign characteristically seen in patients with moderate or severe ulcerative colitis. Other examples of systemic manifestations common in patients with moderate or severe ulcerative colitis include tachycardia, anemia, orthostatic hypotension, and weight loss. A sudden stop in rectal output would be worrisome for development of toxic megacolon. Hematochezia, diarrhea, sacroiliitis, and tenesmus are findings common to almost all patients with ulcerative colitis, including those with mild disease.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 99551,
"questionText": "You are caring for a patient with known ulcerative colitis. Which of the following clinical manifestations is more worrisome for severe or fulminant ulcerative colitis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
Subsets and Splits
No saved queries yet
Save your SQL queries to embed, download, and access them later. Queries will appear here once saved.