article_id
stringlengths 6
7
| url
stringlengths 19
20
| article_data
listlengths 1
7
| questions
listlengths 0
5
|
|---|---|---|---|
936414 | /viewarticle/936414 | [
{
"authors": "Alexandra Scoles, DO; Babe Westlake, DO; Nicholas S. Tedesco, DO",
"content": [
"A 60-year-old woman initially presented to her primary care physician with chronic left lower extremity pain and edema secondary to varicose veins with progressive worsening. After 4 months of conservative management failed, she underwent greater saphenous vein radiofrequency ablation. She had an initial resolution of symptoms but experienced recurrence of swelling and pain in that leg 3 weeks after the procedure.",
"Additional imaging was obtained by her treating vascular surgeon, including a CT scan, which demonstrated a 14-cm multilobulated, intra-articular mass in her left knee with extra-articular extension. She was then referred to our orthopedic oncology clinic. MRI with contrast was ordered and confirmed significant involvement of the popliteal fossa, with compression and displacement of the popliteal vessels (Figures 1-3).",
"Figure 1.",
"Figure 2.",
"Figure 3.",
"MRI revealed a strongly hyperintense, poorly circumscribed, multilobulated mass with septations in the knee joint, with large popliteal fossa extension, popliteal vessel compression, and bone invasion. The mass was markedly heterogeneous on spin echo sequences, with intense gadolinium enhancement and focal areas of contrast voids. Numerous bony erosions were observed in the femoral condyles and proximal tibia due to tumor infiltration.",
"Upon physical examination, a massive, firm, fixed, deep-seated, nontender, soft tissue mass could be palpated in the posterior distal thigh, popliteal fossa, and into the proximal posterior calf. Knee range of motion was +10-110° compared with 0-135° on the contralateral side. Significant ipsilateral pedal edema and multiple large-vessel varicosities were noted. Her skin was otherwise unremarkable."
],
"date": "September 02, 2020",
"figures": [],
"markdown": "# My Strangest Case: A Woman With Varicose Veins and Leg Pain\n\n **Authors:** Alexandra Scoles, DO; Babe Westlake, DO; Nicholas S. Tedesco, DO \n **Date:** September 02, 2020\n\n ## Content\n\n A 60-year-old woman initially presented to her primary care physician with chronic left lower extremity pain and edema secondary to varicose veins with progressive worsening. After 4 months of conservative management failed, she underwent greater saphenous vein radiofrequency ablation. She had an initial resolution of symptoms but experienced recurrence of swelling and pain in that leg 3 weeks after the procedure.\nAdditional imaging was obtained by her treating vascular surgeon, including a CT scan, which demonstrated a 14-cm multilobulated, intra-articular mass in her left knee with extra-articular extension. She was then referred to our orthopedic oncology clinic. MRI with contrast was ordered and confirmed significant involvement of the popliteal fossa, with compression and displacement of the popliteal vessels (Figures 1-3).\nFigure 1.\nFigure 2.\nFigure 3.\nMRI revealed a strongly hyperintense, poorly circumscribed, multilobulated mass with septations in the knee joint, with large popliteal fossa extension, popliteal vessel compression, and bone invasion. The mass was markedly heterogeneous on spin echo sequences, with intense gadolinium enhancement and focal areas of contrast voids. Numerous bony erosions were observed in the femoral condyles and proximal tibia due to tumor infiltration.\nUpon physical examination, a massive, firm, fixed, deep-seated, nontender, soft tissue mass could be palpated in the posterior distal thigh, popliteal fossa, and into the proximal posterior calf. Knee range of motion was +10-110° compared with 0-135° on the contralateral side. Significant ipsilateral pedal edema and multiple large-vessel varicosities were noted. Her skin was otherwise unremarkable.\n\n ## Figures\n\n \n*Page 1 of 3*",
"pagination": {
"current_page": 1,
"total_pages": 3
},
"questionnaire": [],
"title": "My Strangest Case: A Woman With Varicose Veins and Leg Pain"
},
{
"authors": "Alexandra Scoles, DO; Babe Westlake, DO; Nicholas S. Tedesco, DO",
"content": [
"To review, our patient was a 60-year-old woman with a large soft tissue mass that involved both the anterior and posterior compartments of the left knee, with extensive extra-articular extension and osseous erosions present.",
"Her differential diagnosis included both benign and malignant neoplastic disease, massive ganglion cyst, inflammatory arthritis, and crystalline arthritis. We considered inflammatory and crystalline arthritis less likely due to the chronicity of symptoms. Although a benign neoplasm was in the differential diagnosis, we considered the most likely diagnosis to be a primary sarcoma, given the MRI characteristics and apparent aggressiveness of the mass. Inflammatory markers were ordered to rule out inflammatory and autoimmune disease, the results of which were negative. Ultimately, tissue sample for pathologic analysis was needed for diagnosis.",
"After seeing the patient in clinic, she was referred for an ultrasound-guided core needle biopsy. Pathologic findings showed this was a low-grade myxoid lesion; however, not enough tissue was available for final diagnosis. Core needle biopsies have been shown to have acceptable — although definitely lower — diagnostic accuracy compared with open biopsy.[1]",
"The patient returned to the clinic for a discussion of next steps. We advocated for open biopsy. Staging studies were also ordered at this time, including a CT scan of her chest, abdomen, and pelvis, and a bone scan, given the high index of suspicion for sarcoma. The staging study findings were negative.",
"Open biopsy yielded diagnostic tissue. The final pathologic diagnosis was juxta-articular myxoma. This was a surprising diagnosis due to the high-grade features seen on MRI, including bone erosion, infiltrative growth pattern, massive mass size, and heterogeneous MRI spin echo sequencing with central contrast voids. Although myxomas can have some heterogeneity with contrast enhancement, they are typically well circumscribed and homogenous on both T1- and T2-weighted images.[2]",
"Although many soft tissue tumors can arise around the knee, juxta-articular myxomas are rare. They can have many overlapping characteristics, radiographically and histologically, with various tumors, and all factors must be considered when making the diagnosis.[3] Myxomas are a benign, hypocellular, spindle cell neoplasm with myxoid stroma.[4] They lack features of malignancy, such as cellular atypia, frequent mitoses, necrosis, or hyperchromasia. Histologically distinguishing a myxoma from a low-grade myxofibrosarcoma, which will demonstrate features of malignancy, is arguably quite difficult.[5]",
"Macroscopically, myxomas present as cystic formations of soft or friable consistency, are white to yellow in color, and are typically 2-6 cm.[6] Myxomas are more common in men in their third to fifth decade but can occur in all age ranges.[7] Although intramuscular myxomas and juxta-articular myxomas can have similar histologic features, they likely represent distinct entities. Juxta-articular myxomas lack the Gs alpha mutation that is common to sporadic myxomas and those associated with Mazabraud syndrome.[8]",
"Juxta-articular myxomas most commonly occur around the knee but have been reported at other joints.[9,10,11] Other intra-articular pathologic findings are also typically associated with them, such as a meniscal tear or arthritis.[12] Although benign and usually isolated to subcutaneous adipose tissue, juxta-articular myxomas rarely behave more aggressively clinically, with involvement of deeper structures (eg, tendon, bone, capsule, neurovascular structures).[13] Destructive features, infiltrative growth, and massive size are rarely reported.[12,14]",
"In a series of 65 patients with juxta-articular myxoma, Meis and Enzinger[12] reported the mean size to be 3.8 cm and the maximum size to be 12 cm. Although metastasis has not been reported, rapid growth can occur.[11] Juxta-articular myxomas have a recurrence rate of approximately 30% after resection.[15] To our knowledge, our patient's case is one of the largest juxta-articular myxomas reported in the literature. The massive size and destructive features seen on imaging were atypical for this tumor. A careful diagnostic workup ultimately led to the correct diagnosis."
],
"date": "September 02, 2020",
"figures": [],
"markdown": "# My Strangest Case: A Woman With Varicose Veins and Leg Pain\n\n **Authors:** Alexandra Scoles, DO; Babe Westlake, DO; Nicholas S. Tedesco, DO \n **Date:** September 02, 2020\n\n ## Content\n\n To review, our patient was a 60-year-old woman with a large soft tissue mass that involved both the anterior and posterior compartments of the left knee, with extensive extra-articular extension and osseous erosions present.\nHer differential diagnosis included both benign and malignant neoplastic disease, massive ganglion cyst, inflammatory arthritis, and crystalline arthritis. We considered inflammatory and crystalline arthritis less likely due to the chronicity of symptoms. Although a benign neoplasm was in the differential diagnosis, we considered the most likely diagnosis to be a primary sarcoma, given the MRI characteristics and apparent aggressiveness of the mass. Inflammatory markers were ordered to rule out inflammatory and autoimmune disease, the results of which were negative. Ultimately, tissue sample for pathologic analysis was needed for diagnosis.\nAfter seeing the patient in clinic, she was referred for an ultrasound-guided core needle biopsy. Pathologic findings showed this was a low-grade myxoid lesion; however, not enough tissue was available for final diagnosis. Core needle biopsies have been shown to have acceptable — although definitely lower — diagnostic accuracy compared with open biopsy.[1]\nThe patient returned to the clinic for a discussion of next steps. We advocated for open biopsy. Staging studies were also ordered at this time, including a CT scan of her chest, abdomen, and pelvis, and a bone scan, given the high index of suspicion for sarcoma. The staging study findings were negative.\nOpen biopsy yielded diagnostic tissue. The final pathologic diagnosis was juxta-articular myxoma. This was a surprising diagnosis due to the high-grade features seen on MRI, including bone erosion, infiltrative growth pattern, massive mass size, and heterogeneous MRI spin echo sequencing with central contrast voids. Although myxomas can have some heterogeneity with contrast enhancement, they are typically well circumscribed and homogenous on both T1- and T2-weighted images.[2]\nAlthough many soft tissue tumors can arise around the knee, juxta-articular myxomas are rare. They can have many overlapping characteristics, radiographically and histologically, with various tumors, and all factors must be considered when making the diagnosis.[3] Myxomas are a benign, hypocellular, spindle cell neoplasm with myxoid stroma.[4] They lack features of malignancy, such as cellular atypia, frequent mitoses, necrosis, or hyperchromasia. Histologically distinguishing a myxoma from a low-grade myxofibrosarcoma, which will demonstrate features of malignancy, is arguably quite difficult.[5]\nMacroscopically, myxomas present as cystic formations of soft or friable consistency, are white to yellow in color, and are typically 2-6 cm.[6] Myxomas are more common in men in their third to fifth decade but can occur in all age ranges.[7] Although intramuscular myxomas and juxta-articular myxomas can have similar histologic features, they likely represent distinct entities. Juxta-articular myxomas lack the Gs alpha mutation that is common to sporadic myxomas and those associated with Mazabraud syndrome.[8]\nJuxta-articular myxomas most commonly occur around the knee but have been reported at other joints.[9,10,11] Other intra-articular pathologic findings are also typically associated with them, such as a meniscal tear or arthritis.[12] Although benign and usually isolated to subcutaneous adipose tissue, juxta-articular myxomas rarely behave more aggressively clinically, with involvement of deeper structures (eg, tendon, bone, capsule, neurovascular structures).[13] Destructive features, infiltrative growth, and massive size are rarely reported.[12,14]\nIn a series of 65 patients with juxta-articular myxoma, Meis and Enzinger[12] reported the mean size to be 3.8 cm and the maximum size to be 12 cm. Although metastasis has not been reported, rapid growth can occur.[11] Juxta-articular myxomas have a recurrence rate of approximately 30% after resection.[15] To our knowledge, our patient's case is one of the largest juxta-articular myxomas reported in the literature. The massive size and destructive features seen on imaging were atypical for this tumor. A careful diagnostic workup ultimately led to the correct diagnosis.\n\n ## Figures\n\n \n*Page 2 of 3*",
"pagination": {
"current_page": 2,
"total_pages": 3
},
"questionnaire": [],
"title": "My Strangest Case: A Woman With Varicose Veins and Leg Pain"
},
{
"authors": "Alexandra Scoles, DO; Babe Westlake, DO; Nicholas S. Tedesco, DO",
"content": [
"After the pathologic diagnosis was obtained, the patient was given several nonoperative and surgical options for treatment. She ultimately elected for a staged open posterior and arthroscopic anterior intralesional resection (Figures 4, 5).",
"Figure 4.",
"Figure 5.",
"The surgeries occurred 7 weeks apart. During the second arthroscopic stage, she simultaneously underwent chondroplasty and partial medial and lateral meniscectomies. By 6 weeks after her second surgery, she had regained full range of motion and function. She returned to all of her normal activities with no pain. Because of the extensive amount of anatomic tumor infiltration, she remains at high risk for tumor recurrence. However, as of the time of publication, she has demonstrated no such evidence.",
"Follow Medscape on Facebook, Twitter, Instagram, and YouTube"
],
"date": "September 02, 2020",
"figures": [],
"markdown": "# My Strangest Case: A Woman With Varicose Veins and Leg Pain\n\n **Authors:** Alexandra Scoles, DO; Babe Westlake, DO; Nicholas S. Tedesco, DO \n **Date:** September 02, 2020\n\n ## Content\n\n After the pathologic diagnosis was obtained, the patient was given several nonoperative and surgical options for treatment. She ultimately elected for a staged open posterior and arthroscopic anterior intralesional resection (Figures 4, 5).\nFigure 4.\nFigure 5.\nThe surgeries occurred 7 weeks apart. During the second arthroscopic stage, she simultaneously underwent chondroplasty and partial medial and lateral meniscectomies. By 6 weeks after her second surgery, she had regained full range of motion and function. She returned to all of her normal activities with no pain. Because of the extensive amount of anatomic tumor infiltration, she remains at high risk for tumor recurrence. However, as of the time of publication, she has demonstrated no such evidence.\nFollow Medscape on Facebook, Twitter, Instagram, and YouTube\n\n ## Figures\n\n \n*Page 3 of 3*",
"pagination": {
"current_page": 3,
"total_pages": 3
},
"questionnaire": [],
"title": "My Strangest Case: A Woman With Varicose Veins and Leg Pain"
}
] | [] |
935276 | /viewarticle/935276 | [
{
"authors": "Bruce M. Rothschild, MD",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians, but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please email us at [email protected] with the subject line \"Case Challenge Suggestion.\" We look forward to hearing from you.",
"A 60-year-old White woman presented for an annual medical examination. Her past medical history included asthma and several episodes of cough-induced rib fractures over the past 10 years. She was nulligravid and postmenopausal with cessation of menses in her early 50s. The patient reiterated that she was a frequent tea drinker, consuming five or six cups of caffeinated tea each day.",
"Her family history included a mother and sister who died of breast cancer (at age 51 years and 45 years, respectively). Her father had surgically resolved hyperparathyroidism. A review of systems revealed food-induced indigestion, for which she took omeprazole supplemented with aluminum hydroxide and magnesium hydroxide. She did not report any significant back or musculoskeletal pain.",
"She was asked about and denied having:",
"Fatigue, numbness, tingling, weakness, or paralysis",
"Sleep disturbance",
"Weight loss",
"Falls",
"Headaches",
"Blackouts",
"Hair issues",
"Ringing in the ears or trouble hearing",
"Red eyes, double vision, or dry eyes",
"Sores in the nose or mouth, or sinus problems",
"Difficulty swallowing",
"Chest pain, tightness, squeezing or heaviness, shortness of breath, or rapid heartbeat",
"Abdominal pain, diarrhea or constipation, nausea, vomiting, blood or mucus in bowel movements, burning while urinating, or frequent urination",
"Lumps or bumps",
"Fever, chills, sweats, or cold-induced vasospasm",
"Rashes, itching, or sun sensitivity",
"Seizures",
"Alcohol and tobacco use",
"In conversation, the patient casually shared a problem she had with a dress that she had fitted 6 months ago for a wedding. The dress was now too long."
],
"date": "November 13, 2024",
"figures": [],
"markdown": "# Cough-Induced Rib Fractures in a Woman With Indigestion\n\n **Authors:** Bruce M. Rothschild, MD \n **Date:** November 13, 2024\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians, but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please email us at [email protected] with the subject line \"Case Challenge Suggestion.\" We look forward to hearing from you.\nA 60-year-old White woman presented for an annual medical examination. Her past medical history included asthma and several episodes of cough-induced rib fractures over the past 10 years. She was nulligravid and postmenopausal with cessation of menses in her early 50s. The patient reiterated that she was a frequent tea drinker, consuming five or six cups of caffeinated tea each day.\nHer family history included a mother and sister who died of breast cancer (at age 51 years and 45 years, respectively). Her father had surgically resolved hyperparathyroidism. A review of systems revealed food-induced indigestion, for which she took omeprazole supplemented with aluminum hydroxide and magnesium hydroxide. She did not report any significant back or musculoskeletal pain.\nShe was asked about and denied having:\nFatigue, numbness, tingling, weakness, or paralysis\nSleep disturbance\nWeight loss\nFalls\nHeadaches\nBlackouts\nHair issues\nRinging in the ears or trouble hearing\nRed eyes, double vision, or dry eyes\nSores in the nose or mouth, or sinus problems\nDifficulty swallowing\nChest pain, tightness, squeezing or heaviness, shortness of breath, or rapid heartbeat\nAbdominal pain, diarrhea or constipation, nausea, vomiting, blood or mucus in bowel movements, burning while urinating, or frequent urination\nLumps or bumps\nFever, chills, sweats, or cold-induced vasospasm\nRashes, itching, or sun sensitivity\nSeizures\nAlcohol and tobacco use\nIn conversation, the patient casually shared a problem she had with a dress that she had fitted 6 months ago for a wedding. The dress was now too long.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "Cough-Induced Rib Fractures in a Woman With Indigestion"
},
{
"authors": "Bruce M. Rothschild, MD",
"content": [
"A complete physical examination revealed minimal tenderness of the mid-thoracic spine to percussion and a normal range of spinal motion (flexion, extension, lateral bending). No kyphosis was obvious. The remainder of the physical examination was within normal limits except for the reduction of her previously measured height by 1 inch (2.54 cm). Her weight was unchanged at 90 lb (40.82 kg). Both measurements were compared with findings from previous clinical visits that were also taken in the early morning.",
"Overall, a general examination of all systems yielded no significant findings. No masses, rashes, lumps, or bumps were found. A musculoskeletal examination included evaluation for malalignment, asymmetry, crepitation, defects, tenderness, masses, effusion, range of motion, stability, and muscle tone. No abnormalities were found.",
"The workup revealed an erythrocyte sedimentation rate within the reference range. Her hemoglobin level was 15 g/dL, albumin level was 3.5 g/dL, and calcium level was 8.9 mg/dL. Chest radiography was performed (Figure 1).",
"Figure 1.",
"Bone density testing was scheduled but had not yet been performed by her follow-up visit."
],
"date": "November 13, 2024",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/935/276/935276-Thumb1.jpg"
}
],
"markdown": "# Cough-Induced Rib Fractures in a Woman With Indigestion\n\n **Authors:** Bruce M. Rothschild, MD \n **Date:** November 13, 2024\n\n ## Content\n\n A complete physical examination revealed minimal tenderness of the mid-thoracic spine to percussion and a normal range of spinal motion (flexion, extension, lateral bending). No kyphosis was obvious. The remainder of the physical examination was within normal limits except for the reduction of her previously measured height by 1 inch (2.54 cm). Her weight was unchanged at 90 lb (40.82 kg). Both measurements were compared with findings from previous clinical visits that were also taken in the early morning.\nOverall, a general examination of all systems yielded no significant findings. No masses, rashes, lumps, or bumps were found. A musculoskeletal examination included evaluation for malalignment, asymmetry, crepitation, defects, tenderness, masses, effusion, range of motion, stability, and muscle tone. No abnormalities were found.\nThe workup revealed an erythrocyte sedimentation rate within the reference range. Her hemoglobin level was 15 g/dL, albumin level was 3.5 g/dL, and calcium level was 8.9 mg/dL. Chest radiography was performed (Figure 1).\nFigure 1.\nBone density testing was scheduled but had not yet been performed by her follow-up visit.\n\n ## Figures\n\n **Figure 1.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1515784,
"choiceText": "Scheuermann disease",
"correct": false,
"displayOrder": 1,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1515785,
"choiceText": "Metastatic disease",
"correct": false,
"displayOrder": 2,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1515786,
"choiceText": "Osteoporosis",
"correct": true,
"displayOrder": 3,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1515787,
"choiceText": "Hyperparathyroidism",
"correct": false,
"displayOrder": 4,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1515788,
"choiceText": "Tuberculosis",
"correct": false,
"displayOrder": 5,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "",
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": "",
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 485574,
"questionText": "On the basis of only these findings, which is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Cough-Induced Rib Fractures in a Woman With Indigestion"
},
{
"authors": "Bruce M. Rothschild, MD",
"content": [
"The anterior-posterior height differential noted on personal examination of the lateral chest x-ray (Figure 1) identified the presence of compression fractures.",
"Figure 1.",
"The loss of anterior vertebral height explained her height loss. Height loss does not occur with Scheuermann disease. Her history of rib fractures upon minimal exertion and height loss support a diagnosis of osteoporosis <",
"Her 10-year history of repeated fractures with minimal trauma and normal erythrocyte sedimentation rate made metastatic disease and tuberculosis unlikely. Her normal calcium-to-albumin ratio made hyperparathyroidism unlikely. A review of her dental films revealed persistence of the lamina dura (Figure 2), which further ruled out hyperparathyroidism.",
"Figure 2.",
"Chest x-rays revealed osteoporotic compression fractures that had been overlooked in a radiology report.",
"Multiple factors predisposed this woman to osteoporosis. She is nulligravid (risk factor) and had lost estrogen bone protection (postmenopausal). Consistent ingestion of certain medications, including proton pump inhibitors (eg, omeprazole), reduces bone density.[1] Although the patient drew attention to her excess caffeine (theobromine) ingestion, it was probably not a factor in this case.[2] Ingestion of aluminum hydroxide and magnesium hydroxide, rather than calcium carbonate, lessened her calcium intake. The latter is a significant concern. Her history of asthma suggests a possible exposure to corticosteroids, which is also a known cause of bone loss."
],
"date": "November 13, 2024",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/935/276/935276-Thumb1.jpg"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/935/276/935276-Thumb2.jpg"
}
],
"markdown": "# Cough-Induced Rib Fractures in a Woman With Indigestion\n\n **Authors:** Bruce M. Rothschild, MD \n **Date:** November 13, 2024\n\n ## Content\n\n The anterior-posterior height differential noted on personal examination of the lateral chest x-ray (Figure 1) identified the presence of compression fractures.\nFigure 1.\nThe loss of anterior vertebral height explained her height loss. Height loss does not occur with Scheuermann disease. Her history of rib fractures upon minimal exertion and height loss support a diagnosis of osteoporosis <\nHer 10-year history of repeated fractures with minimal trauma and normal erythrocyte sedimentation rate made metastatic disease and tuberculosis unlikely. Her normal calcium-to-albumin ratio made hyperparathyroidism unlikely. A review of her dental films revealed persistence of the lamina dura (Figure 2), which further ruled out hyperparathyroidism.\nFigure 2.\nChest x-rays revealed osteoporotic compression fractures that had been overlooked in a radiology report.\nMultiple factors predisposed this woman to osteoporosis. She is nulligravid (risk factor) and had lost estrogen bone protection (postmenopausal). Consistent ingestion of certain medications, including proton pump inhibitors (eg, omeprazole), reduces bone density.[1] Although the patient drew attention to her excess caffeine (theobromine) ingestion, it was probably not a factor in this case.[2] Ingestion of aluminum hydroxide and magnesium hydroxide, rather than calcium carbonate, lessened her calcium intake. The latter is a significant concern. Her history of asthma suggests a possible exposure to corticosteroids, which is also a known cause of bone loss.\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n\n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1515784,
"choiceText": "Scheuermann disease",
"correct": false,
"displayOrder": 1,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1515785,
"choiceText": "Metastatic disease",
"correct": false,
"displayOrder": 2,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1515786,
"choiceText": "Osteoporosis",
"correct": true,
"displayOrder": 3,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1515787,
"choiceText": "Hyperparathyroidism",
"correct": false,
"displayOrder": 4,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1515788,
"choiceText": "Tuberculosis",
"correct": false,
"displayOrder": 5,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "",
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": "",
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 485574,
"questionText": "On the basis of only these findings, which is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Cough-Induced Rib Fractures in a Woman With Indigestion"
},
{
"authors": "Bruce M. Rothschild, MD",
"content": [
"Osteoporosis is a disorder in which bone integrity is reduced. This is typically measured as decreased bone density, although that is only one component of bone strength. Osteoporosis is associated with microarchitecture deterioration, producing increased bone fragility and the propensity of bone to decompensate and fracture.[3,4] It is the most common metabolic disease with substantial implications.[5,6] More than 53 million Americans either have osteoporosis or are at high risk due to low bone mass.[7] Although more common among White women than in other ethnic groups, men are also affected. Osteoporosis among men is often due to alcohol use (45%-60%), glucocorticosteroid use, or hypogonadism.[8]",
"Many indicators allow the early recognition of osteoporosis.[9] Fractures that occur with minimal trauma or in the absence of identifying trauma, although not an early feature of osteoporosis, are often the first indication and are highly suggestive, as is decreased stature.[10] Fractures that occur with minimal trauma are a sign of skeletal fragility. Although severe coughing can result in fractures, repeated occurrence should raise suspicion of osteoporosis. Height loss is another sign that suggests osteoporotic compression fractures. However, height normally varies daily. Thus, comparing heights at the same time of day is important. Pathologic height alteration may be missed if an abnormal measurement is compared with a previous assessment performed at a different time. Upright posture-related intravertebral disk compression may reduce height by an inch.",
"Osteoporosis is often overlooked during routine clinical assessments. Its possibility must be considered before it can be diagnosed. Fractures that occur with minimal trauma or even in the absence of identifying trauma are often mechanically treated, often without consideration of why they occurred. Such findings are highly suspicious for osteoporosis. Setting or casting a fracture only addresses half the problem. Careful consideration should be given in order to assess the likely presence of an osteoporotic explanation and to prevent future events.",
"Lateral chest x-ray images are an often overlooked, routinely available resource for recognition of osteoporosis. They often contain evidence of compression fractures. However, a radiologist's evaluation of chest radiographs may be so frequent and routine that pathologic findings in the vertebral column (also visible on a chest x-ray, especially the lateral view) is often overlooked.[11] Personal examination of the chest x-ray by the physician is important. Compression fractures are readily recognizable on a lateral chest x-ray and usually identify the presence of osteoporosis.",
"In terms of bone density studies, the range of normal values is predicated on intact vertebrae. Bone density in the presence of vertebral compression fractures is the composite of the original bone and added compression. Bone density measurement in the spine in patients with compression fracture should exclude the vertebrae with fractures. However, bone density of the hips can and should be performed.",
"Whether bone density measurement is essential after identifying osteoporosis through the presence of vertebral compression fractures can be debated. Bisphosphonates are considered the drugs of choice for initial treatment of osteoporosis.[12] When bisphosphonates are used for treatment, bone density screening for effectiveness may be recommended. Alternatively, in patients with fractures, an anabolic agent may be considered. Bone density studies are not used to assess calcitonin efficacy because this agent improves bone strength but does not actually affect density. However, calcitonin is not widely recognized as a primary option for initial treatment."
],
"date": "November 13, 2024",
"figures": [],
"markdown": "# Cough-Induced Rib Fractures in a Woman With Indigestion\n\n **Authors:** Bruce M. Rothschild, MD \n **Date:** November 13, 2024\n\n ## Content\n\n Osteoporosis is a disorder in which bone integrity is reduced. This is typically measured as decreased bone density, although that is only one component of bone strength. Osteoporosis is associated with microarchitecture deterioration, producing increased bone fragility and the propensity of bone to decompensate and fracture.[3,4] It is the most common metabolic disease with substantial implications.[5,6] More than 53 million Americans either have osteoporosis or are at high risk due to low bone mass.[7] Although more common among White women than in other ethnic groups, men are also affected. Osteoporosis among men is often due to alcohol use (45%-60%), glucocorticosteroid use, or hypogonadism.[8]\nMany indicators allow the early recognition of osteoporosis.[9] Fractures that occur with minimal trauma or in the absence of identifying trauma, although not an early feature of osteoporosis, are often the first indication and are highly suggestive, as is decreased stature.[10] Fractures that occur with minimal trauma are a sign of skeletal fragility. Although severe coughing can result in fractures, repeated occurrence should raise suspicion of osteoporosis. Height loss is another sign that suggests osteoporotic compression fractures. However, height normally varies daily. Thus, comparing heights at the same time of day is important. Pathologic height alteration may be missed if an abnormal measurement is compared with a previous assessment performed at a different time. Upright posture-related intravertebral disk compression may reduce height by an inch.\nOsteoporosis is often overlooked during routine clinical assessments. Its possibility must be considered before it can be diagnosed. Fractures that occur with minimal trauma or even in the absence of identifying trauma are often mechanically treated, often without consideration of why they occurred. Such findings are highly suspicious for osteoporosis. Setting or casting a fracture only addresses half the problem. Careful consideration should be given in order to assess the likely presence of an osteoporotic explanation and to prevent future events.\nLateral chest x-ray images are an often overlooked, routinely available resource for recognition of osteoporosis. They often contain evidence of compression fractures. However, a radiologist's evaluation of chest radiographs may be so frequent and routine that pathologic findings in the vertebral column (also visible on a chest x-ray, especially the lateral view) is often overlooked.[11] Personal examination of the chest x-ray by the physician is important. Compression fractures are readily recognizable on a lateral chest x-ray and usually identify the presence of osteoporosis.\nIn terms of bone density studies, the range of normal values is predicated on intact vertebrae. Bone density in the presence of vertebral compression fractures is the composite of the original bone and added compression. Bone density measurement in the spine in patients with compression fracture should exclude the vertebrae with fractures. However, bone density of the hips can and should be performed.\nWhether bone density measurement is essential after identifying osteoporosis through the presence of vertebral compression fractures can be debated. Bisphosphonates are considered the drugs of choice for initial treatment of osteoporosis.[12] When bisphosphonates are used for treatment, bone density screening for effectiveness may be recommended. Alternatively, in patients with fractures, an anabolic agent may be considered. Bone density studies are not used to assess calcitonin efficacy because this agent improves bone strength but does not actually affect density. However, calcitonin is not widely recognized as a primary option for initial treatment.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "Cough-Induced Rib Fractures in a Woman With Indigestion"
},
{
"authors": "Bruce M. Rothschild, MD",
"content": [
"Clinical practice guidelines from the American College of Physicians on the prevention of fractures in patients with low bone density or osteoporosis recommends pharmacologic treatment for women with osteoporosis to reduce their risk for hip and vertebral fractures.[12] Alendronate, risedronate, or zoledronic acid is indicated. Denosumab is suggested as a second-line treatment for postmenopausal women with primary osteoporosis who have contraindications to or experience adverse effects from bisphosphonates. In patients with osteoporotic fractures, an anabolic agent can be considered. Calcitonin, although rarely used, may be an alternative based on patient preference. Supplemental calcium and adequate intake of vitamin D3 are also recommended. In postmenopausal women, the American College of Physicians recommends against use of estrogen, estrogen-progestogen, or raloxifene.",
"Reversible underlying causes of secondary osteoporosis should be addressed to optimize treatment. In this case, the patient's supplemental calcium ingestion was optimized. Her use of aluminum hydroxide and magnesium hydroxide was replaced with calcium carbonate. Bisphosphonates were discussed because they (or anabolic agents) are the option recommended by the guidelines.[12]",
"The patient was concerned about potential jaw necrosis with dental procedures and requirements for subsequent drug holidays. Biologic agents (eg, denosumab), anabolic agents (eg, abaloparatide), and nasally applied calcitonin were also discussed. The patient opted for calcitonin due to its ease of administration. Although her vitamin D (25-hydroxyvitamin D) level was normal, supplemental vitamin D was recommended."
],
"date": "November 13, 2024",
"figures": [],
"markdown": "# Cough-Induced Rib Fractures in a Woman With Indigestion\n\n **Authors:** Bruce M. Rothschild, MD \n **Date:** November 13, 2024\n\n ## Content\n\n Clinical practice guidelines from the American College of Physicians on the prevention of fractures in patients with low bone density or osteoporosis recommends pharmacologic treatment for women with osteoporosis to reduce their risk for hip and vertebral fractures.[12] Alendronate, risedronate, or zoledronic acid is indicated. Denosumab is suggested as a second-line treatment for postmenopausal women with primary osteoporosis who have contraindications to or experience adverse effects from bisphosphonates. In patients with osteoporotic fractures, an anabolic agent can be considered. Calcitonin, although rarely used, may be an alternative based on patient preference. Supplemental calcium and adequate intake of vitamin D3 are also recommended. In postmenopausal women, the American College of Physicians recommends against use of estrogen, estrogen-progestogen, or raloxifene.\nReversible underlying causes of secondary osteoporosis should be addressed to optimize treatment. In this case, the patient's supplemental calcium ingestion was optimized. Her use of aluminum hydroxide and magnesium hydroxide was replaced with calcium carbonate. Bisphosphonates were discussed because they (or anabolic agents) are the option recommended by the guidelines.[12]\nThe patient was concerned about potential jaw necrosis with dental procedures and requirements for subsequent drug holidays. Biologic agents (eg, denosumab), anabolic agents (eg, abaloparatide), and nasally applied calcitonin were also discussed. The patient opted for calcitonin due to its ease of administration. Although her vitamin D (25-hydroxyvitamin D) level was normal, supplemental vitamin D was recommended.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1515789,
"choiceText": "Multiple pregnancies",
"correct": false,
"displayOrder": 1,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1515790,
"choiceText": "Omeprazole use",
"correct": true,
"displayOrder": 2,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1515791,
"choiceText": "Late menopause ",
"correct": false,
"displayOrder": 3,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1515792,
"choiceText": "Excess caffeine intake",
"correct": false,
"displayOrder": 4,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Multiple factors predispose women to osteoporosis. Consistent ingestion of omeprazole reduces bone density. Nulligravid status is a risk factor, as is the loss of estrogen bone protection (postmenopause). Excess caffeine is likely not a significant factor. ",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": "",
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 485575,
"questionText": "Of the following, which is considered the strongest risk factor for osteoporosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1515793,
"choiceText": "Bone density measurement is required for diagnosis in all patients with suspected osteoporosis",
"correct": false,
"displayOrder": 1,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1515794,
"choiceText": "Further workup for osteoporosis is unnecessary in patients with a repetitive fall history",
"correct": false,
"displayOrder": 2,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1515795,
"choiceText": "Parathyroid hormone level assessment should be obtained in all patients with suspected osteoporosis who have a normal calcium-to-albumin ratio",
"correct": false,
"displayOrder": 3,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1515796,
"choiceText": "Compression fractures are recognizable on a lateral chest x-ray and usually identify the presence of osteoporosis",
"correct": true,
"displayOrder": 4,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Lateral chest x-ray images are an often overlooked, routinely available resource for recognition of osteoporosis. They often contain evidence of compression fractures. However, a radiologist's evaluation of chest x-rays may be so frequent and routine that pathologic findings in the vertebral column (also visible on a chest x-ray, especially the lateral view) are overlooked. Having the physician personally examine the x-ray images can be valuable because osteoporosis is so often overlooked that proactive assessment is recommended. Compression fractures are readily recognizable on a lateral chest x-ray and usually identify the presence of osteoporosis. <br><br>\r\n\r\nUnexplained fractures should be prompt investigation for osteoporosis. A repetitive fall history does not exclude osteoporosis and is actually an indicator for its assessment. Bone density studies are not likely to be essential for diagnosis if compression fractures are identified in the absence of severe trauma or bilateral calcaneal fractures. However, they should usually be obtained as a baseline for treatment and as a measure of severity of osteoporosis. Parathyroid hormone levels are unlikely to be clinically relevant if the calcium-to-albumin ratio and levels are normal.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": "",
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 485576,
"questionText": "Which of the following is most accurate regarding the workup for osteoporosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Cough-Induced Rib Fractures in a Woman With Indigestion"
},
{
"authors": "Bruce M. Rothschild, MD",
"content": [],
"date": "November 13, 2024",
"figures": [],
"markdown": "# Cough-Induced Rib Fractures in a Woman With Indigestion\n\n **Authors:** Bruce M. Rothschild, MD \n **Date:** November 13, 2024\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1515789,
"choiceText": "Multiple pregnancies",
"correct": false,
"displayOrder": 1,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1515790,
"choiceText": "Omeprazole use",
"correct": true,
"displayOrder": 2,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1515791,
"choiceText": "Late menopause ",
"correct": false,
"displayOrder": 3,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1515792,
"choiceText": "Excess caffeine intake",
"correct": false,
"displayOrder": 4,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Multiple factors predispose women to osteoporosis. Consistent ingestion of omeprazole reduces bone density. Nulligravid status is a risk factor, as is the loss of estrogen bone protection (postmenopause). Excess caffeine is likely not a significant factor. ",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": "",
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 485575,
"questionText": "Of the following, which is considered the strongest risk factor for osteoporosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1515793,
"choiceText": "Bone density measurement is required for diagnosis in all patients with suspected osteoporosis",
"correct": false,
"displayOrder": 1,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1515794,
"choiceText": "Further workup for osteoporosis is unnecessary in patients with a repetitive fall history",
"correct": false,
"displayOrder": 2,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1515795,
"choiceText": "Parathyroid hormone level assessment should be obtained in all patients with suspected osteoporosis who have a normal calcium-to-albumin ratio",
"correct": false,
"displayOrder": 3,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1515796,
"choiceText": "Compression fractures are recognizable on a lateral chest x-ray and usually identify the presence of osteoporosis",
"correct": true,
"displayOrder": 4,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Lateral chest x-ray images are an often overlooked, routinely available resource for recognition of osteoporosis. They often contain evidence of compression fractures. However, a radiologist's evaluation of chest x-rays may be so frequent and routine that pathologic findings in the vertebral column (also visible on a chest x-ray, especially the lateral view) are overlooked. Having the physician personally examine the x-ray images can be valuable because osteoporosis is so often overlooked that proactive assessment is recommended. Compression fractures are readily recognizable on a lateral chest x-ray and usually identify the presence of osteoporosis. <br><br>\r\n\r\nUnexplained fractures should be prompt investigation for osteoporosis. A repetitive fall history does not exclude osteoporosis and is actually an indicator for its assessment. Bone density studies are not likely to be essential for diagnosis if compression fractures are identified in the absence of severe trauma or bilateral calcaneal fractures. However, they should usually be obtained as a baseline for treatment and as a measure of severity of osteoporosis. Parathyroid hormone levels are unlikely to be clinically relevant if the calcium-to-albumin ratio and levels are normal.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": "",
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 485576,
"questionText": "Which of the following is most accurate regarding the workup for osteoporosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Cough-Induced Rib Fractures in a Woman With Indigestion"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1515784,
"choiceText": "Scheuermann disease",
"correct": false,
"displayOrder": 1,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1515785,
"choiceText": "Metastatic disease",
"correct": false,
"displayOrder": 2,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1515786,
"choiceText": "Osteoporosis",
"correct": true,
"displayOrder": 3,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1515787,
"choiceText": "Hyperparathyroidism",
"correct": false,
"displayOrder": 4,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1515788,
"choiceText": "Tuberculosis",
"correct": false,
"displayOrder": 5,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "",
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": "",
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 485574,
"questionText": "On the basis of only these findings, which is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1515789,
"choiceText": "Multiple pregnancies",
"correct": false,
"displayOrder": 1,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1515790,
"choiceText": "Omeprazole use",
"correct": true,
"displayOrder": 2,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1515791,
"choiceText": "Late menopause ",
"correct": false,
"displayOrder": 3,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1515792,
"choiceText": "Excess caffeine intake",
"correct": false,
"displayOrder": 4,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Multiple factors predispose women to osteoporosis. Consistent ingestion of omeprazole reduces bone density. Nulligravid status is a risk factor, as is the loss of estrogen bone protection (postmenopause). Excess caffeine is likely not a significant factor. ",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": "",
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 485575,
"questionText": "Of the following, which is considered the strongest risk factor for osteoporosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1515793,
"choiceText": "Bone density measurement is required for diagnosis in all patients with suspected osteoporosis",
"correct": false,
"displayOrder": 1,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1515794,
"choiceText": "Further workup for osteoporosis is unnecessary in patients with a repetitive fall history",
"correct": false,
"displayOrder": 2,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1515795,
"choiceText": "Parathyroid hormone level assessment should be obtained in all patients with suspected osteoporosis who have a normal calcium-to-albumin ratio",
"correct": false,
"displayOrder": 3,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1515796,
"choiceText": "Compression fractures are recognizable on a lateral chest x-ray and usually identify the presence of osteoporosis",
"correct": true,
"displayOrder": 4,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Lateral chest x-ray images are an often overlooked, routinely available resource for recognition of osteoporosis. They often contain evidence of compression fractures. However, a radiologist's evaluation of chest x-rays may be so frequent and routine that pathologic findings in the vertebral column (also visible on a chest x-ray, especially the lateral view) are overlooked. Having the physician personally examine the x-ray images can be valuable because osteoporosis is so often overlooked that proactive assessment is recommended. Compression fractures are readily recognizable on a lateral chest x-ray and usually identify the presence of osteoporosis. <br><br>\r\n\r\nUnexplained fractures should be prompt investigation for osteoporosis. A repetitive fall history does not exclude osteoporosis and is actually an indicator for its assessment. Bone density studies are not likely to be essential for diagnosis if compression fractures are identified in the absence of severe trauma or bilateral calcaneal fractures. However, they should usually be obtained as a baseline for treatment and as a measure of severity of osteoporosis. Parathyroid hormone levels are unlikely to be clinically relevant if the calcium-to-albumin ratio and levels are normal.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": "",
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 485576,
"questionText": "Which of the following is most accurate regarding the workup for osteoporosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
934944 | /viewarticle/934944 | [
{
"authors": "Meera Mohan, MD, MS; Paulette Mehta, MD, MPH",
"content": [
"A 63-year-old man was referred to us for recurrence of follicular thyroid carcinoma, with an anaplastic component, after an initial diagnosis of differentiated thyroid cancer 40 years prior. After that initial cancer diagnosis, the patient underwent total thyroidectomy, followed by radioactive iodine ablation and thyroid hormone suppression.",
"When the patient presented to us 40 years later, he described vague symptoms of exertional dyspnea and wheezing. They had lasted for about 4 months before he sought medical help. Imaging studies confirmed a left hilar mass (5.3 × 3.7 × 5.4 cm), with no evidence of disease spread (Figures 1-3). Bronchoscopy with endobronchial ultrasound of the left hilar mass was consistent with follicular thyroid cancer.",
"Figure 1.",
"Figure 2.",
"Figure 3.",
"At the time of presentation to the medical oncology department, the patient had undergone left pneumonectomy and had no evidence of disease on imaging studies, except for a bilobed nodule (1 × 0.7 cm) within the superior segment of the right lower lobe and a nodule (0.7 cm) within the posterior basal segment of the right lower lobe. The risk for complication associated with a biopsy of these subcentimeter lung nodules was substantial. The decision was made to pursue active surveillance with frequent imaging studies.",
"Owing to a lack of consensus or strong evidence-based treatment guidelines, and because of the aggressive nature of this rare cancer, we proceeded with combination chemotherapy with doxorubicin and docetaxel. Molecular study findings, including BRAF V600 and microsatellite instability testing, were unremarkable. The patient also resumed levothyroxine therapy, with the intention of fully suppressing thyroid-stimulating hormone.",
"After the second cycle of chemotherapy, the patient was found to have an incidental drop in cardiac function, from 60% to 39%. The treatment was thus permanently discontinued. At this point, we pursued active surveillance with CT every 6-8 weeks, given the aggressive nature of the anaplastic thyroid cancer."
],
"date": "August 04, 2020",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/934/944/934944-Thumb1.jpg"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/934/944/934944-Thumb2.jpg"
},
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/934/944/934944-Thumb3.jpg"
}
],
"markdown": "# My Strangest Case: The Man With a Heavy Tongue\n\n **Authors:** Meera Mohan, MD, MS; Paulette Mehta, MD, MPH \n **Date:** August 04, 2020\n\n ## Content\n\n A 63-year-old man was referred to us for recurrence of follicular thyroid carcinoma, with an anaplastic component, after an initial diagnosis of differentiated thyroid cancer 40 years prior. After that initial cancer diagnosis, the patient underwent total thyroidectomy, followed by radioactive iodine ablation and thyroid hormone suppression.\nWhen the patient presented to us 40 years later, he described vague symptoms of exertional dyspnea and wheezing. They had lasted for about 4 months before he sought medical help. Imaging studies confirmed a left hilar mass (5.3 × 3.7 × 5.4 cm), with no evidence of disease spread (Figures 1-3). Bronchoscopy with endobronchial ultrasound of the left hilar mass was consistent with follicular thyroid cancer.\nFigure 1.\nFigure 2.\nFigure 3.\nAt the time of presentation to the medical oncology department, the patient had undergone left pneumonectomy and had no evidence of disease on imaging studies, except for a bilobed nodule (1 × 0.7 cm) within the superior segment of the right lower lobe and a nodule (0.7 cm) within the posterior basal segment of the right lower lobe. The risk for complication associated with a biopsy of these subcentimeter lung nodules was substantial. The decision was made to pursue active surveillance with frequent imaging studies.\nOwing to a lack of consensus or strong evidence-based treatment guidelines, and because of the aggressive nature of this rare cancer, we proceeded with combination chemotherapy with doxorubicin and docetaxel. Molecular study findings, including BRAF V600 and microsatellite instability testing, were unremarkable. The patient also resumed levothyroxine therapy, with the intention of fully suppressing thyroid-stimulating hormone.\nAfter the second cycle of chemotherapy, the patient was found to have an incidental drop in cardiac function, from 60% to 39%. The treatment was thus permanently discontinued. At this point, we pursued active surveillance with CT every 6-8 weeks, given the aggressive nature of the anaplastic thyroid cancer.\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n**Figure 3.** \n \n\n\n*Page 1 of 3*",
"pagination": {
"current_page": 1,
"total_pages": 3
},
"questionnaire": [],
"title": "My Strangest Case: The Man With a Heavy Tongue"
},
{
"authors": "Meera Mohan, MD, MS; Paulette Mehta, MD, MPH",
"content": [
"Eight months later, the patient presented with new-onset dysarthria and heaviness of the tongue. Focal neurologic examination was remarkable for a tongue deviation. MRI of the skull base was suggestive of an infiltrative metastatic lesion that involved the clivus and bilateral occipital condyles, with involvement of both hypoglossal canals. The patient underwent 10 sessions of local radiation therapy, with dramatic improvement in his symptoms. On the basis of anecdotal evidence from preclinical and small clinical studies, the patient was started on lenvatinib, a multi–tyrosine kinase inhibitor (TKI). Interim CT scans demonstrated stable disease with no disease progression.",
"Around the same time, the patient developed new lytic bone lesions, mainly in his thoracic spine. He was started on zoledronic acid, which was later switched to denosumab owing to breakthrough lesions despite bisphosphonate therapy. He had stable disease on lenvatinib. Overall treatment was well tolerated, except for hypertension that required a combination of calcium-channel blocker and angiotensin-converting enzyme inhibitors.",
"Tumor tissue was sent for next-generation sequencing to identify a targetable mutation. Results were unrevealing, except for a missense mutation in NTRK1 G18E in exon 1. Nine months later, the patient experienced disease relapse, with an incidental new lytic lesion that compromised weight-bearing on the left femur. He underwent open intramedullary fixation, followed by local radiation therapy for 10 sessions. We proceeded with larotrectinib for NTRK alternations, despite existing literature that reported minimal benefit in this setting. A radioactive iodine scan showed no uptake, suggesting an undifferentiated component at the metastatic sites.",
"Overall, his disease remained stable for 5 months. The patient later presented with a worsening bone lesion and new mediastinal lymph node compression in the right-side bronchi. CT revealed a 4.1 × 3.1 cm bulky subcarinal nodal mass, with mass effect on the right main pulmonary artery and approximately 40% loss of luminal diameter in the right main pulmonary artery. He had ongoing severe pain due to the pelvic lytic lesion. Local radiation therapy was administered for symptom relief. We proceeded with therapy with pembrolizumab as a fourth-line option in this patient with extremely limited treatment choices."
],
"date": "August 04, 2020",
"figures": [],
"markdown": "# My Strangest Case: The Man With a Heavy Tongue\n\n **Authors:** Meera Mohan, MD, MS; Paulette Mehta, MD, MPH \n **Date:** August 04, 2020\n\n ## Content\n\n Eight months later, the patient presented with new-onset dysarthria and heaviness of the tongue. Focal neurologic examination was remarkable for a tongue deviation. MRI of the skull base was suggestive of an infiltrative metastatic lesion that involved the clivus and bilateral occipital condyles, with involvement of both hypoglossal canals. The patient underwent 10 sessions of local radiation therapy, with dramatic improvement in his symptoms. On the basis of anecdotal evidence from preclinical and small clinical studies, the patient was started on lenvatinib, a multi–tyrosine kinase inhibitor (TKI). Interim CT scans demonstrated stable disease with no disease progression.\nAround the same time, the patient developed new lytic bone lesions, mainly in his thoracic spine. He was started on zoledronic acid, which was later switched to denosumab owing to breakthrough lesions despite bisphosphonate therapy. He had stable disease on lenvatinib. Overall treatment was well tolerated, except for hypertension that required a combination of calcium-channel blocker and angiotensin-converting enzyme inhibitors.\nTumor tissue was sent for next-generation sequencing to identify a targetable mutation. Results were unrevealing, except for a missense mutation in NTRK1 G18E in exon 1. Nine months later, the patient experienced disease relapse, with an incidental new lytic lesion that compromised weight-bearing on the left femur. He underwent open intramedullary fixation, followed by local radiation therapy for 10 sessions. We proceeded with larotrectinib for NTRK alternations, despite existing literature that reported minimal benefit in this setting. A radioactive iodine scan showed no uptake, suggesting an undifferentiated component at the metastatic sites.\nOverall, his disease remained stable for 5 months. The patient later presented with a worsening bone lesion and new mediastinal lymph node compression in the right-side bronchi. CT revealed a 4.1 × 3.1 cm bulky subcarinal nodal mass, with mass effect on the right main pulmonary artery and approximately 40% loss of luminal diameter in the right main pulmonary artery. He had ongoing severe pain due to the pelvic lytic lesion. Local radiation therapy was administered for symptom relief. We proceeded with therapy with pembrolizumab as a fourth-line option in this patient with extremely limited treatment choices.\n\n ## Figures\n\n \n*Page 2 of 3*",
"pagination": {
"current_page": 2,
"total_pages": 3
},
"questionnaire": [],
"title": "My Strangest Case: The Man With a Heavy Tongue"
},
{
"authors": "Meera Mohan, MD, MS; Paulette Mehta, MD, MPH",
"content": [
"Four aspects make this case unusual. First, the length of time between the diagnosis and relapse, 40 years, was remarkable. Ordinarily, the prognosis for the patient's original tumor type, follicular thyroid cancer, is excellent; however, recurrence with an undifferentiated or anaplastic component has a dismal outcome. Anaplastic thyroid cancer is a rare, aggressive cancer accounting for 1%-2% of all thyroid cancer, with a median survival of approximately 3 months.[1] Despite reports of a dismal outcome, several years later, the patient in this case report is currently receiving treatment with pembrolizumab.",
"Second, the type of recurrence is unusual. Usually, this cancer is localized to the thyroid gland and neck nodes; however, diffuse metastatic spread to bones, central nervous system, and lymph nodes can happen. This presentation of recurrence at a distant site without any evidence of primary tumor is unusual.",
"Third, the patient's responses, albeit limited, to new molecular therapies are interesting and promising. Lenvatinib is a multi-TKI that targets vascular endothelial growth factor receptors, fibroblast growth factor receptors, platelet-derived growth factor receptor alpha, and the RET and KIT proto-oncogene receptor tyrosine kinase.[2,3] In a recent study, lenvatinib was associated with median overall survival of 4.2 months, compared with 2 months in the palliative care group.[4] In addition, lenvatinib also resulted in a reduction in tumor size ≥ 30% in 31.3% of the patients (n = 5), which was considered to be a clinical partial response.",
"NTRK inhibitors are approved for tumors with NTRK fusion.[5] The patient responded to chemotherapy, lenvatinib, and larotrectinib, each given sequentially. He had response for approximately 8-9 months with each successive line of therapy, which was better than what would be expected without treatment, as predicted by early studies. Agents such as pembrolizumab have shown to be efficacious in this tumor type.[6,7] He is currently on the programmed death ligand 1 inhibitor pembrolizumab and continues to have ongoing response. Although his responses were brief, they are encouraging, and combination treatments are being tested in ongoing clinical trials.",
"Fourth, the typing of tumor to search for targetable mutations is a new direction in the field of oncology treatment. Precision oncology has broadened the therapeutic horizon for patients with cancer. Although no targetable mutations were identified in this patient's tumor, it remains a promising route to determine which drugs may work and which ones probably would not.",
"Follow Medscape on Facebook, Twitter, Instagram, and YouTube"
],
"date": "August 04, 2020",
"figures": [],
"markdown": "# My Strangest Case: The Man With a Heavy Tongue\n\n **Authors:** Meera Mohan, MD, MS; Paulette Mehta, MD, MPH \n **Date:** August 04, 2020\n\n ## Content\n\n Four aspects make this case unusual. First, the length of time between the diagnosis and relapse, 40 years, was remarkable. Ordinarily, the prognosis for the patient's original tumor type, follicular thyroid cancer, is excellent; however, recurrence with an undifferentiated or anaplastic component has a dismal outcome. Anaplastic thyroid cancer is a rare, aggressive cancer accounting for 1%-2% of all thyroid cancer, with a median survival of approximately 3 months.[1] Despite reports of a dismal outcome, several years later, the patient in this case report is currently receiving treatment with pembrolizumab.\nSecond, the type of recurrence is unusual. Usually, this cancer is localized to the thyroid gland and neck nodes; however, diffuse metastatic spread to bones, central nervous system, and lymph nodes can happen. This presentation of recurrence at a distant site without any evidence of primary tumor is unusual.\nThird, the patient's responses, albeit limited, to new molecular therapies are interesting and promising. Lenvatinib is a multi-TKI that targets vascular endothelial growth factor receptors, fibroblast growth factor receptors, platelet-derived growth factor receptor alpha, and the RET and KIT proto-oncogene receptor tyrosine kinase.[2,3] In a recent study, lenvatinib was associated with median overall survival of 4.2 months, compared with 2 months in the palliative care group.[4] In addition, lenvatinib also resulted in a reduction in tumor size ≥ 30% in 31.3% of the patients (n = 5), which was considered to be a clinical partial response.\nNTRK inhibitors are approved for tumors with NTRK fusion.[5] The patient responded to chemotherapy, lenvatinib, and larotrectinib, each given sequentially. He had response for approximately 8-9 months with each successive line of therapy, which was better than what would be expected without treatment, as predicted by early studies. Agents such as pembrolizumab have shown to be efficacious in this tumor type.[6,7] He is currently on the programmed death ligand 1 inhibitor pembrolizumab and continues to have ongoing response. Although his responses were brief, they are encouraging, and combination treatments are being tested in ongoing clinical trials.\nFourth, the typing of tumor to search for targetable mutations is a new direction in the field of oncology treatment. Precision oncology has broadened the therapeutic horizon for patients with cancer. Although no targetable mutations were identified in this patient's tumor, it remains a promising route to determine which drugs may work and which ones probably would not.\nFollow Medscape on Facebook, Twitter, Instagram, and YouTube\n\n ## Figures\n\n \n*Page 3 of 3*",
"pagination": {
"current_page": 3,
"total_pages": 3
},
"questionnaire": [],
"title": "My Strangest Case: The Man With a Heavy Tongue"
}
] | [] |
934162 | /viewarticle/934162 | [
{
"authors": "Elizabeth E. Ginalis, MD; Arthur Carminucci, MD; Nitesh V. Patel, MD; Simon Hanft, MD",
"content": [
"A 68-year-old man presented to us with a 2-month history of progressively worsening bilateral upper-extremity pain. His previous history was significant for renal cell carcinoma (RCC) and right-sided nephrectomy performed 22 years prior.",
"Physical examination findings, including neurologic examination findings, were unremarkable. Subsequently, the patient underwent MRI with gadolinium contrast of the cervical spine (Figure 1).",
"Figure 1.",
"T1-weighted imaging revealed a 1.5-cm contrast-enhancing intradural, extramedullary lesion at the level of C3-C4. The tumor was causing compression of the spinal cord. Ultimately, we offered surgical resection of the tumor.",
"The patient underwent a C3 and C4 laminectomy and surgical resection of the tumor. The tumor had a red appearance on gross inspection. It was present along the left lateral aspect of the spinal cord and was also attached to the lateral aspect of the dura. A biopsy sample was obtained and sent to pathology for frozen section.",
"Frozen section revealed features of a high-grade neoplasm. Consequently, a more aggressive surgical resection was done. We achieved a near gross total resection. Only a part of the tumor capsule remained; it was adherent to the spinal cord, which could be injured with further dissection.",
"The patient did well after the operation. Postoperatively, his physical examination findings were unchanged, and he remained neurologically intact. Fortunately, he also reported complete resolution of his pain from initial presentation. Postoperative MRI with contrast demonstrated excellent resection of the lesion (Figure 2a). The patient was discharged on the third postoperative day.",
"Figure 2.",
"Subsequently, he underwent PET/CT and MRI of the brain, which found several areas of metastatic disease, including the brain, right mediastinum, bilateral pulmonary nodules, and liver lesions. He began systemic chemotherapy with temsirolimus and focused proton therapy to the cervical spine. At 3 months and 6 months of follow-up, he had no evidence of recurrent disease on MRI of the cervical spine with contrast (Figure 2b, 2c).",
"He is currently monitored with MRI of the cervical spine every 6 months. At 3 years of follow-up, he has no evidence of disease recurrence of the intradural spinal metastasis, and his systemic disease is controlled as well."
],
"date": "July 22, 2020",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/934/162/934162-Thumb1.jpg"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/934/162/934162-Thumb2.jpg"
}
],
"markdown": "# My Strangest Case: A Man With Worsening Pain in Both Arms\n\n **Authors:** Elizabeth E. Ginalis, MD; Arthur Carminucci, MD; Nitesh V. Patel, MD; Simon Hanft, MD \n **Date:** July 22, 2020\n\n ## Content\n\n A 68-year-old man presented to us with a 2-month history of progressively worsening bilateral upper-extremity pain. His previous history was significant for renal cell carcinoma (RCC) and right-sided nephrectomy performed 22 years prior.\nPhysical examination findings, including neurologic examination findings, were unremarkable. Subsequently, the patient underwent MRI with gadolinium contrast of the cervical spine (Figure 1).\nFigure 1.\nT1-weighted imaging revealed a 1.5-cm contrast-enhancing intradural, extramedullary lesion at the level of C3-C4. The tumor was causing compression of the spinal cord. Ultimately, we offered surgical resection of the tumor.\nThe patient underwent a C3 and C4 laminectomy and surgical resection of the tumor. The tumor had a red appearance on gross inspection. It was present along the left lateral aspect of the spinal cord and was also attached to the lateral aspect of the dura. A biopsy sample was obtained and sent to pathology for frozen section.\nFrozen section revealed features of a high-grade neoplasm. Consequently, a more aggressive surgical resection was done. We achieved a near gross total resection. Only a part of the tumor capsule remained; it was adherent to the spinal cord, which could be injured with further dissection.\nThe patient did well after the operation. Postoperatively, his physical examination findings were unchanged, and he remained neurologically intact. Fortunately, he also reported complete resolution of his pain from initial presentation. Postoperative MRI with contrast demonstrated excellent resection of the lesion (Figure 2a). The patient was discharged on the third postoperative day.\nFigure 2.\nSubsequently, he underwent PET/CT and MRI of the brain, which found several areas of metastatic disease, including the brain, right mediastinum, bilateral pulmonary nodules, and liver lesions. He began systemic chemotherapy with temsirolimus and focused proton therapy to the cervical spine. At 3 months and 6 months of follow-up, he had no evidence of recurrent disease on MRI of the cervical spine with contrast (Figure 2b, 2c).\nHe is currently monitored with MRI of the cervical spine every 6 months. At 3 years of follow-up, he has no evidence of disease recurrence of the intradural spinal metastasis, and his systemic disease is controlled as well.\n\n ## Figures\n\n **Figure 1.** \n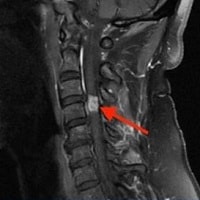 \n\n**Figure 2.** \n \n\n\n*Page 1 of 3*",
"pagination": {
"current_page": 1,
"total_pages": 3
},
"questionnaire": [],
"title": "My Strangest Case: A Man With Worsening Pain in Both Arms"
},
{
"authors": "Elizabeth E. Ginalis, MD; Arthur Carminucci, MD; Nitesh V. Patel, MD; Simon Hanft, MD",
"content": [
"This is a rare case of a 68-year-old man with an intradural extramedullary spinal metastasis to the cervical spinal cord that presented 22 years after treatment for RCC. Ultimately, the pathology of the tumor was confirmed after histologic and immunohistochemical analysis of the surgical resection. Upon microscopic examination, tumor cells demonstrated pleomorphic epithelioid cells in a papillary pattern on hematoxylin and eosin staining (low and high power) (Figure 3a and 3b, respectively).",
"Figure 3.",
"Tumor cells contained pleomorphic nuclei with occasional pseudo-nuclear inclusions. Upon immunohistochemical staining, the tumor stained positive for PAX8 (Figure 3c), CAM5.2 (Figure 3d), vimentin, and EMA. The Ki67 index was 50%.",
"The tumor's profile was consistent with metastatic papillary RCC. Not only is intradural extramedullary spinal metastasis from RCC extremely rare, a latency interval of 22 years to diagnosis of metastasis is among the longest reported in the current literature.",
"As many as 40% of patients with RCC develop metastatic disease.[1,2] RCC most commonly metastasizes to the lungs (50%), bone (49%), lymph nodes (30%), liver (8%), and brain (3%).[1,2,3,4] However, spread to the intradural spinal cord is uncommon. In patients with a history of RCC, considering the possibility of intradural spinal metastasis is important if the patient is experiencing weakness or urinary incontinence.",
"Other common presenting symptoms may include bowel dysfunction, pain, and paresthesias. Physical examination findings may include neurologic deficits in motor and sensory examination or abnormal reflexes. If such signs or symptoms are present, imaging of the spine is indicated, preferably with MRI.",
"Owing to the rarity of intradural metastasis, treatment is often individualized, because management guidelines are not clearly defined. Moreover, intradural metastasis is associated with a poor prognosis and limited life expectancy; thus, any therapy is often palliative, with a goal to improve symptoms. Management options include surgical resection, radiation therapy, chemotherapy, or a combination of these treatments."
],
"date": "July 22, 2020",
"figures": [
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/934/162/934162-Thumb3.jpg"
}
],
"markdown": "# My Strangest Case: A Man With Worsening Pain in Both Arms\n\n **Authors:** Elizabeth E. Ginalis, MD; Arthur Carminucci, MD; Nitesh V. Patel, MD; Simon Hanft, MD \n **Date:** July 22, 2020\n\n ## Content\n\n This is a rare case of a 68-year-old man with an intradural extramedullary spinal metastasis to the cervical spinal cord that presented 22 years after treatment for RCC. Ultimately, the pathology of the tumor was confirmed after histologic and immunohistochemical analysis of the surgical resection. Upon microscopic examination, tumor cells demonstrated pleomorphic epithelioid cells in a papillary pattern on hematoxylin and eosin staining (low and high power) (Figure 3a and 3b, respectively).\nFigure 3.\nTumor cells contained pleomorphic nuclei with occasional pseudo-nuclear inclusions. Upon immunohistochemical staining, the tumor stained positive for PAX8 (Figure 3c), CAM5.2 (Figure 3d), vimentin, and EMA. The Ki67 index was 50%.\nThe tumor's profile was consistent with metastatic papillary RCC. Not only is intradural extramedullary spinal metastasis from RCC extremely rare, a latency interval of 22 years to diagnosis of metastasis is among the longest reported in the current literature.\nAs many as 40% of patients with RCC develop metastatic disease.[1,2] RCC most commonly metastasizes to the lungs (50%), bone (49%), lymph nodes (30%), liver (8%), and brain (3%).[1,2,3,4] However, spread to the intradural spinal cord is uncommon. In patients with a history of RCC, considering the possibility of intradural spinal metastasis is important if the patient is experiencing weakness or urinary incontinence.\nOther common presenting symptoms may include bowel dysfunction, pain, and paresthesias. Physical examination findings may include neurologic deficits in motor and sensory examination or abnormal reflexes. If such signs or symptoms are present, imaging of the spine is indicated, preferably with MRI.\nOwing to the rarity of intradural metastasis, treatment is often individualized, because management guidelines are not clearly defined. Moreover, intradural metastasis is associated with a poor prognosis and limited life expectancy; thus, any therapy is often palliative, with a goal to improve symptoms. Management options include surgical resection, radiation therapy, chemotherapy, or a combination of these treatments.\n\n ## Figures\n\n **Figure 3.** \n \n\n\n*Page 2 of 3*",
"pagination": {
"current_page": 2,
"total_pages": 3
},
"questionnaire": [],
"title": "My Strangest Case: A Man With Worsening Pain in Both Arms"
},
{
"authors": "Elizabeth E. Ginalis, MD; Arthur Carminucci, MD; Nitesh V. Patel, MD; Simon Hanft, MD",
"content": [
"Surgical resection is generally favored because it usually results in the greatest improvement from presenting symptoms.[5,6] In particular, one case series of intradural spinal metastases from RCC found that patients presenting with motor or pain symptoms were more likely to improve postoperatively compared with those who have paresthesia or urinary incontinence.[5] Moreover, early surgical intervention has been associated with longer overall survival and improved prognosis.[7]",
"Unfortunately, not all patients are appropriate surgical candidates because of comorbidities or functional status. These factors must be considered when determining the appropriate treatment for the patient. In these patients who are not deemed appropriate for surgery, radiation therapy, such as stereotactic radiosurgery, can be considered as a second-line option.",
"Unfortunately, RCC is generally radioresistant; however, some studies have found that radiation resulted in improvement of symptoms without an increased survival rate.[8,9,10,11,12] Owing to the rarity of these lesions, management is often dictated by case reports and series in the literature, as well as severity of symptoms. Given that this often presents as an advanced disease process with limited life expectancy, the goal of treatment should be to improve symptoms and enhance quality of life.",
"Only 18 cases of intradural extramedullary spinal metastasis from RCC have been reported to date.[5,6,13,14,15,16,17,18,19,20,21,22,23,24,25] Among these cases, the mean age was 62 years (±14 years) at diagnosis of metastasis. The mean latency period from diagnosis of RCC to diagnosis of metastasis is 3.9 years (±6.2 years), with the longest reported latency being 16 years.[13,14]",
"In the two cases that reported a latency of 16 years, the patients only had a single metastatic tumor to the spinal cord without diffuse disease.[13,14] Similar to these patients, our patient's initial presentation was from the spinal tumor. However, in our case, the patient had diffuse systemic disease not limited to the spinal cord. Despite advanced cancer, our patient described only bilateral upper-extremity pain from the spinal cord tumor, without additional symptoms or physical examination findings from his other metastases.",
"Regarding disease extent, less than one third of patients were found to have metastases to other areas outside the spine, with 17% of patients also having brain metastases. Metastasis to the lumbar spine was most common (59%), especially in the cauda equina. Nearly one quarter of patients presented with metastasis as the initial sign of primary RCC. Half of the patients were managed surgically, five patients (28%) were treated with radiation, and the remaining four patients (22%) received a combination of the two therapies. Nearly all patients (89%) were alive at the last follow-up, with a mean overall survival of 2 years (±2.1 years).",
"Appropriate patient selection for surgical intervention is essential because surgery may provide symptom relief, thus enhancing quality of life in these patients who otherwise have a dismal prognosis. Our patient reported complete resolution of his pain postoperatively and did not have evidence of disease recurrence 3 years after surgery.",
"Although intradural spinal metastasis is an unlikely cause of upper-extremity pain, imaging is essential to identify the underlying pathology. Moreover, given this patient's history of RCC, metastatic disease should certainly be considered despite a remote history of the primary cancer. Pathologic assessment ultimately confirms the diagnosis. Patients should also be evaluated for other areas of metastasis with imaging to assess the extent of tumor spread.",
"Follow Medscape on Facebook, Twitter, Instagram, and YouTube"
],
"date": "July 22, 2020",
"figures": [],
"markdown": "# My Strangest Case: A Man With Worsening Pain in Both Arms\n\n **Authors:** Elizabeth E. Ginalis, MD; Arthur Carminucci, MD; Nitesh V. Patel, MD; Simon Hanft, MD \n **Date:** July 22, 2020\n\n ## Content\n\n Surgical resection is generally favored because it usually results in the greatest improvement from presenting symptoms.[5,6] In particular, one case series of intradural spinal metastases from RCC found that patients presenting with motor or pain symptoms were more likely to improve postoperatively compared with those who have paresthesia or urinary incontinence.[5] Moreover, early surgical intervention has been associated with longer overall survival and improved prognosis.[7]\nUnfortunately, not all patients are appropriate surgical candidates because of comorbidities or functional status. These factors must be considered when determining the appropriate treatment for the patient. In these patients who are not deemed appropriate for surgery, radiation therapy, such as stereotactic radiosurgery, can be considered as a second-line option.\nUnfortunately, RCC is generally radioresistant; however, some studies have found that radiation resulted in improvement of symptoms without an increased survival rate.[8,9,10,11,12] Owing to the rarity of these lesions, management is often dictated by case reports and series in the literature, as well as severity of symptoms. Given that this often presents as an advanced disease process with limited life expectancy, the goal of treatment should be to improve symptoms and enhance quality of life.\nOnly 18 cases of intradural extramedullary spinal metastasis from RCC have been reported to date.[5,6,13,14,15,16,17,18,19,20,21,22,23,24,25] Among these cases, the mean age was 62 years (±14 years) at diagnosis of metastasis. The mean latency period from diagnosis of RCC to diagnosis of metastasis is 3.9 years (±6.2 years), with the longest reported latency being 16 years.[13,14]\nIn the two cases that reported a latency of 16 years, the patients only had a single metastatic tumor to the spinal cord without diffuse disease.[13,14] Similar to these patients, our patient's initial presentation was from the spinal tumor. However, in our case, the patient had diffuse systemic disease not limited to the spinal cord. Despite advanced cancer, our patient described only bilateral upper-extremity pain from the spinal cord tumor, without additional symptoms or physical examination findings from his other metastases.\nRegarding disease extent, less than one third of patients were found to have metastases to other areas outside the spine, with 17% of patients also having brain metastases. Metastasis to the lumbar spine was most common (59%), especially in the cauda equina. Nearly one quarter of patients presented with metastasis as the initial sign of primary RCC. Half of the patients were managed surgically, five patients (28%) were treated with radiation, and the remaining four patients (22%) received a combination of the two therapies. Nearly all patients (89%) were alive at the last follow-up, with a mean overall survival of 2 years (±2.1 years).\nAppropriate patient selection for surgical intervention is essential because surgery may provide symptom relief, thus enhancing quality of life in these patients who otherwise have a dismal prognosis. Our patient reported complete resolution of his pain postoperatively and did not have evidence of disease recurrence 3 years after surgery.\nAlthough intradural spinal metastasis is an unlikely cause of upper-extremity pain, imaging is essential to identify the underlying pathology. Moreover, given this patient's history of RCC, metastatic disease should certainly be considered despite a remote history of the primary cancer. Pathologic assessment ultimately confirms the diagnosis. Patients should also be evaluated for other areas of metastasis with imaging to assess the extent of tumor spread.\nFollow Medscape on Facebook, Twitter, Instagram, and YouTube\n\n ## Figures\n\n \n*Page 3 of 3*",
"pagination": {
"current_page": 3,
"total_pages": 3
},
"questionnaire": [],
"title": "My Strangest Case: A Man With Worsening Pain in Both Arms"
}
] | [] |
933754 | /viewarticle/933754 | [
{
"authors": "Dana Goldenberg, BA; Darrin V. Bann, MD, PhD",
"content": [
"A 53-year-old man presented to us because his shirts were no longer fitting at his collar. In addition, he noted that he had weakness in his right upper arm and new-onset snoring. He reported no other symptoms.",
"His past medical and surgical history were significant for hypertension and the removal of multiple fatty tumors over the past 15 years, as well as excision of a right ankle cyst 30 years previously. A review of his family history was unremarkable. At the time, he was employed as a production worker. He had quit smoking more than a decade prior and consumed alcohol socially.",
"His current medications at the time included hydrochlorothiazide, lisinopril, loratadine, and vitamin D3 supplementation.",
"Upon presentation, the patient was well developed, well nourished, and in no acute distress. He was able to communicate without assistance and had no dyspnea, respiratory distress, stridor, or wheezing. His head was normocephalic and atraumatic, and his face had normal symmetry.",
"Palpation of the neck revealed diffuse fullness but no palpable lymphadenopathy or thyromegaly. Examination of the oral cavity demonstrated no masses or lesions. His cranial nerves were grossly intact, although he did have difficulty abducting his right arm. However, no atrophy of the trapezius was apparent. Direct fiberoptic laryngoscopy was performed and revealed some fullness of the posterior pharyngeal wall and intact vocal cord motion.",
"Although the differential diagnoses for neck masses are vast, few produce seemingly normal physical examination findings, as was the case in this patient. Furthermore, the location of a mass in his retropharyngeal space ruled out many of the more commonly occurring neck masses. Based on his workup, we could not easily determine what was causing this patient's increasing neck size and newly presenting physical restraints.",
"To determine a definitive diagnosis, we chose to obtain MRI, with and without contrast. MRI revealed a large retropharyngeal mass, extending from the level of C1 to C2 in the intervertebral space to the level of T1 in the vertebral body (Figures 1-3).",
"Figure 1.",
"Figure 2.",
"Figure 3.",
"The mass measured 9.2 x 7.7 cm in coronal dimensions and 8 x 3.1 cm in axial dimensions. It contained a predominantly large, solid, poorly enhancing soft-tissue component, as well as lipomatous components that extended from the suprahyoid to the infrahyoid neck. The MRI also discerned no pathologic lymphadenopathy by size or morphologic criteria. The findings also included a mass effect upon the existing cervical roots, no intraspinal extension of the mass, and no adenopathy."
],
"date": "July 16, 2020",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/933/754/933754-Thumb1.png"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/933/754/933754-Thumb2.png"
},
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/933/754/933754-Thumb3.png"
}
],
"markdown": "# My Strangest Case: The Patient Whose Shirts Stopped Fitting\n\n **Authors:** Dana Goldenberg, BA; Darrin V. Bann, MD, PhD \n **Date:** July 16, 2020\n\n ## Content\n\n A 53-year-old man presented to us because his shirts were no longer fitting at his collar. In addition, he noted that he had weakness in his right upper arm and new-onset snoring. He reported no other symptoms.\nHis past medical and surgical history were significant for hypertension and the removal of multiple fatty tumors over the past 15 years, as well as excision of a right ankle cyst 30 years previously. A review of his family history was unremarkable. At the time, he was employed as a production worker. He had quit smoking more than a decade prior and consumed alcohol socially.\nHis current medications at the time included hydrochlorothiazide, lisinopril, loratadine, and vitamin D3 supplementation.\nUpon presentation, the patient was well developed, well nourished, and in no acute distress. He was able to communicate without assistance and had no dyspnea, respiratory distress, stridor, or wheezing. His head was normocephalic and atraumatic, and his face had normal symmetry.\nPalpation of the neck revealed diffuse fullness but no palpable lymphadenopathy or thyromegaly. Examination of the oral cavity demonstrated no masses or lesions. His cranial nerves were grossly intact, although he did have difficulty abducting his right arm. However, no atrophy of the trapezius was apparent. Direct fiberoptic laryngoscopy was performed and revealed some fullness of the posterior pharyngeal wall and intact vocal cord motion.\nAlthough the differential diagnoses for neck masses are vast, few produce seemingly normal physical examination findings, as was the case in this patient. Furthermore, the location of a mass in his retropharyngeal space ruled out many of the more commonly occurring neck masses. Based on his workup, we could not easily determine what was causing this patient's increasing neck size and newly presenting physical restraints.\nTo determine a definitive diagnosis, we chose to obtain MRI, with and without contrast. MRI revealed a large retropharyngeal mass, extending from the level of C1 to C2 in the intervertebral space to the level of T1 in the vertebral body (Figures 1-3).\nFigure 1.\nFigure 2.\nFigure 3.\nThe mass measured 9.2 x 7.7 cm in coronal dimensions and 8 x 3.1 cm in axial dimensions. It contained a predominantly large, solid, poorly enhancing soft-tissue component, as well as lipomatous components that extended from the suprahyoid to the infrahyoid neck. The MRI also discerned no pathologic lymphadenopathy by size or morphologic criteria. The findings also included a mass effect upon the existing cervical roots, no intraspinal extension of the mass, and no adenopathy.\n\n ## Figures\n\n **Figure 1.** \n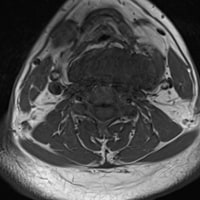 \n\n**Figure 2.** \n \n\n**Figure 3.** \n \n\n\n*Page 1 of 3*",
"pagination": {
"current_page": 1,
"total_pages": 3
},
"questionnaire": [],
"title": "My Strangest Case: The Patient Whose Shirts Stopped Fitting"
},
{
"authors": "Dana Goldenberg, BA; Darrin V. Bann, MD, PhD",
"content": [
"This case proved to be a bit of a mystery to us. Common diagnoses for large, lateral neck masses include lymphadenopathy (benign or malignant), lymphangioma, branchial cleft cyst, and benign or malignant thyroid masses.[1] The location of this patient's mass in the retropharyngeal space ruled out many of these more commonly occurring neck masses. Lesions of the retropharyngeal and prevertebral spaces include[2]:",
"Primary lesions",
"Lipoma",
"Liposarcoma",
"Synovial sarcoma",
"Direct spread",
"Nasopharyngeal carcinoma",
"Squamous cell carcinoma",
"Supraglottic",
"Oropharyngeal",
"Sinonasal",
"Lymphoma",
"Goiter",
"Chordoma",
"Primary spinal tumors",
"Nodal metastasis",
"Squamous cell carcinoma",
"Pharyngeal",
"Larynx",
"Oral cavity",
"Sinonasal",
"Non–squamous cell carcinoma",
"Lymphoma",
"Papillary thyroid carcinoma",
"Melanoma",
"Esthesioneuroblastoma",
"Other",
"Branchial cleft cyst",
"Foregut duplication cyst",
"Ectopic parathyroid adenoma",
"Nerve sheath tumors",
"Vascular malformations",
"Lymphatic malformations",
"Hemangioma",
"Leiomyoma",
"Disk bulge",
"Edema",
"Osteomyelitis",
"Abscess",
"Calcific tendinitis",
"Tortuous carotid artery",
"Considering the location of the mass and the patient's MRI results, our strongest suspicion was that his neck swelling was caused by a retropharyngeal tumor — most likely a large lipoma or liposarcoma — given his unusual presentation.",
"The retropharyngeal space extends from the skull base to the upper mediastinum. Diseases of this space are uncommon but can result in significant morbidity. As in this patient, the lesions are inaccessible to clinical inspection, and diagnosis often hinges on cross-sectional imaging.",
"Liposarcomas are a rare, malignant cancer of the fatty tissue that can occur anywhere in the body. They are most commonly found in the abdomen, thigh, and behind the knee. Liposarcomas of the thyroid are extremely rare, with only approximately 12 cases reported in the English literature.[3] Aside from the rarity of this disease, accurate diagnosis is difficult because of its nonspecific and varied clinical presentation. All patients with liposarcoma of the thyroid have a rapidly growing tumor in the neck, but other symptoms may include dysphagia, respiratory distress (including dyspnea and shortness of breath), alterations in the voice (eg, hoarseness), globus sensation, weight loss, coughing, and deafness in one or both ears.[3] Although our patient had only one of the above symptoms, none are actually required for a diagnosis of liposarcoma.",
"Like liposarcomas, lipomas are also composed of fat tissue. However, they are characterized as benign, soft-tissue masses.[1,4] They tend to be asymptomatic and occur in the head and neck region in 13% of patients. A lipoma can appear as either a sporadic, solitary lesion or as multiple lesions. Lipomas tend to be slow-growing and 2-10 cm in dimension.[4] If a lipoma is larger than 10 cm in size or weighs more than 1000 g, it is classified as giant; only then does a lipoma tend to be symptomatic and require treatment.[1,4]",
"Of note, the literature reflects that lipomas can appear with upper limb paresthesia, which was seen in this patient.[3] Although lipoma seemed to most accurately fit our patient's symptoms and presentation, liposarcoma could not be ruled out. Based on the MRI findings, our primary considerations were a neurogenic tumor, lipoma, or liposarcoma."
],
"date": "July 16, 2020",
"figures": [],
"markdown": "# My Strangest Case: The Patient Whose Shirts Stopped Fitting\n\n **Authors:** Dana Goldenberg, BA; Darrin V. Bann, MD, PhD \n **Date:** July 16, 2020\n\n ## Content\n\n This case proved to be a bit of a mystery to us. Common diagnoses for large, lateral neck masses include lymphadenopathy (benign or malignant), lymphangioma, branchial cleft cyst, and benign or malignant thyroid masses.[1] The location of this patient's mass in the retropharyngeal space ruled out many of these more commonly occurring neck masses. Lesions of the retropharyngeal and prevertebral spaces include[2]:\nPrimary lesions\nLipoma\nLiposarcoma\nSynovial sarcoma\nDirect spread\nNasopharyngeal carcinoma\nSquamous cell carcinoma\nSupraglottic\nOropharyngeal\nSinonasal\nLymphoma\nGoiter\nChordoma\nPrimary spinal tumors\nNodal metastasis\nSquamous cell carcinoma\nPharyngeal\nLarynx\nOral cavity\nSinonasal\nNon–squamous cell carcinoma\nLymphoma\nPapillary thyroid carcinoma\nMelanoma\nEsthesioneuroblastoma\nOther\nBranchial cleft cyst\nForegut duplication cyst\nEctopic parathyroid adenoma\nNerve sheath tumors\nVascular malformations\nLymphatic malformations\nHemangioma\nLeiomyoma\nDisk bulge\nEdema\nOsteomyelitis\nAbscess\nCalcific tendinitis\nTortuous carotid artery\nConsidering the location of the mass and the patient's MRI results, our strongest suspicion was that his neck swelling was caused by a retropharyngeal tumor — most likely a large lipoma or liposarcoma — given his unusual presentation.\nThe retropharyngeal space extends from the skull base to the upper mediastinum. Diseases of this space are uncommon but can result in significant morbidity. As in this patient, the lesions are inaccessible to clinical inspection, and diagnosis often hinges on cross-sectional imaging.\nLiposarcomas are a rare, malignant cancer of the fatty tissue that can occur anywhere in the body. They are most commonly found in the abdomen, thigh, and behind the knee. Liposarcomas of the thyroid are extremely rare, with only approximately 12 cases reported in the English literature.[3] Aside from the rarity of this disease, accurate diagnosis is difficult because of its nonspecific and varied clinical presentation. All patients with liposarcoma of the thyroid have a rapidly growing tumor in the neck, but other symptoms may include dysphagia, respiratory distress (including dyspnea and shortness of breath), alterations in the voice (eg, hoarseness), globus sensation, weight loss, coughing, and deafness in one or both ears.[3] Although our patient had only one of the above symptoms, none are actually required for a diagnosis of liposarcoma.\nLike liposarcomas, lipomas are also composed of fat tissue. However, they are characterized as benign, soft-tissue masses.[1,4] They tend to be asymptomatic and occur in the head and neck region in 13% of patients. A lipoma can appear as either a sporadic, solitary lesion or as multiple lesions. Lipomas tend to be slow-growing and 2-10 cm in dimension.[4] If a lipoma is larger than 10 cm in size or weighs more than 1000 g, it is classified as giant; only then does a lipoma tend to be symptomatic and require treatment.[1,4]\nOf note, the literature reflects that lipomas can appear with upper limb paresthesia, which was seen in this patient.[3] Although lipoma seemed to most accurately fit our patient's symptoms and presentation, liposarcoma could not be ruled out. Based on the MRI findings, our primary considerations were a neurogenic tumor, lipoma, or liposarcoma.\n\n ## Figures\n\n \n*Page 2 of 3*",
"pagination": {
"current_page": 2,
"total_pages": 3
},
"questionnaire": [],
"title": "My Strangest Case: The Patient Whose Shirts Stopped Fitting"
},
{
"authors": "Dana Goldenberg, BA; Darrin V. Bann, MD, PhD",
"content": [
"The patient was informed of the MRI results. Surgery was recommended based on the morphologic criteria seen. The risks and benefits of the surgery and alternatives were explained to him. The patient opted to proceed with surgical resection and was scheduled for a transcervical, possible transmandibular, and possible transoral resection of a large retropharyngeal mass, as well as a possible tracheotomy.",
"The tumor was accessed via a transcervical approach (Figures 4, 5) During surgery, the large tumor was removed from his retropharynx without complication (Figure 6).",
"Figure 4.",
"Figure 5",
"Figure 6.",
"Pathologic findings revealed a lipoma with a central focus of liposarcoma. His follow-up has been uneventful, and he continues to do well.",
"Follow Medscape on Facebook, Twitter, Instagram, and YouTube"
],
"date": "July 16, 2020",
"figures": [
{
"caption": "Figure 4.",
"image_url": "https://img.medscapestatic.com/article/933/754/933754-Thumb4.png"
},
{
"caption": "Figure 5",
"image_url": "https://img.medscapestatic.com/article/933/754/933754-Thumb5.png"
},
{
"caption": "Figure 6.",
"image_url": "https://img.medscapestatic.com/article/933/754/933754-Thumb6.png"
}
],
"markdown": "# My Strangest Case: The Patient Whose Shirts Stopped Fitting\n\n **Authors:** Dana Goldenberg, BA; Darrin V. Bann, MD, PhD \n **Date:** July 16, 2020\n\n ## Content\n\n The patient was informed of the MRI results. Surgery was recommended based on the morphologic criteria seen. The risks and benefits of the surgery and alternatives were explained to him. The patient opted to proceed with surgical resection and was scheduled for a transcervical, possible transmandibular, and possible transoral resection of a large retropharyngeal mass, as well as a possible tracheotomy.\nThe tumor was accessed via a transcervical approach (Figures 4, 5) During surgery, the large tumor was removed from his retropharynx without complication (Figure 6).\nFigure 4.\nFigure 5\nFigure 6.\nPathologic findings revealed a lipoma with a central focus of liposarcoma. His follow-up has been uneventful, and he continues to do well.\nFollow Medscape on Facebook, Twitter, Instagram, and YouTube\n\n ## Figures\n\n **Figure 4.** \n \n\n**Figure 5** \n \n\n**Figure 6.** \n \n\n\n*Page 3 of 3*",
"pagination": {
"current_page": 3,
"total_pages": 3
},
"questionnaire": [],
"title": "My Strangest Case: The Patient Whose Shirts Stopped Fitting"
}
] | [] |
932875 | /viewarticle/932875 | [
{
"authors": "Cameron Nichols, MD; Fnu Nutan, MD",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us .",
"A 69-year-old woman presents with neutropenic fever and painful skin lesions. She has a past medical history of myelodysplastic syndrome, neutropenia, chronic obstructive pulmonary disease, and cirrhosis. The skin lesions began 2 weeks prior as small nodules that progressed to painful, red plaques. At that time, they were present on her back, left breast, and right posterior calf.",
"She also experienced fevers up to 102°F (38.9°C) over the last week. She describes having shortness of breath with myalgias over the last few weeks, in addition to a more recent onset of cough, nausea, and diarrhea. She has no lesions in her mouth or her genitals and reports no vision changes.",
"Her myelodysplastic syndrome has been treated with azacitidine, which she last received 3 weeks prior. She has a right internal jugular port-a-cath, in which a needle was recently dislodged. She has not had any pain since that time."
],
"date": "June 26, 2020",
"figures": [],
"markdown": "# Skin Lesions and Neutropenic Fever in a 69-Year-Old Woman\n\n **Authors:** Cameron Nichols, MD; Fnu Nutan, MD \n **Date:** June 26, 2020\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us .\nA 69-year-old woman presents with neutropenic fever and painful skin lesions. She has a past medical history of myelodysplastic syndrome, neutropenia, chronic obstructive pulmonary disease, and cirrhosis. The skin lesions began 2 weeks prior as small nodules that progressed to painful, red plaques. At that time, they were present on her back, left breast, and right posterior calf.\nShe also experienced fevers up to 102°F (38.9°C) over the last week. She describes having shortness of breath with myalgias over the last few weeks, in addition to a more recent onset of cough, nausea, and diarrhea. She has no lesions in her mouth or her genitals and reports no vision changes.\nHer myelodysplastic syndrome has been treated with azacitidine, which she last received 3 weeks prior. She has a right internal jugular port-a-cath, in which a needle was recently dislodged. She has not had any pain since that time.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "Skin Lesions and Neutropenic Fever in a 69-Year-Old Woman"
},
{
"authors": "Cameron Nichols, MD; Fnu Nutan, MD",
"content": [
"Upon physical examination, the patient was noticeably short of breath. The site around her port was not erythematous, warm, or tender, and no drainage was observed. A 4-5 cm erythematous, edematous plaque was present on her back. It was warm and tender with some overlying crust (Figure 1).",
"Figure 1.",
"A similar plaque was seen on her right posterior calf, and her chest had erythematous and tender papules. She had a low-grade fever (100.4°F [38°C]) and tachypnea (25/min). She was pancytopenic, with a white blood cell count of 1.5 x 109/L and an absolute neutrophil count of 0.3 x 109/L. Her platelet count was 10 x 109/L. Upon admission, her hemoglobin level was 6 g/dL, and she required 2 U packed red blood cells. Chest radiograph findings were unremarkable.",
"A punch biopsy was performed on the plaque on her right calf, and the specimens were sent for histopathologic examination. A portion was sent for tissue cultures. Hematoxylin and eosin stain findings revealed mild spongiosis and acanthosis, as well as papillary dermal edema. The dermis also contained patchy perivascular inflammation, which was variably lymphoplasmacytic and neutrophilic. Abundant extravasated red blood cells were noted. Special stain results were negative for infectious agents.",
"The tissue was cultured for bacteria, fungus, acid-fast bacteria, and nocardia. No growth was observed on any of the culture plates after 1 month."
],
"date": "June 26, 2020",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/932/875/932875-Thumb1.png"
}
],
"markdown": "# Skin Lesions and Neutropenic Fever in a 69-Year-Old Woman\n\n **Authors:** Cameron Nichols, MD; Fnu Nutan, MD \n **Date:** June 26, 2020\n\n ## Content\n\n Upon physical examination, the patient was noticeably short of breath. The site around her port was not erythematous, warm, or tender, and no drainage was observed. A 4-5 cm erythematous, edematous plaque was present on her back. It was warm and tender with some overlying crust (Figure 1).\nFigure 1.\nA similar plaque was seen on her right posterior calf, and her chest had erythematous and tender papules. She had a low-grade fever (100.4°F [38°C]) and tachypnea (25/min). She was pancytopenic, with a white blood cell count of 1.5 x 109/L and an absolute neutrophil count of 0.3 x 109/L. Her platelet count was 10 x 109/L. Upon admission, her hemoglobin level was 6 g/dL, and she required 2 U packed red blood cells. Chest radiograph findings were unremarkable.\nA punch biopsy was performed on the plaque on her right calf, and the specimens were sent for histopathologic examination. A portion was sent for tissue cultures. Hematoxylin and eosin stain findings revealed mild spongiosis and acanthosis, as well as papillary dermal edema. The dermis also contained patchy perivascular inflammation, which was variably lymphoplasmacytic and neutrophilic. Abundant extravasated red blood cells were noted. Special stain results were negative for infectious agents.\nThe tissue was cultured for bacteria, fungus, acid-fast bacteria, and nocardia. No growth was observed on any of the culture plates after 1 month.\n\n ## Figures\n\n **Figure 1.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1505975,
"choiceText": "Pyoderma gangrenosum",
"correct": false,
"displayOrder": 1,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1505976,
"choiceText": "Atypical mycobacterium",
"correct": false,
"displayOrder": 2,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1505977,
"choiceText": "Sweet syndrome",
"correct": true,
"displayOrder": 3,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1505978,
"choiceText": "Deep fungal infection",
"correct": false,
"displayOrder": 4,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1505979,
"choiceText": "Leukemia cutis ",
"correct": false,
"displayOrder": 5,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "",
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": "",
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 482169,
"questionText": "Based only on these findings, which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Skin Lesions and Neutropenic Fever in a 69-Year-Old Woman"
},
{
"authors": "Cameron Nichols, MD; Fnu Nutan, MD",
"content": [
"The patient in this case had tender erythematous plaques in association with fever and myelodysplastic syndrome. The differential diagnoses included infection and Sweet syndrome. The cultures showed no growth, so the patient was diagnosed with Sweet syndrome.",
"Sweet syndrome, also known as acute febrile neutrophilic dermatosis, is an uncommon disease that most commonly affects women aged 30-60 years.[1] It belongs to a group of neutrophilic dermatoses that also includes pyoderma gangrenosum, Behçet disease, and neutrophilic dermatosis of the dorsal hands.",
"The pathogenesis of Sweet syndrome is unknown. It is most commonly associated with underlying diseases.[1] Common conditions and triggers associated with the onset of Sweet syndrome include internal malignancies (15%-30%), preceding infection (25%), causative drugs (10%), and inflammatory bowel disease. The cancers most commonly associated with Sweet syndrome are hematologic, with acute myeloid leukemia and myelodysplastic syndrome being the most frequent.",
"Sweet syndrome can have a wide variety of presentations and mimic many other common skin disorders. Fever often precedes the skin lesions. The classic presentation involves deep red, sharply demarcated plaques or nodules that appear edematous or vesiculated (Figure 1). Ulcers or bullae may be present (Figure 2).",
"Figure 1.",
"Figure 2.",
"The plaques are usually distributed asymmetrically on the face, neck, upper trunk and extremities. Mucosal involvement is not usually seen. Pathergy (disease presenting at sites of trauma or needle sticks) is common and can be seen at intravenous insertion sites or other areas of trauma. The patient in this case had a papule at the site of a previous catheter insertion site on her chest.",
"The plaques often resemble cutaneous infections, such as cellulitis, atypical mycobacterium infection, and erysipelas (Figures 2 and 3). Thus, ruling these infections out prior to initiating therapy is important.",
"Figure 3."
],
"date": "June 26, 2020",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/932/875/932875-Thumb1.png"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/932/875/932875-Thumb2.png"
},
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/932/875/932875-Thumb3.png"
}
],
"markdown": "# Skin Lesions and Neutropenic Fever in a 69-Year-Old Woman\n\n **Authors:** Cameron Nichols, MD; Fnu Nutan, MD \n **Date:** June 26, 2020\n\n ## Content\n\n The patient in this case had tender erythematous plaques in association with fever and myelodysplastic syndrome. The differential diagnoses included infection and Sweet syndrome. The cultures showed no growth, so the patient was diagnosed with Sweet syndrome.\nSweet syndrome, also known as acute febrile neutrophilic dermatosis, is an uncommon disease that most commonly affects women aged 30-60 years.[1] It belongs to a group of neutrophilic dermatoses that also includes pyoderma gangrenosum, Behçet disease, and neutrophilic dermatosis of the dorsal hands.\nThe pathogenesis of Sweet syndrome is unknown. It is most commonly associated with underlying diseases.[1] Common conditions and triggers associated with the onset of Sweet syndrome include internal malignancies (15%-30%), preceding infection (25%), causative drugs (10%), and inflammatory bowel disease. The cancers most commonly associated with Sweet syndrome are hematologic, with acute myeloid leukemia and myelodysplastic syndrome being the most frequent.\nSweet syndrome can have a wide variety of presentations and mimic many other common skin disorders. Fever often precedes the skin lesions. The classic presentation involves deep red, sharply demarcated plaques or nodules that appear edematous or vesiculated (Figure 1). Ulcers or bullae may be present (Figure 2).\nFigure 1.\nFigure 2.\nThe plaques are usually distributed asymmetrically on the face, neck, upper trunk and extremities. Mucosal involvement is not usually seen. Pathergy (disease presenting at sites of trauma or needle sticks) is common and can be seen at intravenous insertion sites or other areas of trauma. The patient in this case had a papule at the site of a previous catheter insertion site on her chest.\nThe plaques often resemble cutaneous infections, such as cellulitis, atypical mycobacterium infection, and erysipelas (Figures 2 and 3). Thus, ruling these infections out prior to initiating therapy is important.\nFigure 3.\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n**Figure 3.** \n \n\n\n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1505975,
"choiceText": "Pyoderma gangrenosum",
"correct": false,
"displayOrder": 1,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1505976,
"choiceText": "Atypical mycobacterium",
"correct": false,
"displayOrder": 2,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1505977,
"choiceText": "Sweet syndrome",
"correct": true,
"displayOrder": 3,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1505978,
"choiceText": "Deep fungal infection",
"correct": false,
"displayOrder": 4,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1505979,
"choiceText": "Leukemia cutis ",
"correct": false,
"displayOrder": 5,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "",
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": "",
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 482169,
"questionText": "Based only on these findings, which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Skin Lesions and Neutropenic Fever in a 69-Year-Old Woman"
},
{
"authors": "Cameron Nichols, MD; Fnu Nutan, MD",
"content": [
"Erythema multiforme can look similar to Sweet syndrome, especially when on the lower extremities, but erythema multiforme classically has a more targetoid appearance. Pyoderma gangrenosum should also be considered, but its features include rolled gray borders and an edge that hangs over an ulceration. Leukemia cutis can be difficult to differentiate, but it typically has a more violaceous appearance. It is best distinguished by biopsy.",
"Sweet syndrome may also have systemic manifestations. Manifestations that are more common include fever, leukocytosis, arthralgias, arthritis (favors knees and elbows), myalgias, and ocular involvement (usually conjunctivitis). Less common manifestations include renal involvement, acute myositis, hepatitis, neutrophilic alveolitis (cough, dyspnea, pleurisy), and aseptic meningitis.[1]",
"Histopathologic findings from a skin biopsy help elucidate a diagnosis of Sweet syndrome. The characteristic finding is neutrophilic infiltration without signs of vasculitis. This finding can also be seen in many infections, so obtaining tissue cultures to rule out infection is important. Histopathologic variants include histiocytoid and lymphocytic Sweet syndrome, as well as giant cellulitis-like and necrotizing fasciitis-like morphologies. Whether or not differing pathologic findings correlate with different underlying causes or disease manifestations is unclear.",
"The diagnostic criteria for Sweet syndrome require two major criteria and two of four minor criteria.[2] Major criteria include:",
"Typical skin lesions: Abrupt onset of painful edematous erythematous plaques and nodules",
"Typical histopathologic findings: Sterile collection of neutrophils without evidence of leukocytoclastic vasculitis",
"Minor criteria include:",
"Fever",
"Excellent response to steroid therapy",
"Association with cancer (especially hematologic), inflammatory disease, pregnancy, or preceding infection",
"Abnormal findings on laboratory tests, including elevated erythrocyte sedimentation rate, positive C-reactive protein level, and leukocytosis with neutrophil predominance",
"Sweet syndrome, when limited to the skin, is a benign condition that usually resolves in 5-12 weeks; however, it recurs in almost 30% of patients and can recur more commonly in patients with hematologic cancer.[1] The patient in this case had previously developed similar lesions that responded well to prednisone. This instance was a recurrence. When the associated lesions resolve, they do not leave scars."
],
"date": "June 26, 2020",
"figures": [],
"markdown": "# Skin Lesions and Neutropenic Fever in a 69-Year-Old Woman\n\n **Authors:** Cameron Nichols, MD; Fnu Nutan, MD \n **Date:** June 26, 2020\n\n ## Content\n\n Erythema multiforme can look similar to Sweet syndrome, especially when on the lower extremities, but erythema multiforme classically has a more targetoid appearance. Pyoderma gangrenosum should also be considered, but its features include rolled gray borders and an edge that hangs over an ulceration. Leukemia cutis can be difficult to differentiate, but it typically has a more violaceous appearance. It is best distinguished by biopsy.\nSweet syndrome may also have systemic manifestations. Manifestations that are more common include fever, leukocytosis, arthralgias, arthritis (favors knees and elbows), myalgias, and ocular involvement (usually conjunctivitis). Less common manifestations include renal involvement, acute myositis, hepatitis, neutrophilic alveolitis (cough, dyspnea, pleurisy), and aseptic meningitis.[1]\nHistopathologic findings from a skin biopsy help elucidate a diagnosis of Sweet syndrome. The characteristic finding is neutrophilic infiltration without signs of vasculitis. This finding can also be seen in many infections, so obtaining tissue cultures to rule out infection is important. Histopathologic variants include histiocytoid and lymphocytic Sweet syndrome, as well as giant cellulitis-like and necrotizing fasciitis-like morphologies. Whether or not differing pathologic findings correlate with different underlying causes or disease manifestations is unclear.\nThe diagnostic criteria for Sweet syndrome require two major criteria and two of four minor criteria.[2] Major criteria include:\nTypical skin lesions: Abrupt onset of painful edematous erythematous plaques and nodules\nTypical histopathologic findings: Sterile collection of neutrophils without evidence of leukocytoclastic vasculitis\nMinor criteria include:\nFever\nExcellent response to steroid therapy\nAssociation with cancer (especially hematologic), inflammatory disease, pregnancy, or preceding infection\nAbnormal findings on laboratory tests, including elevated erythrocyte sedimentation rate, positive C-reactive protein level, and leukocytosis with neutrophil predominance\nSweet syndrome, when limited to the skin, is a benign condition that usually resolves in 5-12 weeks; however, it recurs in almost 30% of patients and can recur more commonly in patients with hematologic cancer.[1] The patient in this case had previously developed similar lesions that responded well to prednisone. This instance was a recurrence. When the associated lesions resolve, they do not leave scars.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "Skin Lesions and Neutropenic Fever in a 69-Year-Old Woman"
},
{
"authors": "Cameron Nichols, MD; Fnu Nutan, MD",
"content": [
"The most effective treatment for Sweet syndrome is oral prednisone (0.5-1 mg/kg/day), tapered over a 4- to 6-week period.[3] In patients who have had recurrence, tapering prednisone over 2-3 months may be necessary. Higher doses can be used for systemic involvement or severe disease.",
"Antibiotics are not effective for this syndrome, but ruling out infection is important because Sweet syndrome can look very similar. Many patients with Sweet syndrome are immunosuppressed secondary to hematologic cancer or treatments for their cancer, which adds to the importance of ruling out infection. Some alternative therapies that have been used include topical corticosteroids (for limited disease), oral dapsone, colchicine, and potassium iodide.[1]",
"Drug-induced Sweet syndrome can appear similar to classic Sweet syndrome; however, a temporal relationship with initiation of medication is required. The most commonly implicated drugs include granulocyte-colony stimulating factors, azathioprine, and all-trans retinoic acid.[4] Lesions typically resolve after drug withdrawal.",
"The patient in this case started taking oral prednisone (1 mg/kg), with plans for a 4-6 week taper. After about 3 weeks of treatment, she demonstrated significant improvement. The dose was gradually tapered."
],
"date": "June 26, 2020",
"figures": [],
"markdown": "# Skin Lesions and Neutropenic Fever in a 69-Year-Old Woman\n\n **Authors:** Cameron Nichols, MD; Fnu Nutan, MD \n **Date:** June 26, 2020\n\n ## Content\n\n The most effective treatment for Sweet syndrome is oral prednisone (0.5-1 mg/kg/day), tapered over a 4- to 6-week period.[3] In patients who have had recurrence, tapering prednisone over 2-3 months may be necessary. Higher doses can be used for systemic involvement or severe disease.\nAntibiotics are not effective for this syndrome, but ruling out infection is important because Sweet syndrome can look very similar. Many patients with Sweet syndrome are immunosuppressed secondary to hematologic cancer or treatments for their cancer, which adds to the importance of ruling out infection. Some alternative therapies that have been used include topical corticosteroids (for limited disease), oral dapsone, colchicine, and potassium iodide.[1]\nDrug-induced Sweet syndrome can appear similar to classic Sweet syndrome; however, a temporal relationship with initiation of medication is required. The most commonly implicated drugs include granulocyte-colony stimulating factors, azathioprine, and all-trans retinoic acid.[4] Lesions typically resolve after drug withdrawal.\nThe patient in this case started taking oral prednisone (1 mg/kg), with plans for a 4-6 week taper. After about 3 weeks of treatment, she demonstrated significant improvement. The dose was gradually tapered.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1505988,
"choiceText": "Abrupt onset of painful erythematous plaques or nodules",
"correct": true,
"displayOrder": 1,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1505989,
"choiceText": "History of acute myeloid leukemia",
"correct": false,
"displayOrder": 2,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1505990,
"choiceText": "Response to oral prednisone",
"correct": false,
"displayOrder": 3,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1505991,
"choiceText": "Elevated erythrocyte sedimentation rate",
"correct": false,
"displayOrder": 4,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "\r\nThe two major criteria for Sweet syndrome are abrupt onset of painful erythematous plaques/nodules and histopathologic evidence of sterile neutrophilic infiltrate without signs of vasculitis. The other answer choices are all minor diagnostic criteria. ",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": "",
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 482181,
"questionText": "Which of the following is considered a major diagnostic criteria for Sweet syndrome?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1505997,
"choiceText": "Antibiotics ",
"correct": false,
"displayOrder": 1,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1505998,
"choiceText": "Antifungal agents",
"correct": false,
"displayOrder": 2,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1505999,
"choiceText": "Oral prednisone",
"correct": true,
"displayOrder": 3,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1506000,
"choiceText": "Methotrexate",
"correct": false,
"displayOrder": 4,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Sweet syndrome is not an infectious process, so antimicrobial agents are not effective. Oral prednisone (approximately 1 mg/kg) has been shown to be the most effective treatment, followed by a 4-6 week taper.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": "",
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 482185,
"questionText": "Which of the following is the most appropriate treatment for Sweet syndrome?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Skin Lesions and Neutropenic Fever in a 69-Year-Old Woman"
},
{
"authors": "Cameron Nichols, MD; Fnu Nutan, MD",
"content": [],
"date": "June 26, 2020",
"figures": [],
"markdown": "# Skin Lesions and Neutropenic Fever in a 69-Year-Old Woman\n\n **Authors:** Cameron Nichols, MD; Fnu Nutan, MD \n **Date:** June 26, 2020\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1505988,
"choiceText": "Abrupt onset of painful erythematous plaques or nodules",
"correct": true,
"displayOrder": 1,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1505989,
"choiceText": "History of acute myeloid leukemia",
"correct": false,
"displayOrder": 2,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1505990,
"choiceText": "Response to oral prednisone",
"correct": false,
"displayOrder": 3,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1505991,
"choiceText": "Elevated erythrocyte sedimentation rate",
"correct": false,
"displayOrder": 4,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "\r\nThe two major criteria for Sweet syndrome are abrupt onset of painful erythematous plaques/nodules and histopathologic evidence of sterile neutrophilic infiltrate without signs of vasculitis. The other answer choices are all minor diagnostic criteria. ",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": "",
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 482181,
"questionText": "Which of the following is considered a major diagnostic criteria for Sweet syndrome?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1505997,
"choiceText": "Antibiotics ",
"correct": false,
"displayOrder": 1,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1505998,
"choiceText": "Antifungal agents",
"correct": false,
"displayOrder": 2,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1505999,
"choiceText": "Oral prednisone",
"correct": true,
"displayOrder": 3,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1506000,
"choiceText": "Methotrexate",
"correct": false,
"displayOrder": 4,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Sweet syndrome is not an infectious process, so antimicrobial agents are not effective. Oral prednisone (approximately 1 mg/kg) has been shown to be the most effective treatment, followed by a 4-6 week taper.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": "",
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 482185,
"questionText": "Which of the following is the most appropriate treatment for Sweet syndrome?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Skin Lesions and Neutropenic Fever in a 69-Year-Old Woman"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1505975,
"choiceText": "Pyoderma gangrenosum",
"correct": false,
"displayOrder": 1,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1505976,
"choiceText": "Atypical mycobacterium",
"correct": false,
"displayOrder": 2,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1505977,
"choiceText": "Sweet syndrome",
"correct": true,
"displayOrder": 3,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1505978,
"choiceText": "Deep fungal infection",
"correct": false,
"displayOrder": 4,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1505979,
"choiceText": "Leukemia cutis ",
"correct": false,
"displayOrder": 5,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "",
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": "",
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 482169,
"questionText": "Based only on these findings, which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1505988,
"choiceText": "Abrupt onset of painful erythematous plaques or nodules",
"correct": true,
"displayOrder": 1,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1505989,
"choiceText": "History of acute myeloid leukemia",
"correct": false,
"displayOrder": 2,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1505990,
"choiceText": "Response to oral prednisone",
"correct": false,
"displayOrder": 3,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1505991,
"choiceText": "Elevated erythrocyte sedimentation rate",
"correct": false,
"displayOrder": 4,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "\r\nThe two major criteria for Sweet syndrome are abrupt onset of painful erythematous plaques/nodules and histopathologic evidence of sterile neutrophilic infiltrate without signs of vasculitis. The other answer choices are all minor diagnostic criteria. ",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": "",
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 482181,
"questionText": "Which of the following is considered a major diagnostic criteria for Sweet syndrome?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1505997,
"choiceText": "Antibiotics ",
"correct": false,
"displayOrder": 1,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1505998,
"choiceText": "Antifungal agents",
"correct": false,
"displayOrder": 2,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1505999,
"choiceText": "Oral prednisone",
"correct": true,
"displayOrder": 3,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1506000,
"choiceText": "Methotrexate",
"correct": false,
"displayOrder": 4,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Sweet syndrome is not an infectious process, so antimicrobial agents are not effective. Oral prednisone (approximately 1 mg/kg) has been shown to be the most effective treatment, followed by a 4-6 week taper.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": "",
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 482185,
"questionText": "Which of the following is the most appropriate treatment for Sweet syndrome?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
931962 | /viewarticle/931962 | [
{
"authors": "Vikramjit S. Kanwar, MBA, MRCP(UK)",
"content": [
"A 3.5-month-old female infant was referred to me by her pediatrician, after her parents noticed lumps in her belly while bathing her. The infant was a born after a full-term, normal vaginal delivery. She was 8.4 lb (2.8 kg) at birth, with no immediate postnatal issues. She was breast-feeding well and had well-formed soft stools. However, both parents felt that she was significantly smaller than her 3-year-old sister had been at the same age.",
"The infant had been diagnosed twice with ear infections at an urgent care center after inconsolable crying. In each case, her symptoms resolved after administration of an oral antibiotic. The infant had acquired a social smile, gurgled and cooed, and happily kicked and moved all limbs. However, her mother had recently noticed intermittent \"jerky\" eye movements and felt that she was not tracking and following movements as well as she used to.",
"She had no fevers, rashes, diarrhea, or any other symptoms of note. The parents were white and from the Midwest. They also had a healthy 3-year-old daughter, with no other siblings or history of stillbirths. The family had no significant history of cancer, blood disorders, or neurologic disorders.",
"Upon examination, the infant appeared small but not emaciated. Her length was 21.25 inches (54 cm), which put her in the lower third percentile. Her weight was 9 lb (4.1 kg), which also put her in the lower third percentile. Her head circumference was 15.75 inches (40 cm), which placed her in the 50th percentile.",
"She had moderate pallor, but no bruising or petechiae. Her oral mucosa were normal, with no ulceration or patches and no rashes or skin nodules. Her anterior fontanelle was 0.98 inch (2.5 cm) and flat. Intermittent nystagmus was noted. The child seemed to have difficulty in tracking and following a bright object. Red reflex was present bilaterally; however, it was not possible to perform fundoscopy.",
"Her remaining neurologic examination findings were normal. She evenly kicked with both legs, brought her hands to midline, and had minimal head lag on dorsal suspension. No palpable adenopathy was noted in her neck, axillae, or groin. Her chest was clear, with no distress. Cardiovascular examination revealed normal heart sounds, with a benign ejection systolic murmur. Palpation of the abdomen was significant for marked hepatomegaly (edge 1.57 inches [4 cm] below the right costal margin) and splenomegaly (edge 1.97 inches [5 cm] below the left costal margin).",
"Her hemoglobin level was 7.8 g/dL. Her total white blood cell count was 28,900 cells/µL, with a differential count of 31% polymorphonuclear cells, 57% lymphocytes, 3% eosinophils, and 9% monocytes; no leukemic blasts were observed on the smear. Her platelet count was 40,000 cells/µL. Liver test and renal function test results were within normal limits. Abdominal ultrasonography confirmed hepatosplenomegaly, but no other masses were seen.",
"At the end of the day, no clear-cut diagnosis was apparent. Although congenital infections, such as cytomegalovirus (CMV) or HIV, could account for some findings, the history simply didn't fit. The child was well at birth and only now developing problems. The degree of hepatosplenomegaly was also unusual. If that was due to CMV, her liver enzyme levels should have been elevated. However, because viral screening tests were easy to obtain, they were pursued, with a follow-up appointment scheduled in 3 days.",
"Other causes, such as storage disorders, a primary immune deficiency, or cancer, would be harder to exclude. We were clearly dealing with a rare condition, but what was it?"
],
"date": "June 10, 2020",
"figures": [],
"markdown": "# My Strangest Case: An Infant With Lumps in Her Belly\n\n **Authors:** Vikramjit S. Kanwar, MBA, MRCP(UK) \n **Date:** June 10, 2020\n\n ## Content\n\n A 3.5-month-old female infant was referred to me by her pediatrician, after her parents noticed lumps in her belly while bathing her. The infant was a born after a full-term, normal vaginal delivery. She was 8.4 lb (2.8 kg) at birth, with no immediate postnatal issues. She was breast-feeding well and had well-formed soft stools. However, both parents felt that she was significantly smaller than her 3-year-old sister had been at the same age.\nThe infant had been diagnosed twice with ear infections at an urgent care center after inconsolable crying. In each case, her symptoms resolved after administration of an oral antibiotic. The infant had acquired a social smile, gurgled and cooed, and happily kicked and moved all limbs. However, her mother had recently noticed intermittent \"jerky\" eye movements and felt that she was not tracking and following movements as well as she used to.\nShe had no fevers, rashes, diarrhea, or any other symptoms of note. The parents were white and from the Midwest. They also had a healthy 3-year-old daughter, with no other siblings or history of stillbirths. The family had no significant history of cancer, blood disorders, or neurologic disorders.\nUpon examination, the infant appeared small but not emaciated. Her length was 21.25 inches (54 cm), which put her in the lower third percentile. Her weight was 9 lb (4.1 kg), which also put her in the lower third percentile. Her head circumference was 15.75 inches (40 cm), which placed her in the 50th percentile.\nShe had moderate pallor, but no bruising or petechiae. Her oral mucosa were normal, with no ulceration or patches and no rashes or skin nodules. Her anterior fontanelle was 0.98 inch (2.5 cm) and flat. Intermittent nystagmus was noted. The child seemed to have difficulty in tracking and following a bright object. Red reflex was present bilaterally; however, it was not possible to perform fundoscopy.\nHer remaining neurologic examination findings were normal. She evenly kicked with both legs, brought her hands to midline, and had minimal head lag on dorsal suspension. No palpable adenopathy was noted in her neck, axillae, or groin. Her chest was clear, with no distress. Cardiovascular examination revealed normal heart sounds, with a benign ejection systolic murmur. Palpation of the abdomen was significant for marked hepatomegaly (edge 1.57 inches [4 cm] below the right costal margin) and splenomegaly (edge 1.97 inches [5 cm] below the left costal margin).\nHer hemoglobin level was 7.8 g/dL. Her total white blood cell count was 28,900 cells/µL, with a differential count of 31% polymorphonuclear cells, 57% lymphocytes, 3% eosinophils, and 9% monocytes; no leukemic blasts were observed on the smear. Her platelet count was 40,000 cells/µL. Liver test and renal function test results were within normal limits. Abdominal ultrasonography confirmed hepatosplenomegaly, but no other masses were seen.\nAt the end of the day, no clear-cut diagnosis was apparent. Although congenital infections, such as cytomegalovirus (CMV) or HIV, could account for some findings, the history simply didn't fit. The child was well at birth and only now developing problems. The degree of hepatosplenomegaly was also unusual. If that was due to CMV, her liver enzyme levels should have been elevated. However, because viral screening tests were easy to obtain, they were pursued, with a follow-up appointment scheduled in 3 days.\nOther causes, such as storage disorders, a primary immune deficiency, or cancer, would be harder to exclude. We were clearly dealing with a rare condition, but what was it?\n\n ## Figures\n\n \n*Page 1 of 3*",
"pagination": {
"current_page": 1,
"total_pages": 3
},
"questionnaire": [],
"title": "My Strangest Case: An Infant With Lumps in Her Belly"
},
{
"authors": "Vikramjit S. Kanwar, MBA, MRCP(UK)",
"content": [
"Reviewing the case, we had an otherwise well-looking infant with growth retardation, blood count changes, significant hepatosplenomegaly, and nystagmus. Because we are not at a major academic center, our access to pediatric subspecialists was limited. We were keen to identify a tentative diagnosis to avoid shuttling the family to different clinicians. The list of differential diagnoses was daunting, and none of them really seemed to fit.",
"Infantile forms of Gaucher disease (Gaucher type 2) can result in hepatosplenomegaly with progressive global neurologic deficits and pancytopenia.[1]Niemann-Pick disease type A in infants can be similar but lacks blood count changes.[2] Glycogen storage disorders can cause hepatomegaly and can occasionally result in neurologic damage due to hypoglycemia, neutropenia, and platelet dysfunction; however, splenomegaly, anemia, and thrombocytopenia are not usually seen.[3]",
"Subacute combined immunodeficiency (SCIDS) is the best-known condition in which infants often look sick, with chronic diarrhea, respiratory symptoms, and repeated infections (commonly viral or fungal).[4] Although hepatosplenomegaly is uncommon, it is frequent in Omenn syndrome, a SCIDS variant accompanied by eosinophilia and rashes, and Duncan syndrome, an X-linked lymphoproliferative syndrome with findings of fulminant Epstein-Barr virus infection.[5] Familial hemophagocytic lymphohistiocytosis is a hyperinflammatory disorder seen in infancy, with splenomegaly and cytopenias; however, it is predominantly associated with fever and toxicity due to marked cytokine release.[6]",
"Infant leukemia is characterized by markedly elevated white blood cell counts, often 400,000-500,000 cells/µL or higher, with numerous leukemic blasts in the blood.[7] Stage 4S infantile neuroblastoma can present with hepatomegaly and \"dancing eyes\" (opsoclonus-myoclonus); however, it is not associated with nystagmus, massive splenomegaly, or thrombocytopenia.[8]Non-Hodgkin lymphoma is rare in infants; blood counts are not usually affected, and nystagmus is not a feature.[9]",
"Faced with a diagnostic dilemma, we revisited each element of the history, physical examination, and testing to identify the underlying pathogenesis. Reviewing the peripheral blood smear provided the key that unlocked this puzzle."
],
"date": "June 10, 2020",
"figures": [],
"markdown": "# My Strangest Case: An Infant With Lumps in Her Belly\n\n **Authors:** Vikramjit S. Kanwar, MBA, MRCP(UK) \n **Date:** June 10, 2020\n\n ## Content\n\n Reviewing the case, we had an otherwise well-looking infant with growth retardation, blood count changes, significant hepatosplenomegaly, and nystagmus. Because we are not at a major academic center, our access to pediatric subspecialists was limited. We were keen to identify a tentative diagnosis to avoid shuttling the family to different clinicians. The list of differential diagnoses was daunting, and none of them really seemed to fit.\nInfantile forms of Gaucher disease (Gaucher type 2) can result in hepatosplenomegaly with progressive global neurologic deficits and pancytopenia.[1]Niemann-Pick disease type A in infants can be similar but lacks blood count changes.[2] Glycogen storage disorders can cause hepatomegaly and can occasionally result in neurologic damage due to hypoglycemia, neutropenia, and platelet dysfunction; however, splenomegaly, anemia, and thrombocytopenia are not usually seen.[3]\nSubacute combined immunodeficiency (SCIDS) is the best-known condition in which infants often look sick, with chronic diarrhea, respiratory symptoms, and repeated infections (commonly viral or fungal).[4] Although hepatosplenomegaly is uncommon, it is frequent in Omenn syndrome, a SCIDS variant accompanied by eosinophilia and rashes, and Duncan syndrome, an X-linked lymphoproliferative syndrome with findings of fulminant Epstein-Barr virus infection.[5] Familial hemophagocytic lymphohistiocytosis is a hyperinflammatory disorder seen in infancy, with splenomegaly and cytopenias; however, it is predominantly associated with fever and toxicity due to marked cytokine release.[6]\nInfant leukemia is characterized by markedly elevated white blood cell counts, often 400,000-500,000 cells/µL or higher, with numerous leukemic blasts in the blood.[7] Stage 4S infantile neuroblastoma can present with hepatomegaly and \"dancing eyes\" (opsoclonus-myoclonus); however, it is not associated with nystagmus, massive splenomegaly, or thrombocytopenia.[8]Non-Hodgkin lymphoma is rare in infants; blood counts are not usually affected, and nystagmus is not a feature.[9]\nFaced with a diagnostic dilemma, we revisited each element of the history, physical examination, and testing to identify the underlying pathogenesis. Reviewing the peripheral blood smear provided the key that unlocked this puzzle.\n\n ## Figures\n\n \n*Page 2 of 3*",
"pagination": {
"current_page": 2,
"total_pages": 3
},
"questionnaire": [],
"title": "My Strangest Case: An Infant With Lumps in Her Belly"
},
{
"authors": "Vikramjit S. Kanwar, MBA, MRCP(UK)",
"content": [
"The laboratory had correctly reported that there were no leukemic blasts. However, the technician had failed to recognize the significance of the scattered immature precursors of red cell and white cell lineage, with occasional giant platelets and red cell anisocytosis that were seen. It was a \"leukoerythroblastic\" blood picture, which is the hallmark of extramedullary hematopoiesis.[10]",
"In adults, this is seen in primary myelofibrosis, in which the marrow is obliterated and the liver and spleen become the sites for blood cell production. This is extremely rare in childhood, and nystagmus is not a reported finding.[11] Nevertheless, we felt confident that the hepatosplenomegaly and deranged blood counts represented extramedullary hematopoiesis. We had excluded all other differential diagnoses.",
"The next step was a bone marrow biopsy. However, before calling the family to schedule such an invasive procedure, it seemed prudent for me to \"phone a friend\" at a large hematology-oncology center and seek her opinion.",
"\"Failure to thrive, hepatosplenomegaly, abnormal eye movements, and leukoerythroblastic peripheral blood smear? That sounds like a case of malignant infantile osteopetrosis,\" was her response. She told me not to bother with a bone marrow biopsy and that the constellation of findings was pathognomonic.[12] She recommended plain skull or chest radiography for \"insurance purposes.\" An example of such imaging is shown below.",
"She also told me that early hematopoietic stem cell treatment (HSCT) is the treatment of choice, and the 5-year survival is almost 80%.[13] As soon as I got off the phone with my colleague, we brought the family back in for counseling and discussion about the child's diagnosis and treatment. They were tearful but grateful that they now had a diagnosis and course of action.",
"The child later underwent an alternate-donor allogeneic HSCT under the care of my colleague. She recovered some of her vision and all of her growth, hearing, and speech with the help of intensive rehabilitation. Today, she is a confident and healthy young woman.",
"Osteopetrosis—from the Greek osteo, meaning \"bone,\" and petros, meaning \"stone\"—is a rare genetic disorder that is characterized by increased bone density on plain radiography, which is diagnostic.[14] The autosomal dominant variant is 10 times more common and is usually mild or asymptomatic. In contrast, the autosomal recessive variant, known as \"malignant infantile osteopetrosis,\" is extremely rare. It has an incidence of 1 in 250,000 population and presents in the first few months of life. Sometimes, it is diagnosed in utero, and it is universally fatal by school age if undiagnosed and untreated.[15]",
"Impairment of osteoclast function, failure of bone remodeling, and disordered bone growth are the key underlying causes. At a molecular level, mutations in TCIRG1 and CLCN7 account for 70% of cases, impairing the ion pump mechanisms that osteoclasts need to create the acidic environment needed for bone resorption[16]; however, in more than 20% of cases, the gene defect remains unidentified. This leads to numerous symptoms, including narrowing of the intramedullary space that impinges on the bone marrow, with compensatory extramedullary hematopoiesis that manifests as hepatosplenomegaly and leukoerythroblastic anemia.",
"Narrowing of skull foramina impinges on the optic nerves and leads to visual impairment, which is a frequent early finding, as well as hearing loss, cranial neuropathies, and obstructive hydrocephalus.[16] Failure to thrive, dental problems, pathologic fractures, osteomyelitis of the mandible, and ear infections are all reported.[17] Patients whose disease goes undiagnosed develop macrocephaly and frontal bossing in the first year of life. Some newborns develop hypocalcemia, with attendant tetanic seizures and secondary hyperparathyroidism.[17]",
"The diagnosis is easily confirmed with skeletal radiography that show diffuse bone sclerosis.[15] CT can also document the narrowing of the optic and auditory canals. Genetic testing does not play a significant role in diagnosis.[16] Osteoclasts are derived from hematopoietic cells, and HSCT is curative for malignant infantile osteopetrosis; however, some complications are common, such as graft failure, pulmonary hypertension, and interstitial fibrosis.[13] Although medical therapies, such as calcitriol and gamma interferon, may palliate the patient for a prolonged duration, they are not curative.[14]",
"In summary, the old adage held true: A careful history, physical examination, and review of tests yielded an accurate diagnosis. For hematologists, review includes the peripheral blood smear, which should always be part of the routine. When confronted with a confusing case, going back to first principles and using the information you have to map out the disease process can help move you forward. Complex or invasive testing should only be used to confirm a diagnosis that is suspected, rather than as a screen. Also, because \"rare things occur rarely,\" you may reduce unnecessary anxiety, tests, and referrals by reaching out to a more experienced colleague, as I did in this case.",
"Follow Medscape on Facebook, Twitter, Instagram, and YouTube"
],
"date": "June 10, 2020",
"figures": [],
"markdown": "# My Strangest Case: An Infant With Lumps in Her Belly\n\n **Authors:** Vikramjit S. Kanwar, MBA, MRCP(UK) \n **Date:** June 10, 2020\n\n ## Content\n\n The laboratory had correctly reported that there were no leukemic blasts. However, the technician had failed to recognize the significance of the scattered immature precursors of red cell and white cell lineage, with occasional giant platelets and red cell anisocytosis that were seen. It was a \"leukoerythroblastic\" blood picture, which is the hallmark of extramedullary hematopoiesis.[10]\nIn adults, this is seen in primary myelofibrosis, in which the marrow is obliterated and the liver and spleen become the sites for blood cell production. This is extremely rare in childhood, and nystagmus is not a reported finding.[11] Nevertheless, we felt confident that the hepatosplenomegaly and deranged blood counts represented extramedullary hematopoiesis. We had excluded all other differential diagnoses.\nThe next step was a bone marrow biopsy. However, before calling the family to schedule such an invasive procedure, it seemed prudent for me to \"phone a friend\" at a large hematology-oncology center and seek her opinion.\n\"Failure to thrive, hepatosplenomegaly, abnormal eye movements, and leukoerythroblastic peripheral blood smear? That sounds like a case of malignant infantile osteopetrosis,\" was her response. She told me not to bother with a bone marrow biopsy and that the constellation of findings was pathognomonic.[12] She recommended plain skull or chest radiography for \"insurance purposes.\" An example of such imaging is shown below.\nShe also told me that early hematopoietic stem cell treatment (HSCT) is the treatment of choice, and the 5-year survival is almost 80%.[13] As soon as I got off the phone with my colleague, we brought the family back in for counseling and discussion about the child's diagnosis and treatment. They were tearful but grateful that they now had a diagnosis and course of action.\nThe child later underwent an alternate-donor allogeneic HSCT under the care of my colleague. She recovered some of her vision and all of her growth, hearing, and speech with the help of intensive rehabilitation. Today, she is a confident and healthy young woman.\nOsteopetrosis—from the Greek osteo, meaning \"bone,\" and petros, meaning \"stone\"—is a rare genetic disorder that is characterized by increased bone density on plain radiography, which is diagnostic.[14] The autosomal dominant variant is 10 times more common and is usually mild or asymptomatic. In contrast, the autosomal recessive variant, known as \"malignant infantile osteopetrosis,\" is extremely rare. It has an incidence of 1 in 250,000 population and presents in the first few months of life. Sometimes, it is diagnosed in utero, and it is universally fatal by school age if undiagnosed and untreated.[15]\nImpairment of osteoclast function, failure of bone remodeling, and disordered bone growth are the key underlying causes. At a molecular level, mutations in TCIRG1 and CLCN7 account for 70% of cases, impairing the ion pump mechanisms that osteoclasts need to create the acidic environment needed for bone resorption[16]; however, in more than 20% of cases, the gene defect remains unidentified. This leads to numerous symptoms, including narrowing of the intramedullary space that impinges on the bone marrow, with compensatory extramedullary hematopoiesis that manifests as hepatosplenomegaly and leukoerythroblastic anemia.\nNarrowing of skull foramina impinges on the optic nerves and leads to visual impairment, which is a frequent early finding, as well as hearing loss, cranial neuropathies, and obstructive hydrocephalus.[16] Failure to thrive, dental problems, pathologic fractures, osteomyelitis of the mandible, and ear infections are all reported.[17] Patients whose disease goes undiagnosed develop macrocephaly and frontal bossing in the first year of life. Some newborns develop hypocalcemia, with attendant tetanic seizures and secondary hyperparathyroidism.[17]\nThe diagnosis is easily confirmed with skeletal radiography that show diffuse bone sclerosis.[15] CT can also document the narrowing of the optic and auditory canals. Genetic testing does not play a significant role in diagnosis.[16] Osteoclasts are derived from hematopoietic cells, and HSCT is curative for malignant infantile osteopetrosis; however, some complications are common, such as graft failure, pulmonary hypertension, and interstitial fibrosis.[13] Although medical therapies, such as calcitriol and gamma interferon, may palliate the patient for a prolonged duration, they are not curative.[14]\nIn summary, the old adage held true: A careful history, physical examination, and review of tests yielded an accurate diagnosis. For hematologists, review includes the peripheral blood smear, which should always be part of the routine. When confronted with a confusing case, going back to first principles and using the information you have to map out the disease process can help move you forward. Complex or invasive testing should only be used to confirm a diagnosis that is suspected, rather than as a screen. Also, because \"rare things occur rarely,\" you may reduce unnecessary anxiety, tests, and referrals by reaching out to a more experienced colleague, as I did in this case.\nFollow Medscape on Facebook, Twitter, Instagram, and YouTube\n\n ## Figures\n\n \n*Page 3 of 3*",
"pagination": {
"current_page": 3,
"total_pages": 3
},
"questionnaire": [],
"title": "My Strangest Case: An Infant With Lumps in Her Belly"
}
] | [] |
844659 | /viewarticle/844659 | [
{
"authors": "Afif Harb, MD; Antoine Saliba, MD",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"A 5-year-old girl presents to the emergency department with a severe headache and projectile vomiting for the past 4 hours. Her mother denies any history of trauma to the head or any recent infection or vaccination, and she states that her daughter did not have a fever or chills. Her mother also reports no diarrhea or signs of abdominal pain accompanying the vomiting episodes. She expresses concern that her daughter is barely using her left arm and seems to have developed a limp in her gait recently, mainly supporting herself with her right leg.",
"The girl's parents are from Kenya. She is the product of a full-term pregnancy and was delivered vaginally, with a negative prenatal and neonatal history. She has been followed by a pediatrician since her delivery and has displayed a normal growth pattern. She has met all developmental milestones with no delays. All vaccinations are up to date.",
"The patient's mother describes pallor ever since infancy. The girl has had anemia since age 3 years that is being treated with iron supplements and vitamins on an ongoing basis. The patient also had recurrent pain attacks that were treated at home with analgesics. Her mother recalls that her daughter once had swollen, painful hands at age 18 months.",
"When asked about the family history, the mother reports that she and her husband are unrelated. She states that the patient's 6-month-old brother died of sudden infant death syndrome. In addition, one of the mother's cousins has a transfusion-dependent anemia."
],
"date": "June 10, 2020",
"figures": [],
"markdown": "# A 5-Year-Old With a Headache, Limp, and Projectile Vomiting\n\n **Authors:** Afif Harb, MD; Antoine Saliba, MD \n **Date:** June 10, 2020\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nA 5-year-old girl presents to the emergency department with a severe headache and projectile vomiting for the past 4 hours. Her mother denies any history of trauma to the head or any recent infection or vaccination, and she states that her daughter did not have a fever or chills. Her mother also reports no diarrhea or signs of abdominal pain accompanying the vomiting episodes. She expresses concern that her daughter is barely using her left arm and seems to have developed a limp in her gait recently, mainly supporting herself with her right leg.\nThe girl's parents are from Kenya. She is the product of a full-term pregnancy and was delivered vaginally, with a negative prenatal and neonatal history. She has been followed by a pediatrician since her delivery and has displayed a normal growth pattern. She has met all developmental milestones with no delays. All vaccinations are up to date.\nThe patient's mother describes pallor ever since infancy. The girl has had anemia since age 3 years that is being treated with iron supplements and vitamins on an ongoing basis. The patient also had recurrent pain attacks that were treated at home with analgesics. Her mother recalls that her daughter once had swollen, painful hands at age 18 months.\nWhen asked about the family history, the mother reports that she and her husband are unrelated. She states that the patient's 6-month-old brother died of sudden infant death syndrome. In addition, one of the mother's cousins has a transfusion-dependent anemia.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 5-Year-Old With a Headache, Limp, and Projectile Vomiting"
},
{
"authors": "Afif Harb, MD; Antoine Saliba, MD",
"content": [
"The patient appears pale and icteric. She displays stupor alternating with irritability. Her heart rate is 136 beats/min, blood pressure is 103/62 mm Hg, temperature is 98.06°F (36.7°C), and respiratory rate is 16 breaths/min.",
"Upon heart examination, regular heart sounds are noted (S1, S2). A grade 2/6 systolic murmur is heard all over the precordium.",
"The abdomen is soft and nontender, and bowel sounds are positive. The spleen is palpated 3 cm below the costal margin.",
"The patient's cranial nerves are intact. She has normal power and sensation over the right side (upper and lower limbs). She has left-sided hemiparesis and hemianesthesia (upper and lower limbs).",
"Laboratory findings are as follows:",
"Hemoglobin level: 6.5 g/dL",
"White blood cell count: 24,000 cells/mcL",
"Platelet count: 500,000 cells/mcL",
"Prothrombin time/partial thromboplastin time: Normal",
"Routine chemistry: Normal",
"Results of blood smear examination, high-performance liquid chromatography, and brain CT scan are shown in Figures 1-3.",
"Figure 1.",
"Figure 2.",
"Figure 3."
],
"date": "June 10, 2020",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/844/659/844659-thumb-1.jpg"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/844/659/844659-thumb-2.jpg"
},
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/844/659/844659-thumb-3.jpg"
}
],
"markdown": "# A 5-Year-Old With a Headache, Limp, and Projectile Vomiting\n\n **Authors:** Afif Harb, MD; Antoine Saliba, MD \n **Date:** June 10, 2020\n\n ## Content\n\n The patient appears pale and icteric. She displays stupor alternating with irritability. Her heart rate is 136 beats/min, blood pressure is 103/62 mm Hg, temperature is 98.06°F (36.7°C), and respiratory rate is 16 breaths/min.\nUpon heart examination, regular heart sounds are noted (S1, S2). A grade 2/6 systolic murmur is heard all over the precordium.\nThe abdomen is soft and nontender, and bowel sounds are positive. The spleen is palpated 3 cm below the costal margin.\nThe patient's cranial nerves are intact. She has normal power and sensation over the right side (upper and lower limbs). She has left-sided hemiparesis and hemianesthesia (upper and lower limbs).\nLaboratory findings are as follows:\nHemoglobin level: 6.5 g/dL\nWhite blood cell count: 24,000 cells/mcL\nPlatelet count: 500,000 cells/mcL\nProthrombin time/partial thromboplastin time: Normal\nRoutine chemistry: Normal\nResults of blood smear examination, high-performance liquid chromatography, and brain CT scan are shown in Figures 1-3.\nFigure 1.\nFigure 2.\nFigure 3.\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n**Figure 3.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 844363,
"choiceText": "Meningitis",
"correct": false,
"displayOrder": 1,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 844365,
"choiceText": "Brain tumor",
"correct": false,
"displayOrder": 2,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 844367,
"choiceText": "Sickle cell anemia with stroke",
"correct": true,
"displayOrder": 3,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 844369,
"choiceText": "Congenital heart disease with brain abscess",
"correct": false,
"displayOrder": 4,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "",
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": "",
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 265479,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 5-Year-Old With a Headache, Limp, and Projectile Vomiting"
},
{
"authors": "Afif Harb, MD; Antoine Saliba, MD",
"content": [
"The patient was diagnosed with sickle cell anemia complicated by a right-sided cerebral stroke. Stroke is one of the leading causes of morbidity and mortality among children with sickle cell anemia. Stroke can affect as many as 30% of children with this condition and can manifest clinically in 11% of all patients with sickle cell anemia.[1] Stroke also manifests as silent cerebral infarction on MRI in another 17%-22% of patients.[2] The risk for overt stroke for children with sickle cell anemia without primary stroke prophylaxis is more than 200-fold higher than in the general population; this risk may be even higher in adults.[3]",
"Silent cerebral infarct can occur as early as the first year of life, and its prevalence increases with age. Approximately 40% of adolescents with sickle cell anemia have silent cerebral infarcts, and adults are likely continue to acquire new or enlarged silent cerebral infarcts.[4] Cerebral infarcts tend to be ischemic in children and hemorrhagic in adult patients.",
"Well-documented risk factors for ischemic stroke in sickle cell anemia include prior transient ischemic attack, low steady-state hemoglobin levels, high white blood cell counts, hypertension, and a history of acute chest syndrome.[1] However, the etiology of stroke in sickle cell anemia remains unclear. Aside from the absence of lipid deposition and plaque formation, many of the histopathologic findings in cerebrovascular lesions in sickle cell anemia resemble those found in stroke patients in the general population.[5]",
"Cerebral infarcts in patients with sickle cell anemia are best detected by transcranial Doppler (TCD) ultrasonography, MRI, and magnetic resonance angiography (MRA). TCD ultrasonography is a reproducible and noninvasive method that aims to find narrowed internal carotid or middle cerebral arteries in asymptomatic children by detecting a signature high-flow pattern. Children with elevated blood flow velocities (≥ 200 cm/sec) have an astonishingly high rate of stroke: approximately 10,000 per 100,000 patient-years. TCD can reveal areas of high flow across the internal carotid artery and middle cerebral arteries that correspond to areas of stenosis; these are risk factors for stroke. Patients with abnormal MRA findings and higher blood-flow velocity on TCD ultrasonography are at an even higher risk for stroke.[6]",
"Cerebral infarcts in patients with sickle cell anemia should be treated by exchange transfusion on diagnosis, with lifelong transfusion therapy. Exchange transfusion is an effective but possibly underutilized therapy in the acute and chronic treatment of sickle cell anemia. The aim is to maintain or reduce the hemoglobin S level to below 30%. It can provide needed oxygen-carrying capacity while reducing the overall viscosity of blood and thus reducing complications. It is mainly indicated in acute infarcted stroke, acute chest syndrome, multiorgan failure syndrome, the right upper quadrant syndrome, and possibly priapism."
],
"date": "June 10, 2020",
"figures": [],
"markdown": "# A 5-Year-Old With a Headache, Limp, and Projectile Vomiting\n\n **Authors:** Afif Harb, MD; Antoine Saliba, MD \n **Date:** June 10, 2020\n\n ## Content\n\n The patient was diagnosed with sickle cell anemia complicated by a right-sided cerebral stroke. Stroke is one of the leading causes of morbidity and mortality among children with sickle cell anemia. Stroke can affect as many as 30% of children with this condition and can manifest clinically in 11% of all patients with sickle cell anemia.[1] Stroke also manifests as silent cerebral infarction on MRI in another 17%-22% of patients.[2] The risk for overt stroke for children with sickle cell anemia without primary stroke prophylaxis is more than 200-fold higher than in the general population; this risk may be even higher in adults.[3]\nSilent cerebral infarct can occur as early as the first year of life, and its prevalence increases with age. Approximately 40% of adolescents with sickle cell anemia have silent cerebral infarcts, and adults are likely continue to acquire new or enlarged silent cerebral infarcts.[4] Cerebral infarcts tend to be ischemic in children and hemorrhagic in adult patients.\nWell-documented risk factors for ischemic stroke in sickle cell anemia include prior transient ischemic attack, low steady-state hemoglobin levels, high white blood cell counts, hypertension, and a history of acute chest syndrome.[1] However, the etiology of stroke in sickle cell anemia remains unclear. Aside from the absence of lipid deposition and plaque formation, many of the histopathologic findings in cerebrovascular lesions in sickle cell anemia resemble those found in stroke patients in the general population.[5]\nCerebral infarcts in patients with sickle cell anemia are best detected by transcranial Doppler (TCD) ultrasonography, MRI, and magnetic resonance angiography (MRA). TCD ultrasonography is a reproducible and noninvasive method that aims to find narrowed internal carotid or middle cerebral arteries in asymptomatic children by detecting a signature high-flow pattern. Children with elevated blood flow velocities (≥ 200 cm/sec) have an astonishingly high rate of stroke: approximately 10,000 per 100,000 patient-years. TCD can reveal areas of high flow across the internal carotid artery and middle cerebral arteries that correspond to areas of stenosis; these are risk factors for stroke. Patients with abnormal MRA findings and higher blood-flow velocity on TCD ultrasonography are at an even higher risk for stroke.[6]\nCerebral infarcts in patients with sickle cell anemia should be treated by exchange transfusion on diagnosis, with lifelong transfusion therapy. Exchange transfusion is an effective but possibly underutilized therapy in the acute and chronic treatment of sickle cell anemia. The aim is to maintain or reduce the hemoglobin S level to below 30%. It can provide needed oxygen-carrying capacity while reducing the overall viscosity of blood and thus reducing complications. It is mainly indicated in acute infarcted stroke, acute chest syndrome, multiorgan failure syndrome, the right upper quadrant syndrome, and possibly priapism.\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 844363,
"choiceText": "Meningitis",
"correct": false,
"displayOrder": 1,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 844365,
"choiceText": "Brain tumor",
"correct": false,
"displayOrder": 2,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 844367,
"choiceText": "Sickle cell anemia with stroke",
"correct": true,
"displayOrder": 3,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 844369,
"choiceText": "Congenital heart disease with brain abscess",
"correct": false,
"displayOrder": 4,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "",
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": "",
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 265479,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 5-Year-Old With a Headache, Limp, and Projectile Vomiting"
},
{
"authors": "Afif Harb, MD; Antoine Saliba, MD",
"content": [
"The Stroke Prevention in Sickle Cell Anemia (STOP 1) trial was performed in 1998 with a goal of assessing the effect of transfusion therapy in preventing the first stroke episode in patients with sickle cell anemia.[7] In this study, 130 children with sickle cell anemia high risk for stroke (two measures on TCD ultrasonography), and comparable baseline characteristics were enrolled. Sixty-three children were randomly assigned to the intervention arm (receipt of transfusions), and 67 children were randomly assigned to the control arm (no receipt of transfusions).",
"The study was terminated early because it demonstrated such a large benefit of transfusion. A clinical alert was issued by the National Heart, Lung, and Blood Institute. The alert stated that screening and prophylactic transfusion must be performed in children with sickle cell anemia aged 2-16 years who are at high risk for stroke and have not yet had a stroke. The conclusions of the STOP 1 trial were clear: Long-term transfusions significantly reduce the risk for stroke in children with sickle cell anemia who have risk factors on TCD ultrasonography.",
"After the remarkable results of the STOP 1 trial, new questions were addressed, including when to start and stop blood transfusions in high-risk patients. The STOP 2 trial sought to determine the effect of discontinuing transfusions in patients with sickle cell anemia who were once at high risk for a stroke, had been receiving transfusions for more than 30 months, and currently had normal blood-flow velocities on TCD ultrasonography.[8] This study was stopped 2 years after initiation, when 79 children of a planned enrollment of 100 had undergone randomization.",
"Among the 41 children in whom transfusions were stopped, high-risk results on TCD ultrasonography developed in 14 and stroke developed in 2 others within an average of 4.5 months of the last transfusion. In contrast, no events of the composite endpoint occurred in the 38 children who continued to receive transfusions. The study team concluded that discontinuation of transfusion for the prevention of stroke in children with sickle cell anemia results in a high rate of reversion to abnormal blood-flow velocities on Doppler studies and stroke. Thus, stopping transfusions in patients who are at high risk of stroke is unsafe.",
"Moreover, an increase in serum ferritin was found, suggesting the development of iron overload in patients who are being treated with lifelong transfusions. Alloimmunization was seen, but no risk for infection was detected.",
"Owing to the conclusive results of the STOP 1 and STOP 2 trials, all patients with sickle cell anemia with abnormal blood-flow velocities on TCD ultrasonography were placed on lifelong transfusion therapy. Because the frequent transfusions cause iron to progressively accumulate in these patients, they began developing complications from iron overload."
],
"date": "June 10, 2020",
"figures": [],
"markdown": "# A 5-Year-Old With a Headache, Limp, and Projectile Vomiting\n\n **Authors:** Afif Harb, MD; Antoine Saliba, MD \n **Date:** June 10, 2020\n\n ## Content\n\n The Stroke Prevention in Sickle Cell Anemia (STOP 1) trial was performed in 1998 with a goal of assessing the effect of transfusion therapy in preventing the first stroke episode in patients with sickle cell anemia.[7] In this study, 130 children with sickle cell anemia high risk for stroke (two measures on TCD ultrasonography), and comparable baseline characteristics were enrolled. Sixty-three children were randomly assigned to the intervention arm (receipt of transfusions), and 67 children were randomly assigned to the control arm (no receipt of transfusions).\nThe study was terminated early because it demonstrated such a large benefit of transfusion. A clinical alert was issued by the National Heart, Lung, and Blood Institute. The alert stated that screening and prophylactic transfusion must be performed in children with sickle cell anemia aged 2-16 years who are at high risk for stroke and have not yet had a stroke. The conclusions of the STOP 1 trial were clear: Long-term transfusions significantly reduce the risk for stroke in children with sickle cell anemia who have risk factors on TCD ultrasonography.\nAfter the remarkable results of the STOP 1 trial, new questions were addressed, including when to start and stop blood transfusions in high-risk patients. The STOP 2 trial sought to determine the effect of discontinuing transfusions in patients with sickle cell anemia who were once at high risk for a stroke, had been receiving transfusions for more than 30 months, and currently had normal blood-flow velocities on TCD ultrasonography.[8] This study was stopped 2 years after initiation, when 79 children of a planned enrollment of 100 had undergone randomization.\nAmong the 41 children in whom transfusions were stopped, high-risk results on TCD ultrasonography developed in 14 and stroke developed in 2 others within an average of 4.5 months of the last transfusion. In contrast, no events of the composite endpoint occurred in the 38 children who continued to receive transfusions. The study team concluded that discontinuation of transfusion for the prevention of stroke in children with sickle cell anemia results in a high rate of reversion to abnormal blood-flow velocities on Doppler studies and stroke. Thus, stopping transfusions in patients who are at high risk of stroke is unsafe.\nMoreover, an increase in serum ferritin was found, suggesting the development of iron overload in patients who are being treated with lifelong transfusions. Alloimmunization was seen, but no risk for infection was detected.\nOwing to the conclusive results of the STOP 1 and STOP 2 trials, all patients with sickle cell anemia with abnormal blood-flow velocities on TCD ultrasonography were placed on lifelong transfusion therapy. Because the frequent transfusions cause iron to progressively accumulate in these patients, they began developing complications from iron overload.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 5-Year-Old With a Headache, Limp, and Projectile Vomiting"
},
{
"authors": "Afif Harb, MD; Antoine Saliba, MD",
"content": [
"All children with homozygous sickle cell anemia or sickle beta zero thalassemia who are younger than 16 years should undergo annual screening with TCD ultrasonography. Values greater than 170 cm/sec are worrisome, and patients with a value higher than 200 cm/sec should be started on indefinite transfusion therapy.",
"Iron overload can be measured using several techniques. Biopsy is the criterion standard for measurement of liver iron concentration, but this technique is invasive. Serial measurement of serum ferritin levels is practical but has questionable accuracy. MRI is a noninvasive and reliable technique for measurement of liver iron concentration. On the basis of the degree of iron overload, these patients should be treated with iron chelation therapy.",
"Three chelators are available: deferoxamine (parenteral), deferiprone (oral), and deferasirox (oral).[9] Most knowledge on the efficacy and safety of these chelators in reducing iron overload comes from studies in patients with thalassemia who had iron overload and in patients with sickle cell disease. Currently, most evidence supports the use of deferasirox in treating iron overload because of its safe profile, easy means of administration, good rate of patient adherence, and efficacy in decreasing excess iron in the body.[10]",
"After diagnosis, the patient in this case underwent acute exchange transfusion and was later moved to lifelong transfusion therapy to prevent stroke recurrence. She was also given deferasirox (30 mg/kg/d) orally once daily to prevent transfusion iron overload and its complications."
],
"date": "June 10, 2020",
"figures": [],
"markdown": "# A 5-Year-Old With a Headache, Limp, and Projectile Vomiting\n\n **Authors:** Afif Harb, MD; Antoine Saliba, MD \n **Date:** June 10, 2020\n\n ## Content\n\n All children with homozygous sickle cell anemia or sickle beta zero thalassemia who are younger than 16 years should undergo annual screening with TCD ultrasonography. Values greater than 170 cm/sec are worrisome, and patients with a value higher than 200 cm/sec should be started on indefinite transfusion therapy.\nIron overload can be measured using several techniques. Biopsy is the criterion standard for measurement of liver iron concentration, but this technique is invasive. Serial measurement of serum ferritin levels is practical but has questionable accuracy. MRI is a noninvasive and reliable technique for measurement of liver iron concentration. On the basis of the degree of iron overload, these patients should be treated with iron chelation therapy.\nThree chelators are available: deferoxamine (parenteral), deferiprone (oral), and deferasirox (oral).[9] Most knowledge on the efficacy and safety of these chelators in reducing iron overload comes from studies in patients with thalassemia who had iron overload and in patients with sickle cell disease. Currently, most evidence supports the use of deferasirox in treating iron overload because of its safe profile, easy means of administration, good rate of patient adherence, and efficacy in decreasing excess iron in the body.[10]\nAfter diagnosis, the patient in this case underwent acute exchange transfusion and was later moved to lifelong transfusion therapy to prevent stroke recurrence. She was also given deferasirox (30 mg/kg/d) orally once daily to prevent transfusion iron overload and its complications.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 844371,
"choiceText": "MRI",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 844373,
"choiceText": "MRA",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 844375,
"choiceText": "Ultrasonography",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 844377,
"choiceText": "TCD ultrasonography",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "TCD ultrasonography is both reproducible and noninvasive. TCD ultrasonography can also detect flow abnormalities that are indicative of stroke before lesions are evident on MRA.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 265481,
"questionText": "Which of the following is the most appropriate study to assess stroke risk in patients with sickle cell anemia?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 844379,
"choiceText": "1 year after initial treatment",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 844381,
"choiceText": "2 years after initial treatment",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 844383,
"choiceText": "5 years after initial treatment",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 844385,
"choiceText": "Treatment is lifelong",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The STOP 2 study found that discontinuing transfusion in children with sickle cell anemia at high risk for stroke results in a high rate of reversion to abnormal blood-flow velocities on Doppler studies and subsequent stroke. Patients who are at high risk for stroke must continue to receive transfusion.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 265483,
"questionText": "When can exchange transfusions reasonably be stopped in patients with sickle cell disease who were at high risk for stroke?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 5-Year-Old With a Headache, Limp, and Projectile Vomiting"
},
{
"authors": "Afif Harb, MD; Antoine Saliba, MD",
"content": [],
"date": "June 10, 2020",
"figures": [],
"markdown": "# A 5-Year-Old With a Headache, Limp, and Projectile Vomiting\n\n **Authors:** Afif Harb, MD; Antoine Saliba, MD \n **Date:** June 10, 2020\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 844371,
"choiceText": "MRI",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 844373,
"choiceText": "MRA",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 844375,
"choiceText": "Ultrasonography",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 844377,
"choiceText": "TCD ultrasonography",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "TCD ultrasonography is both reproducible and noninvasive. TCD ultrasonography can also detect flow abnormalities that are indicative of stroke before lesions are evident on MRA.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 265481,
"questionText": "Which of the following is the most appropriate study to assess stroke risk in patients with sickle cell anemia?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 844379,
"choiceText": "1 year after initial treatment",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 844381,
"choiceText": "2 years after initial treatment",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 844383,
"choiceText": "5 years after initial treatment",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 844385,
"choiceText": "Treatment is lifelong",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The STOP 2 study found that discontinuing transfusion in children with sickle cell anemia at high risk for stroke results in a high rate of reversion to abnormal blood-flow velocities on Doppler studies and subsequent stroke. Patients who are at high risk for stroke must continue to receive transfusion.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 265483,
"questionText": "When can exchange transfusions reasonably be stopped in patients with sickle cell disease who were at high risk for stroke?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 5-Year-Old With a Headache, Limp, and Projectile Vomiting"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 844363,
"choiceText": "Meningitis",
"correct": false,
"displayOrder": 1,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 844365,
"choiceText": "Brain tumor",
"correct": false,
"displayOrder": 2,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 844367,
"choiceText": "Sickle cell anemia with stroke",
"correct": true,
"displayOrder": 3,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 844369,
"choiceText": "Congenital heart disease with brain abscess",
"correct": false,
"displayOrder": 4,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "",
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": "",
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 265479,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 844371,
"choiceText": "MRI",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 844373,
"choiceText": "MRA",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 844375,
"choiceText": "Ultrasonography",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 844377,
"choiceText": "TCD ultrasonography",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "TCD ultrasonography is both reproducible and noninvasive. TCD ultrasonography can also detect flow abnormalities that are indicative of stroke before lesions are evident on MRA.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 265481,
"questionText": "Which of the following is the most appropriate study to assess stroke risk in patients with sickle cell anemia?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 844379,
"choiceText": "1 year after initial treatment",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 844381,
"choiceText": "2 years after initial treatment",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 844383,
"choiceText": "5 years after initial treatment",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 844385,
"choiceText": "Treatment is lifelong",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The STOP 2 study found that discontinuing transfusion in children with sickle cell anemia at high risk for stroke results in a high rate of reversion to abnormal blood-flow velocities on Doppler studies and subsequent stroke. Patients who are at high risk for stroke must continue to receive transfusion.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 265483,
"questionText": "When can exchange transfusions reasonably be stopped in patients with sickle cell disease who were at high risk for stroke?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
840382 | /viewarticle/840382 | [
{
"authors": "Kirti Saxena, MD; Toi Blakley Harris, MD; Angelo P. Giardino, MD, PhD",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"A 14-year-old girl in the fourth month of her freshman year of high school lives in a two-parent household with a younger brother and has a relatively large extended family that lives close by. In middle school, she was described by teachers as \"fretful.\" She worried a great deal about getting assignments in on time and would occasionally voice concerns about the health and well-being of her older relatives.",
"At the start of high school, she maintained good grades, tried out for the track and field team, and developed several close friendships. However, she has lost interest in school, had trouble sleeping, lost her appetite and a small amount of weight, and quit the track team.",
"Her friends report that she is not communicating much. Her teachers describe missed assignments, and her parents state that she stays in her bedroom after school and for most the weekend. When her parents ask her about her behavior, she reportedly responds that \"it's nothing\" or that they should \"just leave her alone.\""
],
"date": "June 09, 2020",
"figures": [],
"markdown": "# A Withdrawn 14-Year-Old With Weight Loss and Sleep Problems\n\n **Authors:** Kirti Saxena, MD; Toi Blakley Harris, MD; Angelo P. Giardino, MD, PhD \n **Date:** June 09, 2020\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nA 14-year-old girl in the fourth month of her freshman year of high school lives in a two-parent household with a younger brother and has a relatively large extended family that lives close by. In middle school, she was described by teachers as \"fretful.\" She worried a great deal about getting assignments in on time and would occasionally voice concerns about the health and well-being of her older relatives.\nAt the start of high school, she maintained good grades, tried out for the track and field team, and developed several close friendships. However, she has lost interest in school, had trouble sleeping, lost her appetite and a small amount of weight, and quit the track team.\nHer friends report that she is not communicating much. Her teachers describe missed assignments, and her parents state that she stays in her bedroom after school and for most the weekend. When her parents ask her about her behavior, she reportedly responds that \"it's nothing\" or that they should \"just leave her alone.\"\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A Withdrawn 14-Year-Old With Weight Loss and Sleep Problems"
},
{
"authors": "Kirti Saxena, MD; Toi Blakley Harris, MD; Angelo P. Giardino, MD, PhD",
"content": [
"The patient was initially taken to her primary care provider (PCP), who conducted a complete physical examination, including blood tests, which ruled out anemia and thyroid problems. With permission, the PCP obtained blood and urine drug screenings and a pregnancy test. All laboratory data were within normal limits, and the PCP made a referral to psychiatrist.",
"During the consultation, the psychiatrist found the patient to be sullen, alert, and oriented to all domains and coherent in her thinking. No hallucinations or delusions are reported or suspected. She did not disclose any thoughts of suicide or hurting anyone else. However, she did describe worrying that her \"wanting to be alone\" was hurting her family. Her memory (both short and long term) was intact. No family history of mental illness was reported.",
"She denied any traumatic events or recent losses. Although carefully investigated, no signs of abuse from her parents or others are apparent. When asked about her recent decline in school performance and her departure from the track and field team, she reports that she simply lost interest and is unable to concentrate on her studies.",
"She became tearful when describing how her behavior was \"disappointing her family.\" She explained that she just wants to stay in bed in her room because she is so tired that she does not have the energy to worry about things or get a good night's sleep."
],
"date": "June 09, 2020",
"figures": [],
"markdown": "# A Withdrawn 14-Year-Old With Weight Loss and Sleep Problems\n\n **Authors:** Kirti Saxena, MD; Toi Blakley Harris, MD; Angelo P. Giardino, MD, PhD \n **Date:** June 09, 2020\n\n ## Content\n\n The patient was initially taken to her primary care provider (PCP), who conducted a complete physical examination, including blood tests, which ruled out anemia and thyroid problems. With permission, the PCP obtained blood and urine drug screenings and a pregnancy test. All laboratory data were within normal limits, and the PCP made a referral to psychiatrist.\nDuring the consultation, the psychiatrist found the patient to be sullen, alert, and oriented to all domains and coherent in her thinking. No hallucinations or delusions are reported or suspected. She did not disclose any thoughts of suicide or hurting anyone else. However, she did describe worrying that her \"wanting to be alone\" was hurting her family. Her memory (both short and long term) was intact. No family history of mental illness was reported.\nShe denied any traumatic events or recent losses. Although carefully investigated, no signs of abuse from her parents or others are apparent. When asked about her recent decline in school performance and her departure from the track and field team, she reports that she simply lost interest and is unable to concentrate on her studies.\nShe became tearful when describing how her behavior was \"disappointing her family.\" She explained that she just wants to stay in bed in her room because she is so tired that she does not have the energy to worry about things or get a good night's sleep.\n\n ## Figures\n\n \n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 817271,
"choiceText": "Generalized anxiety disorder",
"correct": false,
"displayOrder": 1,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 817273,
"choiceText": "Attention-deficit/hyperactivity disorder",
"correct": false,
"displayOrder": 2,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 817275,
"choiceText": "Major depressive disorder",
"correct": true,
"displayOrder": 3,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 817277,
"choiceText": "Conduct disorder ",
"correct": false,
"displayOrder": 4,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "",
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": "",
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 256573,
"questionText": "Of the following conditions, which is the most likely diagnosis? ",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A Withdrawn 14-Year-Old With Weight Loss and Sleep Problems"
},
{
"authors": "Kirti Saxena, MD; Toi Blakley Harris, MD; Angelo P. Giardino, MD, PhD",
"content": [
"The patient appears to be suffering from more than adolescent moodiness. On the basis of the diagnostic criteria described in detail below, she can be diagnosed with major depressive disorder (MDD), which is among the more common psychiatric conditions diagnosed in adolescence.",
"The Diagnostic and Statistical Manual of Mental Disorders, fifth edition (DSM-5), uses the same basic criteria to diagnose depression in children and adolescents as in adults.[1] A few adjustments to the diagnostic criteria are made to account for the differences in age and stage of development in adults vs children. In this case, the patient meets the criteria for MDD, commonly referred to simply as \"depression.\"",
"Although the criteria for depressive disorders are similar in adults and younger individuals, children and adolescents may present with irritable mood as the prominent mood symptom (with or without depressed mood).",
"According to the DSM-5, a child or adolescent meets the diagnostic criteria for MDD when a minimum of five of the following nine symptoms are present during the same 2-week period:",
"Depressed mood: For children and adolescents, this can also be an irritable mood)",
"Diminished interest or loss of pleasure in activities (anhedonia)",
"Weight change or appetite disturbance: For children, this can include failure to achieve expected weight gain",
"Sleep disturbance (insomnia or hypersomnia)",
"Psychomotor agitation or retardation",
"Fatigue or loss of energy",
"Feelings of worthlessness, diminished ability to think or concentrate; indecisiveness",
"Recurrent thoughts of death, recurrent suicidal ideation without a specific plan for committing suicide or a suicide attempt/specific plan for committing suicide",
"At least one of the symptoms must be either depressed/irritable mood or loss of interest or pleasure. The symptoms must represent a change from previous functioning and cause clinically significant distress or impairment in social, occupational, or other important areas of functioning.",
"The patient in this case has more than five of the above symptoms and has experienced them for several months, meeting the diagnostic criteria for MDD."
],
"date": "June 09, 2020",
"figures": [],
"markdown": "# A Withdrawn 14-Year-Old With Weight Loss and Sleep Problems\n\n **Authors:** Kirti Saxena, MD; Toi Blakley Harris, MD; Angelo P. Giardino, MD, PhD \n **Date:** June 09, 2020\n\n ## Content\n\n The patient appears to be suffering from more than adolescent moodiness. On the basis of the diagnostic criteria described in detail below, she can be diagnosed with major depressive disorder (MDD), which is among the more common psychiatric conditions diagnosed in adolescence.\nThe Diagnostic and Statistical Manual of Mental Disorders, fifth edition (DSM-5), uses the same basic criteria to diagnose depression in children and adolescents as in adults.[1] A few adjustments to the diagnostic criteria are made to account for the differences in age and stage of development in adults vs children. In this case, the patient meets the criteria for MDD, commonly referred to simply as \"depression.\"\nAlthough the criteria for depressive disorders are similar in adults and younger individuals, children and adolescents may present with irritable mood as the prominent mood symptom (with or without depressed mood).\nAccording to the DSM-5, a child or adolescent meets the diagnostic criteria for MDD when a minimum of five of the following nine symptoms are present during the same 2-week period:\nDepressed mood: For children and adolescents, this can also be an irritable mood)\nDiminished interest or loss of pleasure in activities (anhedonia)\nWeight change or appetite disturbance: For children, this can include failure to achieve expected weight gain\nSleep disturbance (insomnia or hypersomnia)\nPsychomotor agitation or retardation\nFatigue or loss of energy\nFeelings of worthlessness, diminished ability to think or concentrate; indecisiveness\nRecurrent thoughts of death, recurrent suicidal ideation without a specific plan for committing suicide or a suicide attempt/specific plan for committing suicide\nAt least one of the symptoms must be either depressed/irritable mood or loss of interest or pleasure. The symptoms must represent a change from previous functioning and cause clinically significant distress or impairment in social, occupational, or other important areas of functioning.\nThe patient in this case has more than five of the above symptoms and has experienced them for several months, meeting the diagnostic criteria for MDD.\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 817271,
"choiceText": "Generalized anxiety disorder",
"correct": false,
"displayOrder": 1,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 817273,
"choiceText": "Attention-deficit/hyperactivity disorder",
"correct": false,
"displayOrder": 2,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 817275,
"choiceText": "Major depressive disorder",
"correct": true,
"displayOrder": 3,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 817277,
"choiceText": "Conduct disorder ",
"correct": false,
"displayOrder": 4,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "",
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": "",
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 256573,
"questionText": "Of the following conditions, which is the most likely diagnosis? ",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A Withdrawn 14-Year-Old With Weight Loss and Sleep Problems"
},
{
"authors": "Kirti Saxena, MD; Toi Blakley Harris, MD; Angelo P. Giardino, MD, PhD",
"content": [
"Although this patient has MDD, MDD and dysthymia can be classified into numerous subtypes, including atypical, psychotic, seasonal, catatonic, melancholic, and postpartum depression. Atypical features include an increase in appetite or significant weight gain, increased sleep, feelings of heaviness in the arms or legs, and rejection sensitivity.[2]",
"Psychotic depression, although rare before adolescence, represents a severe form of depression, with psychotic symptoms usually presenting as mood congruent. That congruency means that, if they are sad, their delusions are of \"a world that is doomed.\" Children primarily have auditory hallucinations, whereas adolescents usually have delusions. Hallucinations are false sensations. For example, with auditory hallucinations, one hears voices that are not actually present. In contrast, delusions are false ideas or beliefs, such as \"the FBI is following me.\" Adolescents with psychotic depression tend to do worse, and some later develop bipolar affective disorder.[3]",
"Youth whose symptoms of depression predominantly occur during winter or fall (the season when there is less daylight) are considered to have seasonal affective disorder (SAD). Because school starts in the fall or winter months, distinguishing depression triggered by school stress from SAD is important.[3]",
"Approximately 20% of youth with MDD experience manic episodes by adulthood.[4] Indeed, depression is usually the first symptom of bipolar disorder recognized in children and adolescents. Factors predicting development of mania in depressed youth include a family history of bipolar disorder, a depressive episode characterized by rapid onset, psychomotor retardation, and psychotic features.[3]",
"Although not as debilitating or dramatic as MDD, dysthymia is a long (≥1 year) low-level depression that is marked by pessimism and withdrawal from life. While the patient is depressed or irritable, at least two of the following must be present:",
"Decreased or increased appetite",
"Insomnia or hypersomnia",
"Low energy or fatigue",
"Low self-esteem",
"Poor concentration and/or difficulty making decisions",
"Feelings of hopelessness",
"In addition, symptoms must not resolve for more than 2 months at a time. For both MDD and dysthymia, the symptoms should not be due to substance abuse, use of medications, other psychiatric illness, bereavement, or medical illness.",
"\"Other specified depressive disorders\" involve presentations in which symptoms are characteristic of a depressive disorder and cause clinically significant distress or impairment in social, occupational, or other important areas of functioning but do not meet the full criteria for any of the depressive disorders in that diagnostic class.",
"Examples of presentations that can be specified using the \"other specified\" designation include the following:",
"Recurrent brief depression: This requires concurrent presence of depressed mood and at least four other symptoms of depression for 2-13 days at least once per month (not associated with the menstrual cycle) for at least 12 consecutive months in an individual whose presentation has never met the criteria for any other depressive or bipolar disorder and does not currently meet active or residual criteria for any psychotic disorder.",
"Short-duration depressive episode (4-13 days): This involves depressed affect and at least four of the other eight symptoms of a major depressive episode associated with clinically significant distress or impairment that persists for more than 4 days but less than 14 days. The individual's presentation has never met criteria for any other depressive or bipolar disorder, does not currently meet active or residual criteria for any psychotic disorder, and does not meet criteria for recurrent brief depression.",
"Depressive episode with insufficient symptoms: This requires depressed affect and at least one of the other eight symptoms of a major depressive episode associated with clinically significant distress or impairment that persist for at least 2 weeks. The individual's presentation has never met criteria for any other depressive or bipolar disorder, does not currently meet active or residual criteria for any psychotic disorder, and does not meet criteria for mixed anxiety and depressive disorder symptoms.",
"\"Unspecified depressive disorder\" is a category that applies to presentations in which symptoms characteristic of a depressive disorder that cause clinically significant distress or impairment in social, occupational, or other important areas of functioning predominate but do not meet the full criteria for any of the disorders in the depressive disorders diagnostic class. The unspecified depressive disorder category is used in situations in which the clinician chooses not to specify the reason that the criteria are not met for a specific depressive disorder and includes presentations for which information is insufficient to make a more specific diagnosis (eg, in emergency department settings).",
"Depressive disorder with anxious distress is noted when at least two of the following symptoms are present during most days of a major depressive episode or persistent depressive disorder (dysthymia):",
"Feeling keyed up or tense",
"Feeling unusually restless",
"Difficulty concentrating because of worry",
"Fear that something awful may happen",
"Feeling that the individual might lose control of himself or herself",
"In addition, depressive disorder with anxious distress can be subcategorized by severity on the basis of how many of the above symptoms are present. Current severity is as follows:",
"Mild: Two symptoms",
"Moderate: Three symptoms",
"Moderate to severe: Four or five symptoms",
"Severe: Four or five symptoms, with motor agitation",
"Anxious distress has been noted as a prominent feature of both bipolar disorder and MDD in primary care and specialty mental health settings. High levels of anxiety have been associated with higher suicide risk, longer duration of illness, and greater likelihood of treatment nonresponse. As a result, accurately specifying the presence and severity of anxious distress is important, for treatment planning and monitoring of response to treatment.",
"Depressive disorder with mixed features is diagnosed when the following manic/hypomanic symptoms are present nearly every day during most days of a major depressive episode:",
"Elevated expansive mood",
"Inflated self-esteem or grandiosity, more talkative than usual or pressure to keep talking",
"Flight of ideas, or the subjective experience that thoughts are racing"
],
"date": "June 09, 2020",
"figures": [],
"markdown": "# A Withdrawn 14-Year-Old With Weight Loss and Sleep Problems\n\n **Authors:** Kirti Saxena, MD; Toi Blakley Harris, MD; Angelo P. Giardino, MD, PhD \n **Date:** June 09, 2020\n\n ## Content\n\n Although this patient has MDD, MDD and dysthymia can be classified into numerous subtypes, including atypical, psychotic, seasonal, catatonic, melancholic, and postpartum depression. Atypical features include an increase in appetite or significant weight gain, increased sleep, feelings of heaviness in the arms or legs, and rejection sensitivity.[2]\nPsychotic depression, although rare before adolescence, represents a severe form of depression, with psychotic symptoms usually presenting as mood congruent. That congruency means that, if they are sad, their delusions are of \"a world that is doomed.\" Children primarily have auditory hallucinations, whereas adolescents usually have delusions. Hallucinations are false sensations. For example, with auditory hallucinations, one hears voices that are not actually present. In contrast, delusions are false ideas or beliefs, such as \"the FBI is following me.\" Adolescents with psychotic depression tend to do worse, and some later develop bipolar affective disorder.[3]\nYouth whose symptoms of depression predominantly occur during winter or fall (the season when there is less daylight) are considered to have seasonal affective disorder (SAD). Because school starts in the fall or winter months, distinguishing depression triggered by school stress from SAD is important.[3]\nApproximately 20% of youth with MDD experience manic episodes by adulthood.[4] Indeed, depression is usually the first symptom of bipolar disorder recognized in children and adolescents. Factors predicting development of mania in depressed youth include a family history of bipolar disorder, a depressive episode characterized by rapid onset, psychomotor retardation, and psychotic features.[3]\nAlthough not as debilitating or dramatic as MDD, dysthymia is a long (≥1 year) low-level depression that is marked by pessimism and withdrawal from life. While the patient is depressed or irritable, at least two of the following must be present:\nDecreased or increased appetite\nInsomnia or hypersomnia\nLow energy or fatigue\nLow self-esteem\nPoor concentration and/or difficulty making decisions\nFeelings of hopelessness\nIn addition, symptoms must not resolve for more than 2 months at a time. For both MDD and dysthymia, the symptoms should not be due to substance abuse, use of medications, other psychiatric illness, bereavement, or medical illness.\n\"Other specified depressive disorders\" involve presentations in which symptoms are characteristic of a depressive disorder and cause clinically significant distress or impairment in social, occupational, or other important areas of functioning but do not meet the full criteria for any of the depressive disorders in that diagnostic class.\nExamples of presentations that can be specified using the \"other specified\" designation include the following:\nRecurrent brief depression: This requires concurrent presence of depressed mood and at least four other symptoms of depression for 2-13 days at least once per month (not associated with the menstrual cycle) for at least 12 consecutive months in an individual whose presentation has never met the criteria for any other depressive or bipolar disorder and does not currently meet active or residual criteria for any psychotic disorder.\nShort-duration depressive episode (4-13 days): This involves depressed affect and at least four of the other eight symptoms of a major depressive episode associated with clinically significant distress or impairment that persists for more than 4 days but less than 14 days. The individual's presentation has never met criteria for any other depressive or bipolar disorder, does not currently meet active or residual criteria for any psychotic disorder, and does not meet criteria for recurrent brief depression.\nDepressive episode with insufficient symptoms: This requires depressed affect and at least one of the other eight symptoms of a major depressive episode associated with clinically significant distress or impairment that persist for at least 2 weeks. The individual's presentation has never met criteria for any other depressive or bipolar disorder, does not currently meet active or residual criteria for any psychotic disorder, and does not meet criteria for mixed anxiety and depressive disorder symptoms.\n\"Unspecified depressive disorder\" is a category that applies to presentations in which symptoms characteristic of a depressive disorder that cause clinically significant distress or impairment in social, occupational, or other important areas of functioning predominate but do not meet the full criteria for any of the disorders in the depressive disorders diagnostic class. The unspecified depressive disorder category is used in situations in which the clinician chooses not to specify the reason that the criteria are not met for a specific depressive disorder and includes presentations for which information is insufficient to make a more specific diagnosis (eg, in emergency department settings).\nDepressive disorder with anxious distress is noted when at least two of the following symptoms are present during most days of a major depressive episode or persistent depressive disorder (dysthymia):\nFeeling keyed up or tense\nFeeling unusually restless\nDifficulty concentrating because of worry\nFear that something awful may happen\nFeeling that the individual might lose control of himself or herself\nIn addition, depressive disorder with anxious distress can be subcategorized by severity on the basis of how many of the above symptoms are present. Current severity is as follows:\nMild: Two symptoms\nModerate: Three symptoms\nModerate to severe: Four or five symptoms\nSevere: Four or five symptoms, with motor agitation\nAnxious distress has been noted as a prominent feature of both bipolar disorder and MDD in primary care and specialty mental health settings. High levels of anxiety have been associated with higher suicide risk, longer duration of illness, and greater likelihood of treatment nonresponse. As a result, accurately specifying the presence and severity of anxious distress is important, for treatment planning and monitoring of response to treatment.\nDepressive disorder with mixed features is diagnosed when the following manic/hypomanic symptoms are present nearly every day during most days of a major depressive episode:\nElevated expansive mood\nInflated self-esteem or grandiosity, more talkative than usual or pressure to keep talking\nFlight of ideas, or the subjective experience that thoughts are racing\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A Withdrawn 14-Year-Old With Weight Loss and Sleep Problems"
},
{
"authors": "Kirti Saxena, MD; Toi Blakley Harris, MD; Angelo P. Giardino, MD, PhD",
"content": [
"The choice of initial acute therapy for MDD depends on the following factors:",
"Severity",
"Number of prior episodes",
"Chronicity",
"Subtype",
"Patient age",
"Genetics (eg, family history of response to certain medication)",
"Contextual issues (ie, family conflict, academic problems, exposure to negative life events)",
"Ability to adhere to treatment recommendations",
"Previous response to treatment",
"Patient's and family's motivation for treatment",
"Potential of self-destructive behavior or danger to others, which may indicate a need for hospitalization",
"Patient attitude and willingness to accept treatment",
"In mild cases, psychosocial interventions and psychotherapies are often recommended as first-line treatments. In the most severe cases, medication in addition to psychotherapeutic intervention is often recommended, as well as hospitalization.",
"Cognitive-behavioral therapy (CBT) has been shown in multiple randomized clinical trials to be effective in the treatment of mild MDD in children and adolescents. For moderate-to-severe depression, psychotherapies and psychopharmacologic treatment are recommended. Hospitalization should be considered for severely depressed persons.",
"In CBT for MDD or dysthymia, the focus is on cognitive distortions. These include negative distortions of life experience and a negative view of the self and outside world. CBT teaches new ways of thinking to replace negative thoughts. The therapist is an active participant, and the focus is on the child. CBT often involves keeping a diary and doing homework. The goals are to alleviate the depressive episodes; to prevent recurrences by identifying and testing negative cognitions; and to develop flexible, positive ways of thinking.",
"Factors that appear to be related to the response to psychotherapy include the following:",
"Age at onset of depression",
"Severity of depression;",
"Presence of comorbid psychiatric disorders",
"Presence or absence of social support",
"Parental psychopathology",
"Family conflict",
"Exposure to stressful life events",
"Socioeconomic status",
"Quality of treatment",
"Therapist's expertise",
"Motivation of the patient and therapist",
"Presence or absence of self-destructive behavior",
"Level of insight",
"Safety and stability of home environment and community",
"Studies on pharmacotherapy in youth with MDD are few. Agents that have been used or proposed for use include the following:",
"Tricyclic antidepressants (rarely used now)",
"Selective serotonin reuptake inhibitors",
"Other antidepressants: heterocyclics (eg, amoxapine and maprotiline), monoamine oxidase inhibitors, bupropion, fluoxetine, venlafaxine, and nefazodone (found useful in adults)",
"Mood disorders, such as depression, substantially increase the risk for suicide; suicidal behavior is a matter of serious concern for clinicians who deal with the mental health problems of children and adolescents. The incidence of suicide attempts reaches a peak during the mid-adolescent years, and the mortality rate from suicide increases steadily through the teenage years; suicide is the third leading cause of death in that age group.",
"The US prevalence rates for depression in children and adolescents vary. Studies have demonstrated that depression is not rare and is regularly encountered in pediatric and psychiatric practice. Birmaher and colleagues[5] found the incidence of depression to be approximately 2% in children and 4%-8% in adolescents. Garrison and colleagues[6] conducted a study of adolescents aged 11-16 years in the southeastern United States and found that the 1-year incidence of major depression was 3.3%.",
"The World Health Organization report \"Health for the World's Adolescents: A Second Chance in the Second Decade\" states that depression is the most frequent cause worldwide of illness and disability in persons aged 10-19 years, with the highest rate in females. The report also states that up to one half of all mental disorders arise by age 14 years, but they are usually not recognized.[7]",
"The presentation of some symptoms may change with age. Such symptoms as somatic complaints, irritability, and social withdrawal are more common in children, whereas psychomotor retardation, hypersomnia, and delusions are less common before puberty than they are in adolescence and adulthood.[8,9,10,11]",
"The Substance Abuse and Mental Health Services Administration indicates that sex differences in depression rates emerge at age 12-17 years. Its report states that girls aged 12-17 years are three times more likely than boys aged 12-17 years to have had a major depressive episode in the past year.[12]",
"Whether damaging experiences or biological processes trigger depressive episodes remains the topic of some debate. However, the final common pathways to depression involve biochemical changes in the brain. Sleep, appetite, and memory are commonly disturbed in persons with depression. Reductions in the activity of circuits that use serotonin and norepinephrine may contribute to depression; antidepressants act on one or both of these symptoms.",
"Neurotransmitter system abnormalities are being investigated to understand the biology of depression. Nobile and colleagues[13] found that uptake of human platelet 5-hydroxytryptamine (5-HT) or serotonin, is differentially influenced in nondepressed and depressed children by a common genetic variant of the promoter region of 5-HT (Figures 1 and 2).",
"Figure 1.",
"Figure 1.",
"Figure 2.",
"Figure 2.",
"In summary, the patient's presentation and symptoms meet the diagnostic criteria for MDD. Although there are explicit diagnostic criteria for MDD, it may remain undiagnosed and therefore undertreated in adolescents. In this case, the patient's parents identified the problem early and have begun psychiatric care in a timely manner. The patient is likely to respond well to CBT and an initial course of medication."
],
"date": "June 09, 2020",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/840/382/840382-thumb-1.jpg"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/840/382/840382-thumb-2.jpg"
}
],
"markdown": "# A Withdrawn 14-Year-Old With Weight Loss and Sleep Problems\n\n **Authors:** Kirti Saxena, MD; Toi Blakley Harris, MD; Angelo P. Giardino, MD, PhD \n **Date:** June 09, 2020\n\n ## Content\n\n The choice of initial acute therapy for MDD depends on the following factors:\nSeverity\nNumber of prior episodes\nChronicity\nSubtype\nPatient age\nGenetics (eg, family history of response to certain medication)\nContextual issues (ie, family conflict, academic problems, exposure to negative life events)\nAbility to adhere to treatment recommendations\nPrevious response to treatment\nPatient's and family's motivation for treatment\nPotential of self-destructive behavior or danger to others, which may indicate a need for hospitalization\nPatient attitude and willingness to accept treatment\nIn mild cases, psychosocial interventions and psychotherapies are often recommended as first-line treatments. In the most severe cases, medication in addition to psychotherapeutic intervention is often recommended, as well as hospitalization.\nCognitive-behavioral therapy (CBT) has been shown in multiple randomized clinical trials to be effective in the treatment of mild MDD in children and adolescents. For moderate-to-severe depression, psychotherapies and psychopharmacologic treatment are recommended. Hospitalization should be considered for severely depressed persons.\nIn CBT for MDD or dysthymia, the focus is on cognitive distortions. These include negative distortions of life experience and a negative view of the self and outside world. CBT teaches new ways of thinking to replace negative thoughts. The therapist is an active participant, and the focus is on the child. CBT often involves keeping a diary and doing homework. The goals are to alleviate the depressive episodes; to prevent recurrences by identifying and testing negative cognitions; and to develop flexible, positive ways of thinking.\nFactors that appear to be related to the response to psychotherapy include the following:\nAge at onset of depression\nSeverity of depression;\nPresence of comorbid psychiatric disorders\nPresence or absence of social support\nParental psychopathology\nFamily conflict\nExposure to stressful life events\nSocioeconomic status\nQuality of treatment\nTherapist's expertise\nMotivation of the patient and therapist\nPresence or absence of self-destructive behavior\nLevel of insight\nSafety and stability of home environment and community\nStudies on pharmacotherapy in youth with MDD are few. Agents that have been used or proposed for use include the following:\nTricyclic antidepressants (rarely used now)\nSelective serotonin reuptake inhibitors\nOther antidepressants: heterocyclics (eg, amoxapine and maprotiline), monoamine oxidase inhibitors, bupropion, fluoxetine, venlafaxine, and nefazodone (found useful in adults)\nMood disorders, such as depression, substantially increase the risk for suicide; suicidal behavior is a matter of serious concern for clinicians who deal with the mental health problems of children and adolescents. The incidence of suicide attempts reaches a peak during the mid-adolescent years, and the mortality rate from suicide increases steadily through the teenage years; suicide is the third leading cause of death in that age group.\nThe US prevalence rates for depression in children and adolescents vary. Studies have demonstrated that depression is not rare and is regularly encountered in pediatric and psychiatric practice. Birmaher and colleagues[5] found the incidence of depression to be approximately 2% in children and 4%-8% in adolescents. Garrison and colleagues[6] conducted a study of adolescents aged 11-16 years in the southeastern United States and found that the 1-year incidence of major depression was 3.3%.\nThe World Health Organization report \"Health for the World's Adolescents: A Second Chance in the Second Decade\" states that depression is the most frequent cause worldwide of illness and disability in persons aged 10-19 years, with the highest rate in females. The report also states that up to one half of all mental disorders arise by age 14 years, but they are usually not recognized.[7]\nThe presentation of some symptoms may change with age. Such symptoms as somatic complaints, irritability, and social withdrawal are more common in children, whereas psychomotor retardation, hypersomnia, and delusions are less common before puberty than they are in adolescence and adulthood.[8,9,10,11]\nThe Substance Abuse and Mental Health Services Administration indicates that sex differences in depression rates emerge at age 12-17 years. Its report states that girls aged 12-17 years are three times more likely than boys aged 12-17 years to have had a major depressive episode in the past year.[12]\nWhether damaging experiences or biological processes trigger depressive episodes remains the topic of some debate. However, the final common pathways to depression involve biochemical changes in the brain. Sleep, appetite, and memory are commonly disturbed in persons with depression. Reductions in the activity of circuits that use serotonin and norepinephrine may contribute to depression; antidepressants act on one or both of these symptoms.\nNeurotransmitter system abnormalities are being investigated to understand the biology of depression. Nobile and colleagues[13] found that uptake of human platelet 5-hydroxytryptamine (5-HT) or serotonin, is differentially influenced in nondepressed and depressed children by a common genetic variant of the promoter region of 5-HT (Figures 1 and 2).\nFigure 1.\nFigure 1.\nFigure 2.\nFigure 2.\nIn summary, the patient's presentation and symptoms meet the diagnostic criteria for MDD. Although there are explicit diagnostic criteria for MDD, it may remain undiagnosed and therefore undertreated in adolescents. In this case, the patient's parents identified the problem early and have begun psychiatric care in a timely manner. The patient is likely to respond well to CBT and an initial course of medication.\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n\n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 817289,
"choiceText": "2 weeks",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 817291,
"choiceText": "2 years",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 817293,
"choiceText": "1 year",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 817295,
"choiceText": "2 months",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The DSM-5 criteria for dysthymia require depressed or irritable mood lasting at least 1 year. While the person is depressed or irritable, two of the following symptoms must also be present:\r\n\r\n<ul><li>Decreased or increased appetite;</li>\r\n<li>Insomnia or hypersomnia;</li>\r\n\r\n<li>Low energy or fatigue;</li>\r\n\r\n<li>Low self-esteem;</li>\r\n\r\n<li>Poor concentration; and</li>\r\n\r\n<li>Feelings of hopelessness.</li></ul><br /> ",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 256581,
"questionText": "For dysthymia to be diagnosed in children and adolescents, symptoms must be present for which of the following durations?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 817297,
"choiceText": "Family history of bipolar disorder",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 817299,
"choiceText": "Psychomotor retardation",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 817301,
"choiceText": "Psychotic features",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 817303,
"choiceText": "All of the above ",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Several factors can predict the development of mania in depressed youth. These include a family history of bipolar disorder, a depressive episode characterized by rapid onset, psychomotor retardation, and psychotic features.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 256583,
"questionText": "In a depressed youth, which of the following factors predict the development of mania?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A Withdrawn 14-Year-Old With Weight Loss and Sleep Problems"
},
{
"authors": "Kirti Saxena, MD; Toi Blakley Harris, MD; Angelo P. Giardino, MD, PhD",
"content": [],
"date": "June 09, 2020",
"figures": [],
"markdown": "# A Withdrawn 14-Year-Old With Weight Loss and Sleep Problems\n\n **Authors:** Kirti Saxena, MD; Toi Blakley Harris, MD; Angelo P. Giardino, MD, PhD \n **Date:** June 09, 2020\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 817289,
"choiceText": "2 weeks",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 817291,
"choiceText": "2 years",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 817293,
"choiceText": "1 year",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 817295,
"choiceText": "2 months",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The DSM-5 criteria for dysthymia require depressed or irritable mood lasting at least 1 year. While the person is depressed or irritable, two of the following symptoms must also be present:\r\n\r\n<ul><li>Decreased or increased appetite;</li>\r\n<li>Insomnia or hypersomnia;</li>\r\n\r\n<li>Low energy or fatigue;</li>\r\n\r\n<li>Low self-esteem;</li>\r\n\r\n<li>Poor concentration; and</li>\r\n\r\n<li>Feelings of hopelessness.</li></ul><br /> ",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 256581,
"questionText": "For dysthymia to be diagnosed in children and adolescents, symptoms must be present for which of the following durations?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 817297,
"choiceText": "Family history of bipolar disorder",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 817299,
"choiceText": "Psychomotor retardation",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 817301,
"choiceText": "Psychotic features",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 817303,
"choiceText": "All of the above ",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Several factors can predict the development of mania in depressed youth. These include a family history of bipolar disorder, a depressive episode characterized by rapid onset, psychomotor retardation, and psychotic features.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 256583,
"questionText": "In a depressed youth, which of the following factors predict the development of mania?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A Withdrawn 14-Year-Old With Weight Loss and Sleep Problems"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 817271,
"choiceText": "Generalized anxiety disorder",
"correct": false,
"displayOrder": 1,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 817273,
"choiceText": "Attention-deficit/hyperactivity disorder",
"correct": false,
"displayOrder": 2,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 817275,
"choiceText": "Major depressive disorder",
"correct": true,
"displayOrder": 3,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 817277,
"choiceText": "Conduct disorder ",
"correct": false,
"displayOrder": 4,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "",
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": "",
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 256573,
"questionText": "Of the following conditions, which is the most likely diagnosis? ",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 817289,
"choiceText": "2 weeks",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 817291,
"choiceText": "2 years",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 817293,
"choiceText": "1 year",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 817295,
"choiceText": "2 months",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The DSM-5 criteria for dysthymia require depressed or irritable mood lasting at least 1 year. While the person is depressed or irritable, two of the following symptoms must also be present:\r\n\r\n<ul><li>Decreased or increased appetite;</li>\r\n<li>Insomnia or hypersomnia;</li>\r\n\r\n<li>Low energy or fatigue;</li>\r\n\r\n<li>Low self-esteem;</li>\r\n\r\n<li>Poor concentration; and</li>\r\n\r\n<li>Feelings of hopelessness.</li></ul><br /> ",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 256581,
"questionText": "For dysthymia to be diagnosed in children and adolescents, symptoms must be present for which of the following durations?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 817297,
"choiceText": "Family history of bipolar disorder",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 817299,
"choiceText": "Psychomotor retardation",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 817301,
"choiceText": "Psychotic features",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 817303,
"choiceText": "All of the above ",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Several factors can predict the development of mania in depressed youth. These include a family history of bipolar disorder, a depressive episode characterized by rapid onset, psychomotor retardation, and psychotic features.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 256583,
"questionText": "In a depressed youth, which of the following factors predict the development of mania?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
930202 | /viewarticle/930202 | [
{
"authors": "Monique A. Rainford, MD; Katherine H. Campbell, MD, MPH",
"content": [
"Find the latest COVID-19 news and guidance in Medscape's Coronavirus Resource Center.",
"A 26-year-old pregnant woman (gravida 3 para 1011) at 38 weeks and 3 days' gestation telephoned an acute care service after having a cough for 3 days. She also reported shortness of breath that was worse from baseline when she coughed. She described fatigue but stated that it was no worse than usual during her current pregnancy.",
"The patient's medical record was reviewed. Five days prior to her telephoning acute care services, the patient canceled a scheduled iron infusion because she felt unwell. At that time, she described having nausea and diarrhea without vomiting. She attributed these symptoms to eating an undercooked meat sandwich. The patient visited her obstetrician at a primary care site the next day. She received iron infusion therapy the day following that visit. Her cough began the day after her infusion therapy, 2 days prior to her telephone call to acute services. The patient had taken her temperature on multiple occasions and remained afebrile.",
"The patient's obstetric history was notable for a prior spontaneous vaginal delivery and anemia during her current pregnancy, which was being treated with iron infusions. She had a history of a positive purified protein derivative test result 3 years prior but had a subsequent negative QuantiFERON-TB Gold test result. Her social history was significant for known COVID-19 exposure.",
"Five days prior to the patient phoning acute care services, she attended a religious ceremony. She discovered that one of the attendees tested positive for COVID-19 4 days after that religious event. The patient had subsequent exposures to family members of the individual who tested positive, including a mother and a preschool-aged child. She was within 6 feet of the child and approximately 6 feet from the mother of the child. The patient's husband, who also attended the religious event, developed a cough on the same day as the patient. He developed a fever that reached 100.6° F (38.1° C). Their 20-month-old daughter developed a cough but remained afebrile.",
"Upon receiving the patient's call, acute care triage notified the on-call obstetrician. After further consultation with an established COVID-19 hospital hotline and the team at the outpatient facility, the patient and family were recommended to undergo testing for COVID-19 infection. The acute care physician, the pediatrician, and an obstetrician met the family in the ambulance bay at the outpatient facility the following day. All team members donned full personal protective equipment (PPE). Multiple nasopharyngeal and oropharyngeal swabs were taken for each of the three family members. One set was sent to an institutional investigational lab and another was sent to a commercial lab, both for polymerase chain reaction testing. A third set of samples was sent to the commercial lab for a respiratory viral panel.",
"The obstetrician checked fetal heart tones, which were normal, and verbally assessed the patient. Positive results from the investigational lab for the patient and husband were received the day after the samples were obtained, and the couple was informed. Confirmatory test results were received 5 days later and were also positive. The child's investigational test and confirmatory nasopharyngeal swab results were both negative; however, her oropharyngeal swab was positive. The patient was contacted by telephone and informed of these results."
],
"date": "May 09, 2020",
"figures": [],
"markdown": "# Case Report: COVID-19 in a Third-Trimester Pregnancy\n\n **Authors:** Monique A. Rainford, MD; Katherine H. Campbell, MD, MPH \n **Date:** May 09, 2020\n\n ## Content\n\n Find the latest COVID-19 news and guidance in Medscape's Coronavirus Resource Center.\nA 26-year-old pregnant woman (gravida 3 para 1011) at 38 weeks and 3 days' gestation telephoned an acute care service after having a cough for 3 days. She also reported shortness of breath that was worse from baseline when she coughed. She described fatigue but stated that it was no worse than usual during her current pregnancy.\nThe patient's medical record was reviewed. Five days prior to her telephoning acute care services, the patient canceled a scheduled iron infusion because she felt unwell. At that time, she described having nausea and diarrhea without vomiting. She attributed these symptoms to eating an undercooked meat sandwich. The patient visited her obstetrician at a primary care site the next day. She received iron infusion therapy the day following that visit. Her cough began the day after her infusion therapy, 2 days prior to her telephone call to acute services. The patient had taken her temperature on multiple occasions and remained afebrile.\nThe patient's obstetric history was notable for a prior spontaneous vaginal delivery and anemia during her current pregnancy, which was being treated with iron infusions. She had a history of a positive purified protein derivative test result 3 years prior but had a subsequent negative QuantiFERON-TB Gold test result. Her social history was significant for known COVID-19 exposure.\nFive days prior to the patient phoning acute care services, she attended a religious ceremony. She discovered that one of the attendees tested positive for COVID-19 4 days after that religious event. The patient had subsequent exposures to family members of the individual who tested positive, including a mother and a preschool-aged child. She was within 6 feet of the child and approximately 6 feet from the mother of the child. The patient's husband, who also attended the religious event, developed a cough on the same day as the patient. He developed a fever that reached 100.6° F (38.1° C). Their 20-month-old daughter developed a cough but remained afebrile.\nUpon receiving the patient's call, acute care triage notified the on-call obstetrician. After further consultation with an established COVID-19 hospital hotline and the team at the outpatient facility, the patient and family were recommended to undergo testing for COVID-19 infection. The acute care physician, the pediatrician, and an obstetrician met the family in the ambulance bay at the outpatient facility the following day. All team members donned full personal protective equipment (PPE). Multiple nasopharyngeal and oropharyngeal swabs were taken for each of the three family members. One set was sent to an institutional investigational lab and another was sent to a commercial lab, both for polymerase chain reaction testing. A third set of samples was sent to the commercial lab for a respiratory viral panel.\nThe obstetrician checked fetal heart tones, which were normal, and verbally assessed the patient. Positive results from the investigational lab for the patient and husband were received the day after the samples were obtained, and the couple was informed. Confirmatory test results were received 5 days later and were also positive. The child's investigational test and confirmatory nasopharyngeal swab results were both negative; however, her oropharyngeal swab was positive. The patient was contacted by telephone and informed of these results.\n\n ## Figures\n\n \n*Page 1 of 3*",
"pagination": {
"current_page": 1,
"total_pages": 3
},
"questionnaire": [],
"title": "Case Report: COVID-19 in a Third-Trimester Pregnancy"
},
{
"authors": "Monique A. Rainford, MD; Katherine H. Campbell, MD, MPH",
"content": [
"Eight days after her initial telephone call to acute services, the patient had a follow-up visit with an obstetrician, who wore full PPE in a negative pressure room. A significant cough was noted. Otherwise, the patient felt better. She was informed of the hospital policy requiring a healthy caregiver. She was instructed to contact the obstetrician when she was in labor and to contact the labor-floor charge nurse upon arriving at the hospital. That way, she could be met by labor and delivery staff at the entrance of the hospital, in order to be properly masked. Hospital leadership was made aware that she was a patient under investigation and informed of the positive test results. Plans were established for the patient's labor and delivery.",
"Care management nurses contacted the patient every 48 hours for the remainder of the week and closely monitored her symptoms. Infectious disease specialists were consulted regarding retesting. Fourteen days after symptom onset, the patient continued to experience a mild cough. She was retested 10 days after her initial positive test result. The results of that test were not received until after she delivered her baby. Her spouse was not retested because he had complete symptom resolution and because 14 days had elapsed since his symptom onset. He was considered clinically cleared by the hospital's infection prevention team.",
"Sixteen days after her initial phone call to acute services, the patient's husband contacted the on-call obstetrician. The patient was at 40 weeks and 5 days' gestation and was in labor. The couple was met by the hospital team at the entrance of the hospital and masks were placed on them. The patient was escorted to a negative pressure room, and care was initiated by a small team wearing full PPE. To reduce the amount of direct face-to-face exposure to the patient, care of the patient was supplemented with video conferencing.",
"The delivery of a healthy male baby was uncomplicated and occurred within 40 minutes of arrival to the hospital. The infant was placed in a negative pressure room and was separated from the mother during the course of admission. However, the father was allowed to interact with the infant and wore a mask. Infant feeding was supplemented by soy formula (via syringe) during the course of the admission.",
"The decision was made not to test the infant because the results would not change the course of clinical care. The patient remained in the hospital until postpartum day 2. At that time, results from the second COVID-19 test were received and were positive. No further testing of the mother was recommended by the infectious disease team, as an additional 6 days had elapsed since the test and approximately 3 weeks had passed since symptom onset. As such, the parents were advised that normal contact with the infant was permissible after discharge; however, masks were advised if either developed a cough."
],
"date": "May 09, 2020",
"figures": [],
"markdown": "# Case Report: COVID-19 in a Third-Trimester Pregnancy\n\n **Authors:** Monique A. Rainford, MD; Katherine H. Campbell, MD, MPH \n **Date:** May 09, 2020\n\n ## Content\n\n Eight days after her initial telephone call to acute services, the patient had a follow-up visit with an obstetrician, who wore full PPE in a negative pressure room. A significant cough was noted. Otherwise, the patient felt better. She was informed of the hospital policy requiring a healthy caregiver. She was instructed to contact the obstetrician when she was in labor and to contact the labor-floor charge nurse upon arriving at the hospital. That way, she could be met by labor and delivery staff at the entrance of the hospital, in order to be properly masked. Hospital leadership was made aware that she was a patient under investigation and informed of the positive test results. Plans were established for the patient's labor and delivery.\nCare management nurses contacted the patient every 48 hours for the remainder of the week and closely monitored her symptoms. Infectious disease specialists were consulted regarding retesting. Fourteen days after symptom onset, the patient continued to experience a mild cough. She was retested 10 days after her initial positive test result. The results of that test were not received until after she delivered her baby. Her spouse was not retested because he had complete symptom resolution and because 14 days had elapsed since his symptom onset. He was considered clinically cleared by the hospital's infection prevention team.\nSixteen days after her initial phone call to acute services, the patient's husband contacted the on-call obstetrician. The patient was at 40 weeks and 5 days' gestation and was in labor. The couple was met by the hospital team at the entrance of the hospital and masks were placed on them. The patient was escorted to a negative pressure room, and care was initiated by a small team wearing full PPE. To reduce the amount of direct face-to-face exposure to the patient, care of the patient was supplemented with video conferencing.\nThe delivery of a healthy male baby was uncomplicated and occurred within 40 minutes of arrival to the hospital. The infant was placed in a negative pressure room and was separated from the mother during the course of admission. However, the father was allowed to interact with the infant and wore a mask. Infant feeding was supplemented by soy formula (via syringe) during the course of the admission.\nThe decision was made not to test the infant because the results would not change the course of clinical care. The patient remained in the hospital until postpartum day 2. At that time, results from the second COVID-19 test were received and were positive. No further testing of the mother was recommended by the infectious disease team, as an additional 6 days had elapsed since the test and approximately 3 weeks had passed since symptom onset. As such, the parents were advised that normal contact with the infant was permissible after discharge; however, masks were advised if either developed a cough.\n\n ## Figures\n\n \n*Page 2 of 3*",
"pagination": {
"current_page": 2,
"total_pages": 3
},
"questionnaire": [],
"title": "Case Report: COVID-19 in a Third-Trimester Pregnancy"
},
{
"authors": "Monique A. Rainford, MD; Katherine H. Campbell, MD, MPH",
"content": [
"The infant was treated as presumed positive for the first 14 days of life. On postpartum day 3, the patient had a telephone clinical encounter with the outpatient pediatrician, who assessed the infant. The infant remained well and was breastfeeding. On postpartum day 7, the patient had a postpartum telephone visit. She was doing well, with an Edinburgh depression score of 7. She was bonding with her infant and doing well with breastfeeding, despite the separation in the hospital. Plans were made for a 6-week postpartum telephone visit.",
"On postpartum day 14, the mother opted to replace an in-office infant visit with another telephone visit, in order to further limit the infant's exposure to COVID-19. At that time, the mother reported that the infant was exclusively breastfeeding approximately every 2 hours in the day and approximately every 3 hours at night. The urine output and frequency of bowel movements were deemed appropriate by the pediatric clinician.",
"This case is an example of a successful vaginal delivery of a woman with COVID-19. Despite being separated from her infant during her hospitalization, the patient was successfully able to bond with her baby. She attributes this success to the nurses in the hospital who fed the baby in a manner that did not affect breastfeeding, her prior experience with childbirth, and her husband's ability to interact with the baby during the hospitalization.",
"This case is also noteworthy for the fact that the virus was only detected by an oropharyngeal swab for the patient's 20-month-old child. Although many members were infected with COVID-19, the family had a good outcome, and the newborn has remained symptom-free, despite the timing of the maternal infection.",
"Recommended Reading",
"Guidance on COVID-19 During Pregnancy and the Puerperium (ISUOG, 2020)",
"COVID-19: Diagnosis and Treatment in Pregnancy",
"COVID-19 Registry Tracks Pregnant Women, Newborns",
"Follow Medscape on Facebook, Twitter, Instagram, and YouTube"
],
"date": "May 09, 2020",
"figures": [],
"markdown": "# Case Report: COVID-19 in a Third-Trimester Pregnancy\n\n **Authors:** Monique A. Rainford, MD; Katherine H. Campbell, MD, MPH \n **Date:** May 09, 2020\n\n ## Content\n\n The infant was treated as presumed positive for the first 14 days of life. On postpartum day 3, the patient had a telephone clinical encounter with the outpatient pediatrician, who assessed the infant. The infant remained well and was breastfeeding. On postpartum day 7, the patient had a postpartum telephone visit. She was doing well, with an Edinburgh depression score of 7. She was bonding with her infant and doing well with breastfeeding, despite the separation in the hospital. Plans were made for a 6-week postpartum telephone visit.\nOn postpartum day 14, the mother opted to replace an in-office infant visit with another telephone visit, in order to further limit the infant's exposure to COVID-19. At that time, the mother reported that the infant was exclusively breastfeeding approximately every 2 hours in the day and approximately every 3 hours at night. The urine output and frequency of bowel movements were deemed appropriate by the pediatric clinician.\nThis case is an example of a successful vaginal delivery of a woman with COVID-19. Despite being separated from her infant during her hospitalization, the patient was successfully able to bond with her baby. She attributes this success to the nurses in the hospital who fed the baby in a manner that did not affect breastfeeding, her prior experience with childbirth, and her husband's ability to interact with the baby during the hospitalization.\nThis case is also noteworthy for the fact that the virus was only detected by an oropharyngeal swab for the patient's 20-month-old child. Although many members were infected with COVID-19, the family had a good outcome, and the newborn has remained symptom-free, despite the timing of the maternal infection.\nRecommended Reading\nGuidance on COVID-19 During Pregnancy and the Puerperium (ISUOG, 2020)\nCOVID-19: Diagnosis and Treatment in Pregnancy\nCOVID-19 Registry Tracks Pregnant Women, Newborns\nFollow Medscape on Facebook, Twitter, Instagram, and YouTube\n\n ## Figures\n\n \n*Page 3 of 3*",
"pagination": {
"current_page": 3,
"total_pages": 3
},
"questionnaire": [],
"title": "Case Report: COVID-19 in a Third-Trimester Pregnancy"
}
] | [] |
722293 | /viewarticle/722293 | [
{
"authors": "Christine Kim, MD; Harold Moskowitz, MD",
"content": [
"Editor's Note:\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"A 45-year-old man with a history of HIV infection presents to the emergency department with progressive shortness of breath and dyspnea on exertion for the past month. He recently developed a cough that is productive of white sputum. Today, he awoke with a temperature of 101.0°F (38.3°C). The patient reports no chest pain, hemoptysis, orthopnea, paroxysmal nocturnal dyspnea, palpitations, or lower-extremity swelling. He developed thrush in the previous 1-2 weeks and has also developed anorexia, with a 20-lb weight loss, over the past 3 months.",
"The remainder of his review of systems is negative. He does not report any recent travel or sick contacts. He has taken no medications recently and elected to stop his HIV therapy regimen 10 years ago because of the adverse effect of severe diarrhea. The patient does not smoke, consume alcohol, or use illicit drugs. He is sexually active with 1 partner and uses condoms inconsistently. The patient has no known drug allergies.",
"Upon physical examination, the patient's temperature is 101.0°F (38.3°C) tympanic. His pulse rate is 136 beats/min, with a regular rhythm. His blood pressure is 100/70 mm Hg. The patient's respiratory rate is 30 breaths/min, with an oxygen saturation of 76% while breathing room air. In general, the patient is cachectic and dyspneic. He has dry mucous membranes, with removable white plaques throughout the oropharynx. The remaining head and neck examination is normal. Coarse bibasilar crackles are present on auscultation. His chest is nontender on palpation, is resonant to percussion, and expands symmetrically. Except for tachycardia, cardiac examination findings are normal.",
"The abdominal and neurologic examinations are unremarkable. The patient has no cyanosis, clubbing, or edema of the extremities. His skin is warm, dry, and free of lesions or rashes. He has no palpable lymphadenopathy.",
"An electrocardiogram reveals sinus tachycardia. The patient's arterial blood gas is consistent with respiratory alkalosis and hypoxia. A chest x-ray reveals diffuse, bilateral increased interstitial markings, and CT angiography (CTA) of the chest demonstrates diffuse ground-glass opacities without lymphadenopathy or pulmonary embolus (Figures 1 and 2).",
"Figure 1.",
"Figure 2.",
"The white blood cell count is 5.2 × 103/μL. The CD4 lymphocyte count is 38 cells/μL. The result on quantitative HIV-1 RNA polymerase chain reaction (PCR) is 1,710,000 copies/mL. Routine blood and urine cultures are negative. An induced sputum specimen is sent for analysis."
],
"date": "April 17, 2020",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/722/290/722290-thumb1.png"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/722/290/722290-thumb_fix2.png"
}
],
"markdown": "# A 45-Year-Old Man With Shortness of Breath, Cough, and Fever\n\n **Authors:** Christine Kim, MD; Harold Moskowitz, MD \n **Date:** April 17, 2020\n\n ## Content\n\n Editor's Note:\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nA 45-year-old man with a history of HIV infection presents to the emergency department with progressive shortness of breath and dyspnea on exertion for the past month. He recently developed a cough that is productive of white sputum. Today, he awoke with a temperature of 101.0°F (38.3°C). The patient reports no chest pain, hemoptysis, orthopnea, paroxysmal nocturnal dyspnea, palpitations, or lower-extremity swelling. He developed thrush in the previous 1-2 weeks and has also developed anorexia, with a 20-lb weight loss, over the past 3 months.\nThe remainder of his review of systems is negative. He does not report any recent travel or sick contacts. He has taken no medications recently and elected to stop his HIV therapy regimen 10 years ago because of the adverse effect of severe diarrhea. The patient does not smoke, consume alcohol, or use illicit drugs. He is sexually active with 1 partner and uses condoms inconsistently. The patient has no known drug allergies.\nUpon physical examination, the patient's temperature is 101.0°F (38.3°C) tympanic. His pulse rate is 136 beats/min, with a regular rhythm. His blood pressure is 100/70 mm Hg. The patient's respiratory rate is 30 breaths/min, with an oxygen saturation of 76% while breathing room air. In general, the patient is cachectic and dyspneic. He has dry mucous membranes, with removable white plaques throughout the oropharynx. The remaining head and neck examination is normal. Coarse bibasilar crackles are present on auscultation. His chest is nontender on palpation, is resonant to percussion, and expands symmetrically. Except for tachycardia, cardiac examination findings are normal.\nThe abdominal and neurologic examinations are unremarkable. The patient has no cyanosis, clubbing, or edema of the extremities. His skin is warm, dry, and free of lesions or rashes. He has no palpable lymphadenopathy.\nAn electrocardiogram reveals sinus tachycardia. The patient's arterial blood gas is consistent with respiratory alkalosis and hypoxia. A chest x-ray reveals diffuse, bilateral increased interstitial markings, and CT angiography (CTA) of the chest demonstrates diffuse ground-glass opacities without lymphadenopathy or pulmonary embolus (Figures 1 and 2).\nFigure 1.\nFigure 2.\nThe white blood cell count is 5.2 × 103/μL. The CD4 lymphocyte count is 38 cells/μL. The result on quantitative HIV-1 RNA polymerase chain reaction (PCR) is 1,710,000 copies/mL. Routine blood and urine cultures are negative. An induced sputum specimen is sent for analysis.\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n\n*Page 1 of 5*",
"pagination": {
"current_page": 1,
"total_pages": 5
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 344650,
"choiceText": "Pulmonary tuberculosis",
"correct": false,
"displayOrder": 1,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 344651,
"choiceText": "Kaposi sarcoma",
"correct": false,
"displayOrder": 2,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 344652,
"choiceText": "Lymphoma",
"correct": false,
"displayOrder": 3,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 344653,
"choiceText": "<em>Pneumocystis jiroveci</em> pneumonia",
"correct": true,
"displayOrder": 4,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 344654,
"choiceText": "<em>Cryptococcus neoformans</em> pneumonia",
"correct": false,
"displayOrder": 5,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "",
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": "",
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 97407,
"questionText": "Which of the following is the most likely diagnosis?<br><br>\r\n<em>Hint: Note the distribution of the opacities on the chest x-ray and chest CTA</em>.",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 45-Year-Old Man With Shortness of Breath, Cough, and Fever"
},
{
"authors": "Christine Kim, MD; Harold Moskowitz, MD",
"content": [
"The diagnosis of \nPneumocystis jiroveci pneumonia (PJP) was based on the patient's history, physical examination, chest x-ray, chest CTA (although not required for diagnosis), and microscopic examination. The patient's induced sputum sample showed rare mixed flora; as a result, a bronchoalveolar lavage (BAL) specimen was obtained. Direct fluorescent antibody testing of the BAL specimen demonstrated P jiroveci. Additional testing, including acid-fast bacilli staining of the sputum; \nCryptococcus neoformans\n antigen testing and serologic testing fortoxoplasmosis and syphilis. Sputnum cultures for cytomegalovirus, fungal pathogens, \nLegionella pneumophila, and \nMycoplasma pneumoniae\n were negative thus, ruling out alternative diagnoses.",
"P jiroveci, previously known as P carinii, has been reclassified from a protozoan to a fungal organism and is one of the most prevalent AIDS-defining infections. Although Pneumocystis species resemble protozoa morphologically, the cell wall composition and nucleotide sequences are more characteristic of fungi.[1] The incidence of PJP is 0.5% in HIV-infected individuals with a CD4 count of 201-350 cells/µL and 8% in those with a CD4 count < 200 cells/µL.[2] The Pulmonary Complications of HIV Infection Study Group, which included more than 1100 individuals, found that 95% of patients with P jiroveci, infections had CD4 cell counts < 200 cells/µL. This study also showed that HIV transmission category, smoking history, and age did not predict the development of pneumonia.",
"The risk of developing PJP in black patients was one third of that seen in white patients.[3] Several risk factors for the development of PJP in patients who are not infected with HIV have been identified, including malignancy, immunosuppression, and solid-organ transplantation. Immunomodulators (most commonly, corticosteroids) and monoclonal antibody therapy are also associated with P jiroveci infection.[4]",
"P jiroveci cannot be sustained outside a host lung, and this limits research involving the organism. In addition, unique forms infect different animal species. P jiroveci is the official name for human Pneumocystis, and this organism cannot infect other animal species. Likewise, organisms found in other animals cannot infect humans. Although the transmission of P jiroveci is not well understood, current evidence supports human-to-human acquisition, probably via airborne particles. This route is favored over the previous suggestion that pneumonia develops from reservoirs of colonized P jiroveci acquired during childhood. Evidence supporting this includes the observation that the same strain of organism infects transplant patients in the same hospital.[1] Once inside a host lung, the haploid trophic form of P jiroveci is thought to adhere to type 1 alveolar cell membranes, then cluster to conjugate and progress as cysts. The cysts produce intracystic sporozoites, which are released and differentiate into trophozoites. One survival mechanism within the host lung may involve disabling phagocytic activity and reducing alveolar macrophage activation.[5]"
],
"date": "April 17, 2020",
"figures": [],
"markdown": "# A 45-Year-Old Man With Shortness of Breath, Cough, and Fever\n\n **Authors:** Christine Kim, MD; Harold Moskowitz, MD \n **Date:** April 17, 2020\n\n ## Content\n\n The diagnosis of \nPneumocystis jiroveci pneumonia (PJP) was based on the patient's history, physical examination, chest x-ray, chest CTA (although not required for diagnosis), and microscopic examination. The patient's induced sputum sample showed rare mixed flora; as a result, a bronchoalveolar lavage (BAL) specimen was obtained. Direct fluorescent antibody testing of the BAL specimen demonstrated P jiroveci. Additional testing, including acid-fast bacilli staining of the sputum; \nCryptococcus neoformans\n antigen testing and serologic testing fortoxoplasmosis and syphilis. Sputnum cultures for cytomegalovirus, fungal pathogens, \nLegionella pneumophila, and \nMycoplasma pneumoniae\n were negative thus, ruling out alternative diagnoses.\nP jiroveci, previously known as P carinii, has been reclassified from a protozoan to a fungal organism and is one of the most prevalent AIDS-defining infections. Although Pneumocystis species resemble protozoa morphologically, the cell wall composition and nucleotide sequences are more characteristic of fungi.[1] The incidence of PJP is 0.5% in HIV-infected individuals with a CD4 count of 201-350 cells/µL and 8% in those with a CD4 count < 200 cells/µL.[2] The Pulmonary Complications of HIV Infection Study Group, which included more than 1100 individuals, found that 95% of patients with P jiroveci, infections had CD4 cell counts < 200 cells/µL. This study also showed that HIV transmission category, smoking history, and age did not predict the development of pneumonia.\nThe risk of developing PJP in black patients was one third of that seen in white patients.[3] Several risk factors for the development of PJP in patients who are not infected with HIV have been identified, including malignancy, immunosuppression, and solid-organ transplantation. Immunomodulators (most commonly, corticosteroids) and monoclonal antibody therapy are also associated with P jiroveci infection.[4]\nP jiroveci cannot be sustained outside a host lung, and this limits research involving the organism. In addition, unique forms infect different animal species. P jiroveci is the official name for human Pneumocystis, and this organism cannot infect other animal species. Likewise, organisms found in other animals cannot infect humans. Although the transmission of P jiroveci is not well understood, current evidence supports human-to-human acquisition, probably via airborne particles. This route is favored over the previous suggestion that pneumonia develops from reservoirs of colonized P jiroveci acquired during childhood. Evidence supporting this includes the observation that the same strain of organism infects transplant patients in the same hospital.[1] Once inside a host lung, the haploid trophic form of P jiroveci is thought to adhere to type 1 alveolar cell membranes, then cluster to conjugate and progress as cysts. The cysts produce intracystic sporozoites, which are released and differentiate into trophozoites. One survival mechanism within the host lung may involve disabling phagocytic activity and reducing alveolar macrophage activation.[5]\n\n ## Figures\n\n \n*Page 2 of 5*",
"pagination": {
"current_page": 2,
"total_pages": 5
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 344650,
"choiceText": "Pulmonary tuberculosis",
"correct": false,
"displayOrder": 1,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 344651,
"choiceText": "Kaposi sarcoma",
"correct": false,
"displayOrder": 2,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 344652,
"choiceText": "Lymphoma",
"correct": false,
"displayOrder": 3,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 344653,
"choiceText": "<em>Pneumocystis jiroveci</em> pneumonia",
"correct": true,
"displayOrder": 4,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 344654,
"choiceText": "<em>Cryptococcus neoformans</em> pneumonia",
"correct": false,
"displayOrder": 5,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "",
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": "",
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 97407,
"questionText": "Which of the following is the most likely diagnosis?<br><br>\r\n<em>Hint: Note the distribution of the opacities on the chest x-ray and chest CTA</em>.",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 45-Year-Old Man With Shortness of Breath, Cough, and Fever"
},
{
"authors": "Christine Kim, MD; Harold Moskowitz, MD",
"content": [
"Studies have shown that HIV-infected patients with \nP jiroveci have fewer neutrophils and a higher organism burden than non-HIV-infected immunocompromised patients. This observation may account for the difference in the clinical features of PJP observed in patients with AIDS as compared with those without AIDS. Although the host immune system limits and clears Pneumocystis infections, an exaggerated inflammatory response results in lung injury and alveolar damage, impaired gas exchange, and respiratory failure. Nearly all HIV-infected patients present with a gradual onset of fever, progressive dyspnea, and cough that is usually nonproductive. They may also experience chest pain, chills, weight loss, and fatigue. Some patients are asymptomatic.",
"Physical examination findings include fever, tachypnea, and crackles or rhonchi on auscultation; however, approximately half of patients have normal chest examinations. In contrast to HIV-infected patients, individuals with compromised immune systems resulting from other causes present with acute fulminate respiratory failure that frequently requires mechanical ventilation. Other symptoms include fever and nonproductive cough, which may correlate with immunosuppressant titration or dosage.[4,5] A difference in patient mortality has also been observed. The mortality of patients with AIDS and acute PJP infection is 10%-20% and is significantly higher for those requiring mechanical ventilation. The mortality rate in non-HIV-infected patients with PJP approaches 30%-60%.[6]",
"PJP can be difficult to diagnose initially. The first signs and symptoms are nonspecific and may be subtle or atypical in those receiving prophylactic drugs. In addition, immunocompromised patients may be co-infected with multiple organisms. Because P jiroveci cannot be cultured, microscopic evaluation is required for definitive diagnosis. The first step in diagnosing PJP is obtaining sputum induced by the inhalation of hypertonic saline, which has a 55%-92% yield. The sensitivity may be lower in patients using aerosolized pentamidine for prophylaxis. A negative result should prompt the performance of BAL, a more invasive test with sensitivity ranging from 89% to 98%. Potential complications of BAL include fever and respiratory failure.",
"Transbronchial biopsy is usually not indicated because of the high sensitivity of BAL and the potential complications of hemoptysis and pneumothorax. Similarly, transthoracic needle biopsy is highly sensitive, but should be avoided because of a 30% incidence of pneumothorax. Thoracotomy or video-assisted thoracoscopic surgical biopsies have sensitivities reported as 100%, but they are rarely used because of the expense and invasiveness of the procedure. If a patient is intubated, endotracheal aspirates yield a sensitivity of 92%, usually making BAL unnecessary. Sputum or bronchial aspirates may be stained for the trophic or cystic forms of P jiroveci. Immunofluorescence studies with monoclonal antibodies can detect trophic and cystic forms and may increase the sensitivity of induced sputum specimens. PCR may help differentiate colonization from infection as well as identify drug-resistant strains of P jiroveci. Another possible diagnostic test is measurement of serum lactate dehydrogenase (LDH) levels; although a normal serum level makes P jiroveci unlikely, an elevated level has low specificity.",
"The appropriate initial imaging study for immunocompromised patients with pulmonary or constitutional symptoms is 2-view chest x-ray. Radiographically, PJP classically presents as bilateral perihilar interstitial infiltrates that become more extensive and may progress to consolidations over several days; however, multiple chest x-ray findings, including normal findings, have been described in HIV-positive patients with PJP. Chest x-ray findings resolve in 2-4 weeks with appropriate treatment. Parenchymal injury may result in persistently abnormal radiographic findings. Up to 10%-39% of patients may have a normal chest x-ray. Less common findings include parenchymal nodules, pneumatocele, focal consolidation, bronchiectasis, and pneumothorax. Spontaneous PJP-associated pneumothorax have higher mortality rates. Patients previously treated with aerosolized pentamidine may present with upper lobe infiltrates.",
"High-resolution chest tomography (HRCT) may help with the diagnosis if the chest x-ray is negative and clinical suspicion remains high. The HRCT finding of ground-glass attenuation opacities is noted when exudate fills alveoli without obstruction of the underlying pulmonary architecture.[6] One study reported a sensitivity of 100% with a specificity of 89% in the presence of patchy or nodular ground-glass densities on HRCT.[4] The differential diagnosis of ground-glass opacities includes pulmonary edema, drug toxicity, hypersensitivity pneumonitis, acute respiratory distress syndrome, lymphoma, Kaposi sarcoma, and other opportunistic lung infections. Focal consolidation is a less common finding. Although HRCT does not establish the diagnosis, a negative study may exclude the diagnosis. Another highly sensitive imaging examination is gallium-67 citrate scanning, which demonstrates diffuse, intense uptake in patients with PJP. Low specificity, a 2-day delay in results, and expense limit the utility of this test. P jiroveci infection that occurs despite the use of appropriate prophylactic therapy may result in lower sensitivity and atypical imaging findings.[4,5]"
],
"date": "April 17, 2020",
"figures": [],
"markdown": "# A 45-Year-Old Man With Shortness of Breath, Cough, and Fever\n\n **Authors:** Christine Kim, MD; Harold Moskowitz, MD \n **Date:** April 17, 2020\n\n ## Content\n\n Studies have shown that HIV-infected patients with \nP jiroveci have fewer neutrophils and a higher organism burden than non-HIV-infected immunocompromised patients. This observation may account for the difference in the clinical features of PJP observed in patients with AIDS as compared with those without AIDS. Although the host immune system limits and clears Pneumocystis infections, an exaggerated inflammatory response results in lung injury and alveolar damage, impaired gas exchange, and respiratory failure. Nearly all HIV-infected patients present with a gradual onset of fever, progressive dyspnea, and cough that is usually nonproductive. They may also experience chest pain, chills, weight loss, and fatigue. Some patients are asymptomatic.\nPhysical examination findings include fever, tachypnea, and crackles or rhonchi on auscultation; however, approximately half of patients have normal chest examinations. In contrast to HIV-infected patients, individuals with compromised immune systems resulting from other causes present with acute fulminate respiratory failure that frequently requires mechanical ventilation. Other symptoms include fever and nonproductive cough, which may correlate with immunosuppressant titration or dosage.[4,5] A difference in patient mortality has also been observed. The mortality of patients with AIDS and acute PJP infection is 10%-20% and is significantly higher for those requiring mechanical ventilation. The mortality rate in non-HIV-infected patients with PJP approaches 30%-60%.[6]\nPJP can be difficult to diagnose initially. The first signs and symptoms are nonspecific and may be subtle or atypical in those receiving prophylactic drugs. In addition, immunocompromised patients may be co-infected with multiple organisms. Because P jiroveci cannot be cultured, microscopic evaluation is required for definitive diagnosis. The first step in diagnosing PJP is obtaining sputum induced by the inhalation of hypertonic saline, which has a 55%-92% yield. The sensitivity may be lower in patients using aerosolized pentamidine for prophylaxis. A negative result should prompt the performance of BAL, a more invasive test with sensitivity ranging from 89% to 98%. Potential complications of BAL include fever and respiratory failure.\nTransbronchial biopsy is usually not indicated because of the high sensitivity of BAL and the potential complications of hemoptysis and pneumothorax. Similarly, transthoracic needle biopsy is highly sensitive, but should be avoided because of a 30% incidence of pneumothorax. Thoracotomy or video-assisted thoracoscopic surgical biopsies have sensitivities reported as 100%, but they are rarely used because of the expense and invasiveness of the procedure. If a patient is intubated, endotracheal aspirates yield a sensitivity of 92%, usually making BAL unnecessary. Sputum or bronchial aspirates may be stained for the trophic or cystic forms of P jiroveci. Immunofluorescence studies with monoclonal antibodies can detect trophic and cystic forms and may increase the sensitivity of induced sputum specimens. PCR may help differentiate colonization from infection as well as identify drug-resistant strains of P jiroveci. Another possible diagnostic test is measurement of serum lactate dehydrogenase (LDH) levels; although a normal serum level makes P jiroveci unlikely, an elevated level has low specificity.\nThe appropriate initial imaging study for immunocompromised patients with pulmonary or constitutional symptoms is 2-view chest x-ray. Radiographically, PJP classically presents as bilateral perihilar interstitial infiltrates that become more extensive and may progress to consolidations over several days; however, multiple chest x-ray findings, including normal findings, have been described in HIV-positive patients with PJP. Chest x-ray findings resolve in 2-4 weeks with appropriate treatment. Parenchymal injury may result in persistently abnormal radiographic findings. Up to 10%-39% of patients may have a normal chest x-ray. Less common findings include parenchymal nodules, pneumatocele, focal consolidation, bronchiectasis, and pneumothorax. Spontaneous PJP-associated pneumothorax have higher mortality rates. Patients previously treated with aerosolized pentamidine may present with upper lobe infiltrates.\nHigh-resolution chest tomography (HRCT) may help with the diagnosis if the chest x-ray is negative and clinical suspicion remains high. The HRCT finding of ground-glass attenuation opacities is noted when exudate fills alveoli without obstruction of the underlying pulmonary architecture.[6] One study reported a sensitivity of 100% with a specificity of 89% in the presence of patchy or nodular ground-glass densities on HRCT.[4] The differential diagnosis of ground-glass opacities includes pulmonary edema, drug toxicity, hypersensitivity pneumonitis, acute respiratory distress syndrome, lymphoma, Kaposi sarcoma, and other opportunistic lung infections. Focal consolidation is a less common finding. Although HRCT does not establish the diagnosis, a negative study may exclude the diagnosis. Another highly sensitive imaging examination is gallium-67 citrate scanning, which demonstrates diffuse, intense uptake in patients with PJP. Low specificity, a 2-day delay in results, and expense limit the utility of this test. P jiroveci infection that occurs despite the use of appropriate prophylactic therapy may result in lower sensitivity and atypical imaging findings.[4,5]\n\n ## Figures\n\n \n*Page 3 of 5*",
"pagination": {
"current_page": 3,
"total_pages": 5
},
"questionnaire": [],
"title": "A 45-Year-Old Man With Shortness of Breath, Cough, and Fever"
},
{
"authors": "Christine Kim, MD; Harold Moskowitz, MD",
"content": [
"The treatment of choice for acute PJP infection is trimethoprim-sulfamethoxazole for 21 days. The recommended dose for mild or moderate disease is 320 mg of trimethoprim and 1600 mg of sulfamethoxazole every 8 hours. Severe disease requires parenteral therapy. For patients with sulfa drug allergies, alternative therapy includes parenteral pentamidine, atovaquone, dapsone-trimethoprim, or clindamycin-primaquine. Empiric treatment for PJP is discouraged in the era of highly active antiretroviral therapy (HAART) because of concerns of the lower prevalence of PJP or atypical presentation in those receiving HAART or prophylactic antibiotic therapy and the potential concerns for drug toxicity. Adjunctive therapy with systemic corticosteroids has been shown to improve the clinical outcome and mortality rate in HIV-infected patients with moderate to severe symptoms from PJP without increasing the risk for other opportunistic infections. In particular, HIV-infected patients with a partial pressure of arterial oxygen < 70 mm Hg while breathing room air or an alveolar-arteriolar gradient > 35 have been shown to benefit from the addition of corticosteroids. The recommended prednisone regimen includes 40 mg twice daily for 5 days, 40 mg daily for the next 5 days, and 20 mg for the following 10 days.[7]",
"Prophylaxis against PJP in HIV-infected patients begins when the CD4 count is < 200 cells/µL. Other indications include a history of oropharyngeal candidiasis or PJP. Prophylaxis is discontinued when the CD4 count rises above 200 cells/µL for 3 months, as may occur in response to HAART, and is reinstituted if the CD4 count decreases below the threshold. The prophylactic agent of choice is trimethoprim-sulfamethoxazole. Alternatives include dapsone, aerosolized pentamidine, and atovaquone. For non-HIV-infected patients, immunosuppressive therapy is an indication for prophylaxis.[8]",
"The use of trimethoprim-sulfamethoxazole may be limited for several reasons. Patients with sulfa medication allergies may not tolerate trimethoprim-sulfamethoxazole. Some studies have shown an association between failed trimethoprim-sulfamethoxazole prophylaxis and treatment in patients with mutations of the dihydropteroate synthase (DHPS) gene, which encodes the folate biosynthesis enzyme inhibited by sulfamethoxazole. Resistance to other pharmaceuticals has also emerged in patients receiving prophylactic therapy.[9] Strains of P jiroveci with mutations in cytochrome b, the target of atovaquone, have been observed. Multiple studies have addressed concerns about the use of trimethoprim-sulfamethoxazole by patients treated with methotrexate and failed to show the development of severe myelosuppression with prophylaxis.[5] However, treatment guidelines recommend folate supplementation or leucovorin with complete blood count and monitoring of liver function tests.",
"The patient in this case was admitted and treated with parenteral trimethoprim-sulfamethoxazole, a prednisone taper, and supplemental oxygen. The patient was transitioned to oral trimethoprim-sulfamethoxazole and eventually switched to atovaquone for prophylaxis. He received nystatin rinses and fluconazole for thrush. Since his discharge, a new primary care physician and an infectious disease physician have guided the patient's follow-up care, including the initiation of lamivudine, ritonavir, atazanavir, and abacavir for antiretroviral therapy."
],
"date": "April 17, 2020",
"figures": [],
"markdown": "# A 45-Year-Old Man With Shortness of Breath, Cough, and Fever\n\n **Authors:** Christine Kim, MD; Harold Moskowitz, MD \n **Date:** April 17, 2020\n\n ## Content\n\n The treatment of choice for acute PJP infection is trimethoprim-sulfamethoxazole for 21 days. The recommended dose for mild or moderate disease is 320 mg of trimethoprim and 1600 mg of sulfamethoxazole every 8 hours. Severe disease requires parenteral therapy. For patients with sulfa drug allergies, alternative therapy includes parenteral pentamidine, atovaquone, dapsone-trimethoprim, or clindamycin-primaquine. Empiric treatment for PJP is discouraged in the era of highly active antiretroviral therapy (HAART) because of concerns of the lower prevalence of PJP or atypical presentation in those receiving HAART or prophylactic antibiotic therapy and the potential concerns for drug toxicity. Adjunctive therapy with systemic corticosteroids has been shown to improve the clinical outcome and mortality rate in HIV-infected patients with moderate to severe symptoms from PJP without increasing the risk for other opportunistic infections. In particular, HIV-infected patients with a partial pressure of arterial oxygen < 70 mm Hg while breathing room air or an alveolar-arteriolar gradient > 35 have been shown to benefit from the addition of corticosteroids. The recommended prednisone regimen includes 40 mg twice daily for 5 days, 40 mg daily for the next 5 days, and 20 mg for the following 10 days.[7]\nProphylaxis against PJP in HIV-infected patients begins when the CD4 count is < 200 cells/µL. Other indications include a history of oropharyngeal candidiasis or PJP. Prophylaxis is discontinued when the CD4 count rises above 200 cells/µL for 3 months, as may occur in response to HAART, and is reinstituted if the CD4 count decreases below the threshold. The prophylactic agent of choice is trimethoprim-sulfamethoxazole. Alternatives include dapsone, aerosolized pentamidine, and atovaquone. For non-HIV-infected patients, immunosuppressive therapy is an indication for prophylaxis.[8]\nThe use of trimethoprim-sulfamethoxazole may be limited for several reasons. Patients with sulfa medication allergies may not tolerate trimethoprim-sulfamethoxazole. Some studies have shown an association between failed trimethoprim-sulfamethoxazole prophylaxis and treatment in patients with mutations of the dihydropteroate synthase (DHPS) gene, which encodes the folate biosynthesis enzyme inhibited by sulfamethoxazole. Resistance to other pharmaceuticals has also emerged in patients receiving prophylactic therapy.[9] Strains of P jiroveci with mutations in cytochrome b, the target of atovaquone, have been observed. Multiple studies have addressed concerns about the use of trimethoprim-sulfamethoxazole by patients treated with methotrexate and failed to show the development of severe myelosuppression with prophylaxis.[5] However, treatment guidelines recommend folate supplementation or leucovorin with complete blood count and monitoring of liver function tests.\nThe patient in this case was admitted and treated with parenteral trimethoprim-sulfamethoxazole, a prednisone taper, and supplemental oxygen. The patient was transitioned to oral trimethoprim-sulfamethoxazole and eventually switched to atovaquone for prophylaxis. He received nystatin rinses and fluconazole for thrush. Since his discharge, a new primary care physician and an infectious disease physician have guided the patient's follow-up care, including the initiation of lamivudine, ritonavir, atazanavir, and abacavir for antiretroviral therapy.\n\n ## Figures\n\n \n*Page 4 of 5*",
"pagination": {
"current_page": 4,
"total_pages": 5
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 344655,
"choiceText": "Chest x-ray",
"correct": true,
"displayOrder": 1,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 344656,
"choiceText": "HRCT",
"correct": false,
"displayOrder": 2,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 344657,
"choiceText": "Bronchoalveolar lavage",
"correct": false,
"displayOrder": 3,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 344658,
"choiceText": "MRI with contrast",
"correct": false,
"displayOrder": 4,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 344659,
"choiceText": "Arterial blood gas measurement",
"correct": false,
"displayOrder": 5,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The appropriate initial imaging study for immunocompromised patients with pulmonary or constitutional symptoms is 2-view chest x-ray. Radiographically, PJP classically presents as bilateral perihilar interstitial infiltrates that become more extensive and may progress to consolidations over several days; however, multiple chest x-ray findings, including normal findings, have been described in HIV-positive patients with PJP. HRCT may help with the diagnosis if the chest x-ray is negative and clinical suspicion remains high. The HRCT finding of ground-glass attenuation opacities is noted when exudate fills alveoli without obstruction of the underlying pulmonary architecture. Arterial blood gas may help determine disease severity and guide treatment, but is not a diagnostic test for PJP. Bronchoalveolar lavage and magnetic resonance imaging are not appropriate initial tests.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": "",
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 97408,
"questionText": "An HIV-positive patient who has a CD4 count of 70 cells/µL presents with progressive dyspnea, a low-grade fever, and a nonproductive cough. Which of the following is the most appropriate first diagnostic test in the emergency department setting?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 344660,
"choiceText": "Prophylaxis should be started and continued for the lifetime of the patient",
"correct": false,
"displayOrder": 1,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 344661,
"choiceText": "Prophylaxis should be started and discontinued once the patient's CD4 count is above 100 cells/µL",
"correct": false,
"displayOrder": 2,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 344662,
"choiceText": "Prophylaxis should be started and discontinued once the patient's CD4 count is above 150 cells/µL",
"correct": false,
"displayOrder": 3,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 344663,
"choiceText": "Prophylaxis should be started and discontinued once the patient's CD4 count is above 200 cells/µL for 3 months",
"correct": true,
"displayOrder": 4,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 344664,
"choiceText": "Prophylaxis with trimethoprim-sulfamethoxazole should be avoided in patients using methotrexate because of the risk for severe myelosuppression",
"correct": false,
"displayOrder": 5,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Prophylaxis against <em>P jiroveci</em> should begin when the CD4 count is below 200 cells/µL. Once the CD4 count rises above 200 cells/µL for 3 months, prophylaxis may be discontinued. Prophylaxis should be reinstituted if the cell count decreases below this threshold.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": "",
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 97409,
"questionText": "The patient is diagnosed with PJP and received trimethoprim-sulfamethoxazole therapy for 21 days. Which of the following statements concerning prophylactic therapy for <em>Pneumocystis jiroveci</em> is true?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 45-Year-Old Man With Shortness of Breath, Cough, and Fever"
},
{
"authors": "Christine Kim, MD; Harold Moskowitz, MD",
"content": [],
"date": "April 17, 2020",
"figures": [],
"markdown": "# A 45-Year-Old Man With Shortness of Breath, Cough, and Fever\n\n **Authors:** Christine Kim, MD; Harold Moskowitz, MD \n **Date:** April 17, 2020\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 5 of 5*",
"pagination": {
"current_page": 5,
"total_pages": 5
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 344655,
"choiceText": "Chest x-ray",
"correct": true,
"displayOrder": 1,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 344656,
"choiceText": "HRCT",
"correct": false,
"displayOrder": 2,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 344657,
"choiceText": "Bronchoalveolar lavage",
"correct": false,
"displayOrder": 3,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 344658,
"choiceText": "MRI with contrast",
"correct": false,
"displayOrder": 4,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 344659,
"choiceText": "Arterial blood gas measurement",
"correct": false,
"displayOrder": 5,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The appropriate initial imaging study for immunocompromised patients with pulmonary or constitutional symptoms is 2-view chest x-ray. Radiographically, PJP classically presents as bilateral perihilar interstitial infiltrates that become more extensive and may progress to consolidations over several days; however, multiple chest x-ray findings, including normal findings, have been described in HIV-positive patients with PJP. HRCT may help with the diagnosis if the chest x-ray is negative and clinical suspicion remains high. The HRCT finding of ground-glass attenuation opacities is noted when exudate fills alveoli without obstruction of the underlying pulmonary architecture. Arterial blood gas may help determine disease severity and guide treatment, but is not a diagnostic test for PJP. Bronchoalveolar lavage and magnetic resonance imaging are not appropriate initial tests.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": "",
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 97408,
"questionText": "An HIV-positive patient who has a CD4 count of 70 cells/µL presents with progressive dyspnea, a low-grade fever, and a nonproductive cough. Which of the following is the most appropriate first diagnostic test in the emergency department setting?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 344660,
"choiceText": "Prophylaxis should be started and continued for the lifetime of the patient",
"correct": false,
"displayOrder": 1,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 344661,
"choiceText": "Prophylaxis should be started and discontinued once the patient's CD4 count is above 100 cells/µL",
"correct": false,
"displayOrder": 2,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 344662,
"choiceText": "Prophylaxis should be started and discontinued once the patient's CD4 count is above 150 cells/µL",
"correct": false,
"displayOrder": 3,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 344663,
"choiceText": "Prophylaxis should be started and discontinued once the patient's CD4 count is above 200 cells/µL for 3 months",
"correct": true,
"displayOrder": 4,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 344664,
"choiceText": "Prophylaxis with trimethoprim-sulfamethoxazole should be avoided in patients using methotrexate because of the risk for severe myelosuppression",
"correct": false,
"displayOrder": 5,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Prophylaxis against <em>P jiroveci</em> should begin when the CD4 count is below 200 cells/µL. Once the CD4 count rises above 200 cells/µL for 3 months, prophylaxis may be discontinued. Prophylaxis should be reinstituted if the cell count decreases below this threshold.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": "",
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 97409,
"questionText": "The patient is diagnosed with PJP and received trimethoprim-sulfamethoxazole therapy for 21 days. Which of the following statements concerning prophylactic therapy for <em>Pneumocystis jiroveci</em> is true?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 45-Year-Old Man With Shortness of Breath, Cough, and Fever"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 344650,
"choiceText": "Pulmonary tuberculosis",
"correct": false,
"displayOrder": 1,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 344651,
"choiceText": "Kaposi sarcoma",
"correct": false,
"displayOrder": 2,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 344652,
"choiceText": "Lymphoma",
"correct": false,
"displayOrder": 3,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 344653,
"choiceText": "<em>Pneumocystis jiroveci</em> pneumonia",
"correct": true,
"displayOrder": 4,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 344654,
"choiceText": "<em>Cryptococcus neoformans</em> pneumonia",
"correct": false,
"displayOrder": 5,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "",
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": "",
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 97407,
"questionText": "Which of the following is the most likely diagnosis?<br><br>\r\n<em>Hint: Note the distribution of the opacities on the chest x-ray and chest CTA</em>.",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 344655,
"choiceText": "Chest x-ray",
"correct": true,
"displayOrder": 1,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 344656,
"choiceText": "HRCT",
"correct": false,
"displayOrder": 2,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 344657,
"choiceText": "Bronchoalveolar lavage",
"correct": false,
"displayOrder": 3,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 344658,
"choiceText": "MRI with contrast",
"correct": false,
"displayOrder": 4,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 344659,
"choiceText": "Arterial blood gas measurement",
"correct": false,
"displayOrder": 5,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The appropriate initial imaging study for immunocompromised patients with pulmonary or constitutional symptoms is 2-view chest x-ray. Radiographically, PJP classically presents as bilateral perihilar interstitial infiltrates that become more extensive and may progress to consolidations over several days; however, multiple chest x-ray findings, including normal findings, have been described in HIV-positive patients with PJP. HRCT may help with the diagnosis if the chest x-ray is negative and clinical suspicion remains high. The HRCT finding of ground-glass attenuation opacities is noted when exudate fills alveoli without obstruction of the underlying pulmonary architecture. Arterial blood gas may help determine disease severity and guide treatment, but is not a diagnostic test for PJP. Bronchoalveolar lavage and magnetic resonance imaging are not appropriate initial tests.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": "",
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 97408,
"questionText": "An HIV-positive patient who has a CD4 count of 70 cells/µL presents with progressive dyspnea, a low-grade fever, and a nonproductive cough. Which of the following is the most appropriate first diagnostic test in the emergency department setting?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 344660,
"choiceText": "Prophylaxis should be started and continued for the lifetime of the patient",
"correct": false,
"displayOrder": 1,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 344661,
"choiceText": "Prophylaxis should be started and discontinued once the patient's CD4 count is above 100 cells/µL",
"correct": false,
"displayOrder": 2,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 344662,
"choiceText": "Prophylaxis should be started and discontinued once the patient's CD4 count is above 150 cells/µL",
"correct": false,
"displayOrder": 3,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 344663,
"choiceText": "Prophylaxis should be started and discontinued once the patient's CD4 count is above 200 cells/µL for 3 months",
"correct": true,
"displayOrder": 4,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 344664,
"choiceText": "Prophylaxis with trimethoprim-sulfamethoxazole should be avoided in patients using methotrexate because of the risk for severe myelosuppression",
"correct": false,
"displayOrder": 5,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Prophylaxis against <em>P jiroveci</em> should begin when the CD4 count is below 200 cells/µL. Once the CD4 count rises above 200 cells/µL for 3 months, prophylaxis may be discontinued. Prophylaxis should be reinstituted if the cell count decreases below this threshold.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": "",
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 97409,
"questionText": "The patient is diagnosed with PJP and received trimethoprim-sulfamethoxazole therapy for 21 days. Which of the following statements concerning prophylactic therapy for <em>Pneumocystis jiroveci</em> is true?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
927030 | /viewarticle/927030 | [
{
"authors": "Monica Saini, MD, MBBS",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"A 25-year-old woman presents with left eye pain and double vision that began 3 days prior. She describes throbbing pain that started in the left retro-orbital region and spread to her right forehead within several minutes. This was associated with mild nausea, without vomiting, and photophobia. She went to bed and subsequently noted droopiness of the left eyelid and double vision upon waking up after a few hours. The droopiness and diplopia gradually worsened over 24-48 hours, which prompted her to seek medical attention.",
"She had recovered from an upper respiratory tract infection 1 week before the onset of her current symptoms. She reports no facial numbness, dysphagia, dysarthria, limb numbness, or weakness. Her medical history is significant for infrequent, episodic migraines (without aura), which she treats with simple analgesics. The headache that preceded the droopiness and diplopia was similar to her usual migraines. She reports having had three similar episodes of left-eye droopiness and diplopia associated with headache at age 10 years, 17 years, and 23 years. Each time, she spontaneously recovered over a period of few days. She has not undergone previous brain scanning and has never received steroids. She describes a family history of similar headaches.",
"The patient's physical examination findings are unremarkable. No ocular abnormalities are apparent, and the fundus examination findings are normal. Her neurologic examination is significant for a partial, left oculomotor nerve palsy (left mild ptosis and limited eye adduction), with pupillary sparing. No other cranial nerve, motor, sensory, or cerebellar deficits are noted. Her deep tendon reflexes are normal.",
"The patient's renal, liver, and thyroid panel; erythrocyte sedimentation rate; and antinuclear antibody and anti-extractable nuclear antigen antibody findings are all within reference ranges. All other laboratory findings are also unremarkable. MRI with magnetic resonance angiography is performed (Figure)."
],
"date": "March 20, 2020",
"figures": [],
"markdown": "# A 25-Year-Old Woman With a Droopy Eyelid and Double Vision\n\n **Authors:** Monica Saini, MD, MBBS \n **Date:** March 20, 2020\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nA 25-year-old woman presents with left eye pain and double vision that began 3 days prior. She describes throbbing pain that started in the left retro-orbital region and spread to her right forehead within several minutes. This was associated with mild nausea, without vomiting, and photophobia. She went to bed and subsequently noted droopiness of the left eyelid and double vision upon waking up after a few hours. The droopiness and diplopia gradually worsened over 24-48 hours, which prompted her to seek medical attention.\nShe had recovered from an upper respiratory tract infection 1 week before the onset of her current symptoms. She reports no facial numbness, dysphagia, dysarthria, limb numbness, or weakness. Her medical history is significant for infrequent, episodic migraines (without aura), which she treats with simple analgesics. The headache that preceded the droopiness and diplopia was similar to her usual migraines. She reports having had three similar episodes of left-eye droopiness and diplopia associated with headache at age 10 years, 17 years, and 23 years. Each time, she spontaneously recovered over a period of few days. She has not undergone previous brain scanning and has never received steroids. She describes a family history of similar headaches.\nThe patient's physical examination findings are unremarkable. No ocular abnormalities are apparent, and the fundus examination findings are normal. Her neurologic examination is significant for a partial, left oculomotor nerve palsy (left mild ptosis and limited eye adduction), with pupillary sparing. No other cranial nerve, motor, sensory, or cerebellar deficits are noted. Her deep tendon reflexes are normal.\nThe patient's renal, liver, and thyroid panel; erythrocyte sedimentation rate; and antinuclear antibody and anti-extractable nuclear antigen antibody findings are all within reference ranges. All other laboratory findings are also unremarkable. MRI with magnetic resonance angiography is performed (Figure).\n\n ## Figures\n\n \n*Page 1 of 5*",
"pagination": {
"current_page": 1,
"total_pages": 5
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1480521,
"choiceText": "Recurrent painful ophthalmoplegic neuropathy (RPON)",
"correct": false,
"displayOrder": 1,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1480522,
"choiceText": "Tolosa-Hunt syndrome ",
"correct": false,
"displayOrder": 2,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1480523,
"choiceText": "Posterior communicating artery aneurysm ",
"correct": false,
"displayOrder": 3,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1480524,
"choiceText": "Infiltrative lesion of the left oculomotor nerve ",
"correct": true,
"displayOrder": 4,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "",
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": "",
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 473659,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 25-Year-Old Woman With a Droopy Eyelid and Double Vision"
},
{
"authors": "Monica Saini, MD, MBBS",
"content": [
"Painful third nerve palsy is a neurologic emergency. The patient in this case had recurrent episodes of unilateral ptosis and diplopia, in association with migraine. Clinically, these episodes were thought to be consistent with recurrent painful ophthalmoplegic neuropathy (RPON), which was previously termed \"ophthalmoplegic migraine.\" However, incomplete recovery and persistent neuroimaging abnormalities (Figure) indicated a mass lesion of the left oculomotor nerve, which is believed to be a schwannoma.",
"MRI of the patient's brain revealed an enhancing, nodular lesion (red arrow) at the cisternal segment of the left oculomotor nerve (green arrowheads), just distal to the nerve root exit. The left posterior communicating artery (blue dashed arrow) and the left posterior cerebral artery (short blue arrows) were identified in relation to the left oculomotor nerve.",
"RPON is a controversial disorder and was previously considered to be a migraine variant.[1] After ophthalmoplegic migraine was found to be associated with enhancement of the oculomotor nerve, a neuropathic etiology was put forth. Although ophthalmoplegic migraine has been renamed RPON by the International Headache Society,[2] some researchers still support the classification of RPON as a migraine variant. Whether RPON is truly neuropathic or migrainous is an ongoing subject of debate.",
"Ophthalmoplegic migraine was originally described as one or more ocular cranial nerve palsies (> 60% oculomotor nerve) that occur in association with, or within several days of, episodic headache (usually severe), with gradual resolution of symptoms over time. In a prospective cohort of 62 patients, ophthalmoplegic migraine was reported in predominantly young individuals (mean age 36.4 ± 12.8 years), with 95% of patients developing ophthalmoplegia during a severe attack of migraine without aura.[3] More than 80% of patients reported an increase in migraine severity in the preceding ≥ 2 weeks. Ophthalmoplegia occurred ipsilateral to a unilateral headache in 75% of patients, whereas bilateral headaches were noted in the rest. Only 22.5% patients had more than one attack of ophthalmoplegic migraine. No nerve abnormalities were noted in any of the patients who underwent contrast-enhanced MRI.",
"The presence of nerve enhancement, a relapsing-remitting course of progression, response to steroids, and absence of headache in some patients led to reclassification of ophthalmoplegic migraine as a neuropathic disorder (RPON). The diagnostic criteria for RPON include the following:",
"At least two attacks that include both a unilateral headache and ipsilateral paresis of one, two, or three ocular motor nerves",
"Exclusion of orbital, parasellar, or posterior fossa lesion using appropriate investigation",
"Symptoms not better explained by another diagnosis in the third edition of the International Classification of Headache Disorders (ICHD-3)"
],
"date": "March 20, 2020",
"figures": [],
"markdown": "# A 25-Year-Old Woman With a Droopy Eyelid and Double Vision\n\n **Authors:** Monica Saini, MD, MBBS \n **Date:** March 20, 2020\n\n ## Content\n\n Painful third nerve palsy is a neurologic emergency. The patient in this case had recurrent episodes of unilateral ptosis and diplopia, in association with migraine. Clinically, these episodes were thought to be consistent with recurrent painful ophthalmoplegic neuropathy (RPON), which was previously termed \"ophthalmoplegic migraine.\" However, incomplete recovery and persistent neuroimaging abnormalities (Figure) indicated a mass lesion of the left oculomotor nerve, which is believed to be a schwannoma.\nMRI of the patient's brain revealed an enhancing, nodular lesion (red arrow) at the cisternal segment of the left oculomotor nerve (green arrowheads), just distal to the nerve root exit. The left posterior communicating artery (blue dashed arrow) and the left posterior cerebral artery (short blue arrows) were identified in relation to the left oculomotor nerve.\nRPON is a controversial disorder and was previously considered to be a migraine variant.[1] After ophthalmoplegic migraine was found to be associated with enhancement of the oculomotor nerve, a neuropathic etiology was put forth. Although ophthalmoplegic migraine has been renamed RPON by the International Headache Society,[2] some researchers still support the classification of RPON as a migraine variant. Whether RPON is truly neuropathic or migrainous is an ongoing subject of debate.\nOphthalmoplegic migraine was originally described as one or more ocular cranial nerve palsies (> 60% oculomotor nerve) that occur in association with, or within several days of, episodic headache (usually severe), with gradual resolution of symptoms over time. In a prospective cohort of 62 patients, ophthalmoplegic migraine was reported in predominantly young individuals (mean age 36.4 ± 12.8 years), with 95% of patients developing ophthalmoplegia during a severe attack of migraine without aura.[3] More than 80% of patients reported an increase in migraine severity in the preceding ≥ 2 weeks. Ophthalmoplegia occurred ipsilateral to a unilateral headache in 75% of patients, whereas bilateral headaches were noted in the rest. Only 22.5% patients had more than one attack of ophthalmoplegic migraine. No nerve abnormalities were noted in any of the patients who underwent contrast-enhanced MRI.\nThe presence of nerve enhancement, a relapsing-remitting course of progression, response to steroids, and absence of headache in some patients led to reclassification of ophthalmoplegic migraine as a neuropathic disorder (RPON). The diagnostic criteria for RPON include the following:\nAt least two attacks that include both a unilateral headache and ipsilateral paresis of one, two, or three ocular motor nerves\nExclusion of orbital, parasellar, or posterior fossa lesion using appropriate investigation\nSymptoms not better explained by another diagnosis in the third edition of the International Classification of Headache Disorders (ICHD-3)\n\n ## Figures\n\n \n*Page 2 of 5*",
"pagination": {
"current_page": 2,
"total_pages": 5
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1480521,
"choiceText": "Recurrent painful ophthalmoplegic neuropathy (RPON)",
"correct": false,
"displayOrder": 1,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1480522,
"choiceText": "Tolosa-Hunt syndrome ",
"correct": false,
"displayOrder": 2,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1480523,
"choiceText": "Posterior communicating artery aneurysm ",
"correct": false,
"displayOrder": 3,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1480524,
"choiceText": "Infiltrative lesion of the left oculomotor nerve ",
"correct": true,
"displayOrder": 4,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "",
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": "",
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 473659,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 25-Year-Old Woman With a Droopy Eyelid and Double Vision"
},
{
"authors": "Monica Saini, MD, MBBS",
"content": [
"RPON is a rare disorder and has been reported in patients aged 7 months to 50 years. In most patients, headache occurs around 1.5 days on average before ophthalmoplegia; however, in 25% of patients, episodes may be painless.[4] Headache in RPON is thought to be secondary to irritation of sensory pain fibers of the trigeminal nerve traversing the third cranial nerve, triggering a trigeminovascular response. Complete third nerve palsy with pupil involvement is the most common presentation; however, partial involvement may occur. The involvement of multiple oculomotor nerves is seen in < 10% of cases. Headache may last a few days to a week, and ophthalmoplegia may persist for 2-3 weeks to 2-3 months.[5] Recovery from an attack of RPON may not be complete, especially after multiple episodes.[6]",
"Enhancement of the oculomotor nerve is seen in most patients, usually at the nerve root exit zone in the interpeduncular cistern.[5] MRI abnormalities usually resolve after the episode (< 3 months), but resolution may take months to years; typically, enhancement resolves before improvement in thickening is noted.",
"The pathogenesis of RPON has not yet been determined. Theories include microvascular ischemia secondary to vasa nervosum compromise, release of inflammatory neuropeptides, vasospasm related to blood-neural barrier damage, and structural compromise by vascular anomalies that increase susceptibility of the cranial nerve to an altered trigeminovascular response.[7,8,9]",
"The differential diagnoses of a painful third nerve palsy includes aneurysm, most commonly involving the posterior communicating artery, Tolosa-Hunt syndrome, vasculitic and nonvasculitic arteritic cranial neuropathy, meningeal infiltrative disorders, carotid-cavernous fistula, arterial dissection, cavernous sinus pathologies, and mass lesions.",
"Tolosa-Hunt syndrome presents with periorbital headache and cranial nerve palsy, both of which show excellent response to corticosteroids. Neuroimaging easily differentiates Tolosa-Hunt syndrome from RPON, with evidence of granulomatous inflammation seen in the orbit, superior orbital fissure, and/or the cavernous sinus.[10]",
"Arteritic cranial neuropathy, typically oculomotor neuropathy in patients with diabetes, tends to be painless and is more commonly seen in older individuals with clear vascular risk factors. Giant cell arteritis (GCA) must be considered in patients older than 50 years. A history of systemic symptoms (eg, fever, weight loss), jaw claudication, and arthritis must be present and temporal artery tenderness or loss of pulsatility must also be reported. Evaluation of inflammatory markers, including erythrocyte sedimentation rate and C-reactive protein level, and fundus examination is mandatory in patients with features suggestive of GCA. Depending on associated systemic and neurologic features, investigations for painful third nerve palsy may include spinal fluid examination and meningeal biopsy."
],
"date": "March 20, 2020",
"figures": [],
"markdown": "# A 25-Year-Old Woman With a Droopy Eyelid and Double Vision\n\n **Authors:** Monica Saini, MD, MBBS \n **Date:** March 20, 2020\n\n ## Content\n\n RPON is a rare disorder and has been reported in patients aged 7 months to 50 years. In most patients, headache occurs around 1.5 days on average before ophthalmoplegia; however, in 25% of patients, episodes may be painless.[4] Headache in RPON is thought to be secondary to irritation of sensory pain fibers of the trigeminal nerve traversing the third cranial nerve, triggering a trigeminovascular response. Complete third nerve palsy with pupil involvement is the most common presentation; however, partial involvement may occur. The involvement of multiple oculomotor nerves is seen in < 10% of cases. Headache may last a few days to a week, and ophthalmoplegia may persist for 2-3 weeks to 2-3 months.[5] Recovery from an attack of RPON may not be complete, especially after multiple episodes.[6]\nEnhancement of the oculomotor nerve is seen in most patients, usually at the nerve root exit zone in the interpeduncular cistern.[5] MRI abnormalities usually resolve after the episode (< 3 months), but resolution may take months to years; typically, enhancement resolves before improvement in thickening is noted.\nThe pathogenesis of RPON has not yet been determined. Theories include microvascular ischemia secondary to vasa nervosum compromise, release of inflammatory neuropeptides, vasospasm related to blood-neural barrier damage, and structural compromise by vascular anomalies that increase susceptibility of the cranial nerve to an altered trigeminovascular response.[7,8,9]\nThe differential diagnoses of a painful third nerve palsy includes aneurysm, most commonly involving the posterior communicating artery, Tolosa-Hunt syndrome, vasculitic and nonvasculitic arteritic cranial neuropathy, meningeal infiltrative disorders, carotid-cavernous fistula, arterial dissection, cavernous sinus pathologies, and mass lesions.\nTolosa-Hunt syndrome presents with periorbital headache and cranial nerve palsy, both of which show excellent response to corticosteroids. Neuroimaging easily differentiates Tolosa-Hunt syndrome from RPON, with evidence of granulomatous inflammation seen in the orbit, superior orbital fissure, and/or the cavernous sinus.[10]\nArteritic cranial neuropathy, typically oculomotor neuropathy in patients with diabetes, tends to be painless and is more commonly seen in older individuals with clear vascular risk factors. Giant cell arteritis (GCA) must be considered in patients older than 50 years. A history of systemic symptoms (eg, fever, weight loss), jaw claudication, and arthritis must be present and temporal artery tenderness or loss of pulsatility must also be reported. Evaluation of inflammatory markers, including erythrocyte sedimentation rate and C-reactive protein level, and fundus examination is mandatory in patients with features suggestive of GCA. Depending on associated systemic and neurologic features, investigations for painful third nerve palsy may include spinal fluid examination and meningeal biopsy.\n\n ## Figures\n\n \n*Page 3 of 5*",
"pagination": {
"current_page": 3,
"total_pages": 5
},
"questionnaire": [],
"title": "A 25-Year-Old Woman With a Droopy Eyelid and Double Vision"
},
{
"authors": "Monica Saini, MD, MBBS",
"content": [
"Mass lesions tend to show slow progression. However, tumors that present as RPON, especially schwannomas, have been reported; patients typically show complete resolution of deficits between episodes.[11,12,13] Most reported cases involve the oculomotor nerve, with lesions predominantly seen in the cisternal segment.",
"Suspicion for infiltrative lesions is typically suggested by a subsequent attack with incomplete recovery and/or persistent nerve enhancement on imaging. The mechanism of episodic headache and remitting cranial neuropathy in such cases is unclear. Postulated theories include the release of inflammatory substances from the tumor. The absence of histologic diagnostic confirmation is a limiting factor in almost all such reported cases.[11,12,13] Repeated inflammation in the nerve may itself trigger cellular proliferation and subsequent transformation into a neoplastic process.",
"The patient in this case received simple analgesics for 1 week. Neurologic improvement was noted over 10 weeks. Neuroimaging abnormalities remained stable. She is continuing to undergo follow-up and interval neuroimaging will be performed to monitor progression of the left oculomotor schwannoma.",
"In conclusion, neuroimaging is mandatory in patients who present with painful ocular neuropathies. RPON is a diagnosis of exclusion. Follow-up neuroimaging must be considered in patients with nerve abnormalities and is mandatory in patients who show incomplete recovery, to exclude neural tumors."
],
"date": "March 20, 2020",
"figures": [],
"markdown": "# A 25-Year-Old Woman With a Droopy Eyelid and Double Vision\n\n **Authors:** Monica Saini, MD, MBBS \n **Date:** March 20, 2020\n\n ## Content\n\n Mass lesions tend to show slow progression. However, tumors that present as RPON, especially schwannomas, have been reported; patients typically show complete resolution of deficits between episodes.[11,12,13] Most reported cases involve the oculomotor nerve, with lesions predominantly seen in the cisternal segment.\nSuspicion for infiltrative lesions is typically suggested by a subsequent attack with incomplete recovery and/or persistent nerve enhancement on imaging. The mechanism of episodic headache and remitting cranial neuropathy in such cases is unclear. Postulated theories include the release of inflammatory substances from the tumor. The absence of histologic diagnostic confirmation is a limiting factor in almost all such reported cases.[11,12,13] Repeated inflammation in the nerve may itself trigger cellular proliferation and subsequent transformation into a neoplastic process.\nThe patient in this case received simple analgesics for 1 week. Neurologic improvement was noted over 10 weeks. Neuroimaging abnormalities remained stable. She is continuing to undergo follow-up and interval neuroimaging will be performed to monitor progression of the left oculomotor schwannoma.\nIn conclusion, neuroimaging is mandatory in patients who present with painful ocular neuropathies. RPON is a diagnosis of exclusion. Follow-up neuroimaging must be considered in patients with nerve abnormalities and is mandatory in patients who show incomplete recovery, to exclude neural tumors.\n\n ## Figures\n\n \n*Page 4 of 5*",
"pagination": {
"current_page": 4,
"total_pages": 5
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1480593,
"choiceText": "Cisternal segment at the root exit zone ",
"correct": true,
"displayOrder": 1,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1480594,
"choiceText": "Cavernous sinus ",
"correct": false,
"displayOrder": 2,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1480595,
"choiceText": "Superior orbital fissure ",
"correct": false,
"displayOrder": 3,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1480596,
"choiceText": "Orbit ",
"correct": false,
"displayOrder": 4,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "As many as 75% of patients with RPON have abnormal enhancement of the oculomotor nerve. This is most commonly noted at the nerve root exit zone in the interpeduncular cistern. ",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": "",
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 473683,
"questionText": "Enhancement of the oculomotor nerve in patients with RPON is most commonly noted in which of the following? ",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1480597,
"choiceText": "Bilateral headache ",
"correct": false,
"displayOrder": 1,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1480598,
"choiceText": "A unilateral headache without ipsilateral paresis",
"correct": false,
"displayOrder": 2,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1480599,
"choiceText": "Third, fourth, or sixth ocular cranial nerve palsy ",
"correct": false,
"displayOrder": 3,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1480600,
"choiceText": "Exclusion of orbital, parasellar, or posterior fossa lesion using appropriate neuroimaging ",
"correct": true,
"displayOrder": 4,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The diagnostic criteria for RPON include the following:\r\n<ul>\r\n<li>At least two attacks that include both a unilateral headache and ipsilateral paresis of one, two, or three ocular motor nerves</li>\r\n<li>Exclusion of orbital, parasellar, or posterior fossa lesion using appropriate investigation</li>\r\n<li>Symptoms not better explained by another diagnosis in the third edition of the ICHD-3</li>\r\n</ul>\r\n",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": "",
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 473684,
"questionText": "Which of the following is a diagnostic feature of RPON?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 25-Year-Old Woman With a Droopy Eyelid and Double Vision"
},
{
"authors": "Monica Saini, MD, MBBS",
"content": [],
"date": "March 20, 2020",
"figures": [],
"markdown": "# A 25-Year-Old Woman With a Droopy Eyelid and Double Vision\n\n **Authors:** Monica Saini, MD, MBBS \n **Date:** March 20, 2020\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 5 of 5*",
"pagination": {
"current_page": 5,
"total_pages": 5
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1480593,
"choiceText": "Cisternal segment at the root exit zone ",
"correct": true,
"displayOrder": 1,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1480594,
"choiceText": "Cavernous sinus ",
"correct": false,
"displayOrder": 2,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1480595,
"choiceText": "Superior orbital fissure ",
"correct": false,
"displayOrder": 3,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1480596,
"choiceText": "Orbit ",
"correct": false,
"displayOrder": 4,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "As many as 75% of patients with RPON have abnormal enhancement of the oculomotor nerve. This is most commonly noted at the nerve root exit zone in the interpeduncular cistern. ",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": "",
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 473683,
"questionText": "Enhancement of the oculomotor nerve in patients with RPON is most commonly noted in which of the following? ",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1480597,
"choiceText": "Bilateral headache ",
"correct": false,
"displayOrder": 1,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1480598,
"choiceText": "A unilateral headache without ipsilateral paresis",
"correct": false,
"displayOrder": 2,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1480599,
"choiceText": "Third, fourth, or sixth ocular cranial nerve palsy ",
"correct": false,
"displayOrder": 3,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1480600,
"choiceText": "Exclusion of orbital, parasellar, or posterior fossa lesion using appropriate neuroimaging ",
"correct": true,
"displayOrder": 4,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The diagnostic criteria for RPON include the following:\r\n<ul>\r\n<li>At least two attacks that include both a unilateral headache and ipsilateral paresis of one, two, or three ocular motor nerves</li>\r\n<li>Exclusion of orbital, parasellar, or posterior fossa lesion using appropriate investigation</li>\r\n<li>Symptoms not better explained by another diagnosis in the third edition of the ICHD-3</li>\r\n</ul>\r\n",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": "",
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 473684,
"questionText": "Which of the following is a diagnostic feature of RPON?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 25-Year-Old Woman With a Droopy Eyelid and Double Vision"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1480521,
"choiceText": "Recurrent painful ophthalmoplegic neuropathy (RPON)",
"correct": false,
"displayOrder": 1,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1480522,
"choiceText": "Tolosa-Hunt syndrome ",
"correct": false,
"displayOrder": 2,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1480523,
"choiceText": "Posterior communicating artery aneurysm ",
"correct": false,
"displayOrder": 3,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1480524,
"choiceText": "Infiltrative lesion of the left oculomotor nerve ",
"correct": true,
"displayOrder": 4,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "",
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": "",
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 473659,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1480593,
"choiceText": "Cisternal segment at the root exit zone ",
"correct": true,
"displayOrder": 1,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1480594,
"choiceText": "Cavernous sinus ",
"correct": false,
"displayOrder": 2,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1480595,
"choiceText": "Superior orbital fissure ",
"correct": false,
"displayOrder": 3,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1480596,
"choiceText": "Orbit ",
"correct": false,
"displayOrder": 4,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "As many as 75% of patients with RPON have abnormal enhancement of the oculomotor nerve. This is most commonly noted at the nerve root exit zone in the interpeduncular cistern. ",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": "",
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 473683,
"questionText": "Enhancement of the oculomotor nerve in patients with RPON is most commonly noted in which of the following? ",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1480597,
"choiceText": "Bilateral headache ",
"correct": false,
"displayOrder": 1,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1480598,
"choiceText": "A unilateral headache without ipsilateral paresis",
"correct": false,
"displayOrder": 2,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1480599,
"choiceText": "Third, fourth, or sixth ocular cranial nerve palsy ",
"correct": false,
"displayOrder": 3,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1480600,
"choiceText": "Exclusion of orbital, parasellar, or posterior fossa lesion using appropriate neuroimaging ",
"correct": true,
"displayOrder": 4,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The diagnostic criteria for RPON include the following:\r\n<ul>\r\n<li>At least two attacks that include both a unilateral headache and ipsilateral paresis of one, two, or three ocular motor nerves</li>\r\n<li>Exclusion of orbital, parasellar, or posterior fossa lesion using appropriate investigation</li>\r\n<li>Symptoms not better explained by another diagnosis in the third edition of the ICHD-3</li>\r\n</ul>\r\n",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": "",
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 473684,
"questionText": "Which of the following is a diagnostic feature of RPON?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
898695 | /viewarticle/898695 | [
{
"authors": "Kenneth B.V. Gross, MD",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"A 39-year-old black woman presents to the emergency department with a rash and fever. She has reportedly been ill for about a week, and her fever had reached 103°F (39.4°C). The rash is near her ear. Over the past 2 days, she has noted intermittent facial twitches and tongue extrusions (an example is shown in Figure 1), and attributes that to feeling nervous about her recent illness.",
"Figure 1.",
"The patient has a history of mild hypertension. She admits to occasional marijuana and cocaine use 20 years ago, with no use of any drugs over recent months. Her alcohol use was sporadic over the past 10 years, with limited use (three glasses of wine per week) over the past 6 months. The patient's medications include hydrochlorothiazide for hypertension.",
"The patient has a remote history of depression, for which she had been given duloxetine and citalopram. She has been pregnant four times, with two spontaneous abortions for unknown reasons and two pregnancies carried to delivery, producing two healthy children now in their late teens. The pregnancies that were carried to term were uneventful except for severe morning sickness, for which she was given metoclopramide. She has no known allergies or family history of relevant diseases. The patient works as a federal government clerk.",
"Physical examination reveals a blood pressure of 150/100 mm Hg. Her pulse is 100 beats/min. Her temperature is 101.9°F (38.8°C). Her respiration rate is 12 breaths/min. She appears well-developed and well-nourished.",
"Her skin has bilateral, postauricular scaly patches. She has distal streaks of red macules on her anterior legs. Examination of her head, eyes, ears, nose, and throat revealed mild pharyngeal erythema and small, scattered oral ulcers. She has slight cervical adenopathy in her neck.",
"Scattered bibasilar rales are noted upon chest examination. She has normal S1 and S2 sounds, with I/VI systolic murmur at base. Her abdomen is nontender, with no organomegaly observed. A trace of edema is noted on her ankle.",
"Upon neurological examination, the patient is oriented x 3. Her central nervous findings are normal. She has mild stocking-glove sensory changes. Her muscle power is 5/5. No dysmetria or ataxia is noted. She has orofacial dyskinesia with lip smacking and grimacing, as well as tic-like nodding. No chorea is noted. While in the emergency department, the patient had a 2-minute generalized seizure that began with rhythmic jerking of the right arm. Tongue biting and incontinence were noted after the event. The patient was confused for 5 minutes thereafter but then fully recovered alertness and cognition.",
"Six-channel serum multiple analysis findings are normal. Complete blood cell count is normal, except for mild leukocytosis (10,000 white blood cells [WBC]/µL, with minimal left shift). Her erythrocyte sedimentation rate is 50 mm/hr. No alcohol is found in her system. Total creatinine test results and thyroid panel results are normal. Noncontrast head CT scan findings are normal. Urinalysis reveals WBCs that are too numerous to count, few red cell casts, and a protein level of 30 mg/dL."
],
"date": "March 13, 2020",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/898/695/898695-Thumb2.jpg"
}
],
"markdown": "# A 39-Year-Old Woman With Past Cocaine Use, Rash, and Seizure\n\n **Authors:** Kenneth B.V. Gross, MD \n **Date:** March 13, 2020\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nA 39-year-old black woman presents to the emergency department with a rash and fever. She has reportedly been ill for about a week, and her fever had reached 103°F (39.4°C). The rash is near her ear. Over the past 2 days, she has noted intermittent facial twitches and tongue extrusions (an example is shown in Figure 1), and attributes that to feeling nervous about her recent illness.\nFigure 1.\nThe patient has a history of mild hypertension. She admits to occasional marijuana and cocaine use 20 years ago, with no use of any drugs over recent months. Her alcohol use was sporadic over the past 10 years, with limited use (three glasses of wine per week) over the past 6 months. The patient's medications include hydrochlorothiazide for hypertension.\nThe patient has a remote history of depression, for which she had been given duloxetine and citalopram. She has been pregnant four times, with two spontaneous abortions for unknown reasons and two pregnancies carried to delivery, producing two healthy children now in their late teens. The pregnancies that were carried to term were uneventful except for severe morning sickness, for which she was given metoclopramide. She has no known allergies or family history of relevant diseases. The patient works as a federal government clerk.\nPhysical examination reveals a blood pressure of 150/100 mm Hg. Her pulse is 100 beats/min. Her temperature is 101.9°F (38.8°C). Her respiration rate is 12 breaths/min. She appears well-developed and well-nourished.\nHer skin has bilateral, postauricular scaly patches. She has distal streaks of red macules on her anterior legs. Examination of her head, eyes, ears, nose, and throat revealed mild pharyngeal erythema and small, scattered oral ulcers. She has slight cervical adenopathy in her neck.\nScattered bibasilar rales are noted upon chest examination. She has normal S1 and S2 sounds, with I/VI systolic murmur at base. Her abdomen is nontender, with no organomegaly observed. A trace of edema is noted on her ankle.\nUpon neurological examination, the patient is oriented x 3. Her central nervous findings are normal. She has mild stocking-glove sensory changes. Her muscle power is 5/5. No dysmetria or ataxia is noted. She has orofacial dyskinesia with lip smacking and grimacing, as well as tic-like nodding. No chorea is noted. While in the emergency department, the patient had a 2-minute generalized seizure that began with rhythmic jerking of the right arm. Tongue biting and incontinence were noted after the event. The patient was confused for 5 minutes thereafter but then fully recovered alertness and cognition.\nSix-channel serum multiple analysis findings are normal. Complete blood cell count is normal, except for mild leukocytosis (10,000 white blood cells [WBC]/µL, with minimal left shift). Her erythrocyte sedimentation rate is 50 mm/hr. No alcohol is found in her system. Total creatinine test results and thyroid panel results are normal. Noncontrast head CT scan findings are normal. Urinalysis reveals WBCs that are too numerous to count, few red cell casts, and a protein level of 30 mg/dL.\n\n ## Figures\n\n **Figure 1.** \n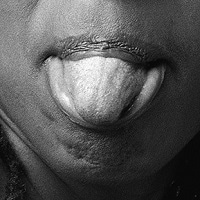 \n\n\n*Page 1 of 5*",
"pagination": {
"current_page": 1,
"total_pages": 5
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1242516,
"choiceText": "Urosepsis with surreptitious cocaine/alcohol abuse",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1242518,
"choiceText": "Neuroleptic-induced seizures and tardive dyskinesia complicating latent schizophrenia",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1242520,
"choiceText": "Systemic lupus erythematosus mimicking tardive dyskinesia",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1242522,
"choiceText": "\r\nAntidepressant-induced tardive dyskinesia with concurrent infection and seizure disorder",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 395255,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 39-Year-Old Woman With Past Cocaine Use, Rash, and Seizure"
},
{
"authors": "Kenneth B.V. Gross, MD",
"content": [
"Movement disorders and seizures coexisting in the same patient generate a broad differential diagnosis or multiple possible diagnoses. Additionally, tardive dyskinesia has many potential causes. The patient in this case has systemic lupus erythematosus (SLE) that is mimicking tardive dyskinesia.",
"Lupus is strongly inserted into the differential diagnosis of a young black woman with fever, a seizure, a movement disorder, and a rash. Certainly, the seizure must be suspected in this context to be part of a cerebral vasculitis associated with lupus. In addition, two spontaneous abortions suggest the presence of preexisting autoantibodies associated with this condition. Although the patient's movement disorder mimics tardive dyskinesia, it is caused here by SLE.",
"Tardive dyskinesia is characterized by numerous movement disorders often tied to chronic use of antipsychotic medication. Tardive dyskinesia refers to a group of delayed-onset iatrogenic movement disorders of various phenomenology that are caused by dopamine receptor-blocking agents, also referred to as neuroleptics. In some cases, the movement disorder may be accompanied by sensory phenomena such as paresthesias, pain, and an inner urge to move.",
"Neuroleptic medications were introduced in the early 1950s and revolutionized the treatment of schizophrenia and other psychiatric disorders. However, just a few years later, neuroleptics were recognized as a cause of abnormal involuntary movements. The first report of orofacial stereotypic involuntary movements, referred to as paroxysmal dyskinesia, in a patient treated with the phenothiazine derivative chlorpromazine, was published in 1957.[1]",
"The term tardive dyskinesia was first introduced in 1964 by Faurbye and colleagues, highlighting the delay between the initiation of treatment with the offending drug and the onset of the abnormal movements; hence the name \"tardive.\" The term is now frequently used to define any tardive hyperkinetic movement disorder, including stereotypy, akathisia, dystonia, tremor, tics, chorea, and myoclonus. However, some physicians reserve the term tardive dyskinesia exclusively for oro-bucco-lingual stereotypy, which has caused confusion in the medical literature. Because many patients present with a combination of different phenomenologies, which may include a movement disorder as well as sensory symptoms, the term \"tardive syndrome\" is more appropriate when referring to all tardive disorders. These disorders are manifested by any combination of hyperkinetic or hypokinetic movement disorders, as well as sensory symptoms that may be clinically distinct but share the same etiological background (recent exposure to dopamine receptor-blocking agents). Because tardive dyskinesia was present before antipsychotics were introduced to those with schizophrenia, a genetic basis compounded by the administration of psychotropics, particularly dopamine blockers, is possible.[1]"
],
"date": "March 13, 2020",
"figures": [],
"markdown": "# A 39-Year-Old Woman With Past Cocaine Use, Rash, and Seizure\n\n **Authors:** Kenneth B.V. Gross, MD \n **Date:** March 13, 2020\n\n ## Content\n\n Movement disorders and seizures coexisting in the same patient generate a broad differential diagnosis or multiple possible diagnoses. Additionally, tardive dyskinesia has many potential causes. The patient in this case has systemic lupus erythematosus (SLE) that is mimicking tardive dyskinesia.\nLupus is strongly inserted into the differential diagnosis of a young black woman with fever, a seizure, a movement disorder, and a rash. Certainly, the seizure must be suspected in this context to be part of a cerebral vasculitis associated with lupus. In addition, two spontaneous abortions suggest the presence of preexisting autoantibodies associated with this condition. Although the patient's movement disorder mimics tardive dyskinesia, it is caused here by SLE.\nTardive dyskinesia is characterized by numerous movement disorders often tied to chronic use of antipsychotic medication. Tardive dyskinesia refers to a group of delayed-onset iatrogenic movement disorders of various phenomenology that are caused by dopamine receptor-blocking agents, also referred to as neuroleptics. In some cases, the movement disorder may be accompanied by sensory phenomena such as paresthesias, pain, and an inner urge to move.\nNeuroleptic medications were introduced in the early 1950s and revolutionized the treatment of schizophrenia and other psychiatric disorders. However, just a few years later, neuroleptics were recognized as a cause of abnormal involuntary movements. The first report of orofacial stereotypic involuntary movements, referred to as paroxysmal dyskinesia, in a patient treated with the phenothiazine derivative chlorpromazine, was published in 1957.[1]\nThe term tardive dyskinesia was first introduced in 1964 by Faurbye and colleagues, highlighting the delay between the initiation of treatment with the offending drug and the onset of the abnormal movements; hence the name \"tardive.\" The term is now frequently used to define any tardive hyperkinetic movement disorder, including stereotypy, akathisia, dystonia, tremor, tics, chorea, and myoclonus. However, some physicians reserve the term tardive dyskinesia exclusively for oro-bucco-lingual stereotypy, which has caused confusion in the medical literature. Because many patients present with a combination of different phenomenologies, which may include a movement disorder as well as sensory symptoms, the term \"tardive syndrome\" is more appropriate when referring to all tardive disorders. These disorders are manifested by any combination of hyperkinetic or hypokinetic movement disorders, as well as sensory symptoms that may be clinically distinct but share the same etiological background (recent exposure to dopamine receptor-blocking agents). Because tardive dyskinesia was present before antipsychotics were introduced to those with schizophrenia, a genetic basis compounded by the administration of psychotropics, particularly dopamine blockers, is possible.[1]\n\n ## Figures\n\n \n*Page 2 of 5*",
"pagination": {
"current_page": 2,
"total_pages": 5
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1242516,
"choiceText": "Urosepsis with surreptitious cocaine/alcohol abuse",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1242518,
"choiceText": "Neuroleptic-induced seizures and tardive dyskinesia complicating latent schizophrenia",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1242520,
"choiceText": "Systemic lupus erythematosus mimicking tardive dyskinesia",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1242522,
"choiceText": "\r\nAntidepressant-induced tardive dyskinesia with concurrent infection and seizure disorder",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 395255,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 39-Year-Old Woman With Past Cocaine Use, Rash, and Seizure"
},
{
"authors": "Kenneth B.V. Gross, MD",
"content": [
"The patient in this case has no history of acute antipsychotic, anxioloytic, or antiemetic use. Her remote history of psychotropic drugs and metachlopramide are not substantial enough to imply their role in the orofacial dyskinesias described. Numerous agents can cause acute and chronic tardive dyskinesias. They include metoclopramide, the aforementioned phenothiazine and others in this class (eg, thioridazine, prochlorperazine), haloperidol, newer atypical psychotics (eg, olanapine, quetiapine, risperidone), selective serotonin reuptake inhibitors (SSRIs), and duloxetine.[2,3] However, the patient in this case used some of these medications long ago, which would not explain her current presentation. Latent schizophrenia is also unlikely in this case, as no evidence suggests a thought disorder. Absent drug-induced tardive dyskinesia, other variants were considered.",
"Neither Tourette syndrome or neuroacanthocytosis feature fever, rash, or possible nephritis; however, they both can have onsets in adulthood and present with oro-facial tics, as noted here. In addition, total creatinine test results are generally high with neuroacanthocytosis, and red cell abnormalities are noted on peripheral smears. Huntington disease can rarely be associated with this type of dyskinesia. However, the patient in this case had no family history. Lesch-Nyhan syndrome is strongly associated with orofacial dyskinesia, if not tongue biting; however, this is a childhood illness of purine metabolism. Thyroid disease, particularly hyperthyroidism can feature hyperkinesia; however, tremor is most common. Isolated oral facial dyskinesia is extremely unusual in thyroid disease. In various tardive dyskinesia-related syndromes, tongue protrusion or blepharospasm can dominate the clinical picture.[4]",
"Returning to autoimmunity and movement disorders, antiphospholipid antiboides may have explained the patient's spontaneous abortions but also may be contributing to her lupus-related neurological syndrome. The patient's elevated erythrocyte sedimentation rate is nonspecific but is clearly linked to her likely positive antinuclear antibody levels and related autoantibodies.",
"More broadly, various tardive dyskinesia-like entities are tied to autoimmune disease.[5] In addition to lupus and antiphospholipid conditions, which can exist without lupus, other disorders are associated with various autoimmune diseases. These include Sydenham chorea, pediatric autoimmune neuropsychiatric disorders associated with streptococcus, SLE, antiphospholipid syndrome, gluten sensitivity, and paraneoplastic and autoimmune encephalopathies. Tremors, dystonia, akathisia, catatonia, chorea, ballism, myoclonus, Parkinsonism, and ataxia may be the initial and even the only presentation of these autoimmune diseases.[6] Although antibodies directed against various cellular components of the central nervous system have been implicated, the pathogenic mechanisms of these autoimmune movement disorders have not yet been fully elucidated. The patient in this case did have a rash, not one that suggested dermatomyositis."
],
"date": "March 13, 2020",
"figures": [],
"markdown": "# A 39-Year-Old Woman With Past Cocaine Use, Rash, and Seizure\n\n **Authors:** Kenneth B.V. Gross, MD \n **Date:** March 13, 2020\n\n ## Content\n\n The patient in this case has no history of acute antipsychotic, anxioloytic, or antiemetic use. Her remote history of psychotropic drugs and metachlopramide are not substantial enough to imply their role in the orofacial dyskinesias described. Numerous agents can cause acute and chronic tardive dyskinesias. They include metoclopramide, the aforementioned phenothiazine and others in this class (eg, thioridazine, prochlorperazine), haloperidol, newer atypical psychotics (eg, olanapine, quetiapine, risperidone), selective serotonin reuptake inhibitors (SSRIs), and duloxetine.[2,3] However, the patient in this case used some of these medications long ago, which would not explain her current presentation. Latent schizophrenia is also unlikely in this case, as no evidence suggests a thought disorder. Absent drug-induced tardive dyskinesia, other variants were considered.\nNeither Tourette syndrome or neuroacanthocytosis feature fever, rash, or possible nephritis; however, they both can have onsets in adulthood and present with oro-facial tics, as noted here. In addition, total creatinine test results are generally high with neuroacanthocytosis, and red cell abnormalities are noted on peripheral smears. Huntington disease can rarely be associated with this type of dyskinesia. However, the patient in this case had no family history. Lesch-Nyhan syndrome is strongly associated with orofacial dyskinesia, if not tongue biting; however, this is a childhood illness of purine metabolism. Thyroid disease, particularly hyperthyroidism can feature hyperkinesia; however, tremor is most common. Isolated oral facial dyskinesia is extremely unusual in thyroid disease. In various tardive dyskinesia-related syndromes, tongue protrusion or blepharospasm can dominate the clinical picture.[4]\nReturning to autoimmunity and movement disorders, antiphospholipid antiboides may have explained the patient's spontaneous abortions but also may be contributing to her lupus-related neurological syndrome. The patient's elevated erythrocyte sedimentation rate is nonspecific but is clearly linked to her likely positive antinuclear antibody levels and related autoantibodies.\nMore broadly, various tardive dyskinesia-like entities are tied to autoimmune disease.[5] In addition to lupus and antiphospholipid conditions, which can exist without lupus, other disorders are associated with various autoimmune diseases. These include Sydenham chorea, pediatric autoimmune neuropsychiatric disorders associated with streptococcus, SLE, antiphospholipid syndrome, gluten sensitivity, and paraneoplastic and autoimmune encephalopathies. Tremors, dystonia, akathisia, catatonia, chorea, ballism, myoclonus, Parkinsonism, and ataxia may be the initial and even the only presentation of these autoimmune diseases.[6] Although antibodies directed against various cellular components of the central nervous system have been implicated, the pathogenic mechanisms of these autoimmune movement disorders have not yet been fully elucidated. The patient in this case did have a rash, not one that suggested dermatomyositis.\n\n ## Figures\n\n \n*Page 3 of 5*",
"pagination": {
"current_page": 3,
"total_pages": 5
},
"questionnaire": [],
"title": "A 39-Year-Old Woman With Past Cocaine Use, Rash, and Seizure"
},
{
"authors": "Kenneth B.V. Gross, MD",
"content": [
"Reports have documented reversal of tardive dyskinesia with steroids. Oxidative stress as an inducer of Tardive dyskinesia has also been implicated.[7] Thus, more research on tardive dyskinesia is indicated to better understand these phenomena. Intriguingly, general anesthesia has also been reported to reverse tardive dyskinesia.[8] The first drug available specifically for tardive dyskinesia, valbenazine, causes a reversible reduction of dopamine release by selectively inhibiting presynaptic human vesicular monoamine transporter type 2. Whether this agent can work for SLE and related immunological disorders presenting with tardive dyskinesia variants is uncertain but warrants research.",
"This patient has subacute orofacial dyskinesias in the context of a febrile illness associated with a seizure, a rash, and possible nephritis. Tardive dyskinesia is traditionally induced by antipsychotics. Other psychotropic or antidopaminergic drugs can manifest in a similar way. In this case, in which a movement disorder consistent with a tardive dyskinesia-like syndrome was noted, the medication history was a red herring, as was a recreational drug and alcohol history that can also trigger tardive dyskinesia variants. The patient used SSRIs briefly many years prior, which could not be pointed to as the cause of her current movement disorder. The patient's drug and alcohol history was also not active and was irrelevant here. SLE has been linked to orofacial dystonia and dyskinesia that can mimic tardive dyskinesia and its variants. Classically, chorea, akathisias, and more general dystonias have been noted in SLE patients; however, Parkinsonism is less common than hyperkinesias.",
"Unfortunately, this patient succumbed to SLE two months after the onset of her neurological syndrome despite several weeks of hydrocorticosteroids and other immunotherapy, including intravenous immunoglobulin and plasmapheresis. The latter two therapies were maximized as nephritis worsened; pericarditis and myocarditis complicated the multisystem involvement. Pericardial tamponade ultimately was the immediate cause of death due to a critically large, lupus-induced pericardial effusion."
],
"date": "March 13, 2020",
"figures": [],
"markdown": "# A 39-Year-Old Woman With Past Cocaine Use, Rash, and Seizure\n\n **Authors:** Kenneth B.V. Gross, MD \n **Date:** March 13, 2020\n\n ## Content\n\n Reports have documented reversal of tardive dyskinesia with steroids. Oxidative stress as an inducer of Tardive dyskinesia has also been implicated.[7] Thus, more research on tardive dyskinesia is indicated to better understand these phenomena. Intriguingly, general anesthesia has also been reported to reverse tardive dyskinesia.[8] The first drug available specifically for tardive dyskinesia, valbenazine, causes a reversible reduction of dopamine release by selectively inhibiting presynaptic human vesicular monoamine transporter type 2. Whether this agent can work for SLE and related immunological disorders presenting with tardive dyskinesia variants is uncertain but warrants research.\nThis patient has subacute orofacial dyskinesias in the context of a febrile illness associated with a seizure, a rash, and possible nephritis. Tardive dyskinesia is traditionally induced by antipsychotics. Other psychotropic or antidopaminergic drugs can manifest in a similar way. In this case, in which a movement disorder consistent with a tardive dyskinesia-like syndrome was noted, the medication history was a red herring, as was a recreational drug and alcohol history that can also trigger tardive dyskinesia variants. The patient used SSRIs briefly many years prior, which could not be pointed to as the cause of her current movement disorder. The patient's drug and alcohol history was also not active and was irrelevant here. SLE has been linked to orofacial dystonia and dyskinesia that can mimic tardive dyskinesia and its variants. Classically, chorea, akathisias, and more general dystonias have been noted in SLE patients; however, Parkinsonism is less common than hyperkinesias.\nUnfortunately, this patient succumbed to SLE two months after the onset of her neurological syndrome despite several weeks of hydrocorticosteroids and other immunotherapy, including intravenous immunoglobulin and plasmapheresis. The latter two therapies were maximized as nephritis worsened; pericarditis and myocarditis complicated the multisystem involvement. Pericardial tamponade ultimately was the immediate cause of death due to a critically large, lupus-induced pericardial effusion.\n\n ## Figures\n\n \n*Page 4 of 5*",
"pagination": {
"current_page": 4,
"total_pages": 5
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1242524,
"choiceText": "Seizures and psychosis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1242526,
"choiceText": "Chorea and related dyskinesias",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1242528,
"choiceText": "Neuropathy and seizures",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1242530,
"choiceText": "Psychosis and peripheral neuropathy",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1242532,
"choiceText": "All of the above\r\n",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "One or more of the neurological or neuropsychiatric features may be present in individuals with SLE. The psychosis is an organic psychosis caused by the lupus immunopathy/vasculitis. However, steroids can also induce a psychosis in patients with SLE who may have received steroids for seizures; whether the lupus or the steroids themselves cause the organic brain syndrome is unclear.<sup>[9]</sup>\r\n",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 395257,
"questionText": "SLE may present with which of the following combinations of neurological and/or neuropsychiatric features?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1242534,
"choiceText": "Dopamine",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1242536,
"choiceText": "Combinations of dopamine and steroids",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1242538,
"choiceText": "Phenobarbital ",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1242540,
"choiceText": "All of the above\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Studies have documented improvement in jaw dystonia and orofacial dyskinesias in patients with SLE upon administration of both dopamine and steroids.<sup>[8]</sup> Traditionally, dopamine has had a role in treating movement disorders noted in lupus. Various central nervous system neuropathies respond to immunosuppressive treatment; thus, immunotherapy beyond steroids may be valuable.<sup>[10,11]</sup>",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 395259,
"questionText": "Treatment for orofacial dyskinesias in SLE has traditionally included which of the following?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 39-Year-Old Woman With Past Cocaine Use, Rash, and Seizure"
},
{
"authors": "Kenneth B.V. Gross, MD",
"content": [],
"date": "March 13, 2020",
"figures": [],
"markdown": "# A 39-Year-Old Woman With Past Cocaine Use, Rash, and Seizure\n\n **Authors:** Kenneth B.V. Gross, MD \n **Date:** March 13, 2020\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 5 of 5*",
"pagination": {
"current_page": 5,
"total_pages": 5
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1242524,
"choiceText": "Seizures and psychosis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1242526,
"choiceText": "Chorea and related dyskinesias",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1242528,
"choiceText": "Neuropathy and seizures",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1242530,
"choiceText": "Psychosis and peripheral neuropathy",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1242532,
"choiceText": "All of the above\r\n",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "One or more of the neurological or neuropsychiatric features may be present in individuals with SLE. The psychosis is an organic psychosis caused by the lupus immunopathy/vasculitis. However, steroids can also induce a psychosis in patients with SLE who may have received steroids for seizures; whether the lupus or the steroids themselves cause the organic brain syndrome is unclear.<sup>[9]</sup>\r\n",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 395257,
"questionText": "SLE may present with which of the following combinations of neurological and/or neuropsychiatric features?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1242534,
"choiceText": "Dopamine",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1242536,
"choiceText": "Combinations of dopamine and steroids",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1242538,
"choiceText": "Phenobarbital ",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1242540,
"choiceText": "All of the above\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Studies have documented improvement in jaw dystonia and orofacial dyskinesias in patients with SLE upon administration of both dopamine and steroids.<sup>[8]</sup> Traditionally, dopamine has had a role in treating movement disorders noted in lupus. Various central nervous system neuropathies respond to immunosuppressive treatment; thus, immunotherapy beyond steroids may be valuable.<sup>[10,11]</sup>",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 395259,
"questionText": "Treatment for orofacial dyskinesias in SLE has traditionally included which of the following?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 39-Year-Old Woman With Past Cocaine Use, Rash, and Seizure"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1242516,
"choiceText": "Urosepsis with surreptitious cocaine/alcohol abuse",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1242518,
"choiceText": "Neuroleptic-induced seizures and tardive dyskinesia complicating latent schizophrenia",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1242520,
"choiceText": "Systemic lupus erythematosus mimicking tardive dyskinesia",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1242522,
"choiceText": "\r\nAntidepressant-induced tardive dyskinesia with concurrent infection and seizure disorder",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 395255,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1242524,
"choiceText": "Seizures and psychosis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1242526,
"choiceText": "Chorea and related dyskinesias",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1242528,
"choiceText": "Neuropathy and seizures",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1242530,
"choiceText": "Psychosis and peripheral neuropathy",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1242532,
"choiceText": "All of the above\r\n",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "One or more of the neurological or neuropsychiatric features may be present in individuals with SLE. The psychosis is an organic psychosis caused by the lupus immunopathy/vasculitis. However, steroids can also induce a psychosis in patients with SLE who may have received steroids for seizures; whether the lupus or the steroids themselves cause the organic brain syndrome is unclear.<sup>[9]</sup>\r\n",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 395257,
"questionText": "SLE may present with which of the following combinations of neurological and/or neuropsychiatric features?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1242534,
"choiceText": "Dopamine",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1242536,
"choiceText": "Combinations of dopamine and steroids",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1242538,
"choiceText": "Phenobarbital ",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1242540,
"choiceText": "All of the above\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Studies have documented improvement in jaw dystonia and orofacial dyskinesias in patients with SLE upon administration of both dopamine and steroids.<sup>[8]</sup> Traditionally, dopamine has had a role in treating movement disorders noted in lupus. Various central nervous system neuropathies respond to immunosuppressive treatment; thus, immunotherapy beyond steroids may be valuable.<sup>[10,11]</sup>",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 395259,
"questionText": "Treatment for orofacial dyskinesias in SLE has traditionally included which of the following?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
828049 | /viewarticle/828049 | [
{
"authors": "Herbert S. Diamond, MD, MACP",
"content": [
"Editor's Note:\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"A 46-year-old white man presents with diffuse musculoskeletal pain and morning stiffness lasting about one hour. Six months ago, he developed a painful, diffusely swollen right fourth toe. The pain was improved with over-the-counter naproxen, but the swelling never completely resolved.",
"Over the next six months, pain spread to involve his cervical and lumbar spine; he also developed swelling and pain affecting his right wrist and forearm, the left fourth distal interphalangeal (DIP) joint, and the right ankle. In addition, tenderness was reported in the area of the greater trochanter.",
"His past medical history was positive only for nasal pollen allergies, acne as a teenager, and dandruff for the past five years. His family history is positive for myocardial infarction in his father (at age 49 years), psoriasis, (also in his father), and stage 1 breast cancer in his mother, who was treated with radiation and a lumpectomy, with good outcome to date.",
"A physical examination confirmed swelling in the joints, as well as tenderness in the areas that the patient had described as painful. Erythema and warmth were found over the involved peripheral joints. The remainder of the physical examination was within normal limits except for a nonpruritic, erythematous rash in the intragluteal cleft and multiple patches of a scaly rash on his scalp that had been diagnosed as seborrhea. He had no rashes on the extensor surfaces and no nail abnormalities.",
"Workup revealed a hemoglobin level of 11.8 g/dL and an otherwise normal complete blood count (CBC). Hepatic and renal chemistry, urine analysis, rheumatoid factor (RF), and antinuclear antibody (ANA) findings were normal. His serum uric acid level was 6.7 mg/dL (normal range ≤7 mg/dL).",
"Radiographs of the hands revealed soft tissue swelling of the right wrist and forearm without erosions or juxta-articular osteoporosis, and soft tissue swelling and an erosion at the left fourth DIP joint."
],
"date": "February 26, 2020",
"figures": [],
"markdown": "# A 46-Year-Old Man With Spine Pain and a Rash\n\n **Authors:** Herbert S. Diamond, MD, MACP \n **Date:** February 26, 2020\n\n ## Content\n\n Editor's Note:\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nA 46-year-old white man presents with diffuse musculoskeletal pain and morning stiffness lasting about one hour. Six months ago, he developed a painful, diffusely swollen right fourth toe. The pain was improved with over-the-counter naproxen, but the swelling never completely resolved.\nOver the next six months, pain spread to involve his cervical and lumbar spine; he also developed swelling and pain affecting his right wrist and forearm, the left fourth distal interphalangeal (DIP) joint, and the right ankle. In addition, tenderness was reported in the area of the greater trochanter.\nHis past medical history was positive only for nasal pollen allergies, acne as a teenager, and dandruff for the past five years. His family history is positive for myocardial infarction in his father (at age 49 years), psoriasis, (also in his father), and stage 1 breast cancer in his mother, who was treated with radiation and a lumpectomy, with good outcome to date.\nA physical examination confirmed swelling in the joints, as well as tenderness in the areas that the patient had described as painful. Erythema and warmth were found over the involved peripheral joints. The remainder of the physical examination was within normal limits except for a nonpruritic, erythematous rash in the intragluteal cleft and multiple patches of a scaly rash on his scalp that had been diagnosed as seborrhea. He had no rashes on the extensor surfaces and no nail abnormalities.\nWorkup revealed a hemoglobin level of 11.8 g/dL and an otherwise normal complete blood count (CBC). Hepatic and renal chemistry, urine analysis, rheumatoid factor (RF), and antinuclear antibody (ANA) findings were normal. His serum uric acid level was 6.7 mg/dL (normal range ≤7 mg/dL).\nRadiographs of the hands revealed soft tissue swelling of the right wrist and forearm without erosions or juxta-articular osteoporosis, and soft tissue swelling and an erosion at the left fourth DIP joint.\n\n ## Figures\n\n \n*Page 1 of 5*",
"pagination": {
"current_page": 1,
"total_pages": 5
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 745991,
"choiceText": "RF-negative rheumatoid arthritis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 745993,
"choiceText": "Gout",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 745995,
"choiceText": "Osteoarthritis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 745997,
"choiceText": "Ankylosing spondylitis",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 745999,
"choiceText": "Psoriatic arthritis",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 233361,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 46-Year-Old Man With Spine Pain and a Rash"
},
{
"authors": "Herbert S. Diamond, MD, MACP",
"content": [
"The patient described the gradual onset of an inflammatory polyarthritis. The condition involved peripheral joints, including a DIP joint; tendon sheaths, as exemplified by the swelling in the forearm; and the lumbar and cervical spine. Physical examination confirmed a pauciarticular, asymmetrical arthritis, and the presence of inflammation was indicated by the erythema and warmth of the involved joints. Although the laboratory studies were unrevealing, the patient’s radiograph showed erosive arthritis of a DIP joint, and the absence of juxta-articular osteoporosis was noted. All of these findings, which also included a rash on the scalp and intragluteal cleft, were consistent with psoriatic arthritis. The patient was started on naproxen 500 mg twice daily and oral methotrexate 15 mg once weekly, with monitoring of blood counts and liver function.",
"Psoriatic arthritis occurs in up to 1% of the adult white population but is less frequent in other racial groups. Onset can occur at any age, including childhood and old age. The sex ratio is equal. The incidence of psoriatic arthritis in patients with psoriasis may be as high as 30%, although some studies have reported a lower incidence. Psoriasis typically precedes the onset of arthritis, usually by no more than 10 years. However, in some patients, the onset is simultaneous, and in a minority of cases, arthritis is the first symptom.[1,2,3]",
"Psoriatic arthritis is associated with a strong genetic predisposition; a family history of psoriasis, arthritis, or both is often present. Multiple genes that predispose to either psoriasis or psoriatic arthritis have been identified, including human leukocyte antigen (HLA)-associated genes and non-HLA genes. Still, most of the genetic predisposition remains to be determined. Although genes that predispose to psoriasis and those that predispose to psoriatic arthritis overlap, important differences among the predisposing genes are observed.",
"The rash in psoriatic arthritis is erythematous and scaling. It most often occurs in plaques but may develop in a guttate pattern or, rarely, as a diffuse erythema. An example is shown in the image below.",
"Figure 1.",
"Figure 1.",
"The scalp is frequently involved, although scalp psoriasis is often misdiagnosed as seborrhea. When the rash involves skin folds, scaling may be absent. An example is shown in the image below.",
"Figure 2.",
"Figure 2.",
"Nail involvement is frequent and can include dystrophic changes, cracking, pitting, and splinter hemorrhages.",
"Early in the disease, joint involvement is most often in the form of an asymmetrical arthritis. The DIP joints are frequently involved, and cervical and lumbar spine involvement develops in 50% of patients. Tendon sheaths are often affected as well, which may result in diffuse swelling of the digits (so-called sausage digits). Diffuse, edematous swelling of a distal extremity may also occur, due to extensive tendon sheath inflammation. As in ankylosing spondylitis, involvement of tendon and ligament insertions (entheseal involvement) is common.",
"A minority of patients with psoriatic arthritis present with a diffuse, symmetrical pattern of arthritis or with isolated spinal involvement. In rare cases, patients have a pattern of severe, highly destructive bone resorption that, when it occurs, often affects the small joints of the hands and feet; this pattern is often refractory to treatment.[1,3]"
],
"date": "February 26, 2020",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/828/049/828049-thumb-1.jpg"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/828/049/828049-thumb-2.jpg"
}
],
"markdown": "# A 46-Year-Old Man With Spine Pain and a Rash\n\n **Authors:** Herbert S. Diamond, MD, MACP \n **Date:** February 26, 2020\n\n ## Content\n\n The patient described the gradual onset of an inflammatory polyarthritis. The condition involved peripheral joints, including a DIP joint; tendon sheaths, as exemplified by the swelling in the forearm; and the lumbar and cervical spine. Physical examination confirmed a pauciarticular, asymmetrical arthritis, and the presence of inflammation was indicated by the erythema and warmth of the involved joints. Although the laboratory studies were unrevealing, the patient’s radiograph showed erosive arthritis of a DIP joint, and the absence of juxta-articular osteoporosis was noted. All of these findings, which also included a rash on the scalp and intragluteal cleft, were consistent with psoriatic arthritis. The patient was started on naproxen 500 mg twice daily and oral methotrexate 15 mg once weekly, with monitoring of blood counts and liver function.\nPsoriatic arthritis occurs in up to 1% of the adult white population but is less frequent in other racial groups. Onset can occur at any age, including childhood and old age. The sex ratio is equal. The incidence of psoriatic arthritis in patients with psoriasis may be as high as 30%, although some studies have reported a lower incidence. Psoriasis typically precedes the onset of arthritis, usually by no more than 10 years. However, in some patients, the onset is simultaneous, and in a minority of cases, arthritis is the first symptom.[1,2,3]\nPsoriatic arthritis is associated with a strong genetic predisposition; a family history of psoriasis, arthritis, or both is often present. Multiple genes that predispose to either psoriasis or psoriatic arthritis have been identified, including human leukocyte antigen (HLA)-associated genes and non-HLA genes. Still, most of the genetic predisposition remains to be determined. Although genes that predispose to psoriasis and those that predispose to psoriatic arthritis overlap, important differences among the predisposing genes are observed.\nThe rash in psoriatic arthritis is erythematous and scaling. It most often occurs in plaques but may develop in a guttate pattern or, rarely, as a diffuse erythema. An example is shown in the image below.\nFigure 1.\nFigure 1.\nThe scalp is frequently involved, although scalp psoriasis is often misdiagnosed as seborrhea. When the rash involves skin folds, scaling may be absent. An example is shown in the image below.\nFigure 2.\nFigure 2.\nNail involvement is frequent and can include dystrophic changes, cracking, pitting, and splinter hemorrhages.\nEarly in the disease, joint involvement is most often in the form of an asymmetrical arthritis. The DIP joints are frequently involved, and cervical and lumbar spine involvement develops in 50% of patients. Tendon sheaths are often affected as well, which may result in diffuse swelling of the digits (so-called sausage digits). Diffuse, edematous swelling of a distal extremity may also occur, due to extensive tendon sheath inflammation. As in ankylosing spondylitis, involvement of tendon and ligament insertions (entheseal involvement) is common.\nA minority of patients with psoriatic arthritis present with a diffuse, symmetrical pattern of arthritis or with isolated spinal involvement. In rare cases, patients have a pattern of severe, highly destructive bone resorption that, when it occurs, often affects the small joints of the hands and feet; this pattern is often refractory to treatment.[1,3]\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n\n*Page 2 of 5*",
"pagination": {
"current_page": 2,
"total_pages": 5
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 745991,
"choiceText": "RF-negative rheumatoid arthritis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 745993,
"choiceText": "Gout",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 745995,
"choiceText": "Osteoarthritis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 745997,
"choiceText": "Ankylosing spondylitis",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 745999,
"choiceText": "Psoriatic arthritis",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 233361,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 46-Year-Old Man With Spine Pain and a Rash"
},
{
"authors": "Herbert S. Diamond, MD, MACP",
"content": [
"Gastrointestinal symptoms are more common in patients with psoriatic arthritis than in the general population. Eye disease and aortic valve disease are less common than in ankylosing spondylitis. In contrast to rheumatoid arthritis, other systemic features are atypical. Subcutaneous nodules do not occur, and other organ involvement is rare.",
"Laboratory studies are not helpful in establishing the presence of psoriatic arthritis, with no tests being diagnostically specific for the disease. A mild anemia may be present, but white blood counts are generally normal. RF and ANA test findings are positive only at the same frequency as in the healthy population. Uric acid levels may be elevated in patients with extensive skin disease but is usually normal. Similarly, the erythrocyte sedimentation rate and C-reactive protein levels also are often normal, although they may be elevated in some cases. Synovial fluid shows nonspecific inflammation with an elevated neutrophil count. Skin biopsy with pathology can establish the diagnosis of psoriasis, but biopsy is not required in most cases.",
"Radiologic findings can help to establish the diagnosis of psoriatic arthritis.[4] Features of the condition that distinguish it from rheumatoid arthritis include asymmetrical joint involvement, DIP joint disease, periosteal new bone formation, pencil-in-cup erosions (Figure 3), joint fusion, sacroiliac and lumbar spine involvement, and absence of periarticular osteoporosis.[1,3,4]",
"Figure 3.",
"Figure 3.",
"In rheumatoid arthritis, symmetrical arthritis, sparing of the DIP joints and lumbosacral spine, and juxta-articular osteoporosis on radiography are common. Less tendon sheath and entheseal involvement is associated with rheumatoid arthritis, patients are usually RF-positive, and skin rash is not a feature. Ankylosing spondylitis, an RF-negative disease, can involve the entire spine; peripheral arthritis is generally limited to large central joints, such as the hip and knee. Wrist, ankle, or DIP joint involvement is unlikely in ankylosing spondylitis, as is rash.",
"The differential diagnosis of psoriatic arthritis also includes gout and osteoarthritis. Gout is characterized by an episodic, self-limited, acute, monoarticular or pauciarticular arthritis; the spine and sacroiliac joints are rarely involved, and no associated rash is present. Uric acid crystals are present in the fluid from affected joints. In osteoarthritis, inflammation is usually absent, wrist and ankle involvement is unusual, inflammation is generally absent, and no rash occurs."
],
"date": "February 26, 2020",
"figures": [
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/828/049/828049-thumb-3.jpg"
}
],
"markdown": "# A 46-Year-Old Man With Spine Pain and a Rash\n\n **Authors:** Herbert S. Diamond, MD, MACP \n **Date:** February 26, 2020\n\n ## Content\n\n Gastrointestinal symptoms are more common in patients with psoriatic arthritis than in the general population. Eye disease and aortic valve disease are less common than in ankylosing spondylitis. In contrast to rheumatoid arthritis, other systemic features are atypical. Subcutaneous nodules do not occur, and other organ involvement is rare.\nLaboratory studies are not helpful in establishing the presence of psoriatic arthritis, with no tests being diagnostically specific for the disease. A mild anemia may be present, but white blood counts are generally normal. RF and ANA test findings are positive only at the same frequency as in the healthy population. Uric acid levels may be elevated in patients with extensive skin disease but is usually normal. Similarly, the erythrocyte sedimentation rate and C-reactive protein levels also are often normal, although they may be elevated in some cases. Synovial fluid shows nonspecific inflammation with an elevated neutrophil count. Skin biopsy with pathology can establish the diagnosis of psoriasis, but biopsy is not required in most cases.\nRadiologic findings can help to establish the diagnosis of psoriatic arthritis.[4] Features of the condition that distinguish it from rheumatoid arthritis include asymmetrical joint involvement, DIP joint disease, periosteal new bone formation, pencil-in-cup erosions (Figure 3), joint fusion, sacroiliac and lumbar spine involvement, and absence of periarticular osteoporosis.[1,3,4]\nFigure 3.\nFigure 3.\nIn rheumatoid arthritis, symmetrical arthritis, sparing of the DIP joints and lumbosacral spine, and juxta-articular osteoporosis on radiography are common. Less tendon sheath and entheseal involvement is associated with rheumatoid arthritis, patients are usually RF-positive, and skin rash is not a feature. Ankylosing spondylitis, an RF-negative disease, can involve the entire spine; peripheral arthritis is generally limited to large central joints, such as the hip and knee. Wrist, ankle, or DIP joint involvement is unlikely in ankylosing spondylitis, as is rash.\nThe differential diagnosis of psoriatic arthritis also includes gout and osteoarthritis. Gout is characterized by an episodic, self-limited, acute, monoarticular or pauciarticular arthritis; the spine and sacroiliac joints are rarely involved, and no associated rash is present. Uric acid crystals are present in the fluid from affected joints. In osteoarthritis, inflammation is usually absent, wrist and ankle involvement is unusual, inflammation is generally absent, and no rash occurs.\n\n ## Figures\n\n **Figure 3.** \n \n\n\n*Page 3 of 5*",
"pagination": {
"current_page": 3,
"total_pages": 5
},
"questionnaire": [],
"title": "A 46-Year-Old Man With Spine Pain and a Rash"
},
{
"authors": "Herbert S. Diamond, MD, MACP",
"content": [
"Tumor necrosis factor (TNF) inhibitors are highly effective for skin and joint disease and are recommended as initial treatment for patients with active psoriatic arthritis. No data support the superiority of any one TNF inhibitor over another.[1,4,5,6,7] If initial TNF inhibitor treatment provides an inadequate response, switching to a different TNF inhibitor is recommended.",
"Nonsteroidal anti-inflammatory drugs (NSAIDs) may be helpful for joint pain in psoriatic arthritis, but they do not alter joint progression or benefit skin disease. Methotrexate in higher doses is effective for psoriasis, but neither it nor other disease-modifying antirheumatic drugs (DMARDs), including sulfasalazine and cyclosporine, have been shown to provide long-term benefits in delaying progression in patients with erosive joint disease. DMARDs may be used as initial treatment in patients without severe disease who prefer starting with oral therapy or have a contraindication to TNF inhibitors.",
"Antimalarials are generally avoided, since they have been associated with a severe, albeit rare, skin reaction. Corticosteroids are used, but tapering of corticosteroids has been associated with flares of skin disease. Local injection of corticosteroids can be useful for the management of individual joints and tendon sheath involvement.",
"Other, more recently approved biologics are beneficial in patients with an unsatisfactory response to TNF inhibitors. These include several inhibitors of interleukin (IL)-17, which are highly effective for psoriasis; ustekinumab, an IL-12/23 inhibitor; abatacept, a CTLA4-Ig inhibitor, tofacitinib, a JAK inhibitor; and apremilast, a phosphodiesterase-4 inhibitor. Other biologic agents for psoriatic arthritis are in clinical trials.[8]",
"After the patient in this case began the aforementioned treatment with naproxen and oral methotrexate, there was some improvement in the skin rash but little change in the arthritis, which spread to additional joints. A TNF inhibitor was added to the treatment, and over the next 3 months gradual improvement was observed in the patient’s arthritis, which became almost inactive. Naproxen was discontinued, and the methotrexate dose was decreased back to 15 mg weekly. The patient remains on this treatment regimen, with continued excellent response."
],
"date": "February 26, 2020",
"figures": [],
"markdown": "# A 46-Year-Old Man With Spine Pain and a Rash\n\n **Authors:** Herbert S. Diamond, MD, MACP \n **Date:** February 26, 2020\n\n ## Content\n\n Tumor necrosis factor (TNF) inhibitors are highly effective for skin and joint disease and are recommended as initial treatment for patients with active psoriatic arthritis. No data support the superiority of any one TNF inhibitor over another.[1,4,5,6,7] If initial TNF inhibitor treatment provides an inadequate response, switching to a different TNF inhibitor is recommended.\nNonsteroidal anti-inflammatory drugs (NSAIDs) may be helpful for joint pain in psoriatic arthritis, but they do not alter joint progression or benefit skin disease. Methotrexate in higher doses is effective for psoriasis, but neither it nor other disease-modifying antirheumatic drugs (DMARDs), including sulfasalazine and cyclosporine, have been shown to provide long-term benefits in delaying progression in patients with erosive joint disease. DMARDs may be used as initial treatment in patients without severe disease who prefer starting with oral therapy or have a contraindication to TNF inhibitors.\nAntimalarials are generally avoided, since they have been associated with a severe, albeit rare, skin reaction. Corticosteroids are used, but tapering of corticosteroids has been associated with flares of skin disease. Local injection of corticosteroids can be useful for the management of individual joints and tendon sheath involvement.\nOther, more recently approved biologics are beneficial in patients with an unsatisfactory response to TNF inhibitors. These include several inhibitors of interleukin (IL)-17, which are highly effective for psoriasis; ustekinumab, an IL-12/23 inhibitor; abatacept, a CTLA4-Ig inhibitor, tofacitinib, a JAK inhibitor; and apremilast, a phosphodiesterase-4 inhibitor. Other biologic agents for psoriatic arthritis are in clinical trials.[8]\nAfter the patient in this case began the aforementioned treatment with naproxen and oral methotrexate, there was some improvement in the skin rash but little change in the arthritis, which spread to additional joints. A TNF inhibitor was added to the treatment, and over the next 3 months gradual improvement was observed in the patient’s arthritis, which became almost inactive. Naproxen was discontinued, and the methotrexate dose was decreased back to 15 mg weekly. The patient remains on this treatment regimen, with continued excellent response.\n\n ## Figures\n\n \n*Page 4 of 5*",
"pagination": {
"current_page": 4,
"total_pages": 5
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 746011,
"choiceText": "DIP joint arthritis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 746013,
"choiceText": "Tendon sheath inflammation",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 746015,
"choiceText": "Lumbar spine involvement",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 746017,
"choiceText": "Juxta-articular osteoporosis",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 746019,
"choiceText": "Asymmetrical arthritis",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Juxta-articular osteoporosis is a feature of rheumatoid arthritis but not of psoriatic arthritis. The other findings are characteristic features of psoriatic arthritis.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 233369,
"questionText": "Which of the following is not a characteristic finding in psoriatic arthritis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 746021,
"choiceText": "Negative RF",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 746023,
"choiceText": "DIP joint and wrist arthritis",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 746025,
"choiceText": "Sacroiliac joint arthritis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 746027,
"choiceText": "Hip and knee arthritis",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 746029,
"choiceText": "Entheseal arthritis",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Patients with either psoriatic arthritis or ankylosing spondylitis often have a negative RF; arthritis of the sacroiliac joints, lumbar spine, hips, and knees; and entheseal involvement. In contrast, DIP joint and wrist disease are frequent in psoriatic arthritis but are uncommon in ankylosing spondylitis.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 233371,
"questionText": "Which of the following suggests psoriatic arthritis rather than ankylosing spondylitis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 46-Year-Old Man With Spine Pain and a Rash"
},
{
"authors": "Herbert S. Diamond, MD, MACP",
"content": [],
"date": "February 26, 2020",
"figures": [],
"markdown": "# A 46-Year-Old Man With Spine Pain and a Rash\n\n **Authors:** Herbert S. Diamond, MD, MACP \n **Date:** February 26, 2020\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 5 of 5*",
"pagination": {
"current_page": 5,
"total_pages": 5
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 746011,
"choiceText": "DIP joint arthritis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 746013,
"choiceText": "Tendon sheath inflammation",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 746015,
"choiceText": "Lumbar spine involvement",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 746017,
"choiceText": "Juxta-articular osteoporosis",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 746019,
"choiceText": "Asymmetrical arthritis",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Juxta-articular osteoporosis is a feature of rheumatoid arthritis but not of psoriatic arthritis. The other findings are characteristic features of psoriatic arthritis.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 233369,
"questionText": "Which of the following is not a characteristic finding in psoriatic arthritis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 746021,
"choiceText": "Negative RF",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 746023,
"choiceText": "DIP joint and wrist arthritis",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 746025,
"choiceText": "Sacroiliac joint arthritis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 746027,
"choiceText": "Hip and knee arthritis",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 746029,
"choiceText": "Entheseal arthritis",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Patients with either psoriatic arthritis or ankylosing spondylitis often have a negative RF; arthritis of the sacroiliac joints, lumbar spine, hips, and knees; and entheseal involvement. In contrast, DIP joint and wrist disease are frequent in psoriatic arthritis but are uncommon in ankylosing spondylitis.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 233371,
"questionText": "Which of the following suggests psoriatic arthritis rather than ankylosing spondylitis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 46-Year-Old Man With Spine Pain and a Rash"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 745991,
"choiceText": "RF-negative rheumatoid arthritis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 745993,
"choiceText": "Gout",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 745995,
"choiceText": "Osteoarthritis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 745997,
"choiceText": "Ankylosing spondylitis",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 745999,
"choiceText": "Psoriatic arthritis",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 233361,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 746011,
"choiceText": "DIP joint arthritis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 746013,
"choiceText": "Tendon sheath inflammation",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 746015,
"choiceText": "Lumbar spine involvement",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 746017,
"choiceText": "Juxta-articular osteoporosis",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 746019,
"choiceText": "Asymmetrical arthritis",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Juxta-articular osteoporosis is a feature of rheumatoid arthritis but not of psoriatic arthritis. The other findings are characteristic features of psoriatic arthritis.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 233369,
"questionText": "Which of the following is not a characteristic finding in psoriatic arthritis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 746021,
"choiceText": "Negative RF",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 746023,
"choiceText": "DIP joint and wrist arthritis",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 746025,
"choiceText": "Sacroiliac joint arthritis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 746027,
"choiceText": "Hip and knee arthritis",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 746029,
"choiceText": "Entheseal arthritis",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Patients with either psoriatic arthritis or ankylosing spondylitis often have a negative RF; arthritis of the sacroiliac joints, lumbar spine, hips, and knees; and entheseal involvement. In contrast, DIP joint and wrist disease are frequent in psoriatic arthritis but are uncommon in ankylosing spondylitis.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 233371,
"questionText": "Which of the following suggests psoriatic arthritis rather than ankylosing spondylitis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
925154 | /viewarticle/925154 | [
{
"authors": "Olusegun John Oluwole, MBBS",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians, but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please email us at [email protected] with the subject line \"Case Challenge Suggestion.\" We look forward to hearing from you.",
"A 33-year-old man is brought to the emergency department owing to sudden onset of severe vertigo, vomiting, and loss of balance, which had begun 2 days earlier. He had been in the kitchen preparing his breakfast when he suddenly felt dizzy, lost his balance, and fell on the floor. He did not hit his head on the floor during the fall.",
"From that time onward, the patient has experienced a constant spinning sensation and had become unable to sit or stand without support. During questioning, he reports several episodes of vomiting that occurred both spontaneously and in association with any head movements, as well as persistent numbness in the left half of his face. In addition, he reports a mild to moderate left-sided occipital headache and ill-defined neck pain. He reports no clouding of consciousness, neck stiffness, or photophobia; however, he was reluctant to open his eyes because of a perception of continuous spinning.",
"The patient denies any prior manipulation of his neck or any recent strenuous physical activity. He reports no seizures nor weakness in any of his limbs. He has not had double vision, blurring of vision, or temporary loss of vision. He has not experienced swallowing difficulty or hoarseness. He denies any hearing loss or tinnitus and reports no recent upper respiratory tract infection. He does not have a previous history of similar attacks of dizziness or vertigo and reports no fever, ear pain, or ear discharge. He has had no prior head or neck trauma and had been generally well until the onset of his symptoms 2 days prior.",
"His past medical history is positive for systemic hypertension diagnosed 5 years earlier, and he has not regularly taken his antihypertensive medications. To his knowledge, no known secondary etiologies have been identified for his hypertension. His father also has hypertension; however, he has no known family history of neurologic disease. He is not a smoker and reports no alcohol abuse or use of any illicit drugs."
],
"date": "December 09, 2024",
"figures": [],
"markdown": "# A 33-Year-Old Man Suddenly Unable to Stand or Sit\n\n **Authors:** Olusegun John Oluwole, MBBS \n **Date:** December 09, 2024\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians, but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please email us at [email protected] with the subject line \"Case Challenge Suggestion.\" We look forward to hearing from you.\nA 33-year-old man is brought to the emergency department owing to sudden onset of severe vertigo, vomiting, and loss of balance, which had begun 2 days earlier. He had been in the kitchen preparing his breakfast when he suddenly felt dizzy, lost his balance, and fell on the floor. He did not hit his head on the floor during the fall.\nFrom that time onward, the patient has experienced a constant spinning sensation and had become unable to sit or stand without support. During questioning, he reports several episodes of vomiting that occurred both spontaneously and in association with any head movements, as well as persistent numbness in the left half of his face. In addition, he reports a mild to moderate left-sided occipital headache and ill-defined neck pain. He reports no clouding of consciousness, neck stiffness, or photophobia; however, he was reluctant to open his eyes because of a perception of continuous spinning.\nThe patient denies any prior manipulation of his neck or any recent strenuous physical activity. He reports no seizures nor weakness in any of his limbs. He has not had double vision, blurring of vision, or temporary loss of vision. He has not experienced swallowing difficulty or hoarseness. He denies any hearing loss or tinnitus and reports no recent upper respiratory tract infection. He does not have a previous history of similar attacks of dizziness or vertigo and reports no fever, ear pain, or ear discharge. He has had no prior head or neck trauma and had been generally well until the onset of his symptoms 2 days prior.\nHis past medical history is positive for systemic hypertension diagnosed 5 years earlier, and he has not regularly taken his antihypertensive medications. To his knowledge, no known secondary etiologies have been identified for his hypertension. His father also has hypertension; however, he has no known family history of neurologic disease. He is not a smoker and reports no alcohol abuse or use of any illicit drugs.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 33-Year-Old Man Suddenly Unable to Stand or Sit"
},
{
"authors": "Olusegun John Oluwole, MBBS",
"content": [
"Upon examination, the patient is alert and oriented; however, he will not open his eyes because of severe vertigo. He is afebrile, with a temperature of 98.8°F (37.1°C). He has no conjunctival pallor but appears mildly dehydrated. He has no pedal edema.",
"A chest examination reveals a respiratory rate of 16 breaths/min, with vesicular breath sounds. Cardiovascular examination reveals a regular pulse, at 90 beats/min; elevated blood pressure, at 240/120 mm Hg; normal jugular venous pulsations; and a heaving, undisplaced cardiac apex with heart sounds S1, S2, and S4.",
"His abdomen is flat and nontender. He has no palpable organ enlargement. Bowel sounds are normoactive. Skin examination is unremarkable.",
"Upon neurologic examination, the patient has no neck stiffness. Kernig sign results are negative. He has mild left-sided ptosis but full ocular range of motion. His left pupil is smaller than the right pupil, and the difference is more noticeable in dim light. Both pupils react to light. He has spontaneous right-beating nystagmus that does not change direction with gaze. Results of head impulse testing and testing of skew are negative. Pinprick testing reveals hypoesthesia over the left half of the face and right-sided limbs. Subtle, left-sided, central-type facioparesis is noted. His gag reflex is normal bilaterally.",
"Manual motor testing reveals normal strength in all limbs, with preserved deep tendon reflexes. Plantar response is downgoing on the right and flat on the left. The left arm shows dysmetria during finger-nose testing as well as clumsiness during rapid alternating hand-movement testing. Heel-shin testing of the left leg also reveals some clumsiness. Gait testing is impossible owing to marked dizziness and postural imbalance. The patient falls back in bed immediately when he is made to sit. Pinprick sensation is reduced on the left side, with a gradient in which sensation is more impaired in the lower limb than in the upper limb.",
"Baseline laboratory analysis—including random blood glucose level, complete blood count, kidney function test results, and electrolyte levels—is unremarkable. Immediate noncontrast head CT reveals the following (Figures 1 and 2).",
"Figure 1.",
"Figure 2."
],
"date": "December 09, 2024",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/925/154/925154-Thumb1.png"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/925/154/925154-Thumb2.png"
}
],
"markdown": "# A 33-Year-Old Man Suddenly Unable to Stand or Sit\n\n **Authors:** Olusegun John Oluwole, MBBS \n **Date:** December 09, 2024\n\n ## Content\n\n Upon examination, the patient is alert and oriented; however, he will not open his eyes because of severe vertigo. He is afebrile, with a temperature of 98.8°F (37.1°C). He has no conjunctival pallor but appears mildly dehydrated. He has no pedal edema.\nA chest examination reveals a respiratory rate of 16 breaths/min, with vesicular breath sounds. Cardiovascular examination reveals a regular pulse, at 90 beats/min; elevated blood pressure, at 240/120 mm Hg; normal jugular venous pulsations; and a heaving, undisplaced cardiac apex with heart sounds S1, S2, and S4.\nHis abdomen is flat and nontender. He has no palpable organ enlargement. Bowel sounds are normoactive. Skin examination is unremarkable.\nUpon neurologic examination, the patient has no neck stiffness. Kernig sign results are negative. He has mild left-sided ptosis but full ocular range of motion. His left pupil is smaller than the right pupil, and the difference is more noticeable in dim light. Both pupils react to light. He has spontaneous right-beating nystagmus that does not change direction with gaze. Results of head impulse testing and testing of skew are negative. Pinprick testing reveals hypoesthesia over the left half of the face and right-sided limbs. Subtle, left-sided, central-type facioparesis is noted. His gag reflex is normal bilaterally.\nManual motor testing reveals normal strength in all limbs, with preserved deep tendon reflexes. Plantar response is downgoing on the right and flat on the left. The left arm shows dysmetria during finger-nose testing as well as clumsiness during rapid alternating hand-movement testing. Heel-shin testing of the left leg also reveals some clumsiness. Gait testing is impossible owing to marked dizziness and postural imbalance. The patient falls back in bed immediately when he is made to sit. Pinprick sensation is reduced on the left side, with a gradient in which sensation is more impaired in the lower limb than in the upper limb.\nBaseline laboratory analysis—including random blood glucose level, complete blood count, kidney function test results, and electrolyte levels—is unremarkable. Immediate noncontrast head CT reveals the following (Figures 1 and 2).\nFigure 1.\nFigure 2.\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1469664,
"choiceText": "Acute vestibular neuronitis",
"correct": false,
"displayOrder": 1,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1469666,
"choiceText": "Aneurysmal subarachnoid hemorrhage",
"correct": false,
"displayOrder": 2,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1469668,
"choiceText": "Lateral medullary syndrome",
"correct": true,
"displayOrder": 3,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1469670,
"choiceText": "Ménière disease\r\n",
"correct": false,
"displayOrder": 4,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "",
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": "",
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 470155,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 33-Year-Old Man Suddenly Unable to Stand or Sit"
},
{
"authors": "Olusegun John Oluwole, MBBS",
"content": [
"Despite normal brain CT findings, the patient was suspected to have lateral medullary infarction on the basis of his constellation of symptoms and signs. Brain MRI was ordered and confirmed an infarct in the left dorsolateral medulla (Figure 3). The patient was hospitalized in the intensive care unit.",
"Figure 3.",
"Lateral medullary syndrome, or Wallenberg syndrome, results from occlusion of the posterior inferior cerebellar artery or the vertebral artery.[1,2] This causes infarction in the lateral medulla with or without involvement of the inferior cerebellar peduncle. Symptoms and signs include vertigo, ipsilateral Horner syndrome, loss of pain and temperature sensation over the ipsilateral face and contralateral body, ipsilateral ataxia, and hoarseness and dysphagia.[3]",
"Vertigo, nystagmus, and vomiting occur due to involvement of the vestibular nuclei or their connections to the cerebellum.[4] Ipsilateral Horner syndrome occurs as a result of involvement of the descending sympathetic pathways in the lateral reticular formation.[5] Ipsilateral facial sensory loss results from involvement of the descending trigeminal tract. Contralateral limb sensory loss occurs owing to effects on the ascending spinothalamic sensory tract. Ipsilateral ataxia is due to involvement of the restiform body or the spinocerebellar pathway. Dysphagia and hoarseness are believed to result from involvement of the nucleus ambiguus.",
"The patient in this case demonstrated several typical features of lateral medullary syndrome, with a few interesting exceptions. He did not display hoarseness or dysphagia. Variations are known to occur in the extent of clinical symptomatology of lateral medullary syndrome, depending on the size and extent of the infarct.[2] In a case series, patients whose infarcts occurred in the caudal part of the medulla tended to have little or no dysphagia and hoarseness.[5] This led to the suggestion that the caudal part of the nucleus ambiguus is not directly involved with visceral efferent fibers. In most cases where dysphagia and hoarseness occur, the lesions extend to the rostral and ventral aspects of the lateral medulla.The second unique feature in this patient's presentation is the occurrence of subtle ipsilateral central facioparesis. Ipsilateral peripheral facioparesis is said to be possible when the lesion extends to the lower pons, whereas involvement of an aberrant looping corticobulbar tract has been suggested as a plausible mechanism for ipsilateral central facioparesis.[5,6] Other reported variations not seen in this patient include contralateral facial sensory loss and bilateral facial sensory loss. Bilateral sensory impairment has been reported in patients with large lesions that involve both the descending and the ascending trigeminal sensory tracts, whereas contralateral facial sensory loss has been observed in lesions involving the ascending trigeminal sensory tract.[5]"
],
"date": "December 09, 2024",
"figures": [
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/925/154/925154-Thumb3.png"
}
],
"markdown": "# A 33-Year-Old Man Suddenly Unable to Stand or Sit\n\n **Authors:** Olusegun John Oluwole, MBBS \n **Date:** December 09, 2024\n\n ## Content\n\n Despite normal brain CT findings, the patient was suspected to have lateral medullary infarction on the basis of his constellation of symptoms and signs. Brain MRI was ordered and confirmed an infarct in the left dorsolateral medulla (Figure 3). The patient was hospitalized in the intensive care unit.\nFigure 3.\nLateral medullary syndrome, or Wallenberg syndrome, results from occlusion of the posterior inferior cerebellar artery or the vertebral artery.[1,2] This causes infarction in the lateral medulla with or without involvement of the inferior cerebellar peduncle. Symptoms and signs include vertigo, ipsilateral Horner syndrome, loss of pain and temperature sensation over the ipsilateral face and contralateral body, ipsilateral ataxia, and hoarseness and dysphagia.[3]\nVertigo, nystagmus, and vomiting occur due to involvement of the vestibular nuclei or their connections to the cerebellum.[4] Ipsilateral Horner syndrome occurs as a result of involvement of the descending sympathetic pathways in the lateral reticular formation.[5] Ipsilateral facial sensory loss results from involvement of the descending trigeminal tract. Contralateral limb sensory loss occurs owing to effects on the ascending spinothalamic sensory tract. Ipsilateral ataxia is due to involvement of the restiform body or the spinocerebellar pathway. Dysphagia and hoarseness are believed to result from involvement of the nucleus ambiguus.\nThe patient in this case demonstrated several typical features of lateral medullary syndrome, with a few interesting exceptions. He did not display hoarseness or dysphagia. Variations are known to occur in the extent of clinical symptomatology of lateral medullary syndrome, depending on the size and extent of the infarct.[2] In a case series, patients whose infarcts occurred in the caudal part of the medulla tended to have little or no dysphagia and hoarseness.[5] This led to the suggestion that the caudal part of the nucleus ambiguus is not directly involved with visceral efferent fibers. In most cases where dysphagia and hoarseness occur, the lesions extend to the rostral and ventral aspects of the lateral medulla.The second unique feature in this patient's presentation is the occurrence of subtle ipsilateral central facioparesis. Ipsilateral peripheral facioparesis is said to be possible when the lesion extends to the lower pons, whereas involvement of an aberrant looping corticobulbar tract has been suggested as a plausible mechanism for ipsilateral central facioparesis.[5,6] Other reported variations not seen in this patient include contralateral facial sensory loss and bilateral facial sensory loss. Bilateral sensory impairment has been reported in patients with large lesions that involve both the descending and the ascending trigeminal sensory tracts, whereas contralateral facial sensory loss has been observed in lesions involving the ascending trigeminal sensory tract.[5]\n\n ## Figures\n\n **Figure 3.** \n \n\n\n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1469664,
"choiceText": "Acute vestibular neuronitis",
"correct": false,
"displayOrder": 1,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1469666,
"choiceText": "Aneurysmal subarachnoid hemorrhage",
"correct": false,
"displayOrder": 2,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1469668,
"choiceText": "Lateral medullary syndrome",
"correct": true,
"displayOrder": 3,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1469670,
"choiceText": "Ménière disease\r\n",
"correct": false,
"displayOrder": 4,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "",
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": "",
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 470155,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 33-Year-Old Man Suddenly Unable to Stand or Sit"
},
{
"authors": "Olusegun John Oluwole, MBBS",
"content": [
"Lateral medullary syndrome is one of the few conditions that must be considered in the emergency department when assessing patients who present with a first-ever acute attack of spontaneous vertigo or dizziness (so-called acute vestibular syndrome).[7] Other conditions to consider in this setting include acute vestibular neuronitis and the first attack of Ménière disease.[7]",
"The features of lateral medullary syndrome are distinctive and should be explored, especially in patients who present with acute dizziness and also have known traditional cardiovascular risk factors. Acute vestibular neuronitis also presents with an acute onset of dizziness, vertigo, disturbance of balance, and vomiting. However, limb ataxia, sensory loss, and Horner syndrome are not expected in peripheral causes of vertigo, such as vestibular neuronitis. Head impulse test findings may also be positive in vestibular neuronitis.[7]",
"Tinnitus and hearing loss are usually present along with vertigo in Ménière disease. No additional neurologic features are typically observed. Aneurysmal subarachnoid hemorrhage due to ruptured posterior communicating artery aneurysm causes a large pupil, rather than the small pupil seen in this patient.[8] The absence of neck stiffness and photophobia, in addition to a normal head CT findings, makes an aneurysmal bleed less likely.",
"Brain MRI is always required for confirmation of diagnosis once the syndrome is clinically suspected. Brain CT has poor sensitivity for imaging posterior fossa structures.[9] Notwithstanding the sensitivity of MRI, no changes may be observed on early imaging studies, and the changes may be missed altogether if suggestive clinical information is not provided to the interpreting radiologist by the requesting clinician.[10]",
"Once the diagnosis is confirmed on imaging, the patient must be investigated for likely risk factors and causative mechanisms. As a rule of thumb, vertebral artery dissection must always be considered in young patients with lateral medullary syndrome. This should always prompt a request for MR/CT angiography. The age of the patient in this case and the presence of a left occipital headache and mild neck pain are known clinical symptoms associated with arterial dissection.",
"Several cases of lateral medullary infarction have been reported in adolescents and young adults after hyperextension neck injuries during sports, as well as after neck manipulation, leading to vertebral artery dissection.[3,10] In older adults, traditional cardiovascular risk factors must be assessed. In a case series of 33 patients, hypertension was reported in 24 cases, hyperlipidemia in nine cases, diabetes mellitus in eight cases, cigarette smoking in eight cases, and cardiac disease in three cases.[5] The cause may be elusive in as many as 10%-15% of cases.[2,5]The patient in this case had CT angiography, which showed no evidence of vertebral artery dissection nor any atherosclerotic changes in the large arteries. Echocardiography revealed hypertensive concentric left ventricular hypertrophy. Electrocardiography showed no cardiac arrhythmias. Fasting glucose and lipid panel findings were unremarkable, as were glycated hemoglobin levels. The underlying mechanism of stroke was thought to be small-vessel disease related to hypertension."
],
"date": "December 09, 2024",
"figures": [],
"markdown": "# A 33-Year-Old Man Suddenly Unable to Stand or Sit\n\n **Authors:** Olusegun John Oluwole, MBBS \n **Date:** December 09, 2024\n\n ## Content\n\n Lateral medullary syndrome is one of the few conditions that must be considered in the emergency department when assessing patients who present with a first-ever acute attack of spontaneous vertigo or dizziness (so-called acute vestibular syndrome).[7] Other conditions to consider in this setting include acute vestibular neuronitis and the first attack of Ménière disease.[7]\nThe features of lateral medullary syndrome are distinctive and should be explored, especially in patients who present with acute dizziness and also have known traditional cardiovascular risk factors. Acute vestibular neuronitis also presents with an acute onset of dizziness, vertigo, disturbance of balance, and vomiting. However, limb ataxia, sensory loss, and Horner syndrome are not expected in peripheral causes of vertigo, such as vestibular neuronitis. Head impulse test findings may also be positive in vestibular neuronitis.[7]\nTinnitus and hearing loss are usually present along with vertigo in Ménière disease. No additional neurologic features are typically observed. Aneurysmal subarachnoid hemorrhage due to ruptured posterior communicating artery aneurysm causes a large pupil, rather than the small pupil seen in this patient.[8] The absence of neck stiffness and photophobia, in addition to a normal head CT findings, makes an aneurysmal bleed less likely.\nBrain MRI is always required for confirmation of diagnosis once the syndrome is clinically suspected. Brain CT has poor sensitivity for imaging posterior fossa structures.[9] Notwithstanding the sensitivity of MRI, no changes may be observed on early imaging studies, and the changes may be missed altogether if suggestive clinical information is not provided to the interpreting radiologist by the requesting clinician.[10]\nOnce the diagnosis is confirmed on imaging, the patient must be investigated for likely risk factors and causative mechanisms. As a rule of thumb, vertebral artery dissection must always be considered in young patients with lateral medullary syndrome. This should always prompt a request for MR/CT angiography. The age of the patient in this case and the presence of a left occipital headache and mild neck pain are known clinical symptoms associated with arterial dissection.\nSeveral cases of lateral medullary infarction have been reported in adolescents and young adults after hyperextension neck injuries during sports, as well as after neck manipulation, leading to vertebral artery dissection.[3,10] In older adults, traditional cardiovascular risk factors must be assessed. In a case series of 33 patients, hypertension was reported in 24 cases, hyperlipidemia in nine cases, diabetes mellitus in eight cases, cigarette smoking in eight cases, and cardiac disease in three cases.[5] The cause may be elusive in as many as 10%-15% of cases.[2,5]The patient in this case had CT angiography, which showed no evidence of vertebral artery dissection nor any atherosclerotic changes in the large arteries. Echocardiography revealed hypertensive concentric left ventricular hypertrophy. Electrocardiography showed no cardiac arrhythmias. Fasting glucose and lipid panel findings were unremarkable, as were glycated hemoglobin levels. The underlying mechanism of stroke was thought to be small-vessel disease related to hypertension.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 33-Year-Old Man Suddenly Unable to Stand or Sit"
},
{
"authors": "Olusegun John Oluwole, MBBS",
"content": [
"The appropriate treatment for lateral medullary syndrome depends on the duration of symptoms at diagnosis and the mechanism of stroke. Confirmation of diagnosis within 3-4.5 hours from onset may allow for thrombolytic treatment for patients with nondissection etiology who have no contraindication to such therapy.[11] Outside of the time window for thrombolytic treatment, antiplatelet treatment should be offered alongside secondary cardiovascular prevention via control of identified risk factors. The risk reduction strategy should also include anticoagulation in those with cardioembolic mechanisms.[11]",
"For patients with extracranial vertebral artery dissection as the underlying etiology, thrombolysis is reasonably safe; however, the risk is unknown in those with intracranial arterial dissection.[12] For those with underlying vertebral artery dissection, anticoagulation or antiplatelet treatments are usually prescribed for 3-6 months; however, surgical intervention in the form of endovascular treatment or arterial repair may be warranted in cases that involve subarachnoid hemorrhage, symptomatic aneurysmal dilatation, or failure of medical management.[12]",
"The patient in this case was diagnosed late and would not benefit from thrombolysis. He had very high blood pressure, exceeding a mean of 140 mm Hg, and was managed on intravenous labetalol in the first few days and later switched to oral antihypertensives. Extreme blood pressure fluctuations were noted during his care, which probably resulted from sympathetic pathway affectation in the lateral medulla. An oral antiplatelet was later added when his blood pressure became reasonably controlled.",
"For most patients with lateral medullary syndrome, prognosis is good. Case reports and large case series have shown good recovery in most patients, with only few residual deficits seen after 3-6 months of follow up.[2,5] In a series of 130 patients, only one patient died in the acute phase.[2]",
"The patient in this case initially had marked ataxia and was unable to sit unsupported. However, he regained independent sitting before he was discharged from the hospital intensive care unit for physical rehabilitation about 7 days after symptom onset. He was still unable to stand independently and tended to fall toward the left side (ipsilateral to the lesion) when he was helped to stand. During his follow-up visit 2 weeks later, he was ambulant, although he displayed a wide-based gait and some balance difficulty. At that time, he had also recovered full sensation in the left side of his face and the right side of his limbs."
],
"date": "December 09, 2024",
"figures": [],
"markdown": "# A 33-Year-Old Man Suddenly Unable to Stand or Sit\n\n **Authors:** Olusegun John Oluwole, MBBS \n **Date:** December 09, 2024\n\n ## Content\n\n The appropriate treatment for lateral medullary syndrome depends on the duration of symptoms at diagnosis and the mechanism of stroke. Confirmation of diagnosis within 3-4.5 hours from onset may allow for thrombolytic treatment for patients with nondissection etiology who have no contraindication to such therapy.[11] Outside of the time window for thrombolytic treatment, antiplatelet treatment should be offered alongside secondary cardiovascular prevention via control of identified risk factors. The risk reduction strategy should also include anticoagulation in those with cardioembolic mechanisms.[11]\nFor patients with extracranial vertebral artery dissection as the underlying etiology, thrombolysis is reasonably safe; however, the risk is unknown in those with intracranial arterial dissection.[12] For those with underlying vertebral artery dissection, anticoagulation or antiplatelet treatments are usually prescribed for 3-6 months; however, surgical intervention in the form of endovascular treatment or arterial repair may be warranted in cases that involve subarachnoid hemorrhage, symptomatic aneurysmal dilatation, or failure of medical management.[12]\nThe patient in this case was diagnosed late and would not benefit from thrombolysis. He had very high blood pressure, exceeding a mean of 140 mm Hg, and was managed on intravenous labetalol in the first few days and later switched to oral antihypertensives. Extreme blood pressure fluctuations were noted during his care, which probably resulted from sympathetic pathway affectation in the lateral medulla. An oral antiplatelet was later added when his blood pressure became reasonably controlled.\nFor most patients with lateral medullary syndrome, prognosis is good. Case reports and large case series have shown good recovery in most patients, with only few residual deficits seen after 3-6 months of follow up.[2,5] In a series of 130 patients, only one patient died in the acute phase.[2]\nThe patient in this case initially had marked ataxia and was unable to sit unsupported. However, he regained independent sitting before he was discharged from the hospital intensive care unit for physical rehabilitation about 7 days after symptom onset. He was still unable to stand independently and tended to fall toward the left side (ipsilateral to the lesion) when he was helped to stand. During his follow-up visit 2 weeks later, he was ambulant, although he displayed a wide-based gait and some balance difficulty. At that time, he had also recovered full sensation in the left side of his face and the right side of his limbs.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1469672,
"choiceText": "Dysphagia and hoarseness are more common with caudal lesions",
"correct": false,
"displayOrder": 1,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1469674,
"choiceText": "Ipsilateral peripheral facioparesis is most often associated with involvement of an aberrant looping corticobulbar tract",
"correct": false,
"displayOrder": 2,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1469676,
"choiceText": "Bilateral sensory impairment is typically linked to lesions that involve the ascending trigeminal sensory tract",
"correct": false,
"displayOrder": 3,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1469678,
"choiceText": "Symptoms and signs include vertigo and ipsilateral ataxia",
"correct": true,
"displayOrder": 4,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Lateral medullary syndrome is caused by occlusion of the posterior inferior cerebellar artery or the vertebral artery.<sup>[1,2]</sup> This leads to infarction in the lateral medulla with or without involvement of the inferior cerebellar peduncle. Among the symptoms and signs are <a href=\"https://emedicine.medscape.com/article/2149881-overview\">vertigo</a>, ipsilateral <a href=\"https://emedicine.medscape.com/article/1220091-overview\">Horner syndrome</a>, loss of both pain and temperature sensation over the ipsilateral face and contralateral body, ipsilateral ataxia, as well as hoarseness and dysphagia.<sup>[3]</sup>\r\n\r\n<br /><br />Variations occur in the clinical symptomatology of lateral medullary syndrome and depend on the infarct size and extent.<sup>[2]</sup> In a case series, patients whose infarcts occurred in the caudal part of the medulla generally had little or no dysphagia and hoarseness.<sup>[5]</sup> In the majority of the cases in which there are dysphagia and hoarseness, the lesions extend to the rostral and ventral aspects of the lateral medulla. Ipsilateral peripheral facioparesis is considered plausible when the lesion extends to the lower pons, whereas involvement of an aberrant looping corticobulbar tract has been proposed as a possible mechanism for ipsilateral central facioparesis.<sup>[5,6]</sup> Among other reported variations are contralateral facial sensory loss and bilateral facial sensory loss. Bilateral sensory impairment has occurred in patients with large lesions that affect both the descending and the ascending trigeminal sensory tracts; in lesions involving the ascending trigeminal sensory tract, contralateral facial sensory loss has been reported.<sup>[5]</sup>",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": "",
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 470157,
"questionText": "Which of the following is most accurate regarding the clinical features of lateral medullary syndrome?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1469748,
"choiceText": "Brain CT is the imaging modality of choice",
"correct": false,
"displayOrder": 1,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1469750,
"choiceText": "Head and neck angiography are recommended in the assessment of older patients but optional in younger patients",
"correct": false,
"displayOrder": 2,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1469752,
"choiceText": "The presence of a left occipital headache and mild neck pain should prompt investigation for arterial dissection",
"correct": true,
"displayOrder": 3,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1469754,
"choiceText": "The absence of findings on imaging studies obtained soon after the condition's onset is sufficient to exclude this diagnosis",
"correct": false,
"displayOrder": 4,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>As noted previously, brain MRI is always necessary to confirm diagnosis when lateral medullary syndrome is clinically suspected. Brain CT has poor sensitivity for imaging posterior fossa structures.<sup>[9]</sup> Regardless of the sensitivity of MRI, no changes may be seen on early imaging studies, and the changes could be missed overall if the requesting clinician does not provide appropriate clinical information to the interpreting radiologist.<sup>[10]</sup><br /><br />Again, after the diagnosis is confirmed on imaging, the risk factors and causative mechanisms should be explored. As a general rule, <a href=\"https://emedicine.medscape.com/article/761451-overview\">vertebral artery dissection</a> should always be considered in young patients with lateral medullary syndrome, prompting a request for MR/CT angiography. In this case, the age of the patient and the presence of a left occipital headache and mild neck pain signaled the need for investigation for arterial dissection.</p>",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": "",
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 470175,
"questionText": "Which of the following options is most accurate regarding the evaluation of suspected lateral medullary syndrome? ",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 33-Year-Old Man Suddenly Unable to Stand or Sit"
},
{
"authors": "Olusegun John Oluwole, MBBS",
"content": [],
"date": "December 09, 2024",
"figures": [],
"markdown": "# A 33-Year-Old Man Suddenly Unable to Stand or Sit\n\n **Authors:** Olusegun John Oluwole, MBBS \n **Date:** December 09, 2024\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1469672,
"choiceText": "Dysphagia and hoarseness are more common with caudal lesions",
"correct": false,
"displayOrder": 1,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1469674,
"choiceText": "Ipsilateral peripheral facioparesis is most often associated with involvement of an aberrant looping corticobulbar tract",
"correct": false,
"displayOrder": 2,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1469676,
"choiceText": "Bilateral sensory impairment is typically linked to lesions that involve the ascending trigeminal sensory tract",
"correct": false,
"displayOrder": 3,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1469678,
"choiceText": "Symptoms and signs include vertigo and ipsilateral ataxia",
"correct": true,
"displayOrder": 4,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Lateral medullary syndrome is caused by occlusion of the posterior inferior cerebellar artery or the vertebral artery.<sup>[1,2]</sup> This leads to infarction in the lateral medulla with or without involvement of the inferior cerebellar peduncle. Among the symptoms and signs are <a href=\"https://emedicine.medscape.com/article/2149881-overview\">vertigo</a>, ipsilateral <a href=\"https://emedicine.medscape.com/article/1220091-overview\">Horner syndrome</a>, loss of both pain and temperature sensation over the ipsilateral face and contralateral body, ipsilateral ataxia, as well as hoarseness and dysphagia.<sup>[3]</sup>\r\n\r\n<br /><br />Variations occur in the clinical symptomatology of lateral medullary syndrome and depend on the infarct size and extent.<sup>[2]</sup> In a case series, patients whose infarcts occurred in the caudal part of the medulla generally had little or no dysphagia and hoarseness.<sup>[5]</sup> In the majority of the cases in which there are dysphagia and hoarseness, the lesions extend to the rostral and ventral aspects of the lateral medulla. Ipsilateral peripheral facioparesis is considered plausible when the lesion extends to the lower pons, whereas involvement of an aberrant looping corticobulbar tract has been proposed as a possible mechanism for ipsilateral central facioparesis.<sup>[5,6]</sup> Among other reported variations are contralateral facial sensory loss and bilateral facial sensory loss. Bilateral sensory impairment has occurred in patients with large lesions that affect both the descending and the ascending trigeminal sensory tracts; in lesions involving the ascending trigeminal sensory tract, contralateral facial sensory loss has been reported.<sup>[5]</sup>",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": "",
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 470157,
"questionText": "Which of the following is most accurate regarding the clinical features of lateral medullary syndrome?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1469748,
"choiceText": "Brain CT is the imaging modality of choice",
"correct": false,
"displayOrder": 1,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1469750,
"choiceText": "Head and neck angiography are recommended in the assessment of older patients but optional in younger patients",
"correct": false,
"displayOrder": 2,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1469752,
"choiceText": "The presence of a left occipital headache and mild neck pain should prompt investigation for arterial dissection",
"correct": true,
"displayOrder": 3,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1469754,
"choiceText": "The absence of findings on imaging studies obtained soon after the condition's onset is sufficient to exclude this diagnosis",
"correct": false,
"displayOrder": 4,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>As noted previously, brain MRI is always necessary to confirm diagnosis when lateral medullary syndrome is clinically suspected. Brain CT has poor sensitivity for imaging posterior fossa structures.<sup>[9]</sup> Regardless of the sensitivity of MRI, no changes may be seen on early imaging studies, and the changes could be missed overall if the requesting clinician does not provide appropriate clinical information to the interpreting radiologist.<sup>[10]</sup><br /><br />Again, after the diagnosis is confirmed on imaging, the risk factors and causative mechanisms should be explored. As a general rule, <a href=\"https://emedicine.medscape.com/article/761451-overview\">vertebral artery dissection</a> should always be considered in young patients with lateral medullary syndrome, prompting a request for MR/CT angiography. In this case, the age of the patient and the presence of a left occipital headache and mild neck pain signaled the need for investigation for arterial dissection.</p>",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": "",
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 470175,
"questionText": "Which of the following options is most accurate regarding the evaluation of suspected lateral medullary syndrome? ",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 33-Year-Old Man Suddenly Unable to Stand or Sit"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1469664,
"choiceText": "Acute vestibular neuronitis",
"correct": false,
"displayOrder": 1,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1469666,
"choiceText": "Aneurysmal subarachnoid hemorrhage",
"correct": false,
"displayOrder": 2,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1469668,
"choiceText": "Lateral medullary syndrome",
"correct": true,
"displayOrder": 3,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1469670,
"choiceText": "Ménière disease\r\n",
"correct": false,
"displayOrder": 4,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "",
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": "",
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 470155,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1469672,
"choiceText": "Dysphagia and hoarseness are more common with caudal lesions",
"correct": false,
"displayOrder": 1,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1469674,
"choiceText": "Ipsilateral peripheral facioparesis is most often associated with involvement of an aberrant looping corticobulbar tract",
"correct": false,
"displayOrder": 2,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1469676,
"choiceText": "Bilateral sensory impairment is typically linked to lesions that involve the ascending trigeminal sensory tract",
"correct": false,
"displayOrder": 3,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1469678,
"choiceText": "Symptoms and signs include vertigo and ipsilateral ataxia",
"correct": true,
"displayOrder": 4,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Lateral medullary syndrome is caused by occlusion of the posterior inferior cerebellar artery or the vertebral artery.<sup>[1,2]</sup> This leads to infarction in the lateral medulla with or without involvement of the inferior cerebellar peduncle. Among the symptoms and signs are <a href=\"https://emedicine.medscape.com/article/2149881-overview\">vertigo</a>, ipsilateral <a href=\"https://emedicine.medscape.com/article/1220091-overview\">Horner syndrome</a>, loss of both pain and temperature sensation over the ipsilateral face and contralateral body, ipsilateral ataxia, as well as hoarseness and dysphagia.<sup>[3]</sup>\r\n\r\n<br /><br />Variations occur in the clinical symptomatology of lateral medullary syndrome and depend on the infarct size and extent.<sup>[2]</sup> In a case series, patients whose infarcts occurred in the caudal part of the medulla generally had little or no dysphagia and hoarseness.<sup>[5]</sup> In the majority of the cases in which there are dysphagia and hoarseness, the lesions extend to the rostral and ventral aspects of the lateral medulla. Ipsilateral peripheral facioparesis is considered plausible when the lesion extends to the lower pons, whereas involvement of an aberrant looping corticobulbar tract has been proposed as a possible mechanism for ipsilateral central facioparesis.<sup>[5,6]</sup> Among other reported variations are contralateral facial sensory loss and bilateral facial sensory loss. Bilateral sensory impairment has occurred in patients with large lesions that affect both the descending and the ascending trigeminal sensory tracts; in lesions involving the ascending trigeminal sensory tract, contralateral facial sensory loss has been reported.<sup>[5]</sup>",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": "",
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 470157,
"questionText": "Which of the following is most accurate regarding the clinical features of lateral medullary syndrome?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1469748,
"choiceText": "Brain CT is the imaging modality of choice",
"correct": false,
"displayOrder": 1,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1469750,
"choiceText": "Head and neck angiography are recommended in the assessment of older patients but optional in younger patients",
"correct": false,
"displayOrder": 2,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1469752,
"choiceText": "The presence of a left occipital headache and mild neck pain should prompt investigation for arterial dissection",
"correct": true,
"displayOrder": 3,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1469754,
"choiceText": "The absence of findings on imaging studies obtained soon after the condition's onset is sufficient to exclude this diagnosis",
"correct": false,
"displayOrder": 4,
"explanation": "",
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>As noted previously, brain MRI is always necessary to confirm diagnosis when lateral medullary syndrome is clinically suspected. Brain CT has poor sensitivity for imaging posterior fossa structures.<sup>[9]</sup> Regardless of the sensitivity of MRI, no changes may be seen on early imaging studies, and the changes could be missed overall if the requesting clinician does not provide appropriate clinical information to the interpreting radiologist.<sup>[10]</sup><br /><br />Again, after the diagnosis is confirmed on imaging, the risk factors and causative mechanisms should be explored. As a general rule, <a href=\"https://emedicine.medscape.com/article/761451-overview\">vertebral artery dissection</a> should always be considered in young patients with lateral medullary syndrome, prompting a request for MR/CT angiography. In this case, the age of the patient and the presence of a left occipital headache and mild neck pain signaled the need for investigation for arterial dissection.</p>",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": "",
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 470175,
"questionText": "Which of the following options is most accurate regarding the evaluation of suspected lateral medullary syndrome? ",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
732961 | /viewarticle/732961 | [
{
"authors": "Roza Chaireti, MD; Mats Pettersson, MD",
"content": [
"Editor's Note:\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"A 46-year-old man presents to the emergency department with a 5-day history of progressively worsening breathlessness on exertion and mild, general flulike symptoms. He also complains about night sweats and an intermittent low-grade fever, both of which started about 2 weeks ago.",
"Upon physical examination, the patient does not appear to be in any acute distress. His vital signs are measured as a pulse of 89 beats/min, blood pressure of 140/85 mm Hg, and a respiratory rate of 19 breaths/min. He is afebrile, with a temperature of 99.8°F (37.7°C).",
"The chest examination reveals nothing out of the ordinary, and his cardiovascular and respiratory examinations, including auscultation, are unremarkable. The abdominal examination reveals no fluid thrill, shifting dullness, or bruit. The liver and the spleen are not enlarged. No lymph nodes are palpable.",
"Multiple areas of hyperpigmentation are noted; otherwise, the skin inspection is unremarkable, with no hematomas, bruises, wounds, or scars noted. Electrocardiography (ECG) reveals a sinus rhythm with a heart rate of 84 beats/min, and the T waves are somewhat flattened in leads V1, aVL, and III, but they are otherwise unremarkable."
],
"date": "January 15, 2020",
"figures": [],
"markdown": "# Dyspnea and Night Sweats in a 46-Year-Old Man\n\n **Authors:** Roza Chaireti, MD; Mats Pettersson, MD \n **Date:** January 15, 2020\n\n ## Content\n\n Editor's Note:\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nA 46-year-old man presents to the emergency department with a 5-day history of progressively worsening breathlessness on exertion and mild, general flulike symptoms. He also complains about night sweats and an intermittent low-grade fever, both of which started about 2 weeks ago.\nUpon physical examination, the patient does not appear to be in any acute distress. His vital signs are measured as a pulse of 89 beats/min, blood pressure of 140/85 mm Hg, and a respiratory rate of 19 breaths/min. He is afebrile, with a temperature of 99.8°F (37.7°C).\nThe chest examination reveals nothing out of the ordinary, and his cardiovascular and respiratory examinations, including auscultation, are unremarkable. The abdominal examination reveals no fluid thrill, shifting dullness, or bruit. The liver and the spleen are not enlarged. No lymph nodes are palpable.\nMultiple areas of hyperpigmentation are noted; otherwise, the skin inspection is unremarkable, with no hematomas, bruises, wounds, or scars noted. Electrocardiography (ECG) reveals a sinus rhythm with a heart rate of 84 beats/min, and the T waves are somewhat flattened in leads V1, aVL, and III, but they are otherwise unremarkable.\n\n ## Figures\n\n \n*Page 1 of 5*",
"pagination": {
"current_page": 1,
"total_pages": 5
},
"questionnaire": [],
"title": "Dyspnea and Night Sweats in a 46-Year-Old Man"
},
{
"authors": "Roza Chaireti, MD; Mats Pettersson, MD",
"content": [
"Significant laboratory findings include a white blood cell count of 9.1 x 103/µL (9.1 x 109/L; reference range, 3.5-8.8 x 109/L), a platelet count of 429 x 103/μL (429 x 109/L; reference range, 140-350 109/L), a C-reactive protein level of 91 mg/L (reference range, < 10 mg/L), a lactate dehydrogenase (LDH) level of 4.7 microkatals (µkat)/L (reference range, < 3.5 µkat/L), an erythrocyte sedimentation rate (ESR) of 30 mm (reference range, 1-12 mm), and a D-dimer of 2.2 mg/L (reference range, < 0.25 mg/L).",
"A spiral CT scan is performed, which shows no pulmonary embolism. It does, however, reveal the presence of a significant pericardial effusion (1 cm ventral x 2.5 cm dorsal) and a multilobular substernal mass occupying the anterior superior mediastinum that is about 2.5 cm in thickness and 7 cm in length, with high absorption. Additionally, the mediastinal lymph nodes are enlarged; some are as large as 2 cm in size. No other pertinent findings on spiral CT are reported.",
"An emergent bedside echocardiogram is obtained. The echocardiographic findings confirm the presence of pericardial effusion, without signs or symptoms of a cardiac tamponade; additionally, a retrosternal mass is detected (see video clips)."
],
"date": "January 15, 2020",
"figures": [],
"markdown": "# Dyspnea and Night Sweats in a 46-Year-Old Man\n\n **Authors:** Roza Chaireti, MD; Mats Pettersson, MD \n **Date:** January 15, 2020\n\n ## Content\n\n Significant laboratory findings include a white blood cell count of 9.1 x 103/µL (9.1 x 109/L; reference range, 3.5-8.8 x 109/L), a platelet count of 429 x 103/μL (429 x 109/L; reference range, 140-350 109/L), a C-reactive protein level of 91 mg/L (reference range, < 10 mg/L), a lactate dehydrogenase (LDH) level of 4.7 microkatals (µkat)/L (reference range, < 3.5 µkat/L), an erythrocyte sedimentation rate (ESR) of 30 mm (reference range, 1-12 mm), and a D-dimer of 2.2 mg/L (reference range, < 0.25 mg/L).\nA spiral CT scan is performed, which shows no pulmonary embolism. It does, however, reveal the presence of a significant pericardial effusion (1 cm ventral x 2.5 cm dorsal) and a multilobular substernal mass occupying the anterior superior mediastinum that is about 2.5 cm in thickness and 7 cm in length, with high absorption. Additionally, the mediastinal lymph nodes are enlarged; some are as large as 2 cm in size. No other pertinent findings on spiral CT are reported.\nAn emergent bedside echocardiogram is obtained. The echocardiographic findings confirm the presence of pericardial effusion, without signs or symptoms of a cardiac tamponade; additionally, a retrosternal mass is detected (see video clips).\n\n ## Figures\n\n \n*Page 2 of 5*",
"pagination": {
"current_page": 2,
"total_pages": 5
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 377672,
"choiceText": "Bruton agammaglobulinemia",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 377673,
"choiceText": "Thymoma",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 377674,
"choiceText": "Pheochromocytoma",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 377675,
"choiceText": "Teratoma",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 108859,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Dyspnea and Night Sweats in a 46-Year-Old Man"
},
{
"authors": "Roza Chaireti, MD; Mats Pettersson, MD",
"content": [
"The patient in this case was eventually diagnosed with thymoma. Symptoms from a thymoma or thymic carcinoma may be due to the presence of a tumor in the mediastinum or they may be a manifestation of a paraneoplastic syndrome. As many as one half of thymomas are diagnosed incidentally on the basis of a radiographic abnormality in an asymptomatic patient. Clinical signs and symptoms are related to both the size of the tumor and its effects on adjacent organs (eg, chest pain, shortness of breath, cough, phrenic nerve palsy, and superior vena cava obstruction). Less commonly, systemic (\"B\") symptoms, including fever, weight loss, and/or night sweats, may be present. Pleural or pericardial effusions are the most common manifestation of metastatic involvement. Extrathoracic metastases occur in less than 7% of patients, most commonly to the kidney, extrathoracic lymph nodes, liver, brain, adrenals, thyroid, and bone. Metastases to the ipsilateral lung are unusual.[1]",
"For patients who present with mediastinal masses, an extensive differential should come to mind. Anterior mediastinal masses include germ cell tumors, lymphoma, thymic carcinoma, and masses arising from the thyroid. Tumors that can arise directly from the thymus include thymoma, lymphoma, carcinoid tumors, thymolipoma, and thymic carcinoma.[1,2] Thymomas account for about 20% of mediastinal neoplasms. Most patients are between age 40 and 80 years; thymomas are more common in males, Asians, and Pacific Islanders. No risk factors have been conclusively identified. Thymic carcinomas account for less than 1% of thymic malignancies.[3] Malignant thymoma is exceptionally rare, with an overall incidence of 0.15 per 100,000 person-years.[4,5]",
"Thymoma is an uncommon tumor that is best known for its association with the neuromuscular disorder myasthenia gravis. Indeed, approximately 70% of symptomatic patients with thymoma have an associated systemic syndrome, such as myasthenia gravis (30-50%), hypogammaglobulinemia, red cell aplasia, dermatomyositis, systemic lupus erythematosus, Cushing syndrome, or syndrome of inappropriate antidiuretic hormone secretion.[6,7]",
"Differentiating thymoma from the more aggressive thymic carcinoma is extremely important. Thymic carcinoma exhibits aggressive cytologic features with evidence of mediastinal invasion in the majority of patients. Histologically, thymic carcinomas exhibit cellular atypia, increased proliferative capacity, and anaplastic features.[8] Radiographically, thymomas are more likely to exhibit smooth contours and a round shape on CT scans (Figure 1) than thymic carcinomas, which more commonly exhibit irregular contours.[9]",
"Figure 1.",
"The histologic diagnosis of thymic neoplasms can be difficult. A continuum of differentiation from thymoma to thymic carcinoma is apparent, and primary thymic epithelial neoplasms can have features of both. Carcinoma and thymoma can occur synchronously or carcinoma can develop within a preexisting thymoma after an interval of years. The World Health Organization (WHO) system is widely used to classify thymomas into 6 types, on the basis of histologic differences. Two main systems for staging thymomas are the Masaoka staging system and the French Groupe d'Etudes des Tumeurs Thymiques (GETT) system. Staging by the Masaoka system has correlated well with overall 5-year survival rates in several large series.[1]",
"Paraneoplastic autoimmune syndromes associated with thymoma include myasthenia gravis, polymyositis, systemic lupus erythematosus, rheumatoid arthritis, thyroiditis, and Sjögren syndrome, among others.[10] Several case reports have described a syndrome of thymoma-associated multiorgan autoimmunity that is similar to graft-vs-host disease. Patients present with variable combinations of a morbilliform skin eruption, chronic diarrhea, and liver enzyme abnormalities. Histopathology of the skin or bowel mucosa is similar to that seen with graft-vs-host disease.[1] Autoimmune pure red cell aplasia and hypogammaglobulinemia affect approximately 5% and 5%-10% of patients with thymoma, respectively. Thymoma-associated autoimmune disease involves an alteration in circulating T-cell subsets."
],
"date": "January 15, 2020",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/732/961/732961-thumb.jpg"
}
],
"markdown": "# Dyspnea and Night Sweats in a 46-Year-Old Man\n\n **Authors:** Roza Chaireti, MD; Mats Pettersson, MD \n **Date:** January 15, 2020\n\n ## Content\n\n The patient in this case was eventually diagnosed with thymoma. Symptoms from a thymoma or thymic carcinoma may be due to the presence of a tumor in the mediastinum or they may be a manifestation of a paraneoplastic syndrome. As many as one half of thymomas are diagnosed incidentally on the basis of a radiographic abnormality in an asymptomatic patient. Clinical signs and symptoms are related to both the size of the tumor and its effects on adjacent organs (eg, chest pain, shortness of breath, cough, phrenic nerve palsy, and superior vena cava obstruction). Less commonly, systemic (\"B\") symptoms, including fever, weight loss, and/or night sweats, may be present. Pleural or pericardial effusions are the most common manifestation of metastatic involvement. Extrathoracic metastases occur in less than 7% of patients, most commonly to the kidney, extrathoracic lymph nodes, liver, brain, adrenals, thyroid, and bone. Metastases to the ipsilateral lung are unusual.[1]\nFor patients who present with mediastinal masses, an extensive differential should come to mind. Anterior mediastinal masses include germ cell tumors, lymphoma, thymic carcinoma, and masses arising from the thyroid. Tumors that can arise directly from the thymus include thymoma, lymphoma, carcinoid tumors, thymolipoma, and thymic carcinoma.[1,2] Thymomas account for about 20% of mediastinal neoplasms. Most patients are between age 40 and 80 years; thymomas are more common in males, Asians, and Pacific Islanders. No risk factors have been conclusively identified. Thymic carcinomas account for less than 1% of thymic malignancies.[3] Malignant thymoma is exceptionally rare, with an overall incidence of 0.15 per 100,000 person-years.[4,5]\nThymoma is an uncommon tumor that is best known for its association with the neuromuscular disorder myasthenia gravis. Indeed, approximately 70% of symptomatic patients with thymoma have an associated systemic syndrome, such as myasthenia gravis (30-50%), hypogammaglobulinemia, red cell aplasia, dermatomyositis, systemic lupus erythematosus, Cushing syndrome, or syndrome of inappropriate antidiuretic hormone secretion.[6,7]\nDifferentiating thymoma from the more aggressive thymic carcinoma is extremely important. Thymic carcinoma exhibits aggressive cytologic features with evidence of mediastinal invasion in the majority of patients. Histologically, thymic carcinomas exhibit cellular atypia, increased proliferative capacity, and anaplastic features.[8] Radiographically, thymomas are more likely to exhibit smooth contours and a round shape on CT scans (Figure 1) than thymic carcinomas, which more commonly exhibit irregular contours.[9]\nFigure 1.\nThe histologic diagnosis of thymic neoplasms can be difficult. A continuum of differentiation from thymoma to thymic carcinoma is apparent, and primary thymic epithelial neoplasms can have features of both. Carcinoma and thymoma can occur synchronously or carcinoma can develop within a preexisting thymoma after an interval of years. The World Health Organization (WHO) system is widely used to classify thymomas into 6 types, on the basis of histologic differences. Two main systems for staging thymomas are the Masaoka staging system and the French Groupe d'Etudes des Tumeurs Thymiques (GETT) system. Staging by the Masaoka system has correlated well with overall 5-year survival rates in several large series.[1]\nParaneoplastic autoimmune syndromes associated with thymoma include myasthenia gravis, polymyositis, systemic lupus erythematosus, rheumatoid arthritis, thyroiditis, and Sjögren syndrome, among others.[10] Several case reports have described a syndrome of thymoma-associated multiorgan autoimmunity that is similar to graft-vs-host disease. Patients present with variable combinations of a morbilliform skin eruption, chronic diarrhea, and liver enzyme abnormalities. Histopathology of the skin or bowel mucosa is similar to that seen with graft-vs-host disease.[1] Autoimmune pure red cell aplasia and hypogammaglobulinemia affect approximately 5% and 5%-10% of patients with thymoma, respectively. Thymoma-associated autoimmune disease involves an alteration in circulating T-cell subsets.\n\n ## Figures\n\n **Figure 1.** \n \n\n\n*Page 3 of 5*",
"pagination": {
"current_page": 3,
"total_pages": 5
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 377672,
"choiceText": "Bruton agammaglobulinemia",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 377673,
"choiceText": "Thymoma",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 377674,
"choiceText": "Pheochromocytoma",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 377675,
"choiceText": "Teratoma",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 108859,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Dyspnea and Night Sweats in a 46-Year-Old Man"
},
{
"authors": "Roza Chaireti, MD; Mats Pettersson, MD",
"content": [
"Most thymomas are diagnosed and staged at the time of surgical intervention. Surgical resection is the preferred treatment for patients who can tolerate surgery and have a mediastinal mass that is suspected of being a thymoma. A total thymectomy with complete resection of all tumor tissue can be achieved in nearly all early stage patients.[11] Later stages are associated with more morbidity and mortality, and postoperative radiation therapy is generally employed in these cases. Most thymomas in the later stages can only rarely be resected completely, and patients are usually offered debulking surgery and postoperative radiation therapy as palliative measures.[10]",
"The likelihood of long-term survival depends on the completeness of surgical resection.[12] (Seven-year survival following a complete resection is about 70%.) Resection of the pericardium and accompanying lung parenchyma is often required to achieve a complete resection with histologically negative margins.[1] Radiation therapy may be useful in the management of patients with microscopic or macroscopic residual thymoma or thymic carcinoma after an incomplete surgical resection, as an adjuvant following complete resection of an invasive tumor, and for those with locally advanced or metastatic unresectable disease.[1] Radiation is also useful in recurrent disease.",
"In patients with locally invasive tumor or large bulky masses, immediate surgical resection may not be technically feasible. Combination chemotherapy followed by radical resection with or without postoperative adjuvant therapy is considered experimental at this point. The most effective agents against thymoma are cisplatin, doxorubicin, cyclophosphamide, ifosfamide, and steroids. Single-agent and combination therapy have been used in adjuvant and neoadjuvant treatment, but combination therapy is more effective; combination therapy that includes cisplatin is perhaps the most effective chemotherapy.",
"Patients with thymoma are also at risk for the development of other malignancies, which have been reported in 17%-28% of patients following thymectomy. In a series of 849 cases of thymoma identified through the Surveillance Epidemiology and End Results (SEER) database, the risk was significantly increased for B-cell non-Hodgkin lymphoma, gastrointestinal cancers, and soft-tissue sarcomas (standardized incidence ratios [SIR] 4.7, 1.8, and 11.1, respectively, compared with the number of cases expected in the general population).[1]",
"In this case, a multilobular tumor was present in the anterior superior section of the mediastinum, which is usually occupied by the thymic cortex. The mass had occasional calcifications surrounded by small tumor nodules. The pericardial infiltration measured up to 3 cm from the base of the heart to the apex area. The lungs and the abdomen were free of tumor infiltrations. The patient was transferred to the nearby university hospital and was admitted to the intensive care unit of the cardiology department, wherein pericardiocentesis was performed; 500 mL of bloody pericardial effusion were drained, samples of which were sent for cytology. A CT scan of the thorax and the abdomen was performed, which showed that the pericardial effusion had almost completely regressed. Serum alpha-fetoprotein and beta–human chorionic gonadotropin findings were negative.",
"Because of the tumor's location, a fine-needle aspiration biopsy or a bronchoscopy (with transbronchial biopsy) was not feasible; therefore, a video-assisted thoracic surgical biopsy under general anesthesia was performed. Further pertinent lab results included a urine electrophoresis with no trace of Bence-Jones proteinuria and a plasma electrophoresis showing signs of an inflammatory process, with normal immunoglobulins and no M components. The cellular analysis of the pericardial effusion showed few lymphoid cells associated with an inflammatory process. The biopsy confirmed the presence of a thymoma.",
"The patient was transferred to the pulmonary medicine department with a combined treatment plan of chemotherapy, radiation treatment, and surgery. At the time of this report, the patient had completed the third chemotherapy cycle with cisplatin, doxorubicin, and cyclophosphamide. The next planned step was surgical extraction of the tumor."
],
"date": "January 15, 2020",
"figures": [],
"markdown": "# Dyspnea and Night Sweats in a 46-Year-Old Man\n\n **Authors:** Roza Chaireti, MD; Mats Pettersson, MD \n **Date:** January 15, 2020\n\n ## Content\n\n Most thymomas are diagnosed and staged at the time of surgical intervention. Surgical resection is the preferred treatment for patients who can tolerate surgery and have a mediastinal mass that is suspected of being a thymoma. A total thymectomy with complete resection of all tumor tissue can be achieved in nearly all early stage patients.[11] Later stages are associated with more morbidity and mortality, and postoperative radiation therapy is generally employed in these cases. Most thymomas in the later stages can only rarely be resected completely, and patients are usually offered debulking surgery and postoperative radiation therapy as palliative measures.[10]\nThe likelihood of long-term survival depends on the completeness of surgical resection.[12] (Seven-year survival following a complete resection is about 70%.) Resection of the pericardium and accompanying lung parenchyma is often required to achieve a complete resection with histologically negative margins.[1] Radiation therapy may be useful in the management of patients with microscopic or macroscopic residual thymoma or thymic carcinoma after an incomplete surgical resection, as an adjuvant following complete resection of an invasive tumor, and for those with locally advanced or metastatic unresectable disease.[1] Radiation is also useful in recurrent disease.\nIn patients with locally invasive tumor or large bulky masses, immediate surgical resection may not be technically feasible. Combination chemotherapy followed by radical resection with or without postoperative adjuvant therapy is considered experimental at this point. The most effective agents against thymoma are cisplatin, doxorubicin, cyclophosphamide, ifosfamide, and steroids. Single-agent and combination therapy have been used in adjuvant and neoadjuvant treatment, but combination therapy is more effective; combination therapy that includes cisplatin is perhaps the most effective chemotherapy.\nPatients with thymoma are also at risk for the development of other malignancies, which have been reported in 17%-28% of patients following thymectomy. In a series of 849 cases of thymoma identified through the Surveillance Epidemiology and End Results (SEER) database, the risk was significantly increased for B-cell non-Hodgkin lymphoma, gastrointestinal cancers, and soft-tissue sarcomas (standardized incidence ratios [SIR] 4.7, 1.8, and 11.1, respectively, compared with the number of cases expected in the general population).[1]\nIn this case, a multilobular tumor was present in the anterior superior section of the mediastinum, which is usually occupied by the thymic cortex. The mass had occasional calcifications surrounded by small tumor nodules. The pericardial infiltration measured up to 3 cm from the base of the heart to the apex area. The lungs and the abdomen were free of tumor infiltrations. The patient was transferred to the nearby university hospital and was admitted to the intensive care unit of the cardiology department, wherein pericardiocentesis was performed; 500 mL of bloody pericardial effusion were drained, samples of which were sent for cytology. A CT scan of the thorax and the abdomen was performed, which showed that the pericardial effusion had almost completely regressed. Serum alpha-fetoprotein and beta–human chorionic gonadotropin findings were negative.\nBecause of the tumor's location, a fine-needle aspiration biopsy or a bronchoscopy (with transbronchial biopsy) was not feasible; therefore, a video-assisted thoracic surgical biopsy under general anesthesia was performed. Further pertinent lab results included a urine electrophoresis with no trace of Bence-Jones proteinuria and a plasma electrophoresis showing signs of an inflammatory process, with normal immunoglobulins and no M components. The cellular analysis of the pericardial effusion showed few lymphoid cells associated with an inflammatory process. The biopsy confirmed the presence of a thymoma.\nThe patient was transferred to the pulmonary medicine department with a combined treatment plan of chemotherapy, radiation treatment, and surgery. At the time of this report, the patient had completed the third chemotherapy cycle with cisplatin, doxorubicin, and cyclophosphamide. The next planned step was surgical extraction of the tumor.\n\n ## Figures\n\n \n*Page 4 of 5*",
"pagination": {
"current_page": 4,
"total_pages": 5
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 377676,
"choiceText": "Radiation therapy",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 377677,
"choiceText": "Chemotherapy",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 377678,
"choiceText": "Surgical excision",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 377679,
"choiceText": "Monoclonal antibodies (rituximab)",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Surgical resection is the preferred treatment for patients who can tolerate it and when the thymoma is amenable to surgery. A total thymectomy with complete resection of all tumor tissue can be achieved in the earlier stages, but the probability of incomplete resection increases in tumors of later stages. Postoperative radiation therapy is generally employed in those cases. Monoclonal antibodies (rituximab) have been employed for the treatment of thymoma-induced myasthenia gravis, although this is controversial. Similarly, chemotherapy is not the first choice for treatment of a surgically resectable tumor.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 108860,
"questionText": "Which of the following is most often the treatment of choice for patients with thymoma?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 377680,
"choiceText": "Myasthenia gravis",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 377681,
"choiceText": "Systemic lupus erythematosus",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 377682,
"choiceText": "Polymyositis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 377683,
"choiceText": "Thyroiditis",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Fifteen percent of patients with myasthenia gravis also develop thymoma, which accounts for about 30%-40% of all thymoma cases.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 108861,
"questionText": "Which of the following autoimmune syndromes is most commonly associated with thymoma?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Dyspnea and Night Sweats in a 46-Year-Old Man"
},
{
"authors": "Roza Chaireti, MD; Mats Pettersson, MD",
"content": [],
"date": "January 15, 2020",
"figures": [],
"markdown": "# Dyspnea and Night Sweats in a 46-Year-Old Man\n\n **Authors:** Roza Chaireti, MD; Mats Pettersson, MD \n **Date:** January 15, 2020\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 5 of 5*",
"pagination": {
"current_page": 5,
"total_pages": 5
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 377676,
"choiceText": "Radiation therapy",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 377677,
"choiceText": "Chemotherapy",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 377678,
"choiceText": "Surgical excision",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 377679,
"choiceText": "Monoclonal antibodies (rituximab)",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Surgical resection is the preferred treatment for patients who can tolerate it and when the thymoma is amenable to surgery. A total thymectomy with complete resection of all tumor tissue can be achieved in the earlier stages, but the probability of incomplete resection increases in tumors of later stages. Postoperative radiation therapy is generally employed in those cases. Monoclonal antibodies (rituximab) have been employed for the treatment of thymoma-induced myasthenia gravis, although this is controversial. Similarly, chemotherapy is not the first choice for treatment of a surgically resectable tumor.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 108860,
"questionText": "Which of the following is most often the treatment of choice for patients with thymoma?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 377680,
"choiceText": "Myasthenia gravis",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 377681,
"choiceText": "Systemic lupus erythematosus",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 377682,
"choiceText": "Polymyositis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 377683,
"choiceText": "Thyroiditis",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Fifteen percent of patients with myasthenia gravis also develop thymoma, which accounts for about 30%-40% of all thymoma cases.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 108861,
"questionText": "Which of the following autoimmune syndromes is most commonly associated with thymoma?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Dyspnea and Night Sweats in a 46-Year-Old Man"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 377672,
"choiceText": "Bruton agammaglobulinemia",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 377673,
"choiceText": "Thymoma",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 377674,
"choiceText": "Pheochromocytoma",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 377675,
"choiceText": "Teratoma",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 108859,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 377676,
"choiceText": "Radiation therapy",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 377677,
"choiceText": "Chemotherapy",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 377678,
"choiceText": "Surgical excision",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 377679,
"choiceText": "Monoclonal antibodies (rituximab)",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Surgical resection is the preferred treatment for patients who can tolerate it and when the thymoma is amenable to surgery. A total thymectomy with complete resection of all tumor tissue can be achieved in the earlier stages, but the probability of incomplete resection increases in tumors of later stages. Postoperative radiation therapy is generally employed in those cases. Monoclonal antibodies (rituximab) have been employed for the treatment of thymoma-induced myasthenia gravis, although this is controversial. Similarly, chemotherapy is not the first choice for treatment of a surgically resectable tumor.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 108860,
"questionText": "Which of the following is most often the treatment of choice for patients with thymoma?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 377680,
"choiceText": "Myasthenia gravis",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 377681,
"choiceText": "Systemic lupus erythematosus",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 377682,
"choiceText": "Polymyositis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 377683,
"choiceText": "Thyroiditis",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Fifteen percent of patients with myasthenia gravis also develop thymoma, which accounts for about 30%-40% of all thymoma cases.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 108861,
"questionText": "Which of the following autoimmune syndromes is most commonly associated with thymoma?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
922201 | /viewarticle/922201 | [
{
"authors": "Sarah Blissett, MD, MHPE; Punag Divanji, MD; Harsh Agrawal, MD; Vaikom S. Mahadevan, MD; Elyse Foster, MD",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"A 63-year-old man with repaired tetralogy of Fallot and repaired subaortic membrane presents with dyspnea upon exertion. He is not experiencing chest pain, pressure, or tightness. He does not report orthopnea, paroxysmal nocturnal dyspnea, palpitations, presyncope, or syncope.",
"Prior surgical procedures included valve-sparing repair of the pulmonic valve at 4 years of age, followed by insertion of a 26-mm pulmonary homograft, resection of a subaortic membrane, and addition of a mechanical aortic valve at 41 years of age. This was complicated by complete heart block, which necessitated implantation of a dual-chamber pacemaker.",
"He also has permanent atrial fibrillation, hypertension, and hyperlipidemia. Medications at the time of presentation include bisoprolol (5 mg daily), lisinopril (20 mg daily), spironolactone (50 mg daily), warfarin (4 mg daily), aspirin (81 mg daily), and atorvastatin (40 mg daily). He has no known allergies.",
"On social history, the patient states that he has never smoked tobacco or used illicit drugs. He has not recently been consuming alcohol. He has no family history of premature coronary artery disease."
],
"date": "December 09, 2019",
"figures": [],
"markdown": "# Dyspnea in a 63-Year-Old Who Had Heart Surgery as a Child\n\n **Authors:** Sarah Blissett, MD, MHPE; Punag Divanji, MD; Harsh Agrawal, MD; Vaikom S. Mahadevan, MD; Elyse Foster, MD \n **Date:** December 09, 2019\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nA 63-year-old man with repaired tetralogy of Fallot and repaired subaortic membrane presents with dyspnea upon exertion. He is not experiencing chest pain, pressure, or tightness. He does not report orthopnea, paroxysmal nocturnal dyspnea, palpitations, presyncope, or syncope.\nPrior surgical procedures included valve-sparing repair of the pulmonic valve at 4 years of age, followed by insertion of a 26-mm pulmonary homograft, resection of a subaortic membrane, and addition of a mechanical aortic valve at 41 years of age. This was complicated by complete heart block, which necessitated implantation of a dual-chamber pacemaker.\nHe also has permanent atrial fibrillation, hypertension, and hyperlipidemia. Medications at the time of presentation include bisoprolol (5 mg daily), lisinopril (20 mg daily), spironolactone (50 mg daily), warfarin (4 mg daily), aspirin (81 mg daily), and atorvastatin (40 mg daily). He has no known allergies.\nOn social history, the patient states that he has never smoked tobacco or used illicit drugs. He has not recently been consuming alcohol. He has no family history of premature coronary artery disease.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "Dyspnea in a 63-Year-Old Who Had Heart Surgery as a Child"
},
{
"authors": "Sarah Blissett, MD, MHPE; Punag Divanji, MD; Harsh Agrawal, MD; Vaikom S. Mahadevan, MD; Elyse Foster, MD",
"content": [
"Upon physical examination, the patient's blood pressure is 90/57 mm Hg. His heart rate is 66 beats/min and regular. His jugular venous pressure is minimally elevated to 6 cm above the sternoclavicular angle. His chest is clear to auscultation. S1 is normal; a crisp, mechanical S2 is observed. A grade 2/6 systolic ejection murmur and a long, grade 2/4 diastolic murmur are auscultated over the left upper sternal border. Trace pedal edema is noted.",
"Laboratory investigations reveal no hematologic, electrolyte, or renal abnormalities. Thyroid-stimulating hormone, creatinine kinase, and ferritin levels are within the reference ranges. His N-terminal prohormone of brain natriuretic peptide level is elevated, at 1480 pg/mL.",
"An ECG reveals a ventricularly paced rhythm (QRS duration, 182 msec) at 70 beats/min with underlying atrial fibrillation. No high-rate episodes are detected on interrogation of the device. Echocardiography reveals an interval decrease in the left ventricular (LV) ejection fraction (EF) to 25%-30%. This is accompanied by global hypokinesis and septal motion consistent with a paced rhythm, normal right ventricular (RV) size and function, mild pulmonic stenosis, mild pulmonic regurgitation, a normally functioning mechanical aortic valve, and an intact ventral septal defect patch. See the video below.",
"The patient undergoes right- and left-heart catheterization, revealing a right atrial pressure of 8 mm Hg, RV systolic pressure of 55 mm Hg, RV end-diastolic pressure of 9 mm Hg, pulmonary pressure of 44/12 mm Hg (mean, 24 mm Hg), pulmonary capillary wedge pressure of 15 mm Hg, and aortic pressure of 100/51 mm Hg (mean, 70 mm Hg). No step-up is noted on saturations obtained in the right-sided heart chambers. Cardiac output is normal (Fick cardiac output, 4.4 L/min). Salient coronary angiography findings are shown in Figure 1.",
"Figure 1."
],
"date": "December 09, 2019",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/922/201/922201-Thumb1.png"
}
],
"markdown": "# Dyspnea in a 63-Year-Old Who Had Heart Surgery as a Child\n\n **Authors:** Sarah Blissett, MD, MHPE; Punag Divanji, MD; Harsh Agrawal, MD; Vaikom S. Mahadevan, MD; Elyse Foster, MD \n **Date:** December 09, 2019\n\n ## Content\n\n Upon physical examination, the patient's blood pressure is 90/57 mm Hg. His heart rate is 66 beats/min and regular. His jugular venous pressure is minimally elevated to 6 cm above the sternoclavicular angle. His chest is clear to auscultation. S1 is normal; a crisp, mechanical S2 is observed. A grade 2/6 systolic ejection murmur and a long, grade 2/4 diastolic murmur are auscultated over the left upper sternal border. Trace pedal edema is noted.\nLaboratory investigations reveal no hematologic, electrolyte, or renal abnormalities. Thyroid-stimulating hormone, creatinine kinase, and ferritin levels are within the reference ranges. His N-terminal prohormone of brain natriuretic peptide level is elevated, at 1480 pg/mL.\nAn ECG reveals a ventricularly paced rhythm (QRS duration, 182 msec) at 70 beats/min with underlying atrial fibrillation. No high-rate episodes are detected on interrogation of the device. Echocardiography reveals an interval decrease in the left ventricular (LV) ejection fraction (EF) to 25%-30%. This is accompanied by global hypokinesis and septal motion consistent with a paced rhythm, normal right ventricular (RV) size and function, mild pulmonic stenosis, mild pulmonic regurgitation, a normally functioning mechanical aortic valve, and an intact ventral septal defect patch. See the video below.\nThe patient undergoes right- and left-heart catheterization, revealing a right atrial pressure of 8 mm Hg, RV systolic pressure of 55 mm Hg, RV end-diastolic pressure of 9 mm Hg, pulmonary pressure of 44/12 mm Hg (mean, 24 mm Hg), pulmonary capillary wedge pressure of 15 mm Hg, and aortic pressure of 100/51 mm Hg (mean, 70 mm Hg). No step-up is noted on saturations obtained in the right-sided heart chambers. Cardiac output is normal (Fick cardiac output, 4.4 L/min). Salient coronary angiography findings are shown in Figure 1.\nFigure 1.\n\n ## Figures\n\n **Figure 1.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1444864,
"choiceText": "Pulmonic valve dysfunction",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1444866,
"choiceText": "Ischemic cardiomyopathy alone",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1444868,
"choiceText": "Nonischemic cardiomyopathy due to atrial arrhythmias ",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1444870,
"choiceText": "Cardiomyopathy due to coronary artery disease and sequelae of repaired tetralogy of Fallot",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 462059,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Dyspnea in a 63-Year-Old Who Had Heart Surgery as a Child"
},
{
"authors": "Sarah Blissett, MD, MHPE; Punag Divanji, MD; Harsh Agrawal, MD; Vaikom S. Mahadevan, MD; Elyse Foster, MD",
"content": [
"In this case, a 63-year-old man with repaired tetralogy of Fallot presented with dyspnea upon exertion. Tetralogy of Fallot consists of four cardiac abnormalities: narrowing of the RV outflow tract, ventricular septal defect, RV hypertrophy, and overriding aorta. Most patients undergo surgical repair to relieve the RV outflow tract narrowing and to repair the ventricular septal defect during childhood. Contemporary data demonstrate that more than 90% of patients with repaired tetralogy of Fallot survive into adulthood.[1] Repaired tetralogy of Fallot is currently the most prevalent complex congenital cardiac lesion in adults, with approximately 72,000 adults in the United States affected.[2,3]",
"The differential diagnosis of dyspnea in a patient with repaired tetralogy of Fallot is broad and includes causes related to congenital heart disease, acquired heart disease, and noncardiac etiologies. Congenital issues include pulmonary stenosis, pulmonary regurgitation, tricuspid regurgitation, RV dysfunction, LV dysfunction, atrial arrhythmias, and ventricular arrhythmias. Acquired heart diseases that may be present include coronary artery disease, diabetic cardiomyopathy, alcohol-related cardiomyopathy, and endocarditis.",
"Although right-sided valvular dysfunction and RV dysfunction are more common than left-sided lesions, approximately 20%-30% of adult patients with repaired tetralogy of Fallot have LV dysfunction, defined as LVEF < 55%.[4,5,6] Of note, the presence of LV systolic dysfunction confers a worse prognosis, with higher rates of cardiac events (death or hospitalization) seen in patients with LVEF < 55% than in patients with normal EFs.[5]"
],
"date": "December 09, 2019",
"figures": [],
"markdown": "# Dyspnea in a 63-Year-Old Who Had Heart Surgery as a Child\n\n **Authors:** Sarah Blissett, MD, MHPE; Punag Divanji, MD; Harsh Agrawal, MD; Vaikom S. Mahadevan, MD; Elyse Foster, MD \n **Date:** December 09, 2019\n\n ## Content\n\n In this case, a 63-year-old man with repaired tetralogy of Fallot presented with dyspnea upon exertion. Tetralogy of Fallot consists of four cardiac abnormalities: narrowing of the RV outflow tract, ventricular septal defect, RV hypertrophy, and overriding aorta. Most patients undergo surgical repair to relieve the RV outflow tract narrowing and to repair the ventricular septal defect during childhood. Contemporary data demonstrate that more than 90% of patients with repaired tetralogy of Fallot survive into adulthood.[1] Repaired tetralogy of Fallot is currently the most prevalent complex congenital cardiac lesion in adults, with approximately 72,000 adults in the United States affected.[2,3]\nThe differential diagnosis of dyspnea in a patient with repaired tetralogy of Fallot is broad and includes causes related to congenital heart disease, acquired heart disease, and noncardiac etiologies. Congenital issues include pulmonary stenosis, pulmonary regurgitation, tricuspid regurgitation, RV dysfunction, LV dysfunction, atrial arrhythmias, and ventricular arrhythmias. Acquired heart diseases that may be present include coronary artery disease, diabetic cardiomyopathy, alcohol-related cardiomyopathy, and endocarditis.\nAlthough right-sided valvular dysfunction and RV dysfunction are more common than left-sided lesions, approximately 20%-30% of adult patients with repaired tetralogy of Fallot have LV dysfunction, defined as LVEF < 55%.[4,5,6] Of note, the presence of LV systolic dysfunction confers a worse prognosis, with higher rates of cardiac events (death or hospitalization) seen in patients with LVEF < 55% than in patients with normal EFs.[5]\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1444864,
"choiceText": "Pulmonic valve dysfunction",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1444866,
"choiceText": "Ischemic cardiomyopathy alone",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1444868,
"choiceText": "Nonischemic cardiomyopathy due to atrial arrhythmias ",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1444870,
"choiceText": "Cardiomyopathy due to coronary artery disease and sequelae of repaired tetralogy of Fallot",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 462059,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Dyspnea in a 63-Year-Old Who Had Heart Surgery as a Child"
},
{
"authors": "Sarah Blissett, MD, MHPE; Punag Divanji, MD; Harsh Agrawal, MD; Vaikom S. Mahadevan, MD; Elyse Foster, MD",
"content": [
"Clinicians should seek to identify the cause of LV dysfunction, considering acquired or congenital etiologies. Acquired causes of LV dysfunction should be evaluated, including coronary artery disease, diabetes, alcohol, thyroid abnormalities, iron overload states, and tachycardia-mediated cardiomyopathy.",
"Coronary artery disease should not be overlooked in patients with congenital heart disease. In a Dutch cohort study, 80% of adults with congenital heart disease also had at least one cardiovascular risk factor.[7] Compared with women, men were more likely to smoke, with 24% using tobacco, and were more likely to have hypertension.[7] Data from Europe and the United Kingdom suggest the prevalence of diabetes is at least similar to the prevalence seen in the general population, with one study demonstrating a higher prevalence of diabetes in patients with congenital heart disease.[8] The rate of myocardial infarction in patients with repaired tetralogy of Fallot is similar to that in the general population.[9]",
"Once acquired causes are excluded, consideration can be given to LV dysfunction as it relates to sequelae of repaired tetralogy of Fallot. Several clinical variables have been associated with LV dysfunction as it relates to repaired tetralogy of Fallot. These include the following[4,5,6]:",
"Male gender",
"Decreased RVEF",
"RV dilation",
"RV or LV late gadolinium enhancement",
"Significant pulmonary regurgitation",
"History of arrhythmia",
"Prolonged QRS duration",
"Presence of an implantable cardiac defibrillator",
"Duration of shunt before repair",
"Prior surgeries",
"The association with decreased RV function, RV dilation, and significant pulmonary regurgitation highlights the ventriculo-ventricular interactions between the RV and LV as a contributor in the pathophysiology of LV dysfunction in repaired tetralogy of Fallot. The QRS duration highlights the contribution of electromechanical dyssynchrony. Longer durations of palliative shunts, particularly Waterston (ascending aorta to pulmonary artery) and Potts (descending aorta to pulmonary artery) shunts, impose a volume load on the LV that could contribute to dysfunction. A greater number of surgical procedures, particularly in older patients who underwent surgeries with older bypass techniques, may contribute to LV owing to inadequate coronary protection during the surgical procedures.",
"Coronary artery disease was considered first in determining the etiology of heart failure for this patient in this case. Invasive coronary angiography was selected to evaluate for ischemia, given the multiple cardiovascular risk factors. Coronary angiography demonstrated a 95% obstructive lesion in the ostium of the left anterior descending artery. The LV dysfunction was felt to be out of proportion to the degree of coronary disease, particularly given the global hypokinesis identified, raising the possibly of a concomitant nonischemic contributor. Contributions from factors associated with repaired tetralogy of Fallot were suspected, given the presence of clinical features described to be involved in the pathophysiology of LV dysfunction in these patients, including male gender, longer QRS duration, and history of arrhythmia. Thus, the patient's LV dysfunction was thought to be due to coronary disease and sequelae of repaired tetralogy of Fallot."
],
"date": "December 09, 2019",
"figures": [],
"markdown": "# Dyspnea in a 63-Year-Old Who Had Heart Surgery as a Child\n\n **Authors:** Sarah Blissett, MD, MHPE; Punag Divanji, MD; Harsh Agrawal, MD; Vaikom S. Mahadevan, MD; Elyse Foster, MD \n **Date:** December 09, 2019\n\n ## Content\n\n Clinicians should seek to identify the cause of LV dysfunction, considering acquired or congenital etiologies. Acquired causes of LV dysfunction should be evaluated, including coronary artery disease, diabetes, alcohol, thyroid abnormalities, iron overload states, and tachycardia-mediated cardiomyopathy.\nCoronary artery disease should not be overlooked in patients with congenital heart disease. In a Dutch cohort study, 80% of adults with congenital heart disease also had at least one cardiovascular risk factor.[7] Compared with women, men were more likely to smoke, with 24% using tobacco, and were more likely to have hypertension.[7] Data from Europe and the United Kingdom suggest the prevalence of diabetes is at least similar to the prevalence seen in the general population, with one study demonstrating a higher prevalence of diabetes in patients with congenital heart disease.[8] The rate of myocardial infarction in patients with repaired tetralogy of Fallot is similar to that in the general population.[9]\nOnce acquired causes are excluded, consideration can be given to LV dysfunction as it relates to sequelae of repaired tetralogy of Fallot. Several clinical variables have been associated with LV dysfunction as it relates to repaired tetralogy of Fallot. These include the following[4,5,6]:\nMale gender\nDecreased RVEF\nRV dilation\nRV or LV late gadolinium enhancement\nSignificant pulmonary regurgitation\nHistory of arrhythmia\nProlonged QRS duration\nPresence of an implantable cardiac defibrillator\nDuration of shunt before repair\nPrior surgeries\nThe association with decreased RV function, RV dilation, and significant pulmonary regurgitation highlights the ventriculo-ventricular interactions between the RV and LV as a contributor in the pathophysiology of LV dysfunction in repaired tetralogy of Fallot. The QRS duration highlights the contribution of electromechanical dyssynchrony. Longer durations of palliative shunts, particularly Waterston (ascending aorta to pulmonary artery) and Potts (descending aorta to pulmonary artery) shunts, impose a volume load on the LV that could contribute to dysfunction. A greater number of surgical procedures, particularly in older patients who underwent surgeries with older bypass techniques, may contribute to LV owing to inadequate coronary protection during the surgical procedures.\nCoronary artery disease was considered first in determining the etiology of heart failure for this patient in this case. Invasive coronary angiography was selected to evaluate for ischemia, given the multiple cardiovascular risk factors. Coronary angiography demonstrated a 95% obstructive lesion in the ostium of the left anterior descending artery. The LV dysfunction was felt to be out of proportion to the degree of coronary disease, particularly given the global hypokinesis identified, raising the possibly of a concomitant nonischemic contributor. Contributions from factors associated with repaired tetralogy of Fallot were suspected, given the presence of clinical features described to be involved in the pathophysiology of LV dysfunction in these patients, including male gender, longer QRS duration, and history of arrhythmia. Thus, the patient's LV dysfunction was thought to be due to coronary disease and sequelae of repaired tetralogy of Fallot.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "Dyspnea in a 63-Year-Old Who Had Heart Surgery as a Child"
},
{
"authors": "Sarah Blissett, MD, MHPE; Punag Divanji, MD; Harsh Agrawal, MD; Vaikom S. Mahadevan, MD; Elyse Foster, MD",
"content": [
"As with patients with acquired heart disease, adult patients with repaired tetralogy of Fallot benefit from treatment of atherosclerotic coronary artery disease and aggressive management of cardiovascular factors. Risk factors should be screened for and treated as in patients without congenital heart disease.[10,11]",
"The patient in this case had a low-density lipoprotein level of 65 mg/dL (target, < 70 mg/dL), high-density lipoprotein level of 32 mg/dL, and triglyceride level of 90 mg/dL. His most recent A1c level was 6.5%. His blood pressure was controlled, at 117/64 mm Hg.",
"Little evidence is available to direct management in patients with repaired tetralogy of Fallot and heart failure symptoms due to LV dysfunction. Clinicians often extrapolate the principles of management that have been extensively researched in patients without congenital heart disease, including the use of beta-blockers, angiotensin-converting enzyme (ACE) inhibitors/angiotensin II receptor blockers, aldosterone antagonists, and cardiac resynchronization therapy. However, the underlying pathophysiology in patients with repaired tetralogy of Fallot is distinct from that in patients with acquired heart disease and may limit the efficacy of these therapies.",
"Symptomatic management with diuretics may be initiated, with judicious use in patients with concomitant pulmonic stenosis or restrictive RV physiology who may be preload-dependent. The use of ACE inhibitors has been investigated, with subtle improvements in LV systolic and diastolic function noted in adult patients with repaired tetralogy of Fallot and at least moderate pulmonary regurgitation.[12] Currently, no data are available to guide the use of beta-blockers, aldosterone antagonists, or neprilysin inhibitors for LV dysfunction in patients with repaired tetralogy of Fallot. Experience with cardiac resynchronization therapy in pediatric patients with repaired tetralogy of Fallot continues to evolve, with most devices implanted in the presence of chronic ventricular pacing and most patients experiencing improvements in symptoms and left ventricular function.[13,14]",
"Given the established increased risk for sudden cardiac death in patients with repaired tetralogy of Fallot and LV dysfunction, considering whether an implantable cardiac defibrillator could be of benefit is prudent. Guidelines for management of patients with adult congenital heart disease highlight that implantable cardiac defibrillators can be considered in patients with LV or RV dysfunction, nonsustained ventricular tachycardia, QRS duration > 180 msec, extensive RV scarring, or inducible sustained ventricular tachycardia on electrophysiologic study.[15] The potential benefit must be balanced with the risks, including inappropriate shocks, lead malfunction, and effect on quality of life.",
"In conclusion, the patient in this case was found to have LV dysfunction that was probably due to coronary artery disease and sequelae of repaired tetralogy of Fallot. His cardiovascular risk factors were managed according to guidelines. He was already taking medications typically used to treat heart failure, including bisoprolol, spironolactone, and lisinopril. He underwent percutaneous coronary intervention with a drug-eluting stent to relieve the stenosis in the ostium of the left anterior descending artery (Figure 2).",
"Figure 2.",
"He was seen for follow-up 3 months after stenting. His dyspnea had improved to New York Heart Association class I, and his LVEF improved to 40%-45% on repeat transthoracic echocardiography.",
"This case illustrates two important take-home messages. First, as many as one third of patients with repaired tetralogy of Fallot develop LV dysfunction due to various pathophysiologic mechanisms in adulthood. Second, considering coronary artery disease as a contributor to LV dysfunction is important in patients with repaired tetralogy of Fallot because revascularization may improve symptoms and LV function."
],
"date": "December 09, 2019",
"figures": [
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/922/201/922201-Thumb2.png"
}
],
"markdown": "# Dyspnea in a 63-Year-Old Who Had Heart Surgery as a Child\n\n **Authors:** Sarah Blissett, MD, MHPE; Punag Divanji, MD; Harsh Agrawal, MD; Vaikom S. Mahadevan, MD; Elyse Foster, MD \n **Date:** December 09, 2019\n\n ## Content\n\n As with patients with acquired heart disease, adult patients with repaired tetralogy of Fallot benefit from treatment of atherosclerotic coronary artery disease and aggressive management of cardiovascular factors. Risk factors should be screened for and treated as in patients without congenital heart disease.[10,11]\nThe patient in this case had a low-density lipoprotein level of 65 mg/dL (target, < 70 mg/dL), high-density lipoprotein level of 32 mg/dL, and triglyceride level of 90 mg/dL. His most recent A1c level was 6.5%. His blood pressure was controlled, at 117/64 mm Hg.\nLittle evidence is available to direct management in patients with repaired tetralogy of Fallot and heart failure symptoms due to LV dysfunction. Clinicians often extrapolate the principles of management that have been extensively researched in patients without congenital heart disease, including the use of beta-blockers, angiotensin-converting enzyme (ACE) inhibitors/angiotensin II receptor blockers, aldosterone antagonists, and cardiac resynchronization therapy. However, the underlying pathophysiology in patients with repaired tetralogy of Fallot is distinct from that in patients with acquired heart disease and may limit the efficacy of these therapies.\nSymptomatic management with diuretics may be initiated, with judicious use in patients with concomitant pulmonic stenosis or restrictive RV physiology who may be preload-dependent. The use of ACE inhibitors has been investigated, with subtle improvements in LV systolic and diastolic function noted in adult patients with repaired tetralogy of Fallot and at least moderate pulmonary regurgitation.[12] Currently, no data are available to guide the use of beta-blockers, aldosterone antagonists, or neprilysin inhibitors for LV dysfunction in patients with repaired tetralogy of Fallot. Experience with cardiac resynchronization therapy in pediatric patients with repaired tetralogy of Fallot continues to evolve, with most devices implanted in the presence of chronic ventricular pacing and most patients experiencing improvements in symptoms and left ventricular function.[13,14]\nGiven the established increased risk for sudden cardiac death in patients with repaired tetralogy of Fallot and LV dysfunction, considering whether an implantable cardiac defibrillator could be of benefit is prudent. Guidelines for management of patients with adult congenital heart disease highlight that implantable cardiac defibrillators can be considered in patients with LV or RV dysfunction, nonsustained ventricular tachycardia, QRS duration > 180 msec, extensive RV scarring, or inducible sustained ventricular tachycardia on electrophysiologic study.[15] The potential benefit must be balanced with the risks, including inappropriate shocks, lead malfunction, and effect on quality of life.\nIn conclusion, the patient in this case was found to have LV dysfunction that was probably due to coronary artery disease and sequelae of repaired tetralogy of Fallot. His cardiovascular risk factors were managed according to guidelines. He was already taking medications typically used to treat heart failure, including bisoprolol, spironolactone, and lisinopril. He underwent percutaneous coronary intervention with a drug-eluting stent to relieve the stenosis in the ostium of the left anterior descending artery (Figure 2).\nFigure 2.\nHe was seen for follow-up 3 months after stenting. His dyspnea had improved to New York Heart Association class I, and his LVEF improved to 40%-45% on repeat transthoracic echocardiography.\nThis case illustrates two important take-home messages. First, as many as one third of patients with repaired tetralogy of Fallot develop LV dysfunction due to various pathophysiologic mechanisms in adulthood. Second, considering coronary artery disease as a contributor to LV dysfunction is important in patients with repaired tetralogy of Fallot because revascularization may improve symptoms and LV function.\n\n ## Figures\n\n **Figure 2.** \n \n\n\n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1444872,
"choiceText": "The prevalence of myocardial infarction is lower in these patients than in patients who do not have congenital heart disease",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1444874,
"choiceText": "LV dysfunction in these patients is associated with a lower risk for death than among the general population",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1444876,
"choiceText": "As many as 30% of these patients develop LV dysfunction",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1444878,
"choiceText": "Cardiovascular risk factors are absent in most of these patients ",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Although RV dysfunction and pulmonary valve abnormalities are often thought to be more prevalent, approximately 20%-30% of adult patients who have undergone tetralogy of Fallot repair develop LV dysfunction, defined as LVEF < 55%. \r\nMost patients with congenital heart disease have at least one cardiovascular risk factor. The prevalence of myocardial infarction is similar among these patients and the general population. An LVEF < 55% has been associated with increased risk for death and hospitalization.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 462061,
"questionText": "Which of the following is most accurate regarding patients with repaired tetralogy of Fallot?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1444880,
"choiceText": "Female gender",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1444882,
"choiceText": "RV narrowing",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1444884,
"choiceText": "Increased RVEF",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1444886,
"choiceText": "Prolonged QRS duration",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The QRS duration highlights the contribution of electromechanical dyssynchrony. Longer durations of palliative shunts, particularly Waterston (ascending aorta to pulmonary artery) and Potts (descending aorta to pulmonary artery) shunts, impose a volume load on the LV that could contribute to dysfunction. A greater number of surgical procedures, particularly in older patients who underwent surgeries with older bypass techniques, may contribute to LV owing to inadequate coronary protection during the surgical procedures. <br><br>\r\nSeveral clinical variables have been associated with LV dysfunction as it relates to repaired tetralogy of Fallot. These include the following:\r\n<ul>\r\n\t<li>Prolonged QRS duration</li>\r\n\t<li>Male gender</li>\r\n\t<li>Decreased RVEF</li>\r\n\t<li>RV dilation</li>\r\n\t<li>RV or LV late gadolinium enhancement</li>\r\n\t<li>Significant pulmonary regurgitation</li>\r\n\t<li>History of arrhythmia</li>\r\n\t<li>Presence of an implantable cardiac defibrillator</li>\r\n\t<li>Duration of shunt before repair</li>\r\n\t<li>Prior surgeries</li>\r\n</ul>\r\n\r\n",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 462063,
"questionText": "Which of the following variables has been more commonly associated with LV dysfunction in patients with repaired tetralogy of Fallot?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Dyspnea in a 63-Year-Old Who Had Heart Surgery as a Child"
},
{
"authors": "Sarah Blissett, MD, MHPE; Punag Divanji, MD; Harsh Agrawal, MD; Vaikom S. Mahadevan, MD; Elyse Foster, MD",
"content": [],
"date": "December 09, 2019",
"figures": [],
"markdown": "# Dyspnea in a 63-Year-Old Who Had Heart Surgery as a Child\n\n **Authors:** Sarah Blissett, MD, MHPE; Punag Divanji, MD; Harsh Agrawal, MD; Vaikom S. Mahadevan, MD; Elyse Foster, MD \n **Date:** December 09, 2019\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1444872,
"choiceText": "The prevalence of myocardial infarction is lower in these patients than in patients who do not have congenital heart disease",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1444874,
"choiceText": "LV dysfunction in these patients is associated with a lower risk for death than among the general population",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1444876,
"choiceText": "As many as 30% of these patients develop LV dysfunction",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1444878,
"choiceText": "Cardiovascular risk factors are absent in most of these patients ",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Although RV dysfunction and pulmonary valve abnormalities are often thought to be more prevalent, approximately 20%-30% of adult patients who have undergone tetralogy of Fallot repair develop LV dysfunction, defined as LVEF < 55%. \r\nMost patients with congenital heart disease have at least one cardiovascular risk factor. The prevalence of myocardial infarction is similar among these patients and the general population. An LVEF < 55% has been associated with increased risk for death and hospitalization.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 462061,
"questionText": "Which of the following is most accurate regarding patients with repaired tetralogy of Fallot?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1444880,
"choiceText": "Female gender",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1444882,
"choiceText": "RV narrowing",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1444884,
"choiceText": "Increased RVEF",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1444886,
"choiceText": "Prolonged QRS duration",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The QRS duration highlights the contribution of electromechanical dyssynchrony. Longer durations of palliative shunts, particularly Waterston (ascending aorta to pulmonary artery) and Potts (descending aorta to pulmonary artery) shunts, impose a volume load on the LV that could contribute to dysfunction. A greater number of surgical procedures, particularly in older patients who underwent surgeries with older bypass techniques, may contribute to LV owing to inadequate coronary protection during the surgical procedures. <br><br>\r\nSeveral clinical variables have been associated with LV dysfunction as it relates to repaired tetralogy of Fallot. These include the following:\r\n<ul>\r\n\t<li>Prolonged QRS duration</li>\r\n\t<li>Male gender</li>\r\n\t<li>Decreased RVEF</li>\r\n\t<li>RV dilation</li>\r\n\t<li>RV or LV late gadolinium enhancement</li>\r\n\t<li>Significant pulmonary regurgitation</li>\r\n\t<li>History of arrhythmia</li>\r\n\t<li>Presence of an implantable cardiac defibrillator</li>\r\n\t<li>Duration of shunt before repair</li>\r\n\t<li>Prior surgeries</li>\r\n</ul>\r\n\r\n",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 462063,
"questionText": "Which of the following variables has been more commonly associated with LV dysfunction in patients with repaired tetralogy of Fallot?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Dyspnea in a 63-Year-Old Who Had Heart Surgery as a Child"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1444864,
"choiceText": "Pulmonic valve dysfunction",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1444866,
"choiceText": "Ischemic cardiomyopathy alone",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1444868,
"choiceText": "Nonischemic cardiomyopathy due to atrial arrhythmias ",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1444870,
"choiceText": "Cardiomyopathy due to coronary artery disease and sequelae of repaired tetralogy of Fallot",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 462059,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1444872,
"choiceText": "The prevalence of myocardial infarction is lower in these patients than in patients who do not have congenital heart disease",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1444874,
"choiceText": "LV dysfunction in these patients is associated with a lower risk for death than among the general population",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1444876,
"choiceText": "As many as 30% of these patients develop LV dysfunction",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1444878,
"choiceText": "Cardiovascular risk factors are absent in most of these patients ",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Although RV dysfunction and pulmonary valve abnormalities are often thought to be more prevalent, approximately 20%-30% of adult patients who have undergone tetralogy of Fallot repair develop LV dysfunction, defined as LVEF < 55%. \r\nMost patients with congenital heart disease have at least one cardiovascular risk factor. The prevalence of myocardial infarction is similar among these patients and the general population. An LVEF < 55% has been associated with increased risk for death and hospitalization.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 462061,
"questionText": "Which of the following is most accurate regarding patients with repaired tetralogy of Fallot?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1444880,
"choiceText": "Female gender",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1444882,
"choiceText": "RV narrowing",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1444884,
"choiceText": "Increased RVEF",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1444886,
"choiceText": "Prolonged QRS duration",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The QRS duration highlights the contribution of electromechanical dyssynchrony. Longer durations of palliative shunts, particularly Waterston (ascending aorta to pulmonary artery) and Potts (descending aorta to pulmonary artery) shunts, impose a volume load on the LV that could contribute to dysfunction. A greater number of surgical procedures, particularly in older patients who underwent surgeries with older bypass techniques, may contribute to LV owing to inadequate coronary protection during the surgical procedures. <br><br>\r\nSeveral clinical variables have been associated with LV dysfunction as it relates to repaired tetralogy of Fallot. These include the following:\r\n<ul>\r\n\t<li>Prolonged QRS duration</li>\r\n\t<li>Male gender</li>\r\n\t<li>Decreased RVEF</li>\r\n\t<li>RV dilation</li>\r\n\t<li>RV or LV late gadolinium enhancement</li>\r\n\t<li>Significant pulmonary regurgitation</li>\r\n\t<li>History of arrhythmia</li>\r\n\t<li>Presence of an implantable cardiac defibrillator</li>\r\n\t<li>Duration of shunt before repair</li>\r\n\t<li>Prior surgeries</li>\r\n</ul>\r\n\r\n",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 462063,
"questionText": "Which of the following variables has been more commonly associated with LV dysfunction in patients with repaired tetralogy of Fallot?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
921730 | /viewarticle/921730 | [
{
"authors": "Jeffrey S. Forrest, MD; Alexander B. Shortridge",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"A 23-year-old woman is brought to the emergency department by her concerned mother after experiencing an apparent fall. The patient's mother reported that she found the patient early that morning. The patient was lying on the floor of the bathroom staring blankly at the ceiling.",
"The patient recounted that she had had fallen asleep several hours earlier while studying for her exams and woke up needing to urinate. She stated that she could not remember what happened after this time. She was unable to get up without assistance from her mother. The patient downplayed her fall and had to be convinced by her mother to come to the emergency department. The patient mentioned that she might have bumped her head on the bathroom sink during the fall, but she reported no pain and did not lose consciousness. She also nonchalantly reported that she could not see out of her left eye and described lower-extremity weakness, also on her left side.",
"When questioned about her medical history, the patient only reported that she has occasional migraines for which she takes sumatriptan. Her mother contributes that her daughter also takes alosetron for irritable bowel syndrome and fluoxetine for depression.",
"The patient appeared to be noticeably unworried about her condition. Her mother mentioned that her daughter has experienced numerous significant life stressors recently, including her father's recent diagnosis of terminal glioblastoma multiforme and increasing pressure from her rigorous nurse practitioner program. When questioned about this, the patient minimized her current situation, simply stating, \"I have my ups and downs.\" The patient denied suicidal ideation, plans, gestures, or intention."
],
"date": "November 27, 2019",
"figures": [],
"markdown": "# A 23-Year-Old With Blindness After a Fall She Can't Remember\n\n **Authors:** Jeffrey S. Forrest, MD; Alexander B. Shortridge \n **Date:** November 27, 2019\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nA 23-year-old woman is brought to the emergency department by her concerned mother after experiencing an apparent fall. The patient's mother reported that she found the patient early that morning. The patient was lying on the floor of the bathroom staring blankly at the ceiling.\nThe patient recounted that she had had fallen asleep several hours earlier while studying for her exams and woke up needing to urinate. She stated that she could not remember what happened after this time. She was unable to get up without assistance from her mother. The patient downplayed her fall and had to be convinced by her mother to come to the emergency department. The patient mentioned that she might have bumped her head on the bathroom sink during the fall, but she reported no pain and did not lose consciousness. She also nonchalantly reported that she could not see out of her left eye and described lower-extremity weakness, also on her left side.\nWhen questioned about her medical history, the patient only reported that she has occasional migraines for which she takes sumatriptan. Her mother contributes that her daughter also takes alosetron for irritable bowel syndrome and fluoxetine for depression.\nThe patient appeared to be noticeably unworried about her condition. Her mother mentioned that her daughter has experienced numerous significant life stressors recently, including her father's recent diagnosis of terminal glioblastoma multiforme and increasing pressure from her rigorous nurse practitioner program. When questioned about this, the patient minimized her current situation, simply stating, \"I have my ups and downs.\" The patient denied suicidal ideation, plans, gestures, or intention.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 23-Year-Old With Blindness After a Fall She Can't Remember"
},
{
"authors": "Jeffrey S. Forrest, MD; Alexander B. Shortridge",
"content": [
"The patient is 5'2\" (1.57 m) and weighs 120 lb (54.43 kg). Her pulse is 80 beats/min. Her oxygen saturation is 95% on room air. Her respiration rate is 14 breaths/min, blood pressure is 128/84 mm Hg, and temperature is 98.3°F (36.8°C). The patient appears to be calm but also detached.",
"Ecchymosis is noted on the right side of her forehead from possible recent trauma. She is normocephalic. Her tympanic membranes appear normal. She has normal pupillary size and intact light reflex. Her visual acuity is 20/250 in her left eye (per Snellen chart test), whereas her visual acuity is 20/20 in her right eye. Optokinetic nystagmus is present.",
"No murmurs, rubs, or gallops are heard during cardiac evaluation. She has a regular heart rhythm. She displays a normal rate of breathing. Her lungs are clear to auscultation bilaterally. Her abdomen is nontender and tympanitic to percussion, with normal bowel sounds. She does not display facial droop, and her cranial nerves are otherwise intact. Hoover sign and Babinski reflex test results are negative. The patient cannot walk unaided upon gait evaluation. Muscle strength is 1/5 in her left lower extremity and 5/5 in all other extremities.",
"When giving her history, the patient acknowledged but downplayed the stresses her mother talked about. She said, \"I can handle it.\" She reported no family history of mental illness. During a mental status evaluation, the patient appears alert and oriented to name, location, date, and time. She is appropriately groomed with good hygiene. Her behavior is detached. Her speech is soft, with slow responses of normal rate, rhythm, and prosody. Her thought process appears logical, linear, and goal oriented. She denies having any delusions, paranoia, or thought insertion/thought broadcasting.",
"She denies out-of-body experiences, although she agrees with the statement \"the world feels like a dream.\" She continues to state, \"I'm fine.\" As noted in her initial examination, the patient denied suicidal ideation, plans, gestures, or intention. Her affect is congruent with her mood; she has a blank, blunted appearance that conveys a distinct lack of concern. She answers questions appropriately. She is able to complete a serial sevens examination without errors and successfully recites the months of the year backward. She scores 3 for 3 on 3-item recall. Her recent and long-term memory is intact. Her insight appears poor, as she does not seem concerned about significant symptoms. The patient seems limited in her decision-making ability at present. When she was asked about proverbs such as \"people who live in glass houses should not throw stones,\" she responded that it was a silly statement.",
"An EKG reveals normal findings. A CT scan of the head without contrast is performed and also reveals normal findings, with no evidence of cerebrovascular accident or mass lesions or bleeds (Figure).",
"Figure 1.",
"Urine toxicology screen results are negative. Urinalysis findings are within normal limits. Her complete blood cell count, comprehensive metabolic profile, and thyroid-stimulating hormone levels are all within reference range values."
],
"date": "November 27, 2019",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/921/730/921730-Thumb1.png"
}
],
"markdown": "# A 23-Year-Old With Blindness After a Fall She Can't Remember\n\n **Authors:** Jeffrey S. Forrest, MD; Alexander B. Shortridge \n **Date:** November 27, 2019\n\n ## Content\n\n The patient is 5'2\" (1.57 m) and weighs 120 lb (54.43 kg). Her pulse is 80 beats/min. Her oxygen saturation is 95% on room air. Her respiration rate is 14 breaths/min, blood pressure is 128/84 mm Hg, and temperature is 98.3°F (36.8°C). The patient appears to be calm but also detached.\nEcchymosis is noted on the right side of her forehead from possible recent trauma. She is normocephalic. Her tympanic membranes appear normal. She has normal pupillary size and intact light reflex. Her visual acuity is 20/250 in her left eye (per Snellen chart test), whereas her visual acuity is 20/20 in her right eye. Optokinetic nystagmus is present.\nNo murmurs, rubs, or gallops are heard during cardiac evaluation. She has a regular heart rhythm. She displays a normal rate of breathing. Her lungs are clear to auscultation bilaterally. Her abdomen is nontender and tympanitic to percussion, with normal bowel sounds. She does not display facial droop, and her cranial nerves are otherwise intact. Hoover sign and Babinski reflex test results are negative. The patient cannot walk unaided upon gait evaluation. Muscle strength is 1/5 in her left lower extremity and 5/5 in all other extremities.\nWhen giving her history, the patient acknowledged but downplayed the stresses her mother talked about. She said, \"I can handle it.\" She reported no family history of mental illness. During a mental status evaluation, the patient appears alert and oriented to name, location, date, and time. She is appropriately groomed with good hygiene. Her behavior is detached. Her speech is soft, with slow responses of normal rate, rhythm, and prosody. Her thought process appears logical, linear, and goal oriented. She denies having any delusions, paranoia, or thought insertion/thought broadcasting.\nShe denies out-of-body experiences, although she agrees with the statement \"the world feels like a dream.\" She continues to state, \"I'm fine.\" As noted in her initial examination, the patient denied suicidal ideation, plans, gestures, or intention. Her affect is congruent with her mood; she has a blank, blunted appearance that conveys a distinct lack of concern. She answers questions appropriately. She is able to complete a serial sevens examination without errors and successfully recites the months of the year backward. She scores 3 for 3 on 3-item recall. Her recent and long-term memory is intact. Her insight appears poor, as she does not seem concerned about significant symptoms. The patient seems limited in her decision-making ability at present. When she was asked about proverbs such as \"people who live in glass houses should not throw stones,\" she responded that it was a silly statement.\nAn EKG reveals normal findings. A CT scan of the head without contrast is performed and also reveals normal findings, with no evidence of cerebrovascular accident or mass lesions or bleeds (Figure).\nFigure 1.\nUrine toxicology screen results are negative. Urinalysis findings are within normal limits. Her complete blood cell count, comprehensive metabolic profile, and thyroid-stimulating hormone levels are all within reference range values.\n\n ## Figures\n\n **Figure 1.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1439676,
"choiceText": "Malingering",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1439678,
"choiceText": "Conversion disorder",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1439680,
"choiceText": "Somatic symptom disorder",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1439682,
"choiceText": "Factitious disorder ",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 460405,
"questionText": "Given the information above, which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 23-Year-Old With Blindness After a Fall She Can't Remember"
},
{
"authors": "Jeffrey S. Forrest, MD; Alexander B. Shortridge",
"content": [
"This patient has an apparent conversion disorder, manifested as both functional visual loss and left-sided lower-extremity weakness. Her neurological workup and laboratory investigations revealed no obvious explanation for symptoms. Furthermore, the patient has been under significant psychological stress. She also demonstrates detachment (la belle indifférence) that is unexpected, given the nature of her condition. Although this finding is not specific to or diagnostic for conversion disorders, it has frequently been associated.",
"Conversion disorder, also called functional neurologic disorder, is an illness of symptoms of altered voluntary motor or sensory function. The motor symptoms of conversion disorder frequently manifest as weakness, paralysis, and/or abnormal movement. The sensory symptoms of this condition may manifest as anesthesia, blindness, and/or hearing loss. Other possible presentations of conversion disorder include nonepileptic seizures, episodes of unresponsiveness, or aphonia (loss of speech).[1] The basic idea of a conversion disorder is that an individual takes a psychological problem, situation, or conflict and \"converts it\" into a physical symptom. What is key is that the patient does so without knowing it.",
"A diagnosis of conversion disorder requires the following[1]:",
"At least one altered or impaired voluntary motor or sensory function",
"Clinical findings demonstrate an incompatibility between symptoms and recognized medical or neurologic disease",
"Symptoms cannot be better explained by another medical or mental disorder",
"Symptoms cause clinically significant distress or impairment in social, occupational, or other important areas of functioning",
"Diagnosing conversion disorder is challenging because it is a diagnosis of exclusion.[2] Clinicians should remember that, although it may appear that symptoms cannot be explained by another medical disorder, eliminating all other possible causes entirely is generally not possible. However, a thorough investigation of any reasonable and relevant potential medical conditions is required before the diagnosis of conversion disorder can be considered.",
"The exact prevalence of conversion disorder is unknown. Estimates suggest that 20%-25% of general hospital patients have individual conversion symptoms.[3] However, only 5% of those experiencing conversion symptoms meet the criteria for full diagnosis. The incidence of conversion disorder is estimated to be 4-12 cases per 100,000 population. In psychiatric settings, this estimate increases to 11-22 cases per 100,000 population.[4]",
"Certain risk factors are associated with the onset of conversion disorders. Female sex is more commonly associated with conversion disorder; it occurs in women 2-10 times more frequently than in men.[3] Low socioeconomic status and low education level are also common among individuals with conversion disorder. This association is further reflected by data that demonstrate higher prevalence of conversion disorder in third-world countries and rural populations. A history of significant psychosocial trauma, such as physical or sexual abuse, is also common in patients who develop conversion disorder.[3]"
],
"date": "November 27, 2019",
"figures": [],
"markdown": "# A 23-Year-Old With Blindness After a Fall She Can't Remember\n\n **Authors:** Jeffrey S. Forrest, MD; Alexander B. Shortridge \n **Date:** November 27, 2019\n\n ## Content\n\n This patient has an apparent conversion disorder, manifested as both functional visual loss and left-sided lower-extremity weakness. Her neurological workup and laboratory investigations revealed no obvious explanation for symptoms. Furthermore, the patient has been under significant psychological stress. She also demonstrates detachment (la belle indifférence) that is unexpected, given the nature of her condition. Although this finding is not specific to or diagnostic for conversion disorders, it has frequently been associated.\nConversion disorder, also called functional neurologic disorder, is an illness of symptoms of altered voluntary motor or sensory function. The motor symptoms of conversion disorder frequently manifest as weakness, paralysis, and/or abnormal movement. The sensory symptoms of this condition may manifest as anesthesia, blindness, and/or hearing loss. Other possible presentations of conversion disorder include nonepileptic seizures, episodes of unresponsiveness, or aphonia (loss of speech).[1] The basic idea of a conversion disorder is that an individual takes a psychological problem, situation, or conflict and \"converts it\" into a physical symptom. What is key is that the patient does so without knowing it.\nA diagnosis of conversion disorder requires the following[1]:\nAt least one altered or impaired voluntary motor or sensory function\nClinical findings demonstrate an incompatibility between symptoms and recognized medical or neurologic disease\nSymptoms cannot be better explained by another medical or mental disorder\nSymptoms cause clinically significant distress or impairment in social, occupational, or other important areas of functioning\nDiagnosing conversion disorder is challenging because it is a diagnosis of exclusion.[2] Clinicians should remember that, although it may appear that symptoms cannot be explained by another medical disorder, eliminating all other possible causes entirely is generally not possible. However, a thorough investigation of any reasonable and relevant potential medical conditions is required before the diagnosis of conversion disorder can be considered.\nThe exact prevalence of conversion disorder is unknown. Estimates suggest that 20%-25% of general hospital patients have individual conversion symptoms.[3] However, only 5% of those experiencing conversion symptoms meet the criteria for full diagnosis. The incidence of conversion disorder is estimated to be 4-12 cases per 100,000 population. In psychiatric settings, this estimate increases to 11-22 cases per 100,000 population.[4]\nCertain risk factors are associated with the onset of conversion disorders. Female sex is more commonly associated with conversion disorder; it occurs in women 2-10 times more frequently than in men.[3] Low socioeconomic status and low education level are also common among individuals with conversion disorder. This association is further reflected by data that demonstrate higher prevalence of conversion disorder in third-world countries and rural populations. A history of significant psychosocial trauma, such as physical or sexual abuse, is also common in patients who develop conversion disorder.[3]\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1439676,
"choiceText": "Malingering",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1439678,
"choiceText": "Conversion disorder",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1439680,
"choiceText": "Somatic symptom disorder",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1439682,
"choiceText": "Factitious disorder ",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 460405,
"questionText": "Given the information above, which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 23-Year-Old With Blindness After a Fall She Can't Remember"
},
{
"authors": "Jeffrey S. Forrest, MD; Alexander B. Shortridge",
"content": [
"The esoteric nature of conversion disorder symptoms makes it difficult to delineate their precise etiology. The onset of such disorders is often described as psychogenic in origin. Two prominent theories may explain the generation of symptoms demonstrated in conversion disorders. The psychoanalytic theory, also known as the Freudian theory, posits that physical symptoms arise from unconscious conflicts between an instinctual impulse (eg, aggression or sexuality) and barriers toward expressing that impulse.[5] As a result of this theoretical unconscious conflict, physical symptoms manifest in a way that symbolically reflects that conflict. An example of this might include a presentation of vaginismus, or involuntary contraction of the vagina, alleged to result from repressed sexual impulses. By contrast, the learning theory focuses on the characterization of physical symptoms as classically conditioned learned behaviors. In this paradigm, physical symptoms serve as a coping mechanism to psychological stressors.[5]",
"Certain biological and neuropsychological factors have been associated with the onset of conversion disorder. An example of such factors include distinct changes in brain structure and function. For instance, impaired hemispheric communication within the brain has been observed in patients with conversion disorder. This impaired hemispheric communication is thought to contribute to excessive cortical arousal, giving rise to discrete physical symptoms.[5] One meta-analysis of neuroimaging studies of patients with conversion disorder found evidence of altered cortical structure compared with healthy control subjects. Additionally, decreased pituitary gland volume was observed in patients with nonepileptic seizures.[6]",
"Conversion disorders are entirely distinct from the similar syndromes of factitious disorders and malingering. Factitious disorders feature the falsification or induction of physical or psychological symptoms for the purpose of presenting an illness or syndrome in the absence of other obvious external rewards. In a sense, a person likes or wants the role of being a patient. By comparison, malingering is the similar false production or exaggeration of symptoms motivated by external incentives (eg, avoiding work, financial compensation, obtaining medications).[7] However, in noting these distinctions, obtaining evidence that symptoms are feigned or not feigned is often difficult and unreliable.",
"The diagnosis of conversion disorder requires that \"clinical findings provide evidence of incompatibility between the symptom and recognized neurologic or medical conditions.\"[1] Such evidence of incompatibility between symptoms present and known conditions may be potentially obtained through several clinical signs. For example, the Hoover sign, an involuntary extension of the leg upon flexion of the contralateral leg, can help distinguish organic from nonorganic hemiparesis. Similarly, the entrainment test can aid in identifying functional tremors.[7]",
"Noting again that conversion disorder is a diagnosis of exclusion, clinicians must consider alternative causes of the symptoms present. In particular, primary neurologic disease must be excluded through the careful examination of the progression of symptoms. Therefore, in the diagnosis, the physician must first rule out any and all possible physical etiologies. This often leads to extensive testing; however, missing a medically treatable cause is likely worse.",
"Conditions that are similar to conversion disorder, such as somatic symptom disorder, must either be ruled out or recognized as being comorbid with the conversion disorder.[8] In both conversion disorder and somatic symptom disorder, the symptoms present do not have an obvious medical cause. A distinction is that the diagnosis of conversion disorder requires that the symptoms present are incompatible with typical medical or neurologic disease. By contrast, somatic symptom disorder symptoms are frequently compatible with possible pathophysiology.[1]",
"Mood disorders, such as major depressive disorder, are frequently comorbid with conversion disorder. Depressive disorders may produce limb weakness similar to what might be seen in some conversion disorders. However, a depressive disorder generally includes more generalized weakness than the focal signs that are often more typical for those associated with a conversion disorder.[9]Anxiety disorders, such as panic disorder, are also known to produce transient neurologic symptoms similar to those that might be seen in a conversion disorder. Such symptoms are typically more acute and short-lived compared with those of conversion disorder.[9]",
"The workup of conversion disorder also may include evoked potentials (EPs). EPs are measurable electrical impulses conducted by the nervous system in response to certain stimuli.[10] Functional abnormalities can alter EP readings in known ways. Such EP alterations can indicate functional abnormalities in visual, auditory, or sensory pathways. However, given the nonorganic nature of conversion symptoms, EP readings in patients with conversion disorder are typically normal.[11]",
"In this patient, visual EPs (VEPs) are needed to confirm the diagnosis of visual loss. VEPs can be measured in several ways. They are often measured with a VEP pattern reversal test that uses a checkerboard stimulus.[12] This, combined with normal neuro-ophthalmic findings, strongly suggests a diagnosis of functional vision loss.[13] Additionally, electromyography tests must also be conducted to evaluate the lower-extremity weakness in presentations similar to the patient's in this case."
],
"date": "November 27, 2019",
"figures": [],
"markdown": "# A 23-Year-Old With Blindness After a Fall She Can't Remember\n\n **Authors:** Jeffrey S. Forrest, MD; Alexander B. Shortridge \n **Date:** November 27, 2019\n\n ## Content\n\n The esoteric nature of conversion disorder symptoms makes it difficult to delineate their precise etiology. The onset of such disorders is often described as psychogenic in origin. Two prominent theories may explain the generation of symptoms demonstrated in conversion disorders. The psychoanalytic theory, also known as the Freudian theory, posits that physical symptoms arise from unconscious conflicts between an instinctual impulse (eg, aggression or sexuality) and barriers toward expressing that impulse.[5] As a result of this theoretical unconscious conflict, physical symptoms manifest in a way that symbolically reflects that conflict. An example of this might include a presentation of vaginismus, or involuntary contraction of the vagina, alleged to result from repressed sexual impulses. By contrast, the learning theory focuses on the characterization of physical symptoms as classically conditioned learned behaviors. In this paradigm, physical symptoms serve as a coping mechanism to psychological stressors.[5]\nCertain biological and neuropsychological factors have been associated with the onset of conversion disorder. An example of such factors include distinct changes in brain structure and function. For instance, impaired hemispheric communication within the brain has been observed in patients with conversion disorder. This impaired hemispheric communication is thought to contribute to excessive cortical arousal, giving rise to discrete physical symptoms.[5] One meta-analysis of neuroimaging studies of patients with conversion disorder found evidence of altered cortical structure compared with healthy control subjects. Additionally, decreased pituitary gland volume was observed in patients with nonepileptic seizures.[6]\nConversion disorders are entirely distinct from the similar syndromes of factitious disorders and malingering. Factitious disorders feature the falsification or induction of physical or psychological symptoms for the purpose of presenting an illness or syndrome in the absence of other obvious external rewards. In a sense, a person likes or wants the role of being a patient. By comparison, malingering is the similar false production or exaggeration of symptoms motivated by external incentives (eg, avoiding work, financial compensation, obtaining medications).[7] However, in noting these distinctions, obtaining evidence that symptoms are feigned or not feigned is often difficult and unreliable.\nThe diagnosis of conversion disorder requires that \"clinical findings provide evidence of incompatibility between the symptom and recognized neurologic or medical conditions.\"[1] Such evidence of incompatibility between symptoms present and known conditions may be potentially obtained through several clinical signs. For example, the Hoover sign, an involuntary extension of the leg upon flexion of the contralateral leg, can help distinguish organic from nonorganic hemiparesis. Similarly, the entrainment test can aid in identifying functional tremors.[7]\nNoting again that conversion disorder is a diagnosis of exclusion, clinicians must consider alternative causes of the symptoms present. In particular, primary neurologic disease must be excluded through the careful examination of the progression of symptoms. Therefore, in the diagnosis, the physician must first rule out any and all possible physical etiologies. This often leads to extensive testing; however, missing a medically treatable cause is likely worse.\nConditions that are similar to conversion disorder, such as somatic symptom disorder, must either be ruled out or recognized as being comorbid with the conversion disorder.[8] In both conversion disorder and somatic symptom disorder, the symptoms present do not have an obvious medical cause. A distinction is that the diagnosis of conversion disorder requires that the symptoms present are incompatible with typical medical or neurologic disease. By contrast, somatic symptom disorder symptoms are frequently compatible with possible pathophysiology.[1]\nMood disorders, such as major depressive disorder, are frequently comorbid with conversion disorder. Depressive disorders may produce limb weakness similar to what might be seen in some conversion disorders. However, a depressive disorder generally includes more generalized weakness than the focal signs that are often more typical for those associated with a conversion disorder.[9]Anxiety disorders, such as panic disorder, are also known to produce transient neurologic symptoms similar to those that might be seen in a conversion disorder. Such symptoms are typically more acute and short-lived compared with those of conversion disorder.[9]\nThe workup of conversion disorder also may include evoked potentials (EPs). EPs are measurable electrical impulses conducted by the nervous system in response to certain stimuli.[10] Functional abnormalities can alter EP readings in known ways. Such EP alterations can indicate functional abnormalities in visual, auditory, or sensory pathways. However, given the nonorganic nature of conversion symptoms, EP readings in patients with conversion disorder are typically normal.[11]\nIn this patient, visual EPs (VEPs) are needed to confirm the diagnosis of visual loss. VEPs can be measured in several ways. They are often measured with a VEP pattern reversal test that uses a checkerboard stimulus.[12] This, combined with normal neuro-ophthalmic findings, strongly suggests a diagnosis of functional vision loss.[13] Additionally, electromyography tests must also be conducted to evaluate the lower-extremity weakness in presentations similar to the patient's in this case.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 23-Year-Old With Blindness After a Fall She Can't Remember"
},
{
"authors": "Jeffrey S. Forrest, MD; Alexander B. Shortridge",
"content": [
"The treatment of conversion disorder requires a comprehensive multidisciplinary approach. An initial neurologic consultation serves to corroborate this possible diagnosis by eliminating organic causes of the symptoms present. Psychiatric consultation serves to address mental health conditions that are commonly comorbid with conversion disorder, including mood disorders, anxiety disorders, posttraumatic stress disorders, somatic symptom disorders, and dissociative disorders. One approach to both diagnosis and treatment for some patients with a conversion disorder is a sodium amytal interview.[14] These interviews have revealed the underlying psychological conflict in some instances. They have also helped in rehabilitation efforts.",
"Antidepressant medication has not been found to treat the conversion disorder itself. No specific antidepressant is widely recognized as effective in the treatment of conversion disorder. Thus, the recommendation is to prescribe medication as needed to treat comorbid psychiatric conditions.[15] Research studies also support cognitive behavioral therapy and hypnotherapy as effective treatments for conversion disorder. Often, psychodynamic-orientated psychotherapy has not been effective in many patients who deny any underlying mental conflicts. However, supportive psychotherapy that emphasizes that physical therapy can be effective has been useful.",
"Physical therapy is indicated to treat the motor symptoms of conversion disorders. Evidence suggests that inpatient physical therapy treatment is highly effective in treating motor symptoms in such conditions. One study found that patients with conversion disorder who were given physical therapy maintained partial or complete symptom remission for up to 1 year after completion of a physical rehabilitation program.[15] The collaboration of specialists in the multidisciplinary team is essential to ensure optimal management for conversion disorder symptoms.",
"The long-term outcome of conversion disorder widely varies depending on the nature of the symptoms. Positive prognostic factors for conversion disorder include sudden symptom onset, short symptom duration, precipitation by an early identifiable stressor, good premorbid functioning, and the absence of comorbid psychiatric disorders.[3] Conversion disorders that have predominant motor features, by contrast, frequently have less favorable outcomes.[16] One meta-analysis demonstrated 66%-100% of patients with such conditions had the same or worse symptoms at follow-up; only 20% demonstrated full remission of symptoms. The same analysis found conversion disorder syndromes that featured visual symptoms had better outcomes, with 46%-78% of patients showing improvement or full remission at follow-up.",
"This patient in this case was treated with a multidisciplinary approach. Psychiatric consultation was obtained for optimization of the patient's antidepressant regimen for her comorbid depressive symptoms. A regimen of intensive outpatient therapy, including cognitive behavioral therapy, was also provided to assist with the myriad of psychological stressors besetting the patient. A physical therapy regimen was initiated to treat her left lower-extremity weakness. After several weeks of treatment, the patient's symptoms resolved."
],
"date": "November 27, 2019",
"figures": [],
"markdown": "# A 23-Year-Old With Blindness After a Fall She Can't Remember\n\n **Authors:** Jeffrey S. Forrest, MD; Alexander B. Shortridge \n **Date:** November 27, 2019\n\n ## Content\n\n The treatment of conversion disorder requires a comprehensive multidisciplinary approach. An initial neurologic consultation serves to corroborate this possible diagnosis by eliminating organic causes of the symptoms present. Psychiatric consultation serves to address mental health conditions that are commonly comorbid with conversion disorder, including mood disorders, anxiety disorders, posttraumatic stress disorders, somatic symptom disorders, and dissociative disorders. One approach to both diagnosis and treatment for some patients with a conversion disorder is a sodium amytal interview.[14] These interviews have revealed the underlying psychological conflict in some instances. They have also helped in rehabilitation efforts.\nAntidepressant medication has not been found to treat the conversion disorder itself. No specific antidepressant is widely recognized as effective in the treatment of conversion disorder. Thus, the recommendation is to prescribe medication as needed to treat comorbid psychiatric conditions.[15] Research studies also support cognitive behavioral therapy and hypnotherapy as effective treatments for conversion disorder. Often, psychodynamic-orientated psychotherapy has not been effective in many patients who deny any underlying mental conflicts. However, supportive psychotherapy that emphasizes that physical therapy can be effective has been useful.\nPhysical therapy is indicated to treat the motor symptoms of conversion disorders. Evidence suggests that inpatient physical therapy treatment is highly effective in treating motor symptoms in such conditions. One study found that patients with conversion disorder who were given physical therapy maintained partial or complete symptom remission for up to 1 year after completion of a physical rehabilitation program.[15] The collaboration of specialists in the multidisciplinary team is essential to ensure optimal management for conversion disorder symptoms.\nThe long-term outcome of conversion disorder widely varies depending on the nature of the symptoms. Positive prognostic factors for conversion disorder include sudden symptom onset, short symptom duration, precipitation by an early identifiable stressor, good premorbid functioning, and the absence of comorbid psychiatric disorders.[3] Conversion disorders that have predominant motor features, by contrast, frequently have less favorable outcomes.[16] One meta-analysis demonstrated 66%-100% of patients with such conditions had the same or worse symptoms at follow-up; only 20% demonstrated full remission of symptoms. The same analysis found conversion disorder syndromes that featured visual symptoms had better outcomes, with 46%-78% of patients showing improvement or full remission at follow-up.\nThis patient in this case was treated with a multidisciplinary approach. Psychiatric consultation was obtained for optimization of the patient's antidepressant regimen for her comorbid depressive symptoms. A regimen of intensive outpatient therapy, including cognitive behavioral therapy, was also provided to assist with the myriad of psychological stressors besetting the patient. A physical therapy regimen was initiated to treat her left lower-extremity weakness. After several weeks of treatment, the patient's symptoms resolved.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1439714,
"choiceText": "Symptoms incompatible with known medical conditions",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1439716,
"choiceText": "Prior or recent psychological trauma",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1439718,
"choiceText": "Significant distress or impairment to daily life",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1439720,
"choiceText": "At least one altered or impaired voluntary motor or sensory function",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Although psychological trauma often precedes conversion disorder onset, it is not required to have occurred in order to make a diagnosis of conversion disorder.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 460413,
"questionText": "Which of the following is <i>not</i> required for a diagnosis of conversion disorder? ",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1439738,
"choiceText": "Ageusia (loss of taste) ",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1439740,
"choiceText": "Ataxia (lack of muscle control)",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1439742,
"choiceText": "Esophageal spasms",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1439744,
"choiceText": "Tonic-clonic pseudoseizures",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Conversion symptoms primarily manifest as alterations or impairments in voluntary motor or sensory functions. The esophagus is primarily under involuntary control. ",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 460419,
"questionText": "Which of the following is <i>least likely</i> to occur as a conversion symptom?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 23-Year-Old With Blindness After a Fall She Can't Remember"
},
{
"authors": "Jeffrey S. Forrest, MD; Alexander B. Shortridge",
"content": [],
"date": "November 27, 2019",
"figures": [],
"markdown": "# A 23-Year-Old With Blindness After a Fall She Can't Remember\n\n **Authors:** Jeffrey S. Forrest, MD; Alexander B. Shortridge \n **Date:** November 27, 2019\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1439714,
"choiceText": "Symptoms incompatible with known medical conditions",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1439716,
"choiceText": "Prior or recent psychological trauma",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1439718,
"choiceText": "Significant distress or impairment to daily life",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1439720,
"choiceText": "At least one altered or impaired voluntary motor or sensory function",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Although psychological trauma often precedes conversion disorder onset, it is not required to have occurred in order to make a diagnosis of conversion disorder.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 460413,
"questionText": "Which of the following is <i>not</i> required for a diagnosis of conversion disorder? ",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1439738,
"choiceText": "Ageusia (loss of taste) ",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1439740,
"choiceText": "Ataxia (lack of muscle control)",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1439742,
"choiceText": "Esophageal spasms",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1439744,
"choiceText": "Tonic-clonic pseudoseizures",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Conversion symptoms primarily manifest as alterations or impairments in voluntary motor or sensory functions. The esophagus is primarily under involuntary control. ",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 460419,
"questionText": "Which of the following is <i>least likely</i> to occur as a conversion symptom?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 23-Year-Old With Blindness After a Fall She Can't Remember"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1439676,
"choiceText": "Malingering",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1439678,
"choiceText": "Conversion disorder",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1439680,
"choiceText": "Somatic symptom disorder",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1439682,
"choiceText": "Factitious disorder ",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 460405,
"questionText": "Given the information above, which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1439714,
"choiceText": "Symptoms incompatible with known medical conditions",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1439716,
"choiceText": "Prior or recent psychological trauma",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1439718,
"choiceText": "Significant distress or impairment to daily life",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1439720,
"choiceText": "At least one altered or impaired voluntary motor or sensory function",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Although psychological trauma often precedes conversion disorder onset, it is not required to have occurred in order to make a diagnosis of conversion disorder.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 460413,
"questionText": "Which of the following is <i>not</i> required for a diagnosis of conversion disorder? ",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1439738,
"choiceText": "Ageusia (loss of taste) ",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1439740,
"choiceText": "Ataxia (lack of muscle control)",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1439742,
"choiceText": "Esophageal spasms",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1439744,
"choiceText": "Tonic-clonic pseudoseizures",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Conversion symptoms primarily manifest as alterations or impairments in voluntary motor or sensory functions. The esophagus is primarily under involuntary control. ",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 460419,
"questionText": "Which of the following is <i>least likely</i> to occur as a conversion symptom?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
920859 | /viewarticle/920859 | [
{
"authors": "James Lee, MD; Stephanie Oh, PhD; Gaurav Gupta, MD",
"content": [
"The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"A 44-year-old, right-handed woman presents to an outpatient clinic 1 month after being diagnosed with influenza, for which she received treatment with oseltamivir. She reports that 2 weeks after her illness, she began experiencing an insidious headache along her left occipital region. She also describes left ear hyperacusis, difficulty furrowing her left eyebrow and closing her left eye, left-sided tongue numbness, and left facial asymmetry. At that time, she presented to her primary care physician, who indicated that it was probably due to the effects of her viral illness. She prescribed the patient prednisone and valganciclovir for 1 week, with no improvement in her symptoms. She was also given an eye patch and eye drops, as well as a prescription for physical therapy.",
"The patient reports having photophobia, phonophobia, and lightheadedness associated with her symptoms. She reports using ibuprofen, with moderate improvement in the aforementioned headache. She denies any history of similar headaches. The patient otherwise also denies any history of recent travel, hiking, or tick exposure, as well as any fevers, rashes, or joint pain.",
"Her medical history is significant for irregular menstrual periods, for which she is on oral estrogen-based contraception. She also has a history of Raynaud syndrome-associated bilateral leg pain, for which she was successfully treated with verapamil and pregabalin, with resolution of her symptoms.",
"She describes no significant surgical history. In terms of her family, she is a married, heterosexual woman with two healthy children. Her parents are both alive, in their 70s, with a history of hyperlipidemia. She denies any known family history of cancer, thyroid issues, or autoimmune disease."
],
"date": "November 13, 2019",
"figures": [],
"markdown": "# A 44-Year-Old With a Headache, Photophobia, and Phonophobia\n\n **Authors:** James Lee, MD; Stephanie Oh, PhD; Gaurav Gupta, MD \n **Date:** November 13, 2019\n\n ## Content\n\n The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nA 44-year-old, right-handed woman presents to an outpatient clinic 1 month after being diagnosed with influenza, for which she received treatment with oseltamivir. She reports that 2 weeks after her illness, she began experiencing an insidious headache along her left occipital region. She also describes left ear hyperacusis, difficulty furrowing her left eyebrow and closing her left eye, left-sided tongue numbness, and left facial asymmetry. At that time, she presented to her primary care physician, who indicated that it was probably due to the effects of her viral illness. She prescribed the patient prednisone and valganciclovir for 1 week, with no improvement in her symptoms. She was also given an eye patch and eye drops, as well as a prescription for physical therapy.\nThe patient reports having photophobia, phonophobia, and lightheadedness associated with her symptoms. She reports using ibuprofen, with moderate improvement in the aforementioned headache. She denies any history of similar headaches. The patient otherwise also denies any history of recent travel, hiking, or tick exposure, as well as any fevers, rashes, or joint pain.\nHer medical history is significant for irregular menstrual periods, for which she is on oral estrogen-based contraception. She also has a history of Raynaud syndrome-associated bilateral leg pain, for which she was successfully treated with verapamil and pregabalin, with resolution of her symptoms.\nShe describes no significant surgical history. In terms of her family, she is a married, heterosexual woman with two healthy children. Her parents are both alive, in their 70s, with a history of hyperlipidemia. She denies any known family history of cancer, thyroid issues, or autoimmune disease.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 44-Year-Old With a Headache, Photophobia, and Phonophobia"
},
{
"authors": "James Lee, MD; Stephanie Oh, PhD; Gaurav Gupta, MD",
"content": [
"Upon physical examination, the patient is noted to be of average build, with normal body mass index. Her head is normocephalic and atraumatic. She is oriented to person, place, and time. Her speech is fluent and clear, and she is able to follow complex commands. Her pupils are equal, round, and reactive to light.",
"The fundi are normal, and spontaneous venous pulsations are present. Extraocular movements are intact, and visual fields are full to visual confrontation. A decreased palpebral fissure is noted in her left eye, along with weakness in left eye closure and an inability to furrow her left eyebrow. She is also noted to have decreased pinprick and cold sensation along the left V2/V3 distribution. Her muscles of mastication are normal. She has prominent left facial asymmetry, with flattening of the nasolabial fold. Her hearing is grossly symmetrical, with subjective hypersensitivity to sound in her left ear. Her palate elevates in the midline, and the tongue has normal motion without fasciculations. Weber and Rinne test results are unremarkable. Finger-to-nose, heel-to-shin, and tandem gait are normal.",
"She has normal muscle bulk and tone; strength and sensation are normal in her upper and lower extremities. Her deep tendon reflexes are normal throughout, except 3+ in her bilateral patellar tendons. Her toes are downgoing bilaterally, with no clonus.",
"Serum findings were unremarkable. Brain MRI with and without contrast (with special attention given to the internal auditory canals) and electromyography (EMG) were performed. An example similar to the MRI findings in this patient is shown in the image below.",
"Figure 1."
],
"date": "November 13, 2019",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/920/859/920859-fig1-thumb.jpg"
}
],
"markdown": "# A 44-Year-Old With a Headache, Photophobia, and Phonophobia\n\n **Authors:** James Lee, MD; Stephanie Oh, PhD; Gaurav Gupta, MD \n **Date:** November 13, 2019\n\n ## Content\n\n Upon physical examination, the patient is noted to be of average build, with normal body mass index. Her head is normocephalic and atraumatic. She is oriented to person, place, and time. Her speech is fluent and clear, and she is able to follow complex commands. Her pupils are equal, round, and reactive to light.\nThe fundi are normal, and spontaneous venous pulsations are present. Extraocular movements are intact, and visual fields are full to visual confrontation. A decreased palpebral fissure is noted in her left eye, along with weakness in left eye closure and an inability to furrow her left eyebrow. She is also noted to have decreased pinprick and cold sensation along the left V2/V3 distribution. Her muscles of mastication are normal. She has prominent left facial asymmetry, with flattening of the nasolabial fold. Her hearing is grossly symmetrical, with subjective hypersensitivity to sound in her left ear. Her palate elevates in the midline, and the tongue has normal motion without fasciculations. Weber and Rinne test results are unremarkable. Finger-to-nose, heel-to-shin, and tandem gait are normal.\nShe has normal muscle bulk and tone; strength and sensation are normal in her upper and lower extremities. Her deep tendon reflexes are normal throughout, except 3+ in her bilateral patellar tendons. Her toes are downgoing bilaterally, with no clonus.\nSerum findings were unremarkable. Brain MRI with and without contrast (with special attention given to the internal auditory canals) and electromyography (EMG) were performed. An example similar to the MRI findings in this patient is shown in the image below.\nFigure 1.\n\n ## Figures\n\n **Figure 1.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1433344,
"choiceText": "Acute ischemic stroke",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1433346,
"choiceText": "Basilar meningitis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1433348,
"choiceText": "Cerebellopontine angle lesion (ie, acoustic neuroma)",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1433350,
"choiceText": "Guillain-Barré syndrome",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1433352,
"choiceText": "Bell palsy",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 458217,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 44-Year-Old With a Headache, Photophobia, and Phonophobia"
},
{
"authors": "James Lee, MD; Stephanie Oh, PhD; Gaurav Gupta, MD",
"content": [
"Named after the anatomist Sir Charles Bell, who first described the facial nerve (cranial nerve [CN] VII), Bell palsy is characterized by acute, idiopathic, peripheral facial paralysis.[1] Bell palsy affects CN VII, a mixed sensory and motor nerve that carries fibers involved in taste, lacrimation, salivation, and sensation of the ear while also innervating the muscles of facial expression. The condition is a common neurologic complaint in both men and women, with an annual incidence of approximately 20-30 cases per 100,000 population.[2,3,4]",
"The facial paralysis seen in Bell palsy is typically unilateral, with either the right or left side of the face affected with equal frequency. Complete palsy is present in most cases.[5] Bilateral paralysis occurs in less than 1% of patients. Although Bell palsy is thought to be a clinically benign diagnosis that typically resolves over time, as many as 30% of patients fail to recover full functionality.[6] In addition, the temporary facial paralysis of Bell palsy can significantly affect quality of life. It can be disfiguring, affect the ability to eat, and even lead to permanent eye injury owing to an inability to completely close the eye.",
"Risk factors for Bell palsy include diabetes, hypertension, immunocompromised status, obesity, pregnancy, and recent upper respiratory viral infection.[5] Although the exact cause of Bell palsy remains unclear, potential etiologies include anatomical factors, viral infection, ischemia, autoimmune/inflammatory demyelination, genetic predisposition, and even acute cold exposure.[7]",
"The anatomical theory posits that CN VII is susceptible to trauma and compression, owing to its long course from the brainstem that abuts many bony structures. Specifically, CN VII emerges from the pons to join the vestibulocochlear nerve (CN VIII) through the internal auditory meatus. CN VII then travels 20-30 mm in the facial canal, exiting through the stylomastoid foramen and passing through the parotid gland, where it divides into its terminal branches that innervate the muscles of facial expression.[8] This course is longer than that of any other cranial nerve, making CN VII particularly vulnerable to anatomical changes.",
"An etiology that is widely accepted is that the edema and inflammation is caused by the reactivation of viruses that target peripheral neurons, such as herpes simplex virus type 1 (HSV-1) and type 2 (HSV-2) and varicella zoster virus.[9,10] Because these viruses can establish latent infection within nerve ganglia, reactivation and subsequent viral-mediated axonal degradation can be incited by immune modulation or immunosuppression.[11] Of note, an inactivated intranasal influenza vaccine has been linked with Bell palsy, possibly owing to reactivation of HSV.[12,13]",
"Another postulated mechanism for Bell palsy is ischemia. Ischemic insults, such as diabetic microangiopathy or cerebral venous thrombosis, are thought to compromise the vasa nervosum of the facial nerve.[14,15] This may lead to vasospasm, increased capillary permeability, edema, and inflammation, with an end result of perivasculitis and endarteritis. Fibrotic nerve sheath scarring left from this process may result in refractory facial paralysis and may require surgical decompression.[16]",
"Proposed inflammatory mechanisms for Bell palsy are numerous, including the idea that it may be a mononeuritic variant of Guillain-Barré syndrome, in which the peripheral nerve myelin is attacked by a cell-mediated immune reaction.[17] In addition, Bell palsy is seen frequently during pregnancy and with severe preeclampsia, suggesting that pregnancy-related fluid retention, perineural edema, and resulting inflammation are possible mechanisms.[18]"
],
"date": "November 13, 2019",
"figures": [],
"markdown": "# A 44-Year-Old With a Headache, Photophobia, and Phonophobia\n\n **Authors:** James Lee, MD; Stephanie Oh, PhD; Gaurav Gupta, MD \n **Date:** November 13, 2019\n\n ## Content\n\n Named after the anatomist Sir Charles Bell, who first described the facial nerve (cranial nerve [CN] VII), Bell palsy is characterized by acute, idiopathic, peripheral facial paralysis.[1] Bell palsy affects CN VII, a mixed sensory and motor nerve that carries fibers involved in taste, lacrimation, salivation, and sensation of the ear while also innervating the muscles of facial expression. The condition is a common neurologic complaint in both men and women, with an annual incidence of approximately 20-30 cases per 100,000 population.[2,3,4]\nThe facial paralysis seen in Bell palsy is typically unilateral, with either the right or left side of the face affected with equal frequency. Complete palsy is present in most cases.[5] Bilateral paralysis occurs in less than 1% of patients. Although Bell palsy is thought to be a clinically benign diagnosis that typically resolves over time, as many as 30% of patients fail to recover full functionality.[6] In addition, the temporary facial paralysis of Bell palsy can significantly affect quality of life. It can be disfiguring, affect the ability to eat, and even lead to permanent eye injury owing to an inability to completely close the eye.\nRisk factors for Bell palsy include diabetes, hypertension, immunocompromised status, obesity, pregnancy, and recent upper respiratory viral infection.[5] Although the exact cause of Bell palsy remains unclear, potential etiologies include anatomical factors, viral infection, ischemia, autoimmune/inflammatory demyelination, genetic predisposition, and even acute cold exposure.[7]\nThe anatomical theory posits that CN VII is susceptible to trauma and compression, owing to its long course from the brainstem that abuts many bony structures. Specifically, CN VII emerges from the pons to join the vestibulocochlear nerve (CN VIII) through the internal auditory meatus. CN VII then travels 20-30 mm in the facial canal, exiting through the stylomastoid foramen and passing through the parotid gland, where it divides into its terminal branches that innervate the muscles of facial expression.[8] This course is longer than that of any other cranial nerve, making CN VII particularly vulnerable to anatomical changes.\nAn etiology that is widely accepted is that the edema and inflammation is caused by the reactivation of viruses that target peripheral neurons, such as herpes simplex virus type 1 (HSV-1) and type 2 (HSV-2) and varicella zoster virus.[9,10] Because these viruses can establish latent infection within nerve ganglia, reactivation and subsequent viral-mediated axonal degradation can be incited by immune modulation or immunosuppression.[11] Of note, an inactivated intranasal influenza vaccine has been linked with Bell palsy, possibly owing to reactivation of HSV.[12,13]\nAnother postulated mechanism for Bell palsy is ischemia. Ischemic insults, such as diabetic microangiopathy or cerebral venous thrombosis, are thought to compromise the vasa nervosum of the facial nerve.[14,15] This may lead to vasospasm, increased capillary permeability, edema, and inflammation, with an end result of perivasculitis and endarteritis. Fibrotic nerve sheath scarring left from this process may result in refractory facial paralysis and may require surgical decompression.[16]\nProposed inflammatory mechanisms for Bell palsy are numerous, including the idea that it may be a mononeuritic variant of Guillain-Barré syndrome, in which the peripheral nerve myelin is attacked by a cell-mediated immune reaction.[17] In addition, Bell palsy is seen frequently during pregnancy and with severe preeclampsia, suggesting that pregnancy-related fluid retention, perineural edema, and resulting inflammation are possible mechanisms.[18]\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1433344,
"choiceText": "Acute ischemic stroke",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1433346,
"choiceText": "Basilar meningitis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1433348,
"choiceText": "Cerebellopontine angle lesion (ie, acoustic neuroma)",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1433350,
"choiceText": "Guillain-Barré syndrome",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1433352,
"choiceText": "Bell palsy",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 458217,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 44-Year-Old With a Headache, Photophobia, and Phonophobia"
},
{
"authors": "James Lee, MD; Stephanie Oh, PhD; Gaurav Gupta, MD",
"content": [
"The diagnosis of Bell palsy is largely one of exclusion and depends on a thorough neurologic examination. It is based on the clinical recognition of symptoms, such as ipsilateral sagging of the eyebrow, which is key in recognizing the peripheral facial palsy of Bell palsy. This is opposed to central facial palsy, which spares the upper third of the contralateral face. With the weakness of all facial muscles seen in Bell palsy, one may also observe ptosis, flattening of the nasolabial fold, and inability to control the lips and mouth. The upward movement of the eye while attempting to close the eye, which is due to weakness of the orbicularis oculi, is a pathognomonic sign termed \"Bell phenomenon.\"[19]These facial motor symptoms develop acutely within hours to 1-2 days and are maximal within 3 weeks or less from symptom onset. Patients may also present with associated hyperacusis due to disruption of fibers to the stapedius muscle; pain around the neck or ear; facial paresthesia; dry eyes and dry mouth; or dysgeusia, especially on the anterior two thirds of the tongue.",
"Once a clinical diagnosis has been established, patients should receive reassurance and counseling that the prognosis is usually favorable. Patients should be educated about the risk for corneal exposure and should use lubricating drops during waking hours, as well as a patch to close the eyelid at night. Evidence-based guidelines from the American Academy of Neurology (AAN) provide a level A recommendation that oral steroids should be offered to patients with acute-onset Bell palsy within 1-3 days of symptom onset.[20] Prednisone (60-80 mg/d) for 1 week is a common regimen. The AAN also provides a level C recommendation that antivirals can be offered in addition to steroids to increase the probability of recovery of facial function; however, the benefit of antivirals has not yet been established.[20] Nevertheless, early combined therapy with prednisone (60-80 mg/d) and valacyclovir (1000 mg three times daily) for 1 week can be offered, especially to those with severe facial palsy.",
"To assess facial function, physician-graded scales, such as the House-Brackmann scale and the Sunnybrook Facial Grading System (FGS), are commonly used. The House-Brackmann scale was intended only to describe recovery after vestibular schwannoma surgery and is thought to lack sensitivity compared with the Sunnybrook FGS.[21] The Sunnybrook FGS was proposed in 1996 by Ross and colleagues[22] and rates the following three categories: (1) resting or static symmetry of the affected side compared with the normal side; (2) dynamic symmetry of voluntary movement of the affected side, as measured by the degree of muscle excursion compared with the normal side; and (3) rate of degree of involuntary muscle contraction (synkinesis) of the affected side. The Sunnybrook FGS ranges from 0 to 100 and has been robustly validated, with reliable reproducibility between observers.[23] Because patients with Bell palsy typically recover within 3 weeks, one randomized trial found the use of Sunnybrook FGS at 1 month to most accurately predict nonrecovery at 12 months.[24]",
"Severe or complete facial paralysis, bilateral paralysis, involvement of other nerves, or nonrecovery within 4 weeks should prompt referral to a specialist; additional evaluation; and consideration of differential diagnosis, such as neoplasms, HIV infection, Lyme disease, Guillain-Barré syndrome, multiple sclerosis, sarcoidosis, Sjögren syndrome, and even rare conditions like Melkersson-Rosenthal syndrome. In these cases, electrodiagnostic and imaging studies may be useful.",
"Electrodiagnostic tests, such as EMG and nerve conduction studies, can provide information on the viability of the nerve, especially in patients with a clinically complete lesion. These tests are most useful when performed within 2 weeks to help guide treatment decisions on surgical management.[25] Within 1-3 months of symptom onset, needle EMG can yield prognostic information on the degree of muscle denervation and axonal damage and can also confirm any subclinical signs of re-innervation and recovery.[26]",
"Imaging studies, such as CT with contrast or gadolinium-enhanced MRI, should be focused on the brain, temporal bone, and parotid glands. High-resolution CT can demonstrate bony erosions, and MRI can evaluate for inflammation, edema, or neoplasm. Imaging can be used if a patient fails to improve within 4 months of symptom onset and then again at 7 months. Pathologic investigation, such as parotid gland biopsy, may be used if symptoms persist and imaging remains negative.[27]"
],
"date": "November 13, 2019",
"figures": [],
"markdown": "# A 44-Year-Old With a Headache, Photophobia, and Phonophobia\n\n **Authors:** James Lee, MD; Stephanie Oh, PhD; Gaurav Gupta, MD \n **Date:** November 13, 2019\n\n ## Content\n\n The diagnosis of Bell palsy is largely one of exclusion and depends on a thorough neurologic examination. It is based on the clinical recognition of symptoms, such as ipsilateral sagging of the eyebrow, which is key in recognizing the peripheral facial palsy of Bell palsy. This is opposed to central facial palsy, which spares the upper third of the contralateral face. With the weakness of all facial muscles seen in Bell palsy, one may also observe ptosis, flattening of the nasolabial fold, and inability to control the lips and mouth. The upward movement of the eye while attempting to close the eye, which is due to weakness of the orbicularis oculi, is a pathognomonic sign termed \"Bell phenomenon.\"[19]These facial motor symptoms develop acutely within hours to 1-2 days and are maximal within 3 weeks or less from symptom onset. Patients may also present with associated hyperacusis due to disruption of fibers to the stapedius muscle; pain around the neck or ear; facial paresthesia; dry eyes and dry mouth; or dysgeusia, especially on the anterior two thirds of the tongue.\nOnce a clinical diagnosis has been established, patients should receive reassurance and counseling that the prognosis is usually favorable. Patients should be educated about the risk for corneal exposure and should use lubricating drops during waking hours, as well as a patch to close the eyelid at night. Evidence-based guidelines from the American Academy of Neurology (AAN) provide a level A recommendation that oral steroids should be offered to patients with acute-onset Bell palsy within 1-3 days of symptom onset.[20] Prednisone (60-80 mg/d) for 1 week is a common regimen. The AAN also provides a level C recommendation that antivirals can be offered in addition to steroids to increase the probability of recovery of facial function; however, the benefit of antivirals has not yet been established.[20] Nevertheless, early combined therapy with prednisone (60-80 mg/d) and valacyclovir (1000 mg three times daily) for 1 week can be offered, especially to those with severe facial palsy.\nTo assess facial function, physician-graded scales, such as the House-Brackmann scale and the Sunnybrook Facial Grading System (FGS), are commonly used. The House-Brackmann scale was intended only to describe recovery after vestibular schwannoma surgery and is thought to lack sensitivity compared with the Sunnybrook FGS.[21] The Sunnybrook FGS was proposed in 1996 by Ross and colleagues[22] and rates the following three categories: (1) resting or static symmetry of the affected side compared with the normal side; (2) dynamic symmetry of voluntary movement of the affected side, as measured by the degree of muscle excursion compared with the normal side; and (3) rate of degree of involuntary muscle contraction (synkinesis) of the affected side. The Sunnybrook FGS ranges from 0 to 100 and has been robustly validated, with reliable reproducibility between observers.[23] Because patients with Bell palsy typically recover within 3 weeks, one randomized trial found the use of Sunnybrook FGS at 1 month to most accurately predict nonrecovery at 12 months.[24]\nSevere or complete facial paralysis, bilateral paralysis, involvement of other nerves, or nonrecovery within 4 weeks should prompt referral to a specialist; additional evaluation; and consideration of differential diagnosis, such as neoplasms, HIV infection, Lyme disease, Guillain-Barré syndrome, multiple sclerosis, sarcoidosis, Sjögren syndrome, and even rare conditions like Melkersson-Rosenthal syndrome. In these cases, electrodiagnostic and imaging studies may be useful.\nElectrodiagnostic tests, such as EMG and nerve conduction studies, can provide information on the viability of the nerve, especially in patients with a clinically complete lesion. These tests are most useful when performed within 2 weeks to help guide treatment decisions on surgical management.[25] Within 1-3 months of symptom onset, needle EMG can yield prognostic information on the degree of muscle denervation and axonal damage and can also confirm any subclinical signs of re-innervation and recovery.[26]\nImaging studies, such as CT with contrast or gadolinium-enhanced MRI, should be focused on the brain, temporal bone, and parotid glands. High-resolution CT can demonstrate bony erosions, and MRI can evaluate for inflammation, edema, or neoplasm. Imaging can be used if a patient fails to improve within 4 months of symptom onset and then again at 7 months. Pathologic investigation, such as parotid gland biopsy, may be used if symptoms persist and imaging remains negative.[27]\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 44-Year-Old With a Headache, Photophobia, and Phonophobia"
},
{
"authors": "James Lee, MD; Stephanie Oh, PhD; Gaurav Gupta, MD",
"content": [
"No consensus has been reached regarding the benefit of or indications for surgery in the treatment of Bell palsy.[28,29] Surgical facial nerve decompression has been used as a management option to theoretically relieve the swelling and entrapment of the nerve, with such complications as seizures, vertigo, unilateral hearing loss, cerebrospinal fluid leak, and facial nerve injury.[30] Observational studies on surgical decompression have had mixed results, with some studies showing statistically significant improvement in surgical groups compared with control groups, and others showing no difference.[31,32] One systematic analysis of two randomized controlled trials found only very low-quality evidence, insufficient to decide whether surgical decompression was useful in the management of Bell palsy.[33]",
"In this case, the patient was advised to continue using eye drops and an eye patch, particularly at bedtime. She was also advised to continue using ibuprofen for symptomatic relief of her headache. After 6 months of conservative medical management, her symptoms persisted with only minor improvement. Serum studies and radiographic images were obtained; the findings were all unremarkable. She was subsequently referred for surgical intervention. She underwent facial nerve decompression, with near-complete resolution of her symptoms.",
"In conclusion, Bell palsy is a clinical diagnosis of exclusion made in patients with acute-onset, peripheral facial palsy. Although most patients experience spontaneous improvement, delay in diagnosis and treatment can significant affect quality of life. It is crucial to recognize key symptoms and initiate early treatment with corticosteroids. Prompt referral to a specialist is also important to assess for differential diagnoses and optimize recovery."
],
"date": "November 13, 2019",
"figures": [],
"markdown": "# A 44-Year-Old With a Headache, Photophobia, and Phonophobia\n\n **Authors:** James Lee, MD; Stephanie Oh, PhD; Gaurav Gupta, MD \n **Date:** November 13, 2019\n\n ## Content\n\n No consensus has been reached regarding the benefit of or indications for surgery in the treatment of Bell palsy.[28,29] Surgical facial nerve decompression has been used as a management option to theoretically relieve the swelling and entrapment of the nerve, with such complications as seizures, vertigo, unilateral hearing loss, cerebrospinal fluid leak, and facial nerve injury.[30] Observational studies on surgical decompression have had mixed results, with some studies showing statistically significant improvement in surgical groups compared with control groups, and others showing no difference.[31,32] One systematic analysis of two randomized controlled trials found only very low-quality evidence, insufficient to decide whether surgical decompression was useful in the management of Bell palsy.[33]\nIn this case, the patient was advised to continue using eye drops and an eye patch, particularly at bedtime. She was also advised to continue using ibuprofen for symptomatic relief of her headache. After 6 months of conservative medical management, her symptoms persisted with only minor improvement. Serum studies and radiographic images were obtained; the findings were all unremarkable. She was subsequently referred for surgical intervention. She underwent facial nerve decompression, with near-complete resolution of her symptoms.\nIn conclusion, Bell palsy is a clinical diagnosis of exclusion made in patients with acute-onset, peripheral facial palsy. Although most patients experience spontaneous improvement, delay in diagnosis and treatment can significant affect quality of life. It is crucial to recognize key symptoms and initiate early treatment with corticosteroids. Prompt referral to a specialist is also important to assess for differential diagnoses and optimize recovery.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1433354,
"choiceText": "Viral-mediated inflammation",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1433356,
"choiceText": "Axonal degradation",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1433358,
"choiceText": "Nerve ischemia",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1433360,
"choiceText": "Overuse of muscles of mastication",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1433362,
"choiceText": "Environmental temperature",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>Although anatomical changes that affect the muscles of facial expression have been cited as potentially reasons for compression of the facial nerve, evidence does not suggest that excess use of the muscles of mastication is a primary cause for the development of Bell palsy.</p>",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 458219,
"questionText": "Which of the following pathophysiologic mechanisms is <em>not</em> typically considered a primary cause of Bell palsy?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1433364,
"choiceText": "Reassurance and observation",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1433366,
"choiceText": "Surgical decompression",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1433368,
"choiceText": "Antivirals alone",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1433370,
"choiceText": "Steroids alone",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1433372,
"choiceText": "Steroids and antivirals",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>The strongest (level A) recommendations from the AAN indicate that steroids alone are the most effective treatment for acute management of Bell palsy. Use of antivirals, although often provided for patients with severe deficits, carries only level C recommendations for efficacy. Although most cases of Bell palsy resolve spontaneously with observation and conservative management, in the acute phase of illness (< 1 week), studies indicate that steroids provide the best opportunity for full functional recovery.</p>",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 458221,
"questionText": "Which of the following is typically recommended as initial treatment for acute Bell palsy (< 1 week from symptom onset)?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 44-Year-Old With a Headache, Photophobia, and Phonophobia"
},
{
"authors": "James Lee, MD; Stephanie Oh, PhD; Gaurav Gupta, MD",
"content": [],
"date": "November 13, 2019",
"figures": [],
"markdown": "# A 44-Year-Old With a Headache, Photophobia, and Phonophobia\n\n **Authors:** James Lee, MD; Stephanie Oh, PhD; Gaurav Gupta, MD \n **Date:** November 13, 2019\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1433354,
"choiceText": "Viral-mediated inflammation",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1433356,
"choiceText": "Axonal degradation",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1433358,
"choiceText": "Nerve ischemia",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1433360,
"choiceText": "Overuse of muscles of mastication",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1433362,
"choiceText": "Environmental temperature",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>Although anatomical changes that affect the muscles of facial expression have been cited as potentially reasons for compression of the facial nerve, evidence does not suggest that excess use of the muscles of mastication is a primary cause for the development of Bell palsy.</p>",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 458219,
"questionText": "Which of the following pathophysiologic mechanisms is <em>not</em> typically considered a primary cause of Bell palsy?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1433364,
"choiceText": "Reassurance and observation",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1433366,
"choiceText": "Surgical decompression",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1433368,
"choiceText": "Antivirals alone",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1433370,
"choiceText": "Steroids alone",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1433372,
"choiceText": "Steroids and antivirals",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>The strongest (level A) recommendations from the AAN indicate that steroids alone are the most effective treatment for acute management of Bell palsy. Use of antivirals, although often provided for patients with severe deficits, carries only level C recommendations for efficacy. Although most cases of Bell palsy resolve spontaneously with observation and conservative management, in the acute phase of illness (< 1 week), studies indicate that steroids provide the best opportunity for full functional recovery.</p>",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 458221,
"questionText": "Which of the following is typically recommended as initial treatment for acute Bell palsy (< 1 week from symptom onset)?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 44-Year-Old With a Headache, Photophobia, and Phonophobia"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1433344,
"choiceText": "Acute ischemic stroke",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1433346,
"choiceText": "Basilar meningitis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1433348,
"choiceText": "Cerebellopontine angle lesion (ie, acoustic neuroma)",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1433350,
"choiceText": "Guillain-Barré syndrome",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1433352,
"choiceText": "Bell palsy",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 458217,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1433354,
"choiceText": "Viral-mediated inflammation",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1433356,
"choiceText": "Axonal degradation",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1433358,
"choiceText": "Nerve ischemia",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1433360,
"choiceText": "Overuse of muscles of mastication",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1433362,
"choiceText": "Environmental temperature",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>Although anatomical changes that affect the muscles of facial expression have been cited as potentially reasons for compression of the facial nerve, evidence does not suggest that excess use of the muscles of mastication is a primary cause for the development of Bell palsy.</p>",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 458219,
"questionText": "Which of the following pathophysiologic mechanisms is <em>not</em> typically considered a primary cause of Bell palsy?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1433364,
"choiceText": "Reassurance and observation",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1433366,
"choiceText": "Surgical decompression",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1433368,
"choiceText": "Antivirals alone",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1433370,
"choiceText": "Steroids alone",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1433372,
"choiceText": "Steroids and antivirals",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>The strongest (level A) recommendations from the AAN indicate that steroids alone are the most effective treatment for acute management of Bell palsy. Use of antivirals, although often provided for patients with severe deficits, carries only level C recommendations for efficacy. Although most cases of Bell palsy resolve spontaneously with observation and conservative management, in the acute phase of illness (< 1 week), studies indicate that steroids provide the best opportunity for full functional recovery.</p>",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 458221,
"questionText": "Which of the following is typically recommended as initial treatment for acute Bell palsy (< 1 week from symptom onset)?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
842851 | /viewarticle/842851 | [
{
"authors": "Kennedy O. Omonuwa, MD; Arunabh Talwar, MD; Lindsay Goodman, MD",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"A 56-year-old African-American woman with a 20-year history of pulmonary sarcoidosis presents to the emergency department with a 6-month history of progressive and worsening dyspnea. The patient's dyspnea is worse during exertion and is associated with decreased exercise tolerance. She denies any coughing, wheezing, fever, chest pain, or leg swelling.",
"She has had multiple presentations to her primary care provider's office; at each visit, the dyspnea was attributed to progression of her underlying pulmonary sarcoidosis, and she was prescribed corticosteroids. Despite steroid therapy, her dyspnea has persisted and has worsened.",
"The patient's only medications are oral prednisone and multivitamins. She has no known drug or food allergies, as well as no history of smoking, alcohol abuse, or illicit drug use."
],
"date": "October 31, 2019",
"figures": [],
"markdown": "# A 56-Year-Old Woman With Worsening Dyspnea and Sarcoidosis\n\n **Authors:** Kennedy O. Omonuwa, MD; Arunabh Talwar, MD; Lindsay Goodman, MD \n **Date:** October 31, 2019\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nA 56-year-old African-American woman with a 20-year history of pulmonary sarcoidosis presents to the emergency department with a 6-month history of progressive and worsening dyspnea. The patient's dyspnea is worse during exertion and is associated with decreased exercise tolerance. She denies any coughing, wheezing, fever, chest pain, or leg swelling.\nShe has had multiple presentations to her primary care provider's office; at each visit, the dyspnea was attributed to progression of her underlying pulmonary sarcoidosis, and she was prescribed corticosteroids. Despite steroid therapy, her dyspnea has persisted and has worsened.\nThe patient's only medications are oral prednisone and multivitamins. She has no known drug or food allergies, as well as no history of smoking, alcohol abuse, or illicit drug use.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 56-Year-Old Woman With Worsening Dyspnea and Sarcoidosis"
},
{
"authors": "Kennedy O. Omonuwa, MD; Arunabh Talwar, MD; Lindsay Goodman, MD",
"content": [
"The physical examination reveals an alert, well-appearing female, with a regular pulse rate of 95 beats/min, blood pressure of 110/70 mm Hg, respiratory rate of 24 breaths/min, and an oxygen saturation of 91% while breathing room air. Her neck is supple and without jugular venous distention.",
"The cardiac examination reveals a normal S1, a loud P2, and no audible murmurs, rubs or gallops. Examination of the chest shows decreased air entry at the lung bases, but no wheezes, rhonchi, or rales are noted. The abdomen is soft, nontender, and without organomegaly. No pedal edema, cyanosis, or clubbing is observed. The peripheral arterial pulses are palpable. The neurologic examination findings are normal.",
"Blood tests, including a complete blood cell count, comprehensive metabolic panel, B-type natriuretic peptide, and serial troponins, are all within normal limits. A blood gas test on room air shows a pH of 7.46, pCO2 of 35 mm Hg, and pO2 of 70 mm Hg.",
"Chest radiography shows bilateral hilar adenopathy, with increased markings bilaterally. Pulmonary function tests (PFTs) performed 1 month before presentation showed an FVC of 1.9 L (63% of predicted), FEV1 of 1.65 L (70% of predicted), FEV1/FVC ratio of 89%, a residual volume of 0.83 L (38% of predicted), total lung capacity of 2.74 L (53% of predicted), and a diffusing capacity (DLCO) of 8.73 mL/min/mm Hg (38% of predicted). These results are unchanged from an examination performed 6 months ago.",
"A 12-lead ECG is obtained (Figure 1). A 2-dimensional (2D) echocardiography examination is subsequently obtained, and right-heart catheterization performed.",
"Figure 1.",
"Figure 1."
],
"date": "October 31, 2019",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/842/851/842851-Thumb1.png"
}
],
"markdown": "# A 56-Year-Old Woman With Worsening Dyspnea and Sarcoidosis\n\n **Authors:** Kennedy O. Omonuwa, MD; Arunabh Talwar, MD; Lindsay Goodman, MD \n **Date:** October 31, 2019\n\n ## Content\n\n The physical examination reveals an alert, well-appearing female, with a regular pulse rate of 95 beats/min, blood pressure of 110/70 mm Hg, respiratory rate of 24 breaths/min, and an oxygen saturation of 91% while breathing room air. Her neck is supple and without jugular venous distention.\nThe cardiac examination reveals a normal S1, a loud P2, and no audible murmurs, rubs or gallops. Examination of the chest shows decreased air entry at the lung bases, but no wheezes, rhonchi, or rales are noted. The abdomen is soft, nontender, and without organomegaly. No pedal edema, cyanosis, or clubbing is observed. The peripheral arterial pulses are palpable. The neurologic examination findings are normal.\nBlood tests, including a complete blood cell count, comprehensive metabolic panel, B-type natriuretic peptide, and serial troponins, are all within normal limits. A blood gas test on room air shows a pH of 7.46, pCO2 of 35 mm Hg, and pO2 of 70 mm Hg.\nChest radiography shows bilateral hilar adenopathy, with increased markings bilaterally. Pulmonary function tests (PFTs) performed 1 month before presentation showed an FVC of 1.9 L (63% of predicted), FEV1 of 1.65 L (70% of predicted), FEV1/FVC ratio of 89%, a residual volume of 0.83 L (38% of predicted), total lung capacity of 2.74 L (53% of predicted), and a diffusing capacity (DLCO) of 8.73 mL/min/mm Hg (38% of predicted). These results are unchanged from an examination performed 6 months ago.\nA 12-lead ECG is obtained (Figure 1). A 2-dimensional (2D) echocardiography examination is subsequently obtained, and right-heart catheterization performed.\nFigure 1.\nFigure 1.\n\n ## Figures\n\n **Figure 1.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 833127,
"choiceText": "Right-sided myocardial infarction",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 833129,
"choiceText": "Pulmonary hypertension",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 833131,
"choiceText": "Pericarditis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 833133,
"choiceText": "Chronic obstructive pulmonary disease",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 262031,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 56-Year-Old Woman With Worsening Dyspnea and Sarcoidosis"
},
{
"authors": "Kennedy O. Omonuwa, MD; Arunabh Talwar, MD; Lindsay Goodman, MD",
"content": [
"An eventual diagnosis of pulmonary hypertension was made in this patient with pulmonary sarcoidosis who presented with persistent dyspnea that was refractory to steroid therapy. The loud P2 detected on the cardiac examination, together with the abnormal ECG findings of right ventricular hypertrophy (tall R wave > 6 mm in V1), right-axis deviation, and a right atrial abnormality (P wave > 2.5 mm in lead II and > 1.5 mm in V1) suggestive of right-heart dysfunction were useful clues to the suspicion of complicating pulmonary hypertension in this patient with a known cause (sarcoidosis) of pulmonary hypertension.",
"These discoveries correlated with the 2D echocardiogram findings, which showed a high estimated peak pulmonary artery systolic pressure of 86 mm Hg (normal range, 18-25 mm Hg at rest). The subsequent right-heart catheterization confirmed the diagnosis of pulmonary hypertension (with an elevated mean pulmonary arterial pressure of 55 mm Hg and elevated pulmonary vascular resistance of 11.13 Wood units). Because the PFT results remained unchanged after 6 months, the progressive dyspnea is less likely to be secondary to worsening pulmonary sarcoidosis itself.",
"Sarcoidosis is a multiorgan disorder of unknown cause characterized by the presence of noncaseating granulomas, most commonly involving the lungs. Sarcoid patients with advanced pulmonary disease, especially end-stage pulmonary fibrosis, are at risk for developing sarcoidosis-associated pulmonary hypertension (SAPH). Among end-stage sarcoid patients awaiting lung transplantation, pulmonary hypertension is documented in approximately 75% of cases, which is predictive of increased mortality. Pulmonary hypertension has also been reported as an early manifestation of sarcoidosis and in patients with sarcoidosis who are not considered candidates for transplant.[1,2]",
"Pulmonary hypertension is defined as a mean pulmonary arterial pressure greater than 25 mm Hg at rest or 30 mm Hg with exercise. Patients with pulmonary hypertension and pulmonary sarcoidosis have a worse prognosis.",
"SAPH is placed in the miscellaneous (Group V) category of the World Health Organization classification of pulmonary hypertension because the mechanism of pulmonary hypertension in sarcoidosis patients is multifactorial. The pathogenesis of SAPH includes fibrotic destruction of the lung vasculature, granulomatous pulmonary angiitis, or extrinsic compression of major pulmonary vessels by mediastinal or hilar adenopathy. In addition to its direct effects on the pulmonary vasculature, left ventricular myocardial infiltration may cause diastolic dysfunction or diminished systolic function and lead to secondary pulmonary hypertension.[1,2]"
],
"date": "October 31, 2019",
"figures": [],
"markdown": "# A 56-Year-Old Woman With Worsening Dyspnea and Sarcoidosis\n\n **Authors:** Kennedy O. Omonuwa, MD; Arunabh Talwar, MD; Lindsay Goodman, MD \n **Date:** October 31, 2019\n\n ## Content\n\n An eventual diagnosis of pulmonary hypertension was made in this patient with pulmonary sarcoidosis who presented with persistent dyspnea that was refractory to steroid therapy. The loud P2 detected on the cardiac examination, together with the abnormal ECG findings of right ventricular hypertrophy (tall R wave > 6 mm in V1), right-axis deviation, and a right atrial abnormality (P wave > 2.5 mm in lead II and > 1.5 mm in V1) suggestive of right-heart dysfunction were useful clues to the suspicion of complicating pulmonary hypertension in this patient with a known cause (sarcoidosis) of pulmonary hypertension.\nThese discoveries correlated with the 2D echocardiogram findings, which showed a high estimated peak pulmonary artery systolic pressure of 86 mm Hg (normal range, 18-25 mm Hg at rest). The subsequent right-heart catheterization confirmed the diagnosis of pulmonary hypertension (with an elevated mean pulmonary arterial pressure of 55 mm Hg and elevated pulmonary vascular resistance of 11.13 Wood units). Because the PFT results remained unchanged after 6 months, the progressive dyspnea is less likely to be secondary to worsening pulmonary sarcoidosis itself.\nSarcoidosis is a multiorgan disorder of unknown cause characterized by the presence of noncaseating granulomas, most commonly involving the lungs. Sarcoid patients with advanced pulmonary disease, especially end-stage pulmonary fibrosis, are at risk for developing sarcoidosis-associated pulmonary hypertension (SAPH). Among end-stage sarcoid patients awaiting lung transplantation, pulmonary hypertension is documented in approximately 75% of cases, which is predictive of increased mortality. Pulmonary hypertension has also been reported as an early manifestation of sarcoidosis and in patients with sarcoidosis who are not considered candidates for transplant.[1,2]\nPulmonary hypertension is defined as a mean pulmonary arterial pressure greater than 25 mm Hg at rest or 30 mm Hg with exercise. Patients with pulmonary hypertension and pulmonary sarcoidosis have a worse prognosis.\nSAPH is placed in the miscellaneous (Group V) category of the World Health Organization classification of pulmonary hypertension because the mechanism of pulmonary hypertension in sarcoidosis patients is multifactorial. The pathogenesis of SAPH includes fibrotic destruction of the lung vasculature, granulomatous pulmonary angiitis, or extrinsic compression of major pulmonary vessels by mediastinal or hilar adenopathy. In addition to its direct effects on the pulmonary vasculature, left ventricular myocardial infiltration may cause diastolic dysfunction or diminished systolic function and lead to secondary pulmonary hypertension.[1,2]\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 833127,
"choiceText": "Right-sided myocardial infarction",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 833129,
"choiceText": "Pulmonary hypertension",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 833131,
"choiceText": "Pericarditis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 833133,
"choiceText": "Chronic obstructive pulmonary disease",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 262031,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 56-Year-Old Woman With Worsening Dyspnea and Sarcoidosis"
},
{
"authors": "Kennedy O. Omonuwa, MD; Arunabh Talwar, MD; Lindsay Goodman, MD",
"content": [
"The development of pulmonary hypertension in patients with pulmonary sarcoidosis is typically insidious and is associated with minimal symptoms and signs in earlier stages. Dyspnea, especially on exertion, is the most common symptom associated with pulmonary hypertension. Because exertional dyspnea is also a symptom in patients with pulmonary sarcoidosis, the presence of coexisting pulmonary hypertension could easily be overlooked in such patients.",
"Advanced stages of pulmonary hypertension (especially in patients with concomitant significant tricuspid valve regurgitation) are easier to recognize and are associated with such symptoms as leg edema, abdominal bloating and distention, angina, and syncope (symptoms that are indicative of right-sided heart failure). In cases of early pulmonary hypertension in which the tricuspid valve remains competent, abnormal physical findings may not be present. Patients with severe or advanced disease may exhibit such signs as a loud pulmonic valve sound (P2), a systolic murmur of tricuspid regurgitation, or a right ventricular gallop and jugular venous distention resulting from right ventricular failure.[1,2]",
"Because the signs and symptoms of pulmonary sarcoidosis and pulmonary hypertension are nonspecific, the occurrence of progressive dyspnea in an adult with pulmonary sarcoidosis should not be attributed to the underlying disease until other causes have been excluded. Dyspnea is a common symptom, with many differential diagnoses. Most cases of dyspnea are caused by cardiac or pulmonary disease. In patients with a pulmonary disease, consider cardiac causes, such as left-sided heart failure, myocardial infarction, valvular dysfunction, and cardiomyopathy. The clinician should also, in the appropriate clinical setting, consider mixed cardiac and pulmonary disorders, such as interstitial lung disease with pulmonary hypertension, cor pulmonale, and pulmonary emboli.[1,2]",
"The progression of pulmonary sarcoidosis to include pulmonary hypertension may be poorly recognized because the clinical presentation tends to be nonspecific, particularly in the presence of underlying pulmonary parenchymal disease. In patients with pulmonary hypertension, the ECG commonly reveals abnormalities of right ventricular hypertrophy, right-axis deviation, right bundle branch block, and right atrial enlargement. An R wave amplitude in lead V1 of more than 1.2 mV is reportedly 94% sensitive for detecting a systolic mean pulmonary arterial pressure of more than 90 mm Hg. Furthermore, a QRS axis of more than 100° has been found to be highly predictive of right ventricular enlargement, and a pulmonary vascular resistance of more than 5 Wood units is indicative of pulmonary hypertension.[2,3]",
"Chest radiography may reveal signs suggestive of pulmonary hypertension, such as enlarged pulmonary arteries, attenuation of the peripheral pulmonary vasculature, and right ventricular enlargement. These abnormalities may also be appreciated on high-resolution CT scanning, which is commonly required for the evaluation of underlying parenchymal lung disease and mediastinal disorders in patients with pulmonary sarcoidosis.",
"PFTs and assessment of the arterial blood oxygenation are necessary to evaluate the degree of pulmonary functional impairment. Transthoracic echocardiography is an essential, noninvasive test for patients with suspected pulmonary hypertension. Assessment of the velocity of the tricuspid regurgitation jet by Doppler echocardiography can help estimate the peak systolic pulmonary arterial pressure, and 2D echocardiography can assess the presence of associated abnormalities, such as right atrial enlargement, right ventricular enlargement or right or left ventricular dysfunction, valvular disease, and pericardial effusion.[1]",
"Right-heart catheterization is the criterion standard for the diagnosis of pulmonary hypertension. In patients with suspected pulmonary hypertension, right-heart catheterization is required to confirm the presence of pulmonary hypertension by measuring the mean pulmonary arterial pressure, pulmonary vascular resistance, right atrial pressure, cardiac output, and the index. Right-heart catheterization may also determine the severity of pulmonary hypertension, assess the prognosis, and guide initial therapy by vasoreactivity testing.",
"The clinician should consider other causes of pulmonary hypertension and not necessarily assume a causal relationship between pulmonary sarcoidosis and suspected pulmonary hypertension. With that in mind, the clinician should perform other tests as appropriate, such as ventilation/perfusion scanning to evaluate for chronic thromboembolic disease, autoantibody tests for collagen vascular disease, and HIV testing."
],
"date": "October 31, 2019",
"figures": [],
"markdown": "# A 56-Year-Old Woman With Worsening Dyspnea and Sarcoidosis\n\n **Authors:** Kennedy O. Omonuwa, MD; Arunabh Talwar, MD; Lindsay Goodman, MD \n **Date:** October 31, 2019\n\n ## Content\n\n The development of pulmonary hypertension in patients with pulmonary sarcoidosis is typically insidious and is associated with minimal symptoms and signs in earlier stages. Dyspnea, especially on exertion, is the most common symptom associated with pulmonary hypertension. Because exertional dyspnea is also a symptom in patients with pulmonary sarcoidosis, the presence of coexisting pulmonary hypertension could easily be overlooked in such patients.\nAdvanced stages of pulmonary hypertension (especially in patients with concomitant significant tricuspid valve regurgitation) are easier to recognize and are associated with such symptoms as leg edema, abdominal bloating and distention, angina, and syncope (symptoms that are indicative of right-sided heart failure). In cases of early pulmonary hypertension in which the tricuspid valve remains competent, abnormal physical findings may not be present. Patients with severe or advanced disease may exhibit such signs as a loud pulmonic valve sound (P2), a systolic murmur of tricuspid regurgitation, or a right ventricular gallop and jugular venous distention resulting from right ventricular failure.[1,2]\nBecause the signs and symptoms of pulmonary sarcoidosis and pulmonary hypertension are nonspecific, the occurrence of progressive dyspnea in an adult with pulmonary sarcoidosis should not be attributed to the underlying disease until other causes have been excluded. Dyspnea is a common symptom, with many differential diagnoses. Most cases of dyspnea are caused by cardiac or pulmonary disease. In patients with a pulmonary disease, consider cardiac causes, such as left-sided heart failure, myocardial infarction, valvular dysfunction, and cardiomyopathy. The clinician should also, in the appropriate clinical setting, consider mixed cardiac and pulmonary disorders, such as interstitial lung disease with pulmonary hypertension, cor pulmonale, and pulmonary emboli.[1,2]\nThe progression of pulmonary sarcoidosis to include pulmonary hypertension may be poorly recognized because the clinical presentation tends to be nonspecific, particularly in the presence of underlying pulmonary parenchymal disease. In patients with pulmonary hypertension, the ECG commonly reveals abnormalities of right ventricular hypertrophy, right-axis deviation, right bundle branch block, and right atrial enlargement. An R wave amplitude in lead V1 of more than 1.2 mV is reportedly 94% sensitive for detecting a systolic mean pulmonary arterial pressure of more than 90 mm Hg. Furthermore, a QRS axis of more than 100° has been found to be highly predictive of right ventricular enlargement, and a pulmonary vascular resistance of more than 5 Wood units is indicative of pulmonary hypertension.[2,3]\nChest radiography may reveal signs suggestive of pulmonary hypertension, such as enlarged pulmonary arteries, attenuation of the peripheral pulmonary vasculature, and right ventricular enlargement. These abnormalities may also be appreciated on high-resolution CT scanning, which is commonly required for the evaluation of underlying parenchymal lung disease and mediastinal disorders in patients with pulmonary sarcoidosis.\nPFTs and assessment of the arterial blood oxygenation are necessary to evaluate the degree of pulmonary functional impairment. Transthoracic echocardiography is an essential, noninvasive test for patients with suspected pulmonary hypertension. Assessment of the velocity of the tricuspid regurgitation jet by Doppler echocardiography can help estimate the peak systolic pulmonary arterial pressure, and 2D echocardiography can assess the presence of associated abnormalities, such as right atrial enlargement, right ventricular enlargement or right or left ventricular dysfunction, valvular disease, and pericardial effusion.[1]\nRight-heart catheterization is the criterion standard for the diagnosis of pulmonary hypertension. In patients with suspected pulmonary hypertension, right-heart catheterization is required to confirm the presence of pulmonary hypertension by measuring the mean pulmonary arterial pressure, pulmonary vascular resistance, right atrial pressure, cardiac output, and the index. Right-heart catheterization may also determine the severity of pulmonary hypertension, assess the prognosis, and guide initial therapy by vasoreactivity testing.\nThe clinician should consider other causes of pulmonary hypertension and not necessarily assume a causal relationship between pulmonary sarcoidosis and suspected pulmonary hypertension. With that in mind, the clinician should perform other tests as appropriate, such as ventilation/perfusion scanning to evaluate for chronic thromboembolic disease, autoantibody tests for collagen vascular disease, and HIV testing.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 56-Year-Old Woman With Worsening Dyspnea and Sarcoidosis"
},
{
"authors": "Kennedy O. Omonuwa, MD; Arunabh Talwar, MD; Lindsay Goodman, MD",
"content": [
"Treatment of SAPH can be divided into primary therapy and advanced therapy. Primary therapy for SAPH consists of treating the underlying lung disease with, most commonly, corticosteroids and other immunosuppressive agents. Corticosteroid therapy has been shown to improve pulmonary hypertension in some patients with sarcoidosis, even in the absence of parenchymal fibrosis.",
"Advanced therapy is directed at the pulmonary hypertension and includes administration of antiproliferative pharmacologic agents that promote vasodilation. These agents include endothelin receptor antagonists (eg, bosentan), phosphodiesterase-5 inhibitors (eg, sildenafil), and prostanoids (eg, epoprostenol).",
"Lung transplantation should be considered in patients who have progressive or severe SAPH, particularly if the patient does not improve after initiation of advanced therapy.[1,4]",
"Pulmonary hypertension is a predictor of poor outcome in sarcoidosis; hence, mortality increases as the severity of the pulmonary hypertension increases. Physicians should have a high index of suspicion for this complication and should consider echocardiographic screening for pulmonary hypertension in the evaluation of persistent or unexplained dyspnea in patients with pulmonary sarcoidosis or other interstitial lung disease known to cause pulmonary hypertension, especially if the ECG shows findings suggestive of right-heart dysfunction. Those patients with elevated pulmonary artery systolic pressure on echocardiography should then be considered for right-heart catheterization as a means to establish the diagnosis and severity of pulmonary hypertension, and to assess the response to vasodilator therapy. Early identification of pulmonary hypertension in patients with pulmonary sarcoidosis may identify a subgroup of patients who require more aggressive vasodilator therapy rather than standard management of pulmonary hypertension.[1,2,4]",
"The patient in this case was admitted and evaluated by a pulmonary specialist and had right-heart catheterization done, which confirmed the diagnosis of pulmonary hypertension. She was started on bosentan and sildenafil for her pulmonary hypertension and continued to receive oral prednisone for her underlying pulmonary sarcoidosis. She was discharged the next day to follow up with a pulmonary hypertension clinic. After several months of outpatient therapy and monitoring, the patient's dyspnea and exercise tolerance considerably improved."
],
"date": "October 31, 2019",
"figures": [],
"markdown": "# A 56-Year-Old Woman With Worsening Dyspnea and Sarcoidosis\n\n **Authors:** Kennedy O. Omonuwa, MD; Arunabh Talwar, MD; Lindsay Goodman, MD \n **Date:** October 31, 2019\n\n ## Content\n\n Treatment of SAPH can be divided into primary therapy and advanced therapy. Primary therapy for SAPH consists of treating the underlying lung disease with, most commonly, corticosteroids and other immunosuppressive agents. Corticosteroid therapy has been shown to improve pulmonary hypertension in some patients with sarcoidosis, even in the absence of parenchymal fibrosis.\nAdvanced therapy is directed at the pulmonary hypertension and includes administration of antiproliferative pharmacologic agents that promote vasodilation. These agents include endothelin receptor antagonists (eg, bosentan), phosphodiesterase-5 inhibitors (eg, sildenafil), and prostanoids (eg, epoprostenol).\nLung transplantation should be considered in patients who have progressive or severe SAPH, particularly if the patient does not improve after initiation of advanced therapy.[1,4]\nPulmonary hypertension is a predictor of poor outcome in sarcoidosis; hence, mortality increases as the severity of the pulmonary hypertension increases. Physicians should have a high index of suspicion for this complication and should consider echocardiographic screening for pulmonary hypertension in the evaluation of persistent or unexplained dyspnea in patients with pulmonary sarcoidosis or other interstitial lung disease known to cause pulmonary hypertension, especially if the ECG shows findings suggestive of right-heart dysfunction. Those patients with elevated pulmonary artery systolic pressure on echocardiography should then be considered for right-heart catheterization as a means to establish the diagnosis and severity of pulmonary hypertension, and to assess the response to vasodilator therapy. Early identification of pulmonary hypertension in patients with pulmonary sarcoidosis may identify a subgroup of patients who require more aggressive vasodilator therapy rather than standard management of pulmonary hypertension.[1,2,4]\nThe patient in this case was admitted and evaluated by a pulmonary specialist and had right-heart catheterization done, which confirmed the diagnosis of pulmonary hypertension. She was started on bosentan and sildenafil for her pulmonary hypertension and continued to receive oral prednisone for her underlying pulmonary sarcoidosis. She was discharged the next day to follow up with a pulmonary hypertension clinic. After several months of outpatient therapy and monitoring, the patient's dyspnea and exercise tolerance considerably improved.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 833135,
"choiceText": "Chest radiography",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 833137,
"choiceText": "PFTs",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 833139,
"choiceText": "Transthoracic Doppler echocardiography",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 833141,
"choiceText": "Right-heart catheterization",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Although radiography, PFTs, and echocardiography are important examinations for evaluating patients with pulmonary hypertension, right-heart catheterization is the criterion standard for obtaining the diagnosis. In patients with suspected pulmonary hypertension, right-heart catheterization is required to confirm diagnosis by measuring the mean pulmonary arterial pressure, pulmonary vascular resistance, right atrial pressure, cardiac output, and the index. It may also determine the severity of pulmonary hypertension, assess prognosis, and guide initial therapy by vasoreactivity testing.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 262033,
"questionText": "Which of the following diagnostic tests is the criterion standard for establishing the diagnosis of pulmonary hypertension?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 833143,
"choiceText": "Corticosteroids",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 833145,
"choiceText": "Endothelin receptor antagonists",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 833147,
"choiceText": "Lung transplantation",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 833149,
"choiceText": "Long-term antibiotic therapy",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Treatment of SAPH can be divided into primary therapy and advanced therapy. Primary therapy of SAPH consists of treating the underlying lung disease with, most commonly, corticosteroids and other immunosuppressive agents. Advanced therapy is directed at the pulmonary hypertension and includes administration of antiproliferative pharmacologic agents that promote vasodilation. These agents include endothelin receptor antagonists (eg, bosentan), phosphodiesterase-5 inhibitors (eg, sildenafil), and prostanoids (eg, epoprostenol).<br><br>Lung transplantation should be considered in patients who have progressive or severe SAPH, particularly if the patient does not improve after initiation of advanced therapy.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 262035,
"questionText": "Which of the following is <i>not</i> typically part of the treatment for a patient with SAPH?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 56-Year-Old Woman With Worsening Dyspnea and Sarcoidosis"
},
{
"authors": "Kennedy O. Omonuwa, MD; Arunabh Talwar, MD; Lindsay Goodman, MD",
"content": [],
"date": "October 31, 2019",
"figures": [],
"markdown": "# A 56-Year-Old Woman With Worsening Dyspnea and Sarcoidosis\n\n **Authors:** Kennedy O. Omonuwa, MD; Arunabh Talwar, MD; Lindsay Goodman, MD \n **Date:** October 31, 2019\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 833135,
"choiceText": "Chest radiography",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 833137,
"choiceText": "PFTs",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 833139,
"choiceText": "Transthoracic Doppler echocardiography",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 833141,
"choiceText": "Right-heart catheterization",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Although radiography, PFTs, and echocardiography are important examinations for evaluating patients with pulmonary hypertension, right-heart catheterization is the criterion standard for obtaining the diagnosis. In patients with suspected pulmonary hypertension, right-heart catheterization is required to confirm diagnosis by measuring the mean pulmonary arterial pressure, pulmonary vascular resistance, right atrial pressure, cardiac output, and the index. It may also determine the severity of pulmonary hypertension, assess prognosis, and guide initial therapy by vasoreactivity testing.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 262033,
"questionText": "Which of the following diagnostic tests is the criterion standard for establishing the diagnosis of pulmonary hypertension?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 833143,
"choiceText": "Corticosteroids",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 833145,
"choiceText": "Endothelin receptor antagonists",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 833147,
"choiceText": "Lung transplantation",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 833149,
"choiceText": "Long-term antibiotic therapy",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Treatment of SAPH can be divided into primary therapy and advanced therapy. Primary therapy of SAPH consists of treating the underlying lung disease with, most commonly, corticosteroids and other immunosuppressive agents. Advanced therapy is directed at the pulmonary hypertension and includes administration of antiproliferative pharmacologic agents that promote vasodilation. These agents include endothelin receptor antagonists (eg, bosentan), phosphodiesterase-5 inhibitors (eg, sildenafil), and prostanoids (eg, epoprostenol).<br><br>Lung transplantation should be considered in patients who have progressive or severe SAPH, particularly if the patient does not improve after initiation of advanced therapy.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 262035,
"questionText": "Which of the following is <i>not</i> typically part of the treatment for a patient with SAPH?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 56-Year-Old Woman With Worsening Dyspnea and Sarcoidosis"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 833127,
"choiceText": "Right-sided myocardial infarction",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 833129,
"choiceText": "Pulmonary hypertension",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 833131,
"choiceText": "Pericarditis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 833133,
"choiceText": "Chronic obstructive pulmonary disease",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 262031,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 833135,
"choiceText": "Chest radiography",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 833137,
"choiceText": "PFTs",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 833139,
"choiceText": "Transthoracic Doppler echocardiography",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 833141,
"choiceText": "Right-heart catheterization",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Although radiography, PFTs, and echocardiography are important examinations for evaluating patients with pulmonary hypertension, right-heart catheterization is the criterion standard for obtaining the diagnosis. In patients with suspected pulmonary hypertension, right-heart catheterization is required to confirm diagnosis by measuring the mean pulmonary arterial pressure, pulmonary vascular resistance, right atrial pressure, cardiac output, and the index. It may also determine the severity of pulmonary hypertension, assess prognosis, and guide initial therapy by vasoreactivity testing.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 262033,
"questionText": "Which of the following diagnostic tests is the criterion standard for establishing the diagnosis of pulmonary hypertension?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 833143,
"choiceText": "Corticosteroids",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 833145,
"choiceText": "Endothelin receptor antagonists",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 833147,
"choiceText": "Lung transplantation",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 833149,
"choiceText": "Long-term antibiotic therapy",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Treatment of SAPH can be divided into primary therapy and advanced therapy. Primary therapy of SAPH consists of treating the underlying lung disease with, most commonly, corticosteroids and other immunosuppressive agents. Advanced therapy is directed at the pulmonary hypertension and includes administration of antiproliferative pharmacologic agents that promote vasodilation. These agents include endothelin receptor antagonists (eg, bosentan), phosphodiesterase-5 inhibitors (eg, sildenafil), and prostanoids (eg, epoprostenol).<br><br>Lung transplantation should be considered in patients who have progressive or severe SAPH, particularly if the patient does not improve after initiation of advanced therapy.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 262035,
"questionText": "Which of the following is <i>not</i> typically part of the treatment for a patient with SAPH?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
920201 | /viewarticle/920201 | [
{
"authors": "Prashanth Rawla, MD; Jeffrey Pradeep Raj, MD",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"A 65-year-old white man presents to the emergency department with a hugely distended abdomen. He states that his abdomen has been progressively distending over the past 4 days. He describes decreased appetite and nausea but no vomiting. He also describes abdominal discomfort and mild diffuse pain. He states that the last time he had a bowel movement was 5 days ago. He is passing flatus.",
"The patient denies any fever, chills, or chest pain. He mentions that he has difficulty breathing. His medical history is significant for hypothyroidism, for which he is taking levothyroxine. He has also been diagnosed with chronic obstructive pulmonary disease (COPD), for which he uses an albuterol inhaler and a budesonide and formoterol inhaler. He also has hypertension, for which he takes amlodipine, and hyperlipidemia, for which he takes atorvastatin.",
"His past surgical history is significant for a meniscal tear repair in his right knee. The patient reports a 50–pack-year smoking history and denies any significant history of alcohol use or illegal drug use.",
"He was recently admitted to the hospital for a COPD exacerbation and acute bronchitis. He had a prolonged stay in the hospital (7 days) and was treated with intravenous methylprednisolone, levofloxacin, albuterol, budesonide, and arformoterol. He was discharged home on a tapering course of oral prednisone 4 days ago."
],
"date": "October 24, 2019",
"figures": [],
"markdown": "# A 65-Year-Old Man With a Hugely Distended Abdomen\n\n **Authors:** Prashanth Rawla, MD; Jeffrey Pradeep Raj, MD \n **Date:** October 24, 2019\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nA 65-year-old white man presents to the emergency department with a hugely distended abdomen. He states that his abdomen has been progressively distending over the past 4 days. He describes decreased appetite and nausea but no vomiting. He also describes abdominal discomfort and mild diffuse pain. He states that the last time he had a bowel movement was 5 days ago. He is passing flatus.\nThe patient denies any fever, chills, or chest pain. He mentions that he has difficulty breathing. His medical history is significant for hypothyroidism, for which he is taking levothyroxine. He has also been diagnosed with chronic obstructive pulmonary disease (COPD), for which he uses an albuterol inhaler and a budesonide and formoterol inhaler. He also has hypertension, for which he takes amlodipine, and hyperlipidemia, for which he takes atorvastatin.\nHis past surgical history is significant for a meniscal tear repair in his right knee. The patient reports a 50–pack-year smoking history and denies any significant history of alcohol use or illegal drug use.\nHe was recently admitted to the hospital for a COPD exacerbation and acute bronchitis. He had a prolonged stay in the hospital (7 days) and was treated with intravenous methylprednisolone, levofloxacin, albuterol, budesonide, and arformoterol. He was discharged home on a tapering course of oral prednisone 4 days ago.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 65-Year-Old Man With a Hugely Distended Abdomen"
},
{
"authors": "Prashanth Rawla, MD; Jeffrey Pradeep Raj, MD",
"content": [
"Upon arrival to the emergency department, the patient has a temperature of 98.9°F (37.16°C), a heart rate of 104 beats/min, a respiratory rate of 22 breaths/min, blood pressure of 112/84 mm Hg, and 92% oxygen saturation on room air.",
"Upon physical examination, he appears uncomfortable and is lying in the supine position; he has dry oral mucosa. Abdominal examination reveals a very large, distended abdomen with apparent mild diffuse tenderness to palpation and without any rebound tenderness or guarding. Bowel sounds are tympanic. His abdominal pain appears out of proportion to the physical examination findings.",
"His laboratory values are as follows:",
"White blood cell count: 14,000 cells/µL (reference range, 4500-11,000 cells/µL)",
"Hemoglobin level: 13.9 g/dL (reference range, 13.5-17.5 g/dL)",
"Hematocrit concentration: 37% (reference range, 45%-52%)",
"Platelet count: 306,000 cells/µL (reference range, 150,000-450,000 cells/µL)",
"Sodium level: 135 mEq/L (reference range, 135-145 mEq/L)",
"Potassium level: 3.5 mmol/L (reference range, 3.6-5.2 mmol/L)",
"Chloride level: 107 mmol/L (reference range, 98-106 mmol/L)",
"Blood urea nitrogen (BUN) level: 43 g/dL (reference range, 7-20 mg/dL)",
"Creatinine level: 1.51 mg/dL (reference range, 0.6-1.2 mg/dL)",
"Glucose level: 102 mg/dL (reference level, < 100 mg/dL)",
"Aspartate aminotransferase level: 46 U/L (reference range, 10-40 U/L)",
"Alanine aminotransferase level: 41 U/L (reference range, 7-56 U/L)",
"Alkaline phosphatase level: 142 IU/L (reference range, 44-147 IU/L)",
"Amylase level: 25 U/L (reference range, 23-85 U/L)",
"Lipase level: 19 U/L (reference range, 0-160 U/L)",
"Lactic acid level: 0.7 mmol/L (reference range, 0.5-1 mmol/L)",
"All other laboratory values are unremarkable. An abdominal x-ray is obtained. He is admitted to the hospital on the basis of its findings. A subsequent CT scan of the abdomen/pelvis without contrast was obtained. Imaging study findings are shown below (Figures 1-4).",
"Figure 1.",
"Figure 2.",
"Figure 3.",
"Figure 4."
],
"date": "October 24, 2019",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/920/201/920201-Thumb1.png"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/920/201/920201-Thumb2.png"
},
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/920/201/920201-Thumb3.png"
},
{
"caption": "Figure 4.",
"image_url": "https://img.medscapestatic.com/article/920/201/920201-Thumb4.png"
}
],
"markdown": "# A 65-Year-Old Man With a Hugely Distended Abdomen\n\n **Authors:** Prashanth Rawla, MD; Jeffrey Pradeep Raj, MD \n **Date:** October 24, 2019\n\n ## Content\n\n Upon arrival to the emergency department, the patient has a temperature of 98.9°F (37.16°C), a heart rate of 104 beats/min, a respiratory rate of 22 breaths/min, blood pressure of 112/84 mm Hg, and 92% oxygen saturation on room air.\nUpon physical examination, he appears uncomfortable and is lying in the supine position; he has dry oral mucosa. Abdominal examination reveals a very large, distended abdomen with apparent mild diffuse tenderness to palpation and without any rebound tenderness or guarding. Bowel sounds are tympanic. His abdominal pain appears out of proportion to the physical examination findings.\nHis laboratory values are as follows:\nWhite blood cell count: 14,000 cells/µL (reference range, 4500-11,000 cells/µL)\nHemoglobin level: 13.9 g/dL (reference range, 13.5-17.5 g/dL)\nHematocrit concentration: 37% (reference range, 45%-52%)\nPlatelet count: 306,000 cells/µL (reference range, 150,000-450,000 cells/µL)\nSodium level: 135 mEq/L (reference range, 135-145 mEq/L)\nPotassium level: 3.5 mmol/L (reference range, 3.6-5.2 mmol/L)\nChloride level: 107 mmol/L (reference range, 98-106 mmol/L)\nBlood urea nitrogen (BUN) level: 43 g/dL (reference range, 7-20 mg/dL)\nCreatinine level: 1.51 mg/dL (reference range, 0.6-1.2 mg/dL)\nGlucose level: 102 mg/dL (reference level, < 100 mg/dL)\nAspartate aminotransferase level: 46 U/L (reference range, 10-40 U/L)\nAlanine aminotransferase level: 41 U/L (reference range, 7-56 U/L)\nAlkaline phosphatase level: 142 IU/L (reference range, 44-147 IU/L)\nAmylase level: 25 U/L (reference range, 23-85 U/L)\nLipase level: 19 U/L (reference range, 0-160 U/L)\nLactic acid level: 0.7 mmol/L (reference range, 0.5-1 mmol/L)\nAll other laboratory values are unremarkable. An abdominal x-ray is obtained. He is admitted to the hospital on the basis of its findings. A subsequent CT scan of the abdomen/pelvis without contrast was obtained. Imaging study findings are shown below (Figures 1-4).\nFigure 1.\nFigure 2.\nFigure 3.\nFigure 4.\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n**Figure 3.** \n \n\n**Figure 4.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1423200,
"choiceText": "Sigmoid volvulus",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1423202,
"choiceText": "Toxic megacolon",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1423204,
"choiceText": "Acute colonic pseudo-obstruction",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1423206,
"choiceText": "Mesenteric ischemia ",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 454933,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 65-Year-Old Man With a Hugely Distended Abdomen"
},
{
"authors": "Prashanth Rawla, MD; Jeffrey Pradeep Raj, MD",
"content": [
"An acute colonic pseudo-obstruction (ACPO) is also often referred to by its historical eponym, Ogilvie syndrome. It is characterized by severe colonic distension in the absence of any mechanical obstruction.[1] The exact pathophysiology of ACPO is not clearly elucidated.[2] However, a hypothesis suggests that an imbalance in the autonomic nervous system leads to either increased sympathetic tone, decreased parasympathetic tone, or both combined.[3]",
"ACPO has many causes; trauma, serious infections, cardiac diseases (eg, myocardial infarction, congestive heart failure), recent surgery, electrolyte imbalance, and severe hypothyroidism are among the more common causes.[4] This patient is an older man with multiple comorbidities and distended intestines on radiologic imaging studies but with no clinical evidence of mechanical obstruction. Thus, his condition fits well with a diagnosis of ACPO.",
"The incidence of ACPO is approximately 100 per 100,000 cases of hospital admissions, although the condition may be underreported.[5] The prevalence was 0.29% in a study that involved 2703 critically ill patients with burns.[6] In another study conducted among 10,468 patients who underwent hip arthroplasty, the prevalence of ACPO during the postoperative period was once again 0.29%.[7] However, the prevalence may be much higher among patients undergoing major orthopedic surgery, with reported rates of 0.65%-1.3%.[8] Approximately 95% of patients have medical or surgical comorbidities; the remaining cases of ACPO are idiopathic[9] with a male to female ratio of 1.5 to 1.[10] The condition usually affects older people in their sixth decade of life[10]; however, recent studies have shown a rise in the mean age to the seventh or eighth decade of life.[7,11]",
"Patients with ACPO typically have obstructive symptoms that include nausea, vomiting, and abdominal pain. However, this differs from mechanical obstruction by the fact that nearly 40%-50% of patients continue to pass flatus.[12] Upon examination, almost all patients have a distended abdomen of sudden onset and a progressive course, abdominal tenderness, and an empty rectum upon digital examination.[13] No major differences are noted between the symptoms of patients with ACPO and patients with ischemic bowel or perforated bowel except for a higher incidence of fever among patients with the latter conditions.[10] Thus, besides the important masquerades (ie, mechanical intestinal obstruction due to any cause), bowel ischemia, and perforation, other differential diagnoses are also noted. These include acute/chronic/toxic megacolon, Hirschsprung disease, diverticulitis, acute/chronic mesenteric ischemia, and tumors.[14]"
],
"date": "October 24, 2019",
"figures": [],
"markdown": "# A 65-Year-Old Man With a Hugely Distended Abdomen\n\n **Authors:** Prashanth Rawla, MD; Jeffrey Pradeep Raj, MD \n **Date:** October 24, 2019\n\n ## Content\n\n An acute colonic pseudo-obstruction (ACPO) is also often referred to by its historical eponym, Ogilvie syndrome. It is characterized by severe colonic distension in the absence of any mechanical obstruction.[1] The exact pathophysiology of ACPO is not clearly elucidated.[2] However, a hypothesis suggests that an imbalance in the autonomic nervous system leads to either increased sympathetic tone, decreased parasympathetic tone, or both combined.[3]\nACPO has many causes; trauma, serious infections, cardiac diseases (eg, myocardial infarction, congestive heart failure), recent surgery, electrolyte imbalance, and severe hypothyroidism are among the more common causes.[4] This patient is an older man with multiple comorbidities and distended intestines on radiologic imaging studies but with no clinical evidence of mechanical obstruction. Thus, his condition fits well with a diagnosis of ACPO.\nThe incidence of ACPO is approximately 100 per 100,000 cases of hospital admissions, although the condition may be underreported.[5] The prevalence was 0.29% in a study that involved 2703 critically ill patients with burns.[6] In another study conducted among 10,468 patients who underwent hip arthroplasty, the prevalence of ACPO during the postoperative period was once again 0.29%.[7] However, the prevalence may be much higher among patients undergoing major orthopedic surgery, with reported rates of 0.65%-1.3%.[8] Approximately 95% of patients have medical or surgical comorbidities; the remaining cases of ACPO are idiopathic[9] with a male to female ratio of 1.5 to 1.[10] The condition usually affects older people in their sixth decade of life[10]; however, recent studies have shown a rise in the mean age to the seventh or eighth decade of life.[7,11]\nPatients with ACPO typically have obstructive symptoms that include nausea, vomiting, and abdominal pain. However, this differs from mechanical obstruction by the fact that nearly 40%-50% of patients continue to pass flatus.[12] Upon examination, almost all patients have a distended abdomen of sudden onset and a progressive course, abdominal tenderness, and an empty rectum upon digital examination.[13] No major differences are noted between the symptoms of patients with ACPO and patients with ischemic bowel or perforated bowel except for a higher incidence of fever among patients with the latter conditions.[10] Thus, besides the important masquerades (ie, mechanical intestinal obstruction due to any cause), bowel ischemia, and perforation, other differential diagnoses are also noted. These include acute/chronic/toxic megacolon, Hirschsprung disease, diverticulitis, acute/chronic mesenteric ischemia, and tumors.[14]\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1423200,
"choiceText": "Sigmoid volvulus",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1423202,
"choiceText": "Toxic megacolon",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1423204,
"choiceText": "Acute colonic pseudo-obstruction",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1423206,
"choiceText": "Mesenteric ischemia ",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 454933,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 65-Year-Old Man With a Hugely Distended Abdomen"
},
{
"authors": "Prashanth Rawla, MD; Jeffrey Pradeep Raj, MD",
"content": [
"The diagnosis of ACPO is usually a diagnosis of exclusion that largely depends on the history, clinical signs and symptoms, and findings from radiographic studies.[15] The most important diagnostic tool is plain radiography, which reveals a massive colonic dilatation that involves the cecum, ascending colon, and transverse colon. Other ACPO features include well-defined colonic septa, preservation of haustral markings, and a smooth contour of the inner lumen.[1,10] This helps to rule out causes of mechanical obstruction, small bowel obstruction, or frank perforation.[1] The cecum is the most common region for perforation,[16] and plain radiography enables measurement of the cecal diameter; a cecal dilatation of approximately 12 cm is suggestive of an impending perforation.[17] Contrast enema may be used if plain radiography does not rule out mechanical obstruction of the colon. Similarly, a CT scan may help in ruling out perforation, obstruction, or toxic megacolon.[18] Laboratory studies may indicate investigation for leukocytosis, electrolyte imbalance, and renal insufficiency or azotemia.[19]",
"The goals of treatment include pain relief, relief of symptoms associated with gut motility, and nutritional support.[20] Thus, management can be classified as conservative, including supportive care and pharmacologic therapy, colonoscopic decompression, and surgical intervention.[20] Patients are advised to eat nothing by mouth. Once perforation and ischemia are ruled out, any underlying cause such as congestive heart failure, sepsis, or respiratory failure should be aggressively treated.[21] Electrolyte imbalance should be corrected by appropriate intravenous management.[21]",
"Nasogastric decompensation is advised using a nasogastric tube, and drugs that are known to inhibit gastric motility should be reduced or discontinued.[21] In cases of impending perforation, incentive spirometry, intermittent positive pressure ventilation, and airway ventilation should be avoided. Other less effective measures include repeated enemas, use of rectal tubes, and rigid sigmoidoscopy.[22] Pharmacologic therapy represents another important part of conservative management. This involves the use of prokinetic agents (eg, metoclopramide, cisapride)[23] and cholinesterase inhibitors (eg, neostigmine).[24] Cathartic agents such as lactulose, low-dose polyethylene glycol, or daily bisacodyl suppositories to induce rectal emptying can facilitate improvement of symptoms and prevent recurrences.[25]",
"A flexible colonoscopy, on the other hand, serves a diagnostic and therapeutic purpose by ruling out mechanical obstruction, performing colonic decompression, and obtaining biopsy if a colonic mass is present.[4,10] This procedure is safe and effective; a retrospective study concluded that colonoscopic decompression is superior to neostigmine administration.[26] However, the procedure has certain technical difficulties when compared with other elective diagnostic colonoscopies. These include an inability to prepare the bowel before the procedure and subsequent lack of visibility, minimal air insufflation to avoid the risk of further cecal dilatation, and risk of perforation.[27] However, recurrence after a single decompression is common (18%-65%)[10,26,28] and can be decreased by placing indwelling decompression tubes in the proximal colon.[29] However, these catheters can get blocked. An alternative approach is to use serial colonic decompression.[27,28,29]"
],
"date": "October 24, 2019",
"figures": [],
"markdown": "# A 65-Year-Old Man With a Hugely Distended Abdomen\n\n **Authors:** Prashanth Rawla, MD; Jeffrey Pradeep Raj, MD \n **Date:** October 24, 2019\n\n ## Content\n\n The diagnosis of ACPO is usually a diagnosis of exclusion that largely depends on the history, clinical signs and symptoms, and findings from radiographic studies.[15] The most important diagnostic tool is plain radiography, which reveals a massive colonic dilatation that involves the cecum, ascending colon, and transverse colon. Other ACPO features include well-defined colonic septa, preservation of haustral markings, and a smooth contour of the inner lumen.[1,10] This helps to rule out causes of mechanical obstruction, small bowel obstruction, or frank perforation.[1] The cecum is the most common region for perforation,[16] and plain radiography enables measurement of the cecal diameter; a cecal dilatation of approximately 12 cm is suggestive of an impending perforation.[17] Contrast enema may be used if plain radiography does not rule out mechanical obstruction of the colon. Similarly, a CT scan may help in ruling out perforation, obstruction, or toxic megacolon.[18] Laboratory studies may indicate investigation for leukocytosis, electrolyte imbalance, and renal insufficiency or azotemia.[19]\nThe goals of treatment include pain relief, relief of symptoms associated with gut motility, and nutritional support.[20] Thus, management can be classified as conservative, including supportive care and pharmacologic therapy, colonoscopic decompression, and surgical intervention.[20] Patients are advised to eat nothing by mouth. Once perforation and ischemia are ruled out, any underlying cause such as congestive heart failure, sepsis, or respiratory failure should be aggressively treated.[21] Electrolyte imbalance should be corrected by appropriate intravenous management.[21]\nNasogastric decompensation is advised using a nasogastric tube, and drugs that are known to inhibit gastric motility should be reduced or discontinued.[21] In cases of impending perforation, incentive spirometry, intermittent positive pressure ventilation, and airway ventilation should be avoided. Other less effective measures include repeated enemas, use of rectal tubes, and rigid sigmoidoscopy.[22] Pharmacologic therapy represents another important part of conservative management. This involves the use of prokinetic agents (eg, metoclopramide, cisapride)[23] and cholinesterase inhibitors (eg, neostigmine).[24] Cathartic agents such as lactulose, low-dose polyethylene glycol, or daily bisacodyl suppositories to induce rectal emptying can facilitate improvement of symptoms and prevent recurrences.[25]\nA flexible colonoscopy, on the other hand, serves a diagnostic and therapeutic purpose by ruling out mechanical obstruction, performing colonic decompression, and obtaining biopsy if a colonic mass is present.[4,10] This procedure is safe and effective; a retrospective study concluded that colonoscopic decompression is superior to neostigmine administration.[26] However, the procedure has certain technical difficulties when compared with other elective diagnostic colonoscopies. These include an inability to prepare the bowel before the procedure and subsequent lack of visibility, minimal air insufflation to avoid the risk of further cecal dilatation, and risk of perforation.[27] However, recurrence after a single decompression is common (18%-65%)[10,26,28] and can be decreased by placing indwelling decompression tubes in the proximal colon.[29] However, these catheters can get blocked. An alternative approach is to use serial colonic decompression.[27,28,29]\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 65-Year-Old Man With a Hugely Distended Abdomen"
},
{
"authors": "Prashanth Rawla, MD; Jeffrey Pradeep Raj, MD",
"content": [
"The patient in this case was initially treated with conservative measures, including giving him nothing by mouth, intravenous fluid hydration, and placement of a nasogastric tube. He was given neostigmine with initial improvement in his condition; however, his abdominal distention worsened the next day. He underwent colonoscopy with decompression twice during this admission, and his symptoms markedly improved. Colonoscopy showed no signs of mechanical obstruction (Figure 5).",
"Figure 5.",
"In patients with ACPO, surgical intervention is the last resort after conservative management if colonic decompression fails or if clinical signs of perforation, ischemia, or abdominal sepsis are present.[10,30] Tube cecostomy is the procedure of choice if ischemic perforation is not present; the tube may be removed later without another surgical intervention.[16,31] However, a cecal or right colon resection is needed if the cecal wall is thin due to severe dilatation.[10,31]",
"On the other hand, if signs suggest perforation or bowel ischemia, an emergency laparotomy should be performed. The affected segment should be resected, followed by primary anastomosis or a diversion procedure, depending on the presence of perforation, fecal contamination, and the extensiveness of the bowel segment affected.[10,32] Total abdominal colectomy may be the preferred intervention in patients with chronic constipation, colonic pseudo-obstruction, and a transitional zone in the left colon, whereas a subtotal colectomy is recommended in some patients with perforation.[33] If surgery is indicated, precautions include perioperative fluid management sufficient enough to ensure intestinal perfusion and prophylactic intravenous antibiotics.[32,33]",
"A 73-year-old woman with mild Alzheimer disease presents to the emergency department with abdominal distention. She has had constipation for 5 days and progressive abdominal distention for the past 3 days. She also describes anorexia and nausea without vomiting.",
"Upon examination, the patient appears to be in mild distress with abdominal distention. Her vital signs are all within the normal limits. Examination of the abdomen reveals a markedly distended abdomen with diminished bowel sounds and moderate discomfort on palpation of the left side without rebound tenderness or guarding. Her laboratory findings are as follows:",
"Sodium level: 137 mmol/L",
"Potassium level: 2.5 mmol/L",
"Chloride level: 108 mmol/L",
"BUN level: 41 g/dL",
"Creatinine level: 1.10 mg/dL",
"Glucose level: 107 mg/dL",
"CT scanning of the abdomen reveals significant cecal dilatation as large as 11 cm in diameter with no signs of peritonitis or obstruction."
],
"date": "October 24, 2019",
"figures": [
{
"caption": "Figure 5.",
"image_url": "https://img.medscapestatic.com/article/920/201/920201-Thumb5.png"
}
],
"markdown": "# A 65-Year-Old Man With a Hugely Distended Abdomen\n\n **Authors:** Prashanth Rawla, MD; Jeffrey Pradeep Raj, MD \n **Date:** October 24, 2019\n\n ## Content\n\n The patient in this case was initially treated with conservative measures, including giving him nothing by mouth, intravenous fluid hydration, and placement of a nasogastric tube. He was given neostigmine with initial improvement in his condition; however, his abdominal distention worsened the next day. He underwent colonoscopy with decompression twice during this admission, and his symptoms markedly improved. Colonoscopy showed no signs of mechanical obstruction (Figure 5).\nFigure 5.\nIn patients with ACPO, surgical intervention is the last resort after conservative management if colonic decompression fails or if clinical signs of perforation, ischemia, or abdominal sepsis are present.[10,30] Tube cecostomy is the procedure of choice if ischemic perforation is not present; the tube may be removed later without another surgical intervention.[16,31] However, a cecal or right colon resection is needed if the cecal wall is thin due to severe dilatation.[10,31]\nOn the other hand, if signs suggest perforation or bowel ischemia, an emergency laparotomy should be performed. The affected segment should be resected, followed by primary anastomosis or a diversion procedure, depending on the presence of perforation, fecal contamination, and the extensiveness of the bowel segment affected.[10,32] Total abdominal colectomy may be the preferred intervention in patients with chronic constipation, colonic pseudo-obstruction, and a transitional zone in the left colon, whereas a subtotal colectomy is recommended in some patients with perforation.[33] If surgery is indicated, precautions include perioperative fluid management sufficient enough to ensure intestinal perfusion and prophylactic intravenous antibiotics.[32,33]\nA 73-year-old woman with mild Alzheimer disease presents to the emergency department with abdominal distention. She has had constipation for 5 days and progressive abdominal distention for the past 3 days. She also describes anorexia and nausea without vomiting.\nUpon examination, the patient appears to be in mild distress with abdominal distention. Her vital signs are all within the normal limits. Examination of the abdomen reveals a markedly distended abdomen with diminished bowel sounds and moderate discomfort on palpation of the left side without rebound tenderness or guarding. Her laboratory findings are as follows:\nSodium level: 137 mmol/L\nPotassium level: 2.5 mmol/L\nChloride level: 108 mmol/L\nBUN level: 41 g/dL\nCreatinine level: 1.10 mg/dL\nGlucose level: 107 mg/dL\nCT scanning of the abdomen reveals significant cecal dilatation as large as 11 cm in diameter with no signs of peritonitis or obstruction.\n\n ## Figures\n\n **Figure 5.** \n \n\n\n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1423182,
"choiceText": "Colonic decompression",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1423184,
"choiceText": "Exploratory laparotomy",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1423186,
"choiceText": "Rectal tube insertion",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1423188,
"choiceText": "Bowel rest, intravenous fluids replacement, and correction of electrolytic abnormalities",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Initial management of ACPO is usually conservative, with bowel rest and intravenous fluid replacement. Electrolyte imbalances should be corrected. Nasogastric decompensation is advised, and drugs that are known to inhibit gastric motility should be reduced or discontinued. Perforation and ischemia must be ruled out. Any underlying causes such as congestive heart failure, sepsis, electrolyte abnormalities, hypothyroidism, or respiratory failure should be aggressively treated.",
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 454929,
"questionText": "Which of the following is the most appropriate initial management of this patient?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1423190,
"choiceText": "Neostigmine",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1423192,
"choiceText": "Atropine",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1423194,
"choiceText": "Meperidine",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1423196,
"choiceText": "Pyridostigmine\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1423198,
"choiceText": "Metoprolol",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "An acetylcholinesterase inhibitor, neostigmine is used in patients with ACPO who have a cecal diameter greater than 12 cm or in patients who do not respond after 24-48 hours of conservative therapy. Use of neostigmine has shown to shorten the time to colonic decompression and decrease the need for invasive interventions in these patients. Patients who have a partial response or recurrence of symptoms after resolution can be given a second dose, if needed. ",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 454931,
"questionText": "After 48 hours of conservative management, the condition of the patient described in the question above remains the same. Which of the following medications is likely to be useful in the management of this patient?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 65-Year-Old Man With a Hugely Distended Abdomen"
},
{
"authors": "Prashanth Rawla, MD; Jeffrey Pradeep Raj, MD",
"content": [],
"date": "October 24, 2019",
"figures": [],
"markdown": "# A 65-Year-Old Man With a Hugely Distended Abdomen\n\n **Authors:** Prashanth Rawla, MD; Jeffrey Pradeep Raj, MD \n **Date:** October 24, 2019\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1423182,
"choiceText": "Colonic decompression",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1423184,
"choiceText": "Exploratory laparotomy",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1423186,
"choiceText": "Rectal tube insertion",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1423188,
"choiceText": "Bowel rest, intravenous fluids replacement, and correction of electrolytic abnormalities",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Initial management of ACPO is usually conservative, with bowel rest and intravenous fluid replacement. Electrolyte imbalances should be corrected. Nasogastric decompensation is advised, and drugs that are known to inhibit gastric motility should be reduced or discontinued. Perforation and ischemia must be ruled out. Any underlying causes such as congestive heart failure, sepsis, electrolyte abnormalities, hypothyroidism, or respiratory failure should be aggressively treated.",
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 454929,
"questionText": "Which of the following is the most appropriate initial management of this patient?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1423190,
"choiceText": "Neostigmine",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1423192,
"choiceText": "Atropine",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1423194,
"choiceText": "Meperidine",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1423196,
"choiceText": "Pyridostigmine\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1423198,
"choiceText": "Metoprolol",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "An acetylcholinesterase inhibitor, neostigmine is used in patients with ACPO who have a cecal diameter greater than 12 cm or in patients who do not respond after 24-48 hours of conservative therapy. Use of neostigmine has shown to shorten the time to colonic decompression and decrease the need for invasive interventions in these patients. Patients who have a partial response or recurrence of symptoms after resolution can be given a second dose, if needed. ",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 454931,
"questionText": "After 48 hours of conservative management, the condition of the patient described in the question above remains the same. Which of the following medications is likely to be useful in the management of this patient?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 65-Year-Old Man With a Hugely Distended Abdomen"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1423200,
"choiceText": "Sigmoid volvulus",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1423202,
"choiceText": "Toxic megacolon",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1423204,
"choiceText": "Acute colonic pseudo-obstruction",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1423206,
"choiceText": "Mesenteric ischemia ",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 454933,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1423182,
"choiceText": "Colonic decompression",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1423184,
"choiceText": "Exploratory laparotomy",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1423186,
"choiceText": "Rectal tube insertion",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1423188,
"choiceText": "Bowel rest, intravenous fluids replacement, and correction of electrolytic abnormalities",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Initial management of ACPO is usually conservative, with bowel rest and intravenous fluid replacement. Electrolyte imbalances should be corrected. Nasogastric decompensation is advised, and drugs that are known to inhibit gastric motility should be reduced or discontinued. Perforation and ischemia must be ruled out. Any underlying causes such as congestive heart failure, sepsis, electrolyte abnormalities, hypothyroidism, or respiratory failure should be aggressively treated.",
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 454929,
"questionText": "Which of the following is the most appropriate initial management of this patient?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1423190,
"choiceText": "Neostigmine",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1423192,
"choiceText": "Atropine",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1423194,
"choiceText": "Meperidine",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1423196,
"choiceText": "Pyridostigmine\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1423198,
"choiceText": "Metoprolol",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "An acetylcholinesterase inhibitor, neostigmine is used in patients with ACPO who have a cecal diameter greater than 12 cm or in patients who do not respond after 24-48 hours of conservative therapy. Use of neostigmine has shown to shorten the time to colonic decompression and decrease the need for invasive interventions in these patients. Patients who have a partial response or recurrence of symptoms after resolution can be given a second dose, if needed. ",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 454931,
"questionText": "After 48 hours of conservative management, the condition of the patient described in the question above remains the same. Which of the following medications is likely to be useful in the management of this patient?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
835742 | /viewarticle/835742 | [
{
"authors": "Jeffrey A. Toretsky, MD; Stefan Zöllner, MD",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to recognize accurately. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"An 11-year-old girl presents with painful, nontender, right facial swelling. Aside from neonatal gastrointestinal intussusception, she has no history of severe disease and receives no long-term medication. She has multiple allergies, including nuts and hay fever.",
"The child developed the swelling, accompanied by recurrent night fever, 3 weeks before admission. Assuming an acute dental abscess, the attending dentist incised and drained the tissue and prescribed antibiotics.",
"The patient also has intermittent, severe, achy right leg pain that is relieved by acetaminophen; this had been diagnosed as a sprain at an outside emergency center. The mother recalls a trauma during a soccer game some weeks ago. The patient has also been experiencing ongoing fatigue over the past month. No other constitutional signs or symptoms, such as weight loss, night sweats, diarrhea, nausea, or vomiting, are noted. Her family history is unremarkable for cancer. Her brother has just recovered from an acute respiratory infection.",
"Because of progressive facial swelling, mild headache, ongoing fever, and persistent pain, the patient is referred to a pediatric specialist."
],
"date": "October 08, 2019",
"figures": [],
"markdown": "# An 11-Year-Old Girl With Facial Swelling and Leg Pain\n\n **Authors:** Jeffrey A. Toretsky, MD; Stefan Zöllner, MD \n **Date:** October 08, 2019\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to recognize accurately. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nAn 11-year-old girl presents with painful, nontender, right facial swelling. Aside from neonatal gastrointestinal intussusception, she has no history of severe disease and receives no long-term medication. She has multiple allergies, including nuts and hay fever.\nThe child developed the swelling, accompanied by recurrent night fever, 3 weeks before admission. Assuming an acute dental abscess, the attending dentist incised and drained the tissue and prescribed antibiotics.\nThe patient also has intermittent, severe, achy right leg pain that is relieved by acetaminophen; this had been diagnosed as a sprain at an outside emergency center. The mother recalls a trauma during a soccer game some weeks ago. The patient has also been experiencing ongoing fatigue over the past month. No other constitutional signs or symptoms, such as weight loss, night sweats, diarrhea, nausea, or vomiting, are noted. Her family history is unremarkable for cancer. Her brother has just recovered from an acute respiratory infection.\nBecause of progressive facial swelling, mild headache, ongoing fever, and persistent pain, the patient is referred to a pediatric specialist.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "An 11-Year-Old Girl With Facial Swelling and Leg Pain"
},
{
"authors": "Jeffrey A. Toretsky, MD; Stefan Zöllner, MD",
"content": [
"Upon admission, the patient appears well nourished (between the 50th and 75th percentile for age) and has a mild fever (temperature, 100.8°F [38.2°C]), a heart rate of 120 beats/min, and blood pressure of 104/63 mm Hg. Intraoral examination reveals a hard, fixed, expansive mass approximately 3.5 cm in diameter, with slight evidence of dental caries on the right side of the mandible. An example similar to the patient in this case is shown in Figure 1.",
"Figure 1.",
"The overlying mucosa is centrally erythematous, without ulceration. The patient has enlarged tonsils with no exudates. Further examination reveals slight numbness of the skin over the swelling. The right eardrum is injected and bulging. The left eardrum is unremarkable. The cranial nerves are otherwise without deficits.",
"The pupils are symmetric and normally reactive, and the conjunctivae are pale. No cervical, axillary, or inguinal lymph nodes are palpated. Mild systolic murmur is noted on cardiac auscultation, with slight basal pulmonary crackles. Upper and lower extremities are bilaterally normal, apart from a slightly pressure-sensitive hematoma in resorption on the left tibia.",
"Blood examination shows the following:",
"White blood cell count: 9630 cells/µL",
"Hemoglobin level: 8.2 g/dL",
"Hematocrit: 35%",
"Mean corpuscular volume: 75 fL",
"Mean cell hemoglobin level: 29 pg",
"Platelet count: 360,000 cells/µL",
"C-reactive protein level: 5.4 mg/L",
"Erythrocyte sedimentation rate: 25 mm/h",
"Alanine aminotransferase level: 265 U/L",
"Aspartate aminotransferase level: 253 U/L",
"Alkaline phosphatase level: 349 U/L",
"Lactate dehydrogenase level: 1095 U/L",
"Uric acid level: 3.2 mg/dL",
"Maxillofacial CT with contrast reveals an enhancing lytic mass measuring 3.6 cm × 2.7 cm × 4.3 cm in the right mandible, with multiple surrounding lytic areas.",
"The patient undergoes an incisional biopsy, and the material is sent to the pathologist for histopathologic analysis. Microscopically, the specimen shows solid sheets of small round cells, exhibiting scant cytoplasm and round and oval hyperchromatic nuclei. Neither mitotic figures nor necrotic areas are seen. Intracytoplasmic glycogen is demonstrated on periodic acid-Schiff (PAS) staining.",
"Immunohistochemistry results are as follows:",
"Cytokeratin: negative",
"Desmin: negative",
"S-100: positive",
"Vimentin: positive",
"Leukocyte common antigen: negative",
"CD99: positive"
],
"date": "October 08, 2019",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/835/742/835742-thumb-1.jpg"
}
],
"markdown": "# An 11-Year-Old Girl With Facial Swelling and Leg Pain\n\n **Authors:** Jeffrey A. Toretsky, MD; Stefan Zöllner, MD \n **Date:** October 08, 2019\n\n ## Content\n\n Upon admission, the patient appears well nourished (between the 50th and 75th percentile for age) and has a mild fever (temperature, 100.8°F [38.2°C]), a heart rate of 120 beats/min, and blood pressure of 104/63 mm Hg. Intraoral examination reveals a hard, fixed, expansive mass approximately 3.5 cm in diameter, with slight evidence of dental caries on the right side of the mandible. An example similar to the patient in this case is shown in Figure 1.\nFigure 1.\nThe overlying mucosa is centrally erythematous, without ulceration. The patient has enlarged tonsils with no exudates. Further examination reveals slight numbness of the skin over the swelling. The right eardrum is injected and bulging. The left eardrum is unremarkable. The cranial nerves are otherwise without deficits.\nThe pupils are symmetric and normally reactive, and the conjunctivae are pale. No cervical, axillary, or inguinal lymph nodes are palpated. Mild systolic murmur is noted on cardiac auscultation, with slight basal pulmonary crackles. Upper and lower extremities are bilaterally normal, apart from a slightly pressure-sensitive hematoma in resorption on the left tibia.\nBlood examination shows the following:\nWhite blood cell count: 9630 cells/µL\nHemoglobin level: 8.2 g/dL\nHematocrit: 35%\nMean corpuscular volume: 75 fL\nMean cell hemoglobin level: 29 pg\nPlatelet count: 360,000 cells/µL\nC-reactive protein level: 5.4 mg/L\nErythrocyte sedimentation rate: 25 mm/h\nAlanine aminotransferase level: 265 U/L\nAspartate aminotransferase level: 253 U/L\nAlkaline phosphatase level: 349 U/L\nLactate dehydrogenase level: 1095 U/L\nUric acid level: 3.2 mg/dL\nMaxillofacial CT with contrast reveals an enhancing lytic mass measuring 3.6 cm × 2.7 cm × 4.3 cm in the right mandible, with multiple surrounding lytic areas.\nThe patient undergoes an incisional biopsy, and the material is sent to the pathologist for histopathologic analysis. Microscopically, the specimen shows solid sheets of small round cells, exhibiting scant cytoplasm and round and oval hyperchromatic nuclei. Neither mitotic figures nor necrotic areas are seen. Intracytoplasmic glycogen is demonstrated on periodic acid-Schiff (PAS) staining.\nImmunohistochemistry results are as follows:\nCytokeratin: negative\nDesmin: negative\nS-100: positive\nVimentin: positive\nLeukocyte common antigen: negative\nCD99: positive\n\n ## Figures\n\n **Figure 1.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 795119,
"choiceText": "Metastatic neuroblastoma",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 795121,
"choiceText": "Burkitt lymphoma",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 795123,
"choiceText": "Ewing sarcoma",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 795125,
"choiceText": "Disseminated pulmonary mesothelioma",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 795127,
"choiceText": "Osteoblastoma-like osteosarcoma",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 249257,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "An 11-Year-Old Girl With Facial Swelling and Leg Pain"
},
{
"authors": "Jeffrey A. Toretsky, MD; Stefan Zöllner, MD",
"content": [
"Facial swelling is a common clinical problem in the pediatric population. The origins of a facial mass or swelling can vary from congenital causes to acquired conditions (eg, infection) and benign tumors or cancer in soft tissue or bone. The clinical history, a thorough physical examination, and knowledge of the location and clinical characteristics of a lesion are the most important factors in the evaluation of facial swelling to formulate a differential diagnosis and plan further diagnostic procedures.",
"The general clinical manifestations of facial swelling can be classified into four groups: acute swelling with inflammation, nonprogressive swelling, slowly progressive swelling, and rapidly progressive swelling.",
"An inflammatory process is the most common type of facial swelling in childhood; lymphadenitis is its most common cause, followed by sinusitis and odontogenic infection. Nonprogressive midfacial swelling is suggestive of a congenital anomaly (eg, frontoethmoidal cephalocele, nasal glioma, nasal dermoid, epidermoid cyst). Slowly progressive swelling may be secondary to an underlying mass, such as neurofibroma, lymphatic or vascular malformation, or hemangioma; it may also be due to an osseous disease, such as fibrous dysplasia.",
"Rapidly progressive lesions with associated cranial nerve deficits are suggestive of cancer.[1,2] The cancer differential diagnosis for rapidly progressive facial swelling in childhood should include rhabdomyosarcoma, Langerhans cell histiocytosis, metastatic neuroblastoma, lymphoma, and Ewing sarcoma (ES).[3]",
"ES is a highly malignant tumor of the bone and soft tissue that is presumed to be of mesenchymal origin; it is typically diagnosed in the second decade of life.[4,5] Histologically, ES consists of small, round to ovoid hyperchromatic cells with minimal cytoplasm (Figure 2). In 75% of cases, PAS staining results are positive. Overexpression of the surface membrane protein CD99 is a universal feature of ES; although similar to PAS staining, it is not wholly specific (Figure 3).[6,7]",
"Figure 2.",
"Figure 3.",
"Molecular biological examinations are often required to confirm diagnosis. In 95% of ES cases, a balanced EWS/ETS translocation is detected.[8] The chimeric fusion protein functions as a powerful transcription regulator. Despite multimodal treatment including definitive local management, 30% of patients with localized disease and 75%-80% of patients with metastatic disease die within 5 years of diagnosis.[9,10]",
"Primary malignant tumors of the jaws are rare; the diagnosis and treatment of ES in this setting can be challenging. In addition to their rarity, morphologic and immunohistochemical overlap is noted between tumors that most commonly arise in the head and neck region. ES comprises only 1%-9% of tumors at this site. Less than 3% of all ESs originate in the maxillofacial region, generally involving the mandible and less frequently the maxilla.[11,12] Most patients are males aged 5-20 years.",
"Clinical findings of ES in the head and neck regions are nonspecific and usually involve rapid growth, swelling, and pain. Moreover, when the mandible is involved, loosening of teeth, middle-ear infection, and paresthesia are not uncommon.[10,13,14] Systemic symptoms, such as slight or moderate fever that is often remittent, weight loss, and anemia, are occasionally observed and correlate with more advanced or metastatic disease.[15,16,17]"
],
"date": "October 08, 2019",
"figures": [
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/835/742/835742-thumb-2.jpg"
},
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/835/742/835742-thumb-3.jpg"
}
],
"markdown": "# An 11-Year-Old Girl With Facial Swelling and Leg Pain\n\n **Authors:** Jeffrey A. Toretsky, MD; Stefan Zöllner, MD \n **Date:** October 08, 2019\n\n ## Content\n\n Facial swelling is a common clinical problem in the pediatric population. The origins of a facial mass or swelling can vary from congenital causes to acquired conditions (eg, infection) and benign tumors or cancer in soft tissue or bone. The clinical history, a thorough physical examination, and knowledge of the location and clinical characteristics of a lesion are the most important factors in the evaluation of facial swelling to formulate a differential diagnosis and plan further diagnostic procedures.\nThe general clinical manifestations of facial swelling can be classified into four groups: acute swelling with inflammation, nonprogressive swelling, slowly progressive swelling, and rapidly progressive swelling.\nAn inflammatory process is the most common type of facial swelling in childhood; lymphadenitis is its most common cause, followed by sinusitis and odontogenic infection. Nonprogressive midfacial swelling is suggestive of a congenital anomaly (eg, frontoethmoidal cephalocele, nasal glioma, nasal dermoid, epidermoid cyst). Slowly progressive swelling may be secondary to an underlying mass, such as neurofibroma, lymphatic or vascular malformation, or hemangioma; it may also be due to an osseous disease, such as fibrous dysplasia.\nRapidly progressive lesions with associated cranial nerve deficits are suggestive of cancer.[1,2] The cancer differential diagnosis for rapidly progressive facial swelling in childhood should include rhabdomyosarcoma, Langerhans cell histiocytosis, metastatic neuroblastoma, lymphoma, and Ewing sarcoma (ES).[3]\nES is a highly malignant tumor of the bone and soft tissue that is presumed to be of mesenchymal origin; it is typically diagnosed in the second decade of life.[4,5] Histologically, ES consists of small, round to ovoid hyperchromatic cells with minimal cytoplasm (Figure 2). In 75% of cases, PAS staining results are positive. Overexpression of the surface membrane protein CD99 is a universal feature of ES; although similar to PAS staining, it is not wholly specific (Figure 3).[6,7]\nFigure 2.\nFigure 3.\nMolecular biological examinations are often required to confirm diagnosis. In 95% of ES cases, a balanced EWS/ETS translocation is detected.[8] The chimeric fusion protein functions as a powerful transcription regulator. Despite multimodal treatment including definitive local management, 30% of patients with localized disease and 75%-80% of patients with metastatic disease die within 5 years of diagnosis.[9,10]\nPrimary malignant tumors of the jaws are rare; the diagnosis and treatment of ES in this setting can be challenging. In addition to their rarity, morphologic and immunohistochemical overlap is noted between tumors that most commonly arise in the head and neck region. ES comprises only 1%-9% of tumors at this site. Less than 3% of all ESs originate in the maxillofacial region, generally involving the mandible and less frequently the maxilla.[11,12] Most patients are males aged 5-20 years.\nClinical findings of ES in the head and neck regions are nonspecific and usually involve rapid growth, swelling, and pain. Moreover, when the mandible is involved, loosening of teeth, middle-ear infection, and paresthesia are not uncommon.[10,13,14] Systemic symptoms, such as slight or moderate fever that is often remittent, weight loss, and anemia, are occasionally observed and correlate with more advanced or metastatic disease.[15,16,17]\n\n ## Figures\n\n **Figure 2.** \n \n\n**Figure 3.** \n \n\n\n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 795119,
"choiceText": "Metastatic neuroblastoma",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 795121,
"choiceText": "Burkitt lymphoma",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 795123,
"choiceText": "Ewing sarcoma",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 795125,
"choiceText": "Disseminated pulmonary mesothelioma",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 795127,
"choiceText": "Osteoblastoma-like osteosarcoma",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 249257,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "An 11-Year-Old Girl With Facial Swelling and Leg Pain"
},
{
"authors": "Jeffrey A. Toretsky, MD; Stefan Zöllner, MD",
"content": [
"Radiographically, ES appears as a poorly defined osteolytic lesion that may be frequently associated with cortical erosion and a soft-tissue mass adjacent to the destructive site.[18,19] Although ES is most often presents osteolytic change with bone destruction, this aspect is not a pathognomonic feature. The differential diagnosis of indistinct osteolytic lesions with a similar image pattern comprises neuroblastoma, osteogenic sarcoma, Langerhans cell histiocytosis, and osteomyelitis.[1] In addition to conventional radiography, MRI and CT are highly recommended for accurate evaluation of expansion of the lesion before treatment is initiated.[19]",
"In this case, correlating the patient's symptoms to the pattern of differential diagnoses of facial swelling and systemic diseases is important. The rapid development of swelling within weeks, localized paresthesia indicating cranial nerve involvement, and progressive growth despite antibiotic treatment should guide the diagnosis toward cancer. Recurrent mild fever, normochromic and normocytic anemia, and fatigue are further nonspecific signs of an underlying malignant condition, possibly metastasized.[20,21]",
"Fever, malaise, and loss of appetite and weight may mimic septicemia or disseminated neuroblastoma. However, a normotensive patient older than 5 years without abdominal mass, autonomic symptoms, or periorbital ecchymosis due to orbital involvement renders neuroblastoma clinically improbable. Clearly, evaluation of urine catecholamines is crucial to rule out this diagnosis.[22,23] The absence of palpable lymph nodes makes lymphoma unlikely. Still, pain, swelling, and sensory disturbance are clinical signs of intraoral lymphomas.[24]",
"Hodgkin lymphoma presents more commonly in adolescents, whereas non-Hodgkin lymphoma (NHL) predominates in children younger than 10 years. Of all the different types of NHL, endemic Burkitt lymphoma presents more commonly with jaw or facial bone tumors. Sporadic, nonendemic Burkitt lymphoma represents the most common subtype of NHL but usually exhibits an abdominal manifestation, such as bleeding, bowel obstruction, and ascites.[25]",
"The leg pain of this particular patient had to be both included in and excluded from the differential diagnosis. It could have been trauma-induced independent of the facial process, because its nocturnal occurrence and pain relief by nonsteroidal anti-inflammatory drugs might indicate an osteoid osteoma transmitted by arthritis. It may also be caused by systemic lupus erythematosus with anemia and pleural effusion.[26,27,28] It could have also been related to the malignant facial focus, such as metastasis or primary tumor.",
"In the context of osteomyelitis, headache and leg pain could be symptoms of infectious emboli to sinus cavernosus and bone marrow.[29,30] Malignant craniofacial tumors can also cause sinus cavernosus thrombus.[31]",
"In addition, the initial presentation of leg and jaw pain is suggestive of multiple bony lesions due to Langerhans cell histiocytosis.[32,33] Craniofacial lesions are the most frequent lesions at presentation in patients with Langerhans cell histiocytosis; the mandible tends to be affected more common in adults than in children.[34] The elevated liver enzyme levels could indicate the liver involvement of sclerosing cholangitis, which is common in many patients with multisystem Langerhans cell histiocytosis.[35] Abdominal ultrasonography should be performed.",
"In light of the patient's pulmonary crackles and fever, and her brother's infection, radiographic imaging of the chest should be provided to detect any infectious or malignant foci."
],
"date": "October 08, 2019",
"figures": [],
"markdown": "# An 11-Year-Old Girl With Facial Swelling and Leg Pain\n\n **Authors:** Jeffrey A. Toretsky, MD; Stefan Zöllner, MD \n **Date:** October 08, 2019\n\n ## Content\n\n Radiographically, ES appears as a poorly defined osteolytic lesion that may be frequently associated with cortical erosion and a soft-tissue mass adjacent to the destructive site.[18,19] Although ES is most often presents osteolytic change with bone destruction, this aspect is not a pathognomonic feature. The differential diagnosis of indistinct osteolytic lesions with a similar image pattern comprises neuroblastoma, osteogenic sarcoma, Langerhans cell histiocytosis, and osteomyelitis.[1] In addition to conventional radiography, MRI and CT are highly recommended for accurate evaluation of expansion of the lesion before treatment is initiated.[19]\nIn this case, correlating the patient's symptoms to the pattern of differential diagnoses of facial swelling and systemic diseases is important. The rapid development of swelling within weeks, localized paresthesia indicating cranial nerve involvement, and progressive growth despite antibiotic treatment should guide the diagnosis toward cancer. Recurrent mild fever, normochromic and normocytic anemia, and fatigue are further nonspecific signs of an underlying malignant condition, possibly metastasized.[20,21]\nFever, malaise, and loss of appetite and weight may mimic septicemia or disseminated neuroblastoma. However, a normotensive patient older than 5 years without abdominal mass, autonomic symptoms, or periorbital ecchymosis due to orbital involvement renders neuroblastoma clinically improbable. Clearly, evaluation of urine catecholamines is crucial to rule out this diagnosis.[22,23] The absence of palpable lymph nodes makes lymphoma unlikely. Still, pain, swelling, and sensory disturbance are clinical signs of intraoral lymphomas.[24]\nHodgkin lymphoma presents more commonly in adolescents, whereas non-Hodgkin lymphoma (NHL) predominates in children younger than 10 years. Of all the different types of NHL, endemic Burkitt lymphoma presents more commonly with jaw or facial bone tumors. Sporadic, nonendemic Burkitt lymphoma represents the most common subtype of NHL but usually exhibits an abdominal manifestation, such as bleeding, bowel obstruction, and ascites.[25]\nThe leg pain of this particular patient had to be both included in and excluded from the differential diagnosis. It could have been trauma-induced independent of the facial process, because its nocturnal occurrence and pain relief by nonsteroidal anti-inflammatory drugs might indicate an osteoid osteoma transmitted by arthritis. It may also be caused by systemic lupus erythematosus with anemia and pleural effusion.[26,27,28] It could have also been related to the malignant facial focus, such as metastasis or primary tumor.\nIn the context of osteomyelitis, headache and leg pain could be symptoms of infectious emboli to sinus cavernosus and bone marrow.[29,30] Malignant craniofacial tumors can also cause sinus cavernosus thrombus.[31]\nIn addition, the initial presentation of leg and jaw pain is suggestive of multiple bony lesions due to Langerhans cell histiocytosis.[32,33] Craniofacial lesions are the most frequent lesions at presentation in patients with Langerhans cell histiocytosis; the mandible tends to be affected more common in adults than in children.[34] The elevated liver enzyme levels could indicate the liver involvement of sclerosing cholangitis, which is common in many patients with multisystem Langerhans cell histiocytosis.[35] Abdominal ultrasonography should be performed.\nIn light of the patient's pulmonary crackles and fever, and her brother's infection, radiographic imaging of the chest should be provided to detect any infectious or malignant foci.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "An 11-Year-Old Girl With Facial Swelling and Leg Pain"
},
{
"authors": "Jeffrey A. Toretsky, MD; Stefan Zöllner, MD",
"content": [
"In the case of a progressive facial lesion, even in the common setting of odontogenic infection with a presumed underlying abscess that might require drainage, imaging is indicated and should precede any invasive procedures. Often, the image pattern both gives diagnostic hints and also elicits the expansion of a mass, and it is needed to plan subsequent biopsy. Image patterns for osteomyelitis and cancer are similar.[18,36] If the clinical picture is indistinct, drainage specimens should be examined by a pathologist.",
"The clinical examination and imaging diagnostics can narrow the diagnosis only to a certain extent. Therefore, histopathologic examination is required, especially to distinguish among different cancers. In this case, metastatic carcinoma, muscular tumors, and various lymphomas were ruled out owing to negative tests for cytokeratin, desmin, and leukocyte common antigen, respectively. CD99 overexpression and expression of various neural markers, such as vimentin and S-100, are likely features of ES. Meanwhile, neuroblastoma does not express CD99 but is consistently positive for neurospecific enolase and chromogranin A. Rhabdomyosarcoma is often positive for smooth-muscle antigen, desmin, and myogenin.[37] Molecular pathologic examination for the chromosomal translocation using fluorescence in situ hybridization, spectral karyotype, or reverse-transcriptase polymerase chain reaction (RT-PCR) should finally confirm ES.",
"Survival rates for patients with ES of the head and neck seem to be significantly better if complete tumor resection can be performed.[14,38] Even when ES appears to be resectable, neoadjuvant chemotherapy is required to eradicate micrometastatic disease.[39] In the craniofacial area, complete resection may be difficult to achieve without significant cosmetic or functional defects.[40] The combination of ablative and reconstructive surgery within a single procedure is desirable to minimize deformity and overall treatment time and to prevent wound contraction and displacement of bony segments.[41,42]",
"Local control using radiation therapy in selected patients with ES was similar to that seen in surgical series reporting local tumor control rates of 90%-95% at 5 years.[43,44,45] Because use of surgery is often limited to patients with resectable lesions and radiation therapy is reserved for unresectable lesions, a direct comparison between the outcomes of the two modalities might be unjustifiable in craniofacial ES. Large tumors are poorly controlled locally by definitive irradiation, regardless of the radiation dose used; however, patients younger than 14 years might benefit most from radiation therapy as the sole local modality.[45]",
"All patients diagnosed with ES should be included in clinical trials and treated according to current therapeutic regimens. Local control is generally carried out after the sixth cycle of induction chemotherapy, with a preference for surgical intervention with or without additional radiotherapy.",
"In this case, staging procedures revealed no signs of systemic disease, and the patient was started on multiagent chemotherapy, including vincristine, ifosfamide, doxorubicin, and etoposide (according to the EURO-EWING 99 protocol). However, after the first chemotherapeutic cycle, the patient developed fever, neutropenia, and posterior sepsis, which was unsuccessfully treated with systemic antimicrobial therapy. A few days later, her systemic condition rapidly deteriorated and culminated with her death.",
"In conclusion, ES is a rare differential diagnosis of facial swelling in the pediatric setting. The fundamental challenge remains proper diagnosis and its confirmation, for adequate and timely treatment of the patient. Multimodal treatment including neoadjuvant chemotherapy is crucial. The choice of definitive local treatment in the craniofacial setting is debatable and must be adjusted to the individual case."
],
"date": "October 08, 2019",
"figures": [],
"markdown": "# An 11-Year-Old Girl With Facial Swelling and Leg Pain\n\n **Authors:** Jeffrey A. Toretsky, MD; Stefan Zöllner, MD \n **Date:** October 08, 2019\n\n ## Content\n\n In the case of a progressive facial lesion, even in the common setting of odontogenic infection with a presumed underlying abscess that might require drainage, imaging is indicated and should precede any invasive procedures. Often, the image pattern both gives diagnostic hints and also elicits the expansion of a mass, and it is needed to plan subsequent biopsy. Image patterns for osteomyelitis and cancer are similar.[18,36] If the clinical picture is indistinct, drainage specimens should be examined by a pathologist.\nThe clinical examination and imaging diagnostics can narrow the diagnosis only to a certain extent. Therefore, histopathologic examination is required, especially to distinguish among different cancers. In this case, metastatic carcinoma, muscular tumors, and various lymphomas were ruled out owing to negative tests for cytokeratin, desmin, and leukocyte common antigen, respectively. CD99 overexpression and expression of various neural markers, such as vimentin and S-100, are likely features of ES. Meanwhile, neuroblastoma does not express CD99 but is consistently positive for neurospecific enolase and chromogranin A. Rhabdomyosarcoma is often positive for smooth-muscle antigen, desmin, and myogenin.[37] Molecular pathologic examination for the chromosomal translocation using fluorescence in situ hybridization, spectral karyotype, or reverse-transcriptase polymerase chain reaction (RT-PCR) should finally confirm ES.\nSurvival rates for patients with ES of the head and neck seem to be significantly better if complete tumor resection can be performed.[14,38] Even when ES appears to be resectable, neoadjuvant chemotherapy is required to eradicate micrometastatic disease.[39] In the craniofacial area, complete resection may be difficult to achieve without significant cosmetic or functional defects.[40] The combination of ablative and reconstructive surgery within a single procedure is desirable to minimize deformity and overall treatment time and to prevent wound contraction and displacement of bony segments.[41,42]\nLocal control using radiation therapy in selected patients with ES was similar to that seen in surgical series reporting local tumor control rates of 90%-95% at 5 years.[43,44,45] Because use of surgery is often limited to patients with resectable lesions and radiation therapy is reserved for unresectable lesions, a direct comparison between the outcomes of the two modalities might be unjustifiable in craniofacial ES. Large tumors are poorly controlled locally by definitive irradiation, regardless of the radiation dose used; however, patients younger than 14 years might benefit most from radiation therapy as the sole local modality.[45]\nAll patients diagnosed with ES should be included in clinical trials and treated according to current therapeutic regimens. Local control is generally carried out after the sixth cycle of induction chemotherapy, with a preference for surgical intervention with or without additional radiotherapy.\nIn this case, staging procedures revealed no signs of systemic disease, and the patient was started on multiagent chemotherapy, including vincristine, ifosfamide, doxorubicin, and etoposide (according to the EURO-EWING 99 protocol). However, after the first chemotherapeutic cycle, the patient developed fever, neutropenia, and posterior sepsis, which was unsuccessfully treated with systemic antimicrobial therapy. A few days later, her systemic condition rapidly deteriorated and culminated with her death.\nIn conclusion, ES is a rare differential diagnosis of facial swelling in the pediatric setting. The fundamental challenge remains proper diagnosis and its confirmation, for adequate and timely treatment of the patient. Multimodal treatment including neoadjuvant chemotherapy is crucial. The choice of definitive local treatment in the craniofacial setting is debatable and must be adjusted to the individual case.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 795129,
"choiceText": "Osteomyelitis with infectious embolism to sinus cavernosus and bone marrow",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 795131,
"choiceText": "Bone-lytic dental abscess\r\n",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 795133,
"choiceText": "Plunging ranula with secondary infection",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 795135,
"choiceText": "Acute mastoiditis",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 795137,
"choiceText": "Malignant tumor of the mandible",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 795139,
"choiceText": "Heerfordt-Waldenström syndrome",
"correct": false,
"displayOrder": 6,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 795141,
"choiceText": "Systemic lupus erythematosus with anemia, pleural effusion, and temporomandibular arthritis",
"correct": false,
"displayOrder": 7,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<a href=\"http://emedicine.medscape.com/article/2056657-overview\">Acute mastoiditis</a> is the most common complication of acute <a href=\"http://emedicine.medscape.com/article/994656-overview\">otitis media</a>. The diagnosis is based on clinical findings and includes recent symptoms of acute otitis media accompanied by signs of retroauricular inflammation, such as erythema, swelling, or pain, or anteroinferior protrusion of the auricle. The most common pathogens causing acute mastoiditis in children are gram-positive organisms, such as <i>Streptococcus pneumonia</i> or <i>Streptococcus pyogenes</i>.<br>\r\n<br>\r\nAcute mastoiditis should be effectively treated without delay because severe intratemporal and potentially fatal intracranial complications can arise from further spread of the disease. Intravenous antimicrobial medication and myringotomy with microbial sampling are the cornerstones of treatment. In the case described, acute mastoiditis is unlikely, given the patient's history and symptoms.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 249259,
"questionText": "Which of the following is <i>not</i> typically included in the differential diagnosis for a patient such as the one described in this article?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 795143,
"choiceText": "Amplified MYCN",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 795145,
"choiceText": "t(11;22) by RT-PCR",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 795147,
"choiceText": "t(2;13) by RT-PCR",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 795149,
"choiceText": "t(1;13) by RT-PCR",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The <a href=\"http://emedicine.medscape.com/article/990378-overview\">ES</a> family of tumors is defined by the characteristic chromosomal translocation t(11:22) and its fusion protein product EWS-FLI1. The translocation, or a related variant, occurs in 95% of tumors between the central exons of the <i>EWSR1</i> gene (Ewing sarcoma breakpoint region 1; chromosome 22) to the central exons of an ETS family gene, either FLI1 (friend leukemia integration 1; chromosome 11) or ERG (v-ets erythroblastosis virus E26 oncogene homolog; chromosome 21)—t(11;22) and t(21;22), respectively.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 249261,
"questionText": "Which of the following provides definitive support for the diagnosis of ES?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "An 11-Year-Old Girl With Facial Swelling and Leg Pain"
},
{
"authors": "Jeffrey A. Toretsky, MD; Stefan Zöllner, MD",
"content": [],
"date": "October 08, 2019",
"figures": [],
"markdown": "# An 11-Year-Old Girl With Facial Swelling and Leg Pain\n\n **Authors:** Jeffrey A. Toretsky, MD; Stefan Zöllner, MD \n **Date:** October 08, 2019\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 795129,
"choiceText": "Osteomyelitis with infectious embolism to sinus cavernosus and bone marrow",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 795131,
"choiceText": "Bone-lytic dental abscess\r\n",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 795133,
"choiceText": "Plunging ranula with secondary infection",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 795135,
"choiceText": "Acute mastoiditis",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 795137,
"choiceText": "Malignant tumor of the mandible",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 795139,
"choiceText": "Heerfordt-Waldenström syndrome",
"correct": false,
"displayOrder": 6,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 795141,
"choiceText": "Systemic lupus erythematosus with anemia, pleural effusion, and temporomandibular arthritis",
"correct": false,
"displayOrder": 7,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<a href=\"http://emedicine.medscape.com/article/2056657-overview\">Acute mastoiditis</a> is the most common complication of acute <a href=\"http://emedicine.medscape.com/article/994656-overview\">otitis media</a>. The diagnosis is based on clinical findings and includes recent symptoms of acute otitis media accompanied by signs of retroauricular inflammation, such as erythema, swelling, or pain, or anteroinferior protrusion of the auricle. The most common pathogens causing acute mastoiditis in children are gram-positive organisms, such as <i>Streptococcus pneumonia</i> or <i>Streptococcus pyogenes</i>.<br>\r\n<br>\r\nAcute mastoiditis should be effectively treated without delay because severe intratemporal and potentially fatal intracranial complications can arise from further spread of the disease. Intravenous antimicrobial medication and myringotomy with microbial sampling are the cornerstones of treatment. In the case described, acute mastoiditis is unlikely, given the patient's history and symptoms.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 249259,
"questionText": "Which of the following is <i>not</i> typically included in the differential diagnosis for a patient such as the one described in this article?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 795143,
"choiceText": "Amplified MYCN",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 795145,
"choiceText": "t(11;22) by RT-PCR",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 795147,
"choiceText": "t(2;13) by RT-PCR",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 795149,
"choiceText": "t(1;13) by RT-PCR",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The <a href=\"http://emedicine.medscape.com/article/990378-overview\">ES</a> family of tumors is defined by the characteristic chromosomal translocation t(11:22) and its fusion protein product EWS-FLI1. The translocation, or a related variant, occurs in 95% of tumors between the central exons of the <i>EWSR1</i> gene (Ewing sarcoma breakpoint region 1; chromosome 22) to the central exons of an ETS family gene, either FLI1 (friend leukemia integration 1; chromosome 11) or ERG (v-ets erythroblastosis virus E26 oncogene homolog; chromosome 21)—t(11;22) and t(21;22), respectively.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 249261,
"questionText": "Which of the following provides definitive support for the diagnosis of ES?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "An 11-Year-Old Girl With Facial Swelling and Leg Pain"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 795119,
"choiceText": "Metastatic neuroblastoma",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 795121,
"choiceText": "Burkitt lymphoma",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 795123,
"choiceText": "Ewing sarcoma",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 795125,
"choiceText": "Disseminated pulmonary mesothelioma",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 795127,
"choiceText": "Osteoblastoma-like osteosarcoma",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 249257,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 795129,
"choiceText": "Osteomyelitis with infectious embolism to sinus cavernosus and bone marrow",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 795131,
"choiceText": "Bone-lytic dental abscess\r\n",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 795133,
"choiceText": "Plunging ranula with secondary infection",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 795135,
"choiceText": "Acute mastoiditis",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 795137,
"choiceText": "Malignant tumor of the mandible",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 795139,
"choiceText": "Heerfordt-Waldenström syndrome",
"correct": false,
"displayOrder": 6,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 795141,
"choiceText": "Systemic lupus erythematosus with anemia, pleural effusion, and temporomandibular arthritis",
"correct": false,
"displayOrder": 7,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<a href=\"http://emedicine.medscape.com/article/2056657-overview\">Acute mastoiditis</a> is the most common complication of acute <a href=\"http://emedicine.medscape.com/article/994656-overview\">otitis media</a>. The diagnosis is based on clinical findings and includes recent symptoms of acute otitis media accompanied by signs of retroauricular inflammation, such as erythema, swelling, or pain, or anteroinferior protrusion of the auricle. The most common pathogens causing acute mastoiditis in children are gram-positive organisms, such as <i>Streptococcus pneumonia</i> or <i>Streptococcus pyogenes</i>.<br>\r\n<br>\r\nAcute mastoiditis should be effectively treated without delay because severe intratemporal and potentially fatal intracranial complications can arise from further spread of the disease. Intravenous antimicrobial medication and myringotomy with microbial sampling are the cornerstones of treatment. In the case described, acute mastoiditis is unlikely, given the patient's history and symptoms.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 249259,
"questionText": "Which of the following is <i>not</i> typically included in the differential diagnosis for a patient such as the one described in this article?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 795143,
"choiceText": "Amplified MYCN",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 795145,
"choiceText": "t(11;22) by RT-PCR",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 795147,
"choiceText": "t(2;13) by RT-PCR",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 795149,
"choiceText": "t(1;13) by RT-PCR",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The <a href=\"http://emedicine.medscape.com/article/990378-overview\">ES</a> family of tumors is defined by the characteristic chromosomal translocation t(11:22) and its fusion protein product EWS-FLI1. The translocation, or a related variant, occurs in 95% of tumors between the central exons of the <i>EWSR1</i> gene (Ewing sarcoma breakpoint region 1; chromosome 22) to the central exons of an ETS family gene, either FLI1 (friend leukemia integration 1; chromosome 11) or ERG (v-ets erythroblastosis virus E26 oncogene homolog; chromosome 21)—t(11;22) and t(21;22), respectively.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 249261,
"questionText": "Which of the following provides definitive support for the diagnosis of ES?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
919074 | /viewarticle/919074 | [
{
"authors": "F. Gerald Wade, MD; Elie Ghoulam, MD; Thomas Fay, MD; George Vasquez Rios, MD; Kamran Qureshi, MD",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"The patient is a 58-year-old man with a medical history of hypertension, untreated chronic hepatitis C virus (HCV) infection, and end-stage renal disease secondary to hypertensive nephropathy. He was on hemodialysis for 3 years while awaiting kidney transplant. After discussion, he agreed that he would accept a kidney from an HCV-positive donor. He underwent deceased donor kidney transplant within a month of agreeing to accept an HCV-positive organ. His surgery was uncomplicated, and he did not need dialysis posttransplant. He followed up with transplant and nephrology specialists for his posttransplant care. About 3 months after transplant, he was noted to have new elevations in his creatinine levels.",
"The patient reported that he felt generally weaker than usual over the past month and had mild bilateral knee pain. He also has a new rash (Figure 1).",
"Figure 1.",
"The patient denied shortness of breath, chest pain, trouble urinating, frequent urination, dysuria, blood in the urine, back or flank pain, lower-extremity swelling, or confusion. He reported drinking plenty of fluids and denied any recent illness. He reportedly adhered to his prescribed medications. His medications include lisinopril, amlodipine, hydrochlorothiazide, tacrolimus, prednisone, and mycophenolate mofetil. He has no known medication allergies. He denied alcohol, cigarette, or illicit drug use."
],
"date": "October 02, 2019",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/919/074/919074-Thumb1.png"
}
],
"markdown": "# A 58-Year-Old Man With a Rash and Elevated Creatinine Levels\n\n **Authors:** F. Gerald Wade, MD; Elie Ghoulam, MD; Thomas Fay, MD; George Vasquez Rios, MD; Kamran Qureshi, MD \n **Date:** October 02, 2019\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nThe patient is a 58-year-old man with a medical history of hypertension, untreated chronic hepatitis C virus (HCV) infection, and end-stage renal disease secondary to hypertensive nephropathy. He was on hemodialysis for 3 years while awaiting kidney transplant. After discussion, he agreed that he would accept a kidney from an HCV-positive donor. He underwent deceased donor kidney transplant within a month of agreeing to accept an HCV-positive organ. His surgery was uncomplicated, and he did not need dialysis posttransplant. He followed up with transplant and nephrology specialists for his posttransplant care. About 3 months after transplant, he was noted to have new elevations in his creatinine levels.\nThe patient reported that he felt generally weaker than usual over the past month and had mild bilateral knee pain. He also has a new rash (Figure 1).\nFigure 1.\nThe patient denied shortness of breath, chest pain, trouble urinating, frequent urination, dysuria, blood in the urine, back or flank pain, lower-extremity swelling, or confusion. He reported drinking plenty of fluids and denied any recent illness. He reportedly adhered to his prescribed medications. His medications include lisinopril, amlodipine, hydrochlorothiazide, tacrolimus, prednisone, and mycophenolate mofetil. He has no known medication allergies. He denied alcohol, cigarette, or illicit drug use.\n\n ## Figures\n\n **Figure 1.** \n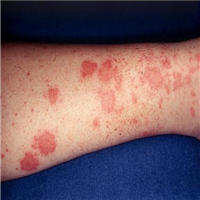 \n\n\n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 58-Year-Old Man With a Rash and Elevated Creatinine Levels"
},
{
"authors": "F. Gerald Wade, MD; Elie Ghoulam, MD; Thomas Fay, MD; George Vasquez Rios, MD; Kamran Qureshi, MD",
"content": [
"On examination, the patient's vital signs were within normal limits. A physical examination revealed normal abdominal findings. His knees had full range of motion bilaterally and were without crepitus or effusion. Lower-extremity examination revealed scattered erythematous, hyperpigmented papules on both legs. Otherwise, physical examination findings were unremarkable.",
"Repeat laboratory assessment was notable for the following:",
"Creatinine level: 2.1 mg/dL (posttransplant creatinine baseline level, 1.36 mg/dL)",
"Blood urea nitrogen level: 22 mg/dL (typical reference range, 7-20 mg/dL)",
"Total protein: 7 g/dL (reference range, 6-8.3 g/dL)",
"Albumin level: 3.2 g/dL (reference range, 3.4-5 g/dL)",
"Dipstick urinalysis with microscopic examination revealed > 100 red blood cells per high-power field and 1+ protein.",
"The following day, the patient's tacrolimus trough level was supratherapeutic, at 11 ng/mL (reference range, 5-10 ng/mL). The tacrolimus dose was adjusted, and the patient was instructed to follow up at a clinic in 1 week. At that visit, the patient's creatinine level had increased to 2.8 mg/dL and his tacrolimus level was 9 ng/mL. In addition, his aspartate aminotransferase and alanine aminotransferase levels were now elevated, at 68 U/L (reference range, 5-34 U/L) and 66 U/L (reference range, 0-55 U/L), respectively.",
"Over the course of another week, while workup for the patient's acute kidney injury and HCV was pursued, his creatinine level continued to rise (3.1 mg/dL). Additional laboratory investigation revealed the following:",
"BK virus: negative",
"Tacrolimus level: 9 ng/mL",
"HCV RNA: 35,591,559 copies/mL",
"HCV genotype: 1a",
"C4 level: 6 mg/dL (reference range, 15-57 mg/dL)",
"C3 level: 86 mg/dL (reference range, 82-193 mg/dL)",
"Antinuclear antibody (Ab): positive",
"Anti-dsDNA Ab: negative",
"Erythrocyte sedimentation rate: 47 mm/h (reference range, 0-30 mm/h)",
"C-reactive protein level: 27 mg/dL (reference range, < 0.5 mg/dL)",
"Rheumatoid factor : 63 IU/mL (reference range, < 14 IU/mL)",
"Anti-RNP Ab: < 10 U (reference range, < 10 U)",
"Antihistone Ab: negative",
"Anti-Smith Ab: negative",
"Renal biopsy of the graft kidney is shown in Figures 2 and 3.",
"Figure 2.",
"Figure 3."
],
"date": "October 02, 2019",
"figures": [
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/919/074/919074-Thumb2.png"
},
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/919/074/919074-Thumb3.png"
}
],
"markdown": "# A 58-Year-Old Man With a Rash and Elevated Creatinine Levels\n\n **Authors:** F. Gerald Wade, MD; Elie Ghoulam, MD; Thomas Fay, MD; George Vasquez Rios, MD; Kamran Qureshi, MD \n **Date:** October 02, 2019\n\n ## Content\n\n On examination, the patient's vital signs were within normal limits. A physical examination revealed normal abdominal findings. His knees had full range of motion bilaterally and were without crepitus or effusion. Lower-extremity examination revealed scattered erythematous, hyperpigmented papules on both legs. Otherwise, physical examination findings were unremarkable.\nRepeat laboratory assessment was notable for the following:\nCreatinine level: 2.1 mg/dL (posttransplant creatinine baseline level, 1.36 mg/dL)\nBlood urea nitrogen level: 22 mg/dL (typical reference range, 7-20 mg/dL)\nTotal protein: 7 g/dL (reference range, 6-8.3 g/dL)\nAlbumin level: 3.2 g/dL (reference range, 3.4-5 g/dL)\nDipstick urinalysis with microscopic examination revealed > 100 red blood cells per high-power field and 1+ protein.\nThe following day, the patient's tacrolimus trough level was supratherapeutic, at 11 ng/mL (reference range, 5-10 ng/mL). The tacrolimus dose was adjusted, and the patient was instructed to follow up at a clinic in 1 week. At that visit, the patient's creatinine level had increased to 2.8 mg/dL and his tacrolimus level was 9 ng/mL. In addition, his aspartate aminotransferase and alanine aminotransferase levels were now elevated, at 68 U/L (reference range, 5-34 U/L) and 66 U/L (reference range, 0-55 U/L), respectively.\nOver the course of another week, while workup for the patient's acute kidney injury and HCV was pursued, his creatinine level continued to rise (3.1 mg/dL). Additional laboratory investigation revealed the following:\nBK virus: negative\nTacrolimus level: 9 ng/mL\nHCV RNA: 35,591,559 copies/mL\nHCV genotype: 1a\nC4 level: 6 mg/dL (reference range, 15-57 mg/dL)\nC3 level: 86 mg/dL (reference range, 82-193 mg/dL)\nAntinuclear antibody (Ab): positive\nAnti-dsDNA Ab: negative\nErythrocyte sedimentation rate: 47 mm/h (reference range, 0-30 mm/h)\nC-reactive protein level: 27 mg/dL (reference range, < 0.5 mg/dL)\nRheumatoid factor : 63 IU/mL (reference range, < 14 IU/mL)\nAnti-RNP Ab: < 10 U (reference range, < 10 U)\nAntihistone Ab: negative\nAnti-Smith Ab: negative\nRenal biopsy of the graft kidney is shown in Figures 2 and 3.\nFigure 2.\nFigure 3.\n\n ## Figures\n\n **Figure 2.** \n \n\n**Figure 3.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1413356,
"choiceText": "Amyloidosis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1413358,
"choiceText": "Cryoglobulinemia ",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1413360,
"choiceText": "Acute rejection",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1413362,
"choiceText": "Tacrolimus toxicity",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1413364,
"choiceText": "Lupus nephritis",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 451489,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 58-Year-Old Man With a Rash and Elevated Creatinine Levels"
},
{
"authors": "F. Gerald Wade, MD; Elie Ghoulam, MD; Thomas Fay, MD; George Vasquez Rios, MD; Kamran Qureshi, MD",
"content": [
"Extrahepatic manifestations of HCV infection are common and increase morbidity and mortality among HCV-infected patients, even in the absence of cirrhosis.[1,2] In addition to the healthcare costs of HCV-associated liver disease, the economic burden of extrahepatic manifestations of HCV was $1.5 billion in 2014.[3] Extrahepatic manifestations of HCV include mixed cryoglobulinemia, Sjögren syndrome, type 2 diabetes, porphyria cutanea tarda, hypothyroidism, lymphoproliferative disorders, and cardiovascular disease, among others.[4,5,6,7,8,9]",
"Chronic HCV infection is highly prevalent in liver and kidney transplant recipients, with an increased incidence of clinically significant extrahepatic manifestations due to concurrent immunosuppression.[10,11,12] Lymphoproliferative disorders and HCV-related kidney disease are serious consequences of untreated HCV posttransplant.[13] Clinically aggressive cases of mixed cryoglobulinemia can lead to rapidly progressive posttransplant renal failure due to immune-complex deposition that damages the glomerulus. HCV infection also further increases the risk for diabetes mellitus in transplant recipients, which can also contribute to progressive renal dysfunction.[10,14]",
"Cryoglobulinemia is most strongly associated with HCV but also occurs less often from hepatitis B virus (HBV), HIV, monoclonal gammopathies, and some autoimmune disorders.[15,16,17,18] Mixed cryoglobulinemia is caused by an immune complex reaction to HCV virion with HCV immunoglobulin G, rheumatoid factor, and complement to form polyclonal cryoglobulins. Immune complex deposition in small-vessel walls and cryoprotein precipitation in vessel lumens lead to cryoglobulinemia syndrome.[19]",
"Patients with HCV commonly have circulating cryoglobulins, with symptomatic disease occurring in about 20% of patients with cryoglobulins in the serum.[16,20] Rates are similar in liver transplant recipients.[21] HCV-associated mixed cryoglobulinemia most often leads to subclinical or mild disease and can present with the Meltzer triad of purpura, arthralgia, and weakness.[22] Aggressive disease course is seen in 15%-20% of patients with HCV who have cryoglobulinemia. Cryoglobulinemia vasculitis syndromes can cause renal disease, digital ischemia, neuropathy, pulmonary disease, gastrointestinal hemorrhage and ischemia, and liver disease.[15] Among such patients, renal failure is the most common cause of death.",
"HCV-cryoglobulinemic renal disease typically presents with microscopic hematuria and mild proteinuria without significant renal dysfunction.[20] Approximately 30% of patients with HCV-associated mixed cryoglobulinemia develop some degree of renal dysfunction, with a small percentage developing fulminant renal failure. Overall mortality is high in patients with mixed cryoglobulinemia and renal insufficiency.[19] Thus, early diagnosis and treatment of HCV in patients with cryoglobulin-related renal disease is important to curb progression of renal dysfunction.[23,24] Patients with HCV who have proteinuria and microscopic hematuria should have renal biopsy to evaluate for cryoglobulinemia or other HCV-related glomerular diseases.[25]"
],
"date": "October 02, 2019",
"figures": [],
"markdown": "# A 58-Year-Old Man With a Rash and Elevated Creatinine Levels\n\n **Authors:** F. Gerald Wade, MD; Elie Ghoulam, MD; Thomas Fay, MD; George Vasquez Rios, MD; Kamran Qureshi, MD \n **Date:** October 02, 2019\n\n ## Content\n\n Extrahepatic manifestations of HCV infection are common and increase morbidity and mortality among HCV-infected patients, even in the absence of cirrhosis.[1,2] In addition to the healthcare costs of HCV-associated liver disease, the economic burden of extrahepatic manifestations of HCV was $1.5 billion in 2014.[3] Extrahepatic manifestations of HCV include mixed cryoglobulinemia, Sjögren syndrome, type 2 diabetes, porphyria cutanea tarda, hypothyroidism, lymphoproliferative disorders, and cardiovascular disease, among others.[4,5,6,7,8,9]\nChronic HCV infection is highly prevalent in liver and kidney transplant recipients, with an increased incidence of clinically significant extrahepatic manifestations due to concurrent immunosuppression.[10,11,12] Lymphoproliferative disorders and HCV-related kidney disease are serious consequences of untreated HCV posttransplant.[13] Clinically aggressive cases of mixed cryoglobulinemia can lead to rapidly progressive posttransplant renal failure due to immune-complex deposition that damages the glomerulus. HCV infection also further increases the risk for diabetes mellitus in transplant recipients, which can also contribute to progressive renal dysfunction.[10,14]\nCryoglobulinemia is most strongly associated with HCV but also occurs less often from hepatitis B virus (HBV), HIV, monoclonal gammopathies, and some autoimmune disorders.[15,16,17,18] Mixed cryoglobulinemia is caused by an immune complex reaction to HCV virion with HCV immunoglobulin G, rheumatoid factor, and complement to form polyclonal cryoglobulins. Immune complex deposition in small-vessel walls and cryoprotein precipitation in vessel lumens lead to cryoglobulinemia syndrome.[19]\nPatients with HCV commonly have circulating cryoglobulins, with symptomatic disease occurring in about 20% of patients with cryoglobulins in the serum.[16,20] Rates are similar in liver transplant recipients.[21] HCV-associated mixed cryoglobulinemia most often leads to subclinical or mild disease and can present with the Meltzer triad of purpura, arthralgia, and weakness.[22] Aggressive disease course is seen in 15%-20% of patients with HCV who have cryoglobulinemia. Cryoglobulinemia vasculitis syndromes can cause renal disease, digital ischemia, neuropathy, pulmonary disease, gastrointestinal hemorrhage and ischemia, and liver disease.[15] Among such patients, renal failure is the most common cause of death.\nHCV-cryoglobulinemic renal disease typically presents with microscopic hematuria and mild proteinuria without significant renal dysfunction.[20] Approximately 30% of patients with HCV-associated mixed cryoglobulinemia develop some degree of renal dysfunction, with a small percentage developing fulminant renal failure. Overall mortality is high in patients with mixed cryoglobulinemia and renal insufficiency.[19] Thus, early diagnosis and treatment of HCV in patients with cryoglobulin-related renal disease is important to curb progression of renal dysfunction.[23,24] Patients with HCV who have proteinuria and microscopic hematuria should have renal biopsy to evaluate for cryoglobulinemia or other HCV-related glomerular diseases.[25]\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1413356,
"choiceText": "Amyloidosis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1413358,
"choiceText": "Cryoglobulinemia ",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1413360,
"choiceText": "Acute rejection",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1413362,
"choiceText": "Tacrolimus toxicity",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1413364,
"choiceText": "Lupus nephritis",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 451489,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 58-Year-Old Man With a Rash and Elevated Creatinine Levels"
},
{
"authors": "F. Gerald Wade, MD; Elie Ghoulam, MD; Thomas Fay, MD; George Vasquez Rios, MD; Kamran Qureshi, MD",
"content": [
"Clinicians should have a high index of suspicion for cryoglobulinemia in patients known to have HCV infection who present with nephritic syndrome, proteinuria, or renal dysfunction.[25] HBV and HIV are also associated with cryoglobulinemia; thus, clinicians also should screen for these viruses in cases of suspected cryoglobulinemia syndromes.[17,18] To test for cryoglobulins, blood is placed in a prewarmed test tube without anticoagulants to prevent false-positive results. It is then cooled to detect precipitation of cryoglobulins.[26] Unfortunately, serologic testing for circulating cryoglobulins is not sensitive for cryoglobulinemic glomerulonephritis, which can obscure the diagnosis. A combination of a positive rheumatoid factor, hypocomplementia with low C4 levels, and typical manifestations of cryoglobulinemia suggests the presence of cryoglobulinemia, even without serologic evidence for cryoprecipitate.[27]",
"Diagnosis of cryoglobulinemia-associated renal disease is confirmed by tissue biopsy with characteristic histologic features of cryoglobulinemia. Light microscopy reveals a membranoproliferative pattern, with mesangial proliferation and hyaline pseudothrombi representing cryoglobulin deposits. Arteriole leukocytoclastic vasculitis and glomerular crescents are often present.[19] Immunofluorescence shows subendothelial and intracapillary deposits with IgM and C3. Electron microscopy reveals a double contour appearance of glomerular basement membrane and foot process effacement. It also reveals subendothelial, luminal, and mesangial deposits, which are typically polyclonal but can sometimes be monoclonal with cylindrical and curvilinear substructure.[19]",
"This patient's clinical triad of weakness, arthralgias, and a new rash, in addition to his initial elevated aminotransferase levels and urinalysis suggestive of nephritis, raised suspicion for HCV-associated mixed cryoglobulinemia. The differential diagnosis for this patient's acute kidney injury was wide, but the main considerations were rejection; tacrolimus toxicity; lupus nephritis; or another HCV-associated vasculitis, such as polyarteritis nodosa or membranoproliferative glomerulonephritis.",
"Suspicion for a vasculitis in this patient was further heightened when his creatinine level continued to rise despite appropriate tacrolimus dose adjustment. Additional laboratory investigation found a low C4 level, positive rheumatoid factor, and negative lupus-associated antibodies. Cryoglobulins were also detected in the patient's serum. Diagnosis was confirmed by renal biopsy. Hematoxylin and eosin stain showed hyaline pseudothrombi (Figure 2). Electron microscopy showed subendothelial, mesangial, and luminal deposits (Figure 3).",
"Figure 2.",
"Figure 3.",
"After successful diagnosis of cryoglobulinemia and/or cryoglobulinemia-associated renal disease, treatment should target the underlying etiology. For mild disease, treatment should focus on eradication of HCV infection. Sustained viral response of HCV reduces proteinuria; improves renal function; and resolves other cryoglobulinemia symptoms, including purpura, neuropathy, and arthralgias.[24,25,28] Treatment of the underlying HCV infection shows benefit for not only mixed cryoglobulinemia but also several other extrahepatic manifestations of HCV infection, including diabetes mellitus, cardiovascular disease, non-Hodgkin lymphoma, and cognitive function.[29,30,31,32] One retrospective study showed that diabetic patients with HCV infection who were treated with interferon gamma and ribavirin had a lower incidence of end-stage renal disease, stroke, and acute coronary syndrome.[30]",
"Moderate or severe cryoglobulinemic vasculitis with rapidly progressing glomerulonephritis, respiratory failure, severe skin ulcers, intestinal vasculitis, or central nervous system involvement requires immunosuppression.[25] HCV treatment is typically delayed until after immunosuppressive therapy is completed. Treatment algorithms for moderate to severe mixed cryoglobulinemia typically include a combination of initial high-dose glucocorticoid therapy with prednisone taper and rituximab.[25] Plasma exchange can be used for patients with life-threatening disease or for those who do not respond to high-dose immunosuppression. Despite these interventions, the prognosis for patients with cryoglobulinemia who present with severe disease and life-threatening organ involvement is poor.[33]"
],
"date": "October 02, 2019",
"figures": [
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/919/074/919074-Thumb2.png"
},
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/919/074/919074-Thumb3.png"
}
],
"markdown": "# A 58-Year-Old Man With a Rash and Elevated Creatinine Levels\n\n **Authors:** F. Gerald Wade, MD; Elie Ghoulam, MD; Thomas Fay, MD; George Vasquez Rios, MD; Kamran Qureshi, MD \n **Date:** October 02, 2019\n\n ## Content\n\n Clinicians should have a high index of suspicion for cryoglobulinemia in patients known to have HCV infection who present with nephritic syndrome, proteinuria, or renal dysfunction.[25] HBV and HIV are also associated with cryoglobulinemia; thus, clinicians also should screen for these viruses in cases of suspected cryoglobulinemia syndromes.[17,18] To test for cryoglobulins, blood is placed in a prewarmed test tube without anticoagulants to prevent false-positive results. It is then cooled to detect precipitation of cryoglobulins.[26] Unfortunately, serologic testing for circulating cryoglobulins is not sensitive for cryoglobulinemic glomerulonephritis, which can obscure the diagnosis. A combination of a positive rheumatoid factor, hypocomplementia with low C4 levels, and typical manifestations of cryoglobulinemia suggests the presence of cryoglobulinemia, even without serologic evidence for cryoprecipitate.[27]\nDiagnosis of cryoglobulinemia-associated renal disease is confirmed by tissue biopsy with characteristic histologic features of cryoglobulinemia. Light microscopy reveals a membranoproliferative pattern, with mesangial proliferation and hyaline pseudothrombi representing cryoglobulin deposits. Arteriole leukocytoclastic vasculitis and glomerular crescents are often present.[19] Immunofluorescence shows subendothelial and intracapillary deposits with IgM and C3. Electron microscopy reveals a double contour appearance of glomerular basement membrane and foot process effacement. It also reveals subendothelial, luminal, and mesangial deposits, which are typically polyclonal but can sometimes be monoclonal with cylindrical and curvilinear substructure.[19]\nThis patient's clinical triad of weakness, arthralgias, and a new rash, in addition to his initial elevated aminotransferase levels and urinalysis suggestive of nephritis, raised suspicion for HCV-associated mixed cryoglobulinemia. The differential diagnosis for this patient's acute kidney injury was wide, but the main considerations were rejection; tacrolimus toxicity; lupus nephritis; or another HCV-associated vasculitis, such as polyarteritis nodosa or membranoproliferative glomerulonephritis.\nSuspicion for a vasculitis in this patient was further heightened when his creatinine level continued to rise despite appropriate tacrolimus dose adjustment. Additional laboratory investigation found a low C4 level, positive rheumatoid factor, and negative lupus-associated antibodies. Cryoglobulins were also detected in the patient's serum. Diagnosis was confirmed by renal biopsy. Hematoxylin and eosin stain showed hyaline pseudothrombi (Figure 2). Electron microscopy showed subendothelial, mesangial, and luminal deposits (Figure 3).\nFigure 2.\nFigure 3.\nAfter successful diagnosis of cryoglobulinemia and/or cryoglobulinemia-associated renal disease, treatment should target the underlying etiology. For mild disease, treatment should focus on eradication of HCV infection. Sustained viral response of HCV reduces proteinuria; improves renal function; and resolves other cryoglobulinemia symptoms, including purpura, neuropathy, and arthralgias.[24,25,28] Treatment of the underlying HCV infection shows benefit for not only mixed cryoglobulinemia but also several other extrahepatic manifestations of HCV infection, including diabetes mellitus, cardiovascular disease, non-Hodgkin lymphoma, and cognitive function.[29,30,31,32] One retrospective study showed that diabetic patients with HCV infection who were treated with interferon gamma and ribavirin had a lower incidence of end-stage renal disease, stroke, and acute coronary syndrome.[30]\nModerate or severe cryoglobulinemic vasculitis with rapidly progressing glomerulonephritis, respiratory failure, severe skin ulcers, intestinal vasculitis, or central nervous system involvement requires immunosuppression.[25] HCV treatment is typically delayed until after immunosuppressive therapy is completed. Treatment algorithms for moderate to severe mixed cryoglobulinemia typically include a combination of initial high-dose glucocorticoid therapy with prednisone taper and rituximab.[25] Plasma exchange can be used for patients with life-threatening disease or for those who do not respond to high-dose immunosuppression. Despite these interventions, the prognosis for patients with cryoglobulinemia who present with severe disease and life-threatening organ involvement is poor.[33]\n\n ## Figures\n\n **Figure 2.** \n \n\n**Figure 3.** \n \n\n\n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 58-Year-Old Man With a Rash and Elevated Creatinine Levels"
},
{
"authors": "F. Gerald Wade, MD; Elie Ghoulam, MD; Thomas Fay, MD; George Vasquez Rios, MD; Kamran Qureshi, MD",
"content": [
"The patient in this case was initially treated with high-dose glucocorticoids and rituximab for his HCV-associated cryoglobulinemic glomerulonephritis. However, his renal function continued to deteriorate after a few days of immunosuppressive therapy; thus, he was also started on plasmapheresis. His creatinine level began to plateau shortly thereafter. As his creatinine level slowly began to improve, paperwork was submitted to his insurance for HCV therapy.",
"He was started on a direct-acting antiviral (DAA) regimen to treat his HCV infection, once his kidney function had stabilized. He completed 12 weeks of ombitasvir, paritaprevir, ritonavir, and dasabuvir combination therapy, with eradication of HCV infection. His liver enzymes normalized, and his creatinine level stabilized around 2 mg/dL. He was continued on immunosuppression with tacrolimus, prednisone, and mycophenolate mofetil.",
"The favorable efficacy and safety profiles of DAA therapy enable more liberal use among nontransplant patients compared with prior interferon therapies.[34] Patients with HCV who receive DAA therapy usually achieve sustained virologic response, which can lead to reduced morbidity, mortality, and nonhepatic HCV complications.[35,36] The decline in liver transplantations for HCV cirrhosis due to DAA therapy has freed up donor livers for other liver diseases, including nonalcoholic steatohepatitis and alcoholic liver disease.[37]",
"DAA therapy is also safe and effective in the posttransplant setting.[38,39] Clearance of HCV after liver transplant is associated with improved outcomes.[40] HCV infection in renal transplant recipients is associated with decreased survival, increased allograft loss, and increased risk for extrahepatic complications compared with patients without HCV infection; thus, clearance of HCV may improve outcomes in this population as well.[41,42] DAA therapy has also expanded the organ donor pool, because HCV-positive recipients can now receive some HCV-positive organs and be cured of HCV posttransplant.[43]",
"In conclusion, HCV infection can lead to significant morbidity and mortality from nonhepatic manifestations, such as mixed cryoglobulinemia. Cryoglobulinemia can present with a clinically aggressive course that requires immunosuppressive therapy. Interferon-free DAA regimens can cure HCV, prevent occurrence of HCV extrahepatic manifestations, and improve outcomes in patients posttransplant."
],
"date": "October 02, 2019",
"figures": [],
"markdown": "# A 58-Year-Old Man With a Rash and Elevated Creatinine Levels\n\n **Authors:** F. Gerald Wade, MD; Elie Ghoulam, MD; Thomas Fay, MD; George Vasquez Rios, MD; Kamran Qureshi, MD \n **Date:** October 02, 2019\n\n ## Content\n\n The patient in this case was initially treated with high-dose glucocorticoids and rituximab for his HCV-associated cryoglobulinemic glomerulonephritis. However, his renal function continued to deteriorate after a few days of immunosuppressive therapy; thus, he was also started on plasmapheresis. His creatinine level began to plateau shortly thereafter. As his creatinine level slowly began to improve, paperwork was submitted to his insurance for HCV therapy.\nHe was started on a direct-acting antiviral (DAA) regimen to treat his HCV infection, once his kidney function had stabilized. He completed 12 weeks of ombitasvir, paritaprevir, ritonavir, and dasabuvir combination therapy, with eradication of HCV infection. His liver enzymes normalized, and his creatinine level stabilized around 2 mg/dL. He was continued on immunosuppression with tacrolimus, prednisone, and mycophenolate mofetil.\nThe favorable efficacy and safety profiles of DAA therapy enable more liberal use among nontransplant patients compared with prior interferon therapies.[34] Patients with HCV who receive DAA therapy usually achieve sustained virologic response, which can lead to reduced morbidity, mortality, and nonhepatic HCV complications.[35,36] The decline in liver transplantations for HCV cirrhosis due to DAA therapy has freed up donor livers for other liver diseases, including nonalcoholic steatohepatitis and alcoholic liver disease.[37]\nDAA therapy is also safe and effective in the posttransplant setting.[38,39] Clearance of HCV after liver transplant is associated with improved outcomes.[40] HCV infection in renal transplant recipients is associated with decreased survival, increased allograft loss, and increased risk for extrahepatic complications compared with patients without HCV infection; thus, clearance of HCV may improve outcomes in this population as well.[41,42] DAA therapy has also expanded the organ donor pool, because HCV-positive recipients can now receive some HCV-positive organs and be cured of HCV posttransplant.[43]\nIn conclusion, HCV infection can lead to significant morbidity and mortality from nonhepatic manifestations, such as mixed cryoglobulinemia. Cryoglobulinemia can present with a clinically aggressive course that requires immunosuppressive therapy. Interferon-free DAA regimens can cure HCV, prevent occurrence of HCV extrahepatic manifestations, and improve outcomes in patients posttransplant.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1413366,
"choiceText": "Steroids with plasma exchange",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1413368,
"choiceText": "DAA therapy",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1413370,
"choiceText": "Rituximab",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1413372,
"choiceText": "Steroids with DAA therapy",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1413374,
"choiceText": "Steroids with rituximab",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "For mild disease, treatment should focus on eradication of HCV. Sustained viral response of HCV reduces proteinuria; improves renal function; and resolves other cryoglobulinemia symptoms, such as purpura, neuropathy, and arthralgias.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 451491,
"questionText": "Which of the following is the most appropriate treatment of HCV-associated cryoglobulinemia in a patient who has developed a rash and glomerulonephritis without acute kidney injury?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1413376,
"choiceText": "Arthralgias, hematuria, and myalgia",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1413378,
"choiceText": "Purpura, hematuria, and fever",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1413380,
"choiceText": "Weakness, purpura, and arthralgia",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1413382,
"choiceText": "Hematuria, abdominal pain, and arthralgia",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1413384,
"choiceText": "Weakness, arthralgia, and fever",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "HCV-associated mixed cryoglobulinemia can present with the Meltzer triad of purpura, arthralgia, and weakness.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 451493,
"questionText": "Which of the following is considered the \"classic\" presentation of cryoglobulinemia?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 58-Year-Old Man With a Rash and Elevated Creatinine Levels"
},
{
"authors": "F. Gerald Wade, MD; Elie Ghoulam, MD; Thomas Fay, MD; George Vasquez Rios, MD; Kamran Qureshi, MD",
"content": [],
"date": "October 02, 2019",
"figures": [],
"markdown": "# A 58-Year-Old Man With a Rash and Elevated Creatinine Levels\n\n **Authors:** F. Gerald Wade, MD; Elie Ghoulam, MD; Thomas Fay, MD; George Vasquez Rios, MD; Kamran Qureshi, MD \n **Date:** October 02, 2019\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1413366,
"choiceText": "Steroids with plasma exchange",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1413368,
"choiceText": "DAA therapy",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1413370,
"choiceText": "Rituximab",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1413372,
"choiceText": "Steroids with DAA therapy",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1413374,
"choiceText": "Steroids with rituximab",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "For mild disease, treatment should focus on eradication of HCV. Sustained viral response of HCV reduces proteinuria; improves renal function; and resolves other cryoglobulinemia symptoms, such as purpura, neuropathy, and arthralgias.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 451491,
"questionText": "Which of the following is the most appropriate treatment of HCV-associated cryoglobulinemia in a patient who has developed a rash and glomerulonephritis without acute kidney injury?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1413376,
"choiceText": "Arthralgias, hematuria, and myalgia",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1413378,
"choiceText": "Purpura, hematuria, and fever",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1413380,
"choiceText": "Weakness, purpura, and arthralgia",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1413382,
"choiceText": "Hematuria, abdominal pain, and arthralgia",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1413384,
"choiceText": "Weakness, arthralgia, and fever",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "HCV-associated mixed cryoglobulinemia can present with the Meltzer triad of purpura, arthralgia, and weakness.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 451493,
"questionText": "Which of the following is considered the \"classic\" presentation of cryoglobulinemia?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 58-Year-Old Man With a Rash and Elevated Creatinine Levels"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1413356,
"choiceText": "Amyloidosis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1413358,
"choiceText": "Cryoglobulinemia ",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1413360,
"choiceText": "Acute rejection",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1413362,
"choiceText": "Tacrolimus toxicity",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1413364,
"choiceText": "Lupus nephritis",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 451489,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1413366,
"choiceText": "Steroids with plasma exchange",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1413368,
"choiceText": "DAA therapy",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1413370,
"choiceText": "Rituximab",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1413372,
"choiceText": "Steroids with DAA therapy",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1413374,
"choiceText": "Steroids with rituximab",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "For mild disease, treatment should focus on eradication of HCV. Sustained viral response of HCV reduces proteinuria; improves renal function; and resolves other cryoglobulinemia symptoms, such as purpura, neuropathy, and arthralgias.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 451491,
"questionText": "Which of the following is the most appropriate treatment of HCV-associated cryoglobulinemia in a patient who has developed a rash and glomerulonephritis without acute kidney injury?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1413376,
"choiceText": "Arthralgias, hematuria, and myalgia",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1413378,
"choiceText": "Purpura, hematuria, and fever",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1413380,
"choiceText": "Weakness, purpura, and arthralgia",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1413382,
"choiceText": "Hematuria, abdominal pain, and arthralgia",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1413384,
"choiceText": "Weakness, arthralgia, and fever",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "HCV-associated mixed cryoglobulinemia can present with the Meltzer triad of purpura, arthralgia, and weakness.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 451493,
"questionText": "Which of the following is considered the \"classic\" presentation of cryoglobulinemia?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
884107 | /viewarticle/884107 | [
{
"authors": "Shatha M. Khatib, MD",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"A 10-year-old boy with a history of multiple fainting spells is brought to an outpatient pediatric clinic by his parents. These spells are sometimes complicated by generalized tonic-clonic \"seizurelike\" activity.",
"The patient first started experiencing these attacks 8 months ago. His mother has noticed that the fainting most often occurs either in the early morning, after the sounding of an alarm clock, or during some type of sports activity. The child states that the seizures occur without warning, and they are sometimes associated with urinary incontinence or vomiting. According to the patient's family, the child remains unconscious for about 1 minute, after which he awakens abruptly, with no evidence of confusion and full recall of all of the events preceding the attack.",
"The patient was born full-term, without any complications. He has no chronic medical conditions and is not on any medications. As a result of experiencing similar symptoms, his father was diagnosed with epilepsy and started on treatment at age 8 years; however, the father has been without treatment and has not had any attacks since age 14 years. The patient's paternal uncle was also diagnosed with epilepsy at age 10 years; he died during a seizure at age 19 years. The patient has an 8-year-old brother who is well, with no history of seizures or fainting spells."
],
"date": "September 25, 2019",
"figures": [],
"markdown": "# A 10-Year-Old Boy With Fainting Spells and Seizure Activity\n\n **Authors:** Shatha M. Khatib, MD \n **Date:** September 25, 2019\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nA 10-year-old boy with a history of multiple fainting spells is brought to an outpatient pediatric clinic by his parents. These spells are sometimes complicated by generalized tonic-clonic \"seizurelike\" activity.\nThe patient first started experiencing these attacks 8 months ago. His mother has noticed that the fainting most often occurs either in the early morning, after the sounding of an alarm clock, or during some type of sports activity. The child states that the seizures occur without warning, and they are sometimes associated with urinary incontinence or vomiting. According to the patient's family, the child remains unconscious for about 1 minute, after which he awakens abruptly, with no evidence of confusion and full recall of all of the events preceding the attack.\nThe patient was born full-term, without any complications. He has no chronic medical conditions and is not on any medications. As a result of experiencing similar symptoms, his father was diagnosed with epilepsy and started on treatment at age 8 years; however, the father has been without treatment and has not had any attacks since age 14 years. The patient's paternal uncle was also diagnosed with epilepsy at age 10 years; he died during a seizure at age 19 years. The patient has an 8-year-old brother who is well, with no history of seizures or fainting spells.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 10-Year-Old Boy With Fainting Spells and Seizure Activity"
},
{
"authors": "Shatha M. Khatib, MD",
"content": [
"Upon physical examination, the patient is a well-appearing and well-developed boy whose weight and height are in the 50th and 60th percentiles, respectively. His oral temperature is 98.6°F (37°C). His pulse is strong at 66 beats/min, with a regular rhythm. His blood pressure is 105/65 mm Hg, and his respiratory rate is 15 breaths/min.",
"Head and neck examination findings are normal. The lungs are clear to auscultation, and normal respiratory effort is noted. Cardiac auscultation reveals normal S1 and S2 heart sounds, and no audible murmurs, rubs, or gallops are heard. His abdomen is soft, with no tenderness. No organomegaly is detected. The neurologic examination reveals intact cranial nerves and intact speech. Sensory and motor functions are normal in all extremities, without any pronator drift. The deep tendon reflexes are brisk and symmetric throughout. The patient's Romberg sign is negative, and his gait is stable.",
"The laboratory analysis, including a complete blood cell count and a basic metabolic panel with serum electrolytes (including calcium and magnesium), is normal. Chest radiography and brain CT findings are also normal. An ECG is obtained (Figure 1).",
"Figure 1."
],
"date": "September 25, 2019",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/884/107/884107-Thumb1.png"
}
],
"markdown": "# A 10-Year-Old Boy With Fainting Spells and Seizure Activity\n\n **Authors:** Shatha M. Khatib, MD \n **Date:** September 25, 2019\n\n ## Content\n\n Upon physical examination, the patient is a well-appearing and well-developed boy whose weight and height are in the 50th and 60th percentiles, respectively. His oral temperature is 98.6°F (37°C). His pulse is strong at 66 beats/min, with a regular rhythm. His blood pressure is 105/65 mm Hg, and his respiratory rate is 15 breaths/min.\nHead and neck examination findings are normal. The lungs are clear to auscultation, and normal respiratory effort is noted. Cardiac auscultation reveals normal S1 and S2 heart sounds, and no audible murmurs, rubs, or gallops are heard. His abdomen is soft, with no tenderness. No organomegaly is detected. The neurologic examination reveals intact cranial nerves and intact speech. Sensory and motor functions are normal in all extremities, without any pronator drift. The deep tendon reflexes are brisk and symmetric throughout. The patient's Romberg sign is negative, and his gait is stable.\nThe laboratory analysis, including a complete blood cell count and a basic metabolic panel with serum electrolytes (including calcium and magnesium), is normal. Chest radiography and brain CT findings are also normal. An ECG is obtained (Figure 1).\nFigure 1.\n\n ## Figures\n\n **Figure 1.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1128132,
"choiceText": "Seizures",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1128134,
"choiceText": "Congenital long QT syndrome",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1128136,
"choiceText": "Brugada syndrome",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1128138,
"choiceText": "Vasovagal syncope",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 357685,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 10-Year-Old Boy With Fainting Spells and Seizure Activity"
},
{
"authors": "Shatha M. Khatib, MD",
"content": [
"The patient's ECG showed a prolonged QT interval (Figure 1). The corrected QT (QTc) was 0.56 second. In addition, a biphasic T wave was seen in the precordial leads. His father's ECG (Figure 2) also showed a prolonged QT interval (QTc, 0.55 second) and a high-amplitude, rounded T wave in leads V2 and V3. On the ECG of the patient's 8-year-old brother (Figure 3), the QTc was 0.52 second, and T/U wave abnormalities in leads V2 and V3 were also detected.",
"Figure 1.",
"Figure 2.",
"Figure 3.",
"In the context of the patient's history of recurrent fainting and the family history of \"seizures\" and sudden death, the prolonged QT intervals noted were very suggestive of intermittent ventricular arrhythmias caused by congenital long QT syndrome (LQTS).",
"LQTS is an electrical disease of the ventricular myocardium that is characterized by prolonged ventricular repolarization, which results in prolongation of the QT interval on the surface ECG and an increased risk for sudden death. It is characteristically associated with the potentially life-threatening cardiac arrhythmia known as torsade de pointes, which is a form of polymorphic ventricular tachycardia.[1]",
"A prolonged QT interval may be acquired (usually resulting from drugs or electrolyte disturbances) or congenital. The congenital form is caused by mutations in the gene coding for the cardiac potassium, sodium, or calcium ion channels; about 400 mutations in 10 gene loci have been identified.[2] The distinct genetic types are designated LQT1-LQT10. LQT1, LQT2, and LQT3 account for over 90% of cases of LQTS, with some estimated prevalences of 45%, 45%, and 7%, respectively.[3] The specific genotype influences the clinical course, the kinds of triggering events that may initiate arrhythmias, the prognosis, and the recommended form of treatment.[3] An underlying genetic predisposition has been identified in some patients with the acquired form of LQTS.",
"Traditionally, congenital LQTS has been characterized as two clinical entities:",
"Romano-Ward syndrome, which is inherited in an autosomal dominant fashion and only has cardiac manifestations.",
"Jervell and Lange-Nielsen syndrome, which is inherited in an autosomal recessive fashion and is associated with sensorineural deafness.",
"Most of the epidemiologic and clinical data on congenital LQTS come from reports from the International LQTS Registry. The registry, which began in 1979 and is still ongoing, is a major source of data on the incidence, natural history, and prognosis of patients with congenital LQTS.[4]",
"The incidence of congenital LQTS is difficult to determine, but it is estimated to be 1 case per 2500-10,000 population, with most estimates around 1 case per 5000 population.[5] The range is broad because a large number of cases go undiagnosed; about 20%-50% of affected patients may not demonstrate QT prolongation on resting ECG. Technical difficulties and methodological controversies in accurately measuring the QT interval are in part to blame. It is nonetheless one of the most common causes of autopsy-negative, unexplained sudden death.",
"Women are more commonly affected than men. Patients with congenital LQTS usually present in childhood, adolescence, or early adulthood, and they usually present with palpitations, syncope or near syncope, seizures, or cardiac arrest. Syncopal episodes associated with secondary seizures may be misdiagnosed as primary seizure disorders. The seizures are probably secondary to hypoperfusion of the brain during arrhythmic events."
],
"date": "September 25, 2019",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/884/107/884107-Thumb1.png"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/884/107/884107-Thumb2.png"
},
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/884/107/884107-Thumb3.png"
}
],
"markdown": "# A 10-Year-Old Boy With Fainting Spells and Seizure Activity\n\n **Authors:** Shatha M. Khatib, MD \n **Date:** September 25, 2019\n\n ## Content\n\n The patient's ECG showed a prolonged QT interval (Figure 1). The corrected QT (QTc) was 0.56 second. In addition, a biphasic T wave was seen in the precordial leads. His father's ECG (Figure 2) also showed a prolonged QT interval (QTc, 0.55 second) and a high-amplitude, rounded T wave in leads V2 and V3. On the ECG of the patient's 8-year-old brother (Figure 3), the QTc was 0.52 second, and T/U wave abnormalities in leads V2 and V3 were also detected.\nFigure 1.\nFigure 2.\nFigure 3.\nIn the context of the patient's history of recurrent fainting and the family history of \"seizures\" and sudden death, the prolonged QT intervals noted were very suggestive of intermittent ventricular arrhythmias caused by congenital long QT syndrome (LQTS).\nLQTS is an electrical disease of the ventricular myocardium that is characterized by prolonged ventricular repolarization, which results in prolongation of the QT interval on the surface ECG and an increased risk for sudden death. It is characteristically associated with the potentially life-threatening cardiac arrhythmia known as torsade de pointes, which is a form of polymorphic ventricular tachycardia.[1]\nA prolonged QT interval may be acquired (usually resulting from drugs or electrolyte disturbances) or congenital. The congenital form is caused by mutations in the gene coding for the cardiac potassium, sodium, or calcium ion channels; about 400 mutations in 10 gene loci have been identified.[2] The distinct genetic types are designated LQT1-LQT10. LQT1, LQT2, and LQT3 account for over 90% of cases of LQTS, with some estimated prevalences of 45%, 45%, and 7%, respectively.[3] The specific genotype influences the clinical course, the kinds of triggering events that may initiate arrhythmias, the prognosis, and the recommended form of treatment.[3] An underlying genetic predisposition has been identified in some patients with the acquired form of LQTS.\nTraditionally, congenital LQTS has been characterized as two clinical entities:\nRomano-Ward syndrome, which is inherited in an autosomal dominant fashion and only has cardiac manifestations.\nJervell and Lange-Nielsen syndrome, which is inherited in an autosomal recessive fashion and is associated with sensorineural deafness.\nMost of the epidemiologic and clinical data on congenital LQTS come from reports from the International LQTS Registry. The registry, which began in 1979 and is still ongoing, is a major source of data on the incidence, natural history, and prognosis of patients with congenital LQTS.[4]\nThe incidence of congenital LQTS is difficult to determine, but it is estimated to be 1 case per 2500-10,000 population, with most estimates around 1 case per 5000 population.[5] The range is broad because a large number of cases go undiagnosed; about 20%-50% of affected patients may not demonstrate QT prolongation on resting ECG. Technical difficulties and methodological controversies in accurately measuring the QT interval are in part to blame. It is nonetheless one of the most common causes of autopsy-negative, unexplained sudden death.\nWomen are more commonly affected than men. Patients with congenital LQTS usually present in childhood, adolescence, or early adulthood, and they usually present with palpitations, syncope or near syncope, seizures, or cardiac arrest. Syncopal episodes associated with secondary seizures may be misdiagnosed as primary seizure disorders. The seizures are probably secondary to hypoperfusion of the brain during arrhythmic events.\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n**Figure 3.** \n \n\n\n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1128132,
"choiceText": "Seizures",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1128134,
"choiceText": "Congenital long QT syndrome",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1128136,
"choiceText": "Brugada syndrome",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1128138,
"choiceText": "Vasovagal syncope",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 357685,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 10-Year-Old Boy With Fainting Spells and Seizure Activity"
},
{
"authors": "Shatha M. Khatib, MD",
"content": [
"Cardiac dysrhythmias can be initiated by an external trigger, such as emotional stress, exercise, or sudden loud noises (ie, an alarm clock or telephone); however, this is not always the case. Ventricular arrhythmias may also occur during sleep, which is commonly seen in patients with the LQT3 genotype. In fact, the kind of triggering event is often linked to the underlying mutation, with certain triggers more commonly associated with certain genotypes.",
"As many as 10% of patients are only diagnosed with LQTS at the time of sudden death.[6] Mortality can be as high as 70% in patients who remain untreated over a 10-year period.[3] This emphasizes the importance of presymptomatic diagnosis and treatment. Important clues indicating that a patient may have LQTS include abnormal ECG findings, a family history of unexplained death, or hearing loss (which is present in around 4% of patients with LQTS).[7]",
"In patients with suspected congenital LQTS, the initial evaluation should be directed at calculating the QTc interval on a resting ECG. The QTc interval is the QT interval corrected for heart rate because, under normal physiologic circumstances, the actual measured QT interval adjusts with the heart rate; in other words, it is longer at slower rates and shorter at faster rates. QTc is calculated by dividing the measured QT by the square root of the R-R interval (the Bazett formula), both of which are measured in seconds.",
"Prolongation of the QTc interval is defined on the basis of the following age-specific and sex-specific criteria (Table)[1]:",
"Table. Definitions of the QTc Interval",
"For practical purposes, any value > 0.45 second should be considered abnormal.",
"To increase accuracy, the QT interval can be manually measured on serial ECGs by using multiple leads, measuring several successive beats, and calculating their average for each ECG.[8] Nevertheless, approximately 10%-15% of patients with congenital LQTS (diagnosed by genetic tests) present with a normal QTc duration[1]; therefore, if suspicion is high despite a normal ECG, Holter monitoring or stress testing may be necessary to try to provoke or unmask the long QTc.",
"Other ECG features that help to establish the diagnosis of congenital LQTS include the following:",
"Abnormal T-wave morphology, including notched or biphasic T waves",
"The presence of T-wave alternans, which is defined as the regular alternation in T wave amplitude or polarity",
"Increased QT dispersion, which is defined as the variability in QT duration among different ECG leads",
"A scoring system for the diagnosis of congenital LQTS was established in 1985 by Schwartz and colleagues[9] and revised in 1993 but still serves as the best guide for clinicians today. It incorporates the ECG criteria (the measured resting QTc interval, history of torsade de pointes, presence of T-wave alternans or notched T wave on ECG, and low heart rate for age), the clinical criteria (syncope or congenital deafness), and family history (family members with definite LQTS or unexplained sudden death at < 30 years of age). Points ranging from 0.5 to 3 are assigned to each of the above criteria, and the points are added to calculate the LQTS score. Depending on the patient's score, the probability of having LQTS is rated as low (< 1 point), intermediate (2-3 points), or high (≥ 4 points). Additional testing, such as cold-water facial immersion or exercise testing, may be applied in patients in whom the diagnosis is still unclear.",
"Genetic testing for congenital LQTS is now available in specialized centers; however, the practical application of genetic testing is limited because of the complexity and heterogeneity of congenital LQTS. In addition, as many as 25% of patients have unknown mutations[10]; therefore, a negative test does not exclude the disease. Once an index case with congenital LQTS is identified, evaluation needs to extend to all first-degree relatives, and treatment must be established where indicated."
],
"date": "September 25, 2019",
"figures": [],
"markdown": "# A 10-Year-Old Boy With Fainting Spells and Seizure Activity\n\n **Authors:** Shatha M. Khatib, MD \n **Date:** September 25, 2019\n\n ## Content\n\n Cardiac dysrhythmias can be initiated by an external trigger, such as emotional stress, exercise, or sudden loud noises (ie, an alarm clock or telephone); however, this is not always the case. Ventricular arrhythmias may also occur during sleep, which is commonly seen in patients with the LQT3 genotype. In fact, the kind of triggering event is often linked to the underlying mutation, with certain triggers more commonly associated with certain genotypes.\nAs many as 10% of patients are only diagnosed with LQTS at the time of sudden death.[6] Mortality can be as high as 70% in patients who remain untreated over a 10-year period.[3] This emphasizes the importance of presymptomatic diagnosis and treatment. Important clues indicating that a patient may have LQTS include abnormal ECG findings, a family history of unexplained death, or hearing loss (which is present in around 4% of patients with LQTS).[7]\nIn patients with suspected congenital LQTS, the initial evaluation should be directed at calculating the QTc interval on a resting ECG. The QTc interval is the QT interval corrected for heart rate because, under normal physiologic circumstances, the actual measured QT interval adjusts with the heart rate; in other words, it is longer at slower rates and shorter at faster rates. QTc is calculated by dividing the measured QT by the square root of the R-R interval (the Bazett formula), both of which are measured in seconds.\nProlongation of the QTc interval is defined on the basis of the following age-specific and sex-specific criteria (Table)[1]:\nTable. Definitions of the QTc Interval\nFor practical purposes, any value > 0.45 second should be considered abnormal.\nTo increase accuracy, the QT interval can be manually measured on serial ECGs by using multiple leads, measuring several successive beats, and calculating their average for each ECG.[8] Nevertheless, approximately 10%-15% of patients with congenital LQTS (diagnosed by genetic tests) present with a normal QTc duration[1]; therefore, if suspicion is high despite a normal ECG, Holter monitoring or stress testing may be necessary to try to provoke or unmask the long QTc.\nOther ECG features that help to establish the diagnosis of congenital LQTS include the following:\nAbnormal T-wave morphology, including notched or biphasic T waves\nThe presence of T-wave alternans, which is defined as the regular alternation in T wave amplitude or polarity\nIncreased QT dispersion, which is defined as the variability in QT duration among different ECG leads\nA scoring system for the diagnosis of congenital LQTS was established in 1985 by Schwartz and colleagues[9] and revised in 1993 but still serves as the best guide for clinicians today. It incorporates the ECG criteria (the measured resting QTc interval, history of torsade de pointes, presence of T-wave alternans or notched T wave on ECG, and low heart rate for age), the clinical criteria (syncope or congenital deafness), and family history (family members with definite LQTS or unexplained sudden death at < 30 years of age). Points ranging from 0.5 to 3 are assigned to each of the above criteria, and the points are added to calculate the LQTS score. Depending on the patient's score, the probability of having LQTS is rated as low (< 1 point), intermediate (2-3 points), or high (≥ 4 points). Additional testing, such as cold-water facial immersion or exercise testing, may be applied in patients in whom the diagnosis is still unclear.\nGenetic testing for congenital LQTS is now available in specialized centers; however, the practical application of genetic testing is limited because of the complexity and heterogeneity of congenital LQTS. In addition, as many as 25% of patients have unknown mutations[10]; therefore, a negative test does not exclude the disease. Once an index case with congenital LQTS is identified, evaluation needs to extend to all first-degree relatives, and treatment must be established where indicated.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 10-Year-Old Boy With Fainting Spells and Seizure Activity"
},
{
"authors": "Shatha M. Khatib, MD",
"content": [
"The guidelines published by the American College of Cardiology, the American Heart Association, and the European Society of Cardiology consider \"lifestyle modifications,\" defined as the contraindication of competitive sports and of all drugs known to prolong the QT interval, as a class I recommendation and an important strategy for the prevention of fatal arrhythmia in patients with congenital LQTS.[11] The mainstay of medical treatment for LQTS is the use of beta-blockers.[12] Beta-blockers shorten the QT interval, which decreases the risk for torsade de pointes arrhythmia and reduces the incidence of syncope and sudden cardiac death. They are effective in approximately 70% of patients.",
"In high-risk patients, implantable cardioverter-defibrillators (ICDs) appear to be the most effective therapy. An ICD may also be considered as a primary therapy if the patient has a strong family history of sudden cardiac death.[13]",
"Another therapeutic measure reserved for patients who continue to have symptoms despite medical therapy and ICD placement is left cervicothoracic sympathectomy (LCTS). LCTS causes sympathetic denervation of the heart and, in some cases, decreases the event rate.[13]",
"Because of the appreciable risk for torsades de pointes arrhythmia and sudden cardiac death without treatment, all symptomatic patients with congenital LQTS should be treated. Treating asymptomatic patients is more controversial; however, because sudden cardiac death can be the first manifestation of LQTS, a safe approach would be to treat even asymptomatic patients with at least medical therapy.[1] Patients who survive a cardiac arrest, those in whom beta-blocker therapy fails, and those with high-risk features based on family history or genetic screening should be considered for an ICD. Any workup for LQTS should be performed in consultation with a cardiologist.",
"Gene-specific therapy is currently an area under investigation, and the management of LQTS is increasingly being guided by gene-specific diagnoses. General recommendations based on knowledge of the patient's genotype that assist in making treatment selections include avoidance of strenuous exercise, stress, and unsupervised swimming or diving in patients with LQT1, because these are common triggers of arrhythmia. Beta-blockers are highly protective in these patients. LQT2 is also induced by exercise, but to a lesser degree than LQT1 is.",
"Among patients with LQT3, events are less commonly induced by exercise; they usually occur during sleep or rest. Beta-blockers are less beneficial in these patients.[3,14] In numerous case reports, mexiletine (a sodium-channel blocker) has been demonstrated to shorten the QT interval in the subgroup of patients with LQT3.",
"In this case, the diagnosis was confirmed by an exertional ECG recording that reproduced torsade de pointes arrhythmia. The patient was started on a beta-blocker and has since remained asymptomatic. Because of the high risk-profile in this family, his younger brother was also started on beta-blocker therapy."
],
"date": "September 25, 2019",
"figures": [],
"markdown": "# A 10-Year-Old Boy With Fainting Spells and Seizure Activity\n\n **Authors:** Shatha M. Khatib, MD \n **Date:** September 25, 2019\n\n ## Content\n\n The guidelines published by the American College of Cardiology, the American Heart Association, and the European Society of Cardiology consider \"lifestyle modifications,\" defined as the contraindication of competitive sports and of all drugs known to prolong the QT interval, as a class I recommendation and an important strategy for the prevention of fatal arrhythmia in patients with congenital LQTS.[11] The mainstay of medical treatment for LQTS is the use of beta-blockers.[12] Beta-blockers shorten the QT interval, which decreases the risk for torsade de pointes arrhythmia and reduces the incidence of syncope and sudden cardiac death. They are effective in approximately 70% of patients.\nIn high-risk patients, implantable cardioverter-defibrillators (ICDs) appear to be the most effective therapy. An ICD may also be considered as a primary therapy if the patient has a strong family history of sudden cardiac death.[13]\nAnother therapeutic measure reserved for patients who continue to have symptoms despite medical therapy and ICD placement is left cervicothoracic sympathectomy (LCTS). LCTS causes sympathetic denervation of the heart and, in some cases, decreases the event rate.[13]\nBecause of the appreciable risk for torsades de pointes arrhythmia and sudden cardiac death without treatment, all symptomatic patients with congenital LQTS should be treated. Treating asymptomatic patients is more controversial; however, because sudden cardiac death can be the first manifestation of LQTS, a safe approach would be to treat even asymptomatic patients with at least medical therapy.[1] Patients who survive a cardiac arrest, those in whom beta-blocker therapy fails, and those with high-risk features based on family history or genetic screening should be considered for an ICD. Any workup for LQTS should be performed in consultation with a cardiologist.\nGene-specific therapy is currently an area under investigation, and the management of LQTS is increasingly being guided by gene-specific diagnoses. General recommendations based on knowledge of the patient's genotype that assist in making treatment selections include avoidance of strenuous exercise, stress, and unsupervised swimming or diving in patients with LQT1, because these are common triggers of arrhythmia. Beta-blockers are highly protective in these patients. LQT2 is also induced by exercise, but to a lesser degree than LQT1 is.\nAmong patients with LQT3, events are less commonly induced by exercise; they usually occur during sleep or rest. Beta-blockers are less beneficial in these patients.[3,14] In numerous case reports, mexiletine (a sodium-channel blocker) has been demonstrated to shorten the QT interval in the subgroup of patients with LQT3.\nIn this case, the diagnosis was confirmed by an exertional ECG recording that reproduced torsade de pointes arrhythmia. The patient was started on a beta-blocker and has since remained asymptomatic. Because of the high risk-profile in this family, his younger brother was also started on beta-blocker therapy.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1128140,
"choiceText": "Congenital sensorineural hearing loss",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1128142,
"choiceText": "Congenital optic atrophy",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1128144,
"choiceText": "Syncopal attacks",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1128146,
"choiceText": "Seizures",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1128148,
"choiceText": "Sudden cardiac death",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Important clues indicating that a patient may have LQTS include abnormal ECG findings, a family history of unexplained death, or hearing loss (which is present in around 4% of patients with LQTS). Patients with congenital LQTS usually present in childhood, adolescence, or early adulthood, and they usually present with palpitations, syncope or near syncope, seizures, or cardiac arrest. LQTS is one of the most common causes of autopsy-negative, unexplained sudden death.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 357687,
"questionText": "Which of the following conditions is <em>not</em> associated with congenital LQTS?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1128150,
"choiceText": "Calcium-channel blockers",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1128152,
"choiceText": "Implantable cardioverter defibrillator",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1128154,
"choiceText": "Amiodarone",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1128156,
"choiceText": "Beta-blockers",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1128158,
"choiceText": "Class 1A antiarrhythmic agents",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The mainstay of therapy in symptomatic and asymptomatic patients with congenital LQTS is beta-blockers. Beta-blockers shorten the QT interval, which decreases the risk for torsade de pointes arrhythmia and reduces the incidence of syncope and sudden cardiac death. They are effective in approximately 70% of patients; however, 30% of patients continue to experience cardiac events. In this group of high-risk patients, ICDs appear to be the most effective therapy. Mexiletine, a sodium-channel blocker, may help in shortening the QT interval in the LQT3 subgroup of patients and improve protection. ",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 357689,
"questionText": "Which of the following choices is the mainstay of therapy for most types of congenital LQTS?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 10-Year-Old Boy With Fainting Spells and Seizure Activity"
},
{
"authors": "Shatha M. Khatib, MD",
"content": [],
"date": "September 25, 2019",
"figures": [],
"markdown": "# A 10-Year-Old Boy With Fainting Spells and Seizure Activity\n\n **Authors:** Shatha M. Khatib, MD \n **Date:** September 25, 2019\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1128140,
"choiceText": "Congenital sensorineural hearing loss",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1128142,
"choiceText": "Congenital optic atrophy",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1128144,
"choiceText": "Syncopal attacks",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1128146,
"choiceText": "Seizures",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1128148,
"choiceText": "Sudden cardiac death",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Important clues indicating that a patient may have LQTS include abnormal ECG findings, a family history of unexplained death, or hearing loss (which is present in around 4% of patients with LQTS). Patients with congenital LQTS usually present in childhood, adolescence, or early adulthood, and they usually present with palpitations, syncope or near syncope, seizures, or cardiac arrest. LQTS is one of the most common causes of autopsy-negative, unexplained sudden death.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 357687,
"questionText": "Which of the following conditions is <em>not</em> associated with congenital LQTS?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1128150,
"choiceText": "Calcium-channel blockers",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1128152,
"choiceText": "Implantable cardioverter defibrillator",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1128154,
"choiceText": "Amiodarone",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1128156,
"choiceText": "Beta-blockers",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1128158,
"choiceText": "Class 1A antiarrhythmic agents",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The mainstay of therapy in symptomatic and asymptomatic patients with congenital LQTS is beta-blockers. Beta-blockers shorten the QT interval, which decreases the risk for torsade de pointes arrhythmia and reduces the incidence of syncope and sudden cardiac death. They are effective in approximately 70% of patients; however, 30% of patients continue to experience cardiac events. In this group of high-risk patients, ICDs appear to be the most effective therapy. Mexiletine, a sodium-channel blocker, may help in shortening the QT interval in the LQT3 subgroup of patients and improve protection. ",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 357689,
"questionText": "Which of the following choices is the mainstay of therapy for most types of congenital LQTS?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 10-Year-Old Boy With Fainting Spells and Seizure Activity"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1128132,
"choiceText": "Seizures",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1128134,
"choiceText": "Congenital long QT syndrome",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1128136,
"choiceText": "Brugada syndrome",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1128138,
"choiceText": "Vasovagal syncope",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 357685,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1128140,
"choiceText": "Congenital sensorineural hearing loss",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1128142,
"choiceText": "Congenital optic atrophy",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1128144,
"choiceText": "Syncopal attacks",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1128146,
"choiceText": "Seizures",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1128148,
"choiceText": "Sudden cardiac death",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Important clues indicating that a patient may have LQTS include abnormal ECG findings, a family history of unexplained death, or hearing loss (which is present in around 4% of patients with LQTS). Patients with congenital LQTS usually present in childhood, adolescence, or early adulthood, and they usually present with palpitations, syncope or near syncope, seizures, or cardiac arrest. LQTS is one of the most common causes of autopsy-negative, unexplained sudden death.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 357687,
"questionText": "Which of the following conditions is <em>not</em> associated with congenital LQTS?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1128150,
"choiceText": "Calcium-channel blockers",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1128152,
"choiceText": "Implantable cardioverter defibrillator",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1128154,
"choiceText": "Amiodarone",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1128156,
"choiceText": "Beta-blockers",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1128158,
"choiceText": "Class 1A antiarrhythmic agents",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The mainstay of therapy in symptomatic and asymptomatic patients with congenital LQTS is beta-blockers. Beta-blockers shorten the QT interval, which decreases the risk for torsade de pointes arrhythmia and reduces the incidence of syncope and sudden cardiac death. They are effective in approximately 70% of patients; however, 30% of patients continue to experience cardiac events. In this group of high-risk patients, ICDs appear to be the most effective therapy. Mexiletine, a sodium-channel blocker, may help in shortening the QT interval in the LQT3 subgroup of patients and improve protection. ",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 357689,
"questionText": "Which of the following choices is the mainstay of therapy for most types of congenital LQTS?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
918183 | /viewarticle/918183 | [
{
"authors": "Ricardo Correa, MD; Gauri Behari, MD",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"A 26-year-old woman with a medical history of calcium pyrophosphate deposition disease presents with severe knee pain. She now requires a cane for walking, owing to hip and knee pain. She also has abnormal dentition with tooth loss that required dental implants a few years ago. For 2 years, she has had a nonhealing small avulsion fracture in her right first metatarsal, which is confirmed by radiography.",
"Upon physical examination, the patient is alert, awake, and oriented. Dental implants in the frontal area are noted. Cardiopulmonary examination reveals regular rate and rhythm, with no murmurs. Her lungs are clear to auscultation. Her abdomen is soft, nondistended, and nontender, with normal bowel sounds.",
"She has no joint swelling but has decreased range of motion of her shoulders, hip, and bilateral knees owing to pain. Her left tibia is tender to palpation.",
"Laboratory findings reveal the following:",
"Calcium level: 9.2 mg/dL",
"Phosphorus level: 3.7 mg/dL",
"Albumin level: 4 g/dL",
"Vitamin D level: 52 ng/mL",
"Vitamin B6 level: 175 ng/mL",
"Alkaline phosphatase (ALP) level: 12 U/L",
"The remainder of her laboratory findings, including complete blood cell count, renal function, and liver function test results, are normal.",
"Imaging studies include the following:",
"Figure 1.",
"Figure 2."
],
"date": "September 13, 2019",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/918/183/918183-Thumb1.png"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/918/183/918183-Thumb2.png"
}
],
"markdown": "# A 26-Year-Old Woman Who Uses a Walking Cane Because of Pain\n\n **Authors:** Ricardo Correa, MD; Gauri Behari, MD \n **Date:** September 13, 2019\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nA 26-year-old woman with a medical history of calcium pyrophosphate deposition disease presents with severe knee pain. She now requires a cane for walking, owing to hip and knee pain. She also has abnormal dentition with tooth loss that required dental implants a few years ago. For 2 years, she has had a nonhealing small avulsion fracture in her right first metatarsal, which is confirmed by radiography.\nUpon physical examination, the patient is alert, awake, and oriented. Dental implants in the frontal area are noted. Cardiopulmonary examination reveals regular rate and rhythm, with no murmurs. Her lungs are clear to auscultation. Her abdomen is soft, nondistended, and nontender, with normal bowel sounds.\nShe has no joint swelling but has decreased range of motion of her shoulders, hip, and bilateral knees owing to pain. Her left tibia is tender to palpation.\nLaboratory findings reveal the following:\nCalcium level: 9.2 mg/dL\nPhosphorus level: 3.7 mg/dL\nAlbumin level: 4 g/dL\nVitamin D level: 52 ng/mL\nVitamin B6 level: 175 ng/mL\nAlkaline phosphatase (ALP) level: 12 U/L\nThe remainder of her laboratory findings, including complete blood cell count, renal function, and liver function test results, are normal.\nImaging studies include the following:\nFigure 1.\nFigure 2.\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n\n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1406188,
"choiceText": "X-Linked hypophosphatemia",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1406190,
"choiceText": "Vitamin D deficiency",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1406192,
"choiceText": "Osteogenesis imperfecta",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1406194,
"choiceText": "Hypophosphatasia",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 449075,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 26-Year-Old Woman Who Uses a Walking Cane Because of Pain"
},
{
"authors": "Ricardo Correa, MD; Gauri Behari, MD",
"content": [
"Because of the elevated vitamin B6 level, low ALP level, dental loss, and nonhealing fractures, genetic testing was performed in this patient. She was found to have a novel heterozygous variant in the ALPL gene (p.Ala105Asp), which encodes for tissue-nonspecific alkaline phosphatase (TNSALP). Three of her family members also have low ALP levels; one of them was tested and found to have the same variant in the ALPL gene, without any signs or symptoms of the disease.",
"Hypophosphatasia is caused by a mutation in the ALPL gene on chromosome 1, which encodes TNSALP. TNSALP is an ectoenzyme bound to the outer surface of osteoblasts. It dephosphorylates several substrates, including inorganic pyrophosphate (PPi), which inhibits bone mineralization produced by osteoblasts and chondrocytes. Accumulation of PPi when TNSALP is deficient impairs calcium/phosphate formation of hydroxyapatite, leading to accumulation of unmineralized osteoid (a feature of rickets and osteomalacia).[1,2]",
"The exact prevalence of hypophosphatasia varies, depending on the form and the region.[3,4] In the United States, it affects approximately 500-600 individuals. Hypophosphatasia disrupts mineralization, in which such minerals as calcium and phosphorus are deposited in developing bones and teeth.[5] Mineralization is critical for the formation of bones that are strong and rigid and teeth that can withstand chewing and grinding.",
"The signs and symptoms of hypophosphatasia vary widely and can appear anytime from before birth to adulthood. The most severe forms of the disorder tend to occur before birth and in early infancy. Hypophosphatasia weakens and softens the bones, causing skeletal abnormalities, similar to rickets. Affected infants are born with short limbs, an abnormally shaped chest, and soft skull bones. Additional complications in infancy include poor feeding and a failure to gain weight; respiratory problems; and high levels of calcium in the blood (hypercalcemia), which can lead to recurrent vomiting and kidney problems. These complications are life-threatening in some cases."
],
"date": "September 13, 2019",
"figures": [],
"markdown": "# A 26-Year-Old Woman Who Uses a Walking Cane Because of Pain\n\n **Authors:** Ricardo Correa, MD; Gauri Behari, MD \n **Date:** September 13, 2019\n\n ## Content\n\n Because of the elevated vitamin B6 level, low ALP level, dental loss, and nonhealing fractures, genetic testing was performed in this patient. She was found to have a novel heterozygous variant in the ALPL gene (p.Ala105Asp), which encodes for tissue-nonspecific alkaline phosphatase (TNSALP). Three of her family members also have low ALP levels; one of them was tested and found to have the same variant in the ALPL gene, without any signs or symptoms of the disease.\nHypophosphatasia is caused by a mutation in the ALPL gene on chromosome 1, which encodes TNSALP. TNSALP is an ectoenzyme bound to the outer surface of osteoblasts. It dephosphorylates several substrates, including inorganic pyrophosphate (PPi), which inhibits bone mineralization produced by osteoblasts and chondrocytes. Accumulation of PPi when TNSALP is deficient impairs calcium/phosphate formation of hydroxyapatite, leading to accumulation of unmineralized osteoid (a feature of rickets and osteomalacia).[1,2]\nThe exact prevalence of hypophosphatasia varies, depending on the form and the region.[3,4] In the United States, it affects approximately 500-600 individuals. Hypophosphatasia disrupts mineralization, in which such minerals as calcium and phosphorus are deposited in developing bones and teeth.[5] Mineralization is critical for the formation of bones that are strong and rigid and teeth that can withstand chewing and grinding.\nThe signs and symptoms of hypophosphatasia vary widely and can appear anytime from before birth to adulthood. The most severe forms of the disorder tend to occur before birth and in early infancy. Hypophosphatasia weakens and softens the bones, causing skeletal abnormalities, similar to rickets. Affected infants are born with short limbs, an abnormally shaped chest, and soft skull bones. Additional complications in infancy include poor feeding and a failure to gain weight; respiratory problems; and high levels of calcium in the blood (hypercalcemia), which can lead to recurrent vomiting and kidney problems. These complications are life-threatening in some cases.\n\n ## Figures\n\n \n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1406188,
"choiceText": "X-Linked hypophosphatemia",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1406190,
"choiceText": "Vitamin D deficiency",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1406192,
"choiceText": "Osteogenesis imperfecta",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1406194,
"choiceText": "Hypophosphatasia",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 449075,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 26-Year-Old Woman Who Uses a Walking Cane Because of Pain"
},
{
"authors": "Ricardo Correa, MD; Gauri Behari, MD",
"content": [
"The forms of hypophosphatasia that appear in childhood or adulthood are typically less severe than those that appear in infancy. Early loss of primary (baby) teeth is one of the first signs of the condition in children. Affected children may have short stature with bowed legs or knock knees, enlarged wrist and ankle joints, and an abnormal skull shape. Adult forms of hypophosphatasia are characterized by osteomalacia. In adults, recurrent fractures in the foot and thigh bones can lead to chronic pain.[6] Affected adults may lose their secondary (adult) teeth prematurely, as was the case with the patient described here. They are also at increased risk for joint pain and inflammation, again as experienced by the patient in this case.",
"The mildest form of this condition, called \"odontohypophosphatasia,\" affects only the teeth. People with this disorder typically experience abnormal tooth development and premature tooth loss but do not have the skeletal abnormalities seen in other forms of hypophosphatasia.[5]",
"The clinical presentation of hypophosphatasia varies from devastating prenatal intrauterine disease to mild manifestations in adulthood. Six clinical forms are identified: perinatal, prenatal benign (with spontaneous improvement of skeletal defects despite prenatal signs of disease), infantile, juvenile, adult, and odontohypophosphatasia.[7]",
"Adults present with early loss of primary or secondary teeth, osteoporosis, bone pain, chondrocalcinosis, chronic muscle pain, reduced muscular strength, and fractures. Joint pain and restricted range of motion may be associated because of chondrocalcinosis.[6]",
"The diagnosis is made on the basis of history and physical examination findings, as well as laboratory tests and imaging, and is confirmed with the use of genetic analysis for the ALPL gene mutations in controversial cases.[7,8] Asymptomatic adult patients are not uncommon; in these cases, the diagnosis is made using laboratory findings, including elevated levels of vitamin B6 and its metabolite in the urine and low ALP levels.[9]",
"In this case, X-linked hypophosphatemia was ruled out because it occurs mostly in younger men. It is an X-linked dominant form of osteomalacia that differs from most cases of rickets in that vitamin D supplementation does not cure the condition. It can cause bone deformity, including short stature and genu varum (bow-leggedness).",
"Vitamin D deficiency is defined as a level < 20 ng/mL. Because this patient had a level of 52 ng/mL, it was ruled out.",
"Osteogenesis imperfecta is a genetic bone disorder characterized by fragile bones that easily break. It is also known as \"brittle bone disease.\" The term literally means \"bone that is imperfectly made from the beginning of life.\" A person born with this disorder is affected throughout his or her lifetime. The patient in this case was in her mid-20s when the symptoms first began. In addition, osteogenesis imperfecta is not associated with elevated vitamin B6 levels."
],
"date": "September 13, 2019",
"figures": [],
"markdown": "# A 26-Year-Old Woman Who Uses a Walking Cane Because of Pain\n\n **Authors:** Ricardo Correa, MD; Gauri Behari, MD \n **Date:** September 13, 2019\n\n ## Content\n\n The forms of hypophosphatasia that appear in childhood or adulthood are typically less severe than those that appear in infancy. Early loss of primary (baby) teeth is one of the first signs of the condition in children. Affected children may have short stature with bowed legs or knock knees, enlarged wrist and ankle joints, and an abnormal skull shape. Adult forms of hypophosphatasia are characterized by osteomalacia. In adults, recurrent fractures in the foot and thigh bones can lead to chronic pain.[6] Affected adults may lose their secondary (adult) teeth prematurely, as was the case with the patient described here. They are also at increased risk for joint pain and inflammation, again as experienced by the patient in this case.\nThe mildest form of this condition, called \"odontohypophosphatasia,\" affects only the teeth. People with this disorder typically experience abnormal tooth development and premature tooth loss but do not have the skeletal abnormalities seen in other forms of hypophosphatasia.[5]\nThe clinical presentation of hypophosphatasia varies from devastating prenatal intrauterine disease to mild manifestations in adulthood. Six clinical forms are identified: perinatal, prenatal benign (with spontaneous improvement of skeletal defects despite prenatal signs of disease), infantile, juvenile, adult, and odontohypophosphatasia.[7]\nAdults present with early loss of primary or secondary teeth, osteoporosis, bone pain, chondrocalcinosis, chronic muscle pain, reduced muscular strength, and fractures. Joint pain and restricted range of motion may be associated because of chondrocalcinosis.[6]\nThe diagnosis is made on the basis of history and physical examination findings, as well as laboratory tests and imaging, and is confirmed with the use of genetic analysis for the ALPL gene mutations in controversial cases.[7,8] Asymptomatic adult patients are not uncommon; in these cases, the diagnosis is made using laboratory findings, including elevated levels of vitamin B6 and its metabolite in the urine and low ALP levels.[9]\nIn this case, X-linked hypophosphatemia was ruled out because it occurs mostly in younger men. It is an X-linked dominant form of osteomalacia that differs from most cases of rickets in that vitamin D supplementation does not cure the condition. It can cause bone deformity, including short stature and genu varum (bow-leggedness).\nVitamin D deficiency is defined as a level < 20 ng/mL. Because this patient had a level of 52 ng/mL, it was ruled out.\nOsteogenesis imperfecta is a genetic bone disorder characterized by fragile bones that easily break. It is also known as \"brittle bone disease.\" The term literally means \"bone that is imperfectly made from the beginning of life.\" A person born with this disorder is affected throughout his or her lifetime. The patient in this case was in her mid-20s when the symptoms first began. In addition, osteogenesis imperfecta is not associated with elevated vitamin B6 levels.\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [],
"title": "A 26-Year-Old Woman Who Uses a Walking Cane Because of Pain"
},
{
"authors": "Ricardo Correa, MD; Gauri Behari, MD",
"content": [
"Data on treatment of hypophosphatasia in the adult population are limited. Hypophosphatasia has a significantly negative impact on quality of life. Almost all adult patients report chronic pain in bone, joints, and muscles; a large majority require daily use of analgesic drugs.[10] Therefore, pain management is a mainstay of therapy. A combination of physiotherapy, occupational therapy, and chronic pain management is most effective.[11]",
"Enzyme replacement therapy using bone-targeting recombinant alkaline phosphatase or asfotase alfa has been used to treat infants, children, and some adults with hypophosphatasia. A long-term study of asfotase alfa demonstrated improvement of bone mineralization, respiratory function, and survival rate in 37 patients with hypophosphatasia compared with 48 historical controls.[12] Suggested indications for treatment of hypophosphatasia in adults with asfotase alfa include history of childhood involvement (< 18 y), and one or more of the following:",
"Musculoskeletal pain requiring prescription pain medications",
"Disabling polyarthropathy or chondrocalcinosis",
"Major low trauma fracture attributable to hypophosphatasia (eg, spine, hip, humerus)",
"Delayed or incomplete fracture healing or fracture nonunion",
"Repeated episodes of orthopedic surgery for complications of hypophosphatasia",
"Disabling functional impairment affecting mobility, gait, and activities of daily living",
"Low bone mineral density on dual-energy x-ray absorptiometry",
"Radiologic evidence of nephrocalcinosis",
"Currently, no guidelines are available for selecting adult patients for treatment, evaluating results of treatment, or determining optimal duration of hypophosphatasia treatment.[11,12,13,14] Teriparatide has also been used in patients with hypophosphatasia. In a study of 10 adult patients, hypophosphatasia was treated with teriparatide to increase osteoblast production of ALP.[15] Effects of treatment on bone mineral density varied; however, the study found improvements in pain, mobility, and fracture repair in some cases.",
"In another study, eight adult patients were treated with anti-sclerostin monoclonal antibody.[16] Treatment for 29 weeks increased bone formation markers and transiently decreased C-telopeptide. Lumbar spine bone mineral density showed a mean increase of 3.9% at the end of the study.",
"A key point in management is that treatment with antiresorptive agents (eg, bisphosphonates) is contraindicated because they may further lower the ALP level and are harmful in patients with hypophosphatasia. Although this disease is physical, several studies underline that mental and emotional health are affected in adult patients with hypophosphatasia.[6,17]"
],
"date": "September 13, 2019",
"figures": [],
"markdown": "# A 26-Year-Old Woman Who Uses a Walking Cane Because of Pain\n\n **Authors:** Ricardo Correa, MD; Gauri Behari, MD \n **Date:** September 13, 2019\n\n ## Content\n\n Data on treatment of hypophosphatasia in the adult population are limited. Hypophosphatasia has a significantly negative impact on quality of life. Almost all adult patients report chronic pain in bone, joints, and muscles; a large majority require daily use of analgesic drugs.[10] Therefore, pain management is a mainstay of therapy. A combination of physiotherapy, occupational therapy, and chronic pain management is most effective.[11]\nEnzyme replacement therapy using bone-targeting recombinant alkaline phosphatase or asfotase alfa has been used to treat infants, children, and some adults with hypophosphatasia. A long-term study of asfotase alfa demonstrated improvement of bone mineralization, respiratory function, and survival rate in 37 patients with hypophosphatasia compared with 48 historical controls.[12] Suggested indications for treatment of hypophosphatasia in adults with asfotase alfa include history of childhood involvement (< 18 y), and one or more of the following:\nMusculoskeletal pain requiring prescription pain medications\nDisabling polyarthropathy or chondrocalcinosis\nMajor low trauma fracture attributable to hypophosphatasia (eg, spine, hip, humerus)\nDelayed or incomplete fracture healing or fracture nonunion\nRepeated episodes of orthopedic surgery for complications of hypophosphatasia\nDisabling functional impairment affecting mobility, gait, and activities of daily living\nLow bone mineral density on dual-energy x-ray absorptiometry\nRadiologic evidence of nephrocalcinosis\nCurrently, no guidelines are available for selecting adult patients for treatment, evaluating results of treatment, or determining optimal duration of hypophosphatasia treatment.[11,12,13,14] Teriparatide has also been used in patients with hypophosphatasia. In a study of 10 adult patients, hypophosphatasia was treated with teriparatide to increase osteoblast production of ALP.[15] Effects of treatment on bone mineral density varied; however, the study found improvements in pain, mobility, and fracture repair in some cases.\nIn another study, eight adult patients were treated with anti-sclerostin monoclonal antibody.[16] Treatment for 29 weeks increased bone formation markers and transiently decreased C-telopeptide. Lumbar spine bone mineral density showed a mean increase of 3.9% at the end of the study.\nA key point in management is that treatment with antiresorptive agents (eg, bisphosphonates) is contraindicated because they may further lower the ALP level and are harmful in patients with hypophosphatasia. Although this disease is physical, several studies underline that mental and emotional health are affected in adult patients with hypophosphatasia.[6,17]\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 26-Year-Old Woman Who Uses a Walking Cane Because of Pain"
},
{
"authors": "Ricardo Correa, MD; Gauri Behari, MD",
"content": [
"In summary, hypophosphatasia is a multisystemic disease with deleterious effects that can appear at different ages and progress over time. In adults, the symptoms of hypophosphatasia are often misdiagnosed and confused with those of other more common bone or rheumatologic diseases, leading to delayed diagnosis and inappropriate treatment. Incorrect treatments, such as high-dose vitamin D, excessive calcium supplementation, and bisphosphonates, may worsen symptoms.[6] Patients who present with abnormal bone features should have their ALP and vitamin B6 levels assessed to rule out this diagnosis.",
"This patient was started on ALP replacement (asfotase alfa) at 40 mg subcutaneously 6 times per week. After 6 months, her pain resolved. She has not had any additional fractures."
],
"date": "September 13, 2019",
"figures": [],
"markdown": "# A 26-Year-Old Woman Who Uses a Walking Cane Because of Pain\n\n **Authors:** Ricardo Correa, MD; Gauri Behari, MD \n **Date:** September 13, 2019\n\n ## Content\n\n In summary, hypophosphatasia is a multisystemic disease with deleterious effects that can appear at different ages and progress over time. In adults, the symptoms of hypophosphatasia are often misdiagnosed and confused with those of other more common bone or rheumatologic diseases, leading to delayed diagnosis and inappropriate treatment. Incorrect treatments, such as high-dose vitamin D, excessive calcium supplementation, and bisphosphonates, may worsen symptoms.[6] Patients who present with abnormal bone features should have their ALP and vitamin B6 levels assessed to rule out this diagnosis.\nThis patient was started on ALP replacement (asfotase alfa) at 40 mg subcutaneously 6 times per week. After 6 months, her pain resolved. She has not had any additional fractures.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1406252,
"choiceText": "<i>P53</i>",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1406254,
"choiceText": "<i>ALPL</i>",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1406256,
"choiceText": "<i>PHEX</i>\r\n",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1406258,
"choiceText": "<i>COLA1</i>\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Mutations in the <i>ALPL</i> gene cause hypophosphatasia. This gene provides instructions for making TNSALP, which plays an essential role in mineralization of the skeleton and teeth. Mutations in <i>ALPL</i> lead to the production of an abnormal version of TNSALP that cannot effectively participate in the mineralization process. A shortage of TNSALP allows several other substances that are normally processed by the enzyme to abnormally build up in the body. Researchers believe that a buildup of one of these compounds (PPi) underlies the defective mineralization of bones and teeth in people with hypophosphatasia.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 449091,
"questionText": "Which of the following genes is mutated in patients with hypophosphatasia?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1406260,
"choiceText": "Vitamin B<sub>6</sub>",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1406262,
"choiceText": "Inorganic pyrophosphate",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1406264,
"choiceText": "Bone-specific ALP",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1406266,
"choiceText": "Magnesium",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Elevated levels of inorganic pyrophosphate can indirectly lead to elevated levels of calcium in the body and insufficient calcification of bone in patients with hypophosphatasia. ",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 449093,
"questionText": "An elevation in which of the following may cause insufficient calcification of the bone in patients with hypophosphatasia?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 26-Year-Old Woman Who Uses a Walking Cane Because of Pain"
},
{
"authors": "Ricardo Correa, MD; Gauri Behari, MD",
"content": [],
"date": "September 13, 2019",
"figures": [],
"markdown": "# A 26-Year-Old Woman Who Uses a Walking Cane Because of Pain\n\n **Authors:** Ricardo Correa, MD; Gauri Behari, MD \n **Date:** September 13, 2019\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1406252,
"choiceText": "<i>P53</i>",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1406254,
"choiceText": "<i>ALPL</i>",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1406256,
"choiceText": "<i>PHEX</i>\r\n",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1406258,
"choiceText": "<i>COLA1</i>\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Mutations in the <i>ALPL</i> gene cause hypophosphatasia. This gene provides instructions for making TNSALP, which plays an essential role in mineralization of the skeleton and teeth. Mutations in <i>ALPL</i> lead to the production of an abnormal version of TNSALP that cannot effectively participate in the mineralization process. A shortage of TNSALP allows several other substances that are normally processed by the enzyme to abnormally build up in the body. Researchers believe that a buildup of one of these compounds (PPi) underlies the defective mineralization of bones and teeth in people with hypophosphatasia.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 449091,
"questionText": "Which of the following genes is mutated in patients with hypophosphatasia?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1406260,
"choiceText": "Vitamin B<sub>6</sub>",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1406262,
"choiceText": "Inorganic pyrophosphate",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1406264,
"choiceText": "Bone-specific ALP",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1406266,
"choiceText": "Magnesium",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Elevated levels of inorganic pyrophosphate can indirectly lead to elevated levels of calcium in the body and insufficient calcification of bone in patients with hypophosphatasia. ",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 449093,
"questionText": "An elevation in which of the following may cause insufficient calcification of the bone in patients with hypophosphatasia?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 26-Year-Old Woman Who Uses a Walking Cane Because of Pain"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1406188,
"choiceText": "X-Linked hypophosphatemia",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1406190,
"choiceText": "Vitamin D deficiency",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1406192,
"choiceText": "Osteogenesis imperfecta",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1406194,
"choiceText": "Hypophosphatasia",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 449075,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1406252,
"choiceText": "<i>P53</i>",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1406254,
"choiceText": "<i>ALPL</i>",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1406256,
"choiceText": "<i>PHEX</i>\r\n",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1406258,
"choiceText": "<i>COLA1</i>\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Mutations in the <i>ALPL</i> gene cause hypophosphatasia. This gene provides instructions for making TNSALP, which plays an essential role in mineralization of the skeleton and teeth. Mutations in <i>ALPL</i> lead to the production of an abnormal version of TNSALP that cannot effectively participate in the mineralization process. A shortage of TNSALP allows several other substances that are normally processed by the enzyme to abnormally build up in the body. Researchers believe that a buildup of one of these compounds (PPi) underlies the defective mineralization of bones and teeth in people with hypophosphatasia.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 449091,
"questionText": "Which of the following genes is mutated in patients with hypophosphatasia?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1406260,
"choiceText": "Vitamin B<sub>6</sub>",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1406262,
"choiceText": "Inorganic pyrophosphate",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1406264,
"choiceText": "Bone-specific ALP",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1406266,
"choiceText": "Magnesium",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Elevated levels of inorganic pyrophosphate can indirectly lead to elevated levels of calcium in the body and insufficient calcification of bone in patients with hypophosphatasia. ",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 449093,
"questionText": "An elevation in which of the following may cause insufficient calcification of the bone in patients with hypophosphatasia?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
917371 | /viewarticle/917371 | [
{
"authors": "Nicole Davey-Ranasinghe, MD; Constantine K. Saadeh, MD",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"A 36-year-old woman with long-standing allergic rhinitis, recurrent sinusitis, atopic dermatitis, and asthma was referred for allergy and asthma evaluation by her primary care physician because of her respiratory symptoms. She developed a cough that has worsened over the past 3 months. She reports that the cough is generally productive and denies hemoptysis. She also reports that her chronic rhinorrhea has been more bothersome. She has also had a decrease in hearing that has been subtle over the past 6 months.",
"She has required antibiotics for sinusitis four times over the past 12 months. She requires use of her rescue inhaler approximately four times per week and has noted an increase in her asthma exacerbations. She received a course of oral prednisone three times over the past 12 months. She also notes new-onset chest pain over the past month that is worse upon moderate exertion.",
"She has no current rash but reports that she has struggled with eczema intermittently since childhood. She is currently taking a daily antihistamine, intranasal fluticasone, and an inhaled combination long-acting beta-agonist and glucocorticoid with mometasone-formoterol (200 µg/5 µg)."
],
"date": "September 05, 2019",
"figures": [],
"markdown": "# A 36-Year-Old Woman With a Worsening Cough and Hearing Loss\n\n **Authors:** Nicole Davey-Ranasinghe, MD; Constantine K. Saadeh, MD \n **Date:** September 05, 2019\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nA 36-year-old woman with long-standing allergic rhinitis, recurrent sinusitis, atopic dermatitis, and asthma was referred for allergy and asthma evaluation by her primary care physician because of her respiratory symptoms. She developed a cough that has worsened over the past 3 months. She reports that the cough is generally productive and denies hemoptysis. She also reports that her chronic rhinorrhea has been more bothersome. She has also had a decrease in hearing that has been subtle over the past 6 months.\nShe has required antibiotics for sinusitis four times over the past 12 months. She requires use of her rescue inhaler approximately four times per week and has noted an increase in her asthma exacerbations. She received a course of oral prednisone three times over the past 12 months. She also notes new-onset chest pain over the past month that is worse upon moderate exertion.\nShe has no current rash but reports that she has struggled with eczema intermittently since childhood. She is currently taking a daily antihistamine, intranasal fluticasone, and an inhaled combination long-acting beta-agonist and glucocorticoid with mometasone-formoterol (200 µg/5 µg).\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 36-Year-Old Woman With a Worsening Cough and Hearing Loss"
},
{
"authors": "Nicole Davey-Ranasinghe, MD; Constantine K. Saadeh, MD",
"content": [
"Upon physical examination, the patient is afebrile. Her respiratory rate is 18 breaths/min, and her heart rate is 105 beats/min. She has atopic dermatitis on the extensor surface of her elbows. She also has bilateral otitis media. Periorbital edema is observed, with darkening of the periorbital skin. She has clear nasal discharge and nasal polyposis. She has bilateral expiratory wheezing and mild crackles upon examination. She has no necrotic nasal lesions and no evidence of cutaneous vasculitis. Her neurologic examination findings are unremarkable. No murmur, rubs, or gallops are noted during her cardiovascular examination; however, mild tachycardia is detected.",
"Sinus radiography reveals findings consistent with chronic sinusitis, including mucosal thickening and air fluid levels in the bilateral maxillary sinuses (Figures 1-3). Near complete opacification of the bilateral frontal sinuses is noted. Chest radiography reveals diffuse interstitial changes (Figures 4 and 5).",
"Figure 1.",
"Figure 2.",
"Figure 3.",
"Figure 4.",
"Figure 5.",
"This prompts high-resolution CT to be ordered, which reveals peribronchial thickening and widely scattered patchy infiltrates. ECG findings reveal sinus tachycardia with no other abnormality. Pulmonary function testing reveals a moderate obstructive defect; lung diffusion capacity is mildly decreased, at 70%.",
"A complete metabolic panel with differential reveals an eosinophil count of 7500 eosinophils/µL. Her erythrocyte sedimentation rate is elevated, at 85 mm. Her C-reactive protein level is elevated, at 5.6 mg/dL. Test results for antineutrophil cytoplasmic antibodies (ANCAs) are positive for myeloperoxidase ANCAs and negative for proteinase-3 ANCAs. Urinalysis is unremarkable. Troponin test results are negative. Rheumatoid factor test results are positive, with a low titer of 1:40. Antinuclear antibody test results are negative. C3 and C4 levels are within normal limits."
],
"date": "September 05, 2019",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/917/371/917371-Thumb1.png"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/917/371/917371-Thumb2.png"
},
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/917/371/917371-Thumb3.png"
},
{
"caption": "Figure 4.",
"image_url": "https://img.medscapestatic.com/article/917/371/917371-Thumb4.png"
},
{
"caption": "Figure 5.",
"image_url": "https://img.medscapestatic.com/article/917/371/917371-Thumb5.png"
}
],
"markdown": "# A 36-Year-Old Woman With a Worsening Cough and Hearing Loss\n\n **Authors:** Nicole Davey-Ranasinghe, MD; Constantine K. Saadeh, MD \n **Date:** September 05, 2019\n\n ## Content\n\n Upon physical examination, the patient is afebrile. Her respiratory rate is 18 breaths/min, and her heart rate is 105 beats/min. She has atopic dermatitis on the extensor surface of her elbows. She also has bilateral otitis media. Periorbital edema is observed, with darkening of the periorbital skin. She has clear nasal discharge and nasal polyposis. She has bilateral expiratory wheezing and mild crackles upon examination. She has no necrotic nasal lesions and no evidence of cutaneous vasculitis. Her neurologic examination findings are unremarkable. No murmur, rubs, or gallops are noted during her cardiovascular examination; however, mild tachycardia is detected.\nSinus radiography reveals findings consistent with chronic sinusitis, including mucosal thickening and air fluid levels in the bilateral maxillary sinuses (Figures 1-3). Near complete opacification of the bilateral frontal sinuses is noted. Chest radiography reveals diffuse interstitial changes (Figures 4 and 5).\nFigure 1.\nFigure 2.\nFigure 3.\nFigure 4.\nFigure 5.\nThis prompts high-resolution CT to be ordered, which reveals peribronchial thickening and widely scattered patchy infiltrates. ECG findings reveal sinus tachycardia with no other abnormality. Pulmonary function testing reveals a moderate obstructive defect; lung diffusion capacity is mildly decreased, at 70%.\nA complete metabolic panel with differential reveals an eosinophil count of 7500 eosinophils/µL. Her erythrocyte sedimentation rate is elevated, at 85 mm. Her C-reactive protein level is elevated, at 5.6 mg/dL. Test results for antineutrophil cytoplasmic antibodies (ANCAs) are positive for myeloperoxidase ANCAs and negative for proteinase-3 ANCAs. Urinalysis is unremarkable. Troponin test results are negative. Rheumatoid factor test results are positive, with a low titer of 1:40. Antinuclear antibody test results are negative. C3 and C4 levels are within normal limits.\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n**Figure 3.** \n \n\n**Figure 4.** \n \n\n**Figure 5.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1401608,
"choiceText": "Granulomatosis with polyangiitis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1401610,
"choiceText": "Aspirin-associated respiratory disease",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1401612,
"choiceText": "Eosinophilic granulomatosis with polyangiitis",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1401614,
"choiceText": "Hypereosinophilic syndrome",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 447507,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 36-Year-Old Woman With a Worsening Cough and Hearing Loss"
},
{
"authors": "Nicole Davey-Ranasinghe, MD; Constantine K. Saadeh, MD",
"content": [
"Eosinophilic granulomatosis with polyangiitis (EGPA)—formerly termed \"Churg-Strauss syndrome\"—is a vasculitis of small and medium vessels. The vasculitis itself is not always clinically apparent; this is especially true early in the disease. The disease usually progresses from a prodromal phase to an eosinophilic phase and then to a vasculitic phase. The disease is multisystem and is characterized by asthma, chronic rhinosinusitis, and peripheral eosinophilia.[1]",
"The lungs, upper airways, and skin are the most commonly involved organs. However, any organ system can be involved, including the cardiovascular, neurologic, renal, gastrointestinal, and musculoskeletal systems. Eosinophilic lymphadenopathy has also been noted in patients with EGPA. As with other vasculitides, the risk for thromboembolic events is increased in patients with EGPA.",
"Asthma is the primary feature of EGPA and is present in the vast majority of patients. The asthma generally precedes the vasculitis and can be present for a decade or longer before the vasculitis is clinically evident. After onset of vasculitis, asthma exacerbations often increase in both frequency and severity. Patients are often taking inhaled or systemic glucocorticoids for underlying asthma; thus, the vasculitis does not become apparent until the glucocorticoids are weaned or discontinued.",
"Upper-airway and ear disease—including ear, nose, and throat involvement, with nasal polyposis—is present in most patients. Sensorineural hearing loss can also occur. Compared with patients with granulomatosis with polyangiitis (GPA), those with EGPA lack necrotizing lesions of the nasopharynx. Cutaneous vasculitis can appear at any time during the course of the disease. Cutaneous granulomas present as tender, subcutaneous nodules on the extensor surfaces."
],
"date": "September 05, 2019",
"figures": [],
"markdown": "# A 36-Year-Old Woman With a Worsening Cough and Hearing Loss\n\n **Authors:** Nicole Davey-Ranasinghe, MD; Constantine K. Saadeh, MD \n **Date:** September 05, 2019\n\n ## Content\n\n Eosinophilic granulomatosis with polyangiitis (EGPA)—formerly termed \"Churg-Strauss syndrome\"—is a vasculitis of small and medium vessels. The vasculitis itself is not always clinically apparent; this is especially true early in the disease. The disease usually progresses from a prodromal phase to an eosinophilic phase and then to a vasculitic phase. The disease is multisystem and is characterized by asthma, chronic rhinosinusitis, and peripheral eosinophilia.[1]\nThe lungs, upper airways, and skin are the most commonly involved organs. However, any organ system can be involved, including the cardiovascular, neurologic, renal, gastrointestinal, and musculoskeletal systems. Eosinophilic lymphadenopathy has also been noted in patients with EGPA. As with other vasculitides, the risk for thromboembolic events is increased in patients with EGPA.\nAsthma is the primary feature of EGPA and is present in the vast majority of patients. The asthma generally precedes the vasculitis and can be present for a decade or longer before the vasculitis is clinically evident. After onset of vasculitis, asthma exacerbations often increase in both frequency and severity. Patients are often taking inhaled or systemic glucocorticoids for underlying asthma; thus, the vasculitis does not become apparent until the glucocorticoids are weaned or discontinued.\nUpper-airway and ear disease—including ear, nose, and throat involvement, with nasal polyposis—is present in most patients. Sensorineural hearing loss can also occur. Compared with patients with granulomatosis with polyangiitis (GPA), those with EGPA lack necrotizing lesions of the nasopharynx. Cutaneous vasculitis can appear at any time during the course of the disease. Cutaneous granulomas present as tender, subcutaneous nodules on the extensor surfaces.\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1401608,
"choiceText": "Granulomatosis with polyangiitis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1401610,
"choiceText": "Aspirin-associated respiratory disease",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1401612,
"choiceText": "Eosinophilic granulomatosis with polyangiitis",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1401614,
"choiceText": "Hypereosinophilic syndrome",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 447507,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 36-Year-Old Woman With a Worsening Cough and Hearing Loss"
},
{
"authors": "Nicole Davey-Ranasinghe, MD; Constantine K. Saadeh, MD",
"content": [
"Suspecting EGPA in patients with peripheral eosinophilia, allergic rhinitis, and asthma is essential. However, confirming the diagnosis can be difficult because vasculitis is not universally clinically evident. The American College of Rheumatology (ACR) has established criteria for the classification of EGPA.[2] However, these classification criteria apply to patients with documented vasculitis. Among patients with EGPA, only 40%-60% have positive ANCA results.",
"Biopsies are also not always conclusive for granulomas or necrotizing vasculitis and may show less specific changes, such as infiltration of the vessel with eosinophils. Therefore, if EGPA is clinically suspected, further evaluation and work-up are needed to establish the diagnosis. The presence of four or more of the ACR criteria has a sensitivity of 85% and a specificity of 99.7% for the diagnosis of EGPA.[2] The criteria include the following:",
"Asthma",
"> 10% eosinophils on differential",
"Mononeuropathy or polyneuropathy",
"Migratory or transient pulmonary opacities detected radiographically",
"Paranasal sinus abnormality",
"Biopsy containing a blood vessel showing accumulation of eosinophils in extravascular areas",
"The Lanham criteria require three criteria for a diagnosis of EGPA[3]: asthma, peak peripheral blood eosinophilia > 1500 cells/µL, and systemic vasculitis involving two or more extrapulmonary organs.",
"Although no laboratory test is specific for EGPA, the presence of myeloperoxidase ANCAs can help raise the clinical likelihood of the diagnosis. In general, evaluation for peripheral eosinophilia with a complete blood cell count including differential is recommended. Inflammatory markers can be elevated or normal. Other laboratory evaluations to evaluate for renal or cardiac involvement may also be considered, if clinically indicated.",
"In this case, the patient had new-onset chest pain. EKG was performed, and troponin levels were checked. Cardiac manifestations for EGPA can vary; rhythm abnormalities, heart failure, and pericarditis have been reported. Endomyocardial biopsy may be obtained if endomyocarditis is suspected. Cardiac involvement may affect the clinical decision whether to start immunosuppressive therapy. Other noninvasive cardiovascular testing—including ECG, echocardiography, and cardiac MRI with gadolinium—is often part of the evaluation for patients with suspected cardiac involvement due to EGPA.",
"The differential diagnosis for EGPA is broad. Aspirin-induced respiratory disease; chronic eosinophilic pneumonia; hypereosinophilic syndrome; and other vasculitides, including GPA and microscopic polyangiitis, should all be considered in a patient with suspected EGPA.",
"In this case, GPA was less likely, on the basis of the patient's history of worsening asthma and lack of necrotic lesions on nasal examination. The presence of myeloperoxidase rather than proteinase-3 ANCA also favors a diagnosis of EGPA. Likewise, the myeloperoxidase antibody is not seen in hypereosinophilic syndrome. Finally, although aspirin-associated respiratory disease can mimic EGPA, the patient reported no significant history of aspirin use."
],
"date": "September 05, 2019",
"figures": [],
"markdown": "# A 36-Year-Old Woman With a Worsening Cough and Hearing Loss\n\n **Authors:** Nicole Davey-Ranasinghe, MD; Constantine K. Saadeh, MD \n **Date:** September 05, 2019\n\n ## Content\n\n Suspecting EGPA in patients with peripheral eosinophilia, allergic rhinitis, and asthma is essential. However, confirming the diagnosis can be difficult because vasculitis is not universally clinically evident. The American College of Rheumatology (ACR) has established criteria for the classification of EGPA.[2] However, these classification criteria apply to patients with documented vasculitis. Among patients with EGPA, only 40%-60% have positive ANCA results.\nBiopsies are also not always conclusive for granulomas or necrotizing vasculitis and may show less specific changes, such as infiltration of the vessel with eosinophils. Therefore, if EGPA is clinically suspected, further evaluation and work-up are needed to establish the diagnosis. The presence of four or more of the ACR criteria has a sensitivity of 85% and a specificity of 99.7% for the diagnosis of EGPA.[2] The criteria include the following:\nAsthma\n> 10% eosinophils on differential\nMononeuropathy or polyneuropathy\nMigratory or transient pulmonary opacities detected radiographically\nParanasal sinus abnormality\nBiopsy containing a blood vessel showing accumulation of eosinophils in extravascular areas\nThe Lanham criteria require three criteria for a diagnosis of EGPA[3]: asthma, peak peripheral blood eosinophilia > 1500 cells/µL, and systemic vasculitis involving two or more extrapulmonary organs.\nAlthough no laboratory test is specific for EGPA, the presence of myeloperoxidase ANCAs can help raise the clinical likelihood of the diagnosis. In general, evaluation for peripheral eosinophilia with a complete blood cell count including differential is recommended. Inflammatory markers can be elevated or normal. Other laboratory evaluations to evaluate for renal or cardiac involvement may also be considered, if clinically indicated.\nIn this case, the patient had new-onset chest pain. EKG was performed, and troponin levels were checked. Cardiac manifestations for EGPA can vary; rhythm abnormalities, heart failure, and pericarditis have been reported. Endomyocardial biopsy may be obtained if endomyocarditis is suspected. Cardiac involvement may affect the clinical decision whether to start immunosuppressive therapy. Other noninvasive cardiovascular testing—including ECG, echocardiography, and cardiac MRI with gadolinium—is often part of the evaluation for patients with suspected cardiac involvement due to EGPA.\nThe differential diagnosis for EGPA is broad. Aspirin-induced respiratory disease; chronic eosinophilic pneumonia; hypereosinophilic syndrome; and other vasculitides, including GPA and microscopic polyangiitis, should all be considered in a patient with suspected EGPA.\nIn this case, GPA was less likely, on the basis of the patient's history of worsening asthma and lack of necrotic lesions on nasal examination. The presence of myeloperoxidase rather than proteinase-3 ANCA also favors a diagnosis of EGPA. Likewise, the myeloperoxidase antibody is not seen in hypereosinophilic syndrome. Finally, although aspirin-associated respiratory disease can mimic EGPA, the patient reported no significant history of aspirin use.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 36-Year-Old Woman With a Worsening Cough and Hearing Loss"
},
{
"authors": "Nicole Davey-Ranasinghe, MD; Constantine K. Saadeh, MD",
"content": [
"The primary treatment for EGPA is systemic glucocorticoids. In addition, an immunosuppressive agent is typically added for advanced refractory disease. Various disease-modifying agents have been used as glucocorticoid-sparing or maintenance therapy in patients with EGPA. Variable responses to methotrexate, azathioprine, mycophenolate mofetil, and leflunomide in the setting of EGPA have been reported; however, evidence is lacking to support these therapies.",
"Other therapies, including intravenous immunoglobulin, rituximab, interferon alpha, and anti-immunoglobulin E, have all been used in EGPA. Anti–interleukin-5 (IL-5) antibodies are available for the treatment of asthma and have affected the treatment options for patients with EGPA. Mepolizumab, a humanized monoclonal antibody to IL-5, is approved by the US Food and Drug Administration (FDA) for the treatment of EGPA. Mepolizumab has been assessed in multiple case reports and in a multicenter randomized, placebo-controlled trial.[4] Mepolizumab resulted in increased frequency of remission and allowed for tapering of prednisolone or prednisone compared with placebo. The dosage of mepolizumab for EGPA is 300 mg every 4 weeks, which is higher than the traditional asthma dosage.",
"This patient was treated with prednisone at a dosage of 60 mg daily (approximately 0.8 mg/kg). Her symptoms improved after 4 weeks, and a slow steroid taper was initiated. Monitoring of her vasculitis, both clinically and with repeat imaging and laboratory testing, is scheduled. An anti–IL-5 regimen may be initiated, if it is needed as a steroid-sparing agent. She concomitantly received Pneumocystis jirovecii pneumonia prophylaxis. Ongoing close monitoring for all patients with EGPA in a multidisciplinary fashion is essential."
],
"date": "September 05, 2019",
"figures": [],
"markdown": "# A 36-Year-Old Woman With a Worsening Cough and Hearing Loss\n\n **Authors:** Nicole Davey-Ranasinghe, MD; Constantine K. Saadeh, MD \n **Date:** September 05, 2019\n\n ## Content\n\n The primary treatment for EGPA is systemic glucocorticoids. In addition, an immunosuppressive agent is typically added for advanced refractory disease. Various disease-modifying agents have been used as glucocorticoid-sparing or maintenance therapy in patients with EGPA. Variable responses to methotrexate, azathioprine, mycophenolate mofetil, and leflunomide in the setting of EGPA have been reported; however, evidence is lacking to support these therapies.\nOther therapies, including intravenous immunoglobulin, rituximab, interferon alpha, and anti-immunoglobulin E, have all been used in EGPA. Anti–interleukin-5 (IL-5) antibodies are available for the treatment of asthma and have affected the treatment options for patients with EGPA. Mepolizumab, a humanized monoclonal antibody to IL-5, is approved by the US Food and Drug Administration (FDA) for the treatment of EGPA. Mepolizumab has been assessed in multiple case reports and in a multicenter randomized, placebo-controlled trial.[4] Mepolizumab resulted in increased frequency of remission and allowed for tapering of prednisolone or prednisone compared with placebo. The dosage of mepolizumab for EGPA is 300 mg every 4 weeks, which is higher than the traditional asthma dosage.\nThis patient was treated with prednisone at a dosage of 60 mg daily (approximately 0.8 mg/kg). Her symptoms improved after 4 weeks, and a slow steroid taper was initiated. Monitoring of her vasculitis, both clinically and with repeat imaging and laboratory testing, is scheduled. An anti–IL-5 regimen may be initiated, if it is needed as a steroid-sparing agent. She concomitantly received Pneumocystis jirovecii pneumonia prophylaxis. Ongoing close monitoring for all patients with EGPA in a multidisciplinary fashion is essential.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1401616,
"choiceText": "Evidence of vasculitis with granulomatous involvement",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1401618,
"choiceText": "Involvement of the ears, nose, and throat",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1401620,
"choiceText": "Necrotic lesions of the upper airway",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1401622,
"choiceText": "Pulmonary involvement with variable radiographic findings\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Distinguishing between EGPA and other types of vasculitis can be difficult. In general, necrotic lesions are not seen in EGPA but are present in GPA. Evidence of vasculitis and upper-airway involvement, as well as pulmonary involvement, can be seen in both GPA and EGPA. Other distinguishing factors include a history of asthma and peripheral eosinophilia, which are seen in EGPA.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 447509,
"questionText": "Which of the following is <i>not</i> seen in both EGPA and GPA?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1401624,
"choiceText": "Azathioprine\r\n\t\r\n",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1401626,
"choiceText": "Methotrexate",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1401628,
"choiceText": "Omalizumab",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1401630,
"choiceText": "Mepolizumab ",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "A study of mepolizumab for EGPA found that treatment resulted in more weeks in remission and a higher proportion of participants in remission than did placebo. This allowed for a greater reduction in total glucocorticoid use. However, approximately half the patients treated with mepolizumab did not achieve protocol-defined remission. Mepolizumab is FDA-approved for the treatment of EGPA.\r\n<br><br>\r\nAlthough other disease-modifying biologic agents can be considered, data favor mepolizumab over other medications in the setting of EGPA. ",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 447511,
"questionText": "Which of the following should be initiated as a steroid-sparing agent in a patient who requires glucocorticoids for the treatment of EGPA?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 36-Year-Old Woman With a Worsening Cough and Hearing Loss"
},
{
"authors": "Nicole Davey-Ranasinghe, MD; Constantine K. Saadeh, MD",
"content": [],
"date": "September 05, 2019",
"figures": [],
"markdown": "# A 36-Year-Old Woman With a Worsening Cough and Hearing Loss\n\n **Authors:** Nicole Davey-Ranasinghe, MD; Constantine K. Saadeh, MD \n **Date:** September 05, 2019\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1401616,
"choiceText": "Evidence of vasculitis with granulomatous involvement",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1401618,
"choiceText": "Involvement of the ears, nose, and throat",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1401620,
"choiceText": "Necrotic lesions of the upper airway",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1401622,
"choiceText": "Pulmonary involvement with variable radiographic findings\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Distinguishing between EGPA and other types of vasculitis can be difficult. In general, necrotic lesions are not seen in EGPA but are present in GPA. Evidence of vasculitis and upper-airway involvement, as well as pulmonary involvement, can be seen in both GPA and EGPA. Other distinguishing factors include a history of asthma and peripheral eosinophilia, which are seen in EGPA.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 447509,
"questionText": "Which of the following is <i>not</i> seen in both EGPA and GPA?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1401624,
"choiceText": "Azathioprine\r\n\t\r\n",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1401626,
"choiceText": "Methotrexate",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1401628,
"choiceText": "Omalizumab",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1401630,
"choiceText": "Mepolizumab ",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "A study of mepolizumab for EGPA found that treatment resulted in more weeks in remission and a higher proportion of participants in remission than did placebo. This allowed for a greater reduction in total glucocorticoid use. However, approximately half the patients treated with mepolizumab did not achieve protocol-defined remission. Mepolizumab is FDA-approved for the treatment of EGPA.\r\n<br><br>\r\nAlthough other disease-modifying biologic agents can be considered, data favor mepolizumab over other medications in the setting of EGPA. ",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 447511,
"questionText": "Which of the following should be initiated as a steroid-sparing agent in a patient who requires glucocorticoids for the treatment of EGPA?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 36-Year-Old Woman With a Worsening Cough and Hearing Loss"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1401608,
"choiceText": "Granulomatosis with polyangiitis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1401610,
"choiceText": "Aspirin-associated respiratory disease",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1401612,
"choiceText": "Eosinophilic granulomatosis with polyangiitis",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1401614,
"choiceText": "Hypereosinophilic syndrome",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 447507,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1401616,
"choiceText": "Evidence of vasculitis with granulomatous involvement",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1401618,
"choiceText": "Involvement of the ears, nose, and throat",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1401620,
"choiceText": "Necrotic lesions of the upper airway",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1401622,
"choiceText": "Pulmonary involvement with variable radiographic findings\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Distinguishing between EGPA and other types of vasculitis can be difficult. In general, necrotic lesions are not seen in EGPA but are present in GPA. Evidence of vasculitis and upper-airway involvement, as well as pulmonary involvement, can be seen in both GPA and EGPA. Other distinguishing factors include a history of asthma and peripheral eosinophilia, which are seen in EGPA.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 447509,
"questionText": "Which of the following is <i>not</i> seen in both EGPA and GPA?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1401624,
"choiceText": "Azathioprine\r\n\t\r\n",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1401626,
"choiceText": "Methotrexate",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1401628,
"choiceText": "Omalizumab",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1401630,
"choiceText": "Mepolizumab ",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "A study of mepolizumab for EGPA found that treatment resulted in more weeks in remission and a higher proportion of participants in remission than did placebo. This allowed for a greater reduction in total glucocorticoid use. However, approximately half the patients treated with mepolizumab did not achieve protocol-defined remission. Mepolizumab is FDA-approved for the treatment of EGPA.\r\n<br><br>\r\nAlthough other disease-modifying biologic agents can be considered, data favor mepolizumab over other medications in the setting of EGPA. ",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 447511,
"questionText": "Which of the following should be initiated as a steroid-sparing agent in a patient who requires glucocorticoids for the treatment of EGPA?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
828903 | /viewarticle/828903 | [
{
"authors": "Sumaira Nabi, MBBS; Sadaf Khattak, MBBS; Mazhar Badshah, MBBS, MD, FCPS",
"content": [
"Editorial Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"A 65-year-old man presents to the emergency department with a 20-day history of sudden, severe, sharp, right-sided retro-orbital pain. The onset of pain was followed two days later by diplopia in all directions of gaze (without visual loss) and by tingling on the upper half of the right side of the face; after an additional two days, the patient developed drooping of his right eyelid.",
"He reports no history of any other such episode or of fever, joint pains, rash, or weight loss. The patient is a mason with five children. He does not use tobacco or other substances. He has had hypertension for the last 11 years and is well controlled on tablet lisinopril 10 mg once daily. The family history is unremarkable."
],
"date": "August 21, 2019",
"figures": [],
"markdown": "# A 65-Year-Old Man With Diplopia and a Drooping Eyelid\n\n **Authors:** Sumaira Nabi, MBBS; Sadaf Khattak, MBBS; Mazhar Badshah, MBBS, MD, FCPS \n **Date:** August 21, 2019\n\n ## Content\n\n Editorial Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nA 65-year-old man presents to the emergency department with a 20-day history of sudden, severe, sharp, right-sided retro-orbital pain. The onset of pain was followed two days later by diplopia in all directions of gaze (without visual loss) and by tingling on the upper half of the right side of the face; after an additional two days, the patient developed drooping of his right eyelid.\nHe reports no history of any other such episode or of fever, joint pains, rash, or weight loss. The patient is a mason with five children. He does not use tobacco or other substances. He has had hypertension for the last 11 years and is well controlled on tablet lisinopril 10 mg once daily. The family history is unremarkable.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 65-Year-Old Man With Diplopia and a Drooping Eyelid"
},
{
"authors": "Sumaira Nabi, MBBS; Sadaf Khattak, MBBS; Mazhar Badshah, MBBS, MD, FCPS",
"content": [
"Upon clinical examination, the patient is an alert, older male oriented in time, place, and person. His vital signs include an oral temperature of 98.6°F (37°C), a regular pulse rate of 70 beats/minute, and a blood pressure of 140/70 mm Hg. His respiratory rate is 14 breaths/min, and his Glasgow Coma Scale is 15/15.",
"Upon ocular examination, the patient has a right-sided, partial ptosis with restriction of horizontal and vertical eye movements. He has no lid swelling, proptosis, or chemosis. The pupillary size and reaction are normal, and visual acuity is intact in both eyes. Upon funduscopic examination, changes are consistent with a grade-1 hypertensive retinopathy. However, the disc margins are clear.",
"Facial sensory loss in the distribution of the first division of right cranial nerve V (CN V1) is also detected. The remaining cranial nerves are intact and symmetrical. No signs suggest meningeal irritation, pyramidal weakness, or cerebellar dysfunction. The patient’s abdomen is soft and nontender, and no clinical evidence suggests organomegaly or ascites. Bowel sounds are audible. The patient's precordial examination reveals normal heart sounds, and auscultation of the lung fields shows normal vesicular breathing.",
"The laboratory analysis demonstrates a normal complete blood count (CBC) and erythrocyte sedimentation rate (ESR). The patient’s liver function test results, renal function test results, serum glucose levels, ECG, and chest radiograph findings are unremarkable. Results for antinuclear antibodies (ANAs), extractable nuclear antigen (ENA) profile, HIV serology, hepatitis B surface antigen (HBsAg), and hepatitis C antibody (anti-HCV) are negative. Serum angiotensin-converting enzyme (ACE) levels are normal, as are the thyroid function tests and routine cerebrospinal fluid (CSF) examination.",
"MRI of the brain and orbit shows a normal cavernous sinus, as well as normal brain parenchyma and orbital contents. No orbital or intracranial mass lesion is seen (Figures 1-3). Repetitive nerve stimulation is normal, and anti-acetylcholinesterase antibodies are negative.",
"Figure 1.",
"Figure 2.",
"Figure 3."
],
"date": "August 21, 2019",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/828/903/828903-thumb-1.jpg"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/828/903/828903-thumb-2.jpg"
},
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/828/903/828903-thumb-3.jpg"
}
],
"markdown": "# A 65-Year-Old Man With Diplopia and a Drooping Eyelid\n\n **Authors:** Sumaira Nabi, MBBS; Sadaf Khattak, MBBS; Mazhar Badshah, MBBS, MD, FCPS \n **Date:** August 21, 2019\n\n ## Content\n\n Upon clinical examination, the patient is an alert, older male oriented in time, place, and person. His vital signs include an oral temperature of 98.6°F (37°C), a regular pulse rate of 70 beats/minute, and a blood pressure of 140/70 mm Hg. His respiratory rate is 14 breaths/min, and his Glasgow Coma Scale is 15/15.\nUpon ocular examination, the patient has a right-sided, partial ptosis with restriction of horizontal and vertical eye movements. He has no lid swelling, proptosis, or chemosis. The pupillary size and reaction are normal, and visual acuity is intact in both eyes. Upon funduscopic examination, changes are consistent with a grade-1 hypertensive retinopathy. However, the disc margins are clear.\nFacial sensory loss in the distribution of the first division of right cranial nerve V (CN V1) is also detected. The remaining cranial nerves are intact and symmetrical. No signs suggest meningeal irritation, pyramidal weakness, or cerebellar dysfunction. The patient’s abdomen is soft and nontender, and no clinical evidence suggests organomegaly or ascites. Bowel sounds are audible. The patient's precordial examination reveals normal heart sounds, and auscultation of the lung fields shows normal vesicular breathing.\nThe laboratory analysis demonstrates a normal complete blood count (CBC) and erythrocyte sedimentation rate (ESR). The patient’s liver function test results, renal function test results, serum glucose levels, ECG, and chest radiograph findings are unremarkable. Results for antinuclear antibodies (ANAs), extractable nuclear antigen (ENA) profile, HIV serology, hepatitis B surface antigen (HBsAg), and hepatitis C antibody (anti-HCV) are negative. Serum angiotensin-converting enzyme (ACE) levels are normal, as are the thyroid function tests and routine cerebrospinal fluid (CSF) examination.\nMRI of the brain and orbit shows a normal cavernous sinus, as well as normal brain parenchyma and orbital contents. No orbital or intracranial mass lesion is seen (Figures 1-3). Repetitive nerve stimulation is normal, and anti-acetylcholinesterase antibodies are negative.\nFigure 1.\nFigure 2.\nFigure 3.\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n**Figure 3.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1398364,
"choiceText": "Parasellar syndrome",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1398366,
"choiceText": "Orbital lymphoma",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1398368,
"choiceText": "Cavernous sinus thrombosis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1398370,
"choiceText": "Tolosa-Hunt syndrome",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 446473,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 65-Year-Old Man With Diplopia and a Drooping Eyelid"
},
{
"authors": "Sumaira Nabi, MBBS; Sadaf Khattak, MBBS; Mazhar Badshah, MBBS, MD, FCPS",
"content": [
"The patient was given a trial of oral prednisolone and experienced significant pain relief within 48 hours. At discharge, after 10 days in the hospital, he had significant improvement in eye movement. He was discharged on a 6-week, tapering dose of oral steroids; the dosage was to be modified on follow-up visit based on his response.",
"Tolosa-Hunt syndrome (THS) is a rare disorder characterized by painful recurrent and relapsing ophthalmoplegia caused by nonspecific inflammation of the cavernous sinus and/or superior orbital fissure.[1] It manifests as recurrent episodes of retro-orbital, periorbital, or hemicranial pain, with ocular motor nerve palsies (ie, the third, fourth and sixth cranial nerves). Sensory loss in the distribution of the ophthalmic and occasionally the maxillary division of the trigeminal nerve may also be observed, occurring in various combinations and with oculosympathetic involvement.",
"In 1954, Tolosa reported THS for the first time, in a patient with painful, left-sided ophthalmoplegia, with involvement of left CN V1.[2] Cerebral angiography revealed segmental narrowing of the carotid siphon. Postmortem surgical exploration disclosed granulomatous inflammation of the left cavernous sinus and carotid artery. In 1961, Hunt et al described similar findings in 6 patients; they also reported the therapeutic efficacy of systemic corticosteroids, with prompt, dramatic improvement of signs and symptoms occurring in two of the patients.[3] In 1966, Smith and Taxdal termed this clinical condition Tolosa-Hunt syndrome.[4]",
"An uncommon disorder, THS affects males and females equally and can occur at virtually any age, although it is rarely seen during the first two decades of life.[1] Almost all patients report pain, which is a characteristic feature of the disease, although the nature of it is described differently by different patients. Usually, however, the pain is described as severe, sharp, stabbing, boring, lancinating, or intense. It may be periorbital in location, but some patients may report pain in different locations involving the retro-orbital, frontal, and temporal regions. In some cases, a severe hemicranial pain may be described. If left untreated, this pain persists, on average, for eight weeks. Either side may be affected; bilateral and/or simultaneous involvement may be present. The etiology of THS remains unknown.",
"Simultaneous onset of pain and ocular motor cranial nerve involvement may be noted, or a lag period as long as 2 weeks may occur between the two clinical features.[5] The oculomotor, trochlear, and abducens nerves may be involved alone or in various combinations. The pupil may have a normal size and reaction, as in our patient, or pupillary dilatation may occur secondary to parasympathetic third nerve involvement or constriction due to sympathetic involvement or Horner syndrome. Occasional optic nerve (CN II) involvement has been reported with THS if the pathologic process affects the orbital apex.[6,7,8] Variable degrees of visual loss may occur if optic nerve dysfunction is present. In rare cases, cranial nerves not located within the cavernous sinus/superior orbital fissure, including the mandibular division of the trigeminal nerve and the facial nerve, may be affected.[6,9,10] Other than nausea and vomiting, systemic or other neurologic involvement is not generally reported."
],
"date": "August 21, 2019",
"figures": [],
"markdown": "# A 65-Year-Old Man With Diplopia and a Drooping Eyelid\n\n **Authors:** Sumaira Nabi, MBBS; Sadaf Khattak, MBBS; Mazhar Badshah, MBBS, MD, FCPS \n **Date:** August 21, 2019\n\n ## Content\n\n The patient was given a trial of oral prednisolone and experienced significant pain relief within 48 hours. At discharge, after 10 days in the hospital, he had significant improvement in eye movement. He was discharged on a 6-week, tapering dose of oral steroids; the dosage was to be modified on follow-up visit based on his response.\nTolosa-Hunt syndrome (THS) is a rare disorder characterized by painful recurrent and relapsing ophthalmoplegia caused by nonspecific inflammation of the cavernous sinus and/or superior orbital fissure.[1] It manifests as recurrent episodes of retro-orbital, periorbital, or hemicranial pain, with ocular motor nerve palsies (ie, the third, fourth and sixth cranial nerves). Sensory loss in the distribution of the ophthalmic and occasionally the maxillary division of the trigeminal nerve may also be observed, occurring in various combinations and with oculosympathetic involvement.\nIn 1954, Tolosa reported THS for the first time, in a patient with painful, left-sided ophthalmoplegia, with involvement of left CN V1.[2] Cerebral angiography revealed segmental narrowing of the carotid siphon. Postmortem surgical exploration disclosed granulomatous inflammation of the left cavernous sinus and carotid artery. In 1961, Hunt et al described similar findings in 6 patients; they also reported the therapeutic efficacy of systemic corticosteroids, with prompt, dramatic improvement of signs and symptoms occurring in two of the patients.[3] In 1966, Smith and Taxdal termed this clinical condition Tolosa-Hunt syndrome.[4]\nAn uncommon disorder, THS affects males and females equally and can occur at virtually any age, although it is rarely seen during the first two decades of life.[1] Almost all patients report pain, which is a characteristic feature of the disease, although the nature of it is described differently by different patients. Usually, however, the pain is described as severe, sharp, stabbing, boring, lancinating, or intense. It may be periorbital in location, but some patients may report pain in different locations involving the retro-orbital, frontal, and temporal regions. In some cases, a severe hemicranial pain may be described. If left untreated, this pain persists, on average, for eight weeks. Either side may be affected; bilateral and/or simultaneous involvement may be present. The etiology of THS remains unknown.\nSimultaneous onset of pain and ocular motor cranial nerve involvement may be noted, or a lag period as long as 2 weeks may occur between the two clinical features.[5] The oculomotor, trochlear, and abducens nerves may be involved alone or in various combinations. The pupil may have a normal size and reaction, as in our patient, or pupillary dilatation may occur secondary to parasympathetic third nerve involvement or constriction due to sympathetic involvement or Horner syndrome. Occasional optic nerve (CN II) involvement has been reported with THS if the pathologic process affects the orbital apex.[6,7,8] Variable degrees of visual loss may occur if optic nerve dysfunction is present. In rare cases, cranial nerves not located within the cavernous sinus/superior orbital fissure, including the mandibular division of the trigeminal nerve and the facial nerve, may be affected.[6,9,10] Other than nausea and vomiting, systemic or other neurologic involvement is not generally reported.\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1398364,
"choiceText": "Parasellar syndrome",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1398366,
"choiceText": "Orbital lymphoma",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1398368,
"choiceText": "Cavernous sinus thrombosis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1398370,
"choiceText": "Tolosa-Hunt syndrome",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 446473,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 65-Year-Old Man With Diplopia and a Drooping Eyelid"
},
{
"authors": "Sumaira Nabi, MBBS; Sadaf Khattak, MBBS; Mazhar Badshah, MBBS, MD, FCPS",
"content": [
"THS has an extensive list of differentials, with many other disease entities having a similar clinical presentation. These include metastases, aspergillosis, sarcoidosis, carotid-cavernous fistulae, vasculopathic cranial neuropathy, Wegener granulomatosis, lymphoma, and ophthalmoplegic migraine.[11] The diagnosis is mainly clinical, based on history and examination, and is essentially one of exclusion.[3] Pertinent laboratory investigations may be done to eliminate other processes.[1] Laboratory workup requires a complete blood picture, ESR, electrolytes, blood glucose levels, thyroid function tests, ANAs, antineutrophil cytoplasmic antibodies (ANCAs), serum protein electrophoresis, Lyme titre, ACE level, and HIV titre to exclude other conditions, which can have significant morbidity associated. Anti-GQ1b antibodies may be helpful in distinguishing THS from Miller Fisher syndrome. Various CSF studies may also be required to eliminate conditions that mimic THS.",
"Neuroimaging must be done to rule out malignancies, vascular pathologies, and non-THS forms of inflammation in the region of the cavernous sinus and/or superior orbital fissure. Contrast-enhanced MRI should be the initial diagnostic study performed. Multiple views must be obtained, particularly coronal sections, which are important in visualizing the area of the cavernous sinus. In some patients with THS, an area of abnormal soft tissue in the region of the cavernous sinus is visualized, with intermediate signal intensity on T1- and intermediate-weighted images; this may show postcontrast enhancement suggestive of an inflammatory process.[12,13,14,15,16,17,18,19] Abnormal convexity of the wall of the cavernous sinus may be seen. At times, focal narrowing of the intracavernous internal carotid artery may be found. However, MRI findings in THS lack specificity, which is a drawback; at times, the MRI scan may even be normal, as in this particular patient.[20,21]",
"The International Headache Society (IHS) formulated specific diagnostic criteria for THS in 1988; the most recent criteria are available in the third edition of the International Classification of Headache Disorders (ICHD-3).[3,22] According to the society, characteristics of THS include the following:",
"Unilateral orbital or periorbital pain that is ipsilateral to granulomatous inflammation",
"Paresis of CN III, IV, and/or VI and demonstration via MRI or biopsy of granulomas present in the cavernous sinus, superior orbital fissure, or orbit",
"Development of cranial nerve palsy/palsies within two weeks of the onset of orbital pain or with onset of pain",
"Exclusion of other causes by appropriate investigation"
],
"date": "August 21, 2019",
"figures": [],
"markdown": "# A 65-Year-Old Man With Diplopia and a Drooping Eyelid\n\n **Authors:** Sumaira Nabi, MBBS; Sadaf Khattak, MBBS; Mazhar Badshah, MBBS, MD, FCPS \n **Date:** August 21, 2019\n\n ## Content\n\n THS has an extensive list of differentials, with many other disease entities having a similar clinical presentation. These include metastases, aspergillosis, sarcoidosis, carotid-cavernous fistulae, vasculopathic cranial neuropathy, Wegener granulomatosis, lymphoma, and ophthalmoplegic migraine.[11] The diagnosis is mainly clinical, based on history and examination, and is essentially one of exclusion.[3] Pertinent laboratory investigations may be done to eliminate other processes.[1] Laboratory workup requires a complete blood picture, ESR, electrolytes, blood glucose levels, thyroid function tests, ANAs, antineutrophil cytoplasmic antibodies (ANCAs), serum protein electrophoresis, Lyme titre, ACE level, and HIV titre to exclude other conditions, which can have significant morbidity associated. Anti-GQ1b antibodies may be helpful in distinguishing THS from Miller Fisher syndrome. Various CSF studies may also be required to eliminate conditions that mimic THS.\nNeuroimaging must be done to rule out malignancies, vascular pathologies, and non-THS forms of inflammation in the region of the cavernous sinus and/or superior orbital fissure. Contrast-enhanced MRI should be the initial diagnostic study performed. Multiple views must be obtained, particularly coronal sections, which are important in visualizing the area of the cavernous sinus. In some patients with THS, an area of abnormal soft tissue in the region of the cavernous sinus is visualized, with intermediate signal intensity on T1- and intermediate-weighted images; this may show postcontrast enhancement suggestive of an inflammatory process.[12,13,14,15,16,17,18,19] Abnormal convexity of the wall of the cavernous sinus may be seen. At times, focal narrowing of the intracavernous internal carotid artery may be found. However, MRI findings in THS lack specificity, which is a drawback; at times, the MRI scan may even be normal, as in this particular patient.[20,21]\nThe International Headache Society (IHS) formulated specific diagnostic criteria for THS in 1988; the most recent criteria are available in the third edition of the International Classification of Headache Disorders (ICHD-3).[3,22] According to the society, characteristics of THS include the following:\nUnilateral orbital or periorbital pain that is ipsilateral to granulomatous inflammation\nParesis of CN III, IV, and/or VI and demonstration via MRI or biopsy of granulomas present in the cavernous sinus, superior orbital fissure, or orbit\nDevelopment of cranial nerve palsy/palsies within two weeks of the onset of orbital pain or with onset of pain\nExclusion of other causes by appropriate investigation\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 65-Year-Old Man With Diplopia and a Drooping Eyelid"
},
{
"authors": "Sumaira Nabi, MBBS; Sadaf Khattak, MBBS; Mazhar Badshah, MBBS, MD, FCPS",
"content": [
"THS is a self-limited illness. A dramatic improvement in painful ophthalmoplegia of THS after administration of steroids for 48 hours is a hallmark feature that differentiates THS from other causes of painful ophthalmoplegia. The usual regimen is a course of steroids tapered over weeks to months. However, some cases may necessitate prolonged therapy. THS still causes significant morbidity, and in some patients residual cranial nerve palsies persist.[5]",
"The onset of THS is usually acute, with an unpredictable clinical course. Although spontaneous remissions do occur, recurrences are common. For refractory cases, azathioprine (Imuran), methotrexate, or radiation therapy has been instituted.[1] Surgical intervention is reserved for cases in which histopathologic evidence is required. The patient in this case made a full recovery and regained complete ocular motility."
],
"date": "August 21, 2019",
"figures": [],
"markdown": "# A 65-Year-Old Man With Diplopia and a Drooping Eyelid\n\n **Authors:** Sumaira Nabi, MBBS; Sadaf Khattak, MBBS; Mazhar Badshah, MBBS, MD, FCPS \n **Date:** August 21, 2019\n\n ## Content\n\n THS is a self-limited illness. A dramatic improvement in painful ophthalmoplegia of THS after administration of steroids for 48 hours is a hallmark feature that differentiates THS from other causes of painful ophthalmoplegia. The usual regimen is a course of steroids tapered over weeks to months. However, some cases may necessitate prolonged therapy. THS still causes significant morbidity, and in some patients residual cranial nerve palsies persist.[5]\nThe onset of THS is usually acute, with an unpredictable clinical course. Although spontaneous remissions do occur, recurrences are common. For refractory cases, azathioprine (Imuran), methotrexate, or radiation therapy has been instituted.[1] Surgical intervention is reserved for cases in which histopathologic evidence is required. The patient in this case made a full recovery and regained complete ocular motility.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1398372,
"choiceText": "CN II",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1398374,
"choiceText": "CN III",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1398376,
"choiceText": "CN XI",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1398378,
"choiceText": "CN IV",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1398380,
"choiceText": "CN VI",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Diagnostic criteria for THS from the ISH include paresis of CN III, IV, and/or VI. Occasional involvement of the optic nerve (CN II) has also been reported in THS if the pathologic process affects the orbital apex. The syndrome does not involve CN XI.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 446475,
"questionText": "Which of the following cranial nerves is not involved in THS?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1398382,
"choiceText": "Viral infection",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1398384,
"choiceText": "Fungal infection",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1398386,
"choiceText": "Bacterial infection",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1398388,
"choiceText": "Unknown",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1398390,
"choiceText": "Demyelination",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "THS involves a nonspecific inflammatory process involving the boundaries of the cavernous sinus and/or superior orbital fissure. Some have reported granulomatous inflammation, and others have identified necrotic tissue on histopathology. However, the exact etiology leading to this cascade of inflammation has not yet been elucidated. No infectious agent has been isolated or identified as the likely cause.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 446477,
"questionText": "What is the etiology of THS?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 65-Year-Old Man With Diplopia and a Drooping Eyelid"
},
{
"authors": "Sumaira Nabi, MBBS; Sadaf Khattak, MBBS; Mazhar Badshah, MBBS, MD, FCPS",
"content": [],
"date": "August 21, 2019",
"figures": [],
"markdown": "# A 65-Year-Old Man With Diplopia and a Drooping Eyelid\n\n **Authors:** Sumaira Nabi, MBBS; Sadaf Khattak, MBBS; Mazhar Badshah, MBBS, MD, FCPS \n **Date:** August 21, 2019\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1398372,
"choiceText": "CN II",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1398374,
"choiceText": "CN III",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1398376,
"choiceText": "CN XI",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1398378,
"choiceText": "CN IV",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1398380,
"choiceText": "CN VI",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Diagnostic criteria for THS from the ISH include paresis of CN III, IV, and/or VI. Occasional involvement of the optic nerve (CN II) has also been reported in THS if the pathologic process affects the orbital apex. The syndrome does not involve CN XI.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 446475,
"questionText": "Which of the following cranial nerves is not involved in THS?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1398382,
"choiceText": "Viral infection",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1398384,
"choiceText": "Fungal infection",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1398386,
"choiceText": "Bacterial infection",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1398388,
"choiceText": "Unknown",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1398390,
"choiceText": "Demyelination",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "THS involves a nonspecific inflammatory process involving the boundaries of the cavernous sinus and/or superior orbital fissure. Some have reported granulomatous inflammation, and others have identified necrotic tissue on histopathology. However, the exact etiology leading to this cascade of inflammation has not yet been elucidated. No infectious agent has been isolated or identified as the likely cause.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 446477,
"questionText": "What is the etiology of THS?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 65-Year-Old Man With Diplopia and a Drooping Eyelid"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1398364,
"choiceText": "Parasellar syndrome",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1398366,
"choiceText": "Orbital lymphoma",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1398368,
"choiceText": "Cavernous sinus thrombosis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1398370,
"choiceText": "Tolosa-Hunt syndrome",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 446473,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1398372,
"choiceText": "CN II",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1398374,
"choiceText": "CN III",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1398376,
"choiceText": "CN XI",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1398378,
"choiceText": "CN IV",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1398380,
"choiceText": "CN VI",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Diagnostic criteria for THS from the ISH include paresis of CN III, IV, and/or VI. Occasional involvement of the optic nerve (CN II) has also been reported in THS if the pathologic process affects the orbital apex. The syndrome does not involve CN XI.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 446475,
"questionText": "Which of the following cranial nerves is not involved in THS?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1398382,
"choiceText": "Viral infection",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1398384,
"choiceText": "Fungal infection",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1398386,
"choiceText": "Bacterial infection",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1398388,
"choiceText": "Unknown",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1398390,
"choiceText": "Demyelination",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "THS involves a nonspecific inflammatory process involving the boundaries of the cavernous sinus and/or superior orbital fissure. Some have reported granulomatous inflammation, and others have identified necrotic tissue on histopathology. However, the exact etiology leading to this cascade of inflammation has not yet been elucidated. No infectious agent has been isolated or identified as the likely cause.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 446477,
"questionText": "What is the etiology of THS?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
916974 | /viewarticle/916974 | [
{
"authors": "Andres Applewhite, MD",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"The patient is a 22-year-old white man who presents after he lost consciousness while climbing a flight of stairs. He states that this is the first such incident. He also states that he does not take any supplements, medications, or illicit drugs. He is a nonsmoker. He claims that his alcohol intake is infrequent, minimal, and limited to social events. He has not had any recent travel, illness, or sick contacts. His family history is only significant for type 1 diabetes in his maternal grandfather.",
"Upon physical examination, the patient has a temperature of 99.5° F (37.5° C). His blood pressure is 115/75 mm Hg, his respiratory rate is 17 breaths/min (with a pulse oximetry of 95% on room air), and his heart rate is 85 beats/min. Examination also reveals a parasternal heave, a pansystolic murmur that is loudest at the left lower sternal edge, and an accompanying thrill in the suprasternal region. His weight is 151.7 lb (68.8 kg), and his height is 5' 7\" (1.7 m).",
"An examination of his neck, lungs, and abdomen is unremarkable. Severe clubbing of his fingers is noted. Muscle strength is 5/5, and reflexes are 2+ throughout. No peripheral edema or jugular venous distention is appreciated. Peripheral pulses are palpable. Finger-to-nose test results are normal. Romberg sign is negative. His gait is normal. Neurological examination is unremarkable. He is calm, cooperative, and in no discomfort.",
"Electrocardiography reveals sinus rhythm, right axis deviation, and evidence of right ventricular hypertrophy with secondary ST-T wave abnormalities. Chest radiography is suggestive of right ventricular hypertrophy.",
"Transthoracic echocardiography reveals a hypokinetic, small-volume left ventricle with an ejection fraction of 40%. It also reveals a hypertrophied septal wall, a membranous ventricular septal defect with a right-to-left shunt, a hypokinetic and severely enlarged right ventricle, dilated right atrium, and subvalvular right ventricular outflow tract obstruction.",
"His complete blood cell count, basic metabolic panel, arterial gas, and cardiac enzyme findings are within the reference range, other than a hematocrit level of 56.8% and lactate levels of 3.4 mmol/L."
],
"date": "August 20, 2019",
"figures": [],
"markdown": "# A 22-Year-Old Man Who Lost Consciousness Climbing Stairs\n\n **Authors:** Andres Applewhite, MD \n **Date:** August 20, 2019\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nThe patient is a 22-year-old white man who presents after he lost consciousness while climbing a flight of stairs. He states that this is the first such incident. He also states that he does not take any supplements, medications, or illicit drugs. He is a nonsmoker. He claims that his alcohol intake is infrequent, minimal, and limited to social events. He has not had any recent travel, illness, or sick contacts. His family history is only significant for type 1 diabetes in his maternal grandfather.\nUpon physical examination, the patient has a temperature of 99.5° F (37.5° C). His blood pressure is 115/75 mm Hg, his respiratory rate is 17 breaths/min (with a pulse oximetry of 95% on room air), and his heart rate is 85 beats/min. Examination also reveals a parasternal heave, a pansystolic murmur that is loudest at the left lower sternal edge, and an accompanying thrill in the suprasternal region. His weight is 151.7 lb (68.8 kg), and his height is 5' 7\" (1.7 m).\nAn examination of his neck, lungs, and abdomen is unremarkable. Severe clubbing of his fingers is noted. Muscle strength is 5/5, and reflexes are 2+ throughout. No peripheral edema or jugular venous distention is appreciated. Peripheral pulses are palpable. Finger-to-nose test results are normal. Romberg sign is negative. His gait is normal. Neurological examination is unremarkable. He is calm, cooperative, and in no discomfort.\nElectrocardiography reveals sinus rhythm, right axis deviation, and evidence of right ventricular hypertrophy with secondary ST-T wave abnormalities. Chest radiography is suggestive of right ventricular hypertrophy.\nTransthoracic echocardiography reveals a hypokinetic, small-volume left ventricle with an ejection fraction of 40%. It also reveals a hypertrophied septal wall, a membranous ventricular septal defect with a right-to-left shunt, a hypokinetic and severely enlarged right ventricle, dilated right atrium, and subvalvular right ventricular outflow tract obstruction.\nHis complete blood cell count, basic metabolic panel, arterial gas, and cardiac enzyme findings are within the reference range, other than a hematocrit level of 56.8% and lactate levels of 3.4 mmol/L.\n\n ## Figures\n\n \n*Page 1 of 5*",
"pagination": {
"current_page": 1,
"total_pages": 5
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1397156,
"choiceText": "Pulmonary atresia",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1397158,
"choiceText": "Tetralogy of Fallot",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1397160,
"choiceText": "Right-sided heart failure",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1397162,
"choiceText": "Hypoplastic left heart syndrome",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1397164,
"choiceText": "Double outlet right ventricle",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 446091,
"questionText": "Which one of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 22-Year-Old Man Who Lost Consciousness Climbing Stairs"
},
{
"authors": "Andres Applewhite, MD",
"content": [
"Tetralogy of Fallot (TOF) is the most common cyanotic heart disease and accounts for 10% of all cases of congenital heart disease.[1] The Centers for Disease Control and Prevention estimates that each year, about 1660 babies in the United States are born with TOF.[2] The vast majority of these patients undergo surgery to correct these anatomical defects before their first year of life. Mortality in unrepaired defects is very high. Left untreated, 66% of patients survive the first year of life, 24% survive 10 years, and only 4% survive to age 30 years.[1]",
"This patient's echocardiogram reports three of the following four essential components of TOF:",
"Ventricular septal defect",
"Hypertrophy of the right ventricle",
"Right ventricular outflow obstruction",
"Overriding aorta",
"The patient was also found to have an overriding aorta. The pathology behind these four defects involves the underdevelopment of the right ventricular infundibulum, resulting in an anterior-leftward misalignment of the infundibular septum. This misalignment defines the amount of right ventricular outflow tract obstruction, with its detrimental consequences.",
"TOF can present with various clinical signs and symptoms, primarily as a result of differences in the basic anatomic morphology of the heart and the type of surgical repair performed in infancy. The pathophysiology of this condition primarily depends on the severity of the right ventricular outflow tract obstruction, which then determines the severity of the right-to-left shunting.",
"Other diseases that can present in a similar fashion to TOF include the following:",
"Pulmonary stenosis presents with a systolic ejection murmur that can be difficult to clinically differentiate from TOF",
"The intensity of the murmur depends on the size of the ventricular septal defect. Smaller ventricular septal defects tend to have a louder murmur because the gradient between right and left ventricles is higher. A large defect usually has softer murmurs because pressures are more equalized between both ventricles.",
"Hypoplastic left heart syndrome, tricuspid atresia, D-transposition, pulmonary atresia, anomalous pulmonary venous connection, truncus arteriosus, and Ebstein anomaly can all be difficult to differentiate clinically from cyanotic newborns with TOF.",
"Double outlet right ventricle cannot be differentiated from TOF based solely on physical examination findings.",
"Other diseases that can present with difficulty breathing and cyanosis in infants include pulmonary diseases (eg, bronchiolitis, acute respiratory distress syndrome, apnea, pneumonia, pneumothorax), foreign body ingestion, and sickle cell anemia.",
"In the case of this patient, all of these diseases were essentially ruled out by visualizing the anatomy of the heart. Because TOF has such particular and specific anatomic defects, its diagnosis is fairly evident once the heart structures can be visualized."
],
"date": "August 20, 2019",
"figures": [],
"markdown": "# A 22-Year-Old Man Who Lost Consciousness Climbing Stairs\n\n **Authors:** Andres Applewhite, MD \n **Date:** August 20, 2019\n\n ## Content\n\n Tetralogy of Fallot (TOF) is the most common cyanotic heart disease and accounts for 10% of all cases of congenital heart disease.[1] The Centers for Disease Control and Prevention estimates that each year, about 1660 babies in the United States are born with TOF.[2] The vast majority of these patients undergo surgery to correct these anatomical defects before their first year of life. Mortality in unrepaired defects is very high. Left untreated, 66% of patients survive the first year of life, 24% survive 10 years, and only 4% survive to age 30 years.[1]\nThis patient's echocardiogram reports three of the following four essential components of TOF:\nVentricular septal defect\nHypertrophy of the right ventricle\nRight ventricular outflow obstruction\nOverriding aorta\nThe patient was also found to have an overriding aorta. The pathology behind these four defects involves the underdevelopment of the right ventricular infundibulum, resulting in an anterior-leftward misalignment of the infundibular septum. This misalignment defines the amount of right ventricular outflow tract obstruction, with its detrimental consequences.\nTOF can present with various clinical signs and symptoms, primarily as a result of differences in the basic anatomic morphology of the heart and the type of surgical repair performed in infancy. The pathophysiology of this condition primarily depends on the severity of the right ventricular outflow tract obstruction, which then determines the severity of the right-to-left shunting.\nOther diseases that can present in a similar fashion to TOF include the following:\nPulmonary stenosis presents with a systolic ejection murmur that can be difficult to clinically differentiate from TOF\nThe intensity of the murmur depends on the size of the ventricular septal defect. Smaller ventricular septal defects tend to have a louder murmur because the gradient between right and left ventricles is higher. A large defect usually has softer murmurs because pressures are more equalized between both ventricles.\nHypoplastic left heart syndrome, tricuspid atresia, D-transposition, pulmonary atresia, anomalous pulmonary venous connection, truncus arteriosus, and Ebstein anomaly can all be difficult to differentiate clinically from cyanotic newborns with TOF.\nDouble outlet right ventricle cannot be differentiated from TOF based solely on physical examination findings.\nOther diseases that can present with difficulty breathing and cyanosis in infants include pulmonary diseases (eg, bronchiolitis, acute respiratory distress syndrome, apnea, pneumonia, pneumothorax), foreign body ingestion, and sickle cell anemia.\nIn the case of this patient, all of these diseases were essentially ruled out by visualizing the anatomy of the heart. Because TOF has such particular and specific anatomic defects, its diagnosis is fairly evident once the heart structures can be visualized.\n\n ## Figures\n\n \n*Page 2 of 5*",
"pagination": {
"current_page": 2,
"total_pages": 5
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1397156,
"choiceText": "Pulmonary atresia",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1397158,
"choiceText": "Tetralogy of Fallot",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1397160,
"choiceText": "Right-sided heart failure",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1397162,
"choiceText": "Hypoplastic left heart syndrome",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1397164,
"choiceText": "Double outlet right ventricle",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 446091,
"questionText": "Which one of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 22-Year-Old Man Who Lost Consciousness Climbing Stairs"
},
{
"authors": "Andres Applewhite, MD",
"content": [
"The vast majority of patients with TOF are diagnosed prenatally or perinatally. When diagnosed prenatally, certain fetal defects are noted during routine imaging; fetal echocardiography can then be requested to confirm the diagnosis. Postnatal diagnosis is usually made when the neonate suffers an episode of turning blue (a \"tet spell\") or by detecting a heart murmur during examination; however, a heart murmur may be absent at first. Again, the diagnosis can be confirmed with echocardiography.",
"The rare patients who go undiagnosed, such as the one in this case, remain relatively asymptomatic because of several factors. This mainly involves a delicate balance between the degree of right ventricular outflow tract obstruction and the ventricular septal defect. A certain equilibrium can be maintained between the pulmonary and systemic circulation until this balance is disrupted later in life.",
"Also, developing left ventricular hypertrophy delays the right-to-left shunting of blood within the ventricles, which helps delay symptoms. Finally, these patients can eventually develop intracardiac shunts, such as patent ductus arteriosus, or extracardiac shunts through internal mammaries, which contribute to maintaining the balance between pressures. All these factors can help keep symptoms to a minimum.",
"Color-flow echocardiography usually yields a vast amount of information when performed on a patient with TOF. Thus, it is among the first tests to be performed. It can assess the overall cardiac function, the status and anatomy of the valves, the presence of a ventricular septal defect or atrial septal defect, shunts, and regurgitant flows. It can also estimate chamber sizes and the grade and severity of right ventricular outflow tract obstruction.",
"MRI is now considered the criterion standard for assessing the right ventricular anatomy and function. It can reveal the chamber size and measure intracardiac pressures, gradients, and blood flow speeds. It can also reveal the status of a right ventricular outflow tract obstruction and the presence of ventricular septal defects.",
"In addition, MRI can reveal the anatomy of the pulmonary valve and quantify regurgitant volume, which is the most common problem later in life for patients who underwent surgery for TOF in childhood. This is the initial test when a problem with the pulmonary valve is suspected.[3] MRI is sensitive at uncovering branch pulmonary artery stenosis, which can contribute to increased pulmonary valve insufficiency and the formation of aortopulmonary collaterals.",
"In patients with TOF, electrocardiography usually reveals the presence of right ventricular hypertrophy, noted as right axis deviation (>90°), tall R-waves in lead V1, deep S-waves in lead V5-V6, and/or a slight increase in QRS duration.[4] Furthermore, when the QRS interval is longer than 180 milliseconds, it is a significant marker for the development of ventricular arrhythmias and sudden death.[5] Another predictor is the rate of change in QRS interval: a fast increase (>3.5 milliseconds/year) is associated with a higher risk for arrhythmias and sudden death.[6]",
"Although not routinely performed, cardiac catheterization can be used in patients with TOF to assess the pressures and status of the right ventricle and pulmonary artery. It can also be performed when some aspects of cardiac anatomy cannot be entirely appreciated using echocardiography, or if an anomalous coronary artery or pulmonary hypertension are suspected.",
"The clinical features of TOF in adults are directly related to the severity of the anatomic defects. These may include right-sided heart failure (eg, elevated jugular venous pressure, ascites, peripheral edema, hepatomegaly) and vulnerability to ventricular arrhythmias (sometimes fatal) secondary to chronic pulmonary regurgitation and subsequent ventricular hypertrophy.[7] Patients with TOF have a lifelong reduction in exercise tolerance, develop palpitations and syncope, and undergo general decline in body functions. These patients develop severe clubbing. They often learn to \"squat\" to increase pulmonary blood flow by increasing systemic vascular resistance, therefore temporarily reducing the amount of intracardiac shunting of blood through the ventricular septal defect. They may be polycythemic and suffer complications associated with long-standing polycythemia, including coagulopathy, intracranial abscess, stroke, hyperuricemia, and neurodevelopmental delay.[8]"
],
"date": "August 20, 2019",
"figures": [],
"markdown": "# A 22-Year-Old Man Who Lost Consciousness Climbing Stairs\n\n **Authors:** Andres Applewhite, MD \n **Date:** August 20, 2019\n\n ## Content\n\n The vast majority of patients with TOF are diagnosed prenatally or perinatally. When diagnosed prenatally, certain fetal defects are noted during routine imaging; fetal echocardiography can then be requested to confirm the diagnosis. Postnatal diagnosis is usually made when the neonate suffers an episode of turning blue (a \"tet spell\") or by detecting a heart murmur during examination; however, a heart murmur may be absent at first. Again, the diagnosis can be confirmed with echocardiography.\nThe rare patients who go undiagnosed, such as the one in this case, remain relatively asymptomatic because of several factors. This mainly involves a delicate balance between the degree of right ventricular outflow tract obstruction and the ventricular septal defect. A certain equilibrium can be maintained between the pulmonary and systemic circulation until this balance is disrupted later in life.\nAlso, developing left ventricular hypertrophy delays the right-to-left shunting of blood within the ventricles, which helps delay symptoms. Finally, these patients can eventually develop intracardiac shunts, such as patent ductus arteriosus, or extracardiac shunts through internal mammaries, which contribute to maintaining the balance between pressures. All these factors can help keep symptoms to a minimum.\nColor-flow echocardiography usually yields a vast amount of information when performed on a patient with TOF. Thus, it is among the first tests to be performed. It can assess the overall cardiac function, the status and anatomy of the valves, the presence of a ventricular septal defect or atrial septal defect, shunts, and regurgitant flows. It can also estimate chamber sizes and the grade and severity of right ventricular outflow tract obstruction.\nMRI is now considered the criterion standard for assessing the right ventricular anatomy and function. It can reveal the chamber size and measure intracardiac pressures, gradients, and blood flow speeds. It can also reveal the status of a right ventricular outflow tract obstruction and the presence of ventricular septal defects.\nIn addition, MRI can reveal the anatomy of the pulmonary valve and quantify regurgitant volume, which is the most common problem later in life for patients who underwent surgery for TOF in childhood. This is the initial test when a problem with the pulmonary valve is suspected.[3] MRI is sensitive at uncovering branch pulmonary artery stenosis, which can contribute to increased pulmonary valve insufficiency and the formation of aortopulmonary collaterals.\nIn patients with TOF, electrocardiography usually reveals the presence of right ventricular hypertrophy, noted as right axis deviation (>90°), tall R-waves in lead V1, deep S-waves in lead V5-V6, and/or a slight increase in QRS duration.[4] Furthermore, when the QRS interval is longer than 180 milliseconds, it is a significant marker for the development of ventricular arrhythmias and sudden death.[5] Another predictor is the rate of change in QRS interval: a fast increase (>3.5 milliseconds/year) is associated with a higher risk for arrhythmias and sudden death.[6]\nAlthough not routinely performed, cardiac catheterization can be used in patients with TOF to assess the pressures and status of the right ventricle and pulmonary artery. It can also be performed when some aspects of cardiac anatomy cannot be entirely appreciated using echocardiography, or if an anomalous coronary artery or pulmonary hypertension are suspected.\nThe clinical features of TOF in adults are directly related to the severity of the anatomic defects. These may include right-sided heart failure (eg, elevated jugular venous pressure, ascites, peripheral edema, hepatomegaly) and vulnerability to ventricular arrhythmias (sometimes fatal) secondary to chronic pulmonary regurgitation and subsequent ventricular hypertrophy.[7] Patients with TOF have a lifelong reduction in exercise tolerance, develop palpitations and syncope, and undergo general decline in body functions. These patients develop severe clubbing. They often learn to \"squat\" to increase pulmonary blood flow by increasing systemic vascular resistance, therefore temporarily reducing the amount of intracardiac shunting of blood through the ventricular septal defect. They may be polycythemic and suffer complications associated with long-standing polycythemia, including coagulopathy, intracranial abscess, stroke, hyperuricemia, and neurodevelopmental delay.[8]\n\n ## Figures\n\n \n*Page 3 of 5*",
"pagination": {
"current_page": 3,
"total_pages": 5
},
"questionnaire": [],
"title": "A 22-Year-Old Man Who Lost Consciousness Climbing Stairs"
},
{
"authors": "Andres Applewhite, MD",
"content": [
"Numerous potential immediate complications are associated with surgical repair of TOF, including electrical disturbances, arrhythmias, leaking valves, residual ventricular septal defect, aneurysm, and endocarditis. The most common long-term complication for these patients is pulmonary valve regurgitation, with its array of consequences.",
"Primary surgical correction in infants with TOF is the ideal treatment. Virtually all affected newborns undergo corrective surgery within months after diagnosis. The aim of surgery is to close the ventricular septal defect and resect the area of infundibular stenosis, thereby relieving right ventricular outflow tract obstruction. This procedure used to be considered the definitive cure for TOF; however, related issues almost inevitably arise years later. These include chronic pulmonary valve insufficiency, obstruction of the right ventricular outflow tract, depressed right ventricular function, residual ventricular septal defect leaks, and arrhythmias. Many experts now consider initial surgical repair to be long-term palliative, as opposed to corrective.[4] Pulmonary regurgitation is the most common complication that occurs in adults with TOF who underwent initial surgical repair. Thus, pulmonary valve replacement is almost always required at some point.[4] Unlike infants with TOF, emergency surgery is rare in adults.",
"As with any surgical procedure, risks must be weighed against benefits. If the patient is in such an advanced state of disease that right ventricular dysfunction is irreversible, nothing short of a heart transplant is lifesaving. The surgical procedure to correct TOF is complex, delicate, and highly technical. It should be performed by a surgeon who specializes in adult congenital heart disease. The operation involves a combination of techniques to fix all four defects and generally involves closing the ventricular septal defect with a patch, widening the infundibulum, and repairing or replacing the pulmonary valve.",
"No medical treatment for pulmonary insufficiency is effective. However, research is being conducted on drugs that can dilate the pulmonary vasculature and lower the pulmonary insufficiency. Sildenafil has been used to treat such patients. However, no long-term studies have established whether this agent can prevent the progression of pulmonary insufficiency.[9]",
"For this patient, a highly specialized multidisciplinary team was consulted. After analyzing results from the battery of tests, surgical repair was decided. The surgical team opted to close the ventricular septal defect with a patch, open the passageway out of the right ventricle, and replace the pulmonary valve. Because the pulmonary arteries to both lungs were enlarged, a tube was placed between the right ventricle and the pulmonary artery to improve blood flow."
],
"date": "August 20, 2019",
"figures": [],
"markdown": "# A 22-Year-Old Man Who Lost Consciousness Climbing Stairs\n\n **Authors:** Andres Applewhite, MD \n **Date:** August 20, 2019\n\n ## Content\n\n Numerous potential immediate complications are associated with surgical repair of TOF, including electrical disturbances, arrhythmias, leaking valves, residual ventricular septal defect, aneurysm, and endocarditis. The most common long-term complication for these patients is pulmonary valve regurgitation, with its array of consequences.\nPrimary surgical correction in infants with TOF is the ideal treatment. Virtually all affected newborns undergo corrective surgery within months after diagnosis. The aim of surgery is to close the ventricular septal defect and resect the area of infundibular stenosis, thereby relieving right ventricular outflow tract obstruction. This procedure used to be considered the definitive cure for TOF; however, related issues almost inevitably arise years later. These include chronic pulmonary valve insufficiency, obstruction of the right ventricular outflow tract, depressed right ventricular function, residual ventricular septal defect leaks, and arrhythmias. Many experts now consider initial surgical repair to be long-term palliative, as opposed to corrective.[4] Pulmonary regurgitation is the most common complication that occurs in adults with TOF who underwent initial surgical repair. Thus, pulmonary valve replacement is almost always required at some point.[4] Unlike infants with TOF, emergency surgery is rare in adults.\nAs with any surgical procedure, risks must be weighed against benefits. If the patient is in such an advanced state of disease that right ventricular dysfunction is irreversible, nothing short of a heart transplant is lifesaving. The surgical procedure to correct TOF is complex, delicate, and highly technical. It should be performed by a surgeon who specializes in adult congenital heart disease. The operation involves a combination of techniques to fix all four defects and generally involves closing the ventricular septal defect with a patch, widening the infundibulum, and repairing or replacing the pulmonary valve.\nNo medical treatment for pulmonary insufficiency is effective. However, research is being conducted on drugs that can dilate the pulmonary vasculature and lower the pulmonary insufficiency. Sildenafil has been used to treat such patients. However, no long-term studies have established whether this agent can prevent the progression of pulmonary insufficiency.[9]\nFor this patient, a highly specialized multidisciplinary team was consulted. After analyzing results from the battery of tests, surgical repair was decided. The surgical team opted to close the ventricular septal defect with a patch, open the passageway out of the right ventricle, and replace the pulmonary valve. Because the pulmonary arteries to both lungs were enlarged, a tube was placed between the right ventricle and the pulmonary artery to improve blood flow.\n\n ## Figures\n\n \n*Page 4 of 5*",
"pagination": {
"current_page": 4,
"total_pages": 5
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1397166,
"choiceText": "CT",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1397168,
"choiceText": "MRI",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1397170,
"choiceText": "Echocardiography",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1397172,
"choiceText": "Cardiac catheterization",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>MRI has become the criterion standard for assessing right ventricular function and size and for quantifying the pulmonary regurgitant volume. MRI can also map the speed of pulmonary regurgitation and provide a good outline of the aortic size and evaluation of the pulmonary arteries and its branches, the status of the right ventricular outflow tract, and the presence of ventricular septal defects and right ventricular hypertrophy. It can also be used to measure intracardiac pressures, gradients, and blood flows.<sup type=\"ref\">[10]</sup></p>",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 446093,
"questionText": "Which one of the following is considered the criterion standard study to evaluate right ventricular function?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1397182,
"choiceText": "To reduce the diameter of the ventricular septal defect",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1397184,
"choiceText": "To improve the forward flow of blood into systemic circulation",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1397186,
"choiceText": "To help force more blood through the pulmonary valve",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1397188,
"choiceText": "To decrease heart rate, and therefore improve cardiac oxygen consumption",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1397190,
"choiceText": "To increase venous return, and therefore improve right ventricular function",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>When patients with TOF have a high grade of right ventricular outflow tract obstruction, the right ventricle must generate increased pressure to get the column of blood through the pulmonary valve. When pressure generated by the right ventricle supersedes the pressure generated by the left ventricle, blood is forced through the ventricular septal defect from the right to left ventricle during systole. This right-to-left shunt allows for deoxygenated blood to enter systemic circulation and cause symptoms (such as \"tet spells\").</p>\r\n\r\n<p>By squatting, systemic vascular resistance is greatly increased, thus requiring the left ventricle to generate much higher pressures to pump the blood into the aorta. When this increase in left ventricular pressure surpasses the already high basal right ventricular pressure, the blood no longer shunts from right to left through the ventricular septal defect; instead, more blood is forced to go through the pulmonary valve into the lungs and increase blood oxygenation, which reduces symptoms.<sup type=\"ref\">[11]</sup></p>",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 446097,
"questionText": "Which one of the following best explains why individuals with TOF learn to squat to improve symptoms?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 22-Year-Old Man Who Lost Consciousness Climbing Stairs"
},
{
"authors": "Andres Applewhite, MD",
"content": [],
"date": "August 20, 2019",
"figures": [],
"markdown": "# A 22-Year-Old Man Who Lost Consciousness Climbing Stairs\n\n **Authors:** Andres Applewhite, MD \n **Date:** August 20, 2019\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 5 of 5*",
"pagination": {
"current_page": 5,
"total_pages": 5
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1397166,
"choiceText": "CT",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1397168,
"choiceText": "MRI",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1397170,
"choiceText": "Echocardiography",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1397172,
"choiceText": "Cardiac catheterization",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>MRI has become the criterion standard for assessing right ventricular function and size and for quantifying the pulmonary regurgitant volume. MRI can also map the speed of pulmonary regurgitation and provide a good outline of the aortic size and evaluation of the pulmonary arteries and its branches, the status of the right ventricular outflow tract, and the presence of ventricular septal defects and right ventricular hypertrophy. It can also be used to measure intracardiac pressures, gradients, and blood flows.<sup type=\"ref\">[10]</sup></p>",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 446093,
"questionText": "Which one of the following is considered the criterion standard study to evaluate right ventricular function?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1397182,
"choiceText": "To reduce the diameter of the ventricular septal defect",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1397184,
"choiceText": "To improve the forward flow of blood into systemic circulation",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1397186,
"choiceText": "To help force more blood through the pulmonary valve",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1397188,
"choiceText": "To decrease heart rate, and therefore improve cardiac oxygen consumption",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1397190,
"choiceText": "To increase venous return, and therefore improve right ventricular function",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>When patients with TOF have a high grade of right ventricular outflow tract obstruction, the right ventricle must generate increased pressure to get the column of blood through the pulmonary valve. When pressure generated by the right ventricle supersedes the pressure generated by the left ventricle, blood is forced through the ventricular septal defect from the right to left ventricle during systole. This right-to-left shunt allows for deoxygenated blood to enter systemic circulation and cause symptoms (such as \"tet spells\").</p>\r\n\r\n<p>By squatting, systemic vascular resistance is greatly increased, thus requiring the left ventricle to generate much higher pressures to pump the blood into the aorta. When this increase in left ventricular pressure surpasses the already high basal right ventricular pressure, the blood no longer shunts from right to left through the ventricular septal defect; instead, more blood is forced to go through the pulmonary valve into the lungs and increase blood oxygenation, which reduces symptoms.<sup type=\"ref\">[11]</sup></p>",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 446097,
"questionText": "Which one of the following best explains why individuals with TOF learn to squat to improve symptoms?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 22-Year-Old Man Who Lost Consciousness Climbing Stairs"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1397156,
"choiceText": "Pulmonary atresia",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1397158,
"choiceText": "Tetralogy of Fallot",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1397160,
"choiceText": "Right-sided heart failure",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1397162,
"choiceText": "Hypoplastic left heart syndrome",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1397164,
"choiceText": "Double outlet right ventricle",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 446091,
"questionText": "Which one of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1397166,
"choiceText": "CT",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1397168,
"choiceText": "MRI",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1397170,
"choiceText": "Echocardiography",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1397172,
"choiceText": "Cardiac catheterization",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>MRI has become the criterion standard for assessing right ventricular function and size and for quantifying the pulmonary regurgitant volume. MRI can also map the speed of pulmonary regurgitation and provide a good outline of the aortic size and evaluation of the pulmonary arteries and its branches, the status of the right ventricular outflow tract, and the presence of ventricular septal defects and right ventricular hypertrophy. It can also be used to measure intracardiac pressures, gradients, and blood flows.<sup type=\"ref\">[10]</sup></p>",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 446093,
"questionText": "Which one of the following is considered the criterion standard study to evaluate right ventricular function?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1397182,
"choiceText": "To reduce the diameter of the ventricular septal defect",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1397184,
"choiceText": "To improve the forward flow of blood into systemic circulation",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1397186,
"choiceText": "To help force more blood through the pulmonary valve",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1397188,
"choiceText": "To decrease heart rate, and therefore improve cardiac oxygen consumption",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1397190,
"choiceText": "To increase venous return, and therefore improve right ventricular function",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>When patients with TOF have a high grade of right ventricular outflow tract obstruction, the right ventricle must generate increased pressure to get the column of blood through the pulmonary valve. When pressure generated by the right ventricle supersedes the pressure generated by the left ventricle, blood is forced through the ventricular septal defect from the right to left ventricle during systole. This right-to-left shunt allows for deoxygenated blood to enter systemic circulation and cause symptoms (such as \"tet spells\").</p>\r\n\r\n<p>By squatting, systemic vascular resistance is greatly increased, thus requiring the left ventricle to generate much higher pressures to pump the blood into the aorta. When this increase in left ventricular pressure surpasses the already high basal right ventricular pressure, the blood no longer shunts from right to left through the ventricular septal defect; instead, more blood is forced to go through the pulmonary valve into the lungs and increase blood oxygenation, which reduces symptoms.<sup type=\"ref\">[11]</sup></p>",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 446097,
"questionText": "Which one of the following best explains why individuals with TOF learn to squat to improve symptoms?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
868650 | /viewarticle/868650 | [
{
"authors": "Mohammad Elbatta, MD; Jason Schairer, MD",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"A 30-year-old woman presents with recurrent abdominal pain and loose stools. She states that she has experienced these symptoms since adolescence, with periods of improvement and worsening over the years. Over the past year, her symptoms have been occurring more frequently and with greater severity. For the past 6 months, she has been bothered by bloating. The bloating seems to worsen with food intake.",
"When questioned about abdominal pain, the patient describes it as 6 (on a scale of 10) at its worst. Acute worsening occurs immediately before defecation, with significant improvement after defecation. She has had this pain at least once every week for the past 6 months.",
"The patient has loose stools approximately one third of the time and often has two or three bowel movements per day (with a baseline of one bowel movement a day). She denied waking up at night to have a bowel movement and denied any blood in the stool. She has had no nausea, vomiting, or change in weight. No relationship was found between her symptoms and her diet, including milk products, spicy foods, alcohol, and processed meats. She denies excessive hunger or anorexia symptoms.",
"Other than her mentioned symptoms, the patient considers herself healthy. She has no chronic illnesses or prior surgeries. She has no family history of organic gastrointestinal diseases, such as inflammatory bowel disease, cancer, or celiac disease."
],
"date": "August 12, 2019",
"figures": [],
"markdown": "# Worsening Abdominal Pain and Bloating in a 30-Year-Old Woman\n\n **Authors:** Mohammad Elbatta, MD; Jason Schairer, MD \n **Date:** August 12, 2019\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nA 30-year-old woman presents with recurrent abdominal pain and loose stools. She states that she has experienced these symptoms since adolescence, with periods of improvement and worsening over the years. Over the past year, her symptoms have been occurring more frequently and with greater severity. For the past 6 months, she has been bothered by bloating. The bloating seems to worsen with food intake.\nWhen questioned about abdominal pain, the patient describes it as 6 (on a scale of 10) at its worst. Acute worsening occurs immediately before defecation, with significant improvement after defecation. She has had this pain at least once every week for the past 6 months.\nThe patient has loose stools approximately one third of the time and often has two or three bowel movements per day (with a baseline of one bowel movement a day). She denied waking up at night to have a bowel movement and denied any blood in the stool. She has had no nausea, vomiting, or change in weight. No relationship was found between her symptoms and her diet, including milk products, spicy foods, alcohol, and processed meats. She denies excessive hunger or anorexia symptoms.\nOther than her mentioned symptoms, the patient considers herself healthy. She has no chronic illnesses or prior surgeries. She has no family history of organic gastrointestinal diseases, such as inflammatory bowel disease, cancer, or celiac disease.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "Worsening Abdominal Pain and Bloating in a 30-Year-Old Woman"
},
{
"authors": "Mohammad Elbatta, MD; Jason Schairer, MD",
"content": [
"Upon physical examination, the patient's height is 5'6\", weight is 160 lb, blood pressure is 108/70 mm Hg, pulse is 70 beats/min, and respiratory rate is 12 breaths/min. The physical examination reveals a comfortable-appearing woman in no acute distress. The head and neck examination findings are normal, and no scleral icterus is noted. She does not appear dehydrated. Her lung sounds are clear to auscultation bilaterally. The S1 and S2 heart sounds are normal, with no audible murmurs, rubs, or gallops.",
"The patient has a soft abdomen that is not distended, with bowel sounds heard in all four quadrants. Palpation reveals no tenderness at the time of examination and no masses are felt. A rectal examination reveals brown, guaiac-negative stool. No neurologic deficits are noted. Basic workup, including complete blood cell count and basic metabolic profile, are within the reference range."
],
"date": "August 12, 2019",
"figures": [],
"markdown": "# Worsening Abdominal Pain and Bloating in a 30-Year-Old Woman\n\n **Authors:** Mohammad Elbatta, MD; Jason Schairer, MD \n **Date:** August 12, 2019\n\n ## Content\n\n Upon physical examination, the patient's height is 5'6\", weight is 160 lb, blood pressure is 108/70 mm Hg, pulse is 70 beats/min, and respiratory rate is 12 breaths/min. The physical examination reveals a comfortable-appearing woman in no acute distress. The head and neck examination findings are normal, and no scleral icterus is noted. She does not appear dehydrated. Her lung sounds are clear to auscultation bilaterally. The S1 and S2 heart sounds are normal, with no audible murmurs, rubs, or gallops.\nThe patient has a soft abdomen that is not distended, with bowel sounds heard in all four quadrants. Palpation reveals no tenderness at the time of examination and no masses are felt. A rectal examination reveals brown, guaiac-negative stool. No neurologic deficits are noted. Basic workup, including complete blood cell count and basic metabolic profile, are within the reference range.\n\n ## Figures\n\n \n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1011331,
"choiceText": "Irritable bowel syndrome",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1011333,
"choiceText": "Small intestinal bacterial overgrowth",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1011335,
"choiceText": "Celiac disease",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1011337,
"choiceText": "Crohn disease\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 319947,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Worsening Abdominal Pain and Bloating in a 30-Year-Old Woman"
},
{
"authors": "Mohammad Elbatta, MD; Jason Schairer, MD",
"content": [
"Abdominal pain is a common issue encountered by primary care physicians. Differentiating functional disorders (eg, irritable bowel syndrome, chronic idiopathic constipation) from other more concerning causes (eg, small intestinal bacterial overgrowth [SIBO], cancer, celiac disease, Crohn disease, ulcerative colitis) is important.",
"Figure 1.",
"Irritable bowel syndrome is a gastrointestinal syndrome characterized by chronic abdominal pain and altered bowel habits in the absence of any organic cause (Figure 1). It is the most commonly diagnosed gastrointestinal condition. The prevalence of irritable bowel syndrome in North America is approximately 10%-15%, and the incidence is 1%-2% of the population per year.[1] Women are two to three times more likely than men to develop irritable bowel syndrome.[2]",
"The hallmark symptoms of irritable bowel syndrome are abdominal pain or discomfort that happens once every week for at least 3 months. This pain or discomfort is noted to have at least two of the following Rome IV criteria for irritable bowel syndrome[3]:",
"Pain or discomfort associated with a change in the form of the stool: These can be loose bowel movements, hard bowel movements, or a combination of both.",
"Pain or discomfort associated with a change in the stool frequency: Bowel movements can either be more frequent or less frequent.",
"The pain or discomfort is relieved by having a bowel movement.",
"The reality of clinical practice is that irritable bowel syndrome can have a wide variety of clinical presentations. In addition to the \"classic\" symptom complex mentioned above, patients often have other abdominal or defecation-related symptoms, such as bloating, a prominent gastrocolic reflex, flatulence, distention, mucus in the stool, tenesmus (a sense of incomplete evacuation), and straining required for defecation."
],
"date": "August 12, 2019",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/868/650/868650-Thumb1.png"
}
],
"markdown": "# Worsening Abdominal Pain and Bloating in a 30-Year-Old Woman\n\n **Authors:** Mohammad Elbatta, MD; Jason Schairer, MD \n **Date:** August 12, 2019\n\n ## Content\n\n Abdominal pain is a common issue encountered by primary care physicians. Differentiating functional disorders (eg, irritable bowel syndrome, chronic idiopathic constipation) from other more concerning causes (eg, small intestinal bacterial overgrowth [SIBO], cancer, celiac disease, Crohn disease, ulcerative colitis) is important.\nFigure 1.\nIrritable bowel syndrome is a gastrointestinal syndrome characterized by chronic abdominal pain and altered bowel habits in the absence of any organic cause (Figure 1). It is the most commonly diagnosed gastrointestinal condition. The prevalence of irritable bowel syndrome in North America is approximately 10%-15%, and the incidence is 1%-2% of the population per year.[1] Women are two to three times more likely than men to develop irritable bowel syndrome.[2]\nThe hallmark symptoms of irritable bowel syndrome are abdominal pain or discomfort that happens once every week for at least 3 months. This pain or discomfort is noted to have at least two of the following Rome IV criteria for irritable bowel syndrome[3]:\nPain or discomfort associated with a change in the form of the stool: These can be loose bowel movements, hard bowel movements, or a combination of both.\nPain or discomfort associated with a change in the stool frequency: Bowel movements can either be more frequent or less frequent.\nThe pain or discomfort is relieved by having a bowel movement.\nThe reality of clinical practice is that irritable bowel syndrome can have a wide variety of clinical presentations. In addition to the \"classic\" symptom complex mentioned above, patients often have other abdominal or defecation-related symptoms, such as bloating, a prominent gastrocolic reflex, flatulence, distention, mucus in the stool, tenesmus (a sense of incomplete evacuation), and straining required for defecation.\n\n ## Figures\n\n **Figure 1.** \n \n\n\n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1011331,
"choiceText": "Irritable bowel syndrome",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1011333,
"choiceText": "Small intestinal bacterial overgrowth",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1011335,
"choiceText": "Celiac disease",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1011337,
"choiceText": "Crohn disease\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 319947,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Worsening Abdominal Pain and Bloating in a 30-Year-Old Woman"
},
{
"authors": "Mohammad Elbatta, MD; Jason Schairer, MD",
"content": [
"The key to diagnosing irritable bowel syndrome is to exclude other conditions that would require further workup. Alarm symptoms or atypical symptoms that are not compatible with irritable bowel syndrome include rectal bleeding, nocturnal bowel movements, progressive abdominal pain, and weight loss.",
"Nocturnal symptoms are an important sign of pathology, because the motility of the gastrointestinal tract is depressed during sleep.[4] As a result, adults should not experience bowel movements during sleep. In addition, osmotic diarrhea usually occurs after the ingestion of a substrate that a person cannot digest or absorb. These patients should have symptoms after meals but be able to sleep through the night. If a person is waking up to have bowel movements, then an inflammatory, infectious, or secretory cause of diarrhea must be investigated.",
"Weight loss is not a feature of irritable bowel syndrome and should always prompt a workup for another etiology. The differential diagnosis includes cancer, inflammatory bowel disease, exocrine pancreatic insufficiency, chronic infections, hyperthyroidism, and severe malabsorption conditions.",
"SIBO is a condition in which native or nonnative bacteria are present in increased numbers, resulting in excessive fermentation, inflammation, or malabsorption. It typically occurs in association with anatomical abnormalities, motility disorders, or multifactorial causes (eg, cirrhosis, chronic pancreatitis). Most patients with SIBO present with nonspecific symptoms of bloating, flatulence, or abdominal discomfort, or they may be asymptomatic. Many patients diagnosed with severe SIBO have diarrhea. Although classic descriptions of SIBO include steatorrhea with greasy or bulky stools, this is uncommon and occurs principally if SIBO is caused by altered anatomy, such as blind loop syndrome. Rarely, patients have weight loss due to severe diarrhea, malabsorption, or poor oral intake. These symptoms do not match irritable bowel syndrome criteria.",
"Chronic idiopathic constipation, or functional constipation, is a common condition that affects the gastrointestinal tract, with an estimated prevalence of 4%-20% of the general population. This functional disorder is defined as the infrequent, persistently difficult passage of stools or seemingly incomplete defecation, which does not meet the criteria for irritable bowel syndrome. These patients usually do not have any physiologic abnormality.",
"In patients with symptoms compatible with irritable bowel syndrome on the basis of the Rome IV criteria, a limited number of diagnostic studies guided by the clinical setting are used to rule out other likely conditions. Appropriate tests for the workup of irritable bowel syndrome include a complete blood count, basic metabolic profile, C-reactive protein, and thyroid-stimulating hormone. If anemia is present, it could be microcytic, due to a chronic illness such as inflammatory bowel disease or iron deficiency associated with celiac disease (for which it is a primary presenting symptom). On the other hand, SIBO can prevent dietary vitamin B12 absorption at the terminal ileum, creating a macrocytic anemia.",
"The initial workup for irritable bowel syndrome does not routinely include colonoscopy, imaging tests (eg, ultrasonography, CT), or breath tests for SIBO. These have been found to be low yield for finding pathology.",
"The pathophysiology of irritable bowel syndrome remains uncertain. However, gastrointestinal motility, intestinal inflammation and food sensitivity, gastrointestinal infections with subsequent bacterial overgrowth and changes in the colonic microflora, genetics, and psychosocial alterations have all been implicated in the pathogenesis of the disease. Although motor abnormalities of the gastrointestinal tract (increased frequency and irregularity of luminal contractions, abnormal transit time) are detectable in some patients with irritable bowel syndrome, no predominant pattern of motor activity has emerged as a marker.",
"Hypersensitization of visceral afferent nerves in the gut has also been observed in these patients. Mucosal immune system activation has been revealed. On the basis of the history of acute diarrheal illness (infectious gastroenteritis) preceding the development of irritable bowel syndrome, malabsorption, increased enteroendocrine cells/lymphocytes, and antibiotic use have been proposed as plausible theories for the development of the entity. Food-specific antibodies, carbohydrate malabsorption, and gluten sensitivity may also play a role in developing the disease.",
"A genetic susceptibility to irritable bowel syndrome has been suggested, although familial patterns may also reflect underlying social factors. Associations between specific genes and irritable bowel syndrome are under investigation. Polymorphisms in the serotonin transporter gene result in altered serotonin reuptake efficacy, affecting intestinal peristalsis."
],
"date": "August 12, 2019",
"figures": [],
"markdown": "# Worsening Abdominal Pain and Bloating in a 30-Year-Old Woman\n\n **Authors:** Mohammad Elbatta, MD; Jason Schairer, MD \n **Date:** August 12, 2019\n\n ## Content\n\n The key to diagnosing irritable bowel syndrome is to exclude other conditions that would require further workup. Alarm symptoms or atypical symptoms that are not compatible with irritable bowel syndrome include rectal bleeding, nocturnal bowel movements, progressive abdominal pain, and weight loss.\nNocturnal symptoms are an important sign of pathology, because the motility of the gastrointestinal tract is depressed during sleep.[4] As a result, adults should not experience bowel movements during sleep. In addition, osmotic diarrhea usually occurs after the ingestion of a substrate that a person cannot digest or absorb. These patients should have symptoms after meals but be able to sleep through the night. If a person is waking up to have bowel movements, then an inflammatory, infectious, or secretory cause of diarrhea must be investigated.\nWeight loss is not a feature of irritable bowel syndrome and should always prompt a workup for another etiology. The differential diagnosis includes cancer, inflammatory bowel disease, exocrine pancreatic insufficiency, chronic infections, hyperthyroidism, and severe malabsorption conditions.\nSIBO is a condition in which native or nonnative bacteria are present in increased numbers, resulting in excessive fermentation, inflammation, or malabsorption. It typically occurs in association with anatomical abnormalities, motility disorders, or multifactorial causes (eg, cirrhosis, chronic pancreatitis). Most patients with SIBO present with nonspecific symptoms of bloating, flatulence, or abdominal discomfort, or they may be asymptomatic. Many patients diagnosed with severe SIBO have diarrhea. Although classic descriptions of SIBO include steatorrhea with greasy or bulky stools, this is uncommon and occurs principally if SIBO is caused by altered anatomy, such as blind loop syndrome. Rarely, patients have weight loss due to severe diarrhea, malabsorption, or poor oral intake. These symptoms do not match irritable bowel syndrome criteria.\nChronic idiopathic constipation, or functional constipation, is a common condition that affects the gastrointestinal tract, with an estimated prevalence of 4%-20% of the general population. This functional disorder is defined as the infrequent, persistently difficult passage of stools or seemingly incomplete defecation, which does not meet the criteria for irritable bowel syndrome. These patients usually do not have any physiologic abnormality.\nIn patients with symptoms compatible with irritable bowel syndrome on the basis of the Rome IV criteria, a limited number of diagnostic studies guided by the clinical setting are used to rule out other likely conditions. Appropriate tests for the workup of irritable bowel syndrome include a complete blood count, basic metabolic profile, C-reactive protein, and thyroid-stimulating hormone. If anemia is present, it could be microcytic, due to a chronic illness such as inflammatory bowel disease or iron deficiency associated with celiac disease (for which it is a primary presenting symptom). On the other hand, SIBO can prevent dietary vitamin B12 absorption at the terminal ileum, creating a macrocytic anemia.\nThe initial workup for irritable bowel syndrome does not routinely include colonoscopy, imaging tests (eg, ultrasonography, CT), or breath tests for SIBO. These have been found to be low yield for finding pathology.\nThe pathophysiology of irritable bowel syndrome remains uncertain. However, gastrointestinal motility, intestinal inflammation and food sensitivity, gastrointestinal infections with subsequent bacterial overgrowth and changes in the colonic microflora, genetics, and psychosocial alterations have all been implicated in the pathogenesis of the disease. Although motor abnormalities of the gastrointestinal tract (increased frequency and irregularity of luminal contractions, abnormal transit time) are detectable in some patients with irritable bowel syndrome, no predominant pattern of motor activity has emerged as a marker.\nHypersensitization of visceral afferent nerves in the gut has also been observed in these patients. Mucosal immune system activation has been revealed. On the basis of the history of acute diarrheal illness (infectious gastroenteritis) preceding the development of irritable bowel syndrome, malabsorption, increased enteroendocrine cells/lymphocytes, and antibiotic use have been proposed as plausible theories for the development of the entity. Food-specific antibodies, carbohydrate malabsorption, and gluten sensitivity may also play a role in developing the disease.\nA genetic susceptibility to irritable bowel syndrome has been suggested, although familial patterns may also reflect underlying social factors. Associations between specific genes and irritable bowel syndrome are under investigation. Polymorphisms in the serotonin transporter gene result in altered serotonin reuptake efficacy, affecting intestinal peristalsis.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "Worsening Abdominal Pain and Bloating in a 30-Year-Old Woman"
},
{
"authors": "Mohammad Elbatta, MD; Jason Schairer, MD",
"content": [
"Four subtypes of irritable bowel syndrome are recognized, on the basis of the predominant symptoms during days with abnormal bowel movements. The Bristol Stool Form Scale should be used to record stool consistency. Subtypes can only confidently be established when the patient is evaluated off medications used to treat bowel habit abnormalities, such as antidiarrheal drugs or laxatives.",
"Irritable bowel syndrome subtypes are defined for clinical practice as follows:",
"Irritable bowel syndrome with constipation.",
"Irritable bowel syndrome with diarrhea.",
"Mixed irritable bowel syndrome: Abnormal bowel movements alternate between constipation and diarrhea.",
"Unclassified irritable bowel syndrome: These are patients who meet diagnostic criteria for irritable bowel syndrome but cannot be accurately categorized into one of the other subtypes.",
"In this case, the patient does not have any alarming symptoms (eg, blood in the stool, anemia, nocturnal symptoms) and has no family history to suggest Crohn disease. Furthermore, a complete blood cell count in the reference range is unlikely in celiac disease. The patient has not had abdominal surgery or other cause of stasis in the small intestine; thus, SIBO is unlikely.",
"The patient meets the Rome IV criteria for irritable bowel syndrome. She has had the pain for more than 3 years, with a recent increase in symptoms. She has weekly symptoms of pain, bloating, loose stools, and more frequent bowel movements. The most likely diagnosis in this case is irritable bowel syndrome. Patients with mild and intermittent symptoms that do not impair the quality of life should undergo lifestyle and dietary modification, rather than treatment with medication. Thus, the first step is validating the patient’s symptoms via a therapeutic clinician/patient relationship. In patients with mild to moderate symptoms that fail to respond to initial management with lifestyle and dietary modifications and in patients with moderate to severe symptoms that affect quality of life, adding pharmacologic therapy is suggested. The choice of medication should be based on patient's predominant symptom (constipation or diarrhea). In patients with diarrhea-predominant symptoms, antidiarrheal medication (eg, loperamide) is indicated initially, with bile acid sequestrants (eg, cholestyramine, colestipol, colesevelam) recommended as a second-line therapy.",
"In patients with irritable bowel syndrome with constipation in whom a trial of soluble fiber (eg, psyllium/ispaghula) has failed, the next step is polyethylene glycol. In patients with persistent constipation despite treatment with polyethylene glycol, lubiprostone or linaclotide may be recommended.",
"In patients with abdominal pain due to irritable bowel syndrome, the authors use antispasmodics (eg, dicyclomine, hyoscyamine) on an as-needed basis. In patients with irritable bowel syndrome with constipation, antispasmodics are initiated only if the abdominal pain persists despite treatment of constipation.",
"Because this patient has symptoms that did not impair quality of life, lifestyle and dietary modifications alone were suggested rather than specific pharmacologic agents, with further follow-up for symptomatic treatment as needed."
],
"date": "August 12, 2019",
"figures": [],
"markdown": "# Worsening Abdominal Pain and Bloating in a 30-Year-Old Woman\n\n **Authors:** Mohammad Elbatta, MD; Jason Schairer, MD \n **Date:** August 12, 2019\n\n ## Content\n\n Four subtypes of irritable bowel syndrome are recognized, on the basis of the predominant symptoms during days with abnormal bowel movements. The Bristol Stool Form Scale should be used to record stool consistency. Subtypes can only confidently be established when the patient is evaluated off medications used to treat bowel habit abnormalities, such as antidiarrheal drugs or laxatives.\nIrritable bowel syndrome subtypes are defined for clinical practice as follows:\nIrritable bowel syndrome with constipation.\nIrritable bowel syndrome with diarrhea.\nMixed irritable bowel syndrome: Abnormal bowel movements alternate between constipation and diarrhea.\nUnclassified irritable bowel syndrome: These are patients who meet diagnostic criteria for irritable bowel syndrome but cannot be accurately categorized into one of the other subtypes.\nIn this case, the patient does not have any alarming symptoms (eg, blood in the stool, anemia, nocturnal symptoms) and has no family history to suggest Crohn disease. Furthermore, a complete blood cell count in the reference range is unlikely in celiac disease. The patient has not had abdominal surgery or other cause of stasis in the small intestine; thus, SIBO is unlikely.\nThe patient meets the Rome IV criteria for irritable bowel syndrome. She has had the pain for more than 3 years, with a recent increase in symptoms. She has weekly symptoms of pain, bloating, loose stools, and more frequent bowel movements. The most likely diagnosis in this case is irritable bowel syndrome. Patients with mild and intermittent symptoms that do not impair the quality of life should undergo lifestyle and dietary modification, rather than treatment with medication. Thus, the first step is validating the patient’s symptoms via a therapeutic clinician/patient relationship. In patients with mild to moderate symptoms that fail to respond to initial management with lifestyle and dietary modifications and in patients with moderate to severe symptoms that affect quality of life, adding pharmacologic therapy is suggested. The choice of medication should be based on patient's predominant symptom (constipation or diarrhea). In patients with diarrhea-predominant symptoms, antidiarrheal medication (eg, loperamide) is indicated initially, with bile acid sequestrants (eg, cholestyramine, colestipol, colesevelam) recommended as a second-line therapy.\nIn patients with irritable bowel syndrome with constipation in whom a trial of soluble fiber (eg, psyllium/ispaghula) has failed, the next step is polyethylene glycol. In patients with persistent constipation despite treatment with polyethylene glycol, lubiprostone or linaclotide may be recommended.\nIn patients with abdominal pain due to irritable bowel syndrome, the authors use antispasmodics (eg, dicyclomine, hyoscyamine) on an as-needed basis. In patients with irritable bowel syndrome with constipation, antispasmodics are initiated only if the abdominal pain persists despite treatment of constipation.\nBecause this patient has symptoms that did not impair quality of life, lifestyle and dietary modifications alone were suggested rather than specific pharmacologic agents, with further follow-up for symptomatic treatment as needed.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1011339,
"choiceText": "Refer to another physician for second opinion",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1011341,
"choiceText": "Schedule patient for colonoscopy",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1011343,
"choiceText": "Establish a therapeutic clinician/patient relationship to validate the patient's symptoms",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1011345,
"choiceText": "Start antidiarrheal medications",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Establishing a therapeutic clinician/patient relationship to validate the patient's symptoms is important.<sup>[5]</sup> Patients should also be counseled that although irritable bowel syndrome does not increase the risk for cancer, it is a chronic disease. The clinician should establish realistic expectations with consistent limits and involve the patient in treatment decisions. In patients with mild and intermittent symptoms that do not impair quality of life, lifestyle and dietary modification alone are recommended, rather than specific pharmacologic agents.<sup>[6]</sup><br><br>\r\nDietary and lifestyle modification include the following:<br>\r\n<ul>\r\n<li>\tA diet low in fermentable oligo-, di-, and monosaccharides and polyols (low FODMAP diet)<sup type=\"ref\">[7]</sup>: These short-chain carbohydrates are poorly absorbed and are osmotically active in the intestinal lumen, where they are rapidly fermented, resulting in abdominal bloating and pain. </li>\r\n\r\n<li>\tExclusion of gas-producing food (eg, beans, onions, celery, carrots, raisins, bananas, apricots, prunes, Brussels sprouts, wheat germ, pretzels, bagels), alcohol, and caffeine.</li>\r\n\r\n<li>\tPhysical activity: This should be advised in patients with irritable bowel syndrome, given a potential benefit with regard to irritable bowel syndrome symptoms and the general health benefits of exercise.</li></ul>",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 319949,
"questionText": "Which of the following is the next best step for a patient with suspected irritable bowel syndrome with diarrhea?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1011347,
"choiceText": "Polyethylene glycol",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1011349,
"choiceText": "Loperamide",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1011351,
"choiceText": "Lubiprostone",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1011353,
"choiceText": "\r\nLinaclotide\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "In patients with mild to moderate symptoms that fail to respond to initial management and in patients with moderate to severe symptoms that affect quality of life, pharmacologic therapy is suggested as adjunctive treatment. The choice of the medication should be based on the patient's predominant symptom (constipation or diarrhea).<br><br>\r\n\r\nIn patients with diarrhea-predominant symptoms, antidiarrheal medication (eg, loperamide) is used as initial treatment, with bile acid sequestrants (eg, cholestyramine, colestipol, colesevelam) indicated as second-line therapy. \r\n",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 319951,
"questionText": "In a patient with no alarming symptoms but in whom diet and lifestyle modifications do not improve diarrhea-predominant symptoms that are now affecting her daily activities, which of the following is indicated?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Worsening Abdominal Pain and Bloating in a 30-Year-Old Woman"
},
{
"authors": "Mohammad Elbatta, MD; Jason Schairer, MD",
"content": [],
"date": "August 12, 2019",
"figures": [],
"markdown": "# Worsening Abdominal Pain and Bloating in a 30-Year-Old Woman\n\n **Authors:** Mohammad Elbatta, MD; Jason Schairer, MD \n **Date:** August 12, 2019\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1011339,
"choiceText": "Refer to another physician for second opinion",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1011341,
"choiceText": "Schedule patient for colonoscopy",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1011343,
"choiceText": "Establish a therapeutic clinician/patient relationship to validate the patient's symptoms",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1011345,
"choiceText": "Start antidiarrheal medications",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Establishing a therapeutic clinician/patient relationship to validate the patient's symptoms is important.<sup>[5]</sup> Patients should also be counseled that although irritable bowel syndrome does not increase the risk for cancer, it is a chronic disease. The clinician should establish realistic expectations with consistent limits and involve the patient in treatment decisions. In patients with mild and intermittent symptoms that do not impair quality of life, lifestyle and dietary modification alone are recommended, rather than specific pharmacologic agents.<sup>[6]</sup><br><br>\r\nDietary and lifestyle modification include the following:<br>\r\n<ul>\r\n<li>\tA diet low in fermentable oligo-, di-, and monosaccharides and polyols (low FODMAP diet)<sup type=\"ref\">[7]</sup>: These short-chain carbohydrates are poorly absorbed and are osmotically active in the intestinal lumen, where they are rapidly fermented, resulting in abdominal bloating and pain. </li>\r\n\r\n<li>\tExclusion of gas-producing food (eg, beans, onions, celery, carrots, raisins, bananas, apricots, prunes, Brussels sprouts, wheat germ, pretzels, bagels), alcohol, and caffeine.</li>\r\n\r\n<li>\tPhysical activity: This should be advised in patients with irritable bowel syndrome, given a potential benefit with regard to irritable bowel syndrome symptoms and the general health benefits of exercise.</li></ul>",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 319949,
"questionText": "Which of the following is the next best step for a patient with suspected irritable bowel syndrome with diarrhea?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1011347,
"choiceText": "Polyethylene glycol",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1011349,
"choiceText": "Loperamide",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1011351,
"choiceText": "Lubiprostone",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1011353,
"choiceText": "\r\nLinaclotide\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "In patients with mild to moderate symptoms that fail to respond to initial management and in patients with moderate to severe symptoms that affect quality of life, pharmacologic therapy is suggested as adjunctive treatment. The choice of the medication should be based on the patient's predominant symptom (constipation or diarrhea).<br><br>\r\n\r\nIn patients with diarrhea-predominant symptoms, antidiarrheal medication (eg, loperamide) is used as initial treatment, with bile acid sequestrants (eg, cholestyramine, colestipol, colesevelam) indicated as second-line therapy. \r\n",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 319951,
"questionText": "In a patient with no alarming symptoms but in whom diet and lifestyle modifications do not improve diarrhea-predominant symptoms that are now affecting her daily activities, which of the following is indicated?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Worsening Abdominal Pain and Bloating in a 30-Year-Old Woman"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1011331,
"choiceText": "Irritable bowel syndrome",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1011333,
"choiceText": "Small intestinal bacterial overgrowth",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1011335,
"choiceText": "Celiac disease",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1011337,
"choiceText": "Crohn disease\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 319947,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1011339,
"choiceText": "Refer to another physician for second opinion",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1011341,
"choiceText": "Schedule patient for colonoscopy",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1011343,
"choiceText": "Establish a therapeutic clinician/patient relationship to validate the patient's symptoms",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1011345,
"choiceText": "Start antidiarrheal medications",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Establishing a therapeutic clinician/patient relationship to validate the patient's symptoms is important.<sup>[5]</sup> Patients should also be counseled that although irritable bowel syndrome does not increase the risk for cancer, it is a chronic disease. The clinician should establish realistic expectations with consistent limits and involve the patient in treatment decisions. In patients with mild and intermittent symptoms that do not impair quality of life, lifestyle and dietary modification alone are recommended, rather than specific pharmacologic agents.<sup>[6]</sup><br><br>\r\nDietary and lifestyle modification include the following:<br>\r\n<ul>\r\n<li>\tA diet low in fermentable oligo-, di-, and monosaccharides and polyols (low FODMAP diet)<sup type=\"ref\">[7]</sup>: These short-chain carbohydrates are poorly absorbed and are osmotically active in the intestinal lumen, where they are rapidly fermented, resulting in abdominal bloating and pain. </li>\r\n\r\n<li>\tExclusion of gas-producing food (eg, beans, onions, celery, carrots, raisins, bananas, apricots, prunes, Brussels sprouts, wheat germ, pretzels, bagels), alcohol, and caffeine.</li>\r\n\r\n<li>\tPhysical activity: This should be advised in patients with irritable bowel syndrome, given a potential benefit with regard to irritable bowel syndrome symptoms and the general health benefits of exercise.</li></ul>",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 319949,
"questionText": "Which of the following is the next best step for a patient with suspected irritable bowel syndrome with diarrhea?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1011347,
"choiceText": "Polyethylene glycol",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1011349,
"choiceText": "Loperamide",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1011351,
"choiceText": "Lubiprostone",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1011353,
"choiceText": "\r\nLinaclotide\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "In patients with mild to moderate symptoms that fail to respond to initial management and in patients with moderate to severe symptoms that affect quality of life, pharmacologic therapy is suggested as adjunctive treatment. The choice of the medication should be based on the patient's predominant symptom (constipation or diarrhea).<br><br>\r\n\r\nIn patients with diarrhea-predominant symptoms, antidiarrheal medication (eg, loperamide) is used as initial treatment, with bile acid sequestrants (eg, cholestyramine, colestipol, colesevelam) indicated as second-line therapy. \r\n",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 319951,
"questionText": "In a patient with no alarming symptoms but in whom diet and lifestyle modifications do not improve diarrhea-predominant symptoms that are now affecting her daily activities, which of the following is indicated?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
916277 | /viewarticle/916277 | [
{
"authors": "Jeffrey S. Forrest, MD; Alexander B. Shortridge",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"A 65-year-old man is brought to the emergency department by military police. The patient, who had been armed with a shotgun, refused to allow his spouse to leave their domicile. During the event, the patient's relatives contacted the authorities. The patient insisted to his relatives that cell phone towers were beaming in rays that were worsening his wife's dementia and rheumatoid arthritis. To counter this, the patient placed tin foil on the walls of his house. The patient would not let his spouse leave because he was concerned that government agents were attempting to attack her. The patient stated that he had been \"reactivated\" to his military duties. After a brief standoff with local police, the patient agreed to cooperate if the military police were contacted. Once the military police arrived, they were able to convince the patient to come out of his home unarmed and brought him to the hospital for treatment.",
"Upon his initial presentation, the patient refused to answer questions. He only provided his name, his military serial number, and his rank. He responded exclusively with this information to all questions posed to him.",
"During a follow-up interview the next day, the patient again reported that he had been \"reactivated\" and had \"found some classified files.\" The patient stated that he had to report this information and had called the commanding general of the US Army. He stated that his orders were to stay in place and secure the area. He acknowledged having a 16-gauge shotgun during the prior day's events.",
"Information was then obtained by telephone from the patient's son. The son reported that, several years prior, his father had taken his mother to a nearby city for safety because he thought \"drones were flying above him\" and that he and his wife were not safe. The patient had rented an apartment and placed his spouse inside a closet for safety. The son reported that the patient had been completely irrational at the time. When the son attempted to speak to his father, his father felt that the conversation was being tracked by phone and ended the call. After that incident, the patient's wife and son came up with a \"code word\" that she could use on the phone with her son should the patient become irrational again. On the day of the patient's admission, the patient's spouse called her son and used that code word."
],
"date": "August 06, 2019",
"figures": [],
"markdown": "# A 65-Year-Old Man Brought to the ED by Military Police\n\n **Authors:** Jeffrey S. Forrest, MD; Alexander B. Shortridge \n **Date:** August 06, 2019\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nA 65-year-old man is brought to the emergency department by military police. The patient, who had been armed with a shotgun, refused to allow his spouse to leave their domicile. During the event, the patient's relatives contacted the authorities. The patient insisted to his relatives that cell phone towers were beaming in rays that were worsening his wife's dementia and rheumatoid arthritis. To counter this, the patient placed tin foil on the walls of his house. The patient would not let his spouse leave because he was concerned that government agents were attempting to attack her. The patient stated that he had been \"reactivated\" to his military duties. After a brief standoff with local police, the patient agreed to cooperate if the military police were contacted. Once the military police arrived, they were able to convince the patient to come out of his home unarmed and brought him to the hospital for treatment.\nUpon his initial presentation, the patient refused to answer questions. He only provided his name, his military serial number, and his rank. He responded exclusively with this information to all questions posed to him.\nDuring a follow-up interview the next day, the patient again reported that he had been \"reactivated\" and had \"found some classified files.\" The patient stated that he had to report this information and had called the commanding general of the US Army. He stated that his orders were to stay in place and secure the area. He acknowledged having a 16-gauge shotgun during the prior day's events.\nInformation was then obtained by telephone from the patient's son. The son reported that, several years prior, his father had taken his mother to a nearby city for safety because he thought \"drones were flying above him\" and that he and his wife were not safe. The patient had rented an apartment and placed his spouse inside a closet for safety. The son reported that the patient had been completely irrational at the time. When the son attempted to speak to his father, his father felt that the conversation was being tracked by phone and ended the call. After that incident, the patient's wife and son came up with a \"code word\" that she could use on the phone with her son should the patient become irrational again. On the day of the patient's admission, the patient's spouse called her son and used that code word.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 65-Year-Old Man Brought to the ED by Military Police"
},
{
"authors": "Jeffrey S. Forrest, MD; Alexander B. Shortridge",
"content": [
"Upon physical examination, the patient is 5'9\" (1.79 m), weighs 243 lb (110.5 kg), and has a body mass index (BMI) of 35.9 kg/m2. His pulse is 94 beats/min, his respirations are 18 breaths/min, his blood oxygen level is 97%, his temperature is 97.8° F (36.6° C), and his blood pressure is 138/104 mm Hg.",
"Initially, the patient continually repeats his name, rank, and serial number. When he eventually begins answering questions, the patient appears alert and oriented to name, location, date, and time. He is appropriately groomed and in no acute distress. In recall, he does well in immediate, recent, and long-term memory. His behavior is calm and attentive, with no excessive body movements and good eye contact. His speech has normal rate, rhythm, volume, and prosody.",
"Substantive delusional thought content is appreciated. He believes that he is a prisoner of war. His emotional tone is consistent with delusional thought constructs. He uses military jargon. He denies suicidal ideation at the time of his interview. He denies being homicidal but says that if he or his wife are in danger, he will \"defend them.\"",
"He states that his mood is good, and his affect is congruent with his stated mood. Neither his wife nor son report having noticed any significant signs or symptoms of depression at any point in his history. The patient neither confirms nor denies any auditory or visual hallucinations, derealization, or depersonalization. Although the patient appears able to attend to the interview, he does not cooperate with the serial sevens examination. His insight is poor, as he demonstrates significant reality-testing impairments. He is unable to assess situations correctly or appropriately, and clearly has evidence of dangerous decision-making.",
"Laboratory findings are as follows:",
"Total bilirubin level: 1.8 μmol/L",
"Blood urea nitrogen level: 21 mg/dL",
"Creatinine level: 1.5 mg/dL",
"Sodium level: 140 mmol/L",
"Potassium level: 3.9 mmol/L",
"Red blood cell count: 5.2 x 1012/L",
"Hemoglobin level: 15.9 g/dL",
"Hematocrit level: 46%",
"White blood cell count: 12.9 x 109/L",
"An ECG reveals marked left axis deviation and incomplete right bundle branch block (QT/QTc 406/41). A CT scan of the head is unrevealing. His urine drug screen results are negative."
],
"date": "August 06, 2019",
"figures": [],
"markdown": "# A 65-Year-Old Man Brought to the ED by Military Police\n\n **Authors:** Jeffrey S. Forrest, MD; Alexander B. Shortridge \n **Date:** August 06, 2019\n\n ## Content\n\n Upon physical examination, the patient is 5'9\" (1.79 m), weighs 243 lb (110.5 kg), and has a body mass index (BMI) of 35.9 kg/m2. His pulse is 94 beats/min, his respirations are 18 breaths/min, his blood oxygen level is 97%, his temperature is 97.8° F (36.6° C), and his blood pressure is 138/104 mm Hg.\nInitially, the patient continually repeats his name, rank, and serial number. When he eventually begins answering questions, the patient appears alert and oriented to name, location, date, and time. He is appropriately groomed and in no acute distress. In recall, he does well in immediate, recent, and long-term memory. His behavior is calm and attentive, with no excessive body movements and good eye contact. His speech has normal rate, rhythm, volume, and prosody.\nSubstantive delusional thought content is appreciated. He believes that he is a prisoner of war. His emotional tone is consistent with delusional thought constructs. He uses military jargon. He denies suicidal ideation at the time of his interview. He denies being homicidal but says that if he or his wife are in danger, he will \"defend them.\"\nHe states that his mood is good, and his affect is congruent with his stated mood. Neither his wife nor son report having noticed any significant signs or symptoms of depression at any point in his history. The patient neither confirms nor denies any auditory or visual hallucinations, derealization, or depersonalization. Although the patient appears able to attend to the interview, he does not cooperate with the serial sevens examination. His insight is poor, as he demonstrates significant reality-testing impairments. He is unable to assess situations correctly or appropriately, and clearly has evidence of dangerous decision-making.\nLaboratory findings are as follows:\nTotal bilirubin level: 1.8 μmol/L\nBlood urea nitrogen level: 21 mg/dL\nCreatinine level: 1.5 mg/dL\nSodium level: 140 mmol/L\nPotassium level: 3.9 mmol/L\nRed blood cell count: 5.2 x 1012/L\nHemoglobin level: 15.9 g/dL\nHematocrit level: 46%\nWhite blood cell count: 12.9 x 109/L\nAn ECG reveals marked left axis deviation and incomplete right bundle branch block (QT/QTc 406/41). A CT scan of the head is unrevealing. His urine drug screen results are negative.\n\n ## Figures\n\n \n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1391798,
"choiceText": "Bipolar disorder",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1391800,
"choiceText": "Borderline personality disorder",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1391802,
"choiceText": "Schizophrenia",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1391804,
"choiceText": "Wernicke-Korsakoff syndrome",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 444445,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 65-Year-Old Man Brought to the ED by Military Police"
},
{
"authors": "Jeffrey S. Forrest, MD; Alexander B. Shortridge",
"content": [
"This patient appears to be experiencing an exacerbation of schizophrenia. This diagnosis is supported by his history of erratic and irrational behavior, delusional constructs, and florid impairment in reality testing. He also lacks any evidence of mental organic impairment, such as orientation and recollection problems. After further questioning, his son recalls his mother telling him that a previous physician had suggested a diagnosis of schizophrenia four decades earlier.",
"Schizophrenia is a lifelong psychotic disorder in which symptoms have been present for at least 6 months.[1] The term \"psychosis\" connotes a syndrome in which a patient's ability to discern reality from fiction is impaired. The core features of schizophrenia include the following:",
"Delusions (fixed, firm beliefs that are false)",
"Hallucinations (any false sensation—including visual, auditory, gustatory, olfactory, and touch—although auditory hallucinations are most common)",
"Disorganized speech (frequent derailment, loose associations, neologism, or incoherence)",
"All cases of schizophrenia feature at least one of these three core features. In addition, grossly disorganized or catatonic behavior and/or negative symptoms (absence of typically present behavior) may be present.",
"Distinguishing between positive symptoms and negative symptoms in schizophrenia is important. Positive symptoms include the presence of abnormal phenomena, such as delusions (eg, this patient's belief that he had orders from the military and was a prisoner of war), hallucinations, ideas of reference (beliefs that innocuous events have personal significance, such as this patient's concern about cell phone towers), or paranoia (eg, this patient's belief that government agents were attempting to harm his spouse). Other psychotic symptoms include a belief that one can broadcast his or her thoughts to others (thought broadcasting) or receive the thoughts of others (thought insertion).[2]",
"By contrast, negative symptoms of schizophrenia describe the absence of typical behaviors. Examples of this might include a flattened affect, avolition (such as poor grooming or eating behaviors), amotivational syndrome, poor eye contact, anhedonia, poverty of speech, and poor social attention. Generally, the presence of negative symptoms is a predictor of a relatively poorer prognosis in schizophrenia. Whereas positive symptoms of schizophrenia frequently respond to antipsychotic medication to at least some extent, negative symptoms do not necessarily respond as readily.[3] Although negative symptoms of schizophrenia are historically more difficult to treat with medication, psychosocial interventions that support individuals building and maintaining social networks have been correlated with reduction of negative symptoms in schizophrenia.[4]"
],
"date": "August 06, 2019",
"figures": [],
"markdown": "# A 65-Year-Old Man Brought to the ED by Military Police\n\n **Authors:** Jeffrey S. Forrest, MD; Alexander B. Shortridge \n **Date:** August 06, 2019\n\n ## Content\n\n This patient appears to be experiencing an exacerbation of schizophrenia. This diagnosis is supported by his history of erratic and irrational behavior, delusional constructs, and florid impairment in reality testing. He also lacks any evidence of mental organic impairment, such as orientation and recollection problems. After further questioning, his son recalls his mother telling him that a previous physician had suggested a diagnosis of schizophrenia four decades earlier.\nSchizophrenia is a lifelong psychotic disorder in which symptoms have been present for at least 6 months.[1] The term \"psychosis\" connotes a syndrome in which a patient's ability to discern reality from fiction is impaired. The core features of schizophrenia include the following:\nDelusions (fixed, firm beliefs that are false)\nHallucinations (any false sensation—including visual, auditory, gustatory, olfactory, and touch—although auditory hallucinations are most common)\nDisorganized speech (frequent derailment, loose associations, neologism, or incoherence)\nAll cases of schizophrenia feature at least one of these three core features. In addition, grossly disorganized or catatonic behavior and/or negative symptoms (absence of typically present behavior) may be present.\nDistinguishing between positive symptoms and negative symptoms in schizophrenia is important. Positive symptoms include the presence of abnormal phenomena, such as delusions (eg, this patient's belief that he had orders from the military and was a prisoner of war), hallucinations, ideas of reference (beliefs that innocuous events have personal significance, such as this patient's concern about cell phone towers), or paranoia (eg, this patient's belief that government agents were attempting to harm his spouse). Other psychotic symptoms include a belief that one can broadcast his or her thoughts to others (thought broadcasting) or receive the thoughts of others (thought insertion).[2]\nBy contrast, negative symptoms of schizophrenia describe the absence of typical behaviors. Examples of this might include a flattened affect, avolition (such as poor grooming or eating behaviors), amotivational syndrome, poor eye contact, anhedonia, poverty of speech, and poor social attention. Generally, the presence of negative symptoms is a predictor of a relatively poorer prognosis in schizophrenia. Whereas positive symptoms of schizophrenia frequently respond to antipsychotic medication to at least some extent, negative symptoms do not necessarily respond as readily.[3] Although negative symptoms of schizophrenia are historically more difficult to treat with medication, psychosocial interventions that support individuals building and maintaining social networks have been correlated with reduction of negative symptoms in schizophrenia.[4]\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1391798,
"choiceText": "Bipolar disorder",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1391800,
"choiceText": "Borderline personality disorder",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1391802,
"choiceText": "Schizophrenia",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1391804,
"choiceText": "Wernicke-Korsakoff syndrome",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 444445,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 65-Year-Old Man Brought to the ED by Military Police"
},
{
"authors": "Jeffrey S. Forrest, MD; Alexander B. Shortridge",
"content": [
"Schizophrenia affects approximately 1 in 100 individuals, with roughly equal incidence between the sexes.[2] The age of onset varies between men and women, however. In men, the typical onset is age 15-25 years. In women, most cases present at age 25-35, but 3%-10% of cases in women present after age 40. Schizophrenia rarely presents before age 15 or after age 55. Monozygotic twin studies suggest a rate of about 50% concordance for development of schizophrenia syndrome.[2] This suggests a strong, but not exclusive, genetic component, with environmental factors also significant in the pathogenesis of the disease process. Thus, an epigenetic mechanism is in play; both hereditary and environmental factors are involved. Numerous studies have demonstrated the role of stress in activating a schizophrenic episode, such as the stress of living in highly populated urban environments.[5]",
"As with the evaluation of most psychiatric conditions, the timely obtainment of collateral and supplemental history is essential in formulating an appropriate diagnosis. In the case presented, essential history from relatives provided critical information in the chronicity of the patient's symptoms and prior psychiatric history.",
"Psychotic symptoms may also arise from medical conditions, medications, or substance abuse. A careful metabolic workup, including a metabolic panel, is appropriate to rule out possible alternative causes. Certain infections and blood conditions may also contribute to psychiatric symptoms, making a complete blood count an appropriate test as well. A CT scan of the head is important to rule out organic or structural precipitants for psychotic symptoms. A urine toxicology screen is also essential to exclude contribution from the effects of illicit substances.",
"In evaluating mental health conditions, a careful evaluation of patient risk to personal safety and that of others is imperative. Should such an evaluation reveal the presence of dangerous delusions, hallucinations, suicidal thoughts, or other severe symptoms that might cause harm (or the inability to avoid harm) to the patient or others, the patient may be temporarily committed on an involuntary basis for a brief period while further evaluation can be conducted. A patient who is suicidal and/or homicidal must not be left unattended. Requirements and durations vary and are based on regional laws.",
"The first-line treatments for schizophrenia symptoms are antipsychotic agents. The primary mechanism of action for the therapeutic effect of antipsychotic agents is blockade at the dopamine receptor D2 in the mesolimbic pathway of the brain.[3] Unfortunately, such agents also may block dopamine receptors in the substantia nigra, resulting in the common side effect of extrapyramidal symptoms (EPS). These include akathisia, dyskinesia, dystonia, and akinesia. The most concerning of these is the syndrome of tardive dyskinesia (TD), wherein a patient may develop chronic and intractable repetitive movements of the face, tongue, or body.[3]",
"The balance of dopaminergic and cholinergic activity is central to the pathogenesis of EPS, including TD. Dopamine typically inhibits acetylcholine release. Blocking D2 receptors reduces dopamine's ability to inhibit acetylcholine release, leading to increased cholinergic activity. For this reason, anticholinergic agents such as benztropine are often administered with antipsychotics to reduce EPS such as TD.[3,6]",
"First-generation antipsychotic agents, such as haloperidol or perphenazine, have a greater risk of inducing EPS because of their overall greater level of D2 receptor blockade (approximately 80%). By contrast, second-generation antipsychotic agents (eg, quetiapine, ziprasidone) have lower rates of TD induction, as D2 blockade varies from 60% to 80%; however, the risk for metabolic side effects is increased.[3,7]",
"Two common tools for assessing TD in patients with schizophrenia are the Abnormal Involuntary Movement Scale (AIMS) and the Extrapyramidal Symptom Rating Scale (ESRS). AIMS categorizes abnormal movements by anatomic location and overall severity. ESRS rates symptoms on the basis of severity and frequency. Subjective and objective measures of parkinsonism, dystonia, akathisia, and dyskinesia are included. A cross-scale comparison found high concordance between AIMS and ESRS.[8]",
"Medication noncompliance is a major challenge to relapse prevention in the treatment of schizophrenia. By nature of the condition, symptoms may impede treatment. Patients with schizophrenia may come to believe that they no longer require medication, may experience hallucinations commanding them to stop taking medication, or may simply become apathetic and avolitional in regard to all activities. One pharmacologic strategy for preventing medication noncompliance includes use of long-acting injectable antipsychotic agents. Depot preparations of antipsychotic medications require administration much less frequently than daily oral antipsychotic medications and have been demonstrated to reduce rehospitalizations.[9] Patients with schizophrenia may stop their medication because of the aforementioned adverse effects of their drugs.",
"In addition to pharmacologic management, psychosocial interventions may be used to improve outcomes in schizophrenia. One example is Assertive Community Treatment, in which a team-based approach to care helps to assure access, delivery, and administration of medications. Such a team may include a psychiatrist, nurse, case manager, social worker, and primary care physician, working in tandem to monitor and provide services to chronic mental health patients. Cognitive-behavioral therapy has also been demonstrated to reduce positive symptoms in schizophrenia.[10] Social skills training for patients with schizophrenia encourages the development and maintenance of social networks, which is correlated with a reduction in negative symptoms.[4]"
],
"date": "August 06, 2019",
"figures": [],
"markdown": "# A 65-Year-Old Man Brought to the ED by Military Police\n\n **Authors:** Jeffrey S. Forrest, MD; Alexander B. Shortridge \n **Date:** August 06, 2019\n\n ## Content\n\n Schizophrenia affects approximately 1 in 100 individuals, with roughly equal incidence between the sexes.[2] The age of onset varies between men and women, however. In men, the typical onset is age 15-25 years. In women, most cases present at age 25-35, but 3%-10% of cases in women present after age 40. Schizophrenia rarely presents before age 15 or after age 55. Monozygotic twin studies suggest a rate of about 50% concordance for development of schizophrenia syndrome.[2] This suggests a strong, but not exclusive, genetic component, with environmental factors also significant in the pathogenesis of the disease process. Thus, an epigenetic mechanism is in play; both hereditary and environmental factors are involved. Numerous studies have demonstrated the role of stress in activating a schizophrenic episode, such as the stress of living in highly populated urban environments.[5]\nAs with the evaluation of most psychiatric conditions, the timely obtainment of collateral and supplemental history is essential in formulating an appropriate diagnosis. In the case presented, essential history from relatives provided critical information in the chronicity of the patient's symptoms and prior psychiatric history.\nPsychotic symptoms may also arise from medical conditions, medications, or substance abuse. A careful metabolic workup, including a metabolic panel, is appropriate to rule out possible alternative causes. Certain infections and blood conditions may also contribute to psychiatric symptoms, making a complete blood count an appropriate test as well. A CT scan of the head is important to rule out organic or structural precipitants for psychotic symptoms. A urine toxicology screen is also essential to exclude contribution from the effects of illicit substances.\nIn evaluating mental health conditions, a careful evaluation of patient risk to personal safety and that of others is imperative. Should such an evaluation reveal the presence of dangerous delusions, hallucinations, suicidal thoughts, or other severe symptoms that might cause harm (or the inability to avoid harm) to the patient or others, the patient may be temporarily committed on an involuntary basis for a brief period while further evaluation can be conducted. A patient who is suicidal and/or homicidal must not be left unattended. Requirements and durations vary and are based on regional laws.\nThe first-line treatments for schizophrenia symptoms are antipsychotic agents. The primary mechanism of action for the therapeutic effect of antipsychotic agents is blockade at the dopamine receptor D2 in the mesolimbic pathway of the brain.[3] Unfortunately, such agents also may block dopamine receptors in the substantia nigra, resulting in the common side effect of extrapyramidal symptoms (EPS). These include akathisia, dyskinesia, dystonia, and akinesia. The most concerning of these is the syndrome of tardive dyskinesia (TD), wherein a patient may develop chronic and intractable repetitive movements of the face, tongue, or body.[3]\nThe balance of dopaminergic and cholinergic activity is central to the pathogenesis of EPS, including TD. Dopamine typically inhibits acetylcholine release. Blocking D2 receptors reduces dopamine's ability to inhibit acetylcholine release, leading to increased cholinergic activity. For this reason, anticholinergic agents such as benztropine are often administered with antipsychotics to reduce EPS such as TD.[3,6]\nFirst-generation antipsychotic agents, such as haloperidol or perphenazine, have a greater risk of inducing EPS because of their overall greater level of D2 receptor blockade (approximately 80%). By contrast, second-generation antipsychotic agents (eg, quetiapine, ziprasidone) have lower rates of TD induction, as D2 blockade varies from 60% to 80%; however, the risk for metabolic side effects is increased.[3,7]\nTwo common tools for assessing TD in patients with schizophrenia are the Abnormal Involuntary Movement Scale (AIMS) and the Extrapyramidal Symptom Rating Scale (ESRS). AIMS categorizes abnormal movements by anatomic location and overall severity. ESRS rates symptoms on the basis of severity and frequency. Subjective and objective measures of parkinsonism, dystonia, akathisia, and dyskinesia are included. A cross-scale comparison found high concordance between AIMS and ESRS.[8]\nMedication noncompliance is a major challenge to relapse prevention in the treatment of schizophrenia. By nature of the condition, symptoms may impede treatment. Patients with schizophrenia may come to believe that they no longer require medication, may experience hallucinations commanding them to stop taking medication, or may simply become apathetic and avolitional in regard to all activities. One pharmacologic strategy for preventing medication noncompliance includes use of long-acting injectable antipsychotic agents. Depot preparations of antipsychotic medications require administration much less frequently than daily oral antipsychotic medications and have been demonstrated to reduce rehospitalizations.[9] Patients with schizophrenia may stop their medication because of the aforementioned adverse effects of their drugs.\nIn addition to pharmacologic management, psychosocial interventions may be used to improve outcomes in schizophrenia. One example is Assertive Community Treatment, in which a team-based approach to care helps to assure access, delivery, and administration of medications. Such a team may include a psychiatrist, nurse, case manager, social worker, and primary care physician, working in tandem to monitor and provide services to chronic mental health patients. Cognitive-behavioral therapy has also been demonstrated to reduce positive symptoms in schizophrenia.[10] Social skills training for patients with schizophrenia encourages the development and maintenance of social networks, which is correlated with a reduction in negative symptoms.[4]\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 65-Year-Old Man Brought to the ED by Military Police"
},
{
"authors": "Jeffrey S. Forrest, MD; Alexander B. Shortridge",
"content": [
"Suicide is the single leading cause of premature death in patients with schizophrenia, making careful monitoring and assessment for suicidal ideation essential in this patient population. As many as 20%-50% of patients with schizophrenia make at least one suicide attempt.[2] Estimates suggest that as many as 10%-13% of patients with schizophrenia ultimately complete suicide. Patients with schizophrenia and a higher intelligence quotient have been found to be at greater risk for suicide, as have patients of younger age.[11] The second-generation antipsychotic clozapine has been associated with reduced suicidality in patients.",
"Substance abuse is another concern in patients with schizophrenia. The estimated prevalence of substance use disorders in patients with schizophrenia is thought to be at least 40%-50%; this has not changed over the past several decades.[12] A meta-analysis from 1990-2017 estimated that at least 26% of patients with schizophrenia are regular users of cannabis.[12] Regular cannabis usage has been postulated to be a risk factor for the development or potentiation of psychotic symptoms, with a potential relationship existing between the dose used and age of the first usage.[13] Increased use of street drugs is also demonstrated in patients with schizophrenia. Agents such as cocaine, methamphetamine, or phencyclidine may cause or exacerbate psychotic symptoms in their own right, making this issue of particular concern. Alcohol abuse is also common in patients with schizophrenia, with prevalence rates as high as 24%, which increases risk for both hospitalization and suicide.[12]",
"The long-term outcome of schizophrenia varies and is influenced by numerous factors, including access to resources and medication compliance.[14] A significant majority of patients require repeated hospitalizations for symptom exacerbations. Only a small minority of patients can be described as having a good outcome. Positive prognostic factors include later age of onset, female gender, rapid onset of symptoms, and presence of positive symptoms (as compared with negative symptoms). Negative prognostic indicators include multiple or severe negative symptoms, male gender, early age of onset, and insidious onset of psychotic symptoms.",
"The patient in this case was found to have self-discontinued an antipsychotic medication. After several weeks of admission and care coordination, he agreed to receive a long-acting injectable form of his medication, with careful plans and family involvement helping to ensure his participation in continued treatment going forward."
],
"date": "August 06, 2019",
"figures": [],
"markdown": "# A 65-Year-Old Man Brought to the ED by Military Police\n\n **Authors:** Jeffrey S. Forrest, MD; Alexander B. Shortridge \n **Date:** August 06, 2019\n\n ## Content\n\n Suicide is the single leading cause of premature death in patients with schizophrenia, making careful monitoring and assessment for suicidal ideation essential in this patient population. As many as 20%-50% of patients with schizophrenia make at least one suicide attempt.[2] Estimates suggest that as many as 10%-13% of patients with schizophrenia ultimately complete suicide. Patients with schizophrenia and a higher intelligence quotient have been found to be at greater risk for suicide, as have patients of younger age.[11] The second-generation antipsychotic clozapine has been associated with reduced suicidality in patients.\nSubstance abuse is another concern in patients with schizophrenia. The estimated prevalence of substance use disorders in patients with schizophrenia is thought to be at least 40%-50%; this has not changed over the past several decades.[12] A meta-analysis from 1990-2017 estimated that at least 26% of patients with schizophrenia are regular users of cannabis.[12] Regular cannabis usage has been postulated to be a risk factor for the development or potentiation of psychotic symptoms, with a potential relationship existing between the dose used and age of the first usage.[13] Increased use of street drugs is also demonstrated in patients with schizophrenia. Agents such as cocaine, methamphetamine, or phencyclidine may cause or exacerbate psychotic symptoms in their own right, making this issue of particular concern. Alcohol abuse is also common in patients with schizophrenia, with prevalence rates as high as 24%, which increases risk for both hospitalization and suicide.[12]\nThe long-term outcome of schizophrenia varies and is influenced by numerous factors, including access to resources and medication compliance.[14] A significant majority of patients require repeated hospitalizations for symptom exacerbations. Only a small minority of patients can be described as having a good outcome. Positive prognostic factors include later age of onset, female gender, rapid onset of symptoms, and presence of positive symptoms (as compared with negative symptoms). Negative prognostic indicators include multiple or severe negative symptoms, male gender, early age of onset, and insidious onset of psychotic symptoms.\nThe patient in this case was found to have self-discontinued an antipsychotic medication. After several weeks of admission and care coordination, he agreed to receive a long-acting injectable form of his medication, with careful plans and family involvement helping to ensure his participation in continued treatment going forward.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1391806,
"choiceText": "Rapid symptom onset",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1391808,
"choiceText": "Flattened affect on exam",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1391810,
"choiceText": "Early age of onset",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1391812,
"choiceText": "Male gender",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>A rapid progression into psychosis suggests a better relative outcome than a slow, gradual onset of symptoms. Other positive prognostic features include the presence of positive symptoms (compared with negative symptoms), female gender, and older age of onset.</p>",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 444447,
"questionText": "Which of the following features suggests a relatively positive prognosis for a patient with schizophrenia?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1391814,
"choiceText": "Decreased dopaminergic activity in the nucleus accumbens",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1391816,
"choiceText": "Decreased cholinergic activity in the striatum",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1391818,
"choiceText": "Increased dopaminergic activity in the nucleus accumbens",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1391820,
"choiceText": "Increased cholinergic activity in the striatum",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1391822,
"choiceText": "Decreased dopaminergic activity in the tuberoinfundibular pathway",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>EPS such as cogwheel rigidity result from dopaminergic block. This, in turn, results in increased activity of acetylcholine in the striatum. Patients with this appear to have symptoms of Parkinson disease. Treatments include anticholinergic agents such as benztropine and withdrawing the offending agents.</p>",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 444449,
"questionText": "Which of the following best explains a finding of cogwheel rigidity in a patient with schizophrenia receiving risperidone?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 65-Year-Old Man Brought to the ED by Military Police"
},
{
"authors": "Jeffrey S. Forrest, MD; Alexander B. Shortridge",
"content": [],
"date": "August 06, 2019",
"figures": [],
"markdown": "# A 65-Year-Old Man Brought to the ED by Military Police\n\n **Authors:** Jeffrey S. Forrest, MD; Alexander B. Shortridge \n **Date:** August 06, 2019\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1391806,
"choiceText": "Rapid symptom onset",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1391808,
"choiceText": "Flattened affect on exam",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1391810,
"choiceText": "Early age of onset",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1391812,
"choiceText": "Male gender",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>A rapid progression into psychosis suggests a better relative outcome than a slow, gradual onset of symptoms. Other positive prognostic features include the presence of positive symptoms (compared with negative symptoms), female gender, and older age of onset.</p>",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 444447,
"questionText": "Which of the following features suggests a relatively positive prognosis for a patient with schizophrenia?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1391814,
"choiceText": "Decreased dopaminergic activity in the nucleus accumbens",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1391816,
"choiceText": "Decreased cholinergic activity in the striatum",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1391818,
"choiceText": "Increased dopaminergic activity in the nucleus accumbens",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1391820,
"choiceText": "Increased cholinergic activity in the striatum",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1391822,
"choiceText": "Decreased dopaminergic activity in the tuberoinfundibular pathway",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>EPS such as cogwheel rigidity result from dopaminergic block. This, in turn, results in increased activity of acetylcholine in the striatum. Patients with this appear to have symptoms of Parkinson disease. Treatments include anticholinergic agents such as benztropine and withdrawing the offending agents.</p>",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 444449,
"questionText": "Which of the following best explains a finding of cogwheel rigidity in a patient with schizophrenia receiving risperidone?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 65-Year-Old Man Brought to the ED by Military Police"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1391798,
"choiceText": "Bipolar disorder",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1391800,
"choiceText": "Borderline personality disorder",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1391802,
"choiceText": "Schizophrenia",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1391804,
"choiceText": "Wernicke-Korsakoff syndrome",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 444445,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1391806,
"choiceText": "Rapid symptom onset",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1391808,
"choiceText": "Flattened affect on exam",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1391810,
"choiceText": "Early age of onset",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1391812,
"choiceText": "Male gender",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>A rapid progression into psychosis suggests a better relative outcome than a slow, gradual onset of symptoms. Other positive prognostic features include the presence of positive symptoms (compared with negative symptoms), female gender, and older age of onset.</p>",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 444447,
"questionText": "Which of the following features suggests a relatively positive prognosis for a patient with schizophrenia?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1391814,
"choiceText": "Decreased dopaminergic activity in the nucleus accumbens",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1391816,
"choiceText": "Decreased cholinergic activity in the striatum",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1391818,
"choiceText": "Increased dopaminergic activity in the nucleus accumbens",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1391820,
"choiceText": "Increased cholinergic activity in the striatum",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1391822,
"choiceText": "Decreased dopaminergic activity in the tuberoinfundibular pathway",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>EPS such as cogwheel rigidity result from dopaminergic block. This, in turn, results in increased activity of acetylcholine in the striatum. Patients with this appear to have symptoms of Parkinson disease. Treatments include anticholinergic agents such as benztropine and withdrawing the offending agents.</p>",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 444449,
"questionText": "Which of the following best explains a finding of cogwheel rigidity in a patient with schizophrenia receiving risperidone?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
915889 | /viewarticle/915889 | [
{
"authors": "Heidi Moawad, MD",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"A 67-year-old man who was diagnosed with Parkinson disease 8 years ago has experienced recent, unintentional weight loss. He lost 30 lb over the course of 12 months. He is 6'1\" tall and his weight is now 136 lb (61.7 kg), giving him a body mass index of 18 kg/m2, which is slightly underweight.",
"The patient has never been overweight. His cholesterol and blood glucose levels were previously in the upper limits of the reference range. However, he has not recently been dieting or trying to lose weight. He states that he has not paid much attention to how much he has been eating lately.",
"Other than Parkinson disease, the patient does not have a significant or relevant medical history. He has been taking a combination of amantadine and carbidopa/levodopa, with good control of his motor symptoms. His Parkinson disease is mainly characterized by a resting tremor in his left arm; muscle rigidity in all four extremities; a flat affect; and a slow, shuffling gait."
],
"date": "July 25, 2019",
"figures": [],
"markdown": "# Dramatic Weight Loss in a Patient With Parkinson Disease\n\n **Authors:** Heidi Moawad, MD \n **Date:** July 25, 2019\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nA 67-year-old man who was diagnosed with Parkinson disease 8 years ago has experienced recent, unintentional weight loss. He lost 30 lb over the course of 12 months. He is 6'1\" tall and his weight is now 136 lb (61.7 kg), giving him a body mass index of 18 kg/m2, which is slightly underweight.\nThe patient has never been overweight. His cholesterol and blood glucose levels were previously in the upper limits of the reference range. However, he has not recently been dieting or trying to lose weight. He states that he has not paid much attention to how much he has been eating lately.\nOther than Parkinson disease, the patient does not have a significant or relevant medical history. He has been taking a combination of amantadine and carbidopa/levodopa, with good control of his motor symptoms. His Parkinson disease is mainly characterized by a resting tremor in his left arm; muscle rigidity in all four extremities; a flat affect; and a slow, shuffling gait.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "Dramatic Weight Loss in a Patient With Parkinson Disease"
},
{
"authors": "Heidi Moawad, MD",
"content": [
"The patient appears to be well-nourished, despite his recent weight loss. He is alert and oriented and in no acute distress. He has dry, flaky skin, as is commonly seen with Parkinson disease-associated seborrheic dermatitis. Otherwise, his skin appears normal. His heart sounds are normal, his respiration is normal, and his abdomen is nondistended and nontender. His blood pressure is 160/85 mm Hg, and his pulse is 86 beats/min.",
"Upon neurologic examination, the patient's mental status and affect are normal. He is able to recall his medical history with great detail. His speech is clear, and he speaks rapidly in a monotone voice. He does not have any tremors during the examination.",
"The patient's extraocular movements are intact, with no nystagmus. He does not have any visual field cut. His pupils are symmetrical and reactive to light, and he does not have papilledema. He does not have facial asymmetry, and his facial sensation is normal.",
"His muscle tone is increased in all four extremities, and his strength is 5/5 in all four extremities. His sensory examination is normal, and his reflexes are normal. He has a shuffling gait but is able to walk without difficulty."
],
"date": "July 25, 2019",
"figures": [],
"markdown": "# Dramatic Weight Loss in a Patient With Parkinson Disease\n\n **Authors:** Heidi Moawad, MD \n **Date:** July 25, 2019\n\n ## Content\n\n The patient appears to be well-nourished, despite his recent weight loss. He is alert and oriented and in no acute distress. He has dry, flaky skin, as is commonly seen with Parkinson disease-associated seborrheic dermatitis. Otherwise, his skin appears normal. His heart sounds are normal, his respiration is normal, and his abdomen is nondistended and nontender. His blood pressure is 160/85 mm Hg, and his pulse is 86 beats/min.\nUpon neurologic examination, the patient's mental status and affect are normal. He is able to recall his medical history with great detail. His speech is clear, and he speaks rapidly in a monotone voice. He does not have any tremors during the examination.\nThe patient's extraocular movements are intact, with no nystagmus. He does not have any visual field cut. His pupils are symmetrical and reactive to light, and he does not have papilledema. He does not have facial asymmetry, and his facial sensation is normal.\nHis muscle tone is increased in all four extremities, and his strength is 5/5 in all four extremities. His sensory examination is normal, and his reflexes are normal. He has a shuffling gait but is able to walk without difficulty.\n\n ## Figures\n\n \n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1386212,
"choiceText": "Brain MRI",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1386214,
"choiceText": "Electromyography",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1386216,
"choiceText": "Abdominal CT",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1386218,
"choiceText": "ECG",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 442705,
"questionText": "Which of the following tests should be obtained first to investigate the cause of this patient's weight loss?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Dramatic Weight Loss in a Patient With Parkinson Disease"
},
{
"authors": "Heidi Moawad, MD",
"content": [
"Although Parkinson disease can cause weight loss, this patient's rapid weight loss is concerning. Abdominal CT may help identify primary or metastatic cancer or another abnormality in the gastrointestinal tract, liver, or kidneys.",
"The patient does not appear to have neurologic changes; thus, brain MRI is unlikely to provide information about his condition. He also does not appear to have any symptoms that would correspond with myopathy or neuropathy, which could be detected by electromyography. No symptoms are inherently suggestive of cardiac dysfunction; thus, ECG would not be helpful in evaluating his weight loss.",
"Because of concern about cancer, a gastrointestinal disorder, or a metabolic illness, abdominal CT was performed. The test results were normal. The patient also underwent a rectal examination to test for occult blood, a complete blood cell count, liver function testing, and chest CT. All findings were normal.",
"After ruling out other possible etiologies of rapid weight loss, the patient's Parkinson disease is deemed to be the cause of his weight loss. Such weight loss is described in approximately 48% of patients who have Parkinson disease.[1]"
],
"date": "July 25, 2019",
"figures": [],
"markdown": "# Dramatic Weight Loss in a Patient With Parkinson Disease\n\n **Authors:** Heidi Moawad, MD \n **Date:** July 25, 2019\n\n ## Content\n\n Although Parkinson disease can cause weight loss, this patient's rapid weight loss is concerning. Abdominal CT may help identify primary or metastatic cancer or another abnormality in the gastrointestinal tract, liver, or kidneys.\nThe patient does not appear to have neurologic changes; thus, brain MRI is unlikely to provide information about his condition. He also does not appear to have any symptoms that would correspond with myopathy or neuropathy, which could be detected by electromyography. No symptoms are inherently suggestive of cardiac dysfunction; thus, ECG would not be helpful in evaluating his weight loss.\nBecause of concern about cancer, a gastrointestinal disorder, or a metabolic illness, abdominal CT was performed. The test results were normal. The patient also underwent a rectal examination to test for occult blood, a complete blood cell count, liver function testing, and chest CT. All findings were normal.\nAfter ruling out other possible etiologies of rapid weight loss, the patient's Parkinson disease is deemed to be the cause of his weight loss. Such weight loss is described in approximately 48% of patients who have Parkinson disease.[1]\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1386212,
"choiceText": "Brain MRI",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1386214,
"choiceText": "Electromyography",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1386216,
"choiceText": "Abdominal CT",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1386218,
"choiceText": "ECG",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 442705,
"questionText": "Which of the following tests should be obtained first to investigate the cause of this patient's weight loss?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Dramatic Weight Loss in a Patient With Parkinson Disease"
},
{
"authors": "Heidi Moawad, MD",
"content": [
"This patient rapidly lost more weight than the average patient with Parkinson disease. Thus, ruling out cancer or other disease processes was an important aspect of his evaluation and management. With other causes eliminated, his medical team focused on identifying and addressing Parkinson disease-related aspects of his weight loss.",
"Hyposmia is a diminished sense of smell. It is commonly seen in neurologic conditions, such as Parkinson disease, Alzheimer disease, Lewy body dementia, and stroke. Because smell is such an important component of taste, the loss of smell sensation can diminish a person's food intake. Of note, altered sense of smell has been reported in association with hyposmia, and some patients may develop an aversion to the smells of certain foods.",
"Parkinson disease itself can induce weight loss, even without diminished food intake. Although the pathophysiology is not well understood, weight loss has been described as part of the Parkinson disease process. In addition, some medications used for Parkinson disease, such as levodopa, may have a side effect of weight loss.[2]",
"Other causes of weight loss in Parkinson disease have also been recognized. Depression is fairly common in Parkinson disease and can result in a loss of appetite and deceased food intake owing to apathy and/or excessive sleep. Gastrointestinal disturbances have also been observed in Parkinson disease, including gastroesophageal reflux disease (GERD), constipation, and sialorrhea (excessive salivation or drooling).[3] All of these may cause difficulty eating and may result in decreased oral intake. Some patients with Parkinson disease may also experience dysphagia, which can cause difficulties eating and/or an aversion to eating. Many patients with Parkinson disease experience more than one of these symptoms, and the additive effects can have a substantial effect on appetite, metabolism, and the ability to safely swallow and eat."
],
"date": "July 25, 2019",
"figures": [],
"markdown": "# Dramatic Weight Loss in a Patient With Parkinson Disease\n\n **Authors:** Heidi Moawad, MD \n **Date:** July 25, 2019\n\n ## Content\n\n This patient rapidly lost more weight than the average patient with Parkinson disease. Thus, ruling out cancer or other disease processes was an important aspect of his evaluation and management. With other causes eliminated, his medical team focused on identifying and addressing Parkinson disease-related aspects of his weight loss.\nHyposmia is a diminished sense of smell. It is commonly seen in neurologic conditions, such as Parkinson disease, Alzheimer disease, Lewy body dementia, and stroke. Because smell is such an important component of taste, the loss of smell sensation can diminish a person's food intake. Of note, altered sense of smell has been reported in association with hyposmia, and some patients may develop an aversion to the smells of certain foods.\nParkinson disease itself can induce weight loss, even without diminished food intake. Although the pathophysiology is not well understood, weight loss has been described as part of the Parkinson disease process. In addition, some medications used for Parkinson disease, such as levodopa, may have a side effect of weight loss.[2]\nOther causes of weight loss in Parkinson disease have also been recognized. Depression is fairly common in Parkinson disease and can result in a loss of appetite and deceased food intake owing to apathy and/or excessive sleep. Gastrointestinal disturbances have also been observed in Parkinson disease, including gastroesophageal reflux disease (GERD), constipation, and sialorrhea (excessive salivation or drooling).[3] All of these may cause difficulty eating and may result in decreased oral intake. Some patients with Parkinson disease may also experience dysphagia, which can cause difficulties eating and/or an aversion to eating. Many patients with Parkinson disease experience more than one of these symptoms, and the additive effects can have a substantial effect on appetite, metabolism, and the ability to safely swallow and eat.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1386220,
"choiceText": "Hyposmia",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1386222,
"choiceText": "The disease process itself",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1386224,
"choiceText": "Medication use",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1386226,
"choiceText": "All of the above",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 442707,
"questionText": "Which of the following aspects of Parkinson disease most likely contributes to weight loss?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Dramatic Weight Loss in a Patient With Parkinson Disease"
},
{
"authors": "Heidi Moawad, MD",
"content": [
"The prediction of weight loss among patients with Parkinson disease has been attempted. The NINDS Exploratory Trials in Parkinson Disease Long-term Study-1 was a large prospective clinical trial cohort of early treated Parkinson disease.[4] The study evaluated numerous factors and their associations with weight loss in Parkinson disease. The results indicated that the following factors were strongly associated with weight loss:",
"Older age",
"Female sex",
"Higher Unified Parkinson Disease Rating Scale score (more advanced disease)",
"Increased postural instability",
"Difficulty eating and drinking",
"Lower cognitive scores",
"Levodopa use",
"The same study showed that dyskinesia, depression, intestinal hypomotility and self-reported nausea, vomiting, or anorexia were associated with weight loss; however, these factors were not as significant as the other factors.",
"Another study found an association between early weight loss in Parkinson disease and poorer prognosis.[5] Patients with Parkinson disease who lost > 5% of their weight in the early stages of disease were more likely to experience sustained weight loss, develop dementia, lose their independence, and experience early death. This research suggests that averting excessive weight loss in Parkinson disease may have a beneficial long-term effect on patient outcome."
],
"date": "July 25, 2019",
"figures": [],
"markdown": "# Dramatic Weight Loss in a Patient With Parkinson Disease\n\n **Authors:** Heidi Moawad, MD \n **Date:** July 25, 2019\n\n ## Content\n\n The prediction of weight loss among patients with Parkinson disease has been attempted. The NINDS Exploratory Trials in Parkinson Disease Long-term Study-1 was a large prospective clinical trial cohort of early treated Parkinson disease.[4] The study evaluated numerous factors and their associations with weight loss in Parkinson disease. The results indicated that the following factors were strongly associated with weight loss:\nOlder age\nFemale sex\nHigher Unified Parkinson Disease Rating Scale score (more advanced disease)\nIncreased postural instability\nDifficulty eating and drinking\nLower cognitive scores\nLevodopa use\nThe same study showed that dyskinesia, depression, intestinal hypomotility and self-reported nausea, vomiting, or anorexia were associated with weight loss; however, these factors were not as significant as the other factors.\nAnother study found an association between early weight loss in Parkinson disease and poorer prognosis.[5] Patients with Parkinson disease who lost > 5% of their weight in the early stages of disease were more likely to experience sustained weight loss, develop dementia, lose their independence, and experience early death. This research suggests that averting excessive weight loss in Parkinson disease may have a beneficial long-term effect on patient outcome.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1386404,
"choiceText": "Reduce his medication dose",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1386406,
"choiceText": "Change to another medication",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1386408,
"choiceText": "Refer him to a dietitian",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1386410,
"choiceText": "Prescribe a medication to increase his appetite (eg, dronabinol or prednisone)",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 442761,
"questionText": "Which of the following is the recommended next step in helping this patient maintain a healthy weight?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Dramatic Weight Loss in a Patient With Parkinson Disease"
},
{
"authors": "Heidi Moawad, MD",
"content": [
"Because many aspects of Parkinson disease could be responsible for this patient's weight loss, changing his medication dose or changing to another medication may not have the desired effect of weight gain. Neither increasing nor decreasing the Parkinson disease medication dose has been consistently associated with weight gain or weight loss. Whether optimal treatment for Parkinson disease could help alleviate weight loss is unclear; however, this patient's Parkinson disease symptoms seem well managed, and he is not experiencing other side effects. Thus, altering his medication to address weight issues may exacerbate his Parkinson disease or cause unnecessary side effects.",
"An appetite stimulant, such as dronabinol (a cannabinoid), is not the recommended next step. This medication is used for aiding patients who are severely ill, such as those with cancer who are experiencing weight loss and/or loss of appetite. However, the patient in this scenario may have other causes for his weight loss besides loss of appetite. Thus, dronabinol may not produce the desired effects. Similarly, although prednisone can cause some patients to gain weight, that may not be the right treatment for the patient at this time.",
"A consultation with a dietitian can help in determining the etiology and the right course of action for this patient. One important step is evaluating the patient's caloric needs and intake. After an assessment, suggestions regarding a change in diet may be needed if hyposmia, loss of appetite, or dysphagia is part of the problem.",
"This patient may also have other Parkinson disease-related issues that are causing his weight loss, and a multidisciplinary evaluation makes sense. If he is found to have dysphagia, swallowing exercises are beneficial. If he has GERD or constipation, appropriate medical therapy for these conditions may help alleviate his weight problem."
],
"date": "July 25, 2019",
"figures": [],
"markdown": "# Dramatic Weight Loss in a Patient With Parkinson Disease\n\n **Authors:** Heidi Moawad, MD \n **Date:** July 25, 2019\n\n ## Content\n\n Because many aspects of Parkinson disease could be responsible for this patient's weight loss, changing his medication dose or changing to another medication may not have the desired effect of weight gain. Neither increasing nor decreasing the Parkinson disease medication dose has been consistently associated with weight gain or weight loss. Whether optimal treatment for Parkinson disease could help alleviate weight loss is unclear; however, this patient's Parkinson disease symptoms seem well managed, and he is not experiencing other side effects. Thus, altering his medication to address weight issues may exacerbate his Parkinson disease or cause unnecessary side effects.\nAn appetite stimulant, such as dronabinol (a cannabinoid), is not the recommended next step. This medication is used for aiding patients who are severely ill, such as those with cancer who are experiencing weight loss and/or loss of appetite. However, the patient in this scenario may have other causes for his weight loss besides loss of appetite. Thus, dronabinol may not produce the desired effects. Similarly, although prednisone can cause some patients to gain weight, that may not be the right treatment for the patient at this time.\nA consultation with a dietitian can help in determining the etiology and the right course of action for this patient. One important step is evaluating the patient's caloric needs and intake. After an assessment, suggestions regarding a change in diet may be needed if hyposmia, loss of appetite, or dysphagia is part of the problem.\nThis patient may also have other Parkinson disease-related issues that are causing his weight loss, and a multidisciplinary evaluation makes sense. If he is found to have dysphagia, swallowing exercises are beneficial. If he has GERD or constipation, appropriate medical therapy for these conditions may help alleviate his weight problem.\n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1386404,
"choiceText": "Reduce his medication dose",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1386406,
"choiceText": "Change to another medication",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1386408,
"choiceText": "Refer him to a dietitian",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1386410,
"choiceText": "Prescribe a medication to increase his appetite (eg, dronabinol or prednisone)",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 442761,
"questionText": "Which of the following is the recommended next step in helping this patient maintain a healthy weight?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Dramatic Weight Loss in a Patient With Parkinson Disease"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1386212,
"choiceText": "Brain MRI",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1386214,
"choiceText": "Electromyography",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1386216,
"choiceText": "Abdominal CT",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1386218,
"choiceText": "ECG",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 442705,
"questionText": "Which of the following tests should be obtained first to investigate the cause of this patient's weight loss?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1386220,
"choiceText": "Hyposmia",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1386222,
"choiceText": "The disease process itself",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1386224,
"choiceText": "Medication use",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1386226,
"choiceText": "All of the above",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 442707,
"questionText": "Which of the following aspects of Parkinson disease most likely contributes to weight loss?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1386404,
"choiceText": "Reduce his medication dose",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1386406,
"choiceText": "Change to another medication",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1386408,
"choiceText": "Refer him to a dietitian",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1386410,
"choiceText": "Prescribe a medication to increase his appetite (eg, dronabinol or prednisone)",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 442761,
"questionText": "Which of the following is the recommended next step in helping this patient maintain a healthy weight?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
829678 | /viewarticle/829678 | [
{
"authors": "Stephen Soreff, MD; Foad Afshar, PsyD, EdM",
"content": [
"Editorial Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"Michael is a 12-year-old male who lives in an intact family, including a sister two years his junior. He is in the fifth grade, and over the course of the last year, his grades have fallen significantly. He avoids school, claiming to be sick, and when he is in school, he is disruptive and frequently visits the nurse. Michael’s parents report that getting their son to do any homework is difficult and that he has “meltdowns” and “tantrums” when confronted with any tasks that are time-consuming or cognitively demanding. His favorite hobby is playing video games.",
"His parents also note that Michael can be very forgetful. At home, by the time he gets to the top of the stairs in the evening to engage in before-bedtime routines, he literally forgets why he is there and instead begin playing with his Legos or entertaining himself with the cat. Moreover, although Michael is interested in helping his father work on mechanical machines and engines, he often forgets the tools he is sent to retrieve. Michael’s parents have sought consultation after meeting with their pediatrician at the urging of Michael’s teachers and guidance counselor."
],
"date": "June 25, 2019",
"figures": [],
"markdown": "# A 12-Year-Old Boy With Falling Grades and Forgetful Behavior\n\n **Authors:** Stephen Soreff, MD; Foad Afshar, PsyD, EdM \n **Date:** June 25, 2019\n\n ## Content\n\n Editorial Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nMichael is a 12-year-old male who lives in an intact family, including a sister two years his junior. He is in the fifth grade, and over the course of the last year, his grades have fallen significantly. He avoids school, claiming to be sick, and when he is in school, he is disruptive and frequently visits the nurse. Michael’s parents report that getting their son to do any homework is difficult and that he has “meltdowns” and “tantrums” when confronted with any tasks that are time-consuming or cognitively demanding. His favorite hobby is playing video games.\nHis parents also note that Michael can be very forgetful. At home, by the time he gets to the top of the stairs in the evening to engage in before-bedtime routines, he literally forgets why he is there and instead begin playing with his Legos or entertaining himself with the cat. Moreover, although Michael is interested in helping his father work on mechanical machines and engines, he often forgets the tools he is sent to retrieve. Michael’s parents have sought consultation after meeting with their pediatrician at the urging of Michael’s teachers and guidance counselor.\n\n ## Figures\n\n \n*Page 1 of 7*",
"pagination": {
"current_page": 1,
"total_pages": 7
},
"questionnaire": [],
"title": "A 12-Year-Old Boy With Falling Grades and Forgetful Behavior"
},
{
"authors": "Stephen Soreff, MD; Foad Afshar, PsyD, EdM",
"content": [
"During the initial interview, in addition to the history taken from both parents, a mental status examination is conducted via a clinical interview with the patient. The examination reveals relatively high anxiety, orientation x 3, coherent thoughts, and an affect that is contextually congruent. Michael shows a good sense of humor and typical mastery of oral expression, with complex sentence structure and age-appropriate vocabulary. Michael’s short- and long-term memory are intact as well. No hallucinations, delusions, or violent behavior are reported. He does not report thoughts of self-harm, although he does note that he feels \"stupid\" and not part of groups sometimes. He has no history of head injuries or any significant medical conditions. No routine laboratory or physical examination findings appear abnormal. Test results also indicate that Michael does not have any hearing or sight problems.",
"Growing up, Michael reached all developmental milestones within normal limits; indeed, Michael is reported to have talked and walked early (and often). In addition, his parents describe him as an “active and outdoors kind of kid.” No history of any major family crises or emotional traumas is reported. The boy’s early school history is significant for reports of classroom disruption and Michael’s need to move about; however, at that time, Michael was able to meet academic demands without having to do his homework. Michael has no family history of mental illness; however, his father states that he himself often starts too many tasks and sometimes does not complete them.",
"During the diagnostic session, Michael becomes visibly fidgety, his speech becomes more stammered, his ears become red, and he turns blotchy red when asked to read a third-grade ̶ level text passage and do basic arithmetic. He later explains that when he has to do tasks like this, he becomes nauseous and feels “hot.” Michael also reports that he often gets headaches as soon as he begins to do tasks that require extended attention over time, especially “if they are boring.” He has no difficulty memorizing dialogue from plays (he is involved in theater), nor does he have trouble reading musical notes during band rehearsal.",
"He admits to feeling “stupid” in school and taking his frustrations out on kids whom he considers to be smart by sabotaging their learning through disruptive behaviors.",
"In a comprehensive psychoeducational evaluation by school personnel (including completion of Conners’ Rating Scales by Michael’s parents and teachers), which was carried out prior to the consultation, his teachers indicated that Michael was unable to focus on tasks; abandoned tasks that he had already started, moving on to new ones; and daydreamed often."
],
"date": "June 25, 2019",
"figures": [],
"markdown": "# A 12-Year-Old Boy With Falling Grades and Forgetful Behavior\n\n **Authors:** Stephen Soreff, MD; Foad Afshar, PsyD, EdM \n **Date:** June 25, 2019\n\n ## Content\n\n During the initial interview, in addition to the history taken from both parents, a mental status examination is conducted via a clinical interview with the patient. The examination reveals relatively high anxiety, orientation x 3, coherent thoughts, and an affect that is contextually congruent. Michael shows a good sense of humor and typical mastery of oral expression, with complex sentence structure and age-appropriate vocabulary. Michael’s short- and long-term memory are intact as well. No hallucinations, delusions, or violent behavior are reported. He does not report thoughts of self-harm, although he does note that he feels \"stupid\" and not part of groups sometimes. He has no history of head injuries or any significant medical conditions. No routine laboratory or physical examination findings appear abnormal. Test results also indicate that Michael does not have any hearing or sight problems.\nGrowing up, Michael reached all developmental milestones within normal limits; indeed, Michael is reported to have talked and walked early (and often). In addition, his parents describe him as an “active and outdoors kind of kid.” No history of any major family crises or emotional traumas is reported. The boy’s early school history is significant for reports of classroom disruption and Michael’s need to move about; however, at that time, Michael was able to meet academic demands without having to do his homework. Michael has no family history of mental illness; however, his father states that he himself often starts too many tasks and sometimes does not complete them.\nDuring the diagnostic session, Michael becomes visibly fidgety, his speech becomes more stammered, his ears become red, and he turns blotchy red when asked to read a third-grade ̶ level text passage and do basic arithmetic. He later explains that when he has to do tasks like this, he becomes nauseous and feels “hot.” Michael also reports that he often gets headaches as soon as he begins to do tasks that require extended attention over time, especially “if they are boring.” He has no difficulty memorizing dialogue from plays (he is involved in theater), nor does he have trouble reading musical notes during band rehearsal.\nHe admits to feeling “stupid” in school and taking his frustrations out on kids whom he considers to be smart by sabotaging their learning through disruptive behaviors.\nIn a comprehensive psychoeducational evaluation by school personnel (including completion of Conners’ Rating Scales by Michael’s parents and teachers), which was carried out prior to the consultation, his teachers indicated that Michael was unable to focus on tasks; abandoned tasks that he had already started, moving on to new ones; and daydreamed often.\n\n ## Figures\n\n \n*Page 2 of 7*",
"pagination": {
"current_page": 2,
"total_pages": 7
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 755865,
"choiceText": "Conduct disorder",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 755867,
"choiceText": "Posttraumatic stress disorder",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 755869,
"choiceText": "Learning disability",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 755871,
"choiceText": "Attention deficit hyperactivity disorder",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 755873,
"choiceText": "Bipolar disorder",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 236513,
"questionText": "Based on the history and evaluation, which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 12-Year-Old Boy With Falling Grades and Forgetful Behavior"
},
{
"authors": "Stephen Soreff, MD; Foad Afshar, PsyD, EdM",
"content": [
"In the school’s psychoeducational evaluation, reports from Michael’s teachers showed clinically significant symptoms of attention deficit hyperactivity disorder (ADHD), and the parents’ reports showed mildly elevated risk in the clinical range of ADHD. Michael’s mother—who, when Michael’s history was being taken, mentioned that she often wondered if her husband “had a touch of ADHD”—rated Michael’s behaviors at a higher level of risk than his father’s. One of the keys in diagnosing ADHD is that the symptoms are present both at school and at home. This is important, as a diagnosis of ADHD requires that the behavior must occur both at home and at school.",
"Michael’s math and reading teachers also ranked his difficulties in these subjects as consistent with symptoms of ADHD. The Woodcock-Johnson Test of Academic Achievement indicated that Michael was in the 37th percentile for reading and mathematics. Although these scores were lower than average, they were within the expected range of functioning, given his IQ score of 102. Many children with ADHD have above-average IQ scores. Also, when provided with academic curricular tests in a 1:1 environment with focusing cues and no distractions, Michael did better on the tests than he did when they were administered in a classroom environment with distractions and time constraints.",
"Treatment recommendations included a family education program on how to best structure Michael's home schedule and initiation of a stimulant medication by the referring pediatrician.[1]",
"With regard to a biologic explanation for ADHD, abnormal activity of the neurotransmitter dopamine—including lower dopamine activity in the limbic system, as revealed on positron emission tomography (PET) scans—has been noted in some persons with ADHD.[2,3] These changes in dopamine activity may explain why medications that act to increase dopamine, such as methylphenidate, have been effective in treating ADHD.[4] Research indicates that dysregulation and changes in the activity of the neurotransmitter norepinephrine are also associated with ADHD. The lower levels of the neurotransmitters (dopamine and norepinephrine) in patients with ADHD helps explain the paradox of providing treatment that includes a stimulant, such as methylphenidate, to persons who are already hyperactive.",
"Evidence has also demonstrated frontostriatal malfunctioning in ADHD,[5] and research has demonstrated the existence of deformations in the basal ganglia (caudate, putamen, globus pallidus) that are normalized by stimulant medications.[6] Another clue to the biologic etiology of ADHD is evidence that it is genetically transmissible.[7] In the case of Michael, for example, his father may have it.",
"In addition to biologic factors, ADHD can be affected by environmental and sociologic ones as well; specifically, stress and labeling. Conflicts, pressure, family dysfunction, and the mere phenomenon of being labeled as having ADHD may exacerbate current symptoms of the condition and produce other symptoms of ADHD that were not initially present.[2]"
],
"date": "June 25, 2019",
"figures": [],
"markdown": "# A 12-Year-Old Boy With Falling Grades and Forgetful Behavior\n\n **Authors:** Stephen Soreff, MD; Foad Afshar, PsyD, EdM \n **Date:** June 25, 2019\n\n ## Content\n\n In the school’s psychoeducational evaluation, reports from Michael’s teachers showed clinically significant symptoms of attention deficit hyperactivity disorder (ADHD), and the parents’ reports showed mildly elevated risk in the clinical range of ADHD. Michael’s mother—who, when Michael’s history was being taken, mentioned that she often wondered if her husband “had a touch of ADHD”—rated Michael’s behaviors at a higher level of risk than his father’s. One of the keys in diagnosing ADHD is that the symptoms are present both at school and at home. This is important, as a diagnosis of ADHD requires that the behavior must occur both at home and at school.\nMichael’s math and reading teachers also ranked his difficulties in these subjects as consistent with symptoms of ADHD. The Woodcock-Johnson Test of Academic Achievement indicated that Michael was in the 37th percentile for reading and mathematics. Although these scores were lower than average, they were within the expected range of functioning, given his IQ score of 102. Many children with ADHD have above-average IQ scores. Also, when provided with academic curricular tests in a 1:1 environment with focusing cues and no distractions, Michael did better on the tests than he did when they were administered in a classroom environment with distractions and time constraints.\nTreatment recommendations included a family education program on how to best structure Michael's home schedule and initiation of a stimulant medication by the referring pediatrician.[1]\nWith regard to a biologic explanation for ADHD, abnormal activity of the neurotransmitter dopamine—including lower dopamine activity in the limbic system, as revealed on positron emission tomography (PET) scans—has been noted in some persons with ADHD.[2,3] These changes in dopamine activity may explain why medications that act to increase dopamine, such as methylphenidate, have been effective in treating ADHD.[4] Research indicates that dysregulation and changes in the activity of the neurotransmitter norepinephrine are also associated with ADHD. The lower levels of the neurotransmitters (dopamine and norepinephrine) in patients with ADHD helps explain the paradox of providing treatment that includes a stimulant, such as methylphenidate, to persons who are already hyperactive.\nEvidence has also demonstrated frontostriatal malfunctioning in ADHD,[5] and research has demonstrated the existence of deformations in the basal ganglia (caudate, putamen, globus pallidus) that are normalized by stimulant medications.[6] Another clue to the biologic etiology of ADHD is evidence that it is genetically transmissible.[7] In the case of Michael, for example, his father may have it.\nIn addition to biologic factors, ADHD can be affected by environmental and sociologic ones as well; specifically, stress and labeling. Conflicts, pressure, family dysfunction, and the mere phenomenon of being labeled as having ADHD may exacerbate current symptoms of the condition and produce other symptoms of ADHD that were not initially present.[2]\n\n ## Figures\n\n \n*Page 3 of 7*",
"pagination": {
"current_page": 3,
"total_pages": 7
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 755865,
"choiceText": "Conduct disorder",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 755867,
"choiceText": "Posttraumatic stress disorder",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 755869,
"choiceText": "Learning disability",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 755871,
"choiceText": "Attention deficit hyperactivity disorder",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 755873,
"choiceText": "Bipolar disorder",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 236513,
"questionText": "Based on the history and evaluation, which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 12-Year-Old Boy With Falling Grades and Forgetful Behavior"
},
{
"authors": "Stephen Soreff, MD; Foad Afshar, PsyD, EdM",
"content": [
"Regarding its epidemiology, ADHD is more common in boys than in girls (13.2% vs 5.6%, respectively). The rate at which the condition is diagnosed has increased dramatically, from 7.8% of children aged 4-17 years in 2003 to 11% in this same age group in 2011.[8] Various theories suggest why this may be the case. For example, a greater focus on early education may mean that behaviors that would frequently be tolerated at home are attracting more notice in preschool or kindergarten classes. Moreover, when medication is available to treat a condition, such as ADHD, then that condition tends to be diagnosed more often; this may itself be associated with academic pressures on children to succeed and the desire to obtain ADHD treatment for students if it will better enable that success.",
"Studies suggest that childhood ADHD is a risk factor for subsequent conduct and substance abuse problems, which can lead to significant morbidity and mortality and to involvement with the criminal justice system. The condition may also lead to academic and employment difficulties and to social problems that greatly impact normal development.[9] The course of ADHD varies among patients. Some persons outgrow their symptoms, while others have lifelong symptoms and require treatment into adulthood.[10] Still others are not diagnosed with and treated for ADHD until adulthood.",
"In diagnosing ADHD, differential diagnoses must be considered first to ensure that a treatable medical condition or another possible psychiatric diagnosis is not missed. The first evaluation phase begins with a complete medical history, a physical examination, and appropriate laboratory tests. Many classic symptoms of ADHD can individually be attributed to numerous other causes (eg, inattention may occur in relation to a learning disability, boredom, or a hearing difficulty). In addition, numerous psychiatric disorders can mimic many ADHD symptoms; these conditions include affective bipolar disorder, schizophrenia, posttraumatic stress disorder (PTSD), and Tourette syndrome. Key symptoms of ADHD may also arise from a learning disability.[9,11]",
"In this specific case, Michael did not appear to have any physical or medical conditions that could manifest as ADHD-like symptoms. He also failed to meet the criteria for any of the major psychoses associated with childhood or with a conduct disorder; also, no significant family or personal disruptions had occurred over the past year that would have caused a change in his behavior.",
"Michael was reported by his teachers and parents to generally be a jovial and friendly child with many friends. He was reported to be helpful in school and to be the \"runner\" for teachers, getting them supplies and delivering messages to the main office regularly and with great reliability. Michael did not exhibit hypomania or any other symptoms associated with mood or anxiety disorders. He did not report racing thoughts, denied having problems sleeping, and also denied being particularly afraid of anything. In addition, his emotional range was congruent with circumstances and situations. Hence, Michael’s problem was less likely to have a psychodynamic etiology. Testing, moreover, suggested that he did not have a learning disorder."
],
"date": "June 25, 2019",
"figures": [],
"markdown": "# A 12-Year-Old Boy With Falling Grades and Forgetful Behavior\n\n **Authors:** Stephen Soreff, MD; Foad Afshar, PsyD, EdM \n **Date:** June 25, 2019\n\n ## Content\n\n Regarding its epidemiology, ADHD is more common in boys than in girls (13.2% vs 5.6%, respectively). The rate at which the condition is diagnosed has increased dramatically, from 7.8% of children aged 4-17 years in 2003 to 11% in this same age group in 2011.[8] Various theories suggest why this may be the case. For example, a greater focus on early education may mean that behaviors that would frequently be tolerated at home are attracting more notice in preschool or kindergarten classes. Moreover, when medication is available to treat a condition, such as ADHD, then that condition tends to be diagnosed more often; this may itself be associated with academic pressures on children to succeed and the desire to obtain ADHD treatment for students if it will better enable that success.\nStudies suggest that childhood ADHD is a risk factor for subsequent conduct and substance abuse problems, which can lead to significant morbidity and mortality and to involvement with the criminal justice system. The condition may also lead to academic and employment difficulties and to social problems that greatly impact normal development.[9] The course of ADHD varies among patients. Some persons outgrow their symptoms, while others have lifelong symptoms and require treatment into adulthood.[10] Still others are not diagnosed with and treated for ADHD until adulthood.\nIn diagnosing ADHD, differential diagnoses must be considered first to ensure that a treatable medical condition or another possible psychiatric diagnosis is not missed. The first evaluation phase begins with a complete medical history, a physical examination, and appropriate laboratory tests. Many classic symptoms of ADHD can individually be attributed to numerous other causes (eg, inattention may occur in relation to a learning disability, boredom, or a hearing difficulty). In addition, numerous psychiatric disorders can mimic many ADHD symptoms; these conditions include affective bipolar disorder, schizophrenia, posttraumatic stress disorder (PTSD), and Tourette syndrome. Key symptoms of ADHD may also arise from a learning disability.[9,11]\nIn this specific case, Michael did not appear to have any physical or medical conditions that could manifest as ADHD-like symptoms. He also failed to meet the criteria for any of the major psychoses associated with childhood or with a conduct disorder; also, no significant family or personal disruptions had occurred over the past year that would have caused a change in his behavior.\nMichael was reported by his teachers and parents to generally be a jovial and friendly child with many friends. He was reported to be helpful in school and to be the \"runner\" for teachers, getting them supplies and delivering messages to the main office regularly and with great reliability. Michael did not exhibit hypomania or any other symptoms associated with mood or anxiety disorders. He did not report racing thoughts, denied having problems sleeping, and also denied being particularly afraid of anything. In addition, his emotional range was congruent with circumstances and situations. Hence, Michael’s problem was less likely to have a psychodynamic etiology. Testing, moreover, suggested that he did not have a learning disorder.\n\n ## Figures\n\n \n*Page 4 of 7*",
"pagination": {
"current_page": 4,
"total_pages": 7
},
"questionnaire": [],
"title": "A 12-Year-Old Boy With Falling Grades and Forgetful Behavior"
},
{
"authors": "Stephen Soreff, MD; Foad Afshar, PsyD, EdM",
"content": [
"The criteria set forth in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5),[12] for the diagnosis of ADHD include those for inattention and for hyperactivity and impulsivity. With regard to inattention, children as old as 16 years must show 6 or more symptoms, whereas patients aged 17 years or older must show 5 or more symptoms. In addition, the symptoms must have been present for at least 6 months and be inappropriate for the patient’s developmental level. The symptoms of inattention are as follows:[12]",
"Often fails to give close attention to details or makes careless mistakes in schoolwork, at work, or in other activities",
"Often has trouble holding attention on tasks or play activities",
"Often does not seem to listen when spoken to directly",
"Often does not follow through on instructions and fails to finish schoolwork, chores, or duties in the workplace (eg, loses focus, sidetracked)",
"Often has trouble organizing tasks and activities",
"Often avoids, dislikes, or is reluctant to do tasks that require mental effort over a long period of time (such as schoolwork or homework)",
"Often loses things necessary for tasks and activities (eg, school materials, pencils, books, tools, wallets, keys, paperwork, eyeglasses, mobile telephones)",
"Is often easily distracted",
"Is often forgetful in daily activities",
"As with the above criteria for inattention, the DSM-5’s criteria for hyperactivity-impulsivity require that children as old as 16 years show 6 or more symptoms and that patients aged 17 years or older show at least 5 symptoms. In addition, the symptoms must have been displayed for at least 6 months and be pronounced enough to be disruptive and inappropriate for the patient’s developmental level. They are as follows:[12]",
"Often fidgets with or taps hands or feet or squirms in seat",
"Often leaves seat in situations in which remaining seated is expected",
"Often runs about or climbs in situations where it is not appropriate (adolescents or adults may be limited to feeling restless)",
"Often unable to play or take part in leisure activities quietly",
"Is often \"on the go,\" acting as if \"driven by a motor\"",
"Often talks excessively",
"Often blurts out an answer before a question has been completed",
"Often has trouble waiting his/her turn",
"Often interrupts or intrudes on others (eg, butts into conversations or games)",
"The following criteria must also be met for a diagnosis of ADHD:[12]",
"Several symptoms of inattention or hyperactivity-impulsivity have been present since before age 12 years",
"Several symptoms are present in two or more settings (eg, at home, school, or work; with friends or relatives; in other activities)",
"Clear evidence must suggest that the symptoms interfere with, or reduce the quality of, social, school, or work functioning",
"The symptoms do not happen only during the course of schizophrenia or another psychotic disorder; the symptoms are not better explained by another mental disorder (eg, mood disorder, anxiety disorders, dissociative disorder, or personality disorder)",
"Depending on the symptoms exhibited, the presentation of ADHD can be classified as follows (although note that because the symptoms can change over time, the presentation may change as well):[8,12]",
"Combined presentation: If enough symptoms to satisfy the criteria for both inattention and hyperactivity-impulsivity have existed for at least 6 months",
"Predominantly inattentive presentation: If enough symptoms to satisfy the criteria for inattention, but not for hyperactivity-impulsivity, have existed for at least 6 months",
"Predominantly hyperactive-impulsive presentation: If enough symptoms to satisfy the criteria for hyperactivity-impulsivity, but not for inattention, have existed for at least 6 months",
"In diagnosing ADHD, the patient must be observed in two or more locations. Generally, the parents do so at home, while a teacher observes the patient in the classroom. The following are examples of observation charting and recording tools:",
"Barkley Adult ADHD Rating Scale ̶ IV (BAARS-IV)[13]",
"Conners’ Rating Scales[14]",
"Vanderbilt ADHD Diagnostic Teacher Rating Scale[15]",
"In this case, indications for ADHD included the fact that Michael is male, had 7 of the possible symptoms for inattention and 6 for hyperactivity-impulsivity (combined presentation), demonstrated the symptoms for more than 6 months, and displayed them in two locations (home and school)."
],
"date": "June 25, 2019",
"figures": [],
"markdown": "# A 12-Year-Old Boy With Falling Grades and Forgetful Behavior\n\n **Authors:** Stephen Soreff, MD; Foad Afshar, PsyD, EdM \n **Date:** June 25, 2019\n\n ## Content\n\n The criteria set forth in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5),[12] for the diagnosis of ADHD include those for inattention and for hyperactivity and impulsivity. With regard to inattention, children as old as 16 years must show 6 or more symptoms, whereas patients aged 17 years or older must show 5 or more symptoms. In addition, the symptoms must have been present for at least 6 months and be inappropriate for the patient’s developmental level. The symptoms of inattention are as follows:[12]\nOften fails to give close attention to details or makes careless mistakes in schoolwork, at work, or in other activities\nOften has trouble holding attention on tasks or play activities\nOften does not seem to listen when spoken to directly\nOften does not follow through on instructions and fails to finish schoolwork, chores, or duties in the workplace (eg, loses focus, sidetracked)\nOften has trouble organizing tasks and activities\nOften avoids, dislikes, or is reluctant to do tasks that require mental effort over a long period of time (such as schoolwork or homework)\nOften loses things necessary for tasks and activities (eg, school materials, pencils, books, tools, wallets, keys, paperwork, eyeglasses, mobile telephones)\nIs often easily distracted\nIs often forgetful in daily activities\nAs with the above criteria for inattention, the DSM-5’s criteria for hyperactivity-impulsivity require that children as old as 16 years show 6 or more symptoms and that patients aged 17 years or older show at least 5 symptoms. In addition, the symptoms must have been displayed for at least 6 months and be pronounced enough to be disruptive and inappropriate for the patient’s developmental level. They are as follows:[12]\nOften fidgets with or taps hands or feet or squirms in seat\nOften leaves seat in situations in which remaining seated is expected\nOften runs about or climbs in situations where it is not appropriate (adolescents or adults may be limited to feeling restless)\nOften unable to play or take part in leisure activities quietly\nIs often \"on the go,\" acting as if \"driven by a motor\"\nOften talks excessively\nOften blurts out an answer before a question has been completed\nOften has trouble waiting his/her turn\nOften interrupts or intrudes on others (eg, butts into conversations or games)\nThe following criteria must also be met for a diagnosis of ADHD:[12]\nSeveral symptoms of inattention or hyperactivity-impulsivity have been present since before age 12 years\nSeveral symptoms are present in two or more settings (eg, at home, school, or work; with friends or relatives; in other activities)\nClear evidence must suggest that the symptoms interfere with, or reduce the quality of, social, school, or work functioning\nThe symptoms do not happen only during the course of schizophrenia or another psychotic disorder; the symptoms are not better explained by another mental disorder (eg, mood disorder, anxiety disorders, dissociative disorder, or personality disorder)\nDepending on the symptoms exhibited, the presentation of ADHD can be classified as follows (although note that because the symptoms can change over time, the presentation may change as well):[8,12]\nCombined presentation: If enough symptoms to satisfy the criteria for both inattention and hyperactivity-impulsivity have existed for at least 6 months\nPredominantly inattentive presentation: If enough symptoms to satisfy the criteria for inattention, but not for hyperactivity-impulsivity, have existed for at least 6 months\nPredominantly hyperactive-impulsive presentation: If enough symptoms to satisfy the criteria for hyperactivity-impulsivity, but not for inattention, have existed for at least 6 months\nIn diagnosing ADHD, the patient must be observed in two or more locations. Generally, the parents do so at home, while a teacher observes the patient in the classroom. The following are examples of observation charting and recording tools:\nBarkley Adult ADHD Rating Scale ̶ IV (BAARS-IV)[13]\nConners’ Rating Scales[14]\nVanderbilt ADHD Diagnostic Teacher Rating Scale[15]\nIn this case, indications for ADHD included the fact that Michael is male, had 7 of the possible symptoms for inattention and 6 for hyperactivity-impulsivity (combined presentation), demonstrated the symptoms for more than 6 months, and displayed them in two locations (home and school).\n\n ## Figures\n\n \n*Page 5 of 7*",
"pagination": {
"current_page": 5,
"total_pages": 7
},
"questionnaire": [],
"title": "A 12-Year-Old Boy With Falling Grades and Forgetful Behavior"
},
{
"authors": "Stephen Soreff, MD; Foad Afshar, PsyD, EdM",
"content": [
"Any treatment program for a patient with ADHD rests on 4 pillars. The first is individual psychotherapeutic work with the patient; this can be behavioral, dynamic, educational, or cognitive.[16] Additionally in this realm, numerous alternative approaches and diets are available, although these need more evaluation.[17]",
"Second, treatment that involves the family includes education, guidelines, instructions, and listening. Third, an individualized education plan in the school should be developed with the patient’s teachers, guidance counselor, and family. The fourth pillar is medication, if the symptoms of ADHD are debilitating and severe and cause impairment in school, at home, or at work. Stimulants (methylphenidate, dextroamphetamine) are first-line therapy, as they are generally the most effective drugs for treating ADHD.[9] More specific, age-related pharmacologic interventions are outlined in guidelines from the US Centers for Disease Control and Prevention (CDC).[8,11] What remains key is that treatment requires all four pillars. Too often, people see medication alone as the basic approach. ADHD can be viewed a dynamic issue within the context of the school and community."
],
"date": "June 25, 2019",
"figures": [],
"markdown": "# A 12-Year-Old Boy With Falling Grades and Forgetful Behavior\n\n **Authors:** Stephen Soreff, MD; Foad Afshar, PsyD, EdM \n **Date:** June 25, 2019\n\n ## Content\n\n Any treatment program for a patient with ADHD rests on 4 pillars. The first is individual psychotherapeutic work with the patient; this can be behavioral, dynamic, educational, or cognitive.[16] Additionally in this realm, numerous alternative approaches and diets are available, although these need more evaluation.[17]\nSecond, treatment that involves the family includes education, guidelines, instructions, and listening. Third, an individualized education plan in the school should be developed with the patient’s teachers, guidance counselor, and family. The fourth pillar is medication, if the symptoms of ADHD are debilitating and severe and cause impairment in school, at home, or at work. Stimulants (methylphenidate, dextroamphetamine) are first-line therapy, as they are generally the most effective drugs for treating ADHD.[9] More specific, age-related pharmacologic interventions are outlined in guidelines from the US Centers for Disease Control and Prevention (CDC).[8,11] What remains key is that treatment requires all four pillars. Too often, people see medication alone as the basic approach. ADHD can be viewed a dynamic issue within the context of the school and community.\n\n ## Figures\n\n \n*Page 6 of 7*",
"pagination": {
"current_page": 6,
"total_pages": 7
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 755875,
"choiceText": "Often does not seem to listen when spoken to directly",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 755877,
"choiceText": "Often picks at one’s skin",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 755879,
"choiceText": "Often easily distracted",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 755881,
"choiceText": "Often avoids, dislikes, or is reluctant to do tasks that require mental effort over a long period of time",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 755883,
"choiceText": "Often forgetful in daily activities",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Criteria set forth in the DSM-5 for the diagnosis of ADHD include those for inattention and for hyperactivity and impulsivity. With regard to inattention, children up to age 16 years must show 6 or more symptoms, whereas patients aged 17 years or older must show at least 5 symptoms. In addition, the symptoms must have been present for at least 6 months and be inappropriate for the patient's developmental level. None of the symptoms involve picking at the skin. Skin picking itself may suggest an allergy, an infection, a response to a substance abuse (eg, cocaine), or a hallucination of bugs under the skin.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 236515,
"questionText": "Which of the following is <i>not</i> typically considered a symptom of inattention?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 755885,
"choiceText": "Norepinephrine",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 755887,
"choiceText": "Dopamine",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 755889,
"choiceText": "Norepinephrine and dopamine",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 755891,
"choiceText": "None of the above",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Research has associated deficiencies of norepinephrine and dopamine with ADHD. Drug therapies to relieve these deficiencies are used in its treatment.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 236517,
"questionText": "ADHD has been associated with which of the following?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 12-Year-Old Boy With Falling Grades and Forgetful Behavior"
},
{
"authors": "Stephen Soreff, MD; Foad Afshar, PsyD, EdM",
"content": [],
"date": "June 25, 2019",
"figures": [],
"markdown": "# A 12-Year-Old Boy With Falling Grades and Forgetful Behavior\n\n **Authors:** Stephen Soreff, MD; Foad Afshar, PsyD, EdM \n **Date:** June 25, 2019\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 7 of 7*",
"pagination": {
"current_page": 7,
"total_pages": 7
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 755875,
"choiceText": "Often does not seem to listen when spoken to directly",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 755877,
"choiceText": "Often picks at one’s skin",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 755879,
"choiceText": "Often easily distracted",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 755881,
"choiceText": "Often avoids, dislikes, or is reluctant to do tasks that require mental effort over a long period of time",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 755883,
"choiceText": "Often forgetful in daily activities",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Criteria set forth in the DSM-5 for the diagnosis of ADHD include those for inattention and for hyperactivity and impulsivity. With regard to inattention, children up to age 16 years must show 6 or more symptoms, whereas patients aged 17 years or older must show at least 5 symptoms. In addition, the symptoms must have been present for at least 6 months and be inappropriate for the patient's developmental level. None of the symptoms involve picking at the skin. Skin picking itself may suggest an allergy, an infection, a response to a substance abuse (eg, cocaine), or a hallucination of bugs under the skin.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 236515,
"questionText": "Which of the following is <i>not</i> typically considered a symptom of inattention?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 755885,
"choiceText": "Norepinephrine",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 755887,
"choiceText": "Dopamine",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 755889,
"choiceText": "Norepinephrine and dopamine",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 755891,
"choiceText": "None of the above",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Research has associated deficiencies of norepinephrine and dopamine with ADHD. Drug therapies to relieve these deficiencies are used in its treatment.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 236517,
"questionText": "ADHD has been associated with which of the following?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 12-Year-Old Boy With Falling Grades and Forgetful Behavior"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 755865,
"choiceText": "Conduct disorder",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 755867,
"choiceText": "Posttraumatic stress disorder",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 755869,
"choiceText": "Learning disability",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 755871,
"choiceText": "Attention deficit hyperactivity disorder",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 755873,
"choiceText": "Bipolar disorder",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 236513,
"questionText": "Based on the history and evaluation, which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 755875,
"choiceText": "Often does not seem to listen when spoken to directly",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 755877,
"choiceText": "Often picks at one’s skin",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 755879,
"choiceText": "Often easily distracted",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 755881,
"choiceText": "Often avoids, dislikes, or is reluctant to do tasks that require mental effort over a long period of time",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 755883,
"choiceText": "Often forgetful in daily activities",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Criteria set forth in the DSM-5 for the diagnosis of ADHD include those for inattention and for hyperactivity and impulsivity. With regard to inattention, children up to age 16 years must show 6 or more symptoms, whereas patients aged 17 years or older must show at least 5 symptoms. In addition, the symptoms must have been present for at least 6 months and be inappropriate for the patient's developmental level. None of the symptoms involve picking at the skin. Skin picking itself may suggest an allergy, an infection, a response to a substance abuse (eg, cocaine), or a hallucination of bugs under the skin.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 236515,
"questionText": "Which of the following is <i>not</i> typically considered a symptom of inattention?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 755885,
"choiceText": "Norepinephrine",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 755887,
"choiceText": "Dopamine",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 755889,
"choiceText": "Norepinephrine and dopamine",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 755891,
"choiceText": "None of the above",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Research has associated deficiencies of norepinephrine and dopamine with ADHD. Drug therapies to relieve these deficiencies are used in its treatment.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 236517,
"questionText": "ADHD has been associated with which of the following?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
914730 | /viewarticle/914730 | [
{
"authors": "Jeffrey S. Forrest, MD; Alexander B. Shortridge",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"A 45-year-old woman presents to her primary care physician stating that she feels \"like a zombie.\" The patient tearfully reports that she has been unable to work or even drive because her mind has not been functioning like it had before a recent illness.",
"The patient reported that her symptoms began 3 weeks earlier, when she was diagnosed with influenza A. She reported several urgent care visits, in which she had been instructed to take a promethazine/codeine cough syrup, albuterol inhaler, oseltamivir for influenza attenuation, doxycycline for antibiotic coverage, prednisone for respiratory symptom control, and guaifenesin/dextromethorphan for cough symptom control. In the first 2 weeks of her presentation, her coughing was constant and productive, keeping her awake at night. The patient states that she believes her thoughts began slowing a few days into her recent upper respiratory illness; however, she was unsure because of how acutely ill she had been feeling. She has also had increased nightmares about war zones and death.",
"The patient reports that she has continued to experience confusion and sedation for weeks, even after her upper respiratory symptoms abated. The patient states that she had not felt this sedated since she had taken trazodone (50 mg) for insomnia a few years before. The patient stated she had been knocked out for most of the day after she took that medication.",
"The patient also reports numbness on the left side of her face and an unusual sensation in her left eyeball. She is worried that she has had a stroke. The patient also reports continuing waves of a \"fast heartbeat\" that she can feel. She further attests to having to change her sheets at night because of night sweats. These night sweats had slowly started to decrease in frequency before her presentation. The patient also stated that she has occasionally been experiencing hallucinations of \"men singing\" at night.",
"In addition to the medications for influenza listed here, the patient reports taking oral contraceptive pills, fluoxetine (60 mg daily) for major depression, topiramate XR (150 mg) daily for migraine prophylaxis, and erenumab injection monthly for migraine prophylaxis.",
"Her past medical history includes major depression that was diagnosed 20 years prior; it has been stable on fluoxetine. She also reports scoliosis (four childhood surgeries), posttraumatic stress disorder, appendectomy, and a cesarean delivery 15 years prior."
],
"date": "June 24, 2019",
"figures": [],
"markdown": "# A 45-Year-Old Woman Who Feels 'Like a Zombie'\n\n **Authors:** Jeffrey S. Forrest, MD; Alexander B. Shortridge \n **Date:** June 24, 2019\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nA 45-year-old woman presents to her primary care physician stating that she feels \"like a zombie.\" The patient tearfully reports that she has been unable to work or even drive because her mind has not been functioning like it had before a recent illness.\nThe patient reported that her symptoms began 3 weeks earlier, when she was diagnosed with influenza A. She reported several urgent care visits, in which she had been instructed to take a promethazine/codeine cough syrup, albuterol inhaler, oseltamivir for influenza attenuation, doxycycline for antibiotic coverage, prednisone for respiratory symptom control, and guaifenesin/dextromethorphan for cough symptom control. In the first 2 weeks of her presentation, her coughing was constant and productive, keeping her awake at night. The patient states that she believes her thoughts began slowing a few days into her recent upper respiratory illness; however, she was unsure because of how acutely ill she had been feeling. She has also had increased nightmares about war zones and death.\nThe patient reports that she has continued to experience confusion and sedation for weeks, even after her upper respiratory symptoms abated. The patient states that she had not felt this sedated since she had taken trazodone (50 mg) for insomnia a few years before. The patient stated she had been knocked out for most of the day after she took that medication.\nThe patient also reports numbness on the left side of her face and an unusual sensation in her left eyeball. She is worried that she has had a stroke. The patient also reports continuing waves of a \"fast heartbeat\" that she can feel. She further attests to having to change her sheets at night because of night sweats. These night sweats had slowly started to decrease in frequency before her presentation. The patient also stated that she has occasionally been experiencing hallucinations of \"men singing\" at night.\nIn addition to the medications for influenza listed here, the patient reports taking oral contraceptive pills, fluoxetine (60 mg daily) for major depression, topiramate XR (150 mg) daily for migraine prophylaxis, and erenumab injection monthly for migraine prophylaxis.\nHer past medical history includes major depression that was diagnosed 20 years prior; it has been stable on fluoxetine. She also reports scoliosis (four childhood surgeries), posttraumatic stress disorder, appendectomy, and a cesarean delivery 15 years prior.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 45-Year-Old Woman Who Feels 'Like a Zombie'"
},
{
"authors": "Jeffrey S. Forrest, MD; Alexander B. Shortridge",
"content": [
"On physical examination, the patient is tearful, anxious, and in mild distress. Her height is 5'0\" (152.4 cm) and her weight is 115 lb (52.2 kg). Her temperature is 98.2°F (36.8°C), her pulse is 108 beats/min, her oxygen level is 95%, and her blood pressure is 128/84 mm Hg.",
"She has no apparent trauma and is normocephalic. Her extraocular movements are intact. A slight saccades motion impairment is noted in her left eye. Her ear canals and tympanic membranes appear normal. Mild injection is noted in her pharynx, likely because of her respiratory illness.",
"No murmurs, rubs, or gallops are heard. Her pulse has a regular rhythm. Mild residual crackles are observed in her lower lung fields bilaterally; these are reduced from her previous examination. Her abdomen is nontender and tympanitic to percussion, with normal bowel sounds. Modest gait impairment is noted. She has mild numbness to sharp touch in the left side of her face. No facial droop is present. Her cranial nerves are otherwise intact, and she has no other focal neurologic signs. She has 5/5 strength in all extremities.",
"On mental status examination, she is alert and oriented to name, location, date, and time. She appears appropriately groomed. She is attentive, with no excessive body movements and good eye contact. Her speech has normal rate, rhythm, volume, and prosody; she does occasionally have slow responses. Her thought process is logical, linear, and goal directed. She does not exhibit delusions or paranoia. She does have concern about her ability to think and concentrate. Her mood is described as poor, and her affect is congruent with her stated mood.",
"She has not had any visual hallucinations, derealization, or depersonalization. The patient struggles to name the months of the year backward, but can attend to the interview and answer questions. Her insight is generally good, and she is aware of possible illness processes. Her judgement appears good. She is aware of the danger associated with driving in her current condition, is aware of limitations resulting from her symptoms, and is appropriately seeking help.",
"Chest x-ray findings are normal. Her ECG findings are notable for tachycardia (108 beats/min) but are otherwise normal. A head CT scan without contrast is normal. She has no evidence of a cerebrovascular accident, mass lesions, or bleeding. Urine screening for illicit drugs is negative. Urinalysis findings are within normal limits. She has a white blood cell count of 15.4 × 109/L. The remainder of her complete blood cell count is within reference range values. Her blood urea nitrogen level is 40 mg/dL. The remainder of her complete metabolic profile is otherwise within reference range values. Her thyroid-stimulating hormone level is within the reference range."
],
"date": "June 24, 2019",
"figures": [],
"markdown": "# A 45-Year-Old Woman Who Feels 'Like a Zombie'\n\n **Authors:** Jeffrey S. Forrest, MD; Alexander B. Shortridge \n **Date:** June 24, 2019\n\n ## Content\n\n On physical examination, the patient is tearful, anxious, and in mild distress. Her height is 5'0\" (152.4 cm) and her weight is 115 lb (52.2 kg). Her temperature is 98.2°F (36.8°C), her pulse is 108 beats/min, her oxygen level is 95%, and her blood pressure is 128/84 mm Hg.\nShe has no apparent trauma and is normocephalic. Her extraocular movements are intact. A slight saccades motion impairment is noted in her left eye. Her ear canals and tympanic membranes appear normal. Mild injection is noted in her pharynx, likely because of her respiratory illness.\nNo murmurs, rubs, or gallops are heard. Her pulse has a regular rhythm. Mild residual crackles are observed in her lower lung fields bilaterally; these are reduced from her previous examination. Her abdomen is nontender and tympanitic to percussion, with normal bowel sounds. Modest gait impairment is noted. She has mild numbness to sharp touch in the left side of her face. No facial droop is present. Her cranial nerves are otherwise intact, and she has no other focal neurologic signs. She has 5/5 strength in all extremities.\nOn mental status examination, she is alert and oriented to name, location, date, and time. She appears appropriately groomed. She is attentive, with no excessive body movements and good eye contact. Her speech has normal rate, rhythm, volume, and prosody; she does occasionally have slow responses. Her thought process is logical, linear, and goal directed. She does not exhibit delusions or paranoia. She does have concern about her ability to think and concentrate. Her mood is described as poor, and her affect is congruent with her stated mood.\nShe has not had any visual hallucinations, derealization, or depersonalization. The patient struggles to name the months of the year backward, but can attend to the interview and answer questions. Her insight is generally good, and she is aware of possible illness processes. Her judgement appears good. She is aware of the danger associated with driving in her current condition, is aware of limitations resulting from her symptoms, and is appropriately seeking help.\nChest x-ray findings are normal. Her ECG findings are notable for tachycardia (108 beats/min) but are otherwise normal. A head CT scan without contrast is normal. She has no evidence of a cerebrovascular accident, mass lesions, or bleeding. Urine screening for illicit drugs is negative. Urinalysis findings are within normal limits. She has a white blood cell count of 15.4 × 109/L. The remainder of her complete blood cell count is within reference range values. Her blood urea nitrogen level is 40 mg/dL. The remainder of her complete metabolic profile is otherwise within reference range values. Her thyroid-stimulating hormone level is within the reference range.\n\n ## Figures\n\n \n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1374212,
"choiceText": "Bell palsy",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1374214,
"choiceText": "Stroke",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1374216,
"choiceText": "Medication toxicity",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1374218,
"choiceText": "Somatic symptom disorder",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1374220,
"choiceText": "Hypothyroidism",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 438735,
"questionText": "Which one of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 45-Year-Old Woman Who Feels 'Like a Zombie'"
},
{
"authors": "Jeffrey S. Forrest, MD; Alexander B. Shortridge",
"content": [
"This patient has most likely experienced adverse effects resulting from numerous drug-to-drug interactions. The most significant of these interactions likely involved topiramate, which is well known for causing cognitive slowing.[1] Topiramate's mechanism of action involves sodium channel blockade. It also has gamma-aminobutyric acid receptor activity and antiglutaminergic receptor activity and is an inhibitor of carbonic anhydrase. Topiramate has been used as an anticonvulsant. More recently, it has been used for migraine prophylaxis (as in this patient). The extended-release formulation of topiramate has a half-life that is estimated to be 57 hours in a typical individual compared with 25 hours for the immediate-release formulation.[2] The patient's acute illness status may have also impaired her clearance of topiramate.",
"During her workup, the patient reported a history of oversedation by trazodone. Trazodone is primarily metabolized by the liver enzyme system cytochrome P450 3A4 (CYP3A4).[3] Of note, topiramate is predominantly metabolized by this same enzyme system. This may suggest that the patient's baseline metabolism may be slower than is typically expected.",
"What leads to poor CYP3A4 metabolism is the subject of debate. CYP3A4 expression may differ among individuals because of factors such as disease states that alter homeostasis (eg, the patient's acute illness in this case), upregulation or downregulation by environmental stimuli (eg, smoking, drug intake, diet), and genetic mutation.[4] In general, five full half-lives must pass before a medication is cleared from the body. On the basis of its 57-hour half-life, standard clearance of topiramate XR could take as long as 12 days. This could be even longer with a slower metabolism. Also, polymorphisms in the CYP3A4 gene could account for differences in drug efficacy and toxicity among different individuals.[5] This may be one contributing factor in the patient's continued cognitive slowing weeks after her illness.",
"The patient's metabolism of topiramate may have been further impaired by her long-standing fluoxetine therapy. Fluoxetine, although predominantly known as a cytochrome P450 2D6 (CYP2D6) system inhibitor, is also known to be a modest inhibitor of CYP3A4.[6] This effect may have also resulted in decreased topiramate clearance, increased topiramate levels, and subsequent significant and protracted cognitive sedation."
],
"date": "June 24, 2019",
"figures": [],
"markdown": "# A 45-Year-Old Woman Who Feels 'Like a Zombie'\n\n **Authors:** Jeffrey S. Forrest, MD; Alexander B. Shortridge \n **Date:** June 24, 2019\n\n ## Content\n\n This patient has most likely experienced adverse effects resulting from numerous drug-to-drug interactions. The most significant of these interactions likely involved topiramate, which is well known for causing cognitive slowing.[1] Topiramate's mechanism of action involves sodium channel blockade. It also has gamma-aminobutyric acid receptor activity and antiglutaminergic receptor activity and is an inhibitor of carbonic anhydrase. Topiramate has been used as an anticonvulsant. More recently, it has been used for migraine prophylaxis (as in this patient). The extended-release formulation of topiramate has a half-life that is estimated to be 57 hours in a typical individual compared with 25 hours for the immediate-release formulation.[2] The patient's acute illness status may have also impaired her clearance of topiramate.\nDuring her workup, the patient reported a history of oversedation by trazodone. Trazodone is primarily metabolized by the liver enzyme system cytochrome P450 3A4 (CYP3A4).[3] Of note, topiramate is predominantly metabolized by this same enzyme system. This may suggest that the patient's baseline metabolism may be slower than is typically expected.\nWhat leads to poor CYP3A4 metabolism is the subject of debate. CYP3A4 expression may differ among individuals because of factors such as disease states that alter homeostasis (eg, the patient's acute illness in this case), upregulation or downregulation by environmental stimuli (eg, smoking, drug intake, diet), and genetic mutation.[4] In general, five full half-lives must pass before a medication is cleared from the body. On the basis of its 57-hour half-life, standard clearance of topiramate XR could take as long as 12 days. This could be even longer with a slower metabolism. Also, polymorphisms in the CYP3A4 gene could account for differences in drug efficacy and toxicity among different individuals.[5] This may be one contributing factor in the patient's continued cognitive slowing weeks after her illness.\nThe patient's metabolism of topiramate may have been further impaired by her long-standing fluoxetine therapy. Fluoxetine, although predominantly known as a cytochrome P450 2D6 (CYP2D6) system inhibitor, is also known to be a modest inhibitor of CYP3A4.[6] This effect may have also resulted in decreased topiramate clearance, increased topiramate levels, and subsequent significant and protracted cognitive sedation.\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1374212,
"choiceText": "Bell palsy",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1374214,
"choiceText": "Stroke",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1374216,
"choiceText": "Medication toxicity",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1374218,
"choiceText": "Somatic symptom disorder",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1374220,
"choiceText": "Hypothyroidism",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 438735,
"questionText": "Which one of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 45-Year-Old Woman Who Feels 'Like a Zombie'"
},
{
"authors": "Jeffrey S. Forrest, MD; Alexander B. Shortridge",
"content": [
"Another important potential contributor to this patient's symptoms may have been substantially increased levels of dextromethorphan. Dextromethorphan is predominantly metabolized by the CYP2D6 system. As mentioned previously, this system is substantively inhibited by numerous antidepressants, including fluoxetine.[6] Supratherapeutic levels of dextromethorphan may result in neurological symptoms such as dystonia, fatigue, drowsiness, and dizziness. Nystagmus, slurred speech, and lightheadedness have also been observed in cases of dextromethorphan toxicity.[7] Auditory hallucinations, such as those described by the patient in this case, have also been reported.[8] An unintentional drug-to-drug interaction may have occurred between the fluoxetine and dextromethorphan in this patient.",
"In addition to possible adverse effects from topiramate and dextromethorphan, sedation from promethazine therapy should also be considered in this patient. As with many antihistamines, promethazine has been demonstrated to have significant anticholinergic activity.[9] Acetylcholine plays a pivotal role in attention and memory.[1] As a result, anticholinergic agents are frequently sedating to a greater or lesser extent.[9] Promethazine is also predominantly metabolized by CYP2D6 in the liver, which may have been inhibited by the patient's long-established fluoxetine treatment.[10] Therefore, along with dextromethorphan, increased promethazine levels may have contributed to the patient's cognitive impairment.",
"Yet another potential contributor to the sedation experienced by this patient may be from increased levels of codeine, which is also metabolized by the CYP2D6 system.[11] Codeine is also known to cause drowsiness. The patient in this case did not report constipation, and was not observed to have respiratory depression; these symptoms may occur with significant codeine toxicity. Their absence suggests possible greater contributions from other medications to the clinical scenario described. Notwithstanding, careful consideration must be exercised in administering codeine-based medications to patients receiving CYP2D6 inhibitors to avoid this possible interaction. Oseltamivir is not a substrate of CYP450 and is not thought to influence metabolism of other drugs by this system.[12] It was likely not a contributor in this patient.",
"Considering other factors that may have complicated this patient's clinical course is also important. For example, patients who are experiencing cognitive sedation may inadvertently take more or less medication than intended, as a result of their symptoms. Unintentional overdosing of any medication may have easily exacerbated this patient's sedation, especially with the drug-to-drug interactions described here."
],
"date": "June 24, 2019",
"figures": [],
"markdown": "# A 45-Year-Old Woman Who Feels 'Like a Zombie'\n\n **Authors:** Jeffrey S. Forrest, MD; Alexander B. Shortridge \n **Date:** June 24, 2019\n\n ## Content\n\n Another important potential contributor to this patient's symptoms may have been substantially increased levels of dextromethorphan. Dextromethorphan is predominantly metabolized by the CYP2D6 system. As mentioned previously, this system is substantively inhibited by numerous antidepressants, including fluoxetine.[6] Supratherapeutic levels of dextromethorphan may result in neurological symptoms such as dystonia, fatigue, drowsiness, and dizziness. Nystagmus, slurred speech, and lightheadedness have also been observed in cases of dextromethorphan toxicity.[7] Auditory hallucinations, such as those described by the patient in this case, have also been reported.[8] An unintentional drug-to-drug interaction may have occurred between the fluoxetine and dextromethorphan in this patient.\nIn addition to possible adverse effects from topiramate and dextromethorphan, sedation from promethazine therapy should also be considered in this patient. As with many antihistamines, promethazine has been demonstrated to have significant anticholinergic activity.[9] Acetylcholine plays a pivotal role in attention and memory.[1] As a result, anticholinergic agents are frequently sedating to a greater or lesser extent.[9] Promethazine is also predominantly metabolized by CYP2D6 in the liver, which may have been inhibited by the patient's long-established fluoxetine treatment.[10] Therefore, along with dextromethorphan, increased promethazine levels may have contributed to the patient's cognitive impairment.\nYet another potential contributor to the sedation experienced by this patient may be from increased levels of codeine, which is also metabolized by the CYP2D6 system.[11] Codeine is also known to cause drowsiness. The patient in this case did not report constipation, and was not observed to have respiratory depression; these symptoms may occur with significant codeine toxicity. Their absence suggests possible greater contributions from other medications to the clinical scenario described. Notwithstanding, careful consideration must be exercised in administering codeine-based medications to patients receiving CYP2D6 inhibitors to avoid this possible interaction. Oseltamivir is not a substrate of CYP450 and is not thought to influence metabolism of other drugs by this system.[12] It was likely not a contributor in this patient.\nConsidering other factors that may have complicated this patient's clinical course is also important. For example, patients who are experiencing cognitive sedation may inadvertently take more or less medication than intended, as a result of their symptoms. Unintentional overdosing of any medication may have easily exacerbated this patient's sedation, especially with the drug-to-drug interactions described here.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 45-Year-Old Woman Who Feels 'Like a Zombie'"
},
{
"authors": "Jeffrey S. Forrest, MD; Alexander B. Shortridge",
"content": [
"This case demonstrates the importance of taking a thorough medication history during an evaluation, even if an initial presentation (such as that for influenza) seems classic. Such factors can be overlooked in busy urgent care or emergency department settings but may result in significant preventable patient morbidity.",
"After the possible medication interactions listed here were discovered, the patient stopped taking topiramate XR, guaifenesin/dextromethorphan, and promethazine/codeine and temporarily reduced her fluoxetine dose to 30 mg daily. At a 1-week follow-up with her neurologist, the patient was found to still have significant topiramate levels, suggesting a slower-than-typical metabolism. The \"unusual sensation\" the patient felt in her eyeball and facial numbness was thought to be either from viral irritation of the nerve by the aggravated upper respiratory virus (as can occur in Bell palsy or similar syndromes) or from topiramate toxicity, which has been demonstrated to result in myopia in some cases.[13] Her bouts of palpitations and night sweats were also found to be the result of medication toxicity.",
"This case scenario further demonstrates the potential dangers of polypharmacy, which may be defined as multiple medications taken concomitantly by a single individual.[14] Risk factors for polypharmacy include age and chronic medical conditions, especially in the context of the onset of a new acute illness (eg, influenza in this patient). Impaired patients may not report, or be able to report, all of the medications that they have been taking. This makes scrutiny for this concern in elderly, intellectually disabled, or otherwise cognitively impaired patients essential.[15,16]",
"The best intervention for medication-related problems is to prevent them from happening in the first place. Careful history taking is essential, as is limiting medication treatment to optimized regimens that have sufficient therapeutic effect while also having minimal redundancy. Systematic medication reviews by multidisciplinary teams may assist in the identification and prevention of potential medication-induced complications before they occur.[15] Should suspicion arise for a medication-induced complication, cessation of the aggravating agents when possible is essential. Toxicology and/or poison control center referral may also be indicated to ensure medication-related events are safely and appropriately managed.",
"The patient in this case was closely monitored with weekly follow-up visits. She achieved complete remission of her symptoms approximately 1 month after cessation of the medications thought to be responsible."
],
"date": "June 24, 2019",
"figures": [],
"markdown": "# A 45-Year-Old Woman Who Feels 'Like a Zombie'\n\n **Authors:** Jeffrey S. Forrest, MD; Alexander B. Shortridge \n **Date:** June 24, 2019\n\n ## Content\n\n This case demonstrates the importance of taking a thorough medication history during an evaluation, even if an initial presentation (such as that for influenza) seems classic. Such factors can be overlooked in busy urgent care or emergency department settings but may result in significant preventable patient morbidity.\nAfter the possible medication interactions listed here were discovered, the patient stopped taking topiramate XR, guaifenesin/dextromethorphan, and promethazine/codeine and temporarily reduced her fluoxetine dose to 30 mg daily. At a 1-week follow-up with her neurologist, the patient was found to still have significant topiramate levels, suggesting a slower-than-typical metabolism. The \"unusual sensation\" the patient felt in her eyeball and facial numbness was thought to be either from viral irritation of the nerve by the aggravated upper respiratory virus (as can occur in Bell palsy or similar syndromes) or from topiramate toxicity, which has been demonstrated to result in myopia in some cases.[13] Her bouts of palpitations and night sweats were also found to be the result of medication toxicity.\nThis case scenario further demonstrates the potential dangers of polypharmacy, which may be defined as multiple medications taken concomitantly by a single individual.[14] Risk factors for polypharmacy include age and chronic medical conditions, especially in the context of the onset of a new acute illness (eg, influenza in this patient). Impaired patients may not report, or be able to report, all of the medications that they have been taking. This makes scrutiny for this concern in elderly, intellectually disabled, or otherwise cognitively impaired patients essential.[15,16]\nThe best intervention for medication-related problems is to prevent them from happening in the first place. Careful history taking is essential, as is limiting medication treatment to optimized regimens that have sufficient therapeutic effect while also having minimal redundancy. Systematic medication reviews by multidisciplinary teams may assist in the identification and prevention of potential medication-induced complications before they occur.[15] Should suspicion arise for a medication-induced complication, cessation of the aggravating agents when possible is essential. Toxicology and/or poison control center referral may also be indicated to ensure medication-related events are safely and appropriately managed.\nThe patient in this case was closely monitored with weekly follow-up visits. She achieved complete remission of her symptoms approximately 1 month after cessation of the medications thought to be responsible.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1374222,
"choiceText": "CYP2D6",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1374224,
"choiceText": "CYP1A2",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1374226,
"choiceText": "CYP2C19",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1374228,
"choiceText": "CYP3A4",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1374230,
"choiceText": "CYP1A4",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Topiramate is predominantly metabolized by the CYP3A4 enzyme system. The immediate-release formulation has a half-life of about 21-25 hours, whereas the XR formulation has a half-life of about 57 hours. Medications that inhibit or partially inhibit the CYP3A4 system (eg, fluoxetine) may result in decreased clearance of this medication, resulting in sedation.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 438737,
"questionText": "Which one of the following enzyme systems predominantly metabolizes topiramate?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1374232,
"choiceText": "Dextromethorphan",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1374234,
"choiceText": "Oseltamivir",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1374236,
"choiceText": "Promethazine",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1374238,
"choiceText": "Codeine",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1374240,
"choiceText": "Topiramate",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Dextromethorphan, codeine, and promethazine are all heavily metabolized by the CYP2D6 system; topiramate is predominantly metabolized by the CYP3A4 system. Both of these systems are inhibited by fluoxetine, although the CYP2D6 system is more significantly impacted. Oseltamivir (Tamiflu) is not thought to be a substrate or modulator of the CYP450 system.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 438739,
"questionText": "Which one of the following drugs is least likely to have its levels increased by fluoxetine treatment?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 45-Year-Old Woman Who Feels 'Like a Zombie'"
},
{
"authors": "Jeffrey S. Forrest, MD; Alexander B. Shortridge",
"content": [],
"date": "June 24, 2019",
"figures": [],
"markdown": "# A 45-Year-Old Woman Who Feels 'Like a Zombie'\n\n **Authors:** Jeffrey S. Forrest, MD; Alexander B. Shortridge \n **Date:** June 24, 2019\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1374222,
"choiceText": "CYP2D6",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1374224,
"choiceText": "CYP1A2",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1374226,
"choiceText": "CYP2C19",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1374228,
"choiceText": "CYP3A4",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1374230,
"choiceText": "CYP1A4",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Topiramate is predominantly metabolized by the CYP3A4 enzyme system. The immediate-release formulation has a half-life of about 21-25 hours, whereas the XR formulation has a half-life of about 57 hours. Medications that inhibit or partially inhibit the CYP3A4 system (eg, fluoxetine) may result in decreased clearance of this medication, resulting in sedation.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 438737,
"questionText": "Which one of the following enzyme systems predominantly metabolizes topiramate?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1374232,
"choiceText": "Dextromethorphan",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1374234,
"choiceText": "Oseltamivir",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1374236,
"choiceText": "Promethazine",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1374238,
"choiceText": "Codeine",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1374240,
"choiceText": "Topiramate",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Dextromethorphan, codeine, and promethazine are all heavily metabolized by the CYP2D6 system; topiramate is predominantly metabolized by the CYP3A4 system. Both of these systems are inhibited by fluoxetine, although the CYP2D6 system is more significantly impacted. Oseltamivir (Tamiflu) is not thought to be a substrate or modulator of the CYP450 system.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 438739,
"questionText": "Which one of the following drugs is least likely to have its levels increased by fluoxetine treatment?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 45-Year-Old Woman Who Feels 'Like a Zombie'"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1374212,
"choiceText": "Bell palsy",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1374214,
"choiceText": "Stroke",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1374216,
"choiceText": "Medication toxicity",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1374218,
"choiceText": "Somatic symptom disorder",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1374220,
"choiceText": "Hypothyroidism",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 438735,
"questionText": "Which one of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1374222,
"choiceText": "CYP2D6",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1374224,
"choiceText": "CYP1A2",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1374226,
"choiceText": "CYP2C19",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1374228,
"choiceText": "CYP3A4",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1374230,
"choiceText": "CYP1A4",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Topiramate is predominantly metabolized by the CYP3A4 enzyme system. The immediate-release formulation has a half-life of about 21-25 hours, whereas the XR formulation has a half-life of about 57 hours. Medications that inhibit or partially inhibit the CYP3A4 system (eg, fluoxetine) may result in decreased clearance of this medication, resulting in sedation.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 438737,
"questionText": "Which one of the following enzyme systems predominantly metabolizes topiramate?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1374232,
"choiceText": "Dextromethorphan",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1374234,
"choiceText": "Oseltamivir",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1374236,
"choiceText": "Promethazine",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1374238,
"choiceText": "Codeine",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1374240,
"choiceText": "Topiramate",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Dextromethorphan, codeine, and promethazine are all heavily metabolized by the CYP2D6 system; topiramate is predominantly metabolized by the CYP3A4 system. Both of these systems are inhibited by fluoxetine, although the CYP2D6 system is more significantly impacted. Oseltamivir (Tamiflu) is not thought to be a substrate or modulator of the CYP450 system.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 438739,
"questionText": "Which one of the following drugs is least likely to have its levels increased by fluoxetine treatment?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
884505 | /viewarticle/884505 | [
{
"authors": "Shah Azmoon, MD; Matthew Budoff, MD; David Atkinson, MD",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"A 14-year-old Japanese male presents with a 2-week history of unrelenting fever and generalized fatigue. He has had been experiencing mild headaches and associated mild neck stiffness, with elevation of his temperature that resolves with antipyretics. He and his family have visited several community emergency departments, where he has had multiple negative lumbar punctures and was last given a diagnosis of aseptic meningitis.",
"He recently developed dry, fissured lips and some skin peeling. He is otherwise without any specific symptoms, such as cough, shortness of breath, abdominal pain, back pain, or urinary complaints. He has no significant medical or family history, and he is not taking any medications."
],
"date": "June 17, 2019",
"figures": [],
"markdown": "# A 14-Year-Old Boy With Unexplained, Recurring Fever\n\n **Authors:** Shah Azmoon, MD; Matthew Budoff, MD; David Atkinson, MD \n **Date:** June 17, 2019\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nA 14-year-old Japanese male presents with a 2-week history of unrelenting fever and generalized fatigue. He has had been experiencing mild headaches and associated mild neck stiffness, with elevation of his temperature that resolves with antipyretics. He and his family have visited several community emergency departments, where he has had multiple negative lumbar punctures and was last given a diagnosis of aseptic meningitis.\nHe recently developed dry, fissured lips and some skin peeling. He is otherwise without any specific symptoms, such as cough, shortness of breath, abdominal pain, back pain, or urinary complaints. He has no significant medical or family history, and he is not taking any medications.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 14-Year-Old Boy With Unexplained, Recurring Fever"
},
{
"authors": "Shah Azmoon, MD; Matthew Budoff, MD; David Atkinson, MD",
"content": [
"On physical examination, the patient is ill-appearing, fatigued, and febrile. He has a blood pressure of 125/66 mm Hg, a heart rate of 116 beats/min with a regular rhythm, a respiratory rate of 19 breaths/min, and an oxygen saturation of 96% while breathing room air. He has mild bilateral conjunctival injection with limbic sparing, enlarged right lymphadenopathy, and a mild desquamating rash between his fingers.",
"His lung sounds are clear bilaterally, and cardiac auscultation reveals a normal first heart sound and a normal physiologically split second heart sound. No murmurs, thrills, rubs, or gallops are appreciated. No significant conduction abnormalities are noted on ECG, but an initial transthoracic echocardiogram (TTE) in the emergency department, although a poorly delineated study, raises suspicion for cardiac motion abnormalities.",
"Laboratory tests are performed that reveal a markedly elevated white blood cell count of 21.6 × 103 cells/µL, with 15% bands; a slightly low hemoglobin level of 12.2 g/dL; and an elevated platelet count of 720 × 103 cells/µL. Blood cultures are obtained and sent to the lab. Intravenous immunoglobulin and antibiotics are empirically initiated for suspicion of Kawasaki disease (KD), scarlet fever, or sepsis, and the patient is admitted for cardiac monitoring.",
"The following day, the patient is referred for coronary CT angiography (CCTA) for better delineation of the cardiac and coronary anatomy. Images are obtained using low-dose protocols, with an estimated radiation dose of 5 mSv (Figure 1).",
"Figure 1."
],
"date": "June 17, 2019",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/884/505/884505-thumb1.jpg"
}
],
"markdown": "# A 14-Year-Old Boy With Unexplained, Recurring Fever\n\n **Authors:** Shah Azmoon, MD; Matthew Budoff, MD; David Atkinson, MD \n **Date:** June 17, 2019\n\n ## Content\n\n On physical examination, the patient is ill-appearing, fatigued, and febrile. He has a blood pressure of 125/66 mm Hg, a heart rate of 116 beats/min with a regular rhythm, a respiratory rate of 19 breaths/min, and an oxygen saturation of 96% while breathing room air. He has mild bilateral conjunctival injection with limbic sparing, enlarged right lymphadenopathy, and a mild desquamating rash between his fingers.\nHis lung sounds are clear bilaterally, and cardiac auscultation reveals a normal first heart sound and a normal physiologically split second heart sound. No murmurs, thrills, rubs, or gallops are appreciated. No significant conduction abnormalities are noted on ECG, but an initial transthoracic echocardiogram (TTE) in the emergency department, although a poorly delineated study, raises suspicion for cardiac motion abnormalities.\nLaboratory tests are performed that reveal a markedly elevated white blood cell count of 21.6 × 103 cells/µL, with 15% bands; a slightly low hemoglobin level of 12.2 g/dL; and an elevated platelet count of 720 × 103 cells/µL. Blood cultures are obtained and sent to the lab. Intravenous immunoglobulin and antibiotics are empirically initiated for suspicion of Kawasaki disease (KD), scarlet fever, or sepsis, and the patient is admitted for cardiac monitoring.\nThe following day, the patient is referred for coronary CT angiography (CCTA) for better delineation of the cardiac and coronary anatomy. Images are obtained using low-dose protocols, with an estimated radiation dose of 5 mSv (Figure 1).\nFigure 1.\n\n ## Figures\n\n **Figure 1.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1130706,
"choiceText": "Pulmonary vein anomaly",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1130708,
"choiceText": "Left atrial thrombus",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1130710,
"choiceText": "Coronary artery aneurysm",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1130712,
"choiceText": "Aortic aneurysm",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 358593,
"questionText": "<p>Which of the following is the most likely diagnosis?</p>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 14-Year-Old Boy With Unexplained, Recurring Fever"
},
{
"authors": "Shah Azmoon, MD; Matthew Budoff, MD; David Atkinson, MD",
"content": [
"The two images below revealed significant aneurysms of the left anterior descending coronary artery (LAD), the first diagonal branch of the LAD (D1), and the right coronary artery (RCA), all of which confirmed the diagnosis of KD.",
"Figure 1.",
"Figure 2.",
"KD, an immune-mediated vasculitis with a self-limited clinical course, was first described in 1967 by Dr Tomisaku Kawasaki.[1] It can affect arteries of any diameter and typically presents in children younger than 5 years; occurrence beyond late childhood is thought to be rare.[2]",
"A diagnosis of KD should be considered when patients present with a core body temperatures > 101.3°F for more than 5 days, along with 4 of the 5 following associated clinical findings[3]:",
"Bilateral conjunctival injection sparing the limbus, without exudate and starting with the onset of fever",
"Cracked or fissured lips, with or without injected oral mucosa or strawberry tongue",
"A generalized polymorphous rash",
"Edema or erythema of the palms and soles, followed by periungual desquamation in the convalescent phase",
"Painful cervical lymph node enlargement > 1.5 cm in diameter",
"In patients with high clinical suspicion who present with < 4 of the diagnostic clinical signs, the term \"incomplete KD\" is used. Patients with incomplete KD, although lacking the epidemiologic definition of KD, present similarly to patients with classic KD and have similar cardiac risk factors.[4,5,6] As many as 10% of patients develop coronary artery aneurysms but never meet the clinical criteria for KD first described by Kawasaki in 1967.[1,7]",
"All children with KD have some form of coronary artery involvement and, without therapy, manifestations of acute inflammation can last up to several weeks, with an increased risk for cardiac complications.[8] In the United States, children of Hispanic, Pacific Island, or Asian descent have been noted to be at increased risk for coronary artery aneurysm.[9]",
"The incidence of coronary artery aneurysms is noted to be 25% in untreated patients, with mortality rates as high as 2%[10]; however, with early detection and treatment protocols, the prevalence and mortality of such complications have been reduced to 5% and < 1%, respectively.",
"Signs and symptoms in the acute phase of cardiac complications may include fever with tachycardia out of proportion to the degree of fever, cervical lymphadenopathy, bilateral conjunctival injection, rash, inflammation of the oral mucosa, muffled heart tones, and gallop heart sounds.[4]"
],
"date": "June 17, 2019",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/884/505/884505-thumb1.jpg"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/884/505/884505-thumb2.jpg"
}
],
"markdown": "# A 14-Year-Old Boy With Unexplained, Recurring Fever\n\n **Authors:** Shah Azmoon, MD; Matthew Budoff, MD; David Atkinson, MD \n **Date:** June 17, 2019\n\n ## Content\n\n The two images below revealed significant aneurysms of the left anterior descending coronary artery (LAD), the first diagonal branch of the LAD (D1), and the right coronary artery (RCA), all of which confirmed the diagnosis of KD.\nFigure 1.\nFigure 2.\nKD, an immune-mediated vasculitis with a self-limited clinical course, was first described in 1967 by Dr Tomisaku Kawasaki.[1] It can affect arteries of any diameter and typically presents in children younger than 5 years; occurrence beyond late childhood is thought to be rare.[2]\nA diagnosis of KD should be considered when patients present with a core body temperatures > 101.3°F for more than 5 days, along with 4 of the 5 following associated clinical findings[3]:\nBilateral conjunctival injection sparing the limbus, without exudate and starting with the onset of fever\nCracked or fissured lips, with or without injected oral mucosa or strawberry tongue\nA generalized polymorphous rash\nEdema or erythema of the palms and soles, followed by periungual desquamation in the convalescent phase\nPainful cervical lymph node enlargement > 1.5 cm in diameter\nIn patients with high clinical suspicion who present with < 4 of the diagnostic clinical signs, the term \"incomplete KD\" is used. Patients with incomplete KD, although lacking the epidemiologic definition of KD, present similarly to patients with classic KD and have similar cardiac risk factors.[4,5,6] As many as 10% of patients develop coronary artery aneurysms but never meet the clinical criteria for KD first described by Kawasaki in 1967.[1,7]\nAll children with KD have some form of coronary artery involvement and, without therapy, manifestations of acute inflammation can last up to several weeks, with an increased risk for cardiac complications.[8] In the United States, children of Hispanic, Pacific Island, or Asian descent have been noted to be at increased risk for coronary artery aneurysm.[9]\nThe incidence of coronary artery aneurysms is noted to be 25% in untreated patients, with mortality rates as high as 2%[10]; however, with early detection and treatment protocols, the prevalence and mortality of such complications have been reduced to 5% and < 1%, respectively.\nSigns and symptoms in the acute phase of cardiac complications may include fever with tachycardia out of proportion to the degree of fever, cervical lymphadenopathy, bilateral conjunctival injection, rash, inflammation of the oral mucosa, muffled heart tones, and gallop heart sounds.[4]\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n\n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1130706,
"choiceText": "Pulmonary vein anomaly",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1130708,
"choiceText": "Left atrial thrombus",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1130710,
"choiceText": "Coronary artery aneurysm",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1130712,
"choiceText": "Aortic aneurysm",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 358593,
"questionText": "<p>Which of the following is the most likely diagnosis?</p>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 14-Year-Old Boy With Unexplained, Recurring Fever"
},
{
"authors": "Shah Azmoon, MD; Matthew Budoff, MD; David Atkinson, MD",
"content": [
"ECG findings in KD may reveal low-voltage R waves and flattening T waves, with possible prolongation of the PR and QT intervals. Although not common, findings indicative of an acute myocardial infarction (MI) may be seen, including Q waves and repolarization abnormalities.[11,12,13] Early echocardiography may help suggest the diagnosis of KD when suspected in patients with nontypical presentations. The associated myocardial inflammation may reveal mild left ventricular dilation along with a decrease in systolic function, mitral regurgitation, pericardial effusion, aortic root dilation, and mild aortic insufficiency.[14,15] These echocardiographic findings tend to spontaneously improve within a few weeks of therapy, although abnormalities may persist several months to years after the diagnosis.",
"In about 50% of children with KD, coronary artery dilation may be seen by the 10th day of the disease process. Male infants have the highest risk for coronary artery aneurysms.[16] Enlargement of the left main coronary artery is usually seen, with ectasia of the left anterior descending and/or circumflex coronary arteries.[4] Aneurysm forms as a result of arterial vessel-wall destruction during the accumulation of cytokines and chemokines in an proinflammatory milieu.[17] An increased risk for thrombosis is noted during the acute phase of the illness, caused by enhanced platelet activity and stasis within the aneurysm. Stenosis can occur at the proximal and distal ends of an aneurysm.",
"Aneurysms that are > 15 mm in length and > 5 mm in diameter are at risk for local vessel stenosis within a remodeled aneurysm.[18] Long-term anticoagulation with antiplatelet agents, such as aspirin and concomitant warfarin (international normalized ratio, 2-2.5), has been used to reduce the risk for vessel occlusion in patients with large (> 6 mm) or giant (> 8 mm) aneurysms.[19] Fortunately, approximately 50% of coronary artery aneurysms tend to regress within the first 2 years via proliferation of intimal cells, but these patients are still at increased risk for accelerated atherosclerosis and are considered at moderate risk for future ischemic disease.[20,21,22] Regression is more common in smaller aneurysms (< 8 mm), younger patients, and fusiform aneurysms (versus saccular).[20,23,24]",
"In patients with KD in whom prompt therapy is initiated with aspirin and gamma-globulin, the prevalence of coronary artery aneurysm is reduced to < 5%.[17,19] Therapy with aspirin has been instrumental in suppression of the anti-inflammatory process, leading to a significant decrease in mortality from 2% to 0.5%.[4] In general, antiplatelet therapy should be continued until documented regression of the coronary aneurysm, with avoidance of high-risk sports activities to minimize the risk for bleeding.[25] The recommended initial dose of aspirin for effective anti-inflammatory action is 80-100 mg/kg, divided into 4 daily doses.[23] Subsequent lower doses of aspirin for effective antiplatelet action may be used.",
"Gamma-globulins also appear to have anti-inflammatory effects, and multiple studies have validated a decrease in the frequency of coronary aneurysms compared with use of aspirin alone, as well as a reduction in morbidity and mortality when gamma-globulins are promptly administered. The recommended doses of intravenous gamma-globulin have been up to 2 g/kg, infused over 12-18 hours.[23]",
"In contrast, when aneurysm is left untreated or treated with aspirin alone, about one half of patients progress to aneurysmal dilatation within a few days, and they have increased risk for hemopericardium and death resulting from rupture. Patients with moderate-sized (> 3 mm) or larger aneurysms require further follow-up because they are at the highest risk for persistence of the aneurysms and future ischemic disease.[3,20,26] Within this group, most deaths occur within the first year of diagnosis, as a result of acute MI within the left coronary distribution; they have a 22% mortality rate with the first MI and up to an 83% mortality with the third MI.[26]",
"As many as 37% of patients may have silent MIs and, when symptomatic, children may not present with chest pain, instead presenting with inconsolable crying, abdominal pain, pallor, and vomiting. Because infants present most often with incomplete KD (resulting in delayed diagnosis), a low threshold for the initiation of therapy in this group is often necessary in the context of unexplained fever with no other clinical findings, in order to minimize the risk for coronary aneurysms.[27,28]"
],
"date": "June 17, 2019",
"figures": [],
"markdown": "# A 14-Year-Old Boy With Unexplained, Recurring Fever\n\n **Authors:** Shah Azmoon, MD; Matthew Budoff, MD; David Atkinson, MD \n **Date:** June 17, 2019\n\n ## Content\n\n ECG findings in KD may reveal low-voltage R waves and flattening T waves, with possible prolongation of the PR and QT intervals. Although not common, findings indicative of an acute myocardial infarction (MI) may be seen, including Q waves and repolarization abnormalities.[11,12,13] Early echocardiography may help suggest the diagnosis of KD when suspected in patients with nontypical presentations. The associated myocardial inflammation may reveal mild left ventricular dilation along with a decrease in systolic function, mitral regurgitation, pericardial effusion, aortic root dilation, and mild aortic insufficiency.[14,15] These echocardiographic findings tend to spontaneously improve within a few weeks of therapy, although abnormalities may persist several months to years after the diagnosis.\nIn about 50% of children with KD, coronary artery dilation may be seen by the 10th day of the disease process. Male infants have the highest risk for coronary artery aneurysms.[16] Enlargement of the left main coronary artery is usually seen, with ectasia of the left anterior descending and/or circumflex coronary arteries.[4] Aneurysm forms as a result of arterial vessel-wall destruction during the accumulation of cytokines and chemokines in an proinflammatory milieu.[17] An increased risk for thrombosis is noted during the acute phase of the illness, caused by enhanced platelet activity and stasis within the aneurysm. Stenosis can occur at the proximal and distal ends of an aneurysm.\nAneurysms that are > 15 mm in length and > 5 mm in diameter are at risk for local vessel stenosis within a remodeled aneurysm.[18] Long-term anticoagulation with antiplatelet agents, such as aspirin and concomitant warfarin (international normalized ratio, 2-2.5), has been used to reduce the risk for vessel occlusion in patients with large (> 6 mm) or giant (> 8 mm) aneurysms.[19] Fortunately, approximately 50% of coronary artery aneurysms tend to regress within the first 2 years via proliferation of intimal cells, but these patients are still at increased risk for accelerated atherosclerosis and are considered at moderate risk for future ischemic disease.[20,21,22] Regression is more common in smaller aneurysms (< 8 mm), younger patients, and fusiform aneurysms (versus saccular).[20,23,24]\nIn patients with KD in whom prompt therapy is initiated with aspirin and gamma-globulin, the prevalence of coronary artery aneurysm is reduced to < 5%.[17,19] Therapy with aspirin has been instrumental in suppression of the anti-inflammatory process, leading to a significant decrease in mortality from 2% to 0.5%.[4] In general, antiplatelet therapy should be continued until documented regression of the coronary aneurysm, with avoidance of high-risk sports activities to minimize the risk for bleeding.[25] The recommended initial dose of aspirin for effective anti-inflammatory action is 80-100 mg/kg, divided into 4 daily doses.[23] Subsequent lower doses of aspirin for effective antiplatelet action may be used.\nGamma-globulins also appear to have anti-inflammatory effects, and multiple studies have validated a decrease in the frequency of coronary aneurysms compared with use of aspirin alone, as well as a reduction in morbidity and mortality when gamma-globulins are promptly administered. The recommended doses of intravenous gamma-globulin have been up to 2 g/kg, infused over 12-18 hours.[23]\nIn contrast, when aneurysm is left untreated or treated with aspirin alone, about one half of patients progress to aneurysmal dilatation within a few days, and they have increased risk for hemopericardium and death resulting from rupture. Patients with moderate-sized (> 3 mm) or larger aneurysms require further follow-up because they are at the highest risk for persistence of the aneurysms and future ischemic disease.[3,20,26] Within this group, most deaths occur within the first year of diagnosis, as a result of acute MI within the left coronary distribution; they have a 22% mortality rate with the first MI and up to an 83% mortality with the third MI.[26]\nAs many as 37% of patients may have silent MIs and, when symptomatic, children may not present with chest pain, instead presenting with inconsolable crying, abdominal pain, pallor, and vomiting. Because infants present most often with incomplete KD (resulting in delayed diagnosis), a low threshold for the initiation of therapy in this group is often necessary in the context of unexplained fever with no other clinical findings, in order to minimize the risk for coronary aneurysms.[27,28]\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 14-Year-Old Boy With Unexplained, Recurring Fever"
},
{
"authors": "Shah Azmoon, MD; Matthew Budoff, MD; David Atkinson, MD",
"content": [
"In this case, a diagnosis of KD was suspected in the emergency department after the initial TTE revealed an 8-mm dilation of the proximal RCA. The following day, the patient underwent CCTA for better evaluation of his coronary anatomy. Reformatted three-dimensional images of the study revealed initial enlargement of the LAD (4.97 mm), first diagonal branch of the LAD (5.12 mm), and RCA (8.90 mm) (Figure 2).",
"Figure 2.",
"On the third day of the patient's admission, a repeat TTE revealed a > 9 mm aneurysm within the RCA, and a second dose of intravenous immunoglobulin was initiated along with antiplatelet therapy using abciximab. By hospital day 4, the RCA aneurysm progressed to 12 mm, with a possible small thrombus, and dilation of the distal LAD to 7 mm was noted. The patient was started on anticoagulation and a 3-day course of methylprednisolone sodium succinate (30 mg/kg), after which he finally became afebrile. The painful lymphadenopathy limiting his movement also decreased, and he regained full range of motion of his head and neck.",
"Unfortunately, daily TTEs failed to note stabilization of his coronary artery enlargement, and the patient was started on tumor necrosis factor-alpha antagonists. Repeat CCTA on day 10 revealed a 9-mm RCA, with segmental 14.16-mm and 12-mm aneurysms; an LAD of 10 mm, with a 14-mm aneurysm; and a 7.69-mm first diagonal branch aneurysm. In the ensuing 13 days, no further progression of coronary enlargement was noticed on repeat imaging, and the patient felt much better. Whole-body CTA was negative for vascular aneurysm or dilatation elsewhere, including the circle of Willis. The patient was discharged on bed rest, warfarin, atenolol, aspirin, lisinopril, and clopidogrel, with monthly cardiology follow-up.",
"Early recognition of KD, in the context of unrelenting fever in children with or without all of the associated features, is vital for prompt suppression of vascular inflammation and prevention of cardiac complications."
],
"date": "June 17, 2019",
"figures": [
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/884/505/884505-thumb2.jpg"
}
],
"markdown": "# A 14-Year-Old Boy With Unexplained, Recurring Fever\n\n **Authors:** Shah Azmoon, MD; Matthew Budoff, MD; David Atkinson, MD \n **Date:** June 17, 2019\n\n ## Content\n\n In this case, a diagnosis of KD was suspected in the emergency department after the initial TTE revealed an 8-mm dilation of the proximal RCA. The following day, the patient underwent CCTA for better evaluation of his coronary anatomy. Reformatted three-dimensional images of the study revealed initial enlargement of the LAD (4.97 mm), first diagonal branch of the LAD (5.12 mm), and RCA (8.90 mm) (Figure 2).\nFigure 2.\nOn the third day of the patient's admission, a repeat TTE revealed a > 9 mm aneurysm within the RCA, and a second dose of intravenous immunoglobulin was initiated along with antiplatelet therapy using abciximab. By hospital day 4, the RCA aneurysm progressed to 12 mm, with a possible small thrombus, and dilation of the distal LAD to 7 mm was noted. The patient was started on anticoagulation and a 3-day course of methylprednisolone sodium succinate (30 mg/kg), after which he finally became afebrile. The painful lymphadenopathy limiting his movement also decreased, and he regained full range of motion of his head and neck.\nUnfortunately, daily TTEs failed to note stabilization of his coronary artery enlargement, and the patient was started on tumor necrosis factor-alpha antagonists. Repeat CCTA on day 10 revealed a 9-mm RCA, with segmental 14.16-mm and 12-mm aneurysms; an LAD of 10 mm, with a 14-mm aneurysm; and a 7.69-mm first diagonal branch aneurysm. In the ensuing 13 days, no further progression of coronary enlargement was noticed on repeat imaging, and the patient felt much better. Whole-body CTA was negative for vascular aneurysm or dilatation elsewhere, including the circle of Willis. The patient was discharged on bed rest, warfarin, atenolol, aspirin, lisinopril, and clopidogrel, with monthly cardiology follow-up.\nEarly recognition of KD, in the context of unrelenting fever in children with or without all of the associated features, is vital for prompt suppression of vascular inflammation and prevention of cardiac complications.\n\n ## Figures\n\n **Figure 2.** \n \n\n\n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1130714,
"choiceText": "Bilateral conjunctival injection",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1130716,
"choiceText": "Cracked or fissured lips",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1130718,
"choiceText": "Generalized polymorphous rash",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1130720,
"choiceText": "Hepatosplenomegaly",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1130722,
"choiceText": "Cervical lymph node enlargement",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>Hepatosplenomegaly is not typically associated with KD and should not necessarily prompt consideration for the diagnosis.</p>",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 358595,
"questionText": "<p>Which of the following conditions is <em>not</em> typically associated with KD?</p>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1130724,
"choiceText": "Fever and tachycardia out of proportion to the degree of fever",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1130726,
"choiceText": "Cervical lymphadenopathy and rash",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1130728,
"choiceText": "Conjunctivitis and mucositis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1130730,
"choiceText": "Muffled heart tones",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1130732,
"choiceText": "All of the above",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>Signs and symptoms in the acute phase of cardiac complications may include fever and tachycardia out of proportion to the degree of fever, cervical lymphadenopathy, bilateral conjunctival injection, rash, inflammation of the oral mucosa, muffled heart tones, and gallop sounds. </p>",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 358597,
"questionText": "<p>Which of the following signs and symptoms may occur during the acute phase of cardiac complications in patients with KD?</p>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 14-Year-Old Boy With Unexplained, Recurring Fever"
},
{
"authors": "Shah Azmoon, MD; Matthew Budoff, MD; David Atkinson, MD",
"content": [],
"date": "June 17, 2019",
"figures": [],
"markdown": "# A 14-Year-Old Boy With Unexplained, Recurring Fever\n\n **Authors:** Shah Azmoon, MD; Matthew Budoff, MD; David Atkinson, MD \n **Date:** June 17, 2019\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1130714,
"choiceText": "Bilateral conjunctival injection",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1130716,
"choiceText": "Cracked or fissured lips",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1130718,
"choiceText": "Generalized polymorphous rash",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1130720,
"choiceText": "Hepatosplenomegaly",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1130722,
"choiceText": "Cervical lymph node enlargement",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>Hepatosplenomegaly is not typically associated with KD and should not necessarily prompt consideration for the diagnosis.</p>",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 358595,
"questionText": "<p>Which of the following conditions is <em>not</em> typically associated with KD?</p>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1130724,
"choiceText": "Fever and tachycardia out of proportion to the degree of fever",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1130726,
"choiceText": "Cervical lymphadenopathy and rash",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1130728,
"choiceText": "Conjunctivitis and mucositis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1130730,
"choiceText": "Muffled heart tones",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1130732,
"choiceText": "All of the above",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>Signs and symptoms in the acute phase of cardiac complications may include fever and tachycardia out of proportion to the degree of fever, cervical lymphadenopathy, bilateral conjunctival injection, rash, inflammation of the oral mucosa, muffled heart tones, and gallop sounds. </p>",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 358597,
"questionText": "<p>Which of the following signs and symptoms may occur during the acute phase of cardiac complications in patients with KD?</p>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 14-Year-Old Boy With Unexplained, Recurring Fever"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1130706,
"choiceText": "Pulmonary vein anomaly",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1130708,
"choiceText": "Left atrial thrombus",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1130710,
"choiceText": "Coronary artery aneurysm",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1130712,
"choiceText": "Aortic aneurysm",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 358593,
"questionText": "<p>Which of the following is the most likely diagnosis?</p>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1130714,
"choiceText": "Bilateral conjunctival injection",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1130716,
"choiceText": "Cracked or fissured lips",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1130718,
"choiceText": "Generalized polymorphous rash",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1130720,
"choiceText": "Hepatosplenomegaly",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1130722,
"choiceText": "Cervical lymph node enlargement",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>Hepatosplenomegaly is not typically associated with KD and should not necessarily prompt consideration for the diagnosis.</p>",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 358595,
"questionText": "<p>Which of the following conditions is <em>not</em> typically associated with KD?</p>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1130724,
"choiceText": "Fever and tachycardia out of proportion to the degree of fever",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1130726,
"choiceText": "Cervical lymphadenopathy and rash",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1130728,
"choiceText": "Conjunctivitis and mucositis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1130730,
"choiceText": "Muffled heart tones",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1130732,
"choiceText": "All of the above",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>Signs and symptoms in the acute phase of cardiac complications may include fever and tachycardia out of proportion to the degree of fever, cervical lymphadenopathy, bilateral conjunctival injection, rash, inflammation of the oral mucosa, muffled heart tones, and gallop sounds. </p>",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 358597,
"questionText": "<p>Which of the following signs and symptoms may occur during the acute phase of cardiac complications in patients with KD?</p>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
913038 | /viewarticle/913038 | [
{
"authors": "Winston Tan, MD; Nicole Ganon, PA; Ashton Ritter, MMS",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"A 64-year-old right-handed man presents to the emergency department with left-sided arm and leg weakness. He reports that over the past 4 weeks, he has had episodes of feeling \"off balance\" when walking but denies any falls or syncopal episodes. Over the past week, he has developed weakness and numbness in his left side, with increased difficulty when walking. The weakness worsened to the point where he could not comfortably walk or lift his left arm.",
"He does not report headaches, visual changes, confusion, facial weakness, or incontinence. He denies any falls or loss of consciousness. He has no pain. He has no recent history of fever, immunocompromised state, or neck stiffness. He has no history of convulsions or seizures. His history does not include stroke, myocardial infarction, or blood clots. He does not take any blood thinners but is on aspirin (81 mg by mouth daily). His past medical history includes well-controlled hypertension, sleep apnea, migraine, and depression. He is a current smoker, with a 30–pack-year history.",
"He has a history of stage I non-small cell lung adenocarcinoma diagnosed 5 years ago, for which he had a right upper lung lobectomy. The tumor was EGFR negative, anaplastic lymphoma kinase (ALK) negative, ROS1 negative, and PD-L1 positive. He did not have any chemotherapy or radiation adjuvantly and has been following up with PET and CT annually, with no evidence of disease on the most recent PET scan 5 months before evaluation."
],
"date": "May 21, 2019",
"figures": [],
"markdown": "# A 64-Year-Old Man Unable to Lift His Arm or Walk\n\n **Authors:** Winston Tan, MD; Nicole Ganon, PA; Ashton Ritter, MMS \n **Date:** May 21, 2019\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nA 64-year-old right-handed man presents to the emergency department with left-sided arm and leg weakness. He reports that over the past 4 weeks, he has had episodes of feeling \"off balance\" when walking but denies any falls or syncopal episodes. Over the past week, he has developed weakness and numbness in his left side, with increased difficulty when walking. The weakness worsened to the point where he could not comfortably walk or lift his left arm.\nHe does not report headaches, visual changes, confusion, facial weakness, or incontinence. He denies any falls or loss of consciousness. He has no pain. He has no recent history of fever, immunocompromised state, or neck stiffness. He has no history of convulsions or seizures. His history does not include stroke, myocardial infarction, or blood clots. He does not take any blood thinners but is on aspirin (81 mg by mouth daily). His past medical history includes well-controlled hypertension, sleep apnea, migraine, and depression. He is a current smoker, with a 30–pack-year history.\nHe has a history of stage I non-small cell lung adenocarcinoma diagnosed 5 years ago, for which he had a right upper lung lobectomy. The tumor was EGFR negative, anaplastic lymphoma kinase (ALK) negative, ROS1 negative, and PD-L1 positive. He did not have any chemotherapy or radiation adjuvantly and has been following up with PET and CT annually, with no evidence of disease on the most recent PET scan 5 months before evaluation.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 64-Year-Old Man Unable to Lift His Arm or Walk"
},
{
"authors": "Winston Tan, MD; Nicole Ganon, PA; Ashton Ritter, MMS",
"content": [
"His vital signs on admission include temperature of 98.3°F (36.8°C), blood pressure of 140/60 mm Hg, heart rate of 92 beats/min, respiratory rate of 18 breaths/min, and SpO2 of 97% on room air.",
"Upon physical examination, he is alert and oriented to person, place, time, and situation. He is in no acute distress. He has normal speech and no facial weakness, and his cranial nerves are intact. He has 5/5 strength in right-side upper and lower extremities and grade 3/5 weakness of the left arm and left leg. He has difficulty with the coordination of movement. He is unable to walk unassisted. He has decreased sensations on the lower right foot to the knee, as well as fingers to mid-forearm on the right side. Cardiac examination findings are negative. Decreased breath sounds are noted in the right upper lobe. He has normal abdominal examination findings.",
"His complete blood cell count and basic metabolic panel are within normal limits. His international normalized ratio is 1.2. Imaging study findings are shown below.",
"Figure 1.",
"Figure 2."
],
"date": "May 21, 2019",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/913/038/913038-Thumb1.png"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/913/038/913038-Thumb2.png"
}
],
"markdown": "# A 64-Year-Old Man Unable to Lift His Arm or Walk\n\n **Authors:** Winston Tan, MD; Nicole Ganon, PA; Ashton Ritter, MMS \n **Date:** May 21, 2019\n\n ## Content\n\n His vital signs on admission include temperature of 98.3°F (36.8°C), blood pressure of 140/60 mm Hg, heart rate of 92 beats/min, respiratory rate of 18 breaths/min, and SpO2 of 97% on room air.\nUpon physical examination, he is alert and oriented to person, place, time, and situation. He is in no acute distress. He has normal speech and no facial weakness, and his cranial nerves are intact. He has 5/5 strength in right-side upper and lower extremities and grade 3/5 weakness of the left arm and left leg. He has difficulty with the coordination of movement. He is unable to walk unassisted. He has decreased sensations on the lower right foot to the knee, as well as fingers to mid-forearm on the right side. Cardiac examination findings are negative. Decreased breath sounds are noted in the right upper lobe. He has normal abdominal examination findings.\nHis complete blood cell count and basic metabolic panel are within normal limits. His international normalized ratio is 1.2. Imaging study findings are shown below.\nFigure 1.\nFigure 2.\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1359764,
"choiceText": "Sarcoidosis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1359766,
"choiceText": "Brain abscess",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1359768,
"choiceText": "Primary tumor of the brain",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1359770,
"choiceText": "Lung cancer metastasis to the brain",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 433863,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 64-Year-Old Man Unable to Lift His Arm or Walk"
},
{
"authors": "Winston Tan, MD; Nicole Ganon, PA; Ashton Ritter, MMS",
"content": [
"The CT findings of a mass with associated edema, along with his history of lung cancer, strongly suggested brain metastasis (Figure 1).",
"Figure 1.",
"In this case, a solitary metastasis must be differentiated from a primary central nervous system neoplasm. MRI of the head revealed the following:",
"Figure 2.",
"Brain metastasis is one of the most significant complications of lung cancer and represents the most common brain tumor in adults. The incidence of brain metastasis is rising with the increase in survival of patients with cancer. MRI with contrast enhancement is currently the procedure of choice because it is more sensitive and specific than other imaging modalities in determining the presence, location, and number of metastases.[1] However, contrast-enhanced CT is widely used because of its accessibility and low cost.",
"Lung cancer is the leading cause of cancer-related mortality in men and women.[2] In 2018, 154,050 lung cancer-related deaths were estimated in the United States.[3] Most lung cancers are classified as either small-cell lung cancer (SCLC) or non-small cell lung cancer (NSCLC). Of the two, NSCLC is the most common form and accounts for approximately 85% of lung cancer cases. Histologic classification of NSCLC is further classified into adenocarcinoma, squamous cell carcinoma, and large cell carcinoma. Classification of lung cancer by histopathologic subtype is important because it provides information about prognosis and guides treatment decisions."
],
"date": "May 21, 2019",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/913/038/913038-Thumb1.png"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/913/038/913038-Thumb2.png"
}
],
"markdown": "# A 64-Year-Old Man Unable to Lift His Arm or Walk\n\n **Authors:** Winston Tan, MD; Nicole Ganon, PA; Ashton Ritter, MMS \n **Date:** May 21, 2019\n\n ## Content\n\n The CT findings of a mass with associated edema, along with his history of lung cancer, strongly suggested brain metastasis (Figure 1).\nFigure 1.\nIn this case, a solitary metastasis must be differentiated from a primary central nervous system neoplasm. MRI of the head revealed the following:\nFigure 2.\nBrain metastasis is one of the most significant complications of lung cancer and represents the most common brain tumor in adults. The incidence of brain metastasis is rising with the increase in survival of patients with cancer. MRI with contrast enhancement is currently the procedure of choice because it is more sensitive and specific than other imaging modalities in determining the presence, location, and number of metastases.[1] However, contrast-enhanced CT is widely used because of its accessibility and low cost.\nLung cancer is the leading cause of cancer-related mortality in men and women.[2] In 2018, 154,050 lung cancer-related deaths were estimated in the United States.[3] Most lung cancers are classified as either small-cell lung cancer (SCLC) or non-small cell lung cancer (NSCLC). Of the two, NSCLC is the most common form and accounts for approximately 85% of lung cancer cases. Histologic classification of NSCLC is further classified into adenocarcinoma, squamous cell carcinoma, and large cell carcinoma. Classification of lung cancer by histopathologic subtype is important because it provides information about prognosis and guides treatment decisions.\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n\n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1359764,
"choiceText": "Sarcoidosis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1359766,
"choiceText": "Brain abscess",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1359768,
"choiceText": "Primary tumor of the brain",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1359770,
"choiceText": "Lung cancer metastasis to the brain",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 433863,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 64-Year-Old Man Unable to Lift His Arm or Walk"
},
{
"authors": "Winston Tan, MD; Nicole Ganon, PA; Ashton Ritter, MMS",
"content": [
"Patients with metastatic NSCLC to the brain have a poor prognosis. At diagnosis, brain metastases are rare and account for only 7%-10% of cases; however, as many as 20%-40% of patients develop brain metastasis during their disease course.[4] Traditionally, because of poor prognosis and limited survival, palliative treatment with corticosteroids and whole-brain radiation has been the standard treatment. In some selected patients with solitary brain metastasis from NSCLC, more aggressive management with surgical resection or radiosurgery has been pursued.",
"The most important risk factor in the development of lung cancer is exposure to tobacco smoke, either from active cigarette smoking or passive smoking. The relative risk for lung cancer in lifetime smokers is estimated to be 10-30 times higher than in nonsmokers.[5] A study to assess the impact of smoking at diagnosis and quitting on 1-year survival of NSCLC reported that 77% of never-smokers were alive at 1 year, compared with 60% of ex-smokers and 57% of current smokers.[6]",
"Other risk factors for development of lung cancer include radiation therapy, occupational and environmental carcinogens, and dietary and genetic factors. Radiation therapy, used to treat such cancers as Hodgkin lymphoma, carries a risk for lung cancer that should be addressed with patients. A review of the literature on long-term complications of the treatment of Hodgkin lymphoma showed that the risk for lung cancer was increased twofold to sevenfold in patients who received radiation therapy.[7]",
"Patients with risk factors for lung cancer or patients with symptoms of cough, shortness of breath, hemoptysis, or unexplained weight loss and fatigue should undergo investigation for lung cancer. These symptoms are usually related to tumor location and may be due to the primary tumor, local or regional spread, or metastatic disease.[1] Physical examination, complete blood cell count, and chest radiography are the first steps in lung cancer screening. Concerning findings on chest radiography include pulmonary masses, nodules, effusions, or infiltrates. Widening of the mediastinum as well as hilar enlargement and atelectasis are other findings of concern.",
"Most patients with lung cancer present with advanced-stage disease. Symptoms of metastasis include bone pain, neurologic changes, or spinal cord impingement; therefore, PET plays an important role. In addition, a wide variety of paraneoplastic syndromes are observed in association with lung cancer.[8] Symptoms may include Trousseau syndrome of hypercoagulability, syndrome of inappropriate antidiuretic hormone secretion (SIADH), clubbing, and hypertrophic osteoarthropathy."
],
"date": "May 21, 2019",
"figures": [],
"markdown": "# A 64-Year-Old Man Unable to Lift His Arm or Walk\n\n **Authors:** Winston Tan, MD; Nicole Ganon, PA; Ashton Ritter, MMS \n **Date:** May 21, 2019\n\n ## Content\n\n Patients with metastatic NSCLC to the brain have a poor prognosis. At diagnosis, brain metastases are rare and account for only 7%-10% of cases; however, as many as 20%-40% of patients develop brain metastasis during their disease course.[4] Traditionally, because of poor prognosis and limited survival, palliative treatment with corticosteroids and whole-brain radiation has been the standard treatment. In some selected patients with solitary brain metastasis from NSCLC, more aggressive management with surgical resection or radiosurgery has been pursued.\nThe most important risk factor in the development of lung cancer is exposure to tobacco smoke, either from active cigarette smoking or passive smoking. The relative risk for lung cancer in lifetime smokers is estimated to be 10-30 times higher than in nonsmokers.[5] A study to assess the impact of smoking at diagnosis and quitting on 1-year survival of NSCLC reported that 77% of never-smokers were alive at 1 year, compared with 60% of ex-smokers and 57% of current smokers.[6]\nOther risk factors for development of lung cancer include radiation therapy, occupational and environmental carcinogens, and dietary and genetic factors. Radiation therapy, used to treat such cancers as Hodgkin lymphoma, carries a risk for lung cancer that should be addressed with patients. A review of the literature on long-term complications of the treatment of Hodgkin lymphoma showed that the risk for lung cancer was increased twofold to sevenfold in patients who received radiation therapy.[7]\nPatients with risk factors for lung cancer or patients with symptoms of cough, shortness of breath, hemoptysis, or unexplained weight loss and fatigue should undergo investigation for lung cancer. These symptoms are usually related to tumor location and may be due to the primary tumor, local or regional spread, or metastatic disease.[1] Physical examination, complete blood cell count, and chest radiography are the first steps in lung cancer screening. Concerning findings on chest radiography include pulmonary masses, nodules, effusions, or infiltrates. Widening of the mediastinum as well as hilar enlargement and atelectasis are other findings of concern.\nMost patients with lung cancer present with advanced-stage disease. Symptoms of metastasis include bone pain, neurologic changes, or spinal cord impingement; therefore, PET plays an important role. In addition, a wide variety of paraneoplastic syndromes are observed in association with lung cancer.[8] Symptoms may include Trousseau syndrome of hypercoagulability, syndrome of inappropriate antidiuretic hormone secretion (SIADH), clubbing, and hypertrophic osteoarthropathy.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 64-Year-Old Man Unable to Lift His Arm or Walk"
},
{
"authors": "Winston Tan, MD; Nicole Ganon, PA; Ashton Ritter, MMS",
"content": [
"The National Comprehensive Cancer Network (NCCN) guidelines outline several options for localized or symptomatic localized recurrent NSCLC.[9] If the patient presents with endobronchial obstruction, options include laser therapy, stenting, surgery, external-beam radiation, brachytherapy, or photodynamic therapy. If a recurrence is resectable, the preferred treatment is re-resection; however, external-beam radiation and stereotactic ablative radiotherapy are also options. If a patient presents with mediastinal lymph node recurrence without prior radiation, the NCCN recommends concurrent chemoradiation. If the patient has received prior radiation, the recommendation is for systemic therapy. If a patient presents with superior vena cava (SVC) obstruction, several options are available, including concurrent chemoradiation with or without SVC stenting, external-beam radiation with or without SVC stenting, or SVC stenting alone. To treat severe hemoptysis, any combination of external-beam radiation, brachytherapy, laser, photodynamic therapy, embolization, or surgery is recommended.",
"After any of these interventions, imaging with CT with contrast, brain MRI with contrast, and PET/CT are indicated to determine whether disseminated disease exists. If no evidence is present, options include observation versus systemic therapy. If present, the NCCN guidelines suggest pursuing systemic therapy.",
"If a patient presents with diffuse distant metastasis to the brain, the recommendation is to pursue palliative external-beam radiation. If lesions are limited, options include stereotactic radiosurgery alone or surgical resection; if symptomatic or warranted for diagnosis, this is followed by stereotactic radiosurgery or whole-brain radiotherapy.",
"Identifying genetic mutations that affect therapy selection in NSCLC is increasingly important. Important gene alterations include EGFR mutation, ALK gene rearrangements, ROS1 gene rearrangements, BRAF point mutation, KRAS point mutation, and PD-L1 mutation. These can be obtained on surgical or biopsy specimen or liquid blood biopsy. Other emerging biomarkers include high-level MET amplification, RET rearrangements, ERBB2 (HER2) mutations, and tumor mutation burden; however, the latter remains controversial owing to a lack of standardization.",
"The patient in this case was evaluated by a neurosurgeon and was started on dexamethasone. Invasive surgery was not attempted. His symptoms improved, and repeat MRI revealed that areas of necrosis and brain edema significantly improved as well. Further imaging studies revealed no clear evidence of disease elsewhere; however, given the patient's high expression of PD-L1 and the fact that cancer had metastasized to his brain, he was started on treatment with a PD-1 inhibitor (pembrolizumab). He was stable at last follow-up."
],
"date": "May 21, 2019",
"figures": [],
"markdown": "# A 64-Year-Old Man Unable to Lift His Arm or Walk\n\n **Authors:** Winston Tan, MD; Nicole Ganon, PA; Ashton Ritter, MMS \n **Date:** May 21, 2019\n\n ## Content\n\n The National Comprehensive Cancer Network (NCCN) guidelines outline several options for localized or symptomatic localized recurrent NSCLC.[9] If the patient presents with endobronchial obstruction, options include laser therapy, stenting, surgery, external-beam radiation, brachytherapy, or photodynamic therapy. If a recurrence is resectable, the preferred treatment is re-resection; however, external-beam radiation and stereotactic ablative radiotherapy are also options. If a patient presents with mediastinal lymph node recurrence without prior radiation, the NCCN recommends concurrent chemoradiation. If the patient has received prior radiation, the recommendation is for systemic therapy. If a patient presents with superior vena cava (SVC) obstruction, several options are available, including concurrent chemoradiation with or without SVC stenting, external-beam radiation with or without SVC stenting, or SVC stenting alone. To treat severe hemoptysis, any combination of external-beam radiation, brachytherapy, laser, photodynamic therapy, embolization, or surgery is recommended.\nAfter any of these interventions, imaging with CT with contrast, brain MRI with contrast, and PET/CT are indicated to determine whether disseminated disease exists. If no evidence is present, options include observation versus systemic therapy. If present, the NCCN guidelines suggest pursuing systemic therapy.\nIf a patient presents with diffuse distant metastasis to the brain, the recommendation is to pursue palliative external-beam radiation. If lesions are limited, options include stereotactic radiosurgery alone or surgical resection; if symptomatic or warranted for diagnosis, this is followed by stereotactic radiosurgery or whole-brain radiotherapy.\nIdentifying genetic mutations that affect therapy selection in NSCLC is increasingly important. Important gene alterations include EGFR mutation, ALK gene rearrangements, ROS1 gene rearrangements, BRAF point mutation, KRAS point mutation, and PD-L1 mutation. These can be obtained on surgical or biopsy specimen or liquid blood biopsy. Other emerging biomarkers include high-level MET amplification, RET rearrangements, ERBB2 (HER2) mutations, and tumor mutation burden; however, the latter remains controversial owing to a lack of standardization.\nThe patient in this case was evaluated by a neurosurgeon and was started on dexamethasone. Invasive surgery was not attempted. His symptoms improved, and repeat MRI revealed that areas of necrosis and brain edema significantly improved as well. Further imaging studies revealed no clear evidence of disease elsewhere; however, given the patient's high expression of PD-L1 and the fact that cancer had metastasized to his brain, he was started on treatment with a PD-1 inhibitor (pembrolizumab). He was stable at last follow-up.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1359772,
"choiceText": "Most patients with lung cancer present with early-stage disease",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1359774,
"choiceText": "Narrowing of the mediastinum on chest radiography is a common finding of concern in patients with lung cancer",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1359776,
"choiceText": "Some treatments for Hodgkin lymphoma are associated with a significantly increased risk for lung cancer",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1359778,
"choiceText": "Paraneoplastic syndromes occur only with SCLC, as opposed to NSCLC",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Radiation therapy, used to treat such cancers as Hodgkin lymphoma, carries a risk for lung cancer that should be addressed with patients. A review of the literature on long-term complications of the treatment of Hodgkin lymphoma showed that the risk for lung cancer was increased twofold to sevenfold in patients who received radiation therapy.<sup>[7]</sup><br><br>\r\n\r\nMost patients with lung cancer present with advanced-stage disease. Concerning findings on chest radiography include pulmonary masses, nodules, effusions, or infiltrates. Widening of the mediastinum as well as hilar enlargement and atelectasis are other findings of concern. A wide variety of paraneoplastic syndromes are observed in association with NSCLC. Symptoms may include Trousseau syndrome of hypercoagulability, SIADH, clubbing, and hypertrophic osteoarthropathy.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 433865,
"questionText": "Which of the following is most accurate regarding lung cancer risk factors, presentation, and diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1359780,
"choiceText": "Chemoradiation is contraindicated in patients with mediastinal lymph node recurrence without prior radiation",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1359782,
"choiceText": "Palliative external-beam radiation is recommended for patients who present with diffuse distant metastasis to the brain ",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1359784,
"choiceText": "External-beam radiation and stereotactic ablative radiotherapy are preferred to re-resection in patients with recurrent lung cancer, even if the recurrence is resectable",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1359786,
"choiceText": "SVC stenting is required in all patients with lung cancer who have an SVC obstruction ",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "If a patient presents with diffuse distant metastasis to the brain, the recommendation is to pursue palliative external-beam radiation. If lesions are limited, options include stereotactic radiosurgery alone or surgical resection; if symptomatic or warranted for diagnosis, this is followed by stereotactic radiosurgery or whole-brain radiotherapy.<br><br>\r\n\r\nIf a patient presents with mediastinal lymph node recurrence without prior radiation, the NCCN recommends concurrent chemoradiation. If a recurrence is resectable, the preferred treatment is re-resection; however, external-beam radiation or stereotactic ablative radiotherapy are also options. If a patient presents with SVC obstruction, several options are available, including concurrent chemoradiation with or without SVC stenting, external-beam radiation with or without SVC stenting, or SVC stenting alone.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 433867,
"questionText": "Which of the following is most accurate regarding the treatment of NSCLC and related metastasis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 64-Year-Old Man Unable to Lift His Arm or Walk"
},
{
"authors": "Winston Tan, MD; Nicole Ganon, PA; Ashton Ritter, MMS",
"content": [],
"date": "May 21, 2019",
"figures": [],
"markdown": "# A 64-Year-Old Man Unable to Lift His Arm or Walk\n\n **Authors:** Winston Tan, MD; Nicole Ganon, PA; Ashton Ritter, MMS \n **Date:** May 21, 2019\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1359772,
"choiceText": "Most patients with lung cancer present with early-stage disease",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1359774,
"choiceText": "Narrowing of the mediastinum on chest radiography is a common finding of concern in patients with lung cancer",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1359776,
"choiceText": "Some treatments for Hodgkin lymphoma are associated with a significantly increased risk for lung cancer",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1359778,
"choiceText": "Paraneoplastic syndromes occur only with SCLC, as opposed to NSCLC",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Radiation therapy, used to treat such cancers as Hodgkin lymphoma, carries a risk for lung cancer that should be addressed with patients. A review of the literature on long-term complications of the treatment of Hodgkin lymphoma showed that the risk for lung cancer was increased twofold to sevenfold in patients who received radiation therapy.<sup>[7]</sup><br><br>\r\n\r\nMost patients with lung cancer present with advanced-stage disease. Concerning findings on chest radiography include pulmonary masses, nodules, effusions, or infiltrates. Widening of the mediastinum as well as hilar enlargement and atelectasis are other findings of concern. A wide variety of paraneoplastic syndromes are observed in association with NSCLC. Symptoms may include Trousseau syndrome of hypercoagulability, SIADH, clubbing, and hypertrophic osteoarthropathy.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 433865,
"questionText": "Which of the following is most accurate regarding lung cancer risk factors, presentation, and diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1359780,
"choiceText": "Chemoradiation is contraindicated in patients with mediastinal lymph node recurrence without prior radiation",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1359782,
"choiceText": "Palliative external-beam radiation is recommended for patients who present with diffuse distant metastasis to the brain ",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1359784,
"choiceText": "External-beam radiation and stereotactic ablative radiotherapy are preferred to re-resection in patients with recurrent lung cancer, even if the recurrence is resectable",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1359786,
"choiceText": "SVC stenting is required in all patients with lung cancer who have an SVC obstruction ",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "If a patient presents with diffuse distant metastasis to the brain, the recommendation is to pursue palliative external-beam radiation. If lesions are limited, options include stereotactic radiosurgery alone or surgical resection; if symptomatic or warranted for diagnosis, this is followed by stereotactic radiosurgery or whole-brain radiotherapy.<br><br>\r\n\r\nIf a patient presents with mediastinal lymph node recurrence without prior radiation, the NCCN recommends concurrent chemoradiation. If a recurrence is resectable, the preferred treatment is re-resection; however, external-beam radiation or stereotactic ablative radiotherapy are also options. If a patient presents with SVC obstruction, several options are available, including concurrent chemoradiation with or without SVC stenting, external-beam radiation with or without SVC stenting, or SVC stenting alone.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 433867,
"questionText": "Which of the following is most accurate regarding the treatment of NSCLC and related metastasis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 64-Year-Old Man Unable to Lift His Arm or Walk"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1359764,
"choiceText": "Sarcoidosis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1359766,
"choiceText": "Brain abscess",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1359768,
"choiceText": "Primary tumor of the brain",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1359770,
"choiceText": "Lung cancer metastasis to the brain",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 433863,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1359772,
"choiceText": "Most patients with lung cancer present with early-stage disease",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1359774,
"choiceText": "Narrowing of the mediastinum on chest radiography is a common finding of concern in patients with lung cancer",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1359776,
"choiceText": "Some treatments for Hodgkin lymphoma are associated with a significantly increased risk for lung cancer",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1359778,
"choiceText": "Paraneoplastic syndromes occur only with SCLC, as opposed to NSCLC",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Radiation therapy, used to treat such cancers as Hodgkin lymphoma, carries a risk for lung cancer that should be addressed with patients. A review of the literature on long-term complications of the treatment of Hodgkin lymphoma showed that the risk for lung cancer was increased twofold to sevenfold in patients who received radiation therapy.<sup>[7]</sup><br><br>\r\n\r\nMost patients with lung cancer present with advanced-stage disease. Concerning findings on chest radiography include pulmonary masses, nodules, effusions, or infiltrates. Widening of the mediastinum as well as hilar enlargement and atelectasis are other findings of concern. A wide variety of paraneoplastic syndromes are observed in association with NSCLC. Symptoms may include Trousseau syndrome of hypercoagulability, SIADH, clubbing, and hypertrophic osteoarthropathy.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 433865,
"questionText": "Which of the following is most accurate regarding lung cancer risk factors, presentation, and diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1359780,
"choiceText": "Chemoradiation is contraindicated in patients with mediastinal lymph node recurrence without prior radiation",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1359782,
"choiceText": "Palliative external-beam radiation is recommended for patients who present with diffuse distant metastasis to the brain ",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1359784,
"choiceText": "External-beam radiation and stereotactic ablative radiotherapy are preferred to re-resection in patients with recurrent lung cancer, even if the recurrence is resectable",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1359786,
"choiceText": "SVC stenting is required in all patients with lung cancer who have an SVC obstruction ",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "If a patient presents with diffuse distant metastasis to the brain, the recommendation is to pursue palliative external-beam radiation. If lesions are limited, options include stereotactic radiosurgery alone or surgical resection; if symptomatic or warranted for diagnosis, this is followed by stereotactic radiosurgery or whole-brain radiotherapy.<br><br>\r\n\r\nIf a patient presents with mediastinal lymph node recurrence without prior radiation, the NCCN recommends concurrent chemoradiation. If a recurrence is resectable, the preferred treatment is re-resection; however, external-beam radiation or stereotactic ablative radiotherapy are also options. If a patient presents with SVC obstruction, several options are available, including concurrent chemoradiation with or without SVC stenting, external-beam radiation with or without SVC stenting, or SVC stenting alone.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 433867,
"questionText": "Which of the following is most accurate regarding the treatment of NSCLC and related metastasis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
825725 | /viewarticle/825725 | [
{
"authors": "Caroline Tschibelu, MD",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to recognize accurately. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"A 29-year-old woman is referred to a rheumatologist for multiple joint and muscle pain, back pain, and increasing fatigue. Her wrists are painful at the end of the day whenever she performs repetitive movements in her job as a research assistant. The patient states that her symptoms started over five years ago with the sudden onset of sharp, left shoulder pain, which she attributed to carrying her shoulder bag. She noted gradual radiation of the pain to both arms and worsening of symptoms with cold temperature. Her primary care provider (PCP) recommended over-the-counter analgesics as needed, to relieve the pain, and these resolved the symptoms at the time.",
"However, the shoulder pain has returned and is associated with diffuse muscle pain. The patient has also recently developed occasional bilateral hip pain and has noticed ascending numbness from her feet whenever she sits down for prolonged periods of time. The numbness usually resolves whenever she stands up. She has become increasingly fatigued and has trouble falling and staying asleep. The patient also reports difficulty with memory and concentration, as well as feelings of depression. She denies having burning or tingling sensations, or urinary or fecal incontinence.",
"The remainder of the review of system is unremarkable. Her medical history includes well-controlled asthma, eczema, seasonal allergies, and endometriosis. She had an appendectomy as a child. Her medications include albuterol as needed, as well as formoterol, fexofenadine, acetaminophen, and a cortisone cream."
],
"date": "May 08, 2019",
"figures": [],
"markdown": "# A 29-Year-Old Woman With Worsening Pain and Memory Issues\n\n **Authors:** Caroline Tschibelu, MD \n **Date:** May 08, 2019\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to recognize accurately. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nA 29-year-old woman is referred to a rheumatologist for multiple joint and muscle pain, back pain, and increasing fatigue. Her wrists are painful at the end of the day whenever she performs repetitive movements in her job as a research assistant. The patient states that her symptoms started over five years ago with the sudden onset of sharp, left shoulder pain, which she attributed to carrying her shoulder bag. She noted gradual radiation of the pain to both arms and worsening of symptoms with cold temperature. Her primary care provider (PCP) recommended over-the-counter analgesics as needed, to relieve the pain, and these resolved the symptoms at the time.\nHowever, the shoulder pain has returned and is associated with diffuse muscle pain. The patient has also recently developed occasional bilateral hip pain and has noticed ascending numbness from her feet whenever she sits down for prolonged periods of time. The numbness usually resolves whenever she stands up. She has become increasingly fatigued and has trouble falling and staying asleep. The patient also reports difficulty with memory and concentration, as well as feelings of depression. She denies having burning or tingling sensations, or urinary or fecal incontinence.\nThe remainder of the review of system is unremarkable. Her medical history includes well-controlled asthma, eczema, seasonal allergies, and endometriosis. She had an appendectomy as a child. Her medications include albuterol as needed, as well as formoterol, fexofenadine, acetaminophen, and a cortisone cream.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 29-Year-Old Woman With Worsening Pain and Memory Issues"
},
{
"authors": "Caroline Tschibelu, MD",
"content": [
"On physical exam, the patient is found to be a thin, tall woman in no acute distress. Her vital signs are within normal limits. Her cranial nerves are grossly intact, and her pupils are 3 mm, equal, and reactive bilaterally. Her reflexes are 2+ throughout. No Romberg sign or pronator drift is noted. Her strength is 4/5 in the upper extremities and 5/5 in the lower extremities. Her gait and range of motion for all extremities are normal. No deformities, swelling, or erythema over the joints are observed.",
"The examination findings are remarkable for extreme tenderness to palpation when pressure is applied over multiple areas of the body, including the occiput; the fourth through sixth intertransverse spaces of the cervical spine; bilaterally down the scapular, supraspinatus and trapezius muscles; and bilaterally on the medial aspect of both knees.",
"Her PCP performs a workup before referral. The patient's complete blood count (CBC), basic metabolic panel (BMP), and urinalysis (UA) are unremarkable. Her erythrocyte sedimentation rate (ESR) is 26 mm/h (reference range = 0-20 mm/h). Her C-reactive protein (CRP) level is 0.5 mg/dL (reference range = 0.0-0.8 mg/dL). Her thyroid stimulating hormone (TSH) level is 4 µU/mL (reference range = 0.5-5.0 µU/mL). Her free thyroxine (FT4) and free triiodothyronine (FT3) levels are within normal limits. Her creatine kinase level is within the reference range. Her antinuclear antibody (ANA) titer is 1:160 (low-positive range = 1:40 to 1:60). Her rheumatoid factor (RF) test result is positive."
],
"date": "May 08, 2019",
"figures": [],
"markdown": "# A 29-Year-Old Woman With Worsening Pain and Memory Issues\n\n **Authors:** Caroline Tschibelu, MD \n **Date:** May 08, 2019\n\n ## Content\n\n On physical exam, the patient is found to be a thin, tall woman in no acute distress. Her vital signs are within normal limits. Her cranial nerves are grossly intact, and her pupils are 3 mm, equal, and reactive bilaterally. Her reflexes are 2+ throughout. No Romberg sign or pronator drift is noted. Her strength is 4/5 in the upper extremities and 5/5 in the lower extremities. Her gait and range of motion for all extremities are normal. No deformities, swelling, or erythema over the joints are observed.\nThe examination findings are remarkable for extreme tenderness to palpation when pressure is applied over multiple areas of the body, including the occiput; the fourth through sixth intertransverse spaces of the cervical spine; bilaterally down the scapular, supraspinatus and trapezius muscles; and bilaterally on the medial aspect of both knees.\nHer PCP performs a workup before referral. The patient's complete blood count (CBC), basic metabolic panel (BMP), and urinalysis (UA) are unremarkable. Her erythrocyte sedimentation rate (ESR) is 26 mm/h (reference range = 0-20 mm/h). Her C-reactive protein (CRP) level is 0.5 mg/dL (reference range = 0.0-0.8 mg/dL). Her thyroid stimulating hormone (TSH) level is 4 µU/mL (reference range = 0.5-5.0 µU/mL). Her free thyroxine (FT4) and free triiodothyronine (FT3) levels are within normal limits. Her creatine kinase level is within the reference range. Her antinuclear antibody (ANA) titer is 1:160 (low-positive range = 1:40 to 1:60). Her rheumatoid factor (RF) test result is positive.\n\n ## Figures\n\n \n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 731703,
"choiceText": "Rheumatoid arthritis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 731705,
"choiceText": "Systemic lupus erythematosus",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 731707,
"choiceText": "Ankylosing spondylitis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 731709,
"choiceText": "Fibromyalgia",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 731711,
"choiceText": "Myositis",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 731713,
"choiceText": "Hypothyroidism",
"correct": false,
"displayOrder": 6,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 228653,
"questionText": "Based on the history, physical examination, and workup, which of the following is the likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 29-Year-Old Woman With Worsening Pain and Memory Issues"
},
{
"authors": "Caroline Tschibelu, MD",
"content": [
"The diagnosis of fibromyalgia (FM), a chronic disorder characterized by widespread pain and tenderness, was made clinically based on the characteristic medical history and physical examination findings.",
"Fibromyalgia affects approximately 5 million Americans, 80%-90% of those who are diagnosed with the disease are women aged 20-55 years.[1] The diagnosis of fibromyalgia should be made based on the specific criteria for the disease and the patient's presentation, as well as on exclusion of differential diagnoses.[2] For the condition to be diagnosed, chronic symptoms must have been present for over three months. Widespread pain and tenderness must be reported both above and below the waist, as well as along the axial spine bilaterally. Associated symptoms include fatigue, sleep disturbances, and cognitive or mood disorders that result in impairment of activities of daily living (ADL).[3] Patients have a lifetime prevalence of depression and anxiety of 74% and 60%, respectively.",
"The etiology of FM is still being elucidated; however, it is multifactorial (Figure 1). The condition seems to involve disordered central pain processing and hypersensitivity that leads to a lower threshold of pain, heat, cold, and other stimuli that register as pain.",
"Figure 1.",
"Quantitative sensory studies using various stimuli and functional magnetic resonance imaging (fMRI) evaluated the pattern of cerebral activation in patients with fibromyalgia compared with controls. The results suggested that fibromyalgia is associated with cortical and subcortical augmentation processing.[4] Pain-processing abnormalities suggested in FM include hypersensitivity of N-methyl-D-aspartate (NMDA) receptors, dysregulation of dopaminergic neurotransmission[5] and of the hypothalamic-pituitary axis, decreased levels of inhibitory neurotransmitters (serotonin, norepinephrine), and increased levels of excitatory neurotransmitters (substance P, glutamate).[6,7]",
"The American College of Rheumatology published criteria that allow physicians to diagnose fibromyalgia with a carefully taken history in addition to a physical examination, without the need for specialized training.[8] The criteria include a Widespread Pain Index (WPI) and a Symptom Severity Index (SSI). The WPI documents whether the patient has had pain or tenderness in 19 different areas of the body over the previous week and quantifies the pain on a scale of 0-19. (Figure 2 shows tender points.)",
"Figure 2.",
"The SSI quantifies severity of fatigue, trouble sleeping, and memory impairment on a scale of 0-12. A diagnosis of fibromyalgia is made if symptoms have been present for at least three months, the WPI score is 7 or higher, and the SSI score is 5 or higher.",
"The initial workup should include a CBC, BMP, and UA, as well as thyroid screening to rule out hypothyroidism as the etiology of the fatigue. Those test values are usually within normal limits in fibromyalgia. Based on the patient's clinical presentation, clinicians should also consider that the patient is suffering from low levels of vitamin D (which cause muscle pain and depression), vitamin B-12 (which cause pain and fatigue), iron (which cause fatigue and restless leg syndrome), or magnesium (which cause fatigue). Imaging modalities are not necessary in the diagnosis of fibromyalgia."
],
"date": "May 08, 2019",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/825/725/825725-thumb-1.jpg"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/825/725/825725-thumb-2.jpg"
}
],
"markdown": "# A 29-Year-Old Woman With Worsening Pain and Memory Issues\n\n **Authors:** Caroline Tschibelu, MD \n **Date:** May 08, 2019\n\n ## Content\n\n The diagnosis of fibromyalgia (FM), a chronic disorder characterized by widespread pain and tenderness, was made clinically based on the characteristic medical history and physical examination findings.\nFibromyalgia affects approximately 5 million Americans, 80%-90% of those who are diagnosed with the disease are women aged 20-55 years.[1] The diagnosis of fibromyalgia should be made based on the specific criteria for the disease and the patient's presentation, as well as on exclusion of differential diagnoses.[2] For the condition to be diagnosed, chronic symptoms must have been present for over three months. Widespread pain and tenderness must be reported both above and below the waist, as well as along the axial spine bilaterally. Associated symptoms include fatigue, sleep disturbances, and cognitive or mood disorders that result in impairment of activities of daily living (ADL).[3] Patients have a lifetime prevalence of depression and anxiety of 74% and 60%, respectively.\nThe etiology of FM is still being elucidated; however, it is multifactorial (Figure 1). The condition seems to involve disordered central pain processing and hypersensitivity that leads to a lower threshold of pain, heat, cold, and other stimuli that register as pain.\nFigure 1.\nQuantitative sensory studies using various stimuli and functional magnetic resonance imaging (fMRI) evaluated the pattern of cerebral activation in patients with fibromyalgia compared with controls. The results suggested that fibromyalgia is associated with cortical and subcortical augmentation processing.[4] Pain-processing abnormalities suggested in FM include hypersensitivity of N-methyl-D-aspartate (NMDA) receptors, dysregulation of dopaminergic neurotransmission[5] and of the hypothalamic-pituitary axis, decreased levels of inhibitory neurotransmitters (serotonin, norepinephrine), and increased levels of excitatory neurotransmitters (substance P, glutamate).[6,7]\nThe American College of Rheumatology published criteria that allow physicians to diagnose fibromyalgia with a carefully taken history in addition to a physical examination, without the need for specialized training.[8] The criteria include a Widespread Pain Index (WPI) and a Symptom Severity Index (SSI). The WPI documents whether the patient has had pain or tenderness in 19 different areas of the body over the previous week and quantifies the pain on a scale of 0-19. (Figure 2 shows tender points.)\nFigure 2.\nThe SSI quantifies severity of fatigue, trouble sleeping, and memory impairment on a scale of 0-12. A diagnosis of fibromyalgia is made if symptoms have been present for at least three months, the WPI score is 7 or higher, and the SSI score is 5 or higher.\nThe initial workup should include a CBC, BMP, and UA, as well as thyroid screening to rule out hypothyroidism as the etiology of the fatigue. Those test values are usually within normal limits in fibromyalgia. Based on the patient's clinical presentation, clinicians should also consider that the patient is suffering from low levels of vitamin D (which cause muscle pain and depression), vitamin B-12 (which cause pain and fatigue), iron (which cause fatigue and restless leg syndrome), or magnesium (which cause fatigue). Imaging modalities are not necessary in the diagnosis of fibromyalgia.\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n\n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 731703,
"choiceText": "Rheumatoid arthritis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 731705,
"choiceText": "Systemic lupus erythematosus",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 731707,
"choiceText": "Ankylosing spondylitis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 731709,
"choiceText": "Fibromyalgia",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 731711,
"choiceText": "Myositis",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 731713,
"choiceText": "Hypothyroidism",
"correct": false,
"displayOrder": 6,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 228653,
"questionText": "Based on the history, physical examination, and workup, which of the following is the likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 29-Year-Old Woman With Worsening Pain and Memory Issues"
},
{
"authors": "Caroline Tschibelu, MD",
"content": [
"Complete and thorough management of the patient implies that fibromyalgia should be high in the differential when a patient has a complaint of widespread pain; however, other possible diagnoses should be excluded if any are clinically suspected.[9] Rheumatic diseases such as systemic lupus erythematosus (SLE), rheumatoid arthritis,[10,11] and Sjögren syndrome (SS) also present with generalized fatigue, arthralgias, and myalgias. Additionally, the prevalence of these diseases is higher among patients with fibromyalgia than it is in the general population.[12,13] However, certain characteristics are more specific to these diseases and are not found in patients with fibromyalgia alone.",
"Serologic tests such as ANA and RF are not useful for differentiating these conditions from fibromyalgia. A prospective study conducted by Dinerman et al evaluated 118 patients with fibromyalgia and found that 14% had a positive ANA finding;[14] however, 5%-10% of healthy women also have a positive ANA finding usually with low titers of the antibody. Similarly, RF is not specific to RA only and is positive in 5% of the general healthy population. Furthermore, a positive RF test result does not suggest the diagnosis of RA if the patient, like the one in this case, does not have signs and symptoms of RA; these primarily include painful, swollen joints or evidence of prior active arthritis, including limited range of motion or radiographic changes. More specific laboratory findings include positive anticyclic citrullinated peptide (anti-CCP) and anti–mutated citrullinated vimentin (anti-MCV) test results. These findings more strongly suggest RA or pre-RA; however, RA cannot be diagnosed in the absence of objective joint inflammation.",
"Patients with SLE may present with a facial butterfly rash; more specific serologic tests, such as anti-ds-DNA and anti-Sm antibodies, should be obtained if clinical suspicion for this disease is high.[15]",
"Patients with SS present with complaints of xerophthalmia and xerostomia (dry mouth and eyes). In addition, serologic test results that are positive for antibodies to anti-SS-A and anti-SS-B are indicative of the disease.[16]",
"Ankylosing spondylitis may present with fatigue and stiffness, but patients have limited axial spine mobility, as well as the characteristic \"bamboo spine\" and fused sacroiliac joints findings on radiography. The inflammatory changes that affect the axial spine can be diagnosed earlier with MRI.[17]",
"Patients with myositis will present with myalgias, but their creatine kinase levels will be elevated. Additional considerations in the differential diagnosis of fibromyalgia that may need to be excluded with specific lab tests or imaging include multiple sclerosis, polymyalgia rheumatica, Lyme disease and other chronic infections (eg, mononucleosis, hepatitis), endocrine disorders (Cushing syndrome, hyperparathyroidism), and myofascial syndrome."
],
"date": "May 08, 2019",
"figures": [],
"markdown": "# A 29-Year-Old Woman With Worsening Pain and Memory Issues\n\n **Authors:** Caroline Tschibelu, MD \n **Date:** May 08, 2019\n\n ## Content\n\n Complete and thorough management of the patient implies that fibromyalgia should be high in the differential when a patient has a complaint of widespread pain; however, other possible diagnoses should be excluded if any are clinically suspected.[9] Rheumatic diseases such as systemic lupus erythematosus (SLE), rheumatoid arthritis,[10,11] and Sjögren syndrome (SS) also present with generalized fatigue, arthralgias, and myalgias. Additionally, the prevalence of these diseases is higher among patients with fibromyalgia than it is in the general population.[12,13] However, certain characteristics are more specific to these diseases and are not found in patients with fibromyalgia alone.\nSerologic tests such as ANA and RF are not useful for differentiating these conditions from fibromyalgia. A prospective study conducted by Dinerman et al evaluated 118 patients with fibromyalgia and found that 14% had a positive ANA finding;[14] however, 5%-10% of healthy women also have a positive ANA finding usually with low titers of the antibody. Similarly, RF is not specific to RA only and is positive in 5% of the general healthy population. Furthermore, a positive RF test result does not suggest the diagnosis of RA if the patient, like the one in this case, does not have signs and symptoms of RA; these primarily include painful, swollen joints or evidence of prior active arthritis, including limited range of motion or radiographic changes. More specific laboratory findings include positive anticyclic citrullinated peptide (anti-CCP) and anti–mutated citrullinated vimentin (anti-MCV) test results. These findings more strongly suggest RA or pre-RA; however, RA cannot be diagnosed in the absence of objective joint inflammation.\nPatients with SLE may present with a facial butterfly rash; more specific serologic tests, such as anti-ds-DNA and anti-Sm antibodies, should be obtained if clinical suspicion for this disease is high.[15]\nPatients with SS present with complaints of xerophthalmia and xerostomia (dry mouth and eyes). In addition, serologic test results that are positive for antibodies to anti-SS-A and anti-SS-B are indicative of the disease.[16]\nAnkylosing spondylitis may present with fatigue and stiffness, but patients have limited axial spine mobility, as well as the characteristic \"bamboo spine\" and fused sacroiliac joints findings on radiography. The inflammatory changes that affect the axial spine can be diagnosed earlier with MRI.[17]\nPatients with myositis will present with myalgias, but their creatine kinase levels will be elevated. Additional considerations in the differential diagnosis of fibromyalgia that may need to be excluded with specific lab tests or imaging include multiple sclerosis, polymyalgia rheumatica, Lyme disease and other chronic infections (eg, mononucleosis, hepatitis), endocrine disorders (Cushing syndrome, hyperparathyroidism), and myofascial syndrome.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 29-Year-Old Woman With Worsening Pain and Memory Issues"
},
{
"authors": "Caroline Tschibelu, MD",
"content": [
"Fibromyalgia is best managed using a patient-centered, multidisciplinary approach initiated in the primary care setting.[18] Multiple studies have shown that the use of a combination of pharmacologic and nonpharmacologic therapies is the most effective approach to treatment and helps to control symptoms for many patients.[19,20] The goal is to reduce symptoms, improve quality of life, and help patients to regain the ability to perform ADL. Acknowledging the disease and showing empathy to patients suffering from fibromyalgia is important, as studies have shown that patients use health care resources to a lesser extent after they have been diagnosed and their illness, they feel, has been validated.[21,22] Patients should be well educated about their diagnosis and the active role they have in managing their symptoms, and should understand that fibromyalgia is a chronic condition that requires long-term management.",
"Important nonpharmacologic therapies include education on good sleep hygiene and a customized exercise plan. Exercise has been shown to decrease pain and help with general well-being.[23] Several studies have reviewed the efficacy of cardiovascular training, low-impact aerobic activities, and yoga, among other types of exercise.[24] Patients should be encouraged to gradually increase the amount of time that they exercise.",
"Drug monotherapy should be initiated if the nonpharmacologic approach is not sufficient. The medications that have shown efficacy for chronic pain include antidepressants and anticonvulsants.[25] Tricyclic antidepressants (TCA) are usually initiated first at a low dose taken at bedtime and titrated up as tolerated. They are effective at reducing pain, fatigue, and sleep disturbances.[26] They are also relatively inexpensive compared with other classes of medications. Most patients with fibromyalgia are started on amitriptyline; however, desipramine is an alternative tricyclic that has fewer anticholinergic side effects and less cardiotoxicity. For patients with moderate symptoms, cyclobenzaprine, a tricyclic-related medication and muscle relaxant, may be an acceptable alternative,[27] but it has minimal antidepressant effect.",
"Patients who do not respond to tricyclics or those who have depression may benefit from selective serotonin and norepinephrine reuptake inhibitors such as duloxetine,[28] venlafaxine, and milnacipran.[29,30] Duloxetine and milnacipran are approved for fibromyalgia; duloxetine has shown the most efficacy in clinical trials.",
"Anticonvulsants that have shown benefits for chronic pain include gabapentin and pregabalin; these are calcium channel modulators that block the release of neurotransmitters, thereby exerting their analgesic effects. They are also preferred in patients with severe sleep problems. In a 2009 meta-analysis by Hauser, five randomized, controlled trials on the treatment of fibromyalgia with gabapentin and pregabalin were reviewed and showed evidence of pain reduction, improved sleep, and improved health-related quality of life.[31]",
"Non-steroidal anti-inflammatory drugs (NSAIDs) and acetaminophen have not been shown to be very effective for chronic fibromyalgia pain but can be used to manage acute flare-ups or nociceptive pain from comorbidities such as arthritis. Based on a few studies, tramadol is an analgesic that effectively improves chronic fibromyalgia pain. This may be due to its combined effect on mu opioid receptors and inhibitory effects on serotonin and norepinephrine reuptake;[32,33] however, tramadol is a narcotic and is addictive. Given the risk of addiction in patients with fibromyalgia, it should be used with caution, in limited doses, and only intermittently.",
"If symptoms persist with a single pharmacotherapy at the highest dose tolerated by the patient, a combination of drugs should be considered. Patients should also be referred to a rheumatologist or to other specialists, to manage a complex drug regimen or comorbidities. Depression should be treated aggressively, and patients should see a psychiatrist or may benefit from psychological intervention, including cognitive behavioral therapy, to help manage stress and anxiety. Additional treatments that can be considered include trigger point injections, acupuncture, physical therapy, and chiropractic treatment.",
"The patient in this case was started on low-dose amitriptyline at bedtime and tramadol as needed. She started exercising regularly and occasionally wears appropriate splints, applied as needed to relieve wrist pain. The patient reports that current management has improved her symptoms by approximately 80%, and her symptoms are well controlled most of the time."
],
"date": "May 08, 2019",
"figures": [],
"markdown": "# A 29-Year-Old Woman With Worsening Pain and Memory Issues\n\n **Authors:** Caroline Tschibelu, MD \n **Date:** May 08, 2019\n\n ## Content\n\n Fibromyalgia is best managed using a patient-centered, multidisciplinary approach initiated in the primary care setting.[18] Multiple studies have shown that the use of a combination of pharmacologic and nonpharmacologic therapies is the most effective approach to treatment and helps to control symptoms for many patients.[19,20] The goal is to reduce symptoms, improve quality of life, and help patients to regain the ability to perform ADL. Acknowledging the disease and showing empathy to patients suffering from fibromyalgia is important, as studies have shown that patients use health care resources to a lesser extent after they have been diagnosed and their illness, they feel, has been validated.[21,22] Patients should be well educated about their diagnosis and the active role they have in managing their symptoms, and should understand that fibromyalgia is a chronic condition that requires long-term management.\nImportant nonpharmacologic therapies include education on good sleep hygiene and a customized exercise plan. Exercise has been shown to decrease pain and help with general well-being.[23] Several studies have reviewed the efficacy of cardiovascular training, low-impact aerobic activities, and yoga, among other types of exercise.[24] Patients should be encouraged to gradually increase the amount of time that they exercise.\nDrug monotherapy should be initiated if the nonpharmacologic approach is not sufficient. The medications that have shown efficacy for chronic pain include antidepressants and anticonvulsants.[25] Tricyclic antidepressants (TCA) are usually initiated first at a low dose taken at bedtime and titrated up as tolerated. They are effective at reducing pain, fatigue, and sleep disturbances.[26] They are also relatively inexpensive compared with other classes of medications. Most patients with fibromyalgia are started on amitriptyline; however, desipramine is an alternative tricyclic that has fewer anticholinergic side effects and less cardiotoxicity. For patients with moderate symptoms, cyclobenzaprine, a tricyclic-related medication and muscle relaxant, may be an acceptable alternative,[27] but it has minimal antidepressant effect.\nPatients who do not respond to tricyclics or those who have depression may benefit from selective serotonin and norepinephrine reuptake inhibitors such as duloxetine,[28] venlafaxine, and milnacipran.[29,30] Duloxetine and milnacipran are approved for fibromyalgia; duloxetine has shown the most efficacy in clinical trials.\nAnticonvulsants that have shown benefits for chronic pain include gabapentin and pregabalin; these are calcium channel modulators that block the release of neurotransmitters, thereby exerting their analgesic effects. They are also preferred in patients with severe sleep problems. In a 2009 meta-analysis by Hauser, five randomized, controlled trials on the treatment of fibromyalgia with gabapentin and pregabalin were reviewed and showed evidence of pain reduction, improved sleep, and improved health-related quality of life.[31]\nNon-steroidal anti-inflammatory drugs (NSAIDs) and acetaminophen have not been shown to be very effective for chronic fibromyalgia pain but can be used to manage acute flare-ups or nociceptive pain from comorbidities such as arthritis. Based on a few studies, tramadol is an analgesic that effectively improves chronic fibromyalgia pain. This may be due to its combined effect on mu opioid receptors and inhibitory effects on serotonin and norepinephrine reuptake;[32,33] however, tramadol is a narcotic and is addictive. Given the risk of addiction in patients with fibromyalgia, it should be used with caution, in limited doses, and only intermittently.\nIf symptoms persist with a single pharmacotherapy at the highest dose tolerated by the patient, a combination of drugs should be considered. Patients should also be referred to a rheumatologist or to other specialists, to manage a complex drug regimen or comorbidities. Depression should be treated aggressively, and patients should see a psychiatrist or may benefit from psychological intervention, including cognitive behavioral therapy, to help manage stress and anxiety. Additional treatments that can be considered include trigger point injections, acupuncture, physical therapy, and chiropractic treatment.\nThe patient in this case was started on low-dose amitriptyline at bedtime and tramadol as needed. She started exercising regularly and occasionally wears appropriate splints, applied as needed to relieve wrist pain. The patient reports that current management has improved her symptoms by approximately 80%, and her symptoms are well controlled most of the time.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 731715,
"choiceText": "Venlafaxine",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 731717,
"choiceText": "Tramadol",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 731719,
"choiceText": "Duloxetine ",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 731721,
"choiceText": "Desipramine",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 731723,
"choiceText": "Milnacipran",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Desipramine is an alternative tricyclic that has fewer anticholinergic side effects and less cardiotoxicity than amitriptyline.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 228655,
"questionText": "A 45-year-old patient with Sjögren syndrome is seen by her PCP with complaints of fatigue, widespread pain, sleep disturbances, and difficulty concentrating at work, for the past 6 months. Which of the following is the most appropriate initial therapy for this patient?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 731725,
"choiceText": "Acetaminophen",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 731727,
"choiceText": "Prednisone course",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 731729,
"choiceText": "Cyclobenzaprine",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 731731,
"choiceText": "Desipramine",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 731733,
"choiceText": "Duloxetine",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 731735,
"choiceText": "Gabapentin",
"correct": false,
"displayOrder": 6,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Patients who do not respond to tricyclics or those who have depression may benefit from selective serotonin and norepinephrine reuptake inhibitors such as duloxetine, venlafaxine, and milnacipran. Duloxetine and milnacipran are approved for fibromyalgia; duloxetine has shown the most efficacy in clinical trials.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 228657,
"questionText": "A 32-year-old patient who was diagnosed with fibromyalgia one year ago presents to her rheumatologist stating that she still feels depressed. She also feels as if her pain is not well controlled anymore, as she has been using tramadol more often than usual. She tries to exercise regularly and is currently on amitriptyline 75 mg. She does not have any other comorbidities. Which of the following is most likely to be beneficial for this patient and an appropriate replacement for amitriptyline?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 29-Year-Old Woman With Worsening Pain and Memory Issues"
},
{
"authors": "Caroline Tschibelu, MD",
"content": [],
"date": "May 08, 2019",
"figures": [],
"markdown": "# A 29-Year-Old Woman With Worsening Pain and Memory Issues\n\n **Authors:** Caroline Tschibelu, MD \n **Date:** May 08, 2019\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 731715,
"choiceText": "Venlafaxine",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 731717,
"choiceText": "Tramadol",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 731719,
"choiceText": "Duloxetine ",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 731721,
"choiceText": "Desipramine",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 731723,
"choiceText": "Milnacipran",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Desipramine is an alternative tricyclic that has fewer anticholinergic side effects and less cardiotoxicity than amitriptyline.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 228655,
"questionText": "A 45-year-old patient with Sjögren syndrome is seen by her PCP with complaints of fatigue, widespread pain, sleep disturbances, and difficulty concentrating at work, for the past 6 months. Which of the following is the most appropriate initial therapy for this patient?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 731725,
"choiceText": "Acetaminophen",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 731727,
"choiceText": "Prednisone course",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 731729,
"choiceText": "Cyclobenzaprine",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 731731,
"choiceText": "Desipramine",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 731733,
"choiceText": "Duloxetine",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 731735,
"choiceText": "Gabapentin",
"correct": false,
"displayOrder": 6,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Patients who do not respond to tricyclics or those who have depression may benefit from selective serotonin and norepinephrine reuptake inhibitors such as duloxetine, venlafaxine, and milnacipran. Duloxetine and milnacipran are approved for fibromyalgia; duloxetine has shown the most efficacy in clinical trials.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 228657,
"questionText": "A 32-year-old patient who was diagnosed with fibromyalgia one year ago presents to her rheumatologist stating that she still feels depressed. She also feels as if her pain is not well controlled anymore, as she has been using tramadol more often than usual. She tries to exercise regularly and is currently on amitriptyline 75 mg. She does not have any other comorbidities. Which of the following is most likely to be beneficial for this patient and an appropriate replacement for amitriptyline?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 29-Year-Old Woman With Worsening Pain and Memory Issues"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 731703,
"choiceText": "Rheumatoid arthritis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 731705,
"choiceText": "Systemic lupus erythematosus",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 731707,
"choiceText": "Ankylosing spondylitis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 731709,
"choiceText": "Fibromyalgia",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 731711,
"choiceText": "Myositis",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 731713,
"choiceText": "Hypothyroidism",
"correct": false,
"displayOrder": 6,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 228653,
"questionText": "Based on the history, physical examination, and workup, which of the following is the likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 731715,
"choiceText": "Venlafaxine",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 731717,
"choiceText": "Tramadol",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 731719,
"choiceText": "Duloxetine ",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 731721,
"choiceText": "Desipramine",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 731723,
"choiceText": "Milnacipran",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Desipramine is an alternative tricyclic that has fewer anticholinergic side effects and less cardiotoxicity than amitriptyline.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 228655,
"questionText": "A 45-year-old patient with Sjögren syndrome is seen by her PCP with complaints of fatigue, widespread pain, sleep disturbances, and difficulty concentrating at work, for the past 6 months. Which of the following is the most appropriate initial therapy for this patient?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 731725,
"choiceText": "Acetaminophen",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 731727,
"choiceText": "Prednisone course",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 731729,
"choiceText": "Cyclobenzaprine",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 731731,
"choiceText": "Desipramine",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 731733,
"choiceText": "Duloxetine",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 731735,
"choiceText": "Gabapentin",
"correct": false,
"displayOrder": 6,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Patients who do not respond to tricyclics or those who have depression may benefit from selective serotonin and norepinephrine reuptake inhibitors such as duloxetine, venlafaxine, and milnacipran. Duloxetine and milnacipran are approved for fibromyalgia; duloxetine has shown the most efficacy in clinical trials.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 228657,
"questionText": "A 32-year-old patient who was diagnosed with fibromyalgia one year ago presents to her rheumatologist stating that she still feels depressed. She also feels as if her pain is not well controlled anymore, as she has been using tramadol more often than usual. She tries to exercise regularly and is currently on amitriptyline 75 mg. She does not have any other comorbidities. Which of the following is most likely to be beneficial for this patient and an appropriate replacement for amitriptyline?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
912292 | /viewarticle/912292 | [
{
"authors": "Heidi Moawad, MD",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"A 31-year-old woman has noticed intermittent tremors in her right hand and arm over the past month. She is left-handed and says that she has noticed that her right arm shakes when she carries grocery bags in each hand. She also says that when she carries large or heavy objects using both hands, her right arm begins to shake even though her left arm is doing most of the lifting. She occasionally develops a fine tremor in her right hand when she drives.",
"She is otherwise in good health and has not needed to see a doctor in years. About a year before her current appointment, she was scheduled to see a doctor but canceled because she got busy and her symptoms resolved. When describing her symptoms at that time, she explains that she had low energy and felt sleepy almost every day after work for several months. This especially bothered her because her bout of low energy occurred during the summer, when she likes to play tennis outdoors. When she regained her energy after a few months, she resumed playing tennis in the fall at an indoor club that she joins for the cold months. Her current tremor has not been a problem for her when she plays tennis, but she says that she has been using her left arm more than usual when she plays."
],
"date": "May 01, 2019",
"figures": [],
"markdown": "# A 31-Year-Old Woman With Right Arm Tremors\n\n **Authors:** Heidi Moawad, MD \n **Date:** May 01, 2019\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nA 31-year-old woman has noticed intermittent tremors in her right hand and arm over the past month. She is left-handed and says that she has noticed that her right arm shakes when she carries grocery bags in each hand. She also says that when she carries large or heavy objects using both hands, her right arm begins to shake even though her left arm is doing most of the lifting. She occasionally develops a fine tremor in her right hand when she drives.\nShe is otherwise in good health and has not needed to see a doctor in years. About a year before her current appointment, she was scheduled to see a doctor but canceled because she got busy and her symptoms resolved. When describing her symptoms at that time, she explains that she had low energy and felt sleepy almost every day after work for several months. This especially bothered her because her bout of low energy occurred during the summer, when she likes to play tennis outdoors. When she regained her energy after a few months, she resumed playing tennis in the fall at an indoor club that she joins for the cold months. Her current tremor has not been a problem for her when she plays tennis, but she says that she has been using her left arm more than usual when she plays.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 31-Year-Old Woman With Right Arm Tremors"
},
{
"authors": "Heidi Moawad, MD",
"content": [
"Upon physical examination, the patient is alert, oriented, and cooperative and does not appear to be in distress. Her affect is normal, and her memory and cognitive functioning is normal.",
"She does not have any rashes, lesions, or discoloration. Her pulse is regular, and her respiratory, cardiac, and abdominal examinations are unremarkable.",
"She does not have any facial asymmetry. Her extraocular movements are full, and she does not have diplopia. She has saccades in both eyes when looking toward the right and left, but not while at rest. Her pupils are equal, round, and reactive to light.",
"Her language and speech are coherent and fluent without any defects. Her strength is slightly decreased in the right biceps and triceps. Otherwise, her motor strength is 5/5, including her right hand. She did not have tremors or involuntary movements during the physical examination.",
"Her muscle tone is slightly diminished in her right upper extremity and is otherwise normal. Her reflexes are normal, with the exception of slightly brisk reflexes in the right upper extremity. Her coordination is impaired. She demonstrates dysmetria on finger-to-nose testing when using her right hand, but her left hand is normal. Similarly, rapid alternating movement test results are slow and imprecise when using her right hand, but normal when using her left hand. Her sensory examination is normal to light touch, pinprick, vibration, position, and temperature. Her gait is normal, and she can do a tandem walk without difficulty."
],
"date": "May 01, 2019",
"figures": [],
"markdown": "# A 31-Year-Old Woman With Right Arm Tremors\n\n **Authors:** Heidi Moawad, MD \n **Date:** May 01, 2019\n\n ## Content\n\n Upon physical examination, the patient is alert, oriented, and cooperative and does not appear to be in distress. Her affect is normal, and her memory and cognitive functioning is normal.\nShe does not have any rashes, lesions, or discoloration. Her pulse is regular, and her respiratory, cardiac, and abdominal examinations are unremarkable.\nShe does not have any facial asymmetry. Her extraocular movements are full, and she does not have diplopia. She has saccades in both eyes when looking toward the right and left, but not while at rest. Her pupils are equal, round, and reactive to light.\nHer language and speech are coherent and fluent without any defects. Her strength is slightly decreased in the right biceps and triceps. Otherwise, her motor strength is 5/5, including her right hand. She did not have tremors or involuntary movements during the physical examination.\nHer muscle tone is slightly diminished in her right upper extremity and is otherwise normal. Her reflexes are normal, with the exception of slightly brisk reflexes in the right upper extremity. Her coordination is impaired. She demonstrates dysmetria on finger-to-nose testing when using her right hand, but her left hand is normal. Similarly, rapid alternating movement test results are slow and imprecise when using her right hand, but normal when using her left hand. Her sensory examination is normal to light touch, pinprick, vibration, position, and temperature. Her gait is normal, and she can do a tandem walk without difficulty.\n\n ## Figures\n\n \n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1353116,
"choiceText": "Cervical spine stenosis ",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1353118,
"choiceText": "Early-onset Parkinson disease ",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1353120,
"choiceText": "Stroke ",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1353122,
"choiceText": "Demyelination\r\n",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 431641,
"questionText": "Which of the following is the most likely responsible for the patient's symptoms?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 31-Year-Old Woman With Right Arm Tremors"
},
{
"authors": "Heidi Moawad, MD",
"content": [
"The patient probably has a demyelinating episode that appears to be a recurrent demyelinating condition of the central nervous system. Given her symptoms, along with saccades and dysmetria, she may have more than one lesion. Her decreased tone in the right arm, along with increased reflexes, is an unusual pattern that suggests multiple lesions.",
"Multiple sclerosis is the most common causes of demyelination. The patient in this case may have relapsing-remitting multiple sclerosis (RRMS), which manifests with symptoms that resemble those of a stroke or cervical spine stenosis. Parkinson disease and multiple sclerosis do not usually present with similar symptoms, but in this instance, the presence of a tremor requires considering a possible diagnosis of Parkinson disease.",
"RRMS is characterized by symptoms caused by episodic demyelination of the brain, spine, or optic nerves, typically of the white matter. These exacerbations can involve one or more lesions at a time and can occur at varying intervals, ranging from several times per year to only a few times in a person's lifetime. Many patients have some residual signs and symptoms between their exacerbations.",
"Multiple sclerosis typically manifests with motor or visual impairment. In addition, cognitive changes, mood changes, fatigue, and low energy can accompany focal symptoms or can occur independently of focal symptoms. The patient's examination findings are consistent with at least one focal lesion, corresponding to her right upper-extremity symptoms, and at least one other lesion, corresponding to the saccades."
],
"date": "May 01, 2019",
"figures": [],
"markdown": "# A 31-Year-Old Woman With Right Arm Tremors\n\n **Authors:** Heidi Moawad, MD \n **Date:** May 01, 2019\n\n ## Content\n\n The patient probably has a demyelinating episode that appears to be a recurrent demyelinating condition of the central nervous system. Given her symptoms, along with saccades and dysmetria, she may have more than one lesion. Her decreased tone in the right arm, along with increased reflexes, is an unusual pattern that suggests multiple lesions.\nMultiple sclerosis is the most common causes of demyelination. The patient in this case may have relapsing-remitting multiple sclerosis (RRMS), which manifests with symptoms that resemble those of a stroke or cervical spine stenosis. Parkinson disease and multiple sclerosis do not usually present with similar symptoms, but in this instance, the presence of a tremor requires considering a possible diagnosis of Parkinson disease.\nRRMS is characterized by symptoms caused by episodic demyelination of the brain, spine, or optic nerves, typically of the white matter. These exacerbations can involve one or more lesions at a time and can occur at varying intervals, ranging from several times per year to only a few times in a person's lifetime. Many patients have some residual signs and symptoms between their exacerbations.\nMultiple sclerosis typically manifests with motor or visual impairment. In addition, cognitive changes, mood changes, fatigue, and low energy can accompany focal symptoms or can occur independently of focal symptoms. The patient's examination findings are consistent with at least one focal lesion, corresponding to her right upper-extremity symptoms, and at least one other lesion, corresponding to the saccades.\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1353116,
"choiceText": "Cervical spine stenosis ",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1353118,
"choiceText": "Early-onset Parkinson disease ",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1353120,
"choiceText": "Stroke ",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1353122,
"choiceText": "Demyelination\r\n",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 431641,
"questionText": "Which of the following is the most likely responsible for the patient's symptoms?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 31-Year-Old Woman With Right Arm Tremors"
},
{
"authors": "Heidi Moawad, MD",
"content": [
"This patient seems to have an episodic condition or a systemic illness. Her bout of fatigue improved without any intervention. Because she was not examined at the time, whether she had focal findings is unclear. Typically, focal symptoms prompt an urgent medical visit. Patients with multiple sclerosis who do not have focal symptoms on the first event may not seek medical attention, delaying the diagnosis. Multiple sclerosis is also often worse during hot weather. The fact that her episode of fatigue and low energy and her episode of tremors both occurred during the warm summer months supports multiple sclerosis as the likely etiology.",
"Tremors are not common in multiple sclerosis, and when they occur, they manifest as intention tremors, rather than resting tremors. Evidence suggests that lesions in the pons and cerebellum are correlated with an intention tremor in multiple sclerosis.[1] Pharmacologic and surgical approaches specifically focused on managing tremors are used to improve quality of life for patients with multiple sclerosis who have persistent tremors, if treatment for an acute multiple sclerosis exacerbation and disease-modifying therapy do not alleviate the tremor.",
"Cervical spine stenosis may cause spinal cord and/or nerve root impingement, which can produce focal symptoms. Symptoms may include weakness and/or sensory loss, with increased or decreased reflexes, depending on peripheral nerve or cervical spine impingement. Some of this patient's physical examination findings may be explained by cervical spine stenosis; however, it would not explain her saccades or intermittent intention tremor. Her unusual bout of fatigue would also not be explained by cervical spine stenosis.",
"Stroke can cause focal weakness and increased reflexes and is a rare cause of tremors as well. The patient's examination findings suggest at least one focal lesion, which could be consistent with one or more ischemic brain lesions. Although she may have several ischemic brain lesions, the bout of fatigue that she experienced more strongly suggests a systemic or episodic condition.",
"Parkinson disease often manifests with tremors. However, this patient clearly has an intention tremor, as opposed to the resting tremors that are more typical of Parkinson disease. Her symptoms and signs are focal, which is not typical of Parkinson disease. Her upper-extremity reflexes are brisk, which is also an atypical finding in Parkinson disease. She also has decreased muscle tone with an absence of the muscle rigidity that would be expected to accompany Parkinson disease. Eye movement abnormalities can occur with Parkinson disease, but they are usually limitations of eye movement, rather than saccades. Her signs and symptoms are suggestive of one or more focal lesions rather than a degenerative disease process, such as Parkinson disease."
],
"date": "May 01, 2019",
"figures": [],
"markdown": "# A 31-Year-Old Woman With Right Arm Tremors\n\n **Authors:** Heidi Moawad, MD \n **Date:** May 01, 2019\n\n ## Content\n\n This patient seems to have an episodic condition or a systemic illness. Her bout of fatigue improved without any intervention. Because she was not examined at the time, whether she had focal findings is unclear. Typically, focal symptoms prompt an urgent medical visit. Patients with multiple sclerosis who do not have focal symptoms on the first event may not seek medical attention, delaying the diagnosis. Multiple sclerosis is also often worse during hot weather. The fact that her episode of fatigue and low energy and her episode of tremors both occurred during the warm summer months supports multiple sclerosis as the likely etiology.\nTremors are not common in multiple sclerosis, and when they occur, they manifest as intention tremors, rather than resting tremors. Evidence suggests that lesions in the pons and cerebellum are correlated with an intention tremor in multiple sclerosis.[1] Pharmacologic and surgical approaches specifically focused on managing tremors are used to improve quality of life for patients with multiple sclerosis who have persistent tremors, if treatment for an acute multiple sclerosis exacerbation and disease-modifying therapy do not alleviate the tremor.\nCervical spine stenosis may cause spinal cord and/or nerve root impingement, which can produce focal symptoms. Symptoms may include weakness and/or sensory loss, with increased or decreased reflexes, depending on peripheral nerve or cervical spine impingement. Some of this patient's physical examination findings may be explained by cervical spine stenosis; however, it would not explain her saccades or intermittent intention tremor. Her unusual bout of fatigue would also not be explained by cervical spine stenosis.\nStroke can cause focal weakness and increased reflexes and is a rare cause of tremors as well. The patient's examination findings suggest at least one focal lesion, which could be consistent with one or more ischemic brain lesions. Although she may have several ischemic brain lesions, the bout of fatigue that she experienced more strongly suggests a systemic or episodic condition.\nParkinson disease often manifests with tremors. However, this patient clearly has an intention tremor, as opposed to the resting tremors that are more typical of Parkinson disease. Her symptoms and signs are focal, which is not typical of Parkinson disease. Her upper-extremity reflexes are brisk, which is also an atypical finding in Parkinson disease. She also has decreased muscle tone with an absence of the muscle rigidity that would be expected to accompany Parkinson disease. Eye movement abnormalities can occur with Parkinson disease, but they are usually limitations of eye movement, rather than saccades. Her signs and symptoms are suggestive of one or more focal lesions rather than a degenerative disease process, such as Parkinson disease.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 31-Year-Old Woman With Right Arm Tremors"
},
{
"authors": "Heidi Moawad, MD",
"content": [
"The patient had brain MRI with contrast, which showed three lesions, two of which were described as small demyelinating lesions in the left subcortical white-matter region and one in the right cerebellum. Similar findings are shown in Figure 1.",
"Figure 1. Axial T2-weighted MRI images of the infratentorial region from three unique patients demonstrating foci of T2-hyperintensity at the brainstem (A), bilateral cerebellar lobes (B), and brainstem and bilateral cerebellar peduncle regions (C). A sagittal T2-weighted MRI image of the cervical spinal cord reveals multifocal regions of T2-hypertensinsity within the parenchyma (D). Image from the National Institute of Health’s US National Library of Medicine",
"She also had lumbar puncture, which showed oligoclonal bands and a high protein level (Figure 2).",
"Figure 2. Immunodetection of oligoclonal bands (OCBs) in serum and cerebrospinal fluid (CSF) of patients with multiple sclerosis. Pattern 1: OCBs in CSF only (positive). Pattern 2: No OCBs seen (negative, polyclonal). Pattern 3: Identical OCBs in both (mirror). Pattern 4: Identical OCBs in both, with extra in CSF. Image from the National Institute of Health’s US National Library of Medicine"
],
"date": "May 01, 2019",
"figures": [
{
"caption": "Figure 1. Axial T2-weighted MRI images of the infratentorial region from three unique patients demonstrating foci of T2-hyperintensity at the brainstem (A), bilateral cerebellar lobes (B), and brainstem and bilateral cerebellar peduncle regions (C). A sagittal T2-weighted MRI image of the cervical spinal cord reveals multifocal regions of T2-hypertensinsity within the parenchyma (D). Image from the National Institute of Health’s US National Library of Medicine",
"image_url": "https://img.medscapestatic.com/article/912/292/912292-Thumb1.jpg"
},
{
"caption": "Figure 2. Immunodetection of oligoclonal bands (OCBs) in serum and cerebrospinal fluid (CSF) of patients with multiple sclerosis. Pattern 1: OCBs in CSF only (positive). Pattern 2: No OCBs seen (negative, polyclonal). Pattern 3: Identical OCBs in both (mirror). Pattern 4: Identical OCBs in both, with extra in CSF. Image from the National Institute of Health’s US National Library of Medicine",
"image_url": "https://img.medscapestatic.com/article/912/292/912292-Thumb2.jpg"
}
],
"markdown": "# A 31-Year-Old Woman With Right Arm Tremors\n\n **Authors:** Heidi Moawad, MD \n **Date:** May 01, 2019\n\n ## Content\n\n The patient had brain MRI with contrast, which showed three lesions, two of which were described as small demyelinating lesions in the left subcortical white-matter region and one in the right cerebellum. Similar findings are shown in Figure 1.\nFigure 1. Axial T2-weighted MRI images of the infratentorial region from three unique patients demonstrating foci of T2-hyperintensity at the brainstem (A), bilateral cerebellar lobes (B), and brainstem and bilateral cerebellar peduncle regions (C). A sagittal T2-weighted MRI image of the cervical spinal cord reveals multifocal regions of T2-hypertensinsity within the parenchyma (D). Image from the National Institute of Health’s US National Library of Medicine\nShe also had lumbar puncture, which showed oligoclonal bands and a high protein level (Figure 2).\nFigure 2. Immunodetection of oligoclonal bands (OCBs) in serum and cerebrospinal fluid (CSF) of patients with multiple sclerosis. Pattern 1: OCBs in CSF only (positive). Pattern 2: No OCBs seen (negative, polyclonal). Pattern 3: Identical OCBs in both (mirror). Pattern 4: Identical OCBs in both, with extra in CSF. Image from the National Institute of Health’s US National Library of Medicine\n\n ## Figures\n\n **Figure 1. Axial T2-weighted MRI images of the infratentorial region from three unique patients demonstrating foci of T2-hyperintensity at the brainstem (A), bilateral cerebellar lobes (B), and brainstem and bilateral cerebellar peduncle regions (C). A sagittal T2-weighted MRI image of the cervical spinal cord reveals multifocal regions of T2-hypertensinsity within the parenchyma (D). Image from the National Institute of Health’s US National Library of Medicine** \n \n\n**Figure 2. Immunodetection of oligoclonal bands (OCBs) in serum and cerebrospinal fluid (CSF) of patients with multiple sclerosis. Pattern 1: OCBs in CSF only (positive). Pattern 2: No OCBs seen (negative, polyclonal). Pattern 3: Identical OCBs in both (mirror). Pattern 4: Identical OCBs in both, with extra in CSF. Image from the National Institute of Health’s US National Library of Medicine** \n \n\n\n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1353140,
"choiceText": "Disease-modifying therapy",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1353142,
"choiceText": "Stem cell transplantation",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1353144,
"choiceText": "A course of intravenous steroids",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1353146,
"choiceText": "Observation without treatment",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The patient's symptoms, physical examination findings, and MRI all suggest that she may be experiencing a demyelinating process. A course of intravenous steroids is recommended for treatment of this episode, which is characterized by symptoms and several corresponding lesions on brain MRI. She may be experiencing an acute exacerbation of multiple sclerosis or another autoimmune or inflammatory event; thus, such lesions warrant acute treatment. \r\n<br><br>\r\nDisease-modifying therapies—including platform injectable immunomodulatory, oral, or infusion immunosuppressive therapies—are not recommended for treatment of an acute exacerbation. They are, however, used for maintenance therapy to reduce relapse rates and prevent clinical progression. Stem cell transplantation is currently being studied and may be an alternative to chronic immunosuppressive in the future. \r\n<br><br>\r\nObserving the patient without starting any treatment is not optimal in this case. Although she may experience spontaneous resolution of her symptoms, she could develop long-term sequelae, and treatment for her acute event may prevent residual sequelae. <br><br>\r\n\r\nThe patient was treated with a course of steroids, and her tremor resolved within the first few days of treatment. Her physical examination findings did not improve by the time her course of steroids was complete. Follow-up brain MRI 2 months later did not show any lesions, and she did not have residual physical deficits at that time.\r\n",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 431647,
"questionText": "Given the findings detailed in this patient, which of the following is most likely the first step in her treatment plan?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1353148,
"choiceText": "Disease-modifying therapy",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1353150,
"choiceText": "Stem cell transplantation",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1353152,
"choiceText": "Regular courses of intravenous steroids",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1353154,
"choiceText": "Deep brain stimulation \r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "This patient has evidence for lesion dissemination in space with lesions in multiple locations and has oligoclonal bands in her cerebrospinal fluid, a marker carrying similar implications to that of clinical or MRI lesion dissemination in time. Even if this patient only has clinically isolated syndrome (CIS), a precursor to RRMS, many neuroimmunologists would recommend disease-modifying therapy to prevent progression to clinically definite multiple sclerosis and prevent progression of disability. Although observation without treatment may be a potential strategy, because this patient fulfills <a href=\"https://www.nationalmssociety.org/About-the-Society/News/Updated-McDonald-Criteria-Expected-to-Speed-the-Di\">the 2017 McDonald Criteria for the diagnosis of multiple sclerosis</a>, and because several disease-modifying therapies have been approved in patients with CIS, starting disease-modifying therapy is the most reasonable plan in this patient.\r\n<br><br>\r\nStem cell transplantation has been used for multiple sclerosis as a disease-modifying approach; however, this patient does not appear to be a good candidate at this time. Stem cell therapy for multiple sclerosis is under investigation.<sup type=\"ref\">[2]</sup> This would expose the patient to potential side effects, including issues with fertility.\r\n<br><br>\r\nDeep brain stimulation has been used to treat severe tremor in certain patients with multiple sclerosis, which was not present in this patient. This patient's tremor resolved after treatment with intravenous steroids for her acute exacerbation. Because her exacerbation has resolved, repeated courses of intravenous steroids are unnecessary. Some patients with multiple sclerosis who experience relapses may benefit when monthly methylprednisolone is added to interferon as a maintenance therapy<sup type=\"ref\">[3]</sup>; however, at this point, the patient does not appear to have an acute relapse that warrants this type of treatment.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 431649,
"questionText": "Given the available information, which of the following is the recommended long-term treatment plan for this patient?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 31-Year-Old Woman With Right Arm Tremors"
},
{
"authors": "Heidi Moawad, MD",
"content": [],
"date": "May 01, 2019",
"figures": [],
"markdown": "# A 31-Year-Old Woman With Right Arm Tremors\n\n **Authors:** Heidi Moawad, MD \n **Date:** May 01, 2019\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1353140,
"choiceText": "Disease-modifying therapy",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1353142,
"choiceText": "Stem cell transplantation",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1353144,
"choiceText": "A course of intravenous steroids",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1353146,
"choiceText": "Observation without treatment",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The patient's symptoms, physical examination findings, and MRI all suggest that she may be experiencing a demyelinating process. A course of intravenous steroids is recommended for treatment of this episode, which is characterized by symptoms and several corresponding lesions on brain MRI. She may be experiencing an acute exacerbation of multiple sclerosis or another autoimmune or inflammatory event; thus, such lesions warrant acute treatment. \r\n<br><br>\r\nDisease-modifying therapies—including platform injectable immunomodulatory, oral, or infusion immunosuppressive therapies—are not recommended for treatment of an acute exacerbation. They are, however, used for maintenance therapy to reduce relapse rates and prevent clinical progression. Stem cell transplantation is currently being studied and may be an alternative to chronic immunosuppressive in the future. \r\n<br><br>\r\nObserving the patient without starting any treatment is not optimal in this case. Although she may experience spontaneous resolution of her symptoms, she could develop long-term sequelae, and treatment for her acute event may prevent residual sequelae. <br><br>\r\n\r\nThe patient was treated with a course of steroids, and her tremor resolved within the first few days of treatment. Her physical examination findings did not improve by the time her course of steroids was complete. Follow-up brain MRI 2 months later did not show any lesions, and she did not have residual physical deficits at that time.\r\n",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 431647,
"questionText": "Given the findings detailed in this patient, which of the following is most likely the first step in her treatment plan?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1353148,
"choiceText": "Disease-modifying therapy",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1353150,
"choiceText": "Stem cell transplantation",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1353152,
"choiceText": "Regular courses of intravenous steroids",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1353154,
"choiceText": "Deep brain stimulation \r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "This patient has evidence for lesion dissemination in space with lesions in multiple locations and has oligoclonal bands in her cerebrospinal fluid, a marker carrying similar implications to that of clinical or MRI lesion dissemination in time. Even if this patient only has clinically isolated syndrome (CIS), a precursor to RRMS, many neuroimmunologists would recommend disease-modifying therapy to prevent progression to clinically definite multiple sclerosis and prevent progression of disability. Although observation without treatment may be a potential strategy, because this patient fulfills <a href=\"https://www.nationalmssociety.org/About-the-Society/News/Updated-McDonald-Criteria-Expected-to-Speed-the-Di\">the 2017 McDonald Criteria for the diagnosis of multiple sclerosis</a>, and because several disease-modifying therapies have been approved in patients with CIS, starting disease-modifying therapy is the most reasonable plan in this patient.\r\n<br><br>\r\nStem cell transplantation has been used for multiple sclerosis as a disease-modifying approach; however, this patient does not appear to be a good candidate at this time. Stem cell therapy for multiple sclerosis is under investigation.<sup type=\"ref\">[2]</sup> This would expose the patient to potential side effects, including issues with fertility.\r\n<br><br>\r\nDeep brain stimulation has been used to treat severe tremor in certain patients with multiple sclerosis, which was not present in this patient. This patient's tremor resolved after treatment with intravenous steroids for her acute exacerbation. Because her exacerbation has resolved, repeated courses of intravenous steroids are unnecessary. Some patients with multiple sclerosis who experience relapses may benefit when monthly methylprednisolone is added to interferon as a maintenance therapy<sup type=\"ref\">[3]</sup>; however, at this point, the patient does not appear to have an acute relapse that warrants this type of treatment.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 431649,
"questionText": "Given the available information, which of the following is the recommended long-term treatment plan for this patient?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 31-Year-Old Woman With Right Arm Tremors"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1353116,
"choiceText": "Cervical spine stenosis ",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1353118,
"choiceText": "Early-onset Parkinson disease ",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1353120,
"choiceText": "Stroke ",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1353122,
"choiceText": "Demyelination\r\n",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 431641,
"questionText": "Which of the following is the most likely responsible for the patient's symptoms?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1353140,
"choiceText": "Disease-modifying therapy",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1353142,
"choiceText": "Stem cell transplantation",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1353144,
"choiceText": "A course of intravenous steroids",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1353146,
"choiceText": "Observation without treatment",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The patient's symptoms, physical examination findings, and MRI all suggest that she may be experiencing a demyelinating process. A course of intravenous steroids is recommended for treatment of this episode, which is characterized by symptoms and several corresponding lesions on brain MRI. She may be experiencing an acute exacerbation of multiple sclerosis or another autoimmune or inflammatory event; thus, such lesions warrant acute treatment. \r\n<br><br>\r\nDisease-modifying therapies—including platform injectable immunomodulatory, oral, or infusion immunosuppressive therapies—are not recommended for treatment of an acute exacerbation. They are, however, used for maintenance therapy to reduce relapse rates and prevent clinical progression. Stem cell transplantation is currently being studied and may be an alternative to chronic immunosuppressive in the future. \r\n<br><br>\r\nObserving the patient without starting any treatment is not optimal in this case. Although she may experience spontaneous resolution of her symptoms, she could develop long-term sequelae, and treatment for her acute event may prevent residual sequelae. <br><br>\r\n\r\nThe patient was treated with a course of steroids, and her tremor resolved within the first few days of treatment. Her physical examination findings did not improve by the time her course of steroids was complete. Follow-up brain MRI 2 months later did not show any lesions, and she did not have residual physical deficits at that time.\r\n",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 431647,
"questionText": "Given the findings detailed in this patient, which of the following is most likely the first step in her treatment plan?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1353148,
"choiceText": "Disease-modifying therapy",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1353150,
"choiceText": "Stem cell transplantation",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1353152,
"choiceText": "Regular courses of intravenous steroids",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1353154,
"choiceText": "Deep brain stimulation \r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "This patient has evidence for lesion dissemination in space with lesions in multiple locations and has oligoclonal bands in her cerebrospinal fluid, a marker carrying similar implications to that of clinical or MRI lesion dissemination in time. Even if this patient only has clinically isolated syndrome (CIS), a precursor to RRMS, many neuroimmunologists would recommend disease-modifying therapy to prevent progression to clinically definite multiple sclerosis and prevent progression of disability. Although observation without treatment may be a potential strategy, because this patient fulfills <a href=\"https://www.nationalmssociety.org/About-the-Society/News/Updated-McDonald-Criteria-Expected-to-Speed-the-Di\">the 2017 McDonald Criteria for the diagnosis of multiple sclerosis</a>, and because several disease-modifying therapies have been approved in patients with CIS, starting disease-modifying therapy is the most reasonable plan in this patient.\r\n<br><br>\r\nStem cell transplantation has been used for multiple sclerosis as a disease-modifying approach; however, this patient does not appear to be a good candidate at this time. Stem cell therapy for multiple sclerosis is under investigation.<sup type=\"ref\">[2]</sup> This would expose the patient to potential side effects, including issues with fertility.\r\n<br><br>\r\nDeep brain stimulation has been used to treat severe tremor in certain patients with multiple sclerosis, which was not present in this patient. This patient's tremor resolved after treatment with intravenous steroids for her acute exacerbation. Because her exacerbation has resolved, repeated courses of intravenous steroids are unnecessary. Some patients with multiple sclerosis who experience relapses may benefit when monthly methylprednisolone is added to interferon as a maintenance therapy<sup type=\"ref\">[3]</sup>; however, at this point, the patient does not appear to have an acute relapse that warrants this type of treatment.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 431649,
"questionText": "Given the available information, which of the following is the recommended long-term treatment plan for this patient?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
911713 | /viewarticle/911713 | [
{
"authors": "Avan Armaghani, MD",
"content": [
"Update: The image originally associated with this Case Challenge was mirrored (reversed) and incorrectly suggested the patient's left breast was affected. The image has been removed to avoid any confusion, as the text correctly describes a right mastectomy.",
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"A 64-year-old, generally healthy man presents with right lower back pain. Approximately 7 years prior, he had presented to his primary care physician after noticing a lump in his right breast 4 months before. Although he did not have any other constitutional symptoms, including fever, chills, chest pain, shortness of breath, nausea, vomiting, or diarrhea, he was concerned because the lump had increased in size.",
"A core needle biopsy of the right breast mass and other investigations revealed he had stage IIA estrogen receptor–positive HER2 negative breast cancer. The patient underwent right mastectomy and then started tamoxifen adjuvantly.",
"Now, 2 years after the completion of 5 years of tamoxifen therapy, the patient describes concerning right lower back pain. The rest of his medical and surgical history is unremarkable. He does not have a family history of breast cancer or other cancers. He works as an electrician and lives in the Cayman Islands with his wife. He has two children. He admits to drinking alcohol, but only in moderation (2-3 drinks 1-2 days per week, maximum)."
],
"date": "April 16, 2019",
"figures": [],
"markdown": "# A 64-Year-Old Man With Back Pain After Mastectomy\n\n **Authors:** Avan Armaghani, MD \n **Date:** April 16, 2019\n\n ## Content\n\n Update: The image originally associated with this Case Challenge was mirrored (reversed) and incorrectly suggested the patient's left breast was affected. The image has been removed to avoid any confusion, as the text correctly describes a right mastectomy.\nEditor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nA 64-year-old, generally healthy man presents with right lower back pain. Approximately 7 years prior, he had presented to his primary care physician after noticing a lump in his right breast 4 months before. Although he did not have any other constitutional symptoms, including fever, chills, chest pain, shortness of breath, nausea, vomiting, or diarrhea, he was concerned because the lump had increased in size.\nA core needle biopsy of the right breast mass and other investigations revealed he had stage IIA estrogen receptor–positive HER2 negative breast cancer. The patient underwent right mastectomy and then started tamoxifen adjuvantly.\nNow, 2 years after the completion of 5 years of tamoxifen therapy, the patient describes concerning right lower back pain. The rest of his medical and surgical history is unremarkable. He does not have a family history of breast cancer or other cancers. He works as an electrician and lives in the Cayman Islands with his wife. He has two children. He admits to drinking alcohol, but only in moderation (2-3 drinks 1-2 days per week, maximum).\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 64-Year-Old Man With Back Pain After Mastectomy"
},
{
"authors": "Avan Armaghani, MD",
"content": [
"On physical examination, the patient appears in no distress. He is awake and alert. He is afebrile, with a blood pressure of 138/87 mm Hg, heart rate of 74 beats/min, and oxygen saturation of 98% on room air. An examination of the oral cavity reveals moist mucus membranes. He has normal heart sounds. His lungs are auscultated and clear bilaterally without wheezes, rales, or rhonchi. The abdomen is soft, without tenderness or masses. The patient has full range of motion of upper and lower extremities.",
"His complete blood count and complete metabolic panel findings are normal. An MRI of the pelvis reveals a complex lytic lesion in the right iliac wing measuring 5.5 × 2.6 × 3.6 cm. A positron emission tomography scan reveals a heterogeneous mass in the inferior right lobe of the liver, with a focus standardized uptake value maximum of 7.1. A focus in the anterior liver has a standardized uptake value maximum of 8.8, and a lesion involving the anterior right iliac wing has a standardized uptake value maximum of 5.6."
],
"date": "April 16, 2019",
"figures": [],
"markdown": "# A 64-Year-Old Man With Back Pain After Mastectomy\n\n **Authors:** Avan Armaghani, MD \n **Date:** April 16, 2019\n\n ## Content\n\n On physical examination, the patient appears in no distress. He is awake and alert. He is afebrile, with a blood pressure of 138/87 mm Hg, heart rate of 74 beats/min, and oxygen saturation of 98% on room air. An examination of the oral cavity reveals moist mucus membranes. He has normal heart sounds. His lungs are auscultated and clear bilaterally without wheezes, rales, or rhonchi. The abdomen is soft, without tenderness or masses. The patient has full range of motion of upper and lower extremities.\nHis complete blood count and complete metabolic panel findings are normal. An MRI of the pelvis reveals a complex lytic lesion in the right iliac wing measuring 5.5 × 2.6 × 3.6 cm. A positron emission tomography scan reveals a heterogeneous mass in the inferior right lobe of the liver, with a focus standardized uptake value maximum of 7.1. A focus in the anterior liver has a standardized uptake value maximum of 8.8, and a lesion involving the anterior right iliac wing has a standardized uptake value maximum of 5.6.\n\n ## Figures\n\n \n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1348942,
"choiceText": "Liver cirrhosis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1348944,
"choiceText": "Sarcoma with metastasis to the liver",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1348946,
"choiceText": "Osteoporosis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1348948,
"choiceText": "Metastatic breast cancer",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 430243,
"questionText": "Which one of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 64-Year-Old Man With Back Pain After Mastectomy"
},
{
"authors": "Avan Armaghani, MD",
"content": [
"Liver and bone biopsies confirmed recurrent, metastatic breast cancer in this patient. Male breast cancer is rare and represents less than 1% of all breast cancer cases.[1] The American Cancer Society estimates 2670 new cases of male breast cancer in 2019.[2] The age distribution in male breast cancer is unimodal, with peak at age 71 years, compared with female breast cancer, which has a bimodal distribution that peaks at ages 52 and 71 years.[1] Because of the rarity of this disease, most information has been obtained from single-institution retrospective studies and extrapolated from clinical trials in female breast cancer.[3,4,5]",
"Several risk factors are recognized as having an association with male breast cancer. Although the patient in this case had no family history, genetic predisposition is a major risk factor for male breast cancer: Approximately 15%-20% of men with breast cancer report a family history of breast or ovarian cancer.[6] In men, BRCA2 mutation accounts for approximately 15% of all breast cancers, and men with a BRCA2 mutation have a 5%-10% lifetime risk of developing breast cancer.[6] BRCA1 mutation is associated with a lower risk of developing male breast cancer, with an estimated lifetime risk of approximately 1%-5%. Other genetic mutations have been shown to be associated with the development of male breast cancer, including PTEN, p53, and CHEK2.",
"In addition to genetic mutations, other risk factors for developing male breast cancers are noted. Klinefelter syndrome is characterized by 47,XXY karyotype, small testes, azoospermia, and gynecomastia. Klinefelter syndrome confers upward of 50-fold increased risk for male breast cancer.[7] Interestingly, 4%-20% of men with breast cancer are estimated to have Klinefelter syndrome. Other risk factors for developing male breast cancer include undescended testes, congenital inguinal hernia, orchiectomy, orchitis, testicular injury, and infertility. Lifestyle risk factors include obesity and alcohol intake."
],
"date": "April 16, 2019",
"figures": [],
"markdown": "# A 64-Year-Old Man With Back Pain After Mastectomy\n\n **Authors:** Avan Armaghani, MD \n **Date:** April 16, 2019\n\n ## Content\n\n Liver and bone biopsies confirmed recurrent, metastatic breast cancer in this patient. Male breast cancer is rare and represents less than 1% of all breast cancer cases.[1] The American Cancer Society estimates 2670 new cases of male breast cancer in 2019.[2] The age distribution in male breast cancer is unimodal, with peak at age 71 years, compared with female breast cancer, which has a bimodal distribution that peaks at ages 52 and 71 years.[1] Because of the rarity of this disease, most information has been obtained from single-institution retrospective studies and extrapolated from clinical trials in female breast cancer.[3,4,5]\nSeveral risk factors are recognized as having an association with male breast cancer. Although the patient in this case had no family history, genetic predisposition is a major risk factor for male breast cancer: Approximately 15%-20% of men with breast cancer report a family history of breast or ovarian cancer.[6] In men, BRCA2 mutation accounts for approximately 15% of all breast cancers, and men with a BRCA2 mutation have a 5%-10% lifetime risk of developing breast cancer.[6] BRCA1 mutation is associated with a lower risk of developing male breast cancer, with an estimated lifetime risk of approximately 1%-5%. Other genetic mutations have been shown to be associated with the development of male breast cancer, including PTEN, p53, and CHEK2.\nIn addition to genetic mutations, other risk factors for developing male breast cancers are noted. Klinefelter syndrome is characterized by 47,XXY karyotype, small testes, azoospermia, and gynecomastia. Klinefelter syndrome confers upward of 50-fold increased risk for male breast cancer.[7] Interestingly, 4%-20% of men with breast cancer are estimated to have Klinefelter syndrome. Other risk factors for developing male breast cancer include undescended testes, congenital inguinal hernia, orchiectomy, orchitis, testicular injury, and infertility. Lifestyle risk factors include obesity and alcohol intake.\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1348942,
"choiceText": "Liver cirrhosis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1348944,
"choiceText": "Sarcoma with metastasis to the liver",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1348946,
"choiceText": "Osteoporosis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1348948,
"choiceText": "Metastatic breast cancer",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 430243,
"questionText": "Which one of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 64-Year-Old Man With Back Pain After Mastectomy"
},
{
"authors": "Avan Armaghani, MD",
"content": [
"Male breast cancer often presents with a painless mass in the breast. Other symptoms may include pain, nipple retraction, and nipple discharge. The differential diagnosis for painless breast mass in a man includes gynecomastia, infection, lipoma, fibromatosis, granular cell tumor, or in rare cases, metastatic disease from a separate primary site.[8]",
"Men often present at later stages than women because there is less awareness of male breast cancer, contributing to worse outcomes.[7] In one database of male breast cancers, the 5-year survival rate from 2005 to 2010 for men with breast cancer was 82.8% compared with 88.5% in female breast cancer.[9] In addition, the risk for death because of breast cancer was 43% higher among men than among women.",
"The workup for a suspicious breast mass in men is similar to that in women. Mammography is the imaging modality of choice. In one study, the sensitivity and specificity of diagnosing malignant breast cancer in men with mammography were 92% and 90%, respectively.[10] A core needle biopsy is required to obtain a pathologic diagnosis.",
"The predominant pathologic subtype in male breast cancer is infiltrating ductal carcinoma, accounting for more than 80% of all cases.[6] The second most common subtype is ductal carcinoma in situ. Other subtypes are less common, including invasive papillary carcinoma and lobular carcinoma. Male breast cancers tend to have higher hormone receptor positivity compared with female breast cancer. An estimated 80% of male breast cancers are estrogen receptor–positive.[4] In one review, more than 80% of male breast cancers were found to be estrogen receptor–positive, and almost 75% were progesterone receptor–positive.[7]",
"The staging system used in male breast cancers is the same that is used for women, and is based on size of primary tumor, nodal status, and presence or absence of distant metastatic disease. The prognostic factors in male breast cancer are also similar to female breast cancer and include axillary lymph node status, tumor size, histologic grade, and hormone receptor status.[7]"
],
"date": "April 16, 2019",
"figures": [],
"markdown": "# A 64-Year-Old Man With Back Pain After Mastectomy\n\n **Authors:** Avan Armaghani, MD \n **Date:** April 16, 2019\n\n ## Content\n\n Male breast cancer often presents with a painless mass in the breast. Other symptoms may include pain, nipple retraction, and nipple discharge. The differential diagnosis for painless breast mass in a man includes gynecomastia, infection, lipoma, fibromatosis, granular cell tumor, or in rare cases, metastatic disease from a separate primary site.[8]\nMen often present at later stages than women because there is less awareness of male breast cancer, contributing to worse outcomes.[7] In one database of male breast cancers, the 5-year survival rate from 2005 to 2010 for men with breast cancer was 82.8% compared with 88.5% in female breast cancer.[9] In addition, the risk for death because of breast cancer was 43% higher among men than among women.\nThe workup for a suspicious breast mass in men is similar to that in women. Mammography is the imaging modality of choice. In one study, the sensitivity and specificity of diagnosing malignant breast cancer in men with mammography were 92% and 90%, respectively.[10] A core needle biopsy is required to obtain a pathologic diagnosis.\nThe predominant pathologic subtype in male breast cancer is infiltrating ductal carcinoma, accounting for more than 80% of all cases.[6] The second most common subtype is ductal carcinoma in situ. Other subtypes are less common, including invasive papillary carcinoma and lobular carcinoma. Male breast cancers tend to have higher hormone receptor positivity compared with female breast cancer. An estimated 80% of male breast cancers are estrogen receptor–positive.[4] In one review, more than 80% of male breast cancers were found to be estrogen receptor–positive, and almost 75% were progesterone receptor–positive.[7]\nThe staging system used in male breast cancers is the same that is used for women, and is based on size of primary tumor, nodal status, and presence or absence of distant metastatic disease. The prognostic factors in male breast cancer are also similar to female breast cancer and include axillary lymph node status, tumor size, histologic grade, and hormone receptor status.[7]\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 64-Year-Old Man With Back Pain After Mastectomy"
},
{
"authors": "Avan Armaghani, MD",
"content": [
"The treatment of male breast cancer is extrapolated from that of female breast cancer. Modified radical mastectomy is the most common surgical modality because of the subareolar location of most male breast cancers.[11] Lumpectomy is generally not performed because of limited breast tissue.[7] Radiation therapy after modified radical mastectomy in male breast cancer remains controversial. Some studies have shown a reduction in local recurrence with radiation therapy, but no improvement in overall survival. However, others suggest that because male breast cancers tend to be more centrally located and close in proximity to the internal mammary nodes, consideration should be given to radiation treatment of the internal mammary lymph nodes.[7]",
"Given the limited data on the use of adjuvant chemotherapy in male breast cancer, the same guidelines used in female breast cancer for adjuvant chemotherapy are also applied in men. The 21-gene recurrence score test is used to guide decision on use of chemotherapy in the adjuvant setting in hormone receptor–positive, HER2-negative, node-negative, or limited node–positive disease.",
"Antiestrogen therapy is the most common systemic therapy that is used in early-stage hormone receptor–positive male breast cancers. Tamoxifen is the most widely studied antiestrogen medication and is associated with an improvement in overall survival.[12,13] There are insufficient data to support the use of aromatase inhibitors (AIs) in male breast cancer. In one retrospective study, AIs were actually found to be detrimental to overall survival.[14] In contrast to postmenopausal women, in whom AIs work to block estrogen production, AIs do not block testicular production of estrogen in men.[8]",
"The treatment of metastatic male breast cancer mirrors the treatment in female breast cancer. Antiestrogen therapy can be used in hormone receptor–positive breast cancer.[1] Systemic chemotherapy is also a treatment option, but often this is used in later lines of therapy, as men generally respond well to antiestrogen therapy.",
"In this case, the patient had breast cancer treated with a modified radical mastectomy followed by 5 years of tamoxifen therapy. He then had recurrent disease in the bone (right iliac) and liver. Both liver and bone biopsies were performed and confirmed recurrent, metastatic disease. The patient was recently started on and continues to receive therapy with fulvestrant."
],
"date": "April 16, 2019",
"figures": [],
"markdown": "# A 64-Year-Old Man With Back Pain After Mastectomy\n\n **Authors:** Avan Armaghani, MD \n **Date:** April 16, 2019\n\n ## Content\n\n The treatment of male breast cancer is extrapolated from that of female breast cancer. Modified radical mastectomy is the most common surgical modality because of the subareolar location of most male breast cancers.[11] Lumpectomy is generally not performed because of limited breast tissue.[7] Radiation therapy after modified radical mastectomy in male breast cancer remains controversial. Some studies have shown a reduction in local recurrence with radiation therapy, but no improvement in overall survival. However, others suggest that because male breast cancers tend to be more centrally located and close in proximity to the internal mammary nodes, consideration should be given to radiation treatment of the internal mammary lymph nodes.[7]\nGiven the limited data on the use of adjuvant chemotherapy in male breast cancer, the same guidelines used in female breast cancer for adjuvant chemotherapy are also applied in men. The 21-gene recurrence score test is used to guide decision on use of chemotherapy in the adjuvant setting in hormone receptor–positive, HER2-negative, node-negative, or limited node–positive disease.\nAntiestrogen therapy is the most common systemic therapy that is used in early-stage hormone receptor–positive male breast cancers. Tamoxifen is the most widely studied antiestrogen medication and is associated with an improvement in overall survival.[12,13] There are insufficient data to support the use of aromatase inhibitors (AIs) in male breast cancer. In one retrospective study, AIs were actually found to be detrimental to overall survival.[14] In contrast to postmenopausal women, in whom AIs work to block estrogen production, AIs do not block testicular production of estrogen in men.[8]\nThe treatment of metastatic male breast cancer mirrors the treatment in female breast cancer. Antiestrogen therapy can be used in hormone receptor–positive breast cancer.[1] Systemic chemotherapy is also a treatment option, but often this is used in later lines of therapy, as men generally respond well to antiestrogen therapy.\nIn this case, the patient had breast cancer treated with a modified radical mastectomy followed by 5 years of tamoxifen therapy. He then had recurrent disease in the bone (right iliac) and liver. Both liver and bone biopsies were performed and confirmed recurrent, metastatic disease. The patient was recently started on and continues to receive therapy with fulvestrant.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1348950,
"choiceText": "<i>BRCA</i> mutation",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1348952,
"choiceText": "Klinefelter syndrome",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1348954,
"choiceText": "Orchiectomy",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1348956,
"choiceText": "Family history of breast or ovarian cancer",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1348958,
"choiceText": "Younger age (<30 years)",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Younger age is not associated with an increased risk for breast cancer among men. Male breast cancer has a unimodal age distribution, with peak at age 71 years. Genetic predisposition does increase risk for male breast cancer; this includes family history and <i>BRCA</i> mutation. Other risk factors include Klinefelter syndrome and orchiectomy.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 430245,
"questionText": "Which one of the following is not a well-recognized risk factor for the development of breast cancer in men? ",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1348960,
"choiceText": "Estrogen receptor–negative, progesterone receptor–negative, <i>HER2</i>-negative (triple negative)",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1348962,
"choiceText": "Estrogen receptor–negative, <i>HER2</i>-positive",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1348964,
"choiceText": "Estrogen receptor–negative, <i>HER2</i>-negative",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1348966,
"choiceText": "Estrogen receptor–positive, <i>HER2</i>-negative",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Certain subtypes are more common in women than men. Triple-negative and <em>HER2</em>-positive breast cancers are more commonly observed among women, whereas most male breast cancers are estrogen receptor–positive.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 430247,
"questionText": "Which one of the following pathologic subtypes is more commonly seen in men with breast cancer?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 64-Year-Old Man With Back Pain After Mastectomy"
},
{
"authors": "Avan Armaghani, MD",
"content": [],
"date": "April 16, 2019",
"figures": [],
"markdown": "# A 64-Year-Old Man With Back Pain After Mastectomy\n\n **Authors:** Avan Armaghani, MD \n **Date:** April 16, 2019\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1348950,
"choiceText": "<i>BRCA</i> mutation",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1348952,
"choiceText": "Klinefelter syndrome",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1348954,
"choiceText": "Orchiectomy",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1348956,
"choiceText": "Family history of breast or ovarian cancer",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1348958,
"choiceText": "Younger age (<30 years)",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Younger age is not associated with an increased risk for breast cancer among men. Male breast cancer has a unimodal age distribution, with peak at age 71 years. Genetic predisposition does increase risk for male breast cancer; this includes family history and <i>BRCA</i> mutation. Other risk factors include Klinefelter syndrome and orchiectomy.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 430245,
"questionText": "Which one of the following is not a well-recognized risk factor for the development of breast cancer in men? ",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1348960,
"choiceText": "Estrogen receptor–negative, progesterone receptor–negative, <i>HER2</i>-negative (triple negative)",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1348962,
"choiceText": "Estrogen receptor–negative, <i>HER2</i>-positive",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1348964,
"choiceText": "Estrogen receptor–negative, <i>HER2</i>-negative",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1348966,
"choiceText": "Estrogen receptor–positive, <i>HER2</i>-negative",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Certain subtypes are more common in women than men. Triple-negative and <em>HER2</em>-positive breast cancers are more commonly observed among women, whereas most male breast cancers are estrogen receptor–positive.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 430247,
"questionText": "Which one of the following pathologic subtypes is more commonly seen in men with breast cancer?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 64-Year-Old Man With Back Pain After Mastectomy"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1348942,
"choiceText": "Liver cirrhosis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1348944,
"choiceText": "Sarcoma with metastasis to the liver",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1348946,
"choiceText": "Osteoporosis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1348948,
"choiceText": "Metastatic breast cancer",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 430243,
"questionText": "Which one of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1348950,
"choiceText": "<i>BRCA</i> mutation",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1348952,
"choiceText": "Klinefelter syndrome",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1348954,
"choiceText": "Orchiectomy",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1348956,
"choiceText": "Family history of breast or ovarian cancer",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1348958,
"choiceText": "Younger age (<30 years)",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Younger age is not associated with an increased risk for breast cancer among men. Male breast cancer has a unimodal age distribution, with peak at age 71 years. Genetic predisposition does increase risk for male breast cancer; this includes family history and <i>BRCA</i> mutation. Other risk factors include Klinefelter syndrome and orchiectomy.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 430245,
"questionText": "Which one of the following is not a well-recognized risk factor for the development of breast cancer in men? ",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1348960,
"choiceText": "Estrogen receptor–negative, progesterone receptor–negative, <i>HER2</i>-negative (triple negative)",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1348962,
"choiceText": "Estrogen receptor–negative, <i>HER2</i>-positive",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1348964,
"choiceText": "Estrogen receptor–negative, <i>HER2</i>-negative",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1348966,
"choiceText": "Estrogen receptor–positive, <i>HER2</i>-negative",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Certain subtypes are more common in women than men. Triple-negative and <em>HER2</em>-positive breast cancers are more commonly observed among women, whereas most male breast cancers are estrogen receptor–positive.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 430247,
"questionText": "Which one of the following pathologic subtypes is more commonly seen in men with breast cancer?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
911040 | /viewarticle/911040 | [
{
"authors": "Gregory Taylor, DO; Adam M. Vieder, DO",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"A 64-year-old man with a significant medical history of chronic obstructive pulmonary disease, alcohol abuse, and depression presented to the intensive care unit as a transfer from an outside hospital for acute hepatic failure. The patient had ingested a large quantity of tablets in an attempted suicide 6 hours before arrival at the previous hospital.",
"His initial pertinent laboratory evaluation from the outside hospital was notable for the following:",
"Ethanol level: 320 mg/dL",
"Acetaminophen level: 430 µg/mL",
"Aspartate aminotransferase (AST) level: 2066 U/L",
"Alanine aminotransferase (ALT) level: 1321 U/L",
"Nonhemolyzed potassium level: 7.6 mEq/L",
"Creatinine phosphokinase (CPK) level: 62,530 IU/L",
"He was placed on a norepinephrine infusion and was intubated secondary to being obtunded. He underwent treatment for hyperkalemia, was started on intravenous N-acetylcysteine (NAC), and was transferred."
],
"date": "March 29, 2019",
"figures": [],
"markdown": "# Acute Liver Failure in a 64-Year-Old Man\n\n **Authors:** Gregory Taylor, DO; Adam M. Vieder, DO \n **Date:** March 29, 2019\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nA 64-year-old man with a significant medical history of chronic obstructive pulmonary disease, alcohol abuse, and depression presented to the intensive care unit as a transfer from an outside hospital for acute hepatic failure. The patient had ingested a large quantity of tablets in an attempted suicide 6 hours before arrival at the previous hospital.\nHis initial pertinent laboratory evaluation from the outside hospital was notable for the following:\nEthanol level: 320 mg/dL\nAcetaminophen level: 430 µg/mL\nAspartate aminotransferase (AST) level: 2066 U/L\nAlanine aminotransferase (ALT) level: 1321 U/L\nNonhemolyzed potassium level: 7.6 mEq/L\nCreatinine phosphokinase (CPK) level: 62,530 IU/L\nHe was placed on a norepinephrine infusion and was intubated secondary to being obtunded. He underwent treatment for hyperkalemia, was started on intravenous N-acetylcysteine (NAC), and was transferred.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "Acute Liver Failure in a 64-Year-Old Man"
},
{
"authors": "Gregory Taylor, DO; Adam M. Vieder, DO",
"content": [
"Upon arrival at the intensive care unit, the patient was afebrile. His blood pressure was 94/57 mm Hg (on norepinephrine at 20 µg/h). His heart rate was 92 beats/min. His respiratory rate was 22 breaths/min. His weight was 196 lb (87 kg). His oxygen saturation was 96% on the ventilator.",
"Upon physical examination, he exhibited diffuse jaundice, smelled of alcohol and tobacco, and appeared much older than his stated age. He was in no acute distress. He had bilateral scleral icterus. His cardiopulmonary examination findings were unremarkable for any acute changes. His abdominal examination revealed a fluid wave with the absence of any peritoneal signs.",
"Pertinent laboratory evaluation findings included the following:",
"Hemoglobin level: 10.5 g/dL",
"Thrombocytopenia (110 bil/L)",
"Sodium level: 146 mmol/L",
"Potassium level: 4.6 mmol/L",
"Carbon dioxide level: 21 mmol/L",
"Blood urea nitrogen level: 26 mg/dL",
"Creatinine level: 3.02 mg/dL",
"AST level: 2094 U/L",
"ALT level: 1685 U/L",
"Total bilirubin level: 0.4 mg/dL",
"International normalized ratio: 2.11",
"Troponin level: 35.25 ng/mL (increasing to 44.66 ng/mL)",
"Acetaminophen level: 237 µg/mL",
"CPK level: 57,000 IU/L",
"Ultrasonography of the abdomen revealed an increased hepatic echogenicity suggesting diffuse hepatocellular disease, cirrhosis, and small amount of perihepatic ascites.",
"Cardiology assessment found that the elevated troponin level was multifactorial, with a type 2 myocardial oxygen supply and/or demand mismatch secondary to coronary endothelial dysfunction, hypotension, volume depletion, rhabdomyolysis, acute renal failure, and hepatic failure. Non–ST-segment elevation myocardial infarction was most likely the result of plaque rupture in the setting of acute renal failure and fulminant hepatic failure.",
"Given the patient's multisystem organ failure and poor prognosis, conservative treatment was recommended. He was started on a low-intensity heparin infusion for 48 hours. The transplant surgery team deemed him to be a poor candidate from a surgical perspective for a liver transplant, with concerns regarding compliance, cardiac status, alcohol intoxication, and a suicide attempt.",
"By day 3, the patient's creatinine level rose to 4.18 mg/dL, and he was anuric. His AST level increased to 3895 U/L, and his ALT level increased to 3215 U/L, despite intravenous NAC administration. His lactic acid level increased from 2.2 to 8.2 mmol/L, with an anion gap of 25. He subsequently underwent dialysis.",
"By day 4, vancomycin and piperacillin/tazobactam were initiated for healthcare-associated pneumonia. Chest radiography revealed hazy opacities in the lung bases (Figure 1).",
"Figure 1.",
"Despite maximal pressor support, the patient continued to decline and remained unresponsive on the ventilator. A family meeting was held, and they elected to withdrawal care. He died shortly thereafter."
],
"date": "March 29, 2019",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/910/911/040/911040-Thumb1.png"
}
],
"markdown": "# Acute Liver Failure in a 64-Year-Old Man\n\n **Authors:** Gregory Taylor, DO; Adam M. Vieder, DO \n **Date:** March 29, 2019\n\n ## Content\n\n Upon arrival at the intensive care unit, the patient was afebrile. His blood pressure was 94/57 mm Hg (on norepinephrine at 20 µg/h). His heart rate was 92 beats/min. His respiratory rate was 22 breaths/min. His weight was 196 lb (87 kg). His oxygen saturation was 96% on the ventilator.\nUpon physical examination, he exhibited diffuse jaundice, smelled of alcohol and tobacco, and appeared much older than his stated age. He was in no acute distress. He had bilateral scleral icterus. His cardiopulmonary examination findings were unremarkable for any acute changes. His abdominal examination revealed a fluid wave with the absence of any peritoneal signs.\nPertinent laboratory evaluation findings included the following:\nHemoglobin level: 10.5 g/dL\nThrombocytopenia (110 bil/L)\nSodium level: 146 mmol/L\nPotassium level: 4.6 mmol/L\nCarbon dioxide level: 21 mmol/L\nBlood urea nitrogen level: 26 mg/dL\nCreatinine level: 3.02 mg/dL\nAST level: 2094 U/L\nALT level: 1685 U/L\nTotal bilirubin level: 0.4 mg/dL\nInternational normalized ratio: 2.11\nTroponin level: 35.25 ng/mL (increasing to 44.66 ng/mL)\nAcetaminophen level: 237 µg/mL\nCPK level: 57,000 IU/L\nUltrasonography of the abdomen revealed an increased hepatic echogenicity suggesting diffuse hepatocellular disease, cirrhosis, and small amount of perihepatic ascites.\nCardiology assessment found that the elevated troponin level was multifactorial, with a type 2 myocardial oxygen supply and/or demand mismatch secondary to coronary endothelial dysfunction, hypotension, volume depletion, rhabdomyolysis, acute renal failure, and hepatic failure. Non–ST-segment elevation myocardial infarction was most likely the result of plaque rupture in the setting of acute renal failure and fulminant hepatic failure.\nGiven the patient's multisystem organ failure and poor prognosis, conservative treatment was recommended. He was started on a low-intensity heparin infusion for 48 hours. The transplant surgery team deemed him to be a poor candidate from a surgical perspective for a liver transplant, with concerns regarding compliance, cardiac status, alcohol intoxication, and a suicide attempt.\nBy day 3, the patient's creatinine level rose to 4.18 mg/dL, and he was anuric. His AST level increased to 3895 U/L, and his ALT level increased to 3215 U/L, despite intravenous NAC administration. His lactic acid level increased from 2.2 to 8.2 mmol/L, with an anion gap of 25. He subsequently underwent dialysis.\nBy day 4, vancomycin and piperacillin/tazobactam were initiated for healthcare-associated pneumonia. Chest radiography revealed hazy opacities in the lung bases (Figure 1).\nFigure 1.\nDespite maximal pressor support, the patient continued to decline and remained unresponsive on the ventilator. A family meeting was held, and they elected to withdrawal care. He died shortly thereafter.\n\n ## Figures\n\n **Figure 1.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1345162,
"choiceText": "Diazepam overdose",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1345164,
"choiceText": "Heroin overdose ",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1345166,
"choiceText": "Acetaminophen overdose",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1345168,
"choiceText": "Tricyclic antidepressant overdose",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 428979,
"questionText": "What was the most likely cause of this patient's death?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Acute Liver Failure in a 64-Year-Old Man"
},
{
"authors": "Gregory Taylor, DO; Adam M. Vieder, DO",
"content": [
"The patient in this case reportedly ingested 50 tablets of hydrocodone/acetaminophen (10-325 mg; 16.2 g of acetaminophen) approximately 6 hours before arrival at the initial outside hospital. Acetaminophen overdose accounts for approximately 50% of overdose-related liver failure cases and as many as 20% of all liver transplants, and acetaminophen is one of the most common pharmaceutical agents to cause drug-induced liver injury.[1]",
"Acetaminophen is considered the most widely utilized antipyretic and analgesic in the United States. The US Food and Drug Administration (FDA) advertises that up to 4000 mg of acetaminophen within a 24-hour period is safe, without toxic effects.[1] Toxic ingestions that result in liver failure usually exceed 150 mg/kg as a single dose. The patient in this case ingested beyond this toxic dose. Various risk prediction tools, including a paracetamol-aminotransferase multiplication product, are available to help identify patients who are likely to develop hepatotoxicity.[2]",
"Acetaminophen metabolism involves conjugation pathways to nontoxic metabolites, using a process called sulfation and glucuronidation, in addition to the cytochrome P450 CYP2E1 through the liver, forming a potentially toxic metabolite called NAPQ1. The body uses glutathione to convert NAPQ1 into nontoxic metabolites. However, with significant overdoses, the regular metabolic pathways are overwhelmed, glutathione stores are depleted, and NAPQ1 accumulates. The result is necrosis of the hepatocytes and fulminant liver failure.[3] Many patients may present with nonspecific symptoms (malaise, fatigue, abdominal pain, nausea/vomiting) similar to those of a viral syndrome at first.[1]"
],
"date": "March 29, 2019",
"figures": [],
"markdown": "# Acute Liver Failure in a 64-Year-Old Man\n\n **Authors:** Gregory Taylor, DO; Adam M. Vieder, DO \n **Date:** March 29, 2019\n\n ## Content\n\n The patient in this case reportedly ingested 50 tablets of hydrocodone/acetaminophen (10-325 mg; 16.2 g of acetaminophen) approximately 6 hours before arrival at the initial outside hospital. Acetaminophen overdose accounts for approximately 50% of overdose-related liver failure cases and as many as 20% of all liver transplants, and acetaminophen is one of the most common pharmaceutical agents to cause drug-induced liver injury.[1]\nAcetaminophen is considered the most widely utilized antipyretic and analgesic in the United States. The US Food and Drug Administration (FDA) advertises that up to 4000 mg of acetaminophen within a 24-hour period is safe, without toxic effects.[1] Toxic ingestions that result in liver failure usually exceed 150 mg/kg as a single dose. The patient in this case ingested beyond this toxic dose. Various risk prediction tools, including a paracetamol-aminotransferase multiplication product, are available to help identify patients who are likely to develop hepatotoxicity.[2]\nAcetaminophen metabolism involves conjugation pathways to nontoxic metabolites, using a process called sulfation and glucuronidation, in addition to the cytochrome P450 CYP2E1 through the liver, forming a potentially toxic metabolite called NAPQ1. The body uses glutathione to convert NAPQ1 into nontoxic metabolites. However, with significant overdoses, the regular metabolic pathways are overwhelmed, glutathione stores are depleted, and NAPQ1 accumulates. The result is necrosis of the hepatocytes and fulminant liver failure.[3] Many patients may present with nonspecific symptoms (malaise, fatigue, abdominal pain, nausea/vomiting) similar to those of a viral syndrome at first.[1]\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1345162,
"choiceText": "Diazepam overdose",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1345164,
"choiceText": "Heroin overdose ",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1345166,
"choiceText": "Acetaminophen overdose",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1345168,
"choiceText": "Tricyclic antidepressant overdose",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 428979,
"questionText": "What was the most likely cause of this patient's death?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Acute Liver Failure in a 64-Year-Old Man"
},
{
"authors": "Gregory Taylor, DO; Adam M. Vieder, DO",
"content": [
"Four stages of hepatotoxicity are recognized, with overlap between them depending on preexisting liver disease, comorbidities, formulation, and the degree of overdose.",
"Stage 1 of hepatotoxicity occurs within the first 24 hours of ingestion. Presentations range from asymptomatic to nausea/vomiting, malaise, and fatigue. AST and ALT levels are often normal during this stage; however, in a severe overdose, as was the case with this patient, AST and ALT levels have been shown to be elevated within 8 hours.[1]",
"Stage 2 of hepatotoxicity occurs 24-72 hours after ingestion. This stage is characterized by either an improvement in symptoms or resolution of the previously mentioned symptoms. Depending on the nature of the overdose, AST and ALT levels begin to increase during this stage. In severe overdoses, patients can present with hepatomegaly, severe right upper-quadrant abdominal pain, coagulopathy, and jaundice.[1]",
"Stage 3 of hepatotoxicity occurs 72-96 hours after ingestion. AST and ALT levels are often significantly elevated (often > 3000 U/L), with associated jaundice, coagulopathy, encephalopathy, and severe lactic acidosis. The highest risk for death occurs during stage 3 secondary to multiorgan failure.[1] Intubation and mechanical ventilation often occur during this stage. Other features of toxicity can include renal failure and pancreatitis.[3]",
"Stage 4 of hepatotoxicity occurs after 96 hours after ingestion and lasts up to 2 weeks. For patients who survive stage 3, this is also referred to as \"the recovery phase.\" The length of this phase depends on the degree of overdose.[1]",
"In a suspected ingestion of an unknown medication, suicide attempt, or altered mental status, obtaining an acetaminophen level is critical. A 4-hour acetaminophen level is of paramount importance because it guides treatment.[1] The Rumack-Matthew nomogram is commonly used when the acuity of the ingestion is within a 24-hour period, in order to calculate the likelihood of hepatic injury. The nomogram should not be used with chronic ingestions or an unknown time since ingestion. Important values within the nomogram are termed the \"probable toxicity line,\" which includes an acetaminophen level of 200 µg/mL at 4 hours since ingestion and 25 µg/mL at 16 hours.[1] Patients with values above this line are at risk for hepatotoxicity. Parallel to the probable toxicity line is the \"high toxicity line,\" which includes an acetaminophen level of at least 300 µg/mL at 4 hours. Mortality at this point has said to approach 30%.[1] To help identify and protect high-risk patients who may have a decreasing acetaminophen level, a third line is used, the \"treatment line,\" which includes an acetaminophen level of 150 µg/mL at 4 hours.[1]",
"Treatment involves use of the antidote, NAC. NAC replenishes glutathione stores, allowing conjugation with NAPQI. If given during the early course of intoxication, hepatic recovery may be possible.[3] If the acetaminophen level or timing since ingestion is in doubt, initiation of NAC is indicated.[1] In one large retrospective study, 11,195 cases of suspected acetaminophen overdose were analyzed. Outcomes of 2540 patients treated with NAC revealed that the efficacy decreases longer than 8 hours after ingestion. However, NAC did decrease the degree of hepatotoxicity when given as long as 24 hours after ingestion.[4]"
],
"date": "March 29, 2019",
"figures": [],
"markdown": "# Acute Liver Failure in a 64-Year-Old Man\n\n **Authors:** Gregory Taylor, DO; Adam M. Vieder, DO \n **Date:** March 29, 2019\n\n ## Content\n\n Four stages of hepatotoxicity are recognized, with overlap between them depending on preexisting liver disease, comorbidities, formulation, and the degree of overdose.\nStage 1 of hepatotoxicity occurs within the first 24 hours of ingestion. Presentations range from asymptomatic to nausea/vomiting, malaise, and fatigue. AST and ALT levels are often normal during this stage; however, in a severe overdose, as was the case with this patient, AST and ALT levels have been shown to be elevated within 8 hours.[1]\nStage 2 of hepatotoxicity occurs 24-72 hours after ingestion. This stage is characterized by either an improvement in symptoms or resolution of the previously mentioned symptoms. Depending on the nature of the overdose, AST and ALT levels begin to increase during this stage. In severe overdoses, patients can present with hepatomegaly, severe right upper-quadrant abdominal pain, coagulopathy, and jaundice.[1]\nStage 3 of hepatotoxicity occurs 72-96 hours after ingestion. AST and ALT levels are often significantly elevated (often > 3000 U/L), with associated jaundice, coagulopathy, encephalopathy, and severe lactic acidosis. The highest risk for death occurs during stage 3 secondary to multiorgan failure.[1] Intubation and mechanical ventilation often occur during this stage. Other features of toxicity can include renal failure and pancreatitis.[3]\nStage 4 of hepatotoxicity occurs after 96 hours after ingestion and lasts up to 2 weeks. For patients who survive stage 3, this is also referred to as \"the recovery phase.\" The length of this phase depends on the degree of overdose.[1]\nIn a suspected ingestion of an unknown medication, suicide attempt, or altered mental status, obtaining an acetaminophen level is critical. A 4-hour acetaminophen level is of paramount importance because it guides treatment.[1] The Rumack-Matthew nomogram is commonly used when the acuity of the ingestion is within a 24-hour period, in order to calculate the likelihood of hepatic injury. The nomogram should not be used with chronic ingestions or an unknown time since ingestion. Important values within the nomogram are termed the \"probable toxicity line,\" which includes an acetaminophen level of 200 µg/mL at 4 hours since ingestion and 25 µg/mL at 16 hours.[1] Patients with values above this line are at risk for hepatotoxicity. Parallel to the probable toxicity line is the \"high toxicity line,\" which includes an acetaminophen level of at least 300 µg/mL at 4 hours. Mortality at this point has said to approach 30%.[1] To help identify and protect high-risk patients who may have a decreasing acetaminophen level, a third line is used, the \"treatment line,\" which includes an acetaminophen level of 150 µg/mL at 4 hours.[1]\nTreatment involves use of the antidote, NAC. NAC replenishes glutathione stores, allowing conjugation with NAPQI. If given during the early course of intoxication, hepatic recovery may be possible.[3] If the acetaminophen level or timing since ingestion is in doubt, initiation of NAC is indicated.[1] In one large retrospective study, 11,195 cases of suspected acetaminophen overdose were analyzed. Outcomes of 2540 patients treated with NAC revealed that the efficacy decreases longer than 8 hours after ingestion. However, NAC did decrease the degree of hepatotoxicity when given as long as 24 hours after ingestion.[4]\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "Acute Liver Failure in a 64-Year-Old Man"
},
{
"authors": "Gregory Taylor, DO; Adam M. Vieder, DO",
"content": [
"Various treatment protocols for acetaminophen toxicity are available, depending on the institution and the poison control center. For intravenous treatment (used in pregnant women, altered mental status, acetaminophen-induced liver failure, and intractable vomiting), the current FDA guidelines follow a 21-hour protocol. This protocol consists of an intravenous loading dose of 150 mg/kg bolus over 60 minutes, followed by 50 mg/kg over the next 4 hours, and 100 mg/kg over the remaining 16 hours.[5] The most serious side effect is anaphylaxis; although rare, it can occur during the initial 1-hour bolus infusion. With anaphylactoid reactions (rash, angioedema, bronchospasm, hypotension), which are similar to anaphylaxis but not immunoglobulin E-mediated, NAC is temporarily stopped, treatment of the reaction is initiated, and a subsequent lower infusion rate is later started.[3,5]",
"For example, for oral administration, the California Poison Control Center uses a 140-mg/kg bolus, with a maintenance dose of 70 mg/kg every 4 hours. Conventional protocol uses 17 doses of oral NAC over a period of 72 hours; however, clinical research has shown success with shorter protocols. As such, the California Poison Control Center uses treatment every 4 hours for 20 hours, totaling five doses. Similarly, the institution in this case uses six doses.[5] If, at the conclusion of the treatment regimen, liver enzyme levels are still elevated or significant acetaminophen levels are still detected, treatment is continued until the toxicity is resolved. Oral NAC treatment smells like rotten eggs and can result in vomiting. The rare anaphylactoid reaction seen with intravenous NAC is not present with oral NAC, and both formulations are equally efficacious.",
"The King's College Criteria for acetaminophen toxicity are used to identify patients who should be emergently transferred for potential liver transplant. Criteria include arterial pH < 7.3, international normalized ratio > 6.5, creatinine level > 3.4 mg/dL, and presence of grade III or IV hepatic encephalopathy.[1] Patients who meet these criteria have a mortality of nearly 90%.[1] This score has excellent specificity; however, it has been criticized for its oversensitivity. A second scoring system that has proven superior is the Sequential Organ Failure Assessment, which takes into consideration PaO2 level, FiO2 level, platelet level, Glasgow Coma Score, bilirubin level, mean arterial pressure, and creatinine level.[1] Acetaminophen toxicity is also amenable to extracorporeal treatments; however, owing to the efficacy of NAC, they are reserved for rare situations.[6]",
"Overall, recognizing acetaminophen overdose is critical because it can result in significant morbidity and mortality."
],
"date": "March 29, 2019",
"figures": [],
"markdown": "# Acute Liver Failure in a 64-Year-Old Man\n\n **Authors:** Gregory Taylor, DO; Adam M. Vieder, DO \n **Date:** March 29, 2019\n\n ## Content\n\n Various treatment protocols for acetaminophen toxicity are available, depending on the institution and the poison control center. For intravenous treatment (used in pregnant women, altered mental status, acetaminophen-induced liver failure, and intractable vomiting), the current FDA guidelines follow a 21-hour protocol. This protocol consists of an intravenous loading dose of 150 mg/kg bolus over 60 minutes, followed by 50 mg/kg over the next 4 hours, and 100 mg/kg over the remaining 16 hours.[5] The most serious side effect is anaphylaxis; although rare, it can occur during the initial 1-hour bolus infusion. With anaphylactoid reactions (rash, angioedema, bronchospasm, hypotension), which are similar to anaphylaxis but not immunoglobulin E-mediated, NAC is temporarily stopped, treatment of the reaction is initiated, and a subsequent lower infusion rate is later started.[3,5]\nFor example, for oral administration, the California Poison Control Center uses a 140-mg/kg bolus, with a maintenance dose of 70 mg/kg every 4 hours. Conventional protocol uses 17 doses of oral NAC over a period of 72 hours; however, clinical research has shown success with shorter protocols. As such, the California Poison Control Center uses treatment every 4 hours for 20 hours, totaling five doses. Similarly, the institution in this case uses six doses.[5] If, at the conclusion of the treatment regimen, liver enzyme levels are still elevated or significant acetaminophen levels are still detected, treatment is continued until the toxicity is resolved. Oral NAC treatment smells like rotten eggs and can result in vomiting. The rare anaphylactoid reaction seen with intravenous NAC is not present with oral NAC, and both formulations are equally efficacious.\nThe King's College Criteria for acetaminophen toxicity are used to identify patients who should be emergently transferred for potential liver transplant. Criteria include arterial pH < 7.3, international normalized ratio > 6.5, creatinine level > 3.4 mg/dL, and presence of grade III or IV hepatic encephalopathy.[1] Patients who meet these criteria have a mortality of nearly 90%.[1] This score has excellent specificity; however, it has been criticized for its oversensitivity. A second scoring system that has proven superior is the Sequential Organ Failure Assessment, which takes into consideration PaO2 level, FiO2 level, platelet level, Glasgow Coma Score, bilirubin level, mean arterial pressure, and creatinine level.[1] Acetaminophen toxicity is also amenable to extracorporeal treatments; however, owing to the efficacy of NAC, they are reserved for rare situations.[6]\nOverall, recognizing acetaminophen overdose is critical because it can result in significant morbidity and mortality.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1345170,
"choiceText": "A patient may be asymptomatic or present with nonspecific symptoms, such as malaise, fatigue, abdominal pain, and nausea/vomiting",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1345172,
"choiceText": "The Rumack-Matthew nomogram can be used for chronic ingestions ",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1345174,
"choiceText": "Glutathione stores are depleted with significant overdoses",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1345176,
"choiceText": "NAC is used to replenish glutathione stores\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The Rumack-Matthew nomogram is commonly used to calculate the likelihood of hepatic injury when the acuity of the ingestion is within a 24-hour period. The nomogram should not be used with chronic ingestions or an unknown time since ingestion. The other answers are accurate.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 428981,
"questionText": "Which of the following statements is false regarding an acetaminophen overdose?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1345178,
"choiceText": "Pregnant women",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1345180,
"choiceText": "Altered mental status",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1345182,
"choiceText": "Intractable vomiting",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1345184,
"choiceText": "Acetaminophen-induced liver failure",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1345186,
"choiceText": "All of the above",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Intravenous NAC treatment is used in pregnant women and patients with altered mental status, acetaminophen-induced liver failure, and intractable vomiting. Oral NAC treatment smells like rotten eggs and can result in vomiting. For this reason, an antiemetic may be given before administration of oral NAC; otherwise, if intractable vomiting occurs, intravenous NAC is recommended.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 428983,
"questionText": "NAC is recommended in which of the following patients?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Acute Liver Failure in a 64-Year-Old Man"
},
{
"authors": "Gregory Taylor, DO; Adam M. Vieder, DO",
"content": [],
"date": "March 29, 2019",
"figures": [],
"markdown": "# Acute Liver Failure in a 64-Year-Old Man\n\n **Authors:** Gregory Taylor, DO; Adam M. Vieder, DO \n **Date:** March 29, 2019\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1345170,
"choiceText": "A patient may be asymptomatic or present with nonspecific symptoms, such as malaise, fatigue, abdominal pain, and nausea/vomiting",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1345172,
"choiceText": "The Rumack-Matthew nomogram can be used for chronic ingestions ",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1345174,
"choiceText": "Glutathione stores are depleted with significant overdoses",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1345176,
"choiceText": "NAC is used to replenish glutathione stores\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The Rumack-Matthew nomogram is commonly used to calculate the likelihood of hepatic injury when the acuity of the ingestion is within a 24-hour period. The nomogram should not be used with chronic ingestions or an unknown time since ingestion. The other answers are accurate.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 428981,
"questionText": "Which of the following statements is false regarding an acetaminophen overdose?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1345178,
"choiceText": "Pregnant women",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1345180,
"choiceText": "Altered mental status",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1345182,
"choiceText": "Intractable vomiting",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1345184,
"choiceText": "Acetaminophen-induced liver failure",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1345186,
"choiceText": "All of the above",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Intravenous NAC treatment is used in pregnant women and patients with altered mental status, acetaminophen-induced liver failure, and intractable vomiting. Oral NAC treatment smells like rotten eggs and can result in vomiting. For this reason, an antiemetic may be given before administration of oral NAC; otherwise, if intractable vomiting occurs, intravenous NAC is recommended.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 428983,
"questionText": "NAC is recommended in which of the following patients?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Acute Liver Failure in a 64-Year-Old Man"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1345162,
"choiceText": "Diazepam overdose",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1345164,
"choiceText": "Heroin overdose ",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1345166,
"choiceText": "Acetaminophen overdose",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1345168,
"choiceText": "Tricyclic antidepressant overdose",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 428979,
"questionText": "What was the most likely cause of this patient's death?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1345170,
"choiceText": "A patient may be asymptomatic or present with nonspecific symptoms, such as malaise, fatigue, abdominal pain, and nausea/vomiting",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1345172,
"choiceText": "The Rumack-Matthew nomogram can be used for chronic ingestions ",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1345174,
"choiceText": "Glutathione stores are depleted with significant overdoses",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1345176,
"choiceText": "NAC is used to replenish glutathione stores\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The Rumack-Matthew nomogram is commonly used to calculate the likelihood of hepatic injury when the acuity of the ingestion is within a 24-hour period. The nomogram should not be used with chronic ingestions or an unknown time since ingestion. The other answers are accurate.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 428981,
"questionText": "Which of the following statements is false regarding an acetaminophen overdose?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1345178,
"choiceText": "Pregnant women",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1345180,
"choiceText": "Altered mental status",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1345182,
"choiceText": "Intractable vomiting",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1345184,
"choiceText": "Acetaminophen-induced liver failure",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1345186,
"choiceText": "All of the above",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Intravenous NAC treatment is used in pregnant women and patients with altered mental status, acetaminophen-induced liver failure, and intractable vomiting. Oral NAC treatment smells like rotten eggs and can result in vomiting. For this reason, an antiemetic may be given before administration of oral NAC; otherwise, if intractable vomiting occurs, intravenous NAC is recommended.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 428983,
"questionText": "NAC is recommended in which of the following patients?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
910490 | /viewarticle/910490 | [
{
"authors": "Jeff S. Healey, MD, MSc, FRCPC; Jonathan P. Piccini, MD, MHS, FACC; Christian T. Ruff, MD, MPH",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"A 79-year-old woman presents for her annual pacemaker follow-up. She has a history of hypertension over the past 20 years, which is currently well controlled on perindopril (8 mg daily). She has no other significant medical or surgical history, no known allergies, and no history of bleeding. Ten years ago she had some transient lightheadedness and was diagnosed in the emergency room with a possible transient ischemic attack. A follow-up visit with a neurologist did not reveal any neurologic deficit, and an MRI of the brain revealed a small, old lacunar infarct in the basal ganglia that did not correlate with her symptoms. Her echocardiogram revealed mild left ventricular hypertrophy and mild left atrial enlargement.",
"Eight years ago the patient presented with a more profound episode of lightheadedness and had a syncopal episode while in the emergency room. The cardiac monitor revealed paroxysmal high-grade atrioventricular (AV) block. She then underwent implantation of a dual-chamber pacemaker, and her symptoms have not recurred. Her pacemaker and leads continue to function as expected. At this year's visit, she is found for the first time to have short-lasting, asymptomatic atrial high-rate episodes (Figure 1).",
"Figure 1.",
"She does not report palpitations, shortness of breath upon exertion, chest pain, or recurrent syncope. She does not appear to have had any further neurologic events. She denies any other symptoms and has not sought medical attention for any issues since her last visit."
],
"date": "March 22, 2019",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/910/490/910490-fig1-thumb.jpg"
}
],
"markdown": "# A 79-Year-Old Woman With a Pacemaker and High Atrial Rates\n\n **Authors:** Jeff S. Healey, MD, MSc, FRCPC; Jonathan P. Piccini, MD, MHS, FACC; Christian T. Ruff, MD, MPH \n **Date:** March 22, 2019\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nA 79-year-old woman presents for her annual pacemaker follow-up. She has a history of hypertension over the past 20 years, which is currently well controlled on perindopril (8 mg daily). She has no other significant medical or surgical history, no known allergies, and no history of bleeding. Ten years ago she had some transient lightheadedness and was diagnosed in the emergency room with a possible transient ischemic attack. A follow-up visit with a neurologist did not reveal any neurologic deficit, and an MRI of the brain revealed a small, old lacunar infarct in the basal ganglia that did not correlate with her symptoms. Her echocardiogram revealed mild left ventricular hypertrophy and mild left atrial enlargement.\nEight years ago the patient presented with a more profound episode of lightheadedness and had a syncopal episode while in the emergency room. The cardiac monitor revealed paroxysmal high-grade atrioventricular (AV) block. She then underwent implantation of a dual-chamber pacemaker, and her symptoms have not recurred. Her pacemaker and leads continue to function as expected. At this year's visit, she is found for the first time to have short-lasting, asymptomatic atrial high-rate episodes (Figure 1).\nFigure 1.\nShe does not report palpitations, shortness of breath upon exertion, chest pain, or recurrent syncope. She does not appear to have had any further neurologic events. She denies any other symptoms and has not sought medical attention for any issues since her last visit.\n\n ## Figures\n\n **Figure 1.** \n \n\n\n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 79-Year-Old Woman With a Pacemaker and High Atrial Rates"
},
{
"authors": "Jeff S. Healey, MD, MSc, FRCPC; Jonathan P. Piccini, MD, MHS, FACC; Christian T. Ruff, MD, MPH",
"content": [
"Upon physical examination, the patient's heart rate is regular at 60 beats/min. Her blood pressure is 135/75 mm Hg. No crackles on auscultation of the chest are heard. The jugular venous pressure is 1 cm above the sternal angle, and no peripheral edema is noted. No vascular bruits are observed, and the heart sounds are normal except for a soft S4 sound. The cardiac apex is neither displaced nor sustained. The left pectoral pacemaker site is well healed, with no bruising of the skin. Her weight is 130 lb (59 kg).",
"A repeat echocardiogram reveals borderline left ventricular hypertrophy, with a left atrial volume index of 35 mL/m2. Left ventricular systolic function is normal, while diastolic functional assessment suggests impaired relaxation. Valvular function is normal. Fasting blood sugar and blood glucose levels are within normal limits.",
"Complete blood count findings are within the reference range, as are standard coagulation tests. Serum electrolyte levels are normal, and the serum creatinine level is 118 µmol/L. A 12-lead ECG shows sinus rhythm at 60 beats/min with atrial sensing and intermittent ventricular pacing. Interrogation of her pacemaker shows three episodes of atrial high-rate episodes, lasting 8 minutes, 12 minutes, and 33 minutes in duration.",
"These were detected over a 1-week period 6 months ago and are the only such episodes detected since the time the patient's pacemaker was implanted. Device and lead function are normal, and she has an estimated 2 years of remaining battery life. She is paced only 10% of the time in the atrium at 60 beats/min and is paced 40% in the ventricle."
],
"date": "March 22, 2019",
"figures": [],
"markdown": "# A 79-Year-Old Woman With a Pacemaker and High Atrial Rates\n\n **Authors:** Jeff S. Healey, MD, MSc, FRCPC; Jonathan P. Piccini, MD, MHS, FACC; Christian T. Ruff, MD, MPH \n **Date:** March 22, 2019\n\n ## Content\n\n Upon physical examination, the patient's heart rate is regular at 60 beats/min. Her blood pressure is 135/75 mm Hg. No crackles on auscultation of the chest are heard. The jugular venous pressure is 1 cm above the sternal angle, and no peripheral edema is noted. No vascular bruits are observed, and the heart sounds are normal except for a soft S4 sound. The cardiac apex is neither displaced nor sustained. The left pectoral pacemaker site is well healed, with no bruising of the skin. Her weight is 130 lb (59 kg).\nA repeat echocardiogram reveals borderline left ventricular hypertrophy, with a left atrial volume index of 35 mL/m2. Left ventricular systolic function is normal, while diastolic functional assessment suggests impaired relaxation. Valvular function is normal. Fasting blood sugar and blood glucose levels are within normal limits.\nComplete blood count findings are within the reference range, as are standard coagulation tests. Serum electrolyte levels are normal, and the serum creatinine level is 118 µmol/L. A 12-lead ECG shows sinus rhythm at 60 beats/min with atrial sensing and intermittent ventricular pacing. Interrogation of her pacemaker shows three episodes of atrial high-rate episodes, lasting 8 minutes, 12 minutes, and 33 minutes in duration.\nThese were detected over a 1-week period 6 months ago and are the only such episodes detected since the time the patient's pacemaker was implanted. Device and lead function are normal, and she has an estimated 2 years of remaining battery life. She is paced only 10% of the time in the atrium at 60 beats/min and is paced 40% in the ventricle.\n\n ## Figures\n\n \n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1338894,
"choiceText": "Atrial flutter",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1338896,
"choiceText": "Atrial premature beats",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1338898,
"choiceText": "Atrial fibrillation",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1338900,
"choiceText": "Subclinical atrial fibrillation",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 427039,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 79-Year-Old Woman With a Pacemaker and High Atrial Rates"
},
{
"authors": "Jeff S. Healey, MD, MSc, FRCPC; Jonathan P. Piccini, MD, MHS, FACC; Christian T. Ruff, MD, MPH",
"content": [
"Atrial fibrillation, detected by surface-ECG methods, is associated with a 4- to 5-fold increase in the risk for stroke,[1] and treatment with oral anticoagulation reduces this risk by more than 60%.[2,3] However, most patients with atrial fibrillation have a high burden of the arrhythmia, and many are in atrial fibrillation all or most of the time.",
"In the 1990s, implanted pacemakers were developed that could detect and document atrial arrhythmias, even if they were asymptomatic and lasted only a few seconds or minutes. Such episodes were termed \"atrial high-rate episodes.\"[4] In many cases, these detections were not actual atrial arrhythmias at all but were the result of noise or other \"false-positive\" detections (Figure 2).",
"Figure 2.",
"Physicians questioned their significance, and it was uncommon that they led to any form of treatment. Two large cohort studies then evaluated the prevalence of atrial high-rate events and their relationship with stroke.[5,6] These studies both found that atrial high-rate episodes occurred in 30%-40% of patients with pacemakers who did not have prior clinical atrial fibrillation. The studies also concluded that the presence of atrial high-rate episodes was associated with a 2- to 2.5-fold increase in the risk for stroke. One of the studies conducted a manual review and adjudication of all episodes to determine that they were in fact atrial arrhythmias, which it described with the term subclinical atrial fibrillation.[6]",
"Although subclinical atrial fibrillation was associated with an increased risk for stroke in two studies,[5,6] the relative risk for stroke was only half of that seen in studies of ECG-detected atrial fibrillation,[1] with an absolute risk that was also substantially lower. This raised questions about the benefits of treating such patients with an oral anticoagulant.[7,8] As a result, many patients with subclinical atrial fibrillation continue to be managed without oral anticoagulation.[9] The use of oral anticoagulation must balance the potential for stroke reduction against the risk of bleeding due to anticoagulation. Most patients with pacemakers are in their mid-70s or older, and the risk of bleeding with all antithrombotic therapies increases in those older than 75 years; it is also higher among individuals with more cardiovascular risk factors (ie, a higher CHA2DS2-VASc score).[10,11] Many different mechanisms are responsible for strokes in older individuals, not all of which can be prevented with oral anticoagulation.[12] Even among anticoagulated patients with subclinical atrial fibrillation, the background rate of stroke is around 1% per year.[3]"
],
"date": "March 22, 2019",
"figures": [
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/910/490/910490-fig2-thumb.jpg"
}
],
"markdown": "# A 79-Year-Old Woman With a Pacemaker and High Atrial Rates\n\n **Authors:** Jeff S. Healey, MD, MSc, FRCPC; Jonathan P. Piccini, MD, MHS, FACC; Christian T. Ruff, MD, MPH \n **Date:** March 22, 2019\n\n ## Content\n\n Atrial fibrillation, detected by surface-ECG methods, is associated with a 4- to 5-fold increase in the risk for stroke,[1] and treatment with oral anticoagulation reduces this risk by more than 60%.[2,3] However, most patients with atrial fibrillation have a high burden of the arrhythmia, and many are in atrial fibrillation all or most of the time.\nIn the 1990s, implanted pacemakers were developed that could detect and document atrial arrhythmias, even if they were asymptomatic and lasted only a few seconds or minutes. Such episodes were termed \"atrial high-rate episodes.\"[4] In many cases, these detections were not actual atrial arrhythmias at all but were the result of noise or other \"false-positive\" detections (Figure 2).\nFigure 2.\nPhysicians questioned their significance, and it was uncommon that they led to any form of treatment. Two large cohort studies then evaluated the prevalence of atrial high-rate events and their relationship with stroke.[5,6] These studies both found that atrial high-rate episodes occurred in 30%-40% of patients with pacemakers who did not have prior clinical atrial fibrillation. The studies also concluded that the presence of atrial high-rate episodes was associated with a 2- to 2.5-fold increase in the risk for stroke. One of the studies conducted a manual review and adjudication of all episodes to determine that they were in fact atrial arrhythmias, which it described with the term subclinical atrial fibrillation.[6]\nAlthough subclinical atrial fibrillation was associated with an increased risk for stroke in two studies,[5,6] the relative risk for stroke was only half of that seen in studies of ECG-detected atrial fibrillation,[1] with an absolute risk that was also substantially lower. This raised questions about the benefits of treating such patients with an oral anticoagulant.[7,8] As a result, many patients with subclinical atrial fibrillation continue to be managed without oral anticoagulation.[9] The use of oral anticoagulation must balance the potential for stroke reduction against the risk of bleeding due to anticoagulation. Most patients with pacemakers are in their mid-70s or older, and the risk of bleeding with all antithrombotic therapies increases in those older than 75 years; it is also higher among individuals with more cardiovascular risk factors (ie, a higher CHA2DS2-VASc score).[10,11] Many different mechanisms are responsible for strokes in older individuals, not all of which can be prevented with oral anticoagulation.[12] Even among anticoagulated patients with subclinical atrial fibrillation, the background rate of stroke is around 1% per year.[3]\n\n ## Figures\n\n **Figure 2.** \n \n\n\n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1338894,
"choiceText": "Atrial flutter",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1338896,
"choiceText": "Atrial premature beats",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1338898,
"choiceText": "Atrial fibrillation",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1338900,
"choiceText": "Subclinical atrial fibrillation",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 427039,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 79-Year-Old Woman With a Pacemaker and High Atrial Rates"
},
{
"authors": "Jeff S. Healey, MD, MSc, FRCPC; Jonathan P. Piccini, MD, MHS, FACC; Christian T. Ruff, MD, MPH",
"content": [
"Clinical stroke risk factors (eg, hypertension, diabetes) are not the only determinants of stroke risk in patients with subclinical atrial fibrillation. Fairly consistent findings suggest that increased duration or burden of subclinical atrial fibrillation also predicts an increased risk for stroke. In the TRENDS study, patients with atrial high-rate events were divided into two groups on the basis of their maximum total daily burden of atrial high-rate events in the prior 30 days.[5] In patients whose burden exceeded 5.5 hours, a borderline significant, 2.2-fold increase in the risk for thromboembolic events was noted. Those with a lesser burden had no measurable increase in the risk for thromboembolism.",
"A subsequent analysis from the ASSERT trial examined patient outcomes according to their longest individual episode of subclinical atrial fibrillation, using a time-dependent survival analysis technique.[13] This analysis concluded that if patients developed episodes of subclinical atrial fibrillation lasting more than 24 continuous hours, their risk for stroke increased approximately 5-fold, reaching an annual risk of nearly 5% per year. This risk is similar in magnitude to clinical atrial fibrillation and is why the authors suggested that it might be reasonable to treat individuals with these longer episodes in a similar fashion to patients with clinically detected atrial fibrillation.[7,13] However, whether individuals with shorter episodes of subclinical atrial fibrillation should receive oral anticoagulation, or even if they are at increased risk for stroke, remains unclear. Over 2.5 years, only about 15% of such patients develop clinically detected atrial fibrillation or episodes longer than 24 hours; thus, watchful waiting may be a reasonable strategy for most patients.[6,11] The use of remote monitoring of a patient's pacemaker or implanted defibrillator can permit prompt recognition of longer episodes when they develop; however, this has not been shown to reduce stroke risk.[14]",
"Two large clinical trials are investigating whether patients with shorter episodes of subclinical atrial fibrillation (in one trial, between 6 minutes and 24 hours) benefit from treatment with an oral anticoagulant compared with a strategy of waiting and only treating if longer-lasting episodes or clinical atrial fibrillation develops.[7,8] Determining the optimal treatment strategy for this large group of patients with pacemakers and defibrillators is not only important for their clinical management but has much broader implications in this era marked by the proliferation of long-term noninvasive monitoring for atrial fibrillation, which includes devices marketed directly to patients.[15,16] Increasingly, physicians must determine how to manage patients with short-lasting episodes of subclinical atrial fibrillation detected incidentally during the investigation of other complaints (eg, syncope), or detected by patients themselves after purchasing devices capable of detecting atrial fibrillation."
],
"date": "March 22, 2019",
"figures": [],
"markdown": "# A 79-Year-Old Woman With a Pacemaker and High Atrial Rates\n\n **Authors:** Jeff S. Healey, MD, MSc, FRCPC; Jonathan P. Piccini, MD, MHS, FACC; Christian T. Ruff, MD, MPH \n **Date:** March 22, 2019\n\n ## Content\n\n Clinical stroke risk factors (eg, hypertension, diabetes) are not the only determinants of stroke risk in patients with subclinical atrial fibrillation. Fairly consistent findings suggest that increased duration or burden of subclinical atrial fibrillation also predicts an increased risk for stroke. In the TRENDS study, patients with atrial high-rate events were divided into two groups on the basis of their maximum total daily burden of atrial high-rate events in the prior 30 days.[5] In patients whose burden exceeded 5.5 hours, a borderline significant, 2.2-fold increase in the risk for thromboembolic events was noted. Those with a lesser burden had no measurable increase in the risk for thromboembolism.\nA subsequent analysis from the ASSERT trial examined patient outcomes according to their longest individual episode of subclinical atrial fibrillation, using a time-dependent survival analysis technique.[13] This analysis concluded that if patients developed episodes of subclinical atrial fibrillation lasting more than 24 continuous hours, their risk for stroke increased approximately 5-fold, reaching an annual risk of nearly 5% per year. This risk is similar in magnitude to clinical atrial fibrillation and is why the authors suggested that it might be reasonable to treat individuals with these longer episodes in a similar fashion to patients with clinically detected atrial fibrillation.[7,13] However, whether individuals with shorter episodes of subclinical atrial fibrillation should receive oral anticoagulation, or even if they are at increased risk for stroke, remains unclear. Over 2.5 years, only about 15% of such patients develop clinically detected atrial fibrillation or episodes longer than 24 hours; thus, watchful waiting may be a reasonable strategy for most patients.[6,11] The use of remote monitoring of a patient's pacemaker or implanted defibrillator can permit prompt recognition of longer episodes when they develop; however, this has not been shown to reduce stroke risk.[14]\nTwo large clinical trials are investigating whether patients with shorter episodes of subclinical atrial fibrillation (in one trial, between 6 minutes and 24 hours) benefit from treatment with an oral anticoagulant compared with a strategy of waiting and only treating if longer-lasting episodes or clinical atrial fibrillation develops.[7,8] Determining the optimal treatment strategy for this large group of patients with pacemakers and defibrillators is not only important for their clinical management but has much broader implications in this era marked by the proliferation of long-term noninvasive monitoring for atrial fibrillation, which includes devices marketed directly to patients.[15,16] Increasingly, physicians must determine how to manage patients with short-lasting episodes of subclinical atrial fibrillation detected incidentally during the investigation of other complaints (eg, syncope), or detected by patients themselves after purchasing devices capable of detecting atrial fibrillation.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 79-Year-Old Woman With a Pacemaker and High Atrial Rates"
},
{
"authors": "Jeff S. Healey, MD, MSc, FRCPC; Jonathan P. Piccini, MD, MHS, FACC; Christian T. Ruff, MD, MPH",
"content": [
"The results of the ARTESiA and NOAH-AF trials will have major implications for the viability and logistics of population-based atrial fibrillation screening.[7,8,15] Short-lasting subclinical atrial fibrillation is much more common than clinical atrial fibrillation that is detected with single time-point, ECG-based screening. However, detecting such episodes is cumbersome and more costly. If treating subclinical atrial fibrillation proves worthwhile, then screening for longer periods of time may prove to be a cost-effective way to prevent stroke in the population.[15] Otherwise, simple intermittent screening or symptom-driven investigations may be preferred.",
"Insights from pacemaker studies and studies in a more general population have shown that subclinical atrial fibrillation is common and associated with stroke.[17] Ongoing studies will determine when and how patients with subclinical atrial fibrillation should be treated and will help clarify whether population-based screening for atrial fibrillation is a cost-effective stroke-prevention strategy."
],
"date": "March 22, 2019",
"figures": [],
"markdown": "# A 79-Year-Old Woman With a Pacemaker and High Atrial Rates\n\n **Authors:** Jeff S. Healey, MD, MSc, FRCPC; Jonathan P. Piccini, MD, MHS, FACC; Christian T. Ruff, MD, MPH \n **Date:** March 22, 2019\n\n ## Content\n\n The results of the ARTESiA and NOAH-AF trials will have major implications for the viability and logistics of population-based atrial fibrillation screening.[7,8,15] Short-lasting subclinical atrial fibrillation is much more common than clinical atrial fibrillation that is detected with single time-point, ECG-based screening. However, detecting such episodes is cumbersome and more costly. If treating subclinical atrial fibrillation proves worthwhile, then screening for longer periods of time may prove to be a cost-effective way to prevent stroke in the population.[15] Otherwise, simple intermittent screening or symptom-driven investigations may be preferred.\nInsights from pacemaker studies and studies in a more general population have shown that subclinical atrial fibrillation is common and associated with stroke.[17] Ongoing studies will determine when and how patients with subclinical atrial fibrillation should be treated and will help clarify whether population-based screening for atrial fibrillation is a cost-effective stroke-prevention strategy.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1338902,
"choiceText": "Treatment with a beta-blocker or calcium channel blocker",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1338904,
"choiceText": "Treatment with amiodarone",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1338906,
"choiceText": "Catheter ablation",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1338908,
"choiceText": "Watchful waiting",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>Currently, the main indication to suppress atrial fibrillation with an antiarrhythmic drug is the control of symptoms. This patient in this case is completely asymptomatic, so this is not required. Rate-controlling agents may be useful in patients with rapid atrial fibrillation (> 110 beats/min at rest) to control symptoms and to prevent the development of heart failure and/or left ventricular dysfunction.<sup type=\"ref\">[18]</sup> However, this patient has clearly gone on to develop persistent complete AV block; thus, her ventricular rate is entirely under the control of the pacemaker and is therefore not at risk of increasing. She does not have a prior history of myocardial infarction or left ventricular systolic dysfunction, so she has no other indication for beta-blocker therapy.</p>\r\n\r\n<p>More recent data from the CASTLE-AF trial suggest that ablation of atrial fibrillation may improve heart failure outcomes in patients with symptomatic heart failure and left ventricular systolic dysfunction.<sup type=\"ref\">[19]</sup> However, the patients in this trial all had a much higher burden with typical clinical atrial fibrillation. Thus, the results of CASTLE-AF do not apply to the patient in this case, who has a markedly lower burden of atrial fibrillation, no history of heart failure, and normal left ventricular systolic function.</p>",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 427041,
"questionText": "Which of the following is the appropriate next step in the treatment of the subclinical atrial fibrillation described in the patient in this case?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1338910,
"choiceText": "Treat with an oral anticoagulant (direct oral anticoagulant or warfarin) immediately",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1338912,
"choiceText": "Treat with long-term antiplatelet medications",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1338914,
"choiceText": "Treat with daily aspirin",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1338916,
"choiceText": "Monitor and treat with an anticoagulant if atrial fibrillation is detected or if longer, device-detected episodes are documented",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>In this case, the atrial high-rate event is indeed an atrial arrhythmia and is termed subclinical atrial fibrillation because it is short-lasting, asymptomatic, and detected only with long-term continuous monitoring. Although the atrial ECG appears regular, this is only the atrial activity at the location of the atrial pacing lead, which often appears regular (due to a prolonged local atrial refractory period), even if the patient's ECG shows atrial fibrillation. An analysis from the ASSERT study suggests that the stroke risk for such highly organized episodes is similar to episodes with disorganized atrial activity<sup type=\"ref\">[13]</sup>; thus, irrespective of apparent atrial activity, these episodes are simply termed subclinical atrial fibrillation.</p>\r\n\r\n<p>The risk-benefit ratio of treating subclinical atrial fibrillation with long-term oral anticoagulation remains unclear. This is particularly true in older individuals (such as the patient in this case) with some degree of renal insufficiency who are at higher risk of bleeding. Current guidelines and position statements give different recommendations for the management of subclinical atrial fibrillation. Some recommend surface-ECG monitoring for atrial fibrillation (and initiation of treatment if detected), whereas others recommend initiation of treatment if longer, device-detected episodes are documented.<sup type=\"ref\">[11]</sup> The definition of \"longer\" also varies, ranging from a burden of 5.5 hours/day<sup type=\"ref\">[20]</sup> to the presence of at least one continuous episode of longer than 24 hours.<sup type=\"ref\">[21]</sup></p>\r\n\r\n<p>Two large randomized trials are ongoing to determine the best strategy to manage patients with pacemaker or defibrillator-detected subclinical atrial fibrillation and are expected to report data within the next 3-4 years.<sup type=\"ref\">[7,8]</sup> Until then, both options described are reasonable and supported by experts.</p>\r\n\r\n<p>In this case, empiric treatment with an oral anticoagulant or antiplatelet medication does not appear justified, as only three episodes were detected over the past 8 years, the longest of which was less than 1 hour in duration. Both the TRENDS and ASSERT trials failed to demonstrate that episodes this brief were even associated with an increased risk for stroke, let alone that such patients would benefit from chronic oral anticoagulation.<sup type=\"ref\">[5,13]</sup> In this patient, the presence of an old lacunar stroke on neuroimaging does not alter this recommendation. Lacunar strokes are common in elderly patients with hypertension and are not felt to be due to cardioembolism nor benefit from chronic treatment with oral anticoagulation.</p>",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 427043,
"questionText": "Which of the following is most appropriate in terms of managing the risk for stroke for the patient in this case?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 79-Year-Old Woman With a Pacemaker and High Atrial Rates"
},
{
"authors": "Jeff S. Healey, MD, MSc, FRCPC; Jonathan P. Piccini, MD, MHS, FACC; Christian T. Ruff, MD, MPH",
"content": [],
"date": "March 22, 2019",
"figures": [],
"markdown": "# A 79-Year-Old Woman With a Pacemaker and High Atrial Rates\n\n **Authors:** Jeff S. Healey, MD, MSc, FRCPC; Jonathan P. Piccini, MD, MHS, FACC; Christian T. Ruff, MD, MPH \n **Date:** March 22, 2019\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1338902,
"choiceText": "Treatment with a beta-blocker or calcium channel blocker",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1338904,
"choiceText": "Treatment with amiodarone",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1338906,
"choiceText": "Catheter ablation",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1338908,
"choiceText": "Watchful waiting",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>Currently, the main indication to suppress atrial fibrillation with an antiarrhythmic drug is the control of symptoms. This patient in this case is completely asymptomatic, so this is not required. Rate-controlling agents may be useful in patients with rapid atrial fibrillation (> 110 beats/min at rest) to control symptoms and to prevent the development of heart failure and/or left ventricular dysfunction.<sup type=\"ref\">[18]</sup> However, this patient has clearly gone on to develop persistent complete AV block; thus, her ventricular rate is entirely under the control of the pacemaker and is therefore not at risk of increasing. She does not have a prior history of myocardial infarction or left ventricular systolic dysfunction, so she has no other indication for beta-blocker therapy.</p>\r\n\r\n<p>More recent data from the CASTLE-AF trial suggest that ablation of atrial fibrillation may improve heart failure outcomes in patients with symptomatic heart failure and left ventricular systolic dysfunction.<sup type=\"ref\">[19]</sup> However, the patients in this trial all had a much higher burden with typical clinical atrial fibrillation. Thus, the results of CASTLE-AF do not apply to the patient in this case, who has a markedly lower burden of atrial fibrillation, no history of heart failure, and normal left ventricular systolic function.</p>",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 427041,
"questionText": "Which of the following is the appropriate next step in the treatment of the subclinical atrial fibrillation described in the patient in this case?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1338910,
"choiceText": "Treat with an oral anticoagulant (direct oral anticoagulant or warfarin) immediately",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1338912,
"choiceText": "Treat with long-term antiplatelet medications",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1338914,
"choiceText": "Treat with daily aspirin",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1338916,
"choiceText": "Monitor and treat with an anticoagulant if atrial fibrillation is detected or if longer, device-detected episodes are documented",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>In this case, the atrial high-rate event is indeed an atrial arrhythmia and is termed subclinical atrial fibrillation because it is short-lasting, asymptomatic, and detected only with long-term continuous monitoring. Although the atrial ECG appears regular, this is only the atrial activity at the location of the atrial pacing lead, which often appears regular (due to a prolonged local atrial refractory period), even if the patient's ECG shows atrial fibrillation. An analysis from the ASSERT study suggests that the stroke risk for such highly organized episodes is similar to episodes with disorganized atrial activity<sup type=\"ref\">[13]</sup>; thus, irrespective of apparent atrial activity, these episodes are simply termed subclinical atrial fibrillation.</p>\r\n\r\n<p>The risk-benefit ratio of treating subclinical atrial fibrillation with long-term oral anticoagulation remains unclear. This is particularly true in older individuals (such as the patient in this case) with some degree of renal insufficiency who are at higher risk of bleeding. Current guidelines and position statements give different recommendations for the management of subclinical atrial fibrillation. Some recommend surface-ECG monitoring for atrial fibrillation (and initiation of treatment if detected), whereas others recommend initiation of treatment if longer, device-detected episodes are documented.<sup type=\"ref\">[11]</sup> The definition of \"longer\" also varies, ranging from a burden of 5.5 hours/day<sup type=\"ref\">[20]</sup> to the presence of at least one continuous episode of longer than 24 hours.<sup type=\"ref\">[21]</sup></p>\r\n\r\n<p>Two large randomized trials are ongoing to determine the best strategy to manage patients with pacemaker or defibrillator-detected subclinical atrial fibrillation and are expected to report data within the next 3-4 years.<sup type=\"ref\">[7,8]</sup> Until then, both options described are reasonable and supported by experts.</p>\r\n\r\n<p>In this case, empiric treatment with an oral anticoagulant or antiplatelet medication does not appear justified, as only three episodes were detected over the past 8 years, the longest of which was less than 1 hour in duration. Both the TRENDS and ASSERT trials failed to demonstrate that episodes this brief were even associated with an increased risk for stroke, let alone that such patients would benefit from chronic oral anticoagulation.<sup type=\"ref\">[5,13]</sup> In this patient, the presence of an old lacunar stroke on neuroimaging does not alter this recommendation. Lacunar strokes are common in elderly patients with hypertension and are not felt to be due to cardioembolism nor benefit from chronic treatment with oral anticoagulation.</p>",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 427043,
"questionText": "Which of the following is most appropriate in terms of managing the risk for stroke for the patient in this case?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 79-Year-Old Woman With a Pacemaker and High Atrial Rates"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1338894,
"choiceText": "Atrial flutter",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1338896,
"choiceText": "Atrial premature beats",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1338898,
"choiceText": "Atrial fibrillation",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1338900,
"choiceText": "Subclinical atrial fibrillation",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 427039,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1338902,
"choiceText": "Treatment with a beta-blocker or calcium channel blocker",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1338904,
"choiceText": "Treatment with amiodarone",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1338906,
"choiceText": "Catheter ablation",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1338908,
"choiceText": "Watchful waiting",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>Currently, the main indication to suppress atrial fibrillation with an antiarrhythmic drug is the control of symptoms. This patient in this case is completely asymptomatic, so this is not required. Rate-controlling agents may be useful in patients with rapid atrial fibrillation (> 110 beats/min at rest) to control symptoms and to prevent the development of heart failure and/or left ventricular dysfunction.<sup type=\"ref\">[18]</sup> However, this patient has clearly gone on to develop persistent complete AV block; thus, her ventricular rate is entirely under the control of the pacemaker and is therefore not at risk of increasing. She does not have a prior history of myocardial infarction or left ventricular systolic dysfunction, so she has no other indication for beta-blocker therapy.</p>\r\n\r\n<p>More recent data from the CASTLE-AF trial suggest that ablation of atrial fibrillation may improve heart failure outcomes in patients with symptomatic heart failure and left ventricular systolic dysfunction.<sup type=\"ref\">[19]</sup> However, the patients in this trial all had a much higher burden with typical clinical atrial fibrillation. Thus, the results of CASTLE-AF do not apply to the patient in this case, who has a markedly lower burden of atrial fibrillation, no history of heart failure, and normal left ventricular systolic function.</p>",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 427041,
"questionText": "Which of the following is the appropriate next step in the treatment of the subclinical atrial fibrillation described in the patient in this case?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1338910,
"choiceText": "Treat with an oral anticoagulant (direct oral anticoagulant or warfarin) immediately",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1338912,
"choiceText": "Treat with long-term antiplatelet medications",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1338914,
"choiceText": "Treat with daily aspirin",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1338916,
"choiceText": "Monitor and treat with an anticoagulant if atrial fibrillation is detected or if longer, device-detected episodes are documented",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>In this case, the atrial high-rate event is indeed an atrial arrhythmia and is termed subclinical atrial fibrillation because it is short-lasting, asymptomatic, and detected only with long-term continuous monitoring. Although the atrial ECG appears regular, this is only the atrial activity at the location of the atrial pacing lead, which often appears regular (due to a prolonged local atrial refractory period), even if the patient's ECG shows atrial fibrillation. An analysis from the ASSERT study suggests that the stroke risk for such highly organized episodes is similar to episodes with disorganized atrial activity<sup type=\"ref\">[13]</sup>; thus, irrespective of apparent atrial activity, these episodes are simply termed subclinical atrial fibrillation.</p>\r\n\r\n<p>The risk-benefit ratio of treating subclinical atrial fibrillation with long-term oral anticoagulation remains unclear. This is particularly true in older individuals (such as the patient in this case) with some degree of renal insufficiency who are at higher risk of bleeding. Current guidelines and position statements give different recommendations for the management of subclinical atrial fibrillation. Some recommend surface-ECG monitoring for atrial fibrillation (and initiation of treatment if detected), whereas others recommend initiation of treatment if longer, device-detected episodes are documented.<sup type=\"ref\">[11]</sup> The definition of \"longer\" also varies, ranging from a burden of 5.5 hours/day<sup type=\"ref\">[20]</sup> to the presence of at least one continuous episode of longer than 24 hours.<sup type=\"ref\">[21]</sup></p>\r\n\r\n<p>Two large randomized trials are ongoing to determine the best strategy to manage patients with pacemaker or defibrillator-detected subclinical atrial fibrillation and are expected to report data within the next 3-4 years.<sup type=\"ref\">[7,8]</sup> Until then, both options described are reasonable and supported by experts.</p>\r\n\r\n<p>In this case, empiric treatment with an oral anticoagulant or antiplatelet medication does not appear justified, as only three episodes were detected over the past 8 years, the longest of which was less than 1 hour in duration. Both the TRENDS and ASSERT trials failed to demonstrate that episodes this brief were even associated with an increased risk for stroke, let alone that such patients would benefit from chronic oral anticoagulation.<sup type=\"ref\">[5,13]</sup> In this patient, the presence of an old lacunar stroke on neuroimaging does not alter this recommendation. Lacunar strokes are common in elderly patients with hypertension and are not felt to be due to cardioembolism nor benefit from chronic treatment with oral anticoagulation.</p>",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 427043,
"questionText": "Which of the following is most appropriate in terms of managing the risk for stroke for the patient in this case?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
908904 | /viewarticle/908904 | [
{
"authors": "Nazem Akoum, MD, MS; Steven A. Lubitz, MD, MPH; Zachary D. Goldberger, MD, MSc, FACC",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to recognize accurately. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a Case Challenge, please contact us.",
"A 67-year-old man presents after experiencing palpitations while having dinner with his wife. He describes his heart as \"flopping around\" in his chest. This sensation lasted for a few minutes and was suddenly followed by lightheadedness and a transient loss of consciousness. His wife reports that he woke up almost right away, within a few seconds. He felt normal afterward. He denies any prodrome and any symptoms of vertigo.",
"During his interview, he discloses a similar episode about 1 month prior, albeit of milder intensity. That episode was not associated with a loss of consciousness. His past medical history includes hypertension, which is controlled with a diuretic and an angiotensin receptor blocker, and Gilbert disease without significant liver dysfunction. He takes a baby aspirin daily.",
"He is active, exercises regularly, and works a full-time job. He has not had any symptoms such as chest pain, shortness of breath, or palpitations, presyncope, or syncope associated with exercise. He does not smoke or consume alcohol. He has no known food or drug allergies. His family history includes hypertension on his maternal side without any history of syncope or unexplained sudden death."
],
"date": "February 19, 2019",
"figures": [],
"markdown": "# A 67-Year-Old Man With Palpitations and Syncope\n\n **Authors:** Nazem Akoum, MD, MS; Steven A. Lubitz, MD, MPH; Zachary D. Goldberger, MD, MSc, FACC \n **Date:** February 19, 2019\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to recognize accurately. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a Case Challenge, please contact us.\nA 67-year-old man presents after experiencing palpitations while having dinner with his wife. He describes his heart as \"flopping around\" in his chest. This sensation lasted for a few minutes and was suddenly followed by lightheadedness and a transient loss of consciousness. His wife reports that he woke up almost right away, within a few seconds. He felt normal afterward. He denies any prodrome and any symptoms of vertigo.\nDuring his interview, he discloses a similar episode about 1 month prior, albeit of milder intensity. That episode was not associated with a loss of consciousness. His past medical history includes hypertension, which is controlled with a diuretic and an angiotensin receptor blocker, and Gilbert disease without significant liver dysfunction. He takes a baby aspirin daily.\nHe is active, exercises regularly, and works a full-time job. He has not had any symptoms such as chest pain, shortness of breath, or palpitations, presyncope, or syncope associated with exercise. He does not smoke or consume alcohol. He has no known food or drug allergies. His family history includes hypertension on his maternal side without any history of syncope or unexplained sudden death.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 67-Year-Old Man With Palpitations and Syncope"
},
{
"authors": "Nazem Akoum, MD, MS; Steven A. Lubitz, MD, MPH; Zachary D. Goldberger, MD, MSc, FACC",
"content": [
"Physical examination in the office revealed a healthy-appearing man in no distress. His vital signs were normal. His physical examination findings were also normal, including a thorough cardiovascular evaluation that revealed no abnormal heart sounds including murmurs, rubs, or gallops. He had normal carotid pulses, as well as in his upper and lower extremities. An ECG revealed normal sinus rhythm with normal conduction intervals, with no evidence of ischemia and no signs of a prior myocardial infarction. An echocardiogram showed mild left ventricular hypertrophy and normal biventricular function, normal valve function, mild atrial enlargement, and Doppler evidence of impaired ventricular relaxation. Complete blood count and comprehensive chemistry panel findings were normal, except for mildly elevated bilirubin. His thyroid-stimulating-hormone level was normal.",
"Ambulatory cardiac monitoring with a 30-day event monitor revealed electrocardiograms such as the one shown in the figure below, followed by resumption of sinus rhythm associated with symptoms of dizziness.",
"Fig. 1"
],
"date": "February 19, 2019",
"figures": [
{
"caption": "Fig. 1",
"image_url": "https://img.medscapestatic.com/article/908/904/908904-figure-1-thumb.jpg"
}
],
"markdown": "# A 67-Year-Old Man With Palpitations and Syncope\n\n **Authors:** Nazem Akoum, MD, MS; Steven A. Lubitz, MD, MPH; Zachary D. Goldberger, MD, MSc, FACC \n **Date:** February 19, 2019\n\n ## Content\n\n Physical examination in the office revealed a healthy-appearing man in no distress. His vital signs were normal. His physical examination findings were also normal, including a thorough cardiovascular evaluation that revealed no abnormal heart sounds including murmurs, rubs, or gallops. He had normal carotid pulses, as well as in his upper and lower extremities. An ECG revealed normal sinus rhythm with normal conduction intervals, with no evidence of ischemia and no signs of a prior myocardial infarction. An echocardiogram showed mild left ventricular hypertrophy and normal biventricular function, normal valve function, mild atrial enlargement, and Doppler evidence of impaired ventricular relaxation. Complete blood count and comprehensive chemistry panel findings were normal, except for mildly elevated bilirubin. His thyroid-stimulating-hormone level was normal.\nAmbulatory cardiac monitoring with a 30-day event monitor revealed electrocardiograms such as the one shown in the figure below, followed by resumption of sinus rhythm associated with symptoms of dizziness.\nFig. 1\n\n ## Figures\n\n **Fig. 1** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1325350,
"choiceText": "Volume depletion",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1325352,
"choiceText": "Neurocardiogenic syncope",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1325354,
"choiceText": "Cardiac arrhythmia",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1325356,
"choiceText": "Liver cirrhosis",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1325358,
"choiceText": "Cerebrovascular ischemia",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 422889,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 67-Year-Old Man With Palpitations and Syncope"
},
{
"authors": "Nazem Akoum, MD, MS; Steven A. Lubitz, MD, MPH; Zachary D. Goldberger, MD, MSc, FACC",
"content": [
"The patient's episodes are not suggestive of volume depletion or neurocardiogenic etiologies. Volume depletion typically causes positional symptoms, which were not present in this case. In the case of neurocardiogenic syncope, excessive vagal stimulation leads to significant bradycardia, vasodilatation, or both. Vagal stimulation is classically associated with triggers such as painful stimuli, which were notably absent in this case. Atypical triggers can be present. Of note, after an episode of neurocardiogenic syncope, a period of fatigue or feeing confused may follow, even for hours. The patient's history of Gilbert disease is unrelated to this presentation. Gilbert disease does not typically cause liver cirrhosis and the patient's symptoms are also not suggestive of advanced liver cirrhosis, transient ischemic attacks, or stroke. Therefore, the most likely diagnosis explaining this patient's symptoms is a cardiac arrhythmia.",
"Arrhythmic causes of syncope include both bradycardic (sinus node or atrioventricular conduction disease) and tachycardic (supraventricular or ventricular) etiologies. As the ambulatory cardiac monitoring suggests, the patient's symptoms of palpitations are due to tachycardia associated with atrial fibrillation (note the irregularly irregular rhythm that dominates most of the strip shown above), whereas his near syncope as well as recent history of true transient loss of consciousness and syncope are likely due to bradycardia and prolonged pauses observed upon termination of the paroxysms of atrial fibrillation.",
"The patient is manifesting classic findings of tachycardia-bradycardia, or \"tachy-brady,\" syndrome, one of the common findings in sinus node dysfunction and a common cause of arrhythmia-related syncope. The tachycardia in this setting is usually due to atrial fibrillation or atrial flutter, and the bradycardia is due to a prolonged sinus node recovery following cessation of the tachyarrhythmia. In addition to tachy-brady syndrome, sinus node dysfunction also includes a broad spectrum of diagnoses, including symptomatic sinus bradycardia, chronotropic incompetence, sinoatrial exit block, and sinus arrhythmia."
],
"date": "February 19, 2019",
"figures": [],
"markdown": "# A 67-Year-Old Man With Palpitations and Syncope\n\n **Authors:** Nazem Akoum, MD, MS; Steven A. Lubitz, MD, MPH; Zachary D. Goldberger, MD, MSc, FACC \n **Date:** February 19, 2019\n\n ## Content\n\n The patient's episodes are not suggestive of volume depletion or neurocardiogenic etiologies. Volume depletion typically causes positional symptoms, which were not present in this case. In the case of neurocardiogenic syncope, excessive vagal stimulation leads to significant bradycardia, vasodilatation, or both. Vagal stimulation is classically associated with triggers such as painful stimuli, which were notably absent in this case. Atypical triggers can be present. Of note, after an episode of neurocardiogenic syncope, a period of fatigue or feeing confused may follow, even for hours. The patient's history of Gilbert disease is unrelated to this presentation. Gilbert disease does not typically cause liver cirrhosis and the patient's symptoms are also not suggestive of advanced liver cirrhosis, transient ischemic attacks, or stroke. Therefore, the most likely diagnosis explaining this patient's symptoms is a cardiac arrhythmia.\nArrhythmic causes of syncope include both bradycardic (sinus node or atrioventricular conduction disease) and tachycardic (supraventricular or ventricular) etiologies. As the ambulatory cardiac monitoring suggests, the patient's symptoms of palpitations are due to tachycardia associated with atrial fibrillation (note the irregularly irregular rhythm that dominates most of the strip shown above), whereas his near syncope as well as recent history of true transient loss of consciousness and syncope are likely due to bradycardia and prolonged pauses observed upon termination of the paroxysms of atrial fibrillation.\nThe patient is manifesting classic findings of tachycardia-bradycardia, or \"tachy-brady,\" syndrome, one of the common findings in sinus node dysfunction and a common cause of arrhythmia-related syncope. The tachycardia in this setting is usually due to atrial fibrillation or atrial flutter, and the bradycardia is due to a prolonged sinus node recovery following cessation of the tachyarrhythmia. In addition to tachy-brady syndrome, sinus node dysfunction also includes a broad spectrum of diagnoses, including symptomatic sinus bradycardia, chronotropic incompetence, sinoatrial exit block, and sinus arrhythmia.\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1325350,
"choiceText": "Volume depletion",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1325352,
"choiceText": "Neurocardiogenic syncope",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1325354,
"choiceText": "Cardiac arrhythmia",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1325356,
"choiceText": "Liver cirrhosis",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1325358,
"choiceText": "Cerebrovascular ischemia",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 422889,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 67-Year-Old Man With Palpitations and Syncope"
},
{
"authors": "Nazem Akoum, MD, MS; Steven A. Lubitz, MD, MPH; Zachary D. Goldberger, MD, MSc, FACC",
"content": [
"Management of sinus node dysfunction in the setting of tachy-brady syndrome can be challenging. Drugs used for rate control during periods of atrial fibrillation (eg, beta-blockers, calcium-channel blockers, digoxin) often exacerbate the bradycardia and pauses that are usually seen upon termination of the tachyarrhythmia. These drugs can also cause slow ventricular conduction during atrial fibrillation.",
"The use of Vaughan Williams Class III antiarrhythmic agents (eg, amiodarone, dronedarone, sotalol) or class IC agents (eg, flecainide or propafenone combined with an atrioventricular nodal blocking agent) may exacerbate the bradycardia and pauses, as seen with rate-control agents.",
"Of note, dofetilide is least likely to cause bradycardia. Like other Class III agents, dofetilide is a potassium-channel blocker but selectively targets the slow inward rectifier potassium current. It prolongs repolarization without affecting the rate of spontaneous depolarization in the sinus node cells. However, it requires a mandatory inpatient admission for initiation, given the potential for QT prolongation with risk for torsades de pointes, necessitating frequent ECG monitoring.",
"The implantation of a permanent pacemaker is often recommended in patients with tachy-brady syndrome.[1] The pacemaker is usually programmed in a demand mode and starts pacing whenever the heart rate drops below a certain threshold, thereby eliminating all pauses and periods of bradycardia that follow termination of atrial arrhythmias.",
"A question that commonly arises in patients with atrial fibrillation is whether suppression of the atrial tachyarrhythmia, using catheter ablation rather than antiarrhythmic drugs, could eliminate the termination pauses and bradycardia associated with the use of antiarrhythmic and rate-control agents and therefore avert the need for a permanent pacemaker. The answer is not straightforward, as the pathophysiology of sinus node dysfunction in the setting of atrial fibrillation is complex.",
"Anatomically, the sinus node is a compact structure located in the superior lateral region of the right atrium near the junction with the superior vena cava. The sinus node is made of specialized pacemaker cells that exhibit spontaneous electrical depolarization. These cells are shielded from neighboring atrial myocytes by connective tissue and transitional cells.[2,3] The sinus node receives parasympathetic and sympathetic autonomic nervous stimulation that determines the rate of firing or spontaneous depolarization.",
"In the setting of atrial arrhythmias including atrial fibrillation and atrial flutter, studies have demonstrated that the rapid electrical rhythm is associated with electrical remodeling that leads to longer sinus node recovery times.[4,5] Electrical remodeling in atrial fibrillation manifests with an increase in the dispersion of action potential durations. This electrical phenomenon is reversed with restoration and maintenance of sinus rhythm.[6,7] Some studies of patients with atrial fibrillation and sinus node disease have demonstrated that some patients who undergo successful catheter ablation demonstrate an improvement in sinus node dysfunction.[8] However, other studies have shown that some patients still require permanent pacing for bradycardia and pauses.[9] The discrepancy in the findings is likely related to fibrotic involvement of the sinus node, or structural remodeling, that leads to the clinical manifestations of sinus node disease. Excessive fibrosis involving the sinus node is not expected to be reversible; hence, for these patients, permanent pacing is unavoidable."
],
"date": "February 19, 2019",
"figures": [],
"markdown": "# A 67-Year-Old Man With Palpitations and Syncope\n\n **Authors:** Nazem Akoum, MD, MS; Steven A. Lubitz, MD, MPH; Zachary D. Goldberger, MD, MSc, FACC \n **Date:** February 19, 2019\n\n ## Content\n\n Management of sinus node dysfunction in the setting of tachy-brady syndrome can be challenging. Drugs used for rate control during periods of atrial fibrillation (eg, beta-blockers, calcium-channel blockers, digoxin) often exacerbate the bradycardia and pauses that are usually seen upon termination of the tachyarrhythmia. These drugs can also cause slow ventricular conduction during atrial fibrillation.\nThe use of Vaughan Williams Class III antiarrhythmic agents (eg, amiodarone, dronedarone, sotalol) or class IC agents (eg, flecainide or propafenone combined with an atrioventricular nodal blocking agent) may exacerbate the bradycardia and pauses, as seen with rate-control agents.\nOf note, dofetilide is least likely to cause bradycardia. Like other Class III agents, dofetilide is a potassium-channel blocker but selectively targets the slow inward rectifier potassium current. It prolongs repolarization without affecting the rate of spontaneous depolarization in the sinus node cells. However, it requires a mandatory inpatient admission for initiation, given the potential for QT prolongation with risk for torsades de pointes, necessitating frequent ECG monitoring.\nThe implantation of a permanent pacemaker is often recommended in patients with tachy-brady syndrome.[1] The pacemaker is usually programmed in a demand mode and starts pacing whenever the heart rate drops below a certain threshold, thereby eliminating all pauses and periods of bradycardia that follow termination of atrial arrhythmias.\nA question that commonly arises in patients with atrial fibrillation is whether suppression of the atrial tachyarrhythmia, using catheter ablation rather than antiarrhythmic drugs, could eliminate the termination pauses and bradycardia associated with the use of antiarrhythmic and rate-control agents and therefore avert the need for a permanent pacemaker. The answer is not straightforward, as the pathophysiology of sinus node dysfunction in the setting of atrial fibrillation is complex.\nAnatomically, the sinus node is a compact structure located in the superior lateral region of the right atrium near the junction with the superior vena cava. The sinus node is made of specialized pacemaker cells that exhibit spontaneous electrical depolarization. These cells are shielded from neighboring atrial myocytes by connective tissue and transitional cells.[2,3] The sinus node receives parasympathetic and sympathetic autonomic nervous stimulation that determines the rate of firing or spontaneous depolarization.\nIn the setting of atrial arrhythmias including atrial fibrillation and atrial flutter, studies have demonstrated that the rapid electrical rhythm is associated with electrical remodeling that leads to longer sinus node recovery times.[4,5] Electrical remodeling in atrial fibrillation manifests with an increase in the dispersion of action potential durations. This electrical phenomenon is reversed with restoration and maintenance of sinus rhythm.[6,7] Some studies of patients with atrial fibrillation and sinus node disease have demonstrated that some patients who undergo successful catheter ablation demonstrate an improvement in sinus node dysfunction.[8] However, other studies have shown that some patients still require permanent pacing for bradycardia and pauses.[9] The discrepancy in the findings is likely related to fibrotic involvement of the sinus node, or structural remodeling, that leads to the clinical manifestations of sinus node disease. Excessive fibrosis involving the sinus node is not expected to be reversible; hence, for these patients, permanent pacing is unavoidable.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 67-Year-Old Man With Palpitations and Syncope"
},
{
"authors": "Nazem Akoum, MD, MS; Steven A. Lubitz, MD, MPH; Zachary D. Goldberger, MD, MSc, FACC",
"content": [
"Advancing age is associated with an increase in the collagen content of the sinus node.[10] Age is also associated with an increase in the sinoatrial conduction time, another manifestation of sinus node dysfunction. Of note, patients with age-related sinus node fibrosis also demonstrated fibrosis involving the atrioventricular node.[10]",
"For patients with tachy-brady syndrome and symptoms of both atrial fibrillation and sinus node dysfunction, treatment of both conditions may be necessary. With prolonged pauses causing presyncope or syncope, a pacemaker implantation is performed first to prevent associated injury; atrial fibrillation is addressed as a subsequent step. For patients without prolonged pauses or syncope but with symptomatic bradycardia associated with the use of antiarrhythmic or rate control agents, considering catheter ablation for atrial fibrillation first to allow for removal of these drugs is reasonable. However, patients with extensive sinus node fibrosis may still require permanent pacing despite successful ablation.[9]"
],
"date": "February 19, 2019",
"figures": [],
"markdown": "# A 67-Year-Old Man With Palpitations and Syncope\n\n **Authors:** Nazem Akoum, MD, MS; Steven A. Lubitz, MD, MPH; Zachary D. Goldberger, MD, MSc, FACC \n **Date:** February 19, 2019\n\n ## Content\n\n Advancing age is associated with an increase in the collagen content of the sinus node.[10] Age is also associated with an increase in the sinoatrial conduction time, another manifestation of sinus node dysfunction. Of note, patients with age-related sinus node fibrosis also demonstrated fibrosis involving the atrioventricular node.[10]\nFor patients with tachy-brady syndrome and symptoms of both atrial fibrillation and sinus node dysfunction, treatment of both conditions may be necessary. With prolonged pauses causing presyncope or syncope, a pacemaker implantation is performed first to prevent associated injury; atrial fibrillation is addressed as a subsequent step. For patients without prolonged pauses or syncope but with symptomatic bradycardia associated with the use of antiarrhythmic or rate control agents, considering catheter ablation for atrial fibrillation first to allow for removal of these drugs is reasonable. However, patients with extensive sinus node fibrosis may still require permanent pacing despite successful ablation.[9]\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1325360,
"choiceText": "A permanent pacemaker implant",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1325362,
"choiceText": "Catheter ablation for atrial fibrillation",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1325364,
"choiceText": "Amiodarone drug therapy",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1325366,
"choiceText": "An implantable cardioverter defibrillator",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "With prolonged pauses associated with syncope, not associated with the use of antiarrhythmic or rate control agents, a permanent pacemaker is the best answer. Catheter ablation may be considered; however, it may not avert the need for permanent pacing. Amiodarone therapy may worsen bradycardia. The patient has no indication for an implanted defibrillator.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 422891,
"questionText": "Given the findings on the ambulatory event monitor described in this patient, which of the following is the most appropriate next step?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1325368,
"choiceText": "Switch to clopidogrel",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1325370,
"choiceText": "Both aspirin and clopidogrel",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1325372,
"choiceText": "Switch to oral anticoagulation (OAC)",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1325374,
"choiceText": "Both aspirin and OAC",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>Guidelines for the management of atrial fibrillation recommend using the CHA<sub>2</sub>DS<sub>2</sub>-VASc score for thromboembolism risk assessment. The patient scores 2 points for his age of 67 years and hypertension on drug therapy.<sup type=\"ref\">[11]</sup> Guidelines support anticoagulation with a vitamin K antagonist (ie, warfarin) or a direct anticoagulant with a CHA<sub>2</sub>DS<sub>2</sub>-VASc score of at least 2.</p>\r\n\r\n<p>Of note, women receive a score of 1 because of their female gender, but they would merit anticoagulation in the context of an additional risk factor. A CHA<sub>2</sub>DS<sub>2</sub>-VASc score of 1 is more challenging, and guidelines allow for using aspirin alone, oral anticoagulation, or neither. This comes with a class IIb recommendation, indicating diverging expert opinion and absence of strong evidence for usefulness or efficacy. Of the options provided, a switch to OAC is the best answer. Clopidogrel in combination with aspirin is inferior to OAC with warfarin in reducing thromboembolism risk, and this combination has been associated with an increased risk for bleeding.<sup type=\"ref\">[12]</sup> The same applies for the combination of aspirin and OAC.<sup type=\"ref\">[13]</sup> Clopidogrel alone has not been studied in thromboembolism prevention in atrial fibrillation. </p>\r\n\r\n<p>The choice of anticoagulation with warfarin, a vitamin K antagonist OAC, or nonvitamin-K-antagonist oral anticoagulants (NOACs), also referred to as direct oral anticoagulants (DOACs) depends on various patient factors. These include renal and hepatic function, the presence of mechanical heart valves, drug–drug interactions, and bleeding risk. The major advantage of NOACs compared with warfarin is ease of use, as the pharmacology of these agents allows for quick onset of action as well as daily or twice daily dosing with a predictable therapeutic effect. This is in contrast to warfarin, which has a slow onset of action and requires monitoring of the prothrombin time. NOACs are not an option for patients with mechanical heart valves. The patient in this case had no contraindication to any of these choices, and his bleeding risk was estimated to be low. He decided on using a NOAC to avoid repeated blood testing.</p>\r\n\r\n<p>The patient in this case was prescribed apixaban and underwent implantation of a permanent dual-chamber pacemaker. He has not had any recurrent syncope during follow-up. However, he continued to experience palpitations because of a progressively increasing burden of atrial fibrillation. These continued despite a trial of antiarrhythmic drug therapy with sotalol. He eventually underwent catheter ablation for atrial fibrillation with pulmonary vein isolation, which effectively suppressed his arrhythmia.</p>",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 422893,
"questionText": "Which of the following is the best strategy to reduce this patient's risk for thromboembolism?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 67-Year-Old Man With Palpitations and Syncope"
},
{
"authors": "Nazem Akoum, MD, MS; Steven A. Lubitz, MD, MPH; Zachary D. Goldberger, MD, MSc, FACC",
"content": [],
"date": "February 19, 2019",
"figures": [],
"markdown": "# A 67-Year-Old Man With Palpitations and Syncope\n\n **Authors:** Nazem Akoum, MD, MS; Steven A. Lubitz, MD, MPH; Zachary D. Goldberger, MD, MSc, FACC \n **Date:** February 19, 2019\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1325360,
"choiceText": "A permanent pacemaker implant",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1325362,
"choiceText": "Catheter ablation for atrial fibrillation",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1325364,
"choiceText": "Amiodarone drug therapy",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1325366,
"choiceText": "An implantable cardioverter defibrillator",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "With prolonged pauses associated with syncope, not associated with the use of antiarrhythmic or rate control agents, a permanent pacemaker is the best answer. Catheter ablation may be considered; however, it may not avert the need for permanent pacing. Amiodarone therapy may worsen bradycardia. The patient has no indication for an implanted defibrillator.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 422891,
"questionText": "Given the findings on the ambulatory event monitor described in this patient, which of the following is the most appropriate next step?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1325368,
"choiceText": "Switch to clopidogrel",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1325370,
"choiceText": "Both aspirin and clopidogrel",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1325372,
"choiceText": "Switch to oral anticoagulation (OAC)",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1325374,
"choiceText": "Both aspirin and OAC",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>Guidelines for the management of atrial fibrillation recommend using the CHA<sub>2</sub>DS<sub>2</sub>-VASc score for thromboembolism risk assessment. The patient scores 2 points for his age of 67 years and hypertension on drug therapy.<sup type=\"ref\">[11]</sup> Guidelines support anticoagulation with a vitamin K antagonist (ie, warfarin) or a direct anticoagulant with a CHA<sub>2</sub>DS<sub>2</sub>-VASc score of at least 2.</p>\r\n\r\n<p>Of note, women receive a score of 1 because of their female gender, but they would merit anticoagulation in the context of an additional risk factor. A CHA<sub>2</sub>DS<sub>2</sub>-VASc score of 1 is more challenging, and guidelines allow for using aspirin alone, oral anticoagulation, or neither. This comes with a class IIb recommendation, indicating diverging expert opinion and absence of strong evidence for usefulness or efficacy. Of the options provided, a switch to OAC is the best answer. Clopidogrel in combination with aspirin is inferior to OAC with warfarin in reducing thromboembolism risk, and this combination has been associated with an increased risk for bleeding.<sup type=\"ref\">[12]</sup> The same applies for the combination of aspirin and OAC.<sup type=\"ref\">[13]</sup> Clopidogrel alone has not been studied in thromboembolism prevention in atrial fibrillation. </p>\r\n\r\n<p>The choice of anticoagulation with warfarin, a vitamin K antagonist OAC, or nonvitamin-K-antagonist oral anticoagulants (NOACs), also referred to as direct oral anticoagulants (DOACs) depends on various patient factors. These include renal and hepatic function, the presence of mechanical heart valves, drug–drug interactions, and bleeding risk. The major advantage of NOACs compared with warfarin is ease of use, as the pharmacology of these agents allows for quick onset of action as well as daily or twice daily dosing with a predictable therapeutic effect. This is in contrast to warfarin, which has a slow onset of action and requires monitoring of the prothrombin time. NOACs are not an option for patients with mechanical heart valves. The patient in this case had no contraindication to any of these choices, and his bleeding risk was estimated to be low. He decided on using a NOAC to avoid repeated blood testing.</p>\r\n\r\n<p>The patient in this case was prescribed apixaban and underwent implantation of a permanent dual-chamber pacemaker. He has not had any recurrent syncope during follow-up. However, he continued to experience palpitations because of a progressively increasing burden of atrial fibrillation. These continued despite a trial of antiarrhythmic drug therapy with sotalol. He eventually underwent catheter ablation for atrial fibrillation with pulmonary vein isolation, which effectively suppressed his arrhythmia.</p>",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 422893,
"questionText": "Which of the following is the best strategy to reduce this patient's risk for thromboembolism?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 67-Year-Old Man With Palpitations and Syncope"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1325350,
"choiceText": "Volume depletion",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1325352,
"choiceText": "Neurocardiogenic syncope",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1325354,
"choiceText": "Cardiac arrhythmia",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1325356,
"choiceText": "Liver cirrhosis",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1325358,
"choiceText": "Cerebrovascular ischemia",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 422889,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1325360,
"choiceText": "A permanent pacemaker implant",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1325362,
"choiceText": "Catheter ablation for atrial fibrillation",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1325364,
"choiceText": "Amiodarone drug therapy",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1325366,
"choiceText": "An implantable cardioverter defibrillator",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "With prolonged pauses associated with syncope, not associated with the use of antiarrhythmic or rate control agents, a permanent pacemaker is the best answer. Catheter ablation may be considered; however, it may not avert the need for permanent pacing. Amiodarone therapy may worsen bradycardia. The patient has no indication for an implanted defibrillator.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 422891,
"questionText": "Given the findings on the ambulatory event monitor described in this patient, which of the following is the most appropriate next step?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1325368,
"choiceText": "Switch to clopidogrel",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1325370,
"choiceText": "Both aspirin and clopidogrel",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1325372,
"choiceText": "Switch to oral anticoagulation (OAC)",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1325374,
"choiceText": "Both aspirin and OAC",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>Guidelines for the management of atrial fibrillation recommend using the CHA<sub>2</sub>DS<sub>2</sub>-VASc score for thromboembolism risk assessment. The patient scores 2 points for his age of 67 years and hypertension on drug therapy.<sup type=\"ref\">[11]</sup> Guidelines support anticoagulation with a vitamin K antagonist (ie, warfarin) or a direct anticoagulant with a CHA<sub>2</sub>DS<sub>2</sub>-VASc score of at least 2.</p>\r\n\r\n<p>Of note, women receive a score of 1 because of their female gender, but they would merit anticoagulation in the context of an additional risk factor. A CHA<sub>2</sub>DS<sub>2</sub>-VASc score of 1 is more challenging, and guidelines allow for using aspirin alone, oral anticoagulation, or neither. This comes with a class IIb recommendation, indicating diverging expert opinion and absence of strong evidence for usefulness or efficacy. Of the options provided, a switch to OAC is the best answer. Clopidogrel in combination with aspirin is inferior to OAC with warfarin in reducing thromboembolism risk, and this combination has been associated with an increased risk for bleeding.<sup type=\"ref\">[12]</sup> The same applies for the combination of aspirin and OAC.<sup type=\"ref\">[13]</sup> Clopidogrel alone has not been studied in thromboembolism prevention in atrial fibrillation. </p>\r\n\r\n<p>The choice of anticoagulation with warfarin, a vitamin K antagonist OAC, or nonvitamin-K-antagonist oral anticoagulants (NOACs), also referred to as direct oral anticoagulants (DOACs) depends on various patient factors. These include renal and hepatic function, the presence of mechanical heart valves, drug–drug interactions, and bleeding risk. The major advantage of NOACs compared with warfarin is ease of use, as the pharmacology of these agents allows for quick onset of action as well as daily or twice daily dosing with a predictable therapeutic effect. This is in contrast to warfarin, which has a slow onset of action and requires monitoring of the prothrombin time. NOACs are not an option for patients with mechanical heart valves. The patient in this case had no contraindication to any of these choices, and his bleeding risk was estimated to be low. He decided on using a NOAC to avoid repeated blood testing.</p>\r\n\r\n<p>The patient in this case was prescribed apixaban and underwent implantation of a permanent dual-chamber pacemaker. He has not had any recurrent syncope during follow-up. However, he continued to experience palpitations because of a progressively increasing burden of atrial fibrillation. These continued despite a trial of antiarrhythmic drug therapy with sotalol. He eventually underwent catheter ablation for atrial fibrillation with pulmonary vein isolation, which effectively suppressed his arrhythmia.</p>",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 422893,
"questionText": "Which of the following is the best strategy to reduce this patient's risk for thromboembolism?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
908341 | /viewarticle/908341 | [
{
"authors": "Jeffrey S. Forrest, MD; Steven Kendell, MD",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"A 66-year-old man was admitted to the hospital for stabilization and workup after sustaining numerous injuries from an apparent fall, including a distal radius (Colles) fracture. Upon admission, the patient was fully oriented. He explained that he fell trying to get out of his bathtub (Figure 1).",
"Figure 1.",
"The patient insisted that he had otherwise been feeling fine and downplayed the need for further assistance. He requested medication for his headache. The patient denied substance abuse.",
"On the second day of admission, the patient developed worsening tremor and diaphoresis. He began to pick at his skin, stating that he wants to get rid of the \"bugs that are crawling on him.\" The patient attempted to pull his intravenous (IV) lines out and turned his head rapidly, stating that he could hear the voice of his deceased spouse. He believed he was in a seafood restaurant.",
"The patient's medical history of note included atrial fibrillation, hypertension, and peripheral vascular disease. During admission, he had stated that he could not remember whether he had been taking his blood pressure medication correctly.",
"The patient stated that he has no family since the passing of his spouse. Family medical history was unobtainable."
],
"date": "January 31, 2019",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/908/341/908341-Thumb1.jpg"
}
],
"markdown": "# A 66-Year-Old Man Who Heard His Deceased Spouse's Voice\n\n **Authors:** Jeffrey S. Forrest, MD; Steven Kendell, MD \n **Date:** January 31, 2019\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nA 66-year-old man was admitted to the hospital for stabilization and workup after sustaining numerous injuries from an apparent fall, including a distal radius (Colles) fracture. Upon admission, the patient was fully oriented. He explained that he fell trying to get out of his bathtub (Figure 1).\nFigure 1.\nThe patient insisted that he had otherwise been feeling fine and downplayed the need for further assistance. He requested medication for his headache. The patient denied substance abuse.\nOn the second day of admission, the patient developed worsening tremor and diaphoresis. He began to pick at his skin, stating that he wants to get rid of the \"bugs that are crawling on him.\" The patient attempted to pull his intravenous (IV) lines out and turned his head rapidly, stating that he could hear the voice of his deceased spouse. He believed he was in a seafood restaurant.\nThe patient's medical history of note included atrial fibrillation, hypertension, and peripheral vascular disease. During admission, he had stated that he could not remember whether he had been taking his blood pressure medication correctly.\nThe patient stated that he has no family since the passing of his spouse. Family medical history was unobtainable.\n\n ## Figures\n\n **Figure 1.** \n \n\n\n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 66-Year-Old Man Who Heard His Deceased Spouse's Voice"
},
{
"authors": "Jeffrey S. Forrest, MD; Steven Kendell, MD",
"content": [
"Upon his admission physical examination, the patient appeared mildly tremulous and anxious and had dry mucous membranes. He had numerous evident ecchymoses on his extremities. Numerous spider angiomata were observed on his skin (Figure 2).",
"Figure 2.",
"A bilateral hand-flapping tremor was observed at his wrist joints before his right wrist was splinted for a Colles fracture (Figure 3).",
"Figure 3.",
"The patient's admission cardiac examination revealed an irregular rate and rhythm, with mild tachycardia evident. Upon abdominal exam, mild hepatomegaly was suspected by percussion. Mild epigastric tenderness was elicited on palpation.",
"The patient's admission mental status examination revealed a fully oriented patient who answered questions tersely and was not forthcoming. The patient answered questions appropriately and demonstrated an anxious speech pattern. His thought processes were logical and linear, and his thought content was devoid of delusions or paranoid ideation. He denied suicidal ideations and auditory or visual hallucinations.",
"On his follow-up mental status examination on the second hospital day, the patient appeared agitated and was picking at his skin. He turned his head periodically, stating that he could hear his deceased spouse talking to him. He complained of bugs crawling on his skin and attempted to pick them off. He also stated that he can see bugs crawling on the walls of his hospital room.",
"Laboratory abnormalities of note included a potassium level of 3.1 mEq/L, magnesium level of 1.5 mEq/L, bicarbonate level of 36 mEq/L, blood urea nitrogen level of 70 mg/dL, aspartate aminotransferase (AST) level of 212 U/L, alanine aminotransferase (ALT) level 109 of U/L, ammonia level of 61 µ/dL, creatinine level of 1.4 mg/dL, and gamma-glutamyltransferase (GGT) level of 62 U/L. EKG depicted atrial fibrillation and tachycardia to 108 beats/min.",
"During the third day of admission, the patient was observed to experience a tonic-clonic seizure by nursing staff and was transported to the intensive care unit for further management."
],
"date": "January 31, 2019",
"figures": [
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/908/341/908341-Thumb2.jpg"
},
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/908/341/908341-Thumb3.jpg"
}
],
"markdown": "# A 66-Year-Old Man Who Heard His Deceased Spouse's Voice\n\n **Authors:** Jeffrey S. Forrest, MD; Steven Kendell, MD \n **Date:** January 31, 2019\n\n ## Content\n\n Upon his admission physical examination, the patient appeared mildly tremulous and anxious and had dry mucous membranes. He had numerous evident ecchymoses on his extremities. Numerous spider angiomata were observed on his skin (Figure 2).\nFigure 2.\nA bilateral hand-flapping tremor was observed at his wrist joints before his right wrist was splinted for a Colles fracture (Figure 3).\nFigure 3.\nThe patient's admission cardiac examination revealed an irregular rate and rhythm, with mild tachycardia evident. Upon abdominal exam, mild hepatomegaly was suspected by percussion. Mild epigastric tenderness was elicited on palpation.\nThe patient's admission mental status examination revealed a fully oriented patient who answered questions tersely and was not forthcoming. The patient answered questions appropriately and demonstrated an anxious speech pattern. His thought processes were logical and linear, and his thought content was devoid of delusions or paranoid ideation. He denied suicidal ideations and auditory or visual hallucinations.\nOn his follow-up mental status examination on the second hospital day, the patient appeared agitated and was picking at his skin. He turned his head periodically, stating that he could hear his deceased spouse talking to him. He complained of bugs crawling on his skin and attempted to pick them off. He also stated that he can see bugs crawling on the walls of his hospital room.\nLaboratory abnormalities of note included a potassium level of 3.1 mEq/L, magnesium level of 1.5 mEq/L, bicarbonate level of 36 mEq/L, blood urea nitrogen level of 70 mg/dL, aspartate aminotransferase (AST) level of 212 U/L, alanine aminotransferase (ALT) level 109 of U/L, ammonia level of 61 µ/dL, creatinine level of 1.4 mg/dL, and gamma-glutamyltransferase (GGT) level of 62 U/L. EKG depicted atrial fibrillation and tachycardia to 108 beats/min.\nDuring the third day of admission, the patient was observed to experience a tonic-clonic seizure by nursing staff and was transported to the intensive care unit for further management.\n\n ## Figures\n\n **Figure 2.** \n \n\n**Figure 3.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1321118,
"choiceText": "Syncope due to atrial fibrillation",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1321120,
"choiceText": "Hepatic encephalopathy",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1321122,
"choiceText": "Alcohol withdrawal delirium",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1321124,
"choiceText": "Medication noncompliance",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1321126,
"choiceText": "Delirium due to wrist fracture\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 421565,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 66-Year-Old Man Who Heard His Deceased Spouse's Voice"
},
{
"authors": "Jeffrey S. Forrest, MD; Steven Kendell, MD",
"content": [
"This patient has delirium secondary to alcohol withdrawal. Alcohol use is frequently denied or underreported by patients secondary to embarrassment or denial. Patients admitted for diagnostic workup of other pathologies commonly develop withdrawal symptoms if they are chronic alcohol users. The clinician must be aware of this possibility because alcohol withdrawal can be lethal if not appropriately managed.",
"Even if a patient is not forthcoming with their alcohol history, as in this case, numerous findings in the history, physical examination, and laboratory studies may suggest chronic alcoholism. The patient reported falling getting out of a bathtub. When considered in the context of numerous bruises, asterixis, spider angiomata, a suggestive AST:ALT ratio > 2:1, hypokalemia, and apparent volume depletion, the index of suspicion for alcohol withdrawal is raised. The presence of comorbid atrial fibrillation adds another potential cause or contribution to the patient's numerous apparent falls. Another laboratory test that may be considered helpful is a GGT assay. At least 70% of individuals with GGT levels > 35 U/L are heavy drinkers. Elevated percentage of carbohydrate-deficient transferrin is another sensitive and specific marker for heavy drinking.[1]",
"Whenever alcohol abuse in a patient is suspected, prompt administration of intravenous (IV) thiamine before glucose administration is essential to reduce the risk for Wernicke-Korsakoff syndrome. Volume and electrolyte repletion may also be necessary, because patients frequently have depleted magnesium and potassium (especially in vomiting patients) and prerenal azotemia. IV magnesium may also be helpful, because chronic alcohol abuse may result in hypomagnesemia. Correction of this abnormality may help prevent the development of seizures."
],
"date": "January 31, 2019",
"figures": [],
"markdown": "# A 66-Year-Old Man Who Heard His Deceased Spouse's Voice\n\n **Authors:** Jeffrey S. Forrest, MD; Steven Kendell, MD \n **Date:** January 31, 2019\n\n ## Content\n\n This patient has delirium secondary to alcohol withdrawal. Alcohol use is frequently denied or underreported by patients secondary to embarrassment or denial. Patients admitted for diagnostic workup of other pathologies commonly develop withdrawal symptoms if they are chronic alcohol users. The clinician must be aware of this possibility because alcohol withdrawal can be lethal if not appropriately managed.\nEven if a patient is not forthcoming with their alcohol history, as in this case, numerous findings in the history, physical examination, and laboratory studies may suggest chronic alcoholism. The patient reported falling getting out of a bathtub. When considered in the context of numerous bruises, asterixis, spider angiomata, a suggestive AST:ALT ratio > 2:1, hypokalemia, and apparent volume depletion, the index of suspicion for alcohol withdrawal is raised. The presence of comorbid atrial fibrillation adds another potential cause or contribution to the patient's numerous apparent falls. Another laboratory test that may be considered helpful is a GGT assay. At least 70% of individuals with GGT levels > 35 U/L are heavy drinkers. Elevated percentage of carbohydrate-deficient transferrin is another sensitive and specific marker for heavy drinking.[1]\nWhenever alcohol abuse in a patient is suspected, prompt administration of intravenous (IV) thiamine before glucose administration is essential to reduce the risk for Wernicke-Korsakoff syndrome. Volume and electrolyte repletion may also be necessary, because patients frequently have depleted magnesium and potassium (especially in vomiting patients) and prerenal azotemia. IV magnesium may also be helpful, because chronic alcohol abuse may result in hypomagnesemia. Correction of this abnormality may help prevent the development of seizures.\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1321118,
"choiceText": "Syncope due to atrial fibrillation",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1321120,
"choiceText": "Hepatic encephalopathy",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1321122,
"choiceText": "Alcohol withdrawal delirium",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1321124,
"choiceText": "Medication noncompliance",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1321126,
"choiceText": "Delirium due to wrist fracture\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 421565,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 66-Year-Old Man Who Heard His Deceased Spouse's Voice"
},
{
"authors": "Jeffrey S. Forrest, MD; Steven Kendell, MD",
"content": [
"Alcohol is a central nervous system depressant that acts to potentiate the action of the inhibitory neurotransmitter gamma-aminobutyric acid (GABA) at its receptors, especially those with delta subunits. Chronic exposure to alcohol is thought to therefore result in downregulation of inhibitory GABA receptors as well as upregulation of excitatory N-methyl-D-aspartate (NMDA) and glutamate receptor activity to maintain homeostasis.[2] As a result, during alcohol withdrawal, a relative increase in excitatory neurotransmitters and their receptor sensitivities results in a clinical syndrome described below.",
"The first physical sign, which is present in virtually all cases of alcohol withdrawal, is tremulousness. Anxiety, nausea, vomiting, headaches, pyrexia, diaphoresis, and autonomic instability are also important potential signs in the first 10-30 hours from the last use of alcohol. Auditory and visual hallucinations may also develop; these tend to appear in the 12- to 48-hour range from last alcohol exposure but are somewhat less common than other findings.",
"The most concerning potential complications from alcohol withdrawal are tonic-clonic seizures and the syndrome of delirium tremens. Seizures peak in incidence in the first 12-48 hours from last drink but can happen for several days after. As many as 5% of alcohol withdrawal seizures progress to status epilepticus.[2]",
"Delirium tremens features severe disorientation, fever, diaphoresis, and auditory and tactile hallucinations, as observed in this case. The peak incidence for delirium tremens is 48-72 hours after the last drink, but it has been reported to occur as long as 3-10 days after. This should be distinguished from isolated alcohol hallucinosis, which does not result in disorientation alone. Seizures and delirium tremens should be closely monitored, as they may result in significant morbidity and even mortality. A prior history of complications from alcohol withdrawal is predictive of potential future withdrawal events and should be ascertained whenever possible.",
"The most commonly used tool for assessing alcohol withdrawal severity is the Clinical Institute Withdrawal Assessment for Alcohol (CIWA-Ar). This 10-item scale assesses for nausea/vomiting, tremor, disorientation, headaches, auditory and visual sensorium changes, sweats, and tactile disturbances. During an admission, CIWA-Ar scores may be monitored to track a patient's withdrawal symptom severity. Scores < 15 are considered mild withdrawal, scores of 15-20 are considered moderate withdrawal, and scores > 20 are considered severe withdrawal. Severe alcohol withdrawal may require management in the intensive care unit."
],
"date": "January 31, 2019",
"figures": [],
"markdown": "# A 66-Year-Old Man Who Heard His Deceased Spouse's Voice\n\n **Authors:** Jeffrey S. Forrest, MD; Steven Kendell, MD \n **Date:** January 31, 2019\n\n ## Content\n\n Alcohol is a central nervous system depressant that acts to potentiate the action of the inhibitory neurotransmitter gamma-aminobutyric acid (GABA) at its receptors, especially those with delta subunits. Chronic exposure to alcohol is thought to therefore result in downregulation of inhibitory GABA receptors as well as upregulation of excitatory N-methyl-D-aspartate (NMDA) and glutamate receptor activity to maintain homeostasis.[2] As a result, during alcohol withdrawal, a relative increase in excitatory neurotransmitters and their receptor sensitivities results in a clinical syndrome described below.\nThe first physical sign, which is present in virtually all cases of alcohol withdrawal, is tremulousness. Anxiety, nausea, vomiting, headaches, pyrexia, diaphoresis, and autonomic instability are also important potential signs in the first 10-30 hours from the last use of alcohol. Auditory and visual hallucinations may also develop; these tend to appear in the 12- to 48-hour range from last alcohol exposure but are somewhat less common than other findings.\nThe most concerning potential complications from alcohol withdrawal are tonic-clonic seizures and the syndrome of delirium tremens. Seizures peak in incidence in the first 12-48 hours from last drink but can happen for several days after. As many as 5% of alcohol withdrawal seizures progress to status epilepticus.[2]\nDelirium tremens features severe disorientation, fever, diaphoresis, and auditory and tactile hallucinations, as observed in this case. The peak incidence for delirium tremens is 48-72 hours after the last drink, but it has been reported to occur as long as 3-10 days after. This should be distinguished from isolated alcohol hallucinosis, which does not result in disorientation alone. Seizures and delirium tremens should be closely monitored, as they may result in significant morbidity and even mortality. A prior history of complications from alcohol withdrawal is predictive of potential future withdrawal events and should be ascertained whenever possible.\nThe most commonly used tool for assessing alcohol withdrawal severity is the Clinical Institute Withdrawal Assessment for Alcohol (CIWA-Ar). This 10-item scale assesses for nausea/vomiting, tremor, disorientation, headaches, auditory and visual sensorium changes, sweats, and tactile disturbances. During an admission, CIWA-Ar scores may be monitored to track a patient's withdrawal symptom severity. Scores < 15 are considered mild withdrawal, scores of 15-20 are considered moderate withdrawal, and scores > 20 are considered severe withdrawal. Severe alcohol withdrawal may require management in the intensive care unit.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 66-Year-Old Man Who Heard His Deceased Spouse's Voice"
},
{
"authors": "Jeffrey S. Forrest, MD; Steven Kendell, MD",
"content": [
"The treatments of choice for alcohol withdrawal are benzodiazepine agents. Benzodiazepine drugs act in a similar manner to alcohol by increasing inhibitory effect of GABA at its receptors.[3] The rebound excitatory state that occurs in the central nervous system (CNS) can thus be countered with a controlled taper of a CNS-inhibiting agent. Longer-acting benzodiazepines, such as diazepam or chlordiazepoxide, have traditionally been favored to provide patients with a smoother release from withdrawal excitation. However, many patients with alcohol withdrawal, such as the patient in this case, may have compromised liver function as a result of chronic abuse. In this scenario, medications that bypass first-phase liver metabolism (eg, lorazepam) may be preferable to avoid potentiated sedation from impaired medication elimination.",
"The appropriate dosing of medication may vary, depending on the clinical scenario. The provider must weigh a need to gain fast control of immediate symptoms (eg, agitation) and prevent subsequent breakthrough symptoms against potential adverse effects of oversedation, respiratory depression, and also medication dependence. Some institutions perform serial CIWA-Ar assessments to help guide the clinician in managing withdrawal symptoms. In severe withdrawal, such as demonstrated in this case, an IV drip of a medication such as lorazepam may be necessary until symptoms are under control. Possible withdrawal symptoms from the benzodiazepine agents used to treat alcohol withdrawal must also be guarded against.",
"Antipsychotic agents, such as haloperidol, may lower the seizure threshold and contribute to cardiac arrhythmias by prolonging the qTc interval on an EKG. They are best avoided or used only with extreme caution in the early stages of alcohol withdrawal delirium. Antipsychotics may be considered in late-stage delirium when severe hallucinations and agitation have not been controlled by benzodiazepine agents.",
"The patient in this case was treated in the intensive care unit for several days with IV lorazepam before being transferred back to the medical floor. He experienced one additional seizure during his 11-day admission. He was referred for substance abuse counseling and offered rehabilitation services upon his discharge."
],
"date": "January 31, 2019",
"figures": [],
"markdown": "# A 66-Year-Old Man Who Heard His Deceased Spouse's Voice\n\n **Authors:** Jeffrey S. Forrest, MD; Steven Kendell, MD \n **Date:** January 31, 2019\n\n ## Content\n\n The treatments of choice for alcohol withdrawal are benzodiazepine agents. Benzodiazepine drugs act in a similar manner to alcohol by increasing inhibitory effect of GABA at its receptors.[3] The rebound excitatory state that occurs in the central nervous system (CNS) can thus be countered with a controlled taper of a CNS-inhibiting agent. Longer-acting benzodiazepines, such as diazepam or chlordiazepoxide, have traditionally been favored to provide patients with a smoother release from withdrawal excitation. However, many patients with alcohol withdrawal, such as the patient in this case, may have compromised liver function as a result of chronic abuse. In this scenario, medications that bypass first-phase liver metabolism (eg, lorazepam) may be preferable to avoid potentiated sedation from impaired medication elimination.\nThe appropriate dosing of medication may vary, depending on the clinical scenario. The provider must weigh a need to gain fast control of immediate symptoms (eg, agitation) and prevent subsequent breakthrough symptoms against potential adverse effects of oversedation, respiratory depression, and also medication dependence. Some institutions perform serial CIWA-Ar assessments to help guide the clinician in managing withdrawal symptoms. In severe withdrawal, such as demonstrated in this case, an IV drip of a medication such as lorazepam may be necessary until symptoms are under control. Possible withdrawal symptoms from the benzodiazepine agents used to treat alcohol withdrawal must also be guarded against.\nAntipsychotic agents, such as haloperidol, may lower the seizure threshold and contribute to cardiac arrhythmias by prolonging the qTc interval on an EKG. They are best avoided or used only with extreme caution in the early stages of alcohol withdrawal delirium. Antipsychotics may be considered in late-stage delirium when severe hallucinations and agitation have not been controlled by benzodiazepine agents.\nThe patient in this case was treated in the intensive care unit for several days with IV lorazepam before being transferred back to the medical floor. He experienced one additional seizure during his 11-day admission. He was referred for substance abuse counseling and offered rehabilitation services upon his discharge.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1321128,
"choiceText": "Diazepam therapy",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1321130,
"choiceText": "Haloperidol therapy",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1321132,
"choiceText": "Four-point restraints",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1321134,
"choiceText": "Morphine therapy",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1321136,
"choiceText": "Ziprasidone therapy\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "This patient is experiencing alcohol withdrawal delirium, as suggested by his autonomic instability from his vital signs, sudden disorientation delayed from initial admission, and hallucinosis. Benzodiazepine agents, such as lorazepam, are first-line treatments. Antipsychotic agents may lower the seizure threshold or worsen arrhythmias; they are best not used or used only adjunctively in severe cases. Restraints are best avoided where possible, because delirious patients may fight against restraints to a dangerous level of exhaustion.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 421567,
"questionText": "A 56-year-old man is admitted to the hospital after a recent fall. Upon admission, his mental status examination findings are normal. During the second day of his admission, the patient becomes rapidly disoriented, confuses the nurse for a loved one, and thinks he is in a movie theater. His vital signs include a blood pressure of 164/99 mm Hg, a pulse of 106 beats/min, and a temperature 101.8°F (38.78°C). He states he is hearing music and begins pulling at his IV lines. Which of the following is the most appropriate next step in management?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1321138,
"choiceText": "They are usually tonic-clonic",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1321140,
"choiceText": "They occur only in the first 12-48 hours",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1321142,
"choiceText": "They may progress to status epilepticus",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1321144,
"choiceText": "Prior alcohol withdrawal seizures increase risk",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1321146,
"choiceText": "They may occur without other withdrawal symptoms",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Alcohol withdrawal seizures may occur at any time during an alcohol withdrawal syndrome, although the peak incidence is in the first 12-48 hours. Seizures are generally tonic-clonic, and about 5% progress to status epilepticus. Previous seizures are a risk factor. They may occur in the absence of other alcohol withdrawal symptoms. ",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 421569,
"questionText": "Which of the following is <em>not</em> accurate about alcohol withdrawal seizures?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 66-Year-Old Man Who Heard His Deceased Spouse's Voice"
},
{
"authors": "Jeffrey S. Forrest, MD; Steven Kendell, MD",
"content": [],
"date": "January 31, 2019",
"figures": [],
"markdown": "# A 66-Year-Old Man Who Heard His Deceased Spouse's Voice\n\n **Authors:** Jeffrey S. Forrest, MD; Steven Kendell, MD \n **Date:** January 31, 2019\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1321128,
"choiceText": "Diazepam therapy",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1321130,
"choiceText": "Haloperidol therapy",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1321132,
"choiceText": "Four-point restraints",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1321134,
"choiceText": "Morphine therapy",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1321136,
"choiceText": "Ziprasidone therapy\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "This patient is experiencing alcohol withdrawal delirium, as suggested by his autonomic instability from his vital signs, sudden disorientation delayed from initial admission, and hallucinosis. Benzodiazepine agents, such as lorazepam, are first-line treatments. Antipsychotic agents may lower the seizure threshold or worsen arrhythmias; they are best not used or used only adjunctively in severe cases. Restraints are best avoided where possible, because delirious patients may fight against restraints to a dangerous level of exhaustion.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 421567,
"questionText": "A 56-year-old man is admitted to the hospital after a recent fall. Upon admission, his mental status examination findings are normal. During the second day of his admission, the patient becomes rapidly disoriented, confuses the nurse for a loved one, and thinks he is in a movie theater. His vital signs include a blood pressure of 164/99 mm Hg, a pulse of 106 beats/min, and a temperature 101.8°F (38.78°C). He states he is hearing music and begins pulling at his IV lines. Which of the following is the most appropriate next step in management?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1321138,
"choiceText": "They are usually tonic-clonic",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1321140,
"choiceText": "They occur only in the first 12-48 hours",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1321142,
"choiceText": "They may progress to status epilepticus",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1321144,
"choiceText": "Prior alcohol withdrawal seizures increase risk",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1321146,
"choiceText": "They may occur without other withdrawal symptoms",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Alcohol withdrawal seizures may occur at any time during an alcohol withdrawal syndrome, although the peak incidence is in the first 12-48 hours. Seizures are generally tonic-clonic, and about 5% progress to status epilepticus. Previous seizures are a risk factor. They may occur in the absence of other alcohol withdrawal symptoms. ",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 421569,
"questionText": "Which of the following is <em>not</em> accurate about alcohol withdrawal seizures?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 66-Year-Old Man Who Heard His Deceased Spouse's Voice"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1321118,
"choiceText": "Syncope due to atrial fibrillation",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1321120,
"choiceText": "Hepatic encephalopathy",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1321122,
"choiceText": "Alcohol withdrawal delirium",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1321124,
"choiceText": "Medication noncompliance",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1321126,
"choiceText": "Delirium due to wrist fracture\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 421565,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1321128,
"choiceText": "Diazepam therapy",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1321130,
"choiceText": "Haloperidol therapy",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1321132,
"choiceText": "Four-point restraints",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1321134,
"choiceText": "Morphine therapy",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1321136,
"choiceText": "Ziprasidone therapy\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "This patient is experiencing alcohol withdrawal delirium, as suggested by his autonomic instability from his vital signs, sudden disorientation delayed from initial admission, and hallucinosis. Benzodiazepine agents, such as lorazepam, are first-line treatments. Antipsychotic agents may lower the seizure threshold or worsen arrhythmias; they are best not used or used only adjunctively in severe cases. Restraints are best avoided where possible, because delirious patients may fight against restraints to a dangerous level of exhaustion.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 421567,
"questionText": "A 56-year-old man is admitted to the hospital after a recent fall. Upon admission, his mental status examination findings are normal. During the second day of his admission, the patient becomes rapidly disoriented, confuses the nurse for a loved one, and thinks he is in a movie theater. His vital signs include a blood pressure of 164/99 mm Hg, a pulse of 106 beats/min, and a temperature 101.8°F (38.78°C). He states he is hearing music and begins pulling at his IV lines. Which of the following is the most appropriate next step in management?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1321138,
"choiceText": "They are usually tonic-clonic",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1321140,
"choiceText": "They occur only in the first 12-48 hours",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1321142,
"choiceText": "They may progress to status epilepticus",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1321144,
"choiceText": "Prior alcohol withdrawal seizures increase risk",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1321146,
"choiceText": "They may occur without other withdrawal symptoms",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Alcohol withdrawal seizures may occur at any time during an alcohol withdrawal syndrome, although the peak incidence is in the first 12-48 hours. Seizures are generally tonic-clonic, and about 5% progress to status epilepticus. Previous seizures are a risk factor. They may occur in the absence of other alcohol withdrawal symptoms. ",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 421569,
"questionText": "Which of the following is <em>not</em> accurate about alcohol withdrawal seizures?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
906864 | /viewarticle/906864 | [
{
"authors": "Heidi Moawad, MD",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"A 56-year-old woman has had worsening paresthesias in her arms for several months. Within the past few weeks, she began to notice that lifting boxes at her job as a store stocker became more difficult. She gets exhausted climbing the stairs in her home, especially when she is carrying a full laundry basket. She does not regularly exercise, so she does not know whether her endurance has changed. She also has noticed that she occasionally coughs and chokes while she is eating and drinking, and she is wondering whether she may have the flu.",
"Her medical history is significant for mild springtime allergies, frequent urinary tract infections, and a kidney stone.",
"She does not smoke and drinks about one alcoholic beverage every few months, at social occasions only. She is not under any unusual stress. She lives with her husband. They have three healthy, adult children. Her husband smokes about one half of a pack of cigarettes per day.",
"Her family history is significant for a sister who has multiple sclerosis. Both of her parents were heavy smokers. Her father died of lung cancer, and her mother, who is living, has severe congestive heart failure."
],
"date": "December 26, 2018",
"figures": [],
"markdown": "# A 56-Year-Old Woman With Paresthesia and Choking\n\n **Authors:** Heidi Moawad, MD \n **Date:** December 26, 2018\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nA 56-year-old woman has had worsening paresthesias in her arms for several months. Within the past few weeks, she began to notice that lifting boxes at her job as a store stocker became more difficult. She gets exhausted climbing the stairs in her home, especially when she is carrying a full laundry basket. She does not regularly exercise, so she does not know whether her endurance has changed. She also has noticed that she occasionally coughs and chokes while she is eating and drinking, and she is wondering whether she may have the flu.\nHer medical history is significant for mild springtime allergies, frequent urinary tract infections, and a kidney stone.\nShe does not smoke and drinks about one alcoholic beverage every few months, at social occasions only. She is not under any unusual stress. She lives with her husband. They have three healthy, adult children. Her husband smokes about one half of a pack of cigarettes per day.\nHer family history is significant for a sister who has multiple sclerosis. Both of her parents were heavy smokers. Her father died of lung cancer, and her mother, who is living, has severe congestive heart failure.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 56-Year-Old Woman With Paresthesia and Choking"
},
{
"authors": "Heidi Moawad, MD",
"content": [
"Upon physical examination, the patient appears well nourished and in no acute distress. Her skin appears normal, without any lesions, rashes, or discoloration. Her breathing is regular, and her chest sounds are clear. She does not have abdominal tenderness or distention. Her pulse is normal, and her heart sounds are regular, without any detectable murmurs during her physical examination.",
"Upon neurologic examination, no spasms or fasciculations are noted. The patient's cranial nerve examination findings are normal, although her pupillary response is slow. Upon motor examination, her biceps and triceps strength are slightly decreased on the left and right sides and are documented as 4+/5. Her strength is 5/5 in her distal arm and hand muscles on both the left and right sides. Her strength is 4/5 in bilateral proximal lower extremities, and 4+/5 in her distal lower extremities.",
"Her reflexes are slightly diminished on the right and left upper and lower extremities, and her toes are down-going. Upon sensory examination, she has normal reaction to light touch, pinprick, vibration, and proprioception. Her coordination is normal as evaluated by rapid alternating moments and finger-to-nose testing. Her gait is normal, and she can perform heel-to-toe walking and Romberg testing without any difficulty.",
"Electrodiagnostic studies reveal a pattern of small compound muscle action potentials (CMAPs) with decreased amplitude in the action potentials. This is followed by an increase in the amplitude of the action potentials with repetitive stimulation."
],
"date": "December 26, 2018",
"figures": [],
"markdown": "# A 56-Year-Old Woman With Paresthesia and Choking\n\n **Authors:** Heidi Moawad, MD \n **Date:** December 26, 2018\n\n ## Content\n\n Upon physical examination, the patient appears well nourished and in no acute distress. Her skin appears normal, without any lesions, rashes, or discoloration. Her breathing is regular, and her chest sounds are clear. She does not have abdominal tenderness or distention. Her pulse is normal, and her heart sounds are regular, without any detectable murmurs during her physical examination.\nUpon neurologic examination, no spasms or fasciculations are noted. The patient's cranial nerve examination findings are normal, although her pupillary response is slow. Upon motor examination, her biceps and triceps strength are slightly decreased on the left and right sides and are documented as 4+/5. Her strength is 5/5 in her distal arm and hand muscles on both the left and right sides. Her strength is 4/5 in bilateral proximal lower extremities, and 4+/5 in her distal lower extremities.\nHer reflexes are slightly diminished on the right and left upper and lower extremities, and her toes are down-going. Upon sensory examination, she has normal reaction to light touch, pinprick, vibration, and proprioception. Her coordination is normal as evaluated by rapid alternating moments and finger-to-nose testing. Her gait is normal, and she can perform heel-to-toe walking and Romberg testing without any difficulty.\nElectrodiagnostic studies reveal a pattern of small compound muscle action potentials (CMAPs) with decreased amplitude in the action potentials. This is followed by an increase in the amplitude of the action potentials with repetitive stimulation.\n\n ## Figures\n\n \n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1312046,
"choiceText": "Myopathy",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1312048,
"choiceText": "Neuropathy",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1312050,
"choiceText": "Lambert-Eaton myasthenic syndrome",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1312052,
"choiceText": "Myasthenia gravis",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 418729,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 56-Year-Old Woman With Paresthesia and Choking"
},
{
"authors": "Heidi Moawad, MD",
"content": [
"Lambert-Eaton myasthenic syndrome (LEMS) is a rare disorder of the neuromuscular junction, which is the synapse between the motor nerve endings and their associated muscle receptors.[1] Antibodies against the voltage-gated calcium channels of the presynaptic nerves interfere with release of calcium in the nerve.[2] Calcium functions to stimulate the release of acetylcholine from the nerve terminal, which serves to bind to the postsynaptic muscle receptor to activate contraction of the muscle.",
"About one half of patients with LEMS do not have a known etiology for the disorder, although cancer is believed to be the etiology in the other half.[1] Cancer can trigger the formation of the autoantibodies that attack a person's own body, resulting in what is described as a group of paraneoplastic syndromes. LEMS is among several paraneoplastic syndromes, and they are all rare. The most common type of cancer associated with LEMS is small cell lung cancer. Yet, less than 5% of those who have lung cancer have LEMS, which gives a sense of how rare it is as a complication of cancer.",
"The symptoms of LEMS include muscle weakness, which is most prominent in the proximal muscles of the extremities and rarely involves the face. Generally, the autonomic nervous system is involved as well, and patients may experience constipation, blood pressure fluctuations (especially low blood pressure), dry mouth, decreased sweating, and dizziness."
],
"date": "December 26, 2018",
"figures": [],
"markdown": "# A 56-Year-Old Woman With Paresthesia and Choking\n\n **Authors:** Heidi Moawad, MD \n **Date:** December 26, 2018\n\n ## Content\n\n Lambert-Eaton myasthenic syndrome (LEMS) is a rare disorder of the neuromuscular junction, which is the synapse between the motor nerve endings and their associated muscle receptors.[1] Antibodies against the voltage-gated calcium channels of the presynaptic nerves interfere with release of calcium in the nerve.[2] Calcium functions to stimulate the release of acetylcholine from the nerve terminal, which serves to bind to the postsynaptic muscle receptor to activate contraction of the muscle.\nAbout one half of patients with LEMS do not have a known etiology for the disorder, although cancer is believed to be the etiology in the other half.[1] Cancer can trigger the formation of the autoantibodies that attack a person's own body, resulting in what is described as a group of paraneoplastic syndromes. LEMS is among several paraneoplastic syndromes, and they are all rare. The most common type of cancer associated with LEMS is small cell lung cancer. Yet, less than 5% of those who have lung cancer have LEMS, which gives a sense of how rare it is as a complication of cancer.\nThe symptoms of LEMS include muscle weakness, which is most prominent in the proximal muscles of the extremities and rarely involves the face. Generally, the autonomic nervous system is involved as well, and patients may experience constipation, blood pressure fluctuations (especially low blood pressure), dry mouth, decreased sweating, and dizziness.\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1312046,
"choiceText": "Myopathy",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1312048,
"choiceText": "Neuropathy",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1312050,
"choiceText": "Lambert-Eaton myasthenic syndrome",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1312052,
"choiceText": "Myasthenia gravis",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 418729,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 56-Year-Old Woman With Paresthesia and Choking"
},
{
"authors": "Heidi Moawad, MD",
"content": [
"Myopathy is muscle disease. Over 100 different myopathies are recognized. The clinical presentation of several myopathies can be similar to that of LEMS. Diagnostic testing, such as electromyography and nerve conduction studies, are often needed to distinguish LEMS from myopathy (Figure 1).",
"Figure 1.",
"In LEMS, electrodiagnostic studies show a pattern of small CMAPs, which is caused by diminished muscle activity. A characteristic feature of LEMS is decreased amplitude in the action potentials, followed by an increase in the amplitude of the action potentials with repetitive stimulation. This results from a buildup of calcium and acetylcholine release that triggers an increase in the action potentials, although CMAPs remain smaller than normal even throughout the period of repetitive stimulation. In the different types of myopathy, the electrodiagnostic studies can vary, and are typically characterized by small CMAPs due to muscle fiber dysfunction, without the increase in amplitude seen in LEMS.",
"Neuropathy is disease of the nerves. Although LEMS is technically a disease of the nerve endings, it manifests differently from neuropathy. Neuropathy affects function of the nerve axons, which can result from demyelination or axonal damage. Neuropathies often manifest with weakness and diminished reflexes, but the weakness is generally more prominent in the peripheral, distal muscles, whereas LEMS causes proximal muscle weakness. Neuropathy often manifests with sensory abnormalities, including diminished peripheral sensation and paresthesias, whereas LEMS can cause paresthesias but does not usually cause sensory deficits. The electrodiagnostic studies of neuropathy generally show low-amplitude action potentials and a delay in the latency.",
"Myasthenia gravis is the condition that is most commonly confused with LEMS. Myasthenia gravis is also a disease of the neuromuscular junction; however, there are a few major differences in the symptoms, etiology, diagnosis, and treatment. Myasthenia gravis is characterized by facial and eyelid weakness and does not include autonomic symptoms. Antibodies that attack the muscular side of the neuromuscular junction cause myasthenia gravis. The symptoms of myasthenia gravis briefly improve with a diagnostic Tensilon (edrophonium) test, whereas symptoms of LEMS worsen with this test.",
"The diagnosis of LEMS is based on clinical symptoms and electrodiagnostic tests. Paraneoplastic antibodies may be present, but their absence does not exclude the diagnosis. A muscle biopsy can be normal or can show muscle atrophy and is not usually helpful in the diagnosis."
],
"date": "December 26, 2018",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/906/864/906864-Figure-1-thumb.png"
}
],
"markdown": "# A 56-Year-Old Woman With Paresthesia and Choking\n\n **Authors:** Heidi Moawad, MD \n **Date:** December 26, 2018\n\n ## Content\n\n Myopathy is muscle disease. Over 100 different myopathies are recognized. The clinical presentation of several myopathies can be similar to that of LEMS. Diagnostic testing, such as electromyography and nerve conduction studies, are often needed to distinguish LEMS from myopathy (Figure 1).\nFigure 1.\nIn LEMS, electrodiagnostic studies show a pattern of small CMAPs, which is caused by diminished muscle activity. A characteristic feature of LEMS is decreased amplitude in the action potentials, followed by an increase in the amplitude of the action potentials with repetitive stimulation. This results from a buildup of calcium and acetylcholine release that triggers an increase in the action potentials, although CMAPs remain smaller than normal even throughout the period of repetitive stimulation. In the different types of myopathy, the electrodiagnostic studies can vary, and are typically characterized by small CMAPs due to muscle fiber dysfunction, without the increase in amplitude seen in LEMS.\nNeuropathy is disease of the nerves. Although LEMS is technically a disease of the nerve endings, it manifests differently from neuropathy. Neuropathy affects function of the nerve axons, which can result from demyelination or axonal damage. Neuropathies often manifest with weakness and diminished reflexes, but the weakness is generally more prominent in the peripheral, distal muscles, whereas LEMS causes proximal muscle weakness. Neuropathy often manifests with sensory abnormalities, including diminished peripheral sensation and paresthesias, whereas LEMS can cause paresthesias but does not usually cause sensory deficits. The electrodiagnostic studies of neuropathy generally show low-amplitude action potentials and a delay in the latency.\nMyasthenia gravis is the condition that is most commonly confused with LEMS. Myasthenia gravis is also a disease of the neuromuscular junction; however, there are a few major differences in the symptoms, etiology, diagnosis, and treatment. Myasthenia gravis is characterized by facial and eyelid weakness and does not include autonomic symptoms. Antibodies that attack the muscular side of the neuromuscular junction cause myasthenia gravis. The symptoms of myasthenia gravis briefly improve with a diagnostic Tensilon (edrophonium) test, whereas symptoms of LEMS worsen with this test.\nThe diagnosis of LEMS is based on clinical symptoms and electrodiagnostic tests. Paraneoplastic antibodies may be present, but their absence does not exclude the diagnosis. A muscle biopsy can be normal or can show muscle atrophy and is not usually helpful in the diagnosis.\n\n ## Figures\n\n **Figure 1.** \n \n\n\n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 56-Year-Old Woman With Paresthesia and Choking"
},
{
"authors": "Heidi Moawad, MD",
"content": [
"The prognosis of LEMS varies, depending on associated cancer. If a patient has a diagnosis of cancer, treatment of the cancer is essential, but it may not reverse the neurologic symptoms.",
"Plasmapheresis or intravenous immunoglobulin, and possibly immunosuppression with prednisone or prednisone plus azathioprine, may be needed to manage the neurologic symptoms by reducing the action of the antibodies. Another symptomatic therapy, amifampridine (3,4-diaminopyridine), is a potassium-channel blocker that can prolong an action potential, maximizing release of acetylcholine from the presynaptic nerve terminal. Even in the absence of cancer, symptoms can be persistent and often interfere with quality of life."
],
"date": "December 26, 2018",
"figures": [],
"markdown": "# A 56-Year-Old Woman With Paresthesia and Choking\n\n **Authors:** Heidi Moawad, MD \n **Date:** December 26, 2018\n\n ## Content\n\n The prognosis of LEMS varies, depending on associated cancer. If a patient has a diagnosis of cancer, treatment of the cancer is essential, but it may not reverse the neurologic symptoms.\nPlasmapheresis or intravenous immunoglobulin, and possibly immunosuppression with prednisone or prednisone plus azathioprine, may be needed to manage the neurologic symptoms by reducing the action of the antibodies. Another symptomatic therapy, amifampridine (3,4-diaminopyridine), is a potassium-channel blocker that can prolong an action potential, maximizing release of acetylcholine from the presynaptic nerve terminal. Even in the absence of cancer, symptoms can be persistent and often interfere with quality of life.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1312054,
"choiceText": "Bronchoscopy ",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1312056,
"choiceText": "Another chest CT in 6 months ",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1312058,
"choiceText": "A chest MRI",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1312060,
"choiceText": "A lung biopsy\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "A negative imaging result is generally not considered sufficient to rule out cancer in LEMS. Although this patient is not a smoker, she has been exposed to secondhand smoke as a child and as an adult. A tissue sample should be obtained using bronchoscopy, because it would be more likely to yield cancerous tissue, if any is present, than a lung biopsy in light of negative chest CT.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 418731,
"questionText": "The patient in this case has chest CT to look for cancer, which is negative. Which of the following choices represents the best next diagnostic step to investigate cancer?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1312062,
"choiceText": "Chemotherapy ",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1312064,
"choiceText": "Plasmapheresis",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1312066,
"choiceText": "Pyridostigmine",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1312068,
"choiceText": "Thymectomy",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Neurologic symptoms of LEMS are best treated with immunosuppression and/or with a potassium-channel blocker. Chemotherapy may be an appropriate treatment if the patient has demonstrated cancer; however, her neurologic symptoms should be addressed as well. Pyridostigmine and thymectomy are treatments for myasthenia gravis, but they do not have a therapeutic effect in LEMS.<br><br>\r\n\r\nThe patient in this case had bronchoscopy, which was positive for small cell lung cancer. She had a local lesion. Owing to the location of the lesion, she was unable to have surgery. She was treated with radiation and chemotherapy. Her neurologic symptoms continued to worsen, and she was treated with plasmapheresis, with moderate improvement of her symptoms. \r\n<br><br>\r\nAt 6-month follow-up, the patient was tumor-free. She developed worsening neurologic symptoms 18 months after her initial complaints, was treated again with plasmapheresis, and had marked improvement of her symptoms. She remained tumor-free at 18-month follow-up. Two years after her initial symptoms, she had not had recurrent neurologic symptoms after her second treatment with plasmapheresis, and no tumor was detectable.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 418733,
"questionText": "The patient in this case develops worsening symptoms of weakness. An appropriate treatment plan should include which of the following?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 56-Year-Old Woman With Paresthesia and Choking"
},
{
"authors": "Heidi Moawad, MD",
"content": [],
"date": "December 26, 2018",
"figures": [],
"markdown": "# A 56-Year-Old Woman With Paresthesia and Choking\n\n **Authors:** Heidi Moawad, MD \n **Date:** December 26, 2018\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1312054,
"choiceText": "Bronchoscopy ",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1312056,
"choiceText": "Another chest CT in 6 months ",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1312058,
"choiceText": "A chest MRI",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1312060,
"choiceText": "A lung biopsy\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "A negative imaging result is generally not considered sufficient to rule out cancer in LEMS. Although this patient is not a smoker, she has been exposed to secondhand smoke as a child and as an adult. A tissue sample should be obtained using bronchoscopy, because it would be more likely to yield cancerous tissue, if any is present, than a lung biopsy in light of negative chest CT.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 418731,
"questionText": "The patient in this case has chest CT to look for cancer, which is negative. Which of the following choices represents the best next diagnostic step to investigate cancer?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1312062,
"choiceText": "Chemotherapy ",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1312064,
"choiceText": "Plasmapheresis",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1312066,
"choiceText": "Pyridostigmine",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1312068,
"choiceText": "Thymectomy",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Neurologic symptoms of LEMS are best treated with immunosuppression and/or with a potassium-channel blocker. Chemotherapy may be an appropriate treatment if the patient has demonstrated cancer; however, her neurologic symptoms should be addressed as well. Pyridostigmine and thymectomy are treatments for myasthenia gravis, but they do not have a therapeutic effect in LEMS.<br><br>\r\n\r\nThe patient in this case had bronchoscopy, which was positive for small cell lung cancer. She had a local lesion. Owing to the location of the lesion, she was unable to have surgery. She was treated with radiation and chemotherapy. Her neurologic symptoms continued to worsen, and she was treated with plasmapheresis, with moderate improvement of her symptoms. \r\n<br><br>\r\nAt 6-month follow-up, the patient was tumor-free. She developed worsening neurologic symptoms 18 months after her initial complaints, was treated again with plasmapheresis, and had marked improvement of her symptoms. She remained tumor-free at 18-month follow-up. Two years after her initial symptoms, she had not had recurrent neurologic symptoms after her second treatment with plasmapheresis, and no tumor was detectable.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 418733,
"questionText": "The patient in this case develops worsening symptoms of weakness. An appropriate treatment plan should include which of the following?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 56-Year-Old Woman With Paresthesia and Choking"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1312046,
"choiceText": "Myopathy",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1312048,
"choiceText": "Neuropathy",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1312050,
"choiceText": "Lambert-Eaton myasthenic syndrome",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1312052,
"choiceText": "Myasthenia gravis",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 418729,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1312054,
"choiceText": "Bronchoscopy ",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1312056,
"choiceText": "Another chest CT in 6 months ",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1312058,
"choiceText": "A chest MRI",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1312060,
"choiceText": "A lung biopsy\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "A negative imaging result is generally not considered sufficient to rule out cancer in LEMS. Although this patient is not a smoker, she has been exposed to secondhand smoke as a child and as an adult. A tissue sample should be obtained using bronchoscopy, because it would be more likely to yield cancerous tissue, if any is present, than a lung biopsy in light of negative chest CT.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 418731,
"questionText": "The patient in this case has chest CT to look for cancer, which is negative. Which of the following choices represents the best next diagnostic step to investigate cancer?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1312062,
"choiceText": "Chemotherapy ",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1312064,
"choiceText": "Plasmapheresis",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1312066,
"choiceText": "Pyridostigmine",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1312068,
"choiceText": "Thymectomy",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Neurologic symptoms of LEMS are best treated with immunosuppression and/or with a potassium-channel blocker. Chemotherapy may be an appropriate treatment if the patient has demonstrated cancer; however, her neurologic symptoms should be addressed as well. Pyridostigmine and thymectomy are treatments for myasthenia gravis, but they do not have a therapeutic effect in LEMS.<br><br>\r\n\r\nThe patient in this case had bronchoscopy, which was positive for small cell lung cancer. She had a local lesion. Owing to the location of the lesion, she was unable to have surgery. She was treated with radiation and chemotherapy. Her neurologic symptoms continued to worsen, and she was treated with plasmapheresis, with moderate improvement of her symptoms. \r\n<br><br>\r\nAt 6-month follow-up, the patient was tumor-free. She developed worsening neurologic symptoms 18 months after her initial complaints, was treated again with plasmapheresis, and had marked improvement of her symptoms. She remained tumor-free at 18-month follow-up. Two years after her initial symptoms, she had not had recurrent neurologic symptoms after her second treatment with plasmapheresis, and no tumor was detectable.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 418733,
"questionText": "The patient in this case develops worsening symptoms of weakness. An appropriate treatment plan should include which of the following?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
905776 | /viewarticle/905776 | [
{
"authors": "Adrian Preda, MD",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to recognize accurately. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a Case Challenge, please contact us.",
"A 24-year-old woman with a past diagnosis of schizoaffective disorder presents with a chief complaint of severe anxiety. She reports that she has been experiencing gradually worsening anxiety and depression over the past 2 months, despite ongoing treatment with a selective serotonin reuptake inhibitor (fluoxetine) and a second-generation neuroleptic (quetiapine).",
"She experienced her first \"mental breakdown\" around age 17 in the context of extreme stress. She was preparing for admission to a competitive college and experiencing relationship stress, the breakup of her first significant romantic relationship. She was unable to sleep for almost a week. Because she was severely agitated, she saw a psychiatrist who recommended admission to a psychiatric facility. She readily accepted inpatient treatment because she felt too unwell to function.",
"She has a poor recollection of her 2-3 weeks in the psychiatric hospital. She remembers taking \"a ton of medications,\" being asleep most of the time, and \"tied to a bed\" during the brief moments when she would wake up. She was discharged on a second-generation antipsychotic (quetiapine), which she has continued at doses ranging from 200 mg to 400 mg total daily dose.",
"Over the past 7 years since her initial episode, she experienced one additional episode, following another romantic disappointment. During that episode, she described her mood quality as \"agitated and wired.\" She experienced psychomotor agitation (\"feeling restless\") and abundant energy despite an inability to sleep for about 3 weeks. Her symptoms culminated with paranoid and grandiose delusions and resulted in a second hospitalization. With treatment that involved antipsychotic medications, her symptoms remitted and she returned to baseline normal functioning, including doing well in her current graduate studies. She is now engaged to be married following a 2-year stable relationship.",
"Unlike prior episodes, during the current episode, her mood is colored by a \"black and unbearable sadness.\" She reports low energy levels and lack of motivation and interest, to the point that she stopped seeing her friends and going to school. Although her past episodes followed significant stress, she says that prior to the current episode, things were \"going well\" and reports no significant stressors.",
"As for her treatment, her prior psychiatrist gradually increased the dose of quetiapine from 200 mg daily to 200 mg twice daily and added fluoxetine, which was gradually escalated over a 4-week span from an initial dose of 10 mg daily to a dose of 40 mg daily. Following the medication changes, the patient experienced some improvement in her sadness, with brief, hours-long, sporadic episodes (2-3 times a week) of suddenly feeling better. She also reported a change in her overall mood quality from \"just feeling sad\" to \"being sick with worries about anything and everything.\" However, she denies any thoughts or plans regarding suicide. Her associated symptoms also changed from decreased motivation to ambivalence (\"I can't start anything\") and from decreased energy to negative energy (\"I am tired but restless\"). Her sleep remained poor. She denies experiencing abnormal perceptions, disorganized or delusional thinking, or any other psychotic symptoms, and no evidence suggests any such elements were present.",
"Her other past psychiatric and developmental history is noncontributory. Regarding substance use, she reports drinking 1-2 glasses of wine \"almost every evening\" as a \"nightcap to fall asleep\" for the past month. She has no other history of alcohol or other substance abuse. Her past medical and surgical history is essentially negative. Her family history is negative for formal diagnoses, but she acknowledges that mental illness was stigmatized in her environment and that her father was \"moody\" but would not seek help. She has no family history of other psychiatric hospitalizations."
],
"date": "December 05, 2018",
"figures": [],
"markdown": "# Anxiety, Depression, and Psychosis in a 24-Year-Old Woman\n\n **Authors:** Adrian Preda, MD \n **Date:** December 05, 2018\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to recognize accurately. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a Case Challenge, please contact us.\nA 24-year-old woman with a past diagnosis of schizoaffective disorder presents with a chief complaint of severe anxiety. She reports that she has been experiencing gradually worsening anxiety and depression over the past 2 months, despite ongoing treatment with a selective serotonin reuptake inhibitor (fluoxetine) and a second-generation neuroleptic (quetiapine).\nShe experienced her first \"mental breakdown\" around age 17 in the context of extreme stress. She was preparing for admission to a competitive college and experiencing relationship stress, the breakup of her first significant romantic relationship. She was unable to sleep for almost a week. Because she was severely agitated, she saw a psychiatrist who recommended admission to a psychiatric facility. She readily accepted inpatient treatment because she felt too unwell to function.\nShe has a poor recollection of her 2-3 weeks in the psychiatric hospital. She remembers taking \"a ton of medications,\" being asleep most of the time, and \"tied to a bed\" during the brief moments when she would wake up. She was discharged on a second-generation antipsychotic (quetiapine), which she has continued at doses ranging from 200 mg to 400 mg total daily dose.\nOver the past 7 years since her initial episode, she experienced one additional episode, following another romantic disappointment. During that episode, she described her mood quality as \"agitated and wired.\" She experienced psychomotor agitation (\"feeling restless\") and abundant energy despite an inability to sleep for about 3 weeks. Her symptoms culminated with paranoid and grandiose delusions and resulted in a second hospitalization. With treatment that involved antipsychotic medications, her symptoms remitted and she returned to baseline normal functioning, including doing well in her current graduate studies. She is now engaged to be married following a 2-year stable relationship.\nUnlike prior episodes, during the current episode, her mood is colored by a \"black and unbearable sadness.\" She reports low energy levels and lack of motivation and interest, to the point that she stopped seeing her friends and going to school. Although her past episodes followed significant stress, she says that prior to the current episode, things were \"going well\" and reports no significant stressors.\nAs for her treatment, her prior psychiatrist gradually increased the dose of quetiapine from 200 mg daily to 200 mg twice daily and added fluoxetine, which was gradually escalated over a 4-week span from an initial dose of 10 mg daily to a dose of 40 mg daily. Following the medication changes, the patient experienced some improvement in her sadness, with brief, hours-long, sporadic episodes (2-3 times a week) of suddenly feeling better. She also reported a change in her overall mood quality from \"just feeling sad\" to \"being sick with worries about anything and everything.\" However, she denies any thoughts or plans regarding suicide. Her associated symptoms also changed from decreased motivation to ambivalence (\"I can't start anything\") and from decreased energy to negative energy (\"I am tired but restless\"). Her sleep remained poor. She denies experiencing abnormal perceptions, disorganized or delusional thinking, or any other psychotic symptoms, and no evidence suggests any such elements were present.\nHer other past psychiatric and developmental history is noncontributory. Regarding substance use, she reports drinking 1-2 glasses of wine \"almost every evening\" as a \"nightcap to fall asleep\" for the past month. She has no other history of alcohol or other substance abuse. Her past medical and surgical history is essentially negative. Her family history is negative for formal diagnoses, but she acknowledges that mental illness was stigmatized in her environment and that her father was \"moody\" but would not seek help. She has no family history of other psychiatric hospitalizations.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "Anxiety, Depression, and Psychosis in a 24-Year-Old Woman"
},
{
"authors": "Adrian Preda, MD",
"content": [
"The patient is in generally good health and had a recent physical examination with normal findings and nonsignificant laboratory test results, including complete blood count, complete metabolic panel, thyroid function tests, vitamin D level, folate level, and thiamine levels.",
"Upon mental status examination, the patient presents as a casually dressed young woman, appearing at her stated age. She has a degree of psychomotor agitation, often shifting positions in her chair and wringing her hands throughout the interview. She speaks with a monotonous voice, and while her speech is not overly pressured, she is hard to interrupt. Her mood and affect are anxious.",
"Her thoughts are circumstantial to tangential. Her thought context is positive for themes of hopelessness and guilt but negative for suicidal or homicidal ideation and abnormal perceptions. She is oriented to time, place, and person and has no problems with her memory during testing for immediate, recent, and distant recall. Although she demonstrates overvalued ideas about people paying attention to her and judging her, she has no overtly delusional ideas. Her cognitive examination findings are significant for focusing deficits. Her insight and judgment are fair. Her intellect appears above average."
],
"date": "December 05, 2018",
"figures": [],
"markdown": "# Anxiety, Depression, and Psychosis in a 24-Year-Old Woman\n\n **Authors:** Adrian Preda, MD \n **Date:** December 05, 2018\n\n ## Content\n\n The patient is in generally good health and had a recent physical examination with normal findings and nonsignificant laboratory test results, including complete blood count, complete metabolic panel, thyroid function tests, vitamin D level, folate level, and thiamine levels.\nUpon mental status examination, the patient presents as a casually dressed young woman, appearing at her stated age. She has a degree of psychomotor agitation, often shifting positions in her chair and wringing her hands throughout the interview. She speaks with a monotonous voice, and while her speech is not overly pressured, she is hard to interrupt. Her mood and affect are anxious.\nHer thoughts are circumstantial to tangential. Her thought context is positive for themes of hopelessness and guilt but negative for suicidal or homicidal ideation and abnormal perceptions. She is oriented to time, place, and person and has no problems with her memory during testing for immediate, recent, and distant recall. Although she demonstrates overvalued ideas about people paying attention to her and judging her, she has no overtly delusional ideas. Her cognitive examination findings are significant for focusing deficits. Her insight and judgment are fair. Her intellect appears above average.\n\n ## Figures\n\n \n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1294302,
"choiceText": "Schizoaffective disorder, most recent episode depressed",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1294304,
"choiceText": "Generalized anxiety disorder",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1294306,
"choiceText": "Major depressive disorder, most recent episode severe",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1294308,
"choiceText": "Bipolar disorder type I, most recent episode mixed, severe",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1294310,
"choiceText": "Alcohol-induced mood disorder, depressed",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 412793,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Anxiety, Depression, and Psychosis in a 24-Year-Old Woman"
},
{
"authors": "Adrian Preda, MD",
"content": [
"This patient has a past diagnosis of schizoaffective disorder. The fact that she has been stable on a maintenance treatment where the mainstay was an antipsychotic may also be an indication that her diagnosis belongs in the realm of psychotic disorders, including schizoaffective disorder and schizophrenia. Her psychiatric disorder started with a severe episode that resulted in psychiatric hospitalization. Although the details of that episode are unclear, she may have been experiencing psychotic symptoms because she was hospitalized, heavily medicated, and placed in restraints. The history suggests that her past diagnosis might be accurate; however, the test-retest reliability of schizoaffective disorder is low,[1] and this is a retrospective diagnosis. Thus, a wary clinician should inquire further instead of assuming the diagnosis is correct.",
"A careful history revealed that her first episode—even if it was psychotic—remitted in a matter of weeks. This is evidence against a diagnosis of schizophrenia. Her subsequent disorder course has been episodic, with both strong affective and psychotic components. Although this can be indicative of a schizoaffective disorder, it is also suggestive of a mood disorder with psychotic features. The diagnostic requirement for schizoaffective disorder requires a period of psychotic symptoms that do not overlap with affective symptoms, which was the case for her first episode. However, a diagnosis of schizoaffective disorder also requires that the total duration of psychosis account for about 50% of the total duration of illness.[2] In essence, schizoaffective disorder is schizophrenia with a significant mood component. Because the patient's only other episode of psychosis appeared in the context of a manic episode, and because she had no other episodes of psychosis, a diagnosis of schizoaffective disorder is less likely than a diagnosis of bipolar disorder type I."
],
"date": "December 05, 2018",
"figures": [],
"markdown": "# Anxiety, Depression, and Psychosis in a 24-Year-Old Woman\n\n **Authors:** Adrian Preda, MD \n **Date:** December 05, 2018\n\n ## Content\n\n This patient has a past diagnosis of schizoaffective disorder. The fact that she has been stable on a maintenance treatment where the mainstay was an antipsychotic may also be an indication that her diagnosis belongs in the realm of psychotic disorders, including schizoaffective disorder and schizophrenia. Her psychiatric disorder started with a severe episode that resulted in psychiatric hospitalization. Although the details of that episode are unclear, she may have been experiencing psychotic symptoms because she was hospitalized, heavily medicated, and placed in restraints. The history suggests that her past diagnosis might be accurate; however, the test-retest reliability of schizoaffective disorder is low,[1] and this is a retrospective diagnosis. Thus, a wary clinician should inquire further instead of assuming the diagnosis is correct.\nA careful history revealed that her first episode—even if it was psychotic—remitted in a matter of weeks. This is evidence against a diagnosis of schizophrenia. Her subsequent disorder course has been episodic, with both strong affective and psychotic components. Although this can be indicative of a schizoaffective disorder, it is also suggestive of a mood disorder with psychotic features. The diagnostic requirement for schizoaffective disorder requires a period of psychotic symptoms that do not overlap with affective symptoms, which was the case for her first episode. However, a diagnosis of schizoaffective disorder also requires that the total duration of psychosis account for about 50% of the total duration of illness.[2] In essence, schizoaffective disorder is schizophrenia with a significant mood component. Because the patient's only other episode of psychosis appeared in the context of a manic episode, and because she had no other episodes of psychosis, a diagnosis of schizoaffective disorder is less likely than a diagnosis of bipolar disorder type I.\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1294302,
"choiceText": "Schizoaffective disorder, most recent episode depressed",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1294304,
"choiceText": "Generalized anxiety disorder",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1294306,
"choiceText": "Major depressive disorder, most recent episode severe",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1294308,
"choiceText": "Bipolar disorder type I, most recent episode mixed, severe",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1294310,
"choiceText": "Alcohol-induced mood disorder, depressed",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 412793,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Anxiety, Depression, and Psychosis in a 24-Year-Old Woman"
},
{
"authors": "Adrian Preda, MD",
"content": [
"The patient's recent symptoms were indicative of significant depression prior to her medication changes. Her social isolation and poor school attendance indicated moderate functional impairment. She thus met diagnostic criteria for a major depressive episode. Based on symptom severity and level of impairment, her episode severity qualified as moderate. Her recent decompensation was treated with an increase in her antidepressant and atypical neuroleptic doses; however, her symptoms worsened and were complicated by a mixed affective presentation. Clinical worsening after initiating or increasing antidepressant should raise the suspicion for bipolar disorder misdiagnosed as depression, which can occur in as many as 40% of patients.[3] Her current presentation is consistent with an episode of mixed mania.[2]",
"Earlier episodes also contain elements that are suggestive of bipolar disorder, especially the grandiose delusions. Although the precise details of her first episode and associated hospitalization are somewhat unclear, decreased sleep with psychomotor agitation were reported. Patients with depression commonly report feeling exhausted and wanting to sleep and yet are unable to either fall asleep or stay asleep. In contrast, during her second episode, the patient reported significantly increased energy while sleeping less.",
"Her history of not feeling tired despite minimal sleep for a period longer than a week is characteristic of hypomania or mania. As a rule of thumb, for patients with mood disorders, it is helpful to differentiate between complaints about an inability to sleep despite a desire to do so, which is usually indicative of depression, and abundant energy despite a lack of sleep, which is usually indicative of a mania/mixed state. The patient's single episode of mania effectively retrospectively rules out a diagnosis of major depressive disorder.",
"Because her past episode of mania was complicated by psychotic symptoms and resulted in hospitalization, a retrospective diagnosis of a severe manic episode with psychotic features is warranted. This suggests that the patient has bipolar disorder type I. Important, although the patient did not experience overt euphoria or an expansive and elevated mood during any of her past episodes, this does not exclude a diagnosis of bipolar disorder, as the mood can be either elevated or irritable during an episode of mania.[2]",
"Although the details of the first episode are unclear, its sudden onset at an early age and relatively rapid resolution are consistent with a bipolar diathesis. When depressed, patients with bipolar disorder are also more likely to be psychotic.[4] Furthermore, during the current episode, the patient experienced a rapid change in her mood quality following an increase in her antidepressant dose. Such rapid mood switches with changes in antidepressants can be indicative of a bipolar diathesis.[5] Of note, although her father never had a formal diagnosis, his mental health issues also suggest a possible bipolar diathesis; this, in turn, increases the risk for his daughter. A diagnosis of bipolar type I disorder, most recent episode depressed, severe, without psychotic features, best explains the total current presentation and past history."
],
"date": "December 05, 2018",
"figures": [],
"markdown": "# Anxiety, Depression, and Psychosis in a 24-Year-Old Woman\n\n **Authors:** Adrian Preda, MD \n **Date:** December 05, 2018\n\n ## Content\n\n The patient's recent symptoms were indicative of significant depression prior to her medication changes. Her social isolation and poor school attendance indicated moderate functional impairment. She thus met diagnostic criteria for a major depressive episode. Based on symptom severity and level of impairment, her episode severity qualified as moderate. Her recent decompensation was treated with an increase in her antidepressant and atypical neuroleptic doses; however, her symptoms worsened and were complicated by a mixed affective presentation. Clinical worsening after initiating or increasing antidepressant should raise the suspicion for bipolar disorder misdiagnosed as depression, which can occur in as many as 40% of patients.[3] Her current presentation is consistent with an episode of mixed mania.[2]\nEarlier episodes also contain elements that are suggestive of bipolar disorder, especially the grandiose delusions. Although the precise details of her first episode and associated hospitalization are somewhat unclear, decreased sleep with psychomotor agitation were reported. Patients with depression commonly report feeling exhausted and wanting to sleep and yet are unable to either fall asleep or stay asleep. In contrast, during her second episode, the patient reported significantly increased energy while sleeping less.\nHer history of not feeling tired despite minimal sleep for a period longer than a week is characteristic of hypomania or mania. As a rule of thumb, for patients with mood disorders, it is helpful to differentiate between complaints about an inability to sleep despite a desire to do so, which is usually indicative of depression, and abundant energy despite a lack of sleep, which is usually indicative of a mania/mixed state. The patient's single episode of mania effectively retrospectively rules out a diagnosis of major depressive disorder.\nBecause her past episode of mania was complicated by psychotic symptoms and resulted in hospitalization, a retrospective diagnosis of a severe manic episode with psychotic features is warranted. This suggests that the patient has bipolar disorder type I. Important, although the patient did not experience overt euphoria or an expansive and elevated mood during any of her past episodes, this does not exclude a diagnosis of bipolar disorder, as the mood can be either elevated or irritable during an episode of mania.[2]\nAlthough the details of the first episode are unclear, its sudden onset at an early age and relatively rapid resolution are consistent with a bipolar diathesis. When depressed, patients with bipolar disorder are also more likely to be psychotic.[4] Furthermore, during the current episode, the patient experienced a rapid change in her mood quality following an increase in her antidepressant dose. Such rapid mood switches with changes in antidepressants can be indicative of a bipolar diathesis.[5] Of note, although her father never had a formal diagnosis, his mental health issues also suggest a possible bipolar diathesis; this, in turn, increases the risk for his daughter. A diagnosis of bipolar type I disorder, most recent episode depressed, severe, without psychotic features, best explains the total current presentation and past history.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "Anxiety, Depression, and Psychosis in a 24-Year-Old Woman"
},
{
"authors": "Adrian Preda, MD",
"content": [
"The patient's diagnosis is also complicated by her severe anxiety, which she has been experiencing over the past 2 months. However, her anxiety is not independent and overlaps with her severe depression. Furthermore, she notes that her depression preceded her anxiety and also that her anxiety followed an increase in her antidepressant dose. The timeline of her anxiety symptoms suggests that her anxiety is not a primary and independent comorbid disorder but likely secondary to her mood disorder.",
"The fact that her anxiety appears to have been precipitated by an increase in the antidepressant is further evidence against a primary, independent, comorbid generalized anxiety disorder at this time and suggests bipolar disorder. Although almost half of patients with bipolar disorder also have a comorbid anxiety disorder,[6] anxiety is commonly present during a depressive or mixed episode. Such overlapping presentations do not warrant an additional diagnosis of an anxiety disorder. Thus, generalized anxiety disorder is ruled out at this time. However, if her anxiety symptoms continue and present a distinct course that is separate from her mood symptoms, a separate diagnosis of generalized anxiety disorder may be warranted in the future.",
"In terms of alcohol as a contributing factor, the National Institute on Alcohol Abuse and Alcoholism defines low-risk drinking as no more than three drinks on any single day and no more than seven drinks per week.[7] With her one-to-two nightly drinks almost every evening, the patient is on the border of concern; however, no other indications suggest that she is misusing alcohol. Although her ongoing use of alcohol and increased risk for drinking should be addressed as part of the treatment plan, a diagnosis of alcohol misuse disorder or alcohol-induced mood disorder is not warranted in this case.",
"After the patient in this case received a diagnosis of bipolar disorder type I, her antidepressant was discontinued. She was continued on quetiapine (400 mg daily). Lorazepam (1 mg every 6 hours), as needed for anxiety, agitation, and/or sleep, was added as a short-term intervention. At a 2-week follow-up, the patient was sleeping well and reported that her depression was significantly improved and her anxiety had resolved. The lorazepam was stopped, and she was continued on quetiapine."
],
"date": "December 05, 2018",
"figures": [],
"markdown": "# Anxiety, Depression, and Psychosis in a 24-Year-Old Woman\n\n **Authors:** Adrian Preda, MD \n **Date:** December 05, 2018\n\n ## Content\n\n The patient's diagnosis is also complicated by her severe anxiety, which she has been experiencing over the past 2 months. However, her anxiety is not independent and overlaps with her severe depression. Furthermore, she notes that her depression preceded her anxiety and also that her anxiety followed an increase in her antidepressant dose. The timeline of her anxiety symptoms suggests that her anxiety is not a primary and independent comorbid disorder but likely secondary to her mood disorder.\nThe fact that her anxiety appears to have been precipitated by an increase in the antidepressant is further evidence against a primary, independent, comorbid generalized anxiety disorder at this time and suggests bipolar disorder. Although almost half of patients with bipolar disorder also have a comorbid anxiety disorder,[6] anxiety is commonly present during a depressive or mixed episode. Such overlapping presentations do not warrant an additional diagnosis of an anxiety disorder. Thus, generalized anxiety disorder is ruled out at this time. However, if her anxiety symptoms continue and present a distinct course that is separate from her mood symptoms, a separate diagnosis of generalized anxiety disorder may be warranted in the future.\nIn terms of alcohol as a contributing factor, the National Institute on Alcohol Abuse and Alcoholism defines low-risk drinking as no more than three drinks on any single day and no more than seven drinks per week.[7] With her one-to-two nightly drinks almost every evening, the patient is on the border of concern; however, no other indications suggest that she is misusing alcohol. Although her ongoing use of alcohol and increased risk for drinking should be addressed as part of the treatment plan, a diagnosis of alcohol misuse disorder or alcohol-induced mood disorder is not warranted in this case.\nAfter the patient in this case received a diagnosis of bipolar disorder type I, her antidepressant was discontinued. She was continued on quetiapine (400 mg daily). Lorazepam (1 mg every 6 hours), as needed for anxiety, agitation, and/or sleep, was added as a short-term intervention. At a 2-week follow-up, the patient was sleeping well and reported that her depression was significantly improved and her anxiety had resolved. The lorazepam was stopped, and she was continued on quetiapine.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1294312,
"choiceText": "1%",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1294314,
"choiceText": "10%",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1294316,
"choiceText": "30%",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1294318,
"choiceText": "40%",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Clinical worsening after initiating or increasing antidepressant should raise the suspicion for bipolar disorder misdiagnosed as depression, which can occur in as many as 40% of patients.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 412795,
"questionText": "Misdiagnosis of bipolar disorder as depression occurs in as many as how many patients?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1294320,
"choiceText": "A family history of bipolar disorder",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1294322,
"choiceText": "A past diagnosis of schizoaffective disorder",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1294324,
"choiceText": "A past history of mood switches while taking antidepressants",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1294326,
"choiceText": "A history of psychotic symptoms",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "A history of psychotic symptoms and family history of bipolar disorder are associated with an increased risk for bipolar diathesis. Mood switches while taking antidepressants are also suggestive of this. A past diagnosis of schizoaffective disorder is not inherently associated with an increased risk for bipolar diathesis.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 412797,
"questionText": "Which of the following is <em>not</em> indicative of an increased risk for bipolar diathesis in patients with depression?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Anxiety, Depression, and Psychosis in a 24-Year-Old Woman"
},
{
"authors": "Adrian Preda, MD",
"content": [],
"date": "December 05, 2018",
"figures": [],
"markdown": "# Anxiety, Depression, and Psychosis in a 24-Year-Old Woman\n\n **Authors:** Adrian Preda, MD \n **Date:** December 05, 2018\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1294312,
"choiceText": "1%",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1294314,
"choiceText": "10%",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1294316,
"choiceText": "30%",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1294318,
"choiceText": "40%",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Clinical worsening after initiating or increasing antidepressant should raise the suspicion for bipolar disorder misdiagnosed as depression, which can occur in as many as 40% of patients.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 412795,
"questionText": "Misdiagnosis of bipolar disorder as depression occurs in as many as how many patients?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1294320,
"choiceText": "A family history of bipolar disorder",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1294322,
"choiceText": "A past diagnosis of schizoaffective disorder",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1294324,
"choiceText": "A past history of mood switches while taking antidepressants",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1294326,
"choiceText": "A history of psychotic symptoms",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "A history of psychotic symptoms and family history of bipolar disorder are associated with an increased risk for bipolar diathesis. Mood switches while taking antidepressants are also suggestive of this. A past diagnosis of schizoaffective disorder is not inherently associated with an increased risk for bipolar diathesis.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 412797,
"questionText": "Which of the following is <em>not</em> indicative of an increased risk for bipolar diathesis in patients with depression?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Anxiety, Depression, and Psychosis in a 24-Year-Old Woman"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1294302,
"choiceText": "Schizoaffective disorder, most recent episode depressed",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1294304,
"choiceText": "Generalized anxiety disorder",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1294306,
"choiceText": "Major depressive disorder, most recent episode severe",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1294308,
"choiceText": "Bipolar disorder type I, most recent episode mixed, severe",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1294310,
"choiceText": "Alcohol-induced mood disorder, depressed",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 412793,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1294312,
"choiceText": "1%",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1294314,
"choiceText": "10%",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1294316,
"choiceText": "30%",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1294318,
"choiceText": "40%",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Clinical worsening after initiating or increasing antidepressant should raise the suspicion for bipolar disorder misdiagnosed as depression, which can occur in as many as 40% of patients.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 412795,
"questionText": "Misdiagnosis of bipolar disorder as depression occurs in as many as how many patients?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1294320,
"choiceText": "A family history of bipolar disorder",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1294322,
"choiceText": "A past diagnosis of schizoaffective disorder",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1294324,
"choiceText": "A past history of mood switches while taking antidepressants",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1294326,
"choiceText": "A history of psychotic symptoms",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "A history of psychotic symptoms and family history of bipolar disorder are associated with an increased risk for bipolar diathesis. Mood switches while taking antidepressants are also suggestive of this. A past diagnosis of schizoaffective disorder is not inherently associated with an increased risk for bipolar diathesis.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 412797,
"questionText": "Which of the following is <em>not</em> indicative of an increased risk for bipolar diathesis in patients with depression?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
904911 | /viewarticle/904911 | [
{
"authors": "Heidi Moawad, MD",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to recognize accurately. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a Challenge, please contact us.",
"A 64-year-old woman presents to her primary care physician's office with blurry vision. Upon further questioning, she also says that she has been using the handrails when she walks up or down the stairs and has occasionally felt off-balance while walking during the past year; however, she has not fallen. Her husband, who has accompanied her, says she has been quiet and unmotivated for the past 2 years since she retired from her job as an administrative assistant. He also says she is not interested in traveling or spending time with her friends, which she had previously said she wanted to do when she retired.",
"She has a past medical history of hypothyroidism, diagnosed at age 35 years, which has been well managed, and endometriosis, which improved after she had menopause in her early 50s."
],
"date": "November 19, 2018",
"figures": [],
"markdown": "# A 64-Year-Old Woman With Blurred Vision and Loss of Balance\n\n **Authors:** Heidi Moawad, MD \n **Date:** November 19, 2018\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to recognize accurately. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a Challenge, please contact us.\nA 64-year-old woman presents to her primary care physician's office with blurry vision. Upon further questioning, she also says that she has been using the handrails when she walks up or down the stairs and has occasionally felt off-balance while walking during the past year; however, she has not fallen. Her husband, who has accompanied her, says she has been quiet and unmotivated for the past 2 years since she retired from her job as an administrative assistant. He also says she is not interested in traveling or spending time with her friends, which she had previously said she wanted to do when she retired.\nShe has a past medical history of hypothyroidism, diagnosed at age 35 years, which has been well managed, and endometriosis, which improved after she had menopause in her early 50s.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 64-Year-Old Woman With Blurred Vision and Loss of Balance"
},
{
"authors": "Heidi Moawad, MD",
"content": [
"Upon physical examination, the patient has normal mental status examination findings. She has a somewhat flat affect and does not volunteer any information or initiate conversation, but she responds appropriately to all questions.",
"Her skin is normal with no rashes, wounds, bruises, or discoloration. She does not have jaundice of the skin or eyes. Her chest is clear and heart sounds are normal. Her abdominal examination findings are normal, with no distension, pain, or tenderness. Her pedal and radial pulse examination findings are regular.",
"Upon neurological examination, no spasms, tremors, or fasciculations are observed. She has normal facial movements during the examination. At rest, she has contraction of the forehead muscles with an expression of surprise.",
"Her conjunctivae appear normal. Her pupils are equal, round, and reactive to light. She has a full range of ocular movements, with vertical saccades upon eye examination, which slow down during the examination. She has 20/20 vision with her reading glasses",
"She has 5/5 motor strength with moderate rigidity of the upper extremities. She also has normal reflexes of the upper and lower extremities, with a positive Babinski on the left side. She has intact sensation to light touch, pinprick, vibration, and proprioception. She has slow movements on her finger-to-nose examination, but her coordination is not impaired and she accurately reaches the target. She has a wide-based gait and appears to be off-balance on heel-to-toe walking."
],
"date": "November 19, 2018",
"figures": [],
"markdown": "# A 64-Year-Old Woman With Blurred Vision and Loss of Balance\n\n **Authors:** Heidi Moawad, MD \n **Date:** November 19, 2018\n\n ## Content\n\n Upon physical examination, the patient has normal mental status examination findings. She has a somewhat flat affect and does not volunteer any information or initiate conversation, but she responds appropriately to all questions.\nHer skin is normal with no rashes, wounds, bruises, or discoloration. She does not have jaundice of the skin or eyes. Her chest is clear and heart sounds are normal. Her abdominal examination findings are normal, with no distension, pain, or tenderness. Her pedal and radial pulse examination findings are regular.\nUpon neurological examination, no spasms, tremors, or fasciculations are observed. She has normal facial movements during the examination. At rest, she has contraction of the forehead muscles with an expression of surprise.\nHer conjunctivae appear normal. Her pupils are equal, round, and reactive to light. She has a full range of ocular movements, with vertical saccades upon eye examination, which slow down during the examination. She has 20/20 vision with her reading glasses\nShe has 5/5 motor strength with moderate rigidity of the upper extremities. She also has normal reflexes of the upper and lower extremities, with a positive Babinski on the left side. She has intact sensation to light touch, pinprick, vibration, and proprioception. She has slow movements on her finger-to-nose examination, but her coordination is not impaired and she accurately reaches the target. She has a wide-based gait and appears to be off-balance on heel-to-toe walking.\n\n ## Figures\n\n \n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1285856,
"choiceText": "Progressive supranuclear palsy",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1285858,
"choiceText": "Lewy body dementia \r\n",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1285860,
"choiceText": "Parkinson disease",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1285862,
"choiceText": "Myasthenia gravis",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 409951,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 64-Year-Old Woman With Blurred Vision and Loss of Balance"
},
{
"authors": "Heidi Moawad, MD",
"content": [
"Progressive supranuclear palsy (PSP) is a neurodegenerative disorder that causes muscle rigidity, limitations of eye movement, apathy, and dementia. The clinical presentation is similar to that of early Parkinson disease, and it can be mistaken for depression, Lewy body dementia, or myasthenia gravis on the initial presentation, depending on which symptoms are most prominent. PSP is less common than any of these other conditions. Mistaking PSP for another illness is more common than misdiagnosing another illness as PSP.",
"Parkinson disease involves more prominent motor features, whereas PSP involves eye movement limitations (palsy). Patients with PSP have rigidity, as do patients with Parkinson disease. The rigidity can affect gait and may lead to falls in patients with either of these conditions. Patients with Parkinson disease almost always have a fine-motor resting tremor, which is not a characteristic of typical PSP and was absent in the patient in this case.",
"Upon physical examination, patients with PSP are more likely to have unilateral or bilateral positive Babinski sign findings, whereas patients with Parkinson disease typically have a negative Babinski sign.[1] Patients with PSP frequently have vertical saccades observed during examination of extraocular movements, whereas patients with Parkinson disease do not. Saccades are movements of the eyes that appear to be jerking or jumping back and forth. The characteristic facial features noted in the patient described in this case, referred to as the Procerus sign, are not typical of patients with Parkinson disease, who may have an expressionless face.[2]",
"As Parkinson disease or PSP progresses, the differences between the two conditions become clearer over time. Patients with Parkinson disease develop tremors and increasing stiffness, whereas patients with PSP develop increasingly limited eye movements, as well as dementia. Mood lability, or pseudobulbar affect, may affect patients with PSP and patients with Parkinson disease, manifesting with sudden bouts of crying or laughter that are often embarrassing for the patient."
],
"date": "November 19, 2018",
"figures": [],
"markdown": "# A 64-Year-Old Woman With Blurred Vision and Loss of Balance\n\n **Authors:** Heidi Moawad, MD \n **Date:** November 19, 2018\n\n ## Content\n\n Progressive supranuclear palsy (PSP) is a neurodegenerative disorder that causes muscle rigidity, limitations of eye movement, apathy, and dementia. The clinical presentation is similar to that of early Parkinson disease, and it can be mistaken for depression, Lewy body dementia, or myasthenia gravis on the initial presentation, depending on which symptoms are most prominent. PSP is less common than any of these other conditions. Mistaking PSP for another illness is more common than misdiagnosing another illness as PSP.\nParkinson disease involves more prominent motor features, whereas PSP involves eye movement limitations (palsy). Patients with PSP have rigidity, as do patients with Parkinson disease. The rigidity can affect gait and may lead to falls in patients with either of these conditions. Patients with Parkinson disease almost always have a fine-motor resting tremor, which is not a characteristic of typical PSP and was absent in the patient in this case.\nUpon physical examination, patients with PSP are more likely to have unilateral or bilateral positive Babinski sign findings, whereas patients with Parkinson disease typically have a negative Babinski sign.[1] Patients with PSP frequently have vertical saccades observed during examination of extraocular movements, whereas patients with Parkinson disease do not. Saccades are movements of the eyes that appear to be jerking or jumping back and forth. The characteristic facial features noted in the patient described in this case, referred to as the Procerus sign, are not typical of patients with Parkinson disease, who may have an expressionless face.[2]\nAs Parkinson disease or PSP progresses, the differences between the two conditions become clearer over time. Patients with Parkinson disease develop tremors and increasing stiffness, whereas patients with PSP develop increasingly limited eye movements, as well as dementia. Mood lability, or pseudobulbar affect, may affect patients with PSP and patients with Parkinson disease, manifesting with sudden bouts of crying or laughter that are often embarrassing for the patient.\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1285856,
"choiceText": "Progressive supranuclear palsy",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1285858,
"choiceText": "Lewy body dementia \r\n",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1285860,
"choiceText": "Parkinson disease",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1285862,
"choiceText": "Myasthenia gravis",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 409951,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 64-Year-Old Woman With Blurred Vision and Loss of Balance"
},
{
"authors": "Heidi Moawad, MD",
"content": [
"Patients who have PSP may also erroneously be diagnosed with depression. Because the symptoms of PSP are subtle and neurological findings are not always present within the first 2-5 years after disease onset, apathy can be the most prominent complaint from families of individuals who have PSP. However, patients with depression report feelings of sadness, whereas patients who have PSP typically are not disturbed by their own apathy. Depression should not normally be accompanied by abnormal neurological examination findings. In this instance, the patient may indeed also have a diagnosis of depression; however, she must also have a neurological cause behind the saccades and muscle rigidity, gait imbalance, and positive Babinski sign. The apathy that her husband is noticing may be related to her PSP. Screening a patient with PSP for concomitant depression involves asking questions about mood, appetite, and sleep.",
"Lewy body dementia is a condition predominantly characterized by rapidly worsening dementia. Lewy body dementia is usually associated with signs and symptoms of a Parkinsonlike movement disorder, including rigidity and gait imbalance, as well as behavioral changes. Movement disorder signs and symptoms are more prominent in Lewy body dementia than they are in PSP. Like Lewy body dementia, PSP causes dementia and behavioral changes; however, these symptoms tend to begin later in the course of disease and tend to progress more slowly in PSP than they do in Lewy body dementia.",
"Myasthenia gravis is a neuromuscular disease that produces symptoms of blurred vision or double vision, eyelid weakness, fatigue, and generalized weakness of the body. Patients with either PSP or myasthenia gravis may be less active than usual and may complain of double vision or blurred vision, as well as diminished control of eyelids and forehead muscles. Muscle fatigue is a prominent early physical examination finding with myasthenia gravis, and patients may appear depressed. Patients with myasthenia gravis have droopy eyelids and weak eye movement, which causes the blurred or double vision. Patients with PSP may have raised eyelids, a decreased ability to move the eyes vertically, and saccades, all of which are not present with myasthenia gravis. The rigidity of PSP, the gait imbalance, and the symptoms of dementia and pseudobulbar affect are not present in myasthenia gravis.",
"PSP is clinical diagnosis based on symptoms. However, because the symptoms are subtle, it is important to rule out other conditions that can also cause subtle neurological symptoms, such as cerebrovascular disease, brain tumors, and multisystem atrophy. These conditions cause changes that can be visualized with a brain CT or MRI scan, whereas PSP rarely causes changes on brain imaging. When PSP does cause changes in brain imaging, it appears as atrophy in the midbrain, reflective of the degeneration of the substantia nigra that is the pathophysiologic basis of the disease (Figure).[3]",
"Figure."
],
"date": "November 19, 2018",
"figures": [
{
"caption": "Figure.",
"image_url": "https://img.medscapestatic.com/article/904/911/904911-Thumb1.jpg"
}
],
"markdown": "# A 64-Year-Old Woman With Blurred Vision and Loss of Balance\n\n **Authors:** Heidi Moawad, MD \n **Date:** November 19, 2018\n\n ## Content\n\n Patients who have PSP may also erroneously be diagnosed with depression. Because the symptoms of PSP are subtle and neurological findings are not always present within the first 2-5 years after disease onset, apathy can be the most prominent complaint from families of individuals who have PSP. However, patients with depression report feelings of sadness, whereas patients who have PSP typically are not disturbed by their own apathy. Depression should not normally be accompanied by abnormal neurological examination findings. In this instance, the patient may indeed also have a diagnosis of depression; however, she must also have a neurological cause behind the saccades and muscle rigidity, gait imbalance, and positive Babinski sign. The apathy that her husband is noticing may be related to her PSP. Screening a patient with PSP for concomitant depression involves asking questions about mood, appetite, and sleep.\nLewy body dementia is a condition predominantly characterized by rapidly worsening dementia. Lewy body dementia is usually associated with signs and symptoms of a Parkinsonlike movement disorder, including rigidity and gait imbalance, as well as behavioral changes. Movement disorder signs and symptoms are more prominent in Lewy body dementia than they are in PSP. Like Lewy body dementia, PSP causes dementia and behavioral changes; however, these symptoms tend to begin later in the course of disease and tend to progress more slowly in PSP than they do in Lewy body dementia.\nMyasthenia gravis is a neuromuscular disease that produces symptoms of blurred vision or double vision, eyelid weakness, fatigue, and generalized weakness of the body. Patients with either PSP or myasthenia gravis may be less active than usual and may complain of double vision or blurred vision, as well as diminished control of eyelids and forehead muscles. Muscle fatigue is a prominent early physical examination finding with myasthenia gravis, and patients may appear depressed. Patients with myasthenia gravis have droopy eyelids and weak eye movement, which causes the blurred or double vision. Patients with PSP may have raised eyelids, a decreased ability to move the eyes vertically, and saccades, all of which are not present with myasthenia gravis. The rigidity of PSP, the gait imbalance, and the symptoms of dementia and pseudobulbar affect are not present in myasthenia gravis.\nPSP is clinical diagnosis based on symptoms. However, because the symptoms are subtle, it is important to rule out other conditions that can also cause subtle neurological symptoms, such as cerebrovascular disease, brain tumors, and multisystem atrophy. These conditions cause changes that can be visualized with a brain CT or MRI scan, whereas PSP rarely causes changes on brain imaging. When PSP does cause changes in brain imaging, it appears as atrophy in the midbrain, reflective of the degeneration of the substantia nigra that is the pathophysiologic basis of the disease (Figure).[3]\nFigure.\n\n ## Figures\n\n **Figure.** \n \n\n\n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 64-Year-Old Woman With Blurred Vision and Loss of Balance"
},
{
"authors": "Heidi Moawad, MD",
"content": [
"The prognosis of PSP is a slow deterioration, with more prominent gait impairment, dementia, and worsening coordination over the course of 10-15 years. Patients are able to survive with a slightly reduced life expectancy and eventually need assistance for mobility and self-care.",
"No treatment is curative for PSP, and symptomatic treatment is only moderately effective. Some patients are given a trial of dopaminergic medications because of the involvement of largely dopaminergic substantia nigra. Antidepressants may be useful for some patients who have significant apathy. As the coordination worsens, physical therapy can be helpful. Speech and swallow therapy may be needed if speech or swallowing becomes impaired. Some patients experience involuntary contraction of the eye muscles, which can interfere with vision, adding to the visual deficits that are caused by the ocular palsy. In these instances, treatment with botulinum toxin injections may be helpful."
],
"date": "November 19, 2018",
"figures": [],
"markdown": "# A 64-Year-Old Woman With Blurred Vision and Loss of Balance\n\n **Authors:** Heidi Moawad, MD \n **Date:** November 19, 2018\n\n ## Content\n\n The prognosis of PSP is a slow deterioration, with more prominent gait impairment, dementia, and worsening coordination over the course of 10-15 years. Patients are able to survive with a slightly reduced life expectancy and eventually need assistance for mobility and self-care.\nNo treatment is curative for PSP, and symptomatic treatment is only moderately effective. Some patients are given a trial of dopaminergic medications because of the involvement of largely dopaminergic substantia nigra. Antidepressants may be useful for some patients who have significant apathy. As the coordination worsens, physical therapy can be helpful. Speech and swallow therapy may be needed if speech or swallowing becomes impaired. Some patients experience involuntary contraction of the eye muscles, which can interfere with vision, adding to the visual deficits that are caused by the ocular palsy. In these instances, treatment with botulinum toxin injections may be helpful.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1285864,
"choiceText": "Anxiety",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1285866,
"choiceText": "Schizophrenia",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1285868,
"choiceText": "Pseudobulbar affect",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1285870,
"choiceText": "Bipolar disorder\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Her forgetfulness is likely caused by progression of her PSP, which causes dementia as the disease progresses. Patients with PSP often develop pseudobulbar affect, which is characterized by bouts of uncontrollable crying or laughing. Anxiety can manifest in numerous ways, and symptoms such as crying or laughing generally occur in response to unease or stressful events rather than occurring unprovoked. <br><br>\r\n\r\nSchizophrenia is often associated with unusual behavior, and hallucinations are the most noticeable symptom, whereas forgetfulness is not a prominent characteristic of schizophrenia. Bipolar disorder, often called manic-depression, is characterized by mood swings that usually last for weeks at a time, and do not necessarily manifest with crying or laughing. Bipolar disorder is not generally associated with forgetfulness.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 409953,
"questionText": "The patient in this case remained stable for 2 years and then began to have increasing signs of forgetfulness, as well as unprovoked bouts of sudden laughing or crying lasting for minutes at a time. Which of the following is the probable cause of her new symptoms? ",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1285916,
"choiceText": "Further increase the dose of carbidopa-levodopa",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1285918,
"choiceText": "Decrease the dose of carbidopa-levodopa",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1285920,
"choiceText": "Wait 4 weeks to see if the carbidopa-levodopa begins to work",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1285922,
"choiceText": "Discontinue carbidopa-levodopa\r\n",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "This patient did not have a response to the medication, and when her dose was increased, she began having side effects and still did not have improvement of her rigidity. Given her side effect of hallucinations, it is unlikely that she can tolerate another increase in dose. She is not expected to have improvement of her rigidity if her dose is decreased again. Waiting to see if improvement occurs is not beneficial, as she has already been on the higher dose for 3 weeks. The best course of action is to discontinue carbidopa-levodopa. ",
"displayOrder": 4,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 409975,
"questionText": "The patient in this case was started on dopaminergic medication (carbidopa-levodopa) for her rigidity, but she did not improve. When her dose was increased, she began to have visual hallucinations after 3 weeks of taking the increased dose, without improvements of her symptoms. Which of the following is the best option as the next step?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 64-Year-Old Woman With Blurred Vision and Loss of Balance"
},
{
"authors": "Heidi Moawad, MD",
"content": [],
"date": "November 19, 2018",
"figures": [],
"markdown": "# A 64-Year-Old Woman With Blurred Vision and Loss of Balance\n\n **Authors:** Heidi Moawad, MD \n **Date:** November 19, 2018\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1285864,
"choiceText": "Anxiety",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1285866,
"choiceText": "Schizophrenia",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1285868,
"choiceText": "Pseudobulbar affect",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1285870,
"choiceText": "Bipolar disorder\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Her forgetfulness is likely caused by progression of her PSP, which causes dementia as the disease progresses. Patients with PSP often develop pseudobulbar affect, which is characterized by bouts of uncontrollable crying or laughing. Anxiety can manifest in numerous ways, and symptoms such as crying or laughing generally occur in response to unease or stressful events rather than occurring unprovoked. <br><br>\r\n\r\nSchizophrenia is often associated with unusual behavior, and hallucinations are the most noticeable symptom, whereas forgetfulness is not a prominent characteristic of schizophrenia. Bipolar disorder, often called manic-depression, is characterized by mood swings that usually last for weeks at a time, and do not necessarily manifest with crying or laughing. Bipolar disorder is not generally associated with forgetfulness.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 409953,
"questionText": "The patient in this case remained stable for 2 years and then began to have increasing signs of forgetfulness, as well as unprovoked bouts of sudden laughing or crying lasting for minutes at a time. Which of the following is the probable cause of her new symptoms? ",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1285916,
"choiceText": "Further increase the dose of carbidopa-levodopa",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1285918,
"choiceText": "Decrease the dose of carbidopa-levodopa",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1285920,
"choiceText": "Wait 4 weeks to see if the carbidopa-levodopa begins to work",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1285922,
"choiceText": "Discontinue carbidopa-levodopa\r\n",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "This patient did not have a response to the medication, and when her dose was increased, she began having side effects and still did not have improvement of her rigidity. Given her side effect of hallucinations, it is unlikely that she can tolerate another increase in dose. She is not expected to have improvement of her rigidity if her dose is decreased again. Waiting to see if improvement occurs is not beneficial, as she has already been on the higher dose for 3 weeks. The best course of action is to discontinue carbidopa-levodopa. ",
"displayOrder": 4,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 409975,
"questionText": "The patient in this case was started on dopaminergic medication (carbidopa-levodopa) for her rigidity, but she did not improve. When her dose was increased, she began to have visual hallucinations after 3 weeks of taking the increased dose, without improvements of her symptoms. Which of the following is the best option as the next step?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 64-Year-Old Woman With Blurred Vision and Loss of Balance"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1285856,
"choiceText": "Progressive supranuclear palsy",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1285858,
"choiceText": "Lewy body dementia \r\n",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1285860,
"choiceText": "Parkinson disease",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1285862,
"choiceText": "Myasthenia gravis",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 409951,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1285864,
"choiceText": "Anxiety",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1285866,
"choiceText": "Schizophrenia",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1285868,
"choiceText": "Pseudobulbar affect",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1285870,
"choiceText": "Bipolar disorder\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Her forgetfulness is likely caused by progression of her PSP, which causes dementia as the disease progresses. Patients with PSP often develop pseudobulbar affect, which is characterized by bouts of uncontrollable crying or laughing. Anxiety can manifest in numerous ways, and symptoms such as crying or laughing generally occur in response to unease or stressful events rather than occurring unprovoked. <br><br>\r\n\r\nSchizophrenia is often associated with unusual behavior, and hallucinations are the most noticeable symptom, whereas forgetfulness is not a prominent characteristic of schizophrenia. Bipolar disorder, often called manic-depression, is characterized by mood swings that usually last for weeks at a time, and do not necessarily manifest with crying or laughing. Bipolar disorder is not generally associated with forgetfulness.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 409953,
"questionText": "The patient in this case remained stable for 2 years and then began to have increasing signs of forgetfulness, as well as unprovoked bouts of sudden laughing or crying lasting for minutes at a time. Which of the following is the probable cause of her new symptoms? ",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1285916,
"choiceText": "Further increase the dose of carbidopa-levodopa",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1285918,
"choiceText": "Decrease the dose of carbidopa-levodopa",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1285920,
"choiceText": "Wait 4 weeks to see if the carbidopa-levodopa begins to work",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1285922,
"choiceText": "Discontinue carbidopa-levodopa\r\n",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "This patient did not have a response to the medication, and when her dose was increased, she began having side effects and still did not have improvement of her rigidity. Given her side effect of hallucinations, it is unlikely that she can tolerate another increase in dose. She is not expected to have improvement of her rigidity if her dose is decreased again. Waiting to see if improvement occurs is not beneficial, as she has already been on the higher dose for 3 weeks. The best course of action is to discontinue carbidopa-levodopa. ",
"displayOrder": 4,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 409975,
"questionText": "The patient in this case was started on dopaminergic medication (carbidopa-levodopa) for her rigidity, but she did not improve. When her dose was increased, she began to have visual hallucinations after 3 weeks of taking the increased dose, without improvements of her symptoms. Which of the following is the best option as the next step?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
903551 | /viewarticle/903551 | [
{
"authors": "Nafisa Kuwajerwala, MD; Angad Pordal, MD",
"content": [
"The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to recognize accurately. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a Case Challenge, please contact us.",
"A 68-year-old woman presents to the emergency department with nausea, vomiting, and diffuse abdominal pain. She reports a 30-lb weight loss over the past 3 to 4 weeks. She has had intermittent nonbloody, nonbilious vomiting and poor oral intake. She reports having bowel movements and flatus. She denies any pruritus, shortness of breath, chest pain, fevers, or chills.",
"Two years prior, the patient was diagnosed with and treated for breast cancer. She was asymptomatic at the time of the mammography screening but had a family history that included two sisters who were diagnosed with breast cancer, at ages 60 and 65 years. Also, both her brother and father had prostate cancer. The screening mammogram showed evidence of calcifications approximately 1.9 cm in the lower-inner quadrant of her right breast, with a rating of BIRADS 4. This finding was new compared with her previous mammogram 1 year prior.",
"The patient subsequently underwent diagnostic mammography with stereotactic core needle biopsy, which yielded pathology consistent with a grade 2 invasive ductal carcinoma with foci of ductal carcinoma in situ; thus, her breast cancer was determined to be clinical stage 1a/T1a/cN0/M0. Hormonal testing revealed strong estrogen receptor (ER) positivity, with 51%-100% of cells staining positive. It also revealed negative progesterone receptor (PR) receptivity, with less than 1% of cells staining, and negative HER2/neu findings.",
"Due to the high risk associated with her family history, the patient underwent diagnostic bilateral breast MRI. A lesion consistent with the calcifications seen on the mammogram was revealed at the 4-o'clock position, with a new suspicious lesion at the 8-o'clock position, measuring 4 mm. Based on her family history of malignancy, the patient underwent further genetic testing. Myriad 25-gene testing, including BRCA1 and BRCA2 testing, yielded negative findings. The patient opted for breast conservation with postoperative radiation and adjuvant systemic treatment.",
"The patient subsequently underwent a 3-needle localized right lumpectomy (2-needle localization bracketing the area of cancer in the additional 8-o'clock position, with additional needle localization of the 4-o'clock mass) with sentinel lymph node biopsy and possible axillary node dissection. The pathology of the surgical specimen was consistent with a 3.5-cm invasive ductal carcinoma, grade 2, with a positive inferior margin and close anterior and lateral margins. Sentinel nodes revealed evidence of one node with 0.1-mm micrometastasis and three negative nodes. The pathology of the lesion at the 8-o'clock position showed findings consistent with intraductal papilloma.",
"The patient was upstaged to a T2/N1mic/M0 stage IIB cancer, with an oncotype score of 26. She subsequently underwent adjuvant chemotherapy with docetaxel anhydrous, cyclophosphamide, and pegfilgrastim and postchemotherapy reexcision of her margins, with all new margins reported as negative. A follow-up mammogram performed 1 year later—approximately 10 months prior to her current presentation for nausea, vomiting, and abdominal pain—revealed no abnormal findings."
],
"date": "October 19, 2018",
"figures": [],
"markdown": "# A 68-Year-Old Woman With Weight Loss and Abdominal Pain\n\n **Authors:** Nafisa Kuwajerwala, MD; Angad Pordal, MD \n **Date:** October 19, 2018\n\n ## Content\n\n The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to recognize accurately. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a Case Challenge, please contact us.\nA 68-year-old woman presents to the emergency department with nausea, vomiting, and diffuse abdominal pain. She reports a 30-lb weight loss over the past 3 to 4 weeks. She has had intermittent nonbloody, nonbilious vomiting and poor oral intake. She reports having bowel movements and flatus. She denies any pruritus, shortness of breath, chest pain, fevers, or chills.\nTwo years prior, the patient was diagnosed with and treated for breast cancer. She was asymptomatic at the time of the mammography screening but had a family history that included two sisters who were diagnosed with breast cancer, at ages 60 and 65 years. Also, both her brother and father had prostate cancer. The screening mammogram showed evidence of calcifications approximately 1.9 cm in the lower-inner quadrant of her right breast, with a rating of BIRADS 4. This finding was new compared with her previous mammogram 1 year prior.\nThe patient subsequently underwent diagnostic mammography with stereotactic core needle biopsy, which yielded pathology consistent with a grade 2 invasive ductal carcinoma with foci of ductal carcinoma in situ; thus, her breast cancer was determined to be clinical stage 1a/T1a/cN0/M0. Hormonal testing revealed strong estrogen receptor (ER) positivity, with 51%-100% of cells staining positive. It also revealed negative progesterone receptor (PR) receptivity, with less than 1% of cells staining, and negative HER2/neu findings.\nDue to the high risk associated with her family history, the patient underwent diagnostic bilateral breast MRI. A lesion consistent with the calcifications seen on the mammogram was revealed at the 4-o'clock position, with a new suspicious lesion at the 8-o'clock position, measuring 4 mm. Based on her family history of malignancy, the patient underwent further genetic testing. Myriad 25-gene testing, including BRCA1 and BRCA2 testing, yielded negative findings. The patient opted for breast conservation with postoperative radiation and adjuvant systemic treatment.\nThe patient subsequently underwent a 3-needle localized right lumpectomy (2-needle localization bracketing the area of cancer in the additional 8-o'clock position, with additional needle localization of the 4-o'clock mass) with sentinel lymph node biopsy and possible axillary node dissection. The pathology of the surgical specimen was consistent with a 3.5-cm invasive ductal carcinoma, grade 2, with a positive inferior margin and close anterior and lateral margins. Sentinel nodes revealed evidence of one node with 0.1-mm micrometastasis and three negative nodes. The pathology of the lesion at the 8-o'clock position showed findings consistent with intraductal papilloma.\nThe patient was upstaged to a T2/N1mic/M0 stage IIB cancer, with an oncotype score of 26. She subsequently underwent adjuvant chemotherapy with docetaxel anhydrous, cyclophosphamide, and pegfilgrastim and postchemotherapy reexcision of her margins, with all new margins reported as negative. A follow-up mammogram performed 1 year later—approximately 10 months prior to her current presentation for nausea, vomiting, and abdominal pain—revealed no abnormal findings.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 68-Year-Old Woman With Weight Loss and Abdominal Pain"
},
{
"authors": "Nafisa Kuwajerwala, MD; Angad Pordal, MD",
"content": [
"Upon physical examination, the patient's vitals are normal. Her lungs are clear to auscultation bilaterally, and she has a regular heart rate and rhythm. Her abdomen is soft, mildly distended, and mildly tender to palpation diffusely, with increased tenderness in the right, upper quadrant.",
"No guarding, rebound, or rigidity is observed. She has a negative Murphy's sign. She is found to have a white blood cell count of 9.4 × 109/L and a hemoglobin level of 13.5 g/dL. Her total bilirubin level is 0.5 mg/dL. Her alkaline phosphatase level is 140 IU/L. Her alanine aminotransferase level is 98 U/L, and her aspartate aminotransferase level is 73 U/L. She states that she previously underwent a CT scan of her abdomen at an outside institution.",
"MRI of the abdomen reveals the findings below.",
"Figure 1.",
"Figure 2.",
"Figure 3.",
"Figure 4."
],
"date": "October 19, 2018",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/903/551/903551-figure-1-thumb.jpg"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/903/551/903551-figure-2-thumb.jpg"
},
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/903/551/903551-figure-3-thumb.jpg"
},
{
"caption": "Figure 4.",
"image_url": "https://img.medscapestatic.com/article/903/551/903551-figure-4-thumb.jpg"
}
],
"markdown": "# A 68-Year-Old Woman With Weight Loss and Abdominal Pain\n\n **Authors:** Nafisa Kuwajerwala, MD; Angad Pordal, MD \n **Date:** October 19, 2018\n\n ## Content\n\n Upon physical examination, the patient's vitals are normal. Her lungs are clear to auscultation bilaterally, and she has a regular heart rate and rhythm. Her abdomen is soft, mildly distended, and mildly tender to palpation diffusely, with increased tenderness in the right, upper quadrant.\nNo guarding, rebound, or rigidity is observed. She has a negative Murphy's sign. She is found to have a white blood cell count of 9.4 × 109/L and a hemoglobin level of 13.5 g/dL. Her total bilirubin level is 0.5 mg/dL. Her alkaline phosphatase level is 140 IU/L. Her alanine aminotransferase level is 98 U/L, and her aspartate aminotransferase level is 73 U/L. She states that she previously underwent a CT scan of her abdomen at an outside institution.\nMRI of the abdomen reveals the findings below.\nFigure 1.\nFigure 2.\nFigure 3.\nFigure 4.\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n**Figure 3.** \n \n\n**Figure 4.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1276942,
"choiceText": "Hepatocellular carcinoma",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1276944,
"choiceText": "Metastatic invasive ductal carcinoma",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1276946,
"choiceText": "Hepatic adenoma",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1276948,
"choiceText": "Metastatic melanoma",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 406883,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 68-Year-Old Woman With Weight Loss and Abdominal Pain"
},
{
"authors": "Nafisa Kuwajerwala, MD; Angad Pordal, MD",
"content": [
"Ductal carcinoma in situ is considered a noninvasive breast neoplasm and accounts for approximately 20% of all breast cancer cases in the United States.[1] It is characterized by abnormal cells lining one or more of the lactiferous ducts of the breast. It is classified by its pathological architecture into four discrete types: papillary, solid, cribriform, and comedo. These architectures are rarely seen alone and are usually of mixed type.",
"Comedo necrosis architecture, as seen in the patient in this case, has a higher grade and carries a larger malignant potential.[1] As the cells inside the ductules grow farther from the blood supply, located in the basement membrane, coagulative necrosis occurs, which results in calcifications that are often seen on mammograms that suggest breast cancer. When the degree of suspension is high for malignancy based on the BIRADS radiographic classification, core needle biopsy should be performed to provide a tissue diagnosis with the cellular architecture intact.",
"Although ductal carcinoma in situ progresses into invasive disease only in approximately 30% of cases, differentiating between cases that will progress into invasive disease and those that do not is impossible. Thus intervention with lumpectomy with 2-mm margins, postoperative radiation, and adjuvant hormonal treatment is warranted.[1]",
"Invasive ductal carcinoma accounts for 50%-70% of all invasive breast malignancies; the remainder consists of lobular, mixed, tubular, medullary, and metaplastic breast cancer. When detected, treatment along with genetic testing—when indicated—is warranted.[1] Invasive ductal carcinoma has varying degrees of estrogen and progesterone hormone receptor expression and HER2/neu tyrosine kinase expression. Tumors with hormone receptor positivity are eligible for hormonal therapy and are associated with better outcomes than those without."
],
"date": "October 19, 2018",
"figures": [],
"markdown": "# A 68-Year-Old Woman With Weight Loss and Abdominal Pain\n\n **Authors:** Nafisa Kuwajerwala, MD; Angad Pordal, MD \n **Date:** October 19, 2018\n\n ## Content\n\n Ductal carcinoma in situ is considered a noninvasive breast neoplasm and accounts for approximately 20% of all breast cancer cases in the United States.[1] It is characterized by abnormal cells lining one or more of the lactiferous ducts of the breast. It is classified by its pathological architecture into four discrete types: papillary, solid, cribriform, and comedo. These architectures are rarely seen alone and are usually of mixed type.\nComedo necrosis architecture, as seen in the patient in this case, has a higher grade and carries a larger malignant potential.[1] As the cells inside the ductules grow farther from the blood supply, located in the basement membrane, coagulative necrosis occurs, which results in calcifications that are often seen on mammograms that suggest breast cancer. When the degree of suspension is high for malignancy based on the BIRADS radiographic classification, core needle biopsy should be performed to provide a tissue diagnosis with the cellular architecture intact.\nAlthough ductal carcinoma in situ progresses into invasive disease only in approximately 30% of cases, differentiating between cases that will progress into invasive disease and those that do not is impossible. Thus intervention with lumpectomy with 2-mm margins, postoperative radiation, and adjuvant hormonal treatment is warranted.[1]\nInvasive ductal carcinoma accounts for 50%-70% of all invasive breast malignancies; the remainder consists of lobular, mixed, tubular, medullary, and metaplastic breast cancer. When detected, treatment along with genetic testing—when indicated—is warranted.[1] Invasive ductal carcinoma has varying degrees of estrogen and progesterone hormone receptor expression and HER2/neu tyrosine kinase expression. Tumors with hormone receptor positivity are eligible for hormonal therapy and are associated with better outcomes than those without.\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1276942,
"choiceText": "Hepatocellular carcinoma",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1276944,
"choiceText": "Metastatic invasive ductal carcinoma",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1276946,
"choiceText": "Hepatic adenoma",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1276948,
"choiceText": "Metastatic melanoma",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 406883,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 68-Year-Old Woman With Weight Loss and Abdominal Pain"
},
{
"authors": "Nafisa Kuwajerwala, MD; Angad Pordal, MD",
"content": [
"Surgical options for invasive ductal carcinoma are tailored to each patient and range from total mastectomy to breast-conserving therapy with sentinel lymph node biopsy and possible axillary lymph node dissection.[2] In some instances, postoperative radiation therapy and systemic chemotherapy with or without hormonal therapy—as determined by their receptor status—are administered as adjuvant therapy.",
"Those who prefer breast conservation undergo preoperative radiologic localization (needle or radiologic seed), as the lesions are often not grossly palpable. The area of concern is surgically excised with negative margins. Although wide margins were originally thought to be associated with better outcomes, margins that are negative on ink for invasive cancer are now presumed to be oncologically adequate. Postoperatively, patients undergo radiation to the tumor bed in addition to systemic chemotherapy if the sentinel nodes are positive for disease or if they are found to have triple negative receptor expression. Those patients whose cancers have hormonal receptor positivity undergo hormonal therapy, with or without trastuzumab, based on their HER2/neu status.[2] Unfortunately, approximately 20% of patients with breast cancer develop metastasis, with hepatic involvement in half of these cases.[3]",
"Patients with hepatic metastasis present with nonspecific abdominal symptoms including nausea, vomiting, and abdominal pain. Weight loss is common, as well as decreased appetite. Liver function test results may be mildly elevated. Bilirubinemia and jaundice can occur based on the involvement of the biliary tree.[3]",
"CT scanning is the modality of choice in evaluating for hepatic metastasis because of the effects of the liver's dual blood supply on the enhancement of metastasis in comparison to the normal parenchyma; however, MRI allows for the effective localization and evaluation of vascular invasion of the lesions.[4]",
"Once the lesions are confirmed through imaging, tissue diagnosis is necessary. Ultrasonography-guided biopsy is the most practical and cost-effective modality for liver biopsy and was the modality used in this case. A biopsy of this patient's liver mass confirmed the presence of ER/PR-negative, HER2-positive invasive ductal carcinoma. Compared with her original breast biopsy findings of ER-positive, PR-negative, HER2-negative invasive ductal carcinoma, these findings make it unclear whether her current metastatic disease is due to an undiagnosed primary tumor or, as is the case in most instances, to the heterogeneity of the tumor."
],
"date": "October 19, 2018",
"figures": [],
"markdown": "# A 68-Year-Old Woman With Weight Loss and Abdominal Pain\n\n **Authors:** Nafisa Kuwajerwala, MD; Angad Pordal, MD \n **Date:** October 19, 2018\n\n ## Content\n\n Surgical options for invasive ductal carcinoma are tailored to each patient and range from total mastectomy to breast-conserving therapy with sentinel lymph node biopsy and possible axillary lymph node dissection.[2] In some instances, postoperative radiation therapy and systemic chemotherapy with or without hormonal therapy—as determined by their receptor status—are administered as adjuvant therapy.\nThose who prefer breast conservation undergo preoperative radiologic localization (needle or radiologic seed), as the lesions are often not grossly palpable. The area of concern is surgically excised with negative margins. Although wide margins were originally thought to be associated with better outcomes, margins that are negative on ink for invasive cancer are now presumed to be oncologically adequate. Postoperatively, patients undergo radiation to the tumor bed in addition to systemic chemotherapy if the sentinel nodes are positive for disease or if they are found to have triple negative receptor expression. Those patients whose cancers have hormonal receptor positivity undergo hormonal therapy, with or without trastuzumab, based on their HER2/neu status.[2] Unfortunately, approximately 20% of patients with breast cancer develop metastasis, with hepatic involvement in half of these cases.[3]\nPatients with hepatic metastasis present with nonspecific abdominal symptoms including nausea, vomiting, and abdominal pain. Weight loss is common, as well as decreased appetite. Liver function test results may be mildly elevated. Bilirubinemia and jaundice can occur based on the involvement of the biliary tree.[3]\nCT scanning is the modality of choice in evaluating for hepatic metastasis because of the effects of the liver's dual blood supply on the enhancement of metastasis in comparison to the normal parenchyma; however, MRI allows for the effective localization and evaluation of vascular invasion of the lesions.[4]\nOnce the lesions are confirmed through imaging, tissue diagnosis is necessary. Ultrasonography-guided biopsy is the most practical and cost-effective modality for liver biopsy and was the modality used in this case. A biopsy of this patient's liver mass confirmed the presence of ER/PR-negative, HER2-positive invasive ductal carcinoma. Compared with her original breast biopsy findings of ER-positive, PR-negative, HER2-negative invasive ductal carcinoma, these findings make it unclear whether her current metastatic disease is due to an undiagnosed primary tumor or, as is the case in most instances, to the heterogeneity of the tumor.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 68-Year-Old Woman With Weight Loss and Abdominal Pain"
},
{
"authors": "Nafisa Kuwajerwala, MD; Angad Pordal, MD",
"content": [
"One of the hallmarks of breast cancer is the heterogeneity of its cell populations.[5] This heterogeneity is classified based on its temporal as well as spatial heterogeneity. As tumor progression occurs, cell populations undergo changes in response to various growth factors expressed from the tumor cells as well as changes in response to various treatments to which they are exposed. This change in cell population over time is referred to as a temporal heterogeneity.[5] However, at any given time in the progression of a breast malignancy, malignant cells of various phenotypes are seen and make up the spatial heterogeneity of breast cancer. This variation in phenotype is a result of genetic and epigenetic alterations that occur in clonal populations but can also involve adaptive responses to levels of proteins involved in signaling pathways.[6] These variations in cell populations thus yield variations in the expression of receptors used to target and treat breast malignancies. This accounts for variability in hormone and trastuzumab therapy response between individuals with similar expression levels seen on biopsy; it can also account for the variability in expression between primary and metastatic lesions. However, certain factors help associate metastatic lesions to their original primary.",
"GATA3 is a zinc-finger binding transcription factor that has been implicated in the differentiation of many tissues, including breast tissue.[7] Previous studies have shown that GATA3 expression in breast tissue is highly specific for carcinoma. In addition, GATA3 is expressed in ER-positive tumors rather than in those that are ER negative. However, studies have shown GATA3 expression is maintained between primary and metastatic lesions.",
"GATA3 test results of the liver lesions in this patient were positive. This suggests that the malignancy was due to an ER-positive breast carcinoma, like the patient's original carcinoma, rather than an undiagnosed cancer. Unfortunately for the patient in this case, during her workup for the hepatic lesions, she was found to have an obstructing renal calculus that required cystoscopic intervention. She subsequently opted to pursue palliative care."
],
"date": "October 19, 2018",
"figures": [],
"markdown": "# A 68-Year-Old Woman With Weight Loss and Abdominal Pain\n\n **Authors:** Nafisa Kuwajerwala, MD; Angad Pordal, MD \n **Date:** October 19, 2018\n\n ## Content\n\n One of the hallmarks of breast cancer is the heterogeneity of its cell populations.[5] This heterogeneity is classified based on its temporal as well as spatial heterogeneity. As tumor progression occurs, cell populations undergo changes in response to various growth factors expressed from the tumor cells as well as changes in response to various treatments to which they are exposed. This change in cell population over time is referred to as a temporal heterogeneity.[5] However, at any given time in the progression of a breast malignancy, malignant cells of various phenotypes are seen and make up the spatial heterogeneity of breast cancer. This variation in phenotype is a result of genetic and epigenetic alterations that occur in clonal populations but can also involve adaptive responses to levels of proteins involved in signaling pathways.[6] These variations in cell populations thus yield variations in the expression of receptors used to target and treat breast malignancies. This accounts for variability in hormone and trastuzumab therapy response between individuals with similar expression levels seen on biopsy; it can also account for the variability in expression between primary and metastatic lesions. However, certain factors help associate metastatic lesions to their original primary.\nGATA3 is a zinc-finger binding transcription factor that has been implicated in the differentiation of many tissues, including breast tissue.[7] Previous studies have shown that GATA3 expression in breast tissue is highly specific for carcinoma. In addition, GATA3 is expressed in ER-positive tumors rather than in those that are ER negative. However, studies have shown GATA3 expression is maintained between primary and metastatic lesions.\nGATA3 test results of the liver lesions in this patient were positive. This suggests that the malignancy was due to an ER-positive breast carcinoma, like the patient's original carcinoma, rather than an undiagnosed cancer. Unfortunately for the patient in this case, during her workup for the hepatic lesions, she was found to have an obstructing renal calculus that required cystoscopic intervention. She subsequently opted to pursue palliative care.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1276950,
"choiceText": "1 cm",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1276952,
"choiceText": "2 mm",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1276954,
"choiceText": "5 mm",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1276956,
"choiceText": "Negative on ink",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Although larger margins were originally thought to provide a more oncologic resection, newer guidelines suggest that margins that are negative on ink are sufficient for invasive ductal carcinoma. A 2-mm margin is necessary for patients who undergo excision of ductal carcinoma in situ with postsurgical radiation therapy.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 406885,
"questionText": "Which of the following margin sizes are considered adequate for excision of invasive ductal carcinoma?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1276962,
"choiceText": "Surgery alone",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1276964,
"choiceText": "Surgery with hormonal therapy",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1276966,
"choiceText": "Surgery with adjuvant radiation and hormonal therapy",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1276968,
"choiceText": "Surgery with adjuvant radiation and trastuzumab therapy",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1276970,
"choiceText": "Surgery with adjuvant radiation, hormonal therapy, and systemic chemotherapy",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Current guidelines indicate that all patients who undergo breast-conserving therapy should receive postoperative radiation therapy to the breast, with strong consideration for radiation to the supraclavicular, infraclavicular, internal mammary, or axillary nodes at risk. Those with hormone receptor positivity and <em>HER2</em>/neu-negative status but with gross node-positive disease, defined as three or more positive nodes, regardless of T status, should receive postoperative adjuvant chemotherapy in addition to hormonal therapy.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 406889,
"questionText": "<p>A 63-year-old woman is found to have a 3-cm area of concern on screening mammography. Core needle biopsy of the lesion reveals invasive ductal carcinoma grade 2/3, ER/PR-positive, <em>HER2</em>/neu-negative. She opts for breast-conservation therapy. A hot, blue 2-cm lymph node is found that is resectable. She undergoes lumpectomy and subsequent axillary lymph node dissection, which yields four positive lymph nodes.</p>\r\n\r\n<p>Which of the following treatment plans is recommended?</p>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 68-Year-Old Woman With Weight Loss and Abdominal Pain"
},
{
"authors": "Nafisa Kuwajerwala, MD; Angad Pordal, MD",
"content": [],
"date": "October 19, 2018",
"figures": [],
"markdown": "# A 68-Year-Old Woman With Weight Loss and Abdominal Pain\n\n **Authors:** Nafisa Kuwajerwala, MD; Angad Pordal, MD \n **Date:** October 19, 2018\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1276950,
"choiceText": "1 cm",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1276952,
"choiceText": "2 mm",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1276954,
"choiceText": "5 mm",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1276956,
"choiceText": "Negative on ink",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Although larger margins were originally thought to provide a more oncologic resection, newer guidelines suggest that margins that are negative on ink are sufficient for invasive ductal carcinoma. A 2-mm margin is necessary for patients who undergo excision of ductal carcinoma in situ with postsurgical radiation therapy.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 406885,
"questionText": "Which of the following margin sizes are considered adequate for excision of invasive ductal carcinoma?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1276962,
"choiceText": "Surgery alone",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1276964,
"choiceText": "Surgery with hormonal therapy",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1276966,
"choiceText": "Surgery with adjuvant radiation and hormonal therapy",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1276968,
"choiceText": "Surgery with adjuvant radiation and trastuzumab therapy",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1276970,
"choiceText": "Surgery with adjuvant radiation, hormonal therapy, and systemic chemotherapy",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Current guidelines indicate that all patients who undergo breast-conserving therapy should receive postoperative radiation therapy to the breast, with strong consideration for radiation to the supraclavicular, infraclavicular, internal mammary, or axillary nodes at risk. Those with hormone receptor positivity and <em>HER2</em>/neu-negative status but with gross node-positive disease, defined as three or more positive nodes, regardless of T status, should receive postoperative adjuvant chemotherapy in addition to hormonal therapy.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 406889,
"questionText": "<p>A 63-year-old woman is found to have a 3-cm area of concern on screening mammography. Core needle biopsy of the lesion reveals invasive ductal carcinoma grade 2/3, ER/PR-positive, <em>HER2</em>/neu-negative. She opts for breast-conservation therapy. A hot, blue 2-cm lymph node is found that is resectable. She undergoes lumpectomy and subsequent axillary lymph node dissection, which yields four positive lymph nodes.</p>\r\n\r\n<p>Which of the following treatment plans is recommended?</p>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 68-Year-Old Woman With Weight Loss and Abdominal Pain"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1276942,
"choiceText": "Hepatocellular carcinoma",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1276944,
"choiceText": "Metastatic invasive ductal carcinoma",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1276946,
"choiceText": "Hepatic adenoma",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1276948,
"choiceText": "Metastatic melanoma",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 406883,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1276950,
"choiceText": "1 cm",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1276952,
"choiceText": "2 mm",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1276954,
"choiceText": "5 mm",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1276956,
"choiceText": "Negative on ink",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Although larger margins were originally thought to provide a more oncologic resection, newer guidelines suggest that margins that are negative on ink are sufficient for invasive ductal carcinoma. A 2-mm margin is necessary for patients who undergo excision of ductal carcinoma in situ with postsurgical radiation therapy.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 406885,
"questionText": "Which of the following margin sizes are considered adequate for excision of invasive ductal carcinoma?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1276962,
"choiceText": "Surgery alone",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1276964,
"choiceText": "Surgery with hormonal therapy",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1276966,
"choiceText": "Surgery with adjuvant radiation and hormonal therapy",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1276968,
"choiceText": "Surgery with adjuvant radiation and trastuzumab therapy",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1276970,
"choiceText": "Surgery with adjuvant radiation, hormonal therapy, and systemic chemotherapy",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Current guidelines indicate that all patients who undergo breast-conserving therapy should receive postoperative radiation therapy to the breast, with strong consideration for radiation to the supraclavicular, infraclavicular, internal mammary, or axillary nodes at risk. Those with hormone receptor positivity and <em>HER2</em>/neu-negative status but with gross node-positive disease, defined as three or more positive nodes, regardless of T status, should receive postoperative adjuvant chemotherapy in addition to hormonal therapy.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 406889,
"questionText": "<p>A 63-year-old woman is found to have a 3-cm area of concern on screening mammography. Core needle biopsy of the lesion reveals invasive ductal carcinoma grade 2/3, ER/PR-positive, <em>HER2</em>/neu-negative. She opts for breast-conservation therapy. A hot, blue 2-cm lymph node is found that is resectable. She undergoes lumpectomy and subsequent axillary lymph node dissection, which yields four positive lymph nodes.</p>\r\n\r\n<p>Which of the following treatment plans is recommended?</p>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
900203 | /viewarticle/900203 | [
{
"authors": "Patricia Ayuk Noumedem, PharmD; Marissa Tabile; Ermias Shikur; Bini Mathews; Anthony L. Huynh; Tracy Kwaku",
"content": [
"The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to recognize accurately. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a Case Challenge, please contact us.",
"A 64-year-old black man is admitted to the emergency department with throat swelling, difficulty swallowing, and drooling (Figure).",
"Figure 1.",
"His past medical history is significant for hypertension, diabetes mellitus, substance abuse (heroin), depression, schizophrenia, and alcohol abuse. He also has a past medical history of tongue swelling and airway compromise.",
"No history of home medications is reported; however, he was alleged to have ingested lisinopril that was prescribed for his mother. Upon admission, he is administered dexamethasone, diphenhydramine, a fentanyl drip, a versed drip, a propofol drip, a phenylephrine drip, amlodipine, carvedilol, hydralazine, albuterol sulfate, dextrose sodium, heparin, famotidine, and acetaminophen, as well as mechanical ventilation."
],
"date": "August 08, 2018",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/900/203/900203-figure-1-thumb.jpg"
}
],
"markdown": "# A 64-Year-Old Man With Throat Swelling\n\n **Authors:** Patricia Ayuk Noumedem, PharmD; Marissa Tabile; Ermias Shikur; Bini Mathews; Anthony L. Huynh; Tracy Kwaku \n **Date:** August 08, 2018\n\n ## Content\n\n The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to recognize accurately. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a Case Challenge, please contact us.\nA 64-year-old black man is admitted to the emergency department with throat swelling, difficulty swallowing, and drooling (Figure).\nFigure 1.\nHis past medical history is significant for hypertension, diabetes mellitus, substance abuse (heroin), depression, schizophrenia, and alcohol abuse. He also has a past medical history of tongue swelling and airway compromise.\nNo history of home medications is reported; however, he was alleged to have ingested lisinopril that was prescribed for his mother. Upon admission, he is administered dexamethasone, diphenhydramine, a fentanyl drip, a versed drip, a propofol drip, a phenylephrine drip, amlodipine, carvedilol, hydralazine, albuterol sulfate, dextrose sodium, heparin, famotidine, and acetaminophen, as well as mechanical ventilation.\n\n ## Figures\n\n **Figure 1.** \n \n\n\n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 64-Year-Old Man With Throat Swelling"
},
{
"authors": "Patricia Ayuk Noumedem, PharmD; Marissa Tabile; Ermias Shikur; Bini Mathews; Anthony L. Huynh; Tracy Kwaku",
"content": [
"Physical examination reveals a middle-aged man with swollen lips, tongue, and throat. The pharynx is edematous. The respiratory and cardiovascular system examinations reveal features of hypertensive heart disease in biventricular failure. Upon further evaluation, the patient has airway compromise and tongue swelling, requiring a tracheostomy.",
"C1- inhibitor (C1-INH) and complement assay levels and function are normal. Other laboratory findings include:",
"Creatinine level : 1 mg/dL (upon admission)",
"Ammonia : 59 mcg/dL",
"Alanine aminotransferase (ALT) level : 80 U/L",
"Aspartate aminotransferase (AST) level : 73 U/L",
"Lactic acid level : 4.9 mmol/L",
"White blood cell count : 15.5 × 109/L",
"Hemoglobin level : 14 g/dL",
"Hematocrit level : 42.5%",
"Platelet count : 131 × 10/mm3",
"Sodium level : 134 mEq/L",
"Potassium level : 5.6 mEq/L",
"Chloride level : 104 mEq/L",
"CO2 level : 20 mEq/L)",
"Blood urea nitrogen level : 21 mg/dL",
"Creatinine level : 2.82 mg/dL",
"Glucose level : 144 mg/dL"
],
"date": "August 08, 2018",
"figures": [],
"markdown": "# A 64-Year-Old Man With Throat Swelling\n\n **Authors:** Patricia Ayuk Noumedem, PharmD; Marissa Tabile; Ermias Shikur; Bini Mathews; Anthony L. Huynh; Tracy Kwaku \n **Date:** August 08, 2018\n\n ## Content\n\n Physical examination reveals a middle-aged man with swollen lips, tongue, and throat. The pharynx is edematous. The respiratory and cardiovascular system examinations reveal features of hypertensive heart disease in biventricular failure. Upon further evaluation, the patient has airway compromise and tongue swelling, requiring a tracheostomy.\nC1- inhibitor (C1-INH) and complement assay levels and function are normal. Other laboratory findings include:\nCreatinine level : 1 mg/dL (upon admission)\nAmmonia : 59 mcg/dL\nAlanine aminotransferase (ALT) level : 80 U/L\nAspartate aminotransferase (AST) level : 73 U/L\nLactic acid level : 4.9 mmol/L\nWhite blood cell count : 15.5 × 109/L\nHemoglobin level : 14 g/dL\nHematocrit level : 42.5%\nPlatelet count : 131 × 10/mm3\nSodium level : 134 mEq/L\nPotassium level : 5.6 mEq/L\nChloride level : 104 mEq/L\nCO2 level : 20 mEq/L)\nBlood urea nitrogen level : 21 mg/dL\nCreatinine level : 2.82 mg/dL\nGlucose level : 144 mg/dL\n\n ## Figures\n\n \n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1254098,
"choiceText": "Pernicious anemia",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1254100,
"choiceText": "C1-INH deficiency",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1254102,
"choiceText": "Drug-induced angioedema",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1254104,
"choiceText": "Hereditary angioedema",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 398985,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 64-Year-Old Man With Throat Swelling"
},
{
"authors": "Patricia Ayuk Noumedem, PharmD; Marissa Tabile; Ermias Shikur; Bini Mathews; Anthony L. Huynh; Tracy Kwaku",
"content": [
"Angiotensin-converting enzyme (ACE)-induced angioedema is a well-recognized side effect of ACE inhibitor therapy. These medications are primarily used in the treatment of hypertension and congestive heart failure. The mechanism of action of ACE inhibitors is blocking the formation of angiotensin II and bradykinin metabolism, which leads to vasodilation and lower blood pressure. A rare but severe side effect of ACE inhibitors is angioedema, which causes swelling of the face, tongue, larynx, and the upper/lower extremities. It can also lead to breathing difficulty due to airway obstruction and, in rare instances, nausea and vomiting due to swelling of the gastrointestinal tract.[1,2,3,4,5]",
"The patient did not present with urticaria; thus, ruling out hereditary angioedema, acquired C1-INH deficiency, and ACE inhibitor-induced angioedema was necessary.[1] A thorough history is the key to differentiating among the etiologies of angioedema. C1-inhibitor level tests and complement assay (C4, C1q) are important in distinguishing ACE inhibitor angioedema from hereditary angioedema. The tryptase level is usually elevated in allergic angioedema. Hereditary angioedema is differentiated from the acquired form by the low levels of C1q.[2]",
"ACE inhibitors are the most common cause of drug-induced angioedema in the United States. Although the chance of developing angioedema from ACE inhibitors is considered rare (< 1%), the risk is greater for certain populations (≤ 5% for black people). Of those who develop angioedema in response to ACE inhibitors, about 25% have the reaction within the first month of treatment.[1,2,3,4,5,6] However, the reaction can develop for the first time months or even years after treatment begins. In fact, doctors believe late-onset angioedema (as an adverse effect of ACE inhibitors) may be more common than previously thought.[2,3]",
"Overall, the incidence of angioedema in the United States is approximately 10%-15%, with ACE inhibitor-related angioedema accounting for approximately 30%-40% of all emergency department visits. Although uncommon (0.1%-2.2%), severe angioedema can be life threatening and can occur anytime during ACE inhibitor therapy.[2,3,4] However, most cases occur during the first week of use. Black women have a higher incidence of angioedema compared with others in the general population, and neither the dose nor duration of treatment has been implicated in instances of reported angioedema. The increased incidence may be a result of low bradykinin that is suddenly increased in patients taking ACE inhibitors.[2]"
],
"date": "August 08, 2018",
"figures": [],
"markdown": "# A 64-Year-Old Man With Throat Swelling\n\n **Authors:** Patricia Ayuk Noumedem, PharmD; Marissa Tabile; Ermias Shikur; Bini Mathews; Anthony L. Huynh; Tracy Kwaku \n **Date:** August 08, 2018\n\n ## Content\n\n Angiotensin-converting enzyme (ACE)-induced angioedema is a well-recognized side effect of ACE inhibitor therapy. These medications are primarily used in the treatment of hypertension and congestive heart failure. The mechanism of action of ACE inhibitors is blocking the formation of angiotensin II and bradykinin metabolism, which leads to vasodilation and lower blood pressure. A rare but severe side effect of ACE inhibitors is angioedema, which causes swelling of the face, tongue, larynx, and the upper/lower extremities. It can also lead to breathing difficulty due to airway obstruction and, in rare instances, nausea and vomiting due to swelling of the gastrointestinal tract.[1,2,3,4,5]\nThe patient did not present with urticaria; thus, ruling out hereditary angioedema, acquired C1-INH deficiency, and ACE inhibitor-induced angioedema was necessary.[1] A thorough history is the key to differentiating among the etiologies of angioedema. C1-inhibitor level tests and complement assay (C4, C1q) are important in distinguishing ACE inhibitor angioedema from hereditary angioedema. The tryptase level is usually elevated in allergic angioedema. Hereditary angioedema is differentiated from the acquired form by the low levels of C1q.[2]\nACE inhibitors are the most common cause of drug-induced angioedema in the United States. Although the chance of developing angioedema from ACE inhibitors is considered rare (< 1%), the risk is greater for certain populations (≤ 5% for black people). Of those who develop angioedema in response to ACE inhibitors, about 25% have the reaction within the first month of treatment.[1,2,3,4,5,6] However, the reaction can develop for the first time months or even years after treatment begins. In fact, doctors believe late-onset angioedema (as an adverse effect of ACE inhibitors) may be more common than previously thought.[2,3]\nOverall, the incidence of angioedema in the United States is approximately 10%-15%, with ACE inhibitor-related angioedema accounting for approximately 30%-40% of all emergency department visits. Although uncommon (0.1%-2.2%), severe angioedema can be life threatening and can occur anytime during ACE inhibitor therapy.[2,3,4] However, most cases occur during the first week of use. Black women have a higher incidence of angioedema compared with others in the general population, and neither the dose nor duration of treatment has been implicated in instances of reported angioedema. The increased incidence may be a result of low bradykinin that is suddenly increased in patients taking ACE inhibitors.[2]\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1254098,
"choiceText": "Pernicious anemia",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1254100,
"choiceText": "C1-INH deficiency",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1254102,
"choiceText": "Drug-induced angioedema",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1254104,
"choiceText": "Hereditary angioedema",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 398985,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 64-Year-Old Man With Throat Swelling"
},
{
"authors": "Patricia Ayuk Noumedem, PharmD; Marissa Tabile; Ermias Shikur; Bini Mathews; Anthony L. Huynh; Tracy Kwaku",
"content": [
"Angioedema is a potential adverse effect of many other drugs, including:",
"Bupropion",
"Vaccines",
"Selective serotonin reuptake inhibitors (SSRIs)",
"Nonsteroidal anti-inflammatory drugs (NSAIDs)",
"Proton pump inhibitors",
"Statins",
"Radiocontrast agents",
"ACE-induced angioedema typically presents with swelling of extremities such as legs, hands, and feet, as well as facial swelling, including the tongue. Internally, ACE inhibitors can cause swelling of the pharynx and larynx, which can only be seen via CT scanning and MRI. Flexible fiberoptic laryngoscopy may be beneficial in patients with head and neck angioedema with any lingual involvement.[7] Notable symptoms include:[1,2]",
"Trouble breathing",
"Tightness in throat",
"Trouble swallowing",
"Nausea and vomiting",
"Cramps or stomach pain",
"Erythema",
"Intubation is indicated in severe cases in which the airway is obstructed. Discontinuation of the ACE inhibitor is required and may be sufficient in less severe cases. A ratings scale and discharge criteria have been proposed by Bonner and colleagues.[8]",
"Pharmacological treatments include:[2]",
"Subcutaneous epinephrine",
"Ecallantide",
"Icatibant",
"Fresh frozen plasma",
"Lisinopril, the drug responsible in this patient, is indicated in adults and pediatric patients (> 6 y) for the treatment of hypertension and also as an adjunct in the therapy of heart failure. It is also indicated for the treatment of acute myocardial infarction. ACE is responsible for the conversion of angiotensin I to angiotensin II, a potent vasoconstrictor that also stimulates aldosterone secretion. ACE inhibitors therefore block the conversion of angiotensin I to angiotensin II, which leads to a decrease in vasoconstriction and aldosterone secretion. Lisinopril also exerts its effect by blocking the breakdown of bradykinin, which is a contributory factor to its vasodilatory effect and adverse effects of angioedema and cough.[1,2,4] Other side effects of lisinopril include dizziness, headache, rash, hypotension, angina, palpitations, and fatigue. Gastrointestinal side effects include nausea, vomiting, diarrhea, and constipation. Taste perversions may also be associated with its use. Hyperkalemia (especially in renal dysfunction) has been reported.[1,2]",
"Drug monitoring should be carried out upon initiation of treatment or dosage increase because of a propensity for renal dysfunction. Contraindications for the use of lisinopril include a history of angioedema, hereditary or idiopathic. Lisinopril is also contraindicated in patients with known hypersensitivity to the drug and in coadministration with aliskiren in patients with diabetes mellitus.[2]"
],
"date": "August 08, 2018",
"figures": [],
"markdown": "# A 64-Year-Old Man With Throat Swelling\n\n **Authors:** Patricia Ayuk Noumedem, PharmD; Marissa Tabile; Ermias Shikur; Bini Mathews; Anthony L. Huynh; Tracy Kwaku \n **Date:** August 08, 2018\n\n ## Content\n\n Angioedema is a potential adverse effect of many other drugs, including:\nBupropion\nVaccines\nSelective serotonin reuptake inhibitors (SSRIs)\nNonsteroidal anti-inflammatory drugs (NSAIDs)\nProton pump inhibitors\nStatins\nRadiocontrast agents\nACE-induced angioedema typically presents with swelling of extremities such as legs, hands, and feet, as well as facial swelling, including the tongue. Internally, ACE inhibitors can cause swelling of the pharynx and larynx, which can only be seen via CT scanning and MRI. Flexible fiberoptic laryngoscopy may be beneficial in patients with head and neck angioedema with any lingual involvement.[7] Notable symptoms include:[1,2]\nTrouble breathing\nTightness in throat\nTrouble swallowing\nNausea and vomiting\nCramps or stomach pain\nErythema\nIntubation is indicated in severe cases in which the airway is obstructed. Discontinuation of the ACE inhibitor is required and may be sufficient in less severe cases. A ratings scale and discharge criteria have been proposed by Bonner and colleagues.[8]\nPharmacological treatments include:[2]\nSubcutaneous epinephrine\nEcallantide\nIcatibant\nFresh frozen plasma\nLisinopril, the drug responsible in this patient, is indicated in adults and pediatric patients (> 6 y) for the treatment of hypertension and also as an adjunct in the therapy of heart failure. It is also indicated for the treatment of acute myocardial infarction. ACE is responsible for the conversion of angiotensin I to angiotensin II, a potent vasoconstrictor that also stimulates aldosterone secretion. ACE inhibitors therefore block the conversion of angiotensin I to angiotensin II, which leads to a decrease in vasoconstriction and aldosterone secretion. Lisinopril also exerts its effect by blocking the breakdown of bradykinin, which is a contributory factor to its vasodilatory effect and adverse effects of angioedema and cough.[1,2,4] Other side effects of lisinopril include dizziness, headache, rash, hypotension, angina, palpitations, and fatigue. Gastrointestinal side effects include nausea, vomiting, diarrhea, and constipation. Taste perversions may also be associated with its use. Hyperkalemia (especially in renal dysfunction) has been reported.[1,2]\nDrug monitoring should be carried out upon initiation of treatment or dosage increase because of a propensity for renal dysfunction. Contraindications for the use of lisinopril include a history of angioedema, hereditary or idiopathic. Lisinopril is also contraindicated in patients with known hypersensitivity to the drug and in coadministration with aliskiren in patients with diabetes mellitus.[2]\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 64-Year-Old Man With Throat Swelling"
},
{
"authors": "Patricia Ayuk Noumedem, PharmD; Marissa Tabile; Ermias Shikur; Bini Mathews; Anthony L. Huynh; Tracy Kwaku",
"content": [
"This patient case was quite rare in that he was hospitalized for about 10 days and heavily sedated due to the severity of his tracheal swelling and the difficulty he experienced with breathing. The patient was not arousable, and his oxygen saturation was about 82% due to the extensive swelling of his tongue, which was blocking his airway passage. Reversibility of angioedema after discontinuation of the offending agent, lisinopril, proved to be an arduous task. In most cases, corticosteroid therapy as well as diphenhydramine therapy is sufficient to resolve the swelling and irritation. Novel therapy (icatibant) was considered because the patient's condition remained unchanged for 10 days.[3] Numerous studies have demonstrated the benefit of new therapies in some patients who did not respond to antihistamines or steroids. However, the cost of these therapies is quite high.[3,5] A study by Sinert and colleagues[9] found that icatibant was ineffective in the treatment of ACE-induced angioedema.",
"This patient received two units of fresh frozen plasma and was then immediately transferred to a nearby hospital, where more invasive care was provided.[10] He was administered a nasotracheal intubation to secure his airway and carefully monitored until symptom resolution. His symptoms improved, and he was discharged home 2 days later. Prior to discharge, the patient received counseling to avoid ACE inhibitors and other medications associated with a risk for angioedema. This patient should also avoid use of angiotensin-receptor blockers, which have demonstrated similar reactions.[11] He was also provided with an epinephrine kit for future emergencies."
],
"date": "August 08, 2018",
"figures": [],
"markdown": "# A 64-Year-Old Man With Throat Swelling\n\n **Authors:** Patricia Ayuk Noumedem, PharmD; Marissa Tabile; Ermias Shikur; Bini Mathews; Anthony L. Huynh; Tracy Kwaku \n **Date:** August 08, 2018\n\n ## Content\n\n This patient case was quite rare in that he was hospitalized for about 10 days and heavily sedated due to the severity of his tracheal swelling and the difficulty he experienced with breathing. The patient was not arousable, and his oxygen saturation was about 82% due to the extensive swelling of his tongue, which was blocking his airway passage. Reversibility of angioedema after discontinuation of the offending agent, lisinopril, proved to be an arduous task. In most cases, corticosteroid therapy as well as diphenhydramine therapy is sufficient to resolve the swelling and irritation. Novel therapy (icatibant) was considered because the patient's condition remained unchanged for 10 days.[3] Numerous studies have demonstrated the benefit of new therapies in some patients who did not respond to antihistamines or steroids. However, the cost of these therapies is quite high.[3,5] A study by Sinert and colleagues[9] found that icatibant was ineffective in the treatment of ACE-induced angioedema.\nThis patient received two units of fresh frozen plasma and was then immediately transferred to a nearby hospital, where more invasive care was provided.[10] He was administered a nasotracheal intubation to secure his airway and carefully monitored until symptom resolution. His symptoms improved, and he was discharged home 2 days later. Prior to discharge, the patient received counseling to avoid ACE inhibitors and other medications associated with a risk for angioedema. This patient should also avoid use of angiotensin-receptor blockers, which have demonstrated similar reactions.[11] He was also provided with an epinephrine kit for future emergencies.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1254106,
"choiceText": "White women",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1254108,
"choiceText": "Black women",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1254110,
"choiceText": "White men",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1254112,
"choiceText": "Black men",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>Because urinary kallikrein is decreased in black persons with hypertension, endogenous bradykinin levels may be lower within this population.<sup type=\"ref\">[12]</sup> They may also be more sensitive to sudden increases in bradykinin levels that occur with ACE inhibitor therapy.</p>",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 398987,
"questionText": "Which of the following groups has the highest incidence of ACE inhibitor-related angioedema?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1254114,
"choiceText": "Lisinopril can be safely coadministered with aliskiren in patients with diabetes mellitus",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1254116,
"choiceText": "Lisinopril is contraindicated in the setting of acute myocardial infarction or in patients with existing heart failure",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1254118,
"choiceText": "In addition to blocking the breakdown of bradykinin, lisinopril decreases aldosterone secretion",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1254120,
"choiceText": "A history of previous angioedema, even if the cause is not known, is considered a contraindication for lisinopril",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>Lisinopril is indicated in adults and pediatric patients (> 6 y) for the treatment of hypertension and also as an adjunct in the therapy of heart failure. It is also indicated for the treatment of acute myocardial infarction. ACE is responsible for the conversion of angiotensin I to angiotensin II, a potent vasoconstrictor that also stimulates aldosterone secretion. Side effects of lisinopril include dizziness, headache, rash, hypotension, angina, palpitations, and fatigue. Gastrointestinal side effects include nausea, vomiting, diarrhea, and constipation. Taste perversions may also be associated with its use. Hyperkalemia (especially in renal dysfunction) has been reported. Contraindications for the use of lisinopril include a history of angioedema, hereditary or idiopathic. Lisinopril is also contraindicated in patients with known hypersensitivity to the drug and in coadministration with aliskiren in patients with diabetes mellitus.</p>",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 398989,
"questionText": "Which of the following is accurate about the use of lisinopril?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 64-Year-Old Man With Throat Swelling"
},
{
"authors": "Patricia Ayuk Noumedem, PharmD; Marissa Tabile; Ermias Shikur; Bini Mathews; Anthony L. Huynh; Tracy Kwaku",
"content": [],
"date": "August 08, 2018",
"figures": [],
"markdown": "# A 64-Year-Old Man With Throat Swelling\n\n **Authors:** Patricia Ayuk Noumedem, PharmD; Marissa Tabile; Ermias Shikur; Bini Mathews; Anthony L. Huynh; Tracy Kwaku \n **Date:** August 08, 2018\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1254106,
"choiceText": "White women",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1254108,
"choiceText": "Black women",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1254110,
"choiceText": "White men",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1254112,
"choiceText": "Black men",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>Because urinary kallikrein is decreased in black persons with hypertension, endogenous bradykinin levels may be lower within this population.<sup type=\"ref\">[12]</sup> They may also be more sensitive to sudden increases in bradykinin levels that occur with ACE inhibitor therapy.</p>",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 398987,
"questionText": "Which of the following groups has the highest incidence of ACE inhibitor-related angioedema?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1254114,
"choiceText": "Lisinopril can be safely coadministered with aliskiren in patients with diabetes mellitus",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1254116,
"choiceText": "Lisinopril is contraindicated in the setting of acute myocardial infarction or in patients with existing heart failure",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1254118,
"choiceText": "In addition to blocking the breakdown of bradykinin, lisinopril decreases aldosterone secretion",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1254120,
"choiceText": "A history of previous angioedema, even if the cause is not known, is considered a contraindication for lisinopril",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>Lisinopril is indicated in adults and pediatric patients (> 6 y) for the treatment of hypertension and also as an adjunct in the therapy of heart failure. It is also indicated for the treatment of acute myocardial infarction. ACE is responsible for the conversion of angiotensin I to angiotensin II, a potent vasoconstrictor that also stimulates aldosterone secretion. Side effects of lisinopril include dizziness, headache, rash, hypotension, angina, palpitations, and fatigue. Gastrointestinal side effects include nausea, vomiting, diarrhea, and constipation. Taste perversions may also be associated with its use. Hyperkalemia (especially in renal dysfunction) has been reported. Contraindications for the use of lisinopril include a history of angioedema, hereditary or idiopathic. Lisinopril is also contraindicated in patients with known hypersensitivity to the drug and in coadministration with aliskiren in patients with diabetes mellitus.</p>",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 398989,
"questionText": "Which of the following is accurate about the use of lisinopril?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 64-Year-Old Man With Throat Swelling"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1254098,
"choiceText": "Pernicious anemia",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1254100,
"choiceText": "C1-INH deficiency",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1254102,
"choiceText": "Drug-induced angioedema",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1254104,
"choiceText": "Hereditary angioedema",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 398985,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1254106,
"choiceText": "White women",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1254108,
"choiceText": "Black women",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1254110,
"choiceText": "White men",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1254112,
"choiceText": "Black men",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>Because urinary kallikrein is decreased in black persons with hypertension, endogenous bradykinin levels may be lower within this population.<sup type=\"ref\">[12]</sup> They may also be more sensitive to sudden increases in bradykinin levels that occur with ACE inhibitor therapy.</p>",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 398987,
"questionText": "Which of the following groups has the highest incidence of ACE inhibitor-related angioedema?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1254114,
"choiceText": "Lisinopril can be safely coadministered with aliskiren in patients with diabetes mellitus",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1254116,
"choiceText": "Lisinopril is contraindicated in the setting of acute myocardial infarction or in patients with existing heart failure",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1254118,
"choiceText": "In addition to blocking the breakdown of bradykinin, lisinopril decreases aldosterone secretion",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1254120,
"choiceText": "A history of previous angioedema, even if the cause is not known, is considered a contraindication for lisinopril",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>Lisinopril is indicated in adults and pediatric patients (> 6 y) for the treatment of hypertension and also as an adjunct in the therapy of heart failure. It is also indicated for the treatment of acute myocardial infarction. ACE is responsible for the conversion of angiotensin I to angiotensin II, a potent vasoconstrictor that also stimulates aldosterone secretion. Side effects of lisinopril include dizziness, headache, rash, hypotension, angina, palpitations, and fatigue. Gastrointestinal side effects include nausea, vomiting, diarrhea, and constipation. Taste perversions may also be associated with its use. Hyperkalemia (especially in renal dysfunction) has been reported. Contraindications for the use of lisinopril include a history of angioedema, hereditary or idiopathic. Lisinopril is also contraindicated in patients with known hypersensitivity to the drug and in coadministration with aliskiren in patients with diabetes mellitus.</p>",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 398989,
"questionText": "Which of the following is accurate about the use of lisinopril?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
899914 | /viewarticle/899914 | [
{
"authors": "Nicholas Bennett, MBBChir, PhD",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to recognize accurately. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a Case Challenge, please contact us.",
"A mother of three young children presents to a pediatrician's office with her oldest child due to concerns regarding an ear infection. Her son has had a fever to 102°F that began 2 days earlier. Copious purulent drainage from his left ear canal was noted today.",
"The patient is a 5-year-old male. A thorough review of his medical chart reveals repeated clinic visits for similar infections since age 1 year. He has been treated for recurrent bacterial pneumonia as an outpatient and twice required inpatient hospitalization. These lower respiratory tract infections were noted to affect the left, right, and bilateral pulmonary fields, at various times. Due to recurrent bacterial infections of the ears and lungs, pressure-equalizing (PE) ear tubes were placed and a referral was made to pediatric pulmonology to investigate the possibility of an anatomic cause for the recurrent pneumonias.",
"Despite these interventions, his infections have continued. Typically, he has required an antibiotic course to treat infections every 1 to 3 weeks. The longest stretch noted between treatment courses was 2 to 3 months.",
"The patient is up to date on his immunizations, including a completed pneumococcal vaccine series and annual influenza vaccinations. His two younger siblings, a brother aged 3 years and a sister aged 2 years (both born to the same parents), are healthy and well developed. His siblings reportedly do not have frequent or recurrent bacterial illnesses. Family history is also unremarkable."
],
"date": "July 30, 2018",
"figures": [],
"markdown": "# Recurrent Infections in a 5-Year-Old Boy\n\n **Authors:** Nicholas Bennett, MBBChir, PhD \n **Date:** July 30, 2018\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to recognize accurately. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a Case Challenge, please contact us.\nA mother of three young children presents to a pediatrician's office with her oldest child due to concerns regarding an ear infection. Her son has had a fever to 102°F that began 2 days earlier. Copious purulent drainage from his left ear canal was noted today.\nThe patient is a 5-year-old male. A thorough review of his medical chart reveals repeated clinic visits for similar infections since age 1 year. He has been treated for recurrent bacterial pneumonia as an outpatient and twice required inpatient hospitalization. These lower respiratory tract infections were noted to affect the left, right, and bilateral pulmonary fields, at various times. Due to recurrent bacterial infections of the ears and lungs, pressure-equalizing (PE) ear tubes were placed and a referral was made to pediatric pulmonology to investigate the possibility of an anatomic cause for the recurrent pneumonias.\nDespite these interventions, his infections have continued. Typically, he has required an antibiotic course to treat infections every 1 to 3 weeks. The longest stretch noted between treatment courses was 2 to 3 months.\nThe patient is up to date on his immunizations, including a completed pneumococcal vaccine series and annual influenza vaccinations. His two younger siblings, a brother aged 3 years and a sister aged 2 years (both born to the same parents), are healthy and well developed. His siblings reportedly do not have frequent or recurrent bacterial illnesses. Family history is also unremarkable.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "Recurrent Infections in a 5-Year-Old Boy"
},
{
"authors": "Nicholas Bennett, MBBChir, PhD",
"content": [
"Upon examination, the patient is slim. He weighs 35.2 lb, which is at the 10th percentile for weight, and is 43.3 inches tall, which is at the 50th percentile for height. He is well nourished, well developed, and in no acute distress. His temperature, taken orally, is 101°F. Heart and respiratory rates are normal. Craniofacial features appear within normal limits.",
"Otic examination reveals an occluded ear canal with purulent drainage. His left tympanic membrane is erythematous, with some purulent fluid draining through the properly positioned PE tube. The right tympanic membrane appears normal. His cardiac, respiratory, abdominal, musculoskeletal, and skin examination findings are within normal limits. No abnormal findings were present as a result of the recurrent pneumonias. He had no hepatosplenomegaly. Palpable, nontender lymph nodes are noted along his anterior cervical chain (1-2 cm in size); none are found elsewhere. To help further define the diagnosis beyond the current presentation, the following laboratory studies were obtained:",
"White blood cell count of 10.4 x 109/L",
"Hemoglobin level of 12.2 g/dL",
"Platelet count of 230 x 109/L",
"Neutrophils 54% (absolute neutrophil count, 230/mcL)",
"Immunoglobulin G (IgG) level of 450 mg/dL (reference range, 500-1000 mg/dL)",
"Immunoglobulin A (IgA) level of 26 mg/dL",
"Immunoglobulin M (IgM) level of 145 mg/dL",
"Extremely high titers (14 µg/mL, 23 µg/mL, and >84 µg/mL) to 3 of 14 pneumococcal serotypes, and lacking protective titers to all other pneumococcal serotypes."
],
"date": "July 30, 2018",
"figures": [],
"markdown": "# Recurrent Infections in a 5-Year-Old Boy\n\n **Authors:** Nicholas Bennett, MBBChir, PhD \n **Date:** July 30, 2018\n\n ## Content\n\n Upon examination, the patient is slim. He weighs 35.2 lb, which is at the 10th percentile for weight, and is 43.3 inches tall, which is at the 50th percentile for height. He is well nourished, well developed, and in no acute distress. His temperature, taken orally, is 101°F. Heart and respiratory rates are normal. Craniofacial features appear within normal limits.\nOtic examination reveals an occluded ear canal with purulent drainage. His left tympanic membrane is erythematous, with some purulent fluid draining through the properly positioned PE tube. The right tympanic membrane appears normal. His cardiac, respiratory, abdominal, musculoskeletal, and skin examination findings are within normal limits. No abnormal findings were present as a result of the recurrent pneumonias. He had no hepatosplenomegaly. Palpable, nontender lymph nodes are noted along his anterior cervical chain (1-2 cm in size); none are found elsewhere. To help further define the diagnosis beyond the current presentation, the following laboratory studies were obtained:\nWhite blood cell count of 10.4 x 109/L\nHemoglobin level of 12.2 g/dL\nPlatelet count of 230 x 109/L\nNeutrophils 54% (absolute neutrophil count, 230/mcL)\nImmunoglobulin G (IgG) level of 450 mg/dL (reference range, 500-1000 mg/dL)\nImmunoglobulin A (IgA) level of 26 mg/dL\nImmunoglobulin M (IgM) level of 145 mg/dL\nExtremely high titers (14 µg/mL, 23 µg/mL, and >84 µg/mL) to 3 of 14 pneumococcal serotypes, and lacking protective titers to all other pneumococcal serotypes.\n\n ## Figures\n\n \n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1249624,
"choiceText": "Common variable immunodeficiency (CVID)",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1249626,
"choiceText": "Transient hypogammaglobulinemia of infancy",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1249628,
"choiceText": "X-linked hypogammaglobulinemia (Bruton agammaglobulinemia)",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1249630,
"choiceText": "22q11.2 deletion syndrome (DiGeorge syndrome, velocardiofacial syndrome)",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 397531,
"questionText": "Which of the following is the most likely diagnosis?\r\n",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Recurrent Infections in a 5-Year-Old Boy"
},
{
"authors": "Nicholas Bennett, MBBChir, PhD",
"content": [
"Common variable immunodeficiency (CVID), also known as common variable hypogammaglobulinemia, is a primary humoral immune deficiency, typically characterized by recurrent upper and lower respiratory tract infections due to bacterial and/or viral pathogens, defects in immune memory (in particular to polysaccharide antigens such as those found on pneumococcus), and an increased risk for autoimmune disease. Laboratory findings often include low total IgG (although this is not an absolute requirement), absent or inadequate vaccine responses, and low switched memory B cells on flow cytometry (CD27+ CD19+ IgM- IgD-).[1,2]",
"Our patient in this case had very high pneumococcal titers in three vaccine strains, which likely represented breakthrough infections (vaccine failures), as their values are too high for a 5-year-old boy. A more typical result at this age would show 7 to 10 serotypes reactive, with titers between 1-5 µg/mL. The loss of overall immunity, recurrent bacterial infections, and the slightly low total IgG indicate that CVID is the most likely diagnosis. CVID also presents with absent vaccine responses, most commonly to polysaccharide antigens; however, even highly immunogenic vaccines such as tetanus may be anergic in some individuals.",
"The diagnosis should be confirmed with either reboosting with pneumococcal vaccine or performing expanded flow-cytometry for switched memory B cells. Standardly, a normal primary vaccine response is a 4-fold increase in titers following immunization. Children who receive a full immunization schedule typically boost much higher, with postimmunization titers of 10-20 µg/mL or more, demonstrating a \"memory\" response. Failures to respond to live viral vaccines or proteins such as tetanus toxoid are much less common. At least two different vaccines from each category should be tested to reduce the risk for an incorrect diagnosis; however, if this finding is seen without vaccine titers in an immunized individual, the diagnosis of a primary immune deficiency is more likely.",
"Plain polysaccharide responses can occur from direct B-cell stimulation; however, responses are much lower in patients younger than 2 years. The conjugated polysaccharide vaccines used in infancy require a coordinated T-cell and B-cell response, and therefore a greater likelihood of vaccine failure as a result of relatively subtle immune defects. Respiratory pathogens, such as Haemophilus influenzae, pneumococcus, and streptococcus, have polysaccharide capsules; this helps explain the propensity for respiratory infections in children with CVID and other humoral immune deficiencies."
],
"date": "July 30, 2018",
"figures": [],
"markdown": "# Recurrent Infections in a 5-Year-Old Boy\n\n **Authors:** Nicholas Bennett, MBBChir, PhD \n **Date:** July 30, 2018\n\n ## Content\n\n Common variable immunodeficiency (CVID), also known as common variable hypogammaglobulinemia, is a primary humoral immune deficiency, typically characterized by recurrent upper and lower respiratory tract infections due to bacterial and/or viral pathogens, defects in immune memory (in particular to polysaccharide antigens such as those found on pneumococcus), and an increased risk for autoimmune disease. Laboratory findings often include low total IgG (although this is not an absolute requirement), absent or inadequate vaccine responses, and low switched memory B cells on flow cytometry (CD27+ CD19+ IgM- IgD-).[1,2]\nOur patient in this case had very high pneumococcal titers in three vaccine strains, which likely represented breakthrough infections (vaccine failures), as their values are too high for a 5-year-old boy. A more typical result at this age would show 7 to 10 serotypes reactive, with titers between 1-5 µg/mL. The loss of overall immunity, recurrent bacterial infections, and the slightly low total IgG indicate that CVID is the most likely diagnosis. CVID also presents with absent vaccine responses, most commonly to polysaccharide antigens; however, even highly immunogenic vaccines such as tetanus may be anergic in some individuals.\nThe diagnosis should be confirmed with either reboosting with pneumococcal vaccine or performing expanded flow-cytometry for switched memory B cells. Standardly, a normal primary vaccine response is a 4-fold increase in titers following immunization. Children who receive a full immunization schedule typically boost much higher, with postimmunization titers of 10-20 µg/mL or more, demonstrating a \"memory\" response. Failures to respond to live viral vaccines or proteins such as tetanus toxoid are much less common. At least two different vaccines from each category should be tested to reduce the risk for an incorrect diagnosis; however, if this finding is seen without vaccine titers in an immunized individual, the diagnosis of a primary immune deficiency is more likely.\nPlain polysaccharide responses can occur from direct B-cell stimulation; however, responses are much lower in patients younger than 2 years. The conjugated polysaccharide vaccines used in infancy require a coordinated T-cell and B-cell response, and therefore a greater likelihood of vaccine failure as a result of relatively subtle immune defects. Respiratory pathogens, such as Haemophilus influenzae, pneumococcus, and streptococcus, have polysaccharide capsules; this helps explain the propensity for respiratory infections in children with CVID and other humoral immune deficiencies.\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1249624,
"choiceText": "Common variable immunodeficiency (CVID)",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1249626,
"choiceText": "Transient hypogammaglobulinemia of infancy",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1249628,
"choiceText": "X-linked hypogammaglobulinemia (Bruton agammaglobulinemia)",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1249630,
"choiceText": "22q11.2 deletion syndrome (DiGeorge syndrome, velocardiofacial syndrome)",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 397531,
"questionText": "Which of the following is the most likely diagnosis?\r\n",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Recurrent Infections in a 5-Year-Old Boy"
},
{
"authors": "Nicholas Bennett, MBBChir, PhD",
"content": [
"Transient hypogammaglobulinemia of infancy (THI) is a form of hypogammaglobulinemia that appears shortly after birth, with decreased levels of IgG and sometimes decreased levels of IgA and IgM. Symptoms are similar to those of CVID in regard to recurrent bacterial infections but present earlier in life (ages 6 to 12 months), and the condition typically resolves without treatment within a year's time. THI is a diagnosis of exclusion that occurs in the absence of other primary immunodeficiencies.",
"X-linked hypogammaglobulinemia, Bruton agammaglobulinemia, or X-linked agammaglobulinemia (XLA) is a rare genetic disorder inherited in an X-linked recessive fashion. XLA primarily affects males due to an X-linked mutation at the Bruton tyrosine kinase (Btk) gene, which blocks B-cell development and markedly reduces immunoglobulin production. Boys typically present in early childhood with recurrent, potentially fatal, infections due to extracellular, encapsulated bacteria. Given the serological detection of IgG in the patient in this case, this diagnosis is unlikely.",
"22q11.2 deletion syndrome, also known as DiGeorge syndrome or velocardiofacial syndrome, is due to a deletion of genetic material on chromosome 22. This microdeletion syndrome leads to a defect in the embryonic development of the third and fourth branchial arches, which can lead to thymic dysplasia and subsequent defects in B-cell immunity due to a lack of helper T-cell activity. Craniofacial findings can include narrow palpebral fissures, cupped ears with narrow tortuous ear canals, broad nasal bridge, wide mouth with a thin upper lip, cleft lip and palate, and weakened dental enamel. Speech may be hypernasal. Patients may have history of congenital heart disease and low serum calcium (hypocalcemia). Developmental delays and learning disabilities are often present. Ear infections are common in early childhood but are more likely due to anatomic defects than humoral immune defects. Lower respiratory infections are uncommon. Although DiGeorge syndrome has a higher prevalence worldwide (around 1 case per 4000 population vs 1 in 25000 for CVID), given the absence of phenotypic findings and the medical history of the patient in this case, the diagnosis of 22q11.2 deletion syndrome is unlikely.",
"Recent analysis of immune function in children with recurrent otitis media have revealed low vaccine titers and antigen-specific memory B-cell responses; however, these observations may reflect a continuum of immune function, from normality to true disease.[3,4,5,6] Etiology for a high rate of vaccine failure is unclear. One theory is that because many of these children are diagnosed with asthma and atopy they are frequently prescribed systemic steroids for treatment, which leads to blunted vaccine responses. Their immune system may also be skewed toward an atopic cytokine milieu, hampering normal B-cell function. The vast majority of these children respond appropriately to booster immunizations with pneumococcal vaccine, with no demonstrable loss of immune memory."
],
"date": "July 30, 2018",
"figures": [],
"markdown": "# Recurrent Infections in a 5-Year-Old Boy\n\n **Authors:** Nicholas Bennett, MBBChir, PhD \n **Date:** July 30, 2018\n\n ## Content\n\n Transient hypogammaglobulinemia of infancy (THI) is a form of hypogammaglobulinemia that appears shortly after birth, with decreased levels of IgG and sometimes decreased levels of IgA and IgM. Symptoms are similar to those of CVID in regard to recurrent bacterial infections but present earlier in life (ages 6 to 12 months), and the condition typically resolves without treatment within a year's time. THI is a diagnosis of exclusion that occurs in the absence of other primary immunodeficiencies.\nX-linked hypogammaglobulinemia, Bruton agammaglobulinemia, or X-linked agammaglobulinemia (XLA) is a rare genetic disorder inherited in an X-linked recessive fashion. XLA primarily affects males due to an X-linked mutation at the Bruton tyrosine kinase (Btk) gene, which blocks B-cell development and markedly reduces immunoglobulin production. Boys typically present in early childhood with recurrent, potentially fatal, infections due to extracellular, encapsulated bacteria. Given the serological detection of IgG in the patient in this case, this diagnosis is unlikely.\n22q11.2 deletion syndrome, also known as DiGeorge syndrome or velocardiofacial syndrome, is due to a deletion of genetic material on chromosome 22. This microdeletion syndrome leads to a defect in the embryonic development of the third and fourth branchial arches, which can lead to thymic dysplasia and subsequent defects in B-cell immunity due to a lack of helper T-cell activity. Craniofacial findings can include narrow palpebral fissures, cupped ears with narrow tortuous ear canals, broad nasal bridge, wide mouth with a thin upper lip, cleft lip and palate, and weakened dental enamel. Speech may be hypernasal. Patients may have history of congenital heart disease and low serum calcium (hypocalcemia). Developmental delays and learning disabilities are often present. Ear infections are common in early childhood but are more likely due to anatomic defects than humoral immune defects. Lower respiratory infections are uncommon. Although DiGeorge syndrome has a higher prevalence worldwide (around 1 case per 4000 population vs 1 in 25000 for CVID), given the absence of phenotypic findings and the medical history of the patient in this case, the diagnosis of 22q11.2 deletion syndrome is unlikely.\nRecent analysis of immune function in children with recurrent otitis media have revealed low vaccine titers and antigen-specific memory B-cell responses; however, these observations may reflect a continuum of immune function, from normality to true disease.[3,4,5,6] Etiology for a high rate of vaccine failure is unclear. One theory is that because many of these children are diagnosed with asthma and atopy they are frequently prescribed systemic steroids for treatment, which leads to blunted vaccine responses. Their immune system may also be skewed toward an atopic cytokine milieu, hampering normal B-cell function. The vast majority of these children respond appropriately to booster immunizations with pneumococcal vaccine, with no demonstrable loss of immune memory.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "Recurrent Infections in a 5-Year-Old Boy"
},
{
"authors": "Nicholas Bennett, MBBChir, PhD",
"content": [
"A primary immune deficiency should be considered in anyone with a history of frequent bacterial infections, infections with atypical organisms, or a family history of a primary immune deficiency. CVID is one of the more prevalent symptomatic primary immunodeficiencies and presents with a wide range of symptoms and severity. Diagnosis of CVID cannot be definitively made in patients younger than 4 years because clinical presentation may be confused with other immunologic defects or with physiologic immaturity. The patient in this case was diagnosed at age 5 years.",
"Interestingly, most cases of CVID are diagnosed in adulthood (age 20 to 40 years), as patients develop symptoms later in life. Recurrent bacterial infections of the ears, sinuses, and lungs occur. Gastrointestinal infections and inflammation can cause recurrent abdominal complaints, weight loss, and intestinal malabsorption. Patients with CVID also have an increased lifetime risk for autoimmune disease (immune thrombocytopenic purpura, autoimmune hemolytic anemia, rheumatoid arthritis), inflammatory bowel disease, and malignancy (non-Hodgkin lymphoma, gastric cancer).[7,8,9]",
"In more than 90% of cases, the etiology of CVID remains unknown; however, for about 10% of patients, a genetic cause can be defined. Several genes have been identified as contributing to the CVID phenotype.[10,11] Generally speaking, the known genes are involved in B-cell signaling or maturation. Testing using whole-exome sequencing or specific gene array panels for genes associated with immunodeficiency may help with providing counseling to the patient and their family about future risks to themselves or their offspring. Once diagnosed, treatment with prophylactic antibiotics or immunoglobulin infusions is recommended.[12]",
"Intravenous (IV) and subcutaneous (SC) immunoglobulin products for replacement therapy are available and are mostly equivalent in reducing the rate of infections in patients with CVID. SC immunoglobulin therapy is preferred by both physicians and patients because of the lower rate of infusion reactions, reduced risk of breakthrough infections at the end of a monthly infusion cycle, and the ease of administration in the home environment without the need for skilled nursing support. Consultation with an immunologist or infectious disease specialist familiar with the management of primary immune deficiencies is recommended to monitor therapy, provide counseling, and address complications or risks.",
"The child in this case was tested using expanded flow cytometry, which revealed low switched memory B cells for age of 0.5% (normal range, 2.9%-17.4%). He was started on IV immunoglobulin replacement therapy. After 3 months, the family agreed to try SC weekly injections of immunoglobulin because of the patient's infusion-related headaches. Since starting SC therapy, the child had a case of otitis media (in the fourth week of the first treatment cycle) but has since been infection-free and is currently tolerating injections, with only mild injection-site reactions."
],
"date": "July 30, 2018",
"figures": [],
"markdown": "# Recurrent Infections in a 5-Year-Old Boy\n\n **Authors:** Nicholas Bennett, MBBChir, PhD \n **Date:** July 30, 2018\n\n ## Content\n\n A primary immune deficiency should be considered in anyone with a history of frequent bacterial infections, infections with atypical organisms, or a family history of a primary immune deficiency. CVID is one of the more prevalent symptomatic primary immunodeficiencies and presents with a wide range of symptoms and severity. Diagnosis of CVID cannot be definitively made in patients younger than 4 years because clinical presentation may be confused with other immunologic defects or with physiologic immaturity. The patient in this case was diagnosed at age 5 years.\nInterestingly, most cases of CVID are diagnosed in adulthood (age 20 to 40 years), as patients develop symptoms later in life. Recurrent bacterial infections of the ears, sinuses, and lungs occur. Gastrointestinal infections and inflammation can cause recurrent abdominal complaints, weight loss, and intestinal malabsorption. Patients with CVID also have an increased lifetime risk for autoimmune disease (immune thrombocytopenic purpura, autoimmune hemolytic anemia, rheumatoid arthritis), inflammatory bowel disease, and malignancy (non-Hodgkin lymphoma, gastric cancer).[7,8,9]\nIn more than 90% of cases, the etiology of CVID remains unknown; however, for about 10% of patients, a genetic cause can be defined. Several genes have been identified as contributing to the CVID phenotype.[10,11] Generally speaking, the known genes are involved in B-cell signaling or maturation. Testing using whole-exome sequencing or specific gene array panels for genes associated with immunodeficiency may help with providing counseling to the patient and their family about future risks to themselves or their offspring. Once diagnosed, treatment with prophylactic antibiotics or immunoglobulin infusions is recommended.[12]\nIntravenous (IV) and subcutaneous (SC) immunoglobulin products for replacement therapy are available and are mostly equivalent in reducing the rate of infections in patients with CVID. SC immunoglobulin therapy is preferred by both physicians and patients because of the lower rate of infusion reactions, reduced risk of breakthrough infections at the end of a monthly infusion cycle, and the ease of administration in the home environment without the need for skilled nursing support. Consultation with an immunologist or infectious disease specialist familiar with the management of primary immune deficiencies is recommended to monitor therapy, provide counseling, and address complications or risks.\nThe child in this case was tested using expanded flow cytometry, which revealed low switched memory B cells for age of 0.5% (normal range, 2.9%-17.4%). He was started on IV immunoglobulin replacement therapy. After 3 months, the family agreed to try SC weekly injections of immunoglobulin because of the patient's infusion-related headaches. Since starting SC therapy, the child had a case of otitis media (in the fourth week of the first treatment cycle) but has since been infection-free and is currently tolerating injections, with only mild injection-site reactions.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1249632,
"choiceText": "Tetanus",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1249634,
"choiceText": "Measles\r\n",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1249636,
"choiceText": "Pneumococcus",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1249638,
"choiceText": "Rotavirus ",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Pneumococcal vaccine is the only conjugated polysaccharide vaccine cited in the answer options. Conjugated polysaccharide vaccines require a coordinated response between B cells and T cells, as well as intact antigen presentation through the major histocompatibility complex proteins.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 397533,
"questionText": "Vaccine failure for which of the following conditions is most likely seen in patients with CVID?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1249640,
"choiceText": "Tetanus",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1249642,
"choiceText": "Human papillomavirus",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1249644,
"choiceText": "Pertussis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1249646,
"choiceText": "Meningococcus",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "A quadrivalent conjugated meningococcal vaccine (covering strains A, C, Y, and W-135) is recommended for preteens (ages 11 to 12 years), with a recommended booster dose 5 years later, prior to attending college. The vaccine response to the meningococcal vaccine is therefore more likely to be interpretable than that of pneumococcus in an older child or teenager, and both assess the immune response to polysaccharide antigens. <br><br>\r\n\r\nTetanus and pertussis vaccines are both strongly immunogenic proteins found in the Tdap vaccine given at around age 11 years. Although they can be tested as part of the evaluation of a teenager, they do not provide insight into the same type of immune response as that for polysaccharide antigens. Human papillomavirus is another protein-based vaccine, but titers are not readily available through commercial laboratories.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 397535,
"questionText": "The pneumococcal vaccine titers may be expected to be low in a teenager simply because of the lapsed time from their last immunization. Response to which of the following vaccines may be tested to assess the same immune mechanism?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Recurrent Infections in a 5-Year-Old Boy"
},
{
"authors": "Nicholas Bennett, MBBChir, PhD",
"content": [],
"date": "July 30, 2018",
"figures": [],
"markdown": "# Recurrent Infections in a 5-Year-Old Boy\n\n **Authors:** Nicholas Bennett, MBBChir, PhD \n **Date:** July 30, 2018\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1249632,
"choiceText": "Tetanus",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1249634,
"choiceText": "Measles\r\n",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1249636,
"choiceText": "Pneumococcus",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1249638,
"choiceText": "Rotavirus ",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Pneumococcal vaccine is the only conjugated polysaccharide vaccine cited in the answer options. Conjugated polysaccharide vaccines require a coordinated response between B cells and T cells, as well as intact antigen presentation through the major histocompatibility complex proteins.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 397533,
"questionText": "Vaccine failure for which of the following conditions is most likely seen in patients with CVID?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1249640,
"choiceText": "Tetanus",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1249642,
"choiceText": "Human papillomavirus",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1249644,
"choiceText": "Pertussis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1249646,
"choiceText": "Meningococcus",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "A quadrivalent conjugated meningococcal vaccine (covering strains A, C, Y, and W-135) is recommended for preteens (ages 11 to 12 years), with a recommended booster dose 5 years later, prior to attending college. The vaccine response to the meningococcal vaccine is therefore more likely to be interpretable than that of pneumococcus in an older child or teenager, and both assess the immune response to polysaccharide antigens. <br><br>\r\n\r\nTetanus and pertussis vaccines are both strongly immunogenic proteins found in the Tdap vaccine given at around age 11 years. Although they can be tested as part of the evaluation of a teenager, they do not provide insight into the same type of immune response as that for polysaccharide antigens. Human papillomavirus is another protein-based vaccine, but titers are not readily available through commercial laboratories.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 397535,
"questionText": "The pneumococcal vaccine titers may be expected to be low in a teenager simply because of the lapsed time from their last immunization. Response to which of the following vaccines may be tested to assess the same immune mechanism?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Recurrent Infections in a 5-Year-Old Boy"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1249624,
"choiceText": "Common variable immunodeficiency (CVID)",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1249626,
"choiceText": "Transient hypogammaglobulinemia of infancy",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1249628,
"choiceText": "X-linked hypogammaglobulinemia (Bruton agammaglobulinemia)",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1249630,
"choiceText": "22q11.2 deletion syndrome (DiGeorge syndrome, velocardiofacial syndrome)",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 397531,
"questionText": "Which of the following is the most likely diagnosis?\r\n",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1249632,
"choiceText": "Tetanus",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1249634,
"choiceText": "Measles\r\n",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1249636,
"choiceText": "Pneumococcus",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1249638,
"choiceText": "Rotavirus ",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Pneumococcal vaccine is the only conjugated polysaccharide vaccine cited in the answer options. Conjugated polysaccharide vaccines require a coordinated response between B cells and T cells, as well as intact antigen presentation through the major histocompatibility complex proteins.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 397533,
"questionText": "Vaccine failure for which of the following conditions is most likely seen in patients with CVID?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1249640,
"choiceText": "Tetanus",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1249642,
"choiceText": "Human papillomavirus",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1249644,
"choiceText": "Pertussis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1249646,
"choiceText": "Meningococcus",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "A quadrivalent conjugated meningococcal vaccine (covering strains A, C, Y, and W-135) is recommended for preteens (ages 11 to 12 years), with a recommended booster dose 5 years later, prior to attending college. The vaccine response to the meningococcal vaccine is therefore more likely to be interpretable than that of pneumococcus in an older child or teenager, and both assess the immune response to polysaccharide antigens. <br><br>\r\n\r\nTetanus and pertussis vaccines are both strongly immunogenic proteins found in the Tdap vaccine given at around age 11 years. Although they can be tested as part of the evaluation of a teenager, they do not provide insight into the same type of immune response as that for polysaccharide antigens. Human papillomavirus is another protein-based vaccine, but titers are not readily available through commercial laboratories.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 397535,
"questionText": "The pneumococcal vaccine titers may be expected to be low in a teenager simply because of the lapsed time from their last immunization. Response to which of the following vaccines may be tested to assess the same immune mechanism?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
899262 | /viewarticle/899262 | [
{
"authors": "Francisco Aguilar, MD; Carla Arellano Pizano, MD",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to recognize accurately. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a Case Challenge, please contact us.",
"A 64-year-old woman presents to the emergency department with shortness of breath while at rest, nonproductive cough, and lower extremity swelling for the past 2 days. She reports that she was able to walk less than one block and climb less than one flight of stairs because of shortness of breath. She explains that she has to use two pillows at home to sleep. She denies any fever, chills, or chest pain. Her urine output was usual. She further explains that since the day before her symptoms started she was feeling \"overwhelmed\" because of news about her husband being diagnosed with metastatic colon cancer.",
"Her past medical history is significant for the following:",
"Type 2 diabetes mellitus",
"Hypertension",
"Hyperlipidemia",
"Obesity",
"Chronic kidney disease (stage 2)",
"Obstructive sleep apnea",
"Her family history is positive for hypertension and diabetes on both her mother's and father's side. She denies smoking cigarettes, illicit drug use, and significant alcohol use."
],
"date": "July 17, 2018",
"figures": [],
"markdown": "# A 64-Year-Old Woman With Extremity Swelling and Cough\n\n **Authors:** Francisco Aguilar, MD; Carla Arellano Pizano, MD \n **Date:** July 17, 2018\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to recognize accurately. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a Case Challenge, please contact us.\nA 64-year-old woman presents to the emergency department with shortness of breath while at rest, nonproductive cough, and lower extremity swelling for the past 2 days. She reports that she was able to walk less than one block and climb less than one flight of stairs because of shortness of breath. She explains that she has to use two pillows at home to sleep. She denies any fever, chills, or chest pain. Her urine output was usual. She further explains that since the day before her symptoms started she was feeling \"overwhelmed\" because of news about her husband being diagnosed with metastatic colon cancer.\nHer past medical history is significant for the following:\nType 2 diabetes mellitus\nHypertension\nHyperlipidemia\nObesity\nChronic kidney disease (stage 2)\nObstructive sleep apnea\nHer family history is positive for hypertension and diabetes on both her mother's and father's side. She denies smoking cigarettes, illicit drug use, and significant alcohol use.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 64-Year-Old Woman With Extremity Swelling and Cough"
},
{
"authors": "Francisco Aguilar, MD; Carla Arellano Pizano, MD",
"content": [
"A physical exam reveals blood pressure of 183/84 mm Hg, heart rate of 77 beats/min, respiratory rate of 20 breaths/min, oxygen saturation of 90% on room air, temperature of 98.4°F (36.9°C), and a body mass index of 35.5 kg/m2. She is awake and alert.",
"Jugular venous distention is noted. Upon auscultation, bilateral crackles are present in the lower two thirds of the thorax. Her heart sounds are audible and regular, with normal S1 and S2. An S3 gallop is present. No audible murmurs are noted. Apex impulse is present and nondisplaced from the midclavicular line. Her abdomen is soft, nondistended, and nontender to palpation. Moderate bilateral pitting edema in the lower extremities is present.",
"Initial laboratory workup reveals a serum creatinine level of 1.2 mg/dL (same as baseline level). Her brain natriuretic peptide (BNP) level is 4,237 pg/mL. Her serum troponin I level is 0.42 ng/mL. Lipase and liver function test results are within normal limits. Her ferritin level is 150 ng/mL. Her plasma and 24-hour urine fractionated metanephrine and catecholamine levels are within normal limits. Urine drug screen results are negative for cocaine.",
"An ECG reveals sinus rhythm with a heart rate of 75 beats/min and new T-wave inversion in the anterolateral leads compared with a previous ECG. Chest radiography reveals diffuse patchy opacities on bilateral lung fields that are suggestive of moderate pulmonary edema and blunting of costophrenic angle, which is suggestive of bilateral pleural effusion. Two-dimensional (2D) transthoracic echocardiography reveals hypokinesis of the septal, lateral, and anterior mid-distal segments with a left ventricular ejection fraction of 30% to 35% (Figures 1-2).",
"Figure 1.",
"Figure 2.",
"Given the patient's symptoms, ECG changes, elevated troponin levels, and 2D echocardiography findings, urgent left heart catheterization with angiography is performed. Coronary angiography reveals 40% obstruction of the left anterior descending artery, 30% obstruction of the left circumflex artery, and 30% to 40% obstruction of the right coronary arteries. Left ventriculography revealing systole is shown in Figure 3.",
"Figure 3."
],
"date": "July 17, 2018",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/899/262/899262-Thumb3.png"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/899/262/899262-Thumb4.png"
},
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/899/262/899262-Thumb5.png"
}
],
"markdown": "# A 64-Year-Old Woman With Extremity Swelling and Cough\n\n **Authors:** Francisco Aguilar, MD; Carla Arellano Pizano, MD \n **Date:** July 17, 2018\n\n ## Content\n\n A physical exam reveals blood pressure of 183/84 mm Hg, heart rate of 77 beats/min, respiratory rate of 20 breaths/min, oxygen saturation of 90% on room air, temperature of 98.4°F (36.9°C), and a body mass index of 35.5 kg/m2. She is awake and alert.\nJugular venous distention is noted. Upon auscultation, bilateral crackles are present in the lower two thirds of the thorax. Her heart sounds are audible and regular, with normal S1 and S2. An S3 gallop is present. No audible murmurs are noted. Apex impulse is present and nondisplaced from the midclavicular line. Her abdomen is soft, nondistended, and nontender to palpation. Moderate bilateral pitting edema in the lower extremities is present.\nInitial laboratory workup reveals a serum creatinine level of 1.2 mg/dL (same as baseline level). Her brain natriuretic peptide (BNP) level is 4,237 pg/mL. Her serum troponin I level is 0.42 ng/mL. Lipase and liver function test results are within normal limits. Her ferritin level is 150 ng/mL. Her plasma and 24-hour urine fractionated metanephrine and catecholamine levels are within normal limits. Urine drug screen results are negative for cocaine.\nAn ECG reveals sinus rhythm with a heart rate of 75 beats/min and new T-wave inversion in the anterolateral leads compared with a previous ECG. Chest radiography reveals diffuse patchy opacities on bilateral lung fields that are suggestive of moderate pulmonary edema and blunting of costophrenic angle, which is suggestive of bilateral pleural effusion. Two-dimensional (2D) transthoracic echocardiography reveals hypokinesis of the septal, lateral, and anterior mid-distal segments with a left ventricular ejection fraction of 30% to 35% (Figures 1-2).\nFigure 1.\nFigure 2.\nGiven the patient's symptoms, ECG changes, elevated troponin levels, and 2D echocardiography findings, urgent left heart catheterization with angiography is performed. Coronary angiography reveals 40% obstruction of the left anterior descending artery, 30% obstruction of the left circumflex artery, and 30% to 40% obstruction of the right coronary arteries. Left ventriculography revealing systole is shown in Figure 3.\nFigure 3.\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n**Figure 3.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1244470,
"choiceText": "Acute-on-chronic heart failure secondary to hypertensive emergency",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1244472,
"choiceText": "Acute-on-chronic heart failure secondary to myocardial ischemia",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1244474,
"choiceText": "Acute-on-chronic heart failure secondary to Takotsubo cardiomyopathy ",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1244476,
"choiceText": "Pulmonary hypertension World Health Organization classification group I",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 395931,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 64-Year-Old Woman With Extremity Swelling and Cough"
},
{
"authors": "Francisco Aguilar, MD; Carla Arellano Pizano, MD",
"content": [
"Two-dimensional echocardiogram revealed left ventricular ejection fraction of 30% to 35%; and severe hypokinesis of the mid-to-distal septum, anterior wall, inferior wall, and apex, with wall motion preserved in the basal segments compatible with Takotsubo cardiomyopathy. No left ventricular outflow obstruction was present, and no intracavitary thrombus was observed. Left ventriculography during systole confirmed the apical ballooning pattern.",
"Takotsubo cardiomyopathy is a transient myocardial dysfunction characterized by left ventricular apical ballooning, with or without dyskinesis/akinesis of the midsegments in the absence of significant coronary artery disease (> 50% stenosis of the left main stem, > 70% stenosis in a major coronary vessel, or 30% to 70% stenosis, with fractional flow reserve ≤ 0.8). Sato et al were the first to describe this syndrome in 1990 and named it Takotsubo, which means \"octopus trap\" in Japanese.[1] They chose this name because of the similarities of the morphology of the left ventricle with an octopus trap.",
"This disorder more commonly affects postmenopausal women, and the symptoms are often preceded by a triggering factor (ie, emotional or physical stress). Common emotional stressors described in the literature include the death of a relative or friend, interpersonal conflict, a severe medical diagnosis, and the sudden loss of money or valuables. Physical stressors include invasive procedures, exacerbation of systemic diseases, and acute infections. However, the absence of a stressor does not rule out this condition; as many as one third of patients report no evident trigger.[2]"
],
"date": "July 17, 2018",
"figures": [],
"markdown": "# A 64-Year-Old Woman With Extremity Swelling and Cough\n\n **Authors:** Francisco Aguilar, MD; Carla Arellano Pizano, MD \n **Date:** July 17, 2018\n\n ## Content\n\n Two-dimensional echocardiogram revealed left ventricular ejection fraction of 30% to 35%; and severe hypokinesis of the mid-to-distal septum, anterior wall, inferior wall, and apex, with wall motion preserved in the basal segments compatible with Takotsubo cardiomyopathy. No left ventricular outflow obstruction was present, and no intracavitary thrombus was observed. Left ventriculography during systole confirmed the apical ballooning pattern.\nTakotsubo cardiomyopathy is a transient myocardial dysfunction characterized by left ventricular apical ballooning, with or without dyskinesis/akinesis of the midsegments in the absence of significant coronary artery disease (> 50% stenosis of the left main stem, > 70% stenosis in a major coronary vessel, or 30% to 70% stenosis, with fractional flow reserve ≤ 0.8). Sato et al were the first to describe this syndrome in 1990 and named it Takotsubo, which means \"octopus trap\" in Japanese.[1] They chose this name because of the similarities of the morphology of the left ventricle with an octopus trap.\nThis disorder more commonly affects postmenopausal women, and the symptoms are often preceded by a triggering factor (ie, emotional or physical stress). Common emotional stressors described in the literature include the death of a relative or friend, interpersonal conflict, a severe medical diagnosis, and the sudden loss of money or valuables. Physical stressors include invasive procedures, exacerbation of systemic diseases, and acute infections. However, the absence of a stressor does not rule out this condition; as many as one third of patients report no evident trigger.[2]\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1244470,
"choiceText": "Acute-on-chronic heart failure secondary to hypertensive emergency",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1244472,
"choiceText": "Acute-on-chronic heart failure secondary to myocardial ischemia",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1244474,
"choiceText": "Acute-on-chronic heart failure secondary to Takotsubo cardiomyopathy ",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1244476,
"choiceText": "Pulmonary hypertension World Health Organization classification group I",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 395931,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 64-Year-Old Woman With Extremity Swelling and Cough"
},
{
"authors": "Francisco Aguilar, MD; Carla Arellano Pizano, MD",
"content": [
"The pathophysiology of Takotsubo cardiomyopathy is unclear. The reason that it more commonly affects postmenopausal women and the left ventricle apex and midsegments is not certain. Several studies have found elevated levels of catecholamines in patients with Takotsubo cardiomyopathy. The relationship with a stressor suggests that this event promotes the release of catecholamines, which cause myocardial stunning that is mediated by coronary artery spasms or direct myocardial toxicity. This theory is supported by an animal study model in which rats that were exposed to physical stressors were found to have elevated catecholamine levels and left ventricular apical ballooning.[3,4] The same authors found that these changes were prevented by alpha-blockade or beta-blockade.",
"Other studies propose microvascular abnormalities and hormonal environment as the main pathophysiologic mechanism that predispose to a transient obliteration of the coronary arteries in the presence of a stressor. The predominance of postmenopausal women has suggested that these changes may be related to decreased estrogenic influence.[2,3,4] The clinical presentation often mimics acute coronary syndrome. The most common presenting symptoms include chest pain and shortness of breath; ventricular arrhythmias and cardiogenic shock are less frequently reported.",
"Ischemic ECG changes commonly include ST elevation/depression and T-wave inversion. Mild troponin leak is also a common finding, which makes this syndrome more challenging to differentiate from acute coronary syndromes.[5] BNP and N-terminal pro-BNP levels are elevated in as many as 82% of cases, with a median value of six times the upper limit of normal values.[6]",
"The most common complication is heart failure, with or without pulmonary edema; however, these patients can also develop cardiogenic shock and left ventricular thrombus. Diagnosis is often challenging because the initial presentation often misleadingly suggests acute coronary syndrome. The modified Mayo Clinic criteria for diagnosis require four elements to be present, including:[7]",
"Transient hypokinesis, dyskinesis, or akinesis of the left ventricle midsegments, with or without apical involvement; the regional wall-motion abnormalities extend beyond a single epicardial vascular distribution",
"Absence of obstructive coronary disease or angiographic evidence of acute plaque rupture",
"New ECG abnormalities (either ST-segment elevation and/or T-wave inversion) or modest elevation in the cardiac troponin level",
"Absence of cerebrovascular disease, pheochromocytoma, or myocarditis",
"Transthoracic echocardiography is an accessible, quick, and reliable diagnostic tool that can reveal the typical wall motion abnormalities described above. Echocardiography is also helpful in detecting the presence of a left ventricular thrombus. As many as 5% of cases have evidence of a left ventricular thrombus.[2] Embolization from this left ventricular thrombus is a potential complication. Coronary angiography is required for diagnosis and helps differentiate this entity from acute coronary syndrome. Most patients with Takotsubo cardiomyopathy have normal coronary arteries or nonsignificant coronary artery disease.[2] Left ventriculography performed during left heart catheterization reveals the characteristic pattern of apical ballooning during systole, providing the best imaging for diagnosis.",
"The primary differential diagnosis of this condition is acute coronary syndrome. Although both conditions can coexist, Takotsubo cardiomyopathy involves a myocardial territory that cannot be explained by the affected coronary artery. Other conditions that can present similarly include cocaine-induced acute coronary syndrome, myocarditis, pheochromocytoma, and acute brain injury."
],
"date": "July 17, 2018",
"figures": [],
"markdown": "# A 64-Year-Old Woman With Extremity Swelling and Cough\n\n **Authors:** Francisco Aguilar, MD; Carla Arellano Pizano, MD \n **Date:** July 17, 2018\n\n ## Content\n\n The pathophysiology of Takotsubo cardiomyopathy is unclear. The reason that it more commonly affects postmenopausal women and the left ventricle apex and midsegments is not certain. Several studies have found elevated levels of catecholamines in patients with Takotsubo cardiomyopathy. The relationship with a stressor suggests that this event promotes the release of catecholamines, which cause myocardial stunning that is mediated by coronary artery spasms or direct myocardial toxicity. This theory is supported by an animal study model in which rats that were exposed to physical stressors were found to have elevated catecholamine levels and left ventricular apical ballooning.[3,4] The same authors found that these changes were prevented by alpha-blockade or beta-blockade.\nOther studies propose microvascular abnormalities and hormonal environment as the main pathophysiologic mechanism that predispose to a transient obliteration of the coronary arteries in the presence of a stressor. The predominance of postmenopausal women has suggested that these changes may be related to decreased estrogenic influence.[2,3,4] The clinical presentation often mimics acute coronary syndrome. The most common presenting symptoms include chest pain and shortness of breath; ventricular arrhythmias and cardiogenic shock are less frequently reported.\nIschemic ECG changes commonly include ST elevation/depression and T-wave inversion. Mild troponin leak is also a common finding, which makes this syndrome more challenging to differentiate from acute coronary syndromes.[5] BNP and N-terminal pro-BNP levels are elevated in as many as 82% of cases, with a median value of six times the upper limit of normal values.[6]\nThe most common complication is heart failure, with or without pulmonary edema; however, these patients can also develop cardiogenic shock and left ventricular thrombus. Diagnosis is often challenging because the initial presentation often misleadingly suggests acute coronary syndrome. The modified Mayo Clinic criteria for diagnosis require four elements to be present, including:[7]\nTransient hypokinesis, dyskinesis, or akinesis of the left ventricle midsegments, with or without apical involvement; the regional wall-motion abnormalities extend beyond a single epicardial vascular distribution\nAbsence of obstructive coronary disease or angiographic evidence of acute plaque rupture\nNew ECG abnormalities (either ST-segment elevation and/or T-wave inversion) or modest elevation in the cardiac troponin level\nAbsence of cerebrovascular disease, pheochromocytoma, or myocarditis\nTransthoracic echocardiography is an accessible, quick, and reliable diagnostic tool that can reveal the typical wall motion abnormalities described above. Echocardiography is also helpful in detecting the presence of a left ventricular thrombus. As many as 5% of cases have evidence of a left ventricular thrombus.[2] Embolization from this left ventricular thrombus is a potential complication. Coronary angiography is required for diagnosis and helps differentiate this entity from acute coronary syndrome. Most patients with Takotsubo cardiomyopathy have normal coronary arteries or nonsignificant coronary artery disease.[2] Left ventriculography performed during left heart catheterization reveals the characteristic pattern of apical ballooning during systole, providing the best imaging for diagnosis.\nThe primary differential diagnosis of this condition is acute coronary syndrome. Although both conditions can coexist, Takotsubo cardiomyopathy involves a myocardial territory that cannot be explained by the affected coronary artery. Other conditions that can present similarly include cocaine-induced acute coronary syndrome, myocarditis, pheochromocytoma, and acute brain injury.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 64-Year-Old Woman With Extremity Swelling and Cough"
},
{
"authors": "Francisco Aguilar, MD; Carla Arellano Pizano, MD",
"content": [
"Treatment of Takotsubo cardiomyopathy is supportive and conservative. Removal of the stressor often leads to an improvement of the symptoms and recovery of the ejection fraction. Special attention is required in cases where complications develop, because treatment must be tailored according to the initial presentation and clinical findings.",
"In cases that include hypotension and shock, left ventricular outflow tract (LVOT) obstruction must be assessed by imaging. If LVOT obstruction is present, the management is similar to hypertrophic obstructive cardiomyopathy; volume resuscitation and beta-blockers are preferred. In the absence of LVOT obstruction, positive inotropics and vasopressors are the best choices. Anticoagulation with vitamin K antagonists to prevent the formation of an intracavitary thrombus is recommended only for cases in which the bleeding risk is low. It is also recommended for patients with a left ventricular thrombus to prevent embolization.",
"Patients with Takotsubo cardiomyopathy generally have a good prognosis. Although in-hospital mortality is low compared with that of patients who have acute coronary syndrome (around 1%), risk for in-hospital complication is similar between the two groups.[2,6] Left ventricular apical ballooning may recur, but is uncommon. Recovery of the ventricular ejection fraction typically occurs within days to weeks, with an average of 18 days.[6]",
"The patient in this case was managed with diuresis and supportive treatment. Her gastrointestinal symptoms resolved after 2 days. Her shortness of breath improved over 5 days. Given the resolution of her symptoms, she was discharged home. She attended a follow-up appointment in the cardiology outpatient clinic 1 month after discharge. Studies performed at that time revealed a left ventricular ejection fraction of 55%. No wall motion abnormalities or diastolic dysfunction were observed."
],
"date": "July 17, 2018",
"figures": [],
"markdown": "# A 64-Year-Old Woman With Extremity Swelling and Cough\n\n **Authors:** Francisco Aguilar, MD; Carla Arellano Pizano, MD \n **Date:** July 17, 2018\n\n ## Content\n\n Treatment of Takotsubo cardiomyopathy is supportive and conservative. Removal of the stressor often leads to an improvement of the symptoms and recovery of the ejection fraction. Special attention is required in cases where complications develop, because treatment must be tailored according to the initial presentation and clinical findings.\nIn cases that include hypotension and shock, left ventricular outflow tract (LVOT) obstruction must be assessed by imaging. If LVOT obstruction is present, the management is similar to hypertrophic obstructive cardiomyopathy; volume resuscitation and beta-blockers are preferred. In the absence of LVOT obstruction, positive inotropics and vasopressors are the best choices. Anticoagulation with vitamin K antagonists to prevent the formation of an intracavitary thrombus is recommended only for cases in which the bleeding risk is low. It is also recommended for patients with a left ventricular thrombus to prevent embolization.\nPatients with Takotsubo cardiomyopathy generally have a good prognosis. Although in-hospital mortality is low compared with that of patients who have acute coronary syndrome (around 1%), risk for in-hospital complication is similar between the two groups.[2,6] Left ventricular apical ballooning may recur, but is uncommon. Recovery of the ventricular ejection fraction typically occurs within days to weeks, with an average of 18 days.[6]\nThe patient in this case was managed with diuresis and supportive treatment. Her gastrointestinal symptoms resolved after 2 days. Her shortness of breath improved over 5 days. Given the resolution of her symptoms, she was discharged home. She attended a follow-up appointment in the cardiology outpatient clinic 1 month after discharge. Studies performed at that time revealed a left ventricular ejection fraction of 55%. No wall motion abnormalities or diastolic dysfunction were observed.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1244478,
"choiceText": "Dobutamine",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1244480,
"choiceText": "Midodrine",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1244482,
"choiceText": "Milrinone",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1244484,
"choiceText": "Furosemide",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1244486,
"choiceText": "Digoxin",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "This case presented as acute-on-chronic heart failure. Initial therapy should be aimed to decrease the preload and congestion of the cardiopulmonary vasculature. Because the blood pressure is elevated, and although chronic kidney disease is present, the creatinine level at baseline with production of urine makes diuresis a good option.<br><br>\r\nDobutamine and milrinone increase contractility and can be used in certain cases of cardiogenic shock or advanced heart failure; however, in this case, the patient is hemodynamically stable. Digoxin can help increase contractility but, just as midodrine, has no indication in this case.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 395933,
"questionText": "Which of the following is the best initial treatment for patients with a similar presentation to the one in this case?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1244488,
"choiceText": "Carvedilol",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1244490,
"choiceText": "Atorvastatin",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1244492,
"choiceText": "Aspirin",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1244494,
"choiceText": "Clopidogrel",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1244496,
"choiceText": "Warfarin",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Patients with a left ventricular thrombus are recommended to receive anticoagulation therapy for at least 3 months. This evidence is extrapolated from studies in patients with left ventricular thrombus after myocardial infarction, in which anticoagulation therapy with a vitamin K antagonist for 4 to 6 months decreased the rate of embolization.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 395935,
"questionText": "A 54-year-old woman with no significant past medical history presents with typical chest pain. She is hemodynamically stable and has ST-segment elevation in the anterior leads. The patient undergoes coronary angiography that reveals no coronary artery disease. Two-dimensional echocardiography reveals left ventricular apical akinesia with dyskinesia of midsegments. A left ventricular apical thrombus is observed. Which of the following is the best management of this patient?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 64-Year-Old Woman With Extremity Swelling and Cough"
},
{
"authors": "Francisco Aguilar, MD; Carla Arellano Pizano, MD",
"content": [],
"date": "July 17, 2018",
"figures": [],
"markdown": "# A 64-Year-Old Woman With Extremity Swelling and Cough\n\n **Authors:** Francisco Aguilar, MD; Carla Arellano Pizano, MD \n **Date:** July 17, 2018\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1244478,
"choiceText": "Dobutamine",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1244480,
"choiceText": "Midodrine",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1244482,
"choiceText": "Milrinone",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1244484,
"choiceText": "Furosemide",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1244486,
"choiceText": "Digoxin",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "This case presented as acute-on-chronic heart failure. Initial therapy should be aimed to decrease the preload and congestion of the cardiopulmonary vasculature. Because the blood pressure is elevated, and although chronic kidney disease is present, the creatinine level at baseline with production of urine makes diuresis a good option.<br><br>\r\nDobutamine and milrinone increase contractility and can be used in certain cases of cardiogenic shock or advanced heart failure; however, in this case, the patient is hemodynamically stable. Digoxin can help increase contractility but, just as midodrine, has no indication in this case.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 395933,
"questionText": "Which of the following is the best initial treatment for patients with a similar presentation to the one in this case?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1244488,
"choiceText": "Carvedilol",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1244490,
"choiceText": "Atorvastatin",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1244492,
"choiceText": "Aspirin",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1244494,
"choiceText": "Clopidogrel",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1244496,
"choiceText": "Warfarin",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Patients with a left ventricular thrombus are recommended to receive anticoagulation therapy for at least 3 months. This evidence is extrapolated from studies in patients with left ventricular thrombus after myocardial infarction, in which anticoagulation therapy with a vitamin K antagonist for 4 to 6 months decreased the rate of embolization.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 395935,
"questionText": "A 54-year-old woman with no significant past medical history presents with typical chest pain. She is hemodynamically stable and has ST-segment elevation in the anterior leads. The patient undergoes coronary angiography that reveals no coronary artery disease. Two-dimensional echocardiography reveals left ventricular apical akinesia with dyskinesia of midsegments. A left ventricular apical thrombus is observed. Which of the following is the best management of this patient?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 64-Year-Old Woman With Extremity Swelling and Cough"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1244470,
"choiceText": "Acute-on-chronic heart failure secondary to hypertensive emergency",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1244472,
"choiceText": "Acute-on-chronic heart failure secondary to myocardial ischemia",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1244474,
"choiceText": "Acute-on-chronic heart failure secondary to Takotsubo cardiomyopathy ",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1244476,
"choiceText": "Pulmonary hypertension World Health Organization classification group I",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 395931,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1244478,
"choiceText": "Dobutamine",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1244480,
"choiceText": "Midodrine",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1244482,
"choiceText": "Milrinone",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1244484,
"choiceText": "Furosemide",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1244486,
"choiceText": "Digoxin",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "This case presented as acute-on-chronic heart failure. Initial therapy should be aimed to decrease the preload and congestion of the cardiopulmonary vasculature. Because the blood pressure is elevated, and although chronic kidney disease is present, the creatinine level at baseline with production of urine makes diuresis a good option.<br><br>\r\nDobutamine and milrinone increase contractility and can be used in certain cases of cardiogenic shock or advanced heart failure; however, in this case, the patient is hemodynamically stable. Digoxin can help increase contractility but, just as midodrine, has no indication in this case.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 395933,
"questionText": "Which of the following is the best initial treatment for patients with a similar presentation to the one in this case?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1244488,
"choiceText": "Carvedilol",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1244490,
"choiceText": "Atorvastatin",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1244492,
"choiceText": "Aspirin",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1244494,
"choiceText": "Clopidogrel",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1244496,
"choiceText": "Warfarin",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Patients with a left ventricular thrombus are recommended to receive anticoagulation therapy for at least 3 months. This evidence is extrapolated from studies in patients with left ventricular thrombus after myocardial infarction, in which anticoagulation therapy with a vitamin K antagonist for 4 to 6 months decreased the rate of embolization.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 395935,
"questionText": "A 54-year-old woman with no significant past medical history presents with typical chest pain. She is hemodynamically stable and has ST-segment elevation in the anterior leads. The patient undergoes coronary angiography that reveals no coronary artery disease. Two-dimensional echocardiography reveals left ventricular apical akinesia with dyskinesia of midsegments. A left ventricular apical thrombus is observed. Which of the following is the best management of this patient?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
897549 | /viewarticle/897549 | [
{
"authors": "Nancy Hammond, MD",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"The patient is a 25-year-old woman with a history of headaches since age 15 years. She is currently 24 weeks pregnant. Her pregnancy so far has been uneventful other than worsening headaches. Hours prior to getting a headache, she has a strong sense of malaise and fatigue with mild nausea. Occasionally, prior to headache onset, she will see a scintillating, zig-zag light in one visual field. This light can be in either visual field. The visual symptoms last 15-20 minutes, and then she experiences a severe unilateral, pulsatile headache.",
"She has associated symptoms of nausea, occasional vomiting, photophobia, and phonophobia. Initially, headaches were occurring about once per month; however, they have increased in frequency since she became pregnant and now occur about once a week. Other than occurring more frequently, the headaches are similar in quality to the headaches she had prior to becoming pregnant. Each headache will last for 6-8 hours, and sleep will relieve the headache.",
"She is otherwise healthy and takes no medications, other than occasional ibuprofen for her headaches. Prior to pregnancy, she had good headache relief with sumatriptan. She works as an accountant and misses work about two times a month due to headaches. She denies any use of tobacco or illicit drugs. She reports occasional alcohol use but also notes that alcohol use can provoke a headache. She has not used alcohol since she has been pregnant. Her family history is remarkable for similar headaches in her mother and brother."
],
"date": "June 07, 2018",
"figures": [],
"markdown": "# A 25-Year-Old Pregnant Woman With Worsening Headaches\n\n **Authors:** Nancy Hammond, MD \n **Date:** June 07, 2018\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nThe patient is a 25-year-old woman with a history of headaches since age 15 years. She is currently 24 weeks pregnant. Her pregnancy so far has been uneventful other than worsening headaches. Hours prior to getting a headache, she has a strong sense of malaise and fatigue with mild nausea. Occasionally, prior to headache onset, she will see a scintillating, zig-zag light in one visual field. This light can be in either visual field. The visual symptoms last 15-20 minutes, and then she experiences a severe unilateral, pulsatile headache.\nShe has associated symptoms of nausea, occasional vomiting, photophobia, and phonophobia. Initially, headaches were occurring about once per month; however, they have increased in frequency since she became pregnant and now occur about once a week. Other than occurring more frequently, the headaches are similar in quality to the headaches she had prior to becoming pregnant. Each headache will last for 6-8 hours, and sleep will relieve the headache.\nShe is otherwise healthy and takes no medications, other than occasional ibuprofen for her headaches. Prior to pregnancy, she had good headache relief with sumatriptan. She works as an accountant and misses work about two times a month due to headaches. She denies any use of tobacco or illicit drugs. She reports occasional alcohol use but also notes that alcohol use can provoke a headache. She has not used alcohol since she has been pregnant. Her family history is remarkable for similar headaches in her mother and brother.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 25-Year-Old Pregnant Woman With Worsening Headaches"
},
{
"authors": "Nancy Hammond, MD",
"content": [
"A general examination reveals a healthy, pregnant woman. General medical examination of the heart and lungs reveals normal findings. Fundoscopic examination reveals normal optic discs and posterior segments. Cranial nerve, motor, reflex, sensory, and coordination examination findings are all normal.",
"An MRI done prior to her becoming pregnant shows small subcortical white matter T2 hyperintensities (Figure 1).",
"Figure 1."
],
"date": "June 07, 2018",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/897/549/897549-Thumb1.png"
}
],
"markdown": "# A 25-Year-Old Pregnant Woman With Worsening Headaches\n\n **Authors:** Nancy Hammond, MD \n **Date:** June 07, 2018\n\n ## Content\n\n A general examination reveals a healthy, pregnant woman. General medical examination of the heart and lungs reveals normal findings. Fundoscopic examination reveals normal optic discs and posterior segments. Cranial nerve, motor, reflex, sensory, and coordination examination findings are all normal.\nAn MRI done prior to her becoming pregnant shows small subcortical white matter T2 hyperintensities (Figure 1).\nFigure 1.\n\n ## Figures\n\n **Figure 1.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1228542,
"choiceText": "Idiopathic intracranial hypertension ",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1228544,
"choiceText": "Tension-type headache",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1228546,
"choiceText": "Cerebral venous thrombosis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1228548,
"choiceText": "Migraine with aura",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1228550,
"choiceText": "Occipital seizures\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 390587,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 25-Year-Old Pregnant Woman With Worsening Headaches"
},
{
"authors": "Nancy Hammond, MD",
"content": [
"This young woman suffers from migraine with aura (classic migraine). The International Headache Society defines migraine with aura as starting with the gradual development of visual, sensory, or speech symptoms lasting less than 1 hour.[1] Symptoms may occur alone or in combination but must be fully reversible. Motor, brainstem, and retinal symptoms should be absent. Headache follows the initial neurologic symptoms within 60 minutes. Patients with migraine often describe a prodrome of fatigue, nausea, irritability, excessive thirst, irritability, or depressed mood that can occur hours to days prior to the headache.",
"Migraine headaches are often moderate to severe in intensity and unilateral, although they can be present bilaterally. The headache is often described as pulsating in quality and aggravated by routine physical activity. Nausea or vomiting and photophobia or phonophobia usually accompany the headache. Without treatment, migraine headaches last hours to days. Estimates of migraine prevalence vary widely throughout the world. In the United States, around 15%-20% of women and 5%-8% of men have migraine.[2] Incidence of migraine with aura peaks between age 12-13 years in females.",
"Tension headaches are usually less severe than migraine. They are often described as a bandlike, squeezing pain around the head. Associated symptoms can include mild nausea and photophobia or phonophobia. Cluster headaches are characterized by sudden onset of unilateral, retro-orbital, stabbing pain accompanied by autonomic symptoms ipsilateral to the pain of ptosis, conjunctival injection, tearing, and rhinorrhea. Occipital seizures can present with a visual aura but are usually shorter in duration than the visual symptoms of migraine with aura."
],
"date": "June 07, 2018",
"figures": [],
"markdown": "# A 25-Year-Old Pregnant Woman With Worsening Headaches\n\n **Authors:** Nancy Hammond, MD \n **Date:** June 07, 2018\n\n ## Content\n\n This young woman suffers from migraine with aura (classic migraine). The International Headache Society defines migraine with aura as starting with the gradual development of visual, sensory, or speech symptoms lasting less than 1 hour.[1] Symptoms may occur alone or in combination but must be fully reversible. Motor, brainstem, and retinal symptoms should be absent. Headache follows the initial neurologic symptoms within 60 minutes. Patients with migraine often describe a prodrome of fatigue, nausea, irritability, excessive thirst, irritability, or depressed mood that can occur hours to days prior to the headache.\nMigraine headaches are often moderate to severe in intensity and unilateral, although they can be present bilaterally. The headache is often described as pulsating in quality and aggravated by routine physical activity. Nausea or vomiting and photophobia or phonophobia usually accompany the headache. Without treatment, migraine headaches last hours to days. Estimates of migraine prevalence vary widely throughout the world. In the United States, around 15%-20% of women and 5%-8% of men have migraine.[2] Incidence of migraine with aura peaks between age 12-13 years in females.\nTension headaches are usually less severe than migraine. They are often described as a bandlike, squeezing pain around the head. Associated symptoms can include mild nausea and photophobia or phonophobia. Cluster headaches are characterized by sudden onset of unilateral, retro-orbital, stabbing pain accompanied by autonomic symptoms ipsilateral to the pain of ptosis, conjunctival injection, tearing, and rhinorrhea. Occipital seizures can present with a visual aura but are usually shorter in duration than the visual symptoms of migraine with aura.\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1228542,
"choiceText": "Idiopathic intracranial hypertension ",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1228544,
"choiceText": "Tension-type headache",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1228546,
"choiceText": "Cerebral venous thrombosis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1228548,
"choiceText": "Migraine with aura",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1228550,
"choiceText": "Occipital seizures\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 390587,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 25-Year-Old Pregnant Woman With Worsening Headaches"
},
{
"authors": "Nancy Hammond, MD",
"content": [
"When a patient presents with classic symptoms of migraine, imaging should only be performed when clinical signs or symptoms are suggestive of a secondary headache. The following symptoms and signs could be a marker for a more serious cause of headache:",
"An abnormal neurologic examination, including presence of papilledema",
"Abnormal mental status",
"Thunderclap headache (a headache that suddenly reaches maximal intensity)",
"Headache associated with positional changes",
"New-onset headaches",
"A substantial change in headache characteristics or pattern",
"Signs of systemic disease (eg, fever or weight loss)",
"Neuroimaging is usually normal in migraine. White-matter T2 and fluid-attenuated inversion recovery (FLAIR) hyperintensities are seen more commonly in patients with migraine with aura. The pathologic mechanisms responsible for these changes are not known. Pathologic studies have shown gliosis in these lesions. These lesions do not correlate with migraine severity or frequency or have any other symptom correlation.[3]",
"This case brings up an interesting dilemma in treatment of migraine in young women who are or may become pregnant. Fortunately, migraine improves for most women during the second and third trimester of pregnancy.[4] Unfortunately, both preventive and acute treatments for migraine are limited during pregnancy due to teratogenic potential.",
"Several causes are responsible for secondary headaches in pregnant women. Cerebral venous thrombosis presents as a nonspecific but constant headache that often worsens over time (Figure 2).",
"Figure 2.",
"Usually, other focal neurologic deficits or seizures are present. When a pregnant woman presents with headache, hypertension, proteinuria, and eclampsia should be considered. The headaches in these cases are typically severe and not responsive to analgesics. Posterior reversible encephalopathy and reversible cerebral vasoconstriction are possible serious complications of eclampsia. Pregnant women are also at risk for ischemic stroke. Ischemic stroke is more common in women with diabetes mellitus, sickle cell disease, hypertension, and pre-existing heart disease.",
"Pituitary apoplexy is a rare cause of sudden-onset severe headache due to infarction and hemorrhage of the pituitary gland. Pituitary apoplexy is typically seen in the presence of pituitary adenoma; however, physiologic pituitary hyperplasia during pregnancy can also result in pituitary apoplexy. Associated symptoms include nausea, vomiting, and visual disturbances.",
"Idiopathic intracranial hypertension can also present during pregnancy. Symptoms of this disorder include headache, transient visual obscurations, particularly when bending forward, and pulsatile tinnitus. Fundoscopic examination reveals papilledema (Figure 3).",
"Figure 3.",
"Once neuroimaging is performed and no space occupying lesion is seen, lumbar puncture with opening pressure should performed. The presence of elevated cerebrospinal fluid pressure is diagnostic of idiopathic intracranial hypertension.",
"The young woman in this case has had headaches that have become more frequent since she became pregnant; however, she had a normal neurologic examination, which is reassuring, and prior neuroimaging did not show any significant findings.",
"Careful consideration should be given to the need for neuroimaging during pregnancy. If neuroimaging is deemed necessary based on history and physical examination, MRI is typically preferred over CT. Use of either iodinated contrast or gadolinium-based contrast is not recommended. If imaging is needed, the risks and benefits should be discussed with the patient."
],
"date": "June 07, 2018",
"figures": [
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/897/549/897549-Thumb2.png"
},
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/897/549/897549-Thumb3.png"
}
],
"markdown": "# A 25-Year-Old Pregnant Woman With Worsening Headaches\n\n **Authors:** Nancy Hammond, MD \n **Date:** June 07, 2018\n\n ## Content\n\n When a patient presents with classic symptoms of migraine, imaging should only be performed when clinical signs or symptoms are suggestive of a secondary headache. The following symptoms and signs could be a marker for a more serious cause of headache:\nAn abnormal neurologic examination, including presence of papilledema\nAbnormal mental status\nThunderclap headache (a headache that suddenly reaches maximal intensity)\nHeadache associated with positional changes\nNew-onset headaches\nA substantial change in headache characteristics or pattern\nSigns of systemic disease (eg, fever or weight loss)\nNeuroimaging is usually normal in migraine. White-matter T2 and fluid-attenuated inversion recovery (FLAIR) hyperintensities are seen more commonly in patients with migraine with aura. The pathologic mechanisms responsible for these changes are not known. Pathologic studies have shown gliosis in these lesions. These lesions do not correlate with migraine severity or frequency or have any other symptom correlation.[3]\nThis case brings up an interesting dilemma in treatment of migraine in young women who are or may become pregnant. Fortunately, migraine improves for most women during the second and third trimester of pregnancy.[4] Unfortunately, both preventive and acute treatments for migraine are limited during pregnancy due to teratogenic potential.\nSeveral causes are responsible for secondary headaches in pregnant women. Cerebral venous thrombosis presents as a nonspecific but constant headache that often worsens over time (Figure 2).\nFigure 2.\nUsually, other focal neurologic deficits or seizures are present. When a pregnant woman presents with headache, hypertension, proteinuria, and eclampsia should be considered. The headaches in these cases are typically severe and not responsive to analgesics. Posterior reversible encephalopathy and reversible cerebral vasoconstriction are possible serious complications of eclampsia. Pregnant women are also at risk for ischemic stroke. Ischemic stroke is more common in women with diabetes mellitus, sickle cell disease, hypertension, and pre-existing heart disease.\nPituitary apoplexy is a rare cause of sudden-onset severe headache due to infarction and hemorrhage of the pituitary gland. Pituitary apoplexy is typically seen in the presence of pituitary adenoma; however, physiologic pituitary hyperplasia during pregnancy can also result in pituitary apoplexy. Associated symptoms include nausea, vomiting, and visual disturbances.\nIdiopathic intracranial hypertension can also present during pregnancy. Symptoms of this disorder include headache, transient visual obscurations, particularly when bending forward, and pulsatile tinnitus. Fundoscopic examination reveals papilledema (Figure 3).\nFigure 3.\nOnce neuroimaging is performed and no space occupying lesion is seen, lumbar puncture with opening pressure should performed. The presence of elevated cerebrospinal fluid pressure is diagnostic of idiopathic intracranial hypertension.\nThe young woman in this case has had headaches that have become more frequent since she became pregnant; however, she had a normal neurologic examination, which is reassuring, and prior neuroimaging did not show any significant findings.\nCareful consideration should be given to the need for neuroimaging during pregnancy. If neuroimaging is deemed necessary based on history and physical examination, MRI is typically preferred over CT. Use of either iodinated contrast or gadolinium-based contrast is not recommended. If imaging is needed, the risks and benefits should be discussed with the patient.\n\n ## Figures\n\n **Figure 2.** \n \n\n**Figure 3.** \n \n\n\n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 25-Year-Old Pregnant Woman With Worsening Headaches"
},
{
"authors": "Nancy Hammond, MD",
"content": [
"Treatment of headache during pregnancy is complicated due to possible teratogenic effects of medications. If a cause of headache is found, treatment should focus on the underlying condition. If a primary headache is diagnosed, nonpharmacologic measures should be the first step. Women should be encouraged to keep a headache journal to identify possible triggers. Common migraine triggers include stress, irregular sleep, erratic meals, dehydration, and either caffeine overuse or withdrawal. Certain foods can also trigger migraine, and a headache journal can be very helpful in determining specific triggers for an individual patient. Relaxation techniques, biofeedback, and acupuncture may all be helpful in reducing headache frequency. Preventive therapy for migraine should be considered if headaches are occurring once a week or more or if headaches are leading to missed work or other disability.",
"Acute treatments for migraine are challenging in pregnant women. Acetaminophen is commonly thought to be safe during pregnancy and can be used for mild-to-moderate headaches. Nonsteroidal anti-inflammatory drugs (NSAIDs) are generally felt to be safe to take in the first and second trimester and are probably more effective for acute migraine.[5] Triptans, such as sumatriptan, are serotonin agonists and are highly effective for migraine. Animal studies have shown teratogenic risk, but pregnancy registries for sumatriptan, naratriptan, and rizatriptan have not shown an increased teratogenic risk.[6] Triptans are a reasonable option if acetaminophen or NSAIDs do not provide adequate headache relief. Antiemetic medications should be considered as an adjunctive treatment for migraine during pregnancy. Metoclopramide and prochlorperazine have not been shown to contribute to fetal malformations.[7] They are effective for both relief of migraine pain and nausea.[8]",
"Preventive medications should be considered when migraines occur once a week or more or if the woman has significant disability due to migraine attacks. The most common migraine preventives include blood pressure medications, antiepileptic medications, and tricyclic antidepressants. When considering a preventive medication, the lowest effective dose should be used in order to minimize any possible teratogenic effects.",
"Propranolol is a beta blocker used for migraine prevention. Human studies have not shown any teratogenic effects; however, propranolol should be discontinued if possible in the third trimester to reduce the risk for fetal bradycardia. Amitriptyline is a tricyclic antidepressant that is widely used for migraine prevention. It is generally believed to be safe to take at low doses during pregnancy. Topiramate is an antiepileptic medication commonly used for migraine prevention; however, topiramate should not be used during pregnancy or in a woman desiring pregnancy because of teratogenic risk. Supplemental oral magnesium, riboflavin, and Co-Q10 may have a role in migraine prevention during pregnancy."
],
"date": "June 07, 2018",
"figures": [],
"markdown": "# A 25-Year-Old Pregnant Woman With Worsening Headaches\n\n **Authors:** Nancy Hammond, MD \n **Date:** June 07, 2018\n\n ## Content\n\n Treatment of headache during pregnancy is complicated due to possible teratogenic effects of medications. If a cause of headache is found, treatment should focus on the underlying condition. If a primary headache is diagnosed, nonpharmacologic measures should be the first step. Women should be encouraged to keep a headache journal to identify possible triggers. Common migraine triggers include stress, irregular sleep, erratic meals, dehydration, and either caffeine overuse or withdrawal. Certain foods can also trigger migraine, and a headache journal can be very helpful in determining specific triggers for an individual patient. Relaxation techniques, biofeedback, and acupuncture may all be helpful in reducing headache frequency. Preventive therapy for migraine should be considered if headaches are occurring once a week or more or if headaches are leading to missed work or other disability.\nAcute treatments for migraine are challenging in pregnant women. Acetaminophen is commonly thought to be safe during pregnancy and can be used for mild-to-moderate headaches. Nonsteroidal anti-inflammatory drugs (NSAIDs) are generally felt to be safe to take in the first and second trimester and are probably more effective for acute migraine.[5] Triptans, such as sumatriptan, are serotonin agonists and are highly effective for migraine. Animal studies have shown teratogenic risk, but pregnancy registries for sumatriptan, naratriptan, and rizatriptan have not shown an increased teratogenic risk.[6] Triptans are a reasonable option if acetaminophen or NSAIDs do not provide adequate headache relief. Antiemetic medications should be considered as an adjunctive treatment for migraine during pregnancy. Metoclopramide and prochlorperazine have not been shown to contribute to fetal malformations.[7] They are effective for both relief of migraine pain and nausea.[8]\nPreventive medications should be considered when migraines occur once a week or more or if the woman has significant disability due to migraine attacks. The most common migraine preventives include blood pressure medications, antiepileptic medications, and tricyclic antidepressants. When considering a preventive medication, the lowest effective dose should be used in order to minimize any possible teratogenic effects.\nPropranolol is a beta blocker used for migraine prevention. Human studies have not shown any teratogenic effects; however, propranolol should be discontinued if possible in the third trimester to reduce the risk for fetal bradycardia. Amitriptyline is a tricyclic antidepressant that is widely used for migraine prevention. It is generally believed to be safe to take at low doses during pregnancy. Topiramate is an antiepileptic medication commonly used for migraine prevention; however, topiramate should not be used during pregnancy or in a woman desiring pregnancy because of teratogenic risk. Supplemental oral magnesium, riboflavin, and Co-Q10 may have a role in migraine prevention during pregnancy.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1228552,
"choiceText": "Dihydroergotamine",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1228554,
"choiceText": "Acetaminophen",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1228556,
"choiceText": "Sumatriptan",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1228558,
"choiceText": "Oxycodone\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Acetaminophen is the safest medication to treat migraine during pregnancy. Sumatriptan is a reasonable option if acetaminophen is not effective. Dihydroergotamine is contraindicated in pregnancy. Opioid medications are not recommended during pregnancy.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 390589,
"questionText": "Which of the following is the safest acute treatment for migraine during pregnancy?\r\n",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1228560,
"choiceText": "Valproic acid",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1228562,
"choiceText": "Topiramate",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1228564,
"choiceText": "Propranolol",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1228566,
"choiceText": "Lisinopril",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Valproic acid, topiramate, and lisinopril are all medications used for migraine prevention that should not be used during pregnancy due to teratogenic risks.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 390591,
"questionText": "Which of the following is an appropriate medication to use for migraine prevention in a pregnant woman?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 25-Year-Old Pregnant Woman With Worsening Headaches"
},
{
"authors": "Nancy Hammond, MD",
"content": [],
"date": "June 07, 2018",
"figures": [],
"markdown": "# A 25-Year-Old Pregnant Woman With Worsening Headaches\n\n **Authors:** Nancy Hammond, MD \n **Date:** June 07, 2018\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1228552,
"choiceText": "Dihydroergotamine",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1228554,
"choiceText": "Acetaminophen",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1228556,
"choiceText": "Sumatriptan",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1228558,
"choiceText": "Oxycodone\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Acetaminophen is the safest medication to treat migraine during pregnancy. Sumatriptan is a reasonable option if acetaminophen is not effective. Dihydroergotamine is contraindicated in pregnancy. Opioid medications are not recommended during pregnancy.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 390589,
"questionText": "Which of the following is the safest acute treatment for migraine during pregnancy?\r\n",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1228560,
"choiceText": "Valproic acid",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1228562,
"choiceText": "Topiramate",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1228564,
"choiceText": "Propranolol",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1228566,
"choiceText": "Lisinopril",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Valproic acid, topiramate, and lisinopril are all medications used for migraine prevention that should not be used during pregnancy due to teratogenic risks.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 390591,
"questionText": "Which of the following is an appropriate medication to use for migraine prevention in a pregnant woman?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 25-Year-Old Pregnant Woman With Worsening Headaches"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1228542,
"choiceText": "Idiopathic intracranial hypertension ",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1228544,
"choiceText": "Tension-type headache",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1228546,
"choiceText": "Cerebral venous thrombosis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1228548,
"choiceText": "Migraine with aura",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1228550,
"choiceText": "Occipital seizures\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 390587,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1228552,
"choiceText": "Dihydroergotamine",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1228554,
"choiceText": "Acetaminophen",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1228556,
"choiceText": "Sumatriptan",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1228558,
"choiceText": "Oxycodone\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Acetaminophen is the safest medication to treat migraine during pregnancy. Sumatriptan is a reasonable option if acetaminophen is not effective. Dihydroergotamine is contraindicated in pregnancy. Opioid medications are not recommended during pregnancy.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 390589,
"questionText": "Which of the following is the safest acute treatment for migraine during pregnancy?\r\n",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1228560,
"choiceText": "Valproic acid",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1228562,
"choiceText": "Topiramate",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1228564,
"choiceText": "Propranolol",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1228566,
"choiceText": "Lisinopril",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Valproic acid, topiramate, and lisinopril are all medications used for migraine prevention that should not be used during pregnancy due to teratogenic risks.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 390591,
"questionText": "Which of the following is an appropriate medication to use for migraine prevention in a pregnant woman?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
896574 | /viewarticle/896574 | [
{
"authors": "Dan Beardmore, DO; Saba Fatima, MD",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"At 6 days of life, an infant is presented to a pediatrician for a scheduled visit. The full-term male infant was born via vaginal delivery after uncomplicated pregnancy and labor. During the newborn examination, a midline, dermal lesion was noted at the L3-L4 spinal segment. It was described as purple-red in hue and slightly raised. The lesion was noted to be irregular; it measured approximately 1.5 cm × 2 cm and had a small, 1-mm central dermal pit. It did not blanch, was not firm to touch, and was seemingly nontender.",
"During the newborn examination, neurology findings were normal, with good lower extremity tone, normal reflexes, and normal sensation. The remainder of the infant's examination findings were unremarkable, including normal results on anal examination and male genitourinary examination. During his nursery stay, he voided and stooled normally. He was discharged with instructions for a scheduled newborn visit. No imaging was done in the nursery.",
"Three days later, the infant is now presented to the pediatrician. He is well-appearing, has been feeding adequately, and is stooling and voiding normally. The lesion has changed slightly, with new, faint, ill-defined, macular erythema that extends from the original cutaneous lesion towards his left flank (Figure 1). The original papular lesion and the central dermal pit are unchanged, and no drainage from the pit has occurred.",
"Figure 1."
],
"date": "May 15, 2018",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/896/574/896574-Thumb1.png"
}
],
"markdown": "# A Newborn Infant With a Lumbar Lesion and Dermal Defect\n\n **Authors:** Dan Beardmore, DO; Saba Fatima, MD \n **Date:** May 15, 2018\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nAt 6 days of life, an infant is presented to a pediatrician for a scheduled visit. The full-term male infant was born via vaginal delivery after uncomplicated pregnancy and labor. During the newborn examination, a midline, dermal lesion was noted at the L3-L4 spinal segment. It was described as purple-red in hue and slightly raised. The lesion was noted to be irregular; it measured approximately 1.5 cm × 2 cm and had a small, 1-mm central dermal pit. It did not blanch, was not firm to touch, and was seemingly nontender.\nDuring the newborn examination, neurology findings were normal, with good lower extremity tone, normal reflexes, and normal sensation. The remainder of the infant's examination findings were unremarkable, including normal results on anal examination and male genitourinary examination. During his nursery stay, he voided and stooled normally. He was discharged with instructions for a scheduled newborn visit. No imaging was done in the nursery.\nThree days later, the infant is now presented to the pediatrician. He is well-appearing, has been feeding adequately, and is stooling and voiding normally. The lesion has changed slightly, with new, faint, ill-defined, macular erythema that extends from the original cutaneous lesion towards his left flank (Figure 1). The original papular lesion and the central dermal pit are unchanged, and no drainage from the pit has occurred.\nFigure 1.\n\n ## Figures\n\n **Figure 1.** \n \n\n\n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A Newborn Infant With a Lumbar Lesion and Dermal Defect"
},
{
"authors": "Dan Beardmore, DO; Saba Fatima, MD",
"content": [
"The remainder of the infant's examination is normal, including normal results on full neurologic examination. The parents are asked to bring the baby to a nearby children's hospital for a first-step ultrasound of the lesion.",
"The spinal canal ultrasonography performed at 8 days of life to evaluate for underlying or associated defects shows that the conus medullaris terminates at spinal segment L3, which is low-lying for a full-term infant but nonspecific. No evidence suggests a tethered cord. No subdermal abnormality is seen at the area of the lumbosacral lesion. Given these results, the parents were told that a neurosurgery referral appointment is less urgent than was previously discussed.",
"At follow-up with the pediatrician at 28 days of life, the infant continues to do well at home according to his parents. The only concern is a change in appearance of the lesion. The parents report that it has enlarged, darkened, and become more raised; however, it stopped changing altogether about 1 week ago (Figure 2).",
"Figure 2.",
"The entirety of the lesion is now approximately 6 cm × 3 cm and darker red, with a well-demarcated but irregular border. It is all now slightly raised and still nonblanching, with no areas of breakdown nor ulceration. The centralmost 2 cm × 3 cm area surrounding the dermal pit remains the most raised and is also the darkest shade of red."
],
"date": "May 15, 2018",
"figures": [
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/896/574/896574-Thumb2.png"
}
],
"markdown": "# A Newborn Infant With a Lumbar Lesion and Dermal Defect\n\n **Authors:** Dan Beardmore, DO; Saba Fatima, MD \n **Date:** May 15, 2018\n\n ## Content\n\n The remainder of the infant's examination is normal, including normal results on full neurologic examination. The parents are asked to bring the baby to a nearby children's hospital for a first-step ultrasound of the lesion.\nThe spinal canal ultrasonography performed at 8 days of life to evaluate for underlying or associated defects shows that the conus medullaris terminates at spinal segment L3, which is low-lying for a full-term infant but nonspecific. No evidence suggests a tethered cord. No subdermal abnormality is seen at the area of the lumbosacral lesion. Given these results, the parents were told that a neurosurgery referral appointment is less urgent than was previously discussed.\nAt follow-up with the pediatrician at 28 days of life, the infant continues to do well at home according to his parents. The only concern is a change in appearance of the lesion. The parents report that it has enlarged, darkened, and become more raised; however, it stopped changing altogether about 1 week ago (Figure 2).\nFigure 2.\nThe entirety of the lesion is now approximately 6 cm × 3 cm and darker red, with a well-demarcated but irregular border. It is all now slightly raised and still nonblanching, with no areas of breakdown nor ulceration. The centralmost 2 cm × 3 cm area surrounding the dermal pit remains the most raised and is also the darkest shade of red.\n\n ## Figures\n\n **Figure 2.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1221178,
"choiceText": "Arteriovenous malformation",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1221180,
"choiceText": "Infantile hemangioma",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1221182,
"choiceText": "Pyogenic granuloma",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1221184,
"choiceText": "Meningocele",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1221186,
"choiceText": "Neurodevelopmental tumor",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 388231,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A Newborn Infant With a Lumbar Lesion and Dermal Defect"
},
{
"authors": "Dan Beardmore, DO; Saba Fatima, MD",
"content": [
"On the basis of examination findings and appearance of the lesion, progressing strawberry hemangioma was diagnosed, with continued concern for an underlying neural tube/spinal cord defect. The baby was admitted for initiation of oral propranolol to treat the hemangioma and for MRI of the lumbar spine.",
"Infantile hemangiomas are the most common vascular tumors of infancy, affecting 5% of infants in the United States.[1] They most commonly occur in the head and neck region; the trunk and extremities are the next most common location.",
"The evolution of infantile hemangiomas is characterized by a rapid proliferation phase, which is followed by prolonged involution. Although most infantile hemangiomas are managed by observation, about 10% are associated with complications and require intervention.[2] These complications may include infection, ulceration, bleeding, pain, and vision or airway compromise."
],
"date": "May 15, 2018",
"figures": [],
"markdown": "# A Newborn Infant With a Lumbar Lesion and Dermal Defect\n\n **Authors:** Dan Beardmore, DO; Saba Fatima, MD \n **Date:** May 15, 2018\n\n ## Content\n\n On the basis of examination findings and appearance of the lesion, progressing strawberry hemangioma was diagnosed, with continued concern for an underlying neural tube/spinal cord defect. The baby was admitted for initiation of oral propranolol to treat the hemangioma and for MRI of the lumbar spine.\nInfantile hemangiomas are the most common vascular tumors of infancy, affecting 5% of infants in the United States.[1] They most commonly occur in the head and neck region; the trunk and extremities are the next most common location.\nThe evolution of infantile hemangiomas is characterized by a rapid proliferation phase, which is followed by prolonged involution. Although most infantile hemangiomas are managed by observation, about 10% are associated with complications and require intervention.[2] These complications may include infection, ulceration, bleeding, pain, and vision or airway compromise.\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1221178,
"choiceText": "Arteriovenous malformation",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1221180,
"choiceText": "Infantile hemangioma",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1221182,
"choiceText": "Pyogenic granuloma",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1221184,
"choiceText": "Meningocele",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1221186,
"choiceText": "Neurodevelopmental tumor",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 388231,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A Newborn Infant With a Lumbar Lesion and Dermal Defect"
},
{
"authors": "Dan Beardmore, DO; Saba Fatima, MD",
"content": [
"Infantile hemangiomas that occur in the lumbosacral region in infants have an increased risk for occult spinal dysraphism, especially those that are large or associated with more than one abnormality (both being the case with this patient).[2] Ulceration and coexisting anomalies have increased risk for predisposition to spinal anomalies. Spinal dysraphisms that are frequently associated with infantile hemangiomas include tethered cord, spinal lipoma, and intraspinal hemangioma.[2] However, multicenter studies have showed that even isolated infantile hemangiomas in this region can be associated with spinal anomalies.[3] This is because the neural tube has five origin centers and closes by meeting at four locations: the forehead, the back of the skull, the nape of the neck, and the lumbosacral spine.[4]",
"A thorough physical examination including genitourinary, anal, lower extremity, and neurologic components is hence recommended, and LUMBAR syndrome (similar to PHACE syndrome in the upper half of the body) should be considered during this evaluation. [5]",
"MRI is recommended if a suspicious cutaneous lesion overlies the spine, in order to fully rule out spinal dysraphism.[6] Other lumbar and lumbosacral abnormalities that are included in the differential diagnosis for this case include, but are not limited to, the following:",
"Meningocele",
"Myelomeningocele",
"Spina bifida",
"Other vascular lesions and malignancies"
],
"date": "May 15, 2018",
"figures": [],
"markdown": "# A Newborn Infant With a Lumbar Lesion and Dermal Defect\n\n **Authors:** Dan Beardmore, DO; Saba Fatima, MD \n **Date:** May 15, 2018\n\n ## Content\n\n Infantile hemangiomas that occur in the lumbosacral region in infants have an increased risk for occult spinal dysraphism, especially those that are large or associated with more than one abnormality (both being the case with this patient).[2] Ulceration and coexisting anomalies have increased risk for predisposition to spinal anomalies. Spinal dysraphisms that are frequently associated with infantile hemangiomas include tethered cord, spinal lipoma, and intraspinal hemangioma.[2] However, multicenter studies have showed that even isolated infantile hemangiomas in this region can be associated with spinal anomalies.[3] This is because the neural tube has five origin centers and closes by meeting at four locations: the forehead, the back of the skull, the nape of the neck, and the lumbosacral spine.[4]\nA thorough physical examination including genitourinary, anal, lower extremity, and neurologic components is hence recommended, and LUMBAR syndrome (similar to PHACE syndrome in the upper half of the body) should be considered during this evaluation. [5]\nMRI is recommended if a suspicious cutaneous lesion overlies the spine, in order to fully rule out spinal dysraphism.[6] Other lumbar and lumbosacral abnormalities that are included in the differential diagnosis for this case include, but are not limited to, the following:\nMeningocele\nMyelomeningocele\nSpina bifida\nOther vascular lesions and malignancies\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A Newborn Infant With a Lumbar Lesion and Dermal Defect"
},
{
"authors": "Dan Beardmore, DO; Saba Fatima, MD",
"content": [
"Propranolol was incidentally discovered in 2008 as a treatment for infantile hemangiomas and has now emerged as first-line therapy.[7] Propranolol's mechanism of action of has been hypothesized to include its effects by vasoconstriction and downregulation of growth factors.[7] It can be administered orally at low doses or applied topically, using drops or gels.",
"The most concerning possible side effects of propranolol include hypotension, bradycardia, hypoglycemia, hyperkalemia, and bronchospasm.[6] For these reasons, many clinicians and institutions choose to initially administer the medication to children during an inpatient visit, especially for younger patients, in whom oral administration is chosen over topical treatment.[7,8]",
"The infant in this case was admitted to a nearby children's hospital for initiation of oral propranolol to treat the infantile hemangioma. Before beginning the treatment, MRI of the spine was successfully obtained (without sedation). The MRI results identified an enhancing tract connecting the skin with the paraspinal soft tissues at the level of L4/L5; however, no definitive communication with the spinal canal was seen (although subtle communication could not be excluded). Surface thickening of the skin above the tract compatible with given history of an infantile hemangioma was noted. Finally, the conus medullaris terminated at L2-L3 spinal level, with no tethering, which was a normal location for the patient's age. No other abnormalities were reported.",
"Propranolol was started, with a 3-day regimen: 0.5 mg/kg/day divided three times on the first day, then 1 mg/kg/day divided three times on the second day, then 2 mg/kg/day divided three times on the third day. Continuous monitoring and frequent vitals revealed a normal heart rate and blood pressure throughout. Electrolyte levels, including glucose levels, were also normal throughout.",
"After tolerating the final goal dose of 2 mg/kg/day divided three times per day, the patient was discharged home with a 1-week follow up to assess the infantile hemangioma in the oncology clinic. A 2-month follow-up with neurosurgery to repeat the MRI of the lumbosacral region was also indicated.",
"At primary care follow-up 2 weeks later, the parents were pleased with the treatment and the interval improvement of the lesion (Figure 3). A single café au lait spot was also noted on his right upper back during this visit.",
"Figure 3.",
"The patient was lost to follow-up for a short while; however, the family returned at 6 months for a check-up. His dose of propranolol was increased by the outpatient oncologists. The lesion continues to change in appearance, with no further growth and lightening and fading almost completely in some peripheral areas (Figure 4).",
"Figure 4.",
"The patient's growth is appropriate, his milestones are on track, and he is otherwise in good health."
],
"date": "May 15, 2018",
"figures": [
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/896/574/896574-Thumb3.png"
},
{
"caption": "Figure 4.",
"image_url": "https://img.medscapestatic.com/article/896/574/896574-Thumb4.png"
}
],
"markdown": "# A Newborn Infant With a Lumbar Lesion and Dermal Defect\n\n **Authors:** Dan Beardmore, DO; Saba Fatima, MD \n **Date:** May 15, 2018\n\n ## Content\n\n Propranolol was incidentally discovered in 2008 as a treatment for infantile hemangiomas and has now emerged as first-line therapy.[7] Propranolol's mechanism of action of has been hypothesized to include its effects by vasoconstriction and downregulation of growth factors.[7] It can be administered orally at low doses or applied topically, using drops or gels.\nThe most concerning possible side effects of propranolol include hypotension, bradycardia, hypoglycemia, hyperkalemia, and bronchospasm.[6] For these reasons, many clinicians and institutions choose to initially administer the medication to children during an inpatient visit, especially for younger patients, in whom oral administration is chosen over topical treatment.[7,8]\nThe infant in this case was admitted to a nearby children's hospital for initiation of oral propranolol to treat the infantile hemangioma. Before beginning the treatment, MRI of the spine was successfully obtained (without sedation). The MRI results identified an enhancing tract connecting the skin with the paraspinal soft tissues at the level of L4/L5; however, no definitive communication with the spinal canal was seen (although subtle communication could not be excluded). Surface thickening of the skin above the tract compatible with given history of an infantile hemangioma was noted. Finally, the conus medullaris terminated at L2-L3 spinal level, with no tethering, which was a normal location for the patient's age. No other abnormalities were reported.\nPropranolol was started, with a 3-day regimen: 0.5 mg/kg/day divided three times on the first day, then 1 mg/kg/day divided three times on the second day, then 2 mg/kg/day divided three times on the third day. Continuous monitoring and frequent vitals revealed a normal heart rate and blood pressure throughout. Electrolyte levels, including glucose levels, were also normal throughout.\nAfter tolerating the final goal dose of 2 mg/kg/day divided three times per day, the patient was discharged home with a 1-week follow up to assess the infantile hemangioma in the oncology clinic. A 2-month follow-up with neurosurgery to repeat the MRI of the lumbosacral region was also indicated.\nAt primary care follow-up 2 weeks later, the parents were pleased with the treatment and the interval improvement of the lesion (Figure 3). A single café au lait spot was also noted on his right upper back during this visit.\nFigure 3.\nThe patient was lost to follow-up for a short while; however, the family returned at 6 months for a check-up. His dose of propranolol was increased by the outpatient oncologists. The lesion continues to change in appearance, with no further growth and lightening and fading almost completely in some peripheral areas (Figure 4).\nFigure 4.\nThe patient's growth is appropriate, his milestones are on track, and he is otherwise in good health.\n\n ## Figures\n\n **Figure 3.** \n \n\n**Figure 4.** \n \n\n\n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1221188,
"choiceText": "To rule out underlying spinal dysraphism",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1221190,
"choiceText": "To rule out associated tethered cord",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1221192,
"choiceText": "To rule out cancer",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1221194,
"choiceText": "All of the above\r\n",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "MRI should be obtained in all infants who present with lumbosacral hemangiomas in order to rule out spinal dysraphism, associated tethered cord, and cancer. An isolated small lesion should also not be ignored, because it may be associated with occult spinal anomalies.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 388233,
"questionText": "Which of the following best explains the purpose of an MRI in the evaluation of a cutaneous lesion over the lumbosacral spine?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1221196,
"choiceText": "Hyperglycemia",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1221198,
"choiceText": "Bronchospasm ",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1221200,
"choiceText": "Tachycardia",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1221202,
"choiceText": "Hypokalemia\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Propranolol has emerged as an effective treatment for infantile hemangiomas predisposed to complications. Owing to potential side effects, inpatient monitoring for hypoglycemia, hypotension, bronchospasm, and bradycardia is usually the method used during initiation of therapy. ",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 388235,
"questionText": "Which of the following is a potential side effect of oral propranolol when used to treat infantile hemangiomas?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A Newborn Infant With a Lumbar Lesion and Dermal Defect"
},
{
"authors": "Dan Beardmore, DO; Saba Fatima, MD",
"content": [],
"date": "May 15, 2018",
"figures": [],
"markdown": "# A Newborn Infant With a Lumbar Lesion and Dermal Defect\n\n **Authors:** Dan Beardmore, DO; Saba Fatima, MD \n **Date:** May 15, 2018\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1221188,
"choiceText": "To rule out underlying spinal dysraphism",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1221190,
"choiceText": "To rule out associated tethered cord",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1221192,
"choiceText": "To rule out cancer",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1221194,
"choiceText": "All of the above\r\n",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "MRI should be obtained in all infants who present with lumbosacral hemangiomas in order to rule out spinal dysraphism, associated tethered cord, and cancer. An isolated small lesion should also not be ignored, because it may be associated with occult spinal anomalies.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 388233,
"questionText": "Which of the following best explains the purpose of an MRI in the evaluation of a cutaneous lesion over the lumbosacral spine?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1221196,
"choiceText": "Hyperglycemia",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1221198,
"choiceText": "Bronchospasm ",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1221200,
"choiceText": "Tachycardia",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1221202,
"choiceText": "Hypokalemia\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Propranolol has emerged as an effective treatment for infantile hemangiomas predisposed to complications. Owing to potential side effects, inpatient monitoring for hypoglycemia, hypotension, bronchospasm, and bradycardia is usually the method used during initiation of therapy. ",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 388235,
"questionText": "Which of the following is a potential side effect of oral propranolol when used to treat infantile hemangiomas?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A Newborn Infant With a Lumbar Lesion and Dermal Defect"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1221178,
"choiceText": "Arteriovenous malformation",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1221180,
"choiceText": "Infantile hemangioma",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1221182,
"choiceText": "Pyogenic granuloma",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1221184,
"choiceText": "Meningocele",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1221186,
"choiceText": "Neurodevelopmental tumor",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 388231,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1221188,
"choiceText": "To rule out underlying spinal dysraphism",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1221190,
"choiceText": "To rule out associated tethered cord",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1221192,
"choiceText": "To rule out cancer",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1221194,
"choiceText": "All of the above\r\n",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "MRI should be obtained in all infants who present with lumbosacral hemangiomas in order to rule out spinal dysraphism, associated tethered cord, and cancer. An isolated small lesion should also not be ignored, because it may be associated with occult spinal anomalies.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 388233,
"questionText": "Which of the following best explains the purpose of an MRI in the evaluation of a cutaneous lesion over the lumbosacral spine?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1221196,
"choiceText": "Hyperglycemia",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1221198,
"choiceText": "Bronchospasm ",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1221200,
"choiceText": "Tachycardia",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1221202,
"choiceText": "Hypokalemia\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Propranolol has emerged as an effective treatment for infantile hemangiomas predisposed to complications. Owing to potential side effects, inpatient monitoring for hypoglycemia, hypotension, bronchospasm, and bradycardia is usually the method used during initiation of therapy. ",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 388235,
"questionText": "Which of the following is a potential side effect of oral propranolol when used to treat infantile hemangiomas?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
896234 | /viewarticle/896234 | [
{
"authors": "Angela Castillo, MD; Alfredo Cabello Buscema, MD; Francisco Valdiviezo, MD",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"A 33-year-old woman presents to her primary care provider owing to a \"lump\" in the volar aspect of her right wrist. The lump started off as painless; however, over the course of 1 year, the patient progressively developed significant pain in the volar aspect of her right hand and wrist. The pain was associated with wrist paresthesias as well as decreased strength. She reports that she was limited at her job, where she works as a receptionist, and also has impaired routine functions of daily life.",
"She has no significant medical history. She does not report taking any medications. She does not describe any family history that is pertinent to her medical condition."
],
"date": "May 10, 2018",
"figures": [],
"markdown": "# A 33-Year-Old Woman With a Lump in Her Wrist\n\n **Authors:** Angela Castillo, MD; Alfredo Cabello Buscema, MD; Francisco Valdiviezo, MD \n **Date:** May 10, 2018\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nA 33-year-old woman presents to her primary care provider owing to a \"lump\" in the volar aspect of her right wrist. The lump started off as painless; however, over the course of 1 year, the patient progressively developed significant pain in the volar aspect of her right hand and wrist. The pain was associated with wrist paresthesias as well as decreased strength. She reports that she was limited at her job, where she works as a receptionist, and also has impaired routine functions of daily life.\nShe has no significant medical history. She does not report taking any medications. She does not describe any family history that is pertinent to her medical condition.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 33-Year-Old Woman With a Lump in Her Wrist"
},
{
"authors": "Angela Castillo, MD; Alfredo Cabello Buscema, MD; Francisco Valdiviezo, MD",
"content": [
"Upon physical examination, the patient appears to have a 2.5 cm × 2 cm immobile mass in the volar aspect of her right wrist (Figure 1).",
"Figure 1.",
"As she described, she demonstrates decreased muscle strength when using that hand. She also has decreased pinprick sensitivity in the fingers of that hand, sparing the fifth finger. The Tinel sign and Phalen maneuver are both positive. Her radial and cubital pulses are both normal. No tenderness is observed at the distal radial styloid process.",
"The remainder of the patient's physical examination is unremarkable."
],
"date": "May 10, 2018",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/896/234/896234-Thumb1.png"
}
],
"markdown": "# A 33-Year-Old Woman With a Lump in Her Wrist\n\n **Authors:** Angela Castillo, MD; Alfredo Cabello Buscema, MD; Francisco Valdiviezo, MD \n **Date:** May 10, 2018\n\n ## Content\n\n Upon physical examination, the patient appears to have a 2.5 cm × 2 cm immobile mass in the volar aspect of her right wrist (Figure 1).\nFigure 1.\nAs she described, she demonstrates decreased muscle strength when using that hand. She also has decreased pinprick sensitivity in the fingers of that hand, sparing the fifth finger. The Tinel sign and Phalen maneuver are both positive. Her radial and cubital pulses are both normal. No tenderness is observed at the distal radial styloid process.\nThe remainder of the patient's physical examination is unremarkable.\n\n ## Figures\n\n **Figure 1.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1218224,
"choiceText": "de Quervain tenosynovitis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1218226,
"choiceText": "Carpal tunnel syndrome",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1218228,
"choiceText": "Cervical radiculopathy ",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1218230,
"choiceText": "Peripheral neuropathy ",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1218232,
"choiceText": "Ulnar compressive neuropathy\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 387347,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 33-Year-Old Woman With a Lump in Her Wrist"
},
{
"authors": "Angela Castillo, MD; Alfredo Cabello Buscema, MD; Francisco Valdiviezo, MD",
"content": [
"Carpal tunnel syndrome (CTS) is the most common entrapment neuropathy of the upper extremities. CTS is caused by chronic and focal compression of the median nerve trunk through the carpal tunnel, causing demyelization.[1] Patients typically present with paresthesias in the wrist and sometimes in the distribution of the median nerve (palmar aspect of the first, second, and third fingers; radial half of the fourth finger). Symptoms are typically worst upon arising in the morning or after repetitive use.",
"CTS is more common in women than in men and has a peak of prevalence after age 55 years.[2] CTS has multiple etiologies and is related to any condition that acts as an \"occupation space\" in this limited anatomical area.",
"CTS may occur with the following:",
"Endocrine conditions: diabetes, hypothyroidism, obesity, acromegaly, mucopolysaccharidoses",
"Rheumatologic conditions: polymyalgia rheumatica, rheumatoid arthritis, amyloidosis, monoclonal gammopathies",
"Congenital disorders: anatomical anomalies, skeletal dysplasia",
"CTS may also be posttraumatic (eg, job or sports-related injuries) or occur concurrent with other nerve compression syndromes (especially ulnar tunnel syndrome).",
"The patient in this case has CTS due to a volar ganglion cyst that acted as an \"occupation space\" lesion from the distal aspect of the radius to the tubercle of scaphoid bone. Another volar ganglion originates from the scaphotrapezium capsule.",
"Diagnosis of CTS is clinical and should include the following[3]:",
"Tinel test: A positive Tinel sign consists of triggering the symptoms (pain or paresthesias in the fingers innervated by the median nerve) after repeatedly tapping the volar surface of the patient's transverse carpal ligament. It has a sensitivity of 36%-50% and a specificity of 77% for CTS.",
"Phalen maneuver: With a positive Phalen maneuver, symptoms are triggered after flexing the patient's wrist 90°, with the elbow in full extension and maintaining this position for 60 seconds. The Phalen maneuver has a sensitivity of 57%-68% and a specificity of 58%-73% for CTS. When performed in combination with the median nerve compression test, the sensitivity increases to 80% and the specificity increases to 92%.",
"Median nerve compression test: The symptoms appear within 30 seconds of applying direct pressure over the transverse carpal ligament. A positive finding has a sensitivity of 64% and a specificity of 83% for CTS.",
"Hand elevation test: A positive test result consists of the appearance of symptoms after the patient raises his or her hands above the head for 1 minute. It has similar sensitivity and specificity to that of the Phalen maneuver and the Tinel test.",
"Flick sign: This sign is positive when the patient wakes with symptoms that are alleviated by shaking the hand. It has 93% sensitivity and 96% specificity for CTS.",
"Thumb abduction weakness test: If positive, the symptoms appear after raising the patient's thumb perpendicular to the palm, followed by downward pressure on the distal phalange while the patient resists. It has a sensitivity of 29%-65% and a specificity of 65%-80% for CTS.",
"Thenar atrophy assessment: The presence of thenar atrophy has a sensitivity of 12%-16% and a specificity of 90%-94% for CTS.",
"Hypoalgesia test: A positive result occurs with decreased sensation to pain on the palmar aspect of the second finger (compared with the fifth finger on the same hand). It has a sensitivity of 39% and a specificity of 88% for CTS."
],
"date": "May 10, 2018",
"figures": [],
"markdown": "# A 33-Year-Old Woman With a Lump in Her Wrist\n\n **Authors:** Angela Castillo, MD; Alfredo Cabello Buscema, MD; Francisco Valdiviezo, MD \n **Date:** May 10, 2018\n\n ## Content\n\n Carpal tunnel syndrome (CTS) is the most common entrapment neuropathy of the upper extremities. CTS is caused by chronic and focal compression of the median nerve trunk through the carpal tunnel, causing demyelization.[1] Patients typically present with paresthesias in the wrist and sometimes in the distribution of the median nerve (palmar aspect of the first, second, and third fingers; radial half of the fourth finger). Symptoms are typically worst upon arising in the morning or after repetitive use.\nCTS is more common in women than in men and has a peak of prevalence after age 55 years.[2] CTS has multiple etiologies and is related to any condition that acts as an \"occupation space\" in this limited anatomical area.\nCTS may occur with the following:\nEndocrine conditions: diabetes, hypothyroidism, obesity, acromegaly, mucopolysaccharidoses\nRheumatologic conditions: polymyalgia rheumatica, rheumatoid arthritis, amyloidosis, monoclonal gammopathies\nCongenital disorders: anatomical anomalies, skeletal dysplasia\nCTS may also be posttraumatic (eg, job or sports-related injuries) or occur concurrent with other nerve compression syndromes (especially ulnar tunnel syndrome).\nThe patient in this case has CTS due to a volar ganglion cyst that acted as an \"occupation space\" lesion from the distal aspect of the radius to the tubercle of scaphoid bone. Another volar ganglion originates from the scaphotrapezium capsule.\nDiagnosis of CTS is clinical and should include the following[3]:\nTinel test: A positive Tinel sign consists of triggering the symptoms (pain or paresthesias in the fingers innervated by the median nerve) after repeatedly tapping the volar surface of the patient's transverse carpal ligament. It has a sensitivity of 36%-50% and a specificity of 77% for CTS.\nPhalen maneuver: With a positive Phalen maneuver, symptoms are triggered after flexing the patient's wrist 90°, with the elbow in full extension and maintaining this position for 60 seconds. The Phalen maneuver has a sensitivity of 57%-68% and a specificity of 58%-73% for CTS. When performed in combination with the median nerve compression test, the sensitivity increases to 80% and the specificity increases to 92%.\nMedian nerve compression test: The symptoms appear within 30 seconds of applying direct pressure over the transverse carpal ligament. A positive finding has a sensitivity of 64% and a specificity of 83% for CTS.\nHand elevation test: A positive test result consists of the appearance of symptoms after the patient raises his or her hands above the head for 1 minute. It has similar sensitivity and specificity to that of the Phalen maneuver and the Tinel test.\nFlick sign: This sign is positive when the patient wakes with symptoms that are alleviated by shaking the hand. It has 93% sensitivity and 96% specificity for CTS.\nThumb abduction weakness test: If positive, the symptoms appear after raising the patient's thumb perpendicular to the palm, followed by downward pressure on the distal phalange while the patient resists. It has a sensitivity of 29%-65% and a specificity of 65%-80% for CTS.\nThenar atrophy assessment: The presence of thenar atrophy has a sensitivity of 12%-16% and a specificity of 90%-94% for CTS.\nHypoalgesia test: A positive result occurs with decreased sensation to pain on the palmar aspect of the second finger (compared with the fifth finger on the same hand). It has a sensitivity of 39% and a specificity of 88% for CTS.\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1218224,
"choiceText": "de Quervain tenosynovitis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1218226,
"choiceText": "Carpal tunnel syndrome",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1218228,
"choiceText": "Cervical radiculopathy ",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1218230,
"choiceText": "Peripheral neuropathy ",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1218232,
"choiceText": "Ulnar compressive neuropathy\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 387347,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 33-Year-Old Woman With a Lump in Her Wrist"
},
{
"authors": "Angela Castillo, MD; Alfredo Cabello Buscema, MD; Francisco Valdiviezo, MD",
"content": [
"Differential diagnoses for CTS include the following:",
"Carpometacarpal arthritis of the thumb: Pain is localized, and grind test results are positive. Diagnosis is confirmed by radiologic findings.",
"Cervical radiculopathy (C6): Neck pain radiates to the fingers with numbness. Only the thumb and index finger are involved.",
"de Quervain tenosynovitis: Tenderness is observed at the distal radial styloid process.",
"Peripheral neuropathy: Bilateral symptoms are observed. The lower extremities are probably involved, as well as additional findings beyond the respective neuropathy.",
"Wrist arthritis: Wrist motion is painful, and radiographic findings are diagnostic.",
"Raynaud syndrome: The fingers change color, precipitated by cold exposure.",
"Ulnar compressive neuropathy: Paresthesias of the fourth and fifth fingers are observed. The Tinel sign is positive. Compression testing at the elbow or wrist canal confirms the diagnosis.",
"CTS is diagnosed by clinical findings. The use of electrophysiologic tools to determine disease extent and prognosis is necessary only in selected cases. These selected cases are probably candidates for surgical intervention. Electrophysiologic studies have a sensitivity of 56%-85% and a specificity of 94%-99% for CTS.[4]",
"Radiographic studies are useful when anatomical abnormalities (eg, associated bone fracture, rheumatologic damage) are suspected. Ultrasonography may be used not only as a diagnostic tool but also as guidance for local steroid injection. MRI is not routinely used because the imaging features are frequently nonspecific and histologic examination is required to establish the final diagnosis. In addition, the cost of the test limits its use. In some cases, it is valuable after surgery."
],
"date": "May 10, 2018",
"figures": [],
"markdown": "# A 33-Year-Old Woman With a Lump in Her Wrist\n\n **Authors:** Angela Castillo, MD; Alfredo Cabello Buscema, MD; Francisco Valdiviezo, MD \n **Date:** May 10, 2018\n\n ## Content\n\n Differential diagnoses for CTS include the following:\nCarpometacarpal arthritis of the thumb: Pain is localized, and grind test results are positive. Diagnosis is confirmed by radiologic findings.\nCervical radiculopathy (C6): Neck pain radiates to the fingers with numbness. Only the thumb and index finger are involved.\nde Quervain tenosynovitis: Tenderness is observed at the distal radial styloid process.\nPeripheral neuropathy: Bilateral symptoms are observed. The lower extremities are probably involved, as well as additional findings beyond the respective neuropathy.\nWrist arthritis: Wrist motion is painful, and radiographic findings are diagnostic.\nRaynaud syndrome: The fingers change color, precipitated by cold exposure.\nUlnar compressive neuropathy: Paresthesias of the fourth and fifth fingers are observed. The Tinel sign is positive. Compression testing at the elbow or wrist canal confirms the diagnosis.\nCTS is diagnosed by clinical findings. The use of electrophysiologic tools to determine disease extent and prognosis is necessary only in selected cases. These selected cases are probably candidates for surgical intervention. Electrophysiologic studies have a sensitivity of 56%-85% and a specificity of 94%-99% for CTS.[4]\nRadiographic studies are useful when anatomical abnormalities (eg, associated bone fracture, rheumatologic damage) are suspected. Ultrasonography may be used not only as a diagnostic tool but also as guidance for local steroid injection. MRI is not routinely used because the imaging features are frequently nonspecific and histologic examination is required to establish the final diagnosis. In addition, the cost of the test limits its use. In some cases, it is valuable after surgery.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 33-Year-Old Woman With a Lump in Her Wrist"
},
{
"authors": "Angela Castillo, MD; Alfredo Cabello Buscema, MD; Francisco Valdiviezo, MD",
"content": [
"Conservative treatment should always be the first option for CTS (eg, splint, steroid injection, physical therapy, yoga). In mild to moderate cases, wrist splinting at night for 3 weeks often results in remission. Local steroid injection can induce remission and often provides relief for 1-2 months. In severe cases, it can delay surgery for as long as 1 year. Nonsteroidal anti-inflammatory drugs, diuretics, and vitamin B6 are typically ineffective.",
"Surgical management is indicated in severe cases without improvement 4-6 months after conservative measures are initiated.[5,6] These patients usually have an inability to distinguish between two points < 6 mm apart, weakness of the thumb abduction, opposition and atrophy of the thenar compartment, and/or any other additional clinical features that complicate the natural course of the disease. The standard treatment approach is surgical decompression of the median nerve in the carpal tunnel by section of the flexor retinaculum. Endoscopic and open approaches are equally effective; however, patients return to work 1 week earlier with endoscopic techniques.",
"Some patients develop postoperative incisional pain that is related to injury of the fat pad situated between the palmar carpal ligament and the flexor retinaculum. The development of neuroma due to injury of the transverse branches of the palmar cutaneous nerves is another concern. Therefore, some surgeons have developed a modified technique that respects the integrity of the subretinacularis fat pad.",
"In this case, through a volar longitudinal approach of the wrist, the surgical team performed a broad resection of the cystic tumor attached to the flexor retinaculum (Figures 2-4).",
"Figure 2.",
"Figure 3.",
"Figure 4.",
"The mass also involved the palmaris longus and flexor carpi radialis tendon. After the resection, compression of the median nerve was released. A 3 cm × 2.5 cm histologic sample of a bland and brown cyst was sent for pathologic examination and was reported to be a synovial cyst with chronic inflammation and vascular congestion. The patient presented for follow-up 3 weeks after the surgical intervention and reported complete resolution of her symptoms."
],
"date": "May 10, 2018",
"figures": [
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/896/234/896234-Thumb2.png"
},
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/896/234/896234-Thumb3.png"
},
{
"caption": "Figure 4.",
"image_url": "https://img.medscapestatic.com/article/896/234/896234-Thumb4.png"
}
],
"markdown": "# A 33-Year-Old Woman With a Lump in Her Wrist\n\n **Authors:** Angela Castillo, MD; Alfredo Cabello Buscema, MD; Francisco Valdiviezo, MD \n **Date:** May 10, 2018\n\n ## Content\n\n Conservative treatment should always be the first option for CTS (eg, splint, steroid injection, physical therapy, yoga). In mild to moderate cases, wrist splinting at night for 3 weeks often results in remission. Local steroid injection can induce remission and often provides relief for 1-2 months. In severe cases, it can delay surgery for as long as 1 year. Nonsteroidal anti-inflammatory drugs, diuretics, and vitamin B6 are typically ineffective.\nSurgical management is indicated in severe cases without improvement 4-6 months after conservative measures are initiated.[5,6] These patients usually have an inability to distinguish between two points < 6 mm apart, weakness of the thumb abduction, opposition and atrophy of the thenar compartment, and/or any other additional clinical features that complicate the natural course of the disease. The standard treatment approach is surgical decompression of the median nerve in the carpal tunnel by section of the flexor retinaculum. Endoscopic and open approaches are equally effective; however, patients return to work 1 week earlier with endoscopic techniques.\nSome patients develop postoperative incisional pain that is related to injury of the fat pad situated between the palmar carpal ligament and the flexor retinaculum. The development of neuroma due to injury of the transverse branches of the palmar cutaneous nerves is another concern. Therefore, some surgeons have developed a modified technique that respects the integrity of the subretinacularis fat pad.\nIn this case, through a volar longitudinal approach of the wrist, the surgical team performed a broad resection of the cystic tumor attached to the flexor retinaculum (Figures 2-4).\nFigure 2.\nFigure 3.\nFigure 4.\nThe mass also involved the palmaris longus and flexor carpi radialis tendon. After the resection, compression of the median nerve was released. A 3 cm × 2.5 cm histologic sample of a bland and brown cyst was sent for pathologic examination and was reported to be a synovial cyst with chronic inflammation and vascular congestion. The patient presented for follow-up 3 weeks after the surgical intervention and reported complete resolution of her symptoms.\n\n ## Figures\n\n **Figure 2.** \n \n\n**Figure 3.** \n \n\n**Figure 4.** \n \n\n\n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1218234,
"choiceText": "MRI",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1218236,
"choiceText": "Radiography",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1218238,
"choiceText": "Ultrasonography",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1218240,
"choiceText": "Electrophysiologic studies\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "CTS is diagnosed by clinical findings. The use of electrophysiologic tools to determine disease extent and prognosis is necessary only in selected cases. Radiographic studies are useful when anatomical abnormalities (eg, associated bone fracture, rheumatologic damage) are suspected. Ultrasonography may be used not only as a diagnostic tool but also as guidance for local steroid injection. MRI is not routinely used because the imaging features are frequently nonspecific and histologic examination is required to establish the final diagnosis. In addition, the cost of the test limits its use.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 387349,
"questionText": "Which of the following is routinely recommended for patients with potential CTS without suspected anatomical abnormalities?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1218242,
"choiceText": "Open approaches are generally preferred to endoscopic approaches",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1218244,
"choiceText": "Endoscopic approaches are generally preferred to open approaches",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1218246,
"choiceText": "Surgical treatment is recommended in patients who have not improved in 3-5 weeks after initiation of conservative treatment measures",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1218248,
"choiceText": "Surgical decompression of the median nerve in the carpal tunnel by section of the flexor retinaculum is the standard approach\r\n",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Surgical management is indicated in severe cases that have not improved 4-6 months after conservative measures are initiated. These patients usually have an inability to distinguish between two points < 6 mm apart, weakness of the thumb abduction, opposition and atrophy of the thenar compartment, and/or any other additional clinical features that complicate the natural course of the disease. The standard treatment approach is surgical decompression of the median nerve in the carpal tunnel by section of the flexor retinaculum. Endoscopic and open approaches are equally effective; however, patients return to work 1 week earlier with endoscopic techniques. ",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 387351,
"questionText": "Which of the following is accurate about surgical approaches to CTS?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 33-Year-Old Woman With a Lump in Her Wrist"
},
{
"authors": "Angela Castillo, MD; Alfredo Cabello Buscema, MD; Francisco Valdiviezo, MD",
"content": [],
"date": "May 10, 2018",
"figures": [],
"markdown": "# A 33-Year-Old Woman With a Lump in Her Wrist\n\n **Authors:** Angela Castillo, MD; Alfredo Cabello Buscema, MD; Francisco Valdiviezo, MD \n **Date:** May 10, 2018\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1218234,
"choiceText": "MRI",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1218236,
"choiceText": "Radiography",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1218238,
"choiceText": "Ultrasonography",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1218240,
"choiceText": "Electrophysiologic studies\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "CTS is diagnosed by clinical findings. The use of electrophysiologic tools to determine disease extent and prognosis is necessary only in selected cases. Radiographic studies are useful when anatomical abnormalities (eg, associated bone fracture, rheumatologic damage) are suspected. Ultrasonography may be used not only as a diagnostic tool but also as guidance for local steroid injection. MRI is not routinely used because the imaging features are frequently nonspecific and histologic examination is required to establish the final diagnosis. In addition, the cost of the test limits its use.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 387349,
"questionText": "Which of the following is routinely recommended for patients with potential CTS without suspected anatomical abnormalities?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1218242,
"choiceText": "Open approaches are generally preferred to endoscopic approaches",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1218244,
"choiceText": "Endoscopic approaches are generally preferred to open approaches",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1218246,
"choiceText": "Surgical treatment is recommended in patients who have not improved in 3-5 weeks after initiation of conservative treatment measures",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1218248,
"choiceText": "Surgical decompression of the median nerve in the carpal tunnel by section of the flexor retinaculum is the standard approach\r\n",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Surgical management is indicated in severe cases that have not improved 4-6 months after conservative measures are initiated. These patients usually have an inability to distinguish between two points < 6 mm apart, weakness of the thumb abduction, opposition and atrophy of the thenar compartment, and/or any other additional clinical features that complicate the natural course of the disease. The standard treatment approach is surgical decompression of the median nerve in the carpal tunnel by section of the flexor retinaculum. Endoscopic and open approaches are equally effective; however, patients return to work 1 week earlier with endoscopic techniques. ",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 387351,
"questionText": "Which of the following is accurate about surgical approaches to CTS?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 33-Year-Old Woman With a Lump in Her Wrist"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1218224,
"choiceText": "de Quervain tenosynovitis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1218226,
"choiceText": "Carpal tunnel syndrome",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1218228,
"choiceText": "Cervical radiculopathy ",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1218230,
"choiceText": "Peripheral neuropathy ",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1218232,
"choiceText": "Ulnar compressive neuropathy\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 387347,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1218234,
"choiceText": "MRI",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1218236,
"choiceText": "Radiography",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1218238,
"choiceText": "Ultrasonography",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1218240,
"choiceText": "Electrophysiologic studies\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "CTS is diagnosed by clinical findings. The use of electrophysiologic tools to determine disease extent and prognosis is necessary only in selected cases. Radiographic studies are useful when anatomical abnormalities (eg, associated bone fracture, rheumatologic damage) are suspected. Ultrasonography may be used not only as a diagnostic tool but also as guidance for local steroid injection. MRI is not routinely used because the imaging features are frequently nonspecific and histologic examination is required to establish the final diagnosis. In addition, the cost of the test limits its use.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 387349,
"questionText": "Which of the following is routinely recommended for patients with potential CTS without suspected anatomical abnormalities?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1218242,
"choiceText": "Open approaches are generally preferred to endoscopic approaches",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1218244,
"choiceText": "Endoscopic approaches are generally preferred to open approaches",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1218246,
"choiceText": "Surgical treatment is recommended in patients who have not improved in 3-5 weeks after initiation of conservative treatment measures",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1218248,
"choiceText": "Surgical decompression of the median nerve in the carpal tunnel by section of the flexor retinaculum is the standard approach\r\n",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Surgical management is indicated in severe cases that have not improved 4-6 months after conservative measures are initiated. These patients usually have an inability to distinguish between two points < 6 mm apart, weakness of the thumb abduction, opposition and atrophy of the thenar compartment, and/or any other additional clinical features that complicate the natural course of the disease. The standard treatment approach is surgical decompression of the median nerve in the carpal tunnel by section of the flexor retinaculum. Endoscopic and open approaches are equally effective; however, patients return to work 1 week earlier with endoscopic techniques. ",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 387351,
"questionText": "Which of the following is accurate about surgical approaches to CTS?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
895449 | /viewarticle/895449 | [
{
"authors": "Rebecca Winderman, MD; Natalie Banniettis, MD; Simon S. Rabinowitz, MD, PhD",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"A 10-year-old boy who emigrated from Guinea, West Africa, 6 months ago presents with splenomegaly lasting several years. His mother reports that he had lived in a small village in Guinea with his grandmother since birth. Starting at age 5 years, the patient began complaining of left-sided abdominal pain. A workup performed in Guinea was inconclusive, according to his grandmother.",
"He was the product of a full-term vaginal delivery and weighed 9 lb (4 kg) at birth. Although the patient has always been small for his age (third to fifth percentile), he has cognitively developed appropriately alongside his peers.",
"He has no significant medical history. The patient has two younger female siblings who are currently residing in the United States; both are reportedly healthy. Routine vaccinations are up to date."
],
"date": "April 23, 2018",
"figures": [],
"markdown": "# Chronic Splenomegaly in a 10-Year-Old Boy\n\n **Authors:** Rebecca Winderman, MD; Natalie Banniettis, MD; Simon S. Rabinowitz, MD, PhD \n **Date:** April 23, 2018\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nA 10-year-old boy who emigrated from Guinea, West Africa, 6 months ago presents with splenomegaly lasting several years. His mother reports that he had lived in a small village in Guinea with his grandmother since birth. Starting at age 5 years, the patient began complaining of left-sided abdominal pain. A workup performed in Guinea was inconclusive, according to his grandmother.\nHe was the product of a full-term vaginal delivery and weighed 9 lb (4 kg) at birth. Although the patient has always been small for his age (third to fifth percentile), he has cognitively developed appropriately alongside his peers.\nHe has no significant medical history. The patient has two younger female siblings who are currently residing in the United States; both are reportedly healthy. Routine vaccinations are up to date.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "Chronic Splenomegaly in a 10-Year-Old Boy"
},
{
"authors": "Rebecca Winderman, MD; Natalie Banniettis, MD; Simon S. Rabinowitz, MD, PhD",
"content": [
"Upon physical examination, a review of systems is negative for fevers, rashes, fatigue, seizures, jaundice, vomiting, diarrhea, behavioral changes, easy bruising, and musculoskeletal or visual disturbances.",
"His weight is 53.5 lb (24.25 kg), which puts him beneath the fifth percentile for his age. His height is 4'1\" (125 cm), which also is beneath the fifth percentile for his age. A head, eyes, ears, nose, and throat examination reveals anicteric, moist pink conjunctiva and shoddy mobile anterior cervical lymphadenopathy (largest, <1 cm). He has no rash, edema, joint swelling, warmth, or tenderness.",
"His lungs are clear to auscultation. Bilateral breath sounds are equal. His abdomen is soft and distended, with normal bowel sounds. His liver is 3 cm below the right costal margin. It is smooth, nontender, and not firm. His spleen is palpable 10 cm below the left costal margin and is 6 cm wide. It is nontender. No other tenderness or peritoneal signs are noted.",
"Laboratory findings are as follows:",
"White blood cell (WBC) count—5.7 x 109/L",
"Hemoglobin level—9.7 g/dL (hemoglobin electrophoresis is normal)",
"Hematocrit level—30.4%",
"Mean corpuscular volume—77.5 fL/red cell",
"Platelet count—128 x 109/L",
"Neutrophil count—41.9%",
"Lymphocyte count—40.1%",
"Eosinophil count—8.9%",
"Reticulocyte count—7%",
"Comprehensive metabolic panel results are as follows:",
"Sodium level—138 mEq/L",
"Potassium level—3.7 mEq/L",
"Chloride level—103 mEq/L",
"Carbon dioxide level—27 mEq/L",
"Glucose level—101 mmol/L",
"Blood urea nitrogen level—9 mmol/L",
"Creatinine level—0.34 mg/dL",
"Total protein level—7.9 g/dL",
"Albumin level—4.5 g/L",
"Calcium level—10.2 mg/dL",
"Total bilirubin level—1.9 mg/dL",
"Direct bilirubin level—0.3 mg/dL",
"Alkaline phosphatase level—206 IU/L",
"Aspartate aminotransferase level—26 IU/L",
"Alanine aminotransferase level—13 IU/L",
"Urinalysis reveals clear urine with a trace of blood. It is negative for urobilinogen, nitrate, leukocyte esterase, glucose, and ketones. Occasional bacteria is noted, with no other organisms seen. A urine culture reveals no growth. Other urinalysis findings are as follows:",
"Specific gravity—1.015",
"pH level—6.5",
"Protein level—100 mg/dL",
"Red blood cell (RBC) count—9 RBC/high-powered field (HPF)",
"WBC count—40 WBC/HPF",
"Stool tests negative for the presence of blood. An immune and metabolic workup is negative for antinuclear antibody and rheumatoid factor. Ferritin level is normal, as is the urine glycosaminoglycan level. A T-cell subset panel reveals normal findings. Other findings are as follows:",
"Erythrocyte sedimentation rate—55 mm/hr",
"C-reactive protein level—<4 mg/L",
"Lactate dehydrogenase level—245 IU/L",
"Immunoglobulin (Ig) M level—156 mg/dL",
"IgG level—2053 mg/dL",
"IgA level—75.9 mg/dL",
"IgE level—355 mg/dL",
"Further evaluation looked at exposure to infectious agents. The patient's Epstein-Barr virus results are negative. Hepatitis C antibody, hepatitis B surface antigen, and HIV tests are negative. Quantiferon testing for latent tuberculosis is negative. A malaria blood smear is also negative. The patient tests negative to the following organisms:",
"Schistosoma",
"Strongyloides",
"Echinococcus",
"Cryptosporidium",
"Giardia",
"By abdominal ultrasonography, the patient's liver is 12.1 cm with normal echogenicity, no nodularity, and no intrahepatic or extrahepatic ductal dilatation. The common bile duct is 2.4 mm. No mass is identified. His spleen is 15 cm, with tortuosity and dilatation of the splenic vasculature (Figure).",
"Figure."
],
"date": "April 23, 2018",
"figures": [
{
"caption": "Figure.",
"image_url": "https://img.medscapestatic.com/article/895/449/895449-Thumb1.png"
}
],
"markdown": "# Chronic Splenomegaly in a 10-Year-Old Boy\n\n **Authors:** Rebecca Winderman, MD; Natalie Banniettis, MD; Simon S. Rabinowitz, MD, PhD \n **Date:** April 23, 2018\n\n ## Content\n\n Upon physical examination, a review of systems is negative for fevers, rashes, fatigue, seizures, jaundice, vomiting, diarrhea, behavioral changes, easy bruising, and musculoskeletal or visual disturbances.\nHis weight is 53.5 lb (24.25 kg), which puts him beneath the fifth percentile for his age. His height is 4'1\" (125 cm), which also is beneath the fifth percentile for his age. A head, eyes, ears, nose, and throat examination reveals anicteric, moist pink conjunctiva and shoddy mobile anterior cervical lymphadenopathy (largest, <1 cm). He has no rash, edema, joint swelling, warmth, or tenderness.\nHis lungs are clear to auscultation. Bilateral breath sounds are equal. His abdomen is soft and distended, with normal bowel sounds. His liver is 3 cm below the right costal margin. It is smooth, nontender, and not firm. His spleen is palpable 10 cm below the left costal margin and is 6 cm wide. It is nontender. No other tenderness or peritoneal signs are noted.\nLaboratory findings are as follows:\nWhite blood cell (WBC) count—5.7 x 109/L\nHemoglobin level—9.7 g/dL (hemoglobin electrophoresis is normal)\nHematocrit level—30.4%\nMean corpuscular volume—77.5 fL/red cell\nPlatelet count—128 x 109/L\nNeutrophil count—41.9%\nLymphocyte count—40.1%\nEosinophil count—8.9%\nReticulocyte count—7%\nComprehensive metabolic panel results are as follows:\nSodium level—138 mEq/L\nPotassium level—3.7 mEq/L\nChloride level—103 mEq/L\nCarbon dioxide level—27 mEq/L\nGlucose level—101 mmol/L\nBlood urea nitrogen level—9 mmol/L\nCreatinine level—0.34 mg/dL\nTotal protein level—7.9 g/dL\nAlbumin level—4.5 g/L\nCalcium level—10.2 mg/dL\nTotal bilirubin level—1.9 mg/dL\nDirect bilirubin level—0.3 mg/dL\nAlkaline phosphatase level—206 IU/L\nAspartate aminotransferase level—26 IU/L\nAlanine aminotransferase level—13 IU/L\nUrinalysis reveals clear urine with a trace of blood. It is negative for urobilinogen, nitrate, leukocyte esterase, glucose, and ketones. Occasional bacteria is noted, with no other organisms seen. A urine culture reveals no growth. Other urinalysis findings are as follows:\nSpecific gravity—1.015\npH level—6.5\nProtein level—100 mg/dL\nRed blood cell (RBC) count—9 RBC/high-powered field (HPF)\nWBC count—40 WBC/HPF\nStool tests negative for the presence of blood. An immune and metabolic workup is negative for antinuclear antibody and rheumatoid factor. Ferritin level is normal, as is the urine glycosaminoglycan level. A T-cell subset panel reveals normal findings. Other findings are as follows:\nErythrocyte sedimentation rate—55 mm/hr\nC-reactive protein level—<4 mg/L\nLactate dehydrogenase level—245 IU/L\nImmunoglobulin (Ig) M level—156 mg/dL\nIgG level—2053 mg/dL\nIgA level—75.9 mg/dL\nIgE level—355 mg/dL\nFurther evaluation looked at exposure to infectious agents. The patient's Epstein-Barr virus results are negative. Hepatitis C antibody, hepatitis B surface antigen, and HIV tests are negative. Quantiferon testing for latent tuberculosis is negative. A malaria blood smear is also negative. The patient tests negative to the following organisms:\nSchistosoma\nStrongyloides\nEchinococcus\nCryptosporidium\nGiardia\nBy abdominal ultrasonography, the patient's liver is 12.1 cm with normal echogenicity, no nodularity, and no intrahepatic or extrahepatic ductal dilatation. The common bile duct is 2.4 mm. No mass is identified. His spleen is 15 cm, with tortuosity and dilatation of the splenic vasculature (Figure).\nFigure.\n\n ## Figures\n\n **Figure.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1213404,
"choiceText": "Sickle cell disease",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1213406,
"choiceText": "Chronic malaria ",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1213408,
"choiceText": "Leukemia ",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1213410,
"choiceText": "Lymphoma ",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1213412,
"choiceText": "Lipid storage disease\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 385851,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Chronic Splenomegaly in a 10-Year-Old Boy"
},
{
"authors": "Rebecca Winderman, MD; Natalie Banniettis, MD; Simon S. Rabinowitz, MD, PhD",
"content": [
"This child was a recent immigrant from Guinea, where malaria is endemic. Despite negative smears for malaria, a high index of suspicion prompted molecular testing (polymerase chain reaction [PCR]), which was positive for Plasmodium falciparum. P falciparum is responsible for more than 85% of endemic malaria species in Guinea. This child was diagnosed with hyperreactive malarial syndrome (HMS), also known as tropical splenomegaly syndrome. HMS is the leading cause of massive splenomegaly in malaria-endemic countries.[1] The incidence ranges from 0.5% to 80% of the adult population in endemic countries. The pathogenesis is unknown but has been postulated to result from chronic antigenic stimulation due to long-term exposure to the malaria parasite in the blood. This parasite is initially obtained via a bite from an infected mosquito. The highest prevalence is in Papua New Guinea (≤80%), with an estimated 3-year fatality rate of 36%.[2]",
"The patient in this case presented with long-standing splenomegaly, anemia, a slightly elevated IgG level, and a history of residing in a highly endemic area, making the diagnosis of HMS most likely.",
"Sickle cell disease is associated with splenomegaly that can evolve into splenic sequestration. However, this child had normal hemoglobin electrophoresis findings and had long-standing splenomegaly. By his age, a patient with sickle cell disease would have likely had infarction of the organ rather than stable enlargement.",
"Leukemia, lymphoma, or other less commonly encountered primary or metastatic pediatric malignancies may present with splenomegaly and an elevated erythrocyte sedimentation rate. However, in this case, the patient's history of a long-standing, chronic process without any clinical progression made this possibility unlikely. Furthermore, the imaging and blood tests did not suggest a heterogeneous organ or reveal any malignant cells.",
"Several storage diseases can present with splenomegaly. The mucopolysaccharidoses are a group of disorders that involve a defect in lysosomal enzymes, which degrade mucopolysaccharides. Patients initially develop normally, with the onset of abnormalities later in infancy or childhood. These patients often have multiorgan involvement, including neurologic, cardiovascular, pulmonary, ophthalmologic, hearing, and musculoskeletal findings. Diagnosis is initially made by measuring urine glycosaminoglycans, which were negative in this case.",
"Other common examples of rarely encountered lipid storage diseases include Gaucher disease or Fabry disease. Affected individuals usually have autosomal recessive inheritance of an enzyme mutation that prevents complete metabolism of a naturally occurring precursor. Abnormal metabolites then accumulate in the reticuloendothelial system, yielding enlargement of the spleen and liver. Oftentimes central nervous system or other organ system involvement is present; however, splenomegaly can be isolated. Definitive diagnosis is made by testing for the individual enzyme that is affected. If the patient in this case did not have a positive PCR result for malaria, peripheral blood leukocytes would have been obtained to measure glucocerebrosidase activity as a test for Gaucher disease.",
"Gaucher disease can present with splenomegaly, usually in the setting of concurrent hepatomegaly, pancytopenia (particularly anemia and thrombocytopenia), and bone involvement. Patients with Gaucher disease often have failure to thrive. In the case of Gaucher type 2 and 3, individuals have neurologic involvement. Although this patient had significant splenomegaly, he did not have the typical pancytopenia or hepatomegaly associated with Gaucher disease and had normal enzyme activity. Furthermore, Gaucher disease type 2 and 3 involves neurologic dysfunction, which the patient did not have."
],
"date": "April 23, 2018",
"figures": [],
"markdown": "# Chronic Splenomegaly in a 10-Year-Old Boy\n\n **Authors:** Rebecca Winderman, MD; Natalie Banniettis, MD; Simon S. Rabinowitz, MD, PhD \n **Date:** April 23, 2018\n\n ## Content\n\n This child was a recent immigrant from Guinea, where malaria is endemic. Despite negative smears for malaria, a high index of suspicion prompted molecular testing (polymerase chain reaction [PCR]), which was positive for Plasmodium falciparum. P falciparum is responsible for more than 85% of endemic malaria species in Guinea. This child was diagnosed with hyperreactive malarial syndrome (HMS), also known as tropical splenomegaly syndrome. HMS is the leading cause of massive splenomegaly in malaria-endemic countries.[1] The incidence ranges from 0.5% to 80% of the adult population in endemic countries. The pathogenesis is unknown but has been postulated to result from chronic antigenic stimulation due to long-term exposure to the malaria parasite in the blood. This parasite is initially obtained via a bite from an infected mosquito. The highest prevalence is in Papua New Guinea (≤80%), with an estimated 3-year fatality rate of 36%.[2]\nThe patient in this case presented with long-standing splenomegaly, anemia, a slightly elevated IgG level, and a history of residing in a highly endemic area, making the diagnosis of HMS most likely.\nSickle cell disease is associated with splenomegaly that can evolve into splenic sequestration. However, this child had normal hemoglobin electrophoresis findings and had long-standing splenomegaly. By his age, a patient with sickle cell disease would have likely had infarction of the organ rather than stable enlargement.\nLeukemia, lymphoma, or other less commonly encountered primary or metastatic pediatric malignancies may present with splenomegaly and an elevated erythrocyte sedimentation rate. However, in this case, the patient's history of a long-standing, chronic process without any clinical progression made this possibility unlikely. Furthermore, the imaging and blood tests did not suggest a heterogeneous organ or reveal any malignant cells.\nSeveral storage diseases can present with splenomegaly. The mucopolysaccharidoses are a group of disorders that involve a defect in lysosomal enzymes, which degrade mucopolysaccharides. Patients initially develop normally, with the onset of abnormalities later in infancy or childhood. These patients often have multiorgan involvement, including neurologic, cardiovascular, pulmonary, ophthalmologic, hearing, and musculoskeletal findings. Diagnosis is initially made by measuring urine glycosaminoglycans, which were negative in this case.\nOther common examples of rarely encountered lipid storage diseases include Gaucher disease or Fabry disease. Affected individuals usually have autosomal recessive inheritance of an enzyme mutation that prevents complete metabolism of a naturally occurring precursor. Abnormal metabolites then accumulate in the reticuloendothelial system, yielding enlargement of the spleen and liver. Oftentimes central nervous system or other organ system involvement is present; however, splenomegaly can be isolated. Definitive diagnosis is made by testing for the individual enzyme that is affected. If the patient in this case did not have a positive PCR result for malaria, peripheral blood leukocytes would have been obtained to measure glucocerebrosidase activity as a test for Gaucher disease.\nGaucher disease can present with splenomegaly, usually in the setting of concurrent hepatomegaly, pancytopenia (particularly anemia and thrombocytopenia), and bone involvement. Patients with Gaucher disease often have failure to thrive. In the case of Gaucher type 2 and 3, individuals have neurologic involvement. Although this patient had significant splenomegaly, he did not have the typical pancytopenia or hepatomegaly associated with Gaucher disease and had normal enzyme activity. Furthermore, Gaucher disease type 2 and 3 involves neurologic dysfunction, which the patient did not have.\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1213404,
"choiceText": "Sickle cell disease",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1213406,
"choiceText": "Chronic malaria ",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1213408,
"choiceText": "Leukemia ",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1213410,
"choiceText": "Lymphoma ",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1213412,
"choiceText": "Lipid storage disease\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 385851,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Chronic Splenomegaly in a 10-Year-Old Boy"
},
{
"authors": "Rebecca Winderman, MD; Natalie Banniettis, MD; Simon S. Rabinowitz, MD, PhD",
"content": [
"HMS is considered a distinct entity from the splenomegaly that results from acute malarial parasitemia. The pathogenesis is believed to result from overproduction of immunoglobulins in response to repeated infection with malaria, particularly IgM, which aggregate in immune complexes within the spleen. As a result of delayed clearance from the reticuloendothelial tissue, splenomegaly develops and persists. Parasitemia is uncommon. The spleen size has been shown to be directly correlated to the serum level of IgM.[2] Genetic predispositions have been noted among individuals with the human leukocyte antigen (HLA)-DR2 haplotype.[3]",
"Although the highest prevalence of HMS is reported at 80%-85% in Papua New Guinea, other locations also report a significant prevalence, including northern Nigeria (40%), Kenya (41%), and Ghana (76%). A diagnosis of HMS should be considered in individuals who present from endemic areas with longstanding splenomegaly.",
"Diagnostic criteria for HMS include the following major criteria:",
"Persistent splenomegaly (>10 cm below the costal margin)",
"Elevated antimalarial antibody levels",
"IgM titer >2 standard deviations above the mean",
"Favorable response to long-term malaria prophylaxis",
"Minor criteria include the following:",
"Hepatic sinusoidal lymphocytosis (liver biopsy histology)",
"Normal humoral immune responses to antigenic challenge (phytohemagglutinin stimulation)",
"Hypersplenism",
"Lymphocyte proliferation",
"Occurrence within family tribes",
"Other laboratory findings suggestive of HMS include hemolytic anemia, direct hyperbilirubinemia, neutropenia, and thrombocytopenia. Definitive diagnosis is made via PCR.",
"Long-standing untreated HMS is often fatal and has been associated with premalignant conditions. It can evolve into chronic lymphocytic leukemia, hairy cell leukemia, or splenic lymphoma with villous lymphocytes (SLVL). At times, HMS is difficult to distinguish from lymphoproliferative disorders. Many serologic markers are similar between SLVL and HMS, including high antimalarial antibodies and IgM levels."
],
"date": "April 23, 2018",
"figures": [],
"markdown": "# Chronic Splenomegaly in a 10-Year-Old Boy\n\n **Authors:** Rebecca Winderman, MD; Natalie Banniettis, MD; Simon S. Rabinowitz, MD, PhD \n **Date:** April 23, 2018\n\n ## Content\n\n HMS is considered a distinct entity from the splenomegaly that results from acute malarial parasitemia. The pathogenesis is believed to result from overproduction of immunoglobulins in response to repeated infection with malaria, particularly IgM, which aggregate in immune complexes within the spleen. As a result of delayed clearance from the reticuloendothelial tissue, splenomegaly develops and persists. Parasitemia is uncommon. The spleen size has been shown to be directly correlated to the serum level of IgM.[2] Genetic predispositions have been noted among individuals with the human leukocyte antigen (HLA)-DR2 haplotype.[3]\nAlthough the highest prevalence of HMS is reported at 80%-85% in Papua New Guinea, other locations also report a significant prevalence, including northern Nigeria (40%), Kenya (41%), and Ghana (76%). A diagnosis of HMS should be considered in individuals who present from endemic areas with longstanding splenomegaly.\nDiagnostic criteria for HMS include the following major criteria:\nPersistent splenomegaly (>10 cm below the costal margin)\nElevated antimalarial antibody levels\nIgM titer >2 standard deviations above the mean\nFavorable response to long-term malaria prophylaxis\nMinor criteria include the following:\nHepatic sinusoidal lymphocytosis (liver biopsy histology)\nNormal humoral immune responses to antigenic challenge (phytohemagglutinin stimulation)\nHypersplenism\nLymphocyte proliferation\nOccurrence within family tribes\nOther laboratory findings suggestive of HMS include hemolytic anemia, direct hyperbilirubinemia, neutropenia, and thrombocytopenia. Definitive diagnosis is made via PCR.\nLong-standing untreated HMS is often fatal and has been associated with premalignant conditions. It can evolve into chronic lymphocytic leukemia, hairy cell leukemia, or splenic lymphoma with villous lymphocytes (SLVL). At times, HMS is difficult to distinguish from lymphoproliferative disorders. Many serologic markers are similar between SLVL and HMS, including high antimalarial antibodies and IgM levels.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "Chronic Splenomegaly in a 10-Year-Old Boy"
},
{
"authors": "Rebecca Winderman, MD; Natalie Banniettis, MD; Simon S. Rabinowitz, MD, PhD",
"content": [
"Treatment regimens for HMS differ by region. If the patient resides in an endemic country with a risk for recurring exposures to Anopheles mosquitoes, chronic treatment is recommended. Although multiple sources recommend chronic antimalarial therapy, in nonendemic countries, a short course (≤1 week) of treatment (chloroquine, atovaquone-proguanil, pyrimethamine-sulfadoxine) is likely sufficient because the risk for re-exposure is low. Splenectomy should be reserved for patients with huge splenomegaly, those with disabling symptoms, or patients who do not respond to medical therapy.",
"The patient in this case was treated with atovaquone-proguanil for 3 days, as per recommendations from the Centers for Disease Control and Prevention. Clinical improvement was noted on examination findings within 2 months of therapy, by which point the patient was no longer complaining of abdominal pain. The spleen had become barely palpable, and the patient was eating better and gaining weight."
],
"date": "April 23, 2018",
"figures": [],
"markdown": "# Chronic Splenomegaly in a 10-Year-Old Boy\n\n **Authors:** Rebecca Winderman, MD; Natalie Banniettis, MD; Simon S. Rabinowitz, MD, PhD \n **Date:** April 23, 2018\n\n ## Content\n\n Treatment regimens for HMS differ by region. If the patient resides in an endemic country with a risk for recurring exposures to Anopheles mosquitoes, chronic treatment is recommended. Although multiple sources recommend chronic antimalarial therapy, in nonendemic countries, a short course (≤1 week) of treatment (chloroquine, atovaquone-proguanil, pyrimethamine-sulfadoxine) is likely sufficient because the risk for re-exposure is low. Splenectomy should be reserved for patients with huge splenomegaly, those with disabling symptoms, or patients who do not respond to medical therapy.\nThe patient in this case was treated with atovaquone-proguanil for 3 days, as per recommendations from the Centers for Disease Control and Prevention. Clinical improvement was noted on examination findings within 2 months of therapy, by which point the patient was no longer complaining of abdominal pain. The spleen had become barely palpable, and the patient was eating better and gaining weight.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1213414,
"choiceText": "Normal humoral immune responses to antigenic challenge (phytohemagglutinin stimulation)",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1213416,
"choiceText": "Hypersplenism",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1213418,
"choiceText": "Lymphocyte proliferation ",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1213420,
"choiceText": "IgM titer >2 standard deviations above the mean ",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Diagnostic criteria for HMS include the following major criteria:<ul>\r\n<li>Persistent splenomegaly (>10 cm below the costal margin) </li>\r\n<li>Elevated antimalarial antibody levels</li>\r\n<li>IgM titer >2 standard deviations above the mean</li>\r\n<li>Favorable response to long-term malaria prophylaxis</li></ul>\r\nMinor criteria include the following:\r\n<br><ul>\r\n<li>Hepatic sinusoidal lymphocytosis (liver biopsy histology) </li>\r\n<li>Normal humoral immune responses to antigenic challenge (phytohemagglutinin stimulation) </li>\r\n<li>Hypersplenism</li>\r\n<li>Lymphocyte proliferation</li>\r\n<li>Occurrence within family tribes</li></ul>",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 385853,
"questionText": "Which of the following is considered a major criteria for HMS diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1213422,
"choiceText": "Splenectomy",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1213424,
"choiceText": "Praziquantel",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1213426,
"choiceText": "Iodoquinol\r\n",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1213428,
"choiceText": "Atovaquone-proguanil",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Treatment regimens for HMS differ by region. If the patient resides in an endemic country with a risk for recurring exposures to <i>Anopheles</i> mosquitoes, chronic treatment is recommended. Although multiple sources recommend chronic antimalarial therapy, in nonendemic countries, a short course (≤1 week) of treatment (chloroquine, atovaquone-proguanil, pyrimethamine-sulfadoxine) is likely sufficient because the risk for re-exposure is low. Splenectomy should be reserved for patients with huge splenomegaly, those with disabling symptoms, or patients who do not respond to medical therapy. ",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 385855,
"questionText": "Which of the following is most suitable as first-line treatment for patients with HMS in nonendemic countries?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Chronic Splenomegaly in a 10-Year-Old Boy"
},
{
"authors": "Rebecca Winderman, MD; Natalie Banniettis, MD; Simon S. Rabinowitz, MD, PhD",
"content": [],
"date": "April 23, 2018",
"figures": [],
"markdown": "# Chronic Splenomegaly in a 10-Year-Old Boy\n\n **Authors:** Rebecca Winderman, MD; Natalie Banniettis, MD; Simon S. Rabinowitz, MD, PhD \n **Date:** April 23, 2018\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1213414,
"choiceText": "Normal humoral immune responses to antigenic challenge (phytohemagglutinin stimulation)",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1213416,
"choiceText": "Hypersplenism",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1213418,
"choiceText": "Lymphocyte proliferation ",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1213420,
"choiceText": "IgM titer >2 standard deviations above the mean ",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Diagnostic criteria for HMS include the following major criteria:<ul>\r\n<li>Persistent splenomegaly (>10 cm below the costal margin) </li>\r\n<li>Elevated antimalarial antibody levels</li>\r\n<li>IgM titer >2 standard deviations above the mean</li>\r\n<li>Favorable response to long-term malaria prophylaxis</li></ul>\r\nMinor criteria include the following:\r\n<br><ul>\r\n<li>Hepatic sinusoidal lymphocytosis (liver biopsy histology) </li>\r\n<li>Normal humoral immune responses to antigenic challenge (phytohemagglutinin stimulation) </li>\r\n<li>Hypersplenism</li>\r\n<li>Lymphocyte proliferation</li>\r\n<li>Occurrence within family tribes</li></ul>",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 385853,
"questionText": "Which of the following is considered a major criteria for HMS diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1213422,
"choiceText": "Splenectomy",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1213424,
"choiceText": "Praziquantel",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1213426,
"choiceText": "Iodoquinol\r\n",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1213428,
"choiceText": "Atovaquone-proguanil",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Treatment regimens for HMS differ by region. If the patient resides in an endemic country with a risk for recurring exposures to <i>Anopheles</i> mosquitoes, chronic treatment is recommended. Although multiple sources recommend chronic antimalarial therapy, in nonendemic countries, a short course (≤1 week) of treatment (chloroquine, atovaquone-proguanil, pyrimethamine-sulfadoxine) is likely sufficient because the risk for re-exposure is low. Splenectomy should be reserved for patients with huge splenomegaly, those with disabling symptoms, or patients who do not respond to medical therapy. ",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 385855,
"questionText": "Which of the following is most suitable as first-line treatment for patients with HMS in nonendemic countries?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Chronic Splenomegaly in a 10-Year-Old Boy"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1213404,
"choiceText": "Sickle cell disease",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1213406,
"choiceText": "Chronic malaria ",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1213408,
"choiceText": "Leukemia ",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1213410,
"choiceText": "Lymphoma ",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1213412,
"choiceText": "Lipid storage disease\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 385851,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1213414,
"choiceText": "Normal humoral immune responses to antigenic challenge (phytohemagglutinin stimulation)",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1213416,
"choiceText": "Hypersplenism",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1213418,
"choiceText": "Lymphocyte proliferation ",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1213420,
"choiceText": "IgM titer >2 standard deviations above the mean ",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Diagnostic criteria for HMS include the following major criteria:<ul>\r\n<li>Persistent splenomegaly (>10 cm below the costal margin) </li>\r\n<li>Elevated antimalarial antibody levels</li>\r\n<li>IgM titer >2 standard deviations above the mean</li>\r\n<li>Favorable response to long-term malaria prophylaxis</li></ul>\r\nMinor criteria include the following:\r\n<br><ul>\r\n<li>Hepatic sinusoidal lymphocytosis (liver biopsy histology) </li>\r\n<li>Normal humoral immune responses to antigenic challenge (phytohemagglutinin stimulation) </li>\r\n<li>Hypersplenism</li>\r\n<li>Lymphocyte proliferation</li>\r\n<li>Occurrence within family tribes</li></ul>",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 385853,
"questionText": "Which of the following is considered a major criteria for HMS diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1213422,
"choiceText": "Splenectomy",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1213424,
"choiceText": "Praziquantel",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1213426,
"choiceText": "Iodoquinol\r\n",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1213428,
"choiceText": "Atovaquone-proguanil",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Treatment regimens for HMS differ by region. If the patient resides in an endemic country with a risk for recurring exposures to <i>Anopheles</i> mosquitoes, chronic treatment is recommended. Although multiple sources recommend chronic antimalarial therapy, in nonendemic countries, a short course (≤1 week) of treatment (chloroquine, atovaquone-proguanil, pyrimethamine-sulfadoxine) is likely sufficient because the risk for re-exposure is low. Splenectomy should be reserved for patients with huge splenomegaly, those with disabling symptoms, or patients who do not respond to medical therapy. ",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 385855,
"questionText": "Which of the following is most suitable as first-line treatment for patients with HMS in nonendemic countries?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
894774 | /viewarticle/894774 | [
{
"authors": "Shahida Badsha, MBBS, FCPS, MCPS",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"A 19-year-old man is transported by wheelchair to a clinic by his older brother due to difficulty with ambulation. His chief complaints are morbid obesity and oozing skin ulcers. The patient was born to consanguineous parents and had a normal, full-term delivery in the hospital, with no antenatal or perinatal complications. His birth weight was reportedly average, and he had no apparent dysmorphic features. When he was circumcised, the small size of his penis was documented. No absence of testis was documented at this time, despite the noted micropenis.",
"The mother reported an uneventful full-term pregnancy and delivery with no history of decreased fetal movements. He was initially exclusively breastfed and later given cow's milk and home food. His mother recalls frequent regurgitation and posseting in his infancy. Within the first 2 months, he developed redness and scaling of the skin; he received treatment but was never completely cured. He has three older siblings, all reportedly healthy. His mother does not report any hypotonia or failure to thrive in early infancy.",
"He has no reported history of seizures, cyanotic spells, or respiratory distress. His motor milestones were delayed when compared with his siblings. He walked and spoke short phrases by age 2 years. By age 4 years, he had a voracious appetite and was larger in size than his cousin, who was about the same age (Figure 1).",
"Figure 1.",
"As a young child, he could not run well and was unable to articulate Rs and Ss in his speech. He had no visual problems. He was admitted to school at age 6 years and was able to study until age 15 years, when he stopped going to school due to poor mobility secondary to his severe obesity. His mother reports that he had frequent outbursts at school as he progressed and attributed it to peers making fun of his obesity.",
"At age 8 years, he was reportedly not growing in height, and his testes were not palpable in the scrotum. Medical advice was sought; however, the family was not told a diagnosis or given treatment approaches. At age 14 years, the family became significantly worried about his gross obesity, snoring while sleeping, absence of facial and axillary hair, and short stature. His growth in height, as expected with a pubertal growth spurt, did not occur. The patient also became increasingly aggressive, which the family attributed to his peers making fun of his obesity. His unrelenting appetite had become a major problem.",
"To treat his irritated skin, topical creams and ointments had been applied. Additionally, three courses of oral antibiotics and steroids had been prescribed for his skin condition, which was diagnosed as a nonspecific dermatitis. He has no significant history of constipation or heat/cold intolerance. His parents and siblings remain healthy. His family history is negative for pubertal delay, short stature, morbid obesity, congenital anomalies, and developmental or learning disabilities. He has a positive family history of asthma among his uncles and aunts. His grandparents are both deceased, with no specific cause of death attributed."
],
"date": "April 09, 2018",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/894/774/894774-Thumb1.png"
}
],
"markdown": "# A 19-Year-Old Man With Life-Threatening Obesity\n\n **Authors:** Shahida Badsha, MBBS, FCPS, MCPS \n **Date:** April 09, 2018\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nA 19-year-old man is transported by wheelchair to a clinic by his older brother due to difficulty with ambulation. His chief complaints are morbid obesity and oozing skin ulcers. The patient was born to consanguineous parents and had a normal, full-term delivery in the hospital, with no antenatal or perinatal complications. His birth weight was reportedly average, and he had no apparent dysmorphic features. When he was circumcised, the small size of his penis was documented. No absence of testis was documented at this time, despite the noted micropenis.\nThe mother reported an uneventful full-term pregnancy and delivery with no history of decreased fetal movements. He was initially exclusively breastfed and later given cow's milk and home food. His mother recalls frequent regurgitation and posseting in his infancy. Within the first 2 months, he developed redness and scaling of the skin; he received treatment but was never completely cured. He has three older siblings, all reportedly healthy. His mother does not report any hypotonia or failure to thrive in early infancy.\nHe has no reported history of seizures, cyanotic spells, or respiratory distress. His motor milestones were delayed when compared with his siblings. He walked and spoke short phrases by age 2 years. By age 4 years, he had a voracious appetite and was larger in size than his cousin, who was about the same age (Figure 1).\nFigure 1.\nAs a young child, he could not run well and was unable to articulate Rs and Ss in his speech. He had no visual problems. He was admitted to school at age 6 years and was able to study until age 15 years, when he stopped going to school due to poor mobility secondary to his severe obesity. His mother reports that he had frequent outbursts at school as he progressed and attributed it to peers making fun of his obesity.\nAt age 8 years, he was reportedly not growing in height, and his testes were not palpable in the scrotum. Medical advice was sought; however, the family was not told a diagnosis or given treatment approaches. At age 14 years, the family became significantly worried about his gross obesity, snoring while sleeping, absence of facial and axillary hair, and short stature. His growth in height, as expected with a pubertal growth spurt, did not occur. The patient also became increasingly aggressive, which the family attributed to his peers making fun of his obesity. His unrelenting appetite had become a major problem.\nTo treat his irritated skin, topical creams and ointments had been applied. Additionally, three courses of oral antibiotics and steroids had been prescribed for his skin condition, which was diagnosed as a nonspecific dermatitis. He has no significant history of constipation or heat/cold intolerance. His parents and siblings remain healthy. His family history is negative for pubertal delay, short stature, morbid obesity, congenital anomalies, and developmental or learning disabilities. He has a positive family history of asthma among his uncles and aunts. His grandparents are both deceased, with no specific cause of death attributed.\n\n ## Figures\n\n **Figure 1.** \n \n\n\n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 19-Year-Old Man With Life-Threatening Obesity"
},
{
"authors": "Shahida Badsha, MBBS, FCPS, MCPS",
"content": [
"Upon physical examination, the patient appears uncomfortable (Figure 2). He speaks very little, and his answers are appropriate but in monosyllables.",
"Figure 2.",
"He has gross, generalized obesity with no obvious differential fat distribution on the face, trunk, or limbs. His eyes may be considered almond-shaped, and he has a fullness in the forehead region and in the cheeks, giving a pear-shaped appearance. His mouth is somewhat fish-shaped, with a thin upper lip. He is alert, well-oriented, and communicative, although he seemingly resents doing so and replies in muffled monosyllables or by nodding. His hearing and vision are within normal limits.",
"His weight is 192 lb (87 kg), which puts him in the 90th percentile, and his height is 51.2 inches (130 cm), which puts him below the fifth percentile. His body mass index (BMI) is 51.5 kg/m2. His occipitofrontal head circumference (OFC) is 22 inches (56 cm). His blood pressure is 100/80 mm Hg. Taking his blood pressure required the largest available cuff. Although it is 100/80 mm Hg at presentation, a consultation note from another doctor recorded it as 183/109 mm Hg. A separate note from a different visit shows a blood pressure of 112/68 mm Hg in an outpatient setting. His pulse is regular at 80 beats/min. His respiration is audible, although not noisy, with 18-20 breaths/min. He cannot lie down on a couch due to the concern of respiratory distress from airway obstruction.",
"He has a plethoric face (Figure 2) and a low frontal hairline. No facial, axillary, or pubic hair is seen. He has some carious teeth; otherwise, the oral cavity and tongue are normal. No goiter is appreciated. Heart sounds and chest auscultation findings are normal. His liver and spleen cannot be satisfactorily palpated. However, liver function test results were reportedly normal on several occasions. CT scan and ultrasonography findings of the abdomen reveal the liver to be normal. Grossly examined, cranial nerves and sensations are intact. Tendon reflexes can be elicited, and plantars are downgoing. He has small hands and feet. A single transverse palmar crease is noted on the right hand.",
"Due to suprapubic fat, his external genitalia are difficult to observe. Palpation of the scrotum for testes is not permitted. His skin is erythematous, dry, and scaly. No pitting edema is noted. He has numerous ulcerative lesions scattered over the lower half of his body (Figure 3).",
"Figure 3.",
"Some ulcers are reported as painless, and some are reported as painful. His hands and feet are small, and his nails are discolored (Figures 4-6).",
"Figure 4.",
"Figure 5.",
"Figure 6.",
"Laboratory studies including complete blood count, peripheral smear, sodium, potassium level, blood urea nitrogen level, calcium level, creatinine level, alanine aminotransferase level, aspartate aminotransferase level, bilirubin level, phosphate level, and alkaline phosphatase level are all within normal limits.",
"Urinalysis reveals normal specific gravity, with no protein, glucose, or red or white cells. His fasting blood glucose level is 7.2 mmol/L, and his postprandial blood glucose level is 8.1 mmol/L. The postprandial glucose level was tested 90 minutes after breakfast.",
"MRI of the brain is performed to investigate the hypothalamic-hypophyseal axis as a cause of obesity and short stature. The MRI reveals mild cerebral atrophy. High-resolution CT scanning of the chest, abdomen, and pelvis are performed to investigate for a hormone-secreting tumor and reveal normal findings. An x-ray of the dorsal spine is unremarkable. His bone age assessed at age 14 years was reportedly delayed to 8-9 years. Skin biopsy reveals nonspecific dermatitis.",
"Hormonal studies reveal the following:",
"Serum cortisol (M) level, <50 nmol/L",
"Serum cortisol (E) level, <20 nmol/L",
"Urinary free cortisol level, 58 nmol/24 h",
"Thyroid-stimulating hormone level, 1.63 mIU/L",
"Free T4 level, 13.5 pmol/L",
"Adrenocorticotropic hormone (ACTH) level, 3.2 pmol/L",
"Fasting growth hormone level, 2.5 ng/mL"
],
"date": "April 09, 2018",
"figures": [
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/894/774/894774-Thumb2.png"
},
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/894/774/894774-Thumb3.png"
},
{
"caption": "Figure 4.",
"image_url": "https://img.medscapestatic.com/article/894/774/894774-Thumb4.png"
},
{
"caption": "Figure 5.",
"image_url": "https://img.medscapestatic.com/article/894/774/894774-Thumb5.png"
},
{
"caption": "Figure 6.",
"image_url": "https://img.medscapestatic.com/article/894/774/894774-Thumb6.png"
}
],
"markdown": "# A 19-Year-Old Man With Life-Threatening Obesity\n\n **Authors:** Shahida Badsha, MBBS, FCPS, MCPS \n **Date:** April 09, 2018\n\n ## Content\n\n Upon physical examination, the patient appears uncomfortable (Figure 2). He speaks very little, and his answers are appropriate but in monosyllables.\nFigure 2.\nHe has gross, generalized obesity with no obvious differential fat distribution on the face, trunk, or limbs. His eyes may be considered almond-shaped, and he has a fullness in the forehead region and in the cheeks, giving a pear-shaped appearance. His mouth is somewhat fish-shaped, with a thin upper lip. He is alert, well-oriented, and communicative, although he seemingly resents doing so and replies in muffled monosyllables or by nodding. His hearing and vision are within normal limits.\nHis weight is 192 lb (87 kg), which puts him in the 90th percentile, and his height is 51.2 inches (130 cm), which puts him below the fifth percentile. His body mass index (BMI) is 51.5 kg/m2. His occipitofrontal head circumference (OFC) is 22 inches (56 cm). His blood pressure is 100/80 mm Hg. Taking his blood pressure required the largest available cuff. Although it is 100/80 mm Hg at presentation, a consultation note from another doctor recorded it as 183/109 mm Hg. A separate note from a different visit shows a blood pressure of 112/68 mm Hg in an outpatient setting. His pulse is regular at 80 beats/min. His respiration is audible, although not noisy, with 18-20 breaths/min. He cannot lie down on a couch due to the concern of respiratory distress from airway obstruction.\nHe has a plethoric face (Figure 2) and a low frontal hairline. No facial, axillary, or pubic hair is seen. He has some carious teeth; otherwise, the oral cavity and tongue are normal. No goiter is appreciated. Heart sounds and chest auscultation findings are normal. His liver and spleen cannot be satisfactorily palpated. However, liver function test results were reportedly normal on several occasions. CT scan and ultrasonography findings of the abdomen reveal the liver to be normal. Grossly examined, cranial nerves and sensations are intact. Tendon reflexes can be elicited, and plantars are downgoing. He has small hands and feet. A single transverse palmar crease is noted on the right hand.\nDue to suprapubic fat, his external genitalia are difficult to observe. Palpation of the scrotum for testes is not permitted. His skin is erythematous, dry, and scaly. No pitting edema is noted. He has numerous ulcerative lesions scattered over the lower half of his body (Figure 3).\nFigure 3.\nSome ulcers are reported as painless, and some are reported as painful. His hands and feet are small, and his nails are discolored (Figures 4-6).\nFigure 4.\nFigure 5.\nFigure 6.\nLaboratory studies including complete blood count, peripheral smear, sodium, potassium level, blood urea nitrogen level, calcium level, creatinine level, alanine aminotransferase level, aspartate aminotransferase level, bilirubin level, phosphate level, and alkaline phosphatase level are all within normal limits.\nUrinalysis reveals normal specific gravity, with no protein, glucose, or red or white cells. His fasting blood glucose level is 7.2 mmol/L, and his postprandial blood glucose level is 8.1 mmol/L. The postprandial glucose level was tested 90 minutes after breakfast.\nMRI of the brain is performed to investigate the hypothalamic-hypophyseal axis as a cause of obesity and short stature. The MRI reveals mild cerebral atrophy. High-resolution CT scanning of the chest, abdomen, and pelvis are performed to investigate for a hormone-secreting tumor and reveal normal findings. An x-ray of the dorsal spine is unremarkable. His bone age assessed at age 14 years was reportedly delayed to 8-9 years. Skin biopsy reveals nonspecific dermatitis.\nHormonal studies reveal the following:\nSerum cortisol (M) level, <50 nmol/L\nSerum cortisol (E) level, <20 nmol/L\nUrinary free cortisol level, 58 nmol/24 h\nThyroid-stimulating hormone level, 1.63 mIU/L\nFree T4 level, 13.5 pmol/L\nAdrenocorticotropic hormone (ACTH) level, 3.2 pmol/L\nFasting growth hormone level, 2.5 ng/mL\n\n ## Figures\n\n **Figure 2.** \n \n\n**Figure 3.** \n \n\n**Figure 4.** \n \n\n**Figure 5.** \n \n\n**Figure 6.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1207490,
"choiceText": "Bardet-Biedl syndrome",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1207492,
"choiceText": "Cushing syndrome",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1207494,
"choiceText": "Exogenous obesity",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1207496,
"choiceText": "Pseudohypoparathyroidism",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1207498,
"choiceText": "Prader-Willi syndrome",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 383955,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 19-Year-Old Man With Life-Threatening Obesity"
},
{
"authors": "Shahida Badsha, MBBS, FCPS, MCPS",
"content": [
"The pathophysiologic basis of different diseases that lead to obesity differs and requires different approaches to diagnosis and management. In addition to metabolic studies, genetic studies are often required to support the correct diagnosis. Exogenous or common obesity has a multifactorial etiology with a polygenic basis. Although no consensus has been established regarding the definition of morbid obesity, a BMI >3 standard deviations for age is considered an acceptable parameter.[1] The patient in this case had a medical history and clinical features suggestive of Prader-Willi syndrome. His history of excessive food intake and past treatment with steroids for his dermatitis may have misled physicians to a diagnosis of either exogenous obesity or iatrogenic Cushing syndrome. The diagnosis of Prader-Willi syndrome was based on the clinical features of a voracious appetite, gross obesity with short stature, disproportionately small hands and feet, and no signs of facial, axillary, or pubic hair.",
"Prader-Willi syndrome is the most common cause of morbid, syndromic obesity, with an estimated worldwide prevalence of 1:10,000 to 1:30,000. Males and females are affected with equal frequency, and the syndrome is seen in all races and ethnicities. It is a genetic disorder primarily caused by gene deletion on the paternal-inherited chromosome 15 in the q11.2-q13 region. Overall, three molecular mechanisms of Prader-Willi syndrome have been described: paternal 15q11.2-q13 deletion, maternal uniparental disomy (UPD) of chromosome 15, and imprinting defect. These mechanisms constitute 65%-75%, 20%-30%, and 1%-3% of cases, respectively. DNA methylation analysis is the technique used to diagnose PWS for all three mechanisms.[2]"
],
"date": "April 09, 2018",
"figures": [],
"markdown": "# A 19-Year-Old Man With Life-Threatening Obesity\n\n **Authors:** Shahida Badsha, MBBS, FCPS, MCPS \n **Date:** April 09, 2018\n\n ## Content\n\n The pathophysiologic basis of different diseases that lead to obesity differs and requires different approaches to diagnosis and management. In addition to metabolic studies, genetic studies are often required to support the correct diagnosis. Exogenous or common obesity has a multifactorial etiology with a polygenic basis. Although no consensus has been established regarding the definition of morbid obesity, a BMI >3 standard deviations for age is considered an acceptable parameter.[1] The patient in this case had a medical history and clinical features suggestive of Prader-Willi syndrome. His history of excessive food intake and past treatment with steroids for his dermatitis may have misled physicians to a diagnosis of either exogenous obesity or iatrogenic Cushing syndrome. The diagnosis of Prader-Willi syndrome was based on the clinical features of a voracious appetite, gross obesity with short stature, disproportionately small hands and feet, and no signs of facial, axillary, or pubic hair.\nPrader-Willi syndrome is the most common cause of morbid, syndromic obesity, with an estimated worldwide prevalence of 1:10,000 to 1:30,000. Males and females are affected with equal frequency, and the syndrome is seen in all races and ethnicities. It is a genetic disorder primarily caused by gene deletion on the paternal-inherited chromosome 15 in the q11.2-q13 region. Overall, three molecular mechanisms of Prader-Willi syndrome have been described: paternal 15q11.2-q13 deletion, maternal uniparental disomy (UPD) of chromosome 15, and imprinting defect. These mechanisms constitute 65%-75%, 20%-30%, and 1%-3% of cases, respectively. DNA methylation analysis is the technique used to diagnose PWS for all three mechanisms.[2]\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1207490,
"choiceText": "Bardet-Biedl syndrome",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1207492,
"choiceText": "Cushing syndrome",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1207494,
"choiceText": "Exogenous obesity",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1207496,
"choiceText": "Pseudohypoparathyroidism",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1207498,
"choiceText": "Prader-Willi syndrome",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 383955,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 19-Year-Old Man With Life-Threatening Obesity"
},
{
"authors": "Shahida Badsha, MBBS, FCPS, MCPS",
"content": [
"Infants with Prader-Willi syndrome present with hypotonia and difficulty feeding due to poor sucking and swallowing reflexes. Failure to thrive can ensue. Typically, an insatiable appetite and chronic food-seeking behaviors (hyperphagia) begin in the preschool years, leading to excessive weight gain. Satiety and energy balance are mainly controlled by the hypothalamus; however, numerous regions in the nervous system play a role. Ghrelin, a 28-amino acid peptide, produced in the stomach, is potentially the hormone responsible for causing obesity in individuals with Prader-Willi syndrome.",
"Children with Prader-Willi syndrome typically have mild-to-moderate intellectual impairment and learning disabilities. Vision problems, such as strabismus and near-sightedness, are common. Behavioral problems are also common, such as temper outbursts, stubbornness, and obsessive-compulsive behaviors described as picking at the skin and skin lesions. Phenotypic features include craniofacial features described as a pear-shaped face with a narrow forehead and almond-shaped eyes, downturned corners to the mouth with a thin upper lip, underdeveloped genitalia, hypogonadism, and small hands and feet. Hypogonadism can be of hypothalamic origin or due to primary gonadal failure.[3,4,5] Puberty is delayed or incomplete. Scoliosis can be a concern.",
"Patients with Prader-Willi syndrome have short stature, and their bone age lags behind their chronological age. Short stature is an important characteristic of Prader-Willi syndrome; these children do not have a growth spurt at pubertal age. This lack of height growth is contrary to exogenous obesity, in which height is gained in the prepubertal and postpubertal years. Growth hormone can help children with Prader-Willi syndrome to normalize height, increase lean body mass and mobility, and decrease fat mass. Structured behavioral modification approaches have also helped these patients with weight management.",
"Morbidity and mortality in Prader-Willi syndrome are related to life-threatening morbid obesity and its associated comorbidities.[2,6,7] These patients are prone to develop type 2 diabetes. Poor circulation can lead to stasis dermatitis nodosum. Other skin lesions have also been reported in association with Prader-Willi syndrome.[8,9] Sleep abnormalities are common. Patients are at high risk of developing sleep-disordered breathing (obstructive sleep apnea) and cor pulmonale due to the comorbidities associated with morbid obesity.[10,11]",
"Bardet-Biedl syndrome is a genetic condition characterized with syndromic central obesity. Common features include learning disabilities, retinitis pigmentosa, hypogonadism, postaxial polydactyly, and structural or functional renal abnormalities. Bardet-Biedl syndrome is inherited as an autosomal recessive trait and can result from mutations in at least 14 different genes, called BBS genes. These mutations lead to abnormal structure and function of cilia. About 25% of all cases of BBS result from mutations in the BBS1 gene; 20% are caused by mutations in the BBS10 gene. The remaining BBS genes account for a small percentage of cases. In 25% of patients diagnosed with BBS, the cause is unknown. Diagnosis is usually based on clinical findings and is confirmed by gene sequencing. No definitive treatment is available. Multidisciplinary surveillance is important to control symptoms and complications.[12,13,14]",
"Cushing syndrome involves a combination of clinical features that result from prolonged exposure to excess cortisol, either exogenous or endogenous. Exogenous Cushing syndrome is often iatrogenic and is caused by administration of glucocorticoids used to treat medical disorders. Endogenous Cushing syndrome is divided into two types: ACTH-dependent and ACTH-independent. The most common ACTH-dependent disease is due to pituitary adenoma. Endogenous ACTH-independent disease is due to ACTH-producing tumors in the chest (eg, small cell lung carcinoma, bronchial and thymic carcinoids, medullary thyroid carcinoma, or other conditions that ectopically produce ACTH (eg, gastroenteropancreatic neuroendocrine tumors, pheochromocytoma).[15] In this case, although the patient had received oral steroids for his skin condition, neither his clinical features nor his laboratory results suggest Cushing syndrome.",
"Pseudohypoparathyroidism, or Albright hereditary osteodystrophy, is characterized by parathyroid hormone-resistant hypocalcemia and hyperphosphatemia, combined with developmental and skeletal defects. Patients present with short stature, obesity, rounded face, dental hypoplasia, shortened fourth metacarpals, and soft tissue calcification or ossification. Pseudohypoparathyroidism is diagnosed by measuring the sera levels of calcium, phosphorus, and parathyroid hormone. A mutation identified in the GNAS1 gene is confirmatory.[16] This patient had normal calcium and phosphate levels."
],
"date": "April 09, 2018",
"figures": [],
"markdown": "# A 19-Year-Old Man With Life-Threatening Obesity\n\n **Authors:** Shahida Badsha, MBBS, FCPS, MCPS \n **Date:** April 09, 2018\n\n ## Content\n\n Infants with Prader-Willi syndrome present with hypotonia and difficulty feeding due to poor sucking and swallowing reflexes. Failure to thrive can ensue. Typically, an insatiable appetite and chronic food-seeking behaviors (hyperphagia) begin in the preschool years, leading to excessive weight gain. Satiety and energy balance are mainly controlled by the hypothalamus; however, numerous regions in the nervous system play a role. Ghrelin, a 28-amino acid peptide, produced in the stomach, is potentially the hormone responsible for causing obesity in individuals with Prader-Willi syndrome.\nChildren with Prader-Willi syndrome typically have mild-to-moderate intellectual impairment and learning disabilities. Vision problems, such as strabismus and near-sightedness, are common. Behavioral problems are also common, such as temper outbursts, stubbornness, and obsessive-compulsive behaviors described as picking at the skin and skin lesions. Phenotypic features include craniofacial features described as a pear-shaped face with a narrow forehead and almond-shaped eyes, downturned corners to the mouth with a thin upper lip, underdeveloped genitalia, hypogonadism, and small hands and feet. Hypogonadism can be of hypothalamic origin or due to primary gonadal failure.[3,4,5] Puberty is delayed or incomplete. Scoliosis can be a concern.\nPatients with Prader-Willi syndrome have short stature, and their bone age lags behind their chronological age. Short stature is an important characteristic of Prader-Willi syndrome; these children do not have a growth spurt at pubertal age. This lack of height growth is contrary to exogenous obesity, in which height is gained in the prepubertal and postpubertal years. Growth hormone can help children with Prader-Willi syndrome to normalize height, increase lean body mass and mobility, and decrease fat mass. Structured behavioral modification approaches have also helped these patients with weight management.\nMorbidity and mortality in Prader-Willi syndrome are related to life-threatening morbid obesity and its associated comorbidities.[2,6,7] These patients are prone to develop type 2 diabetes. Poor circulation can lead to stasis dermatitis nodosum. Other skin lesions have also been reported in association with Prader-Willi syndrome.[8,9] Sleep abnormalities are common. Patients are at high risk of developing sleep-disordered breathing (obstructive sleep apnea) and cor pulmonale due to the comorbidities associated with morbid obesity.[10,11]\nBardet-Biedl syndrome is a genetic condition characterized with syndromic central obesity. Common features include learning disabilities, retinitis pigmentosa, hypogonadism, postaxial polydactyly, and structural or functional renal abnormalities. Bardet-Biedl syndrome is inherited as an autosomal recessive trait and can result from mutations in at least 14 different genes, called BBS genes. These mutations lead to abnormal structure and function of cilia. About 25% of all cases of BBS result from mutations in the BBS1 gene; 20% are caused by mutations in the BBS10 gene. The remaining BBS genes account for a small percentage of cases. In 25% of patients diagnosed with BBS, the cause is unknown. Diagnosis is usually based on clinical findings and is confirmed by gene sequencing. No definitive treatment is available. Multidisciplinary surveillance is important to control symptoms and complications.[12,13,14]\nCushing syndrome involves a combination of clinical features that result from prolonged exposure to excess cortisol, either exogenous or endogenous. Exogenous Cushing syndrome is often iatrogenic and is caused by administration of glucocorticoids used to treat medical disorders. Endogenous Cushing syndrome is divided into two types: ACTH-dependent and ACTH-independent. The most common ACTH-dependent disease is due to pituitary adenoma. Endogenous ACTH-independent disease is due to ACTH-producing tumors in the chest (eg, small cell lung carcinoma, bronchial and thymic carcinoids, medullary thyroid carcinoma, or other conditions that ectopically produce ACTH (eg, gastroenteropancreatic neuroendocrine tumors, pheochromocytoma).[15] In this case, although the patient had received oral steroids for his skin condition, neither his clinical features nor his laboratory results suggest Cushing syndrome.\nPseudohypoparathyroidism, or Albright hereditary osteodystrophy, is characterized by parathyroid hormone-resistant hypocalcemia and hyperphosphatemia, combined with developmental and skeletal defects. Patients present with short stature, obesity, rounded face, dental hypoplasia, shortened fourth metacarpals, and soft tissue calcification or ossification. Pseudohypoparathyroidism is diagnosed by measuring the sera levels of calcium, phosphorus, and parathyroid hormone. A mutation identified in the GNAS1 gene is confirmatory.[16] This patient had normal calcium and phosphate levels.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 19-Year-Old Man With Life-Threatening Obesity"
},
{
"authors": "Shahida Badsha, MBBS, FCPS, MCPS",
"content": [
"After elevated blood glucose levels were found, the patient was referred to an endocrinologist for treatment of type 2 diabetes. He was prescribed an oral hypoglycemic, metformin, with recommended close monitoring of his blood sugar to prevent hypoglycemia.",
"Three weeks later, the patient developed respiratory distress and oliguria. He was admitted to a hospital and diagnosed with pulmonary edema. Oxygen support and diuretic treatment were initiated. Despite these interventions, his respiratory distress persisted. On day 4 of admission, the patient had a cardiopulmonary arrest and later died at age 19 years, 3 months."
],
"date": "April 09, 2018",
"figures": [],
"markdown": "# A 19-Year-Old Man With Life-Threatening Obesity\n\n **Authors:** Shahida Badsha, MBBS, FCPS, MCPS \n **Date:** April 09, 2018\n\n ## Content\n\n After elevated blood glucose levels were found, the patient was referred to an endocrinologist for treatment of type 2 diabetes. He was prescribed an oral hypoglycemic, metformin, with recommended close monitoring of his blood sugar to prevent hypoglycemia.\nThree weeks later, the patient developed respiratory distress and oliguria. He was admitted to a hospital and diagnosed with pulmonary edema. Oxygen support and diuretic treatment were initiated. Despite these interventions, his respiratory distress persisted. On day 4 of admission, the patient had a cardiopulmonary arrest and later died at age 19 years, 3 months.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1207500,
"choiceText": "Autosomal recessive inheritance",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1207502,
"choiceText": "Autosomal dominant inheritance",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1207504,
"choiceText": "Gene deletion",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1207506,
"choiceText": "X-linked recessive inheritance",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The most common etiology of Prader-Willi syndrome is loss of genomic material of the paternal 15q11.2-13 locus. In contrast, a deletion of same genomic material of the maternal 15q11.2-13 causes Angelman syndrome.\r\n<br><br>\r\nThree molecular mechanisms of Prader-Willi syndrome are known: paternal 15q11.2-q13 deletion, maternal UPD of chromosome 15, and imprinting defect. These constitute 65%-75%, 20%-30%, and 1%-3% of cases, respectively. DNA methylation analysis is the technique used to diagnose Prader-Willi syndrome in all three mechanisms.[2] Most cases are sporadic. Less than 1% of patients whose syndrome is due to an imprinting defect carry the risk for recurrence.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 383957,
"questionText": "Which of the following is responsible for most cases of Prader-Willi syndrome?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1207508,
"choiceText": "Leptin",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1207510,
"choiceText": "Ghrelin",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1207512,
"choiceText": "Insulin ",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1207514,
"choiceText": "Hypocretin",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Satiety and energy balance are mainly controlled by the hypothalamus; however, numerous regions in the nervous system play a role. Ghrelin, a 28-amino acid peptide produced in the stomach, is potentially the hormone responsible for causing obesity in individuals with Prader-Willi syndrome. This hormone transmits satiety signals. Its levels are higher in individuals with Prader-Willi syndrome when compared with individuals who have obesity due to other causes.<sup type=\"ref\">[17,18]</sup><br><br>\r\n\r\nObese patients are leptin-resistant. Whereas ghrelin plays a role in meal initiation, leptin mediates long-term regulation of energy balance. It suppresses food intake and induces weight loss. Although the level of the orexigenic hormone, ghrelin, is decreased in obese individuals, the circulating level of the anorexigenic hormone, leptin, is increased.<sup type=\"ref\">[17,18]</sup>\r\n<br><br>\r\n\r\nDiagnostic exclusion of an insulinoma in any patient with morbid obesity is important; however, fasting insulin level was found to be significantly lower in patients with Prader-Willi syndrome, although they had a much higher insulin sensitivity. Although diabetes is increasingly recognized as a complication of Prader-Willi syndrome, it has a different etiology and is not solely a complication of obesity.<sup type=\"ref\">[4,5]</sup>\r\n<br><br>\r\n\r\nHypothalamic neuropeptides called hypocretins, also known as orexins, are thought to play an important role in sleep and wakefulness as well as appetite; however, no significant difference was seen in the total number of hypocretin-containing neurons in patients with Prader-Willi syndrome compared with age-matched controls.<sup type=\"ref\">[10,11]</sup>",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 383959,
"questionText": "Which of the following hormones is likely related to the inefficient satiety and hyperphagia in Prader-Willi syndrome?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 19-Year-Old Man With Life-Threatening Obesity"
},
{
"authors": "Shahida Badsha, MBBS, FCPS, MCPS",
"content": [],
"date": "April 09, 2018",
"figures": [],
"markdown": "# A 19-Year-Old Man With Life-Threatening Obesity\n\n **Authors:** Shahida Badsha, MBBS, FCPS, MCPS \n **Date:** April 09, 2018\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1207500,
"choiceText": "Autosomal recessive inheritance",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1207502,
"choiceText": "Autosomal dominant inheritance",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1207504,
"choiceText": "Gene deletion",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1207506,
"choiceText": "X-linked recessive inheritance",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The most common etiology of Prader-Willi syndrome is loss of genomic material of the paternal 15q11.2-13 locus. In contrast, a deletion of same genomic material of the maternal 15q11.2-13 causes Angelman syndrome.\r\n<br><br>\r\nThree molecular mechanisms of Prader-Willi syndrome are known: paternal 15q11.2-q13 deletion, maternal UPD of chromosome 15, and imprinting defect. These constitute 65%-75%, 20%-30%, and 1%-3% of cases, respectively. DNA methylation analysis is the technique used to diagnose Prader-Willi syndrome in all three mechanisms.[2] Most cases are sporadic. Less than 1% of patients whose syndrome is due to an imprinting defect carry the risk for recurrence.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 383957,
"questionText": "Which of the following is responsible for most cases of Prader-Willi syndrome?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1207508,
"choiceText": "Leptin",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1207510,
"choiceText": "Ghrelin",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1207512,
"choiceText": "Insulin ",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1207514,
"choiceText": "Hypocretin",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Satiety and energy balance are mainly controlled by the hypothalamus; however, numerous regions in the nervous system play a role. Ghrelin, a 28-amino acid peptide produced in the stomach, is potentially the hormone responsible for causing obesity in individuals with Prader-Willi syndrome. This hormone transmits satiety signals. Its levels are higher in individuals with Prader-Willi syndrome when compared with individuals who have obesity due to other causes.<sup type=\"ref\">[17,18]</sup><br><br>\r\n\r\nObese patients are leptin-resistant. Whereas ghrelin plays a role in meal initiation, leptin mediates long-term regulation of energy balance. It suppresses food intake and induces weight loss. Although the level of the orexigenic hormone, ghrelin, is decreased in obese individuals, the circulating level of the anorexigenic hormone, leptin, is increased.<sup type=\"ref\">[17,18]</sup>\r\n<br><br>\r\n\r\nDiagnostic exclusion of an insulinoma in any patient with morbid obesity is important; however, fasting insulin level was found to be significantly lower in patients with Prader-Willi syndrome, although they had a much higher insulin sensitivity. Although diabetes is increasingly recognized as a complication of Prader-Willi syndrome, it has a different etiology and is not solely a complication of obesity.<sup type=\"ref\">[4,5]</sup>\r\n<br><br>\r\n\r\nHypothalamic neuropeptides called hypocretins, also known as orexins, are thought to play an important role in sleep and wakefulness as well as appetite; however, no significant difference was seen in the total number of hypocretin-containing neurons in patients with Prader-Willi syndrome compared with age-matched controls.<sup type=\"ref\">[10,11]</sup>",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 383959,
"questionText": "Which of the following hormones is likely related to the inefficient satiety and hyperphagia in Prader-Willi syndrome?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 19-Year-Old Man With Life-Threatening Obesity"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1207490,
"choiceText": "Bardet-Biedl syndrome",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1207492,
"choiceText": "Cushing syndrome",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1207494,
"choiceText": "Exogenous obesity",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1207496,
"choiceText": "Pseudohypoparathyroidism",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1207498,
"choiceText": "Prader-Willi syndrome",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 383955,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1207500,
"choiceText": "Autosomal recessive inheritance",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1207502,
"choiceText": "Autosomal dominant inheritance",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1207504,
"choiceText": "Gene deletion",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1207506,
"choiceText": "X-linked recessive inheritance",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The most common etiology of Prader-Willi syndrome is loss of genomic material of the paternal 15q11.2-13 locus. In contrast, a deletion of same genomic material of the maternal 15q11.2-13 causes Angelman syndrome.\r\n<br><br>\r\nThree molecular mechanisms of Prader-Willi syndrome are known: paternal 15q11.2-q13 deletion, maternal UPD of chromosome 15, and imprinting defect. These constitute 65%-75%, 20%-30%, and 1%-3% of cases, respectively. DNA methylation analysis is the technique used to diagnose Prader-Willi syndrome in all three mechanisms.[2] Most cases are sporadic. Less than 1% of patients whose syndrome is due to an imprinting defect carry the risk for recurrence.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 383957,
"questionText": "Which of the following is responsible for most cases of Prader-Willi syndrome?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1207508,
"choiceText": "Leptin",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1207510,
"choiceText": "Ghrelin",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1207512,
"choiceText": "Insulin ",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1207514,
"choiceText": "Hypocretin",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Satiety and energy balance are mainly controlled by the hypothalamus; however, numerous regions in the nervous system play a role. Ghrelin, a 28-amino acid peptide produced in the stomach, is potentially the hormone responsible for causing obesity in individuals with Prader-Willi syndrome. This hormone transmits satiety signals. Its levels are higher in individuals with Prader-Willi syndrome when compared with individuals who have obesity due to other causes.<sup type=\"ref\">[17,18]</sup><br><br>\r\n\r\nObese patients are leptin-resistant. Whereas ghrelin plays a role in meal initiation, leptin mediates long-term regulation of energy balance. It suppresses food intake and induces weight loss. Although the level of the orexigenic hormone, ghrelin, is decreased in obese individuals, the circulating level of the anorexigenic hormone, leptin, is increased.<sup type=\"ref\">[17,18]</sup>\r\n<br><br>\r\n\r\nDiagnostic exclusion of an insulinoma in any patient with morbid obesity is important; however, fasting insulin level was found to be significantly lower in patients with Prader-Willi syndrome, although they had a much higher insulin sensitivity. Although diabetes is increasingly recognized as a complication of Prader-Willi syndrome, it has a different etiology and is not solely a complication of obesity.<sup type=\"ref\">[4,5]</sup>\r\n<br><br>\r\n\r\nHypothalamic neuropeptides called hypocretins, also known as orexins, are thought to play an important role in sleep and wakefulness as well as appetite; however, no significant difference was seen in the total number of hypocretin-containing neurons in patients with Prader-Willi syndrome compared with age-matched controls.<sup type=\"ref\">[10,11]</sup>",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 383959,
"questionText": "Which of the following hormones is likely related to the inefficient satiety and hyperphagia in Prader-Willi syndrome?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
893350 | /viewarticle/893350 | [
{
"authors": "Catherine Anastasopoulou, MD, PhD; Kimberly Lessard, DO",
"content": [
"The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"A 67-year-old woman presents to the emergency department for evaluation of progressive bilateral lower extremity edema. The patient is a poor historian and appears confused. She has a medical history of osteoporosis, fibromyalgia, chronic cough, mild intermittent asthma, anemia, and glaucoma. She was also recently involved in a significant motor vehicle accident and sustained a pelvic fracture. The patient's recovery was complicated by the development of acute deep vein thrombosis and pulmonary embolism. She was also discovered to have heparin-induced thrombocytopenia and thrombosis. She has since remained on anticoagulation therapy with rivaroxaban.",
"Discussion with family members reveals that the patient has demonstrated a gradual decline in mental status following her car accident and has complained of frequent headaches, shortness of breath, and progressive leg swelling. She is also reported to have had several recent falls without associated loss of consciousness or significant trauma. She has been compliant with all of her home medications, including escitalopram, albuterol, cholecalciferol, and rivaroxaban as well as recently prescribed daily furosemide."
],
"date": "March 08, 2018",
"figures": [],
"markdown": "# A 67-Year-Old Woman With Orthostatic Hypotension and Edema\n\n **Authors:** Catherine Anastasopoulou, MD, PhD; Kimberly Lessard, DO \n **Date:** March 08, 2018\n\n ## Content\n\n The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nA 67-year-old woman presents to the emergency department for evaluation of progressive bilateral lower extremity edema. The patient is a poor historian and appears confused. She has a medical history of osteoporosis, fibromyalgia, chronic cough, mild intermittent asthma, anemia, and glaucoma. She was also recently involved in a significant motor vehicle accident and sustained a pelvic fracture. The patient's recovery was complicated by the development of acute deep vein thrombosis and pulmonary embolism. She was also discovered to have heparin-induced thrombocytopenia and thrombosis. She has since remained on anticoagulation therapy with rivaroxaban.\nDiscussion with family members reveals that the patient has demonstrated a gradual decline in mental status following her car accident and has complained of frequent headaches, shortness of breath, and progressive leg swelling. She is also reported to have had several recent falls without associated loss of consciousness or significant trauma. She has been compliant with all of her home medications, including escitalopram, albuterol, cholecalciferol, and rivaroxaban as well as recently prescribed daily furosemide.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 67-Year-Old Woman With Orthostatic Hypotension and Edema"
},
{
"authors": "Catherine Anastasopoulou, MD, PhD; Kimberly Lessard, DO",
"content": [
"Upon physical examination, the patient is an ill-appearing, overweight woman in mild distress. Her vital signs upon arrival included a blood pressure of 106/59 mm Hg, a heart rate of 73 beats/min, an oral temperature of 97.3°F (36.3°C), oxygen saturation level of 94% on room air, weight of 150 lb (68 kg), and a body mass index of 29.3. Upon standing, the patient develops signs of orthostatic hypotension with significant dizziness, lightheadedness, and a drop in systolic blood pressure to 92 mm Hg.",
"The examination of the head and neck reveals dry mucus membranes. Cardiopulmonary examination findings are within normal limits, with normal S1 and S2 sounds, a regular heart rate and rhythm, and no appreciable murmur or jugular vein distention. Abdominal examination reveals slightly diffuse abdominal tenderness with normal bowel sounds and no hepatosplenomegaly. A neurologic examination reveals no significant deficit. She is noted to have +1 pitting edema in both lower extremities to the level of the knee with associated tenderness and decreased flexion of both joints. Her skin appears dry without appreciable tenting, bruising, or any lesions.",
"The initial workup includes an EKG, which reveals normal sinus rhythm without ST-T changes. A chest x-ray is noncontributory, and CT scanning of the chest is performed, which is negative for pulmonary embolism. Results of the initial chemistry are as follows:",
"Sodium level—134 mmol/L (reference range, 136-146 mmol/L)",
"Potassium level—4.9 mmol/L (reference range, 3.6-5.1 mmol/L)",
"Chloride level—101 mmol/L (reference range, 98-107 mmol/L)",
"Bicarbonate level—23 mmol/L (reference range, 22-31 mmol/L)",
"Blood urea nitrogen level—22 mg/dL (reference range, 10-20 mg/dL)",
"Creatinine level—0.5 mg/dL (reference range, 0.6-1 mg/dL)",
"Glucose level—143 mg/dL (reference range, 74-99 mg/dL)",
"Magnesium level—1.4 mg/dL (reference range, 1.6-2.6 mg/dL)",
"A complete blood count is significant for normocytic anemia with a hemoglobin level of 10.7 g/dL (reference range, 12-16 g/dL) and mean corpuscular volume of 85.4 fL (reference range, 81-96 fL); all other values are within normal limits. Her international normalized ratio is 2 IU (reference range, 0.9-1.1 IU), her prothrombin time is 22.7 s (reference range, 11.8-14.3 s), and her partial thromboplastin time is 52.1 s (reference range, 25.5-36 s). Serial troponin findings are negative; however, the brain natriuretic peptide is elevated at 150.2 pg/mL (reference range, <100 pg/mL). Her serum osmolality is 273 mOsm/kg, and her urine sodium level is 41 mmol/L.",
"The patient is admitted for further workup and is treated with continuous intravenous (IV) normal saline (0.9% at 120 cc/h) as well as IV magnesium. Despite fluid resuscitation, however, she remains borderline hypotensive (96/59 mm Hg) with persistent orthostatic symptoms. Continuous telemetry monitoring reveals occasional bradycardia. Transthoracic echocardiography reveals intact ejection function and only grade 1 diastolic dysfunction. Subsequent laboratory evaluation is significant for a vitamin B12 level of 512 pg/mL (reference range, 213-816 pg/mL), thyroid-stimulating hormone level of 2.29 mcIU/mL (reference range, 0.35-4.94 mcIU/mL), folate level of 4.6 ng/mL (5.4-20 ng/mL), vitamin D 25-OH level of 45.9 ng/mL (reference range, 30-50 ng/mL), and early (05:00) cortisol level of 0.8 (reference range, 3.7-19.4 µg/dL). An infectious workup, including urine studies and blood cultures, is negative.",
"Repeat chemistry after 24 hours of IV fluids reveals the following:",
"Sodium level—138 mmol/L",
"Potassium level—4.3 mmol/L",
"Chloride level—103 mmol/L",
"Bicarbonate level—25 mmol/L",
"Blood urea nitrogen level—15 mg/dL",
"Creatinine level—0.5 mg/dL",
"Glucose level—115 mg/dL (reference range, 74-99 mg/dL)",
"Magnesium level—1.5 mg/dL",
"The CT pulmonary embolism study from her prior hospitalization is subsequently obtained (see Figures 1-2 below)."
],
"date": "March 08, 2018",
"figures": [
{
"caption": "",
"image_url": "https://img.medscapestatic.com/article/893/350/893350-Image1-thumb.jpg"
},
{
"caption": "",
"image_url": "https://img.medscapestatic.com/article/893/350/893350-Image2-thumb.jpg"
}
],
"markdown": "# A 67-Year-Old Woman With Orthostatic Hypotension and Edema\n\n **Authors:** Catherine Anastasopoulou, MD, PhD; Kimberly Lessard, DO \n **Date:** March 08, 2018\n\n ## Content\n\n Upon physical examination, the patient is an ill-appearing, overweight woman in mild distress. Her vital signs upon arrival included a blood pressure of 106/59 mm Hg, a heart rate of 73 beats/min, an oral temperature of 97.3°F (36.3°C), oxygen saturation level of 94% on room air, weight of 150 lb (68 kg), and a body mass index of 29.3. Upon standing, the patient develops signs of orthostatic hypotension with significant dizziness, lightheadedness, and a drop in systolic blood pressure to 92 mm Hg.\nThe examination of the head and neck reveals dry mucus membranes. Cardiopulmonary examination findings are within normal limits, with normal S1 and S2 sounds, a regular heart rate and rhythm, and no appreciable murmur or jugular vein distention. Abdominal examination reveals slightly diffuse abdominal tenderness with normal bowel sounds and no hepatosplenomegaly. A neurologic examination reveals no significant deficit. She is noted to have +1 pitting edema in both lower extremities to the level of the knee with associated tenderness and decreased flexion of both joints. Her skin appears dry without appreciable tenting, bruising, or any lesions.\nThe initial workup includes an EKG, which reveals normal sinus rhythm without ST-T changes. A chest x-ray is noncontributory, and CT scanning of the chest is performed, which is negative for pulmonary embolism. Results of the initial chemistry are as follows:\nSodium level—134 mmol/L (reference range, 136-146 mmol/L)\nPotassium level—4.9 mmol/L (reference range, 3.6-5.1 mmol/L)\nChloride level—101 mmol/L (reference range, 98-107 mmol/L)\nBicarbonate level—23 mmol/L (reference range, 22-31 mmol/L)\nBlood urea nitrogen level—22 mg/dL (reference range, 10-20 mg/dL)\nCreatinine level—0.5 mg/dL (reference range, 0.6-1 mg/dL)\nGlucose level—143 mg/dL (reference range, 74-99 mg/dL)\nMagnesium level—1.4 mg/dL (reference range, 1.6-2.6 mg/dL)\nA complete blood count is significant for normocytic anemia with a hemoglobin level of 10.7 g/dL (reference range, 12-16 g/dL) and mean corpuscular volume of 85.4 fL (reference range, 81-96 fL); all other values are within normal limits. Her international normalized ratio is 2 IU (reference range, 0.9-1.1 IU), her prothrombin time is 22.7 s (reference range, 11.8-14.3 s), and her partial thromboplastin time is 52.1 s (reference range, 25.5-36 s). Serial troponin findings are negative; however, the brain natriuretic peptide is elevated at 150.2 pg/mL (reference range, <100 pg/mL). Her serum osmolality is 273 mOsm/kg, and her urine sodium level is 41 mmol/L.\nThe patient is admitted for further workup and is treated with continuous intravenous (IV) normal saline (0.9% at 120 cc/h) as well as IV magnesium. Despite fluid resuscitation, however, she remains borderline hypotensive (96/59 mm Hg) with persistent orthostatic symptoms. Continuous telemetry monitoring reveals occasional bradycardia. Transthoracic echocardiography reveals intact ejection function and only grade 1 diastolic dysfunction. Subsequent laboratory evaluation is significant for a vitamin B12 level of 512 pg/mL (reference range, 213-816 pg/mL), thyroid-stimulating hormone level of 2.29 mcIU/mL (reference range, 0.35-4.94 mcIU/mL), folate level of 4.6 ng/mL (5.4-20 ng/mL), vitamin D 25-OH level of 45.9 ng/mL (reference range, 30-50 ng/mL), and early (05:00) cortisol level of 0.8 (reference range, 3.7-19.4 µg/dL). An infectious workup, including urine studies and blood cultures, is negative.\nRepeat chemistry after 24 hours of IV fluids reveals the following:\nSodium level—138 mmol/L\nPotassium level—4.3 mmol/L\nChloride level—103 mmol/L\nBicarbonate level—25 mmol/L\nBlood urea nitrogen level—15 mg/dL\nCreatinine level—0.5 mg/dL\nGlucose level—115 mg/dL (reference range, 74-99 mg/dL)\nMagnesium level—1.5 mg/dL\nThe CT pulmonary embolism study from her prior hospitalization is subsequently obtained (see Figures 1-2 below).\n\n ## Figures\n\n **** \n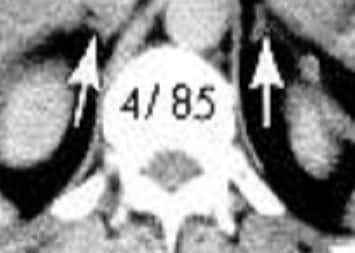 \n\n**** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1194672,
"choiceText": "Secondary adrenal insufficiency",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1194674,
"choiceText": "Primary adrenal insufficiency",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1194676,
"choiceText": "Syndrome of inappropriate antidiuretic hormone secretion (SIADH)",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1194678,
"choiceText": "Decompensated diastolic heart failure",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1194680,
"choiceText": "Autonomic dysfunction",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1194682,
"choiceText": "Symptomatic anemia",
"correct": false,
"displayOrder": 6,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 379775,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 67-Year-Old Woman With Orthostatic Hypotension and Edema"
},
{
"authors": "Catherine Anastasopoulou, MD, PhD; Kimberly Lessard, DO",
"content": [
"The most likely diagnosis for this patient is primary adrenal insufficiency, also known as Addison disease, likely from bilateral adrenal hemorrhage. Given her history of trauma, as well as heparin-induced thrombocytopenia and thrombosis, the changes seen on CT scan at the levels of the adrenals bilaterally are highly suspicious for adrenal hemorrhage. Notable is the markedly low morning cortisol level, which is pathognomonic of adrenal insufficiency. The patient also exhibited several classic signs and symptoms of adrenal insufficiency, including orthostatic hypotension, hyponatremia, and confusion. The hypotension, hyponatremia, and somewhat elevated potassium level are consistent with mineralocorticoid deficiency, as is expected in bilateral adrenal hemorrhage. Normalization of sodium and potassium levels following IV normal saline is also suggestive of adrenal insufficiency.",
"In general, the presentation of adrenal insufficiency varies according to the level of acuity, the degree of adrenal loss/malfunction, and the level of mineralocorticoid involvement. The onset of adrenal insufficiency may be insidious, unless adrenal crisis is precipitated by stress or illness. In chronic adrenal insufficiency, the most common symptoms include fatigue (84%-95%); anorexia and weight loss (66%-76%); nausea, vomiting, and abdominal pain (49%-62%); and musculoskeletal pain (35%-40%). The most common associated laboratory findings include hyponatremia (70%-80%), hyperkalemia (30%-40%), and anemia (11%-15%). In adrenal crisis, on the other hand, the most common presentation is hypotension or shock. Patients with adrenal crisis may also have nonspecific symptoms similar to those with chronic adrenal insufficiency but may also exhibit fever, confusion, or coma.",
"Elements of clinical presentation specific to primary adrenal insufficiency, particularly in individuals with long-standing disease, include skin hyperpigmentation related to increased proopiomelanocortin, postural hypotension, and salt craving. Furthermore, in primary adrenal insufficiency, the overall clinical presentation often depends on the etiology. In primary autoimmune adrenalitis, for example, many features of the clinical presentation are similar to that of septic shock.",
"Bilateral adrenal injury, hemorrhage, or infarction are important diagnoses to consider in the differential etiology of a new primary adrenal insufficiency seen in the setting of blunt trauma or coagulopathy. In bilateral adrenal hemorrhage, most patients develop acute primary adrenal insufficiency that results in hypotension or shock (90%). Other signs and symptoms suggestive of this diagnosis include evidence of occult hemorrhage, such as a drop in hemoglobin level, progressive hyperkalemia and hyponatremia, and refractory volume contraction. Major risk factors for adrenal hemorrhage or infarction include a postoperative state, underlying coagulopathy, and anticoagulant therapy. Bleeding can occur even when anticoagulant therapy is within therapeutic range and can remain isolated to the adrenals."
],
"date": "March 08, 2018",
"figures": [],
"markdown": "# A 67-Year-Old Woman With Orthostatic Hypotension and Edema\n\n **Authors:** Catherine Anastasopoulou, MD, PhD; Kimberly Lessard, DO \n **Date:** March 08, 2018\n\n ## Content\n\n The most likely diagnosis for this patient is primary adrenal insufficiency, also known as Addison disease, likely from bilateral adrenal hemorrhage. Given her history of trauma, as well as heparin-induced thrombocytopenia and thrombosis, the changes seen on CT scan at the levels of the adrenals bilaterally are highly suspicious for adrenal hemorrhage. Notable is the markedly low morning cortisol level, which is pathognomonic of adrenal insufficiency. The patient also exhibited several classic signs and symptoms of adrenal insufficiency, including orthostatic hypotension, hyponatremia, and confusion. The hypotension, hyponatremia, and somewhat elevated potassium level are consistent with mineralocorticoid deficiency, as is expected in bilateral adrenal hemorrhage. Normalization of sodium and potassium levels following IV normal saline is also suggestive of adrenal insufficiency.\nIn general, the presentation of adrenal insufficiency varies according to the level of acuity, the degree of adrenal loss/malfunction, and the level of mineralocorticoid involvement. The onset of adrenal insufficiency may be insidious, unless adrenal crisis is precipitated by stress or illness. In chronic adrenal insufficiency, the most common symptoms include fatigue (84%-95%); anorexia and weight loss (66%-76%); nausea, vomiting, and abdominal pain (49%-62%); and musculoskeletal pain (35%-40%). The most common associated laboratory findings include hyponatremia (70%-80%), hyperkalemia (30%-40%), and anemia (11%-15%). In adrenal crisis, on the other hand, the most common presentation is hypotension or shock. Patients with adrenal crisis may also have nonspecific symptoms similar to those with chronic adrenal insufficiency but may also exhibit fever, confusion, or coma.\nElements of clinical presentation specific to primary adrenal insufficiency, particularly in individuals with long-standing disease, include skin hyperpigmentation related to increased proopiomelanocortin, postural hypotension, and salt craving. Furthermore, in primary adrenal insufficiency, the overall clinical presentation often depends on the etiology. In primary autoimmune adrenalitis, for example, many features of the clinical presentation are similar to that of septic shock.\nBilateral adrenal injury, hemorrhage, or infarction are important diagnoses to consider in the differential etiology of a new primary adrenal insufficiency seen in the setting of blunt trauma or coagulopathy. In bilateral adrenal hemorrhage, most patients develop acute primary adrenal insufficiency that results in hypotension or shock (90%). Other signs and symptoms suggestive of this diagnosis include evidence of occult hemorrhage, such as a drop in hemoglobin level, progressive hyperkalemia and hyponatremia, and refractory volume contraction. Major risk factors for adrenal hemorrhage or infarction include a postoperative state, underlying coagulopathy, and anticoagulant therapy. Bleeding can occur even when anticoagulant therapy is within therapeutic range and can remain isolated to the adrenals.\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1194672,
"choiceText": "Secondary adrenal insufficiency",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1194674,
"choiceText": "Primary adrenal insufficiency",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1194676,
"choiceText": "Syndrome of inappropriate antidiuretic hormone secretion (SIADH)",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1194678,
"choiceText": "Decompensated diastolic heart failure",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1194680,
"choiceText": "Autonomic dysfunction",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1194682,
"choiceText": "Symptomatic anemia",
"correct": false,
"displayOrder": 6,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 379775,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 67-Year-Old Woman With Orthostatic Hypotension and Edema"
},
{
"authors": "Catherine Anastasopoulou, MD, PhD; Kimberly Lessard, DO",
"content": [
"Adrenal hemorrhage has been associated with infectious etiologies. One such condition, Waterhouse-Friderichsen syndrome, involves adrenal hemorrhage secondary to meningococcemia and has hallmark features of fever and petechiae (50%-60%). Additional bacterial causes of sepsis involving bilateral adrenal hemorrhage include Haemophilus influenzae, Escherichia coli, Mycoplasma pneumoniae, Streptococcus pneumoniae, and Staphylococcus aureus. Adrenal hemorrhage/infarction has also been associated with Rocky Mountain spotted fever, murine and epidemic typhus, and viral infections, including cytomegalovirus, parvovirus B19, and Epstein-Barr virus.",
"Because adrenal crisis and insufficiency can be difficult to discern from other conditions in such patients, the diagnosis should be considered when any such risk factor is present. Without appropriate treatment, hypotension often progresses to shock with very high mortality. Furthermore, adrenal function rarely returns to normal in such patients and often requires permanent hormone replacement therapy.",
"In this patient, secondary adrenal insufficiency was less likely given the suspicion for adrenal hemorrhage. Secondary adrenal insufficiency involves adrenocorticotropic hormone (ACTH) deficiency due to pituitary causes or, less often, hypothalamic causes, including suppression from exogenous corticosteroid. In secondary adrenal insufficiency, mineralocorticoid function is typically spared. As a result, secondary adrenal insufficiency generally does not result in hypotension, hyponatremia, hyperkalemia, or hyperpigmentation. Differentiating primary adrenal insufficiency from secondary adrenal insufficiency typically involves measuring ACTH. In the setting of low morning cortisol levels, inappropriately low ACTH levels are diagnostic of secondary adrenal insufficiency. Conversely, ACTH levels are elevated in primary adrenal insufficiency in response to absent cortisol. Secondary adrenal insufficiency often coexists with hypothyroidism and/or growth-hormone deficiency; neither were present in this patient.",
"Tertiary adrenal insufficiency at the level of the hypothalamus was also considered in the differential diagnosis. In tertiary adrenal insufficiency, classic findings include low cortisol levels, low-normal ACTH levels, and reduced corticotropin-releasing hormone levels. Once again, a normal thyroid function makes dysfunction at the level of the hypothalamus less likely.",
"SIADH, although also part of the differential diagnoses for hyponatremia, was less likely in this patient and requires the exclusion of adrenal insufficiency and hypothyroidism. The patient in this case appeared to be hypovolemic and required IV fluid resuscitation for clinical stabilization. The correction of sodium to normal values following administration of IV fluids makes SIADH, which is typically treated with fluid restriction, less likely. Additionally, the patient has several clinical features not explained by SIADH alone, including hypotension, hyperkalemia, and peripheral edema. The sodium level in this patient was also not low enough to explain her neurologic symptoms.",
"Although the patient was noted to have grade 1 diastolic dysfunction as well as peripheral edema, decompensated heart failure was less likely given the patient's relatively normal echocardiography and lack of clinical findings (no jugular venous distention or pulmonary edema) with only slightly elevated brain natriuretic peptide. Although patients with congestive heart failure and/or renal insufficiency do not usually have peripheral edema, they may have difficulty getting rid of fluids due to reduction in free water clearance.",
"Autonomic dysfunction should be considered as a cause of hypotension in the elderly, particularly following trauma, but was unlikely to account for the electrolytic abnormalities in this patient. The patient is noted to be anemic, which is likely multifactorial in origin, with a hemoglobin below the standard threshold for symptom development (approximately 12 g/dL). However, her history of chronic anemia and lack of acute drop in hemoglobin made this less likely to be the cause of her symptoms. Also, it also would not account for her metabolic abnormalities. However, in the context of therapeutic anticoagulation and recent adrenal hemorrhage, hemoglobin should be closely monitored in patients like the one in this case.",
"A morning serum cortisol level <3 µg/dL alone is diagnostic of adrenal insufficiency. Based on clinical presentation and etiology related to adrenal hemorrhage, primary adrenal insufficiency can be presumed. Thus, further testing is not indicated to differentiate between primary, secondary, or tertiary causes of adrenal insufficiency.",
"A morning ACTH level can be used, albeit less commonly, in differentiating between primary, secondary, and tertiary adrenal insufficiency. ACTH is a hormone secreted by the anterior pituitary with the principle effect of promoting cortisol production at the level of the adrenal cortex. In the setting of a decreased morning cortisol level, an appropriately elevated ACTH level is suggestive of dysfunction at the level of the adrenal gland and thus primary adrenal insufficiency. Conversely, an inappropriately low level of ACTH in the setting of decreased cortisol is suggestive of dysfunction at the level of the pituitary (secondary adrenal insufficiency) or potentially at the level of the hypothalamus (tertiary adrenal insufficiency). ACTH measurement was not indicated in this patient due to sufficient evidence of primary adrenal insufficiency.",
"A cosyntropin stimulation test is used to confirm the presence of adrenal insufficiency by evaluating the adrenal response to ACTH. This is done by injection of cosyntropin (synthetic ACTH) and measurement of baseline and subsequent cortisol levels 30 minutes, 60 minutes, and 90 minutes later. The primary use of a cosyntropin stimulation test is to rule out adrenal insufficiency when cortisol levels are intermediate or inconclusive (ie, 3-15 µg/dL). Interpretation of this test may also aid in differentiating between primary and secondary adrenal insufficiency. In healthy individuals with a normal baseline morning cortisol level (20-30 µg/dL), the cortisol level is expected to rise by 200% (ie, double) within 60 minutes of cosyntropin administration. In primary adrenal insufficiency, the baseline cortisol is generally <10 µg/dL and has no more than a 25% increase in response to cosyntropin administration. Secondary adrenal insufficiency can result in variable response to cosyntropin. In general, a failure for cortisol level to rise >18 µg/dL is considered confirmation of adrenal insufficiency. The insulin tolerance test is considered the criterion standard test for diagnosing adrenal insufficiency. However, due to risks and side effects of the insulin tolerance test, the cosyntropin stimulation test is more commonly used. The cosyntropin stimulation test is 97% sensitive (at 95% specificity) for diagnosing primary adrenal insufficiency and is only 57%-61% sensitive for diagnosing secondary adrenal insufficiency. The patient in this case was found to have a significantly reduced morning cortisol level, well below 3 µg/dL. For this reason, no further testing was needed to confirm the presence of adrenal insufficiency.",
"Although aldosterone levels are expected to be low in patients with primary adrenal insufficiency, levels can be normal in secondary and tertiary adrenal insufficiency. Although these levels can be adjunctive to low morning cortisol in confirming mineralocorticoid deficits in primary adrenal insufficiency, they are not routinely measured and are not needed for diagnosis in patients like the one described here. Furthermore, if primary adrenal insufficiency is suspected, treatment with both mineralocorticoid and glucocorticoid replacement should be empirically started and should not be withheld while awaiting serum aldosterone levels.",
"The measurement of 24-hour urine cortisol is used to confirm the presence of hypercortisolism in Cushing disease. Although basal urinary cortisol levels may be low in patients with severe adrenal insufficiency, they may be low-normal in a patient with partial adrenal insufficiency. Thus, urine cortisol is not indicated for assessment of adrenal insufficiency. PM salivary cortisol testing is used to assess for hypercortisolism in Cushing disease and takes into consideration the diurnal release of cortisol. This test has no role in the evaluation of adrenal insufficiency."
],
"date": "March 08, 2018",
"figures": [],
"markdown": "# A 67-Year-Old Woman With Orthostatic Hypotension and Edema\n\n **Authors:** Catherine Anastasopoulou, MD, PhD; Kimberly Lessard, DO \n **Date:** March 08, 2018\n\n ## Content\n\n Adrenal hemorrhage has been associated with infectious etiologies. One such condition, Waterhouse-Friderichsen syndrome, involves adrenal hemorrhage secondary to meningococcemia and has hallmark features of fever and petechiae (50%-60%). Additional bacterial causes of sepsis involving bilateral adrenal hemorrhage include Haemophilus influenzae, Escherichia coli, Mycoplasma pneumoniae, Streptococcus pneumoniae, and Staphylococcus aureus. Adrenal hemorrhage/infarction has also been associated with Rocky Mountain spotted fever, murine and epidemic typhus, and viral infections, including cytomegalovirus, parvovirus B19, and Epstein-Barr virus.\nBecause adrenal crisis and insufficiency can be difficult to discern from other conditions in such patients, the diagnosis should be considered when any such risk factor is present. Without appropriate treatment, hypotension often progresses to shock with very high mortality. Furthermore, adrenal function rarely returns to normal in such patients and often requires permanent hormone replacement therapy.\nIn this patient, secondary adrenal insufficiency was less likely given the suspicion for adrenal hemorrhage. Secondary adrenal insufficiency involves adrenocorticotropic hormone (ACTH) deficiency due to pituitary causes or, less often, hypothalamic causes, including suppression from exogenous corticosteroid. In secondary adrenal insufficiency, mineralocorticoid function is typically spared. As a result, secondary adrenal insufficiency generally does not result in hypotension, hyponatremia, hyperkalemia, or hyperpigmentation. Differentiating primary adrenal insufficiency from secondary adrenal insufficiency typically involves measuring ACTH. In the setting of low morning cortisol levels, inappropriately low ACTH levels are diagnostic of secondary adrenal insufficiency. Conversely, ACTH levels are elevated in primary adrenal insufficiency in response to absent cortisol. Secondary adrenal insufficiency often coexists with hypothyroidism and/or growth-hormone deficiency; neither were present in this patient.\nTertiary adrenal insufficiency at the level of the hypothalamus was also considered in the differential diagnosis. In tertiary adrenal insufficiency, classic findings include low cortisol levels, low-normal ACTH levels, and reduced corticotropin-releasing hormone levels. Once again, a normal thyroid function makes dysfunction at the level of the hypothalamus less likely.\nSIADH, although also part of the differential diagnoses for hyponatremia, was less likely in this patient and requires the exclusion of adrenal insufficiency and hypothyroidism. The patient in this case appeared to be hypovolemic and required IV fluid resuscitation for clinical stabilization. The correction of sodium to normal values following administration of IV fluids makes SIADH, which is typically treated with fluid restriction, less likely. Additionally, the patient has several clinical features not explained by SIADH alone, including hypotension, hyperkalemia, and peripheral edema. The sodium level in this patient was also not low enough to explain her neurologic symptoms.\nAlthough the patient was noted to have grade 1 diastolic dysfunction as well as peripheral edema, decompensated heart failure was less likely given the patient's relatively normal echocardiography and lack of clinical findings (no jugular venous distention or pulmonary edema) with only slightly elevated brain natriuretic peptide. Although patients with congestive heart failure and/or renal insufficiency do not usually have peripheral edema, they may have difficulty getting rid of fluids due to reduction in free water clearance.\nAutonomic dysfunction should be considered as a cause of hypotension in the elderly, particularly following trauma, but was unlikely to account for the electrolytic abnormalities in this patient. The patient is noted to be anemic, which is likely multifactorial in origin, with a hemoglobin below the standard threshold for symptom development (approximately 12 g/dL). However, her history of chronic anemia and lack of acute drop in hemoglobin made this less likely to be the cause of her symptoms. Also, it also would not account for her metabolic abnormalities. However, in the context of therapeutic anticoagulation and recent adrenal hemorrhage, hemoglobin should be closely monitored in patients like the one in this case.\nA morning serum cortisol level <3 µg/dL alone is diagnostic of adrenal insufficiency. Based on clinical presentation and etiology related to adrenal hemorrhage, primary adrenal insufficiency can be presumed. Thus, further testing is not indicated to differentiate between primary, secondary, or tertiary causes of adrenal insufficiency.\nA morning ACTH level can be used, albeit less commonly, in differentiating between primary, secondary, and tertiary adrenal insufficiency. ACTH is a hormone secreted by the anterior pituitary with the principle effect of promoting cortisol production at the level of the adrenal cortex. In the setting of a decreased morning cortisol level, an appropriately elevated ACTH level is suggestive of dysfunction at the level of the adrenal gland and thus primary adrenal insufficiency. Conversely, an inappropriately low level of ACTH in the setting of decreased cortisol is suggestive of dysfunction at the level of the pituitary (secondary adrenal insufficiency) or potentially at the level of the hypothalamus (tertiary adrenal insufficiency). ACTH measurement was not indicated in this patient due to sufficient evidence of primary adrenal insufficiency.\nA cosyntropin stimulation test is used to confirm the presence of adrenal insufficiency by evaluating the adrenal response to ACTH. This is done by injection of cosyntropin (synthetic ACTH) and measurement of baseline and subsequent cortisol levels 30 minutes, 60 minutes, and 90 minutes later. The primary use of a cosyntropin stimulation test is to rule out adrenal insufficiency when cortisol levels are intermediate or inconclusive (ie, 3-15 µg/dL). Interpretation of this test may also aid in differentiating between primary and secondary adrenal insufficiency. In healthy individuals with a normal baseline morning cortisol level (20-30 µg/dL), the cortisol level is expected to rise by 200% (ie, double) within 60 minutes of cosyntropin administration. In primary adrenal insufficiency, the baseline cortisol is generally <10 µg/dL and has no more than a 25% increase in response to cosyntropin administration. Secondary adrenal insufficiency can result in variable response to cosyntropin. In general, a failure for cortisol level to rise >18 µg/dL is considered confirmation of adrenal insufficiency. The insulin tolerance test is considered the criterion standard test for diagnosing adrenal insufficiency. However, due to risks and side effects of the insulin tolerance test, the cosyntropin stimulation test is more commonly used. The cosyntropin stimulation test is 97% sensitive (at 95% specificity) for diagnosing primary adrenal insufficiency and is only 57%-61% sensitive for diagnosing secondary adrenal insufficiency. The patient in this case was found to have a significantly reduced morning cortisol level, well below 3 µg/dL. For this reason, no further testing was needed to confirm the presence of adrenal insufficiency.\nAlthough aldosterone levels are expected to be low in patients with primary adrenal insufficiency, levels can be normal in secondary and tertiary adrenal insufficiency. Although these levels can be adjunctive to low morning cortisol in confirming mineralocorticoid deficits in primary adrenal insufficiency, they are not routinely measured and are not needed for diagnosis in patients like the one described here. Furthermore, if primary adrenal insufficiency is suspected, treatment with both mineralocorticoid and glucocorticoid replacement should be empirically started and should not be withheld while awaiting serum aldosterone levels.\nThe measurement of 24-hour urine cortisol is used to confirm the presence of hypercortisolism in Cushing disease. Although basal urinary cortisol levels may be low in patients with severe adrenal insufficiency, they may be low-normal in a patient with partial adrenal insufficiency. Thus, urine cortisol is not indicated for assessment of adrenal insufficiency. PM salivary cortisol testing is used to assess for hypercortisolism in Cushing disease and takes into consideration the diurnal release of cortisol. This test has no role in the evaluation of adrenal insufficiency.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 67-Year-Old Woman With Orthostatic Hypotension and Edema"
},
{
"authors": "Catherine Anastasopoulou, MD, PhD; Kimberly Lessard, DO",
"content": [
"The patient in this case required treatment with both hydrocortisone and fludrocortisone. Because her morning cortisol confirmed deficient glucocorticoid levels, replacement with hydrocortisone was absolutely necessary. Hydrocortisone is most often initiated twice daily to match the diurnal pattern of cortisol release. Certain elements of this patient's presentation, including hypotension and hyponatremia, are reflective of a significant mineralocorticoid deficiency. As a result, mineralocorticoid replacement therapy with fludrocortisone was also indicated. Fludrocortisone acetate, a prodrug to fludrocortisone, is a synthetic oral adrenocortical steroid with potent mineralocorticoid properties and also moderate-high glucocorticoid effect.",
"In primary adrenal insufficiency, both mineralocorticoid and glucocorticoid deficiency can lead to the development of adrenal crisis. Hypotension can be seen in all forms of adrenal insufficiency; however, primary adrenal insufficiency may also cause significant intravascular volume depletion related to the degree of mineralocorticoid involvement. Mineralocorticoid (ie, aldosterone) deficiency leads to decreased sodium retention and subsequent volume depletion. Insufficient aldosterone also leads to suppressed vasoconstrictive response, causing decreased blood pressure and orthostatic changes. Glucocorticoid deficiency alone can also potentiate hypotension due to decreased vascular response to the effects of angiotensin II and norepinephrine, as well as decreased renin and increased prostacyclin production. In primary adrenal insufficiency, dual therapy with both an oral glucocorticoid (hydrocortisone) and an oral mineralocorticoid (fludrocortisone) is required to achieve adequate and balanced adrenocortical replacement.",
"No male or female hormone replacement, such as dehydroepiandrosterone (DHEA), is required in similar patients because those hormones are not affected by the adrenal dysfunction. Furthermore, replacement with salt tablets is inappropriate in patients such as the one in this case. IV normal saline should be used in the initial treatment of hyponatremia related to primary adrenal insufficiency if signs of significant volume depletion are present. Initiation of fludrocortisone should be performed immediately to improve sodium retention. Patients can be encouraged to liberalize their sodium intake but are unlikely to require sodium tablets to normalize blood sodium levels or blood pressure.",
"Along with IV fluids, the patient in this case was started on twice-daily hydrocortisone (20-mg morning dose, 10-mg evening dose) as well as fludrocortisone (0.05 mg orally daily). Furosemide was discontinued. At the time of discharge, the patient's sodium had improved to 138 mmol/L, and her blood pressure averaged 115/71 mm Hg. At follow-up, she was found to have stable blood pressure (118/73 mm Hg) without orthostasis and was noted to have improvement in her mentation and fatigue. She had no further falls and also had marginal improvement in her lower extremities. Repeat chemistry from this visit revealed a sodium level of 143 mmol/L, a potassium level of 4.1 mmol/L, and a glucose level of 138 mg/dL. At this point, her hydrocortisone was reduced to a once-daily morning dose of 20 mg.",
"The patient will have repeat chemistry as well as a cortisol levels obtained in the upcoming weeks to assess for the potential return of adrenal function. Given the etiology of her primary adrenal failure, significant gain in adrenal function is not expected, and long-term glucocorticoid and mineralocorticoid replacement therapy is likely required."
],
"date": "March 08, 2018",
"figures": [],
"markdown": "# A 67-Year-Old Woman With Orthostatic Hypotension and Edema\n\n **Authors:** Catherine Anastasopoulou, MD, PhD; Kimberly Lessard, DO \n **Date:** March 08, 2018\n\n ## Content\n\n The patient in this case required treatment with both hydrocortisone and fludrocortisone. Because her morning cortisol confirmed deficient glucocorticoid levels, replacement with hydrocortisone was absolutely necessary. Hydrocortisone is most often initiated twice daily to match the diurnal pattern of cortisol release. Certain elements of this patient's presentation, including hypotension and hyponatremia, are reflective of a significant mineralocorticoid deficiency. As a result, mineralocorticoid replacement therapy with fludrocortisone was also indicated. Fludrocortisone acetate, a prodrug to fludrocortisone, is a synthetic oral adrenocortical steroid with potent mineralocorticoid properties and also moderate-high glucocorticoid effect.\nIn primary adrenal insufficiency, both mineralocorticoid and glucocorticoid deficiency can lead to the development of adrenal crisis. Hypotension can be seen in all forms of adrenal insufficiency; however, primary adrenal insufficiency may also cause significant intravascular volume depletion related to the degree of mineralocorticoid involvement. Mineralocorticoid (ie, aldosterone) deficiency leads to decreased sodium retention and subsequent volume depletion. Insufficient aldosterone also leads to suppressed vasoconstrictive response, causing decreased blood pressure and orthostatic changes. Glucocorticoid deficiency alone can also potentiate hypotension due to decreased vascular response to the effects of angiotensin II and norepinephrine, as well as decreased renin and increased prostacyclin production. In primary adrenal insufficiency, dual therapy with both an oral glucocorticoid (hydrocortisone) and an oral mineralocorticoid (fludrocortisone) is required to achieve adequate and balanced adrenocortical replacement.\nNo male or female hormone replacement, such as dehydroepiandrosterone (DHEA), is required in similar patients because those hormones are not affected by the adrenal dysfunction. Furthermore, replacement with salt tablets is inappropriate in patients such as the one in this case. IV normal saline should be used in the initial treatment of hyponatremia related to primary adrenal insufficiency if signs of significant volume depletion are present. Initiation of fludrocortisone should be performed immediately to improve sodium retention. Patients can be encouraged to liberalize their sodium intake but are unlikely to require sodium tablets to normalize blood sodium levels or blood pressure.\nAlong with IV fluids, the patient in this case was started on twice-daily hydrocortisone (20-mg morning dose, 10-mg evening dose) as well as fludrocortisone (0.05 mg orally daily). Furosemide was discontinued. At the time of discharge, the patient's sodium had improved to 138 mmol/L, and her blood pressure averaged 115/71 mm Hg. At follow-up, she was found to have stable blood pressure (118/73 mm Hg) without orthostasis and was noted to have improvement in her mentation and fatigue. She had no further falls and also had marginal improvement in her lower extremities. Repeat chemistry from this visit revealed a sodium level of 143 mmol/L, a potassium level of 4.1 mmol/L, and a glucose level of 138 mg/dL. At this point, her hydrocortisone was reduced to a once-daily morning dose of 20 mg.\nThe patient will have repeat chemistry as well as a cortisol levels obtained in the upcoming weeks to assess for the potential return of adrenal function. Given the etiology of her primary adrenal failure, significant gain in adrenal function is not expected, and long-term glucocorticoid and mineralocorticoid replacement therapy is likely required.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1194684,
"choiceText": "Morning serum ACTH level testing",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1194686,
"choiceText": "Cosyntropin stimulation testing",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1194688,
"choiceText": "Serum aldosterone level testing",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1194690,
"choiceText": "24-hour urine free cortisol testing",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1194692,
"choiceText": "PM salivary cortisol testing",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1194694,
"choiceText": "No further testing",
"correct": true,
"displayOrder": 6,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>A morning serum cortisol level <3 µg/dL alone is diagnostic of adrenal insufficiency. Based on a clinical presentation and etiology related to adrenal hemorrhage, primary adrenal insufficiency can be presumed. Thus, further testing is not indicated to differentiate between primary, secondary, or tertiary causes of adrenal insufficiency.</p>",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 379777,
"questionText": "In a patient who presents in a fashion similar to the patient described in this case, including low morning serum cortisol level, which of the following is indicated for diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1194696,
"choiceText": "Hydrocortisone",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1194698,
"choiceText": "Fludrocortisone",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1194700,
"choiceText": "Hydrocortisone and fludrocortisone",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1194702,
"choiceText": "Hydrocortisone, fludrocortisone, and dehydroepiandrosterone",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1194704,
"choiceText": "Hydrocortisone, fludrocortisone, and salt tablets",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>This patient required treatment with both hydrocortisone and fludrocortisone. Because her morning cortisol confirmed deficient glucocorticoid levels, replacement with hydrocortisone was absolutely necessary. Hydrocortisone is most often initiated as a twice daily to match the diurnal pattern of cortisol release. Certain elements of this patient's presentation, including hypotension and hyponatremia, are reflective of a significant mineralocorticoid deficiency. As a result, mineralocorticoid replacement therapy with fludrocortisone is also indicated. Fludrocortisone acetate, a prodrug to fludrocortisone, is a synthetic oral adrenocortical steroid with potent mineralocorticoid properties and also moderate-high glucocorticoid effect.</p>",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 379779,
"questionText": "In a patient diagnosed with adrenal insufficiency who presents in a fashion similar to the patient described in this case, which of the following is the most appropriate pharmacotherapy?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 67-Year-Old Woman With Orthostatic Hypotension and Edema"
},
{
"authors": "Catherine Anastasopoulou, MD, PhD; Kimberly Lessard, DO",
"content": [
"Adem PV, Montgomery CP, Husain AN, et al. Staphylococcus aureus sepsis and the Waterhouse-Friderichsen syndrome in children. N Engl J Med. 2005;353:1245-1251. Medline",
"Bancos I, Hahner S, Tomlinson J, Arlt W. Diagnosis and management of adrenal insufficiency. Lancet Diabetes Endocrinol. 2015;3:216-226. Medline",
"Bleicken B, Hahner S, Ventz M, Quinkler M. Delayed diagnosis of adrenal insufficiency is common: a cross-sectional study in 216 patients. Am J Med Sci. 2010;339:525-531. Medline",
"Bornstein SR, Allolio B, Arlt W, et al. Diagnosis and treatment of primary adrenal insufficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016;101:364-389. Medline",
"Bouachour G, Tirot P, Varache N, Gouello JP, Harry P, Alquier P. Hemodynamic changes in acute adrenal insufficiency. Intensive Care Med. 1994;20:138-141. Medline",
"Burke CW. Adrenocortical insufficiency. Clin Endocrinol Metab. 1985;14:947-976. Medline",
"Castaldo ET, Guillamondegui OD, Greco JA 3rd, Feurer ID, Miller RS, Morris JA. Are adrenal injuries predictive of adrenal insufficiency in patients sustaining blunt trauma? Am Surg. 2008;74:262-266. Medline",
"Deutschbein T, Unger N, Mann K, Petersenn S. Diagnosis of secondary adrenal insufficiency: unstimulated early morning cortisol in saliva and serum in comparison with the insulin tolerance test. Horm Metab Res. 2009;41:834-839. Medline",
"Dorin RI, Qualls CR, Crapo LM. Diagnosis of adrenal insufficiency. Ann Intern Med. 2003;139:194-204. Medline",
"Feldman RD, Gros R. Vascular effects of aldosterone: sorting out the receptors and the ligands. Clin Exp Pharmacol Physiol. 2013;40:916-921. Medline",
"Hahner S, Loeffler M, Bleicken B, et al. Epidemiology of adrenal crisis in chronic adrenal insufficiency: the need for new prevention strategies. Eur J Endocrinol. 2010;162:597-602. Medline",
"Jacobs TP, Whitlock RT, Edsall J, Holub DA. Addisonian crisis while taking high-dose glucocorticoids. An unusual presentation of primary adrenal failure in two patients with underlying inflammatory diseases. JAMA. 1988;260:2082-2084. Medline",
"Jenkins D, Forsham PH, Laidlaw JC, Reddy WJ, Thorn GW. Use of ACTH in the diagnosis of adrenal cortical insufficiency. Am J Med. 1955;18:3-14. Medline",
"Margaretten W, Nakai H, Landing BH. Septicemic adrenal hemorrhage. Am J Dis Child. 1963;105:346-351. Medline",
"Piedrola G, Casado JL, Lopez E, Moreno A, Perez-Elias MJ, Garcia-Robles R. Clinical features of adrenal insufficiency in patients with acquired immunodeficiency syndrome. Clin Endocrinol (Oxf). 1996;45:97-101. Medline",
"Randeva HS, Schoebel J, Byrne J, Esiri M, Adams CB, Wass JA. Classical pituitary apoplexy: clinical features, management and outcome. Clin Endocrinol (Oxf). 1999;51:181-188. Medline",
"Rao RH, Vagnucci AH, Amico JA. Bilateral massive adrenal hemorrhage: early recognition and treatment. Ann Intern Med. 1989;110:227-235. Medline",
"Warkentin TE, Safyan EL, Linkins LA. Heparin-induced thrombocytopenia presenting as bilateral adrenal hemorrhages. N Engl J Med. 2015;372:492-494. Medline",
"Xarli VP, Steele AA, Davis PJ, Buescher ES, Rios CN, Garcia-Bunuel R. Adrenal hemorrhage in the adult. Medicine (Baltimore). 1978;57:211-221. Medline",
"Zelissen PM, Bast EJ, Croughs RJ. Associated autoimmunity in Addison's disease. J Autoimmun. 1995;8:121-130. Medline"
],
"date": "March 08, 2018",
"figures": [],
"markdown": "# A 67-Year-Old Woman With Orthostatic Hypotension and Edema\n\n **Authors:** Catherine Anastasopoulou, MD, PhD; Kimberly Lessard, DO \n **Date:** March 08, 2018\n\n ## Content\n\n Adem PV, Montgomery CP, Husain AN, et al. Staphylococcus aureus sepsis and the Waterhouse-Friderichsen syndrome in children. N Engl J Med. 2005;353:1245-1251. Medline\nBancos I, Hahner S, Tomlinson J, Arlt W. Diagnosis and management of adrenal insufficiency. Lancet Diabetes Endocrinol. 2015;3:216-226. Medline\nBleicken B, Hahner S, Ventz M, Quinkler M. Delayed diagnosis of adrenal insufficiency is common: a cross-sectional study in 216 patients. Am J Med Sci. 2010;339:525-531. Medline\nBornstein SR, Allolio B, Arlt W, et al. Diagnosis and treatment of primary adrenal insufficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016;101:364-389. Medline\nBouachour G, Tirot P, Varache N, Gouello JP, Harry P, Alquier P. Hemodynamic changes in acute adrenal insufficiency. Intensive Care Med. 1994;20:138-141. Medline\nBurke CW. Adrenocortical insufficiency. Clin Endocrinol Metab. 1985;14:947-976. Medline\nCastaldo ET, Guillamondegui OD, Greco JA 3rd, Feurer ID, Miller RS, Morris JA. Are adrenal injuries predictive of adrenal insufficiency in patients sustaining blunt trauma? Am Surg. 2008;74:262-266. Medline\nDeutschbein T, Unger N, Mann K, Petersenn S. Diagnosis of secondary adrenal insufficiency: unstimulated early morning cortisol in saliva and serum in comparison with the insulin tolerance test. Horm Metab Res. 2009;41:834-839. Medline\nDorin RI, Qualls CR, Crapo LM. Diagnosis of adrenal insufficiency. Ann Intern Med. 2003;139:194-204. Medline\nFeldman RD, Gros R. Vascular effects of aldosterone: sorting out the receptors and the ligands. Clin Exp Pharmacol Physiol. 2013;40:916-921. Medline\nHahner S, Loeffler M, Bleicken B, et al. Epidemiology of adrenal crisis in chronic adrenal insufficiency: the need for new prevention strategies. Eur J Endocrinol. 2010;162:597-602. Medline\nJacobs TP, Whitlock RT, Edsall J, Holub DA. Addisonian crisis while taking high-dose glucocorticoids. An unusual presentation of primary adrenal failure in two patients with underlying inflammatory diseases. JAMA. 1988;260:2082-2084. Medline\nJenkins D, Forsham PH, Laidlaw JC, Reddy WJ, Thorn GW. Use of ACTH in the diagnosis of adrenal cortical insufficiency. Am J Med. 1955;18:3-14. Medline\nMargaretten W, Nakai H, Landing BH. Septicemic adrenal hemorrhage. Am J Dis Child. 1963;105:346-351. Medline\nPiedrola G, Casado JL, Lopez E, Moreno A, Perez-Elias MJ, Garcia-Robles R. Clinical features of adrenal insufficiency in patients with acquired immunodeficiency syndrome. Clin Endocrinol (Oxf). 1996;45:97-101. Medline\nRandeva HS, Schoebel J, Byrne J, Esiri M, Adams CB, Wass JA. Classical pituitary apoplexy: clinical features, management and outcome. Clin Endocrinol (Oxf). 1999;51:181-188. Medline\nRao RH, Vagnucci AH, Amico JA. Bilateral massive adrenal hemorrhage: early recognition and treatment. Ann Intern Med. 1989;110:227-235. Medline\nWarkentin TE, Safyan EL, Linkins LA. Heparin-induced thrombocytopenia presenting as bilateral adrenal hemorrhages. N Engl J Med. 2015;372:492-494. Medline\nXarli VP, Steele AA, Davis PJ, Buescher ES, Rios CN, Garcia-Bunuel R. Adrenal hemorrhage in the adult. Medicine (Baltimore). 1978;57:211-221. Medline\nZelissen PM, Bast EJ, Croughs RJ. Associated autoimmunity in Addison's disease. J Autoimmun. 1995;8:121-130. Medline\n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1194684,
"choiceText": "Morning serum ACTH level testing",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1194686,
"choiceText": "Cosyntropin stimulation testing",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1194688,
"choiceText": "Serum aldosterone level testing",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1194690,
"choiceText": "24-hour urine free cortisol testing",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1194692,
"choiceText": "PM salivary cortisol testing",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1194694,
"choiceText": "No further testing",
"correct": true,
"displayOrder": 6,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>A morning serum cortisol level <3 µg/dL alone is diagnostic of adrenal insufficiency. Based on a clinical presentation and etiology related to adrenal hemorrhage, primary adrenal insufficiency can be presumed. Thus, further testing is not indicated to differentiate between primary, secondary, or tertiary causes of adrenal insufficiency.</p>",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 379777,
"questionText": "In a patient who presents in a fashion similar to the patient described in this case, including low morning serum cortisol level, which of the following is indicated for diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1194696,
"choiceText": "Hydrocortisone",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1194698,
"choiceText": "Fludrocortisone",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1194700,
"choiceText": "Hydrocortisone and fludrocortisone",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1194702,
"choiceText": "Hydrocortisone, fludrocortisone, and dehydroepiandrosterone",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1194704,
"choiceText": "Hydrocortisone, fludrocortisone, and salt tablets",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>This patient required treatment with both hydrocortisone and fludrocortisone. Because her morning cortisol confirmed deficient glucocorticoid levels, replacement with hydrocortisone was absolutely necessary. Hydrocortisone is most often initiated as a twice daily to match the diurnal pattern of cortisol release. Certain elements of this patient's presentation, including hypotension and hyponatremia, are reflective of a significant mineralocorticoid deficiency. As a result, mineralocorticoid replacement therapy with fludrocortisone is also indicated. Fludrocortisone acetate, a prodrug to fludrocortisone, is a synthetic oral adrenocortical steroid with potent mineralocorticoid properties and also moderate-high glucocorticoid effect.</p>",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 379779,
"questionText": "In a patient diagnosed with adrenal insufficiency who presents in a fashion similar to the patient described in this case, which of the following is the most appropriate pharmacotherapy?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 67-Year-Old Woman With Orthostatic Hypotension and Edema"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1194672,
"choiceText": "Secondary adrenal insufficiency",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1194674,
"choiceText": "Primary adrenal insufficiency",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1194676,
"choiceText": "Syndrome of inappropriate antidiuretic hormone secretion (SIADH)",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1194678,
"choiceText": "Decompensated diastolic heart failure",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1194680,
"choiceText": "Autonomic dysfunction",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1194682,
"choiceText": "Symptomatic anemia",
"correct": false,
"displayOrder": 6,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 379775,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1194684,
"choiceText": "Morning serum ACTH level testing",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1194686,
"choiceText": "Cosyntropin stimulation testing",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1194688,
"choiceText": "Serum aldosterone level testing",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1194690,
"choiceText": "24-hour urine free cortisol testing",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1194692,
"choiceText": "PM salivary cortisol testing",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1194694,
"choiceText": "No further testing",
"correct": true,
"displayOrder": 6,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>A morning serum cortisol level <3 µg/dL alone is diagnostic of adrenal insufficiency. Based on a clinical presentation and etiology related to adrenal hemorrhage, primary adrenal insufficiency can be presumed. Thus, further testing is not indicated to differentiate between primary, secondary, or tertiary causes of adrenal insufficiency.</p>",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 379777,
"questionText": "In a patient who presents in a fashion similar to the patient described in this case, including low morning serum cortisol level, which of the following is indicated for diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1194696,
"choiceText": "Hydrocortisone",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1194698,
"choiceText": "Fludrocortisone",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1194700,
"choiceText": "Hydrocortisone and fludrocortisone",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1194702,
"choiceText": "Hydrocortisone, fludrocortisone, and dehydroepiandrosterone",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1194704,
"choiceText": "Hydrocortisone, fludrocortisone, and salt tablets",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>This patient required treatment with both hydrocortisone and fludrocortisone. Because her morning cortisol confirmed deficient glucocorticoid levels, replacement with hydrocortisone was absolutely necessary. Hydrocortisone is most often initiated as a twice daily to match the diurnal pattern of cortisol release. Certain elements of this patient's presentation, including hypotension and hyponatremia, are reflective of a significant mineralocorticoid deficiency. As a result, mineralocorticoid replacement therapy with fludrocortisone is also indicated. Fludrocortisone acetate, a prodrug to fludrocortisone, is a synthetic oral adrenocortical steroid with potent mineralocorticoid properties and also moderate-high glucocorticoid effect.</p>",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 379779,
"questionText": "In a patient diagnosed with adrenal insufficiency who presents in a fashion similar to the patient described in this case, which of the following is the most appropriate pharmacotherapy?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
892220 | /viewarticle/892220 | [
{
"authors": "Basma Abdulhadi, MD",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"A 52-year-old African-American man is brought to the emergency department by his family for evaluation of headache, nausea, blurry vision, and confusion. The patient is somnolent but arousable and oriented to person only.",
"His medical history is significant for morbid obesity, hypertension, hyperlipidemia, and prediabetes. His medications include nifedipine, chlorthalidone, and atorvastatin. His family history is notable for hypertension, peripheral vascular disease, transient ischemic attack (father), and diabetes (mother)."
],
"date": "February 05, 2018",
"figures": [],
"markdown": "# A 52-Year-Old Man With Blurred Vision and Headache\n\n **Authors:** Basma Abdulhadi, MD \n **Date:** February 05, 2018\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nA 52-year-old African-American man is brought to the emergency department by his family for evaluation of headache, nausea, blurry vision, and confusion. The patient is somnolent but arousable and oriented to person only.\nHis medical history is significant for morbid obesity, hypertension, hyperlipidemia, and prediabetes. His medications include nifedipine, chlorthalidone, and atorvastatin. His family history is notable for hypertension, peripheral vascular disease, transient ischemic attack (father), and diabetes (mother).\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 52-Year-Old Man With Blurred Vision and Headache"
},
{
"authors": "Basma Abdulhadi, MD",
"content": [
"Upon physical examination, the patient is obese and noted to be drowsy. His vital signs reveal an oral temperature of 98.4°F, heart rate of 76 beats/min, blood pressure of 220/110 mm Hg, and respiratory rate of 12 breaths/min. His sclerae are anicteric, and his oropharynx is dry.",
"A funduscopic exam reveals retinal hemorrhages and exudates without papilledema (Figure 1).",
"Figure 1.",
"Apart from drowsiness and somnolence causing altered mental status, the rest of his neurologic examination is nonfocal. Respiratory and cardiovascular examination findings are normal. The abdomen is soft and nontender.",
"A complete blood cell count and basic metabolic panel findings are obtained, with no significant findings.",
"An ECG is obtained (Figure 2).",
"Figure 2."
],
"date": "February 05, 2018",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/892/220/892220-Thumb1.png"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/892/220/892220-Thumb2.png"
}
],
"markdown": "# A 52-Year-Old Man With Blurred Vision and Headache\n\n **Authors:** Basma Abdulhadi, MD \n **Date:** February 05, 2018\n\n ## Content\n\n Upon physical examination, the patient is obese and noted to be drowsy. His vital signs reveal an oral temperature of 98.4°F, heart rate of 76 beats/min, blood pressure of 220/110 mm Hg, and respiratory rate of 12 breaths/min. His sclerae are anicteric, and his oropharynx is dry.\nA funduscopic exam reveals retinal hemorrhages and exudates without papilledema (Figure 1).\nFigure 1.\nApart from drowsiness and somnolence causing altered mental status, the rest of his neurologic examination is nonfocal. Respiratory and cardiovascular examination findings are normal. The abdomen is soft and nontender.\nA complete blood cell count and basic metabolic panel findings are obtained, with no significant findings.\nAn ECG is obtained (Figure 2).\nFigure 2.\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1186516,
"choiceText": "Hypertensive emergency",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1186518,
"choiceText": "Hypertensive urgency",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1186520,
"choiceText": "Myocardial infarction",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1186522,
"choiceText": "Acute decompensated heart failure",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 377201,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 52-Year-Old Man With Blurred Vision and Headache"
},
{
"authors": "Basma Abdulhadi, MD",
"content": [
"Hypertension is a medical condition that affects approximately 1 billion people worldwide.[1] Hypertension accounted for more than 44 million emergency department visits in the United States in 2006; in 2009, the direct and indirect medical costs related to hypertension totaled $73 billion.[1] Moreover, an estimated 74% of patients are unaware that they have this condition, which explains why hypertension is often labeled the \"silent killer.\"",
"Hypertension is a known risk factor for cardiovascular disease and is more common than cigarette smoking, dyslipidemia, and diabetes.[1,2] Globally, hypertension accounts for an estimated 47% of all ischemic heart disease events and approximately 54% of all strokes. Hypertension increases the risk for stroke, coronary artery disease, heart failure, atrial fibrillation, and vascular dementia.[3,4] The risk for coronary disease and stroke in patients older than 50 years progressively increases with increasing blood pressure; the risk was shown to double with every 20 mm Hg and 10 mm Hg in systolic and diastolic blood pressure, respectively, beginning at a blood pressure of 115/75 mm Hg.[4] Clinical trials have shown that decreasing systolic blood pressure by 10-12 mm Hg and diastolic blood pressure by 5-6 mm Hg results in decreased cardiovascular morbidity and mortality. Hypertension is considered a modifiable risk factor for cardiovascular disease, and attempts should be made to control blood pressure to reduce the significant morbidity and mortality associated with uncontrolled hypertension.[1,2]",
"Around two thirds of patients receiving hypertension treatment fail to achieve adequate blood pressure control; approximately 1%-2% of patients with hypertension develop hypertensive urgency or hypertensive emergency at one point during the course of their lives.[3,4] Hypertensive emergency is defined as severe hypertension with evidence of impending or progressive end-organ dysfunction; hypertensive urgency is defined as severe hypertension without acute end-organ dysfunction.[4] Systolic blood pressure in these settings is usually above 180 mm Hg and diastolic blood pressure more than 120 mm Hg.[2,5] The incidence of hypertensive emergencies or urgencies is higher in men, elderly persons, and African-American individuals. Medication noncompliance, the use of stimulant drugs (eg, cocaine, amphetamines), and withdrawal from medications (eg, clonidine, beta-blockers) can precipitate hypertensive urgencies and emergencies.",
"The clinical manifestation of hypertensive emergency depends on the target organs involved, but those that are mainly affected are the cardiovascular, neurologic, and renal systems; manifestations include acute coronary syndrome, acute encephalopathy, acute hemorrhagic or ischemic strokes, papilledema, pulmonary edema, aortic dissection, and acute renal failure.[4,5] Acute hypertensive encephalopathy is usually reversible with appropriate management. Acute aortic dissection has a high immediate morbidity and mortality and necessitates more aggressive blood pressure reduction. It usually presents with severe, tearing chest pain that radiates to the back.[5,6]"
],
"date": "February 05, 2018",
"figures": [],
"markdown": "# A 52-Year-Old Man With Blurred Vision and Headache\n\n **Authors:** Basma Abdulhadi, MD \n **Date:** February 05, 2018\n\n ## Content\n\n Hypertension is a medical condition that affects approximately 1 billion people worldwide.[1] Hypertension accounted for more than 44 million emergency department visits in the United States in 2006; in 2009, the direct and indirect medical costs related to hypertension totaled $73 billion.[1] Moreover, an estimated 74% of patients are unaware that they have this condition, which explains why hypertension is often labeled the \"silent killer.\"\nHypertension is a known risk factor for cardiovascular disease and is more common than cigarette smoking, dyslipidemia, and diabetes.[1,2] Globally, hypertension accounts for an estimated 47% of all ischemic heart disease events and approximately 54% of all strokes. Hypertension increases the risk for stroke, coronary artery disease, heart failure, atrial fibrillation, and vascular dementia.[3,4] The risk for coronary disease and stroke in patients older than 50 years progressively increases with increasing blood pressure; the risk was shown to double with every 20 mm Hg and 10 mm Hg in systolic and diastolic blood pressure, respectively, beginning at a blood pressure of 115/75 mm Hg.[4] Clinical trials have shown that decreasing systolic blood pressure by 10-12 mm Hg and diastolic blood pressure by 5-6 mm Hg results in decreased cardiovascular morbidity and mortality. Hypertension is considered a modifiable risk factor for cardiovascular disease, and attempts should be made to control blood pressure to reduce the significant morbidity and mortality associated with uncontrolled hypertension.[1,2]\nAround two thirds of patients receiving hypertension treatment fail to achieve adequate blood pressure control; approximately 1%-2% of patients with hypertension develop hypertensive urgency or hypertensive emergency at one point during the course of their lives.[3,4] Hypertensive emergency is defined as severe hypertension with evidence of impending or progressive end-organ dysfunction; hypertensive urgency is defined as severe hypertension without acute end-organ dysfunction.[4] Systolic blood pressure in these settings is usually above 180 mm Hg and diastolic blood pressure more than 120 mm Hg.[2,5] The incidence of hypertensive emergencies or urgencies is higher in men, elderly persons, and African-American individuals. Medication noncompliance, the use of stimulant drugs (eg, cocaine, amphetamines), and withdrawal from medications (eg, clonidine, beta-blockers) can precipitate hypertensive urgencies and emergencies.\nThe clinical manifestation of hypertensive emergency depends on the target organs involved, but those that are mainly affected are the cardiovascular, neurologic, and renal systems; manifestations include acute coronary syndrome, acute encephalopathy, acute hemorrhagic or ischemic strokes, papilledema, pulmonary edema, aortic dissection, and acute renal failure.[4,5] Acute hypertensive encephalopathy is usually reversible with appropriate management. Acute aortic dissection has a high immediate morbidity and mortality and necessitates more aggressive blood pressure reduction. It usually presents with severe, tearing chest pain that radiates to the back.[5,6]\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1186516,
"choiceText": "Hypertensive emergency",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1186518,
"choiceText": "Hypertensive urgency",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1186520,
"choiceText": "Myocardial infarction",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1186522,
"choiceText": "Acute decompensated heart failure",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 377201,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 52-Year-Old Man With Blurred Vision and Headache"
},
{
"authors": "Basma Abdulhadi, MD",
"content": [
"A thorough history and focused physical examination should be performed for every patient presenting with elevated blood pressure to identify the presence of end-organ dysfunction. The history should include the patient's blood pressure medications, dosages, and compliance. Also, the use of recreational drugs, especially stimulants, should be determined. Blood pressure should be measured in both arms with an appropriately sized blood pressure cuff. Ophthalmoscopy should also be performed to evaluate for papilledema and exudates.",
"A neurologic examination is important for assessing mental status and the presence of focal neurologic deficits. Cardiac and pulmonary examinations are also crucial to assess for the presence of acute pulmonary edema. Patients should undergo electrocardiography upon presentation to evaluate for acute ischemia, myocardial infarction, and left ventricular hypertrophy. Blood tests should include a basic metabolic profile to assess renal function, and measurement of cardiac biomarkers may be necessary if acute coronary syndrome is suspected. Chest radiography should be obtained to assess for pulmonary edema and cardiomegaly. If mental status is altered or focal neurologic signs are present on examination, head imaging is important to obtain, as in this case.",
"In this case, the presence of retinal hemorrhages and exudates upon examination and the presence of left ventricular hypertrophy on the ECG indicates that the patient has end-organ complications from uncontrolled hypertension; his altered mental status points to hypertensive encephalopathy, which is reversible with timely management.[6,7] Brain imaging was obtained and did not show signs of acute cerebrovascular events. The patient was admitted to the medical intensive care unit with a goal of reducing his blood pressure gradually in a monitored setting to prevent the complications from hypoperfusion (eg, tissue ischemia) that occur if blood pressure is more rapidly reduced.[7]",
"Initial therapy for hypertensive emergencies consists of admitting the patient to a monitored unit with an initial therapeutic goal of reducing mean arterial pressure by no more than 10% within the first hour. Blood pressure that decreases more excessively within the first hour puts the patient at risk for worsening renal, coronary, and cerebral perfusion, resulting in ischemia.[2] After the first hour, the blood pressure can be lowered by 10% per hour to a value around 160/100 mm Hg. The blood pressure should then be gradually dropped to the patient's baseline over the next 24-48 hours. Once blood pressure is under control and end-organ dysfunction has ceased, the intravenous antihypertensives can be gradually tapered, and the patient can be transitioned back to oral hypertensive therapy. An important exception is patients with aortic dissection, in whom blood pressure should be reduced to at least 120 mm Hg (systolic blood pressure) within 20 minutes.[2]",
"Hypertensive urgencies can usually be managed by oral antihypertensives, whereas hypertensive emergencies require parenteral medications. The choice of pharmacologic agent to control blood pressure should be individualized on the basis of the patient's comorbidities and the end-organ dysfunction that has resulted.[2,6,7] Intravenous calcium-channel blockers are preferred in the management of hypertensive emergencies in the absence of acute heart failure and cardiac ischemia; in patients with myocardial ischemia or acute pulmonary edema, intravenous nitroglycerin remains the drug of choice. Esmolol is used when beta-blocker withdrawal is thought to contribute to hypertensive emergency. Esmolol should be avoided in acute heart failure. In pregnant patients with uncontrolled hypertension, methyldopa and labetalol are considered safe and are the commonly used medications.[2,6,7]"
],
"date": "February 05, 2018",
"figures": [],
"markdown": "# A 52-Year-Old Man With Blurred Vision and Headache\n\n **Authors:** Basma Abdulhadi, MD \n **Date:** February 05, 2018\n\n ## Content\n\n A thorough history and focused physical examination should be performed for every patient presenting with elevated blood pressure to identify the presence of end-organ dysfunction. The history should include the patient's blood pressure medications, dosages, and compliance. Also, the use of recreational drugs, especially stimulants, should be determined. Blood pressure should be measured in both arms with an appropriately sized blood pressure cuff. Ophthalmoscopy should also be performed to evaluate for papilledema and exudates.\nA neurologic examination is important for assessing mental status and the presence of focal neurologic deficits. Cardiac and pulmonary examinations are also crucial to assess for the presence of acute pulmonary edema. Patients should undergo electrocardiography upon presentation to evaluate for acute ischemia, myocardial infarction, and left ventricular hypertrophy. Blood tests should include a basic metabolic profile to assess renal function, and measurement of cardiac biomarkers may be necessary if acute coronary syndrome is suspected. Chest radiography should be obtained to assess for pulmonary edema and cardiomegaly. If mental status is altered or focal neurologic signs are present on examination, head imaging is important to obtain, as in this case.\nIn this case, the presence of retinal hemorrhages and exudates upon examination and the presence of left ventricular hypertrophy on the ECG indicates that the patient has end-organ complications from uncontrolled hypertension; his altered mental status points to hypertensive encephalopathy, which is reversible with timely management.[6,7] Brain imaging was obtained and did not show signs of acute cerebrovascular events. The patient was admitted to the medical intensive care unit with a goal of reducing his blood pressure gradually in a monitored setting to prevent the complications from hypoperfusion (eg, tissue ischemia) that occur if blood pressure is more rapidly reduced.[7]\nInitial therapy for hypertensive emergencies consists of admitting the patient to a monitored unit with an initial therapeutic goal of reducing mean arterial pressure by no more than 10% within the first hour. Blood pressure that decreases more excessively within the first hour puts the patient at risk for worsening renal, coronary, and cerebral perfusion, resulting in ischemia.[2] After the first hour, the blood pressure can be lowered by 10% per hour to a value around 160/100 mm Hg. The blood pressure should then be gradually dropped to the patient's baseline over the next 24-48 hours. Once blood pressure is under control and end-organ dysfunction has ceased, the intravenous antihypertensives can be gradually tapered, and the patient can be transitioned back to oral hypertensive therapy. An important exception is patients with aortic dissection, in whom blood pressure should be reduced to at least 120 mm Hg (systolic blood pressure) within 20 minutes.[2]\nHypertensive urgencies can usually be managed by oral antihypertensives, whereas hypertensive emergencies require parenteral medications. The choice of pharmacologic agent to control blood pressure should be individualized on the basis of the patient's comorbidities and the end-organ dysfunction that has resulted.[2,6,7] Intravenous calcium-channel blockers are preferred in the management of hypertensive emergencies in the absence of acute heart failure and cardiac ischemia; in patients with myocardial ischemia or acute pulmonary edema, intravenous nitroglycerin remains the drug of choice. Esmolol is used when beta-blocker withdrawal is thought to contribute to hypertensive emergency. Esmolol should be avoided in acute heart failure. In pregnant patients with uncontrolled hypertension, methyldopa and labetalol are considered safe and are the commonly used medications.[2,6,7]\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 52-Year-Old Man With Blurred Vision and Headache"
},
{
"authors": "Basma Abdulhadi, MD",
"content": [
"In this case, the patient's blood pressure was decreased by 20% in the medical intensive care unit over a couple of hours. He slowly became more awake and returned to his baseline mental status over the next 24 hours. He was transitioned back to his oral antihypertensive regimen. Upon further questioning, he reported running out of his blood pressure medications 1 week before his presentation. He was counseled on the importance of taking his blood pressure medications daily and the importance of keeping his blood pressure within normal ranges to avoid progression to cardiovascular and cerebrovascular disease.",
"The presence of left ventricular hypertrophy on ECG for a hypertensive patient is indicative of poor blood pressure control. Left ventricular hypertrophy can occur when the ventricle has to pump against a high afterload from elevated blood pressure. The presence of left ventricular hypertrophy has previously been shown to be associated with worsening outcomes, including sudden cardiac death, cardiovascular events, heart failure, acute stroke/transient ischemic attack, and increased all-cause and cardiac mortality.[5,8,9] Given its prognostic value, the presence of left ventricular hypertrophy should prompt improved blood pressure control to prevent further complications of uncontrolled hypertension.[8,9,10]",
"Addressing the patient's other comorbidities are also important to reduce his overall risk for cardiovascular disease. He now has diabetes and hyperlipidemia. He is on atorvastatin to control his hyperlipidemia. His diabetes should be optimized and monitored regularly, with a goal A1c level of 6%-7%. He was counseled about the importance of exercise and diet. Ensuring outpatient follow-up and risk factor modification is also crucial to prevent further hospitalizations and cardiac complications."
],
"date": "February 05, 2018",
"figures": [],
"markdown": "# A 52-Year-Old Man With Blurred Vision and Headache\n\n **Authors:** Basma Abdulhadi, MD \n **Date:** February 05, 2018\n\n ## Content\n\n In this case, the patient's blood pressure was decreased by 20% in the medical intensive care unit over a couple of hours. He slowly became more awake and returned to his baseline mental status over the next 24 hours. He was transitioned back to his oral antihypertensive regimen. Upon further questioning, he reported running out of his blood pressure medications 1 week before his presentation. He was counseled on the importance of taking his blood pressure medications daily and the importance of keeping his blood pressure within normal ranges to avoid progression to cardiovascular and cerebrovascular disease.\nThe presence of left ventricular hypertrophy on ECG for a hypertensive patient is indicative of poor blood pressure control. Left ventricular hypertrophy can occur when the ventricle has to pump against a high afterload from elevated blood pressure. The presence of left ventricular hypertrophy has previously been shown to be associated with worsening outcomes, including sudden cardiac death, cardiovascular events, heart failure, acute stroke/transient ischemic attack, and increased all-cause and cardiac mortality.[5,8,9] Given its prognostic value, the presence of left ventricular hypertrophy should prompt improved blood pressure control to prevent further complications of uncontrolled hypertension.[8,9,10]\nAddressing the patient's other comorbidities are also important to reduce his overall risk for cardiovascular disease. He now has diabetes and hyperlipidemia. He is on atorvastatin to control his hyperlipidemia. His diabetes should be optimized and monitored regularly, with a goal A1c level of 6%-7%. He was counseled about the importance of exercise and diet. Ensuring outpatient follow-up and risk factor modification is also crucial to prevent further hospitalizations and cardiac complications.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1186524,
"choiceText": "Labetalol",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1186526,
"choiceText": "Hydralazine",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1186528,
"choiceText": "Esmolol",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1186530,
"choiceText": "Nitroglycerin",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Nitroglycerin is a direct venous dilator that reduces preload. It has vasodilatory effects on coronary vessels and should be used in the setting of acute cardiac ischemia and pulmonary edema.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 377203,
"questionText": "A 56-year-old patient is brought to the emergency department with a blood pressure of 200/120 mm Hg. He is experiencing dyspnea and is found to be hypoxemic. Chest radiography is obtained and shows acute pulmonary edema. An ECG shows T-wave inversions in the inferolateral leads.\r\n<br><br>\r\nWhich of the following medications should be used to reduce his blood pressure? ",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1186532,
"choiceText": "Labetalol",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1186534,
"choiceText": "Enalapril",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1186536,
"choiceText": "Nifedipine",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1186538,
"choiceText": "Nitroprusside\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Labetalol is a combined alpha and beta-adrenergic receptor blocker. It is safe for use in pregnancy. It is also used in patients with aortic dissection. ",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 377205,
"questionText": "A 28-year-old woman who is 36 weeks pregnant is brought to the emergency department because of elevated blood pressure (180/90 mm Hg). She is otherwise asymptomatic. She has a history of preeclampsia in a previous pregnancy. <br><br>\r\n\r\nWhich of the following agents should be used to lower her blood pressure?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 52-Year-Old Man With Blurred Vision and Headache"
},
{
"authors": "Basma Abdulhadi, MD",
"content": [],
"date": "February 05, 2018",
"figures": [],
"markdown": "# A 52-Year-Old Man With Blurred Vision and Headache\n\n **Authors:** Basma Abdulhadi, MD \n **Date:** February 05, 2018\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1186524,
"choiceText": "Labetalol",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1186526,
"choiceText": "Hydralazine",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1186528,
"choiceText": "Esmolol",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1186530,
"choiceText": "Nitroglycerin",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Nitroglycerin is a direct venous dilator that reduces preload. It has vasodilatory effects on coronary vessels and should be used in the setting of acute cardiac ischemia and pulmonary edema.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 377203,
"questionText": "A 56-year-old patient is brought to the emergency department with a blood pressure of 200/120 mm Hg. He is experiencing dyspnea and is found to be hypoxemic. Chest radiography is obtained and shows acute pulmonary edema. An ECG shows T-wave inversions in the inferolateral leads.\r\n<br><br>\r\nWhich of the following medications should be used to reduce his blood pressure? ",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1186532,
"choiceText": "Labetalol",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1186534,
"choiceText": "Enalapril",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1186536,
"choiceText": "Nifedipine",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1186538,
"choiceText": "Nitroprusside\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Labetalol is a combined alpha and beta-adrenergic receptor blocker. It is safe for use in pregnancy. It is also used in patients with aortic dissection. ",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 377205,
"questionText": "A 28-year-old woman who is 36 weeks pregnant is brought to the emergency department because of elevated blood pressure (180/90 mm Hg). She is otherwise asymptomatic. She has a history of preeclampsia in a previous pregnancy. <br><br>\r\n\r\nWhich of the following agents should be used to lower her blood pressure?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 52-Year-Old Man With Blurred Vision and Headache"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1186516,
"choiceText": "Hypertensive emergency",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1186518,
"choiceText": "Hypertensive urgency",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1186520,
"choiceText": "Myocardial infarction",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1186522,
"choiceText": "Acute decompensated heart failure",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 377201,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1186524,
"choiceText": "Labetalol",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1186526,
"choiceText": "Hydralazine",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1186528,
"choiceText": "Esmolol",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1186530,
"choiceText": "Nitroglycerin",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Nitroglycerin is a direct venous dilator that reduces preload. It has vasodilatory effects on coronary vessels and should be used in the setting of acute cardiac ischemia and pulmonary edema.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 377203,
"questionText": "A 56-year-old patient is brought to the emergency department with a blood pressure of 200/120 mm Hg. He is experiencing dyspnea and is found to be hypoxemic. Chest radiography is obtained and shows acute pulmonary edema. An ECG shows T-wave inversions in the inferolateral leads.\r\n<br><br>\r\nWhich of the following medications should be used to reduce his blood pressure? ",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1186532,
"choiceText": "Labetalol",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1186534,
"choiceText": "Enalapril",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1186536,
"choiceText": "Nifedipine",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1186538,
"choiceText": "Nitroprusside\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Labetalol is a combined alpha and beta-adrenergic receptor blocker. It is safe for use in pregnancy. It is also used in patients with aortic dissection. ",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 377205,
"questionText": "A 28-year-old woman who is 36 weeks pregnant is brought to the emergency department because of elevated blood pressure (180/90 mm Hg). She is otherwise asymptomatic. She has a history of preeclampsia in a previous pregnancy. <br><br>\r\n\r\nWhich of the following agents should be used to lower her blood pressure?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
889518 | /viewarticle/889518 | [
{
"authors": "Ryland P. Byrd, Jr, MD; Ehab S. Kasasbeh, MD; Jonathan W. Burress, DO",
"content": [
"The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"An 80-year-old man is referred for a preoperative cardiac risk assessment before elective surgery for a hernia repair. He has no personal history of coronary artery disease or myocardial infarction, and no known family history of atherosclerotic heart disease.",
"The patient denies having any episodic chest pain and pressure, palpitations, nausea, vomiting, diaphoresis, or syncope. However, he describes some exertional shortness of breath that he attributes to a long history of habitual cigarette smoking, as well as an established diagnosis of chronic obstructive pulmonary disease (COPD).",
"In addition to the shortness of breath, he has also experienced brief episodes of lightheadedness from time to time. The lightheadedness occurs without warning and without any identifiable precipitating factor, and it abates without intervention. Other than his COPD, the patient has been remarkably healthy his whole life. His review of systems, other than as noted above, is negative."
],
"date": "December 08, 2017",
"figures": [],
"markdown": "# An 80-Year-Old Man With Lightheadedness\n\n **Authors:** Ryland P. Byrd, Jr, MD; Ehab S. Kasasbeh, MD; Jonathan W. Burress, DO \n **Date:** December 08, 2017\n\n ## Content\n\n The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nAn 80-year-old man is referred for a preoperative cardiac risk assessment before elective surgery for a hernia repair. He has no personal history of coronary artery disease or myocardial infarction, and no known family history of atherosclerotic heart disease.\nThe patient denies having any episodic chest pain and pressure, palpitations, nausea, vomiting, diaphoresis, or syncope. However, he describes some exertional shortness of breath that he attributes to a long history of habitual cigarette smoking, as well as an established diagnosis of chronic obstructive pulmonary disease (COPD).\nIn addition to the shortness of breath, he has also experienced brief episodes of lightheadedness from time to time. The lightheadedness occurs without warning and without any identifiable precipitating factor, and it abates without intervention. Other than his COPD, the patient has been remarkably healthy his whole life. His review of systems, other than as noted above, is negative.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "An 80-Year-Old Man With Lightheadedness"
},
{
"authors": "Ryland P. Byrd, Jr, MD; Ehab S. Kasasbeh, MD; Jonathan W. Burress, DO",
"content": [
"Upon physical examination, the patient is afebrile, with a blood pressure of 125/67 mm Hg, a pulse of 75 beats/min, a respiratory rate of 20 breaths/min, and an oxygen saturation of 91% while breathing room air. The patient appears younger than his stated age. He is in no acute distress.",
"The neck examination shows no appreciable jugular venous distention or carotid bruits. His heart sounds are remarkable for a regular heart rhythm, with frequent skipped beats and a slightly accentuated second heart sound. No murmurs, rubs, or gallops are appreciated. Auscultation of his chest reveals distant breath sounds with no wheezing, crackles, or rhonchi. No peripheral edema of the lower extremities is observed. The remainder of the physical examination is unremarkable.",
"A panel of preoperative blood tests and an ECG are ordered. While the patient is waiting in the preoperative holding area, he experiences an episode of lightheadedness. Upon noting a rapid pulse, a technician attaches leads to obtain a cardiac rhythm strip and an ECG. The patient's blood pressure is recorded at 80/46 mm Hg. A 12-lead ECG is obtained (Figure 1).",
"Figure 1."
],
"date": "December 08, 2017",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/889/518/889518-Figure-1-thumb.jpg"
}
],
"markdown": "# An 80-Year-Old Man With Lightheadedness\n\n **Authors:** Ryland P. Byrd, Jr, MD; Ehab S. Kasasbeh, MD; Jonathan W. Burress, DO \n **Date:** December 08, 2017\n\n ## Content\n\n Upon physical examination, the patient is afebrile, with a blood pressure of 125/67 mm Hg, a pulse of 75 beats/min, a respiratory rate of 20 breaths/min, and an oxygen saturation of 91% while breathing room air. The patient appears younger than his stated age. He is in no acute distress.\nThe neck examination shows no appreciable jugular venous distention or carotid bruits. His heart sounds are remarkable for a regular heart rhythm, with frequent skipped beats and a slightly accentuated second heart sound. No murmurs, rubs, or gallops are appreciated. Auscultation of his chest reveals distant breath sounds with no wheezing, crackles, or rhonchi. No peripheral edema of the lower extremities is observed. The remainder of the physical examination is unremarkable.\nA panel of preoperative blood tests and an ECG are ordered. While the patient is waiting in the preoperative holding area, he experiences an episode of lightheadedness. Upon noting a rapid pulse, a technician attaches leads to obtain a cardiac rhythm strip and an ECG. The patient's blood pressure is recorded at 80/46 mm Hg. A 12-lead ECG is obtained (Figure 1).\nFigure 1.\n\n ## Figures\n\n **Figure 1.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1166908,
"choiceText": "Nonsustained ventricular tachycardia",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1166910,
"choiceText": "Supraventricular tachycardia with aberrant conduction",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1166912,
"choiceText": "Atrial flutter",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1166914,
"choiceText": "Torsade de pointes",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 370931,
"questionText": "Which of the following rhythms does the first portion of the ECG demonstrate?<br><em>Hint: Note the association between the P waves and each QRS complex.</em>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "An 80-Year-Old Man With Lightheadedness"
},
{
"authors": "Ryland P. Byrd, Jr, MD; Ehab S. Kasasbeh, MD; Jonathan W. Burress, DO",
"content": [
"A wide-complex tachycardia is a cardiac dysrhythmia with a ventricular rate > 100 beats/min in the setting of a QRS duration ≥ 120 ms. A wide-complex tachycardia can originate from either a ventricular focus or a supraventricular focus associated with a conduction abnormality. In this case, on the basis of the QRS morphology (the QRS width being > 140 ms at the widest leads) and the atrioventricular (AV) dissociation (arrows in Figure 2), the ECG was determined to have the characteristics of ventricular tachycardia.",
"Figure 2.",
"Ventricular tachycardia is the most common cause of wide-complex tachycardias, accounting for as many as 80% of cases. The frequency can be even higher in patients with structural or ischemic heart disease. Ventricular tachycardia also occurs in patients with electrolyte abnormalities, such as hypokalemia and hypomagnesemia, as well as in hypoxemic patients, individuals with acidemia, and patients with mitral valve prolapse. The rhythm can occasionally occur in individuals without any identifiable risk factors.",
"Adverse drug reactions can also induce ventricular tachycardia by prolonging the QT interval. Drugs that are known to increase the risk for ventricular tachycardia include digitalis; phenothiazines; tricyclic antidepressants; some long-acting antihistamines; and, paradoxically, antiarrhythmics. Digitalis toxicity can also cause a rare bidirectional ventricular tachycardia in which the QRS complexes in any given lead alternate in polarity. Finally, common iatrogenic causes of wide-complex tachycardia in certain settings include electronic pacemakers or implantable cardioverter-defibrillators with pacemaker capability.[1]",
"As mentioned above, a tachycardic rhythm with a wide QRS complex can also occur in association with a supraventricular tachycardia with abnormal conduction, which can make differentiation of ventricular tachycardia from supraventricular tachycardia with aberrant conduction difficult in the acute setting (especially because both types of patients may present with similar symptoms)."
],
"date": "December 08, 2017",
"figures": [
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/889/518/889518-Figure-2-thumb.jpg"
}
],
"markdown": "# An 80-Year-Old Man With Lightheadedness\n\n **Authors:** Ryland P. Byrd, Jr, MD; Ehab S. Kasasbeh, MD; Jonathan W. Burress, DO \n **Date:** December 08, 2017\n\n ## Content\n\n A wide-complex tachycardia is a cardiac dysrhythmia with a ventricular rate > 100 beats/min in the setting of a QRS duration ≥ 120 ms. A wide-complex tachycardia can originate from either a ventricular focus or a supraventricular focus associated with a conduction abnormality. In this case, on the basis of the QRS morphology (the QRS width being > 140 ms at the widest leads) and the atrioventricular (AV) dissociation (arrows in Figure 2), the ECG was determined to have the characteristics of ventricular tachycardia.\nFigure 2.\nVentricular tachycardia is the most common cause of wide-complex tachycardias, accounting for as many as 80% of cases. The frequency can be even higher in patients with structural or ischemic heart disease. Ventricular tachycardia also occurs in patients with electrolyte abnormalities, such as hypokalemia and hypomagnesemia, as well as in hypoxemic patients, individuals with acidemia, and patients with mitral valve prolapse. The rhythm can occasionally occur in individuals without any identifiable risk factors.\nAdverse drug reactions can also induce ventricular tachycardia by prolonging the QT interval. Drugs that are known to increase the risk for ventricular tachycardia include digitalis; phenothiazines; tricyclic antidepressants; some long-acting antihistamines; and, paradoxically, antiarrhythmics. Digitalis toxicity can also cause a rare bidirectional ventricular tachycardia in which the QRS complexes in any given lead alternate in polarity. Finally, common iatrogenic causes of wide-complex tachycardia in certain settings include electronic pacemakers or implantable cardioverter-defibrillators with pacemaker capability.[1]\nAs mentioned above, a tachycardic rhythm with a wide QRS complex can also occur in association with a supraventricular tachycardia with abnormal conduction, which can make differentiation of ventricular tachycardia from supraventricular tachycardia with aberrant conduction difficult in the acute setting (especially because both types of patients may present with similar symptoms).\n\n ## Figures\n\n **Figure 2.** \n \n\n\n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1166908,
"choiceText": "Nonsustained ventricular tachycardia",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1166910,
"choiceText": "Supraventricular tachycardia with aberrant conduction",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1166912,
"choiceText": "Atrial flutter",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1166914,
"choiceText": "Torsade de pointes",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 370931,
"questionText": "Which of the following rhythms does the first portion of the ECG demonstrate?<br><em>Hint: Note the association between the P waves and each QRS complex.</em>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "An 80-Year-Old Man With Lightheadedness"
},
{
"authors": "Ryland P. Byrd, Jr, MD; Ehab S. Kasasbeh, MD; Jonathan W. Burress, DO",
"content": [
"The symptoms associated with a wide-complex tachycardia are typically caused by decreased cardiac output; they include orthostasis, hypotension, presyncope, syncope, dyspnea, and exercise limitation. Of note, monomorphic ventricular tachycardia can be asymptomatic, despite the widespread belief that sustained ventricular tachycardia always produces symptoms. Clinical symptomatology is, therefore, of limited use in the differentiation of ventricular tachycardia from supraventricular tachycardia.",
"Accurately diagnosing the underlying rhythm in a patient with a wide-complex tachycardia is critical for determining treatment and management, especially if the patient presents emergently and is hemodynamically unstable. This is of particular concern because the medications routinely used to treat supraventricular tachycardia can cause severe hemodynamic deterioration by inducing the relatively stable rhythm of ventricular tachycardia to degenerate into ventricular fibrillation, with subsequent cardiac arrest. In fact, misdiagnosis of ventricular tachycardia as supraventricular tachycardia with abnormal conduction in patients presenting with a wide-complex tachycardia is not uncommon, especially if the abnormal rhythm is hemodynamically tolerated.",
"In general, if the clinician is unsure, a wide-complex tachycardia should be presumed to be a ventricular tachycardia until the presence of supraventricular tachycardia can be definitively proven. A patient with a wide-complex tachycardia in an unstable condition should receive immediate electrical cardioversion. In patients with a stable ventricular tachycardia or with a wide-complex tachycardia of unclear origin, pharmacologic agents, including amiodarone, procainamide, or lidocaine, may be used in accordance with established advanced cardiovascular life support guidelines. If it is determined that a wide-complex tachycardia is a supraventricular tachycardia with abnormal conduction, a trial of vagal stimulation (carotid massage) or treatment with adenosine may be attempted.[2]",
"Several studies have, by using various criteria or combinations of criteria, attempted to improve the diagnostic accuracy of differentiating ventricular tachycardia from supraventricular tachycardia in the evaluation of wide-complex tachycardia. Although no single algorithm is 100% sensitive and 100% specific, several characteristics and clues can be of use. One of the best-recognized systematic algorithms consists of four differentiating characteristics proposed by Brugada and colleagues,[3] as follows:",
"If an RS complex cannot be identified in any precordial lead, ventricular tachycardia can be diagnosed with 100% specificity and 21% sensitivity.",
"If an RS complex is clearly distinguished in one or more precordial leads, the interval between the onset of the R wave and the deepest part of the S wave (RS interval) is measured (if RS complexes are present in several precordial leads, the longest RS interval is used). If the RS interval is > 100 ms, ventricular tachycardia can be diagnosed with 98% specificity and 66% sensitivity.",
"If the RS interval is < 100 ms, the presence or absence of AV dissociation must be determined. Evidence of AV dissociation is 100% specific and 82% sensitive for ventricular tachycardia; this is because AV dissociation does not occur in supraventricular tachycardia. AV dissociation is characterized by atrial activity that is completely independent of ventricular activity. Although the presence of AV dissociation establishes ventricular tachycardia as the etiology, its absence does not exclude the possibility of ventricular tachycardia with retrograde conduction of ventricular impulses through the AV node producing an atrial rhythm. This phenomenon, called \"retrograde ventriculoatrial conduction,\" is easily misinterpreted as AV conduction because of the presence of P waves.",
"If the RS interval is < 100 ms, and if AV dissociation cannot clearly be demonstrated, the QRS morphology may be evaluated. Morphologic criteria suggestive of ventricular tachycardia are extensive and complex, and they should be evaluated in conjunction with a cardiologist, if necessary. This final step in evaluation moves the algorithm to 96.5% specific and 98.7% sensitive."
],
"date": "December 08, 2017",
"figures": [],
"markdown": "# An 80-Year-Old Man With Lightheadedness\n\n **Authors:** Ryland P. Byrd, Jr, MD; Ehab S. Kasasbeh, MD; Jonathan W. Burress, DO \n **Date:** December 08, 2017\n\n ## Content\n\n The symptoms associated with a wide-complex tachycardia are typically caused by decreased cardiac output; they include orthostasis, hypotension, presyncope, syncope, dyspnea, and exercise limitation. Of note, monomorphic ventricular tachycardia can be asymptomatic, despite the widespread belief that sustained ventricular tachycardia always produces symptoms. Clinical symptomatology is, therefore, of limited use in the differentiation of ventricular tachycardia from supraventricular tachycardia.\nAccurately diagnosing the underlying rhythm in a patient with a wide-complex tachycardia is critical for determining treatment and management, especially if the patient presents emergently and is hemodynamically unstable. This is of particular concern because the medications routinely used to treat supraventricular tachycardia can cause severe hemodynamic deterioration by inducing the relatively stable rhythm of ventricular tachycardia to degenerate into ventricular fibrillation, with subsequent cardiac arrest. In fact, misdiagnosis of ventricular tachycardia as supraventricular tachycardia with abnormal conduction in patients presenting with a wide-complex tachycardia is not uncommon, especially if the abnormal rhythm is hemodynamically tolerated.\nIn general, if the clinician is unsure, a wide-complex tachycardia should be presumed to be a ventricular tachycardia until the presence of supraventricular tachycardia can be definitively proven. A patient with a wide-complex tachycardia in an unstable condition should receive immediate electrical cardioversion. In patients with a stable ventricular tachycardia or with a wide-complex tachycardia of unclear origin, pharmacologic agents, including amiodarone, procainamide, or lidocaine, may be used in accordance with established advanced cardiovascular life support guidelines. If it is determined that a wide-complex tachycardia is a supraventricular tachycardia with abnormal conduction, a trial of vagal stimulation (carotid massage) or treatment with adenosine may be attempted.[2]\nSeveral studies have, by using various criteria or combinations of criteria, attempted to improve the diagnostic accuracy of differentiating ventricular tachycardia from supraventricular tachycardia in the evaluation of wide-complex tachycardia. Although no single algorithm is 100% sensitive and 100% specific, several characteristics and clues can be of use. One of the best-recognized systematic algorithms consists of four differentiating characteristics proposed by Brugada and colleagues,[3] as follows:\nIf an RS complex cannot be identified in any precordial lead, ventricular tachycardia can be diagnosed with 100% specificity and 21% sensitivity.\nIf an RS complex is clearly distinguished in one or more precordial leads, the interval between the onset of the R wave and the deepest part of the S wave (RS interval) is measured (if RS complexes are present in several precordial leads, the longest RS interval is used). If the RS interval is > 100 ms, ventricular tachycardia can be diagnosed with 98% specificity and 66% sensitivity.\nIf the RS interval is < 100 ms, the presence or absence of AV dissociation must be determined. Evidence of AV dissociation is 100% specific and 82% sensitive for ventricular tachycardia; this is because AV dissociation does not occur in supraventricular tachycardia. AV dissociation is characterized by atrial activity that is completely independent of ventricular activity. Although the presence of AV dissociation establishes ventricular tachycardia as the etiology, its absence does not exclude the possibility of ventricular tachycardia with retrograde conduction of ventricular impulses through the AV node producing an atrial rhythm. This phenomenon, called \"retrograde ventriculoatrial conduction,\" is easily misinterpreted as AV conduction because of the presence of P waves.\nIf the RS interval is < 100 ms, and if AV dissociation cannot clearly be demonstrated, the QRS morphology may be evaluated. Morphologic criteria suggestive of ventricular tachycardia are extensive and complex, and they should be evaluated in conjunction with a cardiologist, if necessary. This final step in evaluation moves the algorithm to 96.5% specific and 98.7% sensitive.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "An 80-Year-Old Man With Lightheadedness"
},
{
"authors": "Ryland P. Byrd, Jr, MD; Ehab S. Kasasbeh, MD; Jonathan W. Burress, DO",
"content": [
"Other characteristic ECG findings that can be helpful for quickly differentiating ventricular tachycardia from supraventricular tachycardia include the following[1,4]:",
"An extreme rightward axis (-90° to -180°) is often more suggestive of ventricular tachycardia.",
"A slight irregularity of the RR intervals, especially in the early stages before settling into a regular rhythm, can be suggestive of ventricular tachycardia.",
"The width of the QRS complex can also be useful for distinguishing supraventricular tachycardia from ventricular tachycardia. In general, a wide QRS complex > 140 ms suggests tachycardia; however, a QRS duration < 140 ms is not helpful for excluding ventricular tachycardia, because ventricular tachycardia is sometimes associated with a relatively narrow QRS complex.",
"If the degree of voltage change in the first 40 ms of the QRS complex is less than the degree of voltage change in the last 40 ms of the complex, this finding is suggestive of ventricular tachycardia.",
"Fusion occurs when a supraventricular impulse reaches the AV node simultaneously with a ventricular impulse. The resulting QRS complex has a hybrid morphology that is between a narrow atrial complex and a wide ventricular complex. Intermittent fusion beats during a wide-complex tachycardia indicate AV dissociation and, therefore, also indicate ventricular tachycardia.",
"A capture beat occurs when a supraventricular rhythm briefly conducts in a normal fashion, with a resultant normal QRS complex. The term \"capture beat\" implies that the normal conduction system has momentarily replaced the control of a ventricular focus; hence, ventricular tachycardia is present.",
"As mentioned, the patient in this case was diagnosed with ventricular tachycardia, and elective surgery for repairing the hernia was put on hold. An electrophysiology study was arranged after consultation with a cardiologist. An arrhythmogenic focus of myocardial irritability, which was thought to be caused by scar tissue from an unrecognized previous myocardial infarction, was identified during the study. The patient had an automatic internal cardiac defibrillator placed and, subsequently, his hernia was successfully repaired."
],
"date": "December 08, 2017",
"figures": [],
"markdown": "# An 80-Year-Old Man With Lightheadedness\n\n **Authors:** Ryland P. Byrd, Jr, MD; Ehab S. Kasasbeh, MD; Jonathan W. Burress, DO \n **Date:** December 08, 2017\n\n ## Content\n\n Other characteristic ECG findings that can be helpful for quickly differentiating ventricular tachycardia from supraventricular tachycardia include the following[1,4]:\nAn extreme rightward axis (-90° to -180°) is often more suggestive of ventricular tachycardia.\nA slight irregularity of the RR intervals, especially in the early stages before settling into a regular rhythm, can be suggestive of ventricular tachycardia.\nThe width of the QRS complex can also be useful for distinguishing supraventricular tachycardia from ventricular tachycardia. In general, a wide QRS complex > 140 ms suggests tachycardia; however, a QRS duration < 140 ms is not helpful for excluding ventricular tachycardia, because ventricular tachycardia is sometimes associated with a relatively narrow QRS complex.\nIf the degree of voltage change in the first 40 ms of the QRS complex is less than the degree of voltage change in the last 40 ms of the complex, this finding is suggestive of ventricular tachycardia.\nFusion occurs when a supraventricular impulse reaches the AV node simultaneously with a ventricular impulse. The resulting QRS complex has a hybrid morphology that is between a narrow atrial complex and a wide ventricular complex. Intermittent fusion beats during a wide-complex tachycardia indicate AV dissociation and, therefore, also indicate ventricular tachycardia.\nA capture beat occurs when a supraventricular rhythm briefly conducts in a normal fashion, with a resultant normal QRS complex. The term \"capture beat\" implies that the normal conduction system has momentarily replaced the control of a ventricular focus; hence, ventricular tachycardia is present.\nAs mentioned, the patient in this case was diagnosed with ventricular tachycardia, and elective surgery for repairing the hernia was put on hold. An electrophysiology study was arranged after consultation with a cardiologist. An arrhythmogenic focus of myocardial irritability, which was thought to be caused by scar tissue from an unrecognized previous myocardial infarction, was identified during the study. The patient had an automatic internal cardiac defibrillator placed and, subsequently, his hernia was successfully repaired.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1166916,
"choiceText": "Ventricular tachycardia accounts for 80% of cases of wide-complex tachycardia",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1166918,
"choiceText": "The presumptive diagnosis of ventricular tachycardia guards against inappropriate and potentially dangerous therapies",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1166920,
"choiceText": "Treatment of supraventricular tachycardia as if it were ventricular tachycardia is safe and frequently effective at restoring a sinus rhythm",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1166922,
"choiceText": "The presence of hemodynamic stability should be regarded as diagnostic of supraventricular tachycardia with aberrancy",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1166924,
"choiceText": "Structural heart disease is the most common cause of ventricular tachycardia",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Differentiation of ventricular tachycardia from supraventricular tachycardia with aberrant conduction is difficult in the acute setting (especially because both types of patients may present with similar symptoms). Hemodynamic stability should not be regarded as diagnostic of supraventricular tachycardia with aberrancy.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 370933,
"questionText": "Which of the following statements concerning wide-complex tachycardias is <em>not</em> accurate?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1166926,
"choiceText": "RS complex not present in any precordial lead",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1166928,
"choiceText": "Regularity of the RR interval",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1166930,
"choiceText": "RS interval > 100 ms",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1166932,
"choiceText": "AV dissociation",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1166934,
"choiceText": "Presence of capture beats",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "A slight irregularity of the RR intervals, especially in the early stages before settling into a regular rhythm, can be suggestive of ventricular tachycardia.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 370935,
"questionText": "Which of the following ECG findings is <em>not</em> suggestive of ventricular tachycardia?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "An 80-Year-Old Man With Lightheadedness"
},
{
"authors": "Ryland P. Byrd, Jr, MD; Ehab S. Kasasbeh, MD; Jonathan W. Burress, DO",
"content": [],
"date": "December 08, 2017",
"figures": [],
"markdown": "# An 80-Year-Old Man With Lightheadedness\n\n **Authors:** Ryland P. Byrd, Jr, MD; Ehab S. Kasasbeh, MD; Jonathan W. Burress, DO \n **Date:** December 08, 2017\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1166916,
"choiceText": "Ventricular tachycardia accounts for 80% of cases of wide-complex tachycardia",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1166918,
"choiceText": "The presumptive diagnosis of ventricular tachycardia guards against inappropriate and potentially dangerous therapies",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1166920,
"choiceText": "Treatment of supraventricular tachycardia as if it were ventricular tachycardia is safe and frequently effective at restoring a sinus rhythm",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1166922,
"choiceText": "The presence of hemodynamic stability should be regarded as diagnostic of supraventricular tachycardia with aberrancy",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1166924,
"choiceText": "Structural heart disease is the most common cause of ventricular tachycardia",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Differentiation of ventricular tachycardia from supraventricular tachycardia with aberrant conduction is difficult in the acute setting (especially because both types of patients may present with similar symptoms). Hemodynamic stability should not be regarded as diagnostic of supraventricular tachycardia with aberrancy.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 370933,
"questionText": "Which of the following statements concerning wide-complex tachycardias is <em>not</em> accurate?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1166926,
"choiceText": "RS complex not present in any precordial lead",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1166928,
"choiceText": "Regularity of the RR interval",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1166930,
"choiceText": "RS interval > 100 ms",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1166932,
"choiceText": "AV dissociation",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1166934,
"choiceText": "Presence of capture beats",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "A slight irregularity of the RR intervals, especially in the early stages before settling into a regular rhythm, can be suggestive of ventricular tachycardia.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 370935,
"questionText": "Which of the following ECG findings is <em>not</em> suggestive of ventricular tachycardia?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "An 80-Year-Old Man With Lightheadedness"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1166908,
"choiceText": "Nonsustained ventricular tachycardia",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1166910,
"choiceText": "Supraventricular tachycardia with aberrant conduction",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1166912,
"choiceText": "Atrial flutter",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1166914,
"choiceText": "Torsade de pointes",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 370931,
"questionText": "Which of the following rhythms does the first portion of the ECG demonstrate?<br><em>Hint: Note the association between the P waves and each QRS complex.</em>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1166916,
"choiceText": "Ventricular tachycardia accounts for 80% of cases of wide-complex tachycardia",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1166918,
"choiceText": "The presumptive diagnosis of ventricular tachycardia guards against inappropriate and potentially dangerous therapies",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1166920,
"choiceText": "Treatment of supraventricular tachycardia as if it were ventricular tachycardia is safe and frequently effective at restoring a sinus rhythm",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1166922,
"choiceText": "The presence of hemodynamic stability should be regarded as diagnostic of supraventricular tachycardia with aberrancy",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1166924,
"choiceText": "Structural heart disease is the most common cause of ventricular tachycardia",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Differentiation of ventricular tachycardia from supraventricular tachycardia with aberrant conduction is difficult in the acute setting (especially because both types of patients may present with similar symptoms). Hemodynamic stability should not be regarded as diagnostic of supraventricular tachycardia with aberrancy.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 370933,
"questionText": "Which of the following statements concerning wide-complex tachycardias is <em>not</em> accurate?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1166926,
"choiceText": "RS complex not present in any precordial lead",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1166928,
"choiceText": "Regularity of the RR interval",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1166930,
"choiceText": "RS interval > 100 ms",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1166932,
"choiceText": "AV dissociation",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1166934,
"choiceText": "Presence of capture beats",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "A slight irregularity of the RR intervals, especially in the early stages before settling into a regular rhythm, can be suggestive of ventricular tachycardia.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 370935,
"questionText": "Which of the following ECG findings is <em>not</em> suggestive of ventricular tachycardia?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
887423 | /viewarticle/887423 | [
{
"authors": "Alba Morales Pozzo, MD",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"A previously healthy 4-year-old girl, who was delivered at normal gestation, developed frequent urination, excessive thirst, and new-onset enuresis 4 weeks prior to her presentation at an emergency department (ED) in a children's hospital. Her parents report that on the day of the ED visit, she appeared fatigued, irritable, and pale. The patient also described abdominal pain and has had seven to 10 episodes of emesis over the last 2 days.",
"The patient takes no medications at home and has no significant medical or surgical history. Her development has been normal to this point, and her vaccinations are up to date. Her family history is remarkable for type 2 diabetes mellitus in her maternal grandmother."
],
"date": "March 05, 2019",
"figures": [],
"markdown": "# A 4-Year-Old Girl in Significant Distress\n\n **Authors:** Alba Morales Pozzo, MD \n **Date:** March 05, 2019\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nA previously healthy 4-year-old girl, who was delivered at normal gestation, developed frequent urination, excessive thirst, and new-onset enuresis 4 weeks prior to her presentation at an emergency department (ED) in a children's hospital. Her parents report that on the day of the ED visit, she appeared fatigued, irritable, and pale. The patient also described abdominal pain and has had seven to 10 episodes of emesis over the last 2 days.\nThe patient takes no medications at home and has no significant medical or surgical history. Her development has been normal to this point, and her vaccinations are up to date. Her family history is remarkable for type 2 diabetes mellitus in her maternal grandmother.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 4-Year-Old Girl in Significant Distress"
},
{
"authors": "Alba Morales Pozzo, MD",
"content": [
"Upon physical examination, her vital signs are as follows:",
"Blood pressure: 80/40 mm Hg",
"Pulse: 165 beats/min",
"Temperature: 98.5°F (36.9°C)",
"Weight: 30 lb (13.6 kg; 10th percentile)",
"Height: 40 in (101.6 cm; 65th percentile)",
"The initial examination reveals an ill-appearing, severely dehydrated, and almost obtunded toddler. Her head and neck findings are normal except for dry lips, sunken eyes, and dry oral mucosae. Chest, abdominal, and extremity examinations reveal only a delayed capillary refill of 3-4 seconds. A neurologic examination is remarkable for somnolence. No skin lesions are noted.",
"Her initial laboratory findings are noted in the following table:",
"Table. Initial Laboratory Findings",
"Management for severe diabetic ketoacidosis was rapidly implemented (intravenous rehydration and insulin therapy as per hospital protocol). A right femoral central venous catheter (CVC) was inserted for quick fluid resuscitation while in the ED. Twenty-eight hours after her admission to the intensive care unit, her parents note that she refuses to bear weight on her right leg, which now appears to be swollen.",
"The patient is awake, alert, and answers questions appropriately. She appears much improved compared with her physical examination upon admission, with moist, pink mucosae and brisk capillary refill. Her head and neck examination findings are normal. Her lung and heart sounds are normal. An abdominal examination is unremarkable.",
"Her right lower extremity is noted to be edematous from mid-foot to just above her knee. The pedal, posterior malleolar, and popliteal pulses on the right leg are palpable. The skin over the leg has mild erythema and is warm to the touch, with no visible lesions. She avoids putting weight on her right foot when she tries to stand and appears to be in moderate discomfort as she stands."
],
"date": "March 05, 2019",
"figures": [],
"markdown": "# A 4-Year-Old Girl in Significant Distress\n\n **Authors:** Alba Morales Pozzo, MD \n **Date:** March 05, 2019\n\n ## Content\n\n Upon physical examination, her vital signs are as follows:\nBlood pressure: 80/40 mm Hg\nPulse: 165 beats/min\nTemperature: 98.5°F (36.9°C)\nWeight: 30 lb (13.6 kg; 10th percentile)\nHeight: 40 in (101.6 cm; 65th percentile)\nThe initial examination reveals an ill-appearing, severely dehydrated, and almost obtunded toddler. Her head and neck findings are normal except for dry lips, sunken eyes, and dry oral mucosae. Chest, abdominal, and extremity examinations reveal only a delayed capillary refill of 3-4 seconds. A neurologic examination is remarkable for somnolence. No skin lesions are noted.\nHer initial laboratory findings are noted in the following table:\nTable. Initial Laboratory Findings\nManagement for severe diabetic ketoacidosis was rapidly implemented (intravenous rehydration and insulin therapy as per hospital protocol). A right femoral central venous catheter (CVC) was inserted for quick fluid resuscitation while in the ED. Twenty-eight hours after her admission to the intensive care unit, her parents note that she refuses to bear weight on her right leg, which now appears to be swollen.\nThe patient is awake, alert, and answers questions appropriately. She appears much improved compared with her physical examination upon admission, with moist, pink mucosae and brisk capillary refill. Her head and neck examination findings are normal. Her lung and heart sounds are normal. An abdominal examination is unremarkable.\nHer right lower extremity is noted to be edematous from mid-foot to just above her knee. The pedal, posterior malleolar, and popliteal pulses on the right leg are palpable. The skin over the leg has mild erythema and is warm to the touch, with no visible lesions. She avoids putting weight on her right foot when she tries to stand and appears to be in moderate discomfort as she stands.\n\n ## Figures\n\n \n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1150292,
"choiceText": "Cellulitis ",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1150294,
"choiceText": "Deep vein thrombosis",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1150296,
"choiceText": "Hematoma ",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1150298,
"choiceText": "Head of tibia fracture\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 365339,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 4-Year-Old Girl in Significant Distress"
},
{
"authors": "Alba Morales Pozzo, MD",
"content": [
"This patient has either a deep femoral venous thrombosis or a thrombus within another of the deep veins of the right lower extremity, most likely as a complication of CVC placement in a very young patient with severe diabetic ketoacidosis.",
"The typical signs and symptoms associated with deep venous thrombosis (DVT) of an extremity are persistent extremity edema with or without pain or changes in skin color, prominent superficial veins, tenderness, pain with passive dorsiflexion of the foot (Homans sign), and peripheral cyanosis. Signs and symptoms tend to be nonspecific and can vary in severity depending on the size of the thrombus and extent of venous occlusion. An example in an adult is shown in Figure 1.",
"Figure 1.",
"CVC use in the pediatric population is increasing, which has caused an increase in the incidence of pediatric DVT. CVCs can increase the risk for DVT by causing vascular damage and causing turbulent blood flow in the vessel (Figure 2).",
"Figure 2.",
"When compared with the adult population, thromboembolism in pediatric patients is rare, with most of cases occurring in hospitalized children. In 1845, Virchow described the three important factors in the development of a thrombus as follows:",
"Impairment of blood flow",
"Vascular injury",
"Alterations in the blood (hypercoagulability)",
"Common risk factors for thrombus formation include the following:",
"Presence of a CVC",
"Immobility",
"Heart disease",
"Ventriculoatrial shunt",
"Trauma",
"Cancer",
"Surgery",
"Infection",
"Dehydration",
"Shock",
"Pregnancy",
"Smoking",
"Obesity",
"Persons with poorly controlled diabetes seem to have abnormalities of hemostasis, but the mechanism that causes the problem is not well understood. Clinical studies in patients with diabetic ketoacidosis have demonstrated transient changes in coagulation factors (increased platelet activation, fibrinolytic activity, and endothelial activation).[1,2,3] Diabetic ketoacidosis is also accompanied by an enhanced inflammatory state, with increased levels of cytokines, C-reactive protein, and complement activation. The combination of diabetic ketoacidosis and CVC placement may be a significant risk factor for the development of DVT."
],
"date": "March 05, 2019",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/887/423/887423-Thumb1.png"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/887/423/887423-Thumb2.png"
}
],
"markdown": "# A 4-Year-Old Girl in Significant Distress\n\n **Authors:** Alba Morales Pozzo, MD \n **Date:** March 05, 2019\n\n ## Content\n\n This patient has either a deep femoral venous thrombosis or a thrombus within another of the deep veins of the right lower extremity, most likely as a complication of CVC placement in a very young patient with severe diabetic ketoacidosis.\nThe typical signs and symptoms associated with deep venous thrombosis (DVT) of an extremity are persistent extremity edema with or without pain or changes in skin color, prominent superficial veins, tenderness, pain with passive dorsiflexion of the foot (Homans sign), and peripheral cyanosis. Signs and symptoms tend to be nonspecific and can vary in severity depending on the size of the thrombus and extent of venous occlusion. An example in an adult is shown in Figure 1.\nFigure 1.\nCVC use in the pediatric population is increasing, which has caused an increase in the incidence of pediatric DVT. CVCs can increase the risk for DVT by causing vascular damage and causing turbulent blood flow in the vessel (Figure 2).\nFigure 2.\nWhen compared with the adult population, thromboembolism in pediatric patients is rare, with most of cases occurring in hospitalized children. In 1845, Virchow described the three important factors in the development of a thrombus as follows:\nImpairment of blood flow\nVascular injury\nAlterations in the blood (hypercoagulability)\nCommon risk factors for thrombus formation include the following:\nPresence of a CVC\nImmobility\nHeart disease\nVentriculoatrial shunt\nTrauma\nCancer\nSurgery\nInfection\nDehydration\nShock\nPregnancy\nSmoking\nObesity\nPersons with poorly controlled diabetes seem to have abnormalities of hemostasis, but the mechanism that causes the problem is not well understood. Clinical studies in patients with diabetic ketoacidosis have demonstrated transient changes in coagulation factors (increased platelet activation, fibrinolytic activity, and endothelial activation).[1,2,3] Diabetic ketoacidosis is also accompanied by an enhanced inflammatory state, with increased levels of cytokines, C-reactive protein, and complement activation. The combination of diabetic ketoacidosis and CVC placement may be a significant risk factor for the development of DVT.\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n\n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1150292,
"choiceText": "Cellulitis ",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1150294,
"choiceText": "Deep vein thrombosis",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1150296,
"choiceText": "Hematoma ",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1150298,
"choiceText": "Head of tibia fracture\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 365339,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 4-Year-Old Girl in Significant Distress"
},
{
"authors": "Alba Morales Pozzo, MD",
"content": [
"The presence of CVC is the single most important risk factor for DVT in children, with at least 85% of cases related to CVC use.[4,5]",
"CVC-related thrombosis as a complication of diabetic ketoacidosis in young children has been described in the literature. In 2003, Gutierrez and colleagues[6] published a retrospective case-matched control series in the setting of a pediatric intensive care unit of two university-affiliated hospitals. He described eight pediatric patients with diabetic ketoacidosis who had femoral CVCs placed from 1998 to 2001 and age-matched control patients with femoral venous catheters and circulatory shock. CVC-related DVT was defined as persistent ipsilateral leg swelling after removal of a femoral CVC. Control patients who had other risk factors for DVT were excluded. Four of 8 patients with diabetic ketoacidosis developed CVC-related DVT compared with none of the 16 control patients. All four patients with DVT and diabetic ketoacidosis were younger than 3 years. DVT cases were confirmed by Doppler ultrasound in 3 out of 4 patients. This study suggested that young children with diabetic ketoacidosis have an increased incidence of DVT associated with the placement of CVCs.",
"In 2004, Worly and colleagues[7] described a retrospective cohort study of 113 patients with diabetic ketoacidosis in the pediatric intensive care unit. Six (5.3%) required femoral CVC for initial management; 50% of these patients developed ipsilateral DVT within 48 hours of CVC placement. All three patients required long-term therapy with low-molecular-weight heparin for persistent leg swelling. The patients with diabetic ketoacidosis who developed DVT after CVC use were younger (median age, 10.5 months) than patients with diabetic ketoacidosis and CVC use who did not develop DVT. The authors concluded that femoral CVCs should be avoided in patients with diabetic ketoacidosis or removed as soon as possible and that DVT prophylaxis should be considered if a CVC is needed for management.",
"Potential mechanisms that could explain this increased risk for CVC-related thrombosis in pediatric patients with diabetic ketoacidosis include the following:",
"Increased platelet aggregability during hyperglycemia[8]",
"Erythrocyte wall rigidity increase in the hyperglycemic and acidic bloodstream, increasing viscous resistance and causing poor blood flow[9,10]",
"Dehydration during diabetic ketoacidosis that could increase venous stasis (part of Virchow's triad of pathogenesis of thrombosis)",
"Small caliber of the vessels in toddlers because the median age of the children who developed CVC-related DVTs in the published cases was only 18 months",
"A high index of suspicion for DVT in critically ill children is important, as unrecognized DVT can give rise to pulmonary embolism, can become a nidus for infection, and can lead to stasis dermatitis and ulceration. Clinical signs of DVT can be difficult to assess because critically ill children are often edematous and overloaded with fluid."
],
"date": "March 05, 2019",
"figures": [],
"markdown": "# A 4-Year-Old Girl in Significant Distress\n\n **Authors:** Alba Morales Pozzo, MD \n **Date:** March 05, 2019\n\n ## Content\n\n The presence of CVC is the single most important risk factor for DVT in children, with at least 85% of cases related to CVC use.[4,5]\nCVC-related thrombosis as a complication of diabetic ketoacidosis in young children has been described in the literature. In 2003, Gutierrez and colleagues[6] published a retrospective case-matched control series in the setting of a pediatric intensive care unit of two university-affiliated hospitals. He described eight pediatric patients with diabetic ketoacidosis who had femoral CVCs placed from 1998 to 2001 and age-matched control patients with femoral venous catheters and circulatory shock. CVC-related DVT was defined as persistent ipsilateral leg swelling after removal of a femoral CVC. Control patients who had other risk factors for DVT were excluded. Four of 8 patients with diabetic ketoacidosis developed CVC-related DVT compared with none of the 16 control patients. All four patients with DVT and diabetic ketoacidosis were younger than 3 years. DVT cases were confirmed by Doppler ultrasound in 3 out of 4 patients. This study suggested that young children with diabetic ketoacidosis have an increased incidence of DVT associated with the placement of CVCs.\nIn 2004, Worly and colleagues[7] described a retrospective cohort study of 113 patients with diabetic ketoacidosis in the pediatric intensive care unit. Six (5.3%) required femoral CVC for initial management; 50% of these patients developed ipsilateral DVT within 48 hours of CVC placement. All three patients required long-term therapy with low-molecular-weight heparin for persistent leg swelling. The patients with diabetic ketoacidosis who developed DVT after CVC use were younger (median age, 10.5 months) than patients with diabetic ketoacidosis and CVC use who did not develop DVT. The authors concluded that femoral CVCs should be avoided in patients with diabetic ketoacidosis or removed as soon as possible and that DVT prophylaxis should be considered if a CVC is needed for management.\nPotential mechanisms that could explain this increased risk for CVC-related thrombosis in pediatric patients with diabetic ketoacidosis include the following:\nIncreased platelet aggregability during hyperglycemia[8]\nErythrocyte wall rigidity increase in the hyperglycemic and acidic bloodstream, increasing viscous resistance and causing poor blood flow[9,10]\nDehydration during diabetic ketoacidosis that could increase venous stasis (part of Virchow's triad of pathogenesis of thrombosis)\nSmall caliber of the vessels in toddlers because the median age of the children who developed CVC-related DVTs in the published cases was only 18 months\nA high index of suspicion for DVT in critically ill children is important, as unrecognized DVT can give rise to pulmonary embolism, can become a nidus for infection, and can lead to stasis dermatitis and ulceration. Clinical signs of DVT can be difficult to assess because critically ill children are often edematous and overloaded with fluid.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 4-Year-Old Girl in Significant Distress"
},
{
"authors": "Alba Morales Pozzo, MD",
"content": [
"The problem of DVT in children has become significant, yet evidence-based guidelines for the treatment of pediatric patients with DVT are lacking.[11] Current management recommendations are based on adult data, large tertiary care pediatric center observations and experience, and consensus statements of pediatric specialists.",
"In the acute setting, the goals of therapy for venous thromboembolism include re-establishing flow through the occluded vessel, preventing thrombus extension, and preventing embolism. Heparins (unfractionated and low-molecular-weight) are still used most often to treat DVT in children. Low-molecular-weight heparin has the advantage of lower risks for bleeding and heparin-induced thrombocytopenia compared with unfractionated heparin.[4] Pediatricians have the longest experience with unfractionated heparin, which has the disadvantage of increased bleeding risk and the requirement of normal antithrombin III levels. The suggested duration of anticoagulation is approximately 3 months; however, case reports have documented treatment for as long as 6 months.[7] Because heparin is administered once or twice daily subcutaneously, this places a significant burden and trauma on young children and their caretakers.",
"Children, especially toddlers, who have diabetic ketoacidosis seem to be at an increased risk of developing CVC-related DVT. If placement of a CVC is absolutely required for resuscitation, it should be immediately removed after the acute rehydration goals have been achieved. Prophylaxis with low-molecular-weight heparin should be considered in patients who require prolonged use of CVCs for prevention of DVT. A review of the impact of CVC use on the incidence of pediatric thromboembolism describes the potentially life-threatening complications that can arise from the use of CVCs in critically ill children and highlights the need for clinical studies that can lead to the delineation of guidelines for the safe use of CVCs in this fragile population.[12]",
"The patient in this case underwent Doppler ultrasound of the right lower extremity, which confirmed the diagnosis of DVT. Biochemical markers for coagulation disorders were negative. All of her coagulation factor levels were measured within normal limits. Therapy with subcutaneous low-molecular-weight heparin was initiated on the second to last day of hospitalization. Her signs and symptoms had completely resolved by her 6-week follow-up appointment in the pediatric diabetes clinic. Heparin treatment was continued for 6 months after discharge from the hospital and then discontinued without complications."
],
"date": "March 05, 2019",
"figures": [],
"markdown": "# A 4-Year-Old Girl in Significant Distress\n\n **Authors:** Alba Morales Pozzo, MD \n **Date:** March 05, 2019\n\n ## Content\n\n The problem of DVT in children has become significant, yet evidence-based guidelines for the treatment of pediatric patients with DVT are lacking.[11] Current management recommendations are based on adult data, large tertiary care pediatric center observations and experience, and consensus statements of pediatric specialists.\nIn the acute setting, the goals of therapy for venous thromboembolism include re-establishing flow through the occluded vessel, preventing thrombus extension, and preventing embolism. Heparins (unfractionated and low-molecular-weight) are still used most often to treat DVT in children. Low-molecular-weight heparin has the advantage of lower risks for bleeding and heparin-induced thrombocytopenia compared with unfractionated heparin.[4] Pediatricians have the longest experience with unfractionated heparin, which has the disadvantage of increased bleeding risk and the requirement of normal antithrombin III levels. The suggested duration of anticoagulation is approximately 3 months; however, case reports have documented treatment for as long as 6 months.[7] Because heparin is administered once or twice daily subcutaneously, this places a significant burden and trauma on young children and their caretakers.\nChildren, especially toddlers, who have diabetic ketoacidosis seem to be at an increased risk of developing CVC-related DVT. If placement of a CVC is absolutely required for resuscitation, it should be immediately removed after the acute rehydration goals have been achieved. Prophylaxis with low-molecular-weight heparin should be considered in patients who require prolonged use of CVCs for prevention of DVT. A review of the impact of CVC use on the incidence of pediatric thromboembolism describes the potentially life-threatening complications that can arise from the use of CVCs in critically ill children and highlights the need for clinical studies that can lead to the delineation of guidelines for the safe use of CVCs in this fragile population.[12]\nThe patient in this case underwent Doppler ultrasound of the right lower extremity, which confirmed the diagnosis of DVT. Biochemical markers for coagulation disorders were negative. All of her coagulation factor levels were measured within normal limits. Therapy with subcutaneous low-molecular-weight heparin was initiated on the second to last day of hospitalization. Her signs and symptoms had completely resolved by her 6-week follow-up appointment in the pediatric diabetes clinic. Heparin treatment was continued for 6 months after discharge from the hospital and then discontinued without complications.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1150300,
"choiceText": "Older age",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1150302,
"choiceText": "Placement of a CVC",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1150304,
"choiceText": "Immobilization",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1150306,
"choiceText": "Trauma\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1150308,
"choiceText": "All are equally important",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Even though older age, immobilization, and trauma are risk factors for DVT, placement of a CVC has been proven to cause at least 85% of DVT cases in children.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 365341,
"questionText": "Which of the following is the most important risk factor for the development of DVT in children?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1150318,
"choiceText": "Infection",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1150320,
"choiceText": "Vascular injury",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1150322,
"choiceText": "Obesity",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1150324,
"choiceText": "Coma\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Infection, obesity, and coma are secondary risk factors for the development of thromboembolisms. The original three factors described historically by Virchow in the 1800s are impairment of blood flow, alterations in the blood, and injury to the vessel in question.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 365345,
"questionText": "The three most important factors in the development of a thrombus are impairment of blood flow, alterations in the blood (hypercoagulability), and which of the following?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 4-Year-Old Girl in Significant Distress"
},
{
"authors": "Alba Morales Pozzo, MD",
"content": [],
"date": "March 05, 2019",
"figures": [],
"markdown": "# A 4-Year-Old Girl in Significant Distress\n\n **Authors:** Alba Morales Pozzo, MD \n **Date:** March 05, 2019\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1150300,
"choiceText": "Older age",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1150302,
"choiceText": "Placement of a CVC",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1150304,
"choiceText": "Immobilization",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1150306,
"choiceText": "Trauma\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1150308,
"choiceText": "All are equally important",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Even though older age, immobilization, and trauma are risk factors for DVT, placement of a CVC has been proven to cause at least 85% of DVT cases in children.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 365341,
"questionText": "Which of the following is the most important risk factor for the development of DVT in children?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1150318,
"choiceText": "Infection",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1150320,
"choiceText": "Vascular injury",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1150322,
"choiceText": "Obesity",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1150324,
"choiceText": "Coma\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Infection, obesity, and coma are secondary risk factors for the development of thromboembolisms. The original three factors described historically by Virchow in the 1800s are impairment of blood flow, alterations in the blood, and injury to the vessel in question.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 365345,
"questionText": "The three most important factors in the development of a thrombus are impairment of blood flow, alterations in the blood (hypercoagulability), and which of the following?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 4-Year-Old Girl in Significant Distress"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1150292,
"choiceText": "Cellulitis ",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1150294,
"choiceText": "Deep vein thrombosis",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1150296,
"choiceText": "Hematoma ",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1150298,
"choiceText": "Head of tibia fracture\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 365339,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1150300,
"choiceText": "Older age",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1150302,
"choiceText": "Placement of a CVC",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1150304,
"choiceText": "Immobilization",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1150306,
"choiceText": "Trauma\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1150308,
"choiceText": "All are equally important",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Even though older age, immobilization, and trauma are risk factors for DVT, placement of a CVC has been proven to cause at least 85% of DVT cases in children.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 365341,
"questionText": "Which of the following is the most important risk factor for the development of DVT in children?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1150318,
"choiceText": "Infection",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1150320,
"choiceText": "Vascular injury",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1150322,
"choiceText": "Obesity",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1150324,
"choiceText": "Coma\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Infection, obesity, and coma are secondary risk factors for the development of thromboembolisms. The original three factors described historically by Virchow in the 1800s are impairment of blood flow, alterations in the blood, and injury to the vessel in question.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 365345,
"questionText": "The three most important factors in the development of a thrombus are impairment of blood flow, alterations in the blood (hypercoagulability), and which of the following?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
886748 | /viewarticle/886748 | [
{
"authors": "Winston Tan, MD; Matthew Tan",
"content": [
"Editor's Note:",
"The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"Background",
"A 61-year-old woman with a history of stage III breast cancer (T3N2MX; estrogen receptor-negative/progesterone receptor-negative) 7 years prior presents to a primary care clinic with painful constipation. She had previously undergone bilateral mastectomy for lobular breast cancer (positive in 19 nodes). She received six cycles of cyclophosphamide, doxorubicin (Adriamycin), and 5-fluorouracil (CAF) and has been taking anastrozole for 7 years. She also has a history of multiple sclerosis, with recurrent symptoms every 4-6 months for the past 4-5 years, and is receiving intermittent steroid treatment.",
"The patient presents to the clinic with constipation over the past 3-4 months, with smaller-caliber stools noted particularly in the past few months. She has had a hard time passing her stools, with increasing difficulty and increased pain. Over the past week, she has also found urinating difficult. She has had hesitancy, frequency, and dysuria."
],
"date": "October 12, 2017",
"figures": [],
"markdown": "# A 61-Year-Old Woman With Painful Constipation\n\n **Authors:** Winston Tan, MD; Matthew Tan \n **Date:** October 12, 2017\n\n ## Content\n\n Editor's Note:\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nBackground\nA 61-year-old woman with a history of stage III breast cancer (T3N2MX; estrogen receptor-negative/progesterone receptor-negative) 7 years prior presents to a primary care clinic with painful constipation. She had previously undergone bilateral mastectomy for lobular breast cancer (positive in 19 nodes). She received six cycles of cyclophosphamide, doxorubicin (Adriamycin), and 5-fluorouracil (CAF) and has been taking anastrozole for 7 years. She also has a history of multiple sclerosis, with recurrent symptoms every 4-6 months for the past 4-5 years, and is receiving intermittent steroid treatment.\nThe patient presents to the clinic with constipation over the past 3-4 months, with smaller-caliber stools noted particularly in the past few months. She has had a hard time passing her stools, with increasing difficulty and increased pain. Over the past week, she has also found urinating difficult. She has had hesitancy, frequency, and dysuria.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 61-Year-Old Woman With Painful Constipation"
},
{
"authors": "Winston Tan, MD; Matthew Tan",
"content": [
"Physical Examination and Workup",
"Upon physical examination, the patient is conscious, coherent, and not in respiratory distress. She is 5'3\" tall and weighs 110.2 lb. Her blood pressure is 112/84 mm Hg, heart rate is 76 beats/min, respiratory rate is 14 breaths/min, and temperature is 97.34°F.",
"On head, eye, ear, nose, and throat examination, the pupils are both equally reactive to light. Extraocular muscle movements are intact. The nose and throat are both clear. No neck adenopathy or supraclavicular adenopathy is noted.",
"Cardiovascular examination reveals regular S1 and S2 sounds, with no S3 or S4 sounds. The lungs sound clear. The abdomen is soft and nontender, with no hepatosplenomegaly noted. No edema, cyanosis, or clubbing is observed.",
"Examination of the patient's breasts reveals no mass or nodule in her right breast area. In her left breast area, no mass or nodule is found. No axillary adenopathy is observed. No signs of recurrence are evident.",
"The patient's multiple sclerosis appears to be stable. Rectal examination reveals a mass that is obstructing the rectal outlet (Figure 1). It is nontender, solid, and firm, with no bleeding. Colonoscopy reveals a mass in the rectal sigmoid junction, causing at least 80% obstruction.",
"Figure 1.",
"PET of the head and neck reveals no abnormal hypermetabolic activity. No abnormal hypermetabolic activity is noted in the patient's chest. Hypermetabolic activity is noted in the pelvis, with thickening of the rectal wall. Soft-tissue density is observed adjacent to this rectum thickening. Moderate right hydronephrosis and mild left hydronephrosis are also noted."
],
"date": "October 12, 2017",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/886/748/886748_Figure-1_thumb.jpg"
}
],
"markdown": "# A 61-Year-Old Woman With Painful Constipation\n\n **Authors:** Winston Tan, MD; Matthew Tan \n **Date:** October 12, 2017\n\n ## Content\n\n Physical Examination and Workup\nUpon physical examination, the patient is conscious, coherent, and not in respiratory distress. She is 5'3\" tall and weighs 110.2 lb. Her blood pressure is 112/84 mm Hg, heart rate is 76 beats/min, respiratory rate is 14 breaths/min, and temperature is 97.34°F.\nOn head, eye, ear, nose, and throat examination, the pupils are both equally reactive to light. Extraocular muscle movements are intact. The nose and throat are both clear. No neck adenopathy or supraclavicular adenopathy is noted.\nCardiovascular examination reveals regular S1 and S2 sounds, with no S3 or S4 sounds. The lungs sound clear. The abdomen is soft and nontender, with no hepatosplenomegaly noted. No edema, cyanosis, or clubbing is observed.\nExamination of the patient's breasts reveals no mass or nodule in her right breast area. In her left breast area, no mass or nodule is found. No axillary adenopathy is observed. No signs of recurrence are evident.\nThe patient's multiple sclerosis appears to be stable. Rectal examination reveals a mass that is obstructing the rectal outlet (Figure 1). It is nontender, solid, and firm, with no bleeding. Colonoscopy reveals a mass in the rectal sigmoid junction, causing at least 80% obstruction.\nFigure 1.\nPET of the head and neck reveals no abnormal hypermetabolic activity. No abnormal hypermetabolic activity is noted in the patient's chest. Hypermetabolic activity is noted in the pelvis, with thickening of the rectal wall. Soft-tissue density is observed adjacent to this rectum thickening. Moderate right hydronephrosis and mild left hydronephrosis are also noted.\n\n ## Figures\n\n **Figure 1.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1147756,
"choiceText": "Arteriovenous malformation",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1147758,
"choiceText": "Hemorrhoids",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1147760,
"choiceText": "Metastatic lobular breast cancer",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1147762,
"choiceText": "Rectal polyp",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1147764,
"choiceText": "Diverticulosis",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 364431,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 61-Year-Old Woman With Painful Constipation"
},
{
"authors": "Winston Tan, MD; Matthew Tan",
"content": [
"Because the mass in this patient was solid and obstructing the rectal outlet, biopsy was performed after colonoscopy. The mass was determined to be metastatic invasive lobular carcinoma of the breast. Several immunohistochemical studies were performed. E-cadherin findings were negative, consistent with a diagnosis of metastatic breast cancer. BRST2 findings were strongly positive. The progesterone receptor findings were negative. The HER2/neu finding was 2+, and fluorescence in situ hybridization analysis was negative. The tumor cells were positive for ER, CK7, and AE1/AE3 and were negative for CA125, CK20, MOC-31, CD45, and Ber-EP4. Histologic immunohistochemistries were strongly suggestive of metastatic invasive lobular carcinoma of the breast.",
"Arteriovenous malformations do not typically present as acute obstructions; lower gastrointestinal bleeding is more common. Hemorrhoids most often present with long-term intermittent bleeding. A rectal polyp is usually small and asymptomatic. Diverticulosis presents with intermittent pain in the event of diverticulitis. Of the choices above, only a solid mass, such as metastasis, is consistent with this subacute presentation. Biopsy clarified and confirmed the diagnosis.",
"The most common metastatic sites of breast cancer are well known: the lungs, bones, liver, and brain. Involvement of the gastrointestinal tract remains unusual.[1] Borst and Ingold[2] published a large series that analyzed more than 2500 cases of breast cancer with metastatic disease over 18 years; they found that only 17 patients (less than 1%) had metastasis to the gastrointestinal tract.McLemore and colleagues[3] also reported that infiltrating lobular carcinoma represented 64% of cases of gastrointestinal metastasis among breast cancer metastasis. Even in patients with mixed ductal and lobular primary breast carcinoma, the lobular type is most likely to cause metastatic disease.[3] Metastasis to the gastrointestinal tract, such as the rectum, owing to breast cancer is a rare condition and is difficult diagnose. This diagnosis should be suspected when the patient has a history of lobular breast cancer.",
"This case serves as a reminder that, in a patient with history of cancer, evaluating a mass as a possible recurrence or metastasis is important—especially because constipation is a common problem among patients with cancer, owing to treatment or other conditions. A constipated patient may present with abdominal bloating, pain during defecation, rectal bleeding, diarrhea, low back pain, unexpected weight loss, inability to pass flatus, or vomiting. Understanding the many differential diagnoses associated with constipation is important. Among the factors to consider are the age of patient, acute or chronic presentation, an inherent gastrointestinal problem, comorbid problems (eg, neurologic conditions, endocrinopathies, motility problems), and medication use. Common differential diagnoses associated with constipation include hernias, colon cancer, ileus, and irritable bowel syndrome. Diet-related factors that should be considered include diabetes, lead poisoning, spinal cord injuries, and medications that can cause constipation. Rare causes include Chagas disease, panhypopituitarism, hypothyroidism, or peritonitis.[1]",
"A detailed history of the constipated patient should include questions about normal patterns of defecation, frequency, and laxative use. Understanding how much fluid the patient drinks daily and information about movement and walking are also important. A cancer-related condition is not a likely cause of constipation for patients younger than 50 years and for those who have had the same symptoms for longer than 2 years. Ileus is usually associated with volvulus and an acute presentation. Volvulus is mostly found in young patients or in those with previous abdominal surgery and presents with abdominal pain secondary to a mechanical defect in the colon. Ileus may be secondary to psychiatric conditions, medications, or changes in diet."
],
"date": "October 12, 2017",
"figures": [],
"markdown": "# A 61-Year-Old Woman With Painful Constipation\n\n **Authors:** Winston Tan, MD; Matthew Tan \n **Date:** October 12, 2017\n\n ## Content\n\n Because the mass in this patient was solid and obstructing the rectal outlet, biopsy was performed after colonoscopy. The mass was determined to be metastatic invasive lobular carcinoma of the breast. Several immunohistochemical studies were performed. E-cadherin findings were negative, consistent with a diagnosis of metastatic breast cancer. BRST2 findings were strongly positive. The progesterone receptor findings were negative. The HER2/neu finding was 2+, and fluorescence in situ hybridization analysis was negative. The tumor cells were positive for ER, CK7, and AE1/AE3 and were negative for CA125, CK20, MOC-31, CD45, and Ber-EP4. Histologic immunohistochemistries were strongly suggestive of metastatic invasive lobular carcinoma of the breast.\nArteriovenous malformations do not typically present as acute obstructions; lower gastrointestinal bleeding is more common. Hemorrhoids most often present with long-term intermittent bleeding. A rectal polyp is usually small and asymptomatic. Diverticulosis presents with intermittent pain in the event of diverticulitis. Of the choices above, only a solid mass, such as metastasis, is consistent with this subacute presentation. Biopsy clarified and confirmed the diagnosis.\nThe most common metastatic sites of breast cancer are well known: the lungs, bones, liver, and brain. Involvement of the gastrointestinal tract remains unusual.[1] Borst and Ingold[2] published a large series that analyzed more than 2500 cases of breast cancer with metastatic disease over 18 years; they found that only 17 patients (less than 1%) had metastasis to the gastrointestinal tract.McLemore and colleagues[3] also reported that infiltrating lobular carcinoma represented 64% of cases of gastrointestinal metastasis among breast cancer metastasis. Even in patients with mixed ductal and lobular primary breast carcinoma, the lobular type is most likely to cause metastatic disease.[3] Metastasis to the gastrointestinal tract, such as the rectum, owing to breast cancer is a rare condition and is difficult diagnose. This diagnosis should be suspected when the patient has a history of lobular breast cancer.\nThis case serves as a reminder that, in a patient with history of cancer, evaluating a mass as a possible recurrence or metastasis is important—especially because constipation is a common problem among patients with cancer, owing to treatment or other conditions. A constipated patient may present with abdominal bloating, pain during defecation, rectal bleeding, diarrhea, low back pain, unexpected weight loss, inability to pass flatus, or vomiting. Understanding the many differential diagnoses associated with constipation is important. Among the factors to consider are the age of patient, acute or chronic presentation, an inherent gastrointestinal problem, comorbid problems (eg, neurologic conditions, endocrinopathies, motility problems), and medication use. Common differential diagnoses associated with constipation include hernias, colon cancer, ileus, and irritable bowel syndrome. Diet-related factors that should be considered include diabetes, lead poisoning, spinal cord injuries, and medications that can cause constipation. Rare causes include Chagas disease, panhypopituitarism, hypothyroidism, or peritonitis.[1]\nA detailed history of the constipated patient should include questions about normal patterns of defecation, frequency, and laxative use. Understanding how much fluid the patient drinks daily and information about movement and walking are also important. A cancer-related condition is not a likely cause of constipation for patients younger than 50 years and for those who have had the same symptoms for longer than 2 years. Ileus is usually associated with volvulus and an acute presentation. Volvulus is mostly found in young patients or in those with previous abdominal surgery and presents with abdominal pain secondary to a mechanical defect in the colon. Ileus may be secondary to psychiatric conditions, medications, or changes in diet.\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1147756,
"choiceText": "Arteriovenous malformation",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1147758,
"choiceText": "Hemorrhoids",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1147760,
"choiceText": "Metastatic lobular breast cancer",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1147762,
"choiceText": "Rectal polyp",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1147764,
"choiceText": "Diverticulosis",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 364431,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 61-Year-Old Woman With Painful Constipation"
},
{
"authors": "Winston Tan, MD; Matthew Tan",
"content": [
"A physical examination in patients with constipation reveals abdominal tenderness and the presence or absence of bowel sounds. A rectal examination helps determine whether an anatomical or physiologic cause of obstruction is present. Several diagnostic tests are helpful, including abdominal radiography to look for signs of obstruction. CT is used to check for masses or areas of obstruction. Direct visualization with anoscopy or colonoscopy is often helpful in determining an anatomical etiology for constipation. Laboratory evaluation does not play a large role in the initial assessment of a patient with constipation.[4]",
"Various imaging studies are used to investigate for acute processes that may be causing colonic ileus, and to evaluate for causes of chronic constipation. In patients with acute abdominal pain, fever, leukocytosis, or other symptoms that suggest systemic or intra-abdominal processes, imaging studies are used to rule out sources of sepsis or other intra-abdominal concerns.",
"Colonic transit study, defecography, lower gastrointestinal endoscopy, surface anal electromyography, anorectal manometry, and balloon expulsion may also be used in the evaluation of constipation. In an acute situation with a patient who is usually not constipated and who is not at high risk for a serious condition, sometimes no further evaluation is necessary. Sigmoidoscopy, barium enema, or colonoscopy may be considered for colorectal cancer screening in patients older than 50 years. The current criterion standard is colonoscopy.",
"Diabetic gastroparesis can present with fecal impaction. This may be seen in patients with diabetic neuropathy. Scleroderma and other connective-tissue diseases may be complicated by motor disturbances that mimic colonic obstruction on plain films. Myxedema ileus is a result of hypothyroidism.",
"If no anatomical lesion is noted on rectal examination, abnormal physiology should be suspected. Anorectal manometry documents several parameters, including external anal sphincter and puborectalis function, reflex relaxation of the internal sphincter when the rectum is distended, coordination of these muscles during the bear-down phase of defecation, anorectal pressures during these events, and the threshold at which rectal distention is perceived."
],
"date": "October 12, 2017",
"figures": [],
"markdown": "# A 61-Year-Old Woman With Painful Constipation\n\n **Authors:** Winston Tan, MD; Matthew Tan \n **Date:** October 12, 2017\n\n ## Content\n\n A physical examination in patients with constipation reveals abdominal tenderness and the presence or absence of bowel sounds. A rectal examination helps determine whether an anatomical or physiologic cause of obstruction is present. Several diagnostic tests are helpful, including abdominal radiography to look for signs of obstruction. CT is used to check for masses or areas of obstruction. Direct visualization with anoscopy or colonoscopy is often helpful in determining an anatomical etiology for constipation. Laboratory evaluation does not play a large role in the initial assessment of a patient with constipation.[4]\nVarious imaging studies are used to investigate for acute processes that may be causing colonic ileus, and to evaluate for causes of chronic constipation. In patients with acute abdominal pain, fever, leukocytosis, or other symptoms that suggest systemic or intra-abdominal processes, imaging studies are used to rule out sources of sepsis or other intra-abdominal concerns.\nColonic transit study, defecography, lower gastrointestinal endoscopy, surface anal electromyography, anorectal manometry, and balloon expulsion may also be used in the evaluation of constipation. In an acute situation with a patient who is usually not constipated and who is not at high risk for a serious condition, sometimes no further evaluation is necessary. Sigmoidoscopy, barium enema, or colonoscopy may be considered for colorectal cancer screening in patients older than 50 years. The current criterion standard is colonoscopy.\nDiabetic gastroparesis can present with fecal impaction. This may be seen in patients with diabetic neuropathy. Scleroderma and other connective-tissue diseases may be complicated by motor disturbances that mimic colonic obstruction on plain films. Myxedema ileus is a result of hypothyroidism.\nIf no anatomical lesion is noted on rectal examination, abnormal physiology should be suspected. Anorectal manometry documents several parameters, including external anal sphincter and puborectalis function, reflex relaxation of the internal sphincter when the rectum is distended, coordination of these muscles during the bear-down phase of defecation, anorectal pressures during these events, and the threshold at which rectal distention is perceived.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 61-Year-Old Woman With Painful Constipation"
},
{
"authors": "Winston Tan, MD; Matthew Tan",
"content": [
"After any critical illness associated with constipation has been ruled out, manual disimpaction and transrectal enemas are often used. A well-lubricated, gloved finger may be used for this purpose in patients with lower anorectal impactions. Warm-water enemas are not usually necessary in the emergency department. After these measures, elective evaluation for the cause of the constipation is performed.",
"Enemas, suppositories, and laxatives do not address the underlying problem of constipation. Therefore, medical care should focus on dietary change and exercise. Patients with chronic constipation are at high risk of becoming dependent on laxatives and developing a laxative colon. For most patients, the key to treatment is correction of dietary deficiencies. Encouraging aerobic exercise is a reasonable strategy, although the effectiveness of exercise in patients with constipation is controversial. Brisk walking may help with bowel motility and is unlikely to cause problems in most patients.",
"Surgery is generally restricted to management of complications or evaluation of underlying causes. If an underlying psychiatric cause is suspected, surgical intervention should be avoided. Patients who present with abdominal pain with acute porphyria have undergone inappropriate surgical procedures, usually owing to an incomplete evaluation and history. Patients with a large-bowel obstruction or colonic ileus secondary to an acute intra-abdominal process should undergo surgical consultation. This consultation is also indicated for patients who have anorectal complications of constipation. Anal fissures and symptomatic hemorrhoids are generally considered complications of constipation. Urgent surgical consultation is required in patients with acute hemorrhoidal thrombosis, in order to achieve pain relief and evacuation of the clot. Patients with anal fissures should undergo conservative management because most fissures respond well to programs involving sitz baths, stool softeners, and anesthetic ointment.",
"Dietary fiber may also aid patients with constipation. Natural sources include fruits, vegetables, and cereals. Although natural sources are preferred, advising patients to consume more fruits and vegetables is often unsuccessful. Thus, fiber supplements may be used.",
"If the cause of constipation is opioid use, discontinuation of therapy may be required. Long-term opioid therapy results in opioid-induced constipation in 40%-80% of patients.",
"In this patient, a biopsy resulted in the specific diagnosis of metastatic breast cancer. Chemotherapy with capecitabine and radiation resulted in dramatic improvement of her symptoms and control of her disease. MRI revealed resolution of her perirectal mass (Figure 2).",
"Figure 2.",
"Initial treatment was followed by chemotherapy with 5-fluorouracil, epirubicin, and cyclophosphamide (FEC). Taxane-based chemotherapy was avoided owing to the risk of worsening her neuropathy from multiple sclerosis. The patient continues to be in partial remission 4 years later and is receiving monthly maintenance treatment with fulvestrant."
],
"date": "October 12, 2017",
"figures": [
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/886/748/886748_Figure-2_thumb.jpg"
}
],
"markdown": "# A 61-Year-Old Woman With Painful Constipation\n\n **Authors:** Winston Tan, MD; Matthew Tan \n **Date:** October 12, 2017\n\n ## Content\n\n After any critical illness associated with constipation has been ruled out, manual disimpaction and transrectal enemas are often used. A well-lubricated, gloved finger may be used for this purpose in patients with lower anorectal impactions. Warm-water enemas are not usually necessary in the emergency department. After these measures, elective evaluation for the cause of the constipation is performed.\nEnemas, suppositories, and laxatives do not address the underlying problem of constipation. Therefore, medical care should focus on dietary change and exercise. Patients with chronic constipation are at high risk of becoming dependent on laxatives and developing a laxative colon. For most patients, the key to treatment is correction of dietary deficiencies. Encouraging aerobic exercise is a reasonable strategy, although the effectiveness of exercise in patients with constipation is controversial. Brisk walking may help with bowel motility and is unlikely to cause problems in most patients.\nSurgery is generally restricted to management of complications or evaluation of underlying causes. If an underlying psychiatric cause is suspected, surgical intervention should be avoided. Patients who present with abdominal pain with acute porphyria have undergone inappropriate surgical procedures, usually owing to an incomplete evaluation and history. Patients with a large-bowel obstruction or colonic ileus secondary to an acute intra-abdominal process should undergo surgical consultation. This consultation is also indicated for patients who have anorectal complications of constipation. Anal fissures and symptomatic hemorrhoids are generally considered complications of constipation. Urgent surgical consultation is required in patients with acute hemorrhoidal thrombosis, in order to achieve pain relief and evacuation of the clot. Patients with anal fissures should undergo conservative management because most fissures respond well to programs involving sitz baths, stool softeners, and anesthetic ointment.\nDietary fiber may also aid patients with constipation. Natural sources include fruits, vegetables, and cereals. Although natural sources are preferred, advising patients to consume more fruits and vegetables is often unsuccessful. Thus, fiber supplements may be used.\nIf the cause of constipation is opioid use, discontinuation of therapy may be required. Long-term opioid therapy results in opioid-induced constipation in 40%-80% of patients.\nIn this patient, a biopsy resulted in the specific diagnosis of metastatic breast cancer. Chemotherapy with capecitabine and radiation resulted in dramatic improvement of her symptoms and control of her disease. MRI revealed resolution of her perirectal mass (Figure 2).\nFigure 2.\nInitial treatment was followed by chemotherapy with 5-fluorouracil, epirubicin, and cyclophosphamide (FEC). Taxane-based chemotherapy was avoided owing to the risk of worsening her neuropathy from multiple sclerosis. The patient continues to be in partial remission 4 years later and is receiving monthly maintenance treatment with fulvestrant.\n\n ## Figures\n\n **Figure 2.** \n \n\n\n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1147766,
"choiceText": "Biopsy is generally not indicated",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1147768,
"choiceText": "Surgical treatment should be performed immediately",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1147770,
"choiceText": "Chemotherapy alone is initially preferred to surgical treatment in most cases",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1147772,
"choiceText": "Biopsy is necessary to determine the specific type of treatment needed",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>In patients with constipation in whom a rectal mass is found, biopsy helps guide treatment options. Even if metastatic disease is found, surgery might not be the best next step. Treatment with chemotherapy or radiation may be helpful.</p>",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 364433,
"questionText": "Which of the following is accurate about the next step of management for a patient with constipation in whom a rectal mass found?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1147774,
"choiceText": "Surgery",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1147776,
"choiceText": "Radiation",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1147778,
"choiceText": "Chemoradiation",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1147780,
"choiceText": "Supportive care",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>Breast cancer that metastasizes to the gastrointestinal tract is rare. The acuity of the presentation is an important factor that determines the management of her condition. If acute and rapid obstruction and total obstruction, immediate surgical resection is required to prevent bowel gangrene and death.</p>\r\n\r\n<p>For a subacute presentation like the one in this case, considering such options as radiation or chemoradiation is important. Chemoradiation was chosen because although radiation would be palliative, it would not resolve the systemic portion of the metastasis. Supportive care would lead to progressive disease and was therefore deemed inappropriate.</p>",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 364435,
"questionText": "In a patient similar to the one in this case, with metastatic breast cancer to the gastrointestinal tract, which of the following is the most appropriate next step for treatment?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 61-Year-Old Woman With Painful Constipation"
},
{
"authors": "Winston Tan, MD; Matthew Tan",
"content": [],
"date": "October 12, 2017",
"figures": [],
"markdown": "# A 61-Year-Old Woman With Painful Constipation\n\n **Authors:** Winston Tan, MD; Matthew Tan \n **Date:** October 12, 2017\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1147766,
"choiceText": "Biopsy is generally not indicated",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1147768,
"choiceText": "Surgical treatment should be performed immediately",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1147770,
"choiceText": "Chemotherapy alone is initially preferred to surgical treatment in most cases",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1147772,
"choiceText": "Biopsy is necessary to determine the specific type of treatment needed",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>In patients with constipation in whom a rectal mass is found, biopsy helps guide treatment options. Even if metastatic disease is found, surgery might not be the best next step. Treatment with chemotherapy or radiation may be helpful.</p>",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 364433,
"questionText": "Which of the following is accurate about the next step of management for a patient with constipation in whom a rectal mass found?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1147774,
"choiceText": "Surgery",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1147776,
"choiceText": "Radiation",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1147778,
"choiceText": "Chemoradiation",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1147780,
"choiceText": "Supportive care",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>Breast cancer that metastasizes to the gastrointestinal tract is rare. The acuity of the presentation is an important factor that determines the management of her condition. If acute and rapid obstruction and total obstruction, immediate surgical resection is required to prevent bowel gangrene and death.</p>\r\n\r\n<p>For a subacute presentation like the one in this case, considering such options as radiation or chemoradiation is important. Chemoradiation was chosen because although radiation would be palliative, it would not resolve the systemic portion of the metastasis. Supportive care would lead to progressive disease and was therefore deemed inappropriate.</p>",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 364435,
"questionText": "In a patient similar to the one in this case, with metastatic breast cancer to the gastrointestinal tract, which of the following is the most appropriate next step for treatment?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 61-Year-Old Woman With Painful Constipation"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1147756,
"choiceText": "Arteriovenous malformation",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1147758,
"choiceText": "Hemorrhoids",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1147760,
"choiceText": "Metastatic lobular breast cancer",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1147762,
"choiceText": "Rectal polyp",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1147764,
"choiceText": "Diverticulosis",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 364431,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1147766,
"choiceText": "Biopsy is generally not indicated",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1147768,
"choiceText": "Surgical treatment should be performed immediately",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1147770,
"choiceText": "Chemotherapy alone is initially preferred to surgical treatment in most cases",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1147772,
"choiceText": "Biopsy is necessary to determine the specific type of treatment needed",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>In patients with constipation in whom a rectal mass is found, biopsy helps guide treatment options. Even if metastatic disease is found, surgery might not be the best next step. Treatment with chemotherapy or radiation may be helpful.</p>",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 364433,
"questionText": "Which of the following is accurate about the next step of management for a patient with constipation in whom a rectal mass found?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1147774,
"choiceText": "Surgery",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1147776,
"choiceText": "Radiation",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1147778,
"choiceText": "Chemoradiation",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1147780,
"choiceText": "Supportive care",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>Breast cancer that metastasizes to the gastrointestinal tract is rare. The acuity of the presentation is an important factor that determines the management of her condition. If acute and rapid obstruction and total obstruction, immediate surgical resection is required to prevent bowel gangrene and death.</p>\r\n\r\n<p>For a subacute presentation like the one in this case, considering such options as radiation or chemoradiation is important. Chemoradiation was chosen because although radiation would be palliative, it would not resolve the systemic portion of the metastasis. Supportive care would lead to progressive disease and was therefore deemed inappropriate.</p>",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 364435,
"questionText": "In a patient similar to the one in this case, with metastatic breast cancer to the gastrointestinal tract, which of the following is the most appropriate next step for treatment?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
886156 | /viewarticle/886156 | [
{
"authors": "Shahzad G. Raja, MRCS; Gilles D. Dreyfus, MD, PhD, FRCS",
"content": [
"Editor's Note:",
"The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"Background",
"A 62-year-old man presents to the emergency department with a 3-month history of progressively increasing shortness of breath, fatigue, anorexia, and weight loss. The patient underwent orthotopic heart transplantation for ischemic cardiomyopathy 26 months ago.",
"He received the standard immunosuppression protocol for heart transplantation (\"triple therapy\" consisting of cyclosporine, azathioprine, and prednisolone). In the immediate postoperative period, he was administered prednisolone at 1 mg/kg orally daily, which was tapered over 3 weeks to 20 mg daily and then continued for 3 months. This was decreased to 10 mg for the following 3 months and, finally, reduced to 5 mg daily for the last 6 months (which concluded a year-long taper). His maintenance immunosuppression regimen includes cyclosporine and azathioprine.",
"Upon further inquiry, the patient states that he developed a nonproductive cough and left thigh pain that has lasted for the past month. His history includes coronary artery bypass grafting for triple-vessel coronary artery disease (which occurred 8 years ago), hypertension, hyperlipidemia, and intermittent claudication. He has a smoking history of more than 80 pack-years, but he quit smoking at the time of his heart transplantation."
],
"date": "October 02, 2017",
"figures": [],
"markdown": "# A 62-Year-Old Man With Shortness of Breath Years After Heart Transplantation\n\n **Authors:** Shahzad G. Raja, MRCS; Gilles D. Dreyfus, MD, PhD, FRCS \n **Date:** October 02, 2017\n\n ## Content\n\n Editor's Note:\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nBackground\nA 62-year-old man presents to the emergency department with a 3-month history of progressively increasing shortness of breath, fatigue, anorexia, and weight loss. The patient underwent orthotopic heart transplantation for ischemic cardiomyopathy 26 months ago.\nHe received the standard immunosuppression protocol for heart transplantation (\"triple therapy\" consisting of cyclosporine, azathioprine, and prednisolone). In the immediate postoperative period, he was administered prednisolone at 1 mg/kg orally daily, which was tapered over 3 weeks to 20 mg daily and then continued for 3 months. This was decreased to 10 mg for the following 3 months and, finally, reduced to 5 mg daily for the last 6 months (which concluded a year-long taper). His maintenance immunosuppression regimen includes cyclosporine and azathioprine.\nUpon further inquiry, the patient states that he developed a nonproductive cough and left thigh pain that has lasted for the past month. His history includes coronary artery bypass grafting for triple-vessel coronary artery disease (which occurred 8 years ago), hypertension, hyperlipidemia, and intermittent claudication. He has a smoking history of more than 80 pack-years, but he quit smoking at the time of his heart transplantation.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 62-Year-Old Man With Shortness of Breath Years After Heart Transplantation"
},
{
"authors": "Shahzad G. Raja, MRCS; Gilles D. Dreyfus, MD, PhD, FRCS",
"content": [
"Physical Examination and Workup",
"Upon physical examination, the patient appears pale and cachetic. His oral temperature is 98.6°F. His pulse has a regular rhythm and a rate of 102 beats/min, and his blood pressure is 148/92 mm Hg. He is tachypneic, with a respiratory rate of 30 breaths/min, and his oxygen saturation is 93% while breathing room air.",
"Examination of his head and neck, including a check for icteric sclerae, is normal. A well-healed median sternotomy scar is noted. The patient has reduced air entry at the right lung base, with bilateral, scattered, coarse crepitations noted on auscultation of the lungs. His S1 and S2 heart sounds are normal, and the abdominal examination is unremarkable. The peripheral arterial pulses in the lower extremities are palpable but diminished. Tenderness is elicited on deep palpation of the left mid-thigh. The neurologic examination shows a normal cranial nerve inspection, normal gross motor and sensory function, no pronator drift, and normal speech.",
"A laboratory analysis, including a complete blood cell count and a basic metabolic panel, is normal, except for a hemoglobin level of 9.8 g/dL. A posteroanterior upright chest radiograph (Figure 1; arrow showing the main pathology), followed by a CT scan of the chest (Figure 2), is obtained.",
"Figure 1.",
"Figure 1."
],
"date": "October 02, 2017",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/886/156/886156-figure-1_thumb.jpg"
},
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/886/156/886156-figure-2_thumb.jpg"
}
],
"markdown": "# A 62-Year-Old Man With Shortness of Breath Years After Heart Transplantation\n\n **Authors:** Shahzad G. Raja, MRCS; Gilles D. Dreyfus, MD, PhD, FRCS \n **Date:** October 02, 2017\n\n ## Content\n\n Physical Examination and Workup\nUpon physical examination, the patient appears pale and cachetic. His oral temperature is 98.6°F. His pulse has a regular rhythm and a rate of 102 beats/min, and his blood pressure is 148/92 mm Hg. He is tachypneic, with a respiratory rate of 30 breaths/min, and his oxygen saturation is 93% while breathing room air.\nExamination of his head and neck, including a check for icteric sclerae, is normal. A well-healed median sternotomy scar is noted. The patient has reduced air entry at the right lung base, with bilateral, scattered, coarse crepitations noted on auscultation of the lungs. His S1 and S2 heart sounds are normal, and the abdominal examination is unremarkable. The peripheral arterial pulses in the lower extremities are palpable but diminished. Tenderness is elicited on deep palpation of the left mid-thigh. The neurologic examination shows a normal cranial nerve inspection, normal gross motor and sensory function, no pronator drift, and normal speech.\nA laboratory analysis, including a complete blood cell count and a basic metabolic panel, is normal, except for a hemoglobin level of 9.8 g/dL. A posteroanterior upright chest radiograph (Figure 1; arrow showing the main pathology), followed by a CT scan of the chest (Figure 2), is obtained.\nFigure 1.\nFigure 1.\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 1.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1146310,
"choiceText": "Tuberculosis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1146312,
"choiceText": "Histoplasmosis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1146314,
"choiceText": "Pneumonia",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1146316,
"choiceText": "Bronchogenic carcinoma",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 363909,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 62-Year-Old Man With Shortness of Breath Years After Heart Transplantation"
},
{
"authors": "Shahzad G. Raja, MRCS; Gilles D. Dreyfus, MD, PhD, FRCS",
"content": [
"Discussion",
"In this patient, the posteroanterior upright chest radiograph showed right pleural effusion, a left upper-lobe mass (arrow), and changes consistent with emphysema. The CT scan showed air bronchograms within the left upper-lobe mass; areas of low attenuation throughout the lung parenchyma consistent with emphysema and mediastinal lymphadenopathy were seen as well.",
"Figure 1.",
"Figure 1.",
"Transbronchial biopsy revealed poorly differentiated squamous cell carcinoma, with involvement of the lymphatics and submucosal vessels. A bone scan revealed metastatic disease in the left femur. A review of earlier films, however, showed no evidence or suspicion of early bronchogenic cancer.",
"The risk for cancer after solid-organ transplantation is well recognized in transplant recipients who have received long-term immunosuppression; in this group, the overall incidence of carcinoma increases by up to 100-fold, and the incidence of non-Hodgkin lymphoma increases by up to 40-fold. The incidence of carcinoma may vary with the type of allograft, however, because of differences in the induction therapy and immunosuppressive regimens. The most frequently observed tumors are squamous cell carcinoma of the skin, adenocarcinoma of the lung and gastrointestinal tract, Kaposi sarcoma, acute leukemia, and lymphoma.[1,2,3,4,5]",
"The incidence of bronchogenic carcinoma after solid-organ transplantation remains controversial. Although some authors have reported an incidence similar to that of the overall population, others have found an unexpectedly high incidence in the heart transplant population. In the heart transplant population, the incidence of bronchogenic carcinoma ranges from 0.6% to 4%. The increased incidence of lung cancer is potentially related to the prolonged use of cytotoxic agents for immunosuppression; however, the greatest risk factor for the development of lung cancer in the heart transplant population is probably from a concomitant history of prolonged cigarette smoking. In transplant recipients, the effects of inhaled carcinogens found in mainstream smoking products are believed to act in concert with impaired surveillance mechanisms resulting from long-term immunosuppression to promote the development of cancer. As in the nontransplant population, cardiac transplant recipients with lung carcinoma are predominantly in their sixth or seventh decade of life.[1,2,3,4,6]",
"The interval from transplantation to diagnosis of cancer is usually around 3-5 years. However, neoplasm may be discovered within 6 months of transplantation. The most striking feature is that most patients have advanced disease (stage IIIA or higher) at the time of diagnosis, despite these patients being closely monitored. The advanced stage of disease is mainly responsible for the dismal prognosis in these patients.[2,4,5,6,7]"
],
"date": "October 02, 2017",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/886/156/886156-figure-1_thumb.jpg"
},
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/886/156/886156-figure-2_thumb.jpg"
}
],
"markdown": "# A 62-Year-Old Man With Shortness of Breath Years After Heart Transplantation\n\n **Authors:** Shahzad G. Raja, MRCS; Gilles D. Dreyfus, MD, PhD, FRCS \n **Date:** October 02, 2017\n\n ## Content\n\n Discussion\nIn this patient, the posteroanterior upright chest radiograph showed right pleural effusion, a left upper-lobe mass (arrow), and changes consistent with emphysema. The CT scan showed air bronchograms within the left upper-lobe mass; areas of low attenuation throughout the lung parenchyma consistent with emphysema and mediastinal lymphadenopathy were seen as well.\nFigure 1.\nFigure 1.\nTransbronchial biopsy revealed poorly differentiated squamous cell carcinoma, with involvement of the lymphatics and submucosal vessels. A bone scan revealed metastatic disease in the left femur. A review of earlier films, however, showed no evidence or suspicion of early bronchogenic cancer.\nThe risk for cancer after solid-organ transplantation is well recognized in transplant recipients who have received long-term immunosuppression; in this group, the overall incidence of carcinoma increases by up to 100-fold, and the incidence of non-Hodgkin lymphoma increases by up to 40-fold. The incidence of carcinoma may vary with the type of allograft, however, because of differences in the induction therapy and immunosuppressive regimens. The most frequently observed tumors are squamous cell carcinoma of the skin, adenocarcinoma of the lung and gastrointestinal tract, Kaposi sarcoma, acute leukemia, and lymphoma.[1,2,3,4,5]\nThe incidence of bronchogenic carcinoma after solid-organ transplantation remains controversial. Although some authors have reported an incidence similar to that of the overall population, others have found an unexpectedly high incidence in the heart transplant population. In the heart transplant population, the incidence of bronchogenic carcinoma ranges from 0.6% to 4%. The increased incidence of lung cancer is potentially related to the prolonged use of cytotoxic agents for immunosuppression; however, the greatest risk factor for the development of lung cancer in the heart transplant population is probably from a concomitant history of prolonged cigarette smoking. In transplant recipients, the effects of inhaled carcinogens found in mainstream smoking products are believed to act in concert with impaired surveillance mechanisms resulting from long-term immunosuppression to promote the development of cancer. As in the nontransplant population, cardiac transplant recipients with lung carcinoma are predominantly in their sixth or seventh decade of life.[1,2,3,4,6]\nThe interval from transplantation to diagnosis of cancer is usually around 3-5 years. However, neoplasm may be discovered within 6 months of transplantation. The most striking feature is that most patients have advanced disease (stage IIIA or higher) at the time of diagnosis, despite these patients being closely monitored. The advanced stage of disease is mainly responsible for the dismal prognosis in these patients.[2,4,5,6,7]\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 1.** \n \n\n\n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1146310,
"choiceText": "Tuberculosis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1146312,
"choiceText": "Histoplasmosis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1146314,
"choiceText": "Pneumonia",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1146316,
"choiceText": "Bronchogenic carcinoma",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 363909,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 62-Year-Old Man With Shortness of Breath Years After Heart Transplantation"
},
{
"authors": "Shahzad G. Raja, MRCS; Gilles D. Dreyfus, MD, PhD, FRCS",
"content": [
"The symptoms at presentation vary widely and include cough, unresolved pneumonia, dyspnea, weight loss, and anorexia. Despite routine radiographic surveillance, almost one half of these cancers are incidentally discovered. This observation suggests that these tumors may have rapid doubling times and/or that \"routine surveillance\" chest radiography is inadequate for early diagnosis of lung cancer in this population of patients.",
"In addition, nearly one half of these lesions are initially missed by the reviewing radiologists. Data from studies examining missed and potentially curable lung neoplasms indicate that most lesions are of \"low obviousness.\"[8] Inclusion of the patient's smoking history on the radiographic requisition may help enhance the radiologist's search for possible lung cancer. Immunosuppression further complicates matters because of the likelihood that a new radiographic abnormality, if detected, may first be attributed to an ongoing infectious process rather than a neoplastic one.",
"On chest radiography, a nodule or mass is the most common radiologic manifestation of bronchogenic carcinoma and, in most cases, the tumor size at detection is ≥ 2 cm in diameter. Most radiographically apparent cancers are visible a mean of 12 months before the time of diagnosis. Radiographic recognition of lung cancer in patients with fibrotic lung disease can be particularly problematic because of obscuration by adjacent parenchymal disease. CT evaluation improves the visibility of cancers associated with pulmonary fibrosis, which most commonly manifest as ill-defined lesions mimicking air-space consolidation.[6,9] The diagnosis of bronchogenic carcinoma is established by a bronchoscopic biopsy. The histologic subtypes of the tumors include small cell carcinoma, adenocarcinoma, and anaplastic and squamous cell carcinoma.",
"Surgical intervention is ideally the treatment of choice; when it is performed, medium-term survival is achievable. Optimal surgical treatment might not be possible, however, because of impaired respiratory function. Heart transplantation recipients do not tolerate extensive lung resection in the same manner as nontransplant recipients. As a result of a history of smoking that has caused end-stage failure of one organ, many patients invariably have a degree of chronic lung damage, even in the presence of normal spirometry. The surgical procedures consist of lobectomy, bilobectomy, lobectomy with combined chest-wall resection, and wedge resections. In reality, because most patients have advanced (unresectable) disease, treatment is largely limited to chemotherapy and palliative radiation.[6,9]",
"A high rate of severe postoperative infectious complications, occasionally with fatal consequences, is seen in patients undergoing surgical resection. Postoperative complications are probably favored by the use of immunosuppressive drug therapy. Common postoperative complications include septicemia, pneumonia, prolonged air leak, wound infection, respiratory failure, and chronic renal insufficiency. Some authors have proposed measuring the procalcitonin plasma level to specifically detect bacterial infections in transplant recipients. Earlier treatment of infection may help prevent severe postoperative complications observed in heart transplant recipients undergoing surgery for lung cancer. Preoperative and postoperative repeat sputum cultures must be performed, and careful perioperative management of antibiotic therapy must be adopted.[6,9]",
"According to long-term survival data available in the literature, the median survival of patients who underwent curative surgical treatment is 23 months, with a range of 12-34 months. Survival in heart transplant recipients depends mainly on the quality of their post-transplantation surveillance. The worst survival rate (median survival of 27 days) is observed when standard chest radiography is performed at 6-month intervals during follow-up. The median survival after diagnosis of lung cancer is 3 months, and most deaths are directly related to metastatic disease.[2,6,7,8,10]"
],
"date": "October 02, 2017",
"figures": [],
"markdown": "# A 62-Year-Old Man With Shortness of Breath Years After Heart Transplantation\n\n **Authors:** Shahzad G. Raja, MRCS; Gilles D. Dreyfus, MD, PhD, FRCS \n **Date:** October 02, 2017\n\n ## Content\n\n The symptoms at presentation vary widely and include cough, unresolved pneumonia, dyspnea, weight loss, and anorexia. Despite routine radiographic surveillance, almost one half of these cancers are incidentally discovered. This observation suggests that these tumors may have rapid doubling times and/or that \"routine surveillance\" chest radiography is inadequate for early diagnosis of lung cancer in this population of patients.\nIn addition, nearly one half of these lesions are initially missed by the reviewing radiologists. Data from studies examining missed and potentially curable lung neoplasms indicate that most lesions are of \"low obviousness.\"[8] Inclusion of the patient's smoking history on the radiographic requisition may help enhance the radiologist's search for possible lung cancer. Immunosuppression further complicates matters because of the likelihood that a new radiographic abnormality, if detected, may first be attributed to an ongoing infectious process rather than a neoplastic one.\nOn chest radiography, a nodule or mass is the most common radiologic manifestation of bronchogenic carcinoma and, in most cases, the tumor size at detection is ≥ 2 cm in diameter. Most radiographically apparent cancers are visible a mean of 12 months before the time of diagnosis. Radiographic recognition of lung cancer in patients with fibrotic lung disease can be particularly problematic because of obscuration by adjacent parenchymal disease. CT evaluation improves the visibility of cancers associated with pulmonary fibrosis, which most commonly manifest as ill-defined lesions mimicking air-space consolidation.[6,9] The diagnosis of bronchogenic carcinoma is established by a bronchoscopic biopsy. The histologic subtypes of the tumors include small cell carcinoma, adenocarcinoma, and anaplastic and squamous cell carcinoma.\nSurgical intervention is ideally the treatment of choice; when it is performed, medium-term survival is achievable. Optimal surgical treatment might not be possible, however, because of impaired respiratory function. Heart transplantation recipients do not tolerate extensive lung resection in the same manner as nontransplant recipients. As a result of a history of smoking that has caused end-stage failure of one organ, many patients invariably have a degree of chronic lung damage, even in the presence of normal spirometry. The surgical procedures consist of lobectomy, bilobectomy, lobectomy with combined chest-wall resection, and wedge resections. In reality, because most patients have advanced (unresectable) disease, treatment is largely limited to chemotherapy and palliative radiation.[6,9]\nA high rate of severe postoperative infectious complications, occasionally with fatal consequences, is seen in patients undergoing surgical resection. Postoperative complications are probably favored by the use of immunosuppressive drug therapy. Common postoperative complications include septicemia, pneumonia, prolonged air leak, wound infection, respiratory failure, and chronic renal insufficiency. Some authors have proposed measuring the procalcitonin plasma level to specifically detect bacterial infections in transplant recipients. Earlier treatment of infection may help prevent severe postoperative complications observed in heart transplant recipients undergoing surgery for lung cancer. Preoperative and postoperative repeat sputum cultures must be performed, and careful perioperative management of antibiotic therapy must be adopted.[6,9]\nAccording to long-term survival data available in the literature, the median survival of patients who underwent curative surgical treatment is 23 months, with a range of 12-34 months. Survival in heart transplant recipients depends mainly on the quality of their post-transplantation surveillance. The worst survival rate (median survival of 27 days) is observed when standard chest radiography is performed at 6-month intervals during follow-up. The median survival after diagnosis of lung cancer is 3 months, and most deaths are directly related to metastatic disease.[2,6,7,8,10]\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 62-Year-Old Man With Shortness of Breath Years After Heart Transplantation"
},
{
"authors": "Shahzad G. Raja, MRCS; Gilles D. Dreyfus, MD, PhD, FRCS",
"content": [
"A careful search for bronchogenic carcinoma in recipients with a history of smoking may help improve survival. Standard radiographs miss lung cancer in 50% of patients. Regular CT of the chest is more appropriate for the detection of early-stage lung cancer. The available literature suggests that twice-yearly combined radiography and CT can increase the likelihood of early tumor detection, resection, and improved survival. When a candidate for cardiac transplantation has a 30–pack-year smoking history, an aggressive search for occult intrathoracic cancer is advisable; in this situation, screening CT of the chest may be worth consideration.[1,3,5,6,7,8,9,10]",
"In this case, the patient's condition unfortunately worsened after admission. He underwent endotracheal intubation for progressive respiratory distress and, approximately 1 week after transfer to the intensive care unit, he experienced cardiopulmonary arrest that was probably related to complications from his metastatic bronchogenic carcinoma that resulted in death."
],
"date": "October 02, 2017",
"figures": [],
"markdown": "# A 62-Year-Old Man With Shortness of Breath Years After Heart Transplantation\n\n **Authors:** Shahzad G. Raja, MRCS; Gilles D. Dreyfus, MD, PhD, FRCS \n **Date:** October 02, 2017\n\n ## Content\n\n A careful search for bronchogenic carcinoma in recipients with a history of smoking may help improve survival. Standard radiographs miss lung cancer in 50% of patients. Regular CT of the chest is more appropriate for the detection of early-stage lung cancer. The available literature suggests that twice-yearly combined radiography and CT can increase the likelihood of early tumor detection, resection, and improved survival. When a candidate for cardiac transplantation has a 30–pack-year smoking history, an aggressive search for occult intrathoracic cancer is advisable; in this situation, screening CT of the chest may be worth consideration.[1,3,5,6,7,8,9,10]\nIn this case, the patient's condition unfortunately worsened after admission. He underwent endotracheal intubation for progressive respiratory distress and, approximately 1 week after transfer to the intensive care unit, he experienced cardiopulmonary arrest that was probably related to complications from his metastatic bronchogenic carcinoma that resulted in death.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1146318,
"choiceText": "Chest radiography",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1146320,
"choiceText": "Transesophageal ultrasonography",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1146322,
"choiceText": "Transbronchial biopsy",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1146324,
"choiceText": "CT of the chest",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1146326,
"choiceText": "PET",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>The diagnosis of bronchogenic carcinoma is established by bronchoscopic biopsy. The histologic subtypes of the tumors include small cell carcinoma, adenocarcinoma, and anaplastic and squamous cell carcinoma.</p>",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 363911,
"questionText": "Which of the following diagnostic imaging techniques or procedures definitively establishes the diagnosis of bronchogenic carcinoma?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1146328,
"choiceText": "Bone marrow failure",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1146330,
"choiceText": "Cardiac failure",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1146332,
"choiceText": "Chronic renal failure",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1146334,
"choiceText": "Metastatic disease",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1146336,
"choiceText": "Pneumonia",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>The worst survival rate (median survival of 27 days) is observed when standard chest radiography is performed at 6-month intervals during follow-up. The median survival after diagnosis of lung cancer is 3 months, and most deaths are directly related to metastatic disease.</p>",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 363913,
"questionText": "Which of the following is the most likely cause of death in cardiac transplant recipients with lung cancer?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 62-Year-Old Man With Shortness of Breath Years After Heart Transplantation"
},
{
"authors": "Shahzad G. Raja, MRCS; Gilles D. Dreyfus, MD, PhD, FRCS",
"content": [],
"date": "October 02, 2017",
"figures": [],
"markdown": "# A 62-Year-Old Man With Shortness of Breath Years After Heart Transplantation\n\n **Authors:** Shahzad G. Raja, MRCS; Gilles D. Dreyfus, MD, PhD, FRCS \n **Date:** October 02, 2017\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1146318,
"choiceText": "Chest radiography",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1146320,
"choiceText": "Transesophageal ultrasonography",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1146322,
"choiceText": "Transbronchial biopsy",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1146324,
"choiceText": "CT of the chest",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1146326,
"choiceText": "PET",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>The diagnosis of bronchogenic carcinoma is established by bronchoscopic biopsy. The histologic subtypes of the tumors include small cell carcinoma, adenocarcinoma, and anaplastic and squamous cell carcinoma.</p>",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 363911,
"questionText": "Which of the following diagnostic imaging techniques or procedures definitively establishes the diagnosis of bronchogenic carcinoma?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1146328,
"choiceText": "Bone marrow failure",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1146330,
"choiceText": "Cardiac failure",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1146332,
"choiceText": "Chronic renal failure",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1146334,
"choiceText": "Metastatic disease",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1146336,
"choiceText": "Pneumonia",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>The worst survival rate (median survival of 27 days) is observed when standard chest radiography is performed at 6-month intervals during follow-up. The median survival after diagnosis of lung cancer is 3 months, and most deaths are directly related to metastatic disease.</p>",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 363913,
"questionText": "Which of the following is the most likely cause of death in cardiac transplant recipients with lung cancer?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 62-Year-Old Man With Shortness of Breath Years After Heart Transplantation"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1146310,
"choiceText": "Tuberculosis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1146312,
"choiceText": "Histoplasmosis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1146314,
"choiceText": "Pneumonia",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1146316,
"choiceText": "Bronchogenic carcinoma",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 363909,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1146318,
"choiceText": "Chest radiography",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1146320,
"choiceText": "Transesophageal ultrasonography",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1146322,
"choiceText": "Transbronchial biopsy",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1146324,
"choiceText": "CT of the chest",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1146326,
"choiceText": "PET",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>The diagnosis of bronchogenic carcinoma is established by bronchoscopic biopsy. The histologic subtypes of the tumors include small cell carcinoma, adenocarcinoma, and anaplastic and squamous cell carcinoma.</p>",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 363911,
"questionText": "Which of the following diagnostic imaging techniques or procedures definitively establishes the diagnosis of bronchogenic carcinoma?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1146328,
"choiceText": "Bone marrow failure",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1146330,
"choiceText": "Cardiac failure",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1146332,
"choiceText": "Chronic renal failure",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1146334,
"choiceText": "Metastatic disease",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1146336,
"choiceText": "Pneumonia",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>The worst survival rate (median survival of 27 days) is observed when standard chest radiography is performed at 6-month intervals during follow-up. The median survival after diagnosis of lung cancer is 3 months, and most deaths are directly related to metastatic disease.</p>",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 363913,
"questionText": "Which of the following is the most likely cause of death in cardiac transplant recipients with lung cancer?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
886160 | /viewarticle/886160 | [
{
"authors": "Seina Farshadsefat, DO; Michael H. Piper, MD; Bradley J. Warren, DO",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"A 68-year-old man presents to the hospital with severe pain and swelling in his left foot at the base of his first metatarsophalangeal (MTP) joint. He also reports right knee and medial malleolar pain. He describes vague, mild epigastric pain without nausea, vomiting, diarrhea, melena, or hematochezia. He states that these symptoms initially started several months ago. He reports no fevers or chills. His weight has been stable, and his appetite has been normal. He describes associated painful skin nodules on his lower extremities as well.",
"The patient denies any symptoms of recent viral infection. He has not traveled outside of the country. Surgical history consists of Nissen fundoplication. He reports a history of hypertension, hyperlipidemia, hypothyroidism, recurrent pancreatitis, gastroesophageal reflux disease, and Barrett esophagus. His medications include pantoprazole, aspirin, losartan, tamsulosin, simvastatin, and levothyroxine. He does not take herbal supplements. He has no significant family history. He has a 30–pack-year smoking history and quit 8 years ago. He denies alcoholic beverage intake and illicit drug use. He is a retired plumber."
],
"date": "September 29, 2017",
"figures": [],
"markdown": "# A 68-Year-Old Man With Severe Joint Pain and Nodules\n\n **Authors:** Seina Farshadsefat, DO; Michael H. Piper, MD; Bradley J. Warren, DO \n **Date:** September 29, 2017\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nA 68-year-old man presents to the hospital with severe pain and swelling in his left foot at the base of his first metatarsophalangeal (MTP) joint. He also reports right knee and medial malleolar pain. He describes vague, mild epigastric pain without nausea, vomiting, diarrhea, melena, or hematochezia. He states that these symptoms initially started several months ago. He reports no fevers or chills. His weight has been stable, and his appetite has been normal. He describes associated painful skin nodules on his lower extremities as well.\nThe patient denies any symptoms of recent viral infection. He has not traveled outside of the country. Surgical history consists of Nissen fundoplication. He reports a history of hypertension, hyperlipidemia, hypothyroidism, recurrent pancreatitis, gastroesophageal reflux disease, and Barrett esophagus. His medications include pantoprazole, aspirin, losartan, tamsulosin, simvastatin, and levothyroxine. He does not take herbal supplements. He has no significant family history. He has a 30–pack-year smoking history and quit 8 years ago. He denies alcoholic beverage intake and illicit drug use. He is a retired plumber.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 68-Year-Old Man With Severe Joint Pain and Nodules"
},
{
"authors": "Seina Farshadsefat, DO; Michael H. Piper, MD; Bradley J. Warren, DO",
"content": [
"The patient is alert but appears in pain. He is afebrile and his vital signs are stable. His cardiac and lung examination findings are unremarkable. His abdomen is soft, nontender, and nondistended, and bowel sounds are present.",
"The left first MTP joint is edematous, with surrounding erythema, and is tender to palpation (Figure 1). Multiple, painful, ill-defined, erythematous, reddish-brown nodules are noted on the patient's lower extremities (Figure 2).",
"Figure 1.",
"Figure 2.",
"No neurologic deficits are noted. Laboratory studies reveal a white blood cell count of 12,000 cells/mm3. Complete metabolic panel findings, including liver enzyme levels, are normal. The patient's lipase level is > 16,000 U/L, and his amylase level is > 4700 U/L. His antinuclear antibody and immunoglobulin G4 levels are normal. His C-reactive protein level is 35.6 mg/L. His alcohol, calcium, uric acid, and triglyceride levels are normal.",
"A CT scan of the patient's ankle and foot with contrast is shown in Figure 3. A CT scan of the abdomen with contrast is shown in Figure 4.",
"Figure 3.",
"Figure 4.",
"Aspirate of the left first MTP joint yields 3 mL of purulent material. Subsequent fluid analysis shows a white blood cell count of 6800 cells/mm3, which is predominantly neutrophilic, with no crystals and what appears to be lipids."
],
"date": "September 29, 2017",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/886/160/886160-Thumb1.png"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/886/160/886160-Thumb2a.png"
},
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/886/160/886160-Thumb3a.png"
},
{
"caption": "Figure 4.",
"image_url": "https://img.medscapestatic.com/article/886/160/886160-Thumb4a.png"
}
],
"markdown": "# A 68-Year-Old Man With Severe Joint Pain and Nodules\n\n **Authors:** Seina Farshadsefat, DO; Michael H. Piper, MD; Bradley J. Warren, DO \n **Date:** September 29, 2017\n\n ## Content\n\n The patient is alert but appears in pain. He is afebrile and his vital signs are stable. His cardiac and lung examination findings are unremarkable. His abdomen is soft, nontender, and nondistended, and bowel sounds are present.\nThe left first MTP joint is edematous, with surrounding erythema, and is tender to palpation (Figure 1). Multiple, painful, ill-defined, erythematous, reddish-brown nodules are noted on the patient's lower extremities (Figure 2).\nFigure 1.\nFigure 2.\nNo neurologic deficits are noted. Laboratory studies reveal a white blood cell count of 12,000 cells/mm3. Complete metabolic panel findings, including liver enzyme levels, are normal. The patient's lipase level is > 16,000 U/L, and his amylase level is > 4700 U/L. His antinuclear antibody and immunoglobulin G4 levels are normal. His C-reactive protein level is 35.6 mg/L. His alcohol, calcium, uric acid, and triglyceride levels are normal.\nA CT scan of the patient's ankle and foot with contrast is shown in Figure 3. A CT scan of the abdomen with contrast is shown in Figure 4.\nFigure 3.\nFigure 4.\nAspirate of the left first MTP joint yields 3 mL of purulent material. Subsequent fluid analysis shows a white blood cell count of 6800 cells/mm3, which is predominantly neutrophilic, with no crystals and what appears to be lipids.\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n**Figure 3.** \n \n\n**Figure 4.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1142850,
"choiceText": "Pancreatitis, polyarthritis, and panniculitis syndrome\r\n\r\n",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1142852,
"choiceText": "Sarcoidosis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1142854,
"choiceText": "Caroli disease",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1142856,
"choiceText": "<i>Yersinia</i> infection",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1142858,
"choiceText": "Crohn disease",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 362769,
"questionText": "Which of the following is the most likely diagnosis? \r\n\r\n",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 68-Year-Old Man With Severe Joint Pain and Nodules"
},
{
"authors": "Seina Farshadsefat, DO; Michael H. Piper, MD; Bradley J. Warren, DO",
"content": [
"CT of the abdomen revealed a 1.8 cm × 1.4 cm hypodense lesion within the pancreatic head, with calcifications and mild proximal pancreatic ductal dilatation. Subsequent endoscopic ultrasonography revealed a 16 mm × 17 mm hypoechoic structure in the pancreatic head, with calcifications; it appeared to be communicating with the main pancreatic duct, a possible intraductal papillary mucinous neoplasm. Fine-needle aspiration biopsy revealed acellular material. CT of the left ankle revealed bony cortical destruction in the lateral aspect of the posterior calcaneus.",
"Bone biopsy showed mild chronic inflammation, with no evidence of osteomyelitis. Punch biopsies of the tender, erythematous, hyperpigmented nodules showed lobular panniculitis with extensive necrosis of adipocytes and saponification.",
"Symptomatic treatment with nonsteroidal anti-inflammatory drugs (NSAIDs) and corticosteroids was ineffective. A Whipple procedure was performed to resect the pancreatic head lesion. Postoperative follow-up revealed normalization of the patient's amylase and lipase levels. In addition, resolution of the polyarthritis and panniculitis was noted.",
"Pancreatitis, in an acute setting, typically presents with epigastric or left upper-quadrant pain that classically radiates to the back. The diagnosis of acute pancreatitis is established by the presence of two of the following criteria[1]:",
"Abdominal pain consistent with the disease",
"Serum amylase or lipase levels greater than three times the upper limit of normal",
"Characteristic findings on radiographic imaging",
"The most common causes of acute pancreatitis are gallstones (40%-70%) and alcoholconsumption (25%-35%). Other causes of acute pancreatitis include medications, infectious etiologies, hypertriglyceridemia, hypercalcemia, pancreatic divisum, sphincter of Oddi dysfunction, autoimmune disease, hereditary causes, iatrogenic causes, trauma, and benign or malignant masses or lesions.[1]"
],
"date": "September 29, 2017",
"figures": [],
"markdown": "# A 68-Year-Old Man With Severe Joint Pain and Nodules\n\n **Authors:** Seina Farshadsefat, DO; Michael H. Piper, MD; Bradley J. Warren, DO \n **Date:** September 29, 2017\n\n ## Content\n\n CT of the abdomen revealed a 1.8 cm × 1.4 cm hypodense lesion within the pancreatic head, with calcifications and mild proximal pancreatic ductal dilatation. Subsequent endoscopic ultrasonography revealed a 16 mm × 17 mm hypoechoic structure in the pancreatic head, with calcifications; it appeared to be communicating with the main pancreatic duct, a possible intraductal papillary mucinous neoplasm. Fine-needle aspiration biopsy revealed acellular material. CT of the left ankle revealed bony cortical destruction in the lateral aspect of the posterior calcaneus.\nBone biopsy showed mild chronic inflammation, with no evidence of osteomyelitis. Punch biopsies of the tender, erythematous, hyperpigmented nodules showed lobular panniculitis with extensive necrosis of adipocytes and saponification.\nSymptomatic treatment with nonsteroidal anti-inflammatory drugs (NSAIDs) and corticosteroids was ineffective. A Whipple procedure was performed to resect the pancreatic head lesion. Postoperative follow-up revealed normalization of the patient's amylase and lipase levels. In addition, resolution of the polyarthritis and panniculitis was noted.\nPancreatitis, in an acute setting, typically presents with epigastric or left upper-quadrant pain that classically radiates to the back. The diagnosis of acute pancreatitis is established by the presence of two of the following criteria[1]:\nAbdominal pain consistent with the disease\nSerum amylase or lipase levels greater than three times the upper limit of normal\nCharacteristic findings on radiographic imaging\nThe most common causes of acute pancreatitis are gallstones (40%-70%) and alcoholconsumption (25%-35%). Other causes of acute pancreatitis include medications, infectious etiologies, hypertriglyceridemia, hypercalcemia, pancreatic divisum, sphincter of Oddi dysfunction, autoimmune disease, hereditary causes, iatrogenic causes, trauma, and benign or malignant masses or lesions.[1]\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1142850,
"choiceText": "Pancreatitis, polyarthritis, and panniculitis syndrome\r\n\r\n",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1142852,
"choiceText": "Sarcoidosis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1142854,
"choiceText": "Caroli disease",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1142856,
"choiceText": "<i>Yersinia</i> infection",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1142858,
"choiceText": "Crohn disease",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 362769,
"questionText": "Which of the following is the most likely diagnosis? \r\n\r\n",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 68-Year-Old Man With Severe Joint Pain and Nodules"
},
{
"authors": "Seina Farshadsefat, DO; Michael H. Piper, MD; Bradley J. Warren, DO",
"content": [
"The initial assessment of patients with acute pancreatitis should involve evaluation of the patient's hemodynamic status as well as risk stratification. Poor patient outcomes arise with failure to provide adequate hydration, failure to diagnose and treat cholangitis, and failure to treat early organ failure.[1] Several scoring systems are used to predict the severity of acute pancreatitis, including the Bedside Index of Severity in Acute Pancreatitis score. However, no severity scoring system has been shown to be more accurate than another; thus, careful examination and monitoring of the patient's clinical and hemodynamic status is key.",
"Certain intrinsic risk factors make patients more prone to develop severe pancreatitis. These include the following[1]:",
"Age",
"Body mass index",
"Comorbid health problems",
"Systemic inflammatory response syndrome",
"Elevated blood urea nitrogen and hematocrit levels",
"Altered mental status",
"Pleural effusions",
"Early and aggressive intravenous hydration is the cornerstone in the management of pancreatitis. Hypovolemia is a consequence of vomiting, decreased oral intake, and third-spacing of fluids. Early hydration has been shown to be most beneficial within the first 24 hours of presentation, with aggressive intravenous hydration being defined as 250-500 mL/h of isotonic crystalloid solution (normal saline or lactated Ringer solution), assuming that the patient does not have comorbid cardiovascular or renal disease.[1] For patients who are clinically stable with infected necrosis, drainage by endoscopic, surgical, or radiologic means should be preferably delayed for at least 4 weeks to allow the infected necrosis to wall off. Those who remain symptomatic require surgical necrosectomy.",
"Prophylactic antibiotics in patients with severe acute pancreatitis is not recommended according to guidelines established by the American College of Gastroenterology.[1] Patients who develop necrotizing pancreatitis should be treated with antibiotics once infected necrosis is established. Patients with sterile necrosis should not receive antibiotics. CT-guided fine-needle aspiration is safe and accurate in distinguishing between infected and sterile necrosis.[1]",
"Pancreatitis, polyarthritis, and panniculitis syndrome was first described by Chiari in 1883. The diagnosis is made clinically in patients with acute or chronic pancreatitis that is complicated by panniculitis as well as mono-oligoarthritis. The syndrome is rarely reported, with a frequency of 0.3%-3%.[2,3,4] The syndrome can be seen in patients of any age, with a peak frequency in the fifth decade of life; it is more common in men with a history of significant alcohol intake.[2,3,4,5,6,7]",
"The pathophysiology of the syndrome is hypothesized to involve release of activated pancreatic enzymes into systemic circulation, which promotes lipolysis and inflammation in distant soft-tissue sites.[8,9,10,11] Arthritis may be caused by free fatty acids that are released into the joint, after the lipolytic pancreatic enzymes have hydrolyzed triglycerides in the joint near the bone marrow. Several pancreatic enzymes, including phospholipase A, lipase, amylase, and trypsin, have been implicated.[5,8] Notably, all cases show considerably elevated lipase levels, but not elevated amylase levels. In addition, elevated amylase alone cannot cause panniculitis. Also, although the lipase level does not indicate the severity of acute pancreatitis, it seems to correspond with the extent of extrapancreatic fat-tissue necrosis in pancreatitis, polyarthritis, and panniculitis syndrome.",
"The presence of a pancreatic pseudocyst, or a fistula between the pseudocyst and the superior mesenteric vein, has been described. Although the tiny fistulas are usually difficult to visualize in preoperative imaging, the presence of mesenteric vein thrombosis can be an indirect sign and should always lead to surgical exploration. During surgical management of the underlying pancreatic disease, such fistulae should be corrected, because they apparently drain enzyme-rich pancreatic juice into the portal blood stream.",
"Abdominal pain is characteristically absent or very mild in pancreatitis, polyarthritis, and panniculitis syndrome.",
"Lobular panniculitis presents with erythematous nodules (0.5-5 cm) that typically affect the lower limbs but can spread to the entire body if the disease progresses. Such lesions are tender, and may ulcerate with spontaneous rupture. Proper diagnosis requires biopsy of these lesions. The exudate is sterile and is characterized as an oily, brown, viscous substance as a result of liquefaction necrosis. Histologically, the regions of fat necrosis contain ghostlike fat cells, which is a characteristic finding and is not seen in other causes of panniculitis.",
"Lobular panniculitis can be seen in other diseases, including but not limited to inflammatory bowel disease, gout, Sweet syndrome, and lupus. Joint involvement in pancreatitis, polyarthritis, and panniculitis syndrome typically presents as asymmetric polyarthritis that involves both large and small joints. Monoarthritis and oligoarthritis have been reported.[9,10] The most commonly affected joints include the knees, ankles and wrists. Radiographic findings of the affected joints may include osteolytic lesions, loss of joint space, and bony destruction.[8,10] The differential diagnosis is broad but may include septic arthritis, rheumatoid arthritis, and inflammatory arthritis."
],
"date": "September 29, 2017",
"figures": [],
"markdown": "# A 68-Year-Old Man With Severe Joint Pain and Nodules\n\n **Authors:** Seina Farshadsefat, DO; Michael H. Piper, MD; Bradley J. Warren, DO \n **Date:** September 29, 2017\n\n ## Content\n\n The initial assessment of patients with acute pancreatitis should involve evaluation of the patient's hemodynamic status as well as risk stratification. Poor patient outcomes arise with failure to provide adequate hydration, failure to diagnose and treat cholangitis, and failure to treat early organ failure.[1] Several scoring systems are used to predict the severity of acute pancreatitis, including the Bedside Index of Severity in Acute Pancreatitis score. However, no severity scoring system has been shown to be more accurate than another; thus, careful examination and monitoring of the patient's clinical and hemodynamic status is key.\nCertain intrinsic risk factors make patients more prone to develop severe pancreatitis. These include the following[1]:\nAge\nBody mass index\nComorbid health problems\nSystemic inflammatory response syndrome\nElevated blood urea nitrogen and hematocrit levels\nAltered mental status\nPleural effusions\nEarly and aggressive intravenous hydration is the cornerstone in the management of pancreatitis. Hypovolemia is a consequence of vomiting, decreased oral intake, and third-spacing of fluids. Early hydration has been shown to be most beneficial within the first 24 hours of presentation, with aggressive intravenous hydration being defined as 250-500 mL/h of isotonic crystalloid solution (normal saline or lactated Ringer solution), assuming that the patient does not have comorbid cardiovascular or renal disease.[1] For patients who are clinically stable with infected necrosis, drainage by endoscopic, surgical, or radiologic means should be preferably delayed for at least 4 weeks to allow the infected necrosis to wall off. Those who remain symptomatic require surgical necrosectomy.\nProphylactic antibiotics in patients with severe acute pancreatitis is not recommended according to guidelines established by the American College of Gastroenterology.[1] Patients who develop necrotizing pancreatitis should be treated with antibiotics once infected necrosis is established. Patients with sterile necrosis should not receive antibiotics. CT-guided fine-needle aspiration is safe and accurate in distinguishing between infected and sterile necrosis.[1]\nPancreatitis, polyarthritis, and panniculitis syndrome was first described by Chiari in 1883. The diagnosis is made clinically in patients with acute or chronic pancreatitis that is complicated by panniculitis as well as mono-oligoarthritis. The syndrome is rarely reported, with a frequency of 0.3%-3%.[2,3,4] The syndrome can be seen in patients of any age, with a peak frequency in the fifth decade of life; it is more common in men with a history of significant alcohol intake.[2,3,4,5,6,7]\nThe pathophysiology of the syndrome is hypothesized to involve release of activated pancreatic enzymes into systemic circulation, which promotes lipolysis and inflammation in distant soft-tissue sites.[8,9,10,11] Arthritis may be caused by free fatty acids that are released into the joint, after the lipolytic pancreatic enzymes have hydrolyzed triglycerides in the joint near the bone marrow. Several pancreatic enzymes, including phospholipase A, lipase, amylase, and trypsin, have been implicated.[5,8] Notably, all cases show considerably elevated lipase levels, but not elevated amylase levels. In addition, elevated amylase alone cannot cause panniculitis. Also, although the lipase level does not indicate the severity of acute pancreatitis, it seems to correspond with the extent of extrapancreatic fat-tissue necrosis in pancreatitis, polyarthritis, and panniculitis syndrome.\nThe presence of a pancreatic pseudocyst, or a fistula between the pseudocyst and the superior mesenteric vein, has been described. Although the tiny fistulas are usually difficult to visualize in preoperative imaging, the presence of mesenteric vein thrombosis can be an indirect sign and should always lead to surgical exploration. During surgical management of the underlying pancreatic disease, such fistulae should be corrected, because they apparently drain enzyme-rich pancreatic juice into the portal blood stream.\nAbdominal pain is characteristically absent or very mild in pancreatitis, polyarthritis, and panniculitis syndrome.\nLobular panniculitis presents with erythematous nodules (0.5-5 cm) that typically affect the lower limbs but can spread to the entire body if the disease progresses. Such lesions are tender, and may ulcerate with spontaneous rupture. Proper diagnosis requires biopsy of these lesions. The exudate is sterile and is characterized as an oily, brown, viscous substance as a result of liquefaction necrosis. Histologically, the regions of fat necrosis contain ghostlike fat cells, which is a characteristic finding and is not seen in other causes of panniculitis.\nLobular panniculitis can be seen in other diseases, including but not limited to inflammatory bowel disease, gout, Sweet syndrome, and lupus. Joint involvement in pancreatitis, polyarthritis, and panniculitis syndrome typically presents as asymmetric polyarthritis that involves both large and small joints. Monoarthritis and oligoarthritis have been reported.[9,10] The most commonly affected joints include the knees, ankles and wrists. Radiographic findings of the affected joints may include osteolytic lesions, loss of joint space, and bony destruction.[8,10] The differential diagnosis is broad but may include septic arthritis, rheumatoid arthritis, and inflammatory arthritis.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 68-Year-Old Man With Severe Joint Pain and Nodules"
},
{
"authors": "Seina Farshadsefat, DO; Michael H. Piper, MD; Bradley J. Warren, DO",
"content": [
"Adjunctive therapies for pancreatitis, polyarthritis, and panniculitis syndrome, such as NSAIDs, corticosteroids, plasmapheresis, and subcutaneous octreotide, have been used with varying results but are mostly considered symptomatic therapy. Definitive treatment requires addressing the underlying pancreatic disorder. Prompt diagnosis of this syndrome is critical in order to avoid the associated high morbidity and mortality; the mortality rate can be as high as 24%.",
"In this case, after resection of the pancreatic head lesion that was an intraductal papillary mucinous neoplasm, follow-up laboratory results showed that this patient's pancreatic enzyme levels returned to normal. Follow-up clinical examinations showed eventual resolution of the patient's arthritic pain, as well as the panniculitis in the lower extremities. The patient has had no postoperative complications from the Whipple procedure and has not experienced any long-term sequelae from pancreatitis, polyarthritis, and panniculitis syndrome."
],
"date": "September 29, 2017",
"figures": [],
"markdown": "# A 68-Year-Old Man With Severe Joint Pain and Nodules\n\n **Authors:** Seina Farshadsefat, DO; Michael H. Piper, MD; Bradley J. Warren, DO \n **Date:** September 29, 2017\n\n ## Content\n\n Adjunctive therapies for pancreatitis, polyarthritis, and panniculitis syndrome, such as NSAIDs, corticosteroids, plasmapheresis, and subcutaneous octreotide, have been used with varying results but are mostly considered symptomatic therapy. Definitive treatment requires addressing the underlying pancreatic disorder. Prompt diagnosis of this syndrome is critical in order to avoid the associated high morbidity and mortality; the mortality rate can be as high as 24%.\nIn this case, after resection of the pancreatic head lesion that was an intraductal papillary mucinous neoplasm, follow-up laboratory results showed that this patient's pancreatic enzyme levels returned to normal. Follow-up clinical examinations showed eventual resolution of the patient's arthritic pain, as well as the panniculitis in the lower extremities. The patient has had no postoperative complications from the Whipple procedure and has not experienced any long-term sequelae from pancreatitis, polyarthritis, and panniculitis syndrome.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1142860,
"choiceText": "It is most common in elderly women",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1142862,
"choiceText": "Approximately 10% of patients with pancreatic disease also present with panniculitis and/or arthritis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1142864,
"choiceText": "Panniculitis typically affects the upper limbs",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1142866,
"choiceText": "Typical radiographic findings include multiple osteolytic lesions",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Approximately 0.3%-3% of patients with pancreatic disease present with panniculitis and/or arthritis. This syndrome is most commonly seen during the fifth decade of life and predominantly observed in males. Panniculitis usually affects the lower limbs. Mono-oligoarthritis typically presents as osteolytic lesions on radiographic imaging. ",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 362771,
"questionText": "Which of the following statements is accurate about pancreatitis, polyarthritis, and panniculitis syndrome?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1142886,
"choiceText": "Plasmapheresis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1142888,
"choiceText": "Treatment of the underlying etiology",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1142890,
"choiceText": "Subcutaneous octreotide",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1142892,
"choiceText": "Corticosteroids",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1142894,
"choiceText": "NSAIDs\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The underlying pathophysiology in this syndrome involves release of a high level of pancreatic enzymes (phospholipase A, lipase, amylase, and trypsin) from the pancreas owing to an acute or chronic cause. The systemic release of these enzymes ultimately leads to lobular panniculitis of the lower extremities and peripheral joint damage. Other adjunctive therapies (eg, NSAIDs, corticosteroids, plasmapheresis, subcutaneous octreotide) have been used, but with variable results. Depending on the kind of underlying pancreatic disease, correct treatment can be successful and lead to complete remission.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 362781,
"questionText": "Which of the following is the most appropriate approach to therapy in patients with pancreatitis, polyarthritis, and panniculitis syndrome?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 68-Year-Old Man With Severe Joint Pain and Nodules"
},
{
"authors": "Seina Farshadsefat, DO; Michael H. Piper, MD; Bradley J. Warren, DO",
"content": [],
"date": "September 29, 2017",
"figures": [],
"markdown": "# A 68-Year-Old Man With Severe Joint Pain and Nodules\n\n **Authors:** Seina Farshadsefat, DO; Michael H. Piper, MD; Bradley J. Warren, DO \n **Date:** September 29, 2017\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1142860,
"choiceText": "It is most common in elderly women",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1142862,
"choiceText": "Approximately 10% of patients with pancreatic disease also present with panniculitis and/or arthritis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1142864,
"choiceText": "Panniculitis typically affects the upper limbs",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1142866,
"choiceText": "Typical radiographic findings include multiple osteolytic lesions",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Approximately 0.3%-3% of patients with pancreatic disease present with panniculitis and/or arthritis. This syndrome is most commonly seen during the fifth decade of life and predominantly observed in males. Panniculitis usually affects the lower limbs. Mono-oligoarthritis typically presents as osteolytic lesions on radiographic imaging. ",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 362771,
"questionText": "Which of the following statements is accurate about pancreatitis, polyarthritis, and panniculitis syndrome?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1142886,
"choiceText": "Plasmapheresis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1142888,
"choiceText": "Treatment of the underlying etiology",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1142890,
"choiceText": "Subcutaneous octreotide",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1142892,
"choiceText": "Corticosteroids",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1142894,
"choiceText": "NSAIDs\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The underlying pathophysiology in this syndrome involves release of a high level of pancreatic enzymes (phospholipase A, lipase, amylase, and trypsin) from the pancreas owing to an acute or chronic cause. The systemic release of these enzymes ultimately leads to lobular panniculitis of the lower extremities and peripheral joint damage. Other adjunctive therapies (eg, NSAIDs, corticosteroids, plasmapheresis, subcutaneous octreotide) have been used, but with variable results. Depending on the kind of underlying pancreatic disease, correct treatment can be successful and lead to complete remission.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 362781,
"questionText": "Which of the following is the most appropriate approach to therapy in patients with pancreatitis, polyarthritis, and panniculitis syndrome?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 68-Year-Old Man With Severe Joint Pain and Nodules"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1142850,
"choiceText": "Pancreatitis, polyarthritis, and panniculitis syndrome\r\n\r\n",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1142852,
"choiceText": "Sarcoidosis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1142854,
"choiceText": "Caroli disease",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1142856,
"choiceText": "<i>Yersinia</i> infection",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1142858,
"choiceText": "Crohn disease",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 362769,
"questionText": "Which of the following is the most likely diagnosis? \r\n\r\n",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1142860,
"choiceText": "It is most common in elderly women",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1142862,
"choiceText": "Approximately 10% of patients with pancreatic disease also present with panniculitis and/or arthritis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1142864,
"choiceText": "Panniculitis typically affects the upper limbs",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1142866,
"choiceText": "Typical radiographic findings include multiple osteolytic lesions",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Approximately 0.3%-3% of patients with pancreatic disease present with panniculitis and/or arthritis. This syndrome is most commonly seen during the fifth decade of life and predominantly observed in males. Panniculitis usually affects the lower limbs. Mono-oligoarthritis typically presents as osteolytic lesions on radiographic imaging. ",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 362771,
"questionText": "Which of the following statements is accurate about pancreatitis, polyarthritis, and panniculitis syndrome?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1142886,
"choiceText": "Plasmapheresis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1142888,
"choiceText": "Treatment of the underlying etiology",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1142890,
"choiceText": "Subcutaneous octreotide",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1142892,
"choiceText": "Corticosteroids",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1142894,
"choiceText": "NSAIDs\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The underlying pathophysiology in this syndrome involves release of a high level of pancreatic enzymes (phospholipase A, lipase, amylase, and trypsin) from the pancreas owing to an acute or chronic cause. The systemic release of these enzymes ultimately leads to lobular panniculitis of the lower extremities and peripheral joint damage. Other adjunctive therapies (eg, NSAIDs, corticosteroids, plasmapheresis, subcutaneous octreotide) have been used, but with variable results. Depending on the kind of underlying pancreatic disease, correct treatment can be successful and lead to complete remission.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 362781,
"questionText": "Which of the following is the most appropriate approach to therapy in patients with pancreatitis, polyarthritis, and panniculitis syndrome?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
885234 | /viewarticle/885234 | [
{
"authors": "Jyoti Wadhwa, MBBS, MD; Madhavi Tripathi, MBBS, MD; Madhur Kumar Srivastava, MBBS",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"A 55-year-old man presents to the emergency department with recurrent pain in his right flank. The pain is described as sharp, severe in intensity, and radiating to the groin. Additionally, it increases with urination. He also gives a history of blood-tinged urine that occurred 1 day before the onset of the pain.",
"He denies having any history of fever, vomiting, or dysuria; however, he does report experiencing two similar episodes in the past year, both of which were relieved with oral analgesics. He denies having any bone pain, generalized weakness, change in bowel habits, or history of seizures. No history of similar episodes among his family members is reported.",
"The patient is a nonsmoker and denies heavy alcohol use. He has no known history of chronic medical or psychiatric conditions and is not taking any regular medications."
],
"date": "September 14, 2017",
"figures": [],
"markdown": "# A 55-Year-Old Man With Recurrent Sharp Flank Pain\n\n **Authors:** Jyoti Wadhwa, MBBS, MD; Madhavi Tripathi, MBBS, MD; Madhur Kumar Srivastava, MBBS \n **Date:** September 14, 2017\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nA 55-year-old man presents to the emergency department with recurrent pain in his right flank. The pain is described as sharp, severe in intensity, and radiating to the groin. Additionally, it increases with urination. He also gives a history of blood-tinged urine that occurred 1 day before the onset of the pain.\nHe denies having any history of fever, vomiting, or dysuria; however, he does report experiencing two similar episodes in the past year, both of which were relieved with oral analgesics. He denies having any bone pain, generalized weakness, change in bowel habits, or history of seizures. No history of similar episodes among his family members is reported.\nThe patient is a nonsmoker and denies heavy alcohol use. He has no known history of chronic medical or psychiatric conditions and is not taking any regular medications.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 55-Year-Old Man With Recurrent Sharp Flank Pain"
},
{
"authors": "Jyoti Wadhwa, MBBS, MD; Madhavi Tripathi, MBBS, MD; Madhur Kumar Srivastava, MBBS",
"content": [
"Upon physical examination, his oral temperature is 98.6°F (37°C), his pulse is regular at 90 beats/min, his blood pressure is 136/80 mm Hg, and his respiratory rate is 16 breaths/min. The patient is noted to be uncomfortable as a result of his flank pain but is in no acute distress. The examination of his head and neck is normal. His lungs are clear to auscultation, and he has normal cardiac sounds. His abdomen is soft and nontender, with no palpable masses. Mild costovertebral angle tenderness is present on the right side. A neurologic examination does not reveal any abnormal findings.",
"The initial laboratory studies show hematuria, with 75-100 red blood cells per high power field, and a normal creatinine level of 0.8 mg/dL (70.72 µmol/L). An ultrasound is performed, which reveals a stone of 13 mm in size in the right renal pelvis. After being given intravenous ketorolac, his pain resolves, and he is discharged home.",
"Because of his recurrent nephrolithiasis, a follow-up is arranged with urology, at which time he undergoes further testing. A laboratory workup reveals a serum ionized calcium concentration of 6.1 mg/dL (1.53 mmol/L; reference range, 4.5-5.6 mg/dL) and a 24-hour urinary calcium of 390 mg/24 h (9.75 mmol/24 h; reference range, <300 mg/24 h). Parathyroid scintigraphy is performed (see Figures 1 and 2).",
"Figure 1.",
"Figure 2."
],
"date": "September 14, 2017",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/885/234/885234-thumb1.jpg"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/885/234/885234-thumb2.jpg"
}
],
"markdown": "# A 55-Year-Old Man With Recurrent Sharp Flank Pain\n\n **Authors:** Jyoti Wadhwa, MBBS, MD; Madhavi Tripathi, MBBS, MD; Madhur Kumar Srivastava, MBBS \n **Date:** September 14, 2017\n\n ## Content\n\n Upon physical examination, his oral temperature is 98.6°F (37°C), his pulse is regular at 90 beats/min, his blood pressure is 136/80 mm Hg, and his respiratory rate is 16 breaths/min. The patient is noted to be uncomfortable as a result of his flank pain but is in no acute distress. The examination of his head and neck is normal. His lungs are clear to auscultation, and he has normal cardiac sounds. His abdomen is soft and nontender, with no palpable masses. Mild costovertebral angle tenderness is present on the right side. A neurologic examination does not reveal any abnormal findings.\nThe initial laboratory studies show hematuria, with 75-100 red blood cells per high power field, and a normal creatinine level of 0.8 mg/dL (70.72 µmol/L). An ultrasound is performed, which reveals a stone of 13 mm in size in the right renal pelvis. After being given intravenous ketorolac, his pain resolves, and he is discharged home.\nBecause of his recurrent nephrolithiasis, a follow-up is arranged with urology, at which time he undergoes further testing. A laboratory workup reveals a serum ionized calcium concentration of 6.1 mg/dL (1.53 mmol/L; reference range, 4.5-5.6 mg/dL) and a 24-hour urinary calcium of 390 mg/24 h (9.75 mmol/24 h; reference range, <300 mg/24 h). Parathyroid scintigraphy is performed (see Figures 1 and 2).\nFigure 1.\nFigure 2.\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1134398,
"choiceText": "Parathyroid adenoma",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1134400,
"choiceText": "Ectopic parathyroid gland",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1134402,
"choiceText": "Parathyroid carcinoma",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1134404,
"choiceText": "Sarcoid granuloma",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 359901,
"questionText": "Which of the following is the most likely diagnosis?\r\n\r\n<p><em>Hint: Consider possible underlying causes of hypercalcemia.</em></p>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 55-Year-Old Man With Recurrent Sharp Flank Pain"
},
{
"authors": "Jyoti Wadhwa, MBBS, MD; Madhavi Tripathi, MBBS, MD; Madhur Kumar Srivastava, MBBS",
"content": [
"The patient's technetium 99m-sestamibi scan showed normal uptake in the thyroid gland 20 minutes after administration of the radioisotope. Two hours after administration, once the radioisotope had \"washed off\" of the thyroid glands, uptake was noted in the left inferior parathyroid area, which is consistent with a parathyroid adenoma (see Figures 1 and 2).",
"Figure 1.",
"Figure 2.",
"His serum parathyroid hormone concentration was found to be markedly elevated at 450 pg/mL (450 ng/L; reference range, 10-60 pg/mL), confirming the diagnosis of primary hyperparathyroidism.",
"About 83% of people have four parathyroid glands: two superior glands and two inferior glands. Approximately 13% have more than four glands, whereas 3% have only three glands. The position of the superior glands is fairly constant; ectopic sites are usually seen with the inferior glands but not with the superior glands. The normal thyroid glands are encapsulated, soft, ovoid, yellowish-white organs that are surrounded by fat; each gland weighs approximately 40 mg and measures about 5 x 3 x 1 mm. The branches of the paired inferior thyroid arteries supply most of their blood. They receive blood from the paired superior thyroid arteries, the thyroidea ima artery, and branches of the laryngeal and tracheoesophageal arteries as well.[1,2]",
"The parathyroid glands secrete parathyroid hormone (PTH), which regulates serum calcium and phosphorus levels by raising serum calcium levels while lowering the serum phosphorus concentration. The regulation of PTH secretion occurs through a negative feedback loop; calcium-sensing receptors on the membranes of the parathyroid gland cells decrease PTH production as serum calcium concentrations rise. Hypocalcemia can prompt rapid secretion of the preformed PTH hormone. Extracellular calcium interacts with a calcium sensor to control PTH secretion; stimulation of the receptor by high calcium levels suppresses PTH secretion. The receptor is present in the parathyroid glands, the calcitonin secreting cells (C cells) of the thyroid, the brain, and the kidneys.[1,3]",
"Primary hyperparathyroidism is characterized by an excess of PTH secretion. Primary hyperparathyroidism affects approximately 1 person per 500-1000 population. It is two to four times more common in women than in men. It most commonly affects middle-aged adults and is rarely seen in children. Primary hyperparathyroidism is primarily caused by solitary adenomas (80% of cases) and, less commonly, chief cell hyperplasia (15%), multiple endocrine neoplasia (<5%), and parathyroid carcinoma (rare).[1]"
],
"date": "September 14, 2017",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/885/234/885234-thumb1.jpg"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/885/234/885234-thumb2.jpg"
}
],
"markdown": "# A 55-Year-Old Man With Recurrent Sharp Flank Pain\n\n **Authors:** Jyoti Wadhwa, MBBS, MD; Madhavi Tripathi, MBBS, MD; Madhur Kumar Srivastava, MBBS \n **Date:** September 14, 2017\n\n ## Content\n\n The patient's technetium 99m-sestamibi scan showed normal uptake in the thyroid gland 20 minutes after administration of the radioisotope. Two hours after administration, once the radioisotope had \"washed off\" of the thyroid glands, uptake was noted in the left inferior parathyroid area, which is consistent with a parathyroid adenoma (see Figures 1 and 2).\nFigure 1.\nFigure 2.\nHis serum parathyroid hormone concentration was found to be markedly elevated at 450 pg/mL (450 ng/L; reference range, 10-60 pg/mL), confirming the diagnosis of primary hyperparathyroidism.\nAbout 83% of people have four parathyroid glands: two superior glands and two inferior glands. Approximately 13% have more than four glands, whereas 3% have only three glands. The position of the superior glands is fairly constant; ectopic sites are usually seen with the inferior glands but not with the superior glands. The normal thyroid glands are encapsulated, soft, ovoid, yellowish-white organs that are surrounded by fat; each gland weighs approximately 40 mg and measures about 5 x 3 x 1 mm. The branches of the paired inferior thyroid arteries supply most of their blood. They receive blood from the paired superior thyroid arteries, the thyroidea ima artery, and branches of the laryngeal and tracheoesophageal arteries as well.[1,2]\nThe parathyroid glands secrete parathyroid hormone (PTH), which regulates serum calcium and phosphorus levels by raising serum calcium levels while lowering the serum phosphorus concentration. The regulation of PTH secretion occurs through a negative feedback loop; calcium-sensing receptors on the membranes of the parathyroid gland cells decrease PTH production as serum calcium concentrations rise. Hypocalcemia can prompt rapid secretion of the preformed PTH hormone. Extracellular calcium interacts with a calcium sensor to control PTH secretion; stimulation of the receptor by high calcium levels suppresses PTH secretion. The receptor is present in the parathyroid glands, the calcitonin secreting cells (C cells) of the thyroid, the brain, and the kidneys.[1,3]\nPrimary hyperparathyroidism is characterized by an excess of PTH secretion. Primary hyperparathyroidism affects approximately 1 person per 500-1000 population. It is two to four times more common in women than in men. It most commonly affects middle-aged adults and is rarely seen in children. Primary hyperparathyroidism is primarily caused by solitary adenomas (80% of cases) and, less commonly, chief cell hyperplasia (15%), multiple endocrine neoplasia (<5%), and parathyroid carcinoma (rare).[1]\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n\n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1134398,
"choiceText": "Parathyroid adenoma",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1134400,
"choiceText": "Ectopic parathyroid gland",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1134402,
"choiceText": "Parathyroid carcinoma",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1134404,
"choiceText": "Sarcoid granuloma",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 359901,
"questionText": "Which of the following is the most likely diagnosis?\r\n\r\n<p><em>Hint: Consider possible underlying causes of hypercalcemia.</em></p>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 55-Year-Old Man With Recurrent Sharp Flank Pain"
},
{
"authors": "Jyoti Wadhwa, MBBS, MD; Madhavi Tripathi, MBBS, MD; Madhur Kumar Srivastava, MBBS",
"content": [
"Parathyroid adenomas are usually found in the inferior parathyroid gland; however, in 6%-10% of patients, they may be located in the thyroid, thymus, the pericardium, and behind the esophagus. Adenomas usually weigh 0.5-5 g but may weigh as much as 10-20 g (on average, normal glands weigh about 25 mg).[4]",
"Approximately half of patients with hyperparathyroidism are asymptomatic. The manifestations of hyperparathyroidism primarily involve the skeletal system. Osteitis fibrosa cystica is a constellation of findings pathognomonic of hyperparathyroidism. In osteitis fibrosa cystica, the number of trabeculae on bones is reduced, and the number of giant multinucleated osteoclasts is increased. The surface of the bone may have a scalloped appearance (Howship lacunae). The normal marrow and cellular elements of bones are replaced with fibrous tissue, which weakens the bones and, often, results in pathologic fractures. Other changes include subperiosteal resorption of the phalanges and loss of the lamina dura of the teeth. Tiny punched out lesions (the classic \"salt-and-pepper\" appearance) are seen on the skull.[1,3]",
"Although hyperparathyroidism primarily affects the skeletal system, numerous other organ systems may be affected as well. Historically, kidney involvement has been reported in 60%-70% of cases. However, with improvement in early detection, it is now reported to be present in less than 20% of patients. Kidney involvement either results from the deposition of calcium in the renal parenchyma or from recurrent nephrolithiasis. Renal stones are usually composed of calcium phosphate or calcium oxalate. Recurrent nephrolithiasis can result in renal scarring, urinary tract obstruction, infection, and loss of renal function.[1,3]",
"Muscular manifestations may include proximal muscle weakness, fatigability, and atrophy. Neuropsychiatric findings typically occur only in advanced disease, and they may include confusion, psychosis, agitation, or coma. Band keratopathy is easily detectable in patients undergoing ophthalmologic examination, but these changes are not specific. Gastrointestinal system manifestations are typically subtle and nonspecific; however, they may include significant conditions, such as peptic ulcers, pancreatitis, or paralytic ileus. Chondrocalcinosis and pseudogout are also frequently seen.[1,3]",
"The diagnosis of hyperparathyroidism is typically made by the detection of an elevated immunoreactive PTH level. The suspicion of hyperparathyroidism should arise in patients with hypercalcemia, hypophosphatemia, and raised alkaline phosphatase levels or in those with the abnormal skeletal findings described above. Parathyroid ultrasonography and technetium-99m sestamibi parathyroid scintigraphy are most useful for the preoperative detection of parathyroid adenoma. Parathyroid scintigraphy can be limited by the presence of thyroid nodules or other metabolically active tissues, such as lymph nodes, diffuse hyperplasia, or metastatic thyroid cancer.[5]",
"Primary hyperparathyroidism should be established on the basis of biochemical findings before any imaging studies are performed.[1] The use of preoperative localization imaging is controversial in patients with primary hyperparathyroidism who have not undergone previous neck surgery. Shaha and colleagues[6] indicated that, in the following patients, preoperative imaging studies are warranted:",
"Patients with associated palpable thyroid abnormalities;",
"Patients in hypercalcemic crisis in whom urgent diagnosis is needed;",
"Patients with associated malignancies;",
"Asymptomatic patients with mild hypercalcemia;",
"Obese patients with short necks;",
"High-risk patients whose operative time is crucial;",
"High-risk patients in whom local anesthesia must be used; and",
"Patients with cervical spinal problems with neck extension difficulties."
],
"date": "September 14, 2017",
"figures": [],
"markdown": "# A 55-Year-Old Man With Recurrent Sharp Flank Pain\n\n **Authors:** Jyoti Wadhwa, MBBS, MD; Madhavi Tripathi, MBBS, MD; Madhur Kumar Srivastava, MBBS \n **Date:** September 14, 2017\n\n ## Content\n\n Parathyroid adenomas are usually found in the inferior parathyroid gland; however, in 6%-10% of patients, they may be located in the thyroid, thymus, the pericardium, and behind the esophagus. Adenomas usually weigh 0.5-5 g but may weigh as much as 10-20 g (on average, normal glands weigh about 25 mg).[4]\nApproximately half of patients with hyperparathyroidism are asymptomatic. The manifestations of hyperparathyroidism primarily involve the skeletal system. Osteitis fibrosa cystica is a constellation of findings pathognomonic of hyperparathyroidism. In osteitis fibrosa cystica, the number of trabeculae on bones is reduced, and the number of giant multinucleated osteoclasts is increased. The surface of the bone may have a scalloped appearance (Howship lacunae). The normal marrow and cellular elements of bones are replaced with fibrous tissue, which weakens the bones and, often, results in pathologic fractures. Other changes include subperiosteal resorption of the phalanges and loss of the lamina dura of the teeth. Tiny punched out lesions (the classic \"salt-and-pepper\" appearance) are seen on the skull.[1,3]\nAlthough hyperparathyroidism primarily affects the skeletal system, numerous other organ systems may be affected as well. Historically, kidney involvement has been reported in 60%-70% of cases. However, with improvement in early detection, it is now reported to be present in less than 20% of patients. Kidney involvement either results from the deposition of calcium in the renal parenchyma or from recurrent nephrolithiasis. Renal stones are usually composed of calcium phosphate or calcium oxalate. Recurrent nephrolithiasis can result in renal scarring, urinary tract obstruction, infection, and loss of renal function.[1,3]\nMuscular manifestations may include proximal muscle weakness, fatigability, and atrophy. Neuropsychiatric findings typically occur only in advanced disease, and they may include confusion, psychosis, agitation, or coma. Band keratopathy is easily detectable in patients undergoing ophthalmologic examination, but these changes are not specific. Gastrointestinal system manifestations are typically subtle and nonspecific; however, they may include significant conditions, such as peptic ulcers, pancreatitis, or paralytic ileus. Chondrocalcinosis and pseudogout are also frequently seen.[1,3]\nThe diagnosis of hyperparathyroidism is typically made by the detection of an elevated immunoreactive PTH level. The suspicion of hyperparathyroidism should arise in patients with hypercalcemia, hypophosphatemia, and raised alkaline phosphatase levels or in those with the abnormal skeletal findings described above. Parathyroid ultrasonography and technetium-99m sestamibi parathyroid scintigraphy are most useful for the preoperative detection of parathyroid adenoma. Parathyroid scintigraphy can be limited by the presence of thyroid nodules or other metabolically active tissues, such as lymph nodes, diffuse hyperplasia, or metastatic thyroid cancer.[5]\nPrimary hyperparathyroidism should be established on the basis of biochemical findings before any imaging studies are performed.[1] The use of preoperative localization imaging is controversial in patients with primary hyperparathyroidism who have not undergone previous neck surgery. Shaha and colleagues[6] indicated that, in the following patients, preoperative imaging studies are warranted:\nPatients with associated palpable thyroid abnormalities;\nPatients in hypercalcemic crisis in whom urgent diagnosis is needed;\nPatients with associated malignancies;\nAsymptomatic patients with mild hypercalcemia;\nObese patients with short necks;\nHigh-risk patients whose operative time is crucial;\nHigh-risk patients in whom local anesthesia must be used; and\nPatients with cervical spinal problems with neck extension difficulties.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 55-Year-Old Man With Recurrent Sharp Flank Pain"
},
{
"authors": "Jyoti Wadhwa, MBBS, MD; Madhavi Tripathi, MBBS, MD; Madhur Kumar Srivastava, MBBS",
"content": [
"Whether or not the disease should be treated surgically is the critical question in the management of hyperparathyroidism. The guidelines issued in 2002 for the surgical treatment of patients with asymptomatic hyperparathyroidism include a serum calcium 1 mg/dL above the upper limit of normal, a 24-hour urinary calcium greater than 400 mg, a creatinine clearance reduced by 30% or more, a bone mineral density T score of less than -2.5 at any site, or a patient age of less than 50 years.[7]",
"Parathyroid ultrasonography and technetium-99m sestamibi scans using single-photon emission CT (SPECT) are used preoperatively to predict the location of abnormal glands. Intraoperative sampling of PTH levels before and at 5-minute intervals after the removal of a suspected adenoma is used to confirm a rapid fall (>50%) in PTH to normal levels. Frequent assessment of the patient's calcium level is prudent because postoperative transient hypocalcemia is common. Patients with hyperparathyroidism who do not undergo parathyroid surgery should have their serum calcium levels screened biannually, and their serum creatinine levels and bone density measurements should be checked annually.[8,9,10]",
"The patient in this case was found to have adenoma in the left inferior parathyroid gland, which was surgically resected. His serum calcium and phosphorus levels normalized postoperatively. On a follow-up visit, he reported being asymptomatic and without any recurrent episodes of nephrolithiasis."
],
"date": "September 14, 2017",
"figures": [],
"markdown": "# A 55-Year-Old Man With Recurrent Sharp Flank Pain\n\n **Authors:** Jyoti Wadhwa, MBBS, MD; Madhavi Tripathi, MBBS, MD; Madhur Kumar Srivastava, MBBS \n **Date:** September 14, 2017\n\n ## Content\n\n Whether or not the disease should be treated surgically is the critical question in the management of hyperparathyroidism. The guidelines issued in 2002 for the surgical treatment of patients with asymptomatic hyperparathyroidism include a serum calcium 1 mg/dL above the upper limit of normal, a 24-hour urinary calcium greater than 400 mg, a creatinine clearance reduced by 30% or more, a bone mineral density T score of less than -2.5 at any site, or a patient age of less than 50 years.[7]\nParathyroid ultrasonography and technetium-99m sestamibi scans using single-photon emission CT (SPECT) are used preoperatively to predict the location of abnormal glands. Intraoperative sampling of PTH levels before and at 5-minute intervals after the removal of a suspected adenoma is used to confirm a rapid fall (>50%) in PTH to normal levels. Frequent assessment of the patient's calcium level is prudent because postoperative transient hypocalcemia is common. Patients with hyperparathyroidism who do not undergo parathyroid surgery should have their serum calcium levels screened biannually, and their serum creatinine levels and bone density measurements should be checked annually.[8,9,10]\nThe patient in this case was found to have adenoma in the left inferior parathyroid gland, which was surgically resected. His serum calcium and phosphorus levels normalized postoperatively. On a follow-up visit, he reported being asymptomatic and without any recurrent episodes of nephrolithiasis.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1134406,
"choiceText": "Hypophosphatemia",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1134408,
"choiceText": "Hyperphosphatemia",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1134410,
"choiceText": "Hypocalcemia",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1134412,
"choiceText": "Hypercalcemia",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1134414,
"choiceText": "Hypomagnesemia",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Hypocalcemia can prompt rapid secretion of the preformed PTH hormone. Extracellular calcium interacts with a calcium sensor to control PTH secretion; stimulation of the receptor by high calcium levels suppresses PTH secretion. The receptor is present in the parathyroid glands, the C cells of the thyroid, the brain, and the kidneys.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 359903,
"questionText": "Which of the following conditions is the most rapid stimulant for the secretion of PTH hormone?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1134426,
"choiceText": "Parathyroid carcinoma",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1134428,
"choiceText": "Solitary adenoma",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1134430,
"choiceText": "Parathyroid gland hyperplasia",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1134432,
"choiceText": "Ectopic parathyroid gland",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Primary hyperparathyroidism is primarily caused by solitary adenomas (80% of cases) and, less commonly, chief cell hyperplasia (15%), multiple endocrine neoplasia (<5%), and parathyroid carcinoma (rare).",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 359911,
"questionText": "What is the most common cause of primary hyperparathyroidism?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 55-Year-Old Man With Recurrent Sharp Flank Pain"
},
{
"authors": "Jyoti Wadhwa, MBBS, MD; Madhavi Tripathi, MBBS, MD; Madhur Kumar Srivastava, MBBS",
"content": [],
"date": "September 14, 2017",
"figures": [],
"markdown": "# A 55-Year-Old Man With Recurrent Sharp Flank Pain\n\n **Authors:** Jyoti Wadhwa, MBBS, MD; Madhavi Tripathi, MBBS, MD; Madhur Kumar Srivastava, MBBS \n **Date:** September 14, 2017\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1134406,
"choiceText": "Hypophosphatemia",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1134408,
"choiceText": "Hyperphosphatemia",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1134410,
"choiceText": "Hypocalcemia",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1134412,
"choiceText": "Hypercalcemia",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1134414,
"choiceText": "Hypomagnesemia",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Hypocalcemia can prompt rapid secretion of the preformed PTH hormone. Extracellular calcium interacts with a calcium sensor to control PTH secretion; stimulation of the receptor by high calcium levels suppresses PTH secretion. The receptor is present in the parathyroid glands, the C cells of the thyroid, the brain, and the kidneys.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 359903,
"questionText": "Which of the following conditions is the most rapid stimulant for the secretion of PTH hormone?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1134426,
"choiceText": "Parathyroid carcinoma",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1134428,
"choiceText": "Solitary adenoma",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1134430,
"choiceText": "Parathyroid gland hyperplasia",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1134432,
"choiceText": "Ectopic parathyroid gland",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Primary hyperparathyroidism is primarily caused by solitary adenomas (80% of cases) and, less commonly, chief cell hyperplasia (15%), multiple endocrine neoplasia (<5%), and parathyroid carcinoma (rare).",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 359911,
"questionText": "What is the most common cause of primary hyperparathyroidism?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 55-Year-Old Man With Recurrent Sharp Flank Pain"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1134398,
"choiceText": "Parathyroid adenoma",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1134400,
"choiceText": "Ectopic parathyroid gland",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1134402,
"choiceText": "Parathyroid carcinoma",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1134404,
"choiceText": "Sarcoid granuloma",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 359901,
"questionText": "Which of the following is the most likely diagnosis?\r\n\r\n<p><em>Hint: Consider possible underlying causes of hypercalcemia.</em></p>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1134406,
"choiceText": "Hypophosphatemia",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1134408,
"choiceText": "Hyperphosphatemia",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1134410,
"choiceText": "Hypocalcemia",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1134412,
"choiceText": "Hypercalcemia",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1134414,
"choiceText": "Hypomagnesemia",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Hypocalcemia can prompt rapid secretion of the preformed PTH hormone. Extracellular calcium interacts with a calcium sensor to control PTH secretion; stimulation of the receptor by high calcium levels suppresses PTH secretion. The receptor is present in the parathyroid glands, the C cells of the thyroid, the brain, and the kidneys.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 359903,
"questionText": "Which of the following conditions is the most rapid stimulant for the secretion of PTH hormone?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1134426,
"choiceText": "Parathyroid carcinoma",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1134428,
"choiceText": "Solitary adenoma",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1134430,
"choiceText": "Parathyroid gland hyperplasia",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1134432,
"choiceText": "Ectopic parathyroid gland",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Primary hyperparathyroidism is primarily caused by solitary adenomas (80% of cases) and, less commonly, chief cell hyperplasia (15%), multiple endocrine neoplasia (<5%), and parathyroid carcinoma (rare).",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 359911,
"questionText": "What is the most common cause of primary hyperparathyroidism?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
884108 | /viewarticle/884108 | [
{
"authors": "Derik L. Davis, MD; Harvey Stern, MD; Helen T. Morehouse, MD",
"content": [
"Editor's Note:",
"The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"Background",
"A 57-year-old woman presents to the emergency department (ED) with a 3-day history of colicky lower-abdominal pain and diarrhea. She describes four or five episodes a day of soft diarrhea with blood mixed throughout. She reports no fever, vomiting, or pain with defecation. Her last bowel movement occurred earlier in the day, about 2 hours before her arrival at the ED. She also mentions having had intermittent episodes of bloody diarrhea over the past several months, but none that were this severe.",
"The patient's surgical history is notable only for cesarean deliveries; she reports no other significant medical history or contributory family history. She has no known drug allergies and takes no medications. She admits to smoking tobacco and drinking alcoholic beverages on the weekends."
],
"date": "August 16, 2017",
"figures": [],
"markdown": "# A 57-Year-Old Woman With Bloody Diarrhea\n\n **Authors:** Derik L. Davis, MD; Harvey Stern, MD; Helen T. Morehouse, MD \n **Date:** August 16, 2017\n\n ## Content\n\n Editor's Note:\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nBackground\nA 57-year-old woman presents to the emergency department (ED) with a 3-day history of colicky lower-abdominal pain and diarrhea. She describes four or five episodes a day of soft diarrhea with blood mixed throughout. She reports no fever, vomiting, or pain with defecation. Her last bowel movement occurred earlier in the day, about 2 hours before her arrival at the ED. She also mentions having had intermittent episodes of bloody diarrhea over the past several months, but none that were this severe.\nThe patient's surgical history is notable only for cesarean deliveries; she reports no other significant medical history or contributory family history. She has no known drug allergies and takes no medications. She admits to smoking tobacco and drinking alcoholic beverages on the weekends.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 57-Year-Old Woman With Bloody Diarrhea"
},
{
"authors": "Derik L. Davis, MD; Harvey Stern, MD; Helen T. Morehouse, MD",
"content": [
"Physical Examination and Workup",
"Upon physical examination, the patient has a temperature of 98.8°F. She has a pulse of 98 beats/min and a blood pressure of 114/72 mm Hg. Her respiratory rate is 14 breaths/min. She is well-nourished, well-developed, and in no acute distress.",
"Head and neck examination findings are normal, with moist oral mucosa. The patient's lungs are clear bilaterally, and the heart rate and rhythm are regular, with no murmurs. The breasts are normal.",
"The abdomen is mildly distended, and positive bowel sounds are observed. No guarding, rigidity, or rebound tenderness is noted. Noninflamed and nonswollen external hemorrhoids are present, and the rectum contains feces. The rectal examination reveals no abnormal tenderness, but the stool is strongly guaiac-positive. The rest of the exam is unremarkable.",
"Laboratory analysis demonstrates an abnormal complete blood count, with a low hemoglobin level of 10.4 g/dL (normal range, 12-16 g/dL), a hematocrit of 30.1% (normal range, 42%-51%), and a white blood cell count of 4.1 × 103 cells/µL (normal range, 4.8-10.8 × 103 cells/µL). The platelet count is within the normal range. The basic metabolic panel is normal, except for a low potassium level of 3.3 mEq/L (normal range, 3.5-5 mEq/L). The amylase and lipase levels are elevated, at 186 U/L (normal range, 16-100 U/L) and 75 U/L (normal range < 62 U/L), respectively. Liver function tests, urinalysis, and coagulation studies are in the normal range.",
"Abdominal contrast-enhanced CT is performed because of a high suspicion of intra-abdominal pathology (Figures 1-4).",
"Figure 1.",
"Figure 2.",
"Figure 3.",
"Figure 4."
],
"date": "August 16, 2017",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/884/108/Figure-1_thumb.jpg"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/884/108/Figure-2_thumb.jpg"
},
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/884/108/Figure-3_thumb.jpg"
},
{
"caption": "Figure 4.",
"image_url": "https://img.medscapestatic.com/article/884/108/Figure-4_thumb.jpg"
}
],
"markdown": "# A 57-Year-Old Woman With Bloody Diarrhea\n\n **Authors:** Derik L. Davis, MD; Harvey Stern, MD; Helen T. Morehouse, MD \n **Date:** August 16, 2017\n\n ## Content\n\n Physical Examination and Workup\nUpon physical examination, the patient has a temperature of 98.8°F. She has a pulse of 98 beats/min and a blood pressure of 114/72 mm Hg. Her respiratory rate is 14 breaths/min. She is well-nourished, well-developed, and in no acute distress.\nHead and neck examination findings are normal, with moist oral mucosa. The patient's lungs are clear bilaterally, and the heart rate and rhythm are regular, with no murmurs. The breasts are normal.\nThe abdomen is mildly distended, and positive bowel sounds are observed. No guarding, rigidity, or rebound tenderness is noted. Noninflamed and nonswollen external hemorrhoids are present, and the rectum contains feces. The rectal examination reveals no abnormal tenderness, but the stool is strongly guaiac-positive. The rest of the exam is unremarkable.\nLaboratory analysis demonstrates an abnormal complete blood count, with a low hemoglobin level of 10.4 g/dL (normal range, 12-16 g/dL), a hematocrit of 30.1% (normal range, 42%-51%), and a white blood cell count of 4.1 × 103 cells/µL (normal range, 4.8-10.8 × 103 cells/µL). The platelet count is within the normal range. The basic metabolic panel is normal, except for a low potassium level of 3.3 mEq/L (normal range, 3.5-5 mEq/L). The amylase and lipase levels are elevated, at 186 U/L (normal range, 16-100 U/L) and 75 U/L (normal range < 62 U/L), respectively. Liver function tests, urinalysis, and coagulation studies are in the normal range.\nAbdominal contrast-enhanced CT is performed because of a high suspicion of intra-abdominal pathology (Figures 1-4).\nFigure 1.\nFigure 2.\nFigure 3.\nFigure 4.\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n**Figure 3.** \n \n\n**Figure 4.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1128458,
"choiceText": "Diverticulitis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1128460,
"choiceText": "Pyelonephritis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1128462,
"choiceText": "Volvulus",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1128464,
"choiceText": "Intussusception",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 357791,
"questionText": "Which of the following is the most likely diagnosis?<br/>\r\n<br/>\r\n<em>Hint: The diagnosis is more frequently encountered in children.</em>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 57-Year-Old Woman With Bloody Diarrhea"
},
{
"authors": "Derik L. Davis, MD; Harvey Stern, MD; Helen T. Morehouse, MD",
"content": [
"The axial images of the contrast-enhanced CT scan of the abdomen and pelvis, as well as the associated sagittal reformation, demonstrated a transverse colonic intussusception. Figures 1-4 show the proximal contrast-filled intussusceptum invaginating into the distal intussuscipiens (which is relatively hypodense in appearance, because it does not contain oral contrast) in the left upper quadrant. Mesenteric fat and vessels are noted between the intussusceptum and intussuscipiens, which creates a \"target\" or \"bowel-within-bowel\" appearance. This is a characteristic CT appearance of an intussusception.",
"Figure 1.",
"Figure 2.",
"Figure 3.",
"Figure 4.",
"An adult presenting with intussusception is uncommon. Children constitute most patients affected by intussusception, with adults representing only 5% of cases. Adult intussusception accounts for 1% of bowel obstructions and only 0.002% of all hospital admissions. In 70%-90% of cases of adult intussusception, an underlying organic cause is found. This is in contrast to the pediatric population, which has an idiopathic form in over 95% of cases.[1,2]",
"Intussusception occurs between adjacent segments of bowel. The intussusceptum, a prolapsing segment of bowel, invaginates with its mesentery into a recipient segment of bowel, known as the intussuscipiens, like a telescope. This may be transient, but if it is persistent, it can cause bowel obstruction; if left untreated, it can result in bowel ischemia, eventual sepsis, and death.",
"Intussusception is commonly classified according to location: enteroenteric, ileocolic, ileocecal, or colocolic. Most cases in adults involve the small bowel or a combination of the small and large bowels. In adults, colocolic intussusception occurs in less than 20% of cases.[3,4]"
],
"date": "August 16, 2017",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/884/108/Figure-1_thumb.jpg"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/884/108/Figure-2_thumb.jpg"
},
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/884/108/Figure-3_thumb.jpg"
},
{
"caption": "Figure 4.",
"image_url": "https://img.medscapestatic.com/article/884/108/Figure-4_thumb.jpg"
}
],
"markdown": "# A 57-Year-Old Woman With Bloody Diarrhea\n\n **Authors:** Derik L. Davis, MD; Harvey Stern, MD; Helen T. Morehouse, MD \n **Date:** August 16, 2017\n\n ## Content\n\n The axial images of the contrast-enhanced CT scan of the abdomen and pelvis, as well as the associated sagittal reformation, demonstrated a transverse colonic intussusception. Figures 1-4 show the proximal contrast-filled intussusceptum invaginating into the distal intussuscipiens (which is relatively hypodense in appearance, because it does not contain oral contrast) in the left upper quadrant. Mesenteric fat and vessels are noted between the intussusceptum and intussuscipiens, which creates a \"target\" or \"bowel-within-bowel\" appearance. This is a characteristic CT appearance of an intussusception.\nFigure 1.\nFigure 2.\nFigure 3.\nFigure 4.\nAn adult presenting with intussusception is uncommon. Children constitute most patients affected by intussusception, with adults representing only 5% of cases. Adult intussusception accounts for 1% of bowel obstructions and only 0.002% of all hospital admissions. In 70%-90% of cases of adult intussusception, an underlying organic cause is found. This is in contrast to the pediatric population, which has an idiopathic form in over 95% of cases.[1,2]\nIntussusception occurs between adjacent segments of bowel. The intussusceptum, a prolapsing segment of bowel, invaginates with its mesentery into a recipient segment of bowel, known as the intussuscipiens, like a telescope. This may be transient, but if it is persistent, it can cause bowel obstruction; if left untreated, it can result in bowel ischemia, eventual sepsis, and death.\nIntussusception is commonly classified according to location: enteroenteric, ileocolic, ileocecal, or colocolic. Most cases in adults involve the small bowel or a combination of the small and large bowels. In adults, colocolic intussusception occurs in less than 20% of cases.[3,4]\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n**Figure 3.** \n \n\n**Figure 4.** \n \n\n\n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1128458,
"choiceText": "Diverticulitis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1128460,
"choiceText": "Pyelonephritis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1128462,
"choiceText": "Volvulus",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1128464,
"choiceText": "Intussusception",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 357791,
"questionText": "Which of the following is the most likely diagnosis?<br/>\r\n<br/>\r\n<em>Hint: The diagnosis is more frequently encountered in children.</em>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 57-Year-Old Woman With Bloody Diarrhea"
},
{
"authors": "Derik L. Davis, MD; Harvey Stern, MD; Helen T. Morehouse, MD",
"content": [
"An intraluminal lead point may serve as a point of origin, precipitating the development of intussusception. These lesions may be benign or malignant. Adults with intraluminal lead points may follow a transient course, or they may experience relapsing and remitting obstruction. Children with lead points may present with a similar clinical picture. A high clinical suspicion is usually required to make the diagnosis, given the nonspecific nature of the symptoms.",
"The pathophysiology of cases without relation to a lead point is considered idiopathic, and in adults, it is typically transient. Most children ultimately demonstrate the idiopathic form of intussusception. Most cases of intussusception in adults that involve only the small bowel are also idiopathic. In contrast, intussusception of the large bowel in adults is frequently associated with lead points.",
"The most common causes of colocolic intussusception are lipomas, which are benign lesions. Adenomatous polyps are also benign causes of colocolic intussusception. Nearly 50% of colonic lesions responsible for intussusception in adults are malignant. Adenocarcinoma is the malignant neoplasm most frequently associated with colocolic intussusception. Other, less common causes of malignant lead points are lymphoma and metastasis.[1,3,5]",
"Children with intussusception usually present initially with vomiting, which is often bilious, and recurrent bouts of pain. Intussusception can occur at any age, but the incidence is highest between age 3 months and 6 years, with a peak between age 5 and 9 months. Diarrhea may also be present, and this symptom may lead the clinician astray. Upon physical examination, a sausage-shaped mass, often in the right upper quadrant, may be noted. The findings of the right upper quadrant mass and an \"empty\" right lower quadrant together constitute the classic \"Dance sign.\" A \"currant jelly\" appearance of the stool and lethargy are late findings, but stool may be guaiac-positive early on. An early rectal examination may heighten suspicion and lead to a more timely diagnosis and therapy.",
"Adults with intussusception often experience nonspecific gastrointestinal symptoms. The most common symptoms are abdominal pain and nausea or vomiting. Fewer than 11% of patients have fever, constipation, diarrhea, or weight loss. Patients with the idiopathic form of intussusception may have transient symptoms, compared with those who have an underlying intraluminal lead point and may report intermittent and relapsing symptoms.",
"Physical examination is often nonlocalizing in adults. Only 7% of patients have a palpable mass. Less than 30% have guaiac-positive stools, but this presentation is more likely in patients with malignant colonic lesions. In contrast to the acute presentation of intussusception in children, adults frequently have a more atypical or chronic course. The nonspecific nature of symptoms often presents a confusing clinical picture that leads to an initial misdiagnosis. Adults presenting acutely are often thought to have bowel obstruction, with few patients having a preoperative diagnosis of intussusception.[1,5]",
"Contrast-enhanced CT is the most useful modality for intussusception in the adult. Multiple concentric rings representing the intussusception are seen as a target- or sausage-shaped mass: inner cylinder (canal and wall of intussusceptum), middle cylinder (low-attenuation mesenteric fat, with or without enhancing vessels), and outer cylinder (exiting intussusceptum and intussuscipiens). This bowel-within-bowel appearance on CT scan is pathognomonic for intussusception. A lead point can be identified in some cases, although this is a difficult task in many patients. A rim of contrast material surrounding the intussusceptum may also be seen, and it may help make the diagnosis in cases where mesenteric fat is not readily apparent within the mass. With the increasing use of CT in patients with gastrointestinal symptoms, the number of adults identified with unsuspected intussusception is likely to increase.[2,3,6]",
"Diagnostic studies other than CT are more often used in children when intussusception is suspected. Plain films, abdominal sonograms, and liquid-contrast or air-contrast enemas are the primary imaging modalities in pediatric patients. The contrast enema may be both diagnostic and therapeutic for children. In certain cases, ultrasonography is a good initial test, because it avoids ionizing radiation. Sensitivity is approximately 85%, although it probably varies significantly depending on sonographer experience with this condition. Plain films and sonography are frequently used in the workup of abdominal pain for adults, but they are less accurate than CT for detecting intussusception. The upper gastrointestinal series, flexible sigmoidoscopy, and colonoscopy all have a role in certain clinical situations.[1,7]"
],
"date": "August 16, 2017",
"figures": [],
"markdown": "# A 57-Year-Old Woman With Bloody Diarrhea\n\n **Authors:** Derik L. Davis, MD; Harvey Stern, MD; Helen T. Morehouse, MD \n **Date:** August 16, 2017\n\n ## Content\n\n An intraluminal lead point may serve as a point of origin, precipitating the development of intussusception. These lesions may be benign or malignant. Adults with intraluminal lead points may follow a transient course, or they may experience relapsing and remitting obstruction. Children with lead points may present with a similar clinical picture. A high clinical suspicion is usually required to make the diagnosis, given the nonspecific nature of the symptoms.\nThe pathophysiology of cases without relation to a lead point is considered idiopathic, and in adults, it is typically transient. Most children ultimately demonstrate the idiopathic form of intussusception. Most cases of intussusception in adults that involve only the small bowel are also idiopathic. In contrast, intussusception of the large bowel in adults is frequently associated with lead points.\nThe most common causes of colocolic intussusception are lipomas, which are benign lesions. Adenomatous polyps are also benign causes of colocolic intussusception. Nearly 50% of colonic lesions responsible for intussusception in adults are malignant. Adenocarcinoma is the malignant neoplasm most frequently associated with colocolic intussusception. Other, less common causes of malignant lead points are lymphoma and metastasis.[1,3,5]\nChildren with intussusception usually present initially with vomiting, which is often bilious, and recurrent bouts of pain. Intussusception can occur at any age, but the incidence is highest between age 3 months and 6 years, with a peak between age 5 and 9 months. Diarrhea may also be present, and this symptom may lead the clinician astray. Upon physical examination, a sausage-shaped mass, often in the right upper quadrant, may be noted. The findings of the right upper quadrant mass and an \"empty\" right lower quadrant together constitute the classic \"Dance sign.\" A \"currant jelly\" appearance of the stool and lethargy are late findings, but stool may be guaiac-positive early on. An early rectal examination may heighten suspicion and lead to a more timely diagnosis and therapy.\nAdults with intussusception often experience nonspecific gastrointestinal symptoms. The most common symptoms are abdominal pain and nausea or vomiting. Fewer than 11% of patients have fever, constipation, diarrhea, or weight loss. Patients with the idiopathic form of intussusception may have transient symptoms, compared with those who have an underlying intraluminal lead point and may report intermittent and relapsing symptoms.\nPhysical examination is often nonlocalizing in adults. Only 7% of patients have a palpable mass. Less than 30% have guaiac-positive stools, but this presentation is more likely in patients with malignant colonic lesions. In contrast to the acute presentation of intussusception in children, adults frequently have a more atypical or chronic course. The nonspecific nature of symptoms often presents a confusing clinical picture that leads to an initial misdiagnosis. Adults presenting acutely are often thought to have bowel obstruction, with few patients having a preoperative diagnosis of intussusception.[1,5]\nContrast-enhanced CT is the most useful modality for intussusception in the adult. Multiple concentric rings representing the intussusception are seen as a target- or sausage-shaped mass: inner cylinder (canal and wall of intussusceptum), middle cylinder (low-attenuation mesenteric fat, with or without enhancing vessels), and outer cylinder (exiting intussusceptum and intussuscipiens). This bowel-within-bowel appearance on CT scan is pathognomonic for intussusception. A lead point can be identified in some cases, although this is a difficult task in many patients. A rim of contrast material surrounding the intussusceptum may also be seen, and it may help make the diagnosis in cases where mesenteric fat is not readily apparent within the mass. With the increasing use of CT in patients with gastrointestinal symptoms, the number of adults identified with unsuspected intussusception is likely to increase.[2,3,6]\nDiagnostic studies other than CT are more often used in children when intussusception is suspected. Plain films, abdominal sonograms, and liquid-contrast or air-contrast enemas are the primary imaging modalities in pediatric patients. The contrast enema may be both diagnostic and therapeutic for children. In certain cases, ultrasonography is a good initial test, because it avoids ionizing radiation. Sensitivity is approximately 85%, although it probably varies significantly depending on sonographer experience with this condition. Plain films and sonography are frequently used in the workup of abdominal pain for adults, but they are less accurate than CT for detecting intussusception. The upper gastrointestinal series, flexible sigmoidoscopy, and colonoscopy all have a role in certain clinical situations.[1,7]\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 57-Year-Old Woman With Bloody Diarrhea"
},
{
"authors": "Derik L. Davis, MD; Harvey Stern, MD; Helen T. Morehouse, MD",
"content": [
"Whereas most cases of intussusception are managed nonoperatively in children, surgical management is the treatment of choice for adults. The involved bowel is resected when either diagnosed preoperatively or discovered at the time of an emergency laparotomy. Minimally invasive bowel resection and anastomosis can be safely performed. Laparoscopy also offers an accessible and useful tool for differential diagnosis. Depending on the surgeon's experience and preference, an open procedure is an acceptable alternative.",
"Because many adults with intussusception have lead points, reduction before resection is controversial. Bowel resection without prior reduction is the clear management choice for colocolic intussusception, which has a high risk for malignancy. Avoidance of reduction in these cases may reduce the possibility for the spread of malignant cells. Reduction should also be avoided in patients with an ischemic or inflamed bowel. The risk that mucosal necrosis may extend beyond the resection margins is increased after reduction.[7,8]",
"In this case, after review of the CT images of the abdomen, the physician in the ED consulted a general surgeon, to whose care the patient was admitted. After admission, the patient was brought to the operating room, where the surgical team elected to perform a colonoscopy as the initial procedure. This revealed a large mass completely obstructing the lumen of the transverse colon. Exploratory laparotomy was planned after the endoscopic examination, at which time the diagnosis of colocolic intussusception was confirmed. The involved bowel was resected, and elective cholecystectomy was performed. Pathologic examination for the colonic mass was positive for moderately differentiated adenocarcinoma, which was discovered to arise from a large tubular adenoma in the transverse colon.",
"Postoperatively, the patient fared well, and she was discharged to home on hospital day 6. She was to receive outpatient follow-up care with general surgery and oncology."
],
"date": "August 16, 2017",
"figures": [],
"markdown": "# A 57-Year-Old Woman With Bloody Diarrhea\n\n **Authors:** Derik L. Davis, MD; Harvey Stern, MD; Helen T. Morehouse, MD \n **Date:** August 16, 2017\n\n ## Content\n\n Whereas most cases of intussusception are managed nonoperatively in children, surgical management is the treatment of choice for adults. The involved bowel is resected when either diagnosed preoperatively or discovered at the time of an emergency laparotomy. Minimally invasive bowel resection and anastomosis can be safely performed. Laparoscopy also offers an accessible and useful tool for differential diagnosis. Depending on the surgeon's experience and preference, an open procedure is an acceptable alternative.\nBecause many adults with intussusception have lead points, reduction before resection is controversial. Bowel resection without prior reduction is the clear management choice for colocolic intussusception, which has a high risk for malignancy. Avoidance of reduction in these cases may reduce the possibility for the spread of malignant cells. Reduction should also be avoided in patients with an ischemic or inflamed bowel. The risk that mucosal necrosis may extend beyond the resection margins is increased after reduction.[7,8]\nIn this case, after review of the CT images of the abdomen, the physician in the ED consulted a general surgeon, to whose care the patient was admitted. After admission, the patient was brought to the operating room, where the surgical team elected to perform a colonoscopy as the initial procedure. This revealed a large mass completely obstructing the lumen of the transverse colon. Exploratory laparotomy was planned after the endoscopic examination, at which time the diagnosis of colocolic intussusception was confirmed. The involved bowel was resected, and elective cholecystectomy was performed. Pathologic examination for the colonic mass was positive for moderately differentiated adenocarcinoma, which was discovered to arise from a large tubular adenoma in the transverse colon.\nPostoperatively, the patient fared well, and she was discharged to home on hospital day 6. She was to receive outpatient follow-up care with general surgery and oncology.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1128470,
"choiceText": "Lymphoma",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1128472,
"choiceText": "Metastasis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1128474,
"choiceText": "Adenocarcinoma",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1128476,
"choiceText": "Carcinoid",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1128478,
"choiceText": "Adenoma",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Nearly 50% of colonic lesions responsible for intussusception in adults are malignant. Adenocarcinoma is the malignant neoplasm most frequently associated with colocolic intussusception.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 357795,
"questionText": "Which of the following malignant neoplasms is most frequently associated with colocolic intussusception in adults?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1128742,
"choiceText": "Surgical resection without preoperative reduction",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1128744,
"choiceText": "Surgical resection after preoperative reduction",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1128746,
"choiceText": "Hydrostatic reduction",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1128748,
"choiceText": "Manual reduction without resection at exploratory laparotomy",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1128750,
"choiceText": "Medical management",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Although most cases of intussusception are managed nonoperatively in children, surgical management is the treatment of choice for adults. The involved bowel is resected when the condition either diagnosed preoperatively or discovered at the time of an emergency laparotomy. Currently, most surgeries are open procedures, but in the future, laparoscopic procedures may be performed more often when the diagnosis is known preoperatively. Reduction before resection is controversial. Bowel resection without prior reduction is the clear management choice for colocolic intussusception, which has a high risk for malignancy.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 357893,
"questionText": "Which of the following is the correct approach for managing colocolic intussusception in adults?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 57-Year-Old Woman With Bloody Diarrhea"
},
{
"authors": "Derik L. Davis, MD; Harvey Stern, MD; Helen T. Morehouse, MD",
"content": [],
"date": "August 16, 2017",
"figures": [],
"markdown": "# A 57-Year-Old Woman With Bloody Diarrhea\n\n **Authors:** Derik L. Davis, MD; Harvey Stern, MD; Helen T. Morehouse, MD \n **Date:** August 16, 2017\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1128470,
"choiceText": "Lymphoma",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1128472,
"choiceText": "Metastasis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1128474,
"choiceText": "Adenocarcinoma",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1128476,
"choiceText": "Carcinoid",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1128478,
"choiceText": "Adenoma",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Nearly 50% of colonic lesions responsible for intussusception in adults are malignant. Adenocarcinoma is the malignant neoplasm most frequently associated with colocolic intussusception.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 357795,
"questionText": "Which of the following malignant neoplasms is most frequently associated with colocolic intussusception in adults?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1128742,
"choiceText": "Surgical resection without preoperative reduction",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1128744,
"choiceText": "Surgical resection after preoperative reduction",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1128746,
"choiceText": "Hydrostatic reduction",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1128748,
"choiceText": "Manual reduction without resection at exploratory laparotomy",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1128750,
"choiceText": "Medical management",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Although most cases of intussusception are managed nonoperatively in children, surgical management is the treatment of choice for adults. The involved bowel is resected when the condition either diagnosed preoperatively or discovered at the time of an emergency laparotomy. Currently, most surgeries are open procedures, but in the future, laparoscopic procedures may be performed more often when the diagnosis is known preoperatively. Reduction before resection is controversial. Bowel resection without prior reduction is the clear management choice for colocolic intussusception, which has a high risk for malignancy.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 357893,
"questionText": "Which of the following is the correct approach for managing colocolic intussusception in adults?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 57-Year-Old Woman With Bloody Diarrhea"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1128458,
"choiceText": "Diverticulitis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1128460,
"choiceText": "Pyelonephritis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1128462,
"choiceText": "Volvulus",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1128464,
"choiceText": "Intussusception",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 357791,
"questionText": "Which of the following is the most likely diagnosis?<br/>\r\n<br/>\r\n<em>Hint: The diagnosis is more frequently encountered in children.</em>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1128470,
"choiceText": "Lymphoma",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1128472,
"choiceText": "Metastasis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1128474,
"choiceText": "Adenocarcinoma",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1128476,
"choiceText": "Carcinoid",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1128478,
"choiceText": "Adenoma",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Nearly 50% of colonic lesions responsible for intussusception in adults are malignant. Adenocarcinoma is the malignant neoplasm most frequently associated with colocolic intussusception.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 357795,
"questionText": "Which of the following malignant neoplasms is most frequently associated with colocolic intussusception in adults?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1128742,
"choiceText": "Surgical resection without preoperative reduction",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1128744,
"choiceText": "Surgical resection after preoperative reduction",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1128746,
"choiceText": "Hydrostatic reduction",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1128748,
"choiceText": "Manual reduction without resection at exploratory laparotomy",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1128750,
"choiceText": "Medical management",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Although most cases of intussusception are managed nonoperatively in children, surgical management is the treatment of choice for adults. The involved bowel is resected when the condition either diagnosed preoperatively or discovered at the time of an emergency laparotomy. Currently, most surgeries are open procedures, but in the future, laparoscopic procedures may be performed more often when the diagnosis is known preoperatively. Reduction before resection is controversial. Bowel resection without prior reduction is the clear management choice for colocolic intussusception, which has a high risk for malignancy.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 357893,
"questionText": "Which of the following is the correct approach for managing colocolic intussusception in adults?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
883207 | /viewarticle/883207 | [
{
"authors": "James Robert Brasic, MD, MPH",
"content": [
"Editor's Note:",
"The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"Background",
"A 20-year-old man who no longer speaks is undergoing studies to clarify his condition. Although his parents reported that his early development was normal, they describe unusual behaviors prior to age 5 years. He would spin string and paper from his father's cigarette cartons for hours. His early speech exhibited disarticulation, agrammatism, truncated sentence structures, and a sing-song intonation.",
"When the boy first entered school, he demonstrated attentional and learning disabilities, as well as impaired motor coordination. At age 9 years 5 months, neurologic assessment revealed visual perceptual problems, impaired left-right discrimination, agrammatism, poor memory, and abnormal soft signs. However, his parents denied the presence of abnormalities. They did not continue treatment with a pediatric neurologist.",
"At age 13 years, the boy's teacher told him that he would not be promoted to high school. When he returned home from school, he told his mother he wanted to go to high school. That was the last time that anyone heard him speak. For a few weeks, he made clicking sounds in his throat to communicate. Then verbal communication stopped altogether.",
"After presenting to a psychiatry clinic at age 14 years, he was treated orally with haloperidol (1 mg) and benztropine (1 mg) twice daily for a month. The medications were then discontinued due to lack of response. His father was born in Barbados, and the boy has a paternal family history of mental illness. However, the details are unknown."
],
"date": "July 31, 2017",
"figures": [],
"markdown": "# A 20-Year-Old Man Who Stopped Speaking\n\n **Authors:** James Robert Brasic, MD, MPH \n **Date:** July 31, 2017\n\n ## Content\n\n Editor's Note:\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nBackground\nA 20-year-old man who no longer speaks is undergoing studies to clarify his condition. Although his parents reported that his early development was normal, they describe unusual behaviors prior to age 5 years. He would spin string and paper from his father's cigarette cartons for hours. His early speech exhibited disarticulation, agrammatism, truncated sentence structures, and a sing-song intonation.\nWhen the boy first entered school, he demonstrated attentional and learning disabilities, as well as impaired motor coordination. At age 9 years 5 months, neurologic assessment revealed visual perceptual problems, impaired left-right discrimination, agrammatism, poor memory, and abnormal soft signs. However, his parents denied the presence of abnormalities. They did not continue treatment with a pediatric neurologist.\nAt age 13 years, the boy's teacher told him that he would not be promoted to high school. When he returned home from school, he told his mother he wanted to go to high school. That was the last time that anyone heard him speak. For a few weeks, he made clicking sounds in his throat to communicate. Then verbal communication stopped altogether.\nAfter presenting to a psychiatry clinic at age 14 years, he was treated orally with haloperidol (1 mg) and benztropine (1 mg) twice daily for a month. The medications were then discontinued due to lack of response. His father was born in Barbados, and the boy has a paternal family history of mental illness. However, the details are unknown.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 20-Year-Old Man Who Stopped Speaking"
},
{
"authors": "James Robert Brasic, MD, MPH",
"content": [
"Physical Examination and Workup",
"At age 16 years and 7 months, quantitative electroencephalography (QEEG) demonstrated diminished power in all wave bands (Figure 1).",
"Figure 1.",
"At age 17 years, the patient was hospitalized. He was a well-developed, well-nourished youth in no acute respiratory distress. He exhibited constant grimacing, with continuous eye blinking. He did not respond to questions. His movements were slow. When given a slight push, he moved in the indicated direction. Mild weakness of the right arm and leg were noted. His right foot was dragged on the floor when walking. He could not squeeze his fists. He had a stooped posture when walking.",
"A physical examination at that time was otherwise unremarkable. Findings of a lumbar puncture and MRI of the spinal cord and brain were normal. His serum serology was nonreactive. His antinuclear antibody and HIV antibody titers were negative. His serum ceruloplasmin level was 40 mg/dL. The enzymatic defect of metachromatic leukodystrophy was absent in punch biopsies of the left axilla. Normal mitochondrial enzymes of oxidative phosphorylation were observed. The patient's grimacing ceased for a short time after intravenous injections of lorazepam and three injections of amobarbital.",
"At age 18 years, the patient began requiring assistance for feeding. At age 18 years and 5 months, he required assistance with toilet activities and dressing. Three months later, a left sural nerve biopsy demonstrated loss of axon cylinders (Figure 2), and a left deltoid biopsy revealed perimysial fibrosis and type II fiber predominance (Figure 3).",
"Figure 2.",
"Figure 3.",
"At age 19 years and 2 months, he demonstrated progressively increasing adventitious movements and a decreasing ability to perform activities of daily living. At age 19 years and 8 months, he received a course of 25 electroconvulsive treatments without improvement. Two months later, QEEG revealed increased theta and delta in all brain regions (Figure 4).",
"Figure 4.",
"At age 20 years and 11 months, 2-deoxy-2-[18F]fluoro-D-glucose positron emission tomography ([18F]FDG PET) demonstrated decreased uptake in the right parietal and right temporal regions (Figure 5).",
"Figure 5."
],
"date": "July 31, 2017",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/883/207/883207-Thumb1.jpg"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/883/207/883207-Thumb2.jpg"
},
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/883/207/883207-Thumb5.jpg"
},
{
"caption": "Figure 4.",
"image_url": "https://img.medscapestatic.com/article/883/207/883207-Thumb3.jpg"
},
{
"caption": "Figure 5.",
"image_url": "https://img.medscapestatic.com/article/883/207/883207-Thumb4.jpg"
}
],
"markdown": "# A 20-Year-Old Man Who Stopped Speaking\n\n **Authors:** James Robert Brasic, MD, MPH \n **Date:** July 31, 2017\n\n ## Content\n\n Physical Examination and Workup\nAt age 16 years and 7 months, quantitative electroencephalography (QEEG) demonstrated diminished power in all wave bands (Figure 1).\nFigure 1.\nAt age 17 years, the patient was hospitalized. He was a well-developed, well-nourished youth in no acute respiratory distress. He exhibited constant grimacing, with continuous eye blinking. He did not respond to questions. His movements were slow. When given a slight push, he moved in the indicated direction. Mild weakness of the right arm and leg were noted. His right foot was dragged on the floor when walking. He could not squeeze his fists. He had a stooped posture when walking.\nA physical examination at that time was otherwise unremarkable. Findings of a lumbar puncture and MRI of the spinal cord and brain were normal. His serum serology was nonreactive. His antinuclear antibody and HIV antibody titers were negative. His serum ceruloplasmin level was 40 mg/dL. The enzymatic defect of metachromatic leukodystrophy was absent in punch biopsies of the left axilla. Normal mitochondrial enzymes of oxidative phosphorylation were observed. The patient's grimacing ceased for a short time after intravenous injections of lorazepam and three injections of amobarbital.\nAt age 18 years, the patient began requiring assistance for feeding. At age 18 years and 5 months, he required assistance with toilet activities and dressing. Three months later, a left sural nerve biopsy demonstrated loss of axon cylinders (Figure 2), and a left deltoid biopsy revealed perimysial fibrosis and type II fiber predominance (Figure 3).\nFigure 2.\nFigure 3.\nAt age 19 years and 2 months, he demonstrated progressively increasing adventitious movements and a decreasing ability to perform activities of daily living. At age 19 years and 8 months, he received a course of 25 electroconvulsive treatments without improvement. Two months later, QEEG revealed increased theta and delta in all brain regions (Figure 4).\nFigure 4.\nAt age 20 years and 11 months, 2-deoxy-2-[18F]fluoro-D-glucose positron emission tomography ([18F]FDG PET) demonstrated decreased uptake in the right parietal and right temporal regions (Figure 5).\nFigure 5.\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n**Figure 3.** \n \n\n**Figure 4.** \n \n\n**Figure 5.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1123148,
"choiceText": "Malingering",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1123150,
"choiceText": "Conversion disorder",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1123152,
"choiceText": "Autism with catatonia",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1123154,
"choiceText": "Schizophrenia",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1123156,
"choiceText": "Selective mutism",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 356041,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 20-Year-Old Man Who Stopped Speaking"
},
{
"authors": "James Robert Brasic, MD, MPH",
"content": [
"Discussion",
"The patient in this case has autism spectrum disorder (ASD) complicated by progressive catatonia; he has had persistent facial tics after treatment with a dopamine receptor–blocking drug demonstrated degenerative changes in nerve and muscle biopsies at age 18 years and 8 months.",
"Malingering is a situation in which a person falsely and intentionally reports medical or psychological symptoms[1]; no basis for the symptoms is found upon careful evaluation. Usually, malingering is produced to avoid an unwanted situation (eg, going to school, going to jail) or to gain desired benefits (eg, disability payments). The patient in this case wanted to go to school, and multiple abnormalities were found upon examination and laboratory testing, confirming the presence of neurologic bases for his symptoms.",
"Conversion disorder is a condition in which a person reports symptoms consistent with a neurologic illness.[2] In conversion disorder, the signs and symptoms occur within the voluntary parts of the neuromuscular system (eg, an inability to use one's right arm or hand). Careful examination demonstrates no evidence of a physical basis for the symptoms. Conversion disorder resembles malingering, with the presence of reported symptoms and the absence of examination findings. Malingering represents a situation in which the person deliberately reports symptoms they know to be false. On the other hand, in conversion disorders, the patient reports symptoms that the patient believes are real. In other words, in conversion disorders, the person is not deliberately reporting fabricated symptoms. The key to understanding the underlying basis of the symptom is that an unconscious conflict is converted into a physical symptom. Conversion disorders may occur after environmental stresses, including physical or sexual abuse.[3] An amobarbital interview may possibly reveal the underlying conflict. Again, the examination findings confirmed a neurologic basis in this case.",
"Cessation of speaking can occur in many conditions. Selective mutism refers to a situation in which a person can speak but does not talk under certain circumstances. Some children may be fearful at school, so they never talk while there but speak freely at home. Selective mutism typically occurs when children enter school.[4] They may readily speak at home. The absence of speech at school may reflect high anxiety. Thus, selective mutism may represent an anxiety disorder. This can be a form of social anxiety or social phobia, in which the person anticipates or fears the ridicule of others. The opposite condition may occur when silence at home may reflect an emotional or behavioral situation.[5] An example may be a child who has a parent with an alcohol problem. When that parent drinks, whatever the child says is followed by physical punishment and abuse; thus, that child learns the prudence of silence. The patient in this case did not speak at home or at school after he stopped talking. He never spoke under any circumstances. Although anecdotal case reports describe people who resumed talking after many years of silence, this patient has not displayed that behavior. Also, the abnormal physical and laboratory findings suggest that his silence reflects a degenerative disorder of the nervous system.",
"Schizophrenia is a disorder that typically presents in adolescence or young adulthood, characterized by the presence of hallucinations, perceptions of stimuli absent in the environment, and delusions/fixed false beliefs. Schizophrenia may manifest itself in youth with numerous soft neurologic findings. Auditory hallucinations are the most common perception in schizophrenia. Catatonia can occur with untreated schizophrenia. However, electroconvulsive treatments typically relieve symptoms of catatonia and schizophrenia. This patient demonstrated no response to them."
],
"date": "July 31, 2017",
"figures": [],
"markdown": "# A 20-Year-Old Man Who Stopped Speaking\n\n **Authors:** James Robert Brasic, MD, MPH \n **Date:** July 31, 2017\n\n ## Content\n\n Discussion\nThe patient in this case has autism spectrum disorder (ASD) complicated by progressive catatonia; he has had persistent facial tics after treatment with a dopamine receptor–blocking drug demonstrated degenerative changes in nerve and muscle biopsies at age 18 years and 8 months.\nMalingering is a situation in which a person falsely and intentionally reports medical or psychological symptoms[1]; no basis for the symptoms is found upon careful evaluation. Usually, malingering is produced to avoid an unwanted situation (eg, going to school, going to jail) or to gain desired benefits (eg, disability payments). The patient in this case wanted to go to school, and multiple abnormalities were found upon examination and laboratory testing, confirming the presence of neurologic bases for his symptoms.\nConversion disorder is a condition in which a person reports symptoms consistent with a neurologic illness.[2] In conversion disorder, the signs and symptoms occur within the voluntary parts of the neuromuscular system (eg, an inability to use one's right arm or hand). Careful examination demonstrates no evidence of a physical basis for the symptoms. Conversion disorder resembles malingering, with the presence of reported symptoms and the absence of examination findings. Malingering represents a situation in which the person deliberately reports symptoms they know to be false. On the other hand, in conversion disorders, the patient reports symptoms that the patient believes are real. In other words, in conversion disorders, the person is not deliberately reporting fabricated symptoms. The key to understanding the underlying basis of the symptom is that an unconscious conflict is converted into a physical symptom. Conversion disorders may occur after environmental stresses, including physical or sexual abuse.[3] An amobarbital interview may possibly reveal the underlying conflict. Again, the examination findings confirmed a neurologic basis in this case.\nCessation of speaking can occur in many conditions. Selective mutism refers to a situation in which a person can speak but does not talk under certain circumstances. Some children may be fearful at school, so they never talk while there but speak freely at home. Selective mutism typically occurs when children enter school.[4] They may readily speak at home. The absence of speech at school may reflect high anxiety. Thus, selective mutism may represent an anxiety disorder. This can be a form of social anxiety or social phobia, in which the person anticipates or fears the ridicule of others. The opposite condition may occur when silence at home may reflect an emotional or behavioral situation.[5] An example may be a child who has a parent with an alcohol problem. When that parent drinks, whatever the child says is followed by physical punishment and abuse; thus, that child learns the prudence of silence. The patient in this case did not speak at home or at school after he stopped talking. He never spoke under any circumstances. Although anecdotal case reports describe people who resumed talking after many years of silence, this patient has not displayed that behavior. Also, the abnormal physical and laboratory findings suggest that his silence reflects a degenerative disorder of the nervous system.\nSchizophrenia is a disorder that typically presents in adolescence or young adulthood, characterized by the presence of hallucinations, perceptions of stimuli absent in the environment, and delusions/fixed false beliefs. Schizophrenia may manifest itself in youth with numerous soft neurologic findings. Auditory hallucinations are the most common perception in schizophrenia. Catatonia can occur with untreated schizophrenia. However, electroconvulsive treatments typically relieve symptoms of catatonia and schizophrenia. This patient demonstrated no response to them.\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1123148,
"choiceText": "Malingering",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1123150,
"choiceText": "Conversion disorder",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1123152,
"choiceText": "Autism with catatonia",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1123154,
"choiceText": "Schizophrenia",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1123156,
"choiceText": "Selective mutism",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 356041,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 20-Year-Old Man Who Stopped Speaking"
},
{
"authors": "James Robert Brasic, MD, MPH",
"content": [
"ASD is a broad group of developmental disabilities characterized by the presence of impaired social interactions, atypical communication, and a limited range of interests and activities.[6]",
"The social impairments of people with ASD may include failure to acknowledge the presence of others. Additionally, individuals with ASD may fail to demonstrate the behaviors commonly displayed by people in the general population when interacting with others. Abnormalities in social interactions may take the form of markedly inhibited behaviors with others despite the desire for social interactions. On the other hand, people with ASD may display actions that are generally considered to be inappropriate due to the lack of closeness between the individuals. For example, a child with ASD may hug and kiss a complete stranger. Persons with ASD have difficulty reading other people's emotions and often respond inappropriately in social situations. Additionally, a man with ASD may propose marriage to a woman whom he just met without going through the usual sequence of dating and courtship. People with ASD may be amazed that the general population displays concerns when behaviors generally considered inappropriate are exhibited. For example, a man with ASD began dating and eventually married the adopted daughter of his former wife. He was surprised that the general community was shocked by his behavior.",
"The communication abnormalities of ASD may be idiosyncratic. Pronominal reversal (eg, substitution of \"you\" for \"me\") is common. People with ASD may have their own unique definitions of commonly used words. Additionally, people with ASD may develop neologisms (new words). Unlike other subgroups of ASD, people with Asperger syndrome, which is high-functioning autism that has recently been reclassified as fitting into the ASD spectrum, typically lack the communication abnormalities characteristic of ASD.[7,8,9]",
"The restricted range of activities and interests that are characteristic of ASD can encompass a broad spectrum. People with ASD may exhibit stereotypies and repetitive movements.[10] Rocking back and forth, hand flapping (shaking hands up and down with loose wrists), and other ordinary movements may be repeatedly performed. Waving the fingers back and forth before the eyes may simulate the actions of Venetian blinds. Additionally, people with ASD may engage in self-injurious behaviors.[11,12] These repeated behaviors may represent the efforts of people with ASD to provide self-stimulation in a barren environment.",
"Early diagnosis of ASD is desirable in order to institute treatment as soon as possible. The diagnostic instruments require considerable training and skill to administer and score. Intensive behavioral interventions at home and at school facilitate optimal outcomes.[13] Although imaging is useful for research purposes,[14,15] it may also be useful to rule out treatable structural lesions.",
"Catatonia is a distinctive condition that occurs in the course of many medical, surgical, and other disorders.[16] Catatonia typically manifests as an immobile occurrence. During this state, a patient may demonstrate mutism (the absence of speech), negativism (the refusal to follow commands of others), echolalia (the repetition of the speech of others), echopraxia (the repetition of the movements of others), waxy flexibility (odd postures occurring when others move the limbs of the patient), and withdrawal (marked inactivity and lack of participation in the ongoing activities of others). People with catatonia may alternatively exhibit an excited state, characterized by impulsivity, combativeness, and autonomic instability. In the excited state, people may collapse from exhaustion. This condition may be fatal."
],
"date": "July 31, 2017",
"figures": [],
"markdown": "# A 20-Year-Old Man Who Stopped Speaking\n\n **Authors:** James Robert Brasic, MD, MPH \n **Date:** July 31, 2017\n\n ## Content\n\n ASD is a broad group of developmental disabilities characterized by the presence of impaired social interactions, atypical communication, and a limited range of interests and activities.[6]\nThe social impairments of people with ASD may include failure to acknowledge the presence of others. Additionally, individuals with ASD may fail to demonstrate the behaviors commonly displayed by people in the general population when interacting with others. Abnormalities in social interactions may take the form of markedly inhibited behaviors with others despite the desire for social interactions. On the other hand, people with ASD may display actions that are generally considered to be inappropriate due to the lack of closeness between the individuals. For example, a child with ASD may hug and kiss a complete stranger. Persons with ASD have difficulty reading other people's emotions and often respond inappropriately in social situations. Additionally, a man with ASD may propose marriage to a woman whom he just met without going through the usual sequence of dating and courtship. People with ASD may be amazed that the general population displays concerns when behaviors generally considered inappropriate are exhibited. For example, a man with ASD began dating and eventually married the adopted daughter of his former wife. He was surprised that the general community was shocked by his behavior.\nThe communication abnormalities of ASD may be idiosyncratic. Pronominal reversal (eg, substitution of \"you\" for \"me\") is common. People with ASD may have their own unique definitions of commonly used words. Additionally, people with ASD may develop neologisms (new words). Unlike other subgroups of ASD, people with Asperger syndrome, which is high-functioning autism that has recently been reclassified as fitting into the ASD spectrum, typically lack the communication abnormalities characteristic of ASD.[7,8,9]\nThe restricted range of activities and interests that are characteristic of ASD can encompass a broad spectrum. People with ASD may exhibit stereotypies and repetitive movements.[10] Rocking back and forth, hand flapping (shaking hands up and down with loose wrists), and other ordinary movements may be repeatedly performed. Waving the fingers back and forth before the eyes may simulate the actions of Venetian blinds. Additionally, people with ASD may engage in self-injurious behaviors.[11,12] These repeated behaviors may represent the efforts of people with ASD to provide self-stimulation in a barren environment.\nEarly diagnosis of ASD is desirable in order to institute treatment as soon as possible. The diagnostic instruments require considerable training and skill to administer and score. Intensive behavioral interventions at home and at school facilitate optimal outcomes.[13] Although imaging is useful for research purposes,[14,15] it may also be useful to rule out treatable structural lesions.\nCatatonia is a distinctive condition that occurs in the course of many medical, surgical, and other disorders.[16] Catatonia typically manifests as an immobile occurrence. During this state, a patient may demonstrate mutism (the absence of speech), negativism (the refusal to follow commands of others), echolalia (the repetition of the speech of others), echopraxia (the repetition of the movements of others), waxy flexibility (odd postures occurring when others move the limbs of the patient), and withdrawal (marked inactivity and lack of participation in the ongoing activities of others). People with catatonia may alternatively exhibit an excited state, characterized by impulsivity, combativeness, and autonomic instability. In the excited state, people may collapse from exhaustion. This condition may be fatal.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 20-Year-Old Man Who Stopped Speaking"
},
{
"authors": "James Robert Brasic, MD, MPH",
"content": [
"The patient in this case represents an example of someone with ASD who developed catatonia, apparently in response to the environmental stress of being denied promotion to high school. The administration of a dopamine receptor–blocking drug, haloperidol, may have aggravated his condition. Like other antipsychotic medications, haloperidol is particularly effective in treating the positive signs in persons with schizophrenia, such as hallucinations and delusions. The patient in this case represents a person with ASD who develops progressive catatonia refractory to treatments that are often effective to reverse catatonia, including electroconvulsive treatments. Because his parents fear that medication may worsen his condition, they have refused treatment with lorazepam, an agent often beneficial for people with catatonia.",
"The family history of mental illness in paternal relatives suggests possible familial, genetic components to this condition. This man may have a genetic vulnerability to develop progressive catatonia when the environment adds external forces to challenge his nervous system. Thus, the environmental stress of failing eighth grade may represent an epigenetic influence that triggered the development of progressive catatonia due to an inherited risk of developing this disorder.",
"The onset of catatonia after treatment with haloperidol suggests a striking sensitivity to this dopamine receptor–blocking drug. Degenerative changes in his peripheral nerve and muscle may reflect the immobility that he has exhibited since the onset of his catatonia. Alternatively, the abnormalities in his nerve and muscle may represent effects of an inherited disorder with widespread alterations of the nervous system. He remains unable to perform activities of daily living. Progressive catatonia with grimacing complicating ASD can follow a chronic downhill course.",
"Higher levels of gamma-amino butyric acid (GABA) in children with autism versus typical children suggest dysfunction of GABA neurotransmission.[17,18] Grimaces, a tic-like phenomenon present in the current patient, occurs in many individuals with progressive catatonia in ASD.[19,20] Although people with schizophrenia and catatonia may demonstrate marked remissions with lorazepam and/or electroconvulsive treatment, people with progressive catatonia and ASD may be refractory to those interventions.[17] Nevertheless, lorazepam and electroconvulsive treatments remain the recommended treatments for severe progressive catatonia in ASD.[20,21,22,23]",
"The onset of progressive catatonia in this patient with ASD after treatment with haloperidol and environmental stress suggests a marked sensitivity to a dopamine receptor–blocking drug exacerbated by negative life events. Degenerative changes in his peripheral nerve and muscle likely resulted from the immobility that he has exhibited since the onset of his catatonia. They may also represent a manifestation of an underlying inherited neurologic disorder.",
"The patient is now 42 years old. He is unable to perform activities of daily living, exhibiting the progressive nature of the catatonia.[24,25] He exhibits motoric immobility, mutism, and prominent facial grimacing. He requires the assistance of a full-time home attendant. Because his parents believe that his condition resulted from treatment with haloperidol and benztropine at age 14 years, they refuse treatment with lorazepam and other medications.",
"Note: Figures were reproduced with permission from: Brašicì JR. Clinical manifestations of progressive catatonia. German J Psychiatr. 2000;3:13-24.[24] A previous version of this case was presented at the Third International Scientific Symposium on Tourette Syndrome; June 4-6, 1999; New York, New York, and at the First World Congress on Tourette Syndrome and Tic Disorders; June 24-26, 2015; London, United Kingdom.[26]"
],
"date": "July 31, 2017",
"figures": [],
"markdown": "# A 20-Year-Old Man Who Stopped Speaking\n\n **Authors:** James Robert Brasic, MD, MPH \n **Date:** July 31, 2017\n\n ## Content\n\n The patient in this case represents an example of someone with ASD who developed catatonia, apparently in response to the environmental stress of being denied promotion to high school. The administration of a dopamine receptor–blocking drug, haloperidol, may have aggravated his condition. Like other antipsychotic medications, haloperidol is particularly effective in treating the positive signs in persons with schizophrenia, such as hallucinations and delusions. The patient in this case represents a person with ASD who develops progressive catatonia refractory to treatments that are often effective to reverse catatonia, including electroconvulsive treatments. Because his parents fear that medication may worsen his condition, they have refused treatment with lorazepam, an agent often beneficial for people with catatonia.\nThe family history of mental illness in paternal relatives suggests possible familial, genetic components to this condition. This man may have a genetic vulnerability to develop progressive catatonia when the environment adds external forces to challenge his nervous system. Thus, the environmental stress of failing eighth grade may represent an epigenetic influence that triggered the development of progressive catatonia due to an inherited risk of developing this disorder.\nThe onset of catatonia after treatment with haloperidol suggests a striking sensitivity to this dopamine receptor–blocking drug. Degenerative changes in his peripheral nerve and muscle may reflect the immobility that he has exhibited since the onset of his catatonia. Alternatively, the abnormalities in his nerve and muscle may represent effects of an inherited disorder with widespread alterations of the nervous system. He remains unable to perform activities of daily living. Progressive catatonia with grimacing complicating ASD can follow a chronic downhill course.\nHigher levels of gamma-amino butyric acid (GABA) in children with autism versus typical children suggest dysfunction of GABA neurotransmission.[17,18] Grimaces, a tic-like phenomenon present in the current patient, occurs in many individuals with progressive catatonia in ASD.[19,20] Although people with schizophrenia and catatonia may demonstrate marked remissions with lorazepam and/or electroconvulsive treatment, people with progressive catatonia and ASD may be refractory to those interventions.[17] Nevertheless, lorazepam and electroconvulsive treatments remain the recommended treatments for severe progressive catatonia in ASD.[20,21,22,23]\nThe onset of progressive catatonia in this patient with ASD after treatment with haloperidol and environmental stress suggests a marked sensitivity to a dopamine receptor–blocking drug exacerbated by negative life events. Degenerative changes in his peripheral nerve and muscle likely resulted from the immobility that he has exhibited since the onset of his catatonia. They may also represent a manifestation of an underlying inherited neurologic disorder.\nThe patient is now 42 years old. He is unable to perform activities of daily living, exhibiting the progressive nature of the catatonia.[24,25] He exhibits motoric immobility, mutism, and prominent facial grimacing. He requires the assistance of a full-time home attendant. Because his parents believe that his condition resulted from treatment with haloperidol and benztropine at age 14 years, they refuse treatment with lorazepam and other medications.\nNote: Figures were reproduced with permission from: Brašicì JR. Clinical manifestations of progressive catatonia. German J Psychiatr. 2000;3:13-24.[24] A previous version of this case was presented at the Third International Scientific Symposium on Tourette Syndrome; June 4-6, 1999; New York, New York, and at the First World Congress on Tourette Syndrome and Tic Disorders; June 24-26, 2015; London, United Kingdom.[26]\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1123158,
"choiceText": "A restricted range of interests and activities",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1123160,
"choiceText": "Good eye-hand coordination",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1123162,
"choiceText": "Communication atypicality",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1123164,
"choiceText": "Impaired social skills",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Impaired social skills, communication atypicality, and a restricted range of interests and activities are hallmarks of ASD. Additionally, many people with ASD are clumsy and have limited motor skills.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 356043,
"questionText": "Which of the following is <em>not</em> a characteristic of ASD?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1123166,
"choiceText": "Echolalia",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1123168,
"choiceText": "Echopraxia",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1123170,
"choiceText": "Autonomic instability",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1123172,
"choiceText": "Mutism",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The immobile state of catatonia is characterized by mutism, negativism, echolalia, echopraxia, and waxy flexibility. By contrast, the excited state of catatonia is characterized by autonomic instability, impulsivity, and combativeness. The autonomic instability may represent a medical emergency requiring intensive medical management to prevent death.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 356045,
"questionText": "Which of the following is <em>not</em> a characteristic of the immobile state of catatonia?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 20-Year-Old Man Who Stopped Speaking"
},
{
"authors": "James Robert Brasic, MD, MPH",
"content": [],
"date": "July 31, 2017",
"figures": [],
"markdown": "# A 20-Year-Old Man Who Stopped Speaking\n\n **Authors:** James Robert Brasic, MD, MPH \n **Date:** July 31, 2017\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1123158,
"choiceText": "A restricted range of interests and activities",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1123160,
"choiceText": "Good eye-hand coordination",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1123162,
"choiceText": "Communication atypicality",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1123164,
"choiceText": "Impaired social skills",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Impaired social skills, communication atypicality, and a restricted range of interests and activities are hallmarks of ASD. Additionally, many people with ASD are clumsy and have limited motor skills.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 356043,
"questionText": "Which of the following is <em>not</em> a characteristic of ASD?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1123166,
"choiceText": "Echolalia",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1123168,
"choiceText": "Echopraxia",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1123170,
"choiceText": "Autonomic instability",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1123172,
"choiceText": "Mutism",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The immobile state of catatonia is characterized by mutism, negativism, echolalia, echopraxia, and waxy flexibility. By contrast, the excited state of catatonia is characterized by autonomic instability, impulsivity, and combativeness. The autonomic instability may represent a medical emergency requiring intensive medical management to prevent death.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 356045,
"questionText": "Which of the following is <em>not</em> a characteristic of the immobile state of catatonia?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 20-Year-Old Man Who Stopped Speaking"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1123148,
"choiceText": "Malingering",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1123150,
"choiceText": "Conversion disorder",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1123152,
"choiceText": "Autism with catatonia",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1123154,
"choiceText": "Schizophrenia",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1123156,
"choiceText": "Selective mutism",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 356041,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1123158,
"choiceText": "A restricted range of interests and activities",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1123160,
"choiceText": "Good eye-hand coordination",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1123162,
"choiceText": "Communication atypicality",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1123164,
"choiceText": "Impaired social skills",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Impaired social skills, communication atypicality, and a restricted range of interests and activities are hallmarks of ASD. Additionally, many people with ASD are clumsy and have limited motor skills.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 356043,
"questionText": "Which of the following is <em>not</em> a characteristic of ASD?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1123166,
"choiceText": "Echolalia",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1123168,
"choiceText": "Echopraxia",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1123170,
"choiceText": "Autonomic instability",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1123172,
"choiceText": "Mutism",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The immobile state of catatonia is characterized by mutism, negativism, echolalia, echopraxia, and waxy flexibility. By contrast, the excited state of catatonia is characterized by autonomic instability, impulsivity, and combativeness. The autonomic instability may represent a medical emergency requiring intensive medical management to prevent death.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 356045,
"questionText": "Which of the following is <em>not</em> a characteristic of the immobile state of catatonia?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
883306 | /viewarticle/883306 | [
{
"authors": "Gavin Christian K.W. Koh, MB, BChir, MA, MRCP, DTM&H; Richard J. Maude, BSc, MBChB Hons, MRCP, DTM&H; Pramot Srisamang, MD",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"A 3-year-old boy presents to the emergency department of a regional hospital in northeast Thailand 4 days after falling into a well. The patient's relatives estimate that he was in the water for no longer than 5 minutes and that he may have aspirated a significant amount of water. He did not lose consciousness and appeared to recover completely within minutes. Three days later, however, the boy developed a fever, cough, and increased drowsiness that prompted his family to bring him to the emergency department.",
"The child has never been to a hospital before, is normally fit and well, and is on no regular medications. He comes from a family of rice farmers and often plays in the paddy fields near his home. Both parents and siblings are well-appearing, and the family history does not include diabetes, thalassemia, or renal disease."
],
"date": "July 28, 2017",
"figures": [],
"markdown": "# A 3-Year-Old Boy With Fever and Drowsiness\n\n **Authors:** Gavin Christian K.W. Koh, MB, BChir, MA, MRCP, DTM&H; Richard J. Maude, BSc, MBChB Hons, MRCP, DTM&H; Pramot Srisamang, MD \n **Date:** July 28, 2017\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nA 3-year-old boy presents to the emergency department of a regional hospital in northeast Thailand 4 days after falling into a well. The patient's relatives estimate that he was in the water for no longer than 5 minutes and that he may have aspirated a significant amount of water. He did not lose consciousness and appeared to recover completely within minutes. Three days later, however, the boy developed a fever, cough, and increased drowsiness that prompted his family to bring him to the emergency department.\nThe child has never been to a hospital before, is normally fit and well, and is on no regular medications. He comes from a family of rice farmers and often plays in the paddy fields near his home. Both parents and siblings are well-appearing, and the family history does not include diabetes, thalassemia, or renal disease.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 3-Year-Old Boy With Fever and Drowsiness"
},
{
"authors": "Gavin Christian K.W. Koh, MB, BChir, MA, MRCP, DTM&H; Richard J. Maude, BSc, MBChB Hons, MRCP, DTM&H; Pramot Srisamang, MD",
"content": [
"Upon physical examination, the boy is drowsy, but he is obeying instructions from his mother. His temperature is 102°F, respiratory rate is 36 breaths/min, and pulse rate is 132 beats/min and regular. Owing to lack of equipment, the patient's blood pressure and pulse oximetry are not readily available. He is not cyanotic or jaundiced, and no peripheral signs of chronic disease are noted.",
"The patient has moderately increased work of breathing, with use of the accessory muscles of respiration. Widespread fine crackles are audible over the chest bilaterally. The heart sounds are normal, with no rubs or murmurs. No palpable lymphadenopathy, visible skin lesions, or palpable organomegaly is noted. The abdominal examination is unremarkable.",
"The patient is placed on supplemental oxygen, leading to some improvement in his respiratory status. An intravenous line is placed, and initial laboratory investigations are performed, revealing the following values:",
"Hemoglobin level: 8.7 g/dL",
"Mean corpuscular volume: 60.1 µm3",
"Platelet count: 196 × 103 cells/µL",
"Total white blood cell count: 4.51 × 103 cells/μL (53% neutrophils, 32% lymphocytes , and 12% monocytes)",
"Serum sodium level: 134 mEq/L",
"Potassium level: 3 mEq/L",
"Chloride level: 98 mEq/L",
"Bicarbonate level: 24 mEq/L",
"Blood urea nitrogen level; 8 mg/dL",
"Creatinine level: 0.3 mg/dL",
"No arterial blood gas or glucose measurements are available.",
"The admission chest radiograph (Figure 1) demonstrates bilateral diffuse infiltrates. Broad-spectrum antibiotic therapy with intravenous ceftriaxone and metronidazole is initiated. The patient is admitted to the hospital.",
"Figure 1."
],
"date": "July 28, 2017",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/883/306/883306-thumb1.jpg"
}
],
"markdown": "# A 3-Year-Old Boy With Fever and Drowsiness\n\n **Authors:** Gavin Christian K.W. Koh, MB, BChir, MA, MRCP, DTM&H; Richard J. Maude, BSc, MBChB Hons, MRCP, DTM&H; Pramot Srisamang, MD \n **Date:** July 28, 2017\n\n ## Content\n\n Upon physical examination, the boy is drowsy, but he is obeying instructions from his mother. His temperature is 102°F, respiratory rate is 36 breaths/min, and pulse rate is 132 beats/min and regular. Owing to lack of equipment, the patient's blood pressure and pulse oximetry are not readily available. He is not cyanotic or jaundiced, and no peripheral signs of chronic disease are noted.\nThe patient has moderately increased work of breathing, with use of the accessory muscles of respiration. Widespread fine crackles are audible over the chest bilaterally. The heart sounds are normal, with no rubs or murmurs. No palpable lymphadenopathy, visible skin lesions, or palpable organomegaly is noted. The abdominal examination is unremarkable.\nThe patient is placed on supplemental oxygen, leading to some improvement in his respiratory status. An intravenous line is placed, and initial laboratory investigations are performed, revealing the following values:\nHemoglobin level: 8.7 g/dL\nMean corpuscular volume: 60.1 µm3\nPlatelet count: 196 × 103 cells/µL\nTotal white blood cell count: 4.51 × 103 cells/μL (53% neutrophils, 32% lymphocytes , and 12% monocytes)\nSerum sodium level: 134 mEq/L\nPotassium level: 3 mEq/L\nChloride level: 98 mEq/L\nBicarbonate level: 24 mEq/L\nBlood urea nitrogen level; 8 mg/dL\nCreatinine level: 0.3 mg/dL\nNo arterial blood gas or glucose measurements are available.\nThe admission chest radiograph (Figure 1) demonstrates bilateral diffuse infiltrates. Broad-spectrum antibiotic therapy with intravenous ceftriaxone and metronidazole is initiated. The patient is admitted to the hospital.\nFigure 1.\n\n ## Figures\n\n **Figure 1.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1123120,
"choiceText": "<em>Burkholderia pseudomallei</em>",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1123122,
"choiceText": "<em>Salmonella enterica </em>serotype <em>Typhi</em>",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1123124,
"choiceText": "<em>Vibrio cholerae</em>",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1123126,
"choiceText": "<em>Streptococcus pneumoniae</em>",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1123128,
"choiceText": "<em>Klebsiella pneumoniae</em>",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 356035,
"questionText": "Empirical antimicrobial therapy for this patient should include which of the following bacteria?<br/><br/>\r\n\r\n<em>Hint: Note the contact with surface water and that the location is northeastern Thailand.</em>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 3-Year-Old Boy With Fever and Drowsiness"
},
{
"authors": "Gavin Christian K.W. Koh, MB, BChir, MA, MRCP, DTM&H; Richard J. Maude, BSc, MBChB Hons, MRCP, DTM&H; Pramot Srisamang, MD",
"content": [
"The plain chest radiograph obtained on admission (Figure 1) showed diffuse bilateral infiltrates consistent with bilateral pneumonia.",
"Figure 1.",
"The patient was initially treated with broad-spectrum intravenous antibiotics, including ceftriaxone and metronidazole. Unfortunately, after 3 days of therapy, he continued to have spiking fevers, suggesting treatment failure. The patient was transferred to the pediatric service of the hospital, and empirical treatment with ceftazidime was initiated. On the basis of the geographic location of the case, the clinical history of aspiration of well water, and the nonresponse to standard antibiotic therapy for pneumonia, melioidosis was suspected.",
"During the rainy season, Burkholderia pseudomallei (formerly Pseudomonas pseudomallei) is commonly isolated in cases of community-acquired pneumonia in northeast Thailand and northern Australia.[1] It is an aerobic, motile, gram-negative, saprophytic bacterium commonly found in soil and surface water in southeast Asia and northern Australia. B pseudomallei infection is known as \"melioidosis\" (from the Greek words melis, which means \"distemper of asses,\" and eidos, which means \"the likeness of\"). The disease is probably endemic to the geographic areas between latitudes 20°N and 20°S. Sporadic cases have been reported in Central and South America, the Caribbean, and eastern and western Africa.[2]",
"The clinical manifestations of melioidosis appear to be protean. Definitive diagnosis is made by identifying the causative organism from a clinical specimen. Given the lack of microbiological facilities in many parts of the tropics, the prevalence of melioidosis is almost certainly underestimated. In the United Kingdom, five cases of melioidosis were imported from Bangladesh between 1988 and 1998; however, Bangladesh only reported its first case in 1998.[3] Likewise, two cases of melioidosis imported from Honduras were reported in 2005 in the United States,[4] yet Honduras itself has never reported a case of melioidosis. In Thailand, the first case of melioidosis was reported in 1955, but the disease remained largely unrecognized until the availability of routine microbiological testing in the mid- to late 1970s.[5]"
],
"date": "July 28, 2017",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/883/306/883306-thumb1.jpg"
}
],
"markdown": "# A 3-Year-Old Boy With Fever and Drowsiness\n\n **Authors:** Gavin Christian K.W. Koh, MB, BChir, MA, MRCP, DTM&H; Richard J. Maude, BSc, MBChB Hons, MRCP, DTM&H; Pramot Srisamang, MD \n **Date:** July 28, 2017\n\n ## Content\n\n The plain chest radiograph obtained on admission (Figure 1) showed diffuse bilateral infiltrates consistent with bilateral pneumonia.\nFigure 1.\nThe patient was initially treated with broad-spectrum intravenous antibiotics, including ceftriaxone and metronidazole. Unfortunately, after 3 days of therapy, he continued to have spiking fevers, suggesting treatment failure. The patient was transferred to the pediatric service of the hospital, and empirical treatment with ceftazidime was initiated. On the basis of the geographic location of the case, the clinical history of aspiration of well water, and the nonresponse to standard antibiotic therapy for pneumonia, melioidosis was suspected.\nDuring the rainy season, Burkholderia pseudomallei (formerly Pseudomonas pseudomallei) is commonly isolated in cases of community-acquired pneumonia in northeast Thailand and northern Australia.[1] It is an aerobic, motile, gram-negative, saprophytic bacterium commonly found in soil and surface water in southeast Asia and northern Australia. B pseudomallei infection is known as \"melioidosis\" (from the Greek words melis, which means \"distemper of asses,\" and eidos, which means \"the likeness of\"). The disease is probably endemic to the geographic areas between latitudes 20°N and 20°S. Sporadic cases have been reported in Central and South America, the Caribbean, and eastern and western Africa.[2]\nThe clinical manifestations of melioidosis appear to be protean. Definitive diagnosis is made by identifying the causative organism from a clinical specimen. Given the lack of microbiological facilities in many parts of the tropics, the prevalence of melioidosis is almost certainly underestimated. In the United Kingdom, five cases of melioidosis were imported from Bangladesh between 1988 and 1998; however, Bangladesh only reported its first case in 1998.[3] Likewise, two cases of melioidosis imported from Honduras were reported in 2005 in the United States,[4] yet Honduras itself has never reported a case of melioidosis. In Thailand, the first case of melioidosis was reported in 1955, but the disease remained largely unrecognized until the availability of routine microbiological testing in the mid- to late 1970s.[5]\n\n ## Figures\n\n **Figure 1.** \n \n\n\n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1123120,
"choiceText": "<em>Burkholderia pseudomallei</em>",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1123122,
"choiceText": "<em>Salmonella enterica </em>serotype <em>Typhi</em>",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1123124,
"choiceText": "<em>Vibrio cholerae</em>",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1123126,
"choiceText": "<em>Streptococcus pneumoniae</em>",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1123128,
"choiceText": "<em>Klebsiella pneumoniae</em>",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 356035,
"questionText": "Empirical antimicrobial therapy for this patient should include which of the following bacteria?<br/><br/>\r\n\r\n<em>Hint: Note the contact with surface water and that the location is northeastern Thailand.</em>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 3-Year-Old Boy With Fever and Drowsiness"
},
{
"authors": "Gavin Christian K.W. Koh, MB, BChir, MA, MRCP, DTM&H; Richard J. Maude, BSc, MBChB Hons, MRCP, DTM&H; Pramot Srisamang, MD",
"content": [
"In endemic areas, melioidosis most commonly presents as fulminant sepsis, and the duration of symptoms before presentation is commonly brief. Lesions are usually (but not invariably) found in the lungs (presenting as consolidations or lung abscesses), liver, spleen, kidneys, skin, and joints.[1] The mortality of melioidosis is high (20%-40%), despite adequate and appropriate treatment.[1]",
"Melioidosis also has a less common indolent form, which presents after an extended period of incubation and may be difficult to differentiate from tuberculosis. This is the form most commonly seen in nonendemic areas (eg, United States, Western Europe), where most cases are imported.[6,7]",
"Contact with soil or surface water is usually part of the history. Specific occupations, such as rice farming and gardening, are important risk factors for the disease. The most common route of infection is presumed to be translocation via minor abrasions in the skin, but inhalation is probably an important route, including aspiration of contaminated well water; this has also been described in cases of extreme weather events.[1]",
"Around two thirds of all adult patients with melioidosis have diabetes mellitus as a risk factor, and in endemic areas, melioidosis may be the first presentation of diabetes. As a result, all adult survivors of melioidosis should be evaluated for diabetes after recovery.",
"Other risk factors for melioidosis include immunosuppression (corticosteroids, methotrexate, cancer chemotherapy), chronic renal impairment or renal calculi, thalassemia major, excessive alcohol use, cystic fibrosis, and cancer.[2] A study of over 500 patients with melioidosis found no association with HIV infection.[8]",
"Melioidosis in children differs in numerous ways from that seen in adults. In children, the disease is usually a localized abscess, mortality is low, relapse is uncommon, and contact with soil and water are often the only risk factors.[1] Around one third of all cases of pediatric disease present as parotitis.[1]",
"B pseudomallei is not a normal human commensal, and a carrier state is exceedingly rare. Identification of B pseudomallei in any clinical specimen is diagnostic of melioidosis.[1,9] Specimens of blood, throat, and respiratory secretions (eg, sputum, tracheal aspirate, bronchoalveolar lavage) and urine should be collected from every patient in whom the disease is suspected.[9] In addition, pus from any abscesses or collections and surface swabs from any wounds should be obtained, as should specimens from any other site as clinically indicated. Nasogastric aspirates are useful in young children, who tend to swallow their sputum instead of expectorating it. Throat swabs are a valuable diagnostic tool and are often positive, even in cases where no evidence suggests oropharyngeal or respiratory involvement.[9]",
"B pseudomallei should be handled in biosafety level 3 conditions because of the risk for laboratory-acquired infection.[10] The specimens listed above would normally only be handled in biosafety level 2 conditions; however, the appearance of melioidosis in the differential diagnosis should prompt the clinician to warn the microbiology laboratory before any specimens are sent.",
"The classic textbook appearance of an intracellular bipolar-staining rod (\"safety pin\" appearance) is uncommon, not sensitive, and not dependable for diagnosing B pseudomallei.[11] B pseudomallei is not usually a fastidious organism, allowing for growth in a wide variety of media. Early discussion with the microbiology laboratory permits the use of techniques that maximize the chances of identifying the organism.",
"For blood cultures, lysis centrifugation improves the time to identification, but it requires the blood to be collected because special specimen containers (eg, isolator blood culture tubes) may be required.[12] Specimens obtained from nonsterile sites (eg, sputum and throat swabs) are prone to overgrowth by commensal bacteria, thereby obscuring the presence of B pseudomallei. The use of selective media maximizes the chance of isolating B pseudomallei from these specimens.[13] In endemic areas, Ashdown agar (which contains crystal violet and gentamicin as selective agents) and tryptic soy broth with crystal violet and colistin are routinely used for this purpose. These media are cheap and easy to manufacture, and the ingredients are readily available. Commercially available selective medium for Burkholderia cepacia is an acceptable substitute for microbiology laboratories that no longer make their own media.[14]",
"The colonial morphology of B pseudomallei (classically described as looking like cornflower heads [Figure 2]) is exceedingly variable and may look like environmental contaminants; other environmental species (eg, Pseudomonas, Burkholderia) may produce colonies that are similar in appearance.",
"Figure 2.",
"The laboratory may erroneously report colonies of B pseudomallei as being of no clinical significance, unless appropriately notified. This is unlikely to occur in cases with a heavy, pure growth of the organism from a sterile site (eg, blood or an intraoperative specimen), but it is possible when growth is meager and the specimen is from a nonsterile site. Even if the isolate is recognized as significant, identification of the organism is not straightforward because some commercial systems may erroneously identify the organism as other Burkholderia species, Chromobacterium violaceum, or Pseudomonas species.[15] A 16S polymerase chain reaction assay may be required to properly identify the organism.[16] In the United States, help with identification is available from the Centers for Disease Control and Prevention (CDC)."
],
"date": "July 28, 2017",
"figures": [
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/883/306/883306-thumb2.jpg"
}
],
"markdown": "# A 3-Year-Old Boy With Fever and Drowsiness\n\n **Authors:** Gavin Christian K.W. Koh, MB, BChir, MA, MRCP, DTM&H; Richard J. Maude, BSc, MBChB Hons, MRCP, DTM&H; Pramot Srisamang, MD \n **Date:** July 28, 2017\n\n ## Content\n\n In endemic areas, melioidosis most commonly presents as fulminant sepsis, and the duration of symptoms before presentation is commonly brief. Lesions are usually (but not invariably) found in the lungs (presenting as consolidations or lung abscesses), liver, spleen, kidneys, skin, and joints.[1] The mortality of melioidosis is high (20%-40%), despite adequate and appropriate treatment.[1]\nMelioidosis also has a less common indolent form, which presents after an extended period of incubation and may be difficult to differentiate from tuberculosis. This is the form most commonly seen in nonendemic areas (eg, United States, Western Europe), where most cases are imported.[6,7]\nContact with soil or surface water is usually part of the history. Specific occupations, such as rice farming and gardening, are important risk factors for the disease. The most common route of infection is presumed to be translocation via minor abrasions in the skin, but inhalation is probably an important route, including aspiration of contaminated well water; this has also been described in cases of extreme weather events.[1]\nAround two thirds of all adult patients with melioidosis have diabetes mellitus as a risk factor, and in endemic areas, melioidosis may be the first presentation of diabetes. As a result, all adult survivors of melioidosis should be evaluated for diabetes after recovery.\nOther risk factors for melioidosis include immunosuppression (corticosteroids, methotrexate, cancer chemotherapy), chronic renal impairment or renal calculi, thalassemia major, excessive alcohol use, cystic fibrosis, and cancer.[2] A study of over 500 patients with melioidosis found no association with HIV infection.[8]\nMelioidosis in children differs in numerous ways from that seen in adults. In children, the disease is usually a localized abscess, mortality is low, relapse is uncommon, and contact with soil and water are often the only risk factors.[1] Around one third of all cases of pediatric disease present as parotitis.[1]\nB pseudomallei is not a normal human commensal, and a carrier state is exceedingly rare. Identification of B pseudomallei in any clinical specimen is diagnostic of melioidosis.[1,9] Specimens of blood, throat, and respiratory secretions (eg, sputum, tracheal aspirate, bronchoalveolar lavage) and urine should be collected from every patient in whom the disease is suspected.[9] In addition, pus from any abscesses or collections and surface swabs from any wounds should be obtained, as should specimens from any other site as clinically indicated. Nasogastric aspirates are useful in young children, who tend to swallow their sputum instead of expectorating it. Throat swabs are a valuable diagnostic tool and are often positive, even in cases where no evidence suggests oropharyngeal or respiratory involvement.[9]\nB pseudomallei should be handled in biosafety level 3 conditions because of the risk for laboratory-acquired infection.[10] The specimens listed above would normally only be handled in biosafety level 2 conditions; however, the appearance of melioidosis in the differential diagnosis should prompt the clinician to warn the microbiology laboratory before any specimens are sent.\nThe classic textbook appearance of an intracellular bipolar-staining rod (\"safety pin\" appearance) is uncommon, not sensitive, and not dependable for diagnosing B pseudomallei.[11] B pseudomallei is not usually a fastidious organism, allowing for growth in a wide variety of media. Early discussion with the microbiology laboratory permits the use of techniques that maximize the chances of identifying the organism.\nFor blood cultures, lysis centrifugation improves the time to identification, but it requires the blood to be collected because special specimen containers (eg, isolator blood culture tubes) may be required.[12] Specimens obtained from nonsterile sites (eg, sputum and throat swabs) are prone to overgrowth by commensal bacteria, thereby obscuring the presence of B pseudomallei. The use of selective media maximizes the chance of isolating B pseudomallei from these specimens.[13] In endemic areas, Ashdown agar (which contains crystal violet and gentamicin as selective agents) and tryptic soy broth with crystal violet and colistin are routinely used for this purpose. These media are cheap and easy to manufacture, and the ingredients are readily available. Commercially available selective medium for Burkholderia cepacia is an acceptable substitute for microbiology laboratories that no longer make their own media.[14]\nThe colonial morphology of B pseudomallei (classically described as looking like cornflower heads [Figure 2]) is exceedingly variable and may look like environmental contaminants; other environmental species (eg, Pseudomonas, Burkholderia) may produce colonies that are similar in appearance.\nFigure 2.\nThe laboratory may erroneously report colonies of B pseudomallei as being of no clinical significance, unless appropriately notified. This is unlikely to occur in cases with a heavy, pure growth of the organism from a sterile site (eg, blood or an intraoperative specimen), but it is possible when growth is meager and the specimen is from a nonsterile site. Even if the isolate is recognized as significant, identification of the organism is not straightforward because some commercial systems may erroneously identify the organism as other Burkholderia species, Chromobacterium violaceum, or Pseudomonas species.[15] A 16S polymerase chain reaction assay may be required to properly identify the organism.[16] In the United States, help with identification is available from the Centers for Disease Control and Prevention (CDC).\n\n ## Figures\n\n **Figure 2.** \n \n\n\n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 3-Year-Old Boy With Fever and Drowsiness"
},
{
"authors": "Gavin Christian K.W. Koh, MB, BChir, MA, MRCP, DTM&H; Richard J. Maude, BSc, MBChB Hons, MRCP, DTM&H; Pramot Srisamang, MD",
"content": [
"The organism is intrinsically resistant to a wide range of antimicrobials (eg, ampicillin, ceftriaxone, metronidazole, moxifloxacin, clindamycin), and identification by culture often takes a minimum of 48 hours. A high level of clinical suspicion is required if appropriate therapy is to be initiated in a timely fashion. Although serologic methods are available for the diagnosis of melioidosis, they are of limited value in endemic areas; these methods are more useful for the diagnosis of melioidosis in visitors from nonendemic areas,[17] but seronegativity does not exclude a diagnosis of melioidosis, and culture is still the criterion standard for diagnosis.[16]",
"The treatment of choice is parenteral ceftazidime (120 mg/kg/day in three divided doses; maximum dose, 2 g three times daily). Meropenem and imipenem are alternative treatments.[9] Ceftazidime resistance in B pseudomallei is rare, and resistance to carbapenems has not been described.[9]",
"The recommended minimum duration of parenteral treatment is 10 days, and it should be continued until the patient's clinical findings return to baseline. Usually, patients have a slow clinical response to treatment. The median time to fever clearance is 9 days, despite adequate therapy.[9]",
"Unlike in most other bacterial diseases, failure to respond after 48 hours of appropriate treatment is not an indication to revise antibiotic therapy or consider alternative diagnoses.[1,9] Although resistance can develop during the course of treatment, this is uncommon.[9] However, obtaining repeat cultures from any patient who fails to defervesce after 7 days of therapy is prudent.",
"Adequate supportive treatment is essential and includes fluid resuscitation, artificial ventilation, tight glycemic control, and renal replacement therapy. Adjunctive treatment with granulocyte colony-stimulating factor is ineffective.[18] The efficacy of adjunctive low-dose steroids or activated protein C (drotrecogin alfa) in melioidosis is unknown.",
"Relapse and reinfection are common; therefore, patients require 20 weeks of oral eradication therapy after completing parenteral treatment. Potential choices for adults include trimethoprim/sulfamethoxazole (16/80 mg/kg/day in two divided doses; maximum dose, 320/1600 mg twice daily) and doxycycline (4 mg/kg/day in two divided doses; maximum dose, 100 mg twice daily).[2,9] Children younger than 8 years and pregnant women (for whom doxycycline is relatively contraindicated) should be given amoxicillin/clavulanate (180/45 mg/kg/day in three divided doses).[19] Amoxicillin/clavulanate is also an option for adults unable to tolerate trimethoprim/sulfamethoxazole or doxycycline.[19]",
"Thirteen percent of patients have relapse or reinfection in the 10 years after their primary infection; for this reason, patients with B pseudomallei in Thailand require long-term follow-up.[20] Patients who have no systemic symptoms and only a single localized abscess that has been completely drained may be treated with oral eradication therapy alone.[9]",
"In this case, a nasogastric aspirate obtained on admission returned culture-positive for B pseudomallei. The patient was continued on intravenous ceftazidime. He had spiking fevers of up to 102.2°F until hospital day 5, at which time he defervesced. He was treated with intravenous ceftazidime for 21 days during the hospitalization. The patient made a good recovery and was discharged home on oral amoxicillin/clavulanate for a 5-month follow-up course. He was doing well when seen at follow-up 1 month later."
],
"date": "July 28, 2017",
"figures": [],
"markdown": "# A 3-Year-Old Boy With Fever and Drowsiness\n\n **Authors:** Gavin Christian K.W. Koh, MB, BChir, MA, MRCP, DTM&H; Richard J. Maude, BSc, MBChB Hons, MRCP, DTM&H; Pramot Srisamang, MD \n **Date:** July 28, 2017\n\n ## Content\n\n The organism is intrinsically resistant to a wide range of antimicrobials (eg, ampicillin, ceftriaxone, metronidazole, moxifloxacin, clindamycin), and identification by culture often takes a minimum of 48 hours. A high level of clinical suspicion is required if appropriate therapy is to be initiated in a timely fashion. Although serologic methods are available for the diagnosis of melioidosis, they are of limited value in endemic areas; these methods are more useful for the diagnosis of melioidosis in visitors from nonendemic areas,[17] but seronegativity does not exclude a diagnosis of melioidosis, and culture is still the criterion standard for diagnosis.[16]\nThe treatment of choice is parenteral ceftazidime (120 mg/kg/day in three divided doses; maximum dose, 2 g three times daily). Meropenem and imipenem are alternative treatments.[9] Ceftazidime resistance in B pseudomallei is rare, and resistance to carbapenems has not been described.[9]\nThe recommended minimum duration of parenteral treatment is 10 days, and it should be continued until the patient's clinical findings return to baseline. Usually, patients have a slow clinical response to treatment. The median time to fever clearance is 9 days, despite adequate therapy.[9]\nUnlike in most other bacterial diseases, failure to respond after 48 hours of appropriate treatment is not an indication to revise antibiotic therapy or consider alternative diagnoses.[1,9] Although resistance can develop during the course of treatment, this is uncommon.[9] However, obtaining repeat cultures from any patient who fails to defervesce after 7 days of therapy is prudent.\nAdequate supportive treatment is essential and includes fluid resuscitation, artificial ventilation, tight glycemic control, and renal replacement therapy. Adjunctive treatment with granulocyte colony-stimulating factor is ineffective.[18] The efficacy of adjunctive low-dose steroids or activated protein C (drotrecogin alfa) in melioidosis is unknown.\nRelapse and reinfection are common; therefore, patients require 20 weeks of oral eradication therapy after completing parenteral treatment. Potential choices for adults include trimethoprim/sulfamethoxazole (16/80 mg/kg/day in two divided doses; maximum dose, 320/1600 mg twice daily) and doxycycline (4 mg/kg/day in two divided doses; maximum dose, 100 mg twice daily).[2,9] Children younger than 8 years and pregnant women (for whom doxycycline is relatively contraindicated) should be given amoxicillin/clavulanate (180/45 mg/kg/day in three divided doses).[19] Amoxicillin/clavulanate is also an option for adults unable to tolerate trimethoprim/sulfamethoxazole or doxycycline.[19]\nThirteen percent of patients have relapse or reinfection in the 10 years after their primary infection; for this reason, patients with B pseudomallei in Thailand require long-term follow-up.[20] Patients who have no systemic symptoms and only a single localized abscess that has been completely drained may be treated with oral eradication therapy alone.[9]\nIn this case, a nasogastric aspirate obtained on admission returned culture-positive for B pseudomallei. The patient was continued on intravenous ceftazidime. He had spiking fevers of up to 102.2°F until hospital day 5, at which time he defervesced. He was treated with intravenous ceftazidime for 21 days during the hospitalization. The patient made a good recovery and was discharged home on oral amoxicillin/clavulanate for a 5-month follow-up course. He was doing well when seen at follow-up 1 month later.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1123130,
"choiceText": "Corticosteroid use",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1123132,
"choiceText": "Cystic fibrosis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1123134,
"choiceText": "Diabetes mellitus",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1123136,
"choiceText": "Thalassemia",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1123138,
"choiceText": "HIV",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>Around two thirds of all adult patients with melioidosis have diabetes mellitus as a risk factor,<sup type=\"ref\">[1]</sup> and in endemic areas, melioidosis may be the first presentation of diabetes. As a result, all adult survivors of melioidosis must be tested for diabetes after recovery. Other risk factors for melioidosis include immunosuppression (eg, corticosteroids, methotrexate, cancer chemotherapy), renal disease (chronic renal impairment or renal calculi), thalassemia major, excessive alcohol use, cystic fibrosis, and cancer.<sup type=\"ref\">[2]</sup> A study of over 500 patients with melioidosis found no association with HIV infection.<sup type=\"ref\">[8]</sup></p>\r\n\r\n<p>Melioidosis in children differs in numerous ways from that seen in adults. In children, the disease is usually a localized abscess, mortality is low, relapse is uncommon, and contact with soil and water are often the only risk factors.<sup type=\"ref\">[1]</sup> Around one third of all pediatric disease presents as parotitis.<sup type=\"ref\">[1]</sup></p>",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 356037,
"questionText": "Which of the following is <em>not</em> a recognized risk factor for melioidosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1123140,
"choiceText": "<em>B pseudomallei</em> is fastidious and has special nutritional requirements",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1123142,
"choiceText": "<em>B pseudomallei</em> should be handled in biosafety level 3 conditions",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1123144,
"choiceText": "<em>B pseudomallei</em> may be misidentified or ignored",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1123146,
"choiceText": "The chances of identifying <em>B pseudomallei</em> from a throat swab may be improved by using selective media",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p><em>B pseudomallei</em> easily grows on a wide range of standard media; however, when the specimen is obtained from a nonsterile site (eg, sputum or throat swab), using selective media maximizes the chances that the organism is not missed as a result of overgrowth by commensal organisms. The CDC recommends that the organism should be handled under biosafety level 3 conditions because of the risk for laboratory-acquired infection. If the laboratory is not warned that melioidosis is suspected, <em>B pseudomallei</em> colonies may be mistaken for an environmental contaminant and disregarded. In addition, some commonly used commercial identification systems may misidentify the organism.</p>",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 356039,
"questionText": "Which of the following statements is <em>not</em> accurate?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 3-Year-Old Boy With Fever and Drowsiness"
},
{
"authors": "Gavin Christian K.W. Koh, MB, BChir, MA, MRCP, DTM&H; Richard J. Maude, BSc, MBChB Hons, MRCP, DTM&H; Pramot Srisamang, MD",
"content": [],
"date": "July 28, 2017",
"figures": [],
"markdown": "# A 3-Year-Old Boy With Fever and Drowsiness\n\n **Authors:** Gavin Christian K.W. Koh, MB, BChir, MA, MRCP, DTM&H; Richard J. Maude, BSc, MBChB Hons, MRCP, DTM&H; Pramot Srisamang, MD \n **Date:** July 28, 2017\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1123130,
"choiceText": "Corticosteroid use",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1123132,
"choiceText": "Cystic fibrosis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1123134,
"choiceText": "Diabetes mellitus",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1123136,
"choiceText": "Thalassemia",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1123138,
"choiceText": "HIV",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>Around two thirds of all adult patients with melioidosis have diabetes mellitus as a risk factor,<sup type=\"ref\">[1]</sup> and in endemic areas, melioidosis may be the first presentation of diabetes. As a result, all adult survivors of melioidosis must be tested for diabetes after recovery. Other risk factors for melioidosis include immunosuppression (eg, corticosteroids, methotrexate, cancer chemotherapy), renal disease (chronic renal impairment or renal calculi), thalassemia major, excessive alcohol use, cystic fibrosis, and cancer.<sup type=\"ref\">[2]</sup> A study of over 500 patients with melioidosis found no association with HIV infection.<sup type=\"ref\">[8]</sup></p>\r\n\r\n<p>Melioidosis in children differs in numerous ways from that seen in adults. In children, the disease is usually a localized abscess, mortality is low, relapse is uncommon, and contact with soil and water are often the only risk factors.<sup type=\"ref\">[1]</sup> Around one third of all pediatric disease presents as parotitis.<sup type=\"ref\">[1]</sup></p>",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 356037,
"questionText": "Which of the following is <em>not</em> a recognized risk factor for melioidosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1123140,
"choiceText": "<em>B pseudomallei</em> is fastidious and has special nutritional requirements",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1123142,
"choiceText": "<em>B pseudomallei</em> should be handled in biosafety level 3 conditions",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1123144,
"choiceText": "<em>B pseudomallei</em> may be misidentified or ignored",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1123146,
"choiceText": "The chances of identifying <em>B pseudomallei</em> from a throat swab may be improved by using selective media",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p><em>B pseudomallei</em> easily grows on a wide range of standard media; however, when the specimen is obtained from a nonsterile site (eg, sputum or throat swab), using selective media maximizes the chances that the organism is not missed as a result of overgrowth by commensal organisms. The CDC recommends that the organism should be handled under biosafety level 3 conditions because of the risk for laboratory-acquired infection. If the laboratory is not warned that melioidosis is suspected, <em>B pseudomallei</em> colonies may be mistaken for an environmental contaminant and disregarded. In addition, some commonly used commercial identification systems may misidentify the organism.</p>",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 356039,
"questionText": "Which of the following statements is <em>not</em> accurate?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 3-Year-Old Boy With Fever and Drowsiness"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1123120,
"choiceText": "<em>Burkholderia pseudomallei</em>",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1123122,
"choiceText": "<em>Salmonella enterica </em>serotype <em>Typhi</em>",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1123124,
"choiceText": "<em>Vibrio cholerae</em>",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1123126,
"choiceText": "<em>Streptococcus pneumoniae</em>",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1123128,
"choiceText": "<em>Klebsiella pneumoniae</em>",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 356035,
"questionText": "Empirical antimicrobial therapy for this patient should include which of the following bacteria?<br/><br/>\r\n\r\n<em>Hint: Note the contact with surface water and that the location is northeastern Thailand.</em>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1123130,
"choiceText": "Corticosteroid use",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1123132,
"choiceText": "Cystic fibrosis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1123134,
"choiceText": "Diabetes mellitus",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1123136,
"choiceText": "Thalassemia",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1123138,
"choiceText": "HIV",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>Around two thirds of all adult patients with melioidosis have diabetes mellitus as a risk factor,<sup type=\"ref\">[1]</sup> and in endemic areas, melioidosis may be the first presentation of diabetes. As a result, all adult survivors of melioidosis must be tested for diabetes after recovery. Other risk factors for melioidosis include immunosuppression (eg, corticosteroids, methotrexate, cancer chemotherapy), renal disease (chronic renal impairment or renal calculi), thalassemia major, excessive alcohol use, cystic fibrosis, and cancer.<sup type=\"ref\">[2]</sup> A study of over 500 patients with melioidosis found no association with HIV infection.<sup type=\"ref\">[8]</sup></p>\r\n\r\n<p>Melioidosis in children differs in numerous ways from that seen in adults. In children, the disease is usually a localized abscess, mortality is low, relapse is uncommon, and contact with soil and water are often the only risk factors.<sup type=\"ref\">[1]</sup> Around one third of all pediatric disease presents as parotitis.<sup type=\"ref\">[1]</sup></p>",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 356037,
"questionText": "Which of the following is <em>not</em> a recognized risk factor for melioidosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1123140,
"choiceText": "<em>B pseudomallei</em> is fastidious and has special nutritional requirements",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1123142,
"choiceText": "<em>B pseudomallei</em> should be handled in biosafety level 3 conditions",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1123144,
"choiceText": "<em>B pseudomallei</em> may be misidentified or ignored",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1123146,
"choiceText": "The chances of identifying <em>B pseudomallei</em> from a throat swab may be improved by using selective media",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p><em>B pseudomallei</em> easily grows on a wide range of standard media; however, when the specimen is obtained from a nonsterile site (eg, sputum or throat swab), using selective media maximizes the chances that the organism is not missed as a result of overgrowth by commensal organisms. The CDC recommends that the organism should be handled under biosafety level 3 conditions because of the risk for laboratory-acquired infection. If the laboratory is not warned that melioidosis is suspected, <em>B pseudomallei</em> colonies may be mistaken for an environmental contaminant and disregarded. In addition, some commonly used commercial identification systems may misidentify the organism.</p>",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 356039,
"questionText": "Which of the following statements is <em>not</em> accurate?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
882348 | /viewarticle/882348 | [
{
"authors": "John L. Brusch, MD",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"A 78-year-old man with overall good health, except for symptoms of moderate benign prostatic hypertrophy, presents to the emergency department after becoming unable to pass urine. While watching a football game during a cold autumn afternoon, the man took several drinks of alcohol from a flask to \"keep himself warm.\" After that point, he found himself unable to urinate and was taken to the emergency department. After a traumatic Foley catheter placement, he developed shaking chills and fever. Eventually, he developed septic shock. The patient survived this episode.",
"After a few days, the patient's Foley catheter was removed. During his hospitalization, an underlying vascular dementia became more prominent. He was then transferred to a short-term rehabilitation facility but never regained the ability to return home. He experienced fluctuating levels of delirium, and his confusion worsened."
],
"date": "July 10, 2017",
"figures": [],
"markdown": "# A 78-Year-Old Man With Delirium\n\n **Authors:** John L. Brusch, MD \n **Date:** July 10, 2017\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nA 78-year-old man with overall good health, except for symptoms of moderate benign prostatic hypertrophy, presents to the emergency department after becoming unable to pass urine. While watching a football game during a cold autumn afternoon, the man took several drinks of alcohol from a flask to \"keep himself warm.\" After that point, he found himself unable to urinate and was taken to the emergency department. After a traumatic Foley catheter placement, he developed shaking chills and fever. Eventually, he developed septic shock. The patient survived this episode.\nAfter a few days, the patient's Foley catheter was removed. During his hospitalization, an underlying vascular dementia became more prominent. He was then transferred to a short-term rehabilitation facility but never regained the ability to return home. He experienced fluctuating levels of delirium, and his confusion worsened.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 78-Year-Old Man With Delirium"
},
{
"authors": "John L. Brusch, MD",
"content": [
"Physical examination and neurologic examination findings were unremarkable, except for the patient's change in mental status. He was afebrile. A complete blood cell count and basic metabolic panel findings were normal.",
"A urine culture and urinalysis was performed. The urinalysis revealed white blood cells and was positive for leukocyte esterase and nitrite. The culture findings included 100,000 units of Klebsiella, 10,000-30,000 units of Escherichia coli, and 100,000 units of Proteus. The patient was then placed on oral ciprofloxacin. Within 18 hours of beginning the antibiotic, his confusion cleared. He was treated for a total of 7 days.",
"Three days after the ciprofloxacin was stopped, the patient experienced relapse. He also developed significant urinary incontinence. Because of the relapse in his mental status, the family demanded that ciprofloxacin be restarted. They felt that this deterioration was due to an undertreated urinary tract infection. The physician agreed.",
"The patient remained afebrile, with no significant examination findings. Urine culture and urinalysis were not repeated. No improvement in his mental status was noted 5 days into therapy. Pending the results of a urinalysis and urine culture, the physician switched the patient to intravenous gentamicin. Again, no improvement in the patient's mental status occurred.",
"The urine culture findings revealed extended-spectrum beta-lactamase Klebsiella that was resistant to gentamicin. Ertapenem was begun. Two days later, the patient developed severe diarrhea. Within 48 hours, he died of Clostridium difficile pancolitis complicated by septic shock."
],
"date": "July 10, 2017",
"figures": [],
"markdown": "# A 78-Year-Old Man With Delirium\n\n **Authors:** John L. Brusch, MD \n **Date:** July 10, 2017\n\n ## Content\n\n Physical examination and neurologic examination findings were unremarkable, except for the patient's change in mental status. He was afebrile. A complete blood cell count and basic metabolic panel findings were normal.\nA urine culture and urinalysis was performed. The urinalysis revealed white blood cells and was positive for leukocyte esterase and nitrite. The culture findings included 100,000 units of Klebsiella, 10,000-30,000 units of Escherichia coli, and 100,000 units of Proteus. The patient was then placed on oral ciprofloxacin. Within 18 hours of beginning the antibiotic, his confusion cleared. He was treated for a total of 7 days.\nThree days after the ciprofloxacin was stopped, the patient experienced relapse. He also developed significant urinary incontinence. Because of the relapse in his mental status, the family demanded that ciprofloxacin be restarted. They felt that this deterioration was due to an undertreated urinary tract infection. The physician agreed.\nThe patient remained afebrile, with no significant examination findings. Urine culture and urinalysis were not repeated. No improvement in his mental status was noted 5 days into therapy. Pending the results of a urinalysis and urine culture, the physician switched the patient to intravenous gentamicin. Again, no improvement in the patient's mental status occurred.\nThe urine culture findings revealed extended-spectrum beta-lactamase Klebsiella that was resistant to gentamicin. Ertapenem was begun. Two days later, the patient developed severe diarrhea. Within 48 hours, he died of Clostridium difficile pancolitis complicated by septic shock.\n\n ## Figures\n\n \n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1115986,
"choiceText": "Foley catheter-associated urinary tract infection",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1115988,
"choiceText": "Progression of vascular dementia \r\n",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1115990,
"choiceText": "Cystitis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1115992,
"choiceText": "Depression associated with prolonged hospitalization",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 353685,
"questionText": "Which of the following was the most likely cause of the patient's relapsing dementia? ",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 78-Year-Old Man With Delirium"
},
{
"authors": "John L. Brusch, MD",
"content": [
"This patient's condition was probably due to progression of underlying vascular dementia. Fluctuating mental status is characteristic of vascular dementia, as well as of delirium. No clinical or laboratory evidence suggests that the patient had an underlying urinary tract infection, with or without an association with the Foley catheter.",
"Cystitis, which is a lower urinary tract infection, does not typically produce delirium. Pyelonephritis, which is an upper urinary tract infection, produces fever, delirium, leukocytosis, and hypotension. Male urinary tract infections are considered complex and require 21-28 days of antibiotic treatment to help sterilize the prostate and male bladder. Finally, depression does not lead to significant changes in a patient's mental status.",
"This patient died owing to confusion involving his delirium and his asymptomatic bacteriuria, the latter of which was not responsible for his death.[1] Both of these conditions are common among older individuals. In asymptomatic bacteriuria, urinary tract pathogens are quantitatively isolated from an individual without any localized or systemic symptoms or signs of a urinary tract infection. Clinicians and family members in this case attributed an association between the patient's delirium and his asymptomatic bacteriuria, thinking that an infection was responsible for his declining mental status.",
"Asymptomatic bacteriuria does not require treatment. A Cochrane review of nine studies found no differences in development of symptomatic urinary tract infection, complications, or death between patients with asymptomatic bacteriuria who received antibiotics and those who did not.[2] No clinical benefit was associated with the treatment of asymptomatic bacteriuria in adults.",
"In women, asymptomatic bacteriuria is considered present after two consecutive specimens demonstrate > 100,000 colony-forming units/mL of the same bacterium species without symptoms. In men, a single specimen with isolation of ≥ 100,000 colony-forming units/mL of a single bacterial species is considered diagnostic."
],
"date": "July 10, 2017",
"figures": [],
"markdown": "# A 78-Year-Old Man With Delirium\n\n **Authors:** John L. Brusch, MD \n **Date:** July 10, 2017\n\n ## Content\n\n This patient's condition was probably due to progression of underlying vascular dementia. Fluctuating mental status is characteristic of vascular dementia, as well as of delirium. No clinical or laboratory evidence suggests that the patient had an underlying urinary tract infection, with or without an association with the Foley catheter.\nCystitis, which is a lower urinary tract infection, does not typically produce delirium. Pyelonephritis, which is an upper urinary tract infection, produces fever, delirium, leukocytosis, and hypotension. Male urinary tract infections are considered complex and require 21-28 days of antibiotic treatment to help sterilize the prostate and male bladder. Finally, depression does not lead to significant changes in a patient's mental status.\nThis patient died owing to confusion involving his delirium and his asymptomatic bacteriuria, the latter of which was not responsible for his death.[1] Both of these conditions are common among older individuals. In asymptomatic bacteriuria, urinary tract pathogens are quantitatively isolated from an individual without any localized or systemic symptoms or signs of a urinary tract infection. Clinicians and family members in this case attributed an association between the patient's delirium and his asymptomatic bacteriuria, thinking that an infection was responsible for his declining mental status.\nAsymptomatic bacteriuria does not require treatment. A Cochrane review of nine studies found no differences in development of symptomatic urinary tract infection, complications, or death between patients with asymptomatic bacteriuria who received antibiotics and those who did not.[2] No clinical benefit was associated with the treatment of asymptomatic bacteriuria in adults.\nIn women, asymptomatic bacteriuria is considered present after two consecutive specimens demonstrate > 100,000 colony-forming units/mL of the same bacterium species without symptoms. In men, a single specimen with isolation of ≥ 100,000 colony-forming units/mL of a single bacterial species is considered diagnostic.\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1115986,
"choiceText": "Foley catheter-associated urinary tract infection",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1115988,
"choiceText": "Progression of vascular dementia \r\n",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1115990,
"choiceText": "Cystitis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1115992,
"choiceText": "Depression associated with prolonged hospitalization",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 353685,
"questionText": "Which of the following was the most likely cause of the patient's relapsing dementia? ",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 78-Year-Old Man With Delirium"
},
{
"authors": "John L. Brusch, MD",
"content": [
"Notable prevalences of asymptomatic bacteriuria are as follows[3]:",
"Pregnant women: 11%",
"Diabetic women: 18%",
"Diabetic men: 1.5%-2%",
"Spinal cord injury patients: 70%-100%",
"Renal transplant patients: 20%-40% in the first 2 months; 0.01% after 3 months",
"Patients with short-term Foley catheter placement: 2%-7% per day",
"Women aged 65-80 years: 40%",
"Men aged 65-80 years: 21%",
"The types of organisms that cause asymptomatic bacteriuria are similar to those that are responsible for cystitis and pyelonephritis. However, the organisms in asymptomatic bacteriuria have less pathogenic potential to produce the fimbria that are necessary for the pathogens to attach to the walls of the bladder or to the pelvis of the kidney. Because they are not actively replicating, as they do in an active infection, they are relatively resistant to the bactericidal action of antibiotics. This leads to the development of increasing resistance, such as that seen in this case. It also leads to an inability to sterilize the urine.",
"Any condition that leads to decreased bladder emptying promotes asymptomatic bacteriuria. This may be due to obstruction, such as that caused by benign prostatic hypertrophy (Figure); a stone; or bladder dysfunction, such as that seen in patients with neurogenic bladder.",
"Figure.",
"Evidence of decreased mucosal defenses on the part of the host (eg, decreased toll-like receptor 4) is often present. A major consequence of the repeated use of broad-spectrum antibiotics in asymptomatic bacteriuria is the development of C difficile colitis, which was responsible for the death of this patient. C difficile infection can also occur after just one dose of antibiotics in susceptible patients.",
"The most challenging diagnostic situation is typically seen in patients who undergo Foley catheter placement. Screening for asymptomatic bacteriuria is not justified, with some exceptions, including long-term catheters placed in patients with a significant risk for pyelonephritis, urosepsis, renal stones, vesicoureteral reflux, and renal failure. If a patient requires an indwelling Foley catheter, the old catheter should be removed, and urine for culture should be obtained through a newly inserted Foley catheter. Often, removal of the catheter itself may be definitive treatment. An estimated 30% or more of Foley catheter-associated infections are actually asymptomatic bacteriuria.",
"Asymptomatic bacteriuria in patients with diabetes is usually secondary to autonomic neuropathy of the bladder. Individuals with those symptoms are likely to develop symptomatic urinary tract infection.",
"Glucose control is not impaired in patients with asymptomatic bacteriuria. Screening is not advised in these individuals, because treatment of asymptomatic bacteriuria does not reduce complications.",
"As many as 40% of asymptomatic bacteriuria cases progress to pyelonephritis. This may be associated with premature delivery and low birth weight."
],
"date": "July 10, 2017",
"figures": [
{
"caption": "Figure.",
"image_url": "https://img.medscapestatic.com/article/882/348/882348-Thumb1.jpg"
}
],
"markdown": "# A 78-Year-Old Man With Delirium\n\n **Authors:** John L. Brusch, MD \n **Date:** July 10, 2017\n\n ## Content\n\n Notable prevalences of asymptomatic bacteriuria are as follows[3]:\nPregnant women: 11%\nDiabetic women: 18%\nDiabetic men: 1.5%-2%\nSpinal cord injury patients: 70%-100%\nRenal transplant patients: 20%-40% in the first 2 months; 0.01% after 3 months\nPatients with short-term Foley catheter placement: 2%-7% per day\nWomen aged 65-80 years: 40%\nMen aged 65-80 years: 21%\nThe types of organisms that cause asymptomatic bacteriuria are similar to those that are responsible for cystitis and pyelonephritis. However, the organisms in asymptomatic bacteriuria have less pathogenic potential to produce the fimbria that are necessary for the pathogens to attach to the walls of the bladder or to the pelvis of the kidney. Because they are not actively replicating, as they do in an active infection, they are relatively resistant to the bactericidal action of antibiotics. This leads to the development of increasing resistance, such as that seen in this case. It also leads to an inability to sterilize the urine.\nAny condition that leads to decreased bladder emptying promotes asymptomatic bacteriuria. This may be due to obstruction, such as that caused by benign prostatic hypertrophy (Figure); a stone; or bladder dysfunction, such as that seen in patients with neurogenic bladder.\nFigure.\nEvidence of decreased mucosal defenses on the part of the host (eg, decreased toll-like receptor 4) is often present. A major consequence of the repeated use of broad-spectrum antibiotics in asymptomatic bacteriuria is the development of C difficile colitis, which was responsible for the death of this patient. C difficile infection can also occur after just one dose of antibiotics in susceptible patients.\nThe most challenging diagnostic situation is typically seen in patients who undergo Foley catheter placement. Screening for asymptomatic bacteriuria is not justified, with some exceptions, including long-term catheters placed in patients with a significant risk for pyelonephritis, urosepsis, renal stones, vesicoureteral reflux, and renal failure. If a patient requires an indwelling Foley catheter, the old catheter should be removed, and urine for culture should be obtained through a newly inserted Foley catheter. Often, removal of the catheter itself may be definitive treatment. An estimated 30% or more of Foley catheter-associated infections are actually asymptomatic bacteriuria.\nAsymptomatic bacteriuria in patients with diabetes is usually secondary to autonomic neuropathy of the bladder. Individuals with those symptoms are likely to develop symptomatic urinary tract infection.\nGlucose control is not impaired in patients with asymptomatic bacteriuria. Screening is not advised in these individuals, because treatment of asymptomatic bacteriuria does not reduce complications.\nAs many as 40% of asymptomatic bacteriuria cases progress to pyelonephritis. This may be associated with premature delivery and low birth weight.\n\n ## Figures\n\n **Figure.** \n \n\n\n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 78-Year-Old Man With Delirium"
},
{
"authors": "John L. Brusch, MD",
"content": [
"In patients with spinal cord injuries, treating asymptomatic bacteriuria does not improve survival or frequency of symptomatic urinary tract infections. Overall, intermittent catheterization is not associated with a benefit, because it carries a risk for bacteriuria at a rate of 1%-3% per insertion.",
"In patients who have undergone renal transplantation, screening is indicated in the immediate postoperative period, as long as 6 months after transplantation. Prophylactic antibiotics be used in the perioperative period. This approach seems to prevent the association between asymptomatic bacteriuria and transplant loss.",
"Screening for asymptomatic bacteriuria should be performed only in groups that would significantly benefit from treatment. These include patients receiving renal transplant, pregnant women, and patients undergoing urologic procedures in which significant bleeding is expected. Screening for asymptomatic bacteriuria before implanting prosthetic joints remains controversial.",
"The death of the patient in this case underscores the importance of recognizing asymptomatic bacteriuria, especially among older individuals."
],
"date": "July 10, 2017",
"figures": [],
"markdown": "# A 78-Year-Old Man With Delirium\n\n **Authors:** John L. Brusch, MD \n **Date:** July 10, 2017\n\n ## Content\n\n In patients with spinal cord injuries, treating asymptomatic bacteriuria does not improve survival or frequency of symptomatic urinary tract infections. Overall, intermittent catheterization is not associated with a benefit, because it carries a risk for bacteriuria at a rate of 1%-3% per insertion.\nIn patients who have undergone renal transplantation, screening is indicated in the immediate postoperative period, as long as 6 months after transplantation. Prophylactic antibiotics be used in the perioperative period. This approach seems to prevent the association between asymptomatic bacteriuria and transplant loss.\nScreening for asymptomatic bacteriuria should be performed only in groups that would significantly benefit from treatment. These include patients receiving renal transplant, pregnant women, and patients undergoing urologic procedures in which significant bleeding is expected. Screening for asymptomatic bacteriuria before implanting prosthetic joints remains controversial.\nThe death of the patient in this case underscores the importance of recognizing asymptomatic bacteriuria, especially among older individuals.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1115994,
"choiceText": "Bronchoscopy",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1115996,
"choiceText": "Pregnancy",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1115998,
"choiceText": "Prostatectomy",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1116000,
"choiceText": "Renal transplantation",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Screening for asymptomatic bacteriuria should be performed only in groups that would significantly benefit from treatment. These include patients receiving renal transplant, pregnant women, and patients undergoing urologic procedures in which significant bleeding is expected. Screening for asymptomatic bacteriuria before implanting prosthetic joints remains controversial.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 353687,
"questionText": "In which of the following situations is screening for asymptomatic bacteriuria <i>not</i> justified?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1116002,
"choiceText": "<i>C difficile</i> colitis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1116004,
"choiceText": "Promotion of resistance",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1116006,
"choiceText": "Renal failure",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1116008,
"choiceText": "Increased healthcare cost\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Treating asymptomatic bacteriuria promotes antibiotic resistance and the subsequent development of <i>C difficile</i> colitis, and is associated with increased healthcare costs. Renal failure is not a widely recognized negative consequence of treating asymptomatic bacteriuria.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 353689,
"questionText": "Which of the following is <i>not</i> a generally recognized negative consequence of treating asymptomatic bacteriuria?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 78-Year-Old Man With Delirium"
},
{
"authors": "John L. Brusch, MD",
"content": [],
"date": "July 10, 2017",
"figures": [],
"markdown": "# A 78-Year-Old Man With Delirium\n\n **Authors:** John L. Brusch, MD \n **Date:** July 10, 2017\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1115994,
"choiceText": "Bronchoscopy",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1115996,
"choiceText": "Pregnancy",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1115998,
"choiceText": "Prostatectomy",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1116000,
"choiceText": "Renal transplantation",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Screening for asymptomatic bacteriuria should be performed only in groups that would significantly benefit from treatment. These include patients receiving renal transplant, pregnant women, and patients undergoing urologic procedures in which significant bleeding is expected. Screening for asymptomatic bacteriuria before implanting prosthetic joints remains controversial.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 353687,
"questionText": "In which of the following situations is screening for asymptomatic bacteriuria <i>not</i> justified?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1116002,
"choiceText": "<i>C difficile</i> colitis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1116004,
"choiceText": "Promotion of resistance",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1116006,
"choiceText": "Renal failure",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1116008,
"choiceText": "Increased healthcare cost\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Treating asymptomatic bacteriuria promotes antibiotic resistance and the subsequent development of <i>C difficile</i> colitis, and is associated with increased healthcare costs. Renal failure is not a widely recognized negative consequence of treating asymptomatic bacteriuria.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 353689,
"questionText": "Which of the following is <i>not</i> a generally recognized negative consequence of treating asymptomatic bacteriuria?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 78-Year-Old Man With Delirium"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1115986,
"choiceText": "Foley catheter-associated urinary tract infection",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1115988,
"choiceText": "Progression of vascular dementia \r\n",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1115990,
"choiceText": "Cystitis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1115992,
"choiceText": "Depression associated with prolonged hospitalization",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 353685,
"questionText": "Which of the following was the most likely cause of the patient's relapsing dementia? ",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1115994,
"choiceText": "Bronchoscopy",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1115996,
"choiceText": "Pregnancy",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1115998,
"choiceText": "Prostatectomy",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1116000,
"choiceText": "Renal transplantation",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Screening for asymptomatic bacteriuria should be performed only in groups that would significantly benefit from treatment. These include patients receiving renal transplant, pregnant women, and patients undergoing urologic procedures in which significant bleeding is expected. Screening for asymptomatic bacteriuria before implanting prosthetic joints remains controversial.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 353687,
"questionText": "In which of the following situations is screening for asymptomatic bacteriuria <i>not</i> justified?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1116002,
"choiceText": "<i>C difficile</i> colitis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1116004,
"choiceText": "Promotion of resistance",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1116006,
"choiceText": "Renal failure",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1116008,
"choiceText": "Increased healthcare cost\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Treating asymptomatic bacteriuria promotes antibiotic resistance and the subsequent development of <i>C difficile</i> colitis, and is associated with increased healthcare costs. Renal failure is not a widely recognized negative consequence of treating asymptomatic bacteriuria.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 353689,
"questionText": "Which of the following is <i>not</i> a generally recognized negative consequence of treating asymptomatic bacteriuria?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
881806 | /viewarticle/881806 | [
{
"authors": "Sumaira Nabi, MBBS, FCPS; Sadaf Fayyaz, MBBS; Shahzad Ahmed, MBBS",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"A 49-year-old man presents with a 1-year history of progressive memory loss and forgetfulness. His wife also describes progressive gait impairment; brief, repetitive, generalized jerky movements, with clumsiness using his arms; slurring of speech; and emotional lability for 6 months. He depends on caregivers for most activities of daily living. At times, he wanders off and cannot find his way back to his room.",
"He has no history of fever, loss of consciousness, visual loss, sphincter dysfunction, or sensory symptoms. He is a nonsmoker and denies substance abuse. He has no history of travel abroad or extramarital relationships. His medical and surgical history are unremarkable, and his family history is noncontributory."
],
"date": "June 27, 2017",
"figures": [],
"markdown": "# A 49-Year-Old Man With Forgetfulness and Gait Impairment\n\n **Authors:** Sumaira Nabi, MBBS, FCPS; Sadaf Fayyaz, MBBS; Shahzad Ahmed, MBBS \n **Date:** June 27, 2017\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nA 49-year-old man presents with a 1-year history of progressive memory loss and forgetfulness. His wife also describes progressive gait impairment; brief, repetitive, generalized jerky movements, with clumsiness using his arms; slurring of speech; and emotional lability for 6 months. He depends on caregivers for most activities of daily living. At times, he wanders off and cannot find his way back to his room.\nHe has no history of fever, loss of consciousness, visual loss, sphincter dysfunction, or sensory symptoms. He is a nonsmoker and denies substance abuse. He has no history of travel abroad or extramarital relationships. His medical and surgical history are unremarkable, and his family history is noncontributory.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 49-Year-Old Man With Forgetfulness and Gait Impairment"
},
{
"authors": "Sumaira Nabi, MBBS, FCPS; Sadaf Fayyaz, MBBS; Shahzad Ahmed, MBBS",
"content": [
"Upon clinical examination, the patient is agitated and restless, with generalized myoclonic jerks. His vital signs include an oral temperature of 98.6°F, a regular pulse of 70 beats/min, and a blood pressure of 120/70 mm Hg. His respiratory rate is 14 breaths/min.",
"Upon neurologic examination, the patient is conscious but confused and disoriented to time, place, and person. His speech is limited to brief, incoherent sentences, and he has dysarthria. He scores 14 out of 30 on the Mini-Mental State Examination.",
"The patient has bilateral jerky horizontal nystagmus; however, his cranial nerves are unremarkable. Limb ataxia with titubation is noted. His gait is broad-based and clumsy, with a tendency to fall. Spasticity is noted, along with hyperreflexia and extensor plantar responses. His abdomen is soft and nontender. No organomegaly or ascites is noted. His bowel sounds are audible. The patient's precordial examination reveals normal heart sounds. Auscultation of the lung fields reveals normal vesicular breathing.",
"The laboratory analysis demonstrates a complete blood cell count and erythrocyte sedimentation rate within the reference range. His liver function test results, renal function test results, serum glucose levels, urinalysis findings, ECG findings, and chest radiography findings are unremarkable. Kayser-Fleischer rings are not visualized on slit-lamp examination. HIV serology is negative.",
"Examination of the cerebrospinal fluid (CSF) shows normal protein, glucose, and cell count, and CSF oligoclonal bands are negative. EEG shows diffuse low voltage with slowing. Polyspikes are also noted. MRI of the brain with contrast and diffusion-weighted (DW) imaging are performed, revealing gross cerebral atrophy with asymmetric areas of diffusion restriction in the cerebral cortex (ribbon-like appearance) and left basal ganglia (Figures 1 and 2).",
"Figure 1.",
"Figure 2."
],
"date": "June 27, 2017",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/881/806/881806-Thumb1.png"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/881/806/881806-Thumb2.png"
}
],
"markdown": "# A 49-Year-Old Man With Forgetfulness and Gait Impairment\n\n **Authors:** Sumaira Nabi, MBBS, FCPS; Sadaf Fayyaz, MBBS; Shahzad Ahmed, MBBS \n **Date:** June 27, 2017\n\n ## Content\n\n Upon clinical examination, the patient is agitated and restless, with generalized myoclonic jerks. His vital signs include an oral temperature of 98.6°F, a regular pulse of 70 beats/min, and a blood pressure of 120/70 mm Hg. His respiratory rate is 14 breaths/min.\nUpon neurologic examination, the patient is conscious but confused and disoriented to time, place, and person. His speech is limited to brief, incoherent sentences, and he has dysarthria. He scores 14 out of 30 on the Mini-Mental State Examination.\nThe patient has bilateral jerky horizontal nystagmus; however, his cranial nerves are unremarkable. Limb ataxia with titubation is noted. His gait is broad-based and clumsy, with a tendency to fall. Spasticity is noted, along with hyperreflexia and extensor plantar responses. His abdomen is soft and nontender. No organomegaly or ascites is noted. His bowel sounds are audible. The patient's precordial examination reveals normal heart sounds. Auscultation of the lung fields reveals normal vesicular breathing.\nThe laboratory analysis demonstrates a complete blood cell count and erythrocyte sedimentation rate within the reference range. His liver function test results, renal function test results, serum glucose levels, urinalysis findings, ECG findings, and chest radiography findings are unremarkable. Kayser-Fleischer rings are not visualized on slit-lamp examination. HIV serology is negative.\nExamination of the cerebrospinal fluid (CSF) shows normal protein, glucose, and cell count, and CSF oligoclonal bands are negative. EEG shows diffuse low voltage with slowing. Polyspikes are also noted. MRI of the brain with contrast and diffusion-weighted (DW) imaging are performed, revealing gross cerebral atrophy with asymmetric areas of diffusion restriction in the cerebral cortex (ribbon-like appearance) and left basal ganglia (Figures 1 and 2).\nFigure 1.\nFigure 2.\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1115070,
"choiceText": "Wilson disease",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1115072,
"choiceText": "Subacute sclerosing panencephalitis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1115074,
"choiceText": "Creutzfeldt-Jakob disease",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1115076,
"choiceText": "Acute disseminated encephalomyelitis",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 353425,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": true,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 49-Year-Old Man With Forgetfulness and Gait Impairment"
},
{
"authors": "Sumaira Nabi, MBBS, FCPS; Sadaf Fayyaz, MBBS; Shahzad Ahmed, MBBS",
"content": [
"The patient in this case was diagnosed as having sporadic Creutzfeldt-Jakob disease (sCJD) on the basis of his clinical presentation and typical MRI brain findings. No history of human hormone supplement ingestion is noted. The facility for testing CSF for 14-3-3 protein is not available in Pakistan, where this patient presented. Brain biopsy is conclusive in such cases but was not performed, owing to an elevated risk for disease transmission and lack of family consent.",
"The patient was prescribed symptomatic medication for spasticity and myoclonic jerks and was sent home for rehabilitation. The prognosis and course of disease was explained to the family in detail.",
"CJD is a chronic progressive neurodegenerative disorder and is one of the human prion diseases.[1,2] It was first described by Creutzfeldt and Jakob in the early 1920s. It is a uniformly fatal disease, with an annual incidence rate of 1-2 cases per million population worldwide. CJD accounts for 85% of all human prion diseases.",
"The infectious agent in CJD is a prion that is composed mainly or entirely of an abnormal conformation of a host-encoded glycoprotein called the \"prion protein.\" Its replication involves the recruitment of normally expressed prion protein, which mainly has an alpha-helical structure, into a disease-specific molecule that is rich in beta sheet.",
"CJD principally affects the gray matter of the cerebral cortex, the brainstem, and the molecular layer of the cerebellum.[3] Owing to abnormal prion protein accumulation in the brain, CJD is characterized by spongiform change, neuronal loss, and gliosis. In addition, amyloid protein is detected in cerebral cortex and cerebellum in 10% cases of CJD. These amyloid plaques are unlike Alzheimer disease, because CJD-related plaques are immunoreactive with antibodies directed against prion proteins.",
"Prion disease is transmitted via peripheral routes, either orally or transcutaneously. The prion then replicates in the spleen and peripheral lymph nodes. Only the variant form of CJD is transmissible via blood transfusion from human to human. After peripheral replication, hematogenous spread to the central nervous system occurs and depends on the presence of B lymphocytes. Prions can also reach the central nervous system via vagus parasympathetic nerves.[4,5,6] Familial CJD is rare and is inherited as a mutation in the human prion protein gene (PRNP) on chromosome 20.",
"There are four types of CJD. The most common form is sCJD, which accounts for 85%-90% of the total cases; the others are familial, iatrogenic, and variant forms.[7,8] The mean age of onset in sCJD is 65 years, and most cases occur between age 60 and 80 years.[9] Cases of sCJD in patients younger than 30 years or older than 80 years are rare.[7,8] The etiology of CJD is still unknown, and both genders are almost equally affected.",
"Its clinical features mainly include a rapidly progressive dementia; psychiatric symptoms; and multifocal neurologic findings, such as myoclonus, visual disturbances, and cerebellar and pyramidal/extrapyramidal signs. The disease follows a rapid course, with progression of cognitive and functional impairment toward akinetic mutism in the late stage. Eventually, death occurs, most often within 12 months of the disease onset.[9,10,11]",
"Myoclonus in CJD, especially provoked by startle, is present in more than 90% of patients at some point during their illness; however, it may be absent at presentation, even when dementia is very profound. sCJD should always be considered in the patients who have rapidly progressive dementia with myoclonus."
],
"date": "June 27, 2017",
"figures": [],
"markdown": "# A 49-Year-Old Man With Forgetfulness and Gait Impairment\n\n **Authors:** Sumaira Nabi, MBBS, FCPS; Sadaf Fayyaz, MBBS; Shahzad Ahmed, MBBS \n **Date:** June 27, 2017\n\n ## Content\n\n The patient in this case was diagnosed as having sporadic Creutzfeldt-Jakob disease (sCJD) on the basis of his clinical presentation and typical MRI brain findings. No history of human hormone supplement ingestion is noted. The facility for testing CSF for 14-3-3 protein is not available in Pakistan, where this patient presented. Brain biopsy is conclusive in such cases but was not performed, owing to an elevated risk for disease transmission and lack of family consent.\nThe patient was prescribed symptomatic medication for spasticity and myoclonic jerks and was sent home for rehabilitation. The prognosis and course of disease was explained to the family in detail.\nCJD is a chronic progressive neurodegenerative disorder and is one of the human prion diseases.[1,2] It was first described by Creutzfeldt and Jakob in the early 1920s. It is a uniformly fatal disease, with an annual incidence rate of 1-2 cases per million population worldwide. CJD accounts for 85% of all human prion diseases.\nThe infectious agent in CJD is a prion that is composed mainly or entirely of an abnormal conformation of a host-encoded glycoprotein called the \"prion protein.\" Its replication involves the recruitment of normally expressed prion protein, which mainly has an alpha-helical structure, into a disease-specific molecule that is rich in beta sheet.\nCJD principally affects the gray matter of the cerebral cortex, the brainstem, and the molecular layer of the cerebellum.[3] Owing to abnormal prion protein accumulation in the brain, CJD is characterized by spongiform change, neuronal loss, and gliosis. In addition, amyloid protein is detected in cerebral cortex and cerebellum in 10% cases of CJD. These amyloid plaques are unlike Alzheimer disease, because CJD-related plaques are immunoreactive with antibodies directed against prion proteins.\nPrion disease is transmitted via peripheral routes, either orally or transcutaneously. The prion then replicates in the spleen and peripheral lymph nodes. Only the variant form of CJD is transmissible via blood transfusion from human to human. After peripheral replication, hematogenous spread to the central nervous system occurs and depends on the presence of B lymphocytes. Prions can also reach the central nervous system via vagus parasympathetic nerves.[4,5,6] Familial CJD is rare and is inherited as a mutation in the human prion protein gene (PRNP) on chromosome 20.\nThere are four types of CJD. The most common form is sCJD, which accounts for 85%-90% of the total cases; the others are familial, iatrogenic, and variant forms.[7,8] The mean age of onset in sCJD is 65 years, and most cases occur between age 60 and 80 years.[9] Cases of sCJD in patients younger than 30 years or older than 80 years are rare.[7,8] The etiology of CJD is still unknown, and both genders are almost equally affected.\nIts clinical features mainly include a rapidly progressive dementia; psychiatric symptoms; and multifocal neurologic findings, such as myoclonus, visual disturbances, and cerebellar and pyramidal/extrapyramidal signs. The disease follows a rapid course, with progression of cognitive and functional impairment toward akinetic mutism in the late stage. Eventually, death occurs, most often within 12 months of the disease onset.[9,10,11]\nMyoclonus in CJD, especially provoked by startle, is present in more than 90% of patients at some point during their illness; however, it may be absent at presentation, even when dementia is very profound. sCJD should always be considered in the patients who have rapidly progressive dementia with myoclonus.\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1115070,
"choiceText": "Wilson disease",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1115072,
"choiceText": "Subacute sclerosing panencephalitis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1115074,
"choiceText": "Creutzfeldt-Jakob disease",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1115076,
"choiceText": "Acute disseminated encephalomyelitis",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 353425,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": true,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 49-Year-Old Man With Forgetfulness and Gait Impairment"
},
{
"authors": "Sumaira Nabi, MBBS, FCPS; Sadaf Fayyaz, MBBS; Shahzad Ahmed, MBBS",
"content": [
"Extrapyramidal signs, such as hypokinesia, rigidity, dystonia, and cerebellar manifestations (eg, nystagmus, ataxia), occur in approximately two thirds of patients and are the presenting symptoms in 20%-40%.[12] Corticospinal long tract involvement develops in 40%-80% of patients, including such findings as hyperreflexia, extensor plantar responses (Babinski sign), and spasticity. Iatrogenic CJD, which is related to human gonadotropin, human growth hormone treatment, and dura mater grafts, has a predisposition to manifest as an isolated cerebellar syndrome early during the disease course.[13,14,15,16]",
"All treatable causes of dementia should be ruled out during investigations; these include herpes encephalitis, Hashimoto encephalitis, and steroid-responsive encephalopathy with autoimmune thyroiditis. In addition, diffuse Lewy body disease, chronic meningitis, paraneoplastic syndromes, lithium poisoning, dementia of motor neuron disease, and HIV encephalopathy are included in the differential diagnosis. The workup includes laboratory tests for dementia to rule out other causes, such as toxic or metabolic encephalopathies.",
"In CJD, periodic and pseudoperiodic spikes and waves discharges are observed on EEG with slow background.[17,18] Observations of altered signal in the cerebral cortex and basal ganglia during DW-MRI, and detection of protein 14-3-3 in the CSF, help to confirm the diagnosis of CJD.[19,20,21,22] CSF findings are typically normal, with normal opening pressure in CJD; however, CSF protein may be slightly elevated (not more than 100 mg/dL).",
"Brain biopsy or autopsy is required for a definitive diagnosis of CJD. Brain biopsy, although conclusive in such cases, is usually not performed, owing to an elevated risk for disease transmission.",
"DW-MRI and fluid-attenuated inversion recovery (FLAIR) reveal hyperintense signal in the cortical ribbons, basal ganglia, and thalamus. Two characteristic signs have been described on MRI: the \"hockey stick\" sign, which shows hyperintense signal in the putamen and head of caudate nucleus, and the pulvinar sign, which shows hyperintensity in bilateral pulvinar nuclei of thalamus.",
"MRI has played an increasingly important role in the diagnosis of CJD. Studies on analysis of MRI sequences in CJD have concluded that proton density (PD)-weighted images and DW imaging are superior in identifying signal intensity changes in the basal ganglia compared with T2-weighted or FLAIR images.[23,24,25,26,27,28,29] DW-MRI is also clearly superior in detecting cortical changes. The studies concluded that DW imaging is the most sensitive MRI technique in the diagnosis of CJD."
],
"date": "June 27, 2017",
"figures": [],
"markdown": "# A 49-Year-Old Man With Forgetfulness and Gait Impairment\n\n **Authors:** Sumaira Nabi, MBBS, FCPS; Sadaf Fayyaz, MBBS; Shahzad Ahmed, MBBS \n **Date:** June 27, 2017\n\n ## Content\n\n Extrapyramidal signs, such as hypokinesia, rigidity, dystonia, and cerebellar manifestations (eg, nystagmus, ataxia), occur in approximately two thirds of patients and are the presenting symptoms in 20%-40%.[12] Corticospinal long tract involvement develops in 40%-80% of patients, including such findings as hyperreflexia, extensor plantar responses (Babinski sign), and spasticity. Iatrogenic CJD, which is related to human gonadotropin, human growth hormone treatment, and dura mater grafts, has a predisposition to manifest as an isolated cerebellar syndrome early during the disease course.[13,14,15,16]\nAll treatable causes of dementia should be ruled out during investigations; these include herpes encephalitis, Hashimoto encephalitis, and steroid-responsive encephalopathy with autoimmune thyroiditis. In addition, diffuse Lewy body disease, chronic meningitis, paraneoplastic syndromes, lithium poisoning, dementia of motor neuron disease, and HIV encephalopathy are included in the differential diagnosis. The workup includes laboratory tests for dementia to rule out other causes, such as toxic or metabolic encephalopathies.\nIn CJD, periodic and pseudoperiodic spikes and waves discharges are observed on EEG with slow background.[17,18] Observations of altered signal in the cerebral cortex and basal ganglia during DW-MRI, and detection of protein 14-3-3 in the CSF, help to confirm the diagnosis of CJD.[19,20,21,22] CSF findings are typically normal, with normal opening pressure in CJD; however, CSF protein may be slightly elevated (not more than 100 mg/dL).\nBrain biopsy or autopsy is required for a definitive diagnosis of CJD. Brain biopsy, although conclusive in such cases, is usually not performed, owing to an elevated risk for disease transmission.\nDW-MRI and fluid-attenuated inversion recovery (FLAIR) reveal hyperintense signal in the cortical ribbons, basal ganglia, and thalamus. Two characteristic signs have been described on MRI: the \"hockey stick\" sign, which shows hyperintense signal in the putamen and head of caudate nucleus, and the pulvinar sign, which shows hyperintensity in bilateral pulvinar nuclei of thalamus.\nMRI has played an increasingly important role in the diagnosis of CJD. Studies on analysis of MRI sequences in CJD have concluded that proton density (PD)-weighted images and DW imaging are superior in identifying signal intensity changes in the basal ganglia compared with T2-weighted or FLAIR images.[23,24,25,26,27,28,29] DW-MRI is also clearly superior in detecting cortical changes. The studies concluded that DW imaging is the most sensitive MRI technique in the diagnosis of CJD.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 49-Year-Old Man With Forgetfulness and Gait Impairment"
},
{
"authors": "Sumaira Nabi, MBBS, FCPS; Sadaf Fayyaz, MBBS; Shahzad Ahmed, MBBS",
"content": [
"According to the World Health Organization (WHO), the following are the criteria for definite sCJD[30]",
"Diagnosed by standard neuropathologic techniques",
"and/or immunocytochemically",
"and/or Western blot-confirmed protease-resistant prion protein",
"and/or presence of scrapie-associated fibrils",
"Criteria for probable sCJD are as follows:",
"Progressive dementia",
"At least two of the following four symptoms:",
"Myoclonus",
"Pyramidal/extrapyramidal symptoms",
"Visual or cerebellar symptoms",
"Akinetic mutism",
"Positive EEG (periodic epileptiform discharges) findings and/or a positive 14-3-3 protein result and < 2-year disease duration",
"No alternative diagnosis suggested",
"Criteria for possible sCJD are as follows:",
"Progressive dementia",
"At least two of the following four symptoms:",
"Myoclonus",
"Pyramidal/extrapyramidal symptoms",
"Visual or cerebellar symptoms",
"Akinetic mutism",
"No supportive EEG findings",
"Researchers believe that DW-MRI should be appropriately considered among the diagnostic tests because it is noninvasive and has higher sensitivity and specificity than biopsy.[23,24,25,26,27,28,29]",
"Treatment of prion diseases remains supportive; no specific therapy has been shown to stop the progression of these diseases. Antidementia drugs should be avoided. Pentosan polysulfate, flupirtine, and doxycycline are being investigated for treatment of CJD. The prognosis is dismal; it is an invariably fatal disease, and approximately 90% patients die within 1 year of symptom onset.[31]"
],
"date": "June 27, 2017",
"figures": [],
"markdown": "# A 49-Year-Old Man With Forgetfulness and Gait Impairment\n\n **Authors:** Sumaira Nabi, MBBS, FCPS; Sadaf Fayyaz, MBBS; Shahzad Ahmed, MBBS \n **Date:** June 27, 2017\n\n ## Content\n\n According to the World Health Organization (WHO), the following are the criteria for definite sCJD[30]\nDiagnosed by standard neuropathologic techniques\nand/or immunocytochemically\nand/or Western blot-confirmed protease-resistant prion protein\nand/or presence of scrapie-associated fibrils\nCriteria for probable sCJD are as follows:\nProgressive dementia\nAt least two of the following four symptoms:\nMyoclonus\nPyramidal/extrapyramidal symptoms\nVisual or cerebellar symptoms\nAkinetic mutism\nPositive EEG (periodic epileptiform discharges) findings and/or a positive 14-3-3 protein result and < 2-year disease duration\nNo alternative diagnosis suggested\nCriteria for possible sCJD are as follows:\nProgressive dementia\nAt least two of the following four symptoms:\nMyoclonus\nPyramidal/extrapyramidal symptoms\nVisual or cerebellar symptoms\nAkinetic mutism\nNo supportive EEG findings\nResearchers believe that DW-MRI should be appropriately considered among the diagnostic tests because it is noninvasive and has higher sensitivity and specificity than biopsy.[23,24,25,26,27,28,29]\nTreatment of prion diseases remains supportive; no specific therapy has been shown to stop the progression of these diseases. Antidementia drugs should be avoided. Pentosan polysulfate, flupirtine, and doxycycline are being investigated for treatment of CJD. The prognosis is dismal; it is an invariably fatal disease, and approximately 90% patients die within 1 year of symptom onset.[31]\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1115086,
"choiceText": "Sporadic",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1115088,
"choiceText": "Variant",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1115090,
"choiceText": "Zoonotic",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1115092,
"choiceText": "Familial",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1115094,
"choiceText": "Iatrogenic",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Multiple forms of CJD are recognized. sCJD is the most commonly documented form (85% of cases). Other forms of CJD include variant, familial, and iatrogenic CJD.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 353429,
"questionText": "Which of the following is <em>not</em> a recognized form of CJD?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": true,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1115096,
"choiceText": "<em>TSC1</em> gene on chromosome 9",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1115098,
"choiceText": "<em>TCS2</em> gene on chromosome 16",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1115100,
"choiceText": "<em>NF2</em> gene on chromosome 22",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1115102,
"choiceText": "<em>PRNP</em> gene on chromosome 20",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1115104,
"choiceText": "<em>HTT</em> gene on chromosome 4",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Familial CJD is rare and is inherited as a mutation in the human prion protein gene (<em>PRNP</em>) on chromosome 20.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 353431,
"questionText": "Which of the following genes is implicated in the pathogenesis of familial CJD?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": true,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 49-Year-Old Man With Forgetfulness and Gait Impairment"
},
{
"authors": "Sumaira Nabi, MBBS, FCPS; Sadaf Fayyaz, MBBS; Shahzad Ahmed, MBBS",
"content": [],
"date": "June 27, 2017",
"figures": [],
"markdown": "# A 49-Year-Old Man With Forgetfulness and Gait Impairment\n\n **Authors:** Sumaira Nabi, MBBS, FCPS; Sadaf Fayyaz, MBBS; Shahzad Ahmed, MBBS \n **Date:** June 27, 2017\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1115086,
"choiceText": "Sporadic",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1115088,
"choiceText": "Variant",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1115090,
"choiceText": "Zoonotic",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1115092,
"choiceText": "Familial",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1115094,
"choiceText": "Iatrogenic",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Multiple forms of CJD are recognized. sCJD is the most commonly documented form (85% of cases). Other forms of CJD include variant, familial, and iatrogenic CJD.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 353429,
"questionText": "Which of the following is <em>not</em> a recognized form of CJD?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": true,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1115096,
"choiceText": "<em>TSC1</em> gene on chromosome 9",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1115098,
"choiceText": "<em>TCS2</em> gene on chromosome 16",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1115100,
"choiceText": "<em>NF2</em> gene on chromosome 22",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1115102,
"choiceText": "<em>PRNP</em> gene on chromosome 20",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1115104,
"choiceText": "<em>HTT</em> gene on chromosome 4",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Familial CJD is rare and is inherited as a mutation in the human prion protein gene (<em>PRNP</em>) on chromosome 20.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 353431,
"questionText": "Which of the following genes is implicated in the pathogenesis of familial CJD?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": true,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 49-Year-Old Man With Forgetfulness and Gait Impairment"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1115070,
"choiceText": "Wilson disease",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1115072,
"choiceText": "Subacute sclerosing panencephalitis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1115074,
"choiceText": "Creutzfeldt-Jakob disease",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1115076,
"choiceText": "Acute disseminated encephalomyelitis",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 353425,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": true,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1115086,
"choiceText": "Sporadic",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1115088,
"choiceText": "Variant",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1115090,
"choiceText": "Zoonotic",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1115092,
"choiceText": "Familial",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1115094,
"choiceText": "Iatrogenic",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Multiple forms of CJD are recognized. sCJD is the most commonly documented form (85% of cases). Other forms of CJD include variant, familial, and iatrogenic CJD.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 353429,
"questionText": "Which of the following is <em>not</em> a recognized form of CJD?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": true,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1115096,
"choiceText": "<em>TSC1</em> gene on chromosome 9",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1115098,
"choiceText": "<em>TCS2</em> gene on chromosome 16",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1115100,
"choiceText": "<em>NF2</em> gene on chromosome 22",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1115102,
"choiceText": "<em>PRNP</em> gene on chromosome 20",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1115104,
"choiceText": "<em>HTT</em> gene on chromosome 4",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Familial CJD is rare and is inherited as a mutation in the human prion protein gene (<em>PRNP</em>) on chromosome 20.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 353431,
"questionText": "Which of the following genes is implicated in the pathogenesis of familial CJD?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": true,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
881370 | /viewarticle/881370 | [
{
"authors": "Prasenjit Das, MD, MBBS; Elanthenral Sigamani, MD",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"A 13-year-old boy presents to the orthopedic outpatient department with a 1.5-year history of mild, recurrent pain in his left lower leg, with recent exacerbation of the pain after a trivial trauma during outdoor activity. According to the patient, he has been feeling mild fullness over the left medial malleolus for more than 1 year, without it causing much pain. He has no history of systemic symptoms, such as fever, or any other significant medical history. He also has no significant family medical history."
],
"date": "June 13, 2017",
"figures": [],
"markdown": "# A 13-Year-Old Boy With Recurrent Lower Leg Pain\n\n **Authors:** Prasenjit Das, MD, MBBS; Elanthenral Sigamani, MD \n **Date:** June 13, 2017\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nA 13-year-old boy presents to the orthopedic outpatient department with a 1.5-year history of mild, recurrent pain in his left lower leg, with recent exacerbation of the pain after a trivial trauma during outdoor activity. According to the patient, he has been feeling mild fullness over the left medial malleolus for more than 1 year, without it causing much pain. He has no history of systemic symptoms, such as fever, or any other significant medical history. He also has no significant family medical history.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 13-Year-Old Boy With Recurrent Lower Leg Pain"
},
{
"authors": "Prasenjit Das, MD, MBBS; Elanthenral Sigamani, MD",
"content": [
"The patient is a well-built, athletic-appearing boy. His blood pressure is 116/78 mm Hg, his pulse rate is 68 beats/min, and his body temperature is measured at 98.2°F during his first visit to the orthopedic outpatient department.",
"Examination of the leg reveals severe localized tenderness over the lower left tibial prominence, with soft-tissue swelling and edema. The overlying skin shows mild reddening as well as increased local temperature. The cardiovascular, respiratory, and gastrointestinal organs examined are clinically within normal physiologic limits.",
"The hematologic and biochemical parameters examined are within normal physiologic limits. The erythrocyte sedimentation rate and C-reactive protein levels are also within normal physiologic limits. The hematologic examination shows a hemoglobin level of 11 g/dL, total leukocyte count of 13,000 ×103 cells/μL, and platelet count of 180 ×103 cells/μL.",
"Anteroposterior chest radiography does not show any localized lesions in the lungs. No lymphadenopathy is detected. Anteroposterior-view and lateral-view radiography of the lower left end of the tibia show an eccentrically located, well-demarcated, lytic, bubbly, lobulated lesion in the metaphyseal region (Figure 1). A periosteal reaction is not seen. Focal epiphyseal extension is also noted (Figure 2). A subtle fracture is identified at the lateral margin of the lower tibia (Figures 1 and 2). Axial T2-weighted MRI also shows a hyperintense, solid, lobulated growth (Figure 3).",
"Figure 1.",
"Figure 2.",
"Figure 3.",
"A biopsy is performed, but unfortunately the yield is scant and is composed only of fibrous tissue and traces of cartilaginous material. Curetting and bone grafting is performed, with the corresponding histopathologic examination demonstrating a characteristic biphasic lesion (Figure 4). A frozen section prepared from the curettage shows a possible benign cartilaginous tumor.",
"Figure 4."
],
"date": "June 13, 2017",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/881/370/881370-Thumb1.png"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/881/370/881370-Thumb2.png"
},
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/881/370/881370-Thumb3.png"
},
{
"caption": "Figure 4.",
"image_url": "https://img.medscapestatic.com/article/881/370/881370-Thumb4.png"
}
],
"markdown": "# A 13-Year-Old Boy With Recurrent Lower Leg Pain\n\n **Authors:** Prasenjit Das, MD, MBBS; Elanthenral Sigamani, MD \n **Date:** June 13, 2017\n\n ## Content\n\n The patient is a well-built, athletic-appearing boy. His blood pressure is 116/78 mm Hg, his pulse rate is 68 beats/min, and his body temperature is measured at 98.2°F during his first visit to the orthopedic outpatient department.\nExamination of the leg reveals severe localized tenderness over the lower left tibial prominence, with soft-tissue swelling and edema. The overlying skin shows mild reddening as well as increased local temperature. The cardiovascular, respiratory, and gastrointestinal organs examined are clinically within normal physiologic limits.\nThe hematologic and biochemical parameters examined are within normal physiologic limits. The erythrocyte sedimentation rate and C-reactive protein levels are also within normal physiologic limits. The hematologic examination shows a hemoglobin level of 11 g/dL, total leukocyte count of 13,000 ×103 cells/μL, and platelet count of 180 ×103 cells/μL.\nAnteroposterior chest radiography does not show any localized lesions in the lungs. No lymphadenopathy is detected. Anteroposterior-view and lateral-view radiography of the lower left end of the tibia show an eccentrically located, well-demarcated, lytic, bubbly, lobulated lesion in the metaphyseal region (Figure 1). A periosteal reaction is not seen. Focal epiphyseal extension is also noted (Figure 2). A subtle fracture is identified at the lateral margin of the lower tibia (Figures 1 and 2). Axial T2-weighted MRI also shows a hyperintense, solid, lobulated growth (Figure 3).\nFigure 1.\nFigure 2.\nFigure 3.\nA biopsy is performed, but unfortunately the yield is scant and is composed only of fibrous tissue and traces of cartilaginous material. Curetting and bone grafting is performed, with the corresponding histopathologic examination demonstrating a characteristic biphasic lesion (Figure 4). A frozen section prepared from the curettage shows a possible benign cartilaginous tumor.\nFigure 4.\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n**Figure 3.** \n \n\n**Figure 4.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1103458,
"choiceText": "Chondromyxoid fibroma",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1103460,
"choiceText": "Giant cell tumor of bone",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1103462,
"choiceText": "Aneurysmal bone cyst",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1103464,
"choiceText": "Chondrosarcoma",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 349689,
"questionText": "Which of the following is the most likely diagnosis?<br/><br/>\r\n<em>Hint: The patient is a 13-year-old boy with a well-circumscribed, lytic, bubbly lesion with characteristic histologic findings.</em>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 13-Year-Old Boy With Recurrent Lower Leg Pain"
},
{
"authors": "Prasenjit Das, MD, MBBS; Elanthenral Sigamani, MD",
"content": [
"Radiography and MRI of the left lower tibia demonstrated features of a chondromyxoid fibroma (CMF), with fracture of the lower end of the tibia. Grossly, the specimen demonstrated multiple gray-white fragments, with focally identifiable firm periosteal bone attached over the fragments. The cut surface of the fragments showed a shiny myxoid appearance with intervening firm gray-white areas.",
"Histopathologic examination showed a lobulated lesion. The lobules of chondromyxoid materials were separated by thin cellular septae composed of innocuous-looking spindle cells, with interspersed small vessels and few osteoclastic giant cells. The chondromyxoid component showed varying cellularity. The periphery of the lobules was more cellular in appearance, and the cells were predominantly spindle in shape.",
"In the center, the cells had a moderate amount of eosinophilic cytoplasm, whereas a few others were stellate-shaped. Chondrocytes were also identified. No evidence of multinucleation within the lacunae or significant nuclear pleomorphism was noted. The spindle-cell component also did not show any pleomorphism or mitotic activity (Figure 4). These features, correlated with the radiologic findings, were indicative of CMF.",
"Figure 4.",
"CMF is a benign tumor of bone that was first described by Jaffe and Lichtenstein in 1948.[1,2] This lesion accounts for 1% of all bone tumors and 2% of all benign bone tumors.[3] It is most commonly found in the long tubular bones, especially the tibia and femur near the knee joint; however, the overall most common site is the proximal tibia, which is affected in 30% of cases. Approximately 25% of cases occur in the flat bones.[3,4] The small bones of the lower limbs may also be affected. The skull, spine, and bones of the upper extremity are relatively uncommon sites for this lesion.",
"Patients with this condition range from 3 to 70 years of age; however, most cases are seen in patients aged 10-30 years. A male predominance has been described (male-to-female ratio, 2:1).[3,5] The most common presenting symptoms are pain and swelling, which are noted in 85% and 65% of cases, respectively; the third most common symptom is restriction in joint movement. The pain is usually mild, transient, and of long duration.[6] Approximately 15% of cases are discovered incidentally on radiologic examination. Pathologic fracture may occur in 5% of cases, causing significant morbidity."
],
"date": "June 13, 2017",
"figures": [
{
"caption": "Figure 4.",
"image_url": "https://img.medscapestatic.com/article/881/370/881370-Thumb4.png"
}
],
"markdown": "# A 13-Year-Old Boy With Recurrent Lower Leg Pain\n\n **Authors:** Prasenjit Das, MD, MBBS; Elanthenral Sigamani, MD \n **Date:** June 13, 2017\n\n ## Content\n\n Radiography and MRI of the left lower tibia demonstrated features of a chondromyxoid fibroma (CMF), with fracture of the lower end of the tibia. Grossly, the specimen demonstrated multiple gray-white fragments, with focally identifiable firm periosteal bone attached over the fragments. The cut surface of the fragments showed a shiny myxoid appearance with intervening firm gray-white areas.\nHistopathologic examination showed a lobulated lesion. The lobules of chondromyxoid materials were separated by thin cellular septae composed of innocuous-looking spindle cells, with interspersed small vessels and few osteoclastic giant cells. The chondromyxoid component showed varying cellularity. The periphery of the lobules was more cellular in appearance, and the cells were predominantly spindle in shape.\nIn the center, the cells had a moderate amount of eosinophilic cytoplasm, whereas a few others were stellate-shaped. Chondrocytes were also identified. No evidence of multinucleation within the lacunae or significant nuclear pleomorphism was noted. The spindle-cell component also did not show any pleomorphism or mitotic activity (Figure 4). These features, correlated with the radiologic findings, were indicative of CMF.\nFigure 4.\nCMF is a benign tumor of bone that was first described by Jaffe and Lichtenstein in 1948.[1,2] This lesion accounts for 1% of all bone tumors and 2% of all benign bone tumors.[3] It is most commonly found in the long tubular bones, especially the tibia and femur near the knee joint; however, the overall most common site is the proximal tibia, which is affected in 30% of cases. Approximately 25% of cases occur in the flat bones.[3,4] The small bones of the lower limbs may also be affected. The skull, spine, and bones of the upper extremity are relatively uncommon sites for this lesion.\nPatients with this condition range from 3 to 70 years of age; however, most cases are seen in patients aged 10-30 years. A male predominance has been described (male-to-female ratio, 2:1).[3,5] The most common presenting symptoms are pain and swelling, which are noted in 85% and 65% of cases, respectively; the third most common symptom is restriction in joint movement. The pain is usually mild, transient, and of long duration.[6] Approximately 15% of cases are discovered incidentally on radiologic examination. Pathologic fracture may occur in 5% of cases, causing significant morbidity.\n\n ## Figures\n\n **Figure 4.** \n \n\n\n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1103458,
"choiceText": "Chondromyxoid fibroma",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1103460,
"choiceText": "Giant cell tumor of bone",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1103462,
"choiceText": "Aneurysmal bone cyst",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1103464,
"choiceText": "Chondrosarcoma",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 349689,
"questionText": "Which of the following is the most likely diagnosis?<br/><br/>\r\n<em>Hint: The patient is a 13-year-old boy with a well-circumscribed, lytic, bubbly lesion with characteristic histologic findings.</em>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 13-Year-Old Boy With Recurrent Lower Leg Pain"
},
{
"authors": "Prasenjit Das, MD, MBBS; Elanthenral Sigamani, MD",
"content": [
"Plain radiography is useful when CMF is suspected; however, histopathologic examination is mandatory for confirmation of the diagnosis. Bubbly lesions are typical in the metaphysis, and when seen, a differential diagnosis of giant cell tumor, aneurysmal bone cyst, and CMF should always be kept in mind. The possibility of osteosarcoma should also be considered. The bubbly pattern is usually caused by ridging or endosteal scalloping of the surrounding host bone.",
"Histologically, the possibility of a low-grade chondrosarcoma should also be carefully ruled out. Because of the nodular growth pattern of any cartilaginous tumor, the histologic diagnosis is primarily based on the extent of cellularity and the cytologic atypia of the tumor cells. However, mild to moderate cytologic atypia has also been described in CMF; therefore, the extent of cellularity, multinucleation within the lacunae, and the presence of atypical mitotic activity in the tumor should be relied on as features to rule out chondrosarcoma.",
"In this context, remembering that the periphery of the nodules in CMF show marked cellularity should prompt histopathologists to concentrate on the center of the lobules when assessing the criteria of cellularity. CMF, like osteosarcoma, shows erosion of cortical bone with soft-tissue infiltration. Osteoid production by the tumor cells is also characteristic of this lesion. Radiologic correlation is mandatory because CMFs are typically bubbly and oval, and have a long axis parallel to that of the long bone. Extension into diaphysis or epiphysis is not uncommon.[5,7] Unlike other cartilaginous tumors, calcification is less frequent and the margins are typically scalloped.",
"MRI may help to resolve minute details, such as soft-tissue extension. T2-weighted MRI reveals a hyperintense, homogeneous lesion (Figure 3). CT may help to identify any calcifications within the lesion, which are uncommon and occur in about 2% of cases.[5,7]",
"Figure 3.",
"The histogenesis of CMF is uncertain and is a matter of continuing speculation. The cartilaginous origin, as apparent on the morphologic features, has been supported by ultrastructural studies and by immunohistochemical staining for S100 protein. Because of their occasional immunopositivity for smooth-muscle actin and S100 proteins, possible myofibroblastic, myochondroblastic, and chondrocytic differentiation was suggested.[7] For the same reasons, a possible resemblance with chondroblastoma was cited; however, this finding was not substantiated by other studies. Studies have indicated osteocalcin reactivity, which is seen in more than 50% of CMFs, as a cause to support its link with other bone and cartilage tumors, especially chondroblastomas.[7]",
"A study by Romeo and colleagues[8] examined the DNA microarray of CMF and found that the differential expression of adhesion and extracellular matrix molecules, including CD166, versican, perlecan, and Col4A2, may interfere with normal cartilaginous differentiation and lead to the development of CMF. Some studies indicate a possible cartilaginous derivation by examining the expression of transcription factor SOX9 in the tumor cell nuclei, along with high expression of collagen type II in the cell cytoplasm. A low (< 5%) MIB-1 proliferation index also supports its indolent character.",
"Although many cytogenetic abnormalities have been reported in CMF, t(1;5)(p13;p13) is a novel clonal cytogenetic abnormality in CMF and has diagnostic significance. Other frequent chromosomal abnormalities described are aberrations in chromosomes 2 and 5.[7]"
],
"date": "June 13, 2017",
"figures": [
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/881/370/881370-Thumb3.png"
}
],
"markdown": "# A 13-Year-Old Boy With Recurrent Lower Leg Pain\n\n **Authors:** Prasenjit Das, MD, MBBS; Elanthenral Sigamani, MD \n **Date:** June 13, 2017\n\n ## Content\n\n Plain radiography is useful when CMF is suspected; however, histopathologic examination is mandatory for confirmation of the diagnosis. Bubbly lesions are typical in the metaphysis, and when seen, a differential diagnosis of giant cell tumor, aneurysmal bone cyst, and CMF should always be kept in mind. The possibility of osteosarcoma should also be considered. The bubbly pattern is usually caused by ridging or endosteal scalloping of the surrounding host bone.\nHistologically, the possibility of a low-grade chondrosarcoma should also be carefully ruled out. Because of the nodular growth pattern of any cartilaginous tumor, the histologic diagnosis is primarily based on the extent of cellularity and the cytologic atypia of the tumor cells. However, mild to moderate cytologic atypia has also been described in CMF; therefore, the extent of cellularity, multinucleation within the lacunae, and the presence of atypical mitotic activity in the tumor should be relied on as features to rule out chondrosarcoma.\nIn this context, remembering that the periphery of the nodules in CMF show marked cellularity should prompt histopathologists to concentrate on the center of the lobules when assessing the criteria of cellularity. CMF, like osteosarcoma, shows erosion of cortical bone with soft-tissue infiltration. Osteoid production by the tumor cells is also characteristic of this lesion. Radiologic correlation is mandatory because CMFs are typically bubbly and oval, and have a long axis parallel to that of the long bone. Extension into diaphysis or epiphysis is not uncommon.[5,7] Unlike other cartilaginous tumors, calcification is less frequent and the margins are typically scalloped.\nMRI may help to resolve minute details, such as soft-tissue extension. T2-weighted MRI reveals a hyperintense, homogeneous lesion (Figure 3). CT may help to identify any calcifications within the lesion, which are uncommon and occur in about 2% of cases.[5,7]\nFigure 3.\nThe histogenesis of CMF is uncertain and is a matter of continuing speculation. The cartilaginous origin, as apparent on the morphologic features, has been supported by ultrastructural studies and by immunohistochemical staining for S100 protein. Because of their occasional immunopositivity for smooth-muscle actin and S100 proteins, possible myofibroblastic, myochondroblastic, and chondrocytic differentiation was suggested.[7] For the same reasons, a possible resemblance with chondroblastoma was cited; however, this finding was not substantiated by other studies. Studies have indicated osteocalcin reactivity, which is seen in more than 50% of CMFs, as a cause to support its link with other bone and cartilage tumors, especially chondroblastomas.[7]\nA study by Romeo and colleagues[8] examined the DNA microarray of CMF and found that the differential expression of adhesion and extracellular matrix molecules, including CD166, versican, perlecan, and Col4A2, may interfere with normal cartilaginous differentiation and lead to the development of CMF. Some studies indicate a possible cartilaginous derivation by examining the expression of transcription factor SOX9 in the tumor cell nuclei, along with high expression of collagen type II in the cell cytoplasm. A low (< 5%) MIB-1 proliferation index also supports its indolent character.\nAlthough many cytogenetic abnormalities have been reported in CMF, t(1;5)(p13;p13) is a novel clonal cytogenetic abnormality in CMF and has diagnostic significance. Other frequent chromosomal abnormalities described are aberrations in chromosomes 2 and 5.[7]\n\n ## Figures\n\n **Figure 3.** \n \n\n\n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 13-Year-Old Boy With Recurrent Lower Leg Pain"
},
{
"authors": "Prasenjit Das, MD, MBBS; Elanthenral Sigamani, MD",
"content": [
"The prognosis in cases of CMF is usually excellent, even with recurrence.[5] Treatment of the lesion includes curettage or en bloc excision with bone grafting. Radiation therapy is contraindicated because it causes dedifferentiation and malignancy. In cases of multiple recurrence, radiation therapy may be of some help.[9]",
"Recurrence has been reported in 3%-22% of cases, especially within the first 2 years of surgery, but it has been reported 30 years after surgery as well.[10] Recurrence is more frequently seen in patients younger than 15 years who have a more myxoid tumor with nuclear atypia. Some reports have not found any correlation between the histologic findings and recurrence in CMF. Metastasis has never been reported.[5] All of these facts point to the need for long-term follow-up in these patients.[11]",
"In the patient in this case, curettage of the lesion with bone grafting was performed. Local extension of the lesion into the articular cartilage and the scant yield of the preintervention biopsy dictated the decision to opt for curettage, followed by fracture fixation. Care should be taken to curette the whole lesion, because incomplete curettage is one of the important factors leading to local tumor recurrence. The patient has had two monthly follow-up examinations over 8 months, with no recurrence detected."
],
"date": "June 13, 2017",
"figures": [],
"markdown": "# A 13-Year-Old Boy With Recurrent Lower Leg Pain\n\n **Authors:** Prasenjit Das, MD, MBBS; Elanthenral Sigamani, MD \n **Date:** June 13, 2017\n\n ## Content\n\n The prognosis in cases of CMF is usually excellent, even with recurrence.[5] Treatment of the lesion includes curettage or en bloc excision with bone grafting. Radiation therapy is contraindicated because it causes dedifferentiation and malignancy. In cases of multiple recurrence, radiation therapy may be of some help.[9]\nRecurrence has been reported in 3%-22% of cases, especially within the first 2 years of surgery, but it has been reported 30 years after surgery as well.[10] Recurrence is more frequently seen in patients younger than 15 years who have a more myxoid tumor with nuclear atypia. Some reports have not found any correlation between the histologic findings and recurrence in CMF. Metastasis has never been reported.[5] All of these facts point to the need for long-term follow-up in these patients.[11]\nIn the patient in this case, curettage of the lesion with bone grafting was performed. Local extension of the lesion into the articular cartilage and the scant yield of the preintervention biopsy dictated the decision to opt for curettage, followed by fracture fixation. Care should be taken to curette the whole lesion, because incomplete curettage is one of the important factors leading to local tumor recurrence. The patient has had two monthly follow-up examinations over 8 months, with no recurrence detected.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1103466,
"choiceText": "Conventional radiography",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1103468,
"choiceText": "T2-weighted MRI",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1103470,
"choiceText": "CT",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1103472,
"choiceText": "Histopathologic analysis",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Plain radiography is useful when this lesion is suspected, but histopathologic examination is mandatory for confirmation of the diagnosis. MRI may help in resolving minute details, such as soft-tissue extension. T2-weighted MRI reveals a hyperintense, homogeneous lesion. CT may help to identify any calcifications within the lesion, which are uncommon and occur in about 2% of cases.<sup type=\"ref\">[5,7]</sup>",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 349691,
"questionText": "You are treating a 15-year-old with chronic, mild leg pain. Radiography is performed and, on the basis of the appearance (as well as the patient's clinical presentation), you suspect CMF. Which of the following is necessary to confirm the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1103474,
"choiceText": "Overall prognosis is excellent in older persons",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1103476,
"choiceText": "Prognosis is worse in children whose CMF has a more myxoid component",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1103478,
"choiceText": "Recurrence has been described in < 3% cases",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1103480,
"choiceText": "Metastasis does not occur in CMF",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1103482,
"choiceText": "Radiation therapy worsens the overall prognosis",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Recurrence in CMF is not uncommon and has been described in up to 20%-30% cases. Prognosis is worse in children with a more lobulated tumor showing abundant myxoid material. CMF is essentially a benign bone tumor, and metastasis has not been reported. Radiation therapy is not the treatment of choice and has been shown to induce dedifferentiation and transformation into sarcomas.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 349693,
"questionText": "The patient above is diagnosed with CMF and treated. Which of the following statements about the patient's prognosis is <em>not</em> correct?\r\n\r\n",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 13-Year-Old Boy With Recurrent Lower Leg Pain"
},
{
"authors": "Prasenjit Das, MD, MBBS; Elanthenral Sigamani, MD",
"content": [],
"date": "June 13, 2017",
"figures": [],
"markdown": "# A 13-Year-Old Boy With Recurrent Lower Leg Pain\n\n **Authors:** Prasenjit Das, MD, MBBS; Elanthenral Sigamani, MD \n **Date:** June 13, 2017\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1103466,
"choiceText": "Conventional radiography",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1103468,
"choiceText": "T2-weighted MRI",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1103470,
"choiceText": "CT",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1103472,
"choiceText": "Histopathologic analysis",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Plain radiography is useful when this lesion is suspected, but histopathologic examination is mandatory for confirmation of the diagnosis. MRI may help in resolving minute details, such as soft-tissue extension. T2-weighted MRI reveals a hyperintense, homogeneous lesion. CT may help to identify any calcifications within the lesion, which are uncommon and occur in about 2% of cases.<sup type=\"ref\">[5,7]</sup>",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 349691,
"questionText": "You are treating a 15-year-old with chronic, mild leg pain. Radiography is performed and, on the basis of the appearance (as well as the patient's clinical presentation), you suspect CMF. Which of the following is necessary to confirm the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1103474,
"choiceText": "Overall prognosis is excellent in older persons",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1103476,
"choiceText": "Prognosis is worse in children whose CMF has a more myxoid component",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1103478,
"choiceText": "Recurrence has been described in < 3% cases",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1103480,
"choiceText": "Metastasis does not occur in CMF",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1103482,
"choiceText": "Radiation therapy worsens the overall prognosis",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Recurrence in CMF is not uncommon and has been described in up to 20%-30% cases. Prognosis is worse in children with a more lobulated tumor showing abundant myxoid material. CMF is essentially a benign bone tumor, and metastasis has not been reported. Radiation therapy is not the treatment of choice and has been shown to induce dedifferentiation and transformation into sarcomas.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 349693,
"questionText": "The patient above is diagnosed with CMF and treated. Which of the following statements about the patient's prognosis is <em>not</em> correct?\r\n\r\n",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 13-Year-Old Boy With Recurrent Lower Leg Pain"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1103458,
"choiceText": "Chondromyxoid fibroma",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1103460,
"choiceText": "Giant cell tumor of bone",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1103462,
"choiceText": "Aneurysmal bone cyst",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1103464,
"choiceText": "Chondrosarcoma",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 349689,
"questionText": "Which of the following is the most likely diagnosis?<br/><br/>\r\n<em>Hint: The patient is a 13-year-old boy with a well-circumscribed, lytic, bubbly lesion with characteristic histologic findings.</em>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1103466,
"choiceText": "Conventional radiography",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1103468,
"choiceText": "T2-weighted MRI",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1103470,
"choiceText": "CT",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1103472,
"choiceText": "Histopathologic analysis",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Plain radiography is useful when this lesion is suspected, but histopathologic examination is mandatory for confirmation of the diagnosis. MRI may help in resolving minute details, such as soft-tissue extension. T2-weighted MRI reveals a hyperintense, homogeneous lesion. CT may help to identify any calcifications within the lesion, which are uncommon and occur in about 2% of cases.<sup type=\"ref\">[5,7]</sup>",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 349691,
"questionText": "You are treating a 15-year-old with chronic, mild leg pain. Radiography is performed and, on the basis of the appearance (as well as the patient's clinical presentation), you suspect CMF. Which of the following is necessary to confirm the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1103474,
"choiceText": "Overall prognosis is excellent in older persons",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1103476,
"choiceText": "Prognosis is worse in children whose CMF has a more myxoid component",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1103478,
"choiceText": "Recurrence has been described in < 3% cases",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1103480,
"choiceText": "Metastasis does not occur in CMF",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1103482,
"choiceText": "Radiation therapy worsens the overall prognosis",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Recurrence in CMF is not uncommon and has been described in up to 20%-30% cases. Prognosis is worse in children with a more lobulated tumor showing abundant myxoid material. CMF is essentially a benign bone tumor, and metastasis has not been reported. Radiation therapy is not the treatment of choice and has been shown to induce dedifferentiation and transformation into sarcomas.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 349693,
"questionText": "The patient above is diagnosed with CMF and treated. Which of the following statements about the patient's prognosis is <em>not</em> correct?\r\n\r\n",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
881361 | /viewarticle/881361 | [
{
"authors": "Ryland P. Byrd, Jr, MD; Thomas M. Roy, MD",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"A 42-year-old man presents to the hospital with dull, anterior precordial and retrosternal chest pain that began acutely with a tearing sensation and has lasted for 3 days. He states that during this period he has been unable to \"get comfortable.\" The intensity of the pain increases during inspiration and with body movement.",
"The patient denies any symptoms of recent viral infection, and he has received no recent vaccinations. He has no reported history of hypertension, coronary artery disease, prior cardiac surgery, diabetes mellitus, or hyperlipidemia. He has no family history of cardiovascular disease. The patient is taking no prescribed medication, over-the-counter medication, or herbal remedies, and he denies illicit drug use."
],
"date": "June 12, 2017",
"figures": [],
"markdown": "# Dull Chest Pain in a 42-Year-Old Man\n\n **Authors:** Ryland P. Byrd, Jr, MD; Thomas M. Roy, MD \n **Date:** June 12, 2017\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nA 42-year-old man presents to the hospital with dull, anterior precordial and retrosternal chest pain that began acutely with a tearing sensation and has lasted for 3 days. He states that during this period he has been unable to \"get comfortable.\" The intensity of the pain increases during inspiration and with body movement.\nThe patient denies any symptoms of recent viral infection, and he has received no recent vaccinations. He has no reported history of hypertension, coronary artery disease, prior cardiac surgery, diabetes mellitus, or hyperlipidemia. He has no family history of cardiovascular disease. The patient is taking no prescribed medication, over-the-counter medication, or herbal remedies, and he denies illicit drug use.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "Dull Chest Pain in a 42-Year-Old Man"
},
{
"authors": "Ryland P. Byrd, Jr, MD; Thomas M. Roy, MD",
"content": [
"The patient is alert but appears uncomfortable. He is afebrile. His blood pressure is 160/102 mm Hg in all extremities, with equal and symmetric pulses in both carotid and brachial arteries. His pulse is 103 beats/min and regular. The cardiac examination reveals an early diastolic murmur in the aortic region, with no gallop, pericardial rub, or knock; however, the heart sounds are slightly distant. The patient's lungs are clear to auscultation. The abdominal findings are unremarkable. The patient's cranial nerves are intact, and no neurologic deficits are noted.",
"An ECG is obtained (Figure 1). Serial cardiac isoenzyme tests demonstrate no evidence of myocardial injury. A chest radiograph is obtained (image not available).",
"Figure 1."
],
"date": "June 12, 2017",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/881/361/881361-Thumb1.jpg"
}
],
"markdown": "# Dull Chest Pain in a 42-Year-Old Man\n\n **Authors:** Ryland P. Byrd, Jr, MD; Thomas M. Roy, MD \n **Date:** June 12, 2017\n\n ## Content\n\n The patient is alert but appears uncomfortable. He is afebrile. His blood pressure is 160/102 mm Hg in all extremities, with equal and symmetric pulses in both carotid and brachial arteries. His pulse is 103 beats/min and regular. The cardiac examination reveals an early diastolic murmur in the aortic region, with no gallop, pericardial rub, or knock; however, the heart sounds are slightly distant. The patient's lungs are clear to auscultation. The abdominal findings are unremarkable. The patient's cranial nerves are intact, and no neurologic deficits are noted.\nAn ECG is obtained (Figure 1). Serial cardiac isoenzyme tests demonstrate no evidence of myocardial injury. A chest radiograph is obtained (image not available).\nFigure 1.\n\n ## Figures\n\n **Figure 1.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1103228,
"choiceText": "Pericarditis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1103230,
"choiceText": "Proximal aortic dissection\r\n",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1103232,
"choiceText": "Acute myocardial infarction",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1103234,
"choiceText": "Hyperkalemia",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 349591,
"questionText": "The diastolic murmur found on physical examination in association with acute chest pain requires urgent evaluation for which of the following conditions?<br><br><i>Hint: The findings of the chest radiograph are abnormal.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Dull Chest Pain in a 42-Year-Old Man"
},
{
"authors": "Ryland P. Byrd, Jr, MD; Thomas M. Roy, MD",
"content": [
"This case demonstrates the importance of the history and physical examination in a patient with chest pain. The ECG (Figure 2) demonstrated widespread diffuse ST-segment elevation, an absence of reciprocal ST-segment depression, and depressed PR segments in all leads except aVR and V1.",
"Figure 2.",
"This pattern is characteristic of pericarditis.[1] Although the ECG results are suggestive of pericarditis, other serious conditions can mimic or cause pericarditis and require urgent evaluation and treatment. The patient's history of a sudden tearing sensation at the onset of symptoms is a classic presentation for an acute aortic dissection; it would be atypical for a patient with an acute myocardial infarction. Patients with acute myocardial infarction may have a preceding history of exertional angina, or they may have substernal chest pressure and heaviness that is not positional (which are the classic findings).",
"In addition, this patient's physical examination gave important clues to the diagnosis. The patient was profoundly hypertensive, which in and of itself is a nonspecific finding. However, the finding of a blowing diastolic murmur in the aortic area (upper right sternal border) is always abnormal and requires urgent evaluation for proximal aortic pathology in patients with chest pain and hypertension.",
"Although the patient had equal blood pressures in both arms, this does not rule out an aortic dissection. Rather, equal blood pressures simply suggest that, if a dissection is present, the dissection flap does not impair circulation of either subclavian artery.",
"As part of his initial evaluation, this patient had chest radiography that showed mediastinal widening. Although this is also a nonspecific finding, it should prompt more in-depth cross-sectional imaging on the basis of the details described above.",
"In this case, the emergency department physician pursued further imaging by obtaining CT of the chest with the use of intravenous contrast, which disclosed a proximal dissection of the thoracic aorta."
],
"date": "June 12, 2017",
"figures": [
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/881/361/881361-Thumb2.jpg"
}
],
"markdown": "# Dull Chest Pain in a 42-Year-Old Man\n\n **Authors:** Ryland P. Byrd, Jr, MD; Thomas M. Roy, MD \n **Date:** June 12, 2017\n\n ## Content\n\n This case demonstrates the importance of the history and physical examination in a patient with chest pain. The ECG (Figure 2) demonstrated widespread diffuse ST-segment elevation, an absence of reciprocal ST-segment depression, and depressed PR segments in all leads except aVR and V1.\nFigure 2.\nThis pattern is characteristic of pericarditis.[1] Although the ECG results are suggestive of pericarditis, other serious conditions can mimic or cause pericarditis and require urgent evaluation and treatment. The patient's history of a sudden tearing sensation at the onset of symptoms is a classic presentation for an acute aortic dissection; it would be atypical for a patient with an acute myocardial infarction. Patients with acute myocardial infarction may have a preceding history of exertional angina, or they may have substernal chest pressure and heaviness that is not positional (which are the classic findings).\nIn addition, this patient's physical examination gave important clues to the diagnosis. The patient was profoundly hypertensive, which in and of itself is a nonspecific finding. However, the finding of a blowing diastolic murmur in the aortic area (upper right sternal border) is always abnormal and requires urgent evaluation for proximal aortic pathology in patients with chest pain and hypertension.\nAlthough the patient had equal blood pressures in both arms, this does not rule out an aortic dissection. Rather, equal blood pressures simply suggest that, if a dissection is present, the dissection flap does not impair circulation of either subclavian artery.\nAs part of his initial evaluation, this patient had chest radiography that showed mediastinal widening. Although this is also a nonspecific finding, it should prompt more in-depth cross-sectional imaging on the basis of the details described above.\nIn this case, the emergency department physician pursued further imaging by obtaining CT of the chest with the use of intravenous contrast, which disclosed a proximal dissection of the thoracic aorta.\n\n ## Figures\n\n **Figure 2.** \n \n\n\n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1103228,
"choiceText": "Pericarditis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1103230,
"choiceText": "Proximal aortic dissection\r\n",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1103232,
"choiceText": "Acute myocardial infarction",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1103234,
"choiceText": "Hyperkalemia",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 349591,
"questionText": "The diastolic murmur found on physical examination in association with acute chest pain requires urgent evaluation for which of the following conditions?<br><br><i>Hint: The findings of the chest radiograph are abnormal.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Dull Chest Pain in a 42-Year-Old Man"
},
{
"authors": "Ryland P. Byrd, Jr, MD; Thomas M. Roy, MD",
"content": [
"Aortic dissection is a relatively uncommon but potentially catastrophic condition.[2] The incidence of aortic dissection is estimated to be 5-30 cases per 1 million people per year. Aortic dissection can manifest in numerous ways; it has been described as \"the great mimicker\" because of its myriad of presentations. The symptom complex of aortic dissection depends on the direction and degree of propagation of the initial dissection tear.",
"For example, a dissection of the ascending aorta can propagate in a cephalad fashion to involve either of the carotid arteries, causing acute stroke symptoms. Also, the dissection plane can travel caudally and cause occlusion of either of the main coronary arteries, leading to acute myocardial infarction. If the dissection flap is below the insertion of the pericardial reflection on the ascending aorta, leakage of blood into the pericardial space can cause pericarditis and/or pericardial tamponade.[3] This proximal vascular disruption allows slow penetration of blood into the pericardial space, where it causes inflammatory pericarditis.[3,4] Symptoms of pericarditis may precede fatal aortic rupture by several days.[3] Dissection can also lead to accumulation of blood in the pleural space, with a risk for exsanguination. Finally, aortic dissection can cause aortic valve insufficiency resulting from mechanical disruption of leaflet malcoaptation.",
"The risk factors for aortic dissection include hypertension, Marfan syndrome, and bicuspid aortic valve, but surprisingly, patients with aortic dissection frequently have little coronary atherosclerosis. In addition, an increasing appreciation of genetics in aortic disease has been described.[2] Other variations of aortic dissection include penetrating aortic ulcer and intramural hematoma.",
"Differentiating aortic dissection from other causes of chest pain is important because the therapeutic strategies are completely different. For example, acute pericarditis is treated with anti-inflammatory medications and expectant observation, making it a diagnosis of exclusion. However, acute myocardial infarction is treated with antiplatelet and antithrombin agents, as well as prompt revascularization via either primary angioplasty or pharmacologic thrombolysis.",
"Lack of recognition of aortic dissection can lead to devastating therapeutic decisions, such as anticoagulation for presumed acute myocardial infarction. The use of heparin and/or thrombolytics in an acute aortic dissection can transform a meta-stable, contained dissection into an exsanguinating fatal event. Lack of prompt recognition of an acute aortic dissection may have led to the death of the actor John Ritter.",
"Dissection of the thoracic aorta is a cardiovascular emergency that has a high morbidity and mortality rate when it is not promptly recognized and treated. Untreated, this condition has a mortality rate of 28% within 24 hours, 50% at 48 hours, 70% within 1 week, and 90% within 3 months.[5,6] Most deaths in patients with proximal aortic dissection are caused by rupture, aortic insufficiency, or branch-vessel obstruction.[7]",
"The diagnosis of aortic dissection may not be straightforward and may challenge even experienced physicians. In fact, the diagnosis is missed on initial evaluation in 38% of all patients.[8,9] Further complicating the picture is the fact that at least 1% of patients with aortic dissection have concomitant coronary artery occlusions (from the dissection flap extending into the coronary os), which may precipitate acute myocardial infarction and left ventricular failure[8]; therefore, patients with aortic dissection may have ECG changes consistent with myocardial ischemia or injury, such as marked ST-segment elevation.",
"Other ECG abnormalities occurring with aortic dissection include changes consistent with left ventricular hypertrophy, left-axis deviation, heart blocks, or dysrhythmias.[10] Multiple diagnostic methods can be used to investigate for aortic dissection if the diagnosis is suspected. These diagnostic methods include chest radiography, transthoracic echocardiography, transesophageal echocardiography, MRI, or CT. Each method has its own strengths and drawbacks.",
"Chest radiography is quick and widely available, but it is not sensitive or specific. Transthoracic echocardiography is rapid, can be performed at bedside, and is noninvasive, but it is limited to evaluation of the proximal ascending aorta and the competency of the aortic valve. Transesophageal echocardiography can reliably view the proximal and descending thoracic aorta, but it is invasive, requires sedation of the patient, and may not be available at all institutions 24 hours per day. MRI provides excellent imaging characteristics but may not be available emergently in all institutions. CT is usually rapid and available and has good diagnostic accuracy, but it requires administration of intravenous contrast. In general, CT is usually the most expeditious and easily obtained imaging modality in the absence of contraindications, such as renal insufficiency."
],
"date": "June 12, 2017",
"figures": [],
"markdown": "# Dull Chest Pain in a 42-Year-Old Man\n\n **Authors:** Ryland P. Byrd, Jr, MD; Thomas M. Roy, MD \n **Date:** June 12, 2017\n\n ## Content\n\n Aortic dissection is a relatively uncommon but potentially catastrophic condition.[2] The incidence of aortic dissection is estimated to be 5-30 cases per 1 million people per year. Aortic dissection can manifest in numerous ways; it has been described as \"the great mimicker\" because of its myriad of presentations. The symptom complex of aortic dissection depends on the direction and degree of propagation of the initial dissection tear.\nFor example, a dissection of the ascending aorta can propagate in a cephalad fashion to involve either of the carotid arteries, causing acute stroke symptoms. Also, the dissection plane can travel caudally and cause occlusion of either of the main coronary arteries, leading to acute myocardial infarction. If the dissection flap is below the insertion of the pericardial reflection on the ascending aorta, leakage of blood into the pericardial space can cause pericarditis and/or pericardial tamponade.[3] This proximal vascular disruption allows slow penetration of blood into the pericardial space, where it causes inflammatory pericarditis.[3,4] Symptoms of pericarditis may precede fatal aortic rupture by several days.[3] Dissection can also lead to accumulation of blood in the pleural space, with a risk for exsanguination. Finally, aortic dissection can cause aortic valve insufficiency resulting from mechanical disruption of leaflet malcoaptation.\nThe risk factors for aortic dissection include hypertension, Marfan syndrome, and bicuspid aortic valve, but surprisingly, patients with aortic dissection frequently have little coronary atherosclerosis. In addition, an increasing appreciation of genetics in aortic disease has been described.[2] Other variations of aortic dissection include penetrating aortic ulcer and intramural hematoma.\nDifferentiating aortic dissection from other causes of chest pain is important because the therapeutic strategies are completely different. For example, acute pericarditis is treated with anti-inflammatory medications and expectant observation, making it a diagnosis of exclusion. However, acute myocardial infarction is treated with antiplatelet and antithrombin agents, as well as prompt revascularization via either primary angioplasty or pharmacologic thrombolysis.\nLack of recognition of aortic dissection can lead to devastating therapeutic decisions, such as anticoagulation for presumed acute myocardial infarction. The use of heparin and/or thrombolytics in an acute aortic dissection can transform a meta-stable, contained dissection into an exsanguinating fatal event. Lack of prompt recognition of an acute aortic dissection may have led to the death of the actor John Ritter.\nDissection of the thoracic aorta is a cardiovascular emergency that has a high morbidity and mortality rate when it is not promptly recognized and treated. Untreated, this condition has a mortality rate of 28% within 24 hours, 50% at 48 hours, 70% within 1 week, and 90% within 3 months.[5,6] Most deaths in patients with proximal aortic dissection are caused by rupture, aortic insufficiency, or branch-vessel obstruction.[7]\nThe diagnosis of aortic dissection may not be straightforward and may challenge even experienced physicians. In fact, the diagnosis is missed on initial evaluation in 38% of all patients.[8,9] Further complicating the picture is the fact that at least 1% of patients with aortic dissection have concomitant coronary artery occlusions (from the dissection flap extending into the coronary os), which may precipitate acute myocardial infarction and left ventricular failure[8]; therefore, patients with aortic dissection may have ECG changes consistent with myocardial ischemia or injury, such as marked ST-segment elevation.\nOther ECG abnormalities occurring with aortic dissection include changes consistent with left ventricular hypertrophy, left-axis deviation, heart blocks, or dysrhythmias.[10] Multiple diagnostic methods can be used to investigate for aortic dissection if the diagnosis is suspected. These diagnostic methods include chest radiography, transthoracic echocardiography, transesophageal echocardiography, MRI, or CT. Each method has its own strengths and drawbacks.\nChest radiography is quick and widely available, but it is not sensitive or specific. Transthoracic echocardiography is rapid, can be performed at bedside, and is noninvasive, but it is limited to evaluation of the proximal ascending aorta and the competency of the aortic valve. Transesophageal echocardiography can reliably view the proximal and descending thoracic aorta, but it is invasive, requires sedation of the patient, and may not be available at all institutions 24 hours per day. MRI provides excellent imaging characteristics but may not be available emergently in all institutions. CT is usually rapid and available and has good diagnostic accuracy, but it requires administration of intravenous contrast. In general, CT is usually the most expeditious and easily obtained imaging modality in the absence of contraindications, such as renal insufficiency.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "Dull Chest Pain in a 42-Year-Old Man"
},
{
"authors": "Ryland P. Byrd, Jr, MD; Thomas M. Roy, MD",
"content": [
"Acute medical therapy is directed at stabilizing the patient, preventing further propagation of the dissection, and enabling the patient to survive until definitive operative therapy can be performed. The acute medical therapy is termed \"anti-impulse\" therapy and commonly uses intravenous vasodilators (nitroprusside) and beta-blockers (intravenous esmolol, labetalol, metoprolol) to decrease the force of cardiac contractions.[2] The goal of acute medical therapy is to obtain a blood pressure that is as low as possible while still maintaining mentation and end-organ perfusion.",
"Surgical correction is mandated for any symptomatic aortic dissection. The surgical procedure commonly involves various forms of resection and reapproximation of the dissection flap. The degree of surgical complexity is based on other vascular or valvular structures involved in the dissection. For example, in the case of severe aortic valvular regurgitation complicating a dissection, replacement of the aortic valve may be required. Another nonsurgical approach for the treatment of aortic dissection involves percutaneous placement of an endovascular stent graft to cover the dissection flap and restore the true aortic lumen.",
"The appropriate patient selection and outcomes of patients who receive stent grafts are areas of current investigation. The experience of the surgeon and surgical center is critical in assessing the appropriate type of surgical repair and ensuring a good outcome. In high-volume centers, the survival rate in acute aortic dissection is over 85%.[2]",
"The patient in this case was treated with propranolol and nitroprusside in preparation for surgery. He subsequently underwent surgical repair of a proximal aortic dissection with extension into the pericardium and did well postoperatively.",
"Acknowledgment: Special thanks are extended to John Vozenilek, MD, for his contributions to the publication of this case."
],
"date": "June 12, 2017",
"figures": [],
"markdown": "# Dull Chest Pain in a 42-Year-Old Man\n\n **Authors:** Ryland P. Byrd, Jr, MD; Thomas M. Roy, MD \n **Date:** June 12, 2017\n\n ## Content\n\n Acute medical therapy is directed at stabilizing the patient, preventing further propagation of the dissection, and enabling the patient to survive until definitive operative therapy can be performed. The acute medical therapy is termed \"anti-impulse\" therapy and commonly uses intravenous vasodilators (nitroprusside) and beta-blockers (intravenous esmolol, labetalol, metoprolol) to decrease the force of cardiac contractions.[2] The goal of acute medical therapy is to obtain a blood pressure that is as low as possible while still maintaining mentation and end-organ perfusion.\nSurgical correction is mandated for any symptomatic aortic dissection. The surgical procedure commonly involves various forms of resection and reapproximation of the dissection flap. The degree of surgical complexity is based on other vascular or valvular structures involved in the dissection. For example, in the case of severe aortic valvular regurgitation complicating a dissection, replacement of the aortic valve may be required. Another nonsurgical approach for the treatment of aortic dissection involves percutaneous placement of an endovascular stent graft to cover the dissection flap and restore the true aortic lumen.\nThe appropriate patient selection and outcomes of patients who receive stent grafts are areas of current investigation. The experience of the surgeon and surgical center is critical in assessing the appropriate type of surgical repair and ensuring a good outcome. In high-volume centers, the survival rate in acute aortic dissection is over 85%.[2]\nThe patient in this case was treated with propranolol and nitroprusside in preparation for surgery. He subsequently underwent surgical repair of a proximal aortic dissection with extension into the pericardium and did well postoperatively.\nAcknowledgment: Special thanks are extended to John Vozenilek, MD, for his contributions to the publication of this case.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1103236,
"choiceText": "Beta-blockers",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1103238,
"choiceText": "Heparin\r\n",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1103240,
"choiceText": "Surgical consultation",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1103242,
"choiceText": "Intravenous nitroprusside",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "In a patient with aortic dissection, heparin and other anticoagulants can precipitate severe bleeding and dissection extension and, therefore, should not be given.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 349593,
"questionText": "Which of the following is <i>not</i> associated with acute therapy for aortic dissection?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1103244,
"choiceText": "CT ",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1103246,
"choiceText": "MRI",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1103248,
"choiceText": "Transthoracic echocardiography",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1103250,
"choiceText": "Transesophageal echocardiography\r\n",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Both CT and MRI are relatively contraindicated in patients with moderate renal impairment (because of the iodinated contrast used in CT and the association of MRI with gadolinium-induced systemic sclerosis). Transthoracic imaging is too insensitive in a patient with a high likelihood of an acute dissection.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 349595,
"questionText": "A 75-year-old man with moderate chronic renal insufficiency presents with ripping chest pain radiating to the back and unequal blood pressures in his arms. Which of the following diagnostic tests is appropriate in this setting to evaluate for aortic dissection?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Dull Chest Pain in a 42-Year-Old Man"
},
{
"authors": "Ryland P. Byrd, Jr, MD; Thomas M. Roy, MD",
"content": [],
"date": "June 12, 2017",
"figures": [],
"markdown": "# Dull Chest Pain in a 42-Year-Old Man\n\n **Authors:** Ryland P. Byrd, Jr, MD; Thomas M. Roy, MD \n **Date:** June 12, 2017\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1103236,
"choiceText": "Beta-blockers",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1103238,
"choiceText": "Heparin\r\n",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1103240,
"choiceText": "Surgical consultation",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1103242,
"choiceText": "Intravenous nitroprusside",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "In a patient with aortic dissection, heparin and other anticoagulants can precipitate severe bleeding and dissection extension and, therefore, should not be given.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 349593,
"questionText": "Which of the following is <i>not</i> associated with acute therapy for aortic dissection?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1103244,
"choiceText": "CT ",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1103246,
"choiceText": "MRI",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1103248,
"choiceText": "Transthoracic echocardiography",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1103250,
"choiceText": "Transesophageal echocardiography\r\n",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Both CT and MRI are relatively contraindicated in patients with moderate renal impairment (because of the iodinated contrast used in CT and the association of MRI with gadolinium-induced systemic sclerosis). Transthoracic imaging is too insensitive in a patient with a high likelihood of an acute dissection.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 349595,
"questionText": "A 75-year-old man with moderate chronic renal insufficiency presents with ripping chest pain radiating to the back and unequal blood pressures in his arms. Which of the following diagnostic tests is appropriate in this setting to evaluate for aortic dissection?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Dull Chest Pain in a 42-Year-Old Man"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1103228,
"choiceText": "Pericarditis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1103230,
"choiceText": "Proximal aortic dissection\r\n",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1103232,
"choiceText": "Acute myocardial infarction",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1103234,
"choiceText": "Hyperkalemia",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 349591,
"questionText": "The diastolic murmur found on physical examination in association with acute chest pain requires urgent evaluation for which of the following conditions?<br><br><i>Hint: The findings of the chest radiograph are abnormal.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1103236,
"choiceText": "Beta-blockers",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1103238,
"choiceText": "Heparin\r\n",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1103240,
"choiceText": "Surgical consultation",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1103242,
"choiceText": "Intravenous nitroprusside",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "In a patient with aortic dissection, heparin and other anticoagulants can precipitate severe bleeding and dissection extension and, therefore, should not be given.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 349593,
"questionText": "Which of the following is <i>not</i> associated with acute therapy for aortic dissection?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1103244,
"choiceText": "CT ",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1103246,
"choiceText": "MRI",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1103248,
"choiceText": "Transthoracic echocardiography",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1103250,
"choiceText": "Transesophageal echocardiography\r\n",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Both CT and MRI are relatively contraindicated in patients with moderate renal impairment (because of the iodinated contrast used in CT and the association of MRI with gadolinium-induced systemic sclerosis). Transthoracic imaging is too insensitive in a patient with a high likelihood of an acute dissection.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 349595,
"questionText": "A 75-year-old man with moderate chronic renal insufficiency presents with ripping chest pain radiating to the back and unequal blood pressures in his arms. Which of the following diagnostic tests is appropriate in this setting to evaluate for aortic dissection?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
880611 | /viewarticle/880611 | [
{
"authors": "Djamil Fertikh, MD",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"A 31-year-old woman presents with a 5-month history of progressive right wrist pain following delivery of her baby girl. The patient claims that the pain has become so severe and disabling that it has significantly affected her ability to perform daily activities. The pain is particularly intense when she picks up her child.",
"The patient is a software engineer by training, but she is currently on maternity leave and is staying at home to care for her newborn baby. She lives in a single-family house with her husband and their two dogs.",
"She denies having any fever, chills, or any other associated systemic symptoms. She has no remarkable past medical history; her surgical history is only remarkable for appendicitis when she was 13 years old. She is not taking any medications except for recent daily acetaminophen to alleviate her wrist pain. The acetaminophen has provided only mild and inconsistent improvement of her pain. She denies smoking cigarettes but does admit to drinking a glass of wine on occasion. She does not use any illicit drugs."
],
"date": "May 31, 2017",
"figures": [],
"markdown": "# Wrist Pain After Pregnancy in a 31-Year-Old Woman\n\n **Authors:** Djamil Fertikh, MD \n **Date:** May 31, 2017\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nA 31-year-old woman presents with a 5-month history of progressive right wrist pain following delivery of her baby girl. The patient claims that the pain has become so severe and disabling that it has significantly affected her ability to perform daily activities. The pain is particularly intense when she picks up her child.\nThe patient is a software engineer by training, but she is currently on maternity leave and is staying at home to care for her newborn baby. She lives in a single-family house with her husband and their two dogs.\nShe denies having any fever, chills, or any other associated systemic symptoms. She has no remarkable past medical history; her surgical history is only remarkable for appendicitis when she was 13 years old. She is not taking any medications except for recent daily acetaminophen to alleviate her wrist pain. The acetaminophen has provided only mild and inconsistent improvement of her pain. She denies smoking cigarettes but does admit to drinking a glass of wine on occasion. She does not use any illicit drugs.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "Wrist Pain After Pregnancy in a 31-Year-Old Woman"
},
{
"authors": "Djamil Fertikh, MD",
"content": [
"Upon physical examination, the patient is not in any apparent discomfort. Her oral temperature is 96.3° F (35.7° C). Her pulse has a regular sinus rhythm, with a rate of 88 beats/min. Her blood pressure is measured at 145/75 mm Hg. No skin rash or discoloration is noted.",
"Her head and neck examination is unremarkable. Her lungs are clear to auscultation, with normal respiratory effort. Her abdomen is soft and nontender to deep palpation. No evidence of organomegaly is present. The patient's reflexes are normal and symmetrical. Close examination of her right wrist shows mild but definite soft-tissue swelling just proximal and posterior to the radial styloid. No associated erythema is noted over the area of pain, and no pain is elicited on palpation. Exquisite pain at the thumb occurs with the Finkelstein maneuver (a specific maneuver in which the thumb is placed in the closed fist and the affected hand is tilted towards the little finger, into ulnar deviation).",
"Her laboratory workup includes a complete blood count, with normal red and white blood cell counts and no thrombocytopenia. The erythrocyte sedimentation rate (ESR) is mildly elevated. Her basic metabolic panel is normal. Her serum rheumatoid factor is within normal limits. The antinuclear antibodies (ANA) are within the normal range. Radiographs of the right wrist fail to reveal any bony abnormalities.",
"An MRI scan of her affected wrist is performed (Figure).",
"Figure."
],
"date": "May 31, 2017",
"figures": [
{
"caption": "Figure.",
"image_url": "https://img.medscapestatic.com/article/880/611/880611-Thumb1.png"
}
],
"markdown": "# Wrist Pain After Pregnancy in a 31-Year-Old Woman\n\n **Authors:** Djamil Fertikh, MD \n **Date:** May 31, 2017\n\n ## Content\n\n Upon physical examination, the patient is not in any apparent discomfort. Her oral temperature is 96.3° F (35.7° C). Her pulse has a regular sinus rhythm, with a rate of 88 beats/min. Her blood pressure is measured at 145/75 mm Hg. No skin rash or discoloration is noted.\nHer head and neck examination is unremarkable. Her lungs are clear to auscultation, with normal respiratory effort. Her abdomen is soft and nontender to deep palpation. No evidence of organomegaly is present. The patient's reflexes are normal and symmetrical. Close examination of her right wrist shows mild but definite soft-tissue swelling just proximal and posterior to the radial styloid. No associated erythema is noted over the area of pain, and no pain is elicited on palpation. Exquisite pain at the thumb occurs with the Finkelstein maneuver (a specific maneuver in which the thumb is placed in the closed fist and the affected hand is tilted towards the little finger, into ulnar deviation).\nHer laboratory workup includes a complete blood count, with normal red and white blood cell counts and no thrombocytopenia. The erythrocyte sedimentation rate (ESR) is mildly elevated. Her basic metabolic panel is normal. Her serum rheumatoid factor is within normal limits. The antinuclear antibodies (ANA) are within the normal range. Radiographs of the right wrist fail to reveal any bony abnormalities.\nAn MRI scan of her affected wrist is performed (Figure).\nFigure.\n\n ## Figures\n\n **Figure.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1098858,
"choiceText": "Carpal tunnel syndrome",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1098860,
"choiceText": "Stress fracture of the scaphoid",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1098862,
"choiceText": "Bursitis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1098864,
"choiceText": "de Quervain tenosynovitis",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 348183,
"questionText": "Which of the following is the most likely diagnosis?<br><br><i>\r\n\r\nHint: The condition is caused by overuse, classically among mothers of infants who are repetitively picking up their children.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Wrist Pain After Pregnancy in a 31-Year-Old Woman"
},
{
"authors": "Djamil Fertikh, MD",
"content": [
"The coronal T2-weighted MRI image sequence showed abnormal segmental tendinous thickening involving the first dorsal extensor component of the wrist just proximal to the radial styloid process, with a mild amount of fluid within the tendon sheath. No evidence of significant bony abnormality or soft-tissue masses or collections is noted. These findings are consistent with a diagnosis of de Quervain tenosynovitis, an inflammatory process that typically involves the first dorsal extensor components of the wrist (namely, the abductor pollicis longus [APL] and the extensor pollicis brevis [EPB]) within the narrow fibro-osseous tunnel through which they normally pass. Although MRI is not typically necessary for the diagnosis of de Quervain tenosynovitis, the image in this case nicely shows the inflammatory changes characteristic of the condition.",
"De Quervain tenosynovitis was first described in 1895 by a Swiss surgeon, Fritz de Quervain, who reported five cases of patients with a tender, thickened first dorsal wrist compartment. The condition has subsequently named after him. It is considered to be the second most common entrapment tendinitis of the hand and wrist (after trigger digit syndrome). In 1930, Finkelstein reviewed the literature and reported 24 additional cases. He felt that chronic trauma should be considered as the principal cause of de Quervain tenosynovitis. From his work, the author derived the well-known Finkelstein sign, which is used to diagnose the disease. The Finkelstein sign classically occurs in mothers of infants aged 6-12 months. The cause is believed to be principally endocrine and related to fluid retention in breastfeeding mothers, and not solely as a result of repetitive lifting motion. De Quervain tenosynovitis has also been described in fathers, however, suggesting that the condition can occur in the absence of postpartum endocrine changes. Repetitive trauma in manual laborers is also a common cause. The pain itself may appear either gradually or suddenly.[1,2,3]",
"In most cases, the patient often complains of weeks to months of gradually worsening pain proximal and posterior to the radial styloid process, especially when using the thumb. The patient experiences difficulties with gripping and pinching, and in severe cases, the affected hand may be too painful to use. The pain may radiate into the thumb or it may radiate proximally into the ipsilateral forearm or shoulder.",
"In the vast majority of cases, a discrete soft-tissue mass corresponding to the affected tendon sheath thickening of the first extensor compartment at the level of the distal dorsolateral aspect of the radial styloid process is encountered on clinical examination. The presence of multiple tendon slips and variable insertions of the abductor pollicis longus, as well as the presence of a separate compartment for the extensor pollicis brevis, have been noted in patients who have de Quervain tenosynovitis.",
"Anatomical studies involving human cadavers have shown great variability in the incidence of separate compartments for the extensor pollicis brevis and abductor pollicis longus tendons. In a large series, a separate compartment for the extensor pollicis brevis was found in 120 (40%) of 300 dissected wrists. In addition, several reports have described anatomical variations of the first dorsal wrist compartment and suggested that normal variations of the tendons of this compartment could be related to a cause of de Quervain tenosynovitis. No associated skin changes or discoloration are typically present.[3,4,5,6]"
],
"date": "May 31, 2017",
"figures": [],
"markdown": "# Wrist Pain After Pregnancy in a 31-Year-Old Woman\n\n **Authors:** Djamil Fertikh, MD \n **Date:** May 31, 2017\n\n ## Content\n\n The coronal T2-weighted MRI image sequence showed abnormal segmental tendinous thickening involving the first dorsal extensor component of the wrist just proximal to the radial styloid process, with a mild amount of fluid within the tendon sheath. No evidence of significant bony abnormality or soft-tissue masses or collections is noted. These findings are consistent with a diagnosis of de Quervain tenosynovitis, an inflammatory process that typically involves the first dorsal extensor components of the wrist (namely, the abductor pollicis longus [APL] and the extensor pollicis brevis [EPB]) within the narrow fibro-osseous tunnel through which they normally pass. Although MRI is not typically necessary for the diagnosis of de Quervain tenosynovitis, the image in this case nicely shows the inflammatory changes characteristic of the condition.\nDe Quervain tenosynovitis was first described in 1895 by a Swiss surgeon, Fritz de Quervain, who reported five cases of patients with a tender, thickened first dorsal wrist compartment. The condition has subsequently named after him. It is considered to be the second most common entrapment tendinitis of the hand and wrist (after trigger digit syndrome). In 1930, Finkelstein reviewed the literature and reported 24 additional cases. He felt that chronic trauma should be considered as the principal cause of de Quervain tenosynovitis. From his work, the author derived the well-known Finkelstein sign, which is used to diagnose the disease. The Finkelstein sign classically occurs in mothers of infants aged 6-12 months. The cause is believed to be principally endocrine and related to fluid retention in breastfeeding mothers, and not solely as a result of repetitive lifting motion. De Quervain tenosynovitis has also been described in fathers, however, suggesting that the condition can occur in the absence of postpartum endocrine changes. Repetitive trauma in manual laborers is also a common cause. The pain itself may appear either gradually or suddenly.[1,2,3]\nIn most cases, the patient often complains of weeks to months of gradually worsening pain proximal and posterior to the radial styloid process, especially when using the thumb. The patient experiences difficulties with gripping and pinching, and in severe cases, the affected hand may be too painful to use. The pain may radiate into the thumb or it may radiate proximally into the ipsilateral forearm or shoulder.\nIn the vast majority of cases, a discrete soft-tissue mass corresponding to the affected tendon sheath thickening of the first extensor compartment at the level of the distal dorsolateral aspect of the radial styloid process is encountered on clinical examination. The presence of multiple tendon slips and variable insertions of the abductor pollicis longus, as well as the presence of a separate compartment for the extensor pollicis brevis, have been noted in patients who have de Quervain tenosynovitis.\nAnatomical studies involving human cadavers have shown great variability in the incidence of separate compartments for the extensor pollicis brevis and abductor pollicis longus tendons. In a large series, a separate compartment for the extensor pollicis brevis was found in 120 (40%) of 300 dissected wrists. In addition, several reports have described anatomical variations of the first dorsal wrist compartment and suggested that normal variations of the tendons of this compartment could be related to a cause of de Quervain tenosynovitis. No associated skin changes or discoloration are typically present.[3,4,5,6]\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1098858,
"choiceText": "Carpal tunnel syndrome",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1098860,
"choiceText": "Stress fracture of the scaphoid",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1098862,
"choiceText": "Bursitis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1098864,
"choiceText": "de Quervain tenosynovitis",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 348183,
"questionText": "Which of the following is the most likely diagnosis?<br><br><i>\r\n\r\nHint: The condition is caused by overuse, classically among mothers of infants who are repetitively picking up their children.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Wrist Pain After Pregnancy in a 31-Year-Old Woman"
},
{
"authors": "Djamil Fertikh, MD",
"content": [
"De Quervain tenosynovitis is clinically diagnosed and rarely requires laboratory testing or imaging for confirmation (such as occurred in this case); however, imaging abnormalities have been described, and MRI is currently the preferred imaging modality. It is most often ordered in atypical cases in which an alternative diagnosis is being entertained or in cases that are recurrent or resistant to conventional treatment. An MRI typically shows thickening and edema of the involved segment of the tendon, which is best depicted on the long TR sequences (mainly T2-weighted images).",
"Hyperintense fluid signal-intensity surrounding or within the affected tendon sheath may be noted. A normal-caliber tendon does not exclude the diagnosis. Intravenous (IV) administration of gadolinium is not usually necessary, but when administered, enhancement within the tendons and surrounding soft tissue consistent with tenosynovitis may be seen. Additionally, in atypical cases, an MRI scan is helpful in excluding other etiologies of wrist pain, such as ganglion cysts and nerve sheath tumors.[3,7]",
"Several studies have shown that ultrasonography is also a reliable and noninvasive method for detecting tenosynovitis changes. Ultrasonography of the symptomatic tendon typically shows distention in the tendon sheath, with surrounding fluid that is hypoechoic or anechoic. An axial scan of the tendon exhibits a so-called \"double target\" appearance. Ultrasonography is also used to guide the delivery of therapeutic agents into the affected tendon sheath (as injections into the tendon should be avoided) and to follow the response to treatment. It can also help to determine the segment with the most inflammatory changes, allowing for precise administration of the steroids.",
"Studies have shown a significant decrease in the thickness of the affected tendon sheath at 1 week after a local corticosteroid injection, with complete relief of the clinical symptoms and signs observed at 6 weeks and 12 weeks post-injection. Conventional radiography is typically of limited usefulness, but it may show the area of soft-tissue swelling proximal to the styloid process. Some clinical series have shown focal radial styloid process abnormality at the first dorsal wrist compartment that is significantly associated with de Quervain tenosynovitis. Moreover, in clinical conditions that may mimic de Quervain tenosynovitis, radiography can be helpful in ruling out offending bony pathology, such as degenerative bony changes.[8,9]",
"Histologic examination may reveal myxoid degeneration, which is responsible for the thickening observed in the sheath, and intramural deposits of mucopolysaccharides predominantly within the subsynovial regions. Macroscopically, classic de Quervain tenosynovitis demonstrates chronic inflammation, with scar formation and stenosis of the approximately 1-cm section of the fibro-osseous tunnel of the first dorsal compartment.[10]"
],
"date": "May 31, 2017",
"figures": [],
"markdown": "# Wrist Pain After Pregnancy in a 31-Year-Old Woman\n\n **Authors:** Djamil Fertikh, MD \n **Date:** May 31, 2017\n\n ## Content\n\n De Quervain tenosynovitis is clinically diagnosed and rarely requires laboratory testing or imaging for confirmation (such as occurred in this case); however, imaging abnormalities have been described, and MRI is currently the preferred imaging modality. It is most often ordered in atypical cases in which an alternative diagnosis is being entertained or in cases that are recurrent or resistant to conventional treatment. An MRI typically shows thickening and edema of the involved segment of the tendon, which is best depicted on the long TR sequences (mainly T2-weighted images).\nHyperintense fluid signal-intensity surrounding or within the affected tendon sheath may be noted. A normal-caliber tendon does not exclude the diagnosis. Intravenous (IV) administration of gadolinium is not usually necessary, but when administered, enhancement within the tendons and surrounding soft tissue consistent with tenosynovitis may be seen. Additionally, in atypical cases, an MRI scan is helpful in excluding other etiologies of wrist pain, such as ganglion cysts and nerve sheath tumors.[3,7]\nSeveral studies have shown that ultrasonography is also a reliable and noninvasive method for detecting tenosynovitis changes. Ultrasonography of the symptomatic tendon typically shows distention in the tendon sheath, with surrounding fluid that is hypoechoic or anechoic. An axial scan of the tendon exhibits a so-called \"double target\" appearance. Ultrasonography is also used to guide the delivery of therapeutic agents into the affected tendon sheath (as injections into the tendon should be avoided) and to follow the response to treatment. It can also help to determine the segment with the most inflammatory changes, allowing for precise administration of the steroids.\nStudies have shown a significant decrease in the thickness of the affected tendon sheath at 1 week after a local corticosteroid injection, with complete relief of the clinical symptoms and signs observed at 6 weeks and 12 weeks post-injection. Conventional radiography is typically of limited usefulness, but it may show the area of soft-tissue swelling proximal to the styloid process. Some clinical series have shown focal radial styloid process abnormality at the first dorsal wrist compartment that is significantly associated with de Quervain tenosynovitis. Moreover, in clinical conditions that may mimic de Quervain tenosynovitis, radiography can be helpful in ruling out offending bony pathology, such as degenerative bony changes.[8,9]\nHistologic examination may reveal myxoid degeneration, which is responsible for the thickening observed in the sheath, and intramural deposits of mucopolysaccharides predominantly within the subsynovial regions. Macroscopically, classic de Quervain tenosynovitis demonstrates chronic inflammation, with scar formation and stenosis of the approximately 1-cm section of the fibro-osseous tunnel of the first dorsal compartment.[10]\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "Wrist Pain After Pregnancy in a 31-Year-Old Woman"
},
{
"authors": "Djamil Fertikh, MD",
"content": [
"The goal of treatment for de Quervain tenosynovitis is to relieve the pain caused by irritation and swelling. Treatment includes conservative measures such as splinting of the affected thumb and wrist, which is sometimes ineffective (pain may return as soon as the splint is discontinued). In most cases, however, the disease is largely self-limiting and usually resolves after cessation of breastfeeding.",
"Injection of corticosteroids into the tendon sheath usually results in pain relief in about 82%-95% of the cases. Symptomatic improvement from corticosteroid injection is usually observed within a few days after injection. The prognosis is generally good. A patient generally returns to full function after the inflammation quiets down with treatment. If 4-6 months of conservative treatment fails to alleviate the symptoms, then surgical release of the first dorsal compartment may be considered. The procedure is usually done on an outpatient basis. The surgery typically involves identification and cutting of the diseased tendon sheath segment under local anesthesia. Patients commonly return to their normal activities within 2-3 weeks. The procedure has been reported to be successful in about 90% of cases.[5,11]",
"The patient in this case did not respond to conservative treatment (approximately 4 weeks) with splinting; she had only very mild improvement of her symptoms. Intralesional injection of steroid using a 25-gauge needle was performed. The patient regained function and experienced pain relief within 3-4 days following the injection. No symptom recurrence was reported."
],
"date": "May 31, 2017",
"figures": [],
"markdown": "# Wrist Pain After Pregnancy in a 31-Year-Old Woman\n\n **Authors:** Djamil Fertikh, MD \n **Date:** May 31, 2017\n\n ## Content\n\n The goal of treatment for de Quervain tenosynovitis is to relieve the pain caused by irritation and swelling. Treatment includes conservative measures such as splinting of the affected thumb and wrist, which is sometimes ineffective (pain may return as soon as the splint is discontinued). In most cases, however, the disease is largely self-limiting and usually resolves after cessation of breastfeeding.\nInjection of corticosteroids into the tendon sheath usually results in pain relief in about 82%-95% of the cases. Symptomatic improvement from corticosteroid injection is usually observed within a few days after injection. The prognosis is generally good. A patient generally returns to full function after the inflammation quiets down with treatment. If 4-6 months of conservative treatment fails to alleviate the symptoms, then surgical release of the first dorsal compartment may be considered. The procedure is usually done on an outpatient basis. The surgery typically involves identification and cutting of the diseased tendon sheath segment under local anesthesia. Patients commonly return to their normal activities within 2-3 weeks. The procedure has been reported to be successful in about 90% of cases.[5,11]\nThe patient in this case did not respond to conservative treatment (approximately 4 weeks) with splinting; she had only very mild improvement of her symptoms. Intralesional injection of steroid using a 25-gauge needle was performed. The patient regained function and experienced pain relief within 3-4 days following the injection. No symptom recurrence was reported.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1098882,
"choiceText": "The process can be bilateral",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1098884,
"choiceText": "It is usually a self-limited process",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1098886,
"choiceText": "The diagnosis is typically made clinically and rarely requires confirmation with imaging studies",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1098888,
"choiceText": "The pain typically occurs with repetitive lifting and radial deviation at the wrist",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The process can be bilateral. Treatment includes conservative measures such as splinting of the affected thumb and wrist, which is sometimes ineffective (pain may return as soon as the splint is discontinued). In most cases, however, the disease is largely self-limiting and usually resolves after cessation of breastfeeding. de Quervain tenosynovitis is clinically diagnosed and rarely requires laboratory testing or imaging for confirmation (such as occurred in this case); however, imaging abnormalities have been described, and MRI is currently the preferred imaging modality. ",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 348189,
"questionText": "Which of the following statements regarding de Quervain tenosynovitis is <i>not</i> accurate?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1098890,
"choiceText": "Skin discoloration on the hand",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1098892,
"choiceText": "Pain with use of the thumb",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1098894,
"choiceText": "Loss of sensation in the hand",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1098896,
"choiceText": "Bony abnormalities noted on imaging\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "In most cases, the patient often complains of weeks to months of gradually worsening pain proximal and posterior to the radial styloid process, especially when using the thumb. The patient experiences difficulties with gripping and pinching and, in severe cases, the affected hand may be too painful to use. The pain may radiate into the thumb, or it may radiate proximally into the ipsilateral forearm or shoulder.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 348191,
"questionText": "Which of the following symptoms is most commonly seen in cases of de Quervain tenosynovitis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Wrist Pain After Pregnancy in a 31-Year-Old Woman"
},
{
"authors": "Djamil Fertikh, MD",
"content": [],
"date": "May 31, 2017",
"figures": [],
"markdown": "# Wrist Pain After Pregnancy in a 31-Year-Old Woman\n\n **Authors:** Djamil Fertikh, MD \n **Date:** May 31, 2017\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1098882,
"choiceText": "The process can be bilateral",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1098884,
"choiceText": "It is usually a self-limited process",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1098886,
"choiceText": "The diagnosis is typically made clinically and rarely requires confirmation with imaging studies",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1098888,
"choiceText": "The pain typically occurs with repetitive lifting and radial deviation at the wrist",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The process can be bilateral. Treatment includes conservative measures such as splinting of the affected thumb and wrist, which is sometimes ineffective (pain may return as soon as the splint is discontinued). In most cases, however, the disease is largely self-limiting and usually resolves after cessation of breastfeeding. de Quervain tenosynovitis is clinically diagnosed and rarely requires laboratory testing or imaging for confirmation (such as occurred in this case); however, imaging abnormalities have been described, and MRI is currently the preferred imaging modality. ",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 348189,
"questionText": "Which of the following statements regarding de Quervain tenosynovitis is <i>not</i> accurate?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1098890,
"choiceText": "Skin discoloration on the hand",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1098892,
"choiceText": "Pain with use of the thumb",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1098894,
"choiceText": "Loss of sensation in the hand",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1098896,
"choiceText": "Bony abnormalities noted on imaging\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "In most cases, the patient often complains of weeks to months of gradually worsening pain proximal and posterior to the radial styloid process, especially when using the thumb. The patient experiences difficulties with gripping and pinching and, in severe cases, the affected hand may be too painful to use. The pain may radiate into the thumb, or it may radiate proximally into the ipsilateral forearm or shoulder.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 348191,
"questionText": "Which of the following symptoms is most commonly seen in cases of de Quervain tenosynovitis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Wrist Pain After Pregnancy in a 31-Year-Old Woman"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1098858,
"choiceText": "Carpal tunnel syndrome",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1098860,
"choiceText": "Stress fracture of the scaphoid",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1098862,
"choiceText": "Bursitis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1098864,
"choiceText": "de Quervain tenosynovitis",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 348183,
"questionText": "Which of the following is the most likely diagnosis?<br><br><i>\r\n\r\nHint: The condition is caused by overuse, classically among mothers of infants who are repetitively picking up their children.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1098882,
"choiceText": "The process can be bilateral",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1098884,
"choiceText": "It is usually a self-limited process",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1098886,
"choiceText": "The diagnosis is typically made clinically and rarely requires confirmation with imaging studies",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1098888,
"choiceText": "The pain typically occurs with repetitive lifting and radial deviation at the wrist",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The process can be bilateral. Treatment includes conservative measures such as splinting of the affected thumb and wrist, which is sometimes ineffective (pain may return as soon as the splint is discontinued). In most cases, however, the disease is largely self-limiting and usually resolves after cessation of breastfeeding. de Quervain tenosynovitis is clinically diagnosed and rarely requires laboratory testing or imaging for confirmation (such as occurred in this case); however, imaging abnormalities have been described, and MRI is currently the preferred imaging modality. ",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 348189,
"questionText": "Which of the following statements regarding de Quervain tenosynovitis is <i>not</i> accurate?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1098890,
"choiceText": "Skin discoloration on the hand",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1098892,
"choiceText": "Pain with use of the thumb",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1098894,
"choiceText": "Loss of sensation in the hand",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1098896,
"choiceText": "Bony abnormalities noted on imaging\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "In most cases, the patient often complains of weeks to months of gradually worsening pain proximal and posterior to the radial styloid process, especially when using the thumb. The patient experiences difficulties with gripping and pinching and, in severe cases, the affected hand may be too painful to use. The pain may radiate into the thumb, or it may radiate proximally into the ipsilateral forearm or shoulder.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 348191,
"questionText": "Which of the following symptoms is most commonly seen in cases of de Quervain tenosynovitis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
878994 | /viewarticle/878994 | [
{
"authors": "",
"content": [],
"date": "",
"figures": [],
"markdown": "# \n\n **Authors:** \n **Date:** \n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 1 of 1*",
"pagination": {
"current_page": 1,
"total_pages": 1
},
"questionnaire": [],
"title": ""
}
] | [] |
878995 | /viewarticle/878995 | [
{
"authors": "",
"content": [],
"date": "",
"figures": [],
"markdown": "# \n\n **Authors:** \n **Date:** \n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 1 of 1*",
"pagination": {
"current_page": 1,
"total_pages": 1
},
"questionnaire": [],
"title": ""
}
] | [] |
878933 | /viewarticle/878933 | [
{
"authors": "Craig Johnson, DO; Zachary Redus; Michael Mader, MD; Frederick Eruo, MD, MPH",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"A 30-year-old gravida 3, para 2 patient who is 23 weeks pregnant presents to the maternal/fetal medicine department. She was referred to the department at the request of her primary obstetrician after an obstetric ultrasound demonstrated complete placenta previa.",
"The patient had two prior pregnancies that were complicated by complete placenta previa. Both of these pregnancies were delivered by low transverse cesarean section.",
"The patient has no other surgical history or significant medical history. She has no known allergies and is not currently taking medications. She does not report any vaginal bleeding. She has no abdominal pain, cramps, or contractions.",
"Quickening was first noted at 19 weeks' gestation, and she continues to feel regular fetal movement. She denies having any vaginal discharge or leakage of fluid. She has no other symptoms."
],
"date": "April 25, 2017",
"figures": [],
"markdown": "# A 30-Year-Old Woman With an Abnormal Fetal Ultrasound\n\n **Authors:** Craig Johnson, DO; Zachary Redus; Michael Mader, MD; Frederick Eruo, MD, MPH \n **Date:** April 25, 2017\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nA 30-year-old gravida 3, para 2 patient who is 23 weeks pregnant presents to the maternal/fetal medicine department. She was referred to the department at the request of her primary obstetrician after an obstetric ultrasound demonstrated complete placenta previa.\nThe patient had two prior pregnancies that were complicated by complete placenta previa. Both of these pregnancies were delivered by low transverse cesarean section.\nThe patient has no other surgical history or significant medical history. She has no known allergies and is not currently taking medications. She does not report any vaginal bleeding. She has no abdominal pain, cramps, or contractions.\nQuickening was first noted at 19 weeks' gestation, and she continues to feel regular fetal movement. She denies having any vaginal discharge or leakage of fluid. She has no other symptoms.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 30-Year-Old Woman With an Abnormal Fetal Ultrasound"
},
{
"authors": "Craig Johnson, DO; Zachary Redus; Michael Mader, MD; Frederick Eruo, MD, MPH",
"content": [
"The patient is well-appearing on physical examination. Her blood pressure is 90/60 mm Hg, and her heart rate is 90 beats/min. She has normal respirations at a rate of 12 breaths/min, with an oxygen saturation of 96% while breathing room air and a tympanically obtained temperature of 99°F. She weighs 165 lb and has a body mass index of 28.3 kg/m2.",
"Cardiovascular and respiratory examinations are unremarkable. The abdomen is gravid, with a fundal height of 24 cm. The patient has positive bowel sounds in four quadrants and no tenderness to palpation. No peripheral edema or rash is present.",
"The patient is blood type A-positive, with a negative antibody screen. The rapid plasma reagin test is nonreactive for syphilis, and the hepatitis B surface antigen test is negative. A diabetes screen result is normal at 23.4 weeks of gestation (however, this is typically done at 26-28 weeks of gestation). The antibody screen result is negative.",
"The complete blood cell count demonstrates a hemoglobin level of 14 g/dL (normal range, 12-15 g/dL) and a platelet count of 290 × 103 cells/µL (normal range, 100-450 × 103 cells/µL). Urinalysis demonstrates no evidence of hematuria or proteinuria.",
"Figure 1.",
"Figure 2.",
"Figure 3.",
"Follow-up ultrasonography is performed (Figures 1 and 2), followed by MRI (Figure 3)."
],
"date": "April 25, 2017",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/878/933/878933-Thumb1.jpg"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/878/933/878933-Thumb2.jpg"
},
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/878/933/878933-Thumb3.jpg"
}
],
"markdown": "# A 30-Year-Old Woman With an Abnormal Fetal Ultrasound\n\n **Authors:** Craig Johnson, DO; Zachary Redus; Michael Mader, MD; Frederick Eruo, MD, MPH \n **Date:** April 25, 2017\n\n ## Content\n\n The patient is well-appearing on physical examination. Her blood pressure is 90/60 mm Hg, and her heart rate is 90 beats/min. She has normal respirations at a rate of 12 breaths/min, with an oxygen saturation of 96% while breathing room air and a tympanically obtained temperature of 99°F. She weighs 165 lb and has a body mass index of 28.3 kg/m2.\nCardiovascular and respiratory examinations are unremarkable. The abdomen is gravid, with a fundal height of 24 cm. The patient has positive bowel sounds in four quadrants and no tenderness to palpation. No peripheral edema or rash is present.\nThe patient is blood type A-positive, with a negative antibody screen. The rapid plasma reagin test is nonreactive for syphilis, and the hepatitis B surface antigen test is negative. A diabetes screen result is normal at 23.4 weeks of gestation (however, this is typically done at 26-28 weeks of gestation). The antibody screen result is negative.\nThe complete blood cell count demonstrates a hemoglobin level of 14 g/dL (normal range, 12-15 g/dL) and a platelet count of 290 × 103 cells/µL (normal range, 100-450 × 103 cells/µL). Urinalysis demonstrates no evidence of hematuria or proteinuria.\nFigure 1.\nFigure 2.\nFigure 3.\nFollow-up ultrasonography is performed (Figures 1 and 2), followed by MRI (Figure 3).\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n**Figure 3.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1088772,
"choiceText": "Concurrent placenta previa and placenta accreta",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1088774,
"choiceText": "Abruptio placentae",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1088776,
"choiceText": "Premature rupture of membranes",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1088778,
"choiceText": "Vasa previa\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 344955,
"questionText": "On the basis of the history, physical examination, and workup, what is the likely diagnosis?<br><br><i>\r\n\r\nHint: Previous uterine surgery increases the risk for this condition.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 30-Year-Old Woman With an Abnormal Fetal Ultrasound"
},
{
"authors": "Craig Johnson, DO; Zachary Redus; Michael Mader, MD; Frederick Eruo, MD, MPH",
"content": [
"The ultrasound demonstrated complete placenta previa (Figure 1). In addition, at the site of previous cesarean delivery, significant thinning of the uterus and loss of the uterine/placental interface was noted (Figure 2). It was also difficult to visualize a plane between the bladder and the uterus; because of this, placenta accreta or placenta percreta was suspected.",
"Figure 1.",
"Figure 2.",
"Figure 3.",
"Figure 4.",
"Additional Doppler imaging did not demonstrate serosal extension of the placental vascularity. The subsequent MRI scan (Figure 3) also demonstrated focal loss of the uterine/placental interface (best visualized on the T2-weighted sequences) within the lower uterine segment at the site of previous cesarean section (Figure 4), which is consistent with at least placenta accreta. No placental invasion was visualized, but the fat plane between the bladder and the uterus was not clearly identified to rule out bladder invasion.",
"Placenta accreta was once a rare condition but is becoming increasingly more common. This is believed to be due to a rising cesarean delivery rate.[1]Other risk factors for placenta accreta include multiparity; increasing maternal age; endometrial defects; scarring of the uterus (Asherman syndrome); and, most significantly, placenta previa.",
"Placenta previa is implantation of the placenta in the immediate vicinity of the cervical canal. In complete placenta previa, the placenta covers the entire opening of the internal cervical os. In partial placenta previa, the placenta partially covers the internal cervical os. Marginal placenta previa and low-lying placenta are also described; these occur when the placenta extends to the edge of the internal cervical os or within 2 cm of the internal cervical os, respectively. An important point is that most cases of complete placenta previa early in the second trimester resolve during pregnancy.[1]",
"Placenta accreta is one of three different types of abnormal placentation. Placenta accreta, which accounts for 75% of cases, is the most common presenting type. Placenta accreta occurs when the placental villi adheres directly to the myometrium but does not penetrate the muscular layer, with complete or partial absence of the decidua basalis. Placenta increta accounts for 15% of all cases and is characterized by the adherence of placental villi directly to the myometrium and demonstrates penetration within the myometrium. Placenta percreta, the least common of the three, involves the penetration of the placental villi into the serosal layer of the uterus. Direct attachment to adjacent organs may also occur.",
"Although these three types of abnormal placentation are characteristically different, the literature often refers to them collectively as \"placenta accreta.\"",
"The clinical history in this particular case is extremely important, because the risk for placenta accreta is approximately 40% in a patient with placenta previa and a history of two previous cesarean deliveries. Imaging can help raise or lower the concern for placenta accreta in high-risk patients, but it is not usually definitive, which limits its ability to change management in these patients."
],
"date": "April 25, 2017",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/878/933/878933-Thumb1.jpg"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/878/933/878933-Thumb2.jpg"
},
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/878/933/878933-Thumb3.jpg"
},
{
"caption": "Figure 4.",
"image_url": "https://img.medscapestatic.com/article/878/933/878933-Thumb4.jpg"
}
],
"markdown": "# A 30-Year-Old Woman With an Abnormal Fetal Ultrasound\n\n **Authors:** Craig Johnson, DO; Zachary Redus; Michael Mader, MD; Frederick Eruo, MD, MPH \n **Date:** April 25, 2017\n\n ## Content\n\n The ultrasound demonstrated complete placenta previa (Figure 1). In addition, at the site of previous cesarean delivery, significant thinning of the uterus and loss of the uterine/placental interface was noted (Figure 2). It was also difficult to visualize a plane between the bladder and the uterus; because of this, placenta accreta or placenta percreta was suspected.\nFigure 1.\nFigure 2.\nFigure 3.\nFigure 4.\nAdditional Doppler imaging did not demonstrate serosal extension of the placental vascularity. The subsequent MRI scan (Figure 3) also demonstrated focal loss of the uterine/placental interface (best visualized on the T2-weighted sequences) within the lower uterine segment at the site of previous cesarean section (Figure 4), which is consistent with at least placenta accreta. No placental invasion was visualized, but the fat plane between the bladder and the uterus was not clearly identified to rule out bladder invasion.\nPlacenta accreta was once a rare condition but is becoming increasingly more common. This is believed to be due to a rising cesarean delivery rate.[1]Other risk factors for placenta accreta include multiparity; increasing maternal age; endometrial defects; scarring of the uterus (Asherman syndrome); and, most significantly, placenta previa.\nPlacenta previa is implantation of the placenta in the immediate vicinity of the cervical canal. In complete placenta previa, the placenta covers the entire opening of the internal cervical os. In partial placenta previa, the placenta partially covers the internal cervical os. Marginal placenta previa and low-lying placenta are also described; these occur when the placenta extends to the edge of the internal cervical os or within 2 cm of the internal cervical os, respectively. An important point is that most cases of complete placenta previa early in the second trimester resolve during pregnancy.[1]\nPlacenta accreta is one of three different types of abnormal placentation. Placenta accreta, which accounts for 75% of cases, is the most common presenting type. Placenta accreta occurs when the placental villi adheres directly to the myometrium but does not penetrate the muscular layer, with complete or partial absence of the decidua basalis. Placenta increta accounts for 15% of all cases and is characterized by the adherence of placental villi directly to the myometrium and demonstrates penetration within the myometrium. Placenta percreta, the least common of the three, involves the penetration of the placental villi into the serosal layer of the uterus. Direct attachment to adjacent organs may also occur.\nAlthough these three types of abnormal placentation are characteristically different, the literature often refers to them collectively as \"placenta accreta.\"\nThe clinical history in this particular case is extremely important, because the risk for placenta accreta is approximately 40% in a patient with placenta previa and a history of two previous cesarean deliveries. Imaging can help raise or lower the concern for placenta accreta in high-risk patients, but it is not usually definitive, which limits its ability to change management in these patients.\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n**Figure 3.** \n \n\n**Figure 4.** \n \n\n\n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1088772,
"choiceText": "Concurrent placenta previa and placenta accreta",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1088774,
"choiceText": "Abruptio placentae",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1088776,
"choiceText": "Premature rupture of membranes",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1088778,
"choiceText": "Vasa previa\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 344955,
"questionText": "On the basis of the history, physical examination, and workup, what is the likely diagnosis?<br><br><i>\r\n\r\nHint: Previous uterine surgery increases the risk for this condition.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 30-Year-Old Woman With an Abnormal Fetal Ultrasound"
},
{
"authors": "Craig Johnson, DO; Zachary Redus; Michael Mader, MD; Frederick Eruo, MD, MPH",
"content": [
"Transabdominal and transvaginal ultrasonography remain the imaging modalities of choice in detecting placenta accreta. In women with a history of cesarean delivery, second-trimester sonographic evaluation of placenta accreta may be helpful. The location of the previous cesarean section should be closely examined, because the placenta has a tendency to adhere to this area.",
"MRI is commonly used when ultrasonography remains uncertain. Although ultrasonography and MRI provide a high degree of suspicion for placenta accreta, no imaging study can diagnose placenta accreta with absolute accuracy. This has been demonstrated in numerous studies that have compared the imaging findings of placenta accreta with the pathologic evaluation. Sonographic findings that have a high index of suspicion for placenta accreta include, but are not limited to, loss of the hypoechoic retroplacental myometrial zone; thinned, hyperechoic uterine/bladder interface; presence of placental lacunae; and focal exophytic masses within the bladder.",
"Color Doppler imaging also aids in the evaluation of placenta accreta. The use of color Doppler has improved the sensitivity of gray-scale ultrasonography because it depicts the local vascular anatomy within the uterus and related organs.[2]",
"MRI has come to the forefront in the evaluation of placenta accreta. MRI findings that are suggestive of placenta accreta include uterine bulging, heterogeneous signal intensity within the placenta, and the presence of dark intraplacental bands on T2-weighted imaging.[3]",
"Studies have compared the accuracy of MRI and ultrasonography in the diagnosis of placenta accreta. One study found that ultrasonography had a sensitivity of 77% and a specificity of 96%. In comparison, MRI had a sensitivity of 88% and a specificity of 100%. The superiority of MRI over ultrasonography, however, was not statistically significant.[1] In this study, all patients with ultrasonographic examinations suggestive of placenta accreta underwent MRI. If placenta accreta was suspected, the patient also underwent a dynamic gadolinium-enhanced MRI series. This confirmed the presence of deep invasion.",
"Palacios Jaraquemada and Bruno[4] studied 300 patients with suspected placenta accreta using gadolinium contrast in an attempt to classify the depth and topographic areas in relation to the posterior bladder wall. The MRI scans were compared with pathologic findings. The results of this study, however, were not used to further define the screening characteristics of placenta accreta, but rather to establish a modified surgical technique. The aim was to reduce complications at the time of surgery. Because gadolinium crosses the placenta and its half-life and safety profile in pregnancy have not been established, the American College of Radiology recommends its use only if the benefits outweigh the potential risks.",
"Kim and Narra[5] defined the appearance of the placental myometrial interface using the half-Fourier acquisition single-shot turbo spin-echo (HASTE) sequence. The HASTE sequence has three layers: an inner low-signal-intensity layer, a middle high-signal-intensity layer, and an outer low-signal-intensity layer. In placenta accreta, focal nonvisualization of the inner layer was noted. The limitations of this study were that only five patients were included, all of whom had prior cesarean deliveries, and no comparative studies could be made.",
"Because a limited amount of literature describes the normal anatomy of this area with the use of fast sequences, MRI remains only an adjuvant to inconclusive ultrasonography findings or can be used to evaluate placenta accreta when adherence occurs in the posterior or fundal portions of the uterus.[1,2,3]"
],
"date": "April 25, 2017",
"figures": [],
"markdown": "# A 30-Year-Old Woman With an Abnormal Fetal Ultrasound\n\n **Authors:** Craig Johnson, DO; Zachary Redus; Michael Mader, MD; Frederick Eruo, MD, MPH \n **Date:** April 25, 2017\n\n ## Content\n\n Transabdominal and transvaginal ultrasonography remain the imaging modalities of choice in detecting placenta accreta. In women with a history of cesarean delivery, second-trimester sonographic evaluation of placenta accreta may be helpful. The location of the previous cesarean section should be closely examined, because the placenta has a tendency to adhere to this area.\nMRI is commonly used when ultrasonography remains uncertain. Although ultrasonography and MRI provide a high degree of suspicion for placenta accreta, no imaging study can diagnose placenta accreta with absolute accuracy. This has been demonstrated in numerous studies that have compared the imaging findings of placenta accreta with the pathologic evaluation. Sonographic findings that have a high index of suspicion for placenta accreta include, but are not limited to, loss of the hypoechoic retroplacental myometrial zone; thinned, hyperechoic uterine/bladder interface; presence of placental lacunae; and focal exophytic masses within the bladder.\nColor Doppler imaging also aids in the evaluation of placenta accreta. The use of color Doppler has improved the sensitivity of gray-scale ultrasonography because it depicts the local vascular anatomy within the uterus and related organs.[2]\nMRI has come to the forefront in the evaluation of placenta accreta. MRI findings that are suggestive of placenta accreta include uterine bulging, heterogeneous signal intensity within the placenta, and the presence of dark intraplacental bands on T2-weighted imaging.[3]\nStudies have compared the accuracy of MRI and ultrasonography in the diagnosis of placenta accreta. One study found that ultrasonography had a sensitivity of 77% and a specificity of 96%. In comparison, MRI had a sensitivity of 88% and a specificity of 100%. The superiority of MRI over ultrasonography, however, was not statistically significant.[1] In this study, all patients with ultrasonographic examinations suggestive of placenta accreta underwent MRI. If placenta accreta was suspected, the patient also underwent a dynamic gadolinium-enhanced MRI series. This confirmed the presence of deep invasion.\nPalacios Jaraquemada and Bruno[4] studied 300 patients with suspected placenta accreta using gadolinium contrast in an attempt to classify the depth and topographic areas in relation to the posterior bladder wall. The MRI scans were compared with pathologic findings. The results of this study, however, were not used to further define the screening characteristics of placenta accreta, but rather to establish a modified surgical technique. The aim was to reduce complications at the time of surgery. Because gadolinium crosses the placenta and its half-life and safety profile in pregnancy have not been established, the American College of Radiology recommends its use only if the benefits outweigh the potential risks.\nKim and Narra[5] defined the appearance of the placental myometrial interface using the half-Fourier acquisition single-shot turbo spin-echo (HASTE) sequence. The HASTE sequence has three layers: an inner low-signal-intensity layer, a middle high-signal-intensity layer, and an outer low-signal-intensity layer. In placenta accreta, focal nonvisualization of the inner layer was noted. The limitations of this study were that only five patients were included, all of whom had prior cesarean deliveries, and no comparative studies could be made.\nBecause a limited amount of literature describes the normal anatomy of this area with the use of fast sequences, MRI remains only an adjuvant to inconclusive ultrasonography findings or can be used to evaluate placenta accreta when adherence occurs in the posterior or fundal portions of the uterus.[1,2,3]\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 30-Year-Old Woman With an Abnormal Fetal Ultrasound"
},
{
"authors": "Craig Johnson, DO; Zachary Redus; Michael Mader, MD; Frederick Eruo, MD, MPH",
"content": [
"Placenta accreta is potentially life-threatening to both mother and fetus. The immediate clinical consequence of placenta accreta is massive hemorrhage at the time of placenta removal, and it is the most common indication for emergency intra- or postpartum hysterectomy.[1]",
"Because placenta previa is a risk factor for placental accreta, patients commonly present with third-trimester painless vaginal bleeding. Such complications as hematuria may occur if placenta percreta is present, resulting from direct penetration into the bladder. Because of this, urinalysis is sometimes performed.",
"In a patient with imaging findings highly suggestive of placenta accreta, management must begin in the prenatal period. During this period, a multidisciplinary approach must be used to fully inform the patient and family of the potential outcomes and management options.",
"The risks and benefits of extirpative vs conservative management should be adequately discussed with the patient. Extirpative management, with a scheduled cesarean delivery and hysterectomy at 35-37 weeks of gestation, may be performed. If the patient opts for uterine conservation to allow subsequent pregnancy, measures can be implemented. Commonly, cesarean deliveries alone can be successful because most cases of placenta accreta are focal. Methotrexate administration and uterine artery embolization have been reported, and the literature has either demonstrated various results (with methotrexate) or suggested no benefit (with uterine artery embolization).[6]",
"Whether extirpative or uterine-conserving management is chosen, the delivery must be performed in a center with staff trained for complicated surgeries potentially involving the bowel or bladder; appropriate equipment and resources should be available to handle the many significant complications that may be encountered during the procedure. A blood bank capable of providing large volumes of blood in emergency situations is particularly important.[1,6]",
"Given the patient's findings on ultrasonography and MRI, a cesarean delivery was scheduled, with a high probability of hysterectomy. At the time of the cesarean delivery, the patient was at 36 weeks and 5 days of intrauterine gestation by ultrasonographic dating. As a result of the close approximation of the ureters and the increased risk for trauma during this procedure, the patient underwent bilateral ureteral stent placement for direct visualization of the ureters. After delivery of the fetus, a portion of the placenta remained tightly adhered to the uterus. The decision was made to proceed with cesarean supracervical hysterectomy.",
"The patient tolerated the procedure well. No complications were noted, and the patient was taken to the recovery room in stable condition. The pathologic findings were an area of thinning consistent with previous low transverse incision, with scar formation on the anterior surface of the uterus. This scarred area was focally and markedly thinned to 2 mm. The placenta was adherent to this area. Examination of the surgical specimen displayed a uterus with focal changes of placenta accreta. The patient was discharged to home from the hospital 4 days after surgery."
],
"date": "April 25, 2017",
"figures": [],
"markdown": "# A 30-Year-Old Woman With an Abnormal Fetal Ultrasound\n\n **Authors:** Craig Johnson, DO; Zachary Redus; Michael Mader, MD; Frederick Eruo, MD, MPH \n **Date:** April 25, 2017\n\n ## Content\n\n Placenta accreta is potentially life-threatening to both mother and fetus. The immediate clinical consequence of placenta accreta is massive hemorrhage at the time of placenta removal, and it is the most common indication for emergency intra- or postpartum hysterectomy.[1]\nBecause placenta previa is a risk factor for placental accreta, patients commonly present with third-trimester painless vaginal bleeding. Such complications as hematuria may occur if placenta percreta is present, resulting from direct penetration into the bladder. Because of this, urinalysis is sometimes performed.\nIn a patient with imaging findings highly suggestive of placenta accreta, management must begin in the prenatal period. During this period, a multidisciplinary approach must be used to fully inform the patient and family of the potential outcomes and management options.\nThe risks and benefits of extirpative vs conservative management should be adequately discussed with the patient. Extirpative management, with a scheduled cesarean delivery and hysterectomy at 35-37 weeks of gestation, may be performed. If the patient opts for uterine conservation to allow subsequent pregnancy, measures can be implemented. Commonly, cesarean deliveries alone can be successful because most cases of placenta accreta are focal. Methotrexate administration and uterine artery embolization have been reported, and the literature has either demonstrated various results (with methotrexate) or suggested no benefit (with uterine artery embolization).[6]\nWhether extirpative or uterine-conserving management is chosen, the delivery must be performed in a center with staff trained for complicated surgeries potentially involving the bowel or bladder; appropriate equipment and resources should be available to handle the many significant complications that may be encountered during the procedure. A blood bank capable of providing large volumes of blood in emergency situations is particularly important.[1,6]\nGiven the patient's findings on ultrasonography and MRI, a cesarean delivery was scheduled, with a high probability of hysterectomy. At the time of the cesarean delivery, the patient was at 36 weeks and 5 days of intrauterine gestation by ultrasonographic dating. As a result of the close approximation of the ureters and the increased risk for trauma during this procedure, the patient underwent bilateral ureteral stent placement for direct visualization of the ureters. After delivery of the fetus, a portion of the placenta remained tightly adhered to the uterus. The decision was made to proceed with cesarean supracervical hysterectomy.\nThe patient tolerated the procedure well. No complications were noted, and the patient was taken to the recovery room in stable condition. The pathologic findings were an area of thinning consistent with previous low transverse incision, with scar formation on the anterior surface of the uterus. This scarred area was focally and markedly thinned to 2 mm. The placenta was adherent to this area. Examination of the surgical specimen displayed a uterus with focal changes of placenta accreta. The patient was discharged to home from the hospital 4 days after surgery.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1088780,
"choiceText": "Placenta accreta\r\n",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1088782,
"choiceText": "Placenta percreta",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1088784,
"choiceText": "Placenta increta",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1088786,
"choiceText": "Placenta previa\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Placenta percreta involves the penetration of the placental villi into the serosal layer of the uterus. Direct attachment to adjacent organs may also occur.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 344957,
"questionText": "You are treating a patient whose sagittal T2-weighted HASTE sequence demonstrates loss of the placental myometrial interface, with placental extension through the serosa and into the adjacent bladder wall. Which of the following conditions is most likely in this patient?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1088788,
"choiceText": "Uterine surgery",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1088790,
"choiceText": "Adenomyosis",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1088792,
"choiceText": "Prior dilation and curettage",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1088794,
"choiceText": "Prior cesarean delivery",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "A history of uterine surgery increases the risk for placenta percreta, as does prior dilation and curettage and previous cesarean delivery. Placenta accreta was once a rare condition but is becoming increasingly more common. This is believed to be due to a rising cesarean delivery rate. Other risk factors for placenta accreta include multiparity; increasing maternal age; endometrial defects; scarring of the uterus (Asherman syndrome); and most significantly, placenta previa. ",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 344959,
"questionText": "You receive MRI confirmation that one of your patients has placenta percreta. Which of the following conditions, if part of the patient's history, would not have increased this patient's risk of developing placenta percreta?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 30-Year-Old Woman With an Abnormal Fetal Ultrasound"
},
{
"authors": "Craig Johnson, DO; Zachary Redus; Michael Mader, MD; Frederick Eruo, MD, MPH",
"content": [],
"date": "April 25, 2017",
"figures": [],
"markdown": "# A 30-Year-Old Woman With an Abnormal Fetal Ultrasound\n\n **Authors:** Craig Johnson, DO; Zachary Redus; Michael Mader, MD; Frederick Eruo, MD, MPH \n **Date:** April 25, 2017\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1088780,
"choiceText": "Placenta accreta\r\n",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1088782,
"choiceText": "Placenta percreta",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1088784,
"choiceText": "Placenta increta",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1088786,
"choiceText": "Placenta previa\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Placenta percreta involves the penetration of the placental villi into the serosal layer of the uterus. Direct attachment to adjacent organs may also occur.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 344957,
"questionText": "You are treating a patient whose sagittal T2-weighted HASTE sequence demonstrates loss of the placental myometrial interface, with placental extension through the serosa and into the adjacent bladder wall. Which of the following conditions is most likely in this patient?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1088788,
"choiceText": "Uterine surgery",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1088790,
"choiceText": "Adenomyosis",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1088792,
"choiceText": "Prior dilation and curettage",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1088794,
"choiceText": "Prior cesarean delivery",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "A history of uterine surgery increases the risk for placenta percreta, as does prior dilation and curettage and previous cesarean delivery. Placenta accreta was once a rare condition but is becoming increasingly more common. This is believed to be due to a rising cesarean delivery rate. Other risk factors for placenta accreta include multiparity; increasing maternal age; endometrial defects; scarring of the uterus (Asherman syndrome); and most significantly, placenta previa. ",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 344959,
"questionText": "You receive MRI confirmation that one of your patients has placenta percreta. Which of the following conditions, if part of the patient's history, would not have increased this patient's risk of developing placenta percreta?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 30-Year-Old Woman With an Abnormal Fetal Ultrasound"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1088772,
"choiceText": "Concurrent placenta previa and placenta accreta",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1088774,
"choiceText": "Abruptio placentae",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1088776,
"choiceText": "Premature rupture of membranes",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1088778,
"choiceText": "Vasa previa\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 344955,
"questionText": "On the basis of the history, physical examination, and workup, what is the likely diagnosis?<br><br><i>\r\n\r\nHint: Previous uterine surgery increases the risk for this condition.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1088780,
"choiceText": "Placenta accreta\r\n",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1088782,
"choiceText": "Placenta percreta",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1088784,
"choiceText": "Placenta increta",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1088786,
"choiceText": "Placenta previa\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Placenta percreta involves the penetration of the placental villi into the serosal layer of the uterus. Direct attachment to adjacent organs may also occur.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 344957,
"questionText": "You are treating a patient whose sagittal T2-weighted HASTE sequence demonstrates loss of the placental myometrial interface, with placental extension through the serosa and into the adjacent bladder wall. Which of the following conditions is most likely in this patient?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1088788,
"choiceText": "Uterine surgery",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1088790,
"choiceText": "Adenomyosis",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1088792,
"choiceText": "Prior dilation and curettage",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1088794,
"choiceText": "Prior cesarean delivery",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "A history of uterine surgery increases the risk for placenta percreta, as does prior dilation and curettage and previous cesarean delivery. Placenta accreta was once a rare condition but is becoming increasingly more common. This is believed to be due to a rising cesarean delivery rate. Other risk factors for placenta accreta include multiparity; increasing maternal age; endometrial defects; scarring of the uterus (Asherman syndrome); and most significantly, placenta previa. ",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 344959,
"questionText": "You receive MRI confirmation that one of your patients has placenta percreta. Which of the following conditions, if part of the patient's history, would not have increased this patient's risk of developing placenta percreta?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
877714 | /viewarticle/877714 | [
{
"authors": "Sumaira Nabi, MBBS; Shahzad Ahmed, MBBS; Mazhar Badshah, MBBS",
"content": [
"Editor's Note:\n\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"A 20-year-old woman presents with a 2-week history of bilateral flank pain and episodic vomiting. She has no history of fever, dysuria, polyuria, hematuria, or urinary urgency. She has had epilepsy since early childhood and has been on multiple antiepileptic drugs with variable status of control.",
"She is taking the tablet form of levetiracetam (500 mg) three times a day and tablet carbamazepine (400 mg) three times a day. She is single and unemployed. She is a nonsmoker and denies substance abuse. Her family history is noncontributory."
],
"date": "March 28, 2017",
"figures": [],
"markdown": "# A 20-Year-Old Woman With Flank Pain and Vomiting\n\n **Authors:** Sumaira Nabi, MBBS; Shahzad Ahmed, MBBS; Mazhar Badshah, MBBS \n **Date:** March 28, 2017\n\n ## Content\n\n Editor's Note:\n\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nA 20-year-old woman presents with a 2-week history of bilateral flank pain and episodic vomiting. She has no history of fever, dysuria, polyuria, hematuria, or urinary urgency. She has had epilepsy since early childhood and has been on multiple antiepileptic drugs with variable status of control.\nShe is taking the tablet form of levetiracetam (500 mg) three times a day and tablet carbamazepine (400 mg) three times a day. She is single and unemployed. She is a nonsmoker and denies substance abuse. Her family history is noncontributory.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 20-Year-Old Woman With Flank Pain and Vomiting"
},
{
"authors": "Sumaira Nabi, MBBS; Shahzad Ahmed, MBBS; Mazhar Badshah, MBBS",
"content": [
"Upon clinical examination, she is an alert young female oriented to time, place, and person. Her vital signs include an oral temperature of 98.6°F, a regular pulse of 76 beats/min, and a blood pressure of 110/70 mm Hg. Her respiratory rate is 16 breaths/min. Her Glasgow coma scale score is 15/15, and her higher mental functions are intact.",
"Figure 1.",
"Figure 1.",
"Figure 2.",
"Figure 2.",
"Figure 3.",
"Figure 3.",
"Figure 4.",
"Figure 4.",
"Upon general physical examination, she has malar angiofibromas on the face (Figure 1). She has a leathery patch on her lower back. She also has periungual fibromas on her hands and toes (Figure 2, Figure 3, Figure 4).",
"Upon neurologic examination, her cranial nerves are intact and symmetric. No signs suggest meningeal irritation, pyramidal weakness, or incoordination. Her abdomen is soft, with flank fullness. She has bimanually palpable, nontender, cystic-to-firm masses that extend from the hypochondrium to the lumbar region on both sides. Her bowel sounds are audible. The patient's precordial examination reveals normal heart sounds. Auscultation of the lung fields shows normal vesicular breathing.",
"The laboratory analysis demonstrates a complete blood cell count that reveals anemia, with a hemoglobin of 10 mg/dL; the remainder of the cell counts and erythrocyte sedimentation rate results are normal. Her liver function test results, renal function test results, serum glucose levels, ECG findings, echocardiography findings, and chest x-ray findings are unremarkable.",
"Figure 5.",
"Figure 5.",
"Figure 6.",
"Figure 6.",
"Figure 7.",
"Figure 7.",
"Urine examination reveals albuminuria and microscopic hematuria. CT scan of the abdomen shows kidneys replaced by heterogeneously enhancing, large, well-defined lobulated mass lesions, with variable proportions of soft tissue and fat density areas as well as angioid tissue, consistent with bilateral renal angiomyolipomas (Figure 5, Figure 6). It measures 11 cm by 10 cm on the right side, whereas on the left side it measures 7 cm by 5 cm. The rest of the viscera are normal.",
"Intravenous urography shows an almost nonfunctioning right kidney and a left kidney with near-normal functioning. A non-contrast-enhanced CT scan of the brain reveals subependymal calcified nodules in the bilateral lateral ventricles (Figure 7)."
],
"date": "March 28, 2017",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/877/714/877714-Thumb1.png"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/877/714/877714-Thumb2.png"
},
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/877/714/877714-Thumb3.png"
},
{
"caption": "Figure 4.",
"image_url": "https://img.medscapestatic.com/article/877/714/877714-Thumb4.png"
},
{
"caption": "Figure 5.",
"image_url": "https://img.medscapestatic.com/article/877/714/877714-Thumb5.png"
},
{
"caption": "Figure 6.",
"image_url": "https://img.medscapestatic.com/article/877/714/877714-Thumb6.png"
},
{
"caption": "Figure 7.",
"image_url": "https://img.medscapestatic.com/article/877/714/877714-Thumb7.png"
}
],
"markdown": "# A 20-Year-Old Woman With Flank Pain and Vomiting\n\n **Authors:** Sumaira Nabi, MBBS; Shahzad Ahmed, MBBS; Mazhar Badshah, MBBS \n **Date:** March 28, 2017\n\n ## Content\n\n Upon clinical examination, she is an alert young female oriented to time, place, and person. Her vital signs include an oral temperature of 98.6°F, a regular pulse of 76 beats/min, and a blood pressure of 110/70 mm Hg. Her respiratory rate is 16 breaths/min. Her Glasgow coma scale score is 15/15, and her higher mental functions are intact.\nFigure 1.\nFigure 1.\nFigure 2.\nFigure 2.\nFigure 3.\nFigure 3.\nFigure 4.\nFigure 4.\nUpon general physical examination, she has malar angiofibromas on the face (Figure 1). She has a leathery patch on her lower back. She also has periungual fibromas on her hands and toes (Figure 2, Figure 3, Figure 4).\nUpon neurologic examination, her cranial nerves are intact and symmetric. No signs suggest meningeal irritation, pyramidal weakness, or incoordination. Her abdomen is soft, with flank fullness. She has bimanually palpable, nontender, cystic-to-firm masses that extend from the hypochondrium to the lumbar region on both sides. Her bowel sounds are audible. The patient's precordial examination reveals normal heart sounds. Auscultation of the lung fields shows normal vesicular breathing.\nThe laboratory analysis demonstrates a complete blood cell count that reveals anemia, with a hemoglobin of 10 mg/dL; the remainder of the cell counts and erythrocyte sedimentation rate results are normal. Her liver function test results, renal function test results, serum glucose levels, ECG findings, echocardiography findings, and chest x-ray findings are unremarkable.\nFigure 5.\nFigure 5.\nFigure 6.\nFigure 6.\nFigure 7.\nFigure 7.\nUrine examination reveals albuminuria and microscopic hematuria. CT scan of the abdomen shows kidneys replaced by heterogeneously enhancing, large, well-defined lobulated mass lesions, with variable proportions of soft tissue and fat density areas as well as angioid tissue, consistent with bilateral renal angiomyolipomas (Figure 5, Figure 6). It measures 11 cm by 10 cm on the right side, whereas on the left side it measures 7 cm by 5 cm. The rest of the viscera are normal.\nIntravenous urography shows an almost nonfunctioning right kidney and a left kidney with near-normal functioning. A non-contrast-enhanced CT scan of the brain reveals subependymal calcified nodules in the bilateral lateral ventricles (Figure 7).\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n**Figure 3.** \n \n\n**Figure 4.** \n \n\n**Figure 5.** \n \n\n**Figure 6.** \n \n\n**Figure 7.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1082034,
"choiceText": "Tuberous sclerosis",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1082036,
"choiceText": "Neurofibromatosis type 1",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1082038,
"choiceText": "Sturge-Weber syndrome",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1082040,
"choiceText": "Neurofibromatosis type 2\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 342737,
"questionText": "Which of the following is the likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 20-Year-Old Woman With Flank Pain and Vomiting"
},
{
"authors": "Sumaira Nabi, MBBS; Shahzad Ahmed, MBBS; Mazhar Badshah, MBBS",
"content": [
"This patient was diagnosed with tuberous sclerosis (TS) with renal angiomyolipomas. TS or tuberous sclerosis complex (TSC) is an autosomal dominant neurocutaneous disorder. It was first described in 1880 by Bourneville and is also known as Bourneville disease. It is characterized by multisystem involvement, including the brain, skin, eyes, heart, lung, liver, and kidneys in different combinations with varying severity.[1] The estimated prevalence varies; birth incidence is 1 case per 6000 population, with a prevalence of 1 case per 10,000 population in the United States, whereas prevalence is 0.7-3.8 cases per 100,000 population in the United Kingdom.[2] All races, ethnic groups, and sexes are equally affected.[2] TSC is caused by mutations of the TSC1 gene on chromosome 9 and TSC2 gene on chromosome 16; these encode for the proteins hamartin and tuberin, respectively.[3,4] These proteins are tumor suppressors that regulate cell division, growth, and differentiation.",
"Figure 1.",
"Figure 1.",
"Figure 2.",
"Figure 2.",
"TSC is characterized by the development of various benign tumors in different organ systems. The phenotypic expression widely varies. Almost all patients have one or more of the characteristic skin lesions.[5] The typical dermatologic features include ash-leaf macules, which are elliptical, 0.5- to 2-cm, hypopigmented spots that are most common on the trunk and lower extremities. These lesions may be present since birth. Angiofibromas, previously called adenoma sebaceum, typically involve the malar aspects of the face, as seen in this patient. These erythematous lesions have increased growth during puberty and are sometimes misdiagnosed as acne. Shagreen patches have an orange-peel or leathery texture. These connective tissue hamartomas are often seen in children aged 2-6 years, on the lower trunk, as was seen in this case. Periungual and subungual fibromas develop later on and occur more commonly on the toenails.[6]",
"Brain lesions typical of TSC include cortical tubers, subependymal nodules, subependymal giant cell astrocytomas (SEGAs), and dysplastic, dysmyelinated white matter lesions.[7,8] Subependymal nodules are areas of rounded hypertrophic tissue distributed along the walls of the lateral ventricles and are usually seen as discrete or confluent calcified spots on CT scans of the brain. These nodules and cortical tubers are visualized in nearly 90% of patients with TSC on MRI. SEGAs are proposed to arise from pre-existing subependymal nodules, thus are periventricular in location, and are seen in 5%-20% of cases.",
"The severity of neurologic manifestations is proportional to the burden of radiologic disease. Symptoms include seizures, mental retardation, and behavioral dysfunction including autism. Seizures are encountered in almost 90% of cases and are typically difficult to treat."
],
"date": "March 28, 2017",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/877/714/877714-Thumb1.png"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/877/714/877714-Thumb2.png"
}
],
"markdown": "# A 20-Year-Old Woman With Flank Pain and Vomiting\n\n **Authors:** Sumaira Nabi, MBBS; Shahzad Ahmed, MBBS; Mazhar Badshah, MBBS \n **Date:** March 28, 2017\n\n ## Content\n\n This patient was diagnosed with tuberous sclerosis (TS) with renal angiomyolipomas. TS or tuberous sclerosis complex (TSC) is an autosomal dominant neurocutaneous disorder. It was first described in 1880 by Bourneville and is also known as Bourneville disease. It is characterized by multisystem involvement, including the brain, skin, eyes, heart, lung, liver, and kidneys in different combinations with varying severity.[1] The estimated prevalence varies; birth incidence is 1 case per 6000 population, with a prevalence of 1 case per 10,000 population in the United States, whereas prevalence is 0.7-3.8 cases per 100,000 population in the United Kingdom.[2] All races, ethnic groups, and sexes are equally affected.[2] TSC is caused by mutations of the TSC1 gene on chromosome 9 and TSC2 gene on chromosome 16; these encode for the proteins hamartin and tuberin, respectively.[3,4] These proteins are tumor suppressors that regulate cell division, growth, and differentiation.\nFigure 1.\nFigure 1.\nFigure 2.\nFigure 2.\nTSC is characterized by the development of various benign tumors in different organ systems. The phenotypic expression widely varies. Almost all patients have one or more of the characteristic skin lesions.[5] The typical dermatologic features include ash-leaf macules, which are elliptical, 0.5- to 2-cm, hypopigmented spots that are most common on the trunk and lower extremities. These lesions may be present since birth. Angiofibromas, previously called adenoma sebaceum, typically involve the malar aspects of the face, as seen in this patient. These erythematous lesions have increased growth during puberty and are sometimes misdiagnosed as acne. Shagreen patches have an orange-peel or leathery texture. These connective tissue hamartomas are often seen in children aged 2-6 years, on the lower trunk, as was seen in this case. Periungual and subungual fibromas develop later on and occur more commonly on the toenails.[6]\nBrain lesions typical of TSC include cortical tubers, subependymal nodules, subependymal giant cell astrocytomas (SEGAs), and dysplastic, dysmyelinated white matter lesions.[7,8] Subependymal nodules are areas of rounded hypertrophic tissue distributed along the walls of the lateral ventricles and are usually seen as discrete or confluent calcified spots on CT scans of the brain. These nodules and cortical tubers are visualized in nearly 90% of patients with TSC on MRI. SEGAs are proposed to arise from pre-existing subependymal nodules, thus are periventricular in location, and are seen in 5%-20% of cases.\nThe severity of neurologic manifestations is proportional to the burden of radiologic disease. Symptoms include seizures, mental retardation, and behavioral dysfunction including autism. Seizures are encountered in almost 90% of cases and are typically difficult to treat.\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n\n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1082034,
"choiceText": "Tuberous sclerosis",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1082036,
"choiceText": "Neurofibromatosis type 1",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1082038,
"choiceText": "Sturge-Weber syndrome",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1082040,
"choiceText": "Neurofibromatosis type 2\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 342737,
"questionText": "Which of the following is the likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 20-Year-Old Woman With Flank Pain and Vomiting"
},
{
"authors": "Sumaira Nabi, MBBS; Shahzad Ahmed, MBBS; Mazhar Badshah, MBBS",
"content": [
"Renal involvement is frequently seen in TS. Angiomyolipoma is the most common renal manifestation of TSC and is seen in 50%-80% of cases. These may arise from the renal cortex or medulla. Angiomyolipomas are benign in nature and are usually bilateral and multiple. An increase in the size of angiomyolipomas can lead to pain, hematuria, and disturbance in renal function. Other lesions include benign cysts, lymphangiomas, and renal cell carcinoma.[9] Eye involvement may be retinal, in the form of astrocytic hamartomas. Nonretinal eye involvement may include coloboma, eyelid angiofibroma, nonparalytic strabismus, refractive errors, and papilledema.[10]",
"Multiple skeletal manifestations have been listed in literature, including sclerotic lesions, scoliosis, and bone cysts in the metatarsals, metacarpals, and phalanges.[11] The most characteristic cardiovascular feature is a rhabdomyoma,[12] which is found in approximately 50% of TSC cases on echocardiography. These can lead to valvular dysfunction, outflow obstruction, cardiomyopathy, and cardiac arrhythmias but typically regress over time. Coarctation of the aorta and aortic aneurysm have also been reported. The pulmonary manifestation of TSC is lymphangioleiomyomatosis, which presents with dyspnea and pneumothorax. Rarely, liver leiomyomas or adenomas may be seen.",
"The diagnosis is based on clinical evaluation, laboratory studies, and genetic testing. A thorough history of symptoms associated with TSC should be elicited. A detailed family history is also important. Obtaining a three-generation family history is recommended by international guidelines.[13] A detailed and careful physical examination should be performed, with skin and neurologic systems thoroughly examined. One should look for characteristic dermatologic manifestations of TSC, including ash-leaf macules, facial fibroadenomas, shagreen patches, and periungual fibromas.",
"To support the diagnosis, meticulous neurologic examination and ophthalmic evaluation should be performed. MRI of the brain should be performed to detect cortical tubers, subependymal nodules, SEGAs, or cerebral white matter changes. An electroencephalogram should be obtained to assess for subclinical seizures; whether early antiseizure treatment may affect neurodevelopmental outcome is increasingly under consideration. Ultrasonography and CT scanning of the abdomen is recommended to screen for the presence of renal angiomyolipomas or cysts. Echocardiography, CT chest scanning, and bone x-ray are performed, depending on the history and physical examination findings."
],
"date": "March 28, 2017",
"figures": [],
"markdown": "# A 20-Year-Old Woman With Flank Pain and Vomiting\n\n **Authors:** Sumaira Nabi, MBBS; Shahzad Ahmed, MBBS; Mazhar Badshah, MBBS \n **Date:** March 28, 2017\n\n ## Content\n\n Renal involvement is frequently seen in TS. Angiomyolipoma is the most common renal manifestation of TSC and is seen in 50%-80% of cases. These may arise from the renal cortex or medulla. Angiomyolipomas are benign in nature and are usually bilateral and multiple. An increase in the size of angiomyolipomas can lead to pain, hematuria, and disturbance in renal function. Other lesions include benign cysts, lymphangiomas, and renal cell carcinoma.[9] Eye involvement may be retinal, in the form of astrocytic hamartomas. Nonretinal eye involvement may include coloboma, eyelid angiofibroma, nonparalytic strabismus, refractive errors, and papilledema.[10]\nMultiple skeletal manifestations have been listed in literature, including sclerotic lesions, scoliosis, and bone cysts in the metatarsals, metacarpals, and phalanges.[11] The most characteristic cardiovascular feature is a rhabdomyoma,[12] which is found in approximately 50% of TSC cases on echocardiography. These can lead to valvular dysfunction, outflow obstruction, cardiomyopathy, and cardiac arrhythmias but typically regress over time. Coarctation of the aorta and aortic aneurysm have also been reported. The pulmonary manifestation of TSC is lymphangioleiomyomatosis, which presents with dyspnea and pneumothorax. Rarely, liver leiomyomas or adenomas may be seen.\nThe diagnosis is based on clinical evaluation, laboratory studies, and genetic testing. A thorough history of symptoms associated with TSC should be elicited. A detailed family history is also important. Obtaining a three-generation family history is recommended by international guidelines.[13] A detailed and careful physical examination should be performed, with skin and neurologic systems thoroughly examined. One should look for characteristic dermatologic manifestations of TSC, including ash-leaf macules, facial fibroadenomas, shagreen patches, and periungual fibromas.\nTo support the diagnosis, meticulous neurologic examination and ophthalmic evaluation should be performed. MRI of the brain should be performed to detect cortical tubers, subependymal nodules, SEGAs, or cerebral white matter changes. An electroencephalogram should be obtained to assess for subclinical seizures; whether early antiseizure treatment may affect neurodevelopmental outcome is increasingly under consideration. Ultrasonography and CT scanning of the abdomen is recommended to screen for the presence of renal angiomyolipomas or cysts. Echocardiography, CT chest scanning, and bone x-ray are performed, depending on the history and physical examination findings.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 20-Year-Old Woman With Flank Pain and Vomiting"
},
{
"authors": "Sumaira Nabi, MBBS; Shahzad Ahmed, MBBS; Mazhar Badshah, MBBS",
"content": [
"In 2012, new criteria for the diagnosis of TSC were published by the International Tuberous Sclerosis Complex Consensus Conference.[13] They include the following 11 major and six minor features. The major features are as follows:",
"Hypomelanotic macules (three, at least 5 mm diameter)",
"Angiofibromas (three) or fibrous cephalic plaque",
"Ungual fibromas (two)",
"Shagreen patch",
"Multiple retinal hamartomas",
"Cortical dysplasias",
"Subependymal nodules",
"SEGA",
"Cardiac rhabdomyoma",
"Lymphangioleiomyomatosis",
"Angiomyolipomas (two)",
"Minor features include the following:",
"\"Confetti\" skin lesions",
"Dental enamel pits (more than three)",
"Intraoral fibromas (two)",
"Retinal achromic patch",
"Multiple renal cysts",
"Nonrenal hamartomas",
"Definite diagnosis is established by the presence of two major features or one major feature with two minor features. A possible diagnosis is established by either one major feature or two minor features.",
"The classic Vogt triad of seizures, mental retardation, and adenoma sebaceum is present in less than one third of patients and has become obsolete. Almost half of patients with TSC can have normal intellect. Molecular genetic testing for TSC gene mutations may be required and helpful for genetic counseling, although the clinical signatures are typically specific enough for confirmation of diagnosis. On molecular genetic testing, 10%-25% of patients with TSC have no mutation.",
"Management is directed at symptoms and on monitoring for progression of complications. Treatment of epilepsy in patients with TSC is difficult and often requires polytherapy. Depending on the type of seizures, appropriate and adequate antiepileptics should be prescribed. Nearly 63% cases of epilepsy in TSC are medically refractory.[14] Ketogenic diet or resective surgery of epileptogenic tubers are options for these patients.[15] Suggested frequency of monitoring tests has been documented by the 2012 International Tuberous Sclerosis Complex Consensus Conference, including brain MRI every 1-3 years until age 25 years; an annual clinical assessment of renal function and blood pressure; abdominal MRI, CT, or ultrasound; echocardiography every 1-3 years in asymptomatic patients until regression of cardiac rhabdomyomas is documented; 12-lead ECG every 3-5 years; and high-resolution chest CT scanning every 5-10 years in asymptomatic females ≥18 years and every 2-3 years in patients with lung cysts.[16]",
"Treatment options for brain tumors associated with TSC include surgical resection or medical therapy with mechanistic target of rapamycin inhibitors, such as everolimus. Renal, pulmonary, and cardiac disease, if present, require referral and management by specialists. The prognosis varies and depends on the number and severity of organ systems involved. It is a progressive disorder, and complications lead to morbidity and mortality. Status epilepticus, SEGA, and renal disease are the most common causes of death.",
"The patient in this case was referred to the urology department, who performed a right-sided nephrectomy. Her antiepileptic medication was also readjusted."
],
"date": "March 28, 2017",
"figures": [],
"markdown": "# A 20-Year-Old Woman With Flank Pain and Vomiting\n\n **Authors:** Sumaira Nabi, MBBS; Shahzad Ahmed, MBBS; Mazhar Badshah, MBBS \n **Date:** March 28, 2017\n\n ## Content\n\n In 2012, new criteria for the diagnosis of TSC were published by the International Tuberous Sclerosis Complex Consensus Conference.[13] They include the following 11 major and six minor features. The major features are as follows:\nHypomelanotic macules (three, at least 5 mm diameter)\nAngiofibromas (three) or fibrous cephalic plaque\nUngual fibromas (two)\nShagreen patch\nMultiple retinal hamartomas\nCortical dysplasias\nSubependymal nodules\nSEGA\nCardiac rhabdomyoma\nLymphangioleiomyomatosis\nAngiomyolipomas (two)\nMinor features include the following:\n\"Confetti\" skin lesions\nDental enamel pits (more than three)\nIntraoral fibromas (two)\nRetinal achromic patch\nMultiple renal cysts\nNonrenal hamartomas\nDefinite diagnosis is established by the presence of two major features or one major feature with two minor features. A possible diagnosis is established by either one major feature or two minor features.\nThe classic Vogt triad of seizures, mental retardation, and adenoma sebaceum is present in less than one third of patients and has become obsolete. Almost half of patients with TSC can have normal intellect. Molecular genetic testing for TSC gene mutations may be required and helpful for genetic counseling, although the clinical signatures are typically specific enough for confirmation of diagnosis. On molecular genetic testing, 10%-25% of patients with TSC have no mutation.\nManagement is directed at symptoms and on monitoring for progression of complications. Treatment of epilepsy in patients with TSC is difficult and often requires polytherapy. Depending on the type of seizures, appropriate and adequate antiepileptics should be prescribed. Nearly 63% cases of epilepsy in TSC are medically refractory.[14] Ketogenic diet or resective surgery of epileptogenic tubers are options for these patients.[15] Suggested frequency of monitoring tests has been documented by the 2012 International Tuberous Sclerosis Complex Consensus Conference, including brain MRI every 1-3 years until age 25 years; an annual clinical assessment of renal function and blood pressure; abdominal MRI, CT, or ultrasound; echocardiography every 1-3 years in asymptomatic patients until regression of cardiac rhabdomyomas is documented; 12-lead ECG every 3-5 years; and high-resolution chest CT scanning every 5-10 years in asymptomatic females ≥18 years and every 2-3 years in patients with lung cysts.[16]\nTreatment options for brain tumors associated with TSC include surgical resection or medical therapy with mechanistic target of rapamycin inhibitors, such as everolimus. Renal, pulmonary, and cardiac disease, if present, require referral and management by specialists. The prognosis varies and depends on the number and severity of organ systems involved. It is a progressive disorder, and complications lead to morbidity and mortality. Status epilepticus, SEGA, and renal disease are the most common causes of death.\nThe patient in this case was referred to the urology department, who performed a right-sided nephrectomy. Her antiepileptic medication was also readjusted.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1082046,
"choiceText": "Cortical tubers",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1082048,
"choiceText": "Subependymal nodules",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1082050,
"choiceText": "Pituitary mass",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1082052,
"choiceText": "SEGA",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1082054,
"choiceText": "White matter changes\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Brain lesions typical of TSC include cortical tubers, subependymal nodules, SEGA, and dysplastic, dysmyelinated white matter lesions.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 342741,
"questionText": "Which of the following is <i>not</i> typically found on MRI of the brain in patients with TSC?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1082056,
"choiceText": "Renal cell carcinoma",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1082058,
"choiceText": "Glomerulonephritis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1082060,
"choiceText": "Renal cysts\r\n",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1082062,
"choiceText": "Angiomyolipoma",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1082064,
"choiceText": "Light chain nephropathy",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Renal involvement is frequently seen in TSC. Angiomyolipoma is the most common renal manifestation and is seen in 50%-80% of cases.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 342743,
"questionText": "Which is the most common renal manifestation of TSC?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 20-Year-Old Woman With Flank Pain and Vomiting"
},
{
"authors": "Sumaira Nabi, MBBS; Shahzad Ahmed, MBBS; Mazhar Badshah, MBBS",
"content": [],
"date": "March 28, 2017",
"figures": [],
"markdown": "# A 20-Year-Old Woman With Flank Pain and Vomiting\n\n **Authors:** Sumaira Nabi, MBBS; Shahzad Ahmed, MBBS; Mazhar Badshah, MBBS \n **Date:** March 28, 2017\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1082046,
"choiceText": "Cortical tubers",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1082048,
"choiceText": "Subependymal nodules",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1082050,
"choiceText": "Pituitary mass",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1082052,
"choiceText": "SEGA",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1082054,
"choiceText": "White matter changes\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Brain lesions typical of TSC include cortical tubers, subependymal nodules, SEGA, and dysplastic, dysmyelinated white matter lesions.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 342741,
"questionText": "Which of the following is <i>not</i> typically found on MRI of the brain in patients with TSC?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1082056,
"choiceText": "Renal cell carcinoma",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1082058,
"choiceText": "Glomerulonephritis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1082060,
"choiceText": "Renal cysts\r\n",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1082062,
"choiceText": "Angiomyolipoma",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1082064,
"choiceText": "Light chain nephropathy",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Renal involvement is frequently seen in TSC. Angiomyolipoma is the most common renal manifestation and is seen in 50%-80% of cases.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 342743,
"questionText": "Which is the most common renal manifestation of TSC?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 20-Year-Old Woman With Flank Pain and Vomiting"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1082034,
"choiceText": "Tuberous sclerosis",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1082036,
"choiceText": "Neurofibromatosis type 1",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1082038,
"choiceText": "Sturge-Weber syndrome",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1082040,
"choiceText": "Neurofibromatosis type 2\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 342737,
"questionText": "Which of the following is the likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1082046,
"choiceText": "Cortical tubers",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1082048,
"choiceText": "Subependymal nodules",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1082050,
"choiceText": "Pituitary mass",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1082052,
"choiceText": "SEGA",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1082054,
"choiceText": "White matter changes\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Brain lesions typical of TSC include cortical tubers, subependymal nodules, SEGA, and dysplastic, dysmyelinated white matter lesions.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 342741,
"questionText": "Which of the following is <i>not</i> typically found on MRI of the brain in patients with TSC?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1082056,
"choiceText": "Renal cell carcinoma",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1082058,
"choiceText": "Glomerulonephritis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1082060,
"choiceText": "Renal cysts\r\n",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1082062,
"choiceText": "Angiomyolipoma",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1082064,
"choiceText": "Light chain nephropathy",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Renal involvement is frequently seen in TSC. Angiomyolipoma is the most common renal manifestation and is seen in 50%-80% of cases.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 342743,
"questionText": "Which is the most common renal manifestation of TSC?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
877034 | /viewarticle/877034 | [
{
"authors": "James J. McCombie, MB ChB",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"An 82-year-old woman presents to the emergency department from a nursing home. She had been visited by her general practitioner because she was experiencing abdominal pain. Because the patient has dementia, the history is limited and is provided by a caregiver from the nursing home.",
"The patient has a medical history of severe chronic obstructive pulmonary disease, hypertension, and osteoarthritis of multiple axial skeletal joints that is treated with scheduled codeine for analgesia. She has variable oral intake and is frequently constipated, with up to 4 days between bowel movements. She has used various cathartic and stool-softening agents, without significant improvement. A review of her medical chart reveals that the patient had two previous admissions for fecal impaction."
],
"date": "March 13, 2017",
"figures": [],
"markdown": "# An 82-Year-Old Woman With Abdominal Pain\n\n **Authors:** James J. McCombie, MB ChB \n **Date:** March 13, 2017\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nAn 82-year-old woman presents to the emergency department from a nursing home. She had been visited by her general practitioner because she was experiencing abdominal pain. Because the patient has dementia, the history is limited and is provided by a caregiver from the nursing home.\nThe patient has a medical history of severe chronic obstructive pulmonary disease, hypertension, and osteoarthritis of multiple axial skeletal joints that is treated with scheduled codeine for analgesia. She has variable oral intake and is frequently constipated, with up to 4 days between bowel movements. She has used various cathartic and stool-softening agents, without significant improvement. A review of her medical chart reveals that the patient had two previous admissions for fecal impaction.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "An 82-Year-Old Woman With Abdominal Pain"
},
{
"authors": "James J. McCombie, MB ChB",
"content": [
"The patient is afebrile, with a heart rate of 75 beats/min and a blood pressure of 145/88 mm Hg. Her respiratory rate is 17 breaths/min, and she has an oxygen saturation of 94% on room air. Her tongue is dry, and her skin turgor is reduced.",
"Figure1.",
"Auscultation of the lungs reveals globally poor air entry, with some scattered wheezes. The heart sounds are normal. Inspection of the abdomen reveals massive distention. No scars are noted. The abdomen is soft, tympanitic, and without shifting dullness; no tenderness is elicited. No evidence suggests hernia. Bowel sounds are high-pitched, and digital rectal examination reveals no stool in the vault.",
"Initial interventions in the emergency department include intravenous fluid resuscitation and nebulized bronchodilators. Laboratory testing reveals a normal white blood cell count and differential, a hemoglobin value of 13.6%, a C-reactive protein level of 4 mg/L, sodium level of 134 mEq/L, potassium level of 4.1 mEq/L, urea level of 30.1 mEq/L, and creatinine level of 1.5 mg/dL.",
"An upright anteroposterior abdominal radiograph is obtained (Figure), which demonstrates a greatly dilated loop of sigmoid bowel extending into the upper abdomen, with an absence of rectal gas. Emergency treatment is rendered before admission, and the patient is admitted for observation. Surgical consultation and evaluation are arranged to determine the most appropriate course of further management."
],
"date": "March 13, 2017",
"figures": [
{
"caption": "Figure1.",
"image_url": "https://img.medscapestatic.com/article/877/034/877034-Thumb1.jpg"
}
],
"markdown": "# An 82-Year-Old Woman With Abdominal Pain\n\n **Authors:** James J. McCombie, MB ChB \n **Date:** March 13, 2017\n\n ## Content\n\n The patient is afebrile, with a heart rate of 75 beats/min and a blood pressure of 145/88 mm Hg. Her respiratory rate is 17 breaths/min, and she has an oxygen saturation of 94% on room air. Her tongue is dry, and her skin turgor is reduced.\nFigure1.\nAuscultation of the lungs reveals globally poor air entry, with some scattered wheezes. The heart sounds are normal. Inspection of the abdomen reveals massive distention. No scars are noted. The abdomen is soft, tympanitic, and without shifting dullness; no tenderness is elicited. No evidence suggests hernia. Bowel sounds are high-pitched, and digital rectal examination reveals no stool in the vault.\nInitial interventions in the emergency department include intravenous fluid resuscitation and nebulized bronchodilators. Laboratory testing reveals a normal white blood cell count and differential, a hemoglobin value of 13.6%, a C-reactive protein level of 4 mg/L, sodium level of 134 mEq/L, potassium level of 4.1 mEq/L, urea level of 30.1 mEq/L, and creatinine level of 1.5 mg/dL.\nAn upright anteroposterior abdominal radiograph is obtained (Figure), which demonstrates a greatly dilated loop of sigmoid bowel extending into the upper abdomen, with an absence of rectal gas. Emergency treatment is rendered before admission, and the patient is admitted for observation. Surgical consultation and evaluation are arranged to determine the most appropriate course of further management.\n\n ## Figures\n\n **Figure1.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1073392,
"choiceText": "Mesenteric ischemia",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1073394,
"choiceText": "Stercoral perforation",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1073396,
"choiceText": "Pseudo-obstruction",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1073398,
"choiceText": "Sigmoid volvulus",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 339871,
"questionText": "Which of the following is the most likely diagnosis?<br><br><i>\r\nHint: Pay attention to the patient's history and the characteristic radiography findings.</i>\r\n",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "An 82-Year-Old Woman With Abdominal Pain"
},
{
"authors": "James J. McCombie, MB ChB",
"content": [
"This patient was diagnosed with sigmoid volvulus. Volvulus is a rotation of part of the intestine around an axis somewhat perpendicular to the line of its mesentery, resulting in partial or complete obstruction of the lumen. If not relieved, the condition can lead to bowel ischemia, gangrene, and perforation, often with significant mortality.",
"In general, volvulus is responsible for 1%-7% of large-bowel obstructions.[1] In a series of large-bowel volvulus cases, the sigmoid was involved in 76% of patients, the cecum in 22%, and the transverse colon in 2%.[2] Volvulus of the splenic flexure accounts for 1% of cases.[3] Because volvulus involves twisting around a pedicle formed by the mesentery, a sigmoid mesocolon that is vertically longer (from its root) than it is wide is frequently cited as a predisposing factor.[4]",
"Rotation leading to clinical symptoms is generally between 180° and 720°, in either a clockwise or counterclockwise direction. Lesser degrees of rotation may produce either no symptoms or transient and spontaneously resolving abdominal pain. Rotation of 360° or more generally leads to strangulation and/or obstruction.",
"The incidence of sigmoid volvulus is not well established. It is a relatively uncommon cause of intestinal obstruction, generally accounting for < 10% of cases in most series.[5,6] More than 50% of patients are older than 70 years. Many patients have neuropsychiatric illness or are bedridden and have chronic constipation. A high-residue and low-fiber Western diet is implicated in the genesis of chronic constipation and provides an educational and therapeutic target to reduce the incidence of constipation and subsequent volvulus.[7,8]",
"Sigmoid volvulus also occurs as a complication of megacolon, and this association is particularly identified in areas where Chagas disease is endemic, such as Brazil.[9] Another interesting association is that volvulus (sigmoid more often than cecal) accounts for 25% of cases of intestinal obstruction in pregnancy, occurring more frequently in the third trimester, perhaps as a result of uterine displacement of the colon.",
"Typical presentations of sigmoid volvulus may include some or all of the following: colicky abdominal pain (often with persistence of pain between spasms), constipation, obstipation, and subjective complaints of abdominal bloating or fullness. Examination frequently reveals massive distention (that is often asymmetric) and generalized tympany.",
"Severe pain, focal tenderness, peritoneal signs, tachycardia, and hypotension should raise the possibility of strangulation, necrosis, or perforation. In addition, an elevated white blood cell count and high anion gap metabolic acidosis with increased lactate levels also suggest intra-abdominal complications, as identified above. The medical history often includes previous episodes of nonadhesive colonic obstruction as well as fecal impaction, which is reported in 40%-60% of cases.[10]",
"A plain radiograph of the abdomen is often diagnostic and typically reveals a greatly distended loop of large bowel that has lost its haustral markings. This loop rises out of the pelvis towards the transverse colon. A distinct vertical crease is often present, which results from apposition of the walls of adjacent dilated bowel and can be traced downward to the point of torsion. This has been called the \"coffee bean\" sign. Rectal gas is usually absent. If a barium enema is performed, the contrast column tapers at the site of obstruction, forming an \"ace of spades\" or \"bird's beak\" appearance.",
"CT confirms the distended loop of sigmoid colon, possibly demonstrating a \"whirl sign,\" in which the afferent and efferent loops of bowel are rotated around a tightly twisted mesentery. In addition, there are two transition points in sigmoid volvulus, and both of these transition points can sometimes be detected with CT. In these cases, the transition points are typically in proximity and oriented in opposite directions.[11]"
],
"date": "March 13, 2017",
"figures": [],
"markdown": "# An 82-Year-Old Woman With Abdominal Pain\n\n **Authors:** James J. McCombie, MB ChB \n **Date:** March 13, 2017\n\n ## Content\n\n This patient was diagnosed with sigmoid volvulus. Volvulus is a rotation of part of the intestine around an axis somewhat perpendicular to the line of its mesentery, resulting in partial or complete obstruction of the lumen. If not relieved, the condition can lead to bowel ischemia, gangrene, and perforation, often with significant mortality.\nIn general, volvulus is responsible for 1%-7% of large-bowel obstructions.[1] In a series of large-bowel volvulus cases, the sigmoid was involved in 76% of patients, the cecum in 22%, and the transverse colon in 2%.[2] Volvulus of the splenic flexure accounts for 1% of cases.[3] Because volvulus involves twisting around a pedicle formed by the mesentery, a sigmoid mesocolon that is vertically longer (from its root) than it is wide is frequently cited as a predisposing factor.[4]\nRotation leading to clinical symptoms is generally between 180° and 720°, in either a clockwise or counterclockwise direction. Lesser degrees of rotation may produce either no symptoms or transient and spontaneously resolving abdominal pain. Rotation of 360° or more generally leads to strangulation and/or obstruction.\nThe incidence of sigmoid volvulus is not well established. It is a relatively uncommon cause of intestinal obstruction, generally accounting for < 10% of cases in most series.[5,6] More than 50% of patients are older than 70 years. Many patients have neuropsychiatric illness or are bedridden and have chronic constipation. A high-residue and low-fiber Western diet is implicated in the genesis of chronic constipation and provides an educational and therapeutic target to reduce the incidence of constipation and subsequent volvulus.[7,8]\nSigmoid volvulus also occurs as a complication of megacolon, and this association is particularly identified in areas where Chagas disease is endemic, such as Brazil.[9] Another interesting association is that volvulus (sigmoid more often than cecal) accounts for 25% of cases of intestinal obstruction in pregnancy, occurring more frequently in the third trimester, perhaps as a result of uterine displacement of the colon.\nTypical presentations of sigmoid volvulus may include some or all of the following: colicky abdominal pain (often with persistence of pain between spasms), constipation, obstipation, and subjective complaints of abdominal bloating or fullness. Examination frequently reveals massive distention (that is often asymmetric) and generalized tympany.\nSevere pain, focal tenderness, peritoneal signs, tachycardia, and hypotension should raise the possibility of strangulation, necrosis, or perforation. In addition, an elevated white blood cell count and high anion gap metabolic acidosis with increased lactate levels also suggest intra-abdominal complications, as identified above. The medical history often includes previous episodes of nonadhesive colonic obstruction as well as fecal impaction, which is reported in 40%-60% of cases.[10]\nA plain radiograph of the abdomen is often diagnostic and typically reveals a greatly distended loop of large bowel that has lost its haustral markings. This loop rises out of the pelvis towards the transverse colon. A distinct vertical crease is often present, which results from apposition of the walls of adjacent dilated bowel and can be traced downward to the point of torsion. This has been called the \"coffee bean\" sign. Rectal gas is usually absent. If a barium enema is performed, the contrast column tapers at the site of obstruction, forming an \"ace of spades\" or \"bird's beak\" appearance.\nCT confirms the distended loop of sigmoid colon, possibly demonstrating a \"whirl sign,\" in which the afferent and efferent loops of bowel are rotated around a tightly twisted mesentery. In addition, there are two transition points in sigmoid volvulus, and both of these transition points can sometimes be detected with CT. In these cases, the transition points are typically in proximity and oriented in opposite directions.[11]\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1073392,
"choiceText": "Mesenteric ischemia",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1073394,
"choiceText": "Stercoral perforation",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1073396,
"choiceText": "Pseudo-obstruction",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1073398,
"choiceText": "Sigmoid volvulus",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 339871,
"questionText": "Which of the following is the most likely diagnosis?<br><br><i>\r\nHint: Pay attention to the patient's history and the characteristic radiography findings.</i>\r\n",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "An 82-Year-Old Woman With Abdominal Pain"
},
{
"authors": "James J. McCombie, MB ChB",
"content": [
"Historically, immediate treatment of sigmoid volvulus has been mainly surgical. This approach should still be used if complications are believed to be present. In simple cases, derotation and decompression are the mainstay of acute therapy.",
"Selection of the immediately applied therapy depends on local institutional resources, practitioner availability, and physician preference. Both radiologic and endoscopic therapies are used in treating the patient with nonperforated, nongangrenous sigmoid volvulus. A radiologist may pass a 30- to 36-Fr, 50-cm long, large-bore, flexible tube through the anus and rectum to the point of obstruction under fluoroscopic guidance. Contrast medium is then introduced into the tube, generally using either gravity or gentle hand injection. In successful cases, hydrostatic pressure alone opens the lumen of the twisted segment, thereby decompressing it; a flush of stool and flatus is often explosively obtained. At that point, the transanal, large-bore catheter may be advanced into the proximal bowel to serve as a stent to prevent rerotation and reobstruction.",
"This same effect can be achieved with rigid sigmoidoscopy. The scope is passed along the lumen of the bowel under direct vision until the site of the volvulus is identified. The volvulated segment often derotates around the rigid scope; again, relief is identified by a sudden gush of feces and flatus, of which the operator should be aware. A well-lubricated tube may then be passed through the scope and manipulated gently into the sigmoid.",
"A flexible sigmoidoscope can also be placed under vision into the obstructed sigmoid to achieve the same effect, although it is more difficult to enable derotation around the flexible scope; therefore, this is a distant third choice for bedside decompression.",
"Failure to decompress the volvulated sigmoid should prompt surgical consultation for another attempt in the operating room with the aid of general anesthesia or deep sedation; surgical therapy may be directly undertaken if ischemia is found on the previous endoscopy or if a repeat attempt leads to iatrogenic perforation or identifies existing perforation after decompression (often found at the point of volvulus). A surgeon may be involved in the decision-making process upfront, rather than waiting until a potential complication occurs.",
"If successfully decompressed without the need for surgical therapy, patients can often be discharged home with dietary and medication regimen modification. However, the clinician should know that sigmoid volvulus is often a recurrent problem. One study demonstrated that of 48 patients discharged after one episode of volvulus, 61% were subsequently readmitted with recurrence.[12] With this in mind, many surgeons advocate an elective procedure after the second episode of volvulus, if the patient's condition allows this. This can be in the form of the procedures described later under emergency surgery or percutaneous endoscopic colostomy.",
"Percutaneous endoscopic colostomy has emerged as a possible treatment option for frail patients unfit to undergo surgery and general anesthesia.[13] A colostomy tube is inserted under endoscopic guidance, forming a decompressant or irrigant channel between the colonic lumen and the skin. In a series of 33 patients, fecal peritonitis (the most feared complication) developed in 8%, and minor complications developed in 30%, with an overall mortality of 3%.[14]",
"Clinicians should note that although percutaneous endoscopic colostomy is a novel therapy, percutaneous colonic intubation may be limited by tube clogging; tube dislodgement; tube-related mucosal hemorrhage; and the fact that the volvulated segment often demonstrates impaired motility, leading to failure of the distal colonic cleansing event with irrigation. Alternatively, sigmoid colostomy may be performed under local anesthesia in selected patients.",
"Emergency surgical treatment is generally reserved for patients with evidence of complications—that is, ischemia, necrosis, or perforation—or when conservative treatment has failed. One series reported conservative management to be successful in 74.6% of cases.[12] If surgery is indicated, one should be mindful that most patients are elderly and have comorbidities, often with a grade 4 American Society of Anesthesiologists classification.[15]",
"Once entry has been made into the abdomen, sigmoid derotation aids in determining therapy. Three management strategies are available: (1) sigmoid resection with primary anastomosis, (2) sigmoid resection and proximal end-colostomy with the creation of a distal stump (Hartmann procedure), and (3) sigmoidopexy. Sigmoidopexy (namely, fixing the sigmoid to the abdominal wall) may be considered in a frail patient with a viable colon after derotation who is suspected not to tolerate resection. Any suggestion of nonviable colon should prompt resection.",
"Although classically performed as an open procedure, laparoscopic techniques may be equally well applied, but these should be undertaken with care to abrogate abdominal insufflation-associated hypovolemia because this patient population is at risk for cardiovascular collapse from hypovolemia."
],
"date": "March 13, 2017",
"figures": [],
"markdown": "# An 82-Year-Old Woman With Abdominal Pain\n\n **Authors:** James J. McCombie, MB ChB \n **Date:** March 13, 2017\n\n ## Content\n\n Historically, immediate treatment of sigmoid volvulus has been mainly surgical. This approach should still be used if complications are believed to be present. In simple cases, derotation and decompression are the mainstay of acute therapy.\nSelection of the immediately applied therapy depends on local institutional resources, practitioner availability, and physician preference. Both radiologic and endoscopic therapies are used in treating the patient with nonperforated, nongangrenous sigmoid volvulus. A radiologist may pass a 30- to 36-Fr, 50-cm long, large-bore, flexible tube through the anus and rectum to the point of obstruction under fluoroscopic guidance. Contrast medium is then introduced into the tube, generally using either gravity or gentle hand injection. In successful cases, hydrostatic pressure alone opens the lumen of the twisted segment, thereby decompressing it; a flush of stool and flatus is often explosively obtained. At that point, the transanal, large-bore catheter may be advanced into the proximal bowel to serve as a stent to prevent rerotation and reobstruction.\nThis same effect can be achieved with rigid sigmoidoscopy. The scope is passed along the lumen of the bowel under direct vision until the site of the volvulus is identified. The volvulated segment often derotates around the rigid scope; again, relief is identified by a sudden gush of feces and flatus, of which the operator should be aware. A well-lubricated tube may then be passed through the scope and manipulated gently into the sigmoid.\nA flexible sigmoidoscope can also be placed under vision into the obstructed sigmoid to achieve the same effect, although it is more difficult to enable derotation around the flexible scope; therefore, this is a distant third choice for bedside decompression.\nFailure to decompress the volvulated sigmoid should prompt surgical consultation for another attempt in the operating room with the aid of general anesthesia or deep sedation; surgical therapy may be directly undertaken if ischemia is found on the previous endoscopy or if a repeat attempt leads to iatrogenic perforation or identifies existing perforation after decompression (often found at the point of volvulus). A surgeon may be involved in the decision-making process upfront, rather than waiting until a potential complication occurs.\nIf successfully decompressed without the need for surgical therapy, patients can often be discharged home with dietary and medication regimen modification. However, the clinician should know that sigmoid volvulus is often a recurrent problem. One study demonstrated that of 48 patients discharged after one episode of volvulus, 61% were subsequently readmitted with recurrence.[12] With this in mind, many surgeons advocate an elective procedure after the second episode of volvulus, if the patient's condition allows this. This can be in the form of the procedures described later under emergency surgery or percutaneous endoscopic colostomy.\nPercutaneous endoscopic colostomy has emerged as a possible treatment option for frail patients unfit to undergo surgery and general anesthesia.[13] A colostomy tube is inserted under endoscopic guidance, forming a decompressant or irrigant channel between the colonic lumen and the skin. In a series of 33 patients, fecal peritonitis (the most feared complication) developed in 8%, and minor complications developed in 30%, with an overall mortality of 3%.[14]\nClinicians should note that although percutaneous endoscopic colostomy is a novel therapy, percutaneous colonic intubation may be limited by tube clogging; tube dislodgement; tube-related mucosal hemorrhage; and the fact that the volvulated segment often demonstrates impaired motility, leading to failure of the distal colonic cleansing event with irrigation. Alternatively, sigmoid colostomy may be performed under local anesthesia in selected patients.\nEmergency surgical treatment is generally reserved for patients with evidence of complications—that is, ischemia, necrosis, or perforation—or when conservative treatment has failed. One series reported conservative management to be successful in 74.6% of cases.[12] If surgery is indicated, one should be mindful that most patients are elderly and have comorbidities, often with a grade 4 American Society of Anesthesiologists classification.[15]\nOnce entry has been made into the abdomen, sigmoid derotation aids in determining therapy. Three management strategies are available: (1) sigmoid resection with primary anastomosis, (2) sigmoid resection and proximal end-colostomy with the creation of a distal stump (Hartmann procedure), and (3) sigmoidopexy. Sigmoidopexy (namely, fixing the sigmoid to the abdominal wall) may be considered in a frail patient with a viable colon after derotation who is suspected not to tolerate resection. Any suggestion of nonviable colon should prompt resection.\nAlthough classically performed as an open procedure, laparoscopic techniques may be equally well applied, but these should be undertaken with care to abrogate abdominal insufflation-associated hypovolemia because this patient population is at risk for cardiovascular collapse from hypovolemia.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "An 82-Year-Old Woman With Abdominal Pain"
},
{
"authors": "James J. McCombie, MB ChB",
"content": [
"Primary anastomosis may be considered if there is minimal or no contamination of the abdominal cavity (no perforation or a contained perforation) and the patient is well-nourished and likely to return to near-normal physiologic status; this is rather uncommon in the patient population at risk for sigmoid volvulus. The major risk in this patient population is anastomotic failure. Anastomotic leak rates after colorectal anastomosis range from 4% to 26%[16] and are often devastating complications; the risk is higher in patients with preexisting, severe, protein-calorie malnutrition as well as functional distal obstruction. A Hartmann procedure is generally performed for all others and is particularly indicated when there is perforation, significant peritoneal soilage, malnutrition, or a high likelihood of dysmotile colon.",
"Patients typically fare worse when the disease has progressed to necrosis and perforation. A retrospective study found that in patients treated with derotation and sigmoidopexy, the mortality rate was 0%, whereas those with the condition who required resection had a mortality rate of 44%.[17] This probably reflects significant progression of disease that necessitated surgery involving resection as well as a systemic inflammatory state (sepsis, severe sepsis, and septic shock) rather than the anesthesia and surgery per se. To minimize the mortality of patients presenting with this disease, timely diagnosis and management are paramount. Of course, primary prevention strategies are of even greater importance in disease prevention because the therapies outlined above are reactive and not proactive.",
"The patient above was treated successfully with fluoroscopy-guided barium enema, and after an uneventful hospital observation, she was discharged to her nursing home 2 days later. Follow-up was arranged for consideration of an elective surgical procedure to prevent recurrence."
],
"date": "March 13, 2017",
"figures": [],
"markdown": "# An 82-Year-Old Woman With Abdominal Pain\n\n **Authors:** James J. McCombie, MB ChB \n **Date:** March 13, 2017\n\n ## Content\n\n Primary anastomosis may be considered if there is minimal or no contamination of the abdominal cavity (no perforation or a contained perforation) and the patient is well-nourished and likely to return to near-normal physiologic status; this is rather uncommon in the patient population at risk for sigmoid volvulus. The major risk in this patient population is anastomotic failure. Anastomotic leak rates after colorectal anastomosis range from 4% to 26%[16] and are often devastating complications; the risk is higher in patients with preexisting, severe, protein-calorie malnutrition as well as functional distal obstruction. A Hartmann procedure is generally performed for all others and is particularly indicated when there is perforation, significant peritoneal soilage, malnutrition, or a high likelihood of dysmotile colon.\nPatients typically fare worse when the disease has progressed to necrosis and perforation. A retrospective study found that in patients treated with derotation and sigmoidopexy, the mortality rate was 0%, whereas those with the condition who required resection had a mortality rate of 44%.[17] This probably reflects significant progression of disease that necessitated surgery involving resection as well as a systemic inflammatory state (sepsis, severe sepsis, and septic shock) rather than the anesthesia and surgery per se. To minimize the mortality of patients presenting with this disease, timely diagnosis and management are paramount. Of course, primary prevention strategies are of even greater importance in disease prevention because the therapies outlined above are reactive and not proactive.\nThe patient above was treated successfully with fluoroscopy-guided barium enema, and after an uneventful hospital observation, she was discharged to her nursing home 2 days later. Follow-up was arranged for consideration of an elective surgical procedure to prevent recurrence.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1073410,
"choiceText": "Patients may be safely discharged home from the emergency department and follow up as needed with their primary care practitioner, because recurrence is rare",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1073414,
"choiceText": "Most cases of sigmoid volvulus recur; although immediate surgical treatment is rarely necessary, patients should follow up with a general surgeon promptly after a period of inpatient observation",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1073418,
"choiceText": "Risk for recurrence is exceedingly high in elderly persons; conservative management is only a short-term solution, and surgical treatment is recommended before discharge from the hospital",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1073422,
"choiceText": "Patients with uncomplicated sigmoid volvulus who improve after conservative management rarely have recurrence; if after a period of inpatient observation the patient remains well, surgical consultation is probably unnecessary",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "After a period of observation, definitive surgical treatment should be considered, given the high recurrence rate. Many surgeons opt not to treat surgically until a second episode, but this decision should be left to the surgeon, because many factors must be considered.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 339875,
"questionText": "An 87-year-old patient presents with a first episode of sigmoid volvulus that is treated conservatively, resulting in rapid improvement in symptoms. Which of the following statements is correct regarding patient disposition and expectations for possible recurrence?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1073428,
"choiceText": "Sigmoidopexy",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1073430,
"choiceText": "Sigmoidectomy and primary anastomosis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1073432,
"choiceText": "Hartmann procedure\r\n",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Studies have demonstrated a mortality rate of 0% for sigmoidopexy vs 44% for patients who required resection in cases of sigmoid volvulus and obstruction. This can be explained by the fact that resection is indicated when colonic viability is threatened or apparent. This indicates that prompt diagnosis, conservative therapy, and a swift decision to operate when complications are seen are needed in this condition.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 339879,
"questionText": "Of all patients requiring surgery for sigmoid volvulus, which procedure (after decompression) is associated with the lowest postoperative mortality?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "An 82-Year-Old Woman With Abdominal Pain"
},
{
"authors": "James J. McCombie, MB ChB",
"content": [],
"date": "March 13, 2017",
"figures": [],
"markdown": "# An 82-Year-Old Woman With Abdominal Pain\n\n **Authors:** James J. McCombie, MB ChB \n **Date:** March 13, 2017\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1073410,
"choiceText": "Patients may be safely discharged home from the emergency department and follow up as needed with their primary care practitioner, because recurrence is rare",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1073414,
"choiceText": "Most cases of sigmoid volvulus recur; although immediate surgical treatment is rarely necessary, patients should follow up with a general surgeon promptly after a period of inpatient observation",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1073418,
"choiceText": "Risk for recurrence is exceedingly high in elderly persons; conservative management is only a short-term solution, and surgical treatment is recommended before discharge from the hospital",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1073422,
"choiceText": "Patients with uncomplicated sigmoid volvulus who improve after conservative management rarely have recurrence; if after a period of inpatient observation the patient remains well, surgical consultation is probably unnecessary",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "After a period of observation, definitive surgical treatment should be considered, given the high recurrence rate. Many surgeons opt not to treat surgically until a second episode, but this decision should be left to the surgeon, because many factors must be considered.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 339875,
"questionText": "An 87-year-old patient presents with a first episode of sigmoid volvulus that is treated conservatively, resulting in rapid improvement in symptoms. Which of the following statements is correct regarding patient disposition and expectations for possible recurrence?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1073428,
"choiceText": "Sigmoidopexy",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1073430,
"choiceText": "Sigmoidectomy and primary anastomosis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1073432,
"choiceText": "Hartmann procedure\r\n",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Studies have demonstrated a mortality rate of 0% for sigmoidopexy vs 44% for patients who required resection in cases of sigmoid volvulus and obstruction. This can be explained by the fact that resection is indicated when colonic viability is threatened or apparent. This indicates that prompt diagnosis, conservative therapy, and a swift decision to operate when complications are seen are needed in this condition.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 339879,
"questionText": "Of all patients requiring surgery for sigmoid volvulus, which procedure (after decompression) is associated with the lowest postoperative mortality?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "An 82-Year-Old Woman With Abdominal Pain"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1073392,
"choiceText": "Mesenteric ischemia",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1073394,
"choiceText": "Stercoral perforation",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1073396,
"choiceText": "Pseudo-obstruction",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1073398,
"choiceText": "Sigmoid volvulus",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 339871,
"questionText": "Which of the following is the most likely diagnosis?<br><br><i>\r\nHint: Pay attention to the patient's history and the characteristic radiography findings.</i>\r\n",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1073410,
"choiceText": "Patients may be safely discharged home from the emergency department and follow up as needed with their primary care practitioner, because recurrence is rare",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1073414,
"choiceText": "Most cases of sigmoid volvulus recur; although immediate surgical treatment is rarely necessary, patients should follow up with a general surgeon promptly after a period of inpatient observation",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1073418,
"choiceText": "Risk for recurrence is exceedingly high in elderly persons; conservative management is only a short-term solution, and surgical treatment is recommended before discharge from the hospital",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1073422,
"choiceText": "Patients with uncomplicated sigmoid volvulus who improve after conservative management rarely have recurrence; if after a period of inpatient observation the patient remains well, surgical consultation is probably unnecessary",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "After a period of observation, definitive surgical treatment should be considered, given the high recurrence rate. Many surgeons opt not to treat surgically until a second episode, but this decision should be left to the surgeon, because many factors must be considered.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 339875,
"questionText": "An 87-year-old patient presents with a first episode of sigmoid volvulus that is treated conservatively, resulting in rapid improvement in symptoms. Which of the following statements is correct regarding patient disposition and expectations for possible recurrence?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1073428,
"choiceText": "Sigmoidopexy",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1073430,
"choiceText": "Sigmoidectomy and primary anastomosis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1073432,
"choiceText": "Hartmann procedure\r\n",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Studies have demonstrated a mortality rate of 0% for sigmoidopexy vs 44% for patients who required resection in cases of sigmoid volvulus and obstruction. This can be explained by the fact that resection is indicated when colonic viability is threatened or apparent. This indicates that prompt diagnosis, conservative therapy, and a swift decision to operate when complications are seen are needed in this condition.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 339879,
"questionText": "Of all patients requiring surgery for sigmoid volvulus, which procedure (after decompression) is associated with the lowest postoperative mortality?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
875521 | /viewarticle/875521 | [
{
"authors": "Arunabh Talwar, MD; Craig E. Devoe, MD; Nick Patel, DO",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"A 74-year-old man presents with easy bruising, fatigue, and generalized weakness. The patient's symptoms have been progressing during the past 3 weeks. He denies having any other symptoms. The patient has no significant past medical history and denies any recent travel, tobacco use, or use of illicit drugs. The patient is retired from an office administration position.",
"He is admitted to the hospital for evaluation of his weakness and easy bruising. On admission, a chest radiograph is obtained but no abnormalities are noted. An initial complete blood cell count is ordered, which shows thrombocytopenia, with a platelet count of 12.0 × 103/μL (12.0 × 109/L), and anemia, with a hemoglobin of 6.8 g/dL (68 g/L) and a hematocrit of 19% (0.19). His peripheral blood smear demonstrates 12% blasts.",
"The patient undergoes a subsequent bone marrow biopsy, which confirms acute myelocytic leukemia. He then receives induction chemotherapy with cytarabine and daunorubicin. Following induction, the patient becomes pancytopenic, with a 21-day bone marrow biopsy showing chemotherapeutic effect. The patient's hospital course is then complicated by febrile neutropenia and vancomycin-resistant Enterococcus bacteremia from a central venous catheter infection. He is treated with intravenous linezolid, which clears his bacteremia based on repeat blood cultures; however, he still suffers persistent fevers.",
"On day 30 post-induction, the patient develops shortness of breath and complains of a progressive, nonproductive cough."
],
"date": "February 09, 2017",
"figures": [],
"markdown": "# A 74-Year-Old Man With Easy Bruising and Fatigue\n\n **Authors:** Arunabh Talwar, MD; Craig E. Devoe, MD; Nick Patel, DO \n **Date:** February 09, 2017\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nA 74-year-old man presents with easy bruising, fatigue, and generalized weakness. The patient's symptoms have been progressing during the past 3 weeks. He denies having any other symptoms. The patient has no significant past medical history and denies any recent travel, tobacco use, or use of illicit drugs. The patient is retired from an office administration position.\nHe is admitted to the hospital for evaluation of his weakness and easy bruising. On admission, a chest radiograph is obtained but no abnormalities are noted. An initial complete blood cell count is ordered, which shows thrombocytopenia, with a platelet count of 12.0 × 103/μL (12.0 × 109/L), and anemia, with a hemoglobin of 6.8 g/dL (68 g/L) and a hematocrit of 19% (0.19). His peripheral blood smear demonstrates 12% blasts.\nThe patient undergoes a subsequent bone marrow biopsy, which confirms acute myelocytic leukemia. He then receives induction chemotherapy with cytarabine and daunorubicin. Following induction, the patient becomes pancytopenic, with a 21-day bone marrow biopsy showing chemotherapeutic effect. The patient's hospital course is then complicated by febrile neutropenia and vancomycin-resistant Enterococcus bacteremia from a central venous catheter infection. He is treated with intravenous linezolid, which clears his bacteremia based on repeat blood cultures; however, he still suffers persistent fevers.\nOn day 30 post-induction, the patient develops shortness of breath and complains of a progressive, nonproductive cough.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 74-Year-Old Man With Easy Bruising and Fatigue"
},
{
"authors": "Arunabh Talwar, MD; Craig E. Devoe, MD; Nick Patel, DO",
"content": [
"Upon physical examination, the patient has a blood pressure of 136/60 mm Hg. His heart rate is 92 beats/min, he is febrile, with a temperature of 101.2°F (38.4°C), has a respiratory rate of 28 breaths/min, and his pulse oximetry reading is 93% on a nonrebreather face mask. The patient appears to have increased respiratory effort and diminished breath sounds; additionally, rhonchi are detected in the right upper lung field. He also has minimally decreased breath sounds at the lung bases bilaterally. The remainder of his examination is unremarkable.",
"Figure 1.",
"Figure 2.",
"Figure 3.",
"Figure 4.",
"New laboratory studies are obtained which show that the patient remains pancytopenic. He has 27.0 × 103/μL (27.0 × 109/L) platelets and an absolute neutrophil count of 800 cells/mm3. Plain-film radiography and CT scanning of the chest (Figures 1 and 2) show a dense right upper-lobe consolidation, with narrowing of the right upper-lobe bronchus and mediastinal lymphadenopathy.",
"The patient then undergoes flexible bronchoscopy that reveals a necrotic endobronchial lesion completely obstructing the anterior segment of the right upper lobe (Figure 3). Endobronchial biopsies are taken of this lesion. Histology slides are provided (Figure 4). Rigid bronchoscopy is subsequently performed, with electrocautery of the endobronchial lesion and near-complete reestablishment of the right upper lobe anterior segment lumen, without complications."
],
"date": "February 09, 2017",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/875/521/875521-Thumb1.jpg"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/875/521/875521-Thumb2.jpg"
},
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/875/521/875521-Thumb3.jpg"
},
{
"caption": "Figure 4.",
"image_url": "https://img.medscapestatic.com/article/875/521/875521-Thumb4.jpg"
}
],
"markdown": "# A 74-Year-Old Man With Easy Bruising and Fatigue\n\n **Authors:** Arunabh Talwar, MD; Craig E. Devoe, MD; Nick Patel, DO \n **Date:** February 09, 2017\n\n ## Content\n\n Upon physical examination, the patient has a blood pressure of 136/60 mm Hg. His heart rate is 92 beats/min, he is febrile, with a temperature of 101.2°F (38.4°C), has a respiratory rate of 28 breaths/min, and his pulse oximetry reading is 93% on a nonrebreather face mask. The patient appears to have increased respiratory effort and diminished breath sounds; additionally, rhonchi are detected in the right upper lung field. He also has minimally decreased breath sounds at the lung bases bilaterally. The remainder of his examination is unremarkable.\nFigure 1.\nFigure 2.\nFigure 3.\nFigure 4.\nNew laboratory studies are obtained which show that the patient remains pancytopenic. He has 27.0 × 103/μL (27.0 × 109/L) platelets and an absolute neutrophil count of 800 cells/mm3. Plain-film radiography and CT scanning of the chest (Figures 1 and 2) show a dense right upper-lobe consolidation, with narrowing of the right upper-lobe bronchus and mediastinal lymphadenopathy.\nThe patient then undergoes flexible bronchoscopy that reveals a necrotic endobronchial lesion completely obstructing the anterior segment of the right upper lobe (Figure 3). Endobronchial biopsies are taken of this lesion. Histology slides are provided (Figure 4). Rigid bronchoscopy is subsequently performed, with electrocautery of the endobronchial lesion and near-complete reestablishment of the right upper lobe anterior segment lumen, without complications.\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n**Figure 3.** \n \n\n**Figure 4.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1065960,
"choiceText": "Invasive pulmonary aspergillosis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1065962,
"choiceText": "Non–small cell lung cancer",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1065964,
"choiceText": "Endobronchial mucormycosis",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1065966,
"choiceText": "Non-Hodgkin lymphoma",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1065968,
"choiceText": "Necrotizing bacterial pneumonia\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 337517,
"questionText": "Which of the following is the most likely diagnosis?\r\n<br><br><i>\r\nHint: Look closely at the biopsy specimen.</i>\r\n",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 74-Year-Old Man With Easy Bruising and Fatigue"
},
{
"authors": "Arunabh Talwar, MD; Craig E. Devoe, MD; Nick Patel, DO",
"content": [
"Zygomycetes are a ubiquitously distributed group of fungi found commonly in soil, decaying organic matter, and on fruit and bread. The members of this group of fungi that infect humans include Mucor, Rhizopus, Absidia, and the Cunninghamella species. Given the wide distribution of these fungi, most humans are exposed to these organisms on a daily or weekly basis.[1,2,3]",
"Although first described by Paltauf in 1885, these infections were virtually unrecognized in the lungs until the advent of antimicrobial, immunosuppressive, and antineoplastic therapy. They rarely cause disease in immunocompetent hosts because of the low virulence of the organisms; however, they are now the third most common cause of invasive fungal infection in immunocompromised patients, especially stem-cell transplant recipients and patients with underlying hematologic malignancies.[4]",
"These spore-forming saprophytes infect hosts via inhalation of aerosolized spores or from hematogenous spread. Colonization may be transient or persistent and depends upon host mucosal defense mechanisms and fungal virulence. After colonization, local disease may form and can progress to disseminated illness in susceptible hosts. Mucor are molds in the environment that become hyphal forms in the body tissues. Once the spores begin to grow, fungal hyphae invade blood vessels and produce tissue infarction, necrosis, and thrombosis. Neutrophils are the key host defense against these fungi; therefore, individuals with neutropenia or neutrophil dysfunction (diabetes, steroid use) are at highest risk. Few cases of mucormycosis have been reported in patients with AIDS, suggesting that the host defense against this infection is not primarily mediated by cellular immunity.[2,3,5]",
"The clinical manifestations of infection have traditionally been divided into six separate syndromes: rhinocerebral, pulmonary, cutaneous, gastrointestinal, central nervous system, and disseminated disease. Rhinocerebral and pulmonary involvement are the two most common syndromes. Rhinocerebral disease, the most common form, is most often seen in persons with diabetes, particularly those suffering from diabetic ketoacidosis. Between 50% and 75% of pulmonary mucormycosis infections occur in patients with hematologic malignancies.[1]"
],
"date": "February 09, 2017",
"figures": [],
"markdown": "# A 74-Year-Old Man With Easy Bruising and Fatigue\n\n **Authors:** Arunabh Talwar, MD; Craig E. Devoe, MD; Nick Patel, DO \n **Date:** February 09, 2017\n\n ## Content\n\n Zygomycetes are a ubiquitously distributed group of fungi found commonly in soil, decaying organic matter, and on fruit and bread. The members of this group of fungi that infect humans include Mucor, Rhizopus, Absidia, and the Cunninghamella species. Given the wide distribution of these fungi, most humans are exposed to these organisms on a daily or weekly basis.[1,2,3]\nAlthough first described by Paltauf in 1885, these infections were virtually unrecognized in the lungs until the advent of antimicrobial, immunosuppressive, and antineoplastic therapy. They rarely cause disease in immunocompetent hosts because of the low virulence of the organisms; however, they are now the third most common cause of invasive fungal infection in immunocompromised patients, especially stem-cell transplant recipients and patients with underlying hematologic malignancies.[4]\nThese spore-forming saprophytes infect hosts via inhalation of aerosolized spores or from hematogenous spread. Colonization may be transient or persistent and depends upon host mucosal defense mechanisms and fungal virulence. After colonization, local disease may form and can progress to disseminated illness in susceptible hosts. Mucor are molds in the environment that become hyphal forms in the body tissues. Once the spores begin to grow, fungal hyphae invade blood vessels and produce tissue infarction, necrosis, and thrombosis. Neutrophils are the key host defense against these fungi; therefore, individuals with neutropenia or neutrophil dysfunction (diabetes, steroid use) are at highest risk. Few cases of mucormycosis have been reported in patients with AIDS, suggesting that the host defense against this infection is not primarily mediated by cellular immunity.[2,3,5]\nThe clinical manifestations of infection have traditionally been divided into six separate syndromes: rhinocerebral, pulmonary, cutaneous, gastrointestinal, central nervous system, and disseminated disease. Rhinocerebral and pulmonary involvement are the two most common syndromes. Rhinocerebral disease, the most common form, is most often seen in persons with diabetes, particularly those suffering from diabetic ketoacidosis. Between 50% and 75% of pulmonary mucormycosis infections occur in patients with hematologic malignancies.[1]\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1065960,
"choiceText": "Invasive pulmonary aspergillosis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1065962,
"choiceText": "Non–small cell lung cancer",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1065964,
"choiceText": "Endobronchial mucormycosis",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1065966,
"choiceText": "Non-Hodgkin lymphoma",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1065968,
"choiceText": "Necrotizing bacterial pneumonia\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 337517,
"questionText": "Which of the following is the most likely diagnosis?\r\n<br><br><i>\r\nHint: Look closely at the biopsy specimen.</i>\r\n",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 74-Year-Old Man With Easy Bruising and Fatigue"
},
{
"authors": "Arunabh Talwar, MD; Craig E. Devoe, MD; Nick Patel, DO",
"content": [
"Overall, mucormycosis remains extremely rare. A review of mucormycosis cases at one US cancer center found that 0.7% of patients were found to have mucormycosis at autopsy, and that 20 patients per 100,000 admissions had the disease.[2,5,6] Mucormycosis was found in 1% of patients with acute leukemia in an Italian multicenter review.[7]",
"Mucormycosis carries a very high mortality rate of 50%-85%. Pulmonary and gastrointestinal diseases carry an even higher mortality rate because these forms are typically diagnosed late in the disease course. Rhinocerebral disease causes significant morbidity in patients who survive because treatment usually requires extensive—and often disfiguring—facial surgery.[3]",
"The vast majority of pulmonary mucormycosis infections present with diffuse lung involvement and a rapidly progressive clinical course. Patients report symptoms of rapidly progressive cough, fever, and pleuritic chest pain. Patients are often profoundly ill, with marked gas-exchange abnormalities and rapidly advancing respiratory failure despite therapy with conventional antibiotics.",
"Reported chest abnormalities found on radiography include nodular, lobar, or wedge-shaped infiltrates, which may be seen in 58% of cases. Other signs include mediastinal widening, bronchopneumonia, solitary nodule, miliary pattern, cavitation, air crescent sign, bronchopleural fistula, pulmonary artery pseudoaneurysm, and pleural effusions. The CT halo sign has been shown to represent pulmonary infarcts with surrounding hemorrhage and edema.",
"More typical findings seen on chest radiographs are those of fungal vascular invasion. The invasion of large vascular structures leads to thrombosis and infarction of the lung parenchyma. This may begin as a nodular or nonspecific acinar infiltrate, which subsequently develops into a wedge-shaped density. In some instances, it progresses to lobar consolidation. The major complication associated with pulmonary mucormycosis is massive hemoptysis; therefore, treatment of pulmonary Zygomycetes infections is usually surgical. Despite this, the radiographic variability is vast, and pulmonary mucormycosis cannot be diagnosed or excluded on radiographic grounds alone.[3,4,8]",
"The results of sputum cultures are usually negative, but a positive culture for Mucor from sputum is highly suggestive of invasive infection. A definitive diagnosis requires a histologic investigation. Tissue invasion with characteristic broad (5-50 µm), nonseptate hyphae with right-angle branching and blood vessel invasion must be seen. Culture of histologic material will give variable results as well. Percutaneous needle biopsy, open lung biopsy, and pleural fluid culture have been successful methods to obtain these samples. Bronchoscopic examination is often chosen as a relatively safe and less invasive technique to obtain histologic material for diagnosis; however, the results may be negative in patients with large areas of lung infarction secondary to mucormycosis vascular invasion.[1,3,4]"
],
"date": "February 09, 2017",
"figures": [],
"markdown": "# A 74-Year-Old Man With Easy Bruising and Fatigue\n\n **Authors:** Arunabh Talwar, MD; Craig E. Devoe, MD; Nick Patel, DO \n **Date:** February 09, 2017\n\n ## Content\n\n Overall, mucormycosis remains extremely rare. A review of mucormycosis cases at one US cancer center found that 0.7% of patients were found to have mucormycosis at autopsy, and that 20 patients per 100,000 admissions had the disease.[2,5,6] Mucormycosis was found in 1% of patients with acute leukemia in an Italian multicenter review.[7]\nMucormycosis carries a very high mortality rate of 50%-85%. Pulmonary and gastrointestinal diseases carry an even higher mortality rate because these forms are typically diagnosed late in the disease course. Rhinocerebral disease causes significant morbidity in patients who survive because treatment usually requires extensive—and often disfiguring—facial surgery.[3]\nThe vast majority of pulmonary mucormycosis infections present with diffuse lung involvement and a rapidly progressive clinical course. Patients report symptoms of rapidly progressive cough, fever, and pleuritic chest pain. Patients are often profoundly ill, with marked gas-exchange abnormalities and rapidly advancing respiratory failure despite therapy with conventional antibiotics.\nReported chest abnormalities found on radiography include nodular, lobar, or wedge-shaped infiltrates, which may be seen in 58% of cases. Other signs include mediastinal widening, bronchopneumonia, solitary nodule, miliary pattern, cavitation, air crescent sign, bronchopleural fistula, pulmonary artery pseudoaneurysm, and pleural effusions. The CT halo sign has been shown to represent pulmonary infarcts with surrounding hemorrhage and edema.\nMore typical findings seen on chest radiographs are those of fungal vascular invasion. The invasion of large vascular structures leads to thrombosis and infarction of the lung parenchyma. This may begin as a nodular or nonspecific acinar infiltrate, which subsequently develops into a wedge-shaped density. In some instances, it progresses to lobar consolidation. The major complication associated with pulmonary mucormycosis is massive hemoptysis; therefore, treatment of pulmonary Zygomycetes infections is usually surgical. Despite this, the radiographic variability is vast, and pulmonary mucormycosis cannot be diagnosed or excluded on radiographic grounds alone.[3,4,8]\nThe results of sputum cultures are usually negative, but a positive culture for Mucor from sputum is highly suggestive of invasive infection. A definitive diagnosis requires a histologic investigation. Tissue invasion with characteristic broad (5-50 µm), nonseptate hyphae with right-angle branching and blood vessel invasion must be seen. Culture of histologic material will give variable results as well. Percutaneous needle biopsy, open lung biopsy, and pleural fluid culture have been successful methods to obtain these samples. Bronchoscopic examination is often chosen as a relatively safe and less invasive technique to obtain histologic material for diagnosis; however, the results may be negative in patients with large areas of lung infarction secondary to mucormycosis vascular invasion.[1,3,4]\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 74-Year-Old Man With Easy Bruising and Fatigue"
},
{
"authors": "Arunabh Talwar, MD; Craig E. Devoe, MD; Nick Patel, DO",
"content": [
"Treatment generally starts with control of the patient's underlying disease, which is often difficult to accomplish. Withdrawal of immunosuppressive agents and corticosteroid drugs, glycemic control, and correction of acidosis are all advisable. Amphotericin B remains the only antimicrobial agent with evidence of antifungal efficacy in mucormycosis. Sensitivity to amphotericin varies between isolates. Host factors, such as infarcted tissue, and poor host immunocompetence will affect the success of treatment. Early and aggressive surgery for localized disease appears to offer the best chance of recovery, especially in diabetics with hemoptysis.[3,5]",
"Presentation with endobronchial disease is distinctly rare. Even less common is presentation with endobronchial disease confined to a well-localized area. Again, treatment of endobronchial mucormycosis is primarily surgical. The high degree of vascular invasion and resultant infarction make it unlikely that amphotericin will be effectively delivered to the involved areas.[9] Recognizing that the presentation of pulmonary mucormycosis depends on the associated clinical disorder is important.",
"Patients with leukemia and lymphoma often have diffuse parenchymal disease refractory to medical and surgical therapy. Some patients have localized disease amenable to surgery with or without amphotericin. Overall, early consideration of pulmonary mucormycosis should lead to earlier diagnosis, appropriate medical and surgical therapy, and an increased survival rate.[3]",
"The patient in this case was treated with intravenous amphotericin B for 4 weeks along with endobronchial resection of the lesions. Radiographically and clinically he completely improved after this treatment. This is a rather interesting exception to the normal treatment for endobronchial disease, as he was not primarily managed with surgery. His antifungal regimen did not appear to affect his leukemia treatment, as his induction chemotherapy with cytarabine and daunorubicin showed chemotherapeutic effect at day 21 post-induction, which was demonstrated with a bone marrow biopsy. The patient recovered bone marrow function in a timely fashion."
],
"date": "February 09, 2017",
"figures": [],
"markdown": "# A 74-Year-Old Man With Easy Bruising and Fatigue\n\n **Authors:** Arunabh Talwar, MD; Craig E. Devoe, MD; Nick Patel, DO \n **Date:** February 09, 2017\n\n ## Content\n\n Treatment generally starts with control of the patient's underlying disease, which is often difficult to accomplish. Withdrawal of immunosuppressive agents and corticosteroid drugs, glycemic control, and correction of acidosis are all advisable. Amphotericin B remains the only antimicrobial agent with evidence of antifungal efficacy in mucormycosis. Sensitivity to amphotericin varies between isolates. Host factors, such as infarcted tissue, and poor host immunocompetence will affect the success of treatment. Early and aggressive surgery for localized disease appears to offer the best chance of recovery, especially in diabetics with hemoptysis.[3,5]\nPresentation with endobronchial disease is distinctly rare. Even less common is presentation with endobronchial disease confined to a well-localized area. Again, treatment of endobronchial mucormycosis is primarily surgical. The high degree of vascular invasion and resultant infarction make it unlikely that amphotericin will be effectively delivered to the involved areas.[9] Recognizing that the presentation of pulmonary mucormycosis depends on the associated clinical disorder is important.\nPatients with leukemia and lymphoma often have diffuse parenchymal disease refractory to medical and surgical therapy. Some patients have localized disease amenable to surgery with or without amphotericin. Overall, early consideration of pulmonary mucormycosis should lead to earlier diagnosis, appropriate medical and surgical therapy, and an increased survival rate.[3]\nThe patient in this case was treated with intravenous amphotericin B for 4 weeks along with endobronchial resection of the lesions. Radiographically and clinically he completely improved after this treatment. This is a rather interesting exception to the normal treatment for endobronchial disease, as he was not primarily managed with surgery. His antifungal regimen did not appear to affect his leukemia treatment, as his induction chemotherapy with cytarabine and daunorubicin showed chemotherapeutic effect at day 21 post-induction, which was demonstrated with a bone marrow biopsy. The patient recovered bone marrow function in a timely fashion.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1065970,
"choiceText": "Endocarditis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1065972,
"choiceText": "Intravenous drug use",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1065974,
"choiceText": "Hematologic malignancies",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1065976,
"choiceText": "Diabetes",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1065978,
"choiceText": "Hypertension",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Rhinocerebral and pulmonary involvement are the two most common syndromes. Rhinocerebral disease, the most common form, is most often seen in persons with diabetes, particularly those suffering from diabetic ketoacidosis. Between 50% and 75% of pulmonary mucormycosis infections occur in patients with hematologic malignancies.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 337519,
"questionText": "Which of the following conditions is commonly associated with rhinocerebral mucormycosis and should prompt an investigation?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1066048,
"choiceText": "Radiography of the head and chest",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1066050,
"choiceText": "CT scanning of the head",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1066052,
"choiceText": "Histologic examination",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1066054,
"choiceText": "Sputum culture\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1066056,
"choiceText": "Hypertension\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The results of sputum cultures are usually negative, but a positive culture for <i>Mucor</i> from sputum is highly suggestive of invasive infection. A definitive diagnosis requires a histologic investigation. Tissue invasion with characteristic broad (5-50 µm), nonseptate hyphae with right-angle branching and blood vessel invasion must be seen. Culture of histologic material will give variable results as well. Percutaneous needle biopsy, open lung biopsy, and pleural fluid culture have been successful methods to obtain these samples. Bronchoscopic examination is often chosen as a relatively safe and less invasive technique to obtain histologic material for diagnosis; however, the results may be negative in patients with large areas of lung infarction secondary to mucormycosis vascular invasion.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 337541,
"questionText": "Which of the following investigations definitively establishes the diagnosis of mucormycosis in a patient with rhinocerebral mucormycosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 74-Year-Old Man With Easy Bruising and Fatigue"
},
{
"authors": "Arunabh Talwar, MD; Craig E. Devoe, MD; Nick Patel, DO",
"content": [],
"date": "February 09, 2017",
"figures": [],
"markdown": "# A 74-Year-Old Man With Easy Bruising and Fatigue\n\n **Authors:** Arunabh Talwar, MD; Craig E. Devoe, MD; Nick Patel, DO \n **Date:** February 09, 2017\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1065970,
"choiceText": "Endocarditis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1065972,
"choiceText": "Intravenous drug use",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1065974,
"choiceText": "Hematologic malignancies",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1065976,
"choiceText": "Diabetes",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1065978,
"choiceText": "Hypertension",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Rhinocerebral and pulmonary involvement are the two most common syndromes. Rhinocerebral disease, the most common form, is most often seen in persons with diabetes, particularly those suffering from diabetic ketoacidosis. Between 50% and 75% of pulmonary mucormycosis infections occur in patients with hematologic malignancies.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 337519,
"questionText": "Which of the following conditions is commonly associated with rhinocerebral mucormycosis and should prompt an investigation?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1066048,
"choiceText": "Radiography of the head and chest",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1066050,
"choiceText": "CT scanning of the head",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1066052,
"choiceText": "Histologic examination",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1066054,
"choiceText": "Sputum culture\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1066056,
"choiceText": "Hypertension\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The results of sputum cultures are usually negative, but a positive culture for <i>Mucor</i> from sputum is highly suggestive of invasive infection. A definitive diagnosis requires a histologic investigation. Tissue invasion with characteristic broad (5-50 µm), nonseptate hyphae with right-angle branching and blood vessel invasion must be seen. Culture of histologic material will give variable results as well. Percutaneous needle biopsy, open lung biopsy, and pleural fluid culture have been successful methods to obtain these samples. Bronchoscopic examination is often chosen as a relatively safe and less invasive technique to obtain histologic material for diagnosis; however, the results may be negative in patients with large areas of lung infarction secondary to mucormycosis vascular invasion.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 337541,
"questionText": "Which of the following investigations definitively establishes the diagnosis of mucormycosis in a patient with rhinocerebral mucormycosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 74-Year-Old Man With Easy Bruising and Fatigue"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1065960,
"choiceText": "Invasive pulmonary aspergillosis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1065962,
"choiceText": "Non–small cell lung cancer",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1065964,
"choiceText": "Endobronchial mucormycosis",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1065966,
"choiceText": "Non-Hodgkin lymphoma",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1065968,
"choiceText": "Necrotizing bacterial pneumonia\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 337517,
"questionText": "Which of the following is the most likely diagnosis?\r\n<br><br><i>\r\nHint: Look closely at the biopsy specimen.</i>\r\n",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1065970,
"choiceText": "Endocarditis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1065972,
"choiceText": "Intravenous drug use",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1065974,
"choiceText": "Hematologic malignancies",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1065976,
"choiceText": "Diabetes",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1065978,
"choiceText": "Hypertension",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Rhinocerebral and pulmonary involvement are the two most common syndromes. Rhinocerebral disease, the most common form, is most often seen in persons with diabetes, particularly those suffering from diabetic ketoacidosis. Between 50% and 75% of pulmonary mucormycosis infections occur in patients with hematologic malignancies.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 337519,
"questionText": "Which of the following conditions is commonly associated with rhinocerebral mucormycosis and should prompt an investigation?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1066048,
"choiceText": "Radiography of the head and chest",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1066050,
"choiceText": "CT scanning of the head",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1066052,
"choiceText": "Histologic examination",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1066054,
"choiceText": "Sputum culture\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1066056,
"choiceText": "Hypertension\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The results of sputum cultures are usually negative, but a positive culture for <i>Mucor</i> from sputum is highly suggestive of invasive infection. A definitive diagnosis requires a histologic investigation. Tissue invasion with characteristic broad (5-50 µm), nonseptate hyphae with right-angle branching and blood vessel invasion must be seen. Culture of histologic material will give variable results as well. Percutaneous needle biopsy, open lung biopsy, and pleural fluid culture have been successful methods to obtain these samples. Bronchoscopic examination is often chosen as a relatively safe and less invasive technique to obtain histologic material for diagnosis; however, the results may be negative in patients with large areas of lung infarction secondary to mucormycosis vascular invasion.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 337541,
"questionText": "Which of the following investigations definitively establishes the diagnosis of mucormycosis in a patient with rhinocerebral mucormycosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
875000 | /viewarticle/875000 | [
{
"authors": "Thomas N. Helm, MD; Stefanos Haddad, MD; Robert E. Kalb, MD",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"A 75-year-old woman with a history of hypertension, Parkinson disease, osteoporosis, and trigeminal neuralgia was admitted to the hospital for evaluation of fever, rash, and skin pain. Her widespread skin eruption started 3 days ago on her chest and spread centrifugally. She also reported a dull headache and that her eyes felt \"sore and scratchy.\"",
"Her medications included hydrochlorothiazide (25 mg/d), two extended-release capsules (carbidopa [23.75 mg] and levodopa [95 mg]) three times daily, and vitamin D (800 IU daily). She had been started on carbamazepine (100 mg twice daily) for her trigeminal neuralgia 6 weeks ago; this had recently been increased to 200 mg twice daily."
],
"date": "January 30, 2017",
"figures": [],
"markdown": "# A 75-Year-Old Woman With Fever, Rash, and Skin Pain\n\n **Authors:** Thomas N. Helm, MD; Stefanos Haddad, MD; Robert E. Kalb, MD \n **Date:** January 30, 2017\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nA 75-year-old woman with a history of hypertension, Parkinson disease, osteoporosis, and trigeminal neuralgia was admitted to the hospital for evaluation of fever, rash, and skin pain. Her widespread skin eruption started 3 days ago on her chest and spread centrifugally. She also reported a dull headache and that her eyes felt \"sore and scratchy.\"\nHer medications included hydrochlorothiazide (25 mg/d), two extended-release capsules (carbidopa [23.75 mg] and levodopa [95 mg]) three times daily, and vitamin D (800 IU daily). She had been started on carbamazepine (100 mg twice daily) for her trigeminal neuralgia 6 weeks ago; this had recently been increased to 200 mg twice daily.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 75-Year-Old Woman With Fever, Rash, and Skin Pain"
},
{
"authors": "Thomas N. Helm, MD; Stefanos Haddad, MD; Robert E. Kalb, MD",
"content": [
"Physical examination revealed an anxious and uncomfortable woman with a widespread eruption (Figures 1-3). Erythematous macules on the face, back, chest, and arms blanched with diascopy; sporadic lesions on the lower legs did not blanch entirely but were purpuric. No annular lesions were identified.",
"Figure 1.",
"Figure 2.",
"Figure 3.",
"The patient's temperature was 102.2°F. Cervical and axillary lymphadenopathy were noted. Her laboratory studies were remarkable for atypical lymphocytosis and an elevated serum alanine aminotransferase (ALT) level of 1375 U/L (reference range, 0-41 U/L). Her white blood cell count was > 12,000 cells/µL, and her absolute eosinophil count was > 900 eosinophils per µL of blood. Acute and chronic hepatitis serologies were negative for hepatitis A, hepatitis B, and hepatitis C. Electrocardiography and echocardiography did not reveal any abnormalities.",
"Figure 4.",
"Figure 5."
],
"date": "January 30, 2017",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/875/000/875000-Figure1a-thumb.png"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/875/000/875000-Thumb2.png"
},
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/875/000/875000-Thumb3.png"
},
{
"caption": "Figure 4.",
"image_url": "https://img.medscapestatic.com/article/875/000/875000-Thumb4.png"
},
{
"caption": "Figure 5.",
"image_url": "https://img.medscapestatic.com/article/875/000/875000-Thumb5.png"
}
],
"markdown": "# A 75-Year-Old Woman With Fever, Rash, and Skin Pain\n\n **Authors:** Thomas N. Helm, MD; Stefanos Haddad, MD; Robert E. Kalb, MD \n **Date:** January 30, 2017\n\n ## Content\n\n Physical examination revealed an anxious and uncomfortable woman with a widespread eruption (Figures 1-3). Erythematous macules on the face, back, chest, and arms blanched with diascopy; sporadic lesions on the lower legs did not blanch entirely but were purpuric. No annular lesions were identified.\nFigure 1.\nFigure 2.\nFigure 3.\nThe patient's temperature was 102.2°F. Cervical and axillary lymphadenopathy were noted. Her laboratory studies were remarkable for atypical lymphocytosis and an elevated serum alanine aminotransferase (ALT) level of 1375 U/L (reference range, 0-41 U/L). Her white blood cell count was > 12,000 cells/µL, and her absolute eosinophil count was > 900 eosinophils per µL of blood. Acute and chronic hepatitis serologies were negative for hepatitis A, hepatitis B, and hepatitis C. Electrocardiography and echocardiography did not reveal any abnormalities.\nFigure 4.\nFigure 5.\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n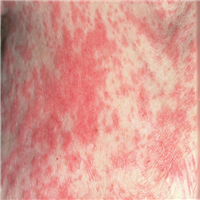 \n\n**Figure 3.** \n \n\n**Figure 4.** \n \n\n**Figure 5.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1063694,
"choiceText": "Psoriasis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1063696,
"choiceText": "Leukocytoclastic vasculitis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1063698,
"choiceText": "Drug eruption\r\n",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1063700,
"choiceText": "Urticaria",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 336827,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 75-Year-Old Woman With Fever, Rash, and Skin Pain"
},
{
"authors": "Thomas N. Helm, MD; Stefanos Haddad, MD; Robert E. Kalb, MD",
"content": [
"Drug eruptions can present in many ways, and drugs can trigger psoriasis, leukocytoclastic vasculitis, and urticaria. Symptoms of \"skin pain\" and \"scratchy eyes\" associated with fever and lymphadenopathy should raise concern for a severe drug reaction.",
"The elevated ALT level, presence of atypical lymphocytosis, elevated eosinophil count on peripheral blood analysis, and skin biopsy findings all support a diagnosis of a drug reaction with eosinophilia and systemic symptoms (DRESS), which is also known as \"drug-induced hypersensitivity syndrome\".[1,2,3] The timing of the eruption 6 weeks after starting use of carbamazepine in this patient is typical.[1] The onset of DRESS is often several weeks after a new medication is started, and the interval is often longer than that for other drug reactions encountered in clinical practice.",
"DRESS is a serious and potentially life-threatening drug-induced hypersensitivity reaction characterized by skin lesions, eosinophilia, lymphadenopathy, and internal organ involvement (lung, liver, and kidney).[1,2,3] Most deaths are related to liver failure, but cardiac necrosis and renal failure can occur. The eruption usually begins 2-8 weeks after drug exposure.",
"Certain human leukocyte antigen (HLA) haplotypes are particularly associated with DRESS.[1,4] Individuals of Han Chinese ancestry and those who have HLA-B*5801 may develop a severe drug reaction after initiation of allopurinol treatment, with some individuals progressing to DRESS.[4]",
"Carbamazepine, allopurinol, lamotrigine, phenobarbital, sulfasalazine, phenytoin, vancomycin, and abacavir are common causes, in descending order of frequency, respectively.[1] Many other agents (eg, minocycline, trimethoprim/sulfamethoxazole) can also cause this reaction pattern; thus, carefully reviewing all medications is important.",
"Reactivation of human herpesvirus 6 infection is associated with DRESS.[1] Whether the drug reaction triggers viral reactivation or whether viral reactivation somehow triggers a cross-reaction and tissue damage via CD8+ lymphocytes is uncertain."
],
"date": "January 30, 2017",
"figures": [],
"markdown": "# A 75-Year-Old Woman With Fever, Rash, and Skin Pain\n\n **Authors:** Thomas N. Helm, MD; Stefanos Haddad, MD; Robert E. Kalb, MD \n **Date:** January 30, 2017\n\n ## Content\n\n Drug eruptions can present in many ways, and drugs can trigger psoriasis, leukocytoclastic vasculitis, and urticaria. Symptoms of \"skin pain\" and \"scratchy eyes\" associated with fever and lymphadenopathy should raise concern for a severe drug reaction.\nThe elevated ALT level, presence of atypical lymphocytosis, elevated eosinophil count on peripheral blood analysis, and skin biopsy findings all support a diagnosis of a drug reaction with eosinophilia and systemic symptoms (DRESS), which is also known as \"drug-induced hypersensitivity syndrome\".[1,2,3] The timing of the eruption 6 weeks after starting use of carbamazepine in this patient is typical.[1] The onset of DRESS is often several weeks after a new medication is started, and the interval is often longer than that for other drug reactions encountered in clinical practice.\nDRESS is a serious and potentially life-threatening drug-induced hypersensitivity reaction characterized by skin lesions, eosinophilia, lymphadenopathy, and internal organ involvement (lung, liver, and kidney).[1,2,3] Most deaths are related to liver failure, but cardiac necrosis and renal failure can occur. The eruption usually begins 2-8 weeks after drug exposure.\nCertain human leukocyte antigen (HLA) haplotypes are particularly associated with DRESS.[1,4] Individuals of Han Chinese ancestry and those who have HLA-B*5801 may develop a severe drug reaction after initiation of allopurinol treatment, with some individuals progressing to DRESS.[4]\nCarbamazepine, allopurinol, lamotrigine, phenobarbital, sulfasalazine, phenytoin, vancomycin, and abacavir are common causes, in descending order of frequency, respectively.[1] Many other agents (eg, minocycline, trimethoprim/sulfamethoxazole) can also cause this reaction pattern; thus, carefully reviewing all medications is important.\nReactivation of human herpesvirus 6 infection is associated with DRESS.[1] Whether the drug reaction triggers viral reactivation or whether viral reactivation somehow triggers a cross-reaction and tissue damage via CD8+ lymphocytes is uncertain.\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1063694,
"choiceText": "Psoriasis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1063696,
"choiceText": "Leukocytoclastic vasculitis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1063698,
"choiceText": "Drug eruption\r\n",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1063700,
"choiceText": "Urticaria",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 336827,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 75-Year-Old Woman With Fever, Rash, and Skin Pain"
},
{
"authors": "Thomas N. Helm, MD; Stefanos Haddad, MD; Robert E. Kalb, MD",
"content": [
"Biopsy reveals spongiosis, acanthosis, and an infiltrate with a variable amount of eosinophils, as well as dermal edema. The eruption usually begins in a morbilliform pattern and then becomes confluent. When facial edema, indurated skin lesions, scaling, and purpura are noted, DRESS must be suspected.",
"Acute and eruptive psoriasis is typically associated with pustular lesions. Biopsy of psoriasis should reveal spongiform pustules. Leukocytoclastic vasculitis is associated with purpuric lesions, and biopsy reveals damage of blood vessel walls not encountered in typical lesions of DRESS.",
"Elderly individuals may have some purpuric lesions in dependent areas, such as the lower legs; however, this should not prompt a reflexive diagnosis of vasculitis. Urticaria is associated with transient wheals and not the fixed and widespread macular and papular eruption, as is seen in this patient.",
"The workup should include serum chemistries; analysis of peripheral blood; and possibly a skin biopsy, which can exclude other dermatoses, such as psoriasis, leukocytoclastic vasculitis, and urticaria. Peripheral eosinophilia, atypical lymphocytes on peripheral smear, or an elevated absolute lymphocyte count of greater than 4500 cells/µL are often found in DRESS.[1] Serum ALT levels are often twice the upper limit of normal, and alkaline phosphatase levels are often 1.5 times greater than the upper limit of normal.",
"Proteinuria and abnormal urinalysis are also often associated with DRESS. According to Bocquet and colleagues,[5] diagnosis requires the following:[1]",
"Drug rash",
"Hematologic abnormalities: eosinophilia (> 1500 cells/µL), presence of atypical lymphocytes",
"Systemic involvement: swollen lymph nodes (> 2 cm in diameter), hepatitis (aminotransferase elevation of at least twice the normal values), interstitial nephritis, pneumonitis, or carditis",
"Newer criteria (eg, SCAR-J) have been proposed but have not yet been universally accepted. Much more research is needed in terms of how to best identify and classify cases. Skin lesions may have various different presentations that include a morbilliform eruption, exfoliative erythroderma, urticarial morbilliform presentation, or erythema multiforme-like lesions.[6] The erythema multiforme-like presentation of DRESS seems to be associated with more severe hepatic involvement."
],
"date": "January 30, 2017",
"figures": [],
"markdown": "# A 75-Year-Old Woman With Fever, Rash, and Skin Pain\n\n **Authors:** Thomas N. Helm, MD; Stefanos Haddad, MD; Robert E. Kalb, MD \n **Date:** January 30, 2017\n\n ## Content\n\n Biopsy reveals spongiosis, acanthosis, and an infiltrate with a variable amount of eosinophils, as well as dermal edema. The eruption usually begins in a morbilliform pattern and then becomes confluent. When facial edema, indurated skin lesions, scaling, and purpura are noted, DRESS must be suspected.\nAcute and eruptive psoriasis is typically associated with pustular lesions. Biopsy of psoriasis should reveal spongiform pustules. Leukocytoclastic vasculitis is associated with purpuric lesions, and biopsy reveals damage of blood vessel walls not encountered in typical lesions of DRESS.\nElderly individuals may have some purpuric lesions in dependent areas, such as the lower legs; however, this should not prompt a reflexive diagnosis of vasculitis. Urticaria is associated with transient wheals and not the fixed and widespread macular and papular eruption, as is seen in this patient.\nThe workup should include serum chemistries; analysis of peripheral blood; and possibly a skin biopsy, which can exclude other dermatoses, such as psoriasis, leukocytoclastic vasculitis, and urticaria. Peripheral eosinophilia, atypical lymphocytes on peripheral smear, or an elevated absolute lymphocyte count of greater than 4500 cells/µL are often found in DRESS.[1] Serum ALT levels are often twice the upper limit of normal, and alkaline phosphatase levels are often 1.5 times greater than the upper limit of normal.\nProteinuria and abnormal urinalysis are also often associated with DRESS. According to Bocquet and colleagues,[5] diagnosis requires the following:[1]\nDrug rash\nHematologic abnormalities: eosinophilia (> 1500 cells/µL), presence of atypical lymphocytes\nSystemic involvement: swollen lymph nodes (> 2 cm in diameter), hepatitis (aminotransferase elevation of at least twice the normal values), interstitial nephritis, pneumonitis, or carditis\nNewer criteria (eg, SCAR-J) have been proposed but have not yet been universally accepted. Much more research is needed in terms of how to best identify and classify cases. Skin lesions may have various different presentations that include a morbilliform eruption, exfoliative erythroderma, urticarial morbilliform presentation, or erythema multiforme-like lesions.[6] The erythema multiforme-like presentation of DRESS seems to be associated with more severe hepatic involvement.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 75-Year-Old Woman With Fever, Rash, and Skin Pain"
},
{
"authors": "Thomas N. Helm, MD; Stefanos Haddad, MD; Robert E. Kalb, MD",
"content": [
"Treatment of DRESS involves withdrawing the offending drug. Skin lesions are often treated with high-potency topical corticosteroids. When severe organ involvement is identified, moderate- to high-dose systemic corticosteroids may be considered and are often prescribed, with a taper over many months. One report suggests that a 7-day course of cyclosporine may be helpful in treating DRESS.[7] Further studies are needed to confirm the efficacy of this approach.",
"In this patient, carbamazepine was discontinued at the time of consultation. Because of the marked elevation in the patient's liver enzyme values, she was placed on prednisone (1 mg/kg/day). She was also given a high-potency corticosteroid cream to apply to her skin lesions three times daily for 1 week.",
"The patient's liver function normalized over the next 2-3 weeks, and her skin eruption resolved, as did her lymphadenopathy. She declined further treatment of her trigeminal neuralgia."
],
"date": "January 30, 2017",
"figures": [],
"markdown": "# A 75-Year-Old Woman With Fever, Rash, and Skin Pain\n\n **Authors:** Thomas N. Helm, MD; Stefanos Haddad, MD; Robert E. Kalb, MD \n **Date:** January 30, 2017\n\n ## Content\n\n Treatment of DRESS involves withdrawing the offending drug. Skin lesions are often treated with high-potency topical corticosteroids. When severe organ involvement is identified, moderate- to high-dose systemic corticosteroids may be considered and are often prescribed, with a taper over many months. One report suggests that a 7-day course of cyclosporine may be helpful in treating DRESS.[7] Further studies are needed to confirm the efficacy of this approach.\nIn this patient, carbamazepine was discontinued at the time of consultation. Because of the marked elevation in the patient's liver enzyme values, she was placed on prednisone (1 mg/kg/day). She was also given a high-potency corticosteroid cream to apply to her skin lesions three times daily for 1 week.\nThe patient's liver function normalized over the next 2-3 weeks, and her skin eruption resolved, as did her lymphadenopathy. She declined further treatment of her trigeminal neuralgia.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1063702,
"choiceText": "Presence of drug rash",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1063704,
"choiceText": "Hematologic abnormalities (eosinophilia > 1500/µL, presence of atypical lymphocytes)",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1063706,
"choiceText": "Systemic involvement (adenopathy [> 2 cm in diameter], hepatitis [aminotransferase elevation of at least twice the normal values], interstitial nephritis, pneumonitis, carditis)",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1063708,
"choiceText": "All of the above\r\n",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1063710,
"choiceText": "None of the above",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "These have all been suggested by Bocquet and colleagues<sup type=\"ref\">[5]</sup> as important diagnostic criteria. Although other sets of criteria have been proposed, all of the listed features are generally considered most important in identifying and establishing a diagnosis of DRESS.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 336829,
"questionText": "Which of the following criteria are useful in establishing the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1063712,
"choiceText": "Human herpesvirus 8",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1063714,
"choiceText": "Human herpesvirus 6",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1063716,
"choiceText": "Varicella zoster virus ",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1063718,
"choiceText": "Human papillomavirus",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "\r\n\r\nAlthough DRESS has been associated with many viruses and is seen in greater frequency in persons infected with HIV, reactivation of human herpesvirus 6 seems to be particularly important in triggering the autoimmune phenomena associated with DRESS.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 336831,
"questionText": "Reactivation of which of the following viruses has been most closely associated with DRESS?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 75-Year-Old Woman With Fever, Rash, and Skin Pain"
},
{
"authors": "Thomas N. Helm, MD; Stefanos Haddad, MD; Robert E. Kalb, MD",
"content": [],
"date": "January 30, 2017",
"figures": [],
"markdown": "# A 75-Year-Old Woman With Fever, Rash, and Skin Pain\n\n **Authors:** Thomas N. Helm, MD; Stefanos Haddad, MD; Robert E. Kalb, MD \n **Date:** January 30, 2017\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1063702,
"choiceText": "Presence of drug rash",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1063704,
"choiceText": "Hematologic abnormalities (eosinophilia > 1500/µL, presence of atypical lymphocytes)",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1063706,
"choiceText": "Systemic involvement (adenopathy [> 2 cm in diameter], hepatitis [aminotransferase elevation of at least twice the normal values], interstitial nephritis, pneumonitis, carditis)",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1063708,
"choiceText": "All of the above\r\n",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1063710,
"choiceText": "None of the above",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "These have all been suggested by Bocquet and colleagues<sup type=\"ref\">[5]</sup> as important diagnostic criteria. Although other sets of criteria have been proposed, all of the listed features are generally considered most important in identifying and establishing a diagnosis of DRESS.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 336829,
"questionText": "Which of the following criteria are useful in establishing the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1063712,
"choiceText": "Human herpesvirus 8",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1063714,
"choiceText": "Human herpesvirus 6",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1063716,
"choiceText": "Varicella zoster virus ",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1063718,
"choiceText": "Human papillomavirus",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "\r\n\r\nAlthough DRESS has been associated with many viruses and is seen in greater frequency in persons infected with HIV, reactivation of human herpesvirus 6 seems to be particularly important in triggering the autoimmune phenomena associated with DRESS.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 336831,
"questionText": "Reactivation of which of the following viruses has been most closely associated with DRESS?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 75-Year-Old Woman With Fever, Rash, and Skin Pain"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1063694,
"choiceText": "Psoriasis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1063696,
"choiceText": "Leukocytoclastic vasculitis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1063698,
"choiceText": "Drug eruption\r\n",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1063700,
"choiceText": "Urticaria",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 336827,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1063702,
"choiceText": "Presence of drug rash",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1063704,
"choiceText": "Hematologic abnormalities (eosinophilia > 1500/µL, presence of atypical lymphocytes)",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1063706,
"choiceText": "Systemic involvement (adenopathy [> 2 cm in diameter], hepatitis [aminotransferase elevation of at least twice the normal values], interstitial nephritis, pneumonitis, carditis)",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1063708,
"choiceText": "All of the above\r\n",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1063710,
"choiceText": "None of the above",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "These have all been suggested by Bocquet and colleagues<sup type=\"ref\">[5]</sup> as important diagnostic criteria. Although other sets of criteria have been proposed, all of the listed features are generally considered most important in identifying and establishing a diagnosis of DRESS.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 336829,
"questionText": "Which of the following criteria are useful in establishing the diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1063712,
"choiceText": "Human herpesvirus 8",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1063714,
"choiceText": "Human herpesvirus 6",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1063716,
"choiceText": "Varicella zoster virus ",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1063718,
"choiceText": "Human papillomavirus",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "\r\n\r\nAlthough DRESS has been associated with many viruses and is seen in greater frequency in persons infected with HIV, reactivation of human herpesvirus 6 seems to be particularly important in triggering the autoimmune phenomena associated with DRESS.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 336831,
"questionText": "Reactivation of which of the following viruses has been most closely associated with DRESS?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
873352 | /viewarticle/873352 | [
{
"authors": "Buraa Kubaisi, MD; Nakhoul Nakhoul, MD; C. Stephen Foster, MD",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"A 2-year-old boy presented to the emergency department (ED) with fever and malaise that started 4 days earlier. His mother treated him with children's ibuprofen (100 mg). Six hours later, the fever and malaise persisted; his mother gave him additional ibuprofen.",
"The next day, the child awakened with a rash, initially on his face and upper part of his body. His mother took him to the ED of a local hospital. The child was diagnosed with chickenpox, and the mother was instructed to keep him at rest and treat with hydration and ibuprofen as needed for fever.",
"His mother observed worsening symptoms within 24 hours and brought him back to the ED."
],
"date": "January 22, 2019",
"figures": [],
"markdown": "# A 2-Year-Old-Boy With an Alarming Facial Rash\n\n **Authors:** Buraa Kubaisi, MD; Nakhoul Nakhoul, MD; C. Stephen Foster, MD \n **Date:** January 22, 2019\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nA 2-year-old boy presented to the emergency department (ED) with fever and malaise that started 4 days earlier. His mother treated him with children's ibuprofen (100 mg). Six hours later, the fever and malaise persisted; his mother gave him additional ibuprofen.\nThe next day, the child awakened with a rash, initially on his face and upper part of his body. His mother took him to the ED of a local hospital. The child was diagnosed with chickenpox, and the mother was instructed to keep him at rest and treat with hydration and ibuprofen as needed for fever.\nHis mother observed worsening symptoms within 24 hours and brought him back to the ED.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 2-Year-Old-Boy With an Alarming Facial Rash"
},
{
"authors": "Buraa Kubaisi, MD; Nakhoul Nakhoul, MD; C. Stephen Foster, MD",
"content": [
"Upon physical examination, the child appeared ill, with generalized aches, malaise, and a fever (103°F [39.44°C]). A generalized erythema with targetlike lesions and associated central purpura and bullae was found mainly on his trunk and extremities. An example of a similar appearance in an adult is shown in Figure 1. The child also had ulcerations on the surface of his lips and his tongue. The ulcers were tender on palpation and hemorrhagic in appearance.",
"Figure 1.",
"Ocular examination revealed acute conjunctivitis, with subconjunctival hemorrhage in both eyes associated with hemorrhagic ulcerations of the eyelids, and watering and yellow discharge from both eyes.",
"The child was hospitalized for supportive care. Blood and urine samples were obtained. Laboratory workup was done and revealed mild anemia, nonspecific low-grade leukocytosis, and elevated interleukin-6 (IL-6) and C-reactive protein (CRP) levels. Electrolytes and other chemistries were normal. Urine analysis revealed no pathology.",
"A dermatologist was consulted, and a skin biopsy was performed. Skin biopsy histopathology revealed full-thickness necrosis of the epidermis with mild dermal inflammatory cell infiltration (Figure 2).",
"Figure 2."
],
"date": "January 22, 2019",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/873/352/873352-Thumb1.png"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/873/352/873352-Thumb2.png"
}
],
"markdown": "# A 2-Year-Old-Boy With an Alarming Facial Rash\n\n **Authors:** Buraa Kubaisi, MD; Nakhoul Nakhoul, MD; C. Stephen Foster, MD \n **Date:** January 22, 2019\n\n ## Content\n\n Upon physical examination, the child appeared ill, with generalized aches, malaise, and a fever (103°F [39.44°C]). A generalized erythema with targetlike lesions and associated central purpura and bullae was found mainly on his trunk and extremities. An example of a similar appearance in an adult is shown in Figure 1. The child also had ulcerations on the surface of his lips and his tongue. The ulcers were tender on palpation and hemorrhagic in appearance.\nFigure 1.\nOcular examination revealed acute conjunctivitis, with subconjunctival hemorrhage in both eyes associated with hemorrhagic ulcerations of the eyelids, and watering and yellow discharge from both eyes.\nThe child was hospitalized for supportive care. Blood and urine samples were obtained. Laboratory workup was done and revealed mild anemia, nonspecific low-grade leukocytosis, and elevated interleukin-6 (IL-6) and C-reactive protein (CRP) levels. Electrolytes and other chemistries were normal. Urine analysis revealed no pathology.\nA dermatologist was consulted, and a skin biopsy was performed. Skin biopsy histopathology revealed full-thickness necrosis of the epidermis with mild dermal inflammatory cell infiltration (Figure 2).\nFigure 2.\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1052878,
"choiceText": "Cicatrizing pemphigoid",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1052880,
"choiceText": "Stevens-Johnson syndrome",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1052882,
"choiceText": "Toxic shock syndrome",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1052884,
"choiceText": "Exfoliative dermatitis\r\n\r\n\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 333313,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 2-Year-Old-Boy With an Alarming Facial Rash"
},
{
"authors": "Buraa Kubaisi, MD; Nakhoul Nakhoul, MD; C. Stephen Foster, MD",
"content": [
"Stevens-Johnson syndrome (SJS) is an immune complex-mediated hypersensitivity reaction in the skin and mucous membranes. SJS and toxic epidermal necrolysis are similar, with variable involvement. When less than 10% of body surface area is involved, the condition is considered SJS; when more than 30% of body surface area is involved, it is considered TEN. When 10%-30% of body surface area is involved, it is considered overlapping SJS/TEN. SJS can involve the skin, eyes, and nose and the gastrointestinal, respiratory, and genitourinary systems, leading to severe morbidity and, in some instances, eventual death.[1]",
"The pathophysiology of SJS has been thoroughly studied. It involves antigen presentation and production of tumor necrosis factor alpha by dendrocytes, activating T lymphocytes that induce epidermal cell apoptosis. The death of keratinocytes causes separation of the epidermis from the dermis. The dying cells provoke recruitment of more chemokines, leading to a vicious cycle of more inflammation and more cell death with extensive epidermal necrolysis. This is compatible with the histopathologic findings in this patient, in whom necrosis of the epidermis was seen, along with mild dermal inflammatory cell infiltration.[2]",
"SJS can affect persons of all races and ages and both sexes. Various etiologies have been implicated in SJS, including the following[3,4]:",
"Drugs (most commonly recognized cause in adults): antibiotics, anticonvulsants, nonsteroidal anti-inflammatory drugs, and analgesics",
"Infections: Viral (herpes simplex virus, AIDS), bacterial (streptococcal, mycobacterial), fungal species (coccidioidomycosis, histoplasmosis)",
"Cancer",
"Autoimmune conditions",
"Trauma",
"Vaccination",
"Idiopathic (25%-50% of cases)",
"The differential diagnosis of SJS includes exfoliative dermatitis, toxic shock syndrome, staphylococcal scalded skin syndrome, bullous systemic lupus erythematosus, epidermolysis bullosa acquisita, and paraneoplastic pemphigus."
],
"date": "January 22, 2019",
"figures": [],
"markdown": "# A 2-Year-Old-Boy With an Alarming Facial Rash\n\n **Authors:** Buraa Kubaisi, MD; Nakhoul Nakhoul, MD; C. Stephen Foster, MD \n **Date:** January 22, 2019\n\n ## Content\n\n Stevens-Johnson syndrome (SJS) is an immune complex-mediated hypersensitivity reaction in the skin and mucous membranes. SJS and toxic epidermal necrolysis are similar, with variable involvement. When less than 10% of body surface area is involved, the condition is considered SJS; when more than 30% of body surface area is involved, it is considered TEN. When 10%-30% of body surface area is involved, it is considered overlapping SJS/TEN. SJS can involve the skin, eyes, and nose and the gastrointestinal, respiratory, and genitourinary systems, leading to severe morbidity and, in some instances, eventual death.[1]\nThe pathophysiology of SJS has been thoroughly studied. It involves antigen presentation and production of tumor necrosis factor alpha by dendrocytes, activating T lymphocytes that induce epidermal cell apoptosis. The death of keratinocytes causes separation of the epidermis from the dermis. The dying cells provoke recruitment of more chemokines, leading to a vicious cycle of more inflammation and more cell death with extensive epidermal necrolysis. This is compatible with the histopathologic findings in this patient, in whom necrosis of the epidermis was seen, along with mild dermal inflammatory cell infiltration.[2]\nSJS can affect persons of all races and ages and both sexes. Various etiologies have been implicated in SJS, including the following[3,4]:\nDrugs (most commonly recognized cause in adults): antibiotics, anticonvulsants, nonsteroidal anti-inflammatory drugs, and analgesics\nInfections: Viral (herpes simplex virus, AIDS), bacterial (streptococcal, mycobacterial), fungal species (coccidioidomycosis, histoplasmosis)\nCancer\nAutoimmune conditions\nTrauma\nVaccination\nIdiopathic (25%-50% of cases)\nThe differential diagnosis of SJS includes exfoliative dermatitis, toxic shock syndrome, staphylococcal scalded skin syndrome, bullous systemic lupus erythematosus, epidermolysis bullosa acquisita, and paraneoplastic pemphigus.\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1052878,
"choiceText": "Cicatrizing pemphigoid",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1052880,
"choiceText": "Stevens-Johnson syndrome",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1052882,
"choiceText": "Toxic shock syndrome",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1052884,
"choiceText": "Exfoliative dermatitis\r\n\r\n\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 333313,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 2-Year-Old-Boy With an Alarming Facial Rash"
},
{
"authors": "Buraa Kubaisi, MD; Nakhoul Nakhoul, MD; C. Stephen Foster, MD",
"content": [
"Certain human leukocyte antigen (HLA) alleles are associated with an increased probability of developing SJS upon exposure to specific drugs; these includes HLA-B*1502 in patients of southeastern Asian ethnicity, which increases the risk of developing SJS while on carbamazepine therapy, and HLA-B*44 in white patients. The HLA-A*0206 and HLA-DQB1*0601 alleles are strongly associated with SJS with ocular involvement.[5]",
"SJS usually starts with a prodromal phase, including fever, malaise, chills, headaches, nausea, and vomiting. The prodromal phase usually lasts less than 2 weeks and is followed by the eruptive phase. Skin lesions may begin as erythema of the face, neck, and upper chest. They may progress into bullae and skin denudation. The palms, soles, dorsum of the hands, and extensor surfaces are most commonly affected. Lesions that involve the upper gastrointestinal system may cause difficulty eating and drinking, in addition to difficulty swallowing.[6]",
"The typical skin lesion has the appearance of a target with central purpura. It develops into a bulla and ruptures, leaving an eroded area (Figure 3).",
"Figure 3.",
"Ocular involvement is not uncommon; the patient may present with red eyes, foreign body sensation, burning and itching, and gross pain. Ocular signs vary, with a wide spectrum of severity, ranging from simple conjunctivitis, blepharitis, and epithelial defects to more severe signs, such as conjunctival membranes, stromal ulcers, and eventual perforation, which is sight-threatening. If not treated early, these may lead to trichiasis, conjunctival shrinkage, symblepharon, corneal scarring, and eventual blindness.",
"Mucosal involvement may include erythema, edema, sloughing, blistering, ulceration, and necrosis. Severe systemic complications may ensue if early diagnosis is not made and the offending drug is not promptly discontinued. Scarring and cosmetic deformity, superinfections, esophageal strictures, and renal failure are a few of these eventual complications. As mentioned above, SJS may lead to death.",
"Early diagnosis is crucial in order to prevent permanent damage and death, especially because SJS at initial presentation my not appear severe. Early diagnosis is achieved by anamnesis and determination of drug exposure or an infection before the onset of symptoms. No specific laboratory studies (other than biopsy) can definitively establish the diagnosis of SJS."
],
"date": "January 22, 2019",
"figures": [
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/873/352/873352-Thumb3.png"
}
],
"markdown": "# A 2-Year-Old-Boy With an Alarming Facial Rash\n\n **Authors:** Buraa Kubaisi, MD; Nakhoul Nakhoul, MD; C. Stephen Foster, MD \n **Date:** January 22, 2019\n\n ## Content\n\n Certain human leukocyte antigen (HLA) alleles are associated with an increased probability of developing SJS upon exposure to specific drugs; these includes HLA-B*1502 in patients of southeastern Asian ethnicity, which increases the risk of developing SJS while on carbamazepine therapy, and HLA-B*44 in white patients. The HLA-A*0206 and HLA-DQB1*0601 alleles are strongly associated with SJS with ocular involvement.[5]\nSJS usually starts with a prodromal phase, including fever, malaise, chills, headaches, nausea, and vomiting. The prodromal phase usually lasts less than 2 weeks and is followed by the eruptive phase. Skin lesions may begin as erythema of the face, neck, and upper chest. They may progress into bullae and skin denudation. The palms, soles, dorsum of the hands, and extensor surfaces are most commonly affected. Lesions that involve the upper gastrointestinal system may cause difficulty eating and drinking, in addition to difficulty swallowing.[6]\nThe typical skin lesion has the appearance of a target with central purpura. It develops into a bulla and ruptures, leaving an eroded area (Figure 3).\nFigure 3.\nOcular involvement is not uncommon; the patient may present with red eyes, foreign body sensation, burning and itching, and gross pain. Ocular signs vary, with a wide spectrum of severity, ranging from simple conjunctivitis, blepharitis, and epithelial defects to more severe signs, such as conjunctival membranes, stromal ulcers, and eventual perforation, which is sight-threatening. If not treated early, these may lead to trichiasis, conjunctival shrinkage, symblepharon, corneal scarring, and eventual blindness.\nMucosal involvement may include erythema, edema, sloughing, blistering, ulceration, and necrosis. Severe systemic complications may ensue if early diagnosis is not made and the offending drug is not promptly discontinued. Scarring and cosmetic deformity, superinfections, esophageal strictures, and renal failure are a few of these eventual complications. As mentioned above, SJS may lead to death.\nEarly diagnosis is crucial in order to prevent permanent damage and death, especially because SJS at initial presentation my not appear severe. Early diagnosis is achieved by anamnesis and determination of drug exposure or an infection before the onset of symptoms. No specific laboratory studies (other than biopsy) can definitively establish the diagnosis of SJS.\n\n ## Figures\n\n **Figure 3.** \n \n\n\n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 2-Year-Old-Boy With an Alarming Facial Rash"
},
{
"authors": "Buraa Kubaisi, MD; Nakhoul Nakhoul, MD; C. Stephen Foster, MD",
"content": [
"The SCORTEN score calculates the risk for death in both SJS and toxic epidermal necrolysis, on the basis of the following variables[7]:",
"Age > 40 years",
"Cancer",
"Heart rate > 120 beats/min",
"Initial percentage of epidermal detachment > 10%",
"Blood urea nitrogen level > 10 mmol/L",
"Serum glucose level > 14 mmol/L",
"Bicarbonate level < 20 mmol/L",
"Each variable is assigned a value of 1 point. Mortality rates are as follows:",
"1 point: ≥ 3.2%",
"2 points: ≥ 12.1%",
"3 points: ≥ 35.3%",
"4 points: ≥ 58.3%",
"≥ 5 points: ≥90%",
"In a matter of a few days, individuals with SJS lose large areas of epidermis. Although this progresses rapidly, it usually ceases suddenly and the reepithelialization process starts. It is impossible to predict the course of a patient at presentation. The reepithelialization phase usually lasts 3-4 weeks.",
"Management of patients with SJS is usually provided in intensive care units or burn centers. Supportive care must include management of fluid and electrolyte requirements. Withdrawal of the suspected offending agent is critically important. The earlier the drug is withdrawn, the better the prognosis.[8]",
"Routine antibiotics are not usually indicated unless infection is evident because fever may be part of the disease. Debridement of necrotic skin is not recommended before disease activity ceases.",
"The use of corticosteroids in the management of SJS is controversial. Some physicians believe that these agents can delay wound healing and increase the chance of infection. If steroids are used, they should be initiated during the initial stage with a prophylactic antibiotic and rapidly tapered off.[9,10]",
"Plasmapheresis, immunosuppressive therapy, and intravenous immunoglobulin have been used, with variably successful results.[11,12]",
"The patient in this case was kept in the intensive care unit for 2 weeks, with special attention given to his hemodynamic stability and fluid status. The causative drug was withdrawn early. He was started on steroids, with a prophylactic antibiotic, and rapidly tapered off within 2 weeks. He had significant healing of the oral and skin lesions at the end of 2 weeks and was discharged afterward."
],
"date": "January 22, 2019",
"figures": [],
"markdown": "# A 2-Year-Old-Boy With an Alarming Facial Rash\n\n **Authors:** Buraa Kubaisi, MD; Nakhoul Nakhoul, MD; C. Stephen Foster, MD \n **Date:** January 22, 2019\n\n ## Content\n\n The SCORTEN score calculates the risk for death in both SJS and toxic epidermal necrolysis, on the basis of the following variables[7]:\nAge > 40 years\nCancer\nHeart rate > 120 beats/min\nInitial percentage of epidermal detachment > 10%\nBlood urea nitrogen level > 10 mmol/L\nSerum glucose level > 14 mmol/L\nBicarbonate level < 20 mmol/L\nEach variable is assigned a value of 1 point. Mortality rates are as follows:\n1 point: ≥ 3.2%\n2 points: ≥ 12.1%\n3 points: ≥ 35.3%\n4 points: ≥ 58.3%\n≥ 5 points: ≥90%\nIn a matter of a few days, individuals with SJS lose large areas of epidermis. Although this progresses rapidly, it usually ceases suddenly and the reepithelialization process starts. It is impossible to predict the course of a patient at presentation. The reepithelialization phase usually lasts 3-4 weeks.\nManagement of patients with SJS is usually provided in intensive care units or burn centers. Supportive care must include management of fluid and electrolyte requirements. Withdrawal of the suspected offending agent is critically important. The earlier the drug is withdrawn, the better the prognosis.[8]\nRoutine antibiotics are not usually indicated unless infection is evident because fever may be part of the disease. Debridement of necrotic skin is not recommended before disease activity ceases.\nThe use of corticosteroids in the management of SJS is controversial. Some physicians believe that these agents can delay wound healing and increase the chance of infection. If steroids are used, they should be initiated during the initial stage with a prophylactic antibiotic and rapidly tapered off.[9,10]\nPlasmapheresis, immunosuppressive therapy, and intravenous immunoglobulin have been used, with variably successful results.[11,12]\nThe patient in this case was kept in the intensive care unit for 2 weeks, with special attention given to his hemodynamic stability and fluid status. The causative drug was withdrawn early. He was started on steroids, with a prophylactic antibiotic, and rapidly tapered off within 2 weeks. He had significant healing of the oral and skin lesions at the end of 2 weeks and was discharged afterward.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1052904,
"choiceText": "Diagnosis is primarily clinical, although some tests may help with confirmation",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1052906,
"choiceText": "Chest radiography and blood and skin cultures are used to confirm the diagnosis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1052908,
"choiceText": "Skin and mucosa biopsy is routinely required for diagnosis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1052910,
"choiceText": "Elevated white blood cell count and blood IL-6, interleukin-2 receptor, and CRP levels indicate the diagnosis",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "SJS is a clinical diagnosis; although skin or mucosa biopsy may aid in assuring the diagnosis, it is not required to diagnose SJS. <br><br>\r\n\r\nSerum levels of IL-6, interleukin-2 receptor, and CRP are usually elevated in SJS, but the white blood cell count is usually within normal limits. Skin and blood cultures are usually required to rule out infections and sepsis.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 333327,
"questionText": "Which of the following is accurate regarding SJS diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1052928,
"choiceText": "Reassurance alone is indicated because the disease is self-limited",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1052930,
"choiceText": "The patient can be treated as an outpatient, with topical anesthetics for pain reduction",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1052932,
"choiceText": "The patient should be hospitalized, with special attention to airway and hemodynamic stability, fluid status, wound/burn care, and pain control",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1052934,
"choiceText": "Withdrawal of the causative drug does not make any difference",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Management of patients with SJS is usually provided in intensive care units or burn centers. Supportive care must include the management of fluid and electrolyte requirements. Withdrawal of the suspected offending agent is critically important. The earlier the drug is withdrawn, the better the prognosis.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 333333,
"questionText": "Which of the following is accurate regarding the treatment and management of the acute phase of SJS?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 2-Year-Old-Boy With an Alarming Facial Rash"
},
{
"authors": "Buraa Kubaisi, MD; Nakhoul Nakhoul, MD; C. Stephen Foster, MD",
"content": [],
"date": "January 22, 2019",
"figures": [],
"markdown": "# A 2-Year-Old-Boy With an Alarming Facial Rash\n\n **Authors:** Buraa Kubaisi, MD; Nakhoul Nakhoul, MD; C. Stephen Foster, MD \n **Date:** January 22, 2019\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1052904,
"choiceText": "Diagnosis is primarily clinical, although some tests may help with confirmation",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1052906,
"choiceText": "Chest radiography and blood and skin cultures are used to confirm the diagnosis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1052908,
"choiceText": "Skin and mucosa biopsy is routinely required for diagnosis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1052910,
"choiceText": "Elevated white blood cell count and blood IL-6, interleukin-2 receptor, and CRP levels indicate the diagnosis",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "SJS is a clinical diagnosis; although skin or mucosa biopsy may aid in assuring the diagnosis, it is not required to diagnose SJS. <br><br>\r\n\r\nSerum levels of IL-6, interleukin-2 receptor, and CRP are usually elevated in SJS, but the white blood cell count is usually within normal limits. Skin and blood cultures are usually required to rule out infections and sepsis.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 333327,
"questionText": "Which of the following is accurate regarding SJS diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1052928,
"choiceText": "Reassurance alone is indicated because the disease is self-limited",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1052930,
"choiceText": "The patient can be treated as an outpatient, with topical anesthetics for pain reduction",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1052932,
"choiceText": "The patient should be hospitalized, with special attention to airway and hemodynamic stability, fluid status, wound/burn care, and pain control",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1052934,
"choiceText": "Withdrawal of the causative drug does not make any difference",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Management of patients with SJS is usually provided in intensive care units or burn centers. Supportive care must include the management of fluid and electrolyte requirements. Withdrawal of the suspected offending agent is critically important. The earlier the drug is withdrawn, the better the prognosis.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 333333,
"questionText": "Which of the following is accurate regarding the treatment and management of the acute phase of SJS?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 2-Year-Old-Boy With an Alarming Facial Rash"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1052878,
"choiceText": "Cicatrizing pemphigoid",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1052880,
"choiceText": "Stevens-Johnson syndrome",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1052882,
"choiceText": "Toxic shock syndrome",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1052884,
"choiceText": "Exfoliative dermatitis\r\n\r\n\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 333313,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1052904,
"choiceText": "Diagnosis is primarily clinical, although some tests may help with confirmation",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1052906,
"choiceText": "Chest radiography and blood and skin cultures are used to confirm the diagnosis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1052908,
"choiceText": "Skin and mucosa biopsy is routinely required for diagnosis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1052910,
"choiceText": "Elevated white blood cell count and blood IL-6, interleukin-2 receptor, and CRP levels indicate the diagnosis",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "SJS is a clinical diagnosis; although skin or mucosa biopsy may aid in assuring the diagnosis, it is not required to diagnose SJS. <br><br>\r\n\r\nSerum levels of IL-6, interleukin-2 receptor, and CRP are usually elevated in SJS, but the white blood cell count is usually within normal limits. Skin and blood cultures are usually required to rule out infections and sepsis.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 333327,
"questionText": "Which of the following is accurate regarding SJS diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1052928,
"choiceText": "Reassurance alone is indicated because the disease is self-limited",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1052930,
"choiceText": "The patient can be treated as an outpatient, with topical anesthetics for pain reduction",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1052932,
"choiceText": "The patient should be hospitalized, with special attention to airway and hemodynamic stability, fluid status, wound/burn care, and pain control",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1052934,
"choiceText": "Withdrawal of the causative drug does not make any difference",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Management of patients with SJS is usually provided in intensive care units or burn centers. Supportive care must include the management of fluid and electrolyte requirements. Withdrawal of the suspected offending agent is critically important. The earlier the drug is withdrawn, the better the prognosis.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 333333,
"questionText": "Which of the following is accurate regarding the treatment and management of the acute phase of SJS?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
872439 | /viewarticle/872439 | [
{
"authors": "Jitendra Gohil, MD; Pramod Gupta, MD",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"A 45-year-old man presents to his primary care physician with gradual swelling in his anterior neck over the past 6 months. At first, he thought nothing of the swelling, expecting it go away on its own; however, over the past 2 months, it has become more noticeable. The patient has become concerned that the swelling may be caused by cancer.",
"He has not experienced any pain in the area of the swelling, nor has he experienced fever. In addition, he denies any difficulty in swallowing and any alteration of his voice. He has no problems with his breathing.",
"The patient has no history of trauma, and he denies any significant personal medical history. His family history is unremarkable. He is not currently taking any medications, does not smoke, and does not use any illicit substances. He is a social drinker."
],
"date": "November 30, 2016",
"figures": [],
"markdown": "# A 45-Year-Old Man With Gradual Neck Swelling\n\n **Authors:** Jitendra Gohil, MD; Pramod Gupta, MD \n **Date:** November 30, 2016\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nA 45-year-old man presents to his primary care physician with gradual swelling in his anterior neck over the past 6 months. At first, he thought nothing of the swelling, expecting it go away on its own; however, over the past 2 months, it has become more noticeable. The patient has become concerned that the swelling may be caused by cancer.\nHe has not experienced any pain in the area of the swelling, nor has he experienced fever. In addition, he denies any difficulty in swallowing and any alteration of his voice. He has no problems with his breathing.\nThe patient has no history of trauma, and he denies any significant personal medical history. His family history is unremarkable. He is not currently taking any medications, does not smoke, and does not use any illicit substances. He is a social drinker.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 45-Year-Old Man With Gradual Neck Swelling"
},
{
"authors": "Jitendra Gohil, MD; Pramod Gupta, MD",
"content": [
"On physical examination, the patient is afebrile and has a pulse of 72 beats/min, a blood pressure of 130/82 mm Hg, a respiratory rate of 12 breaths/min, and a normal oxygen saturation while breathing room air. He is well-developed and well-appearing.",
"Examination of the anterior neck reveals a nontender, nonerythematous, fluctuant mass measuring approximately 10 × 8 cm in the midline of the lower neck, with slight extension to the right side of the midline. The mass moves up and down when the patient swallows, and it slightly displaces anteriorly with protrusion of the tongue.",
"Figure 1.",
"Figure 2.",
"No cervical lymphadenopathy is appreciated. The lung fields are clear bilaterally, without evidence of stridor or wheeze. The heart has a regular rate and rhythm, without murmurs, and the abdomen is soft and nontender, without evidence of masses. The cranial nerves are intact, and the remainder of the neurologic exam is unrevealing.",
"Routine laboratory blood tests, including a complete blood cell count and an electrolyte panel, and a rapid assay for thyroid function are obtained. All laboratory investigations, including the thyroid studies, are within normal limits.",
"Ultrasound of the neck is obtained (Figure 1). CT of the neck is also performed for further evaluation (Figure 2)."
],
"date": "November 30, 2016",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/872/439/872439-Thumb1.png"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/872/439/872439-Thumb2.png"
}
],
"markdown": "# A 45-Year-Old Man With Gradual Neck Swelling\n\n **Authors:** Jitendra Gohil, MD; Pramod Gupta, MD \n **Date:** November 30, 2016\n\n ## Content\n\n On physical examination, the patient is afebrile and has a pulse of 72 beats/min, a blood pressure of 130/82 mm Hg, a respiratory rate of 12 breaths/min, and a normal oxygen saturation while breathing room air. He is well-developed and well-appearing.\nExamination of the anterior neck reveals a nontender, nonerythematous, fluctuant mass measuring approximately 10 × 8 cm in the midline of the lower neck, with slight extension to the right side of the midline. The mass moves up and down when the patient swallows, and it slightly displaces anteriorly with protrusion of the tongue.\nFigure 1.\nFigure 2.\nNo cervical lymphadenopathy is appreciated. The lung fields are clear bilaterally, without evidence of stridor or wheeze. The heart has a regular rate and rhythm, without murmurs, and the abdomen is soft and nontender, without evidence of masses. The cranial nerves are intact, and the remainder of the neurologic exam is unrevealing.\nRoutine laboratory blood tests, including a complete blood cell count and an electrolyte panel, and a rapid assay for thyroid function are obtained. All laboratory investigations, including the thyroid studies, are within normal limits.\nUltrasound of the neck is obtained (Figure 1). CT of the neck is also performed for further evaluation (Figure 2).\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1041174,
"choiceText": "Cystic vascular abnormality",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1041176,
"choiceText": "Cervical teratoma",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1041178,
"choiceText": "Ectopic thyroid",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1041180,
"choiceText": "Thyroglossal duct cyst",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 329537,
"questionText": "Which of the following is the most likely diagnosis?\r\n<br><br><i>\r\nHint: This is the most common congenital anomaly resulting in a midline neck mass.</i>\r\n",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 45-Year-Old Man With Gradual Neck Swelling"
},
{
"authors": "Jitendra Gohil, MD; Pramod Gupta, MD",
"content": [
"The ultrasound image in Figure 1 shows a large cystic mass anterior to the thyroid gland (arrowheads). The contrast-enhanced CT scan (Figure 2) demonstrates the same predominantly midline cystic mass extending anteriorly to the thyroid gland and under the strap muscles, without evidence of ectopic thyroid tissue. The findings are consistent with the diagnosis of a thyroglossal duct cyst.",
"Figure 1.",
"Figure 2.",
"Thyroglossal duct cysts are the most common cause of a midline neck mass. They usually occur between the hyoid bone and the thyroid gland and represent up to 70% of congenital neck anomalies. Thyroglossal duct cysts are second only to lymphadenopathy as the most common cause of a neck mass.[1]",
"The cysts usually appear in the midline and can be present anywhere along the line of fetal descent from the foramen cecum to the level of the thyroid gland. From an embryologic perspective, the thyroid gland develops during the third week of life as an outgrowth of the floor of the primitive pharynx. The primitive thyroid then descends from the foramen cecum to its mature position in the anterior neck through the thyroglossal duct. The thyroglossal duct is normally resorbed by 7-10 weeks of fetal life.",
"Abnormal persistence of the thyroglossal tract accompanied by mucus production from the endothelial lining of the tract leads to the development of a thyroglossal duct cyst. Approximately 7% of the population has thyroglossal duct remnants, and the distribution is equal among males and females. The cysts are usually found in children or adults younger than 30 years, but they can develop in adults of any age. Recently, numerous older patients, including patients in their 80s and 90s, have presented with thyroglossal duct cysts.",
"The four general types of thyroglossal duct cysts are thyrohyoid (61% of cases), suprahyoid (24%), suprasternal (13%), and intralingual (2%).[1,2,3]"
],
"date": "November 30, 2016",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/872/439/872439-Thumb1.png"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/872/439/872439-Thumb2.png"
}
],
"markdown": "# A 45-Year-Old Man With Gradual Neck Swelling\n\n **Authors:** Jitendra Gohil, MD; Pramod Gupta, MD \n **Date:** November 30, 2016\n\n ## Content\n\n The ultrasound image in Figure 1 shows a large cystic mass anterior to the thyroid gland (arrowheads). The contrast-enhanced CT scan (Figure 2) demonstrates the same predominantly midline cystic mass extending anteriorly to the thyroid gland and under the strap muscles, without evidence of ectopic thyroid tissue. The findings are consistent with the diagnosis of a thyroglossal duct cyst.\nFigure 1.\nFigure 2.\nThyroglossal duct cysts are the most common cause of a midline neck mass. They usually occur between the hyoid bone and the thyroid gland and represent up to 70% of congenital neck anomalies. Thyroglossal duct cysts are second only to lymphadenopathy as the most common cause of a neck mass.[1]\nThe cysts usually appear in the midline and can be present anywhere along the line of fetal descent from the foramen cecum to the level of the thyroid gland. From an embryologic perspective, the thyroid gland develops during the third week of life as an outgrowth of the floor of the primitive pharynx. The primitive thyroid then descends from the foramen cecum to its mature position in the anterior neck through the thyroglossal duct. The thyroglossal duct is normally resorbed by 7-10 weeks of fetal life.\nAbnormal persistence of the thyroglossal tract accompanied by mucus production from the endothelial lining of the tract leads to the development of a thyroglossal duct cyst. Approximately 7% of the population has thyroglossal duct remnants, and the distribution is equal among males and females. The cysts are usually found in children or adults younger than 30 years, but they can develop in adults of any age. Recently, numerous older patients, including patients in their 80s and 90s, have presented with thyroglossal duct cysts.\nThe four general types of thyroglossal duct cysts are thyrohyoid (61% of cases), suprahyoid (24%), suprasternal (13%), and intralingual (2%).[1,2,3]\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n\n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1041174,
"choiceText": "Cystic vascular abnormality",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1041176,
"choiceText": "Cervical teratoma",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1041178,
"choiceText": "Ectopic thyroid",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1041180,
"choiceText": "Thyroglossal duct cyst",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 329537,
"questionText": "Which of the following is the most likely diagnosis?\r\n<br><br><i>\r\nHint: This is the most common congenital anomaly resulting in a midline neck mass.</i>\r\n",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 45-Year-Old Man With Gradual Neck Swelling"
},
{
"authors": "Jitendra Gohil, MD; Pramod Gupta, MD",
"content": [
"The differential diagnosis of neck masses can be categorized by the location of the mass itself; the most common categorization is between lateral and midline masses. The most frequent causes of lateral masses are lymphadenopathy, branchial cleft cyst malignancy, cystic lymphangioma, and dermoid and teratoid cysts. Although thyroglossal duct cysts are the most common cause of midline masses, the differential diagnosis also includes dermoid and teratoid cysts, ectopic thyroid tissue, cancer, and cystic lymphangiomas.[1]",
"On radiologic imaging, thyroglossal duct cysts appear as a cystlike mass along the course of the thyroglossal duct. They must be differentiated from dermoid cysts and lymphangiomas. A dermoid cyst usually contains fat; lymphangioma is most common in infancy or early childhood, and it usually occurs in the posterior triangle of the neck, behind the sternocleidomastoid muscle.",
"A thyroglossal duct cyst must also be differentiated from an ectopic thyroid gland. If an ectopic thyroid gland is mistakenly removed, the patient may require long-term thyroid treatment for hypothyroidism. Often, patients with an ectopic thyroid gland also have hypothyroidism, and they have an elevated thyroid-stimulating hormone level.[1]",
"Thyroglossal duct cysts are diagnosed on the basis of the clinical history and confirmed with diagnostic imaging. Most patients with thyroglossal duct cysts present with either a history of a slowly growing, asymptomatic mass or a relatively rapidly growing mass (if the cyst is infected) in the anterior midline of the neck. Frequently, the swelling is exacerbated during an upper respiratory infection. The pathognomonic sign of a thyroglossal duct cyst is that it moves with swallowing and with protrusion of the tongue; however, the mobility of larger cysts may be restricted.",
"Imaging studies, including ultrasound and CT of the neck, confirm the diagnosis and help to rule out a possible ectopic thyroid gland. Thyroid function tests should be obtained to confirm normal thyroid function.[3]"
],
"date": "November 30, 2016",
"figures": [],
"markdown": "# A 45-Year-Old Man With Gradual Neck Swelling\n\n **Authors:** Jitendra Gohil, MD; Pramod Gupta, MD \n **Date:** November 30, 2016\n\n ## Content\n\n The differential diagnosis of neck masses can be categorized by the location of the mass itself; the most common categorization is between lateral and midline masses. The most frequent causes of lateral masses are lymphadenopathy, branchial cleft cyst malignancy, cystic lymphangioma, and dermoid and teratoid cysts. Although thyroglossal duct cysts are the most common cause of midline masses, the differential diagnosis also includes dermoid and teratoid cysts, ectopic thyroid tissue, cancer, and cystic lymphangiomas.[1]\nOn radiologic imaging, thyroglossal duct cysts appear as a cystlike mass along the course of the thyroglossal duct. They must be differentiated from dermoid cysts and lymphangiomas. A dermoid cyst usually contains fat; lymphangioma is most common in infancy or early childhood, and it usually occurs in the posterior triangle of the neck, behind the sternocleidomastoid muscle.\nA thyroglossal duct cyst must also be differentiated from an ectopic thyroid gland. If an ectopic thyroid gland is mistakenly removed, the patient may require long-term thyroid treatment for hypothyroidism. Often, patients with an ectopic thyroid gland also have hypothyroidism, and they have an elevated thyroid-stimulating hormone level.[1]\nThyroglossal duct cysts are diagnosed on the basis of the clinical history and confirmed with diagnostic imaging. Most patients with thyroglossal duct cysts present with either a history of a slowly growing, asymptomatic mass or a relatively rapidly growing mass (if the cyst is infected) in the anterior midline of the neck. Frequently, the swelling is exacerbated during an upper respiratory infection. The pathognomonic sign of a thyroglossal duct cyst is that it moves with swallowing and with protrusion of the tongue; however, the mobility of larger cysts may be restricted.\nImaging studies, including ultrasound and CT of the neck, confirm the diagnosis and help to rule out a possible ectopic thyroid gland. Thyroid function tests should be obtained to confirm normal thyroid function.[3]\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 45-Year-Old Man With Gradual Neck Swelling"
},
{
"authors": "Jitendra Gohil, MD; Pramod Gupta, MD",
"content": [
"Once diagnosed, thyroglossal duct cysts are removed because they are cosmetically undesirable and have the potential to become infected and undergo malignant transformation. The treatment of choice is the Sistrunk procedure, named after Walter Ellis Sistrunk and first described in an article in 1920.[4] The procedure, rather than being simple excision of the cyst, involves dissecting the central portion of the hyoid bone, with extension of the excision up to the base of the tongue to include excision of a small block of muscle around the foramen cecum.",
"Because of the increased risk for thyroglossal duct carcinoma, some practitioners further recommend the addition of thyroid suppression therapy or complete thyroidectomy; however, this practice remains controversial.",
"The recurrence rate associated with simple excision of a thyroglossal duct cyst is approximately 50%, whereas the recurrence rate associated with a formal Sistrunk procedure is approximately 5%. The rate of recurrence after a Sistrunk procedure is increased, however, when a thyroglossal duct is ruptured during dissection. A history of previous infection of the cyst, previous incision and drainage procedures, and adherence of the cyst to the skin all increase the risk for rupture during dissection. If the cyst is infected at the time of diagnosis, treatment with antibiotics, such as ampicillin/sulbactam, amoxicillin/clavulanate, or clindamycin, is indicated before surgical excision.[1,2,4]",
"The most common complications of thyroglossal duct cysts are infection with the possibility for abscess formation, spontaneous rupture, and formation of a secondary sinus tract. A Sistrunk procedure mistakenly performed for thyroid ectopia that removes thyroid tissue can cause hypothyroidism. The cysts can compress the trachea and lead to respiratory distress, especially if they are rapidly expanding (although this is uncommon).",
"Carcinoma is the most feared complication of thyroglossal duct cysts, occurring in about 1% of all cases; papillary carcinoma accounts for 85%-92% of cancers and follicular carcinoma accounting for the rest. Most patients who develop carcinoma tend to present at a later age. Cancer in a thyroglossal duct cyst seems to be more common in females than in males. Carcinoma arising in a thyroglossal duct cyst is typically diagnosed postoperatively by histology.[1]",
"Because the patient in our case presented with a thyroglossal duct cyst that (on clinical grounds) was not infected, he was not given antibiotics. The ultrasound study confirmed the presence of a thyroglossal duct cyst and ruled out the possibility of an ectopic thyroid gland.",
"The patient underwent an elective Sistrunk procedure, with no rupture during dissection. No complications occurred. He was discharged from the hospital the following day. Postoperative histologic analysis did not reveal any evidence of cancer. On follow-up in the clinic 2 weeks later, the patient was noted to be doing well."
],
"date": "November 30, 2016",
"figures": [],
"markdown": "# A 45-Year-Old Man With Gradual Neck Swelling\n\n **Authors:** Jitendra Gohil, MD; Pramod Gupta, MD \n **Date:** November 30, 2016\n\n ## Content\n\n Once diagnosed, thyroglossal duct cysts are removed because they are cosmetically undesirable and have the potential to become infected and undergo malignant transformation. The treatment of choice is the Sistrunk procedure, named after Walter Ellis Sistrunk and first described in an article in 1920.[4] The procedure, rather than being simple excision of the cyst, involves dissecting the central portion of the hyoid bone, with extension of the excision up to the base of the tongue to include excision of a small block of muscle around the foramen cecum.\nBecause of the increased risk for thyroglossal duct carcinoma, some practitioners further recommend the addition of thyroid suppression therapy or complete thyroidectomy; however, this practice remains controversial.\nThe recurrence rate associated with simple excision of a thyroglossal duct cyst is approximately 50%, whereas the recurrence rate associated with a formal Sistrunk procedure is approximately 5%. The rate of recurrence after a Sistrunk procedure is increased, however, when a thyroglossal duct is ruptured during dissection. A history of previous infection of the cyst, previous incision and drainage procedures, and adherence of the cyst to the skin all increase the risk for rupture during dissection. If the cyst is infected at the time of diagnosis, treatment with antibiotics, such as ampicillin/sulbactam, amoxicillin/clavulanate, or clindamycin, is indicated before surgical excision.[1,2,4]\nThe most common complications of thyroglossal duct cysts are infection with the possibility for abscess formation, spontaneous rupture, and formation of a secondary sinus tract. A Sistrunk procedure mistakenly performed for thyroid ectopia that removes thyroid tissue can cause hypothyroidism. The cysts can compress the trachea and lead to respiratory distress, especially if they are rapidly expanding (although this is uncommon).\nCarcinoma is the most feared complication of thyroglossal duct cysts, occurring in about 1% of all cases; papillary carcinoma accounts for 85%-92% of cancers and follicular carcinoma accounting for the rest. Most patients who develop carcinoma tend to present at a later age. Cancer in a thyroglossal duct cyst seems to be more common in females than in males. Carcinoma arising in a thyroglossal duct cyst is typically diagnosed postoperatively by histology.[1]\nBecause the patient in our case presented with a thyroglossal duct cyst that (on clinical grounds) was not infected, he was not given antibiotics. The ultrasound study confirmed the presence of a thyroglossal duct cyst and ruled out the possibility of an ectopic thyroid gland.\nThe patient underwent an elective Sistrunk procedure, with no rupture during dissection. No complications occurred. He was discharged from the hospital the following day. Postoperative histologic analysis did not reveal any evidence of cancer. On follow-up in the clinic 2 weeks later, the patient was noted to be doing well.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1041182,
"choiceText": "Complete thyroidectomy",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1041184,
"choiceText": "Sistrunk procedure for cyst excision",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1041186,
"choiceText": "Antibiotic therapy",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1041188,
"choiceText": "Thyroid suppression therapy",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1041190,
"choiceText": "Fine-needle aspiration",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Once diagnosed, thyroglossal duct cysts are removed because they are cosmetically undesirable and have the potential to become infected and undergo malignant transformation. The treatment of choice is the Sistrunk procedure, named after Walter Ellis Sistrunk and first described in an article in 1920. The procedure, rather than being simple excision of the cyst, involves dissecting the central portion of the hyoid bone, with extension of the excision up to the base of the tongue to include excision of a small block of muscle around the foramen cecum. ",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 329539,
"questionText": "Which of the following is the treatment of choice for a thyroglossal duct cyst?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1041192,
"choiceText": "Most cysts are found in adults aged 50 years or older",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1041194,
"choiceText": "If untreated, most will progress to papillary carcinoma",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1041196,
"choiceText": "The swelling is not exacerbated by upper respiratory infections",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1041198,
"choiceText": "They frequently lead to airway compromise",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1041200,
"choiceText": "They can move with tongue protrusion\r\n",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The pathognomonic sign of a thyroglossal duct cyst is that it moves with swallowing and with protrusion of the tongue; however, the mobility of larger cysts may be restricted.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 329541,
"questionText": "Which of the following is a feature of thyroglossal duct cysts?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 45-Year-Old Man With Gradual Neck Swelling"
},
{
"authors": "Jitendra Gohil, MD; Pramod Gupta, MD",
"content": [],
"date": "November 30, 2016",
"figures": [],
"markdown": "# A 45-Year-Old Man With Gradual Neck Swelling\n\n **Authors:** Jitendra Gohil, MD; Pramod Gupta, MD \n **Date:** November 30, 2016\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1041182,
"choiceText": "Complete thyroidectomy",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1041184,
"choiceText": "Sistrunk procedure for cyst excision",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1041186,
"choiceText": "Antibiotic therapy",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1041188,
"choiceText": "Thyroid suppression therapy",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1041190,
"choiceText": "Fine-needle aspiration",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Once diagnosed, thyroglossal duct cysts are removed because they are cosmetically undesirable and have the potential to become infected and undergo malignant transformation. The treatment of choice is the Sistrunk procedure, named after Walter Ellis Sistrunk and first described in an article in 1920. The procedure, rather than being simple excision of the cyst, involves dissecting the central portion of the hyoid bone, with extension of the excision up to the base of the tongue to include excision of a small block of muscle around the foramen cecum. ",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 329539,
"questionText": "Which of the following is the treatment of choice for a thyroglossal duct cyst?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1041192,
"choiceText": "Most cysts are found in adults aged 50 years or older",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1041194,
"choiceText": "If untreated, most will progress to papillary carcinoma",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1041196,
"choiceText": "The swelling is not exacerbated by upper respiratory infections",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1041198,
"choiceText": "They frequently lead to airway compromise",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1041200,
"choiceText": "They can move with tongue protrusion\r\n",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The pathognomonic sign of a thyroglossal duct cyst is that it moves with swallowing and with protrusion of the tongue; however, the mobility of larger cysts may be restricted.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 329541,
"questionText": "Which of the following is a feature of thyroglossal duct cysts?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 45-Year-Old Man With Gradual Neck Swelling"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1041174,
"choiceText": "Cystic vascular abnormality",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1041176,
"choiceText": "Cervical teratoma",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1041178,
"choiceText": "Ectopic thyroid",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1041180,
"choiceText": "Thyroglossal duct cyst",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 329537,
"questionText": "Which of the following is the most likely diagnosis?\r\n<br><br><i>\r\nHint: This is the most common congenital anomaly resulting in a midline neck mass.</i>\r\n",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1041182,
"choiceText": "Complete thyroidectomy",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1041184,
"choiceText": "Sistrunk procedure for cyst excision",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1041186,
"choiceText": "Antibiotic therapy",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1041188,
"choiceText": "Thyroid suppression therapy",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1041190,
"choiceText": "Fine-needle aspiration",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Once diagnosed, thyroglossal duct cysts are removed because they are cosmetically undesirable and have the potential to become infected and undergo malignant transformation. The treatment of choice is the Sistrunk procedure, named after Walter Ellis Sistrunk and first described in an article in 1920. The procedure, rather than being simple excision of the cyst, involves dissecting the central portion of the hyoid bone, with extension of the excision up to the base of the tongue to include excision of a small block of muscle around the foramen cecum. ",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 329539,
"questionText": "Which of the following is the treatment of choice for a thyroglossal duct cyst?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1041192,
"choiceText": "Most cysts are found in adults aged 50 years or older",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1041194,
"choiceText": "If untreated, most will progress to papillary carcinoma",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1041196,
"choiceText": "The swelling is not exacerbated by upper respiratory infections",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1041198,
"choiceText": "They frequently lead to airway compromise",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1041200,
"choiceText": "They can move with tongue protrusion\r\n",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The pathognomonic sign of a thyroglossal duct cyst is that it moves with swallowing and with protrusion of the tongue; however, the mobility of larger cysts may be restricted.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 329541,
"questionText": "Which of the following is a feature of thyroglossal duct cysts?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
872440 | /viewarticle/872440 | [
{
"authors": "Giovanni Volpicelli, MD",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"A 75-year-old man presents to the emergency department (ED) with a dry cough and dyspnea on exertion. The cough began 2 weeks ago and has been progressively worsening. The patient began experiencing dyspnea approximately 1 week ago. He also notes left-sided chest pain.",
"The patient has a history of coronary artery disease with recent coronary bypass surgery, recurrent episodes of bilateral spontaneous pneumothoraces, and chronic obstructive pulmonary disease (COPD). He has smoked an average of 15 cigarettes daily for 40 years. His symptoms have drastically worsened by the time of presentation to the ED."
],
"date": "November 30, 2016",
"figures": [],
"markdown": "# A 75-Year-Old Man With Dyspnea and Chest Pain\n\n **Authors:** Giovanni Volpicelli, MD \n **Date:** November 30, 2016\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nA 75-year-old man presents to the emergency department (ED) with a dry cough and dyspnea on exertion. The cough began 2 weeks ago and has been progressively worsening. The patient began experiencing dyspnea approximately 1 week ago. He also notes left-sided chest pain.\nThe patient has a history of coronary artery disease with recent coronary bypass surgery, recurrent episodes of bilateral spontaneous pneumothoraces, and chronic obstructive pulmonary disease (COPD). He has smoked an average of 15 cigarettes daily for 40 years. His symptoms have drastically worsened by the time of presentation to the ED.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 75-Year-Old Man With Dyspnea and Chest Pain"
},
{
"authors": "Giovanni Volpicelli, MD",
"content": [
"Figure 1",
"Figure 2",
"Upon physical examination, the patient has significantly abnormal vital signs, including a heart rate of 140 beats/min (sinus rhythm), a respiratory rate of 40 breaths/min, a blood pressure of 170/100 mm Hg, and a pulse oximetry reading showing an oxygen saturation of 60% while breathing room air. He appears agitated, uncomfortable, and in respiratory distress. His airway is patent, and he speaks single words at a time. His breathing is labored, and peripheral cyanosis is appreciated.",
"The patient has no breath sounds in the left hemithorax, but normal air movement is noted in the right hemithorax. In addition, no murmurs or friction rubs are appreciated. The left hemithorax is tympanic to percussion, and there is no tenderness to palpation. No tracheal deviation is noted.",
"The patient is immediately placed on supplemental oxygen, and he maintains an SPO2 of approximately 90%. While chest radiography is urgently performed, preparations are made for emergency tube thoracostomy placement. The radiograph (Figure 1) reveals a left-sided tension pneumothorax.",
"An emergency left-sided tube thoracostomy is inserted under local anesthesia in the left fourth intercostal space at the midaxillary line. A large rush of air is appreciated. After the pneumothorax is drained, the clinical picture rapidly improves. At this point, the patient becomes much less dyspneic, with a respiratory rate of 22 breaths/min, a blood pressure of 124/63 mm Hg, a heart rate of 90 beats/min (normal sinus rhythm), and 92% SPO2 via face mask.",
"Approximately 10 minutes later, the patient experiences dramatic worsening of his condition, including significant shortness of breath. The respiratory rate rises to 32 breaths/min, and the SPO2 drops to 80% despite administration of oxygen via a nonrebreather mask. The blood pressure is maintained at 120/74 mm Hg, with a heart rate of 118 beats/min (sinus rhythm). Crackles are now heard over the left lung, most prominently at the base.",
"Blood gas analysis reveals a pH level of 7.30, a PCO2 of 35.4 mm Hg, a PO2 of 52.8 mm Hg, and a bicarbonate level of 17.2 mEq/L, with a base excess of -7.9 mEq/L. As the patient is prepared for a new chest radiograph (Figure 2), empirical treatment is initiated with medications."
],
"date": "November 30, 2016",
"figures": [
{
"caption": "Figure 1",
"image_url": "https://img.medscapestatic.com/article/872/440/872440-Thumb1.png"
},
{
"caption": "Figure 2",
"image_url": "https://img.medscapestatic.com/article/872/440/872440-Thumb2.png"
}
],
"markdown": "# A 75-Year-Old Man With Dyspnea and Chest Pain\n\n **Authors:** Giovanni Volpicelli, MD \n **Date:** November 30, 2016\n\n ## Content\n\n Figure 1\nFigure 2\nUpon physical examination, the patient has significantly abnormal vital signs, including a heart rate of 140 beats/min (sinus rhythm), a respiratory rate of 40 breaths/min, a blood pressure of 170/100 mm Hg, and a pulse oximetry reading showing an oxygen saturation of 60% while breathing room air. He appears agitated, uncomfortable, and in respiratory distress. His airway is patent, and he speaks single words at a time. His breathing is labored, and peripheral cyanosis is appreciated.\nThe patient has no breath sounds in the left hemithorax, but normal air movement is noted in the right hemithorax. In addition, no murmurs or friction rubs are appreciated. The left hemithorax is tympanic to percussion, and there is no tenderness to palpation. No tracheal deviation is noted.\nThe patient is immediately placed on supplemental oxygen, and he maintains an SPO2 of approximately 90%. While chest radiography is urgently performed, preparations are made for emergency tube thoracostomy placement. The radiograph (Figure 1) reveals a left-sided tension pneumothorax.\nAn emergency left-sided tube thoracostomy is inserted under local anesthesia in the left fourth intercostal space at the midaxillary line. A large rush of air is appreciated. After the pneumothorax is drained, the clinical picture rapidly improves. At this point, the patient becomes much less dyspneic, with a respiratory rate of 22 breaths/min, a blood pressure of 124/63 mm Hg, a heart rate of 90 beats/min (normal sinus rhythm), and 92% SPO2 via face mask.\nApproximately 10 minutes later, the patient experiences dramatic worsening of his condition, including significant shortness of breath. The respiratory rate rises to 32 breaths/min, and the SPO2 drops to 80% despite administration of oxygen via a nonrebreather mask. The blood pressure is maintained at 120/74 mm Hg, with a heart rate of 118 beats/min (sinus rhythm). Crackles are now heard over the left lung, most prominently at the base.\nBlood gas analysis reveals a pH level of 7.30, a PCO2 of 35.4 mm Hg, a PO2 of 52.8 mm Hg, and a bicarbonate level of 17.2 mEq/L, with a base excess of -7.9 mEq/L. As the patient is prepared for a new chest radiograph (Figure 2), empirical treatment is initiated with medications.\n\n ## Figures\n\n **Figure 1** \n \n\n**Figure 2** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1041090,
"choiceText": "Atelectasis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1041092,
"choiceText": "Pulmonary embolus",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1041094,
"choiceText": "Recurrent pneumothorax",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1041096,
"choiceText": "Reexpansion pulmonary edema",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1041098,
"choiceText": "Myocardial infarction",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 329469,
"questionText": "What is the most likely etiology of the patient's deterioration?<br/><br/>\r\n<em>Hint: Pay attention to the new physical examination findings and the radiographic features of the left lung in Figure 2.</em>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 75-Year-Old Man With Dyspnea and Chest Pain"
},
{
"authors": "Giovanni Volpicelli, MD",
"content": [
"Figure 2",
"The clinical presentation and chest radiograph obtained in the ED immediately after the patient's arrival allowed diagnosis of left-sided tension pneumothorax. This condition rapidly improved after drainage via a tube thoracostomy.",
"Tension pneumothorax is a clinical diagnosis; the combination of severe hypoxia and hemodynamic instability is typically sufficient clinical evidence to institute needle decompression without a confirmatory chest radiograph. Delays in getting a chest radiograph can result in increases in morbidity and mortality when treating tension pneumothorax, which is one of the true clinical emergencies.",
"The sudden, dramatic worsening of the clinical picture 10 minutes postprocedure was unexpected. The second chest radiograph (Figure 2) showed florid left-sided pulmonary edema consistent with reexpansion pulmonary edema. This is a rare complication of the treatment of lung collapse secondary to atelectasis, pleural effusion, or pneumothorax.[1,2,3]",
"This case demonstrates that reexpansion pulmonary edema is a risk and should be kept in mind when treating a patient requiring tube thoracostomy. Many cases of unilateral reexpansion pulmonary edema have been reported, and the condition is associated with high mortality. It seems to be a relatively rare complication, but the actual incidence is unknown because many cases do not manifest clinically.",
"Reexpansion pulmonary edema generally occurs after a prolonged period of total lung collapse, or when the reexpansion treatment occurs too rapidly. This complication generally manifests early after reexpansion.[1,3]",
"The pathogenesis of reexpansion pulmonary edema is controversial because the causes are unclear and probably multifactorial. A relative lack of surfactant has been suggested as a causative factor, which could account for unilateral pulmonary edema occurring after long-standing total collapse of the lung. The condition can also develop after a very short time of lung collapse. Some evidence suggests other possible mechanisms, but increased pulmonary microvascular permeability is the only pathogenesis that has actually been studied and proven.",
"Another potential factor in the pathogenesis of reexpansion pulmonary edema is the speed of reexpansion after chest tube insertion. The thought is that rapid reexpansion perhaps enhances the inflow of fluid from the capillaries. This theory is debatable because case reports have demonstrated pulmonary edema occurring in lungs that have reexpanded without suction or in episodes of atelectasis that have reexpanded spontaneously. For pleural effusions, some evidence suggests no more than about 1.5 L should be drained at one time, or extreme caution should be exercised if removal of more than 1.5 L is planned; however, other evidence suggests that much larger volumes of fluid can be safely drained.",
"Cases of reexpansion pulmonary edema occurring with less than 1.5 L that have been caused by unknown factors have also been documented[4]; these factors could possibly be negative intrapleural pressure, the amount of time that the lung has been down, and the age of the patient. Some evidence suggests that only underwater seal and not suction should be used in larger pneumothoraces that last longer than 3 days.",
"Caution should be applied irrespective of the amount of fluid drained, and the patient must be monitored for the development of respiratory symptoms. This could be accomplished, for example, by avoiding excessively negative intrapleural pressures. If no symptoms occur, then little evidence contraindicates draining an effusion to dryness.[1,3,5,6,7] Tarver and colleagues[8] also suggest stopping air or fluid removal when the patient coughs, which is the first sign of reexpansion pulmonary edema."
],
"date": "November 30, 2016",
"figures": [
{
"caption": "Figure 2",
"image_url": "https://img.medscapestatic.com/article/872/440/872440-Thumb2.png"
}
],
"markdown": "# A 75-Year-Old Man With Dyspnea and Chest Pain\n\n **Authors:** Giovanni Volpicelli, MD \n **Date:** November 30, 2016\n\n ## Content\n\n Figure 2\nThe clinical presentation and chest radiograph obtained in the ED immediately after the patient's arrival allowed diagnosis of left-sided tension pneumothorax. This condition rapidly improved after drainage via a tube thoracostomy.\nTension pneumothorax is a clinical diagnosis; the combination of severe hypoxia and hemodynamic instability is typically sufficient clinical evidence to institute needle decompression without a confirmatory chest radiograph. Delays in getting a chest radiograph can result in increases in morbidity and mortality when treating tension pneumothorax, which is one of the true clinical emergencies.\nThe sudden, dramatic worsening of the clinical picture 10 minutes postprocedure was unexpected. The second chest radiograph (Figure 2) showed florid left-sided pulmonary edema consistent with reexpansion pulmonary edema. This is a rare complication of the treatment of lung collapse secondary to atelectasis, pleural effusion, or pneumothorax.[1,2,3]\nThis case demonstrates that reexpansion pulmonary edema is a risk and should be kept in mind when treating a patient requiring tube thoracostomy. Many cases of unilateral reexpansion pulmonary edema have been reported, and the condition is associated with high mortality. It seems to be a relatively rare complication, but the actual incidence is unknown because many cases do not manifest clinically.\nReexpansion pulmonary edema generally occurs after a prolonged period of total lung collapse, or when the reexpansion treatment occurs too rapidly. This complication generally manifests early after reexpansion.[1,3]\nThe pathogenesis of reexpansion pulmonary edema is controversial because the causes are unclear and probably multifactorial. A relative lack of surfactant has been suggested as a causative factor, which could account for unilateral pulmonary edema occurring after long-standing total collapse of the lung. The condition can also develop after a very short time of lung collapse. Some evidence suggests other possible mechanisms, but increased pulmonary microvascular permeability is the only pathogenesis that has actually been studied and proven.\nAnother potential factor in the pathogenesis of reexpansion pulmonary edema is the speed of reexpansion after chest tube insertion. The thought is that rapid reexpansion perhaps enhances the inflow of fluid from the capillaries. This theory is debatable because case reports have demonstrated pulmonary edema occurring in lungs that have reexpanded without suction or in episodes of atelectasis that have reexpanded spontaneously. For pleural effusions, some evidence suggests no more than about 1.5 L should be drained at one time, or extreme caution should be exercised if removal of more than 1.5 L is planned; however, other evidence suggests that much larger volumes of fluid can be safely drained.\nCases of reexpansion pulmonary edema occurring with less than 1.5 L that have been caused by unknown factors have also been documented[4]; these factors could possibly be negative intrapleural pressure, the amount of time that the lung has been down, and the age of the patient. Some evidence suggests that only underwater seal and not suction should be used in larger pneumothoraces that last longer than 3 days.\nCaution should be applied irrespective of the amount of fluid drained, and the patient must be monitored for the development of respiratory symptoms. This could be accomplished, for example, by avoiding excessively negative intrapleural pressures. If no symptoms occur, then little evidence contraindicates draining an effusion to dryness.[1,3,5,6,7] Tarver and colleagues[8] also suggest stopping air or fluid removal when the patient coughs, which is the first sign of reexpansion pulmonary edema.\n\n ## Figures\n\n **Figure 2** \n \n\n\n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1041090,
"choiceText": "Atelectasis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1041092,
"choiceText": "Pulmonary embolus",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1041094,
"choiceText": "Recurrent pneumothorax",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1041096,
"choiceText": "Reexpansion pulmonary edema",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1041098,
"choiceText": "Myocardial infarction",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 329469,
"questionText": "What is the most likely etiology of the patient's deterioration?<br/><br/>\r\n<em>Hint: Pay attention to the new physical examination findings and the radiographic features of the left lung in Figure 2.</em>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 75-Year-Old Man With Dyspnea and Chest Pain"
},
{
"authors": "Giovanni Volpicelli, MD",
"content": [
"The most likely common factor in the pathologic process that leads to reexpansion pulmonary edema seems to be enhanced endothelial permeability. This process may result from alveolar-capillary membrane disruption and ischemia-reperfusion injury caused by stretching, and from increased pulmonary flow occurring in pulmonary reexpansion. This enhanced endothelial permeability may then propagate and worsen as a result of local cellular delivery of free radicals (the main basis of reperfusion injury) and inflammatory mediators.",
"Another potential factor in the pathogenesis of reexpansion pulmonary edema is increased hydrostatic pressure from vascular flooding of the reexpanded lung caused by negative intrapleural pressure. Although draining larger effusions to dryness in the absence of respiratory symptoms is reasonable, caution should be taken to avoid highly negative intrapleural pressures. Patients who appear to be at higher risk and warrant more gradual evacuation include those who have had a large pneumothorax, younger patients, those in whom the lung has not reexpanded for more than 7 days, and possibly those who have had more than 3 L of pleural fluid drained.[1,5,7,9,10]",
"The clinical presentation of reexpansion pulmonary edema can vary widely, ranging from asymptomatic radiologic findings to a combination of severe cardiac and respiratory insufficiency and circulatory shock. Detecting initial signs of unilateral pulmonary edema early is important, in order to begin treatment and prevent the above-mentioned cascade that can lead to severe respiratory failure and death.[3,5,11]",
"Therapy involves, in principle, an increase in the intra-alveolar pressure (to redirect the fluid into the interstitium and capillaries) together with adequate oxygenation and hemodynamic support. The goals of treatment include adequate oxygenation, diuresis (as hemodynamics allow),[12] hemodynamic support, and mechanical ventilation with positive end-expiratory pressure (when necessary).",
"A few reports have detailed the treatment of unilateral reexpansion pulmonary edema with noninvasive continuous positive airway pressure (CPAP). When clinical conditions allow for it, noninvasive ventilation with CPAP is a reasonable therapeutic alternative. The use of CPAP in cardiogenic pulmonary edema is an effective and accepted therapy. Data in the literature support the use of CPAP in other types of pulmonary edema and respiratory failure.",
"The use of noninvasive CPAP in the treatment of reexpansion pulmonary edema, is still considered controversial, especially when it manifests as a unilateral process and the patient is hemodynamically unstable.[2,5] The drop in blood pressure and the hemodynamic instability that are often seen in reexpansion pulmonary edema should not, however, be considered an absolute contraindication to the use of CPAP because the benefits of correcting hypoxemia and increasing the mean airway pressure probably outweigh the contraindications, provided that careful attention is paid to any hemodynamic alterations that may occur with this modality.[2]"
],
"date": "November 30, 2016",
"figures": [],
"markdown": "# A 75-Year-Old Man With Dyspnea and Chest Pain\n\n **Authors:** Giovanni Volpicelli, MD \n **Date:** November 30, 2016\n\n ## Content\n\n The most likely common factor in the pathologic process that leads to reexpansion pulmonary edema seems to be enhanced endothelial permeability. This process may result from alveolar-capillary membrane disruption and ischemia-reperfusion injury caused by stretching, and from increased pulmonary flow occurring in pulmonary reexpansion. This enhanced endothelial permeability may then propagate and worsen as a result of local cellular delivery of free radicals (the main basis of reperfusion injury) and inflammatory mediators.\nAnother potential factor in the pathogenesis of reexpansion pulmonary edema is increased hydrostatic pressure from vascular flooding of the reexpanded lung caused by negative intrapleural pressure. Although draining larger effusions to dryness in the absence of respiratory symptoms is reasonable, caution should be taken to avoid highly negative intrapleural pressures. Patients who appear to be at higher risk and warrant more gradual evacuation include those who have had a large pneumothorax, younger patients, those in whom the lung has not reexpanded for more than 7 days, and possibly those who have had more than 3 L of pleural fluid drained.[1,5,7,9,10]\nThe clinical presentation of reexpansion pulmonary edema can vary widely, ranging from asymptomatic radiologic findings to a combination of severe cardiac and respiratory insufficiency and circulatory shock. Detecting initial signs of unilateral pulmonary edema early is important, in order to begin treatment and prevent the above-mentioned cascade that can lead to severe respiratory failure and death.[3,5,11]\nTherapy involves, in principle, an increase in the intra-alveolar pressure (to redirect the fluid into the interstitium and capillaries) together with adequate oxygenation and hemodynamic support. The goals of treatment include adequate oxygenation, diuresis (as hemodynamics allow),[12] hemodynamic support, and mechanical ventilation with positive end-expiratory pressure (when necessary).\nA few reports have detailed the treatment of unilateral reexpansion pulmonary edema with noninvasive continuous positive airway pressure (CPAP). When clinical conditions allow for it, noninvasive ventilation with CPAP is a reasonable therapeutic alternative. The use of CPAP in cardiogenic pulmonary edema is an effective and accepted therapy. Data in the literature support the use of CPAP in other types of pulmonary edema and respiratory failure.\nThe use of noninvasive CPAP in the treatment of reexpansion pulmonary edema, is still considered controversial, especially when it manifests as a unilateral process and the patient is hemodynamically unstable.[2,5] The drop in blood pressure and the hemodynamic instability that are often seen in reexpansion pulmonary edema should not, however, be considered an absolute contraindication to the use of CPAP because the benefits of correcting hypoxemia and increasing the mean airway pressure probably outweigh the contraindications, provided that careful attention is paid to any hemodynamic alterations that may occur with this modality.[2]\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 75-Year-Old Man With Dyspnea and Chest Pain"
},
{
"authors": "Giovanni Volpicelli, MD",
"content": [
"Figure 3",
"The patient in the above case underwent treatment with intravenous diuretics (furosemide 40 mg) and steroids (methylprednisolone 125 mg). Despite adequate diuresis of approximately 60 mL/h, the clinical picture did not improve. His blood pressure dropped further to 90/50 mm Hg, and it was impossible to maintain an ideal SPO2 even with maximum oxygen supplementation via a nonrebreather mask.",
"The decision was made to stop diuretics and begin treatment with CPAP, and to add an inotropic agent to his treatment regimen. CPAP was initiated via a helmet, with a positive end-expiratory pressure of 7.5 cm H2O and a FiO2 of 0.5, along with the addition of dopamine (5µg/kg/min).",
"The clinical picture improved progressively once CPAP was initiated, with the pulse oximetry consistently above 95% and normalization of blood pressure (permanently over 110/70 mm Hg), heart rate, and respiratory rate. A blood gas analysis obtained 10 hours later revealed a pH of 7.47, a PCO2 of 25.9 mm Hg, and a PO2 of 73.4 mm Hg. CPAP treatment was discontinued after 36 hours, with a repeat arterial blood gas analysis showing a pH of 7.47, a PCO2 of 33.5 mm Hg, and a PO2 of 75 mm Hg on 4 L of oxygen via a nasal cannula.",
"A new follow-up chest radiograph was obtained (Figure 3), which showed marked improvement of the pulmonary edema."
],
"date": "November 30, 2016",
"figures": [
{
"caption": "Figure 3",
"image_url": "https://img.medscapestatic.com/article/872/440/872440-Thumb3.png"
}
],
"markdown": "# A 75-Year-Old Man With Dyspnea and Chest Pain\n\n **Authors:** Giovanni Volpicelli, MD \n **Date:** November 30, 2016\n\n ## Content\n\n Figure 3\nThe patient in the above case underwent treatment with intravenous diuretics (furosemide 40 mg) and steroids (methylprednisolone 125 mg). Despite adequate diuresis of approximately 60 mL/h, the clinical picture did not improve. His blood pressure dropped further to 90/50 mm Hg, and it was impossible to maintain an ideal SPO2 even with maximum oxygen supplementation via a nonrebreather mask.\nThe decision was made to stop diuretics and begin treatment with CPAP, and to add an inotropic agent to his treatment regimen. CPAP was initiated via a helmet, with a positive end-expiratory pressure of 7.5 cm H2O and a FiO2 of 0.5, along with the addition of dopamine (5µg/kg/min).\nThe clinical picture improved progressively once CPAP was initiated, with the pulse oximetry consistently above 95% and normalization of blood pressure (permanently over 110/70 mm Hg), heart rate, and respiratory rate. A blood gas analysis obtained 10 hours later revealed a pH of 7.47, a PCO2 of 25.9 mm Hg, and a PO2 of 73.4 mm Hg. CPAP treatment was discontinued after 36 hours, with a repeat arterial blood gas analysis showing a pH of 7.47, a PCO2 of 33.5 mm Hg, and a PO2 of 75 mm Hg on 4 L of oxygen via a nasal cannula.\nA new follow-up chest radiograph was obtained (Figure 3), which showed marked improvement of the pulmonary edema.\n\n ## Figures\n\n **Figure 3** \n \n\n\n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1041100,
"choiceText": "After drainage of the pneumothorax, chest radiography shows unilateral florid edema of the reexpanded lung",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1041102,
"choiceText": "The patient has a history of congestive heart failure and has mild bilateral pulmonary edema",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1041104,
"choiceText": "After drainage of the pneumothorax, the affected lung does not fully reexpand and the patient does not improve",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1041106,
"choiceText": "Auscultation of the affected lung does not change after drainage of the pneumothorax",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Florid left-sided pulmonary edema is consistent with reexpansion pulmonary edema. This is a rare complication of the treatment of lung collapse secondary to atelectasis, pleural effusion, or pneumothorax.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 329471,
"questionText": "A patient presents with chest pain, severe respiratory compromise, and mild cyanosis. The patient describes a history of recent worsening shortness of breath. Imaging reveals pneumothorax, and the patient is treated by drainage with a chest tube. Which of the following events would lead you to suspect the patient has reexpansion pulmonary edema?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1041108,
"choiceText": "Repositioning the chest tube and increasing the negative pressure of the suction drainage",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1041110,
"choiceText": "Lowering the negative pressure of the suction drainage",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1041112,
"choiceText": "Administering diuretics and removing the drainage chest tube",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1041114,
"choiceText": "Maintaining good diuresis and providing supplemental oxygen and ventilatory support",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The goals of treatment include adequate oxygenation, diuresis (as hemodynamics allow), hemodynamic support, and mechanical ventilation with positive end-expiratory pressure (when necessary).",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 329473,
"questionText": "Which of the following is the next best step in management once reexpansion pulmonary edema is diagnosed and the patient experiences worsening symptoms?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 75-Year-Old Man With Dyspnea and Chest Pain"
},
{
"authors": "Giovanni Volpicelli, MD",
"content": [],
"date": "November 30, 2016",
"figures": [],
"markdown": "# A 75-Year-Old Man With Dyspnea and Chest Pain\n\n **Authors:** Giovanni Volpicelli, MD \n **Date:** November 30, 2016\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1041100,
"choiceText": "After drainage of the pneumothorax, chest radiography shows unilateral florid edema of the reexpanded lung",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1041102,
"choiceText": "The patient has a history of congestive heart failure and has mild bilateral pulmonary edema",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1041104,
"choiceText": "After drainage of the pneumothorax, the affected lung does not fully reexpand and the patient does not improve",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1041106,
"choiceText": "Auscultation of the affected lung does not change after drainage of the pneumothorax",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Florid left-sided pulmonary edema is consistent with reexpansion pulmonary edema. This is a rare complication of the treatment of lung collapse secondary to atelectasis, pleural effusion, or pneumothorax.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 329471,
"questionText": "A patient presents with chest pain, severe respiratory compromise, and mild cyanosis. The patient describes a history of recent worsening shortness of breath. Imaging reveals pneumothorax, and the patient is treated by drainage with a chest tube. Which of the following events would lead you to suspect the patient has reexpansion pulmonary edema?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1041108,
"choiceText": "Repositioning the chest tube and increasing the negative pressure of the suction drainage",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1041110,
"choiceText": "Lowering the negative pressure of the suction drainage",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1041112,
"choiceText": "Administering diuretics and removing the drainage chest tube",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1041114,
"choiceText": "Maintaining good diuresis and providing supplemental oxygen and ventilatory support",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The goals of treatment include adequate oxygenation, diuresis (as hemodynamics allow), hemodynamic support, and mechanical ventilation with positive end-expiratory pressure (when necessary).",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 329473,
"questionText": "Which of the following is the next best step in management once reexpansion pulmonary edema is diagnosed and the patient experiences worsening symptoms?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 75-Year-Old Man With Dyspnea and Chest Pain"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1041090,
"choiceText": "Atelectasis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1041092,
"choiceText": "Pulmonary embolus",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1041094,
"choiceText": "Recurrent pneumothorax",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1041096,
"choiceText": "Reexpansion pulmonary edema",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1041098,
"choiceText": "Myocardial infarction",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 329469,
"questionText": "What is the most likely etiology of the patient's deterioration?<br/><br/>\r\n<em>Hint: Pay attention to the new physical examination findings and the radiographic features of the left lung in Figure 2.</em>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1041100,
"choiceText": "After drainage of the pneumothorax, chest radiography shows unilateral florid edema of the reexpanded lung",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1041102,
"choiceText": "The patient has a history of congestive heart failure and has mild bilateral pulmonary edema",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1041104,
"choiceText": "After drainage of the pneumothorax, the affected lung does not fully reexpand and the patient does not improve",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1041106,
"choiceText": "Auscultation of the affected lung does not change after drainage of the pneumothorax",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Florid left-sided pulmonary edema is consistent with reexpansion pulmonary edema. This is a rare complication of the treatment of lung collapse secondary to atelectasis, pleural effusion, or pneumothorax.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 329471,
"questionText": "A patient presents with chest pain, severe respiratory compromise, and mild cyanosis. The patient describes a history of recent worsening shortness of breath. Imaging reveals pneumothorax, and the patient is treated by drainage with a chest tube. Which of the following events would lead you to suspect the patient has reexpansion pulmonary edema?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1041108,
"choiceText": "Repositioning the chest tube and increasing the negative pressure of the suction drainage",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1041110,
"choiceText": "Lowering the negative pressure of the suction drainage",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1041112,
"choiceText": "Administering diuretics and removing the drainage chest tube",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1041114,
"choiceText": "Maintaining good diuresis and providing supplemental oxygen and ventilatory support",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The goals of treatment include adequate oxygenation, diuresis (as hemodynamics allow), hemodynamic support, and mechanical ventilation with positive end-expiratory pressure (when necessary).",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 329473,
"questionText": "Which of the following is the next best step in management once reexpansion pulmonary edema is diagnosed and the patient experiences worsening symptoms?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
871746 | /viewarticle/871746 | [
{
"authors": "Maribel Munoz, MD",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"A 50-year-old man with a history of AIDS presents to the emergency department (ED) after a fall. He states that he has been experiencing progressive right leg weakness that began approximately 2 weeks ago. Initially, he only noticed difficulty getting up and down the stairs of his home; however, it has progressed to difficulty ambulating on level ground. He also complains of burning pain, cramps, and paresthesia in his left leg, which began around the same time.",
"Although the patient has AIDS, there is no history of major opportunistic infections or hospitalizations. The patient stopped taking his antiretroviral regimen 6 months ago. One month before presentation, he developed a painful vesicular rash extending from his right chest wall to his back and corresponding to the T2-3 dermatomes. He did not seek medical attention at that time, and the rash eventually resolved without specific treatment. He reports that he began noticing leg weakness approximately 1 week after the resolution of the rash. The patient has no known drug allergies."
],
"date": "November 16, 2016",
"figures": [],
"markdown": "# A 50-Year-Old Patient With AIDS and Leg Weakness\n\n **Authors:** Maribel Munoz, MD \n **Date:** November 16, 2016\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nA 50-year-old man with a history of AIDS presents to the emergency department (ED) after a fall. He states that he has been experiencing progressive right leg weakness that began approximately 2 weeks ago. Initially, he only noticed difficulty getting up and down the stairs of his home; however, it has progressed to difficulty ambulating on level ground. He also complains of burning pain, cramps, and paresthesia in his left leg, which began around the same time.\nAlthough the patient has AIDS, there is no history of major opportunistic infections or hospitalizations. The patient stopped taking his antiretroviral regimen 6 months ago. One month before presentation, he developed a painful vesicular rash extending from his right chest wall to his back and corresponding to the T2-3 dermatomes. He did not seek medical attention at that time, and the rash eventually resolved without specific treatment. He reports that he began noticing leg weakness approximately 1 week after the resolution of the rash. The patient has no known drug allergies.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 50-Year-Old Patient With AIDS and Leg Weakness"
},
{
"authors": "Maribel Munoz, MD",
"content": [
"On physical examination, the patient is a thin, alert male who appears to be in no acute discomfort. His vital signs reveal an oral temperature of 97.9°F (36.6°C), a pulse of 60 beats/min, blood pressure of 132/60 mm Hg, respiratory rate of 12 breaths/min, and an oxygen saturation of 99% while breathing room air.",
"Figure.",
"On neurologic examination, the patient is alert and oriented to person, place, and time, and cranial nerves II-XII are noted as intact. Examination of his motor function is significant for 0/5-1/5 strength at his right hip, right knee, and right ankle; it is 5/5 in all of his other muscle groups. Sensory examination is significant for decreased proprioception and vibratory sense in the right foot to the T3 level on the right leg. Both proprioception and vibratory sense are intact on the left leg. However, temperature sensation is decreased, accentuated pinprick sensation is noted, and burning paresthesia is present on the left side, from the foot up to the T3 level. Normal muscle bulk and tone are present, without fasciculations. His coordination is intact; however, the patient is unable to ambulate as a result of the right leg weakness.",
"A laboratory analysis is significant for leukopenia (3.3 × 103/µL [3.3 × 109/L] leukocytes; normal range, 3.5-12.5 × 103/µL) and a low lymphocyte percentage of 7.2% (0.072; normal range, 14%-49%). The patient's CD4 count is 4 cells/mm3 (normal range, 500-1600 cells/mm3), and the HIV polymerase chain reaction (PCR) shows a viral load of 341,493 copies/mL. Serum rapid plasma reagent, Treponema pallidum particle agglutination, and cryptococcus antigen test findings are negative; however, the serum cytomegalovirus antibody test result is positive.",
"A chest x-ray is obtained and has normal findings. A lumbar puncture is performed; the cerebrospinal fluid (CSF) studies reveal a leukocyte count of 0 cells/µL (0 × 106 cells/L), a red blood cell count of 2 cells/µL, a glucose concentration of 54 mg/dL (3.0 mmol/L), a protein concentration of 37 mg/dL (370 mg/L), a negative Gram stain finding, and a negative cryptococcal antigen result. Evaluation of the CSF for cytomegalovirus, herpes simplex virus, and West Nile virus is negative. MRI of the thoracic spine shows an abnormal T2-weighted hyperintense signal involving the upper thoracic cord at approximately the level of T3-4. It predominantly involves the right side of the cord (Figure)."
],
"date": "November 16, 2016",
"figures": [
{
"caption": "Figure.",
"image_url": "https://img.medscapestatic.com/article/871/746/871746-thumb-1.jpg"
}
],
"markdown": "# A 50-Year-Old Patient With AIDS and Leg Weakness\n\n **Authors:** Maribel Munoz, MD \n **Date:** November 16, 2016\n\n ## Content\n\n On physical examination, the patient is a thin, alert male who appears to be in no acute discomfort. His vital signs reveal an oral temperature of 97.9°F (36.6°C), a pulse of 60 beats/min, blood pressure of 132/60 mm Hg, respiratory rate of 12 breaths/min, and an oxygen saturation of 99% while breathing room air.\nFigure.\nOn neurologic examination, the patient is alert and oriented to person, place, and time, and cranial nerves II-XII are noted as intact. Examination of his motor function is significant for 0/5-1/5 strength at his right hip, right knee, and right ankle; it is 5/5 in all of his other muscle groups. Sensory examination is significant for decreased proprioception and vibratory sense in the right foot to the T3 level on the right leg. Both proprioception and vibratory sense are intact on the left leg. However, temperature sensation is decreased, accentuated pinprick sensation is noted, and burning paresthesia is present on the left side, from the foot up to the T3 level. Normal muscle bulk and tone are present, without fasciculations. His coordination is intact; however, the patient is unable to ambulate as a result of the right leg weakness.\nA laboratory analysis is significant for leukopenia (3.3 × 103/µL [3.3 × 109/L] leukocytes; normal range, 3.5-12.5 × 103/µL) and a low lymphocyte percentage of 7.2% (0.072; normal range, 14%-49%). The patient's CD4 count is 4 cells/mm3 (normal range, 500-1600 cells/mm3), and the HIV polymerase chain reaction (PCR) shows a viral load of 341,493 copies/mL. Serum rapid plasma reagent, Treponema pallidum particle agglutination, and cryptococcus antigen test findings are negative; however, the serum cytomegalovirus antibody test result is positive.\nA chest x-ray is obtained and has normal findings. A lumbar puncture is performed; the cerebrospinal fluid (CSF) studies reveal a leukocyte count of 0 cells/µL (0 × 106 cells/L), a red blood cell count of 2 cells/µL, a glucose concentration of 54 mg/dL (3.0 mmol/L), a protein concentration of 37 mg/dL (370 mg/L), a negative Gram stain finding, and a negative cryptococcal antigen result. Evaluation of the CSF for cytomegalovirus, herpes simplex virus, and West Nile virus is negative. MRI of the thoracic spine shows an abnormal T2-weighted hyperintense signal involving the upper thoracic cord at approximately the level of T3-4. It predominantly involves the right side of the cord (Figure).\n\n ## Figures\n\n **Figure.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1036008,
"choiceText": "Anterior cord syndrome",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1036010,
"choiceText": "Brown-Sequard syndrome",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1036012,
"choiceText": "Posterior cord syndrome",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1036014,
"choiceText": "Myelopathy with bilateral cord involvement",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1036016,
"choiceText": "Central cord syndrome",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 327891,
"questionText": "<p>Which of the following is the most likely diagnosis?</p>\r\n\r\n<p><em>Hint: Consider the spinal tracts responsible for the patient's neurologic findings.</em></p>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 50-Year-Old Patient With AIDS and Leg Weakness"
},
{
"authors": "Maribel Munoz, MD",
"content": [
"The patient's right leg weakness, decreased proprioception, and decreased sensitivity to vibration suggested damage to the corticospinal tract and the dorsal column on the right. Decreased temperature sensation in the left leg suggested damage to the spinothalamic tract on the right. When taken together, the physical examination and MRI findings of focal myelitis on the right side of the spinal cord were consistent with Brown-Sequard syndrome. Based on his history of a painful, dermatomal rash, CSF varicella-zoster virus DNA PCR was obtained and confirmed varicella-zoster virus as the causative agent for his myelitis.",
"Because of this patient's impaired immune response, the onset of the paralysis was subacute (occurring over the course of 2 weeks). Cessation of the patient's highly active antiretroviral therapy (HAART) resulted in severe compromise of his immune system, with a dramatic decrease in his CD4 count (4 cells/mm3). This allowed reactivation of the varicella-zoster virus, as exhibited by the symptoms of a dermatomal, vesicular rash and subsequent involvement of the entire right side of his thoracic spinal cord. The resulting myelitis led to the clinical deficits of ipsilateral leg weakness, proprioception and vibration deficits in the right leg, and contralateral decreased temperature sensation. Although the CSF demonstrated a normal leukocyte count, a positive CSF varicella-zoster virus DNA PCR confirmed the presence of the virus and suggested that this was the cause of the myelitis. Varicella-zoster virus immunoglobulin G (IgG) is a more sensitive and preferred test for reactivation in the CSF. Varicella-zoster virus PCR, although confirmatory, is a less sensitive test.[1,2] Although not present in this case, an elevated CSF protein level may be seen in cases of viral myelitis; however, this finding is not necessary for establishing the diagnosis.[3]",
"The true incidence and prevalence of Brown-Sequard syndrome is not known. The Office of Rare Disease Research at the National Institutes of Health considers it a rare disease because the prevalence in the United States is currently less than 200,000 affected individuals. Brown-Sequard syndrome was first described by Dr Charles Edouard Brown-Sequard in 1840. This syndrome involves a unilateral lesion of the spinal cord affecting the corticospinal tract, resulting in an ipsilateral spastic paralysis. Also affected are the posterior white column (dorsal column), with loss of proprioception and vibratory sensation ipsilaterally; and the spinothalamic tract, which leads to a contralateral loss of pain and temperature sensation (because this tract decussates within two spinal levels). All of the deficits are seen below the level of the lesion. A presentation of pure hemisection is rare; an incomplete presentation of the syndrome or the full syndrome with a few additional deficits resulting from bilateral cord involvement is more commonly seen. Pure syndromes are more commonly seen in cases of traumatic injury."
],
"date": "November 16, 2016",
"figures": [],
"markdown": "# A 50-Year-Old Patient With AIDS and Leg Weakness\n\n **Authors:** Maribel Munoz, MD \n **Date:** November 16, 2016\n\n ## Content\n\n The patient's right leg weakness, decreased proprioception, and decreased sensitivity to vibration suggested damage to the corticospinal tract and the dorsal column on the right. Decreased temperature sensation in the left leg suggested damage to the spinothalamic tract on the right. When taken together, the physical examination and MRI findings of focal myelitis on the right side of the spinal cord were consistent with Brown-Sequard syndrome. Based on his history of a painful, dermatomal rash, CSF varicella-zoster virus DNA PCR was obtained and confirmed varicella-zoster virus as the causative agent for his myelitis.\nBecause of this patient's impaired immune response, the onset of the paralysis was subacute (occurring over the course of 2 weeks). Cessation of the patient's highly active antiretroviral therapy (HAART) resulted in severe compromise of his immune system, with a dramatic decrease in his CD4 count (4 cells/mm3). This allowed reactivation of the varicella-zoster virus, as exhibited by the symptoms of a dermatomal, vesicular rash and subsequent involvement of the entire right side of his thoracic spinal cord. The resulting myelitis led to the clinical deficits of ipsilateral leg weakness, proprioception and vibration deficits in the right leg, and contralateral decreased temperature sensation. Although the CSF demonstrated a normal leukocyte count, a positive CSF varicella-zoster virus DNA PCR confirmed the presence of the virus and suggested that this was the cause of the myelitis. Varicella-zoster virus immunoglobulin G (IgG) is a more sensitive and preferred test for reactivation in the CSF. Varicella-zoster virus PCR, although confirmatory, is a less sensitive test.[1,2] Although not present in this case, an elevated CSF protein level may be seen in cases of viral myelitis; however, this finding is not necessary for establishing the diagnosis.[3]\nThe true incidence and prevalence of Brown-Sequard syndrome is not known. The Office of Rare Disease Research at the National Institutes of Health considers it a rare disease because the prevalence in the United States is currently less than 200,000 affected individuals. Brown-Sequard syndrome was first described by Dr Charles Edouard Brown-Sequard in 1840. This syndrome involves a unilateral lesion of the spinal cord affecting the corticospinal tract, resulting in an ipsilateral spastic paralysis. Also affected are the posterior white column (dorsal column), with loss of proprioception and vibratory sensation ipsilaterally; and the spinothalamic tract, which leads to a contralateral loss of pain and temperature sensation (because this tract decussates within two spinal levels). All of the deficits are seen below the level of the lesion. A presentation of pure hemisection is rare; an incomplete presentation of the syndrome or the full syndrome with a few additional deficits resulting from bilateral cord involvement is more commonly seen. Pure syndromes are more commonly seen in cases of traumatic injury.\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1036008,
"choiceText": "Anterior cord syndrome",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1036010,
"choiceText": "Brown-Sequard syndrome",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1036012,
"choiceText": "Posterior cord syndrome",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1036014,
"choiceText": "Myelopathy with bilateral cord involvement",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1036016,
"choiceText": "Central cord syndrome",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 327891,
"questionText": "<p>Which of the following is the most likely diagnosis?</p>\r\n\r\n<p><em>Hint: Consider the spinal tracts responsible for the patient's neurologic findings.</em></p>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 50-Year-Old Patient With AIDS and Leg Weakness"
},
{
"authors": "Maribel Munoz, MD",
"content": [
"The spinal cord lesions found in Brown-Sequard syndrome can arise from various diseases. Trauma, whether caused by transection of the cord or impingement as a result of disc herniation, is the most common cause. Other causes include neoplasms (such as non-Hodgkin lymphoma) or vasculitis (such as systemic lupus erythematosus). Infarction or hemorrhage around the cord can cause these symptoms as well. Infectious diseases may result in localized myelitis of the cord, leading to asymmetric motor and sensory symptoms or extrinsic compression by epidural abscess.",
"Nonviral pathogens include bacteria (Mycoplasma pneumoniae, Borrelia burgdorferi, T pallidum, Mycobacterium tuberculosis), fungi (Actinomyces, Blastomyces, Aspergillus species), and parasites (Schistosoma mansoni). Aseptic meningitis with associated myelitis resulting from viral causes is much more common; implicated pathogens include poliovirus, coxsackievirus, enterovirus, flavivirus (including West Nile virus), HIV, cytomegalovirus, herpes simplex virus types 1 and 2, and varicella-zoster virus.[4] Multiple sclerosis can also cause an incomplete transverse myelitis presenting as Brown-Sequard syndrome.",
"Most people in the United States first encounter varicella-zoster virus in the form of chicken pox as a child; however, with the recent use of varicella vaccinations (a live attenuated virus that becomes latent after vaccination), this is becoming less frequent. The initial infection is exhibited as a widespread outbreak of a vesicular exanthema. Once this resolves, the virus enters a latent period wherein it resides in the peripheral nervous system ganglia of almost every infected host for the lifetime of that individual. In the latent phase, little evidence of inflammation or host changes resulting from the virus is present. Upon reactivation, the virus typically causes a dermatomal, painful, vesicular rash that spreads in a transaxonal manner. Herpes zoster occurs in 1 million individuals in the United States annually. It is more prevalent in the elderly, occurring eight to ten times more frequently in those over the age of 60 years than in those who are younger.",
"Immunocompromised patients also have a higher incidence of herpes zoster.[3] Myelitis caused by varicella-zoster virus reactivation is very rare; but, when it does occur, it is almost always in an immunocompromised host. Young-Barbee and colleagues[5] described a similar case of Brown-Sequard syndrome following zoster reactivation in a 33-year-old immunocompetent man. Myelitis also occurs more commonly in the elderly or in immunocompromised persons. In fact, \"the Advisory Committee for Immunization Practices (ACIP) recommends a single dose of zoster (shingles) vaccine for adults 60 years old or older, whether or not the patient reported a prior episode of shingles. Persons with chronic medical conditions may be vaccinated unless a contraindication or precaution exists for their condition.\"[6] The most common condition associated with varicella-zoster virus myelitis is AIDS (as was seen in this patient). Compared with patients who are HIV-negative, patients with HIV/AIDS not only have a higher incidence of severe disease with herpes zoster but also a longer duration of symptoms with a higher rate of complications.[7]"
],
"date": "November 16, 2016",
"figures": [],
"markdown": "# A 50-Year-Old Patient With AIDS and Leg Weakness\n\n **Authors:** Maribel Munoz, MD \n **Date:** November 16, 2016\n\n ## Content\n\n The spinal cord lesions found in Brown-Sequard syndrome can arise from various diseases. Trauma, whether caused by transection of the cord or impingement as a result of disc herniation, is the most common cause. Other causes include neoplasms (such as non-Hodgkin lymphoma) or vasculitis (such as systemic lupus erythematosus). Infarction or hemorrhage around the cord can cause these symptoms as well. Infectious diseases may result in localized myelitis of the cord, leading to asymmetric motor and sensory symptoms or extrinsic compression by epidural abscess.\nNonviral pathogens include bacteria (Mycoplasma pneumoniae, Borrelia burgdorferi, T pallidum, Mycobacterium tuberculosis), fungi (Actinomyces, Blastomyces, Aspergillus species), and parasites (Schistosoma mansoni). Aseptic meningitis with associated myelitis resulting from viral causes is much more common; implicated pathogens include poliovirus, coxsackievirus, enterovirus, flavivirus (including West Nile virus), HIV, cytomegalovirus, herpes simplex virus types 1 and 2, and varicella-zoster virus.[4] Multiple sclerosis can also cause an incomplete transverse myelitis presenting as Brown-Sequard syndrome.\nMost people in the United States first encounter varicella-zoster virus in the form of chicken pox as a child; however, with the recent use of varicella vaccinations (a live attenuated virus that becomes latent after vaccination), this is becoming less frequent. The initial infection is exhibited as a widespread outbreak of a vesicular exanthema. Once this resolves, the virus enters a latent period wherein it resides in the peripheral nervous system ganglia of almost every infected host for the lifetime of that individual. In the latent phase, little evidence of inflammation or host changes resulting from the virus is present. Upon reactivation, the virus typically causes a dermatomal, painful, vesicular rash that spreads in a transaxonal manner. Herpes zoster occurs in 1 million individuals in the United States annually. It is more prevalent in the elderly, occurring eight to ten times more frequently in those over the age of 60 years than in those who are younger.\nImmunocompromised patients also have a higher incidence of herpes zoster.[3] Myelitis caused by varicella-zoster virus reactivation is very rare; but, when it does occur, it is almost always in an immunocompromised host. Young-Barbee and colleagues[5] described a similar case of Brown-Sequard syndrome following zoster reactivation in a 33-year-old immunocompetent man. Myelitis also occurs more commonly in the elderly or in immunocompromised persons. In fact, \"the Advisory Committee for Immunization Practices (ACIP) recommends a single dose of zoster (shingles) vaccine for adults 60 years old or older, whether or not the patient reported a prior episode of shingles. Persons with chronic medical conditions may be vaccinated unless a contraindication or precaution exists for their condition.\"[6] The most common condition associated with varicella-zoster virus myelitis is AIDS (as was seen in this patient). Compared with patients who are HIV-negative, patients with HIV/AIDS not only have a higher incidence of severe disease with herpes zoster but also a longer duration of symptoms with a higher rate of complications.[7]\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 50-Year-Old Patient With AIDS and Leg Weakness"
},
{
"authors": "Maribel Munoz, MD",
"content": [
"The first-line treatment for neurotropic varicella-zoster virus infection is intravenous acyclovir (10 mg/kg three times a day for 10-14 days). Alternatives to acyclovir include valacyclovir (1000 mg every 8 hours for 7 days) or famciclovir (500 mg every 8 hours for 7 days). In cases of acyclovir resistance, intravenous foscarnet can be used at a dose of 40 mg/kg two to three times a day for 2-3 weeks.[8] If treated early, varicella-zoster virus myelitis has a variable prognosis.",
"Another presentation of reactivation of varicella zoster in the central nervous system (CNS) is vasculitis, which usually affects the vertebrobasilar circulation and can cause recurrent brainstem and cerebellar infarcts. In this situation, steroids may be combined with antiviral therapy.",
"Clark and colleagues[9] presented a case of a patient who developed multilevel transverse myelitis associated with varicella-zoster virus infection after achieving a virologic response to HAART. This was associated with activated lymphocytes in the CSF, without a change in the CD4-positive T-cell count or the presence of activated CD8-positive T cells in the blood.[9] As stated earlier, these findings suggest that myelitis often results from a host's immune response to varicella-zoster virus (localized to the spinal cord) rather than simply the viral infection itself.",
"Given a concern for possible worsening neurologic deficit resulting from immune reconstitution, reintroduction of HAART therapy was delayed in this patient until he had received up to 3 months of zoster therapy. Possibly as a result of his delay in seeking medical attention, the patient in this case had a less favorable outcome. After an initial week of intravenous acyclovir treatment, he was able to walk slowly for short distances, but only with the assistance of a walker. Unfortunately, following discharge from the hospital, the patient did not return to the clinic and was lost to follow-up."
],
"date": "November 16, 2016",
"figures": [],
"markdown": "# A 50-Year-Old Patient With AIDS and Leg Weakness\n\n **Authors:** Maribel Munoz, MD \n **Date:** November 16, 2016\n\n ## Content\n\n The first-line treatment for neurotropic varicella-zoster virus infection is intravenous acyclovir (10 mg/kg three times a day for 10-14 days). Alternatives to acyclovir include valacyclovir (1000 mg every 8 hours for 7 days) or famciclovir (500 mg every 8 hours for 7 days). In cases of acyclovir resistance, intravenous foscarnet can be used at a dose of 40 mg/kg two to three times a day for 2-3 weeks.[8] If treated early, varicella-zoster virus myelitis has a variable prognosis.\nAnother presentation of reactivation of varicella zoster in the central nervous system (CNS) is vasculitis, which usually affects the vertebrobasilar circulation and can cause recurrent brainstem and cerebellar infarcts. In this situation, steroids may be combined with antiviral therapy.\nClark and colleagues[9] presented a case of a patient who developed multilevel transverse myelitis associated with varicella-zoster virus infection after achieving a virologic response to HAART. This was associated with activated lymphocytes in the CSF, without a change in the CD4-positive T-cell count or the presence of activated CD8-positive T cells in the blood.[9] As stated earlier, these findings suggest that myelitis often results from a host's immune response to varicella-zoster virus (localized to the spinal cord) rather than simply the viral infection itself.\nGiven a concern for possible worsening neurologic deficit resulting from immune reconstitution, reintroduction of HAART therapy was delayed in this patient until he had received up to 3 months of zoster therapy. Possibly as a result of his delay in seeking medical attention, the patient in this case had a less favorable outcome. After an initial week of intravenous acyclovir treatment, he was able to walk slowly for short distances, but only with the assistance of a walker. Unfortunately, following discharge from the hospital, the patient did not return to the clinic and was lost to follow-up.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1036028,
"choiceText": "Ipsilateral weakness; loss of vibratory sense and of proprioception; contralateral loss of pain and of temperature sensation",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1036030,
"choiceText": "Ipsilateral loss of pain and of temperature sensation; contralateral weakness; loss of vibratory sense and of proprioception",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1036032,
"choiceText": "Ipsilateral weakness; contralateral loss of vibratory sense and of proprioception",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1036034,
"choiceText": "Ipsilateral loss of vibratory sense and of proprioception; contralateral weakness.",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "This syndrome involves a unilateral lesion of the spinal cord affecting the corticospinal tract, resulting in an ipsilateral spastic paralysis. Also affected are the posterior white column (dorsal column), with loss of proprioception and vibratory sensation ipsilaterally, and the spinothalamic tract, which leads to a contralateral loss of pain and temperature sensation (because this tract decussates within two spinal levels).",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 327895,
"questionText": "A patient presents to the ED with acute-onset neurologic deficits that raise concern for Brown-Sequard syndrome. Which of the following groups of findings would have caused you to suspect this condition?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1036046,
"choiceText": "CSF IgG",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1036048,
"choiceText": "CSF culture",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1036050,
"choiceText": "CSF PCR",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1036052,
"choiceText": "India ink staining",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Varicella-zoster virus PCR of the CSF is specific but not particularly sensitive in detecting reactivation of the virus in the CNS. In contrast, varicella-zoster virus IgG levels are more sensitive but less specific.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 327899,
"questionText": "The patient mentioned above is noted to have a resolving dermatomal rash consistent with herpes zoster. A lumbar puncture is performed to evaluate for CNS infection. What is the most specific test for detecting varicella-zoster virus infection in the CNS?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 50-Year-Old Patient With AIDS and Leg Weakness"
},
{
"authors": "Maribel Munoz, MD",
"content": [],
"date": "November 16, 2016",
"figures": [],
"markdown": "# A 50-Year-Old Patient With AIDS and Leg Weakness\n\n **Authors:** Maribel Munoz, MD \n **Date:** November 16, 2016\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1036028,
"choiceText": "Ipsilateral weakness; loss of vibratory sense and of proprioception; contralateral loss of pain and of temperature sensation",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1036030,
"choiceText": "Ipsilateral loss of pain and of temperature sensation; contralateral weakness; loss of vibratory sense and of proprioception",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1036032,
"choiceText": "Ipsilateral weakness; contralateral loss of vibratory sense and of proprioception",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1036034,
"choiceText": "Ipsilateral loss of vibratory sense and of proprioception; contralateral weakness.",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "This syndrome involves a unilateral lesion of the spinal cord affecting the corticospinal tract, resulting in an ipsilateral spastic paralysis. Also affected are the posterior white column (dorsal column), with loss of proprioception and vibratory sensation ipsilaterally, and the spinothalamic tract, which leads to a contralateral loss of pain and temperature sensation (because this tract decussates within two spinal levels).",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 327895,
"questionText": "A patient presents to the ED with acute-onset neurologic deficits that raise concern for Brown-Sequard syndrome. Which of the following groups of findings would have caused you to suspect this condition?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1036046,
"choiceText": "CSF IgG",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1036048,
"choiceText": "CSF culture",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1036050,
"choiceText": "CSF PCR",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1036052,
"choiceText": "India ink staining",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Varicella-zoster virus PCR of the CSF is specific but not particularly sensitive in detecting reactivation of the virus in the CNS. In contrast, varicella-zoster virus IgG levels are more sensitive but less specific.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 327899,
"questionText": "The patient mentioned above is noted to have a resolving dermatomal rash consistent with herpes zoster. A lumbar puncture is performed to evaluate for CNS infection. What is the most specific test for detecting varicella-zoster virus infection in the CNS?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 50-Year-Old Patient With AIDS and Leg Weakness"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1036008,
"choiceText": "Anterior cord syndrome",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1036010,
"choiceText": "Brown-Sequard syndrome",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1036012,
"choiceText": "Posterior cord syndrome",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1036014,
"choiceText": "Myelopathy with bilateral cord involvement",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1036016,
"choiceText": "Central cord syndrome",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 327891,
"questionText": "<p>Which of the following is the most likely diagnosis?</p>\r\n\r\n<p><em>Hint: Consider the spinal tracts responsible for the patient's neurologic findings.</em></p>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1036028,
"choiceText": "Ipsilateral weakness; loss of vibratory sense and of proprioception; contralateral loss of pain and of temperature sensation",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1036030,
"choiceText": "Ipsilateral loss of pain and of temperature sensation; contralateral weakness; loss of vibratory sense and of proprioception",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1036032,
"choiceText": "Ipsilateral weakness; contralateral loss of vibratory sense and of proprioception",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1036034,
"choiceText": "Ipsilateral loss of vibratory sense and of proprioception; contralateral weakness.",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "This syndrome involves a unilateral lesion of the spinal cord affecting the corticospinal tract, resulting in an ipsilateral spastic paralysis. Also affected are the posterior white column (dorsal column), with loss of proprioception and vibratory sensation ipsilaterally, and the spinothalamic tract, which leads to a contralateral loss of pain and temperature sensation (because this tract decussates within two spinal levels).",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 327895,
"questionText": "A patient presents to the ED with acute-onset neurologic deficits that raise concern for Brown-Sequard syndrome. Which of the following groups of findings would have caused you to suspect this condition?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1036046,
"choiceText": "CSF IgG",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1036048,
"choiceText": "CSF culture",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1036050,
"choiceText": "CSF PCR",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1036052,
"choiceText": "India ink staining",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Varicella-zoster virus PCR of the CSF is specific but not particularly sensitive in detecting reactivation of the virus in the CNS. In contrast, varicella-zoster virus IgG levels are more sensitive but less specific.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 327899,
"questionText": "The patient mentioned above is noted to have a resolving dermatomal rash consistent with herpes zoster. A lumbar puncture is performed to evaluate for CNS infection. What is the most specific test for detecting varicella-zoster virus infection in the CNS?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
871732 | /viewarticle/871732 | [
{
"authors": "Yasmine S. Ali, MD",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"A 32-year-old white woman with a history of severe mitral stenosis presents to the emergency department with sudden onset of acute, severe respiratory distress. Other than her history of severe mitral stenosis, she has no other significant past medical history. She states that she noted the onset of palpitations prior to becoming acutely short of breath at rest."
],
"date": "November 14, 2016",
"figures": [],
"markdown": "# Respiratory Distress in a 32-Year-Old Woman\n\n **Authors:** Yasmine S. Ali, MD \n **Date:** November 14, 2016\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nA 32-year-old white woman with a history of severe mitral stenosis presents to the emergency department with sudden onset of acute, severe respiratory distress. Other than her history of severe mitral stenosis, she has no other significant past medical history. She states that she noted the onset of palpitations prior to becoming acutely short of breath at rest.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "Respiratory Distress in a 32-Year-Old Woman"
},
{
"authors": "Yasmine S. Ali, MD",
"content": [
"On physical examination, her vital signs are as follows:",
"Respiratory rate: 30 breaths/min",
"Heart rate: 140 beats/min",
"Temperature: 98.7° F",
"Oxygen saturation on room air: 88%",
"Blood pressure: 150/90 mm Hg",
"The patient appears slightly older than her stated age and is in acute respiratory distress. Pupils are equal, round, and reactive to light. Nares are patent. Trachea is midline.",
"On lung examination, the patient is noted to be tachypneic with labored breathing. Crackles are heard bilaterally across all lung fields. Dullness to percussion is observed at the lung bases bilaterally.",
"Figure 1.",
"Figure 2.",
"On cardiac examination, her heart rate is tachycardic and the rhythm is irregularly irregular. A II/VI holosystolic murmur is heard across the precordium, loudest at the apex. No gallop or rub is detected.",
"Abdominal examination findings are normal.",
"On extremity examination, there is no evidence to suggest clubbing, cyanosis, or edema.",
"A plain chest x-ray is ordered and shows pulmonary edema with a normal-sized heart (Figure 1).",
"A 12-lead ECG is also obtained (Figure 2)."
],
"date": "November 14, 2016",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/871/732/871732-Thumb1.png"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/871/732/871732-thumb2a.jpg"
}
],
"markdown": "# Respiratory Distress in a 32-Year-Old Woman\n\n **Authors:** Yasmine S. Ali, MD \n **Date:** November 14, 2016\n\n ## Content\n\n On physical examination, her vital signs are as follows:\nRespiratory rate: 30 breaths/min\nHeart rate: 140 beats/min\nTemperature: 98.7° F\nOxygen saturation on room air: 88%\nBlood pressure: 150/90 mm Hg\nThe patient appears slightly older than her stated age and is in acute respiratory distress. Pupils are equal, round, and reactive to light. Nares are patent. Trachea is midline.\nOn lung examination, the patient is noted to be tachypneic with labored breathing. Crackles are heard bilaterally across all lung fields. Dullness to percussion is observed at the lung bases bilaterally.\nFigure 1.\nFigure 2.\nOn cardiac examination, her heart rate is tachycardic and the rhythm is irregularly irregular. A II/VI holosystolic murmur is heard across the precordium, loudest at the apex. No gallop or rub is detected.\nAbdominal examination findings are normal.\nOn extremity examination, there is no evidence to suggest clubbing, cyanosis, or edema.\nA plain chest x-ray is ordered and shows pulmonary edema with a normal-sized heart (Figure 1).\nA 12-lead ECG is also obtained (Figure 2).\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1034528,
"choiceText": "Pulmonary hemorrhage",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1034530,
"choiceText": "Acute fungal pneumonia",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1034532,
"choiceText": "Severe left ventricular dysfunction ",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1034534,
"choiceText": "New-onset atrial fibrillation",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 327409,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Respiratory Distress in a 32-Year-Old Woman"
},
{
"authors": "Yasmine S. Ali, MD",
"content": [
"In a young patient with known severe, chronic mitral stenosis, it is not uncommon to find the patient to be asymptomatic until the onset of atrial fibrillation, which can be devastating, particularly in pregnant patients, and can rapidly cause severe respiratory distress.[1] This is due to the enlarged left atrium and pulmonary hypertension that so often accompanies chronic mitral stenosis, and the dependence on the atrial kick (lost in atrial fibrillation) to help move blood across the stenotic mitral valve.[2]",
"The diagnostic triad for mitral stenosis on chest radiography is pulmonary artery revascularization, a large left atrium, and a normal-sized left ventricle. The normal size of the left ventricle is key in distinguishing acute pulmonary edema due to mitral stenosis from pulmonary edema caused by left ventricular failure (systolic heart failure).",
"Recognizing that patients with severe mitral stenosis are at risk for decompensation with the onset of atrial fibrillation is important to rapid diagnosis and management. In any patient with an unstable arrhythmia, emergent cardioversion is recommended. An arrhythmia that causes severe acute respiratory distress certainly qualifies as an unstable arrhythmia for which emergent cardioversion could be life-saving. A return to sinus rhythm, in combination with a loop diuretic such as furosemide, often alleviates the respiratory compensation almost immediately.",
"Atrial fibrillation as a cause of respiratory decompensation often presents in pregnant patients with known severe mitral stenosis; electrical (direct-current) cardioversion, procainamide, and verapamil are all safe to use during pregnancy. Also keep in mind that stenotic valvular lesions in general are much more problematic during pregnancy than are regurgitant lesions, which tend to be better tolerated."
],
"date": "November 14, 2016",
"figures": [],
"markdown": "# Respiratory Distress in a 32-Year-Old Woman\n\n **Authors:** Yasmine S. Ali, MD \n **Date:** November 14, 2016\n\n ## Content\n\n In a young patient with known severe, chronic mitral stenosis, it is not uncommon to find the patient to be asymptomatic until the onset of atrial fibrillation, which can be devastating, particularly in pregnant patients, and can rapidly cause severe respiratory distress.[1] This is due to the enlarged left atrium and pulmonary hypertension that so often accompanies chronic mitral stenosis, and the dependence on the atrial kick (lost in atrial fibrillation) to help move blood across the stenotic mitral valve.[2]\nThe diagnostic triad for mitral stenosis on chest radiography is pulmonary artery revascularization, a large left atrium, and a normal-sized left ventricle. The normal size of the left ventricle is key in distinguishing acute pulmonary edema due to mitral stenosis from pulmonary edema caused by left ventricular failure (systolic heart failure).\nRecognizing that patients with severe mitral stenosis are at risk for decompensation with the onset of atrial fibrillation is important to rapid diagnosis and management. In any patient with an unstable arrhythmia, emergent cardioversion is recommended. An arrhythmia that causes severe acute respiratory distress certainly qualifies as an unstable arrhythmia for which emergent cardioversion could be life-saving. A return to sinus rhythm, in combination with a loop diuretic such as furosemide, often alleviates the respiratory compensation almost immediately.\nAtrial fibrillation as a cause of respiratory decompensation often presents in pregnant patients with known severe mitral stenosis; electrical (direct-current) cardioversion, procainamide, and verapamil are all safe to use during pregnancy. Also keep in mind that stenotic valvular lesions in general are much more problematic during pregnancy than are regurgitant lesions, which tend to be better tolerated.\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1034528,
"choiceText": "Pulmonary hemorrhage",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1034530,
"choiceText": "Acute fungal pneumonia",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1034532,
"choiceText": "Severe left ventricular dysfunction ",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1034534,
"choiceText": "New-onset atrial fibrillation",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 327409,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Respiratory Distress in a 32-Year-Old Woman"
},
{
"authors": "Yasmine S. Ali, MD",
"content": [
"Being able to recognize atrial fibrillation on a 12-lead ECG or on a rhythm strip is obviously of utmost importance. Atrial fibrillation is best recognized on a 12-lead ECG by an irregularly irregular rhythm characterized by an absence of P waves. The absence of P waves is essential to the diagnosis of atrial fibrillation and confirms the absence of any sinus node activity.",
"The ventricular response rate to atrial fibrillation, which corresponds to the heart rate and usually corresponds to the palpated pulse rate, depends on how rapidly fibrillatory signals from the atrium are conducted through the atrioventricular (AV) node.[1] In this patient, who was not taking any AV nodal blocking agents (such as a beta-blocker or calcium channel blocker) and who, at her relatively young age, could be expected to have a normally functioning AV node, the ventricular response rate is rapid; thus, the full diagnosis is \"new-onset atrial fibrillation with rapid ventricular response.\" On the examination, a holosystolic murmur was loudest at the apex. This is suggestive of mitral regurgitation. Patients with mitral stenosis can have some regurgitation; however, the primary murmur of mitral stenosis is a diatonic murmur (which may not be heard at a heart rate of 140 beats/min).",
"Treatment of atrial fibrillation differs depending on whether the atrial fibrillation is caused by a valvular lesion (known as valvular atrial fibrillation) or not (known as nonvalvular atrial fibrillation). This is particularly true when it comes to the use of the novel oral anticoagulants (NOACs).",
"First, to determine which patients should receive anticoagulation for atrial fibrillation, the CHA2DS2-VASc score must be determined. This score (which replaces the old CHADS2 score) helps to identify which patients are at risk for stroke due to atrial fibrillation and, therefore, which patients should receive anticoagulation to reduce their stroke risk.[3]",
"CHA2DS2-VASc stands for the following[4]:",
"Congestive heart failure - 1 point",
"Hypertension - 1 point",
"Age 75 years or older - 2 points",
"Diabetes - 1 point",
"Stroke or transient ischemic attack - 2 points",
"Vascular disease (including coronary, aortic, carotid, or other peripheral arterial disease) - 1 point",
"Age 65-74 years – 1 point",
"Sex category (female) – 1 point",
"Note that women are at greater risk for stroke, and thus 1 point is added for the sex category component of the score if the patient is female.",
"Patients with a CHA2DS2-VASc score of 0 can be managed with aspirin at a dose of 81-325 mg per day (81 mg being recommended due to lower bleeding risk). Patients with a CHA2DS2-VASc score of 2 or more require anticoagulation, and those with a score of 1 can be on aspirin or an oral anticoagulant; however, consensus is building among experts, due to clinical trial results, that a CHA2DS2-VASc score of 1 should trigger anticoagulation because using aspirin alone in this group has not been found to decrease stroke risk as much as using an oral anticoagulant such as warfarin.[2] In patients with mitral stenosis and chronic atrial fibrillation, anticoagulation with warfarin should be initiated regardless of the CHA2DS2-VASc score."
],
"date": "November 14, 2016",
"figures": [],
"markdown": "# Respiratory Distress in a 32-Year-Old Woman\n\n **Authors:** Yasmine S. Ali, MD \n **Date:** November 14, 2016\n\n ## Content\n\n Being able to recognize atrial fibrillation on a 12-lead ECG or on a rhythm strip is obviously of utmost importance. Atrial fibrillation is best recognized on a 12-lead ECG by an irregularly irregular rhythm characterized by an absence of P waves. The absence of P waves is essential to the diagnosis of atrial fibrillation and confirms the absence of any sinus node activity.\nThe ventricular response rate to atrial fibrillation, which corresponds to the heart rate and usually corresponds to the palpated pulse rate, depends on how rapidly fibrillatory signals from the atrium are conducted through the atrioventricular (AV) node.[1] In this patient, who was not taking any AV nodal blocking agents (such as a beta-blocker or calcium channel blocker) and who, at her relatively young age, could be expected to have a normally functioning AV node, the ventricular response rate is rapid; thus, the full diagnosis is \"new-onset atrial fibrillation with rapid ventricular response.\" On the examination, a holosystolic murmur was loudest at the apex. This is suggestive of mitral regurgitation. Patients with mitral stenosis can have some regurgitation; however, the primary murmur of mitral stenosis is a diatonic murmur (which may not be heard at a heart rate of 140 beats/min).\nTreatment of atrial fibrillation differs depending on whether the atrial fibrillation is caused by a valvular lesion (known as valvular atrial fibrillation) or not (known as nonvalvular atrial fibrillation). This is particularly true when it comes to the use of the novel oral anticoagulants (NOACs).\nFirst, to determine which patients should receive anticoagulation for atrial fibrillation, the CHA2DS2-VASc score must be determined. This score (which replaces the old CHADS2 score) helps to identify which patients are at risk for stroke due to atrial fibrillation and, therefore, which patients should receive anticoagulation to reduce their stroke risk.[3]\nCHA2DS2-VASc stands for the following[4]:\nCongestive heart failure - 1 point\nHypertension - 1 point\nAge 75 years or older - 2 points\nDiabetes - 1 point\nStroke or transient ischemic attack - 2 points\nVascular disease (including coronary, aortic, carotid, or other peripheral arterial disease) - 1 point\nAge 65-74 years – 1 point\nSex category (female) – 1 point\nNote that women are at greater risk for stroke, and thus 1 point is added for the sex category component of the score if the patient is female.\nPatients with a CHA2DS2-VASc score of 0 can be managed with aspirin at a dose of 81-325 mg per day (81 mg being recommended due to lower bleeding risk). Patients with a CHA2DS2-VASc score of 2 or more require anticoagulation, and those with a score of 1 can be on aspirin or an oral anticoagulant; however, consensus is building among experts, due to clinical trial results, that a CHA2DS2-VASc score of 1 should trigger anticoagulation because using aspirin alone in this group has not been found to decrease stroke risk as much as using an oral anticoagulant such as warfarin.[2] In patients with mitral stenosis and chronic atrial fibrillation, anticoagulation with warfarin should be initiated regardless of the CHA2DS2-VASc score.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "Respiratory Distress in a 32-Year-Old Woman"
},
{
"authors": "Yasmine S. Ali, MD",
"content": [
"The only oral anticoagulant that has been approved for use in patients with valvular atrial fibrillation (as in this case of mitral stenosis) is warfarin.",
"For nonvalvular atrial fibrillation, however, any of the following oral anticoagulants may be used: warfarin, dabigatran, rivaroxaban, apixaban, or edoxaban.",
"Management should also focus on controlling ventricular response rate because sustained tachycardia can result in tachycardia-induced cardiomyopathy. First-line agents that can be used to manage ventricular response rate commonly include beta-blockers (eg, metoprolol) and calcium-channel blockers (eg, diltiazem, verapamil). Due to its potential for toxicity and its multiple drug-drug interactions, digoxin, which used to be in common use, is now reserved as an agent to be used when others are contraindicated or are inadequate for rate control.",
"Other than valvular heart disease—particularly mitral valve lesions that can result in an enlarged left atrium—other common risk factors for the development of atrial fibrillation include left ventricular dysfunction, heart failure with preserved ejection fraction, heart failure with reduced ejection fraction, prior cardiac surgery, myocardial ischemia or infarction (current or prior), diabetes, hypertension, congenital heart disease, chronic lung disease, untreated atrial flutter, thyroid disease, obstructive sleep apnea, obesity, and age over 60 years. Also keep in mind that excessive use of alcohol or stimulants can result in atrial fibrillation, as can the presence of a serious systemic illness or infection (eg, sepsis, malignancy).",
"All patients who present with a first episode of atrial fibrillation should undergo workup with a thorough history and physical, 12-lead ECG, transthoracic echocardiogram to identify and characterize any structural heart disease, complete metabolic panel, thyroid function panel, and possibly a chest x-ray if other tests are unrevealing as to the underlying cause.",
"The patient in this case had known severe mitral stenosis, and a 12-lead ECG revealed atrial fibrillation with rapid ventricular response. Because she was unstable upon presentation, in acute respiratory distress, she underwent immediate electrical cardioversion. Her respiratory symptoms subsided within minutes of return to normal sinus rhythm. She was referred to cardiology for a transthoracic echocardiogram and possibly a transesophageal echocardiogram to reassess the severity of her mitral stenosis and to evaluate the need for procedural intervention (either percutaneous mitral commissurotomy, which is preferred, or surgical valve replacement)."
],
"date": "November 14, 2016",
"figures": [],
"markdown": "# Respiratory Distress in a 32-Year-Old Woman\n\n **Authors:** Yasmine S. Ali, MD \n **Date:** November 14, 2016\n\n ## Content\n\n The only oral anticoagulant that has been approved for use in patients with valvular atrial fibrillation (as in this case of mitral stenosis) is warfarin.\nFor nonvalvular atrial fibrillation, however, any of the following oral anticoagulants may be used: warfarin, dabigatran, rivaroxaban, apixaban, or edoxaban.\nManagement should also focus on controlling ventricular response rate because sustained tachycardia can result in tachycardia-induced cardiomyopathy. First-line agents that can be used to manage ventricular response rate commonly include beta-blockers (eg, metoprolol) and calcium-channel blockers (eg, diltiazem, verapamil). Due to its potential for toxicity and its multiple drug-drug interactions, digoxin, which used to be in common use, is now reserved as an agent to be used when others are contraindicated or are inadequate for rate control.\nOther than valvular heart disease—particularly mitral valve lesions that can result in an enlarged left atrium—other common risk factors for the development of atrial fibrillation include left ventricular dysfunction, heart failure with preserved ejection fraction, heart failure with reduced ejection fraction, prior cardiac surgery, myocardial ischemia or infarction (current or prior), diabetes, hypertension, congenital heart disease, chronic lung disease, untreated atrial flutter, thyroid disease, obstructive sleep apnea, obesity, and age over 60 years. Also keep in mind that excessive use of alcohol or stimulants can result in atrial fibrillation, as can the presence of a serious systemic illness or infection (eg, sepsis, malignancy).\nAll patients who present with a first episode of atrial fibrillation should undergo workup with a thorough history and physical, 12-lead ECG, transthoracic echocardiogram to identify and characterize any structural heart disease, complete metabolic panel, thyroid function panel, and possibly a chest x-ray if other tests are unrevealing as to the underlying cause.\nThe patient in this case had known severe mitral stenosis, and a 12-lead ECG revealed atrial fibrillation with rapid ventricular response. Because she was unstable upon presentation, in acute respiratory distress, she underwent immediate electrical cardioversion. Her respiratory symptoms subsided within minutes of return to normal sinus rhythm. She was referred to cardiology for a transthoracic echocardiogram and possibly a transesophageal echocardiogram to reassess the severity of her mitral stenosis and to evaluate the need for procedural intervention (either percutaneous mitral commissurotomy, which is preferred, or surgical valve replacement).\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1034536,
"choiceText": "0",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1034538,
"choiceText": "1",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1034540,
"choiceText": "2",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1034542,
"choiceText": "4",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1034544,
"choiceText": "5\r\n",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "At 82 years of age, this patient scores 2 points for age 75 years or older. She has 1 point for a history of hypertension, 1 point for congestive heart failure, and 1 point for being female. This results in a total CHA<sub>2</sub>DS2-VASc score of 5, which puts her in a high-risk category for stroke, and she thus merits oral anticoagulation as part of her management strategy.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 327411,
"questionText": "An 82-year-old woman with a history of hypertension and systolic congestive heart failure (heart failure with reduced ejection fraction) presents with atrial fibrillation. What is her CHA<sub>2</sub>DS2-VASc score?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1034556,
"choiceText": "Edoxaban",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1034558,
"choiceText": "Warfarin",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1034560,
"choiceText": "Dabigatran",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1034562,
"choiceText": "Rivaroxaban\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Recall that in a patient with atrial fibrillation and any sort of valve disease, including a prior valve replacement, warfarin is currently the only oral anticoagulant that is recommended and approved by the US Food and Drug Administration for this indication.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 327419,
"questionText": "If the aforementioned 82-year-old patient was also known to have a mechanical aortic valve, what oral anticoagulant would be recommended?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Respiratory Distress in a 32-Year-Old Woman"
},
{
"authors": "Yasmine S. Ali, MD",
"content": [],
"date": "November 14, 2016",
"figures": [],
"markdown": "# Respiratory Distress in a 32-Year-Old Woman\n\n **Authors:** Yasmine S. Ali, MD \n **Date:** November 14, 2016\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1034536,
"choiceText": "0",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1034538,
"choiceText": "1",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1034540,
"choiceText": "2",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1034542,
"choiceText": "4",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1034544,
"choiceText": "5\r\n",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "At 82 years of age, this patient scores 2 points for age 75 years or older. She has 1 point for a history of hypertension, 1 point for congestive heart failure, and 1 point for being female. This results in a total CHA<sub>2</sub>DS2-VASc score of 5, which puts her in a high-risk category for stroke, and she thus merits oral anticoagulation as part of her management strategy.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 327411,
"questionText": "An 82-year-old woman with a history of hypertension and systolic congestive heart failure (heart failure with reduced ejection fraction) presents with atrial fibrillation. What is her CHA<sub>2</sub>DS2-VASc score?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1034556,
"choiceText": "Edoxaban",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1034558,
"choiceText": "Warfarin",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1034560,
"choiceText": "Dabigatran",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1034562,
"choiceText": "Rivaroxaban\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Recall that in a patient with atrial fibrillation and any sort of valve disease, including a prior valve replacement, warfarin is currently the only oral anticoagulant that is recommended and approved by the US Food and Drug Administration for this indication.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 327419,
"questionText": "If the aforementioned 82-year-old patient was also known to have a mechanical aortic valve, what oral anticoagulant would be recommended?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Respiratory Distress in a 32-Year-Old Woman"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1034528,
"choiceText": "Pulmonary hemorrhage",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1034530,
"choiceText": "Acute fungal pneumonia",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1034532,
"choiceText": "Severe left ventricular dysfunction ",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1034534,
"choiceText": "New-onset atrial fibrillation",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 327409,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1034536,
"choiceText": "0",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1034538,
"choiceText": "1",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1034540,
"choiceText": "2",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1034542,
"choiceText": "4",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1034544,
"choiceText": "5\r\n",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "At 82 years of age, this patient scores 2 points for age 75 years or older. She has 1 point for a history of hypertension, 1 point for congestive heart failure, and 1 point for being female. This results in a total CHA<sub>2</sub>DS2-VASc score of 5, which puts her in a high-risk category for stroke, and she thus merits oral anticoagulation as part of her management strategy.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 327411,
"questionText": "An 82-year-old woman with a history of hypertension and systolic congestive heart failure (heart failure with reduced ejection fraction) presents with atrial fibrillation. What is her CHA<sub>2</sub>DS2-VASc score?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1034556,
"choiceText": "Edoxaban",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1034558,
"choiceText": "Warfarin",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1034560,
"choiceText": "Dabigatran",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1034562,
"choiceText": "Rivaroxaban\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Recall that in a patient with atrial fibrillation and any sort of valve disease, including a prior valve replacement, warfarin is currently the only oral anticoagulant that is recommended and approved by the US Food and Drug Administration for this indication.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 327419,
"questionText": "If the aforementioned 82-year-old patient was also known to have a mechanical aortic valve, what oral anticoagulant would be recommended?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
871257 | /viewarticle/871257 | [
{
"authors": "Ronald C Gentile, MD; Brooke Nesmith, MD",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"A 66-year-old woman presents with new-onset decreased vision in the right eye with distortion. She experienced similar symptoms in the left eye more than 6 months earlier, which progressed to a central scotoma, but she did not seek medical attention at that time. She reports no eye pain, diplopia, or other ocular symptoms.",
"Otherwise, her ocular history is unremarkable, but her medical history is positive for hypertension, controlled on atenolol (50 mg/d) and amlodipine (10 mg/d). Her social and family history is unremarkable and negative for anemia. A review of symptoms reveals occasional headaches with difficulty hearing on the left side. She recently required a cane to ambulate, but she attributes this to arthritis in the knees and hips. She reports the inability to wear hats, especially her prized Easter bonnets."
],
"date": "November 02, 2016",
"figures": [],
"markdown": "# A 66-Year-Old Woman With Central Vision Loss\n\n **Authors:** Ronald C Gentile, MD; Brooke Nesmith, MD \n **Date:** November 02, 2016\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nA 66-year-old woman presents with new-onset decreased vision in the right eye with distortion. She experienced similar symptoms in the left eye more than 6 months earlier, which progressed to a central scotoma, but she did not seek medical attention at that time. She reports no eye pain, diplopia, or other ocular symptoms.\nOtherwise, her ocular history is unremarkable, but her medical history is positive for hypertension, controlled on atenolol (50 mg/d) and amlodipine (10 mg/d). Her social and family history is unremarkable and negative for anemia. A review of symptoms reveals occasional headaches with difficulty hearing on the left side. She recently required a cane to ambulate, but she attributes this to arthritis in the knees and hips. She reports the inability to wear hats, especially her prized Easter bonnets.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 66-Year-Old Woman With Central Vision Loss"
},
{
"authors": "Ronald C Gentile, MD; Brooke Nesmith, MD",
"content": [
"Figure 1",
"Figure 2",
"On examination, her vision is 20/80 in the right eye and 20/400 in the left eye. Findings on pupil examination are normal, with no relative afferent pupillary defect. Findings on anterior segment slit-lamp examination are unremarkable, except she has mild nuclear sclerotic cataracts in both eyes.",
"Dilated funduscopic examination of her right eye reveals a pinkish-red elevation in the fovea, located under the retina, and surrounding subretinal hemorrhage (Figure 1). The fovea of the left eye has white subretinal fibrosis, with reactive pigmentation and accentuation of the overlying yellow luteal pigment centrally and subretinal hemorrhage inferiorly (Figure 2). The fundus in both eyes has a spotted orange appearance with pigmentary mottling. The optic discs in both eyes are pink, with peripapillary changes that have jagged gray lines radiating from them located deep to the retina.",
"Figure 3",
"Figure 4",
"Fluorescein angiography demonstrates increased hyperfluorescence, with leakage in the macula of the right eye (Figure 3), late staining of the subretinal fibrosis in the left eye (Figure 4), and blockage of fluorescence from the subretinal hemorrhages in both eyes. Spotted areas of increased and decreased fluorescence are observed outside and surrounding the fovea in both eyes. Hyperfluorescence accentuation of the peripapillary linear streaks is noted in both eyes.",
"Figure 5",
"Figure 6",
"Optical CT scanning of the macula in the right eye reveals elevation of the retinal pigment epithelium (RPE), with overlying and adjacent separation of the neurosensory retina and disruption of the photoreceptor layer (Figure 5). Optical CT of the macula in the left eye reveals elevation of the hyper-reflective subretinal fibrosis, total loss of the overlying photoreceptor layer, cystic changes, and edema of the overlying neurosensory retina (Figure 6)."
],
"date": "November 02, 2016",
"figures": [
{
"caption": "Figure 1",
"image_url": "https://img.medscapestatic.com/article/871/257/871257-Thumb-1.png"
},
{
"caption": "Figure 2",
"image_url": "https://img.medscapestatic.com/article/871/257/871257-Thumb-2.png"
},
{
"caption": "Figure 3",
"image_url": "https://img.medscapestatic.com/article/871/257/871257-Thumb-3.png"
},
{
"caption": "Figure 4",
"image_url": "https://img.medscapestatic.com/article/871/257/871257-Thumb-4.png"
},
{
"caption": "Figure 5",
"image_url": "https://img.medscapestatic.com/article/871/257/871257-Thumb-5.png"
},
{
"caption": "Figure 6",
"image_url": "https://img.medscapestatic.com/article/871/257/871257-Thumb-6.png"
}
],
"markdown": "# A 66-Year-Old Woman With Central Vision Loss\n\n **Authors:** Ronald C Gentile, MD; Brooke Nesmith, MD \n **Date:** November 02, 2016\n\n ## Content\n\n Figure 1\nFigure 2\nOn examination, her vision is 20/80 in the right eye and 20/400 in the left eye. Findings on pupil examination are normal, with no relative afferent pupillary defect. Findings on anterior segment slit-lamp examination are unremarkable, except she has mild nuclear sclerotic cataracts in both eyes.\nDilated funduscopic examination of her right eye reveals a pinkish-red elevation in the fovea, located under the retina, and surrounding subretinal hemorrhage (Figure 1). The fovea of the left eye has white subretinal fibrosis, with reactive pigmentation and accentuation of the overlying yellow luteal pigment centrally and subretinal hemorrhage inferiorly (Figure 2). The fundus in both eyes has a spotted orange appearance with pigmentary mottling. The optic discs in both eyes are pink, with peripapillary changes that have jagged gray lines radiating from them located deep to the retina.\nFigure 3\nFigure 4\nFluorescein angiography demonstrates increased hyperfluorescence, with leakage in the macula of the right eye (Figure 3), late staining of the subretinal fibrosis in the left eye (Figure 4), and blockage of fluorescence from the subretinal hemorrhages in both eyes. Spotted areas of increased and decreased fluorescence are observed outside and surrounding the fovea in both eyes. Hyperfluorescence accentuation of the peripapillary linear streaks is noted in both eyes.\nFigure 5\nFigure 6\nOptical CT scanning of the macula in the right eye reveals elevation of the retinal pigment epithelium (RPE), with overlying and adjacent separation of the neurosensory retina and disruption of the photoreceptor layer (Figure 5). Optical CT of the macula in the left eye reveals elevation of the hyper-reflective subretinal fibrosis, total loss of the overlying photoreceptor layer, cystic changes, and edema of the overlying neurosensory retina (Figure 6).\n\n ## Figures\n\n **Figure 1** \n \n\n**Figure 2** \n \n\n**Figure 3** \n \n\n**Figure 4** \n \n\n**Figure 5** \n \n\n**Figure 6** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1031254,
"choiceText": "Exudative age-related macular degeneration",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1031256,
"choiceText": "Idiopathic polypoidal choroidal vasculopathy",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1031258,
"choiceText": "Lacquer cracks with choroidal neovascularization secondary to pathologic myopia",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1031260,
"choiceText": "Choroidal neovascularization associated with angioid streaks",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 326371,
"questionText": "Which of the following diagnoses is most likely?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 66-Year-Old Woman With Central Vision Loss"
},
{
"authors": "Ronald C Gentile, MD; Brooke Nesmith, MD",
"content": [
"Choroidal neovascularization (CNV) is a sight-threatening complication of many retinal diseases that causes degeneration of the macula. CNV is the pathologic growth of new abnormal blood vessels that originate from the choroidal circulation. These new blood vessels grow through breaks in the Bruch membrane and enter the sub-RPE and/or subretinal space.[1]",
"The exact cause of CNV is not known, but it is believed to be the result of an imbalance in the homeostasis of the RPE–Bruch membrane–choriocapillaris complex that results from tissue expression of molecules that favor angiogenesis and the development of new blood vessels.[2] CNV contains blood vessels that grow abnormally and are prone to leak, bleed, and fibrose, which results in damage to the overlying neurosensory retina and photoreceptor layer, and is a cause of significant and permanent vision loss.",
"One primary stimulus for the development of CNV is believed to be hypoxia, which induces a complex angiogenic process that involves the interplay and interaction of different molecules.[3] The most well-known proangiogenic molecule at this time is vascular endothelial growth factor (VEGF), and anti-VEGF intravitreal injections are the current standard treatment for CNV.[3] Additional molecules have been implicated in the development of CNV, including insulin-like growth factor 1, fibroblast growth factor 2, pigment epithelium-derived factor, and endostatin.[4] Research is currently underway to develop CNV therapies that target these molecules.[4]",
"Many retinal diseases are complicated by CNV. To determine the underlying etiology of CNV, a good history must be obtained and the patient's complete ocular examination findings, as well as other systemic and physical features, must be considered.",
"The most common cause of an elderly patient presenting with CNV in the United States is exudative age-related macular degeneration (AMD), or wet AMD. Exudative AMD accounts for only 10% to 20% of all cases of AMD; the other 80% to 90% are nonexudative, or dry, AMD. However, exudative AMD is responsible for more severe vision loss.[5] In this patient, however, dilated fundus examination of both eyes did not reveal any of the hallmark features associated with AMD, such as drusen (yellow subretinal deposits) and RPE irregularities, such as hyperpigmentary or hypopigmentary changes.",
"Another form of macular degeneration, idiopathic polypoidal choroidal vasculopathy (IPCV), is more common in Asian and African American patients, such as the patient in this case. IPCV is associated with a characteristic choroidal lesion that consists of a network of choroidal vessels ending in an aneurysmal bulge.[6] This characteristic vascular lesion is best seen on indocyanine green angiography. Clinically, these appear as reddish-orange polyp-like lesions. However, the patient in this case did not present with any of these features."
],
"date": "November 02, 2016",
"figures": [],
"markdown": "# A 66-Year-Old Woman With Central Vision Loss\n\n **Authors:** Ronald C Gentile, MD; Brooke Nesmith, MD \n **Date:** November 02, 2016\n\n ## Content\n\n Choroidal neovascularization (CNV) is a sight-threatening complication of many retinal diseases that causes degeneration of the macula. CNV is the pathologic growth of new abnormal blood vessels that originate from the choroidal circulation. These new blood vessels grow through breaks in the Bruch membrane and enter the sub-RPE and/or subretinal space.[1]\nThe exact cause of CNV is not known, but it is believed to be the result of an imbalance in the homeostasis of the RPE–Bruch membrane–choriocapillaris complex that results from tissue expression of molecules that favor angiogenesis and the development of new blood vessels.[2] CNV contains blood vessels that grow abnormally and are prone to leak, bleed, and fibrose, which results in damage to the overlying neurosensory retina and photoreceptor layer, and is a cause of significant and permanent vision loss.\nOne primary stimulus for the development of CNV is believed to be hypoxia, which induces a complex angiogenic process that involves the interplay and interaction of different molecules.[3] The most well-known proangiogenic molecule at this time is vascular endothelial growth factor (VEGF), and anti-VEGF intravitreal injections are the current standard treatment for CNV.[3] Additional molecules have been implicated in the development of CNV, including insulin-like growth factor 1, fibroblast growth factor 2, pigment epithelium-derived factor, and endostatin.[4] Research is currently underway to develop CNV therapies that target these molecules.[4]\nMany retinal diseases are complicated by CNV. To determine the underlying etiology of CNV, a good history must be obtained and the patient's complete ocular examination findings, as well as other systemic and physical features, must be considered.\nThe most common cause of an elderly patient presenting with CNV in the United States is exudative age-related macular degeneration (AMD), or wet AMD. Exudative AMD accounts for only 10% to 20% of all cases of AMD; the other 80% to 90% are nonexudative, or dry, AMD. However, exudative AMD is responsible for more severe vision loss.[5] In this patient, however, dilated fundus examination of both eyes did not reveal any of the hallmark features associated with AMD, such as drusen (yellow subretinal deposits) and RPE irregularities, such as hyperpigmentary or hypopigmentary changes.\nAnother form of macular degeneration, idiopathic polypoidal choroidal vasculopathy (IPCV), is more common in Asian and African American patients, such as the patient in this case. IPCV is associated with a characteristic choroidal lesion that consists of a network of choroidal vessels ending in an aneurysmal bulge.[6] This characteristic vascular lesion is best seen on indocyanine green angiography. Clinically, these appear as reddish-orange polyp-like lesions. However, the patient in this case did not present with any of these features.\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1031254,
"choiceText": "Exudative age-related macular degeneration",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1031256,
"choiceText": "Idiopathic polypoidal choroidal vasculopathy",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1031258,
"choiceText": "Lacquer cracks with choroidal neovascularization secondary to pathologic myopia",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1031260,
"choiceText": "Choroidal neovascularization associated with angioid streaks",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 326371,
"questionText": "Which of the following diagnoses is most likely?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 66-Year-Old Woman With Central Vision Loss"
},
{
"authors": "Ronald C Gentile, MD; Brooke Nesmith, MD",
"content": [
"Other entities associated with CNV include pathologic myopia, traumatic choroidal rupture, and presumed ocular histoplasmosis syndrome (POHS). These entities require specific findings and can usually be diagnosed with clinical examination and history. CNV related to myopia is associated with axial myopia, or a longer axial globe. A healthy eye with no refractive error is considered emmetropic, and has an axial length of about 24 mm. Eyes with degenerative myopia that are prone to myopic CNV have a refractive error of more than –6 diopters and an axial length of more than 26 mm. CNV in degenerative myopia originates from cracks in the Bruch membrane, called lacquer cracks, which tend to be small and well-circumscribed.",
"Choroidal ruptures can also be complicated by CNV. The ruptures are caused by a traumatic break in the Bruch membrane and are crescent-shaped and concentric to the optic disc. In these cases, CNV can occur years after a trauma. POHS can also cause CNV. Although seen around the world, it is endemic in the United States and is found in states that encompass the Ohio and Mississippi river valleys. It is believed to be secondary to a previous infection with the yeast form of Histoplasma capsulatum. The triad of POHS involves the macular lesion with CNV, atrophic chorioretinal scars, and peripapillary atrophy. Eyes with POHS typically have an absence of vitritis, and patients do not become symptomatic until CNV complicates a previously punched out macular scar.,/p>",
"Angioid streaks are breaks in an abnormally thickened and calcified Bruch membrane.[5] They appear as reddish-brown to gray narrow lines that spread radially from the optic nerve head.[5] They are associated with various systemic disorders, including pseudoxanthoma elasticum, beta thalassemia, sickle cell disease, Ehlers-Danlos syndrome, and Paget disease.[5]",
"On physical examination, the patient in this case was noted to have enlargement of her skull, which was the reason for her headaches and her inability to wear fitted hats. It also explains her arthritis and hearing loss, which were related to bone growth and pagetic lesions in her legs and skull. After a complete workup and radiologic findings of both lytic and dense bone formation with elevated bone-specific alkaline phosphatase, the diagnosis of Paget disease was confirmed."
],
"date": "November 02, 2016",
"figures": [],
"markdown": "# A 66-Year-Old Woman With Central Vision Loss\n\n **Authors:** Ronald C Gentile, MD; Brooke Nesmith, MD \n **Date:** November 02, 2016\n\n ## Content\n\n Other entities associated with CNV include pathologic myopia, traumatic choroidal rupture, and presumed ocular histoplasmosis syndrome (POHS). These entities require specific findings and can usually be diagnosed with clinical examination and history. CNV related to myopia is associated with axial myopia, or a longer axial globe. A healthy eye with no refractive error is considered emmetropic, and has an axial length of about 24 mm. Eyes with degenerative myopia that are prone to myopic CNV have a refractive error of more than –6 diopters and an axial length of more than 26 mm. CNV in degenerative myopia originates from cracks in the Bruch membrane, called lacquer cracks, which tend to be small and well-circumscribed.\nChoroidal ruptures can also be complicated by CNV. The ruptures are caused by a traumatic break in the Bruch membrane and are crescent-shaped and concentric to the optic disc. In these cases, CNV can occur years after a trauma. POHS can also cause CNV. Although seen around the world, it is endemic in the United States and is found in states that encompass the Ohio and Mississippi river valleys. It is believed to be secondary to a previous infection with the yeast form of Histoplasma capsulatum. The triad of POHS involves the macular lesion with CNV, atrophic chorioretinal scars, and peripapillary atrophy. Eyes with POHS typically have an absence of vitritis, and patients do not become symptomatic until CNV complicates a previously punched out macular scar.,/p>\nAngioid streaks are breaks in an abnormally thickened and calcified Bruch membrane.[5] They appear as reddish-brown to gray narrow lines that spread radially from the optic nerve head.[5] They are associated with various systemic disorders, including pseudoxanthoma elasticum, beta thalassemia, sickle cell disease, Ehlers-Danlos syndrome, and Paget disease.[5]\nOn physical examination, the patient in this case was noted to have enlargement of her skull, which was the reason for her headaches and her inability to wear fitted hats. It also explains her arthritis and hearing loss, which were related to bone growth and pagetic lesions in her legs and skull. After a complete workup and radiologic findings of both lytic and dense bone formation with elevated bone-specific alkaline phosphatase, the diagnosis of Paget disease was confirmed.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 66-Year-Old Woman With Central Vision Loss"
},
{
"authors": "Ronald C Gentile, MD; Brooke Nesmith, MD",
"content": [
"Paget disease of the bone, formerly known as osteitis deformans, is characterized by an accelerated rate of bone remodeling. The resultant overgrowth and impaired integrity of bone can lead to pain related to osteoarthritis or nerve impingement and fractures.[6] Complications associated with Paget disease include deafness, cranial nerve compression syndromes, and spinal stenosis.[7] Osteosarcoma is a rare complication.[7]",
"Angioid streaks do not have an adverse effect on visual function, as long as the overlying retinal layers remain intact. Thus, initially, patients should be closely watched. However, CNV that complicates angioid streaks can develop, leading to retinal hemorrhage and edema, and sometimes fibrotic retinal scaring and permanent vision loss.",
"Treatment for CNV secondary to angioid streaks is the same as current treatment for CNV secondary to exudative AMD. This initially consisted of laser photocoagulation or photodynamic therapy, but the current standard for CNV in exudative AMD, as well as for CNV secondary to angioid streaks, is anti-VEGF therapy, which has been shown to stabilize or improve visual acuity.[7] The purpose of treatment is to limit functional loss; these agents do not cure this chronically active disease.",
"The patient in this case was treated with monthly intravitreal anti-VEGF injections in both eyes, and resolution of retinal fluid and hemorrhage in both eyes was achieved. Vision in the right eye improved to 20/50, and in the left eye remained stable at 20/400."
],
"date": "November 02, 2016",
"figures": [],
"markdown": "# A 66-Year-Old Woman With Central Vision Loss\n\n **Authors:** Ronald C Gentile, MD; Brooke Nesmith, MD \n **Date:** November 02, 2016\n\n ## Content\n\n Paget disease of the bone, formerly known as osteitis deformans, is characterized by an accelerated rate of bone remodeling. The resultant overgrowth and impaired integrity of bone can lead to pain related to osteoarthritis or nerve impingement and fractures.[6] Complications associated with Paget disease include deafness, cranial nerve compression syndromes, and spinal stenosis.[7] Osteosarcoma is a rare complication.[7]\nAngioid streaks do not have an adverse effect on visual function, as long as the overlying retinal layers remain intact. Thus, initially, patients should be closely watched. However, CNV that complicates angioid streaks can develop, leading to retinal hemorrhage and edema, and sometimes fibrotic retinal scaring and permanent vision loss.\nTreatment for CNV secondary to angioid streaks is the same as current treatment for CNV secondary to exudative AMD. This initially consisted of laser photocoagulation or photodynamic therapy, but the current standard for CNV in exudative AMD, as well as for CNV secondary to angioid streaks, is anti-VEGF therapy, which has been shown to stabilize or improve visual acuity.[7] The purpose of treatment is to limit functional loss; these agents do not cure this chronically active disease.\nThe patient in this case was treated with monthly intravitreal anti-VEGF injections in both eyes, and resolution of retinal fluid and hemorrhage in both eyes was achieved. Vision in the right eye improved to 20/50, and in the left eye remained stable at 20/400.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1031262,
"choiceText": "Angioid streaks",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1031264,
"choiceText": "Cranial nerve compression syndromes",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1031266,
"choiceText": "Osteosarcoma",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1031268,
"choiceText": "Bone overgrowth ",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1031320,
"choiceText": "Deafness",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Complications associated with Paget disease include deafness, cranial nerve compression syndromes, and spinal stenosis. Osteosarcoma is a rare complication of Paget disease.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 326373,
"questionText": "Which of the following is a rare complication of Paget disease?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1031270,
"choiceText": "Insulin-like growth factor 1",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1031272,
"choiceText": "Fibroblast-growth factor 2",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1031274,
"choiceText": "Pigment epithelium-derived factor",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1031276,
"choiceText": "VEGF",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1031278,
"choiceText": "Endostatin",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The most well-known proangiogenic molecule at this time is VEGF, and anti-VEGF intravitreal injections are the current standard treatment for CNV.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 326375,
"questionText": "Which of the following is the most well-known proangiogenic molecule involved in the development of CVN?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 66-Year-Old Woman With Central Vision Loss"
},
{
"authors": "Ronald C Gentile, MD; Brooke Nesmith, MD",
"content": [],
"date": "November 02, 2016",
"figures": [],
"markdown": "# A 66-Year-Old Woman With Central Vision Loss\n\n **Authors:** Ronald C Gentile, MD; Brooke Nesmith, MD \n **Date:** November 02, 2016\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1031262,
"choiceText": "Angioid streaks",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1031264,
"choiceText": "Cranial nerve compression syndromes",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1031266,
"choiceText": "Osteosarcoma",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1031268,
"choiceText": "Bone overgrowth ",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1031320,
"choiceText": "Deafness",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Complications associated with Paget disease include deafness, cranial nerve compression syndromes, and spinal stenosis. Osteosarcoma is a rare complication of Paget disease.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 326373,
"questionText": "Which of the following is a rare complication of Paget disease?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1031270,
"choiceText": "Insulin-like growth factor 1",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1031272,
"choiceText": "Fibroblast-growth factor 2",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1031274,
"choiceText": "Pigment epithelium-derived factor",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1031276,
"choiceText": "VEGF",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1031278,
"choiceText": "Endostatin",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The most well-known proangiogenic molecule at this time is VEGF, and anti-VEGF intravitreal injections are the current standard treatment for CNV.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 326375,
"questionText": "Which of the following is the most well-known proangiogenic molecule involved in the development of CVN?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 66-Year-Old Woman With Central Vision Loss"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1031254,
"choiceText": "Exudative age-related macular degeneration",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1031256,
"choiceText": "Idiopathic polypoidal choroidal vasculopathy",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1031258,
"choiceText": "Lacquer cracks with choroidal neovascularization secondary to pathologic myopia",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1031260,
"choiceText": "Choroidal neovascularization associated with angioid streaks",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 326371,
"questionText": "Which of the following diagnoses is most likely?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1031262,
"choiceText": "Angioid streaks",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1031264,
"choiceText": "Cranial nerve compression syndromes",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1031266,
"choiceText": "Osteosarcoma",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1031268,
"choiceText": "Bone overgrowth ",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1031320,
"choiceText": "Deafness",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Complications associated with Paget disease include deafness, cranial nerve compression syndromes, and spinal stenosis. Osteosarcoma is a rare complication of Paget disease.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 326373,
"questionText": "Which of the following is a rare complication of Paget disease?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1031270,
"choiceText": "Insulin-like growth factor 1",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1031272,
"choiceText": "Fibroblast-growth factor 2",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1031274,
"choiceText": "Pigment epithelium-derived factor",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1031276,
"choiceText": "VEGF",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1031278,
"choiceText": "Endostatin",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The most well-known proangiogenic molecule at this time is VEGF, and anti-VEGF intravitreal injections are the current standard treatment for CNV.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 326375,
"questionText": "Which of the following is the most well-known proangiogenic molecule involved in the development of CVN?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
869986 | /viewarticle/869986 | [
{
"authors": "Pramod Gupta, MD; Jitendra Gohil, MD",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"A 60-year-old man presents to the emergency department with a 1-day history of crampy, moderately intense, left-sided abdominal pain. The pain is constant and is exacerbated by movement; it is relieved by lying still. The patient has not experienced anorexia and has not eaten since the evening before.",
"He has had several loose brown stools but denies any nausea or vomiting. The stool in his bowel movements is not blood-streaked and does not appear tarry. He denies any recent travel or camping and has not eaten any uncooked or undercooked foods. He reports feeling febrile, sweaty, and generally fatigued. No urinary symptoms, such as dysuria or increased frequency, are reported. He has not had any recent contact with sick people.",
"He denies having had similar episodes in the past. His medical and surgical histories are unremarkable, although he did have a screening barium enema examination 3 years ago. He is a nonsmoker and denies any heavy or regular alcohol consumption. He does not take any prescription or over-the-counter medications."
],
"date": "October 12, 2016",
"figures": [],
"markdown": "# A 60-Year-Old Man With Intense Left-Sided Abdominal Pain\n\n **Authors:** Pramod Gupta, MD; Jitendra Gohil, MD \n **Date:** October 12, 2016\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nA 60-year-old man presents to the emergency department with a 1-day history of crampy, moderately intense, left-sided abdominal pain. The pain is constant and is exacerbated by movement; it is relieved by lying still. The patient has not experienced anorexia and has not eaten since the evening before.\nHe has had several loose brown stools but denies any nausea or vomiting. The stool in his bowel movements is not blood-streaked and does not appear tarry. He denies any recent travel or camping and has not eaten any uncooked or undercooked foods. He reports feeling febrile, sweaty, and generally fatigued. No urinary symptoms, such as dysuria or increased frequency, are reported. He has not had any recent contact with sick people.\nHe denies having had similar episodes in the past. His medical and surgical histories are unremarkable, although he did have a screening barium enema examination 3 years ago. He is a nonsmoker and denies any heavy or regular alcohol consumption. He does not take any prescription or over-the-counter medications.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 60-Year-Old Man With Intense Left-Sided Abdominal Pain"
},
{
"authors": "Pramod Gupta, MD; Jitendra Gohil, MD",
"content": [
"Figure 1",
"Figure 2",
"Figure 3",
"On physical examination, the patient has an elevated temperature of 101.3°F, blood pressure of 130/76 mm Hg, pulse of 110 beats/min, and respiratory rate of 20 breaths/min. The patient is not in acute distress, but he is mildly ill-appearing and diaphoretic. His oropharynx is clear, with slightly dry mucous membranes. His lungs are clear to auscultation, and his heart rate is regular, without murmurs.",
"The abdominal examination reveals moderate tenderness in the left lower quadrant, with voluntary guarding. No rebound tenderness is noted. No costovertebral angle tenderness or inguinal hernias are appreciated, and his genital examination findings are normal. Upon digital rectal examination, the patient is tender on the left side of the rectal vault, and the stool is noted to guaiac-negative. The remainder of the physical examination findings are unremarkable.",
"Serum laboratory testing is remarkable only for an elevated white blood cell count of 16 × 103 cells/µL, with a neutrophil predominance; the urinalysis is unremarkable. A standard radiograph of the abdomen is obtained, which does not show any significant abnormalities (Figure 1). The patient then underwent CT of the abdomen and pelvis (Figures 2 and 3)."
],
"date": "October 12, 2016",
"figures": [
{
"caption": "Figure 1",
"image_url": "https://img.medscapestatic.com/article/869/986/869986-Thumb1.png"
},
{
"caption": "Figure 2",
"image_url": "https://img.medscapestatic.com/article/869/986/869986-Thumb2.png"
},
{
"caption": "Figure 3",
"image_url": "https://img.medscapestatic.com/article/869/986/869986-Thumb3.png"
}
],
"markdown": "# A 60-Year-Old Man With Intense Left-Sided Abdominal Pain\n\n **Authors:** Pramod Gupta, MD; Jitendra Gohil, MD \n **Date:** October 12, 2016\n\n ## Content\n\n Figure 1\nFigure 2\nFigure 3\nOn physical examination, the patient has an elevated temperature of 101.3°F, blood pressure of 130/76 mm Hg, pulse of 110 beats/min, and respiratory rate of 20 breaths/min. The patient is not in acute distress, but he is mildly ill-appearing and diaphoretic. His oropharynx is clear, with slightly dry mucous membranes. His lungs are clear to auscultation, and his heart rate is regular, without murmurs.\nThe abdominal examination reveals moderate tenderness in the left lower quadrant, with voluntary guarding. No rebound tenderness is noted. No costovertebral angle tenderness or inguinal hernias are appreciated, and his genital examination findings are normal. Upon digital rectal examination, the patient is tender on the left side of the rectal vault, and the stool is noted to guaiac-negative. The remainder of the physical examination findings are unremarkable.\nSerum laboratory testing is remarkable only for an elevated white blood cell count of 16 × 103 cells/µL, with a neutrophil predominance; the urinalysis is unremarkable. A standard radiograph of the abdomen is obtained, which does not show any significant abnormalities (Figure 1). The patient then underwent CT of the abdomen and pelvis (Figures 2 and 3).\n\n ## Figures\n\n **Figure 1** \n \n\n**Figure 2** \n \n\n**Figure 3** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1023756,
"choiceText": "Acute diverticulitis",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1023758,
"choiceText": "Colon cancer",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1023760,
"choiceText": "Acute bacterial peritonitis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1023762,
"choiceText": "Acute appendicitis",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 323917,
"questionText": "What is the diagnosis?<br/><br/>\r\n<em>Hint: This is the most common acute condition related to the sigmoid colon.</em>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 60-Year-Old Man With Intense Left-Sided Abdominal Pain"
},
{
"authors": "Pramod Gupta, MD; Jitendra Gohil, MD",
"content": [
"Acute diverticulitis results from inflammation of a diverticulum (small mucosal and submucosal herniations through the circular muscle layer of the colonic wall) secondary to fecal obstruction. The obstruction typically occurs at the neck of the diverticulum; solidified stool, which typically forms a fecalith, abrades the mucosa within or at the neck of the diverticulum.",
"In uncomplicated cases (typically characterized by a well-appearing patient without peritonitis and systemic signs/symptoms), the inflammatory process is confined to the colonic wall; however, the obstruction, with subsequent high intraluminal pressure within the diverticula, can lead to a microperforation that in turn allows translocation of bacteria through the colonic wall, formation of a pericolic abscess, and diffuse peritonitis.",
"Only 2%-4% of patients diagnosed with diverticulitis are younger than 40 years; the condition is predominantly found in elderly populations.[1,2]",
"Diverticulosis is an intestinal disorder that is characterized by the presence of many diverticula; it occurs equally in men and women, with a higher prevalence in cultures with a low-fiber diet (which is believed to decrease stool transit time, thereby causing increased intraluminal pressure and resulting in mucosal herniations). The colonic diverticula themselves are most commonly found in the sigmoid and descending colon, although less commonly, patients (particularly those of Asian descent) develop diverticula of the right colon. Approximately one third of the population has diverticulosis by age 50 years, and about two thirds have it by age 85 years. Approximately 10%-25% of patients with known diverticulosis go on to develop diverticulitis.[1,2]",
"The classic presentation of diverticulitis is steady, deep abdominal pain that is often initially diffuse and vague, but later localizes in the left lower quadrant of the abdomen. Abdominal bloating; stool changes, such as diarrhea or constipation; and flatulence frequently accompany acute diverticulitis. Fever, fatigue, and anorexia are also common symptoms. Colonic inflammation may irritate the bladder or the ureters, leading to urinary frequency and dysuria.",
"Physical examination may reveal fever; localized, left lower quadrant abdominal tenderness; mild abdominal distention; and, at times, a left lower quadrant mass. The palpated mass is likely to be inflamed loops of bowel or, possibly, an abscess. Digital rectal examination may demonstrate left-sided tenderness and occult blood in the stool.[1,2]"
],
"date": "October 12, 2016",
"figures": [],
"markdown": "# A 60-Year-Old Man With Intense Left-Sided Abdominal Pain\n\n **Authors:** Pramod Gupta, MD; Jitendra Gohil, MD \n **Date:** October 12, 2016\n\n ## Content\n\n Acute diverticulitis results from inflammation of a diverticulum (small mucosal and submucosal herniations through the circular muscle layer of the colonic wall) secondary to fecal obstruction. The obstruction typically occurs at the neck of the diverticulum; solidified stool, which typically forms a fecalith, abrades the mucosa within or at the neck of the diverticulum.\nIn uncomplicated cases (typically characterized by a well-appearing patient without peritonitis and systemic signs/symptoms), the inflammatory process is confined to the colonic wall; however, the obstruction, with subsequent high intraluminal pressure within the diverticula, can lead to a microperforation that in turn allows translocation of bacteria through the colonic wall, formation of a pericolic abscess, and diffuse peritonitis.\nOnly 2%-4% of patients diagnosed with diverticulitis are younger than 40 years; the condition is predominantly found in elderly populations.[1,2]\nDiverticulosis is an intestinal disorder that is characterized by the presence of many diverticula; it occurs equally in men and women, with a higher prevalence in cultures with a low-fiber diet (which is believed to decrease stool transit time, thereby causing increased intraluminal pressure and resulting in mucosal herniations). The colonic diverticula themselves are most commonly found in the sigmoid and descending colon, although less commonly, patients (particularly those of Asian descent) develop diverticula of the right colon. Approximately one third of the population has diverticulosis by age 50 years, and about two thirds have it by age 85 years. Approximately 10%-25% of patients with known diverticulosis go on to develop diverticulitis.[1,2]\nThe classic presentation of diverticulitis is steady, deep abdominal pain that is often initially diffuse and vague, but later localizes in the left lower quadrant of the abdomen. Abdominal bloating; stool changes, such as diarrhea or constipation; and flatulence frequently accompany acute diverticulitis. Fever, fatigue, and anorexia are also common symptoms. Colonic inflammation may irritate the bladder or the ureters, leading to urinary frequency and dysuria.\nPhysical examination may reveal fever; localized, left lower quadrant abdominal tenderness; mild abdominal distention; and, at times, a left lower quadrant mass. The palpated mass is likely to be inflamed loops of bowel or, possibly, an abscess. Digital rectal examination may demonstrate left-sided tenderness and occult blood in the stool.[1,2]\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1023756,
"choiceText": "Acute diverticulitis",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1023758,
"choiceText": "Colon cancer",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1023760,
"choiceText": "Acute bacterial peritonitis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1023762,
"choiceText": "Acute appendicitis",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 323917,
"questionText": "What is the diagnosis?<br/><br/>\r\n<em>Hint: This is the most common acute condition related to the sigmoid colon.</em>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 60-Year-Old Man With Intense Left-Sided Abdominal Pain"
},
{
"authors": "Pramod Gupta, MD; Jitendra Gohil, MD",
"content": [
"The differential diagnosis of acute sigmoid diverticulitis is broad and includes inflammatory bowel disease; irritable bowel syndrome; appendicitis; ischemic colitis; colon cancer; urolithiasis; urinary tract infection; and in women, numerous obstetric/gynecologic conditions, such as tubo-ovarian abscesses and ovarian cysts.",
"The complications of acute diverticulitis include formation of a pericolic abscess, frank colonic perforation leading to free intra-abdominal air, local adhesions, purulent or fecal peritonitis, sepsis, bowel obstruction, and fistula formation between the colon and the bladder or vagina. Fistula formation is more common in the setting of recurrent diverticulitis, with the most common type being a colovesicular fistula that is characterized by fecaluria, pneumaturia, or typical urinary tract infection symptoms.[1,2]",
"The initial evaluation of a patient with suspected acute diverticulitis generally includes physical examination; complete blood cell count; urinalysis; and, when indicated by the presence of peritonitis, plain radiography of the abdomen to rule out colonic perforation. Plain films are of limited value; however, they may show colonic obstruction, mild ileus, or bowel distention. Leukocytosis is found in only 36% of cases of acute diverticulitis.",
"The preferred imaging modality for diagnosis of acute diverticulitis is CT, because it both determines the extent of the disease and detects complications. Abdominal ultrasonography can also be used, but it lacks specificity and is operator-dependent. Barium contrast studies and colonoscopy/sigmoidoscopy should be avoided in the setting of acute diverticulitis because of the risk for bowel perforation; however, these examinations are often performed after resolution of the acute stage to evaluate for the presence of complications, such as fistula formation or other colonic abnormalities.[1,2]"
],
"date": "October 12, 2016",
"figures": [],
"markdown": "# A 60-Year-Old Man With Intense Left-Sided Abdominal Pain\n\n **Authors:** Pramod Gupta, MD; Jitendra Gohil, MD \n **Date:** October 12, 2016\n\n ## Content\n\n The differential diagnosis of acute sigmoid diverticulitis is broad and includes inflammatory bowel disease; irritable bowel syndrome; appendicitis; ischemic colitis; colon cancer; urolithiasis; urinary tract infection; and in women, numerous obstetric/gynecologic conditions, such as tubo-ovarian abscesses and ovarian cysts.\nThe complications of acute diverticulitis include formation of a pericolic abscess, frank colonic perforation leading to free intra-abdominal air, local adhesions, purulent or fecal peritonitis, sepsis, bowel obstruction, and fistula formation between the colon and the bladder or vagina. Fistula formation is more common in the setting of recurrent diverticulitis, with the most common type being a colovesicular fistula that is characterized by fecaluria, pneumaturia, or typical urinary tract infection symptoms.[1,2]\nThe initial evaluation of a patient with suspected acute diverticulitis generally includes physical examination; complete blood cell count; urinalysis; and, when indicated by the presence of peritonitis, plain radiography of the abdomen to rule out colonic perforation. Plain films are of limited value; however, they may show colonic obstruction, mild ileus, or bowel distention. Leukocytosis is found in only 36% of cases of acute diverticulitis.\nThe preferred imaging modality for diagnosis of acute diverticulitis is CT, because it both determines the extent of the disease and detects complications. Abdominal ultrasonography can also be used, but it lacks specificity and is operator-dependent. Barium contrast studies and colonoscopy/sigmoidoscopy should be avoided in the setting of acute diverticulitis because of the risk for bowel perforation; however, these examinations are often performed after resolution of the acute stage to evaluate for the presence of complications, such as fistula formation or other colonic abnormalities.[1,2]\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 60-Year-Old Man With Intense Left-Sided Abdominal Pain"
},
{
"authors": "Pramod Gupta, MD; Jitendra Gohil, MD",
"content": [
"The management of patients with acute diverticulitis depends on the severity of the illness, but medical management alone is commonly successful. Well-appearing patients who are able to tolerate oral intake and do not have systemic symptoms, peritonitis, or complications seen on CT may be treated as outpatients.",
"Figure 2",
"Figure 3",
"Some nontoxic-appearing patients with a history of diverticulitis who present with their typical symptoms may be treated empirically as outpatients, without repeat imaging, if no significant comorbidities (eg, an immunocompromised state, diabetes, or cancer) are noted. All patients treated at home require close follow-up care and reexamination, and they should be given detailed return precautions for worsening pain or systemic illness.",
"Treatment of uncomplicated acute diverticulitis consists of bowel rest, broad-spectrum antibiotics, and pain control. Outpatients may be instructed to begin with a clear liquid diet and advance slowly as tolerated, whereas inpatients should be kept hydrated with intravenous fluids. Antibiotic regimens should cover gram-negative bacteria and anaerobes. A combination of either trimethoprim/sulfamethoxazole or ciprofloxacin, with either metronidazole or clindamycin, is the primary recommended treatment regimen. Monotherapy with amoxicillin/clavulanic acid is an acceptable alternative regimen.[1,2]",
"Figure 4",
"Figure 5",
"Figure 6",
"Patients should be admitted to the hospital if they cannot tolerate oral intake of fluids, are immunocompromised, demonstrate signs of systemic toxicity (such as tachycardia and fever), or have developed evidence of peritonitis or intra-abdominal complications. These patients should receive nothing by mouth and be given intravenous antibiotics. Ciprofloxacin or an aminoglycoside may be paired with metronidazole or clindamycin as the recommended antibiotic regimen. A monotherapeutic agent, such as piperacillin/tazobactam, ampicillin/sulbactam, or ertapenem, may also be used.[1]",
"Selected abscesses detected by ultrasonography or abdominal CT may be drained percutaneously, whereas perforations, fecal peritonitis, and fistula formation all require surgical consultation. Abscesses less than 5 cm in diameter can be treated with antibiotics alone, although evaluation by a surgeon should still be sought. Recurrent diverticulitis and complicated diverticulitis are indications for partial colonic resection.",
"Approximately 10%-25% of patients who are medically managed have recurrent attacks and are at an increased risk for subsequent complications. Of note, patients younger than 40 years are more likely to have recurrences and are more likely to benefit from elective sigmoid resection.[1,2]",
"In this case, the axial CT images of the abdomen at the level of the pelvis (Figures 2 and 3) show acute diverticulitis of the sigmoid colon. Multiple diverticula (arrowheads) and wall thickening are noted (Figure 4), and inflammatory stranding is seen in the sigmoid mesentery (Figure 5). No free air or abscess formation is evident. The screening barium enema performed 3 years ago (Figure 6) shows multiple diverticula in the sigmoid and descending colon (arrowheads).",
"Because of systemic signs and symptoms of infection, this patient was admitted to the hospital. He was placed on bowel rest and started on intravenous metronidazole and ciprofloxacin. Over the next 2 days, the patient defervesced and his leukocytosis resolved. His diet was advanced to a full diet, and he was discharged from the hospital on a 10-day course of amoxicillin/clavulanic acid."
],
"date": "October 12, 2016",
"figures": [
{
"caption": "Figure 2",
"image_url": "https://img.medscapestatic.com/article/869/986/869986-Thumb2.png"
},
{
"caption": "Figure 3",
"image_url": "https://img.medscapestatic.com/article/869/986/869986-Thumb3.png"
},
{
"caption": "Figure 4",
"image_url": "https://img.medscapestatic.com/article/869/986/869986-Thumb4.png"
},
{
"caption": "Figure 5",
"image_url": "https://img.medscapestatic.com/article/869/986/869986-Thumb5.png"
},
{
"caption": "Figure 6",
"image_url": "https://img.medscapestatic.com/article/869/986/869986-Thumb6.png"
}
],
"markdown": "# A 60-Year-Old Man With Intense Left-Sided Abdominal Pain\n\n **Authors:** Pramod Gupta, MD; Jitendra Gohil, MD \n **Date:** October 12, 2016\n\n ## Content\n\n The management of patients with acute diverticulitis depends on the severity of the illness, but medical management alone is commonly successful. Well-appearing patients who are able to tolerate oral intake and do not have systemic symptoms, peritonitis, or complications seen on CT may be treated as outpatients.\nFigure 2\nFigure 3\nSome nontoxic-appearing patients with a history of diverticulitis who present with their typical symptoms may be treated empirically as outpatients, without repeat imaging, if no significant comorbidities (eg, an immunocompromised state, diabetes, or cancer) are noted. All patients treated at home require close follow-up care and reexamination, and they should be given detailed return precautions for worsening pain or systemic illness.\nTreatment of uncomplicated acute diverticulitis consists of bowel rest, broad-spectrum antibiotics, and pain control. Outpatients may be instructed to begin with a clear liquid diet and advance slowly as tolerated, whereas inpatients should be kept hydrated with intravenous fluids. Antibiotic regimens should cover gram-negative bacteria and anaerobes. A combination of either trimethoprim/sulfamethoxazole or ciprofloxacin, with either metronidazole or clindamycin, is the primary recommended treatment regimen. Monotherapy with amoxicillin/clavulanic acid is an acceptable alternative regimen.[1,2]\nFigure 4\nFigure 5\nFigure 6\nPatients should be admitted to the hospital if they cannot tolerate oral intake of fluids, are immunocompromised, demonstrate signs of systemic toxicity (such as tachycardia and fever), or have developed evidence of peritonitis or intra-abdominal complications. These patients should receive nothing by mouth and be given intravenous antibiotics. Ciprofloxacin or an aminoglycoside may be paired with metronidazole or clindamycin as the recommended antibiotic regimen. A monotherapeutic agent, such as piperacillin/tazobactam, ampicillin/sulbactam, or ertapenem, may also be used.[1]\nSelected abscesses detected by ultrasonography or abdominal CT may be drained percutaneously, whereas perforations, fecal peritonitis, and fistula formation all require surgical consultation. Abscesses less than 5 cm in diameter can be treated with antibiotics alone, although evaluation by a surgeon should still be sought. Recurrent diverticulitis and complicated diverticulitis are indications for partial colonic resection.\nApproximately 10%-25% of patients who are medically managed have recurrent attacks and are at an increased risk for subsequent complications. Of note, patients younger than 40 years are more likely to have recurrences and are more likely to benefit from elective sigmoid resection.[1,2]\nIn this case, the axial CT images of the abdomen at the level of the pelvis (Figures 2 and 3) show acute diverticulitis of the sigmoid colon. Multiple diverticula (arrowheads) and wall thickening are noted (Figure 4), and inflammatory stranding is seen in the sigmoid mesentery (Figure 5). No free air or abscess formation is evident. The screening barium enema performed 3 years ago (Figure 6) shows multiple diverticula in the sigmoid and descending colon (arrowheads).\nBecause of systemic signs and symptoms of infection, this patient was admitted to the hospital. He was placed on bowel rest and started on intravenous metronidazole and ciprofloxacin. Over the next 2 days, the patient defervesced and his leukocytosis resolved. His diet was advanced to a full diet, and he was discharged from the hospital on a 10-day course of amoxicillin/clavulanic acid.\n\n ## Figures\n\n **Figure 2** \n \n\n**Figure 3** \n \n\n**Figure 4** \n \n\n**Figure 5** \n \n\n**Figure 6** \n \n\n\n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1023788,
"choiceText": "A relevant history and physical examination",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1023790,
"choiceText": "Complete blood cell count",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1023792,
"choiceText": "MRI of the abdomen",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1023794,
"choiceText": "CT of the abdomen",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1023796,
"choiceText": "Urinalysis",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The initial evaluation of a patient with suspected acute diverticulitis generally includes physical examination; complete blood cell count; urinalysis; and, when indicated by the presence of peritonitis, plain radiography of the abdomen to rule out <a href=\"http://emedicine.medscape.com/article/195537-overview\">colonic perforation</a>.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 323925,
"questionText": "Which of the below examinations is <em>not</em> performed as part of the evaluation for a patient presenting with acute sigmoid diverticulitis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1023802,
"choiceText": "Perforation, with free air",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1023804,
"choiceText": "Peritonitis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1023806,
"choiceText": "Adhesions",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1023808,
"choiceText": "Fistula formation",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1023810,
"choiceText": "All of the above",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The complications of acute diverticulitis include abscess formation, colonic perforation leading to free intra-abdominal air, local adhesions, purulent or fecal peritonitis, sepsis, bowel obstruction, and fistula formation between the colon and the bladder or vagina. Selected abscesses detected by ultrasonography or CT may be drained percutaneously, whereas perforations, fecal peritonitis, and fistula formation require a surgical consultation. Recurrent diverticulitis or complicated diverticulitis are indications for partial colonic resection.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 323929,
"questionText": "Which of the following conditions is a known complication of acute sigmoid diverticulitis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 60-Year-Old Man With Intense Left-Sided Abdominal Pain"
},
{
"authors": "Pramod Gupta, MD; Jitendra Gohil, MD",
"content": [],
"date": "October 12, 2016",
"figures": [],
"markdown": "# A 60-Year-Old Man With Intense Left-Sided Abdominal Pain\n\n **Authors:** Pramod Gupta, MD; Jitendra Gohil, MD \n **Date:** October 12, 2016\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1023788,
"choiceText": "A relevant history and physical examination",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1023790,
"choiceText": "Complete blood cell count",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1023792,
"choiceText": "MRI of the abdomen",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1023794,
"choiceText": "CT of the abdomen",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1023796,
"choiceText": "Urinalysis",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The initial evaluation of a patient with suspected acute diverticulitis generally includes physical examination; complete blood cell count; urinalysis; and, when indicated by the presence of peritonitis, plain radiography of the abdomen to rule out <a href=\"http://emedicine.medscape.com/article/195537-overview\">colonic perforation</a>.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 323925,
"questionText": "Which of the below examinations is <em>not</em> performed as part of the evaluation for a patient presenting with acute sigmoid diverticulitis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1023802,
"choiceText": "Perforation, with free air",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1023804,
"choiceText": "Peritonitis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1023806,
"choiceText": "Adhesions",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1023808,
"choiceText": "Fistula formation",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1023810,
"choiceText": "All of the above",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The complications of acute diverticulitis include abscess formation, colonic perforation leading to free intra-abdominal air, local adhesions, purulent or fecal peritonitis, sepsis, bowel obstruction, and fistula formation between the colon and the bladder or vagina. Selected abscesses detected by ultrasonography or CT may be drained percutaneously, whereas perforations, fecal peritonitis, and fistula formation require a surgical consultation. Recurrent diverticulitis or complicated diverticulitis are indications for partial colonic resection.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 323929,
"questionText": "Which of the following conditions is a known complication of acute sigmoid diverticulitis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 60-Year-Old Man With Intense Left-Sided Abdominal Pain"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1023756,
"choiceText": "Acute diverticulitis",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1023758,
"choiceText": "Colon cancer",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1023760,
"choiceText": "Acute bacterial peritonitis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1023762,
"choiceText": "Acute appendicitis",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 323917,
"questionText": "What is the diagnosis?<br/><br/>\r\n<em>Hint: This is the most common acute condition related to the sigmoid colon.</em>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1023788,
"choiceText": "A relevant history and physical examination",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1023790,
"choiceText": "Complete blood cell count",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1023792,
"choiceText": "MRI of the abdomen",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1023794,
"choiceText": "CT of the abdomen",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1023796,
"choiceText": "Urinalysis",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The initial evaluation of a patient with suspected acute diverticulitis generally includes physical examination; complete blood cell count; urinalysis; and, when indicated by the presence of peritonitis, plain radiography of the abdomen to rule out <a href=\"http://emedicine.medscape.com/article/195537-overview\">colonic perforation</a>.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 323925,
"questionText": "Which of the below examinations is <em>not</em> performed as part of the evaluation for a patient presenting with acute sigmoid diverticulitis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1023802,
"choiceText": "Perforation, with free air",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1023804,
"choiceText": "Peritonitis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1023806,
"choiceText": "Adhesions",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1023808,
"choiceText": "Fistula formation",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1023810,
"choiceText": "All of the above",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The complications of acute diverticulitis include abscess formation, colonic perforation leading to free intra-abdominal air, local adhesions, purulent or fecal peritonitis, sepsis, bowel obstruction, and fistula formation between the colon and the bladder or vagina. Selected abscesses detected by ultrasonography or CT may be drained percutaneously, whereas perforations, fecal peritonitis, and fistula formation require a surgical consultation. Recurrent diverticulitis or complicated diverticulitis are indications for partial colonic resection.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 323929,
"questionText": "Which of the following conditions is a known complication of acute sigmoid diverticulitis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
869984 | /viewarticle/869984 | [
{
"authors": "Sumaira Nabi, MBBS; Shahzad Ahmed, MBBS; Mazhar Badshah, MBBS",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"A 23-year-old Pakistani man presents to an outpatient department with a 3-year history of bilateral progressive hearing loss, tinnitus, and vertigo. At the time of presentation, he also describes a diffuse headache, blurring of vision, and swaying from side to side upon walking over the past 4 months.",
"The patient has no history of limb weakness, sensory symptoms, or seizures. He is single and unemployed. He is a nonsmoker and denies substance abuse. He reports that his mother died after being operated on for a brain neoplasm. However, he does not have any relevant documents with him."
],
"date": "October 12, 2016",
"figures": [],
"markdown": "# Bilateral Deafness and Skin Lesions in a 23-Year-Old Man\n\n **Authors:** Sumaira Nabi, MBBS; Shahzad Ahmed, MBBS; Mazhar Badshah, MBBS \n **Date:** October 12, 2016\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nA 23-year-old Pakistani man presents to an outpatient department with a 3-year history of bilateral progressive hearing loss, tinnitus, and vertigo. At the time of presentation, he also describes a diffuse headache, blurring of vision, and swaying from side to side upon walking over the past 4 months.\nThe patient has no history of limb weakness, sensory symptoms, or seizures. He is single and unemployed. He is a nonsmoker and denies substance abuse. He reports that his mother died after being operated on for a brain neoplasm. However, he does not have any relevant documents with him.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "Bilateral Deafness and Skin Lesions in a 23-Year-Old Man"
},
{
"authors": "Sumaira Nabi, MBBS; Shahzad Ahmed, MBBS; Mazhar Badshah, MBBS",
"content": [
"Figure 1",
"Figure 2",
"Figure 3",
"Upon clinical examination, the patient is an alert young man who is oriented to time, place, and person. His vital signs include an oral temperature of 98.6°F, a regular pulse of 70 beats/min, and a blood pressure of 120/70 mm Hg. His respiratory rate is 14 breaths/min.",
"His Glasgow Coma Score is 15/15, and his higher mental functions are intact. Upon general physical examination, he has subcutaneous swelling on the right side of his face and his back (Figure 1). Neurologic examination reveals visual acuity, in measurement of meters, of 6/18 on his right side and 6/36 on his left side (6/6 is equivalent to 20/20 in US customary units). Loss of the corneal reflex is noted on the left side, as well as a bilateral lower motor neuron type of facial palsy and bilateral sensorineural hearing loss.",
"Funduscopic examination reveals bilateral papilledema. The patient has ataxia with bilateral cerebellar signs. No signs of meningeal irritation or pyramidal weakness are observed.",
"The patient's abdomen is soft and nontender, without evidence of organomegaly or ascites. His bowel sounds are audible. Precordial examination reveals normal heart sounds. Auscultation of the lung fields reveals normal vesicular breathing.",
"On laboratory analysis, the complete blood cell count and erythrocyte sedimentation rate are within the reference range. Liver function test results, renal function test results, serum glucose levels, ECG findings, and chest radiograph are unremarkable. Nerve conduction studies are also normal.",
"MRI of the brain with contrast is performed and reveals bilateral, rounded, well-circumscribed masses at both cerebellopontine angles (Figures 2 and 3). These are isointense on T1-weighted imaging and hyperintense on T2-weighted imaging and fluid attenuation inversion recovery sequences, with patchy postcontrast enhancement. Obstructive hydrocephalus is also seen."
],
"date": "October 12, 2016",
"figures": [
{
"caption": "Figure 1",
"image_url": "https://img.medscapestatic.com/article/869/984/869984-Thumb1.png"
},
{
"caption": "Figure 2",
"image_url": "https://img.medscapestatic.com/article/869/984/869984-Thumb2.png"
},
{
"caption": "Figure 3",
"image_url": "https://img.medscapestatic.com/article/869/984/869984-Thumb3.png"
}
],
"markdown": "# Bilateral Deafness and Skin Lesions in a 23-Year-Old Man\n\n **Authors:** Sumaira Nabi, MBBS; Shahzad Ahmed, MBBS; Mazhar Badshah, MBBS \n **Date:** October 12, 2016\n\n ## Content\n\n Figure 1\nFigure 2\nFigure 3\nUpon clinical examination, the patient is an alert young man who is oriented to time, place, and person. His vital signs include an oral temperature of 98.6°F, a regular pulse of 70 beats/min, and a blood pressure of 120/70 mm Hg. His respiratory rate is 14 breaths/min.\nHis Glasgow Coma Score is 15/15, and his higher mental functions are intact. Upon general physical examination, he has subcutaneous swelling on the right side of his face and his back (Figure 1). Neurologic examination reveals visual acuity, in measurement of meters, of 6/18 on his right side and 6/36 on his left side (6/6 is equivalent to 20/20 in US customary units). Loss of the corneal reflex is noted on the left side, as well as a bilateral lower motor neuron type of facial palsy and bilateral sensorineural hearing loss.\nFunduscopic examination reveals bilateral papilledema. The patient has ataxia with bilateral cerebellar signs. No signs of meningeal irritation or pyramidal weakness are observed.\nThe patient's abdomen is soft and nontender, without evidence of organomegaly or ascites. His bowel sounds are audible. Precordial examination reveals normal heart sounds. Auscultation of the lung fields reveals normal vesicular breathing.\nOn laboratory analysis, the complete blood cell count and erythrocyte sedimentation rate are within the reference range. Liver function test results, renal function test results, serum glucose levels, ECG findings, and chest radiograph are unremarkable. Nerve conduction studies are also normal.\nMRI of the brain with contrast is performed and reveals bilateral, rounded, well-circumscribed masses at both cerebellopontine angles (Figures 2 and 3). These are isointense on T1-weighted imaging and hyperintense on T2-weighted imaging and fluid attenuation inversion recovery sequences, with patchy postcontrast enhancement. Obstructive hydrocephalus is also seen.\n\n ## Figures\n\n **Figure 1** \n \n\n**Figure 2** \n \n\n**Figure 3** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1024136,
"choiceText": "Tuberous sclerosis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1024138,
"choiceText": "Neurofibromatosis type 1 (NF1)",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1024140,
"choiceText": "Sturge-Weber syndrome",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1024142,
"choiceText": "Neurofibromatosis type 2 (NF2)",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 324043,
"questionText": "What is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Bilateral Deafness and Skin Lesions in a 23-Year-Old Man"
},
{
"authors": "Sumaira Nabi, MBBS; Shahzad Ahmed, MBBS; Mazhar Badshah, MBBS",
"content": [
"NF2 was diagnosed on the basis of bilateral vestibular schwannomas. The typical subcutaneous lesions along with a positive family history further supported the diagnosis. The patient was given intravenous steroids and mannitol and was referred to the neurosurgery department for tumor resection.",
"Neurofibromatosis is a set of three distinct genetic syndromes: NF1, also known as von Recklinghausen disease; NF2; and schwannomatosis.[1] These are characterized by a variable number of cutaneous lesions coupled with various tumors of the central and peripheral nervous system.",
"NF2 has an autosomal dominant mode of inheritance, with variable expressivity and incomplete penetrance.[2] The disease was first described by Wishart[3] in 1822 and was further elaborated by von Recklinghausen and Cushing.[4] Affected individuals have multiple tumors of the nervous system. NF2 is associated with a paucity of skin lesions, unlike NF1. Bilateral vestibular schwannomas, intracranial and spinal meningiomas, and spine tumors are the most frequent neoplasms seen in these patients.[5,6]",
"The underlying genetic defect is mutation of the NF2 gene, which is located on chromosome 22; the NF2 gene normally produces merlin, a tumor suppressor protein.[7,8] The mutation is present in over 93% families affected by the syndrome. The estimated incidence ranges from 1 in 25,000 to 1 in 37,000.[9,10]",
"The age of onset varies; however, affected individuals usually present around age 20 years.[11] NF2 is a multisystem disorder, and the clinical features involve the central and peripheral nervous system and eyes, with a relative lack of involvement of skin.",
"Neurologic manifestations are secondary to various types of tumors. Vestibular schwannomas are benign but lead to progressive sensorineural deafness, vertigo, tinnitus, and balance dysfunction in different combinations and cause significant morbidity.[12,13] In untreated cases, these can extend and cause brainstem compression and hydrocephalus. Bilateral vestibular schwannomas that arise from the vestibular branch of the eighth cranial nerve are the diagnostic hallmark of NF2. Other cranial nerves are also involved, commonly cranial nerves V and VII.",
"Intracranial meningiomas, ependymomas, and gliomas lead to focal neurologic signs, headache, visual impairment, and seizures.[14] Meningiomas may be single or multiple and arise much earlier than sporadic ones.[15] These tend to be more aggressive, with higher chances of anaplasia. Cranial and peripheral nerve schwannomas are also seen.[16] Those ramifying from the dorsal root tend to assume a characteristic \"dumbbell\" shape. Extra- and intramedullary spinal tumors present with paresthesias, limb weakness, and sphincter dysfunction. Neuropathy can present as mononeuropathy that involves a single nerve, or peripheral polyneuropathy with loss of reflexes and muscle wasting.[17]",
"Ophthalmologic involvement is seen in the form of cataracts, posterior subcapsular lenticular opacities, optic nerve meningioma, and retinal hamartomas.[10] Cataracts are the most frequently encountered ocular manifestation. All of these can lead to visual dysfunction.",
"Cutaneous manifestations are less frequent and subtle in NF2 compared with NF1.[13,18,19] Subcutaneous neurofibromas are usually seen in 50%-70% of patients. Intracutaneous schwannomas are occasionally seen. Some patients may also have pigmented plaques often with excess hair. Café au lait spots are rarely seen. Axillary or inguinal freckling, Lisch nodules, and malignant transformation of tumors very rarely occur in NF2, unlike in NF1, which is associated with florid skin lesions."
],
"date": "October 12, 2016",
"figures": [],
"markdown": "# Bilateral Deafness and Skin Lesions in a 23-Year-Old Man\n\n **Authors:** Sumaira Nabi, MBBS; Shahzad Ahmed, MBBS; Mazhar Badshah, MBBS \n **Date:** October 12, 2016\n\n ## Content\n\n NF2 was diagnosed on the basis of bilateral vestibular schwannomas. The typical subcutaneous lesions along with a positive family history further supported the diagnosis. The patient was given intravenous steroids and mannitol and was referred to the neurosurgery department for tumor resection.\nNeurofibromatosis is a set of three distinct genetic syndromes: NF1, also known as von Recklinghausen disease; NF2; and schwannomatosis.[1] These are characterized by a variable number of cutaneous lesions coupled with various tumors of the central and peripheral nervous system.\nNF2 has an autosomal dominant mode of inheritance, with variable expressivity and incomplete penetrance.[2] The disease was first described by Wishart[3] in 1822 and was further elaborated by von Recklinghausen and Cushing.[4] Affected individuals have multiple tumors of the nervous system. NF2 is associated with a paucity of skin lesions, unlike NF1. Bilateral vestibular schwannomas, intracranial and spinal meningiomas, and spine tumors are the most frequent neoplasms seen in these patients.[5,6]\nThe underlying genetic defect is mutation of the NF2 gene, which is located on chromosome 22; the NF2 gene normally produces merlin, a tumor suppressor protein.[7,8] The mutation is present in over 93% families affected by the syndrome. The estimated incidence ranges from 1 in 25,000 to 1 in 37,000.[9,10]\nThe age of onset varies; however, affected individuals usually present around age 20 years.[11] NF2 is a multisystem disorder, and the clinical features involve the central and peripheral nervous system and eyes, with a relative lack of involvement of skin.\nNeurologic manifestations are secondary to various types of tumors. Vestibular schwannomas are benign but lead to progressive sensorineural deafness, vertigo, tinnitus, and balance dysfunction in different combinations and cause significant morbidity.[12,13] In untreated cases, these can extend and cause brainstem compression and hydrocephalus. Bilateral vestibular schwannomas that arise from the vestibular branch of the eighth cranial nerve are the diagnostic hallmark of NF2. Other cranial nerves are also involved, commonly cranial nerves V and VII.\nIntracranial meningiomas, ependymomas, and gliomas lead to focal neurologic signs, headache, visual impairment, and seizures.[14] Meningiomas may be single or multiple and arise much earlier than sporadic ones.[15] These tend to be more aggressive, with higher chances of anaplasia. Cranial and peripheral nerve schwannomas are also seen.[16] Those ramifying from the dorsal root tend to assume a characteristic \"dumbbell\" shape. Extra- and intramedullary spinal tumors present with paresthesias, limb weakness, and sphincter dysfunction. Neuropathy can present as mononeuropathy that involves a single nerve, or peripheral polyneuropathy with loss of reflexes and muscle wasting.[17]\nOphthalmologic involvement is seen in the form of cataracts, posterior subcapsular lenticular opacities, optic nerve meningioma, and retinal hamartomas.[10] Cataracts are the most frequently encountered ocular manifestation. All of these can lead to visual dysfunction.\nCutaneous manifestations are less frequent and subtle in NF2 compared with NF1.[13,18,19] Subcutaneous neurofibromas are usually seen in 50%-70% of patients. Intracutaneous schwannomas are occasionally seen. Some patients may also have pigmented plaques often with excess hair. Café au lait spots are rarely seen. Axillary or inguinal freckling, Lisch nodules, and malignant transformation of tumors very rarely occur in NF2, unlike in NF1, which is associated with florid skin lesions.\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1024136,
"choiceText": "Tuberous sclerosis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1024138,
"choiceText": "Neurofibromatosis type 1 (NF1)",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1024140,
"choiceText": "Sturge-Weber syndrome",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1024142,
"choiceText": "Neurofibromatosis type 2 (NF2)",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 324043,
"questionText": "What is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Bilateral Deafness and Skin Lesions in a 23-Year-Old Man"
},
{
"authors": "Sumaira Nabi, MBBS; Shahzad Ahmed, MBBS; Mazhar Badshah, MBBS",
"content": [
"The diagnosis of NF2 was initially based on the National Institutes of Health (NIH) criteria, which are as follows:",
"Bilateral masses of the eighth cranial nerve, seen with appropriate imaging techniques, plus",
"A first-degree relative with NF2 and",
"Either a unilateral mass of the eighth cranial nerve or any two of neurofibroma, meningioma, glioma, schwannoma, or juvenile posterior subcapsular lenticular opacity.[20]",
"Subsequently, the more sensitive Manchester criteria were formulated as a modification of the NIH criteria. The Manchester criteria are more widely used. The main criteria are as follows[16]:",
"Bilateral vestibular schwannomas or family history of NF2, plus",
"Unilateral vestibular schwannoma, or",
"Any two of the following:",
"Meningioma",
"Glioma",
"Neurofibroma",
"Schwannoma",
"Posterior subcapsular lenticular opacities",
"Additional criteria include the following:",
"Unilateral vestibular schwannoma, plus any two of the following:",
"Meningioma",
"Glioma",
"Neurofibroma",
"Schwannoma",
"Posterior subcapsular opacities, or",
"Multiple meningiomas (≥ 2) plus unilateral vestibular schwannoma or any two of the following:",
"Glioma",
"Neurofibroma",
"Schwannoma",
"Cataracts",
"The clinical diagnosis can be then confirmed by molecular genetic testing for the NF2 gene on chromosome 22. Neuroimaging aids in delineating cranial and spinal tumors. Audiometry and brain stem auditory evoked responses are also abnormal. Nerve conduction studies may reveal polyneuropathy in around 40% of cases. Nerve biopsy may demonstrate onion bulb formation.[16]",
"Presymptomatic molecular genetic testing is a vital component of management of the families of patients with NF2. Individuals with a first-degree relative with NF2, those with multiple spinal tumors, and those with cutaneous schwannomas must be screened for NF2.[21,22] Prenatal diagnosis is also important. Prompt recognition of patients with NF2 is important to minimize subsequent complications.",
"Once the NF2 mutation is identified, these patients should receive routine follow-up. MRI of the brain and spine should be repeated every 2 years for individuals younger than 20 years and every 3-5 years after age 20 years. Yearly assessment of hearing should be performed, along with evaluation of eyes and skin. No further tests or follow-up is warranted in individuals who are negative for the affected gene."
],
"date": "October 12, 2016",
"figures": [],
"markdown": "# Bilateral Deafness and Skin Lesions in a 23-Year-Old Man\n\n **Authors:** Sumaira Nabi, MBBS; Shahzad Ahmed, MBBS; Mazhar Badshah, MBBS \n **Date:** October 12, 2016\n\n ## Content\n\n The diagnosis of NF2 was initially based on the National Institutes of Health (NIH) criteria, which are as follows:\nBilateral masses of the eighth cranial nerve, seen with appropriate imaging techniques, plus\nA first-degree relative with NF2 and\nEither a unilateral mass of the eighth cranial nerve or any two of neurofibroma, meningioma, glioma, schwannoma, or juvenile posterior subcapsular lenticular opacity.[20]\nSubsequently, the more sensitive Manchester criteria were formulated as a modification of the NIH criteria. The Manchester criteria are more widely used. The main criteria are as follows[16]:\nBilateral vestibular schwannomas or family history of NF2, plus\nUnilateral vestibular schwannoma, or\nAny two of the following:\nMeningioma\nGlioma\nNeurofibroma\nSchwannoma\nPosterior subcapsular lenticular opacities\nAdditional criteria include the following:\nUnilateral vestibular schwannoma, plus any two of the following:\nMeningioma\nGlioma\nNeurofibroma\nSchwannoma\nPosterior subcapsular opacities, or\nMultiple meningiomas (≥ 2) plus unilateral vestibular schwannoma or any two of the following:\nGlioma\nNeurofibroma\nSchwannoma\nCataracts\nThe clinical diagnosis can be then confirmed by molecular genetic testing for the NF2 gene on chromosome 22. Neuroimaging aids in delineating cranial and spinal tumors. Audiometry and brain stem auditory evoked responses are also abnormal. Nerve conduction studies may reveal polyneuropathy in around 40% of cases. Nerve biopsy may demonstrate onion bulb formation.[16]\nPresymptomatic molecular genetic testing is a vital component of management of the families of patients with NF2. Individuals with a first-degree relative with NF2, those with multiple spinal tumors, and those with cutaneous schwannomas must be screened for NF2.[21,22] Prenatal diagnosis is also important. Prompt recognition of patients with NF2 is important to minimize subsequent complications.\nOnce the NF2 mutation is identified, these patients should receive routine follow-up. MRI of the brain and spine should be repeated every 2 years for individuals younger than 20 years and every 3-5 years after age 20 years. Yearly assessment of hearing should be performed, along with evaluation of eyes and skin. No further tests or follow-up is warranted in individuals who are negative for the affected gene.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "Bilateral Deafness and Skin Lesions in a 23-Year-Old Man"
},
{
"authors": "Sumaira Nabi, MBBS; Shahzad Ahmed, MBBS; Mazhar Badshah, MBBS",
"content": [
"The management of NF2 is challenging and must involve a multidisciplinary approach. The treatment of choice for vestibular schwannomas is surgical resection.[20] Tumors that lead to brainstem compression, hydrocephalus, hearing loss, or facial nerve paresis are best managed surgically. Tumors found incidentally on neuroimaging are better managed conservatively and monitored.",
"The timing and procedure of surgery are important. In addition to open surgery, stereotactic surgery, radiosurgery, and radiation therapy are alternative options.[23] Radiation therapy has shown variable outcomes, and its use is controversial.[24] Cochlear implants may be offered to patients with severe deafness.[25] Meningiomas are also surgically managed.",
"Targeted drug therapy is the probable future treatment for NF2.[26] Bevacizumab, a monoclonal antibody against vascular endothelial growth factor, has been reported in the literature to cause regression in tumor size, with resultant hearing improvement in patients with NF2. Other experimental therapies include everolimus, lapatinib, sorafenib, and erlotinib, which have all demonstrated variable results.[27]",
"The prognosis is dismal, with numerous patients dying in their youth. Despite the advent of advanced imaging techniques and treatment modalities, morbidity and mortality are still significant. Young age at onset and a greater number of meningiomas are poor prognostic markers. Drug therapies working at the molecular and genetic levels are the breakthrough expected in years to come and are anticipated to remarkably improve outcomes in patients with NF2.[26]"
],
"date": "October 12, 2016",
"figures": [],
"markdown": "# Bilateral Deafness and Skin Lesions in a 23-Year-Old Man\n\n **Authors:** Sumaira Nabi, MBBS; Shahzad Ahmed, MBBS; Mazhar Badshah, MBBS \n **Date:** October 12, 2016\n\n ## Content\n\n The management of NF2 is challenging and must involve a multidisciplinary approach. The treatment of choice for vestibular schwannomas is surgical resection.[20] Tumors that lead to brainstem compression, hydrocephalus, hearing loss, or facial nerve paresis are best managed surgically. Tumors found incidentally on neuroimaging are better managed conservatively and monitored.\nThe timing and procedure of surgery are important. In addition to open surgery, stereotactic surgery, radiosurgery, and radiation therapy are alternative options.[23] Radiation therapy has shown variable outcomes, and its use is controversial.[24] Cochlear implants may be offered to patients with severe deafness.[25] Meningiomas are also surgically managed.\nTargeted drug therapy is the probable future treatment for NF2.[26] Bevacizumab, a monoclonal antibody against vascular endothelial growth factor, has been reported in the literature to cause regression in tumor size, with resultant hearing improvement in patients with NF2. Other experimental therapies include everolimus, lapatinib, sorafenib, and erlotinib, which have all demonstrated variable results.[27]\nThe prognosis is dismal, with numerous patients dying in their youth. Despite the advent of advanced imaging techniques and treatment modalities, morbidity and mortality are still significant. Young age at onset and a greater number of meningiomas are poor prognostic markers. Drug therapies working at the molecular and genetic levels are the breakthrough expected in years to come and are anticipated to remarkably improve outcomes in patients with NF2.[26]\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1024146,
"choiceText": "Intracranial meningioma",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1024148,
"choiceText": "Spinal meningioma",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1024150,
"choiceText": "Bilateral vestibular schwannomas",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1024152,
"choiceText": "Peripheral nerve schwannoma",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1024154,
"choiceText": "Optic nerve meningioma",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The hallmark of NF2 is the development of bilateral vestibular schwannomas. These usually present with hearing loss, tinnitus, vertigo, and imbalance.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 324045,
"questionText": "Which of the following tumors is the hallmark lesion of NF2?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1024156,
"choiceText": "Cranial nerve II",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1024158,
"choiceText": "Cranial nerve V",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1024160,
"choiceText": "Cranial nerve VII",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1024162,
"choiceText": "Cranial nerve VIII",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1024164,
"choiceText": "Cranial nerve X",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Cranial nerve VIII is most commonly affected by NF2. Vestibular schwannomas arise from the vestibular branch of the eighth cranial nerve. Other cranial nerves frequently involved are cranial nerves V and VII.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 324047,
"questionText": "Which cranial nerve is most commonly affected by NF2?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Bilateral Deafness and Skin Lesions in a 23-Year-Old Man"
},
{
"authors": "Sumaira Nabi, MBBS; Shahzad Ahmed, MBBS; Mazhar Badshah, MBBS",
"content": [],
"date": "October 12, 2016",
"figures": [],
"markdown": "# Bilateral Deafness and Skin Lesions in a 23-Year-Old Man\n\n **Authors:** Sumaira Nabi, MBBS; Shahzad Ahmed, MBBS; Mazhar Badshah, MBBS \n **Date:** October 12, 2016\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1024146,
"choiceText": "Intracranial meningioma",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1024148,
"choiceText": "Spinal meningioma",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1024150,
"choiceText": "Bilateral vestibular schwannomas",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1024152,
"choiceText": "Peripheral nerve schwannoma",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1024154,
"choiceText": "Optic nerve meningioma",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The hallmark of NF2 is the development of bilateral vestibular schwannomas. These usually present with hearing loss, tinnitus, vertigo, and imbalance.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 324045,
"questionText": "Which of the following tumors is the hallmark lesion of NF2?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1024156,
"choiceText": "Cranial nerve II",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1024158,
"choiceText": "Cranial nerve V",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1024160,
"choiceText": "Cranial nerve VII",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1024162,
"choiceText": "Cranial nerve VIII",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1024164,
"choiceText": "Cranial nerve X",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Cranial nerve VIII is most commonly affected by NF2. Vestibular schwannomas arise from the vestibular branch of the eighth cranial nerve. Other cranial nerves frequently involved are cranial nerves V and VII.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 324047,
"questionText": "Which cranial nerve is most commonly affected by NF2?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Bilateral Deafness and Skin Lesions in a 23-Year-Old Man"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1024136,
"choiceText": "Tuberous sclerosis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1024138,
"choiceText": "Neurofibromatosis type 1 (NF1)",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1024140,
"choiceText": "Sturge-Weber syndrome",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1024142,
"choiceText": "Neurofibromatosis type 2 (NF2)",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 324043,
"questionText": "What is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1024146,
"choiceText": "Intracranial meningioma",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1024148,
"choiceText": "Spinal meningioma",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1024150,
"choiceText": "Bilateral vestibular schwannomas",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1024152,
"choiceText": "Peripheral nerve schwannoma",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1024154,
"choiceText": "Optic nerve meningioma",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The hallmark of NF2 is the development of bilateral vestibular schwannomas. These usually present with hearing loss, tinnitus, vertigo, and imbalance.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 324045,
"questionText": "Which of the following tumors is the hallmark lesion of NF2?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1024156,
"choiceText": "Cranial nerve II",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1024158,
"choiceText": "Cranial nerve V",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1024160,
"choiceText": "Cranial nerve VII",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1024162,
"choiceText": "Cranial nerve VIII",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1024164,
"choiceText": "Cranial nerve X",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Cranial nerve VIII is most commonly affected by NF2. Vestibular schwannomas arise from the vestibular branch of the eighth cranial nerve. Other cranial nerves frequently involved are cranial nerves V and VII.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 324047,
"questionText": "Which cranial nerve is most commonly affected by NF2?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
869222 | /viewarticle/869222 | [
{
"authors": "Amin Esfahani, MD; Mary E. Lohman, BA; Anne Laumann, MBChB, MRCP(UK)",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"A 55-year-old African American woman presents with a 6-month history of \"bumps\" on her cheeks. She reports that the spots initially appeared on both cheeks without any specific precipitating factors. She states that the spots generally last approximately 10 days; however, as they disappear, new ones occur. Associated symptoms include an occasional sensation of warmth and burning in the affected areas, especially after exposure to sunlight. These symptoms are aggravated by heat.",
"She has treated her face with tea tree oil without relief. She had no previous episodes, prior to 6 months ago, and has no history of eczema or acne. She had not used any new facial products, detergents, or medications prior to onset of the eruption. She also reports no recent travel.",
"A review of her systems is significant for occasional flushing, which has occurred for approximately 3 years. She states that she has occasional dryness and a \"gritty\" sensation in her eyes. She reports no fever, recent illness, night sweats, unintentional weight loss, shortness of breath, chest tightness, cough, nausea, vomiting, diarrhea, or dysuria."
],
"date": "October 11, 2017",
"figures": [],
"markdown": "# A 55-Year-Old Woman With Bumps on Her Face\n\n **Authors:** Amin Esfahani, MD; Mary E. Lohman, BA; Anne Laumann, MBChB, MRCP(UK) \n **Date:** October 11, 2017\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nA 55-year-old African American woman presents with a 6-month history of \"bumps\" on her cheeks. She reports that the spots initially appeared on both cheeks without any specific precipitating factors. She states that the spots generally last approximately 10 days; however, as they disappear, new ones occur. Associated symptoms include an occasional sensation of warmth and burning in the affected areas, especially after exposure to sunlight. These symptoms are aggravated by heat.\nShe has treated her face with tea tree oil without relief. She had no previous episodes, prior to 6 months ago, and has no history of eczema or acne. She had not used any new facial products, detergents, or medications prior to onset of the eruption. She also reports no recent travel.\nA review of her systems is significant for occasional flushing, which has occurred for approximately 3 years. She states that she has occasional dryness and a \"gritty\" sensation in her eyes. She reports no fever, recent illness, night sweats, unintentional weight loss, shortness of breath, chest tightness, cough, nausea, vomiting, diarrhea, or dysuria.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 55-Year-Old Woman With Bumps on Her Face"
},
{
"authors": "Amin Esfahani, MD; Mary E. Lohman, BA; Anne Laumann, MBChB, MRCP(UK)",
"content": [
"On examination, the patient, who has Fitzpatrick skin phototype 5, appears well. Relevant skin findings include multiple dome-shaped erythematous to violaceous papules (2–4 mm) and a few superficial pustules on a background of faint erythema on both cheeks (Figures 1 and 2).",
"Figure 1.",
"Figure 2.",
"Also present are scattered hyperpigmented macules.",
"Examination of the back, chest, neck, upper extremities, and fingernails reveals no abnormalities.",
"Recent complete blood count and complete metabolic panel results are within the reference range."
],
"date": "October 11, 2017",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/869/222/869222-Thumb1.png"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/869/222/869222-Thumb2.png"
}
],
"markdown": "# A 55-Year-Old Woman With Bumps on Her Face\n\n **Authors:** Amin Esfahani, MD; Mary E. Lohman, BA; Anne Laumann, MBChB, MRCP(UK) \n **Date:** October 11, 2017\n\n ## Content\n\n On examination, the patient, who has Fitzpatrick skin phototype 5, appears well. Relevant skin findings include multiple dome-shaped erythematous to violaceous papules (2–4 mm) and a few superficial pustules on a background of faint erythema on both cheeks (Figures 1 and 2).\nFigure 1.\nFigure 2.\nAlso present are scattered hyperpigmented macules.\nExamination of the back, chest, neck, upper extremities, and fingernails reveals no abnormalities.\nRecent complete blood count and complete metabolic panel results are within the reference range.\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1018742,
"choiceText": "Cutaneous sarcoidosis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1018744,
"choiceText": "Cutaneous lupus erythematous ",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1018746,
"choiceText": "Seborrheic dermatitis ",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1018748,
"choiceText": "Rosacea (papulopustular)",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1018750,
"choiceText": "Periorificial dermatitis\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 322425,
"questionText": "What is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 55-Year-Old Woman With Bumps on Her Face"
},
{
"authors": "Amin Esfahani, MD; Mary E. Lohman, BA; Anne Laumann, MBChB, MRCP(UK)",
"content": [
"The case described presents a common dermatosis in an atypical skin type for this disease. Rosacea is a chronic skin condition that is characterized by wide range of signs and symptoms. Four types of rosacea are recognized: erythematotelangiectatic, papulopustular, phymatous, and ocular.[1] Rosacea is most common in women older than 30 years, with the exception of phymatous rosacea, which is more common in men. Given the diverse presentation, prevalence is not well defined. Studies, however, show that it is found more frequently in Caucasians and in those with fair, sun-sensitive (phototypes 1 and 2) skin, with a prevalence of approximately 10%.[1–3] Rosacea can affect patients with darker skin tones, but this is uncommon and, as in this case, represents a diagnostic challenge.[4]",
"The pathogenesis of rosacea is not well understood. Current evidence suggests an interplay of immune dysfunction (eg, abnormal cathelicidin, kallikrein 5), microorganisms (eg, Demodex folliculorum, Bacillus oleronius, Helicobacter pylori), ultraviolet radiation, and vascular hyperactivity. Genetic susceptibility has also been demonstrated in some studies.[1,5,6]",
"Each of the four types of rosacea is associated with its own unique features, although overlap between types is common. The hallmarks of erythematotelangiectatic rosacea include centrofacial erythema, flushing, and telangiectasia (enlarged cutaneous blood vessels). An example is shown in Figure 3. Other features include sensitivity to the sun, fine flaking, and, in some individuals, stinging and burning in affected areas. The clinical diagnosis of erythematotelangiectatic rosacea can be a challenge, given that the appearance is similar to chronic actinically (sun) damaged skin. The diagnosis is a challenge in patients with darker skin tones, in particular, because erythema is more violaceous in quality.[1]",
"Papulopustular rosacea is distinguished by a centrofacial eruption of small, dome-shaped, erythematous papules and pustules (as in this case). An example is shown in Figure 4. Each individual lesion generally lasts 2 weeks and heals without scarring (unless manipulated by the individual). Unlike acne, comedones are not present. Patients can be asymptomatic or have a range of symptoms, such as tenderness and/or pruritus.[1]",
"Figure 4.",
"Phymatous rosacea is characterized by tissue hypertrophy, including the sebaceous unit, leading to thickened skin. An example is shown in Figure 5. It is the only type of rosacea that is more common in men. It typically occurs on the nose (rhinophyma), but can also be found on the chin (gnathophyma), ears (otophyma), central forehead (metophyma), and eyelids (blepharophyma). Phymatous rosacea can occur de novo or in the setting of mild rosacea, acne, or chronic sun damage. Its presence does not correlate with the severity of other types of rosacea.[1,7]",
"Figure 5.",
"Ocular rosacea occurs in more than 50% of patients with cutaneous rosacea. Ocular symptoms precede cutaneous findings in approximately 20% of these patients (as in this case), and occur after cutaneous presentation in about 50%. The clinical findings are diverse and nonspecific. They include blepharitis, conjunctival injection, conical dandruff (tiny concretions at the bases of the cilia), scurf (scaling at eyelid margins), keratitis, abnormal tearing, cicatricial conjunctivitis, and chalazion or hordeolum (stye) formation. Symptoms include photophobia, blurry vision, itching, tearing, dryness, and a gritty or foreign-body sensation. A detailed review of ocular symptoms and patient awareness about ocular rosacea are important for early detection. Referral to an ophthalmologist is recommended if a patient exhibits signs or symptoms of ocular rosacea.[1,8,9]"
],
"date": "October 11, 2017",
"figures": [
{
"caption": "Figure 4.",
"image_url": "https://img.medscapestatic.com/article/869/222/869222-Thumb4.png"
},
{
"caption": "Figure 5.",
"image_url": "https://img.medscapestatic.com/article/869/222/869222-Thumb5.png"
}
],
"markdown": "# A 55-Year-Old Woman With Bumps on Her Face\n\n **Authors:** Amin Esfahani, MD; Mary E. Lohman, BA; Anne Laumann, MBChB, MRCP(UK) \n **Date:** October 11, 2017\n\n ## Content\n\n The case described presents a common dermatosis in an atypical skin type for this disease. Rosacea is a chronic skin condition that is characterized by wide range of signs and symptoms. Four types of rosacea are recognized: erythematotelangiectatic, papulopustular, phymatous, and ocular.[1] Rosacea is most common in women older than 30 years, with the exception of phymatous rosacea, which is more common in men. Given the diverse presentation, prevalence is not well defined. Studies, however, show that it is found more frequently in Caucasians and in those with fair, sun-sensitive (phototypes 1 and 2) skin, with a prevalence of approximately 10%.[1–3] Rosacea can affect patients with darker skin tones, but this is uncommon and, as in this case, represents a diagnostic challenge.[4]\nThe pathogenesis of rosacea is not well understood. Current evidence suggests an interplay of immune dysfunction (eg, abnormal cathelicidin, kallikrein 5), microorganisms (eg, Demodex folliculorum, Bacillus oleronius, Helicobacter pylori), ultraviolet radiation, and vascular hyperactivity. Genetic susceptibility has also been demonstrated in some studies.[1,5,6]\nEach of the four types of rosacea is associated with its own unique features, although overlap between types is common. The hallmarks of erythematotelangiectatic rosacea include centrofacial erythema, flushing, and telangiectasia (enlarged cutaneous blood vessels). An example is shown in Figure 3. Other features include sensitivity to the sun, fine flaking, and, in some individuals, stinging and burning in affected areas. The clinical diagnosis of erythematotelangiectatic rosacea can be a challenge, given that the appearance is similar to chronic actinically (sun) damaged skin. The diagnosis is a challenge in patients with darker skin tones, in particular, because erythema is more violaceous in quality.[1]\nPapulopustular rosacea is distinguished by a centrofacial eruption of small, dome-shaped, erythematous papules and pustules (as in this case). An example is shown in Figure 4. Each individual lesion generally lasts 2 weeks and heals without scarring (unless manipulated by the individual). Unlike acne, comedones are not present. Patients can be asymptomatic or have a range of symptoms, such as tenderness and/or pruritus.[1]\nFigure 4.\nPhymatous rosacea is characterized by tissue hypertrophy, including the sebaceous unit, leading to thickened skin. An example is shown in Figure 5. It is the only type of rosacea that is more common in men. It typically occurs on the nose (rhinophyma), but can also be found on the chin (gnathophyma), ears (otophyma), central forehead (metophyma), and eyelids (blepharophyma). Phymatous rosacea can occur de novo or in the setting of mild rosacea, acne, or chronic sun damage. Its presence does not correlate with the severity of other types of rosacea.[1,7]\nFigure 5.\nOcular rosacea occurs in more than 50% of patients with cutaneous rosacea. Ocular symptoms precede cutaneous findings in approximately 20% of these patients (as in this case), and occur after cutaneous presentation in about 50%. The clinical findings are diverse and nonspecific. They include blepharitis, conjunctival injection, conical dandruff (tiny concretions at the bases of the cilia), scurf (scaling at eyelid margins), keratitis, abnormal tearing, cicatricial conjunctivitis, and chalazion or hordeolum (stye) formation. Symptoms include photophobia, blurry vision, itching, tearing, dryness, and a gritty or foreign-body sensation. A detailed review of ocular symptoms and patient awareness about ocular rosacea are important for early detection. Referral to an ophthalmologist is recommended if a patient exhibits signs or symptoms of ocular rosacea.[1,8,9]\n\n ## Figures\n\n **Figure 4.** \n \n\n**Figure 5.** \n \n\n\n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1018742,
"choiceText": "Cutaneous sarcoidosis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1018744,
"choiceText": "Cutaneous lupus erythematous ",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1018746,
"choiceText": "Seborrheic dermatitis ",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1018748,
"choiceText": "Rosacea (papulopustular)",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1018750,
"choiceText": "Periorificial dermatitis\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 322425,
"questionText": "What is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 55-Year-Old Woman With Bumps on Her Face"
},
{
"authors": "Amin Esfahani, MD; Mary E. Lohman, BA; Anne Laumann, MBChB, MRCP(UK)",
"content": [
"Clinical exacerbation of the flushing associated with rosacea has been linked to numerous \"triggers.\" This extensive list includes sun exposure, alcohol consumption, exercise, exposure to extremes of temperature, various emotions, and spicy foods.",
"Rosacea is a clinical diagnosis. Diagnostic studies, including skin biopsy, are rarely indicated. The differential diagnosis varies, depending on the type of rosacea. For the erythematotelangiectatic type, the differential diagnosis includes chronic sun-damaged skin. Location can be used to distinguish rosacea from sun damage. Rosacea is generally limited to the centrofacial region, whereas chronic sun damage usually includes the lateral cheeks and neck. Seborrheic dermatitis can present with similar eyelid findings; however, perinasal erythema, the presence of greasy, yellow scale and the involvement of eyebrows, ears (retroauricular), and scalp are distinguishing features.",
"When evaluating patients with centrofacial erythema, it is important to keep in mind the malar erythema seen in patients with systemic lupus erythematosus. Patients with lupus may or may not be aware of photosensitivity; however, their malar lesions are often more floridly red in character, with well-demarcated borders. A clinical history and a detailed review of systems are useful when distinguishing these entities. This is especially important in people with darker skin tones because erythema in darker skin tones can appear violaceous. In general, unless suspicion is high, an autoimmune panel and other laboratory tests are not indicated. Other flushing disorders are included in the differential diagnosis, such as medication-induced flushing menopausal hot flashes and malignancies, like carcinoid syndrome, pheochromocytoma, and medullary thyroid carcinoma.[1]",
"The differential diagnosis for papulopustular rosacea includes acne vulgaris, periorificial dermatitis, steroid-induced rosacea, Demodex folliculitis, papulopustular eruptions due to epidermal growth-factor receptor (EGFR) inhibitors, and keratosis pilaris rubra faciei. Unlike acne, papulopustular rosacea lacks comedones. Periorificial dermatitis can be distinguished from rosacea by location (surrounding mouth, eyes) and papulopustules at the same stage of development. Patients with periorificial dermatitis can have a history of topical or inhaled corticosteroid use, and might describe symptoms of sun intolerance or sensitivity to cosmetics and hot water. No relapse typically occurs after successful treatment; in contrast, in patients with rosacea, flaring is seen after the discontinuation of therapy.",
"The use of potent topical steroids can lead to steroid-induced rosacea. An example is shown in Figure 6. Papulopustular lesions in this entity are monomorphic. However, given that the appearance is similar to rosacea, a detailed history of medications is crucial. The appearance of Demodex folliculitis (demodicosis) and papulopustular rosacea is similar.",
"Figure 6.",
"Immunocompromised patients are at increased risk for demodicosis. Diagnosis can be made on the basis of clinical history and a potassium hydroxide (KOH) preparation that demonstrates many mites. Demodex mites are also present in those with normal skin, but at a lower concentration.",
"Similar lesions have been seen in patients treated with EGFR inhibitors, so a detailed disease-course, medication, and medical history is necessary. Keratosis pilaris rubra faciei can occur as a manifestation of keratosis pilaris, with rough, erythematous, symmetric triangular patches of skin on both cheeks, at times with overlying 1 to 2 mm follicular-based papules. The presence of keratosis pilaris and a lack of pustules can guide diagnosis."
],
"date": "October 11, 2017",
"figures": [
{
"caption": "Figure 6.",
"image_url": "https://img.medscapestatic.com/article/869/222/869222-Thumb6.png"
}
],
"markdown": "# A 55-Year-Old Woman With Bumps on Her Face\n\n **Authors:** Amin Esfahani, MD; Mary E. Lohman, BA; Anne Laumann, MBChB, MRCP(UK) \n **Date:** October 11, 2017\n\n ## Content\n\n Clinical exacerbation of the flushing associated with rosacea has been linked to numerous \"triggers.\" This extensive list includes sun exposure, alcohol consumption, exercise, exposure to extremes of temperature, various emotions, and spicy foods.\nRosacea is a clinical diagnosis. Diagnostic studies, including skin biopsy, are rarely indicated. The differential diagnosis varies, depending on the type of rosacea. For the erythematotelangiectatic type, the differential diagnosis includes chronic sun-damaged skin. Location can be used to distinguish rosacea from sun damage. Rosacea is generally limited to the centrofacial region, whereas chronic sun damage usually includes the lateral cheeks and neck. Seborrheic dermatitis can present with similar eyelid findings; however, perinasal erythema, the presence of greasy, yellow scale and the involvement of eyebrows, ears (retroauricular), and scalp are distinguishing features.\nWhen evaluating patients with centrofacial erythema, it is important to keep in mind the malar erythema seen in patients with systemic lupus erythematosus. Patients with lupus may or may not be aware of photosensitivity; however, their malar lesions are often more floridly red in character, with well-demarcated borders. A clinical history and a detailed review of systems are useful when distinguishing these entities. This is especially important in people with darker skin tones because erythema in darker skin tones can appear violaceous. In general, unless suspicion is high, an autoimmune panel and other laboratory tests are not indicated. Other flushing disorders are included in the differential diagnosis, such as medication-induced flushing menopausal hot flashes and malignancies, like carcinoid syndrome, pheochromocytoma, and medullary thyroid carcinoma.[1]\nThe differential diagnosis for papulopustular rosacea includes acne vulgaris, periorificial dermatitis, steroid-induced rosacea, Demodex folliculitis, papulopustular eruptions due to epidermal growth-factor receptor (EGFR) inhibitors, and keratosis pilaris rubra faciei. Unlike acne, papulopustular rosacea lacks comedones. Periorificial dermatitis can be distinguished from rosacea by location (surrounding mouth, eyes) and papulopustules at the same stage of development. Patients with periorificial dermatitis can have a history of topical or inhaled corticosteroid use, and might describe symptoms of sun intolerance or sensitivity to cosmetics and hot water. No relapse typically occurs after successful treatment; in contrast, in patients with rosacea, flaring is seen after the discontinuation of therapy.\nThe use of potent topical steroids can lead to steroid-induced rosacea. An example is shown in Figure 6. Papulopustular lesions in this entity are monomorphic. However, given that the appearance is similar to rosacea, a detailed history of medications is crucial. The appearance of Demodex folliculitis (demodicosis) and papulopustular rosacea is similar.\nFigure 6.\nImmunocompromised patients are at increased risk for demodicosis. Diagnosis can be made on the basis of clinical history and a potassium hydroxide (KOH) preparation that demonstrates many mites. Demodex mites are also present in those with normal skin, but at a lower concentration.\nSimilar lesions have been seen in patients treated with EGFR inhibitors, so a detailed disease-course, medication, and medical history is necessary. Keratosis pilaris rubra faciei can occur as a manifestation of keratosis pilaris, with rough, erythematous, symmetric triangular patches of skin on both cheeks, at times with overlying 1 to 2 mm follicular-based papules. The presence of keratosis pilaris and a lack of pustules can guide diagnosis.\n\n ## Figures\n\n **Figure 6.** \n \n\n\n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 55-Year-Old Woman With Bumps on Her Face"
},
{
"authors": "Amin Esfahani, MD; Mary E. Lohman, BA; Anne Laumann, MBChB, MRCP(UK)",
"content": [
"Treatment for rosacea depends on the type of clinical signs and symptoms and their severity. In general, behavioral modifications are necessary to lessen flares. Patients should avoid triggers (if they exist), undertake gentle skin care—with the frequent use of emollients (which help protect skin barrier and minimize symptoms of irritation) and with lukewarm water and mild cleansers—and avoid irritating topical products, such as toners, astringents, and chemical exfoliating agents. Sun protection is important because it can help minimize flares of facial redness.[1]",
"The initial approach for the treatment of erythematotelangiectatic rosacea includes the aforementioned behavioral modifications. However, for patients who fail to improve or who have more severe involvement, pharmacologic and laser/light therapy are options. Laser and light therapy target the vascular features of rosacea. The most commonly used devices are pulsed dye and potassium titanyl phosphate (KTP) lasers and intense pulsed light. Retreatment with lasers might be required. Currently, evidence supporting the pharmacologic treatment of facial erythema in rosacea is limited. Topical brimonidine, a vasoconstrictive alpha-2 adrenergic receptor agonist, has shown benefit in a small percentage of patients. However, some patients have reported severe rebound erythema several hours after application.[1,10,11] Topically applied oxymetazoline cream is another option to treat erythema.",
"In addition to behavioral modifications, papulopustular rosacea requires topical and systemic medications. For mild to moderate disease, first-line therapy includes topical metronidazole, azelaic acid, or ivermectin. Topical metronidazole once daily has been shown to be effective because of a combination of antimicrobial, anti-inflammatory, and antioxidant properties. The efficacy of treatment appears to be similar for different vehicles and strengths (0.75% vs 1%). Azelaic acid is a naturally occurring dicarboxylic acid with antioxidant and anti-inflammatory properties. Recommended dosing is twice daily, but evidence suggests that once-daily use can be effective. Ivermectin 1% cream once daily has been shown to be effective in the management of papulopustular rosacea. Topical ivermectin has both antiparasitic and anti-inflammatory properties.",
"In addition to the aforementioned agents, sulfacetamide–sulfur wash is often recommended, although the mechanism of action is not known. For moderate to severe disease, first-line therapy is an oral tetracycline. Traditionally, the most common treatment has been doxycycline or minocycline 100 mg twice daily. Response time is generally 4-12 weeks. Sub-antimicrobial dosing, such as doxycycline 20 mg twice daily, can be effective. Macrolide antibiotics have also been used, albeit less frequently.",
"For those with refractive disease, isotretinoin is an option. However, given the chronic nature of rosacea, relapse is frequent, necessitating maintenance therapy. This is usually achieved with topical or systemic therapy (either sub-antimicrobial doses of tetracyclines or short full-dose bursts during flares). Similarly, ocular rosacea can be treated with a combination of topical and systemic antibiotics.[1,12,13]",
"Treatment of phymatous rosacea includes ablative laser or surgical debulking.[1]",
"Rosacea is not considered to be a cutaneous manifestation of a systemic disorder. A few studies have linked rosacea to systemic disorders, such as dyslipidemia, hypertension, migraines and other central nervous system disorders, and autoimmune disease. However, the current evidence is not strong enough to recommend further workups in patients with rosacea.",
"This case was a diagnostic challenge because rosacea is rarely seen in skin of color and is, therefore, often overlooked. This can lead to unnecessary workups and referrals. In this patient, a diagnosis of lupus was unlikely due to a lack of associated signs and the presence of papules and pustules. Although seborrheic dermatitis is commonly seen in skin of color, this patient did not have the typical greasy, yellow scale or perinasal, retroauricular, scalp, or eyebrow involvement. Similarly, the history and location of the lesions did not support periorificial dermatitis. One diagnosis that should not be overlooked in a middle-aged African-American patient presenting with facial papules is sarcoidosis. But the pustules, the relapsing and superficial nature of lesions, and the history contradict this diagnosis."
],
"date": "October 11, 2017",
"figures": [],
"markdown": "# A 55-Year-Old Woman With Bumps on Her Face\n\n **Authors:** Amin Esfahani, MD; Mary E. Lohman, BA; Anne Laumann, MBChB, MRCP(UK) \n **Date:** October 11, 2017\n\n ## Content\n\n Treatment for rosacea depends on the type of clinical signs and symptoms and their severity. In general, behavioral modifications are necessary to lessen flares. Patients should avoid triggers (if they exist), undertake gentle skin care—with the frequent use of emollients (which help protect skin barrier and minimize symptoms of irritation) and with lukewarm water and mild cleansers—and avoid irritating topical products, such as toners, astringents, and chemical exfoliating agents. Sun protection is important because it can help minimize flares of facial redness.[1]\nThe initial approach for the treatment of erythematotelangiectatic rosacea includes the aforementioned behavioral modifications. However, for patients who fail to improve or who have more severe involvement, pharmacologic and laser/light therapy are options. Laser and light therapy target the vascular features of rosacea. The most commonly used devices are pulsed dye and potassium titanyl phosphate (KTP) lasers and intense pulsed light. Retreatment with lasers might be required. Currently, evidence supporting the pharmacologic treatment of facial erythema in rosacea is limited. Topical brimonidine, a vasoconstrictive alpha-2 adrenergic receptor agonist, has shown benefit in a small percentage of patients. However, some patients have reported severe rebound erythema several hours after application.[1,10,11] Topically applied oxymetazoline cream is another option to treat erythema.\nIn addition to behavioral modifications, papulopustular rosacea requires topical and systemic medications. For mild to moderate disease, first-line therapy includes topical metronidazole, azelaic acid, or ivermectin. Topical metronidazole once daily has been shown to be effective because of a combination of antimicrobial, anti-inflammatory, and antioxidant properties. The efficacy of treatment appears to be similar for different vehicles and strengths (0.75% vs 1%). Azelaic acid is a naturally occurring dicarboxylic acid with antioxidant and anti-inflammatory properties. Recommended dosing is twice daily, but evidence suggests that once-daily use can be effective. Ivermectin 1% cream once daily has been shown to be effective in the management of papulopustular rosacea. Topical ivermectin has both antiparasitic and anti-inflammatory properties.\nIn addition to the aforementioned agents, sulfacetamide–sulfur wash is often recommended, although the mechanism of action is not known. For moderate to severe disease, first-line therapy is an oral tetracycline. Traditionally, the most common treatment has been doxycycline or minocycline 100 mg twice daily. Response time is generally 4-12 weeks. Sub-antimicrobial dosing, such as doxycycline 20 mg twice daily, can be effective. Macrolide antibiotics have also been used, albeit less frequently.\nFor those with refractive disease, isotretinoin is an option. However, given the chronic nature of rosacea, relapse is frequent, necessitating maintenance therapy. This is usually achieved with topical or systemic therapy (either sub-antimicrobial doses of tetracyclines or short full-dose bursts during flares). Similarly, ocular rosacea can be treated with a combination of topical and systemic antibiotics.[1,12,13]\nTreatment of phymatous rosacea includes ablative laser or surgical debulking.[1]\nRosacea is not considered to be a cutaneous manifestation of a systemic disorder. A few studies have linked rosacea to systemic disorders, such as dyslipidemia, hypertension, migraines and other central nervous system disorders, and autoimmune disease. However, the current evidence is not strong enough to recommend further workups in patients with rosacea.\nThis case was a diagnostic challenge because rosacea is rarely seen in skin of color and is, therefore, often overlooked. This can lead to unnecessary workups and referrals. In this patient, a diagnosis of lupus was unlikely due to a lack of associated signs and the presence of papules and pustules. Although seborrheic dermatitis is commonly seen in skin of color, this patient did not have the typical greasy, yellow scale or perinasal, retroauricular, scalp, or eyebrow involvement. Similarly, the history and location of the lesions did not support periorificial dermatitis. One diagnosis that should not be overlooked in a middle-aged African-American patient presenting with facial papules is sarcoidosis. But the pustules, the relapsing and superficial nature of lesions, and the history contradict this diagnosis.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1018752,
"choiceText": "It is considered \"end-stage\" rosacea",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1018754,
"choiceText": "It involves nodular hypertrophy of sebaceous glands",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1018756,
"choiceText": "It occurs more commonly in postmenopausal women",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1018758,
"choiceText": "Treatment includes topical and systemic antimicrobials\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Rhinophyma is the most common presentation of phymatous rosacea. It is more common in men, and results in hypertrophy of the skin, including sebaceous glands. Rhinophyma does not represent end-stage rosacea; it can occur de novo and, in many cases, in mild disease. Phymatous rosacea is difficult to treat because it is not responsive to topical or systemic antimicrobials. Early lesions might respond to isotretinoin, but advanced lesions require treatment with ablative laser therapy or surgical debulking.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 322427,
"questionText": "Which of the following is accurate regarding rhinophyma?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1018760,
"choiceText": "Perform a skin biopsy to rule out cutaneous metastasis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1018762,
"choiceText": "Contact the patient's oncologist to discuss changing the treatment regimen",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1018764,
"choiceText": "Treat the patient with doxycycline 100 mg twice daily",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1018766,
"choiceText": "Recommend fumigation for possible infestation\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "On the basis of the clinical information available, this patient is suffering from papulopustular eruption secondary to cetuximab (an EGFR inhibitor). More than 90% of patients undergoing treatment develop a similar eruption 1 to 3 weeks after the start of therapy. Evidence suggests that the eruption correlates with the medication's antitumor activity; thus, it is not appropriate to change treatment. Cutaneous metastasis generally does not appear as pustules. Lesions are typically infiltrated and do not appear to be superficial. On the basis of location, morphology, and history, the current eruption does not support reaction to an arthropod bite. Given the severity of rash (which interferes with daily activities), if no contraindications exist, the patient can be treated with topical or systemic antimicrobials.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 322429,
"questionText": "A 54-year-old male with metastatic colorectal cancer currently being treated with cetuximab develops a superficial papulopustular eruption across the face and torso 2 weeks into therapy. The patient is uncomfortable and states that the rash interferes with daily activities. Which of the following is an appropriate next step?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 55-Year-Old Woman With Bumps on Her Face"
},
{
"authors": "Amin Esfahani, MD; Mary E. Lohman, BA; Anne Laumann, MBChB, MRCP(UK)",
"content": [],
"date": "October 11, 2017",
"figures": [],
"markdown": "# A 55-Year-Old Woman With Bumps on Her Face\n\n **Authors:** Amin Esfahani, MD; Mary E. Lohman, BA; Anne Laumann, MBChB, MRCP(UK) \n **Date:** October 11, 2017\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1018752,
"choiceText": "It is considered \"end-stage\" rosacea",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1018754,
"choiceText": "It involves nodular hypertrophy of sebaceous glands",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1018756,
"choiceText": "It occurs more commonly in postmenopausal women",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1018758,
"choiceText": "Treatment includes topical and systemic antimicrobials\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Rhinophyma is the most common presentation of phymatous rosacea. It is more common in men, and results in hypertrophy of the skin, including sebaceous glands. Rhinophyma does not represent end-stage rosacea; it can occur de novo and, in many cases, in mild disease. Phymatous rosacea is difficult to treat because it is not responsive to topical or systemic antimicrobials. Early lesions might respond to isotretinoin, but advanced lesions require treatment with ablative laser therapy or surgical debulking.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 322427,
"questionText": "Which of the following is accurate regarding rhinophyma?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1018760,
"choiceText": "Perform a skin biopsy to rule out cutaneous metastasis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1018762,
"choiceText": "Contact the patient's oncologist to discuss changing the treatment regimen",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1018764,
"choiceText": "Treat the patient with doxycycline 100 mg twice daily",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1018766,
"choiceText": "Recommend fumigation for possible infestation\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "On the basis of the clinical information available, this patient is suffering from papulopustular eruption secondary to cetuximab (an EGFR inhibitor). More than 90% of patients undergoing treatment develop a similar eruption 1 to 3 weeks after the start of therapy. Evidence suggests that the eruption correlates with the medication's antitumor activity; thus, it is not appropriate to change treatment. Cutaneous metastasis generally does not appear as pustules. Lesions are typically infiltrated and do not appear to be superficial. On the basis of location, morphology, and history, the current eruption does not support reaction to an arthropod bite. Given the severity of rash (which interferes with daily activities), if no contraindications exist, the patient can be treated with topical or systemic antimicrobials.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 322429,
"questionText": "A 54-year-old male with metastatic colorectal cancer currently being treated with cetuximab develops a superficial papulopustular eruption across the face and torso 2 weeks into therapy. The patient is uncomfortable and states that the rash interferes with daily activities. Which of the following is an appropriate next step?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 55-Year-Old Woman With Bumps on Her Face"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1018742,
"choiceText": "Cutaneous sarcoidosis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1018744,
"choiceText": "Cutaneous lupus erythematous ",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1018746,
"choiceText": "Seborrheic dermatitis ",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1018748,
"choiceText": "Rosacea (papulopustular)",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1018750,
"choiceText": "Periorificial dermatitis\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 322425,
"questionText": "What is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1018752,
"choiceText": "It is considered \"end-stage\" rosacea",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1018754,
"choiceText": "It involves nodular hypertrophy of sebaceous glands",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1018756,
"choiceText": "It occurs more commonly in postmenopausal women",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1018758,
"choiceText": "Treatment includes topical and systemic antimicrobials\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Rhinophyma is the most common presentation of phymatous rosacea. It is more common in men, and results in hypertrophy of the skin, including sebaceous glands. Rhinophyma does not represent end-stage rosacea; it can occur de novo and, in many cases, in mild disease. Phymatous rosacea is difficult to treat because it is not responsive to topical or systemic antimicrobials. Early lesions might respond to isotretinoin, but advanced lesions require treatment with ablative laser therapy or surgical debulking.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 322427,
"questionText": "Which of the following is accurate regarding rhinophyma?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1018760,
"choiceText": "Perform a skin biopsy to rule out cutaneous metastasis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1018762,
"choiceText": "Contact the patient's oncologist to discuss changing the treatment regimen",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1018764,
"choiceText": "Treat the patient with doxycycline 100 mg twice daily",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1018766,
"choiceText": "Recommend fumigation for possible infestation\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "On the basis of the clinical information available, this patient is suffering from papulopustular eruption secondary to cetuximab (an EGFR inhibitor). More than 90% of patients undergoing treatment develop a similar eruption 1 to 3 weeks after the start of therapy. Evidence suggests that the eruption correlates with the medication's antitumor activity; thus, it is not appropriate to change treatment. Cutaneous metastasis generally does not appear as pustules. Lesions are typically infiltrated and do not appear to be superficial. On the basis of location, morphology, and history, the current eruption does not support reaction to an arthropod bite. Given the severity of rash (which interferes with daily activities), if no contraindications exist, the patient can be treated with topical or systemic antimicrobials.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 322429,
"questionText": "A 54-year-old male with metastatic colorectal cancer currently being treated with cetuximab develops a superficial papulopustular eruption across the face and torso 2 weeks into therapy. The patient is uncomfortable and states that the rash interferes with daily activities. Which of the following is an appropriate next step?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
868590 | /viewarticle/868590 | [
{
"authors": "Roshen Mathew, MBBS; Mathew Abraham, MD, DM",
"content": [
"Editor's Note:\n\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.",
"A 33-year-old woman presents to the emergency department with a severe headache that started suddenly 6 hours earlier. She states that it is the worst headache of her life, that she does not usually get headaches, and that the pain has been worsening despite her use of oral analgesic medication (namely, acetaminophen). She has had associated vomiting, both at home and since arrival at the emergency department.",
"Her headache is associated with mild blurred vision and weakness in her left leg that started about an hour ago. She denies having any fever, loose stools, seizures, or other visual symptoms (such as floaters, fortification, or loss in visual acuity).",
"Her medical history is unremarkable, with no hospitalizations other than for an uncomplicated delivery 8 years ago. Her only medication, other than the recent use of acetaminophen, is an oral contraceptive pill that she has taken for the past 4 years. She denies using any tobacco, alcohol, or recreational drugs."
],
"date": "September 12, 2016",
"figures": [],
"markdown": "# Sudden Headache and Vomiting in a 33-Year-Old Woman\n\n **Authors:** Roshen Mathew, MBBS; Mathew Abraham, MD, DM \n **Date:** September 12, 2016\n\n ## Content\n\n Editor's Note:\n\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.\nA 33-year-old woman presents to the emergency department with a severe headache that started suddenly 6 hours earlier. She states that it is the worst headache of her life, that she does not usually get headaches, and that the pain has been worsening despite her use of oral analgesic medication (namely, acetaminophen). She has had associated vomiting, both at home and since arrival at the emergency department.\nHer headache is associated with mild blurred vision and weakness in her left leg that started about an hour ago. She denies having any fever, loose stools, seizures, or other visual symptoms (such as floaters, fortification, or loss in visual acuity).\nHer medical history is unremarkable, with no hospitalizations other than for an uncomplicated delivery 8 years ago. Her only medication, other than the recent use of acetaminophen, is an oral contraceptive pill that she has taken for the past 4 years. She denies using any tobacco, alcohol, or recreational drugs.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "Sudden Headache and Vomiting in a 33-Year-Old Woman"
},
{
"authors": "Roshen Mathew, MBBS; Mathew Abraham, MD, DM",
"content": [
"Upon physical examination, the patient appears well nourished but mildly dehydrated. She is tearful and states that the intensity of her headache is currently 7 on a scale of 1-10 (with 1 being no pain and 10 being the highest intensity). She is alert and oriented to her surroundings. Her vitals show a temperature of 98.7°F(37.1°C), a blood pressure of 140/90 mm Hg, and a heart rate of 90 beats/min.",
"Figure 1.",
"Figure 1.",
"Examination of the head and neck is normal, with good range of motion, no focal tenderness to palpation, and no meningeal signs. The pupils show a slightly sluggish reaction bilaterally to light. The patient's lungs are clear to auscultation, with normal breath sounds. She has normal S1 and S2 heart sounds. No murmurs or clicks are heard on auscultation. She has a soft and nontender abdomen.",
"Upon neurologic examination, the patient's mental status is normal. The cranial nerves are normal, except for bilateral papilledema noted on funduscopic examination. In the extremities, motor strength is rated at 4 of 5 in the left lower extremity and 5 of 5 in the right lower and bilateral upper extremities (on a scale of 1-5, with 5 being normal strength). Deep tendon reflexes are difficult to elicit, but they are symmetric bilaterally, with normal plantar reflexes. Her sensation is intact bilaterally to pain, touch, and vibration. Finger-to-nose testing does not show past pointing, and her gait is not ataxic.",
"Laboratory investigations, including a complete blood cell count, erythrocyte sedimentation rate, and basic metabolic profile, are all within normal limits. A urine pregnancy test is negative. CT and MRI of the brain are normal. A magnetic resonance venogram (MRV) is obtained (Figure 1)."
],
"date": "September 12, 2016",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/868/590/868590-Thumb1.png"
}
],
"markdown": "# Sudden Headache and Vomiting in a 33-Year-Old Woman\n\n **Authors:** Roshen Mathew, MBBS; Mathew Abraham, MD, DM \n **Date:** September 12, 2016\n\n ## Content\n\n Upon physical examination, the patient appears well nourished but mildly dehydrated. She is tearful and states that the intensity of her headache is currently 7 on a scale of 1-10 (with 1 being no pain and 10 being the highest intensity). She is alert and oriented to her surroundings. Her vitals show a temperature of 98.7°F(37.1°C), a blood pressure of 140/90 mm Hg, and a heart rate of 90 beats/min.\nFigure 1.\nFigure 1.\nExamination of the head and neck is normal, with good range of motion, no focal tenderness to palpation, and no meningeal signs. The pupils show a slightly sluggish reaction bilaterally to light. The patient's lungs are clear to auscultation, with normal breath sounds. She has normal S1 and S2 heart sounds. No murmurs or clicks are heard on auscultation. She has a soft and nontender abdomen.\nUpon neurologic examination, the patient's mental status is normal. The cranial nerves are normal, except for bilateral papilledema noted on funduscopic examination. In the extremities, motor strength is rated at 4 of 5 in the left lower extremity and 5 of 5 in the right lower and bilateral upper extremities (on a scale of 1-5, with 5 being normal strength). Deep tendon reflexes are difficult to elicit, but they are symmetric bilaterally, with normal plantar reflexes. Her sensation is intact bilaterally to pain, touch, and vibration. Finger-to-nose testing does not show past pointing, and her gait is not ataxic.\nLaboratory investigations, including a complete blood cell count, erythrocyte sedimentation rate, and basic metabolic profile, are all within normal limits. A urine pregnancy test is negative. CT and MRI of the brain are normal. A magnetic resonance venogram (MRV) is obtained (Figure 1).\n\n ## Figures\n\n **Figure 1.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1010425,
"choiceText": "Brain tumor",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1010427,
"choiceText": "Bacterial meningitis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1010429,
"choiceText": "Cerebral venous thrombosis (CVT)",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1010431,
"choiceText": "Subarachnoid hemorrhage\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 319657,
"questionText": "What is the most likely diagnosis? <br><br><i>\r\nHint: Oral contraceptives are a recognized risk factor for this condition.</i>\r\n",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Sudden Headache and Vomiting in a 33-Year-Old Woman"
},
{
"authors": "Roshen Mathew, MBBS; Mathew Abraham, MD, DM",
"content": [
"This patient had an MRV that showed a superior sagittal sinus thrombosis. Lack of signal from the distal portion of the superior sagittal sinus was consistent with cerebral venous thrombosis (Figures 2 and 3), as was the patient's clinical presentation. Workup for a hypercoagulable state was negative.",
"Figure 2.",
"Figure 2.",
"Figure 3.",
"Figure 3.",
"CVT should be considered in any patient presenting with severe headache and vomiting when no other cause is identified, especially when papilledema is identified on ophthalmoscopy. Risk factors for CVT include:",
"Trauma (head injury)",
"Iatrogenic causes (craniotomy, jugular catheterization, lumbar puncture)",
"Systemic conditions (pregnancy, puerperium, dehydration)",
"Infections (otitis, sinusitis)",
"Inherited or acquired hypercoagulable states (protein C or S deficiency, antiphospholipid antibody syndrome, antithrombin III deficiency, factor V Leiden)",
"Collagen vascular diseases (systemic lupus erythematosus, Wegener granulomatosis)",
"Hematologic conditions (polycythemia, paroxysmal nocturnal hemoglobinuria, sickle cell disease)",
"Medications (oral contraceptives, hormone therapy, steroids)",
"However, no risk factors are identified in 14% of patients diagnosed with CVT.[1,2,3]",
"The typical pathophysiology in CVT is partial or complete occlusion of a cerebral venous sinus, which causes increased intracranial pressure and papilledema. The most frequently involved venous sinuses are the superior sagittal sinus or either of the paired transverse sinuses. If a thrombus extends into the cortical veins, dilatation of veins and capillary beds can occur, which then can lead to venous infarction, cerebral edema, and intraparenchymal hematoma formation. Seizures or cerebral herniation are potentially fatal complications.[2]",
"The clinical presentation of CVT is usually a severe headache that is aggravated by head movements, sneezing, or coughing. The headache is typically subacute and gradual in onset, although thunderclap headache occurs in 2%-10% of cases (as in this patient). Nausea and vomiting are often present, and severe cases are marked by a decreasing level of consciousness that may result from either a postictal state due to seizures or from cerebral swelling with or without herniation. Focal neurologic findings, including hemiparesis or isolated unilateral lower-extremity weakness, can be seen when a thrombus extends into the cortical veins. This is especially common in CVT of the superior sagittal sinus.",
"If the cavernous sinus is involved (which is associated almost exclusively with infection of the paranasal sinuses), proptosis and chemosis with ipsilateral periorbital edema, retinal hemorrhages, papilledema, extraocular movement abnormalities, and sensory loss in the V1 or V2 distribution of the trigeminal nerve may be seen. Isolated or multiple cranial nerve palsies (III, VII, VIII) have been reported in patients with unilateral occlusion of the transverse or sigmoid sinuses. This unusual presentation has been explained by venous congestion of the ventral pontine and lateral medullary veins. If the thrombus extends into the jugular bulb, a jugular foramen syndrome, with involvement of cranial nerves IX, X and XI, may be seen."
],
"date": "September 12, 2016",
"figures": [
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/868/590/868590-Thumb2.png"
},
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/868/590/868590-Thumb3.png"
}
],
"markdown": "# Sudden Headache and Vomiting in a 33-Year-Old Woman\n\n **Authors:** Roshen Mathew, MBBS; Mathew Abraham, MD, DM \n **Date:** September 12, 2016\n\n ## Content\n\n This patient had an MRV that showed a superior sagittal sinus thrombosis. Lack of signal from the distal portion of the superior sagittal sinus was consistent with cerebral venous thrombosis (Figures 2 and 3), as was the patient's clinical presentation. Workup for a hypercoagulable state was negative.\nFigure 2.\nFigure 2.\nFigure 3.\nFigure 3.\nCVT should be considered in any patient presenting with severe headache and vomiting when no other cause is identified, especially when papilledema is identified on ophthalmoscopy. Risk factors for CVT include:\nTrauma (head injury)\nIatrogenic causes (craniotomy, jugular catheterization, lumbar puncture)\nSystemic conditions (pregnancy, puerperium, dehydration)\nInfections (otitis, sinusitis)\nInherited or acquired hypercoagulable states (protein C or S deficiency, antiphospholipid antibody syndrome, antithrombin III deficiency, factor V Leiden)\nCollagen vascular diseases (systemic lupus erythematosus, Wegener granulomatosis)\nHematologic conditions (polycythemia, paroxysmal nocturnal hemoglobinuria, sickle cell disease)\nMedications (oral contraceptives, hormone therapy, steroids)\nHowever, no risk factors are identified in 14% of patients diagnosed with CVT.[1,2,3]\nThe typical pathophysiology in CVT is partial or complete occlusion of a cerebral venous sinus, which causes increased intracranial pressure and papilledema. The most frequently involved venous sinuses are the superior sagittal sinus or either of the paired transverse sinuses. If a thrombus extends into the cortical veins, dilatation of veins and capillary beds can occur, which then can lead to venous infarction, cerebral edema, and intraparenchymal hematoma formation. Seizures or cerebral herniation are potentially fatal complications.[2]\nThe clinical presentation of CVT is usually a severe headache that is aggravated by head movements, sneezing, or coughing. The headache is typically subacute and gradual in onset, although thunderclap headache occurs in 2%-10% of cases (as in this patient). Nausea and vomiting are often present, and severe cases are marked by a decreasing level of consciousness that may result from either a postictal state due to seizures or from cerebral swelling with or without herniation. Focal neurologic findings, including hemiparesis or isolated unilateral lower-extremity weakness, can be seen when a thrombus extends into the cortical veins. This is especially common in CVT of the superior sagittal sinus.\nIf the cavernous sinus is involved (which is associated almost exclusively with infection of the paranasal sinuses), proptosis and chemosis with ipsilateral periorbital edema, retinal hemorrhages, papilledema, extraocular movement abnormalities, and sensory loss in the V1 or V2 distribution of the trigeminal nerve may be seen. Isolated or multiple cranial nerve palsies (III, VII, VIII) have been reported in patients with unilateral occlusion of the transverse or sigmoid sinuses. This unusual presentation has been explained by venous congestion of the ventral pontine and lateral medullary veins. If the thrombus extends into the jugular bulb, a jugular foramen syndrome, with involvement of cranial nerves IX, X and XI, may be seen.\n\n ## Figures\n\n **Figure 2.** \n \n\n**Figure 3.** \n \n\n\n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1010425,
"choiceText": "Brain tumor",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1010427,
"choiceText": "Bacterial meningitis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1010429,
"choiceText": "Cerebral venous thrombosis (CVT)",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1010431,
"choiceText": "Subarachnoid hemorrhage\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 319657,
"questionText": "What is the most likely diagnosis? <br><br><i>\r\nHint: Oral contraceptives are a recognized risk factor for this condition.</i>\r\n",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Sudden Headache and Vomiting in a 33-Year-Old Woman"
},
{
"authors": "Roshen Mathew, MBBS; Mathew Abraham, MD, DM",
"content": [
"The physical examination is normal in 15%-30% of patients, although careful examination may reveal loss of normal spontaneous retinal venous pulsations. It has been reported that headache (89%), paresis (37%), generalized (30%) or focal (20%) seizures, papilledema (28%), and mental status changes (22%) are the most frequent presenting symptoms and signs associated with CVT.[1,2,3,4]",
"The differential diagnosis of CVT includes subarachnoid hemorrhage, intracranial hemorrhage, ischemic stroke, and bacterial meningitis. In patients presenting with the more typical subacute onset, pseudotumor cerebri, cavernous sinus syndromes, intracranial abscess, subdural empyema, and brain tumor should be considered.[2,3]",
"CVT is definitively diagnosed by demonstration of a thrombus on neuroimaging. Non–contrast-enhanced CT of the head is normal in 25% of patients with a normal examination and 10% of patients with focal neurologic findings (including papilledema). The delta sign is a dense triangle in the superior sagittal sinus caused by the thrombus, and it may be seen on CT.",
"MRV is considered the radiographic investigation of choice and will demonstrate areas of lack of signal where the thrombus involves the venous sinuses. Cerebral angiography may be required if MRV is nondiagnostic and suspicion remains high. CT venography is increasingly being used to diagnose CVT, and it has been shown to be very sensitive and specific. The empty delta sign is a triangular filling defect in the superior sagittal sinus that complements the delta sign on non–contrast-enhanced CT.",
"Lumbar punctures are sometimes performed in patients with no evidence of mass effect on CT. About 50% of patients have abnormal cerebrospinal fluid (CSF) findings, including mild lymphocytic pleocytosis; elevated protein levels; the presence of red blood cells; and, more commonly, an elevated CSF opening pressure (which must be measured with the patient as relaxed as possible in the lateral decubitus position).[1,3,5,6]"
],
"date": "September 12, 2016",
"figures": [],
"markdown": "# Sudden Headache and Vomiting in a 33-Year-Old Woman\n\n **Authors:** Roshen Mathew, MBBS; Mathew Abraham, MD, DM \n **Date:** September 12, 2016\n\n ## Content\n\n The physical examination is normal in 15%-30% of patients, although careful examination may reveal loss of normal spontaneous retinal venous pulsations. It has been reported that headache (89%), paresis (37%), generalized (30%) or focal (20%) seizures, papilledema (28%), and mental status changes (22%) are the most frequent presenting symptoms and signs associated with CVT.[1,2,3,4]\nThe differential diagnosis of CVT includes subarachnoid hemorrhage, intracranial hemorrhage, ischemic stroke, and bacterial meningitis. In patients presenting with the more typical subacute onset, pseudotumor cerebri, cavernous sinus syndromes, intracranial abscess, subdural empyema, and brain tumor should be considered.[2,3]\nCVT is definitively diagnosed by demonstration of a thrombus on neuroimaging. Non–contrast-enhanced CT of the head is normal in 25% of patients with a normal examination and 10% of patients with focal neurologic findings (including papilledema). The delta sign is a dense triangle in the superior sagittal sinus caused by the thrombus, and it may be seen on CT.\nMRV is considered the radiographic investigation of choice and will demonstrate areas of lack of signal where the thrombus involves the venous sinuses. Cerebral angiography may be required if MRV is nondiagnostic and suspicion remains high. CT venography is increasingly being used to diagnose CVT, and it has been shown to be very sensitive and specific. The empty delta sign is a triangular filling defect in the superior sagittal sinus that complements the delta sign on non–contrast-enhanced CT.\nLumbar punctures are sometimes performed in patients with no evidence of mass effect on CT. About 50% of patients have abnormal cerebrospinal fluid (CSF) findings, including mild lymphocytic pleocytosis; elevated protein levels; the presence of red blood cells; and, more commonly, an elevated CSF opening pressure (which must be measured with the patient as relaxed as possible in the lateral decubitus position).[1,3,5,6]\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "Sudden Headache and Vomiting in a 33-Year-Old Woman"
},
{
"authors": "Roshen Mathew, MBBS; Mathew Abraham, MD, DM",
"content": [
"The mainstay of treatment for CVT, even in patients with CT evidence of cerebral ischemia or hemorrhage, is anticoagulation. In two retrospective studies of patients with CVT and moderate-sized hematomas, anticoagulation was not associated with increased hemorrhage volume, neurologic deterioration, or a worse outcome. Heparin should be started initially, with a goal of maintaining an activated partial thromboplastin time that is twice the control value. Heparin therapy is gradually transitioned to warfarin, which is then continued for 4-6 months (longer in patients with an identified predisposition to clotting).",
"Endovascular thrombolytic therapy with urokinase or tissue plasminogen activator may be effective in patients who deteriorate despite adequate anticoagulation with heparin, but this therapy is limited to specialized centers. Surgical intervention, in the form of thrombectomy using a microsnare or rheolytic thrombectomy catheter with local thrombolytic therapy, has been used with variable success in the setting of severe neurologic deterioration.",
"Adjunctive therapy for seizures, cerebral edema, or co-occurring infections may also be required on a case-by-case basis. Headaches related to increased intracranial pressure may respond to elevation of the head of the bed or to acetazolamide, which decreases CSF production (thereby lowering intracranial pressure). When complicated by visual deterioration unresponsive to these measures, lumboperitoneal shunting or optic nerve sheath fenestration should be considered.[1,2,6]",
"Complications of CVT may include coma (resulting from status epilepticus, critically elevated intracranial pressure, or impending cerebral herniation) and pulmonary embolism. In patients who are comatose as a result of increased intracranial pressure, prompt intervention is critical. Initial measures include maintaining the patient's head at 30°-40° elevation, keeping the neck in a neutral position to avoid kinking of the jugular veins, and using mannitol or hyperventilation. Additional therapy, such as ventriculostomy and blood pressure titration with vasoactive agents, should be guided by direct monitoring of intracranial pressure.",
"Pulmonary embolisms occur in up to 11% of cases and may originate from the thrombosed jugular veins or from other sites (such as the legs). This complication carries a high mortality rate.",
"The prognosis is good for patients with CVT that is recognized early. Full recovery is expected in about 70% of cases. Of the remaining 30% of patients, about one third die and two thirds are left with persistent mild to moderate neurologic deficits. Coma, seizures, or underlying disease have no significant effect on the short-term outcome and should not preclude any type of therapeutic intervention. The long-term recurrence rate of CVT is approximately 20%.",
"Oral contraceptives are a recognized risk factor for CVT; this is supported by the increasing rate of CVT in women of childbearing age since the introduction of oral contraceptives, with stable rates seen in men of similar age.[2]",
"In the case presented above, the patient was treated with analgesics and low-molecular-weight heparin, followed by warfarin. She discontinued use of her oral contraceptive pills. The patient recovered completely, with no residual deficits, and she continues to do well on long-term follow-up."
],
"date": "September 12, 2016",
"figures": [],
"markdown": "# Sudden Headache and Vomiting in a 33-Year-Old Woman\n\n **Authors:** Roshen Mathew, MBBS; Mathew Abraham, MD, DM \n **Date:** September 12, 2016\n\n ## Content\n\n The mainstay of treatment for CVT, even in patients with CT evidence of cerebral ischemia or hemorrhage, is anticoagulation. In two retrospective studies of patients with CVT and moderate-sized hematomas, anticoagulation was not associated with increased hemorrhage volume, neurologic deterioration, or a worse outcome. Heparin should be started initially, with a goal of maintaining an activated partial thromboplastin time that is twice the control value. Heparin therapy is gradually transitioned to warfarin, which is then continued for 4-6 months (longer in patients with an identified predisposition to clotting).\nEndovascular thrombolytic therapy with urokinase or tissue plasminogen activator may be effective in patients who deteriorate despite adequate anticoagulation with heparin, but this therapy is limited to specialized centers. Surgical intervention, in the form of thrombectomy using a microsnare or rheolytic thrombectomy catheter with local thrombolytic therapy, has been used with variable success in the setting of severe neurologic deterioration.\nAdjunctive therapy for seizures, cerebral edema, or co-occurring infections may also be required on a case-by-case basis. Headaches related to increased intracranial pressure may respond to elevation of the head of the bed or to acetazolamide, which decreases CSF production (thereby lowering intracranial pressure). When complicated by visual deterioration unresponsive to these measures, lumboperitoneal shunting or optic nerve sheath fenestration should be considered.[1,2,6]\nComplications of CVT may include coma (resulting from status epilepticus, critically elevated intracranial pressure, or impending cerebral herniation) and pulmonary embolism. In patients who are comatose as a result of increased intracranial pressure, prompt intervention is critical. Initial measures include maintaining the patient's head at 30°-40° elevation, keeping the neck in a neutral position to avoid kinking of the jugular veins, and using mannitol or hyperventilation. Additional therapy, such as ventriculostomy and blood pressure titration with vasoactive agents, should be guided by direct monitoring of intracranial pressure.\nPulmonary embolisms occur in up to 11% of cases and may originate from the thrombosed jugular veins or from other sites (such as the legs). This complication carries a high mortality rate.\nThe prognosis is good for patients with CVT that is recognized early. Full recovery is expected in about 70% of cases. Of the remaining 30% of patients, about one third die and two thirds are left with persistent mild to moderate neurologic deficits. Coma, seizures, or underlying disease have no significant effect on the short-term outcome and should not preclude any type of therapeutic intervention. The long-term recurrence rate of CVT is approximately 20%.\nOral contraceptives are a recognized risk factor for CVT; this is supported by the increasing rate of CVT in women of childbearing age since the introduction of oral contraceptives, with stable rates seen in men of similar age.[2]\nIn the case presented above, the patient was treated with analgesics and low-molecular-weight heparin, followed by warfarin. She discontinued use of her oral contraceptive pills. The patient recovered completely, with no residual deficits, and she continues to do well on long-term follow-up.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1010441,
"choiceText": "Aspirin and warfarin",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1010443,
"choiceText": "Measures to reduce intracranial pressure",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1010445,
"choiceText": "Heparin and warfarin",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1010447,
"choiceText": "Immediate rheolytic thrombectomy",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1010449,
"choiceText": "Close observation in a critical care unit without pharmacologic intervention",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Heparin should be started initially, with a goal of maintaining an activated partial thromboplastin time that is twice the control value. Heparin therapy is gradually transitioned to warfarin, which is then continued for 4-6 months (longer in patients with an identified predisposition to clotting).",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 319661,
"questionText": "What is the treatment of choice in patients with CVT who have a hemorrhagic infarct on CT/MRI?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1010467,
"choiceText": "Non–contrast-enhanced CT of the head",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1010469,
"choiceText": "MRV",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1010471,
"choiceText": "Cerebral angiography",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1010473,
"choiceText": "Lumbar puncture",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1010475,
"choiceText": "EEG\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "MRV is considered the radiographic investigation of choice and will demonstrate areas of lack of signal where the thrombus involves the venous sinuses.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 319667,
"questionText": "What is the investigation of choice for the diagnosis of CVT?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Sudden Headache and Vomiting in a 33-Year-Old Woman"
},
{
"authors": "Roshen Mathew, MBBS; Mathew Abraham, MD, DM",
"content": [],
"date": "September 12, 2016",
"figures": [],
"markdown": "# Sudden Headache and Vomiting in a 33-Year-Old Woman\n\n **Authors:** Roshen Mathew, MBBS; Mathew Abraham, MD, DM \n **Date:** September 12, 2016\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1010441,
"choiceText": "Aspirin and warfarin",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1010443,
"choiceText": "Measures to reduce intracranial pressure",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1010445,
"choiceText": "Heparin and warfarin",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1010447,
"choiceText": "Immediate rheolytic thrombectomy",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1010449,
"choiceText": "Close observation in a critical care unit without pharmacologic intervention",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Heparin should be started initially, with a goal of maintaining an activated partial thromboplastin time that is twice the control value. Heparin therapy is gradually transitioned to warfarin, which is then continued for 4-6 months (longer in patients with an identified predisposition to clotting).",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 319661,
"questionText": "What is the treatment of choice in patients with CVT who have a hemorrhagic infarct on CT/MRI?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1010467,
"choiceText": "Non–contrast-enhanced CT of the head",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1010469,
"choiceText": "MRV",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1010471,
"choiceText": "Cerebral angiography",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1010473,
"choiceText": "Lumbar puncture",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1010475,
"choiceText": "EEG\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "MRV is considered the radiographic investigation of choice and will demonstrate areas of lack of signal where the thrombus involves the venous sinuses.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 319667,
"questionText": "What is the investigation of choice for the diagnosis of CVT?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Sudden Headache and Vomiting in a 33-Year-Old Woman"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1010425,
"choiceText": "Brain tumor",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1010427,
"choiceText": "Bacterial meningitis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1010429,
"choiceText": "Cerebral venous thrombosis (CVT)",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1010431,
"choiceText": "Subarachnoid hemorrhage\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 319657,
"questionText": "What is the most likely diagnosis? <br><br><i>\r\nHint: Oral contraceptives are a recognized risk factor for this condition.</i>\r\n",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1010441,
"choiceText": "Aspirin and warfarin",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1010443,
"choiceText": "Measures to reduce intracranial pressure",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1010445,
"choiceText": "Heparin and warfarin",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1010447,
"choiceText": "Immediate rheolytic thrombectomy",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1010449,
"choiceText": "Close observation in a critical care unit without pharmacologic intervention",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Heparin should be started initially, with a goal of maintaining an activated partial thromboplastin time that is twice the control value. Heparin therapy is gradually transitioned to warfarin, which is then continued for 4-6 months (longer in patients with an identified predisposition to clotting).",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 319661,
"questionText": "What is the treatment of choice in patients with CVT who have a hemorrhagic infarct on CT/MRI?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1010467,
"choiceText": "Non–contrast-enhanced CT of the head",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1010469,
"choiceText": "MRV",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1010471,
"choiceText": "Cerebral angiography",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1010473,
"choiceText": "Lumbar puncture",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1010475,
"choiceText": "EEG\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "MRV is considered the radiographic investigation of choice and will demonstrate areas of lack of signal where the thrombus involves the venous sinuses.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 319667,
"questionText": "What is the investigation of choice for the diagnosis of CVT?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
868042 | /viewarticle/868042 | [
{
"authors": "Sara W. Nelson, MD; Daniel M. Lindberg, MD",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"Background",
"A 26-year-old man with an unknown past medical history arrives to the emergency department by ambulance. He had been driving his car while unrestrained and was involved in a high-speed motor vehicle collision. Airbags were deployed, and the vehicle experienced significant front-end damage, with intrusion into the passenger compartment of the car. The patient was extricated from the vehicle and placed on a backboard, and a cervical collar was placed by EMS. A nonrebreather facemask and one peripheral intravenous line were placed in the field."
],
"date": "August 31, 2016",
"figures": [],
"markdown": "# A 26-Year-Old Man Who Has Been in a Motor Vehicle Collision\n\n **Authors:** Sara W. Nelson, MD; Daniel M. Lindberg, MD \n **Date:** August 31, 2016\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nBackground\nA 26-year-old man with an unknown past medical history arrives to the emergency department by ambulance. He had been driving his car while unrestrained and was involved in a high-speed motor vehicle collision. Airbags were deployed, and the vehicle experienced significant front-end damage, with intrusion into the passenger compartment of the car. The patient was extricated from the vehicle and placed on a backboard, and a cervical collar was placed by EMS. A nonrebreather facemask and one peripheral intravenous line were placed in the field.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 26-Year-Old Man Who Has Been in a Motor Vehicle Collision"
},
{
"authors": "Sara W. Nelson, MD; Daniel M. Lindberg, MD",
"content": [
"Physical Examination and Workup",
"On arrival to the hospital, the patient is ill-appearing and combative. His initial vital signs are a heart rate of 117 beats/min, a blood pressure of 85/50 mm Hg, a respiratory rate of 32 breaths/min, and an oxygen saturation of 91% on the nonrebreather mask. Upon primary survey, his oropharynx is clear, his airway is patent, and his trachea appears to be shifted to the right of midline. Upon auscultation, the patient's breath sounds are decreased over the left chest. Percussion of the left chest demonstrates hyperresonance. His carotid pulse is weakly palpable, and his jugular venous pulse is elevated. The patient has a Glasgow Coma Scale score of 12. The patient's clothing is removed, revealing no obvious deformities or areas of bleeding. The patient's abdomen is soft, without any tenderness to palpation. His pelvis is stable. Standard trauma radiographs, including an anteroposterior chest and pelvis scan, are performed after the primary survey. A complete secondary survey is postponed because of the patient's poor clinical condition.",
"Figure 1.",
"A second large-bore peripheral intravenous line is placed, and the patient begins to receive a bolus of 1000 cc of normal saline under pressure. A decision to perform an emergent procedure is made. Immediately after the procedure is performed, the patient is noted to have a dramatic clinical improvement. Subsequent to the procedure, the patient has a pulse of 105 beats/min, a blood pressure of 95/60 mm Hg, a respiratory rate of 22 breaths/min, and an oxygen saturation of 98% on the nonrebreather mask.",
"The secondary survey is completed, revealing no major injuries. Additionally, the chest radiograph (Figure) confirms the suspected clinical diagnosis that prompted the emergent procedure."
],
"date": "August 31, 2016",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/868/042/868042-thumb.jpg"
}
],
"markdown": "# A 26-Year-Old Man Who Has Been in a Motor Vehicle Collision\n\n **Authors:** Sara W. Nelson, MD; Daniel M. Lindberg, MD \n **Date:** August 31, 2016\n\n ## Content\n\n Physical Examination and Workup\nOn arrival to the hospital, the patient is ill-appearing and combative. His initial vital signs are a heart rate of 117 beats/min, a blood pressure of 85/50 mm Hg, a respiratory rate of 32 breaths/min, and an oxygen saturation of 91% on the nonrebreather mask. Upon primary survey, his oropharynx is clear, his airway is patent, and his trachea appears to be shifted to the right of midline. Upon auscultation, the patient's breath sounds are decreased over the left chest. Percussion of the left chest demonstrates hyperresonance. His carotid pulse is weakly palpable, and his jugular venous pulse is elevated. The patient has a Glasgow Coma Scale score of 12. The patient's clothing is removed, revealing no obvious deformities or areas of bleeding. The patient's abdomen is soft, without any tenderness to palpation. His pelvis is stable. Standard trauma radiographs, including an anteroposterior chest and pelvis scan, are performed after the primary survey. A complete secondary survey is postponed because of the patient's poor clinical condition.\nFigure 1.\nA second large-bore peripheral intravenous line is placed, and the patient begins to receive a bolus of 1000 cc of normal saline under pressure. A decision to perform an emergent procedure is made. Immediately after the procedure is performed, the patient is noted to have a dramatic clinical improvement. Subsequent to the procedure, the patient has a pulse of 105 beats/min, a blood pressure of 95/60 mm Hg, a respiratory rate of 22 breaths/min, and an oxygen saturation of 98% on the nonrebreather mask.\nThe secondary survey is completed, revealing no major injuries. Additionally, the chest radiograph (Figure) confirms the suspected clinical diagnosis that prompted the emergent procedure.\n\n ## Figures\n\n **Figure 1.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1007553,
"choiceText": "Upper airway obstruction; cricothyrotomy",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1007555,
"choiceText": "Tension pneumothorax; needle thoracostomy",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1007557,
"choiceText": "Hypovolumic shock; central line placement",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1007559,
"choiceText": "Pericardial tamponade; pericardiocentesis",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 318829,
"questionText": "<p>What is the underlying pathophysiology, and what procedure was performed?</p>\r\n\r\n<p><em>Hint: The cause of this patient's hypotension and hypoxia is a clinical diagnosis, and although portable chest radiography was performed in this case, this condition should not typically require imaging.</em> </p>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 26-Year-Old Man Who Has Been in a Motor Vehicle Collision"
},
{
"authors": "Sara W. Nelson, MD; Daniel M. Lindberg, MD",
"content": [
"Discussion",
"Pneumothorax occurs when air enters the potential space between the visceral and parietal pleura, leading to lung collapse on the affected side. Pneumothoraces may occur spontaneously, especially in the setting of lung disease, or they may result from accidental or iatrogenic trauma. A tension pneumothorax is a life-threatening condition that occurs when the air in the pleural space is under pressure, displacing mediastinal structures and compromising cardiopulmonary function.",
"Tension pneumothoraces result from injuries to the lung parenchyma or bronchial tree and can act as one-way valves such that air enters the pleural space but cannot escape. The trapped air in a tension pneumothorax causes increased intrathoracic pressure, pushing mediastinal structures contralaterally and reducing venous return and cardiac output. These patients are hypoxic and become difficult to ventilate, with potentially rapid progress to cardiorespiratory collapse and death. If sufficient clues are evident (eg, tracheal deviation, hypotension, decreased breath sounds, hyperresonance), chest decompression should begin without a delay for imaging.",
"Hemothorax is defined by blood in the pleural space and occurs when the lung parenchyma and the intercostal or mammary vessels are injured. Massive hemothoraces arise with hilar injuries, aortic ruptures, or myocardial ruptures. A tension hemopneumothorax develops when both blood and air are under tension in the pleural space.",
"A pneumothorax in any patient who has sustained thoracic trauma should arouse suspicion. The patient may complain of an acute onset of sharp pleuritic chest pain, with radiation to the ipsilateral shoulder and associated dyspnea and anxiety. Typical physical findings in pneumothorax include unilaterally decreased breath sounds, hyperresonance to percussion over the affected lung, and asymmetric chest rise. In tension pneumothorax, the patient displays respiratory distress, tachypnea, and tachycardia, and the patient may also experience cyanosis, jugular venous distention, tracheal deviation away from the affected lung, and a pulsus paradoxus."
],
"date": "August 31, 2016",
"figures": [],
"markdown": "# A 26-Year-Old Man Who Has Been in a Motor Vehicle Collision\n\n **Authors:** Sara W. Nelson, MD; Daniel M. Lindberg, MD \n **Date:** August 31, 2016\n\n ## Content\n\n Discussion\nPneumothorax occurs when air enters the potential space between the visceral and parietal pleura, leading to lung collapse on the affected side. Pneumothoraces may occur spontaneously, especially in the setting of lung disease, or they may result from accidental or iatrogenic trauma. A tension pneumothorax is a life-threatening condition that occurs when the air in the pleural space is under pressure, displacing mediastinal structures and compromising cardiopulmonary function.\nTension pneumothoraces result from injuries to the lung parenchyma or bronchial tree and can act as one-way valves such that air enters the pleural space but cannot escape. The trapped air in a tension pneumothorax causes increased intrathoracic pressure, pushing mediastinal structures contralaterally and reducing venous return and cardiac output. These patients are hypoxic and become difficult to ventilate, with potentially rapid progress to cardiorespiratory collapse and death. If sufficient clues are evident (eg, tracheal deviation, hypotension, decreased breath sounds, hyperresonance), chest decompression should begin without a delay for imaging.\nHemothorax is defined by blood in the pleural space and occurs when the lung parenchyma and the intercostal or mammary vessels are injured. Massive hemothoraces arise with hilar injuries, aortic ruptures, or myocardial ruptures. A tension hemopneumothorax develops when both blood and air are under tension in the pleural space.\nA pneumothorax in any patient who has sustained thoracic trauma should arouse suspicion. The patient may complain of an acute onset of sharp pleuritic chest pain, with radiation to the ipsilateral shoulder and associated dyspnea and anxiety. Typical physical findings in pneumothorax include unilaterally decreased breath sounds, hyperresonance to percussion over the affected lung, and asymmetric chest rise. In tension pneumothorax, the patient displays respiratory distress, tachypnea, and tachycardia, and the patient may also experience cyanosis, jugular venous distention, tracheal deviation away from the affected lung, and a pulsus paradoxus.\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1007553,
"choiceText": "Upper airway obstruction; cricothyrotomy",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1007555,
"choiceText": "Tension pneumothorax; needle thoracostomy",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1007557,
"choiceText": "Hypovolumic shock; central line placement",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1007559,
"choiceText": "Pericardial tamponade; pericardiocentesis",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 318829,
"questionText": "<p>What is the underlying pathophysiology, and what procedure was performed?</p>\r\n\r\n<p><em>Hint: The cause of this patient's hypotension and hypoxia is a clinical diagnosis, and although portable chest radiography was performed in this case, this condition should not typically require imaging.</em> </p>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 26-Year-Old Man Who Has Been in a Motor Vehicle Collision"
},
{
"authors": "Sara W. Nelson, MD; Daniel M. Lindberg, MD",
"content": [
"The epidemiology of traumatic pneumothoraces has not been well characterized. In the United States, trauma is the leading cause of death in persons younger than 45 years and accounts for approximately 150,000 deaths annually.[1] The overall mortality for thoracic trauma is 10%, and chest injuries cause approximately 1 in 4 trauma deaths in North America.[2] Pneumothorax is a serious complication of thoracic trauma and has been described in 1 in 5 patients that survive major trauma.[3] Of note, in one study, 12% of patients with asymptomatic chest stab wounds had a delayed pneumothorax or hemothorax.[4]",
"Although pneumothoraces in stable patients can be confirmed radiographically, a tension pneumothorax that causes hemodynamic compromise should be diagnosed clinically, and treatment should never be delayed in favor of diagnostic imaging. A chest radiograph may reveal a linear shadow of visceral pleura, without lateral lung markings. An upright chest radiograph is more sensitive than a supine radiograph, as air tends to accumulate at the lung apex. In recumbent patients, air often accumulates in the anterior portion of the inferior chest and manifests radiographically as a \"deep sulcus.\" If a pneumothorax without tension physiology is suspected but not seen on the initial upright chest radiography, a repeat film during exhalation may reveal it.",
"Increasingly, ultrasound is being used as a rapid bedside modality for diagnosing pneumothoraces; the literature suggests that chest ultrasonography by an emergency department physician is sufficient to rule out or rule in a pneumothorax.[5,6,7,8] CT scanning is more sensitive and specific than chest radiography or ultrasonography for the evaluation of small pneumothoraces and hemothoraces.",
"Occult pneumothoraces may be present in 2%-55% of trauma patients, although the clinical significance of occult pneumothoraces in patients who are not mechanically ventilated under positive pressure is unclear.[9] Making the diagnosis of hemothorax may be more challenging. A minimum of 200-300 mL of blood is needed in the pleural space for blunting of the costophrenic angle to be visible on an upright chest radiograph. Blood is more difficult to appreciate on a supine radiograph because it will typically layer posteriorly, and ever larger volumes (up to 1000 mL) of blood may produce only a mild diffuse radiodensity.",
"Lateral chest films may help differentiate hemothoraces from pulmonary contusions, and ultrasonography may also be useful for detecting fluid above the diaphragm. As with pneumothoraces, CT scanning is the most sensitive modality for diagnosing hemothoraces, although patients with massive hemothoraces may be too unstable for the scan."
],
"date": "August 31, 2016",
"figures": [],
"markdown": "# A 26-Year-Old Man Who Has Been in a Motor Vehicle Collision\n\n **Authors:** Sara W. Nelson, MD; Daniel M. Lindberg, MD \n **Date:** August 31, 2016\n\n ## Content\n\n The epidemiology of traumatic pneumothoraces has not been well characterized. In the United States, trauma is the leading cause of death in persons younger than 45 years and accounts for approximately 150,000 deaths annually.[1] The overall mortality for thoracic trauma is 10%, and chest injuries cause approximately 1 in 4 trauma deaths in North America.[2] Pneumothorax is a serious complication of thoracic trauma and has been described in 1 in 5 patients that survive major trauma.[3] Of note, in one study, 12% of patients with asymptomatic chest stab wounds had a delayed pneumothorax or hemothorax.[4]\nAlthough pneumothoraces in stable patients can be confirmed radiographically, a tension pneumothorax that causes hemodynamic compromise should be diagnosed clinically, and treatment should never be delayed in favor of diagnostic imaging. A chest radiograph may reveal a linear shadow of visceral pleura, without lateral lung markings. An upright chest radiograph is more sensitive than a supine radiograph, as air tends to accumulate at the lung apex. In recumbent patients, air often accumulates in the anterior portion of the inferior chest and manifests radiographically as a \"deep sulcus.\" If a pneumothorax without tension physiology is suspected but not seen on the initial upright chest radiography, a repeat film during exhalation may reveal it.\nIncreasingly, ultrasound is being used as a rapid bedside modality for diagnosing pneumothoraces; the literature suggests that chest ultrasonography by an emergency department physician is sufficient to rule out or rule in a pneumothorax.[5,6,7,8] CT scanning is more sensitive and specific than chest radiography or ultrasonography for the evaluation of small pneumothoraces and hemothoraces.\nOccult pneumothoraces may be present in 2%-55% of trauma patients, although the clinical significance of occult pneumothoraces in patients who are not mechanically ventilated under positive pressure is unclear.[9] Making the diagnosis of hemothorax may be more challenging. A minimum of 200-300 mL of blood is needed in the pleural space for blunting of the costophrenic angle to be visible on an upright chest radiograph. Blood is more difficult to appreciate on a supine radiograph because it will typically layer posteriorly, and ever larger volumes (up to 1000 mL) of blood may produce only a mild diffuse radiodensity.\nLateral chest films may help differentiate hemothoraces from pulmonary contusions, and ultrasonography may also be useful for detecting fluid above the diaphragm. As with pneumothoraces, CT scanning is the most sensitive modality for diagnosing hemothoraces, although patients with massive hemothoraces may be too unstable for the scan.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 26-Year-Old Man Who Has Been in a Motor Vehicle Collision"
},
{
"authors": "Sara W. Nelson, MD; Daniel M. Lindberg, MD",
"content": [
"The treatment of traumatic pneumothoraces and hemothoraces depends on the volume of blood or air that has accumulated and on the condition of the patient. Hemodynamically stable patients who are not intubated and have a relatively small pneumothorax (ie, < 1 cm wide) can be placed under observation. A repeat film should be obtained after 4-6 hours; if the pneumothorax is unchanged in size, the patient can continue to be observed without the need for decompression or tube thoracostomy. These patients should always be placed on 100% oxygen to increase the rate of reabsorption of the air in the pleural space.",
"In unstable patients who, on clinical grounds, are suspected of having a pneumothorax, a needle thoracostomy may be performed to quickly decompress the pleural space. A 14-gauge Angiocath (18-gauge or 20-gauge in an infant) should be placed immediately superior to the rib in the second intercostal space, midclavicular line on the affected side. Once in place, the needle is removed and the Angiocath is secured. A rush of air may be appreciated as the Angiocath enters the pleural space.",
"Pneumothoraces should preferentially be decompressed either by needle decompression or placement of a tube thoracostomy before the patient is intubated, because positive pressure ventilation exacerbates a pneumothorax; however, definitive management of the airway should never be delayed when indicated. Needle thoracostomy generally necessitates the subsequent placement of a chest tube, but stable patients who do not require a chest tube may be observed. In simple spontaneous pneumothoraces, a 20F or 22F chest tube may be used; however, larger-caliber chest tubes (28F to 40F) should be used in most traumatic pneumothoraces and hemothoraces to ensure adequate drainage of any fluid. Chest tubes are placed in the fourth or fifth intercostal space in the anterior axillary or midaxillary line, and they should be directed posteriorly and toward the apex of the lung.",
"After the tube is secured, it should be connected to a water seal and vacuum device, and placement should be confirmed by chest radiography. In the case of a hemothorax, immediate drainage of more than 1500-2000 mL (or 20 mL/kg) of blood, or ongoing hemorrhage exceeding 600-1200 mL/6 hours (or > 3 mL/kg/hr) after the initial drainage, constitute the definition of a massive hemothorax and are generally indications for a thoracotomy. Occasionally, placement of an additional chest tube may be necessary to assist in draining of the hemothorax. Additionally, the possibility of a bronchial injury should be considered if a continuing air leak is observed after several chest tubes and an unexpanded lung. In hemothorax, chest tubes should be directed posteriorly and inferiorly to arrive posterior to the diaphragm (as opposed to the placement for a simple pneumothorax).",
"In this case, the junior emergency medicine resident placed a 14-gauge Angiocath in the second intercostal space, midclavicular line of the left chest. A rush of air was appreciated, and the patient's blood pressure (as previously noted in the case presentation) improved to 95/60 mm Hg. The resident then prepared the left chest and placed a 38F chest tube in the fifth intercostal space, midaxillary line. Immediate drainage of 1600 mL of bloody fluid through the chest tube was noted. Un-crossmatched blood was administered, and the surgical team was consulted for the massive hemothorax. The patient was intubated and transported to the operating room (OR). In the OR, the surgery team performed a thoracotomy, repaired the injured lung parenchyma, and ligated several small arteries that were actively bleeding. The patient was transported to the surgical intensive care unit and extubated the following day. The chest tube was removed 48 hours later, and the patient was discharged on hospital day 4 in stable condition."
],
"date": "August 31, 2016",
"figures": [],
"markdown": "# A 26-Year-Old Man Who Has Been in a Motor Vehicle Collision\n\n **Authors:** Sara W. Nelson, MD; Daniel M. Lindberg, MD \n **Date:** August 31, 2016\n\n ## Content\n\n The treatment of traumatic pneumothoraces and hemothoraces depends on the volume of blood or air that has accumulated and on the condition of the patient. Hemodynamically stable patients who are not intubated and have a relatively small pneumothorax (ie, < 1 cm wide) can be placed under observation. A repeat film should be obtained after 4-6 hours; if the pneumothorax is unchanged in size, the patient can continue to be observed without the need for decompression or tube thoracostomy. These patients should always be placed on 100% oxygen to increase the rate of reabsorption of the air in the pleural space.\nIn unstable patients who, on clinical grounds, are suspected of having a pneumothorax, a needle thoracostomy may be performed to quickly decompress the pleural space. A 14-gauge Angiocath (18-gauge or 20-gauge in an infant) should be placed immediately superior to the rib in the second intercostal space, midclavicular line on the affected side. Once in place, the needle is removed and the Angiocath is secured. A rush of air may be appreciated as the Angiocath enters the pleural space.\nPneumothoraces should preferentially be decompressed either by needle decompression or placement of a tube thoracostomy before the patient is intubated, because positive pressure ventilation exacerbates a pneumothorax; however, definitive management of the airway should never be delayed when indicated. Needle thoracostomy generally necessitates the subsequent placement of a chest tube, but stable patients who do not require a chest tube may be observed. In simple spontaneous pneumothoraces, a 20F or 22F chest tube may be used; however, larger-caliber chest tubes (28F to 40F) should be used in most traumatic pneumothoraces and hemothoraces to ensure adequate drainage of any fluid. Chest tubes are placed in the fourth or fifth intercostal space in the anterior axillary or midaxillary line, and they should be directed posteriorly and toward the apex of the lung.\nAfter the tube is secured, it should be connected to a water seal and vacuum device, and placement should be confirmed by chest radiography. In the case of a hemothorax, immediate drainage of more than 1500-2000 mL (or 20 mL/kg) of blood, or ongoing hemorrhage exceeding 600-1200 mL/6 hours (or > 3 mL/kg/hr) after the initial drainage, constitute the definition of a massive hemothorax and are generally indications for a thoracotomy. Occasionally, placement of an additional chest tube may be necessary to assist in draining of the hemothorax. Additionally, the possibility of a bronchial injury should be considered if a continuing air leak is observed after several chest tubes and an unexpanded lung. In hemothorax, chest tubes should be directed posteriorly and inferiorly to arrive posterior to the diaphragm (as opposed to the placement for a simple pneumothorax).\nIn this case, the junior emergency medicine resident placed a 14-gauge Angiocath in the second intercostal space, midclavicular line of the left chest. A rush of air was appreciated, and the patient's blood pressure (as previously noted in the case presentation) improved to 95/60 mm Hg. The resident then prepared the left chest and placed a 38F chest tube in the fifth intercostal space, midaxillary line. Immediate drainage of 1600 mL of bloody fluid through the chest tube was noted. Un-crossmatched blood was administered, and the surgical team was consulted for the massive hemothorax. The patient was intubated and transported to the operating room (OR). In the OR, the surgery team performed a thoracotomy, repaired the injured lung parenchyma, and ligated several small arteries that were actively bleeding. The patient was transported to the surgical intensive care unit and extubated the following day. The chest tube was removed 48 hours later, and the patient was discharged on hospital day 4 in stable condition.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1007561,
"choiceText": "Immediate drainage of > 2000 mL of bloody fluid\r\n",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1007563,
"choiceText": "Persistent drainage of more than 2 mL/kg/hr of bloody fluid over 6 hours\r\n",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1007565,
"choiceText": "Hemothorax that is complicated by a tension pneumothorax\r\n",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1007567,
"choiceText": "Patient is intubated\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1007569,
"choiceText": "Placement of bilateral chest tubes \r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>Indications for thoracotomy after chest tube placement in a hemothorax include immediate drainage of more than 1500-2000 mL (or 20 mL/kg) of blood, evidence of ongoing hemorrhage after initial drainage exceeding 600-1200 mL over 6 hours (or > 3 mL/kg/hr), and persistent hemodynamic instability and shock. </p>",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 318831,
"questionText": "In the case of a hemothorax, which of the following is an indication for thoracotomy?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1007571,
"choiceText": "Decreased venous return\r\n",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1007573,
"choiceText": "Increased resistance when providing assisted mechanical ventilation\r\n",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1007575,
"choiceText": "Decreased cardiac output\r\n",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1007577,
"choiceText": "Increased sympathetic tone\r\n",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1007579,
"choiceText": "Decreased oxygenation secondary to pulmonary compromise\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>Trapped air in a tension pneumothorax causes a mass effect, pushing mediastinal structures to the opposite side and increasing intrathoracic pressure. The ipsilateral lung collapses and the contralateral lung is impinged, causing hypoxia. Increased intrathoracic tone leads to cardiorespiratory compromise by decreasing venous return, reducing cardiac output, and increasing resistance to assisted ventilation with a bag-valve-mask in both intubated and nonintubated patients. </p>",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 318833,
"questionText": "Which of the following conditions does <i>not</i> lead to possible rapid cardiorespiratory decompensation in tension pneumothorax?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 26-Year-Old Man Who Has Been in a Motor Vehicle Collision"
},
{
"authors": "Sara W. Nelson, MD; Daniel M. Lindberg, MD",
"content": [],
"date": "August 31, 2016",
"figures": [],
"markdown": "# A 26-Year-Old Man Who Has Been in a Motor Vehicle Collision\n\n **Authors:** Sara W. Nelson, MD; Daniel M. Lindberg, MD \n **Date:** August 31, 2016\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1007561,
"choiceText": "Immediate drainage of > 2000 mL of bloody fluid\r\n",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1007563,
"choiceText": "Persistent drainage of more than 2 mL/kg/hr of bloody fluid over 6 hours\r\n",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1007565,
"choiceText": "Hemothorax that is complicated by a tension pneumothorax\r\n",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1007567,
"choiceText": "Patient is intubated\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1007569,
"choiceText": "Placement of bilateral chest tubes \r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>Indications for thoracotomy after chest tube placement in a hemothorax include immediate drainage of more than 1500-2000 mL (or 20 mL/kg) of blood, evidence of ongoing hemorrhage after initial drainage exceeding 600-1200 mL over 6 hours (or > 3 mL/kg/hr), and persistent hemodynamic instability and shock. </p>",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 318831,
"questionText": "In the case of a hemothorax, which of the following is an indication for thoracotomy?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1007571,
"choiceText": "Decreased venous return\r\n",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1007573,
"choiceText": "Increased resistance when providing assisted mechanical ventilation\r\n",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1007575,
"choiceText": "Decreased cardiac output\r\n",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1007577,
"choiceText": "Increased sympathetic tone\r\n",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1007579,
"choiceText": "Decreased oxygenation secondary to pulmonary compromise\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>Trapped air in a tension pneumothorax causes a mass effect, pushing mediastinal structures to the opposite side and increasing intrathoracic pressure. The ipsilateral lung collapses and the contralateral lung is impinged, causing hypoxia. Increased intrathoracic tone leads to cardiorespiratory compromise by decreasing venous return, reducing cardiac output, and increasing resistance to assisted ventilation with a bag-valve-mask in both intubated and nonintubated patients. </p>",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 318833,
"questionText": "Which of the following conditions does <i>not</i> lead to possible rapid cardiorespiratory decompensation in tension pneumothorax?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 26-Year-Old Man Who Has Been in a Motor Vehicle Collision"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1007553,
"choiceText": "Upper airway obstruction; cricothyrotomy",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1007555,
"choiceText": "Tension pneumothorax; needle thoracostomy",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1007557,
"choiceText": "Hypovolumic shock; central line placement",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1007559,
"choiceText": "Pericardial tamponade; pericardiocentesis",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 318829,
"questionText": "<p>What is the underlying pathophysiology, and what procedure was performed?</p>\r\n\r\n<p><em>Hint: The cause of this patient's hypotension and hypoxia is a clinical diagnosis, and although portable chest radiography was performed in this case, this condition should not typically require imaging.</em> </p>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1007561,
"choiceText": "Immediate drainage of > 2000 mL of bloody fluid\r\n",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1007563,
"choiceText": "Persistent drainage of more than 2 mL/kg/hr of bloody fluid over 6 hours\r\n",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1007565,
"choiceText": "Hemothorax that is complicated by a tension pneumothorax\r\n",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1007567,
"choiceText": "Patient is intubated\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1007569,
"choiceText": "Placement of bilateral chest tubes \r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>Indications for thoracotomy after chest tube placement in a hemothorax include immediate drainage of more than 1500-2000 mL (or 20 mL/kg) of blood, evidence of ongoing hemorrhage after initial drainage exceeding 600-1200 mL over 6 hours (or > 3 mL/kg/hr), and persistent hemodynamic instability and shock. </p>",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 318831,
"questionText": "In the case of a hemothorax, which of the following is an indication for thoracotomy?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1007571,
"choiceText": "Decreased venous return\r\n",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1007573,
"choiceText": "Increased resistance when providing assisted mechanical ventilation\r\n",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1007575,
"choiceText": "Decreased cardiac output\r\n",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1007577,
"choiceText": "Increased sympathetic tone\r\n",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1007579,
"choiceText": "Decreased oxygenation secondary to pulmonary compromise\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>Trapped air in a tension pneumothorax causes a mass effect, pushing mediastinal structures to the opposite side and increasing intrathoracic pressure. The ipsilateral lung collapses and the contralateral lung is impinged, causing hypoxia. Increased intrathoracic tone leads to cardiorespiratory compromise by decreasing venous return, reducing cardiac output, and increasing resistance to assisted ventilation with a bag-valve-mask in both intubated and nonintubated patients. </p>",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 318833,
"questionText": "Which of the following conditions does <i>not</i> lead to possible rapid cardiorespiratory decompensation in tension pneumothorax?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
868040 | /viewarticle/868040 | [
{
"authors": "Christopher Redmond, MD, MSc; Kristine M. Lohr, MD, MS",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"A healthy 35-year-old man presents to the emergency department with dizziness and palpitations. He has been experiencing sudden episodes of dizziness intermittently over the past 2 days without provocation. His dizziness is not positional. He denies difficulty hearing, feeling of fullness in his ears, and vertigo.",
"He reports mild, intermittent knee swelling of each knee over the past several months. The effusion lasts for approximately 1 week and spontaneously resolves. He does not recall any recent rash or bug bites. He denies any chest, abdominal, or back pain, and any sensory or focal motor deficits. He has no history of cardiovascular or neurodegenerative disease or of an immunocompromised state. He lives in Western Massachusetts and is an avid mountain biker but has been unable to participate in any outdoor activities for a few weeks because of knee swelling and dizziness.",
"He is single and has had three female sexual partners in the past 6 months. He uses barrier contraception intermittently, but denies dysuria, genital lesions, and penile discharge. He drinks two alcoholic beverages a night and has never used tobacco or any illicit drugs. His father has hypertension. His mother and sister both have systemic lupus erythematosus."
],
"date": "August 31, 2016",
"figures": [],
"markdown": "# A 35-Year-Old Man With Palpitations and Dizziness\n\n **Authors:** Christopher Redmond, MD, MSc; Kristine M. Lohr, MD, MS \n **Date:** August 31, 2016\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nA healthy 35-year-old man presents to the emergency department with dizziness and palpitations. He has been experiencing sudden episodes of dizziness intermittently over the past 2 days without provocation. His dizziness is not positional. He denies difficulty hearing, feeling of fullness in his ears, and vertigo.\nHe reports mild, intermittent knee swelling of each knee over the past several months. The effusion lasts for approximately 1 week and spontaneously resolves. He does not recall any recent rash or bug bites. He denies any chest, abdominal, or back pain, and any sensory or focal motor deficits. He has no history of cardiovascular or neurodegenerative disease or of an immunocompromised state. He lives in Western Massachusetts and is an avid mountain biker but has been unable to participate in any outdoor activities for a few weeks because of knee swelling and dizziness.\nHe is single and has had three female sexual partners in the past 6 months. He uses barrier contraception intermittently, but denies dysuria, genital lesions, and penile discharge. He drinks two alcoholic beverages a night and has never used tobacco or any illicit drugs. His father has hypertension. His mother and sister both have systemic lupus erythematosus.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 35-Year-Old Man With Palpitations and Dizziness"
},
{
"authors": "Christopher Redmond, MD, MSc; Kristine M. Lohr, MD, MS",
"content": [
"The patient appears fatigued but well-developed and well-nourished. Vital signs are all within the reference range. No significant change in blood pressure is noted when he goes from the supine to seated or seated to standing positions. His skin is warm and dry without rash. His head is atraumatic. He has no conjunctival injection or exudate. Pupils are equal, round, and reactive to light. No deficit in extraocular movement is observed.",
"Figure 1.",
"Oral examination reveals no lesions, retropharyngeal erythema, or exudate. No cervical or axillary lymphadenopathy is identified. Cardiac examination is significant for bradycardia (50 beats/min at a regular rate with no murmur, rubs, or gallops). His point of maximum impulse is within the midclavicular line. Radial and posterior tibialis pulses are regular and equal bilaterally. He has no increased work of breathing or accessory muscle use with respiration. Lung fields are clear to auscultation bilaterally with no crackles or wheezing.",
"No swelling of his knees or any other joints is observed. He has unrestricted passive and active range of motion in all four extremities. Assessment of his strength and sensation in upper and lower extremities reveals no deficits. Cranial nerve examination findings are normal bilaterally. He has no appreciable photophobia, neck stiffness, or neck pain with flexion of his legs. Genitourinary examination findings are negative for scrotal edema/tenderness, genital lesion, and penile discharge.",
"Electrocardiography reveals normal sinus rhythm, with a prolonged PR interval of 280 msec. The PR interval is the same length throughout. The QRS interval is narrow with no significant changes in amplitude. No acute ST- or T-wave abnormalities are noted (Figure 1).",
"Blood work is unremarkable. No leukocytosis, anemia, or thrombocytopenia is observed. Sodium, potassium, calcium, and magnesium levels are all within the reference range. Creatinine and estimated glomerular filtration rate reveals normal kidney function."
],
"date": "August 31, 2016",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/868/040/868040-Thumb1.png"
}
],
"markdown": "# A 35-Year-Old Man With Palpitations and Dizziness\n\n **Authors:** Christopher Redmond, MD, MSc; Kristine M. Lohr, MD, MS \n **Date:** August 31, 2016\n\n ## Content\n\n The patient appears fatigued but well-developed and well-nourished. Vital signs are all within the reference range. No significant change in blood pressure is noted when he goes from the supine to seated or seated to standing positions. His skin is warm and dry without rash. His head is atraumatic. He has no conjunctival injection or exudate. Pupils are equal, round, and reactive to light. No deficit in extraocular movement is observed.\nFigure 1.\nOral examination reveals no lesions, retropharyngeal erythema, or exudate. No cervical or axillary lymphadenopathy is identified. Cardiac examination is significant for bradycardia (50 beats/min at a regular rate with no murmur, rubs, or gallops). His point of maximum impulse is within the midclavicular line. Radial and posterior tibialis pulses are regular and equal bilaterally. He has no increased work of breathing or accessory muscle use with respiration. Lung fields are clear to auscultation bilaterally with no crackles or wheezing.\nNo swelling of his knees or any other joints is observed. He has unrestricted passive and active range of motion in all four extremities. Assessment of his strength and sensation in upper and lower extremities reveals no deficits. Cranial nerve examination findings are normal bilaterally. He has no appreciable photophobia, neck stiffness, or neck pain with flexion of his legs. Genitourinary examination findings are negative for scrotal edema/tenderness, genital lesion, and penile discharge.\nElectrocardiography reveals normal sinus rhythm, with a prolonged PR interval of 280 msec. The PR interval is the same length throughout. The QRS interval is narrow with no significant changes in amplitude. No acute ST- or T-wave abnormalities are noted (Figure 1).\nBlood work is unremarkable. No leukocytosis, anemia, or thrombocytopenia is observed. Sodium, potassium, calcium, and magnesium levels are all within the reference range. Creatinine and estimated glomerular filtration rate reveals normal kidney function.\n\n ## Figures\n\n **Figure 1.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1006451,
"choiceText": "Atrial fibrillation ",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1006453,
"choiceText": "Gonococcal infection ",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1006455,
"choiceText": "Lyme disease ",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1006457,
"choiceText": "Systemic lupus erythematosus \r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 318577,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 35-Year-Old Man With Palpitations and Dizziness"
},
{
"authors": "Christopher Redmond, MD, MSc; Kristine M. Lohr, MD, MS",
"content": [
"Lyme disease is caused by an infection from the spirochete Borrelia burgdorferi. The symptoms of Lyme disease can vary widely in severity and in affected physiologic systems, which can often make it an extremely difficult diagnosis. Reports of suspected tick-borne illnesses with some of the hallmark symptoms of Lyme disease date back to the 1800s in Germany.[1] However, the term Lyme disease was not coined until 1975, when a group of children from the towns of Lyme and Old Lyme, Connecticut with suspected juvenile rheumatoid arthritis were identified as having a tick-borne infection with symptoms similar to those described in European manuscripts hundreds of years earlier.[2] By 2014, the number of cases of Lyme disease had grown to over 23,000 cases per year in the United States, with 96% of cases occurring in the Northeastern United States.[3]",
"Figure 2.",
"The most common vector for infection is Ixodes scapularis, also known as the deer tick. Humans typically come into contact with the ticks when walking through high grasses or other heavily wooded areas. The tick attaches to the human and begins its blood meal. B burgdorferi spirochete is not transmitted until the tick has fed for 24 to 48 hours.[4]",
"Lyme disease typically presents with a macular erythematous rash with central clearing, known as erythema migrans, and commonly referred to as a bull's eye rash (Figure 2). The rash appears at the site of the tick bite 3 to 30 days after the bite. The unique appearance of the rash is caused by migration of the spirochetes from the area of inoculation outward, which creates an area of central clearing. Although erythema migrans is typically considered pathognomonic for Lyme disease, an absence of rash does not exclude the diagnosis. Approximately 20% of cases of Lyme disease do not present with rash, typically because the rash goes unnoticed at the time of infection and erythema migrans typically fades within 1 month of infection.[5]",
"Several less common rashes have been seen in patients with Lyme disease, including borrelial lymphocytoma, which is a blue rash of the ear lobe typically only seen in European cases of Lyme disease.[6] Another rash more commonly seen in Europe is acrodermatitis chronica atrophicans, a bluish, poorly demarcated rash that typically starts on the distal extremities and spreads proximally. In addition to cutaneous symptoms, early-onset Lyme disease is also associated with fatigue, fevers, and chills in 20% to 30% of patients.[5] If patients present with erythema migrans or strong suspicion for Lyme disease, empiric treatment with doxycycline or amoxicillin is typically initiated without further serologic investigation.",
"If the diagnosis of Lyme disease is uncertain, serologic testing is the mainstay to confirm B burgdorferi infection. Serologic testing should only be performed in patients with symptoms and/or signs characteristic of Lyme disease because, even with two-tier testing, false positives are common. The current serologic testing for Lyme disease is called standardized two-tier testing (STTT). It was established by the Centers for Disease Control and Prevention in 1995 and uses enzyme immunoassays (EIAs) of immunofluorescence assays (IFA) to look for immunoglobulin (Ig)M and IgG antibodies to a standard set of B burgdorferi antigens. To be considered positive, a patient's IgM EIA/IFA must be reactive to two of three antigens, and for IgG to be considered positive, it must react to five of the 10 Lyme antigens. Several common Lyme antigens—p21 [OspC], p39 [BmpA], and p41 [flagellin B]—are used in both IgM and IgG testing.[7] IgM testing is limited to patients who have had symptoms for less than 30 days.[7] Although the STTT has significantly improved the sensitivity and specificity of serologic testing for Lyme disease, it is by no means perfect. Only 17% to 40% of patients with signs and symptoms of early Lyme disease, such as erythema migrans rash or recent tick bite, have positive STTT findings. This gradually improves to nearly 100% sensitivity for late-stage disease, but emphasizes the fact that the decision to treat or not to treat for Lyme disease should be based on both laboratory data and clinical judgement."
],
"date": "August 31, 2016",
"figures": [
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/868/040/868040-Thumb2.png"
}
],
"markdown": "# A 35-Year-Old Man With Palpitations and Dizziness\n\n **Authors:** Christopher Redmond, MD, MSc; Kristine M. Lohr, MD, MS \n **Date:** August 31, 2016\n\n ## Content\n\n Lyme disease is caused by an infection from the spirochete Borrelia burgdorferi. The symptoms of Lyme disease can vary widely in severity and in affected physiologic systems, which can often make it an extremely difficult diagnosis. Reports of suspected tick-borne illnesses with some of the hallmark symptoms of Lyme disease date back to the 1800s in Germany.[1] However, the term Lyme disease was not coined until 1975, when a group of children from the towns of Lyme and Old Lyme, Connecticut with suspected juvenile rheumatoid arthritis were identified as having a tick-borne infection with symptoms similar to those described in European manuscripts hundreds of years earlier.[2] By 2014, the number of cases of Lyme disease had grown to over 23,000 cases per year in the United States, with 96% of cases occurring in the Northeastern United States.[3]\nFigure 2.\nThe most common vector for infection is Ixodes scapularis, also known as the deer tick. Humans typically come into contact with the ticks when walking through high grasses or other heavily wooded areas. The tick attaches to the human and begins its blood meal. B burgdorferi spirochete is not transmitted until the tick has fed for 24 to 48 hours.[4]\nLyme disease typically presents with a macular erythematous rash with central clearing, known as erythema migrans, and commonly referred to as a bull's eye rash (Figure 2). The rash appears at the site of the tick bite 3 to 30 days after the bite. The unique appearance of the rash is caused by migration of the spirochetes from the area of inoculation outward, which creates an area of central clearing. Although erythema migrans is typically considered pathognomonic for Lyme disease, an absence of rash does not exclude the diagnosis. Approximately 20% of cases of Lyme disease do not present with rash, typically because the rash goes unnoticed at the time of infection and erythema migrans typically fades within 1 month of infection.[5]\nSeveral less common rashes have been seen in patients with Lyme disease, including borrelial lymphocytoma, which is a blue rash of the ear lobe typically only seen in European cases of Lyme disease.[6] Another rash more commonly seen in Europe is acrodermatitis chronica atrophicans, a bluish, poorly demarcated rash that typically starts on the distal extremities and spreads proximally. In addition to cutaneous symptoms, early-onset Lyme disease is also associated with fatigue, fevers, and chills in 20% to 30% of patients.[5] If patients present with erythema migrans or strong suspicion for Lyme disease, empiric treatment with doxycycline or amoxicillin is typically initiated without further serologic investigation.\nIf the diagnosis of Lyme disease is uncertain, serologic testing is the mainstay to confirm B burgdorferi infection. Serologic testing should only be performed in patients with symptoms and/or signs characteristic of Lyme disease because, even with two-tier testing, false positives are common. The current serologic testing for Lyme disease is called standardized two-tier testing (STTT). It was established by the Centers for Disease Control and Prevention in 1995 and uses enzyme immunoassays (EIAs) of immunofluorescence assays (IFA) to look for immunoglobulin (Ig)M and IgG antibodies to a standard set of B burgdorferi antigens. To be considered positive, a patient's IgM EIA/IFA must be reactive to two of three antigens, and for IgG to be considered positive, it must react to five of the 10 Lyme antigens. Several common Lyme antigens—p21 [OspC], p39 [BmpA], and p41 [flagellin B]—are used in both IgM and IgG testing.[7] IgM testing is limited to patients who have had symptoms for less than 30 days.[7] Although the STTT has significantly improved the sensitivity and specificity of serologic testing for Lyme disease, it is by no means perfect. Only 17% to 40% of patients with signs and symptoms of early Lyme disease, such as erythema migrans rash or recent tick bite, have positive STTT findings. This gradually improves to nearly 100% sensitivity for late-stage disease, but emphasizes the fact that the decision to treat or not to treat for Lyme disease should be based on both laboratory data and clinical judgement.\n\n ## Figures\n\n **Figure 2.** \n \n\n\n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1006451,
"choiceText": "Atrial fibrillation ",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1006453,
"choiceText": "Gonococcal infection ",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1006455,
"choiceText": "Lyme disease ",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1006457,
"choiceText": "Systemic lupus erythematosus \r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 318577,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 35-Year-Old Man With Palpitations and Dizziness"
},
{
"authors": "Christopher Redmond, MD, MSc; Kristine M. Lohr, MD, MS",
"content": [
"If left untreated, two-thirds of patients develop late-stage symptoms of Lyme disease.[5] These late-stage symptoms have a typical time course of presentation after inoculation with B burgdorferi. Although certain symptoms are typically seen at specific time intervals of infection, patients do not necessarily display every symptom of late-stage Lyme disease. For example, some patients with Lyme disease have no neurologic symptoms but may develop severe arthralgias and myalgias several weeks later. The variability in presentation and the multitude of symptoms associated with Lyme disease can make the diagnosis of later-stage disease more difficult.",
"Figure 3.",
"After early constitutional signs of infection, neurologic symptoms of Lyme disease, also known as neuroborreliosis, occur in 5% to 20% of patients. It typically develops 1 to 2 months after initial infection.[5] These neurologic symptoms can affect the peripheral nervous system, as in mononeuritis multiplex and Bell palsy, or, in rare instances, can affect the central nervous system, as in meningitis.[8] In some patients, pseudotumor-like symptoms can develop in conjunction with B burgdorferi meningitis. Cerebrospinal fluid (CSF) in patients with neuroborreliosis reveals pleocytosis with mild elevation of protein. In the past, neuroborreliosis has resulted in false-positive Wassermann tests for CSF syphilis infection. CSF cultures and polymerase chain reaction (PCR) findings for B burgdorferi are only positive in approximately 10% of patients with neuroborreliosis.[8]",
"Arthralgias occur several months after B burgdorferi infection. Joint involvement typically presents with large suprapatellar, oligoarticular joint effusions (Figure 3). The most common joints affected are the knees, but multiple small or large joints may be affected. Untreated synovial effusions last approximately 1 week. If left untreated, patients can develop recurrent synovitis in the same or different joints.[4] Recurrent episodes of synovitis can occur months apart. Synovial cultures of B burgdorferi are not typically sensitive for the detection of Lyme arthritis, but PCR findings are often positive for the spirochete. Synovial fluid usually becomes negative after antibiotic treatment, and synovial fluid analysis can have variable levels of leukocytes, ranging from 500 cells/mm3 to more than 25,000 cells/mm3.[7]",
"Although dermatologic, neurologic, and articular symptoms are the most common presentations of Lyme disease, in approximately 1% of patients, the infection can disseminate to the myocardium, causing Lyme carditis.[9] When B burgdorferi enters the heart, it can infiltrate any layer of heart tissue. Biopsies of early Lyme carditis show a primary immune response with primarily neutrophils and macrophages.[9] Spirochetes can be seen within myocytes and in fibrous connective tissue. Later stages of myocardial infection can result in lymphoid infiltration with fibrous plaque formation and myocyte necrosis. Physiologically, B burgdorferi infection of the myocardium results in abnormal myocardial conduction.",
"The most common abnormality seen in Lyme carditis is first-degree atrioventricular block. One distinguishing feature of atrioventricular block caused by Lyme disease is that it can rapidly progress to second-degree or even complete heart block, typically much faster than heart block caused by primarily cardiac etiologies.[9] The length of the initial PR interval seen with Lyme carditis has been shown to be directly proportional to the risk of developing third-degree heart block (i.e., the longer the PR interval, the greater risk of developing complete heart block).[9]"
],
"date": "August 31, 2016",
"figures": [
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/868/040/868040-Thumb3.png"
}
],
"markdown": "# A 35-Year-Old Man With Palpitations and Dizziness\n\n **Authors:** Christopher Redmond, MD, MSc; Kristine M. Lohr, MD, MS \n **Date:** August 31, 2016\n\n ## Content\n\n If left untreated, two-thirds of patients develop late-stage symptoms of Lyme disease.[5] These late-stage symptoms have a typical time course of presentation after inoculation with B burgdorferi. Although certain symptoms are typically seen at specific time intervals of infection, patients do not necessarily display every symptom of late-stage Lyme disease. For example, some patients with Lyme disease have no neurologic symptoms but may develop severe arthralgias and myalgias several weeks later. The variability in presentation and the multitude of symptoms associated with Lyme disease can make the diagnosis of later-stage disease more difficult.\nFigure 3.\nAfter early constitutional signs of infection, neurologic symptoms of Lyme disease, also known as neuroborreliosis, occur in 5% to 20% of patients. It typically develops 1 to 2 months after initial infection.[5] These neurologic symptoms can affect the peripheral nervous system, as in mononeuritis multiplex and Bell palsy, or, in rare instances, can affect the central nervous system, as in meningitis.[8] In some patients, pseudotumor-like symptoms can develop in conjunction with B burgdorferi meningitis. Cerebrospinal fluid (CSF) in patients with neuroborreliosis reveals pleocytosis with mild elevation of protein. In the past, neuroborreliosis has resulted in false-positive Wassermann tests for CSF syphilis infection. CSF cultures and polymerase chain reaction (PCR) findings for B burgdorferi are only positive in approximately 10% of patients with neuroborreliosis.[8]\nArthralgias occur several months after B burgdorferi infection. Joint involvement typically presents with large suprapatellar, oligoarticular joint effusions (Figure 3). The most common joints affected are the knees, but multiple small or large joints may be affected. Untreated synovial effusions last approximately 1 week. If left untreated, patients can develop recurrent synovitis in the same or different joints.[4] Recurrent episodes of synovitis can occur months apart. Synovial cultures of B burgdorferi are not typically sensitive for the detection of Lyme arthritis, but PCR findings are often positive for the spirochete. Synovial fluid usually becomes negative after antibiotic treatment, and synovial fluid analysis can have variable levels of leukocytes, ranging from 500 cells/mm3 to more than 25,000 cells/mm3.[7]\nAlthough dermatologic, neurologic, and articular symptoms are the most common presentations of Lyme disease, in approximately 1% of patients, the infection can disseminate to the myocardium, causing Lyme carditis.[9] When B burgdorferi enters the heart, it can infiltrate any layer of heart tissue. Biopsies of early Lyme carditis show a primary immune response with primarily neutrophils and macrophages.[9] Spirochetes can be seen within myocytes and in fibrous connective tissue. Later stages of myocardial infection can result in lymphoid infiltration with fibrous plaque formation and myocyte necrosis. Physiologically, B burgdorferi infection of the myocardium results in abnormal myocardial conduction.\nThe most common abnormality seen in Lyme carditis is first-degree atrioventricular block. One distinguishing feature of atrioventricular block caused by Lyme disease is that it can rapidly progress to second-degree or even complete heart block, typically much faster than heart block caused by primarily cardiac etiologies.[9] The length of the initial PR interval seen with Lyme carditis has been shown to be directly proportional to the risk of developing third-degree heart block (i.e., the longer the PR interval, the greater risk of developing complete heart block).[9]\n\n ## Figures\n\n **Figure 3.** \n \n\n\n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 35-Year-Old Man With Palpitations and Dizziness"
},
{
"authors": "Christopher Redmond, MD, MSc; Kristine M. Lohr, MD, MS",
"content": [
"Treatment of Lyme carditis involves ensuring that the patient is hemodynamically stable and then treating the underlying infection. If the patient is symptomatic with chest pain, palpitations, dyspnea, or any other cardiac symptom, he or she should be admitted for observation and antibiotic treatment should be initiated. Symptomatic bradycardia from Lyme carditis does not typically respond to treatment with atropine and tends to require transcutaneous or transvenous pacing.[9] Once the patient is stable, treatment should focus on antibiotic therapy to eradicate the B burgdorferi spirochetes from the myocardium.",
"The current recommendation for treatment of asymptomatic first-degree or second-degree heart block is parenteral treatment with penicillin G, ceftriaxone, or cefotaxime for 4 weeks. Oral therapy can be completed with doxycycline or amoxicillin for 2 to 3 weeks.[9] Most cases of Lyme carditis resolve within the first week of treatment. Occasionally, asymptomatic first-degree heart block persists for months; however, these cases of heart block typically resolve without further treatment. In rare circumstances, Lyme carditis has been linked to irreversible cardiomyopathy. Fewer than 10 cases of Lyme carditis evolving into irreversible and fatal cardiomyopathy have been reported.[10] Ruling out Lyme carditis is important in patients presenting with atrioventricular block of unknown etiology, particularly in younger individuals and those with a history consistent with Lyme disease.[10] The diagnosis and treatment of Lyme carditis could prevent patients from receiving unnecessary procedures, such as permanent pacemaker installation.",
"The patient discussed in this case was admitted to the hospital for monitoring on telemetry. STTT serologies were positive for Lyme disease. He was treated with intravenous ceftriaxone for 1 week and his atrioventricular block resolved. He was transitioned to oral doxycycline and was discharged. Follow-up 1 month later showed that the patient remained in normal sinus rhythm and had resolution of all other tertiary Lyme symptoms, including his intermittent arthralgias."
],
"date": "August 31, 2016",
"figures": [],
"markdown": "# A 35-Year-Old Man With Palpitations and Dizziness\n\n **Authors:** Christopher Redmond, MD, MSc; Kristine M. Lohr, MD, MS \n **Date:** August 31, 2016\n\n ## Content\n\n Treatment of Lyme carditis involves ensuring that the patient is hemodynamically stable and then treating the underlying infection. If the patient is symptomatic with chest pain, palpitations, dyspnea, or any other cardiac symptom, he or she should be admitted for observation and antibiotic treatment should be initiated. Symptomatic bradycardia from Lyme carditis does not typically respond to treatment with atropine and tends to require transcutaneous or transvenous pacing.[9] Once the patient is stable, treatment should focus on antibiotic therapy to eradicate the B burgdorferi spirochetes from the myocardium.\nThe current recommendation for treatment of asymptomatic first-degree or second-degree heart block is parenteral treatment with penicillin G, ceftriaxone, or cefotaxime for 4 weeks. Oral therapy can be completed with doxycycline or amoxicillin for 2 to 3 weeks.[9] Most cases of Lyme carditis resolve within the first week of treatment. Occasionally, asymptomatic first-degree heart block persists for months; however, these cases of heart block typically resolve without further treatment. In rare circumstances, Lyme carditis has been linked to irreversible cardiomyopathy. Fewer than 10 cases of Lyme carditis evolving into irreversible and fatal cardiomyopathy have been reported.[10] Ruling out Lyme carditis is important in patients presenting with atrioventricular block of unknown etiology, particularly in younger individuals and those with a history consistent with Lyme disease.[10] The diagnosis and treatment of Lyme carditis could prevent patients from receiving unnecessary procedures, such as permanent pacemaker installation.\nThe patient discussed in this case was admitted to the hospital for monitoring on telemetry. STTT serologies were positive for Lyme disease. He was treated with intravenous ceftriaxone for 1 week and his atrioventricular block resolved. He was transitioned to oral doxycycline and was discharged. Follow-up 1 month later showed that the patient remained in normal sinus rhythm and had resolution of all other tertiary Lyme symptoms, including his intermittent arthralgias.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1006459,
"choiceText": "Multisystemic symptoms, including neurologic symptoms, of Lyme disease ",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1006461,
"choiceText": "Length of time from primary infection \r\n",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1006463,
"choiceText": "PR interval length",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1006465,
"choiceText": "Age of the patient at time of presentation",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The length of the PR interval has been shown to be directly proportional to the severity of Lyme carditis.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 318579,
"questionText": "You admit a patient from the emergency department to the medicine service. He presents with palpitations, Bell palsy, and right knee synovitis. Synovial fluid analysis reveals leukocytosis. The patient states he went hiking in Vermont several months earlier and had several tick bites but did not seek healthcare. Electrocardiography reveals first-degree atrioventricular block, with a PR interval of 300. Previous electrocardiography findings revealed no block. You suspect that the patient has Lyme carditis. <br><br>\r\nWhich of the following signs or symptoms predict worsening Lyme carditis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1006467,
"choiceText": "Cardiac symptoms resolve with antibiotic treatment in most patients",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1006469,
"choiceText": "The chance of having persistent first-degree atrioventricular block is 20%",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1006471,
"choiceText": "The risk for myocardial infarction is 40% higher than if the patient had Lyme carditis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1006473,
"choiceText": "The patient has a high risk for cardiomyopathy and eventual heart transplant",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Most patients with Lyme carditis experience a complete resolution of symptoms with antibiotic treatment. Persistent first-degree atrioventricular block typically self-resolves without further treatment. Although a few cases of myocardial infarction associated with Lyme carditis have been reported, it does not increase the risk for myocardial infarction by 40%. Additionally, only a few rare cases of Lyme cardiomyopathy have been reported.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 318581,
"questionText": "The patient is concerned that he will have lasting heart issues from his Lyme carditis. Which of the following is the most accurate response?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 35-Year-Old Man With Palpitations and Dizziness"
},
{
"authors": "Christopher Redmond, MD, MSc; Kristine M. Lohr, MD, MS",
"content": [],
"date": "August 31, 2016",
"figures": [],
"markdown": "# A 35-Year-Old Man With Palpitations and Dizziness\n\n **Authors:** Christopher Redmond, MD, MSc; Kristine M. Lohr, MD, MS \n **Date:** August 31, 2016\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1006459,
"choiceText": "Multisystemic symptoms, including neurologic symptoms, of Lyme disease ",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1006461,
"choiceText": "Length of time from primary infection \r\n",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1006463,
"choiceText": "PR interval length",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1006465,
"choiceText": "Age of the patient at time of presentation",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The length of the PR interval has been shown to be directly proportional to the severity of Lyme carditis.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 318579,
"questionText": "You admit a patient from the emergency department to the medicine service. He presents with palpitations, Bell palsy, and right knee synovitis. Synovial fluid analysis reveals leukocytosis. The patient states he went hiking in Vermont several months earlier and had several tick bites but did not seek healthcare. Electrocardiography reveals first-degree atrioventricular block, with a PR interval of 300. Previous electrocardiography findings revealed no block. You suspect that the patient has Lyme carditis. <br><br>\r\nWhich of the following signs or symptoms predict worsening Lyme carditis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1006467,
"choiceText": "Cardiac symptoms resolve with antibiotic treatment in most patients",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1006469,
"choiceText": "The chance of having persistent first-degree atrioventricular block is 20%",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1006471,
"choiceText": "The risk for myocardial infarction is 40% higher than if the patient had Lyme carditis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1006473,
"choiceText": "The patient has a high risk for cardiomyopathy and eventual heart transplant",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Most patients with Lyme carditis experience a complete resolution of symptoms with antibiotic treatment. Persistent first-degree atrioventricular block typically self-resolves without further treatment. Although a few cases of myocardial infarction associated with Lyme carditis have been reported, it does not increase the risk for myocardial infarction by 40%. Additionally, only a few rare cases of Lyme cardiomyopathy have been reported.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 318581,
"questionText": "The patient is concerned that he will have lasting heart issues from his Lyme carditis. Which of the following is the most accurate response?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 35-Year-Old Man With Palpitations and Dizziness"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1006451,
"choiceText": "Atrial fibrillation ",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1006453,
"choiceText": "Gonococcal infection ",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1006455,
"choiceText": "Lyme disease ",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1006457,
"choiceText": "Systemic lupus erythematosus \r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 318577,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1006459,
"choiceText": "Multisystemic symptoms, including neurologic symptoms, of Lyme disease ",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1006461,
"choiceText": "Length of time from primary infection \r\n",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1006463,
"choiceText": "PR interval length",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1006465,
"choiceText": "Age of the patient at time of presentation",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The length of the PR interval has been shown to be directly proportional to the severity of Lyme carditis.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 318579,
"questionText": "You admit a patient from the emergency department to the medicine service. He presents with palpitations, Bell palsy, and right knee synovitis. Synovial fluid analysis reveals leukocytosis. The patient states he went hiking in Vermont several months earlier and had several tick bites but did not seek healthcare. Electrocardiography reveals first-degree atrioventricular block, with a PR interval of 300. Previous electrocardiography findings revealed no block. You suspect that the patient has Lyme carditis. <br><br>\r\nWhich of the following signs or symptoms predict worsening Lyme carditis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 1006467,
"choiceText": "Cardiac symptoms resolve with antibiotic treatment in most patients",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1006469,
"choiceText": "The chance of having persistent first-degree atrioventricular block is 20%",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1006471,
"choiceText": "The risk for myocardial infarction is 40% higher than if the patient had Lyme carditis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 1006473,
"choiceText": "The patient has a high risk for cardiomyopathy and eventual heart transplant",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Most patients with Lyme carditis experience a complete resolution of symptoms with antibiotic treatment. Persistent first-degree atrioventricular block typically self-resolves without further treatment. Although a few cases of myocardial infarction associated with Lyme carditis have been reported, it does not increase the risk for myocardial infarction by 40%. Additionally, only a few rare cases of Lyme cardiomyopathy have been reported.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 318581,
"questionText": "The patient is concerned that he will have lasting heart issues from his Lyme carditis. Which of the following is the most accurate response?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
867091 | /viewarticle/867091 | [
{
"authors": "Ehab H. Youssef, MD, FRCR",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.",
"A 70-year-old woman presents to the emergency department (ED) with progressive abdominal pain. This is her second episode; she recently presented to the ED with similar complaints. Over the past few years she has experienced multiple short periods of abdominal pain that always resolve spontaneously a few hours after taking over-the-counter analgesics or with the administration of intravenous analgesics, bowel rest, intravenous fluid, and supportive treatment at visits to her primary care provider and other medical venues. At these previous visits, all laboratory and radiologic investigations have always been unremarkable, and the patient has always been discharged home.",
"At this visit, as a result of increased intensity of the abdominal pain and the development of mild distention, she is admitted to the hospital. She is noted to have two episodes of vomiting and a normal bowel movement while in the ED. Her past medical history is remarkable for insulin-dependent diabetes and hypertension, for which she is on antihypertensive medications. She also takes daily aspirin. She underwent a laparoscopic cholecystectomy 15 years ago but has no other history of surgery."
],
"date": "August 09, 2016",
"figures": [],
"markdown": "# A 70-Year-Old Woman With Progressive Abdominal Pain\n\n **Authors:** Ehab H. Youssef, MD, FRCR \n **Date:** August 09, 2016\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.\nA 70-year-old woman presents to the emergency department (ED) with progressive abdominal pain. This is her second episode; she recently presented to the ED with similar complaints. Over the past few years she has experienced multiple short periods of abdominal pain that always resolve spontaneously a few hours after taking over-the-counter analgesics or with the administration of intravenous analgesics, bowel rest, intravenous fluid, and supportive treatment at visits to her primary care provider and other medical venues. At these previous visits, all laboratory and radiologic investigations have always been unremarkable, and the patient has always been discharged home.\nAt this visit, as a result of increased intensity of the abdominal pain and the development of mild distention, she is admitted to the hospital. She is noted to have two episodes of vomiting and a normal bowel movement while in the ED. Her past medical history is remarkable for insulin-dependent diabetes and hypertension, for which she is on antihypertensive medications. She also takes daily aspirin. She underwent a laparoscopic cholecystectomy 15 years ago but has no other history of surgery.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 70-Year-Old Woman With Progressive Abdominal Pain"
},
{
"authors": "Ehab H. Youssef, MD, FRCR",
"content": [
"Figure 1.",
"Figure 2.",
"Upon physical examination, her oral temperature is 98.6° F (37° C), her pulse rate is 94 beats/min, and her blood pressure is 168/92 mm Hg. Overall, she is noted to be uncomfortable secondary to colicky pain.",
"The head and neck examination findings are normal. Her lungs are clear when auscultated, with normal respiratory effort, and her heart examination is normal except for occasional premature ventricular beats. Upon abdominal examination, some fullness of the epigastric and left upper quadrant regions is noted, with mild tenderness on deep palpation. The rest of the abdomen is soft, with no abnormal pigmentations. Small scars from the previous laparoscopic cholecystectomy are noted. Rectal examination is unremarkable. No edema is observed in the extremities.",
"The laboratory investigations show an elevated blood glucose level of 140.54 mg/dL (7.8 mmol/L; normal range, 75-115 mg/dL), but otherwise the patient is noted to have a normal extended metabolic panel, including lipase and liver enzymes, and a normal complete blood count (CBC), with no evidence of leukocytosis or anemia. A urinalysis is negative for blood, nitrites, and leukocytes.",
"Plain abdominal radiographs reveal a nonspecific bowel gas pattern (not shown) and no signs of bowel obstruction. The patient is transferred to the hospital floor for bowel rest, intravenous fluids, and antiemetics. On hospital day 2, she is noted to be in increasing pain; a general surgery consultation is requested and abdominal CT scanning is performed (Figures 1 and 2)."
],
"date": "August 09, 2016",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/867/091/867091-thumb-1.jpg"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/867/091/867091-thumb-2.jpg"
}
],
"markdown": "# A 70-Year-Old Woman With Progressive Abdominal Pain\n\n **Authors:** Ehab H. Youssef, MD, FRCR \n **Date:** August 09, 2016\n\n ## Content\n\n Figure 1.\nFigure 2.\nUpon physical examination, her oral temperature is 98.6° F (37° C), her pulse rate is 94 beats/min, and her blood pressure is 168/92 mm Hg. Overall, she is noted to be uncomfortable secondary to colicky pain.\nThe head and neck examination findings are normal. Her lungs are clear when auscultated, with normal respiratory effort, and her heart examination is normal except for occasional premature ventricular beats. Upon abdominal examination, some fullness of the epigastric and left upper quadrant regions is noted, with mild tenderness on deep palpation. The rest of the abdomen is soft, with no abnormal pigmentations. Small scars from the previous laparoscopic cholecystectomy are noted. Rectal examination is unremarkable. No edema is observed in the extremities.\nThe laboratory investigations show an elevated blood glucose level of 140.54 mg/dL (7.8 mmol/L; normal range, 75-115 mg/dL), but otherwise the patient is noted to have a normal extended metabolic panel, including lipase and liver enzymes, and a normal complete blood count (CBC), with no evidence of leukocytosis or anemia. A urinalysis is negative for blood, nitrites, and leukocytes.\nPlain abdominal radiographs reveal a nonspecific bowel gas pattern (not shown) and no signs of bowel obstruction. The patient is transferred to the hospital floor for bowel rest, intravenous fluids, and antiemetics. On hospital day 2, she is noted to be in increasing pain; a general surgery consultation is requested and abdominal CT scanning is performed (Figures 1 and 2).\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 997065,
"choiceText": "Acute splenic injury",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 997067,
"choiceText": "Pancreatitis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 997069,
"choiceText": "Left paraduodenal hernia",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 997071,
"choiceText": "Cecal volvulus",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 315687,
"questionText": "<p>What is the cause of the patient's abdominal pain and distention?</p>\r\n\r\n<p><em>Hint: Look carefully at the bowel loops.</em></p>\r\n",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 70-Year-Old Woman With Progressive Abdominal Pain"
},
{
"authors": "Ehab H. Youssef, MD, FRCR",
"content": [
"The abdominal CT images revealed a cluster of prominent bowel loops, likely jejunal, in the left upper quadrant region, lateral to the ascending duodenum pointing inwardly. The duodenum demonstrated an abrupt caliber change. Mesenteric congestion and stranding were noted. Associated mass effect was noted, with displacement of the posterior stomach wall anteriorly. Also noted was displacement of the duodenojejunal junction inferomedially and the transverse colon inferiorly (not shown). These findings were consistent with a diagnosis of paraduodenal hernia.",
"A paraduodenal hernia is a type of internal hernia, further classified as right- or left-sided. An internal hernia is defined as a protrusion of bowel loops through a congenital or acquired defect of the mesentery contained within the abdominal cavity. They are relatively uncommon; paraduodenal hernias are the most common type, making up 53% of all internal hernias. Internal hernias occur secondary to either a congenital defect (eg, paraduodenal) or an acquired defect (eg, following transmesenteric surgery [ie, mesocolic hernia]).",
"Paraduodenal hernias occur when bowel prolapses through the Landzert fossa (also known as the left paraduodenal fossa), an aperture present in approximately 2% of the population. These hernias are therefore classified as congenital internal hernias. The Landzert fossa is located behind the ascending or fourth part of the duodenum and is formed by the lifting up of a peritoneal fold by the inferior mesenteric vein and ascending left colic artery as they run along the lateral side of the fossa. Small-bowel loops prolapse posteroinferiorly through the fossa to the left of the fourth part of the duodenum into the left upper quadrant. A left paraduodenal hernia is a cluster of bowel loops in the left upper quadrant lateral to the ascending duodenum. A right paraduodenal hernia is a cluster of bowel loops that appears in the right upper quadrant region lateral and inferior to the descending duodenum.",
"Mesenteric vessel abnormalities, including distortion, enlargement, stretching, and anterior displacement of the main mesenteric trunks (especially the inferior mesenteric vein) to the left, are also helpful findings. Paraduodenal hernia occurs in 2%-4% of the population and usually occurs between the fourth and sixth decades of life. This condition, however, has also been reported in children, with a male predominance (male-to-female ratio, 3:1).[1,2,3,4,5,6]",
"Patients may be asymptomatic or may complain of vague discomfort, abdominal distention, colicky epigastralgia, or periumbilical pain. A palpable mass may be present, with local tenderness on physical examination. If a bowel obstruction exists, it is usually of a low grade, chronic, and recurrent; however, high-grade, acute, and sudden obstructions have also been found. These hernias have a propensity to reduce on their own; therefore, patients should be imaged when they do exhibit symptoms.[1,4]"
],
"date": "August 09, 2016",
"figures": [],
"markdown": "# A 70-Year-Old Woman With Progressive Abdominal Pain\n\n **Authors:** Ehab H. Youssef, MD, FRCR \n **Date:** August 09, 2016\n\n ## Content\n\n The abdominal CT images revealed a cluster of prominent bowel loops, likely jejunal, in the left upper quadrant region, lateral to the ascending duodenum pointing inwardly. The duodenum demonstrated an abrupt caliber change. Mesenteric congestion and stranding were noted. Associated mass effect was noted, with displacement of the posterior stomach wall anteriorly. Also noted was displacement of the duodenojejunal junction inferomedially and the transverse colon inferiorly (not shown). These findings were consistent with a diagnosis of paraduodenal hernia.\nA paraduodenal hernia is a type of internal hernia, further classified as right- or left-sided. An internal hernia is defined as a protrusion of bowel loops through a congenital or acquired defect of the mesentery contained within the abdominal cavity. They are relatively uncommon; paraduodenal hernias are the most common type, making up 53% of all internal hernias. Internal hernias occur secondary to either a congenital defect (eg, paraduodenal) or an acquired defect (eg, following transmesenteric surgery [ie, mesocolic hernia]).\nParaduodenal hernias occur when bowel prolapses through the Landzert fossa (also known as the left paraduodenal fossa), an aperture present in approximately 2% of the population. These hernias are therefore classified as congenital internal hernias. The Landzert fossa is located behind the ascending or fourth part of the duodenum and is formed by the lifting up of a peritoneal fold by the inferior mesenteric vein and ascending left colic artery as they run along the lateral side of the fossa. Small-bowel loops prolapse posteroinferiorly through the fossa to the left of the fourth part of the duodenum into the left upper quadrant. A left paraduodenal hernia is a cluster of bowel loops in the left upper quadrant lateral to the ascending duodenum. A right paraduodenal hernia is a cluster of bowel loops that appears in the right upper quadrant region lateral and inferior to the descending duodenum.\nMesenteric vessel abnormalities, including distortion, enlargement, stretching, and anterior displacement of the main mesenteric trunks (especially the inferior mesenteric vein) to the left, are also helpful findings. Paraduodenal hernia occurs in 2%-4% of the population and usually occurs between the fourth and sixth decades of life. This condition, however, has also been reported in children, with a male predominance (male-to-female ratio, 3:1).[1,2,3,4,5,6]\nPatients may be asymptomatic or may complain of vague discomfort, abdominal distention, colicky epigastralgia, or periumbilical pain. A palpable mass may be present, with local tenderness on physical examination. If a bowel obstruction exists, it is usually of a low grade, chronic, and recurrent; however, high-grade, acute, and sudden obstructions have also been found. These hernias have a propensity to reduce on their own; therefore, patients should be imaged when they do exhibit symptoms.[1,4]\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 997065,
"choiceText": "Acute splenic injury",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 997067,
"choiceText": "Pancreatitis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 997069,
"choiceText": "Left paraduodenal hernia",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 997071,
"choiceText": "Cecal volvulus",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 315687,
"questionText": "<p>What is the cause of the patient's abdominal pain and distention?</p>\r\n\r\n<p><em>Hint: Look carefully at the bowel loops.</em></p>\r\n",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 70-Year-Old Woman With Progressive Abdominal Pain"
},
{
"authors": "Ehab H. Youssef, MD, FRCR",
"content": [
"Differentiating between clinically similar conditions that can cause abdominal pain, distention, and an intestinal obstruction is important. Adhesive bands are the most common cause for adult intestinal obstruction. Imaging often reveals markedly distended fluid-filled small bowel loops, with a distinct transition to adjacent collapsed loops distal to the band responsible for the obstruction. Additionally, no specific orientation of the bowel loops is noted, as seen in cases of paraduodenal hernia.",
"A volvulus demonstrates a closed dilated segment of the small intestine, with a \"C\"- or \"U\"-shaped configuration of the bowel loops and fusiform tapering at the point of torsion. Other types of hernias may be either external or internal. External hernias are diagnosed by demonstrating the hernial sac bulging through the specific abdominal wall defect for each hernia, which can contain omentum only, bowel, or even solid organ outside the abdominal cavity. Common types include inguinal hernia, in which the hernial sac bulges into the inguinal canal; femoral hernia, in which the hernial sac bulges into the femoral canal; obturator hernia, in which the hernial sac bulges into the obturator canal; ventral hernia, in which the hernial sac bulges through the anterior or lateral abdominal wall; and spigelian hernia, in which the hernial sac bulges intramuscularly between the external abdominal aponeurosis (which remains intact) and internal abdominal muscles, lateral to the rectus sheath muscle.",
"Internal hernias, as described above, are diagnosed as protrusions of bowel loops through a congenital or acquired defect of mesentery within the abdominal cavity. In addition to paraduodenal hernias, another type of internal hernia is a transmesenteric hernia, in which the bowel loops are seen in the right iliac fossa adjacent to the ileocecal valve.[3,5,6,7] Hernias in the mesentery, mesocolon, mesosigmoid, Winslow foramen, and defects of the falciform ligament have been described.",
"Imaging modalities reveal specific findings in the setting of a paraduodenal hernia. Plain abdominal radiographs may show markedly distended segments of bowel in cases wherein the hernia is associated with a small bowel obstruction (ie, closed loop). Fluoroscopic-guided small-bowel follow-through reveals crowding bowel loops in an abnormal location either to the right or left of the colon. In a left paraduodenal hernia, it reveals a circumscribed ovoid mass of jejunal loops in the left upper quadrant region lateral to the ascending duodenum. In a right paraduodenal hernia, it reveals a circumscribed ovoid mass of jejunal loops lateral and inferior to the descending duodenum.",
"Varying degrees of small-bowel obstruction may be present, and a transition point may also be observed. The mass of bowel loops are fixed; the clustered loops cannot be separated or displaced by manual palpation or change in position. The preferred imaging modality, however, is abdominal CT scanning, which reveals evidence of any degree of intestinal obstruction and/or an encapsulated cluster of bowel loops (usually jejunal) in the left upper quadrant region lateral to the ascending duodenum. Displacement of the stomach anteriorly is common, with inferior displacement of the transverse colon and inferomedial displacement of the duodenojejunal junction. The mesenteric vessels are often crowded and engorged, with associated mesenteric inflammatory changes (stranding).[1,2,3,5,7]"
],
"date": "August 09, 2016",
"figures": [],
"markdown": "# A 70-Year-Old Woman With Progressive Abdominal Pain\n\n **Authors:** Ehab H. Youssef, MD, FRCR \n **Date:** August 09, 2016\n\n ## Content\n\n Differentiating between clinically similar conditions that can cause abdominal pain, distention, and an intestinal obstruction is important. Adhesive bands are the most common cause for adult intestinal obstruction. Imaging often reveals markedly distended fluid-filled small bowel loops, with a distinct transition to adjacent collapsed loops distal to the band responsible for the obstruction. Additionally, no specific orientation of the bowel loops is noted, as seen in cases of paraduodenal hernia.\nA volvulus demonstrates a closed dilated segment of the small intestine, with a \"C\"- or \"U\"-shaped configuration of the bowel loops and fusiform tapering at the point of torsion. Other types of hernias may be either external or internal. External hernias are diagnosed by demonstrating the hernial sac bulging through the specific abdominal wall defect for each hernia, which can contain omentum only, bowel, or even solid organ outside the abdominal cavity. Common types include inguinal hernia, in which the hernial sac bulges into the inguinal canal; femoral hernia, in which the hernial sac bulges into the femoral canal; obturator hernia, in which the hernial sac bulges into the obturator canal; ventral hernia, in which the hernial sac bulges through the anterior or lateral abdominal wall; and spigelian hernia, in which the hernial sac bulges intramuscularly between the external abdominal aponeurosis (which remains intact) and internal abdominal muscles, lateral to the rectus sheath muscle.\nInternal hernias, as described above, are diagnosed as protrusions of bowel loops through a congenital or acquired defect of mesentery within the abdominal cavity. In addition to paraduodenal hernias, another type of internal hernia is a transmesenteric hernia, in which the bowel loops are seen in the right iliac fossa adjacent to the ileocecal valve.[3,5,6,7] Hernias in the mesentery, mesocolon, mesosigmoid, Winslow foramen, and defects of the falciform ligament have been described.\nImaging modalities reveal specific findings in the setting of a paraduodenal hernia. Plain abdominal radiographs may show markedly distended segments of bowel in cases wherein the hernia is associated with a small bowel obstruction (ie, closed loop). Fluoroscopic-guided small-bowel follow-through reveals crowding bowel loops in an abnormal location either to the right or left of the colon. In a left paraduodenal hernia, it reveals a circumscribed ovoid mass of jejunal loops in the left upper quadrant region lateral to the ascending duodenum. In a right paraduodenal hernia, it reveals a circumscribed ovoid mass of jejunal loops lateral and inferior to the descending duodenum.\nVarying degrees of small-bowel obstruction may be present, and a transition point may also be observed. The mass of bowel loops are fixed; the clustered loops cannot be separated or displaced by manual palpation or change in position. The preferred imaging modality, however, is abdominal CT scanning, which reveals evidence of any degree of intestinal obstruction and/or an encapsulated cluster of bowel loops (usually jejunal) in the left upper quadrant region lateral to the ascending duodenum. Displacement of the stomach anteriorly is common, with inferior displacement of the transverse colon and inferomedial displacement of the duodenojejunal junction. The mesenteric vessels are often crowded and engorged, with associated mesenteric inflammatory changes (stranding).[1,2,3,5,7]\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 70-Year-Old Woman With Progressive Abdominal Pain"
},
{
"authors": "Ehab H. Youssef, MD, FRCR",
"content": [
"The prognosis for a paraduodenal hernia is usually good with early diagnosis and surgical repair, but missed diagnosis or delayed surgical repair can lead to complications, such as the development of volvulus, mesenteric ischemia, bowel strangulation, gangrene, and potential bowel rupture and peritonitis. The treatment is usually by laparotomy with incision of the inclosing mesentery, correction of the mesenteric defect, and decompression of the bowel loops. Special attention should be devoted to avoid injury to the superior and inferior mesenteric arteries.",
"In this patient, after the completion of the abdominal CT scan, she experienced increasing abdominal pain with worsening distention. A decision was made to take the patient to the operating room for laparotomy. At laparotomy, a 40-cm loop of small bowel was found to have herniated through the left paraduodenal fossa. A 20-cm segment of the herniated bowel loop appeared dull and dusky. This segment was resected with a primary anastomosis and reduction of the remaining herniated bowel. The mesenteric defect was closed.",
"The patient recovered well in the postoperative period and, after a short stay in the surgical intensive care unit, she was transferred to the hospital floor. She continued to do well after the procedure and was discharged to home on postoperative day 5. On a 1-month follow-up visit, the incision was noted to be healing well, and the patient was generally doing well, with no abdominal pain."
],
"date": "August 09, 2016",
"figures": [],
"markdown": "# A 70-Year-Old Woman With Progressive Abdominal Pain\n\n **Authors:** Ehab H. Youssef, MD, FRCR \n **Date:** August 09, 2016\n\n ## Content\n\n The prognosis for a paraduodenal hernia is usually good with early diagnosis and surgical repair, but missed diagnosis or delayed surgical repair can lead to complications, such as the development of volvulus, mesenteric ischemia, bowel strangulation, gangrene, and potential bowel rupture and peritonitis. The treatment is usually by laparotomy with incision of the inclosing mesentery, correction of the mesenteric defect, and decompression of the bowel loops. Special attention should be devoted to avoid injury to the superior and inferior mesenteric arteries.\nIn this patient, after the completion of the abdominal CT scan, she experienced increasing abdominal pain with worsening distention. A decision was made to take the patient to the operating room for laparotomy. At laparotomy, a 40-cm loop of small bowel was found to have herniated through the left paraduodenal fossa. A 20-cm segment of the herniated bowel loop appeared dull and dusky. This segment was resected with a primary anastomosis and reduction of the remaining herniated bowel. The mesenteric defect was closed.\nThe patient recovered well in the postoperative period and, after a short stay in the surgical intensive care unit, she was transferred to the hospital floor. She continued to do well after the procedure and was discharged to home on postoperative day 5. On a 1-month follow-up visit, the incision was noted to be healing well, and the patient was generally doing well, with no abdominal pain.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 997073,
"choiceText": "Internal hernia",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 997075,
"choiceText": "External hernia",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 997077,
"choiceText": "Adhesive bands",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 997079,
"choiceText": "Neoplasm",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 997081,
"choiceText": "Volvulus",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>Differentiating between clinically similar conditions that can cause abdominal pain, distention, and an intestinal obstruction is important. Adhesive bands are the most common cause for adult intestinal obstruction. </p>",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 315689,
"questionText": "Which of the following conditions would be the most likely cause of an intestinal obstruction in this patient?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 997083,
"choiceText": "Barium enema",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 997085,
"choiceText": "Abdominal CT scan",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 997087,
"choiceText": "Abdominal radiography",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 997089,
"choiceText": "Transesophageal ultrasonography",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>Imaging modalities demonstrate specific findings. Plain abdominal radiographs may show markedly distended segments of bowel in cases wherein the hernia is associated with a small-bowel obstruction (ie, closed loop). The preferred imaging modality, however, is abdominal CT scanning, which reveals evidence of any degree of intestinal obstruction and/or an encapsulated cluster of bowel loops (usually jejunal) in the left upper quadrant region lateral to the ascending duodenum. There is also usually displacement of the stomach anteriorly, with inferior displacement of the transverse colon and inferomedial displacement of the duodenojejunal junction. The mesenteric vessels are often crowded and engorged, with associated mesenteric inflammatory changes (stranding). The remaining two imaging examinations have not been shown to be of benefit. </p>",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 315691,
"questionText": "<p>Upon further investigation, you suspect that the case patient may actually be suffering from an internal hernia. Which of the following imaging examinations is the most sensitive test for identifying an internal hernia, such as a paraduodenal hernia?</p>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 70-Year-Old Woman With Progressive Abdominal Pain"
},
{
"authors": "Ehab H. Youssef, MD, FRCR",
"content": [],
"date": "August 09, 2016",
"figures": [],
"markdown": "# A 70-Year-Old Woman With Progressive Abdominal Pain\n\n **Authors:** Ehab H. Youssef, MD, FRCR \n **Date:** August 09, 2016\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 997073,
"choiceText": "Internal hernia",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 997075,
"choiceText": "External hernia",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 997077,
"choiceText": "Adhesive bands",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 997079,
"choiceText": "Neoplasm",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 997081,
"choiceText": "Volvulus",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>Differentiating between clinically similar conditions that can cause abdominal pain, distention, and an intestinal obstruction is important. Adhesive bands are the most common cause for adult intestinal obstruction. </p>",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 315689,
"questionText": "Which of the following conditions would be the most likely cause of an intestinal obstruction in this patient?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 997083,
"choiceText": "Barium enema",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 997085,
"choiceText": "Abdominal CT scan",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 997087,
"choiceText": "Abdominal radiography",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 997089,
"choiceText": "Transesophageal ultrasonography",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>Imaging modalities demonstrate specific findings. Plain abdominal radiographs may show markedly distended segments of bowel in cases wherein the hernia is associated with a small-bowel obstruction (ie, closed loop). The preferred imaging modality, however, is abdominal CT scanning, which reveals evidence of any degree of intestinal obstruction and/or an encapsulated cluster of bowel loops (usually jejunal) in the left upper quadrant region lateral to the ascending duodenum. There is also usually displacement of the stomach anteriorly, with inferior displacement of the transverse colon and inferomedial displacement of the duodenojejunal junction. The mesenteric vessels are often crowded and engorged, with associated mesenteric inflammatory changes (stranding). The remaining two imaging examinations have not been shown to be of benefit. </p>",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 315691,
"questionText": "<p>Upon further investigation, you suspect that the case patient may actually be suffering from an internal hernia. Which of the following imaging examinations is the most sensitive test for identifying an internal hernia, such as a paraduodenal hernia?</p>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 70-Year-Old Woman With Progressive Abdominal Pain"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 997065,
"choiceText": "Acute splenic injury",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 997067,
"choiceText": "Pancreatitis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 997069,
"choiceText": "Left paraduodenal hernia",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 997071,
"choiceText": "Cecal volvulus",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 315687,
"questionText": "<p>What is the cause of the patient's abdominal pain and distention?</p>\r\n\r\n<p><em>Hint: Look carefully at the bowel loops.</em></p>\r\n",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 997073,
"choiceText": "Internal hernia",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 997075,
"choiceText": "External hernia",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 997077,
"choiceText": "Adhesive bands",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 997079,
"choiceText": "Neoplasm",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 997081,
"choiceText": "Volvulus",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>Differentiating between clinically similar conditions that can cause abdominal pain, distention, and an intestinal obstruction is important. Adhesive bands are the most common cause for adult intestinal obstruction. </p>",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 315689,
"questionText": "Which of the following conditions would be the most likely cause of an intestinal obstruction in this patient?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 997083,
"choiceText": "Barium enema",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 997085,
"choiceText": "Abdominal CT scan",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 997087,
"choiceText": "Abdominal radiography",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 997089,
"choiceText": "Transesophageal ultrasonography",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>Imaging modalities demonstrate specific findings. Plain abdominal radiographs may show markedly distended segments of bowel in cases wherein the hernia is associated with a small-bowel obstruction (ie, closed loop). The preferred imaging modality, however, is abdominal CT scanning, which reveals evidence of any degree of intestinal obstruction and/or an encapsulated cluster of bowel loops (usually jejunal) in the left upper quadrant region lateral to the ascending duodenum. There is also usually displacement of the stomach anteriorly, with inferior displacement of the transverse colon and inferomedial displacement of the duodenojejunal junction. The mesenteric vessels are often crowded and engorged, with associated mesenteric inflammatory changes (stranding). The remaining two imaging examinations have not been shown to be of benefit. </p>",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 315691,
"questionText": "<p>Upon further investigation, you suspect that the case patient may actually be suffering from an internal hernia. Which of the following imaging examinations is the most sensitive test for identifying an internal hernia, such as a paraduodenal hernia?</p>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
866401 | /viewarticle/866401 | [
{
"authors": "Tarah Ballinger, MD; Kathy D. Miller, MD",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.",
"A 72-year-old woman presents to her primary care physician because of worsening lower back pain. She has a known history of osteoarthritis and chronic back pain, which is usually helped with steroid injections and over-the-counter nonsteroidal anti-inflammatory medications. However, she had injections the week before her presentation and is taking multiple doses of naproxen each day without the usual relief. She denies any weakness or numbness of the lower extremities. She is otherwise feeling well and is a very active woman who regularly exercises.",
"The patient's medical history is significant for right knee, right hand, and left shoulder surgeries owing to osteoarthritis. Ten years ago, she was diagnosed with stage I estrogen receptor (ER)-positive, human epidermal growth factor receptor (HER2)-negative invasive ductal carcinoma of the breast. Her breast cancer was treated with lumpectomy and axillary lymph node dissection. She had an intermediate risk score for recurrence and proceeded with adjuvant cyclophosphamide, methotrexate, and 5-fluorouracil (CMF) and radiation therapy. She completed 5 years of adjuvant antiestrogen therapy with the aromatase inhibitor anastrozole. She has been compliant in her follow-up, with normal mammogram findings approximately 6 months ago."
],
"date": "July 27, 2016",
"figures": [],
"markdown": "# A 72-Year-Old Woman With Back Pain and Hypercalcemia\n\n **Authors:** Tarah Ballinger, MD; Kathy D. Miller, MD \n **Date:** July 27, 2016\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.\nA 72-year-old woman presents to her primary care physician because of worsening lower back pain. She has a known history of osteoarthritis and chronic back pain, which is usually helped with steroid injections and over-the-counter nonsteroidal anti-inflammatory medications. However, she had injections the week before her presentation and is taking multiple doses of naproxen each day without the usual relief. She denies any weakness or numbness of the lower extremities. She is otherwise feeling well and is a very active woman who regularly exercises.\nThe patient's medical history is significant for right knee, right hand, and left shoulder surgeries owing to osteoarthritis. Ten years ago, she was diagnosed with stage I estrogen receptor (ER)-positive, human epidermal growth factor receptor (HER2)-negative invasive ductal carcinoma of the breast. Her breast cancer was treated with lumpectomy and axillary lymph node dissection. She had an intermediate risk score for recurrence and proceeded with adjuvant cyclophosphamide, methotrexate, and 5-fluorouracil (CMF) and radiation therapy. She completed 5 years of adjuvant antiestrogen therapy with the aromatase inhibitor anastrozole. She has been compliant in her follow-up, with normal mammogram findings approximately 6 months ago.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 72-Year-Old Woman With Back Pain and Hypercalcemia"
},
{
"authors": "Tarah Ballinger, MD; Kathy D. Miller, MD",
"content": [
"The physical examination reveals a healthy woman who appears younger than her stated age. She is well-appearing and in no distress. Vital signs include a temperature of 96.4°F (35.8°C), heart rate of 75 beats/min, blood pressure of 152/78 mm Hg, and oxygen saturation of 98% on room air. Her body weight is 158.7 lb, which is stable from 160.9 lb 3 months ago.",
"Figure 1.",
"Figure 2.",
"Her physical examination is otherwise unremarkable except for well-healed surgical scars on the left breast and left axilla. No masses or skin changes are noted in either breast. Her musculoskeletal examination is unremarkable, with no pain upon palpation of the spine or paraspinal muscles. Her strength is normal and symmetrical in the bilateral lower extremities, and sensation is intact.",
"Routine laboratory testing is significant for a calcium level of 14 mg/dL and a creatinine level of 1.4 mg/dL (baseline, 0.8 mg/dL). The patient's electrolyte levels are within the normal ranges, and her albumin level is 4 mg/dL. Her complete blood count is unremarkable, except for mild anemia with a hemoglobin level of 11.6 g/dL (which is her baseline).",
"She was given intravenous fluids, and MRI of the lumbar spine with contrast was performed to further evaluate her pain (Figure 1). This was followed by CT of the chest, abdomen, and pelvis owing to multiple bony lesions seen on MRI (Figure 2)."
],
"date": "July 27, 2016",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/866/401/866401-Thumb1.png"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/866/401/866401-Thumb2.png"
}
],
"markdown": "# A 72-Year-Old Woman With Back Pain and Hypercalcemia\n\n **Authors:** Tarah Ballinger, MD; Kathy D. Miller, MD \n **Date:** July 27, 2016\n\n ## Content\n\n The physical examination reveals a healthy woman who appears younger than her stated age. She is well-appearing and in no distress. Vital signs include a temperature of 96.4°F (35.8°C), heart rate of 75 beats/min, blood pressure of 152/78 mm Hg, and oxygen saturation of 98% on room air. Her body weight is 158.7 lb, which is stable from 160.9 lb 3 months ago.\nFigure 1.\nFigure 2.\nHer physical examination is otherwise unremarkable except for well-healed surgical scars on the left breast and left axilla. No masses or skin changes are noted in either breast. Her musculoskeletal examination is unremarkable, with no pain upon palpation of the spine or paraspinal muscles. Her strength is normal and symmetrical in the bilateral lower extremities, and sensation is intact.\nRoutine laboratory testing is significant for a calcium level of 14 mg/dL and a creatinine level of 1.4 mg/dL (baseline, 0.8 mg/dL). The patient's electrolyte levels are within the normal ranges, and her albumin level is 4 mg/dL. Her complete blood count is unremarkable, except for mild anemia with a hemoglobin level of 11.6 g/dL (which is her baseline).\nShe was given intravenous fluids, and MRI of the lumbar spine with contrast was performed to further evaluate her pain (Figure 1). This was followed by CT of the chest, abdomen, and pelvis owing to multiple bony lesions seen on MRI (Figure 2).\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 990249,
"choiceText": "Perform additional staging imaging with brain MRI and PET CT",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990251,
"choiceText": "Begin antiestrogen therapy with tamoxifen",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990253,
"choiceText": "Start chemotherapy for visceral crisis with a taxane-based regimen",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990255,
"choiceText": "Perform a biopsy of one of the liver lesions",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990257,
"choiceText": "Consult with radiation oncology\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 313457,
"questionText": "On the basis of the case information and images, what is the best next step in management?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 72-Year-Old Woman With Back Pain and Hypercalcemia"
},
{
"authors": "Tarah Ballinger, MD; Kathy D. Miller, MD",
"content": [
"A liver biopsy was performed, which showed diffuse proliferation of atypical cells (Figure 3). Immunostaining was positive for CD10, CD20, BCL-6, and MUM-1, and negative for HER2, S100, CD30, and cytokeratin.",
"Figure 3.",
"The imaging performed in this patient showed multiple enlarged lymph nodes as well as lesions in the bones and liver, which were initially concerning for new metastases of her prior breast cancer. However, morphologic findings on biopsy showed large, atypical B cells destroying the normal lymph node architecture. The tumor cells were large, with prominent nucleoli and basophilic cytoplasm. This could be consistent with multiple possible cancers, and the differential diagnosis was narrowed using further information provided by immunostaining.",
"The cells are positive for the B-cell markers CD10 and CD20, as well as the B-cell lymphoma proteins BCL-6 and MUM-1. This morphology and immunophenotype is consistent with a diagnosis of diffuse large B-cell lymphoma (DLBCL). Her hypercalcemia was probably due to production of parathyroid-related protein and 1,25-dihydroxyvitamin D (calcitriol) by the lymphoma cells.[1]",
"This case illustrates the importance of biopsy for first recurrence of breast cancer after primary treatment. The National Comprehensive Cancer Network (NCCN) guidelines recommend that the first recurrence of breast cancer or metastatic disease be biopsied, to accurately determine the diagnosis and tumor histology. In addition, if the biopsy is consistent with recurrent breast cancer, this also allows redetermination of ER, PR, and HER2 status, which can change between primary and metastatic breast cancers.",
"Numerous retrospective studies have reported various ranges of receptor status changes between primary tumors and metastatic or local recurrences: as much as 40% for ER receptor status and as much as 14% for HER2 receptor status, with one study reporting HER2 discordance of 27%.[2,3,4,5,6,7] For hormone receptors, it is more common to lose receptor positivity than to gain it. Literature is conflicting on whether it is more common to gain HER2 expression or to lose it; however, loss of HER2 positivity in tumors previously exposed to chemotherapy has been documented.[3,8] Many reasons have been proposed for these changes. The biology of the tumor may change; for example, giving hormone therapy to an ER-positive tumor could select for any ER-negative tumor cells to grow and metastasize.",
"Alternatively, errors in pathologic determination of receptor status or heterogeneity within the tumor may occur, meaning that different parts of the tumor have varying expression of a receptor.[9] Therefore, owing to the possibility for sampling error, taking the patient's clinical course into consideration is important when interpreting biopsy results. For example, in patients who have an indolent clinical course consistent with ER-positive breast cancer and whose primary cancer was ER-positive, testing a course of antiestrogen therapy is reasonable, even if the metastatic disease is ER-negative."
],
"date": "July 27, 2016",
"figures": [
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/866/401/866401-Thumb3.png"
}
],
"markdown": "# A 72-Year-Old Woman With Back Pain and Hypercalcemia\n\n **Authors:** Tarah Ballinger, MD; Kathy D. Miller, MD \n **Date:** July 27, 2016\n\n ## Content\n\n A liver biopsy was performed, which showed diffuse proliferation of atypical cells (Figure 3). Immunostaining was positive for CD10, CD20, BCL-6, and MUM-1, and negative for HER2, S100, CD30, and cytokeratin.\nFigure 3.\nThe imaging performed in this patient showed multiple enlarged lymph nodes as well as lesions in the bones and liver, which were initially concerning for new metastases of her prior breast cancer. However, morphologic findings on biopsy showed large, atypical B cells destroying the normal lymph node architecture. The tumor cells were large, with prominent nucleoli and basophilic cytoplasm. This could be consistent with multiple possible cancers, and the differential diagnosis was narrowed using further information provided by immunostaining.\nThe cells are positive for the B-cell markers CD10 and CD20, as well as the B-cell lymphoma proteins BCL-6 and MUM-1. This morphology and immunophenotype is consistent with a diagnosis of diffuse large B-cell lymphoma (DLBCL). Her hypercalcemia was probably due to production of parathyroid-related protein and 1,25-dihydroxyvitamin D (calcitriol) by the lymphoma cells.[1]\nThis case illustrates the importance of biopsy for first recurrence of breast cancer after primary treatment. The National Comprehensive Cancer Network (NCCN) guidelines recommend that the first recurrence of breast cancer or metastatic disease be biopsied, to accurately determine the diagnosis and tumor histology. In addition, if the biopsy is consistent with recurrent breast cancer, this also allows redetermination of ER, PR, and HER2 status, which can change between primary and metastatic breast cancers.\nNumerous retrospective studies have reported various ranges of receptor status changes between primary tumors and metastatic or local recurrences: as much as 40% for ER receptor status and as much as 14% for HER2 receptor status, with one study reporting HER2 discordance of 27%.[2,3,4,5,6,7] For hormone receptors, it is more common to lose receptor positivity than to gain it. Literature is conflicting on whether it is more common to gain HER2 expression or to lose it; however, loss of HER2 positivity in tumors previously exposed to chemotherapy has been documented.[3,8] Many reasons have been proposed for these changes. The biology of the tumor may change; for example, giving hormone therapy to an ER-positive tumor could select for any ER-negative tumor cells to grow and metastasize.\nAlternatively, errors in pathologic determination of receptor status or heterogeneity within the tumor may occur, meaning that different parts of the tumor have varying expression of a receptor.[9] Therefore, owing to the possibility for sampling error, taking the patient's clinical course into consideration is important when interpreting biopsy results. For example, in patients who have an indolent clinical course consistent with ER-positive breast cancer and whose primary cancer was ER-positive, testing a course of antiestrogen therapy is reasonable, even if the metastatic disease is ER-negative.\n\n ## Figures\n\n **Figure 3.** \n \n\n\n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 990249,
"choiceText": "Perform additional staging imaging with brain MRI and PET CT",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990251,
"choiceText": "Begin antiestrogen therapy with tamoxifen",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990253,
"choiceText": "Start chemotherapy for visceral crisis with a taxane-based regimen",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990255,
"choiceText": "Perform a biopsy of one of the liver lesions",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990257,
"choiceText": "Consult with radiation oncology\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 313457,
"questionText": "On the basis of the case information and images, what is the best next step in management?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 72-Year-Old Woman With Back Pain and Hypercalcemia"
},
{
"authors": "Tarah Ballinger, MD; Kathy D. Miller, MD",
"content": [
"A change in ER/PR or HER2 status can have implications for both prognosis and treatment. In general, survival decreases after recurrence, and overall survival decreases for patients with discordant receptor results between the primary tumor and the recurrent tumor. The poorest outcome is seen in patients who develop a triple-negative phenotype, those who lose ER positivity, or those who lose HER2 positivity.[4,5,7,10] This is probably due to inappropriate therapy choices and lack of effectiveness of targeted therapies.",
"Another important reason for repeat biopsy of recurrent disease is the possibility of a secondary cancer, as was the case for this patient. Secondary cancers are a significant cause of morbidity and mortality in cancer survivors, and patients should be monitored for their occurrence. Patients with breast cancer who have been previously treated with chemotherapy or radiation are at higher risk for numerous long-term treatment-related complications, including secondary cancers. One source of this increased risk comes from adjuvant radiation, use of which has dramatically increased after randomized trials showing similar outcomes for breast-conserving surgery followed by radiation, compared with total mastectomy.[11]",
"Prior large retrospective studies have found an increased risk for acute leukemia, sarcoma, and lung cancer in patients with breast cancer who received radiation therapy.[12,13] Chemotherapy also increases the risk for secondary cancers, particularly bone marrow neoplasms, such as acute myelogenous leukemia (AML) and myelodysplastic syndrome (MDS). Therapy-related AML/MDS accounts for approximately 20% of cases.",
"Two variants of this type of AML/MDS are noted. The first type results from topoisomerase II inhibitors (ie, anthracyclines, such as doxorubicin), with a lifetime risk of less than 1% and a latency period of 1-3 years after treatment; disease is characterized by a chromosome 11q23 translocation. The other results from DNA-alkylating agents (such as cyclophosphamide), with a lifetime incidence of 1%-5% and a latency period of 4-6 years; disease is characterized by deletions of chromosome 5 or 7. This is meaningful for patients with breast cancer, many of whom receive four cycles of both doxorubicin plus cyclophosphamide in the neoadjuvant or adjuvant setting.[14]",
"A large prospective study of 20,000 patients with early-stage breast cancer found a 10-year risk of 0.5% for the development of AML or MDS.[15] These therapy-related leukemias are very refractory to treatment and have a high mortality rate. For the patient in this case, who had an intermediate probability of recurrent disease and therefore derives an uncertain benefit from adjuvant chemotherapy, this was an important risk to take into account."
],
"date": "July 27, 2016",
"figures": [],
"markdown": "# A 72-Year-Old Woman With Back Pain and Hypercalcemia\n\n **Authors:** Tarah Ballinger, MD; Kathy D. Miller, MD \n **Date:** July 27, 2016\n\n ## Content\n\n A change in ER/PR or HER2 status can have implications for both prognosis and treatment. In general, survival decreases after recurrence, and overall survival decreases for patients with discordant receptor results between the primary tumor and the recurrent tumor. The poorest outcome is seen in patients who develop a triple-negative phenotype, those who lose ER positivity, or those who lose HER2 positivity.[4,5,7,10] This is probably due to inappropriate therapy choices and lack of effectiveness of targeted therapies.\nAnother important reason for repeat biopsy of recurrent disease is the possibility of a secondary cancer, as was the case for this patient. Secondary cancers are a significant cause of morbidity and mortality in cancer survivors, and patients should be monitored for their occurrence. Patients with breast cancer who have been previously treated with chemotherapy or radiation are at higher risk for numerous long-term treatment-related complications, including secondary cancers. One source of this increased risk comes from adjuvant radiation, use of which has dramatically increased after randomized trials showing similar outcomes for breast-conserving surgery followed by radiation, compared with total mastectomy.[11]\nPrior large retrospective studies have found an increased risk for acute leukemia, sarcoma, and lung cancer in patients with breast cancer who received radiation therapy.[12,13] Chemotherapy also increases the risk for secondary cancers, particularly bone marrow neoplasms, such as acute myelogenous leukemia (AML) and myelodysplastic syndrome (MDS). Therapy-related AML/MDS accounts for approximately 20% of cases.\nTwo variants of this type of AML/MDS are noted. The first type results from topoisomerase II inhibitors (ie, anthracyclines, such as doxorubicin), with a lifetime risk of less than 1% and a latency period of 1-3 years after treatment; disease is characterized by a chromosome 11q23 translocation. The other results from DNA-alkylating agents (such as cyclophosphamide), with a lifetime incidence of 1%-5% and a latency period of 4-6 years; disease is characterized by deletions of chromosome 5 or 7. This is meaningful for patients with breast cancer, many of whom receive four cycles of both doxorubicin plus cyclophosphamide in the neoadjuvant or adjuvant setting.[14]\nA large prospective study of 20,000 patients with early-stage breast cancer found a 10-year risk of 0.5% for the development of AML or MDS.[15] These therapy-related leukemias are very refractory to treatment and have a high mortality rate. For the patient in this case, who had an intermediate probability of recurrent disease and therefore derives an uncertain benefit from adjuvant chemotherapy, this was an important risk to take into account.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 72-Year-Old Woman With Back Pain and Hypercalcemia"
},
{
"authors": "Tarah Ballinger, MD; Kathy D. Miller, MD",
"content": [
"Patients with breast cancer may also be at increased risk for other solid-tumor malignancies owing to an underlying germline mutation, independent of what cancer treatments they have received. For example, patients with a BRCA1 or BRCA2 mutation are also at increased risk for ovarian, colon, pancreatic, and prostate cancers (the latter two are more commonly associated with BRCA2). Patients with breast cancer who are receiving a selective estrogen receptor modulator, such as tamoxifen, are at increased risk for endometrial cancers, with a lifetime risk of 1%-2%.[16]",
"DLBCL is a rarer secondary cancer. Whether this patient's prior therapy for breast cancer contributed to the development of her lymphoma is unclear. DLBCL is the most common type of lymphoma and accounts for approximately 25% of all non-Hodgkin lymphomas.[17] This patient's presentation is atypical, and patients usually present with a rapidly enlarging nodal mass or \"B\" symptoms, including fevers, drenching night sweats, and weight loss. Like this patient, approximately 40% of patients have involvement outside of the lymph nodes.",
"The patient's lymphoma was considered advanced stage, or stage IV by the Lugano staging system, owing to diffuse involvement of organs outside the lymphatic system and the fact that the disease could not be contained in a single field for radiation therapy.[11] She was therefore treated with six cycles of cyclophosphamide, vincristine, doxorubicin, and prednisone, along with the anti-CD20 antibody rituximab (R-CHOP). In addition, her hypercalcemia was treated with the bisphosphonate zoledronic acid. She tolerated her therapy well and achieved complete remission."
],
"date": "July 27, 2016",
"figures": [],
"markdown": "# A 72-Year-Old Woman With Back Pain and Hypercalcemia\n\n **Authors:** Tarah Ballinger, MD; Kathy D. Miller, MD \n **Date:** July 27, 2016\n\n ## Content\n\n Patients with breast cancer may also be at increased risk for other solid-tumor malignancies owing to an underlying germline mutation, independent of what cancer treatments they have received. For example, patients with a BRCA1 or BRCA2 mutation are also at increased risk for ovarian, colon, pancreatic, and prostate cancers (the latter two are more commonly associated with BRCA2). Patients with breast cancer who are receiving a selective estrogen receptor modulator, such as tamoxifen, are at increased risk for endometrial cancers, with a lifetime risk of 1%-2%.[16]\nDLBCL is a rarer secondary cancer. Whether this patient's prior therapy for breast cancer contributed to the development of her lymphoma is unclear. DLBCL is the most common type of lymphoma and accounts for approximately 25% of all non-Hodgkin lymphomas.[17] This patient's presentation is atypical, and patients usually present with a rapidly enlarging nodal mass or \"B\" symptoms, including fevers, drenching night sweats, and weight loss. Like this patient, approximately 40% of patients have involvement outside of the lymph nodes.\nThe patient's lymphoma was considered advanced stage, or stage IV by the Lugano staging system, owing to diffuse involvement of organs outside the lymphatic system and the fact that the disease could not be contained in a single field for radiation therapy.[11] She was therefore treated with six cycles of cyclophosphamide, vincristine, doxorubicin, and prednisone, along with the anti-CD20 antibody rituximab (R-CHOP). In addition, her hypercalcemia was treated with the bisphosphonate zoledronic acid. She tolerated her therapy well and achieved complete remission.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 990259,
"choiceText": "Endometrial cancer",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990261,
"choiceText": "Local recurrence of her breast cancer",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990263,
"choiceText": "Coronary artery disease",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990265,
"choiceText": "Hypothyroidism",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990267,
"choiceText": "Chronic obstructive pulmonary disease\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The patient had prior radiation therapy to the chest and prior cytotoxic chemotherapy that place her at an increased lifetime risk for coronary artery disease. Aromatase inhibitors increase the risk for osteoporosis, but not for cancer. Breast radiation decreases the risk for local breast cancer recurrence, rather than increasing it.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 313459,
"questionText": "Which of the following medical conditions does the patient in this case have an increased risk for, on the basis of her prior breast cancer therapy (aromatase inhibitor; radiation; and chemotherapy with cyclophosphamide, methotrexate, and 5-fluorouracil)?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 990457,
"choiceText": "Bilateral mammograms or breast MRI every 6 months",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990459,
"choiceText": "Gynecologic exam and transvaginal ultrasound every 2 years",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990461,
"choiceText": "PET CT every year",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990463,
"choiceText": "Echocardiogram every 2 years",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990465,
"choiceText": "Complete blood count every year",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Patients who have been previously exposed to topoisomerase inhibitors or alkylating agents should have a complete blood count every 6-12 months, owing to the risk for bone marrow neoplasms. The patient should otherwise continue annual mammograms for follow-up of her breast cancer and other age-appropriate screening. Routine PET imaging has no role in patients with DLBCL. Screening echocardiograms in patients who have received anthracyclines have not yet been recommended. ",
"displayOrder": 4,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 313527,
"questionText": "Taking into account the patient's history of breast cancer, lymphoma, and prior treatments, which of the following screening tests should be performed?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 72-Year-Old Woman With Back Pain and Hypercalcemia"
},
{
"authors": "Tarah Ballinger, MD; Kathy D. Miller, MD",
"content": [],
"date": "July 27, 2016",
"figures": [],
"markdown": "# A 72-Year-Old Woman With Back Pain and Hypercalcemia\n\n **Authors:** Tarah Ballinger, MD; Kathy D. Miller, MD \n **Date:** July 27, 2016\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 990259,
"choiceText": "Endometrial cancer",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990261,
"choiceText": "Local recurrence of her breast cancer",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990263,
"choiceText": "Coronary artery disease",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990265,
"choiceText": "Hypothyroidism",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990267,
"choiceText": "Chronic obstructive pulmonary disease\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The patient had prior radiation therapy to the chest and prior cytotoxic chemotherapy that place her at an increased lifetime risk for coronary artery disease. Aromatase inhibitors increase the risk for osteoporosis, but not for cancer. Breast radiation decreases the risk for local breast cancer recurrence, rather than increasing it.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 313459,
"questionText": "Which of the following medical conditions does the patient in this case have an increased risk for, on the basis of her prior breast cancer therapy (aromatase inhibitor; radiation; and chemotherapy with cyclophosphamide, methotrexate, and 5-fluorouracil)?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 990457,
"choiceText": "Bilateral mammograms or breast MRI every 6 months",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990459,
"choiceText": "Gynecologic exam and transvaginal ultrasound every 2 years",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990461,
"choiceText": "PET CT every year",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990463,
"choiceText": "Echocardiogram every 2 years",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990465,
"choiceText": "Complete blood count every year",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Patients who have been previously exposed to topoisomerase inhibitors or alkylating agents should have a complete blood count every 6-12 months, owing to the risk for bone marrow neoplasms. The patient should otherwise continue annual mammograms for follow-up of her breast cancer and other age-appropriate screening. Routine PET imaging has no role in patients with DLBCL. Screening echocardiograms in patients who have received anthracyclines have not yet been recommended. ",
"displayOrder": 4,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 313527,
"questionText": "Taking into account the patient's history of breast cancer, lymphoma, and prior treatments, which of the following screening tests should be performed?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 72-Year-Old Woman With Back Pain and Hypercalcemia"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 990249,
"choiceText": "Perform additional staging imaging with brain MRI and PET CT",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990251,
"choiceText": "Begin antiestrogen therapy with tamoxifen",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990253,
"choiceText": "Start chemotherapy for visceral crisis with a taxane-based regimen",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990255,
"choiceText": "Perform a biopsy of one of the liver lesions",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990257,
"choiceText": "Consult with radiation oncology\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 313457,
"questionText": "On the basis of the case information and images, what is the best next step in management?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 990259,
"choiceText": "Endometrial cancer",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990261,
"choiceText": "Local recurrence of her breast cancer",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990263,
"choiceText": "Coronary artery disease",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990265,
"choiceText": "Hypothyroidism",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990267,
"choiceText": "Chronic obstructive pulmonary disease\r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The patient had prior radiation therapy to the chest and prior cytotoxic chemotherapy that place her at an increased lifetime risk for coronary artery disease. Aromatase inhibitors increase the risk for osteoporosis, but not for cancer. Breast radiation decreases the risk for local breast cancer recurrence, rather than increasing it.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 313459,
"questionText": "Which of the following medical conditions does the patient in this case have an increased risk for, on the basis of her prior breast cancer therapy (aromatase inhibitor; radiation; and chemotherapy with cyclophosphamide, methotrexate, and 5-fluorouracil)?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 990457,
"choiceText": "Bilateral mammograms or breast MRI every 6 months",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990459,
"choiceText": "Gynecologic exam and transvaginal ultrasound every 2 years",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990461,
"choiceText": "PET CT every year",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990463,
"choiceText": "Echocardiogram every 2 years",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990465,
"choiceText": "Complete blood count every year",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Patients who have been previously exposed to topoisomerase inhibitors or alkylating agents should have a complete blood count every 6-12 months, owing to the risk for bone marrow neoplasms. The patient should otherwise continue annual mammograms for follow-up of her breast cancer and other age-appropriate screening. Routine PET imaging has no role in patients with DLBCL. Screening echocardiograms in patients who have received anthracyclines have not yet been recommended. ",
"displayOrder": 4,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 313527,
"questionText": "Taking into account the patient's history of breast cancer, lymphoma, and prior treatments, which of the following screening tests should be performed?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
866409 | /viewarticle/866409 | [
{
"authors": "Mauricio E. Pons, MD; Maria Silvana Horenstein, MD",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.",
"A 5-year-old boy presents to his primary care provider with a \"gleam\" that was first noticed in his left eye in dim light 1 week earlier. His parents do not report any fever or concurrent illnesses.",
"The patient has taken no recent medications (except for multivitamins). His parents deny a history of allergies, and his vaccination schedule is up to date. The patient was born full term and has no siblings. The family history is significant only for maternal gestational diabetes and hypothyroidism. No parental consanguinity is noted."
],
"date": "July 22, 2016",
"figures": [],
"markdown": "# A 'Gleam' in the Left Eye of a 5-Year-Old Boy\n\n **Authors:** Mauricio E. Pons, MD; Maria Silvana Horenstein, MD \n **Date:** July 22, 2016\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.\nA 5-year-old boy presents to his primary care provider with a \"gleam\" that was first noticed in his left eye in dim light 1 week earlier. His parents do not report any fever or concurrent illnesses.\nThe patient has taken no recent medications (except for multivitamins). His parents deny a history of allergies, and his vaccination schedule is up to date. The patient was born full term and has no siblings. The family history is significant only for maternal gestational diabetes and hypothyroidism. No parental consanguinity is noted.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 'Gleam' in the Left Eye of a 5-Year-Old Boy"
},
{
"authors": "Mauricio E. Pons, MD; Maria Silvana Horenstein, MD",
"content": [
"The physical examination reveals a well-nourished child in no distress. The patient's height and weight are at the 60th and 50th percentiles, respectively. His oral temperature is 98.6°F. His pulse is regular, with a rate of 80 beats/min, and his blood pressure is 110/65 mm Hg. His lungs are clear to auscultation, with normal respiratory effort. The S1 and S2 heart sounds are normal. The abdomen is soft and nontender. The peripheral arterial pulses in the upper and lower extremities are normal.",
"Figure 1",
"Figure 2",
"Examination of the patient's head and neck shows mild esotropia, normal lids, clear corneas, and moderate conjunctival congestion in the left eye. The left eye is tender to the touch and, as a result, the child does not cooperate with a complete examination of the eye. He is scheduled for another examination under anesthesia by a pediatric ophthalmologist.",
"During the second examination, the eyes are noted to have anisocoric pupils that are round and regular, with an afferent pupillary defect in the left eye. The red reflex is normal in the right eye, but absent in the left. Funduscopic examination with dilated pupils reveals normal fundus in the right eye; in the left eye, a yellowish mass with dilated vessels and total retinal detachment are noted. The crystalline lens is clear in both eyes.",
"Fluorescein angiography is performed, which shows fluorescein leakage from neovascularization of the iris. Gonioscopy reveals a closed anterior chamber angle, with no view of any angle landmarks. The retina exhibits telangiectatic vessels, microaneurysms, and irregular and dilated vessels.",
"Intraocular pressure is measured at 15 mm Hg in the right eye, which is within the reference range, and 50 mm Hg in the left eye, which is elevated. B-scan ultrasonography is performed, which shows total serous retinal detachment in the left eye.",
"A review of family photographs reveals that the abnormal red reflex has been present for several months.",
"Figures 1 and 2 show the patient's dilated left eye and the fluorescein angiography."
],
"date": "July 22, 2016",
"figures": [
{
"caption": "Figure 1",
"image_url": "https://img.medscapestatic.com/article/866/409/866409-Thumb1.png"
},
{
"caption": "Figure 2",
"image_url": "https://img.medscapestatic.com/article/866/409/866409-Thumb2.png"
}
],
"markdown": "# A 'Gleam' in the Left Eye of a 5-Year-Old Boy\n\n **Authors:** Mauricio E. Pons, MD; Maria Silvana Horenstein, MD \n **Date:** July 22, 2016\n\n ## Content\n\n The physical examination reveals a well-nourished child in no distress. The patient's height and weight are at the 60th and 50th percentiles, respectively. His oral temperature is 98.6°F. His pulse is regular, with a rate of 80 beats/min, and his blood pressure is 110/65 mm Hg. His lungs are clear to auscultation, with normal respiratory effort. The S1 and S2 heart sounds are normal. The abdomen is soft and nontender. The peripheral arterial pulses in the upper and lower extremities are normal.\nFigure 1\nFigure 2\nExamination of the patient's head and neck shows mild esotropia, normal lids, clear corneas, and moderate conjunctival congestion in the left eye. The left eye is tender to the touch and, as a result, the child does not cooperate with a complete examination of the eye. He is scheduled for another examination under anesthesia by a pediatric ophthalmologist.\nDuring the second examination, the eyes are noted to have anisocoric pupils that are round and regular, with an afferent pupillary defect in the left eye. The red reflex is normal in the right eye, but absent in the left. Funduscopic examination with dilated pupils reveals normal fundus in the right eye; in the left eye, a yellowish mass with dilated vessels and total retinal detachment are noted. The crystalline lens is clear in both eyes.\nFluorescein angiography is performed, which shows fluorescein leakage from neovascularization of the iris. Gonioscopy reveals a closed anterior chamber angle, with no view of any angle landmarks. The retina exhibits telangiectatic vessels, microaneurysms, and irregular and dilated vessels.\nIntraocular pressure is measured at 15 mm Hg in the right eye, which is within the reference range, and 50 mm Hg in the left eye, which is elevated. B-scan ultrasonography is performed, which shows total serous retinal detachment in the left eye.\nA review of family photographs reveals that the abnormal red reflex has been present for several months.\nFigures 1 and 2 show the patient's dilated left eye and the fluorescein angiography.\n\n ## Figures\n\n **Figure 1** \n \n\n**Figure 2** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 990609,
"choiceText": "Endophalmitis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990611,
"choiceText": "Coats disease (exudative retinitis)",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990613,
"choiceText": "Orbital pseudotumor",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990615,
"choiceText": "Retinoblastoma",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 313591,
"questionText": "What is the underlying cause of the child's total retinal detachment?<br/><br/>\r\n<em>Hint: Look closely at the vascular pattern of the lesions in this child.</em>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 'Gleam' in the Left Eye of a 5-Year-Old Boy"
},
{
"authors": "Mauricio E. Pons, MD; Maria Silvana Horenstein, MD",
"content": [
"Coats disease is a rare idiopathic retinal condition, first described by George Coats in 1908, in which abnormal telangiectatic retinal vessels cause intra- and subretinal exudates, leading to serous retinal detachment.[1] The most common presentations are decreased visual acuity, strabismus, or leukocoria, which is generally more yellow than white owing to the presence of subretinal lipid. Most cases that present with leukocoria are diagnosed at age 5-9 years, which is older than most patients with retinoblastoma.",
"No racial or laterality predilection is noted for Coats disease. The incidence and prevalence are unknown.",
"Clinical examination typically reveals subretinal lipid and abnormal telangiectatic vessels. In advanced cases, ocular ultrasonography demonstrates complete retinal detachment with massive subretinal lipid. Intraocular calcification is very rarely present in Coats disease, another feature that helps to distinguish it from retinoblastoma.",
"Coats disease is presumed to have a vascular etiology, postulated to be a somatic mutation in the NDP gene, resulting in a deficiency of norrin (the protein product of the NDP gene) within the developing retina. It is unilateral in 95% of children, with a 4-to-1 male predominance and a bimodal distribution in males younger than 18 years and middle-aged men, often as a secondary response to a vascular event.",
"The mean age at diagnosis is 5 years. It may be seen as early as age 1 month, and most cases are diagnosed before 10 years of age. The condition is more severe in younger patients (especially those < 3 years), in whom the disease progresses more rapidly.",
"Coats disease has a sporadic occurrence and is not known to be associated with other organ abnormalities, ocular conditions, or systemic conditions. No evidence suggests genetic predisposition; however, it has been associated with retinitis pigmentosa, muscular dystrophy, deafness, and Norrie disease.[2]",
"Clinically, Coats disease presents with decreased vision, strabismus, leukocoria (white pupillary reflex or \"cat's eye reflex\"), and redness. It may, however, be detected incidentally.[3] It is not generally associated with pain, except in advanced cases with secondary associated neovascular glaucoma. It has not been associated with infectious or inflammatory conditions, and most patients have no associated systemic medical problems."
],
"date": "July 22, 2016",
"figures": [],
"markdown": "# A 'Gleam' in the Left Eye of a 5-Year-Old Boy\n\n **Authors:** Mauricio E. Pons, MD; Maria Silvana Horenstein, MD \n **Date:** July 22, 2016\n\n ## Content\n\n Coats disease is a rare idiopathic retinal condition, first described by George Coats in 1908, in which abnormal telangiectatic retinal vessels cause intra- and subretinal exudates, leading to serous retinal detachment.[1] The most common presentations are decreased visual acuity, strabismus, or leukocoria, which is generally more yellow than white owing to the presence of subretinal lipid. Most cases that present with leukocoria are diagnosed at age 5-9 years, which is older than most patients with retinoblastoma.\nNo racial or laterality predilection is noted for Coats disease. The incidence and prevalence are unknown.\nClinical examination typically reveals subretinal lipid and abnormal telangiectatic vessels. In advanced cases, ocular ultrasonography demonstrates complete retinal detachment with massive subretinal lipid. Intraocular calcification is very rarely present in Coats disease, another feature that helps to distinguish it from retinoblastoma.\nCoats disease is presumed to have a vascular etiology, postulated to be a somatic mutation in the NDP gene, resulting in a deficiency of norrin (the protein product of the NDP gene) within the developing retina. It is unilateral in 95% of children, with a 4-to-1 male predominance and a bimodal distribution in males younger than 18 years and middle-aged men, often as a secondary response to a vascular event.\nThe mean age at diagnosis is 5 years. It may be seen as early as age 1 month, and most cases are diagnosed before 10 years of age. The condition is more severe in younger patients (especially those < 3 years), in whom the disease progresses more rapidly.\nCoats disease has a sporadic occurrence and is not known to be associated with other organ abnormalities, ocular conditions, or systemic conditions. No evidence suggests genetic predisposition; however, it has been associated with retinitis pigmentosa, muscular dystrophy, deafness, and Norrie disease.[2]\nClinically, Coats disease presents with decreased vision, strabismus, leukocoria (white pupillary reflex or \"cat's eye reflex\"), and redness. It may, however, be detected incidentally.[3] It is not generally associated with pain, except in advanced cases with secondary associated neovascular glaucoma. It has not been associated with infectious or inflammatory conditions, and most patients have no associated systemic medical problems.\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 990609,
"choiceText": "Endophalmitis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990611,
"choiceText": "Coats disease (exudative retinitis)",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990613,
"choiceText": "Orbital pseudotumor",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990615,
"choiceText": "Retinoblastoma",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 313591,
"questionText": "What is the underlying cause of the child's total retinal detachment?<br/><br/>\r\n<em>Hint: Look closely at the vascular pattern of the lesions in this child.</em>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 'Gleam' in the Left Eye of a 5-Year-Old Boy"
},
{
"authors": "Mauricio E. Pons, MD; Maria Silvana Horenstein, MD",
"content": [
"The clinical presentation of Coats disease varies widely. Typically, ophthalmoscopic examination reveals a localized area of subretinal exudates associated with vascular abnormalities. Vascular changes may include peripheral retinal telangiectasia, capillary and small-vessel dilatation and tortuosity, sheathing, capillary nonperfusion, and small aneurysms located at the equator of the eye and the ora serrata (most commonly, inferotemporal). The posterior pole is less frequently affected.",
"Exudation is a common feature that presents in most cases as flat intraretinal and subretinal exudates. These exudates initially appear in areas of telangiectasia and progress to become more widespread. Macular protein accumulation can occur directly from macular telangiectasia or indirectly from peripheral disease. A dense exudate or white nodule in the macula can progress to a disciform lesion that indicates a poor visual prognosis. Retinal hemorrhages may be seen. The vitreous remains clear until the advanced stages. Retinal cysts may be seen and are common in chronic retinal detachments of different etiologies.[2]",
"The diagnosis may be suspected clinically by indirect ophthalmoscopic evaluation of both eyes, but ancillary testing is extremely helpful to differentiate Coats disease from retinoblastoma, which is a more feared disease. A high percentage of patients with this condition observed by primary care providers are referred to ophthalmologists specifically to rule out retinoblastoma.",
"A- and B-scan ultrasonography reveal low internal reflectivity consistent with an exudative retinal detachment and rules out the presence of a solid tumor. Intravenous fluorescein angiography helps to visualize the telangiectatic vessels as irregular dilated tortuous vessels filling in the late arterial and early venous phases. Microaneurysms are seen as \"lightbulb aneurysms.\" Angiography reveals progressive leakage from abnormal vessels, adjacent areas of capillary dropout, and late staining of intraretinal exudates.[2,4]",
"On the basis of the clinical appearance and progression, Coats disease may be classified in five grades, as follows[5]:",
"Grade I: Isolated focal exudates",
"Grade II: Massive elevated exudation",
"Grade III: Partial retinal detachment",
"Grade IV: Total retinal detachment",
"Grade V: Secondary complications",
"A more recent classification system has emerged, which is based on the prognosis. It grades the disease in the following manner[6]:",
"Grade I: Telangiectasias only",
"Grade II: Telangiectasias and exudation",
"Grade III: Exudative retinal detachment",
"Grade IV: Total retinal detachment with secondary glaucoma",
"Grade V: End-stage disease",
"Spiral CT has been used to rule out intraocular calcifications that more commonly present in retinoblastoma. The clinician must consider and rule out the possibility of retinoblastoma because, if left untreated, it can be fatal. As a result of concerns about radiation exposure, CT is less frequently used today for diagnosing intraocular pediatric tumors. MRI with gadolinium enhancement is helpful in evaluating the optic nerve and the globe. It is not, however, as valuable as CT or ultrasonography for detecting the intraocular calcium deposits commonly found in association with retinoblastoma tumors.",
"MRI is also very helpful for evaluating the pineal gland in cases of familial retinoblastoma. Subretinal fluid appears hyperintense on T1- and T2-weighted images. Solid tumors are hyperintense on T1-weighted images but hypointense in T2-weighted images.",
"The differential diagnosis of Coats disease is similar to that of leukocoria (which is extensive). It includes retinoblastoma; persistent fetal vasculature; retinopathy of prematurity; rhegmatogenous, exudative, or tractional retinal detachment; ocular toxocariasis, familial exudative vitreoretinopathy; retinal capillary hemangiomatosis (von Hippel-Lindau disease); cataract; glaucoma; uveitis; vitreous hemorrhage; and colobomas of the choroid and optic disc. Leber miliary aneurysm disease is an early or nonprogressive form of Coats disease.[2,4]"
],
"date": "July 22, 2016",
"figures": [],
"markdown": "# A 'Gleam' in the Left Eye of a 5-Year-Old Boy\n\n **Authors:** Mauricio E. Pons, MD; Maria Silvana Horenstein, MD \n **Date:** July 22, 2016\n\n ## Content\n\n The clinical presentation of Coats disease varies widely. Typically, ophthalmoscopic examination reveals a localized area of subretinal exudates associated with vascular abnormalities. Vascular changes may include peripheral retinal telangiectasia, capillary and small-vessel dilatation and tortuosity, sheathing, capillary nonperfusion, and small aneurysms located at the equator of the eye and the ora serrata (most commonly, inferotemporal). The posterior pole is less frequently affected.\nExudation is a common feature that presents in most cases as flat intraretinal and subretinal exudates. These exudates initially appear in areas of telangiectasia and progress to become more widespread. Macular protein accumulation can occur directly from macular telangiectasia or indirectly from peripheral disease. A dense exudate or white nodule in the macula can progress to a disciform lesion that indicates a poor visual prognosis. Retinal hemorrhages may be seen. The vitreous remains clear until the advanced stages. Retinal cysts may be seen and are common in chronic retinal detachments of different etiologies.[2]\nThe diagnosis may be suspected clinically by indirect ophthalmoscopic evaluation of both eyes, but ancillary testing is extremely helpful to differentiate Coats disease from retinoblastoma, which is a more feared disease. A high percentage of patients with this condition observed by primary care providers are referred to ophthalmologists specifically to rule out retinoblastoma.\nA- and B-scan ultrasonography reveal low internal reflectivity consistent with an exudative retinal detachment and rules out the presence of a solid tumor. Intravenous fluorescein angiography helps to visualize the telangiectatic vessels as irregular dilated tortuous vessels filling in the late arterial and early venous phases. Microaneurysms are seen as \"lightbulb aneurysms.\" Angiography reveals progressive leakage from abnormal vessels, adjacent areas of capillary dropout, and late staining of intraretinal exudates.[2,4]\nOn the basis of the clinical appearance and progression, Coats disease may be classified in five grades, as follows[5]:\nGrade I: Isolated focal exudates\nGrade II: Massive elevated exudation\nGrade III: Partial retinal detachment\nGrade IV: Total retinal detachment\nGrade V: Secondary complications\nA more recent classification system has emerged, which is based on the prognosis. It grades the disease in the following manner[6]:\nGrade I: Telangiectasias only\nGrade II: Telangiectasias and exudation\nGrade III: Exudative retinal detachment\nGrade IV: Total retinal detachment with secondary glaucoma\nGrade V: End-stage disease\nSpiral CT has been used to rule out intraocular calcifications that more commonly present in retinoblastoma. The clinician must consider and rule out the possibility of retinoblastoma because, if left untreated, it can be fatal. As a result of concerns about radiation exposure, CT is less frequently used today for diagnosing intraocular pediatric tumors. MRI with gadolinium enhancement is helpful in evaluating the optic nerve and the globe. It is not, however, as valuable as CT or ultrasonography for detecting the intraocular calcium deposits commonly found in association with retinoblastoma tumors.\nMRI is also very helpful for evaluating the pineal gland in cases of familial retinoblastoma. Subretinal fluid appears hyperintense on T1- and T2-weighted images. Solid tumors are hyperintense on T1-weighted images but hypointense in T2-weighted images.\nThe differential diagnosis of Coats disease is similar to that of leukocoria (which is extensive). It includes retinoblastoma; persistent fetal vasculature; retinopathy of prematurity; rhegmatogenous, exudative, or tractional retinal detachment; ocular toxocariasis, familial exudative vitreoretinopathy; retinal capillary hemangiomatosis (von Hippel-Lindau disease); cataract; glaucoma; uveitis; vitreous hemorrhage; and colobomas of the choroid and optic disc. Leber miliary aneurysm disease is an early or nonprogressive form of Coats disease.[2,4]\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 'Gleam' in the Left Eye of a 5-Year-Old Boy"
},
{
"authors": "Mauricio E. Pons, MD; Maria Silvana Horenstein, MD",
"content": [
"If untreated, Coats disease worsens progressively in most cases, with variable speed; therefore, early treatment is recommended. The disease does not respond to steroid or antibiotic treatment. Recommended treatments depend mainly on the stage of the disease; these include observation, laser photocoagulation, cryotherapy, retinal detachment repair with pars plana vitrectomy and/or scleral buckle, and enucleation.",
"Because treatment involves general anesthesia and frequent follow-up, discussions with a patient's parents (if the patient is a minor) regarding diagnosis, prognosis, and treatment goals are very important. Laser treatment has been used with a high success rate in cases with less exudation. Treatment is directed to areas of vascular leakage and nonperfusion, which decreases or eliminates further exudation and leads to resolution of the exudates and serous detachment. Fluorescein angiography is very useful in guiding laser treatment. Peripheral lesions are better treated with cryotherapy or a combination of laser and cryotherapy. Treatment may have to be repeated, because recurrences may follow.",
"In more advanced cases, drainage of the subretinal fluid may be necessary, with or without the use of a scleral buckle. Pars plana vitrectomy has been recently used to treat exudative and tractional retinal detachments.",
"Despite resolution of exudates, subretinal fibrosis and scarring may permanently impair vision. Because the disease is unilateral in most cases, patients may decide that this is not a major quality-of-life issue and use polycarbonate glasses for protection of the fellow eye, especially during sports activities.[2,6]",
"Associated complications of Coats disease include neovascular glaucoma (approximately 10% of patients), angle-closure glaucoma, anterior chamber cholesterolosis (3%), retinal/disc neovascularization, vitreous hemorrhage, secondary retinal vasoproliferative tumor, and intraretinal cysts.[6]",
"In this patient, enucleation was the preferred treatment because of the poor visual prognosis, advanced stage of the disease, presence of neovascular glaucoma, elevated intraocular pressure, closed anterior chamber angle, and strabismus. The eye was sent for pathologic examination, and the pathology report confirmed the clinical diagnosis.",
"During surgery, the eye was replaced with a hydroxylapatite implant. The four rectus muscles were attached to the implant. Six weeks later, the patient was fitted with a prosthetic, with excellent cosmetic results and good motility."
],
"date": "July 22, 2016",
"figures": [],
"markdown": "# A 'Gleam' in the Left Eye of a 5-Year-Old Boy\n\n **Authors:** Mauricio E. Pons, MD; Maria Silvana Horenstein, MD \n **Date:** July 22, 2016\n\n ## Content\n\n If untreated, Coats disease worsens progressively in most cases, with variable speed; therefore, early treatment is recommended. The disease does not respond to steroid or antibiotic treatment. Recommended treatments depend mainly on the stage of the disease; these include observation, laser photocoagulation, cryotherapy, retinal detachment repair with pars plana vitrectomy and/or scleral buckle, and enucleation.\nBecause treatment involves general anesthesia and frequent follow-up, discussions with a patient's parents (if the patient is a minor) regarding diagnosis, prognosis, and treatment goals are very important. Laser treatment has been used with a high success rate in cases with less exudation. Treatment is directed to areas of vascular leakage and nonperfusion, which decreases or eliminates further exudation and leads to resolution of the exudates and serous detachment. Fluorescein angiography is very useful in guiding laser treatment. Peripheral lesions are better treated with cryotherapy or a combination of laser and cryotherapy. Treatment may have to be repeated, because recurrences may follow.\nIn more advanced cases, drainage of the subretinal fluid may be necessary, with or without the use of a scleral buckle. Pars plana vitrectomy has been recently used to treat exudative and tractional retinal detachments.\nDespite resolution of exudates, subretinal fibrosis and scarring may permanently impair vision. Because the disease is unilateral in most cases, patients may decide that this is not a major quality-of-life issue and use polycarbonate glasses for protection of the fellow eye, especially during sports activities.[2,6]\nAssociated complications of Coats disease include neovascular glaucoma (approximately 10% of patients), angle-closure glaucoma, anterior chamber cholesterolosis (3%), retinal/disc neovascularization, vitreous hemorrhage, secondary retinal vasoproliferative tumor, and intraretinal cysts.[6]\nIn this patient, enucleation was the preferred treatment because of the poor visual prognosis, advanced stage of the disease, presence of neovascular glaucoma, elevated intraocular pressure, closed anterior chamber angle, and strabismus. The eye was sent for pathologic examination, and the pathology report confirmed the clinical diagnosis.\nDuring surgery, the eye was replaced with a hydroxylapatite implant. The four rectus muscles were attached to the implant. Six weeks later, the patient was fitted with a prosthetic, with excellent cosmetic results and good motility.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 990617,
"choiceText": "Intravenous fluorescein angiography",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990619,
"choiceText": "A- and B-scan ultrasonography",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990621,
"choiceText": "Intraocular pressure measurement",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990623,
"choiceText": "Indirect ophthalmoscopy",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990625,
"choiceText": "Biopsy",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Biopsy is not usually recommended in the initial evaluation because of the possibility of retinoblastoma and metastatic spread. Indirect ophthalmoscopy and A- and B-scan ultrasonography are noninvasive tests that can be performed in the office to quickly narrow down the differential diagnosis.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 313593,
"questionText": "Which of the following tests is not recommended for the initial diagnosis of Coats disease?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 990627,
"choiceText": "Prevalence in females",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990629,
"choiceText": "Leukocoria",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990631,
"choiceText": "Affected family members",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990633,
"choiceText": "Bilaterality",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990635,
"choiceText": "All of the above",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Decreased vision, strabismus, and leukocoria are common presenting symptoms. Coats disease is more prevalent in males and is generally unilateral, and family members are not usually affected",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 313595,
"questionText": "Which of the following features is commonly seen in Coats disease?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 'Gleam' in the Left Eye of a 5-Year-Old Boy"
},
{
"authors": "Mauricio E. Pons, MD; Maria Silvana Horenstein, MD",
"content": [],
"date": "July 22, 2016",
"figures": [],
"markdown": "# A 'Gleam' in the Left Eye of a 5-Year-Old Boy\n\n **Authors:** Mauricio E. Pons, MD; Maria Silvana Horenstein, MD \n **Date:** July 22, 2016\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 990617,
"choiceText": "Intravenous fluorescein angiography",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990619,
"choiceText": "A- and B-scan ultrasonography",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990621,
"choiceText": "Intraocular pressure measurement",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990623,
"choiceText": "Indirect ophthalmoscopy",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990625,
"choiceText": "Biopsy",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Biopsy is not usually recommended in the initial evaluation because of the possibility of retinoblastoma and metastatic spread. Indirect ophthalmoscopy and A- and B-scan ultrasonography are noninvasive tests that can be performed in the office to quickly narrow down the differential diagnosis.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 313593,
"questionText": "Which of the following tests is not recommended for the initial diagnosis of Coats disease?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 990627,
"choiceText": "Prevalence in females",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990629,
"choiceText": "Leukocoria",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990631,
"choiceText": "Affected family members",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990633,
"choiceText": "Bilaterality",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990635,
"choiceText": "All of the above",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Decreased vision, strabismus, and leukocoria are common presenting symptoms. Coats disease is more prevalent in males and is generally unilateral, and family members are not usually affected",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 313595,
"questionText": "Which of the following features is commonly seen in Coats disease?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 'Gleam' in the Left Eye of a 5-Year-Old Boy"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 990609,
"choiceText": "Endophalmitis",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990611,
"choiceText": "Coats disease (exudative retinitis)",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990613,
"choiceText": "Orbital pseudotumor",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990615,
"choiceText": "Retinoblastoma",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 313591,
"questionText": "What is the underlying cause of the child's total retinal detachment?<br/><br/>\r\n<em>Hint: Look closely at the vascular pattern of the lesions in this child.</em>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 990617,
"choiceText": "Intravenous fluorescein angiography",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990619,
"choiceText": "A- and B-scan ultrasonography",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990621,
"choiceText": "Intraocular pressure measurement",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990623,
"choiceText": "Indirect ophthalmoscopy",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990625,
"choiceText": "Biopsy",
"correct": true,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Biopsy is not usually recommended in the initial evaluation because of the possibility of retinoblastoma and metastatic spread. Indirect ophthalmoscopy and A- and B-scan ultrasonography are noninvasive tests that can be performed in the office to quickly narrow down the differential diagnosis.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 313593,
"questionText": "Which of the following tests is not recommended for the initial diagnosis of Coats disease?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 990627,
"choiceText": "Prevalence in females",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990629,
"choiceText": "Leukocoria",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990631,
"choiceText": "Affected family members",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990633,
"choiceText": "Bilaterality",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990635,
"choiceText": "All of the above",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Decreased vision, strabismus, and leukocoria are common presenting symptoms. Coats disease is more prevalent in males and is generally unilateral, and family members are not usually affected",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 313595,
"questionText": "Which of the following features is commonly seen in Coats disease?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
866408 | /viewarticle/866408 | [
{
"authors": "Talib A. Najjar, DMD, MDS, PhD; Prabhjot Singh, DDS",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.",
"A 78-year-old man presents to the oral surgery clinic with discomfort along the left lateral border of his tongue. He states that he first noticed a lesion on his tongue approximately 4 weeks ago, and since that time, it has progressively become larger and more bothersome. The patient denies having any fever, chills, drainage at the site of the lesion, or generalized swelling of the head and neck.",
"A blade implant was placed in the patient's left mandible 15 years ago to restore the lower left first and second premolar teeth. (A blade implant is an older system to replace missing teeth; this has been replaced by the use of single or multiple titanium implants). He has no known chronic medical conditions. He has no allergies and does not take any medications. He reports a 30–pack-year history of smoking, as well as occasional alcohol use."
],
"date": "July 21, 2016",
"figures": [],
"markdown": "# A 78-Year-Old Man With a Lingual Ulcerative Lesion\n\n **Authors:** Talib A. Najjar, DMD, MDS, PhD; Prabhjot Singh, DDS \n **Date:** July 21, 2016\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.\nA 78-year-old man presents to the oral surgery clinic with discomfort along the left lateral border of his tongue. He states that he first noticed a lesion on his tongue approximately 4 weeks ago, and since that time, it has progressively become larger and more bothersome. The patient denies having any fever, chills, drainage at the site of the lesion, or generalized swelling of the head and neck.\nA blade implant was placed in the patient's left mandible 15 years ago to restore the lower left first and second premolar teeth. (A blade implant is an older system to replace missing teeth; this has been replaced by the use of single or multiple titanium implants). He has no known chronic medical conditions. He has no allergies and does not take any medications. He reports a 30–pack-year history of smoking, as well as occasional alcohol use.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 78-Year-Old Man With a Lingual Ulcerative Lesion"
},
{
"authors": "Talib A. Najjar, DMD, MDS, PhD; Prabhjot Singh, DDS",
"content": [
"Upon physical examination, the patient appears well-nourished and in no apparent discomfort. His vital signs include an oral temperature of 99.5°F, heart rate of 78 beats/min, blood pressure of 134/88 mm Hg, respiratory rate of 14 breaths/min, and oxygen saturation of 98% while breathing room air. The cardiovascular, respiratory, and abdominal examination findings are normal. Head and neck examination is negative for any gross swelling or lymphadenopathy.",
"Figure 1.",
"Figure 2.",
"Figure 3.",
"Intraoral examination reveals an indurated nodular mass 1 × 1 cm in diameter on the left lateral margin of the tongue, with two separate areas of ulceration both superior and inferior to the mass (Figure 1). The mass is not tender to palpation and lacks bleeding or drainage. No sublingual elevation, induration, or asymmetry is noted, and the patient's maximal oral opening is measured at 45 mm. Well-healed incisional wounds from prior restorative work of the left mandible are noted.",
"The initial workup consists of a panoramic radiograph, a culture swab, and an incisional wedge biopsy of the lesion. The panoramic radiograph shows the blade implant in place in the left mandible (Figure 2). Minimal bone destruction is noted around the blade implant, and no other bony pathology can be seen. The biopsy specimen is sent for histopathologic diagnosis (Figure 3)."
],
"date": "July 21, 2016",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/866/408/866408-Thumb1.png"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/866/408/866408Thumb2.png"
},
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/866/408/866408-Thumb3.png"
}
],
"markdown": "# A 78-Year-Old Man With a Lingual Ulcerative Lesion\n\n **Authors:** Talib A. Najjar, DMD, MDS, PhD; Prabhjot Singh, DDS \n **Date:** July 21, 2016\n\n ## Content\n\n Upon physical examination, the patient appears well-nourished and in no apparent discomfort. His vital signs include an oral temperature of 99.5°F, heart rate of 78 beats/min, blood pressure of 134/88 mm Hg, respiratory rate of 14 breaths/min, and oxygen saturation of 98% while breathing room air. The cardiovascular, respiratory, and abdominal examination findings are normal. Head and neck examination is negative for any gross swelling or lymphadenopathy.\nFigure 1.\nFigure 2.\nFigure 3.\nIntraoral examination reveals an indurated nodular mass 1 × 1 cm in diameter on the left lateral margin of the tongue, with two separate areas of ulceration both superior and inferior to the mass (Figure 1). The mass is not tender to palpation and lacks bleeding or drainage. No sublingual elevation, induration, or asymmetry is noted, and the patient's maximal oral opening is measured at 45 mm. Well-healed incisional wounds from prior restorative work of the left mandible are noted.\nThe initial workup consists of a panoramic radiograph, a culture swab, and an incisional wedge biopsy of the lesion. The panoramic radiograph shows the blade implant in place in the left mandible (Figure 2). Minimal bone destruction is noted around the blade implant, and no other bony pathology can be seen. The biopsy specimen is sent for histopathologic diagnosis (Figure 3).\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n**Figure 3.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 990289,
"choiceText": "Thyroglossal cyst",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990291,
"choiceText": "Aphthous ulcer",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990293,
"choiceText": "Lingual carcinoma",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990295,
"choiceText": "Actinomycosis\r\n",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 313475,
"questionText": "What is the most likely diagnosis? <br><br><i>\r\nHint: Definitive diagnosis requires close examination of the microscopic biopsy specimen.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 78-Year-Old Man With a Lingual Ulcerative Lesion"
},
{
"authors": "Talib A. Najjar, DMD, MDS, PhD; Prabhjot Singh, DDS",
"content": [
"The diagnosis of actinomycosis was made on the basis of the patient's physical examination as well as the histologic and microbiologic evaluation. The wedge biopsy demonstrated marked acute inflammation, microabscess formation, and visible organisms morphologically consistent with actinomycosis (Figure 3).",
"Figure 3.",
"This case is an atypical presentation of actinomycosis of the head and neck. A more common presentation consists of chronic submandibular swelling, usually brawny induration with fistula formation and purulent drainage. The presentation of actinomycosis on the tongue itself is also unusual. Firm masses with associated ulceration on the lateral border of the tongue in the presence of a significant tobacco and alcohol history are usually associated with cancer, typically squamous cell carcinoma.",
"Although the presence of a chronic, firm swelling is consistent with actinomycosis infection, cancer must also be considered. Therefore, in addition to the microbiologic analysis, an incisional biopsy of the lesion must be obtained.",
"No evidence suggested other cervicofacial, abdominal, pelvic, or pulmonary actinomycosis in this patient. The frequency of cervicofacial, abdominal/pelvic, and pulmonary actinomycosis is 55%, 25%, and 15%, respectively; subcutaneous actinomycosis as well as actinomycosis at other sites accounts for the remaining 5% of cases.",
"Although the pathogenesis is unclear, the two primary predisposing factors for development of an actinomycosis infection are the presence of an introductory pathway into the tissue, and a suitable environment for the bacteria to thrive. Trauma seems to play an important role in most cases by initiating the portal of entry for the organism. In this case, trauma associated with the blade implant may have been involved. Some investigators have proposed that other microorganisms, such as Staphylococcus aureus, act in a synergistic fashion to create an anaerobic environment for the Actinomyces organisms to multiply.",
"In the cervicofacial area, the infection is frequently of odontogenic origin and can be the result of oromaxillofacial trauma, dental intervention, or poor oral hygiene. Actinomyces israelii reproduces easily in the presence of necrotic tissue and can lead to clinical emergence of the disease. The presence of dental or periodontal disease and devitalized tissue after trauma or surgery provides an adequate environment for Actinomyces species to flourish.",
"Actinomyces require the presence of many other types of bacteria to proliferate; the specific ecosystem thus formed has a low oxidoreduction potential that is favorable to anaerobic growth. This ecosystem is formed with polymicrobic \"associate\" flora working in a synergistic fashion. It destroys local tissue, which is highly vascularized and aerobic, and replaces it with poorly vascularized granulomatous tissue, thereby permitting development of an anaerobic milieu that is essential to Actinomyces growth.[1,2,3,4]"
],
"date": "July 21, 2016",
"figures": [
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/866/408/866408-Thumb3.png"
}
],
"markdown": "# A 78-Year-Old Man With a Lingual Ulcerative Lesion\n\n **Authors:** Talib A. Najjar, DMD, MDS, PhD; Prabhjot Singh, DDS \n **Date:** July 21, 2016\n\n ## Content\n\n The diagnosis of actinomycosis was made on the basis of the patient's physical examination as well as the histologic and microbiologic evaluation. The wedge biopsy demonstrated marked acute inflammation, microabscess formation, and visible organisms morphologically consistent with actinomycosis (Figure 3).\nFigure 3.\nThis case is an atypical presentation of actinomycosis of the head and neck. A more common presentation consists of chronic submandibular swelling, usually brawny induration with fistula formation and purulent drainage. The presentation of actinomycosis on the tongue itself is also unusual. Firm masses with associated ulceration on the lateral border of the tongue in the presence of a significant tobacco and alcohol history are usually associated with cancer, typically squamous cell carcinoma.\nAlthough the presence of a chronic, firm swelling is consistent with actinomycosis infection, cancer must also be considered. Therefore, in addition to the microbiologic analysis, an incisional biopsy of the lesion must be obtained.\nNo evidence suggested other cervicofacial, abdominal, pelvic, or pulmonary actinomycosis in this patient. The frequency of cervicofacial, abdominal/pelvic, and pulmonary actinomycosis is 55%, 25%, and 15%, respectively; subcutaneous actinomycosis as well as actinomycosis at other sites accounts for the remaining 5% of cases.\nAlthough the pathogenesis is unclear, the two primary predisposing factors for development of an actinomycosis infection are the presence of an introductory pathway into the tissue, and a suitable environment for the bacteria to thrive. Trauma seems to play an important role in most cases by initiating the portal of entry for the organism. In this case, trauma associated with the blade implant may have been involved. Some investigators have proposed that other microorganisms, such as Staphylococcus aureus, act in a synergistic fashion to create an anaerobic environment for the Actinomyces organisms to multiply.\nIn the cervicofacial area, the infection is frequently of odontogenic origin and can be the result of oromaxillofacial trauma, dental intervention, or poor oral hygiene. Actinomyces israelii reproduces easily in the presence of necrotic tissue and can lead to clinical emergence of the disease. The presence of dental or periodontal disease and devitalized tissue after trauma or surgery provides an adequate environment for Actinomyces species to flourish.\nActinomyces require the presence of many other types of bacteria to proliferate; the specific ecosystem thus formed has a low oxidoreduction potential that is favorable to anaerobic growth. This ecosystem is formed with polymicrobic \"associate\" flora working in a synergistic fashion. It destroys local tissue, which is highly vascularized and aerobic, and replaces it with poorly vascularized granulomatous tissue, thereby permitting development of an anaerobic milieu that is essential to Actinomyces growth.[1,2,3,4]\n\n ## Figures\n\n **Figure 3.** \n \n\n\n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 990289,
"choiceText": "Thyroglossal cyst",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990291,
"choiceText": "Aphthous ulcer",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990293,
"choiceText": "Lingual carcinoma",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990295,
"choiceText": "Actinomycosis\r\n",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 313475,
"questionText": "What is the most likely diagnosis? <br><br><i>\r\nHint: Definitive diagnosis requires close examination of the microscopic biopsy specimen.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 78-Year-Old Man With a Lingual Ulcerative Lesion"
},
{
"authors": "Talib A. Najjar, DMD, MDS, PhD; Prabhjot Singh, DDS",
"content": [
"Clinical findings that are commonly seen in actinomycosis infection include suppuration, draining sinuses, fistulas, and the presence of \"sulfur granules\" (small, white-yellow granular aggregates of bacterial filaments) in exudates or tissues. In patients with cervicofacial actinomycosis, there is a high tendency for poor dental hygiene, caries, oral trauma, dental extraction, or dental abscess. The oral condition in this patient was conducive to the development of actinomycosis.",
"A typical clinical presentation is a hard, \"woody\" swelling in the mandible and neck; an associated abscess may be present in contiguous soft tissue. Ulceration and induration in the tongue were noted in this patient, but no evidence suggested abscess formation, fistula tract, or sulfur-granule exudate. Pain and mild pyrexia are usually present in these cases, and sinus tracts may occur in long-standing disease.",
"Classically, the infection presents as a slowly enlarging and slightly tender swelling that may become indurated as a result of fibrosis and scar formation. The fibrosis may play an important role in the pathogenicity of the infection, because the microorganisms are virtually protected from the host defense; therefore, they are more resistant to antibiotics.",
"The pathogenicity ranges from an acute form, with rapid onset and purulent drainage from multiple sinus tracts, to a slowly progressing chronic form characterized by indurated fibrosis with little suppuration. The lesion in this patient fell into the latter category, with induration, fibrosis, and ulceration of the lingual mucosa. The infection usually occurs from a few weeks to a few months after the organism penetrates through the oral portal of entry (such as a facial bone fracture, periodontal socket, extraction site, pericoronitis, periapical inflammation, root canal treatment, or periodontal surgery).[1,2,3,4]",
"Histologically, actinomycotic lesions are characterized by mixed suppurative and granulomatous inflammatory changes. Proliferation of the connective tissues and the presence of sulfur granules are noted. Under the microscope, sulfur granules may appear cauliflower-like at low magnification, whereas at higher magnification the inflammatory reaction can be seen. These granules may be visualized in preparation of the biopsy specimen while the test tubes are being rotated manually. They can also be identified by washing sampled material and crushing it between a slide and coverglass after it has been Gram-stained. With Gram staining, these microcolonies contain gram-positive, filamentous or branching bacteria.",
"Occasionally, companion bacteria may be observed. Stressing that similar granules can be found in other bacterial infections, such as Nocardia infection and botryomycosis, is important. Botryomycosis is an S aureus infection that mimics actinomycosis. Infection with Nocardia may be differentiated from that with Actinomyces because it is acid-fast when stained. Gel diffusion methods and fluorescent antibody tests (immunofluorescence with fluorescein isothiocyanate antiserum) are also helpful because they can differentiate A israelii from other filamentous anaerobes that produce granules in tissue. They can be used retrospectively with formalin- and paraffin-embedded biopsy specimens.",
"Biopsies of the granulomatous lesion or of the fistula are especially useful when no purulent material is present and when the diagnosis remains unclear despite laboratory evaluation. In the absence of absolute bacterial identification from culture, the diagnosis must rely on the clinical presentation and histopathologic findings.[4]"
],
"date": "July 21, 2016",
"figures": [],
"markdown": "# A 78-Year-Old Man With a Lingual Ulcerative Lesion\n\n **Authors:** Talib A. Najjar, DMD, MDS, PhD; Prabhjot Singh, DDS \n **Date:** July 21, 2016\n\n ## Content\n\n Clinical findings that are commonly seen in actinomycosis infection include suppuration, draining sinuses, fistulas, and the presence of \"sulfur granules\" (small, white-yellow granular aggregates of bacterial filaments) in exudates or tissues. In patients with cervicofacial actinomycosis, there is a high tendency for poor dental hygiene, caries, oral trauma, dental extraction, or dental abscess. The oral condition in this patient was conducive to the development of actinomycosis.\nA typical clinical presentation is a hard, \"woody\" swelling in the mandible and neck; an associated abscess may be present in contiguous soft tissue. Ulceration and induration in the tongue were noted in this patient, but no evidence suggested abscess formation, fistula tract, or sulfur-granule exudate. Pain and mild pyrexia are usually present in these cases, and sinus tracts may occur in long-standing disease.\nClassically, the infection presents as a slowly enlarging and slightly tender swelling that may become indurated as a result of fibrosis and scar formation. The fibrosis may play an important role in the pathogenicity of the infection, because the microorganisms are virtually protected from the host defense; therefore, they are more resistant to antibiotics.\nThe pathogenicity ranges from an acute form, with rapid onset and purulent drainage from multiple sinus tracts, to a slowly progressing chronic form characterized by indurated fibrosis with little suppuration. The lesion in this patient fell into the latter category, with induration, fibrosis, and ulceration of the lingual mucosa. The infection usually occurs from a few weeks to a few months after the organism penetrates through the oral portal of entry (such as a facial bone fracture, periodontal socket, extraction site, pericoronitis, periapical inflammation, root canal treatment, or periodontal surgery).[1,2,3,4]\nHistologically, actinomycotic lesions are characterized by mixed suppurative and granulomatous inflammatory changes. Proliferation of the connective tissues and the presence of sulfur granules are noted. Under the microscope, sulfur granules may appear cauliflower-like at low magnification, whereas at higher magnification the inflammatory reaction can be seen. These granules may be visualized in preparation of the biopsy specimen while the test tubes are being rotated manually. They can also be identified by washing sampled material and crushing it between a slide and coverglass after it has been Gram-stained. With Gram staining, these microcolonies contain gram-positive, filamentous or branching bacteria.\nOccasionally, companion bacteria may be observed. Stressing that similar granules can be found in other bacterial infections, such as Nocardia infection and botryomycosis, is important. Botryomycosis is an S aureus infection that mimics actinomycosis. Infection with Nocardia may be differentiated from that with Actinomyces because it is acid-fast when stained. Gel diffusion methods and fluorescent antibody tests (immunofluorescence with fluorescein isothiocyanate antiserum) are also helpful because they can differentiate A israelii from other filamentous anaerobes that produce granules in tissue. They can be used retrospectively with formalin- and paraffin-embedded biopsy specimens.\nBiopsies of the granulomatous lesion or of the fistula are especially useful when no purulent material is present and when the diagnosis remains unclear despite laboratory evaluation. In the absence of absolute bacterial identification from culture, the diagnosis must rely on the clinical presentation and histopathologic findings.[4]\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 78-Year-Old Man With a Lingual Ulcerative Lesion"
},
{
"authors": "Talib A. Najjar, DMD, MDS, PhD; Prabhjot Singh, DDS",
"content": [
"Surgical management of actinomycosis includes excision/debridement of abnormal or devitalized tissue, such as infected masses and curettage of osteomyelitic bone lesions. Drainage of any existing abscesses or fistula tracts is also necessary, when present. Surgical treatment alone, however, is not sufficient for treatment.",
"Figure 4.",
"Depending on the type of infection, prolonged antibiotic therapy is the cornerstone of management. Classically, these infections are treated with penicillin-type antibiotics; the duration of therapy is targeted to the patient's clinical condition and response to treatment. Penicillin resistance is uncommonly observed. Certain antibiotics (namely, metronidazole, aminoglycosides, cotrimoxazole, or cephalexin) have no role in treating this infection. Limited case reports have reported success with fluoroquinolones, such as levofloxacin or moxifloxacin. This infection may respond to some second- or third-generation cephalosporin, macrolide, or tetracycline antibiotics.[1,2,4]",
"The patient in this case was started on a course of oral penicillin VK at 500 mg every 6 hours for 3 weeks. Because it was felt that the blade implant and associated prosthesis could be harboring actinomycotic colonies and could seed more bacteria in the left lateral tongue, it was decided to remove the blade implant along with the prosthesis supported by the implant. The patient was followed for approximately 1 month, at which time total resolution of the lesion was observed (Figure 4)."
],
"date": "July 21, 2016",
"figures": [
{
"caption": "Figure 4.",
"image_url": "https://img.medscapestatic.com/article/866/408/866408-Thumb4.png"
}
],
"markdown": "# A 78-Year-Old Man With a Lingual Ulcerative Lesion\n\n **Authors:** Talib A. Najjar, DMD, MDS, PhD; Prabhjot Singh, DDS \n **Date:** July 21, 2016\n\n ## Content\n\n Surgical management of actinomycosis includes excision/debridement of abnormal or devitalized tissue, such as infected masses and curettage of osteomyelitic bone lesions. Drainage of any existing abscesses or fistula tracts is also necessary, when present. Surgical treatment alone, however, is not sufficient for treatment.\nFigure 4.\nDepending on the type of infection, prolonged antibiotic therapy is the cornerstone of management. Classically, these infections are treated with penicillin-type antibiotics; the duration of therapy is targeted to the patient's clinical condition and response to treatment. Penicillin resistance is uncommonly observed. Certain antibiotics (namely, metronidazole, aminoglycosides, cotrimoxazole, or cephalexin) have no role in treating this infection. Limited case reports have reported success with fluoroquinolones, such as levofloxacin or moxifloxacin. This infection may respond to some second- or third-generation cephalosporin, macrolide, or tetracycline antibiotics.[1,2,4]\nThe patient in this case was started on a course of oral penicillin VK at 500 mg every 6 hours for 3 weeks. Because it was felt that the blade implant and associated prosthesis could be harboring actinomycotic colonies and could seed more bacteria in the left lateral tongue, it was decided to remove the blade implant along with the prosthesis supported by the implant. The patient was followed for approximately 1 month, at which time total resolution of the lesion was observed (Figure 4).\n\n ## Figures\n\n **Figure 4.** \n \n\n\n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 990297,
"choiceText": "Trauma",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990299,
"choiceText": "Local bacterial coinfection",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990301,
"choiceText": "Poor oral hygiene\r\n",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990303,
"choiceText": "All of the above",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Although the pathogenesis is unclear, the two primary predisposing factors for the development of an actinomycotic infection are the presence of an introductory pathway into the tissue, and a suitable environment for the bacteria to thrive. Trauma seems to play an important role in most cases by initiating the portal of entry for the organism. Some investigators have proposed that other microorganisms, such as <i>S aureus</i>, act in a synergistic fashion to create an anaerobic environment for <i>Actinomyces</i> to multiply. In the cervicofacial area, the infection is frequently of odontogenic origin and can be the result of oromaxillofacial trauma, dental intervention, or poor oral hygiene.\r\n",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 313477,
"questionText": "You are examining a patient whom you suspect may have an actinomycotic infection. As part of the history, the patient mentions that he had dental surgery earlier this year. He states that he often has gum infections, probably as a result of his admittedly poor oral hygiene habits. Which of the following factors in this patient's history could contribute to the development of actinomycosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 990305,
"choiceText": "Laser surgery",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990307,
"choiceText": "Surgical resection\r\n",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990309,
"choiceText": "Antibiotic therapy",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990311,
"choiceText": "Cryosurgery\r\n\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Depending on the type of infection, prolonged antibiotic therapy is the cornerstone of management. Classically, these infections are treated with penicillin-type antibiotics; the duration of therapy is targeted to the patient's clinical condition and response to treatment. Penicillin resistance is uncommonly observed. Certain antibiotics (namely, metronidazole, aminoglycosides, cotrimoxazole, or cephalexin) have no role in treating this infection. Limited case reports have reported success with fluoroquinolones, such as levofloxacin or moxifloxacin. This infection may respond to some second- or third-generation cephalosporin, macrolide, or tetracycline antibiotics.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 313479,
"questionText": "If the patient described above were to be diagnosed with actinomycosis, which of the following choices would be the most appropriate initial course of treatment?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 78-Year-Old Man With a Lingual Ulcerative Lesion"
},
{
"authors": "Talib A. Najjar, DMD, MDS, PhD; Prabhjot Singh, DDS",
"content": [],
"date": "July 21, 2016",
"figures": [],
"markdown": "# A 78-Year-Old Man With a Lingual Ulcerative Lesion\n\n **Authors:** Talib A. Najjar, DMD, MDS, PhD; Prabhjot Singh, DDS \n **Date:** July 21, 2016\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 990297,
"choiceText": "Trauma",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990299,
"choiceText": "Local bacterial coinfection",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990301,
"choiceText": "Poor oral hygiene\r\n",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990303,
"choiceText": "All of the above",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Although the pathogenesis is unclear, the two primary predisposing factors for the development of an actinomycotic infection are the presence of an introductory pathway into the tissue, and a suitable environment for the bacteria to thrive. Trauma seems to play an important role in most cases by initiating the portal of entry for the organism. Some investigators have proposed that other microorganisms, such as <i>S aureus</i>, act in a synergistic fashion to create an anaerobic environment for <i>Actinomyces</i> to multiply. In the cervicofacial area, the infection is frequently of odontogenic origin and can be the result of oromaxillofacial trauma, dental intervention, or poor oral hygiene.\r\n",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 313477,
"questionText": "You are examining a patient whom you suspect may have an actinomycotic infection. As part of the history, the patient mentions that he had dental surgery earlier this year. He states that he often has gum infections, probably as a result of his admittedly poor oral hygiene habits. Which of the following factors in this patient's history could contribute to the development of actinomycosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 990305,
"choiceText": "Laser surgery",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990307,
"choiceText": "Surgical resection\r\n",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990309,
"choiceText": "Antibiotic therapy",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990311,
"choiceText": "Cryosurgery\r\n\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Depending on the type of infection, prolonged antibiotic therapy is the cornerstone of management. Classically, these infections are treated with penicillin-type antibiotics; the duration of therapy is targeted to the patient's clinical condition and response to treatment. Penicillin resistance is uncommonly observed. Certain antibiotics (namely, metronidazole, aminoglycosides, cotrimoxazole, or cephalexin) have no role in treating this infection. Limited case reports have reported success with fluoroquinolones, such as levofloxacin or moxifloxacin. This infection may respond to some second- or third-generation cephalosporin, macrolide, or tetracycline antibiotics.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 313479,
"questionText": "If the patient described above were to be diagnosed with actinomycosis, which of the following choices would be the most appropriate initial course of treatment?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 78-Year-Old Man With a Lingual Ulcerative Lesion"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 990289,
"choiceText": "Thyroglossal cyst",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990291,
"choiceText": "Aphthous ulcer",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990293,
"choiceText": "Lingual carcinoma",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990295,
"choiceText": "Actinomycosis\r\n",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 313475,
"questionText": "What is the most likely diagnosis? <br><br><i>\r\nHint: Definitive diagnosis requires close examination of the microscopic biopsy specimen.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 990297,
"choiceText": "Trauma",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990299,
"choiceText": "Local bacterial coinfection",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990301,
"choiceText": "Poor oral hygiene\r\n",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990303,
"choiceText": "All of the above",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Although the pathogenesis is unclear, the two primary predisposing factors for the development of an actinomycotic infection are the presence of an introductory pathway into the tissue, and a suitable environment for the bacteria to thrive. Trauma seems to play an important role in most cases by initiating the portal of entry for the organism. Some investigators have proposed that other microorganisms, such as <i>S aureus</i>, act in a synergistic fashion to create an anaerobic environment for <i>Actinomyces</i> to multiply. In the cervicofacial area, the infection is frequently of odontogenic origin and can be the result of oromaxillofacial trauma, dental intervention, or poor oral hygiene.\r\n",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 313477,
"questionText": "You are examining a patient whom you suspect may have an actinomycotic infection. As part of the history, the patient mentions that he had dental surgery earlier this year. He states that he often has gum infections, probably as a result of his admittedly poor oral hygiene habits. Which of the following factors in this patient's history could contribute to the development of actinomycosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 990305,
"choiceText": "Laser surgery",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990307,
"choiceText": "Surgical resection\r\n",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990309,
"choiceText": "Antibiotic therapy",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 990311,
"choiceText": "Cryosurgery\r\n\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Depending on the type of infection, prolonged antibiotic therapy is the cornerstone of management. Classically, these infections are treated with penicillin-type antibiotics; the duration of therapy is targeted to the patient's clinical condition and response to treatment. Penicillin resistance is uncommonly observed. Certain antibiotics (namely, metronidazole, aminoglycosides, cotrimoxazole, or cephalexin) have no role in treating this infection. Limited case reports have reported success with fluoroquinolones, such as levofloxacin or moxifloxacin. This infection may respond to some second- or third-generation cephalosporin, macrolide, or tetracycline antibiotics.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 313479,
"questionText": "If the patient described above were to be diagnosed with actinomycosis, which of the following choices would be the most appropriate initial course of treatment?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
865301 | /viewarticle/865301 | [
{
"authors": "Tuyyab Hassan, MD",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"An 84-year-old woman presents with a blistering rash. The rash began 1 week ago on her right hand, but it has progressed to her arm, upper chest, and abdomen.",
"Figure 1.",
"Figure 2.",
"The rash is painless but intensely itchy, and it limits her ability to complete routine tasks. She has had no fever, no history of trauma, and no recent travel. She lives alone and uses an electric scooter to assist her with mobility. The patient relies on help from family and caretakers for activities of daily living.",
"She has a medical history of 100% estrogen-receptor-positive nonmetastatic breast cancer, ischemic heart disease, osteoporosis, and chronic renal insufficiency. The patient takes aspirin, isosorbide mononitrate, ramipril, simvastatin alendronate, and a calcium supplement. Her breast cancer is treated with an aromatase inhibitor. She has had no recent changes in her medication regimen and does not take any over-the-counter or herbal medications."
],
"date": "April 03, 2019",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/865/301/865301-thumb1.png"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/865/301/865301-thumb2.png"
}
],
"markdown": "# An 84-Year-Old With a Blistering Rash That Is Spreading\n\n **Authors:** Tuyyab Hassan, MD \n **Date:** April 03, 2019\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nAn 84-year-old woman presents with a blistering rash. The rash began 1 week ago on her right hand, but it has progressed to her arm, upper chest, and abdomen.\nFigure 1.\nFigure 2.\nThe rash is painless but intensely itchy, and it limits her ability to complete routine tasks. She has had no fever, no history of trauma, and no recent travel. She lives alone and uses an electric scooter to assist her with mobility. The patient relies on help from family and caretakers for activities of daily living.\nShe has a medical history of 100% estrogen-receptor-positive nonmetastatic breast cancer, ischemic heart disease, osteoporosis, and chronic renal insufficiency. The patient takes aspirin, isosorbide mononitrate, ramipril, simvastatin alendronate, and a calcium supplement. Her breast cancer is treated with an aromatase inhibitor. She has had no recent changes in her medication regimen and does not take any over-the-counter or herbal medications.\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n\n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "An 84-Year-Old With a Blistering Rash That Is Spreading"
},
{
"authors": "Tuyyab Hassan, MD",
"content": [
"Upon physical examination, the patient appears frail but in no distress. Her temperature is 98.4°F (36.89°C). Her heart has a regular rhythm, with a rate of 88 beats/min. Her blood pressure is 146/88 mm Hg, and her respiratory rate is timed at 16 breaths/min. She has clear, equal bilateral breath sounds. No heart murmurs are noted, but a loud S2 heart sound is heard. Her apical impulse is not displaced.",
"The patient's abdomen is soft and nontender, and no appreciable organomegaly is detected. She has a deep, fixed, 3-cm mass underlying the nipple of her right breast, with overlying skin puckering. She does not have any axillary lymphadenopathy. Her current breast examination is unchanged from her last documented examination.",
"Examination of the skin reveals several tense bullae, excoriated papules, and vesicles ranging in size from a few millimeters to 3 cm over her hand, right arm, anterior chest, and abdomen (Figures 1 and 2).",
"Figure 1.",
"Figure 2.",
"These bullae and vesicles rest on an erythematous base, and they contain clear fluid. No involvement of the mucous membranes is noted, and the Nikolsky sign (blistering of healthy-appearing skin when it is rubbed) is not elicited.",
"Laboratory tests demonstrate a normal erythrocyte sedimentation rate, antinuclear antibody titer, and C-reactive protein level. The patient has a hemoglobin level of 12.7 g/dL, with mild eosinophilia measured at 0.48 × 109 cells/L. Her blood urea nitrogen level is 28 mg/dL, and her creatinine level is 2.1 mg/dL. Her liver function test results are unremarkable."
],
"date": "April 03, 2019",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/865/301/865301-thumb1.png"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/865/301/865301-thumb2.png"
}
],
"markdown": "# An 84-Year-Old With a Blistering Rash That Is Spreading\n\n **Authors:** Tuyyab Hassan, MD \n **Date:** April 03, 2019\n\n ## Content\n\n Upon physical examination, the patient appears frail but in no distress. Her temperature is 98.4°F (36.89°C). Her heart has a regular rhythm, with a rate of 88 beats/min. Her blood pressure is 146/88 mm Hg, and her respiratory rate is timed at 16 breaths/min. She has clear, equal bilateral breath sounds. No heart murmurs are noted, but a loud S2 heart sound is heard. Her apical impulse is not displaced.\nThe patient's abdomen is soft and nontender, and no appreciable organomegaly is detected. She has a deep, fixed, 3-cm mass underlying the nipple of her right breast, with overlying skin puckering. She does not have any axillary lymphadenopathy. Her current breast examination is unchanged from her last documented examination.\nExamination of the skin reveals several tense bullae, excoriated papules, and vesicles ranging in size from a few millimeters to 3 cm over her hand, right arm, anterior chest, and abdomen (Figures 1 and 2).\nFigure 1.\nFigure 2.\nThese bullae and vesicles rest on an erythematous base, and they contain clear fluid. No involvement of the mucous membranes is noted, and the Nikolsky sign (blistering of healthy-appearing skin when it is rubbed) is not elicited.\nLaboratory tests demonstrate a normal erythrocyte sedimentation rate, antinuclear antibody titer, and C-reactive protein level. The patient has a hemoglobin level of 12.7 g/dL, with mild eosinophilia measured at 0.48 × 109 cells/L. Her blood urea nitrogen level is 28 mg/dL, and her creatinine level is 2.1 mg/dL. Her liver function test results are unremarkable.\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 983429,
"choiceText": "Bullous pemphigoid",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 983431,
"choiceText": "Herpes zoster (shingles)",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 983433,
"choiceText": "Pemphigus vulgaris",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 983435,
"choiceText": "Fixed drug eruption",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 983437,
"choiceText": "Bullous impetigo",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 311239,
"questionText": "<p>Which of the following is the most likely diagnosis?</p>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "An 84-Year-Old With a Blistering Rash That Is Spreading"
},
{
"authors": "Tuyyab Hassan, MD",
"content": [
"The patient in this case exhibited many factors suggestive of bullous pemphigoid. Her advanced age; the clinical appearance of the skin lesions; and the subacute onset of an intensely pruritic, nonpainful rash were consistent with the diagnosis of bullous pemphigoid. A skin biopsy sample sent for routine histologic, direct immunofluorescence, and indirect immunofluorescence studies confirmed the diagnosis. Histopathologic examination of a subepidermal blister demonstrated an eosinophil-rich inflammatory infiltrate in the dermis (Figure 3). Viral and bacterial culture swabs from the blister fluid were negative, which excluded an infectious process (eg, herpes zoster).",
"Figure 3.",
"Bullous pemphigoid is the most common blistering autoimmune disorder in the Western world, with an estimated incidence of 6-7 cases per 1 million persons in France and Germany, and an unknown incidence in the United States. It is a relatively benign condition that tends to run a waxing and waning course, with recurrent episodes of remission and relapse. It most commonly affects elderly persons but can also rarely occur in younger patients and infants.[1,2,3]",
"In cases of bullous pemphigoid, immunoglobulin G autoantibodies attack the skin basement membrane zone, which induces an inflammatory response and activates the complement system. The two main antigenic targets that have been identified are bullous pemphigoid antigen 1 (a 230-kd protein) and bullous pemphigoid antigen 2 (a 180-kd protein). They are both components of the hemidesmosome and allow linkage of intermediate filaments to the basement membrane.",
"Of these antigens, bullous pemphigoid antigen 2 is thought to be the major contributor to the pathophysiology of bullous pemphigoid. Animal studies have shown that blistering does not occur with antibodies induced solely against bullous pemphigoid antigen 1.[2] In these cases, eosinophils are characteristically found on skin histopathologic studies. They migrate to the area of injury by the activity of eotaxin, an eosinophil-specific chemokine.[2]",
"Interleukin-16 is another cytokine that has been found in high concentrations in the serum of patients with bullous pemphigoid. Interleukin-16 stimulates CD-4 helper cells and upregulates interleukin-2 receptors.",
"Bullous pemphigoid can present acutely or subacutely with tense, round or oval blisters and vesicles. Intensely itchy urticarial lesions can precede the bullae by days, weeks, or even months. The lesions of bullous pemphigoid may be either localized or generalized throughout the body, and they often affect the flexor areas of the limbs, as well as the abdomen, chest, and medial thighs. Mucous membrane involvement may occur, but it is uncommon. The lesions heal without any scarring.[1,2]",
"Although more commonly seen in pemphigus, the Nikolsky sign is also occasionally seen in cases of pemphigoid. The Nikolsky sign is the separation of superficial skin from the deeper dermis with application of gentle pressure."
],
"date": "April 03, 2019",
"figures": [
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/865/301/865301-thumb3.png"
}
],
"markdown": "# An 84-Year-Old With a Blistering Rash That Is Spreading\n\n **Authors:** Tuyyab Hassan, MD \n **Date:** April 03, 2019\n\n ## Content\n\n The patient in this case exhibited many factors suggestive of bullous pemphigoid. Her advanced age; the clinical appearance of the skin lesions; and the subacute onset of an intensely pruritic, nonpainful rash were consistent with the diagnosis of bullous pemphigoid. A skin biopsy sample sent for routine histologic, direct immunofluorescence, and indirect immunofluorescence studies confirmed the diagnosis. Histopathologic examination of a subepidermal blister demonstrated an eosinophil-rich inflammatory infiltrate in the dermis (Figure 3). Viral and bacterial culture swabs from the blister fluid were negative, which excluded an infectious process (eg, herpes zoster).\nFigure 3.\nBullous pemphigoid is the most common blistering autoimmune disorder in the Western world, with an estimated incidence of 6-7 cases per 1 million persons in France and Germany, and an unknown incidence in the United States. It is a relatively benign condition that tends to run a waxing and waning course, with recurrent episodes of remission and relapse. It most commonly affects elderly persons but can also rarely occur in younger patients and infants.[1,2,3]\nIn cases of bullous pemphigoid, immunoglobulin G autoantibodies attack the skin basement membrane zone, which induces an inflammatory response and activates the complement system. The two main antigenic targets that have been identified are bullous pemphigoid antigen 1 (a 230-kd protein) and bullous pemphigoid antigen 2 (a 180-kd protein). They are both components of the hemidesmosome and allow linkage of intermediate filaments to the basement membrane.\nOf these antigens, bullous pemphigoid antigen 2 is thought to be the major contributor to the pathophysiology of bullous pemphigoid. Animal studies have shown that blistering does not occur with antibodies induced solely against bullous pemphigoid antigen 1.[2] In these cases, eosinophils are characteristically found on skin histopathologic studies. They migrate to the area of injury by the activity of eotaxin, an eosinophil-specific chemokine.[2]\nInterleukin-16 is another cytokine that has been found in high concentrations in the serum of patients with bullous pemphigoid. Interleukin-16 stimulates CD-4 helper cells and upregulates interleukin-2 receptors.\nBullous pemphigoid can present acutely or subacutely with tense, round or oval blisters and vesicles. Intensely itchy urticarial lesions can precede the bullae by days, weeks, or even months. The lesions of bullous pemphigoid may be either localized or generalized throughout the body, and they often affect the flexor areas of the limbs, as well as the abdomen, chest, and medial thighs. Mucous membrane involvement may occur, but it is uncommon. The lesions heal without any scarring.[1,2]\nAlthough more commonly seen in pemphigus, the Nikolsky sign is also occasionally seen in cases of pemphigoid. The Nikolsky sign is the separation of superficial skin from the deeper dermis with application of gentle pressure.\n\n ## Figures\n\n **Figure 3.** \n \n\n\n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 983429,
"choiceText": "Bullous pemphigoid",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 983431,
"choiceText": "Herpes zoster (shingles)",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 983433,
"choiceText": "Pemphigus vulgaris",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 983435,
"choiceText": "Fixed drug eruption",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 983437,
"choiceText": "Bullous impetigo",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 311239,
"questionText": "<p>Which of the following is the most likely diagnosis?</p>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "An 84-Year-Old With a Blistering Rash That Is Spreading"
},
{
"authors": "Tuyyab Hassan, MD",
"content": [
"A full history and physical examination is essential for recognizing the diagnosis and determining the etiology of bullous pemphigoid. Drug-induced bullous pemphigoid is well recognized and has many causes, including diuretics, antibiotics, and angiotensin-converting enzyme inhibitors. Cessation of the drug normally prevents recurrence.[4]",
"Some evidence suggests an association between bullous pemphigoid and cancer; however, bullous pemphigoid itself has not been shown to increase the incidence of cancer in age- and sex-matched controls.[1] Of note, Gül and colleagues[5] described breast cancer with bone metastases presenting with bullous pemphigoid in a 62-year-old woman. Bullous pemphigoid is a disease of the elderly and, of course, this population is much more prone to developing cancer as well; therefore, a thorough examination for possible associated cancer is prudent.",
"In patients with autoimmune subepidermal blistering disease, bullous pemphigoid is the most likely diagnosis if three of the following four criteria are present:",
"Age > 70 years",
"Absence of atrophic scars",
"Absence of mucosal involvement",
"Absence of predominant bullous lesions on the neck and head",
"These criteria have a sensitivity of 90%, specificity of 83%, and positive predictive value of 95% when validated using immunoelectron microscopy and a sensitivity of 86%, specificity of 90%, and positive predictive value of over 95% when validated using immunoblotting as the criterion standard.",
"The clinical differential diagnosis for bullous pemphigoid is broad and could include pemphigus vulgaris, linear immunoglobulin A disease, epidermolysis bullosa acquisita, bullous lupus erythematosus, and dermatitis herpetiformis. Blistering rashes can also be seen with impetigo, acute viral infections, drug eruptions, and herpes zoster. The patient's history and physical examination will help narrow down the differential diagnosis. The patient's age, vital signs, the distribution of the blisters, and the nature of the blister (ie, tense or flaccid) all provide clues; however, testing is ultimately necessary and includes immunofluorescence studies, histopathologic examination, and viral and bacterial swabs.[1,3]",
"In bullous pemphigoid, indirect immunofluorescence using serum demonstrates circulating antibodies to the basement membrane zone of human skin or monkey esophagus substrate in a linear pattern in 70% of cases.[2] Skin biopsy of a blister often reveals an eosinophilic inflammatory infiltrate, whereas direct immunofluorescence of normal skin adjacent to the lesion shows linear deposits of immunoglobulin G and C3 at the basement membrane zone."
],
"date": "April 03, 2019",
"figures": [],
"markdown": "# An 84-Year-Old With a Blistering Rash That Is Spreading\n\n **Authors:** Tuyyab Hassan, MD \n **Date:** April 03, 2019\n\n ## Content\n\n A full history and physical examination is essential for recognizing the diagnosis and determining the etiology of bullous pemphigoid. Drug-induced bullous pemphigoid is well recognized and has many causes, including diuretics, antibiotics, and angiotensin-converting enzyme inhibitors. Cessation of the drug normally prevents recurrence.[4]\nSome evidence suggests an association between bullous pemphigoid and cancer; however, bullous pemphigoid itself has not been shown to increase the incidence of cancer in age- and sex-matched controls.[1] Of note, Gül and colleagues[5] described breast cancer with bone metastases presenting with bullous pemphigoid in a 62-year-old woman. Bullous pemphigoid is a disease of the elderly and, of course, this population is much more prone to developing cancer as well; therefore, a thorough examination for possible associated cancer is prudent.\nIn patients with autoimmune subepidermal blistering disease, bullous pemphigoid is the most likely diagnosis if three of the following four criteria are present:\nAge > 70 years\nAbsence of atrophic scars\nAbsence of mucosal involvement\nAbsence of predominant bullous lesions on the neck and head\nThese criteria have a sensitivity of 90%, specificity of 83%, and positive predictive value of 95% when validated using immunoelectron microscopy and a sensitivity of 86%, specificity of 90%, and positive predictive value of over 95% when validated using immunoblotting as the criterion standard.\nThe clinical differential diagnosis for bullous pemphigoid is broad and could include pemphigus vulgaris, linear immunoglobulin A disease, epidermolysis bullosa acquisita, bullous lupus erythematosus, and dermatitis herpetiformis. Blistering rashes can also be seen with impetigo, acute viral infections, drug eruptions, and herpes zoster. The patient's history and physical examination will help narrow down the differential diagnosis. The patient's age, vital signs, the distribution of the blisters, and the nature of the blister (ie, tense or flaccid) all provide clues; however, testing is ultimately necessary and includes immunofluorescence studies, histopathologic examination, and viral and bacterial swabs.[1,3]\nIn bullous pemphigoid, indirect immunofluorescence using serum demonstrates circulating antibodies to the basement membrane zone of human skin or monkey esophagus substrate in a linear pattern in 70% of cases.[2] Skin biopsy of a blister often reveals an eosinophilic inflammatory infiltrate, whereas direct immunofluorescence of normal skin adjacent to the lesion shows linear deposits of immunoglobulin G and C3 at the basement membrane zone.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "An 84-Year-Old With a Blistering Rash That Is Spreading"
},
{
"authors": "Tuyyab Hassan, MD",
"content": [
"Other variants of bullous pemphigoid include gestational pemphigoid and cicatricial pemphigoid. Gestational pemphigoid occurs during pregnancy and leads to bullae or urticarial lesions on the abdomen, trunk, and extremities, with mucous membrane sparing. Fetal loss is not increased; however, infants are often born prematurely and small for gestational age. Cicatricial pemphigoid rarely involves the skin and tends to affect mucous membranes, with ocular and oropharyngeal involvement, and it can lead to scarring and significant morbidity from blindness and airway obstruction.[3]",
"Treatment options for bullous pemphigoid include high-potency topical steroids, oral steroids (prednisone 0.5-1 mg/kg/d), antibiotics, steroid-sparing immunosuppressants (eg, azathioprine, mycophenolate mofetil, and methotrexate), plasmapheresis, and immunoglobulin infusions. Calcium and vitamin D supplements with a bisphosphonate should be given when treating with systemic steroids.[2] For acutely ill patients or those with extensive skin involvement, fluid replacement, thermoregulation, and infection management may be necessary.",
"Most patients with bullous pemphigoid are elderly; they may have multiple comorbidities and may be taking multiple medications. Treatment should be carefully tailored to minimize side effects yet control disease adequately.",
"A systematic review by Khumalo and colleagues[6] found that potent topical steroids were safe and effective in patients with bullous pemphigoid, with less systemic side effects than oral steroid treatment. Topical clobetasol propionate 0.05% (40 g/d) has been shown to be superior to oral prednisolone (0.5 mg/kg/d) in terms of overall survival, disease control, and adverse event profile for patients with extensive bullous pemphigoid. It is equally effective for patients with moderate bullous pemphigoid, as shown in a randomized controlled trial.[7] For this reason, topical clobetasol may be used as first-line treatment for bullous pemphigoid.",
"Side effects of steroid treatment can include diabetes mellitus, peptic ulcer disease, glaucoma, cataract formation, and agranulocytosis. The prognosis of patients with bullous pemphigoid is generally good. Disease is usually self-limited over 5-6 years.",
"The patient in this case was treated with high-potency topical steroids and experienced a good result. The medication was tapered over several weeks, and she regularly followed up with her healthcare provider."
],
"date": "April 03, 2019",
"figures": [],
"markdown": "# An 84-Year-Old With a Blistering Rash That Is Spreading\n\n **Authors:** Tuyyab Hassan, MD \n **Date:** April 03, 2019\n\n ## Content\n\n Other variants of bullous pemphigoid include gestational pemphigoid and cicatricial pemphigoid. Gestational pemphigoid occurs during pregnancy and leads to bullae or urticarial lesions on the abdomen, trunk, and extremities, with mucous membrane sparing. Fetal loss is not increased; however, infants are often born prematurely and small for gestational age. Cicatricial pemphigoid rarely involves the skin and tends to affect mucous membranes, with ocular and oropharyngeal involvement, and it can lead to scarring and significant morbidity from blindness and airway obstruction.[3]\nTreatment options for bullous pemphigoid include high-potency topical steroids, oral steroids (prednisone 0.5-1 mg/kg/d), antibiotics, steroid-sparing immunosuppressants (eg, azathioprine, mycophenolate mofetil, and methotrexate), plasmapheresis, and immunoglobulin infusions. Calcium and vitamin D supplements with a bisphosphonate should be given when treating with systemic steroids.[2] For acutely ill patients or those with extensive skin involvement, fluid replacement, thermoregulation, and infection management may be necessary.\nMost patients with bullous pemphigoid are elderly; they may have multiple comorbidities and may be taking multiple medications. Treatment should be carefully tailored to minimize side effects yet control disease adequately.\nA systematic review by Khumalo and colleagues[6] found that potent topical steroids were safe and effective in patients with bullous pemphigoid, with less systemic side effects than oral steroid treatment. Topical clobetasol propionate 0.05% (40 g/d) has been shown to be superior to oral prednisolone (0.5 mg/kg/d) in terms of overall survival, disease control, and adverse event profile for patients with extensive bullous pemphigoid. It is equally effective for patients with moderate bullous pemphigoid, as shown in a randomized controlled trial.[7] For this reason, topical clobetasol may be used as first-line treatment for bullous pemphigoid.\nSide effects of steroid treatment can include diabetes mellitus, peptic ulcer disease, glaucoma, cataract formation, and agranulocytosis. The prognosis of patients with bullous pemphigoid is generally good. Disease is usually self-limited over 5-6 years.\nThe patient in this case was treated with high-potency topical steroids and experienced a good result. The medication was tapered over several weeks, and she regularly followed up with her healthcare provider.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 983439,
"choiceText": "Mucosal involvement",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 983441,
"choiceText": "Tense blisters",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 983443,
"choiceText": "Healing, scarlike lesions",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 983445,
"choiceText": "Pyrexia",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 983447,
"choiceText": "Dermatomal distribution",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>Tense blisters are most likely to be found in cases of bullous pemphigoid; they may be localized or generalized. The blisters may be preceded by urticarial plaques or pruritus, and they are most often seen in the flexor areas of the limbs, medial thighs, abdomen, groin, and chest wall. Mucosal involvement is uncommon. The lesions of bullous pemphigoid typically do not form scars. Pyrexia may be present if bullae are infected, but this is not typical. A dermatomal distribution points to herpes zoster infection.</p>",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 311241,
"questionText": "<p>You are examining a patient and suspect that she has bullous pemphigoid. Which of the following is most likely to be found? </p>\r\n",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 983449,
"choiceText": "Steroids",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 983451,
"choiceText": "Methotrexate",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 983453,
"choiceText": "Plasmapheresis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 983455,
"choiceText": "Intravenous immunoglobulin",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 983457,
"choiceText": "Azathioprine",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>Although many treatment modalities are available, steroids are the mainstay of treatment. The treatment should be tailored to each individual patient, and any underlying medical conditions and medications should be taken into account.</p>",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 311243,
"questionText": "<p>You are examining a 76-year-old patient who presented with a vesiculobullous rash on his elbows and knees that began 2 months ago. The rash is painful and limits his activities. His history reveals that he has experienced similar episodes in the past. You diagnose this patient with bullous pemphigoid, which is confirmed by histopathologic analysis and direct immunofluorescence testing. Which of the following choices is the mainstay of treatment for this patient? </p>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "An 84-Year-Old With a Blistering Rash That Is Spreading"
},
{
"authors": "Tuyyab Hassan, MD",
"content": [],
"date": "April 03, 2019",
"figures": [],
"markdown": "# An 84-Year-Old With a Blistering Rash That Is Spreading\n\n **Authors:** Tuyyab Hassan, MD \n **Date:** April 03, 2019\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 983439,
"choiceText": "Mucosal involvement",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 983441,
"choiceText": "Tense blisters",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 983443,
"choiceText": "Healing, scarlike lesions",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 983445,
"choiceText": "Pyrexia",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 983447,
"choiceText": "Dermatomal distribution",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>Tense blisters are most likely to be found in cases of bullous pemphigoid; they may be localized or generalized. The blisters may be preceded by urticarial plaques or pruritus, and they are most often seen in the flexor areas of the limbs, medial thighs, abdomen, groin, and chest wall. Mucosal involvement is uncommon. The lesions of bullous pemphigoid typically do not form scars. Pyrexia may be present if bullae are infected, but this is not typical. A dermatomal distribution points to herpes zoster infection.</p>",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 311241,
"questionText": "<p>You are examining a patient and suspect that she has bullous pemphigoid. Which of the following is most likely to be found? </p>\r\n",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 983449,
"choiceText": "Steroids",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 983451,
"choiceText": "Methotrexate",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 983453,
"choiceText": "Plasmapheresis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 983455,
"choiceText": "Intravenous immunoglobulin",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 983457,
"choiceText": "Azathioprine",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>Although many treatment modalities are available, steroids are the mainstay of treatment. The treatment should be tailored to each individual patient, and any underlying medical conditions and medications should be taken into account.</p>",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 311243,
"questionText": "<p>You are examining a 76-year-old patient who presented with a vesiculobullous rash on his elbows and knees that began 2 months ago. The rash is painful and limits his activities. His history reveals that he has experienced similar episodes in the past. You diagnose this patient with bullous pemphigoid, which is confirmed by histopathologic analysis and direct immunofluorescence testing. Which of the following choices is the mainstay of treatment for this patient? </p>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "An 84-Year-Old With a Blistering Rash That Is Spreading"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 983429,
"choiceText": "Bullous pemphigoid",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 983431,
"choiceText": "Herpes zoster (shingles)",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 983433,
"choiceText": "Pemphigus vulgaris",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 983435,
"choiceText": "Fixed drug eruption",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 983437,
"choiceText": "Bullous impetigo",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 311239,
"questionText": "<p>Which of the following is the most likely diagnosis?</p>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 983439,
"choiceText": "Mucosal involvement",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 983441,
"choiceText": "Tense blisters",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 983443,
"choiceText": "Healing, scarlike lesions",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 983445,
"choiceText": "Pyrexia",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 983447,
"choiceText": "Dermatomal distribution",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>Tense blisters are most likely to be found in cases of bullous pemphigoid; they may be localized or generalized. The blisters may be preceded by urticarial plaques or pruritus, and they are most often seen in the flexor areas of the limbs, medial thighs, abdomen, groin, and chest wall. Mucosal involvement is uncommon. The lesions of bullous pemphigoid typically do not form scars. Pyrexia may be present if bullae are infected, but this is not typical. A dermatomal distribution points to herpes zoster infection.</p>",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 311241,
"questionText": "<p>You are examining a patient and suspect that she has bullous pemphigoid. Which of the following is most likely to be found? </p>\r\n",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 983449,
"choiceText": "Steroids",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 983451,
"choiceText": "Methotrexate",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 983453,
"choiceText": "Plasmapheresis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 983455,
"choiceText": "Intravenous immunoglobulin",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 983457,
"choiceText": "Azathioprine",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "<p>Although many treatment modalities are available, steroids are the mainstay of treatment. The treatment should be tailored to each individual patient, and any underlying medical conditions and medications should be taken into account.</p>",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 311243,
"questionText": "<p>You are examining a 76-year-old patient who presented with a vesiculobullous rash on his elbows and knees that began 2 months ago. The rash is painful and limits his activities. His history reveals that he has experienced similar episodes in the past. You diagnose this patient with bullous pemphigoid, which is confirmed by histopathologic analysis and direct immunofluorescence testing. Which of the following choices is the mainstay of treatment for this patient? </p>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
865302 | /viewarticle/865302 | [
{
"authors": "Percy G. Balderia, MD; Sian Y. Lim, MD; Tejus A. Bale, MD, PhD; Gregory J. Czuczman, MD; Anthony A. Amato, MD; Sonali P. Desai, MPH",
"content": [
"Editor's Note:\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.",
"A 56-year-old man was diagnosed with low-grade follicular lymphoma 7 years ago and received involved-field radiation therapy. After 3 years of remission, he was found to have diffuse large B-cell lymphoma.",
"The patient was started on rituximab, cyclophosphamide, doxorubicin, and prednisone (R-CHOP), followed by autologous stem cell transplantation. Although his lymphoma went in remission, he developed pancytopenia and was diagnosed with myelodysplastic syndrome. He underwent stem cell transplantation from a matched, unrelated donor 1 year ago, and his myelodysplastic syndrome was confirmed to be in remission.",
"Five months after the second transplant, the patient developed blanching erythematous macules and patches on his extremities, oral ulcers, and elevated aminotransferase levels as the tacrolimus for graft-vs-host disease (GVHD) prophylaxis was being tapered. He was started on prednisone 30 mg daily. Tacrolimus was subsequently discontinued because of hemolytic anemia 9 months after the second transplant. The prednisone dosage was increased because the rash and oral ulcers persisted; he was taking 60 mg daily 1 year after the second transplant.",
"The patient's rash and oral ulcers started to improve, but he started having difficulty in climbing stairs. The weakness was initially attributed to steroid-induced myopathy, so the daily prednisone dosage was decreased by 5 mg every week. Meanwhile, mycophenolate mofetil at 1000 mg twice daily was started for the skin rash and oral ulcers. Unfortunately, the weakness gradually worsened. Fifteen months after the second transplant, the patient had exertional dyspnea and difficulty arising from a chair, leading to admission."
],
"date": "June 27, 2016",
"figures": [],
"markdown": "# A 56-Year-Old Man With Dyspnea and Difficulty Standing\n\n **Authors:** Percy G. Balderia, MD; Sian Y. Lim, MD; Tejus A. Bale, MD, PhD; Gregory J. Czuczman, MD; Anthony A. Amato, MD; Sonali P. Desai, MPH \n **Date:** June 27, 2016\n\n ## Content\n\n Editor's Note:\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.\nA 56-year-old man was diagnosed with low-grade follicular lymphoma 7 years ago and received involved-field radiation therapy. After 3 years of remission, he was found to have diffuse large B-cell lymphoma.\nThe patient was started on rituximab, cyclophosphamide, doxorubicin, and prednisone (R-CHOP), followed by autologous stem cell transplantation. Although his lymphoma went in remission, he developed pancytopenia and was diagnosed with myelodysplastic syndrome. He underwent stem cell transplantation from a matched, unrelated donor 1 year ago, and his myelodysplastic syndrome was confirmed to be in remission.\nFive months after the second transplant, the patient developed blanching erythematous macules and patches on his extremities, oral ulcers, and elevated aminotransferase levels as the tacrolimus for graft-vs-host disease (GVHD) prophylaxis was being tapered. He was started on prednisone 30 mg daily. Tacrolimus was subsequently discontinued because of hemolytic anemia 9 months after the second transplant. The prednisone dosage was increased because the rash and oral ulcers persisted; he was taking 60 mg daily 1 year after the second transplant.\nThe patient's rash and oral ulcers started to improve, but he started having difficulty in climbing stairs. The weakness was initially attributed to steroid-induced myopathy, so the daily prednisone dosage was decreased by 5 mg every week. Meanwhile, mycophenolate mofetil at 1000 mg twice daily was started for the skin rash and oral ulcers. Unfortunately, the weakness gradually worsened. Fifteen months after the second transplant, the patient had exertional dyspnea and difficulty arising from a chair, leading to admission.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 56-Year-Old Man With Dyspnea and Difficulty Standing"
},
{
"authors": "Percy G. Balderia, MD; Sian Y. Lim, MD; Tejus A. Bale, MD, PhD; Gregory J. Czuczman, MD; Anthony A. Amato, MD; Sonali P. Desai, MPH",
"content": [
"Upon examination, the patient did not have oral ulcers or skin rash. He had decreased breath sounds on the right lung base. Neurologic examination revealed symmetric grade 3 strength of the proximal upper and lower extremities and grade 4 strength of the distal upper and lower extremities. Neck flexion and extension were preserved. The patient's serum creatinine kinase level was elevated, at 1488 U/L. The antinuclear and myositis-specific antibody findings were negative.",
"Chest CT showed right lower-lobe consolidation consistent with aspiration pneumonia. Blood culture findings were negative. Swallowing video fluoroscopy confirmed the oropharyngeal dysphagia and aspiration risk. The patient's negative inspiratory force was only -25 cm H2O, and he could not perform well during spirometry.",
"Figure 1.",
"Figure 1.",
"Figure 2.",
"Figure 2.",
"Figure 3.",
"Figure 3.",
"Figure 4.",
"Figure 4.",
"MRI of the left thigh demonstrated extensive multifocal subcutaneous, muscular, and fascial edema (Figure 1), with relatively mild patchy enhancement after administration of gadolinium. A left quadriceps biopsy revealed scattered necrotic and regenerating muscle fibers and scant inflammatory cell infiltration in the epimysium and perimysium (Figure 2). Immunohistochemistry for CD3 showed focal collections of T cells, with no infiltration by CD20-positive B cells (Figure 3). Moreover, major histocompatibility antigen 1 immunostaining was positive in scattered nonnecrotic and nonregenerating muscle fibers (Figure 4). Appropriate controls were performed for these studies. Because of the scant infiltrate, CD4 vs CD8 predominance was not evaluated."
],
"date": "June 27, 2016",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/865/302/865302-Thumb1.png"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/865/302/865302-Thumb2.png"
},
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/865/302/865302-Thumb3.png"
},
{
"caption": "Figure 4.",
"image_url": "https://img.medscapestatic.com/article/865/302/865302-Thumb4.png"
}
],
"markdown": "# A 56-Year-Old Man With Dyspnea and Difficulty Standing\n\n **Authors:** Percy G. Balderia, MD; Sian Y. Lim, MD; Tejus A. Bale, MD, PhD; Gregory J. Czuczman, MD; Anthony A. Amato, MD; Sonali P. Desai, MPH \n **Date:** June 27, 2016\n\n ## Content\n\n Upon examination, the patient did not have oral ulcers or skin rash. He had decreased breath sounds on the right lung base. Neurologic examination revealed symmetric grade 3 strength of the proximal upper and lower extremities and grade 4 strength of the distal upper and lower extremities. Neck flexion and extension were preserved. The patient's serum creatinine kinase level was elevated, at 1488 U/L. The antinuclear and myositis-specific antibody findings were negative.\nChest CT showed right lower-lobe consolidation consistent with aspiration pneumonia. Blood culture findings were negative. Swallowing video fluoroscopy confirmed the oropharyngeal dysphagia and aspiration risk. The patient's negative inspiratory force was only -25 cm H2O, and he could not perform well during spirometry.\nFigure 1.\nFigure 1.\nFigure 2.\nFigure 2.\nFigure 3.\nFigure 3.\nFigure 4.\nFigure 4.\nMRI of the left thigh demonstrated extensive multifocal subcutaneous, muscular, and fascial edema (Figure 1), with relatively mild patchy enhancement after administration of gadolinium. A left quadriceps biopsy revealed scattered necrotic and regenerating muscle fibers and scant inflammatory cell infiltration in the epimysium and perimysium (Figure 2). Immunohistochemistry for CD3 showed focal collections of T cells, with no infiltration by CD20-positive B cells (Figure 3). Moreover, major histocompatibility antigen 1 immunostaining was positive in scattered nonnecrotic and nonregenerating muscle fibers (Figure 4). Appropriate controls were performed for these studies. Because of the scant infiltrate, CD4 vs CD8 predominance was not evaluated.\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n**Figure 3.** \n \n\n**Figure 4.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 982389,
"choiceText": "Steroid-induced myopathy",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 982391,
"choiceText": "Chronic GVHD (cGVHD)-associated myositis",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 982393,
"choiceText": "Bacterial myositis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 982395,
"choiceText": "Polymyositis\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 310935,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 56-Year-Old Man With Dyspnea and Difficulty Standing"
},
{
"authors": "Percy G. Balderia, MD; Sian Y. Lim, MD; Tejus A. Bale, MD, PhD; Gregory J. Czuczman, MD; Anthony A. Amato, MD; Sonali P. Desai, MPH",
"content": [
"The constellation of clinical, laboratory, and pathologic findings indicates that the patient has cGVHD-associated myositis. Steroid-induced myopathy does not cause major increases in creatine kinase levels. The biopsy findings are consistent with cGVHD-associated myositis, and the MRI findings are typical for this disorder. The absence of other signs of infection and negative blood cultures make the diagnosis of bacterial myositis unlikely.",
"The biopsy results are consistent with polymyositis. The absence of antinuclear or myositis-specific antibodies is less likely in this condition but does not exclude it, and given that the patient already has known cGVHD, GVHD myositis is the more likely diagnosis.",
"In cGVHD, immunologically competent cells in the graft react against host cells.[1] Myositis occurs in 0.65% to 3.5% of patients with cGVHD and is less common than involvement of the skin (75%), oral mucosa (51%-63%), liver (21%-51%), and eyes (22%-33%).[2] Although rare, the incidence of myositis in cGVHD is still much higher than in the general population. In a series by Stevens and colleagues,[3] the incidence of myositis in cGVHD was estimated to be 49 per 100,000 person-years.",
"The onset of cGVHD-associated myositis occurs 4-70 months after transplant (mean, 23.8 months).",
"The characteristics and manifestations of 44 patients with cGVHD-associated myositis are summarized as follows:[3,4,5,6,7,8,9,10,11,12,13,14,15,16,17]",
"Age at transplant: 18-56 years (mean, 33.6 years)",
"Male-to-female ratio: 23:21 (52.3% and 47.7%, respectively)",
"Time to myositis onset after transplant: 4-70 months (mean, 23.8 months)",
"Time to cGVHD onset after transplant: 2-65 months (mean, 11.3 months)",
"Creatine kinase level: 28-14420 U/L (mean, 2104.4 U/L)",
"Aldolase level: 4.6-320 U/L (mean, 51.7 U/L)",
"Lactate dehydrogenase level: 169-1143 U/L (mean, 379.1 U/L)",
"Aspartate aminotransferase level: 34-471 U/L (mean, 179.6 U/L)",
"A retrospective study of 1967 Japanese patients who underwent allogeneic stem cell transplant was unable to identify significant risk factors for cGVHD myositis.[8] Myositis is usually a late manifestation of cGVHD, occurring after other symptoms have been present for some time. However, myositis can occur earlier than and have a course independent of the other cGVHD manifestations. Three patients (6.8%) were reported to have isolated myositis as a manifestation of cGVHD.[7,16,17] Moreover, patients who started having skin and mucous membrane manifestations up to 10 months after the onset of muscle involvement have been reported.",
"Among reported cases, only four patients (9.1%) did not experience weakness.[7,8,12] These patients experienced myalgia and were found to have myositis on muscle biopsy. These cases may have been detected early, with patients receiving treatment before the onset of weakness. The weakness from cGVHD-associated myositis usually affects the proximal muscles. Only two cases (4.5%) were reported to have predominantly distal muscle involvement.[10,13]",
"Other rare manifestations of myositis include dysphagia (one patient [2.3%]) and ventilatory muscle weakness (three patients [6.8%]).[4,5,6] Among those with ventilatory muscle weakness, two required mechanical ventilation. The skin and mucous membranes were the most common cGVHD-affected organs at the time of myositis onset."
],
"date": "June 27, 2016",
"figures": [],
"markdown": "# A 56-Year-Old Man With Dyspnea and Difficulty Standing\n\n **Authors:** Percy G. Balderia, MD; Sian Y. Lim, MD; Tejus A. Bale, MD, PhD; Gregory J. Czuczman, MD; Anthony A. Amato, MD; Sonali P. Desai, MPH \n **Date:** June 27, 2016\n\n ## Content\n\n The constellation of clinical, laboratory, and pathologic findings indicates that the patient has cGVHD-associated myositis. Steroid-induced myopathy does not cause major increases in creatine kinase levels. The biopsy findings are consistent with cGVHD-associated myositis, and the MRI findings are typical for this disorder. The absence of other signs of infection and negative blood cultures make the diagnosis of bacterial myositis unlikely.\nThe biopsy results are consistent with polymyositis. The absence of antinuclear or myositis-specific antibodies is less likely in this condition but does not exclude it, and given that the patient already has known cGVHD, GVHD myositis is the more likely diagnosis.\nIn cGVHD, immunologically competent cells in the graft react against host cells.[1] Myositis occurs in 0.65% to 3.5% of patients with cGVHD and is less common than involvement of the skin (75%), oral mucosa (51%-63%), liver (21%-51%), and eyes (22%-33%).[2] Although rare, the incidence of myositis in cGVHD is still much higher than in the general population. In a series by Stevens and colleagues,[3] the incidence of myositis in cGVHD was estimated to be 49 per 100,000 person-years.\nThe onset of cGVHD-associated myositis occurs 4-70 months after transplant (mean, 23.8 months).\nThe characteristics and manifestations of 44 patients with cGVHD-associated myositis are summarized as follows:[3,4,5,6,7,8,9,10,11,12,13,14,15,16,17]\nAge at transplant: 18-56 years (mean, 33.6 years)\nMale-to-female ratio: 23:21 (52.3% and 47.7%, respectively)\nTime to myositis onset after transplant: 4-70 months (mean, 23.8 months)\nTime to cGVHD onset after transplant: 2-65 months (mean, 11.3 months)\nCreatine kinase level: 28-14420 U/L (mean, 2104.4 U/L)\nAldolase level: 4.6-320 U/L (mean, 51.7 U/L)\nLactate dehydrogenase level: 169-1143 U/L (mean, 379.1 U/L)\nAspartate aminotransferase level: 34-471 U/L (mean, 179.6 U/L)\nA retrospective study of 1967 Japanese patients who underwent allogeneic stem cell transplant was unable to identify significant risk factors for cGVHD myositis.[8] Myositis is usually a late manifestation of cGVHD, occurring after other symptoms have been present for some time. However, myositis can occur earlier than and have a course independent of the other cGVHD manifestations. Three patients (6.8%) were reported to have isolated myositis as a manifestation of cGVHD.[7,16,17] Moreover, patients who started having skin and mucous membrane manifestations up to 10 months after the onset of muscle involvement have been reported.\nAmong reported cases, only four patients (9.1%) did not experience weakness.[7,8,12] These patients experienced myalgia and were found to have myositis on muscle biopsy. These cases may have been detected early, with patients receiving treatment before the onset of weakness. The weakness from cGVHD-associated myositis usually affects the proximal muscles. Only two cases (4.5%) were reported to have predominantly distal muscle involvement.[10,13]\nOther rare manifestations of myositis include dysphagia (one patient [2.3%]) and ventilatory muscle weakness (three patients [6.8%]).[4,5,6] Among those with ventilatory muscle weakness, two required mechanical ventilation. The skin and mucous membranes were the most common cGVHD-affected organs at the time of myositis onset.\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 982389,
"choiceText": "Steroid-induced myopathy",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 982391,
"choiceText": "Chronic GVHD (cGVHD)-associated myositis",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 982393,
"choiceText": "Bacterial myositis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 982395,
"choiceText": "Polymyositis\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 310935,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 56-Year-Old Man With Dyspnea and Difficulty Standing"
},
{
"authors": "Percy G. Balderia, MD; Sian Y. Lim, MD; Tejus A. Bale, MD, PhD; Gregory J. Czuczman, MD; Anthony A. Amato, MD; Sonali P. Desai, MPH",
"content": [
"MRI findings of cGVHD-associated myositisinclude patchy or diffuse subcutaneous, fascial, and muscular edema on T2-weighted images, with enhancement on fat-saturated gadolinium-enhanced T1-weighted images. Moreover, areas of intramuscular precontrast T1 hyperintense fatty infiltration can also occur with prolonged disease. However, these findings are not specific for cGVHD and can also be seen in the setting of myositis secondary to other etiologies as well as from muscle injury, such as rhabdomyolysis.[18,19,20] A small study suggested that MRI may be used longitudinally to evaluate the response of myositis to immunosuppressive therapy.[19]",
"Donor-derived CD4+ and CD8+ T lymphocytes are considered to be the active mediators in cGVHD. However, studies point to complex interactions of dendritic and B cells, with T lymphocytes responsible for cGVHD.[21,22] This observation may account for cGVHD cases that are refractory to T-cell–based therapy and for the various muscle biopsy results in cases with myositis.",
"Most reported cases of cGVHD-associated myositis have been reported as polymyositis. However, the definition of polymyositis is controversial, and most cases were diagnosed using the outdated Bohan and Peter criteria. In a review of 44 cases, three patients (6.8%) had biopsy findings consistent with dermatomyositis, and one had findings consistent with granulomatous myositis (2.3%).[13,15] Among these cases, antinuclear antibody was positive in only two patients (titers of 1:320 and 1:640).[8,15] As-yet unidentified antibodies may be responsible for cGVHD myositis."
],
"date": "June 27, 2016",
"figures": [],
"markdown": "# A 56-Year-Old Man With Dyspnea and Difficulty Standing\n\n **Authors:** Percy G. Balderia, MD; Sian Y. Lim, MD; Tejus A. Bale, MD, PhD; Gregory J. Czuczman, MD; Anthony A. Amato, MD; Sonali P. Desai, MPH \n **Date:** June 27, 2016\n\n ## Content\n\n MRI findings of cGVHD-associated myositisinclude patchy or diffuse subcutaneous, fascial, and muscular edema on T2-weighted images, with enhancement on fat-saturated gadolinium-enhanced T1-weighted images. Moreover, areas of intramuscular precontrast T1 hyperintense fatty infiltration can also occur with prolonged disease. However, these findings are not specific for cGVHD and can also be seen in the setting of myositis secondary to other etiologies as well as from muscle injury, such as rhabdomyolysis.[18,19,20] A small study suggested that MRI may be used longitudinally to evaluate the response of myositis to immunosuppressive therapy.[19]\nDonor-derived CD4+ and CD8+ T lymphocytes are considered to be the active mediators in cGVHD. However, studies point to complex interactions of dendritic and B cells, with T lymphocytes responsible for cGVHD.[21,22] This observation may account for cGVHD cases that are refractory to T-cell–based therapy and for the various muscle biopsy results in cases with myositis.\nMost reported cases of cGVHD-associated myositis have been reported as polymyositis. However, the definition of polymyositis is controversial, and most cases were diagnosed using the outdated Bohan and Peter criteria. In a review of 44 cases, three patients (6.8%) had biopsy findings consistent with dermatomyositis, and one had findings consistent with granulomatous myositis (2.3%).[13,15] Among these cases, antinuclear antibody was positive in only two patients (titers of 1:320 and 1:640).[8,15] As-yet unidentified antibodies may be responsible for cGVHD myositis.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 56-Year-Old Man With Dyspnea and Difficulty Standing"
},
{
"authors": "Percy G. Balderia, MD; Sian Y. Lim, MD; Tejus A. Bale, MD, PhD; Gregory J. Czuczman, MD; Anthony A. Amato, MD; Sonali P. Desai, MPH",
"content": [
"Early diagnosis and management are important. Other possible causes of skeletal muscle weakness in a patient who underwent hematopoietic stem cell transplant need to be excluded, such as steroid-induced myopathy, disuse atrophy, poor nutritional status, electrolyte disorders, drug-induced muscle weakness (from statins, colchicine, antimalarials), myasthenia gravis, and Lambert-Eaton myasthenic syndrome.[5,14] Most cases of cGVHD-associated myositis improved with immunotherapy: 80% of patients (35 of 44) responded completely, whereas 15.9% (7 of 44) had a partial response. The time to complete resolution varied from a few weeks to 2 years of treatment. Complications were rare but included relapsing myositis (1 of 44 patients), residual muscle atrophy (2 of 44), and respiratory failure (three of 44).",
"Treatment is based on experience with idiopathic myositis. The treatment of choice is corticosteroids, with subsequent addition of methotrexate, azathioprine, intravenous immunoglobulin, and even rituximab in refractory cases.[3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,23]",
"Dysphagia is not a common manifestation of cGVHD myositis. In patients with idiopathic myositis, dysphagia improves with immunosuppressive treatment but can take many weeks or months, depending on the severity.[24]",
"Myositis is a rare manifestation of cGVHD and usually occurs after other signs have been present for some time. Having an increased index of suspicion for this rare manifestation in patients with cGVHD is important, given the common use of steroids for management of cGVHD and the risk for steroid myopathy, which can also present with muscle weakness. Proximal muscles are more commonly affected, but distal muscle weakness is also seen. Muscle biopsy findings consistent with polymyositis, dermatomyositis, or granulomatous myositis have been reported. Early diagnosis and treatment are important because fixed weakness from irreversible muscle damage, dysphagia, and ventilatory failure can occur. Most patients respond to increase in immunosuppression, but complete response usually takes several months.",
"The patient received intravenous immunoglobulin; pulse intravenous methylprednisolone for 3 days, followed by prednisone 60 mg daily; and weekly subcutaneous methotrexate at 20 mg. Mycophenolate mofetil was discontinued because it was deemed ineffective. Because the patient failed a swallowing evaluation and continued to have ventilatory weakness, he underwent percutaneous endoscopic gastrostomy and tracheostomy. The late diagnosis probably resulted in significant atrophy and weakness of his muscles. The patient was discharged to a rehabilitation facility."
],
"date": "June 27, 2016",
"figures": [],
"markdown": "# A 56-Year-Old Man With Dyspnea and Difficulty Standing\n\n **Authors:** Percy G. Balderia, MD; Sian Y. Lim, MD; Tejus A. Bale, MD, PhD; Gregory J. Czuczman, MD; Anthony A. Amato, MD; Sonali P. Desai, MPH \n **Date:** June 27, 2016\n\n ## Content\n\n Early diagnosis and management are important. Other possible causes of skeletal muscle weakness in a patient who underwent hematopoietic stem cell transplant need to be excluded, such as steroid-induced myopathy, disuse atrophy, poor nutritional status, electrolyte disorders, drug-induced muscle weakness (from statins, colchicine, antimalarials), myasthenia gravis, and Lambert-Eaton myasthenic syndrome.[5,14] Most cases of cGVHD-associated myositis improved with immunotherapy: 80% of patients (35 of 44) responded completely, whereas 15.9% (7 of 44) had a partial response. The time to complete resolution varied from a few weeks to 2 years of treatment. Complications were rare but included relapsing myositis (1 of 44 patients), residual muscle atrophy (2 of 44), and respiratory failure (three of 44).\nTreatment is based on experience with idiopathic myositis. The treatment of choice is corticosteroids, with subsequent addition of methotrexate, azathioprine, intravenous immunoglobulin, and even rituximab in refractory cases.[3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,23]\nDysphagia is not a common manifestation of cGVHD myositis. In patients with idiopathic myositis, dysphagia improves with immunosuppressive treatment but can take many weeks or months, depending on the severity.[24]\nMyositis is a rare manifestation of cGVHD and usually occurs after other signs have been present for some time. Having an increased index of suspicion for this rare manifestation in patients with cGVHD is important, given the common use of steroids for management of cGVHD and the risk for steroid myopathy, which can also present with muscle weakness. Proximal muscles are more commonly affected, but distal muscle weakness is also seen. Muscle biopsy findings consistent with polymyositis, dermatomyositis, or granulomatous myositis have been reported. Early diagnosis and treatment are important because fixed weakness from irreversible muscle damage, dysphagia, and ventilatory failure can occur. Most patients respond to increase in immunosuppression, but complete response usually takes several months.\nThe patient received intravenous immunoglobulin; pulse intravenous methylprednisolone for 3 days, followed by prednisone 60 mg daily; and weekly subcutaneous methotrexate at 20 mg. Mycophenolate mofetil was discontinued because it was deemed ineffective. Because the patient failed a swallowing evaluation and continued to have ventilatory weakness, he underwent percutaneous endoscopic gastrostomy and tracheostomy. The late diagnosis probably resulted in significant atrophy and weakness of his muscles. The patient was discharged to a rehabilitation facility.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 982397,
"choiceText": "Both proximal and distal muscles can be affected",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 982399,
"choiceText": "Myositis occurs only in the presence of other manifestations of cGVHD",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 982401,
"choiceText": "MRI findings of muscular edema on T2-weighted images, with enhancement on fat-saturated gadolinium-enhanced T1-weighted images, are specific",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 982403,
"choiceText": "A decrease in steroid dose is necessary for treatment\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Proximal muscles are more commonly affected, but distal muscle weakness is also seen.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 310937,
"questionText": "Which of the following is true about cGVHD-associated myositis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 982413,
"choiceText": "Steroids",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 982415,
"choiceText": "Methotrexate",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 982417,
"choiceText": "Azathioprine",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 982419,
"choiceText": "Intravenous immunoglobulin\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 982421,
"choiceText": "Rituximab \r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "\r\nTreatment is based on experience with idiopathic myositis. The treatment of choice is corticosteroids, with subsequent addition of methotrexate, azathioprine, intravenous immunoglobulin, and even rituximab in refractory cases.\r\n",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 310941,
"questionText": "Which of the following is the treatment of choice for cGVHD-associated myositis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 56-Year-Old Man With Dyspnea and Difficulty Standing"
},
{
"authors": "Percy G. Balderia, MD; Sian Y. Lim, MD; Tejus A. Bale, MD, PhD; Gregory J. Czuczman, MD; Anthony A. Amato, MD; Sonali P. Desai, MPH",
"content": [],
"date": "June 27, 2016",
"figures": [],
"markdown": "# A 56-Year-Old Man With Dyspnea and Difficulty Standing\n\n **Authors:** Percy G. Balderia, MD; Sian Y. Lim, MD; Tejus A. Bale, MD, PhD; Gregory J. Czuczman, MD; Anthony A. Amato, MD; Sonali P. Desai, MPH \n **Date:** June 27, 2016\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 982397,
"choiceText": "Both proximal and distal muscles can be affected",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 982399,
"choiceText": "Myositis occurs only in the presence of other manifestations of cGVHD",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 982401,
"choiceText": "MRI findings of muscular edema on T2-weighted images, with enhancement on fat-saturated gadolinium-enhanced T1-weighted images, are specific",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 982403,
"choiceText": "A decrease in steroid dose is necessary for treatment\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Proximal muscles are more commonly affected, but distal muscle weakness is also seen.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 310937,
"questionText": "Which of the following is true about cGVHD-associated myositis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 982413,
"choiceText": "Steroids",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 982415,
"choiceText": "Methotrexate",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 982417,
"choiceText": "Azathioprine",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 982419,
"choiceText": "Intravenous immunoglobulin\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 982421,
"choiceText": "Rituximab \r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "\r\nTreatment is based on experience with idiopathic myositis. The treatment of choice is corticosteroids, with subsequent addition of methotrexate, azathioprine, intravenous immunoglobulin, and even rituximab in refractory cases.\r\n",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 310941,
"questionText": "Which of the following is the treatment of choice for cGVHD-associated myositis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 56-Year-Old Man With Dyspnea and Difficulty Standing"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 982389,
"choiceText": "Steroid-induced myopathy",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 982391,
"choiceText": "Chronic GVHD (cGVHD)-associated myositis",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 982393,
"choiceText": "Bacterial myositis",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 982395,
"choiceText": "Polymyositis\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 310935,
"questionText": "Which of the following is the most likely diagnosis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 982397,
"choiceText": "Both proximal and distal muscles can be affected",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 982399,
"choiceText": "Myositis occurs only in the presence of other manifestations of cGVHD",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 982401,
"choiceText": "MRI findings of muscular edema on T2-weighted images, with enhancement on fat-saturated gadolinium-enhanced T1-weighted images, are specific",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 982403,
"choiceText": "A decrease in steroid dose is necessary for treatment\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Proximal muscles are more commonly affected, but distal muscle weakness is also seen.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 310937,
"questionText": "Which of the following is true about cGVHD-associated myositis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 982413,
"choiceText": "Steroids",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 982415,
"choiceText": "Methotrexate",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 982417,
"choiceText": "Azathioprine",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 982419,
"choiceText": "Intravenous immunoglobulin\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 982421,
"choiceText": "Rituximab \r\n",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "\r\nTreatment is based on experience with idiopathic myositis. The treatment of choice is corticosteroids, with subsequent addition of methotrexate, azathioprine, intravenous immunoglobulin, and even rituximab in refractory cases.\r\n",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 310941,
"questionText": "Which of the following is the treatment of choice for cGVHD-associated myositis?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
864715 | /viewarticle/864715 | [
{
"authors": "James J. McCombie, MB ChB; Erik D. Schraga, MD",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.",
"A 77-year-old man presents to the emergency department in the early morning with a 4-hour history of severe, generalized abdominal pain. He describes having had some cramplike abdominal pain and bilious vomiting yesterday, but states that he simply \"got on with things.\" His condition had worsened considerably by late evening. He describes the sudden onset of generalized, constant, intense abdominal pain necessitating an ambulance call.",
"On presentation to the emergency department, the patient has no current vomiting. He describes episodic \"indigestion\" that has occurred off and on for the past few months. On further questioning, the patient reports experiencing infrequent but quite painful episodes of upper abdominal pain after meals, which sometimes feels as if it is \"going to his right upper back\" and is associated with intermittent vomiting. This lasts for minutes to hours after the intake of meals. He states that antacid preparations do little good in controlling these symptoms, but he takes them anyway.",
"His medical history includes hypertension, ischemic heart disease, chronic obstructive pulmonary disease (COPD), and gout. He has no significant surgical history, and his currently prescribed medications include aspirin, atenolol, furosemide, a glyceryl trinitrate spray, and two inhalers for his COPD (the names of which the patient does not know)."
],
"date": "March 28, 2017",
"figures": [],
"markdown": "# Cramplike Pain and Vomiting in a 77-Year-Old Man\n\n **Authors:** James J. McCombie, MB ChB; Erik D. Schraga, MD \n **Date:** March 28, 2017\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.\nA 77-year-old man presents to the emergency department in the early morning with a 4-hour history of severe, generalized abdominal pain. He describes having had some cramplike abdominal pain and bilious vomiting yesterday, but states that he simply \"got on with things.\" His condition had worsened considerably by late evening. He describes the sudden onset of generalized, constant, intense abdominal pain necessitating an ambulance call.\nOn presentation to the emergency department, the patient has no current vomiting. He describes episodic \"indigestion\" that has occurred off and on for the past few months. On further questioning, the patient reports experiencing infrequent but quite painful episodes of upper abdominal pain after meals, which sometimes feels as if it is \"going to his right upper back\" and is associated with intermittent vomiting. This lasts for minutes to hours after the intake of meals. He states that antacid preparations do little good in controlling these symptoms, but he takes them anyway.\nHis medical history includes hypertension, ischemic heart disease, chronic obstructive pulmonary disease (COPD), and gout. He has no significant surgical history, and his currently prescribed medications include aspirin, atenolol, furosemide, a glyceryl trinitrate spray, and two inhalers for his COPD (the names of which the patient does not know).\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "Cramplike Pain and Vomiting in a 77-Year-Old Man"
},
{
"authors": "James J. McCombie, MB ChB; Erik D. Schraga, MD",
"content": [
"Upon examination, the patient is lying quite still. He does not appear cachectic, but does seem clinically dehydrated. His heart rate is 80 beats/min, his blood pressure is 102/65 mm Hg, his capillary refill time is prolonged, and cool extremities are noted. He is afebrile. His lungs are clear to auscultation and his heart sounds are normal, with no added sounds.",
"Figure 1.",
"Figure 2.",
"His abdomen is mildly distended, without visible scars, and there is no discoloration of the skin. When asked to cough, the patient winces in pain. No hernias are appreciated on examination. Palpation of the abdomen reveals generalized, diffuse tenderness and boardlike rigidity. The abdomen is tender to percussion throughout all four quadrants, with a tympanitic note that is associated with loss of liver dullness. A rectal examination reveals a small amount of normal stool. Both femoral pulses are palpable and equal. The neurologic examination reveals no abnormalities. The peripheral examination is normal except for cool extremities.",
"A fluid challenge of 500 mL 0.9% saline is given along with analgesia, and the patient's vital signs improve. Laboratory investigations yield the following:",
"White blood cell count: 15.8 × 103 cells/µL",
"C-reactive protein level: 247 mg/L",
"Sodium level: 148 mEq/L",
"Potassium level: 3.1 mEq/L",
"Urea level: 28.6 mg/dL",
"Creatinine level: 1.5 mg/dL",
"Blood gas analysis reveals a pH level of 7.31, an bicarbonate level of 20 mEq/L, a PCO2 of 4.1 kPa, and lactate level of 23.4 mg/dL.",
"Erect chest and supine abdominal radiographs are obtained (Figures 1 and 2). A nasogastric tube is inserted, and instructions are given for the patient to remain \"nil by mouth.\" He is catheterized, and urinary output is monitored along with the vitals. A further 1000 mL of 0.9% saline is initiated. Cefuroxime and metronidazole are started intravenously, and after urgent surgical consultation, the patient is taken to the operating room for emergency laparotomy."
],
"date": "March 28, 2017",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/864/715/864715-Thumb1.jpg"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/864/715/864715-Thumb2.jpg"
}
],
"markdown": "# Cramplike Pain and Vomiting in a 77-Year-Old Man\n\n **Authors:** James J. McCombie, MB ChB; Erik D. Schraga, MD \n **Date:** March 28, 2017\n\n ## Content\n\n Upon examination, the patient is lying quite still. He does not appear cachectic, but does seem clinically dehydrated. His heart rate is 80 beats/min, his blood pressure is 102/65 mm Hg, his capillary refill time is prolonged, and cool extremities are noted. He is afebrile. His lungs are clear to auscultation and his heart sounds are normal, with no added sounds.\nFigure 1.\nFigure 2.\nHis abdomen is mildly distended, without visible scars, and there is no discoloration of the skin. When asked to cough, the patient winces in pain. No hernias are appreciated on examination. Palpation of the abdomen reveals generalized, diffuse tenderness and boardlike rigidity. The abdomen is tender to percussion throughout all four quadrants, with a tympanitic note that is associated with loss of liver dullness. A rectal examination reveals a small amount of normal stool. Both femoral pulses are palpable and equal. The neurologic examination reveals no abnormalities. The peripheral examination is normal except for cool extremities.\nA fluid challenge of 500 mL 0.9% saline is given along with analgesia, and the patient's vital signs improve. Laboratory investigations yield the following:\nWhite blood cell count: 15.8 × 103 cells/µL\nC-reactive protein level: 247 mg/L\nSodium level: 148 mEq/L\nPotassium level: 3.1 mEq/L\nUrea level: 28.6 mg/dL\nCreatinine level: 1.5 mg/dL\nBlood gas analysis reveals a pH level of 7.31, an bicarbonate level of 20 mEq/L, a PCO2 of 4.1 kPa, and lactate level of 23.4 mg/dL.\nErect chest and supine abdominal radiographs are obtained (Figures 1 and 2). A nasogastric tube is inserted, and instructions are given for the patient to remain \"nil by mouth.\" He is catheterized, and urinary output is monitored along with the vitals. A further 1000 mL of 0.9% saline is initiated. Cefuroxime and metronidazole are started intravenously, and after urgent surgical consultation, the patient is taken to the operating room for emergency laparotomy.\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 975371,
"choiceText": "Cholelithiasis",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 975373,
"choiceText": "Peptic ulcer disease",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 975375,
"choiceText": "Intestinal adhesions",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 975377,
"choiceText": "Chronic pancreatitis\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 308655,
"questionText": "What is the likely index pathology responsible for this patient's current problems?<br><br><i>Hint: Review the patient's medical history and the chronology of the symptoms in his presentation.</i>\r\n",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Cramplike Pain and Vomiting in a 77-Year-Old Man"
},
{
"authors": "James J. McCombie, MB ChB; Erik D. Schraga, MD",
"content": [
"This patient had mechanical small-bowel obstruction complicated by perforation and subsequent peritonitis. His history and examination findings were consistent with this sequence of events. Loss of liver dullness on right upper quadrant abdominal percussion is known as Jobert’s sign and is considered a pathognomonic sign of large visceral perforation. Further support for this was found on the erect chest and supine abdominal radiographs; a large amount of free intraperitoneal air and dilated loops of small bowel were noted. Some authors refer to this radiological finding as Chilaiditi's sign, although the original description by the radiologist Dimitrius Chilaiditi in 1910 described it, not as free air over the liver, but rather the overlapping of a segment of intestine (usually the hepatic flexure of the colon) between the liver and the right diaphragm. Regardless of the underlying etiology, the initial treatment for intra-abdominal free air with peritonitis is emergency laparotomy.",
"After resuscitation, the patient was transferred to the operating room for exploratory laparotomy. During the procedure, it was revealed that the peritoneal cavity was grossly contaminated with intestinal contents spilling out from a small orifice in the terminal ileum. The cause of the obstruction was found to be a gallstone 1.18 in in diameter that was impacted at the ileo-cecal valve. The gallbladder was adherent to the duodenum. The entire length of the bowel was examined for other stones, but none were found.",
"The stone was gently massaged in order to move it proximally, and it was removed via enterolithotomy. The perforation was closed with interrupted sutures and, after copious lavage, the abdomen was closed.",
"Cholelithiasis is a common disorder prevalent in 10% of the population, with symptomatic manifestation in 20%-30% of those affected.[1,2] Gallstone disease may present with an assortment of complications that are usually the result of stones within the gallbladder and biliary tree, with the most common presentation being biliary colic. Extrabiliary problems are rare; however, some 3%-5% of patients with cholelithiasis have a cholecystoenteric fistula as part of the spectrum of their disease, most commonly occurring between the gallbladder and duodenum (71.4%), followed by a fistula with the stomach (14.3%) or the colon (6.3%).[3]",
"In addition, fistulae may arise between the common bile duct and the intestinal tract, and other organs and the abdominal wall, have also been reported as being involved. The possibility of concurrent Mirizzi syndrome (a rare condition in which gallstones lodged in the Hartmann pouch or cystic duct externally compress the common hepatic duct or even erode into the common bile duct or bowel) should be ruled out, because an association between these has been suggested.",
"Gallstones may migrate into the gastrointestinal tract through such a fistula (as in the case of this patient), but most cases pass without incident. Stones larger than 0.7-1.0 in, however, are at risk of becoming impacted.[4] Gallstones can grow in diameter as they pass through the intestinal lumen and sediment from the bowel contents is deposited onto them.",
"In a series of 40 patients, the site of impaction was found to be the ileum in 25 patients, jejunum in nine, duodenum in three, and colon in one.[5] A further review of 1001 patient cases delineated these sites further, with the terminal ileum and ileocecal valve reportedly being most commonly involved, followed by the ligament of Treitz and the pylorus; the duodenum and sigmoid colon were relatively rare locations for impaction of a gallstone within the enteric tract.[6] These sites represent regions of anatomically smaller luminal diameter. Colonic obstruction would probably occur only if preexisting pathology, such as a colonic stricture, were present."
],
"date": "March 28, 2017",
"figures": [],
"markdown": "# Cramplike Pain and Vomiting in a 77-Year-Old Man\n\n **Authors:** James J. McCombie, MB ChB; Erik D. Schraga, MD \n **Date:** March 28, 2017\n\n ## Content\n\n This patient had mechanical small-bowel obstruction complicated by perforation and subsequent peritonitis. His history and examination findings were consistent with this sequence of events. Loss of liver dullness on right upper quadrant abdominal percussion is known as Jobert’s sign and is considered a pathognomonic sign of large visceral perforation. Further support for this was found on the erect chest and supine abdominal radiographs; a large amount of free intraperitoneal air and dilated loops of small bowel were noted. Some authors refer to this radiological finding as Chilaiditi's sign, although the original description by the radiologist Dimitrius Chilaiditi in 1910 described it, not as free air over the liver, but rather the overlapping of a segment of intestine (usually the hepatic flexure of the colon) between the liver and the right diaphragm. Regardless of the underlying etiology, the initial treatment for intra-abdominal free air with peritonitis is emergency laparotomy.\nAfter resuscitation, the patient was transferred to the operating room for exploratory laparotomy. During the procedure, it was revealed that the peritoneal cavity was grossly contaminated with intestinal contents spilling out from a small orifice in the terminal ileum. The cause of the obstruction was found to be a gallstone 1.18 in in diameter that was impacted at the ileo-cecal valve. The gallbladder was adherent to the duodenum. The entire length of the bowel was examined for other stones, but none were found.\nThe stone was gently massaged in order to move it proximally, and it was removed via enterolithotomy. The perforation was closed with interrupted sutures and, after copious lavage, the abdomen was closed.\nCholelithiasis is a common disorder prevalent in 10% of the population, with symptomatic manifestation in 20%-30% of those affected.[1,2] Gallstone disease may present with an assortment of complications that are usually the result of stones within the gallbladder and biliary tree, with the most common presentation being biliary colic. Extrabiliary problems are rare; however, some 3%-5% of patients with cholelithiasis have a cholecystoenteric fistula as part of the spectrum of their disease, most commonly occurring between the gallbladder and duodenum (71.4%), followed by a fistula with the stomach (14.3%) or the colon (6.3%).[3]\nIn addition, fistulae may arise between the common bile duct and the intestinal tract, and other organs and the abdominal wall, have also been reported as being involved. The possibility of concurrent Mirizzi syndrome (a rare condition in which gallstones lodged in the Hartmann pouch or cystic duct externally compress the common hepatic duct or even erode into the common bile duct or bowel) should be ruled out, because an association between these has been suggested.\nGallstones may migrate into the gastrointestinal tract through such a fistula (as in the case of this patient), but most cases pass without incident. Stones larger than 0.7-1.0 in, however, are at risk of becoming impacted.[4] Gallstones can grow in diameter as they pass through the intestinal lumen and sediment from the bowel contents is deposited onto them.\nIn a series of 40 patients, the site of impaction was found to be the ileum in 25 patients, jejunum in nine, duodenum in three, and colon in one.[5] A further review of 1001 patient cases delineated these sites further, with the terminal ileum and ileocecal valve reportedly being most commonly involved, followed by the ligament of Treitz and the pylorus; the duodenum and sigmoid colon were relatively rare locations for impaction of a gallstone within the enteric tract.[6] These sites represent regions of anatomically smaller luminal diameter. Colonic obstruction would probably occur only if preexisting pathology, such as a colonic stricture, were present.\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 975371,
"choiceText": "Cholelithiasis",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 975373,
"choiceText": "Peptic ulcer disease",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 975375,
"choiceText": "Intestinal adhesions",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 975377,
"choiceText": "Chronic pancreatitis\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 308655,
"questionText": "What is the likely index pathology responsible for this patient's current problems?<br><br><i>Hint: Review the patient's medical history and the chronology of the symptoms in his presentation.</i>\r\n",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Cramplike Pain and Vomiting in a 77-Year-Old Man"
},
{
"authors": "James J. McCombie, MB ChB; Erik D. Schraga, MD",
"content": [
"Mechanical intestinal obstruction with a gallstone was first described by Bartholin in 1654, with the term \"gallstone ileus\"; however, this is a misnomer. The symptoms vary depending on the site of impaction and mirror those of intestinal obstruction resulting from any etiology. Classically, the patient presents with subacute episodes of obstruction resulting in abdominal pain and vomiting that subside as the stone spontaneously disimpacts. The symptoms will then recur as the stone becomes larger (because of accrued bowel sediment) and reobstructs the bowel lumen.",
"On a side note, high duodenal or pyloric impaction produces a clinical picture more akin to gastric outlet obstruction and is known as \"Bouveret syndrome.\" Gallstones account for 1%-4% of all the causes of mechanical intestinal obstruction (up to 25% if considering only patients older than 65 y with nonstrangulated obstruction) and occur more commonly in women.[6] Diagnostic confirmation is notoriously difficult, and as many as 50% of diagnoses are made at laparotomy.[4] Patients often have no previous history of biliary symptoms, and other causes of obstruction are more common, which can lead to clinicians overlooking this condition.",
"Radiographic clues are responsible for most early diagnoses. On plain abdominal radiography, diagnosis of gallstone ileus is suggested by the Rigler triad (pneumobilia, small-bowel obstruction, and a gallstone in the right iliac fossa). Only 10% of gallstones are visible on radiographs, and gas in the biliary tree and gallbladder has many causes (most commonly iatrogenic).",
"The Rigler triad can be seen in 15% of patients on abdominal radiography, 11% on ultrasound, and 78% on CT scanning; in fact, CT is often able to identify the fistula itself.[7] Studies support the role of CT scanning in the evaluation of patients with gallstone ileus by highlighting its ability to detect the size, location, and exact number of ectopic stones, and laud its value in the differential diagnosis of acute abdomen.[8]",
"Upper GI studies, albeit less frequently used, have had some success defining the site of obstruction and demonstrating the fistula via retrograde flow into the biliary tree. CT scanning is also effective, avoiding recurrent obstruction if overlooked.",
"If a fistula is present (cholecystohepatic, cholecystocholedochal, or bilioenteric), it should be simultaneously repaired and/or controlled during the initial procedure. The case described here was further complicated by an ileal perforation proximal to the site of obstruction. Only a handful of cases of gallstone ileus report such a complication, which normally arises as a result of the pressure of the stone causing necrosis of the bowel wall at the site of obstruction, or increased wall tension caused by distention proximal to the obstruction.[9]",
"This patient's complication occurred because of a delay in presentation; a delay in the diagnosis also adds to the already significant mortality and morbidity of this condition by allowing the development of further complications (including abdominal sepsis) and decreasing the physiologic reserve of the patient prior to surgery. Patients are typically elderly and are more likely to have comorbidities; these factors explain the overall perioperative mortality of 12%-17%.[6]"
],
"date": "March 28, 2017",
"figures": [],
"markdown": "# Cramplike Pain and Vomiting in a 77-Year-Old Man\n\n **Authors:** James J. McCombie, MB ChB; Erik D. Schraga, MD \n **Date:** March 28, 2017\n\n ## Content\n\n Mechanical intestinal obstruction with a gallstone was first described by Bartholin in 1654, with the term \"gallstone ileus\"; however, this is a misnomer. The symptoms vary depending on the site of impaction and mirror those of intestinal obstruction resulting from any etiology. Classically, the patient presents with subacute episodes of obstruction resulting in abdominal pain and vomiting that subside as the stone spontaneously disimpacts. The symptoms will then recur as the stone becomes larger (because of accrued bowel sediment) and reobstructs the bowel lumen.\nOn a side note, high duodenal or pyloric impaction produces a clinical picture more akin to gastric outlet obstruction and is known as \"Bouveret syndrome.\" Gallstones account for 1%-4% of all the causes of mechanical intestinal obstruction (up to 25% if considering only patients older than 65 y with nonstrangulated obstruction) and occur more commonly in women.[6] Diagnostic confirmation is notoriously difficult, and as many as 50% of diagnoses are made at laparotomy.[4] Patients often have no previous history of biliary symptoms, and other causes of obstruction are more common, which can lead to clinicians overlooking this condition.\nRadiographic clues are responsible for most early diagnoses. On plain abdominal radiography, diagnosis of gallstone ileus is suggested by the Rigler triad (pneumobilia, small-bowel obstruction, and a gallstone in the right iliac fossa). Only 10% of gallstones are visible on radiographs, and gas in the biliary tree and gallbladder has many causes (most commonly iatrogenic).\nThe Rigler triad can be seen in 15% of patients on abdominal radiography, 11% on ultrasound, and 78% on CT scanning; in fact, CT is often able to identify the fistula itself.[7] Studies support the role of CT scanning in the evaluation of patients with gallstone ileus by highlighting its ability to detect the size, location, and exact number of ectopic stones, and laud its value in the differential diagnosis of acute abdomen.[8]\nUpper GI studies, albeit less frequently used, have had some success defining the site of obstruction and demonstrating the fistula via retrograde flow into the biliary tree. CT scanning is also effective, avoiding recurrent obstruction if overlooked.\nIf a fistula is present (cholecystohepatic, cholecystocholedochal, or bilioenteric), it should be simultaneously repaired and/or controlled during the initial procedure. The case described here was further complicated by an ileal perforation proximal to the site of obstruction. Only a handful of cases of gallstone ileus report such a complication, which normally arises as a result of the pressure of the stone causing necrosis of the bowel wall at the site of obstruction, or increased wall tension caused by distention proximal to the obstruction.[9]\nThis patient's complication occurred because of a delay in presentation; a delay in the diagnosis also adds to the already significant mortality and morbidity of this condition by allowing the development of further complications (including abdominal sepsis) and decreasing the physiologic reserve of the patient prior to surgery. Patients are typically elderly and are more likely to have comorbidities; these factors explain the overall perioperative mortality of 12%-17%.[6]\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "Cramplike Pain and Vomiting in a 77-Year-Old Man"
},
{
"authors": "James J. McCombie, MB ChB; Erik D. Schraga, MD",
"content": [
"The highest chance for survival in patients with gallstone ileus is achieved by prompt resuscitation and surgery. Surgical treatment is feasible by laparotomy or laparoscopy. Minimally invasive advocators state the benefits of its diagnostic as well as therapeutic capability and the well-known advantages of such approach.",
"Laparoscopy, however, can result in decreased venous return due to increased intra-abdominal pressure, causing hypotension in an already hemodynamically unstable patient.[10,11] The choice should be based on the surgeon's skill and preference in close communication with the anesthesiologist.",
"Whether definitive biliary surgery should be performed simultaneously is another matter of debate. Relief of the intestinal obstruction is the first priority. Thus, enterolithotomy alone is believed to be the best course of action in the typical patient with gallstone ileus.[12,13] Closure of the fistula and cholecystectomy can then be performed electively at a later date. A \"one-stage procedure\" should be performed only in a highly selected group of low-risk patients, because a small but significant increase in perioperative mortality has been observed (from 11% to 16.7%).[12,14]",
"Although surgery is the treatment of choice, endoscopic sphincterotomy and common bile duct stone extraction have been shown to cause spontaneous healing of fistulas. Extracorporeal and electrohydraulic lithotripsy have also been described.",
"Endoscopic stone removal from the duodenum and colon has been used. Such procedures are reserved for patients unable to undergo surgery.[15,16] The need to examine the entire length of bowel during surgery cannot be overemphasized.",
"In conclusion, mechanical obstruction of the intestine with a gallstone is an uncommon complication of biliary stone disease, occurring in less than 0.5% of patients.[6] Diagnosis is difficult due to its rarity and large proportion of misleading symptoms. Careful history-taking and physical examination is paramount to establish the diagnosis, supported by the appropriate imaging studies, preferably CT scanning. Of course, such time-consuming studies should be done after adequate resuscitative measures and should not delay operative intervention when perforation and peritonitis are present. During the procedure, keep in mind that relief of obstruction is the first priority."
],
"date": "March 28, 2017",
"figures": [],
"markdown": "# Cramplike Pain and Vomiting in a 77-Year-Old Man\n\n **Authors:** James J. McCombie, MB ChB; Erik D. Schraga, MD \n **Date:** March 28, 2017\n\n ## Content\n\n The highest chance for survival in patients with gallstone ileus is achieved by prompt resuscitation and surgery. Surgical treatment is feasible by laparotomy or laparoscopy. Minimally invasive advocators state the benefits of its diagnostic as well as therapeutic capability and the well-known advantages of such approach.\nLaparoscopy, however, can result in decreased venous return due to increased intra-abdominal pressure, causing hypotension in an already hemodynamically unstable patient.[10,11] The choice should be based on the surgeon's skill and preference in close communication with the anesthesiologist.\nWhether definitive biliary surgery should be performed simultaneously is another matter of debate. Relief of the intestinal obstruction is the first priority. Thus, enterolithotomy alone is believed to be the best course of action in the typical patient with gallstone ileus.[12,13] Closure of the fistula and cholecystectomy can then be performed electively at a later date. A \"one-stage procedure\" should be performed only in a highly selected group of low-risk patients, because a small but significant increase in perioperative mortality has been observed (from 11% to 16.7%).[12,14]\nAlthough surgery is the treatment of choice, endoscopic sphincterotomy and common bile duct stone extraction have been shown to cause spontaneous healing of fistulas. Extracorporeal and electrohydraulic lithotripsy have also been described.\nEndoscopic stone removal from the duodenum and colon has been used. Such procedures are reserved for patients unable to undergo surgery.[15,16] The need to examine the entire length of bowel during surgery cannot be overemphasized.\nIn conclusion, mechanical obstruction of the intestine with a gallstone is an uncommon complication of biliary stone disease, occurring in less than 0.5% of patients.[6] Diagnosis is difficult due to its rarity and large proportion of misleading symptoms. Careful history-taking and physical examination is paramount to establish the diagnosis, supported by the appropriate imaging studies, preferably CT scanning. Of course, such time-consuming studies should be done after adequate resuscitative measures and should not delay operative intervention when perforation and peritonitis are present. During the procedure, keep in mind that relief of obstruction is the first priority.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 975379,
"choiceText": "Radiography",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 975381,
"choiceText": "Endoscopy",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 975383,
"choiceText": "Ultrasonography",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 975385,
"choiceText": "CT scanning\r\n",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Evidence indicates that the Rigler triad can be seen in 15% of patients on abdominal radiography, 11% on ultrasound, and 78% on CT; in fact, CT is often able to show the fistula itself. Further studies support the role of CT in the evaluation of patients with gallstone ileus by highlighting its ability to detect the size, location, and exact number of ectopic stones, and its overall value in the diagnosis of many cases of acute abdomen has also been lauded.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 308657,
"questionText": "You are examining a patient with a recent history of intermittent abdominal pain and vomiting. The pain has now become constant and radiates to the back and right side. You suspect an abdominal obstruction, possibly gallstone ileus. Which of the following imaging modalities is most sensitive for detecting gallstone ileus?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 975387,
"choiceText": "Surgical relief of the obstruction, with gallstone removal and intestinal repair",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 975389,
"choiceText": "Dietary change",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 975391,
"choiceText": "Lithotripsy\r\n\r\n",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 975393,
"choiceText": "Administration of antacids with monitoring",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The highest chance for survival in patients with gallstone ileus is achieved by prompt resuscitation and surgery. Surgical treatment is feasible by laparotomy or laparoscopy. Minimally invasive advocators state the benefits of its diagnostic as well as therapeutic capability and the well-known advantages of such approach.<br><br>\r\nLaparoscopy, however, can result in decreased venous return due to increased intra-abdominal pressure, causing hypotension in an already hemodynamically unstable patient.<sup type=\"ref\">[10,11]</sup> The choice should be based on the surgeon's skill and preference in close communication with the anesthesiologist.\r\n",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 308659,
"questionText": "Imaging studies reveal gallstone ileus in the patient described above. Which of the following would be the treatment of choice?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Cramplike Pain and Vomiting in a 77-Year-Old Man"
},
{
"authors": "James J. McCombie, MB ChB; Erik D. Schraga, MD",
"content": [],
"date": "March 28, 2017",
"figures": [],
"markdown": "# Cramplike Pain and Vomiting in a 77-Year-Old Man\n\n **Authors:** James J. McCombie, MB ChB; Erik D. Schraga, MD \n **Date:** March 28, 2017\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 975379,
"choiceText": "Radiography",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 975381,
"choiceText": "Endoscopy",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 975383,
"choiceText": "Ultrasonography",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 975385,
"choiceText": "CT scanning\r\n",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Evidence indicates that the Rigler triad can be seen in 15% of patients on abdominal radiography, 11% on ultrasound, and 78% on CT; in fact, CT is often able to show the fistula itself. Further studies support the role of CT in the evaluation of patients with gallstone ileus by highlighting its ability to detect the size, location, and exact number of ectopic stones, and its overall value in the diagnosis of many cases of acute abdomen has also been lauded.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 308657,
"questionText": "You are examining a patient with a recent history of intermittent abdominal pain and vomiting. The pain has now become constant and radiates to the back and right side. You suspect an abdominal obstruction, possibly gallstone ileus. Which of the following imaging modalities is most sensitive for detecting gallstone ileus?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 975387,
"choiceText": "Surgical relief of the obstruction, with gallstone removal and intestinal repair",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 975389,
"choiceText": "Dietary change",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 975391,
"choiceText": "Lithotripsy\r\n\r\n",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 975393,
"choiceText": "Administration of antacids with monitoring",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The highest chance for survival in patients with gallstone ileus is achieved by prompt resuscitation and surgery. Surgical treatment is feasible by laparotomy or laparoscopy. Minimally invasive advocators state the benefits of its diagnostic as well as therapeutic capability and the well-known advantages of such approach.<br><br>\r\nLaparoscopy, however, can result in decreased venous return due to increased intra-abdominal pressure, causing hypotension in an already hemodynamically unstable patient.<sup type=\"ref\">[10,11]</sup> The choice should be based on the surgeon's skill and preference in close communication with the anesthesiologist.\r\n",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 308659,
"questionText": "Imaging studies reveal gallstone ileus in the patient described above. Which of the following would be the treatment of choice?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Cramplike Pain and Vomiting in a 77-Year-Old Man"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 975371,
"choiceText": "Cholelithiasis",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 975373,
"choiceText": "Peptic ulcer disease",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 975375,
"choiceText": "Intestinal adhesions",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 975377,
"choiceText": "Chronic pancreatitis\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 308655,
"questionText": "What is the likely index pathology responsible for this patient's current problems?<br><br><i>Hint: Review the patient's medical history and the chronology of the symptoms in his presentation.</i>\r\n",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 975379,
"choiceText": "Radiography",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 975381,
"choiceText": "Endoscopy",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 975383,
"choiceText": "Ultrasonography",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 975385,
"choiceText": "CT scanning\r\n",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Evidence indicates that the Rigler triad can be seen in 15% of patients on abdominal radiography, 11% on ultrasound, and 78% on CT; in fact, CT is often able to show the fistula itself. Further studies support the role of CT in the evaluation of patients with gallstone ileus by highlighting its ability to detect the size, location, and exact number of ectopic stones, and its overall value in the diagnosis of many cases of acute abdomen has also been lauded.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 308657,
"questionText": "You are examining a patient with a recent history of intermittent abdominal pain and vomiting. The pain has now become constant and radiates to the back and right side. You suspect an abdominal obstruction, possibly gallstone ileus. Which of the following imaging modalities is most sensitive for detecting gallstone ileus?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 975387,
"choiceText": "Surgical relief of the obstruction, with gallstone removal and intestinal repair",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 975389,
"choiceText": "Dietary change",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 975391,
"choiceText": "Lithotripsy\r\n\r\n",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 975393,
"choiceText": "Administration of antacids with monitoring",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The highest chance for survival in patients with gallstone ileus is achieved by prompt resuscitation and surgery. Surgical treatment is feasible by laparotomy or laparoscopy. Minimally invasive advocators state the benefits of its diagnostic as well as therapeutic capability and the well-known advantages of such approach.<br><br>\r\nLaparoscopy, however, can result in decreased venous return due to increased intra-abdominal pressure, causing hypotension in an already hemodynamically unstable patient.<sup type=\"ref\">[10,11]</sup> The choice should be based on the surgeon's skill and preference in close communication with the anesthesiologist.\r\n",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 308659,
"questionText": "Imaging studies reveal gallstone ileus in the patient described above. Which of the following would be the treatment of choice?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
864717 | /viewarticle/864717 | [
{
"authors": "D. Brady Pregerson, MD",
"content": [
"Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.",
"A 30-year-old woman with no significant medical history presents to the emergency department with intermittent nonradiating pain in the lower left part of her abdomen. The pain started approximately 5 days ago and has been steadily worsening since then. She has also been having fever.",
"At presentation, the patient describes the pain as \"severe\" and notes that it increases with any motion. No abnormal urinary symptoms are reported (ie, pain during urination or increased frequency of urination). She has not had any changes in her bowel habits, including no constipation or diarrhea.",
"She is married and monogamous, and her last sexual intercourse was 2 weeks ago. She has regular menses, with her last menstrual period occurring approximately 3 weeks ago. No vaginal discharge is observed. She has no history of sexually transmitted diseases (STDs). She does not smoke and does not use any illicit substances."
],
"date": "June 15, 2016",
"figures": [],
"markdown": "# A 30-Year-Old Woman With Lower Abdominal Pain\n\n **Authors:** D. Brady Pregerson, MD \n **Date:** June 15, 2016\n\n ## Content\n\n Editor's Note: The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.\nA 30-year-old woman with no significant medical history presents to the emergency department with intermittent nonradiating pain in the lower left part of her abdomen. The pain started approximately 5 days ago and has been steadily worsening since then. She has also been having fever.\nAt presentation, the patient describes the pain as \"severe\" and notes that it increases with any motion. No abnormal urinary symptoms are reported (ie, pain during urination or increased frequency of urination). She has not had any changes in her bowel habits, including no constipation or diarrhea.\nShe is married and monogamous, and her last sexual intercourse was 2 weeks ago. She has regular menses, with her last menstrual period occurring approximately 3 weeks ago. No vaginal discharge is observed. She has no history of sexually transmitted diseases (STDs). She does not smoke and does not use any illicit substances.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 30-Year-Old Woman With Lower Abdominal Pain"
},
{
"authors": "D. Brady Pregerson, MD",
"content": [
"Figure 1.",
"Upon physical examination, the patient has a temperature of 100.2°F and a blood pressure of 116/63 mm Hg. She is tachycardic, with a heart rate of 120 beats/min but a regular rhythm. Her respiratory rate is normal, at 10 breaths/min. She is clearly uncomfortable but does not appear to be in immediate distress. Except for the noted tachycardia, the cardiac and respiratory examinations are unremarkable.",
"The abdominal examination reveals tenderness in the lower abdomen, specifically in the left lower quadrant, but no rebound or guarding is noted. A pelvic examination is performed that reveals scant blood in the vagina and cervical motion tenderness. An 8-cm mass is palpated in the left adnexa, with marked tenderness. The uterus is tender and normal in size. The right adnexa is tender, but no palpable masses are detected.",
"Initial laboratory investigations are ordered. A complete blood cell count reveals an elevated white blood cell count of 13.5 × 103 cells/µL, a hematocrit of 38%, and platelet count of 256 × 103 cells/µL. A basic chemistry panel and a coagulation profile are unremarkable. A urine test for beta-human chorionic gonadotropin is negative, and urine analysis is negative for evidence of a urinary tract infection.",
"Transvaginal and transabdominal pelvic ultrasound are performed (Figure 1)."
],
"date": "June 15, 2016",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/864/717/864717-Thumb1.png"
}
],
"markdown": "# A 30-Year-Old Woman With Lower Abdominal Pain\n\n **Authors:** D. Brady Pregerson, MD \n **Date:** June 15, 2016\n\n ## Content\n\n Figure 1.\nUpon physical examination, the patient has a temperature of 100.2°F and a blood pressure of 116/63 mm Hg. She is tachycardic, with a heart rate of 120 beats/min but a regular rhythm. Her respiratory rate is normal, at 10 breaths/min. She is clearly uncomfortable but does not appear to be in immediate distress. Except for the noted tachycardia, the cardiac and respiratory examinations are unremarkable.\nThe abdominal examination reveals tenderness in the lower abdomen, specifically in the left lower quadrant, but no rebound or guarding is noted. A pelvic examination is performed that reveals scant blood in the vagina and cervical motion tenderness. An 8-cm mass is palpated in the left adnexa, with marked tenderness. The uterus is tender and normal in size. The right adnexa is tender, but no palpable masses are detected.\nInitial laboratory investigations are ordered. A complete blood cell count reveals an elevated white blood cell count of 13.5 × 103 cells/µL, a hematocrit of 38%, and platelet count of 256 × 103 cells/µL. A basic chemistry panel and a coagulation profile are unremarkable. A urine test for beta-human chorionic gonadotropin is negative, and urine analysis is negative for evidence of a urinary tract infection.\nTransvaginal and transabdominal pelvic ultrasound are performed (Figure 1).\n\n ## Figures\n\n **Figure 1.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 975551,
"choiceText": "Tubo-ovarian abscess",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 975553,
"choiceText": "Ovarian cyst",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 975555,
"choiceText": "Ovarian torsion",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 975557,
"choiceText": "Appendicitis",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 308703,
"questionText": "What is the diagnosis?<br/><br/>\r\n<em>Hint: Observe the abnormality in the left adnexa.</em>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 30-Year-Old Woman With Lower Abdominal Pain"
},
{
"authors": "D. Brady Pregerson, MD",
"content": [
"Figure 1.",
"Figure 2.",
"The patient's sonograms (Figures 1 and 2) showed a multiloculated, complex mass in the left adnexa measuring 8 × 6 × 5 cm, which suggested the diagnosis of tubo-ovarian abscess (see the centimeter scale on the left side of the image to estimate the size of the abnormalities).",
"This condition usually occurs after recurrent, chronic, or refractory pelvic inflammatory disease (PID). It can also occur during an initial episode of PID, as well as result from other etiologies, such as following pelvic surgery or other procedures (eg, endometrial biopsy or sonohysterogram). PID is an infection of the female upper genital tract that includes a broad category of diseases, including endometritis salpingitis, salpingo-oophoritis, tubo-ovarian abscess, and pelvic peritonitis.",
"To underscore the close relationship between PID and tubo-ovarian abscess, in some case series as many as one third of patients who were diagnosed with PID went on to develop tubo-ovarian abscess. In patients who have been previously diagnosed with PID, the condition shares the same epidemiology and risk factor profile; it usually occurs in sexually active women between 20 and 40 years of age, with risk factors including a history of STD and new or multiple sexual partners.",
"Although a tubo-ovarian abscess may occur as a complication of an acute initial episode of salpingitis, it is usually the result of recurrent infections superimposed on damaged tissue; fibrinous attachments to nearby organs from previous episodes of PID may serve as the entry point for the extension of infection. Rarely, a secondary tubo-ovarian abscess may result from intraperitoneal spread of infection due to bowel perforation, or it may occur in association with pelvic cancer.[1]",
"In the most women with a tubo-ovarian abscess, abdominal or pelvic pain is the primary presenting symptom. The clinical picture of tubo-ovarian abscess is one of pelvic pain, a tender adnexal mass, fever, and tachycardia. On pelvic examination, excessive mucopurulent cervicitis may be found, and abscesses may be bilateral. Other PID findings, including uterine and bilateral adnexal tenderness, are usually present.[1]"
],
"date": "June 15, 2016",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/864/717/864717-Thumb1.png"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/864/717/864717-Thumb2.png"
}
],
"markdown": "# A 30-Year-Old Woman With Lower Abdominal Pain\n\n **Authors:** D. Brady Pregerson, MD \n **Date:** June 15, 2016\n\n ## Content\n\n Figure 1.\nFigure 2.\nThe patient's sonograms (Figures 1 and 2) showed a multiloculated, complex mass in the left adnexa measuring 8 × 6 × 5 cm, which suggested the diagnosis of tubo-ovarian abscess (see the centimeter scale on the left side of the image to estimate the size of the abnormalities).\nThis condition usually occurs after recurrent, chronic, or refractory pelvic inflammatory disease (PID). It can also occur during an initial episode of PID, as well as result from other etiologies, such as following pelvic surgery or other procedures (eg, endometrial biopsy or sonohysterogram). PID is an infection of the female upper genital tract that includes a broad category of diseases, including endometritis salpingitis, salpingo-oophoritis, tubo-ovarian abscess, and pelvic peritonitis.\nTo underscore the close relationship between PID and tubo-ovarian abscess, in some case series as many as one third of patients who were diagnosed with PID went on to develop tubo-ovarian abscess. In patients who have been previously diagnosed with PID, the condition shares the same epidemiology and risk factor profile; it usually occurs in sexually active women between 20 and 40 years of age, with risk factors including a history of STD and new or multiple sexual partners.\nAlthough a tubo-ovarian abscess may occur as a complication of an acute initial episode of salpingitis, it is usually the result of recurrent infections superimposed on damaged tissue; fibrinous attachments to nearby organs from previous episodes of PID may serve as the entry point for the extension of infection. Rarely, a secondary tubo-ovarian abscess may result from intraperitoneal spread of infection due to bowel perforation, or it may occur in association with pelvic cancer.[1]\nIn the most women with a tubo-ovarian abscess, abdominal or pelvic pain is the primary presenting symptom. The clinical picture of tubo-ovarian abscess is one of pelvic pain, a tender adnexal mass, fever, and tachycardia. On pelvic examination, excessive mucopurulent cervicitis may be found, and abscesses may be bilateral. Other PID findings, including uterine and bilateral adnexal tenderness, are usually present.[1]\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n\n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 975551,
"choiceText": "Tubo-ovarian abscess",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 975553,
"choiceText": "Ovarian cyst",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 975555,
"choiceText": "Ovarian torsion",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 975557,
"choiceText": "Appendicitis",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 308703,
"questionText": "What is the diagnosis?<br/><br/>\r\n<em>Hint: Observe the abnormality in the left adnexa.</em>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 30-Year-Old Woman With Lower Abdominal Pain"
},
{
"authors": "D. Brady Pregerson, MD",
"content": [
"The typical tubo-ovarian abscess is polymicrobial, with a preponderance of gram-negative and anaerobic organisms; the inciting organism may be Chlamydia trachomatis, an intracellular bacterial pathogen and the predominant STD-related organism that causes PID or tubo-ovarian abscess. Neisseria gonorrhea as the primary cause of PID or tubo-ovarian abscess has decreased in the United States. The polymicrobial mix in a tubo-ovarian abscess is generally composed of organisms usually found as normal flora in the gastrointestinal and genitourinary tracts. It may specifically include additional organisms, such as Peptococcus species, Streptococcus agalactiae, enteric gram-negative organisms (eg, Escherichia coli ), and resistant gram-negative anaerobic organisms (eg, Bacteroides fragilis and Bacteroides bivius).[1]",
"The differential diagnosis of lower abdominal pain in young women is broad, and caution is recommended in ruling out other infectious and inflammatory conditions. Specifically, diagnoses related to the gastrointestinal tract, such as appendicitis, periappendiceal abscess, and diverticulitis, should be considered. Genitourinary etiologies, such as cystitis or pyelonephritis, should be considered as well. In addition, other reproductive tract problems, including endometriomas, ectopic pregnancies, hemorrhagic cysts, and ovarian tumors, may present in a similar fashion to a tubo-ovarian abscess.[1]",
"Ultrasonography is the diagnostic imaging modality of choice because of its lack of ionizing radiation, noninvasive nature, and accuracy. A tubo-ovarian abscess appears on ultrasound as a complex adnexal mass, with thickened walls and central fluid. The findings, however, may be nonspecific, and they should be distinguished from possible alternative reproductive tract diagnoses (as detailed above).",
"Transvaginal ultrasound allows detailed visualization of the uterus and adnexa, including the ovaries. Transabdominal ultrasound is not required but can be a useful complementary study to endovaginal examination because it provides a global view of the pelvic contents.",
"For patients who refuse transvaginal ultrasound, a limited transabdominal study may be performed. Some studies have also suggested a high specificity for the diagnosis of tubo-ovarian abscess when performed at the bedside by clinicians.[2] CT should also be considered because it has the notable advantage of improving the accuracy of diagnosing appendiceal pathology (which can mimic PID or tubo-ovarian abscess).",
"In suspected cases of tubo-ovarian abscess with equivocal ultrasound findings, MRI is an excellent imaging modality. A tubo-ovarian abscess on MRI often appears as a thick-walled mass, with low signal intensity on T1-weighted images and high signal intensity on T2-weighted images. Occasionally, the tubo-ovarian abscess may be isointense or hyperintense on T1-weighted images, and it may have heterogeneous signal intensity on T2-weighted images. Some findings have suggested that MRI is as effective as or possibly more accurate than other studies for diagnosing tubo-ovarian abscess and PID.[1,3,4]"
],
"date": "June 15, 2016",
"figures": [],
"markdown": "# A 30-Year-Old Woman With Lower Abdominal Pain\n\n **Authors:** D. Brady Pregerson, MD \n **Date:** June 15, 2016\n\n ## Content\n\n The typical tubo-ovarian abscess is polymicrobial, with a preponderance of gram-negative and anaerobic organisms; the inciting organism may be Chlamydia trachomatis, an intracellular bacterial pathogen and the predominant STD-related organism that causes PID or tubo-ovarian abscess. Neisseria gonorrhea as the primary cause of PID or tubo-ovarian abscess has decreased in the United States. The polymicrobial mix in a tubo-ovarian abscess is generally composed of organisms usually found as normal flora in the gastrointestinal and genitourinary tracts. It may specifically include additional organisms, such as Peptococcus species, Streptococcus agalactiae, enteric gram-negative organisms (eg, Escherichia coli ), and resistant gram-negative anaerobic organisms (eg, Bacteroides fragilis and Bacteroides bivius).[1]\nThe differential diagnosis of lower abdominal pain in young women is broad, and caution is recommended in ruling out other infectious and inflammatory conditions. Specifically, diagnoses related to the gastrointestinal tract, such as appendicitis, periappendiceal abscess, and diverticulitis, should be considered. Genitourinary etiologies, such as cystitis or pyelonephritis, should be considered as well. In addition, other reproductive tract problems, including endometriomas, ectopic pregnancies, hemorrhagic cysts, and ovarian tumors, may present in a similar fashion to a tubo-ovarian abscess.[1]\nUltrasonography is the diagnostic imaging modality of choice because of its lack of ionizing radiation, noninvasive nature, and accuracy. A tubo-ovarian abscess appears on ultrasound as a complex adnexal mass, with thickened walls and central fluid. The findings, however, may be nonspecific, and they should be distinguished from possible alternative reproductive tract diagnoses (as detailed above).\nTransvaginal ultrasound allows detailed visualization of the uterus and adnexa, including the ovaries. Transabdominal ultrasound is not required but can be a useful complementary study to endovaginal examination because it provides a global view of the pelvic contents.\nFor patients who refuse transvaginal ultrasound, a limited transabdominal study may be performed. Some studies have also suggested a high specificity for the diagnosis of tubo-ovarian abscess when performed at the bedside by clinicians.[2] CT should also be considered because it has the notable advantage of improving the accuracy of diagnosing appendiceal pathology (which can mimic PID or tubo-ovarian abscess).\nIn suspected cases of tubo-ovarian abscess with equivocal ultrasound findings, MRI is an excellent imaging modality. A tubo-ovarian abscess on MRI often appears as a thick-walled mass, with low signal intensity on T1-weighted images and high signal intensity on T2-weighted images. Occasionally, the tubo-ovarian abscess may be isointense or hyperintense on T1-weighted images, and it may have heterogeneous signal intensity on T2-weighted images. Some findings have suggested that MRI is as effective as or possibly more accurate than other studies for diagnosing tubo-ovarian abscess and PID.[1,3,4]\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 30-Year-Old Woman With Lower Abdominal Pain"
},
{
"authors": "D. Brady Pregerson, MD",
"content": [
"The initial treatment consists of broad-spectrum antibiotic therapy that covers gram-negative and anaerobic organisms, gonorrhea, and chlamydia. Several regimens, such as cefotetan or cefoxitin in combination with doxycycline, or clindamycin in combination with gentamicin, have been suggested.[5] Most cases of tubo-ovarian abscess (60%-80%) resolve with antibiotics alone; conservative therapy may be attempted if the antimicrobial agents can penetrate the abscess and remain active within the abscess environment.",
"In cases that do not respond to intravenous antibiotic therapy, laparoscopy or imaging-guided drainage using CT or ultrasound may be used to identify and drain any contained pus loculations. A transvaginal ultrasound-guided or CT-guided aspiration technique, in combination with intravenous antibiotic therapy or placement of intracavitary antibiotic therapy, has also been described in several case series as being effective for draining tubo-ovarian abscesses.[3,4] Additional studies have suggested that imaging-guided drainage may be effective as a salvage technique in patients who are nonresponsive to treatment with primary antibiotics, and that patients undergoing primary drainage may have shorter hospital stays and faster resolutions.[6]",
"A small percentage of cases require open laparotomy for failed conservative management or other rare complications, such as abscess rupture, acute hemorrhage (caused by the erosion of adjacent blood vessels), and sepsis. Despite treatment, permanent tubal damage usually occurs, and long-term fertility is substantially reduced (even after a tubo-ovarian abscess has been sterilized). Patients who become pregnant are at a substantially increased risk for ectopic pregnancy in the future, and they must be counseled regarding this risk.",
"The patient in this case had a relatively large tubo-ovarian abscess in her left adnexa. She was admitted to the gynecology service and started on parenteral cefotetan and oral doxycycline, with the plan to monitor her for a response within the first 48 hours before a possible invasive intervention (which would have included laparoscopy or imaging-guided drainage of the abscess).",
"On hospital day 2, the patient was noted to have defervesced and to have decreased tenderness in the area over her abscess. She was continued on the parenteral antibiotic regimen and, on hospital day 6, she underwent repeat pelvic ultrasound that demonstrated interval improvement of the tubo-ovarian abscess (specifically, a reduction in size).",
"After her continued clinical improvement was confirmed and an appropriate follow-up examination was scheduled, the patient was discharged to home with the plan to complete a 14-day course of oral broad-spectrum antibiotics, which included clindamycin and doxycycline. On a follow-up visit to the gynecology clinic at about 8 weeks after discharge from the hospital, she was noted to be feeling well; pelvic ultrasound performed in the clinic showed almost complete resolution.",
"Patients treated for tubo-ovarian abscess should be offered screening for STDs. It is unknown whether the patient in this case was tested for HIV or other STDs."
],
"date": "June 15, 2016",
"figures": [],
"markdown": "# A 30-Year-Old Woman With Lower Abdominal Pain\n\n **Authors:** D. Brady Pregerson, MD \n **Date:** June 15, 2016\n\n ## Content\n\n The initial treatment consists of broad-spectrum antibiotic therapy that covers gram-negative and anaerobic organisms, gonorrhea, and chlamydia. Several regimens, such as cefotetan or cefoxitin in combination with doxycycline, or clindamycin in combination with gentamicin, have been suggested.[5] Most cases of tubo-ovarian abscess (60%-80%) resolve with antibiotics alone; conservative therapy may be attempted if the antimicrobial agents can penetrate the abscess and remain active within the abscess environment.\nIn cases that do not respond to intravenous antibiotic therapy, laparoscopy or imaging-guided drainage using CT or ultrasound may be used to identify and drain any contained pus loculations. A transvaginal ultrasound-guided or CT-guided aspiration technique, in combination with intravenous antibiotic therapy or placement of intracavitary antibiotic therapy, has also been described in several case series as being effective for draining tubo-ovarian abscesses.[3,4] Additional studies have suggested that imaging-guided drainage may be effective as a salvage technique in patients who are nonresponsive to treatment with primary antibiotics, and that patients undergoing primary drainage may have shorter hospital stays and faster resolutions.[6]\nA small percentage of cases require open laparotomy for failed conservative management or other rare complications, such as abscess rupture, acute hemorrhage (caused by the erosion of adjacent blood vessels), and sepsis. Despite treatment, permanent tubal damage usually occurs, and long-term fertility is substantially reduced (even after a tubo-ovarian abscess has been sterilized). Patients who become pregnant are at a substantially increased risk for ectopic pregnancy in the future, and they must be counseled regarding this risk.\nThe patient in this case had a relatively large tubo-ovarian abscess in her left adnexa. She was admitted to the gynecology service and started on parenteral cefotetan and oral doxycycline, with the plan to monitor her for a response within the first 48 hours before a possible invasive intervention (which would have included laparoscopy or imaging-guided drainage of the abscess).\nOn hospital day 2, the patient was noted to have defervesced and to have decreased tenderness in the area over her abscess. She was continued on the parenteral antibiotic regimen and, on hospital day 6, she underwent repeat pelvic ultrasound that demonstrated interval improvement of the tubo-ovarian abscess (specifically, a reduction in size).\nAfter her continued clinical improvement was confirmed and an appropriate follow-up examination was scheduled, the patient was discharged to home with the plan to complete a 14-day course of oral broad-spectrum antibiotics, which included clindamycin and doxycycline. On a follow-up visit to the gynecology clinic at about 8 weeks after discharge from the hospital, she was noted to be feeling well; pelvic ultrasound performed in the clinic showed almost complete resolution.\nPatients treated for tubo-ovarian abscess should be offered screening for STDs. It is unknown whether the patient in this case was tested for HIV or other STDs.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 975567,
"choiceText": "The disease usually occurs after recurrent, chronic, or PID",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 975569,
"choiceText": "Risk factors include a history of STD and new or multiple sexual partners",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 975571,
"choiceText": "Secondary tubo-ovarian abscess may occur from intraperitoneal spread of infection from a bowel perforation or in association with pelvic cancer",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 975573,
"choiceText": "Tubo-ovarian abscess does not occur during an initial, acute episode of an STD",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 975575,
"choiceText": "Various treatment options are available",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "To underscore the close relationship between PID and tubo-ovarian abscess, in some case series as many as one third of patients who were diagnosed with PID went on to develop tubo-ovarian abscess. In patients who have been previously diagnosed with PID, the condition shares the same epidemiology and risk factor profile; it usually occurs in sexually active women between 20 and 40 years of age, with risk factors including a history of STD and new or multiple sexual partners. ",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 308707,
"questionText": "Which of the following statements regarding tubo-ovarian abscess is <em>not</em> true?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 975577,
"choiceText": "Intravenous antibiotics",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 975579,
"choiceText": "CT-guided drainage",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 975581,
"choiceText": "A trial of oral antibiotics",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 975583,
"choiceText": "Ultrasound-guided drainage",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 975585,
"choiceText": "Surgery",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Tubo-ovarian abscess may respond to antibiotics alone, but operative treatment or percutaneous drainage may be required in cases that are severe or do not respond to purely medical management. The initial medical therapy should include intravenous antibiotics to cover gonorrhea and chlamydia, as well as anaerobes (which are often also present). Oral antibiotics are not appropriate for initial therapy.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 308709,
"questionText": "Which of the following choices is <em>not</em> a recommended treatment for tubo-ovarian abscess?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 30-Year-Old Woman With Lower Abdominal Pain"
},
{
"authors": "D. Brady Pregerson, MD",
"content": [],
"date": "June 15, 2016",
"figures": [],
"markdown": "# A 30-Year-Old Woman With Lower Abdominal Pain\n\n **Authors:** D. Brady Pregerson, MD \n **Date:** June 15, 2016\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 975567,
"choiceText": "The disease usually occurs after recurrent, chronic, or PID",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 975569,
"choiceText": "Risk factors include a history of STD and new or multiple sexual partners",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 975571,
"choiceText": "Secondary tubo-ovarian abscess may occur from intraperitoneal spread of infection from a bowel perforation or in association with pelvic cancer",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 975573,
"choiceText": "Tubo-ovarian abscess does not occur during an initial, acute episode of an STD",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 975575,
"choiceText": "Various treatment options are available",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "To underscore the close relationship between PID and tubo-ovarian abscess, in some case series as many as one third of patients who were diagnosed with PID went on to develop tubo-ovarian abscess. In patients who have been previously diagnosed with PID, the condition shares the same epidemiology and risk factor profile; it usually occurs in sexually active women between 20 and 40 years of age, with risk factors including a history of STD and new or multiple sexual partners. ",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 308707,
"questionText": "Which of the following statements regarding tubo-ovarian abscess is <em>not</em> true?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 975577,
"choiceText": "Intravenous antibiotics",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 975579,
"choiceText": "CT-guided drainage",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 975581,
"choiceText": "A trial of oral antibiotics",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 975583,
"choiceText": "Ultrasound-guided drainage",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 975585,
"choiceText": "Surgery",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Tubo-ovarian abscess may respond to antibiotics alone, but operative treatment or percutaneous drainage may be required in cases that are severe or do not respond to purely medical management. The initial medical therapy should include intravenous antibiotics to cover gonorrhea and chlamydia, as well as anaerobes (which are often also present). Oral antibiotics are not appropriate for initial therapy.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 308709,
"questionText": "Which of the following choices is <em>not</em> a recommended treatment for tubo-ovarian abscess?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 30-Year-Old Woman With Lower Abdominal Pain"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 975551,
"choiceText": "Tubo-ovarian abscess",
"correct": true,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 975553,
"choiceText": "Ovarian cyst",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 975555,
"choiceText": "Ovarian torsion",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 975557,
"choiceText": "Appendicitis",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 308703,
"questionText": "What is the diagnosis?<br/><br/>\r\n<em>Hint: Observe the abnormality in the left adnexa.</em>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 975567,
"choiceText": "The disease usually occurs after recurrent, chronic, or PID",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 975569,
"choiceText": "Risk factors include a history of STD and new or multiple sexual partners",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 975571,
"choiceText": "Secondary tubo-ovarian abscess may occur from intraperitoneal spread of infection from a bowel perforation or in association with pelvic cancer",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 975573,
"choiceText": "Tubo-ovarian abscess does not occur during an initial, acute episode of an STD",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 975575,
"choiceText": "Various treatment options are available",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "To underscore the close relationship between PID and tubo-ovarian abscess, in some case series as many as one third of patients who were diagnosed with PID went on to develop tubo-ovarian abscess. In patients who have been previously diagnosed with PID, the condition shares the same epidemiology and risk factor profile; it usually occurs in sexually active women between 20 and 40 years of age, with risk factors including a history of STD and new or multiple sexual partners. ",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 308707,
"questionText": "Which of the following statements regarding tubo-ovarian abscess is <em>not</em> true?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 975577,
"choiceText": "Intravenous antibiotics",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 975579,
"choiceText": "CT-guided drainage",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 975581,
"choiceText": "A trial of oral antibiotics",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 975583,
"choiceText": "Ultrasound-guided drainage",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 975585,
"choiceText": "Surgery",
"correct": false,
"displayOrder": 5,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Tubo-ovarian abscess may respond to antibiotics alone, but operative treatment or percutaneous drainage may be required in cases that are severe or do not respond to purely medical management. The initial medical therapy should include intravenous antibiotics to cover gonorrhea and chlamydia, as well as anaerobes (which are often also present). Oral antibiotics are not appropriate for initial therapy.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 308709,
"questionText": "Which of the following choices is <em>not</em> a recommended treatment for tubo-ovarian abscess?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
864616 | /viewarticle/864616 | [
{
"authors": "Monique Bethel, MD; Laura D. Carbone, MD, MS",
"content": [
"Editor's Note:\n\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.",
"A 74-year-old man with a past medical history of myasthenia gravis treated with chronic corticosteroids, but never treated with radiation therapy, presents with osteoporosis, likely secondary to his chronic steroid use. About 3 months prior, he was referred to an orthopedic surgeon for evaluation of severe left hip pain and bilateral knee pain. The patient is on alendronic acid (70 mg orally, once a week) and has been for over 10 years. He is a current smoker; his smoking history exceeds 50 years.",
"Figure 1.",
"Figure 1.",
"At the time of presentation for hip and knee pain, the patient had left groin pain and decreased range of motion in his left hip. Plain radiography of the pelvis and left and right hip revealed severe arthritic changes, and his left femoral head was shattered. On the right side, a fracture of the proximal femur was noted (Figure 1). His initial treatment was surgical stabilization of his left hip with total hip arthroplasty, and he also had an intramedullary nail placed in his right femur. Today, he presents to a metabolic bone clinic for advice on how to treat his osteoporosis."
],
"date": "June 14, 2016",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/864/616/864616-Thumb1.png"
}
],
"markdown": "# Bilateral Hip Fractures in an Elderly Man\n\n **Authors:** Monique Bethel, MD; Laura D. Carbone, MD, MS \n **Date:** June 14, 2016\n\n ## Content\n\n Editor's Note:\n\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us.\nA 74-year-old man with a past medical history of myasthenia gravis treated with chronic corticosteroids, but never treated with radiation therapy, presents with osteoporosis, likely secondary to his chronic steroid use. About 3 months prior, he was referred to an orthopedic surgeon for evaluation of severe left hip pain and bilateral knee pain. The patient is on alendronic acid (70 mg orally, once a week) and has been for over 10 years. He is a current smoker; his smoking history exceeds 50 years.\nFigure 1.\nFigure 1.\nAt the time of presentation for hip and knee pain, the patient had left groin pain and decreased range of motion in his left hip. Plain radiography of the pelvis and left and right hip revealed severe arthritic changes, and his left femoral head was shattered. On the right side, a fracture of the proximal femur was noted (Figure 1). His initial treatment was surgical stabilization of his left hip with total hip arthroplasty, and he also had an intramedullary nail placed in his right femur. Today, he presents to a metabolic bone clinic for advice on how to treat his osteoporosis.\n\n ## Figures\n\n **Figure 1.** \n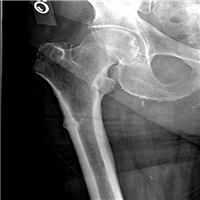 \n\n\n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "Bilateral Hip Fractures in an Elderly Man"
},
{
"authors": "Monique Bethel, MD; Laura D. Carbone, MD, MS",
"content": [
"Upon physical examination, the patient is pleasant and conversant. He is afebrile, his blood pressure is 141/75 mm Hg, his pulse 65 beats/min and body mass index (BMI) is 29.5. He is ambulating with a cane. He has crepitus in both knees and a well-healed scar over the area of his left hip, consistent with his recent hip replacement.",
"The following laboratory tests were obtained to assess secondary causes of osteoporosis:",
"Free testosterone level: 3.1 ng/mL (reference range, 1.9-8.4 ng/mL)",
"25-OH vitamin D level: 33 ng/mL (reference range, 30-50 ng/mL)",
"Creatinine level: 1.2 mg/dL (reference range, 0.6-1.2 mg/dL)",
"Calcium level: 9.2 mg/dL (reference range, 8.5-10.2 mg/dL)",
"Ionized Ca2+ level: 4.2 mg/dL (reference range, 4.5-5.3 mg/dL)",
"Magnesium level: 2.1 mg/dL (reference range, 1.3-2.7 mg/dL)",
"Albumin level: 3.5 g/dL (reference range, 3.5-5 g/dL)",
"Thyroid-stimulating hormone level: 1.88 mIU/mL (reference range, 0.4-4.2 mIU/mL)",
"Alkaline phosphatase level: 65 IU/L (reference range, 44-147 IU/L)",
"Serum protein electrophoresis level: No monoclonal spike"
],
"date": "June 14, 2016",
"figures": [],
"markdown": "# Bilateral Hip Fractures in an Elderly Man\n\n **Authors:** Monique Bethel, MD; Laura D. Carbone, MD, MS \n **Date:** June 14, 2016\n\n ## Content\n\n Upon physical examination, the patient is pleasant and conversant. He is afebrile, his blood pressure is 141/75 mm Hg, his pulse 65 beats/min and body mass index (BMI) is 29.5. He is ambulating with a cane. He has crepitus in both knees and a well-healed scar over the area of his left hip, consistent with his recent hip replacement.\nThe following laboratory tests were obtained to assess secondary causes of osteoporosis:\nFree testosterone level: 3.1 ng/mL (reference range, 1.9-8.4 ng/mL)\n25-OH vitamin D level: 33 ng/mL (reference range, 30-50 ng/mL)\nCreatinine level: 1.2 mg/dL (reference range, 0.6-1.2 mg/dL)\nCalcium level: 9.2 mg/dL (reference range, 8.5-10.2 mg/dL)\nIonized Ca2+ level: 4.2 mg/dL (reference range, 4.5-5.3 mg/dL)\nMagnesium level: 2.1 mg/dL (reference range, 1.3-2.7 mg/dL)\nAlbumin level: 3.5 g/dL (reference range, 3.5-5 g/dL)\nThyroid-stimulating hormone level: 1.88 mIU/mL (reference range, 0.4-4.2 mIU/mL)\nAlkaline phosphatase level: 65 IU/L (reference range, 44-147 IU/L)\nSerum protein electrophoresis level: No monoclonal spike\n\n ## Figures\n\n \n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 974375,
"choiceText": "Age",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 974377,
"choiceText": "Bisphosphonate use ",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 974379,
"choiceText": "Chronic use of corticosteroids",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 974381,
"choiceText": "Extensive smoking history\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 308349,
"questionText": "Which clinical or demographic factor most contributed to his right femur fracture?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Bilateral Hip Fractures in an Elderly Man"
},
{
"authors": "Monique Bethel, MD; Laura D. Carbone, MD, MS",
"content": [
"Although osteoporosis is a common disease among older individuals, it remains less common in men than in women. Therefore, clinicians may not readily identify men at high risk for osteoporosis and its major complications, including hip fractures.",
"When evaluating male patients with known osteoporosis who present with bilateral fractures, such as in this case, ruling out secondary causes is important.[1] Secondary causes include any specific diseases or medications that may cause bone loss. Numerous conditions can induce bone loss, but important causes to consider in an elderly man include testosterone deficiency, vitamin D deficiency, hyperparathyroidism, hypercalciuria, underlying kidney disease, hyperthyroidism, multiple myeloma, and a history of prostate cancer (with use of antiandrogen therapy).",
"The laboratory test results obtained in this case indicated that a hypogonadal state, kidney disease, osteogenesis imperfecta, multiple myeloma, hyperparathyroidism, hypercalciuria, or hyperthyroidism were unlikely causes. Thus, the most likely cause of osteoporosis in this case is chronic steroid use; furthermore, glucocorticoid-induced osteoporosis has been cited as an import risk factor for hip fractures in men.[2]",
"Although osteoporosis is less common in men, at least one third of all hip fractures happen in men. Furthermore, the incidence of hip fractures in men is expected to increase at a faster rate than in women.[3] Despite this, men are less likely to receive treatment for osteoporosis than women, even after sustaining a fracture.[4]",
"Bisphosphonates are commonly used in the treatment of osteoporosis. Hip fractures are on the decline in the United States and in other industrialized countries, a fact that has been attributed, in part, to the use of bisphosphonates to treat osteoporosis and osteopenia.[5] In the mid-2000s, multiple case reports of \"atypical\" femur fractures (AFFs) began to emerge in patients receiving routine bisphosphonate therapy; this called into question the safety of the long-term use of bisphosphonate medications. One of the first case reports was in a series of nine patients receiving chronic alendronate therapy (ranging in duration from 1 to 8 years), with ages ranging from 49 to 76 years, including one man.[6]"
],
"date": "June 14, 2016",
"figures": [],
"markdown": "# Bilateral Hip Fractures in an Elderly Man\n\n **Authors:** Monique Bethel, MD; Laura D. Carbone, MD, MS \n **Date:** June 14, 2016\n\n ## Content\n\n Although osteoporosis is a common disease among older individuals, it remains less common in men than in women. Therefore, clinicians may not readily identify men at high risk for osteoporosis and its major complications, including hip fractures.\nWhen evaluating male patients with known osteoporosis who present with bilateral fractures, such as in this case, ruling out secondary causes is important.[1] Secondary causes include any specific diseases or medications that may cause bone loss. Numerous conditions can induce bone loss, but important causes to consider in an elderly man include testosterone deficiency, vitamin D deficiency, hyperparathyroidism, hypercalciuria, underlying kidney disease, hyperthyroidism, multiple myeloma, and a history of prostate cancer (with use of antiandrogen therapy).\nThe laboratory test results obtained in this case indicated that a hypogonadal state, kidney disease, osteogenesis imperfecta, multiple myeloma, hyperparathyroidism, hypercalciuria, or hyperthyroidism were unlikely causes. Thus, the most likely cause of osteoporosis in this case is chronic steroid use; furthermore, glucocorticoid-induced osteoporosis has been cited as an import risk factor for hip fractures in men.[2]\nAlthough osteoporosis is less common in men, at least one third of all hip fractures happen in men. Furthermore, the incidence of hip fractures in men is expected to increase at a faster rate than in women.[3] Despite this, men are less likely to receive treatment for osteoporosis than women, even after sustaining a fracture.[4]\nBisphosphonates are commonly used in the treatment of osteoporosis. Hip fractures are on the decline in the United States and in other industrialized countries, a fact that has been attributed, in part, to the use of bisphosphonates to treat osteoporosis and osteopenia.[5] In the mid-2000s, multiple case reports of \"atypical\" femur fractures (AFFs) began to emerge in patients receiving routine bisphosphonate therapy; this called into question the safety of the long-term use of bisphosphonate medications. One of the first case reports was in a series of nine patients receiving chronic alendronate therapy (ranging in duration from 1 to 8 years), with ages ranging from 49 to 76 years, including one man.[6]\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 974375,
"choiceText": "Age",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 974377,
"choiceText": "Bisphosphonate use ",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 974379,
"choiceText": "Chronic use of corticosteroids",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 974381,
"choiceText": "Extensive smoking history\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 308349,
"questionText": "Which clinical or demographic factor most contributed to his right femur fracture?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Bilateral Hip Fractures in an Elderly Man"
},
{
"authors": "Monique Bethel, MD; Laura D. Carbone, MD, MS",
"content": [
"Bisphosphonates are \"antiresorptive\" osteoporosis medications that work by inhibiting osteoclast resorptive activity and increasing osteoclast apoptosis.[7] Some evidence suggests that bisphosphonates may inhibit osteoblast apoptosis, although this is controversial.[8] Together, these actions may lead to increased bone mass and reduced risk for fracture, which has made these drugs a popular choice to treat osteoporosis.",
"However, osteoclast activity is needed to repair \"microcracks\" or tiny imperfections in bone that develop over time.[9] Because these microcracks accumulate, bone strength declines, whereas bone mass may be stable or even increase.[10,11] The accumulation of these microcracks may contribute to AFFs in individuals on bisphosphates. This finding has been demonstrated in animal models, but a strong correlation has not been established in humans.[12,13]",
"AFFs typically occur in the subtrochanteric/proximal diaphysis of the femur after minimal or no trauma. These fractures have a characteristic radiographic appearance. Typically, a transversely oriented fracture line (0°-30° from the horizontal) originates in the lateral cortex, accompanied by focal cortical thickening.[14] If the fracture involves the medial cortex, the angle may become more oblique.",
"Of note, no comminution is observed, even with a fracture involving both cortices. A prodrome of thigh pain, occurring days to weeks preceding fracture, has been described; however, patients may have minimal symptoms or may be completely asymptomatic. Therefore, in patients on long-term bisphosphonate therapy, any reports of unexplained thigh pain should raise suspicion. Also, the appearance of one AFF is associated with a high incidence of AFF on the contralateral side; therefore, in patients who have sustained an AFF, radiographs and further evaluation of the contralateral femur should be obtained.",
"To date, the only known risk factor is use of bisphosphonates. Of note, scattered reports have described atypical fractures with the use of denosumab,[15] a receptor activator NF kappa RANK ligand (RANK-L) antibody with a mechanism of action similar to that of bisphosphonates, suggesting that other antiresorptive osteoporosis medications may raise the risk for this complication."
],
"date": "June 14, 2016",
"figures": [],
"markdown": "# Bilateral Hip Fractures in an Elderly Man\n\n **Authors:** Monique Bethel, MD; Laura D. Carbone, MD, MS \n **Date:** June 14, 2016\n\n ## Content\n\n Bisphosphonates are \"antiresorptive\" osteoporosis medications that work by inhibiting osteoclast resorptive activity and increasing osteoclast apoptosis.[7] Some evidence suggests that bisphosphonates may inhibit osteoblast apoptosis, although this is controversial.[8] Together, these actions may lead to increased bone mass and reduced risk for fracture, which has made these drugs a popular choice to treat osteoporosis.\nHowever, osteoclast activity is needed to repair \"microcracks\" or tiny imperfections in bone that develop over time.[9] Because these microcracks accumulate, bone strength declines, whereas bone mass may be stable or even increase.[10,11] The accumulation of these microcracks may contribute to AFFs in individuals on bisphosphates. This finding has been demonstrated in animal models, but a strong correlation has not been established in humans.[12,13]\nAFFs typically occur in the subtrochanteric/proximal diaphysis of the femur after minimal or no trauma. These fractures have a characteristic radiographic appearance. Typically, a transversely oriented fracture line (0°-30° from the horizontal) originates in the lateral cortex, accompanied by focal cortical thickening.[14] If the fracture involves the medial cortex, the angle may become more oblique.\nOf note, no comminution is observed, even with a fracture involving both cortices. A prodrome of thigh pain, occurring days to weeks preceding fracture, has been described; however, patients may have minimal symptoms or may be completely asymptomatic. Therefore, in patients on long-term bisphosphonate therapy, any reports of unexplained thigh pain should raise suspicion. Also, the appearance of one AFF is associated with a high incidence of AFF on the contralateral side; therefore, in patients who have sustained an AFF, radiographs and further evaluation of the contralateral femur should be obtained.\nTo date, the only known risk factor is use of bisphosphonates. Of note, scattered reports have described atypical fractures with the use of denosumab,[15] a receptor activator NF kappa RANK ligand (RANK-L) antibody with a mechanism of action similar to that of bisphosphonates, suggesting that other antiresorptive osteoporosis medications may raise the risk for this complication.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "Bilateral Hip Fractures in an Elderly Man"
},
{
"authors": "Monique Bethel, MD; Laura D. Carbone, MD, MS",
"content": [
"In an individual who has sustained an AFF, bisphosphonates should be discontinued immediately and not used again, as was the case with the patient described above. However, these individuals may remain at significant risk for future fragility fractures or delayed fracture healing/nonunion and may require additional pharmacotherapy to increase bone mass and/or promote healing.",
"In such refractory cases, teriparatide, a parathyroid hormone analogue, has shown efficacy in increasing bone mass.[16] Teriparatide is a daily injectable drug but it is not appropriate in those who are at increased risk for osteosarcoma, such as those who have had radiation exposure or have Paget disease. It is also not indicated in children or in patients with hyperparathyroidism or hypercalcemia. Use of teriparatide is limited to 2 years.",
"Bisphosphonates accumulate in bone and may be slowly released for up to 7 years after discontinuing the drug; therefore, many clinicians are reassessing the use of bisphosphonate after approximately 5 years of therapy. Patients who are still at high risk for fracture may be continued on bisphosphonate therapy, whereas those at lower risk may stop and resume the medicine after a drug holiday.",
"Bisphosphonates, a cornerstone in the treatment of osteoporosis, may come with severe side effects, including AFFs. Clinicians should be cognizant of this severe complication, the heralding signs and symptoms, and periodically assess the risk-benefit ratio of continued use of bisphosphonates in patients over time."
],
"date": "June 14, 2016",
"figures": [],
"markdown": "# Bilateral Hip Fractures in an Elderly Man\n\n **Authors:** Monique Bethel, MD; Laura D. Carbone, MD, MS \n **Date:** June 14, 2016\n\n ## Content\n\n In an individual who has sustained an AFF, bisphosphonates should be discontinued immediately and not used again, as was the case with the patient described above. However, these individuals may remain at significant risk for future fragility fractures or delayed fracture healing/nonunion and may require additional pharmacotherapy to increase bone mass and/or promote healing.\nIn such refractory cases, teriparatide, a parathyroid hormone analogue, has shown efficacy in increasing bone mass.[16] Teriparatide is a daily injectable drug but it is not appropriate in those who are at increased risk for osteosarcoma, such as those who have had radiation exposure or have Paget disease. It is also not indicated in children or in patients with hyperparathyroidism or hypercalcemia. Use of teriparatide is limited to 2 years.\nBisphosphonates accumulate in bone and may be slowly released for up to 7 years after discontinuing the drug; therefore, many clinicians are reassessing the use of bisphosphonate after approximately 5 years of therapy. Patients who are still at high risk for fracture may be continued on bisphosphonate therapy, whereas those at lower risk may stop and resume the medicine after a drug holiday.\nBisphosphonates, a cornerstone in the treatment of osteoporosis, may come with severe side effects, including AFFs. Clinicians should be cognizant of this severe complication, the heralding signs and symptoms, and periodically assess the risk-benefit ratio of continued use of bisphosphonates in patients over time.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 974383,
"choiceText": "Calcitonin",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 974385,
"choiceText": "Teriparatide",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 974387,
"choiceText": "Denosumab\r\n",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 974389,
"choiceText": "Another bisphosphate",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Calcitonin is a second-line treatment for osteoporosis. It also inhibits osteoclastic bone resorption, but it is not as effective as the bisphosphonates. It is unlikely to be beneficial in this patient. <br><br>\r\n\r\nDenosumab is a RANK-L antibody (RANK-L is a key osteoclast differentiation factor) and also inhibits osteoclasts via a mechanism similar to bisphosphates. Reports of AFFs have also been associated with this medication; however, many fewer cases have been noted because it is still relatively new to the market. More AFFs are expected to be observed as the use of this medication increases; therefore, this medication is also not recommended for use in this patient. <br><br>\r\n\r\nDevelopment of AFFs is a contraindication to further use of any bisphosphonate. Teriparatide, a parathyroid hormone analogue administered daily via subcutaneous injections, works by stimulating osteoblastic bone formation and is an appropriate choice to build bone mass in this patient. Some case reports also suggest that teriparatide may help to heal AFFs.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 308351,
"questionText": "In addition to surgical fixation, what pharmacotherapy could be considered for treatment of osteoporosis in a patient who has had a bisphosphonate-associated AFF?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 974391,
"choiceText": "Tobacco abuse",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 974393,
"choiceText": "Hypertension",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 974395,
"choiceText": "Chronic liver disease\r\n",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 974397,
"choiceText": "Prior radiation exposure",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Teriparatide has been associated with the development of osteosarcoma in animal models and therefore should not be used in any patient with a history of bone cancer or Paget disease (which is also associated with a higher risk for osteosarcoma). \r\n<br><br>\r\nSimilarly, prior radiation exposure sensitizes bones to the formation of osteosarcoma; therefore, teriparatide is contraindicated in patients who have received radiation therapy or had any other significant radiation exposure. Due to the concern for potentiating osteosarcoma, a maximum duration of 2 years is also observed. Tobacco use, hypertension, and chronic liver disease are not associated with adverse events from teriparatide use.\r\n",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 308353,
"questionText": "Which of the following is a contraindication for the use of teriparatide?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Bilateral Hip Fractures in an Elderly Man"
},
{
"authors": "Monique Bethel, MD; Laura D. Carbone, MD, MS",
"content": [],
"date": "June 14, 2016",
"figures": [],
"markdown": "# Bilateral Hip Fractures in an Elderly Man\n\n **Authors:** Monique Bethel, MD; Laura D. Carbone, MD, MS \n **Date:** June 14, 2016\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 974383,
"choiceText": "Calcitonin",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 974385,
"choiceText": "Teriparatide",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 974387,
"choiceText": "Denosumab\r\n",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 974389,
"choiceText": "Another bisphosphate",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Calcitonin is a second-line treatment for osteoporosis. It also inhibits osteoclastic bone resorption, but it is not as effective as the bisphosphonates. It is unlikely to be beneficial in this patient. <br><br>\r\n\r\nDenosumab is a RANK-L antibody (RANK-L is a key osteoclast differentiation factor) and also inhibits osteoclasts via a mechanism similar to bisphosphates. Reports of AFFs have also been associated with this medication; however, many fewer cases have been noted because it is still relatively new to the market. More AFFs are expected to be observed as the use of this medication increases; therefore, this medication is also not recommended for use in this patient. <br><br>\r\n\r\nDevelopment of AFFs is a contraindication to further use of any bisphosphonate. Teriparatide, a parathyroid hormone analogue administered daily via subcutaneous injections, works by stimulating osteoblastic bone formation and is an appropriate choice to build bone mass in this patient. Some case reports also suggest that teriparatide may help to heal AFFs.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 308351,
"questionText": "In addition to surgical fixation, what pharmacotherapy could be considered for treatment of osteoporosis in a patient who has had a bisphosphonate-associated AFF?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 974391,
"choiceText": "Tobacco abuse",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 974393,
"choiceText": "Hypertension",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 974395,
"choiceText": "Chronic liver disease\r\n",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 974397,
"choiceText": "Prior radiation exposure",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Teriparatide has been associated with the development of osteosarcoma in animal models and therefore should not be used in any patient with a history of bone cancer or Paget disease (which is also associated with a higher risk for osteosarcoma). \r\n<br><br>\r\nSimilarly, prior radiation exposure sensitizes bones to the formation of osteosarcoma; therefore, teriparatide is contraindicated in patients who have received radiation therapy or had any other significant radiation exposure. Due to the concern for potentiating osteosarcoma, a maximum duration of 2 years is also observed. Tobacco use, hypertension, and chronic liver disease are not associated with adverse events from teriparatide use.\r\n",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 308353,
"questionText": "Which of the following is a contraindication for the use of teriparatide?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "Bilateral Hip Fractures in an Elderly Man"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 974375,
"choiceText": "Age",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 974377,
"choiceText": "Bisphosphonate use ",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 974379,
"choiceText": "Chronic use of corticosteroids",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 974381,
"choiceText": "Extensive smoking history\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 308349,
"questionText": "Which clinical or demographic factor most contributed to his right femur fracture?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 974383,
"choiceText": "Calcitonin",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 974385,
"choiceText": "Teriparatide",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 974387,
"choiceText": "Denosumab\r\n",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 974389,
"choiceText": "Another bisphosphate",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Calcitonin is a second-line treatment for osteoporosis. It also inhibits osteoclastic bone resorption, but it is not as effective as the bisphosphonates. It is unlikely to be beneficial in this patient. <br><br>\r\n\r\nDenosumab is a RANK-L antibody (RANK-L is a key osteoclast differentiation factor) and also inhibits osteoclasts via a mechanism similar to bisphosphates. Reports of AFFs have also been associated with this medication; however, many fewer cases have been noted because it is still relatively new to the market. More AFFs are expected to be observed as the use of this medication increases; therefore, this medication is also not recommended for use in this patient. <br><br>\r\n\r\nDevelopment of AFFs is a contraindication to further use of any bisphosphonate. Teriparatide, a parathyroid hormone analogue administered daily via subcutaneous injections, works by stimulating osteoblastic bone formation and is an appropriate choice to build bone mass in this patient. Some case reports also suggest that teriparatide may help to heal AFFs.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 308351,
"questionText": "In addition to surgical fixation, what pharmacotherapy could be considered for treatment of osteoporosis in a patient who has had a bisphosphonate-associated AFF?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 974391,
"choiceText": "Tobacco abuse",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 974393,
"choiceText": "Hypertension",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 974395,
"choiceText": "Chronic liver disease\r\n",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 974397,
"choiceText": "Prior radiation exposure",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "Teriparatide has been associated with the development of osteosarcoma in animal models and therefore should not be used in any patient with a history of bone cancer or Paget disease (which is also associated with a higher risk for osteosarcoma). \r\n<br><br>\r\nSimilarly, prior radiation exposure sensitizes bones to the formation of osteosarcoma; therefore, teriparatide is contraindicated in patients who have received radiation therapy or had any other significant radiation exposure. Due to the concern for potentiating osteosarcoma, a maximum duration of 2 years is also observed. Tobacco use, hypertension, and chronic liver disease are not associated with adverse events from teriparatide use.\r\n",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 308353,
"questionText": "Which of the following is a contraindication for the use of teriparatide?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
863882 | /viewarticle/863882 | [
{
"authors": "James J. McCombie, MB ChB; Erik D. Schraga, MD",
"content": [
"Editor's Note:\n\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.",
"A 61-year-old woman presents to the emergency department after rapid onset of severe upper abdominal pain while watching TV and eating dinner. The pain was constant and radiating to the right shoulder.",
"The patient used effervescent antacid/analgesic tablets and, subsequently, a glycerin suppository; however, despite having a normal bowel movement, she did not have any relief. She also used hydrocodone and diazepam that she had at home for back spasms, but she continued to experience worsening pain. She felt nauseous and, when bending over to the toilet to vomit, she became lightheaded, had a syncopal episode, and hit her head on the bathtub. She has not had any bloody or dark stools.",
"Currently, any attempt at movement causes severe abdominal pain. The patient continues to have nausea but has not had any vomiting. She feels slightly lightheaded. She denies having any chest pain or palpitations. She has not had any fever.",
"The patient's medical history is significant for hepatitis C, for which she received treatment with interferon alpha 3 years ago. She also has a history of anxiety, chronic lower back pain, and hypertension. Her surgical history includes an appendectomy that was performed 15 years ago and endometrial ablation.",
"The patient's medications include lisinopril, diazepam, and hydrocodone. She was previously a heavy drinker but has not used alcohol in over 5 years."
],
"date": "June 01, 2016",
"figures": [],
"markdown": "# A 61-Year-Old Woman With Abdominal Pain and Syncope\n\n **Authors:** James J. McCombie, MB ChB; Erik D. Schraga, MD \n **Date:** June 01, 2016\n\n ## Content\n\n Editor's Note:\n\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.\nA 61-year-old woman presents to the emergency department after rapid onset of severe upper abdominal pain while watching TV and eating dinner. The pain was constant and radiating to the right shoulder.\nThe patient used effervescent antacid/analgesic tablets and, subsequently, a glycerin suppository; however, despite having a normal bowel movement, she did not have any relief. She also used hydrocodone and diazepam that she had at home for back spasms, but she continued to experience worsening pain. She felt nauseous and, when bending over to the toilet to vomit, she became lightheaded, had a syncopal episode, and hit her head on the bathtub. She has not had any bloody or dark stools.\nCurrently, any attempt at movement causes severe abdominal pain. The patient continues to have nausea but has not had any vomiting. She feels slightly lightheaded. She denies having any chest pain or palpitations. She has not had any fever.\nThe patient's medical history is significant for hepatitis C, for which she received treatment with interferon alpha 3 years ago. She also has a history of anxiety, chronic lower back pain, and hypertension. Her surgical history includes an appendectomy that was performed 15 years ago and endometrial ablation.\nThe patient's medications include lisinopril, diazepam, and hydrocodone. She was previously a heavy drinker but has not used alcohol in over 5 years.\n\n ## Figures\n\n \n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 61-Year-Old Woman With Abdominal Pain and Syncope"
},
{
"authors": "James J. McCombie, MB ChB; Erik D. Schraga, MD",
"content": [
"The patient appears uncomfortable on physical examination. Her vital signs are remarkable for a rapid heart rate of 112 beats/min and a low blood pressure of 96/48 mm Hg. Her other vital signs include a temperature of 96.4°F, a respiratory rate of 18 breaths/min, and an oxygen saturation of 98% while breathing room air. Minimal soft-tissue swelling of the right forehead is noted, with a small ecchymosis. The heart sounds are rapid but without murmurs. The lungs are clear to auscultation.",
"The abdomen is diffusely tender to palpation, which is greatest in the right upper quadrant, where there is positive guarding and rebound tenderness. Palpation of the lower quadrants results in increased right upper-quadrant pain. No distention or ascites are appreciated. No hepatomegaly, palpable pulsatile masses, or bruits are appreciated. The radial and dorsalis pedis pulses are equal bilaterally.",
"Figure 1.",
"Figure 1.",
"Intravenous access is obtained, and the patient is given 1 L of normal saline, which leads to improvement in her heart rate and blood pressure. Multiple doses of intravenous hydromorphone are given, with temporary relief of her pain but no improvement in tenderness.",
"An upright chest radiograph and three-view abdominal radiographs are unremarkable, with no visible free air or bowel abnormalities. Laboratory tests reveal a borderline low hemoglobin level of 11.5 g/dL and a normal white blood cell count of 6.8 x 103 cells/µL. Aminotransferase, bilirubin, and lipase levels are all normal.",
"CT of the abdomen and pelvis with intravenous contrast is performed (Figure 1)."
],
"date": "June 01, 2016",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/863/882/863882-Thumb1.jpg"
}
],
"markdown": "# A 61-Year-Old Woman With Abdominal Pain and Syncope\n\n **Authors:** James J. McCombie, MB ChB; Erik D. Schraga, MD \n **Date:** June 01, 2016\n\n ## Content\n\n The patient appears uncomfortable on physical examination. Her vital signs are remarkable for a rapid heart rate of 112 beats/min and a low blood pressure of 96/48 mm Hg. Her other vital signs include a temperature of 96.4°F, a respiratory rate of 18 breaths/min, and an oxygen saturation of 98% while breathing room air. Minimal soft-tissue swelling of the right forehead is noted, with a small ecchymosis. The heart sounds are rapid but without murmurs. The lungs are clear to auscultation.\nThe abdomen is diffusely tender to palpation, which is greatest in the right upper quadrant, where there is positive guarding and rebound tenderness. Palpation of the lower quadrants results in increased right upper-quadrant pain. No distention or ascites are appreciated. No hepatomegaly, palpable pulsatile masses, or bruits are appreciated. The radial and dorsalis pedis pulses are equal bilaterally.\nFigure 1.\nFigure 1.\nIntravenous access is obtained, and the patient is given 1 L of normal saline, which leads to improvement in her heart rate and blood pressure. Multiple doses of intravenous hydromorphone are given, with temporary relief of her pain but no improvement in tenderness.\nAn upright chest radiograph and three-view abdominal radiographs are unremarkable, with no visible free air or bowel abnormalities. Laboratory tests reveal a borderline low hemoglobin level of 11.5 g/dL and a normal white blood cell count of 6.8 x 103 cells/µL. Aminotransferase, bilirubin, and lipase levels are all normal.\nCT of the abdomen and pelvis with intravenous contrast is performed (Figure 1).\n\n ## Figures\n\n **Figure 1.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 970883,
"choiceText": "Ruptured abdominal aortic aneurysm",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 970885,
"choiceText": "Cholecystitis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 970887,
"choiceText": "Spontaneous rupture of hepatoma",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 970889,
"choiceText": "Small-bowel obstruction\r\n ",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 307163,
"questionText": "What is the cause of the patient's pain? <br><br>\r\n<i>Hint: The patient's medical history includes a likely underlying cause.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 61-Year-Old Woman With Abdominal Pain and Syncope"
},
{
"authors": "James J. McCombie, MB ChB; Erik D. Schraga, MD",
"content": [
"CT of the abdomen and pelvis revealed a heterogeneous 4-cm mass within the dome of the right lobe of the liver, with a small high-attenuation area believed to represent an actively bleeding site (Figure 1). High-density material around the liver and under the right hemidiaphragm (Figure 2), which extended down along the right pericolic gutter into the pelvis and was consistent with blood, was also noted. The liver had a heterogeneous appearance suggestive of cirrhosis.",
"Figure 1.",
"Figure 1.",
"Figure 2.",
"Figure 2.",
"Hepatoma, or hepatocellular carcinoma (HCC), is a primary cancer of the liver and has an incidence of 5.2 per 100,000 population in the United States. In 80% of patients, some degree of underlying cirrhosis is present, with alcoholic cirrhosis being the major cause in the developed world. Other causes of hepatoma include chronic hepatitis B or hepatitis C infection, as well as alpha-1 antitrypsin deficiency, hemochromatosis, and primary biliary cirrhosis.",
"Drugs, such as anabolic steroids, and toxins, such as aflatoxin (which are produced by a species of Aspergillus that grows on stored grains and peanuts), have also been implicated in HCC. Finally, parasitic infestations, such as schistosomiasis, have been known to be responsible.",
"HCC is three to four times more common in males. The clinical manifestations are seldom distinctive and are often masked by those related to the background of cirrhosis or chronic hepatitis. HCC should be suspected if a patient with cirrhosis deteriorates without an alternative explanation.[1]",
"Surveillance should be considered for certain high-risk groups, including persons with chronic viral hepatitis, because this has been shown to improve survival. In a series of patients with chronic hepatitis B infection,[2] HCC tumor size was smaller (4.2 cm vs 7.7 cm) and less frequently present (2.6 vs 3.8) in the surveillance group than in the nonsurveillance group. Definitive treatment with surgery (20% vs 10%) and local ablative therapy (46% vs 19%) was more likely to be able to be performed in the surveillance group than in the nonsurveillance group.",
"Median survival was significantly longer in the surveillance group than the nonsurveillance group (88 weeks vs 26 weeks). The adjusted cumulative survival at 2 years was significantly longer in the surveillance group if the tumor volume doubling time was < 90 days.[2]",
"The presence of a mass > 2 cm on ultrasonography in a patient with underlying cirrhosis is 95% predictive of hepatoma. In addition, an increased alpha-fetoprotein (AFP) level virtually confirms the diagnosis, and the patient should be evaluated for surgery when appropriate.[2] If the AFP level is normal, further imaging with CT, MRI, or angiography should be used to help clarify the diagnosis. Biopsy is indicated only in cases of major diagnostic doubt.[3]"
],
"date": "June 01, 2016",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/863/882/863882-Thumb1.jpg"
},
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/863/882/863882-Thumb2.jpg"
}
],
"markdown": "# A 61-Year-Old Woman With Abdominal Pain and Syncope\n\n **Authors:** James J. McCombie, MB ChB; Erik D. Schraga, MD \n **Date:** June 01, 2016\n\n ## Content\n\n CT of the abdomen and pelvis revealed a heterogeneous 4-cm mass within the dome of the right lobe of the liver, with a small high-attenuation area believed to represent an actively bleeding site (Figure 1). High-density material around the liver and under the right hemidiaphragm (Figure 2), which extended down along the right pericolic gutter into the pelvis and was consistent with blood, was also noted. The liver had a heterogeneous appearance suggestive of cirrhosis.\nFigure 1.\nFigure 1.\nFigure 2.\nFigure 2.\nHepatoma, or hepatocellular carcinoma (HCC), is a primary cancer of the liver and has an incidence of 5.2 per 100,000 population in the United States. In 80% of patients, some degree of underlying cirrhosis is present, with alcoholic cirrhosis being the major cause in the developed world. Other causes of hepatoma include chronic hepatitis B or hepatitis C infection, as well as alpha-1 antitrypsin deficiency, hemochromatosis, and primary biliary cirrhosis.\nDrugs, such as anabolic steroids, and toxins, such as aflatoxin (which are produced by a species of Aspergillus that grows on stored grains and peanuts), have also been implicated in HCC. Finally, parasitic infestations, such as schistosomiasis, have been known to be responsible.\nHCC is three to four times more common in males. The clinical manifestations are seldom distinctive and are often masked by those related to the background of cirrhosis or chronic hepatitis. HCC should be suspected if a patient with cirrhosis deteriorates without an alternative explanation.[1]\nSurveillance should be considered for certain high-risk groups, including persons with chronic viral hepatitis, because this has been shown to improve survival. In a series of patients with chronic hepatitis B infection,[2] HCC tumor size was smaller (4.2 cm vs 7.7 cm) and less frequently present (2.6 vs 3.8) in the surveillance group than in the nonsurveillance group. Definitive treatment with surgery (20% vs 10%) and local ablative therapy (46% vs 19%) was more likely to be able to be performed in the surveillance group than in the nonsurveillance group.\nMedian survival was significantly longer in the surveillance group than the nonsurveillance group (88 weeks vs 26 weeks). The adjusted cumulative survival at 2 years was significantly longer in the surveillance group if the tumor volume doubling time was < 90 days.[2]\nThe presence of a mass > 2 cm on ultrasonography in a patient with underlying cirrhosis is 95% predictive of hepatoma. In addition, an increased alpha-fetoprotein (AFP) level virtually confirms the diagnosis, and the patient should be evaluated for surgery when appropriate.[2] If the AFP level is normal, further imaging with CT, MRI, or angiography should be used to help clarify the diagnosis. Biopsy is indicated only in cases of major diagnostic doubt.[3]\n\n ## Figures\n\n **Figure 1.** \n \n\n**Figure 2.** \n \n\n\n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 970883,
"choiceText": "Ruptured abdominal aortic aneurysm",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 970885,
"choiceText": "Cholecystitis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 970887,
"choiceText": "Spontaneous rupture of hepatoma",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 970889,
"choiceText": "Small-bowel obstruction\r\n ",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 307163,
"questionText": "What is the cause of the patient's pain? <br><br>\r\n<i>Hint: The patient's medical history includes a likely underlying cause.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 61-Year-Old Woman With Abdominal Pain and Syncope"
},
{
"authors": "James J. McCombie, MB ChB; Erik D. Schraga, MD",
"content": [
"Spontaneous rupture of a hepatoma is potentially life-threatening. The lifetime incidence of rupture is < 3% in patients with a diagnosis of hepatoma in Western countries; however, mortality was reported as being close to 50% among Asian patients in another series, where the incidence of hepatoma is as high as 12%-14%.[4]André[5] first reported hemoperitoneum associated with the rupture of primary hepatic tumors in 1851. Rupture is preceded by a rapid increase in the size of the tumor from bleeding within its substance.[6]",
"Any patient with known cirrhosis or hepatoma who presents with acute abdominal pain, particularly when associated with hypovolemia or evidence of peritoneal free fluid, should be approached with suspicion of hepatoma rupture. The diagnosis may be made by ultrasonography, CT, or angiography. When imaging is not readily available, deep peritoneal lavage can be performed as an alternative. In the absence of trauma, hemoperitoneum in a patient with a history of cirrhosis should be considered highly likely to have resulted from hepatoma rupture. Many cases, however, are diagnosed at laparotomy.[7]",
"Broadly speaking, three avenues of treatment exist: transcatheter arterial embolization (TAE), hepatectomy, and conservative management. In the acute phase, TAE for hemostasis has a high success rate (53%-100%). It has a lower 30-day mortality rate than open surgical methods (0-37% vs 28%-75%).",
"For definitive treatment, staged liver resection has a higher resection rate (21%-56% vs 13%-31%) and a lower in-hospital mortality rate (0%-9% vs 17%-100%) than one-stage emergency liver resection. Staged liver resection has a good survival rate (1 year, 54.2%-100%; 3 years, 21.2%-48%; 5 years, 15%-21.2%).[7,8]Emergency hepatectomy should be reserved for patients with an easily resectable lesion who are in a stable cardiovascular condition.",
"Conservative therapy may be used for selected patients in extremely poor condition. The recommended treatment for most patients with ruptured HCC is TAE, followed by hepatectomy (if the lesion is resectable).[8]",
"As long as a patient with rupture undergoes TAE and the tumor is amenable to resection, survival rates are good. In one study, cumulative survival rates at 1, 5, and 10 years among patients treated with elective hepatectomy after ruptured hepatoma and initial TAE were 90.0%, 67.5%, and 20.3%, respectively.[8]However, reports have described intraperitoneal seeding of hepatoma after rupture occurring as early as 3 months after the event.[9]",
"Sorafenib, an inhibitor of several protein kinases implicated in carcinogenesis, was approved by the US Food and Drug Administration for the treatment of advanced HCC in 2007. Results from the Sorafenib Hepatocellular Carcinoma Assessment Randomized Protocol (SHARP) trial demonstrated an increase of 3 months in both median survival time and time to radiographic progression of the lesion in patients given sorafenib over placebo.[10]"
],
"date": "June 01, 2016",
"figures": [],
"markdown": "# A 61-Year-Old Woman With Abdominal Pain and Syncope\n\n **Authors:** James J. McCombie, MB ChB; Erik D. Schraga, MD \n **Date:** June 01, 2016\n\n ## Content\n\n Spontaneous rupture of a hepatoma is potentially life-threatening. The lifetime incidence of rupture is < 3% in patients with a diagnosis of hepatoma in Western countries; however, mortality was reported as being close to 50% among Asian patients in another series, where the incidence of hepatoma is as high as 12%-14%.[4]André[5] first reported hemoperitoneum associated with the rupture of primary hepatic tumors in 1851. Rupture is preceded by a rapid increase in the size of the tumor from bleeding within its substance.[6]\nAny patient with known cirrhosis or hepatoma who presents with acute abdominal pain, particularly when associated with hypovolemia or evidence of peritoneal free fluid, should be approached with suspicion of hepatoma rupture. The diagnosis may be made by ultrasonography, CT, or angiography. When imaging is not readily available, deep peritoneal lavage can be performed as an alternative. In the absence of trauma, hemoperitoneum in a patient with a history of cirrhosis should be considered highly likely to have resulted from hepatoma rupture. Many cases, however, are diagnosed at laparotomy.[7]\nBroadly speaking, three avenues of treatment exist: transcatheter arterial embolization (TAE), hepatectomy, and conservative management. In the acute phase, TAE for hemostasis has a high success rate (53%-100%). It has a lower 30-day mortality rate than open surgical methods (0-37% vs 28%-75%).\nFor definitive treatment, staged liver resection has a higher resection rate (21%-56% vs 13%-31%) and a lower in-hospital mortality rate (0%-9% vs 17%-100%) than one-stage emergency liver resection. Staged liver resection has a good survival rate (1 year, 54.2%-100%; 3 years, 21.2%-48%; 5 years, 15%-21.2%).[7,8]Emergency hepatectomy should be reserved for patients with an easily resectable lesion who are in a stable cardiovascular condition.\nConservative therapy may be used for selected patients in extremely poor condition. The recommended treatment for most patients with ruptured HCC is TAE, followed by hepatectomy (if the lesion is resectable).[8]\nAs long as a patient with rupture undergoes TAE and the tumor is amenable to resection, survival rates are good. In one study, cumulative survival rates at 1, 5, and 10 years among patients treated with elective hepatectomy after ruptured hepatoma and initial TAE were 90.0%, 67.5%, and 20.3%, respectively.[8]However, reports have described intraperitoneal seeding of hepatoma after rupture occurring as early as 3 months after the event.[9]\nSorafenib, an inhibitor of several protein kinases implicated in carcinogenesis, was approved by the US Food and Drug Administration for the treatment of advanced HCC in 2007. Results from the Sorafenib Hepatocellular Carcinoma Assessment Randomized Protocol (SHARP) trial demonstrated an increase of 3 months in both median survival time and time to radiographic progression of the lesion in patients given sorafenib over placebo.[10]\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 61-Year-Old Woman With Abdominal Pain and Syncope"
},
{
"authors": "James J. McCombie, MB ChB; Erik D. Schraga, MD",
"content": [
"The patient in this case continued to have recurrent pain and only transiently responded to fluid resuscitation; therefore, it was suspected that she had active bleeding. She was emergently transferred to the operating room for exploratory laparotomy and wedge resection of her hepatic tumor. Surgical resection was favored over TAE because of the peripheral location of the lesion. The patient was noted to have a small left lobe of the liver, and it was felt that she would not survive if a partial right hepatic lobectomy was performed.",
"Over 1 L of blood and clots were removed from the abdominal cavity. A visibly bleeding tumor was seen on the dome of the liver. Ultrasonography was used to check the margins of the tumor, and these margins were marked. A wedge resection was performed, and the major vessels were ligated. The patient received 2 units of packed red blood cells during the procedure and had an estimated blood loss of approximately 600 mL.",
"The tumor was sent to the pathology laboratory and was identified as HCC. The patient remained stable immediately after the procedure and was extubated in the intensive care unit. She was transfused with several units of packed red blood cells. Her hospital course was complicated by episodes of rapid atrial fibrillation, which responded to an intravenous diltiazem drip. She ultimately improved, with spontaneous resolution of her atrial fibrillation, and was discharged to home with instructions to follow up in both the general surgery and gastroenterology clinics."
],
"date": "June 01, 2016",
"figures": [],
"markdown": "# A 61-Year-Old Woman With Abdominal Pain and Syncope\n\n **Authors:** James J. McCombie, MB ChB; Erik D. Schraga, MD \n **Date:** June 01, 2016\n\n ## Content\n\n The patient in this case continued to have recurrent pain and only transiently responded to fluid resuscitation; therefore, it was suspected that she had active bleeding. She was emergently transferred to the operating room for exploratory laparotomy and wedge resection of her hepatic tumor. Surgical resection was favored over TAE because of the peripheral location of the lesion. The patient was noted to have a small left lobe of the liver, and it was felt that she would not survive if a partial right hepatic lobectomy was performed.\nOver 1 L of blood and clots were removed from the abdominal cavity. A visibly bleeding tumor was seen on the dome of the liver. Ultrasonography was used to check the margins of the tumor, and these margins were marked. A wedge resection was performed, and the major vessels were ligated. The patient received 2 units of packed red blood cells during the procedure and had an estimated blood loss of approximately 600 mL.\nThe tumor was sent to the pathology laboratory and was identified as HCC. The patient remained stable immediately after the procedure and was extubated in the intensive care unit. She was transfused with several units of packed red blood cells. Her hospital course was complicated by episodes of rapid atrial fibrillation, which responded to an intravenous diltiazem drip. She ultimately improved, with spontaneous resolution of her atrial fibrillation, and was discharged to home with instructions to follow up in both the general surgery and gastroenterology clinics.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 970891,
"choiceText": "Raised AFP level",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 970893,
"choiceText": "Lesion > 2 cm in any one direction on ultrasonography",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 970895,
"choiceText": "Deterioration of a patient with known cirrhosis of the liver",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 970897,
"choiceText": "All of the above\r\n",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The presence of a mass > 2 cm on ultrasonography in a patient with underlying cirrhosis is 95% predictive of hepatoma. In addition, a raised AFP level virtually confirms the diagnosis, and the patient should be evaluated for surgery when appropriate. If the AFP level is normal, further imaging with CT, MRI, or angiography should be used to help clarify the diagnosis. Biopsy is indicated only in cases of major diagnostic doubt. The clinical manifestations are seldom distinctive and are often masked by those related to the background of cirrhosis or chronic hepatitis. HCC should be suspected if a patient with cirrhosis deteriorates without an alternative explanation.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 307165,
"questionText": "A patient with a known history of hepatitis C that you have been following on a regular basis is concerned about his risk of developing cancer. Which of the following choices is part of the criteria for diagnosing hepatoma?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 970899,
"choiceText": "Hepatitis B",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 970901,
"choiceText": "Alcoholic cirrhosis",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 970903,
"choiceText": "Diabetes",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 970905,
"choiceText": "Familial adenomatous polyposis\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "In 80% of patients, there is some degree of underlying cirrhosis, with alcoholic cirrhosis being the major cause in the developed world.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 307167,
"questionText": "A patient presents to the emergency department with acute onset of right upper-quadrant abdominal pain, which is determined to be caused by spontaneous rupture of an HCC. Which of the following is known to be the most common cause of HCC in developed countries?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 61-Year-Old Woman With Abdominal Pain and Syncope"
},
{
"authors": "James J. McCombie, MB ChB; Erik D. Schraga, MD",
"content": [],
"date": "June 01, 2016",
"figures": [],
"markdown": "# A 61-Year-Old Woman With Abdominal Pain and Syncope\n\n **Authors:** James J. McCombie, MB ChB; Erik D. Schraga, MD \n **Date:** June 01, 2016\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 970891,
"choiceText": "Raised AFP level",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 970893,
"choiceText": "Lesion > 2 cm in any one direction on ultrasonography",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 970895,
"choiceText": "Deterioration of a patient with known cirrhosis of the liver",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 970897,
"choiceText": "All of the above\r\n",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The presence of a mass > 2 cm on ultrasonography in a patient with underlying cirrhosis is 95% predictive of hepatoma. In addition, a raised AFP level virtually confirms the diagnosis, and the patient should be evaluated for surgery when appropriate. If the AFP level is normal, further imaging with CT, MRI, or angiography should be used to help clarify the diagnosis. Biopsy is indicated only in cases of major diagnostic doubt. The clinical manifestations are seldom distinctive and are often masked by those related to the background of cirrhosis or chronic hepatitis. HCC should be suspected if a patient with cirrhosis deteriorates without an alternative explanation.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 307165,
"questionText": "A patient with a known history of hepatitis C that you have been following on a regular basis is concerned about his risk of developing cancer. Which of the following choices is part of the criteria for diagnosing hepatoma?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 970899,
"choiceText": "Hepatitis B",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 970901,
"choiceText": "Alcoholic cirrhosis",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 970903,
"choiceText": "Diabetes",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 970905,
"choiceText": "Familial adenomatous polyposis\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "In 80% of patients, there is some degree of underlying cirrhosis, with alcoholic cirrhosis being the major cause in the developed world.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 307167,
"questionText": "A patient presents to the emergency department with acute onset of right upper-quadrant abdominal pain, which is determined to be caused by spontaneous rupture of an HCC. Which of the following is known to be the most common cause of HCC in developed countries?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 61-Year-Old Woman With Abdominal Pain and Syncope"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 970883,
"choiceText": "Ruptured abdominal aortic aneurysm",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 970885,
"choiceText": "Cholecystitis",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 970887,
"choiceText": "Spontaneous rupture of hepatoma",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 970889,
"choiceText": "Small-bowel obstruction\r\n ",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 307163,
"questionText": "What is the cause of the patient's pain? <br><br>\r\n<i>Hint: The patient's medical history includes a likely underlying cause.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 970891,
"choiceText": "Raised AFP level",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 970893,
"choiceText": "Lesion > 2 cm in any one direction on ultrasonography",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 970895,
"choiceText": "Deterioration of a patient with known cirrhosis of the liver",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 970897,
"choiceText": "All of the above\r\n",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "The presence of a mass > 2 cm on ultrasonography in a patient with underlying cirrhosis is 95% predictive of hepatoma. In addition, a raised AFP level virtually confirms the diagnosis, and the patient should be evaluated for surgery when appropriate. If the AFP level is normal, further imaging with CT, MRI, or angiography should be used to help clarify the diagnosis. Biopsy is indicated only in cases of major diagnostic doubt. The clinical manifestations are seldom distinctive and are often masked by those related to the background of cirrhosis or chronic hepatitis. HCC should be suspected if a patient with cirrhosis deteriorates without an alternative explanation.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 307165,
"questionText": "A patient with a known history of hepatitis C that you have been following on a regular basis is concerned about his risk of developing cancer. Which of the following choices is part of the criteria for diagnosing hepatoma?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 970899,
"choiceText": "Hepatitis B",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 970901,
"choiceText": "Alcoholic cirrhosis",
"correct": true,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 970903,
"choiceText": "Diabetes",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 970905,
"choiceText": "Familial adenomatous polyposis\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "In 80% of patients, there is some degree of underlying cirrhosis, with alcoholic cirrhosis being the major cause in the developed world.",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 307167,
"questionText": "A patient presents to the emergency department with acute onset of right upper-quadrant abdominal pain, which is determined to be caused by spontaneous rupture of an HCC. Which of the following is known to be the most common cause of HCC in developed countries?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
863884 | /viewarticle/863884 | [
{
"authors": "Carolyn R. Lyon, MD",
"content": [
"Editor's Note:\n\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.",
"A 62-year-old man with a history of hypertension, coronary artery disease, and hemodialysis-dependent end-stage renal disease presents to the emergency department with right shoulder pain that has been worsening over the past 2 months, with associated progressive swelling over the acromioclavicular (AC) joint (Figure 1).",
"Figure 1.",
"Figure 1.",
"The patient denies any recent heavy lifting or trauma. He also denies fever, erythema, or warmth of the right shoulder. No drainage has emerged from the mass.",
"The patient's medications include clonidine, carvedilol, sevelamer, amlodipine/benazepril, cinacalcet, and lanthanum carbonate. His surgical history is notable for a left brachiocephalic fistula. The patient has no allergies to medications and denies any tobacco, alcohol, and illicit drug use. The family history is noncontributory."
],
"date": "June 01, 2016",
"figures": [
{
"caption": "Figure 1.",
"image_url": "https://img.medscapestatic.com/article/863/884/863884-Thumb1.png"
}
],
"markdown": "# A 62-Year-Old Man With Worsening Shoulder Pain\n\n **Authors:** Carolyn R. Lyon, MD \n **Date:** June 01, 2016\n\n ## Content\n\n Editor's Note:\n\nThe Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case you would like to suggest for a future Case Challenge, please contact us.\nA 62-year-old man with a history of hypertension, coronary artery disease, and hemodialysis-dependent end-stage renal disease presents to the emergency department with right shoulder pain that has been worsening over the past 2 months, with associated progressive swelling over the acromioclavicular (AC) joint (Figure 1).\nFigure 1.\nFigure 1.\nThe patient denies any recent heavy lifting or trauma. He also denies fever, erythema, or warmth of the right shoulder. No drainage has emerged from the mass.\nThe patient's medications include clonidine, carvedilol, sevelamer, amlodipine/benazepril, cinacalcet, and lanthanum carbonate. His surgical history is notable for a left brachiocephalic fistula. The patient has no allergies to medications and denies any tobacco, alcohol, and illicit drug use. The family history is noncontributory.\n\n ## Figures\n\n **Figure 1.** \n \n\n\n*Page 1 of 6*",
"pagination": {
"current_page": 1,
"total_pages": 6
},
"questionnaire": [],
"title": "A 62-Year-Old Man With Worsening Shoulder Pain"
},
{
"authors": "Carolyn R. Lyon, MD",
"content": [
"The physical examination shows a well-appearing, middle-aged man in no acute distress. His temperature is 98.9°F, blood pressure is 130/88 mm Hg, heart rate is 62 beats/min, and respiratory rate is 18 breaths/min.",
"On examination of the right shoulder, a large, tense, fluctuant mass is noted over the right AC joint. The mass is superficial and measures 10 x 7 x 4 cm. Significant tenderness to palpation in this area is observed. The skin is intact, without erythema or warmth of the shoulder area.",
"Internal and external rotation of the shoulder are significantly impaired owing to pain, and there is weakness on rotator cuff testing. The patient is unable to lift his arm past 30° of forward flexion or abduction. The distal motor, sensation, and reflex examinations are otherwise unremarkable. The extremity pulses are normal. Besides a left arm brachiocephalic fistula with a positive bruit and palpable thrill, the remainder of the physical examination is unremarkable.",
"Figure 2.",
"Figure 2.",
"Figure 3.",
"Figure 3.",
"The patient is given oral analgesics for pain control, and a radiograph of the right shoulder is subsequently obtained. The right shoulder radiograph does not demonstrate fracture (Figure 2) but does show evidence of a high-riding humerus and advanced deformity of the humeral head. A large soft-tissue mass protrudes above the AC joint. The patient had laboratory examinations performed at an outside clinic that morning, which demonstrated a C-reactive protein level, erythrocyte sedimentation rate, and complete blood count within the reference range.",
"After reviewing the above films, the orthopedist on call is consulted to assist with patient management. The orthopedic surgeon recommends MRI of the right shoulder (Figure 3) to provide further information on the shoulder mass."
],
"date": "June 01, 2016",
"figures": [
{
"caption": "Figure 2.",
"image_url": "https://img.medscapestatic.com/article/863/884/863884-Thumb2.png"
},
{
"caption": "Figure 3.",
"image_url": "https://img.medscapestatic.com/article/863/884/863884-Thumb3.png"
}
],
"markdown": "# A 62-Year-Old Man With Worsening Shoulder Pain\n\n **Authors:** Carolyn R. Lyon, MD \n **Date:** June 01, 2016\n\n ## Content\n\n The physical examination shows a well-appearing, middle-aged man in no acute distress. His temperature is 98.9°F, blood pressure is 130/88 mm Hg, heart rate is 62 beats/min, and respiratory rate is 18 breaths/min.\nOn examination of the right shoulder, a large, tense, fluctuant mass is noted over the right AC joint. The mass is superficial and measures 10 x 7 x 4 cm. Significant tenderness to palpation in this area is observed. The skin is intact, without erythema or warmth of the shoulder area.\nInternal and external rotation of the shoulder are significantly impaired owing to pain, and there is weakness on rotator cuff testing. The patient is unable to lift his arm past 30° of forward flexion or abduction. The distal motor, sensation, and reflex examinations are otherwise unremarkable. The extremity pulses are normal. Besides a left arm brachiocephalic fistula with a positive bruit and palpable thrill, the remainder of the physical examination is unremarkable.\nFigure 2.\nFigure 2.\nFigure 3.\nFigure 3.\nThe patient is given oral analgesics for pain control, and a radiograph of the right shoulder is subsequently obtained. The right shoulder radiograph does not demonstrate fracture (Figure 2) but does show evidence of a high-riding humerus and advanced deformity of the humeral head. A large soft-tissue mass protrudes above the AC joint. The patient had laboratory examinations performed at an outside clinic that morning, which demonstrated a C-reactive protein level, erythrocyte sedimentation rate, and complete blood count within the reference range.\nAfter reviewing the above films, the orthopedist on call is consulted to assist with patient management. The orthopedic surgeon recommends MRI of the right shoulder (Figure 3) to provide further information on the shoulder mass.\n\n ## Figures\n\n **Figure 2.** \n \n\n**Figure 3.** \n \n\n\n*Page 2 of 6*",
"pagination": {
"current_page": 2,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 970907,
"choiceText": "Tumor",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 970909,
"choiceText": "Aneurysm",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 970911,
"choiceText": "AC joint cyst ",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 970913,
"choiceText": "Abscess\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 307169,
"questionText": "What is the diagnosis? <br><br><i>\r\nHint: Consider the history of a slowly enlarging mass for 2 months without associated fever, warmth, or erythema in an otherwise well patient.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 62-Year-Old Man With Worsening Shoulder Pain"
},
{
"authors": "Carolyn R. Lyon, MD",
"content": [
"An AC joint cyst was diagnosed on the basis of the patient's history and the clinical findings on physical examination. MRI of the right shoulder, which demonstrated a complete tear of the supraspinatus tendon with a 5-cm gap between the tendon and the insertion site, supported the diagnosis of AC joint cyst.",
"A large amount of fluid was present in the subacromial subdeltoid space. This fluid freely communicated with the glenohumeral joint. Abnormal signal intensity within the articular surface of the humeral head was consistent with avascular necrosis. The humeral head was subluxed superiorly. A large cystic mass projecting superiorly to the AC joint and measuring 7 cm was believed to communicate with the joint space.",
"The diagnosis of AC joint cyst was supported by palpation of a fluid-filled mass on the physical examination and by its location directly over the AC joint. The lack of warmth or erythema of the mass in a healthy-appearing, afebrile patient, coupled with the chronic, indolent nature of the mass, made a diagnosis of infection less likely. Fluid seen within the cyst on MRI made cancer unlikely. The presence of a geyser sign supported the diagnosis as well.",
"Finally, 110 mL of gelatinous bloody fluid was aspirated in the orthopedics clinic, providing almost complete relief of pain. This was followed by administration of cortisone into the subacromial space. The fluid showed no evidence of infection upon microscopic examination.",
"AC joint cysts are rare complications of complete rotator cuff tears. The first case report of these cysts occurring in association with rotator cuff tears was made by Dr Edward Craig in 1984, and again in 1986.[1,2] He presented a case of an 86-year-old man with left shoulder pain and a recurrent subcutaneous mass over the left AC joint for more than 2 years. On examination, the patient had a 4 x 4 cm mass over the AC joint on the left that was tender to palpation and transilluminated well. Both the supraspinatus and infraspinatus muscles exhibited atrophy, and the patient had difficulty with arm elevation and external rotation.",
"Dr Craig's patient had the mass drained repeatedly, but the fluid always reaccumulated several weeks after aspiration. He even underwent surgical resection of the mass, which failed to eradicate the cyst. Subsequent imaging studies confirmed a rotator cuff tear along with an AC joint cyst. Marino and colleagues[3] reviewed the literature in 1998 and could find only 16 accounts of AC joint cysts, of which 13 were associated with complete rotator cuff tears."
],
"date": "June 01, 2016",
"figures": [],
"markdown": "# A 62-Year-Old Man With Worsening Shoulder Pain\n\n **Authors:** Carolyn R. Lyon, MD \n **Date:** June 01, 2016\n\n ## Content\n\n An AC joint cyst was diagnosed on the basis of the patient's history and the clinical findings on physical examination. MRI of the right shoulder, which demonstrated a complete tear of the supraspinatus tendon with a 5-cm gap between the tendon and the insertion site, supported the diagnosis of AC joint cyst.\nA large amount of fluid was present in the subacromial subdeltoid space. This fluid freely communicated with the glenohumeral joint. Abnormal signal intensity within the articular surface of the humeral head was consistent with avascular necrosis. The humeral head was subluxed superiorly. A large cystic mass projecting superiorly to the AC joint and measuring 7 cm was believed to communicate with the joint space.\nThe diagnosis of AC joint cyst was supported by palpation of a fluid-filled mass on the physical examination and by its location directly over the AC joint. The lack of warmth or erythema of the mass in a healthy-appearing, afebrile patient, coupled with the chronic, indolent nature of the mass, made a diagnosis of infection less likely. Fluid seen within the cyst on MRI made cancer unlikely. The presence of a geyser sign supported the diagnosis as well.\nFinally, 110 mL of gelatinous bloody fluid was aspirated in the orthopedics clinic, providing almost complete relief of pain. This was followed by administration of cortisone into the subacromial space. The fluid showed no evidence of infection upon microscopic examination.\nAC joint cysts are rare complications of complete rotator cuff tears. The first case report of these cysts occurring in association with rotator cuff tears was made by Dr Edward Craig in 1984, and again in 1986.[1,2] He presented a case of an 86-year-old man with left shoulder pain and a recurrent subcutaneous mass over the left AC joint for more than 2 years. On examination, the patient had a 4 x 4 cm mass over the AC joint on the left that was tender to palpation and transilluminated well. Both the supraspinatus and infraspinatus muscles exhibited atrophy, and the patient had difficulty with arm elevation and external rotation.\nDr Craig's patient had the mass drained repeatedly, but the fluid always reaccumulated several weeks after aspiration. He even underwent surgical resection of the mass, which failed to eradicate the cyst. Subsequent imaging studies confirmed a rotator cuff tear along with an AC joint cyst. Marino and colleagues[3] reviewed the literature in 1998 and could find only 16 accounts of AC joint cysts, of which 13 were associated with complete rotator cuff tears.\n\n ## Figures\n\n \n*Page 3 of 6*",
"pagination": {
"current_page": 3,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 970907,
"choiceText": "Tumor",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 970909,
"choiceText": "Aneurysm",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 970911,
"choiceText": "AC joint cyst ",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 970913,
"choiceText": "Abscess\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 307169,
"questionText": "What is the diagnosis? <br><br><i>\r\nHint: Consider the history of a slowly enlarging mass for 2 months without associated fever, warmth, or erythema in an otherwise well patient.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 62-Year-Old Man With Worsening Shoulder Pain"
},
{
"authors": "Carolyn R. Lyon, MD",
"content": [
"The finding of an AC joint cyst on physical examination almost always signifies a complete rupture of the rotator cuff because an isolated AC joint cyst is extremely rare. The AC joint is a diarthrodial joint with fibrocartilage that lines the opposing bony surfaces, and with synovial fluid in the synovial cavity that lubricates the joint. The AC joint, along with the sternoclavicular joint, connects the axial skeleton to the upper extremity. Both the AC and coracoclavicular ligaments, as well as the trapezius and deltoid muscles, provide support and stabilization of the joint.",
"AC joint cysts generally occur in elderly patients who have a progressively enlarging subcutaneous mass on the shoulder. The cyst is typically painless in the beginning because the torn rotator cuff is initially compensated for functionally. It is the presence of this painless mass that often raises concern about a tumor.",
"The etiology of these cysts is mechanical. The supraspinatus tendon is located directly below the AC joint. Whenever the arm is elevated, this muscle contracts and the tendon comes to lie directly beneath the AC joint. Mechanical wear and tear (ie, osteophyte impingement) causes significant tearing of the rotator cuff. Because of shoulder instability, the humeral head migrates superiorly, which allows its articulation with the undersurface of the AC joint and causes narrowing of the subacromial space. Over time, chronic friction leads to AC joint capsule degeneration. A cyst forms when the AC capsule ruptures, allowing the AC and glenohumeral joints to communicate, with subsequent leakage of synovial fluid. Clinical evidence of extensive rotator cuff damage includes impairment of external rotation, difficulty with elevation of the arm, spinatus atrophy, and pain with passive arm elevation.[2,4]",
"On physical examination, an AC joint cyst is a palpable fluid-filled mass. Unenhanced MRI scan showing a large rotator cuff tear, a degenerated AC joint, and a large subcutaneous cyst adjacent to the AC joint is virtually pathognomonic for the disease.[5] Contrast administration into the glenohumeral joint results in the geyser sign, in which the fluid flows from the glenohumeral joint into the subacromial bursa, into the AC joint, and then into the cyst above.",
"The differential diagnosis of an AC joint cyst includes cancer, fracture, arthritis, infection, and aneurysm."
],
"date": "June 01, 2016",
"figures": [],
"markdown": "# A 62-Year-Old Man With Worsening Shoulder Pain\n\n **Authors:** Carolyn R. Lyon, MD \n **Date:** June 01, 2016\n\n ## Content\n\n The finding of an AC joint cyst on physical examination almost always signifies a complete rupture of the rotator cuff because an isolated AC joint cyst is extremely rare. The AC joint is a diarthrodial joint with fibrocartilage that lines the opposing bony surfaces, and with synovial fluid in the synovial cavity that lubricates the joint. The AC joint, along with the sternoclavicular joint, connects the axial skeleton to the upper extremity. Both the AC and coracoclavicular ligaments, as well as the trapezius and deltoid muscles, provide support and stabilization of the joint.\nAC joint cysts generally occur in elderly patients who have a progressively enlarging subcutaneous mass on the shoulder. The cyst is typically painless in the beginning because the torn rotator cuff is initially compensated for functionally. It is the presence of this painless mass that often raises concern about a tumor.\nThe etiology of these cysts is mechanical. The supraspinatus tendon is located directly below the AC joint. Whenever the arm is elevated, this muscle contracts and the tendon comes to lie directly beneath the AC joint. Mechanical wear and tear (ie, osteophyte impingement) causes significant tearing of the rotator cuff. Because of shoulder instability, the humeral head migrates superiorly, which allows its articulation with the undersurface of the AC joint and causes narrowing of the subacromial space. Over time, chronic friction leads to AC joint capsule degeneration. A cyst forms when the AC capsule ruptures, allowing the AC and glenohumeral joints to communicate, with subsequent leakage of synovial fluid. Clinical evidence of extensive rotator cuff damage includes impairment of external rotation, difficulty with elevation of the arm, spinatus atrophy, and pain with passive arm elevation.[2,4]\nOn physical examination, an AC joint cyst is a palpable fluid-filled mass. Unenhanced MRI scan showing a large rotator cuff tear, a degenerated AC joint, and a large subcutaneous cyst adjacent to the AC joint is virtually pathognomonic for the disease.[5] Contrast administration into the glenohumeral joint results in the geyser sign, in which the fluid flows from the glenohumeral joint into the subacromial bursa, into the AC joint, and then into the cyst above.\nThe differential diagnosis of an AC joint cyst includes cancer, fracture, arthritis, infection, and aneurysm.\n\n ## Figures\n\n \n*Page 4 of 6*",
"pagination": {
"current_page": 4,
"total_pages": 6
},
"questionnaire": [],
"title": "A 62-Year-Old Man With Worsening Shoulder Pain"
},
{
"authors": "Carolyn R. Lyon, MD",
"content": [
"Initial treatment is conservative and involves physical therapy (with judicious use of pain and anti-inflammatory medications) along with aspiration. When treating AC joint cysts, the integrity of the patient's rotator cuff must also be evaluated, because these cysts will generally recur if the root of the problem is not corrected (ie, rotator cuff tear).[6] MRI or a shoulder arthrogram should be obtained to evaluate the shoulder.",
"Noncontrast MRI is the imaging modality of choice to diagnose both the AC joint cyst and rotator cuff tear, although it will not demonstrate the presence of a communication between the joint and the cyst. A shoulder arthrogram, in contrast, will show the dye penetrating the bursa and invariably leaking into the AC joint when a full-thickness rotator cuff tear is present.",
"In patients with functional impairment or chronic shoulder pain in whom conservative management has failed, surgical treatment is recommended for both excision of the cyst and repair of the rotator cuff tear. Surgical options include shoulder hemiarthroplasty, acromioplasty, and rotator cuff repair along with cyst excision, as well as cyst resection, resection of the distal clavicle, and repair of the torn rotator cuff. With the latter procedure, removing the lateral end of the clavicle increases exposure of the damaged supraspinatus tendon.[7]",
"The patient in this case was told by his orthopedic surgeon he would most likely need total shoulder arthroplasty for definitive management. He was started on conservative therapy in the interim, with physical therapy and pain control, and a follow-up appointment in 2 months."
],
"date": "June 01, 2016",
"figures": [],
"markdown": "# A 62-Year-Old Man With Worsening Shoulder Pain\n\n **Authors:** Carolyn R. Lyon, MD \n **Date:** June 01, 2016\n\n ## Content\n\n Initial treatment is conservative and involves physical therapy (with judicious use of pain and anti-inflammatory medications) along with aspiration. When treating AC joint cysts, the integrity of the patient's rotator cuff must also be evaluated, because these cysts will generally recur if the root of the problem is not corrected (ie, rotator cuff tear).[6] MRI or a shoulder arthrogram should be obtained to evaluate the shoulder.\nNoncontrast MRI is the imaging modality of choice to diagnose both the AC joint cyst and rotator cuff tear, although it will not demonstrate the presence of a communication between the joint and the cyst. A shoulder arthrogram, in contrast, will show the dye penetrating the bursa and invariably leaking into the AC joint when a full-thickness rotator cuff tear is present.\nIn patients with functional impairment or chronic shoulder pain in whom conservative management has failed, surgical treatment is recommended for both excision of the cyst and repair of the rotator cuff tear. Surgical options include shoulder hemiarthroplasty, acromioplasty, and rotator cuff repair along with cyst excision, as well as cyst resection, resection of the distal clavicle, and repair of the torn rotator cuff. With the latter procedure, removing the lateral end of the clavicle increases exposure of the damaged supraspinatus tendon.[7]\nThe patient in this case was told by his orthopedic surgeon he would most likely need total shoulder arthroplasty for definitive management. He was started on conservative therapy in the interim, with physical therapy and pain control, and a follow-up appointment in 2 months.\n\n ## Figures\n\n \n*Page 5 of 6*",
"pagination": {
"current_page": 5,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 970915,
"choiceText": "Conventional radiography",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 970917,
"choiceText": "CT ",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 970919,
"choiceText": "MRI",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 970921,
"choiceText": "Needle aspiration\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "MRI is the imaging modality of choice for the diagnosis of an AC joint cyst. MRI can demonstrate the AC joint cyst and also provide valuable information on the integrity and stability of the rotator cuff muscles. Both radiography and CT are good for looking at the bones but give little information on the musculature and ligaments. Needle aspiration has no role in the diagnosis of AC joint cysts, but is essential to rule out infection. An AC joint cyst is diagnosed after life- and limb-threatening pathologies, such as infection, are excluded.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 307171,
"questionText": "You are examining an elderly patient who has difficulty elevating and externally rotating his arm. He is afebrile; has had no trauma; and has a large, painful, fluctuant swelling on his lateral shoulder that has developed over a period of months. You suspect an AC joint cyst. Which of the following examinations would most likely definitively diagnose an AC joint cyst?\r\n",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 970923,
"choiceText": "Systemic corticosteroid",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 970925,
"choiceText": "Resection of the mass",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 970927,
"choiceText": "Antibiotics",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 970929,
"choiceText": "Right shoulder hemiarthroplasty\r\n",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "This patient has an AC joint cyst overlying the right shoulder and a probable complete rotator cuff tear. The overwhelming majority of AC joint cysts occur in association with a full-thickness tear of the rotator cuff; therefore, if the rotator cuff is not repaired, the AC joint cyst will continue to recur despite measures taken to eradicate it (ie, drainage, resection).",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 307173,
"questionText": "A 69-year-old woman presents with a mass on her right shoulder that has been getting progressively larger over the past 5 weeks. She also reports a history of right shoulder weakness and increasing pain over the past 6 months, along with an inability to elevate the right arm. On examination, there is a palpable, fluid-filled, 3 × 4 cm mass on the right shoulder directly overlying the AC joint that transilluminates. The patient exhibits weakness of external rotation and cannot fully elevate her right arm. Assuming that conservative treatment with physical therapy, aspiration, pain control, and injections fails, what is the definitive management for this patient's condition?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 62-Year-Old Man With Worsening Shoulder Pain"
},
{
"authors": "Carolyn R. Lyon, MD",
"content": [],
"date": "June 01, 2016",
"figures": [],
"markdown": "# A 62-Year-Old Man With Worsening Shoulder Pain\n\n **Authors:** Carolyn R. Lyon, MD \n **Date:** June 01, 2016\n\n ## Content\n\n \n\n ## Figures\n\n \n*Page 6 of 6*",
"pagination": {
"current_page": 6,
"total_pages": 6
},
"questionnaire": [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 970915,
"choiceText": "Conventional radiography",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 970917,
"choiceText": "CT ",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 970919,
"choiceText": "MRI",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 970921,
"choiceText": "Needle aspiration\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "MRI is the imaging modality of choice for the diagnosis of an AC joint cyst. MRI can demonstrate the AC joint cyst and also provide valuable information on the integrity and stability of the rotator cuff muscles. Both radiography and CT are good for looking at the bones but give little information on the musculature and ligaments. Needle aspiration has no role in the diagnosis of AC joint cysts, but is essential to rule out infection. An AC joint cyst is diagnosed after life- and limb-threatening pathologies, such as infection, are excluded.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 307171,
"questionText": "You are examining an elderly patient who has difficulty elevating and externally rotating his arm. He is afebrile; has had no trauma; and has a large, painful, fluctuant swelling on his lateral shoulder that has developed over a period of months. You suspect an AC joint cyst. Which of the following examinations would most likely definitively diagnose an AC joint cyst?\r\n",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 970923,
"choiceText": "Systemic corticosteroid",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 970925,
"choiceText": "Resection of the mass",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 970927,
"choiceText": "Antibiotics",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 970929,
"choiceText": "Right shoulder hemiarthroplasty\r\n",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "This patient has an AC joint cyst overlying the right shoulder and a probable complete rotator cuff tear. The overwhelming majority of AC joint cysts occur in association with a full-thickness tear of the rotator cuff; therefore, if the rotator cuff is not repaired, the AC joint cyst will continue to recur despite measures taken to eradicate it (ie, drainage, resection).",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 307173,
"questionText": "A 69-year-old woman presents with a mass on her right shoulder that has been getting progressively larger over the past 5 weeks. She also reports a history of right shoulder weakness and increasing pain over the past 6 months, along with an inability to elevate the right arm. On examination, there is a palpable, fluid-filled, 3 × 4 cm mass on the right shoulder directly overlying the AC joint that transilluminates. The patient exhibits weakness of external rotation and cannot fully elevate her right arm. Assuming that conservative treatment with physical therapy, aspiration, pain control, and injections fails, what is the definitive management for this patient's condition?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
],
"title": "A 62-Year-Old Man With Worsening Shoulder Pain"
}
] | [
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 970907,
"choiceText": "Tumor",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 970909,
"choiceText": "Aneurysm",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 970911,
"choiceText": "AC joint cyst ",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 970913,
"choiceText": "Abscess\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": null,
"displayOrder": 1,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 307169,
"questionText": "What is the diagnosis? <br><br><i>\r\nHint: Consider the history of a slowly enlarging mass for 2 months without associated fever, warmth, or erythema in an otherwise well patient.</i>",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 970915,
"choiceText": "Conventional radiography",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 970917,
"choiceText": "CT ",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 970919,
"choiceText": "MRI",
"correct": true,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 970921,
"choiceText": "Needle aspiration\r\n",
"correct": false,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "MRI is the imaging modality of choice for the diagnosis of an AC joint cyst. MRI can demonstrate the AC joint cyst and also provide valuable information on the integrity and stability of the rotator cuff muscles. Both radiography and CT are good for looking at the bones but give little information on the musculature and ligaments. Needle aspiration has no role in the diagnosis of AC joint cysts, but is essential to rule out infection. An AC joint cyst is diagnosed after life- and limb-threatening pathologies, such as infection, are excluded.",
"displayOrder": 2,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 307171,
"questionText": "You are examining an elderly patient who has difficulty elevating and externally rotating his arm. He is afebrile; has had no trauma; and has a large, painful, fluctuant swelling on his lateral shoulder that has developed over a period of months. You suspect an AC joint cyst. Which of the following examinations would most likely definitively diagnose an AC joint cyst?\r\n",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
},
{
"answered": false,
"answeredCorrectly": false,
"branch": false,
"choices": [
{
"branchPath": null,
"choiceId": 970923,
"choiceText": "Systemic corticosteroid",
"correct": false,
"displayOrder": 1,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 970925,
"choiceText": "Resection of the mass",
"correct": false,
"displayOrder": 2,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 970927,
"choiceText": "Antibiotics",
"correct": false,
"displayOrder": 3,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
},
{
"branchPath": null,
"choiceId": 970929,
"choiceText": "Right shoulder hemiarthroplasty\r\n",
"correct": true,
"displayOrder": 4,
"explanation": null,
"hideLabel": false,
"selected": false,
"totalAbsoluteResponseCount": 0,
"totalResponses": "0"
}
],
"discussion": "This patient has an AC joint cyst overlying the right shoulder and a probable complete rotator cuff tear. The overwhelming majority of AC joint cysts occur in association with a full-thickness tear of the rotator cuff; therefore, if the rotator cuff is not repaired, the AC joint cyst will continue to recur despite measures taken to eradicate it (ie, drainage, resection).",
"displayOrder": 3,
"displayType": 1,
"horizontal": false,
"introduction": null,
"matrixQuestions": [],
"mutuallyExclusive": false,
"poll": true,
"professions": [],
"questionId": 307173,
"questionText": "A 69-year-old woman presents with a mass on her right shoulder that has been getting progressively larger over the past 5 weeks. She also reports a history of right shoulder weakness and increasing pain over the past 6 months, along with an inability to elevate the right arm. On examination, there is a palpable, fluid-filled, 3 × 4 cm mass on the right shoulder directly overlying the AC joint that transilluminates. The patient exhibits weakness of external rotation and cannot fully elevate her right arm. Assuming that conservative treatment with physical therapy, aspiration, pain control, and injections fails, what is the definitive management for this patient's condition?",
"questionTypeId": 1,
"required": false,
"responseText": null,
"score": false,
"showAnsTable": true,
"showQuestion": true,
"showResult": true,
"specialties": [],
"totalResponses": 0,
"viewResults": false
}
] |
Subsets and Splits
No saved queries yet
Save your SQL queries to embed, download, and access them later. Queries will appear here once saved.